User login
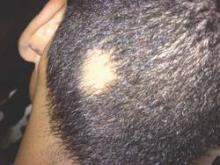
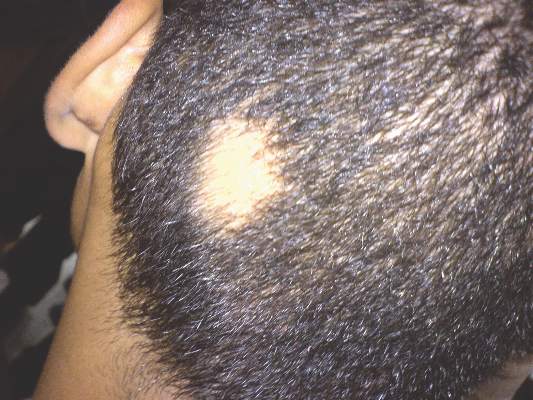
Corticosteroids far outpaced minoxidil use for alopecia areata
Alopecia areata sends “hundreds of thousands” of patients to the doctor every year in the United States, and six in ten of those visits end with a corticosteroid prescription, investigators reported in the Journal of Drugs in Dermatology.
In contrast, “minoxidil appears either underreported or underutilized in this population of patients, which suggests the need to educate both dermatologists and patients on the potential usefulness of this medication in alopecia areata,” wrote Michael Farhangian and his associates at Wake Forest University in Winston-Salem, N.C.
About 2% of individuals develop alopecia areata during their lives, but there are no consensus guidelines for disease in the United States. To better understand treatment patterns here, the investigators analyzed data on about 2.6 outpatient visits for alopecia areata between 2001 and 2010. The data came from two national ambulatory health care surveys (J Drugs Dermatol. 2015;14[9]:1012-14).
Patients with alopecia areata most often sought care from dermatologists (85%), the researchers reported. Providers prescribed topical and injected corticosteroids far more often (61%) than other drugs, such as minoxidil (5.9%), topical tacrolimus (5.7%), topical retinoid (3.3%), oral steroids (1.8%), or anthralin (1.8%).
The British Association of Dermatologists recommends corticosteroids for localized alopecia areata, but long-term use can lead to skin atrophy, hypopigmentation, and telangiectasia, the researchers warned. “This risk may be increased in patients who are prescribed both topical and injected corticosteroids, as was observed in 9.9% of patients,” they added.
Frequencies of minoxidil and tacrolimus use were nearly identical even though tacrolimus has been found ineffectivein alopecia areata, according to the researchers.
“Patients may be hesitant to use minoxidil since it is only FDA-approved for androgenetic alopecia and not for alopecia areata,” they wrote. Minoxidil also is available over-the-counter, which could explain its scarcity in the dataset, they added.
Galderma Laboratories helped fund the work through an unrestricted educational grant. Mr. Farhangian declared no competing interests. Senior author Dr. Steven Feldman reported relationships with Galderma, Janssen, Taro, Abbott Labs, and a number of other pharmaceutical companies. Dr. Feldman also reported holding stock in Causa Research and Medical Quality Enhancement Corporation. Another coauthor reported relationships with several pharmaceutical companies.
Alopecia areata sends “hundreds of thousands” of patients to the doctor every year in the United States, and six in ten of those visits end with a corticosteroid prescription, investigators reported in the Journal of Drugs in Dermatology.
In contrast, “minoxidil appears either underreported or underutilized in this population of patients, which suggests the need to educate both dermatologists and patients on the potential usefulness of this medication in alopecia areata,” wrote Michael Farhangian and his associates at Wake Forest University in Winston-Salem, N.C.
About 2% of individuals develop alopecia areata during their lives, but there are no consensus guidelines for disease in the United States. To better understand treatment patterns here, the investigators analyzed data on about 2.6 outpatient visits for alopecia areata between 2001 and 2010. The data came from two national ambulatory health care surveys (J Drugs Dermatol. 2015;14[9]:1012-14).
Patients with alopecia areata most often sought care from dermatologists (85%), the researchers reported. Providers prescribed topical and injected corticosteroids far more often (61%) than other drugs, such as minoxidil (5.9%), topical tacrolimus (5.7%), topical retinoid (3.3%), oral steroids (1.8%), or anthralin (1.8%).
The British Association of Dermatologists recommends corticosteroids for localized alopecia areata, but long-term use can lead to skin atrophy, hypopigmentation, and telangiectasia, the researchers warned. “This risk may be increased in patients who are prescribed both topical and injected corticosteroids, as was observed in 9.9% of patients,” they added.
Frequencies of minoxidil and tacrolimus use were nearly identical even though tacrolimus has been found ineffectivein alopecia areata, according to the researchers.
“Patients may be hesitant to use minoxidil since it is only FDA-approved for androgenetic alopecia and not for alopecia areata,” they wrote. Minoxidil also is available over-the-counter, which could explain its scarcity in the dataset, they added.
Galderma Laboratories helped fund the work through an unrestricted educational grant. Mr. Farhangian declared no competing interests. Senior author Dr. Steven Feldman reported relationships with Galderma, Janssen, Taro, Abbott Labs, and a number of other pharmaceutical companies. Dr. Feldman also reported holding stock in Causa Research and Medical Quality Enhancement Corporation. Another coauthor reported relationships with several pharmaceutical companies.
Alopecia areata sends “hundreds of thousands” of patients to the doctor every year in the United States, and six in ten of those visits end with a corticosteroid prescription, investigators reported in the Journal of Drugs in Dermatology.
In contrast, “minoxidil appears either underreported or underutilized in this population of patients, which suggests the need to educate both dermatologists and patients on the potential usefulness of this medication in alopecia areata,” wrote Michael Farhangian and his associates at Wake Forest University in Winston-Salem, N.C.
About 2% of individuals develop alopecia areata during their lives, but there are no consensus guidelines for disease in the United States. To better understand treatment patterns here, the investigators analyzed data on about 2.6 outpatient visits for alopecia areata between 2001 and 2010. The data came from two national ambulatory health care surveys (J Drugs Dermatol. 2015;14[9]:1012-14).
Patients with alopecia areata most often sought care from dermatologists (85%), the researchers reported. Providers prescribed topical and injected corticosteroids far more often (61%) than other drugs, such as minoxidil (5.9%), topical tacrolimus (5.7%), topical retinoid (3.3%), oral steroids (1.8%), or anthralin (1.8%).
The British Association of Dermatologists recommends corticosteroids for localized alopecia areata, but long-term use can lead to skin atrophy, hypopigmentation, and telangiectasia, the researchers warned. “This risk may be increased in patients who are prescribed both topical and injected corticosteroids, as was observed in 9.9% of patients,” they added.
Frequencies of minoxidil and tacrolimus use were nearly identical even though tacrolimus has been found ineffectivein alopecia areata, according to the researchers.
“Patients may be hesitant to use minoxidil since it is only FDA-approved for androgenetic alopecia and not for alopecia areata,” they wrote. Minoxidil also is available over-the-counter, which could explain its scarcity in the dataset, they added.
Galderma Laboratories helped fund the work through an unrestricted educational grant. Mr. Farhangian declared no competing interests. Senior author Dr. Steven Feldman reported relationships with Galderma, Janssen, Taro, Abbott Labs, and a number of other pharmaceutical companies. Dr. Feldman also reported holding stock in Causa Research and Medical Quality Enhancement Corporation. Another coauthor reported relationships with several pharmaceutical companies.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Key clinical point: Topical and injected corticosteroids were by far the most commonly recorded treatment for alopecia areata in the United States.
Major finding: Providers prescribed topical or injected corticosteroids during 61% of visits – far more often than minoxidil (5.9%), topical tacrolimus (5.7%), or other drugs.
Data source: Retrospective analysis of about 2.6 million visits for alopecia areata in the United States between 2001 and 2010.
Disclosures: Galderma Laboratories helped fund the work through an unrestricted educational grant. Mr. Farhangian declared no competing interests. Senior author Dr. Steven Feldman reported relationships with Galderma, Janssen, Taro, Abbott Labs, and a number of other pharmaceutical companies. Dr. Feldman also reported holding stock in Causa Research and Medical Quality Enhancement Corporation. Another coauthor reported relationships with several pharmaceutical companies.
Oral methylprednisolone found noninferior to IV steroids for MS relapse
High-dose oral methylprednisolone was noninferior to intravenous steroids for improving disability after multiple sclerosis relapse, according to a study published online in The Lancet.
The findings “support the use of oral methylprednisolone 1,000 mg per day for 3 days to treat MS relapses,” wrote Dr. Emmanuelle Le Page of Rennes (France) University Hospital and her associates. “Oral delivery is simpler and less invasive, more convenient for a quick primary and community care response, and allows obvious savings in cost and logistics,” they added. This also was the first trial with enough power to detect a difference between comparable doses of steroids begun early after relapse onset, they said.
Intravenous methylprednisolone is recommended as first-line treatment for MS relapses, but can be expensive and inconvenient, the investigators said. Authors of a recent Cochrane review found no differences in recovery between patients given high-dose oral or IV methylprednisolone, but concluded that the studies were underpowered. Based on their suggestions, Dr. Le Page and associates randomized 199 adults with relapsing-remitting MS to receive 1,000 mg oral or IV methylprednisolone once daily for 3 days, starting within a week of relapse onset. The researchers powered the study based on a predetermined efficacy margin of 15%. The primary efficacy endpoint was the percentage of patients who improved on day 28 by at least 1 point on their most affected score of the Kurtzke Functional System Scale, without needing more steroids (Lancet 2015;386[9997]:974-81).
