User login
Advocacy Update: Ringing in 2023
New Year, New Codes: A Win-Win for Digital Pathology
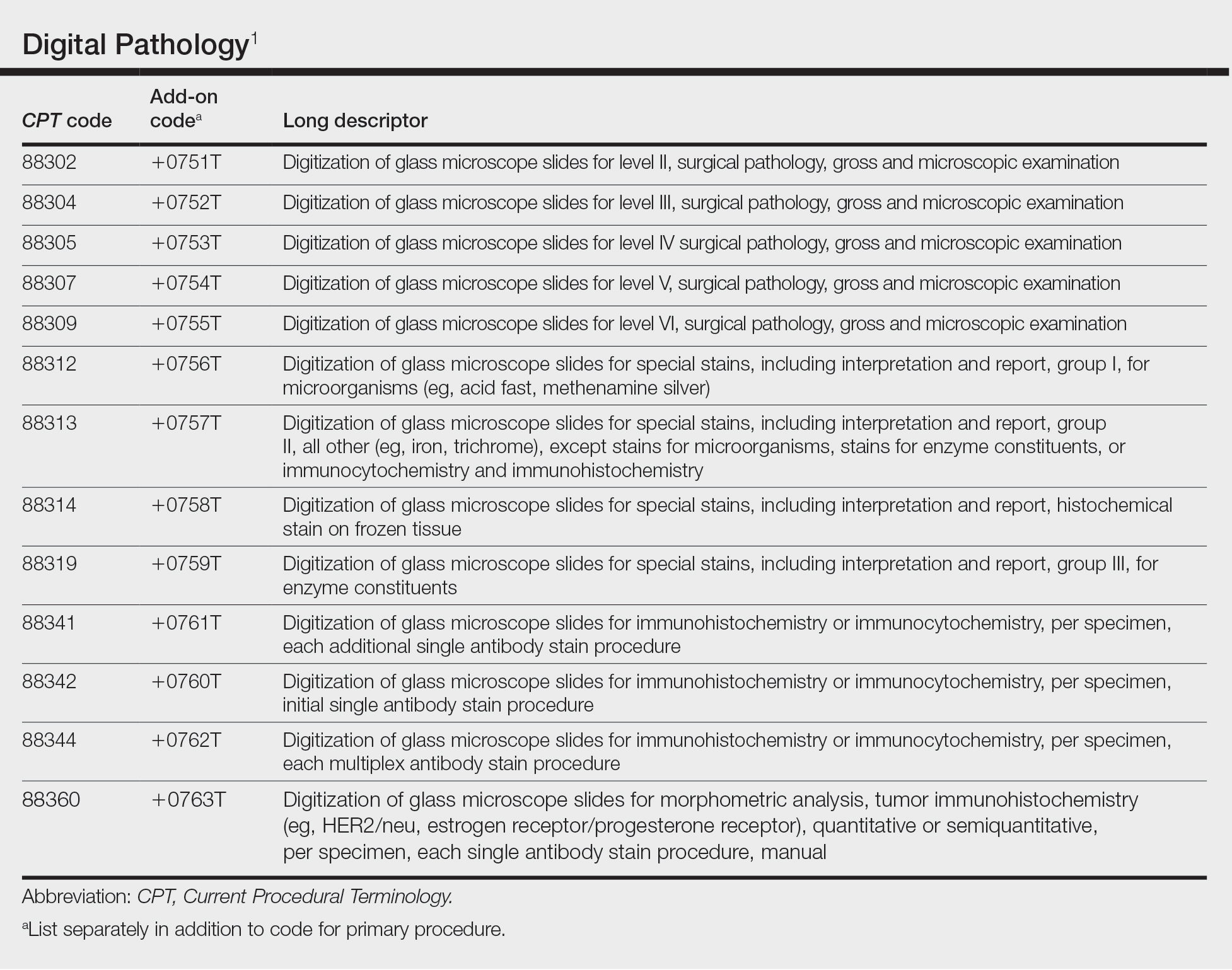
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.
Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
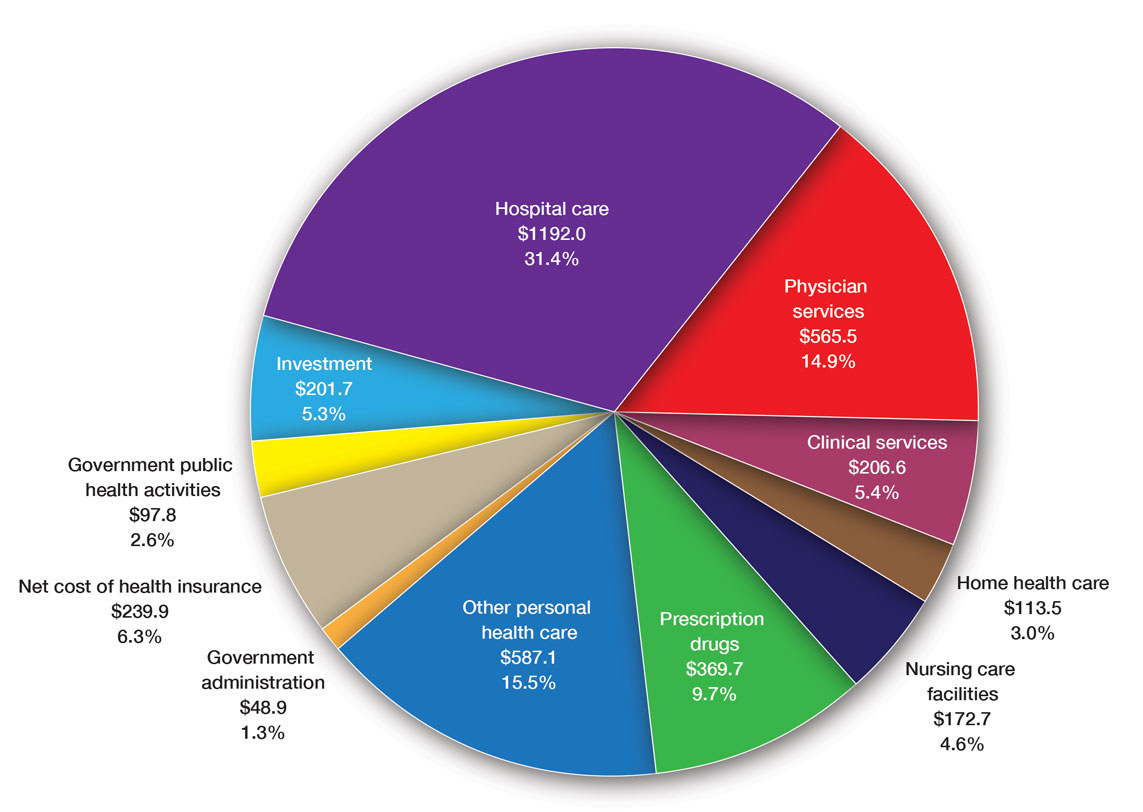
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2
Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
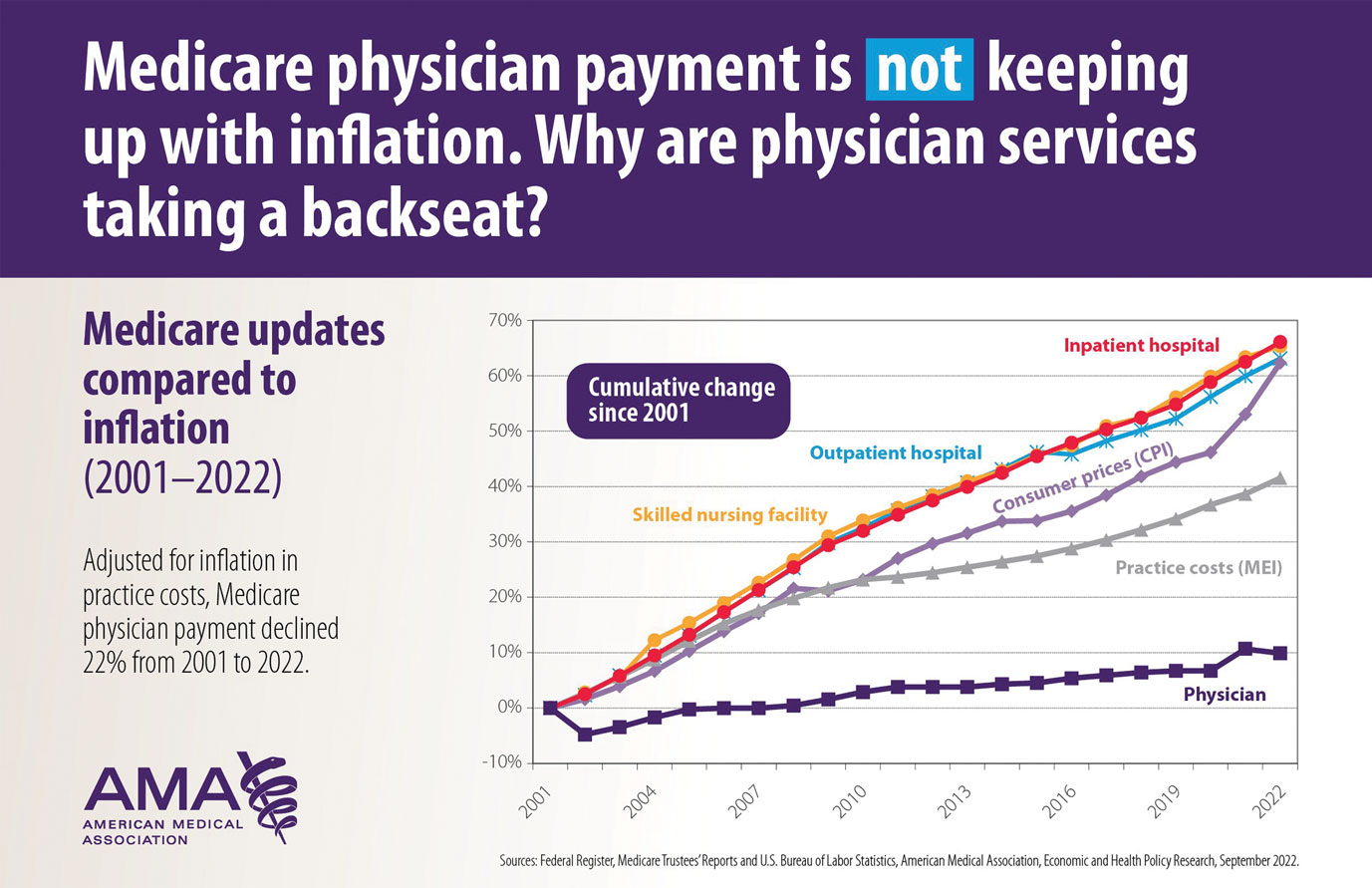
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5
The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
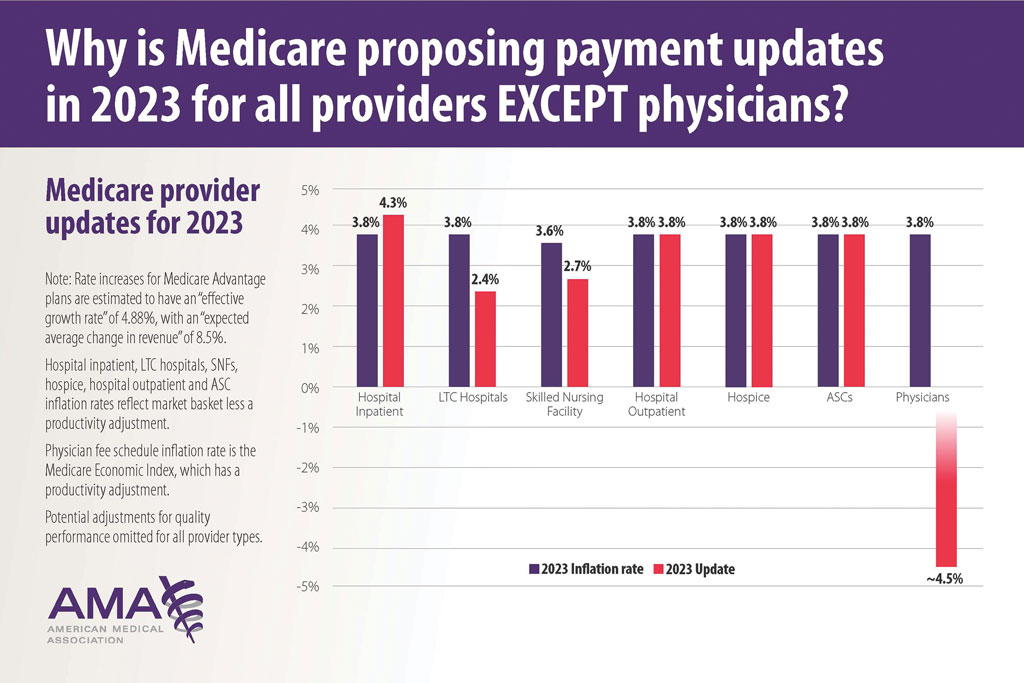
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.
In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
Practice Points
- New digital pathology codes proposed by the American Medical Association can be used starting January 1, 2023.
- A proposed 2023 fee schedule negatively impacting dermatology practices was published by the Centers for Medicare & Medicaid Services in July 2022.
- Advocacy involvement provides a collaborative collective voice for our specialty to help our patients improve their care.
Nurturing a Satisfying Career in Dermatology
The residents of our program asked me to serve as their commencement speaker in June. Since I was retiring from my position as department chair, this touching honor seemed a fitting capstone for my career. It gave me the opportunity to reflect on the enormity of the changes that have occurred between my graduation from residency in 1983 and the current time, which is marked by disruption from the digital revolution and the COVID-19 pandemic. Throughout this 40-year period, there were times of external global turmoil, economic instability, significant changes in the business of medicine, stressful changes in documentation of competency and certification, and the difficult transition to electronic medical records. Another epidemic—AIDS—changed surgical practices. During my residency, we did biopsies without wearing gloves or masks. Gloves were added to protect the person doing the procedure as well as to prevent spread of disease to other patients, not to reduce the infection rate for the patient undergoing the procedure. Of course, change in the last 40 years also occurred outside of work and included various familial stresses. The irritations of daily life easily mounted up to being overwhelming. However, I had gone to work every day for 40 years, seeking to do my best for my patients and my colleagues and the staff with whom I worked, sometimes feeling successful and sometimes feeling incompetent. Some days went smoothly, and some days were filled with challenges that I could not begin to imagine how I would solve. I have a habit of seeing problems rather than successes, which creates its own difficulties. I did, however, grab opportunities that continually improved my practice of medicine and allowed me to serve in several professional positions as well as in leadership positions of multiple professional societies. As I prepared the commencement address, I realized that the totality of my career was very satisfying.
The Merriam-Webster dictionary definition of satisfying is “producing pleasure or contentment by providing what is needed or wanted.”1 My use of the word means that my career over the long term has pleased me—maybe not some of the people I reported to, but rather me.
My approach to my career can be summarized in 3 words: purpose, serendipity, and curiosity.
The first element is purpose. Job satisfaction generally is associated with work being aligned with values, an appreciation that you are accomplishing the purpose with which you set out on your journey. It is not associated with every day being wonderful and problem free or every task being completed without setbacks or complications. The reality of working is not that every moment brings pure happiness or that every task fulfills a passion. How does a person ensure that the days add up to be satisfying? Start with values. Why did you decide to pursue medical school? Some may have chosen it for economic security, but there are many ways to achieve economic security. Maybe being a physician feeds into the family lore, but families generally have broad ranges of acceptable careers. Maybe it appealed scientifically, but a PhD in biology also fulfills that interest. Maybe it is that you noticed respect for physicians in the community when you were growing up, but that is changing and does not represent an internal value anyway. Consider your values carefully, write them down, and keep them at the forefront of the day. Go back to them consciously any time you have a rough day and understand why you are doing what you are doing. When you are 55 years old and going through your umpteenth change in reimbursement process, go back to the day you decided on medicine as a career. Focus on your values as the grounding for your purpose. Also note that purpose is different than goals. Some goals will be reached, and some will not. Goals change with external realities and/ or internal factors. Purpose and values remain the same if we have thoughtfully identified them.
The second element is serendipity. Serendipity often is thought of as luck, as karma, as being in the right place at the right time. It feels random, and at first glance it appears that purpose and serendipity are complete opposites and do not intersect. Serendipity is, however, not just luck. It is an ability to distinguish events and observations in meaningful ways. It is a close relative of creativity and benefits from sloppiness, playfulness, tinkering, and discussion. It cannot exist in a vacuum. History is replete with serendipitous discoveries. It is thought that James Watson and Francis Crick would never have been able to elucidate the nature of DNA without sharing offices with people with whom they argued daily. In fact, figuring out the DNA structure was not even the main focus of their laboratories. It was just a side angle that several people loved to think about. Appreciating serendipity by being truly open to opportunities that are out on the wings brings experiences that are deeply rewarding even if not planned. I had no idea at all, no plan, no goal of serving as president of the American Academy of Dermatology or as Department Chair, and yet these happened. These experiences have allowed me to work on my purpose as I have defined it. How can you harness serendipity in your own life? My philosophy may be somewhat simple, but I think if you show up every day doing the best job you can at the tasks on hand, doors will appear, at odd intervals and in odd directions. You must be open enough and in tune with your purpose to an extent that you can sense the direction in which to turn and what doorways through which to walk.
The third element is curiosity. One definition is that curiosity is the motivation to learn new information. Another definition is that curiosity is a special form of information seeking distinguished by the fact that it is internally motivated. We are all familiar with intellectual curiosity. For example, a patient has a basal cell carcinoma on the upper back. What does the literature say about the cure rates of various treatments for that particular tumor? In addition, we can be curious about other things as well. Is it a really small tumor? How was it found and why is the patient anxious? Why does it make me irritated that the patient is worried about such a small, easily treated tumor? Or is it a large neglected tumor? Why was it not treated before? Why does it make me sad that it is so large? Why does it annoy me that I have a difficult situation to manage? Being able to define an emotional reaction by being curious about its presence helps us manage destructive responses and promote more positive outcomes. This curiosity is related to emotional intelligence and is mindfully harnessed by effective leaders. Curiosity will get you through tough days when your office team is stressed and the tough years that are complicated by professional and personal challenges.
Curiosity also will help you identify your purpose and harness serendipity, and so we come full circle with our 3 elements: purpose, serendipity, and curiosity.
My wish for all of you is that when you are at the tail end of your career, you will look back and say, “This has been a great ride.” I am very grateful that I can acknowledge this for myself. I have been so fortunate to have found dermatology, where I can go to work every day making a difference for patients in a stimulating environment with good colleagues. One of my values is to try and make life better in some way for everyone around me, even if it is just a smile at the start of the workday. As I look back, this value has allowed me to meet interesting people, hear fascinating stories, make good friends, and have enduring relationships. I have held onto fellow travelers, and we have supported each other through tough times as well as celebrated together the good times.
Nurturing a satisfying career includes these essential fundamentals. First, accept the reality of constant change. Second, develop productive relationships with fellow travelers. And third and most importantly, go forth with purpose, serendipity, and curiosity.
- Merriam-Webster. Satisfying. Merriam-Webster.com Dictionary. Accessed November 18, 2022. https://www.merriam-webster.com/dictionary/satisfying
The residents of our program asked me to serve as their commencement speaker in June. Since I was retiring from my position as department chair, this touching honor seemed a fitting capstone for my career. It gave me the opportunity to reflect on the enormity of the changes that have occurred between my graduation from residency in 1983 and the current time, which is marked by disruption from the digital revolution and the COVID-19 pandemic. Throughout this 40-year period, there were times of external global turmoil, economic instability, significant changes in the business of medicine, stressful changes in documentation of competency and certification, and the difficult transition to electronic medical records. Another epidemic—AIDS—changed surgical practices. During my residency, we did biopsies without wearing gloves or masks. Gloves were added to protect the person doing the procedure as well as to prevent spread of disease to other patients, not to reduce the infection rate for the patient undergoing the procedure. Of course, change in the last 40 years also occurred outside of work and included various familial stresses. The irritations of daily life easily mounted up to being overwhelming. However, I had gone to work every day for 40 years, seeking to do my best for my patients and my colleagues and the staff with whom I worked, sometimes feeling successful and sometimes feeling incompetent. Some days went smoothly, and some days were filled with challenges that I could not begin to imagine how I would solve. I have a habit of seeing problems rather than successes, which creates its own difficulties. I did, however, grab opportunities that continually improved my practice of medicine and allowed me to serve in several professional positions as well as in leadership positions of multiple professional societies. As I prepared the commencement address, I realized that the totality of my career was very satisfying.
The Merriam-Webster dictionary definition of satisfying is “producing pleasure or contentment by providing what is needed or wanted.”1 My use of the word means that my career over the long term has pleased me—maybe not some of the people I reported to, but rather me.
My approach to my career can be summarized in 3 words: purpose, serendipity, and curiosity.
The first element is purpose. Job satisfaction generally is associated with work being aligned with values, an appreciation that you are accomplishing the purpose with which you set out on your journey. It is not associated with every day being wonderful and problem free or every task being completed without setbacks or complications. The reality of working is not that every moment brings pure happiness or that every task fulfills a passion. How does a person ensure that the days add up to be satisfying? Start with values. Why did you decide to pursue medical school? Some may have chosen it for economic security, but there are many ways to achieve economic security. Maybe being a physician feeds into the family lore, but families generally have broad ranges of acceptable careers. Maybe it appealed scientifically, but a PhD in biology also fulfills that interest. Maybe it is that you noticed respect for physicians in the community when you were growing up, but that is changing and does not represent an internal value anyway. Consider your values carefully, write them down, and keep them at the forefront of the day. Go back to them consciously any time you have a rough day and understand why you are doing what you are doing. When you are 55 years old and going through your umpteenth change in reimbursement process, go back to the day you decided on medicine as a career. Focus on your values as the grounding for your purpose. Also note that purpose is different than goals. Some goals will be reached, and some will not. Goals change with external realities and/ or internal factors. Purpose and values remain the same if we have thoughtfully identified them.
The second element is serendipity. Serendipity often is thought of as luck, as karma, as being in the right place at the right time. It feels random, and at first glance it appears that purpose and serendipity are complete opposites and do not intersect. Serendipity is, however, not just luck. It is an ability to distinguish events and observations in meaningful ways. It is a close relative of creativity and benefits from sloppiness, playfulness, tinkering, and discussion. It cannot exist in a vacuum. History is replete with serendipitous discoveries. It is thought that James Watson and Francis Crick would never have been able to elucidate the nature of DNA without sharing offices with people with whom they argued daily. In fact, figuring out the DNA structure was not even the main focus of their laboratories. It was just a side angle that several people loved to think about. Appreciating serendipity by being truly open to opportunities that are out on the wings brings experiences that are deeply rewarding even if not planned. I had no idea at all, no plan, no goal of serving as president of the American Academy of Dermatology or as Department Chair, and yet these happened. These experiences have allowed me to work on my purpose as I have defined it. How can you harness serendipity in your own life? My philosophy may be somewhat simple, but I think if you show up every day doing the best job you can at the tasks on hand, doors will appear, at odd intervals and in odd directions. You must be open enough and in tune with your purpose to an extent that you can sense the direction in which to turn and what doorways through which to walk.
The third element is curiosity. One definition is that curiosity is the motivation to learn new information. Another definition is that curiosity is a special form of information seeking distinguished by the fact that it is internally motivated. We are all familiar with intellectual curiosity. For example, a patient has a basal cell carcinoma on the upper back. What does the literature say about the cure rates of various treatments for that particular tumor? In addition, we can be curious about other things as well. Is it a really small tumor? How was it found and why is the patient anxious? Why does it make me irritated that the patient is worried about such a small, easily treated tumor? Or is it a large neglected tumor? Why was it not treated before? Why does it make me sad that it is so large? Why does it annoy me that I have a difficult situation to manage? Being able to define an emotional reaction by being curious about its presence helps us manage destructive responses and promote more positive outcomes. This curiosity is related to emotional intelligence and is mindfully harnessed by effective leaders. Curiosity will get you through tough days when your office team is stressed and the tough years that are complicated by professional and personal challenges.
