User login
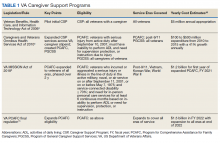
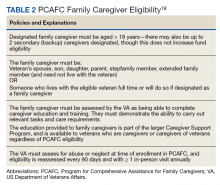
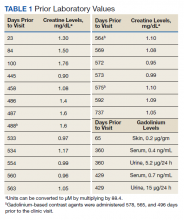
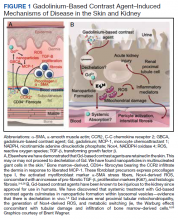
Stories of the Heart: Illness Narratives of Veterans Living With Heart Failure
Heart failure (HF) is a costly and burdensome illness and is the top reason for hospital admissions for the US Department of Veterans Affairs (VA) and Medicare.1 The cost of HF to the United States is estimated to grow to $3 billion annually by 2030.2 People living with HF have a high symptom burden and poor quality of life.3,4 Symptoms include shortness of breath, fatigue, depression, and decreases in psychosocial, existential, and spiritual well-being.5-9
Veterans in the US are a unique cultural group with distinct contextual considerations around their experiences.10 Different groups of veterans require unique cultural considerations, such as the experiences of veterans who served during the Vietnam war and during Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF). The extent of unmet needs of people living with HF, the number of veterans living with this illness, and the unique contextual components related to living with HF among veterans require further exploration into this illness experience for this distinct population. Research should explore innovative ways of managing both the number of people living with the illness and the significant impact of HF in people’s lives due to the high symptom burden and poor quality of life.3
This study used the model of adjustment to illness to explore the psychosocial adjustment to illness and the experience of US veterans living with HF, with a focus on the domains of meaning creation, self-schema, and world schema.11 The model of adjustment to illness describes how people learn to adjust to living with an illness, which can lead to positive health outcomes. Meaning creation is defined as the process in which people create meaning from their experience living with illness. Self-schema is how people living with illness see themselves, and world schema is how people living with chronic illness see their place in the world. These domains shift as part of the adjustment to living with an illness described in this model.11 This foundation allowed the investigators to explore the experience of living with HF among veterans with a focus on these domains. Our study aimed to cocreate illness narratives among veterans living with HF and to explore components of psychosocial adjustment informed by the model.
Methods
This study used narrative inquiry with a focus on illness narratives.12-17 Narrative inquiry as defined by Catherine Riessman involves the generation of socially constructed and cocreated meanings between the researcher and narrator. The researcher is an active participant in narrative creation as the narrator chooses which events to include in the stories based on the social, historical, and cultural context of both the narrator (study participant) and audience (researcher). Riessman describes the importance of contextual factors and meaning creation as an important aspect of narrative inquiry.12-14,16,17 It is important in narrative inquiry to consider how cultural, social, and historical factors influence narrative creation, constriction, and/or elimination.
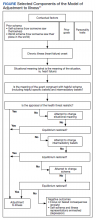
This study prospectively created and collected data at a single time point. Semi-structured interviews explored psychosocial adjustment for people living with HF using an opening question modified from previous illness narrative research: Why do you think you got heart failure?18 Probes included the domains of psychosocial adjustment informed by the model of adjustment to illness domains (Figure). Emergent probes were used to illicit additional data around psychosocial adjustment to illness. Data were created and collected in accordance with narrative inquiry during the cocreation of the illness narratives between the researcher and study participants. This interview guide was tested by the first author in preliminary work to prepare for this study.
Allowing for emergent probes and acknowledging the role of the researcher as audience is key to the cocreation of narratives using this methodological framework. Narrators shape their narrative with the audience in mind; they cocreate their narrative with their audience using this type of narrative inquiry.12,16 What the narrator chooses to include and exclude from their story provides a window into how they see themselves and their world.19 Audio recordings were used to capture data, allowing for the researcher to take contemporaneous notes exploring contextual considerations to the narrative cocreation process and to be used later in analysis. Analytic notes were completed during the interviews as well as later in analysis as part of the contextual reflection.
Setting
Research was conducted in the Rocky Mountain Regional VA Medical Center, Aurora, Colorado. Participants were recruited through the outpatient cardiology clinic where the interviews also took place. This study was approved through the Colorado Institutional Review Board and Rocky Mountain Regional VA Medical Center (IRB: 19-1064). Participants were identified by the treating cardiologist who was a part of the study team. Interested veterans were introduced to the first author who was stationed in an empty clinic room. The study cardiologist screened to ensure all participants were ≥ 18 years of age and had a diagnosis of HF for > 1 year. Persons with an impairment that could interfere with their ability to construct a narrative were also excluded.
Recruitment took place from October 2019 to January 2020. Three veterans refused participation. Five study participants provided informed consent and were enrolled and interviewed. All interviews were completed in the clinic at the time of consent per participant preference. One-hour long semi-structured interviews were conducted and audio recorded. A demographic form was administered at the end of each interview to capture contextual data. The researcher also kept a reflexive journal and audit trail.
Narrative Analysis
Riessman described general steps to conduct narrative analysis, including transcription, narrative clean-up, consideration of contextual factors, exploration of thematic threads, consideration of larger social narratives, and positioning.12 The first author read transcripts while listening to the audio recordings to ensure accuracy. With narrative clean-up each narrative was organized to cocreate overall meaning, changed to protect anonymity, and refined to only include the illness narrative. For example, if a narrator told a story about childhood and then later in the interview remembered another detail to add to their story, narrative clean-up reordered events to make cohesive sense of the story. Demographic, historical, cultural, and social contexts of both the narrator and audience were reflected on during analysis to explore how these components may have shaped and influenced cocreation. Context was also considered within the larger VA setting.
Emergent themes were explored for convergence, divergence, and points of tension within and across each narrative. Larger social narratives were also considered for their influence on possible inclusion/exclusion of experience, such as how gender identity may have influenced study participants’ descriptions of their roles in social systems. These themes and narratives were then shared with our team, and we worked through decision points during the analysis process and discussed interpretation of the data to reach consensus.
Results
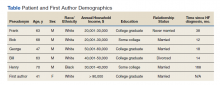
Five veterans living with HF were recruited and consented to participate in the study. Demographics of the participants and first author are included in the Table. Five illness narratives were cocreated, entitled: Blame the Cheese: Frank’s Illness Narrative; Love is Love: Bob’s Illness Narrative; The Brighter Things in Life is My Family: George’s Illness Narrative; We Never Know When Our Time is Coming: Bill’s Illness Narrative; and A Dream Deferred: Henry’s Illness Narrative.
Each narrative was explored focusing on the domains of the model of adjustment to illness. An emergent theme was also identified with multiple subthemes: being a veteran is unique. Related subthemes included: financial benefits, intersectionality of government and health care, the intersectionality of masculinity and military service, and the dichotomy of military experience.
The search for meaning creation after the experience of chronic illness emerged across interviews. One example of meaning creation was in Frank's illness narrative. Frank was unsure why he got HF: “Probably because I ate too much cheese…I mean, that’s gotta be it. It can’t be anything else.” By tying HF to his diet, he found meaning through his health behaviors.
Model of Adjustment to Illness
The narratives illustrate components of the model of adjustment to illness and describe how each of the participants either shifted their self-schema and world schema or reinforced their previously established schemas. It also demonstrates how people use narratives to create meaning and illness understanding from their illness experience, reflecting, and emphasizing different parts creating meaning from their experience.
A commonality across the narratives was a shift in self-schema, including the shift from being a provider to being reliant on others. In accordance with the dominant social narrative around men as providers, each narrator talked about their identity as a provider for themselves and their families. Often keeping their provider identity required modifications of the definition, from physical abilities and employment to financial security and stability. George made all his health care decisions based on his goal of providing for his family and protecting them from having to care for him: “I’m always thinking about the future, always trying to figure out how my family, if something should happen to me, how my family would cope, and how my family would be able to support themselves.” Bob’s health care goals were to stay alive long enough for his wife to get financial benefits as a surviving spouse: “That’s why I’m trying to make everything for her, you know. I’m not worried about myself. I’m not. Her I am, you know. And love is love.” Both of their health care decisions are shaped by their identity as a provider shifting to financial support.
Some narrators changed the way they saw their world, or world schema, while others felt their illness experience just reinforced the way they had already experienced the world. Frank was able to reprioritize what was important to him after his diagnosis and accept his own mortality: “I might as well chill out, no more stress, and just enjoy things ’cause you could die…” For Henry, getting HF was only part of the experience of systemic oppression that had impacted his and his family’s lives for generations. He saw how his oppression by the military and US government led to his father’s exposure to chemicals that Henry believed he inherited and caused his illness. Henry’s illness experience reinforced his distrust in the institutions that were oppressed him and his family.
Veteran Status
Being a veteran in the Veterans Health Administration (VHA) system impacted how a narrative understanding of illness was created. Veterans are a unique cultural population with aspects of their illness experience that are important to understand.10 Institutions such as the VA also enable and constrain components of narrative creation.20 The illness narratives in this study were cocreated within the institutional setting of the VA. Part of the analysis included exploring how the institutional setting impacted the narrative creation. Emergent subthemes of the uniqueness of the veteran experience include financial benefits, intersectionality of government and health care, intersectionality of masculinity and military service, and the dichotomy of military experience.
In the US it is unique to the VA that the government both treats and assesses the severity of medical conditions to determine eligibility for health care and financial benefits. The VA’s financial benefits are intended to help compensate veterans who are experiencing illness as a result of their military experience.21 However, because the VA administers them the Veterans Benefits Administration and the VHA, veterans see both as interconnected. The perceived tie between illness severity and financial compensation could influence or bias how veterans understand their illness severity and experience. This may inadvertently encourage veterans to see their illness as being tied to their military service. This shaping of narratives should be considered as a contextual component as veterans obtain financial compensation and health insurance from the same larger organization that provides their health care and management.
George was a young man who during his service had chest pains and felt tired during physical training. He was surprised when his cardiologist explained his heart was enlarged. “All I know is when I initially joined the military, I was perfectly fine, you know, and when I was in the military, graduating, all that stuff, there was a glitch on the [electrocardiogram] they gave me after one day of doing [physical training] and then they’re like, oh, that’s fine. Come to find out it was mitral valve prolapse. And the doctors didn’t catch it then.” George feels the stress of the military caused his heart problems: “It wasn’t there before… so I’d have to say the strain from the military had to have caused it.” George’s medical history noted that he has a genetic connective tissue disorder that can lead to HF and likely was underlying cause of his illness. This example of how George pruned his narrative experience to highlight the cause as his military experience instead of a genetic disorder could have multiple financial and health benefits. The financial incentive for George to see his illness as caused by his military service could potentially bias his illness narrative to find his illness cause as tied to his service.
Government/Health Care Intersectionality
Veterans who may have experienced trust-breaking events with the government, like Agent Orange exposure or intergenerational racial trauma, may apply that experience to all government agencies. Bob felt the government had purposefully used him to create a military weapon. The army “knew I was angry and they used that for their advantage,” he said. Bob learned that he was exposed to Agent Orange in Vietnam, which is presumed to be associated with HF. Bob felt betrayed that the VHA had not figured out his health problems earlier. “I didn’t know anything about it until 6 months ago… Our government knew about it when they used it, and they didn’t care. They just wanted to win the war, and a whole lot of GIs like me suffered because of that, and I was like my government killed me? And I was fighting for them?”
Henry learned to distrust the government and the health care system because of a long history of systematic oppression and exploitation. These institutions’ erosion of trust has impact beyond the trust-breaking event itself but reverberates into how communities view organizations and institutions for generations. For Black Americans, who have historically been experimented on without consent by the US government and health care systems, this can make it especially hard to trust and build working relationships with those institutions. Health care professionals (HCPs) need to build collaborative partnerships with patients to provide effective care while understanding why some patients may have difficulty trusting health care systems, especially government-led systems.
The nature of HF as an illness can also make it difficult to predict and manage.22 This uncertainty and difficulty in managing HF can make it especially hard for people to establish trust with their HCPs whom they want to see as experts in their illness. HCPs in these narratives were often portrayed as incompetent or neglectful. The unpredictable nature of the illness itself was not reflected in the narrator’s experience.
Masculinity/Military Service Intersectionality
For the veteran narrators, tied into the identity of being a provider are social messages about masculinity. There is a unique intersectionality of being a man, the military culture, and living with chronic illnesses. Dominant social messages around being a man include being tough, not expressing emotion, self-reliance, and having power. This overlaps with social messages on military culture, including self-reliance, toughness, persistence in the face of adversity, limited expression of emotions, and the recognition of power and respect.23
People who internalize these social messages on masculinity may be less likely to access mental health treatment.23 This stigmatizing barrier to mental health treatment could impact how positive narratives are constructed around the experience of chronic illness for narrators who identify as masculine. Military and masculine identity could exclude or constrain stories about a veteran who did not “solider on” or who had to rely on others in a team to get things done. This shift can especially impact veterans experiencing chronic illnesses like HF, which often impact their physical abilities. Veterans may feel pressured to think of and portray themselves as being strong by limiting their expression of pain and other symptoms to remain in alignment with the dominant narrative. By not being open about the full experience of their illness both positive and negative, veterans may have unaddressed aspects of their illness experience or HCPs may not be able have all the information they need before the concern becomes a more serious health problem.
Dichotomy of Military Experience
Some narrators in this study talked about their military experience as both traumatic and beneficial. These dichotomous viewpoints can be difficult for veterans to construct a narrative understanding around. How can an inherently painful potentially traumatic experience, such as war, have benefits? This way of looking at the world may require a large narrative shift in their world and self-schemas to accept.
Bob hurt people in Vietnam as part of his job. “I did a lot of killing.” Bob met a village elder who stopped him from hurting people in the village and “in my spare time, I would go back to the village and he would teach me, how to be a better man,” Bob shared. “He taught me about life and everything, and he was awesome, just to this day, he’s like a father to me.” Bob tried to change his life and learned how to live a life full of love and care because of his experience in Vietnam. Though Bob hurt a lot of people in Vietnam, which still haunts him, he found meaning through his life lessons from the village elder. “I’m ashamed of what I did in Vietnam. I did some really bad stuff, but ever since then, I’ve always tried to do good to help people.”
Discussion
Exploring a person’s illness experience from a truly holistic pathway allows HCPs to see how the ripples of illness echo into the interconnection of surrounding systems and even across time. These stories suggest that veterans may experience their illness and construct their illness narratives based on the distinct contextual considerations of veteran culture.10 Research exploring how veterans see their illness and its potential impact on their health care access and choices could benefit from exploration into narrative understanding and meaning creation as a potentially contributing factor to health care decision making. As veterans are treated across health care systems, this has implications not only for VHA care, but community care as well.
These narratives also demonstrate how veterans create health care goals woven into their narrative understanding of their illness and its cause, lending insight into understanding health care decision making. This change in self-schema shapes how veterans see themselves and their role which shapes other aspects of their health care. These findings also contribute to our understanding of meaning creation. By exploring meaning making and narrative understanding, this work adds to our knowledge of the importance of spirituality as a component of the holistic experience of illness. There have been previous studies exploring the spiritual aspects of HF and the importance of meaning making.24,25 Exploring meaning making as an aspect of illness narratives can have important implications. Future research could explore the connections between meaning creation and illness narratives.
Limitations
The sample of veterans who participated in this study and are not generalizable to all veteran populations. The sample also only reflects people who were willing to participate and may exclude experience of people who may not have felt comfortable talking to a VA employee about their experience. It is also important to note that the small sample size included primarily male and White participants. In narrative inquiry, the number of participants is not as essential as diving into the depth of the interviews with the participants.
It is also important to note the position of the interviewer. As a White cisgender, heterosexual, middle-aged, middle class female who was raised in rural Kansas in a predominantly Protestant community, the positionality of the interviewer as a cocreator of the data inherently shaped and influenced the narratives created during this study. This contextual understanding of narratives created within the research relationship is an essential component to narrative inquiry and understanding.
Conclusions
Exploring these veterans’ narrative understanding of their experience of illness has many potential implications for health care systems, HCPs, and our military and veteran populations described in this article. Thinking about how the impact of racism, the influence of incentives to remain ill, and the complex intersection of identity and health brings light to how these domains may influence how people see themselves and engage in health care. These domains from these stories of the heart may help millions of people living with chronic illnesses like HF to not only live with their illness but inform how their experience is shaped by the systems surrounding them, including health care, government, and systems of power and oppression.
1. Ashton CM, Bozkurt B, Colucci WB, et al. Veterans Affairs quality enhancement research initiative in chronic heart failure. Medical care. 2000;38(6):I-26-I-37.
2. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi:10.1161/CIR.0000000000000350
3. Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35(6):594-603. doi:10.1016/j.jpainsymman.2007.06.007
4. Zambroski CH. Qualitative analysis of living with heart failure. Heart Lung. 2003;32(1):32-40. doi:10.1067/mhl.2003.10
5. Walthall H, Jenkinson C, Boulton M. Living with breathlessness in chronic heart failure: a qualitative study. J Clin Nurs. 2017;26(13-14):2036-2044. doi:10.1111/jocn.13615
6. Francis GS, Greenberg BH, Hsu DT, et al. ACCF/AHA/ACP/HFSA/ISHLT 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant: a report of the ACCF/AHA/ACP Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2010;56(5):424-453. doi:10.1016/j.jacc.2010.04.014
7. Rumsfeld JS, Havranek E, Masoudi FA, et al. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol. 2003;42(10):1811-1817. doi:10.1016/j.jacc.2003.07.013
8. Leeming A, Murray SA, Kendall M. The impact of advanced heart failure on social, psychological and existential aspects and personhood. Eur J Cardiovasc Nurs. 2014;13(2):162-167. doi:10.1177/1474515114520771
9. Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643-648. doi:10.1016/j.cardfail.2007.05.005
10. Weiss E, Coll JE. The influence of military culture and veteran worldviews on mental health treatment: practice implications for combat veteran help-seeking and wellness. Int J Health, Wellness Society. 2011;1(2):75-86. doi:10.18848/2156-8960/CGP/v01i02/41168
11. Sharpe L, Curran L. Understanding the process of adjustment to illness. Soc Sci Med. 2006;62(5):1153-1166. doi:10.1016/j.socscimed.2005.07.010
12. Riessman CK. Narrative Methods for the Human Sciences. SAGE Publications; 2008.
13. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qualitative Research. 2003;3(1):5-33. doi:10.1177/146879410300300101
14. Riessman CK. Strategic uses of narrative in the presentation of self and illness: a research note. Soc Sci Med. 1990;30(11):1195-1200. doi:10.1016/0277-9536(90)90259-U
15. Riessman CK. Analysis of personal narratives. In: Handbook of Interview Research. Sage; 2002:695-710.
16. Riessman CK. Illness Narratives: Positioned Identities. Invited Annual Lecture. Cardiff University. May 2002. Accessed April 14 2022. https://www.researchgate.net/publication/241501264_Illness_Narratives_Positioned_Identities
17. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qual Res. 2003;3(1):5-33. doi:10.1177/146879410300300101
18. Williams G. The genesis of chronic illness: narrative re‐construction. Sociol Health Illn. 1984;6(2):175-200. doi:10.1111/1467-9566.ep10778250
19. White M, Epston D. Narrative Means to Therapeutic Ends. WW Norton & Company; 1990.
20. Burchardt M. Illness Narratives as Theory and Method. SAGE Publications; 2020.
21. Sayer NA, Spoont M, Nelson D. Veterans seeking disability benefits for post-traumatic stress disorder: who applies and the self-reported meaning of disability compensation. Soc Sci Med. 2004;58(11):2133-2143. doi:10.1016/j.socscimed.2003.08.009
22. Winters CA. Heart failure: living with uncertainty. Prog Cardiovasc Nurs. 1999;14(3):85.
23. Plys E, Smith R, Jacobs ML. Masculinity and military culture in VA hospice and palliative care: a narrative review with clinical recommendations. J Palliat Care. 2020;35(2):120-126. doi:10.1177/0825859719851483
24. Johnson LS. Facilitating spiritual meaning‐making for the individual with a diagnosis of a terminal illness. Counseling and Values. 2003;47(3):230-240. doi:10.1002/j.2161-007X.2003.tb00269.x
25. Shahrbabaki PM, Nouhi E, Kazemi M, Ahmadi F. Defective support network: a major obstacle to coping for patients with heart failure: a qualitative study. Glob Health Action. 2016;9:30767. Published 2016 Apr 1. doi:10.3402/gha.v9.30767
Heart failure (HF) is a costly and burdensome illness and is the top reason for hospital admissions for the US Department of Veterans Affairs (VA) and Medicare.1 The cost of HF to the United States is estimated to grow to $3 billion annually by 2030.2 People living with HF have a high symptom burden and poor quality of life.3,4 Symptoms include shortness of breath, fatigue, depression, and decreases in psychosocial, existential, and spiritual well-being.5-9
Veterans in the US are a unique cultural group with distinct contextual considerations around their experiences.10 Different groups of veterans require unique cultural considerations, such as the experiences of veterans who served during the Vietnam war and during Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF). The extent of unmet needs of people living with HF, the number of veterans living with this illness, and the unique contextual components related to living with HF among veterans require further exploration into this illness experience for this distinct population. Research should explore innovative ways of managing both the number of people living with the illness and the significant impact of HF in people’s lives due to the high symptom burden and poor quality of life.3
This study used the model of adjustment to illness to explore the psychosocial adjustment to illness and the experience of US veterans living with HF, with a focus on the domains of meaning creation, self-schema, and world schema.11 The model of adjustment to illness describes how people learn to adjust to living with an illness, which can lead to positive health outcomes. Meaning creation is defined as the process in which people create meaning from their experience living with illness. Self-schema is how people living with illness see themselves, and world schema is how people living with chronic illness see their place in the world. These domains shift as part of the adjustment to living with an illness described in this model.11 This foundation allowed the investigators to explore the experience of living with HF among veterans with a focus on these domains. Our study aimed to cocreate illness narratives among veterans living with HF and to explore components of psychosocial adjustment informed by the model.
Methods
This study used narrative inquiry with a focus on illness narratives.12-17 Narrative inquiry as defined by Catherine Riessman involves the generation of socially constructed and cocreated meanings between the researcher and narrator. The researcher is an active participant in narrative creation as the narrator chooses which events to include in the stories based on the social, historical, and cultural context of both the narrator (study participant) and audience (researcher). Riessman describes the importance of contextual factors and meaning creation as an important aspect of narrative inquiry.12-14,16,17 It is important in narrative inquiry to consider how cultural, social, and historical factors influence narrative creation, constriction, and/or elimination.
This study prospectively created and collected data at a single time point. Semi-structured interviews explored psychosocial adjustment for people living with HF using an opening question modified from previous illness narrative research: Why do you think you got heart failure?18 Probes included the domains of psychosocial adjustment informed by the model of adjustment to illness domains (Figure). Emergent probes were used to illicit additional data around psychosocial adjustment to illness. Data were created and collected in accordance with narrative inquiry during the cocreation of the illness narratives between the researcher and study participants. This interview guide was tested by the first author in preliminary work to prepare for this study.
Allowing for emergent probes and acknowledging the role of the researcher as audience is key to the cocreation of narratives using this methodological framework. Narrators shape their narrative with the audience in mind; they cocreate their narrative with their audience using this type of narrative inquiry.12,16 What the narrator chooses to include and exclude from their story provides a window into how they see themselves and their world.19 Audio recordings were used to capture data, allowing for the researcher to take contemporaneous notes exploring contextual considerations to the narrative cocreation process and to be used later in analysis. Analytic notes were completed during the interviews as well as later in analysis as part of the contextual reflection.
Setting
Research was conducted in the Rocky Mountain Regional VA Medical Center, Aurora, Colorado. Participants were recruited through the outpatient cardiology clinic where the interviews also took place. This study was approved through the Colorado Institutional Review Board and Rocky Mountain Regional VA Medical Center (IRB: 19-1064). Participants were identified by the treating cardiologist who was a part of the study team. Interested veterans were introduced to the first author who was stationed in an empty clinic room. The study cardiologist screened to ensure all participants were ≥ 18 years of age and had a diagnosis of HF for > 1 year. Persons with an impairment that could interfere with their ability to construct a narrative were also excluded.
Recruitment took place from October 2019 to January 2020. Three veterans refused participation. Five study participants provided informed consent and were enrolled and interviewed. All interviews were completed in the clinic at the time of consent per participant preference. One-hour long semi-structured interviews were conducted and audio recorded. A demographic form was administered at the end of each interview to capture contextual data. The researcher also kept a reflexive journal and audit trail.
Narrative Analysis
Riessman described general steps to conduct narrative analysis, including transcription, narrative clean-up, consideration of contextual factors, exploration of thematic threads, consideration of larger social narratives, and positioning.12 The first author read transcripts while listening to the audio recordings to ensure accuracy. With narrative clean-up each narrative was organized to cocreate overall meaning, changed to protect anonymity, and refined to only include the illness narrative. For example, if a narrator told a story about childhood and then later in the interview remembered another detail to add to their story, narrative clean-up reordered events to make cohesive sense of the story. Demographic, historical, cultural, and social contexts of both the narrator and audience were reflected on during analysis to explore how these components may have shaped and influenced cocreation. Context was also considered within the larger VA setting.
Emergent themes were explored for convergence, divergence, and points of tension within and across each narrative. Larger social narratives were also considered for their influence on possible inclusion/exclusion of experience, such as how gender identity may have influenced study participants’ descriptions of their roles in social systems. These themes and narratives were then shared with our team, and we worked through decision points during the analysis process and discussed interpretation of the data to reach consensus.
Results
Five veterans living with HF were recruited and consented to participate in the study. Demographics of the participants and first author are included in the Table. Five illness narratives were cocreated, entitled: Blame the Cheese: Frank’s Illness Narrative; Love is Love: Bob’s Illness Narrative; The Brighter Things in Life is My Family: George’s Illness Narrative; We Never Know When Our Time is Coming: Bill’s Illness Narrative; and A Dream Deferred: Henry’s Illness Narrative.
Each narrative was explored focusing on the domains of the model of adjustment to illness. An emergent theme was also identified with multiple subthemes: being a veteran is unique. Related subthemes included: financial benefits, intersectionality of government and health care, the intersectionality of masculinity and military service, and the dichotomy of military experience.
The search for meaning creation after the experience of chronic illness emerged across interviews. One example of meaning creation was in Frank's illness narrative. Frank was unsure why he got HF: “Probably because I ate too much cheese…I mean, that’s gotta be it. It can’t be anything else.” By tying HF to his diet, he found meaning through his health behaviors.
Model of Adjustment to Illness
The narratives illustrate components of the model of adjustment to illness and describe how each of the participants either shifted their self-schema and world schema or reinforced their previously established schemas. It also demonstrates how people use narratives to create meaning and illness understanding from their illness experience, reflecting, and emphasizing different parts creating meaning from their experience.
A commonality across the narratives was a shift in self-schema, including the shift from being a provider to being reliant on others. In accordance with the dominant social narrative around men as providers, each narrator talked about their identity as a provider for themselves and their families. Often keeping their provider identity required modifications of the definition, from physical abilities and employment to financial security and stability. George made all his health care decisions based on his goal of providing for his family and protecting them from having to care for him: “I’m always thinking about the future, always trying to figure out how my family, if something should happen to me, how my family would cope, and how my family would be able to support themselves.” Bob’s health care goals were to stay alive long enough for his wife to get financial benefits as a surviving spouse: “That’s why I’m trying to make everything for her, you know. I’m not worried about myself. I’m not. Her I am, you know. And love is love.” Both of their health care decisions are shaped by their identity as a provider shifting to financial support.
Some narrators changed the way they saw their world, or world schema, while others felt their illness experience just reinforced the way they had already experienced the world. Frank was able to reprioritize what was important to him after his diagnosis and accept his own mortality: “I might as well chill out, no more stress, and just enjoy things ’cause you could die…” For Henry, getting HF was only part of the experience of systemic oppression that had impacted his and his family’s lives for generations. He saw how his oppression by the military and US government led to his father’s exposure to chemicals that Henry believed he inherited and caused his illness. Henry’s illness experience reinforced his distrust in the institutions that were oppressed him and his family.
Veteran Status
Being a veteran in the Veterans Health Administration (VHA) system impacted how a narrative understanding of illness was created. Veterans are a unique cultural population with aspects of their illness experience that are important to understand.10 Institutions such as the VA also enable and constrain components of narrative creation.20 The illness narratives in this study were cocreated within the institutional setting of the VA. Part of the analysis included exploring how the institutional setting impacted the narrative creation. Emergent subthemes of the uniqueness of the veteran experience include financial benefits, intersectionality of government and health care, intersectionality of masculinity and military service, and the dichotomy of military experience.
In the US it is unique to the VA that the government both treats and assesses the severity of medical conditions to determine eligibility for health care and financial benefits. The VA’s financial benefits are intended to help compensate veterans who are experiencing illness as a result of their military experience.21 However, because the VA administers them the Veterans Benefits Administration and the VHA, veterans see both as interconnected. The perceived tie between illness severity and financial compensation could influence or bias how veterans understand their illness severity and experience. This may inadvertently encourage veterans to see their illness as being tied to their military service. This shaping of narratives should be considered as a contextual component as veterans obtain financial compensation and health insurance from the same larger organization that provides their health care and management.
George was a young man who during his service had chest pains and felt tired during physical training. He was surprised when his cardiologist explained his heart was enlarged. “All I know is when I initially joined the military, I was perfectly fine, you know, and when I was in the military, graduating, all that stuff, there was a glitch on the [electrocardiogram] they gave me after one day of doing [physical training] and then they’re like, oh, that’s fine. Come to find out it was mitral valve prolapse. And the doctors didn’t catch it then.” George feels the stress of the military caused his heart problems: “It wasn’t there before… so I’d have to say the strain from the military had to have caused it.” George’s medical history noted that he has a genetic connective tissue disorder that can lead to HF and likely was underlying cause of his illness. This example of how George pruned his narrative experience to highlight the cause as his military experience instead of a genetic disorder could have multiple financial and health benefits. The financial incentive for George to see his illness as caused by his military service could potentially bias his illness narrative to find his illness cause as tied to his service.
Government/Health Care Intersectionality
Veterans who may have experienced trust-breaking events with the government, like Agent Orange exposure or intergenerational racial trauma, may apply that experience to all government agencies. Bob felt the government had purposefully used him to create a military weapon. The army “knew I was angry and they used that for their advantage,” he said. Bob learned that he was exposed to Agent Orange in Vietnam, which is presumed to be associated with HF. Bob felt betrayed that the VHA had not figured out his health problems earlier. “I didn’t know anything about it until 6 months ago… Our government knew about it when they used it, and they didn’t care. They just wanted to win the war, and a whole lot of GIs like me suffered because of that, and I was like my government killed me? And I was fighting for them?”
Henry learned to distrust the government and the health care system because of a long history of systematic oppression and exploitation. These institutions’ erosion of trust has impact beyond the trust-breaking event itself but reverberates into how communities view organizations and institutions for generations. For Black Americans, who have historically been experimented on without consent by the US government and health care systems, this can make it especially hard to trust and build working relationships with those institutions. Health care professionals (HCPs) need to build collaborative partnerships with patients to provide effective care while understanding why some patients may have difficulty trusting health care systems, especially government-led systems.
The nature of HF as an illness can also make it difficult to predict and manage.22 This uncertainty and difficulty in managing HF can make it especially hard for people to establish trust with their HCPs whom they want to see as experts in their illness. HCPs in these narratives were often portrayed as incompetent or neglectful. The unpredictable nature of the illness itself was not reflected in the narrator’s experience.
Masculinity/Military Service Intersectionality
For the veteran narrators, tied into the identity of being a provider are social messages about masculinity. There is a unique intersectionality of being a man, the military culture, and living with chronic illnesses. Dominant social messages around being a man include being tough, not expressing emotion, self-reliance, and having power. This overlaps with social messages on military culture, including self-reliance, toughness, persistence in the face of adversity, limited expression of emotions, and the recognition of power and respect.23
People who internalize these social messages on masculinity may be less likely to access mental health treatment.23 This stigmatizing barrier to mental health treatment could impact how positive narratives are constructed around the experience of chronic illness for narrators who identify as masculine. Military and masculine identity could exclude or constrain stories about a veteran who did not “solider on” or who had to rely on others in a team to get things done. This shift can especially impact veterans experiencing chronic illnesses like HF, which often impact their physical abilities. Veterans may feel pressured to think of and portray themselves as being strong by limiting their expression of pain and other symptoms to remain in alignment with the dominant narrative. By not being open about the full experience of their illness both positive and negative, veterans may have unaddressed aspects of their illness experience or HCPs may not be able have all the information they need before the concern becomes a more serious health problem.
Dichotomy of Military Experience
Some narrators in this study talked about their military experience as both traumatic and beneficial. These dichotomous viewpoints can be difficult for veterans to construct a narrative understanding around. How can an inherently painful potentially traumatic experience, such as war, have benefits? This way of looking at the world may require a large narrative shift in their world and self-schemas to accept.
Bob hurt people in Vietnam as part of his job. “I did a lot of killing.” Bob met a village elder who stopped him from hurting people in the village and “in my spare time, I would go back to the village and he would teach me, how to be a better man,” Bob shared. “He taught me about life and everything, and he was awesome, just to this day, he’s like a father to me.” Bob tried to change his life and learned how to live a life full of love and care because of his experience in Vietnam. Though Bob hurt a lot of people in Vietnam, which still haunts him, he found meaning through his life lessons from the village elder. “I’m ashamed of what I did in Vietnam. I did some really bad stuff, but ever since then, I’ve always tried to do good to help people.”
Discussion
Exploring a person’s illness experience from a truly holistic pathway allows HCPs to see how the ripples of illness echo into the interconnection of surrounding systems and even across time. These stories suggest that veterans may experience their illness and construct their illness narratives based on the distinct contextual considerations of veteran culture.10 Research exploring how veterans see their illness and its potential impact on their health care access and choices could benefit from exploration into narrative understanding and meaning creation as a potentially contributing factor to health care decision making. As veterans are treated across health care systems, this has implications not only for VHA care, but community care as well.
These narratives also demonstrate how veterans create health care goals woven into their narrative understanding of their illness and its cause, lending insight into understanding health care decision making. This change in self-schema shapes how veterans see themselves and their role which shapes other aspects of their health care. These findings also contribute to our understanding of meaning creation. By exploring meaning making and narrative understanding, this work adds to our knowledge of the importance of spirituality as a component of the holistic experience of illness. There have been previous studies exploring the spiritual aspects of HF and the importance of meaning making.24,25 Exploring meaning making as an aspect of illness narratives can have important implications. Future research could explore the connections between meaning creation and illness narratives.
Limitations
The sample of veterans who participated in this study and are not generalizable to all veteran populations. The sample also only reflects people who were willing to participate and may exclude experience of people who may not have felt comfortable talking to a VA employee about their experience. It is also important to note that the small sample size included primarily male and White participants. In narrative inquiry, the number of participants is not as essential as diving into the depth of the interviews with the participants.
