User login
Field Cancerization in Dermatology: Updates on Treatment Considerations and Emerging Therapies
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
Safety Concerns with CGRP Monoclonal Antibodies
Editors’ note: For the April edition of Expert Perspectives, we asked 2 leading neurologists to present differing views on the use of calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) for the treatment of migraine. Here, Lawrence Robbins, MD, of the Robbins Headache Clinic in Riverwoods, IL, discusses potential safety concerns associated with this drug class. To read a counterargument in which Jack D. Schim, MD, of the Neurology Center of Southern California, discusses the observed benefits of CGRP mAbs, click here.
Lawrence Robbins, MD is an associate professor of neurology at Chicago Medical School and is in private practice in Riverwoods, IL.
Dr. Robbins discloses speaker’s bureau remuneration from AbbVie, Amgen, Biohaven, Impel NeuroPharma, Lundbeck, and Teva.
The calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) were introduced in 2018 as efficacious with few adverse effects. Unfortunately, the phase 3 trials failed to indicate the considerable number of adverse effects that have since been identified. This is a common occurrence with new drugs and mAbs.
There are few adverse events identified in the package insert (PI) for any of the 4 CGRP mAbs, but again that is not restricted to this class. Post-approval, it frequently takes time to piece together the true adverse effect profile. Reasons that phase 3 studies may miss adverse effects include 1) The studies are powered for efficacy but are not powered for adverse effects in terms of both the number of patients and the length of the study; 2) Studies do not use a checklist of likely adverse effects; and 3) Adverse effects become “disaggregated” (for example, 1 person states they have malaise, another tiredness, and another fatigue).
In the ensuing years since the launch of the CGRP mAbs, various lines of evidence pointed to the adverse effects attributed to these mAbs. These include the US Food and Drug Administration (FDA)/FDA Adverse Event Reporting System (FAERS) website, published studies, the collective experience of high prescribers, and filtered comments from patient chat boards. In addition, I have received hundreds of letters from providers and patients regarding serious adverse effects, which are detailed further in this article.
As of March 2022, the FDA/FAERS website has listed approximately 50,000 adverse events in connection with the CGRP mAbs. The number of serious events (hospitalization or life-threatening issues) was about 7000. These large numbers, just 3.75 years after launch, are like those listed for onabotulinumtoxin A after 30 years! Most of the reports involve erenumab. This is because erenumab was approved first and is the most prescribed drug in this class. I do not believe it is more dangerous than the others. Considering that the vast majority of adverse events go unreported, these are staggering numbers. Regarding serious adverse events, only 1% to 10% are actually reported to the FDA. Nobody knows the true percentage of milder adverse events that are actually reported, but in my experience, it is extremely low.
Unfortunately, the FDA/FAERS website lists on only adverse events, not adverse effects, which are just as important to discuss. In my small practice I have observed 4 serious adverse effects in women I believe are attributable to CGRP mAbs. These include a cerebrovascular accident in a 21-year-old patient, a case of reversible cerebral vasoconstrictive syndrome in a 61-year-old patient, severe joint pain in a 66-year-old patient, and a constellation of symptoms that resembled multiple sclerosis in a 30-year-old patient. I have administered onabotulinumtoxinA to thousands of patients for 25 years, with no serious adverse effects.
There have been a number of post-approval articles, studies, and case reports published since the launch of the CGRP mAbs. Many “review” or “meta-analysis” articles tend to repeat the results of the pharma-sponsored studies. They typically characterize the CGRP mAbs as safe, with few adverse events. Long-term safety extension studies almost always conclude that the drug is safe. In my opinion, the results from these studies are not reliable because of their possible ties to phrama.
Other studies tell a different tale. One observational study of erenumab concluded that adverse effects contributed to 33% of the discontinuations. Another study reported that 63.3% of patients taking mAbs described at least 1 adverse effect. A study of patients who had been prescribed erenumab indicated that 48% reported a non-serious adverse event after 3 months.
Neurologists are generally unaware of the dangers posed by CGRP mAbs. In September 2021, I engaged in a debate on this topic during the International 15th World Congress on Controversies in Neurology (CONy). Prior to the debate, the audience members were polled. 94% believed the CGRP mAbs to be safe. After our debate, only 40% felt that these mAbs were safe. I think that the audience, primarily consisting of neurologists, was not informed as to the potential dangers of the CGRP mAbs.
In our practice
For refractory patients, I do prescribe these CGRP mAbs. However, I feel they should only be prescribed after several other, safer options have failed. These include the natural approaches (butterbur, magnesium, riboflavin), several of the standard medications (amitriptyline, beta blockers, topiramate, valproate, angiotensin II receptor blockers), and onabotulinumtoxinA. Additionally, the newer gepants for prevention (rimegepant and atogepant) appear to be safer options than the CGRP mAbs, although we cannot say this definitively at this time.
In 2018, our clinic began a retrospective study that lasted until January 2020. We assessed 119 patients with chronic migraine who had been prescribed one of the CGRP mAbs. This study incorporated the use of a checklist of possible adverse effects. Each of these adverse effects had created a “signal.” We initially asked the patients, “Have you experienced any issues, problems, or side effects due to the CGRP monoclonal antibody?” The patients subsequently were interviewed and asked about each possible adverse effect included on the checklist. The patient and physician determined whether any adverse effect mentioned by the patient was due to the CGRP mAb. After discussing the checklist, 66% of the patients concluded they had experienced 1 additional adverse effect that they attributed to the CGRP mAb and had not originally disclosed in response to the initial question. Most of these patients identified more than 1 additional adverse effect through use of the checklist.
Gathering data
To determine the true adverse event profile post-approval, we rely upon the input of high prescribers, who often can provide this necessary feedback. I have assessed input from headache provider chat boards, private correspondence with many providers, and discussions with colleagues at conferences. The opinions do vary, with some headache providers arguing that there are not that many adverse effects arising from the CGRP mAbs. Many others believe, as I do, that there are a large number of adverse events. In my observations, there is not a consensus among headache providers.
The CGRP patient chat boards are another valuable line of evidence. I have screened 2800 comments from patients regarding adverse events. I filtered these down into 490 “highly believable” comments. Among those, the adverse events described align very well with our other lines of evidence.
If we put all the post-approval lines of evidence together, we come up with the following list of “adverse effect signals” attribututed to the CGRP mAbs. These include constipation (it may be severe; hence the warning in the erenumab PI), injection site reactions, joint pain, anxiety, muscle pain or cramps, hypertension or worsening hypertension (there is a warning in the erenumab PI), nausea (it may be severe; “area postrema syndrome” has occurred), rash, increased headache, fatigue, depression, insomnia, hair loss, tachycardia (and other cardiac arrhythmias), stroke, angina and myocardial infarction, weight gain or loss, irritability, and sexual dysfunction. There are also other adverse events. In his review of the CGRP mAbs, Thomas Moore, a leading expert in adverse events, cited the “sheer number of case reports, and it is likely that AEs of this migraine preventive were underestimated in the clinical trials.”
This discussion has focused on short-term adverse events. We have no idea regarding long-term effects, but I suspect that we will encounter serious ones. Evolution has deemed CGRP to be vital for 450 million years. We ignore evolution at our peril.
CGRP is a powerful vasodilator and protects our cardiovascular and cerebrovascular systems. CGRP resists the onset of hypertension. Wound and burn healing, as well as tissue repair, require CGRP. Bony metabolism and bone healing are partly dependent upon CGRP. CGRP protects from gastrointestinal (GI) ulcers and aids GI motility. CGRP mitigates the effects of sepsis.
CGRP is also involved with flushing, thermoregulation, cold hypersensitivity, protecting the kidneys when under stress, helping regulate insulin release, and mediating the adrenal glucocorticoid response to acute stress (particularly in the mature fetus). Effects on the hypothalamic-pituitary-adrenal axis are worrisome but unknown. CGRP is important as a vasodilator during stress, and the CGRP mAbs have not yet been tested under stress.
The CGRP mAbs have been terrific for many patients, and their efficacy is on par with onabotulinumtoxinA. However, in a short period of time we have witnessed a plethora of serious (and non-serious) adverse effects from short-term use. CGRP plays an important role in many physiologic processes. We have no idea as to the long-term consequences of blocking CGRP and we should proceed with caution.
Editors’ note: For the April edition of Expert Perspectives, we asked 2 leading neurologists to present differing views on the use of calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) for the treatment of migraine. Here, Lawrence Robbins, MD, of the Robbins Headache Clinic in Riverwoods, IL, discusses potential safety concerns associated with this drug class. To read a counterargument in which Jack D. Schim, MD, of the Neurology Center of Southern California, discusses the observed benefits of CGRP mAbs, click here.
Lawrence Robbins, MD is an associate professor of neurology at Chicago Medical School and is in private practice in Riverwoods, IL.
Dr. Robbins discloses speaker’s bureau remuneration from AbbVie, Amgen, Biohaven, Impel NeuroPharma, Lundbeck, and Teva.
The calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) were introduced in 2018 as efficacious with few adverse effects. Unfortunately, the phase 3 trials failed to indicate the considerable number of adverse effects that have since been identified. This is a common occurrence with new drugs and mAbs.
There are few adverse events identified in the package insert (PI) for any of the 4 CGRP mAbs, but again that is not restricted to this class. Post-approval, it frequently takes time to piece together the true adverse effect profile. Reasons that phase 3 studies may miss adverse effects include 1) The studies are powered for efficacy but are not powered for adverse effects in terms of both the number of patients and the length of the study; 2) Studies do not use a checklist of likely adverse effects; and 3) Adverse effects become “disaggregated” (for example, 1 person states they have malaise, another tiredness, and another fatigue).
In the ensuing years since the launch of the CGRP mAbs, various lines of evidence pointed to the adverse effects attributed to these mAbs. These include the US Food and Drug Administration (FDA)/FDA Adverse Event Reporting System (FAERS) website, published studies, the collective experience of high prescribers, and filtered comments from patient chat boards. In addition, I have received hundreds of letters from providers and patients regarding serious adverse effects, which are detailed further in this article.
As of March 2022, the FDA/FAERS website has listed approximately 50,000 adverse events in connection with the CGRP mAbs. The number of serious events (hospitalization or life-threatening issues) was about 7000. These large numbers, just 3.75 years after launch, are like those listed for onabotulinumtoxin A after 30 years! Most of the reports involve erenumab. This is because erenumab was approved first and is the most prescribed drug in this class. I do not believe it is more dangerous than the others. Considering that the vast majority of adverse events go unreported, these are staggering numbers. Regarding serious adverse events, only 1% to 10% are actually reported to the FDA. Nobody knows the true percentage of milder adverse events that are actually reported, but in my experience, it is extremely low.
Unfortunately, the FDA/FAERS website lists on only adverse events, not adverse effects, which are just as important to discuss. In my small practice I have observed 4 serious adverse effects in women I believe are attributable to CGRP mAbs. These include a cerebrovascular accident in a 21-year-old patient, a case of reversible cerebral vasoconstrictive syndrome in a 61-year-old patient, severe joint pain in a 66-year-old patient, and a constellation of symptoms that resembled multiple sclerosis in a 30-year-old patient. I have administered onabotulinumtoxinA to thousands of patients for 25 years, with no serious adverse effects.
