User login
Home BP monitoring is essential
I believe that the most important recommendation from the American Heart Association in recent years is to confirm office blood pressure (BP) readings with repeated home BP measurements, for both diagnosis and management of hypertension. Office BPs are notoriously inaccurate, because it is exceedingly difficult to measure BP properly in a busy office setting. Even when measured correctly, the office BP does not accurately reflect a person’s BP throughout the day, which is the best predictor of cardiovascular damage from hypertension.
Among the problems with relying on office BP readings:We would treat many people for hypertension who are not hypertensive, because 15% to 30% of those with elevated office BP readings have “white-coat” hypertension, which does not require medication.1 White-coat hypertension can only be diagnosed with home BP readings or 24-hour ambulatory BP monitoring.
We would miss the diagnosis of hypertension in patients with “masked” hypertension—that is, people who have normal BP in the office but elevated ambulatory BP. It is estimated that 12% of US adults have masked hypertension.2
We would overtreat some patients who have hypertension and undertreat others, since office BP measurements can underestimate BP by an average of 24/14 mm Hg and overestimate BP by an average of 33/23 mm Hg.3
In this issue of JFP, Spaulding and colleagues4 provide an extensive summary of the research that supports the recommendation for home BP measurements. Here are 3 key takeaways:
- Use an automated BP monitor to measure BP in the office. Automated BP monitors that take repeated BPs over the course of about 5 minutes and average the results provide a much better estimate of 24-hour BP. It is worth the extra time and may be the only basis for making decisions about medications if a patient is unwilling or unable to take home BP readings.
- Provide training to patients who are willing to monitor their BP at home. Explain how to take their BP properly and instruct them to record at least 12 readings over the course of 3 days prior to office visits.
- Recommend patients use a validated BP monitor that uses the brachial artery for measurement, not the wrist (visit www.stridebp.org/bp-monitors and choose “Home”).
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
3. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
4. Spaulding J, Kasper RE, Viera AJ. Hypertension—or not? Looking beyond office BP readings. J Fam Pract. 2022;71:151-158. doi: 10.12788/jfp.0399
I believe that the most important recommendation from the American Heart Association in recent years is to confirm office blood pressure (BP) readings with repeated home BP measurements, for both diagnosis and management of hypertension. Office BPs are notoriously inaccurate, because it is exceedingly difficult to measure BP properly in a busy office setting. Even when measured correctly, the office BP does not accurately reflect a person’s BP throughout the day, which is the best predictor of cardiovascular damage from hypertension.
Among the problems with relying on office BP readings:We would treat many people for hypertension who are not hypertensive, because 15% to 30% of those with elevated office BP readings have “white-coat” hypertension, which does not require medication.1 White-coat hypertension can only be diagnosed with home BP readings or 24-hour ambulatory BP monitoring.
We would miss the diagnosis of hypertension in patients with “masked” hypertension—that is, people who have normal BP in the office but elevated ambulatory BP. It is estimated that 12% of US adults have masked hypertension.2
We would overtreat some patients who have hypertension and undertreat others, since office BP measurements can underestimate BP by an average of 24/14 mm Hg and overestimate BP by an average of 33/23 mm Hg.3
In this issue of JFP, Spaulding and colleagues4 provide an extensive summary of the research that supports the recommendation for home BP measurements. Here are 3 key takeaways:
- Use an automated BP monitor to measure BP in the office. Automated BP monitors that take repeated BPs over the course of about 5 minutes and average the results provide a much better estimate of 24-hour BP. It is worth the extra time and may be the only basis for making decisions about medications if a patient is unwilling or unable to take home BP readings.
- Provide training to patients who are willing to monitor their BP at home. Explain how to take their BP properly and instruct them to record at least 12 readings over the course of 3 days prior to office visits.
- Recommend patients use a validated BP monitor that uses the brachial artery for measurement, not the wrist (visit www.stridebp.org/bp-monitors and choose “Home”).
I believe that the most important recommendation from the American Heart Association in recent years is to confirm office blood pressure (BP) readings with repeated home BP measurements, for both diagnosis and management of hypertension. Office BPs are notoriously inaccurate, because it is exceedingly difficult to measure BP properly in a busy office setting. Even when measured correctly, the office BP does not accurately reflect a person’s BP throughout the day, which is the best predictor of cardiovascular damage from hypertension.
Among the problems with relying on office BP readings:We would treat many people for hypertension who are not hypertensive, because 15% to 30% of those with elevated office BP readings have “white-coat” hypertension, which does not require medication.1 White-coat hypertension can only be diagnosed with home BP readings or 24-hour ambulatory BP monitoring.
We would miss the diagnosis of hypertension in patients with “masked” hypertension—that is, people who have normal BP in the office but elevated ambulatory BP. It is estimated that 12% of US adults have masked hypertension.2
We would overtreat some patients who have hypertension and undertreat others, since office BP measurements can underestimate BP by an average of 24/14 mm Hg and overestimate BP by an average of 33/23 mm Hg.3
In this issue of JFP, Spaulding and colleagues4 provide an extensive summary of the research that supports the recommendation for home BP measurements. Here are 3 key takeaways:
- Use an automated BP monitor to measure BP in the office. Automated BP monitors that take repeated BPs over the course of about 5 minutes and average the results provide a much better estimate of 24-hour BP. It is worth the extra time and may be the only basis for making decisions about medications if a patient is unwilling or unable to take home BP readings.
- Provide training to patients who are willing to monitor their BP at home. Explain how to take their BP properly and instruct them to record at least 12 readings over the course of 3 days prior to office visits.
- Recommend patients use a validated BP monitor that uses the brachial artery for measurement, not the wrist (visit www.stridebp.org/bp-monitors and choose “Home”).
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
3. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
4. Spaulding J, Kasper RE, Viera AJ. Hypertension—or not? Looking beyond office BP readings. J Fam Pract. 2022;71:151-158. doi: 10.12788/jfp.0399
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
3. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
4. Spaulding J, Kasper RE, Viera AJ. Hypertension—or not? Looking beyond office BP readings. J Fam Pract. 2022;71:151-158. doi: 10.12788/jfp.0399
USPSTF recommendation roundup
In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
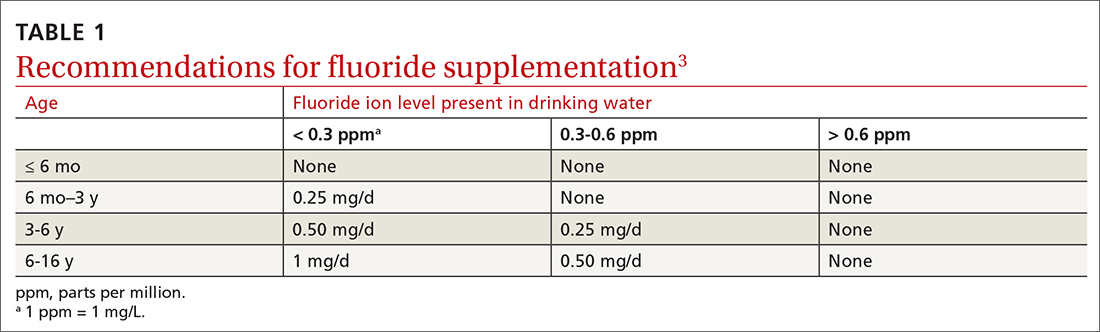
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2
In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
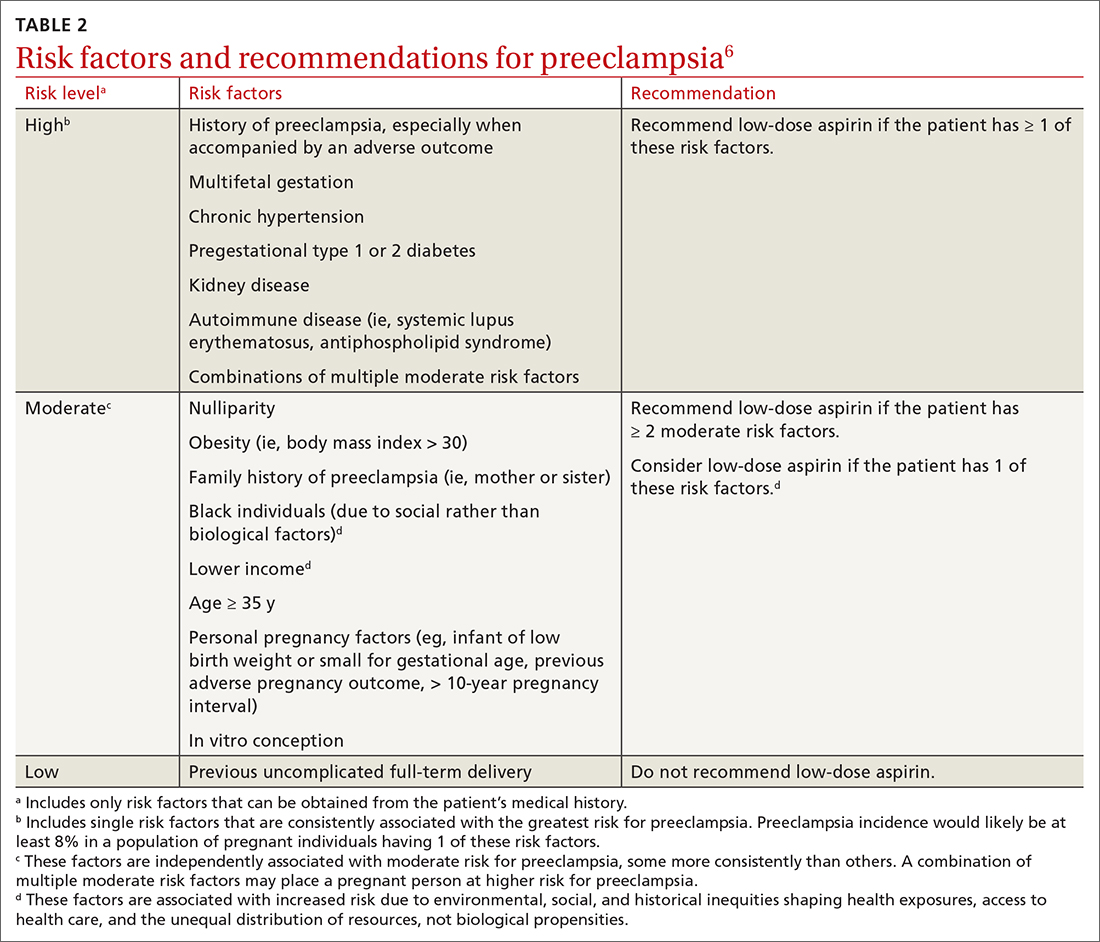
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.
Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
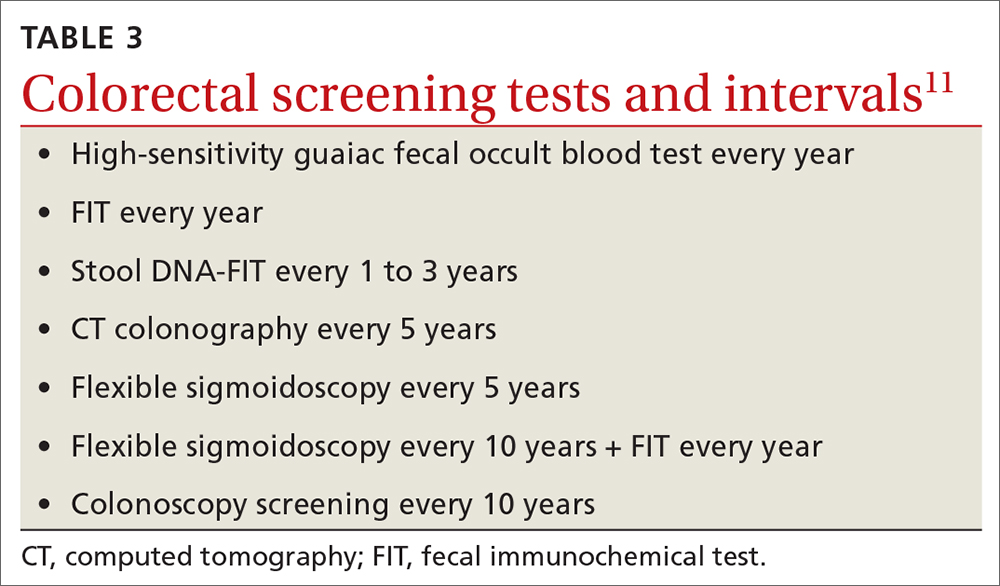
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11
For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
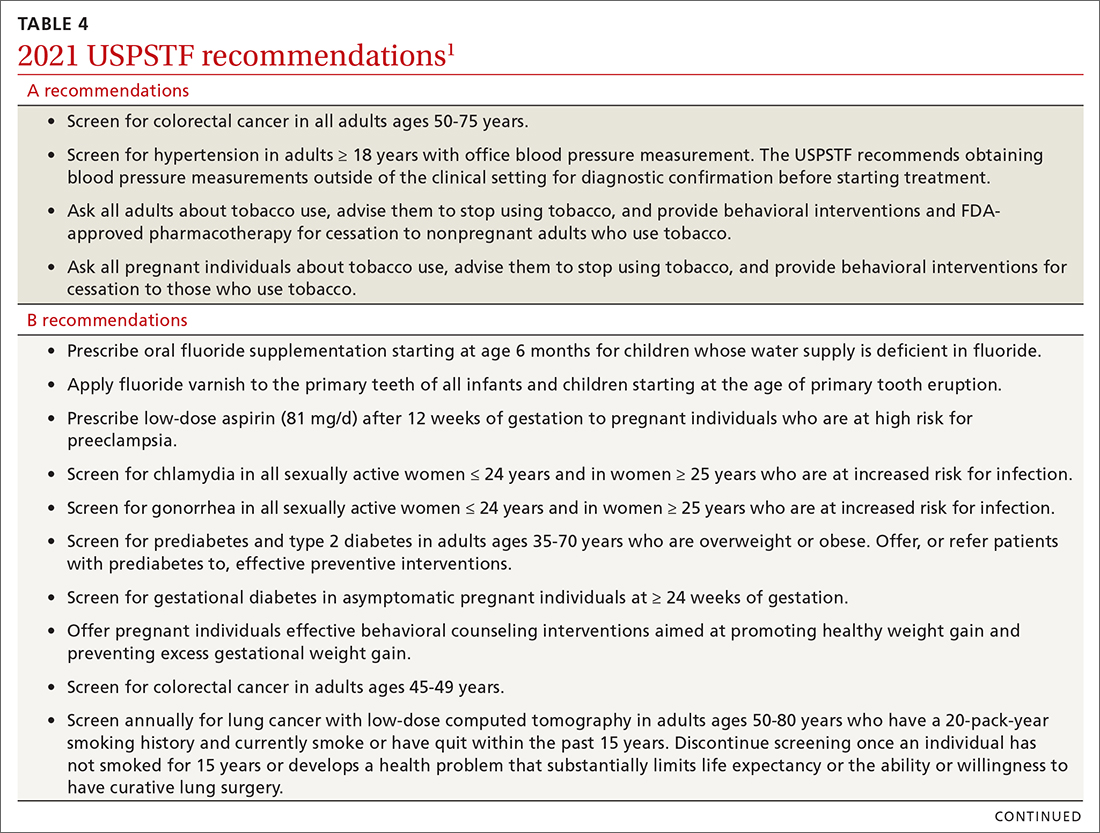
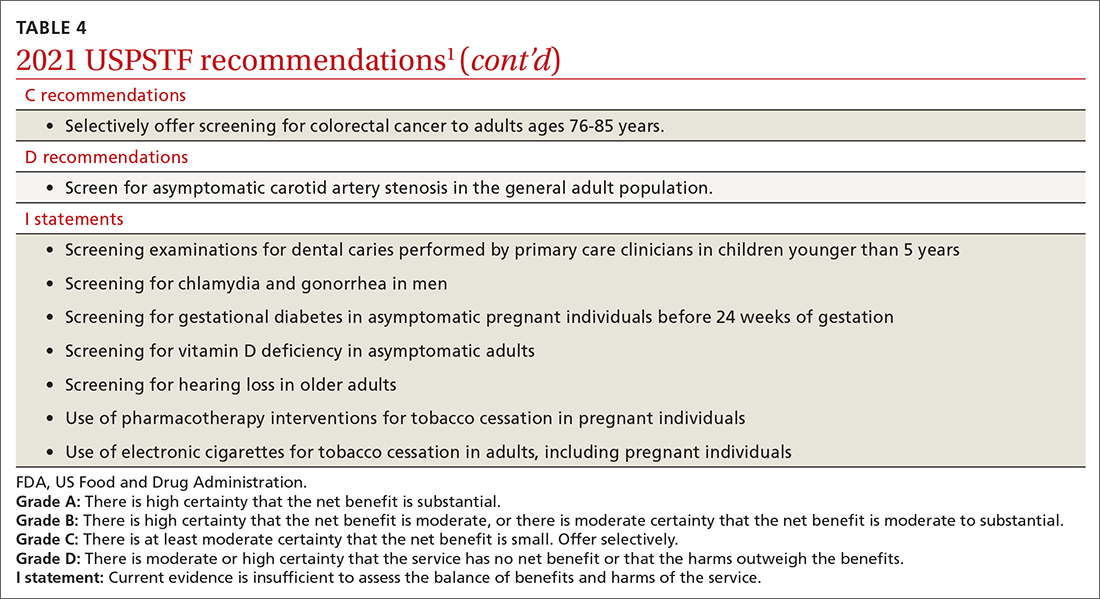
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1
1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2

In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.

Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11

For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1


In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2

In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.

Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11

For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1


1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
Hypertension—or not? Looking beyond office BP readings
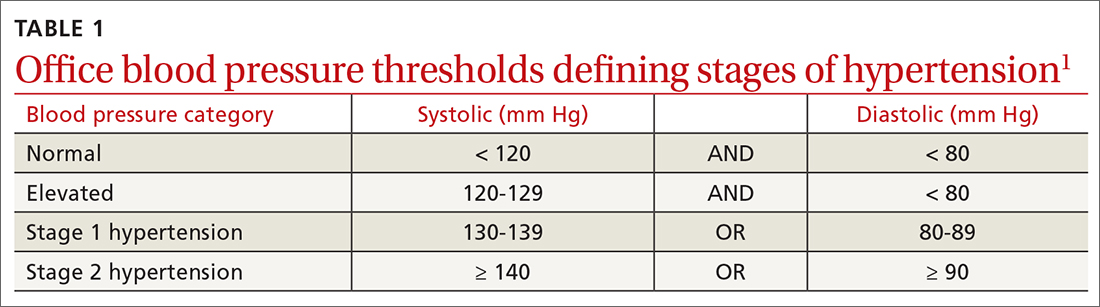
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1
Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
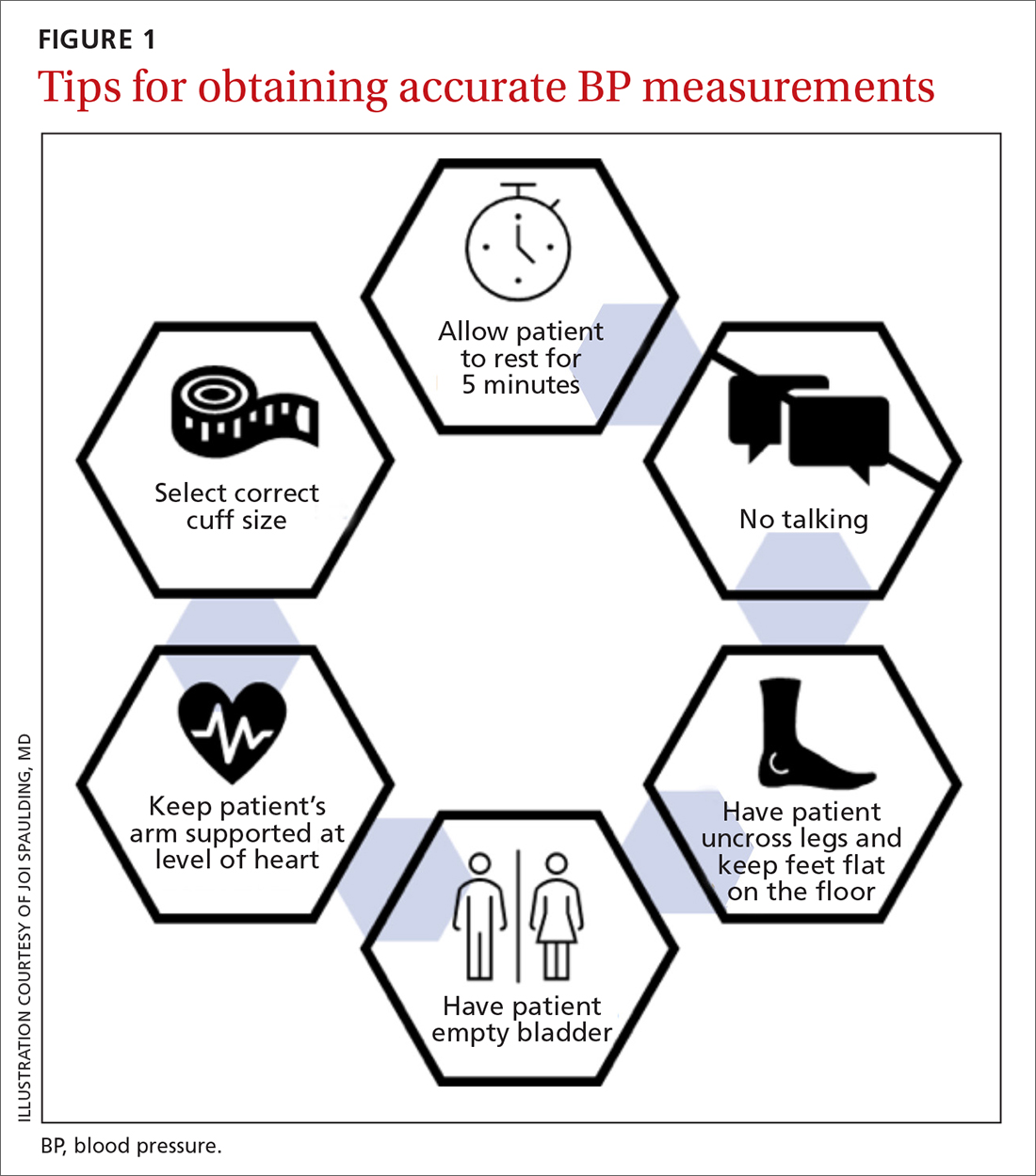
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.
Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
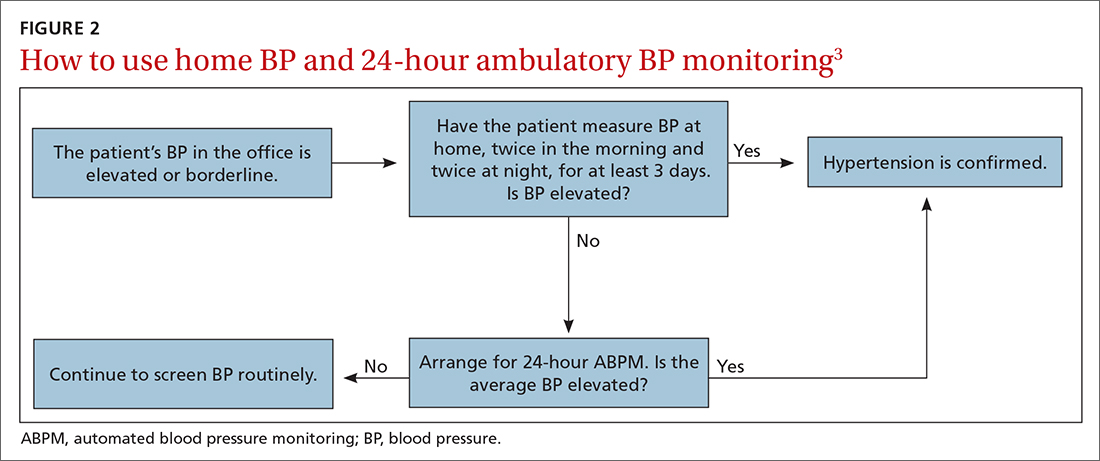
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).
Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
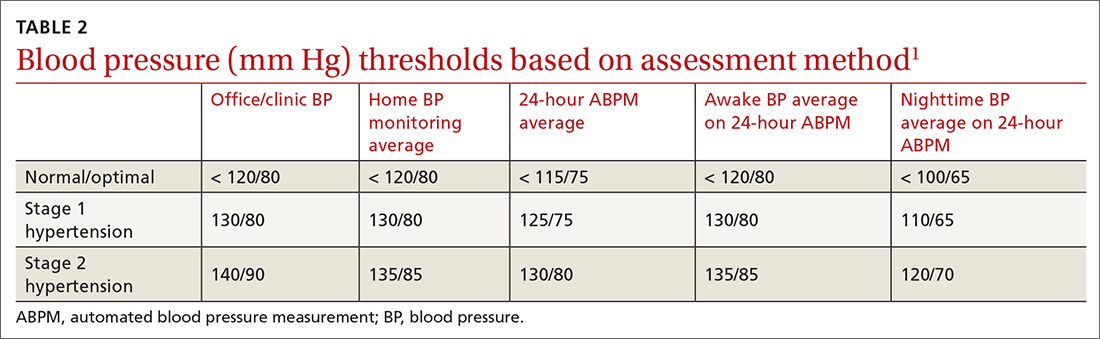
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.
Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
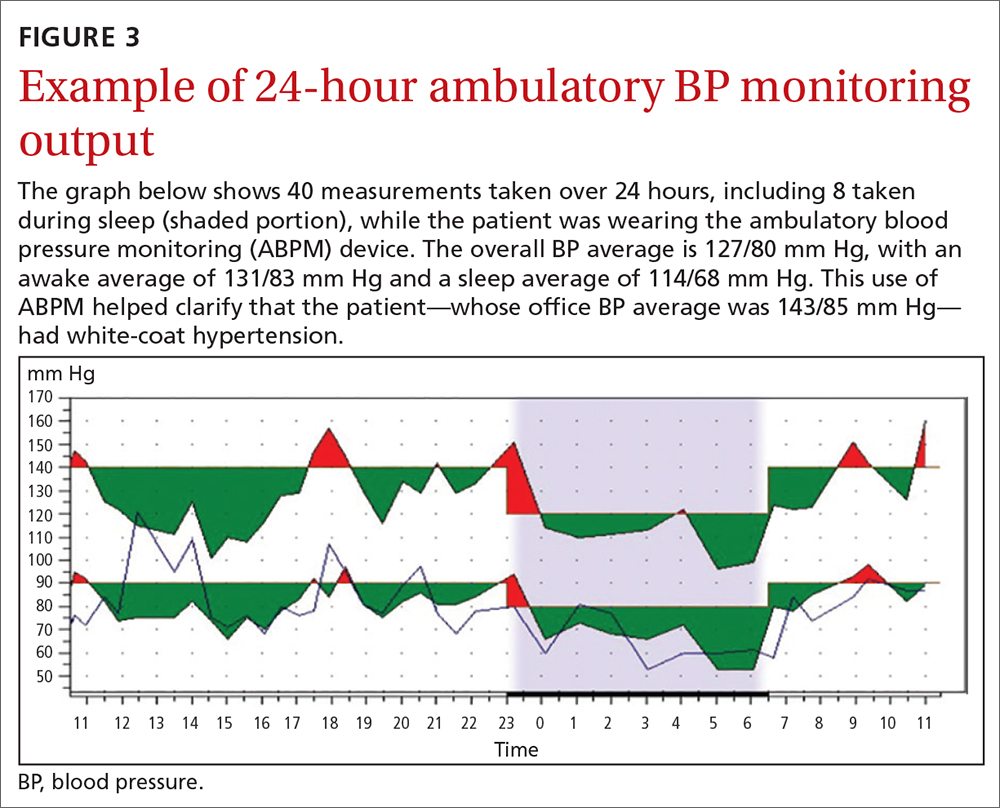
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.
Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.
Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; [email protected]
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1

Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.

Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).

Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.

Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.

Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.

Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; [email protected]
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1

Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.

Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).

Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.

Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.

Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.

Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; [email protected]
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
PRACTICE RECOMMENDATIONS
› Use home blood pressure measurement (HBPM) for initial out-of-office evaluation to confirm hypertension. A
› Use 24-hour ambulatory measurement only when the results between office and HBPM are discordant. A
› Instruct patients to record their home BP measurements twice in the morning and twice at night for a minimum of 3 days. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Retiform Purpura on the Legs
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2
Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
A 70-year-old woman with a medical history of Takayasu arteritis, end-stage renal disease on peritoneal dialysis, coronary artery disease, hypertension, hypothyroidism, and anemia of chronic disease presented to the emergency department with enlarging painful stellate eschars of the legs with associated edema of 3 weeks’ duration. She denied a history of similar-appearing skin lesions. She initially thought the lesions were burns secondary to frequent hot showers for relief of uremic pruritus. For the treatment of these suspected burns prior to hospitalization, she had been applying over-the-counter antibiotic ointments to the affected areas and had completed a 2-week course of oral cephalexin without notable improvement. Physical examination revealed retiform purpura of the legs with large stellate eschars overlying the anteromedial thighs and right medial calf. Computed tomography angiogram of the abdomen and pelvis demonstrated diffuse calcifications of the aortic wall and its associated branches that were most pronounced in the legs without evidence of vessel wall thickening. Punch biopsies were performed, and nephrology, rheumatology, and wound care services were consulted.
Melanoma
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
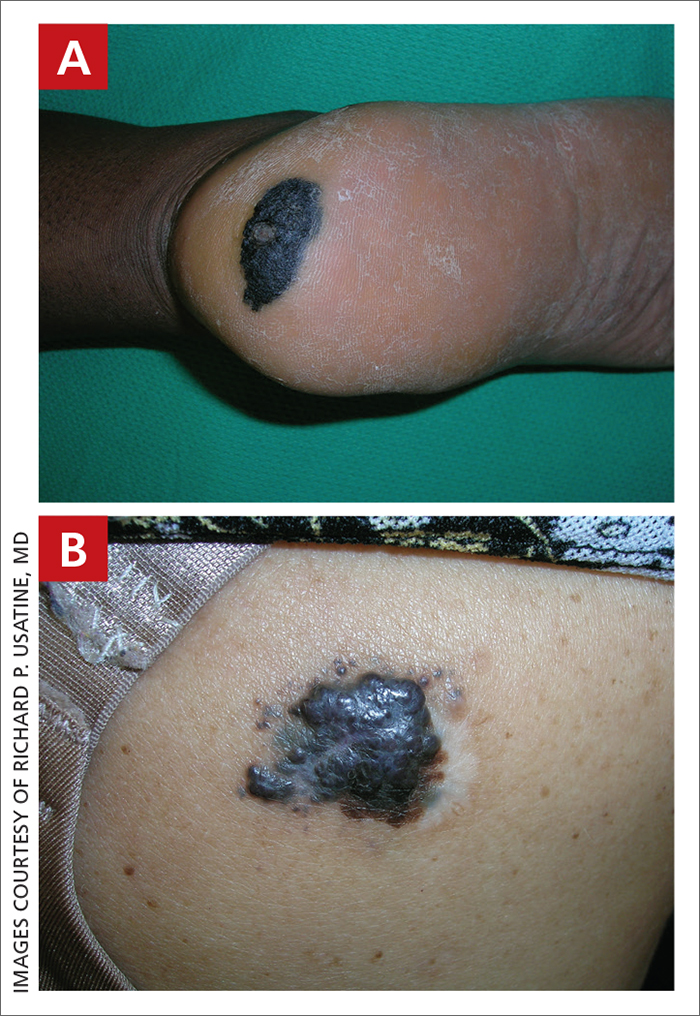
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.

Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.

Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
Mental Health Support of Frontline Medical Personnel in the Javits New York Medical Station Federal COVID-19 Treatment Center
New York City (NYC) was the early epicenter of the COVID-19 pandemic in the United States. By late March 2020, NYC hospitals were overwhelmed, leading to the development of a 452-bed field hospital that became the Javits New York Medical Station (JNYMS).1,2 More than 600 uniformed and other federal personnel, including medical personnel from US Army, Navy, and Public Health Service Commissioned Corps, mobilized to provide medical support to the JNYMS in late March 2020, leading to the treatment of more than 1000 patients with COVID-19 within a 30-day period.1
Literature from the SARS, Ebola, and HIV epidemics indicate that adverse mental health consequences, including burnout, depression, and posttraumatic stress disorder symptoms are common in frontline medical workers.3,4 Emerging data shows a similar trend occurring during the COVID-19 pandemic.5 A recent publication detailed the role of a federal force health protection program created to enhance resiliency of deployed officers during the COVID-19 pandemic, but this focused primarily on providing remote services to frontline workers.6 Another report addressed mental health interventions for health care workers in an academic health care system in NYC during COVID-19.7 However, there has been little published on real-time mental health support for deployed personnel during the pandemic.
Prior publications have described the patient flow, infection control measures, and development of a Consultation-Liaison Psychiatry Service in the JNYMS.2,8,9 Here, we detail the establishment of preventative and responsive mental health services for frontline workers at the JNYMS and explore lessons learned through the outpatient and general support experiences.
Development of Outpatient Mental Health Support Services
At the end of March 2020, the Jacob K. Javits Convention Center was repurposed into the 452-bed JNYMS field hospital, where exposition rooms were transformed into a medical unit and intensive care unit.2 While the majority of personnel providing direct clinical care were specialists, the station also was staffed with uniformed and other federal mental health clinicians, including 5 licensed clinical social workers (LCSWs), 3 psychiatrists, 1 dual-trained internal medicine–psychiatry physician, 1 psychiatric nurse, and 2 behavioral health technicians. To standardize processes early in the deployment, standard operating procedures for behavioral health support of personnel were developed and disseminated within the first few days of the deployment.
The initial mission of the behavioral health team was to establish comprehensive mental health services, as the rapidly shifting mission and unfamiliar environment increased the risk of new-onset stress responses and exacerbating pre-existing stressors in personnel. Behavioral health leadership established operations in conference rooms within the convention center, focusing on identifying, prioritizing, and staffing high-traffic areas. A resiliency center was also established adjacent to the changing room, where all staff would enter and leave the units, and to the dining facility, further increasing traffic. This center was staffed 24 hours a day by at least 1 LCSW and a behavioral health technician with 2 shifts: one from 0630 to 1830 and another from 1830 to 0630. Psychiatrists were available during the day for psychiatry intervention and evaluations, and an on-call schedule was developed for off-hours to provide time-sensitive responses.
The resiliency center was developed to provide a welcoming atmosphere to meet basic needs, including nourishment, healthy social interaction, and a calm environment. Water and food were made available free to personnel, bolstering morale for staff working 12-hour shifts in a pandemic treatment floor where personal protective equipment prevented intake of food or water. Mental health staff were also available to counsel and provide social support to personnel. If personnel wished to discuss stressors or appeared to be in distress, a mental health clinician would provide a real-time intervention or schedule an appointment with the behavioral health team. Resources were made available, including brochures and other reading materials on resilience, stress management, and other mental health topics. Uniformed services and state and federal JNYMS leadership were encouraged to visit the resiliency center to normalize interactions and encourage participation in a behavioral health environment. Signage was placed throughout JNYMS to direct personnel to behavioral health services.
The behavioral health interventions and influence spread from the resiliency center nexus. Initially, therapeutic interventions occurred where and when necessary. One psychiatrist provided crisis intervention to a bereaved soldier in the stairwell within 2 hours of arrival to the JNYMS. Leadership and the behavioral health team recognized that the need for privacy was essential for timely therapeutic interventions, leading to the development of a private individual counseling room. As the area became generally accepted as the central hub of behavioral health activity, space was provided to establish a quiet space and a meditation room. The quiet area provided a cool dark space for personnel to sit quietly in solitude; many were grateful for this reprieve after an overstimulating medical shift. The meditation room supplied sterilized yoga mats for personal mindfulness interventions. The behavioral health team also liaised with military chaplains, who established a spiritual service room near the resiliency center. The chaplains held regular religious services and were available 24 hours a day for timely spiritual interventions.
Rapid notification and movement of uniformed personnel to JNYMS resulted in limited ability for personnel to schedule medical appointments and refill medications. Psychiatrists also had limited access to relevant electronic health record systems. This led to a delay in nonurgent care to evaluate personnel records and confirm prescriptions, especially controlled medications. Local pharmacies filled prescriptions, psychiatrists placed electronic health profiles, and command teams were notified in accordance with US Army and federal regulations.
Medical Unit Support Services
Although a robust outpatient behavioral health service was laid out in the JNYMS, the behavioral health team recognized the need to provide mental health interventions within the main patient care areas as well. The intention was to maximize availability and support while minimizing interference to patient care. As previously described, a psychiatric consultation-liaison (CL) team was organized and operated 24 hours a day by early April 2020.9 Indeed, CL psychiatrists have played a valuable role in supporting the unique patient and staff needs in other COVID-19 treatment environments.10 The CL team at JNYMS observed that medical staff were exposed to multiple stressors, including fear of acquiring COVID-19, treating patients with significant medical comorbidities, practicing outside of clinical specialty, working with unfamiliar and limited equipment, and adjusting to frequently shifting changes in personnel and work schedules. Moreover, psychological stress was compounded by long shifts, jetlag, and continuous wear of extensive personal protective equipment, as has been documented in other COVID-19 treatment centers.11
The team of psychiatrists conducted informal rounds to nursing stations to evaluate the morale and develop relationships with the medical team, including nurses, physicians, medics, and other personnel. Areas of high stress and increased interpersonal conflict were identified for more frequent check-ins by mental health clinicians. The psychiatrists and LCSWs were available for informal walk-in therapy when requested by personnel. When the acuity increased, personnel could be accompanied to the individual counseling room for rapid therapeutic interventions. The CL psychiatrists developed professional relationships with the command and medical leadership teams. Through these relationships and sensitive awareness of morale in the medical work environment, psychiatrists were able to advocate for alterations in the nursing work schedule. Leadership was receptive and resultant changes decreased the hours per shift and number of shifts for most nurses. Morale quickly improved, likely resulting in improved quality of patient care and prevention of burnout.
Mental Health Care Beyond JNYMS
Uniformed services and other federal personnel further supplemented health care operations beyond JYNMS. In April 2020, Urban Augmentation Medical Task Forces were organized and distributed throughout regions where COVID-19–related hospitalizations had significantly overwhelmed the local health care force. Urban Augmentation Medical Task Forces often included a psychiatrist, psychologist, and behavioral health technician with the mission to provide mental health support and interventions to patients and medical staff. Combat Operational Stress Control units from US Army medical brigades operated in NYC and the greater northeast region, providing mental health support and resiliency training to military personnel working in civilian hospitals, medical centers, and other health care or support environments. In addition, a LCSW and behavioral health technician worked with New York Army Reserve personnel assigned to mortuary affairs, providing point-of-care interventions at or near the worksite.
A collaborative federal, uniformed services, and state operation led to the development of the HERO-NY: Healing, Education, Resilience, and Opportunity for New York’s Frontline Workforce “Train the Trainer” Series.12 The series was intended to use uniformed services expertise to address mental health challenges related to the COVID-19 epidemic. Psychiatrists and mental health clinicians from JNYMS modeled small group trainings for future medical trainers. In lieu of traditional unidirectional lecturing, which yields limited retention and learning, the panelists demonstrated how to lead interactive small group training with resiliency topics, including goal setting, communication, anger management, and sleep hygiene.
Transition
After the last patient was discharged from JNYMS in May 2020, personnel were quickly redeployed to their duty stations. At the time of mission completion, the JNYMS behavioral health team had been supplemented with psychiatrists, social workers, behavioral health technicians, psychiatric nurse practitioners, psychiatric nurses, and psychologists representing US Public Health Service Commissioned Corps, Army, Air Force, and Navy, and provided comprehensive support to the nearly 1100 patients with COVID-19 and 600 deployed federal and state medical and support personnel.
Lessons Learned and Future Considerations
Behavioral health care provided at JNYMS offers insight into support of frontline workers in pandemic settings, as literature is limited in this area.13 TheJNYMS behavioral health team used strategies similar to military medical interventions in limited and unpredictable environments, such as rapid formalization of team structure and establishment of standard operating procedures to facilitate uniformity across interventions. Physical space was necessary to create an environment conducive to productive mental health interventions, including therapy rooms and quiet and spiritual spaces. Placing behavioral resources in high-traffic areas normalized mental health and maximized accessibility to interventions. Mental health personnel also addressed issues in the work environment, such as providing informal support and crisis interventions to frontline workers. Finally, Urban Augmentation Medical Task Forces mental health personnel and Combat Operational Stress Control units provided therapeutic interventions and resiliency training for military and civilian personnel throughout burdened medical systems beyond JNYMS.
Future operations should consider what equipment and logistic access are necessary to provide psychiatric and psychological care to mobilized federal and uniformed personnel, such as access to frontline worker electronic health records. Given that prior work has found that provision of resources alone is inadequate, frontline medical workers must be aware of where resources are available (eg, signage) and have easy access to material (eg, brochures) focusing on resiliency and psychological health.14 The spaces can be used for formal psychiatric and psychological interventions, such as assessment, therapy, and medication management. Equally important, these spaces serve as a safe place for healthy social interaction and fulfillment of basic needs (eg, nourishment) and a peaceful environment free of stimulation.
Since mental health personnel provide varied services ranging from basic human interaction to complex crisis interventions, mental health personnel should supplement pandemic medical operations. Evidence supports the notion that effective communication and cohesion throughout the entire leadership and health care team structure can improve resilience and implementation of mental health interventions.15 Incorporating mental health personnel into leadership planning meetings would allow for timely recommendations to improve medical logistics and planning of deployment of behavioral health resources. As a general rule, providing behavioral health experts with a seat at the table enhances advocacy and command awareness of the morale and mental health of frontline personnel.
Conclusions
We present the experience of developing mental health support services for deployed personnel during the COVID-19 pandemic and address the real-time mental health treatment and support of deployed uniformed services and federal personnel in the COVID-19 response environment. Timely and effective interventions included securing safe therapeutic space in high-traffic areas, developing relationships with leadership and frontline workers in their own work environments, and disseminating such services throughout the civilian medical system.
Mental health supplementation during the medical response mission strengthened morale in frontline workers in a disaster scenario. We hope that this report and others like it will provide information to improve mental health responses, reinforce mental health support, and encourage research in evidence-based interventions in challenging pandemic and disaster settings.
Acknowledgments
We would like to acknowledge and thank those serving on the frontlines of the COVID-19 pandemic.
1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. Published 2020 Apr 17. doi:10.15585/mmwr.mm6915e4
2. Brady K, Milzman D, Walton E, Sommer D, Neustadtl A, Napoli A. Uniformed services and the field hospital experience during Coronovirus Disease 2019 (SARS-CoV-2) pandemic: open to closure in 30 days with 1,100 patients: the Javits New York Medical Station [published online ahead of print, 2021 Feb 13]. Mil Med. 2021;usab003. doi:10.1093/milmed/usab003
3. Tucci V, Moukaddam N, Meadows J, Shah S, Galwankar SC, Kapur GB. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151-156. doi:10.4103/jgid.jgid_66_17
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311. doi:10.1177/070674370905400504
5. Panchal N, Kamal R, Cox C, Garfield R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed April 7, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
6. Myles IA, Johnson DR, Pham H, et al. USPHS Corps Care: force health protection for public health officers during the Ebola and COVID-19 responses. Public Health Rep. 2021;136(2):148-153. doi:10.1177/0033354920984775
7. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. 2020;95(8):1136-1139. doi:10.1097/ACM.0000000000003414
8. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):308-311. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009a3
9. Kaplan A, Smith CM, Toukolehto O, van Schalkwyk G. Psychiatric care in a novel federal COVID-19 treatment center: development of a consultation-liaison psychiatry service at the Javits New York Medical Station. Mil Med. 2021;186(5-6):129-131. doi:10.1093/milmed/usaa557
10. Shalev D, Shapiro PA. Epidemic psychiatry: The opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68-71. doi:10.1016/j.genhosppsych.2020.03.009
11. Horn M, Granon B, Vaiva G, Fovet T, Amad A. Role and importance of consultation-liaison psychiatry during the Covid-19 epidemic [published online ahead of print, 2020 Aug 5]. J Psychosom Res. 2020;137:110214. doi:10.1016/j.jpsychores.2020.110214
12. Wei EK, Segall J, Linn-Walton R, et al. Combat stress management and resilience: adapting department of defense combat lessons learned to civilian healthcare during the COVID-19 pandemic [published online ahead of print, 2020 Jul 17]. Health Secur. 2020;10.1089/hs.2020.0091. doi:10.1089/hs.2020.0091
13. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11(11):CD013779. Published 2020 Nov 5. doi:10.1002/14651858.CD013779
14. Schreiber M, Cates DS, Formanski S, King M. Maximizing the Resilience of Healthcare Workers in Multi-hazard Events: Lessons from the 2014-2015 Ebola Response in Africa. Mil Med. 2019;184(suppl 1):114-120. doi:10.1093/milmed/usy400
15. Klomp RW, Jones L, Watanabe E, Thompson WW. CDC’s multiple approaches to safeguard the health, safety, and resilience of Ebola responders. Prehosp Disaster Med. 2020;35(1):69-75. doi:10.1017/S1049023X19005144
New York City (NYC) was the early epicenter of the COVID-19 pandemic in the United States. By late March 2020, NYC hospitals were overwhelmed, leading to the development of a 452-bed field hospital that became the Javits New York Medical Station (JNYMS).1,2 More than 600 uniformed and other federal personnel, including medical personnel from US Army, Navy, and Public Health Service Commissioned Corps, mobilized to provide medical support to the JNYMS in late March 2020, leading to the treatment of more than 1000 patients with COVID-19 within a 30-day period.1
Literature from the SARS, Ebola, and HIV epidemics indicate that adverse mental health consequences, including burnout, depression, and posttraumatic stress disorder symptoms are common in frontline medical workers.3,4 Emerging data shows a similar trend occurring during the COVID-19 pandemic.5 A recent publication detailed the role of a federal force health protection program created to enhance resiliency of deployed officers during the COVID-19 pandemic, but this focused primarily on providing remote services to frontline workers.6 Another report addressed mental health interventions for health care workers in an academic health care system in NYC during COVID-19.7 However, there has been little published on real-time mental health support for deployed personnel during the pandemic.
Prior publications have described the patient flow, infection control measures, and development of a Consultation-Liaison Psychiatry Service in the JNYMS.2,8,9 Here, we detail the establishment of preventative and responsive mental health services for frontline workers at the JNYMS and explore lessons learned through the outpatient and general support experiences.
Development of Outpatient Mental Health Support Services
At the end of March 2020, the Jacob K. Javits Convention Center was repurposed into the 452-bed JNYMS field hospital, where exposition rooms were transformed into a medical unit and intensive care unit.2 While the majority of personnel providing direct clinical care were specialists, the station also was staffed with uniformed and other federal mental health clinicians, including 5 licensed clinical social workers (LCSWs), 3 psychiatrists, 1 dual-trained internal medicine–psychiatry physician, 1 psychiatric nurse, and 2 behavioral health technicians. To standardize processes early in the deployment, standard operating procedures for behavioral health support of personnel were developed and disseminated within the first few days of the deployment.
The initial mission of the behavioral health team was to establish comprehensive mental health services, as the rapidly shifting mission and unfamiliar environment increased the risk of new-onset stress responses and exacerbating pre-existing stressors in personnel. Behavioral health leadership established operations in conference rooms within the convention center, focusing on identifying, prioritizing, and staffing high-traffic areas. A resiliency center was also established adjacent to the changing room, where all staff would enter and leave the units, and to the dining facility, further increasing traffic. This center was staffed 24 hours a day by at least 1 LCSW and a behavioral health technician with 2 shifts: one from 0630 to 1830 and another from 1830 to 0630. Psychiatrists were available during the day for psychiatry intervention and evaluations, and an on-call schedule was developed for off-hours to provide time-sensitive responses.
The resiliency center was developed to provide a welcoming atmosphere to meet basic needs, including nourishment, healthy social interaction, and a calm environment. Water and food were made available free to personnel, bolstering morale for staff working 12-hour shifts in a pandemic treatment floor where personal protective equipment prevented intake of food or water. Mental health staff were also available to counsel and provide social support to personnel. If personnel wished to discuss stressors or appeared to be in distress, a mental health clinician would provide a real-time intervention or schedule an appointment with the behavioral health team. Resources were made available, including brochures and other reading materials on resilience, stress management, and other mental health topics. Uniformed services and state and federal JNYMS leadership were encouraged to visit the resiliency center to normalize interactions and encourage participation in a behavioral health environment. Signage was placed throughout JNYMS to direct personnel to behavioral health services.
The behavioral health interventions and influence spread from the resiliency center nexus. Initially, therapeutic interventions occurred where and when necessary. One psychiatrist provided crisis intervention to a bereaved soldier in the stairwell within 2 hours of arrival to the JNYMS. Leadership and the behavioral health team recognized that the need for privacy was essential for timely therapeutic interventions, leading to the development of a private individual counseling room. As the area became generally accepted as the central hub of behavioral health activity, space was provided to establish a quiet space and a meditation room. The quiet area provided a cool dark space for personnel to sit quietly in solitude; many were grateful for this reprieve after an overstimulating medical shift. The meditation room supplied sterilized yoga mats for personal mindfulness interventions. The behavioral health team also liaised with military chaplains, who established a spiritual service room near the resiliency center. The chaplains held regular religious services and were available 24 hours a day for timely spiritual interventions.
Rapid notification and movement of uniformed personnel to JNYMS resulted in limited ability for personnel to schedule medical appointments and refill medications. Psychiatrists also had limited access to relevant electronic health record systems. This led to a delay in nonurgent care to evaluate personnel records and confirm prescriptions, especially controlled medications. Local pharmacies filled prescriptions, psychiatrists placed electronic health profiles, and command teams were notified in accordance with US Army and federal regulations.
Medical Unit Support Services
Although a robust outpatient behavioral health service was laid out in the JNYMS, the behavioral health team recognized the need to provide mental health interventions within the main patient care areas as well. The intention was to maximize availability and support while minimizing interference to patient care. As previously described, a psychiatric consultation-liaison (CL) team was organized and operated 24 hours a day by early April 2020.9 Indeed, CL psychiatrists have played a valuable role in supporting the unique patient and staff needs in other COVID-19 treatment environments.10 The CL team at JNYMS observed that medical staff were exposed to multiple stressors, including fear of acquiring COVID-19, treating patients with significant medical comorbidities, practicing outside of clinical specialty, working with unfamiliar and limited equipment, and adjusting to frequently shifting changes in personnel and work schedules. Moreover, psychological stress was compounded by long shifts, jetlag, and continuous wear of extensive personal protective equipment, as has been documented in other COVID-19 treatment centers.11
The team of psychiatrists conducted informal rounds to nursing stations to evaluate the morale and develop relationships with the medical team, including nurses, physicians, medics, and other personnel. Areas of high stress and increased interpersonal conflict were identified for more frequent check-ins by mental health clinicians. The psychiatrists and LCSWs were available for informal walk-in therapy when requested by personnel. When the acuity increased, personnel could be accompanied to the individual counseling room for rapid therapeutic interventions. The CL psychiatrists developed professional relationships with the command and medical leadership teams. Through these relationships and sensitive awareness of morale in the medical work environment, psychiatrists were able to advocate for alterations in the nursing work schedule. Leadership was receptive and resultant changes decreased the hours per shift and number of shifts for most nurses. Morale quickly improved, likely resulting in improved quality of patient care and prevention of burnout.
Mental Health Care Beyond JNYMS
Uniformed services and other federal personnel further supplemented health care operations beyond JYNMS. In April 2020, Urban Augmentation Medical Task Forces were organized and distributed throughout regions where COVID-19–related hospitalizations had significantly overwhelmed the local health care force. Urban Augmentation Medical Task Forces often included a psychiatrist, psychologist, and behavioral health technician with the mission to provide mental health support and interventions to patients and medical staff. Combat Operational Stress Control units from US Army medical brigades operated in NYC and the greater northeast region, providing mental health support and resiliency training to military personnel working in civilian hospitals, medical centers, and other health care or support environments. In addition, a LCSW and behavioral health technician worked with New York Army Reserve personnel assigned to mortuary affairs, providing point-of-care interventions at or near the worksite.
A collaborative federal, uniformed services, and state operation led to the development of the HERO-NY: Healing, Education, Resilience, and Opportunity for New York’s Frontline Workforce “Train the Trainer” Series.12 The series was intended to use uniformed services expertise to address mental health challenges related to the COVID-19 epidemic. Psychiatrists and mental health clinicians from JNYMS modeled small group trainings for future medical trainers. In lieu of traditional unidirectional lecturing, which yields limited retention and learning, the panelists demonstrated how to lead interactive small group training with resiliency topics, including goal setting, communication, anger management, and sleep hygiene.
Transition
After the last patient was discharged from JNYMS in May 2020, personnel were quickly redeployed to their duty stations. At the time of mission completion, the JNYMS behavioral health team had been supplemented with psychiatrists, social workers, behavioral health technicians, psychiatric nurse practitioners, psychiatric nurses, and psychologists representing US Public Health Service Commissioned Corps, Army, Air Force, and Navy, and provided comprehensive support to the nearly 1100 patients with COVID-19 and 600 deployed federal and state medical and support personnel.
Lessons Learned and Future Considerations
Behavioral health care provided at JNYMS offers insight into support of frontline workers in pandemic settings, as literature is limited in this area.13 TheJNYMS behavioral health team used strategies similar to military medical interventions in limited and unpredictable environments, such as rapid formalization of team structure and establishment of standard operating procedures to facilitate uniformity across interventions. Physical space was necessary to create an environment conducive to productive mental health interventions, including therapy rooms and quiet and spiritual spaces. Placing behavioral resources in high-traffic areas normalized mental health and maximized accessibility to interventions. Mental health personnel also addressed issues in the work environment, such as providing informal support and crisis interventions to frontline workers. Finally, Urban Augmentation Medical Task Forces mental health personnel and Combat Operational Stress Control units provided therapeutic interventions and resiliency training for military and civilian personnel throughout burdened medical systems beyond JNYMS.
Future operations should consider what equipment and logistic access are necessary to provide psychiatric and psychological care to mobilized federal and uniformed personnel, such as access to frontline worker electronic health records. Given that prior work has found that provision of resources alone is inadequate, frontline medical workers must be aware of where resources are available (eg, signage) and have easy access to material (eg, brochures) focusing on resiliency and psychological health.14 The spaces can be used for formal psychiatric and psychological interventions, such as assessment, therapy, and medication management. Equally important, these spaces serve as a safe place for healthy social interaction and fulfillment of basic needs (eg, nourishment) and a peaceful environment free of stimulation.
Since mental health personnel provide varied services ranging from basic human interaction to complex crisis interventions, mental health personnel should supplement pandemic medical operations. Evidence supports the notion that effective communication and cohesion throughout the entire leadership and health care team structure can improve resilience and implementation of mental health interventions.15 Incorporating mental health personnel into leadership planning meetings would allow for timely recommendations to improve medical logistics and planning of deployment of behavioral health resources. As a general rule, providing behavioral health experts with a seat at the table enhances advocacy and command awareness of the morale and mental health of frontline personnel.
Conclusions
We present the experience of developing mental health support services for deployed personnel during the COVID-19 pandemic and address the real-time mental health treatment and support of deployed uniformed services and federal personnel in the COVID-19 response environment. Timely and effective interventions included securing safe therapeutic space in high-traffic areas, developing relationships with leadership and frontline workers in their own work environments, and disseminating such services throughout the civilian medical system.
Mental health supplementation during the medical response mission strengthened morale in frontline workers in a disaster scenario. We hope that this report and others like it will provide information to improve mental health responses, reinforce mental health support, and encourage research in evidence-based interventions in challenging pandemic and disaster settings.
Acknowledgments
We would like to acknowledge and thank those serving on the frontlines of the COVID-19 pandemic.
New York City (NYC) was the early epicenter of the COVID-19 pandemic in the United States. By late March 2020, NYC hospitals were overwhelmed, leading to the development of a 452-bed field hospital that became the Javits New York Medical Station (JNYMS).1,2 More than 600 uniformed and other federal personnel, including medical personnel from US Army, Navy, and Public Health Service Commissioned Corps, mobilized to provide medical support to the JNYMS in late March 2020, leading to the treatment of more than 1000 patients with COVID-19 within a 30-day period.1
Literature from the SARS, Ebola, and HIV epidemics indicate that adverse mental health consequences, including burnout, depression, and posttraumatic stress disorder symptoms are common in frontline medical workers.3,4 Emerging data shows a similar trend occurring during the COVID-19 pandemic.5 A recent publication detailed the role of a federal force health protection program created to enhance resiliency of deployed officers during the COVID-19 pandemic, but this focused primarily on providing remote services to frontline workers.6 Another report addressed mental health interventions for health care workers in an academic health care system in NYC during COVID-19.7 However, there has been little published on real-time mental health support for deployed personnel during the pandemic.
Prior publications have described the patient flow, infection control measures, and development of a Consultation-Liaison Psychiatry Service in the JNYMS.2,8,9 Here, we detail the establishment of preventative and responsive mental health services for frontline workers at the JNYMS and explore lessons learned through the outpatient and general support experiences.
Development of Outpatient Mental Health Support Services
At the end of March 2020, the Jacob K. Javits Convention Center was repurposed into the 452-bed JNYMS field hospital, where exposition rooms were transformed into a medical unit and intensive care unit.2 While the majority of personnel providing direct clinical care were specialists, the station also was staffed with uniformed and other federal mental health clinicians, including 5 licensed clinical social workers (LCSWs), 3 psychiatrists, 1 dual-trained internal medicine–psychiatry physician, 1 psychiatric nurse, and 2 behavioral health technicians. To standardize processes early in the deployment, standard operating procedures for behavioral health support of personnel were developed and disseminated within the first few days of the deployment.
The initial mission of the behavioral health team was to establish comprehensive mental health services, as the rapidly shifting mission and unfamiliar environment increased the risk of new-onset stress responses and exacerbating pre-existing stressors in personnel. Behavioral health leadership established operations in conference rooms within the convention center, focusing on identifying, prioritizing, and staffing high-traffic areas. A resiliency center was also established adjacent to the changing room, where all staff would enter and leave the units, and to the dining facility, further increasing traffic. This center was staffed 24 hours a day by at least 1 LCSW and a behavioral health technician with 2 shifts: one from 0630 to 1830 and another from 1830 to 0630. Psychiatrists were available during the day for psychiatry intervention and evaluations, and an on-call schedule was developed for off-hours to provide time-sensitive responses.
The resiliency center was developed to provide a welcoming atmosphere to meet basic needs, including nourishment, healthy social interaction, and a calm environment. Water and food were made available free to personnel, bolstering morale for staff working 12-hour shifts in a pandemic treatment floor where personal protective equipment prevented intake of food or water. Mental health staff were also available to counsel and provide social support to personnel. If personnel wished to discuss stressors or appeared to be in distress, a mental health clinician would provide a real-time intervention or schedule an appointment with the behavioral health team. Resources were made available, including brochures and other reading materials on resilience, stress management, and other mental health topics. Uniformed services and state and federal JNYMS leadership were encouraged to visit the resiliency center to normalize interactions and encourage participation in a behavioral health environment. Signage was placed throughout JNYMS to direct personnel to behavioral health services.
The behavioral health interventions and influence spread from the resiliency center nexus. Initially, therapeutic interventions occurred where and when necessary. One psychiatrist provided crisis intervention to a bereaved soldier in the stairwell within 2 hours of arrival to the JNYMS. Leadership and the behavioral health team recognized that the need for privacy was essential for timely therapeutic interventions, leading to the development of a private individual counseling room. As the area became generally accepted as the central hub of behavioral health activity, space was provided to establish a quiet space and a meditation room. The quiet area provided a cool dark space for personnel to sit quietly in solitude; many were grateful for this reprieve after an overstimulating medical shift. The meditation room supplied sterilized yoga mats for personal mindfulness interventions. The behavioral health team also liaised with military chaplains, who established a spiritual service room near the resiliency center. The chaplains held regular religious services and were available 24 hours a day for timely spiritual interventions.
Rapid notification and movement of uniformed personnel to JNYMS resulted in limited ability for personnel to schedule medical appointments and refill medications. Psychiatrists also had limited access to relevant electronic health record systems. This led to a delay in nonurgent care to evaluate personnel records and confirm prescriptions, especially controlled medications. Local pharmacies filled prescriptions, psychiatrists placed electronic health profiles, and command teams were notified in accordance with US Army and federal regulations.
Medical Unit Support Services
Although a robust outpatient behavioral health service was laid out in the JNYMS, the behavioral health team recognized the need to provide mental health interventions within the main patient care areas as well. The intention was to maximize availability and support while minimizing interference to patient care. As previously described, a psychiatric consultation-liaison (CL) team was organized and operated 24 hours a day by early April 2020.9 Indeed, CL psychiatrists have played a valuable role in supporting the unique patient and staff needs in other COVID-19 treatment environments.10 The CL team at JNYMS observed that medical staff were exposed to multiple stressors, including fear of acquiring COVID-19, treating patients with significant medical comorbidities, practicing outside of clinical specialty, working with unfamiliar and limited equipment, and adjusting to frequently shifting changes in personnel and work schedules. Moreover, psychological stress was compounded by long shifts, jetlag, and continuous wear of extensive personal protective equipment, as has been documented in other COVID-19 treatment centers.11
The team of psychiatrists conducted informal rounds to nursing stations to evaluate the morale and develop relationships with the medical team, including nurses, physicians, medics, and other personnel. Areas of high stress and increased interpersonal conflict were identified for more frequent check-ins by mental health clinicians. The psychiatrists and LCSWs were available for informal walk-in therapy when requested by personnel. When the acuity increased, personnel could be accompanied to the individual counseling room for rapid therapeutic interventions. The CL psychiatrists developed professional relationships with the command and medical leadership teams. Through these relationships and sensitive awareness of morale in the medical work environment, psychiatrists were able to advocate for alterations in the nursing work schedule. Leadership was receptive and resultant changes decreased the hours per shift and number of shifts for most nurses. Morale quickly improved, likely resulting in improved quality of patient care and prevention of burnout.
Mental Health Care Beyond JNYMS
Uniformed services and other federal personnel further supplemented health care operations beyond JYNMS. In April 2020, Urban Augmentation Medical Task Forces were organized and distributed throughout regions where COVID-19–related hospitalizations had significantly overwhelmed the local health care force. Urban Augmentation Medical Task Forces often included a psychiatrist, psychologist, and behavioral health technician with the mission to provide mental health support and interventions to patients and medical staff. Combat Operational Stress Control units from US Army medical brigades operated in NYC and the greater northeast region, providing mental health support and resiliency training to military personnel working in civilian hospitals, medical centers, and other health care or support environments. In addition, a LCSW and behavioral health technician worked with New York Army Reserve personnel assigned to mortuary affairs, providing point-of-care interventions at or near the worksite.
A collaborative federal, uniformed services, and state operation led to the development of the HERO-NY: Healing, Education, Resilience, and Opportunity for New York’s Frontline Workforce “Train the Trainer” Series.12 The series was intended to use uniformed services expertise to address mental health challenges related to the COVID-19 epidemic. Psychiatrists and mental health clinicians from JNYMS modeled small group trainings for future medical trainers. In lieu of traditional unidirectional lecturing, which yields limited retention and learning, the panelists demonstrated how to lead interactive small group training with resiliency topics, including goal setting, communication, anger management, and sleep hygiene.
Transition
After the last patient was discharged from JNYMS in May 2020, personnel were quickly redeployed to their duty stations. At the time of mission completion, the JNYMS behavioral health team had been supplemented with psychiatrists, social workers, behavioral health technicians, psychiatric nurse practitioners, psychiatric nurses, and psychologists representing US Public Health Service Commissioned Corps, Army, Air Force, and Navy, and provided comprehensive support to the nearly 1100 patients with COVID-19 and 600 deployed federal and state medical and support personnel.
Lessons Learned and Future Considerations
Behavioral health care provided at JNYMS offers insight into support of frontline workers in pandemic settings, as literature is limited in this area.13 TheJNYMS behavioral health team used strategies similar to military medical interventions in limited and unpredictable environments, such as rapid formalization of team structure and establishment of standard operating procedures to facilitate uniformity across interventions. Physical space was necessary to create an environment conducive to productive mental health interventions, including therapy rooms and quiet and spiritual spaces. Placing behavioral resources in high-traffic areas normalized mental health and maximized accessibility to interventions. Mental health personnel also addressed issues in the work environment, such as providing informal support and crisis interventions to frontline workers. Finally, Urban Augmentation Medical Task Forces mental health personnel and Combat Operational Stress Control units provided therapeutic interventions and resiliency training for military and civilian personnel throughout burdened medical systems beyond JNYMS.
Future operations should consider what equipment and logistic access are necessary to provide psychiatric and psychological care to mobilized federal and uniformed personnel, such as access to frontline worker electronic health records. Given that prior work has found that provision of resources alone is inadequate, frontline medical workers must be aware of where resources are available (eg, signage) and have easy access to material (eg, brochures) focusing on resiliency and psychological health.14 The spaces can be used for formal psychiatric and psychological interventions, such as assessment, therapy, and medication management. Equally important, these spaces serve as a safe place for healthy social interaction and fulfillment of basic needs (eg, nourishment) and a peaceful environment free of stimulation.
Since mental health personnel provide varied services ranging from basic human interaction to complex crisis interventions, mental health personnel should supplement pandemic medical operations. Evidence supports the notion that effective communication and cohesion throughout the entire leadership and health care team structure can improve resilience and implementation of mental health interventions.15 Incorporating mental health personnel into leadership planning meetings would allow for timely recommendations to improve medical logistics and planning of deployment of behavioral health resources. As a general rule, providing behavioral health experts with a seat at the table enhances advocacy and command awareness of the morale and mental health of frontline personnel.
Conclusions
We present the experience of developing mental health support services for deployed personnel during the COVID-19 pandemic and address the real-time mental health treatment and support of deployed uniformed services and federal personnel in the COVID-19 response environment. Timely and effective interventions included securing safe therapeutic space in high-traffic areas, developing relationships with leadership and frontline workers in their own work environments, and disseminating such services throughout the civilian medical system.
Mental health supplementation during the medical response mission strengthened morale in frontline workers in a disaster scenario. We hope that this report and others like it will provide information to improve mental health responses, reinforce mental health support, and encourage research in evidence-based interventions in challenging pandemic and disaster settings.
Acknowledgments
We would like to acknowledge and thank those serving on the frontlines of the COVID-19 pandemic.
1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. Published 2020 Apr 17. doi:10.15585/mmwr.mm6915e4
2. Brady K, Milzman D, Walton E, Sommer D, Neustadtl A, Napoli A. Uniformed services and the field hospital experience during Coronovirus Disease 2019 (SARS-CoV-2) pandemic: open to closure in 30 days with 1,100 patients: the Javits New York Medical Station [published online ahead of print, 2021 Feb 13]. Mil Med. 2021;usab003. doi:10.1093/milmed/usab003
3. Tucci V, Moukaddam N, Meadows J, Shah S, Galwankar SC, Kapur GB. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151-156. doi:10.4103/jgid.jgid_66_17
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311. doi:10.1177/070674370905400504
5. Panchal N, Kamal R, Cox C, Garfield R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed April 7, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
6. Myles IA, Johnson DR, Pham H, et al. USPHS Corps Care: force health protection for public health officers during the Ebola and COVID-19 responses. Public Health Rep. 2021;136(2):148-153. doi:10.1177/0033354920984775
7. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. 2020;95(8):1136-1139. doi:10.1097/ACM.0000000000003414
8. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):308-311. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009a3
9. Kaplan A, Smith CM, Toukolehto O, van Schalkwyk G. Psychiatric care in a novel federal COVID-19 treatment center: development of a consultation-liaison psychiatry service at the Javits New York Medical Station. Mil Med. 2021;186(5-6):129-131. doi:10.1093/milmed/usaa557
10. Shalev D, Shapiro PA. Epidemic psychiatry: The opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68-71. doi:10.1016/j.genhosppsych.2020.03.009
11. Horn M, Granon B, Vaiva G, Fovet T, Amad A. Role and importance of consultation-liaison psychiatry during the Covid-19 epidemic [published online ahead of print, 2020 Aug 5]. J Psychosom Res. 2020;137:110214. doi:10.1016/j.jpsychores.2020.110214
12. Wei EK, Segall J, Linn-Walton R, et al. Combat stress management and resilience: adapting department of defense combat lessons learned to civilian healthcare during the COVID-19 pandemic [published online ahead of print, 2020 Jul 17]. Health Secur. 2020;10.1089/hs.2020.0091. doi:10.1089/hs.2020.0091
13. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11(11):CD013779. Published 2020 Nov 5. doi:10.1002/14651858.CD013779
14. Schreiber M, Cates DS, Formanski S, King M. Maximizing the Resilience of Healthcare Workers in Multi-hazard Events: Lessons from the 2014-2015 Ebola Response in Africa. Mil Med. 2019;184(suppl 1):114-120. doi:10.1093/milmed/usy400
15. Klomp RW, Jones L, Watanabe E, Thompson WW. CDC’s multiple approaches to safeguard the health, safety, and resilience of Ebola responders. Prehosp Disaster Med. 2020;35(1):69-75. doi:10.1017/S1049023X19005144
1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. Published 2020 Apr 17. doi:10.15585/mmwr.mm6915e4
2. Brady K, Milzman D, Walton E, Sommer D, Neustadtl A, Napoli A. Uniformed services and the field hospital experience during Coronovirus Disease 2019 (SARS-CoV-2) pandemic: open to closure in 30 days with 1,100 patients: the Javits New York Medical Station [published online ahead of print, 2021 Feb 13]. Mil Med. 2021;usab003. doi:10.1093/milmed/usab003
3. Tucci V, Moukaddam N, Meadows J, Shah S, Galwankar SC, Kapur GB. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151-156. doi:10.4103/jgid.jgid_66_17
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311. doi:10.1177/070674370905400504
5. Panchal N, Kamal R, Cox C, Garfield R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed April 7, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
6. Myles IA, Johnson DR, Pham H, et al. USPHS Corps Care: force health protection for public health officers during the Ebola and COVID-19 responses. Public Health Rep. 2021;136(2):148-153. doi:10.1177/0033354920984775
7. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. 2020;95(8):1136-1139. doi:10.1097/ACM.0000000000003414
8. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):308-311. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009a3
9. Kaplan A, Smith CM, Toukolehto O, van Schalkwyk G. Psychiatric care in a novel federal COVID-19 treatment center: development of a consultation-liaison psychiatry service at the Javits New York Medical Station. Mil Med. 2021;186(5-6):129-131. doi:10.1093/milmed/usaa557
10. Shalev D, Shapiro PA. Epidemic psychiatry: The opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68-71. doi:10.1016/j.genhosppsych.2020.03.009
11. Horn M, Granon B, Vaiva G, Fovet T, Amad A. Role and importance of consultation-liaison psychiatry during the Covid-19 epidemic [published online ahead of print, 2020 Aug 5]. J Psychosom Res. 2020;137:110214. doi:10.1016/j.jpsychores.2020.110214
12. Wei EK, Segall J, Linn-Walton R, et al. Combat stress management and resilience: adapting department of defense combat lessons learned to civilian healthcare during the COVID-19 pandemic [published online ahead of print, 2020 Jul 17]. Health Secur. 2020;10.1089/hs.2020.0091. doi:10.1089/hs.2020.0091
13. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11(11):CD013779. Published 2020 Nov 5. doi:10.1002/14651858.CD013779
14. Schreiber M, Cates DS, Formanski S, King M. Maximizing the Resilience of Healthcare Workers in Multi-hazard Events: Lessons from the 2014-2015 Ebola Response in Africa. Mil Med. 2019;184(suppl 1):114-120. doi:10.1093/milmed/usy400
15. Klomp RW, Jones L, Watanabe E, Thompson WW. CDC’s multiple approaches to safeguard the health, safety, and resilience of Ebola responders. Prehosp Disaster Med. 2020;35(1):69-75. doi:10.1017/S1049023X19005144
43-year-old male • fatigue • unintentional weight loss • pancytopenia • Dx?
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.
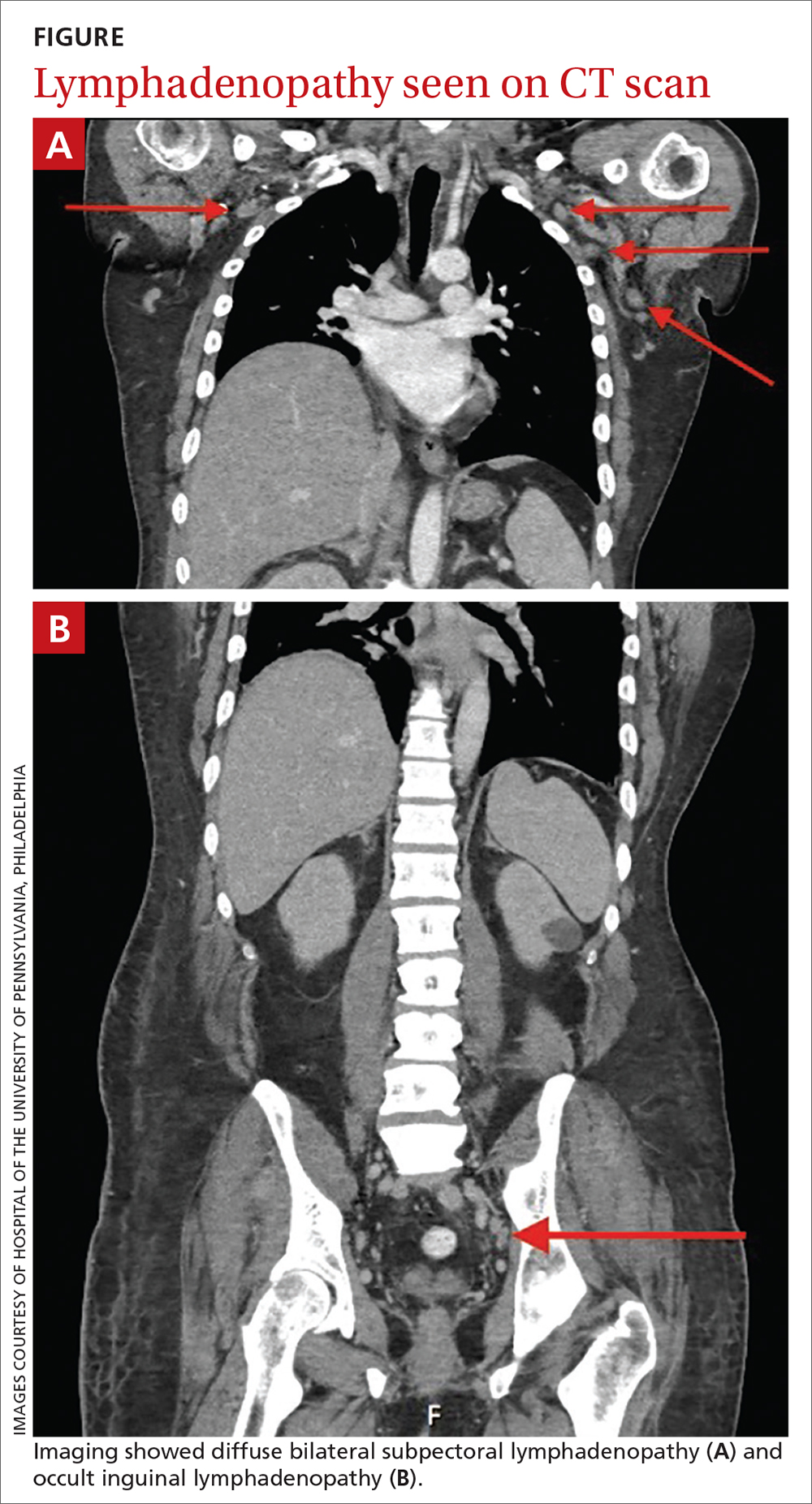
Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.

Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.

Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
Atypical knee pain
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.
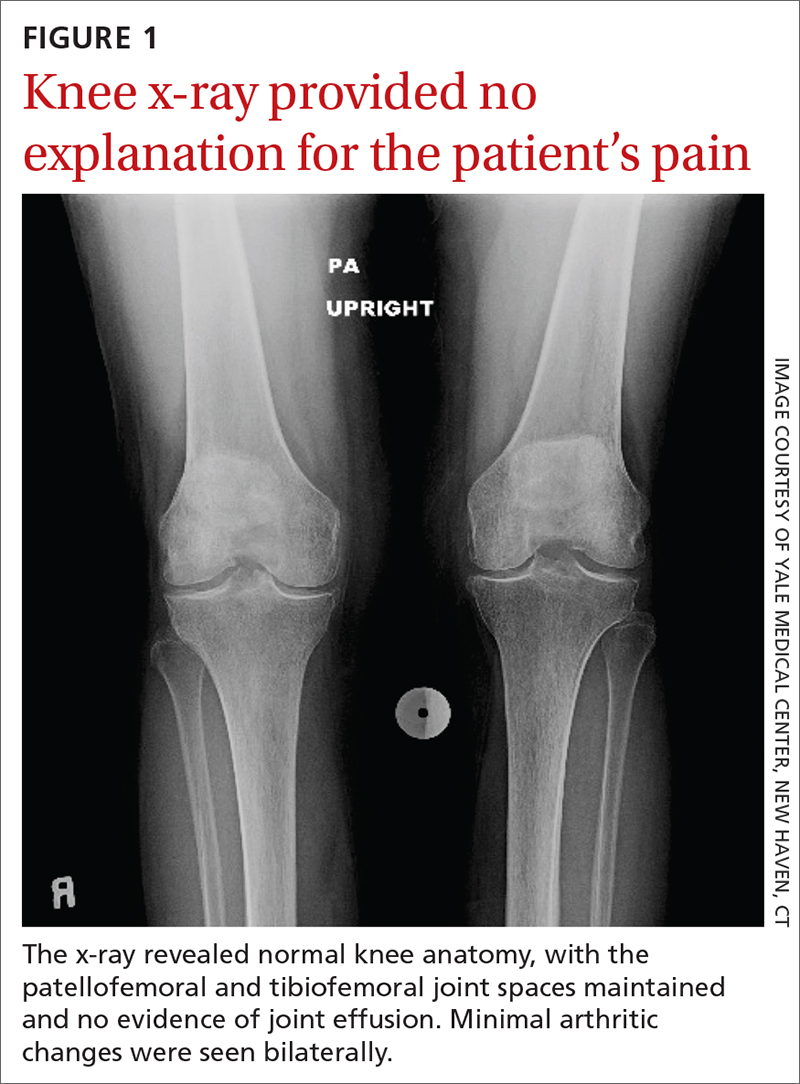
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.
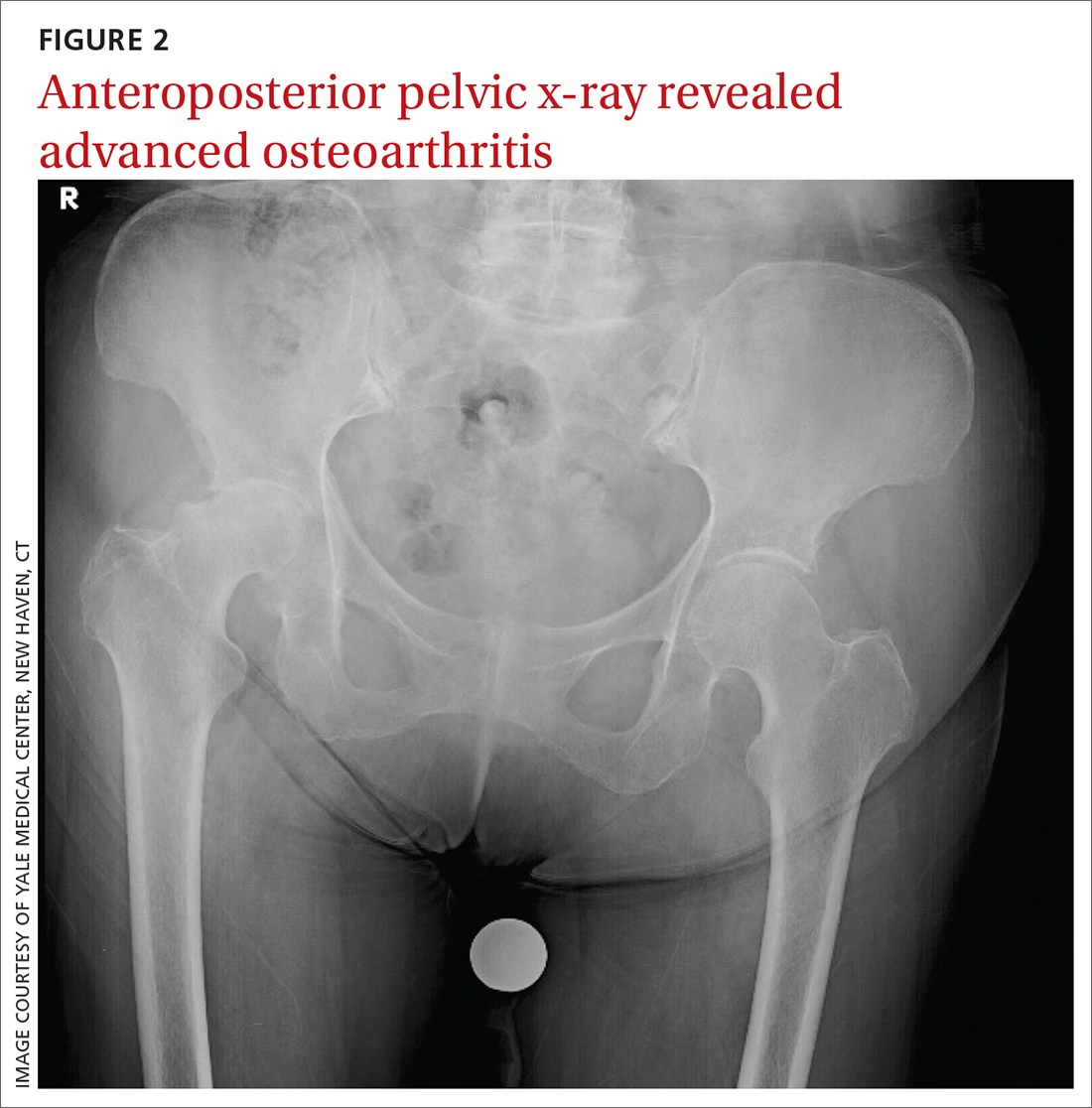
Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.
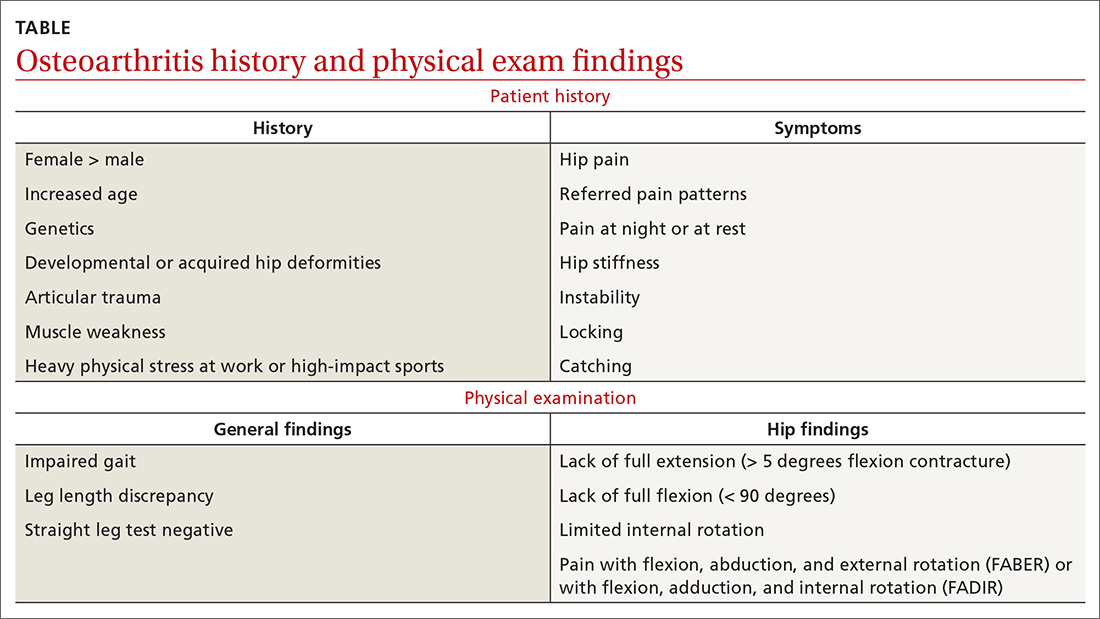
The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.

Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.

The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.

An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.

Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.

The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.

