User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
Case series suggests biologics, JAK inhibitors safe during pandemic
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Yale’s COVID-19 inpatient protocol: Hydroxychloroquine plus/minus tocilizumab
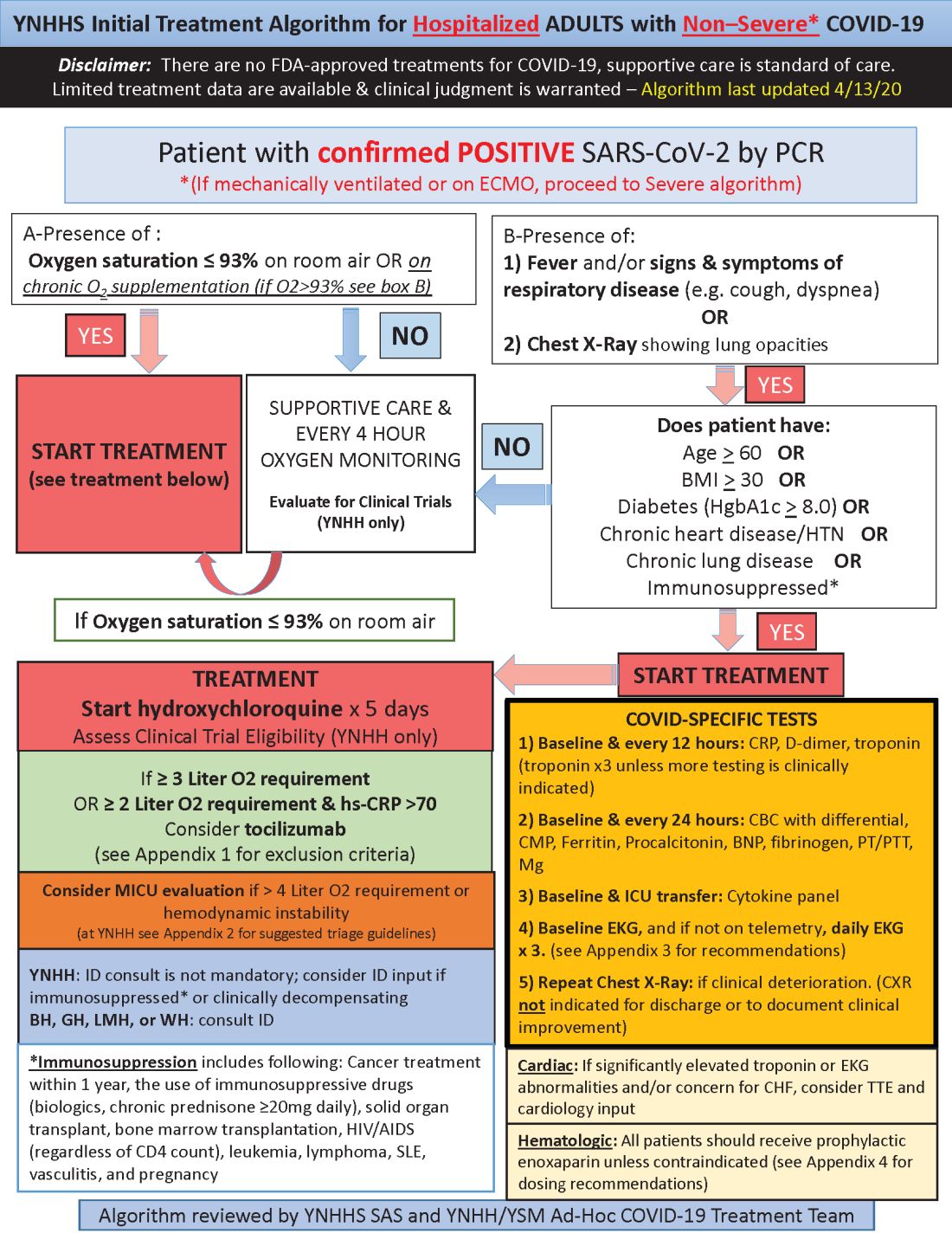
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.
Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
[email protected]
This article was updated with the latest treatment algorithm on 5/6/2020.
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
[email protected]
This article was updated with the latest treatment algorithm on 5/6/2020.
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
[email protected]
This article was updated with the latest treatment algorithm on 5/6/2020.
Evidence on spironolactone safety, COVID-19 reassuring for acne patients
according to a report in the Journal of the American Academy of Dermatology.
The virus needs androgens to infect cells, and uses androgen-dependent transmembrane protease serine 2 to prime viral protein spikes to anchor onto ACE2 receptors. Without that step, the virus can’t enter cells. Androgens are the only known activator in humans, so androgen blockers like spironolactone probably short-circuit the process, said the report’s lead author Carlos Wambier, MD, PhD, of the department of dermatology at Brown University, Providence, R.I (J Am Acad Dermatol. 2020 Apr 10. doi: 10.1016/j.jaad.2020.04.032).
The lack of androgens could be a possible explanation as to why mortality is so rare among children with COVID-19, and why fatalities among men are higher than among women with COVID-19, he said in an interview.
There are a lot of androgen blocker candidates, but he said spironolactone – a mainstay of acne treatment – might well be the best for the pandemic because of its concomitant lung and heart benefits.
The message counters a post on Instagram in March from a New York City dermatologist in private practice, Ellen Marmur, MD, that raised a question about spironolactone. Concerned about the situation in New York, she reviewed the literature and found a 2005 study that reported that macrophages drawn from 10 heart failure patients had increased ACE2 activity and increased messenger RNA expression after the subjects had been on spironolactone 25 mg per day for a month.
In an interview, she said she has been sharing her concerns with patients on spironolactone and offering them an alternative, such as minocycline, until this issue is better elucidated. To date, she has had one young patient who declined to switch to another treatment, and about six patients who were comfortable switching to another treatment for 1-2 months. She said that she is “clearly cautious yet uncertain about the influence of chronic spironolactone for acne on COVID infection in acne patients,” and that eventually she would be interested in seeing retrospective data on outcomes of patients on spironolactone for hypertension versus acne during the pandemic.
Dr. Marmur’s post was spread on social media and was picked up by a few online news outlets.
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said he’s been addressing concerns about spironolactone in educational webinars because of it.
He tells his audience that “you can’t make any claims” for COVID-19 based on the 2005 study. It was a small clinical study in heart failure patients and only assessed ACE2 expression on macrophages, not respiratory, cardiac, or mesangial cells, which are the relevant locations for viral invasion and damage. In fact, there are studies showing that spironolactone reduced ACE2 in renal mesangial cells. Also of note, spironolactone has been used with no indication of virus risk since the 1950s, he pointed out. The American Academy of Dermatology has not said to stop spironolactone.
