User login
Embezzlement: It can happen to you
In November, the office manager of a San Antonio dermatology practice was sentenced to 46 months in prison for defrauding the practice of nearly $350,000 from patient billings and employee profit sharing accounts.
Per the indictment, the practice conducted a nonprofit educational symposium in 2012. A bank account was established to collect contributions for that event, which was supposed to be closed at its conclusion; but the office manager kept it open, and deposited practice receipts into it. She then used the account as her slush fund for travel, property payments, meal purchases, and other personal expenses on credit cards she fraudulently opened in the practice’s name. This continued for several years.
Because this case has received national attention, I am republishing my column on embezzlement, which includes recommendations that could prevent such unfortunate situations from occurring.
Few crimes are more easily overlooked than theft from within. who think everything is fine. Most embezzlers are not skillful or discreet; but their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: the person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Over a year ago, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.”
Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
Ask about computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and should have safeguards built into your system. Ask about them. If they aren’t there, ask why.
Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information websites. (See my previous columns on hiring at http://www.mdedge.com/dermatology/managing-your-practice.)
Look for “red flags.” Examples are employees who refuse to take vacations, because someone else will have do their work; or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and you will be assured of some measure of recovery should your safeguards fail. In addition, the mere knowledge that your staff is bonded will frighten off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In November, the office manager of a San Antonio dermatology practice was sentenced to 46 months in prison for defrauding the practice of nearly $350,000 from patient billings and employee profit sharing accounts.
Per the indictment, the practice conducted a nonprofit educational symposium in 2012. A bank account was established to collect contributions for that event, which was supposed to be closed at its conclusion; but the office manager kept it open, and deposited practice receipts into it. She then used the account as her slush fund for travel, property payments, meal purchases, and other personal expenses on credit cards she fraudulently opened in the practice’s name. This continued for several years.
Because this case has received national attention, I am republishing my column on embezzlement, which includes recommendations that could prevent such unfortunate situations from occurring.
Few crimes are more easily overlooked than theft from within. who think everything is fine. Most embezzlers are not skillful or discreet; but their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: the person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Over a year ago, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.”
Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
Ask about computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and should have safeguards built into your system. Ask about them. If they aren’t there, ask why.
Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information websites. (See my previous columns on hiring at http://www.mdedge.com/dermatology/managing-your-practice.)
Look for “red flags.” Examples are employees who refuse to take vacations, because someone else will have do their work; or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and you will be assured of some measure of recovery should your safeguards fail. In addition, the mere knowledge that your staff is bonded will frighten off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In November, the office manager of a San Antonio dermatology practice was sentenced to 46 months in prison for defrauding the practice of nearly $350,000 from patient billings and employee profit sharing accounts.
Per the indictment, the practice conducted a nonprofit educational symposium in 2012. A bank account was established to collect contributions for that event, which was supposed to be closed at its conclusion; but the office manager kept it open, and deposited practice receipts into it. She then used the account as her slush fund for travel, property payments, meal purchases, and other personal expenses on credit cards she fraudulently opened in the practice’s name. This continued for several years.
Because this case has received national attention, I am republishing my column on embezzlement, which includes recommendations that could prevent such unfortunate situations from occurring.
Few crimes are more easily overlooked than theft from within. who think everything is fine. Most embezzlers are not skillful or discreet; but their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: the person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Over a year ago, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.”
Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
Ask about computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and should have safeguards built into your system. Ask about them. If they aren’t there, ask why.
Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information websites. (See my previous columns on hiring at http://www.mdedge.com/dermatology/managing-your-practice.)
Look for “red flags.” Examples are employees who refuse to take vacations, because someone else will have do their work; or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and you will be assured of some measure of recovery should your safeguards fail. In addition, the mere knowledge that your staff is bonded will frighten off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Sea buckthorn: What is it and what is it good for?
To avoid jumping on the bandwagon of another ingredient trend, we sought to examine the scientific background and properties of sea buckthorn oil and it’s utility for the skin.
Sea buckthorn (Hippophae rhamnoides) – also known as a Siberian pineapple tree, and as sandthorn, sallowthorn, or seaberry – is a thorny, dioecious shrub (or tree) in the oleaster family. It can grow up to 23 feet high and is found in coastal sea cliff areas and on mountain slopes of Western Europe, and in dry sandy areas of Asia Minor and Central Asia, Siberia, China, and Tibet. Common sea buckthorn flowers in late April and early May, producing a large number of small, green and brown flowers, turning into edible, usually yellow or orange round berries. The berries have a bitter, sour taste and have a mild aroma, resembling that of a pineapple. The fruit contains a small stone that covers an oily seed.
The berries are a source of antioxidant vitamins, flavonoids, and organic acids, and when pressed, produce a juice that separates into three layers: a thick cream (upper layer), a combination of saturated and unsaturated fatty acids (middle layer), and juice that is a source of fat (lower layer). The berries contain mainly vitamin C, but also vitamin A (alpha- and beta-carotene) and a mixture of other carotenoids, as well as varying concentrations of tocopherols (vitamin E), folic acid, and vitamin B complex–group vitamins.
In addition to flavonoids, the berries contain catechins and procyanidins, cyclitols, phospholipids, tannins, sugars (galactose, fructose, xylose), organic acids (maleic acid, oxalic acid, malic acid, tartaric acid), phenolic acids (such as ferulic acid), and fatty oil. The amount of vitamin C content varies with the variety of the plant and where it is found. The oil of sea buckthorn may be extracted from two parts of the plant, with mechanical cold pressing of seeds (up to 12.5% weight as oil content) and fruit pulp (8%-12% oil content).
Among vegetable oils, sea buckthorn fruit oil has the highest content of palmitoleic acid (omega-7).
Fruit and seed oils contain tocotrienols and plant sterols. Pulp sea buckthorn oil has a high carotenoid content, as opposed to seed oil, and in Mongolia, Russia, and China, is used as a topical therapy for skin burns.
Other significant fatty acids found in sea buckthorn oil are saturated fatty acids (palmitic acid and stearic acid) and polyunsaturated fatty acids, which include alpha-linolenic acid (omega-3), gamma-linolenic acid (omega-6), linolic acid (omega-6), oleic acid (omega-9), and eicosanoic acid (omega-9). Gamma-linoleic acid in particular is reduced in dry skin conditions, such as aging and atopic dermatitis. The human body can produce some gamma-linolenic acid, oleic acid, and palmitoleic acid, but not linolic acid and alpha-linolenic acid. The addition of these substances to diet or skin care has been found to be beneficial in improving dryness and the skin barrier.
In addition, linolic acid, a natural component of human sebum, has been noted to be decreased in the sebum of people with acne-prone skin. Preliminary evidence indicates that dietary supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of atopic dermatitis.
Besides use in topical skin care and cosmetic preparations, sea buckthorn has also been used successfully in the treatment of chronic gastric ulcer disease, inflammation of the vagina and cervix, and cervical erosion. The bark and leaves of sea buckthorn used to be applied to treat diarrhea and dermatologic conditions, while berry oil has been applied topically or taken orally to soften the skin.
In traditional Indian, Chinese, and Tibetan medicines, sea buckthorn berries are used for medicinal purposes, as their ingredients were thought to have a beneficial effect on the function of the alimentary, respiratory, and circulatory systems. Current studies and uses are now confirming their utility experienced over hundreds of years.
Harvesting sea buckthorn fruit is difficult because of dense thorn arrangement among the berries. Therefore, sometimes the only way to obtain fruit is to remove the entire branch of the shrub, which reduces future crops. For this reason berries can only be harvested once every 2 years.
Sea buckthorn has interesting properties and could be of benefit in topical skin care, as long as it is not overharvested or harvested in a way that has a detrimental impact on the environment.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References
United States Department of Agriculture. PLANTS Profile for Hippophae rhamnoides (seaberry). 2007.
Zielińska A and Nowak I. Lipids Health Dis. 2017 May 19;16(1):95.
Reynolds KA et al. Int J Dermatol. 2019 Dec;58(12):1371-6.
To avoid jumping on the bandwagon of another ingredient trend, we sought to examine the scientific background and properties of sea buckthorn oil and it’s utility for the skin.
Sea buckthorn (Hippophae rhamnoides) – also known as a Siberian pineapple tree, and as sandthorn, sallowthorn, or seaberry – is a thorny, dioecious shrub (or tree) in the oleaster family. It can grow up to 23 feet high and is found in coastal sea cliff areas and on mountain slopes of Western Europe, and in dry sandy areas of Asia Minor and Central Asia, Siberia, China, and Tibet. Common sea buckthorn flowers in late April and early May, producing a large number of small, green and brown flowers, turning into edible, usually yellow or orange round berries. The berries have a bitter, sour taste and have a mild aroma, resembling that of a pineapple. The fruit contains a small stone that covers an oily seed.
The berries are a source of antioxidant vitamins, flavonoids, and organic acids, and when pressed, produce a juice that separates into three layers: a thick cream (upper layer), a combination of saturated and unsaturated fatty acids (middle layer), and juice that is a source of fat (lower layer). The berries contain mainly vitamin C, but also vitamin A (alpha- and beta-carotene) and a mixture of other carotenoids, as well as varying concentrations of tocopherols (vitamin E), folic acid, and vitamin B complex–group vitamins.
In addition to flavonoids, the berries contain catechins and procyanidins, cyclitols, phospholipids, tannins, sugars (galactose, fructose, xylose), organic acids (maleic acid, oxalic acid, malic acid, tartaric acid), phenolic acids (such as ferulic acid), and fatty oil. The amount of vitamin C content varies with the variety of the plant and where it is found. The oil of sea buckthorn may be extracted from two parts of the plant, with mechanical cold pressing of seeds (up to 12.5% weight as oil content) and fruit pulp (8%-12% oil content).
Among vegetable oils, sea buckthorn fruit oil has the highest content of palmitoleic acid (omega-7).
Fruit and seed oils contain tocotrienols and plant sterols. Pulp sea buckthorn oil has a high carotenoid content, as opposed to seed oil, and in Mongolia, Russia, and China, is used as a topical therapy for skin burns.
Other significant fatty acids found in sea buckthorn oil are saturated fatty acids (palmitic acid and stearic acid) and polyunsaturated fatty acids, which include alpha-linolenic acid (omega-3), gamma-linolenic acid (omega-6), linolic acid (omega-6), oleic acid (omega-9), and eicosanoic acid (omega-9). Gamma-linoleic acid in particular is reduced in dry skin conditions, such as aging and atopic dermatitis. The human body can produce some gamma-linolenic acid, oleic acid, and palmitoleic acid, but not linolic acid and alpha-linolenic acid. The addition of these substances to diet or skin care has been found to be beneficial in improving dryness and the skin barrier.
In addition, linolic acid, a natural component of human sebum, has been noted to be decreased in the sebum of people with acne-prone skin. Preliminary evidence indicates that dietary supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of atopic dermatitis.
Besides use in topical skin care and cosmetic preparations, sea buckthorn has also been used successfully in the treatment of chronic gastric ulcer disease, inflammation of the vagina and cervix, and cervical erosion. The bark and leaves of sea buckthorn used to be applied to treat diarrhea and dermatologic conditions, while berry oil has been applied topically or taken orally to soften the skin.
In traditional Indian, Chinese, and Tibetan medicines, sea buckthorn berries are used for medicinal purposes, as their ingredients were thought to have a beneficial effect on the function of the alimentary, respiratory, and circulatory systems. Current studies and uses are now confirming their utility experienced over hundreds of years.
Harvesting sea buckthorn fruit is difficult because of dense thorn arrangement among the berries. Therefore, sometimes the only way to obtain fruit is to remove the entire branch of the shrub, which reduces future crops. For this reason berries can only be harvested once every 2 years.
Sea buckthorn has interesting properties and could be of benefit in topical skin care, as long as it is not overharvested or harvested in a way that has a detrimental impact on the environment.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References
United States Department of Agriculture. PLANTS Profile for Hippophae rhamnoides (seaberry). 2007.
Zielińska A and Nowak I. Lipids Health Dis. 2017 May 19;16(1):95.
Reynolds KA et al. Int J Dermatol. 2019 Dec;58(12):1371-6.
To avoid jumping on the bandwagon of another ingredient trend, we sought to examine the scientific background and properties of sea buckthorn oil and it’s utility for the skin.
Sea buckthorn (Hippophae rhamnoides) – also known as a Siberian pineapple tree, and as sandthorn, sallowthorn, or seaberry – is a thorny, dioecious shrub (or tree) in the oleaster family. It can grow up to 23 feet high and is found in coastal sea cliff areas and on mountain slopes of Western Europe, and in dry sandy areas of Asia Minor and Central Asia, Siberia, China, and Tibet. Common sea buckthorn flowers in late April and early May, producing a large number of small, green and brown flowers, turning into edible, usually yellow or orange round berries. The berries have a bitter, sour taste and have a mild aroma, resembling that of a pineapple. The fruit contains a small stone that covers an oily seed.