It is also important to note the position of the interviewer. As a White cisgender, heterosexual, middle-aged, middle class female who was raised in rural Kansas in a predominantly Protestant community, the positionality of the interviewer as a cocreator of the data inherently shaped and influenced the narratives created during this study. This contextual understanding of narratives created within the research relationship is an essential component to narrative inquiry and understanding.
Conclusions
Exploring these veterans’ narrative understanding of their experience of illness has many potential implications for health care systems, HCPs, and our military and veteran populations described in this article. Thinking about how the impact of racism, the influence of incentives to remain ill, and the complex intersection of identity and health brings light to how these domains may influence how people see themselves and engage in health care. These domains from these stories of the heart may help millions of people living with chronic illnesses like HF to not only live with their illness but inform how their experience is shaped by the systems surrounding them, including health care, government, and systems of power and oppression.
Heart failure (HF) is a costly and burdensome illness and is the top reason for hospital admissions for the US Department of Veterans Affairs (VA) and Medicare.1 The cost of HF to the United States is estimated to grow to $3 billion annually by 2030.2 People living with HF have a high symptom burden and poor quality of life.3,4 Symptoms include shortness of breath, fatigue, depression, and decreases in psychosocial, existential, and spiritual well-being.5-9
Veterans in the US are a unique cultural group with distinct contextual considerations around their experiences.10 Different groups of veterans require unique cultural considerations, such as the experiences of veterans who served during the Vietnam war and during Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF). The extent of unmet needs of people living with HF, the number of veterans living with this illness, and the unique contextual components related to living with HF among veterans require further exploration into this illness experience for this distinct population. Research should explore innovative ways of managing both the number of people living with the illness and the significant impact of HF in people’s lives due to the high symptom burden and poor quality of life.3
This study used the model of adjustment to illness to explore the psychosocial adjustment to illness and the experience of US veterans living with HF, with a focus on the domains of meaning creation, self-schema, and world schema.11 The model of adjustment to illness describes how people learn to adjust to living with an illness, which can lead to positive health outcomes. Meaning creation is defined as the process in which people create meaning from their experience living with illness. Self-schema is how people living with illness see themselves, and world schema is how people living with chronic illness see their place in the world. These domains shift as part of the adjustment to living with an illness described in this model.11 This foundation allowed the investigators to explore the experience of living with HF among veterans with a focus on these domains. Our study aimed to cocreate illness narratives among veterans living with HF and to explore components of psychosocial adjustment informed by the model.
Methods
This study used narrative inquiry with a focus on illness narratives.12-17 Narrative inquiry as defined by Catherine Riessman involves the generation of socially constructed and cocreated meanings between the researcher and narrator. The researcher is an active participant in narrative creation as the narrator chooses which events to include in the stories based on the social, historical, and cultural context of both the narrator (study participant) and audience (researcher). Riessman describes the importance of contextual factors and meaning creation as an important aspect of narrative inquiry.12-14,16,17 It is important in narrative inquiry to consider how cultural, social, and historical factors influence narrative creation, constriction, and/or elimination.
This study prospectively created and collected data at a single time point. Semi-structured interviews explored psychosocial adjustment for people living with HF using an opening question modified from previous illness narrative research: Why do you think you got heart failure?18 Probes included the domains of psychosocial adjustment informed by the model of adjustment to illness domains (Figure). Emergent probes were used to illicit additional data around psychosocial adjustment to illness. Data were created and collected in accordance with narrative inquiry during the cocreation of the illness narratives between the researcher and study participants. This interview guide was tested by the first author in preliminary work to prepare for this study.
Allowing for emergent probes and acknowledging the role of the researcher as audience is key to the cocreation of narratives using this methodological framework. Narrators shape their narrative with the audience in mind; they cocreate their narrative with their audience using this type of narrative inquiry.12,16 What the narrator chooses to include and exclude from their story provides a window into how they see themselves and their world.19 Audio recordings were used to capture data, allowing for the researcher to take contemporaneous notes exploring contextual considerations to the narrative cocreation process and to be used later in analysis. Analytic notes were completed during the interviews as well as later in analysis as part of the contextual reflection.
Setting
Research was conducted in the Rocky Mountain Regional VA Medical Center, Aurora, Colorado. Participants were recruited through the outpatient cardiology clinic where the interviews also took place. This study was approved through the Colorado Institutional Review Board and Rocky Mountain Regional VA Medical Center (IRB: 19-1064). Participants were identified by the treating cardiologist who was a part of the study team. Interested veterans were introduced to the first author who was stationed in an empty clinic room. The study cardiologist screened to ensure all participants were ≥ 18 years of age and had a diagnosis of HF for > 1 year. Persons with an impairment that could interfere with their ability to construct a narrative were also excluded.
Recruitment took place from October 2019 to January 2020. Three veterans refused participation. Five study participants provided informed consent and were enrolled and interviewed. All interviews were completed in the clinic at the time of consent per participant preference. One-hour long semi-structured interviews were conducted and audio recorded. A demographic form was administered at the end of each interview to capture contextual data. The researcher also kept a reflexive journal and audit trail.
Narrative Analysis
Riessman described general steps to conduct narrative analysis, including transcription, narrative clean-up, consideration of contextual factors, exploration of thematic threads, consideration of larger social narratives, and positioning.12 The first author read transcripts while listening to the audio recordings to ensure accuracy. With narrative clean-up each narrative was organized to cocreate overall meaning, changed to protect anonymity, and refined to only include the illness narrative. For example, if a narrator told a story about childhood and then later in the interview remembered another detail to add to their story, narrative clean-up reordered events to make cohesive sense of the story. Demographic, historical, cultural, and social contexts of both the narrator and audience were reflected on during analysis to explore how these components may have shaped and influenced cocreation. Context was also considered within the larger VA setting.
Emergent themes were explored for convergence, divergence, and points of tension within and across each narrative. Larger social narratives were also considered for their influence on possible inclusion/exclusion of experience, such as how gender identity may have influenced study participants’ descriptions of their roles in social systems. These themes and narratives were then shared with our team, and we worked through decision points during the analysis process and discussed interpretation of the data to reach consensus.
Results
Five veterans living with HF were recruited and consented to participate in the study. Demographics of the participants and first author are included in the Table. Five illness narratives were cocreated, entitled: Blame the Cheese: Frank’s Illness Narrative; Love is Love: Bob’s Illness Narrative; The Brighter Things in Life is My Family: George’s Illness Narrative; We Never Know When Our Time is Coming: Bill’s Illness Narrative; and A Dream Deferred: Henry’s Illness Narrative.
Each narrative was explored focusing on the domains of the model of adjustment to illness. An emergent theme was also identified with multiple subthemes: being a veteran is unique. Related subthemes included: financial benefits, intersectionality of government and health care, the intersectionality of masculinity and military service, and the dichotomy of military experience.
The search for meaning creation after the experience of chronic illness emerged across interviews. One example of meaning creation was in Frank's illness narrative. Frank was unsure why he got HF: “Probably because I ate too much cheese…I mean, that’s gotta be it. It can’t be anything else.” By tying HF to his diet, he found meaning through his health behaviors.
Model of Adjustment to Illness
The narratives illustrate components of the model of adjustment to illness and describe how each of the participants either shifted their self-schema and world schema or reinforced their previously established schemas. It also demonstrates how people use narratives to create meaning and illness understanding from their illness experience, reflecting, and emphasizing different parts creating meaning from their experience.
A commonality across the narratives was a shift in self-schema, including the shift from being a provider to being reliant on others. In accordance with the dominant social narrative around men as providers, each narrator talked about their identity as a provider for themselves and their families. Often keeping their provider identity required modifications of the definition, from physical abilities and employment to financial security and stability. George made all his health care decisions based on his goal of providing for his family and protecting them from having to care for him: “I’m always thinking about the future, always trying to figure out how my family, if something should happen to me, how my family would cope, and how my family would be able to support themselves.” Bob’s health care goals were to stay alive long enough for his wife to get financial benefits as a surviving spouse: “That’s why I’m trying to make everything for her, you know. I’m not worried about myself. I’m not. Her I am, you know. And love is love.” Both of their health care decisions are shaped by their identity as a provider shifting to financial support.
Some narrators changed the way they saw their world, or world schema, while others felt their illness experience just reinforced the way they had already experienced the world. Frank was able to reprioritize what was important to him after his diagnosis and accept his own mortality: “I might as well chill out, no more stress, and just enjoy things ’cause you could die…” For Henry, getting HF was only part of the experience of systemic oppression that had impacted his and his family’s lives for generations. He saw how his oppression by the military and US government led to his father’s exposure to chemicals that Henry believed he inherited and caused his illness. Henry’s illness experience reinforced his distrust in the institutions that were oppressed him and his family.
Veteran Status
Being a veteran in the Veterans Health Administration (VHA) system impacted how a narrative understanding of illness was created. Veterans are a unique cultural population with aspects of their illness experience that are important to understand.10 Institutions such as the VA also enable and constrain components of narrative creation.20 The illness narratives in this study were cocreated within the institutional setting of the VA. Part of the analysis included exploring how the institutional setting impacted the narrative creation. Emergent subthemes of the uniqueness of the veteran experience include financial benefits, intersectionality of government and health care, intersectionality of masculinity and military service, and the dichotomy of military experience.
In the US it is unique to the VA that the government both treats and assesses the severity of medical conditions to determine eligibility for health care and financial benefits. The VA’s financial benefits are intended to help compensate veterans who are experiencing illness as a result of their military experience.21 However, because the VA administers them the Veterans Benefits Administration and the VHA, veterans see both as interconnected. The perceived tie between illness severity and financial compensation could influence or bias how veterans understand their illness severity and experience. This may inadvertently encourage veterans to see their illness as being tied to their military service. This shaping of narratives should be considered as a contextual component as veterans obtain financial compensation and health insurance from the same larger organization that provides their health care and management.
George was a young man who during his service had chest pains and felt tired during physical training. He was surprised when his cardiologist explained his heart was enlarged. “All I know is when I initially joined the military, I was perfectly fine, you know, and when I was in the military, graduating, all that stuff, there was a glitch on the [electrocardiogram] they gave me after one day of doing [physical training] and then they’re like, oh, that’s fine. Come to find out it was mitral valve prolapse. And the doctors didn’t catch it then.” George feels the stress of the military caused his heart problems: “It wasn’t there before… so I’d have to say the strain from the military had to have caused it.” George’s medical history noted that he has a genetic connective tissue disorder that can lead to HF and likely was underlying cause of his illness. This example of how George pruned his narrative experience to highlight the cause as his military experience instead of a genetic disorder could have multiple financial and health benefits. The financial incentive for George to see his illness as caused by his military service could potentially bias his illness narrative to find his illness cause as tied to his service.
Government/Health Care Intersectionality
Veterans who may have experienced trust-breaking events with the government, like Agent Orange exposure or intergenerational racial trauma, may apply that experience to all government agencies. Bob felt the government had purposefully used him to create a military weapon. The army “knew I was angry and they used that for their advantage,” he said. Bob learned that he was exposed to Agent Orange in Vietnam, which is presumed to be associated with HF. Bob felt betrayed that the VHA had not figured out his health problems earlier. “I didn’t know anything about it until 6 months ago… Our government knew about it when they used it, and they didn’t care. They just wanted to win the war, and a whole lot of GIs like me suffered because of that, and I was like my government killed me? And I was fighting for them?”
Henry learned to distrust the government and the health care system because of a long history of systematic oppression and exploitation. These institutions’ erosion of trust has impact beyond the trust-breaking event itself but reverberates into how communities view organizations and institutions for generations. For Black Americans, who have historically been experimented on without consent by the US government and health care systems, this can make it especially hard to trust and build working relationships with those institutions. Health care professionals (HCPs) need to build collaborative partnerships with patients to provide effective care while understanding why some patients may have difficulty trusting health care systems, especially government-led systems.
The nature of HF as an illness can also make it difficult to predict and manage.22 This uncertainty and difficulty in managing HF can make it especially hard for people to establish trust with their HCPs whom they want to see as experts in their illness. HCPs in these narratives were often portrayed as incompetent or neglectful. The unpredictable nature of the illness itself was not reflected in the narrator’s experience.
Masculinity/Military Service Intersectionality
For the veteran narrators, tied into the identity of being a provider are social messages about masculinity. There is a unique intersectionality of being a man, the military culture, and living with chronic illnesses. Dominant social messages around being a man include being tough, not expressing emotion, self-reliance, and having power. This overlaps with social messages on military culture, including self-reliance, toughness, persistence in the face of adversity, limited expression of emotions, and the recognition of power and respect.23
People who internalize these social messages on masculinity may be less likely to access mental health treatment.23 This stigmatizing barrier to mental health treatment could impact how positive narratives are constructed around the experience of chronic illness for narrators who identify as masculine. Military and masculine identity could exclude or constrain stories about a veteran who did not “solider on” or who had to rely on others in a team to get things done. This shift can especially impact veterans experiencing chronic illnesses like HF, which often impact their physical abilities. Veterans may feel pressured to think of and portray themselves as being strong by limiting their expression of pain and other symptoms to remain in alignment with the dominant narrative. By not being open about the full experience of their illness both positive and negative, veterans may have unaddressed aspects of their illness experience or HCPs may not be able have all the information they need before the concern becomes a more serious health problem.
Dichotomy of Military Experience
Some narrators in this study talked about their military experience as both traumatic and beneficial. These dichotomous viewpoints can be difficult for veterans to construct a narrative understanding around. How can an inherently painful potentially traumatic experience, such as war, have benefits? This way of looking at the world may require a large narrative shift in their world and self-schemas to accept.
Bob hurt people in Vietnam as part of his job. “I did a lot of killing.” Bob met a village elder who stopped him from hurting people in the village and “in my spare time, I would go back to the village and he would teach me, how to be a better man,” Bob shared. “He taught me about life and everything, and he was awesome, just to this day, he’s like a father to me.” Bob tried to change his life and learned how to live a life full of love and care because of his experience in Vietnam. Though Bob hurt a lot of people in Vietnam, which still haunts him, he found meaning through his life lessons from the village elder. “I’m ashamed of what I did in Vietnam. I did some really bad stuff, but ever since then, I’ve always tried to do good to help people.”
Discussion
Exploring a person’s illness experience from a truly holistic pathway allows HCPs to see how the ripples of illness echo into the interconnection of surrounding systems and even across time. These stories suggest that veterans may experience their illness and construct their illness narratives based on the distinct contextual considerations of veteran culture.10 Research exploring how veterans see their illness and its potential impact on their health care access and choices could benefit from exploration into narrative understanding and meaning creation as a potentially contributing factor to health care decision making. As veterans are treated across health care systems, this has implications not only for VHA care, but community care as well.
These narratives also demonstrate how veterans create health care goals woven into their narrative understanding of their illness and its cause, lending insight into understanding health care decision making. This change in self-schema shapes how veterans see themselves and their role which shapes other aspects of their health care. These findings also contribute to our understanding of meaning creation. By exploring meaning making and narrative understanding, this work adds to our knowledge of the importance of spirituality as a component of the holistic experience of illness. There have been previous studies exploring the spiritual aspects of HF and the importance of meaning making.24,25 Exploring meaning making as an aspect of illness narratives can have important implications. Future research could explore the connections between meaning creation and illness narratives.
Limitations
The sample of veterans who participated in this study and are not generalizable to all veteran populations. The sample also only reflects people who were willing to participate and may exclude experience of people who may not have felt comfortable talking to a VA employee about their experience. It is also important to note that the small sample size included primarily male and White participants. In narrative inquiry, the number of participants is not as essential as diving into the depth of the interviews with the participants.
It is also important to note the position of the interviewer. As a White cisgender, heterosexual, middle-aged, middle class female who was raised in rural Kansas in a predominantly Protestant community, the positionality of the interviewer as a cocreator of the data inherently shaped and influenced the narratives created during this study. This contextual understanding of narratives created within the research relationship is an essential component to narrative inquiry and understanding.
Conclusions
Exploring these veterans’ narrative understanding of their experience of illness has many potential implications for health care systems, HCPs, and our military and veteran populations described in this article. Thinking about how the impact of racism, the influence of incentives to remain ill, and the complex intersection of identity and health brings light to how these domains may influence how people see themselves and engage in health care. These domains from these stories of the heart may help millions of people living with chronic illnesses like HF to not only live with their illness but inform how their experience is shaped by the systems surrounding them, including health care, government, and systems of power and oppression.
1. Ashton CM, Bozkurt B, Colucci WB, et al. Veterans Affairs quality enhancement research initiative in chronic heart failure. Medical care. 2000;38(6):I-26-I-37.
2. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi:10.1161/CIR.0000000000000350
3. Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35(6):594-603. doi:10.1016/j.jpainsymman.2007.06.007
4. Zambroski CH. Qualitative analysis of living with heart failure. Heart Lung. 2003;32(1):32-40. doi:10.1067/mhl.2003.10
5. Walthall H, Jenkinson C, Boulton M. Living with breathlessness in chronic heart failure: a qualitative study. J Clin Nurs. 2017;26(13-14):2036-2044. doi:10.1111/jocn.13615
6. Francis GS, Greenberg BH, Hsu DT, et al. ACCF/AHA/ACP/HFSA/ISHLT 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant: a report of the ACCF/AHA/ACP Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2010;56(5):424-453. doi:10.1016/j.jacc.2010.04.014
7. Rumsfeld JS, Havranek E, Masoudi FA, et al. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol. 2003;42(10):1811-1817. doi:10.1016/j.jacc.2003.07.013
8. Leeming A, Murray SA, Kendall M. The impact of advanced heart failure on social, psychological and existential aspects and personhood. Eur J Cardiovasc Nurs. 2014;13(2):162-167. doi:10.1177/1474515114520771
9. Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643-648. doi:10.1016/j.cardfail.2007.05.005
10. Weiss E, Coll JE. The influence of military culture and veteran worldviews on mental health treatment: practice implications for combat veteran help-seeking and wellness. Int J Health, Wellness Society. 2011;1(2):75-86. doi:10.18848/2156-8960/CGP/v01i02/41168
11. Sharpe L, Curran L. Understanding the process of adjustment to illness. Soc Sci Med. 2006;62(5):1153-1166. doi:10.1016/j.socscimed.2005.07.010
12. Riessman CK. Narrative Methods for the Human Sciences. SAGE Publications; 2008.
13. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qualitative Research. 2003;3(1):5-33. doi:10.1177/146879410300300101
14. Riessman CK. Strategic uses of narrative in the presentation of self and illness: a research note. Soc Sci Med. 1990;30(11):1195-1200. doi:10.1016/0277-9536(90)90259-U
15. Riessman CK. Analysis of personal narratives. In: Handbook of Interview Research. Sage; 2002:695-710.
16. Riessman CK. Illness Narratives: Positioned Identities. Invited Annual Lecture. Cardiff University. May 2002. Accessed April 14 2022. https://www.researchgate.net/publication/241501264_Illness_Narratives_Positioned_Identities
17. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qual Res. 2003;3(1):5-33. doi:10.1177/146879410300300101
18. Williams G. The genesis of chronic illness: narrative re‐construction. Sociol Health Illn. 1984;6(2):175-200. doi:10.1111/1467-9566.ep10778250
19. White M, Epston D. Narrative Means to Therapeutic Ends. WW Norton & Company; 1990.
20. Burchardt M. Illness Narratives as Theory and Method. SAGE Publications; 2020.
21. Sayer NA, Spoont M, Nelson D. Veterans seeking disability benefits for post-traumatic stress disorder: who applies and the self-reported meaning of disability compensation. Soc Sci Med. 2004;58(11):2133-2143. doi:10.1016/j.socscimed.2003.08.009
22. Winters CA. Heart failure: living with uncertainty. Prog Cardiovasc Nurs. 1999;14(3):85.
23. Plys E, Smith R, Jacobs ML. Masculinity and military culture in VA hospice and palliative care: a narrative review with clinical recommendations. J Palliat Care. 2020;35(2):120-126. doi:10.1177/0825859719851483
24. Johnson LS. Facilitating spiritual meaning‐making for the individual with a diagnosis of a terminal illness. Counseling and Values. 2003;47(3):230-240. doi:10.1002/j.2161-007X.2003.tb00269.x
25. Shahrbabaki PM, Nouhi E, Kazemi M, Ahmadi F. Defective support network: a major obstacle to coping for patients with heart failure: a qualitative study. Glob Health Action. 2016;9:30767. Published 2016 Apr 1. doi:10.3402/gha.v9.30767
1. Ashton CM, Bozkurt B, Colucci WB, et al. Veterans Affairs quality enhancement research initiative in chronic heart failure. Medical care. 2000;38(6):I-26-I-37.
2. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi:10.1161/CIR.0000000000000350
3. Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35(6):594-603. doi:10.1016/j.jpainsymman.2007.06.007
4. Zambroski CH. Qualitative analysis of living with heart failure. Heart Lung. 2003;32(1):32-40. doi:10.1067/mhl.2003.10
5. Walthall H, Jenkinson C, Boulton M. Living with breathlessness in chronic heart failure: a qualitative study. J Clin Nurs. 2017;26(13-14):2036-2044. doi:10.1111/jocn.13615
6. Francis GS, Greenberg BH, Hsu DT, et al. ACCF/AHA/ACP/HFSA/ISHLT 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant: a report of the ACCF/AHA/ACP Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2010;56(5):424-453. doi:10.1016/j.jacc.2010.04.014
7. Rumsfeld JS, Havranek E, Masoudi FA, et al. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol. 2003;42(10):1811-1817. doi:10.1016/j.jacc.2003.07.013
8. Leeming A, Murray SA, Kendall M. The impact of advanced heart failure on social, psychological and existential aspects and personhood. Eur J Cardiovasc Nurs. 2014;13(2):162-167. doi:10.1177/1474515114520771
9. Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643-648. doi:10.1016/j.cardfail.2007.05.005
10. Weiss E, Coll JE. The influence of military culture and veteran worldviews on mental health treatment: practice implications for combat veteran help-seeking and wellness. Int J Health, Wellness Society. 2011;1(2):75-86. doi:10.18848/2156-8960/CGP/v01i02/41168
11. Sharpe L, Curran L. Understanding the process of adjustment to illness. Soc Sci Med. 2006;62(5):1153-1166. doi:10.1016/j.socscimed.2005.07.010
12. Riessman CK. Narrative Methods for the Human Sciences. SAGE Publications; 2008.
13. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qualitative Research. 2003;3(1):5-33. doi:10.1177/146879410300300101
14. Riessman CK. Strategic uses of narrative in the presentation of self and illness: a research note. Soc Sci Med. 1990;30(11):1195-1200. doi:10.1016/0277-9536(90)90259-U
15. Riessman CK. Analysis of personal narratives. In: Handbook of Interview Research. Sage; 2002:695-710.
16. Riessman CK. Illness Narratives: Positioned Identities. Invited Annual Lecture. Cardiff University. May 2002. Accessed April 14 2022. https://www.researchgate.net/publication/241501264_Illness_Narratives_Positioned_Identities
17. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qual Res. 2003;3(1):5-33. doi:10.1177/146879410300300101
18. Williams G. The genesis of chronic illness: narrative re‐construction. Sociol Health Illn. 1984;6(2):175-200. doi:10.1111/1467-9566.ep10778250
19. White M, Epston D. Narrative Means to Therapeutic Ends. WW Norton & Company; 1990.
20. Burchardt M. Illness Narratives as Theory and Method. SAGE Publications; 2020.
21. Sayer NA, Spoont M, Nelson D. Veterans seeking disability benefits for post-traumatic stress disorder: who applies and the self-reported meaning of disability compensation. Soc Sci Med. 2004;58(11):2133-2143. doi:10.1016/j.socscimed.2003.08.009
22. Winters CA. Heart failure: living with uncertainty. Prog Cardiovasc Nurs. 1999;14(3):85.
23. Plys E, Smith R, Jacobs ML. Masculinity and military culture in VA hospice and palliative care: a narrative review with clinical recommendations. J Palliat Care. 2020;35(2):120-126. doi:10.1177/0825859719851483
24. Johnson LS. Facilitating spiritual meaning‐making for the individual with a diagnosis of a terminal illness. Counseling and Values. 2003;47(3):230-240. doi:10.1002/j.2161-007X.2003.tb00269.x
25. Shahrbabaki PM, Nouhi E, Kazemi M, Ahmadi F. Defective support network: a major obstacle to coping for patients with heart failure: a qualitative study. Glob Health Action. 2016;9:30767. Published 2016 Apr 1. doi:10.3402/gha.v9.30767
Inverted Appendix in a Patient With Weakness and Occult Bleeding
Appendiceal mucinous neoplasms (AMNs) are rare tumors of the appendix that can be asymptomatic or present with acute right lower quadrant (RLQ) pain mimicking appendicitis. Due to their potential to cause either no symptoms or nonspecific symptoms, such as abdominal pain, nausea, or vomiting, AMNs are often found incidentally during appendectomies or, even more rarely, colonoscopies. Most AMNs grow slowly and have little metastatic potential. However, due to potential complications, such as bowel obstruction and rupture, timely detection and removal of AMN is essential. We describe the case of a patient who appeared to have acute appendicitis complicated by rupture on imaging who was found instead to have a perforated low-grade AMN during surgery.
Case Presentation
A male patient aged 72 years with a history of type 2 diabetes mellitus, hypertension, and aortic stenosis, but no prior abdominal surgery, presented with a chief concern of generalized weakness. As part of the workup for his weakness, a computed tomography (CT) scan of the abdomen was performed which showed an RLQ phlegmon and mild fat stranding in the area. Imaging also revealed an asymptomatic gallstone measuring 1.5 cm with no evidence of cholecystitis. The patient had no fever and reported no abdominal pain, nausea, vomiting, or change in bowel habits. On physical examination, the patient’s abdomen was soft, nontender, and nondistended with normoactive bowel sounds and no rebound or guarding.
To manage the appendicitis, the patient started a 2-week course of amoxicillin clavulanate 875 mg twice daily and was instructed to schedule an interval appendectomy in the coming months. Four days later, during a follow-up with his primary care physician, he was found to be asymptomatic. However, at this visit his stool was found to be positive for occult blood. Given this finding and the lack of a previous colonoscopy, the patient underwent a colonoscopy, which revealed bulging at the appendiceal orifice, consistent with an inverted appendix. Portions of the appendix were biopsied (Figure 1). Histologic analysis of the appendiceal biopsies revealed no dysplasia or malignancy. The colonoscopy also revealed an 8-mm sessile polyp in the ascending colon which was resected, and histologic analysis of this polyp revealed a low-grade tubular adenoma. Additionally, a large angiodysplastic lesion was found in the ascending colon as well as external and medium-sized internal hemorrhoids.
Six weeks after the colonoscopy, the patient was taken to the operating room for a laparoscopic appendectomy. Upon entry of the abdomen, extensive adhesions throughout the RLQ were found which required adhesiolysis. A calcified fecalith adherent to the mesentery of the small intestine in the RLQ was also found and resected. After lysis of the adhesions, the appendix and fibrotic tissue surrounding it could be seen (Figure 2). The appendix was dilated and the tip showed perforation. During dissection of the appendix, clear gelatinous material was found coming from the appendiceal lumen as well as from the fibrotic tissue around the appendix. On postoperative day 1 the appendix was resected and the patient was discharged.
Histologic specimens of the appendix were notable for evidence of perforation and neoplasia leading to a diagnosis of low-grade AMN. The presence of atypical mucinous epithelial cells on the serosal surface of the appendix, confirmed with a positive pancytokeratin stain, provided histologic evidence of appendiceal perforation (Figure 3). The presence of nuclear atypia demonstrated that the appendix was involved by a neoplastic process. Additionally, attenuation of the normal appendiceal epithelium, evidence of a chronic process, further helped to differentiate the AMN from complicated appendicitis. The presence of mucin involving the serosa of the appendix led to the classification of this patient’s neoplasm as grade pT4a. Of note, histologic examination demonstrated that the surgical margins contained tumor cells.
Given the positive margins of the resected AMN and the relatively large size of the neoplasm, a laparoscopic right hemicolectomy was performed 2 months later. Although multiple adhesions were found in the terminal ileum, cecum, and ascending colon during the hemicolectomy, no mucinous lesions were observed grossly. Histologic analysis showed no residual neoplasm as well as no lymph node involvement. On postoperative day 3 the patient was discharged and had an uneventful recovery. At his first surveillance visit 6 months after his hemicolectomy, the patient appeared to be doing well and reported no abdominal pain, nausea, vomiting, change in bowel habits, or any blood in the stool.
Discussion
AMNs are rare tumors with an annual age-adjusted incidence of approximately 0.12 per 1,000,000 people.1 These neoplasms can present as acute or chronic abdominal pain, gastrointestinal bleeding, intestinal obstruction, or acute abdomen.2-4 Most AMNs, however, are asymptomatic and are usually found incidentally during appendectomies for appendicitis, and can even be found during colonoscopies,such as in this case.5,6
Low-grade AMNs are distinguished from appendiceal mucinous adenocarcinomas by their lack of wall invasion.7 Additionally, low-grade AMNs have a very good prognosis as even neoplasms that have spread outside of the appendix have a 5-year overall survival rate of 79 to 86%.8 These low-grade neoplasms also have extremely low rates of recurrence after resection.9 In contrast, appendiceal mucinous adenocarcinomas have a much worse prognosis with a 5-year overall survival rate of 53.6%.10
Treatment of AMNs depends on the extent of their spread. Neoplasms that are confined to the appendix can typically be treated with appendectomy alone, while those that have spread beyond the appendix may require cytoreductive surgery and chemotherapy, namely, hyperthermic intraperitoneal chemotherapy (HIPEC), in addition to appendectomy.11 Cases in which neoplasms are not confined to the appendix also require more frequent surveillance for recurrence as compared to appendix-restricted neoplasms.11
Appendiceal inversion is a rare finding in adults with an estimated prevalence of 0.01%.6 Not only is appendiceal inversion rare in and of itself, it is even more rarely found in combination with appendiceal neoplasms.6 Other causes of appendiceal inversion include intussusception, acute appendicitis, appendiceal nodule, or even iatrogenic due to appendectomy.12-14 While appendiceal inversion can be completely benign, because these morphological changes of the appendix can resemble a polyp, these lesions are often biopsied and/or resected.15 However, lesion resection may be quite problematic due to high risk of bleeding and perforation.15 In order to avoid the risks associated with resection of a potentially benign finding, biopsy should be performed prior to any attempted resection of inverted appendices.15
Another interesting aspect of this case is the finding of fecal occult blood. The differential for fecal occult blood is quite broad and the patient had multiple conditions that could have led to the finding of occult blood in his stool. Hemorrhoids can cause a positive result on a fecal occult blood test (FOBT) although this is relatively uncommon, and hemorrhoids are more likely to cause frank blood to be seen.16 The sessile polyp found in the patient’s colon may also have caused the FOBT to be positive. This patient was also found to have an angiodysplasia (a finding that is associated with aortic stenosis, which this patient has a history of) which can also cause gastrointestinal bleeding.17 Lastly, AMNs may also cause gastrointestinal bleeding and thus a positive FOBT, although bleeding is a relatively uncommon presentation of AMNs, especially those that are low-grade as in this case.18
This case also highlights the association between appendiceal neoplasms and colonic neoplastic lesions. Patients with appendiceal neoplasms are more likely to have colonic neoplastic lesions than patients without appendiceal neoplasms.19 Studies have found that approximately 13 to 42% of patients with appendiceal neoplasms also have colonic neoplastic lesions.19 The majority of these lesions in the colon were right-sided and this finding was also seen in this case as the patient’s polyp was located in the ascending colon.19 Due to this association between appendiceal and colorectal neoplasia, the American Society of Colon and Rectal Surgeons strongly recommends that patients with appendiceal neoplasms or who are suspected of having them receive a colonoscopy.19
Additionally, perforation of an AMN, as was seen in this case, is a finding that should raise significant concern. Perforation of an AMN allows for the spread of malignant mucinous epithelial cells throughout the abdomen. The finding of extensive adhesions throughout the patient’s RLQ was unexpected as abdominal adhesions are most often seen in patients with a history of abdominal surgeries. Considering the lack of any prior abdominal surgeries in this patient, these adhesions were most likely the result of the spread and proliferation of malignant mucinous epithelial cells from the perforated AMN in the RLQ.20 The adhesiolysis performed in this case was thus not only important in order to visualize the appendix, but also for preventing future complications of abdominal adhesions such as bowel obstruction.20 Perforated AMN is also so concerning because it can potentially lead to pseudomyxoma peritonei—a condition in which malignant mucinous epithelial cells accumulate in the abdomen.21 Pseudomyxoma peritonei is extremely rare with an incidence of approximately 1 to 2 cases per million per year.22 Early recognition of AMNs and surgical referral are critically important as pseudomyxoma peritonei is difficult to treat, has a high rate of recurrence, and can be fatal.23
Lastly, this case highlights how findings of a ruptured appendix and/or mucin surrounding the appendix on imaging should warrant laparoscopy because only pathologic analysis of the appendix can definitively rule out AMNs. The utility of laparoscopic evaluation of the appendix is especially apparent as nonsurgical treatment of appendicitis using antibiotics is gaining favor for treating even complicated appendicitis.24 Appendicitis is much more common than AMNs. However, had the patient in this case only been given antibiotics for his suspected complicated appendicitis without any colonoscopy or appendectomy, the neoplasm in his appendix would have gone undetected and continued to grow, causing significant complications. The patient’s age at presentation in this case also necessitated laparoscopic evaluation of the appendix as the incidence of AMNs is highest among patients aged > 60 years.25 Additionally, because appendiceal inversion may be seen with AMNs,the patient’s inverted appendix seen during his colonoscopy was another compelling reason for laparoscopic evaluation of his appendix.6
Conclusions
AMNs can present with nonspecific symptoms or can be completely asymptomatic and are often found incidentally during colonoscopies or appendectomies for acute appendicitis. While it is true that AMNs have low metastatic potential and grow slowly, AMNs can rupture leading to pseudomyxoma peritonei or even cause bowel obstruction warranting timely identification and removal of these neoplasms. Laparoscopic evaluation in cases of ruptured appendices is critical not only for treatment, but also for determining the presence of a potential underlying appendiceal malignancy. Although AMNs are a rare pathology, physicians should still consider the possibility of these neoplasms even when imaging findings suggest appendicitis. Having AMNs as part of the differential diagnosis is especially necessary in cases, such as this one, in which the patient has appendiceal inversion, is aged > 50 years, and has concurrent colorectal neoplasms.
1. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40(6):569-573. doi:10.1097/COC.0000000000000210
2. Kehagias I, Zygomalas A, Markopoulos G, Papandreou T, Kraniotis P. Diagnosis and treatment of mucinous appendiceal neoplasm presented as acute appendicitis. Case Rep Oncol Med. 2016;3:1-6. doi:10.1155/2016/2161952
3. Karatas M, Simsek C, Gunay S, et al. Acute lower gastrointestinal bleeding due to low-grade mucinous neoplasm of appendix. Acta Chir Belg. 2020;120(4):1-4. doi:10.1080/00015458.2020.1860397
4. Mourad FH, Hussein M, Bahlawan M, Haddad M, Tawil A. Intestinal obstruction secondary to appendiceal mucocele. Dig Dis Sci. 1999;44(8):1594-1599. doi:10.1023/a:1026615010989
5. Benabe SH, Leeman R, Brady AC, Hirzel A, Langshaw AH. Low-grade appendiceal mucinous neoplasm in an adolescent patient with untreated Crohn’s disease. ACG Case Reports J. 2020;7(3). doi:10.14309/crj.0000000000000338
6. Liu X, Liu G, Liu Y, et al. Complete appendiceal inversion with local high-grade intraepithelial neoplasia in an adult female: A case report. BMC Surg. 2019;19(1). doi:10.1186/s12893-019-0632-3
7. Gündog˘ar ÖS, Kımılog˘lu ES, Komut NS, et al. The evaluation of appendiceal mucinous neoplasms with a new classification system. Turk J Gastroenterol. 2018;29(5):532-542. doi:10.5152/tjg.2018.17605
8. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089-1103. doi:10.1097/00000478-200308000-00006
9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425-1439. doi:10.1097/PAS.0b013e3181af6067
10. Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2015;122(2):213-221. doi:10.1002/cncr.29744
11. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2018;23(1):137. doi:10.1634/theoncologist.2017-0081erratum
12. Tran C, Sakioka J, Nguyen E, Beutler BD, Hsu J. An inverted appendix found on routine colonoscopy: a case report with discussion of imaging findings. Radiol Case Rep. 2019;14(8):952-955. doi:10.1016/j.radcr.2019.05.022
13. Shafi A, Azab M. A case of everted appendix with benign appendiceal nodule masquerading as appendiceal mucocele: a case report. Am J Gastroenterol. 2018;113:S1436. doi:10.14309/00000434-201810001-02585
14. Pokhrel B, Chang M, Anand G, Savides T, Fehmi S. Appendiceal mucinous neoplasm in an inverted appendix found on prior colonoscopy. VideoGIE. 2020;5(1):34-36. doi:10.1016/j.vgie.2019.09.013
15. Johnson EK, Arcila ME, Steele SR. Appendiceal inversion: a diagnostic and therapeutic dilemma. JSLS. 2009;13(1):92-95.
16. van Turenhout ST, Oort FA, sive Droste JST, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76(1):136-143. doi:10.1016/j.gie.2012.03.169
17. Hudzik B, Wilczek K, Gasior M. Heyde syndrome: Gastrointestinal bleeding and aortic stenosis. CMAJ. 2016;188(2):135-138. doi:10.1503/cmaj.150194
18. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. RadioGraphics. 2017;37(4):1059-1083. doi:10.1148/rg.2017160150
19. Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425-1438. doi:10.1097/DCR.0000000000001530
20. Panagopoulos P, Tsokaki T, Misiakos E, et al. Low-grade appendiceal mucinous neoplasm presenting as an adnexal mass. Case Reports in Obstetrics and Gynecology. 2017;2017:1-3. doi:10.1155/2017/7165321
21. Ramaswamy V. Pathology of mucinous appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol. 2016;7(2):258-267. doi:10.1007/s13193-016-0516-2.
22. Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50. doi:10.4251/wjgo.v2.i1.44
23. Mercier F, Dagbert F, Pocard M, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. 2018;3(2):195-202. doi:10.1002/bjs5.97
24. David A, Dodgion C, Eddine SBZ, Davila D, Webb TP, Trevino CM. Perforated appendicitis: Short duration antibiotics are noninferior to traditional long duration antibiotics. Surgery. 2020;167(2):475-477. doi:10.1016/j.surg.2019.08.007
25. Raijman I, Leong S, Hassaram S, Marcon NE. Appendiceal mucocele: Endoscopic appearance. Endoscopy. 1994;26(3):326-328. doi:10.1055/s-2007-1008979
Appendiceal mucinous neoplasms (AMNs) are rare tumors of the appendix that can be asymptomatic or present with acute right lower quadrant (RLQ) pain mimicking appendicitis. Due to their potential to cause either no symptoms or nonspecific symptoms, such as abdominal pain, nausea, or vomiting, AMNs are often found incidentally during appendectomies or, even more rarely, colonoscopies. Most AMNs grow slowly and have little metastatic potential. However, due to potential complications, such as bowel obstruction and rupture, timely detection and removal of AMN is essential. We describe the case of a patient who appeared to have acute appendicitis complicated by rupture on imaging who was found instead to have a perforated low-grade AMN during surgery.
Case Presentation
A male patient aged 72 years with a history of type 2 diabetes mellitus, hypertension, and aortic stenosis, but no prior abdominal surgery, presented with a chief concern of generalized weakness. As part of the workup for his weakness, a computed tomography (CT) scan of the abdomen was performed which showed an RLQ phlegmon and mild fat stranding in the area. Imaging also revealed an asymptomatic gallstone measuring 1.5 cm with no evidence of cholecystitis. The patient had no fever and reported no abdominal pain, nausea, vomiting, or change in bowel habits. On physical examination, the patient’s abdomen was soft, nontender, and nondistended with normoactive bowel sounds and no rebound or guarding.
To manage the appendicitis, the patient started a 2-week course of amoxicillin clavulanate 875 mg twice daily and was instructed to schedule an interval appendectomy in the coming months. Four days later, during a follow-up with his primary care physician, he was found to be asymptomatic. However, at this visit his stool was found to be positive for occult blood. Given this finding and the lack of a previous colonoscopy, the patient underwent a colonoscopy, which revealed bulging at the appendiceal orifice, consistent with an inverted appendix. Portions of the appendix were biopsied (Figure 1). Histologic analysis of the appendiceal biopsies revealed no dysplasia or malignancy. The colonoscopy also revealed an 8-mm sessile polyp in the ascending colon which was resected, and histologic analysis of this polyp revealed a low-grade tubular adenoma. Additionally, a large angiodysplastic lesion was found in the ascending colon as well as external and medium-sized internal hemorrhoids.
Six weeks after the colonoscopy, the patient was taken to the operating room for a laparoscopic appendectomy. Upon entry of the abdomen, extensive adhesions throughout the RLQ were found which required adhesiolysis. A calcified fecalith adherent to the mesentery of the small intestine in the RLQ was also found and resected. After lysis of the adhesions, the appendix and fibrotic tissue surrounding it could be seen (Figure 2). The appendix was dilated and the tip showed perforation. During dissection of the appendix, clear gelatinous material was found coming from the appendiceal lumen as well as from the fibrotic tissue around the appendix. On postoperative day 1 the appendix was resected and the patient was discharged.
Histologic specimens of the appendix were notable for evidence of perforation and neoplasia leading to a diagnosis of low-grade AMN. The presence of atypical mucinous epithelial cells on the serosal surface of the appendix, confirmed with a positive pancytokeratin stain, provided histologic evidence of appendiceal perforation (Figure 3). The presence of nuclear atypia demonstrated that the appendix was involved by a neoplastic process. Additionally, attenuation of the normal appendiceal epithelium, evidence of a chronic process, further helped to differentiate the AMN from complicated appendicitis. The presence of mucin involving the serosa of the appendix led to the classification of this patient’s neoplasm as grade pT4a. Of note, histologic examination demonstrated that the surgical margins contained tumor cells.
Given the positive margins of the resected AMN and the relatively large size of the neoplasm, a laparoscopic right hemicolectomy was performed 2 months later. Although multiple adhesions were found in the terminal ileum, cecum, and ascending colon during the hemicolectomy, no mucinous lesions were observed grossly. Histologic analysis showed no residual neoplasm as well as no lymph node involvement. On postoperative day 3 the patient was discharged and had an uneventful recovery. At his first surveillance visit 6 months after his hemicolectomy, the patient appeared to be doing well and reported no abdominal pain, nausea, vomiting, change in bowel habits, or any blood in the stool.
Discussion
AMNs are rare tumors with an annual age-adjusted incidence of approximately 0.12 per 1,000,000 people.1 These neoplasms can present as acute or chronic abdominal pain, gastrointestinal bleeding, intestinal obstruction, or acute abdomen.2-4 Most AMNs, however, are asymptomatic and are usually found incidentally during appendectomies for appendicitis, and can even be found during colonoscopies,such as in this case.5,6
Low-grade AMNs are distinguished from appendiceal mucinous adenocarcinomas by their lack of wall invasion.7 Additionally, low-grade AMNs have a very good prognosis as even neoplasms that have spread outside of the appendix have a 5-year overall survival rate of 79 to 86%.8 These low-grade neoplasms also have extremely low rates of recurrence after resection.9 In contrast, appendiceal mucinous adenocarcinomas have a much worse prognosis with a 5-year overall survival rate of 53.6%.10
Treatment of AMNs depends on the extent of their spread. Neoplasms that are confined to the appendix can typically be treated with appendectomy alone, while those that have spread beyond the appendix may require cytoreductive surgery and chemotherapy, namely, hyperthermic intraperitoneal chemotherapy (HIPEC), in addition to appendectomy.11 Cases in which neoplasms are not confined to the appendix also require more frequent surveillance for recurrence as compared to appendix-restricted neoplasms.11
Appendiceal inversion is a rare finding in adults with an estimated prevalence of 0.01%.6 Not only is appendiceal inversion rare in and of itself, it is even more rarely found in combination with appendiceal neoplasms.6 Other causes of appendiceal inversion include intussusception, acute appendicitis, appendiceal nodule, or even iatrogenic due to appendectomy.12-14 While appendiceal inversion can be completely benign, because these morphological changes of the appendix can resemble a polyp, these lesions are often biopsied and/or resected.15 However, lesion resection may be quite problematic due to high risk of bleeding and perforation.15 In order to avoid the risks associated with resection of a potentially benign finding, biopsy should be performed prior to any attempted resection of inverted appendices.15
Another interesting aspect of this case is the finding of fecal occult blood. The differential for fecal occult blood is quite broad and the patient had multiple conditions that could have led to the finding of occult blood in his stool. Hemorrhoids can cause a positive result on a fecal occult blood test (FOBT) although this is relatively uncommon, and hemorrhoids are more likely to cause frank blood to be seen.16 The sessile polyp found in the patient’s colon may also have caused the FOBT to be positive. This patient was also found to have an angiodysplasia (a finding that is associated with aortic stenosis, which this patient has a history of) which can also cause gastrointestinal bleeding.17 Lastly, AMNs may also cause gastrointestinal bleeding and thus a positive FOBT, although bleeding is a relatively uncommon presentation of AMNs, especially those that are low-grade as in this case.18
This case also highlights the association between appendiceal neoplasms and colonic neoplastic lesions. Patients with appendiceal neoplasms are more likely to have colonic neoplastic lesions than patients without appendiceal neoplasms.19 Studies have found that approximately 13 to 42% of patients with appendiceal neoplasms also have colonic neoplastic lesions.19 The majority of these lesions in the colon were right-sided and this finding was also seen in this case as the patient’s polyp was located in the ascending colon.19 Due to this association between appendiceal and colorectal neoplasia, the American Society of Colon and Rectal Surgeons strongly recommends that patients with appendiceal neoplasms or who are suspected of having them receive a colonoscopy.19
Additionally, perforation of an AMN, as was seen in this case, is a finding that should raise significant concern. Perforation of an AMN allows for the spread of malignant mucinous epithelial cells throughout the abdomen. The finding of extensive adhesions throughout the patient’s RLQ was unexpected as abdominal adhesions are most often seen in patients with a history of abdominal surgeries. Considering the lack of any prior abdominal surgeries in this patient, these adhesions were most likely the result of the spread and proliferation of malignant mucinous epithelial cells from the perforated AMN in the RLQ.20 The adhesiolysis performed in this case was thus not only important in order to visualize the appendix, but also for preventing future complications of abdominal adhesions such as bowel obstruction.20 Perforated AMN is also so concerning because it can potentially lead to pseudomyxoma peritonei—a condition in which malignant mucinous epithelial cells accumulate in the abdomen.21 Pseudomyxoma peritonei is extremely rare with an incidence of approximately 1 to 2 cases per million per year.22 Early recognition of AMNs and surgical referral are critically important as pseudomyxoma peritonei is difficult to treat, has a high rate of recurrence, and can be fatal.23
Lastly, this case highlights how findings of a ruptured appendix and/or mucin surrounding the appendix on imaging should warrant laparoscopy because only pathologic analysis of the appendix can definitively rule out AMNs. The utility of laparoscopic evaluation of the appendix is especially apparent as nonsurgical treatment of appendicitis using antibiotics is gaining favor for treating even complicated appendicitis.24 Appendicitis is much more common than AMNs. However, had the patient in this case only been given antibiotics for his suspected complicated appendicitis without any colonoscopy or appendectomy, the neoplasm in his appendix would have gone undetected and continued to grow, causing significant complications. The patient’s age at presentation in this case also necessitated laparoscopic evaluation of the appendix as the incidence of AMNs is highest among patients aged > 60 years.25 Additionally, because appendiceal inversion may be seen with AMNs,the patient’s inverted appendix seen during his colonoscopy was another compelling reason for laparoscopic evaluation of his appendix.6
Conclusions
AMNs can present with nonspecific symptoms or can be completely asymptomatic and are often found incidentally during colonoscopies or appendectomies for acute appendicitis. While it is true that AMNs have low metastatic potential and grow slowly, AMNs can rupture leading to pseudomyxoma peritonei or even cause bowel obstruction warranting timely identification and removal of these neoplasms. Laparoscopic evaluation in cases of ruptured appendices is critical not only for treatment, but also for determining the presence of a potential underlying appendiceal malignancy. Although AMNs are a rare pathology, physicians should still consider the possibility of these neoplasms even when imaging findings suggest appendicitis. Having AMNs as part of the differential diagnosis is especially necessary in cases, such as this one, in which the patient has appendiceal inversion, is aged > 50 years, and has concurrent colorectal neoplasms.
Appendiceal mucinous neoplasms (AMNs) are rare tumors of the appendix that can be asymptomatic or present with acute right lower quadrant (RLQ) pain mimicking appendicitis. Due to their potential to cause either no symptoms or nonspecific symptoms, such as abdominal pain, nausea, or vomiting, AMNs are often found incidentally during appendectomies or, even more rarely, colonoscopies. Most AMNs grow slowly and have little metastatic potential. However, due to potential complications, such as bowel obstruction and rupture, timely detection and removal of AMN is essential. We describe the case of a patient who appeared to have acute appendicitis complicated by rupture on imaging who was found instead to have a perforated low-grade AMN during surgery.
Case Presentation
A male patient aged 72 years with a history of type 2 diabetes mellitus, hypertension, and aortic stenosis, but no prior abdominal surgery, presented with a chief concern of generalized weakness. As part of the workup for his weakness, a computed tomography (CT) scan of the abdomen was performed which showed an RLQ phlegmon and mild fat stranding in the area. Imaging also revealed an asymptomatic gallstone measuring 1.5 cm with no evidence of cholecystitis. The patient had no fever and reported no abdominal pain, nausea, vomiting, or change in bowel habits. On physical examination, the patient’s abdomen was soft, nontender, and nondistended with normoactive bowel sounds and no rebound or guarding.
To manage the appendicitis, the patient started a 2-week course of amoxicillin clavulanate 875 mg twice daily and was instructed to schedule an interval appendectomy in the coming months. Four days later, during a follow-up with his primary care physician, he was found to be asymptomatic. However, at this visit his stool was found to be positive for occult blood. Given this finding and the lack of a previous colonoscopy, the patient underwent a colonoscopy, which revealed bulging at the appendiceal orifice, consistent with an inverted appendix. Portions of the appendix were biopsied (Figure 1). Histologic analysis of the appendiceal biopsies revealed no dysplasia or malignancy. The colonoscopy also revealed an 8-mm sessile polyp in the ascending colon which was resected, and histologic analysis of this polyp revealed a low-grade tubular adenoma. Additionally, a large angiodysplastic lesion was found in the ascending colon as well as external and medium-sized internal hemorrhoids.
Six weeks after the colonoscopy, the patient was taken to the operating room for a laparoscopic appendectomy. Upon entry of the abdomen, extensive adhesions throughout the RLQ were found which required adhesiolysis. A calcified fecalith adherent to the mesentery of the small intestine in the RLQ was also found and resected. After lysis of the adhesions, the appendix and fibrotic tissue surrounding it could be seen (Figure 2). The appendix was dilated and the tip showed perforation. During dissection of the appendix, clear gelatinous material was found coming from the appendiceal lumen as well as from the fibrotic tissue around the appendix. On postoperative day 1 the appendix was resected and the patient was discharged.
Histologic specimens of the appendix were notable for evidence of perforation and neoplasia leading to a diagnosis of low-grade AMN. The presence of atypical mucinous epithelial cells on the serosal surface of the appendix, confirmed with a positive pancytokeratin stain, provided histologic evidence of appendiceal perforation (Figure 3). The presence of nuclear atypia demonstrated that the appendix was involved by a neoplastic process. Additionally, attenuation of the normal appendiceal epithelium, evidence of a chronic process, further helped to differentiate the AMN from complicated appendicitis. The presence of mucin involving the serosa of the appendix led to the classification of this patient’s neoplasm as grade pT4a. Of note, histologic examination demonstrated that the surgical margins contained tumor cells.
Given the positive margins of the resected AMN and the relatively large size of the neoplasm, a laparoscopic right hemicolectomy was performed 2 months later. Although multiple adhesions were found in the terminal ileum, cecum, and ascending colon during the hemicolectomy, no mucinous lesions were observed grossly. Histologic analysis showed no residual neoplasm as well as no lymph node involvement. On postoperative day 3 the patient was discharged and had an uneventful recovery. At his first surveillance visit 6 months after his hemicolectomy, the patient appeared to be doing well and reported no abdominal pain, nausea, vomiting, change in bowel habits, or any blood in the stool.
Discussion
AMNs are rare tumors with an annual age-adjusted incidence of approximately 0.12 per 1,000,000 people.1 These neoplasms can present as acute or chronic abdominal pain, gastrointestinal bleeding, intestinal obstruction, or acute abdomen.2-4 Most AMNs, however, are asymptomatic and are usually found incidentally during appendectomies for appendicitis, and can even be found during colonoscopies,such as in this case.5,6
Low-grade AMNs are distinguished from appendiceal mucinous adenocarcinomas by their lack of wall invasion.7 Additionally, low-grade AMNs have a very good prognosis as even neoplasms that have spread outside of the appendix have a 5-year overall survival rate of 79 to 86%.8 These low-grade neoplasms also have extremely low rates of recurrence after resection.9 In contrast, appendiceal mucinous adenocarcinomas have a much worse prognosis with a 5-year overall survival rate of 53.6%.10
Treatment of AMNs depends on the extent of their spread. Neoplasms that are confined to the appendix can typically be treated with appendectomy alone, while those that have spread beyond the appendix may require cytoreductive surgery and chemotherapy, namely, hyperthermic intraperitoneal chemotherapy (HIPEC), in addition to appendectomy.11 Cases in which neoplasms are not confined to the appendix also require more frequent surveillance for recurrence as compared to appendix-restricted neoplasms.11
Appendiceal inversion is a rare finding in adults with an estimated prevalence of 0.01%.6 Not only is appendiceal inversion rare in and of itself, it is even more rarely found in combination with appendiceal neoplasms.6 Other causes of appendiceal inversion include intussusception, acute appendicitis, appendiceal nodule, or even iatrogenic due to appendectomy.12-14 While appendiceal inversion can be completely benign, because these morphological changes of the appendix can resemble a polyp, these lesions are often biopsied and/or resected.15 However, lesion resection may be quite problematic due to high risk of bleeding and perforation.15 In order to avoid the risks associated with resection of a potentially benign finding, biopsy should be performed prior to any attempted resection of inverted appendices.15
Another interesting aspect of this case is the finding of fecal occult blood. The differential for fecal occult blood is quite broad and the patient had multiple conditions that could have led to the finding of occult blood in his stool. Hemorrhoids can cause a positive result on a fecal occult blood test (FOBT) although this is relatively uncommon, and hemorrhoids are more likely to cause frank blood to be seen.16 The sessile polyp found in the patient’s colon may also have caused the FOBT to be positive. This patient was also found to have an angiodysplasia (a finding that is associated with aortic stenosis, which this patient has a history of) which can also cause gastrointestinal bleeding.17 Lastly, AMNs may also cause gastrointestinal bleeding and thus a positive FOBT, although bleeding is a relatively uncommon presentation of AMNs, especially those that are low-grade as in this case.18
This case also highlights the association between appendiceal neoplasms and colonic neoplastic lesions. Patients with appendiceal neoplasms are more likely to have colonic neoplastic lesions than patients without appendiceal neoplasms.19 Studies have found that approximately 13 to 42% of patients with appendiceal neoplasms also have colonic neoplastic lesions.19 The majority of these lesions in the colon were right-sided and this finding was also seen in this case as the patient’s polyp was located in the ascending colon.19 Due to this association between appendiceal and colorectal neoplasia, the American Society of Colon and Rectal Surgeons strongly recommends that patients with appendiceal neoplasms or who are suspected of having them receive a colonoscopy.19
Additionally, perforation of an AMN, as was seen in this case, is a finding that should raise significant concern. Perforation of an AMN allows for the spread of malignant mucinous epithelial cells throughout the abdomen. The finding of extensive adhesions throughout the patient’s RLQ was unexpected as abdominal adhesions are most often seen in patients with a history of abdominal surgeries. Considering the lack of any prior abdominal surgeries in this patient, these adhesions were most likely the result of the spread and proliferation of malignant mucinous epithelial cells from the perforated AMN in the RLQ.20 The adhesiolysis performed in this case was thus not only important in order to visualize the appendix, but also for preventing future complications of abdominal adhesions such as bowel obstruction.20 Perforated AMN is also so concerning because it can potentially lead to pseudomyxoma peritonei—a condition in which malignant mucinous epithelial cells accumulate in the abdomen.21 Pseudomyxoma peritonei is extremely rare with an incidence of approximately 1 to 2 cases per million per year.22 Early recognition of AMNs and surgical referral are critically important as pseudomyxoma peritonei is difficult to treat, has a high rate of recurrence, and can be fatal.23
Lastly, this case highlights how findings of a ruptured appendix and/or mucin surrounding the appendix on imaging should warrant laparoscopy because only pathologic analysis of the appendix can definitively rule out AMNs. The utility of laparoscopic evaluation of the appendix is especially apparent as nonsurgical treatment of appendicitis using antibiotics is gaining favor for treating even complicated appendicitis.24 Appendicitis is much more common than AMNs. However, had the patient in this case only been given antibiotics for his suspected complicated appendicitis without any colonoscopy or appendectomy, the neoplasm in his appendix would have gone undetected and continued to grow, causing significant complications. The patient’s age at presentation in this case also necessitated laparoscopic evaluation of the appendix as the incidence of AMNs is highest among patients aged > 60 years.25 Additionally, because appendiceal inversion may be seen with AMNs,the patient’s inverted appendix seen during his colonoscopy was another compelling reason for laparoscopic evaluation of his appendix.6
Conclusions
AMNs can present with nonspecific symptoms or can be completely asymptomatic and are often found incidentally during colonoscopies or appendectomies for acute appendicitis. While it is true that AMNs have low metastatic potential and grow slowly, AMNs can rupture leading to pseudomyxoma peritonei or even cause bowel obstruction warranting timely identification and removal of these neoplasms. Laparoscopic evaluation in cases of ruptured appendices is critical not only for treatment, but also for determining the presence of a potential underlying appendiceal malignancy. Although AMNs are a rare pathology, physicians should still consider the possibility of these neoplasms even when imaging findings suggest appendicitis. Having AMNs as part of the differential diagnosis is especially necessary in cases, such as this one, in which the patient has appendiceal inversion, is aged > 50 years, and has concurrent colorectal neoplasms.
1. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40(6):569-573. doi:10.1097/COC.0000000000000210
2. Kehagias I, Zygomalas A, Markopoulos G, Papandreou T, Kraniotis P. Diagnosis and treatment of mucinous appendiceal neoplasm presented as acute appendicitis. Case Rep Oncol Med. 2016;3:1-6. doi:10.1155/2016/2161952
3. Karatas M, Simsek C, Gunay S, et al. Acute lower gastrointestinal bleeding due to low-grade mucinous neoplasm of appendix. Acta Chir Belg. 2020;120(4):1-4. doi:10.1080/00015458.2020.1860397
4. Mourad FH, Hussein M, Bahlawan M, Haddad M, Tawil A. Intestinal obstruction secondary to appendiceal mucocele. Dig Dis Sci. 1999;44(8):1594-1599. doi:10.1023/a:1026615010989
5. Benabe SH, Leeman R, Brady AC, Hirzel A, Langshaw AH. Low-grade appendiceal mucinous neoplasm in an adolescent patient with untreated Crohn’s disease. ACG Case Reports J. 2020;7(3). doi:10.14309/crj.0000000000000338
6. Liu X, Liu G, Liu Y, et al. Complete appendiceal inversion with local high-grade intraepithelial neoplasia in an adult female: A case report. BMC Surg. 2019;19(1). doi:10.1186/s12893-019-0632-3
7. Gündog˘ar ÖS, Kımılog˘lu ES, Komut NS, et al. The evaluation of appendiceal mucinous neoplasms with a new classification system. Turk J Gastroenterol. 2018;29(5):532-542. doi:10.5152/tjg.2018.17605
8. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089-1103. doi:10.1097/00000478-200308000-00006
9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425-1439. doi:10.1097/PAS.0b013e3181af6067
10. Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2015;122(2):213-221. doi:10.1002/cncr.29744
11. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2018;23(1):137. doi:10.1634/theoncologist.2017-0081erratum
12. Tran C, Sakioka J, Nguyen E, Beutler BD, Hsu J. An inverted appendix found on routine colonoscopy: a case report with discussion of imaging findings. Radiol Case Rep. 2019;14(8):952-955. doi:10.1016/j.radcr.2019.05.022
13. Shafi A, Azab M. A case of everted appendix with benign appendiceal nodule masquerading as appendiceal mucocele: a case report. Am J Gastroenterol. 2018;113:S1436. doi:10.14309/00000434-201810001-02585
14. Pokhrel B, Chang M, Anand G, Savides T, Fehmi S. Appendiceal mucinous neoplasm in an inverted appendix found on prior colonoscopy. VideoGIE. 2020;5(1):34-36. doi:10.1016/j.vgie.2019.09.013
15. Johnson EK, Arcila ME, Steele SR. Appendiceal inversion: a diagnostic and therapeutic dilemma. JSLS. 2009;13(1):92-95.
16. van Turenhout ST, Oort FA, sive Droste JST, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76(1):136-143. doi:10.1016/j.gie.2012.03.169
17. Hudzik B, Wilczek K, Gasior M. Heyde syndrome: Gastrointestinal bleeding and aortic stenosis. CMAJ. 2016;188(2):135-138. doi:10.1503/cmaj.150194
18. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. RadioGraphics. 2017;37(4):1059-1083. doi:10.1148/rg.2017160150
19. Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425-1438. doi:10.1097/DCR.0000000000001530
20. Panagopoulos P, Tsokaki T, Misiakos E, et al. Low-grade appendiceal mucinous neoplasm presenting as an adnexal mass. Case Reports in Obstetrics and Gynecology. 2017;2017:1-3. doi:10.1155/2017/7165321
21. Ramaswamy V. Pathology of mucinous appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol. 2016;7(2):258-267. doi:10.1007/s13193-016-0516-2.
22. Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50. doi:10.4251/wjgo.v2.i1.44
23. Mercier F, Dagbert F, Pocard M, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. 2018;3(2):195-202. doi:10.1002/bjs5.97
24. David A, Dodgion C, Eddine SBZ, Davila D, Webb TP, Trevino CM. Perforated appendicitis: Short duration antibiotics are noninferior to traditional long duration antibiotics. Surgery. 2020;167(2):475-477. doi:10.1016/j.surg.2019.08.007
25. Raijman I, Leong S, Hassaram S, Marcon NE. Appendiceal mucocele: Endoscopic appearance. Endoscopy. 1994;26(3):326-328. doi:10.1055/s-2007-1008979
1. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a SEER analysis. Am J Clin Oncol. 2017;40(6):569-573. doi:10.1097/COC.0000000000000210
2. Kehagias I, Zygomalas A, Markopoulos G, Papandreou T, Kraniotis P. Diagnosis and treatment of mucinous appendiceal neoplasm presented as acute appendicitis. Case Rep Oncol Med. 2016;3:1-6. doi:10.1155/2016/2161952
3. Karatas M, Simsek C, Gunay S, et al. Acute lower gastrointestinal bleeding due to low-grade mucinous neoplasm of appendix. Acta Chir Belg. 2020;120(4):1-4. doi:10.1080/00015458.2020.1860397
4. Mourad FH, Hussein M, Bahlawan M, Haddad M, Tawil A. Intestinal obstruction secondary to appendiceal mucocele. Dig Dis Sci. 1999;44(8):1594-1599. doi:10.1023/a:1026615010989
5. Benabe SH, Leeman R, Brady AC, Hirzel A, Langshaw AH. Low-grade appendiceal mucinous neoplasm in an adolescent patient with untreated Crohn’s disease. ACG Case Reports J. 2020;7(3). doi:10.14309/crj.0000000000000338
6. Liu X, Liu G, Liu Y, et al. Complete appendiceal inversion with local high-grade intraepithelial neoplasia in an adult female: A case report. BMC Surg. 2019;19(1). doi:10.1186/s12893-019-0632-3
7. Gündog˘ar ÖS, Kımılog˘lu ES, Komut NS, et al. The evaluation of appendiceal mucinous neoplasms with a new classification system. Turk J Gastroenterol. 2018;29(5):532-542. doi:10.5152/tjg.2018.17605
8. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089-1103. doi:10.1097/00000478-200308000-00006
9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33(10):1425-1439. doi:10.1097/PAS.0b013e3181af6067
10. Asare EA, Compton CC, Hanna NN, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer. 2015;122(2):213-221. doi:10.1002/cncr.29744
11. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. 2018;23(1):137. doi:10.1634/theoncologist.2017-0081erratum
12. Tran C, Sakioka J, Nguyen E, Beutler BD, Hsu J. An inverted appendix found on routine colonoscopy: a case report with discussion of imaging findings. Radiol Case Rep. 2019;14(8):952-955. doi:10.1016/j.radcr.2019.05.022
13. Shafi A, Azab M. A case of everted appendix with benign appendiceal nodule masquerading as appendiceal mucocele: a case report. Am J Gastroenterol. 2018;113:S1436. doi:10.14309/00000434-201810001-02585
14. Pokhrel B, Chang M, Anand G, Savides T, Fehmi S. Appendiceal mucinous neoplasm in an inverted appendix found on prior colonoscopy. VideoGIE. 2020;5(1):34-36. doi:10.1016/j.vgie.2019.09.013
15. Johnson EK, Arcila ME, Steele SR. Appendiceal inversion: a diagnostic and therapeutic dilemma. JSLS. 2009;13(1):92-95.
16. van Turenhout ST, Oort FA, sive Droste JST, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc. 2012;76(1):136-143. doi:10.1016/j.gie.2012.03.169
17. Hudzik B, Wilczek K, Gasior M. Heyde syndrome: Gastrointestinal bleeding and aortic stenosis. CMAJ. 2016;188(2):135-138. doi:10.1503/cmaj.150194
18. Leonards LM, Pahwa A, Patel MK, Petersen J, Nguyen MJ, Jude CM. Neoplasms of the appendix: pictorial review with clinical and pathologic correlation. RadioGraphics. 2017;37(4):1059-1083. doi:10.1148/rg.2017160150
19. Glasgow SC, Gaertner W, Stewart D, et al. The American Society of Colon and Rectal Surgeons, clinical practice guidelines for the management of appendiceal neoplasms. Dis Colon Rectum. 2019;62(12):1425-1438. doi:10.1097/DCR.0000000000001530
20. Panagopoulos P, Tsokaki T, Misiakos E, et al. Low-grade appendiceal mucinous neoplasm presenting as an adnexal mass. Case Reports in Obstetrics and Gynecology. 2017;2017:1-3. doi:10.1155/2017/7165321
21. Ramaswamy V. Pathology of mucinous appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol. 2016;7(2):258-267. doi:10.1007/s13193-016-0516-2.
22. Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50. doi:10.4251/wjgo.v2.i1.44
23. Mercier F, Dagbert F, Pocard M, et al. Recurrence of pseudomyxoma peritonei after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BJS Open. 2018;3(2):195-202. doi:10.1002/bjs5.97
24. David A, Dodgion C, Eddine SBZ, Davila D, Webb TP, Trevino CM. Perforated appendicitis: Short duration antibiotics are noninferior to traditional long duration antibiotics. Surgery. 2020;167(2):475-477. doi:10.1016/j.surg.2019.08.007
25. Raijman I, Leong S, Hassaram S, Marcon NE. Appendiceal mucocele: Endoscopic appearance. Endoscopy. 1994;26(3):326-328. doi:10.1055/s-2007-1008979
Caregiver Support in a Case of Posttraumatic Stress Disorder and Lewy Body Dementia
Caregiving for a person with dementia in the community can be extremely difficult work. Much of this work falls on unpaid or informal caregivers. Sixty-three percent of older adults with dementia depend completely on unpaid caregivers, and an additional 26% receive some combination of paid and unpaid support, together comprising nearly 90% of the more than 3 million older Americans with dementia.1 In-home care is preferable for these patients. For veterans, the Caregiver Support Program (CSP) is the only US Department of Veterans Affairs (VA) program that exclusively supports caregivers. Although the CSP is not a nursing home diversion or cost savings program, successfully enabling at-home living in lieu of facility living also has the potential to reduce overall cost of care, and most importantly, to enable veterans who desire it to age at home.2,3
The CSP has 2 unique programs for caregivers of eligible veterans. The Program of General Caregiver Support Services (PGCSS) provides resources, education, and support to caregivers of all veterans enrolled in the Veterans Health Administration (VHA). The Program of Comprehensive Assistance for Family Caregivers (PCAFC) provides education and training, access to health care insurance if eligible, mental health counseling, access to a monthly caregiver stipend, enhanced respite care, wellness contacts, and travel compensation for VA health care appointments (Table 1).4,5
Patients undergo a rigorous assessment and highly specialized and individualized clinical decision-making process to confirm the service is appropriate for the patient. PCAFC was restructured and expanded on October 1, 2020.6 Currently, veterans who incurred or aggravated a serious injury (defined by a single or combined service-connection rating of ≥ 70%) in active military service before May 8, 1975, or after September 10, 2001, are eligible for PCAFC.6 Most notably, these changes opened eligibility in the PCAFC to caregivers of veterans from the Vietnam, Korean, and World War II eras of conflict and veterans with dependence in activities of daily living (ADL) due to a wider variety of illnesses, including dementia.6 The PCAFC is set to further expand to caregivers of otherwise eligible veterans of all eras of service on October 1, 2022, 2 years after the initial expansion, as laid out in the 2018 VA MISSION Act.6 Additional information on the history of the PGCSS and PCAFC and eligibility criteria for veterans and their family caregivers can be found in Tables 2 and 3.