Curiosity also will help you identify your purpose and harness serendipity, and so we come full circle with our 3 elements: purpose, serendipity, and curiosity.
My wish for all of you is that when you are at the tail end of your career, you will look back and say, “This has been a great ride.” I am very grateful that I can acknowledge this for myself. I have been so fortunate to have found dermatology, where I can go to work every day making a difference for patients in a stimulating environment with good colleagues. One of my values is to try and make life better in some way for everyone around me, even if it is just a smile at the start of the workday. As I look back, this value has allowed me to meet interesting people, hear fascinating stories, make good friends, and have enduring relationships. I have held onto fellow travelers, and we have supported each other through tough times as well as celebrated together the good times.
Nurturing a satisfying career includes these essential fundamentals. First, accept the reality of constant change. Second, develop productive relationships with fellow travelers. And third and most importantly, go forth with purpose, serendipity, and curiosity.
The residents of our program asked me to serve as their commencement speaker in June. Since I was retiring from my position as department chair, this touching honor seemed a fitting capstone for my career. It gave me the opportunity to reflect on the enormity of the changes that have occurred between my graduation from residency in 1983 and the current time, which is marked by disruption from the digital revolution and the COVID-19 pandemic. Throughout this 40-year period, there were times of external global turmoil, economic instability, significant changes in the business of medicine, stressful changes in documentation of competency and certification, and the difficult transition to electronic medical records. Another epidemic—AIDS—changed surgical practices. During my residency, we did biopsies without wearing gloves or masks. Gloves were added to protect the person doing the procedure as well as to prevent spread of disease to other patients, not to reduce the infection rate for the patient undergoing the procedure. Of course, change in the last 40 years also occurred outside of work and included various familial stresses. The irritations of daily life easily mounted up to being overwhelming. However, I had gone to work every day for 40 years, seeking to do my best for my patients and my colleagues and the staff with whom I worked, sometimes feeling successful and sometimes feeling incompetent. Some days went smoothly, and some days were filled with challenges that I could not begin to imagine how I would solve. I have a habit of seeing problems rather than successes, which creates its own difficulties. I did, however, grab opportunities that continually improved my practice of medicine and allowed me to serve in several professional positions as well as in leadership positions of multiple professional societies. As I prepared the commencement address, I realized that the totality of my career was very satisfying.
The Merriam-Webster dictionary definition of satisfying is “producing pleasure or contentment by providing what is needed or wanted.”1 My use of the word means that my career over the long term has pleased me—maybe not some of the people I reported to, but rather me.
My approach to my career can be summarized in 3 words: purpose, serendipity, and curiosity.
The first element is purpose. Job satisfaction generally is associated with work being aligned with values, an appreciation that you are accomplishing the purpose with which you set out on your journey. It is not associated with every day being wonderful and problem free or every task being completed without setbacks or complications. The reality of working is not that every moment brings pure happiness or that every task fulfills a passion. How does a person ensure that the days add up to be satisfying? Start with values. Why did you decide to pursue medical school? Some may have chosen it for economic security, but there are many ways to achieve economic security. Maybe being a physician feeds into the family lore, but families generally have broad ranges of acceptable careers. Maybe it appealed scientifically, but a PhD in biology also fulfills that interest. Maybe it is that you noticed respect for physicians in the community when you were growing up, but that is changing and does not represent an internal value anyway. Consider your values carefully, write them down, and keep them at the forefront of the day. Go back to them consciously any time you have a rough day and understand why you are doing what you are doing. When you are 55 years old and going through your umpteenth change in reimbursement process, go back to the day you decided on medicine as a career. Focus on your values as the grounding for your purpose. Also note that purpose is different than goals. Some goals will be reached, and some will not. Goals change with external realities and/ or internal factors. Purpose and values remain the same if we have thoughtfully identified them.
The second element is serendipity. Serendipity often is thought of as luck, as karma, as being in the right place at the right time. It feels random, and at first glance it appears that purpose and serendipity are complete opposites and do not intersect. Serendipity is, however, not just luck. It is an ability to distinguish events and observations in meaningful ways. It is a close relative of creativity and benefits from sloppiness, playfulness, tinkering, and discussion. It cannot exist in a vacuum. History is replete with serendipitous discoveries. It is thought that James Watson and Francis Crick would never have been able to elucidate the nature of DNA without sharing offices with people with whom they argued daily. In fact, figuring out the DNA structure was not even the main focus of their laboratories. It was just a side angle that several people loved to think about. Appreciating serendipity by being truly open to opportunities that are out on the wings brings experiences that are deeply rewarding even if not planned. I had no idea at all, no plan, no goal of serving as president of the American Academy of Dermatology or as Department Chair, and yet these happened. These experiences have allowed me to work on my purpose as I have defined it. How can you harness serendipity in your own life? My philosophy may be somewhat simple, but I think if you show up every day doing the best job you can at the tasks on hand, doors will appear, at odd intervals and in odd directions. You must be open enough and in tune with your purpose to an extent that you can sense the direction in which to turn and what doorways through which to walk.
The third element is curiosity. One definition is that curiosity is the motivation to learn new information. Another definition is that curiosity is a special form of information seeking distinguished by the fact that it is internally motivated. We are all familiar with intellectual curiosity. For example, a patient has a basal cell carcinoma on the upper back. What does the literature say about the cure rates of various treatments for that particular tumor? In addition, we can be curious about other things as well. Is it a really small tumor? How was it found and why is the patient anxious? Why does it make me irritated that the patient is worried about such a small, easily treated tumor? Or is it a large neglected tumor? Why was it not treated before? Why does it make me sad that it is so large? Why does it annoy me that I have a difficult situation to manage? Being able to define an emotional reaction by being curious about its presence helps us manage destructive responses and promote more positive outcomes. This curiosity is related to emotional intelligence and is mindfully harnessed by effective leaders. Curiosity will get you through tough days when your office team is stressed and the tough years that are complicated by professional and personal challenges.
Curiosity also will help you identify your purpose and harness serendipity, and so we come full circle with our 3 elements: purpose, serendipity, and curiosity.
My wish for all of you is that when you are at the tail end of your career, you will look back and say, “This has been a great ride.” I am very grateful that I can acknowledge this for myself. I have been so fortunate to have found dermatology, where I can go to work every day making a difference for patients in a stimulating environment with good colleagues. One of my values is to try and make life better in some way for everyone around me, even if it is just a smile at the start of the workday. As I look back, this value has allowed me to meet interesting people, hear fascinating stories, make good friends, and have enduring relationships. I have held onto fellow travelers, and we have supported each other through tough times as well as celebrated together the good times.
Nurturing a satisfying career includes these essential fundamentals. First, accept the reality of constant change. Second, develop productive relationships with fellow travelers. And third and most importantly, go forth with purpose, serendipity, and curiosity.
- Merriam-Webster. Satisfying. Merriam-Webster.com Dictionary. Accessed November 18, 2022. https://www.merriam-webster.com/dictionary/satisfying
- Merriam-Webster. Satisfying. Merriam-Webster.com Dictionary. Accessed November 18, 2022. https://www.merriam-webster.com/dictionary/satisfying
The Universal Dermatology Bandage Kit: A Succinct Collection of Supplies
Practice Gap
Biopsies, excisions, and other invasive cutaneous procedures are performed regularly in dermatology clinics and require placement of a bandage after the procedure. Postprocedural bandaging varies by the type of procedure performed, anatomic site, and the physician’s preference of materials. Dermatologists can be left with an overwhelming choice of supplies and little practical education, as bandaging methods are not routinely addressed in residency curricula. To address this concern, we provide a succinct list of basic materials that are versatile and easily adapted to encompass all bandaging needs for dermatology procedures (Table).
With these few components, one can create an array of distinct bandages to cover wounds as small as a shave biopsy to linear closures and basic flaps or grafts. Even traditionally difficult-to-bandage areas are easily addressed. Simple modifications of the basic materials are required for each bandage adaptation, as outlined below.
The Techniques
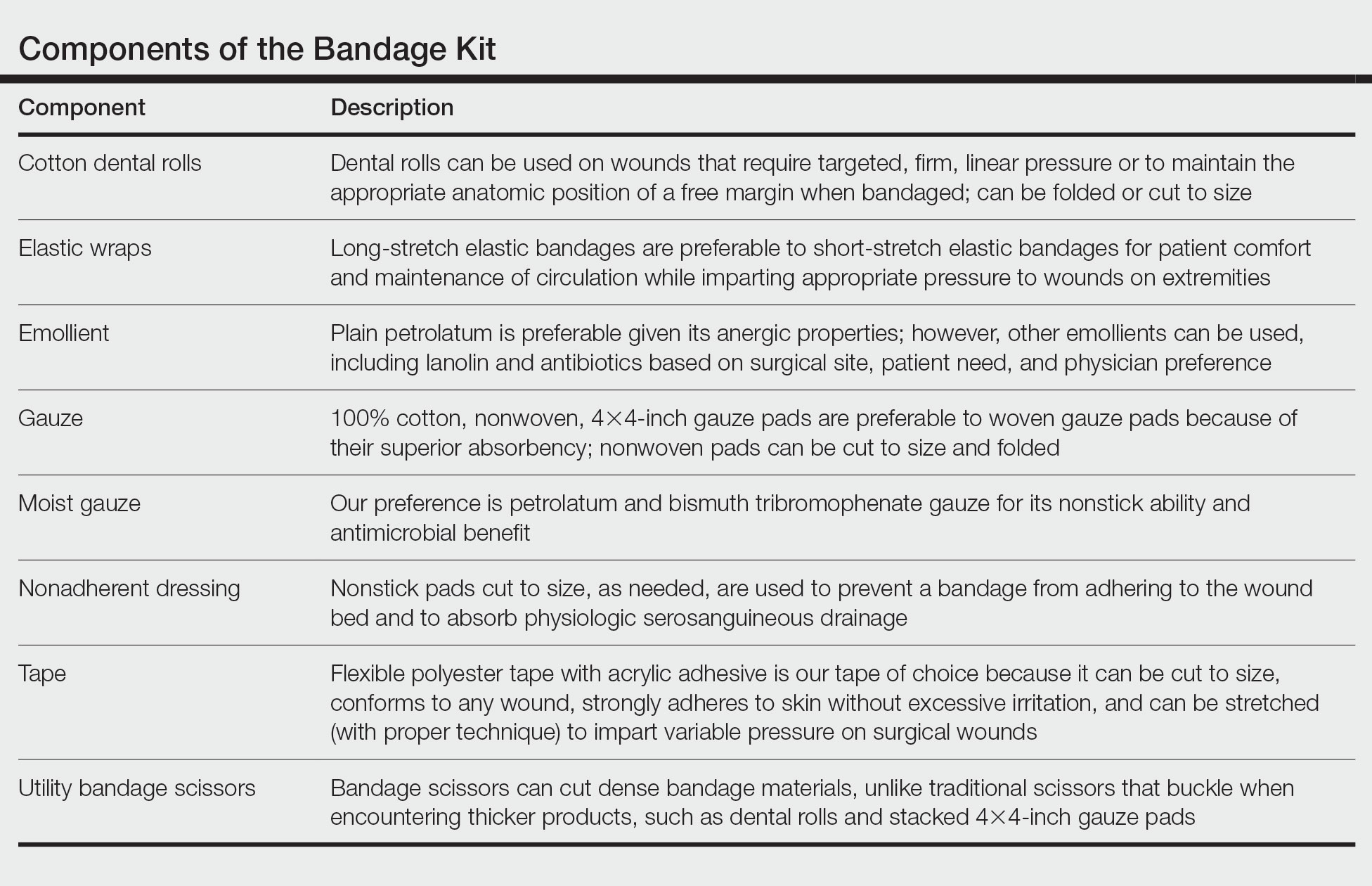
Shave and Punch Biopsy Sites—Layer (from bottom to top) the emollient of choice, a cut 4×4-inch gauze pad, and flexible polyester tape cut to the appropriate size (Figure 1). This simple bandage conforms well to any anatomic site and can replace an adhesive bandage, if desired.
Cutaneous Surgery Sites—Pressure bandages are recommended on cutaneous surgery sites. One of the most common closures performed in dermatology is the layered closure with dissolvable subcutaneous sutures and nondissolvable cutaneous sutures. When this closure is performed on the trunk and proximal extremities, undermining often is required to adequately approximate skin. This technique eliminates tension on the wound but can increase the risk for hematoma.1 A pressure bandage left in place and kept dry for 48 hours after surgery helps eliminate the risk for postoperative bleeding.
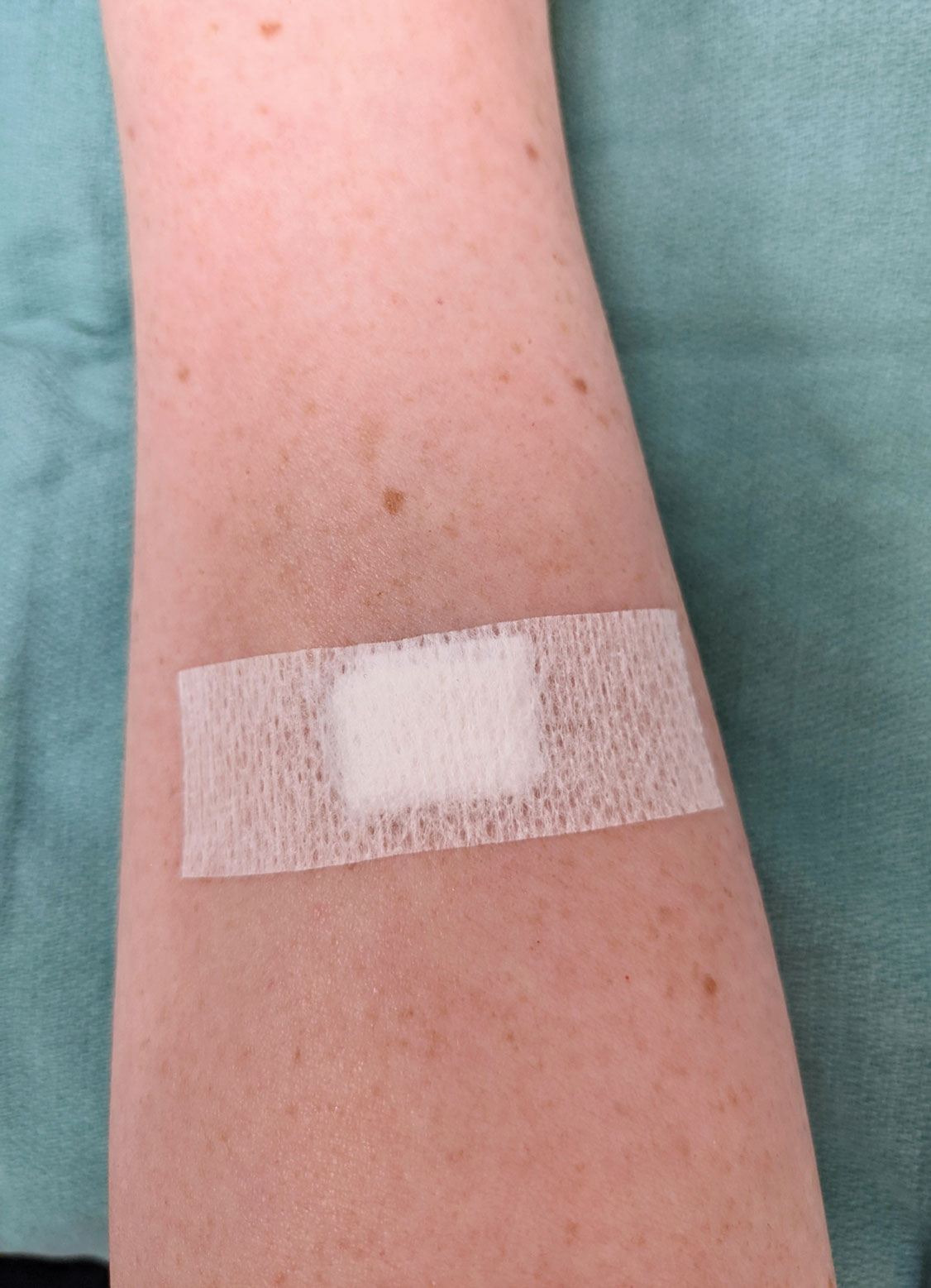
To make a pressure bandage, layer (from bottom to top) the emollient of choice, a nonstick pad cut to size, folded 4×4-inch gauze pads, and flexible polyester tape (Figure 2). Our practice routinely utilizes the tape fanning technique2 to impart equal and firm pressure over the wound.
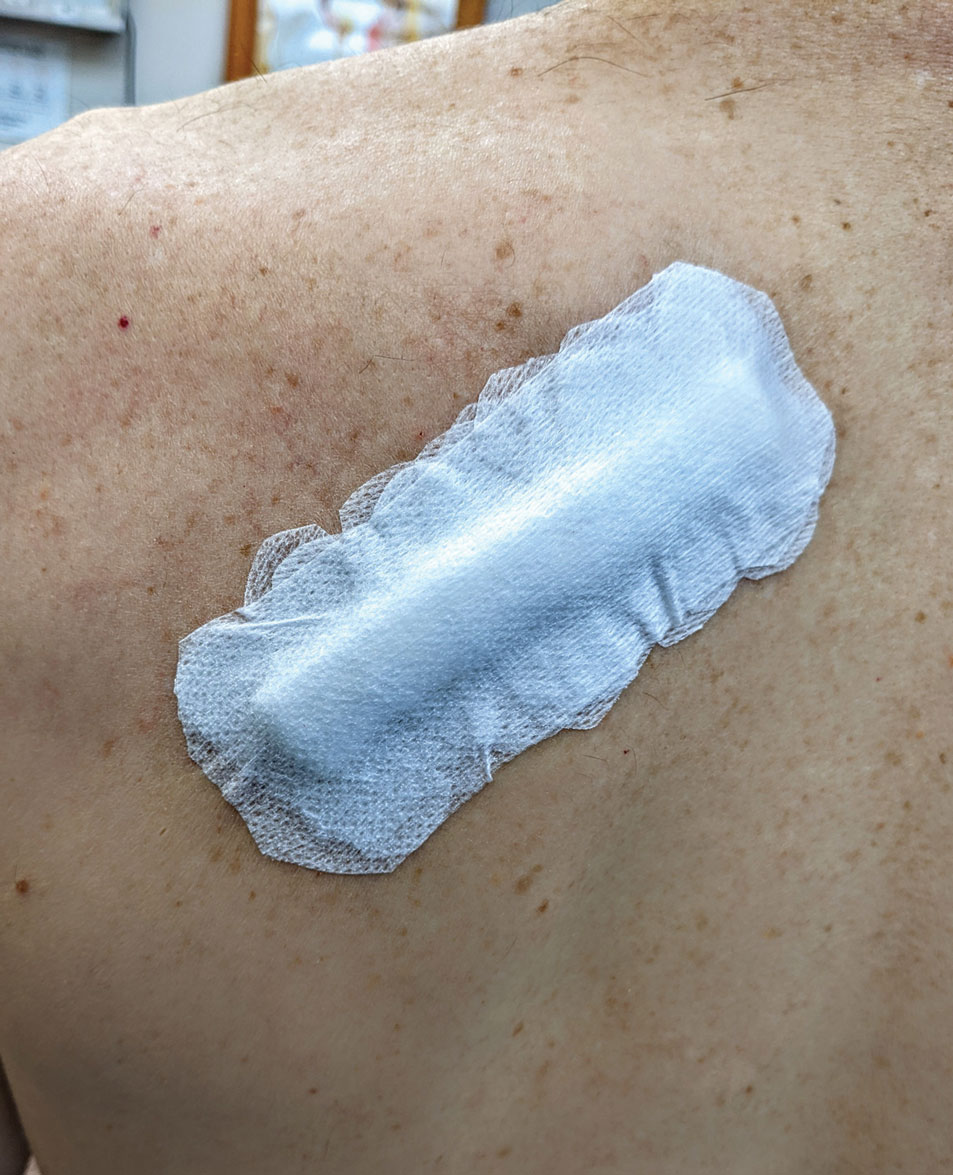
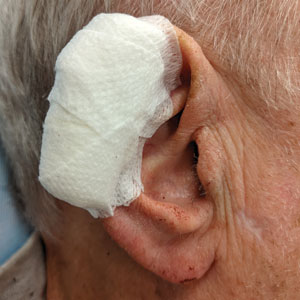
Complex Sites—When making a pressure bandage for an anatomically complex site—the ear, nose, or lip—nonstick pads and 4×4-inch gauze pads can be cut and folded or rolled to match the size and shape of the wound. Flexible polyester tape then conforms to these custom bandage shapes, allowing maintenance of targeted wound pressure (Figure 3).
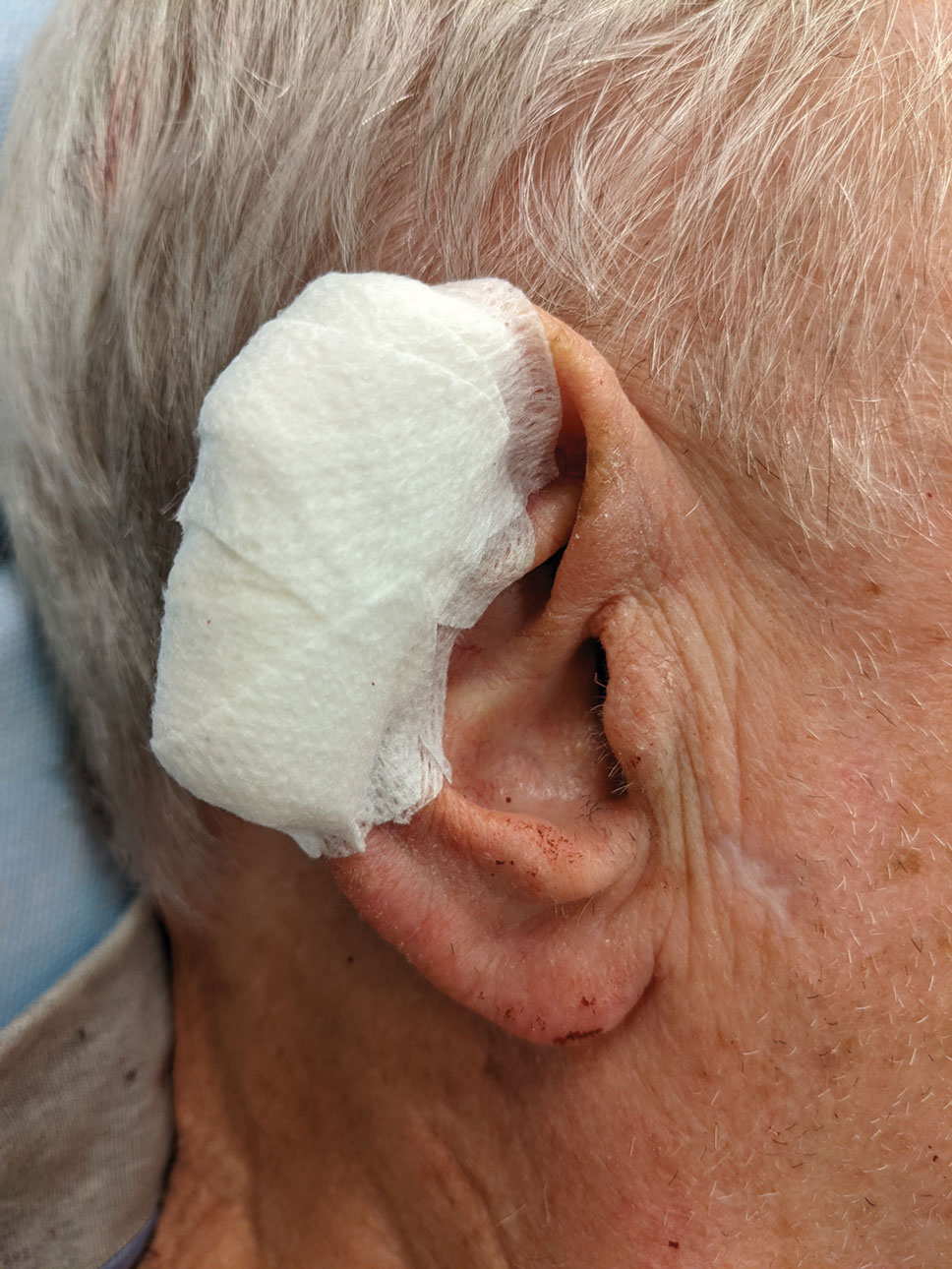
Dental rolls can be of assistance on these sites. For example, a dental roll placed in the postauricular sulcus prior to bandaging an ear maintains comfortable anatomic positioning. Rolls can be placed in the nose, maintaining its architecture while the wound heals and providing counterpressure for added hemostasis of wounds on the lateral nasal sidewall and ala. We recommend coating dental rolls in petrolatum prior to placement in the nares for ease of removal and patient comfort.
Distal Arms and Legs—Another layer of compression is added to pressure bandages on the distal upper and lower extremities using a fabric and elastic wrap (Figure 4). The extra layer keeps the bandage in place on the upper extremities while the patient continues their daily activities. It also helps prevent edema and pain in the lower extremities.
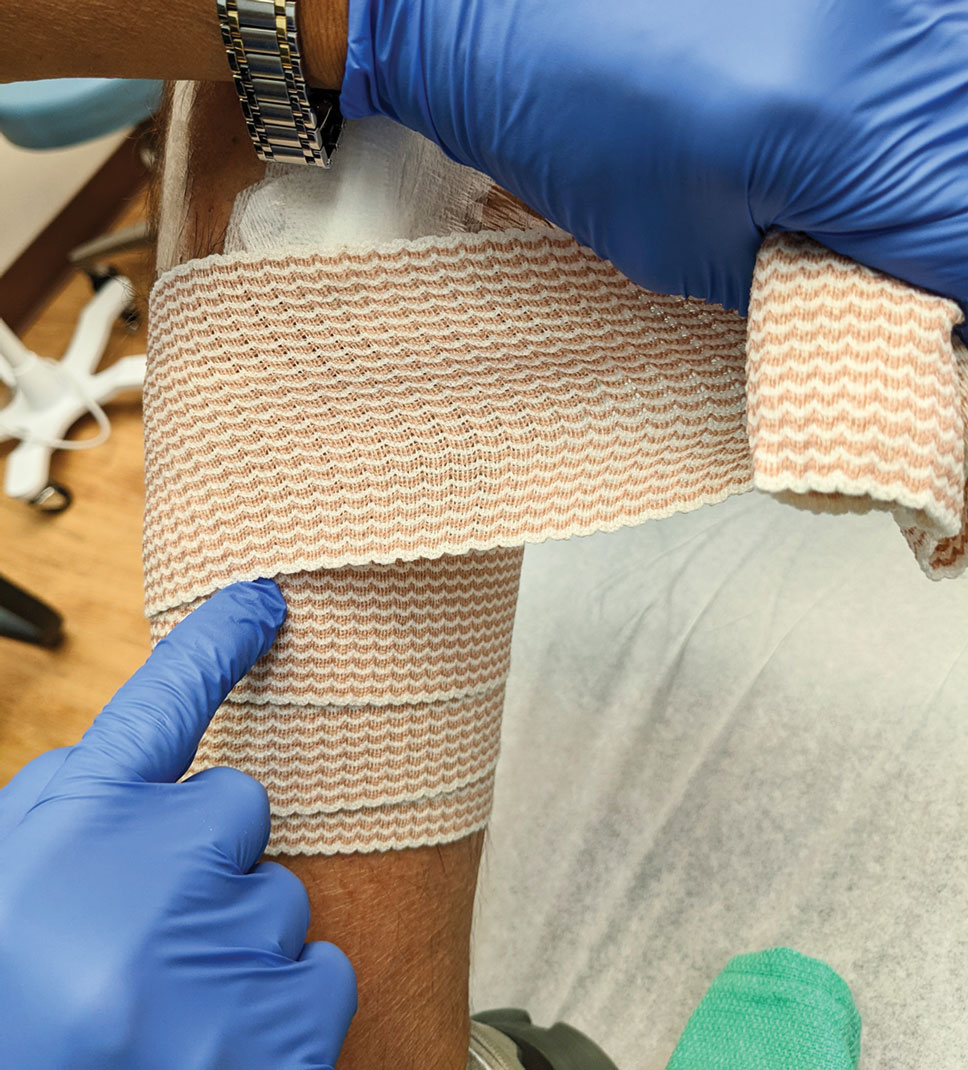
The degree of postoperative lower extremity swelling varies by patient and procedure performed but largely is inevitable with surgery on the leg, given the potential for superficial lymphatic disruption and the dependent position of the leg when standing. Elevation is always advised, but a well-wrapped, long-stretch elastic bandage provides extra support, especially if the patient has baseline venous insufficiency or needs to be on their feet during the day. The wrap is applied from the distal to the proximal leg with graded compression, overlapping by half with each rotation. The wrap is tightest near the ankle, with gradual and subtle easing of tension as it is placed superiorly.
Healing by Secondary Intention, Full-Thickness and Split-Thickness Skin Grafts, and Partial Wound Closure—These postoperative scenarios require bandages with appropriate pressure; however, dressings need to remain moist against the patient’s skin for comfortable removal, which can be accomplished with petrolatum-impregnated gauze with or without antibacterial properties. The gauze is folded to the appropriate size and placed directly on the wound or sutured in place (Figure 5). A pressure bandage is then applied on top of the gauze.
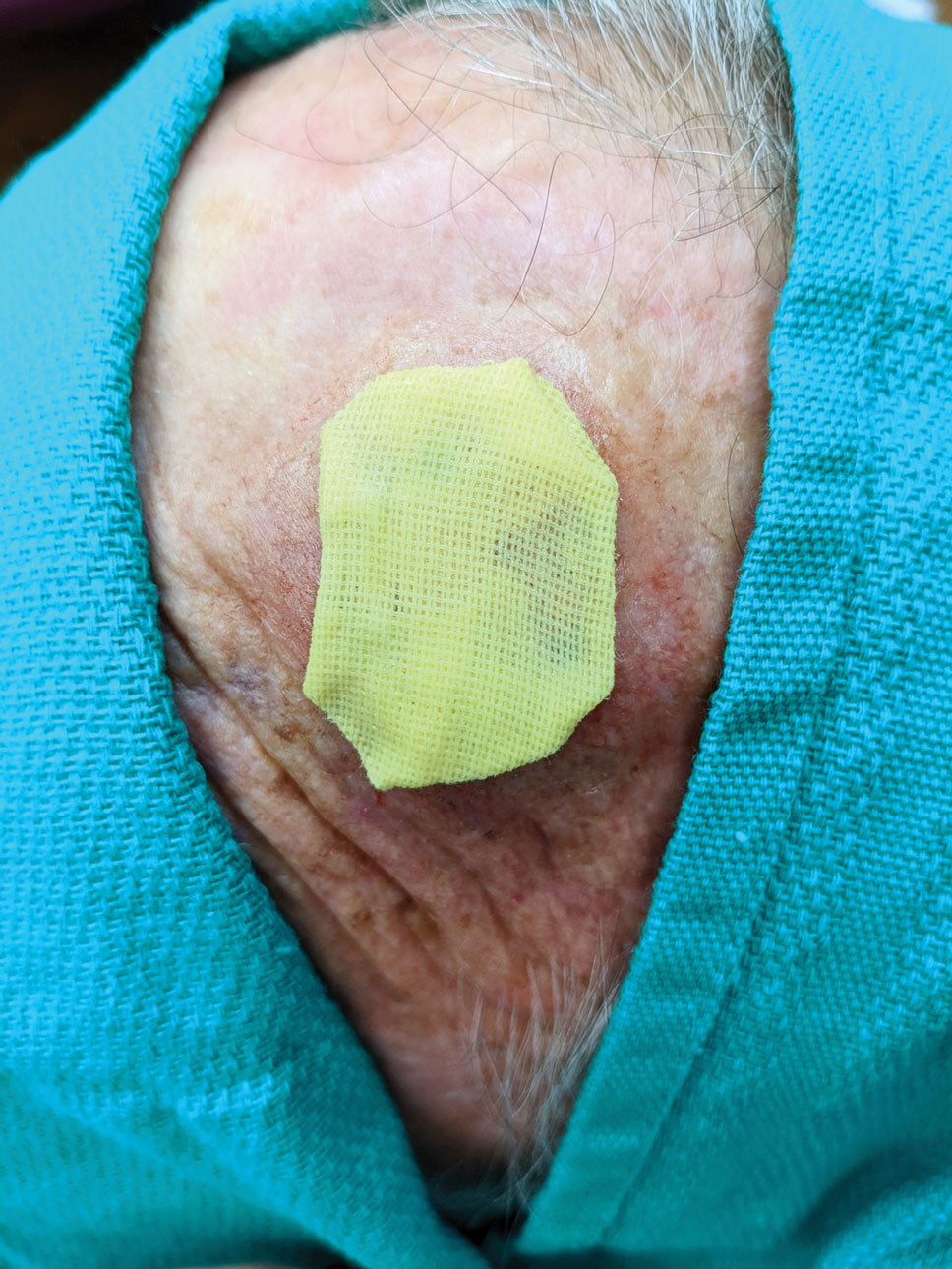
Practice Implications
The universal bandage kit and instructions for its adaptation to accommodate multiple clinical needs can serve as a helpful resource for dermatologists and their staff.
- Bunick CG, Aasi SZ. Hemorrhagic complications in dermatologic surgery. Dermatol Ther. 2011;24:537-550. doi:10.1111/j.1529-8019.2012.01454.x
- Ardilla C, Tarantino I, Goldberg LH, et al. Improved postoperative bleeding control using the fanning pressure dressing technique [published May 31, 2021]. J Am Acad Dermatol. 2021:S0190-9622(21)01040-9. doi:10.1016/j.jaad.2021.05.045
Practice Gap
Biopsies, excisions, and other invasive cutaneous procedures are performed regularly in dermatology clinics and require placement of a bandage after the procedure. Postprocedural bandaging varies by the type of procedure performed, anatomic site, and the physician’s preference of materials. Dermatologists can be left with an overwhelming choice of supplies and little practical education, as bandaging methods are not routinely addressed in residency curricula. To address this concern, we provide a succinct list of basic materials that are versatile and easily adapted to encompass all bandaging needs for dermatology procedures (Table).