1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
Time to consider topical capsaicin for acute trauma pain?
ILLUSTRATIVE CASE
A 23-year-old man with no significant past medical history presents to an urgent care center after a fall on his right arm while playing football. He reports a pain level of 6 using the visual analog scale (VAS). Physical exam reveals minor erythema and edema of his forearm with pain to palpation. Range of motion, strength, and sensation are intact. No lacerations are present. His vital signs are normal. No fracture is found on imaging. The physician decides that treatment with a topical analgesic is reasonable for this uncomplicated contusion of the right forearm. Is there a role for topical capsaicin in the treatment of this patient’s pain?
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are effective for the treatment of acute non–low back pain musculoskeletal injuries.2 They are generally well tolerated and just as effective as oral NSAIDS or acetaminophen for localized injuries. Their ubiquitous availability, affordability, and low adverse effect profile make them an attractive first-line treatment option for acute musculoskeletal pain.
Capsaicin, a topical agent derived from a genus of red peppers, has been used for the treatment of neuropathic and chronic pain via its interactions with substance P, transient receptor potential vanilloid subtype 1 (TRPV1), and nociceptive nerve fibers.3,4 It has demonstrated effectiveness in the management of diabetic neuropathy, knee osteoarthritis, and postherpetic neuralgia, as well as various causes of pruritus.5,6
Although many studies have compared oral and topical NSAIDs, opiates, and acetaminophen, few studies have directly compared topical NSAIDs and capsaicin. This study compared the topical NSAID piroxicam with topical capsaicin.
STUDY SUMMARY
Topical capsaicin demonstrated superior pain reduction
This prospective, double-blind RCT compared the efficacy of topical capsaicin vs topical piroxicam for the treatment of acute pain following upper extremity blunt trauma. Patients (ages ≥ 18 years) who presented to a Turkish emergency department within 2 hours of upper extremity injury were randomized to receive either 0.05% capsaicin gel (n = 69) or 0.5% piroxicam gel (n = 67). Patients reported level 5 or higher pain on the VAS. Those with fractures, dislocations, skin disruption, or other trauma were excluded. Age, gender, pain duration, and mechanism of injury did not differ significantly between study groups.1
Blinding was ensured by placing the gels in opaque containers containing 30 mg of either capsaicin or piroxicam and dyeing the medicine with red and yellow food coloring. A thin layer of medication was applied to an area no larger than 5 × 5 cm on the upper extremity and rubbed for 1 minute. Patients were observed in the emergency department for 2 hours and discharged with instructions to apply the medication 3 times daily for 72 hours.
The investigators measured pain using VAS scores at 1 hour, 2 hours, 24 hours, and 72 hours after treatment. Topical capsaicin was superior to topical piroxicam at achieving both primary outcomes: a VAS score of ≤ 4 (85.5% vs 50.7%; number needed to treat [NNT] = 2.9; P < .001) and a > 50% reduction in VAS score (87% vs 62.7%; NNT = 4.1; P < .01) at the end of treatment.1 (These outcomes were based on earlier determinations of the minimal clinically important difference.7,8)
Additionally, capsaicin was more effective than piroxicam at each time interval. This difference was most pronounced at 72 hours, with a mean difference of delta VAS scores of 1.53 (95% CI, 0.85-2.221) and a mean percentage of the reduction in VAS scores of 19.7% (95% CI, 12.4%-27.2%) (P < .001).1
Reported adverse effects, such as burning, itching, and rash, were mild and infrequent and showed no significant difference between the treatment groups.
WHAT’S NEW
First study comparing topical capsaicin and a topical NSAID in acute trauma
Although both capsaicin and topical piroxicam have proven efficacy for the treatment of pain, this RCT is the first study to directly compare these agents in the setting of acute upper extremity blunt trauma. Capsaicin is currently more commonly prescribed as a treatment for chronic neuropathic pain.4,9 In this study, capsaicin demonstrated superior results in pain reduction at each assessed time interval and at the primary end point of 72 hours.
CAVEATS
Limited generalizability to lower extremity and truncal trauma
This RCT included a relatively small sample size (136 patients). Researchers evaluated only blunt upper extremity injuries; as such, the generalizability of the effectiveness of topical capsaicin in blunt lower extremity and truncal trauma is limited, especially over larger surface areas.
CHALLENGES TO IMPLEMENTATION
No major challenges found
There are no major challenges to implementing this inexpensive treatment.
1. Kocak AO, Dogruyol S, Akbas I, et al. Comparison of topical capsaicin and topical piroxicam in the treatment of acute trauma-induced pain: a randomized double-blind trial. Am J Emerg Med. 2020;38:1767-1771. doi: 10.1016/j.ajem.2020.05.104
2. Busse JW, Sadeghirad B, Oparin Y, et al. Management of acute pain from non–low back, musculoskeletal injuries: a systematic review and network meta-analysis of randomized trials. Ann Intern Med. 2020;173:730-738. doi: 10.7326/M19-3601
3. Chrubasik S, Weiser T, Beime B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother Res. 2010;24:1877-1885. doi: 10.1002/ptr.3335
4. Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2012(9):CD010111. doi: 10.1002/14651858.CD010111
5. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42-53. doi: 10.1016/j.jpain.2016.09.008
6. Papoiu ADP, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi: 10.1517/14656566.2010.481670
7. Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013;13:497-503. doi: 10.1111/papr.12013
8. Kocak AO, Ahiskalioglu A, Sengun E, et al. Comparison of intravenous NSAIDs and trigger point injection for low back pain in ED: a prospective randomized study. Am J Emerg Med. 2019;37:1927-1931. doi: 10.1016/j.ajem.2019.01.015
9. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1(1):CD007393. doi: 10.1002/14651858.CD007393.pub4
ILLUSTRATIVE CASE
A 23-year-old man with no significant past medical history presents to an urgent care center after a fall on his right arm while playing football. He reports a pain level of 6 using the visual analog scale (VAS). Physical exam reveals minor erythema and edema of his forearm with pain to palpation. Range of motion, strength, and sensation are intact. No lacerations are present. His vital signs are normal. No fracture is found on imaging. The physician decides that treatment with a topical analgesic is reasonable for this uncomplicated contusion of the right forearm. Is there a role for topical capsaicin in the treatment of this patient’s pain?
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are effective for the treatment of acute non–low back pain musculoskeletal injuries.2 They are generally well tolerated and just as effective as oral NSAIDS or acetaminophen for localized injuries. Their ubiquitous availability, affordability, and low adverse effect profile make them an attractive first-line treatment option for acute musculoskeletal pain.
Capsaicin, a topical agent derived from a genus of red peppers, has been used for the treatment of neuropathic and chronic pain via its interactions with substance P, transient receptor potential vanilloid subtype 1 (TRPV1), and nociceptive nerve fibers.3,4 It has demonstrated effectiveness in the management of diabetic neuropathy, knee osteoarthritis, and postherpetic neuralgia, as well as various causes of pruritus.5,6
Although many studies have compared oral and topical NSAIDs, opiates, and acetaminophen, few studies have directly compared topical NSAIDs and capsaicin. This study compared the topical NSAID piroxicam with topical capsaicin.
STUDY SUMMARY
Topical capsaicin demonstrated superior pain reduction
This prospective, double-blind RCT compared the efficacy of topical capsaicin vs topical piroxicam for the treatment of acute pain following upper extremity blunt trauma. Patients (ages ≥ 18 years) who presented to a Turkish emergency department within 2 hours of upper extremity injury were randomized to receive either 0.05% capsaicin gel (n = 69) or 0.5% piroxicam gel (n = 67). Patients reported level 5 or higher pain on the VAS. Those with fractures, dislocations, skin disruption, or other trauma were excluded. Age, gender, pain duration, and mechanism of injury did not differ significantly between study groups.1
Blinding was ensured by placing the gels in opaque containers containing 30 mg of either capsaicin or piroxicam and dyeing the medicine with red and yellow food coloring. A thin layer of medication was applied to an area no larger than 5 × 5 cm on the upper extremity and rubbed for 1 minute. Patients were observed in the emergency department for 2 hours and discharged with instructions to apply the medication 3 times daily for 72 hours.
The investigators measured pain using VAS scores at 1 hour, 2 hours, 24 hours, and 72 hours after treatment. Topical capsaicin was superior to topical piroxicam at achieving both primary outcomes: a VAS score of ≤ 4 (85.5% vs 50.7%; number needed to treat [NNT] = 2.9; P < .001) and a > 50% reduction in VAS score (87% vs 62.7%; NNT = 4.1; P < .01) at the end of treatment.1 (These outcomes were based on earlier determinations of the minimal clinically important difference.7,8)
Additionally, capsaicin was more effective than piroxicam at each time interval. This difference was most pronounced at 72 hours, with a mean difference of delta VAS scores of 1.53 (95% CI, 0.85-2.221) and a mean percentage of the reduction in VAS scores of 19.7% (95% CI, 12.4%-27.2%) (P < .001).1
Reported adverse effects, such as burning, itching, and rash, were mild and infrequent and showed no significant difference between the treatment groups.
WHAT’S NEW
First study comparing topical capsaicin and a topical NSAID in acute trauma
Although both capsaicin and topical piroxicam have proven efficacy for the treatment of pain, this RCT is the first study to directly compare these agents in the setting of acute upper extremity blunt trauma. Capsaicin is currently more commonly prescribed as a treatment for chronic neuropathic pain.4,9 In this study, capsaicin demonstrated superior results in pain reduction at each assessed time interval and at the primary end point of 72 hours.
CAVEATS
Limited generalizability to lower extremity and truncal trauma
This RCT included a relatively small sample size (136 patients). Researchers evaluated only blunt upper extremity injuries; as such, the generalizability of the effectiveness of topical capsaicin in blunt lower extremity and truncal trauma is limited, especially over larger surface areas.
CHALLENGES TO IMPLEMENTATION
No major challenges found
There are no major challenges to implementing this inexpensive treatment.
ILLUSTRATIVE CASE
A 23-year-old man with no significant past medical history presents to an urgent care center after a fall on his right arm while playing football. He reports a pain level of 6 using the visual analog scale (VAS). Physical exam reveals minor erythema and edema of his forearm with pain to palpation. Range of motion, strength, and sensation are intact. No lacerations are present. His vital signs are normal. No fracture is found on imaging. The physician decides that treatment with a topical analgesic is reasonable for this uncomplicated contusion of the right forearm. Is there a role for topical capsaicin in the treatment of this patient’s pain?
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are effective for the treatment of acute non–low back pain musculoskeletal injuries.2 They are generally well tolerated and just as effective as oral NSAIDS or acetaminophen for localized injuries. Their ubiquitous availability, affordability, and low adverse effect profile make them an attractive first-line treatment option for acute musculoskeletal pain.
Capsaicin, a topical agent derived from a genus of red peppers, has been used for the treatment of neuropathic and chronic pain via its interactions with substance P, transient receptor potential vanilloid subtype 1 (TRPV1), and nociceptive nerve fibers.3,4 It has demonstrated effectiveness in the management of diabetic neuropathy, knee osteoarthritis, and postherpetic neuralgia, as well as various causes of pruritus.5,6
Although many studies have compared oral and topical NSAIDs, opiates, and acetaminophen, few studies have directly compared topical NSAIDs and capsaicin. This study compared the topical NSAID piroxicam with topical capsaicin.
STUDY SUMMARY
Topical capsaicin demonstrated superior pain reduction
This prospective, double-blind RCT compared the efficacy of topical capsaicin vs topical piroxicam for the treatment of acute pain following upper extremity blunt trauma. Patients (ages ≥ 18 years) who presented to a Turkish emergency department within 2 hours of upper extremity injury were randomized to receive either 0.05% capsaicin gel (n = 69) or 0.5% piroxicam gel (n = 67). Patients reported level 5 or higher pain on the VAS. Those with fractures, dislocations, skin disruption, or other trauma were excluded. Age, gender, pain duration, and mechanism of injury did not differ significantly between study groups.1
Blinding was ensured by placing the gels in opaque containers containing 30 mg of either capsaicin or piroxicam and dyeing the medicine with red and yellow food coloring. A thin layer of medication was applied to an area no larger than 5 × 5 cm on the upper extremity and rubbed for 1 minute. Patients were observed in the emergency department for 2 hours and discharged with instructions to apply the medication 3 times daily for 72 hours.
The investigators measured pain using VAS scores at 1 hour, 2 hours, 24 hours, and 72 hours after treatment. Topical capsaicin was superior to topical piroxicam at achieving both primary outcomes: a VAS score of ≤ 4 (85.5% vs 50.7%; number needed to treat [NNT] = 2.9; P < .001) and a > 50% reduction in VAS score (87% vs 62.7%; NNT = 4.1; P < .01) at the end of treatment.1 (These outcomes were based on earlier determinations of the minimal clinically important difference.7,8)
Additionally, capsaicin was more effective than piroxicam at each time interval. This difference was most pronounced at 72 hours, with a mean difference of delta VAS scores of 1.53 (95% CI, 0.85-2.221) and a mean percentage of the reduction in VAS scores of 19.7% (95% CI, 12.4%-27.2%) (P < .001).1
Reported adverse effects, such as burning, itching, and rash, were mild and infrequent and showed no significant difference between the treatment groups.
WHAT’S NEW
First study comparing topical capsaicin and a topical NSAID in acute trauma
Although both capsaicin and topical piroxicam have proven efficacy for the treatment of pain, this RCT is the first study to directly compare these agents in the setting of acute upper extremity blunt trauma. Capsaicin is currently more commonly prescribed as a treatment for chronic neuropathic pain.4,9 In this study, capsaicin demonstrated superior results in pain reduction at each assessed time interval and at the primary end point of 72 hours.
CAVEATS
Limited generalizability to lower extremity and truncal trauma
This RCT included a relatively small sample size (136 patients). Researchers evaluated only blunt upper extremity injuries; as such, the generalizability of the effectiveness of topical capsaicin in blunt lower extremity and truncal trauma is limited, especially over larger surface areas.
CHALLENGES TO IMPLEMENTATION
No major challenges found
There are no major challenges to implementing this inexpensive treatment.
1. Kocak AO, Dogruyol S, Akbas I, et al. Comparison of topical capsaicin and topical piroxicam in the treatment of acute trauma-induced pain: a randomized double-blind trial. Am J Emerg Med. 2020;38:1767-1771. doi: 10.1016/j.ajem.2020.05.104
2. Busse JW, Sadeghirad B, Oparin Y, et al. Management of acute pain from non–low back, musculoskeletal injuries: a systematic review and network meta-analysis of randomized trials. Ann Intern Med. 2020;173:730-738. doi: 10.7326/M19-3601
3. Chrubasik S, Weiser T, Beime B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother Res. 2010;24:1877-1885. doi: 10.1002/ptr.3335
4. Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2012(9):CD010111. doi: 10.1002/14651858.CD010111
5. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42-53. doi: 10.1016/j.jpain.2016.09.008
6. Papoiu ADP, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi: 10.1517/14656566.2010.481670
7. Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013;13:497-503. doi: 10.1111/papr.12013
8. Kocak AO, Ahiskalioglu A, Sengun E, et al. Comparison of intravenous NSAIDs and trigger point injection for low back pain in ED: a prospective randomized study. Am J Emerg Med. 2019;37:1927-1931. doi: 10.1016/j.ajem.2019.01.015
9. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1(1):CD007393. doi: 10.1002/14651858.CD007393.pub4
1. Kocak AO, Dogruyol S, Akbas I, et al. Comparison of topical capsaicin and topical piroxicam in the treatment of acute trauma-induced pain: a randomized double-blind trial. Am J Emerg Med. 2020;38:1767-1771. doi: 10.1016/j.ajem.2020.05.104
2. Busse JW, Sadeghirad B, Oparin Y, et al. Management of acute pain from non–low back, musculoskeletal injuries: a systematic review and network meta-analysis of randomized trials. Ann Intern Med. 2020;173:730-738. doi: 10.7326/M19-3601
3. Chrubasik S, Weiser T, Beime B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother Res. 2010;24:1877-1885. doi: 10.1002/ptr.3335
4. Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2012(9):CD010111. doi: 10.1002/14651858.CD010111
5. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42-53. doi: 10.1016/j.jpain.2016.09.008
6. Papoiu ADP, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi: 10.1517/14656566.2010.481670
7. Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013;13:497-503. doi: 10.1111/papr.12013
8. Kocak AO, Ahiskalioglu A, Sengun E, et al. Comparison of intravenous NSAIDs and trigger point injection for low back pain in ED: a prospective randomized study. Am J Emerg Med. 2019;37:1927-1931. doi: 10.1016/j.ajem.2019.01.015
9. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1(1):CD007393. doi: 10.1002/14651858.CD007393.pub4
PRACTICE CHANGER
Use topical capsaicin gel 0.05% for pain reduction in patients with isolated blunt injuries of the upper extremity without fracture.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial (RCT)1
Kocak AO, Dogruyol S, Akbas I, et al. Comparison of topical capsaicin and topical piroxicam in the treatment of acute trauma-induced pain: a randomized double-blind trial. Am J Emerg Med. 2020;38:1767-1771.
Managing TIA: Early action and essential risk-reduction steps
As many as 240,000 people per year in the United States experience a transient ischemic attack (TIA),1,2 which is now defined by the American Heart Association and American Stroke Association as a “transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction.”3 An older definition of TIA was based on the duration of the event (ie, resolution of symptoms at 24 hours); in the updated (2009) definition, the diagnostic criterion is the extent of focal tissue damage.3 Using the 2009 definition might mean a decrease in the number of patients who have a diagnosis of a TIA and an increase in the number who are determined to have had a stroke because an infarction is found on initial imaging.
Guided by the 2009 revised definition of a TIA, we review here the work-up and treatment of TIA, emphasizing immediacy of management to (1) prevent further tissue damage and (2) decrease the risk of a second event.
CASE
Martin L, 69 years old, retired, a nonsmoker, and with a history of peripheral arterial disease and hypercholesterolemia, presents to the emergency department (ED) of a rural hospital complaining of slurred speech and left-side facial numbness. He had an episode of facial numbness that lasted 30 minutes, then resolved, each of the 2 previous evenings; he did not seek care at those times. Now, in the ED, Mr. L is normotensive.
The patient’s medication history includes a selective serotonin reuptake inhibitor and melatonin to improve sleep. He reports having discontinued a statin because he could not tolerate its adverse effects.
What immediate steps are recommended for Mr. L’s care?
Common event callsfor quick action
A TIA is the strongest predictor of subsequent stroke and stroke-related death; the highest period of risk of these devastating outcomes is immediately following a TIA.1,2,4,5 It is essential, therefore, for the physician who sees a patient with a current complaint or recent history of suspected focal neurologic deficits to direct that patient to an ED for an accurate diagnosis and, as appropriate, early treatment for the best possible outcome.
Imaging—preferably, diffusion-weighted magnetic resonance imaging (DW-MRI), the gold standard for diagnosing stroke (see “Diagnosis includes ruling out mimics”)2,3—should be performed as soon as the patient with a suspected TIA arrives in the ED. Imaging should not be held while waiting for a stroke to declare itself—ie, by allowing symptoms to persist for longer than 24 hours. 6
Continue to: Late presentation
Late presentation. Some patients present ≥ 48 hours after onset of early symptoms of a TIA; for them, the work-up is the same as for prompt presentation but can be completed in the outpatient clinic—as long as the patient is stable clinically and imaging is accessible there. DW-MRI should be completed within 48 hours after late presentation. In such cases, the patient should be cautioned regarding risks and any recurrence of symptoms.7,8
Diagnosis includes ruling out mimics
All patients in whom a stroke is suspected should be evaluated on an emergency basis with brain imaging upon arrival at the hospital, before any therapy is initiated. As noted, DW-MRI is the preferred modality; noncontrast computed tomography (CT) or CT angiography can be used if MRI is unavailable.2,3
Mimics. Stroke has many mimics; quickly eliminating them from the differential diagnosis is important so that appropriate therapy can be initiated. Mimics usually have a prolonged presentation of symptoms, whereas the presentation of a TIA is usually abrupt. The 3 more common diagnoses that mimic a TIA are migraine with aura, seizure, and syncope.9,10 Symptoms that generally are not associated with a TIA are chest pain, generalized weakness, and confusion.11 A complete history and physical exam provide the path to the imaging, laboratory, and cardiac testing that is needed to differentiate these diagnoses from a TIA.
A thorough history is best obtained from the patient and a witness, if available, and should include identification of any focal neurologic deficits and the duration and time to resolution of symptoms. Obtain a history of risk factors for ischemia—tobacco use, diabetes, obesity, dyslipidemia, hypertension, previous TIA or stroke, atrial fibrillation, and any coagulopathy. Ask questions about a family history of TIA, stroke, and coagulopathy.11
A comprehensive physical exam, including vital signs, cardiac exam, a check for carotid bruits, and complete neurologic exam, should be performed. Most patients present with concerns for unilateral weakness and changes in speech, which are usually associated with infarction on DW-MRI.12 The most common findings on physical exam include cranial nerve abnormalities, such as diplopia, hemianopia, monocular blindness, disconjugate gaze, facial drooping, lateral tongue movement, dysphagia, and vestibular dysfunction. Cerebellar abnormalities are also often noted, including past pointing, dystaxia, ataxia, nystagmus, and motor abnormalities (eg, spasticity, clonus, or unilateral weakness in the face or extremities).11
Electrocardiography at the bedside can confirm atrial fibrillation or another arrhythmia quickly.
Essential laboratory testing includes measurement of blood glucose and serum electrolytes to determine if these particular imbalances are the cause of symptoms. The presence of a hypercoaguable state is determined by a complete blood count and coagulation studies.3,13 Urine toxicology should also be obtained to rule out other causes of symptoms. A lipid profile is beneficial for making long-term treatment decisions.
Continue to: ABCD2 score
ABCD2 score. Patients who have had a TIA and present within 72 hours after symptoms have resolved should be hospitalized if they have an ABCD2 (Age, Blood pressure [BP], Clinical presentation, Diabetes mellitus [type 1 or 2], Duration of symptoms) prediction system score > 3.14 ABCD2 criteria can be used to help identify patients who are at higher risk of stroke or need further therapy (TABLE 1).14,15
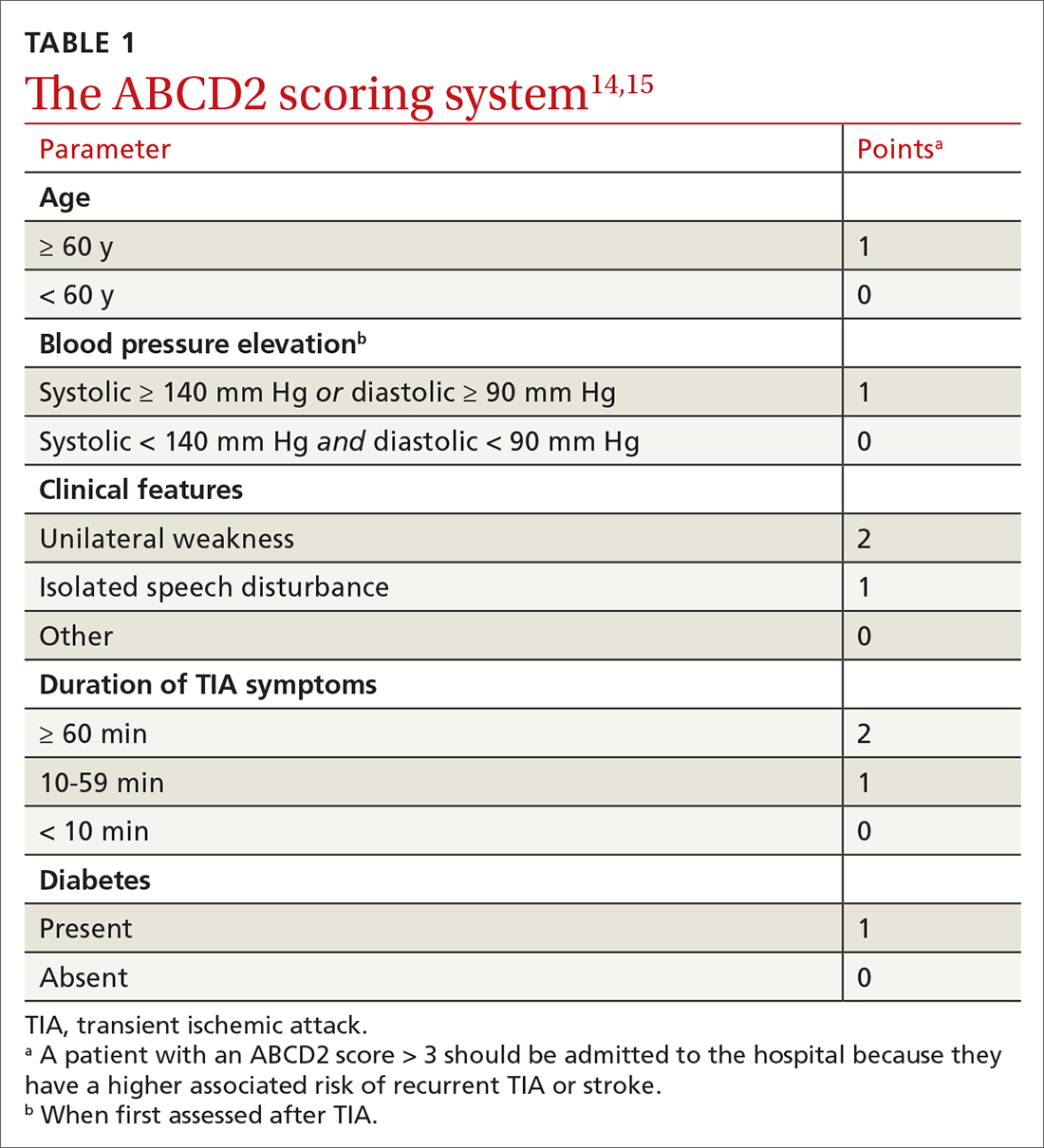
The ABCD2 score is also used to determine whether a patient needs dual antiplatelet therapy. Patients who score at the higher end of the ABCD2 system usually have an increased risk of stroke, longer hospitalization, and greater disability.
CASE
In the ED, Mr. L is immediately assessed and airlifted to a larger regional medical center, where MRI confirms a stroke.
Management
Initial management of a TIA is aimed at reducing the risk of recurrent TIA or stroke. Early medical and possibly surgical treatment are key for preventing stroke and improving outcomes. The first 48 hours after a TIA are the most critical because the incidence of recurrent TIA or stroke is highest during this period.16-18
What is the accepted strategy for early treatment?
Initial treatment must include antiplatelet therapy, BP management, anticoagulation, statin therapy, and carotid endarterectomy as indicated.2,19,20 Control of hypertension and anticoagulation decrease the risk of recurrent stroke by the largest margin20; both are “A”-level Strength of Recommendation Taxonomy interventions.2,3
Step 1: Antiplatelet therapy. After initial imaging is complete and if there are no contraindications, antiplatelet agents are recommended for patients who have had a noncardioembolic TIA. The American Heart Association and American Stroke Association recommend either aspirin, clopidogrel, dipyridamole + aspirin (available in a single capsule [Aggrenox]), or clopidogrel + aspirin as first-line therapy.2,20 The choice of agent needs to be individualized, based on tolerability and adverse effects (TABLE 22,20,21).
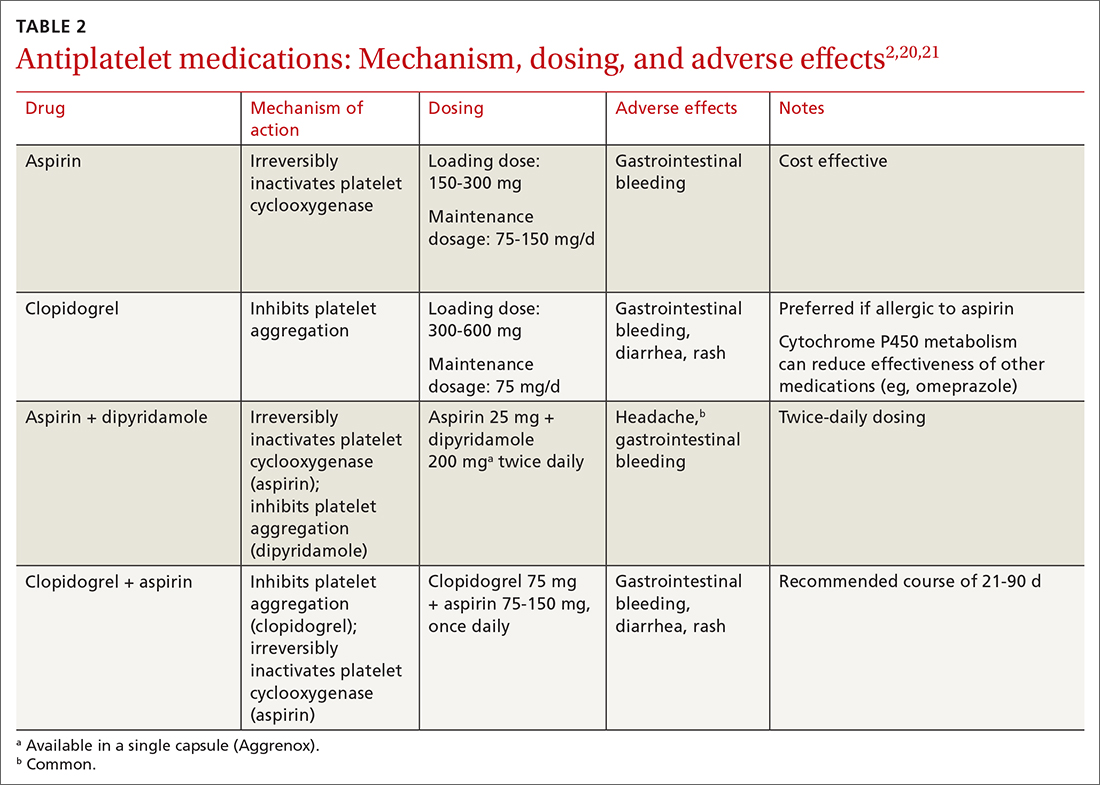
A meta-analysis of antiplatelet therapy reviewed the optimum dosing of each medication.21,22 Reduction of the risk of ischemic stroke with aspirin is 21% to 22% at the optimal dosing of 75 to 150 mg/d, which also reduces the risk of gastrointestinal bleeding.
Continue to: For a patient who has...
For a patient who has an ABCD2 score ≥ 4, has had a prior TIA, or has large-vessel disease, dual antiplatelet therapy is recommended for the first 21 days, with a subsequent return to monotherapy. Dual antiplatelet therapy of clopidogrel + aspirin increases the risk of adverse reactions and has not been shown to have greater long-term benefit23-25 (TABLE 22,20,21).
Step 2: BP management. This is the next immediate step. As many as 80% of patients who present with a TIA have elevated BP upon admission. BP needs to be treated and carefully monitored during this early treatment phase. The recommendation is for a systolic BP < 185 mm Hg and a diastolic BP < 110 mm Hg.24
Step 3: Anticoagulation. Treatment with warfarin or a direct oral anticoagulant (DOAC) is recommended for patients who have the potential for forming emboli—eg, in the setting of atrial fibrillation, ventricular thrombus, mechanical heart valve, or venous thromboembolism.
Step 4. High-intensity statin. A statin agent is recommended as part of immediate and long-term medical management, regardless of the low-density lipoprotein cholesterol (LDL-C) level, to reduce the risk of stroke.2,24
Carotid artery management. Surgical intervention is not always considered a component of immediate medical management. However, guidelines recommend that carotid endarterectomy or stenting be considered in patients who have stenosis > 70%.2
CASE
Mr. L is admitted to the hospital and undergoes neurosurgical intervention. Medical management is instituted.
Long-term management and secondary prevention
The main risk factors for stroke can be divided into modifiable, vascular, and unmodifiable. Addressing both modifiable and vascular risks is important for secondary prevention.
Continue to: Modifiable and vascular risk factors
Modifiable and vascular risk factors
Modifiable risk factors for stroke include hypertension, diabetes, dyslipidemia, smoking, and physical activity; the most important of these, for preventing subsequent stroke after an initial TIA, is hypertension.26
The 2 more significant vascular risk factors for stroke are carotid artery stenosis and atrial fibrillation.
Hypertension. Improving control of hypertension can improve secondary risk reduction for recurrent stroke. Control of both systolic and diastolic BP is important in this regard, with larger systolic BP reductions having a greater impact on decreasing the risk of recurrent stroke.24 Evidence supports lowering BP to improve secondary risk reduction in people with and without diagnosed hypertension: The goal is to lower systolic BP by ≥ 10 mm Hg and diastolic BP by 5 mm Hg.24 No particular class of antihypertensive is recommended in the first line, although preliminary evidence shows that a diuretic, with or without an angiotensin-converting enzyme inhibitor, might be more beneficial than other options.24
Diabetes. The risk of cardiovascular disease, including stroke, is higher in people with diabetes. Evidence shows that various (but not all) agents in 2 pharmaceutical classes—glucagon-like peptide-1 (GLP-1) receptor agonists and the sodium glucose-2 cotransporter (SGLT2) inhibitors—reduce the risk of major cardiovascular events and improve secondary prevention of recurrent stroke:
- EMPA-REG OUTCOME (ClinicalTrials.gov Identifier: NCT01131676) was the first trial to show cardiovascular benefit from an SGLT2 inhibitor (empagliflozin); subsequent studies confirmed the cardiovascular benefits found in EMPA-REG OUTCOME.27,28
- The ELIXA trial (ClinicalTrials.gov Identifier: NCT01147250) was the first to show cardiovascular benefit from a GLP-1 receptor agonist (lixisenatide); subsequent studies supported this finding.29,30
Appropriate agents in these 2 classes should be considered as first-line or adjunctive in patients with both diabetes and known cardiovascular disease, as long as there are no contraindications.27,28
Pioglitazone, a thiazolidinedione-class antidiabetic agent, was once considered a potential option to improve secondary prevention of stroke. However, the thiazolidinediones are generally no longer considered; instead, the SGLT2 inhibitors and GLP-1 receptor agonists are favored.31
Evidence demonstrates the effect of hyperglycemia on cardiovascular events; however, it is important to note that hypoglycemia can result in symptoms and focal changes that mimic a stroke. In addition, some evidence suggests that hypoglycemia can increase cardiovascular risk—thereby supporting the importance of strict control of diabetes and maintenance of euglycemia in reducing overall cardiovascular risk.32
Continue to: Lipids
Lipids. The SPARCL trial (ClinicalTrials.gov Identifier: NCT00147602) was the first study to demonstrate the benefit of high-intensity statin therapy—specifically, atorvastatin 80 mg/d—for secondary prevention for recurrent stroke.33 The recommendation is to use high-intensity statin therapy to decrease the risk of recurrent stroke by reducing the level of LDL-C—by ≥ 50% or to < 70 mg/dL, for maximum risk reduction.24,34
The IMPROVE-IT trial (ClinicalTrials.gov Identifier: NCT00202878) demonstrated the benefit of adding ezetimibe, 10 mg/d, to a moderate-to-high-intensity statin (simvastatin, 40-80 mg/d) to reduce the risk of recurrent stroke.35
Results of recent studies support the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for regulating levels of LDL-C, as an additional option to consider—if needed to further reduce the LDL-C level or if statins are contraindicated in a particular patient.34
Smoking cessation. Cigarette smoking is known to increase the risk of ischemic stroke; newer evidence shows that second-hand exposure to smoke also increases the risk of ischemic stroke.36,37 Although these studies focused on primary prevention of ischemic stroke, the data can reasonably be applied to secondary prevention.38 The recommendation for secondary prevention is to quit smoking and avoid secondhand smoke.24
Alcohol. Evidence demonstrates that heavy alcohol consumption and alcoholism increase the risk of stroke; similar to what is known about smoking, most available data relate to primary prevention.38 The recommendation for providing secondary stroke prevention is to stop or decrease alcohol intake.24
Weight reduction. Obesity (body mass index > 30) increases the risk of ischemic stroke. However, there is, as yet, no evidence that weight loss diminishes the risk of subsequent stroke for secondary prevention.24
Physical activity. Aerobic exercise and strength-training programs after a stroke improve cardiovascular health and mobility. There is no evidence that exercise leads to a reduction in the risk of subsequent stroke.24
Continue to: Nutrition
Nutrition. No current randomized controlled trials are focused on the relationship between diet and recurrent stroke for purposes of prevention; however, evidence for both BP and lipid control incorporate dietary guidance. Recommendations include reducing intake of saturated fats and of sodium (the latter, to < 2.3 g/d) and increasing intake of fruits and vegetables, both of which are beneficial for controlling BP and lipid levels and promoting overall cardiovascular health.38
Carotid artery stenosis. Several randomized controlled trials have demonstrated benefit from treating carotid stenosis (> 70% stenosis but not < 50%) with carotid endarterectomy to reduce the risk of recurrent stroke after TIA.2 The ideal timing of carotid endarterectomy is still being studied; however, available evidence supports intervention within 2 to 6 weeks after TIA or stroke.25 Studies are ongoing that compare carotid angioplasty and stenting against carotid endarterectomy. Medical therapy, with antiplatelet agents and statins, is recommended after carotid endarterectomy.25
Atrial fibrillation increases the risk of recurrent stroke after a TIA, and is the most important indication for secondary stroke prevention with anticoagulation therapy:
- Warfarin. Several studies have shown that warfarin provides a 68% relative risk reduction and a 1.4% absolute risk reduction in the annual stroke rate.24 To achieve this reduction in risk, the optimal international normalized ratio is 2.5 (range, 2-3).24
- Aspirin provides a 13% relative risk reduction for recurrent stroke, although there is evidence that long-term anticoagulation provides more benefit than aspirin after a TIA.39-41 Optimal dosing of aspirin ranges from 75-100 mg/d; greatest benefit is likely in the 12 weeks after stroke, when the risk of recurrent stroke is highest.31,41,42
- DOACs have similar efficacy to warfarin but more rapid onset, lower risk of bleeding, fewer drug interactions, and no requirement for monitoring—often making them a more tolerable long-term choice. Options are rivaroxaban 20 mg/d, dabigatran 150 mg twice daily, apixaban 5 mg twice daily, and edoxaban 60 mg/d.39
When to start anticoagulation and the choice of agent should be weighed against a risk of bleeding, which is highest after the initial stroke. Cost is also a consideration: DOACs are more expensive than warfarin.
CASE
Mr. L is discharged 3 days after carotid endarterectomy and free of residual deficits. He is started on dual antiplatelet therapy (aspirin + clopidogrel) for 21 days, to be followed by a return to monotherapy. He is restarted on a high-intensity statin. He is instructed to resume taking the selective serotonin reuptake inhibitor and melatonin for sleep, as needed. Last, he is told to schedule follow-up with his primary care physician in 7 to 10 days to begin post-stroke care.
Final thoughts
Primary care physicians are often the first point of contact for patients with current or remote TIA symptoms. Based on that provider–patient relationship, evidence supports several recommendations for diagnosing and treating a TIA and for reducing the risk of recurrent stroke after TIA. Addressing each of these areas, in this order, is imperative to reduce the risk of recurrent stroke and improve overall cardiovascular outcomes:
- Obtain an accurate diagnosis of a TIA, using DW-MRI or comparable brain imaging, to allow for prompt intervention.
- Initiate BP management promptly in the acute setting and establish optimal BP control over the long term.
- Begin appropriate antiplatelet therapy.
- When indicated (eg, atrial fibrillation), begin anticoagulation therapy with a DOAC or warfarin.
- Begin high-intensity statin therapy.
- Consider treating patients with diabetes using an SGLT2 inhibitor or GLP-1 receptor agonist.
- Encourage smoking cessation, prescribe quit-smoking medications, or refer a smoker for behavioral support.
Education. Last, it is important to educate patients—especially those who have risk factors for a TIA or stroke—about the presentation of events, so that they know to seek immediate medical attention.
CORRESPONDENCE
Kristen Rundell, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, 655 North Alvernon Way, Suite 228, Tucson, AZ 85711; [email protected]
1. Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720-723. doi: 10.1161/01.STR.0000158917.59233.b7
2. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467. doi: 10.1161/STR.0000000000000375
3. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293. doi: 10.1161/STROKEAHA.108.192218
4. Thacker EL, Wiggins KL, Rice KM, et al. Short-term and long-term risk of incident ischemic stroke after transient ischemic attack. Stroke. 2010;41:239-243. doi: 10.1161/STROKEAHA.109.569707
5. Hill MD, Yiannakoulias N, Jeerakathil T, et al. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015-2020. doi: 10.1212/01.wnl.0000129482.70315.2f
6. Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology. 2011;77:1222-1228. doi: 10.1212/WNL.0b013e3182309f91
7. Cucchiara BL, Kasner SE. All patients should be admitted to the hospital after a transient ischemic attack. Stroke. 2012;43:1446-1447. doi: 10.1161/STROKEAHA.111.636746
8. Amarenco P. Not all patients should be admitted to the hospital for observation after a transient ischemic attack. Stroke. 2012;43:1448-1449. doi: 10.1161/STROKEAHA.111.636753
9. Amort M, Fluri F, Schäfer J, et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc Dis. 2011;32:57-64. doi: 10.1159/000327034
10. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: The Brain Attack Study. Stroke. 2006;37:769-775. doi: 10.1161/01.STR.0000204041.13466.4c
11. Shah KH, Edlow JA. Transient ischemic attack: review for the emergency physician. Ann Emerg Med. 2004;43:592-604. doi: 10.1016/S0196064404000058
12. Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke. 2003;34:932-937. doi: 10.1161/01.STR.0000061496.00669.5E
13. Adams HP Jr, del Zoppo G, Alberts MJ, et al; ; ; ; ; . Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711. doi: 10.1161/STROKEAHA.107.181486
14. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283-292. doi: 10.1016/S0140-6736(07)60150-0
15. Cucchiara BL, Messe SR, Taylor RA, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack? Stroke. 2006;37:1710-1714. doi: 10.1161/01.STR.0000227195.46336.93
16. Amarenco P, Lavallée PC, Labreuche J, et al; . One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542. doi: 10.1056/NEJMoa1412981
17. Wu CM, McLaughlin K, Lorenzetti DL, et al. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167:2417-2422. doi: 10.1001/archinte.167.22.2417
18. Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817-820. doi: 10.1212/01.WNL.0000152985.32732.EE
19. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236. doi: 10.1161/STR.0000000000000024
20. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442. doi: 10.1016/S0140-6736(07)61448-2
21. Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack: an overview of major trials and meta-analyses. Stroke. 2019;50:773-778. doi: c10.1161/STROKEAHA.118.023954
22. Bhatia K, Jain V, Aggarwal D, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: meta-analysis of randomized controlled trials. Stroke. 2021;52:e217-e223. doi: 10.1161/STROKEAHA.120.033033
23. Wang Y, Pan Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: one-year outcomes. Circulation. 2015;132:40-46. doi: 10.1161/CIRCULATIONAHA.114.014791
24. Furie KL, Kasner SE, Adams RJ, et al; . Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276. doi: 10.1161/STR.0b013e3181f7d043
25. Powers WJ, Rabinstein AA, Ackerson T, et al; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. doi: 10.1161/STR.0000000000000158
26. O’Donnell MJ, Chin SL, Rangarajan S, et al; INTERSTROKE Investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761-775. doi: 10.1016/S0140-6736(16)30506-2
27. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. doi:10.1016/S2213-8587(19)30249-9
28. Bertoccini L, Baroni MG. GLP-1 receptor agonists and SGLT2 inhibitors for the treatment of type 2 diabetes: new insights and opportunities for cardiovascular protection. Adv Exp Med Biol. 2021;1307:193-212. doi:10.1007/5584_2020_494
29. Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome N Engl J Med. 2015;373:2247-2257. doi: 10.1056/NEJMoa1509225
30. Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96:156-161. doi:10.1136/postgradmedj-2019-137186
31. Kim AS. Medical management for secondary stroke prevention. Continuum (Minneap Minn). 2020;26:435-456. doi:10.1212/CON.0000000000000849
32. Smith L, Chakraborty D, Bhattacharya P, et al. Exposure to hypoglycemia and risk of stroke. Ann N Y Acad Sci. 2018;1431:25-34. doi:10.1111/nyas.13872
33. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; . High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559. doi:10.1056/NEJMoa061894
34. Castilla-Guerra, L, Fernandez-Moreno M, Leon-Jimenez D, et al. Statins in ischemic stroke prevention: what have we learned in the post-SPARCL (The Stroke Prevention by Aggressive Reduction in Cholesterol Levels) decade? Curr Treat Options Neurol. 2019;21:22. doi: 10.1007/s11940-019-0563-4
35. Bohula EA, Wiviott SD, Giugliano RP, et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2017;136:2440-2450. doi:10.1161/CIRCULATIONAHA.117.029095
36. Moritsugu KP. The 2006 report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 20067;32:542-543. doi: 10.1016/j.amepre.2007.02.026
37. Wolf PA, D’Agostino RB, Kannel WB, et al. Cigarette smoking as a risk factor for stroke: the Framingham Study. JAMA. 1988;259:1025-1029.
38. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583-1633. doi: 10.1161/01.STR.0000223048.70103.F1
39. Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur Stroke J. 2019;4:198-223. doi:10.1177/2396987319841187
40. Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860. doi:10.1016/S0140-6736(09)60503-1
41. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):546S–592S. doi: 10.1378/chest.08-0678
42. Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365-375. doi:10.1016/S0140-6736(16)30468-8
As many as 240,000 people per year in the United States experience a transient ischemic attack (TIA),1,2 which is now defined by the American Heart Association and American Stroke Association as a “transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction.”3 An older definition of TIA was based on the duration of the event (ie, resolution of symptoms at 24 hours); in the updated (2009) definition, the diagnostic criterion is the extent of focal tissue damage.3 Using the 2009 definition might mean a decrease in the number of patients who have a diagnosis of a TIA and an increase in the number who are determined to have had a stroke because an infarction is found on initial imaging.
Guided by the 2009 revised definition of a TIA, we review here the work-up and treatment of TIA, emphasizing immediacy of management to (1) prevent further tissue damage and (2) decrease the risk of a second event.

