User login
Absurdity
Absurdity is everywhere you look. Or don’t look.
As the old comedian Henny Youngman might have said:
Take my prior authorizations. Please!
Prior authorizations
1. Marissa had been taking isotretinoin for 2 months. She learned that three 20-mg capsules would cost her less than the two 30-mg capsules she’d been on.
My secretary called them. The insurance representative (Pharmacist? Clerk? Gal who stopped by to read the gas meter?) couldn’t help. “When I input your information, it issues a denial.” (Who is “it”? Watson’s evil twin Jensen?)
I got on the phone.
“Forgive me,” I said, gently, “but Marissa has been taking isotretinoin for 2 months, 60-mg per day. She was taking two 30’s. I want her to have three 20’s. They both add up to 60 mg. It’s the same dose. Why do you need to authorize it again?”
“Let me input that,” she replied. “Oh, now it’s accepting it. Your patient can have up to four pills a day.”
I was going to say she only needs three, but kept my mouth shut. Maybe Jensen only authorizes even numbers. 2. Danny has the worst atopic dermatitis I’ve ever seen. It’s all over him and never lets up. Topical steroids don’t touch it. Even prednisone – he’s had plenty over the years – barely makes a dent. I put in a Prior Authorization request for dupilumab.
“Your request for dupilumab has been denied,” read the insurer’s reply. “You have not shown failure with tacrolimus ointment or crisaborole.”
Say what? Prednisone didn’t help, and they expect tacrolimus or crisaborole to do the job?
I prescribed tacrolimus (which doesn’t come in a big enough tube to cover Daniel’s affected area anyway). It failed. Amazing.
“Your request for dupilumab has been denied,” said the insurer. “You have not also shown failure with crisaborole.”
Really? OK, I prescribed crisaborole. They denied coverage for it.
Now I was really getting into this. I wrote them. “I prescribed crisaborole.” I observed, “because you asked me to.”
They approved crisaborole. It failed. I reapplied for dupilumab. No response.
I called the insurer’s medical director, on a mobile with a Missouri exchange. After some telephone tag, he called back. “I cannot discuss this case with you,” he said, “because I have already made my determination.” Then he hung up without telling me what his determination was.
Further phone calls went unanswered. I thought Missouri was the Show-Me state.
The patient remained miserable. I decided to try one last time and wrote a long, sarcastic letter, detailing the whole episode. My secretary sent it off.
They approved dupilumab within the hour.
Malice? Nah, that’s giving them too much credit.
Now all Daniel has to do is improve.
Patient privacy!
Some news from abroad: what we know as HIPAA is called the “Data Protection Act” in the United Kingdom.
“We are obliged to conform to the demands of the Data Protection Act, and this specifically applies to the rabbi publicly mentioning the names of individuals who are unwell. The rabbi can only mention specific individuals with their permission or that of a relative designated by the sick person to do so. Anyone wishing for the rabbi to say a public prayer on their behalf must contact him directly by phone, text, or e-mail. To do anything else is breaking the law.”
If someone breaks this law, perhaps the rabbi can assist with atonement.
In any event, henceforth all entreaties to the Almighty must be encrypted. At least in the U.K.
What???!!!
Marina showed me her sunscreen. The label read, “Protects against UVA and UVB rays.”
“What’s the problem?” I asked.
She showed me our American Academy of Dermatology-produced sunscreen handout, which recommends “a broad-spectrum sunscreen that protects against both UVA and UVB rays, both of which cause cancer.”
“Does this mean my sunscreen causes cancer?” asked Marina.
“Not to worry,” I assured her.
I sighed and wafted a small prayer heavenward. Encrypted, of course.
Lo, the answer from above may tarry, but He will never forget His password.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Absurdity is everywhere you look. Or don’t look.
As the old comedian Henny Youngman might have said:
Take my prior authorizations. Please!
Prior authorizations
1. Marissa had been taking isotretinoin for 2 months. She learned that three 20-mg capsules would cost her less than the two 30-mg capsules she’d been on.
My secretary called them. The insurance representative (Pharmacist? Clerk? Gal who stopped by to read the gas meter?) couldn’t help. “When I input your information, it issues a denial.” (Who is “it”? Watson’s evil twin Jensen?)
I got on the phone.
“Forgive me,” I said, gently, “but Marissa has been taking isotretinoin for 2 months, 60-mg per day. She was taking two 30’s. I want her to have three 20’s. They both add up to 60 mg. It’s the same dose. Why do you need to authorize it again?”
“Let me input that,” she replied. “Oh, now it’s accepting it. Your patient can have up to four pills a day.”
I was going to say she only needs three, but kept my mouth shut. Maybe Jensen only authorizes even numbers. 2. Danny has the worst atopic dermatitis I’ve ever seen. It’s all over him and never lets up. Topical steroids don’t touch it. Even prednisone – he’s had plenty over the years – barely makes a dent. I put in a Prior Authorization request for dupilumab.
“Your request for dupilumab has been denied,” read the insurer’s reply. “You have not shown failure with tacrolimus ointment or crisaborole.”
Say what? Prednisone didn’t help, and they expect tacrolimus or crisaborole to do the job?
I prescribed tacrolimus (which doesn’t come in a big enough tube to cover Daniel’s affected area anyway). It failed. Amazing.
“Your request for dupilumab has been denied,” said the insurer. “You have not also shown failure with crisaborole.”
Really? OK, I prescribed crisaborole. They denied coverage for it.
Now I was really getting into this. I wrote them. “I prescribed crisaborole.” I observed, “because you asked me to.”
They approved crisaborole. It failed. I reapplied for dupilumab. No response.
I called the insurer’s medical director, on a mobile with a Missouri exchange. After some telephone tag, he called back. “I cannot discuss this case with you,” he said, “because I have already made my determination.” Then he hung up without telling me what his determination was.
Further phone calls went unanswered. I thought Missouri was the Show-Me state.
The patient remained miserable. I decided to try one last time and wrote a long, sarcastic letter, detailing the whole episode. My secretary sent it off.
They approved dupilumab within the hour.
Malice? Nah, that’s giving them too much credit.
Now all Daniel has to do is improve.
Patient privacy!
Some news from abroad: what we know as HIPAA is called the “Data Protection Act” in the United Kingdom.
“We are obliged to conform to the demands of the Data Protection Act, and this specifically applies to the rabbi publicly mentioning the names of individuals who are unwell. The rabbi can only mention specific individuals with their permission or that of a relative designated by the sick person to do so. Anyone wishing for the rabbi to say a public prayer on their behalf must contact him directly by phone, text, or e-mail. To do anything else is breaking the law.”
If someone breaks this law, perhaps the rabbi can assist with atonement.
In any event, henceforth all entreaties to the Almighty must be encrypted. At least in the U.K.
What???!!!
Marina showed me her sunscreen. The label read, “Protects against UVA and UVB rays.”
“What’s the problem?” I asked.
She showed me our American Academy of Dermatology-produced sunscreen handout, which recommends “a broad-spectrum sunscreen that protects against both UVA and UVB rays, both of which cause cancer.”
“Does this mean my sunscreen causes cancer?” asked Marina.
“Not to worry,” I assured her.
I sighed and wafted a small prayer heavenward. Encrypted, of course.
Lo, the answer from above may tarry, but He will never forget His password.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Absurdity is everywhere you look. Or don’t look.
As the old comedian Henny Youngman might have said:
Take my prior authorizations. Please!
Prior authorizations
1. Marissa had been taking isotretinoin for 2 months. She learned that three 20-mg capsules would cost her less than the two 30-mg capsules she’d been on.
My secretary called them. The insurance representative (Pharmacist? Clerk? Gal who stopped by to read the gas meter?) couldn’t help. “When I input your information, it issues a denial.” (Who is “it”? Watson’s evil twin Jensen?)
I got on the phone.
“Forgive me,” I said, gently, “but Marissa has been taking isotretinoin for 2 months, 60-mg per day. She was taking two 30’s. I want her to have three 20’s. They both add up to 60 mg. It’s the same dose. Why do you need to authorize it again?”
“Let me input that,” she replied. “Oh, now it’s accepting it. Your patient can have up to four pills a day.”
I was going to say she only needs three, but kept my mouth shut. Maybe Jensen only authorizes even numbers. 2. Danny has the worst atopic dermatitis I’ve ever seen. It’s all over him and never lets up. Topical steroids don’t touch it. Even prednisone – he’s had plenty over the years – barely makes a dent. I put in a Prior Authorization request for dupilumab.
“Your request for dupilumab has been denied,” read the insurer’s reply. “You have not shown failure with tacrolimus ointment or crisaborole.”
Say what? Prednisone didn’t help, and they expect tacrolimus or crisaborole to do the job?
I prescribed tacrolimus (which doesn’t come in a big enough tube to cover Daniel’s affected area anyway). It failed. Amazing.
“Your request for dupilumab has been denied,” said the insurer. “You have not also shown failure with crisaborole.”
Really? OK, I prescribed crisaborole. They denied coverage for it.
Now I was really getting into this. I wrote them. “I prescribed crisaborole.” I observed, “because you asked me to.”
They approved crisaborole. It failed. I reapplied for dupilumab. No response.
I called the insurer’s medical director, on a mobile with a Missouri exchange. After some telephone tag, he called back. “I cannot discuss this case with you,” he said, “because I have already made my determination.” Then he hung up without telling me what his determination was.
Further phone calls went unanswered. I thought Missouri was the Show-Me state.
The patient remained miserable. I decided to try one last time and wrote a long, sarcastic letter, detailing the whole episode. My secretary sent it off.
They approved dupilumab within the hour.
Malice? Nah, that’s giving them too much credit.
Now all Daniel has to do is improve.
Patient privacy!
Some news from abroad: what we know as HIPAA is called the “Data Protection Act” in the United Kingdom.
“We are obliged to conform to the demands of the Data Protection Act, and this specifically applies to the rabbi publicly mentioning the names of individuals who are unwell. The rabbi can only mention specific individuals with their permission or that of a relative designated by the sick person to do so. Anyone wishing for the rabbi to say a public prayer on their behalf must contact him directly by phone, text, or e-mail. To do anything else is breaking the law.”
If someone breaks this law, perhaps the rabbi can assist with atonement.
In any event, henceforth all entreaties to the Almighty must be encrypted. At least in the U.K.
What???!!!
Marina showed me her sunscreen. The label read, “Protects against UVA and UVB rays.”
“What’s the problem?” I asked.
She showed me our American Academy of Dermatology-produced sunscreen handout, which recommends “a broad-spectrum sunscreen that protects against both UVA and UVB rays, both of which cause cancer.”
“Does this mean my sunscreen causes cancer?” asked Marina.
“Not to worry,” I assured her.
I sighed and wafted a small prayer heavenward. Encrypted, of course.
Lo, the answer from above may tarry, but He will never forget His password.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at [email protected].
Mixin’ it up
What percentage of your office visits are a response to an acute complaint? What percentage are prescheduled well-child visits? And how many are follow-ups to manage chronic conditions and behavioral problems?
You probably have a sense of how you are spending your time in the office, but do you really have the numbers to support your guesstimate of the patient mix? Does anyone in your organization have that data? You probably could come up with some numbers in a few hours with a pencil and your office schedule for the last 2 months. However, learning how much of your income is generated by each category of visit would be more difficult.
Before you run out to the front desk and ask the receptionist to delete your same-day slots and replace them with a few preventive and chronic care visits, we should question a few of Mr. Hart’s assertions.
Of course, like you, I never spent the time to learn which categories of office visit were driving my income. However, I do know that I saw a stimulating mix of acute and chronic visits, and the most important number, the bottom line, was more than adequate for my needs. To achieve this profitable balance of visits meant that I needed to be as efficient as the patients’ complaints would allow. There is an often-repeated myth that there is a direct correlation between the length of time a physician spends with the patient and the quality of the visit. In my experience, patients are more impressed by the physician’s level of attention and concern than the amount of time he spends in the exam room.
You might argue that you just don’t have the time to fit in all those acute visits. But have you had the courage to open up your schedule, maybe hire more staff, and give it a try? It takes a bit of shift in mindset and the acknowledgment that a large part of what we call preventive care has not proved effective. Immunizations? Yes, but the rest, not so much.
I know this is a heretical proposition but I found that I knew my patients better after seeing them when they were in need rather than in the less frequent but longer encounters of a health maintenance visit. It takes work, but there is room for both kinds of visit.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
What percentage of your office visits are a response to an acute complaint? What percentage are prescheduled well-child visits? And how many are follow-ups to manage chronic conditions and behavioral problems?
You probably have a sense of how you are spending your time in the office, but do you really have the numbers to support your guesstimate of the patient mix? Does anyone in your organization have that data? You probably could come up with some numbers in a few hours with a pencil and your office schedule for the last 2 months. However, learning how much of your income is generated by each category of visit would be more difficult.
Before you run out to the front desk and ask the receptionist to delete your same-day slots and replace them with a few preventive and chronic care visits, we should question a few of Mr. Hart’s assertions.
Of course, like you, I never spent the time to learn which categories of office visit were driving my income. However, I do know that I saw a stimulating mix of acute and chronic visits, and the most important number, the bottom line, was more than adequate for my needs. To achieve this profitable balance of visits meant that I needed to be as efficient as the patients’ complaints would allow. There is an often-repeated myth that there is a direct correlation between the length of time a physician spends with the patient and the quality of the visit. In my experience, patients are more impressed by the physician’s level of attention and concern than the amount of time he spends in the exam room.
You might argue that you just don’t have the time to fit in all those acute visits. But have you had the courage to open up your schedule, maybe hire more staff, and give it a try? It takes a bit of shift in mindset and the acknowledgment that a large part of what we call preventive care has not proved effective. Immunizations? Yes, but the rest, not so much.
I know this is a heretical proposition but I found that I knew my patients better after seeing them when they were in need rather than in the less frequent but longer encounters of a health maintenance visit. It takes work, but there is room for both kinds of visit.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
What percentage of your office visits are a response to an acute complaint? What percentage are prescheduled well-child visits? And how many are follow-ups to manage chronic conditions and behavioral problems?
You probably have a sense of how you are spending your time in the office, but do you really have the numbers to support your guesstimate of the patient mix? Does anyone in your organization have that data? You probably could come up with some numbers in a few hours with a pencil and your office schedule for the last 2 months. However, learning how much of your income is generated by each category of visit would be more difficult.
Before you run out to the front desk and ask the receptionist to delete your same-day slots and replace them with a few preventive and chronic care visits, we should question a few of Mr. Hart’s assertions.
Of course, like you, I never spent the time to learn which categories of office visit were driving my income. However, I do know that I saw a stimulating mix of acute and chronic visits, and the most important number, the bottom line, was more than adequate for my needs. To achieve this profitable balance of visits meant that I needed to be as efficient as the patients’ complaints would allow. There is an often-repeated myth that there is a direct correlation between the length of time a physician spends with the patient and the quality of the visit. In my experience, patients are more impressed by the physician’s level of attention and concern than the amount of time he spends in the exam room.
You might argue that you just don’t have the time to fit in all those acute visits. But have you had the courage to open up your schedule, maybe hire more staff, and give it a try? It takes a bit of shift in mindset and the acknowledgment that a large part of what we call preventive care has not proved effective. Immunizations? Yes, but the rest, not so much.
I know this is a heretical proposition but I found that I knew my patients better after seeing them when they were in need rather than in the less frequent but longer encounters of a health maintenance visit. It takes work, but there is room for both kinds of visit.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Improving survival in older AML patients
The prognosis of AML in the elderly is very poor, with 5-year survival rates less than 10% in patients aged 65 years and older. However, Have we begun to witness an improvement in the survival of these patients?
Several clinical trials and observational registration studies have made it very clear that, without treatment, the survival in AML is very short – ranging from 11-16 weeks (for patients enrolled in therapeutic trials who received best supportive care only) to only 6-8 weeks in the “real-world” setting, based upon observational studies.1,2,3,4
These data are very meaningful because older AML patients often do not receive active therapy. As recently as 2009, SEER data indicate that 50% of patients aged 65 years or older receive no treatment for AML. This trend appears to be changing, based upon data from the AMLSG in 2012-2014, in which only a minority of patients in this age range received best supportive care only for their AML.
