User login
Erythrasma
THE COMPARISON
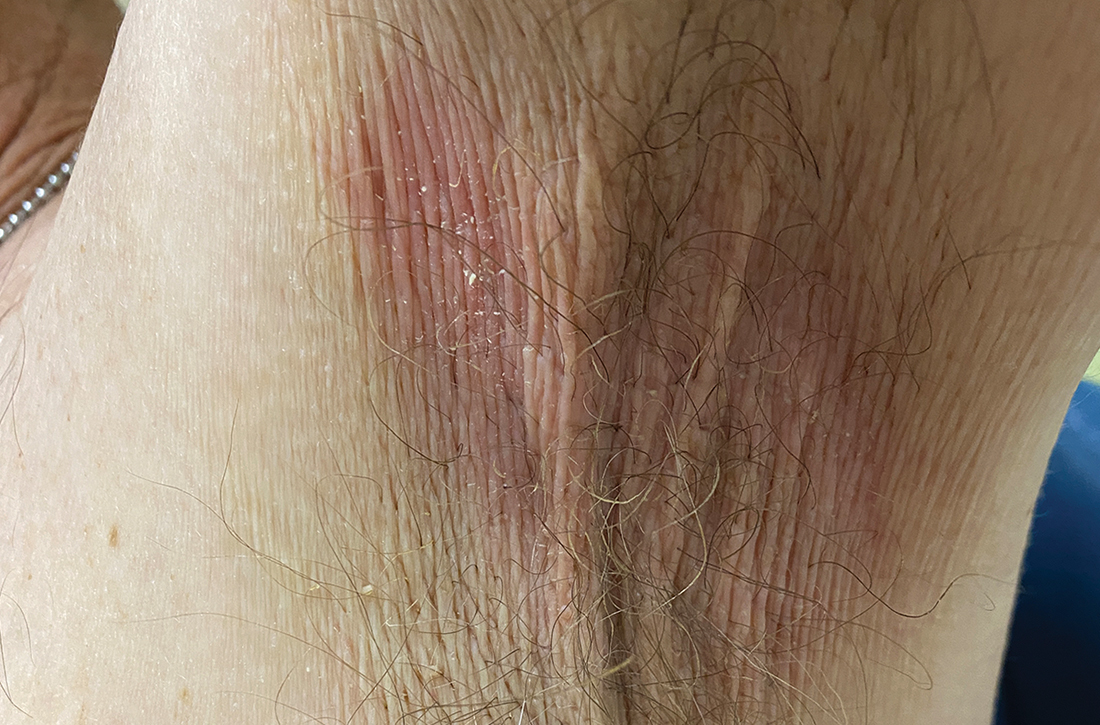
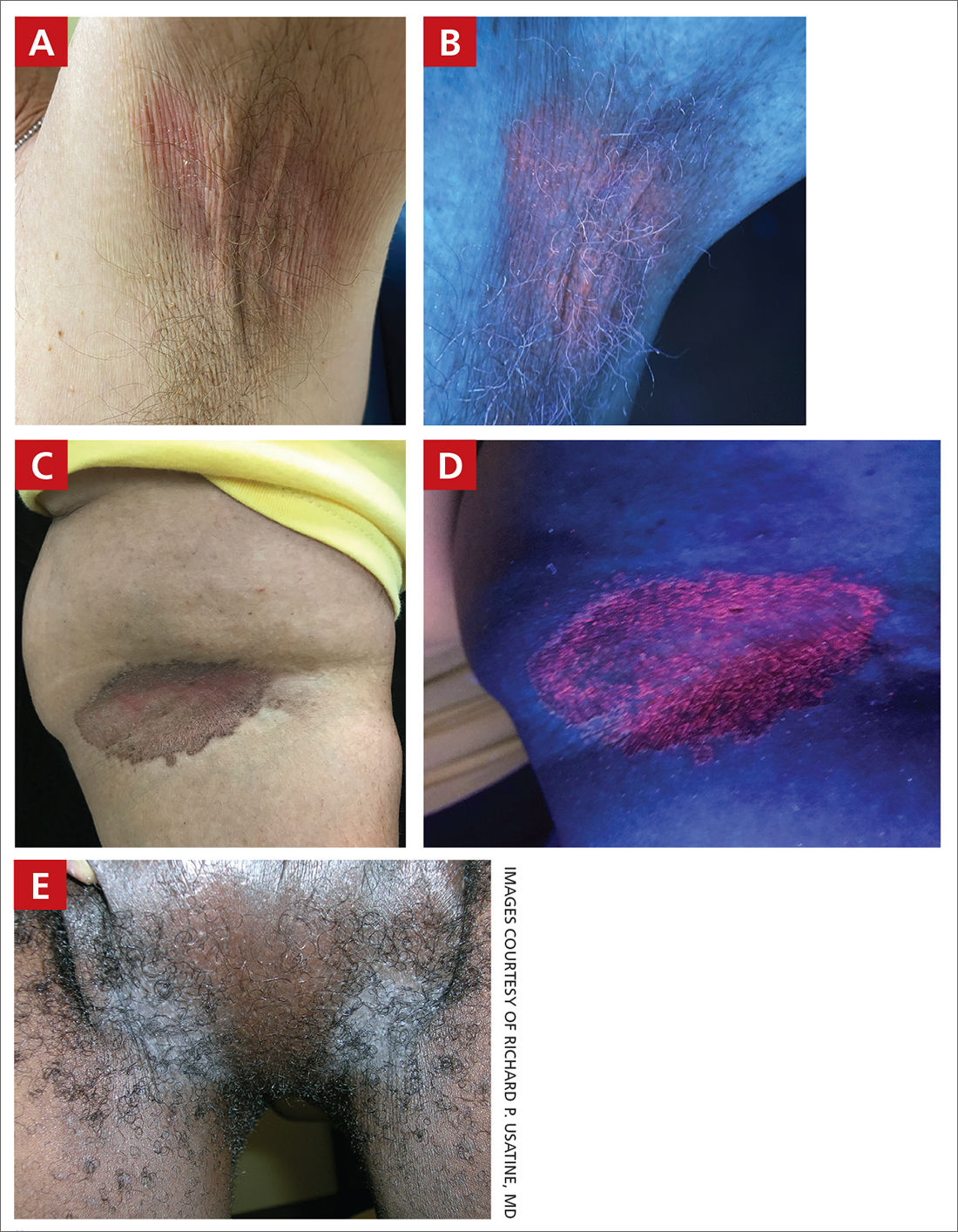
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood-lamp examination of the area showed characteristic bright coral red fluorescence (B).