At least one study is underway to see if spironolactone is beneficial: 100 mg twice a day for 5 days is being pitted against placebo in Turkey among people hospitalized with acute respiratory distress. The study will evaluate the effect of spironolactone on oxygenation.
“There’s no evidence to show spironolactone can increase mortality levels,” Dr. Wambier said. He is using it more now in patients with acne – a sign of androgen hyperactivity – convinced that it will protect against COVID-19. He even started his sister on it to help with androgenic hair loss, and maybe the virus.
Observations in Spain – increased prevalence of androgenic alopecia among hospitalized patients – support the androgen link; 29 of 41 men (71%) hospitalized with bilateral pneumonia had male pattern baldness, which was severe in 16 (39%), according to a recent report (J Cosmet Dermatol. 2020 Apr 16. doi: 10.1111/jocd.13443). The expected prevalence in a similar age-matched population is 31%-53%.
“Based on the scientific rationale combined with this preliminary observation, we believe investigating the potential association between androgens and COVID‐19 disease severity warrants further merit,” concluded the authors, who included Dr. Wambier, and other dermatologists from the United States, as well as Spain, Australia, Croatia, and Switzerland. “If such an association is confirmed, antiandrogens could be evaluated as a potential treatment for COVID‐19 infection,” they wrote.
The numbers are holding up in a larger series from three Spanish hospitals, and also showing a greater prevalence of androgenic hair loss among hospitalized women, Dr. Wambier said in the interview.
Authors of the two studies include an employee of Applied Biology. No conflicts were declared in the Journal of Cosmetic Dermatology study; no disclosures were listed in the JAAD study. Dr. Friedman had no disclosures.
according to a report in the Journal of the American Academy of Dermatology.
The virus needs androgens to infect cells, and uses androgen-dependent transmembrane protease serine 2 to prime viral protein spikes to anchor onto ACE2 receptors. Without that step, the virus can’t enter cells. Androgens are the only known activator in humans, so androgen blockers like spironolactone probably short-circuit the process, said the report’s lead author Carlos Wambier, MD, PhD, of the department of dermatology at Brown University, Providence, R.I (J Am Acad Dermatol. 2020 Apr 10. doi: 10.1016/j.jaad.2020.04.032).
The lack of androgens could be a possible explanation as to why mortality is so rare among children with COVID-19, and why fatalities among men are higher than among women with COVID-19, he said in an interview.
There are a lot of androgen blocker candidates, but he said spironolactone – a mainstay of acne treatment – might well be the best for the pandemic because of its concomitant lung and heart benefits.
The message counters a post on Instagram in March from a New York City dermatologist in private practice, Ellen Marmur, MD, that raised a question about spironolactone. Concerned about the situation in New York, she reviewed the literature and found a 2005 study that reported that macrophages drawn from 10 heart failure patients had increased ACE2 activity and increased messenger RNA expression after the subjects had been on spironolactone 25 mg per day for a month.
In an interview, she said she has been sharing her concerns with patients on spironolactone and offering them an alternative, such as minocycline, until this issue is better elucidated. To date, she has had one young patient who declined to switch to another treatment, and about six patients who were comfortable switching to another treatment for 1-2 months. She said that she is “clearly cautious yet uncertain about the influence of chronic spironolactone for acne on COVID infection in acne patients,” and that eventually she would be interested in seeing retrospective data on outcomes of patients on spironolactone for hypertension versus acne during the pandemic.
Dr. Marmur’s post was spread on social media and was picked up by a few online news outlets.
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said he’s been addressing concerns about spironolactone in educational webinars because of it.
He tells his audience that “you can’t make any claims” for COVID-19 based on the 2005 study. It was a small clinical study in heart failure patients and only assessed ACE2 expression on macrophages, not respiratory, cardiac, or mesangial cells, which are the relevant locations for viral invasion and damage. In fact, there are studies showing that spironolactone reduced ACE2 in renal mesangial cells. Also of note, spironolactone has been used with no indication of virus risk since the 1950s, he pointed out. The American Academy of Dermatology has not said to stop spironolactone.
At least one study is underway to see if spironolactone is beneficial: 100 mg twice a day for 5 days is being pitted against placebo in Turkey among people hospitalized with acute respiratory distress. The study will evaluate the effect of spironolactone on oxygenation.
“There’s no evidence to show spironolactone can increase mortality levels,” Dr. Wambier said. He is using it more now in patients with acne – a sign of androgen hyperactivity – convinced that it will protect against COVID-19. He even started his sister on it to help with androgenic hair loss, and maybe the virus.
Observations in Spain – increased prevalence of androgenic alopecia among hospitalized patients – support the androgen link; 29 of 41 men (71%) hospitalized with bilateral pneumonia had male pattern baldness, which was severe in 16 (39%), according to a recent report (J Cosmet Dermatol. 2020 Apr 16. doi: 10.1111/jocd.13443). The expected prevalence in a similar age-matched population is 31%-53%.
“Based on the scientific rationale combined with this preliminary observation, we believe investigating the potential association between androgens and COVID‐19 disease severity warrants further merit,” concluded the authors, who included Dr. Wambier, and other dermatologists from the United States, as well as Spain, Australia, Croatia, and Switzerland. “If such an association is confirmed, antiandrogens could be evaluated as a potential treatment for COVID‐19 infection,” they wrote.
The numbers are holding up in a larger series from three Spanish hospitals, and also showing a greater prevalence of androgenic hair loss among hospitalized women, Dr. Wambier said in the interview.
Authors of the two studies include an employee of Applied Biology. No conflicts were declared in the Journal of Cosmetic Dermatology study; no disclosures were listed in the JAAD study. Dr. Friedman had no disclosures.
according to a report in the Journal of the American Academy of Dermatology.
The virus needs androgens to infect cells, and uses androgen-dependent transmembrane protease serine 2 to prime viral protein spikes to anchor onto ACE2 receptors. Without that step, the virus can’t enter cells. Androgens are the only known activator in humans, so androgen blockers like spironolactone probably short-circuit the process, said the report’s lead author Carlos Wambier, MD, PhD, of the department of dermatology at Brown University, Providence, R.I (J Am Acad Dermatol. 2020 Apr 10. doi: 10.1016/j.jaad.2020.04.032).
The lack of androgens could be a possible explanation as to why mortality is so rare among children with COVID-19, and why fatalities among men are higher than among women with COVID-19, he said in an interview.
There are a lot of androgen blocker candidates, but he said spironolactone – a mainstay of acne treatment – might well be the best for the pandemic because of its concomitant lung and heart benefits.
The message counters a post on Instagram in March from a New York City dermatologist in private practice, Ellen Marmur, MD, that raised a question about spironolactone. Concerned about the situation in New York, she reviewed the literature and found a 2005 study that reported that macrophages drawn from 10 heart failure patients had increased ACE2 activity and increased messenger RNA expression after the subjects had been on spironolactone 25 mg per day for a month.