In all, 81% of patients who received oral steroids and 80% of those who received IV therapy met this endpoint. Rates of treatment-associated adverse events also were similar between the two groups, except that patients were more likely to report insomnia on oral therapy (77% vs. 64%; P = .039). “This was also reported in the meta-analysis, and might be due to prolonged bioavailability,” the researchers wrote. They recommended giving oral methylprednisolone in the morning to help avert the problem.
The French Health Ministry, Ligue Française contre la Sclérose En Plaques, and Teva Pharmaceutical Industries funded the study. Dr. Le Page reported receiving consulting fees and grant funding from Novartis, Biogen, Teva, and Genzyme Sanofi Aventis. Five coauthors reported financial relationships with several public entities, foundations, and pharmaceutical companies. The other four coauthors declared no competing interests.
This study robustly addressed a question of high importance in clinical practice and with important implications for patients. Moreover, the time window chosen for this study, of only 14 days between onset of symptoms and treatment, compared with the longer time periods of previous studies, is more suitable to establish the role of route administration in relapse recovery, allowing exploration of the effects of steroids on acute inflammation. Windows longer than 14 days reduce the potential beneficial effects on inflammation (the period of enhancement in a new lesion is about 4 weeks) and increase inter-patient variability.
The major limitation of Dr. Le Page and colleagues’ study was the absence of MRI data to support the clinical findings, considering the established modest intra-rater and inter-rater concordance. The use of the same assessor for each patient throughout the study significantly reduced but did not eliminate this bias.
Even with some limitations, the study by Dr. Le Page and colleagues is a milestone in the history of treatment for multiple sclerosis and will lead to a change in clinical practice with relevant advantages for both patients and the community, with potential applications in many other disorders when short courses of high-dose steroids are needed. Despite accumulating evidence for the equal effects of intravenous and oral high-dose steroids, oral administration has not entered clinical practice, except in a few countries, and is mostly not considered as a treatment scheme for relapses in clinical trials, perhaps because in most countries formulations of high-dose steroids for oral administration are not available. In the study by Dr. Le Page and colleagues, 10 tablets were needed to reach the cumulative daily dosage of 1,000 mg of methylprednisolone. More efforts are needed to obtain adequate oral formulations, and we hope that the clear results of this clinical trial will promote actions to address this issue.
Dr. Giancarlo Comi and Dr. Marta Radaelli are with the department of neurology at Vita-Salute San Raffaele University and Hospital San Raffaele in Milan. Dr. Comi declared having received consulting fees from Novartis, Teva, Sanofi, Genzyme, Merck Serono, Biogen, Bayer, Serono Symposia International Foundation, Roche, Almirall, Chugai, and Receptos. Dr. Radaelli declared no competing interests. These comments were taken from their accompanying editorial(Lancet 2015;386[9997];937-9).
This study robustly addressed a question of high importance in clinical practice and with important implications for patients. Moreover, the time window chosen for this study, of only 14 days between onset of symptoms and treatment, compared with the longer time periods of previous studies, is more suitable to establish the role of route administration in relapse recovery, allowing exploration of the effects of steroids on acute inflammation. Windows longer than 14 days reduce the potential beneficial effects on inflammation (the period of enhancement in a new lesion is about 4 weeks) and increase inter-patient variability.
The major limitation of Dr. Le Page and colleagues’ study was the absence of MRI data to support the clinical findings, considering the established modest intra-rater and inter-rater concordance. The use of the same assessor for each patient throughout the study significantly reduced but did not eliminate this bias.
Even with some limitations, the study by Dr. Le Page and colleagues is a milestone in the history of treatment for multiple sclerosis and will lead to a change in clinical practice with relevant advantages for both patients and the community, with potential applications in many other disorders when short courses of high-dose steroids are needed. Despite accumulating evidence for the equal effects of intravenous and oral high-dose steroids, oral administration has not entered clinical practice, except in a few countries, and is mostly not considered as a treatment scheme for relapses in clinical trials, perhaps because in most countries formulations of high-dose steroids for oral administration are not available. In the study by Dr. Le Page and colleagues, 10 tablets were needed to reach the cumulative daily dosage of 1,000 mg of methylprednisolone. More efforts are needed to obtain adequate oral formulations, and we hope that the clear results of this clinical trial will promote actions to address this issue.
Dr. Giancarlo Comi and Dr. Marta Radaelli are with the department of neurology at Vita-Salute San Raffaele University and Hospital San Raffaele in Milan. Dr. Comi declared having received consulting fees from Novartis, Teva, Sanofi, Genzyme, Merck Serono, Biogen, Bayer, Serono Symposia International Foundation, Roche, Almirall, Chugai, and Receptos. Dr. Radaelli declared no competing interests. These comments were taken from their accompanying editorial(Lancet 2015;386[9997];937-9).
This study robustly addressed a question of high importance in clinical practice and with important implications for patients. Moreover, the time window chosen for this study, of only 14 days between onset of symptoms and treatment, compared with the longer time periods of previous studies, is more suitable to establish the role of route administration in relapse recovery, allowing exploration of the effects of steroids on acute inflammation. Windows longer than 14 days reduce the potential beneficial effects on inflammation (the period of enhancement in a new lesion is about 4 weeks) and increase inter-patient variability.
The major limitation of Dr. Le Page and colleagues’ study was the absence of MRI data to support the clinical findings, considering the established modest intra-rater and inter-rater concordance. The use of the same assessor for each patient throughout the study significantly reduced but did not eliminate this bias.
Even with some limitations, the study by Dr. Le Page and colleagues is a milestone in the history of treatment for multiple sclerosis and will lead to a change in clinical practice with relevant advantages for both patients and the community, with potential applications in many other disorders when short courses of high-dose steroids are needed. Despite accumulating evidence for the equal effects of intravenous and oral high-dose steroids, oral administration has not entered clinical practice, except in a few countries, and is mostly not considered as a treatment scheme for relapses in clinical trials, perhaps because in most countries formulations of high-dose steroids for oral administration are not available. In the study by Dr. Le Page and colleagues, 10 tablets were needed to reach the cumulative daily dosage of 1,000 mg of methylprednisolone. More efforts are needed to obtain adequate oral formulations, and we hope that the clear results of this clinical trial will promote actions to address this issue.
Dr. Giancarlo Comi and Dr. Marta Radaelli are with the department of neurology at Vita-Salute San Raffaele University and Hospital San Raffaele in Milan. Dr. Comi declared having received consulting fees from Novartis, Teva, Sanofi, Genzyme, Merck Serono, Biogen, Bayer, Serono Symposia International Foundation, Roche, Almirall, Chugai, and Receptos. Dr. Radaelli declared no competing interests. These comments were taken from their accompanying editorial(Lancet 2015;386[9997];937-9).
High-dose oral methylprednisolone was noninferior to intravenous steroids for improving disability after multiple sclerosis relapse, according to a study published online in The Lancet.
The findings “support the use of oral methylprednisolone 1,000 mg per day for 3 days to treat MS relapses,” wrote Dr. Emmanuelle Le Page of Rennes (France) University Hospital and her associates. “Oral delivery is simpler and less invasive, more convenient for a quick primary and community care response, and allows obvious savings in cost and logistics,” they added. This also was the first trial with enough power to detect a difference between comparable doses of steroids begun early after relapse onset, they said.
Intravenous methylprednisolone is recommended as first-line treatment for MS relapses, but can be expensive and inconvenient, the investigators said. Authors of a recent Cochrane review found no differences in recovery between patients given high-dose oral or IV methylprednisolone, but concluded that the studies were underpowered. Based on their suggestions, Dr. Le Page and associates randomized 199 adults with relapsing-remitting MS to receive 1,000 mg oral or IV methylprednisolone once daily for 3 days, starting within a week of relapse onset. The researchers powered the study based on a predetermined efficacy margin of 15%. The primary efficacy endpoint was the percentage of patients who improved on day 28 by at least 1 point on their most affected score of the Kurtzke Functional System Scale, without needing more steroids (Lancet 2015;386[9997]:974-81).
In all, 81% of patients who received oral steroids and 80% of those who received IV therapy met this endpoint. Rates of treatment-associated adverse events also were similar between the two groups, except that patients were more likely to report insomnia on oral therapy (77% vs. 64%; P = .039). “This was also reported in the meta-analysis, and might be due to prolonged bioavailability,” the researchers wrote. They recommended giving oral methylprednisolone in the morning to help avert the problem.
The French Health Ministry, Ligue Française contre la Sclérose En Plaques, and Teva Pharmaceutical Industries funded the study. Dr. Le Page reported receiving consulting fees and grant funding from Novartis, Biogen, Teva, and Genzyme Sanofi Aventis. Five coauthors reported financial relationships with several public entities, foundations, and pharmaceutical companies. The other four coauthors declared no competing interests.
High-dose oral methylprednisolone was noninferior to intravenous steroids for improving disability after multiple sclerosis relapse, according to a study published online in The Lancet.
The findings “support the use of oral methylprednisolone 1,000 mg per day for 3 days to treat MS relapses,” wrote Dr. Emmanuelle Le Page of Rennes (France) University Hospital and her associates. “Oral delivery is simpler and less invasive, more convenient for a quick primary and community care response, and allows obvious savings in cost and logistics,” they added. This also was the first trial with enough power to detect a difference between comparable doses of steroids begun early after relapse onset, they said.