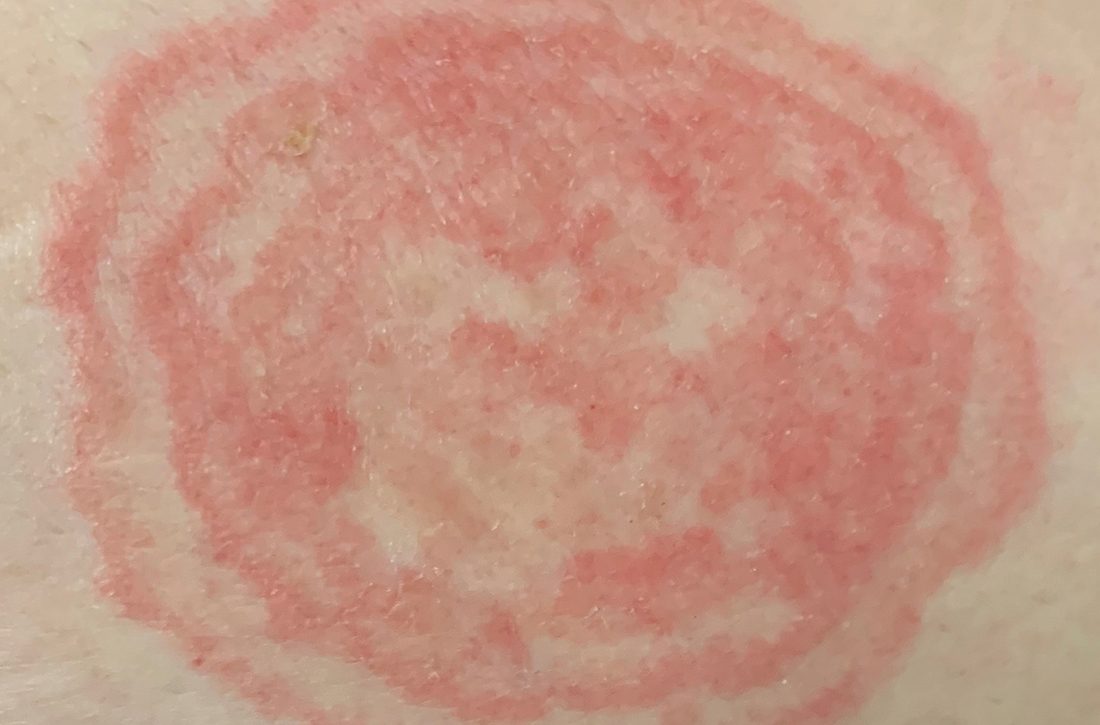
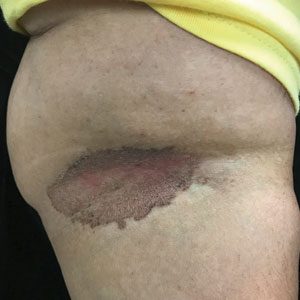
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood-lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches (with pruritus) in the groin of a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.
Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (FIGURES A and C) or as a hypopigmented patch (FIGURE E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
- C minutissimum produces coproporphyrin III, which glows fluorescent red under Wood-lamp examination (FIGURES B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
- Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
- The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
- There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
- Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non-Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes, likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
1. Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
2. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
3. Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
4. Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
5. Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
6. Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
7. Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls. Stat- Pearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood-lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood-lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches (with pruritus) in the groin of a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (FIGURES A and C) or as a hypopigmented patch (FIGURE E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
- C minutissimum produces coproporphyrin III, which glows fluorescent red under Wood-lamp examination (FIGURES B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
- Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
- The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
- There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
- Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non-Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes, likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood-lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood-lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches (with pruritus) in the groin of a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (FIGURES A and C) or as a hypopigmented patch (FIGURE E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
- C minutissimum produces coproporphyrin III, which glows fluorescent red under Wood-lamp examination (FIGURES B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
- Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
- The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
- There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
- Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non-Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes, likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
1. Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
2. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
3. Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
4. Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
5. Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
6. Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
7. Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls. Stat- Pearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
1. Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
2. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
3. Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
4. Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
5. Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
6. Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
7. Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls. Stat- Pearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
A practical guide to hidradenitis suppurativa
Hidradenitis suppurativa (HS), also known as acne inversa or Verneuil disease, is a chronic, recurrent, inflammatory occlusive disease affecting the terminal follicular epithelium in apocrine gland–bearing skin areas.1 HS manifests as painful nodules, abscesses, fistulas, and scarring and often has a severe psychological impact on the affected patient.2
When HS was first identified in the 1800s, it was believed to result from a dysfunction of the sweat glands.3 In 1939, scientists identified the true cause: follicular occlusion.3
Due to its chronic nature, heterogeneity in presentation, and apparent low prevalence,4 HS is considered an orphan disease.5 Over the past 10 years, there has been a surge in HS research—particularly in medical management—which has provided a better understanding of this condition.6,7
In this review, we discuss the most updated evidence regarding the diagnosis and treatment of HS to guide the family physician (FP)’s approach to managing this debilitating disease. But first, we offer a word about the etiology and pathophysiology of the condition.
3 events set the stage for hidradenitis suppurativa
Although the exact cause of HS is still unknown, some researchers have hypothesized that HS results from a combination of genetic predisposition and environmental and lifestyle factors.8-12 The primary mechanism of HS is the obstruction of the terminal follicular epithelium by a keratin plug.1,13,14 A systematic review of molecular inflammatory pathways involved in HS divides the pathogenesis of HS into 3 events: follicular occlusion followed by dilation, follicular rupture and inflammatory response, and chronic inflammatory state with sinus tracts.8
An underreported condition
HS is often underreported and misdiagnosed.4,15 Globally, the prevalence of HS varies from < 1% to 4%.15,16 A systematic review with meta-analysis showed a higher prevalence of HS in females compared to males in American and European populations.17 In the United States, the overall frequency of HS is 0.1%, or 98 per 100,000 persons.16 The prevalence of HS is highest among patients ages 30 to 39 years; there is decreased prevalence in patients ages 55 years and older.16,18
Who is at heightened risk?