In an interview, she said she has been sharing her concerns with patients on spironolactone and offering them an alternative, such as minocycline, until this issue is better elucidated. To date, she has had one young patient who declined to switch to another treatment, and about six patients who were comfortable switching to another treatment for 1-2 months. She said that she is “clearly cautious yet uncertain about the influence of chronic spironolactone for acne on COVID infection in acne patients,” and that eventually she would be interested in seeing retrospective data on outcomes of patients on spironolactone for hypertension versus acne during the pandemic.
Dr. Marmur’s post was spread on social media and was picked up by a few online news outlets.
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said he’s been addressing concerns about spironolactone in educational webinars because of it.
He tells his audience that “you can’t make any claims” for COVID-19 based on the 2005 study. It was a small clinical study in heart failure patients and only assessed ACE2 expression on macrophages, not respiratory, cardiac, or mesangial cells, which are the relevant locations for viral invasion and damage. In fact, there are studies showing that spironolactone reduced ACE2 in renal mesangial cells. Also of note, spironolactone has been used with no indication of virus risk since the 1950s, he pointed out. The American Academy of Dermatology has not said to stop spironolactone.
At least one study is underway to see if spironolactone is beneficial: 100 mg twice a day for 5 days is being pitted against placebo in Turkey among people hospitalized with acute respiratory distress. The study will evaluate the effect of spironolactone on oxygenation.
“There’s no evidence to show spironolactone can increase mortality levels,” Dr. Wambier said. He is using it more now in patients with acne – a sign of androgen hyperactivity – convinced that it will protect against COVID-19. He even started his sister on it to help with androgenic hair loss, and maybe the virus.
Observations in Spain – increased prevalence of androgenic alopecia among hospitalized patients – support the androgen link; 29 of 41 men (71%) hospitalized with bilateral pneumonia had male pattern baldness, which was severe in 16 (39%), according to a recent report (J Cosmet Dermatol. 2020 Apr 16. doi: 10.1111/jocd.13443). The expected prevalence in a similar age-matched population is 31%-53%.
“Based on the scientific rationale combined with this preliminary observation, we believe investigating the potential association between androgens and COVID‐19 disease severity warrants further merit,” concluded the authors, who included Dr. Wambier, and other dermatologists from the United States, as well as Spain, Australia, Croatia, and Switzerland. “If such an association is confirmed, antiandrogens could be evaluated as a potential treatment for COVID‐19 infection,” they wrote.
The numbers are holding up in a larger series from three Spanish hospitals, and also showing a greater prevalence of androgenic hair loss among hospitalized women, Dr. Wambier said in the interview.
Authors of the two studies include an employee of Applied Biology. No conflicts were declared in the Journal of Cosmetic Dermatology study; no disclosures were listed in the JAAD study. Dr. Friedman had no disclosures.
Global registry collects data on pediatric cancer patients with COVID-19
A week after its launch, a new online registry has information on more than 2 dozen cases of pediatric cancer patients with COVID-19.
The registry, created by St. Jude Children’s Research Hospital in Memphis, Tenn., and the International Society of Paediatric Oncology, is the first global COVID-19 registry for children with cancer.
Clinicians enter cases through an online form, then complete 30- and 60-day follow-up reports via email. St. Jude compiles the data and releases regularly updated summaries, including the number of cases by country and by treatment. Eventually, researchers might be able to apply for access to the raw data for their own projects.
It’s all free of charge, said Carlos Rodriguez-Galindo, MD, chair of the department of global pediatric medicine at St. Jude.
The registry is hosted on a website called “The Global COVID-19 Observatory and Resource Center for Childhood Cancer.” In addition to the registry, the website has a resource library and a discussion forum where clinicians can exchange information.
Other COVID-19 cancer registries have launched recently as well, including registries created by the COVID-19 and Cancer Consortium and the American Society of Clinical Oncology. The idea is to compile and disseminate best practices and other information quickly amid concerns that immunosuppressed cancer patients might be especially vulnerable.
So far, that doesn’t seem to be the case for children. Their relative protection from the disease and serious complications seems to hold even when they have cancer, Dr. Rodriguez-Galindo said.
“When we talk with the people in China” the number of COVID-19 cases in children with cancer is “very small,” he said. There are a couple of reports from Europe finding the same thing, and the severity of COVID-19 also “seems to be lower than you would expect,” he added.
The new registry will help better define the situation, according to Dr. Rodriguez-Galindo.
St. Jude is working with European countries that have their own national pediatric cancer COVID-19 registries to share information. St. Jude’s ties with lower- and middle-income countries, established via the department of global pediatric medicine, should help populate the global registry as well.
Furthermore, international surveys are being planned to gauge the impact of COVID-19 on children with cancer and their access to care.
A week after its launch, a new online registry has information on more than 2 dozen cases of pediatric cancer patients with COVID-19.
The registry, created by St. Jude Children’s Research Hospital in Memphis, Tenn., and the International Society of Paediatric Oncology, is the first global COVID-19 registry for children with cancer.
Clinicians enter cases through an online form, then complete 30- and 60-day follow-up reports via email. St. Jude compiles the data and releases regularly updated summaries, including the number of cases by country and by treatment. Eventually, researchers might be able to apply for access to the raw data for their own projects.
It’s all free of charge, said Carlos Rodriguez-Galindo, MD, chair of the department of global pediatric medicine at St. Jude.
The registry is hosted on a website called “The Global COVID-19 Observatory and Resource Center for Childhood Cancer.” In addition to the registry, the website has a resource library and a discussion forum where clinicians can exchange information.
Other COVID-19 cancer registries have launched recently as well, including registries created by the COVID-19 and Cancer Consortium and the American Society of Clinical Oncology. The idea is to compile and disseminate best practices and other information quickly amid concerns that immunosuppressed cancer patients might be especially vulnerable.
So far, that doesn’t seem to be the case for children. Their relative protection from the disease and serious complications seems to hold even when they have cancer, Dr. Rodriguez-Galindo said.
“When we talk with the people in China” the number of COVID-19 cases in children with cancer is “very small,” he said. There are a couple of reports from Europe finding the same thing, and the severity of COVID-19 also “seems to be lower than you would expect,” he added.
The new registry will help better define the situation, according to Dr. Rodriguez-Galindo.
St. Jude is working with European countries that have their own national pediatric cancer COVID-19 registries to share information. St. Jude’s ties with lower- and middle-income countries, established via the department of global pediatric medicine, should help populate the global registry as well.
Furthermore, international surveys are being planned to gauge the impact of COVID-19 on children with cancer and their access to care.
A week after its launch, a new online registry has information on more than 2 dozen cases of pediatric cancer patients with COVID-19.