Posttraumatic stress disorder (PTSD) and cognitive impairment are 2 common causes of disability among veterans who receive VHA care. Among older veterans, rates of lifetime development of PTSD reach up to 30%.7 Dementia diagnosis is also more common in older veterans compared with age-matched civilians.8 Furthermore, a prior diagnosis of PTSD has been associated with nearly a 2-fold increase in risk of development of dementia in older age.7 These conditions are also linked to high degrees of service connection. PTSD is the third most prevalent service-connected disability for veterans receiving compensation and cognitive limitation is the third most prevalent category of service-connected disability among veterans.9
We present a case of a Vietnam-era veteran with a history of combat exposure and service-connected PTSD, and a later diagnosis of Lewy body dementia (LBD). Through combination of VHA geriatric services, the CSP, and the expanded PCAFC, the veteran’s primary family caregiver received the materials, support, and financial resources necessary to enable at-home living for the veteran, despite his illness and later complications.
Case Presentation
A male combat veteran presented to his primary care practitioner (PCP) with concerns of several years of progressive changes in gait, forgetfulness, and a gradual decline in the ability to live independently without assistance. At that time, his medical history was notable for PTSD (50% service connection), which had been diagnosed over a decade prior (but for which the veteran had refused medication or therapy on multiple occasions, stating he preferred to “breathe through” his intrusive symptom flare-ups), localized prostate cancer with a radical prostatectomy (100% service connection), multiple kidney stones with persistent left ureteral inflammation, and arteriosclerotic heart disease (10% service connection). A Saint Louis University Mental Status Exam (SLUMS) performed by the PCP was notable for a score of 9/30, in the dementia range. A computed tomography of the brain demonstrated scattered foci of hypoattenuation attributable to normal aging without any other pathology noted.
The veteran was referred to the Cognitive Care clinic, a local longitudinal multidisciplinary dementia care clinic, along with his spouse/caregiver. Cognitive testing was performed by a licensed clinical psychologist in the clinic and was notable for a Mini-Mental State Exam (MMSE) score of 18/30, also in the dementia range, and a more robust neuropsychiatric battery demonstrated borderline intact memory and language function but impairments in executive function and visuospatial skills. The patient’s clinical history included functional loss over time, with total dependence in instrumental activities of daily living (IADL), or tasks necessary to be fully independent or manage a household, including inability to manage finances, and some need for assistance in ADL, or personal care tasks such as dressing or grooming, including bathing. Physical examination was notable for bradykinesia, a shuffling gait, and rare episodes of speaking to someone who was not in the room, thought to be due to mild nondistressing hallucinations.
A diagnosis of LBD was made. At time of diagnosis, the patient met criteria for probable dementia with Lewy bodies, with 2 of 4 core clinical features (hallucinations and Parkinsonism), and multiple supportive features (gait disturbance, sensory disturbance, and altered mood).10,11 The veteran continued to develop more supportive features for diagnosis of LBD over time, including evidence of autonomic instability.
The veteran and his caregiver were educated on his diagnosis, and longitudinal support was offered. The veteran was no longer driving, and due to the severity of his symptoms, the importance of driving cessation was reinforced by the care team. Over the course of the next year, his illness progressed, with more frequent behaviors and psychological symptoms of dementia (BPSD). He began to exhibit nighttime wandering throughout the house and became more anxious and restless during the day. He lost the ability to make his own health care decisions, and his spouse became his activated health care power of attorney (HCPOA). His BPSD became more disruptive to daily life and was accompanied by a change in the character of his hallucinations, with prior nondistressing visions of other people being replaced with visions of war, burning bodies, and violence, much of it related to combat experiences in Vietnam. The BPSD began to include hiding behind furniture, running out of the house, and shouting and crying in response to hallucinations. At times, his BPSD became violent, lashing out in fear against his hallucinations and caregiver.
The veteran’s change in BPSD was concerning for a new baseline, rather than being clearly related to an underlying unmet physical need, such as pain, hunger, sleep, or discomfort. Multiple hospital admissions during that year involved IV hydration and treatment for urinary tract infections (UTI) for several days of inpatient stay at a time, but these behaviors persisted despite infection treatment and hydration. The patient’s changes in BPSD were thought to be secondary to uncovered and intensified PTSD in the setting of progressive dementia. Due to the clear danger the patient posed to himself and others, potential treatment options for these PTSD-related hallucinations were discussed with his caregiver. The caregiver shared that the patient’s BPSD and hallucinations were so distressing that “he would never want to live like this,” and that things had progressed to the point that “he has no quality of life.”
Oral aripiprazole 2 mg twice daily was prescribed after the risks of infection, cardiac complications, and exacerbation of movement disorder symptoms, such as increased stiffness and falls, were discussed with the caregiver. The caregiver was employed and relied on continued employment for income, but the patient could not be safely left alone. As the patient and his caregiver had reached a crisis point and living at home no longer appeared to be safe, the patient was referred to a VA-contracted skilled nursing facility (SNF) for long-term care. The patient’s caregiver was also referred to CSP for support during this transition. Due to the patient’s level of service connection and personal needs, as well as the patient and caregiver’s preference for the veteran to remain in his home, they were evaluated for the PCAFC for enhanced support to enable home as an alternative to facility living, should the patient respond to the antipsychotic therapy sufficiently, which was evaluated on a regular basis.
After several months, the patient’s BPSD had improved significantly, and he was no longer experiencing distressing hallucinations. However, his mobility also declined, and he became fully dependent in most ADL, including transfers, hygiene, and toileting. Due to the COVID-19 pandemic, visitation was limited, which was difficult for both the patient and his caregiver. The veteran and caregiver were approved for PCAFC due to the veteran’s combination of service-connected illnesses > 70%, dependence for most ADLs, and need for continuous supervision. A transfer home from the SNF was arranged.
The PCAFC allowed the veteran’s caregiver and family members to provide in-home full-time caregiving, as an alternative to facility placement. The caregiver received a variety of support, including access to peer support, instruction on ways to assist in his toileting, hygiene, and transfers, and a caregiving stipend. In addition to offsetting lost wages, the stipend also helped offset the cost of care supplies which were not provided or were not readily available from the VA, which at the time included the patient’s preferred nutritional supplement and some supplies for personal care.
The veteran’s care needs continued to escalate. A fall at home resulted in a hip fracture, which was treated with surgical pinning. Postfracture physical therapy in a facility was considered, but ultimately was provided at home. The patient also experienced multiple UTIs and resulting delirium, with accompanying agitation and hallucinations. These episodes improved with IV antibiotics and hydration during short hospital stays. Ultimately, a computed tomography demonstrated overflow incontinence likely related to urologic damage from prior kidney stones and stent placement was recommended.
Visiting skilled nurses for the patient’s area were difficult to coordinate but were eventually arranged. The patient continued residing in his home with the support of his caregiver, the PCAFC, and the local VA medical center geriatric and transitional care services. The patient was also referred to the palliative care outpatient specialty clinic for discussion of goals of care and assistance with advance care planning as his illness progressed. Mental health and geriatric psychiatry consult teams were considered for this case but not utilized.
Discussion
Older adult Americans are at high risk of poor financial wellbeing, with nearly one quarter of Americans aged > 62 years experiencing financial insecurity.12 Even in this case with health care provided by the VA, successful in-home care was challenging and required a dedicated live-in caregiver, care coordination resources, and financial support. As part of its mission of caring for veterans, the VA has instituted CSP, whose mission is to promote the health and well-being of family caregivers through education, support, and services.
PCAFC offers enhanced clinical support for caregivers of eligible veterans who are seriously injured. This includes resources, education, support, financial stipends, health insurance (if eligible), and beneficiary travel (if eligible) to primary caregivers of eligible veterans. PCAFC was originally reserved for veterans who had onset of service-related disability after September 11, 2001, with an associated personal care need. In this population, PCAFC demonstrated an increased usage of clinical resources, likely related to increased ease in accessing care.13
The cohort of post-9/11 veterans is very different from the cohort of veterans and their caregivers who may now qualify for the PCAFC after its October 2020 expansion. Veterans from the Vietnam, Korean, and World War II eras of conflict have rates of service-connected disability 2 to 3 times higher than those of post-9/11 era veterans and are at greater risk for dementia.9 Veterans aged ≥ 75 years who have service connection also report higher rates of difficulty with independent living and self-care compared with their younger peers.9 Since dementia and PTSD are common causes of service connection and disability it is likely that a significant proportion of older veterans will be eligible to apply for the newly expanded PCAFC.
To be eligible for PCAFC, a veteran must have a service-connected disability rating of ≥ 70% and must need in-person care services for ≥ 6 continuous months, based on either an inability to perform an ADL, or a need for supervision, protection, or instruction.
It is not clear how CSP use or increased access to PCAFC will impact costs. However, the PCAFC monthly stipend is scaled to the median wage of a home health aide and to the location of the caregiver, which is considerably less than the cost of recurrent hospitalization or a year of facility-level care.15 The CSP may eventually be a successful long-term investment in cost savings. In order to ensure the process for PCAFC approval is uniform and prompt as the program expands, CSP has restructured, increasing the number of employees, improving the patient review process, and expanding staff training.16 The VA plans to continually re-assess CSP using the infrastructure of the Caregiver Record Management Application as it continues to expand.17
Conclusions
Dementia and PTSD commonly coexist and are a significant source of disability in the service-connected veteran population. This case brings attention to the recent expansion of PCAFC, which now has the potential to support eligible veterans from the World War II, Korean, and Vietnam-era conflicts, in whom these illnesses are more common. In this case, in-home care was preferred by the veteran and primary caregiver but would not have been possible without a complex intervention. There is still more research to be done on the best way to meet the needs of older adults with dementia, the impact of in-home care, and the system-wide implications of PCAFC, especially as the program grows. However, in-home care is preferable to SNF living for many veterans and caregivers, and CSP will continue to be an essential element of providing care for this population.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. The authors would also like to thank the members of the Veterans Affairs Central Office and National Caregiver Support Program Office, including Elyse Kaplan, Melinda Hogue, Beth Wolfsohn, Colleen Richardson, and Timothy Jobin, for their thorough review of the work and edits to ensure accurate program description.
1. Chi W, Graf E, Hughes L, et al. Community-dwelling older adults with dementia and their caregivers: key indicators from the National Health and Aging Trends study. Published January 29, 2019. Accessed February 16, 2022. https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//186501/DemChartbook.pdf
2. Rapaport P, Burton A, Leverton M, et al. “I just keep thinking that I don’t want to rely on people.” A qualitative study of how people living with dementia achieve and maintain independence at home: stakeholder perspectives. BMC Geriatr. 2020;20(1):1-11. doi:10.1186/s12877-019-1406-6
3. Miller EA, Gidmark S, Gadbois E, Rudolph JL, Intrator O. Nursing home referral within the Veterans Health Administration: practice variation by payment source and facility type. Res Aging. 2018;40(7):687-711. doi:10.1177/0164027517730383
4. Veterans Benefits, Health Care, and Information Technology Act of 2006, Pub L No. 109-461, 120 Stat. 3403.
5. Caregivers and Veterans Omnibus Health Services Act of 2010, Pub L No. 111-163, 115 Stat 552.
6. VA MISSION Act of 2018. 38 CFR § 17.
7. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
8. Krishnan LL, Petersen NJ, Snow AL, et al. Prevalence of dementia among Veterans Affairs medical care system users. Dement Geriatr Cogn Disord. 2005;20(4):245-253. doi:10.1159/000087345
9. Holder, KA. The Disability of Veterans. Social, Economic, and Housing Statistics Division, US Census Bureau; 2014. Accessed February 9, 2022. https://www.census.gov/content/dam/Census/library/working-papers/2016/demo/Holder-2016-01.pdf
10. Sanford AM. Lewy body dementia. Clin Geriatr Med. 2018;34(4):603-615. doi:10.1016/j.cger.2018.06.007
11. Armstrong MJ. Lewy body dementias. Continuum (Minneap Minn). 2019;25(1):128-146. doi:10.1212/CON.0000000000000685
12. Bureau of Consumer Financial Protection. Financial well-being of older Americans. Published December 2018. Accessed February 17, 2022. https://files.consumerfinance.gov/f/documents/bcfp_financial-well-being-older-americans_report.pdf
13. Van Houtven CH, Smith VA, Stechuchak KM, et al. Comprehensive support for family caregivers: impact on veteran health care utilization and costs. Med Care Res Rev. 2019;76(1):89-114. doi:10.1177/1077558717697015
14. Boland L, Légaré F, Perez MMB, et al. Impact of home care versus alternative locations of care on elder health outcomes: an overview of systematic reviews. BMC Geriatr. 2017;17(1):20. doi:10.1186/s12877-016-0395-y
15. Program of Comprehensive Assistance for Family Caregivers Improvements and Amendments Under the VA MISSION Act of 2018. 85 FR § 13356.
16. Extension of Program of Comprehensive Assistance for Family Caregivers Eligibility for Legacy Participants and Legacy Applicants. 86 FR § 52614.
17. US Department of Veterans Affairs, 2020. Certification of the Implementation of the Caregiver Records Management Application (CARMA). 85 FR § 63358.
18. Sussman, JS. Department of Veterans Affairs: Caregiver Support. Congressional Research Service Report No. R46282. Published March 24, 2020. Accessed February 16, 2022. https://www.everycrsreport.com/files/20200324_R46282_656f1e8338af12a2a676c471be3b3c13b2fcb0bb.pdf
19. US Department of Veterans Affairs. Veterans Affairs Program of Comprehensive Assistance for Family Caregivers Eligibility Criteria Fact Sheet. Washington, DC: U.S. Department of Veterans Affairs; 2020. Accessed February 9, 2022. https://www.caregiver.va.gov/pdfs/MissionAct/EligibilityCriteriaFactsheet_Chapter2_Launch_Approved_Final_100120.pdf
Caregiving for a person with dementia in the community can be extremely difficult work. Much of this work falls on unpaid or informal caregivers. Sixty-three percent of older adults with dementia depend completely on unpaid caregivers, and an additional 26% receive some combination of paid and unpaid support, together comprising nearly 90% of the more than 3 million older Americans with dementia.1 In-home care is preferable for these patients. For veterans, the Caregiver Support Program (CSP) is the only US Department of Veterans Affairs (VA) program that exclusively supports caregivers. Although the CSP is not a nursing home diversion or cost savings program, successfully enabling at-home living in lieu of facility living also has the potential to reduce overall cost of care, and most importantly, to enable veterans who desire it to age at home.2,3
The CSP has 2 unique programs for caregivers of eligible veterans. The Program of General Caregiver Support Services (PGCSS) provides resources, education, and support to caregivers of all veterans enrolled in the Veterans Health Administration (VHA). The Program of Comprehensive Assistance for Family Caregivers (PCAFC) provides education and training, access to health care insurance if eligible, mental health counseling, access to a monthly caregiver stipend, enhanced respite care, wellness contacts, and travel compensation for VA health care appointments (Table 1).4,5
Patients undergo a rigorous assessment and highly specialized and individualized clinical decision-making process to confirm the service is appropriate for the patient. PCAFC was restructured and expanded on October 1, 2020.6 Currently, veterans who incurred or aggravated a serious injury (defined by a single or combined service-connection rating of ≥ 70%) in active military service before May 8, 1975, or after September 10, 2001, are eligible for PCAFC.6 Most notably, these changes opened eligibility in the PCAFC to caregivers of veterans from the Vietnam, Korean, and World War II eras of conflict and veterans with dependence in activities of daily living (ADL) due to a wider variety of illnesses, including dementia.6 The PCAFC is set to further expand to caregivers of otherwise eligible veterans of all eras of service on October 1, 2022, 2 years after the initial expansion, as laid out in the 2018 VA MISSION Act.6 Additional information on the history of the PGCSS and PCAFC and eligibility criteria for veterans and their family caregivers can be found in Tables 2 and 3.
Posttraumatic stress disorder (PTSD) and cognitive impairment are 2 common causes of disability among veterans who receive VHA care. Among older veterans, rates of lifetime development of PTSD reach up to 30%.7 Dementia diagnosis is also more common in older veterans compared with age-matched civilians.8 Furthermore, a prior diagnosis of PTSD has been associated with nearly a 2-fold increase in risk of development of dementia in older age.7 These conditions are also linked to high degrees of service connection. PTSD is the third most prevalent service-connected disability for veterans receiving compensation and cognitive limitation is the third most prevalent category of service-connected disability among veterans.9
We present a case of a Vietnam-era veteran with a history of combat exposure and service-connected PTSD, and a later diagnosis of Lewy body dementia (LBD). Through combination of VHA geriatric services, the CSP, and the expanded PCAFC, the veteran’s primary family caregiver received the materials, support, and financial resources necessary to enable at-home living for the veteran, despite his illness and later complications.
Case Presentation
A male combat veteran presented to his primary care practitioner (PCP) with concerns of several years of progressive changes in gait, forgetfulness, and a gradual decline in the ability to live independently without assistance. At that time, his medical history was notable for PTSD (50% service connection), which had been diagnosed over a decade prior (but for which the veteran had refused medication or therapy on multiple occasions, stating he preferred to “breathe through” his intrusive symptom flare-ups), localized prostate cancer with a radical prostatectomy (100% service connection), multiple kidney stones with persistent left ureteral inflammation, and arteriosclerotic heart disease (10% service connection). A Saint Louis University Mental Status Exam (SLUMS) performed by the PCP was notable for a score of 9/30, in the dementia range. A computed tomography of the brain demonstrated scattered foci of hypoattenuation attributable to normal aging without any other pathology noted.
The veteran was referred to the Cognitive Care clinic, a local longitudinal multidisciplinary dementia care clinic, along with his spouse/caregiver. Cognitive testing was performed by a licensed clinical psychologist in the clinic and was notable for a Mini-Mental State Exam (MMSE) score of 18/30, also in the dementia range, and a more robust neuropsychiatric battery demonstrated borderline intact memory and language function but impairments in executive function and visuospatial skills. The patient’s clinical history included functional loss over time, with total dependence in instrumental activities of daily living (IADL), or tasks necessary to be fully independent or manage a household, including inability to manage finances, and some need for assistance in ADL, or personal care tasks such as dressing or grooming, including bathing. Physical examination was notable for bradykinesia, a shuffling gait, and rare episodes of speaking to someone who was not in the room, thought to be due to mild nondistressing hallucinations.
A diagnosis of LBD was made. At time of diagnosis, the patient met criteria for probable dementia with Lewy bodies, with 2 of 4 core clinical features (hallucinations and Parkinsonism), and multiple supportive features (gait disturbance, sensory disturbance, and altered mood).10,11 The veteran continued to develop more supportive features for diagnosis of LBD over time, including evidence of autonomic instability.
The veteran and his caregiver were educated on his diagnosis, and longitudinal support was offered. The veteran was no longer driving, and due to the severity of his symptoms, the importance of driving cessation was reinforced by the care team. Over the course of the next year, his illness progressed, with more frequent behaviors and psychological symptoms of dementia (BPSD). He began to exhibit nighttime wandering throughout the house and became more anxious and restless during the day. He lost the ability to make his own health care decisions, and his spouse became his activated health care power of attorney (HCPOA). His BPSD became more disruptive to daily life and was accompanied by a change in the character of his hallucinations, with prior nondistressing visions of other people being replaced with visions of war, burning bodies, and violence, much of it related to combat experiences in Vietnam. The BPSD began to include hiding behind furniture, running out of the house, and shouting and crying in response to hallucinations. At times, his BPSD became violent, lashing out in fear against his hallucinations and caregiver.
The veteran’s change in BPSD was concerning for a new baseline, rather than being clearly related to an underlying unmet physical need, such as pain, hunger, sleep, or discomfort. Multiple hospital admissions during that year involved IV hydration and treatment for urinary tract infections (UTI) for several days of inpatient stay at a time, but these behaviors persisted despite infection treatment and hydration. The patient’s changes in BPSD were thought to be secondary to uncovered and intensified PTSD in the setting of progressive dementia. Due to the clear danger the patient posed to himself and others, potential treatment options for these PTSD-related hallucinations were discussed with his caregiver. The caregiver shared that the patient’s BPSD and hallucinations were so distressing that “he would never want to live like this,” and that things had progressed to the point that “he has no quality of life.”
Oral aripiprazole 2 mg twice daily was prescribed after the risks of infection, cardiac complications, and exacerbation of movement disorder symptoms, such as increased stiffness and falls, were discussed with the caregiver. The caregiver was employed and relied on continued employment for income, but the patient could not be safely left alone. As the patient and his caregiver had reached a crisis point and living at home no longer appeared to be safe, the patient was referred to a VA-contracted skilled nursing facility (SNF) for long-term care. The patient’s caregiver was also referred to CSP for support during this transition. Due to the patient’s level of service connection and personal needs, as well as the patient and caregiver’s preference for the veteran to remain in his home, they were evaluated for the PCAFC for enhanced support to enable home as an alternative to facility living, should the patient respond to the antipsychotic therapy sufficiently, which was evaluated on a regular basis.
After several months, the patient’s BPSD had improved significantly, and he was no longer experiencing distressing hallucinations. However, his mobility also declined, and he became fully dependent in most ADL, including transfers, hygiene, and toileting. Due to the COVID-19 pandemic, visitation was limited, which was difficult for both the patient and his caregiver. The veteran and caregiver were approved for PCAFC due to the veteran’s combination of service-connected illnesses > 70%, dependence for most ADLs, and need for continuous supervision. A transfer home from the SNF was arranged.
The PCAFC allowed the veteran’s caregiver and family members to provide in-home full-time caregiving, as an alternative to facility placement. The caregiver received a variety of support, including access to peer support, instruction on ways to assist in his toileting, hygiene, and transfers, and a caregiving stipend. In addition to offsetting lost wages, the stipend also helped offset the cost of care supplies which were not provided or were not readily available from the VA, which at the time included the patient’s preferred nutritional supplement and some supplies for personal care.
The veteran’s care needs continued to escalate. A fall at home resulted in a hip fracture, which was treated with surgical pinning. Postfracture physical therapy in a facility was considered, but ultimately was provided at home. The patient also experienced multiple UTIs and resulting delirium, with accompanying agitation and hallucinations. These episodes improved with IV antibiotics and hydration during short hospital stays. Ultimately, a computed tomography demonstrated overflow incontinence likely related to urologic damage from prior kidney stones and stent placement was recommended.
Visiting skilled nurses for the patient’s area were difficult to coordinate but were eventually arranged. The patient continued residing in his home with the support of his caregiver, the PCAFC, and the local VA medical center geriatric and transitional care services. The patient was also referred to the palliative care outpatient specialty clinic for discussion of goals of care and assistance with advance care planning as his illness progressed. Mental health and geriatric psychiatry consult teams were considered for this case but not utilized.
Discussion
Older adult Americans are at high risk of poor financial wellbeing, with nearly one quarter of Americans aged > 62 years experiencing financial insecurity.12 Even in this case with health care provided by the VA, successful in-home care was challenging and required a dedicated live-in caregiver, care coordination resources, and financial support. As part of its mission of caring for veterans, the VA has instituted CSP, whose mission is to promote the health and well-being of family caregivers through education, support, and services.
PCAFC offers enhanced clinical support for caregivers of eligible veterans who are seriously injured. This includes resources, education, support, financial stipends, health insurance (if eligible), and beneficiary travel (if eligible) to primary caregivers of eligible veterans. PCAFC was originally reserved for veterans who had onset of service-related disability after September 11, 2001, with an associated personal care need. In this population, PCAFC demonstrated an increased usage of clinical resources, likely related to increased ease in accessing care.13
The cohort of post-9/11 veterans is very different from the cohort of veterans and their caregivers who may now qualify for the PCAFC after its October 2020 expansion. Veterans from the Vietnam, Korean, and World War II eras of conflict have rates of service-connected disability 2 to 3 times higher than those of post-9/11 era veterans and are at greater risk for dementia.9 Veterans aged ≥ 75 years who have service connection also report higher rates of difficulty with independent living and self-care compared with their younger peers.9 Since dementia and PTSD are common causes of service connection and disability it is likely that a significant proportion of older veterans will be eligible to apply for the newly expanded PCAFC.
To be eligible for PCAFC, a veteran must have a service-connected disability rating of ≥ 70% and must need in-person care services for ≥ 6 continuous months, based on either an inability to perform an ADL, or a need for supervision, protection, or instruction.
It is not clear how CSP use or increased access to PCAFC will impact costs. However, the PCAFC monthly stipend is scaled to the median wage of a home health aide and to the location of the caregiver, which is considerably less than the cost of recurrent hospitalization or a year of facility-level care.15 The CSP may eventually be a successful long-term investment in cost savings. In order to ensure the process for PCAFC approval is uniform and prompt as the program expands, CSP has restructured, increasing the number of employees, improving the patient review process, and expanding staff training.16 The VA plans to continually re-assess CSP using the infrastructure of the Caregiver Record Management Application as it continues to expand.17
Conclusions
Dementia and PTSD commonly coexist and are a significant source of disability in the service-connected veteran population. This case brings attention to the recent expansion of PCAFC, which now has the potential to support eligible veterans from the World War II, Korean, and Vietnam-era conflicts, in whom these illnesses are more common. In this case, in-home care was preferred by the veteran and primary caregiver but would not have been possible without a complex intervention. There is still more research to be done on the best way to meet the needs of older adults with dementia, the impact of in-home care, and the system-wide implications of PCAFC, especially as the program grows. However, in-home care is preferable to SNF living for many veterans and caregivers, and CSP will continue to be an essential element of providing care for this population.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. The authors would also like to thank the members of the Veterans Affairs Central Office and National Caregiver Support Program Office, including Elyse Kaplan, Melinda Hogue, Beth Wolfsohn, Colleen Richardson, and Timothy Jobin, for their thorough review of the work and edits to ensure accurate program description.
Caregiving for a person with dementia in the community can be extremely difficult work. Much of this work falls on unpaid or informal caregivers. Sixty-three percent of older adults with dementia depend completely on unpaid caregivers, and an additional 26% receive some combination of paid and unpaid support, together comprising nearly 90% of the more than 3 million older Americans with dementia.1 In-home care is preferable for these patients. For veterans, the Caregiver Support Program (CSP) is the only US Department of Veterans Affairs (VA) program that exclusively supports caregivers. Although the CSP is not a nursing home diversion or cost savings program, successfully enabling at-home living in lieu of facility living also has the potential to reduce overall cost of care, and most importantly, to enable veterans who desire it to age at home.2,3
The CSP has 2 unique programs for caregivers of eligible veterans. The Program of General Caregiver Support Services (PGCSS) provides resources, education, and support to caregivers of all veterans enrolled in the Veterans Health Administration (VHA). The Program of Comprehensive Assistance for Family Caregivers (PCAFC) provides education and training, access to health care insurance if eligible, mental health counseling, access to a monthly caregiver stipend, enhanced respite care, wellness contacts, and travel compensation for VA health care appointments (Table 1).4,5
Patients undergo a rigorous assessment and highly specialized and individualized clinical decision-making process to confirm the service is appropriate for the patient. PCAFC was restructured and expanded on October 1, 2020.6 Currently, veterans who incurred or aggravated a serious injury (defined by a single or combined service-connection rating of ≥ 70%) in active military service before May 8, 1975, or after September 10, 2001, are eligible for PCAFC.6 Most notably, these changes opened eligibility in the PCAFC to caregivers of veterans from the Vietnam, Korean, and World War II eras of conflict and veterans with dependence in activities of daily living (ADL) due to a wider variety of illnesses, including dementia.6 The PCAFC is set to further expand to caregivers of otherwise eligible veterans of all eras of service on October 1, 2022, 2 years after the initial expansion, as laid out in the 2018 VA MISSION Act.6 Additional information on the history of the PGCSS and PCAFC and eligibility criteria for veterans and their family caregivers can be found in Tables 2 and 3.
Posttraumatic stress disorder (PTSD) and cognitive impairment are 2 common causes of disability among veterans who receive VHA care. Among older veterans, rates of lifetime development of PTSD reach up to 30%.7 Dementia diagnosis is also more common in older veterans compared with age-matched civilians.8 Furthermore, a prior diagnosis of PTSD has been associated with nearly a 2-fold increase in risk of development of dementia in older age.7 These conditions are also linked to high degrees of service connection. PTSD is the third most prevalent service-connected disability for veterans receiving compensation and cognitive limitation is the third most prevalent category of service-connected disability among veterans.9
We present a case of a Vietnam-era veteran with a history of combat exposure and service-connected PTSD, and a later diagnosis of Lewy body dementia (LBD). Through combination of VHA geriatric services, the CSP, and the expanded PCAFC, the veteran’s primary family caregiver received the materials, support, and financial resources necessary to enable at-home living for the veteran, despite his illness and later complications.
Case Presentation
A male combat veteran presented to his primary care practitioner (PCP) with concerns of several years of progressive changes in gait, forgetfulness, and a gradual decline in the ability to live independently without assistance. At that time, his medical history was notable for PTSD (50% service connection), which had been diagnosed over a decade prior (but for which the veteran had refused medication or therapy on multiple occasions, stating he preferred to “breathe through” his intrusive symptom flare-ups), localized prostate cancer with a radical prostatectomy (100% service connection), multiple kidney stones with persistent left ureteral inflammation, and arteriosclerotic heart disease (10% service connection). A Saint Louis University Mental Status Exam (SLUMS) performed by the PCP was notable for a score of 9/30, in the dementia range. A computed tomography of the brain demonstrated scattered foci of hypoattenuation attributable to normal aging without any other pathology noted.
The veteran was referred to the Cognitive Care clinic, a local longitudinal multidisciplinary dementia care clinic, along with his spouse/caregiver. Cognitive testing was performed by a licensed clinical psychologist in the clinic and was notable for a Mini-Mental State Exam (MMSE) score of 18/30, also in the dementia range, and a more robust neuropsychiatric battery demonstrated borderline intact memory and language function but impairments in executive function and visuospatial skills. The patient’s clinical history included functional loss over time, with total dependence in instrumental activities of daily living (IADL), or tasks necessary to be fully independent or manage a household, including inability to manage finances, and some need for assistance in ADL, or personal care tasks such as dressing or grooming, including bathing. Physical examination was notable for bradykinesia, a shuffling gait, and rare episodes of speaking to someone who was not in the room, thought to be due to mild nondistressing hallucinations.
A diagnosis of LBD was made. At time of diagnosis, the patient met criteria for probable dementia with Lewy bodies, with 2 of 4 core clinical features (hallucinations and Parkinsonism), and multiple supportive features (gait disturbance, sensory disturbance, and altered mood).10,11 The veteran continued to develop more supportive features for diagnosis of LBD over time, including evidence of autonomic instability.
The veteran and his caregiver were educated on his diagnosis, and longitudinal support was offered. The veteran was no longer driving, and due to the severity of his symptoms, the importance of driving cessation was reinforced by the care team. Over the course of the next year, his illness progressed, with more frequent behaviors and psychological symptoms of dementia (BPSD). He began to exhibit nighttime wandering throughout the house and became more anxious and restless during the day. He lost the ability to make his own health care decisions, and his spouse became his activated health care power of attorney (HCPOA). His BPSD became more disruptive to daily life and was accompanied by a change in the character of his hallucinations, with prior nondistressing visions of other people being replaced with visions of war, burning bodies, and violence, much of it related to combat experiences in Vietnam. The BPSD began to include hiding behind furniture, running out of the house, and shouting and crying in response to hallucinations. At times, his BPSD became violent, lashing out in fear against his hallucinations and caregiver.
The veteran’s change in BPSD was concerning for a new baseline, rather than being clearly related to an underlying unmet physical need, such as pain, hunger, sleep, or discomfort. Multiple hospital admissions during that year involved IV hydration and treatment for urinary tract infections (UTI) for several days of inpatient stay at a time, but these behaviors persisted despite infection treatment and hydration. The patient’s changes in BPSD were thought to be secondary to uncovered and intensified PTSD in the setting of progressive dementia. Due to the clear danger the patient posed to himself and others, potential treatment options for these PTSD-related hallucinations were discussed with his caregiver. The caregiver shared that the patient’s BPSD and hallucinations were so distressing that “he would never want to live like this,” and that things had progressed to the point that “he has no quality of life.”
Oral aripiprazole 2 mg twice daily was prescribed after the risks of infection, cardiac complications, and exacerbation of movement disorder symptoms, such as increased stiffness and falls, were discussed with the caregiver. The caregiver was employed and relied on continued employment for income, but the patient could not be safely left alone. As the patient and his caregiver had reached a crisis point and living at home no longer appeared to be safe, the patient was referred to a VA-contracted skilled nursing facility (SNF) for long-term care. The patient’s caregiver was also referred to CSP for support during this transition. Due to the patient’s level of service connection and personal needs, as well as the patient and caregiver’s preference for the veteran to remain in his home, they were evaluated for the PCAFC for enhanced support to enable home as an alternative to facility living, should the patient respond to the antipsychotic therapy sufficiently, which was evaluated on a regular basis.
After several months, the patient’s BPSD had improved significantly, and he was no longer experiencing distressing hallucinations. However, his mobility also declined, and he became fully dependent in most ADL, including transfers, hygiene, and toileting. Due to the COVID-19 pandemic, visitation was limited, which was difficult for both the patient and his caregiver. The veteran and caregiver were approved for PCAFC due to the veteran’s combination of service-connected illnesses > 70%, dependence for most ADLs, and need for continuous supervision. A transfer home from the SNF was arranged.
The PCAFC allowed the veteran’s caregiver and family members to provide in-home full-time caregiving, as an alternative to facility placement. The caregiver received a variety of support, including access to peer support, instruction on ways to assist in his toileting, hygiene, and transfers, and a caregiving stipend. In addition to offsetting lost wages, the stipend also helped offset the cost of care supplies which were not provided or were not readily available from the VA, which at the time included the patient’s preferred nutritional supplement and some supplies for personal care.
The veteran’s care needs continued to escalate. A fall at home resulted in a hip fracture, which was treated with surgical pinning. Postfracture physical therapy in a facility was considered, but ultimately was provided at home. The patient also experienced multiple UTIs and resulting delirium, with accompanying agitation and hallucinations. These episodes improved with IV antibiotics and hydration during short hospital stays. Ultimately, a computed tomography demonstrated overflow incontinence likely related to urologic damage from prior kidney stones and stent placement was recommended.
Visiting skilled nurses for the patient’s area were difficult to coordinate but were eventually arranged. The patient continued residing in his home with the support of his caregiver, the PCAFC, and the local VA medical center geriatric and transitional care services. The patient was also referred to the palliative care outpatient specialty clinic for discussion of goals of care and assistance with advance care planning as his illness progressed. Mental health and geriatric psychiatry consult teams were considered for this case but not utilized.
Discussion
Older adult Americans are at high risk of poor financial wellbeing, with nearly one quarter of Americans aged > 62 years experiencing financial insecurity.12 Even in this case with health care provided by the VA, successful in-home care was challenging and required a dedicated live-in caregiver, care coordination resources, and financial support. As part of its mission of caring for veterans, the VA has instituted CSP, whose mission is to promote the health and well-being of family caregivers through education, support, and services.
