User login
Fetal exposure to depression: How does ‘dose’ figure in?
The last two decades have seen an ever-growing number of reports on risks of fetal exposure to medicines used to treat depression during pregnancy. These reports have described issues ranging from estimated risk of congenital malformations following fetal exposure to various psychotropics such as SSRIs or atypical antipsychotics to adverse neonatal effects such as poor neonatal adaptation syndrome. More recent reports, derived primarily from large administrative databases, have focused on concerns regarding both risk for later childhood psychopathology such as autism or ADHD or neurobehavioral sequelae such as motor or speech delay following fetal exposure to antidepressants.
When considering the potential risks of fetal exposure to antidepressants on the spectrum of relevant outcomes, it is important to keep in mind the risks of not receiving antidepressant treatment. Data on known risks of antidepressant use during pregnancy are well described, but the literature supporting adverse effects of untreated depression during pregnancy has also grown substantially. For example, accumulated data over the last several years supports heightened risk for obstetrical and neonatal complications among women who suffer from untreated depression and recent data is inconclusive regarding the effects of untreated maternal depression on gene expression in the CNS during gestation and the effects of depression during pregnancy on development of actual brain structures in areas of the brain that modulate emotion and behavior.
The ability to factor “dose and duration” of exposure to perinatal psychiatric illness into a model predicting risk for a number of obstetrical or neonatal outcomes allows for a more refined risk-benefit decision with respect to use of antidepressants during pregnancy. For example, there may be a threshold over which it’s even more imperative to treat depression during pregnancy than in women who do not suffer from such severe histories of psychiatric disorder.
Research along these lines has been published in a study in Nursing Research in which the question of the effect of maternal mood on infant outcomes was examined, specifically looking at stress, depression, and intimate partner violence, and not just the presence of these elements, but their duration and intensity both before and during the pregnancy (2015 Sep-Oct;64[5]:331-41).
To do this, researchers examined survey data from Utah’s Pregnancy Risk Assessment Monitoring System of 4,296 women who gave birth during 2009-2011. Stress, depression, and intimate partner violence, and the duration and severity of each, were determined by questionnaire. Those determinations were compared with the outcomes of gestational age, birth weight, neonatal ICU admission, and the symptoms and diagnosis of postpartum depression.
Results of the study included the following: Increased duration of depression was associated with a greater risk of neonatal ICU admission, particularly in women who were depressed both before and during their pregnancy (adjusted odds ratio, 2.48), compared with women who had no depression.
We’ve known for a long time that a history of depression predicts increased risk for postpartum depression. In this particular study, it was actually shown that not just a history of depression, but the duration of experienced depression influenced the risk for postpartum depression.
For example, compared with women with no depression, women who were depressed before but not during their pregnancy had an aOR of 7.67, women depressed during pregnancy but not before had an aOR of 17.65, and women depressed both before and during pregnancy had an aOR of 58.35 – an extraordinary stratification of risk, basically.
What these data begin to suggest is that there may be a continuum of risk when it comes to the effects of exposure to depression (factoring in now dose and duration of exposure) during pregnancy. If risk of adverse outcome increases with greater severity of perinatal psychiatric illness, then a mandate to treat depression during pregnancy, whether with pharmacologic or nonpharmacologic interventions (or, commonly, a combination of the two) becomes that much more imperative. Regardless of the treatment interventions that are used, Such a recommendation dovetails with the literature showing the intergenerational effects of untreated depression. Maternal depression is one of the strongest predictors of later childhood psychopathology. With current national trends moving toward mandating screening initiatives for postpartum depression, the appreciation of the extent to which depression before and during pregnancy drives risk for postpartum mood disorder broadens how we think about mitigating risk for puerperal mood disturbance. Specifically, mitigating the effects of postpartum depression on women, their children, and their families must include more effective management of depression both before and during pregnancy.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
The last two decades have seen an ever-growing number of reports on risks of fetal exposure to medicines used to treat depression during pregnancy. These reports have described issues ranging from estimated risk of congenital malformations following fetal exposure to various psychotropics such as SSRIs or atypical antipsychotics to adverse neonatal effects such as poor neonatal adaptation syndrome. More recent reports, derived primarily from large administrative databases, have focused on concerns regarding both risk for later childhood psychopathology such as autism or ADHD or neurobehavioral sequelae such as motor or speech delay following fetal exposure to antidepressants.
When considering the potential risks of fetal exposure to antidepressants on the spectrum of relevant outcomes, it is important to keep in mind the risks of not receiving antidepressant treatment. Data on known risks of antidepressant use during pregnancy are well described, but the literature supporting adverse effects of untreated depression during pregnancy has also grown substantially. For example, accumulated data over the last several years supports heightened risk for obstetrical and neonatal complications among women who suffer from untreated depression and recent data is inconclusive regarding the effects of untreated maternal depression on gene expression in the CNS during gestation and the effects of depression during pregnancy on development of actual brain structures in areas of the brain that modulate emotion and behavior.
The ability to factor “dose and duration” of exposure to perinatal psychiatric illness into a model predicting risk for a number of obstetrical or neonatal outcomes allows for a more refined risk-benefit decision with respect to use of antidepressants during pregnancy. For example, there may be a threshold over which it’s even more imperative to treat depression during pregnancy than in women who do not suffer from such severe histories of psychiatric disorder.
Research along these lines has been published in a study in Nursing Research in which the question of the effect of maternal mood on infant outcomes was examined, specifically looking at stress, depression, and intimate partner violence, and not just the presence of these elements, but their duration and intensity both before and during the pregnancy (2015 Sep-Oct;64[5]:331-41).
To do this, researchers examined survey data from Utah’s Pregnancy Risk Assessment Monitoring System of 4,296 women who gave birth during 2009-2011. Stress, depression, and intimate partner violence, and the duration and severity of each, were determined by questionnaire. Those determinations were compared with the outcomes of gestational age, birth weight, neonatal ICU admission, and the symptoms and diagnosis of postpartum depression.
Results of the study included the following: Increased duration of depression was associated with a greater risk of neonatal ICU admission, particularly in women who were depressed both before and during their pregnancy (adjusted odds ratio, 2.48), compared with women who had no depression.
We’ve known for a long time that a history of depression predicts increased risk for postpartum depression. In this particular study, it was actually shown that not just a history of depression, but the duration of experienced depression influenced the risk for postpartum depression.
For example, compared with women with no depression, women who were depressed before but not during their pregnancy had an aOR of 7.67, women depressed during pregnancy but not before had an aOR of 17.65, and women depressed both before and during pregnancy had an aOR of 58.35 – an extraordinary stratification of risk, basically.
What these data begin to suggest is that there may be a continuum of risk when it comes to the effects of exposure to depression (factoring in now dose and duration of exposure) during pregnancy. If risk of adverse outcome increases with greater severity of perinatal psychiatric illness, then a mandate to treat depression during pregnancy, whether with pharmacologic or nonpharmacologic interventions (or, commonly, a combination of the two) becomes that much more imperative. Regardless of the treatment interventions that are used, Such a recommendation dovetails with the literature showing the intergenerational effects of untreated depression. Maternal depression is one of the strongest predictors of later childhood psychopathology. With current national trends moving toward mandating screening initiatives for postpartum depression, the appreciation of the extent to which depression before and during pregnancy drives risk for postpartum mood disorder broadens how we think about mitigating risk for puerperal mood disturbance. Specifically, mitigating the effects of postpartum depression on women, their children, and their families must include more effective management of depression both before and during pregnancy.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
The last two decades have seen an ever-growing number of reports on risks of fetal exposure to medicines used to treat depression during pregnancy. These reports have described issues ranging from estimated risk of congenital malformations following fetal exposure to various psychotropics such as SSRIs or atypical antipsychotics to adverse neonatal effects such as poor neonatal adaptation syndrome. More recent reports, derived primarily from large administrative databases, have focused on concerns regarding both risk for later childhood psychopathology such as autism or ADHD or neurobehavioral sequelae such as motor or speech delay following fetal exposure to antidepressants.
When considering the potential risks of fetal exposure to antidepressants on the spectrum of relevant outcomes, it is important to keep in mind the risks of not receiving antidepressant treatment. Data on known risks of antidepressant use during pregnancy are well described, but the literature supporting adverse effects of untreated depression during pregnancy has also grown substantially. For example, accumulated data over the last several years supports heightened risk for obstetrical and neonatal complications among women who suffer from untreated depression and recent data is inconclusive regarding the effects of untreated maternal depression on gene expression in the CNS during gestation and the effects of depression during pregnancy on development of actual brain structures in areas of the brain that modulate emotion and behavior.
The ability to factor “dose and duration” of exposure to perinatal psychiatric illness into a model predicting risk for a number of obstetrical or neonatal outcomes allows for a more refined risk-benefit decision with respect to use of antidepressants during pregnancy. For example, there may be a threshold over which it’s even more imperative to treat depression during pregnancy than in women who do not suffer from such severe histories of psychiatric disorder.
Research along these lines has been published in a study in Nursing Research in which the question of the effect of maternal mood on infant outcomes was examined, specifically looking at stress, depression, and intimate partner violence, and not just the presence of these elements, but their duration and intensity both before and during the pregnancy (2015 Sep-Oct;64[5]:331-41).
To do this, researchers examined survey data from Utah’s Pregnancy Risk Assessment Monitoring System of 4,296 women who gave birth during 2009-2011. Stress, depression, and intimate partner violence, and the duration and severity of each, were determined by questionnaire. Those determinations were compared with the outcomes of gestational age, birth weight, neonatal ICU admission, and the symptoms and diagnosis of postpartum depression.
Results of the study included the following: Increased duration of depression was associated with a greater risk of neonatal ICU admission, particularly in women who were depressed both before and during their pregnancy (adjusted odds ratio, 2.48), compared with women who had no depression.