Recent research has shown a relationship between ethnicity and HS.16,19,20 African American and biracial groups (defined as African American and White) have a 3-fold and 2-fold greater prevalence of HS, respectively, compared to White patients.16 However, the prevalence of HS in non-White ethnic groups may be underestimated in clinical trials due to a lack of representation and subgroup analyses based on ethnicity, which may affect generalizability in HS recommendations.21
Continue to: Genetic predisposition
Genetic predisposition. As many as 40% of patients with HS report having at least 1 affected family member. A positive family history of HS is associated with earlier onset, longer disease duration, and severe disease.22 HS is genetically heterogeneous, and several mutations (eg, gamma secretase, PSTPIP1, PSEN1 genes) have been identified in patients and in vitro as the cause of dysregulation of epidermal proliferation and differentiation, immune dysregulation, and promotion of amyloid formation.8,23-25
Obesity and metabolic risk factors. There is a strong relationship between HS and obesity. As many as 70% of patients with HS are obese, and 9% to 40% have metabolic syndrome.12,18,26-28 Obesity is associated with maceration and mechanical stress, increased fragility of the dermo-epidermal junction, changes in cutaneous blood flow, and subdermal fat inflammation—all of which favor the pathophysiology of HS.29,30
Smoking. Tobacco smoking is associated with severe HS and a lower chance of remission.12 Population-based studies have shown that as many as 90% of patients with HS have a history of smoking ≥ 20 packs of cigarettes per year.1,12,18,31,32 The nicotine and thousands of other chemicals present in cigarettes trigger keratinocytes and fibroblasts, resulting in epidermal hyperplasia, infundibular hyperkeratosis, excessive cornification, and dysbiosis.8,23,24