PCAFC offers enhanced clinical support for caregivers of eligible veterans who are seriously injured. This includes resources, education, support, financial stipends, health insurance (if eligible), and beneficiary travel (if eligible) to primary caregivers of eligible veterans. PCAFC was originally reserved for veterans who had onset of service-related disability after September 11, 2001, with an associated personal care need. In this population, PCAFC demonstrated an increased usage of clinical resources, likely related to increased ease in accessing care.13
The cohort of post-9/11 veterans is very different from the cohort of veterans and their caregivers who may now qualify for the PCAFC after its October 2020 expansion. Veterans from the Vietnam, Korean, and World War II eras of conflict have rates of service-connected disability 2 to 3 times higher than those of post-9/11 era veterans and are at greater risk for dementia.9 Veterans aged ≥ 75 years who have service connection also report higher rates of difficulty with independent living and self-care compared with their younger peers.9 Since dementia and PTSD are common causes of service connection and disability it is likely that a significant proportion of older veterans will be eligible to apply for the newly expanded PCAFC.
To be eligible for PCAFC, a veteran must have a service-connected disability rating of ≥ 70% and must need in-person care services for ≥ 6 continuous months, based on either an inability to perform an ADL, or a need for supervision, protection, or instruction.
It is not clear how CSP use or increased access to PCAFC will impact costs. However, the PCAFC monthly stipend is scaled to the median wage of a home health aide and to the location of the caregiver, which is considerably less than the cost of recurrent hospitalization or a year of facility-level care.15 The CSP may eventually be a successful long-term investment in cost savings. In order to ensure the process for PCAFC approval is uniform and prompt as the program expands, CSP has restructured, increasing the number of employees, improving the patient review process, and expanding staff training.16 The VA plans to continually re-assess CSP using the infrastructure of the Caregiver Record Management Application as it continues to expand.17
Conclusions
Dementia and PTSD commonly coexist and are a significant source of disability in the service-connected veteran population. This case brings attention to the recent expansion of PCAFC, which now has the potential to support eligible veterans from the World War II, Korean, and Vietnam-era conflicts, in whom these illnesses are more common. In this case, in-home care was preferred by the veteran and primary caregiver but would not have been possible without a complex intervention. There is still more research to be done on the best way to meet the needs of older adults with dementia, the impact of in-home care, and the system-wide implications of PCAFC, especially as the program grows. However, in-home care is preferable to SNF living for many veterans and caregivers, and CSP will continue to be an essential element of providing care for this population.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. The authors would also like to thank the members of the Veterans Affairs Central Office and National Caregiver Support Program Office, including Elyse Kaplan, Melinda Hogue, Beth Wolfsohn, Colleen Richardson, and Timothy Jobin, for their thorough review of the work and edits to ensure accurate program description.
1. Chi W, Graf E, Hughes L, et al. Community-dwelling older adults with dementia and their caregivers: key indicators from the National Health and Aging Trends study. Published January 29, 2019. Accessed February 16, 2022. https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//186501/DemChartbook.pdf
2. Rapaport P, Burton A, Leverton M, et al. “I just keep thinking that I don’t want to rely on people.” A qualitative study of how people living with dementia achieve and maintain independence at home: stakeholder perspectives. BMC Geriatr. 2020;20(1):1-11. doi:10.1186/s12877-019-1406-6
3. Miller EA, Gidmark S, Gadbois E, Rudolph JL, Intrator O. Nursing home referral within the Veterans Health Administration: practice variation by payment source and facility type. Res Aging. 2018;40(7):687-711. doi:10.1177/0164027517730383
4. Veterans Benefits, Health Care, and Information Technology Act of 2006, Pub L No. 109-461, 120 Stat. 3403.
5. Caregivers and Veterans Omnibus Health Services Act of 2010, Pub L No. 111-163, 115 Stat 552.
6. VA MISSION Act of 2018. 38 CFR § 17.
7. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
8. Krishnan LL, Petersen NJ, Snow AL, et al. Prevalence of dementia among Veterans Affairs medical care system users. Dement Geriatr Cogn Disord. 2005;20(4):245-253. doi:10.1159/000087345
9. Holder, KA. The Disability of Veterans. Social, Economic, and Housing Statistics Division, US Census Bureau; 2014. Accessed February 9, 2022. https://www.census.gov/content/dam/Census/library/working-papers/2016/demo/Holder-2016-01.pdf
10. Sanford AM. Lewy body dementia. Clin Geriatr Med. 2018;34(4):603-615. doi:10.1016/j.cger.2018.06.007
11. Armstrong MJ. Lewy body dementias. Continuum (Minneap Minn). 2019;25(1):128-146. doi:10.1212/CON.0000000000000685
12. Bureau of Consumer Financial Protection. Financial well-being of older Americans. Published December 2018. Accessed February 17, 2022. https://files.consumerfinance.gov/f/documents/bcfp_financial-well-being-older-americans_report.pdf
13. Van Houtven CH, Smith VA, Stechuchak KM, et al. Comprehensive support for family caregivers: impact on veteran health care utilization and costs. Med Care Res Rev. 2019;76(1):89-114. doi:10.1177/1077558717697015
14. Boland L, Légaré F, Perez MMB, et al. Impact of home care versus alternative locations of care on elder health outcomes: an overview of systematic reviews. BMC Geriatr. 2017;17(1):20. doi:10.1186/s12877-016-0395-y
15. Program of Comprehensive Assistance for Family Caregivers Improvements and Amendments Under the VA MISSION Act of 2018. 85 FR § 13356.
16. Extension of Program of Comprehensive Assistance for Family Caregivers Eligibility for Legacy Participants and Legacy Applicants. 86 FR § 52614.
17. US Department of Veterans Affairs, 2020. Certification of the Implementation of the Caregiver Records Management Application (CARMA). 85 FR § 63358.
18. Sussman, JS. Department of Veterans Affairs: Caregiver Support. Congressional Research Service Report No. R46282. Published March 24, 2020. Accessed February 16, 2022. https://www.everycrsreport.com/files/20200324_R46282_656f1e8338af12a2a676c471be3b3c13b2fcb0bb.pdf
19. US Department of Veterans Affairs. Veterans Affairs Program of Comprehensive Assistance for Family Caregivers Eligibility Criteria Fact Sheet. Washington, DC: U.S. Department of Veterans Affairs; 2020. Accessed February 9, 2022. https://www.caregiver.va.gov/pdfs/MissionAct/EligibilityCriteriaFactsheet_Chapter2_Launch_Approved_Final_100120.pdf
1. Chi W, Graf E, Hughes L, et al. Community-dwelling older adults with dementia and their caregivers: key indicators from the National Health and Aging Trends study. Published January 29, 2019. Accessed February 16, 2022. https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//186501/DemChartbook.pdf
2. Rapaport P, Burton A, Leverton M, et al. “I just keep thinking that I don’t want to rely on people.” A qualitative study of how people living with dementia achieve and maintain independence at home: stakeholder perspectives. BMC Geriatr. 2020;20(1):1-11. doi:10.1186/s12877-019-1406-6
3. Miller EA, Gidmark S, Gadbois E, Rudolph JL, Intrator O. Nursing home referral within the Veterans Health Administration: practice variation by payment source and facility type. Res Aging. 2018;40(7):687-711. doi:10.1177/0164027517730383
4. Veterans Benefits, Health Care, and Information Technology Act of 2006, Pub L No. 109-461, 120 Stat. 3403.
5. Caregivers and Veterans Omnibus Health Services Act of 2010, Pub L No. 111-163, 115 Stat 552.
6. VA MISSION Act of 2018. 38 CFR § 17.
7. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
8. Krishnan LL, Petersen NJ, Snow AL, et al. Prevalence of dementia among Veterans Affairs medical care system users. Dement Geriatr Cogn Disord. 2005;20(4):245-253. doi:10.1159/000087345
9. Holder, KA. The Disability of Veterans. Social, Economic, and Housing Statistics Division, US Census Bureau; 2014. Accessed February 9, 2022. https://www.census.gov/content/dam/Census/library/working-papers/2016/demo/Holder-2016-01.pdf
10. Sanford AM. Lewy body dementia. Clin Geriatr Med. 2018;34(4):603-615. doi:10.1016/j.cger.2018.06.007
11. Armstrong MJ. Lewy body dementias. Continuum (Minneap Minn). 2019;25(1):128-146. doi:10.1212/CON.0000000000000685
12. Bureau of Consumer Financial Protection. Financial well-being of older Americans. Published December 2018. Accessed February 17, 2022. https://files.consumerfinance.gov/f/documents/bcfp_financial-well-being-older-americans_report.pdf
13. Van Houtven CH, Smith VA, Stechuchak KM, et al. Comprehensive support for family caregivers: impact on veteran health care utilization and costs. Med Care Res Rev. 2019;76(1):89-114. doi:10.1177/1077558717697015
14. Boland L, Légaré F, Perez MMB, et al. Impact of home care versus alternative locations of care on elder health outcomes: an overview of systematic reviews. BMC Geriatr. 2017;17(1):20. doi:10.1186/s12877-016-0395-y
15. Program of Comprehensive Assistance for Family Caregivers Improvements and Amendments Under the VA MISSION Act of 2018. 85 FR § 13356.
16. Extension of Program of Comprehensive Assistance for Family Caregivers Eligibility for Legacy Participants and Legacy Applicants. 86 FR § 52614.
17. US Department of Veterans Affairs, 2020. Certification of the Implementation of the Caregiver Records Management Application (CARMA). 85 FR § 63358.
18. Sussman, JS. Department of Veterans Affairs: Caregiver Support. Congressional Research Service Report No. R46282. Published March 24, 2020. Accessed February 16, 2022. https://www.everycrsreport.com/files/20200324_R46282_656f1e8338af12a2a676c471be3b3c13b2fcb0bb.pdf
19. US Department of Veterans Affairs. Veterans Affairs Program of Comprehensive Assistance for Family Caregivers Eligibility Criteria Fact Sheet. Washington, DC: U.S. Department of Veterans Affairs; 2020. Accessed February 9, 2022. https://www.caregiver.va.gov/pdfs/MissionAct/EligibilityCriteriaFactsheet_Chapter2_Launch_Approved_Final_100120.pdf
Gadolinium Deposition Disease: A Case Report and the Prevalence of Enhanced MRI Procedures Within the Veterans Health Administration
Gadolinium (Gd)-based contrast agents are frequently used in health care for enhancing magnetic resonance image (MRI) signals at low concentrations. Contrary to popular opinion, this widely used heavy metal is not biologically inert. Once notable for its safety profile, there is mounting evidence for Gd deposition in various organ systems of the body, even in those with normal renal function. A large knowledge gap remains concerning the potential harms of Gd deposition and the factors determining its elimination from the body. However, the findings of deposited Gd throughout various organs and their intracellular compartments even years after the initial exposure have been established. Here, we describe a case of a Vietnam-era veteran whose presentation, clinical, and laboratory findings were consistent within the spectrum of Gd deposition disease.
Case Presentation
A Vietnam-era veteran aged > 70 years presented for evaluation of Gd-based contrast agent–induced chronic multisymptomatic illness His medical history was significant for chronic low back pain, chronic hypertension, type 2 diabetes mellitus, and hypogonadism. Surgical history was notable for back surgery (24 years prior), laminectomy (2 years prior), shoulder replacement (2 years prior), and an epidural complicated by a hematoma (1 year prior). His presenting concerns included a painful and pruritic rash that worsened with showering, pain originating at the right Achilles tendon with migration to the knee, and shoulder pain. His symptoms started shortly after receiving multiple exposures to Gd-based contrast agents to enhance MRIs during his clinical care (Omniscan 20 mL, Omniscan 20 mL, and Gadovist 10 mL, administered 578, 565, and 496 days prior to the clinic visit, respectively). New onset headaches coincided with the timeline of symptom onset, in addition to hoarseness and liberation of an “oily substance” from the skin. More than one year prior to this clinic visit, he was considered for having polymyalgia rheumatica given the ambiguity of symptoms. Functional status remained impaired despite treatment with prednisone and methotrexate.
The patient’s military service was in the mid-1960s. He was deployed to Japan and had no knowledge of an Agent Orange exposure. His tobacco history was distant, and he reported no tattoos, prior transfusions, or occupational metal exposure
Clinical Findings
The patient was afebrile, normotensive (146/88 mmHg), and normocardic. His weight was 100 kg. He was well nourished and in no acute distress. The thought process was attentive, and his affect pleasant. Ocular examination was notable for arcus senilus. The fundoscopic examination was limited on the left, but there was no neovascularization on the right. Jugular venous pulsation was normal at 8 cm. Right ventricular impulse was slightly hyperdynamic, the rhythm was regular, and there was no abnormal splitting of S2. A soft-grade I/VI crescendo/decrescendo murmur was auscultated along the apex. Radial pulses were 2/2. He was not in respiratory distress, with equally resonant fields bilaterally. Lung sounds were clear bilaterally. A papular, erythematous rash was present in a general distribution over the chest, with few telangiectasias and some varicosity along his left arm. The skin had normal elasticity, although the skin of the hands and legs was papyraceous.
Gd levels were measured in the blood and urine (Table 1). Gd was detectable in the skin (0.2 µg/g) nearly 400 days after the last exposure. Gd was still detectable in the patient’s blood and urine (0.2 ng/mL and 0.5 µg/24 h, respectively) more than 3 years after his last exposure.
Discussion
In the United States, there are 40.44 MRI units per million people and 40 million MRIs are conducted annually. From 30 to 50% of these are enhanced with Gd-based contrast agents. In the past 30 years, there have been > 450 million contrast-enhanced MRI procedures.1
Gd is a rare earth metal. Among commercially available elements Gd has exceptional properties for enhancing MRI signals at low concentrations.1 The nonphysiologic metal is detoxified by chelation with proprietary multidentate formulations that enhance (primarily renal) elimination while retaining the paramagnetic and chemical properties for imaging. Gd exposure was found to be associated to iatrogenic nephrogenic systemic fibrosis in 2006 and later confirmed via multiple systematic reviews.2 Gd is retained in every vital organ after exposure.3 Gd-based contrast agents stimulate bone marrow–derived fibrocytes in mediating fibrosis, and bone marrow develop a memory of prior contrast exposure (Figure 1).4-6 Systemic fibrosis is mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor 2.6,7 Even in the setting of normal renal function, Gd-based contrast induces the formation of Gd-rich nanoparticles in the skin and kidney.7,8 Far from being inert, Gd-based contrast agents induce systemic metabolic changes such as hypertriglyceridemia, elevations in low-density lipoprotein cholesterol, insulin resistance, and the Warburg effect (glycolytic/energy switching) in the renal cortex concomitant with profound mitochondrial abnormalities.8
We have discovered that the rate of Gd-enhanced procedures has increased immensely within the Veterans Health Administration (VHA) system in a subset of patients with designated kidney disease (Table 2). Although a substantial number of procedures are dedicated to head and brain imaging within the VHA, the indications for Gd-enhanced diagnoses (eg, cardiac) are increasing (Figure 2).
Retention of Gd can be modeled as a function of time (t) by the half-lives of the fast, intermediate, and slow phases of elimination (Ta, Tb, and Tc, respectively):9
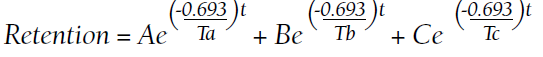
A, B, and C are the proportions (adding to 100%) that represent each of the compartments:
Numerous patients with normal renal function developed similar or novel symptoms that have been attributed to Gd concomitant with detectable urinary Gd years after exposure.11 Gd-based contrast agents are increasingly associated with cutaneous abnormalities even outside of nephrogenic systemic fibrosis. Gd-associated plaques develop in patients without kidney disease—these range from asymptomatic, pruritic, to burning.13 Histologic specimens reveal CD68 and factor XIIIa–positive spindle-shaped myeloid cells (the same mediators of iatrogenic systemic fibrosis) or CD34-positive cells. CD68 and factor XIIIa are distinctive for histologic specimens from patients with systemic fibrosis, and these markers have been detected in our preclinical models that demonstrated that bone marrow–derived cells are involved in mediating fibrosis.3,4,14-19 Similarly, CD34-positive cells have been historically associated with systemic fibrosis lesions.15,16,18-23 Plump osteocyte-appearing cells have also been noted (note that extraosseous metaplasia makes the histologic diagnosis of systemic fibrosis).14 Nephrogenic systemic fibrosis is an iatrogenic disease that can manifest years after exposure to Gd.5 Gd induces the recruitment of bone marrow–derived cells to the affected sites.4
The VA Health Service Research and Development Evidence Synthesis Program reviewed the safety of Gd-based contrast agents in patients with impaired kidney function.24,25 The group found only a single study of Gd and veterans. “Awareness and concern are growing about the long-term deposition of gadolinium in [the] brain and other tissues among patients with normal kidney function,” according to Lunyera and colleagues.25 The largest knowledge gap was that a comprehensive review “of all potential harms associated with gadolinium exposure” was not addressed. Furthermore, the group advised “caution in the use of [Gd-based contrast agents] in patients with severely impaired kidney function and acute kidney injury remains prudent, because the exact clinical factors contributing to [nephrogenic systemic fibrosis] risk in these subpopulations are still unknown.”25
Gd-based contrast agents—contrary to a widely held misconception—are not biologically inert.1 Gd-based contrast agents have a long history of association with acute renal injury. We have demonstrated that systemic treatment with MRI contrast agents leads to vacuolization of the proximal tubule and tubular injury.7,8 Kidney injury may be mediated by the generation of reactive oxygen species from NADPH oxidase 4 (Nox4).26
Gd retention, Gd-induced multisymptomatic illnesses, Gd-associated plaques, Gd-induced neurotoxicity, and nephrogenic systemic fibrosis are part of a continuum (with Gd as the common thread)—a theme of the September 8, 2017, US Food and Drug Administration (FDA) Medical Imaging Drugs Advisory Committee meeting.27 Patients, patient advocacy groups, and regulating agencies are concerned about long-term retention of a nonphysiologic rare earth element such as Gd.28-30 A patient advocacy group, The Lighthouse Project, collected information from patients linking the last date of Gd-based contrast agent exposure and urinary Gd.11 Data from their report suggest that the rate constants (valuable for the elimination equation above) are obtainable from 24-hour urine collections. Conceptually, Gd-induced diseases may represent a continuum that results from the retention of a nonphysiologic, toxic heavy rare earth metal.
As a heavy metal, Gd is not a natural physiologic trace element. Similar to numerous nonphysiologic metals, Gd is toxic. Inhaled Gd oxide (Gd2O3) dust leads to a number of time-dependent pathologies. Animal lung studies demonstrate reduced elasticity, enlarged cells, thickened lung walls, and recruitment of immune cells.31 Symptoms of acute IV Gd toxicity include decreased respiration, lethargy, abdominal cramps, and diarrhea.32 Pharmacologically, Gd concentrates in the liver and kidney and accumulates in the bone.32 Animals demonstrate intestinal depression and low blood pressure in response to Gd and, with higher doses, cardiovascular collapse.32 IV Gd chloride leads to metal deposition in the small blood vessels diffusely throughout the body, particularly in the lung and kidney and the metal is absorbed by the scavenging white blood cells.33 Gd chloride induces severe damage to the liver, spleen, and the digestive tract.33 Furthermore, this form of the toxicant metal markedly impacted functions associated with bleeding and clotting, ie, decreased platelet numbers and an increase in the laboratory-measured coagulation parameters.33 Semelka and colleagues have characterized chronic symptoms attributed to Gd-based contrast agents (not limited to chronic pain, headache, bone pain, skin thickening, and clouded mentation).34,35 Because Gd-induced conditions are underrecognized and ill-defined, disinherited patients often resort to untested (and potentially dangerous) chelation therapies.36
This patient presented with numerous symptoms that arose after Gd exposure. It is well established that Gd-based contrast agents (of any class) are retained in multiple organs (including the brain), for months to years. Gd-based contrast agents enter the cerebrospinal fluid within minutes of IV administration.37 Gd was found in the cerebrospinal fluid 9 months after administration in a case presented to the FDA Medical Imaging Drugs Advisory Committee.38 We know from intentional and accidental intrathecal administrations that Gd-based contrast agents are neurotoxic.39 Runge and colleagues demonstrated that Gd-based contrast agents exert mitochondrial toxicity in cultured neurons in vitro.40 McDonald and his team found Gd-rich nanoparticles within the brain neurons (cytoplasm and nuclei) from patients exposed to MRI contrast in the normal course of care.41 These nanoparticles are similar to what we have found in rodent models of Gd-induced disease.7,8,42
Prolonged elimination of Gd after MRI contrast administration (months to years) may be universal.10 Gd compartmentalizes into leukocytes and erythrocytes and into the cerebrospinal fluid within minutes.37,43 Patients with multisymptomatic illnesses attributed to Gd (Gd deposition disease) have perturbations in cytokine levels, many inflammatory.44,45 The results are concerning: Gd is retained intracellularly in vital organs, including brain neurons. It is inarguable that Gd is an alien, nonphysiologic element. With mounting evidence that Gd retention has clinical consequences, patients should be provided proper informed consent. Complications of renal insufficiency (ie, hyperkalemia, hyperphosphatemia, renal osteodystrophy, hyponatremia, anemia, immunosuppression, etc) follow a smooth, curvilinear slope as the true (not estimated) glomerular filtration declines; the worst iatrogenic complication from Gd—systemic fibrosis—is likely no different.
Patient Perspective
“Seems like it’s one thing after another. My family doctor said that once I had the gadolinium exposures, I have had problems ever since that I don’t recover from.” This includes chronic numbness from the rectum to the bilateral lower extremities and an indolent worsening kidney function; “I have already developed stage 3B chronic kidney disease.” Similar to many suffering with gadolinium retention, the patient was concerned about the long-term consequences. Gadolinium “is a toxic metal that is going through my body for 4 years. That has to be a problem. How come we don’t have that answer?” Clinician ignorance of Gd-induced complications and long-term retention is frustrating. “Not one of my doctors has taken gadolinium retention seriously. Where else are patients supposed to go?”
Conclusions
Health care professionals should be considering subclinical manifestations of nephrogenic systemic fibrosis or open to considering that intracellular neuronal retention of Gd may correlate with symptoms arising after MRI contrast exposures. The science concerning the mechanisms of how Gd exerts its pathologic effects is lagging behind the commercialization of enhancing Gd elimination (ie, chelation therapies) and other untested remedies. Practitioners need to acknowledge the unknown potential consequences of Gd and listen to patients who suspect chronic adverse effects.
1. Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28(2):154-162. doi:10.1097/MNH.0000000000000475
2. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006;21(4):1104-1108. doi:10.1093/ndt/gfk062
3. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol. 2014;307(7):F844-F855. doi:10.1152/ajprenal.00379.2014
4. Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
5. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F1-F11. doi:10.1152/ajprenal.00166.2016
6. Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
7. Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143.e2. doi:10.1016/j.jid.2019.03.1145
8. Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
9. Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85-95. doi:10.1289/ehp.96104s185
10. Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol. 2019;15(2):121-127. doi:10.1007/s13181-018-0693-1
11. Williams S, Grimm H. gadolinium toxicity: shedding light on the effects of retained gadolinium from contrast MRI. Accessed April 11, 2022. https://gdtoxicity.files.wordpress.com/2018/12/gadolinium-clearance-times-for-135-contrast-mri-cases-final-v1-1.pdf
12. DeBevits JJ, Reshma M, Bageac D, et al. Gray matter nucleus hyperintensity after monthly triple-dose gadopentetate dimeglumine with long-term magnetic resonance imaging. Invest Radiol. 2020;55(10):629-635. doi:10.1097/RLI.0000000000000663
13. Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151(3):316-319. doi:10.1001/jamadermatol.2014.2660
14. Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095-1106 e7. doi:10.1016/j.jaad.2010.08.041
15. Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis. 2005;46(4):754-759. doi:10.1053/j.ajkd.2005.06.024
16. Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150(5):1050-1052. doi:10.1111/j.1365-2133.2004.05900.x
17. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-49. doi:10.1016/j.semarthrit.2005.08.002
18. Lewis KG, Lester BW, Pan TD, Robinson-Bostom L. Nephrogenic fibrosing dermopathyand calciphylaxis with pseudoxanthoma elasticum-like changes. J Cutan Pathol. 2006;33(10):695-700. doi:10.1111/j.1600-0560.2006.00490.x
19. Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130(2):209-212. doi:10.5858/2006-130-209-MIINFD
20. Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45(1):56-59. doi:10.1111/j.1365-4632.2005.02701.x
21. Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54(suppl 2):S31-S34. doi:10.1016/j.jaad.2005.04.024
22. Sanyal S, Marckmann P, Scherer S, Abraham JL. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant. 2011;26(11):3616-3626. doi:10.1093/ndt/gfr085
23. Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005;32(7):484-490. doi:10.1111/j.0303-6987.2005.00365.x
24. Goldstein KM, Lunyera J, Mohottige D, et al. Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Washington (DC): Department of Veterans Affairs (US); October 2019. https://www.ncbi.nlm.nih.gov/books/NBK559376/25. Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173(2):110-119. doi:10.7326/M20-0299
26. Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH Oxidase 4 (Nox4) for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
27. Wagner B. The pathophysiology and retention of gadolinium. United States Food & Drug Administration Medical Imaging Drugs Advisory Committee. 2017:1-23. https://www.fda.gov/advisory-committees/medical-imaging-drugs-advisory-committee/2017-meeting-materials-medical-imaging-drugs-advisory-committee?msclkid=6b5764ccbaa611ec95e35dddf8db57af
28. Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52(6):317-323. doi:10.1097/RLI.0000000000000374
29. Wagner B. Scared to the marrow: pitfalls and pearls in renal imaging. Adv Chronic Kidney Dis. 2017;24(3):136-137. doi:10.1053/j.ackd.2017.03.008
30. US Food and Drug Administration. Transcript for the September 8, 2017 Meeting of the Medical Imaging Drugs Advisory Committee (MIDAC). September 8, 2017. Accessed April 11, 2022. https://www.fda.gov/media/108935/download
31. Abel M, Talbot RB. Gadolinium oxide inhalation by guinea pigs: a correlative functional and histopathologic study. J Pharmacol Exp Ther. 1967;157(1):207-213.
32. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br J Pharmacol Chemother. 1961;17(3):526-532. doi:10.1111/j.1476-5381.1961.tb01139.x

33. Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25(3):245-255. doi:10.1177/019262339702500301
34. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229-233. doi:10.2214/AJR.15.15842
35. Semelka RC, Ramalho M. Physicians with self-diagnosed gadolinium deposition disease: a case series. Radiol Bras. 2021;54(4):238-242. doi:10.1590/0100-3984.2020.0073
36. Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents—what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). 2020;58(3):151-160. doi:10.1080/15563650.2019.1681442
37. Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288(2):416-423. doi:10.1148/radiol.2018171105
38. US Food and Drug Administration. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. 2017. Accessed April 12, 2022. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf
39. Calvo N, Jamil M, Feldman S, Shah A, Nauman F, Ferrara J. Neurotoxicity from intrathecal gadolinium administration: case presentation and brief review. Neurol Clin Pract. 2020;10(1):e7-e10. doi:10.1212/CPJ.0000000000000696
40. Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54(8):453-463. doi:10.1097/RLI.0000000000000567
41. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
42. Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. doi:10.34067/KID.0000272019
43. Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S. Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol. 2020;55(1):30-37. doi:10.1097/RLI.0000000000000608
44. Maecker HT, Siebert JC, Rosenberg-Hasson Y, Koran LM, Ramalho M, Semelka RC. Acute chelation therapy-associated changes in urine gadolinium, self-reported flare severity, and serum cytokines in gadolinium deposition disease. Invest Radiol. 2021;56(6):374-384. doi:10.1097/RLI.0000000000000752
45. Maecker HT, Wang W, Rosenberg-Hasson Y, Semelka RC, Hickey J, Koran LM. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol Bras. 2020;53(5):306-313. doi:10.1590/0100-3984.2019.0075
46. Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87(6):3321-3328. doi:10.1021/ac504488k
Gadolinium (Gd)-based contrast agents are frequently used in health care for enhancing magnetic resonance image (MRI) signals at low concentrations. Contrary to popular opinion, this widely used heavy metal is not biologically inert. Once notable for its safety profile, there is mounting evidence for Gd deposition in various organ systems of the body, even in those with normal renal function. A large knowledge gap remains concerning the potential harms of Gd deposition and the factors determining its elimination from the body. However, the findings of deposited Gd throughout various organs and their intracellular compartments even years after the initial exposure have been established. Here, we describe a case of a Vietnam-era veteran whose presentation, clinical, and laboratory findings were consistent within the spectrum of Gd deposition disease.
Case Presentation
A Vietnam-era veteran aged > 70 years presented for evaluation of Gd-based contrast agent–induced chronic multisymptomatic illness His medical history was significant for chronic low back pain, chronic hypertension, type 2 diabetes mellitus, and hypogonadism. Surgical history was notable for back surgery (24 years prior), laminectomy (2 years prior), shoulder replacement (2 years prior), and an epidural complicated by a hematoma (1 year prior). His presenting concerns included a painful and pruritic rash that worsened with showering, pain originating at the right Achilles tendon with migration to the knee, and shoulder pain. His symptoms started shortly after receiving multiple exposures to Gd-based contrast agents to enhance MRIs during his clinical care (Omniscan 20 mL, Omniscan 20 mL, and Gadovist 10 mL, administered 578, 565, and 496 days prior to the clinic visit, respectively). New onset headaches coincided with the timeline of symptom onset, in addition to hoarseness and liberation of an “oily substance” from the skin. More than one year prior to this clinic visit, he was considered for having polymyalgia rheumatica given the ambiguity of symptoms. Functional status remained impaired despite treatment with prednisone and methotrexate.
The patient’s military service was in the mid-1960s. He was deployed to Japan and had no knowledge of an Agent Orange exposure. His tobacco history was distant, and he reported no tattoos, prior transfusions, or occupational metal exposure
Clinical Findings
The patient was afebrile, normotensive (146/88 mmHg), and normocardic. His weight was 100 kg. He was well nourished and in no acute distress. The thought process was attentive, and his affect pleasant. Ocular examination was notable for arcus senilus. The fundoscopic examination was limited on the left, but there was no neovascularization on the right. Jugular venous pulsation was normal at 8 cm. Right ventricular impulse was slightly hyperdynamic, the rhythm was regular, and there was no abnormal splitting of S2. A soft-grade I/VI crescendo/decrescendo murmur was auscultated along the apex. Radial pulses were 2/2. He was not in respiratory distress, with equally resonant fields bilaterally. Lung sounds were clear bilaterally. A papular, erythematous rash was present in a general distribution over the chest, with few telangiectasias and some varicosity along his left arm. The skin had normal elasticity, although the skin of the hands and legs was papyraceous.
Gd levels were measured in the blood and urine (Table 1). Gd was detectable in the skin (0.2 µg/g) nearly 400 days after the last exposure. Gd was still detectable in the patient’s blood and urine (0.2 ng/mL and 0.5 µg/24 h, respectively) more than 3 years after his last exposure.
Discussion
In the United States, there are 40.44 MRI units per million people and 40 million MRIs are conducted annually. From 30 to 50% of these are enhanced with Gd-based contrast agents. In the past 30 years, there have been > 450 million contrast-enhanced MRI procedures.1
Gd is a rare earth metal. Among commercially available elements Gd has exceptional properties for enhancing MRI signals at low concentrations.1 The nonphysiologic metal is detoxified by chelation with proprietary multidentate formulations that enhance (primarily renal) elimination while retaining the paramagnetic and chemical properties for imaging. Gd exposure was found to be associated to iatrogenic nephrogenic systemic fibrosis in 2006 and later confirmed via multiple systematic reviews.2 Gd is retained in every vital organ after exposure.3 Gd-based contrast agents stimulate bone marrow–derived fibrocytes in mediating fibrosis, and bone marrow develop a memory of prior contrast exposure (Figure 1).4-6 Systemic fibrosis is mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor 2.6,7 Even in the setting of normal renal function, Gd-based contrast induces the formation of Gd-rich nanoparticles in the skin and kidney.7,8 Far from being inert, Gd-based contrast agents induce systemic metabolic changes such as hypertriglyceridemia, elevations in low-density lipoprotein cholesterol, insulin resistance, and the Warburg effect (glycolytic/energy switching) in the renal cortex concomitant with profound mitochondrial abnormalities.8
We have discovered that the rate of Gd-enhanced procedures has increased immensely within the Veterans Health Administration (VHA) system in a subset of patients with designated kidney disease (Table 2). Although a substantial number of procedures are dedicated to head and brain imaging within the VHA, the indications for Gd-enhanced diagnoses (eg, cardiac) are increasing (Figure 2).
Retention of Gd can be modeled as a function of time (t) by the half-lives of the fast, intermediate, and slow phases of elimination (Ta, Tb, and Tc, respectively):9
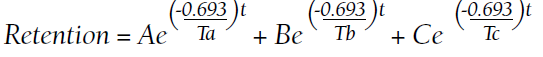
A, B, and C are the proportions (adding to 100%) that represent each of the compartments:
Numerous patients with normal renal function developed similar or novel symptoms that have been attributed to Gd concomitant with detectable urinary Gd years after exposure.11 Gd-based contrast agents are increasingly associated with cutaneous abnormalities even outside of nephrogenic systemic fibrosis. Gd-associated plaques develop in patients without kidney disease—these range from asymptomatic, pruritic, to burning.13 Histologic specimens reveal CD68 and factor XIIIa–positive spindle-shaped myeloid cells (the same mediators of iatrogenic systemic fibrosis) or CD34-positive cells. CD68 and factor XIIIa are distinctive for histologic specimens from patients with systemic fibrosis, and these markers have been detected in our preclinical models that demonstrated that bone marrow–derived cells are involved in mediating fibrosis.3,4,14-19 Similarly, CD34-positive cells have been historically associated with systemic fibrosis lesions.15,16,18-23 Plump osteocyte-appearing cells have also been noted (note that extraosseous metaplasia makes the histologic diagnosis of systemic fibrosis).14 Nephrogenic systemic fibrosis is an iatrogenic disease that can manifest years after exposure to Gd.5 Gd induces the recruitment of bone marrow–derived cells to the affected sites.4
The VA Health Service Research and Development Evidence Synthesis Program reviewed the safety of Gd-based contrast agents in patients with impaired kidney function.24,25 The group found only a single study of Gd and veterans. “Awareness and concern are growing about the long-term deposition of gadolinium in [the] brain and other tissues among patients with normal kidney function,” according to Lunyera and colleagues.25 The largest knowledge gap was that a comprehensive review “of all potential harms associated with gadolinium exposure” was not addressed. Furthermore, the group advised “caution in the use of [Gd-based contrast agents] in patients with severely impaired kidney function and acute kidney injury remains prudent, because the exact clinical factors contributing to [nephrogenic systemic fibrosis] risk in these subpopulations are still unknown.”25
Gd-based contrast agents—contrary to a widely held misconception—are not biologically inert.1 Gd-based contrast agents have a long history of association with acute renal injury. We have demonstrated that systemic treatment with MRI contrast agents leads to vacuolization of the proximal tubule and tubular injury.7,8 Kidney injury may be mediated by the generation of reactive oxygen species from NADPH oxidase 4 (Nox4).26
Gd retention, Gd-induced multisymptomatic illnesses, Gd-associated plaques, Gd-induced neurotoxicity, and nephrogenic systemic fibrosis are part of a continuum (with Gd as the common thread)—a theme of the September 8, 2017, US Food and Drug Administration (FDA) Medical Imaging Drugs Advisory Committee meeting.27 Patients, patient advocacy groups, and regulating agencies are concerned about long-term retention of a nonphysiologic rare earth element such as Gd.28-30 A patient advocacy group, The Lighthouse Project, collected information from patients linking the last date of Gd-based contrast agent exposure and urinary Gd.11 Data from their report suggest that the rate constants (valuable for the elimination equation above) are obtainable from 24-hour urine collections. Conceptually, Gd-induced diseases may represent a continuum that results from the retention of a nonphysiologic, toxic heavy rare earth metal.