We’ve known for a long time that a history of depression predicts increased risk for postpartum depression. In this particular study, it was actually shown that not just a history of depression, but the duration of experienced depression influenced the risk for postpartum depression.
For example, compared with women with no depression, women who were depressed before but not during their pregnancy had an aOR of 7.67, women depressed during pregnancy but not before had an aOR of 17.65, and women depressed both before and during pregnancy had an aOR of 58.35 – an extraordinary stratification of risk, basically.
What these data begin to suggest is that there may be a continuum of risk when it comes to the effects of exposure to depression (factoring in now dose and duration of exposure) during pregnancy. If risk of adverse outcome increases with greater severity of perinatal psychiatric illness, then a mandate to treat depression during pregnancy, whether with pharmacologic or nonpharmacologic interventions (or, commonly, a combination of the two) becomes that much more imperative. Regardless of the treatment interventions that are used, Such a recommendation dovetails with the literature showing the intergenerational effects of untreated depression. Maternal depression is one of the strongest predictors of later childhood psychopathology. With current national trends moving toward mandating screening initiatives for postpartum depression, the appreciation of the extent to which depression before and during pregnancy drives risk for postpartum mood disorder broadens how we think about mitigating risk for puerperal mood disturbance. Specifically, mitigating the effects of postpartum depression on women, their children, and their families must include more effective management of depression both before and during pregnancy.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
What is HIPEC?
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
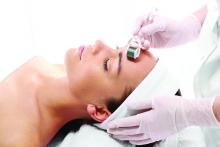
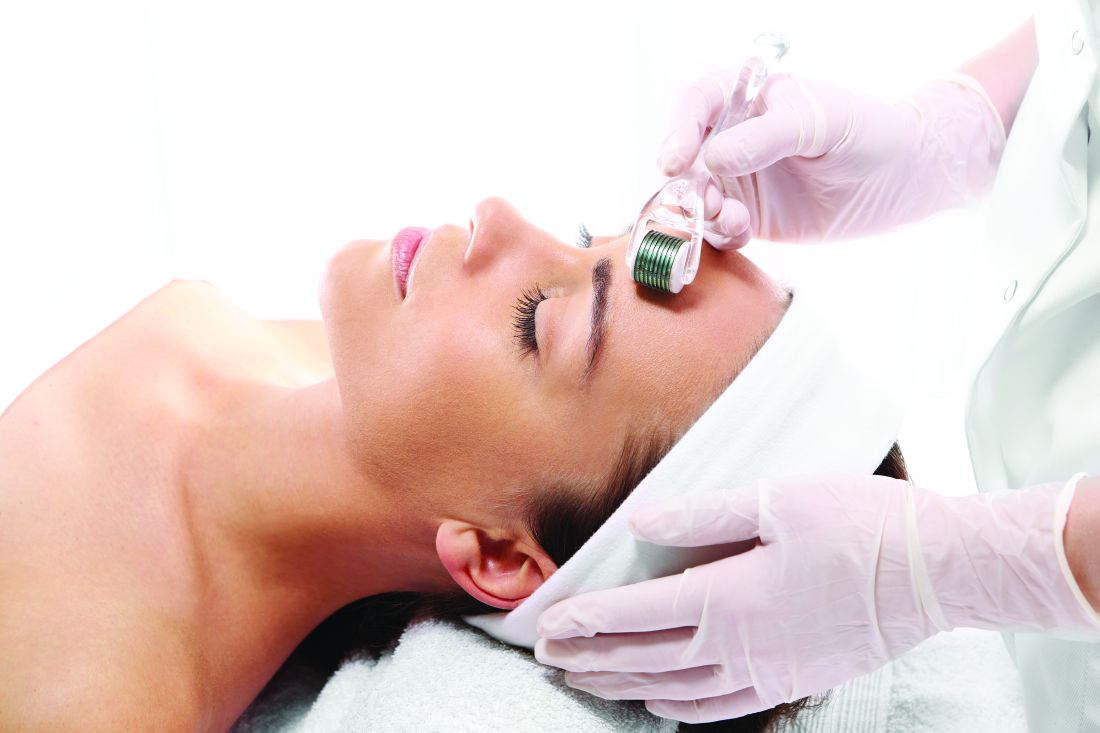
Skin rollers
, but only a few actually have any scientific data or clinical studies supporting their claims. In general, these rollers promise to increase collagen, depuff the skin, lift and firm, increase circulation, increase oxygenation, and decrease inflammation. But no clinically significant results have been reported with most of these over-the-counter devices. Furthermore, not every roller is meant for every skin type – and some should stay within the hands of an experienced professional.
Ice rollers have been used for many years and are very effective to cool the skin for in-office procedures. They are drum-shaped stainless steel rollers that are left in the freezer and cool the epidermis upon application. At-home ice rollers cause immediate vasoconstriction and are a quick fix for periorbital edema or skin erythema. Three-dimensional roller face massagers are simply a massage tool and can be used on any skin type to increase facial circulation; they do not provide any visible clinical benefits. Nanocurrent or vibrating rollers use nanocurrents and vibration alongside a conductor gel to glide across the skin; they massage the skin and help topically applied agents penetrate into the stratum corneum.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Orentreich DS et al. Dermatol Surg. 1995;21(6):543-9.
Aust MC et al. Plast Reconstr Surg. 2008;21(4):1421-9.Fernandes D et al. Clin Dermatol. 2008 Mar-Apr;26(2):192-9.
Nair PA et al. GMJ. 2014;69:24-7.
, but only a few actually have any scientific data or clinical studies supporting their claims. In general, these rollers promise to increase collagen, depuff the skin, lift and firm, increase circulation, increase oxygenation, and decrease inflammation. But no clinically significant results have been reported with most of these over-the-counter devices. Furthermore, not every roller is meant for every skin type – and some should stay within the hands of an experienced professional.
Ice rollers have been used for many years and are very effective to cool the skin for in-office procedures. They are drum-shaped stainless steel rollers that are left in the freezer and cool the epidermis upon application. At-home ice rollers cause immediate vasoconstriction and are a quick fix for periorbital edema or skin erythema. Three-dimensional roller face massagers are simply a massage tool and can be used on any skin type to increase facial circulation; they do not provide any visible clinical benefits. Nanocurrent or vibrating rollers use nanocurrents and vibration alongside a conductor gel to glide across the skin; they massage the skin and help topically applied agents penetrate into the stratum corneum.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Orentreich DS et al. Dermatol Surg. 1995;21(6):543-9.
Aust MC et al. Plast Reconstr Surg. 2008;21(4):1421-9.Fernandes D et al. Clin Dermatol. 2008 Mar-Apr;26(2):192-9.
Nair PA et al. GMJ. 2014;69:24-7.
, but only a few actually have any scientific data or clinical studies supporting their claims. In general, these rollers promise to increase collagen, depuff the skin, lift and firm, increase circulation, increase oxygenation, and decrease inflammation. But no clinically significant results have been reported with most of these over-the-counter devices. Furthermore, not every roller is meant for every skin type – and some should stay within the hands of an experienced professional.
Ice rollers have been used for many years and are very effective to cool the skin for in-office procedures. They are drum-shaped stainless steel rollers that are left in the freezer and cool the epidermis upon application. At-home ice rollers cause immediate vasoconstriction and are a quick fix for periorbital edema or skin erythema. Three-dimensional roller face massagers are simply a massage tool and can be used on any skin type to increase facial circulation; they do not provide any visible clinical benefits. Nanocurrent or vibrating rollers use nanocurrents and vibration alongside a conductor gel to glide across the skin; they massage the skin and help topically applied agents penetrate into the stratum corneum.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Orentreich DS et al. Dermatol Surg. 1995;21(6):543-9.
Aust MC et al. Plast Reconstr Surg. 2008;21(4):1421-9.Fernandes D et al. Clin Dermatol. 2008 Mar-Apr;26(2):192-9.
Nair PA et al. GMJ. 2014;69:24-7.
Grind it out
“And five more, four more, three more, two more, one more, and done!” Just when you thought you could not stand the searing pain any longer, it ends. Your spin instructor is not only helping you be fit, she is also teaching you an important lesson for life: Sometimes you just need to grind it out.
. College basketball teams need to simply grind it out to advance in the NCAA championship tournament. How might Tiger Woods recover from a disastrous few holes at the Masters? “He’ll just have to grind it out on the back nine.” How will you finally finish your PhD thesis? You’ll have to grind it out this month. It’s how I’m writing this column, how I got my taxes in on time, and, sometimes, how I get through clinic.
The phrase is used to describe something which needs to be done that is tedious, laborious, or joyless. Although the outcome of grinding it out is always pleasant, the task is often considered arduous.
In my dermatology practice, patient demand came in like a lion this March, and to meet our awesome access goals, we needed to add clinics on Saturdays, early mornings, and even a few nights. We met our goal, with supply to spare, and felt proud of our accomplishments. Physician wellness gurus (this author not included) say that, to avoid burnout from such excess work, you must find meaning in your work. Be grateful to help that 24-year-old with acne at 8:15 p.m. Think about how lucky you are to serve that lawyer with hand dermatitis at 8:45 p.m. Celebrate the mom’s cancer-free skin screening at 9:00 p.m. By finding meaning in our work, we’re told, we can achieve clinic nirvana. Except it doesn’t always work, and sometimes it serves us badly.
For the long days that ended in night clinic last month, I found myself counting down those last few patients – “four more, three more, two more, and last one.” I love my work and care about my patients, but sometimes I just have to grind it out. I’m proud of what I’ve accomplished.
Now it’s on to spin class.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“And five more, four more, three more, two more, one more, and done!” Just when you thought you could not stand the searing pain any longer, it ends. Your spin instructor is not only helping you be fit, she is also teaching you an important lesson for life: Sometimes you just need to grind it out.