Knowing the very poor outcomes of patients who are not treated for AML, along with a high number of patients who are not treated, we must next ask whether any treatment at all is superior to no treatment. The data appear relatively clear on this question, with two representative publications highlighting the superiority of treatment vs. no treatment. First, in the SEER registry analysis by Medeiros et al., treated patients had a median survival of 5 months, compared with 2.5 months in untreated patients, and there was an unequivocal survival advantage attributed to treatment after adjustment for covariates and propensity score matching. Treatment included both traditional induction regimens and hypomethylating agent (HMA) therapy. Similarly, a phase 3 clinical trial testing low-dose cytarabine (LDAC) vs. best supportive care demonstrated survival improvement with LDAC (odds ratio, 0.60).
Recognizing that treatment improves survival in older adults with AML and that there is an upward trend in the percent of patients who receive active therapy, we can reasonably ask next whether survival has begun to trend upward over the past several years. This, of course, is a challenging question, but one that can be at least partially addressed through analyses of registration cohorts.
SEER data regarding AML patients aged 65 and older from the 1970s to 2013 suggest modestly improved 2-year survival, from less than 10% in the 1970s to 10%-15% since the early 2000s. The Moffitt Cancer Center database of patients aged 70 years and older also indicates a strong trend toward modestly improved survival after 2005, compared with prior to 2005 (unpublished data). Although the precise reason for trending improvements in overall survival of these patients over time is not clear, it is reasonable to suggest that a greater proportion of patients who receive actual therapy for AML could explain the modest improvements being observed. Improvements in supportive care through the years could also contribute to survival improvement trends over time, though this hypothesis has not been formally tested.
Next, we should ask about the most effective currently available therapy for older adults with AML. Standard treatment options for these patients, as mentioned previously, include high-intensity (traditional induction chemotherapy) and lower-intensity (LDAC, HMAs) regimens. Unfortunately, a prospective, randomized comparison between such regimens has not been undertaken, so it is impossible to declare with any certainty as to the superiority of one approach versus another. Larger database analyses, utilizing multivariate cox regression analyses, have been performed, suggesting that HMAs and intensive therapies perform similarly, such that offering an older adult with AML frontline therapy with a lower-intensity regimen is very reasonable.5
It is quite important to address the possibility that newer therapies in AML are changing the natural history of the disease. First of all, strategies utilizing HMA therapy with 5-azacitidine or decitabine have been widely studied. Unfortunately, a clear and convincing signal of survival benefit of frontline HMA therapy, compared with conventional care regimens (most commonly LDAC) has not been demonstrated, although trends toward a very modest survival advantage favoring HMAs were observed.6,7
Interestingly, in the AZA-AML-001 study, only the subgroup of patients who were preselected to receive best supportive care achieved survival benefit from 5-azacitidine, again suggesting that treatment vs. no treatment is among the most important factors leading to survival improvement in elderly AML.
Other novel agents are coming to the forefront, with the potential to change the natural history of AML in elderly patients. CPX-351 is a liposomal product that encapsulates cytarabine and daunorubicin in a fixed and synergistic molar ratio, thereby allowing delivery of both agents to the leukemic cell in the optimal fashion for cell kill. A recently completed phase 3 trial in older adults with secondary AML demonstrated statistically significant survival improvement with CPX-351 as compared with traditional daunorubicin plus cytarabine induction. A substantial minority of patients on this trial went to allogeneic hematopoietic cell transplant during first remission, and a landmark analysis performed at the time of transplant indicated better survival among patients who had received initial therapy with CPX-351.8
These data suggest that, in selected older adults with secondary AML who are fit enough to receive induction chemotherapy, CPX-351 offers a survival advantage, even among traditionally higher-risk subgroups, including patients with adverse karyotype of above age 70 years. As such, CPX-351 (Vyxeos) received FDA approval as frontline therapy for secondary AML in 2017.
Newer targeted therapies for older adults with AML also appear to hold promise. Glasdegib, an inhibitor of SMO (part of the hedgehog signaling pathway) was recently studied in combination with LDAC versus LDAC alone in a randomized phase 2 trial in older patients considered unfit for intensive induction chemotherapy. In this trial, patients assigned to glasdegib plus LDAC had longer median and overall survival than patients treated with LDAC alone, suggesting a promising novel agent on the horizon.9
Another example of a promising and novel targeted agent for AML is venetoclax, an inhibitor of BCL-2. Encouragingly high response rates and overall survival in phase 2 trials that combined venetoclax with LDAC or HMAs have driven randomized trials to definitively ascertain a survival advantage in older patients considered unfit for intensive therapy.10,11
The question also arises as to whether therapeutic outcomes can be optimized by better selection of currently available therapies for any given. This concept requires development of a decision analysis model that can be used to accurately predict outcomes among older patients with newly diagnosed AML. At Moffitt Cancer Center, such a model is being developed using a systematic review of the literature, followed by validation in a large institutional database. To date, there is the strong initial suggestion that initial therapy selection can be optimized for best outcome, taking into account variables including ECOG performance status, Charlson Comorbidity Index, and cytogenetic risk.12
The goal of improving survival in older adults with AML remains elusive. The decision to treat (regardless of high vs. low intensity) appears critical toward achieving this goal. New therapies such as CPX-351, glasdegib, and venetoclax also hold promise in further improving survival in subgroups of older patients. Finally, development of accurate predictive models to optimize initial therapy will be of critical importance for improving survival in this very heterogeneous disease that afflicts a very heterogeneous group of patients.
Dr. Lancet is chair of the department of malignant hematology at H. Lee Moffitt Cancer Center in Tampa. He has received consulting fees from Astellas, BioSight, Celgene, Janssen R&D, and Jazz Pharmaceuticals.
References
1. Burnett AK et al. Cancer. 2007 Mar 15;109(6):1114-24.
2. Harousseau JL et al. Blood. 2009 Aug 6;114(6):1166-73.
3. Medeiros BC et al. Ann Hematol. 2015 Mar 20; 94(7):1127-38.
4. Oran B et al. Haematologica. 2012 Dec;97(12):1916-24.
5. Lancet JE et al. J Clin Oncol. 2017. doi: 10.1200/JCO.2017.35.15_suppl.7031.
6. Dombret H et al. Blood. 2015 Jul 16;126(3):291-9.
7. Kantarjian HM et al. J Clin Oncol. 2012 Jul 20;30(21):2670-7.
8. Lancet JE et al. Blood 2016 128:906.
9. Cortes JE et al. Blood 2016 128:99.
10. DiNardo CD et al. Blood 2017 130:2628.
11. Wei A et al. Blood 2017 130:890.
12. Extermann M et al. SIOG 2017.
The prognosis of AML in the elderly is very poor, with 5-year survival rates less than 10% in patients aged 65 years and older. However, Have we begun to witness an improvement in the survival of these patients?
Several clinical trials and observational registration studies have made it very clear that, without treatment, the survival in AML is very short – ranging from 11-16 weeks (for patients enrolled in therapeutic trials who received best supportive care only) to only 6-8 weeks in the “real-world” setting, based upon observational studies.1,2,3,4
These data are very meaningful because older AML patients often do not receive active therapy. As recently as 2009, SEER data indicate that 50% of patients aged 65 years or older receive no treatment for AML. This trend appears to be changing, based upon data from the AMLSG in 2012-2014, in which only a minority of patients in this age range received best supportive care only for their AML.
Knowing the very poor outcomes of patients who are not treated for AML, along with a high number of patients who are not treated, we must next ask whether any treatment at all is superior to no treatment. The data appear relatively clear on this question, with two representative publications highlighting the superiority of treatment vs. no treatment. First, in the SEER registry analysis by Medeiros et al., treated patients had a median survival of 5 months, compared with 2.5 months in untreated patients, and there was an unequivocal survival advantage attributed to treatment after adjustment for covariates and propensity score matching. Treatment included both traditional induction regimens and hypomethylating agent (HMA) therapy. Similarly, a phase 3 clinical trial testing low-dose cytarabine (LDAC) vs. best supportive care demonstrated survival improvement with LDAC (odds ratio, 0.60).
Recognizing that treatment improves survival in older adults with AML and that there is an upward trend in the percent of patients who receive active therapy, we can reasonably ask next whether survival has begun to trend upward over the past several years. This, of course, is a challenging question, but one that can be at least partially addressed through analyses of registration cohorts.
SEER data regarding AML patients aged 65 and older from the 1970s to 2013 suggest modestly improved 2-year survival, from less than 10% in the 1970s to 10%-15% since the early 2000s. The Moffitt Cancer Center database of patients aged 70 years and older also indicates a strong trend toward modestly improved survival after 2005, compared with prior to 2005 (unpublished data). Although the precise reason for trending improvements in overall survival of these patients over time is not clear, it is reasonable to suggest that a greater proportion of patients who receive actual therapy for AML could explain the modest improvements being observed. Improvements in supportive care through the years could also contribute to survival improvement trends over time, though this hypothesis has not been formally tested.
Next, we should ask about the most effective currently available therapy for older adults with AML. Standard treatment options for these patients, as mentioned previously, include high-intensity (traditional induction chemotherapy) and lower-intensity (LDAC, HMAs) regimens. Unfortunately, a prospective, randomized comparison between such regimens has not been undertaken, so it is impossible to declare with any certainty as to the superiority of one approach versus another. Larger database analyses, utilizing multivariate cox regression analyses, have been performed, suggesting that HMAs and intensive therapies perform similarly, such that offering an older adult with AML frontline therapy with a lower-intensity regimen is very reasonable.5
It is quite important to address the possibility that newer therapies in AML are changing the natural history of the disease. First of all, strategies utilizing HMA therapy with 5-azacitidine or decitabine have been widely studied. Unfortunately, a clear and convincing signal of survival benefit of frontline HMA therapy, compared with conventional care regimens (most commonly LDAC) has not been demonstrated, although trends toward a very modest survival advantage favoring HMAs were observed.6,7
Interestingly, in the AZA-AML-001 study, only the subgroup of patients who were preselected to receive best supportive care achieved survival benefit from 5-azacitidine, again suggesting that treatment vs. no treatment is among the most important factors leading to survival improvement in elderly AML.
Other novel agents are coming to the forefront, with the potential to change the natural history of AML in elderly patients. CPX-351 is a liposomal product that encapsulates cytarabine and daunorubicin in a fixed and synergistic molar ratio, thereby allowing delivery of both agents to the leukemic cell in the optimal fashion for cell kill. A recently completed phase 3 trial in older adults with secondary AML demonstrated statistically significant survival improvement with CPX-351 as compared with traditional daunorubicin plus cytarabine induction. A substantial minority of patients on this trial went to allogeneic hematopoietic cell transplant during first remission, and a landmark analysis performed at the time of transplant indicated better survival among patients who had received initial therapy with CPX-351.8
These data suggest that, in selected older adults with secondary AML who are fit enough to receive induction chemotherapy, CPX-351 offers a survival advantage, even among traditionally higher-risk subgroups, including patients with adverse karyotype of above age 70 years. As such, CPX-351 (Vyxeos) received FDA approval as frontline therapy for secondary AML in 2017.
Newer targeted therapies for older adults with AML also appear to hold promise. Glasdegib, an inhibitor of SMO (part of the hedgehog signaling pathway) was recently studied in combination with LDAC versus LDAC alone in a randomized phase 2 trial in older patients considered unfit for intensive induction chemotherapy. In this trial, patients assigned to glasdegib plus LDAC had longer median and overall survival than patients treated with LDAC alone, suggesting a promising novel agent on the horizon.9
Another example of a promising and novel targeted agent for AML is venetoclax, an inhibitor of BCL-2. Encouragingly high response rates and overall survival in phase 2 trials that combined venetoclax with LDAC or HMAs have driven randomized trials to definitively ascertain a survival advantage in older patients considered unfit for intensive therapy.10,11
The question also arises as to whether therapeutic outcomes can be optimized by better selection of currently available therapies for any given. This concept requires development of a decision analysis model that can be used to accurately predict outcomes among older patients with newly diagnosed AML. At Moffitt Cancer Center, such a model is being developed using a systematic review of the literature, followed by validation in a large institutional database. To date, there is the strong initial suggestion that initial therapy selection can be optimized for best outcome, taking into account variables including ECOG performance status, Charlson Comorbidity Index, and cytogenetic risk.12
The goal of improving survival in older adults with AML remains elusive. The decision to treat (regardless of high vs. low intensity) appears critical toward achieving this goal. New therapies such as CPX-351, glasdegib, and venetoclax also hold promise in further improving survival in subgroups of older patients. Finally, development of accurate predictive models to optimize initial therapy will be of critical importance for improving survival in this very heterogeneous disease that afflicts a very heterogeneous group of patients.
Dr. Lancet is chair of the department of malignant hematology at H. Lee Moffitt Cancer Center in Tampa. He has received consulting fees from Astellas, BioSight, Celgene, Janssen R&D, and Jazz Pharmaceuticals.
References
1. Burnett AK et al. Cancer. 2007 Mar 15;109(6):1114-24.
2. Harousseau JL et al. Blood. 2009 Aug 6;114(6):1166-73.
3. Medeiros BC et al. Ann Hematol. 2015 Mar 20; 94(7):1127-38.
4. Oran B et al. Haematologica. 2012 Dec;97(12):1916-24.
5. Lancet JE et al. J Clin Oncol. 2017. doi: 10.1200/JCO.2017.35.15_suppl.7031.
6. Dombret H et al. Blood. 2015 Jul 16;126(3):291-9.
7. Kantarjian HM et al. J Clin Oncol. 2012 Jul 20;30(21):2670-7.
8. Lancet JE et al. Blood 2016 128:906.
9. Cortes JE et al. Blood 2016 128:99.
10. DiNardo CD et al. Blood 2017 130:2628.
11. Wei A et al. Blood 2017 130:890.
12. Extermann M et al. SIOG 2017.
The prognosis of AML in the elderly is very poor, with 5-year survival rates less than 10% in patients aged 65 years and older. However, Have we begun to witness an improvement in the survival of these patients?
Several clinical trials and observational registration studies have made it very clear that, without treatment, the survival in AML is very short – ranging from 11-16 weeks (for patients enrolled in therapeutic trials who received best supportive care only) to only 6-8 weeks in the “real-world” setting, based upon observational studies.1,2,3,4
These data are very meaningful because older AML patients often do not receive active therapy. As recently as 2009, SEER data indicate that 50% of patients aged 65 years or older receive no treatment for AML. This trend appears to be changing, based upon data from the AMLSG in 2012-2014, in which only a minority of patients in this age range received best supportive care only for their AML.
Knowing the very poor outcomes of patients who are not treated for AML, along with a high number of patients who are not treated, we must next ask whether any treatment at all is superior to no treatment. The data appear relatively clear on this question, with two representative publications highlighting the superiority of treatment vs. no treatment. First, in the SEER registry analysis by Medeiros et al., treated patients had a median survival of 5 months, compared with 2.5 months in untreated patients, and there was an unequivocal survival advantage attributed to treatment after adjustment for covariates and propensity score matching. Treatment included both traditional induction regimens and hypomethylating agent (HMA) therapy. Similarly, a phase 3 clinical trial testing low-dose cytarabine (LDAC) vs. best supportive care demonstrated survival improvement with LDAC (odds ratio, 0.60).
Recognizing that treatment improves survival in older adults with AML and that there is an upward trend in the percent of patients who receive active therapy, we can reasonably ask next whether survival has begun to trend upward over the past several years. This, of course, is a challenging question, but one that can be at least partially addressed through analyses of registration cohorts.
SEER data regarding AML patients aged 65 and older from the 1970s to 2013 suggest modestly improved 2-year survival, from less than 10% in the 1970s to 10%-15% since the early 2000s. The Moffitt Cancer Center database of patients aged 70 years and older also indicates a strong trend toward modestly improved survival after 2005, compared with prior to 2005 (unpublished data). Although the precise reason for trending improvements in overall survival of these patients over time is not clear, it is reasonable to suggest that a greater proportion of patients who receive actual therapy for AML could explain the modest improvements being observed. Improvements in supportive care through the years could also contribute to survival improvement trends over time, though this hypothesis has not been formally tested.