As a heavy metal, Gd is not a natural physiologic trace element. Similar to numerous nonphysiologic metals, Gd is toxic. Inhaled Gd oxide (Gd2O3) dust leads to a number of time-dependent pathologies. Animal lung studies demonstrate reduced elasticity, enlarged cells, thickened lung walls, and recruitment of immune cells.31 Symptoms of acute IV Gd toxicity include decreased respiration, lethargy, abdominal cramps, and diarrhea.32 Pharmacologically, Gd concentrates in the liver and kidney and accumulates in the bone.32 Animals demonstrate intestinal depression and low blood pressure in response to Gd and, with higher doses, cardiovascular collapse.32 IV Gd chloride leads to metal deposition in the small blood vessels diffusely throughout the body, particularly in the lung and kidney and the metal is absorbed by the scavenging white blood cells.33 Gd chloride induces severe damage to the liver, spleen, and the digestive tract.33 Furthermore, this form of the toxicant metal markedly impacted functions associated with bleeding and clotting, ie, decreased platelet numbers and an increase in the laboratory-measured coagulation parameters.33 Semelka and colleagues have characterized chronic symptoms attributed to Gd-based contrast agents (not limited to chronic pain, headache, bone pain, skin thickening, and clouded mentation).34,35 Because Gd-induced conditions are underrecognized and ill-defined, disinherited patients often resort to untested (and potentially dangerous) chelation therapies.36
This patient presented with numerous symptoms that arose after Gd exposure. It is well established that Gd-based contrast agents (of any class) are retained in multiple organs (including the brain), for months to years. Gd-based contrast agents enter the cerebrospinal fluid within minutes of IV administration.37 Gd was found in the cerebrospinal fluid 9 months after administration in a case presented to the FDA Medical Imaging Drugs Advisory Committee.38 We know from intentional and accidental intrathecal administrations that Gd-based contrast agents are neurotoxic.39 Runge and colleagues demonstrated that Gd-based contrast agents exert mitochondrial toxicity in cultured neurons in vitro.40 McDonald and his team found Gd-rich nanoparticles within the brain neurons (cytoplasm and nuclei) from patients exposed to MRI contrast in the normal course of care.41 These nanoparticles are similar to what we have found in rodent models of Gd-induced disease.7,8,42
Prolonged elimination of Gd after MRI contrast administration (months to years) may be universal.10 Gd compartmentalizes into leukocytes and erythrocytes and into the cerebrospinal fluid within minutes.37,43 Patients with multisymptomatic illnesses attributed to Gd (Gd deposition disease) have perturbations in cytokine levels, many inflammatory.44,45 The results are concerning: Gd is retained intracellularly in vital organs, including brain neurons. It is inarguable that Gd is an alien, nonphysiologic element. With mounting evidence that Gd retention has clinical consequences, patients should be provided proper informed consent. Complications of renal insufficiency (ie, hyperkalemia, hyperphosphatemia, renal osteodystrophy, hyponatremia, anemia, immunosuppression, etc) follow a smooth, curvilinear slope as the true (not estimated) glomerular filtration declines; the worst iatrogenic complication from Gd—systemic fibrosis—is likely no different.
Patient Perspective
“Seems like it’s one thing after another. My family doctor said that once I had the gadolinium exposures, I have had problems ever since that I don’t recover from.” This includes chronic numbness from the rectum to the bilateral lower extremities and an indolent worsening kidney function; “I have already developed stage 3B chronic kidney disease.” Similar to many suffering with gadolinium retention, the patient was concerned about the long-term consequences. Gadolinium “is a toxic metal that is going through my body for 4 years. That has to be a problem. How come we don’t have that answer?” Clinician ignorance of Gd-induced complications and long-term retention is frustrating. “Not one of my doctors has taken gadolinium retention seriously. Where else are patients supposed to go?”
Conclusions
Health care professionals should be considering subclinical manifestations of nephrogenic systemic fibrosis or open to considering that intracellular neuronal retention of Gd may correlate with symptoms arising after MRI contrast exposures. The science concerning the mechanisms of how Gd exerts its pathologic effects is lagging behind the commercialization of enhancing Gd elimination (ie, chelation therapies) and other untested remedies. Practitioners need to acknowledge the unknown potential consequences of Gd and listen to patients who suspect chronic adverse effects.
Gadolinium (Gd)-based contrast agents are frequently used in health care for enhancing magnetic resonance image (MRI) signals at low concentrations. Contrary to popular opinion, this widely used heavy metal is not biologically inert. Once notable for its safety profile, there is mounting evidence for Gd deposition in various organ systems of the body, even in those with normal renal function. A large knowledge gap remains concerning the potential harms of Gd deposition and the factors determining its elimination from the body. However, the findings of deposited Gd throughout various organs and their intracellular compartments even years after the initial exposure have been established. Here, we describe a case of a Vietnam-era veteran whose presentation, clinical, and laboratory findings were consistent within the spectrum of Gd deposition disease.
Case Presentation
A Vietnam-era veteran aged > 70 years presented for evaluation of Gd-based contrast agent–induced chronic multisymptomatic illness His medical history was significant for chronic low back pain, chronic hypertension, type 2 diabetes mellitus, and hypogonadism. Surgical history was notable for back surgery (24 years prior), laminectomy (2 years prior), shoulder replacement (2 years prior), and an epidural complicated by a hematoma (1 year prior). His presenting concerns included a painful and pruritic rash that worsened with showering, pain originating at the right Achilles tendon with migration to the knee, and shoulder pain. His symptoms started shortly after receiving multiple exposures to Gd-based contrast agents to enhance MRIs during his clinical care (Omniscan 20 mL, Omniscan 20 mL, and Gadovist 10 mL, administered 578, 565, and 496 days prior to the clinic visit, respectively). New onset headaches coincided with the timeline of symptom onset, in addition to hoarseness and liberation of an “oily substance” from the skin. More than one year prior to this clinic visit, he was considered for having polymyalgia rheumatica given the ambiguity of symptoms. Functional status remained impaired despite treatment with prednisone and methotrexate.
The patient’s military service was in the mid-1960s. He was deployed to Japan and had no knowledge of an Agent Orange exposure. His tobacco history was distant, and he reported no tattoos, prior transfusions, or occupational metal exposure
Clinical Findings
The patient was afebrile, normotensive (146/88 mmHg), and normocardic. His weight was 100 kg. He was well nourished and in no acute distress. The thought process was attentive, and his affect pleasant. Ocular examination was notable for arcus senilus. The fundoscopic examination was limited on the left, but there was no neovascularization on the right. Jugular venous pulsation was normal at 8 cm. Right ventricular impulse was slightly hyperdynamic, the rhythm was regular, and there was no abnormal splitting of S2. A soft-grade I/VI crescendo/decrescendo murmur was auscultated along the apex. Radial pulses were 2/2. He was not in respiratory distress, with equally resonant fields bilaterally. Lung sounds were clear bilaterally. A papular, erythematous rash was present in a general distribution over the chest, with few telangiectasias and some varicosity along his left arm. The skin had normal elasticity, although the skin of the hands and legs was papyraceous.
Gd levels were measured in the blood and urine (Table 1). Gd was detectable in the skin (0.2 µg/g) nearly 400 days after the last exposure. Gd was still detectable in the patient’s blood and urine (0.2 ng/mL and 0.5 µg/24 h, respectively) more than 3 years after his last exposure.
Discussion
In the United States, there are 40.44 MRI units per million people and 40 million MRIs are conducted annually. From 30 to 50% of these are enhanced with Gd-based contrast agents. In the past 30 years, there have been > 450 million contrast-enhanced MRI procedures.1
Gd is a rare earth metal. Among commercially available elements Gd has exceptional properties for enhancing MRI signals at low concentrations.1 The nonphysiologic metal is detoxified by chelation with proprietary multidentate formulations that enhance (primarily renal) elimination while retaining the paramagnetic and chemical properties for imaging. Gd exposure was found to be associated to iatrogenic nephrogenic systemic fibrosis in 2006 and later confirmed via multiple systematic reviews.2 Gd is retained in every vital organ after exposure.3 Gd-based contrast agents stimulate bone marrow–derived fibrocytes in mediating fibrosis, and bone marrow develop a memory of prior contrast exposure (Figure 1).4-6 Systemic fibrosis is mediated by the monocyte chemoattractant protein 1/C-C chemokine receptor 2.6,7 Even in the setting of normal renal function, Gd-based contrast induces the formation of Gd-rich nanoparticles in the skin and kidney.7,8 Far from being inert, Gd-based contrast agents induce systemic metabolic changes such as hypertriglyceridemia, elevations in low-density lipoprotein cholesterol, insulin resistance, and the Warburg effect (glycolytic/energy switching) in the renal cortex concomitant with profound mitochondrial abnormalities.8
We have discovered that the rate of Gd-enhanced procedures has increased immensely within the Veterans Health Administration (VHA) system in a subset of patients with designated kidney disease (Table 2). Although a substantial number of procedures are dedicated to head and brain imaging within the VHA, the indications for Gd-enhanced diagnoses (eg, cardiac) are increasing (Figure 2).
Retention of Gd can be modeled as a function of time (t) by the half-lives of the fast, intermediate, and slow phases of elimination (Ta, Tb, and Tc, respectively):9
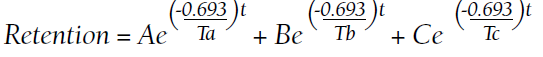
A, B, and C are the proportions (adding to 100%) that represent each of the compartments:
Numerous patients with normal renal function developed similar or novel symptoms that have been attributed to Gd concomitant with detectable urinary Gd years after exposure.11 Gd-based contrast agents are increasingly associated with cutaneous abnormalities even outside of nephrogenic systemic fibrosis. Gd-associated plaques develop in patients without kidney disease—these range from asymptomatic, pruritic, to burning.13 Histologic specimens reveal CD68 and factor XIIIa–positive spindle-shaped myeloid cells (the same mediators of iatrogenic systemic fibrosis) or CD34-positive cells. CD68 and factor XIIIa are distinctive for histologic specimens from patients with systemic fibrosis, and these markers have been detected in our preclinical models that demonstrated that bone marrow–derived cells are involved in mediating fibrosis.3,4,14-19 Similarly, CD34-positive cells have been historically associated with systemic fibrosis lesions.15,16,18-23 Plump osteocyte-appearing cells have also been noted (note that extraosseous metaplasia makes the histologic diagnosis of systemic fibrosis).14 Nephrogenic systemic fibrosis is an iatrogenic disease that can manifest years after exposure to Gd.5 Gd induces the recruitment of bone marrow–derived cells to the affected sites.4
The VA Health Service Research and Development Evidence Synthesis Program reviewed the safety of Gd-based contrast agents in patients with impaired kidney function.24,25 The group found only a single study of Gd and veterans. “Awareness and concern are growing about the long-term deposition of gadolinium in [the] brain and other tissues among patients with normal kidney function,” according to Lunyera and colleagues.25 The largest knowledge gap was that a comprehensive review “of all potential harms associated with gadolinium exposure” was not addressed. Furthermore, the group advised “caution in the use of [Gd-based contrast agents] in patients with severely impaired kidney function and acute kidney injury remains prudent, because the exact clinical factors contributing to [nephrogenic systemic fibrosis] risk in these subpopulations are still unknown.”25
Gd-based contrast agents—contrary to a widely held misconception—are not biologically inert.1 Gd-based contrast agents have a long history of association with acute renal injury. We have demonstrated that systemic treatment with MRI contrast agents leads to vacuolization of the proximal tubule and tubular injury.7,8 Kidney injury may be mediated by the generation of reactive oxygen species from NADPH oxidase 4 (Nox4).26
Gd retention, Gd-induced multisymptomatic illnesses, Gd-associated plaques, Gd-induced neurotoxicity, and nephrogenic systemic fibrosis are part of a continuum (with Gd as the common thread)—a theme of the September 8, 2017, US Food and Drug Administration (FDA) Medical Imaging Drugs Advisory Committee meeting.27 Patients, patient advocacy groups, and regulating agencies are concerned about long-term retention of a nonphysiologic rare earth element such as Gd.28-30 A patient advocacy group, The Lighthouse Project, collected information from patients linking the last date of Gd-based contrast agent exposure and urinary Gd.11 Data from their report suggest that the rate constants (valuable for the elimination equation above) are obtainable from 24-hour urine collections. Conceptually, Gd-induced diseases may represent a continuum that results from the retention of a nonphysiologic, toxic heavy rare earth metal.
As a heavy metal, Gd is not a natural physiologic trace element. Similar to numerous nonphysiologic metals, Gd is toxic. Inhaled Gd oxide (Gd2O3) dust leads to a number of time-dependent pathologies. Animal lung studies demonstrate reduced elasticity, enlarged cells, thickened lung walls, and recruitment of immune cells.31 Symptoms of acute IV Gd toxicity include decreased respiration, lethargy, abdominal cramps, and diarrhea.32 Pharmacologically, Gd concentrates in the liver and kidney and accumulates in the bone.32 Animals demonstrate intestinal depression and low blood pressure in response to Gd and, with higher doses, cardiovascular collapse.32 IV Gd chloride leads to metal deposition in the small blood vessels diffusely throughout the body, particularly in the lung and kidney and the metal is absorbed by the scavenging white blood cells.33 Gd chloride induces severe damage to the liver, spleen, and the digestive tract.33 Furthermore, this form of the toxicant metal markedly impacted functions associated with bleeding and clotting, ie, decreased platelet numbers and an increase in the laboratory-measured coagulation parameters.33 Semelka and colleagues have characterized chronic symptoms attributed to Gd-based contrast agents (not limited to chronic pain, headache, bone pain, skin thickening, and clouded mentation).34,35 Because Gd-induced conditions are underrecognized and ill-defined, disinherited patients often resort to untested (and potentially dangerous) chelation therapies.36
This patient presented with numerous symptoms that arose after Gd exposure. It is well established that Gd-based contrast agents (of any class) are retained in multiple organs (including the brain), for months to years. Gd-based contrast agents enter the cerebrospinal fluid within minutes of IV administration.37 Gd was found in the cerebrospinal fluid 9 months after administration in a case presented to the FDA Medical Imaging Drugs Advisory Committee.38 We know from intentional and accidental intrathecal administrations that Gd-based contrast agents are neurotoxic.39 Runge and colleagues demonstrated that Gd-based contrast agents exert mitochondrial toxicity in cultured neurons in vitro.40 McDonald and his team found Gd-rich nanoparticles within the brain neurons (cytoplasm and nuclei) from patients exposed to MRI contrast in the normal course of care.41 These nanoparticles are similar to what we have found in rodent models of Gd-induced disease.7,8,42
Prolonged elimination of Gd after MRI contrast administration (months to years) may be universal.10 Gd compartmentalizes into leukocytes and erythrocytes and into the cerebrospinal fluid within minutes.37,43 Patients with multisymptomatic illnesses attributed to Gd (Gd deposition disease) have perturbations in cytokine levels, many inflammatory.44,45 The results are concerning: Gd is retained intracellularly in vital organs, including brain neurons. It is inarguable that Gd is an alien, nonphysiologic element. With mounting evidence that Gd retention has clinical consequences, patients should be provided proper informed consent. Complications of renal insufficiency (ie, hyperkalemia, hyperphosphatemia, renal osteodystrophy, hyponatremia, anemia, immunosuppression, etc) follow a smooth, curvilinear slope as the true (not estimated) glomerular filtration declines; the worst iatrogenic complication from Gd—systemic fibrosis—is likely no different.
Patient Perspective
“Seems like it’s one thing after another. My family doctor said that once I had the gadolinium exposures, I have had problems ever since that I don’t recover from.” This includes chronic numbness from the rectum to the bilateral lower extremities and an indolent worsening kidney function; “I have already developed stage 3B chronic kidney disease.” Similar to many suffering with gadolinium retention, the patient was concerned about the long-term consequences. Gadolinium “is a toxic metal that is going through my body for 4 years. That has to be a problem. How come we don’t have that answer?” Clinician ignorance of Gd-induced complications and long-term retention is frustrating. “Not one of my doctors has taken gadolinium retention seriously. Where else are patients supposed to go?”
Conclusions
Health care professionals should be considering subclinical manifestations of nephrogenic systemic fibrosis or open to considering that intracellular neuronal retention of Gd may correlate with symptoms arising after MRI contrast exposures. The science concerning the mechanisms of how Gd exerts its pathologic effects is lagging behind the commercialization of enhancing Gd elimination (ie, chelation therapies) and other untested remedies. Practitioners need to acknowledge the unknown potential consequences of Gd and listen to patients who suspect chronic adverse effects.
1. Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28(2):154-162. doi:10.1097/MNH.0000000000000475
2. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006;21(4):1104-1108. doi:10.1093/ndt/gfk062
3. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol. 2014;307(7):F844-F855. doi:10.1152/ajprenal.00379.2014
4. Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
5. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F1-F11. doi:10.1152/ajprenal.00166.2016
6. Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
7. Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143.e2. doi:10.1016/j.jid.2019.03.1145
8. Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
9. Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85-95. doi:10.1289/ehp.96104s185
10. Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol. 2019;15(2):121-127. doi:10.1007/s13181-018-0693-1
11. Williams S, Grimm H. gadolinium toxicity: shedding light on the effects of retained gadolinium from contrast MRI. Accessed April 11, 2022. https://gdtoxicity.files.wordpress.com/2018/12/gadolinium-clearance-times-for-135-contrast-mri-cases-final-v1-1.pdf
12. DeBevits JJ, Reshma M, Bageac D, et al. Gray matter nucleus hyperintensity after monthly triple-dose gadopentetate dimeglumine with long-term magnetic resonance imaging. Invest Radiol. 2020;55(10):629-635. doi:10.1097/RLI.0000000000000663
13. Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151(3):316-319. doi:10.1001/jamadermatol.2014.2660
14. Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095-1106 e7. doi:10.1016/j.jaad.2010.08.041
15. Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis. 2005;46(4):754-759. doi:10.1053/j.ajkd.2005.06.024
16. Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150(5):1050-1052. doi:10.1111/j.1365-2133.2004.05900.x
17. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-49. doi:10.1016/j.semarthrit.2005.08.002
18. Lewis KG, Lester BW, Pan TD, Robinson-Bostom L. Nephrogenic fibrosing dermopathyand calciphylaxis with pseudoxanthoma elasticum-like changes. J Cutan Pathol. 2006;33(10):695-700. doi:10.1111/j.1600-0560.2006.00490.x
19. Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130(2):209-212. doi:10.5858/2006-130-209-MIINFD
20. Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45(1):56-59. doi:10.1111/j.1365-4632.2005.02701.x
21. Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54(suppl 2):S31-S34. doi:10.1016/j.jaad.2005.04.024
22. Sanyal S, Marckmann P, Scherer S, Abraham JL. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant. 2011;26(11):3616-3626. doi:10.1093/ndt/gfr085
23. Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005;32(7):484-490. doi:10.1111/j.0303-6987.2005.00365.x
24. Goldstein KM, Lunyera J, Mohottige D, et al. Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Washington (DC): Department of Veterans Affairs (US); October 2019. https://www.ncbi.nlm.nih.gov/books/NBK559376/25. Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173(2):110-119. doi:10.7326/M20-0299
26. Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH Oxidase 4 (Nox4) for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
27. Wagner B. The pathophysiology and retention of gadolinium. United States Food & Drug Administration Medical Imaging Drugs Advisory Committee. 2017:1-23. https://www.fda.gov/advisory-committees/medical-imaging-drugs-advisory-committee/2017-meeting-materials-medical-imaging-drugs-advisory-committee?msclkid=6b5764ccbaa611ec95e35dddf8db57af
28. Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52(6):317-323. doi:10.1097/RLI.0000000000000374
29. Wagner B. Scared to the marrow: pitfalls and pearls in renal imaging. Adv Chronic Kidney Dis. 2017;24(3):136-137. doi:10.1053/j.ackd.2017.03.008
30. US Food and Drug Administration. Transcript for the September 8, 2017 Meeting of the Medical Imaging Drugs Advisory Committee (MIDAC). September 8, 2017. Accessed April 11, 2022. https://www.fda.gov/media/108935/download
31. Abel M, Talbot RB. Gadolinium oxide inhalation by guinea pigs: a correlative functional and histopathologic study. J Pharmacol Exp Ther. 1967;157(1):207-213.
32. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br J Pharmacol Chemother. 1961;17(3):526-532. doi:10.1111/j.1476-5381.1961.tb01139.x

33. Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25(3):245-255. doi:10.1177/019262339702500301
34. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229-233. doi:10.2214/AJR.15.15842
35. Semelka RC, Ramalho M. Physicians with self-diagnosed gadolinium deposition disease: a case series. Radiol Bras. 2021;54(4):238-242. doi:10.1590/0100-3984.2020.0073
36. Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents—what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). 2020;58(3):151-160. doi:10.1080/15563650.2019.1681442
37. Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288(2):416-423. doi:10.1148/radiol.2018171105
38. US Food and Drug Administration. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. 2017. Accessed April 12, 2022. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf
39. Calvo N, Jamil M, Feldman S, Shah A, Nauman F, Ferrara J. Neurotoxicity from intrathecal gadolinium administration: case presentation and brief review. Neurol Clin Pract. 2020;10(1):e7-e10. doi:10.1212/CPJ.0000000000000696
40. Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54(8):453-463. doi:10.1097/RLI.0000000000000567
41. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
42. Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. doi:10.34067/KID.0000272019
43. Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S. Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol. 2020;55(1):30-37. doi:10.1097/RLI.0000000000000608
44. Maecker HT, Siebert JC, Rosenberg-Hasson Y, Koran LM, Ramalho M, Semelka RC. Acute chelation therapy-associated changes in urine gadolinium, self-reported flare severity, and serum cytokines in gadolinium deposition disease. Invest Radiol. 2021;56(6):374-384. doi:10.1097/RLI.0000000000000752
45. Maecker HT, Wang W, Rosenberg-Hasson Y, Semelka RC, Hickey J, Koran LM. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol Bras. 2020;53(5):306-313. doi:10.1590/0100-3984.2019.0075
46. Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87(6):3321-3328. doi:10.1021/ac504488k
1. Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28(2):154-162. doi:10.1097/MNH.0000000000000475
2. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?. Nephrol Dial Transplant. 2006;21(4):1104-1108. doi:10.1093/ndt/gfk062
3. Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol. 2014;307(7):F844-F855. doi:10.1152/ajprenal.00379.2014
4. Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
5. Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F1-F11. doi:10.1152/ajprenal.00166.2016
6. Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
7. Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143.e2. doi:10.1016/j.jid.2019.03.1145
8. Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
9. Hirano S, Suzuki KT. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect. 1996;104(suppl 1):85-95. doi:10.1289/ehp.96104s185
10. Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol. 2019;15(2):121-127. doi:10.1007/s13181-018-0693-1
11. Williams S, Grimm H. gadolinium toxicity: shedding light on the effects of retained gadolinium from contrast MRI. Accessed April 11, 2022. https://gdtoxicity.files.wordpress.com/2018/12/gadolinium-clearance-times-for-135-contrast-mri-cases-final-v1-1.pdf
12. DeBevits JJ, Reshma M, Bageac D, et al. Gray matter nucleus hyperintensity after monthly triple-dose gadopentetate dimeglumine with long-term magnetic resonance imaging. Invest Radiol. 2020;55(10):629-635. doi:10.1097/RLI.0000000000000663
13. Gathings RM, Reddy R, Santa Cruz D, Brodell RT. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151(3):316-319. doi:10.1001/jamadermatol.2014.2660
14. Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65(6):1095-1106 e7. doi:10.1016/j.jaad.2010.08.041
15. Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis. 2005;46(4):754-759. doi:10.1053/j.ajkd.2005.06.024
16. Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150(5):1050-1052. doi:10.1111/j.1365-2133.2004.05900.x
17. Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35(4):238-49. doi:10.1016/j.semarthrit.2005.08.002
18. Lewis KG, Lester BW, Pan TD, Robinson-Bostom L. Nephrogenic fibrosing dermopathyand calciphylaxis with pseudoxanthoma elasticum-like changes. J Cutan Pathol. 2006;33(10):695-700. doi:10.1111/j.1600-0560.2006.00490.x
19. Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med. 2006;130(2):209-212. doi:10.5858/2006-130-209-MIINFD
20. Cassis TB, Jackson JM, Sonnier GB, Callen JP. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45(1):56-59. doi:10.1111/j.1365-4632.2005.02701.x
21. Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol. 2006;54(suppl 2):S31-S34. doi:10.1016/j.jaad.2005.04.024
22. Sanyal S, Marckmann P, Scherer S, Abraham JL. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant. 2011;26(11):3616-3626. doi:10.1093/ndt/gfr085
23. Kucher C, Xu X, Pasha T, Elenitsas R. Histopathologic comparison of nephrogenic fibrosing dermopathy and scleromyxedema. J Cutan Pathol. 2005;32(7):484-490. doi:10.1111/j.0303-6987.2005.00365.x
24. Goldstein KM, Lunyera J, Mohottige D, et al. Risk of Nephrogenic Systemic Fibrosis after Exposure to Newer Gadolinium Agents. Washington (DC): Department of Veterans Affairs (US); October 2019. https://www.ncbi.nlm.nih.gov/books/NBK559376/25. Lunyera J, Mohottige D, Alexopoulos AS, et al. Risk for nephrogenic systemic fibrosis after exposure to newer gadolinium agents: a systematic review. Ann Intern Med. 2020;173(2):110-119. doi:10.7326/M20-0299
26. Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH Oxidase 4 (Nox4) for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
27. Wagner B. The pathophysiology and retention of gadolinium. United States Food & Drug Administration Medical Imaging Drugs Advisory Committee. 2017:1-23. https://www.fda.gov/advisory-committees/medical-imaging-drugs-advisory-committee/2017-meeting-materials-medical-imaging-drugs-advisory-committee?msclkid=6b5764ccbaa611ec95e35dddf8db57af
28. Runge VM. Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 2017;52(6):317-323. doi:10.1097/RLI.0000000000000374
29. Wagner B. Scared to the marrow: pitfalls and pearls in renal imaging. Adv Chronic Kidney Dis. 2017;24(3):136-137. doi:10.1053/j.ackd.2017.03.008
30. US Food and Drug Administration. Transcript for the September 8, 2017 Meeting of the Medical Imaging Drugs Advisory Committee (MIDAC). September 8, 2017. Accessed April 11, 2022. https://www.fda.gov/media/108935/download
31. Abel M, Talbot RB. Gadolinium oxide inhalation by guinea pigs: a correlative functional and histopathologic study. J Pharmacol Exp Ther. 1967;157(1):207-213.
32. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of gadolinium and samarium chlorides. Br J Pharmacol Chemother. 1961;17(3):526-532. doi:10.1111/j.1476-5381.1961.tb01139.x

33. Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. Gadolinium chloride toxicity in the rat. Toxicol Pathol. 1997;25(3):245-255. doi:10.1177/019262339702500301
34. Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in humans: a family of disorders. AJR Am J Roentgenol. 2016;207(2):229-233. doi:10.2214/AJR.15.15842
35. Semelka RC, Ramalho M. Physicians with self-diagnosed gadolinium deposition disease: a case series. Radiol Bras. 2021;54(4):238-242. doi:10.1590/0100-3984.2020.0073
36. Layne KA, Wood DM, Dargan PI. Gadolinium-based contrast agents—what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). 2020;58(3):151-160. doi:10.1080/15563650.2019.1681442
37. Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: a prospective observational cohort study. Radiology. 2018;288(2):416-423. doi:10.1148/radiol.2018171105
38. US Food and Drug Administration. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. 2017. Accessed April 12, 2022. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf
39. Calvo N, Jamil M, Feldman S, Shah A, Nauman F, Ferrara J. Neurotoxicity from intrathecal gadolinium administration: case presentation and brief review. Neurol Clin Pract. 2020;10(1):e7-e10. doi:10.1212/CPJ.0000000000000696
40. Bower DV, Richter JK, von Tengg-Kobligk H, Heverhagen JT, Runge VM. Gadolinium-based MRI contrast agents induce mitochondrial toxicity and cell death in human neurons, and toxicity increases with reduced kinetic stability of the agent. Invest Radiol. 2019;54(8):453-463. doi:10.1097/RLI.0000000000000567
41. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
42. Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1(6):561-568. doi:10.34067/KID.0000272019
43. Di Gregorio E, Furlan C, Atlante S, Stefania R, Gianolio E, Aime S. Gadolinium retention in erythrocytes and leukocytes from human and murine blood upon treatment with gadolinium-based contrast agents for magnetic resonance imaging. Invest Radiol. 2020;55(1):30-37. doi:10.1097/RLI.0000000000000608
44. Maecker HT, Siebert JC, Rosenberg-Hasson Y, Koran LM, Ramalho M, Semelka RC. Acute chelation therapy-associated changes in urine gadolinium, self-reported flare severity, and serum cytokines in gadolinium deposition disease. Invest Radiol. 2021;56(6):374-384. doi:10.1097/RLI.0000000000000752
45. Maecker HT, Wang W, Rosenberg-Hasson Y, Semelka RC, Hickey J, Koran LM. An initial investigation of serum cytokine levels in patients with gadolinium retention. Radiol Bras. 2020;53(5):306-313. doi:10.1590/0100-3984.2019.0075
46. Birka M, Wentker KS, Lusmöller E, et al. Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem. 2015;87(6):3321-3328. doi:10.1021/ac504488k
Is There a Relationship Between Facility Peer Review Findings and Quality in the Veterans Health Administration?
Hospital leaders report the most common aim of peer review (PR) is to improve quality and patient safety, thus it is a potentially powerful quality improvement (QI) driver.1 “When conducted systematically and credibly, peer review for quality management can result in both short-term and long-term improvements in patient care by revealing areas for improvement in the provision of care,” Veterans Health Administration (VHA) Directive 1190 states. “This ultimately contributes to organizational improvements.” At the same time, there are anecdotal concerns that PR may be used punitively and driven by case outcomes rather than by accepted best practices supporting QI.
Studies of the PR process suggest these concerns are valid. A key tenet of QI is standardization. PR is problematic in that regard; studies show poor interrater reliability for judgments on care, as well as hindsight bias—the fact that raters are strongly influenced by the outcome of care, not the process of care.2-5 There are concerns that case selection or review process when not standardized may be wielded as punitive too.6 In this study, we sought to identify the relationship between PR findings and subsequent institution quality metrics. If PR does lead to an improvement in quality, or if quality concerns are managed within the PR committee, it should be possible to identify a measurable relationship between the PR process and a facility’s subsequent quality measures.
A handful of studies describe the association between PR and quality of care. Itri and colleagues noted that random, not standardized PR in radiology does not achieve reductions in diagnostic error rate.7 However, adoption of just culture principles in PR resulted in a significant improvement in facility leaders’ self-reports of quality measures at surveyed institutions.8 The same author reported that increases in PR standardization and integration with performance improvement activities could explain up to 18% of objective quality measure variation.9
We sought to determine whether a specific aspect of the PR process, the PR committee judgment of quality of care by clinicians, was related to medical center quality in a cross-sectional study of 136 Veterans Health Administration (VHA) medical centers. The VHA is a good source of study because there are standardized PR processes and training for committee members and reviewers. Our hypothesis was that medical centers with a higher number of Level 2 (“most experienced and competent clinicians might have managed the case differently”) and Level 3 (“most experienced and competent providers would have managed the case differently”) PR findings would also have lower quality metric scores for processes and outcomes of care.
Methods
We used PR data from fiscal year 2018 and 2019. VHA PR data are available quarterly and are self-reported by each facility to the VHA Office of Clinical Risk Management. These data are broken down by facility. The following data, when available in both fiscal years 2018 and 2019, were used for this analysis: percent and number of PR that are ranked as level 1, 2, or 3; medical center group (MCG) acuity measure assigned by the VHA (1 is highest, 3 is lowest); and number of PR per 100,000 unique veteran encounters in 2019. Measures of facility quality are drawn from Strategic Analytics for Improvement and Learning (SAIL) data from 2019, which are available quarterly by facility and are rolling for 12 months. SAIL measures processes and outcomes of care. Table 1 indicates which measures are focused on outcomes vs quality processes.
SAS Version 9.2 was used to perform statistical analyses. We used Spearman correlation to estimate the PR and quality relationship.
Results
There were 136 facilities with 2 years of PR data available. The majority of these facilities (89) were highest complexity MCG 1 facilities; 19 were MCG 2, and 28 were MCG 3. Of 13,515 PRs, most of the 9555 PR findings were level 1 (70.7%). The between-facility range of level 2 and 3 findings was large, varying from 3.5% to nearly 70% in 2019 (Table 2). Findings were similar in 2018; facilities level 2 and 3 ratings ranged from 3.6% to 73.5% of all PR findings.
There was no correlation between most quality measures and facility PR findings (Table 3). The only exception was for Global Measures (GM90), an inpatient process of care measure. Unexpectedly, the correlation was positive—facilities with a higher percentage of level 2 and 3 PR findings had better inpatient processes of care SAIL score. The strongest correlation was between 2018 and 2019 PR findings.
Discussion
We hypothesized that a high percentage of level 2 and 3 PR findings would be negatively associated with objective facility measures of care processes in SAIL but we did not see this association. The only quality measure associated with PR findings was GM90, a score of inpatient care processes. However, the association was positive, with better performance associated with more level 2 and 3 PR findings.
The best predictor of the proportion of a facility’s PR findings is the previous year’s PR findings. With an R = 0.59, the previous year findings explain about 35% of the variability in level assignment. Our analysis may describe a new bias in PR, in which committees consistently assign either low or high proportions of level 2 and 3 findings. This correlation could be due to individual PR committee culture or composition, but it does not relate to objective quality measures.
Strengths
For this study we use objective measures of PR processes, the assignment of levels of care.
Limitations
Facilities self-report PR outcomes, so there could be errors in reporting. In addition, this study was cross sectional and not longitudinal and it is possible that change in quality measures over time are correlated with PR findings. Future studies using the VHA PR and SAIL data could evaluate whether changes over time, and perhaps in response to level 2 and 3 findings, would be a more sensitive indicator of the impact of the PR process on quality metrics. Future studies could incorporate the relationship between findings from the All Employee Survey, which is conducted annually, such as psychologic safety, as well as the distance the facility has gone on the high reliability organization journey, with PR findings and SAIL metrics. Finally, PR is focused on the practice of an individual clinician, while SAIL quality metrics reflect facility performance. Interventions possibly stay at the clinician level and do not drive subsequent QI processes.