Intravenous methylprednisolone is recommended as first-line treatment for MS relapses, but can be expensive and inconvenient, the investigators said. Authors of a recent Cochrane review found no differences in recovery between patients given high-dose oral or IV methylprednisolone, but concluded that the studies were underpowered. Based on their suggestions, Dr. Le Page and associates randomized 199 adults with relapsing-remitting MS to receive 1,000 mg oral or IV methylprednisolone once daily for 3 days, starting within a week of relapse onset. The researchers powered the study based on a predetermined efficacy margin of 15%. The primary efficacy endpoint was the percentage of patients who improved on day 28 by at least 1 point on their most affected score of the Kurtzke Functional System Scale, without needing more steroids (Lancet 2015;386[9997]:974-81).
In all, 81% of patients who received oral steroids and 80% of those who received IV therapy met this endpoint. Rates of treatment-associated adverse events also were similar between the two groups, except that patients were more likely to report insomnia on oral therapy (77% vs. 64%; P = .039). “This was also reported in the meta-analysis, and might be due to prolonged bioavailability,” the researchers wrote. They recommended giving oral methylprednisolone in the morning to help avert the problem.
The French Health Ministry, Ligue Française contre la Sclérose En Plaques, and Teva Pharmaceutical Industries funded the study. Dr. Le Page reported receiving consulting fees and grant funding from Novartis, Biogen, Teva, and Genzyme Sanofi Aventis. Five coauthors reported financial relationships with several public entities, foundations, and pharmaceutical companies. The other four coauthors declared no competing interests.
FROM THE LANCET
Key clinical point: High-dose oral methylprednisolone was noninferior to IV steroids in the treatment of MS relapse.
Major finding: On day 28, 81% of the oral group and 80% of the IV group had improved by at least 1 point on the most affected score of the Kurtzke Functional System Scale.
Data source: Randomized, double-blind, noninferiority trial of 199 adults with relapsing-remitting MS.
Disclosures: The French Health Ministry, Ligue Française contre la Sclérose En Plaques, and Teva Pharmaceutical Industries funded the study. Dr. Le Page reported receiving consulting fees and grant funding from Novartis, Biogen, Teva, and Genzyme Sanofi Aventis. Five coauthors reported financial relationships with several public entities, foundations, and pharmaceutical companies. The other four coauthors declared no competing interests.
Eculizumab benefited pregnant women with paroxysmal nocturnal hemoglobinuria
Eculizumab therapy led to “acceptable” outcomes among pregnant women with paroxysmal nocturnal hemoglobinuria, investigators reported Sept. 9 in the New England Journal of Medicine.
No woman who received eculizumab died while pregnant or within 6 months of delivery; historic mortality rates for these patients are 8%-20%, said Dr. Richard Kelly at St. James’s University Hospital in Leeds, England, and his associates. Treatment with the monoclonal antibody, “has reduced mortality and morbidity associated with PNH and has allowed patients who were previously severely affected to lead a relatively normal life.”
The fetal death rate was 4%, resembling rates of 4%-9% from the era before eculizumab, the researchers said. The rate of premature births also was high (29%) as a result of preeclampsia, suspected intrauterine growth retardation, maternal thrombocytopenia, and slowed fetal movements, they said.
Paroxysmal nocturnal hemoglobinuria is a rare, chronic stem-cell disease in which complement-mediated intravascular hemolysis causes anemia, fatigue, and venous thromboembolism (Adv Exp Med Biol. 2013;735:155-72.). Increased complement activation during pregnancy intensifies the risk of severe hemolytic anemia, fetal morbidity, and fetal mortality for women with PNH. Eculizumab blocks terminal complement activation by binding complement protein C5, and has improved PNH symptoms to the extent that treated women are more likely to consider pregnancy than in the past.
Dr. Kelly and his associates surveyed members of the International PNH Interest Group to study pregnancy outcomes among these women (N Engl J. Med. 2015;373:1032-9). A total of 80% of clinicians responded, reporting data for 75 pregnancies among 61 women, the investigators said. All patients had PNH diagnosed by flow cytometry, and 61% had begun eculizumab therapy before conception. Median age at first pregnancy was 29 years, with a range of 18 to 40 years. The patients received weekly 600-mg IV infusions for 4 weeks, followed by 900 mg every 14 days. Clinicians increased the dose or treatment frequency at their own discretion if patients showed signs of breakthrough intravascular hemolysis.
Two women experienced thrombotic events during treatment, both of which happened soon after delivery. One was a lower-limb deep venous thrombosis, but the other occurred after a patient received a plasma infusion for postpartum hemorrhage. “Plasma contains high levels of complement and can overcome the effects of eculizumab and thereby render the patient susceptible to complications of PNH,” noted the investigators. “The use of plasma should thus be avoided if possible.” Ten samples of breast milk were negative for eculizumab, they added.
Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
Eculizumab therapy led to “acceptable” outcomes among pregnant women with paroxysmal nocturnal hemoglobinuria, investigators reported Sept. 9 in the New England Journal of Medicine.
No woman who received eculizumab died while pregnant or within 6 months of delivery; historic mortality rates for these patients are 8%-20%, said Dr. Richard Kelly at St. James’s University Hospital in Leeds, England, and his associates. Treatment with the monoclonal antibody, “has reduced mortality and morbidity associated with PNH and has allowed patients who were previously severely affected to lead a relatively normal life.”
The fetal death rate was 4%, resembling rates of 4%-9% from the era before eculizumab, the researchers said. The rate of premature births also was high (29%) as a result of preeclampsia, suspected intrauterine growth retardation, maternal thrombocytopenia, and slowed fetal movements, they said.
Paroxysmal nocturnal hemoglobinuria is a rare, chronic stem-cell disease in which complement-mediated intravascular hemolysis causes anemia, fatigue, and venous thromboembolism (Adv Exp Med Biol. 2013;735:155-72.). Increased complement activation during pregnancy intensifies the risk of severe hemolytic anemia, fetal morbidity, and fetal mortality for women with PNH. Eculizumab blocks terminal complement activation by binding complement protein C5, and has improved PNH symptoms to the extent that treated women are more likely to consider pregnancy than in the past.
Dr. Kelly and his associates surveyed members of the International PNH Interest Group to study pregnancy outcomes among these women (N Engl J. Med. 2015;373:1032-9). A total of 80% of clinicians responded, reporting data for 75 pregnancies among 61 women, the investigators said. All patients had PNH diagnosed by flow cytometry, and 61% had begun eculizumab therapy before conception. Median age at first pregnancy was 29 years, with a range of 18 to 40 years. The patients received weekly 600-mg IV infusions for 4 weeks, followed by 900 mg every 14 days. Clinicians increased the dose or treatment frequency at their own discretion if patients showed signs of breakthrough intravascular hemolysis.
Two women experienced thrombotic events during treatment, both of which happened soon after delivery. One was a lower-limb deep venous thrombosis, but the other occurred after a patient received a plasma infusion for postpartum hemorrhage. “Plasma contains high levels of complement and can overcome the effects of eculizumab and thereby render the patient susceptible to complications of PNH,” noted the investigators. “The use of plasma should thus be avoided if possible.” Ten samples of breast milk were negative for eculizumab, they added.
Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
Eculizumab therapy led to “acceptable” outcomes among pregnant women with paroxysmal nocturnal hemoglobinuria, investigators reported Sept. 9 in the New England Journal of Medicine.
No woman who received eculizumab died while pregnant or within 6 months of delivery; historic mortality rates for these patients are 8%-20%, said Dr. Richard Kelly at St. James’s University Hospital in Leeds, England, and his associates. Treatment with the monoclonal antibody, “has reduced mortality and morbidity associated with PNH and has allowed patients who were previously severely affected to lead a relatively normal life.”
The fetal death rate was 4%, resembling rates of 4%-9% from the era before eculizumab, the researchers said. The rate of premature births also was high (29%) as a result of preeclampsia, suspected intrauterine growth retardation, maternal thrombocytopenia, and slowed fetal movements, they said.
Paroxysmal nocturnal hemoglobinuria is a rare, chronic stem-cell disease in which complement-mediated intravascular hemolysis causes anemia, fatigue, and venous thromboembolism (Adv Exp Med Biol. 2013;735:155-72.). Increased complement activation during pregnancy intensifies the risk of severe hemolytic anemia, fetal morbidity, and fetal mortality for women with PNH. Eculizumab blocks terminal complement activation by binding complement protein C5, and has improved PNH symptoms to the extent that treated women are more likely to consider pregnancy than in the past.
Dr. Kelly and his associates surveyed members of the International PNH Interest Group to study pregnancy outcomes among these women (N Engl J. Med. 2015;373:1032-9). A total of 80% of clinicians responded, reporting data for 75 pregnancies among 61 women, the investigators said. All patients had PNH diagnosed by flow cytometry, and 61% had begun eculizumab therapy before conception. Median age at first pregnancy was 29 years, with a range of 18 to 40 years. The patients received weekly 600-mg IV infusions for 4 weeks, followed by 900 mg every 14 days. Clinicians increased the dose or treatment frequency at their own discretion if patients showed signs of breakthrough intravascular hemolysis.
Two women experienced thrombotic events during treatment, both of which happened soon after delivery. One was a lower-limb deep venous thrombosis, but the other occurred after a patient received a plasma infusion for postpartum hemorrhage. “Plasma contains high levels of complement and can overcome the effects of eculizumab and thereby render the patient susceptible to complications of PNH,” noted the investigators. “The use of plasma should thus be avoided if possible.” Ten samples of breast milk were negative for eculizumab, they added.
Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: None of the pregnant women with paroxysmal nocturnal hemoglobinuria died on eculizumab therapy.
Major finding: The fetal death rate was 4%, and the premature birth rate was 29%.
Data source: A retrospective, survey-based study of 75 pregnancies among 61 women with PNH.