. College basketball teams need to simply grind it out to advance in the NCAA championship tournament. How might Tiger Woods recover from a disastrous few holes at the Masters? “He’ll just have to grind it out on the back nine.” How will you finally finish your PhD thesis? You’ll have to grind it out this month. It’s how I’m writing this column, how I got my taxes in on time, and, sometimes, how I get through clinic.
The phrase is used to describe something which needs to be done that is tedious, laborious, or joyless. Although the outcome of grinding it out is always pleasant, the task is often considered arduous.
In my dermatology practice, patient demand came in like a lion this March, and to meet our awesome access goals, we needed to add clinics on Saturdays, early mornings, and even a few nights. We met our goal, with supply to spare, and felt proud of our accomplishments. Physician wellness gurus (this author not included) say that, to avoid burnout from such excess work, you must find meaning in your work. Be grateful to help that 24-year-old with acne at 8:15 p.m. Think about how lucky you are to serve that lawyer with hand dermatitis at 8:45 p.m. Celebrate the mom’s cancer-free skin screening at 9:00 p.m. By finding meaning in our work, we’re told, we can achieve clinic nirvana. Except it doesn’t always work, and sometimes it serves us badly.
For the long days that ended in night clinic last month, I found myself counting down those last few patients – “four more, three more, two more, and last one.” I love my work and care about my patients, but sometimes I just have to grind it out. I’m proud of what I’ve accomplished.
Now it’s on to spin class.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“And five more, four more, three more, two more, one more, and done!” Just when you thought you could not stand the searing pain any longer, it ends. Your spin instructor is not only helping you be fit, she is also teaching you an important lesson for life: Sometimes you just need to grind it out.
. College basketball teams need to simply grind it out to advance in the NCAA championship tournament. How might Tiger Woods recover from a disastrous few holes at the Masters? “He’ll just have to grind it out on the back nine.” How will you finally finish your PhD thesis? You’ll have to grind it out this month. It’s how I’m writing this column, how I got my taxes in on time, and, sometimes, how I get through clinic.
The phrase is used to describe something which needs to be done that is tedious, laborious, or joyless. Although the outcome of grinding it out is always pleasant, the task is often considered arduous.
In my dermatology practice, patient demand came in like a lion this March, and to meet our awesome access goals, we needed to add clinics on Saturdays, early mornings, and even a few nights. We met our goal, with supply to spare, and felt proud of our accomplishments. Physician wellness gurus (this author not included) say that, to avoid burnout from such excess work, you must find meaning in your work. Be grateful to help that 24-year-old with acne at 8:15 p.m. Think about how lucky you are to serve that lawyer with hand dermatitis at 8:45 p.m. Celebrate the mom’s cancer-free skin screening at 9:00 p.m. By finding meaning in our work, we’re told, we can achieve clinic nirvana. Except it doesn’t always work, and sometimes it serves us badly.
For the long days that ended in night clinic last month, I found myself counting down those last few patients – “four more, three more, two more, and last one.” I love my work and care about my patients, but sometimes I just have to grind it out. I’m proud of what I’ve accomplished.
Now it’s on to spin class.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Advanced practice nurses and physician assistants are not the same
Looking across a hospital ward, emergency department, or primary care clinic aligned side by side, you may not see any differences between an advanced practice nurse (APN) or physician assistant (PA). However, if you took a closer look at their education programs and credentialing, you would find considerable differences.
Although both professions hold advanced degrees, the approach to patient care differs, as well as the training they receive, including different models of practice. The APN is trained according to the nursing model, while the PA attends programs that are more in line with the medical model. The APN has a patient-centered model, while the PA adheres to a disease-centered model. Consequently, their approach to caring for the same patient population differs in viewpoint and philosophy.
Entry into the APN programs requires a nursing degree or related field from an accredited college or university. The curriculum includes coursework in health care policy, advocacy, outcomes, advanced assessment, diagnosis, and practice skills as well as, pharmacology, pathophysiology, and a final capstone project.
There are six specialty APN tracks including pediatrics, women’s/gender health, family practice, adult-gerontology, psychiatric, and neonatal. Additionally, there are three additional advanced practice registered nurses tracks: certified nurse anesthesia, certified nurse midwife, and clinical nurse leader. In addition to academic hours, there is a minimum of 1,000 supervised, direct patient care clinical hours in a variety of locations covering all populations specific to the identified specialty.
The Bureau of Labor Statistics defines the role of physician assistant as follows: “Physician assistants practice medicine under the supervision of physicians and surgeons. PAs are formally trained to provide diagnostic, therapeutic, and preventive health care services, as delegated by a physician.” The physician assistant program is a master’s prepared education.
School requirements include completing 2 years of pre-physician assistant undergraduate studies prior to applying to the School of Biomedical Sciences. Many programs have a 200-hour health care experience requirement, which can be either paid or unpaid. However, unlike the APN program, this is not required by all PA programs, but it is strongly encouraged.
Accredited PA programs require completing a 3-year graduate program that includes clinical rotations and results in a Master of Science in Physician Assistant Studies. Physician assistant programs typically involve 1,000 classroom hours and 2,000 or more hours in a clinical setting. The course work focuses on biochemistry, pathology, anatomy and physiology, ethics, and biology.
Both the APN and PA practices are regulated by the state through licensure laws and policy that determine the scope of practice and allow prescriptive authority.
Both programs began in 1965 in response to a shortage of primary care physicians, yet each program took a different route to address this need. According to the May 2017 Bureau of Labor Statistics, there were more than 109,000 physician assistants and more than 166,000 nurse practitioners practicing in the United States.
With the enactment of the Affordable Care Act in 2010, the mandate for APN’s and PA’s to lead patient-centered medical homes continued to grow to meet the demand. Both roles provide direct patient care under the sponsorship of a physician, yet both roles have gained a greater level of independence as state and federal requirements have relaxed restrictive physician collaboration and oversight rules, which has allowed both roles to practice at the highest level of their training. These relaxed restrictions come at a time when a growing physician shortage is met by increased demands placed on the health care system.
Ms. Thew is a certified family nurse practitioner in the division of adolescent medicine at the Medical College of Wisconsin, Milwaukee. Email her at [email protected]
Looking across a hospital ward, emergency department, or primary care clinic aligned side by side, you may not see any differences between an advanced practice nurse (APN) or physician assistant (PA). However, if you took a closer look at their education programs and credentialing, you would find considerable differences.
Although both professions hold advanced degrees, the approach to patient care differs, as well as the training they receive, including different models of practice. The APN is trained according to the nursing model, while the PA attends programs that are more in line with the medical model. The APN has a patient-centered model, while the PA adheres to a disease-centered model. Consequently, their approach to caring for the same patient population differs in viewpoint and philosophy.
Entry into the APN programs requires a nursing degree or related field from an accredited college or university. The curriculum includes coursework in health care policy, advocacy, outcomes, advanced assessment, diagnosis, and practice skills as well as, pharmacology, pathophysiology, and a final capstone project.
There are six specialty APN tracks including pediatrics, women’s/gender health, family practice, adult-gerontology, psychiatric, and neonatal. Additionally, there are three additional advanced practice registered nurses tracks: certified nurse anesthesia, certified nurse midwife, and clinical nurse leader. In addition to academic hours, there is a minimum of 1,000 supervised, direct patient care clinical hours in a variety of locations covering all populations specific to the identified specialty.
The Bureau of Labor Statistics defines the role of physician assistant as follows: “Physician assistants practice medicine under the supervision of physicians and surgeons. PAs are formally trained to provide diagnostic, therapeutic, and preventive health care services, as delegated by a physician.” The physician assistant program is a master’s prepared education.
School requirements include completing 2 years of pre-physician assistant undergraduate studies prior to applying to the School of Biomedical Sciences. Many programs have a 200-hour health care experience requirement, which can be either paid or unpaid. However, unlike the APN program, this is not required by all PA programs, but it is strongly encouraged.
Accredited PA programs require completing a 3-year graduate program that includes clinical rotations and results in a Master of Science in Physician Assistant Studies. Physician assistant programs typically involve 1,000 classroom hours and 2,000 or more hours in a clinical setting. The course work focuses on biochemistry, pathology, anatomy and physiology, ethics, and biology.
Both the APN and PA practices are regulated by the state through licensure laws and policy that determine the scope of practice and allow prescriptive authority.
Both programs began in 1965 in response to a shortage of primary care physicians, yet each program took a different route to address this need. According to the May 2017 Bureau of Labor Statistics, there were more than 109,000 physician assistants and more than 166,000 nurse practitioners practicing in the United States.
With the enactment of the Affordable Care Act in 2010, the mandate for APN’s and PA’s to lead patient-centered medical homes continued to grow to meet the demand. Both roles provide direct patient care under the sponsorship of a physician, yet both roles have gained a greater level of independence as state and federal requirements have relaxed restrictive physician collaboration and oversight rules, which has allowed both roles to practice at the highest level of their training. These relaxed restrictions come at a time when a growing physician shortage is met by increased demands placed on the health care system.
Ms. Thew is a certified family nurse practitioner in the division of adolescent medicine at the Medical College of Wisconsin, Milwaukee. Email her at [email protected]
Looking across a hospital ward, emergency department, or primary care clinic aligned side by side, you may not see any differences between an advanced practice nurse (APN) or physician assistant (PA). However, if you took a closer look at their education programs and credentialing, you would find considerable differences.
Although both professions hold advanced degrees, the approach to patient care differs, as well as the training they receive, including different models of practice. The APN is trained according to the nursing model, while the PA attends programs that are more in line with the medical model. The APN has a patient-centered model, while the PA adheres to a disease-centered model. Consequently, their approach to caring for the same patient population differs in viewpoint and philosophy.
Entry into the APN programs requires a nursing degree or related field from an accredited college or university. The curriculum includes coursework in health care policy, advocacy, outcomes, advanced assessment, diagnosis, and practice skills as well as, pharmacology, pathophysiology, and a final capstone project.