What does this mean for PR? Since the early 1990s, there have been exhortations from experts to improve PR, by adopting a QI model, or for a deeper integration of PR and QI.1,2,10 Just culture tools, which include QI, are promoted as a means to improve PR.8,11,12 Other studies show PR remains problematic in terms of standardization, incorporation of best practices, redesigning systems of care, or demonstrable improvements to facility safety and care quality.1,4,6,8 Several publications have described interventions to improve PR. Deyo-Svedson discussed a program with standardized training and triggers, much like VHA.13 Itri and colleagues standardized PR in radiology to target areas of known diagnostic error, as well as use the issues assessed in PR to perform QI and education. One example of a successful QI effort involved changing the radiology reporting template to make sure areas that are prone to diagnostic error are addressed.7
Conclusions
Since 35% of PR level variance is correlated with prior year’s results, PR committees should look at increased standardization in reviews and findings. We endorse a strong focus on standardization, application of just culture tools to case reviews, and tighter linkage between process and outcome metrics measured by SAIL and PR case finding. Studies should be performed to pilot interventions to improve the linkage between PR and quality, so that greater and faster gains can be made in quality processes and, leading from this, outcomes. Additionally, future research should investigate why some facilities consistently choose higher or lower PR ratings.
Acknowledgments
We acknowledge Dr. George “Web” Ross for his helpful edits.
1. Edwards MT. In pursuit of quality and safety: an 8-year study of clinical peer review best practices in US hospitals. Int J Qual Health Care. 2018;30(8):602-607. doi:10.1093/intqhc/mzy069
2. Dans PE. Clinical peer Review: burnishing a tarnished icon. Ann Intern Med. 1993;118(7):566-568. doi:10.7326/0003-4819-118-7-199304010-00014
3. Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958-960. doi:10.1001/jama.1992.03480070074034
4. Swaroop R. Disrupting physician clinical practice peer review. Perm J. 2019;23:18-207. doi:10.7812/TPP/18-207
5. Caplan RA, Posner KL, Cheney FW. Effect of outcome on physician judgments of appropriateness of care. JAMA. 1991;265(15):1957–1960. doi:10.1001/jama.1991.03460150061024
6. Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20(21):6357-6363. doi:10.3748/wjg.v20.i21.6357
7. Itri JN, Donithan A, Patel SH. Random versus nonrandom peer review: a case for more meaningful peer review. J Am Coll Radiol. 2018;15(7):1045-1052. doi:10.1016/j.jacr.2018.03.054
8. Edwards MT. An assessment of the impact of just culture on quality and safety in US hospitals. Am J Med Qual. 2018; 33(5):502-508. doi:10.1177/1062860618768057
9. Edwards MT. The objective impact of clinical peer review on hospital quality and safety. Am J Med Qual. 2011;26(2);110-119. doi:10.1177/1062860610380732
10. Berwick DM. Peer review and quality management: are they compatible?. QRB Qual Rev Bull. 1990;16(7):246-251. doi:10.1016/s0097-5990(16)30377-3
11. Volkar JK, Phrampus P, English D, et al. Institution of just culture physician peer review in an academic medical center. J Patient Saf. 2021;17(7):e689-e693. doi:10.1097/PTS.0000000000000449
12. Burns J, Miller T, Weiss JM, Erdfarb A, Silber D, Goldberg-Stein S. Just culture: practical implementation for radiologist peer review. J Am Coll Radiol. 2019;16(3):384-388. doi:10.1016/j.jacr.2018.10.021
13. Deyo-Svendsen ME, Phillips MR, Albright JK, et al. A systematic approach to clinical peer review in a critical access hospital. Qual Manag Health Care. 2016;25(4):213-218. doi:10.1097/QMH.0000000000000113
Hospital leaders report the most common aim of peer review (PR) is to improve quality and patient safety, thus it is a potentially powerful quality improvement (QI) driver.1 “When conducted systematically and credibly, peer review for quality management can result in both short-term and long-term improvements in patient care by revealing areas for improvement in the provision of care,” Veterans Health Administration (VHA) Directive 1190 states. “This ultimately contributes to organizational improvements.” At the same time, there are anecdotal concerns that PR may be used punitively and driven by case outcomes rather than by accepted best practices supporting QI.
Studies of the PR process suggest these concerns are valid. A key tenet of QI is standardization. PR is problematic in that regard; studies show poor interrater reliability for judgments on care, as well as hindsight bias—the fact that raters are strongly influenced by the outcome of care, not the process of care.2-5 There are concerns that case selection or review process when not standardized may be wielded as punitive too.6 In this study, we sought to identify the relationship between PR findings and subsequent institution quality metrics. If PR does lead to an improvement in quality, or if quality concerns are managed within the PR committee, it should be possible to identify a measurable relationship between the PR process and a facility’s subsequent quality measures.
A handful of studies describe the association between PR and quality of care. Itri and colleagues noted that random, not standardized PR in radiology does not achieve reductions in diagnostic error rate.7 However, adoption of just culture principles in PR resulted in a significant improvement in facility leaders’ self-reports of quality measures at surveyed institutions.8 The same author reported that increases in PR standardization and integration with performance improvement activities could explain up to 18% of objective quality measure variation.9
We sought to determine whether a specific aspect of the PR process, the PR committee judgment of quality of care by clinicians, was related to medical center quality in a cross-sectional study of 136 Veterans Health Administration (VHA) medical centers. The VHA is a good source of study because there are standardized PR processes and training for committee members and reviewers. Our hypothesis was that medical centers with a higher number of Level 2 (“most experienced and competent clinicians might have managed the case differently”) and Level 3 (“most experienced and competent providers would have managed the case differently”) PR findings would also have lower quality metric scores for processes and outcomes of care.
Methods
We used PR data from fiscal year 2018 and 2019. VHA PR data are available quarterly and are self-reported by each facility to the VHA Office of Clinical Risk Management. These data are broken down by facility. The following data, when available in both fiscal years 2018 and 2019, were used for this analysis: percent and number of PR that are ranked as level 1, 2, or 3; medical center group (MCG) acuity measure assigned by the VHA (1 is highest, 3 is lowest); and number of PR per 100,000 unique veteran encounters in 2019. Measures of facility quality are drawn from Strategic Analytics for Improvement and Learning (SAIL) data from 2019, which are available quarterly by facility and are rolling for 12 months. SAIL measures processes and outcomes of care. Table 1 indicates which measures are focused on outcomes vs quality processes.
SAS Version 9.2 was used to perform statistical analyses. We used Spearman correlation to estimate the PR and quality relationship.
Results
There were 136 facilities with 2 years of PR data available. The majority of these facilities (89) were highest complexity MCG 1 facilities; 19 were MCG 2, and 28 were MCG 3. Of 13,515 PRs, most of the 9555 PR findings were level 1 (70.7%). The between-facility range of level 2 and 3 findings was large, varying from 3.5% to nearly 70% in 2019 (Table 2). Findings were similar in 2018; facilities level 2 and 3 ratings ranged from 3.6% to 73.5% of all PR findings.
There was no correlation between most quality measures and facility PR findings (Table 3). The only exception was for Global Measures (GM90), an inpatient process of care measure. Unexpectedly, the correlation was positive—facilities with a higher percentage of level 2 and 3 PR findings had better inpatient processes of care SAIL score. The strongest correlation was between 2018 and 2019 PR findings.
Discussion
We hypothesized that a high percentage of level 2 and 3 PR findings would be negatively associated with objective facility measures of care processes in SAIL but we did not see this association. The only quality measure associated with PR findings was GM90, a score of inpatient care processes. However, the association was positive, with better performance associated with more level 2 and 3 PR findings.
The best predictor of the proportion of a facility’s PR findings is the previous year’s PR findings. With an R = 0.59, the previous year findings explain about 35% of the variability in level assignment. Our analysis may describe a new bias in PR, in which committees consistently assign either low or high proportions of level 2 and 3 findings. This correlation could be due to individual PR committee culture or composition, but it does not relate to objective quality measures.
Strengths
For this study we use objective measures of PR processes, the assignment of levels of care.
Limitations
Facilities self-report PR outcomes, so there could be errors in reporting. In addition, this study was cross sectional and not longitudinal and it is possible that change in quality measures over time are correlated with PR findings. Future studies using the VHA PR and SAIL data could evaluate whether changes over time, and perhaps in response to level 2 and 3 findings, would be a more sensitive indicator of the impact of the PR process on quality metrics. Future studies could incorporate the relationship between findings from the All Employee Survey, which is conducted annually, such as psychologic safety, as well as the distance the facility has gone on the high reliability organization journey, with PR findings and SAIL metrics. Finally, PR is focused on the practice of an individual clinician, while SAIL quality metrics reflect facility performance. Interventions possibly stay at the clinician level and do not drive subsequent QI processes.
What does this mean for PR? Since the early 1990s, there have been exhortations from experts to improve PR, by adopting a QI model, or for a deeper integration of PR and QI.1,2,10 Just culture tools, which include QI, are promoted as a means to improve PR.8,11,12 Other studies show PR remains problematic in terms of standardization, incorporation of best practices, redesigning systems of care, or demonstrable improvements to facility safety and care quality.1,4,6,8 Several publications have described interventions to improve PR. Deyo-Svedson discussed a program with standardized training and triggers, much like VHA.13 Itri and colleagues standardized PR in radiology to target areas of known diagnostic error, as well as use the issues assessed in PR to perform QI and education. One example of a successful QI effort involved changing the radiology reporting template to make sure areas that are prone to diagnostic error are addressed.7
Conclusions
Since 35% of PR level variance is correlated with prior year’s results, PR committees should look at increased standardization in reviews and findings. We endorse a strong focus on standardization, application of just culture tools to case reviews, and tighter linkage between process and outcome metrics measured by SAIL and PR case finding. Studies should be performed to pilot interventions to improve the linkage between PR and quality, so that greater and faster gains can be made in quality processes and, leading from this, outcomes. Additionally, future research should investigate why some facilities consistently choose higher or lower PR ratings.
Acknowledgments
We acknowledge Dr. George “Web” Ross for his helpful edits.
Hospital leaders report the most common aim of peer review (PR) is to improve quality and patient safety, thus it is a potentially powerful quality improvement (QI) driver.1 “When conducted systematically and credibly, peer review for quality management can result in both short-term and long-term improvements in patient care by revealing areas for improvement in the provision of care,” Veterans Health Administration (VHA) Directive 1190 states. “This ultimately contributes to organizational improvements.” At the same time, there are anecdotal concerns that PR may be used punitively and driven by case outcomes rather than by accepted best practices supporting QI.
Studies of the PR process suggest these concerns are valid. A key tenet of QI is standardization. PR is problematic in that regard; studies show poor interrater reliability for judgments on care, as well as hindsight bias—the fact that raters are strongly influenced by the outcome of care, not the process of care.2-5 There are concerns that case selection or review process when not standardized may be wielded as punitive too.6 In this study, we sought to identify the relationship between PR findings and subsequent institution quality metrics. If PR does lead to an improvement in quality, or if quality concerns are managed within the PR committee, it should be possible to identify a measurable relationship between the PR process and a facility’s subsequent quality measures.
A handful of studies describe the association between PR and quality of care. Itri and colleagues noted that random, not standardized PR in radiology does not achieve reductions in diagnostic error rate.7 However, adoption of just culture principles in PR resulted in a significant improvement in facility leaders’ self-reports of quality measures at surveyed institutions.8 The same author reported that increases in PR standardization and integration with performance improvement activities could explain up to 18% of objective quality measure variation.9
We sought to determine whether a specific aspect of the PR process, the PR committee judgment of quality of care by clinicians, was related to medical center quality in a cross-sectional study of 136 Veterans Health Administration (VHA) medical centers. The VHA is a good source of study because there are standardized PR processes and training for committee members and reviewers. Our hypothesis was that medical centers with a higher number of Level 2 (“most experienced and competent clinicians might have managed the case differently”) and Level 3 (“most experienced and competent providers would have managed the case differently”) PR findings would also have lower quality metric scores for processes and outcomes of care.
Methods
We used PR data from fiscal year 2018 and 2019. VHA PR data are available quarterly and are self-reported by each facility to the VHA Office of Clinical Risk Management. These data are broken down by facility. The following data, when available in both fiscal years 2018 and 2019, were used for this analysis: percent and number of PR that are ranked as level 1, 2, or 3; medical center group (MCG) acuity measure assigned by the VHA (1 is highest, 3 is lowest); and number of PR per 100,000 unique veteran encounters in 2019. Measures of facility quality are drawn from Strategic Analytics for Improvement and Learning (SAIL) data from 2019, which are available quarterly by facility and are rolling for 12 months. SAIL measures processes and outcomes of care. Table 1 indicates which measures are focused on outcomes vs quality processes.
SAS Version 9.2 was used to perform statistical analyses. We used Spearman correlation to estimate the PR and quality relationship.
Results
There were 136 facilities with 2 years of PR data available. The majority of these facilities (89) were highest complexity MCG 1 facilities; 19 were MCG 2, and 28 were MCG 3. Of 13,515 PRs, most of the 9555 PR findings were level 1 (70.7%). The between-facility range of level 2 and 3 findings was large, varying from 3.5% to nearly 70% in 2019 (Table 2). Findings were similar in 2018; facilities level 2 and 3 ratings ranged from 3.6% to 73.5% of all PR findings.
There was no correlation between most quality measures and facility PR findings (Table 3). The only exception was for Global Measures (GM90), an inpatient process of care measure. Unexpectedly, the correlation was positive—facilities with a higher percentage of level 2 and 3 PR findings had better inpatient processes of care SAIL score. The strongest correlation was between 2018 and 2019 PR findings.
Discussion
We hypothesized that a high percentage of level 2 and 3 PR findings would be negatively associated with objective facility measures of care processes in SAIL but we did not see this association. The only quality measure associated with PR findings was GM90, a score of inpatient care processes. However, the association was positive, with better performance associated with more level 2 and 3 PR findings.
The best predictor of the proportion of a facility’s PR findings is the previous year’s PR findings. With an R = 0.59, the previous year findings explain about 35% of the variability in level assignment. Our analysis may describe a new bias in PR, in which committees consistently assign either low or high proportions of level 2 and 3 findings. This correlation could be due to individual PR committee culture or composition, but it does not relate to objective quality measures.
Strengths
For this study we use objective measures of PR processes, the assignment of levels of care.
Limitations
Facilities self-report PR outcomes, so there could be errors in reporting. In addition, this study was cross sectional and not longitudinal and it is possible that change in quality measures over time are correlated with PR findings. Future studies using the VHA PR and SAIL data could evaluate whether changes over time, and perhaps in response to level 2 and 3 findings, would be a more sensitive indicator of the impact of the PR process on quality metrics. Future studies could incorporate the relationship between findings from the All Employee Survey, which is conducted annually, such as psychologic safety, as well as the distance the facility has gone on the high reliability organization journey, with PR findings and SAIL metrics. Finally, PR is focused on the practice of an individual clinician, while SAIL quality metrics reflect facility performance. Interventions possibly stay at the clinician level and do not drive subsequent QI processes.
What does this mean for PR? Since the early 1990s, there have been exhortations from experts to improve PR, by adopting a QI model, or for a deeper integration of PR and QI.1,2,10 Just culture tools, which include QI, are promoted as a means to improve PR.8,11,12 Other studies show PR remains problematic in terms of standardization, incorporation of best practices, redesigning systems of care, or demonstrable improvements to facility safety and care quality.1,4,6,8 Several publications have described interventions to improve PR. Deyo-Svedson discussed a program with standardized training and triggers, much like VHA.13 Itri and colleagues standardized PR in radiology to target areas of known diagnostic error, as well as use the issues assessed in PR to perform QI and education. One example of a successful QI effort involved changing the radiology reporting template to make sure areas that are prone to diagnostic error are addressed.7
Conclusions
Since 35% of PR level variance is correlated with prior year’s results, PR committees should look at increased standardization in reviews and findings. We endorse a strong focus on standardization, application of just culture tools to case reviews, and tighter linkage between process and outcome metrics measured by SAIL and PR case finding. Studies should be performed to pilot interventions to improve the linkage between PR and quality, so that greater and faster gains can be made in quality processes and, leading from this, outcomes. Additionally, future research should investigate why some facilities consistently choose higher or lower PR ratings.
Acknowledgments
We acknowledge Dr. George “Web” Ross for his helpful edits.
1. Edwards MT. In pursuit of quality and safety: an 8-year study of clinical peer review best practices in US hospitals. Int J Qual Health Care. 2018;30(8):602-607. doi:10.1093/intqhc/mzy069
2. Dans PE. Clinical peer Review: burnishing a tarnished icon. Ann Intern Med. 1993;118(7):566-568. doi:10.7326/0003-4819-118-7-199304010-00014
3. Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958-960. doi:10.1001/jama.1992.03480070074034
4. Swaroop R. Disrupting physician clinical practice peer review. Perm J. 2019;23:18-207. doi:10.7812/TPP/18-207
5. Caplan RA, Posner KL, Cheney FW. Effect of outcome on physician judgments of appropriateness of care. JAMA. 1991;265(15):1957–1960. doi:10.1001/jama.1991.03460150061024
6. Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20(21):6357-6363. doi:10.3748/wjg.v20.i21.6357
7. Itri JN, Donithan A, Patel SH. Random versus nonrandom peer review: a case for more meaningful peer review. J Am Coll Radiol. 2018;15(7):1045-1052. doi:10.1016/j.jacr.2018.03.054
8. Edwards MT. An assessment of the impact of just culture on quality and safety in US hospitals. Am J Med Qual. 2018; 33(5):502-508. doi:10.1177/1062860618768057
9. Edwards MT. The objective impact of clinical peer review on hospital quality and safety. Am J Med Qual. 2011;26(2);110-119. doi:10.1177/1062860610380732
10. Berwick DM. Peer review and quality management: are they compatible?. QRB Qual Rev Bull. 1990;16(7):246-251. doi:10.1016/s0097-5990(16)30377-3
11. Volkar JK, Phrampus P, English D, et al. Institution of just culture physician peer review in an academic medical center. J Patient Saf. 2021;17(7):e689-e693. doi:10.1097/PTS.0000000000000449
12. Burns J, Miller T, Weiss JM, Erdfarb A, Silber D, Goldberg-Stein S. Just culture: practical implementation for radiologist peer review. J Am Coll Radiol. 2019;16(3):384-388. doi:10.1016/j.jacr.2018.10.021
13. Deyo-Svendsen ME, Phillips MR, Albright JK, et al. A systematic approach to clinical peer review in a critical access hospital. Qual Manag Health Care. 2016;25(4):213-218. doi:10.1097/QMH.0000000000000113
1. Edwards MT. In pursuit of quality and safety: an 8-year study of clinical peer review best practices in US hospitals. Int J Qual Health Care. 2018;30(8):602-607. doi:10.1093/intqhc/mzy069
2. Dans PE. Clinical peer Review: burnishing a tarnished icon. Ann Intern Med. 1993;118(7):566-568. doi:10.7326/0003-4819-118-7-199304010-00014
3. Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958-960. doi:10.1001/jama.1992.03480070074034
4. Swaroop R. Disrupting physician clinical practice peer review. Perm J. 2019;23:18-207. doi:10.7812/TPP/18-207
5. Caplan RA, Posner KL, Cheney FW. Effect of outcome on physician judgments of appropriateness of care. JAMA. 1991;265(15):1957–1960. doi:10.1001/jama.1991.03460150061024
6. Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20(21):6357-6363. doi:10.3748/wjg.v20.i21.6357
7. Itri JN, Donithan A, Patel SH. Random versus nonrandom peer review: a case for more meaningful peer review. J Am Coll Radiol. 2018;15(7):1045-1052. doi:10.1016/j.jacr.2018.03.054
8. Edwards MT. An assessment of the impact of just culture on quality and safety in US hospitals. Am J Med Qual. 2018; 33(5):502-508. doi:10.1177/1062860618768057
9. Edwards MT. The objective impact of clinical peer review on hospital quality and safety. Am J Med Qual. 2011;26(2);110-119. doi:10.1177/1062860610380732
10. Berwick DM. Peer review and quality management: are they compatible?. QRB Qual Rev Bull. 1990;16(7):246-251. doi:10.1016/s0097-5990(16)30377-3
11. Volkar JK, Phrampus P, English D, et al. Institution of just culture physician peer review in an academic medical center. J Patient Saf. 2021;17(7):e689-e693. doi:10.1097/PTS.0000000000000449
12. Burns J, Miller T, Weiss JM, Erdfarb A, Silber D, Goldberg-Stein S. Just culture: practical implementation for radiologist peer review. J Am Coll Radiol. 2019;16(3):384-388. doi:10.1016/j.jacr.2018.10.021
13. Deyo-Svendsen ME, Phillips MR, Albright JK, et al. A systematic approach to clinical peer review in a critical access hospital. Qual Manag Health Care. 2016;25(4):213-218. doi:10.1097/QMH.0000000000000113
Green Alerts: Balancing Suicide Risk and Privacy
Contemporary critiques of Memorial and Veterans Day celebrations have emphasized that while ceremonies and celebrations are culturally requisite means of demonstrating a society’s respect and gratitude for those who gave their lives and health in the country’s cause—it is not enough. These holidays have immense symbolic significance to remind the nation of the sacrifice of those who bore arms in its service. An enduring and substantive impact on veterans will require real work done on their behalf. Through its representative institutions, such as the US Departments of Defense (DoD) and Veterans Affairs (VA) and citizens’ voluntary efforts, the public must provide practical assistance to veterans and their families.2
Memorial Day honors our sacred dead who lost their lives defending freedom. In federal practice and the larger community, we are duty-bound to try and restore the things war took from these wounded warriors and in whatever measure is possible to return them to the land of the fully living. Except in memory, we cannot bring back the dead. And while life is the most precious gift, those who survived the battlefield too often lose much that matters to a meaningful human life—friends, family, livelihood, housing, self-worth, peace of heart, soundness of mind, and health of the body.
One such recent initiative of reclamation is the Green Alert. Readers are likely familiar with Amber alerts for abducted children and Silver Alerts for older adults often with cognitive impairment who are lost. The Green Alert is a similar program deploying media and law enforcement to search for missing veterans believed to be vulnerable to harm because of a medical or psychiatric illness related to their service.
In 2017 Wisconsin became the first state to pass Green Alert legislation. The Missing Veterans at Risk Act lists 2 criteria that trigger a Green Alert: There is a reason to believe that the veteran at risk is missing due to a physical or mental health condition or that the veteran at risk is missing due to a physical or mental health condition. Relevant to the readers of Federal Practitioner, in Wisconsin, Green Alerts can be issued on behalf of missing veterans, and active-duty guard and reserve members and thus cover almost all the ranks of US military service.3 When law enforcement receives a report of a missing veteran as defined in the act within 72 hours of their disappearance, a Green Alert is issued. The statute directs the US Department of Justice to permit law enforcement to access the crime notification network to notify the media to broadcast pertinent information about the missing veteran.
As of this writing, Delaware, Kentucky, Connecticut, and Texas have passed similar laws, and legislatures in other states are considering bills, as is Congress.4 The sponsorship of the National Green Alert Act is bipartisan. Its stated purpose is: to develop interagency Green Alert systems that would be used when a veteran “goes missing” and “for other purposes.”5
The program’s potential to reduce the number of veterans who die by suicide every day has understandably attracted the attention of legislators and the public.6 The Cost of War project disclosed the terrible irony that at least 4 times as many post-9/11 service members died by suicide as perished in the combat that Memorial Day traditionally commemorates.7 As with many veteran-related laws, the initial Green Alert in Wisconsin was borne out of tragedy and passed through the heroic advocacy of bereaved and outraged family members.8 The DoD and VA, Congress, veterans service organizations, and the loved ones of servicemembers desperately want to turn this devastating tide of self-destruction through any means possible.
It seems almost a blasphemous betrayal of our public trust to raise ethical questions about Green Alerts. Yet that must happen if we ensure that these laws achieve their intended aims of preventing harm. For many veterans, these laws may indeed be lifesaving. However, a 2019 National Public Radio report suggested that these laws may, in some cases, result in several unintended harms.9 On first reading, it is worthy, even our duty, to extend the public health safety net for children who are victims of abduction and individuals with dementia to vulnerable veterans secondary to the mental and physical wounds of service.
When the service member is located, the alert is canceled. Nevertheless, their data remains in all the protean forms of media now available. In these searches for service members thought to be lost, there is a risk of violating their privacy if too much protected health information is made widely public. These breaches of confidentiality can further exacerbate the already too prevalent stigmatization of mental illness in the military, which has been a formidable obstacle to persuading those in uniform to seek treatment.10 As J.R.R. Tolkien has noted, not every person who “wanders” is lost.1 A veteran may leave his home for some period, even without notifying anyone, without being in grave and imminent danger. The diagnoses we health care professionals assign to patients are wide conceptual nets full of empirical holes: they are poorly predictive and protective mechanisms.11 A broadly written or vague law leaves latitude for bias, discrimination, liability, and fear to drive decisions that to be ethically justifiable require consistency, transparency, equity, and expertise. Much more research is needed to develop situational awareness, scientific accuracy, and clinical reliability to understand when, how, and for whom Green Alerts are genuinely beneficial.
These are not insurmountable questions. The experts and stakeholders appointed to the interagency committee the national Green Alert proposes will work to address these problems. Yet, unless they and we look bravely at the thorny issues these laudable laws present, it will be challenging to achieve their purpose to safeguard the dignity, safety, as well as autonomy and well-being of service members.
1. Tolkien JRR. The Fellowship of the Ring. Ballantine Books; 1974.
2. Constantine J. Here’s how to thank veterans for their service. Accessed April 25, 2022. https://www.military.com/veterans-day/heres-how-to-actually-thank-veterans-for-their-service.html
3. 2017 Wisconsin Act 275. Accessed April 25, 2022. https://docs.legis.wisconsin.gov/2017/related/acts/175
4. Thayer RL. Texas is the third state to approve an alert that helps locate missing vets and service members. Stars and Stripes. August 14, 2019. Accessed April 25, 2022. https://www.stripes.com/texas-is-third-state-to-approve-alert-that-helps-locate-missing-vets-servicemembers-1.594348
5. National Green Alert Act of 2021. HR 2797, 117th Cong (2021). Accessed April 25, 2022. https://www.govinfo.gov/app/details/BILLS-117hr2797ih
6. Suitt TH III. High suicide rates among United States service members and veterans of the post 9/11 wars. June 21, 2021. Accessed April 25, 2022. https://watson.brown.edu/costsofwar/files/cow/imce/papers/2021/Suitt_Suicides_Costs%20of%20War_June%2021%202021.pdf
7. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 annual report. September 2021. Accessed April 25, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
8. Chamberlin K. Wisconsin becomes the first state with “green alerts” for vulnerable vets. Military Times. March 31, 2018. Accessed April 25, 2022. https://www.militarytimes.com/veterans/2018/03/31/wisconsin-becomes-first-state-with-green-alerts-for-vulnerable-vets/
9. Lawrence Q. Balancing safety and privacy when a veteran goes missing. All Things Considered. National Public Radio. April 9, 2019. Accessed April 25, 2022. https://www.npr.org/2019/04/09/711040850/balancing-safety-and-privacy-when-a-veteran-goes-missing
10. Kim PJ, Thomas JL, Wilk JE, Castro CA, Hoge CW. Stigma, barriers to care, and use of mental health services among active duty and national guard soldiers after combat. Psychiatric Services. 2010;61(6):582-588. doi:10.1176/ps.2010.61.6.582
11. Peterson K, Anderson J, Bourne D. Evidence Brief: Suicide Prevention in Veterans. Department of Veterans Affairs; 2018. Accessed April 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK535971/
Contemporary critiques of Memorial and Veterans Day celebrations have emphasized that while ceremonies and celebrations are culturally requisite means of demonstrating a society’s respect and gratitude for those who gave their lives and health in the country’s cause—it is not enough. These holidays have immense symbolic significance to remind the nation of the sacrifice of those who bore arms in its service. An enduring and substantive impact on veterans will require real work done on their behalf. Through its representative institutions, such as the US Departments of Defense (DoD) and Veterans Affairs (VA) and citizens’ voluntary efforts, the public must provide practical assistance to veterans and their families.2
Memorial Day honors our sacred dead who lost their lives defending freedom. In federal practice and the larger community, we are duty-bound to try and restore the things war took from these wounded warriors and in whatever measure is possible to return them to the land of the fully living. Except in memory, we cannot bring back the dead. And while life is the most precious gift, those who survived the battlefield too often lose much that matters to a meaningful human life—friends, family, livelihood, housing, self-worth, peace of heart, soundness of mind, and health of the body.
One such recent initiative of reclamation is the Green Alert. Readers are likely familiar with Amber alerts for abducted children and Silver Alerts for older adults often with cognitive impairment who are lost. The Green Alert is a similar program deploying media and law enforcement to search for missing veterans believed to be vulnerable to harm because of a medical or psychiatric illness related to their service.
In 2017 Wisconsin became the first state to pass Green Alert legislation. The Missing Veterans at Risk Act lists 2 criteria that trigger a Green Alert: There is a reason to believe that the veteran at risk is missing due to a physical or mental health condition or that the veteran at risk is missing due to a physical or mental health condition. Relevant to the readers of Federal Practitioner, in Wisconsin, Green Alerts can be issued on behalf of missing veterans, and active-duty guard and reserve members and thus cover almost all the ranks of US military service.3 When law enforcement receives a report of a missing veteran as defined in the act within 72 hours of their disappearance, a Green Alert is issued. The statute directs the US Department of Justice to permit law enforcement to access the crime notification network to notify the media to broadcast pertinent information about the missing veteran.
As of this writing, Delaware, Kentucky, Connecticut, and Texas have passed similar laws, and legislatures in other states are considering bills, as is Congress.4 The sponsorship of the National Green Alert Act is bipartisan. Its stated purpose is: to develop interagency Green Alert systems that would be used when a veteran “goes missing” and “for other purposes.”5
The program’s potential to reduce the number of veterans who die by suicide every day has understandably attracted the attention of legislators and the public.6 The Cost of War project disclosed the terrible irony that at least 4 times as many post-9/11 service members died by suicide as perished in the combat that Memorial Day traditionally commemorates.7 As with many veteran-related laws, the initial Green Alert in Wisconsin was borne out of tragedy and passed through the heroic advocacy of bereaved and outraged family members.8 The DoD and VA, Congress, veterans service organizations, and the loved ones of servicemembers desperately want to turn this devastating tide of self-destruction through any means possible.
It seems almost a blasphemous betrayal of our public trust to raise ethical questions about Green Alerts. Yet that must happen if we ensure that these laws achieve their intended aims of preventing harm. For many veterans, these laws may indeed be lifesaving. However, a 2019 National Public Radio report suggested that these laws may, in some cases, result in several unintended harms.9 On first reading, it is worthy, even our duty, to extend the public health safety net for children who are victims of abduction and individuals with dementia to vulnerable veterans secondary to the mental and physical wounds of service.
When the service member is located, the alert is canceled. Nevertheless, their data remains in all the protean forms of media now available. In these searches for service members thought to be lost, there is a risk of violating their privacy if too much protected health information is made widely public. These breaches of confidentiality can further exacerbate the already too prevalent stigmatization of mental illness in the military, which has been a formidable obstacle to persuading those in uniform to seek treatment.10 As J.R.R. Tolkien has noted, not every person who “wanders” is lost.1 A veteran may leave his home for some period, even without notifying anyone, without being in grave and imminent danger. The diagnoses we health care professionals assign to patients are wide conceptual nets full of empirical holes: they are poorly predictive and protective mechanisms.11 A broadly written or vague law leaves latitude for bias, discrimination, liability, and fear to drive decisions that to be ethically justifiable require consistency, transparency, equity, and expertise. Much more research is needed to develop situational awareness, scientific accuracy, and clinical reliability to understand when, how, and for whom Green Alerts are genuinely beneficial.
These are not insurmountable questions. The experts and stakeholders appointed to the interagency committee the national Green Alert proposes will work to address these problems. Yet, unless they and we look bravely at the thorny issues these laudable laws present, it will be challenging to achieve their purpose to safeguard the dignity, safety, as well as autonomy and well-being of service members.
Contemporary critiques of Memorial and Veterans Day celebrations have emphasized that while ceremonies and celebrations are culturally requisite means of demonstrating a society’s respect and gratitude for those who gave their lives and health in the country’s cause—it is not enough. These holidays have immense symbolic significance to remind the nation of the sacrifice of those who bore arms in its service. An enduring and substantive impact on veterans will require real work done on their behalf. Through its representative institutions, such as the US Departments of Defense (DoD) and Veterans Affairs (VA) and citizens’ voluntary efforts, the public must provide practical assistance to veterans and their families.2
Memorial Day honors our sacred dead who lost their lives defending freedom. In federal practice and the larger community, we are duty-bound to try and restore the things war took from these wounded warriors and in whatever measure is possible to return them to the land of the fully living. Except in memory, we cannot bring back the dead. And while life is the most precious gift, those who survived the battlefield too often lose much that matters to a meaningful human life—friends, family, livelihood, housing, self-worth, peace of heart, soundness of mind, and health of the body.
One such recent initiative of reclamation is the Green Alert. Readers are likely familiar with Amber alerts for abducted children and Silver Alerts for older adults often with cognitive impairment who are lost. The Green Alert is a similar program deploying media and law enforcement to search for missing veterans believed to be vulnerable to harm because of a medical or psychiatric illness related to their service.
In 2017 Wisconsin became the first state to pass Green Alert legislation. The Missing Veterans at Risk Act lists 2 criteria that trigger a Green Alert: There is a reason to believe that the veteran at risk is missing due to a physical or mental health condition or that the veteran at risk is missing due to a physical or mental health condition. Relevant to the readers of Federal Practitioner, in Wisconsin, Green Alerts can be issued on behalf of missing veterans, and active-duty guard and reserve members and thus cover almost all the ranks of US military service.3 When law enforcement receives a report of a missing veteran as defined in the act within 72 hours of their disappearance, a Green Alert is issued. The statute directs the US Department of Justice to permit law enforcement to access the crime notification network to notify the media to broadcast pertinent information about the missing veteran.
As of this writing, Delaware, Kentucky, Connecticut, and Texas have passed similar laws, and legislatures in other states are considering bills, as is Congress.4 The sponsorship of the National Green Alert Act is bipartisan. Its stated purpose is: to develop interagency Green Alert systems that would be used when a veteran “goes missing” and “for other purposes.”5
The program’s potential to reduce the number of veterans who die by suicide every day has understandably attracted the attention of legislators and the public.6 The Cost of War project disclosed the terrible irony that at least 4 times as many post-9/11 service members died by suicide as perished in the combat that Memorial Day traditionally commemorates.7 As with many veteran-related laws, the initial Green Alert in Wisconsin was borne out of tragedy and passed through the heroic advocacy of bereaved and outraged family members.8 The DoD and VA, Congress, veterans service organizations, and the loved ones of servicemembers desperately want to turn this devastating tide of self-destruction through any means possible.