The berries are a source of antioxidant vitamins, flavonoids, and organic acids, and when pressed, produce a juice that separates into three layers: a thick cream (upper layer), a combination of saturated and unsaturated fatty acids (middle layer), and juice that is a source of fat (lower layer). The berries contain mainly vitamin C, but also vitamin A (alpha- and beta-carotene) and a mixture of other carotenoids, as well as varying concentrations of tocopherols (vitamin E), folic acid, and vitamin B complex–group vitamins.
In addition to flavonoids, the berries contain catechins and procyanidins, cyclitols, phospholipids, tannins, sugars (galactose, fructose, xylose), organic acids (maleic acid, oxalic acid, malic acid, tartaric acid), phenolic acids (such as ferulic acid), and fatty oil. The amount of vitamin C content varies with the variety of the plant and where it is found. The oil of sea buckthorn may be extracted from two parts of the plant, with mechanical cold pressing of seeds (up to 12.5% weight as oil content) and fruit pulp (8%-12% oil content).
Among vegetable oils, sea buckthorn fruit oil has the highest content of palmitoleic acid (omega-7).
Fruit and seed oils contain tocotrienols and plant sterols. Pulp sea buckthorn oil has a high carotenoid content, as opposed to seed oil, and in Mongolia, Russia, and China, is used as a topical therapy for skin burns.
Other significant fatty acids found in sea buckthorn oil are saturated fatty acids (palmitic acid and stearic acid) and polyunsaturated fatty acids, which include alpha-linolenic acid (omega-3), gamma-linolenic acid (omega-6), linolic acid (omega-6), oleic acid (omega-9), and eicosanoic acid (omega-9). Gamma-linoleic acid in particular is reduced in dry skin conditions, such as aging and atopic dermatitis. The human body can produce some gamma-linolenic acid, oleic acid, and palmitoleic acid, but not linolic acid and alpha-linolenic acid. The addition of these substances to diet or skin care has been found to be beneficial in improving dryness and the skin barrier.
In addition, linolic acid, a natural component of human sebum, has been noted to be decreased in the sebum of people with acne-prone skin. Preliminary evidence indicates that dietary supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of atopic dermatitis.
Besides use in topical skin care and cosmetic preparations, sea buckthorn has also been used successfully in the treatment of chronic gastric ulcer disease, inflammation of the vagina and cervix, and cervical erosion. The bark and leaves of sea buckthorn used to be applied to treat diarrhea and dermatologic conditions, while berry oil has been applied topically or taken orally to soften the skin.
In traditional Indian, Chinese, and Tibetan medicines, sea buckthorn berries are used for medicinal purposes, as their ingredients were thought to have a beneficial effect on the function of the alimentary, respiratory, and circulatory systems. Current studies and uses are now confirming their utility experienced over hundreds of years.
Harvesting sea buckthorn fruit is difficult because of dense thorn arrangement among the berries. Therefore, sometimes the only way to obtain fruit is to remove the entire branch of the shrub, which reduces future crops. For this reason berries can only be harvested once every 2 years.
Sea buckthorn has interesting properties and could be of benefit in topical skin care, as long as it is not overharvested or harvested in a way that has a detrimental impact on the environment.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References
United States Department of Agriculture. PLANTS Profile for Hippophae rhamnoides (seaberry). 2007.
Zielińska A and Nowak I. Lipids Health Dis. 2017 May 19;16(1):95.
Reynolds KA et al. Int J Dermatol. 2019 Dec;58(12):1371-6.
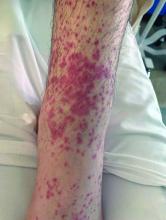
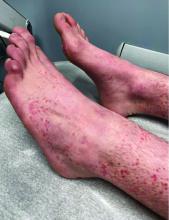
What is the diagnosis?
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
What does it mean to be a trustworthy male ally?
“If you want to be trusted, be trustworthy” – Stephen Covey
A few years ago, while working in my office, a female colleague stopped by for a casual chat. During the course of the conversation, she noticed that I did not have any diplomas or certificates hanging on my office walls. Instead, there were clusters of pictures drawn by my children, family photos, and a white board with my “to-do” list. The only wall art was a print of Banksy’s “The Thinker Monkey,” which depicts a monkey with its fist to its chin similar to Rodin’s famous sculpture, “Le Penseur.”
When asked why I didn’t hang any diplomas or awards, I replied that I preferred to keep my office atmosphere light and fun, and to focus on future goals rather than past accomplishments. I could see her jaw tense. Her frustration appeared deep, but it was for reasons beyond just my self-righteous tone. She said, “You know, I appreciate your focus on future goals, but it’s a pretty privileged position to not have to worry about sharing your accomplishments publicly.”
What followed was a discussion that was generative, enlightening, uncomfortable, and necessary. I had never considered what I chose to hang (or not hang) on my office walls as a privilege, and that was exactly the point. She described numerous episodes when her accomplishments were overlooked or (worse) attributed to a male colleague because she was a woman. I began to understand that graceful self-promotion is not optional for many women in medicine, it is a necessary skill.
This is just one example of how my privilege as a male in medicine contributed to my ignorance of the gender inequities that my female coworkers have faced throughout their careers. My colleague showed a lot of grace by taking the time to help me navigate my male privilege in a constructive manner. I decided to learn more about gender inequities, and eventually determined that I was woefully inadequate as a male ally, not by refusal but by ignorance. I wanted to start earning my colleague’s trust that I would be an ally that she could count on.
Trustworthiness
I wanted to be a trustworthy ally, but what does that entail? Perhaps we can learn from medical education. Trust is a complex construct that is increasingly used as a framework for assessing medical students and residents, such as with entrustable professional activities (EPAs).1,2 Multiple studies have examined the characteristics that make a learner “trustworthy” when determining how much supervision is required.3-8 Ten Cate and Chen performed an interpretivist, narrative review to synthesize the medical education literature on learner trustworthiness in the past 15 years,9 developing five major themes that contribute to trustworthiness: Humility, Capability, Agency, Reliability, and Integrity. Let’s examine each of these through the lens of male allyship.
Humility
Humility involves knowing one’s limits, asking for help, and being receptive to feedback.9 The first thing men need to do is to put their egos in check and recognize that women do not need rescuing; they need partnership. Systemic inequities have led to men holding the majority of leadership positions and significant sociopolitical capital, and correcting these inequities is more feasible when those in leadership and positions of power contribute. Women don’t need knights in shining armor, they need collaborative activism.
Humility also means a willingness to admit fallibility and to ask for help. Men often don’t know what they don’t know (see my foibles in the opening). As David G. Smith, PhD, and W. Brad Johnson, PhD, write in their book, “Good Guys,” “There are no perfect allies. As you work to become a better ally for the women around you, you will undoubtedly make a mistake.”10 Men must accept feedback on their shortcomings as allies without feeling as though they are losing their sociopolitical standing. Allyship for women does not mean there is a devaluing of men. We must escape a “zero-sum” mindset. Mistakes are where growth happens, but only if we approach our missteps with humility.
Capability
Capability entails having the necessary knowledge, skills, and attitudes to be a strong ally. Allyship is not intuitive for most men for several reasons. Many men do not experience the same biases or systemic inequities that women do, and therefore perceive them less frequently. I want to acknowledge that men can be victims of other systemic biases such as those against one’s race, ethnicity, gender identity, sexual orientation, religion, or any number of factors. Men who face inequities for these other reasons may be more cognizant of the biases women face. Even so, allyship is a skill that few men have been explicitly taught. Even if taught, few standard or organized mechanisms for feedback on allyship capability exist. How, then, can men become capable allies?
Just like in medical education, men must become self-directed learners who seek to build capability and receive feedback on their performance as allies. Men should seek allyship training through local women-in-medicine programs or organizations, or through the increasing number of national education options such as the recent ADVANCE PHM Gender Equity Symposium. As with learning any skill, men should go to the literature, seeking knowledge from experts in the field. I recommend starting with “Good Guys: How Men Can Be Better Allies for Women in the Workplace10 or “Athena Rising: How and Why Men Should Mentor Women.”11 Both books, by Dr. Smith and Dr. Johnson, are great entry points into the gender allyship literature. Seek out other resources from local experts on gender equity and allyship. Both aforementioned books were recommended to me by a friend and gender equity expert; without her guidance I would not have known where to start.
Agency
Agency involves being proactive and engaged rather than passive or apathetic. Men must be enthusiastic allies who seek out opportunities to mentor and sponsor women rather than waiting for others to ask. Agency requires being curious and passionate about improving. Most men in medicine are not openly and explicitly misogynistic or sexist, but many are only passive when it comes to gender equity and allyship. Trustworthy allyship entails turning passive support into active change. Not sure how to start? A good first step is to ask female colleagues questions such as, “What can I do to be a better ally for you in the workplace?” or “What are some things at work that are most challenging to you, but I might not notice because I’m a man?” Curiosity is the springboard toward agency.
Reliability
Reliability means being conscientious, accountable, and doing what we say we will do. Nothing undermines trustworthiness faster than making a commitment and not following through. Allyship cannot be a show or an attempt to get public plaudits. It is a longitudinal commitment to supporting women through individual mentorship and sponsorship, and to work toward institutional and systems change.
Reliability also means taking an equitable approach to what Dr. Smith and Dr. Johnson call “office housework.” They define this as “administrative work that is necessary but undervalued, unlikely to lead to promotion, and disproportionately assigned to women.”10 In medicine, these tasks include organizing meetings, taking notes, planning social events, and remembering to celebrate colleagues’ achievements and milestones. Men should take on more of these tasks and advocate for change when the distribution of office housework in their workplace is inequitably directed toward women.
Integrity
Integrity involves honesty, professionalism, and benevolence. It is about making the morally correct choice even if there is potential risk. When men see gender inequity, they have an obligation to speak up. Whether it is overtly misogynistic behavior, subtle sexism, use of gendered language, inequitable distribution of office housework, lack of inclusivity and recognition for women, or another form of inequity, men must act with integrity and make it clear that they are partnering with women for change. Integrity means being an ally even when women are not present, and advocating that women be “at the table” for important conversations.
Beyond the individual
Allyship cannot end with individual actions; systems changes that build trustworthy institutions are necessary. Organizational leaders must approach gender conversations with humility to critically examine inequities and agency to implement meaningful changes. Workplace cultures and institutional policies should be reviewed with an eye toward system-level integrity and reliability for promoting and supporting women. Ongoing faculty and staff development programs must provide men with the knowledge, skills, and attitudes (capability) to be strong allies. We have a long history of male-dominated institutions that are unfair or (worse) unsafe for women. Many systems are designed in a way that disadvantages women. These systems must be redesigned through an equity lens to start building trust with women in medicine.
Becoming trustworthy is a process
Even the best male allies have room to improve their trustworthiness. Many men (myself included) have a LOT of room to improve, but they should not get discouraged by the amount of ground to be gained. Steady, deliberate improvement in men’s humility, capability, agency, reliability, and integrity can build the foundation of trust with female colleagues. Trust takes time. It takes effort. It takes vulnerability. It is an ongoing, developmental process that requires deliberate practice, frequent reflection, and feedback from our female colleagues.
Dr. Kinnear is associate professor of internal medicine and pediatrics in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. He is associate program director for the Med-Peds and Internal Medicine residency programs.
References
1. Ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013 Mar;5(1):157-8. doi: 10.4300/JGME-D-12-00380.1.
2. Ten Cate O. Entrustment decisions: Bringing the patient into the assessment equation. Acad Med. 2017 Jun;92(6):736-8. doi: 10.1097/ACM.0000000000001623.
3. Kennedy TJT et al. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008 Oct;83(10 Suppl):S89-92. doi: 10.1097/ACM.0b013e318183c8b7.
4. Choo KJ et al. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014 Mar;9(3):169-75. doi: 10.1002/jhm.2150.
5. Hauer KE et al. How clinical supervisors develop trust in their trainees: A qualitative study. Med Educ. 2015 Aug;49(8):783-95. doi: 10.1111/medu.12745.
6. Sterkenburg A et al. When do supervising physicians decide to entrust residents with unsupervised tasks? Acad Med. 2010 Sep;85(9):1408-17. doi: 10.1097/ACM.0b013e3181eab0ec.
7. Sheu L et al. How supervisor experience influences trust, supervision, and trainee learning: A qualitative study. Acad Med. 2017 Sep;92(9):1320-7. doi: 10.1097/ACM.0000000000001560.
8. Pingree EW et al. Encouraging entrustment: A qualitative study of resident behaviors that promote entrustment. Acad Med. 2020 Nov;95(11):1718-25. doi: 10.1097/ACM.0000000000003487.
9. Ten Cate O, Chen HC. The ingredients of a rich entrustment decision. Med Teach. 2020 Dec;42(12):1413-20. doi: 10.1080/0142159X.2020.1817348.
10. Smith DG, Johnson WB. Good guys: How men can be better allies for women in the workplace: Harvard Business School Publishing Corporation 2020.