Disclosures: Dr. Kelly and 14 of 15 coauthors reported financial relationships outside this work with Alexion Pharmaceuticals, the maker of eculizumab. One coauthor also reported grant support outside this work from Alnylam Pharmaceuticals, Novartis, and Ra Pharma.
CTL019 induced a durable complete response in refractory MM
The first recipient of CTL019 for advanced refractory multiple myeloma achieved a durable complete response without developing cytokine release syndrome, according to a study published Sept. 9 in the New England Journal of Medicine.
“Twelve months after transplantation, the patient had no evidence of monoclonal immunoglobulin on serum and urine immunofixation and no clinical signs or symptoms of multiple myeloma,” said Dr. Alfred Garfall and his associates at the University of Pennsylvania in Philadelphia. “This response was achieved despite the absence of CD19 expression in 99.95% of the patient’s neoplastic plasma cells.”
CTL019 consists of autologous T cells modified to express an anti-CD19 chimeric antigen receptor (CAR) from a lentiviral vector. The cell therapy has yielded promising results in relapsed/refractory CLL and ALL,but was overlooked in MM because it was thought to infrequently express CD19, the researchers said.
“Several reports, however, have suggested that a minor component of the MM clone with drug-resistant, disease-propagating properties has a B-cell (i.e., CD19-positive) phenotype,” they noted. “In addition, our unpublished observations suggest that neoplastic plasma cells express low levels of CD19” (N Engl J Med. 2015 Sep 9;373:1040-7).
In response, they designed a pilot trial of adults whose MM relapsed or progressed within a year after initial autologous stem cell transplant. The first participant, a 43-year-old woman with IgA kappa MM, partially responded to lenalidomide, bortezomib, and dexamethasone but progressed when therapy was paused to collect stem cells for transplant. She then partially responded to cisplatin, doxorubicin, cyclophosphamide, and etoposide followed by high-dose melphalan and ASCT, but progressed again and continued to worsen despite a total of nine lines of therapy. A bone marrow sample revealed more than 95% plasma cells when the patient began the CTL019 trial, the researchers said.
For the study, the patient received a lower melphalan dose (140 mg/m2 of body surface area), followed by ASCT, CTL019 starting 2 weeks later, and maintenance lenalidomide. On day 100, her tumor burden had dropped by 5-log10, the researchers said. She also did not develop cytokine release syndrome, they added.
So far, 10 patients have been treated on study, of whom six remain progression free, according to the investigators. “The only additional CTL019-attributable toxic effects observed have been one instance of grade 1 cytokine release syndrome and one instance of grade 3 enterocolitis due to autologous graft-versus-host disease,” they reported.
Novartis supported the study and approved the manuscript. The work was also funded by the National Institutes of Health, the International Society for Advancement of Cytometry, the University of Pennsylvania Institute or Translational Medicine and Therapeutics, and a Conquer Cancer Foundation Young Investigator Award. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Several scientists involved in this trial hold patents for these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially or may benefit in the future.
The first recipient of CTL019 for advanced refractory multiple myeloma achieved a durable complete response without developing cytokine release syndrome, according to a study published Sept. 9 in the New England Journal of Medicine.
“Twelve months after transplantation, the patient had no evidence of monoclonal immunoglobulin on serum and urine immunofixation and no clinical signs or symptoms of multiple myeloma,” said Dr. Alfred Garfall and his associates at the University of Pennsylvania in Philadelphia. “This response was achieved despite the absence of CD19 expression in 99.95% of the patient’s neoplastic plasma cells.”
CTL019 consists of autologous T cells modified to express an anti-CD19 chimeric antigen receptor (CAR) from a lentiviral vector. The cell therapy has yielded promising results in relapsed/refractory CLL and ALL,but was overlooked in MM because it was thought to infrequently express CD19, the researchers said.
“Several reports, however, have suggested that a minor component of the MM clone with drug-resistant, disease-propagating properties has a B-cell (i.e., CD19-positive) phenotype,” they noted. “In addition, our unpublished observations suggest that neoplastic plasma cells express low levels of CD19” (N Engl J Med. 2015 Sep 9;373:1040-7).
In response, they designed a pilot trial of adults whose MM relapsed or progressed within a year after initial autologous stem cell transplant. The first participant, a 43-year-old woman with IgA kappa MM, partially responded to lenalidomide, bortezomib, and dexamethasone but progressed when therapy was paused to collect stem cells for transplant. She then partially responded to cisplatin, doxorubicin, cyclophosphamide, and etoposide followed by high-dose melphalan and ASCT, but progressed again and continued to worsen despite a total of nine lines of therapy. A bone marrow sample revealed more than 95% plasma cells when the patient began the CTL019 trial, the researchers said.
For the study, the patient received a lower melphalan dose (140 mg/m2 of body surface area), followed by ASCT, CTL019 starting 2 weeks later, and maintenance lenalidomide. On day 100, her tumor burden had dropped by 5-log10, the researchers said. She also did not develop cytokine release syndrome, they added.
So far, 10 patients have been treated on study, of whom six remain progression free, according to the investigators. “The only additional CTL019-attributable toxic effects observed have been one instance of grade 1 cytokine release syndrome and one instance of grade 3 enterocolitis due to autologous graft-versus-host disease,” they reported.
Novartis supported the study and approved the manuscript. The work was also funded by the National Institutes of Health, the International Society for Advancement of Cytometry, the University of Pennsylvania Institute or Translational Medicine and Therapeutics, and a Conquer Cancer Foundation Young Investigator Award. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Several scientists involved in this trial hold patents for these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially or may benefit in the future.
The first recipient of CTL019 for advanced refractory multiple myeloma achieved a durable complete response without developing cytokine release syndrome, according to a study published Sept. 9 in the New England Journal of Medicine.
“Twelve months after transplantation, the patient had no evidence of monoclonal immunoglobulin on serum and urine immunofixation and no clinical signs or symptoms of multiple myeloma,” said Dr. Alfred Garfall and his associates at the University of Pennsylvania in Philadelphia. “This response was achieved despite the absence of CD19 expression in 99.95% of the patient’s neoplastic plasma cells.”
CTL019 consists of autologous T cells modified to express an anti-CD19 chimeric antigen receptor (CAR) from a lentiviral vector. The cell therapy has yielded promising results in relapsed/refractory CLL and ALL,but was overlooked in MM because it was thought to infrequently express CD19, the researchers said.
“Several reports, however, have suggested that a minor component of the MM clone with drug-resistant, disease-propagating properties has a B-cell (i.e., CD19-positive) phenotype,” they noted. “In addition, our unpublished observations suggest that neoplastic plasma cells express low levels of CD19” (N Engl J Med. 2015 Sep 9;373:1040-7).
In response, they designed a pilot trial of adults whose MM relapsed or progressed within a year after initial autologous stem cell transplant. The first participant, a 43-year-old woman with IgA kappa MM, partially responded to lenalidomide, bortezomib, and dexamethasone but progressed when therapy was paused to collect stem cells for transplant. She then partially responded to cisplatin, doxorubicin, cyclophosphamide, and etoposide followed by high-dose melphalan and ASCT, but progressed again and continued to worsen despite a total of nine lines of therapy. A bone marrow sample revealed more than 95% plasma cells when the patient began the CTL019 trial, the researchers said.
For the study, the patient received a lower melphalan dose (140 mg/m2 of body surface area), followed by ASCT, CTL019 starting 2 weeks later, and maintenance lenalidomide. On day 100, her tumor burden had dropped by 5-log10, the researchers said. She also did not develop cytokine release syndrome, they added.
So far, 10 patients have been treated on study, of whom six remain progression free, according to the investigators. “The only additional CTL019-attributable toxic effects observed have been one instance of grade 1 cytokine release syndrome and one instance of grade 3 enterocolitis due to autologous graft-versus-host disease,” they reported.
Novartis supported the study and approved the manuscript. The work was also funded by the National Institutes of Health, the International Society for Advancement of Cytometry, the University of Pennsylvania Institute or Translational Medicine and Therapeutics, and a Conquer Cancer Foundation Young Investigator Award. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Several scientists involved in this trial hold patents for these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially or may benefit in the future.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: CTL019 cell therapy after stem cell transplant led to a durable complete response that persisted at 12 months in a patient with advanced, refractory multiple myeloma.
Major finding: The patient received a lower melphalan dose (140 mg/m2 of body surface area), followed by ASCT, CTL019 starting 2 weeks later, and maintenance lenalidomide. On day 100, her tumor burden had dropped by 5-log10.
Data source: Ongoing pilot trial of CTL019 for patients with MM who relapsed within 12 months of their first transplant.
Disclosures: Novartis funded the study and approved the manuscript. The work was also funded by the National Institutes of Health, the International Society for Advancement of Cytometry, the University of Pennsylvania Institute or Translational Medicine and Therapeutics, and a Conquer Cancer Foundation Young Investigator Award. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Several scientists involved in this trial hold patents for these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially or may benefit in the future.
Siblings were most common source of infant pertussis
Infants most often acquired pertussis from siblings, not their mothers as was previously found, researchers reported online in Pediatrics.
The trend reflects the changing epidemiology of pertussis in the United States as immunity to acellular Tdap vaccination wanes among older children and adolescents, who then develop clinical or subclinical pertussis and infect younger siblings, said Tami Skoff at the Centers for Disease Control and Prevention in Atlanta, and her associates. “Prevention efforts should focus on increasing Tdap coverage during pregnancy, because this is currently our best strategy for providing direct protection to the infant, regardless of the changing source of infant infection.”