It seems almost a blasphemous betrayal of our public trust to raise ethical questions about Green Alerts. Yet that must happen if we ensure that these laws achieve their intended aims of preventing harm. For many veterans, these laws may indeed be lifesaving. However, a 2019 National Public Radio report suggested that these laws may, in some cases, result in several unintended harms.9 On first reading, it is worthy, even our duty, to extend the public health safety net for children who are victims of abduction and individuals with dementia to vulnerable veterans secondary to the mental and physical wounds of service.
When the service member is located, the alert is canceled. Nevertheless, their data remains in all the protean forms of media now available. In these searches for service members thought to be lost, there is a risk of violating their privacy if too much protected health information is made widely public. These breaches of confidentiality can further exacerbate the already too prevalent stigmatization of mental illness in the military, which has been a formidable obstacle to persuading those in uniform to seek treatment.10 As J.R.R. Tolkien has noted, not every person who “wanders” is lost.1 A veteran may leave his home for some period, even without notifying anyone, without being in grave and imminent danger. The diagnoses we health care professionals assign to patients are wide conceptual nets full of empirical holes: they are poorly predictive and protective mechanisms.11 A broadly written or vague law leaves latitude for bias, discrimination, liability, and fear to drive decisions that to be ethically justifiable require consistency, transparency, equity, and expertise. Much more research is needed to develop situational awareness, scientific accuracy, and clinical reliability to understand when, how, and for whom Green Alerts are genuinely beneficial.
These are not insurmountable questions. The experts and stakeholders appointed to the interagency committee the national Green Alert proposes will work to address these problems. Yet, unless they and we look bravely at the thorny issues these laudable laws present, it will be challenging to achieve their purpose to safeguard the dignity, safety, as well as autonomy and well-being of service members.
1. Tolkien JRR. The Fellowship of the Ring. Ballantine Books; 1974.
2. Constantine J. Here’s how to thank veterans for their service. Accessed April 25, 2022. https://www.military.com/veterans-day/heres-how-to-actually-thank-veterans-for-their-service.html
3. 2017 Wisconsin Act 275. Accessed April 25, 2022. https://docs.legis.wisconsin.gov/2017/related/acts/175
4. Thayer RL. Texas is the third state to approve an alert that helps locate missing vets and service members. Stars and Stripes. August 14, 2019. Accessed April 25, 2022. https://www.stripes.com/texas-is-third-state-to-approve-alert-that-helps-locate-missing-vets-servicemembers-1.594348
5. National Green Alert Act of 2021. HR 2797, 117th Cong (2021). Accessed April 25, 2022. https://www.govinfo.gov/app/details/BILLS-117hr2797ih
6. Suitt TH III. High suicide rates among United States service members and veterans of the post 9/11 wars. June 21, 2021. Accessed April 25, 2022. https://watson.brown.edu/costsofwar/files/cow/imce/papers/2021/Suitt_Suicides_Costs%20of%20War_June%2021%202021.pdf
7. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 annual report. September 2021. Accessed April 25, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
8. Chamberlin K. Wisconsin becomes the first state with “green alerts” for vulnerable vets. Military Times. March 31, 2018. Accessed April 25, 2022. https://www.militarytimes.com/veterans/2018/03/31/wisconsin-becomes-first-state-with-green-alerts-for-vulnerable-vets/
9. Lawrence Q. Balancing safety and privacy when a veteran goes missing. All Things Considered. National Public Radio. April 9, 2019. Accessed April 25, 2022. https://www.npr.org/2019/04/09/711040850/balancing-safety-and-privacy-when-a-veteran-goes-missing
10. Kim PJ, Thomas JL, Wilk JE, Castro CA, Hoge CW. Stigma, barriers to care, and use of mental health services among active duty and national guard soldiers after combat. Psychiatric Services. 2010;61(6):582-588. doi:10.1176/ps.2010.61.6.582
11. Peterson K, Anderson J, Bourne D. Evidence Brief: Suicide Prevention in Veterans. Department of Veterans Affairs; 2018. Accessed April 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK535971/
1. Tolkien JRR. The Fellowship of the Ring. Ballantine Books; 1974.
2. Constantine J. Here’s how to thank veterans for their service. Accessed April 25, 2022. https://www.military.com/veterans-day/heres-how-to-actually-thank-veterans-for-their-service.html
3. 2017 Wisconsin Act 275. Accessed April 25, 2022. https://docs.legis.wisconsin.gov/2017/related/acts/175
4. Thayer RL. Texas is the third state to approve an alert that helps locate missing vets and service members. Stars and Stripes. August 14, 2019. Accessed April 25, 2022. https://www.stripes.com/texas-is-third-state-to-approve-alert-that-helps-locate-missing-vets-servicemembers-1.594348
5. National Green Alert Act of 2021. HR 2797, 117th Cong (2021). Accessed April 25, 2022. https://www.govinfo.gov/app/details/BILLS-117hr2797ih
6. Suitt TH III. High suicide rates among United States service members and veterans of the post 9/11 wars. June 21, 2021. Accessed April 25, 2022. https://watson.brown.edu/costsofwar/files/cow/imce/papers/2021/Suitt_Suicides_Costs%20of%20War_June%2021%202021.pdf
7. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2021 annual report. September 2021. Accessed April 25, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2021/2021-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-9-8-21.pdf
8. Chamberlin K. Wisconsin becomes the first state with “green alerts” for vulnerable vets. Military Times. March 31, 2018. Accessed April 25, 2022. https://www.militarytimes.com/veterans/2018/03/31/wisconsin-becomes-first-state-with-green-alerts-for-vulnerable-vets/
9. Lawrence Q. Balancing safety and privacy when a veteran goes missing. All Things Considered. National Public Radio. April 9, 2019. Accessed April 25, 2022. https://www.npr.org/2019/04/09/711040850/balancing-safety-and-privacy-when-a-veteran-goes-missing
10. Kim PJ, Thomas JL, Wilk JE, Castro CA, Hoge CW. Stigma, barriers to care, and use of mental health services among active duty and national guard soldiers after combat. Psychiatric Services. 2010;61(6):582-588. doi:10.1176/ps.2010.61.6.582
11. Peterson K, Anderson J, Bourne D. Evidence Brief: Suicide Prevention in Veterans. Department of Veterans Affairs; 2018. Accessed April 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK535971/
Patients asking about APOE gene test results? Here’s what to tell them
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.
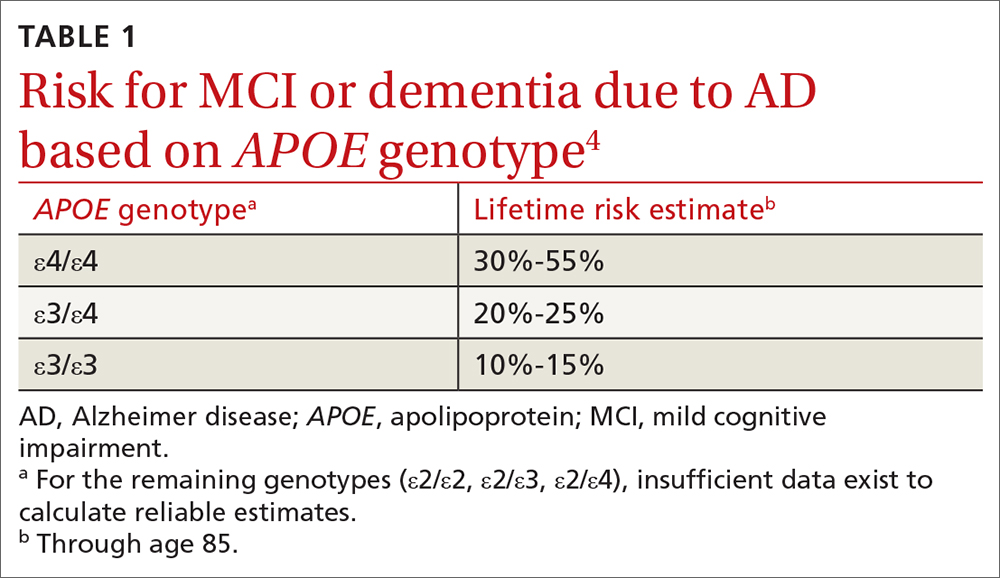
The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.
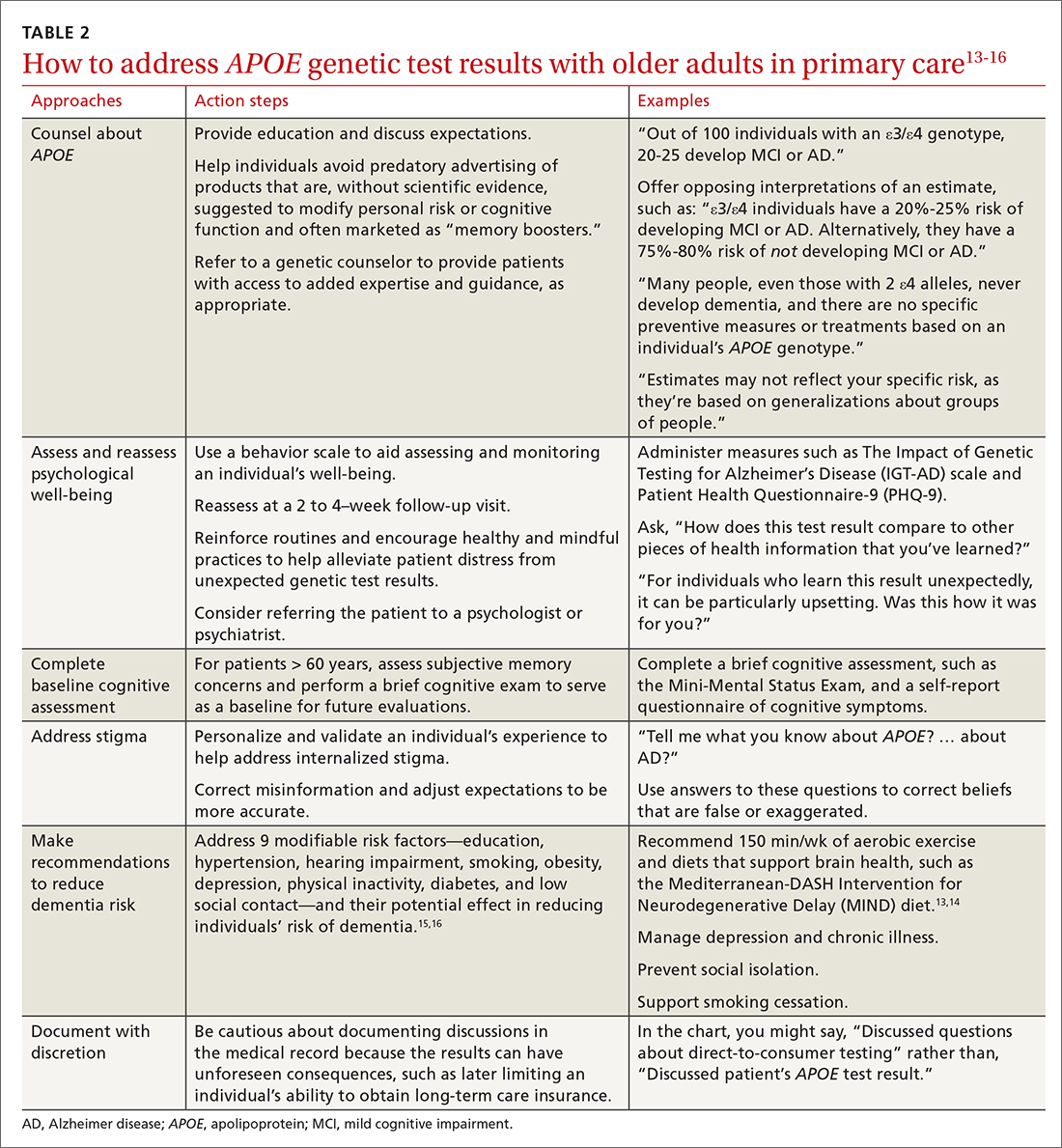
Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.

The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.

Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.

The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.

Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
Substantially enlarged cardiac silhouette
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.
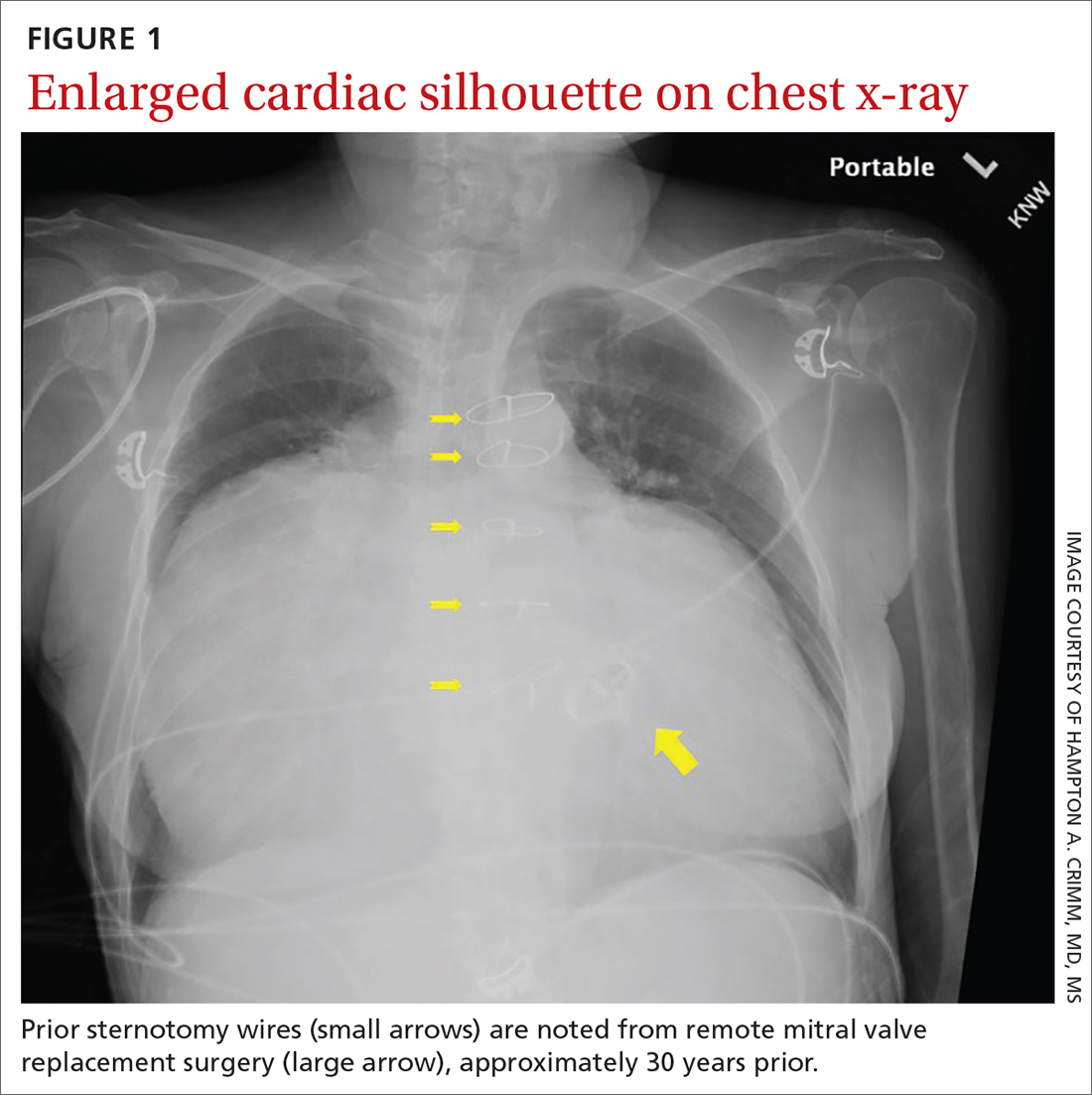
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.
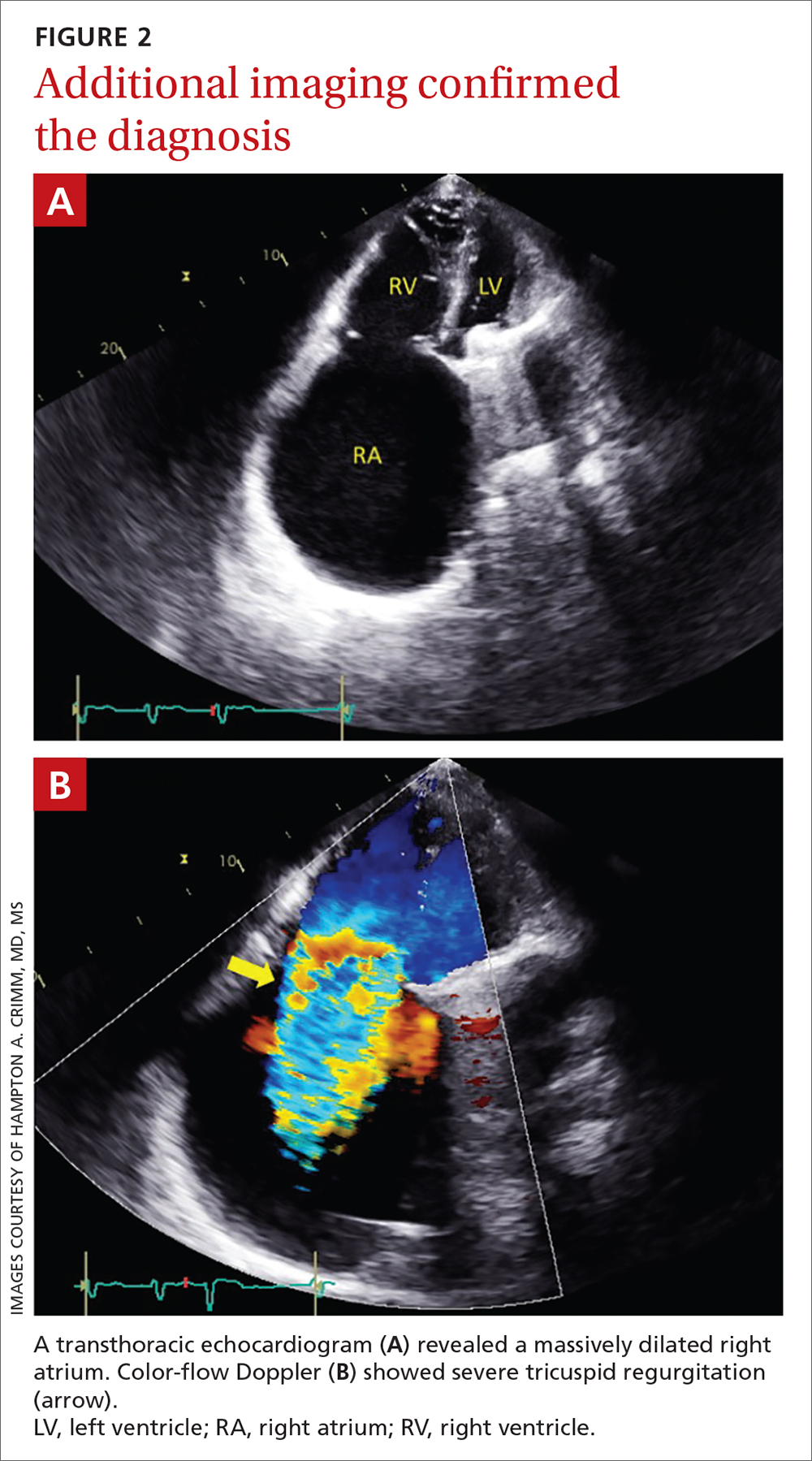
RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.

RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
A 63-YEAR-OLD SOUTHEAST ASIAN WOMAN presented with early satiety, mild swelling of her lower extremities, and several months of progressive shortness of breath that had become severe (provoked by activities of daily living). She had a history of longstanding, rate-controlled atrial fibrillation on oral anticoagulation. She also had a history of mitral valve stenosis that was treated 30 years earlier with mechanical valve replacement. The patient had previously been treated out of state and prior records were not available.
Chest radiography (CXR) was performed as part of the initial work-up (FIGURE 1) and demonstrated a substantially enlarged cardiac silhouette spanning the entire width of the chest without significant pleural effusion or evidence of airspace disease. Suspecting a primary cardiac pathology in this patient, we explored clinical findings of heart failure with transthoracic echocardiography.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Severe tricuspid valve regurgitation secondary to rheumatic heart disease
A transthoracic echocardiogram (FIGURE 2A) revealed cardiomegaly with massive right atrial enlargement; a color-flow Doppler (FIGURE 2B) revealed severe tricuspid regurgitation, reduced right ventricular systolic function, and preserved left ventricular systolic function. All of these findings pointed to the diagnosis of rheumatic heart disease (RHD), especially in the context of prior mitral valve stenosis.

RHD affects more than 33 million people annually and remains a significant problem globally.1 It’s associated with a relatively poor prognosis, especially if heart failure is present (as it was in this case).2,3 Although the mitral and aortic valves are most commonly affected, approximately 34% of patients will develop tricuspid regurgitation.4 Right-side cardiac manifestations of RHD may lead to clinical heart failure with chronic venous congestion and, ultimately, cirrhosis.
Suspect RHD when encountering a new murmur in a patient with prior history of acute rheumatic fever, especially if they are living in or are from a country where rheumatic disease is endemic (most of the developing world).
The diagnosis is confirmed when echocardiographic findings demonstrate characteristic pathologic valve changes (eg, thickening of the anterior mitral valve leaflet, especially the leaflet tips and subvalvular apparatus).
The differential for an enlarged cardiac silhouette
The differential diagnosis for an enlarged cardiac silhouette on CXR includes cardiomegaly (as in this case), pericardial effusion, or a thoracic mass (either mediastinal or pericardial). Imaging artifact from patient orientation may also yield the appearance of an enlarged cardiac silhouette. Distinguishing between these entities may be accomplished by incorporating the history with selection of more definitive imaging (eg, echocardiogram or computed tomography).
Continue to: Management depends on the severity and symptoms
Management depends on the severity and symptoms
Percutaneous or surgical intervention may be required with RHD, depending on the clinical scenario. If the patient also has atrial fibrillation, medical management includes oral anticoagulation (with a vitamin K antagonist). Additionally, secondary prophylaxis with long-term antibiotics (directed against recurrent group A Streptococcus infection) is recommended for RHD patients with mitral stenosis.5 If the patient in this case had engaged in more regular cardiology follow-up, the progression of her tricuspid regurgitation may have been mitigated by surgical intervention and aggressive medical management (although the progression of RHD can eclipse standard treatments).5
In this case, a liver biopsy was pursued for prognostication. Unfortunately, the biopsy demonstrated cirrhosis with perisinusoidal fibrosis suggesting an advanced, end-stage clinical state. This diagnosis precluded the patient’s eligibility for advanced therapies such as right ventricular assist device implantation or cardiac transplantation. Surgical intervention (repair or replacement) was also deemed likely to be futile due to right ventricular dilatation and systolic dysfunction in the context of antecedent left-side valve intervention.
The patient elected to pursue palliative care and died at home several months later. In the years since this case occurred, less invasive tricuspid valve interventions have been explored, offering promise of amelioration of such cases in the future.6
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
1. Watkins DA, Johnson CO, Colquhoun SM, et. al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017; 377:713-722. doi: 10.1056/NEJMoa1603693
2. Zühlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: 2-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456-1466. doi: 10.1161/CIRCULATIONAHA
3. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297-309. doi: 10.1038/nrcardio.2012.7
4. Sriharibabu M, Himabindu Y, Kabir, et al. Rheumatic heart disease in rural south India: a clinico-observational study. J Cardiovasc Dis Res. 2013;4:25-29. doi: 10.1016/j.jcdr.2013.02.011
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:4:e25-e197. doi: 10.1016/j.jacc.2020.11.018
6. Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE System: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501-511. doi: 10.1016/j.jcin.2020.11.045
Does platelet-rich plasma improve patellar tendinopathy symptoms?
Evidence summary
Symptoms improve with PRP—but not significantly
A 2014 double-blind RCT (n = 23) explored recovery outcomes in patients with patellar tendinopathy who received either 1 injection of leukocyte-rich PRP or ultrasound-guided dry needling.1 Both groups also completed standardized eccentric exercises. Participants were predominantly men, ages ≥ 18 years. Symptomatic improvement was assessed using the Victorian Institute of Sport Assessment–Patella (VISA-P), an 8-item subjective questionnaire of functionality with a range of 0 to 100, with 100 as the maximum score for an asymptomatic individual.
At 12 weeks posttreatment, VISA-P scores improved in both groups. However, the improvement in the dry needling group was not statistically significant (5.2 points; 95% CI, –2.2 to 12.6; P = .20), while in the PRP group it was statistically significant (25.4 points; 95% CI, 10.3 to 40.6; P = .01). At ≥ 26 weeks, statistically significant improvement was observed in both treatment groups: scores improved by 33.2 points (95% CI, 24.1 to 42.4; P = .001) in the dry needling group and by 28.9 points (95% CI, 11.4 to 46.3; P = .01) in the PRP group. However, the difference between the groups’ VISA-P scores at ≥ 26 weeks was not significant (P = .66).1
No significant differences observed for PRP vs placebo or physical therapy
A 2019 single-blind RCT (n = 57) involved patients who were treated with 1 injection of either leukocyte-rich PRP, leukocyte-poor PRP, or saline, all in combination with 6 weeks of physical therapy.2 Participants were predominantly men, ages 18 to 50 years, and engaged in recreational sporting activities. There was no statistically significant difference in mean change in VISA-P score at any timepoint of the 2-year study period. P values were not reported.2
A 2010 RCT (n = 31) compared PRP (unspecified whether leukocyte-rich or -poor) in combination with physical therapy to physical therapy alone.3 Groups were matched for sex, age, and sports activity level; patients in the PRP group were required to have failed previous treatment, while control subjects must not have received any treatment for at least 2 months. Subjects were evaluated pretreatment, immediately posttreatment, and 6 months posttreatment. Clinical evaluation was aided by use of the Tegner activity score, a 1-item score that grades activity level on a scale of 0 to 10; the EuroQol-visual analog scale (EQ-VAS), which evaluates subjective rating of overall health; and pain level scores.
At 6 months posttreatment, no statistically significant differences were observed between groups in EQ-VAS and pain level scores. However, Tegner activity scores among PRP recipients showed significant percent improvement over controls at 6 months posttreatment (39% vs 20%; P = .048).3
Recommendations from others
Currently, national orthopedic and professional athletic medical associations have recommended that further research be conducted in order to make a strong statement in favor of or against PRP.4,5
Editor’s takeaway
Existing data regarding PRP fails, again, to show consistent benefits. These small sample sizes, inconsistent comparators, and heterogeneous results limit our certainty. This lack of quality evidence does not prove a lack of effect, but it raises serious doubts.
1. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416
2. Scott A, LaPrade R, Harmon K, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
3. Filardo G, Kon E, Villa S Della, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909. doi: 10.1007/s00264-009-0845-7
4. LaPrade R, Dragoo J, Koh J, et al. AAOS Research Symposium updates and consensus: biologic treatment of orthopaedic injuries. J Am Acad Orthop Surg. 2016;24:e62-e78. doi: 10.5435/JAAOS-D-16-00086
5. Rodeo SA, Bedi A. 2019-2020 NFL and NFL Physician Society orthobiologics consensus statement. Sports Health. 2020;12:58-60. doi: 10.1177/1941738119889013
Evidence summary
Symptoms improve with PRP—but not significantly
A 2014 double-blind RCT (n = 23) explored recovery outcomes in patients with patellar tendinopathy who received either 1 injection of leukocyte-rich PRP or ultrasound-guided dry needling.1 Both groups also completed standardized eccentric exercises. Participants were predominantly men, ages ≥ 18 years. Symptomatic improvement was assessed using the Victorian Institute of Sport Assessment–Patella (VISA-P), an 8-item subjective questionnaire of functionality with a range of 0 to 100, with 100 as the maximum score for an asymptomatic individual.
At 12 weeks posttreatment, VISA-P scores improved in both groups. However, the improvement in the dry needling group was not statistically significant (5.2 points; 95% CI, –2.2 to 12.6; P = .20), while in the PRP group it was statistically significant (25.4 points; 95% CI, 10.3 to 40.6; P = .01). At ≥ 26 weeks, statistically significant improvement was observed in both treatment groups: scores improved by 33.2 points (95% CI, 24.1 to 42.4; P = .001) in the dry needling group and by 28.9 points (95% CI, 11.4 to 46.3; P = .01) in the PRP group. However, the difference between the groups’ VISA-P scores at ≥ 26 weeks was not significant (P = .66).1
No significant differences observed for PRP vs placebo or physical therapy
A 2019 single-blind RCT (n = 57) involved patients who were treated with 1 injection of either leukocyte-rich PRP, leukocyte-poor PRP, or saline, all in combination with 6 weeks of physical therapy.2 Participants were predominantly men, ages 18 to 50 years, and engaged in recreational sporting activities. There was no statistically significant difference in mean change in VISA-P score at any timepoint of the 2-year study period. P values were not reported.2
A 2010 RCT (n = 31) compared PRP (unspecified whether leukocyte-rich or -poor) in combination with physical therapy to physical therapy alone.3 Groups were matched for sex, age, and sports activity level; patients in the PRP group were required to have failed previous treatment, while control subjects must not have received any treatment for at least 2 months. Subjects were evaluated pretreatment, immediately posttreatment, and 6 months posttreatment. Clinical evaluation was aided by use of the Tegner activity score, a 1-item score that grades activity level on a scale of 0 to 10; the EuroQol-visual analog scale (EQ-VAS), which evaluates subjective rating of overall health; and pain level scores.
At 6 months posttreatment, no statistically significant differences were observed between groups in EQ-VAS and pain level scores. However, Tegner activity scores among PRP recipients showed significant percent improvement over controls at 6 months posttreatment (39% vs 20%; P = .048).3
Recommendations from others
Currently, national orthopedic and professional athletic medical associations have recommended that further research be conducted in order to make a strong statement in favor of or against PRP.4,5
Editor’s takeaway
Existing data regarding PRP fails, again, to show consistent benefits. These small sample sizes, inconsistent comparators, and heterogeneous results limit our certainty. This lack of quality evidence does not prove a lack of effect, but it raises serious doubts.
Evidence summary
Symptoms improve with PRP—but not significantly
A 2014 double-blind RCT (n = 23) explored recovery outcomes in patients with patellar tendinopathy who received either 1 injection of leukocyte-rich PRP or ultrasound-guided dry needling.1 Both groups also completed standardized eccentric exercises. Participants were predominantly men, ages ≥ 18 years. Symptomatic improvement was assessed using the Victorian Institute of Sport Assessment–Patella (VISA-P), an 8-item subjective questionnaire of functionality with a range of 0 to 100, with 100 as the maximum score for an asymptomatic individual.
At 12 weeks posttreatment, VISA-P scores improved in both groups. However, the improvement in the dry needling group was not statistically significant (5.2 points; 95% CI, –2.2 to 12.6; P = .20), while in the PRP group it was statistically significant (25.4 points; 95% CI, 10.3 to 40.6; P = .01). At ≥ 26 weeks, statistically significant improvement was observed in both treatment groups: scores improved by 33.2 points (95% CI, 24.1 to 42.4; P = .001) in the dry needling group and by 28.9 points (95% CI, 11.4 to 46.3; P = .01) in the PRP group. However, the difference between the groups’ VISA-P scores at ≥ 26 weeks was not significant (P = .66).1
No significant differences observed for PRP vs placebo or physical therapy
A 2019 single-blind RCT (n = 57) involved patients who were treated with 1 injection of either leukocyte-rich PRP, leukocyte-poor PRP, or saline, all in combination with 6 weeks of physical therapy.2 Participants were predominantly men, ages 18 to 50 years, and engaged in recreational sporting activities. There was no statistically significant difference in mean change in VISA-P score at any timepoint of the 2-year study period. P values were not reported.2
A 2010 RCT (n = 31) compared PRP (unspecified whether leukocyte-rich or -poor) in combination with physical therapy to physical therapy alone.3 Groups were matched for sex, age, and sports activity level; patients in the PRP group were required to have failed previous treatment, while control subjects must not have received any treatment for at least 2 months. Subjects were evaluated pretreatment, immediately posttreatment, and 6 months posttreatment. Clinical evaluation was aided by use of the Tegner activity score, a 1-item score that grades activity level on a scale of 0 to 10; the EuroQol-visual analog scale (EQ-VAS), which evaluates subjective rating of overall health; and pain level scores.
At 6 months posttreatment, no statistically significant differences were observed between groups in EQ-VAS and pain level scores. However, Tegner activity scores among PRP recipients showed significant percent improvement over controls at 6 months posttreatment (39% vs 20%; P = .048).3
Recommendations from others
Currently, national orthopedic and professional athletic medical associations have recommended that further research be conducted in order to make a strong statement in favor of or against PRP.4,5
Editor’s takeaway
Existing data regarding PRP fails, again, to show consistent benefits. These small sample sizes, inconsistent comparators, and heterogeneous results limit our certainty. This lack of quality evidence does not prove a lack of effect, but it raises serious doubts.
1. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416
2. Scott A, LaPrade R, Harmon K, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
3. Filardo G, Kon E, Villa S Della, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909. doi: 10.1007/s00264-009-0845-7
4. LaPrade R, Dragoo J, Koh J, et al. AAOS Research Symposium updates and consensus: biologic treatment of orthopaedic injuries. J Am Acad Orthop Surg. 2016;24:e62-e78. doi: 10.5435/JAAOS-D-16-00086
5. Rodeo SA, Bedi A. 2019-2020 NFL and NFL Physician Society orthobiologics consensus statement. Sports Health. 2020;12:58-60. doi: 10.1177/1941738119889013
1. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416
2. Scott A, LaPrade R, Harmon K, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
3. Filardo G, Kon E, Villa S Della, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909. doi: 10.1007/s00264-009-0845-7
4. LaPrade R, Dragoo J, Koh J, et al. AAOS Research Symposium updates and consensus: biologic treatment of orthopaedic injuries. J Am Acad Orthop Surg. 2016;24:e62-e78. doi: 10.5435/JAAOS-D-16-00086
5. Rodeo SA, Bedi A. 2019-2020 NFL and NFL Physician Society orthobiologics consensus statement. Sports Health. 2020;12:58-60. doi: 10.1177/1941738119889013
EVIDENCE-BASED ANSWER:
IT’S UNCLEAR. High-quality data have not consistently established the effectiveness of platelet-rich plasma (PRP) injections to improve symptomatic recovery in patellar tendinopathy, compared to placebo (strength of recommendation [SOR]: A, based on 3 small randomized controlled trials [RCTs]). The 3 small RCTs included only 111 patients, total. One found no evidence of significant improvement with PRP compared to controls. The other 2 studies showed mixed results, with different outcome measures favoring different treatment groups and heterogeneous results depending on follow-up duration.
Skull Base Regeneration During Treatment With Chemoradiation for Nasopharyngeal Carcinoma: A Case Report
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.