11. Johnson WB, Smith D. Athena rising: How and why men should mentor women: Routledge 2016.
“If you want to be trusted, be trustworthy” – Stephen Covey
A few years ago, while working in my office, a female colleague stopped by for a casual chat. During the course of the conversation, she noticed that I did not have any diplomas or certificates hanging on my office walls. Instead, there were clusters of pictures drawn by my children, family photos, and a white board with my “to-do” list. The only wall art was a print of Banksy’s “The Thinker Monkey,” which depicts a monkey with its fist to its chin similar to Rodin’s famous sculpture, “Le Penseur.”
When asked why I didn’t hang any diplomas or awards, I replied that I preferred to keep my office atmosphere light and fun, and to focus on future goals rather than past accomplishments. I could see her jaw tense. Her frustration appeared deep, but it was for reasons beyond just my self-righteous tone. She said, “You know, I appreciate your focus on future goals, but it’s a pretty privileged position to not have to worry about sharing your accomplishments publicly.”
What followed was a discussion that was generative, enlightening, uncomfortable, and necessary. I had never considered what I chose to hang (or not hang) on my office walls as a privilege, and that was exactly the point. She described numerous episodes when her accomplishments were overlooked or (worse) attributed to a male colleague because she was a woman. I began to understand that graceful self-promotion is not optional for many women in medicine, it is a necessary skill.
This is just one example of how my privilege as a male in medicine contributed to my ignorance of the gender inequities that my female coworkers have faced throughout their careers. My colleague showed a lot of grace by taking the time to help me navigate my male privilege in a constructive manner. I decided to learn more about gender inequities, and eventually determined that I was woefully inadequate as a male ally, not by refusal but by ignorance. I wanted to start earning my colleague’s trust that I would be an ally that she could count on.
Trustworthiness
I wanted to be a trustworthy ally, but what does that entail? Perhaps we can learn from medical education. Trust is a complex construct that is increasingly used as a framework for assessing medical students and residents, such as with entrustable professional activities (EPAs).1,2 Multiple studies have examined the characteristics that make a learner “trustworthy” when determining how much supervision is required.3-8 Ten Cate and Chen performed an interpretivist, narrative review to synthesize the medical education literature on learner trustworthiness in the past 15 years,9 developing five major themes that contribute to trustworthiness: Humility, Capability, Agency, Reliability, and Integrity. Let’s examine each of these through the lens of male allyship.
Humility
Humility involves knowing one’s limits, asking for help, and being receptive to feedback.9 The first thing men need to do is to put their egos in check and recognize that women do not need rescuing; they need partnership. Systemic inequities have led to men holding the majority of leadership positions and significant sociopolitical capital, and correcting these inequities is more feasible when those in leadership and positions of power contribute. Women don’t need knights in shining armor, they need collaborative activism.
Humility also means a willingness to admit fallibility and to ask for help. Men often don’t know what they don’t know (see my foibles in the opening). As David G. Smith, PhD, and W. Brad Johnson, PhD, write in their book, “Good Guys,” “There are no perfect allies. As you work to become a better ally for the women around you, you will undoubtedly make a mistake.”10 Men must accept feedback on their shortcomings as allies without feeling as though they are losing their sociopolitical standing. Allyship for women does not mean there is a devaluing of men. We must escape a “zero-sum” mindset. Mistakes are where growth happens, but only if we approach our missteps with humility.
Capability
Capability entails having the necessary knowledge, skills, and attitudes to be a strong ally. Allyship is not intuitive for most men for several reasons. Many men do not experience the same biases or systemic inequities that women do, and therefore perceive them less frequently. I want to acknowledge that men can be victims of other systemic biases such as those against one’s race, ethnicity, gender identity, sexual orientation, religion, or any number of factors. Men who face inequities for these other reasons may be more cognizant of the biases women face. Even so, allyship is a skill that few men have been explicitly taught. Even if taught, few standard or organized mechanisms for feedback on allyship capability exist. How, then, can men become capable allies?
Just like in medical education, men must become self-directed learners who seek to build capability and receive feedback on their performance as allies. Men should seek allyship training through local women-in-medicine programs or organizations, or through the increasing number of national education options such as the recent ADVANCE PHM Gender Equity Symposium. As with learning any skill, men should go to the literature, seeking knowledge from experts in the field. I recommend starting with “Good Guys: How Men Can Be Better Allies for Women in the Workplace10 or “Athena Rising: How and Why Men Should Mentor Women.”11 Both books, by Dr. Smith and Dr. Johnson, are great entry points into the gender allyship literature. Seek out other resources from local experts on gender equity and allyship. Both aforementioned books were recommended to me by a friend and gender equity expert; without her guidance I would not have known where to start.
Agency
Agency involves being proactive and engaged rather than passive or apathetic. Men must be enthusiastic allies who seek out opportunities to mentor and sponsor women rather than waiting for others to ask. Agency requires being curious and passionate about improving. Most men in medicine are not openly and explicitly misogynistic or sexist, but many are only passive when it comes to gender equity and allyship. Trustworthy allyship entails turning passive support into active change. Not sure how to start? A good first step is to ask female colleagues questions such as, “What can I do to be a better ally for you in the workplace?” or “What are some things at work that are most challenging to you, but I might not notice because I’m a man?” Curiosity is the springboard toward agency.
Reliability
Reliability means being conscientious, accountable, and doing what we say we will do. Nothing undermines trustworthiness faster than making a commitment and not following through. Allyship cannot be a show or an attempt to get public plaudits. It is a longitudinal commitment to supporting women through individual mentorship and sponsorship, and to work toward institutional and systems change.
Reliability also means taking an equitable approach to what Dr. Smith and Dr. Johnson call “office housework.” They define this as “administrative work that is necessary but undervalued, unlikely to lead to promotion, and disproportionately assigned to women.”10 In medicine, these tasks include organizing meetings, taking notes, planning social events, and remembering to celebrate colleagues’ achievements and milestones. Men should take on more of these tasks and advocate for change when the distribution of office housework in their workplace is inequitably directed toward women.
Integrity
Integrity involves honesty, professionalism, and benevolence. It is about making the morally correct choice even if there is potential risk. When men see gender inequity, they have an obligation to speak up. Whether it is overtly misogynistic behavior, subtle sexism, use of gendered language, inequitable distribution of office housework, lack of inclusivity and recognition for women, or another form of inequity, men must act with integrity and make it clear that they are partnering with women for change. Integrity means being an ally even when women are not present, and advocating that women be “at the table” for important conversations.
Beyond the individual
Allyship cannot end with individual actions; systems changes that build trustworthy institutions are necessary. Organizational leaders must approach gender conversations with humility to critically examine inequities and agency to implement meaningful changes. Workplace cultures and institutional policies should be reviewed with an eye toward system-level integrity and reliability for promoting and supporting women. Ongoing faculty and staff development programs must provide men with the knowledge, skills, and attitudes (capability) to be strong allies. We have a long history of male-dominated institutions that are unfair or (worse) unsafe for women. Many systems are designed in a way that disadvantages women. These systems must be redesigned through an equity lens to start building trust with women in medicine.
Becoming trustworthy is a process
Even the best male allies have room to improve their trustworthiness. Many men (myself included) have a LOT of room to improve, but they should not get discouraged by the amount of ground to be gained. Steady, deliberate improvement in men’s humility, capability, agency, reliability, and integrity can build the foundation of trust with female colleagues. Trust takes time. It takes effort. It takes vulnerability. It is an ongoing, developmental process that requires deliberate practice, frequent reflection, and feedback from our female colleagues.
Dr. Kinnear is associate professor of internal medicine and pediatrics in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. He is associate program director for the Med-Peds and Internal Medicine residency programs.
References
1. Ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013 Mar;5(1):157-8. doi: 10.4300/JGME-D-12-00380.1.
2. Ten Cate O. Entrustment decisions: Bringing the patient into the assessment equation. Acad Med. 2017 Jun;92(6):736-8. doi: 10.1097/ACM.0000000000001623.
3. Kennedy TJT et al. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008 Oct;83(10 Suppl):S89-92. doi: 10.1097/ACM.0b013e318183c8b7.
4. Choo KJ et al. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014 Mar;9(3):169-75. doi: 10.1002/jhm.2150.
5. Hauer KE et al. How clinical supervisors develop trust in their trainees: A qualitative study. Med Educ. 2015 Aug;49(8):783-95. doi: 10.1111/medu.12745.
6. Sterkenburg A et al. When do supervising physicians decide to entrust residents with unsupervised tasks? Acad Med. 2010 Sep;85(9):1408-17. doi: 10.1097/ACM.0b013e3181eab0ec.
7. Sheu L et al. How supervisor experience influences trust, supervision, and trainee learning: A qualitative study. Acad Med. 2017 Sep;92(9):1320-7. doi: 10.1097/ACM.0000000000001560.
8. Pingree EW et al. Encouraging entrustment: A qualitative study of resident behaviors that promote entrustment. Acad Med. 2020 Nov;95(11):1718-25. doi: 10.1097/ACM.0000000000003487.
9. Ten Cate O, Chen HC. The ingredients of a rich entrustment decision. Med Teach. 2020 Dec;42(12):1413-20. doi: 10.1080/0142159X.2020.1817348.
10. Smith DG, Johnson WB. Good guys: How men can be better allies for women in the workplace: Harvard Business School Publishing Corporation 2020.
11. Johnson WB, Smith D. Athena rising: How and why men should mentor women: Routledge 2016.
“If you want to be trusted, be trustworthy” – Stephen Covey
A few years ago, while working in my office, a female colleague stopped by for a casual chat. During the course of the conversation, she noticed that I did not have any diplomas or certificates hanging on my office walls. Instead, there were clusters of pictures drawn by my children, family photos, and a white board with my “to-do” list. The only wall art was a print of Banksy’s “The Thinker Monkey,” which depicts a monkey with its fist to its chin similar to Rodin’s famous sculpture, “Le Penseur.”
When asked why I didn’t hang any diplomas or awards, I replied that I preferred to keep my office atmosphere light and fun, and to focus on future goals rather than past accomplishments. I could see her jaw tense. Her frustration appeared deep, but it was for reasons beyond just my self-righteous tone. She said, “You know, I appreciate your focus on future goals, but it’s a pretty privileged position to not have to worry about sharing your accomplishments publicly.”
What followed was a discussion that was generative, enlightening, uncomfortable, and necessary. I had never considered what I chose to hang (or not hang) on my office walls as a privilege, and that was exactly the point. She described numerous episodes when her accomplishments were overlooked or (worse) attributed to a male colleague because she was a woman. I began to understand that graceful self-promotion is not optional for many women in medicine, it is a necessary skill.
This is just one example of how my privilege as a male in medicine contributed to my ignorance of the gender inequities that my female coworkers have faced throughout their careers. My colleague showed a lot of grace by taking the time to help me navigate my male privilege in a constructive manner. I decided to learn more about gender inequities, and eventually determined that I was woefully inadequate as a male ally, not by refusal but by ignorance. I wanted to start earning my colleague’s trust that I would be an ally that she could count on.
Trustworthiness
I wanted to be a trustworthy ally, but what does that entail? Perhaps we can learn from medical education. Trust is a complex construct that is increasingly used as a framework for assessing medical students and residents, such as with entrustable professional activities (EPAs).1,2 Multiple studies have examined the characteristics that make a learner “trustworthy” when determining how much supervision is required.3-8 Ten Cate and Chen performed an interpretivist, narrative review to synthesize the medical education literature on learner trustworthiness in the past 15 years,9 developing five major themes that contribute to trustworthiness: Humility, Capability, Agency, Reliability, and Integrity. Let’s examine each of these through the lens of male allyship.
Humility
Humility involves knowing one’s limits, asking for help, and being receptive to feedback.9 The first thing men need to do is to put their egos in check and recognize that women do not need rescuing; they need partnership. Systemic inequities have led to men holding the majority of leadership positions and significant sociopolitical capital, and correcting these inequities is more feasible when those in leadership and positions of power contribute. Women don’t need knights in shining armor, they need collaborative activism.
Humility also means a willingness to admit fallibility and to ask for help. Men often don’t know what they don’t know (see my foibles in the opening). As David G. Smith, PhD, and W. Brad Johnson, PhD, write in their book, “Good Guys,” “There are no perfect allies. As you work to become a better ally for the women around you, you will undoubtedly make a mistake.”10 Men must accept feedback on their shortcomings as allies without feeling as though they are losing their sociopolitical standing. Allyship for women does not mean there is a devaluing of men. We must escape a “zero-sum” mindset. Mistakes are where growth happens, but only if we approach our missteps with humility.
Capability
Capability entails having the necessary knowledge, skills, and attitudes to be a strong ally. Allyship is not intuitive for most men for several reasons. Many men do not experience the same biases or systemic inequities that women do, and therefore perceive them less frequently. I want to acknowledge that men can be victims of other systemic biases such as those against one’s race, ethnicity, gender identity, sexual orientation, religion, or any number of factors. Men who face inequities for these other reasons may be more cognizant of the biases women face. Even so, allyship is a skill that few men have been explicitly taught. Even if taught, few standard or organized mechanisms for feedback on allyship capability exist. How, then, can men become capable allies?