Next, we should ask about the most effective currently available therapy for older adults with AML. Standard treatment options for these patients, as mentioned previously, include high-intensity (traditional induction chemotherapy) and lower-intensity (LDAC, HMAs) regimens. Unfortunately, a prospective, randomized comparison between such regimens has not been undertaken, so it is impossible to declare with any certainty as to the superiority of one approach versus another. Larger database analyses, utilizing multivariate cox regression analyses, have been performed, suggesting that HMAs and intensive therapies perform similarly, such that offering an older adult with AML frontline therapy with a lower-intensity regimen is very reasonable.5
It is quite important to address the possibility that newer therapies in AML are changing the natural history of the disease. First of all, strategies utilizing HMA therapy with 5-azacitidine or decitabine have been widely studied. Unfortunately, a clear and convincing signal of survival benefit of frontline HMA therapy, compared with conventional care regimens (most commonly LDAC) has not been demonstrated, although trends toward a very modest survival advantage favoring HMAs were observed.6,7
Interestingly, in the AZA-AML-001 study, only the subgroup of patients who were preselected to receive best supportive care achieved survival benefit from 5-azacitidine, again suggesting that treatment vs. no treatment is among the most important factors leading to survival improvement in elderly AML.
Other novel agents are coming to the forefront, with the potential to change the natural history of AML in elderly patients. CPX-351 is a liposomal product that encapsulates cytarabine and daunorubicin in a fixed and synergistic molar ratio, thereby allowing delivery of both agents to the leukemic cell in the optimal fashion for cell kill. A recently completed phase 3 trial in older adults with secondary AML demonstrated statistically significant survival improvement with CPX-351 as compared with traditional daunorubicin plus cytarabine induction. A substantial minority of patients on this trial went to allogeneic hematopoietic cell transplant during first remission, and a landmark analysis performed at the time of transplant indicated better survival among patients who had received initial therapy with CPX-351.8
These data suggest that, in selected older adults with secondary AML who are fit enough to receive induction chemotherapy, CPX-351 offers a survival advantage, even among traditionally higher-risk subgroups, including patients with adverse karyotype of above age 70 years. As such, CPX-351 (Vyxeos) received FDA approval as frontline therapy for secondary AML in 2017.
Newer targeted therapies for older adults with AML also appear to hold promise. Glasdegib, an inhibitor of SMO (part of the hedgehog signaling pathway) was recently studied in combination with LDAC versus LDAC alone in a randomized phase 2 trial in older patients considered unfit for intensive induction chemotherapy. In this trial, patients assigned to glasdegib plus LDAC had longer median and overall survival than patients treated with LDAC alone, suggesting a promising novel agent on the horizon.9
Another example of a promising and novel targeted agent for AML is venetoclax, an inhibitor of BCL-2. Encouragingly high response rates and overall survival in phase 2 trials that combined venetoclax with LDAC or HMAs have driven randomized trials to definitively ascertain a survival advantage in older patients considered unfit for intensive therapy.10,11
The question also arises as to whether therapeutic outcomes can be optimized by better selection of currently available therapies for any given. This concept requires development of a decision analysis model that can be used to accurately predict outcomes among older patients with newly diagnosed AML. At Moffitt Cancer Center, such a model is being developed using a systematic review of the literature, followed by validation in a large institutional database. To date, there is the strong initial suggestion that initial therapy selection can be optimized for best outcome, taking into account variables including ECOG performance status, Charlson Comorbidity Index, and cytogenetic risk.12
The goal of improving survival in older adults with AML remains elusive. The decision to treat (regardless of high vs. low intensity) appears critical toward achieving this goal. New therapies such as CPX-351, glasdegib, and venetoclax also hold promise in further improving survival in subgroups of older patients. Finally, development of accurate predictive models to optimize initial therapy will be of critical importance for improving survival in this very heterogeneous disease that afflicts a very heterogeneous group of patients.
Dr. Lancet is chair of the department of malignant hematology at H. Lee Moffitt Cancer Center in Tampa. He has received consulting fees from Astellas, BioSight, Celgene, Janssen R&D, and Jazz Pharmaceuticals.
References
1. Burnett AK et al. Cancer. 2007 Mar 15;109(6):1114-24.
2. Harousseau JL et al. Blood. 2009 Aug 6;114(6):1166-73.
3. Medeiros BC et al. Ann Hematol. 2015 Mar 20; 94(7):1127-38.
4. Oran B et al. Haematologica. 2012 Dec;97(12):1916-24.
5. Lancet JE et al. J Clin Oncol. 2017. doi: 10.1200/JCO.2017.35.15_suppl.7031.
6. Dombret H et al. Blood. 2015 Jul 16;126(3):291-9.
7. Kantarjian HM et al. J Clin Oncol. 2012 Jul 20;30(21):2670-7.
8. Lancet JE et al. Blood 2016 128:906.
9. Cortes JE et al. Blood 2016 128:99.
10. DiNardo CD et al. Blood 2017 130:2628.
11. Wei A et al. Blood 2017 130:890.
12. Extermann M et al. SIOG 2017.
International travel updates
It’s that time of year again. Many of your patients will join the 80.2 million Americans with plans for international travel this summer.
In 2016, Mexico (31.2 million) and Canada (13.9 million) were the top two destinations of U.S. residents. Based on 2016 U.S. Commerce data, an additional 35.1 million Americans headed to overseas destinations, including 9% who traveled with children. Vacation and visiting friends and relatives accounted for 55% and 27% of the reasons for all travel, respectively. Education accounted for 4% of travelers.
Required versus recommended vaccines
The goal of a required vaccine is to prevent international spread of disease. The host country is protecting its citizens from visitors importing and facilitating the spread of a disease. Yellow fever and meningococcal disease are the only vaccines required for entry into any country. Entry requirements vary by country. Yellow fever may be an entry requirement for all travelers or it may be limited to those who have been in, or have had transit through, a country where yellow fever can be transmitted at least 6 days prior to the arrival at their final destination – a reminder that the sequence of the patient’s itinerary is important. In addition, just because a vaccine is not required for entry does not mean the risk for exposure and acquisition is nonexistent.
In contrast, recommended vaccines are for the protection of the individual. Travelers may be exposed to vaccine-preventable diseases that do not exist in their country (such as measles, typhoid fever, and yellow fever). They are at risk for acquisition and may return home infected, which could create the potential to spread the disease to susceptible contacts.
Most travelers comprehend required vaccines but often fail to understand the importance of receiving recommended vaccines. Lammert et al. reported that, of 24,478 persons who received pretravel advice between July 2012 and June 2014 through Global TravEpiNet, a national consortium of U.S. clinics, 97% were eligible for at least one vaccine. The majority were eligible for typhoid (n = 20,092) and hepatitis A (n = 12,990). Of patients included in the study, 25% (6,573) refused one or more vaccines. The most common reason cited for refusal was a lack of concern about the illness. Travelers visiting friends and relatives were less likely to accept all recommended vaccines, compared with those who were not visiting friends and relatives (odds ratio, 0.74) (J Trav Med. 2017 Jan. doi: 10.1093/jtm/taw075). In the United States, international travel remains the most common risk factor for acquisition of both typhoid fever and hepatitis A.
What’s new
The U.S. Advisory Committee on Immunization Practices recommends administering the hepatitis A vaccine to infants aged 6-11 months with travel to or living in developing countries and areas with high to moderate risk for hepatitis A virus transmission. Any dose received at less than 12 months of age does not count, and the administration of two age-appropriate doses should occur following this dose.
Old but still relevant
Measles: The Advisory Committee on Immunization Practices recommends all infants aged 6-11 months receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Prior to administering, determine whether your patient will travel to a yellow fever–endemic area because both are live vaccines and should be received the same day. Otherwise, administer MMR doses 28 days apart; coordination between facilities or receipt of both at one facility may be necessary.
Yellow fever vaccine: The U.S. supplies of YF-Vax by Sanofi Pasteur are not expected to be available again until the end of 2018. To provide vaccines for U.S. travelers, Stamaril – a yellow fever vaccine produced by Sanofi Pasteur in France – has been made available at more than 250 sites through an Expanded Access Investigational New Drug Program.
Since Stamaril is offered at a limited number of locations, persons with anticipated travel to a country where receipt of yellow fever vaccine is either required for entry or recommended for their protection should not wait until the last minute to obtain it. Postponing a trip or changing a destination is preferred if vaccine is not received, especially when the person is traveling to countries with an ongoing outbreak.
The vaccination does not become valid until 10 days after receipt. Infants aged at least 9 months may receive the vaccine. Since the yellow fever vaccine is a live vaccine, administration may be contraindicated in certain individuals. Exemption letters are provided for those with medical contraindication.
To locate a Stamaril site in your area: https://wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
Current disease outbreaks
Yellow fever: Brazil
Since Dec. 2017, more than 1,100 laboratory-confirmed cases of yellow fever have been reported, including 17 reported in unvaccinated international travelers. Fatal cases also have been reported. In addition to areas in Brazil where yellow fever vaccination had been recommended prior to the recent outbreaks, the vaccine now also is recommended for people who are traveling to or living in all of Espírito Santo State, São Paulo State, and Rio de Janeiro State, as well as several cities in Bahia State. Unvaccinated travelers should avoid travel to areas where vaccination is recommended. Those previously vaccinated at 10 years ago or longer should consider a booster.
Listeria: South Africa
An ongoing outbreak has been reported since Jan. 2017. Around 1,000 people have been infected. Avoid consumption of processed meats including “Polony” (South African bologna).
Measles: Belarus, Japan, Liberia, and Taiwan
All countries have reported an increase in cases since April 2018. Measles outbreaks have been reported in an additional 13 countries since Jan. 2018, including France, Ireland, Italy, the Philippines, and the United Kingdom.
Norovirus: Canada
More than 120 cases have been linked to consumption of raw or lightly cooked oysters from western Canada.
For more country-specific information and up to date travel alerts, visit http://www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and the director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It’s that time of year again. Many of your patients will join the 80.2 million Americans with plans for international travel this summer.
In 2016, Mexico (31.2 million) and Canada (13.9 million) were the top two destinations of U.S. residents. Based on 2016 U.S. Commerce data, an additional 35.1 million Americans headed to overseas destinations, including 9% who traveled with children. Vacation and visiting friends and relatives accounted for 55% and 27% of the reasons for all travel, respectively. Education accounted for 4% of travelers.
Required versus recommended vaccines
The goal of a required vaccine is to prevent international spread of disease. The host country is protecting its citizens from visitors importing and facilitating the spread of a disease. Yellow fever and meningococcal disease are the only vaccines required for entry into any country. Entry requirements vary by country. Yellow fever may be an entry requirement for all travelers or it may be limited to those who have been in, or have had transit through, a country where yellow fever can be transmitted at least 6 days prior to the arrival at their final destination – a reminder that the sequence of the patient’s itinerary is important. In addition, just because a vaccine is not required for entry does not mean the risk for exposure and acquisition is nonexistent.
In contrast, recommended vaccines are for the protection of the individual. Travelers may be exposed to vaccine-preventable diseases that do not exist in their country (such as measles, typhoid fever, and yellow fever). They are at risk for acquisition and may return home infected, which could create the potential to spread the disease to susceptible contacts.
Most travelers comprehend required vaccines but often fail to understand the importance of receiving recommended vaccines. Lammert et al. reported that, of 24,478 persons who received pretravel advice between July 2012 and June 2014 through Global TravEpiNet, a national consortium of U.S. clinics, 97% were eligible for at least one vaccine. The majority were eligible for typhoid (n = 20,092) and hepatitis A (n = 12,990). Of patients included in the study, 25% (6,573) refused one or more vaccines. The most common reason cited for refusal was a lack of concern about the illness. Travelers visiting friends and relatives were less likely to accept all recommended vaccines, compared with those who were not visiting friends and relatives (odds ratio, 0.74) (J Trav Med. 2017 Jan. doi: 10.1093/jtm/taw075). In the United States, international travel remains the most common risk factor for acquisition of both typhoid fever and hepatitis A.
What’s new
The U.S. Advisory Committee on Immunization Practices recommends administering the hepatitis A vaccine to infants aged 6-11 months with travel to or living in developing countries and areas with high to moderate risk for hepatitis A virus transmission. Any dose received at less than 12 months of age does not count, and the administration of two age-appropriate doses should occur following this dose.
Old but still relevant
Measles: The Advisory Committee on Immunization Practices recommends all infants aged 6-11 months receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Prior to administering, determine whether your patient will travel to a yellow fever–endemic area because both are live vaccines and should be received the same day. Otherwise, administer MMR doses 28 days apart; coordination between facilities or receipt of both at one facility may be necessary.
Yellow fever vaccine: The U.S. supplies of YF-Vax by Sanofi Pasteur are not expected to be available again until the end of 2018. To provide vaccines for U.S. travelers, Stamaril – a yellow fever vaccine produced by Sanofi Pasteur in France – has been made available at more than 250 sites through an Expanded Access Investigational New Drug Program.
Since Stamaril is offered at a limited number of locations, persons with anticipated travel to a country where receipt of yellow fever vaccine is either required for entry or recommended for their protection should not wait until the last minute to obtain it. Postponing a trip or changing a destination is preferred if vaccine is not received, especially when the person is traveling to countries with an ongoing outbreak.
The vaccination does not become valid until 10 days after receipt. Infants aged at least 9 months may receive the vaccine. Since the yellow fever vaccine is a live vaccine, administration may be contraindicated in certain individuals. Exemption letters are provided for those with medical contraindication.
To locate a Stamaril site in your area: https://wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
Current disease outbreaks
Yellow fever: Brazil
Since Dec. 2017, more than 1,100 laboratory-confirmed cases of yellow fever have been reported, including 17 reported in unvaccinated international travelers. Fatal cases also have been reported. In addition to areas in Brazil where yellow fever vaccination had been recommended prior to the recent outbreaks, the vaccine now also is recommended for people who are traveling to or living in all of Espírito Santo State, São Paulo State, and Rio de Janeiro State, as well as several cities in Bahia State. Unvaccinated travelers should avoid travel to areas where vaccination is recommended. Those previously vaccinated at 10 years ago or longer should consider a booster.
Listeria: South Africa
An ongoing outbreak has been reported since Jan. 2017. Around 1,000 people have been infected. Avoid consumption of processed meats including “Polony” (South African bologna).
Measles: Belarus, Japan, Liberia, and Taiwan
All countries have reported an increase in cases since April 2018. Measles outbreaks have been reported in an additional 13 countries since Jan. 2018, including France, Ireland, Italy, the Philippines, and the United Kingdom.
Norovirus: Canada
More than 120 cases have been linked to consumption of raw or lightly cooked oysters from western Canada.
For more country-specific information and up to date travel alerts, visit http://www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and the director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It’s that time of year again. Many of your patients will join the 80.2 million Americans with plans for international travel this summer.
In 2016, Mexico (31.2 million) and Canada (13.9 million) were the top two destinations of U.S. residents. Based on 2016 U.S. Commerce data, an additional 35.1 million Americans headed to overseas destinations, including 9% who traveled with children. Vacation and visiting friends and relatives accounted for 55% and 27% of the reasons for all travel, respectively. Education accounted for 4% of travelers.
Required versus recommended vaccines
The goal of a required vaccine is to prevent international spread of disease. The host country is protecting its citizens from visitors importing and facilitating the spread of a disease. Yellow fever and meningococcal disease are the only vaccines required for entry into any country. Entry requirements vary by country. Yellow fever may be an entry requirement for all travelers or it may be limited to those who have been in, or have had transit through, a country where yellow fever can be transmitted at least 6 days prior to the arrival at their final destination – a reminder that the sequence of the patient’s itinerary is important. In addition, just because a vaccine is not required for entry does not mean the risk for exposure and acquisition is nonexistent.
In contrast, recommended vaccines are for the protection of the individual. Travelers may be exposed to vaccine-preventable diseases that do not exist in their country (such as measles, typhoid fever, and yellow fever). They are at risk for acquisition and may return home infected, which could create the potential to spread the disease to susceptible contacts.
Most travelers comprehend required vaccines but often fail to understand the importance of receiving recommended vaccines. Lammert et al. reported that, of 24,478 persons who received pretravel advice between July 2012 and June 2014 through Global TravEpiNet, a national consortium of U.S. clinics, 97% were eligible for at least one vaccine. The majority were eligible for typhoid (n = 20,092) and hepatitis A (n = 12,990). Of patients included in the study, 25% (6,573) refused one or more vaccines. The most common reason cited for refusal was a lack of concern about the illness. Travelers visiting friends and relatives were less likely to accept all recommended vaccines, compared with those who were not visiting friends and relatives (odds ratio, 0.74) (J Trav Med. 2017 Jan. doi: 10.1093/jtm/taw075). In the United States, international travel remains the most common risk factor for acquisition of both typhoid fever and hepatitis A.