CASE
Martin L, 69 years old, retired, a nonsmoker, and with a history of peripheral arterial disease and hypercholesterolemia, presents to the emergency department (ED) of a rural hospital complaining of slurred speech and left-side facial numbness. He had an episode of facial numbness that lasted 30 minutes, then resolved, each of the 2 previous evenings; he did not seek care at those times. Now, in the ED, Mr. L is normotensive.
The patient’s medication history includes a selective serotonin reuptake inhibitor and melatonin to improve sleep. He reports having discontinued a statin because he could not tolerate its adverse effects.
What immediate steps are recommended for Mr. L’s care?
Common event callsfor quick action
A TIA is the strongest predictor of subsequent stroke and stroke-related death; the highest period of risk of these devastating outcomes is immediately following a TIA.1,2,4,5 It is essential, therefore, for the physician who sees a patient with a current complaint or recent history of suspected focal neurologic deficits to direct that patient to an ED for an accurate diagnosis and, as appropriate, early treatment for the best possible outcome.
Imaging—preferably, diffusion-weighted magnetic resonance imaging (DW-MRI), the gold standard for diagnosing stroke (see “Diagnosis includes ruling out mimics”)2,3—should be performed as soon as the patient with a suspected TIA arrives in the ED. Imaging should not be held while waiting for a stroke to declare itself—ie, by allowing symptoms to persist for longer than 24 hours. 6
Continue to: Late presentation
Late presentation. Some patients present ≥ 48 hours after onset of early symptoms of a TIA; for them, the work-up is the same as for prompt presentation but can be completed in the outpatient clinic—as long as the patient is stable clinically and imaging is accessible there. DW-MRI should be completed within 48 hours after late presentation. In such cases, the patient should be cautioned regarding risks and any recurrence of symptoms.7,8
Diagnosis includes ruling out mimics
All patients in whom a stroke is suspected should be evaluated on an emergency basis with brain imaging upon arrival at the hospital, before any therapy is initiated. As noted, DW-MRI is the preferred modality; noncontrast computed tomography (CT) or CT angiography can be used if MRI is unavailable.2,3
Mimics. Stroke has many mimics; quickly eliminating them from the differential diagnosis is important so that appropriate therapy can be initiated. Mimics usually have a prolonged presentation of symptoms, whereas the presentation of a TIA is usually abrupt. The 3 more common diagnoses that mimic a TIA are migraine with aura, seizure, and syncope.9,10 Symptoms that generally are not associated with a TIA are chest pain, generalized weakness, and confusion.11 A complete history and physical exam provide the path to the imaging, laboratory, and cardiac testing that is needed to differentiate these diagnoses from a TIA.
A thorough history is best obtained from the patient and a witness, if available, and should include identification of any focal neurologic deficits and the duration and time to resolution of symptoms. Obtain a history of risk factors for ischemia—tobacco use, diabetes, obesity, dyslipidemia, hypertension, previous TIA or stroke, atrial fibrillation, and any coagulopathy. Ask questions about a family history of TIA, stroke, and coagulopathy.11
A comprehensive physical exam, including vital signs, cardiac exam, a check for carotid bruits, and complete neurologic exam, should be performed. Most patients present with concerns for unilateral weakness and changes in speech, which are usually associated with infarction on DW-MRI.12 The most common findings on physical exam include cranial nerve abnormalities, such as diplopia, hemianopia, monocular blindness, disconjugate gaze, facial drooping, lateral tongue movement, dysphagia, and vestibular dysfunction. Cerebellar abnormalities are also often noted, including past pointing, dystaxia, ataxia, nystagmus, and motor abnormalities (eg, spasticity, clonus, or unilateral weakness in the face or extremities).11
Electrocardiography at the bedside can confirm atrial fibrillation or another arrhythmia quickly.
Essential laboratory testing includes measurement of blood glucose and serum electrolytes to determine if these particular imbalances are the cause of symptoms. The presence of a hypercoaguable state is determined by a complete blood count and coagulation studies.3,13 Urine toxicology should also be obtained to rule out other causes of symptoms. A lipid profile is beneficial for making long-term treatment decisions.
Continue to: ABCD2 score
ABCD2 score. Patients who have had a TIA and present within 72 hours after symptoms have resolved should be hospitalized if they have an ABCD2 (Age, Blood pressure [BP], Clinical presentation, Diabetes mellitus [type 1 or 2], Duration of symptoms) prediction system score > 3.14 ABCD2 criteria can be used to help identify patients who are at higher risk of stroke or need further therapy (TABLE 1).14,15