Hormones. The exact role sex hormones play in the pathogenesis of HS remains unclear.8,32 Most information is based primarily on small studies looking at antiandrogen treatments, HS activity during the menstrual cycle and pregnancy, HS exacerbation related to androgenic effects of hormonal contraception, and the association of HS with metabolic-endocrine disorders (eg, polycystic ovary syndrome [PCOS]).8,33
Androgens induce hyperkeratosis that may lead to follicular occlusion—the hallmark of HS pathology.34 A systematic review looking at the role of androgen and estrogen in HS found that while some patients with HS have elevated androgen levels, most have androgen and estrogen levels within normal range.35 Therefore, increased peripheral androgen receptor sensitivity has been hypothesized as the mechanism of action contributing to HS manifestation.34
Continue to: Host-defense defects
Host-defense defects. HS shares a similar cytokine profile with other well-established immune-mediated inflammatory diseases, including pyoderma gangrenosum (PG)36,37 and Crohn disease.38-40 HS is characterized by the expression of several immune mediators, including tumor necrosis factor-alpha (TNF-alpha), interleukin-1 alpha (IL-1 alpha), IL-1 beta, IL-8, IL-17, and the IL-23/T helper 17 pathway, all of which are upregulated in other inflammatory diseases and also result in an abnormal innate immune response.8,24 The recently described clinical triad of PG, acne, and HS (PASH) and the tetrad of pyogenic arthritis, PG, acne, and HS (PAPASH) further support the role of immune dysregulation in the pathogenesis of HS.40 Nonetheless, further studies are needed to determine the exact pathways of cytokine effect in HS.41
Use these criteria to make the diagnosis
The US and Canadian Hidradenitis Suppurativa Foundations (HSF) guidelines base the clinical diagnosis of HS on the following criteria2:
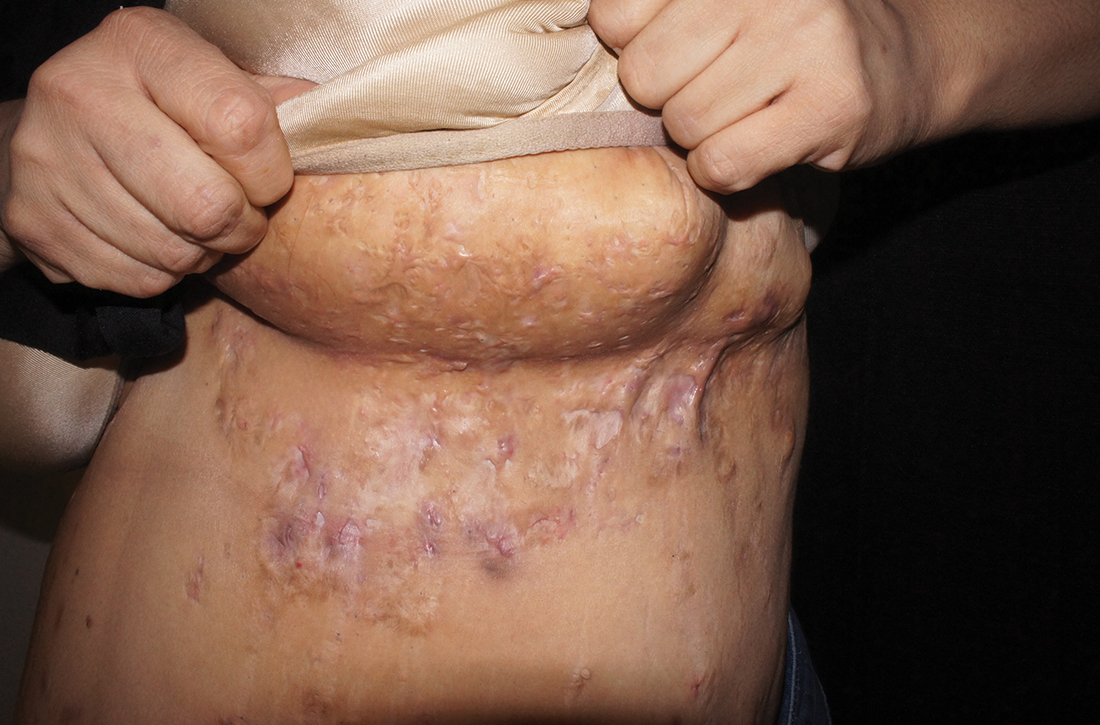
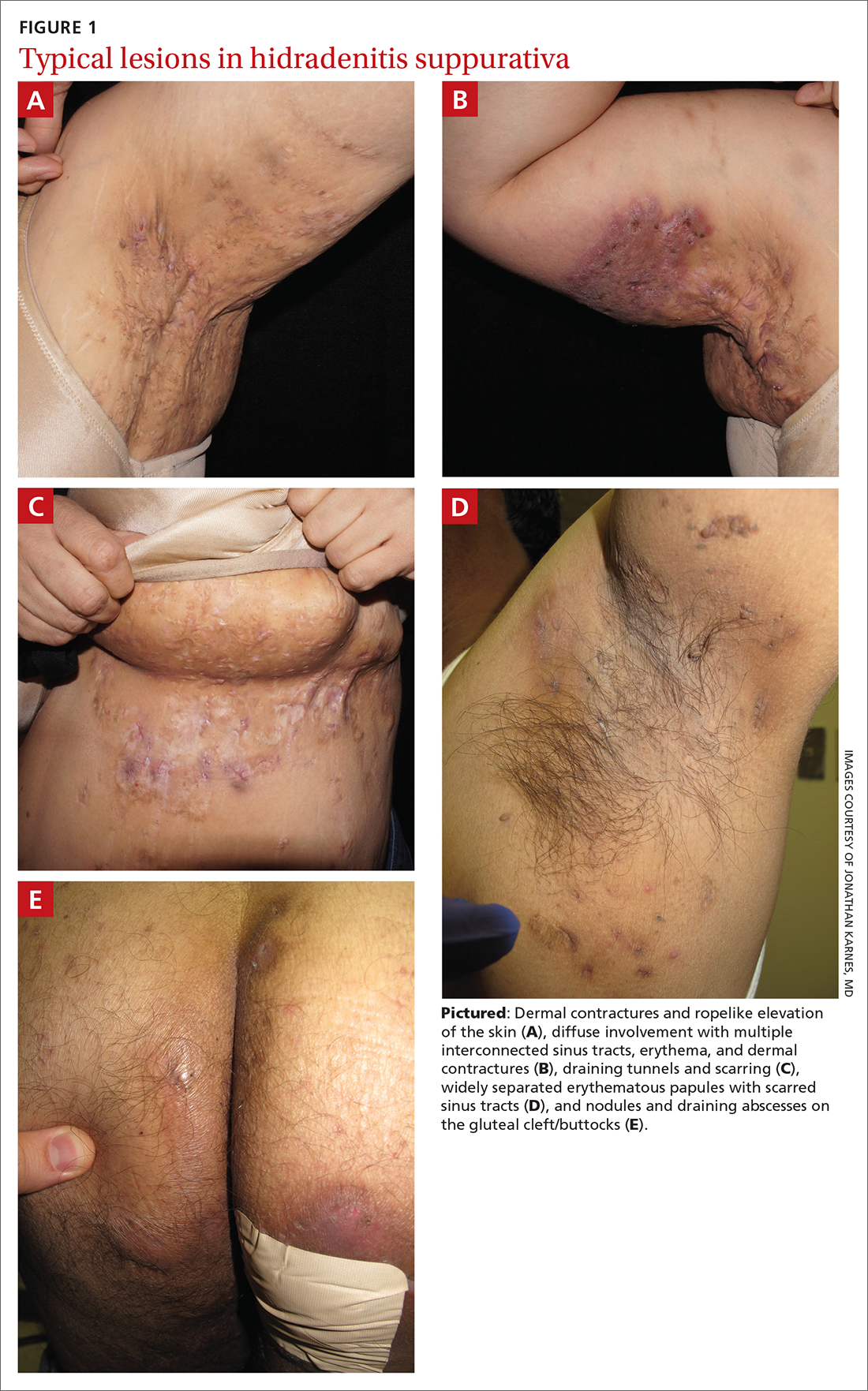
- Typical HS lesions: Erythematous skin lesions; inflamed, deep-seated painful nodules; “tombstone” double-ended comedones; sinus tracts; scarring; deformity. FIGURES 1A-1E show typical lesions seen in patients with HS.