What’s new
The U.S. Advisory Committee on Immunization Practices recommends administering the hepatitis A vaccine to infants aged 6-11 months with travel to or living in developing countries and areas with high to moderate risk for hepatitis A virus transmission. Any dose received at less than 12 months of age does not count, and the administration of two age-appropriate doses should occur following this dose.
Old but still relevant
Measles: The Advisory Committee on Immunization Practices recommends all infants aged 6-11 months receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Prior to administering, determine whether your patient will travel to a yellow fever–endemic area because both are live vaccines and should be received the same day. Otherwise, administer MMR doses 28 days apart; coordination between facilities or receipt of both at one facility may be necessary.
Yellow fever vaccine: The U.S. supplies of YF-Vax by Sanofi Pasteur are not expected to be available again until the end of 2018. To provide vaccines for U.S. travelers, Stamaril – a yellow fever vaccine produced by Sanofi Pasteur in France – has been made available at more than 250 sites through an Expanded Access Investigational New Drug Program.
Since Stamaril is offered at a limited number of locations, persons with anticipated travel to a country where receipt of yellow fever vaccine is either required for entry or recommended for their protection should not wait until the last minute to obtain it. Postponing a trip or changing a destination is preferred if vaccine is not received, especially when the person is traveling to countries with an ongoing outbreak.
The vaccination does not become valid until 10 days after receipt. Infants aged at least 9 months may receive the vaccine. Since the yellow fever vaccine is a live vaccine, administration may be contraindicated in certain individuals. Exemption letters are provided for those with medical contraindication.
To locate a Stamaril site in your area: https://wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
Current disease outbreaks
Yellow fever: Brazil
Since Dec. 2017, more than 1,100 laboratory-confirmed cases of yellow fever have been reported, including 17 reported in unvaccinated international travelers. Fatal cases also have been reported. In addition to areas in Brazil where yellow fever vaccination had been recommended prior to the recent outbreaks, the vaccine now also is recommended for people who are traveling to or living in all of Espírito Santo State, São Paulo State, and Rio de Janeiro State, as well as several cities in Bahia State. Unvaccinated travelers should avoid travel to areas where vaccination is recommended. Those previously vaccinated at 10 years ago or longer should consider a booster.
Listeria: South Africa
An ongoing outbreak has been reported since Jan. 2017. Around 1,000 people have been infected. Avoid consumption of processed meats including “Polony” (South African bologna).
Measles: Belarus, Japan, Liberia, and Taiwan
All countries have reported an increase in cases since April 2018. Measles outbreaks have been reported in an additional 13 countries since Jan. 2018, including France, Ireland, Italy, the Philippines, and the United Kingdom.
Norovirus: Canada
More than 120 cases have been linked to consumption of raw or lightly cooked oysters from western Canada.
For more country-specific information and up to date travel alerts, visit http://www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and the director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Privacy, propaganda, and polarization
Because I rarely perform surgery, my primary product is providing relevant information delivered with competence, compassion, and commitment. I must make the correct diagnosis and prescribe the correct treatment. I deliver that information with compassion to meet the emotional and spiritual needs of my patients and their parents. Parents can trust that I am committed to providing the best possible care for their child, rather than primarily seeking to enrich myself. After all, I chose pediatrics.
Three years ago I wrote a column about the use of Google as an alternative to physicians. The public can access a massive amount of medical information through the Internet. That information has been growing exponentially. But let’s look at what else has happened in the past 3 years that reflects the difference between my professionalism and the merchandising of the Internet.
I am a medical professional committed to my patients. The purveyors of information via the Internet are primarily dedicated to increased advertising revenue through click baiting and profiling. Apple’s CEO Tim Cook put it this way: “A few years ago, users of Internet services began to realize that when an online service is free, you’re not the customer. You’re the product.”
Facebook and Google learn from the content of people’s messages and search terms to build a personal profile that is valuable to advertisers. Soon that profile could include health information. Recently, 300,000 users were tempted to download a survey app via Facebook. The app developer used Facebook tools to scrape profile information not just on those 300,000 users, but on 87,000,000 contacts who did not give explicit consent. This massive leak of privacy was used to target people’s votes. Similar profiles could be used in focused advertising of health care products and services.
I have a professional and legal responsibility to provide accurate information to my patients. Years ago, Internet service providers lobbied for and obtained legal protections saying that they were not responsible for content transmitted over their networks. That idea made some sense when Facebook was primarily sharing information within families and friends. But then Facebook began a news feed without reporters vetting information and without the ethics of journalism and the fourth estate. A generation ago, three television broadcasting companies competed to provide daily evening news programs consisting of four to six stories carefully chosen to be important and relevant. Now a myriad of polarized blogs on unaccountable social media are designed to solicit clicks, spread advertising, and influence shoppers. The result has been a massive, toxic spill of false information into the noosphere. Given the already poor state of health literacy, this fake news contributes to ongoing problems with vaccine hesitancy, worthless cures, and distrust of the medical profession.
It makes the BP/Deep Horizon oil spill into the Gulf look small by comparison. The cleanup of this social media mess is going to be costly and require new technology. Chemical companies used to dump vast quantities of toxic waste and byproducts into rivers and landfills. Superfund sites involve billion dollar cleanups. Efforts are made to trace where the chemicals came from and to bill the original companies. Under a “cradle to grave” concept, a chemical company cannot avoid liability by giving toxic waste to a fly-by-night waste disposal company. Two years ago, Volkswagen stock lost $15 billion overnight when fraud was exposed in diesel emissions testing. Fines and compensation exceeded $25 billion. It has gained it all back. Facebook stock is worth five times more that Volkswagen. So even billion dollar fines would be a small cost of doing business within social media.
One information technology that has resisted pollution is Wikipedia. Google has been featuring Wikipedia websites in its search engine results for many years. Now even Facebook is contemplating using Wikipedia to combat fake news. I would not treat a patient solely based on information I found on Wikipedia. But I do find it convenient to remind me of information I had learned in the past and to reassure me that my memory is neither faulty nor outdated.
One senator said Facebook had problems with privacy and propaganda.He missed a third issue – polarization. Internet apps are designed to affirm people’s biases.By targeting Facebook users with news feeds and advertisements tailored to their prior search terms, likes, sites visited, and friends, Facebook provides news feeds that support people’s current beliefs. My own use of Google to search for health information is similarly tainted. Social media also has contaminated the ability of government to solicit public comments on legislative proposals.Similar issues make product reviews unreliable.
Overall, it is clear that the public’s ability to use the Internet to improve their health has been markedly compromised over the past 3 years. Professionalism is important. Three years ago I asked who you were going to believe – me or billionaire Elizabeth Holmes, CEO of Theranos? Since then, one of us has not signed an agreement with the Securities and Exchange Commission involving massive fraud.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He said he had no relevant financial disclosures. Email him at [email protected].
Because I rarely perform surgery, my primary product is providing relevant information delivered with competence, compassion, and commitment. I must make the correct diagnosis and prescribe the correct treatment. I deliver that information with compassion to meet the emotional and spiritual needs of my patients and their parents. Parents can trust that I am committed to providing the best possible care for their child, rather than primarily seeking to enrich myself. After all, I chose pediatrics.
Three years ago I wrote a column about the use of Google as an alternative to physicians. The public can access a massive amount of medical information through the Internet. That information has been growing exponentially. But let’s look at what else has happened in the past 3 years that reflects the difference between my professionalism and the merchandising of the Internet.
I am a medical professional committed to my patients. The purveyors of information via the Internet are primarily dedicated to increased advertising revenue through click baiting and profiling. Apple’s CEO Tim Cook put it this way: “A few years ago, users of Internet services began to realize that when an online service is free, you’re not the customer. You’re the product.”
Facebook and Google learn from the content of people’s messages and search terms to build a personal profile that is valuable to advertisers. Soon that profile could include health information. Recently, 300,000 users were tempted to download a survey app via Facebook. The app developer used Facebook tools to scrape profile information not just on those 300,000 users, but on 87,000,000 contacts who did not give explicit consent. This massive leak of privacy was used to target people’s votes. Similar profiles could be used in focused advertising of health care products and services.
I have a professional and legal responsibility to provide accurate information to my patients. Years ago, Internet service providers lobbied for and obtained legal protections saying that they were not responsible for content transmitted over their networks. That idea made some sense when Facebook was primarily sharing information within families and friends. But then Facebook began a news feed without reporters vetting information and without the ethics of journalism and the fourth estate. A generation ago, three television broadcasting companies competed to provide daily evening news programs consisting of four to six stories carefully chosen to be important and relevant. Now a myriad of polarized blogs on unaccountable social media are designed to solicit clicks, spread advertising, and influence shoppers. The result has been a massive, toxic spill of false information into the noosphere. Given the already poor state of health literacy, this fake news contributes to ongoing problems with vaccine hesitancy, worthless cures, and distrust of the medical profession.
It makes the BP/Deep Horizon oil spill into the Gulf look small by comparison. The cleanup of this social media mess is going to be costly and require new technology. Chemical companies used to dump vast quantities of toxic waste and byproducts into rivers and landfills. Superfund sites involve billion dollar cleanups. Efforts are made to trace where the chemicals came from and to bill the original companies. Under a “cradle to grave” concept, a chemical company cannot avoid liability by giving toxic waste to a fly-by-night waste disposal company. Two years ago, Volkswagen stock lost $15 billion overnight when fraud was exposed in diesel emissions testing. Fines and compensation exceeded $25 billion. It has gained it all back. Facebook stock is worth five times more that Volkswagen. So even billion dollar fines would be a small cost of doing business within social media.
One information technology that has resisted pollution is Wikipedia. Google has been featuring Wikipedia websites in its search engine results for many years. Now even Facebook is contemplating using Wikipedia to combat fake news. I would not treat a patient solely based on information I found on Wikipedia. But I do find it convenient to remind me of information I had learned in the past and to reassure me that my memory is neither faulty nor outdated.
One senator said Facebook had problems with privacy and propaganda.He missed a third issue – polarization. Internet apps are designed to affirm people’s biases.By targeting Facebook users with news feeds and advertisements tailored to their prior search terms, likes, sites visited, and friends, Facebook provides news feeds that support people’s current beliefs. My own use of Google to search for health information is similarly tainted. Social media also has contaminated the ability of government to solicit public comments on legislative proposals.Similar issues make product reviews unreliable.
Overall, it is clear that the public’s ability to use the Internet to improve their health has been markedly compromised over the past 3 years. Professionalism is important. Three years ago I asked who you were going to believe – me or billionaire Elizabeth Holmes, CEO of Theranos? Since then, one of us has not signed an agreement with the Securities and Exchange Commission involving massive fraud.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He said he had no relevant financial disclosures. Email him at [email protected].
Because I rarely perform surgery, my primary product is providing relevant information delivered with competence, compassion, and commitment. I must make the correct diagnosis and prescribe the correct treatment. I deliver that information with compassion to meet the emotional and spiritual needs of my patients and their parents. Parents can trust that I am committed to providing the best possible care for their child, rather than primarily seeking to enrich myself. After all, I chose pediatrics.
Three years ago I wrote a column about the use of Google as an alternative to physicians. The public can access a massive amount of medical information through the Internet. That information has been growing exponentially. But let’s look at what else has happened in the past 3 years that reflects the difference between my professionalism and the merchandising of the Internet.
I am a medical professional committed to my patients. The purveyors of information via the Internet are primarily dedicated to increased advertising revenue through click baiting and profiling. Apple’s CEO Tim Cook put it this way: “A few years ago, users of Internet services began to realize that when an online service is free, you’re not the customer. You’re the product.”
Facebook and Google learn from the content of people’s messages and search terms to build a personal profile that is valuable to advertisers. Soon that profile could include health information. Recently, 300,000 users were tempted to download a survey app via Facebook. The app developer used Facebook tools to scrape profile information not just on those 300,000 users, but on 87,000,000 contacts who did not give explicit consent. This massive leak of privacy was used to target people’s votes. Similar profiles could be used in focused advertising of health care products and services.
I have a professional and legal responsibility to provide accurate information to my patients. Years ago, Internet service providers lobbied for and obtained legal protections saying that they were not responsible for content transmitted over their networks. That idea made some sense when Facebook was primarily sharing information within families and friends. But then Facebook began a news feed without reporters vetting information and without the ethics of journalism and the fourth estate. A generation ago, three television broadcasting companies competed to provide daily evening news programs consisting of four to six stories carefully chosen to be important and relevant. Now a myriad of polarized blogs on unaccountable social media are designed to solicit clicks, spread advertising, and influence shoppers. The result has been a massive, toxic spill of false information into the noosphere. Given the already poor state of health literacy, this fake news contributes to ongoing problems with vaccine hesitancy, worthless cures, and distrust of the medical profession.
It makes the BP/Deep Horizon oil spill into the Gulf look small by comparison. The cleanup of this social media mess is going to be costly and require new technology. Chemical companies used to dump vast quantities of toxic waste and byproducts into rivers and landfills. Superfund sites involve billion dollar cleanups. Efforts are made to trace where the chemicals came from and to bill the original companies. Under a “cradle to grave” concept, a chemical company cannot avoid liability by giving toxic waste to a fly-by-night waste disposal company. Two years ago, Volkswagen stock lost $15 billion overnight when fraud was exposed in diesel emissions testing. Fines and compensation exceeded $25 billion. It has gained it all back. Facebook stock is worth five times more that Volkswagen. So even billion dollar fines would be a small cost of doing business within social media.
One information technology that has resisted pollution is Wikipedia. Google has been featuring Wikipedia websites in its search engine results for many years. Now even Facebook is contemplating using Wikipedia to combat fake news. I would not treat a patient solely based on information I found on Wikipedia. But I do find it convenient to remind me of information I had learned in the past and to reassure me that my memory is neither faulty nor outdated.
One senator said Facebook had problems with privacy and propaganda.He missed a third issue – polarization. Internet apps are designed to affirm people’s biases.By targeting Facebook users with news feeds and advertisements tailored to their prior search terms, likes, sites visited, and friends, Facebook provides news feeds that support people’s current beliefs. My own use of Google to search for health information is similarly tainted. Social media also has contaminated the ability of government to solicit public comments on legislative proposals.Similar issues make product reviews unreliable.
Overall, it is clear that the public’s ability to use the Internet to improve their health has been markedly compromised over the past 3 years. Professionalism is important. Three years ago I asked who you were going to believe – me or billionaire Elizabeth Holmes, CEO of Theranos? Since then, one of us has not signed an agreement with the Securities and Exchange Commission involving massive fraud.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He said he had no relevant financial disclosures. Email him at [email protected].
Walking the walk
In March 2018, the Human Rights Campaign (HRC), an advocacy organization dedicated to improving the lives of LGBTQ people, released its 11th Annual Healthcare Equality Index. The HEI is an indicator of how inclusive and equitable health care facilities are in providing care for their LGBTQ patients. My own institution, Children’s Hospital of Pittsburgh, scored very high on this index and received the “Leader in LGBTQ Healthcare Equality” designation. The process of receiving this designation is very rigorous, and I am proud of my institution for making great strides in expanding health care access for LGBTQ patients, especially transgender patients. However, this is no time to rest on one’s laurels, as many transgender people still experience challenges and barriers in navigating the health care system.
Insurance access continues to be a problem. I wrote a column in June 2017 about obtaining health care insurance for transgender patients. Preauthorization is common for obtaining cross-sex hormones or pubertal blockers even for insurance companies that are willing to pay for them – a process that can take weeks, even months, to complete. This creates delays in obtaining necessary care for transgender patients. This is just one of the many barriers transgender people face in navigating the health care system.
Increasing access to health care services for transgender patients is more about improving health outcomes than patient satisfaction. Even the smallest policy change may have a meaningful impact on the lives of transgender individuals. A study by Russell et al., in the April 2018 issue of Journal of Adolescent Health found that transgender youth allowed to use their chosen name (instead of the name assigned to them by their parents at birth) were more likely to have fewer depressive symptoms and lower rates of suicidal ideation and suicidal behavior.3 These findings highlight that even a small change can have a huge impact on the health and well-being of this patient population.