With these few components, one can create an array of distinct bandages to cover wounds as small as a shave biopsy to linear closures and basic flaps or grafts. Even traditionally difficult-to-bandage areas are easily addressed. Simple modifications of the basic materials are required for each bandage adaptation, as outlined below.
The Techniques
Shave and Punch Biopsy Sites—Layer (from bottom to top) the emollient of choice, a cut 4×4-inch gauze pad, and flexible polyester tape cut to the appropriate size (Figure 1). This simple bandage conforms well to any anatomic site and can replace an adhesive bandage, if desired.

Cutaneous Surgery Sites—Pressure bandages are recommended on cutaneous surgery sites. One of the most common closures performed in dermatology is the layered closure with dissolvable subcutaneous sutures and nondissolvable cutaneous sutures. When this closure is performed on the trunk and proximal extremities, undermining often is required to adequately approximate skin. This technique eliminates tension on the wound but can increase the risk for hematoma.1 A pressure bandage left in place and kept dry for 48 hours after surgery helps eliminate the risk for postoperative bleeding.
To make a pressure bandage, layer (from bottom to top) the emollient of choice, a nonstick pad cut to size, folded 4×4-inch gauze pads, and flexible polyester tape (Figure 2). Our practice routinely utilizes the tape fanning technique2 to impart equal and firm pressure over the wound.