Bordetella pertussis infects up to 80% of exposed, naive individuals and is particularly risky for infants, the researchers noted. To examine infection sources for this age group, they studied 1,306 pertussis cases among children less than 1 year old that were reported to the Enhanced Pertussis Surveillance program between 2006 and 2013. The program tracks pertussis in Colorado, Connecticut, Massachusetts, Minnesota, New Mexico, New York, and Oregon. For each case, the researchers attempted to identify the infection source – a person with suspected pertussis who had contact with the infant 7-20 days before cough onset (Pediatrics 2015 Sep 7. doi: 10.1542/peds.2015-1120). A source of infection was identified in 43.6% of cases.
Siblings were the infection source for 36% of infants, followed by mothers (21%), fathers (10%), grandparents (8%), and aunts and uncles (7%), the investigators reported. The source was unspecified for 6% of cases, while ill day care contacts, cousins, friends, babysitters, and other contacts accounted for less than 5% of infections each.
“Consistent with previous studies, our analysis of 8 years of enhanced surveillance data identified a source of infection for less than half of reported infant pertussis cases,” the researchers emphasized. Such a high proportion of unknown infection sources limits the efficacy of the cocooning strategy, in which a Tdap booster dose is given to adults and adolescents who are in close contact with infants, the investigators added. “The cocooning strategy is less than ideal, and strong support of vaccination during pregnancy is needed to maximize the protection of infants in the first critical months of life.”
The Enhanced Pertussis Surveillance Program is funded through a cooperative agreement with the Centers for Disease Control and Prevention. The investigators reported no financial disclosures.
Infants most often acquired pertussis from siblings, not their mothers as was previously found, researchers reported online in Pediatrics.
The trend reflects the changing epidemiology of pertussis in the United States as immunity to acellular Tdap vaccination wanes among older children and adolescents, who then develop clinical or subclinical pertussis and infect younger siblings, said Tami Skoff at the Centers for Disease Control and Prevention in Atlanta, and her associates. “Prevention efforts should focus on increasing Tdap coverage during pregnancy, because this is currently our best strategy for providing direct protection to the infant, regardless of the changing source of infant infection.”
Bordetella pertussis infects up to 80% of exposed, naive individuals and is particularly risky for infants, the researchers noted. To examine infection sources for this age group, they studied 1,306 pertussis cases among children less than 1 year old that were reported to the Enhanced Pertussis Surveillance program between 2006 and 2013. The program tracks pertussis in Colorado, Connecticut, Massachusetts, Minnesota, New Mexico, New York, and Oregon. For each case, the researchers attempted to identify the infection source – a person with suspected pertussis who had contact with the infant 7-20 days before cough onset (Pediatrics 2015 Sep 7. doi: 10.1542/peds.2015-1120). A source of infection was identified in 43.6% of cases.
Siblings were the infection source for 36% of infants, followed by mothers (21%), fathers (10%), grandparents (8%), and aunts and uncles (7%), the investigators reported. The source was unspecified for 6% of cases, while ill day care contacts, cousins, friends, babysitters, and other contacts accounted for less than 5% of infections each.
“Consistent with previous studies, our analysis of 8 years of enhanced surveillance data identified a source of infection for less than half of reported infant pertussis cases,” the researchers emphasized. Such a high proportion of unknown infection sources limits the efficacy of the cocooning strategy, in which a Tdap booster dose is given to adults and adolescents who are in close contact with infants, the investigators added. “The cocooning strategy is less than ideal, and strong support of vaccination during pregnancy is needed to maximize the protection of infants in the first critical months of life.”
The Enhanced Pertussis Surveillance Program is funded through a cooperative agreement with the Centers for Disease Control and Prevention. The investigators reported no financial disclosures.
Infants most often acquired pertussis from siblings, not their mothers as was previously found, researchers reported online in Pediatrics.
The trend reflects the changing epidemiology of pertussis in the United States as immunity to acellular Tdap vaccination wanes among older children and adolescents, who then develop clinical or subclinical pertussis and infect younger siblings, said Tami Skoff at the Centers for Disease Control and Prevention in Atlanta, and her associates. “Prevention efforts should focus on increasing Tdap coverage during pregnancy, because this is currently our best strategy for providing direct protection to the infant, regardless of the changing source of infant infection.”
Bordetella pertussis infects up to 80% of exposed, naive individuals and is particularly risky for infants, the researchers noted. To examine infection sources for this age group, they studied 1,306 pertussis cases among children less than 1 year old that were reported to the Enhanced Pertussis Surveillance program between 2006 and 2013. The program tracks pertussis in Colorado, Connecticut, Massachusetts, Minnesota, New Mexico, New York, and Oregon. For each case, the researchers attempted to identify the infection source – a person with suspected pertussis who had contact with the infant 7-20 days before cough onset (Pediatrics 2015 Sep 7. doi: 10.1542/peds.2015-1120). A source of infection was identified in 43.6% of cases.
Siblings were the infection source for 36% of infants, followed by mothers (21%), fathers (10%), grandparents (8%), and aunts and uncles (7%), the investigators reported. The source was unspecified for 6% of cases, while ill day care contacts, cousins, friends, babysitters, and other contacts accounted for less than 5% of infections each.
“Consistent with previous studies, our analysis of 8 years of enhanced surveillance data identified a source of infection for less than half of reported infant pertussis cases,” the researchers emphasized. Such a high proportion of unknown infection sources limits the efficacy of the cocooning strategy, in which a Tdap booster dose is given to adults and adolescents who are in close contact with infants, the investigators added. “The cocooning strategy is less than ideal, and strong support of vaccination during pregnancy is needed to maximize the protection of infants in the first critical months of life.”
The Enhanced Pertussis Surveillance Program is funded through a cooperative agreement with the Centers for Disease Control and Prevention. The investigators reported no financial disclosures.
FROM PEDIATRICS
Key clinical point: Siblings were the most common source of infant pertussis in a national surveillance study.
Major finding: Siblings were the infection source for 36% of cases, followed by mothers (21%), fathers (10%), and grandparents (8%).
Data source: Analysis of 1,306 infant pertussis cases identified through the Enhanced Pertussis Surveillance program between 2006 and 2013.
Disclosures: The Enhanced Pertussis Surveillance Program is funded through a cooperative agreement with the Centers for Disease Control and Prevention. The investigators reported no financial disclosures.
Better outcomes seen for some extremely preterm infants
There has been a significant increase in survival without major neonatal morbidity for infants born at 25-28 weeks’ gestation in the United States in the past 2 decades, researchers reported online Sept. 8 in JAMA.
Survival without major complications improved by about 2% per year for these babies, said Dr. Barbara Stoll of Emory University in Atlanta and her associates. But outcomes for earlier preterm births were mixed. “Although overall survival increased for infants aged 23 and 24 weeks, few infants younger than 25 weeks’ gestational age survived without major neonatal morbidity, underscoring the continued need for interventions to improve outcomes for the most immature infants,” the investigators said.
Despite advances in perinatal care, preterm infants face disproportionate rates of morbidity and mortality. The investigators studied outcomes for 34,636 such babies who were born between 1993 and 2012 at 26 U.S. Neonatal Research Network centers, including 8 that participated for the entire study. All the infants were born at 22-28 weeks’ gestation and weighed 401-1,500 g at birth (JAMA 2015;314[10]:1039-51).
“The percent of infants from a multiple birth increased from 18% in 1993 to 27% in 1998 (P less than .001),with no further increase noted,” Dr. Stoll and her associates reported.
Between 2009 and 2012, survival improved the most for infants born at 23 weeks’ gestation (from 27% to 33%) and at 24 weeks’ gestation (from 63% to 65%). But survival increased only slightly for infants born at 25 and 27 weeks’ gestation, and remained static for infants born at 22, 26, and 28 weeks. Furthermore, although survival without major morbidity improved markedly for infants born at 25-28 weeks, it did not change for those born at 24 weeks.
Rates of late-onset sepsis fell among all gestational age groups, but bronchopulmonary dysplasia rose significantly (P less than .001). The latter “may partly be explained by increased active resuscitation, intensive care, and increased survival, especially for the most immature infants,” the investigators said. Use of prenatal corticosteroids rose from 24% to 87% during the study period (P less than .001), as did rates of cesarean delivery (from 44% to 64%; P less than .001), they added.
The preterm registry used in the study was supported by the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Research Resources, and National Center for Advancing Translational Sciences. The investigators reported no financial disclosures.
This article provides an important historical perspective over the last 2 decades in neonatal-perinatal medicine and the most recent update on trends in neonatal care. Although there has been progress, it is clear that there are still a substantial number of extremely preterm infants who either die or survive after experiencing one or more major neonatal morbidities known to be associated with both short- and long-term adverse consequences. Hence, an additional commitment must be made to further improvements.
There is no obvious breakthrough therapy emerging in the coming years. Perhaps cellular therapy, such as mesenchymal stem cells, will be an important advance in the care of these fragile infants. However, it is more likely that incremental change, such as applying quality improvement practices to outcomes other than nosocomial infection, will lead to improved outcomes.
Dr. Roger F. Soll is at the neonatal-perinatal medicine division, department of pediatrics, University of Vermont Medical Center, Burlington. These comments were excerpted from his accompanying editorial (JAMA 2015;314[10]:1007-8). He reported receiving personal fees from the Vermont Oxford Network outside this work.
This article provides an important historical perspective over the last 2 decades in neonatal-perinatal medicine and the most recent update on trends in neonatal care. Although there has been progress, it is clear that there are still a substantial number of extremely preterm infants who either die or survive after experiencing one or more major neonatal morbidities known to be associated with both short- and long-term adverse consequences. Hence, an additional commitment must be made to further improvements.