There have been a number of post-approval articles, studies, and case reports published since the launch of the CGRP mAbs. Many “review” or “meta-analysis” articles tend to repeat the results of the pharma-sponsored studies. They typically characterize the CGRP mAbs as safe, with few adverse events. Long-term safety extension studies almost always conclude that the drug is safe. In my opinion, the results from these studies are not reliable because of their possible ties to phrama.
Other studies tell a different tale. One observational study of erenumab concluded that adverse effects contributed to 33% of the discontinuations. Another study reported that 63.3% of patients taking mAbs described at least 1 adverse effect. A study of patients who had been prescribed erenumab indicated that 48% reported a non-serious adverse event after 3 months.
Neurologists are generally unaware of the dangers posed by CGRP mAbs. In September 2021, I engaged in a debate on this topic during the International 15th World Congress on Controversies in Neurology (CONy). Prior to the debate, the audience members were polled. 94% believed the CGRP mAbs to be safe. After our debate, only 40% felt that these mAbs were safe. I think that the audience, primarily consisting of neurologists, was not informed as to the potential dangers of the CGRP mAbs.
In our practice
For refractory patients, I do prescribe these CGRP mAbs. However, I feel they should only be prescribed after several other, safer options have failed. These include the natural approaches (butterbur, magnesium, riboflavin), several of the standard medications (amitriptyline, beta blockers, topiramate, valproate, angiotensin II receptor blockers), and onabotulinumtoxinA. Additionally, the newer gepants for prevention (rimegepant and atogepant) appear to be safer options than the CGRP mAbs, although we cannot say this definitively at this time.
In 2018, our clinic began a retrospective study that lasted until January 2020. We assessed 119 patients with chronic migraine who had been prescribed one of the CGRP mAbs. This study incorporated the use of a checklist of possible adverse effects. Each of these adverse effects had created a “signal.” We initially asked the patients, “Have you experienced any issues, problems, or side effects due to the CGRP monoclonal antibody?” The patients subsequently were interviewed and asked about each possible adverse effect included on the checklist. The patient and physician determined whether any adverse effect mentioned by the patient was due to the CGRP mAb. After discussing the checklist, 66% of the patients concluded they had experienced 1 additional adverse effect that they attributed to the CGRP mAb and had not originally disclosed in response to the initial question. Most of these patients identified more than 1 additional adverse effect through use of the checklist.
Gathering data
To determine the true adverse event profile post-approval, we rely upon the input of high prescribers, who often can provide this necessary feedback. I have assessed input from headache provider chat boards, private correspondence with many providers, and discussions with colleagues at conferences. The opinions do vary, with some headache providers arguing that there are not that many adverse effects arising from the CGRP mAbs. Many others believe, as I do, that there are a large number of adverse events. In my observations, there is not a consensus among headache providers.
The CGRP patient chat boards are another valuable line of evidence. I have screened 2800 comments from patients regarding adverse events. I filtered these down into 490 “highly believable” comments. Among those, the adverse events described align very well with our other lines of evidence.
If we put all the post-approval lines of evidence together, we come up with the following list of “adverse effect signals” attribututed to the CGRP mAbs. These include constipation (it may be severe; hence the warning in the erenumab PI), injection site reactions, joint pain, anxiety, muscle pain or cramps, hypertension or worsening hypertension (there is a warning in the erenumab PI), nausea (it may be severe; “area postrema syndrome” has occurred), rash, increased headache, fatigue, depression, insomnia, hair loss, tachycardia (and other cardiac arrhythmias), stroke, angina and myocardial infarction, weight gain or loss, irritability, and sexual dysfunction. There are also other adverse events. In his review of the CGRP mAbs, Thomas Moore, a leading expert in adverse events, cited the “sheer number of case reports, and it is likely that AEs of this migraine preventive were underestimated in the clinical trials.”
This discussion has focused on short-term adverse events. We have no idea regarding long-term effects, but I suspect that we will encounter serious ones. Evolution has deemed CGRP to be vital for 450 million years. We ignore evolution at our peril.
CGRP is a powerful vasodilator and protects our cardiovascular and cerebrovascular systems. CGRP resists the onset of hypertension. Wound and burn healing, as well as tissue repair, require CGRP. Bony metabolism and bone healing are partly dependent upon CGRP. CGRP protects from gastrointestinal (GI) ulcers and aids GI motility. CGRP mitigates the effects of sepsis.
CGRP is also involved with flushing, thermoregulation, cold hypersensitivity, protecting the kidneys when under stress, helping regulate insulin release, and mediating the adrenal glucocorticoid response to acute stress (particularly in the mature fetus). Effects on the hypothalamic-pituitary-adrenal axis are worrisome but unknown. CGRP is important as a vasodilator during stress, and the CGRP mAbs have not yet been tested under stress.
The CGRP mAbs have been terrific for many patients, and their efficacy is on par with onabotulinumtoxinA. However, in a short period of time we have witnessed a plethora of serious (and non-serious) adverse effects from short-term use. CGRP plays an important role in many physiologic processes. We have no idea as to the long-term consequences of blocking CGRP and we should proceed with caution.
Editors’ note: For the April edition of Expert Perspectives, we asked 2 leading neurologists to present differing views on the use of calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) for the treatment of migraine. Here, Lawrence Robbins, MD, of the Robbins Headache Clinic in Riverwoods, IL, discusses potential safety concerns associated with this drug class. To read a counterargument in which Jack D. Schim, MD, of the Neurology Center of Southern California, discusses the observed benefits of CGRP mAbs, click here.
Lawrence Robbins, MD is an associate professor of neurology at Chicago Medical School and is in private practice in Riverwoods, IL.
Dr. Robbins discloses speaker’s bureau remuneration from AbbVie, Amgen, Biohaven, Impel NeuroPharma, Lundbeck, and Teva.
The calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) were introduced in 2018 as efficacious with few adverse effects. Unfortunately, the phase 3 trials failed to indicate the considerable number of adverse effects that have since been identified. This is a common occurrence with new drugs and mAbs.
There are few adverse events identified in the package insert (PI) for any of the 4 CGRP mAbs, but again that is not restricted to this class. Post-approval, it frequently takes time to piece together the true adverse effect profile. Reasons that phase 3 studies may miss adverse effects include 1) The studies are powered for efficacy but are not powered for adverse effects in terms of both the number of patients and the length of the study; 2) Studies do not use a checklist of likely adverse effects; and 3) Adverse effects become “disaggregated” (for example, 1 person states they have malaise, another tiredness, and another fatigue).
In the ensuing years since the launch of the CGRP mAbs, various lines of evidence pointed to the adverse effects attributed to these mAbs. These include the US Food and Drug Administration (FDA)/FDA Adverse Event Reporting System (FAERS) website, published studies, the collective experience of high prescribers, and filtered comments from patient chat boards. In addition, I have received hundreds of letters from providers and patients regarding serious adverse effects, which are detailed further in this article.
As of March 2022, the FDA/FAERS website has listed approximately 50,000 adverse events in connection with the CGRP mAbs. The number of serious events (hospitalization or life-threatening issues) was about 7000. These large numbers, just 3.75 years after launch, are like those listed for onabotulinumtoxin A after 30 years! Most of the reports involve erenumab. This is because erenumab was approved first and is the most prescribed drug in this class. I do not believe it is more dangerous than the others. Considering that the vast majority of adverse events go unreported, these are staggering numbers. Regarding serious adverse events, only 1% to 10% are actually reported to the FDA. Nobody knows the true percentage of milder adverse events that are actually reported, but in my experience, it is extremely low.
Unfortunately, the FDA/FAERS website lists on only adverse events, not adverse effects, which are just as important to discuss. In my small practice I have observed 4 serious adverse effects in women I believe are attributable to CGRP mAbs. These include a cerebrovascular accident in a 21-year-old patient, a case of reversible cerebral vasoconstrictive syndrome in a 61-year-old patient, severe joint pain in a 66-year-old patient, and a constellation of symptoms that resembled multiple sclerosis in a 30-year-old patient. I have administered onabotulinumtoxinA to thousands of patients for 25 years, with no serious adverse effects.
There have been a number of post-approval articles, studies, and case reports published since the launch of the CGRP mAbs. Many “review” or “meta-analysis” articles tend to repeat the results of the pharma-sponsored studies. They typically characterize the CGRP mAbs as safe, with few adverse events. Long-term safety extension studies almost always conclude that the drug is safe. In my opinion, the results from these studies are not reliable because of their possible ties to phrama.
Other studies tell a different tale. One observational study of erenumab concluded that adverse effects contributed to 33% of the discontinuations. Another study reported that 63.3% of patients taking mAbs described at least 1 adverse effect. A study of patients who had been prescribed erenumab indicated that 48% reported a non-serious adverse event after 3 months.
Neurologists are generally unaware of the dangers posed by CGRP mAbs. In September 2021, I engaged in a debate on this topic during the International 15th World Congress on Controversies in Neurology (CONy). Prior to the debate, the audience members were polled. 94% believed the CGRP mAbs to be safe. After our debate, only 40% felt that these mAbs were safe. I think that the audience, primarily consisting of neurologists, was not informed as to the potential dangers of the CGRP mAbs.
In our practice
For refractory patients, I do prescribe these CGRP mAbs. However, I feel they should only be prescribed after several other, safer options have failed. These include the natural approaches (butterbur, magnesium, riboflavin), several of the standard medications (amitriptyline, beta blockers, topiramate, valproate, angiotensin II receptor blockers), and onabotulinumtoxinA. Additionally, the newer gepants for prevention (rimegepant and atogepant) appear to be safer options than the CGRP mAbs, although we cannot say this definitively at this time.
In 2018, our clinic began a retrospective study that lasted until January 2020. We assessed 119 patients with chronic migraine who had been prescribed one of the CGRP mAbs. This study incorporated the use of a checklist of possible adverse effects. Each of these adverse effects had created a “signal.” We initially asked the patients, “Have you experienced any issues, problems, or side effects due to the CGRP monoclonal antibody?” The patients subsequently were interviewed and asked about each possible adverse effect included on the checklist. The patient and physician determined whether any adverse effect mentioned by the patient was due to the CGRP mAb. After discussing the checklist, 66% of the patients concluded they had experienced 1 additional adverse effect that they attributed to the CGRP mAb and had not originally disclosed in response to the initial question. Most of these patients identified more than 1 additional adverse effect through use of the checklist.
Gathering data
To determine the true adverse event profile post-approval, we rely upon the input of high prescribers, who often can provide this necessary feedback. I have assessed input from headache provider chat boards, private correspondence with many providers, and discussions with colleagues at conferences. The opinions do vary, with some headache providers arguing that there are not that many adverse effects arising from the CGRP mAbs. Many others believe, as I do, that there are a large number of adverse events. In my observations, there is not a consensus among headache providers.
The CGRP patient chat boards are another valuable line of evidence. I have screened 2800 comments from patients regarding adverse events. I filtered these down into 490 “highly believable” comments. Among those, the adverse events described align very well with our other lines of evidence.