The registry, created by St. Jude Children’s Research Hospital in Memphis, Tenn., and the International Society of Paediatric Oncology, is the first global COVID-19 registry for children with cancer.
Clinicians enter cases through an online form, then complete 30- and 60-day follow-up reports via email. St. Jude compiles the data and releases regularly updated summaries, including the number of cases by country and by treatment. Eventually, researchers might be able to apply for access to the raw data for their own projects.
It’s all free of charge, said Carlos Rodriguez-Galindo, MD, chair of the department of global pediatric medicine at St. Jude.
The registry is hosted on a website called “The Global COVID-19 Observatory and Resource Center for Childhood Cancer.” In addition to the registry, the website has a resource library and a discussion forum where clinicians can exchange information.
Other COVID-19 cancer registries have launched recently as well, including registries created by the COVID-19 and Cancer Consortium and the American Society of Clinical Oncology. The idea is to compile and disseminate best practices and other information quickly amid concerns that immunosuppressed cancer patients might be especially vulnerable.
So far, that doesn’t seem to be the case for children. Their relative protection from the disease and serious complications seems to hold even when they have cancer, Dr. Rodriguez-Galindo said.
“When we talk with the people in China” the number of COVID-19 cases in children with cancer is “very small,” he said. There are a couple of reports from Europe finding the same thing, and the severity of COVID-19 also “seems to be lower than you would expect,” he added.
The new registry will help better define the situation, according to Dr. Rodriguez-Galindo.
St. Jude is working with European countries that have their own national pediatric cancer COVID-19 registries to share information. St. Jude’s ties with lower- and middle-income countries, established via the department of global pediatric medicine, should help populate the global registry as well.
Furthermore, international surveys are being planned to gauge the impact of COVID-19 on children with cancer and their access to care.
Six million childhood cancer deaths could be prevented over the next 30 years
Unless global investments are made to improve care worldwide, 11.1 million children will die from cancer over the next 30 years; 9.3 million of them (84%) will be in low- and lower-middle–income countries, according to a report in Lancet Oncology.
The report suggests that one in two new cases of childhood cancer are undiagnosed in low- and middle-income countries. If that trend continues, the number of children with cancer who are never diagnosed over the next 3 decades will exceed the number of those who are diagnosed.
Childhood cancer “is not complex, expensive, difficult to diagnose, or complicated to treat,” yet there’s a “worldwide inequity and a bleak picture for children with cancer” in low-income and middle-income countries, according to the report authors. The authors are 44 oncologists, pediatricians, and global health experts from around the world, led by Rifat Atun, MD, a professor of global health systems at Harvard University in Boston.
“For too long, there has been a widespread misconception that caring for children with cancer in low- and middle-income countries is expensive, unattainable, and inappropriate because of competing health priorities. Nothing could be further from the truth,” Dr. Atun said in a statement.
Dr. Atun and colleagues argued that the burden of childhood cancer “could be vastly reduced with new funding to scale up cost-effective interventions.” In fact, the authors estimated that scaling up interventions could prevent 6.2 million childhood cancer deaths between 2020 and 2050.
The reduction in deaths would translate to 318.4 million life-years gained, which would, in turn, translate to a global lifetime productivity gain of $2,580 billion, four times greater than the cumulative cost of $594 billion. This would mean a net return of $3 for every $1 spent.
Potential funders include governments, professional organizations, philanthropic groups, and industry, according to the authors. They also laid out the following six-pronged framework on how to proceed:
- Include childhood cancer in universal health coverage.
- Develop national cancer control plans for low-income and middle-income countries.
- End out-of-pocket costs for childhood cancer.
- Establish national and regional cancer networks to increase access to care.
- Expand population-based cancer registries to include children.
- Invest in research and innovations in low-income and middle-income countries.
“Success will be attained through political leadership, global solidarity, collective action, inclusive participation of all major stakeholders, and alignment of national and global efforts to expand access to effective and sustainable care for children with cancer,” the authors wrote.
No funding sources were reported. The authors didn’t have any disclosures.
SOURCE: Atun R et al. Lancet Oncol. 2020 Apr;21(4):e185-224.
Unless global investments are made to improve care worldwide, 11.1 million children will die from cancer over the next 30 years; 9.3 million of them (84%) will be in low- and lower-middle–income countries, according to a report in Lancet Oncology.
The report suggests that one in two new cases of childhood cancer are undiagnosed in low- and middle-income countries. If that trend continues, the number of children with cancer who are never diagnosed over the next 3 decades will exceed the number of those who are diagnosed.
Childhood cancer “is not complex, expensive, difficult to diagnose, or complicated to treat,” yet there’s a “worldwide inequity and a bleak picture for children with cancer” in low-income and middle-income countries, according to the report authors. The authors are 44 oncologists, pediatricians, and global health experts from around the world, led by Rifat Atun, MD, a professor of global health systems at Harvard University in Boston.
“For too long, there has been a widespread misconception that caring for children with cancer in low- and middle-income countries is expensive, unattainable, and inappropriate because of competing health priorities. Nothing could be further from the truth,” Dr. Atun said in a statement.
Dr. Atun and colleagues argued that the burden of childhood cancer “could be vastly reduced with new funding to scale up cost-effective interventions.” In fact, the authors estimated that scaling up interventions could prevent 6.2 million childhood cancer deaths between 2020 and 2050.
The reduction in deaths would translate to 318.4 million life-years gained, which would, in turn, translate to a global lifetime productivity gain of $2,580 billion, four times greater than the cumulative cost of $594 billion. This would mean a net return of $3 for every $1 spent.
Potential funders include governments, professional organizations, philanthropic groups, and industry, according to the authors. They also laid out the following six-pronged framework on how to proceed:
- Include childhood cancer in universal health coverage.
- Develop national cancer control plans for low-income and middle-income countries.
- End out-of-pocket costs for childhood cancer.
- Establish national and regional cancer networks to increase access to care.
- Expand population-based cancer registries to include children.
- Invest in research and innovations in low-income and middle-income countries.
“Success will be attained through political leadership, global solidarity, collective action, inclusive participation of all major stakeholders, and alignment of national and global efforts to expand access to effective and sustainable care for children with cancer,” the authors wrote.
No funding sources were reported. The authors didn’t have any disclosures.
SOURCE: Atun R et al. Lancet Oncol. 2020 Apr;21(4):e185-224.
Unless global investments are made to improve care worldwide, 11.1 million children will die from cancer over the next 30 years; 9.3 million of them (84%) will be in low- and lower-middle–income countries, according to a report in Lancet Oncology.
The report suggests that one in two new cases of childhood cancer are undiagnosed in low- and middle-income countries. If that trend continues, the number of children with cancer who are never diagnosed over the next 3 decades will exceed the number of those who are diagnosed.