Just like in medical education, men must become self-directed learners who seek to build capability and receive feedback on their performance as allies. Men should seek allyship training through local women-in-medicine programs or organizations, or through the increasing number of national education options such as the recent ADVANCE PHM Gender Equity Symposium. As with learning any skill, men should go to the literature, seeking knowledge from experts in the field. I recommend starting with “Good Guys: How Men Can Be Better Allies for Women in the Workplace10 or “Athena Rising: How and Why Men Should Mentor Women.”11 Both books, by Dr. Smith and Dr. Johnson, are great entry points into the gender allyship literature. Seek out other resources from local experts on gender equity and allyship. Both aforementioned books were recommended to me by a friend and gender equity expert; without her guidance I would not have known where to start.
Agency
Agency involves being proactive and engaged rather than passive or apathetic. Men must be enthusiastic allies who seek out opportunities to mentor and sponsor women rather than waiting for others to ask. Agency requires being curious and passionate about improving. Most men in medicine are not openly and explicitly misogynistic or sexist, but many are only passive when it comes to gender equity and allyship. Trustworthy allyship entails turning passive support into active change. Not sure how to start? A good first step is to ask female colleagues questions such as, “What can I do to be a better ally for you in the workplace?” or “What are some things at work that are most challenging to you, but I might not notice because I’m a man?” Curiosity is the springboard toward agency.
Reliability
Reliability means being conscientious, accountable, and doing what we say we will do. Nothing undermines trustworthiness faster than making a commitment and not following through. Allyship cannot be a show or an attempt to get public plaudits. It is a longitudinal commitment to supporting women through individual mentorship and sponsorship, and to work toward institutional and systems change.
Reliability also means taking an equitable approach to what Dr. Smith and Dr. Johnson call “office housework.” They define this as “administrative work that is necessary but undervalued, unlikely to lead to promotion, and disproportionately assigned to women.”10 In medicine, these tasks include organizing meetings, taking notes, planning social events, and remembering to celebrate colleagues’ achievements and milestones. Men should take on more of these tasks and advocate for change when the distribution of office housework in their workplace is inequitably directed toward women.
Integrity
Integrity involves honesty, professionalism, and benevolence. It is about making the morally correct choice even if there is potential risk. When men see gender inequity, they have an obligation to speak up. Whether it is overtly misogynistic behavior, subtle sexism, use of gendered language, inequitable distribution of office housework, lack of inclusivity and recognition for women, or another form of inequity, men must act with integrity and make it clear that they are partnering with women for change. Integrity means being an ally even when women are not present, and advocating that women be “at the table” for important conversations.
Beyond the individual
Allyship cannot end with individual actions; systems changes that build trustworthy institutions are necessary. Organizational leaders must approach gender conversations with humility to critically examine inequities and agency to implement meaningful changes. Workplace cultures and institutional policies should be reviewed with an eye toward system-level integrity and reliability for promoting and supporting women. Ongoing faculty and staff development programs must provide men with the knowledge, skills, and attitudes (capability) to be strong allies. We have a long history of male-dominated institutions that are unfair or (worse) unsafe for women. Many systems are designed in a way that disadvantages women. These systems must be redesigned through an equity lens to start building trust with women in medicine.
Becoming trustworthy is a process
Even the best male allies have room to improve their trustworthiness. Many men (myself included) have a LOT of room to improve, but they should not get discouraged by the amount of ground to be gained. Steady, deliberate improvement in men’s humility, capability, agency, reliability, and integrity can build the foundation of trust with female colleagues. Trust takes time. It takes effort. It takes vulnerability. It is an ongoing, developmental process that requires deliberate practice, frequent reflection, and feedback from our female colleagues.
Dr. Kinnear is associate professor of internal medicine and pediatrics in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. He is associate program director for the Med-Peds and Internal Medicine residency programs.
References
1. Ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013 Mar;5(1):157-8. doi: 10.4300/JGME-D-12-00380.1.
2. Ten Cate O. Entrustment decisions: Bringing the patient into the assessment equation. Acad Med. 2017 Jun;92(6):736-8. doi: 10.1097/ACM.0000000000001623.
3. Kennedy TJT et al. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008 Oct;83(10 Suppl):S89-92. doi: 10.1097/ACM.0b013e318183c8b7.
4. Choo KJ et al. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014 Mar;9(3):169-75. doi: 10.1002/jhm.2150.
5. Hauer KE et al. How clinical supervisors develop trust in their trainees: A qualitative study. Med Educ. 2015 Aug;49(8):783-95. doi: 10.1111/medu.12745.
6. Sterkenburg A et al. When do supervising physicians decide to entrust residents with unsupervised tasks? Acad Med. 2010 Sep;85(9):1408-17. doi: 10.1097/ACM.0b013e3181eab0ec.
7. Sheu L et al. How supervisor experience influences trust, supervision, and trainee learning: A qualitative study. Acad Med. 2017 Sep;92(9):1320-7. doi: 10.1097/ACM.0000000000001560.
8. Pingree EW et al. Encouraging entrustment: A qualitative study of resident behaviors that promote entrustment. Acad Med. 2020 Nov;95(11):1718-25. doi: 10.1097/ACM.0000000000003487.
9. Ten Cate O, Chen HC. The ingredients of a rich entrustment decision. Med Teach. 2020 Dec;42(12):1413-20. doi: 10.1080/0142159X.2020.1817348.
10. Smith DG, Johnson WB. Good guys: How men can be better allies for women in the workplace: Harvard Business School Publishing Corporation 2020.
11. Johnson WB, Smith D. Athena rising: How and why men should mentor women: Routledge 2016.
A pediatrician’s guide to screening for and treating depression
On Oct. 19, the American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and the Children’s Hospital Association jointly declared a “national emergency in children’s mental health,” calling upon policy makers to take actions that could help address “soaring rates” of anxiety and depression.
Knowing that increasing the work force or creating new programs will come slowly if at all, they called for the integration of mental health care into primary care pediatrics and efforts to reduce the risk of suicide in children and adolescents.
Our clinical experience suggests that adolescent depression, which can lead to profoundly impaired function, impaired development, and even suicide, is a major concern in your practice. We hope to do our part by reviewing the screening, diagnosis, and management of depression that can reasonably happen in the pediatrician’s office.
Depression
Depression affects as many as 20% of adolescents, with girls experiencing major depressive disorder (MDD) twice as often as boys. The incidence of depression increases fourfold after puberty, and there is substantial evidence, but no clear cause, that it has increased by nearly 50% over the past decade, rising from a rate of 8% of U.S. adolescents in 2007 to 13% in 2017.1 In that same time period, the rate of completed suicides among U.S. youth aged 10-24 increased 57.4%, after being stable for the prior decade.2 Adolescent depression is also linked to increased substance use and high-risk behaviors such as drunk driving. In 2020, mental health–related emergency department visits by adolescents aged 12-17 increased by 31%. Visits for suicide attempts among adolescent girls in 2021 jumped by 51% from 2019.3 Clearly, MDD in adolescence is a common, potentially life-threatening problem
.
Screening and assessment
At annual checkups with patients 12 and older or at sick visits of patients with emotional, sleep, or vague somatic concerns, it should be standard practice to screen for depression. The Patient Health Questionnaire 9 modified for Adolescents (PHQ9-A) is a reliable, validated, and free screening instrument that your patients can fill out in the waiting room. (The PHQ9 can be used for your patients who are 18 and older.) It takes only 5 minutes to complete and is very easy to score. It establishes whether your patient meets DSM-5 criteria for MDD, and the degree of severity (5-9 is mild, 10-14 is moderate, 15-19 is moderately severe, and 20-27 is severe). It also screens for thoughts about suicide and past suicide attempts. You might add the more comprehensive parent-completed Pediatric Symptom Checklist, which includes a depression screen.4
These screening instruments can be completed electronically prior to or at the visit and should have a preamble explaining why depression screening is relevant. If screening is positive, interview your adolescent patients alone. This will give you the time to gather more detail about how impaired their function is at school, with friends, and in family relationships. Have they been missing school? Have their grades changed? Are they failing to hand in homework? Have they withdrawn from sports or activities? Are they less likely to hang out with friends? Do they participate in family activities? Have others noticed any changes? You should also check for associated anxiety symptoms (ruminative worries, panic attacks) and drug and alcohol use. Of course, you should ask about any suicidal thoughts (from vague morbid thoughts to specific plans, with intent and factors that have prevented them) and actual attempts. Remember, asking about suicidal thoughts and attempts will not cause or worsen them. On the contrary, your patients may feel shame, but will be relieved to not be alone with these thoughts. And this knowledge will be essential as you decide what to do next. When you meet with the parents, ask them about a family history of depression or suicide attempts, and then offer supportive interventions.
Supportive interventions
For all adolescents with depression, supportive interventions are helpful, and for those with mild symptoms, they are often adequate treatment. This begins with education for your patient and their parents about depression. It is an illness, not a problem of character or discipline. Advise your patients that adequate, restful sleep every night is critical to recovery. Regular exercise (daily is best, but at least three times weekly for 30 minutes) is often effective in mild to moderate depression. Patience and compassion for feelings of sadness, irritability, or disinterest are important at home, and maintaining connections with those people who offer support (friends, coaches, parents, etc.) is essential. They should also be told that “depression lies.” Feelings of guilt and self-reproach are a normal part of the illness, not facts. Organizations such as the National Alliance on Mental Illness (NAMI) and the American Academy of Child and Adolescent Psychiatry (AACAP) offer written materials through their websites that are very helpful educational resources. Connect them with sources of counseling support (through school, for example). For those with mild, brief, and uncomplicated depression, supportive interventions alone should offer relief within 4-6 weeks. It is hard to predict the trajectory of depression, so follow-up visits are relevant to determine if they are improving or worsening.
Psychotherapy
For your patients with moderate depression, or with hopelessness or suicidality, a referral for evidence-based psychotherapy is indicated. Both cognitive behavioral therapy and interpersonal therapy have demonstrated efficacy in treating depression in adolescents. If there is a history of trauma or high family conflict, supportive psychotherapy that will enhance communication skills within the family is very important to recovery. Identify various sources for high-quality psychotherapy services (individual, family, and group) in your community. While this may sound easier said than done, online services such as Psychology Today’s therapist locator can help. If your local university has a graduate program in social work or psychology, connect with them as they may have easier access to high-quality services through their training programs. If there is a group practice of therapists in your community, invite them to meet with your team to learn about whether they use evidence-based therapies and can support families as well as individual youth.
Pharmacologic options
For those adolescents with moderate to severe depression, psychotherapy alone is usually inadequate. Indeed, they may be so impaired that they simply cannot meaningfully engage in the work of psychotherapy. These patients require psychopharmacologic treatment first. First-line treatment is with selective serotonin reuptake inhibitors (SSRIs) (both fluoxetine and escitalopram are approved for use in adolescent depression). While many pediatricians remain reluctant about initiating SSRI treatment of depression since the Food and Drug Administration’s 2004 boxed warning was issued, the risks of untreated severe depression are more marked than are the risks of SSRI treatment. As prescription rates dipped in the following decade, rates of suicide attempts in adolescents with severe depression climbed. Subsequent research on the nature of the risk of “increased suicidality” indicated it is substantially lower than originally thought.
The AAP’s Guidelines for Adolescent Depression in Primary Care offer reassuring guidance: They recommend that pediatricians initiate treatment at a very low dose of SSRI (5 mg of fluoxetine, 12.5 mg of sertraline, or 5 mg of escitalopram) and aim to get to a therapeutic dose within 4 weeks.5 Educate the patient and parent about likely side effects (gastrointestinal upset, sleep disruption, akathisia or restlessness, and activation), which indicate the dose should be held steady until the side effects subside. Patients should be seen weekly until they get to a therapeutic dose, then biweekly to monitor for response. At these regular check-ins, the PHQ9A can follow symptom severity. You should monitor changes in function and for any change in suicidal thoughts. If your patient does not respond with at least energy improvement within 4 weeks, you should cross-taper to a different SSRI.
Managing risk
Suicidal thoughts are a common symptom of depression and an important marker of severity. Adolescents have more limited impulse control than do adults, elevating their risk for impulsively acting on these thoughts. Adolescents who are using alcohol or other substances, or who have a history of impulsivity, are at higher risk. Further compounding the degree of risk are a history of suicide attempts, impulsive aggression or psychotic symptoms, or a family history of completed suicide. In managing risk, it is critical that you assess and discuss these risk factors and discuss the need to have a safety plan.
This planning should include both patient and parent. Help the parent to identify lethal means at home (guns, rope, medications, and knives or box cutters) and make plans to secure or remove them. It includes helping your patient list those strategies that can be helpful if they are feeling more distressed (distracting with music or television, exercise, or connecting with select friends). A safety plan is not a promise or a contract to not do something, rather it is a practical set of strategies the patient and family can employ if they are feeling worse. It depends on the adolescent having a secure, trusting connection with the adults at home and with your office.
If your patient fails to improve, if the diagnosis appears complicated, or if you feel the patient is not safe, you should refer to child psychiatry or, if needed, a local emergency department. If you cannot find access to a psychiatrist, start with your state’s child psychiatric consultation hotline for access to telephone support: www.nncpap.org.