There are six specialty APN tracks including pediatrics, women’s/gender health, family practice, adult-gerontology, psychiatric, and neonatal. Additionally, there are three additional advanced practice registered nurses tracks: certified nurse anesthesia, certified nurse midwife, and clinical nurse leader. In addition to academic hours, there is a minimum of 1,000 supervised, direct patient care clinical hours in a variety of locations covering all populations specific to the identified specialty.
The Bureau of Labor Statistics defines the role of physician assistant as follows: “Physician assistants practice medicine under the supervision of physicians and surgeons. PAs are formally trained to provide diagnostic, therapeutic, and preventive health care services, as delegated by a physician.” The physician assistant program is a master’s prepared education.
School requirements include completing 2 years of pre-physician assistant undergraduate studies prior to applying to the School of Biomedical Sciences. Many programs have a 200-hour health care experience requirement, which can be either paid or unpaid. However, unlike the APN program, this is not required by all PA programs, but it is strongly encouraged.
Accredited PA programs require completing a 3-year graduate program that includes clinical rotations and results in a Master of Science in Physician Assistant Studies. Physician assistant programs typically involve 1,000 classroom hours and 2,000 or more hours in a clinical setting. The course work focuses on biochemistry, pathology, anatomy and physiology, ethics, and biology.
Both the APN and PA practices are regulated by the state through licensure laws and policy that determine the scope of practice and allow prescriptive authority.
Both programs began in 1965 in response to a shortage of primary care physicians, yet each program took a different route to address this need. According to the May 2017 Bureau of Labor Statistics, there were more than 109,000 physician assistants and more than 166,000 nurse practitioners practicing in the United States.
With the enactment of the Affordable Care Act in 2010, the mandate for APN’s and PA’s to lead patient-centered medical homes continued to grow to meet the demand. Both roles provide direct patient care under the sponsorship of a physician, yet both roles have gained a greater level of independence as state and federal requirements have relaxed restrictive physician collaboration and oversight rules, which has allowed both roles to practice at the highest level of their training. These relaxed restrictions come at a time when a growing physician shortage is met by increased demands placed on the health care system.
Ms. Thew is a certified family nurse practitioner in the division of adolescent medicine at the Medical College of Wisconsin, Milwaukee. Email her at [email protected]
Website improvements
Nowadays, almost half of all Americans (and nearly all millennials) seek out doctors online. But your practice website should be doing a lot more than simply describing your practice. How many visitors to your site actually schedule an appointment? With a few relatively simple but important modifications, you can convert casual website viewers to patients.
Start with a good title, one that not only describes your practice but also anticipates how prospective patients will search for you – usually by specialty plus geographic location. My site’s title, for example, is “Belleville Dermatology Center,” so when someone searches for a dermatologist near Belleville, N.J., my site will invariably rank near the top of their search results.
Follow with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “bellevilledermatology[dot]com/?p=89021” is meaningless to anyone except programmers; but “bellevilledermatology[dot]com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly, answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise. Aggressive marketers will sometimes pad their descriptions with a wide variety of other specialties, services, and locations, hoping to gain inclusion in a larger pool of search results. That tactic – “keyword stuffing” in IT parlance – is not only ineffective, but search engines tend to ignore sites that use it. An accurate, honest description works best.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best…?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. Besides, prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference.
Include photos. Especially yours; new patients are more comfortable when they know what you look like. Although some disagree, I feel family photos are also important; they help to present you as a person, as well as a doctor. Photos of your office – professional ones, not casual snapshots – will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Provide online appointment scheduling. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. You should also have a separate “contact” page, listing all of the ways people can reach you, along with a map. Finally, list which insurance plans you accept as a courtesy to patients and to decrease unnecessary calls for your staff.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Nowadays, almost half of all Americans (and nearly all millennials) seek out doctors online. But your practice website should be doing a lot more than simply describing your practice. How many visitors to your site actually schedule an appointment? With a few relatively simple but important modifications, you can convert casual website viewers to patients.
Start with a good title, one that not only describes your practice but also anticipates how prospective patients will search for you – usually by specialty plus geographic location. My site’s title, for example, is “Belleville Dermatology Center,” so when someone searches for a dermatologist near Belleville, N.J., my site will invariably rank near the top of their search results.
Follow with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “bellevilledermatology[dot]com/?p=89021” is meaningless to anyone except programmers; but “bellevilledermatology[dot]com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly, answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise. Aggressive marketers will sometimes pad their descriptions with a wide variety of other specialties, services, and locations, hoping to gain inclusion in a larger pool of search results. That tactic – “keyword stuffing” in IT parlance – is not only ineffective, but search engines tend to ignore sites that use it. An accurate, honest description works best.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best…?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. Besides, prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference.
Include photos. Especially yours; new patients are more comfortable when they know what you look like. Although some disagree, I feel family photos are also important; they help to present you as a person, as well as a doctor. Photos of your office – professional ones, not casual snapshots – will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Provide online appointment scheduling. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. You should also have a separate “contact” page, listing all of the ways people can reach you, along with a map. Finally, list which insurance plans you accept as a courtesy to patients and to decrease unnecessary calls for your staff.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Nowadays, almost half of all Americans (and nearly all millennials) seek out doctors online. But your practice website should be doing a lot more than simply describing your practice. How many visitors to your site actually schedule an appointment? With a few relatively simple but important modifications, you can convert casual website viewers to patients.
Start with a good title, one that not only describes your practice but also anticipates how prospective patients will search for you – usually by specialty plus geographic location. My site’s title, for example, is “Belleville Dermatology Center,” so when someone searches for a dermatologist near Belleville, N.J., my site will invariably rank near the top of their search results.
Follow with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “bellevilledermatology[dot]com/?p=89021” is meaningless to anyone except programmers; but “bellevilledermatology[dot]com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly, answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise. Aggressive marketers will sometimes pad their descriptions with a wide variety of other specialties, services, and locations, hoping to gain inclusion in a larger pool of search results. That tactic – “keyword stuffing” in IT parlance – is not only ineffective, but search engines tend to ignore sites that use it. An accurate, honest description works best.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best…?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. Besides, prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference.