- Typical locations: Intertriginous regions—apocrine gland–containing areas in axilla, groin, perineal region, buttocks, gluteal cleft, and mammary folds; beltline and waistband areas; areas of skin compression and friction.
- Recurrence and chronicity: Recurrent painful or suppurating lesions that appear more than twice in a 6-month period.2,41-43
Patients with HS usually present with painful recurrent abscesses and scarring and often report multiple visits to the emergency department for drainage or failed antibiotic treatment for abscesses.15,44
Ask patients these 2 questions. Vinding et al45 developed a survey for the diagnosis of HS using 2 simple questions based on the 3 criteria established by the HSF:
- “Have you had an outbreak of boils during the last 6 months?” and
- “Where and how many boils have you had?” (This question includes a list of the typical HS locations—eg, axilla, groin, genitals, area under breast.)
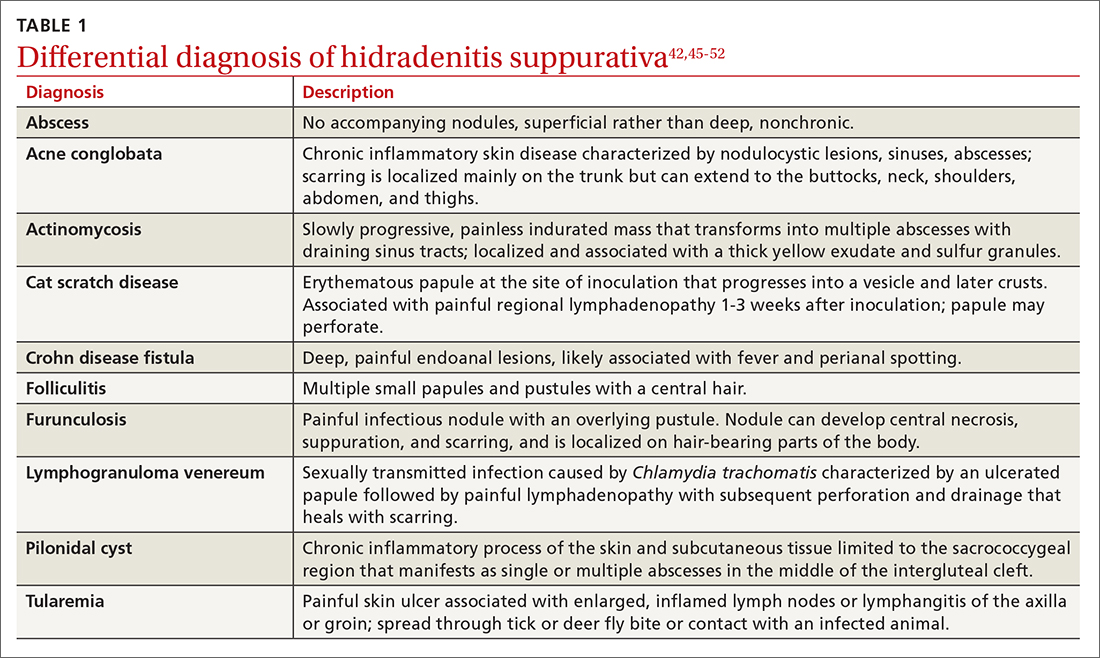
In their questionnaire, Vinding et al45 found that an affirmative answer to Question 1 and reports of > 2 boils in response to Question 2 correlated to a sensitivity of 90%, specificity of 97%, positive predictive value of 96%, and negative predictive value of 92% for the diagnosis of HS. The differential diagnosis of HS is summarized in TABLE 1.42,45-52
Continue to: These tools can help you to stage hidradenitis suppurativa
These tools can help you to stage hidradenitis suppurativa
Multiple tools are available to assess the severity of HS.53 We will describe the Hurley staging system and the International Hidradenitis Suppurativa Severity Score System (IHS4). Other diagnostic tools, such as the Sartorius score and the Hidradenitis Suppurativa Physician’s Global Assessment Scale (HS-PGA), can be time-consuming and challenging to interpret, limiting their use in the clinical setting.2,54
Hurley staging system (available at www.hsdiseasesource.com/hs-disease-staging) considers the presence of nodules, abscesses, sinus tracts, and scarring affecting an entire anatomical area.13,55 This system is most useful as a rapid classification tool for patients with HS in the clinical setting but should not be used to assess clinical response.2,13,56
The IHS4 (available at https://online library.wiley.com/doi/10.1111/bjd.15748) is a validated and easy-to-use tool for assessing HS and guiding the therapeutic strategy in clinical practice.54 With IHS4, the clinician must calculate the following:
- total number of nodules > 10 mm in diameter
- total number of abscesses multiplied by 2, and
- total number of draining tunnels (fistulae/sinuses) multiplied by 4.