Although the suggestions outlined above are grounded in evidence and need, treating moderate to severe depression is likely a new challenge for many pediatricians. Managing the risk of suicide can be stressful, without a doubt. In our own work as child psychiatrists, we recognize that there is no single, reliable method to predict suicide and therefore no specific approach to ensuring prevention. We appreciate this burden of worry when treating a severely depressed adolescent, and follow the rule, “never worry alone” – share your concerns with parents and/or a mental health consultant (hopefully co-located in your office), or obtain a second opinion, even consult a child psychiatrist on a hotline. Offering supportive care for those with mild depression can prevent it from becoming severe, and beginning treatment for those with severe depression can make a profound difference in the course of a young person’s illness.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Pew Research Center. National Survey on Drug Use and Health (2017).
2. Curtin SC. Natl Vital Stat Rep. 2020 Sep;69(11):1-10.
3. Yard E et al. MMWR Morb Mortal Wkly Rep. 2021 Jun 18;70(24):888-94.
4. Jellinek M et al. J Pediatr. 2021 Jun;233:220-6.e1.
5. Zuckerbrot RA et al. Pediatrics. 2018 Mar;141(3):e20174081.
On Oct. 19, the American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and the Children’s Hospital Association jointly declared a “national emergency in children’s mental health,” calling upon policy makers to take actions that could help address “soaring rates” of anxiety and depression.
Knowing that increasing the work force or creating new programs will come slowly if at all, they called for the integration of mental health care into primary care pediatrics and efforts to reduce the risk of suicide in children and adolescents.
Our clinical experience suggests that adolescent depression, which can lead to profoundly impaired function, impaired development, and even suicide, is a major concern in your practice. We hope to do our part by reviewing the screening, diagnosis, and management of depression that can reasonably happen in the pediatrician’s office.
Depression
Depression affects as many as 20% of adolescents, with girls experiencing major depressive disorder (MDD) twice as often as boys. The incidence of depression increases fourfold after puberty, and there is substantial evidence, but no clear cause, that it has increased by nearly 50% over the past decade, rising from a rate of 8% of U.S. adolescents in 2007 to 13% in 2017.1 In that same time period, the rate of completed suicides among U.S. youth aged 10-24 increased 57.4%, after being stable for the prior decade.2 Adolescent depression is also linked to increased substance use and high-risk behaviors such as drunk driving. In 2020, mental health–related emergency department visits by adolescents aged 12-17 increased by 31%. Visits for suicide attempts among adolescent girls in 2021 jumped by 51% from 2019.3 Clearly, MDD in adolescence is a common, potentially life-threatening problem
.
Screening and assessment
At annual checkups with patients 12 and older or at sick visits of patients with emotional, sleep, or vague somatic concerns, it should be standard practice to screen for depression. The Patient Health Questionnaire 9 modified for Adolescents (PHQ9-A) is a reliable, validated, and free screening instrument that your patients can fill out in the waiting room. (The PHQ9 can be used for your patients who are 18 and older.) It takes only 5 minutes to complete and is very easy to score. It establishes whether your patient meets DSM-5 criteria for MDD, and the degree of severity (5-9 is mild, 10-14 is moderate, 15-19 is moderately severe, and 20-27 is severe). It also screens for thoughts about suicide and past suicide attempts. You might add the more comprehensive parent-completed Pediatric Symptom Checklist, which includes a depression screen.4
These screening instruments can be completed electronically prior to or at the visit and should have a preamble explaining why depression screening is relevant. If screening is positive, interview your adolescent patients alone. This will give you the time to gather more detail about how impaired their function is at school, with friends, and in family relationships. Have they been missing school? Have their grades changed? Are they failing to hand in homework? Have they withdrawn from sports or activities? Are they less likely to hang out with friends? Do they participate in family activities? Have others noticed any changes? You should also check for associated anxiety symptoms (ruminative worries, panic attacks) and drug and alcohol use. Of course, you should ask about any suicidal thoughts (from vague morbid thoughts to specific plans, with intent and factors that have prevented them) and actual attempts. Remember, asking about suicidal thoughts and attempts will not cause or worsen them. On the contrary, your patients may feel shame, but will be relieved to not be alone with these thoughts. And this knowledge will be essential as you decide what to do next. When you meet with the parents, ask them about a family history of depression or suicide attempts, and then offer supportive interventions.
Supportive interventions
For all adolescents with depression, supportive interventions are helpful, and for those with mild symptoms, they are often adequate treatment. This begins with education for your patient and their parents about depression. It is an illness, not a problem of character or discipline. Advise your patients that adequate, restful sleep every night is critical to recovery. Regular exercise (daily is best, but at least three times weekly for 30 minutes) is often effective in mild to moderate depression. Patience and compassion for feelings of sadness, irritability, or disinterest are important at home, and maintaining connections with those people who offer support (friends, coaches, parents, etc.) is essential. They should also be told that “depression lies.” Feelings of guilt and self-reproach are a normal part of the illness, not facts. Organizations such as the National Alliance on Mental Illness (NAMI) and the American Academy of Child and Adolescent Psychiatry (AACAP) offer written materials through their websites that are very helpful educational resources. Connect them with sources of counseling support (through school, for example). For those with mild, brief, and uncomplicated depression, supportive interventions alone should offer relief within 4-6 weeks. It is hard to predict the trajectory of depression, so follow-up visits are relevant to determine if they are improving or worsening.
Psychotherapy
For your patients with moderate depression, or with hopelessness or suicidality, a referral for evidence-based psychotherapy is indicated. Both cognitive behavioral therapy and interpersonal therapy have demonstrated efficacy in treating depression in adolescents. If there is a history of trauma or high family conflict, supportive psychotherapy that will enhance communication skills within the family is very important to recovery. Identify various sources for high-quality psychotherapy services (individual, family, and group) in your community. While this may sound easier said than done, online services such as Psychology Today’s therapist locator can help. If your local university has a graduate program in social work or psychology, connect with them as they may have easier access to high-quality services through their training programs. If there is a group practice of therapists in your community, invite them to meet with your team to learn about whether they use evidence-based therapies and can support families as well as individual youth.
Pharmacologic options
For those adolescents with moderate to severe depression, psychotherapy alone is usually inadequate. Indeed, they may be so impaired that they simply cannot meaningfully engage in the work of psychotherapy. These patients require psychopharmacologic treatment first. First-line treatment is with selective serotonin reuptake inhibitors (SSRIs) (both fluoxetine and escitalopram are approved for use in adolescent depression). While many pediatricians remain reluctant about initiating SSRI treatment of depression since the Food and Drug Administration’s 2004 boxed warning was issued, the risks of untreated severe depression are more marked than are the risks of SSRI treatment. As prescription rates dipped in the following decade, rates of suicide attempts in adolescents with severe depression climbed. Subsequent research on the nature of the risk of “increased suicidality” indicated it is substantially lower than originally thought.
The AAP’s Guidelines for Adolescent Depression in Primary Care offer reassuring guidance: They recommend that pediatricians initiate treatment at a very low dose of SSRI (5 mg of fluoxetine, 12.5 mg of sertraline, or 5 mg of escitalopram) and aim to get to a therapeutic dose within 4 weeks.5 Educate the patient and parent about likely side effects (gastrointestinal upset, sleep disruption, akathisia or restlessness, and activation), which indicate the dose should be held steady until the side effects subside. Patients should be seen weekly until they get to a therapeutic dose, then biweekly to monitor for response. At these regular check-ins, the PHQ9A can follow symptom severity. You should monitor changes in function and for any change in suicidal thoughts. If your patient does not respond with at least energy improvement within 4 weeks, you should cross-taper to a different SSRI.
Managing risk
Suicidal thoughts are a common symptom of depression and an important marker of severity. Adolescents have more limited impulse control than do adults, elevating their risk for impulsively acting on these thoughts. Adolescents who are using alcohol or other substances, or who have a history of impulsivity, are at higher risk. Further compounding the degree of risk are a history of suicide attempts, impulsive aggression or psychotic symptoms, or a family history of completed suicide. In managing risk, it is critical that you assess and discuss these risk factors and discuss the need to have a safety plan.
This planning should include both patient and parent. Help the parent to identify lethal means at home (guns, rope, medications, and knives or box cutters) and make plans to secure or remove them. It includes helping your patient list those strategies that can be helpful if they are feeling more distressed (distracting with music or television, exercise, or connecting with select friends). A safety plan is not a promise or a contract to not do something, rather it is a practical set of strategies the patient and family can employ if they are feeling worse. It depends on the adolescent having a secure, trusting connection with the adults at home and with your office.
If your patient fails to improve, if the diagnosis appears complicated, or if you feel the patient is not safe, you should refer to child psychiatry or, if needed, a local emergency department. If you cannot find access to a psychiatrist, start with your state’s child psychiatric consultation hotline for access to telephone support: www.nncpap.org.
Although the suggestions outlined above are grounded in evidence and need, treating moderate to severe depression is likely a new challenge for many pediatricians. Managing the risk of suicide can be stressful, without a doubt. In our own work as child psychiatrists, we recognize that there is no single, reliable method to predict suicide and therefore no specific approach to ensuring prevention. We appreciate this burden of worry when treating a severely depressed adolescent, and follow the rule, “never worry alone” – share your concerns with parents and/or a mental health consultant (hopefully co-located in your office), or obtain a second opinion, even consult a child psychiatrist on a hotline. Offering supportive care for those with mild depression can prevent it from becoming severe, and beginning treatment for those with severe depression can make a profound difference in the course of a young person’s illness.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Pew Research Center. National Survey on Drug Use and Health (2017).
2. Curtin SC. Natl Vital Stat Rep. 2020 Sep;69(11):1-10.
3. Yard E et al. MMWR Morb Mortal Wkly Rep. 2021 Jun 18;70(24):888-94.
4. Jellinek M et al. J Pediatr. 2021 Jun;233:220-6.e1.
5. Zuckerbrot RA et al. Pediatrics. 2018 Mar;141(3):e20174081.
On Oct. 19, the American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and the Children’s Hospital Association jointly declared a “national emergency in children’s mental health,” calling upon policy makers to take actions that could help address “soaring rates” of anxiety and depression.
Knowing that increasing the work force or creating new programs will come slowly if at all, they called for the integration of mental health care into primary care pediatrics and efforts to reduce the risk of suicide in children and adolescents.
Our clinical experience suggests that adolescent depression, which can lead to profoundly impaired function, impaired development, and even suicide, is a major concern in your practice. We hope to do our part by reviewing the screening, diagnosis, and management of depression that can reasonably happen in the pediatrician’s office.
Depression
Depression affects as many as 20% of adolescents, with girls experiencing major depressive disorder (MDD) twice as often as boys. The incidence of depression increases fourfold after puberty, and there is substantial evidence, but no clear cause, that it has increased by nearly 50% over the past decade, rising from a rate of 8% of U.S. adolescents in 2007 to 13% in 2017.1 In that same time period, the rate of completed suicides among U.S. youth aged 10-24 increased 57.4%, after being stable for the prior decade.2 Adolescent depression is also linked to increased substance use and high-risk behaviors such as drunk driving. In 2020, mental health–related emergency department visits by adolescents aged 12-17 increased by 31%. Visits for suicide attempts among adolescent girls in 2021 jumped by 51% from 2019.3 Clearly, MDD in adolescence is a common, potentially life-threatening problem
.
Screening and assessment
At annual checkups with patients 12 and older or at sick visits of patients with emotional, sleep, or vague somatic concerns, it should be standard practice to screen for depression. The Patient Health Questionnaire 9 modified for Adolescents (PHQ9-A) is a reliable, validated, and free screening instrument that your patients can fill out in the waiting room. (The PHQ9 can be used for your patients who are 18 and older.) It takes only 5 minutes to complete and is very easy to score. It establishes whether your patient meets DSM-5 criteria for MDD, and the degree of severity (5-9 is mild, 10-14 is moderate, 15-19 is moderately severe, and 20-27 is severe). It also screens for thoughts about suicide and past suicide attempts. You might add the more comprehensive parent-completed Pediatric Symptom Checklist, which includes a depression screen.4
These screening instruments can be completed electronically prior to or at the visit and should have a preamble explaining why depression screening is relevant. If screening is positive, interview your adolescent patients alone. This will give you the time to gather more detail about how impaired their function is at school, with friends, and in family relationships. Have they been missing school? Have their grades changed? Are they failing to hand in homework? Have they withdrawn from sports or activities? Are they less likely to hang out with friends? Do they participate in family activities? Have others noticed any changes? You should also check for associated anxiety symptoms (ruminative worries, panic attacks) and drug and alcohol use. Of course, you should ask about any suicidal thoughts (from vague morbid thoughts to specific plans, with intent and factors that have prevented them) and actual attempts. Remember, asking about suicidal thoughts and attempts will not cause or worsen them. On the contrary, your patients may feel shame, but will be relieved to not be alone with these thoughts. And this knowledge will be essential as you decide what to do next. When you meet with the parents, ask them about a family history of depression or suicide attempts, and then offer supportive interventions.