What can you do to expand access? First, you must educate yourself and teach others. Many providers report never having received education on LGBTQ health during their training,4 and most barriers for transgender patients stem from this lack of training. Second, work with the transgender community – it is very tempting to see your institution’s name on the HEI and think all the work is done, but the lived experiences of transgender patients sometimes are different than what is seen on paper (or online). Team up with local organizations such as PFLAG (formerly known as Parents and Families of Lesbians and Gays) that can create support groups for both transgender youth and their families. Help create a network of referral systems for your transgender patients – the community is often small enough that they know which providers or establishments are safe for transgender individuals. Many transgender patients find this extremely helpful.5 You still wield significant influence in the community, so work with the health care and insurance systems to improve access and coverage for gender-related services. The HRC HEI is becoming coveted by health care institutions. This is a prime opportunity to be involved in committees seeking to improve health care access for transgender individuals. Finally, as there are champions for transgender health in your clinic, there also are champions for transgender health in insurance companies. They often are well known in the community, so find that individual for counseling on how to navigate the insurance system for your transgender patients.
Although an increasing number of health care institutions and clinics are recognizing the health care needs of transgender patients and providing appropriate care, the health care system remains challenging for transgender individuals to navigate. Small policy changes may have a substantial impact on the health and well-being of transgender individuals. Although creating change within an institution may seem like a monumental task, you do have the agency to help create this type change within the system to expand health care access for transgender patients.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
Resources
- HRC HEI: If you’re interested in learning what policies are inclusive and equitable for LGBT patients, check out the HRC HEI scoring criteria. It’s a good place to start if you want to expand health care access for transgender individuals.
- To find out more about the health care legal protections transgender individuals are entitled to, check out the National Center for Transgender Equality.
References
1. Psychol Bull. 2003 Sep;129(5):674-97.
2. “Injustice at every turn: A report of the National Transgender Discrimination Survey.” (Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011.)
3. J. Adolesc Health. 2018 Apr. doi: 10.1016/j.jadohealth.2018.02.003.
4. Int J Transgenderism. 2008. doi: 10.1300/J485v08n02_08.
5. Transgend Health. 2016 Nov 1;1(1):238-49.
In March 2018, the Human Rights Campaign (HRC), an advocacy organization dedicated to improving the lives of LGBTQ people, released its 11th Annual Healthcare Equality Index. The HEI is an indicator of how inclusive and equitable health care facilities are in providing care for their LGBTQ patients. My own institution, Children’s Hospital of Pittsburgh, scored very high on this index and received the “Leader in LGBTQ Healthcare Equality” designation. The process of receiving this designation is very rigorous, and I am proud of my institution for making great strides in expanding health care access for LGBTQ patients, especially transgender patients. However, this is no time to rest on one’s laurels, as many transgender people still experience challenges and barriers in navigating the health care system.
Insurance access continues to be a problem. I wrote a column in June 2017 about obtaining health care insurance for transgender patients. Preauthorization is common for obtaining cross-sex hormones or pubertal blockers even for insurance companies that are willing to pay for them – a process that can take weeks, even months, to complete. This creates delays in obtaining necessary care for transgender patients. This is just one of the many barriers transgender people face in navigating the health care system.
Increasing access to health care services for transgender patients is more about improving health outcomes than patient satisfaction. Even the smallest policy change may have a meaningful impact on the lives of transgender individuals. A study by Russell et al., in the April 2018 issue of Journal of Adolescent Health found that transgender youth allowed to use their chosen name (instead of the name assigned to them by their parents at birth) were more likely to have fewer depressive symptoms and lower rates of suicidal ideation and suicidal behavior.3 These findings highlight that even a small change can have a huge impact on the health and well-being of this patient population.
What can you do to expand access? First, you must educate yourself and teach others. Many providers report never having received education on LGBTQ health during their training,4 and most barriers for transgender patients stem from this lack of training. Second, work with the transgender community – it is very tempting to see your institution’s name on the HEI and think all the work is done, but the lived experiences of transgender patients sometimes are different than what is seen on paper (or online). Team up with local organizations such as PFLAG (formerly known as Parents and Families of Lesbians and Gays) that can create support groups for both transgender youth and their families. Help create a network of referral systems for your transgender patients – the community is often small enough that they know which providers or establishments are safe for transgender individuals. Many transgender patients find this extremely helpful.5 You still wield significant influence in the community, so work with the health care and insurance systems to improve access and coverage for gender-related services. The HRC HEI is becoming coveted by health care institutions. This is a prime opportunity to be involved in committees seeking to improve health care access for transgender individuals. Finally, as there are champions for transgender health in your clinic, there also are champions for transgender health in insurance companies. They often are well known in the community, so find that individual for counseling on how to navigate the insurance system for your transgender patients.
Although an increasing number of health care institutions and clinics are recognizing the health care needs of transgender patients and providing appropriate care, the health care system remains challenging for transgender individuals to navigate. Small policy changes may have a substantial impact on the health and well-being of transgender individuals. Although creating change within an institution may seem like a monumental task, you do have the agency to help create this type change within the system to expand health care access for transgender patients.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
Resources
- HRC HEI: If you’re interested in learning what policies are inclusive and equitable for LGBT patients, check out the HRC HEI scoring criteria. It’s a good place to start if you want to expand health care access for transgender individuals.
- To find out more about the health care legal protections transgender individuals are entitled to, check out the National Center for Transgender Equality.
References
1. Psychol Bull. 2003 Sep;129(5):674-97.
2. “Injustice at every turn: A report of the National Transgender Discrimination Survey.” (Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011.)
3. J. Adolesc Health. 2018 Apr. doi: 10.1016/j.jadohealth.2018.02.003.
4. Int J Transgenderism. 2008. doi: 10.1300/J485v08n02_08.
5. Transgend Health. 2016 Nov 1;1(1):238-49.
In March 2018, the Human Rights Campaign (HRC), an advocacy organization dedicated to improving the lives of LGBTQ people, released its 11th Annual Healthcare Equality Index. The HEI is an indicator of how inclusive and equitable health care facilities are in providing care for their LGBTQ patients. My own institution, Children’s Hospital of Pittsburgh, scored very high on this index and received the “Leader in LGBTQ Healthcare Equality” designation. The process of receiving this designation is very rigorous, and I am proud of my institution for making great strides in expanding health care access for LGBTQ patients, especially transgender patients. However, this is no time to rest on one’s laurels, as many transgender people still experience challenges and barriers in navigating the health care system.
Insurance access continues to be a problem. I wrote a column in June 2017 about obtaining health care insurance for transgender patients. Preauthorization is common for obtaining cross-sex hormones or pubertal blockers even for insurance companies that are willing to pay for them – a process that can take weeks, even months, to complete. This creates delays in obtaining necessary care for transgender patients. This is just one of the many barriers transgender people face in navigating the health care system.
Increasing access to health care services for transgender patients is more about improving health outcomes than patient satisfaction. Even the smallest policy change may have a meaningful impact on the lives of transgender individuals. A study by Russell et al., in the April 2018 issue of Journal of Adolescent Health found that transgender youth allowed to use their chosen name (instead of the name assigned to them by their parents at birth) were more likely to have fewer depressive symptoms and lower rates of suicidal ideation and suicidal behavior.3 These findings highlight that even a small change can have a huge impact on the health and well-being of this patient population.
What can you do to expand access? First, you must educate yourself and teach others. Many providers report never having received education on LGBTQ health during their training,4 and most barriers for transgender patients stem from this lack of training. Second, work with the transgender community – it is very tempting to see your institution’s name on the HEI and think all the work is done, but the lived experiences of transgender patients sometimes are different than what is seen on paper (or online). Team up with local organizations such as PFLAG (formerly known as Parents and Families of Lesbians and Gays) that can create support groups for both transgender youth and their families. Help create a network of referral systems for your transgender patients – the community is often small enough that they know which providers or establishments are safe for transgender individuals. Many transgender patients find this extremely helpful.5 You still wield significant influence in the community, so work with the health care and insurance systems to improve access and coverage for gender-related services. The HRC HEI is becoming coveted by health care institutions. This is a prime opportunity to be involved in committees seeking to improve health care access for transgender individuals. Finally, as there are champions for transgender health in your clinic, there also are champions for transgender health in insurance companies. They often are well known in the community, so find that individual for counseling on how to navigate the insurance system for your transgender patients.
Although an increasing number of health care institutions and clinics are recognizing the health care needs of transgender patients and providing appropriate care, the health care system remains challenging for transgender individuals to navigate. Small policy changes may have a substantial impact on the health and well-being of transgender individuals. Although creating change within an institution may seem like a monumental task, you do have the agency to help create this type change within the system to expand health care access for transgender patients.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
Resources
- HRC HEI: If you’re interested in learning what policies are inclusive and equitable for LGBT patients, check out the HRC HEI scoring criteria. It’s a good place to start if you want to expand health care access for transgender individuals.
- To find out more about the health care legal protections transgender individuals are entitled to, check out the National Center for Transgender Equality.
References
1. Psychol Bull. 2003 Sep;129(5):674-97.
2. “Injustice at every turn: A report of the National Transgender Discrimination Survey.” (Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011.)
3. J. Adolesc Health. 2018 Apr. doi: 10.1016/j.jadohealth.2018.02.003.
4. Int J Transgenderism. 2008. doi: 10.1300/J485v08n02_08.
5. Transgend Health. 2016 Nov 1;1(1):238-49.
Ketamine formulation study is ‘groundbreaking’
It is remarkable to consider that we now have more than 30 medications approved by the Food and Drug Administration as monotherapy or augmentation for the treatment of major depressive disorder. And yet, we know very little about how these medications perform for patients at high risk for suicide.
Historically, suicidal patients have been excluded from phase 3 antidepressant trials, which provide the basis for regulatory approval. Even studies in treatment-resistant depression (TRD) have tended to exclude patients with the highest risk of suicide. Further, the FDA does not mandate that a new antidepressant medication demonstrate any benefit for suicidal ideation.
Focus on high-risk patients
The recent report by Canuso et al.3 in the American Journal of Psychiatry is a groundbreaking study: Previous placebo-controlled trials of intravenous ketamine in depressed patients with clinically significant suicidal ideation have used only one-time dose administrations4,5,6.
This phase 2, proof-of-concept trial randomized 68 adults with MDD at 11 U.S. sites, which were primarily academic medical centers. In contrast to previous ketamine studies, which recruited patients via advertisement or clinician referral, patients were identified and screened in an emergency department or an inpatient psychiatric unit. Participants had to voluntarily agree to hospitalization for 5 days following randomization, with the remainder of the study conducted on an outpatient basis. Intranasal esketamine (84 mg) or placebo was administered twice per week over 4 weeks, in addition to standard-of-care antidepressant treatment. The primary outcome was the change in the Montgomery Åsberg Depression Rating Scale (MADRS) score from baseline to 4 hours after first dose of study medication.
For the primary MADRS outcome, esketamine statistically separated from placebo at 4 hours and 24 hours, with moderate effect sizes (0.61 to 0.65). There were no significant differences at the end of the double-blind period at day 25 and at posttreatment follow-up at day 81. For the suicidal thoughts item of the MADRS, esketamine’s efficacy was greater than placebo at 4 hours, but not at 24 hours or at day 25. Clinician global judgment of suicide risk was not statistically different between groups at any time point, although the esketamine group had numerically greater improvements at 4 hours and 24 hours. There were no group differences in self-report measures (Beck Scale for Suicidal Ideation or Beck Hopelessness Scale) at any time point.
Regarding safety and tolerability, adverse events led to early termination for 5 patients in the esketamine group, compared with one in the placebo group. The most common adverse events were nausea, dizziness, dissociation, unpleasant taste, and headache, which were more frequent in the esketamine group. Transient elevations in blood pressure and dissociative symptoms generally peaked at 40 minutes after dosing and returned to baseline by 2 hours.
Putting findings in perspective
Several aspects of the trial are noteworthy. First, enrolled patients were markedly depressed, and half required additional suicide precautions in addition to hospitalization. Three patients (all in the placebo group) made suicide attempts during the follow-up period, further evidence that these patients were extremely high risk. Second, the sample was significantly more racially diverse (38% black or African American) than most previous ketamine studies. Third, psychiatric hospitalization plus the initiation of standard antidepressant medication resulted in substantial improvements for many patients randomized to intranasal placebo spray. Inflated short-term placebo responses are commonly seen even in severely depressed patients, making signal detection especially challenging for new drugs. Finally, it is difficult to compare the results of this study with the few placebo-controlled trials of intravenous ketamine for patients with MDD and significant suicidal ideation, because of differences in outcomes measures, patient populations, doses, and route of administration. This study used the Suicide Ideation and Behavior Assessment Tool, a computerized, modular instrument with patient-reported and clinician-reported assessments, which was developed specifically to measure rapid changes in suicidality and awaits further validation in ongoing studies.
Limitations of this study include the absence of reported plasma esketamine levels. Is it possible that higher doses of esketamine, or a different dosing schedule, would have had resulted in greater efficacy? The 84-mg dose used in this trial recently was found to be safe and effective in patients with TRD2, and was reported to have similar plasma levels as IV esketamine 0.2 mg/kg2. This dose, in turn, corresponds to a racemic ketamine dose of approximately 0.31 mg/kg1. Future studies will need to examine the antisuicidal and antidepressant effects of the most commonly used racemic ketamine dose (0.5 mg/kg), compared with 84 mg intranasal esketamine. The twice per week dosing schedule was supported empirically from a previous study of intravenous ketamine showing that twice weekly infusions were equally effective to thrice weekly administrations7. It is unknown, however, whether even less-frequent administrations (such as once weekly) would have been more effective than twice-weekly over the 4-week, double-blind period. Finally, the authors raise the possibility of functional unblinding, which always is a concern in ketamine studies. Although the placebo solution contained a bittering agent to simulate the taste of esketamine intranasal solution, the integrity of the blind was not reported.
Conclusion
Overall, this study is a promising start. In my view, the risk to benefit ratio for this approach is acceptable, given the morbidity and mortality associated with suicidal depression. The fact that esketamine nasal spray would be administered only under the observation of a clinician in a medical setting, and not be dispensed for at-home use, is reassuring and would mitigate the potential for abuse. In the meantime, our field awaits the results of larger phase 3 studies for patients with MDD at imminent risk for suicide.
Dr. Mathew is affiliated with the Michael E. Debakey VA Medical Center, and the Menninger Department of Psychiatry and Behavioral Sciences at the Baylor College of Medicine in Houston. Over the last 12 months, he has served as a paid consultant to Alkermes and Fortress Biotech. He also has served as an investigator on clinical trials sponsored by Janssen Research and Development, the manufacturer of intranasal esketamine, and as an investigator on a trial sponsored by NeuroRx.
References
1. Biol Psychiatry. 2016 Sep 15;80(6):424-31.
2. JAMA Psychiatry. 2018 Feb 1;75(2):139-48.
3. Am J Psychiatry. 2018 Apr 16. doi: 10.1176/appi.ajp.2018.17060720.
4. Am J Psychiatry. 2018 Apr 1;175(4]):327-35.
5. Psychol Med. 2015 Dec;45(16):3571-80.
6. Am J Psychiatry. 2018 Feb 1;175(2):150-8.
7. Am J Psychiatry. 2016 Aug 1;173(8):816-26.
It is remarkable to consider that we now have more than 30 medications approved by the Food and Drug Administration as monotherapy or augmentation for the treatment of major depressive disorder. And yet, we know very little about how these medications perform for patients at high risk for suicide.
Historically, suicidal patients have been excluded from phase 3 antidepressant trials, which provide the basis for regulatory approval. Even studies in treatment-resistant depression (TRD) have tended to exclude patients with the highest risk of suicide. Further, the FDA does not mandate that a new antidepressant medication demonstrate any benefit for suicidal ideation.
Focus on high-risk patients
The recent report by Canuso et al.3 in the American Journal of Psychiatry is a groundbreaking study: Previous placebo-controlled trials of intravenous ketamine in depressed patients with clinically significant suicidal ideation have used only one-time dose administrations4,5,6.
This phase 2, proof-of-concept trial randomized 68 adults with MDD at 11 U.S. sites, which were primarily academic medical centers. In contrast to previous ketamine studies, which recruited patients via advertisement or clinician referral, patients were identified and screened in an emergency department or an inpatient psychiatric unit. Participants had to voluntarily agree to hospitalization for 5 days following randomization, with the remainder of the study conducted on an outpatient basis. Intranasal esketamine (84 mg) or placebo was administered twice per week over 4 weeks, in addition to standard-of-care antidepressant treatment. The primary outcome was the change in the Montgomery Åsberg Depression Rating Scale (MADRS) score from baseline to 4 hours after first dose of study medication.