Childhood cancer “is not complex, expensive, difficult to diagnose, or complicated to treat,” yet there’s a “worldwide inequity and a bleak picture for children with cancer” in low-income and middle-income countries, according to the report authors. The authors are 44 oncologists, pediatricians, and global health experts from around the world, led by Rifat Atun, MD, a professor of global health systems at Harvard University in Boston.
“For too long, there has been a widespread misconception that caring for children with cancer in low- and middle-income countries is expensive, unattainable, and inappropriate because of competing health priorities. Nothing could be further from the truth,” Dr. Atun said in a statement.
Dr. Atun and colleagues argued that the burden of childhood cancer “could be vastly reduced with new funding to scale up cost-effective interventions.” In fact, the authors estimated that scaling up interventions could prevent 6.2 million childhood cancer deaths between 2020 and 2050.
The reduction in deaths would translate to 318.4 million life-years gained, which would, in turn, translate to a global lifetime productivity gain of $2,580 billion, four times greater than the cumulative cost of $594 billion. This would mean a net return of $3 for every $1 spent.
Potential funders include governments, professional organizations, philanthropic groups, and industry, according to the authors. They also laid out the following six-pronged framework on how to proceed:
- Include childhood cancer in universal health coverage.
- Develop national cancer control plans for low-income and middle-income countries.
- End out-of-pocket costs for childhood cancer.
- Establish national and regional cancer networks to increase access to care.
- Expand population-based cancer registries to include children.
- Invest in research and innovations in low-income and middle-income countries.
“Success will be attained through political leadership, global solidarity, collective action, inclusive participation of all major stakeholders, and alignment of national and global efforts to expand access to effective and sustainable care for children with cancer,” the authors wrote.
No funding sources were reported. The authors didn’t have any disclosures.
SOURCE: Atun R et al. Lancet Oncol. 2020 Apr;21(4):e185-224.
FROM LANCET ONCOLOGY
Protean manifestations of COVID-19: “Our ignorance is profound”
Although a cause-and-effect relationship is unknown, people with the virus have presented with or developed heart disease, acute liver injury, ongoing GI issues, skin manifestations, neurologic damage, and other problems, especially among sicker people.
For example, French physicians described an association with encephalopathy, agitation, confusion, and corticospinal tract signs among 58 people hospitalized with acute respiratory distress (N Engl J Med. 2020 Apr 15. doi: 10.1056/NEJMc2008597).
In particular, Yale New Haven (Conn.) Hospital is dealing with unexpected complications up close. Almost half of the beds there are occupied by COVID-19 patients. Over 100 people are in the ICU, and almost 70 intubated. Of the more than 750 COVID admissions so far, only about 350 have been discharged. “Even in a bad flu season, you never see something like this; it’s just unheard of,” said Harlan Krumholz, MD, a Yale cardiologist and professor of medicine helping lead the efforts there.
Kidney injuries prominent
“When they get to the ICU, we are seeing lots of people with acute kidney injuries; lots of people developing endocrine problems; people having blood sugar control issues, coagulation issues, blood clots. We are just waking up to the wide range of ways this virus can affect people. Our ignorance is profound,” Dr. Krumholz said, but physicians “recognize that this thing has the capability of attacking almost every single organ system, and it may or may not present with respiratory symptoms.”
It’s a similar story at Mt. Sinai South Nassau, a hospital in Oceanside, N.Y. “We’ve seen a lot of renal injury in people having complications, a lot of acute dialysis,” but it’s unclear how much is caused by the virus and how much is simply because people are so sick, said Aaron Glatt, MD, infectious disease professor and chair of medicine at the hospital. However, he said things are looking brighter than at Yale.
“We are not seeing the same level of increase in cases that we had previously, and we are starting to see extubations and discharges. We’ve treated a number of patients with plasma therapy, and hopefully that will be of benefit. We’ve seen some response to” the immunosuppressive “tocilizumab [Actemra], and a lot of response to very good respiratory therapy. I think we are starting to flatten the curve,” Dr. Glatt said.
“Look for tricky symptoms”
The growing awareness of COVID’s protean manifestations is evident in Medscape’s Consult forum, an online community where physicians and medical students share information and seek advice; there’s been over 200 COVID-19 cases and questions since January.
Early on, traffic was mostly about typical pulmonary presentations, but lately it’s shifted to nonrespiratory involvement. Physicians want to know if what they are seeing is related to the virus, and if other people are seeing the same things.
There’s a case on Consult of a 37-year-old man with stomach pain, vomiting, and diarrhea, but no respiratory symptoms and a positive COVID test. A chest CT incidental to his abdominal scan revealed significant bilateral lung involvement.
A 69-year-old woman with a history of laparotomy and new onset intestinal subocclusion had only adhesions on a subsequent exploratory laparotomy, and was doing okay otherwise. She suddenly went into respiratory failure with progressive bradycardia and died 3 days later. Aspiration pneumonia, pulmonary embolism, and MI had been ruled out. “The pattern of cardiovascular failure was in favor of myocarditis, but we don’t have any other clue,” the physician said after describing a second similar case.
Another doctor on the forum reported elevated cardiac enzymes without coronary artery obstruction in a positive patient who went into shock, with an ejection fraction of 40% and markedly increased heart wall thickness, but no lung involvement. There are also two cases of idiopathic thrombocytopenia without fever of hypoxia.
An Italian gastroenterologist said: “Look for tricky symptoms.” Expand “patient history, asking about the sudden occurrence of dysgeusia and/or anosmia. These symptoms have become my guiding diagnostic light” in Verona. “Most patients become nauseated, [and] the taste of any food is unbearable. When I find these symptoms by history, the patient is COVID positive 100%.”
‘Make sure that they didn’t die in vain’
There was interest in those and other reports on Consult, and comments from physicians who have theories, but no certain answers about what is, and is not, caused by the virus.
Direct viral attack is likely a part of it, said Stanley Perlman, MD, PhD, a professor of microbiology and immunology at the University of Iowa, Iowa City.
The ACE2 receptor the virus uses to enter cells is common in many organs, plus there were extrapulmonary manifestations with severe acute respiratory syndrome (SARS), another pandemic caused by a zoonotic coronavirus almost 20 years ago. At least with SARS, “many organs were infected when examined at autopsy,” he said.
The body’s inflammatory response is almost certainly also in play. Progressive derangements in inflammatory markers – C-reactive protein, D-dimer, ferritin – correlate with worse prognosis, and “the cytokine storm that occurs in these patients can lead to a degree of encephalopathy, myocarditis, liver impairment, and kidney impairment; multiorgan dysfunction, in other words,” said William Shaffner, MD, a professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
But in some cases, the virus might simply be a bystander to an unrelated disease process; in others, the experimental treatments being used might cause problems. Indeed, cardiology groups recently warned of torsade de pointes – a dangerously abnormal heart rhythm – with hydroxychloroquine and azithromycin.