Supportive interventions
For all adolescents with depression, supportive interventions are helpful, and for those with mild symptoms, they are often adequate treatment. This begins with education for your patient and their parents about depression. It is an illness, not a problem of character or discipline. Advise your patients that adequate, restful sleep every night is critical to recovery. Regular exercise (daily is best, but at least three times weekly for 30 minutes) is often effective in mild to moderate depression. Patience and compassion for feelings of sadness, irritability, or disinterest are important at home, and maintaining connections with those people who offer support (friends, coaches, parents, etc.) is essential. They should also be told that “depression lies.” Feelings of guilt and self-reproach are a normal part of the illness, not facts. Organizations such as the National Alliance on Mental Illness (NAMI) and the American Academy of Child and Adolescent Psychiatry (AACAP) offer written materials through their websites that are very helpful educational resources. Connect them with sources of counseling support (through school, for example). For those with mild, brief, and uncomplicated depression, supportive interventions alone should offer relief within 4-6 weeks. It is hard to predict the trajectory of depression, so follow-up visits are relevant to determine if they are improving or worsening.
Psychotherapy
For your patients with moderate depression, or with hopelessness or suicidality, a referral for evidence-based psychotherapy is indicated. Both cognitive behavioral therapy and interpersonal therapy have demonstrated efficacy in treating depression in adolescents. If there is a history of trauma or high family conflict, supportive psychotherapy that will enhance communication skills within the family is very important to recovery. Identify various sources for high-quality psychotherapy services (individual, family, and group) in your community. While this may sound easier said than done, online services such as Psychology Today’s therapist locator can help. If your local university has a graduate program in social work or psychology, connect with them as they may have easier access to high-quality services through their training programs. If there is a group practice of therapists in your community, invite them to meet with your team to learn about whether they use evidence-based therapies and can support families as well as individual youth.
Pharmacologic options
For those adolescents with moderate to severe depression, psychotherapy alone is usually inadequate. Indeed, they may be so impaired that they simply cannot meaningfully engage in the work of psychotherapy. These patients require psychopharmacologic treatment first. First-line treatment is with selective serotonin reuptake inhibitors (SSRIs) (both fluoxetine and escitalopram are approved for use in adolescent depression). While many pediatricians remain reluctant about initiating SSRI treatment of depression since the Food and Drug Administration’s 2004 boxed warning was issued, the risks of untreated severe depression are more marked than are the risks of SSRI treatment. As prescription rates dipped in the following decade, rates of suicide attempts in adolescents with severe depression climbed. Subsequent research on the nature of the risk of “increased suicidality” indicated it is substantially lower than originally thought.
The AAP’s Guidelines for Adolescent Depression in Primary Care offer reassuring guidance: They recommend that pediatricians initiate treatment at a very low dose of SSRI (5 mg of fluoxetine, 12.5 mg of sertraline, or 5 mg of escitalopram) and aim to get to a therapeutic dose within 4 weeks.5 Educate the patient and parent about likely side effects (gastrointestinal upset, sleep disruption, akathisia or restlessness, and activation), which indicate the dose should be held steady until the side effects subside. Patients should be seen weekly until they get to a therapeutic dose, then biweekly to monitor for response. At these regular check-ins, the PHQ9A can follow symptom severity. You should monitor changes in function and for any change in suicidal thoughts. If your patient does not respond with at least energy improvement within 4 weeks, you should cross-taper to a different SSRI.
Managing risk
Suicidal thoughts are a common symptom of depression and an important marker of severity. Adolescents have more limited impulse control than do adults, elevating their risk for impulsively acting on these thoughts. Adolescents who are using alcohol or other substances, or who have a history of impulsivity, are at higher risk. Further compounding the degree of risk are a history of suicide attempts, impulsive aggression or psychotic symptoms, or a family history of completed suicide. In managing risk, it is critical that you assess and discuss these risk factors and discuss the need to have a safety plan.
This planning should include both patient and parent. Help the parent to identify lethal means at home (guns, rope, medications, and knives or box cutters) and make plans to secure or remove them. It includes helping your patient list those strategies that can be helpful if they are feeling more distressed (distracting with music or television, exercise, or connecting with select friends). A safety plan is not a promise or a contract to not do something, rather it is a practical set of strategies the patient and family can employ if they are feeling worse. It depends on the adolescent having a secure, trusting connection with the adults at home and with your office.
If your patient fails to improve, if the diagnosis appears complicated, or if you feel the patient is not safe, you should refer to child psychiatry or, if needed, a local emergency department. If you cannot find access to a psychiatrist, start with your state’s child psychiatric consultation hotline for access to telephone support: www.nncpap.org.
Although the suggestions outlined above are grounded in evidence and need, treating moderate to severe depression is likely a new challenge for many pediatricians. Managing the risk of suicide can be stressful, without a doubt. In our own work as child psychiatrists, we recognize that there is no single, reliable method to predict suicide and therefore no specific approach to ensuring prevention. We appreciate this burden of worry when treating a severely depressed adolescent, and follow the rule, “never worry alone” – share your concerns with parents and/or a mental health consultant (hopefully co-located in your office), or obtain a second opinion, even consult a child psychiatrist on a hotline. Offering supportive care for those with mild depression can prevent it from becoming severe, and beginning treatment for those with severe depression can make a profound difference in the course of a young person’s illness.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Pew Research Center. National Survey on Drug Use and Health (2017).
2. Curtin SC. Natl Vital Stat Rep. 2020 Sep;69(11):1-10.
3. Yard E et al. MMWR Morb Mortal Wkly Rep. 2021 Jun 18;70(24):888-94.
4. Jellinek M et al. J Pediatr. 2021 Jun;233:220-6.e1.
5. Zuckerbrot RA et al. Pediatrics. 2018 Mar;141(3):e20174081.
A house divided cannot stand
The United States of America are not united. Politics have polarized the competing monologues and the policy making around vaccines, masks, children returning to school, what children are taught in school, and whether the federal government (or the National Football League) can or should create universal mandates enforcing one extreme of any of those policy disputes. Public health and health care have become so entangled in polarized politics that the role of science has often been pushed aside.
Polarization is not a novel event in the history of governments. The partition of India in 1947 divided most of its Hindu and Muslim inhabitants into separate countries, but that hasn’t stopped the recent resurgence of Hindu nationalism in India. The Thirty Years’ War in Europe sought to decide whether Catholics or Protestants would dominate Western Christianity. Those two sides decided in 1648 that coexistence was wiser than continuing into the abyss of mutual annihilation. Current conflicts between Israelis and Palestinians, between Shia and Sunni Arab states, between China and the Uyghurs, and within Sudan and Ethiopia together demonstrate that polarization to the point of genocide can occur regardless of religion, race, and nationality.
Abraham Lincoln, a lawyer in Illinois with a habit of losing elections, was nominated in 1858 to be the Republican nominee in the U.S. Senate race. His speech accepting the nomination spoke a truth that resonated across the nation and across time. It is known as the House Divided speech. He said: “A house divided against itself cannot stand. I believe this government cannot endure, permanently half slave and half free. I do not expect the Union to be dissolved – I do not expect the house to fall – but I do expect it will cease to be divided. It will become all one thing or all the other.”
The Republican Lincoln, supported by antislavery groups, lost that election to the Democrat Stephen A. Douglas, whose party espoused popular sovereignty and local decision-making about slavery. Lincoln’s acceptance speech propelled him 2 years later to be nominated for and elected President of the United States. Lincoln’s first inaugural address as the President of the United States on March 4, 1861, focused on the issue of division and secession. This time, Lincoln placed much more emphasis on preserving the Union. He specifically renounced any federal efforts to use force to abolish slavery in the states that permitted it. He declared: “I have no purpose, directly or indirectly, to interfere with the institution of slavery in the States where it exists. I believe I have no lawful right to do so, and I have no inclination to do so.”
President Lincoln’s approach might not meet muster in today’s cancel culture. He was facing a precariously divided nation not unlike the current day, so his speech contains insights and wisdom important for today. Lincoln saw government as “a majority held in restraint by constitutional checks and limitations.” I am loath to further quote out of context or paraphrase his masterful words. Go read the original, in its balanced entirety.
I have written previous columns about the importance of taking time to reflect on one’s life and one’s career. Reflection is both a wellness check and a moral compass check. Some call it mindfulness. I lean toward calling it thankfulness and gratitude. Hence, November is a convenient time for pediatricians if flu and respiratory syncytial virus seasons haven’t started.
The Gettysburg Address extols the virtue of dedication. Lincoln’s second inaugural address promotes mercy and forgiveness. His Farewell Address to Springfield in 1861 in a single paragraph captures grief, faith, and hope. Those speeches are my perennial favorites. But this year it is the two aforementioned addresses that must be mined for wisdom.
I advocate vaccine and mask mandates, but I am not enamored with the idea of President Biden using the unchecked power of the executive branch to promulgate a single federal regulation that overreaches into every moderate-size business nationwide. The 1861 inaugural address concurs. Lincoln’s prophecy that division will be solved when one side ultimately wins is not the model I seek. It hasn’t worked for gun control. It hasn’t worked for abortion as we approach the 50th anniversary of Roe v. Wade. The present 50+1 vote majority in the U.S. Senate does not have a mandate to overhaul society, especially when those majorities are transient. One should have the courage to seek change, but beware of creating large divisions with small majorities.
Facebook profits when you meditate in the echo chambers of large, outraged groups. Avoid that. Hebrew tradition has some reflection occurring in groups of two or three, rather than solo. Truth is revealed in community. Voltaire said: “Cherish those who seek the truth but beware of those who find it.” As a scientist, my experience is that humility, skepticism, and a dedication to finding truth have served me well for a lifetime.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
The United States of America are not united. Politics have polarized the competing monologues and the policy making around vaccines, masks, children returning to school, what children are taught in school, and whether the federal government (or the National Football League) can or should create universal mandates enforcing one extreme of any of those policy disputes. Public health and health care have become so entangled in polarized politics that the role of science has often been pushed aside.
Polarization is not a novel event in the history of governments. The partition of India in 1947 divided most of its Hindu and Muslim inhabitants into separate countries, but that hasn’t stopped the recent resurgence of Hindu nationalism in India. The Thirty Years’ War in Europe sought to decide whether Catholics or Protestants would dominate Western Christianity. Those two sides decided in 1648 that coexistence was wiser than continuing into the abyss of mutual annihilation. Current conflicts between Israelis and Palestinians, between Shia and Sunni Arab states, between China and the Uyghurs, and within Sudan and Ethiopia together demonstrate that polarization to the point of genocide can occur regardless of religion, race, and nationality.
Abraham Lincoln, a lawyer in Illinois with a habit of losing elections, was nominated in 1858 to be the Republican nominee in the U.S. Senate race. His speech accepting the nomination spoke a truth that resonated across the nation and across time. It is known as the House Divided speech. He said: “A house divided against itself cannot stand. I believe this government cannot endure, permanently half slave and half free. I do not expect the Union to be dissolved – I do not expect the house to fall – but I do expect it will cease to be divided. It will become all one thing or all the other.”
The Republican Lincoln, supported by antislavery groups, lost that election to the Democrat Stephen A. Douglas, whose party espoused popular sovereignty and local decision-making about slavery. Lincoln’s acceptance speech propelled him 2 years later to be nominated for and elected President of the United States. Lincoln’s first inaugural address as the President of the United States on March 4, 1861, focused on the issue of division and secession. This time, Lincoln placed much more emphasis on preserving the Union. He specifically renounced any federal efforts to use force to abolish slavery in the states that permitted it. He declared: “I have no purpose, directly or indirectly, to interfere with the institution of slavery in the States where it exists. I believe I have no lawful right to do so, and I have no inclination to do so.”
President Lincoln’s approach might not meet muster in today’s cancel culture. He was facing a precariously divided nation not unlike the current day, so his speech contains insights and wisdom important for today. Lincoln saw government as “a majority held in restraint by constitutional checks and limitations.” I am loath to further quote out of context or paraphrase his masterful words. Go read the original, in its balanced entirety.
I have written previous columns about the importance of taking time to reflect on one’s life and one’s career. Reflection is both a wellness check and a moral compass check. Some call it mindfulness. I lean toward calling it thankfulness and gratitude. Hence, November is a convenient time for pediatricians if flu and respiratory syncytial virus seasons haven’t started.
The Gettysburg Address extols the virtue of dedication. Lincoln’s second inaugural address promotes mercy and forgiveness. His Farewell Address to Springfield in 1861 in a single paragraph captures grief, faith, and hope. Those speeches are my perennial favorites. But this year it is the two aforementioned addresses that must be mined for wisdom.
I advocate vaccine and mask mandates, but I am not enamored with the idea of President Biden using the unchecked power of the executive branch to promulgate a single federal regulation that overreaches into every moderate-size business nationwide. The 1861 inaugural address concurs. Lincoln’s prophecy that division will be solved when one side ultimately wins is not the model I seek. It hasn’t worked for gun control. It hasn’t worked for abortion as we approach the 50th anniversary of Roe v. Wade. The present 50+1 vote majority in the U.S. Senate does not have a mandate to overhaul society, especially when those majorities are transient. One should have the courage to seek change, but beware of creating large divisions with small majorities.