Mild HS is defined as a score ≤ 3 points; moderate HS, 4 to 10 points; and severe HS, ≥ 11 points.54
No diagnostic tests, but ultrasound may be helpful
There are currently no established biological markers or specific tests for diagnosing HS.15 Ultrasound is emerging as a tool to assess dermal thickness, hair follicle morphology, and number and extent of fluid collections. Two recent studies showed that pairing clinical assessment with ultrasound findings improves accuracy of scoring in 84% of cases.57,58 For patients with severe HS, skin biopsy can be considered to rule out squamous cell carcinoma. Cultures, however, have limited utility except for suspected superimposed bacterial infection.2
Continue to: Screening for comorbidities
Screening for comorbidities
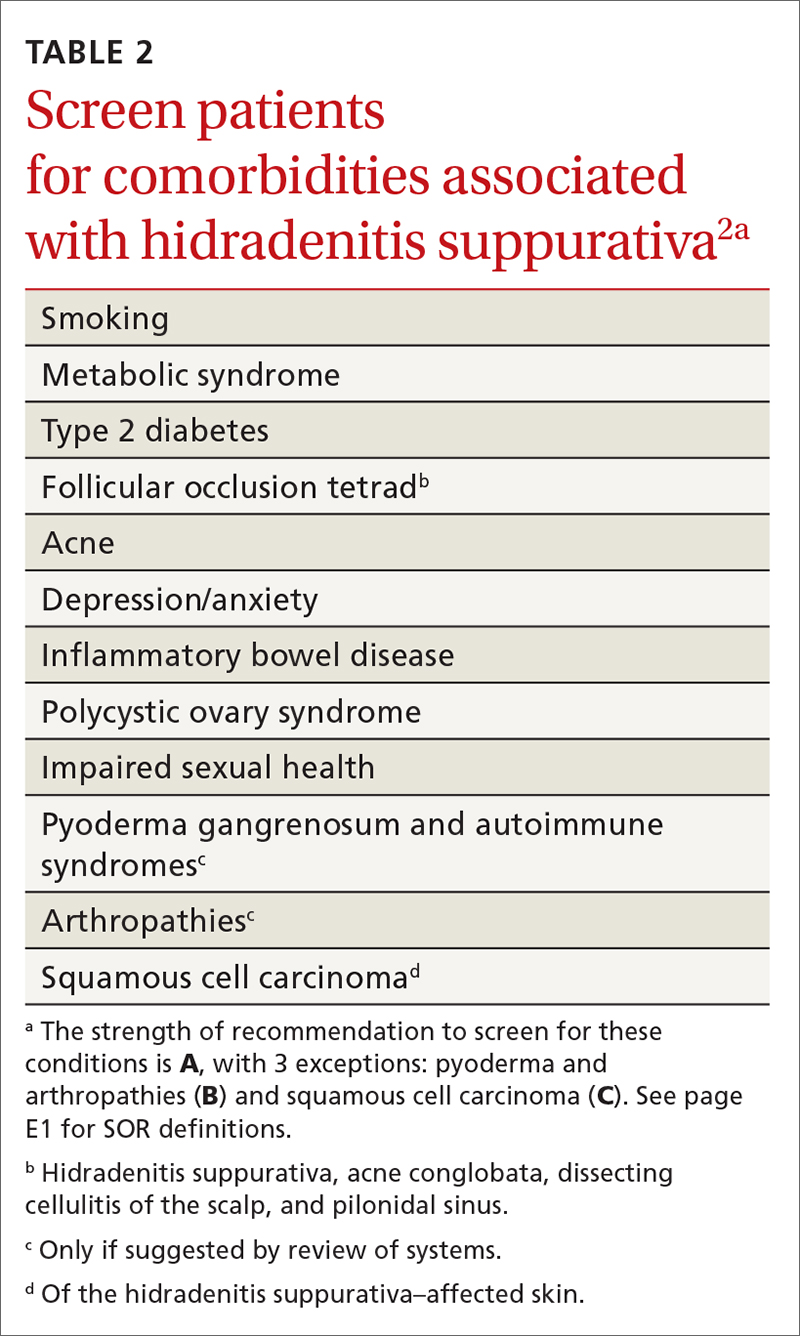
HSF recommends clinicians screen patients for comorbidities associated with HS (TABLE 2).2 Overall, screening patients for active and past history of smoking is strongly recommended, as is screening for metabolic syndrome, hyperlipidemia, type 2 diabetes (1.5- to 3-fold greater risk of type 2 diabetes in HS patients), and PCOS (3-fold greater risk).2,26,27,59 Screening patients for depression and anxiety is also routinely recommended.2 However, the authors of this article strongly recommend screening all patients with HS for psychiatric comorbidities, as research has shown a 2-fold greater risk of depression and anxiety, social isolation, and low self-esteem that severely limits quality of life (QOL) in this patient population.60,61
Management
Treat existing lesions, reduce formation of new ones
The main goals of treatment for patients with HS are to treat existing lesions and reduce associated symptoms, reduce the formation of new lesions, and minimize associated psychological morbidity.15 FPs play an important role in the early diagnosis, treatment, and comprehensive care of patients with HS. This includes monitoring patients, managing comorbidities, making appropriate referrals to dermatologists, and coordinating the multidisciplinary care that patients with HS require.