If we put all the post-approval lines of evidence together, we come up with the following list of “adverse effect signals” attribututed to the CGRP mAbs. These include constipation (it may be severe; hence the warning in the erenumab PI), injection site reactions, joint pain, anxiety, muscle pain or cramps, hypertension or worsening hypertension (there is a warning in the erenumab PI), nausea (it may be severe; “area postrema syndrome” has occurred), rash, increased headache, fatigue, depression, insomnia, hair loss, tachycardia (and other cardiac arrhythmias), stroke, angina and myocardial infarction, weight gain or loss, irritability, and sexual dysfunction. There are also other adverse events. In his review of the CGRP mAbs, Thomas Moore, a leading expert in adverse events, cited the “sheer number of case reports, and it is likely that AEs of this migraine preventive were underestimated in the clinical trials.”
This discussion has focused on short-term adverse events. We have no idea regarding long-term effects, but I suspect that we will encounter serious ones. Evolution has deemed CGRP to be vital for 450 million years. We ignore evolution at our peril.
CGRP is a powerful vasodilator and protects our cardiovascular and cerebrovascular systems. CGRP resists the onset of hypertension. Wound and burn healing, as well as tissue repair, require CGRP. Bony metabolism and bone healing are partly dependent upon CGRP. CGRP protects from gastrointestinal (GI) ulcers and aids GI motility. CGRP mitigates the effects of sepsis.
CGRP is also involved with flushing, thermoregulation, cold hypersensitivity, protecting the kidneys when under stress, helping regulate insulin release, and mediating the adrenal glucocorticoid response to acute stress (particularly in the mature fetus). Effects on the hypothalamic-pituitary-adrenal axis are worrisome but unknown. CGRP is important as a vasodilator during stress, and the CGRP mAbs have not yet been tested under stress.
The CGRP mAbs have been terrific for many patients, and their efficacy is on par with onabotulinumtoxinA. However, in a short period of time we have witnessed a plethora of serious (and non-serious) adverse effects from short-term use. CGRP plays an important role in many physiologic processes. We have no idea as to the long-term consequences of blocking CGRP and we should proceed with caution.
CGRPs: They’ve Been a Long Time Coming
Editors’ note: For the April edition of Expert Perspectives, we asked two leading neurologists to present differing views on the use of calcitonin gene-related peptide (CGRPs) monoclonal antibodies (mAbs) for the treatment of migraine. Here, Jack D. Schim, MD, of the Neurology Center of Southern California, discusses the observed benefits of the CGRP mAbs. To read a counter-argument in which Lawrence Robbins, MD, of the Robbins Headache Clinic in Riverwoods, IL, discusses potential safety concerns associated with this drug class, click here.
Dr. Schim is Co-Director of The Headache Center of Southern California, The Neurology Center. He is Board Member and past President of the Headache Consortium of the Pacific, Board Member and past President, American Heart Association San Diego, a Past President of the California Neurologic Society, and an active member of the American Academy of Neurology, American Stroke Association, and American Headache Society.
The identification of CGRP as a crucial pain signaling molecule in the trigeminal pathway, thereby establishing its link to migraine pain, has transformed care for patients with episodic and chronic migraine. Since 2018, the FDA has approved 4 monoclonal antibodies (mAbs) that either block the CGRP receptor or bind its ligand to prevent attachment to the receptor.
These medications are helping patients with medication overuse headache (MOH) and chronic migraine; one study involving 139 patients showed that half the patients saw their headache days per month cut by half, and migraine days per month cut by 62%. In another small study, 23 patients (47%) with medication overuse headache reported having no MOH issues after 3 months. Most of these patients had a history of medication overuse, but no specific diagnosis of MOH.
In a survey, 277 physicians said that nearly half of their patients had resistant migraine, and 29% had refractory migraine. For these patients, the mAbs have helped restore some normalcy in their lives.
But are these medications safe? Some clinicians posed this question even before the 2018 approval.
It is a valid question. The CGRP neuropeptide is a powerful dilator, establishing vascular homeostasis, organ development in utero, wound healing, and more. It is expressed in the peripheral and central nervous system and found abundantly in neurons and the unmyelinated A-fibers of the peripheral trigeminovascular system and trigeminal ganglion.
So, would blocking CGRP function in one area affect its function in another? So far, I have to say the answer is no. Neither the literature nor my observations say otherwise.
Trials vs the real world
Finding an answer to this question takes more than reading clinical trials data. Participants in these trials do not necessarily represent the real world – no complicated morbidities, no pregnant or lactating women. And trial lengths are generally short.
Thus, it is important to assess the safety of these medications in the real world.
In the trials of the subcutaneous mAbs, local injection site reactions were the most common adverse event. More specifically, erenumab showed increased incidence of constipation, and post-marketing surveillance has revealed some risk of hypertension; pooled analysis from 4 trial phases showed that across treatment groups, 20 people out of 2443 began treatment for hypertension. Some patients enrolled in trials for atogepant, an oral small molecule CGRP receptor agonist, also reported constipation and nausea, suggesting that receptor blockade may result in a higher incidence of GI disturbance. In addition, these medicines on occasion have caused alopecia, fatigue, or achiness.
Eptinezumab is administered intravenously, every 3 months, and thus does not have injection site reactions as a safety concern. In clinical trials, the most common adverse event was nasopharyngitis and hypersensitivity; Datta et al have provided a summary of safety and efficacy.
Raynaud’s and cluster headaches
A retrospective chart review study from the Mayo Clinic looking at individuals with Raynaud’s disease who were treated with CGRP antagonists showed that 5.3% of 169 patients had microvascular complications such as gangrene or autoimmune necrosis. There was no significant difference in demographic characteristics or rheumatologic history among those with Raynaud's who did or did not experience complications. In addition, microvascular complications of migraine therapies have preceded the use of CGRP modulators, as this has been documented in the past with other vasoactive substances such as ergots, triptans, and beta blockers.
In a tolerability and safety study of galcanezumab in patients with chronic cluster headache, with up to 15 months of treatment, the most common treatment emergent adverse events were nasopharyngitis and injection site pain. In this population with 11% to 12% of individuals having baseline hypertension and nearly 63% currently using tobacco, less than 2.5% had any abnormalities on ECG.
Vascular complications including pulmonary embolism, TIA, myocardial infarction, and atrial fibrillation have been reported but with no apparent relation between galcanezumab dosing and onset, with onset following the second to up to the eleventh monthly dose.
Antidrug antibodies can also occur as a complication of exposure to therapeutic antibodies of this class and have been detected in up to 12% of individuals treated. This might lead to therapeutic failure but would not likely be a safety issue.
Anaphylaxis or serious hypersensitivity reactions are uncommon, and in general are the only contraindication to the use of these agents.
Pregnancy registry information is just starting to become available. The number of reports on adverse drug reactions remains limited, and thus it is wise to avoid pregnancy and breastfeeding exposures as best as possible.
FAERS
The FDA adverse event reporting system (FAERS) database does contain reports on adverse events of approved medications. However, even the FDA website warns that the information provided regarding individual cases is unverified and wouldn’t establish causation, considering the confounding variables involved, and this information can be duplicated as well as incomplete. In addition, rates of occurrence cannot be established with reports, as the denominator of exposure is uncertain.
With those caveats established, the most common reports in FAERS concern injection site reactions, more frequent migraine or headache, or drug ineffectiveness. While constipation has been the second most common AE for erenumab, it did not make the top 10 for fremanezumab or galcanezumab, and cardiovascular events have not ranked in the top 10 for any product. Reasons for discontinuing treatment included withdrawal by the patient (147 of 1,890 [8%]) and lack of efficacy (77 of 1,890 [4%]).
As the reader can ascertain, different studies point out different reasons for discontinuing CGRP therapies. Other studies note that the most frequent reasons for discontinuation are lack of efficacy, constipation, and lack of insurance .
Conclusions
These medications have been well received; I believe. Fremanezumab, manufactured by Teva Pharmaceuticals, was approved in September 2018. According to Teva’s 2021 annual report, Ajovy, fremanezumab’s brand name, reached 21% market share in the US and 21% in Europe.
Aimovig, or erenumab, has been prescribed 620,000 times since its approval, according to the Novartis 2021 annual report. Galcanezumab, or Emgality, was approved in 2019, and had 65,300 prescriptions written in the since then. . Adherence to treatment has generally been very high in clinical practice, with the most common reason for a patient to switch being lack of effectiveness, rather than adverse events.
Of note, adverse events of other migraine therapeutics are not insignificant, and considering the pain that people with migraine endure, side effects must be hard to contend with for individuals to stop taking them. Case in point: A year before erenumab was approved, a study was published that looked at medical records for medication persistence in 8,700 chronic migraine patients, who all had been prescribed beta blockers, anti-seizure medications, or antidepressants. By 6 months, only 1 out of 4 patients were still taking the prescribed medicines. By 12 months, that was down to 14%. And about one-third of patients who stopped treatment stayed untreated for at least a year.
The presumed reasons these patients stopped their medications: side effects and-or lack of efficacy.
Migraine is a disabling disorder, for which the mAb class of medications has been highly beneficial for large numbers of patients, with far greater tolerability than prior oral preventives. While no treatments have absolute safety, the overall safety of this class of medications has been very high, leading to much improved clinical outcomes.
Editors’ note: For the April edition of Expert Perspectives, we asked two leading neurologists to present differing views on the use of calcitonin gene-related peptide (CGRPs) monoclonal antibodies (mAbs) for the treatment of migraine. Here, Jack D. Schim, MD, of the Neurology Center of Southern California, discusses the observed benefits of the CGRP mAbs. To read a counter-argument in which Lawrence Robbins, MD, of the Robbins Headache Clinic in Riverwoods, IL, discusses potential safety concerns associated with this drug class, click here.
Dr. Schim is Co-Director of The Headache Center of Southern California, The Neurology Center. He is Board Member and past President of the Headache Consortium of the Pacific, Board Member and past President, American Heart Association San Diego, a Past President of the California Neurologic Society, and an active member of the American Academy of Neurology, American Stroke Association, and American Headache Society.
The identification of CGRP as a crucial pain signaling molecule in the trigeminal pathway, thereby establishing its link to migraine pain, has transformed care for patients with episodic and chronic migraine. Since 2018, the FDA has approved 4 monoclonal antibodies (mAbs) that either block the CGRP receptor or bind its ligand to prevent attachment to the receptor.
These medications are helping patients with medication overuse headache (MOH) and chronic migraine; one study involving 139 patients showed that half the patients saw their headache days per month cut by half, and migraine days per month cut by 62%. In another small study, 23 patients (47%) with medication overuse headache reported having no MOH issues after 3 months. Most of these patients had a history of medication overuse, but no specific diagnosis of MOH.
In a survey, 277 physicians said that nearly half of their patients had resistant migraine, and 29% had refractory migraine. For these patients, the mAbs have helped restore some normalcy in their lives.
But are these medications safe? Some clinicians posed this question even before the 2018 approval.
It is a valid question. The CGRP neuropeptide is a powerful dilator, establishing vascular homeostasis, organ development in utero, wound healing, and more. It is expressed in the peripheral and central nervous system and found abundantly in neurons and the unmyelinated A-fibers of the peripheral trigeminovascular system and trigeminal ganglion.
So, would blocking CGRP function in one area affect its function in another? So far, I have to say the answer is no. Neither the literature nor my observations say otherwise.
Trials vs the real world
Finding an answer to this question takes more than reading clinical trials data. Participants in these trials do not necessarily represent the real world – no complicated morbidities, no pregnant or lactating women. And trial lengths are generally short.