Complex Sites—When making a pressure bandage for an anatomically complex site—the ear, nose, or lip—nonstick pads and 4×4-inch gauze pads can be cut and folded or rolled to match the size and shape of the wound. Flexible polyester tape then conforms to these custom bandage shapes, allowing maintenance of targeted wound pressure (Figure 3).

Dental rolls can be of assistance on these sites. For example, a dental roll placed in the postauricular sulcus prior to bandaging an ear maintains comfortable anatomic positioning. Rolls can be placed in the nose, maintaining its architecture while the wound heals and providing counterpressure for added hemostasis of wounds on the lateral nasal sidewall and ala. We recommend coating dental rolls in petrolatum prior to placement in the nares for ease of removal and patient comfort.
Distal Arms and Legs—Another layer of compression is added to pressure bandages on the distal upper and lower extremities using a fabric and elastic wrap (Figure 4). The extra layer keeps the bandage in place on the upper extremities while the patient continues their daily activities. It also helps prevent edema and pain in the lower extremities.

The degree of postoperative lower extremity swelling varies by patient and procedure performed but largely is inevitable with surgery on the leg, given the potential for superficial lymphatic disruption and the dependent position of the leg when standing. Elevation is always advised, but a well-wrapped, long-stretch elastic bandage provides extra support, especially if the patient has baseline venous insufficiency or needs to be on their feet during the day. The wrap is applied from the distal to the proximal leg with graded compression, overlapping by half with each rotation. The wrap is tightest near the ankle, with gradual and subtle easing of tension as it is placed superiorly.
Healing by Secondary Intention, Full-Thickness and Split-Thickness Skin Grafts, and Partial Wound Closure—These postoperative scenarios require bandages with appropriate pressure; however, dressings need to remain moist against the patient’s skin for comfortable removal, which can be accomplished with petrolatum-impregnated gauze with or without antibacterial properties. The gauze is folded to the appropriate size and placed directly on the wound or sutured in place (Figure 5). A pressure bandage is then applied on top of the gauze.

Practice Implications
The universal bandage kit and instructions for its adaptation to accommodate multiple clinical needs can serve as a helpful resource for dermatologists and their staff.
Practice Gap
Biopsies, excisions, and other invasive cutaneous procedures are performed regularly in dermatology clinics and require placement of a bandage after the procedure. Postprocedural bandaging varies by the type of procedure performed, anatomic site, and the physician’s preference of materials. Dermatologists can be left with an overwhelming choice of supplies and little practical education, as bandaging methods are not routinely addressed in residency curricula. To address this concern, we provide a succinct list of basic materials that are versatile and easily adapted to encompass all bandaging needs for dermatology procedures (Table).

With these few components, one can create an array of distinct bandages to cover wounds as small as a shave biopsy to linear closures and basic flaps or grafts. Even traditionally difficult-to-bandage areas are easily addressed. Simple modifications of the basic materials are required for each bandage adaptation, as outlined below.
The Techniques
Shave and Punch Biopsy Sites—Layer (from bottom to top) the emollient of choice, a cut 4×4-inch gauze pad, and flexible polyester tape cut to the appropriate size (Figure 1). This simple bandage conforms well to any anatomic site and can replace an adhesive bandage, if desired.

Cutaneous Surgery Sites—Pressure bandages are recommended on cutaneous surgery sites. One of the most common closures performed in dermatology is the layered closure with dissolvable subcutaneous sutures and nondissolvable cutaneous sutures. When this closure is performed on the trunk and proximal extremities, undermining often is required to adequately approximate skin. This technique eliminates tension on the wound but can increase the risk for hematoma.1 A pressure bandage left in place and kept dry for 48 hours after surgery helps eliminate the risk for postoperative bleeding.
To make a pressure bandage, layer (from bottom to top) the emollient of choice, a nonstick pad cut to size, folded 4×4-inch gauze pads, and flexible polyester tape (Figure 2). Our practice routinely utilizes the tape fanning technique2 to impart equal and firm pressure over the wound.

Complex Sites—When making a pressure bandage for an anatomically complex site—the ear, nose, or lip—nonstick pads and 4×4-inch gauze pads can be cut and folded or rolled to match the size and shape of the wound. Flexible polyester tape then conforms to these custom bandage shapes, allowing maintenance of targeted wound pressure (Figure 3).

Dental rolls can be of assistance on these sites. For example, a dental roll placed in the postauricular sulcus prior to bandaging an ear maintains comfortable anatomic positioning. Rolls can be placed in the nose, maintaining its architecture while the wound heals and providing counterpressure for added hemostasis of wounds on the lateral nasal sidewall and ala. We recommend coating dental rolls in petrolatum prior to placement in the nares for ease of removal and patient comfort.
Distal Arms and Legs—Another layer of compression is added to pressure bandages on the distal upper and lower extremities using a fabric and elastic wrap (Figure 4). The extra layer keeps the bandage in place on the upper extremities while the patient continues their daily activities. It also helps prevent edema and pain in the lower extremities.

The degree of postoperative lower extremity swelling varies by patient and procedure performed but largely is inevitable with surgery on the leg, given the potential for superficial lymphatic disruption and the dependent position of the leg when standing. Elevation is always advised, but a well-wrapped, long-stretch elastic bandage provides extra support, especially if the patient has baseline venous insufficiency or needs to be on their feet during the day. The wrap is applied from the distal to the proximal leg with graded compression, overlapping by half with each rotation. The wrap is tightest near the ankle, with gradual and subtle easing of tension as it is placed superiorly.
Healing by Secondary Intention, Full-Thickness and Split-Thickness Skin Grafts, and Partial Wound Closure—These postoperative scenarios require bandages with appropriate pressure; however, dressings need to remain moist against the patient’s skin for comfortable removal, which can be accomplished with petrolatum-impregnated gauze with or without antibacterial properties. The gauze is folded to the appropriate size and placed directly on the wound or sutured in place (Figure 5). A pressure bandage is then applied on top of the gauze.