Include photos. Especially yours; new patients are more comfortable when they know what you look like. Although some disagree, I feel family photos are also important; they help to present you as a person, as well as a doctor. Photos of your office – professional ones, not casual snapshots – will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Provide online appointment scheduling. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. You should also have a separate “contact” page, listing all of the ways people can reach you, along with a map. Finally, list which insurance plans you accept as a courtesy to patients and to decrease unnecessary calls for your staff.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
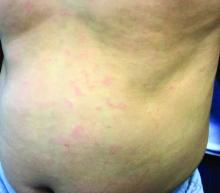
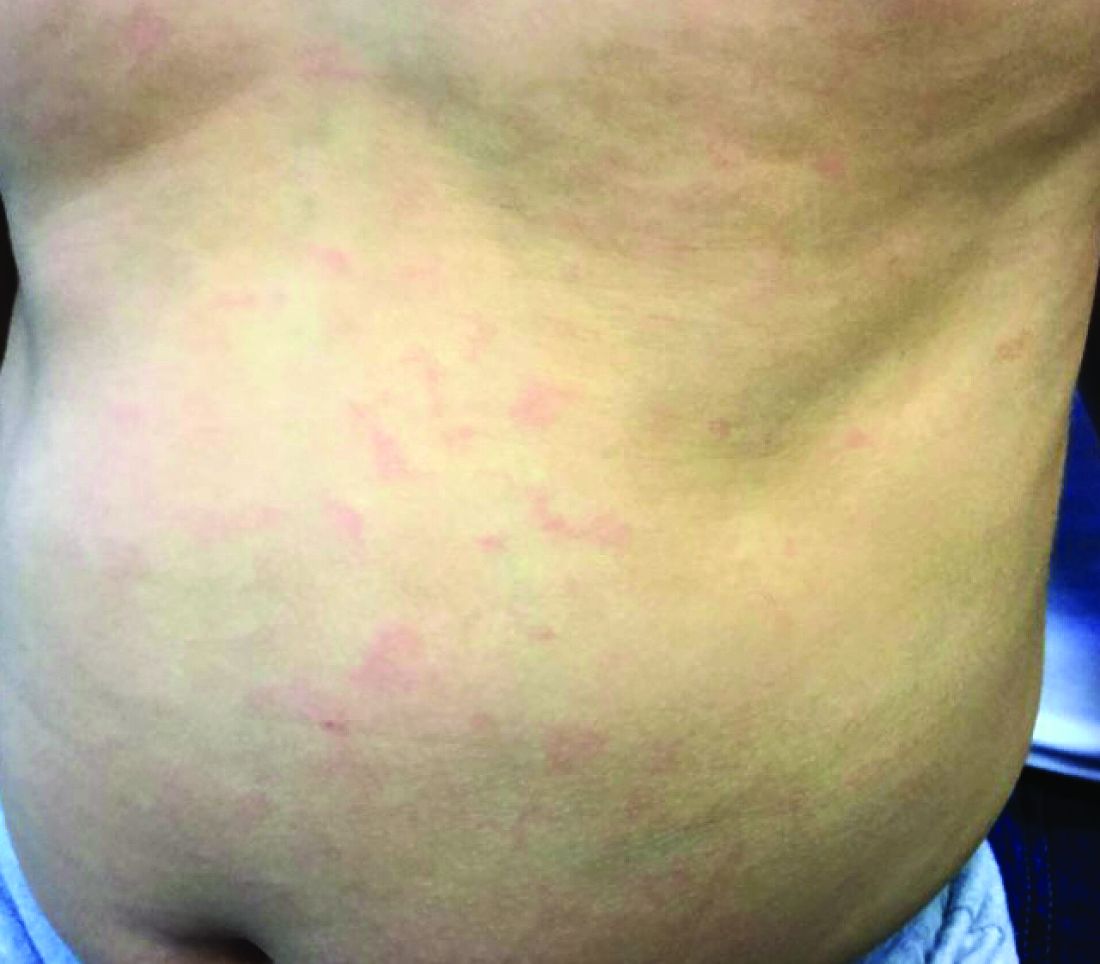
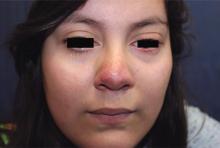
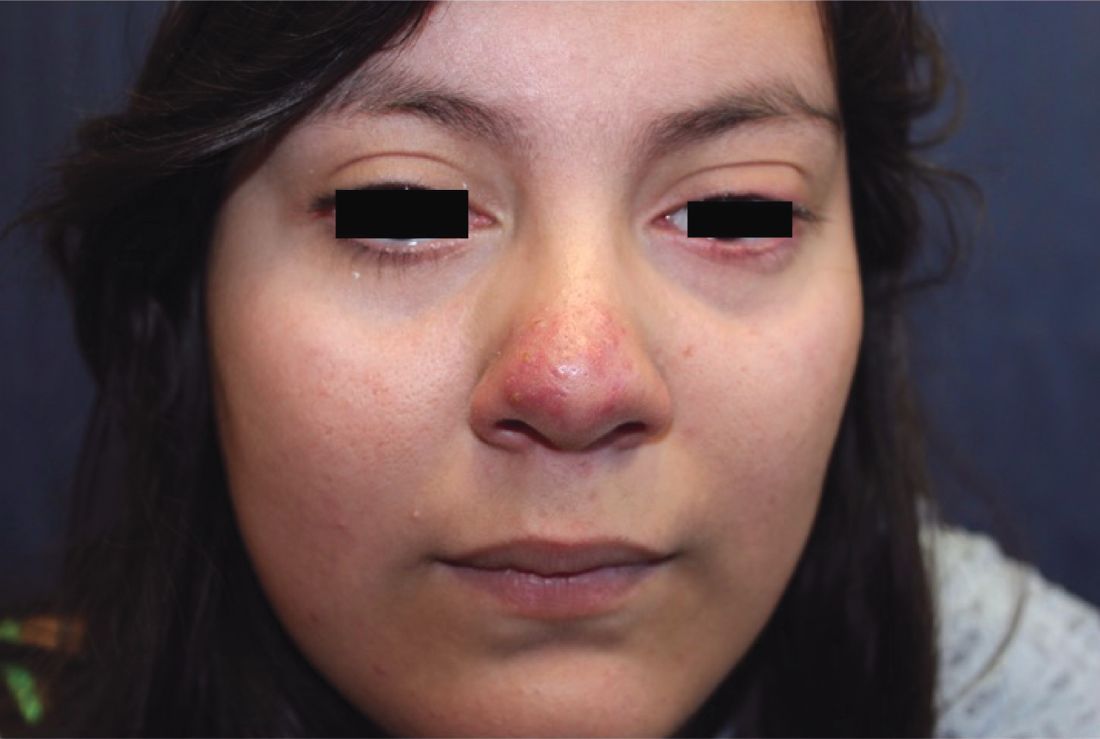
Make the Diagnosis - May 2018
Generally, school-aged children are most often affected. Infections are more likely in late winter and early spring. The virus is spread via respiratory secretions, blood products, and transmission from mother to fetus. The cutaneous findings occur about 10 days after exposure to the virus. By that time, the risk of being contagious is low.
Healthy individuals have no sequelae from fifth disease and require no treatment. However, in patients with hemoglobinopathies, such as sickle cell disease, an aplastic crisis can be triggered. In patients with deficient immune systems, parvovirus B19 may cause infection and anemia, requiring hospitalization. Pregnant women exposed to parvovirus B19 are at risk for hydrops fetalis and rarely, fetal malformations or fetal demise. Other uncommon associations include hepatitis, vasculitides, and neurologic disease.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected]. This case and photo were submitted by Dr. Bilu Martin.
Generally, school-aged children are most often affected. Infections are more likely in late winter and early spring. The virus is spread via respiratory secretions, blood products, and transmission from mother to fetus. The cutaneous findings occur about 10 days after exposure to the virus. By that time, the risk of being contagious is low.
Healthy individuals have no sequelae from fifth disease and require no treatment. However, in patients with hemoglobinopathies, such as sickle cell disease, an aplastic crisis can be triggered. In patients with deficient immune systems, parvovirus B19 may cause infection and anemia, requiring hospitalization. Pregnant women exposed to parvovirus B19 are at risk for hydrops fetalis and rarely, fetal malformations or fetal demise. Other uncommon associations include hepatitis, vasculitides, and neurologic disease.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected]. This case and photo were submitted by Dr. Bilu Martin.
Generally, school-aged children are most often affected. Infections are more likely in late winter and early spring. The virus is spread via respiratory secretions, blood products, and transmission from mother to fetus. The cutaneous findings occur about 10 days after exposure to the virus. By that time, the risk of being contagious is low.
Healthy individuals have no sequelae from fifth disease and require no treatment. However, in patients with hemoglobinopathies, such as sickle cell disease, an aplastic crisis can be triggered. In patients with deficient immune systems, parvovirus B19 may cause infection and anemia, requiring hospitalization. Pregnant women exposed to parvovirus B19 are at risk for hydrops fetalis and rarely, fetal malformations or fetal demise. Other uncommon associations include hepatitis, vasculitides, and neurologic disease.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected]. This case and photo were submitted by Dr. Bilu Martin.
Why do people act that way?
You can try all you want, but you never really figure people out.
Take Brendan. He spent a good few minutes chewing out my front desk staffer Linda. “You’re incompetent!” he shouted at her.
Linda tried to explain. “We can’t administer the Botox for your sweating today,” she said, “because it hasn’t come in yet.”
No, Brendan certainly didn’t want to hear that. Nor did he want to hear that we had called in advance to ask him to actually wait until we let him know that the Botox was in. But here he was, hyperinflating his lungs.
“You’re just damned useless!” he explained.
OK, we all have unreasonable patients from time to time. But what I’m describing is behavior that’s not just aggressive and unpleasant but incomprehensible. Would you like to know why I call it incomprehensible?
Because Brendan has been coming in for axillary Botox injections every 6 months for 5 years! He knows the drill. He knows the staff. He’s always pleasant as punch. Just not today. Why? Who knows?
What’s true for patients can of course also be true for employees. Model workers, superb team players, reliable folks who show up in snowstorms, who come right back to work after major surgery, who deliver heartfelt speeches at holiday parties about their good luck in overcoming adversity to be able to work. Contributors who share their pleasant disposition and can-do energy year after year ...
Until one day that they don’t show up, send a text to say they quit, no warning or explanation, then apply for Workmen’s Compensation, and at the magistrate’s hearing to which they’ve dragged my staff and our HR attorney, lie right to the face of the manager who was their best friend and confidante until the day before yesterday.
Get it? I don’t. Never will.
Two pieces of advice: 1) Don’t ever take things like that personally; and 2) Hire a good human resources attorney.
**********************
On the other hand ...
Jeralyn is 27 years old. She lists her chief complaint as “dark spots on my back.”
“How did you notice these?” I ask. “Did you see them, or did you doctor point them out?”
“It was when I tried on my wedding dress,” she says.
I understand, or think I do. Her dress exposed her back, her mother noticed the spots ...
“When is the wedding?” I ask.
“The wedding? Oh, the wedding already was. A year and a half ago.”
“You saw the spots when you tried on your wedding dress, and you’re coming in a year and half later?” I ask, trying not to sound incredulous. I’ve met enough nervous brides – and brides’ mothers – to find her account astonishing. “You must be a very patient person,” I say.
“Well,” says Jeralyn, “the spots didn’t spread or get any worse. And I am patient. I teach kindergarten. You have to be patient with little ones.”
“If you decide to have children, they’ll benefit from that,” I reply.
“That’s actually why I’m here,” says Jeralyn. “My husband and I want to start a family, and we want to be sure my skin condition wouldn’t affect that.”
A bride so unconcerned with herself that she wears a wedding dress that reveals a rash she doesn’t go running to a doctor to fix? Who comes only when the rash might affect someone else?
What is the matter with Jeralyn? Doesn’t she know she’s a millennial?
**********************
Kidding aside, wouldn’t it be nice if everyone acted like Jeralyn, and acted in a measured manner, appropriate to the concerns and circumstances? Wouldn’t it be nice if no one acted like Brendan?
That’s not how it is, though, is it? As professionals who deal with the public (as patients, coworkers, employees), we take all comers and roll with them: tolerate the annoyances and celebrate the (much larger, if less individually memorable) group who are pleasant, often delightful, sometimes inspiring.
And every once in a while, someone like Jeralyn comes along, upending all those negative expectations and reminding us that even if you can never really figure people out, it’s still worth the effort to keep trying.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
You can try all you want, but you never really figure people out.
Take Brendan. He spent a good few minutes chewing out my front desk staffer Linda. “You’re incompetent!” he shouted at her.
Linda tried to explain. “We can’t administer the Botox for your sweating today,” she said, “because it hasn’t come in yet.”
No, Brendan certainly didn’t want to hear that. Nor did he want to hear that we had called in advance to ask him to actually wait until we let him know that the Botox was in. But here he was, hyperinflating his lungs.
“You’re just damned useless!” he explained.
OK, we all have unreasonable patients from time to time. But what I’m describing is behavior that’s not just aggressive and unpleasant but incomprehensible. Would you like to know why I call it incomprehensible?
Because Brendan has been coming in for axillary Botox injections every 6 months for 5 years! He knows the drill. He knows the staff. He’s always pleasant as punch. Just not today. Why? Who knows?
What’s true for patients can of course also be true for employees. Model workers, superb team players, reliable folks who show up in snowstorms, who come right back to work after major surgery, who deliver heartfelt speeches at holiday parties about their good luck in overcoming adversity to be able to work. Contributors who share their pleasant disposition and can-do energy year after year ...