“We think it’s some combination,” but don’t really know, Dr. Krumholz said. In the meantime, “we are forced to treat patients by instinct and first principles,” and long-term sequelae are unknown. “We don’t want to be in this position for long.”
To that end, he said, “this is the time for us all to hold hands and be together because we need to learn rapidly from each other. Our job is both to care for the people in front of us and make sure that they didn’t die in vain, that the experience they had is funneled into a larger set of data to make sure the next person is better off.”
Although a cause-and-effect relationship is unknown, people with the virus have presented with or developed heart disease, acute liver injury, ongoing GI issues, skin manifestations, neurologic damage, and other problems, especially among sicker people.
For example, French physicians described an association with encephalopathy, agitation, confusion, and corticospinal tract signs among 58 people hospitalized with acute respiratory distress (N Engl J Med. 2020 Apr 15. doi: 10.1056/NEJMc2008597).
In particular, Yale New Haven (Conn.) Hospital is dealing with unexpected complications up close. Almost half of the beds there are occupied by COVID-19 patients. Over 100 people are in the ICU, and almost 70 intubated. Of the more than 750 COVID admissions so far, only about 350 have been discharged. “Even in a bad flu season, you never see something like this; it’s just unheard of,” said Harlan Krumholz, MD, a Yale cardiologist and professor of medicine helping lead the efforts there.
Kidney injuries prominent
“When they get to the ICU, we are seeing lots of people with acute kidney injuries; lots of people developing endocrine problems; people having blood sugar control issues, coagulation issues, blood clots. We are just waking up to the wide range of ways this virus can affect people. Our ignorance is profound,” Dr. Krumholz said, but physicians “recognize that this thing has the capability of attacking almost every single organ system, and it may or may not present with respiratory symptoms.”
It’s a similar story at Mt. Sinai South Nassau, a hospital in Oceanside, N.Y. “We’ve seen a lot of renal injury in people having complications, a lot of acute dialysis,” but it’s unclear how much is caused by the virus and how much is simply because people are so sick, said Aaron Glatt, MD, infectious disease professor and chair of medicine at the hospital. However, he said things are looking brighter than at Yale.
“We are not seeing the same level of increase in cases that we had previously, and we are starting to see extubations and discharges. We’ve treated a number of patients with plasma therapy, and hopefully that will be of benefit. We’ve seen some response to” the immunosuppressive “tocilizumab [Actemra], and a lot of response to very good respiratory therapy. I think we are starting to flatten the curve,” Dr. Glatt said.
“Look for tricky symptoms”
The growing awareness of COVID’s protean manifestations is evident in Medscape’s Consult forum, an online community where physicians and medical students share information and seek advice; there’s been over 200 COVID-19 cases and questions since January.
Early on, traffic was mostly about typical pulmonary presentations, but lately it’s shifted to nonrespiratory involvement. Physicians want to know if what they are seeing is related to the virus, and if other people are seeing the same things.
There’s a case on Consult of a 37-year-old man with stomach pain, vomiting, and diarrhea, but no respiratory symptoms and a positive COVID test. A chest CT incidental to his abdominal scan revealed significant bilateral lung involvement.
A 69-year-old woman with a history of laparotomy and new onset intestinal subocclusion had only adhesions on a subsequent exploratory laparotomy, and was doing okay otherwise. She suddenly went into respiratory failure with progressive bradycardia and died 3 days later. Aspiration pneumonia, pulmonary embolism, and MI had been ruled out. “The pattern of cardiovascular failure was in favor of myocarditis, but we don’t have any other clue,” the physician said after describing a second similar case.
Another doctor on the forum reported elevated cardiac enzymes without coronary artery obstruction in a positive patient who went into shock, with an ejection fraction of 40% and markedly increased heart wall thickness, but no lung involvement. There are also two cases of idiopathic thrombocytopenia without fever of hypoxia.
An Italian gastroenterologist said: “Look for tricky symptoms.” Expand “patient history, asking about the sudden occurrence of dysgeusia and/or anosmia. These symptoms have become my guiding diagnostic light” in Verona. “Most patients become nauseated, [and] the taste of any food is unbearable. When I find these symptoms by history, the patient is COVID positive 100%.”
‘Make sure that they didn’t die in vain’
There was interest in those and other reports on Consult, and comments from physicians who have theories, but no certain answers about what is, and is not, caused by the virus.
Direct viral attack is likely a part of it, said Stanley Perlman, MD, PhD, a professor of microbiology and immunology at the University of Iowa, Iowa City.
The ACE2 receptor the virus uses to enter cells is common in many organs, plus there were extrapulmonary manifestations with severe acute respiratory syndrome (SARS), another pandemic caused by a zoonotic coronavirus almost 20 years ago. At least with SARS, “many organs were infected when examined at autopsy,” he said.
The body’s inflammatory response is almost certainly also in play. Progressive derangements in inflammatory markers – C-reactive protein, D-dimer, ferritin – correlate with worse prognosis, and “the cytokine storm that occurs in these patients can lead to a degree of encephalopathy, myocarditis, liver impairment, and kidney impairment; multiorgan dysfunction, in other words,” said William Shaffner, MD, a professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
But in some cases, the virus might simply be a bystander to an unrelated disease process; in others, the experimental treatments being used might cause problems. Indeed, cardiology groups recently warned of torsade de pointes – a dangerously abnormal heart rhythm – with hydroxychloroquine and azithromycin.
“We think it’s some combination,” but don’t really know, Dr. Krumholz said. In the meantime, “we are forced to treat patients by instinct and first principles,” and long-term sequelae are unknown. “We don’t want to be in this position for long.”
To that end, he said, “this is the time for us all to hold hands and be together because we need to learn rapidly from each other. Our job is both to care for the people in front of us and make sure that they didn’t die in vain, that the experience they had is funneled into a larger set of data to make sure the next person is better off.”
Although a cause-and-effect relationship is unknown, people with the virus have presented with or developed heart disease, acute liver injury, ongoing GI issues, skin manifestations, neurologic damage, and other problems, especially among sicker people.
For example, French physicians described an association with encephalopathy, agitation, confusion, and corticospinal tract signs among 58 people hospitalized with acute respiratory distress (N Engl J Med. 2020 Apr 15. doi: 10.1056/NEJMc2008597).
In particular, Yale New Haven (Conn.) Hospital is dealing with unexpected complications up close. Almost half of the beds there are occupied by COVID-19 patients. Over 100 people are in the ICU, and almost 70 intubated. Of the more than 750 COVID admissions so far, only about 350 have been discharged. “Even in a bad flu season, you never see something like this; it’s just unheard of,” said Harlan Krumholz, MD, a Yale cardiologist and professor of medicine helping lead the efforts there.