Facebook profits when you meditate in the echo chambers of large, outraged groups. Avoid that. Hebrew tradition has some reflection occurring in groups of two or three, rather than solo. Truth is revealed in community. Voltaire said: “Cherish those who seek the truth but beware of those who find it.” As a scientist, my experience is that humility, skepticism, and a dedication to finding truth have served me well for a lifetime.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
The United States of America are not united. Politics have polarized the competing monologues and the policy making around vaccines, masks, children returning to school, what children are taught in school, and whether the federal government (or the National Football League) can or should create universal mandates enforcing one extreme of any of those policy disputes. Public health and health care have become so entangled in polarized politics that the role of science has often been pushed aside.
Polarization is not a novel event in the history of governments. The partition of India in 1947 divided most of its Hindu and Muslim inhabitants into separate countries, but that hasn’t stopped the recent resurgence of Hindu nationalism in India. The Thirty Years’ War in Europe sought to decide whether Catholics or Protestants would dominate Western Christianity. Those two sides decided in 1648 that coexistence was wiser than continuing into the abyss of mutual annihilation. Current conflicts between Israelis and Palestinians, between Shia and Sunni Arab states, between China and the Uyghurs, and within Sudan and Ethiopia together demonstrate that polarization to the point of genocide can occur regardless of religion, race, and nationality.
Abraham Lincoln, a lawyer in Illinois with a habit of losing elections, was nominated in 1858 to be the Republican nominee in the U.S. Senate race. His speech accepting the nomination spoke a truth that resonated across the nation and across time. It is known as the House Divided speech. He said: “A house divided against itself cannot stand. I believe this government cannot endure, permanently half slave and half free. I do not expect the Union to be dissolved – I do not expect the house to fall – but I do expect it will cease to be divided. It will become all one thing or all the other.”
The Republican Lincoln, supported by antislavery groups, lost that election to the Democrat Stephen A. Douglas, whose party espoused popular sovereignty and local decision-making about slavery. Lincoln’s acceptance speech propelled him 2 years later to be nominated for and elected President of the United States. Lincoln’s first inaugural address as the President of the United States on March 4, 1861, focused on the issue of division and secession. This time, Lincoln placed much more emphasis on preserving the Union. He specifically renounced any federal efforts to use force to abolish slavery in the states that permitted it. He declared: “I have no purpose, directly or indirectly, to interfere with the institution of slavery in the States where it exists. I believe I have no lawful right to do so, and I have no inclination to do so.”
President Lincoln’s approach might not meet muster in today’s cancel culture. He was facing a precariously divided nation not unlike the current day, so his speech contains insights and wisdom important for today. Lincoln saw government as “a majority held in restraint by constitutional checks and limitations.” I am loath to further quote out of context or paraphrase his masterful words. Go read the original, in its balanced entirety.
I have written previous columns about the importance of taking time to reflect on one’s life and one’s career. Reflection is both a wellness check and a moral compass check. Some call it mindfulness. I lean toward calling it thankfulness and gratitude. Hence, November is a convenient time for pediatricians if flu and respiratory syncytial virus seasons haven’t started.
The Gettysburg Address extols the virtue of dedication. Lincoln’s second inaugural address promotes mercy and forgiveness. His Farewell Address to Springfield in 1861 in a single paragraph captures grief, faith, and hope. Those speeches are my perennial favorites. But this year it is the two aforementioned addresses that must be mined for wisdom.
I advocate vaccine and mask mandates, but I am not enamored with the idea of President Biden using the unchecked power of the executive branch to promulgate a single federal regulation that overreaches into every moderate-size business nationwide. The 1861 inaugural address concurs. Lincoln’s prophecy that division will be solved when one side ultimately wins is not the model I seek. It hasn’t worked for gun control. It hasn’t worked for abortion as we approach the 50th anniversary of Roe v. Wade. The present 50+1 vote majority in the U.S. Senate does not have a mandate to overhaul society, especially when those majorities are transient. One should have the courage to seek change, but beware of creating large divisions with small majorities.
Facebook profits when you meditate in the echo chambers of large, outraged groups. Avoid that. Hebrew tradition has some reflection occurring in groups of two or three, rather than solo. Truth is revealed in community. Voltaire said: “Cherish those who seek the truth but beware of those who find it.” As a scientist, my experience is that humility, skepticism, and a dedication to finding truth have served me well for a lifetime.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Aaron Beck: An appreciation
He always dressed the same at conferences: dark suit, white shirt, bright red bow tie.
For all his fame, he was very kind, warmly greeting those who wanted to see him and immediately turning attention toward their research rather than his own. Aaron Beck actually didn’t lecture much; he preferred to roleplay cognitive therapy with an audience member acting as the patient. He would engage in what he called Socratic questioning, or more formally, cognitive restructuring, with warmth and true curiosity:
- What might be another explanation or viewpoint?
- What are the effects of thinking this way?
- Can you think of any evidence that supports the opposite view?
The audience member/patient would benefit not only from thinking about things differently, but also from the captivating interaction with the man, Aaron Temkin Beck, MD, (who went by Tim), youngest child of Jewish immigrants from the Ukraine.
When written up in treatment manuals, cognitive restructuring can seem cold and overly logical, but in person, Dr. Beck made it come to life. This ability to nurture curiosity was a special talent; his friend and fellow cognitive psychologist Donald Meichenbaum, PhD, recalls that even over lunch, he never stopped asking questions, personal and professional, on a wide range of topics.
It is widely accepted that Dr. Beck, who died Nov. 1 at the age of 100 in suburban Philadelphia, was the most important figure in the field of cognitive-behavioral therapy (CBT).
He didn’t invent the field. Behaviorism predated him by generations, founded by figures such as John Watson and B.F. Skinner. Those psychologists set up behaviorism as an alternative to the reigning power of Freudian psychoanalysis, but they ran a distant second.
It wasn’t until Dr. Beck added a new approach, cognitive therapy, to the behavioristic movement that the new mélange, CBT, began to gain traction with clinicians and researchers. Dr. Beck, who had trained in psychiatry, developed his ideas in the 1960s while observing what he believed were limitations in the classic Freudian methods. He recognized that patients had “automatic thoughts,” not just unconscious emotions, when they engaged in Freudian free association, saying whatever came to their minds.
These thoughts often distorted reality, he observed; they were “maladaptive beliefs,” and when they changed, patients’ emotional states improved.
Dr. Beck wasn’t alone. The psychologist Albert Ellis, PhD, in New York, had come to similar conclusions a decade earlier, though with a more coldly logical and challenging style. The prominent British psychologist Hans Eysenck, PhD, had argued strongly that Freudian psychoanalysis was ineffective and that behavioral approaches were better.
Dr. Beck turned the Freudian equation around: Instead of emotion as cause and thought as effect, it was thought which affected emotion, for better or worse. Once you connected behavior as the outcome, you had the essence of CBT: thought, emotion, and behavior – each affecting the other, with thought being the strongest axis of change.
The process wasn’t bloodless. Behaviorists defended their turf against cognitivists, just as much as Freudians rejected both. At one point the behaviorists in the Association for the Advancement of Behavior Therapy tried to expel the advocates of a cognitive approach. Dr. Beck responded by leading the cognitivists in creating a new journal; he emphasized the importance of research being the main mechanism to decide what treatments worked the best.
Putting these ideas out in the 1960s and 1970s, Dr. Beck garnered support from researchers when he manualized the approach. Freudian psychoanalysis was idiosyncratic; it was almost impossible to study empirically, because the therapist would be responding to the unpredictable dreams and memories of patients engaged in free association. Each case was unique.
But CBT was systematic: The same general approach was taken to all patients; the same negative cognitions were found in depression, for instance, like all-or-nothing thinking or overgeneralization. Once manualized, CBT became the standard method of psychotherapy studied with the newly developed method of randomized controlled trials (RCTs).
By the 1980s, RCTs had proven the efficacy of CBT in depression, and the approach took off.
Dr. Beck already had developed a series of rating scales: the Beck Depression Inventory, the Beck Scale for Suicidal Ideation, the Beck Anxiety Inventory, the Beck Hopelessness Scale. Widely used, these scales extended his influence enormously. Copyrighted, they created a new industry of psychological research.
Dr. Beck’s own work was mainly in depression, but his followers extended it everywhere else: anxiety disorders and phobias, eating disorders, substance abuse, bipolar illness, even schizophrenia. Meanwhile, Freudian psychoanalysis fell into a steep decline from which it never recovered.
Some argued that it was abetted by insurance restrictions on psychotherapy, which favored shorter-term CBT; others that its research was biased in its favor because psychotherapy treatments, unlike medications, cannot be blinded; others that its efficacy could not be shown to be specific to its theory, as opposed to the interpersonal relationship between therapist and client.
Still, CBT has transformed psychotherapy and continues to expand its influence. Computer-based CBT has been proven effective, and digital CBT has become a standard approach in many smartphone applications and is central to the claims of multiple new biotechnology companies advocating for digital psychotherapy.
Aaron Beck continued publishing scientific articles to age 98. His last papers reviewed his life’s work. He characteristically gave credit to others, calmly recollected how he traveled away from psychoanalysis, described how his work started and ended in schizophrenia, and noted that the “working relationship with the therapist” remained a key factor for the success of CBT.
That parting comment reminds us that behind all the technology and research stands the kindly man in the dark suit, white shirt, and bright red bow tie, looking at you warmly, asking about your thoughts, and curiously wondering what might be another explanation or viewpoint you hadn’t considered.
Nassir Ghaemi, MD, MPH, is a professor of psychiatry at Tufts Medical Center and a lecturer in psychiatry at Harvard Medical School. He is the author of several general-interest books on psychiatry. A version of this article first appeared on Medscape.com.
He always dressed the same at conferences: dark suit, white shirt, bright red bow tie.
For all his fame, he was very kind, warmly greeting those who wanted to see him and immediately turning attention toward their research rather than his own. Aaron Beck actually didn’t lecture much; he preferred to roleplay cognitive therapy with an audience member acting as the patient. He would engage in what he called Socratic questioning, or more formally, cognitive restructuring, with warmth and true curiosity:
- What might be another explanation or viewpoint?
- What are the effects of thinking this way?
- Can you think of any evidence that supports the opposite view?
The audience member/patient would benefit not only from thinking about things differently, but also from the captivating interaction with the man, Aaron Temkin Beck, MD, (who went by Tim), youngest child of Jewish immigrants from the Ukraine.
When written up in treatment manuals, cognitive restructuring can seem cold and overly logical, but in person, Dr. Beck made it come to life. This ability to nurture curiosity was a special talent; his friend and fellow cognitive psychologist Donald Meichenbaum, PhD, recalls that even over lunch, he never stopped asking questions, personal and professional, on a wide range of topics.
It is widely accepted that Dr. Beck, who died Nov. 1 at the age of 100 in suburban Philadelphia, was the most important figure in the field of cognitive-behavioral therapy (CBT).
He didn’t invent the field. Behaviorism predated him by generations, founded by figures such as John Watson and B.F. Skinner. Those psychologists set up behaviorism as an alternative to the reigning power of Freudian psychoanalysis, but they ran a distant second.
It wasn’t until Dr. Beck added a new approach, cognitive therapy, to the behavioristic movement that the new mélange, CBT, began to gain traction with clinicians and researchers. Dr. Beck, who had trained in psychiatry, developed his ideas in the 1960s while observing what he believed were limitations in the classic Freudian methods. He recognized that patients had “automatic thoughts,” not just unconscious emotions, when they engaged in Freudian free association, saying whatever came to their minds.
These thoughts often distorted reality, he observed; they were “maladaptive beliefs,” and when they changed, patients’ emotional states improved.
Dr. Beck wasn’t alone. The psychologist Albert Ellis, PhD, in New York, had come to similar conclusions a decade earlier, though with a more coldly logical and challenging style. The prominent British psychologist Hans Eysenck, PhD, had argued strongly that Freudian psychoanalysis was ineffective and that behavioral approaches were better.
Dr. Beck turned the Freudian equation around: Instead of emotion as cause and thought as effect, it was thought which affected emotion, for better or worse. Once you connected behavior as the outcome, you had the essence of CBT: thought, emotion, and behavior – each affecting the other, with thought being the strongest axis of change.
The process wasn’t bloodless. Behaviorists defended their turf against cognitivists, just as much as Freudians rejected both. At one point the behaviorists in the Association for the Advancement of Behavior Therapy tried to expel the advocates of a cognitive approach. Dr. Beck responded by leading the cognitivists in creating a new journal; he emphasized the importance of research being the main mechanism to decide what treatments worked the best.
Putting these ideas out in the 1960s and 1970s, Dr. Beck garnered support from researchers when he manualized the approach. Freudian psychoanalysis was idiosyncratic; it was almost impossible to study empirically, because the therapist would be responding to the unpredictable dreams and memories of patients engaged in free association. Each case was unique.