Practice Implications
The universal bandage kit and instructions for its adaptation to accommodate multiple clinical needs can serve as a helpful resource for dermatologists and their staff.
- Bunick CG, Aasi SZ. Hemorrhagic complications in dermatologic surgery. Dermatol Ther. 2011;24:537-550. doi:10.1111/j.1529-8019.2012.01454.x
- Ardilla C, Tarantino I, Goldberg LH, et al. Improved postoperative bleeding control using the fanning pressure dressing technique [published May 31, 2021]. J Am Acad Dermatol. 2021:S0190-9622(21)01040-9. doi:10.1016/j.jaad.2021.05.045
- Bunick CG, Aasi SZ. Hemorrhagic complications in dermatologic surgery. Dermatol Ther. 2011;24:537-550. doi:10.1111/j.1529-8019.2012.01454.x
- Ardilla C, Tarantino I, Goldberg LH, et al. Improved postoperative bleeding control using the fanning pressure dressing technique [published May 31, 2021]. J Am Acad Dermatol. 2021:S0190-9622(21)01040-9. doi:10.1016/j.jaad.2021.05.045
New Razor Technology Improves Appearance and Quality of Life in Men With Pseudofolliculitis Barbae
Pseudofolliculitis barbae (PFB)(also known as razor bumps or shaving bumps)1 is a skin condition that consists of papules resulting from ingrown hairs.2 In more severe cases, papules become pustules, then abscesses, which can cause scarring.1,2 The condition can be distressing for patients, with considerable negative impact on their daily lives.3 The condition also is associated with shaving-related stinging, burning, pruritus, and cuts on the skin.4
Pseudofolliculitis barbae is most common in men of African descent due to the curved nature of the hair follicle,2,5,6 with an estimated prevalence in this population of 45% to 83%,1,6 but it can affect men of other ethnicities.7 A genetic polymorphism in a gene encoding a keratin specific to the hair follicle also has been found to predispose some individuals to PFB.5 When hair from a curved or destabilized hair follicle is cut to form a sharp tip, it is susceptible to extrafollicular and/or transfollicular penetration,5,6,8 as illustrated in Figure 1.
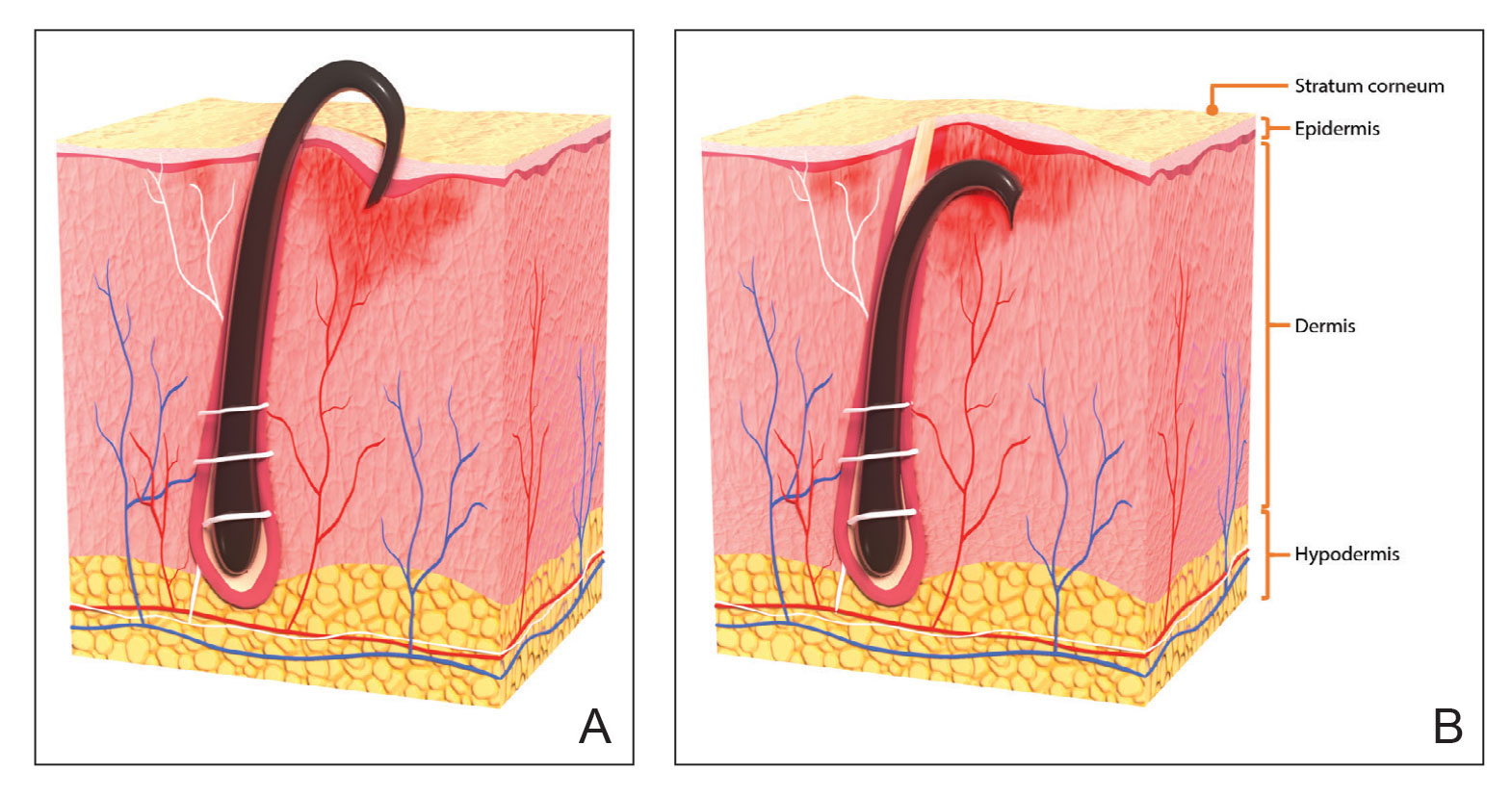
With extrafollicular or transfollicular penetration, the hair shaft re-enters or retracts into the dermis, triggering an inflammatory response that may be exacerbated by subsequent shaving.2 Few studies have been published that aim to identify potential shaving solutions for individuals with PFB who elect to or need to continue shaving.
A new razor technology comprising 2 blades separated by a bridge feature has been designed specifically for men with razor bumps (SkinGuard [Procter & Gamble]). The SkinGuard razor redistributes shaving pressure so that there is less force from the blades on the skin and inflamed lesions than without the bridge, as seen in Figure 2. The razor has been designed to protect the skin from the blades, thereby minimizing the occurrence of new lesions and allowing existing lesions to heal.
![Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company. Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company.](https://cdn.mdedge.com/files/s3fs-public/Moran_2.jpg)
The primary purpose of this study was to assess the appearance of males with razor bumps and shaving irritation when using the new razor technology in a regular shaving routine. The secondary objective was to measure satisfaction of the shaving experience when using the new razor by means of assessing itching, burning, and stinging using the participant global severity assessment (PGSA) and the impact on quality of life (QOL) measures.
Methods
Participants—Eligible participants were male, aged 20 to 60 years, and had clinically diagnosed PFB as well as symptoms of skin irritation from shaving. Participants were recruited from a dermatology clinic and via institutional review board–approved advertising.
Those eligible for inclusion in the study had a shaving routine that comprised shaving at least 3 times a week using a wet-shave, blade-razor technique accompanied by only a shave gel or foam. In addition, eligible participants had mild to moderate symptoms of skin irritation (a minimum of 10 razor bumps) from shaving based on investigator global severity assessment (IGSA) rating scales and were willing to shave at least 5 times a week during the study period. Participants could continue certain topical and systemic interventions for their skin.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
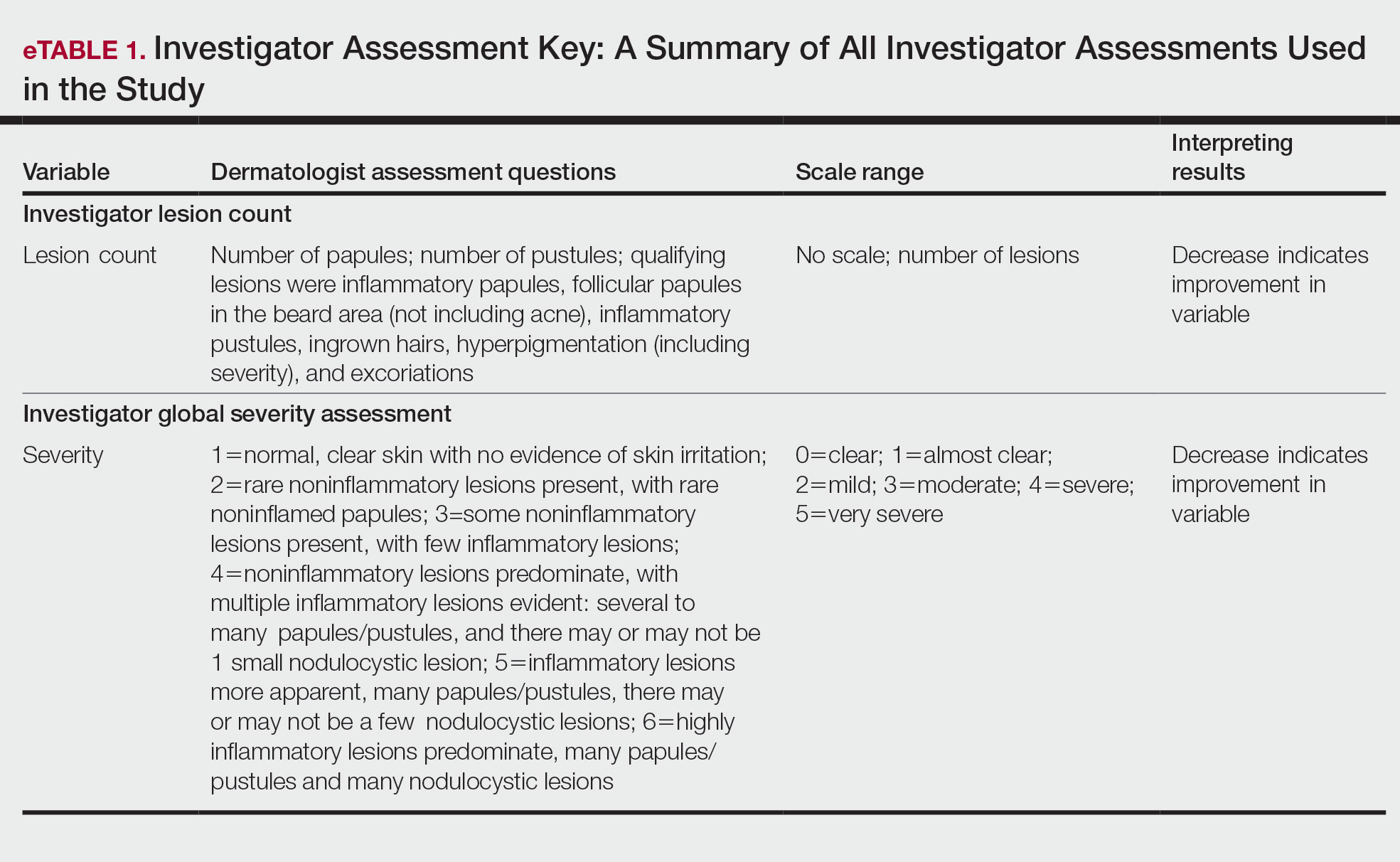
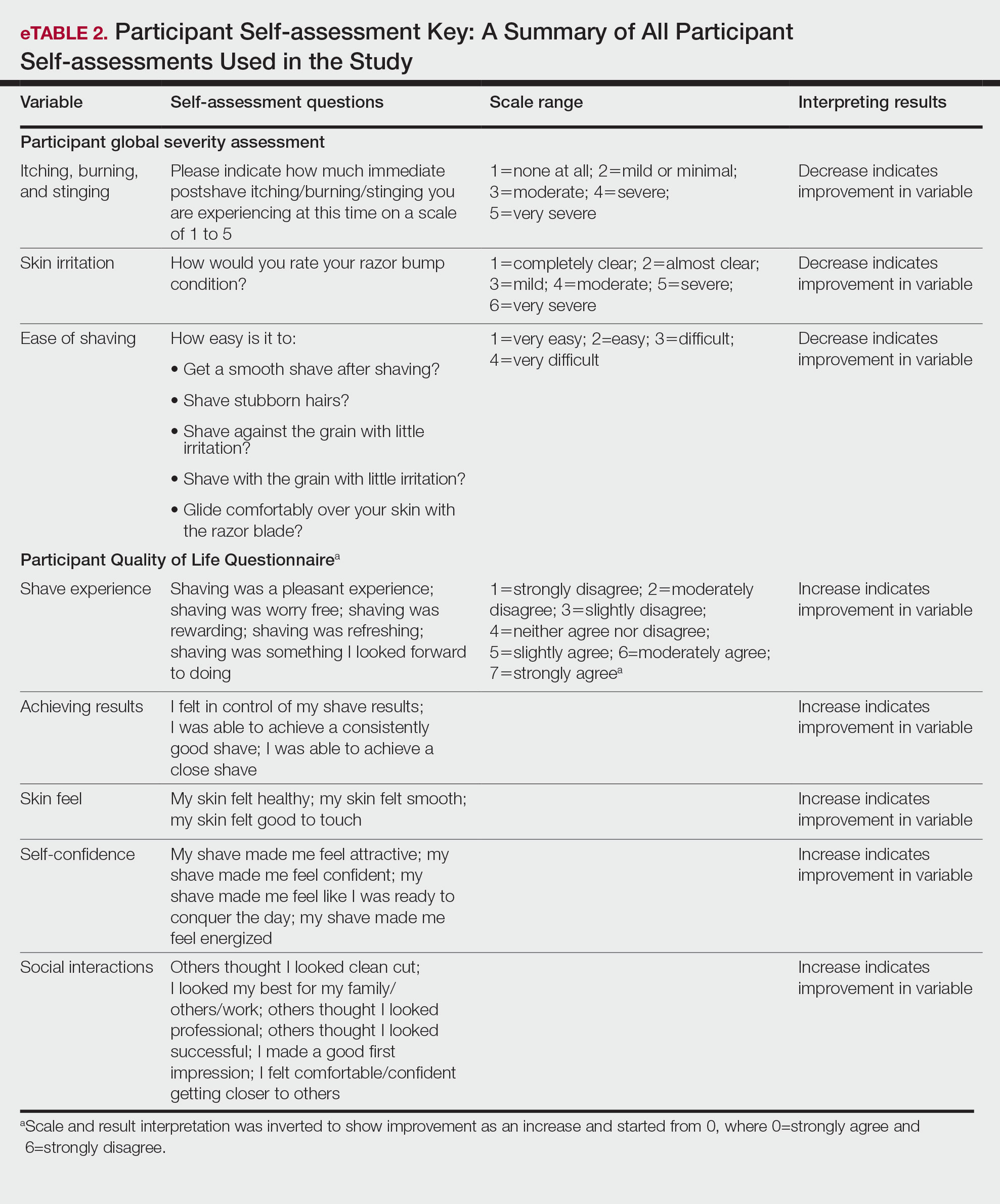
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.
The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.
Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.
Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.
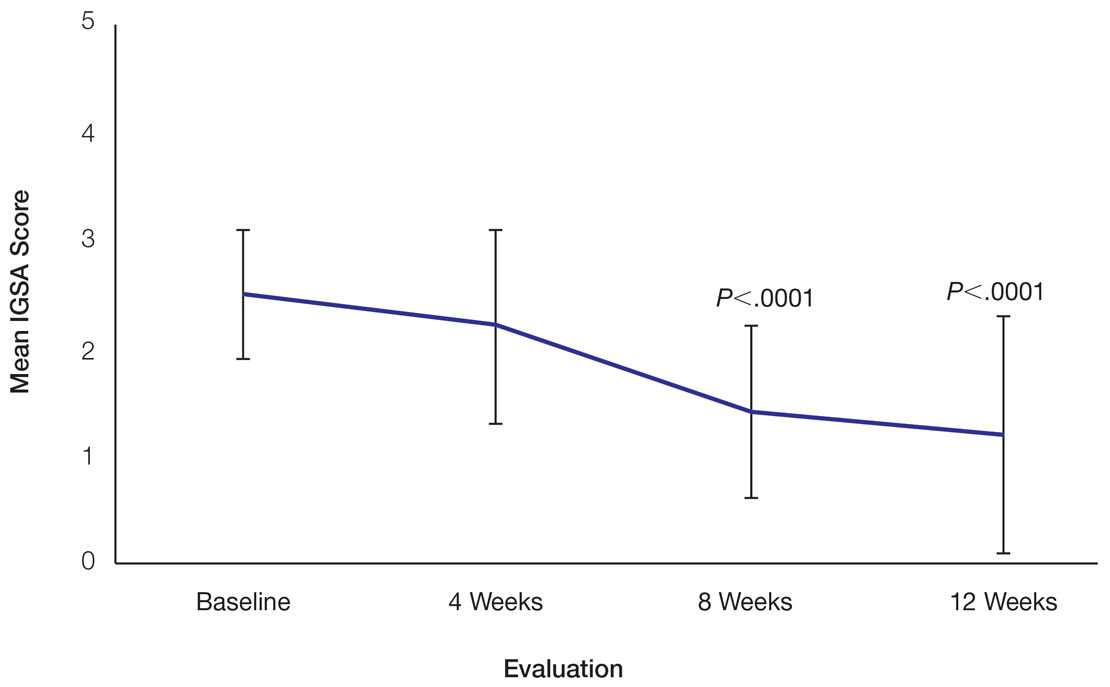
Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.
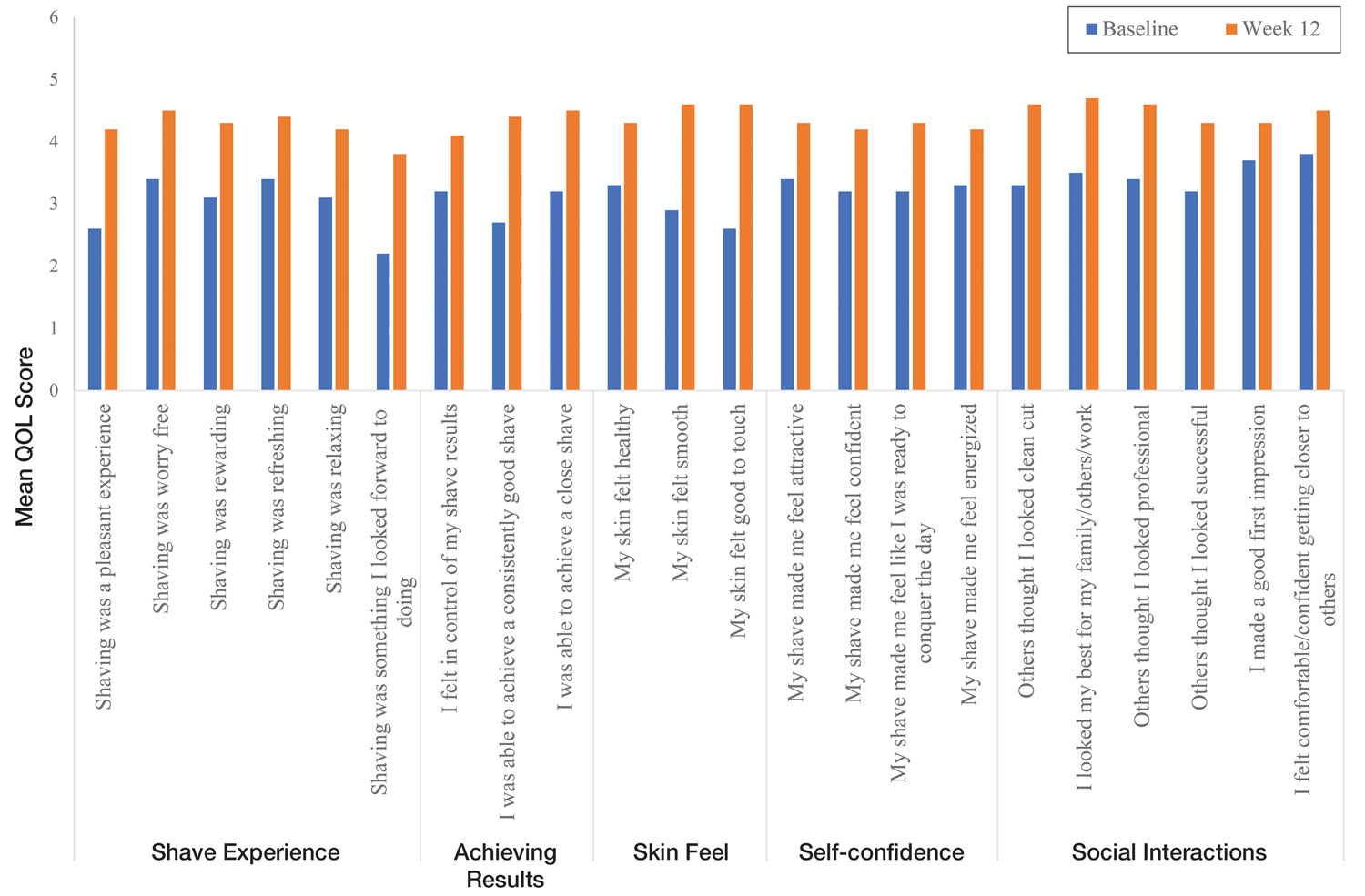
Comment
Improvement With Novel Razor Technology—For the first time, frequent use of a novel razor technology designed specifically for men with PFB was found to significantly improve skin appearance, shave satisfaction, and QOL after 12 weeks vs baseline in participants clinically diagnosed with PFB. In men with shave-related skin irritation and razor bumps who typically wet-shaved with a razor at least 3 times a week, use of the test razor with their regular shaving preparation product 5 or more times per week for 12 weeks was associated with significant improvements from baseline in investigator lesion count, IGSA, PGSA, and Participant Quality of Life Questionnaire measurements.
Study strengths included the quantification of the change in the number of lesions and the degree of severity by a trained investigator in a prospective clinical study along with an assessment of the impact on participant QOL. A lack of a control arm could be considered a limitation of the study; however, study end points were evaluated compared with baseline, with each participant serving as their own control. Spontaneous resolution of the condition with their standard routine was considered highly unlikely in these participants; therefore, in the absence of any other changes, improvements were attributed to regular use of the test product over the course of the study. The results presented here provide strong support for the effectiveness of the new razor technology in improving the appearance of men with razor bumps and shaving irritation.
Hair Removal Tools for the Management of PFB—Although various tools and techniques have been proposed in the past for men with PFB, the current test razor technology provided unique benefits, including improvements in appearance and severity of the condition as well as a positive impact on QOL. In 1979, Conte and Lawrence9 evaluated the effect of using an electric hair clipper and twice-daily use of a skin-cleansing pad on the occurrence of PFB. Participants (n=96) allowed their beards to grow out for 1 month, after which they started shaving with an electric clipper with a triple O head. The authors reported a favorable response in 95% (91/96) of cases. However, the electric clippers left 1 mm of beard at the skin level,9 which may not be acceptable for those who prefer a clean-shaven appearance.6
A prospective survey of 22 men of African descent with PFB found use of a safety razor was preferred over an electric razor.10 The single-arm study evaluated use of a foil-guarded shaver (single-razor blade) in the management of PFB based on investigator lesion counts and a participant questionnaire. Participants were asked to shave at least every other day and use a specially designed preshave brush. A mean reduction in lesion counts was observed at 2 weeks (29.6%), 4 weeks (38.1%), and 6 weeks (47.1%); statistical significance was not reported. At 6 weeks, 77.3% (17/22) of participants judged the foil-guarded shaver to be superior to other shaving devices in controlling their razor bumps, and 90.9% (20/22) indicated they would recommend the shaver to others with PFB. The authors hypothesized that the guard buffered the skin from the blade, which might otherwise facilitate the penetration of ingrowing hairs and cause trauma to existing lesions.
The mean reduction in lesion count from baseline observed at week 4 was greater in the study with the foil-guarded shaver and preshave brush (38% reduction)10 than in our study (19% reduction in papule count). Different methodologies, use of a preshave brush in the earlier study, and a difference in lesion severity at baseline may have contributed to this difference. The study with the foil-guarded shaver concluded after 6 weeks, and there was a 47.1% reduction in lesion counts vs baseline.10 In contrast, the current study continued for 12 weeks, and a 59.6% reduction in lesion counts was reported. Participants from both studies reported an improved shaving experience compared with their usual practice,10 though only the current study explored the positive impact of the new razor technology on participant QOL.
Preventing Hairs From Being Cut Too Close—The closeness of the shave is believed to be a contributory factor in the development and persistence of PFB6,8,11 based on a tendency for the distal portion of tightly curled hair shafts to re-enter the skin after shaving via transfollicular penetration.12 Inclusion of a buffer in the razor between the sharp blades and the skin has been proposed to prevent hairs from being cut too close and causing transfollicular penetration.12
In the test razor used in the current study, the bridge technology acted as the buffer to prevent hairs from being cut too close to the skin and to reduce blade contact with the skin (Figure 2). Having only 2 blades also reduced the closeness of the shave compared with 5-bladed technologies,13 as each hair can only be pulled and cut up to a maximum of 2 times per shaving stroke. Notably, this did not impact the participants’ QOL scores related to achieving a close shave or skin feeling smooth; both attributes were significantly improved at 12 weeks vs baseline (Figure 4).
By reducing blade contact with the skin, the bridge technology in the test razor was designed to prevent excessive force from being applied to the skin through the blades. Reduced blade loading minimizes contact with and impact on sensitive skin.14 Additional design features of the test razor to minimize the impact of shaving on the skin include treatment of the 2 blades with low-friction coatings, which allows the blades to cut through the beard hair with minimal force, helping to reduce the tug-and-pull effect that may otherwise result in irritation and inflammation.13,15 Lubrication strips before and after the blades in the test razor reduce friction between the blades and the skin to further protect the skin from the blades.15
Shaving With Multiblade Razors Does Not Exacerbate PFB—In a 1-week, split-faced, randomized study of 45 Black men, shaving with a manual 3-bladed razor was compared with use of 3 different chemical depilatory formulations.16 Shaving every other day for 1 week with the manual razor resulted in more papule formation but less irritation than use of the depilatories. The authors concluded that a study with longer duration was needed to explore the impact of shaving on papule formation in participants with a history of PFB.16
In 2013, an investigator-blinded study of 90 African American men with PFB compared the impact of different shaving regimens on the signs and symptoms of PFB over a 12-week period.4 Participants were randomized to 1 of 3 arms: (1) shaving 2 to 3 times per week with a triple-blade razor and standard products (control group); (2) shaving daily with a 5-bladed razor and standard products; and (3) shaving daily with a 5-bladed razor and “advanced” specific pre- and postshave products. The researchers found that the mean papule measurement significantly decreased from baseline in the advanced (P=.01) and control (P=.016) groups. Between-group comparison revealed no significant differences for papule or pustule count among each arm. For the investigator-graded severity, the change from baseline was significant for all 3 groups (P≤.04); however, the differences among groups were not significant. Importantly, these data demonstrated that PFB was not exacerbated by multiblade razors used as part of a daily shaving regimen.4
The findings of the current study were consistent with those of Daniel et al4 in that there was no exacerbation of the signs and symptoms of PFB associated with daily shaving. However, rather than requiring participants to change their entire shaving regimen, the present study only required a change of razor type. Moreover, the use of the new razor technology significantly decreased papule counts at week 12 vs the baseline measurement (P<.0001) and was associated with an improvement in subjective skin severity measurements. The participants in the present study reported significantly less burning, stinging, and itching after using the test product for 12 weeks (P<.0001).
Impact of Treatment on QOL—The current study further expanded on prior findings by combining these clinical end points with the QOL results to assess the test razor’s impact on participants’ lives. Results showed that over the course of 12 weeks, the new razor technology significantly improved the participants’ QOL in all questions related to shaving experience, achieving results, skin feel, self-confidence, and social interactions. The significant improvement in QOL included statements such as “shaving was a pleasant experience,” “I was able to achieve a consistently good shave,” and “my skin felt smooth.” Participants also reported improvements in meaningful categories such as “my shave made me feel attractive” and “I felt comfortable/confident getting closer to others.” As the current study showed, a shave regimen has the potential to change participants’ overall assessment of their QOL, a variable that must not be overlooked.
Conclusion
In men with clinically diagnosed PFB, regular shaving with a razor designed to protect the skin was found to significantly decrease lesion counts, increase shave satisfaction, and improve QOL after 12 weeks compared with their usual shaving practice (baseline measures). This razor technology provides another option to help manage PFB for men who wish to or need to continue shaving.
Acknowledgments—The clinical study was funded by the Procter & Gamble Company. Editorial writing assistance, supported financially by the Procter & Gamble Company, was provided by Gill McFeat, PhD, of McFeat Science Ltd (Devon, United Kingdom).
- Alexander AM, Delph WI. Pseudofolliculitis barbae in the military. a medical, administrative and social problem. J Natl Med Assoc. 1974;66:459-464, 479.
- Kligman AM, Strauss JS. Pseudofolliculitis of the beard. AMA Arch Derm. 1956;74:533-542.
- Banta J, Bowen C, Wong E, et al. Perceptions of shaving profiles and their potential impacts on career progression in the United States Air Force. Mil Med. 2021;186:187-189.
- Daniel A, Gustafson CJ, Zupkosky PJ, et al. Shave frequency and regimen variation effects on the management of pseudofolliculitis barbae. J Drugs Dermatol. 2013;12:410-418.
- Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652-657.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21:629-644.
- Ribera M, Fernández-Chico N, Casals M. Pseudofolliculitis barbae [in Spanish]. Actas Dermosifiliogr. 2010;101:749-757.
- Conte MS, Lawrence JE. Pseudofolliculitis barbae. no ‘pseudoproblem.’ JAMA. 1979;241:53-54.
- Alexander AM. Evaluation of a foil-guarded shaver in the management of pseudofolliculitis barbae. Cutis. 1981;27:534-537, 540-542.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328;331-333.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Cowley K, Vanoosthuyze K, Ertel K, et al. Blade shaving. In: Draelos ZD, ed. Cosmetic Dermatology: Products and Procedures. 2nd ed. John Wiley & Sons; 2015:166-173.
- Cowley K, Vanoosthuyze K. Insights into shaving and its impact on skin. Br J Dermatol. 2012;166(suppl 1):6-12.
- Cowley K, Vanoosthuyze K. The biomechanics of blade shaving. Int J Cosmet Sci. 2016;38(suppl 1):17-23.
- Kindred C, Oresajo CO, Yatskayer M, et al. Comparative evaluation of men’s depilatory composition versus razor in black men. Cutis. 2011;88:98-103.
Pseudofolliculitis barbae (PFB)(also known as razor bumps or shaving bumps)1 is a skin condition that consists of papules resulting from ingrown hairs.2 In more severe cases, papules become pustules, then abscesses, which can cause scarring.1,2 The condition can be distressing for patients, with considerable negative impact on their daily lives.3 The condition also is associated with shaving-related stinging, burning, pruritus, and cuts on the skin.4
Pseudofolliculitis barbae is most common in men of African descent due to the curved nature of the hair follicle,2,5,6 with an estimated prevalence in this population of 45% to 83%,1,6 but it can affect men of other ethnicities.7 A genetic polymorphism in a gene encoding a keratin specific to the hair follicle also has been found to predispose some individuals to PFB.5 When hair from a curved or destabilized hair follicle is cut to form a sharp tip, it is susceptible to extrafollicular and/or transfollicular penetration,5,6,8 as illustrated in Figure 1.