Thus, it is important to assess the safety of these medications in the real world.
In the trials of the subcutaneous mAbs, local injection site reactions were the most common adverse event. More specifically, erenumab showed increased incidence of constipation, and post-marketing surveillance has revealed some risk of hypertension; pooled analysis from 4 trial phases showed that across treatment groups, 20 people out of 2443 began treatment for hypertension. Some patients enrolled in trials for atogepant, an oral small molecule CGRP receptor agonist, also reported constipation and nausea, suggesting that receptor blockade may result in a higher incidence of GI disturbance. In addition, these medicines on occasion have caused alopecia, fatigue, or achiness.
Eptinezumab is administered intravenously, every 3 months, and thus does not have injection site reactions as a safety concern. In clinical trials, the most common adverse event was nasopharyngitis and hypersensitivity; Datta et al have provided a summary of safety and efficacy.
Raynaud’s and cluster headaches
A retrospective chart review study from the Mayo Clinic looking at individuals with Raynaud’s disease who were treated with CGRP antagonists showed that 5.3% of 169 patients had microvascular complications such as gangrene or autoimmune necrosis. There was no significant difference in demographic characteristics or rheumatologic history among those with Raynaud's who did or did not experience complications. In addition, microvascular complications of migraine therapies have preceded the use of CGRP modulators, as this has been documented in the past with other vasoactive substances such as ergots, triptans, and beta blockers.
In a tolerability and safety study of galcanezumab in patients with chronic cluster headache, with up to 15 months of treatment, the most common treatment emergent adverse events were nasopharyngitis and injection site pain. In this population with 11% to 12% of individuals having baseline hypertension and nearly 63% currently using tobacco, less than 2.5% had any abnormalities on ECG.
Vascular complications including pulmonary embolism, TIA, myocardial infarction, and atrial fibrillation have been reported but with no apparent relation between galcanezumab dosing and onset, with onset following the second to up to the eleventh monthly dose.
Antidrug antibodies can also occur as a complication of exposure to therapeutic antibodies of this class and have been detected in up to 12% of individuals treated. This might lead to therapeutic failure but would not likely be a safety issue.
Anaphylaxis or serious hypersensitivity reactions are uncommon, and in general are the only contraindication to the use of these agents.
Pregnancy registry information is just starting to become available. The number of reports on adverse drug reactions remains limited, and thus it is wise to avoid pregnancy and breastfeeding exposures as best as possible.
FAERS
The FDA adverse event reporting system (FAERS) database does contain reports on adverse events of approved medications. However, even the FDA website warns that the information provided regarding individual cases is unverified and wouldn’t establish causation, considering the confounding variables involved, and this information can be duplicated as well as incomplete. In addition, rates of occurrence cannot be established with reports, as the denominator of exposure is uncertain.
With those caveats established, the most common reports in FAERS concern injection site reactions, more frequent migraine or headache, or drug ineffectiveness. While constipation has been the second most common AE for erenumab, it did not make the top 10 for fremanezumab or galcanezumab, and cardiovascular events have not ranked in the top 10 for any product. Reasons for discontinuing treatment included withdrawal by the patient (147 of 1,890 [8%]) and lack of efficacy (77 of 1,890 [4%]).
As the reader can ascertain, different studies point out different reasons for discontinuing CGRP therapies. Other studies note that the most frequent reasons for discontinuation are lack of efficacy, constipation, and lack of insurance .
Conclusions
These medications have been well received; I believe. Fremanezumab, manufactured by Teva Pharmaceuticals, was approved in September 2018. According to Teva’s 2021 annual report, Ajovy, fremanezumab’s brand name, reached 21% market share in the US and 21% in Europe.
Aimovig, or erenumab, has been prescribed 620,000 times since its approval, according to the Novartis 2021 annual report. Galcanezumab, or Emgality, was approved in 2019, and had 65,300 prescriptions written in the since then. . Adherence to treatment has generally been very high in clinical practice, with the most common reason for a patient to switch being lack of effectiveness, rather than adverse events.
Of note, adverse events of other migraine therapeutics are not insignificant, and considering the pain that people with migraine endure, side effects must be hard to contend with for individuals to stop taking them. Case in point: A year before erenumab was approved, a study was published that looked at medical records for medication persistence in 8,700 chronic migraine patients, who all had been prescribed beta blockers, anti-seizure medications, or antidepressants. By 6 months, only 1 out of 4 patients were still taking the prescribed medicines. By 12 months, that was down to 14%. And about one-third of patients who stopped treatment stayed untreated for at least a year.
The presumed reasons these patients stopped their medications: side effects and-or lack of efficacy.
Migraine is a disabling disorder, for which the mAb class of medications has been highly beneficial for large numbers of patients, with far greater tolerability than prior oral preventives. While no treatments have absolute safety, the overall safety of this class of medications has been very high, leading to much improved clinical outcomes.
Editors’ note: For the April edition of Expert Perspectives, we asked two leading neurologists to present differing views on the use of calcitonin gene-related peptide (CGRPs) monoclonal antibodies (mAbs) for the treatment of migraine. Here, Jack D. Schim, MD, of the Neurology Center of Southern California, discusses the observed benefits of the CGRP mAbs. To read a counter-argument in which Lawrence Robbins, MD, of the Robbins Headache Clinic in Riverwoods, IL, discusses potential safety concerns associated with this drug class, click here.
Dr. Schim is Co-Director of The Headache Center of Southern California, The Neurology Center. He is Board Member and past President of the Headache Consortium of the Pacific, Board Member and past President, American Heart Association San Diego, a Past President of the California Neurologic Society, and an active member of the American Academy of Neurology, American Stroke Association, and American Headache Society.
The identification of CGRP as a crucial pain signaling molecule in the trigeminal pathway, thereby establishing its link to migraine pain, has transformed care for patients with episodic and chronic migraine. Since 2018, the FDA has approved 4 monoclonal antibodies (mAbs) that either block the CGRP receptor or bind its ligand to prevent attachment to the receptor.
These medications are helping patients with medication overuse headache (MOH) and chronic migraine; one study involving 139 patients showed that half the patients saw their headache days per month cut by half, and migraine days per month cut by 62%. In another small study, 23 patients (47%) with medication overuse headache reported having no MOH issues after 3 months. Most of these patients had a history of medication overuse, but no specific diagnosis of MOH.
In a survey, 277 physicians said that nearly half of their patients had resistant migraine, and 29% had refractory migraine. For these patients, the mAbs have helped restore some normalcy in their lives.
But are these medications safe? Some clinicians posed this question even before the 2018 approval.
It is a valid question. The CGRP neuropeptide is a powerful dilator, establishing vascular homeostasis, organ development in utero, wound healing, and more. It is expressed in the peripheral and central nervous system and found abundantly in neurons and the unmyelinated A-fibers of the peripheral trigeminovascular system and trigeminal ganglion.
So, would blocking CGRP function in one area affect its function in another? So far, I have to say the answer is no. Neither the literature nor my observations say otherwise.
Trials vs the real world
Finding an answer to this question takes more than reading clinical trials data. Participants in these trials do not necessarily represent the real world – no complicated morbidities, no pregnant or lactating women. And trial lengths are generally short.
Thus, it is important to assess the safety of these medications in the real world.
In the trials of the subcutaneous mAbs, local injection site reactions were the most common adverse event. More specifically, erenumab showed increased incidence of constipation, and post-marketing surveillance has revealed some risk of hypertension; pooled analysis from 4 trial phases showed that across treatment groups, 20 people out of 2443 began treatment for hypertension. Some patients enrolled in trials for atogepant, an oral small molecule CGRP receptor agonist, also reported constipation and nausea, suggesting that receptor blockade may result in a higher incidence of GI disturbance. In addition, these medicines on occasion have caused alopecia, fatigue, or achiness.
Eptinezumab is administered intravenously, every 3 months, and thus does not have injection site reactions as a safety concern. In clinical trials, the most common adverse event was nasopharyngitis and hypersensitivity; Datta et al have provided a summary of safety and efficacy.
Raynaud’s and cluster headaches
A retrospective chart review study from the Mayo Clinic looking at individuals with Raynaud’s disease who were treated with CGRP antagonists showed that 5.3% of 169 patients had microvascular complications such as gangrene or autoimmune necrosis. There was no significant difference in demographic characteristics or rheumatologic history among those with Raynaud's who did or did not experience complications. In addition, microvascular complications of migraine therapies have preceded the use of CGRP modulators, as this has been documented in the past with other vasoactive substances such as ergots, triptans, and beta blockers.
In a tolerability and safety study of galcanezumab in patients with chronic cluster headache, with up to 15 months of treatment, the most common treatment emergent adverse events were nasopharyngitis and injection site pain. In this population with 11% to 12% of individuals having baseline hypertension and nearly 63% currently using tobacco, less than 2.5% had any abnormalities on ECG.
Vascular complications including pulmonary embolism, TIA, myocardial infarction, and atrial fibrillation have been reported but with no apparent relation between galcanezumab dosing and onset, with onset following the second to up to the eleventh monthly dose.
Antidrug antibodies can also occur as a complication of exposure to therapeutic antibodies of this class and have been detected in up to 12% of individuals treated. This might lead to therapeutic failure but would not likely be a safety issue.
Anaphylaxis or serious hypersensitivity reactions are uncommon, and in general are the only contraindication to the use of these agents.
Pregnancy registry information is just starting to become available. The number of reports on adverse drug reactions remains limited, and thus it is wise to avoid pregnancy and breastfeeding exposures as best as possible.
FAERS
The FDA adverse event reporting system (FAERS) database does contain reports on adverse events of approved medications. However, even the FDA website warns that the information provided regarding individual cases is unverified and wouldn’t establish causation, considering the confounding variables involved, and this information can be duplicated as well as incomplete. In addition, rates of occurrence cannot be established with reports, as the denominator of exposure is uncertain.
With those caveats established, the most common reports in FAERS concern injection site reactions, more frequent migraine or headache, or drug ineffectiveness. While constipation has been the second most common AE for erenumab, it did not make the top 10 for fremanezumab or galcanezumab, and cardiovascular events have not ranked in the top 10 for any product. Reasons for discontinuing treatment included withdrawal by the patient (147 of 1,890 [8%]) and lack of efficacy (77 of 1,890 [4%]).
As the reader can ascertain, different studies point out different reasons for discontinuing CGRP therapies. Other studies note that the most frequent reasons for discontinuation are lack of efficacy, constipation, and lack of insurance .
Conclusions
These medications have been well received; I believe. Fremanezumab, manufactured by Teva Pharmaceuticals, was approved in September 2018. According to Teva’s 2021 annual report, Ajovy, fremanezumab’s brand name, reached 21% market share in the US and 21% in Europe.
Aimovig, or erenumab, has been prescribed 620,000 times since its approval, according to the Novartis 2021 annual report. Galcanezumab, or Emgality, was approved in 2019, and had 65,300 prescriptions written in the since then. . Adherence to treatment has generally been very high in clinical practice, with the most common reason for a patient to switch being lack of effectiveness, rather than adverse events.