Until one day that they don’t show up, send a text to say they quit, no warning or explanation, then apply for Workmen’s Compensation, and at the magistrate’s hearing to which they’ve dragged my staff and our HR attorney, lie right to the face of the manager who was their best friend and confidante until the day before yesterday.
Get it? I don’t. Never will.
Two pieces of advice: 1) Don’t ever take things like that personally; and 2) Hire a good human resources attorney.
**********************
On the other hand ...
Jeralyn is 27 years old. She lists her chief complaint as “dark spots on my back.”
“How did you notice these?” I ask. “Did you see them, or did you doctor point them out?”
“It was when I tried on my wedding dress,” she says.
I understand, or think I do. Her dress exposed her back, her mother noticed the spots ...
“When is the wedding?” I ask.
“The wedding? Oh, the wedding already was. A year and a half ago.”
“You saw the spots when you tried on your wedding dress, and you’re coming in a year and half later?” I ask, trying not to sound incredulous. I’ve met enough nervous brides – and brides’ mothers – to find her account astonishing. “You must be a very patient person,” I say.
“Well,” says Jeralyn, “the spots didn’t spread or get any worse. And I am patient. I teach kindergarten. You have to be patient with little ones.”
“If you decide to have children, they’ll benefit from that,” I reply.
“That’s actually why I’m here,” says Jeralyn. “My husband and I want to start a family, and we want to be sure my skin condition wouldn’t affect that.”
A bride so unconcerned with herself that she wears a wedding dress that reveals a rash she doesn’t go running to a doctor to fix? Who comes only when the rash might affect someone else?
What is the matter with Jeralyn? Doesn’t she know she’s a millennial?
**********************
Kidding aside, wouldn’t it be nice if everyone acted like Jeralyn, and acted in a measured manner, appropriate to the concerns and circumstances? Wouldn’t it be nice if no one acted like Brendan?
That’s not how it is, though, is it? As professionals who deal with the public (as patients, coworkers, employees), we take all comers and roll with them: tolerate the annoyances and celebrate the (much larger, if less individually memorable) group who are pleasant, often delightful, sometimes inspiring.
And every once in a while, someone like Jeralyn comes along, upending all those negative expectations and reminding us that even if you can never really figure people out, it’s still worth the effort to keep trying.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
You can try all you want, but you never really figure people out.
Take Brendan. He spent a good few minutes chewing out my front desk staffer Linda. “You’re incompetent!” he shouted at her.
Linda tried to explain. “We can’t administer the Botox for your sweating today,” she said, “because it hasn’t come in yet.”
No, Brendan certainly didn’t want to hear that. Nor did he want to hear that we had called in advance to ask him to actually wait until we let him know that the Botox was in. But here he was, hyperinflating his lungs.
“You’re just damned useless!” he explained.
OK, we all have unreasonable patients from time to time. But what I’m describing is behavior that’s not just aggressive and unpleasant but incomprehensible. Would you like to know why I call it incomprehensible?
Because Brendan has been coming in for axillary Botox injections every 6 months for 5 years! He knows the drill. He knows the staff. He’s always pleasant as punch. Just not today. Why? Who knows?
What’s true for patients can of course also be true for employees. Model workers, superb team players, reliable folks who show up in snowstorms, who come right back to work after major surgery, who deliver heartfelt speeches at holiday parties about their good luck in overcoming adversity to be able to work. Contributors who share their pleasant disposition and can-do energy year after year ...
Until one day that they don’t show up, send a text to say they quit, no warning or explanation, then apply for Workmen’s Compensation, and at the magistrate’s hearing to which they’ve dragged my staff and our HR attorney, lie right to the face of the manager who was their best friend and confidante until the day before yesterday.
Get it? I don’t. Never will.
Two pieces of advice: 1) Don’t ever take things like that personally; and 2) Hire a good human resources attorney.
**********************
On the other hand ...
Jeralyn is 27 years old. She lists her chief complaint as “dark spots on my back.”
“How did you notice these?” I ask. “Did you see them, or did you doctor point them out?”
“It was when I tried on my wedding dress,” she says.
I understand, or think I do. Her dress exposed her back, her mother noticed the spots ...
“When is the wedding?” I ask.
“The wedding? Oh, the wedding already was. A year and a half ago.”
“You saw the spots when you tried on your wedding dress, and you’re coming in a year and half later?” I ask, trying not to sound incredulous. I’ve met enough nervous brides – and brides’ mothers – to find her account astonishing. “You must be a very patient person,” I say.
“Well,” says Jeralyn, “the spots didn’t spread or get any worse. And I am patient. I teach kindergarten. You have to be patient with little ones.”
“If you decide to have children, they’ll benefit from that,” I reply.
“That’s actually why I’m here,” says Jeralyn. “My husband and I want to start a family, and we want to be sure my skin condition wouldn’t affect that.”
A bride so unconcerned with herself that she wears a wedding dress that reveals a rash she doesn’t go running to a doctor to fix? Who comes only when the rash might affect someone else?
What is the matter with Jeralyn? Doesn’t she know she’s a millennial?
**********************
Kidding aside, wouldn’t it be nice if everyone acted like Jeralyn, and acted in a measured manner, appropriate to the concerns and circumstances? Wouldn’t it be nice if no one acted like Brendan?
That’s not how it is, though, is it? As professionals who deal with the public (as patients, coworkers, employees), we take all comers and roll with them: tolerate the annoyances and celebrate the (much larger, if less individually memorable) group who are pleasant, often delightful, sometimes inspiring.
And every once in a while, someone like Jeralyn comes along, upending all those negative expectations and reminding us that even if you can never really figure people out, it’s still worth the effort to keep trying.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
AHRQ Practice Toolbox: Practice facilitation
This is the eighth in a series of articles from the National Center for Excellence in Primary Care Research (NCEPCR) in the Agency for Healthcare Research and Quality (AHRQ). This series introduces sets of tools and resources designed to help your practice.
Last month’s article discussed AHRQ’s tool and resources for practice transformation, or how to help make your practice ready for advances such as shared decision making, team-based care, and integrating behavioral health and primary care. Use of practice facilitation, an evidence-based approach to quality improvement, can be a key element to helping primary care practices make the necessary changes for transformation. Practice facilitators (also known as practice coaches, quality improvement coaches, or practice enhancement assistants) are specially trained individuals who assist primary care clinicians in research and quality improvement projects. They are distinguished from consultants through specialized training, broad scope of practice, and long-term relationships with an organization, its staff and clinicians, and its patients.
If you are wondering how a practice facilitator can help you, AHRQ offers How a Practice Facilitator Can Support Your Practice. This tip sheet provides ideas and techniques for primary care practices interested in getting started with quality improvement activities and describes the benefits of working with a practice facilitator. The tip sheet is distilled from a more extensive white paper on Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators, which presents a framework for engaging primary care practices in quality improvement and provides strategies for sustained involvement in the undertaking.
For those with greater interest in implementation of practice facilitation, AHRQ offers the Practice Facilitation Handbook: Training Modules for New Facilitators and Their Trainers. The Handbook is designed to assist in the training of new practice facilitators as they begin to develop the knowledge and skills needed to support meaningful improvement in primary care practices. AHRQ built upon the modules in the Handbook with the Primary Care Practice Facilitation Curriculum. The Curriculum updates the modules in the Handbook and adds an additional twelve modules for a total of 32 modules. More importantly, some of the modules can be used by clinicians and staff as a sort of Quality Improvement 101. Modules on appreciative inquiry, approaches to quality improvement, redesigning work flow, root cause analysis, and measuring clinical performance can be useful to anyone interested in increasing their knowledge and skills for quality improvement.
Mr. McNellis is senior adviser for primary care at AHRQ and Dr. Ganiats is director of the National Center for Excellence in Primary Care Research at AHRQ.
Links from the AHRQ Web site:
How a Practice Facilitator Can Support Your Practice
Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators
Primary Care Practice Facilitation Curriculum
Practice Facilitation Handbook
A How-To Guide on Developing and Running a Practice Facilitation Program
These and other tools can be found at the NCEPCR Web site.
This is the eighth in a series of articles from the National Center for Excellence in Primary Care Research (NCEPCR) in the Agency for Healthcare Research and Quality (AHRQ). This series introduces sets of tools and resources designed to help your practice.
Last month’s article discussed AHRQ’s tool and resources for practice transformation, or how to help make your practice ready for advances such as shared decision making, team-based care, and integrating behavioral health and primary care. Use of practice facilitation, an evidence-based approach to quality improvement, can be a key element to helping primary care practices make the necessary changes for transformation. Practice facilitators (also known as practice coaches, quality improvement coaches, or practice enhancement assistants) are specially trained individuals who assist primary care clinicians in research and quality improvement projects. They are distinguished from consultants through specialized training, broad scope of practice, and long-term relationships with an organization, its staff and clinicians, and its patients.
If you are wondering how a practice facilitator can help you, AHRQ offers How a Practice Facilitator Can Support Your Practice. This tip sheet provides ideas and techniques for primary care practices interested in getting started with quality improvement activities and describes the benefits of working with a practice facilitator. The tip sheet is distilled from a more extensive white paper on Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators, which presents a framework for engaging primary care practices in quality improvement and provides strategies for sustained involvement in the undertaking.
For those with greater interest in implementation of practice facilitation, AHRQ offers the Practice Facilitation Handbook: Training Modules for New Facilitators and Their Trainers. The Handbook is designed to assist in the training of new practice facilitators as they begin to develop the knowledge and skills needed to support meaningful improvement in primary care practices. AHRQ built upon the modules in the Handbook with the Primary Care Practice Facilitation Curriculum. The Curriculum updates the modules in the Handbook and adds an additional twelve modules for a total of 32 modules. More importantly, some of the modules can be used by clinicians and staff as a sort of Quality Improvement 101. Modules on appreciative inquiry, approaches to quality improvement, redesigning work flow, root cause analysis, and measuring clinical performance can be useful to anyone interested in increasing their knowledge and skills for quality improvement.