There is no obvious breakthrough therapy emerging in the coming years. Perhaps cellular therapy, such as mesenchymal stem cells, will be an important advance in the care of these fragile infants. However, it is more likely that incremental change, such as applying quality improvement practices to outcomes other than nosocomial infection, will lead to improved outcomes.
Dr. Roger F. Soll is at the neonatal-perinatal medicine division, department of pediatrics, University of Vermont Medical Center, Burlington. These comments were excerpted from his accompanying editorial (JAMA 2015;314[10]:1007-8). He reported receiving personal fees from the Vermont Oxford Network outside this work.
This article provides an important historical perspective over the last 2 decades in neonatal-perinatal medicine and the most recent update on trends in neonatal care. Although there has been progress, it is clear that there are still a substantial number of extremely preterm infants who either die or survive after experiencing one or more major neonatal morbidities known to be associated with both short- and long-term adverse consequences. Hence, an additional commitment must be made to further improvements.
There is no obvious breakthrough therapy emerging in the coming years. Perhaps cellular therapy, such as mesenchymal stem cells, will be an important advance in the care of these fragile infants. However, it is more likely that incremental change, such as applying quality improvement practices to outcomes other than nosocomial infection, will lead to improved outcomes.
Dr. Roger F. Soll is at the neonatal-perinatal medicine division, department of pediatrics, University of Vermont Medical Center, Burlington. These comments were excerpted from his accompanying editorial (JAMA 2015;314[10]:1007-8). He reported receiving personal fees from the Vermont Oxford Network outside this work.
There has been a significant increase in survival without major neonatal morbidity for infants born at 25-28 weeks’ gestation in the United States in the past 2 decades, researchers reported online Sept. 8 in JAMA.
Survival without major complications improved by about 2% per year for these babies, said Dr. Barbara Stoll of Emory University in Atlanta and her associates. But outcomes for earlier preterm births were mixed. “Although overall survival increased for infants aged 23 and 24 weeks, few infants younger than 25 weeks’ gestational age survived without major neonatal morbidity, underscoring the continued need for interventions to improve outcomes for the most immature infants,” the investigators said.
Despite advances in perinatal care, preterm infants face disproportionate rates of morbidity and mortality. The investigators studied outcomes for 34,636 such babies who were born between 1993 and 2012 at 26 U.S. Neonatal Research Network centers, including 8 that participated for the entire study. All the infants were born at 22-28 weeks’ gestation and weighed 401-1,500 g at birth (JAMA 2015;314[10]:1039-51).
“The percent of infants from a multiple birth increased from 18% in 1993 to 27% in 1998 (P less than .001),with no further increase noted,” Dr. Stoll and her associates reported.
Between 2009 and 2012, survival improved the most for infants born at 23 weeks’ gestation (from 27% to 33%) and at 24 weeks’ gestation (from 63% to 65%). But survival increased only slightly for infants born at 25 and 27 weeks’ gestation, and remained static for infants born at 22, 26, and 28 weeks. Furthermore, although survival without major morbidity improved markedly for infants born at 25-28 weeks, it did not change for those born at 24 weeks.
Rates of late-onset sepsis fell among all gestational age groups, but bronchopulmonary dysplasia rose significantly (P less than .001). The latter “may partly be explained by increased active resuscitation, intensive care, and increased survival, especially for the most immature infants,” the investigators said. Use of prenatal corticosteroids rose from 24% to 87% during the study period (P less than .001), as did rates of cesarean delivery (from 44% to 64%; P less than .001), they added.
The preterm registry used in the study was supported by the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Research Resources, and National Center for Advancing Translational Sciences. The investigators reported no financial disclosures.
There has been a significant increase in survival without major neonatal morbidity for infants born at 25-28 weeks’ gestation in the United States in the past 2 decades, researchers reported online Sept. 8 in JAMA.
Survival without major complications improved by about 2% per year for these babies, said Dr. Barbara Stoll of Emory University in Atlanta and her associates. But outcomes for earlier preterm births were mixed. “Although overall survival increased for infants aged 23 and 24 weeks, few infants younger than 25 weeks’ gestational age survived without major neonatal morbidity, underscoring the continued need for interventions to improve outcomes for the most immature infants,” the investigators said.
Despite advances in perinatal care, preterm infants face disproportionate rates of morbidity and mortality. The investigators studied outcomes for 34,636 such babies who were born between 1993 and 2012 at 26 U.S. Neonatal Research Network centers, including 8 that participated for the entire study. All the infants were born at 22-28 weeks’ gestation and weighed 401-1,500 g at birth (JAMA 2015;314[10]:1039-51).
“The percent of infants from a multiple birth increased from 18% in 1993 to 27% in 1998 (P less than .001),with no further increase noted,” Dr. Stoll and her associates reported.
Between 2009 and 2012, survival improved the most for infants born at 23 weeks’ gestation (from 27% to 33%) and at 24 weeks’ gestation (from 63% to 65%). But survival increased only slightly for infants born at 25 and 27 weeks’ gestation, and remained static for infants born at 22, 26, and 28 weeks. Furthermore, although survival without major morbidity improved markedly for infants born at 25-28 weeks, it did not change for those born at 24 weeks.
Rates of late-onset sepsis fell among all gestational age groups, but bronchopulmonary dysplasia rose significantly (P less than .001). The latter “may partly be explained by increased active resuscitation, intensive care, and increased survival, especially for the most immature infants,” the investigators said. Use of prenatal corticosteroids rose from 24% to 87% during the study period (P less than .001), as did rates of cesarean delivery (from 44% to 64%; P less than .001), they added.
The preterm registry used in the study was supported by the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Research Resources, and National Center for Advancing Translational Sciences. The investigators reported no financial disclosures.
FROM JAMA
Key clinical point: There has been a significant increase in survival without major neonatal morbidity for infants born at 25-28 weeks’ gestation in the United States in the past 2 decades.
Major finding: Survival without major morbidity rose by about 2% per year for infants born at 25-28 weeks’ gestation.
Data source: A prospective registry study of 34,636 extremely preterm infants born at U.S. academic centers between 1993 and 2012.
Disclosures: The preterm registry used in the study was supported by the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Research Resources, and National Center for Advancing Translational Sciences. The investigators reported no financial disclosures.
H Pylori Resistance Highlights Need for Guided Therapy
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Gluten Exposure Tied to Worsened GI and Extraintestinal Symptoms in Gluten Sensitivity
Consuming the equivalent of two slices of bread a day for just 1 week significantly worsened abdominal pain, bloating, foggy mind, depression, and aphthous stomatitis among patients with self-diagnosed gluten intolerance, according to a double-blind, placebo-controlled, crossover study reported in the September issue of Clinical Gastroenterology and Hepatology.
The study is unique because its patients were referred for intestinal and extraintestinal symptoms of gluten sensitivity, rather than irritable bowel syndrome, said Dr. Antonio Di Sabatino at the University of Pavia (Italy) and his associates. The study also used gluten capsules that had been shown to be indistinguishable from placebo, excluded patients with minimal baseline symptoms, and used global response as the primary outcome measure because that is patients’ major concern, the investigators said.
The existence and definition of nonceliac gluten sensitivity (NCGS) remains debatable even as patients in “crowded online forums” diagnose themselves with it, the researchers noted.
To test if NCGS exists and if so, the amount of gluten needed to cause symptoms, they conducted a randomized, double-blind, placebo-controlled trial of 61 self-diagnosed patients with no history of wheat allergy or celiac disease. Patients ingested either 4.375 g of gluten – the equivalent of two slices of wheat bread – or placebo rice starch in capsule form every day for 1 week. Then they followed a gluten-free diet for a week before crossing over to the other study arm. Each day, patients were asked to grade 15 intestinal symptoms and 13 extraintestinal symptoms on a scale of 0 (absent) to 3 (severe and interfering with daily activities). The group averaged 39 years of age, and 87% were female, the investigators said (Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.01.029).
Among 59 patients who finished the trial, gluten intake was tied to significantly higher overall symptom scores, compared with placebo (median score, 55 vs. 33; P = .034), said the researchers. Patients also reported significantly worse abdominal bloating (P = .04), abdominal pain (P = .047), aphthous stomatitis (P = .025), foggy mind (P = .019), and depression (P = .02) when they ingested gluten, compared with placebo.
“The observation that short-term exposure to gluten induced depression is remarkable,” Dr. Di Sabatino and his associates commented. “This result was supported by a recent double-blind, placebo-controlled, crossover study in which depression was assessed by an ad hoc psychiatric score (Aliment Pharmacol Ther. 2014 May;39:1104-12). The direct, highly significant correlation between symptom score and symptom prevalence at both the intestinal and extraintestinal levels is indirect proof of the validity of our findings.”
The study did not yield data on identifiable biomarkers, nor did it pinpoint pathogenic mechanisms for NCGS, the investigators acknowledged.
“Self-prescription of gluten withdrawal is becoming increasingly common, but this behavior should be strongly discouraged because it may lead to the consequent preclusion of a proper diagnosis of celiac disease and to a high and unjustified economic burden,” they emphasized. “Because a reliable marker of NCGS is not readily available at present, double-blind, placebo-controlled trials are mandatory to ascertain this condition.” Their laboratory is working to identify cytokines in the duodenal mucosa of patients in the trial, and early results have not implicated innate or adaptive immune mechanisms for NCGS, they said.
St. Matteo Hospital Foundation supported the research, and Giuliani Pharma provided the capsules used in the study. The investigators reported having no conflicts of interest.