The ABCD2 score is also used to determine whether a patient needs dual antiplatelet therapy. Patients who score at the higher end of the ABCD2 system usually have an increased risk of stroke, longer hospitalization, and greater disability.
CASE
In the ED, Mr. L is immediately assessed and airlifted to a larger regional medical center, where MRI confirms a stroke.
Management
Initial management of a TIA is aimed at reducing the risk of recurrent TIA or stroke. Early medical and possibly surgical treatment are key for preventing stroke and improving outcomes. The first 48 hours after a TIA are the most critical because the incidence of recurrent TIA or stroke is highest during this period.16-18
What is the accepted strategy for early treatment?
Initial treatment must include antiplatelet therapy, BP management, anticoagulation, statin therapy, and carotid endarterectomy as indicated.2,19,20 Control of hypertension and anticoagulation decrease the risk of recurrent stroke by the largest margin20; both are “A”-level Strength of Recommendation Taxonomy interventions.2,3
Step 1: Antiplatelet therapy. After initial imaging is complete and if there are no contraindications, antiplatelet agents are recommended for patients who have had a noncardioembolic TIA. The American Heart Association and American Stroke Association recommend either aspirin, clopidogrel, dipyridamole + aspirin (available in a single capsule [Aggrenox]), or clopidogrel + aspirin as first-line therapy.2,20 The choice of agent needs to be individualized, based on tolerability and adverse effects (TABLE 22,20,21).

A meta-analysis of antiplatelet therapy reviewed the optimum dosing of each medication.21,22 Reduction of the risk of ischemic stroke with aspirin is 21% to 22% at the optimal dosing of 75 to 150 mg/d, which also reduces the risk of gastrointestinal bleeding.
Continue to: For a patient who has...
For a patient who has an ABCD2 score ≥ 4, has had a prior TIA, or has large-vessel disease, dual antiplatelet therapy is recommended for the first 21 days, with a subsequent return to monotherapy. Dual antiplatelet therapy of clopidogrel + aspirin increases the risk of adverse reactions and has not been shown to have greater long-term benefit23-25 (TABLE 22,20,21).
Step 2: BP management. This is the next immediate step. As many as 80% of patients who present with a TIA have elevated BP upon admission. BP needs to be treated and carefully monitored during this early treatment phase. The recommendation is for a systolic BP < 185 mm Hg and a diastolic BP < 110 mm Hg.24
Step 3: Anticoagulation. Treatment with warfarin or a direct oral anticoagulant (DOAC) is recommended for patients who have the potential for forming emboli—eg, in the setting of atrial fibrillation, ventricular thrombus, mechanical heart valve, or venous thromboembolism.
Step 4. High-intensity statin. A statin agent is recommended as part of immediate and long-term medical management, regardless of the low-density lipoprotein cholesterol (LDL-C) level, to reduce the risk of stroke.2,24
Carotid artery management. Surgical intervention is not always considered a component of immediate medical management. However, guidelines recommend that carotid endarterectomy or stenting be considered in patients who have stenosis > 70%.2
CASE
Mr. L is admitted to the hospital and undergoes neurosurgical intervention. Medical management is instituted.
Long-term management and secondary prevention
The main risk factors for stroke can be divided into modifiable, vascular, and unmodifiable. Addressing both modifiable and vascular risks is important for secondary prevention.
Continue to: Modifiable and vascular risk factors
Modifiable and vascular risk factors
Modifiable risk factors for stroke include hypertension, diabetes, dyslipidemia, smoking, and physical activity; the most important of these, for preventing subsequent stroke after an initial TIA, is hypertension.26
The 2 more significant vascular risk factors for stroke are carotid artery stenosis and atrial fibrillation.
Hypertension. Improving control of hypertension can improve secondary risk reduction for recurrent stroke. Control of both systolic and diastolic BP is important in this regard, with larger systolic BP reductions having a greater impact on decreasing the risk of recurrent stroke.24 Evidence supports lowering BP to improve secondary risk reduction in people with and without diagnosed hypertension: The goal is to lower systolic BP by ≥ 10 mm Hg and diastolic BP by 5 mm Hg.24 No particular class of antihypertensive is recommended in the first line, although preliminary evidence shows that a diuretic, with or without an angiotensin-converting enzyme inhibitor, might be more beneficial than other options.24
Diabetes. The risk of cardiovascular disease, including stroke, is higher in people with diabetes. Evidence shows that various (but not all) agents in 2 pharmaceutical classes—glucagon-like peptide-1 (GLP-1) receptor agonists and the sodium glucose-2 cotransporter (SGLT2) inhibitors—reduce the risk of major cardiovascular events and improve secondary prevention of recurrent stroke:
- EMPA-REG OUTCOME (ClinicalTrials.gov Identifier: NCT01131676) was the first trial to show cardiovascular benefit from an SGLT2 inhibitor (empagliflozin); subsequent studies confirmed the cardiovascular benefits found in EMPA-REG OUTCOME.27,28
- The ELIXA trial (ClinicalTrials.gov Identifier: NCT01147250) was the first to show cardiovascular benefit from a GLP-1 receptor agonist (lixisenatide); subsequent studies supported this finding.29,30
Appropriate agents in these 2 classes should be considered as first-line or adjunctive in patients with both diabetes and known cardiovascular disease, as long as there are no contraindications.27,28
Pioglitazone, a thiazolidinedione-class antidiabetic agent, was once considered a potential option to improve secondary prevention of stroke. However, the thiazolidinediones are generally no longer considered; instead, the SGLT2 inhibitors and GLP-1 receptor agonists are favored.31
Evidence demonstrates the effect of hyperglycemia on cardiovascular events; however, it is important to note that hypoglycemia can result in symptoms and focal changes that mimic a stroke. In addition, some evidence suggests that hypoglycemia can increase cardiovascular risk—thereby supporting the importance of strict control of diabetes and maintenance of euglycemia in reducing overall cardiovascular risk.32
Continue to: Lipids
Lipids. The SPARCL trial (ClinicalTrials.gov Identifier: NCT00147602) was the first study to demonstrate the benefit of high-intensity statin therapy—specifically, atorvastatin 80 mg/d—for secondary prevention for recurrent stroke.33 The recommendation is to use high-intensity statin therapy to decrease the risk of recurrent stroke by reducing the level of LDL-C—by ≥ 50% or to < 70 mg/dL, for maximum risk reduction.24,34
The IMPROVE-IT trial (ClinicalTrials.gov Identifier: NCT00202878) demonstrated the benefit of adding ezetimibe, 10 mg/d, to a moderate-to-high-intensity statin (simvastatin, 40-80 mg/d) to reduce the risk of recurrent stroke.35
Results of recent studies support the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for regulating levels of LDL-C, as an additional option to consider—if needed to further reduce the LDL-C level or if statins are contraindicated in a particular patient.34
Smoking cessation. Cigarette smoking is known to increase the risk of ischemic stroke; newer evidence shows that second-hand exposure to smoke also increases the risk of ischemic stroke.36,37 Although these studies focused on primary prevention of ischemic stroke, the data can reasonably be applied to secondary prevention.38 The recommendation for secondary prevention is to quit smoking and avoid secondhand smoke.24
Alcohol. Evidence demonstrates that heavy alcohol consumption and alcoholism increase the risk of stroke; similar to what is known about smoking, most available data relate to primary prevention.38 The recommendation for providing secondary stroke prevention is to stop or decrease alcohol intake.24
Weight reduction. Obesity (body mass index > 30) increases the risk of ischemic stroke. However, there is, as yet, no evidence that weight loss diminishes the risk of subsequent stroke for secondary prevention.24
Physical activity. Aerobic exercise and strength-training programs after a stroke improve cardiovascular health and mobility. There is no evidence that exercise leads to a reduction in the risk of subsequent stroke.24
Continue to: Nutrition
Nutrition. No current randomized controlled trials are focused on the relationship between diet and recurrent stroke for purposes of prevention; however, evidence for both BP and lipid control incorporate dietary guidance. Recommendations include reducing intake of saturated fats and of sodium (the latter, to < 2.3 g/d) and increasing intake of fruits and vegetables, both of which are beneficial for controlling BP and lipid levels and promoting overall cardiovascular health.38
Carotid artery stenosis. Several randomized controlled trials have demonstrated benefit from treating carotid stenosis (> 70% stenosis but not < 50%) with carotid endarterectomy to reduce the risk of recurrent stroke after TIA.2 The ideal timing of carotid endarterectomy is still being studied; however, available evidence supports intervention within 2 to 6 weeks after TIA or stroke.25 Studies are ongoing that compare carotid angioplasty and stenting against carotid endarterectomy. Medical therapy, with antiplatelet agents and statins, is recommended after carotid endarterectomy.25
Atrial fibrillation increases the risk of recurrent stroke after a TIA, and is the most important indication for secondary stroke prevention with anticoagulation therapy:
- Warfarin. Several studies have shown that warfarin provides a 68% relative risk reduction and a 1.4% absolute risk reduction in the annual stroke rate.24 To achieve this reduction in risk, the optimal international normalized ratio is 2.5 (range, 2-3).24
- Aspirin provides a 13% relative risk reduction for recurrent stroke, although there is evidence that long-term anticoagulation provides more benefit than aspirin after a TIA.39-41 Optimal dosing of aspirin ranges from 75-100 mg/d; greatest benefit is likely in the 12 weeks after stroke, when the risk of recurrent stroke is highest.31,41,42
- DOACs have similar efficacy to warfarin but more rapid onset, lower risk of bleeding, fewer drug interactions, and no requirement for monitoring—often making them a more tolerable long-term choice. Options are rivaroxaban 20 mg/d, dabigatran 150 mg twice daily, apixaban 5 mg twice daily, and edoxaban 60 mg/d.39
When to start anticoagulation and the choice of agent should be weighed against a risk of bleeding, which is highest after the initial stroke. Cost is also a consideration: DOACs are more expensive than warfarin.
CASE
Mr. L is discharged 3 days after carotid endarterectomy and free of residual deficits. He is started on dual antiplatelet therapy (aspirin + clopidogrel) for 21 days, to be followed by a return to monotherapy. He is restarted on a high-intensity statin. He is instructed to resume taking the selective serotonin reuptake inhibitor and melatonin for sleep, as needed. Last, he is told to schedule follow-up with his primary care physician in 7 to 10 days to begin post-stroke care.
Final thoughts
Primary care physicians are often the first point of contact for patients with current or remote TIA symptoms. Based on that provider–patient relationship, evidence supports several recommendations for diagnosing and treating a TIA and for reducing the risk of recurrent stroke after TIA. Addressing each of these areas, in this order, is imperative to reduce the risk of recurrent stroke and improve overall cardiovascular outcomes:
- Obtain an accurate diagnosis of a TIA, using DW-MRI or comparable brain imaging, to allow for prompt intervention.
- Initiate BP management promptly in the acute setting and establish optimal BP control over the long term.
- Begin appropriate antiplatelet therapy.
- When indicated (eg, atrial fibrillation), begin anticoagulation therapy with a DOAC or warfarin.
- Begin high-intensity statin therapy.
- Consider treating patients with diabetes using an SGLT2 inhibitor or GLP-1 receptor agonist.
- Encourage smoking cessation, prescribe quit-smoking medications, or refer a smoker for behavioral support.
Education. Last, it is important to educate patients—especially those who have risk factors for a TIA or stroke—about the presentation of events, so that they know to seek immediate medical attention.
CORRESPONDENCE
Kristen Rundell, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, 655 North Alvernon Way, Suite 228, Tucson, AZ 85711; [email protected]
As many as 240,000 people per year in the United States experience a transient ischemic attack (TIA),1,2 which is now defined by the American Heart Association and American Stroke Association as a “transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction.”3 An older definition of TIA was based on the duration of the event (ie, resolution of symptoms at 24 hours); in the updated (2009) definition, the diagnostic criterion is the extent of focal tissue damage.3 Using the 2009 definition might mean a decrease in the number of patients who have a diagnosis of a TIA and an increase in the number who are determined to have had a stroke because an infarction is found on initial imaging.
Guided by the 2009 revised definition of a TIA, we review here the work-up and treatment of TIA, emphasizing immediacy of management to (1) prevent further tissue damage and (2) decrease the risk of a second event.

CASE
Martin L, 69 years old, retired, a nonsmoker, and with a history of peripheral arterial disease and hypercholesterolemia, presents to the emergency department (ED) of a rural hospital complaining of slurred speech and left-side facial numbness. He had an episode of facial numbness that lasted 30 minutes, then resolved, each of the 2 previous evenings; he did not seek care at those times. Now, in the ED, Mr. L is normotensive.
The patient’s medication history includes a selective serotonin reuptake inhibitor and melatonin to improve sleep. He reports having discontinued a statin because he could not tolerate its adverse effects.
What immediate steps are recommended for Mr. L’s care?
Common event callsfor quick action
A TIA is the strongest predictor of subsequent stroke and stroke-related death; the highest period of risk of these devastating outcomes is immediately following a TIA.1,2,4,5 It is essential, therefore, for the physician who sees a patient with a current complaint or recent history of suspected focal neurologic deficits to direct that patient to an ED for an accurate diagnosis and, as appropriate, early treatment for the best possible outcome.
Imaging—preferably, diffusion-weighted magnetic resonance imaging (DW-MRI), the gold standard for diagnosing stroke (see “Diagnosis includes ruling out mimics”)2,3—should be performed as soon as the patient with a suspected TIA arrives in the ED. Imaging should not be held while waiting for a stroke to declare itself—ie, by allowing symptoms to persist for longer than 24 hours. 6
Continue to: Late presentation
Late presentation. Some patients present ≥ 48 hours after onset of early symptoms of a TIA; for them, the work-up is the same as for prompt presentation but can be completed in the outpatient clinic—as long as the patient is stable clinically and imaging is accessible there. DW-MRI should be completed within 48 hours after late presentation. In such cases, the patient should be cautioned regarding risks and any recurrence of symptoms.7,8
Diagnosis includes ruling out mimics
All patients in whom a stroke is suspected should be evaluated on an emergency basis with brain imaging upon arrival at the hospital, before any therapy is initiated. As noted, DW-MRI is the preferred modality; noncontrast computed tomography (CT) or CT angiography can be used if MRI is unavailable.2,3
Mimics. Stroke has many mimics; quickly eliminating them from the differential diagnosis is important so that appropriate therapy can be initiated. Mimics usually have a prolonged presentation of symptoms, whereas the presentation of a TIA is usually abrupt. The 3 more common diagnoses that mimic a TIA are migraine with aura, seizure, and syncope.9,10 Symptoms that generally are not associated with a TIA are chest pain, generalized weakness, and confusion.11 A complete history and physical exam provide the path to the imaging, laboratory, and cardiac testing that is needed to differentiate these diagnoses from a TIA.
A thorough history is best obtained from the patient and a witness, if available, and should include identification of any focal neurologic deficits and the duration and time to resolution of symptoms. Obtain a history of risk factors for ischemia—tobacco use, diabetes, obesity, dyslipidemia, hypertension, previous TIA or stroke, atrial fibrillation, and any coagulopathy. Ask questions about a family history of TIA, stroke, and coagulopathy.11
A comprehensive physical exam, including vital signs, cardiac exam, a check for carotid bruits, and complete neurologic exam, should be performed. Most patients present with concerns for unilateral weakness and changes in speech, which are usually associated with infarction on DW-MRI.12 The most common findings on physical exam include cranial nerve abnormalities, such as diplopia, hemianopia, monocular blindness, disconjugate gaze, facial drooping, lateral tongue movement, dysphagia, and vestibular dysfunction. Cerebellar abnormalities are also often noted, including past pointing, dystaxia, ataxia, nystagmus, and motor abnormalities (eg, spasticity, clonus, or unilateral weakness in the face or extremities).11
Electrocardiography at the bedside can confirm atrial fibrillation or another arrhythmia quickly.
Essential laboratory testing includes measurement of blood glucose and serum electrolytes to determine if these particular imbalances are the cause of symptoms. The presence of a hypercoaguable state is determined by a complete blood count and coagulation studies.3,13 Urine toxicology should also be obtained to rule out other causes of symptoms. A lipid profile is beneficial for making long-term treatment decisions.
Continue to: ABCD2 score
ABCD2 score. Patients who have had a TIA and present within 72 hours after symptoms have resolved should be hospitalized if they have an ABCD2 (Age, Blood pressure [BP], Clinical presentation, Diabetes mellitus [type 1 or 2], Duration of symptoms) prediction system score > 3.14 ABCD2 criteria can be used to help identify patients who are at higher risk of stroke or need further therapy (TABLE 1).14,15

The ABCD2 score is also used to determine whether a patient needs dual antiplatelet therapy. Patients who score at the higher end of the ABCD2 system usually have an increased risk of stroke, longer hospitalization, and greater disability.
CASE
In the ED, Mr. L is immediately assessed and airlifted to a larger regional medical center, where MRI confirms a stroke.
Management
Initial management of a TIA is aimed at reducing the risk of recurrent TIA or stroke. Early medical and possibly surgical treatment are key for preventing stroke and improving outcomes. The first 48 hours after a TIA are the most critical because the incidence of recurrent TIA or stroke is highest during this period.16-18
What is the accepted strategy for early treatment?
Initial treatment must include antiplatelet therapy, BP management, anticoagulation, statin therapy, and carotid endarterectomy as indicated.2,19,20 Control of hypertension and anticoagulation decrease the risk of recurrent stroke by the largest margin20; both are “A”-level Strength of Recommendation Taxonomy interventions.2,3
Step 1: Antiplatelet therapy. After initial imaging is complete and if there are no contraindications, antiplatelet agents are recommended for patients who have had a noncardioembolic TIA. The American Heart Association and American Stroke Association recommend either aspirin, clopidogrel, dipyridamole + aspirin (available in a single capsule [Aggrenox]), or clopidogrel + aspirin as first-line therapy.2,20 The choice of agent needs to be individualized, based on tolerability and adverse effects (TABLE 22,20,21).