Mr. McNellis is senior adviser for primary care at AHRQ and Dr. Ganiats is director of the National Center for Excellence in Primary Care Research at AHRQ.
Links from the AHRQ Web site:
How a Practice Facilitator Can Support Your Practice
Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators
Primary Care Practice Facilitation Curriculum
Practice Facilitation Handbook
A How-To Guide on Developing and Running a Practice Facilitation Program
These and other tools can be found at the NCEPCR Web site.
This is the eighth in a series of articles from the National Center for Excellence in Primary Care Research (NCEPCR) in the Agency for Healthcare Research and Quality (AHRQ). This series introduces sets of tools and resources designed to help your practice.
Last month’s article discussed AHRQ’s tool and resources for practice transformation, or how to help make your practice ready for advances such as shared decision making, team-based care, and integrating behavioral health and primary care. Use of practice facilitation, an evidence-based approach to quality improvement, can be a key element to helping primary care practices make the necessary changes for transformation. Practice facilitators (also known as practice coaches, quality improvement coaches, or practice enhancement assistants) are specially trained individuals who assist primary care clinicians in research and quality improvement projects. They are distinguished from consultants through specialized training, broad scope of practice, and long-term relationships with an organization, its staff and clinicians, and its patients.
If you are wondering how a practice facilitator can help you, AHRQ offers How a Practice Facilitator Can Support Your Practice. This tip sheet provides ideas and techniques for primary care practices interested in getting started with quality improvement activities and describes the benefits of working with a practice facilitator. The tip sheet is distilled from a more extensive white paper on Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators, which presents a framework for engaging primary care practices in quality improvement and provides strategies for sustained involvement in the undertaking.
For those with greater interest in implementation of practice facilitation, AHRQ offers the Practice Facilitation Handbook: Training Modules for New Facilitators and Their Trainers. The Handbook is designed to assist in the training of new practice facilitators as they begin to develop the knowledge and skills needed to support meaningful improvement in primary care practices. AHRQ built upon the modules in the Handbook with the Primary Care Practice Facilitation Curriculum. The Curriculum updates the modules in the Handbook and adds an additional twelve modules for a total of 32 modules. More importantly, some of the modules can be used by clinicians and staff as a sort of Quality Improvement 101. Modules on appreciative inquiry, approaches to quality improvement, redesigning work flow, root cause analysis, and measuring clinical performance can be useful to anyone interested in increasing their knowledge and skills for quality improvement.
Mr. McNellis is senior adviser for primary care at AHRQ and Dr. Ganiats is director of the National Center for Excellence in Primary Care Research at AHRQ.
Links from the AHRQ Web site:
How a Practice Facilitator Can Support Your Practice
Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators
Primary Care Practice Facilitation Curriculum
Practice Facilitation Handbook
A How-To Guide on Developing and Running a Practice Facilitation Program
These and other tools can be found at the NCEPCR Web site.
Pediatric Dermatology Consult - March 2018
Rosacea is a chronic inflammatory skin disorder characterized by flushing, telangiectasia, erythema, papules, and pustules, most classically of the central face. Fair-skinned individuals and women are more commonly affected than are men, with age of onset typically around 30 years and older.1 In children and adolescents, rosacea is rare, especially among prepubertal children, so other papulopustular disorders should be considered when a rosacealike picture is present.2 Recurrent chalazia are seen with ocular rosacea and may be a clue to the diagnosis of acne rosacea. An individual’s subtype of rosacea also may transform with time to a different or an additional subtype.
Papulopustular rosacea (subtype II) is characterized by the presence of erythematous, dome-shaped papules distributed in crops in the central facial region. Cheeks, nasolabial folds, and the chin are most commonly affected. Pustules may or may not be present, but comedones are notably absent in an exclusively rosacea disease process. If comedones are present, a diagnosis of acne vulgaris should be considered instead of, or in addition to, rosacea. Pediatric patients with rosacea frequently present with papules and/or pustules, following the development of flushing.2
Ocular rosacea (subtype IV) may range in severity from mild blepharitis to severe keratitis and corneal vascularization. Patients may complain of a foreign body sensation. On external exam, lid margin telangiectasias, blepharitis, conjunctivitis, conjunctival injection, and recurrent chalazia may be frequently seen.3 Ocular rosacea may present without any signs of cutaneous disease; it may be the only form of rosacea (15% of patients in one study of 20 patients had only ocular rosacea)4 or may herald the development of cutaneous involvement. In fact, in children, ocular rosacea is frequently the first sign of disease. (A total of 55% of patients in the same study had both ocular and cutaneous rosacea, with ocular symptoms manifesting before the cutaneous disease). Thus an index of suspicion for rosacea should be maintained when a child presents with ocular findings.2
Other dermatitides resembling rosacea include steroid rosacea, perioral dermatitis, and idiopathic facial aseptic granuloma. Steroid rosacea, also known as iatrosacea, describes an eruption of erythema, papules, and telangiectasias that is clinically indistinguishable from rosacea.6 It results from chronic use of topical steroids, generally high potency, or abrupt withdrawal of steroids. Steroid rosacea should be treated by discontinuation of the steroid via tapered withdrawal.7 Perioral dermatitis, also known as periorificial dermatitis, may also appear rosacea-form. It usually is located around the perioral and perinasal areas, but may extend to the periocular area.8 Idiopathic facial septic granuloma describes erythematous to violaceous nodules of the cheeks and eyelid in children, with chalazia frequently present; it is thought to be associated with rosacea.9
Although the exact pathophysiology of rosacea is unknown, it is clear that the dysregulation of the innate immune system plays a key role in the pathogenesis of rosacea. Studies have found that patients with rosacea have increased expression of cathelicidin, and its activating serine protease, kallikrein.5 Interestingly, UV light, a known trigger of rosacea, induces expression of cathelicidin and its inflammatory cascade.5 Neurovascular signaling is also aberrantly upregulated; vanilloid and ankyrin receptors have been shown to be active in rosacea, and are activated by rosacea-exacerbating stimuli, such as heat, inflammation, and spices. Higher levels of Demodex folliculorum and Staphylococcus epidermis also have been consistently found on the skin of rosacea patients, compared with healthy subjects, though it is unclear what role these pathogens play in the development of rosacea.
Treatment of rosacea is very important given its profound impact on quality of life; one study found that the odds ratio for depression in individuals with rosacea is 4.81.10 Patient education is essential, and patients should be encouraged to identify specific triggers so they can minimize exposure when feasible. Common triggers include hot and cold temperature, sunlight, wind, spicy foods, alcohol, exercise, emotional stress, and certain medications such as niacin. Topical steroids frequently are exacerbating, so patients should be encouraged to avoid them and use moisturizers often, given their skin’s increased transepidermal water loss and susceptibility to irritation. In addition, sunscreens are essential to reduce inflammation from reactive oxygen species, which aggravate rosacea.11 For pharmaceutical therapeutics, topical sodium sulfacetamide, metronidazole, and azelaic acid have been shown to be effective in rosacea. For persistent erythema, topical alpha-adrenergic receptor agonists including brimonidine tartrate and oxymetazoline have been shown to reduce erythema by vasoconstricting blood vessels, although some products are associated with a rebound erythema on treatment discontinuation. For moderate to severe rosacea, low-dose oral doxycycline at anti-inflammatory doses (less than 50 mg daily) is the mainstay of therapy. Other oral antibiotics and topical permethrin have been used, and topical ivermectin 1% cream has been approved for inflammatory rosacea.11 Oral beta-blockers also have been successfully used in some patients to reduce erythema and flushing, as well as isotretinoin for refractory, severe rosacea with improvement.
Allison Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Han or Dr. Eichenfield.
References
1. J Am Acad Dermatol. 2018 Jan;78(1):148-55.
2. Cutis. 2016 Jul;98(1):49-53.
3. J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1732-8.
4. J Fr Ophtalmol. 2011 Dec;34(10):703-10.
5. J Am Acad Dermatol. 2015 May;72(5):749-58.
6. Indian J Dermatol. 2011 Jan;56(1):30-2.
7. Cutis, 2004. 74(2):99-103.
8. Pediatr Dermatol. 1992 Mar;9(1):22-6.
9. Pediatr Dermatol. 2015 Jul-Aug;32(4):e136-9.
10. Br J Dermatol. 2005 Dec;153(6):1176-81.
11. J Am Acad Dermatol. 2015 May;72(5):761-70.
Rosacea is a chronic inflammatory skin disorder characterized by flushing, telangiectasia, erythema, papules, and pustules, most classically of the central face. Fair-skinned individuals and women are more commonly affected than are men, with age of onset typically around 30 years and older.1 In children and adolescents, rosacea is rare, especially among prepubertal children, so other papulopustular disorders should be considered when a rosacealike picture is present.2 Recurrent chalazia are seen with ocular rosacea and may be a clue to the diagnosis of acne rosacea. An individual’s subtype of rosacea also may transform with time to a different or an additional subtype.