Of note, adverse events of other migraine therapeutics are not insignificant, and considering the pain that people with migraine endure, side effects must be hard to contend with for individuals to stop taking them. Case in point: A year before erenumab was approved, a study was published that looked at medical records for medication persistence in 8,700 chronic migraine patients, who all had been prescribed beta blockers, anti-seizure medications, or antidepressants. By 6 months, only 1 out of 4 patients were still taking the prescribed medicines. By 12 months, that was down to 14%. And about one-third of patients who stopped treatment stayed untreated for at least a year.
The presumed reasons these patients stopped their medications: side effects and-or lack of efficacy.
Migraine is a disabling disorder, for which the mAb class of medications has been highly beneficial for large numbers of patients, with far greater tolerability than prior oral preventives. While no treatments have absolute safety, the overall safety of this class of medications has been very high, leading to much improved clinical outcomes.
Natriuretic Peptide Screening for Primary Prevention or Early Detection of Heart Failure: A Pharmacist-Driven Team-Based Approach
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
Heart failure (HF) is one of the leading causes of hospitalizations and the most expensive Medicare diagnosis. Its prevalence continues to rise with a projected increase of 46% from 2012 to 2030 resulting in > 8 million people aged ≥ 18 years with HF in the United States. Despite improvements in therapy, mortality remains unacceptably high with a 50% mortality rate within 5 years. Early detection strategies are needed to identify patients at risk of developing HF to delay the disease course and improve survival.1,2
Emerging data indicates that natriuretic peptide biomarker-based screening using B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) and early intervention for patients at risk of HF could prevent development of left ventricular dysfunction or new-onset HF.3-5 The 2013 St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial is the largest study to date to evaluate BNP as a screening tool for patients at risk for HF.4 Patients at risk of HF who did not have established left ventricular systolic dysfunction or symptomatic HF were assigned randomly to usual primary care or BNP screening. Patients with BNP levels ≥ 50 pg/mL underwent echocardiogram and were referred to a cardiovascular specialty service for management. The cardiovascular specialty clinic included a team of registered nurses, nurse practitioners, pharmacists, dieticians, palliative care specialists, and cardiologists. Individuals in the intervention group showed increased renin-angiotensin system (RAS) inhibitor use at follow-up (control, 49.6%; intervention, 59.6%; P = .01). All patients received coaching by a nurse who emphasized individual risk, importance of medication adherence, and healthy lifestyle behaviors. After a mean follow-up of 4.2 years, 59 of 677 participants (8.7%) in the control group and 37 of 697 (5.3%) in the intervention group (odds ratio [OR], 0.55; 95% CI, 0.37 to 0.82; P = .003) met the primary end point of left ventricular dysfunction with or without HF. BNP-based screening in conjunction with collaborative care reduced rates of left ventricular dysfunction and HF.
In the 2013 PONTIAC trial, patients with type 2 diabetes mellitus (T2DM) without cardiac disease but with NT-proBNP levels > 125 pg/mL were randomized to usual diabetes care or intensified care at a cardiac outpatient clinic for initiation and increase of RAS inhibitors and β blockers.5 After 2 years, patients randomized to the intensified care group showed a 65% risk reduction of the primary endpoint of hospitalization or death from cardiac disease (P = .04).
Based on this evidence, the 2017 focused update of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guideline for managing HF added a IIa recommendation for natriuretic peptide biomarker screening in those at risk of developing HF.6 The guideline recommends biomarker screening in conjunction with team-based care, including a cardiovascular specialist, and guideline-directed management and therapy to prevent development of left ventricular dysfunction or new-onset HF.
Although ordering a natriuretic peptide biomarker laboratory test is straightforward, the variability of team-based care across institutions and health systems makes it difficult to standardize screening and interventions for patients at risk for HF. We developed and piloted a process using clinical pharmacists in primary care for natriuretic peptide biomarker screening and risk factor reduction within the established patient aligned care team (PACT) framework at a US Department of Veterans Affairs (VA) medical center. In this paper, we describe our implementation process including descriptive preliminary outcomes.
Methods
The PACT team-based approach in primary care clinics is similar to the patient-centered medical home framework. A PACT includes the veteran patient and an interdisciplinary team of health professionals composed of their primary care practitioner (PCP), registered nurse care manager, clinical pharmacist, and other clinical and administrative staff. The PACT clinical pharmacist has prescriptive authority within a scope of practice to provide postdiagnostic chronic disease state management including management of T2DM, hypertension, HF, chronic obstructive pulmonary disease, anticoagulation, tobacco cessation, and atherosclerotic cardiovascular disease (ASCVD) risk reduction. Clinical pharmacists can prescribe and adjust medications and order laboratory tests.
Our institution, Clement J. Zablocki VA Medical Center (CJZVAMC) in Milwaukee, Wisconsin, has a specialty HF clinic that primarily manages ACC/AHA Stage C HF patients. The HF clinic uses a team-based approach to collaborate and coordinate care for the veteran. The HF team is comprised of cardiology specialists, registered nurses, clinical pharmacists, dietitians, and administrative staff. Two PACT clinical pharmacists also staff the HF clinic at CJZVAMC and work collaboratively to initiate, adjust, and optimize veterans’ HF medication regimens.
Two primary care PACT panels were selected for this project. Before implementation, a pharmacy resident and 3 PACT clinical pharmacists (2 of whom also staff the HF clinic) met with a HF cardiology specialist and 2 PACT PCPs to finalize the team-based process and workflow. PCPs were presented with the evidence-based background, purpose, and project design, which included patient identification, NT-proBNP laboratory test ordering, medication adjustment schedules, and protocol for ordering echocardiograms (Figure). Templated notes were created to allow for consistent documentation in patients’ electronic health record. A telephone script also was written for the initial telephone call to patients to explain in patient-friendly terms the implications of an elevated NT-proBNP level, the echocardiogram procedure, and recommendations for risk reduction.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.
The percentage of patients in this project with risk factors for HF and an elevated NT-proBNP were similar to the elevated levels described in the STOP-HF trial. In our project, 32.9% of patients had elevated NT-proBNP levels, similar to the 41.6% of patients in STOP-HF. Among the completed echocardiograms, 16% revealed mildly reduced or reduced EF. These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
In addition to early identification of reduced EF, this project allowed a targeted approach to identifying patients for risk factor reduction. Between the 2 PACT teams, 246 patients with T2DM and/or hypertension were seen from September 2019 to January 2020. By using natriuretic peptide screening, the clinical pharmacists were able to prioritize and focus risk factor management on patients at higher risk. Pharmacists were then able to intervene for all risk factors assessed: hypertension, T2DM, ASCVD risk reduction, NSAID use reduction, and tobacco cessation.
During the implementation period, VA criteria of use of the angiotensin receptor-neprilysin inhibitor, sacubitril/valsartan, was restricted to VA cardiology. For patients with reduced EF, it was up to the PCP’s discretion to consult cardiology for further follow-up. In November 2020, the VA removed the restriction to cardiology and PCPs were able to order sacubitril/valsartan. Although not included in the Figure at the time of project implementation, the clinical pharmacist could now transition a patient with reduced EF from a RAS inhibitor to sacubitril/valsartan and adjust to target dosages.
Clinical pharmacists involved in this project had established working relationships with each of the PACT members before project initiation. The PACT employed the clinical pharmacists regularly for chronic disease state management. This facilitated adoption of the natriuretic peptide screening process and PCP buy-in and support. The PCPs agreed to discuss adding a NT-proBNP laboratory test with the patient, when possible, during their in-person appointment and informed the patient that a pharmacist would call if the result was elevated. This warm hand-off facilitated the patient’s reception to the clinical pharmacists’ recommendations after an elevated NT-proBNP result. We also reported PCPs’ high acceptance rate of pharmacist recommendations and interventions for disease state management. These high acceptance rates reflect the established working relationships between clinical pharmacists and the PACT.
Development of templated notes, medication adjustment schedules, and telephone script allowed for consistent implementation into the PACT panels. This process could be duplicated and adopted into other PACTs who want to use a clinical pharmacist to facilitate natriuretic peptide screening and risk factor reduction. The findings from this project can be extrapolated to other team-based care such as the patient-centered medical home model because these programs exhibit many similarities. Both health care models centralize patient care and use interdisciplinary care teams to promote continuity, care coordination, and access to achieve optimized patient outcomes.
Cost was an important factor to consider when implementing this project. With an increase in prescriptions and elective, outpatient echocardiograms, higher outpatient cost is expected. A cost-effectiveness analysis in the STOP-HF trial found an overall cost benefit by reducing the number of patients diagnosed with left ventricular dysfunction or HF and emergency hospitalizations for cardiac events in those who received collaborative care after natriuretic peptide testing.8 These cost savings offset increased outpatient costs.
Limitations
Participants were identified initially through a computer-generated list of patients with hypertension or T2DM without a HF diagnosis documented in their problem list. This problem list is manually updated by PCPs. Although we reviewed records for exclusion criteria, eligible patients might have been excluded. The use and interpretation of an NT-proBNP level is not specific to cardiac disease. Elevations can be seen with increased age, kidney dysfunction, and pulmonary disease. Additionally, an NT-proBNP level might be falsely low in patients who are overweight or obese. Because of the relatively short period of time, we could not analyze associations with HF diagnosis or progression, hospitalizations due to HF, or mortality. Regarding external validity, because of the pre-established interdisciplinary clinic settings and VA pharmacists’ scope of practice with prescriptive authority, implementing this project might have been better received by PCPs and allowed for higher acceptance rates of pharmacist interventions at the VA compared with a community setting.
Conclusions
The ACC/AHA/HFSA guidelines recommended use of natriuretic peptide biomarker screening in conjunction with team-based care for those at risk of developing HF. We describe our process for implementing team-based care using clinical pharmacists in primary care. Our process provides a targeted approach to identifying patients for risk factor reduction through comprehensive medication management and could be replicated by other primary care clinics using a patient-centered medical home model.
Acknowledgments
We would like to acknowledge Dr. Sara Hariman, Dr. Payal Sanghani, and Dr. Cecilia Scholcoff for their support and collaboration with the project.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
1. Braunwald E. Heart failure. J Am Coll Cardiol HF. 2013;1(1):1-20. doi: 10.1016/j.jchf.2012.10.002
2. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a
3. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1900.
4. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66-74. doi:10.1001/jama.2013.7588
5. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365-1372. doi:10.1016/j.jacc.2013.05.069
6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
7. Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eu J Heart Fail. 2019;21:715-731. doi:10.1002/ejhf.1494
8. Ledwidge MT, O’Connell E, Gallagher J, et al; Heart Failure Association of the European Society of Cardiology. Cost-effectiveness of natriuretic peptide-based screening and collaborative care: a report from the STOP-HF (St. Vincent’s Screening to Prevent Heart Failure) study. Eur J Heart Fail. 2015;17(7):672-679.
BRAF V600E Expression in Primary Melanoma and Its Association With Death: A Population-Based, Retrospective, Cross-Sectional Study
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.
Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).
BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.

Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).

BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.

Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).

BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
Practice Points
- Approximately 50% of melanomas contain BRAF mutations; the effects on survival are unclear.
- Women with BRAF-mutated melanoma are at increased risk for death from melanoma.
- BRAF expression is associated with death of any cause for adults aged 18 to 39 years.
Impact of the COVID-19 Pandemic on Characteristics of Cutaneous Tumors Treated by Mohs Micrographic Surgery
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
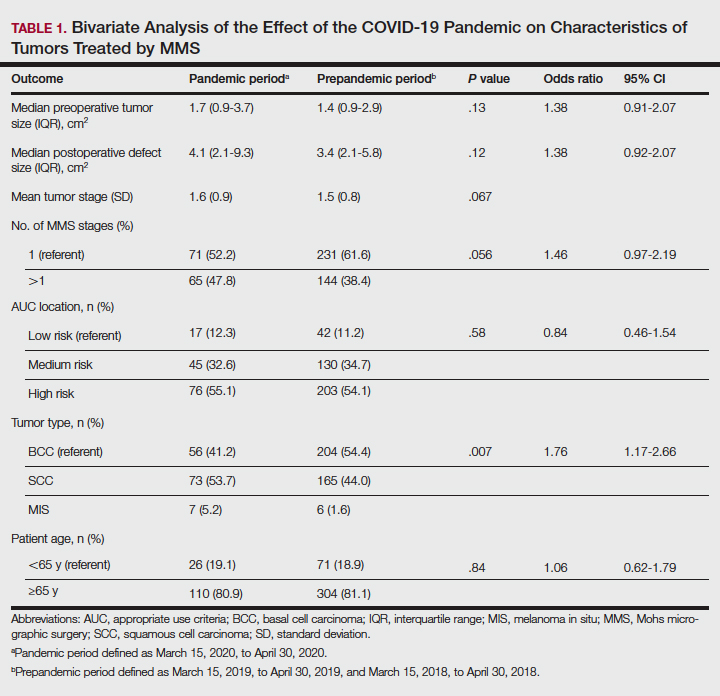
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
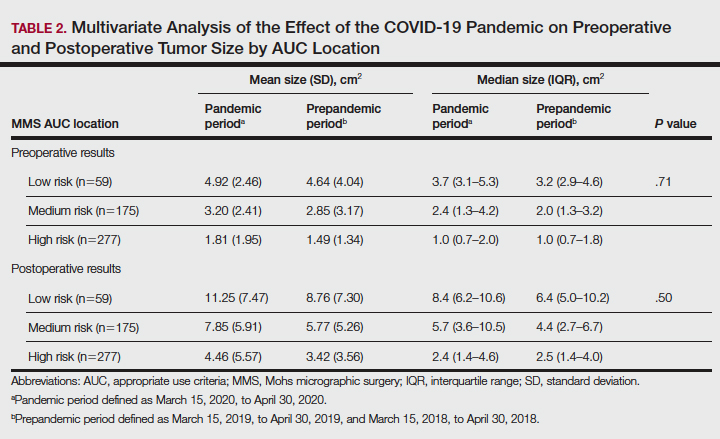
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
Practice Points
- Mohs surgeons should follow best practice guidelines dictated by our governing professional societies in selecting skin cancers for treatment by Mohs micrographic surgery (MMS) during the COVID-19 pandemic and beyond.
- The COVID-19 pandemic has impacted the characteristics of skin cancers treated by MMS, largely driven by new guidelines.
- Changing characteristics of skin cancers treated by MMS are of clinical significance, potentially affecting the extent of reconstructive surgery, cost, operating time, and future tumor characteristics.
Reflectance Confocal Microscopy Findings in a Small-Diameter Invasive Melanoma
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.
Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.
Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.
Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).
Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.
The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.

Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.

Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.

Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).

Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
Melanomas have been designated as small melanomas or micromelanomas according to their long-axis diameter (<6 mm and ≤3 mm, respectively).1-3 Because small-diameter melanomas also have the potential to metastasize, particularly if nodular, early diagnosis can be highly rewarding. Deep melanomas with small diameters may have the same potential for metastasis as large-diameter melanomas. In this context, dermoscopy, digital dermoscopic monitoring, and total-body photography are useful in clinical practice. However, these techniques are of limited utility for small, dermoscopic feature–poor melanomas. Conversely, less than 10% of changing lesions, which are spotted via digital dermoscopic surveillance, turn out to be melanomas; therefore, simply removing all changing lesions may result in many unnecessary excisions of benign lesions.4
In vivo reflectance confocal microscopy (RCM) is an advanced technique that allows recognition of the architectural and cellular details of pigmented lesions. Reflectance confocal microscopy has the potential to reduce the rate of unnecessary excisions and to diminish the risk for missing a melanoma.5-7 In meta-analyses, RCM sensitivity was reported as 90% to 93% and specificity was reported as 78% to 82% in detecting melanoma.8,9
We describe a case that highlights the potential role of RCM in the diagnosis of small-diameter melanomas.

Case Report
A 57-year-old man with Fitzpatrick skin type III presented to the dermato-oncology unit for evaluation of multiple nevi. He was otherwise healthy and denied a history of skin cancer. Total-body skin examination with dermoscopy was performed, and several mildly atypical lesions were identified. We decided to perform digital dermoscopic monitoring. The patient’s 6-month monitoring appointment had been scheduled, but he did not arrive for the follow-up visit until 10 months after the initial examination. A lesion on the left arm, which initially was 1.5 mm in diameter, had enlarged. It was now a dark brown–gray papule with a 2.5-mm diameter (Figure 1). Dermoscopy revealed grayish globules/dots at the center of the lesion, reticular gray-blue areas, and few milialike cysts; at the periphery, a narrow rim of brownish delicate pigment network also was seen (Figure 2). The clinical and dermoscopic differential diagnosis was either an atypical nevus or an early melanoma. For a more precise diagnosis before excision, the lesion was evaluated with RCM, which takes 10 to 15 minutes to perform.

Under RCM at the epidermis level, there was a cobblestone pattern that showed a focus with mild disarrangement and few small, roundish, nucleated cells (Figure 3). A mosaic image, akin to low-magnification microscopy that enables overview of the entire lesion, at the level of the dermoepidermal junction (DEJ) showed an overall irregular meshwork pattern. Higher-magnification optical sections showed marked and diffuse (extending >10% of lesion area) architectural disorder with confluent junctional nests that were irregular to bizarre in shape and uneven in size and spacing as well as edged and nonedged papillae. At the superficial dermal level, atypical bright nucleated cells (>5 cells/mm2) were observed (Figure 4). Bright dots and/or plump bright cells within papillae also were observed. These RCM findings were highly suggestive for melanoma.

Histopathology showed an asymmetric, junctional, lentiginous, and nested proliferation of atypical epithelioid melanocytes, with few melanocytes in a pagetoid spread. There were small nests of atypical epithelioid melanocytes at the superficial dermis extending to a depth of 0.3 mm. The atypical epithelioid melanocytes displayed angulated hyperchromatic nuclei with conspicuous nucleoli and dusty brown cytoplasm. There was notable inflammation and pigment incontinence at the dermis. There was no evidence of ulceration or mitosis at the dermal component. The diagnosis of a pT1a malignant melanoma was reported (Figure 5).