Kidney injuries prominent
“When they get to the ICU, we are seeing lots of people with acute kidney injuries; lots of people developing endocrine problems; people having blood sugar control issues, coagulation issues, blood clots. We are just waking up to the wide range of ways this virus can affect people. Our ignorance is profound,” Dr. Krumholz said, but physicians “recognize that this thing has the capability of attacking almost every single organ system, and it may or may not present with respiratory symptoms.”
It’s a similar story at Mt. Sinai South Nassau, a hospital in Oceanside, N.Y. “We’ve seen a lot of renal injury in people having complications, a lot of acute dialysis,” but it’s unclear how much is caused by the virus and how much is simply because people are so sick, said Aaron Glatt, MD, infectious disease professor and chair of medicine at the hospital. However, he said things are looking brighter than at Yale.
“We are not seeing the same level of increase in cases that we had previously, and we are starting to see extubations and discharges. We’ve treated a number of patients with plasma therapy, and hopefully that will be of benefit. We’ve seen some response to” the immunosuppressive “tocilizumab [Actemra], and a lot of response to very good respiratory therapy. I think we are starting to flatten the curve,” Dr. Glatt said.
“Look for tricky symptoms”
The growing awareness of COVID’s protean manifestations is evident in Medscape’s Consult forum, an online community where physicians and medical students share information and seek advice; there’s been over 200 COVID-19 cases and questions since January.
Early on, traffic was mostly about typical pulmonary presentations, but lately it’s shifted to nonrespiratory involvement. Physicians want to know if what they are seeing is related to the virus, and if other people are seeing the same things.
There’s a case on Consult of a 37-year-old man with stomach pain, vomiting, and diarrhea, but no respiratory symptoms and a positive COVID test. A chest CT incidental to his abdominal scan revealed significant bilateral lung involvement.
A 69-year-old woman with a history of laparotomy and new onset intestinal subocclusion had only adhesions on a subsequent exploratory laparotomy, and was doing okay otherwise. She suddenly went into respiratory failure with progressive bradycardia and died 3 days later. Aspiration pneumonia, pulmonary embolism, and MI had been ruled out. “The pattern of cardiovascular failure was in favor of myocarditis, but we don’t have any other clue,” the physician said after describing a second similar case.
Another doctor on the forum reported elevated cardiac enzymes without coronary artery obstruction in a positive patient who went into shock, with an ejection fraction of 40% and markedly increased heart wall thickness, but no lung involvement. There are also two cases of idiopathic thrombocytopenia without fever of hypoxia.
An Italian gastroenterologist said: “Look for tricky symptoms.” Expand “patient history, asking about the sudden occurrence of dysgeusia and/or anosmia. These symptoms have become my guiding diagnostic light” in Verona. “Most patients become nauseated, [and] the taste of any food is unbearable. When I find these symptoms by history, the patient is COVID positive 100%.”
‘Make sure that they didn’t die in vain’
There was interest in those and other reports on Consult, and comments from physicians who have theories, but no certain answers about what is, and is not, caused by the virus.
Direct viral attack is likely a part of it, said Stanley Perlman, MD, PhD, a professor of microbiology and immunology at the University of Iowa, Iowa City.
The ACE2 receptor the virus uses to enter cells is common in many organs, plus there were extrapulmonary manifestations with severe acute respiratory syndrome (SARS), another pandemic caused by a zoonotic coronavirus almost 20 years ago. At least with SARS, “many organs were infected when examined at autopsy,” he said.
The body’s inflammatory response is almost certainly also in play. Progressive derangements in inflammatory markers – C-reactive protein, D-dimer, ferritin – correlate with worse prognosis, and “the cytokine storm that occurs in these patients can lead to a degree of encephalopathy, myocarditis, liver impairment, and kidney impairment; multiorgan dysfunction, in other words,” said William Shaffner, MD, a professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
But in some cases, the virus might simply be a bystander to an unrelated disease process; in others, the experimental treatments being used might cause problems. Indeed, cardiology groups recently warned of torsade de pointes – a dangerously abnormal heart rhythm – with hydroxychloroquine and azithromycin.
“We think it’s some combination,” but don’t really know, Dr. Krumholz said. In the meantime, “we are forced to treat patients by instinct and first principles,” and long-term sequelae are unknown. “We don’t want to be in this position for long.”
To that end, he said, “this is the time for us all to hold hands and be together because we need to learn rapidly from each other. Our job is both to care for the people in front of us and make sure that they didn’t die in vain, that the experience they had is funneled into a larger set of data to make sure the next person is better off.”
COVID-19 PPE-related skin effects described in survey of Chinese doctors, nurses
Almost 75% of doctors and nurses in and around Wuhan, China, where the outbreak first emerged, reported skin problems during a single week in early February 2020, in a survey of health care workers (HCW) caring for COVID-19 patients at five university and five regional hospitals. Hands, cheeks, and the nasal bridge were the most commonly affected areas, with skin dryness, maceration, papules, and erythema the most common problems, according to research published in the British Journal of Dermatology.
In New York City, masks in particular are “really an issue,” said Ellen Marmur, MD, a dermatologist in private practice and an associate clinical professor at the Mount Sinai School of Medicine, New York.
She’s dealing with patients who have abrasions and skin infections at the tip of the nose, bruising from the metal strap that goes across the bridge of the nose, and skin irritation from the straps. “Rosacea is [also] definitely flaring up, [and] people’s acne is definitely flaring up, not only because of the stress, but because of the sweat and humidity” that builds up under the masks, she said.
“It’s not a life-threatening thing, but it’s definitely something we’ve been helping people with,” she said. This includes her husband, a cardiologist pulling 12-hour shifts in a New York City hospital wearing an N95 mask; when he comes home, the tip of his nose is red and abraded.
Treatment entails first aid skin care: a dab of a gentle ointment like Aquaphor to prevent abrasions while the mask is on and to help them heal after it’s off, and bacitracin if infection is a worry. For acne and rosacea flares, a course of minocycline or topical clindamycin might help, Dr. Marmur said.
Although almost 75% of the doctors and nurses in the Chinese study reported skin problems, the response rate was low, just 376 of the 1,000 surveyed (37.6%). That might have tilted the results to providers who actually ran into problems, wrote the investigators, led by Ping Lin of the department of dermatology and venereology at Peking University First Hospital, Beijing.
Still, 280 (74.5%) reported adverse skin reactions from caring for COVID-19 patients. “Of note, this rate was much higher than the rate of occupational contact dermatitis (31.5%) in HCWs under normal working condition[s], and that of adverse skin reactions (21.4%-35.5%)” during the outbreak of another coronavirus in 2003, severe acute respiratory syndrome, they wrote.
Most providers in the study washed their hands more than 10 times a day, but only about 22% applied hand cream afterwards, they reported.