Papulopustular rosacea (subtype II) is characterized by the presence of erythematous, dome-shaped papules distributed in crops in the central facial region. Cheeks, nasolabial folds, and the chin are most commonly affected. Pustules may or may not be present, but comedones are notably absent in an exclusively rosacea disease process. If comedones are present, a diagnosis of acne vulgaris should be considered instead of, or in addition to, rosacea. Pediatric patients with rosacea frequently present with papules and/or pustules, following the development of flushing.2
Ocular rosacea (subtype IV) may range in severity from mild blepharitis to severe keratitis and corneal vascularization. Patients may complain of a foreign body sensation. On external exam, lid margin telangiectasias, blepharitis, conjunctivitis, conjunctival injection, and recurrent chalazia may be frequently seen.3 Ocular rosacea may present without any signs of cutaneous disease; it may be the only form of rosacea (15% of patients in one study of 20 patients had only ocular rosacea)4 or may herald the development of cutaneous involvement. In fact, in children, ocular rosacea is frequently the first sign of disease. (A total of 55% of patients in the same study had both ocular and cutaneous rosacea, with ocular symptoms manifesting before the cutaneous disease). Thus an index of suspicion for rosacea should be maintained when a child presents with ocular findings.2
Other dermatitides resembling rosacea include steroid rosacea, perioral dermatitis, and idiopathic facial aseptic granuloma. Steroid rosacea, also known as iatrosacea, describes an eruption of erythema, papules, and telangiectasias that is clinically indistinguishable from rosacea.6 It results from chronic use of topical steroids, generally high potency, or abrupt withdrawal of steroids. Steroid rosacea should be treated by discontinuation of the steroid via tapered withdrawal.7 Perioral dermatitis, also known as periorificial dermatitis, may also appear rosacea-form. It usually is located around the perioral and perinasal areas, but may extend to the periocular area.8 Idiopathic facial septic granuloma describes erythematous to violaceous nodules of the cheeks and eyelid in children, with chalazia frequently present; it is thought to be associated with rosacea.9
Although the exact pathophysiology of rosacea is unknown, it is clear that the dysregulation of the innate immune system plays a key role in the pathogenesis of rosacea. Studies have found that patients with rosacea have increased expression of cathelicidin, and its activating serine protease, kallikrein.5 Interestingly, UV light, a known trigger of rosacea, induces expression of cathelicidin and its inflammatory cascade.5 Neurovascular signaling is also aberrantly upregulated; vanilloid and ankyrin receptors have been shown to be active in rosacea, and are activated by rosacea-exacerbating stimuli, such as heat, inflammation, and spices. Higher levels of Demodex folliculorum and Staphylococcus epidermis also have been consistently found on the skin of rosacea patients, compared with healthy subjects, though it is unclear what role these pathogens play in the development of rosacea.
Treatment of rosacea is very important given its profound impact on quality of life; one study found that the odds ratio for depression in individuals with rosacea is 4.81.10 Patient education is essential, and patients should be encouraged to identify specific triggers so they can minimize exposure when feasible. Common triggers include hot and cold temperature, sunlight, wind, spicy foods, alcohol, exercise, emotional stress, and certain medications such as niacin. Topical steroids frequently are exacerbating, so patients should be encouraged to avoid them and use moisturizers often, given their skin’s increased transepidermal water loss and susceptibility to irritation. In addition, sunscreens are essential to reduce inflammation from reactive oxygen species, which aggravate rosacea.11 For pharmaceutical therapeutics, topical sodium sulfacetamide, metronidazole, and azelaic acid have been shown to be effective in rosacea. For persistent erythema, topical alpha-adrenergic receptor agonists including brimonidine tartrate and oxymetazoline have been shown to reduce erythema by vasoconstricting blood vessels, although some products are associated with a rebound erythema on treatment discontinuation. For moderate to severe rosacea, low-dose oral doxycycline at anti-inflammatory doses (less than 50 mg daily) is the mainstay of therapy. Other oral antibiotics and topical permethrin have been used, and topical ivermectin 1% cream has been approved for inflammatory rosacea.11 Oral beta-blockers also have been successfully used in some patients to reduce erythema and flushing, as well as isotretinoin for refractory, severe rosacea with improvement.
Allison Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Han or Dr. Eichenfield.
References
1. J Am Acad Dermatol. 2018 Jan;78(1):148-55.
2. Cutis. 2016 Jul;98(1):49-53.
3. J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1732-8.
4. J Fr Ophtalmol. 2011 Dec;34(10):703-10.
5. J Am Acad Dermatol. 2015 May;72(5):749-58.
6. Indian J Dermatol. 2011 Jan;56(1):30-2.
7. Cutis, 2004. 74(2):99-103.
8. Pediatr Dermatol. 1992 Mar;9(1):22-6.
9. Pediatr Dermatol. 2015 Jul-Aug;32(4):e136-9.
10. Br J Dermatol. 2005 Dec;153(6):1176-81.
11. J Am Acad Dermatol. 2015 May;72(5):761-70.
Rosacea is a chronic inflammatory skin disorder characterized by flushing, telangiectasia, erythema, papules, and pustules, most classically of the central face. Fair-skinned individuals and women are more commonly affected than are men, with age of onset typically around 30 years and older.1 In children and adolescents, rosacea is rare, especially among prepubertal children, so other papulopustular disorders should be considered when a rosacealike picture is present.2 Recurrent chalazia are seen with ocular rosacea and may be a clue to the diagnosis of acne rosacea. An individual’s subtype of rosacea also may transform with time to a different or an additional subtype.
Papulopustular rosacea (subtype II) is characterized by the presence of erythematous, dome-shaped papules distributed in crops in the central facial region. Cheeks, nasolabial folds, and the chin are most commonly affected. Pustules may or may not be present, but comedones are notably absent in an exclusively rosacea disease process. If comedones are present, a diagnosis of acne vulgaris should be considered instead of, or in addition to, rosacea. Pediatric patients with rosacea frequently present with papules and/or pustules, following the development of flushing.2
Ocular rosacea (subtype IV) may range in severity from mild blepharitis to severe keratitis and corneal vascularization. Patients may complain of a foreign body sensation. On external exam, lid margin telangiectasias, blepharitis, conjunctivitis, conjunctival injection, and recurrent chalazia may be frequently seen.3 Ocular rosacea may present without any signs of cutaneous disease; it may be the only form of rosacea (15% of patients in one study of 20 patients had only ocular rosacea)4 or may herald the development of cutaneous involvement. In fact, in children, ocular rosacea is frequently the first sign of disease. (A total of 55% of patients in the same study had both ocular and cutaneous rosacea, with ocular symptoms manifesting before the cutaneous disease). Thus an index of suspicion for rosacea should be maintained when a child presents with ocular findings.2
Other dermatitides resembling rosacea include steroid rosacea, perioral dermatitis, and idiopathic facial aseptic granuloma. Steroid rosacea, also known as iatrosacea, describes an eruption of erythema, papules, and telangiectasias that is clinically indistinguishable from rosacea.6 It results from chronic use of topical steroids, generally high potency, or abrupt withdrawal of steroids. Steroid rosacea should be treated by discontinuation of the steroid via tapered withdrawal.7 Perioral dermatitis, also known as periorificial dermatitis, may also appear rosacea-form. It usually is located around the perioral and perinasal areas, but may extend to the periocular area.8 Idiopathic facial septic granuloma describes erythematous to violaceous nodules of the cheeks and eyelid in children, with chalazia frequently present; it is thought to be associated with rosacea.9
Although the exact pathophysiology of rosacea is unknown, it is clear that the dysregulation of the innate immune system plays a key role in the pathogenesis of rosacea. Studies have found that patients with rosacea have increased expression of cathelicidin, and its activating serine protease, kallikrein.5 Interestingly, UV light, a known trigger of rosacea, induces expression of cathelicidin and its inflammatory cascade.5 Neurovascular signaling is also aberrantly upregulated; vanilloid and ankyrin receptors have been shown to be active in rosacea, and are activated by rosacea-exacerbating stimuli, such as heat, inflammation, and spices. Higher levels of Demodex folliculorum and Staphylococcus epidermis also have been consistently found on the skin of rosacea patients, compared with healthy subjects, though it is unclear what role these pathogens play in the development of rosacea.
Treatment of rosacea is very important given its profound impact on quality of life; one study found that the odds ratio for depression in individuals with rosacea is 4.81.10 Patient education is essential, and patients should be encouraged to identify specific triggers so they can minimize exposure when feasible. Common triggers include hot and cold temperature, sunlight, wind, spicy foods, alcohol, exercise, emotional stress, and certain medications such as niacin. Topical steroids frequently are exacerbating, so patients should be encouraged to avoid them and use moisturizers often, given their skin’s increased transepidermal water loss and susceptibility to irritation. In addition, sunscreens are essential to reduce inflammation from reactive oxygen species, which aggravate rosacea.11 For pharmaceutical therapeutics, topical sodium sulfacetamide, metronidazole, and azelaic acid have been shown to be effective in rosacea. For persistent erythema, topical alpha-adrenergic receptor agonists including brimonidine tartrate and oxymetazoline have been shown to reduce erythema by vasoconstricting blood vessels, although some products are associated with a rebound erythema on treatment discontinuation. For moderate to severe rosacea, low-dose oral doxycycline at anti-inflammatory doses (less than 50 mg daily) is the mainstay of therapy. Other oral antibiotics and topical permethrin have been used, and topical ivermectin 1% cream has been approved for inflammatory rosacea.11 Oral beta-blockers also have been successfully used in some patients to reduce erythema and flushing, as well as isotretinoin for refractory, severe rosacea with improvement.
Allison Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Ms. Han or Dr. Eichenfield.
References
1. J Am Acad Dermatol. 2018 Jan;78(1):148-55.
2. Cutis. 2016 Jul;98(1):49-53.
3. J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1732-8.
4. J Fr Ophtalmol. 2011 Dec;34(10):703-10.
5. J Am Acad Dermatol. 2015 May;72(5):749-58.
6. Indian J Dermatol. 2011 Jan;56(1):30-2.
7. Cutis, 2004. 74(2):99-103.
8. Pediatr Dermatol. 1992 Mar;9(1):22-6.
9. Pediatr Dermatol. 2015 Jul-Aug;32(4):e136-9.
10. Br J Dermatol. 2005 Dec;153(6):1176-81.
11. J Am Acad Dermatol. 2015 May;72(5):761-70.
A 16-year-old girl presented with a 6-month history of an erythematous eruption of small papules and pustules around the cheeks and nose. She states the erythema had started first, with periods of feeling flushed that became worse with sun exposure. She saw her primary care physician who prescribed topical steroids. After using the steroids, the rash became worse, and she developed papules and pustules.