With extrafollicular or transfollicular penetration, the hair shaft re-enters or retracts into the dermis, triggering an inflammatory response that may be exacerbated by subsequent shaving.2 Few studies have been published that aim to identify potential shaving solutions for individuals with PFB who elect to or need to continue shaving.
A new razor technology comprising 2 blades separated by a bridge feature has been designed specifically for men with razor bumps (SkinGuard [Procter & Gamble]). The SkinGuard razor redistributes shaving pressure so that there is less force from the blades on the skin and inflamed lesions than without the bridge, as seen in Figure 2. The razor has been designed to protect the skin from the blades, thereby minimizing the occurrence of new lesions and allowing existing lesions to heal.
![Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company. Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company.](https://cdn.mdedge.com/files/s3fs-public/Moran_2.jpg)
The primary purpose of this study was to assess the appearance of males with razor bumps and shaving irritation when using the new razor technology in a regular shaving routine. The secondary objective was to measure satisfaction of the shaving experience when using the new razor by means of assessing itching, burning, and stinging using the participant global severity assessment (PGSA) and the impact on quality of life (QOL) measures.
Methods
Participants—Eligible participants were male, aged 20 to 60 years, and had clinically diagnosed PFB as well as symptoms of skin irritation from shaving. Participants were recruited from a dermatology clinic and via institutional review board–approved advertising.
Those eligible for inclusion in the study had a shaving routine that comprised shaving at least 3 times a week using a wet-shave, blade-razor technique accompanied by only a shave gel or foam. In addition, eligible participants had mild to moderate symptoms of skin irritation (a minimum of 10 razor bumps) from shaving based on investigator global severity assessment (IGSA) rating scales and were willing to shave at least 5 times a week during the study period. Participants could continue certain topical and systemic interventions for their skin.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.

The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.

Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.
Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.

Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.

Comment
Improvement With Novel Razor Technology—For the first time, frequent use of a novel razor technology designed specifically for men with PFB was found to significantly improve skin appearance, shave satisfaction, and QOL after 12 weeks vs baseline in participants clinically diagnosed with PFB. In men with shave-related skin irritation and razor bumps who typically wet-shaved with a razor at least 3 times a week, use of the test razor with their regular shaving preparation product 5 or more times per week for 12 weeks was associated with significant improvements from baseline in investigator lesion count, IGSA, PGSA, and Participant Quality of Life Questionnaire measurements.
Study strengths included the quantification of the change in the number of lesions and the degree of severity by a trained investigator in a prospective clinical study along with an assessment of the impact on participant QOL. A lack of a control arm could be considered a limitation of the study; however, study end points were evaluated compared with baseline, with each participant serving as their own control. Spontaneous resolution of the condition with their standard routine was considered highly unlikely in these participants; therefore, in the absence of any other changes, improvements were attributed to regular use of the test product over the course of the study. The results presented here provide strong support for the effectiveness of the new razor technology in improving the appearance of men with razor bumps and shaving irritation.
Hair Removal Tools for the Management of PFB—Although various tools and techniques have been proposed in the past for men with PFB, the current test razor technology provided unique benefits, including improvements in appearance and severity of the condition as well as a positive impact on QOL. In 1979, Conte and Lawrence9 evaluated the effect of using an electric hair clipper and twice-daily use of a skin-cleansing pad on the occurrence of PFB. Participants (n=96) allowed their beards to grow out for 1 month, after which they started shaving with an electric clipper with a triple O head. The authors reported a favorable response in 95% (91/96) of cases. However, the electric clippers left 1 mm of beard at the skin level,9 which may not be acceptable for those who prefer a clean-shaven appearance.6
A prospective survey of 22 men of African descent with PFB found use of a safety razor was preferred over an electric razor.10 The single-arm study evaluated use of a foil-guarded shaver (single-razor blade) in the management of PFB based on investigator lesion counts and a participant questionnaire. Participants were asked to shave at least every other day and use a specially designed preshave brush. A mean reduction in lesion counts was observed at 2 weeks (29.6%), 4 weeks (38.1%), and 6 weeks (47.1%); statistical significance was not reported. At 6 weeks, 77.3% (17/22) of participants judged the foil-guarded shaver to be superior to other shaving devices in controlling their razor bumps, and 90.9% (20/22) indicated they would recommend the shaver to others with PFB. The authors hypothesized that the guard buffered the skin from the blade, which might otherwise facilitate the penetration of ingrowing hairs and cause trauma to existing lesions.
The mean reduction in lesion count from baseline observed at week 4 was greater in the study with the foil-guarded shaver and preshave brush (38% reduction)10 than in our study (19% reduction in papule count). Different methodologies, use of a preshave brush in the earlier study, and a difference in lesion severity at baseline may have contributed to this difference. The study with the foil-guarded shaver concluded after 6 weeks, and there was a 47.1% reduction in lesion counts vs baseline.10 In contrast, the current study continued for 12 weeks, and a 59.6% reduction in lesion counts was reported. Participants from both studies reported an improved shaving experience compared with their usual practice,10 though only the current study explored the positive impact of the new razor technology on participant QOL.
Preventing Hairs From Being Cut Too Close—The closeness of the shave is believed to be a contributory factor in the development and persistence of PFB6,8,11 based on a tendency for the distal portion of tightly curled hair shafts to re-enter the skin after shaving via transfollicular penetration.12 Inclusion of a buffer in the razor between the sharp blades and the skin has been proposed to prevent hairs from being cut too close and causing transfollicular penetration.12
In the test razor used in the current study, the bridge technology acted as the buffer to prevent hairs from being cut too close to the skin and to reduce blade contact with the skin (Figure 2). Having only 2 blades also reduced the closeness of the shave compared with 5-bladed technologies,13 as each hair can only be pulled and cut up to a maximum of 2 times per shaving stroke. Notably, this did not impact the participants’ QOL scores related to achieving a close shave or skin feeling smooth; both attributes were significantly improved at 12 weeks vs baseline (Figure 4).
By reducing blade contact with the skin, the bridge technology in the test razor was designed to prevent excessive force from being applied to the skin through the blades. Reduced blade loading minimizes contact with and impact on sensitive skin.14 Additional design features of the test razor to minimize the impact of shaving on the skin include treatment of the 2 blades with low-friction coatings, which allows the blades to cut through the beard hair with minimal force, helping to reduce the tug-and-pull effect that may otherwise result in irritation and inflammation.13,15 Lubrication strips before and after the blades in the test razor reduce friction between the blades and the skin to further protect the skin from the blades.15
Shaving With Multiblade Razors Does Not Exacerbate PFB—In a 1-week, split-faced, randomized study of 45 Black men, shaving with a manual 3-bladed razor was compared with use of 3 different chemical depilatory formulations.16 Shaving every other day for 1 week with the manual razor resulted in more papule formation but less irritation than use of the depilatories. The authors concluded that a study with longer duration was needed to explore the impact of shaving on papule formation in participants with a history of PFB.16
In 2013, an investigator-blinded study of 90 African American men with PFB compared the impact of different shaving regimens on the signs and symptoms of PFB over a 12-week period.4 Participants were randomized to 1 of 3 arms: (1) shaving 2 to 3 times per week with a triple-blade razor and standard products (control group); (2) shaving daily with a 5-bladed razor and standard products; and (3) shaving daily with a 5-bladed razor and “advanced” specific pre- and postshave products. The researchers found that the mean papule measurement significantly decreased from baseline in the advanced (P=.01) and control (P=.016) groups. Between-group comparison revealed no significant differences for papule or pustule count among each arm. For the investigator-graded severity, the change from baseline was significant for all 3 groups (P≤.04); however, the differences among groups were not significant. Importantly, these data demonstrated that PFB was not exacerbated by multiblade razors used as part of a daily shaving regimen.4
The findings of the current study were consistent with those of Daniel et al4 in that there was no exacerbation of the signs and symptoms of PFB associated with daily shaving. However, rather than requiring participants to change their entire shaving regimen, the present study only required a change of razor type. Moreover, the use of the new razor technology significantly decreased papule counts at week 12 vs the baseline measurement (P<.0001) and was associated with an improvement in subjective skin severity measurements. The participants in the present study reported significantly less burning, stinging, and itching after using the test product for 12 weeks (P<.0001).
Impact of Treatment on QOL—The current study further expanded on prior findings by combining these clinical end points with the QOL results to assess the test razor’s impact on participants’ lives. Results showed that over the course of 12 weeks, the new razor technology significantly improved the participants’ QOL in all questions related to shaving experience, achieving results, skin feel, self-confidence, and social interactions. The significant improvement in QOL included statements such as “shaving was a pleasant experience,” “I was able to achieve a consistently good shave,” and “my skin felt smooth.” Participants also reported improvements in meaningful categories such as “my shave made me feel attractive” and “I felt comfortable/confident getting closer to others.” As the current study showed, a shave regimen has the potential to change participants’ overall assessment of their QOL, a variable that must not be overlooked.
Conclusion
In men with clinically diagnosed PFB, regular shaving with a razor designed to protect the skin was found to significantly decrease lesion counts, increase shave satisfaction, and improve QOL after 12 weeks compared with their usual shaving practice (baseline measures). This razor technology provides another option to help manage PFB for men who wish to or need to continue shaving.
Acknowledgments—The clinical study was funded by the Procter & Gamble Company. Editorial writing assistance, supported financially by the Procter & Gamble Company, was provided by Gill McFeat, PhD, of McFeat Science Ltd (Devon, United Kingdom).
Pseudofolliculitis barbae (PFB)(also known as razor bumps or shaving bumps)1 is a skin condition that consists of papules resulting from ingrown hairs.2 In more severe cases, papules become pustules, then abscesses, which can cause scarring.1,2 The condition can be distressing for patients, with considerable negative impact on their daily lives.3 The condition also is associated with shaving-related stinging, burning, pruritus, and cuts on the skin.4
Pseudofolliculitis barbae is most common in men of African descent due to the curved nature of the hair follicle,2,5,6 with an estimated prevalence in this population of 45% to 83%,1,6 but it can affect men of other ethnicities.7 A genetic polymorphism in a gene encoding a keratin specific to the hair follicle also has been found to predispose some individuals to PFB.5 When hair from a curved or destabilized hair follicle is cut to form a sharp tip, it is susceptible to extrafollicular and/or transfollicular penetration,5,6,8 as illustrated in Figure 1.

With extrafollicular or transfollicular penetration, the hair shaft re-enters or retracts into the dermis, triggering an inflammatory response that may be exacerbated by subsequent shaving.2 Few studies have been published that aim to identify potential shaving solutions for individuals with PFB who elect to or need to continue shaving.
A new razor technology comprising 2 blades separated by a bridge feature has been designed specifically for men with razor bumps (SkinGuard [Procter & Gamble]). The SkinGuard razor redistributes shaving pressure so that there is less force from the blades on the skin and inflamed lesions than without the bridge, as seen in Figure 2. The razor has been designed to protect the skin from the blades, thereby minimizing the occurrence of new lesions and allowing existing lesions to heal.
![Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company. Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company.](https://cdn.mdedge.com/files/s3fs-public/Moran_2.jpg)
The primary purpose of this study was to assess the appearance of males with razor bumps and shaving irritation when using the new razor technology in a regular shaving routine. The secondary objective was to measure satisfaction of the shaving experience when using the new razor by means of assessing itching, burning, and stinging using the participant global severity assessment (PGSA) and the impact on quality of life (QOL) measures.
Methods
Participants—Eligible participants were male, aged 20 to 60 years, and had clinically diagnosed PFB as well as symptoms of skin irritation from shaving. Participants were recruited from a dermatology clinic and via institutional review board–approved advertising.
Those eligible for inclusion in the study had a shaving routine that comprised shaving at least 3 times a week using a wet-shave, blade-razor technique accompanied by only a shave gel or foam. In addition, eligible participants had mild to moderate symptoms of skin irritation (a minimum of 10 razor bumps) from shaving based on investigator global severity assessment (IGSA) rating scales and were willing to shave at least 5 times a week during the study period. Participants could continue certain topical and systemic interventions for their skin.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.

The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.

Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.
Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.

Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.