But CBT was systematic: The same general approach was taken to all patients; the same negative cognitions were found in depression, for instance, like all-or-nothing thinking or overgeneralization. Once manualized, CBT became the standard method of psychotherapy studied with the newly developed method of randomized controlled trials (RCTs).
By the 1980s, RCTs had proven the efficacy of CBT in depression, and the approach took off.
Dr. Beck already had developed a series of rating scales: the Beck Depression Inventory, the Beck Scale for Suicidal Ideation, the Beck Anxiety Inventory, the Beck Hopelessness Scale. Widely used, these scales extended his influence enormously. Copyrighted, they created a new industry of psychological research.
Dr. Beck’s own work was mainly in depression, but his followers extended it everywhere else: anxiety disorders and phobias, eating disorders, substance abuse, bipolar illness, even schizophrenia. Meanwhile, Freudian psychoanalysis fell into a steep decline from which it never recovered.
Some argued that it was abetted by insurance restrictions on psychotherapy, which favored shorter-term CBT; others that its research was biased in its favor because psychotherapy treatments, unlike medications, cannot be blinded; others that its efficacy could not be shown to be specific to its theory, as opposed to the interpersonal relationship between therapist and client.
Still, CBT has transformed psychotherapy and continues to expand its influence. Computer-based CBT has been proven effective, and digital CBT has become a standard approach in many smartphone applications and is central to the claims of multiple new biotechnology companies advocating for digital psychotherapy.
Aaron Beck continued publishing scientific articles to age 98. His last papers reviewed his life’s work. He characteristically gave credit to others, calmly recollected how he traveled away from psychoanalysis, described how his work started and ended in schizophrenia, and noted that the “working relationship with the therapist” remained a key factor for the success of CBT.
That parting comment reminds us that behind all the technology and research stands the kindly man in the dark suit, white shirt, and bright red bow tie, looking at you warmly, asking about your thoughts, and curiously wondering what might be another explanation or viewpoint you hadn’t considered.
Nassir Ghaemi, MD, MPH, is a professor of psychiatry at Tufts Medical Center and a lecturer in psychiatry at Harvard Medical School. He is the author of several general-interest books on psychiatry. A version of this article first appeared on Medscape.com.
He always dressed the same at conferences: dark suit, white shirt, bright red bow tie.
For all his fame, he was very kind, warmly greeting those who wanted to see him and immediately turning attention toward their research rather than his own. Aaron Beck actually didn’t lecture much; he preferred to roleplay cognitive therapy with an audience member acting as the patient. He would engage in what he called Socratic questioning, or more formally, cognitive restructuring, with warmth and true curiosity:
- What might be another explanation or viewpoint?
- What are the effects of thinking this way?
- Can you think of any evidence that supports the opposite view?
The audience member/patient would benefit not only from thinking about things differently, but also from the captivating interaction with the man, Aaron Temkin Beck, MD, (who went by Tim), youngest child of Jewish immigrants from the Ukraine.
When written up in treatment manuals, cognitive restructuring can seem cold and overly logical, but in person, Dr. Beck made it come to life. This ability to nurture curiosity was a special talent; his friend and fellow cognitive psychologist Donald Meichenbaum, PhD, recalls that even over lunch, he never stopped asking questions, personal and professional, on a wide range of topics.
It is widely accepted that Dr. Beck, who died Nov. 1 at the age of 100 in suburban Philadelphia, was the most important figure in the field of cognitive-behavioral therapy (CBT).
He didn’t invent the field. Behaviorism predated him by generations, founded by figures such as John Watson and B.F. Skinner. Those psychologists set up behaviorism as an alternative to the reigning power of Freudian psychoanalysis, but they ran a distant second.
It wasn’t until Dr. Beck added a new approach, cognitive therapy, to the behavioristic movement that the new mélange, CBT, began to gain traction with clinicians and researchers. Dr. Beck, who had trained in psychiatry, developed his ideas in the 1960s while observing what he believed were limitations in the classic Freudian methods. He recognized that patients had “automatic thoughts,” not just unconscious emotions, when they engaged in Freudian free association, saying whatever came to their minds.
These thoughts often distorted reality, he observed; they were “maladaptive beliefs,” and when they changed, patients’ emotional states improved.
Dr. Beck wasn’t alone. The psychologist Albert Ellis, PhD, in New York, had come to similar conclusions a decade earlier, though with a more coldly logical and challenging style. The prominent British psychologist Hans Eysenck, PhD, had argued strongly that Freudian psychoanalysis was ineffective and that behavioral approaches were better.
Dr. Beck turned the Freudian equation around: Instead of emotion as cause and thought as effect, it was thought which affected emotion, for better or worse. Once you connected behavior as the outcome, you had the essence of CBT: thought, emotion, and behavior – each affecting the other, with thought being the strongest axis of change.
The process wasn’t bloodless. Behaviorists defended their turf against cognitivists, just as much as Freudians rejected both. At one point the behaviorists in the Association for the Advancement of Behavior Therapy tried to expel the advocates of a cognitive approach. Dr. Beck responded by leading the cognitivists in creating a new journal; he emphasized the importance of research being the main mechanism to decide what treatments worked the best.
Putting these ideas out in the 1960s and 1970s, Dr. Beck garnered support from researchers when he manualized the approach. Freudian psychoanalysis was idiosyncratic; it was almost impossible to study empirically, because the therapist would be responding to the unpredictable dreams and memories of patients engaged in free association. Each case was unique.
But CBT was systematic: The same general approach was taken to all patients; the same negative cognitions were found in depression, for instance, like all-or-nothing thinking or overgeneralization. Once manualized, CBT became the standard method of psychotherapy studied with the newly developed method of randomized controlled trials (RCTs).
By the 1980s, RCTs had proven the efficacy of CBT in depression, and the approach took off.
Dr. Beck already had developed a series of rating scales: the Beck Depression Inventory, the Beck Scale for Suicidal Ideation, the Beck Anxiety Inventory, the Beck Hopelessness Scale. Widely used, these scales extended his influence enormously. Copyrighted, they created a new industry of psychological research.
Dr. Beck’s own work was mainly in depression, but his followers extended it everywhere else: anxiety disorders and phobias, eating disorders, substance abuse, bipolar illness, even schizophrenia. Meanwhile, Freudian psychoanalysis fell into a steep decline from which it never recovered.
Some argued that it was abetted by insurance restrictions on psychotherapy, which favored shorter-term CBT; others that its research was biased in its favor because psychotherapy treatments, unlike medications, cannot be blinded; others that its efficacy could not be shown to be specific to its theory, as opposed to the interpersonal relationship between therapist and client.
Still, CBT has transformed psychotherapy and continues to expand its influence. Computer-based CBT has been proven effective, and digital CBT has become a standard approach in many smartphone applications and is central to the claims of multiple new biotechnology companies advocating for digital psychotherapy.
Aaron Beck continued publishing scientific articles to age 98. His last papers reviewed his life’s work. He characteristically gave credit to others, calmly recollected how he traveled away from psychoanalysis, described how his work started and ended in schizophrenia, and noted that the “working relationship with the therapist” remained a key factor for the success of CBT.
That parting comment reminds us that behind all the technology and research stands the kindly man in the dark suit, white shirt, and bright red bow tie, looking at you warmly, asking about your thoughts, and curiously wondering what might be another explanation or viewpoint you hadn’t considered.
Nassir Ghaemi, MD, MPH, is a professor of psychiatry at Tufts Medical Center and a lecturer in psychiatry at Harvard Medical School. He is the author of several general-interest books on psychiatry. A version of this article first appeared on Medscape.com.
From bored to springboard
A weekend, for most of us in solo practice, doesn’t really signify time off from work. It just means we’re not seeing patients at the office.
There’s always business stuff to do like payroll and paying bills, records to review, the never-ending forms for a million things, and all the other stuff there never seems to be enough time to do on weekdays.
This weekend I started attacking the pile after dinner on Friday and found myself done by Saturday afternoon, which is rare. Usually I spend the better part of a weekend at my desk.
And then, unexpectedly faced with an empty desk, I found myself wondering what to do next.
Boredom is one of the odder human conditions. I have no idea if any other animal experiences it. Certainly, at least for us, there are more ways to entertain ourselves now than there ever have been – TV, Netflix, phone games, TikTok, books, just to name a few.
But do we always have to be entertained? Many great scientists have said that world-changing ideas have come to them when they weren’t working, such as while showering or riding to work. Leo Szilard was crossing a London street in 1933 when he suddenly saw how a nuclear chain reaction would be self-sustaining once initiated. Fortunately he wasn’t hit by a car in the process.
But I’m not Szilard. So I rationalized a reason not to exercise and sat on the couch with a book.
The remarkable human brain doesn’t shut down easily. With nothing else to do, most mammals tend do doze off. But not us. Our brains are always on, trying to think of the next goal, the next move, the next whatever.
Having nothing to do sounds like a great idea, until you have nothing to do. It may be fine for a few days, but after a while you realize there’s only so long you can stare at the waves or mountains before your mind turns back to “what’s next.” Many patients tell me how retirement sounded good until they got there and then found themselves volunteering or taking new jobs just to keep busy.
This isn’t a bad thing. Being bored is probably constructive. Without realizing it we use it to form new ideas and start new plans.
Maybe this is why we are where we are. Perhaps it’s this feature that pushed the development of intelligence further and led us to form civilizations.
It’s how we keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A weekend, for most of us in solo practice, doesn’t really signify time off from work. It just means we’re not seeing patients at the office.
There’s always business stuff to do like payroll and paying bills, records to review, the never-ending forms for a million things, and all the other stuff there never seems to be enough time to do on weekdays.
This weekend I started attacking the pile after dinner on Friday and found myself done by Saturday afternoon, which is rare. Usually I spend the better part of a weekend at my desk.
And then, unexpectedly faced with an empty desk, I found myself wondering what to do next.
Boredom is one of the odder human conditions. I have no idea if any other animal experiences it. Certainly, at least for us, there are more ways to entertain ourselves now than there ever have been – TV, Netflix, phone games, TikTok, books, just to name a few.
But do we always have to be entertained? Many great scientists have said that world-changing ideas have come to them when they weren’t working, such as while showering or riding to work. Leo Szilard was crossing a London street in 1933 when he suddenly saw how a nuclear chain reaction would be self-sustaining once initiated. Fortunately he wasn’t hit by a car in the process.
But I’m not Szilard. So I rationalized a reason not to exercise and sat on the couch with a book.
The remarkable human brain doesn’t shut down easily. With nothing else to do, most mammals tend do doze off. But not us. Our brains are always on, trying to think of the next goal, the next move, the next whatever.
Having nothing to do sounds like a great idea, until you have nothing to do. It may be fine for a few days, but after a while you realize there’s only so long you can stare at the waves or mountains before your mind turns back to “what’s next.” Many patients tell me how retirement sounded good until they got there and then found themselves volunteering or taking new jobs just to keep busy.
This isn’t a bad thing. Being bored is probably constructive. Without realizing it we use it to form new ideas and start new plans.
Maybe this is why we are where we are. Perhaps it’s this feature that pushed the development of intelligence further and led us to form civilizations.
It’s how we keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A weekend, for most of us in solo practice, doesn’t really signify time off from work. It just means we’re not seeing patients at the office.
There’s always business stuff to do like payroll and paying bills, records to review, the never-ending forms for a million things, and all the other stuff there never seems to be enough time to do on weekdays.
This weekend I started attacking the pile after dinner on Friday and found myself done by Saturday afternoon, which is rare. Usually I spend the better part of a weekend at my desk.
And then, unexpectedly faced with an empty desk, I found myself wondering what to do next.
Boredom is one of the odder human conditions. I have no idea if any other animal experiences it. Certainly, at least for us, there are more ways to entertain ourselves now than there ever have been – TV, Netflix, phone games, TikTok, books, just to name a few.
But do we always have to be entertained? Many great scientists have said that world-changing ideas have come to them when they weren’t working, such as while showering or riding to work. Leo Szilard was crossing a London street in 1933 when he suddenly saw how a nuclear chain reaction would be self-sustaining once initiated. Fortunately he wasn’t hit by a car in the process.
But I’m not Szilard. So I rationalized a reason not to exercise and sat on the couch with a book.
The remarkable human brain doesn’t shut down easily. With nothing else to do, most mammals tend do doze off. But not us. Our brains are always on, trying to think of the next goal, the next move, the next whatever.
Having nothing to do sounds like a great idea, until you have nothing to do. It may be fine for a few days, but after a while you realize there’s only so long you can stare at the waves or mountains before your mind turns back to “what’s next.” Many patients tell me how retirement sounded good until they got there and then found themselves volunteering or taking new jobs just to keep busy.
This isn’t a bad thing. Being bored is probably constructive. Without realizing it we use it to form new ideas and start new plans.
Maybe this is why we are where we are. Perhaps it’s this feature that pushed the development of intelligence further and led us to form civilizations.
It’s how we keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Does the use of frankincense make sense in dermatology?
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
Q&A: Meeting the challenge of giving COVID vaccines to younger kids
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.