On multivariate analysis, working in hospitals harder hit by the pandemic (odds ratio, 2.41; P = .001), working on inpatient wards (OR, 2.44; P = .003), wearing full-body personal protective equipment over 6 hours (OR, 4.26; P < .001), and female sex (OR, 1.87; P = .038) increased the risk of adverse skin reactions. The team suggested moisturizers would help to protect against hand dermatitis, and alcohol-based products instead of soaps “as the former show high antimicrobial activity and low risks of skin damage.” Also, “restricting duration of wearing” of protection gear “to no more than 6 hours would help.”
The study investigators reported that they had no conflicts of interest.
SOURCE: Lin P et al. Br J Dermatol. 2020 Apr 7. doi: 10.1111/bjd.19089.
Almost 75% of doctors and nurses in and around Wuhan, China, where the outbreak first emerged, reported skin problems during a single week in early February 2020, in a survey of health care workers (HCW) caring for COVID-19 patients at five university and five regional hospitals. Hands, cheeks, and the nasal bridge were the most commonly affected areas, with skin dryness, maceration, papules, and erythema the most common problems, according to research published in the British Journal of Dermatology.
In New York City, masks in particular are “really an issue,” said Ellen Marmur, MD, a dermatologist in private practice and an associate clinical professor at the Mount Sinai School of Medicine, New York.
She’s dealing with patients who have abrasions and skin infections at the tip of the nose, bruising from the metal strap that goes across the bridge of the nose, and skin irritation from the straps. “Rosacea is [also] definitely flaring up, [and] people’s acne is definitely flaring up, not only because of the stress, but because of the sweat and humidity” that builds up under the masks, she said.
“It’s not a life-threatening thing, but it’s definitely something we’ve been helping people with,” she said. This includes her husband, a cardiologist pulling 12-hour shifts in a New York City hospital wearing an N95 mask; when he comes home, the tip of his nose is red and abraded.
Treatment entails first aid skin care: a dab of a gentle ointment like Aquaphor to prevent abrasions while the mask is on and to help them heal after it’s off, and bacitracin if infection is a worry. For acne and rosacea flares, a course of minocycline or topical clindamycin might help, Dr. Marmur said.
Although almost 75% of the doctors and nurses in the Chinese study reported skin problems, the response rate was low, just 376 of the 1,000 surveyed (37.6%). That might have tilted the results to providers who actually ran into problems, wrote the investigators, led by Ping Lin of the department of dermatology and venereology at Peking University First Hospital, Beijing.
Still, 280 (74.5%) reported adverse skin reactions from caring for COVID-19 patients. “Of note, this rate was much higher than the rate of occupational contact dermatitis (31.5%) in HCWs under normal working condition[s], and that of adverse skin reactions (21.4%-35.5%)” during the outbreak of another coronavirus in 2003, severe acute respiratory syndrome, they wrote.
Most providers in the study washed their hands more than 10 times a day, but only about 22% applied hand cream afterwards, they reported.
On multivariate analysis, working in hospitals harder hit by the pandemic (odds ratio, 2.41; P = .001), working on inpatient wards (OR, 2.44; P = .003), wearing full-body personal protective equipment over 6 hours (OR, 4.26; P < .001), and female sex (OR, 1.87; P = .038) increased the risk of adverse skin reactions. The team suggested moisturizers would help to protect against hand dermatitis, and alcohol-based products instead of soaps “as the former show high antimicrobial activity and low risks of skin damage.” Also, “restricting duration of wearing” of protection gear “to no more than 6 hours would help.”
The study investigators reported that they had no conflicts of interest.
SOURCE: Lin P et al. Br J Dermatol. 2020 Apr 7. doi: 10.1111/bjd.19089.
Almost 75% of doctors and nurses in and around Wuhan, China, where the outbreak first emerged, reported skin problems during a single week in early February 2020, in a survey of health care workers (HCW) caring for COVID-19 patients at five university and five regional hospitals. Hands, cheeks, and the nasal bridge were the most commonly affected areas, with skin dryness, maceration, papules, and erythema the most common problems, according to research published in the British Journal of Dermatology.
In New York City, masks in particular are “really an issue,” said Ellen Marmur, MD, a dermatologist in private practice and an associate clinical professor at the Mount Sinai School of Medicine, New York.
She’s dealing with patients who have abrasions and skin infections at the tip of the nose, bruising from the metal strap that goes across the bridge of the nose, and skin irritation from the straps. “Rosacea is [also] definitely flaring up, [and] people’s acne is definitely flaring up, not only because of the stress, but because of the sweat and humidity” that builds up under the masks, she said.
“It’s not a life-threatening thing, but it’s definitely something we’ve been helping people with,” she said. This includes her husband, a cardiologist pulling 12-hour shifts in a New York City hospital wearing an N95 mask; when he comes home, the tip of his nose is red and abraded.
Treatment entails first aid skin care: a dab of a gentle ointment like Aquaphor to prevent abrasions while the mask is on and to help them heal after it’s off, and bacitracin if infection is a worry. For acne and rosacea flares, a course of minocycline or topical clindamycin might help, Dr. Marmur said.
Although almost 75% of the doctors and nurses in the Chinese study reported skin problems, the response rate was low, just 376 of the 1,000 surveyed (37.6%). That might have tilted the results to providers who actually ran into problems, wrote the investigators, led by Ping Lin of the department of dermatology and venereology at Peking University First Hospital, Beijing.
Still, 280 (74.5%) reported adverse skin reactions from caring for COVID-19 patients. “Of note, this rate was much higher than the rate of occupational contact dermatitis (31.5%) in HCWs under normal working condition[s], and that of adverse skin reactions (21.4%-35.5%)” during the outbreak of another coronavirus in 2003, severe acute respiratory syndrome, they wrote.
Most providers in the study washed their hands more than 10 times a day, but only about 22% applied hand cream afterwards, they reported.
On multivariate analysis, working in hospitals harder hit by the pandemic (odds ratio, 2.41; P = .001), working on inpatient wards (OR, 2.44; P = .003), wearing full-body personal protective equipment over 6 hours (OR, 4.26; P < .001), and female sex (OR, 1.87; P = .038) increased the risk of adverse skin reactions. The team suggested moisturizers would help to protect against hand dermatitis, and alcohol-based products instead of soaps “as the former show high antimicrobial activity and low risks of skin damage.” Also, “restricting duration of wearing” of protection gear “to no more than 6 hours would help.”
The study investigators reported that they had no conflicts of interest.
SOURCE: Lin P et al. Br J Dermatol. 2020 Apr 7. doi: 10.1111/bjd.19089.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
CDC: Screen nearly all adults, including pregnant women, for HCV
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
Cardiology groups push back on hydroxychloroquine, azithromycin for COVID-19
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
NCCN panel: Defer nonurgent skin cancer care during pandemic
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.