Comment
A small but enlarging dark gray papule with reticular gray-blue areas under dermoscopy in a 57-year-old man is obviously suspicious for melanoma. In daily practice, this type of small-diameter melanoma is difficult to diagnose with high confidence. We balance our aim to diagnose melanomas early with the need to reduce unnecessary excisions. Reflectance confocal microscopy may allow the clinician to arrive at the correct diagnosis and management decision with confidence before excision of the lesion.

The distinction of a small-diameter melanoma from a nevus via RCM relies on evaluation of the architectural and cellular features. Findings on RCM in small-diameter melanomas have been scarcely reported in the literature; Pupelli et al10 evaluated small melanomas with a diameter of 2 to 5 mm. Among these small-diameter melanomas, the RCM features suggestive for melanomas were the presence of cytologic atypia with cellular pleomorphism, architectural disorder with irregular nests, at least 5 pagetoid cells/mm2, dendrites or tangled lines (ie, short fine lines with no visible nucleus interlacing with the adjacent keratinocytes) within the epidermis, and atypical roundish cells at the DEJ.10
The distinction between an atypical nevus and a small-diameter melanoma using RCM occasionally may be challenging.11 Pellacani et al12 reported an algorithm to distinguish melanoma from atypical nevi. According to this algorithm, when at least 1 of the architectural atypia features (irregular junctional nests, short interconnections between junctional nests, and nonhomogeneous cellularity within junctional nests) and at least 1 of the cytologic atypia features (round pagetoid cells or atypical cells at the DEJ) are observed simultaneously, the lesion is diagnosed as a dysplastic nevus or a melanoma in the first step. In the second step, the RCM diagnosis of melanoma requires at least 1 of 3 parameters: roundish pagetoid cells encompassing at least 50% of the lesional area at the spinous layer, atypical cells involving at least 50% of the lesional area at the DEJ level, and nonedged papillae involving at least 10% of the lesional area.12 Accordingly, our case corresponded with these RCM criteria for a melanoma, given that there were irregular junctional nests, atypical cells at the DEJ, and nonedged papillae involving at least 10% of the lesion.
The current limitations of RCM are the high cost of the device (approximately $58,125–$139,400 for different models), the amount of time needed to train staff in RCM units (seminars, congresses, and special courses organized by the International Confocal Working Group), and the amount of time needed for evaluation of individual lesions (15–20 minutes). However, RCM can be valuable in the clinical diagnosis of difficult lesions, as seen in our case.
Conclusion
Our case highlights the benefit of RCM in allowing the confident diagnosis and correct management of a small-diameter melanoma that turned out to be a melanoma with 0.3-mm Breslow thickness. Even so, histopathologic evaluation remains the gold standard for the diagnosis of melanoma.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Bergman R, Katz I, Lichtig C, et al. Malignant melanomas with histologic diameters less than 6 mm. J Am Acad Dermatol. 1992;26:462-466.
- Bono A, Tolomio E, Trincone S, et al. Micro-melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol. 2006;155:570-573.
- Bono A, Bartoli C, Baldi M, et al. Micro-melanoma detection. a clinical study on 22 cases of melanoma with a diameter equal to or less than 3 mm. Tumori. 2004;90:128-131.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Ferrari B, Pupelli G, Farnetani F, et al. Dermoscopic difficult lesions: an objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135-1140.
- Dinnes J, Deeks JJ, Saleh D, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Pupelli G, Longo C, Veneziano L, et al. Small-diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027-1033.
- Carrera C, Marghoob AA. Discriminating nevi from melanomas: clues and pitfalls. Dermatol Clin. 2016;34:395-409.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
Practice Points
- Melanomas with a long-axis diameter smaller than 6 mm are considered small melanomas, and those with diameters of 3 mm and smaller are considered micromelanomas; both are difficult to detect.
- Digital dermoscopic monitoring and reflectance confocal microscopy are important tools in detecting small melanomas.
Surgical Planning for Mohs Defect Reconstruction in the Digital Age
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.

Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).

Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.

Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).

Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.

Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).

Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
How Dermatology Residents Can Best Serve the Needs of the LGBT Community
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.
Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.

Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.

Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
Resident Pearl
- Because of the longitudinal relationships dermatology residents make with their patients, they have a unique opportunity to provide a safe space and life-changing care to patients within the lesbian, gay, bisexual, and transgender community.
Expanded Treatment Options for Lupus Nephritis
Lupus nephritis (LN) affects approximately 25%-60% of patients with systemic lupus erythematosus. Currently, guideline-directed therapy recommends a combination of steroids plus either mycophenolate mofetil or cyclophosphamide. Despite treatment, about 10%-30% of LN patients will progress to end-stage kidney disease.
Fortunately, over the past 2 years, the FDA approved two novel agents that expand treatment options for patients with active disease who have received standard-of-care therapy.
In this ReCAP, Dr Joan Merrill, of the University of Oklahoma Health Sciences Center, reports on the B-lymphocyte stimulator–specific inhibitor belimumab, which was evaluated in the BLISS-LN trial, and the oral calcineurin inhibitor voclosporin, which was assessed in the AURORA trials.
Dr Merrill discusses how these recently approved medications fit into the standard of care for LN patients.
--
Joan Merrill, MD, Professor, Department of Medicine, Division of Rheumatology, University of Oklahoma Health Sciences Center; Director of Clinical Projects, Arthritis & Clinical Immunology Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma
Joan Merrill, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Biogen
Received research grant from: Bristol-Myers Squibb; GlaxoSmithKline
Received income in an amount equal to or greater than $250 from: AbbVie; Alexion; Amgen; AstraZeneca; Aurinia; Bristol-Myers Squibb; EMD Serono; Genentech; Gilead; GlaxoSmithKline; Lilly; Merck; Provention; RemeGen; Sanofi; UCB; Zenas
Received research grant from: AbbVie; AstraZeneca; BeiGene; Pharmacyclics; TG Therapeutics
Lupus nephritis (LN) affects approximately 25%-60% of patients with systemic lupus erythematosus. Currently, guideline-directed therapy recommends a combination of steroids plus either mycophenolate mofetil or cyclophosphamide. Despite treatment, about 10%-30% of LN patients will progress to end-stage kidney disease.
Fortunately, over the past 2 years, the FDA approved two novel agents that expand treatment options for patients with active disease who have received standard-of-care therapy.
In this ReCAP, Dr Joan Merrill, of the University of Oklahoma Health Sciences Center, reports on the B-lymphocyte stimulator–specific inhibitor belimumab, which was evaluated in the BLISS-LN trial, and the oral calcineurin inhibitor voclosporin, which was assessed in the AURORA trials.
Dr Merrill discusses how these recently approved medications fit into the standard of care for LN patients.
--
Joan Merrill, MD, Professor, Department of Medicine, Division of Rheumatology, University of Oklahoma Health Sciences Center; Director of Clinical Projects, Arthritis & Clinical Immunology Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma
Joan Merrill, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Biogen
Received research grant from: Bristol-Myers Squibb; GlaxoSmithKline
Received income in an amount equal to or greater than $250 from: AbbVie; Alexion; Amgen; AstraZeneca; Aurinia; Bristol-Myers Squibb; EMD Serono; Genentech; Gilead; GlaxoSmithKline; Lilly; Merck; Provention; RemeGen; Sanofi; UCB; Zenas
Received research grant from: AbbVie; AstraZeneca; BeiGene; Pharmacyclics; TG Therapeutics
Lupus nephritis (LN) affects approximately 25%-60% of patients with systemic lupus erythematosus. Currently, guideline-directed therapy recommends a combination of steroids plus either mycophenolate mofetil or cyclophosphamide. Despite treatment, about 10%-30% of LN patients will progress to end-stage kidney disease.
Fortunately, over the past 2 years, the FDA approved two novel agents that expand treatment options for patients with active disease who have received standard-of-care therapy.
In this ReCAP, Dr Joan Merrill, of the University of Oklahoma Health Sciences Center, reports on the B-lymphocyte stimulator–specific inhibitor belimumab, which was evaluated in the BLISS-LN trial, and the oral calcineurin inhibitor voclosporin, which was assessed in the AURORA trials.
Dr Merrill discusses how these recently approved medications fit into the standard of care for LN patients.
--
Joan Merrill, MD, Professor, Department of Medicine, Division of Rheumatology, University of Oklahoma Health Sciences Center; Director of Clinical Projects, Arthritis & Clinical Immunology Program, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma
Joan Merrill, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Biogen
Received research grant from: Bristol-Myers Squibb; GlaxoSmithKline
Received income in an amount equal to or greater than $250 from: AbbVie; Alexion; Amgen; AstraZeneca; Aurinia; Bristol-Myers Squibb; EMD Serono; Genentech; Gilead; GlaxoSmithKline; Lilly; Merck; Provention; RemeGen; Sanofi; UCB; Zenas
Received research grant from: AbbVie; AstraZeneca; BeiGene; Pharmacyclics; TG Therapeutics