For the primary MADRS outcome, esketamine statistically separated from placebo at 4 hours and 24 hours, with moderate effect sizes (0.61 to 0.65). There were no significant differences at the end of the double-blind period at day 25 and at posttreatment follow-up at day 81. For the suicidal thoughts item of the MADRS, esketamine’s efficacy was greater than placebo at 4 hours, but not at 24 hours or at day 25. Clinician global judgment of suicide risk was not statistically different between groups at any time point, although the esketamine group had numerically greater improvements at 4 hours and 24 hours. There were no group differences in self-report measures (Beck Scale for Suicidal Ideation or Beck Hopelessness Scale) at any time point.
Regarding safety and tolerability, adverse events led to early termination for 5 patients in the esketamine group, compared with one in the placebo group. The most common adverse events were nausea, dizziness, dissociation, unpleasant taste, and headache, which were more frequent in the esketamine group. Transient elevations in blood pressure and dissociative symptoms generally peaked at 40 minutes after dosing and returned to baseline by 2 hours.
Putting findings in perspective
Several aspects of the trial are noteworthy. First, enrolled patients were markedly depressed, and half required additional suicide precautions in addition to hospitalization. Three patients (all in the placebo group) made suicide attempts during the follow-up period, further evidence that these patients were extremely high risk. Second, the sample was significantly more racially diverse (38% black or African American) than most previous ketamine studies. Third, psychiatric hospitalization plus the initiation of standard antidepressant medication resulted in substantial improvements for many patients randomized to intranasal placebo spray. Inflated short-term placebo responses are commonly seen even in severely depressed patients, making signal detection especially challenging for new drugs. Finally, it is difficult to compare the results of this study with the few placebo-controlled trials of intravenous ketamine for patients with MDD and significant suicidal ideation, because of differences in outcomes measures, patient populations, doses, and route of administration. This study used the Suicide Ideation and Behavior Assessment Tool, a computerized, modular instrument with patient-reported and clinician-reported assessments, which was developed specifically to measure rapid changes in suicidality and awaits further validation in ongoing studies.
Limitations of this study include the absence of reported plasma esketamine levels. Is it possible that higher doses of esketamine, or a different dosing schedule, would have had resulted in greater efficacy? The 84-mg dose used in this trial recently was found to be safe and effective in patients with TRD2, and was reported to have similar plasma levels as IV esketamine 0.2 mg/kg2. This dose, in turn, corresponds to a racemic ketamine dose of approximately 0.31 mg/kg1. Future studies will need to examine the antisuicidal and antidepressant effects of the most commonly used racemic ketamine dose (0.5 mg/kg), compared with 84 mg intranasal esketamine. The twice per week dosing schedule was supported empirically from a previous study of intravenous ketamine showing that twice weekly infusions were equally effective to thrice weekly administrations7. It is unknown, however, whether even less-frequent administrations (such as once weekly) would have been more effective than twice-weekly over the 4-week, double-blind period. Finally, the authors raise the possibility of functional unblinding, which always is a concern in ketamine studies. Although the placebo solution contained a bittering agent to simulate the taste of esketamine intranasal solution, the integrity of the blind was not reported.
Conclusion
Overall, this study is a promising start. In my view, the risk to benefit ratio for this approach is acceptable, given the morbidity and mortality associated with suicidal depression. The fact that esketamine nasal spray would be administered only under the observation of a clinician in a medical setting, and not be dispensed for at-home use, is reassuring and would mitigate the potential for abuse. In the meantime, our field awaits the results of larger phase 3 studies for patients with MDD at imminent risk for suicide.
Dr. Mathew is affiliated with the Michael E. Debakey VA Medical Center, and the Menninger Department of Psychiatry and Behavioral Sciences at the Baylor College of Medicine in Houston. Over the last 12 months, he has served as a paid consultant to Alkermes and Fortress Biotech. He also has served as an investigator on clinical trials sponsored by Janssen Research and Development, the manufacturer of intranasal esketamine, and as an investigator on a trial sponsored by NeuroRx.
References
1. Biol Psychiatry. 2016 Sep 15;80(6):424-31.
2. JAMA Psychiatry. 2018 Feb 1;75(2):139-48.
3. Am J Psychiatry. 2018 Apr 16. doi: 10.1176/appi.ajp.2018.17060720.
4. Am J Psychiatry. 2018 Apr 1;175(4]):327-35.
5. Psychol Med. 2015 Dec;45(16):3571-80.
6. Am J Psychiatry. 2018 Feb 1;175(2):150-8.
7. Am J Psychiatry. 2016 Aug 1;173(8):816-26.
It is remarkable to consider that we now have more than 30 medications approved by the Food and Drug Administration as monotherapy or augmentation for the treatment of major depressive disorder. And yet, we know very little about how these medications perform for patients at high risk for suicide.
Historically, suicidal patients have been excluded from phase 3 antidepressant trials, which provide the basis for regulatory approval. Even studies in treatment-resistant depression (TRD) have tended to exclude patients with the highest risk of suicide. Further, the FDA does not mandate that a new antidepressant medication demonstrate any benefit for suicidal ideation.
Focus on high-risk patients
The recent report by Canuso et al.3 in the American Journal of Psychiatry is a groundbreaking study: Previous placebo-controlled trials of intravenous ketamine in depressed patients with clinically significant suicidal ideation have used only one-time dose administrations4,5,6.
This phase 2, proof-of-concept trial randomized 68 adults with MDD at 11 U.S. sites, which were primarily academic medical centers. In contrast to previous ketamine studies, which recruited patients via advertisement or clinician referral, patients were identified and screened in an emergency department or an inpatient psychiatric unit. Participants had to voluntarily agree to hospitalization for 5 days following randomization, with the remainder of the study conducted on an outpatient basis. Intranasal esketamine (84 mg) or placebo was administered twice per week over 4 weeks, in addition to standard-of-care antidepressant treatment. The primary outcome was the change in the Montgomery Åsberg Depression Rating Scale (MADRS) score from baseline to 4 hours after first dose of study medication.
For the primary MADRS outcome, esketamine statistically separated from placebo at 4 hours and 24 hours, with moderate effect sizes (0.61 to 0.65). There were no significant differences at the end of the double-blind period at day 25 and at posttreatment follow-up at day 81. For the suicidal thoughts item of the MADRS, esketamine’s efficacy was greater than placebo at 4 hours, but not at 24 hours or at day 25. Clinician global judgment of suicide risk was not statistically different between groups at any time point, although the esketamine group had numerically greater improvements at 4 hours and 24 hours. There were no group differences in self-report measures (Beck Scale for Suicidal Ideation or Beck Hopelessness Scale) at any time point.
Regarding safety and tolerability, adverse events led to early termination for 5 patients in the esketamine group, compared with one in the placebo group. The most common adverse events were nausea, dizziness, dissociation, unpleasant taste, and headache, which were more frequent in the esketamine group. Transient elevations in blood pressure and dissociative symptoms generally peaked at 40 minutes after dosing and returned to baseline by 2 hours.
Putting findings in perspective
Several aspects of the trial are noteworthy. First, enrolled patients were markedly depressed, and half required additional suicide precautions in addition to hospitalization. Three patients (all in the placebo group) made suicide attempts during the follow-up period, further evidence that these patients were extremely high risk. Second, the sample was significantly more racially diverse (38% black or African American) than most previous ketamine studies. Third, psychiatric hospitalization plus the initiation of standard antidepressant medication resulted in substantial improvements for many patients randomized to intranasal placebo spray. Inflated short-term placebo responses are commonly seen even in severely depressed patients, making signal detection especially challenging for new drugs. Finally, it is difficult to compare the results of this study with the few placebo-controlled trials of intravenous ketamine for patients with MDD and significant suicidal ideation, because of differences in outcomes measures, patient populations, doses, and route of administration. This study used the Suicide Ideation and Behavior Assessment Tool, a computerized, modular instrument with patient-reported and clinician-reported assessments, which was developed specifically to measure rapid changes in suicidality and awaits further validation in ongoing studies.
Limitations of this study include the absence of reported plasma esketamine levels. Is it possible that higher doses of esketamine, or a different dosing schedule, would have had resulted in greater efficacy? The 84-mg dose used in this trial recently was found to be safe and effective in patients with TRD2, and was reported to have similar plasma levels as IV esketamine 0.2 mg/kg2. This dose, in turn, corresponds to a racemic ketamine dose of approximately 0.31 mg/kg1. Future studies will need to examine the antisuicidal and antidepressant effects of the most commonly used racemic ketamine dose (0.5 mg/kg), compared with 84 mg intranasal esketamine. The twice per week dosing schedule was supported empirically from a previous study of intravenous ketamine showing that twice weekly infusions were equally effective to thrice weekly administrations7. It is unknown, however, whether even less-frequent administrations (such as once weekly) would have been more effective than twice-weekly over the 4-week, double-blind period. Finally, the authors raise the possibility of functional unblinding, which always is a concern in ketamine studies. Although the placebo solution contained a bittering agent to simulate the taste of esketamine intranasal solution, the integrity of the blind was not reported.
Conclusion
Overall, this study is a promising start. In my view, the risk to benefit ratio for this approach is acceptable, given the morbidity and mortality associated with suicidal depression. The fact that esketamine nasal spray would be administered only under the observation of a clinician in a medical setting, and not be dispensed for at-home use, is reassuring and would mitigate the potential for abuse. In the meantime, our field awaits the results of larger phase 3 studies for patients with MDD at imminent risk for suicide.
Dr. Mathew is affiliated with the Michael E. Debakey VA Medical Center, and the Menninger Department of Psychiatry and Behavioral Sciences at the Baylor College of Medicine in Houston. Over the last 12 months, he has served as a paid consultant to Alkermes and Fortress Biotech. He also has served as an investigator on clinical trials sponsored by Janssen Research and Development, the manufacturer of intranasal esketamine, and as an investigator on a trial sponsored by NeuroRx.
References
1. Biol Psychiatry. 2016 Sep 15;80(6):424-31.
2. JAMA Psychiatry. 2018 Feb 1;75(2):139-48.
3. Am J Psychiatry. 2018 Apr 16. doi: 10.1176/appi.ajp.2018.17060720.
4. Am J Psychiatry. 2018 Apr 1;175(4]):327-35.
5. Psychol Med. 2015 Dec;45(16):3571-80.
6. Am J Psychiatry. 2018 Feb 1;175(2):150-8.
7. Am J Psychiatry. 2016 Aug 1;173(8):816-26.
Self-harm
Nonsuicidal self-injury (NSSI) has become more prevalent in youth over recent years and has many inherent risks. In the Diagnostic and Statistical Manual, Fifth Edition (DSM-5), NSSI is a diagnosis suggested for further study, and criteria include engaging in self-injury for 5 or more days without suicidal intent as well as self-injury associated with at least 1 of the following: obtaining relief from negative thoughts or feelings, resolving interpersonal challenges, inducing positive feelings. It is associated with interpersonal difficulties or negative thoughts/feelings. The behavior causes significant impairment in functioning and is not better explained by another condition.1
Estimates of lifetime prevalence in community-based samples of youth range from 15% to 20%. Individuals often start during early adolescence. It can pose many risks including infection, permanent scarring or disfigurement, decreased self-esteem, interpersonal conflict, severe injury, or death. Reasons for engaging in self-harm can vary and include attempts to regulate negative affect, to manage feelings of emptiness/numbness, regain a sense of control over body, feelings, etc., or to provide a consequence for perceived faults. Youth often may start to engage in self-harm covertly, and it may first become apparent in emergency or primary care settings. However, upon discovery, the response given also may affect future behavior.
Efforts also have been underway to distinguish between youth who engage in self-harm with and without suicidal ideation. Girls are more likely than are boys to report NSSI, although male NSSI may present differently. In addition to cutting or more stereotypical self-injury, they may punch walls or engage in fights or other risky behaviors as a proxy for self-harm. Risk factors for boys with regard to suicide attempts include hopelessness and history of sexual abuse. Maladaptive eating patterns and hopelessness were the two most significant factors for girls.4
With regard to issues of confidentiality, it will be important to carefully gauge level of safety and to clearly communicate with the patient (and family) limits of confidentiality. This may result in working within shades of gray to help maintain the therapeutic relationship and the patient’s comfort in being able to disclose potentially sensitive information.
Families can struggle with how to manage this, and it can generate fear as well as other strong emotions.
Tips for parents and guardians
- Validate the underlying emotions while not validating the behavior. Self-injury is a coping strategy. Focus on the driving forces for the actions rather than the actions themselves.
- Approach your child from a nonjudgmental stance.
- Recognize that change may not happen overnight, and that there may be periods of regression.
- Acknowledge successes when they occur.
- Make yourself available for open communication. Open-ended questions may facilitate more dialogue.
- Take care of yourself as well. Ensure you use your supports and are engaging in healthy self-care.
- Take the behavior seriously. While this behavior is relatively common, do not assume it is “just a phase.”
- While remaining supportive, it is important to maintain a parental role and to keep expectations rather than “walking on eggshells.”
- Involve the child in identifying what can be of support.
- Become aware of local crisis resources in your community. National resources include Call 1-800-273-TALK for the national suicide hotline or Text 741741 to connect with a crisis counselor.
Things to avoid
- Avoid taking a punitive stance. While the behavior can be provocative, most likely the primary purpose is not for attention.
- Avoid engaging in power struggles.
- Avoid creating increased isolation for the child. This can be a delicate balance with regard to peer groups, but encouraging healthy social interactions and activities is a way to help build resilience.
- Avoid taking the behavior personally.5
In working with youth who engage in self-harm, it is important to work within a team, which may include family, primary care, mental health support, school, and potentially other community supports. Treatment evidence is relatively limited, but there is some evidence to support use of cognitive behavioral therapy, dialectical behavior therapy, and mentalization-based therapy. Regardless, work will likely be long term and at times intensive in addressing the problems leading to self-harm behavior.6
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures.
References
1. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (Arlington, Va.: American Psychiatric Association Publishing, 2013)
2. J Adolesc. 2014 Dec;37(8):1335-44.
3. Behav Ther. 2017 May; 48(3):366-79.
4. Acad Pediatr. 2012 May-Jun;12(3):205-13.
5. “Information for parents: What you need to know about self-injury.” The Fact Sheet Series, Cornell Research Program on Self-Injury and Recovery. 2009.
6. Clin Pediatr. 2016 Sep 13;55(11):1012-9.
Nonsuicidal self-injury (NSSI) has become more prevalent in youth over recent years and has many inherent risks. In the Diagnostic and Statistical Manual, Fifth Edition (DSM-5), NSSI is a diagnosis suggested for further study, and criteria include engaging in self-injury for 5 or more days without suicidal intent as well as self-injury associated with at least 1 of the following: obtaining relief from negative thoughts or feelings, resolving interpersonal challenges, inducing positive feelings. It is associated with interpersonal difficulties or negative thoughts/feelings. The behavior causes significant impairment in functioning and is not better explained by another condition.1
Estimates of lifetime prevalence in community-based samples of youth range from 15% to 20%. Individuals often start during early adolescence. It can pose many risks including infection, permanent scarring or disfigurement, decreased self-esteem, interpersonal conflict, severe injury, or death. Reasons for engaging in self-harm can vary and include attempts to regulate negative affect, to manage feelings of emptiness/numbness, regain a sense of control over body, feelings, etc., or to provide a consequence for perceived faults. Youth often may start to engage in self-harm covertly, and it may first become apparent in emergency or primary care settings. However, upon discovery, the response given also may affect future behavior.
Efforts also have been underway to distinguish between youth who engage in self-harm with and without suicidal ideation. Girls are more likely than are boys to report NSSI, although male NSSI may present differently. In addition to cutting or more stereotypical self-injury, they may punch walls or engage in fights or other risky behaviors as a proxy for self-harm. Risk factors for boys with regard to suicide attempts include hopelessness and history of sexual abuse. Maladaptive eating patterns and hopelessness were the two most significant factors for girls.4
With regard to issues of confidentiality, it will be important to carefully gauge level of safety and to clearly communicate with the patient (and family) limits of confidentiality. This may result in working within shades of gray to help maintain the therapeutic relationship and the patient’s comfort in being able to disclose potentially sensitive information.
Families can struggle with how to manage this, and it can generate fear as well as other strong emotions.
Tips for parents and guardians
- Validate the underlying emotions while not validating the behavior. Self-injury is a coping strategy. Focus on the driving forces for the actions rather than the actions themselves.