Comment
Improvement With Novel Razor Technology—For the first time, frequent use of a novel razor technology designed specifically for men with PFB was found to significantly improve skin appearance, shave satisfaction, and QOL after 12 weeks vs baseline in participants clinically diagnosed with PFB. In men with shave-related skin irritation and razor bumps who typically wet-shaved with a razor at least 3 times a week, use of the test razor with their regular shaving preparation product 5 or more times per week for 12 weeks was associated with significant improvements from baseline in investigator lesion count, IGSA, PGSA, and Participant Quality of Life Questionnaire measurements.
Study strengths included the quantification of the change in the number of lesions and the degree of severity by a trained investigator in a prospective clinical study along with an assessment of the impact on participant QOL. A lack of a control arm could be considered a limitation of the study; however, study end points were evaluated compared with baseline, with each participant serving as their own control. Spontaneous resolution of the condition with their standard routine was considered highly unlikely in these participants; therefore, in the absence of any other changes, improvements were attributed to regular use of the test product over the course of the study. The results presented here provide strong support for the effectiveness of the new razor technology in improving the appearance of men with razor bumps and shaving irritation.
Hair Removal Tools for the Management of PFB—Although various tools and techniques have been proposed in the past for men with PFB, the current test razor technology provided unique benefits, including improvements in appearance and severity of the condition as well as a positive impact on QOL. In 1979, Conte and Lawrence9 evaluated the effect of using an electric hair clipper and twice-daily use of a skin-cleansing pad on the occurrence of PFB. Participants (n=96) allowed their beards to grow out for 1 month, after which they started shaving with an electric clipper with a triple O head. The authors reported a favorable response in 95% (91/96) of cases. However, the electric clippers left 1 mm of beard at the skin level,9 which may not be acceptable for those who prefer a clean-shaven appearance.6
A prospective survey of 22 men of African descent with PFB found use of a safety razor was preferred over an electric razor.10 The single-arm study evaluated use of a foil-guarded shaver (single-razor blade) in the management of PFB based on investigator lesion counts and a participant questionnaire. Participants were asked to shave at least every other day and use a specially designed preshave brush. A mean reduction in lesion counts was observed at 2 weeks (29.6%), 4 weeks (38.1%), and 6 weeks (47.1%); statistical significance was not reported. At 6 weeks, 77.3% (17/22) of participants judged the foil-guarded shaver to be superior to other shaving devices in controlling their razor bumps, and 90.9% (20/22) indicated they would recommend the shaver to others with PFB. The authors hypothesized that the guard buffered the skin from the blade, which might otherwise facilitate the penetration of ingrowing hairs and cause trauma to existing lesions.
The mean reduction in lesion count from baseline observed at week 4 was greater in the study with the foil-guarded shaver and preshave brush (38% reduction)10 than in our study (19% reduction in papule count). Different methodologies, use of a preshave brush in the earlier study, and a difference in lesion severity at baseline may have contributed to this difference. The study with the foil-guarded shaver concluded after 6 weeks, and there was a 47.1% reduction in lesion counts vs baseline.10 In contrast, the current study continued for 12 weeks, and a 59.6% reduction in lesion counts was reported. Participants from both studies reported an improved shaving experience compared with their usual practice,10 though only the current study explored the positive impact of the new razor technology on participant QOL.
Preventing Hairs From Being Cut Too Close—The closeness of the shave is believed to be a contributory factor in the development and persistence of PFB6,8,11 based on a tendency for the distal portion of tightly curled hair shafts to re-enter the skin after shaving via transfollicular penetration.12 Inclusion of a buffer in the razor between the sharp blades and the skin has been proposed to prevent hairs from being cut too close and causing transfollicular penetration.12
In the test razor used in the current study, the bridge technology acted as the buffer to prevent hairs from being cut too close to the skin and to reduce blade contact with the skin (Figure 2). Having only 2 blades also reduced the closeness of the shave compared with 5-bladed technologies,13 as each hair can only be pulled and cut up to a maximum of 2 times per shaving stroke. Notably, this did not impact the participants’ QOL scores related to achieving a close shave or skin feeling smooth; both attributes were significantly improved at 12 weeks vs baseline (Figure 4).
By reducing blade contact with the skin, the bridge technology in the test razor was designed to prevent excessive force from being applied to the skin through the blades. Reduced blade loading minimizes contact with and impact on sensitive skin.14 Additional design features of the test razor to minimize the impact of shaving on the skin include treatment of the 2 blades with low-friction coatings, which allows the blades to cut through the beard hair with minimal force, helping to reduce the tug-and-pull effect that may otherwise result in irritation and inflammation.13,15 Lubrication strips before and after the blades in the test razor reduce friction between the blades and the skin to further protect the skin from the blades.15
Shaving With Multiblade Razors Does Not Exacerbate PFB—In a 1-week, split-faced, randomized study of 45 Black men, shaving with a manual 3-bladed razor was compared with use of 3 different chemical depilatory formulations.16 Shaving every other day for 1 week with the manual razor resulted in more papule formation but less irritation than use of the depilatories. The authors concluded that a study with longer duration was needed to explore the impact of shaving on papule formation in participants with a history of PFB.16
In 2013, an investigator-blinded study of 90 African American men with PFB compared the impact of different shaving regimens on the signs and symptoms of PFB over a 12-week period.4 Participants were randomized to 1 of 3 arms: (1) shaving 2 to 3 times per week with a triple-blade razor and standard products (control group); (2) shaving daily with a 5-bladed razor and standard products; and (3) shaving daily with a 5-bladed razor and “advanced” specific pre- and postshave products. The researchers found that the mean papule measurement significantly decreased from baseline in the advanced (P=.01) and control (P=.016) groups. Between-group comparison revealed no significant differences for papule or pustule count among each arm. For the investigator-graded severity, the change from baseline was significant for all 3 groups (P≤.04); however, the differences among groups were not significant. Importantly, these data demonstrated that PFB was not exacerbated by multiblade razors used as part of a daily shaving regimen.4
The findings of the current study were consistent with those of Daniel et al4 in that there was no exacerbation of the signs and symptoms of PFB associated with daily shaving. However, rather than requiring participants to change their entire shaving regimen, the present study only required a change of razor type. Moreover, the use of the new razor technology significantly decreased papule counts at week 12 vs the baseline measurement (P<.0001) and was associated with an improvement in subjective skin severity measurements. The participants in the present study reported significantly less burning, stinging, and itching after using the test product for 12 weeks (P<.0001).
Impact of Treatment on QOL—The current study further expanded on prior findings by combining these clinical end points with the QOL results to assess the test razor’s impact on participants’ lives. Results showed that over the course of 12 weeks, the new razor technology significantly improved the participants’ QOL in all questions related to shaving experience, achieving results, skin feel, self-confidence, and social interactions. The significant improvement in QOL included statements such as “shaving was a pleasant experience,” “I was able to achieve a consistently good shave,” and “my skin felt smooth.” Participants also reported improvements in meaningful categories such as “my shave made me feel attractive” and “I felt comfortable/confident getting closer to others.” As the current study showed, a shave regimen has the potential to change participants’ overall assessment of their QOL, a variable that must not be overlooked.
Conclusion
In men with clinically diagnosed PFB, regular shaving with a razor designed to protect the skin was found to significantly decrease lesion counts, increase shave satisfaction, and improve QOL after 12 weeks compared with their usual shaving practice (baseline measures). This razor technology provides another option to help manage PFB for men who wish to or need to continue shaving.
Acknowledgments—The clinical study was funded by the Procter & Gamble Company. Editorial writing assistance, supported financially by the Procter & Gamble Company, was provided by Gill McFeat, PhD, of McFeat Science Ltd (Devon, United Kingdom).
- Alexander AM, Delph WI. Pseudofolliculitis barbae in the military. a medical, administrative and social problem. J Natl Med Assoc. 1974;66:459-464, 479.
- Kligman AM, Strauss JS. Pseudofolliculitis of the beard. AMA Arch Derm. 1956;74:533-542.
- Banta J, Bowen C, Wong E, et al. Perceptions of shaving profiles and their potential impacts on career progression in the United States Air Force. Mil Med. 2021;186:187-189.
- Daniel A, Gustafson CJ, Zupkosky PJ, et al. Shave frequency and regimen variation effects on the management of pseudofolliculitis barbae. J Drugs Dermatol. 2013;12:410-418.
- Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652-657.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21:629-644.
- Ribera M, Fernández-Chico N, Casals M. Pseudofolliculitis barbae [in Spanish]. Actas Dermosifiliogr. 2010;101:749-757.
- Conte MS, Lawrence JE. Pseudofolliculitis barbae. no ‘pseudoproblem.’ JAMA. 1979;241:53-54.
- Alexander AM. Evaluation of a foil-guarded shaver in the management of pseudofolliculitis barbae. Cutis. 1981;27:534-537, 540-542.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328;331-333.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Cowley K, Vanoosthuyze K, Ertel K, et al. Blade shaving. In: Draelos ZD, ed. Cosmetic Dermatology: Products and Procedures. 2nd ed. John Wiley & Sons; 2015:166-173.
- Cowley K, Vanoosthuyze K. Insights into shaving and its impact on skin. Br J Dermatol. 2012;166(suppl 1):6-12.
- Cowley K, Vanoosthuyze K. The biomechanics of blade shaving. Int J Cosmet Sci. 2016;38(suppl 1):17-23.
- Kindred C, Oresajo CO, Yatskayer M, et al. Comparative evaluation of men’s depilatory composition versus razor in black men. Cutis. 2011;88:98-103.
- Alexander AM, Delph WI. Pseudofolliculitis barbae in the military. a medical, administrative and social problem. J Natl Med Assoc. 1974;66:459-464, 479.
- Kligman AM, Strauss JS. Pseudofolliculitis of the beard. AMA Arch Derm. 1956;74:533-542.
- Banta J, Bowen C, Wong E, et al. Perceptions of shaving profiles and their potential impacts on career progression in the United States Air Force. Mil Med. 2021;186:187-189.
- Daniel A, Gustafson CJ, Zupkosky PJ, et al. Shave frequency and regimen variation effects on the management of pseudofolliculitis barbae. J Drugs Dermatol. 2013;12:410-418.
- Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652-657.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21:629-644.
- Ribera M, Fernández-Chico N, Casals M. Pseudofolliculitis barbae [in Spanish]. Actas Dermosifiliogr. 2010;101:749-757.
- Conte MS, Lawrence JE. Pseudofolliculitis barbae. no ‘pseudoproblem.’ JAMA. 1979;241:53-54.
- Alexander AM. Evaluation of a foil-guarded shaver in the management of pseudofolliculitis barbae. Cutis. 1981;27:534-537, 540-542.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328;331-333.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Cowley K, Vanoosthuyze K, Ertel K, et al. Blade shaving. In: Draelos ZD, ed. Cosmetic Dermatology: Products and Procedures. 2nd ed. John Wiley & Sons; 2015:166-173.
- Cowley K, Vanoosthuyze K. Insights into shaving and its impact on skin. Br J Dermatol. 2012;166(suppl 1):6-12.
- Cowley K, Vanoosthuyze K. The biomechanics of blade shaving. Int J Cosmet Sci. 2016;38(suppl 1):17-23.
- Kindred C, Oresajo CO, Yatskayer M, et al. Comparative evaluation of men’s depilatory composition versus razor in black men. Cutis. 2011;88:98-103.
Practice Points
- Pseudofolliculitis barbae (PFB) is a common follicular inflammatory disorder associated with shaving, most commonly seen in men of African ancestry. It can be distressing and cause a substantial impact on quality of life (QOL).
- Frequent use of a novel razor technology designed specifically for men with PFB was found to improve skin appearance and QOL after 12 weeks vs baseline.
- This razor technology provides an alternative approach to help manage PFB for men who wish to or need to continue shaving.
Commentary: Prevention in AD, December 2022
We are in the golden age of atopic dermatitis (AD) drug development. We are fortunate to have numerous topicals, oral systemics, and biologics recently approved or in late-stage clinical development. Yet, we are still lacking effective strategies for primary prevention of incident AD and secondary prevention of AD exacerbations.
Kottner and colleagues published the results from the ADAPI study of 150 infants who were at an enhanced risk for AD. The children were randomly assigned to receive either a skincare regimen that was standardized or unstandardized skincare preferred by parents. They found that in the first year of life, the overall cumulative incidence rate of AD was similar between standardized skincare and skincare preferred by parents (P = .999).
Bradshaw and colleagues also published results from the BEEP study (a 5-year prospective study) of 1394 infants who were at high risk for AD. The children were randomly assigned to receive either emollient for the first year plus standard skincare or standard skincare alone. They found a similar proportion of children were clinically diagnosed with AD between 12 and 60 months in the emollient plus skincare group vs skincare alone group (31% vs 28%; adjusted relative risk 1.10; 95% CI 0.93-1.30). Unfortunately, the results from both studies are consistent with earlier results from BEEP, as well as other studies, and did not show that early application of emollients successfully prevent AD.
The use of applying emollients for primary prevention is unclear. However, proactive application of topical corticosteroids (TCS) and other topical nonsteroidal agents is well accepted in AD treatment guidelines for secondary prevention of AD exacerbations.1 Although, a recent study from Kamiya and colleagues suggested that proactive application of topical corticosteroids may not work as well as we think. They conducted an open-label, active-controlled, parallel-group study of 49 pediatric patients with moderate to severe AD who achieved remission with potent TCS. The children were then randomly assigned to receive proactive therapy with or discontinuation of TCS. The authors found no significant decrease in relapse rates with proactive vs no proactive treatment groups (8.33% vs 20.0%; P = .0859). I don't think these results will change our guidelines. But I do think these results raise important questions about the myriad aspects of proactive therapy that require appropriate counseling, including frequency of application per week (1-3 times), choice of therapies (corticosteroid or nonsteroidal agent), additional emollient use, bathing practice, etc. I personally would strongly recommend use of proactive therapy in clinical practice, but these results highlight that it is not a magic bullet for all patients either.
Additional Reference
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. Doi: 10.1016/j.anai.2017.10.039
We are in the golden age of atopic dermatitis (AD) drug development. We are fortunate to have numerous topicals, oral systemics, and biologics recently approved or in late-stage clinical development. Yet, we are still lacking effective strategies for primary prevention of incident AD and secondary prevention of AD exacerbations.
Kottner and colleagues published the results from the ADAPI study of 150 infants who were at an enhanced risk for AD. The children were randomly assigned to receive either a skincare regimen that was standardized or unstandardized skincare preferred by parents. They found that in the first year of life, the overall cumulative incidence rate of AD was similar between standardized skincare and skincare preferred by parents (P = .999).
Bradshaw and colleagues also published results from the BEEP study (a 5-year prospective study) of 1394 infants who were at high risk for AD. The children were randomly assigned to receive either emollient for the first year plus standard skincare or standard skincare alone. They found a similar proportion of children were clinically diagnosed with AD between 12 and 60 months in the emollient plus skincare group vs skincare alone group (31% vs 28%; adjusted relative risk 1.10; 95% CI 0.93-1.30). Unfortunately, the results from both studies are consistent with earlier results from BEEP, as well as other studies, and did not show that early application of emollients successfully prevent AD.
The use of applying emollients for primary prevention is unclear. However, proactive application of topical corticosteroids (TCS) and other topical nonsteroidal agents is well accepted in AD treatment guidelines for secondary prevention of AD exacerbations.1 Although, a recent study from Kamiya and colleagues suggested that proactive application of topical corticosteroids may not work as well as we think. They conducted an open-label, active-controlled, parallel-group study of 49 pediatric patients with moderate to severe AD who achieved remission with potent TCS. The children were then randomly assigned to receive proactive therapy with or discontinuation of TCS. The authors found no significant decrease in relapse rates with proactive vs no proactive treatment groups (8.33% vs 20.0%; P = .0859). I don't think these results will change our guidelines. But I do think these results raise important questions about the myriad aspects of proactive therapy that require appropriate counseling, including frequency of application per week (1-3 times), choice of therapies (corticosteroid or nonsteroidal agent), additional emollient use, bathing practice, etc. I personally would strongly recommend use of proactive therapy in clinical practice, but these results highlight that it is not a magic bullet for all patients either.
Additional Reference
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. Doi: 10.1016/j.anai.2017.10.039
We are in the golden age of atopic dermatitis (AD) drug development. We are fortunate to have numerous topicals, oral systemics, and biologics recently approved or in late-stage clinical development. Yet, we are still lacking effective strategies for primary prevention of incident AD and secondary prevention of AD exacerbations.
Kottner and colleagues published the results from the ADAPI study of 150 infants who were at an enhanced risk for AD. The children were randomly assigned to receive either a skincare regimen that was standardized or unstandardized skincare preferred by parents. They found that in the first year of life, the overall cumulative incidence rate of AD was similar between standardized skincare and skincare preferred by parents (P = .999).
Bradshaw and colleagues also published results from the BEEP study (a 5-year prospective study) of 1394 infants who were at high risk for AD. The children were randomly assigned to receive either emollient for the first year plus standard skincare or standard skincare alone. They found a similar proportion of children were clinically diagnosed with AD between 12 and 60 months in the emollient plus skincare group vs skincare alone group (31% vs 28%; adjusted relative risk 1.10; 95% CI 0.93-1.30). Unfortunately, the results from both studies are consistent with earlier results from BEEP, as well as other studies, and did not show that early application of emollients successfully prevent AD.
The use of applying emollients for primary prevention is unclear. However, proactive application of topical corticosteroids (TCS) and other topical nonsteroidal agents is well accepted in AD treatment guidelines for secondary prevention of AD exacerbations.1 Although, a recent study from Kamiya and colleagues suggested that proactive application of topical corticosteroids may not work as well as we think. They conducted an open-label, active-controlled, parallel-group study of 49 pediatric patients with moderate to severe AD who achieved remission with potent TCS. The children were then randomly assigned to receive proactive therapy with or discontinuation of TCS. The authors found no significant decrease in relapse rates with proactive vs no proactive treatment groups (8.33% vs 20.0%; P = .0859). I don't think these results will change our guidelines. But I do think these results raise important questions about the myriad aspects of proactive therapy that require appropriate counseling, including frequency of application per week (1-3 times), choice of therapies (corticosteroid or nonsteroidal agent), additional emollient use, bathing practice, etc. I personally would strongly recommend use of proactive therapy in clinical practice, but these results highlight that it is not a magic bullet for all patients either.
Additional Reference
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. Doi: 10.1016/j.anai.2017.10.039
What is the diagnosis?
Syphilis
Although extremely rare, we checked a Venereal Disease Research Laboratory PCR, the results of which came back positive. A Treponema pallidum hemagglutination assay also returned positive with titers 1:5120 (ULN, < 1:80), confirming the diagnosis of syphilis. During his hospitalization, the patient developed a syphilitic skin rash on his back, chest, palms, feet, and soles (Figure C). The patient was started on penicillin G, 4 million units intravenously every 4 hours. The fever broke 36 hours after antibiotic initiation and 48 hours later, his bilirubin started to downtrend, followed by the alkaline phosphatase and GGT 3 days later. His rash completely disappeared 5 days after antibiotic initiation. He received a total of 2 weeks of penicillin G intravenously at 24 million units a day and his liver enzymes normalized 7 weeks later.
Syphilitic hepatitis is extremely rare and occurs in 0.2% of patients with secondary syphilis.1 There are few cases of syphilitic hepatitis in HIV carriers reported in the literature. Of the described cases, only two patients had an undetectable viral load.2,3 The clinical presentation of syphilitic hepatitis includes jaundice, pruritus, nausea, and vomiting, in addition to generalized symptoms of fatigue, malaise, and weight loss. Biochemically, alkaline phosphatase and GGT are predominantly elevated with mild elevation in the transaminases. Few cases describe an elevation in the bilirubin. Diagnosis is made based on treponemal testing and/or evaluation of tissue for spirochetes on liver biopsy. The majority of cases used penicillin G with excellent response. Doxycycline was also used in one case and ceftriaxone was used in another.
In our case, the patient had several other possible reasons for his liver enzyme elevation, including drug-induced liver injury, cocaine, and alcohol use, which could have contributed to his disturbed liver enzymes. The steady improvement in his cholestatic liver enzymes, fever, and rash, shortly after the initiation of penicillin G indicates that syphilis was the cause of his hepatitis. Given the improvement in his symptoms and biochemical markers, we refrained from obtaining a liver biopsy.
References
1. Lee M., Wang C., Dorer R. et al. A great masquerader: Acute syphilitic hepatitis. Dig Dis Sci. 2013;58:923-5.
2. Mullick CJ. Liappis A.P. Benator D.A. et al. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis. 2004;39:e100-e105.
3. German MN. Matkowskyj K.A. Hoffman R.J. et al. A case of syphilitic hepatitis in an HIV-infected patient. Hum Pathol. 2018;79:184-7.
Syphilis
Although extremely rare, we checked a Venereal Disease Research Laboratory PCR, the results of which came back positive. A Treponema pallidum hemagglutination assay also returned positive with titers 1:5120 (ULN, < 1:80), confirming the diagnosis of syphilis. During his hospitalization, the patient developed a syphilitic skin rash on his back, chest, palms, feet, and soles (Figure C). The patient was started on penicillin G, 4 million units intravenously every 4 hours. The fever broke 36 hours after antibiotic initiation and 48 hours later, his bilirubin started to downtrend, followed by the alkaline phosphatase and GGT 3 days later. His rash completely disappeared 5 days after antibiotic initiation. He received a total of 2 weeks of penicillin G intravenously at 24 million units a day and his liver enzymes normalized 7 weeks later.
Syphilitic hepatitis is extremely rare and occurs in 0.2% of patients with secondary syphilis.1 There are few cases of syphilitic hepatitis in HIV carriers reported in the literature. Of the described cases, only two patients had an undetectable viral load.2,3 The clinical presentation of syphilitic hepatitis includes jaundice, pruritus, nausea, and vomiting, in addition to generalized symptoms of fatigue, malaise, and weight loss. Biochemically, alkaline phosphatase and GGT are predominantly elevated with mild elevation in the transaminases. Few cases describe an elevation in the bilirubin. Diagnosis is made based on treponemal testing and/or evaluation of tissue for spirochetes on liver biopsy. The majority of cases used penicillin G with excellent response. Doxycycline was also used in one case and ceftriaxone was used in another.
In our case, the patient had several other possible reasons for his liver enzyme elevation, including drug-induced liver injury, cocaine, and alcohol use, which could have contributed to his disturbed liver enzymes. The steady improvement in his cholestatic liver enzymes, fever, and rash, shortly after the initiation of penicillin G indicates that syphilis was the cause of his hepatitis. Given the improvement in his symptoms and biochemical markers, we refrained from obtaining a liver biopsy.
References
1. Lee M., Wang C., Dorer R. et al. A great masquerader: Acute syphilitic hepatitis. Dig Dis Sci. 2013;58:923-5.
2. Mullick CJ. Liappis A.P. Benator D.A. et al. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis. 2004;39:e100-e105.
3. German MN. Matkowskyj K.A. Hoffman R.J. et al. A case of syphilitic hepatitis in an HIV-infected patient. Hum Pathol. 2018;79:184-7.