Consuming the equivalent of two slices of bread a day for just 1 week significantly worsened abdominal pain, bloating, foggy mind, depression, and aphthous stomatitis among patients with self-diagnosed gluten intolerance, according to a double-blind, placebo-controlled, crossover study reported in the September issue of Clinical Gastroenterology and Hepatology.
The study is unique because its patients were referred for intestinal and extraintestinal symptoms of gluten sensitivity, rather than irritable bowel syndrome, said Dr. Antonio Di Sabatino at the University of Pavia (Italy) and his associates. The study also used gluten capsules that had been shown to be indistinguishable from placebo, excluded patients with minimal baseline symptoms, and used global response as the primary outcome measure because that is patients’ major concern, the investigators said.
The existence and definition of nonceliac gluten sensitivity (NCGS) remains debatable even as patients in “crowded online forums” diagnose themselves with it, the researchers noted.
To test if NCGS exists and if so, the amount of gluten needed to cause symptoms, they conducted a randomized, double-blind, placebo-controlled trial of 61 self-diagnosed patients with no history of wheat allergy or celiac disease. Patients ingested either 4.375 g of gluten – the equivalent of two slices of wheat bread – or placebo rice starch in capsule form every day for 1 week. Then they followed a gluten-free diet for a week before crossing over to the other study arm. Each day, patients were asked to grade 15 intestinal symptoms and 13 extraintestinal symptoms on a scale of 0 (absent) to 3 (severe and interfering with daily activities). The group averaged 39 years of age, and 87% were female, the investigators said (Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.01.029).
Among 59 patients who finished the trial, gluten intake was tied to significantly higher overall symptom scores, compared with placebo (median score, 55 vs. 33; P = .034), said the researchers. Patients also reported significantly worse abdominal bloating (P = .04), abdominal pain (P = .047), aphthous stomatitis (P = .025), foggy mind (P = .019), and depression (P = .02) when they ingested gluten, compared with placebo.
“The observation that short-term exposure to gluten induced depression is remarkable,” Dr. Di Sabatino and his associates commented. “This result was supported by a recent double-blind, placebo-controlled, crossover study in which depression was assessed by an ad hoc psychiatric score (Aliment Pharmacol Ther. 2014 May;39:1104-12). The direct, highly significant correlation between symptom score and symptom prevalence at both the intestinal and extraintestinal levels is indirect proof of the validity of our findings.”
The study did not yield data on identifiable biomarkers, nor did it pinpoint pathogenic mechanisms for NCGS, the investigators acknowledged.
“Self-prescription of gluten withdrawal is becoming increasingly common, but this behavior should be strongly discouraged because it may lead to the consequent preclusion of a proper diagnosis of celiac disease and to a high and unjustified economic burden,” they emphasized. “Because a reliable marker of NCGS is not readily available at present, double-blind, placebo-controlled trials are mandatory to ascertain this condition.” Their laboratory is working to identify cytokines in the duodenal mucosa of patients in the trial, and early results have not implicated innate or adaptive immune mechanisms for NCGS, they said.
St. Matteo Hospital Foundation supported the research, and Giuliani Pharma provided the capsules used in the study. The investigators reported having no conflicts of interest.
Consuming the equivalent of two slices of bread a day for just 1 week significantly worsened abdominal pain, bloating, foggy mind, depression, and aphthous stomatitis among patients with self-diagnosed gluten intolerance, according to a double-blind, placebo-controlled, crossover study reported in the September issue of Clinical Gastroenterology and Hepatology.
The study is unique because its patients were referred for intestinal and extraintestinal symptoms of gluten sensitivity, rather than irritable bowel syndrome, said Dr. Antonio Di Sabatino at the University of Pavia (Italy) and his associates. The study also used gluten capsules that had been shown to be indistinguishable from placebo, excluded patients with minimal baseline symptoms, and used global response as the primary outcome measure because that is patients’ major concern, the investigators said.
The existence and definition of nonceliac gluten sensitivity (NCGS) remains debatable even as patients in “crowded online forums” diagnose themselves with it, the researchers noted.
To test if NCGS exists and if so, the amount of gluten needed to cause symptoms, they conducted a randomized, double-blind, placebo-controlled trial of 61 self-diagnosed patients with no history of wheat allergy or celiac disease. Patients ingested either 4.375 g of gluten – the equivalent of two slices of wheat bread – or placebo rice starch in capsule form every day for 1 week. Then they followed a gluten-free diet for a week before crossing over to the other study arm. Each day, patients were asked to grade 15 intestinal symptoms and 13 extraintestinal symptoms on a scale of 0 (absent) to 3 (severe and interfering with daily activities). The group averaged 39 years of age, and 87% were female, the investigators said (Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.01.029).
Among 59 patients who finished the trial, gluten intake was tied to significantly higher overall symptom scores, compared with placebo (median score, 55 vs. 33; P = .034), said the researchers. Patients also reported significantly worse abdominal bloating (P = .04), abdominal pain (P = .047), aphthous stomatitis (P = .025), foggy mind (P = .019), and depression (P = .02) when they ingested gluten, compared with placebo.
“The observation that short-term exposure to gluten induced depression is remarkable,” Dr. Di Sabatino and his associates commented. “This result was supported by a recent double-blind, placebo-controlled, crossover study in which depression was assessed by an ad hoc psychiatric score (Aliment Pharmacol Ther. 2014 May;39:1104-12). The direct, highly significant correlation between symptom score and symptom prevalence at both the intestinal and extraintestinal levels is indirect proof of the validity of our findings.”
The study did not yield data on identifiable biomarkers, nor did it pinpoint pathogenic mechanisms for NCGS, the investigators acknowledged.
“Self-prescription of gluten withdrawal is becoming increasingly common, but this behavior should be strongly discouraged because it may lead to the consequent preclusion of a proper diagnosis of celiac disease and to a high and unjustified economic burden,” they emphasized. “Because a reliable marker of NCGS is not readily available at present, double-blind, placebo-controlled trials are mandatory to ascertain this condition.” Their laboratory is working to identify cytokines in the duodenal mucosa of patients in the trial, and early results have not implicated innate or adaptive immune mechanisms for NCGS, they said.
St. Matteo Hospital Foundation supported the research, and Giuliani Pharma provided the capsules used in the study. The investigators reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
H. pylori resistance highlights need for guided therapy
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Because H. pylori showed high rates of resistance to clarithromycin, metronidazole, and levofloxacin, they should be excluded from triple therapy regimens for helicobacteriosis.
Major finding: Half of strains were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin, 20% were resistant to metronidazole, 16% were resistant to clarithromycin, 0.8% were resistant to tetracycline, and none were resistant to amoxicillin.
Data source: Analysis of gastric biopsies from 656 U.S. veterans who underwent esophagogastroduodenoscopy in Texas between 2009 and 2013.
Disclosures: The National Institutes of Health and the VA Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Ledipasvir-sofosbuvir plus ribavirin in advanced HCV does well
Combination treatment with ledipasvir, sofosbuvir, and ribavirin for chronic hepatitis C virus infection led to sustained viral response (SVR) rates of 86%-89% among nontransplanted patients and 60%-100% among transplant recipients, according to a study published in the September issue of Gastroenterology.
“Rates of SVR were over 85% in every group of patients with Child-Pugh class [CPC] B decompensated cirrhosis – in those who had and had not undergone liver transplantation, as well as those who were receiving 12 and 24 weeks of treatment,” said Dr. Michael Charlton of Intermountain Medical Center in Salt Lake City, and his associates. “Similar response rates were observed in Child-Pugh class C patients who had not undergone liver transplantation. Rates of response were numerically lower in liver transplant recipients with Child-Pugh class C disease, but the small sample size ... makes this a preliminary observation.”
Patients with chronic HCV infection and advanced liver disease have a poor prognosis because of their risk of liver failure and hepatocellular carcinoma and because they have very limited treatment options. Chances of reinfection are 100% if patients have detectable virus at transplantation, and reinfection increases rates of subsequent graft loss, morbidity, and mortality, the investigators noted.
To assess the recently approved sofosbuvir-ledipasvir regimen, they conducted an open-label, phase II trial (SOLAR-1) of the combination plus ribavirin in 337 HCV-infected patients with advanced liver disease, including transplant recipients. Nearly all (99%) of the patients had HCV genotype 1 infection, while 1% had genotype 4 infection. The investigators randomized patients to receive 90 mg ledipasvir and 400 mg sofosbuvir in a fixed-dose daily regimen for either 12 or 24 weeks in combination with ribavirin, which was dosed based on body weight, tolerance of side effects, and extent of liver disease. Patients with CPC C cirrhosis received lower doses of ribavirin because of increased risk of hemotoxicity (Gastroenterology 2015 May 15. doi:10.1053/j.gastro.2015.05.010).
Nontransplanted patients who had decompensated cirrhosis achieved SVR12 or SVR24 rates of 86%-89%, even if they had CPC C decompensated disease, the researchers reported. “Our results also demonstrate that SVR12 in patients with decompensated cirrhosis is associated with early improvements in CPC and MELD [Model for End-Stage Liver Disease] scores, suggesting that eradication of the virus can rapidly improve hepatic function by attenuating the injury and inflammation due to HCV replication,” they said. Patients with CPC B disease also had significant improvements in albumin and bilirubin levels after 4 weeks of treatment.