- Approach your child from a nonjudgmental stance.
- Recognize that change may not happen overnight, and that there may be periods of regression.
- Acknowledge successes when they occur.
- Make yourself available for open communication. Open-ended questions may facilitate more dialogue.
- Take care of yourself as well. Ensure you use your supports and are engaging in healthy self-care.
- Take the behavior seriously. While this behavior is relatively common, do not assume it is “just a phase.”
- While remaining supportive, it is important to maintain a parental role and to keep expectations rather than “walking on eggshells.”
- Involve the child in identifying what can be of support.
- Become aware of local crisis resources in your community. National resources include Call 1-800-273-TALK for the national suicide hotline or Text 741741 to connect with a crisis counselor.
Things to avoid
- Avoid taking a punitive stance. While the behavior can be provocative, most likely the primary purpose is not for attention.
- Avoid engaging in power struggles.
- Avoid creating increased isolation for the child. This can be a delicate balance with regard to peer groups, but encouraging healthy social interactions and activities is a way to help build resilience.
- Avoid taking the behavior personally.5
In working with youth who engage in self-harm, it is important to work within a team, which may include family, primary care, mental health support, school, and potentially other community supports. Treatment evidence is relatively limited, but there is some evidence to support use of cognitive behavioral therapy, dialectical behavior therapy, and mentalization-based therapy. Regardless, work will likely be long term and at times intensive in addressing the problems leading to self-harm behavior.6
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures.
References
1. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (Arlington, Va.: American Psychiatric Association Publishing, 2013)
2. J Adolesc. 2014 Dec;37(8):1335-44.
3. Behav Ther. 2017 May; 48(3):366-79.
4. Acad Pediatr. 2012 May-Jun;12(3):205-13.
5. “Information for parents: What you need to know about self-injury.” The Fact Sheet Series, Cornell Research Program on Self-Injury and Recovery. 2009.
6. Clin Pediatr. 2016 Sep 13;55(11):1012-9.
Nonsuicidal self-injury (NSSI) has become more prevalent in youth over recent years and has many inherent risks. In the Diagnostic and Statistical Manual, Fifth Edition (DSM-5), NSSI is a diagnosis suggested for further study, and criteria include engaging in self-injury for 5 or more days without suicidal intent as well as self-injury associated with at least 1 of the following: obtaining relief from negative thoughts or feelings, resolving interpersonal challenges, inducing positive feelings. It is associated with interpersonal difficulties or negative thoughts/feelings. The behavior causes significant impairment in functioning and is not better explained by another condition.1
Estimates of lifetime prevalence in community-based samples of youth range from 15% to 20%. Individuals often start during early adolescence. It can pose many risks including infection, permanent scarring or disfigurement, decreased self-esteem, interpersonal conflict, severe injury, or death. Reasons for engaging in self-harm can vary and include attempts to regulate negative affect, to manage feelings of emptiness/numbness, regain a sense of control over body, feelings, etc., or to provide a consequence for perceived faults. Youth often may start to engage in self-harm covertly, and it may first become apparent in emergency or primary care settings. However, upon discovery, the response given also may affect future behavior.
Efforts also have been underway to distinguish between youth who engage in self-harm with and without suicidal ideation. Girls are more likely than are boys to report NSSI, although male NSSI may present differently. In addition to cutting or more stereotypical self-injury, they may punch walls or engage in fights or other risky behaviors as a proxy for self-harm. Risk factors for boys with regard to suicide attempts include hopelessness and history of sexual abuse. Maladaptive eating patterns and hopelessness were the two most significant factors for girls.4
With regard to issues of confidentiality, it will be important to carefully gauge level of safety and to clearly communicate with the patient (and family) limits of confidentiality. This may result in working within shades of gray to help maintain the therapeutic relationship and the patient’s comfort in being able to disclose potentially sensitive information.
Families can struggle with how to manage this, and it can generate fear as well as other strong emotions.
Tips for parents and guardians
- Validate the underlying emotions while not validating the behavior. Self-injury is a coping strategy. Focus on the driving forces for the actions rather than the actions themselves.
- Approach your child from a nonjudgmental stance.
- Recognize that change may not happen overnight, and that there may be periods of regression.
- Acknowledge successes when they occur.
- Make yourself available for open communication. Open-ended questions may facilitate more dialogue.
- Take care of yourself as well. Ensure you use your supports and are engaging in healthy self-care.
- Take the behavior seriously. While this behavior is relatively common, do not assume it is “just a phase.”
- While remaining supportive, it is important to maintain a parental role and to keep expectations rather than “walking on eggshells.”
- Involve the child in identifying what can be of support.
- Become aware of local crisis resources in your community. National resources include Call 1-800-273-TALK for the national suicide hotline or Text 741741 to connect with a crisis counselor.
Things to avoid
- Avoid taking a punitive stance. While the behavior can be provocative, most likely the primary purpose is not for attention.
- Avoid engaging in power struggles.
- Avoid creating increased isolation for the child. This can be a delicate balance with regard to peer groups, but encouraging healthy social interactions and activities is a way to help build resilience.
- Avoid taking the behavior personally.5
In working with youth who engage in self-harm, it is important to work within a team, which may include family, primary care, mental health support, school, and potentially other community supports. Treatment evidence is relatively limited, but there is some evidence to support use of cognitive behavioral therapy, dialectical behavior therapy, and mentalization-based therapy. Regardless, work will likely be long term and at times intensive in addressing the problems leading to self-harm behavior.6
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures.
References
1. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (Arlington, Va.: American Psychiatric Association Publishing, 2013)
2. J Adolesc. 2014 Dec;37(8):1335-44.
3. Behav Ther. 2017 May; 48(3):366-79.
4. Acad Pediatr. 2012 May-Jun;12(3):205-13.
5. “Information for parents: What you need to know about self-injury.” The Fact Sheet Series, Cornell Research Program on Self-Injury and Recovery. 2009.
6. Clin Pediatr. 2016 Sep 13;55(11):1012-9.
Hints of altered microRNA expression in women exposed to EDCs
Endocrine-disrupting chemicals (EDCs) are structurally similar to endogenous hormones and are therefore capable of mimicking these natural hormones, interfering with their biosynthesis, transport, binding action, and/or elimination. In animal studies and human clinical observational and epidemiologic studies of various EDCs, these chemicals have consistently been associated with diabetes mellitus, obesity, hormone-sensitive cancers, neurodevelopmental disorders in children exposed prenatally, and reproductive health.
In 2009, the Endocrine Society published a scientific statement in which it called EDCs a significant concern to human health (Endocr Rev. 2009;30[4]:293-342). Several years later, the American College of Obstetricians and Gynecologists and the American Society for Reproductive Medicine issued a Committee Opinion on Exposure to Toxic Environmental Agents, warning that patient exposure to EDCs and other toxic environmental agents can have a “profound and lasting effect” on reproductive health outcomes across the life course and calling the reduction of exposure a “critical area of intervention” for ob.gyns. and other reproductive health care professionals (Obstet Gynecol. 2013;122[4]:931-5).
Despite strong calls by each of these organizations to not overlook EDCs in the clinical arena, as well as emerging evidence that EDCs may be a risk factor for gestational diabetes (GDM), EDC exposure may not be on the practicing ob.gyn.’s radar. Clinicians should know what these chemicals are and how to talk about them in preconception and prenatal visits. We should carefully consider their known – and potential – risks, and encourage our patients to identify and reduce exposure without being alarmist.
Low-dose effects
EDCs are used in the manufacture of pesticides, industrial chemicals, plastics and plasticizers, hand sanitizers, medical equipment, dental sealants, a variety of personal care products, cosmetics, and other common consumer and household products. They’re found, for example, in sunscreens, canned foods and beverages, food-packaging materials, baby bottles, flame-retardant furniture, stain-resistant carpet, and shoes. We are all ingesting and breathing them in to some degree.
Bisphenol A (BPA), one of the most extensively studied EDCs, is found in the thermal receipt paper routinely used by gas stations, supermarkets, and other stores. In a small study we conducted at Harvard, we found that urinary BPA concentrations increased after continual handling of receipts for 2 hours without gloves but did not increase significantly when gloves were used (JAMA. 2014 Feb 26;311[8]:859-60).

Informed consumers can then affect the market through their purchasing choices, but the removal of concerning chemicals from products takes a long time, and it’s not always immediately clear that replacement chemicals are safer. For instance, the BPA in “BPA-free” water bottles and canned foods has been replaced by bisphenol S (BPS), which has a very similar molecular structure to BPA. The potential adverse health effects of these replacement chemicals are now being examined in experimental and epidemiologic studies.
Through its National Health and Nutrition Examination Survey, the Centers for Disease Control and Prevention has reported detection rates of between 75% and 99% for different EDCs in urine samples collected from a representative sample of the U.S. population. In other human research, several EDCs have been shown to cross the placenta and have been measured in maternal blood and urine and in cord blood and amniotic fluid, as well as in placental tissue at birth.
It is interesting to note that BPA’s structure is similar to that of diethylstilbestrol (DES). BPA was first shown to have estrogenic activity in 1936 and was originally considered for use in pharmaceuticals to prevent miscarriages, spontaneous abortions, and premature labor but was put aside in favor of DES. (DES was eventually found to be carcinogenic and was taken off the market.) In the 1950s, the use of BPA was resuscitated though not in pharmaceuticals.
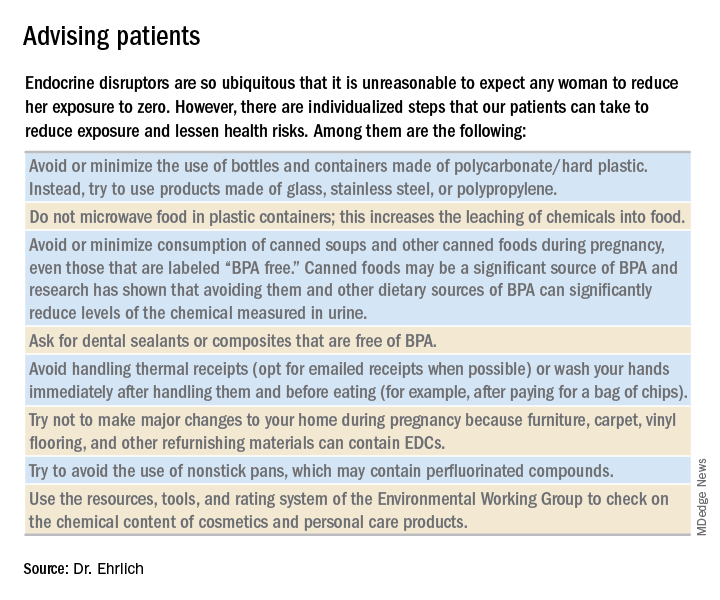
A better understanding about the mechanisms of action and dose-response patterns of EDCs has indicated that EDCs can act at low doses, and in many cases a nonmonotonic dose-response association has been demonstrated. This is a paradigm shift for traditional toxicology in which it is “the dose that makes the poison,” and some toxicologists have been critical of the claims of low-dose potency for EDCs.
A team of epidemiologists, toxicologists, and other scientists, including myself, critically analyzed in vitro, animal, and epidemiologic studies as part of a National Institute of Environmental Health Sciences working group on BPA to determine the strength of the evidence for low-dose effects (doses lower than those tested in traditional toxicology assessments) of BPA. We found that consistent, reproducible, and often adverse low-dose effects have been demonstrated for BPA in cell lines, primary cells and tissues, laboratory animals, and human populations. We also concluded that EDCs can pose the greatest threats when exposure occurs during early development, organogenesis, and during critical postnatal periods when tissues are differentiating (Endocr Disruptors [Austin, Tex.]. 2013 Sep;1:e25078-1-13).
A potential risk factor for GDM
Quite a lot of research has been done on EDCs and the risk of type 2 diabetes. A recent meta-analysis that included 41 cross-sectional and 8 prospective studies found that serum concentrations of dioxins, polychlorinated biphenyls, and chlorinated pesticides – and urine concentrations of BPA and phthalates – were significantly associated with type 2 diabetes risk. Comparing the highest and lowest concentration categories, the pooled relative risk was 1.45 for BPA and phthalates. EDC concentrations also were associated with indicators of impaired fasting glucose and insulin resistance (J Diabetes. 2016 Jul;8[4]:516-32).
Despite the mounting evidence for an association between BPA and type 2 diabetes, and despite the fact that the increased incidence of GDM in the past 20 years has mirrored the increasing use of EDCs, there has been a dearth of research examining the possible relationship between EDCs and GDM. The effects of BPA on GDM were identified as a knowledge gap by the National Institute of Environmental Health Sciences after a review of the literature from 2007 to 2013 (Environ Health Perspect. 2014 Aug:122[8]:775-86).
To understand the association between EDCs and GDM and the underlying mechanistic pathway of EDCs, we are conducting research that uses a growing body of evidence that suggests that environmental toxins are involved in the control of microRNA (miRNA) expression in trophoblast cells.
MiRNA, a single-stranded, short, noncoding RNA that is involved in posttranslational gene expression, can be packaged along with other signaling molecules inside extracellular vesicles in the placenta called exosomes. These exosomes appear to be shed from the placenta into the maternal circulation as early as 6-7 weeks into pregnancy. Once released into the maternal circulation, research has shown that the exosomes can target and reprogram other cells via the transfer of noncoding miRNAs, thereby changing the gene expression in these cells.
Such an exosome-mediated signaling pathway provides us with the opportunity to isolate exosomes, sequence the miRNAs, and look at whether women who are exposed to higher levels of EDCs (as indicated in urine concentration) have a particular miRNA signature that correlates with GDM. In other words, we’re working to determine whether particular EDCs and exposure levels affect the miRNA placental profiles, and if these profiles are predictive of GDM.
Thus far, in a pilot prospective cohort study of pregnant women, we are seeing hints of altered miRNA expression in relation to GDM. We have selected study participants who are at high risk of developing GDM (for example, prepregnancy body mass index greater than 30, past pregnancy with GDM, or macrosomia) because we suspect that, in many women, EDCs are a tipping point for the development of GDM rather than a sole causative factor. In addition to understanding the impact of EDCs on GDM, it is our hope that miRNAs in maternal circulation will serve as a noninvasive biomarker for early detection of GDM development or susceptibility.
Dr. Ehrlich is an assistant professor of pediatrics and environmental health at Cincinnati Children’s Hospital Medical Center.
Endocrine-disrupting chemicals (EDCs) are structurally similar to endogenous hormones and are therefore capable of mimicking these natural hormones, interfering with their biosynthesis, transport, binding action, and/or elimination. In animal studies and human clinical observational and epidemiologic studies of various EDCs, these chemicals have consistently been associated with diabetes mellitus, obesity, hormone-sensitive cancers, neurodevelopmental disorders in children exposed prenatally, and reproductive health.
In 2009, the Endocrine Society published a scientific statement in which it called EDCs a significant concern to human health (Endocr Rev. 2009;30[4]:293-342). Several years later, the American College of Obstetricians and Gynecologists and the American Society for Reproductive Medicine issued a Committee Opinion on Exposure to Toxic Environmental Agents, warning that patient exposure to EDCs and other toxic environmental agents can have a “profound and lasting effect” on reproductive health outcomes across the life course and calling the reduction of exposure a “critical area of intervention” for ob.gyns. and other reproductive health care professionals (Obstet Gynecol. 2013;122[4]:931-5).
Despite strong calls by each of these organizations to not overlook EDCs in the clinical arena, as well as emerging evidence that EDCs may be a risk factor for gestational diabetes (GDM), EDC exposure may not be on the practicing ob.gyn.’s radar. Clinicians should know what these chemicals are and how to talk about them in preconception and prenatal visits. We should carefully consider their known – and potential – risks, and encourage our patients to identify and reduce exposure without being alarmist.
Low-dose effects
EDCs are used in the manufacture of pesticides, industrial chemicals, plastics and plasticizers, hand sanitizers, medical equipment, dental sealants, a variety of personal care products, cosmetics, and other common consumer and household products. They’re found, for example, in sunscreens, canned foods and beverages, food-packaging materials, baby bottles, flame-retardant furniture, stain-resistant carpet, and shoes. We are all ingesting and breathing them in to some degree.