A meta-analysis of antiplatelet therapy reviewed the optimum dosing of each medication.21,22 Reduction of the risk of ischemic stroke with aspirin is 21% to 22% at the optimal dosing of 75 to 150 mg/d, which also reduces the risk of gastrointestinal bleeding.
Continue to: For a patient who has...
For a patient who has an ABCD2 score ≥ 4, has had a prior TIA, or has large-vessel disease, dual antiplatelet therapy is recommended for the first 21 days, with a subsequent return to monotherapy. Dual antiplatelet therapy of clopidogrel + aspirin increases the risk of adverse reactions and has not been shown to have greater long-term benefit23-25 (TABLE 22,20,21).
Step 2: BP management. This is the next immediate step. As many as 80% of patients who present with a TIA have elevated BP upon admission. BP needs to be treated and carefully monitored during this early treatment phase. The recommendation is for a systolic BP < 185 mm Hg and a diastolic BP < 110 mm Hg.24
Step 3: Anticoagulation. Treatment with warfarin or a direct oral anticoagulant (DOAC) is recommended for patients who have the potential for forming emboli—eg, in the setting of atrial fibrillation, ventricular thrombus, mechanical heart valve, or venous thromboembolism.
Step 4. High-intensity statin. A statin agent is recommended as part of immediate and long-term medical management, regardless of the low-density lipoprotein cholesterol (LDL-C) level, to reduce the risk of stroke.2,24
Carotid artery management. Surgical intervention is not always considered a component of immediate medical management. However, guidelines recommend that carotid endarterectomy or stenting be considered in patients who have stenosis > 70%.2
CASE
Mr. L is admitted to the hospital and undergoes neurosurgical intervention. Medical management is instituted.
Long-term management and secondary prevention
The main risk factors for stroke can be divided into modifiable, vascular, and unmodifiable. Addressing both modifiable and vascular risks is important for secondary prevention.
Continue to: Modifiable and vascular risk factors
Modifiable and vascular risk factors
Modifiable risk factors for stroke include hypertension, diabetes, dyslipidemia, smoking, and physical activity; the most important of these, for preventing subsequent stroke after an initial TIA, is hypertension.26
The 2 more significant vascular risk factors for stroke are carotid artery stenosis and atrial fibrillation.
Hypertension. Improving control of hypertension can improve secondary risk reduction for recurrent stroke. Control of both systolic and diastolic BP is important in this regard, with larger systolic BP reductions having a greater impact on decreasing the risk of recurrent stroke.24 Evidence supports lowering BP to improve secondary risk reduction in people with and without diagnosed hypertension: The goal is to lower systolic BP by ≥ 10 mm Hg and diastolic BP by 5 mm Hg.24 No particular class of antihypertensive is recommended in the first line, although preliminary evidence shows that a diuretic, with or without an angiotensin-converting enzyme inhibitor, might be more beneficial than other options.24
Diabetes. The risk of cardiovascular disease, including stroke, is higher in people with diabetes. Evidence shows that various (but not all) agents in 2 pharmaceutical classes—glucagon-like peptide-1 (GLP-1) receptor agonists and the sodium glucose-2 cotransporter (SGLT2) inhibitors—reduce the risk of major cardiovascular events and improve secondary prevention of recurrent stroke:
- EMPA-REG OUTCOME (ClinicalTrials.gov Identifier: NCT01131676) was the first trial to show cardiovascular benefit from an SGLT2 inhibitor (empagliflozin); subsequent studies confirmed the cardiovascular benefits found in EMPA-REG OUTCOME.27,28
- The ELIXA trial (ClinicalTrials.gov Identifier: NCT01147250) was the first to show cardiovascular benefit from a GLP-1 receptor agonist (lixisenatide); subsequent studies supported this finding.29,30
Appropriate agents in these 2 classes should be considered as first-line or adjunctive in patients with both diabetes and known cardiovascular disease, as long as there are no contraindications.27,28
Pioglitazone, a thiazolidinedione-class antidiabetic agent, was once considered a potential option to improve secondary prevention of stroke. However, the thiazolidinediones are generally no longer considered; instead, the SGLT2 inhibitors and GLP-1 receptor agonists are favored.31
Evidence demonstrates the effect of hyperglycemia on cardiovascular events; however, it is important to note that hypoglycemia can result in symptoms and focal changes that mimic a stroke. In addition, some evidence suggests that hypoglycemia can increase cardiovascular risk—thereby supporting the importance of strict control of diabetes and maintenance of euglycemia in reducing overall cardiovascular risk.32
Continue to: Lipids
Lipids. The SPARCL trial (ClinicalTrials.gov Identifier: NCT00147602) was the first study to demonstrate the benefit of high-intensity statin therapy—specifically, atorvastatin 80 mg/d—for secondary prevention for recurrent stroke.33 The recommendation is to use high-intensity statin therapy to decrease the risk of recurrent stroke by reducing the level of LDL-C—by ≥ 50% or to < 70 mg/dL, for maximum risk reduction.24,34
The IMPROVE-IT trial (ClinicalTrials.gov Identifier: NCT00202878) demonstrated the benefit of adding ezetimibe, 10 mg/d, to a moderate-to-high-intensity statin (simvastatin, 40-80 mg/d) to reduce the risk of recurrent stroke.35
Results of recent studies support the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for regulating levels of LDL-C, as an additional option to consider—if needed to further reduce the LDL-C level or if statins are contraindicated in a particular patient.34
Smoking cessation. Cigarette smoking is known to increase the risk of ischemic stroke; newer evidence shows that second-hand exposure to smoke also increases the risk of ischemic stroke.36,37 Although these studies focused on primary prevention of ischemic stroke, the data can reasonably be applied to secondary prevention.38 The recommendation for secondary prevention is to quit smoking and avoid secondhand smoke.24
Alcohol. Evidence demonstrates that heavy alcohol consumption and alcoholism increase the risk of stroke; similar to what is known about smoking, most available data relate to primary prevention.38 The recommendation for providing secondary stroke prevention is to stop or decrease alcohol intake.24
Weight reduction. Obesity (body mass index > 30) increases the risk of ischemic stroke. However, there is, as yet, no evidence that weight loss diminishes the risk of subsequent stroke for secondary prevention.24
Physical activity. Aerobic exercise and strength-training programs after a stroke improve cardiovascular health and mobility. There is no evidence that exercise leads to a reduction in the risk of subsequent stroke.24
Continue to: Nutrition
Nutrition. No current randomized controlled trials are focused on the relationship between diet and recurrent stroke for purposes of prevention; however, evidence for both BP and lipid control incorporate dietary guidance. Recommendations include reducing intake of saturated fats and of sodium (the latter, to < 2.3 g/d) and increasing intake of fruits and vegetables, both of which are beneficial for controlling BP and lipid levels and promoting overall cardiovascular health.38
Carotid artery stenosis. Several randomized controlled trials have demonstrated benefit from treating carotid stenosis (> 70% stenosis but not < 50%) with carotid endarterectomy to reduce the risk of recurrent stroke after TIA.2 The ideal timing of carotid endarterectomy is still being studied; however, available evidence supports intervention within 2 to 6 weeks after TIA or stroke.25 Studies are ongoing that compare carotid angioplasty and stenting against carotid endarterectomy. Medical therapy, with antiplatelet agents and statins, is recommended after carotid endarterectomy.25
Atrial fibrillation increases the risk of recurrent stroke after a TIA, and is the most important indication for secondary stroke prevention with anticoagulation therapy:
- Warfarin. Several studies have shown that warfarin provides a 68% relative risk reduction and a 1.4% absolute risk reduction in the annual stroke rate.24 To achieve this reduction in risk, the optimal international normalized ratio is 2.5 (range, 2-3).24
- Aspirin provides a 13% relative risk reduction for recurrent stroke, although there is evidence that long-term anticoagulation provides more benefit than aspirin after a TIA.39-41 Optimal dosing of aspirin ranges from 75-100 mg/d; greatest benefit is likely in the 12 weeks after stroke, when the risk of recurrent stroke is highest.31,41,42
- DOACs have similar efficacy to warfarin but more rapid onset, lower risk of bleeding, fewer drug interactions, and no requirement for monitoring—often making them a more tolerable long-term choice. Options are rivaroxaban 20 mg/d, dabigatran 150 mg twice daily, apixaban 5 mg twice daily, and edoxaban 60 mg/d.39
When to start anticoagulation and the choice of agent should be weighed against a risk of bleeding, which is highest after the initial stroke. Cost is also a consideration: DOACs are more expensive than warfarin.
CASE
Mr. L is discharged 3 days after carotid endarterectomy and free of residual deficits. He is started on dual antiplatelet therapy (aspirin + clopidogrel) for 21 days, to be followed by a return to monotherapy. He is restarted on a high-intensity statin. He is instructed to resume taking the selective serotonin reuptake inhibitor and melatonin for sleep, as needed. Last, he is told to schedule follow-up with his primary care physician in 7 to 10 days to begin post-stroke care.
Final thoughts
Primary care physicians are often the first point of contact for patients with current or remote TIA symptoms. Based on that provider–patient relationship, evidence supports several recommendations for diagnosing and treating a TIA and for reducing the risk of recurrent stroke after TIA. Addressing each of these areas, in this order, is imperative to reduce the risk of recurrent stroke and improve overall cardiovascular outcomes:
- Obtain an accurate diagnosis of a TIA, using DW-MRI or comparable brain imaging, to allow for prompt intervention.
- Initiate BP management promptly in the acute setting and establish optimal BP control over the long term.
- Begin appropriate antiplatelet therapy.
- When indicated (eg, atrial fibrillation), begin anticoagulation therapy with a DOAC or warfarin.
- Begin high-intensity statin therapy.
- Consider treating patients with diabetes using an SGLT2 inhibitor or GLP-1 receptor agonist.
- Encourage smoking cessation, prescribe quit-smoking medications, or refer a smoker for behavioral support.
Education. Last, it is important to educate patients—especially those who have risk factors for a TIA or stroke—about the presentation of events, so that they know to seek immediate medical attention.
CORRESPONDENCE
Kristen Rundell, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, 655 North Alvernon Way, Suite 228, Tucson, AZ 85711; [email protected]
1. Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720-723. doi: 10.1161/01.STR.0000158917.59233.b7
2. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467. doi: 10.1161/STR.0000000000000375
3. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293. doi: 10.1161/STROKEAHA.108.192218
4. Thacker EL, Wiggins KL, Rice KM, et al. Short-term and long-term risk of incident ischemic stroke after transient ischemic attack. Stroke. 2010;41:239-243. doi: 10.1161/STROKEAHA.109.569707
5. Hill MD, Yiannakoulias N, Jeerakathil T, et al. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015-2020. doi: 10.1212/01.wnl.0000129482.70315.2f
6. Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology. 2011;77:1222-1228. doi: 10.1212/WNL.0b013e3182309f91
7. Cucchiara BL, Kasner SE. All patients should be admitted to the hospital after a transient ischemic attack. Stroke. 2012;43:1446-1447. doi: 10.1161/STROKEAHA.111.636746
8. Amarenco P. Not all patients should be admitted to the hospital for observation after a transient ischemic attack. Stroke. 2012;43:1448-1449. doi: 10.1161/STROKEAHA.111.636753
9. Amort M, Fluri F, Schäfer J, et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc Dis. 2011;32:57-64. doi: 10.1159/000327034
10. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: The Brain Attack Study. Stroke. 2006;37:769-775. doi: 10.1161/01.STR.0000204041.13466.4c
11. Shah KH, Edlow JA. Transient ischemic attack: review for the emergency physician. Ann Emerg Med. 2004;43:592-604. doi: 10.1016/S0196064404000058
12. Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke. 2003;34:932-937. doi: 10.1161/01.STR.0000061496.00669.5E
13. Adams HP Jr, del Zoppo G, Alberts MJ, et al; ; ; ; ; . Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711. doi: 10.1161/STROKEAHA.107.181486
14. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283-292. doi: 10.1016/S0140-6736(07)60150-0
15. Cucchiara BL, Messe SR, Taylor RA, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack? Stroke. 2006;37:1710-1714. doi: 10.1161/01.STR.0000227195.46336.93
16. Amarenco P, Lavallée PC, Labreuche J, et al; . One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542. doi: 10.1056/NEJMoa1412981
17. Wu CM, McLaughlin K, Lorenzetti DL, et al. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167:2417-2422. doi: 10.1001/archinte.167.22.2417
18. Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817-820. doi: 10.1212/01.WNL.0000152985.32732.EE
19. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236. doi: 10.1161/STR.0000000000000024
20. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442. doi: 10.1016/S0140-6736(07)61448-2
21. Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack: an overview of major trials and meta-analyses. Stroke. 2019;50:773-778. doi: c10.1161/STROKEAHA.118.023954
22. Bhatia K, Jain V, Aggarwal D, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: meta-analysis of randomized controlled trials. Stroke. 2021;52:e217-e223. doi: 10.1161/STROKEAHA.120.033033
23. Wang Y, Pan Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: one-year outcomes. Circulation. 2015;132:40-46. doi: 10.1161/CIRCULATIONAHA.114.014791
24. Furie KL, Kasner SE, Adams RJ, et al; . Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276. doi: 10.1161/STR.0b013e3181f7d043
25. Powers WJ, Rabinstein AA, Ackerson T, et al; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. doi: 10.1161/STR.0000000000000158
26. O’Donnell MJ, Chin SL, Rangarajan S, et al; INTERSTROKE Investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761-775. doi: 10.1016/S0140-6736(16)30506-2
27. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. doi:10.1016/S2213-8587(19)30249-9
28. Bertoccini L, Baroni MG. GLP-1 receptor agonists and SGLT2 inhibitors for the treatment of type 2 diabetes: new insights and opportunities for cardiovascular protection. Adv Exp Med Biol. 2021;1307:193-212. doi:10.1007/5584_2020_494
29. Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome N Engl J Med. 2015;373:2247-2257. doi: 10.1056/NEJMoa1509225
30. Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96:156-161. doi:10.1136/postgradmedj-2019-137186
31. Kim AS. Medical management for secondary stroke prevention. Continuum (Minneap Minn). 2020;26:435-456. doi:10.1212/CON.0000000000000849
32. Smith L, Chakraborty D, Bhattacharya P, et al. Exposure to hypoglycemia and risk of stroke. Ann N Y Acad Sci. 2018;1431:25-34. doi:10.1111/nyas.13872
33. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; . High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559. doi:10.1056/NEJMoa061894
34. Castilla-Guerra, L, Fernandez-Moreno M, Leon-Jimenez D, et al. Statins in ischemic stroke prevention: what have we learned in the post-SPARCL (The Stroke Prevention by Aggressive Reduction in Cholesterol Levels) decade? Curr Treat Options Neurol. 2019;21:22. doi: 10.1007/s11940-019-0563-4
35. Bohula EA, Wiviott SD, Giugliano RP, et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2017;136:2440-2450. doi:10.1161/CIRCULATIONAHA.117.029095
36. Moritsugu KP. The 2006 report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 20067;32:542-543. doi: 10.1016/j.amepre.2007.02.026
37. Wolf PA, D’Agostino RB, Kannel WB, et al. Cigarette smoking as a risk factor for stroke: the Framingham Study. JAMA. 1988;259:1025-1029.
38. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583-1633. doi: 10.1161/01.STR.0000223048.70103.F1
39. Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur Stroke J. 2019;4:198-223. doi:10.1177/2396987319841187
40. Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860. doi:10.1016/S0140-6736(09)60503-1
41. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):546S–592S. doi: 10.1378/chest.08-0678
42. Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365-375. doi:10.1016/S0140-6736(16)30468-8
1. Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720-723. doi: 10.1161/01.STR.0000158917.59233.b7
2. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467. doi: 10.1161/STR.0000000000000375
3. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293. doi: 10.1161/STROKEAHA.108.192218
4. Thacker EL, Wiggins KL, Rice KM, et al. Short-term and long-term risk of incident ischemic stroke after transient ischemic attack. Stroke. 2010;41:239-243. doi: 10.1161/STROKEAHA.109.569707
5. Hill MD, Yiannakoulias N, Jeerakathil T, et al. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015-2020. doi: 10.1212/01.wnl.0000129482.70315.2f
6. Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology. 2011;77:1222-1228. doi: 10.1212/WNL.0b013e3182309f91
7. Cucchiara BL, Kasner SE. All patients should be admitted to the hospital after a transient ischemic attack. Stroke. 2012;43:1446-1447. doi: 10.1161/STROKEAHA.111.636746
8. Amarenco P. Not all patients should be admitted to the hospital for observation after a transient ischemic attack. Stroke. 2012;43:1448-1449. doi: 10.1161/STROKEAHA.111.636753
9. Amort M, Fluri F, Schäfer J, et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc Dis. 2011;32:57-64. doi: 10.1159/000327034
10. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: The Brain Attack Study. Stroke. 2006;37:769-775. doi: 10.1161/01.STR.0000204041.13466.4c
11. Shah KH, Edlow JA. Transient ischemic attack: review for the emergency physician. Ann Emerg Med. 2004;43:592-604. doi: 10.1016/S0196064404000058
12. Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke. 2003;34:932-937. doi: 10.1161/01.STR.0000061496.00669.5E
13. Adams HP Jr, del Zoppo G, Alberts MJ, et al; ; ; ; ; . Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711. doi: 10.1161/STROKEAHA.107.181486
14. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283-292. doi: 10.1016/S0140-6736(07)60150-0
15. Cucchiara BL, Messe SR, Taylor RA, et al. Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack? Stroke. 2006;37:1710-1714. doi: 10.1161/01.STR.0000227195.46336.93
16. Amarenco P, Lavallée PC, Labreuche J, et al; . One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533-1542. doi: 10.1056/NEJMoa1412981
17. Wu CM, McLaughlin K, Lorenzetti DL, et al. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167:2417-2422. doi: 10.1001/archinte.167.22.2417
18. Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817-820. doi: 10.1212/01.WNL.0000152985.32732.EE
19. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236. doi: 10.1161/STR.0000000000000024
20. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432-1442. doi: 10.1016/S0140-6736(07)61448-2
21. Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack: an overview of major trials and meta-analyses. Stroke. 2019;50:773-778. doi: c10.1161/STROKEAHA.118.023954
22. Bhatia K, Jain V, Aggarwal D, et al. Dual antiplatelet therapy versus aspirin in patients with stroke or transient ischemic attack: meta-analysis of randomized controlled trials. Stroke. 2021;52:e217-e223. doi: 10.1161/STROKEAHA.120.033033
23. Wang Y, Pan Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: one-year outcomes. Circulation. 2015;132:40-46. doi: 10.1161/CIRCULATIONAHA.114.014791
24. Furie KL, Kasner SE, Adams RJ, et al; . Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276. doi: 10.1161/STR.0b013e3181f7d043
25. Powers WJ, Rabinstein AA, Ackerson T, et al; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. doi: 10.1161/STR.0000000000000158
26. O’Donnell MJ, Chin SL, Rangarajan S, et al; INTERSTROKE Investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761-775. doi: 10.1016/S0140-6736(16)30506-2
27. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. doi:10.1016/S2213-8587(19)30249-9
28. Bertoccini L, Baroni MG. GLP-1 receptor agonists and SGLT2 inhibitors for the treatment of type 2 diabetes: new insights and opportunities for cardiovascular protection. Adv Exp Med Biol. 2021;1307:193-212. doi:10.1007/5584_2020_494
29. Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome N Engl J Med. 2015;373:2247-2257. doi: 10.1056/NEJMoa1509225
30. Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96:156-161. doi:10.1136/postgradmedj-2019-137186
31. Kim AS. Medical management for secondary stroke prevention. Continuum (Minneap Minn). 2020;26:435-456. doi:10.1212/CON.0000000000000849
32. Smith L, Chakraborty D, Bhattacharya P, et al. Exposure to hypoglycemia and risk of stroke. Ann N Y Acad Sci. 2018;1431:25-34. doi:10.1111/nyas.13872
33. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; . High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559. doi:10.1056/NEJMoa061894
34. Castilla-Guerra, L, Fernandez-Moreno M, Leon-Jimenez D, et al. Statins in ischemic stroke prevention: what have we learned in the post-SPARCL (The Stroke Prevention by Aggressive Reduction in Cholesterol Levels) decade? Curr Treat Options Neurol. 2019;21:22. doi: 10.1007/s11940-019-0563-4
35. Bohula EA, Wiviott SD, Giugliano RP, et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2017;136:2440-2450. doi:10.1161/CIRCULATIONAHA.117.029095
36. Moritsugu KP. The 2006 report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 20067;32:542-543. doi: 10.1016/j.amepre.2007.02.026
37. Wolf PA, D’Agostino RB, Kannel WB, et al. Cigarette smoking as a risk factor for stroke: the Framingham Study. JAMA. 1988;259:1025-1029.
38. Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583-1633. doi: 10.1161/01.STR.0000223048.70103.F1
39. Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur Stroke J. 2019;4:198-223. doi:10.1177/2396987319841187
40. Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860. doi:10.1016/S0140-6736(09)60503-1
41. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):546S–592S. doi: 10.1378/chest.08-0678
42. Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365-375. doi:10.1016/S0140-6736(16)30468-8
PRACTICE RECOMMENDATIONS
In the hospital, the treating physician should:
› Immediately initiate brain imaging with diffusion-weighted magnetic resonance imaging when TIA is suspected, upon the patient’s arrival at the hospital. A
› Control blood pressure when a TIA is confirmed, to decrease the risk of recurrent stroke. A
› Initiate antiplatelet therapy, to decrease the risk of recurrent stroke. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series