Among transplant recipients who were noncirrhotic or were in compensated cirrhosis, SVR rates ranged from 96% to 98%, regardless of treatment duration or presence of cirrhosis, Dr. Charlton and associates reported. Among transplant recipients with decompensated cirrhosis, SVR rates were 86%-88% for the subgroup with CPC B disease, but were 60% for CPC C patients who were treated for 12 weeks, and were 75% for CPC C patients who were treated for 24 weeks. In contrast, all six patients with fibrosing cholestatic hepatitis achieved SVR and also experienced large drops in total bilirubin levels within 2-4 weeks of starting treatment, the researchers said. Finally, among the seven patients who underwent liver transplantation either during treatment or before posttreatment week 12, six achieved virologic response after surgery, and one died of multiorgan failure and septic shock 1 day after testing negative for HCV RNA.
In all, 13 patients (4%) discontinued treatment because of adverse events, primarily liver decompensation, the researchers reported. Adverse events that led more than one patient to stop treatment included sepsis, renal failure, dyspnea, and gastrointestinal hemorrhage. Patients were more likely to stop taking ribavirin if they were treated for 24 weeks or had worsening disease. Ten patients died on study, but none of the deaths were thought to be related to treatment, the researchers wrote.
Dr. Charlton disclosed ties with Gilead Sciences, maker of sofosbuvir and ledipasvir. Gilead funded the study. Four coauthors reported having no conflicts; the other 25 investigators reported employment or other financial ties with numerous pharmaceutical companies.
Combination treatment with ledipasvir, sofosbuvir, and ribavirin for chronic hepatitis C virus infection led to sustained viral response (SVR) rates of 86%-89% among nontransplanted patients and 60%-100% among transplant recipients, according to a study published in the September issue of Gastroenterology.
“Rates of SVR were over 85% in every group of patients with Child-Pugh class [CPC] B decompensated cirrhosis – in those who had and had not undergone liver transplantation, as well as those who were receiving 12 and 24 weeks of treatment,” said Dr. Michael Charlton of Intermountain Medical Center in Salt Lake City, and his associates. “Similar response rates were observed in Child-Pugh class C patients who had not undergone liver transplantation. Rates of response were numerically lower in liver transplant recipients with Child-Pugh class C disease, but the small sample size ... makes this a preliminary observation.”
Patients with chronic HCV infection and advanced liver disease have a poor prognosis because of their risk of liver failure and hepatocellular carcinoma and because they have very limited treatment options. Chances of reinfection are 100% if patients have detectable virus at transplantation, and reinfection increases rates of subsequent graft loss, morbidity, and mortality, the investigators noted.
To assess the recently approved sofosbuvir-ledipasvir regimen, they conducted an open-label, phase II trial (SOLAR-1) of the combination plus ribavirin in 337 HCV-infected patients with advanced liver disease, including transplant recipients. Nearly all (99%) of the patients had HCV genotype 1 infection, while 1% had genotype 4 infection. The investigators randomized patients to receive 90 mg ledipasvir and 400 mg sofosbuvir in a fixed-dose daily regimen for either 12 or 24 weeks in combination with ribavirin, which was dosed based on body weight, tolerance of side effects, and extent of liver disease. Patients with CPC C cirrhosis received lower doses of ribavirin because of increased risk of hemotoxicity (Gastroenterology 2015 May 15. doi:10.1053/j.gastro.2015.05.010).
Nontransplanted patients who had decompensated cirrhosis achieved SVR12 or SVR24 rates of 86%-89%, even if they had CPC C decompensated disease, the researchers reported. “Our results also demonstrate that SVR12 in patients with decompensated cirrhosis is associated with early improvements in CPC and MELD [Model for End-Stage Liver Disease] scores, suggesting that eradication of the virus can rapidly improve hepatic function by attenuating the injury and inflammation due to HCV replication,” they said. Patients with CPC B disease also had significant improvements in albumin and bilirubin levels after 4 weeks of treatment.
Among transplant recipients who were noncirrhotic or were in compensated cirrhosis, SVR rates ranged from 96% to 98%, regardless of treatment duration or presence of cirrhosis, Dr. Charlton and associates reported. Among transplant recipients with decompensated cirrhosis, SVR rates were 86%-88% for the subgroup with CPC B disease, but were 60% for CPC C patients who were treated for 12 weeks, and were 75% for CPC C patients who were treated for 24 weeks. In contrast, all six patients with fibrosing cholestatic hepatitis achieved SVR and also experienced large drops in total bilirubin levels within 2-4 weeks of starting treatment, the researchers said. Finally, among the seven patients who underwent liver transplantation either during treatment or before posttreatment week 12, six achieved virologic response after surgery, and one died of multiorgan failure and septic shock 1 day after testing negative for HCV RNA.
In all, 13 patients (4%) discontinued treatment because of adverse events, primarily liver decompensation, the researchers reported. Adverse events that led more than one patient to stop treatment included sepsis, renal failure, dyspnea, and gastrointestinal hemorrhage. Patients were more likely to stop taking ribavirin if they were treated for 24 weeks or had worsening disease. Ten patients died on study, but none of the deaths were thought to be related to treatment, the researchers wrote.
Dr. Charlton disclosed ties with Gilead Sciences, maker of sofosbuvir and ledipasvir. Gilead funded the study. Four coauthors reported having no conflicts; the other 25 investigators reported employment or other financial ties with numerous pharmaceutical companies.
Combination treatment with ledipasvir, sofosbuvir, and ribavirin for chronic hepatitis C virus infection led to sustained viral response (SVR) rates of 86%-89% among nontransplanted patients and 60%-100% among transplant recipients, according to a study published in the September issue of Gastroenterology.
“Rates of SVR were over 85% in every group of patients with Child-Pugh class [CPC] B decompensated cirrhosis – in those who had and had not undergone liver transplantation, as well as those who were receiving 12 and 24 weeks of treatment,” said Dr. Michael Charlton of Intermountain Medical Center in Salt Lake City, and his associates. “Similar response rates were observed in Child-Pugh class C patients who had not undergone liver transplantation. Rates of response were numerically lower in liver transplant recipients with Child-Pugh class C disease, but the small sample size ... makes this a preliminary observation.”
Patients with chronic HCV infection and advanced liver disease have a poor prognosis because of their risk of liver failure and hepatocellular carcinoma and because they have very limited treatment options. Chances of reinfection are 100% if patients have detectable virus at transplantation, and reinfection increases rates of subsequent graft loss, morbidity, and mortality, the investigators noted.
To assess the recently approved sofosbuvir-ledipasvir regimen, they conducted an open-label, phase II trial (SOLAR-1) of the combination plus ribavirin in 337 HCV-infected patients with advanced liver disease, including transplant recipients. Nearly all (99%) of the patients had HCV genotype 1 infection, while 1% had genotype 4 infection. The investigators randomized patients to receive 90 mg ledipasvir and 400 mg sofosbuvir in a fixed-dose daily regimen for either 12 or 24 weeks in combination with ribavirin, which was dosed based on body weight, tolerance of side effects, and extent of liver disease. Patients with CPC C cirrhosis received lower doses of ribavirin because of increased risk of hemotoxicity (Gastroenterology 2015 May 15. doi:10.1053/j.gastro.2015.05.010).
Nontransplanted patients who had decompensated cirrhosis achieved SVR12 or SVR24 rates of 86%-89%, even if they had CPC C decompensated disease, the researchers reported. “Our results also demonstrate that SVR12 in patients with decompensated cirrhosis is associated with early improvements in CPC and MELD [Model for End-Stage Liver Disease] scores, suggesting that eradication of the virus can rapidly improve hepatic function by attenuating the injury and inflammation due to HCV replication,” they said. Patients with CPC B disease also had significant improvements in albumin and bilirubin levels after 4 weeks of treatment.
Among transplant recipients who were noncirrhotic or were in compensated cirrhosis, SVR rates ranged from 96% to 98%, regardless of treatment duration or presence of cirrhosis, Dr. Charlton and associates reported. Among transplant recipients with decompensated cirrhosis, SVR rates were 86%-88% for the subgroup with CPC B disease, but were 60% for CPC C patients who were treated for 12 weeks, and were 75% for CPC C patients who were treated for 24 weeks. In contrast, all six patients with fibrosing cholestatic hepatitis achieved SVR and also experienced large drops in total bilirubin levels within 2-4 weeks of starting treatment, the researchers said. Finally, among the seven patients who underwent liver transplantation either during treatment or before posttreatment week 12, six achieved virologic response after surgery, and one died of multiorgan failure and septic shock 1 day after testing negative for HCV RNA.
In all, 13 patients (4%) discontinued treatment because of adverse events, primarily liver decompensation, the researchers reported. Adverse events that led more than one patient to stop treatment included sepsis, renal failure, dyspnea, and gastrointestinal hemorrhage. Patients were more likely to stop taking ribavirin if they were treated for 24 weeks or had worsening disease. Ten patients died on study, but none of the deaths were thought to be related to treatment, the researchers wrote.
Dr. Charlton disclosed ties with Gilead Sciences, maker of sofosbuvir and ledipasvir. Gilead funded the study. Four coauthors reported having no conflicts; the other 25 investigators reported employment or other financial ties with numerous pharmaceutical companies.
FROM GASTROENTEROLOGY
Key clinical point: Ledipasvir-sofosbuvir plus ribavirin for 12 weeks achieved high SVR rates among patients with hepatitis C virus infection and advanced liver disease.
Major finding: Rates of SVR exceeded 85% in every cohort of Child-Pugh class B decompensated cirrhosis, regardless of treatment duration or transplantation status.
Data source: Randomized open-label phase II study of 337 patients with chronic HCV infection and advanced liver disease.
Disclosures: Dr. Charlton disclosed ties with Gilead Sciences, maker of sofosbuvir and ledipasvir. Gilead funded the study. Four coauthors reported having no conflicts; the other 25 investigators reported employment or other financial ties with numerous pharmaceutical companies.