Syphilis
Although extremely rare, we checked a Venereal Disease Research Laboratory PCR, the results of which came back positive. A Treponema pallidum hemagglutination assay also returned positive with titers 1:5120 (ULN, < 1:80), confirming the diagnosis of syphilis. During his hospitalization, the patient developed a syphilitic skin rash on his back, chest, palms, feet, and soles (Figure C). The patient was started on penicillin G, 4 million units intravenously every 4 hours. The fever broke 36 hours after antibiotic initiation and 48 hours later, his bilirubin started to downtrend, followed by the alkaline phosphatase and GGT 3 days later. His rash completely disappeared 5 days after antibiotic initiation. He received a total of 2 weeks of penicillin G intravenously at 24 million units a day and his liver enzymes normalized 7 weeks later.
Syphilitic hepatitis is extremely rare and occurs in 0.2% of patients with secondary syphilis.1 There are few cases of syphilitic hepatitis in HIV carriers reported in the literature. Of the described cases, only two patients had an undetectable viral load.2,3 The clinical presentation of syphilitic hepatitis includes jaundice, pruritus, nausea, and vomiting, in addition to generalized symptoms of fatigue, malaise, and weight loss. Biochemically, alkaline phosphatase and GGT are predominantly elevated with mild elevation in the transaminases. Few cases describe an elevation in the bilirubin. Diagnosis is made based on treponemal testing and/or evaluation of tissue for spirochetes on liver biopsy. The majority of cases used penicillin G with excellent response. Doxycycline was also used in one case and ceftriaxone was used in another.
In our case, the patient had several other possible reasons for his liver enzyme elevation, including drug-induced liver injury, cocaine, and alcohol use, which could have contributed to his disturbed liver enzymes. The steady improvement in his cholestatic liver enzymes, fever, and rash, shortly after the initiation of penicillin G indicates that syphilis was the cause of his hepatitis. Given the improvement in his symptoms and biochemical markers, we refrained from obtaining a liver biopsy.
References
1. Lee M., Wang C., Dorer R. et al. A great masquerader: Acute syphilitic hepatitis. Dig Dis Sci. 2013;58:923-5.
2. Mullick CJ. Liappis A.P. Benator D.A. et al. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis. 2004;39:e100-e105.
3. German MN. Matkowskyj K.A. Hoffman R.J. et al. A case of syphilitic hepatitis in an HIV-infected patient. Hum Pathol. 2018;79:184-7.
A 48-year-old man with HIV infection (PCR undetectable CD4 483) who used cocaine and was a heavy user of alcohol presented with jaundice, fever, and acute-onset left-upper quadrant abdominal pain. The pain was exacerbated by breathing. He had associated intermittent fevers and weight loss starting 3 weeks before presentation. He denied chest pain, nausea, vomiting, or a change in bowel habits. Home medications included dolutegravir, emtricitabine, tenofovir disoproxil fumarate, and recent intake of tamoxifen, clomiphene, and chorionic gonadotropin to counteract the effects of anabolic steroids that were used 4 months before presentation.
On examination, his temperature was 39°C, he was jaundiced, and he had icteric sclera. The abdomen was soft and nondistended with minimal left-upper quadrant tenderness. Blood work showed a white blood cell count of 7,800/mcL, hemoglobin of 12.2 g/dL, platelets of 378,000/mcL, alanine aminotransferase 236 IU/L (upper limit of normal [ULN], 65 IU/L), aspartate aminotransferase 166 IU/L (ULN, 50 IU/L), total bilirubin 3.4 mg/dL (ULN, 1.2 mg/dL), direct bilirubin 2.6 mg/dL (ULN, 0.3 mg/dL), alkaline phosphatase 1,064 IU/L (ULN, 120 IU/L), gamma-glutamyl transferase (GGT) of 655 (ULN, 50 IU/L), protein of 73 g/L, and albumin of 34 g/L. Lipase, lactate dehydrogenase, and international normalized ratio were normal. Blood smear was unrevealing. A contrasted computed tomography scan showed multiple subcentimetric mesenteric and multiple retroperitoneal lymph nodes, the largest of which was 1.3 cm in the aortocaval area. All medications were discontinued. Hepatitis A, B, and C serologies were negative, including hepatitis B and C PCR. Epstein-Barr virus IgM was negative and cytomegalovirus IgM was equivocal.
During this hospitalization, his cholestatic liver enzymes continued to rise, reaching a maximum value of total bilirubin of 7.8 mg/dL, direct bilirubin of 6.5 mg/dL, and 3 days later, alkaline phosphatase of 1,637 IU/L and GGT of 1,171 IU/L. Alanine aminotransferase and aspartate aminotransferase slowly downtrended during the hospitalization. Magnetic resonance cholangiopancreatography showed an edematous enlarged liver with minimal peripheral intrahepatic dilatation without an obstructing mass or extrahepatic biliary ductal dilatation (Figure A). Comprehensive autoimmune hepatic serology, iron studies, ceruloplasmin, and alpha-1 antitrypsin labs were negative. The patient remained febrile, so a positron emission tomography computed tomography scan was done and it showed active and enlarged (2.8-cm) portocaval and porta hepatis lymph nodes. Bone marrow biopsy showed no lymphoproliferative disorder, but there was a small poorly formed granuloma (Figure B, between the arrows).
What other testing would you obtain to evaluate this patient's fever and abnormal liver enzymes?
Previously published in Gastroenterology
Multiple Annular Erythematous Plaques
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
A 59-year-old man was admitted to the medical ward with multiple annular erythematous plaques and polyarthralgia of several months’ duration. His medical history included low-grade stage IIA follicular lymphoma diagnosed 6 months prior to presentation, substance abuse with opiates and cocaine, coronary artery disease, ascending aortic aneurysm, and chronic lower back pain. Physical examination revealed multiple red to red-brown papules and plaques, some in an annular configuration, that were distributed on the cheeks, left wrist, knees, dorsal feet, and soles. Bilateral inguinal lymphadenopathy also was noted. Serological testing for HIV, hepatitis B and C viruses, Treponema pallidum, Borrelia burgdorferi, and tuberculosis assay were negative. Arthrocentesis of the left wrist 1 week prior to admission noted 5333 nucleated cells/μL (reference range, <3000 cells/μL) and no crystals; culture of the fluid was sterile. Skin biopsies of plaques on the left wrist, left dorsal foot, and right knee were obtained for histopathologic analysis.
Commentary: Risk factors and treatment for pediatric migraine, December 2022
This month, we will take a look at three new studies investigating risk factors and treatments for headache in children.
Stress has long been noted to be one of the most consistent triggers for migraine attacks. Much has been written and studied regarding the effect of migraine on mood in adults; however, few studies have done the same in the pediatric and adolescent population. Childhood trauma has been associated with the development of chronic migraine as an adult, and behavioral treatments, such as cognitive-behavioral therapy and biofeedback, are considered as effective or more effective for migraine prevention in children compared with preventive medications. Falla and colleagues have quantified the risk for anxiety and depression in children and adolescents with migraine.
The "internalization of symptoms" is defined by the authors as an individual's tendency to react to stress with physical symptoms, including anxiety and depression. These are thought to be elevated in children and adolescents with many effects, including migraine. However, no correlation has yet been shown. Beyond the internalization of symptoms, specific psychiatric diagnoses may also be more prominent in this population.
This study was a meta-analysis of data pooled from studies that assessed migraine-related symptoms as they relate to disorders on the spectrum of anxiety, depression, and trauma-related disorders. Any studies with participants older than 18 years were excluded from this analysis. A total of 80 studies were included. Anxiety symptoms were seen to be significantly higher in children and adolescents with migraine compared to controls and the odds of having an anxiety disorder were higher among those with migraine compared with controls. Depressive symptoms were also significantly higher; however, this effect size was much smaller. The incidence of migraine was not different than that of other headache disorders.
Many patients describe stress as a trigger for migraine and other headache attacks. Mood disorders and childhood trauma are associated with the development of chronic migraine as an adult. This study reveals a two-way connection between mood disorders and headache diagnoses in children. Screening for the underlying symptoms of depression and anxiety should be done when evaluating children and adolescents for headache disorders, and there should be a focus on the treatment of these conditions in addition to treating the headache symptoms.
There are, unfortunately, very few acute pediatric migraine trials. Only a handful of medications have actually been investigated for the treatment of migraine in children younger than 12 years, and only one migraine-specific medication, rizatriptan, is approved in the United States for pediatric use. Because of this, many argue that children and adolescents with migraines end up overusing over-the-counter medication options, increasing the risk for medication overuse headache. In addition, many patients need nonoral acute migraine treatments due to nausea and vomiting or rapid onset migraine attacks.
Yonker and colleagues conducted a phase 3, randomized, double-blind, multicenter trial that only enrolled patients aged 6-11 years, all of whom had a diagnosis of episodic migraine (< 15 days of migraine per month). Children that weighed < 50 kg were given a randomly assigned lower dose of 1 mg or 2.5 mg zolmitriptan nasal spray, and those who weighed > 50 kg were randomly assigned to either 2.5 mg or 5 mg, which is the standard adult dose.
The primary outcome was a standard 2-hour pain freedom level; secondary outcomes included the proportion of improvement at 0.5, 1, and 24 hours post-dose, as well as sustained headache response for the following 2-24 hours and time to rescue medication use. Although 300 patients were enrolled and taken through the run-in process, 114 were discontinued either due to placebo response or because they had no treated migraine during the run-in phase. The mean age was 9.2 years and half of the patients were girls (of note, this is considered an appropriate proportion for pediatric migraine).
The primary endpoint of 2-hour pain freedom was not met; however, more patients in the high-dose treatment group were pain-free after 2 hours than in the placebo group. Several secondary endpoints did achieve statistical significance, including pain-free status at 1 hour post-dose, as well as headache response at 0.5, 1, and 2 hours — all of which were lower in the high-dose treatment group. The lower-dose treatment group was statistically similar to placebo for all the timepoints noted above. Treatment-related adverse events were rare in all groups.
This study did not meet its primary efficacy endpoint. However, it does show safety and effectiveness, enough at least to broaden the use of zolmitriptan for pediatric migraine. Many more effective medications for migraine treatment in adults should follow the lead of this group to find better and more specific treatments for children with migraine.
As we noted above, childhood trauma is associated with the development of chronic migraine in adulthood. Prior studies have defined childhood trauma as physical or emotional abuse primarily, and the correlation between childhood illnesses and headache disorder has not previously been determined. Davidsson and colleagues published a study in the Journal of Cancer Epidemiology that reviewed nationwide database registers longitudinally to better understand this potential risk factor.
The Danish Cancer Register was reviewed for the purpose of identifying anyone in the general Danish population who developed a diagnosis of cancer before age 20 years. The individual identification numbers in that register were linked to data in other nationwide registers for the purpose of determining whether those individuals initiated migraine-specific medications or were admitted for an inpatient hospitalization for migraine. Study participants were also grouped based on cancer type, specifically hematologic cancers involving chemotherapy and central nervous system-directed therapies, brain tumors needing intracranial surgery or radiation to the brain, blastomas or other solid tumors outside of the central nervous system, sarcomas treated with an alkylating chemotherapy, and all other carcinomas.
Among all individuals diagnosed with a childhood cancer, there was a significant increase in overall risk for the need to initiate antimigraine medication. Of interest, this was higher in those diagnosed in their teenage years. Migraine hospitalization was also noted to be higher in nearly all strata, with the exception of those diagnosed with cancer prior to the age of 5 years. The highest risk was also noted in individuals with hematologic cancers, blastomas, and brain tumors as opposed to those with sarcomas and other carcinomas. The highest cumulative risk for migraine remains in those who were diagnosed with cancer between ages 15 and 19 years.
This month, we will take a look at three new studies investigating risk factors and treatments for headache in children.
Stress has long been noted to be one of the most consistent triggers for migraine attacks. Much has been written and studied regarding the effect of migraine on mood in adults; however, few studies have done the same in the pediatric and adolescent population. Childhood trauma has been associated with the development of chronic migraine as an adult, and behavioral treatments, such as cognitive-behavioral therapy and biofeedback, are considered as effective or more effective for migraine prevention in children compared with preventive medications. Falla and colleagues have quantified the risk for anxiety and depression in children and adolescents with migraine.
The "internalization of symptoms" is defined by the authors as an individual's tendency to react to stress with physical symptoms, including anxiety and depression. These are thought to be elevated in children and adolescents with many effects, including migraine. However, no correlation has yet been shown. Beyond the internalization of symptoms, specific psychiatric diagnoses may also be more prominent in this population.
This study was a meta-analysis of data pooled from studies that assessed migraine-related symptoms as they relate to disorders on the spectrum of anxiety, depression, and trauma-related disorders. Any studies with participants older than 18 years were excluded from this analysis. A total of 80 studies were included. Anxiety symptoms were seen to be significantly higher in children and adolescents with migraine compared to controls and the odds of having an anxiety disorder were higher among those with migraine compared with controls. Depressive symptoms were also significantly higher; however, this effect size was much smaller. The incidence of migraine was not different than that of other headache disorders.
Many patients describe stress as a trigger for migraine and other headache attacks. Mood disorders and childhood trauma are associated with the development of chronic migraine as an adult. This study reveals a two-way connection between mood disorders and headache diagnoses in children. Screening for the underlying symptoms of depression and anxiety should be done when evaluating children and adolescents for headache disorders, and there should be a focus on the treatment of these conditions in addition to treating the headache symptoms.
There are, unfortunately, very few acute pediatric migraine trials. Only a handful of medications have actually been investigated for the treatment of migraine in children younger than 12 years, and only one migraine-specific medication, rizatriptan, is approved in the United States for pediatric use. Because of this, many argue that children and adolescents with migraines end up overusing over-the-counter medication options, increasing the risk for medication overuse headache. In addition, many patients need nonoral acute migraine treatments due to nausea and vomiting or rapid onset migraine attacks.
Yonker and colleagues conducted a phase 3, randomized, double-blind, multicenter trial that only enrolled patients aged 6-11 years, all of whom had a diagnosis of episodic migraine (< 15 days of migraine per month). Children that weighed < 50 kg were given a randomly assigned lower dose of 1 mg or 2.5 mg zolmitriptan nasal spray, and those who weighed > 50 kg were randomly assigned to either 2.5 mg or 5 mg, which is the standard adult dose.
The primary outcome was a standard 2-hour pain freedom level; secondary outcomes included the proportion of improvement at 0.5, 1, and 24 hours post-dose, as well as sustained headache response for the following 2-24 hours and time to rescue medication use. Although 300 patients were enrolled and taken through the run-in process, 114 were discontinued either due to placebo response or because they had no treated migraine during the run-in phase. The mean age was 9.2 years and half of the patients were girls (of note, this is considered an appropriate proportion for pediatric migraine).
The primary endpoint of 2-hour pain freedom was not met; however, more patients in the high-dose treatment group were pain-free after 2 hours than in the placebo group. Several secondary endpoints did achieve statistical significance, including pain-free status at 1 hour post-dose, as well as headache response at 0.5, 1, and 2 hours — all of which were lower in the high-dose treatment group. The lower-dose treatment group was statistically similar to placebo for all the timepoints noted above. Treatment-related adverse events were rare in all groups.
This study did not meet its primary efficacy endpoint. However, it does show safety and effectiveness, enough at least to broaden the use of zolmitriptan for pediatric migraine. Many more effective medications for migraine treatment in adults should follow the lead of this group to find better and more specific treatments for children with migraine.
As we noted above, childhood trauma is associated with the development of chronic migraine in adulthood. Prior studies have defined childhood trauma as physical or emotional abuse primarily, and the correlation between childhood illnesses and headache disorder has not previously been determined. Davidsson and colleagues published a study in the Journal of Cancer Epidemiology that reviewed nationwide database registers longitudinally to better understand this potential risk factor.
The Danish Cancer Register was reviewed for the purpose of identifying anyone in the general Danish population who developed a diagnosis of cancer before age 20 years. The individual identification numbers in that register were linked to data in other nationwide registers for the purpose of determining whether those individuals initiated migraine-specific medications or were admitted for an inpatient hospitalization for migraine. Study participants were also grouped based on cancer type, specifically hematologic cancers involving chemotherapy and central nervous system-directed therapies, brain tumors needing intracranial surgery or radiation to the brain, blastomas or other solid tumors outside of the central nervous system, sarcomas treated with an alkylating chemotherapy, and all other carcinomas.
Among all individuals diagnosed with a childhood cancer, there was a significant increase in overall risk for the need to initiate antimigraine medication. Of interest, this was higher in those diagnosed in their teenage years. Migraine hospitalization was also noted to be higher in nearly all strata, with the exception of those diagnosed with cancer prior to the age of 5 years. The highest risk was also noted in individuals with hematologic cancers, blastomas, and brain tumors as opposed to those with sarcomas and other carcinomas. The highest cumulative risk for migraine remains in those who were diagnosed with cancer between ages 15 and 19 years.
This month, we will take a look at three new studies investigating risk factors and treatments for headache in children.
Stress has long been noted to be one of the most consistent triggers for migraine attacks. Much has been written and studied regarding the effect of migraine on mood in adults; however, few studies have done the same in the pediatric and adolescent population. Childhood trauma has been associated with the development of chronic migraine as an adult, and behavioral treatments, such as cognitive-behavioral therapy and biofeedback, are considered as effective or more effective for migraine prevention in children compared with preventive medications. Falla and colleagues have quantified the risk for anxiety and depression in children and adolescents with migraine.
The "internalization of symptoms" is defined by the authors as an individual's tendency to react to stress with physical symptoms, including anxiety and depression. These are thought to be elevated in children and adolescents with many effects, including migraine. However, no correlation has yet been shown. Beyond the internalization of symptoms, specific psychiatric diagnoses may also be more prominent in this population.
This study was a meta-analysis of data pooled from studies that assessed migraine-related symptoms as they relate to disorders on the spectrum of anxiety, depression, and trauma-related disorders. Any studies with participants older than 18 years were excluded from this analysis. A total of 80 studies were included. Anxiety symptoms were seen to be significantly higher in children and adolescents with migraine compared to controls and the odds of having an anxiety disorder were higher among those with migraine compared with controls. Depressive symptoms were also significantly higher; however, this effect size was much smaller. The incidence of migraine was not different than that of other headache disorders.
Many patients describe stress as a trigger for migraine and other headache attacks. Mood disorders and childhood trauma are associated with the development of chronic migraine as an adult. This study reveals a two-way connection between mood disorders and headache diagnoses in children. Screening for the underlying symptoms of depression and anxiety should be done when evaluating children and adolescents for headache disorders, and there should be a focus on the treatment of these conditions in addition to treating the headache symptoms.
There are, unfortunately, very few acute pediatric migraine trials. Only a handful of medications have actually been investigated for the treatment of migraine in children younger than 12 years, and only one migraine-specific medication, rizatriptan, is approved in the United States for pediatric use. Because of this, many argue that children and adolescents with migraines end up overusing over-the-counter medication options, increasing the risk for medication overuse headache. In addition, many patients need nonoral acute migraine treatments due to nausea and vomiting or rapid onset migraine attacks.
Yonker and colleagues conducted a phase 3, randomized, double-blind, multicenter trial that only enrolled patients aged 6-11 years, all of whom had a diagnosis of episodic migraine (< 15 days of migraine per month). Children that weighed < 50 kg were given a randomly assigned lower dose of 1 mg or 2.5 mg zolmitriptan nasal spray, and those who weighed > 50 kg were randomly assigned to either 2.5 mg or 5 mg, which is the standard adult dose.
The primary outcome was a standard 2-hour pain freedom level; secondary outcomes included the proportion of improvement at 0.5, 1, and 24 hours post-dose, as well as sustained headache response for the following 2-24 hours and time to rescue medication use. Although 300 patients were enrolled and taken through the run-in process, 114 were discontinued either due to placebo response or because they had no treated migraine during the run-in phase. The mean age was 9.2 years and half of the patients were girls (of note, this is considered an appropriate proportion for pediatric migraine).
The primary endpoint of 2-hour pain freedom was not met; however, more patients in the high-dose treatment group were pain-free after 2 hours than in the placebo group. Several secondary endpoints did achieve statistical significance, including pain-free status at 1 hour post-dose, as well as headache response at 0.5, 1, and 2 hours — all of which were lower in the high-dose treatment group. The lower-dose treatment group was statistically similar to placebo for all the timepoints noted above. Treatment-related adverse events were rare in all groups.
This study did not meet its primary efficacy endpoint. However, it does show safety and effectiveness, enough at least to broaden the use of zolmitriptan for pediatric migraine. Many more effective medications for migraine treatment in adults should follow the lead of this group to find better and more specific treatments for children with migraine.
As we noted above, childhood trauma is associated with the development of chronic migraine in adulthood. Prior studies have defined childhood trauma as physical or emotional abuse primarily, and the correlation between childhood illnesses and headache disorder has not previously been determined. Davidsson and colleagues published a study in the Journal of Cancer Epidemiology that reviewed nationwide database registers longitudinally to better understand this potential risk factor.
The Danish Cancer Register was reviewed for the purpose of identifying anyone in the general Danish population who developed a diagnosis of cancer before age 20 years. The individual identification numbers in that register were linked to data in other nationwide registers for the purpose of determining whether those individuals initiated migraine-specific medications or were admitted for an inpatient hospitalization for migraine. Study participants were also grouped based on cancer type, specifically hematologic cancers involving chemotherapy and central nervous system-directed therapies, brain tumors needing intracranial surgery or radiation to the brain, blastomas or other solid tumors outside of the central nervous system, sarcomas treated with an alkylating chemotherapy, and all other carcinomas.
Among all individuals diagnosed with a childhood cancer, there was a significant increase in overall risk for the need to initiate antimigraine medication. Of interest, this was higher in those diagnosed in their teenage years. Migraine hospitalization was also noted to be higher in nearly all strata, with the exception of those diagnosed with cancer prior to the age of 5 years. The highest risk was also noted in individuals with hematologic cancers, blastomas, and brain tumors as opposed to those with sarcomas and other carcinomas. The highest cumulative risk for migraine remains in those who were diagnosed with cancer between ages 15 and 19 years.