Bisphenol A (BPA), one of the most extensively studied EDCs, is found in the thermal receipt paper routinely used by gas stations, supermarkets, and other stores. In a small study we conducted at Harvard, we found that urinary BPA concentrations increased after continual handling of receipts for 2 hours without gloves but did not increase significantly when gloves were used (JAMA. 2014 Feb 26;311[8]:859-60).

Informed consumers can then affect the market through their purchasing choices, but the removal of concerning chemicals from products takes a long time, and it’s not always immediately clear that replacement chemicals are safer. For instance, the BPA in “BPA-free” water bottles and canned foods has been replaced by bisphenol S (BPS), which has a very similar molecular structure to BPA. The potential adverse health effects of these replacement chemicals are now being examined in experimental and epidemiologic studies.
Through its National Health and Nutrition Examination Survey, the Centers for Disease Control and Prevention has reported detection rates of between 75% and 99% for different EDCs in urine samples collected from a representative sample of the U.S. population. In other human research, several EDCs have been shown to cross the placenta and have been measured in maternal blood and urine and in cord blood and amniotic fluid, as well as in placental tissue at birth.
It is interesting to note that BPA’s structure is similar to that of diethylstilbestrol (DES). BPA was first shown to have estrogenic activity in 1936 and was originally considered for use in pharmaceuticals to prevent miscarriages, spontaneous abortions, and premature labor but was put aside in favor of DES. (DES was eventually found to be carcinogenic and was taken off the market.) In the 1950s, the use of BPA was resuscitated though not in pharmaceuticals.

A better understanding about the mechanisms of action and dose-response patterns of EDCs has indicated that EDCs can act at low doses, and in many cases a nonmonotonic dose-response association has been demonstrated. This is a paradigm shift for traditional toxicology in which it is “the dose that makes the poison,” and some toxicologists have been critical of the claims of low-dose potency for EDCs.
A team of epidemiologists, toxicologists, and other scientists, including myself, critically analyzed in vitro, animal, and epidemiologic studies as part of a National Institute of Environmental Health Sciences working group on BPA to determine the strength of the evidence for low-dose effects (doses lower than those tested in traditional toxicology assessments) of BPA. We found that consistent, reproducible, and often adverse low-dose effects have been demonstrated for BPA in cell lines, primary cells and tissues, laboratory animals, and human populations. We also concluded that EDCs can pose the greatest threats when exposure occurs during early development, organogenesis, and during critical postnatal periods when tissues are differentiating (Endocr Disruptors [Austin, Tex.]. 2013 Sep;1:e25078-1-13).
A potential risk factor for GDM
Quite a lot of research has been done on EDCs and the risk of type 2 diabetes. A recent meta-analysis that included 41 cross-sectional and 8 prospective studies found that serum concentrations of dioxins, polychlorinated biphenyls, and chlorinated pesticides – and urine concentrations of BPA and phthalates – were significantly associated with type 2 diabetes risk. Comparing the highest and lowest concentration categories, the pooled relative risk was 1.45 for BPA and phthalates. EDC concentrations also were associated with indicators of impaired fasting glucose and insulin resistance (J Diabetes. 2016 Jul;8[4]:516-32).
Despite the mounting evidence for an association between BPA and type 2 diabetes, and despite the fact that the increased incidence of GDM in the past 20 years has mirrored the increasing use of EDCs, there has been a dearth of research examining the possible relationship between EDCs and GDM. The effects of BPA on GDM were identified as a knowledge gap by the National Institute of Environmental Health Sciences after a review of the literature from 2007 to 2013 (Environ Health Perspect. 2014 Aug:122[8]:775-86).
To understand the association between EDCs and GDM and the underlying mechanistic pathway of EDCs, we are conducting research that uses a growing body of evidence that suggests that environmental toxins are involved in the control of microRNA (miRNA) expression in trophoblast cells.
MiRNA, a single-stranded, short, noncoding RNA that is involved in posttranslational gene expression, can be packaged along with other signaling molecules inside extracellular vesicles in the placenta called exosomes. These exosomes appear to be shed from the placenta into the maternal circulation as early as 6-7 weeks into pregnancy. Once released into the maternal circulation, research has shown that the exosomes can target and reprogram other cells via the transfer of noncoding miRNAs, thereby changing the gene expression in these cells.
Such an exosome-mediated signaling pathway provides us with the opportunity to isolate exosomes, sequence the miRNAs, and look at whether women who are exposed to higher levels of EDCs (as indicated in urine concentration) have a particular miRNA signature that correlates with GDM. In other words, we’re working to determine whether particular EDCs and exposure levels affect the miRNA placental profiles, and if these profiles are predictive of GDM.
Thus far, in a pilot prospective cohort study of pregnant women, we are seeing hints of altered miRNA expression in relation to GDM. We have selected study participants who are at high risk of developing GDM (for example, prepregnancy body mass index greater than 30, past pregnancy with GDM, or macrosomia) because we suspect that, in many women, EDCs are a tipping point for the development of GDM rather than a sole causative factor. In addition to understanding the impact of EDCs on GDM, it is our hope that miRNAs in maternal circulation will serve as a noninvasive biomarker for early detection of GDM development or susceptibility.
Dr. Ehrlich is an assistant professor of pediatrics and environmental health at Cincinnati Children’s Hospital Medical Center.
Endocrine-disrupting chemicals (EDCs) are structurally similar to endogenous hormones and are therefore capable of mimicking these natural hormones, interfering with their biosynthesis, transport, binding action, and/or elimination. In animal studies and human clinical observational and epidemiologic studies of various EDCs, these chemicals have consistently been associated with diabetes mellitus, obesity, hormone-sensitive cancers, neurodevelopmental disorders in children exposed prenatally, and reproductive health.
In 2009, the Endocrine Society published a scientific statement in which it called EDCs a significant concern to human health (Endocr Rev. 2009;30[4]:293-342). Several years later, the American College of Obstetricians and Gynecologists and the American Society for Reproductive Medicine issued a Committee Opinion on Exposure to Toxic Environmental Agents, warning that patient exposure to EDCs and other toxic environmental agents can have a “profound and lasting effect” on reproductive health outcomes across the life course and calling the reduction of exposure a “critical area of intervention” for ob.gyns. and other reproductive health care professionals (Obstet Gynecol. 2013;122[4]:931-5).
Despite strong calls by each of these organizations to not overlook EDCs in the clinical arena, as well as emerging evidence that EDCs may be a risk factor for gestational diabetes (GDM), EDC exposure may not be on the practicing ob.gyn.’s radar. Clinicians should know what these chemicals are and how to talk about them in preconception and prenatal visits. We should carefully consider their known – and potential – risks, and encourage our patients to identify and reduce exposure without being alarmist.
Low-dose effects
EDCs are used in the manufacture of pesticides, industrial chemicals, plastics and plasticizers, hand sanitizers, medical equipment, dental sealants, a variety of personal care products, cosmetics, and other common consumer and household products. They’re found, for example, in sunscreens, canned foods and beverages, food-packaging materials, baby bottles, flame-retardant furniture, stain-resistant carpet, and shoes. We are all ingesting and breathing them in to some degree.
Bisphenol A (BPA), one of the most extensively studied EDCs, is found in the thermal receipt paper routinely used by gas stations, supermarkets, and other stores. In a small study we conducted at Harvard, we found that urinary BPA concentrations increased after continual handling of receipts for 2 hours without gloves but did not increase significantly when gloves were used (JAMA. 2014 Feb 26;311[8]:859-60).

Informed consumers can then affect the market through their purchasing choices, but the removal of concerning chemicals from products takes a long time, and it’s not always immediately clear that replacement chemicals are safer. For instance, the BPA in “BPA-free” water bottles and canned foods has been replaced by bisphenol S (BPS), which has a very similar molecular structure to BPA. The potential adverse health effects of these replacement chemicals are now being examined in experimental and epidemiologic studies.
Through its National Health and Nutrition Examination Survey, the Centers for Disease Control and Prevention has reported detection rates of between 75% and 99% for different EDCs in urine samples collected from a representative sample of the U.S. population. In other human research, several EDCs have been shown to cross the placenta and have been measured in maternal blood and urine and in cord blood and amniotic fluid, as well as in placental tissue at birth.
It is interesting to note that BPA’s structure is similar to that of diethylstilbestrol (DES). BPA was first shown to have estrogenic activity in 1936 and was originally considered for use in pharmaceuticals to prevent miscarriages, spontaneous abortions, and premature labor but was put aside in favor of DES. (DES was eventually found to be carcinogenic and was taken off the market.) In the 1950s, the use of BPA was resuscitated though not in pharmaceuticals.

A better understanding about the mechanisms of action and dose-response patterns of EDCs has indicated that EDCs can act at low doses, and in many cases a nonmonotonic dose-response association has been demonstrated. This is a paradigm shift for traditional toxicology in which it is “the dose that makes the poison,” and some toxicologists have been critical of the claims of low-dose potency for EDCs.
A team of epidemiologists, toxicologists, and other scientists, including myself, critically analyzed in vitro, animal, and epidemiologic studies as part of a National Institute of Environmental Health Sciences working group on BPA to determine the strength of the evidence for low-dose effects (doses lower than those tested in traditional toxicology assessments) of BPA. We found that consistent, reproducible, and often adverse low-dose effects have been demonstrated for BPA in cell lines, primary cells and tissues, laboratory animals, and human populations. We also concluded that EDCs can pose the greatest threats when exposure occurs during early development, organogenesis, and during critical postnatal periods when tissues are differentiating (Endocr Disruptors [Austin, Tex.]. 2013 Sep;1:e25078-1-13).
A potential risk factor for GDM
Quite a lot of research has been done on EDCs and the risk of type 2 diabetes. A recent meta-analysis that included 41 cross-sectional and 8 prospective studies found that serum concentrations of dioxins, polychlorinated biphenyls, and chlorinated pesticides – and urine concentrations of BPA and phthalates – were significantly associated with type 2 diabetes risk. Comparing the highest and lowest concentration categories, the pooled relative risk was 1.45 for BPA and phthalates. EDC concentrations also were associated with indicators of impaired fasting glucose and insulin resistance (J Diabetes. 2016 Jul;8[4]:516-32).
Despite the mounting evidence for an association between BPA and type 2 diabetes, and despite the fact that the increased incidence of GDM in the past 20 years has mirrored the increasing use of EDCs, there has been a dearth of research examining the possible relationship between EDCs and GDM. The effects of BPA on GDM were identified as a knowledge gap by the National Institute of Environmental Health Sciences after a review of the literature from 2007 to 2013 (Environ Health Perspect. 2014 Aug:122[8]:775-86).
To understand the association between EDCs and GDM and the underlying mechanistic pathway of EDCs, we are conducting research that uses a growing body of evidence that suggests that environmental toxins are involved in the control of microRNA (miRNA) expression in trophoblast cells.
MiRNA, a single-stranded, short, noncoding RNA that is involved in posttranslational gene expression, can be packaged along with other signaling molecules inside extracellular vesicles in the placenta called exosomes. These exosomes appear to be shed from the placenta into the maternal circulation as early as 6-7 weeks into pregnancy. Once released into the maternal circulation, research has shown that the exosomes can target and reprogram other cells via the transfer of noncoding miRNAs, thereby changing the gene expression in these cells.
Such an exosome-mediated signaling pathway provides us with the opportunity to isolate exosomes, sequence the miRNAs, and look at whether women who are exposed to higher levels of EDCs (as indicated in urine concentration) have a particular miRNA signature that correlates with GDM. In other words, we’re working to determine whether particular EDCs and exposure levels affect the miRNA placental profiles, and if these profiles are predictive of GDM.
Thus far, in a pilot prospective cohort study of pregnant women, we are seeing hints of altered miRNA expression in relation to GDM. We have selected study participants who are at high risk of developing GDM (for example, prepregnancy body mass index greater than 30, past pregnancy with GDM, or macrosomia) because we suspect that, in many women, EDCs are a tipping point for the development of GDM rather than a sole causative factor. In addition to understanding the impact of EDCs on GDM, it is our hope that miRNAs in maternal circulation will serve as a noninvasive biomarker for early detection of GDM development or susceptibility.
Dr. Ehrlich is an assistant professor of pediatrics and environmental health at Cincinnati Children’s Hospital Medical Center.
Studying the gestational diabetes risk associated with endocrine-disrupting chemicals
Pregnancy presents a unique opportunity for ob.gyns. to counsel their patients on the benefits of adopting healthy lifestyle habits. Women routinely seek care from a practitioner on a regular basis. Expectant mothers are highly motivated to take care of themselves for the sake of their developing babies. Patients can be much more receptive to recommendations from their health care teams during pregnancy than they might be outside of pregnancy. Frequent biometric analyses allow ob.gyns. to monitor patients’ progress and let them know, in a supportive manner, where they might be “falling short” of their health goals.
There are a number of chemicals with which we come in contact every day, sometimes multiple times in a day, which may deeply affect our health. This month’s Master Class highlights one such group of compounds, endocrine-disrupting chemicals, the most widely known of which is bisphenol A (BPA).
Several years ago, our guest author, Dr. Shelley Ehrlich of the University of Cincinnati, spoke at a diabetes in pregnancy meeting about her research on BPA and its potential association with the development of gestational diabetes mellitus (GDM). As a perinatologist who worked for many years with patients who had diabetes in pregnancy, I was particularly struck by her preliminary findings which indicated that BPA might be altering gene expression, thereby leading to pregnancy-related disorders. At the time, Dr. Ehrlich’s research was still in the very early stages. However, her results were a new way of answering the age-old question of why some women, including those without other overt risk factors, might develop GDM.
Therefore, I’m delighted that Dr. Ehrlich agreed to author this month’s class to provide an overview of where her last few years of research has taken her.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Pregnancy presents a unique opportunity for ob.gyns. to counsel their patients on the benefits of adopting healthy lifestyle habits. Women routinely seek care from a practitioner on a regular basis. Expectant mothers are highly motivated to take care of themselves for the sake of their developing babies. Patients can be much more receptive to recommendations from their health care teams during pregnancy than they might be outside of pregnancy. Frequent biometric analyses allow ob.gyns. to monitor patients’ progress and let them know, in a supportive manner, where they might be “falling short” of their health goals.
There are a number of chemicals with which we come in contact every day, sometimes multiple times in a day, which may deeply affect our health. This month’s Master Class highlights one such group of compounds, endocrine-disrupting chemicals, the most widely known of which is bisphenol A (BPA).
Several years ago, our guest author, Dr. Shelley Ehrlich of the University of Cincinnati, spoke at a diabetes in pregnancy meeting about her research on BPA and its potential association with the development of gestational diabetes mellitus (GDM). As a perinatologist who worked for many years with patients who had diabetes in pregnancy, I was particularly struck by her preliminary findings which indicated that BPA might be altering gene expression, thereby leading to pregnancy-related disorders. At the time, Dr. Ehrlich’s research was still in the very early stages. However, her results were a new way of answering the age-old question of why some women, including those without other overt risk factors, might develop GDM.
Therefore, I’m delighted that Dr. Ehrlich agreed to author this month’s class to provide an overview of where her last few years of research has taken her.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Pregnancy presents a unique opportunity for ob.gyns. to counsel their patients on the benefits of adopting healthy lifestyle habits. Women routinely seek care from a practitioner on a regular basis. Expectant mothers are highly motivated to take care of themselves for the sake of their developing babies. Patients can be much more receptive to recommendations from their health care teams during pregnancy than they might be outside of pregnancy. Frequent biometric analyses allow ob.gyns. to monitor patients’ progress and let them know, in a supportive manner, where they might be “falling short” of their health goals.
There are a number of chemicals with which we come in contact every day, sometimes multiple times in a day, which may deeply affect our health. This month’s Master Class highlights one such group of compounds, endocrine-disrupting chemicals, the most widely known of which is bisphenol A (BPA).
Several years ago, our guest author, Dr. Shelley Ehrlich of the University of Cincinnati, spoke at a diabetes in pregnancy meeting about her research on BPA and its potential association with the development of gestational diabetes mellitus (GDM). As a perinatologist who worked for many years with patients who had diabetes in pregnancy, I was particularly struck by her preliminary findings which indicated that BPA might be altering gene expression, thereby leading to pregnancy-related disorders. At the time, Dr. Ehrlich’s research was still in the very early stages. However, her results were a new way of answering the age-old question of why some women, including those without other overt risk factors, might develop GDM.
Therefore, I’m delighted that Dr. Ehrlich agreed to author this month’s class to provide an overview of where her last few years of research has taken her.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].