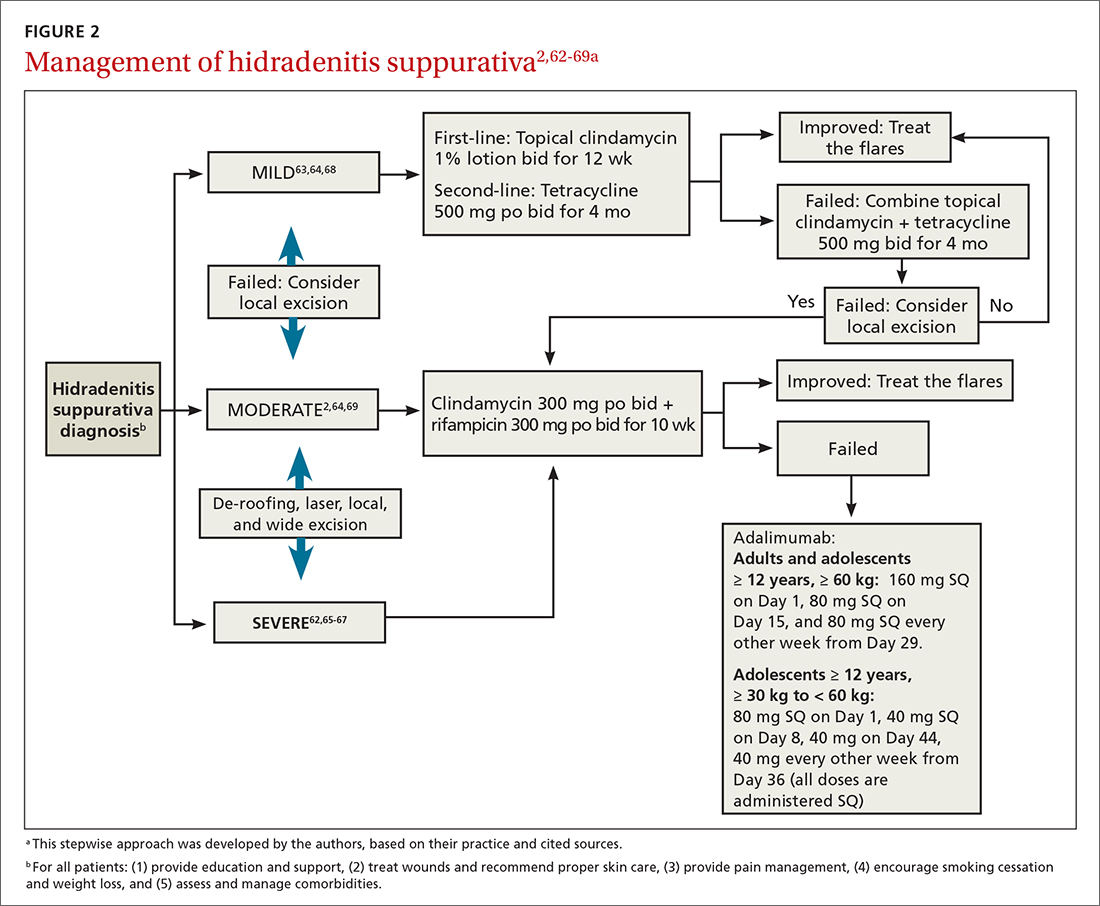
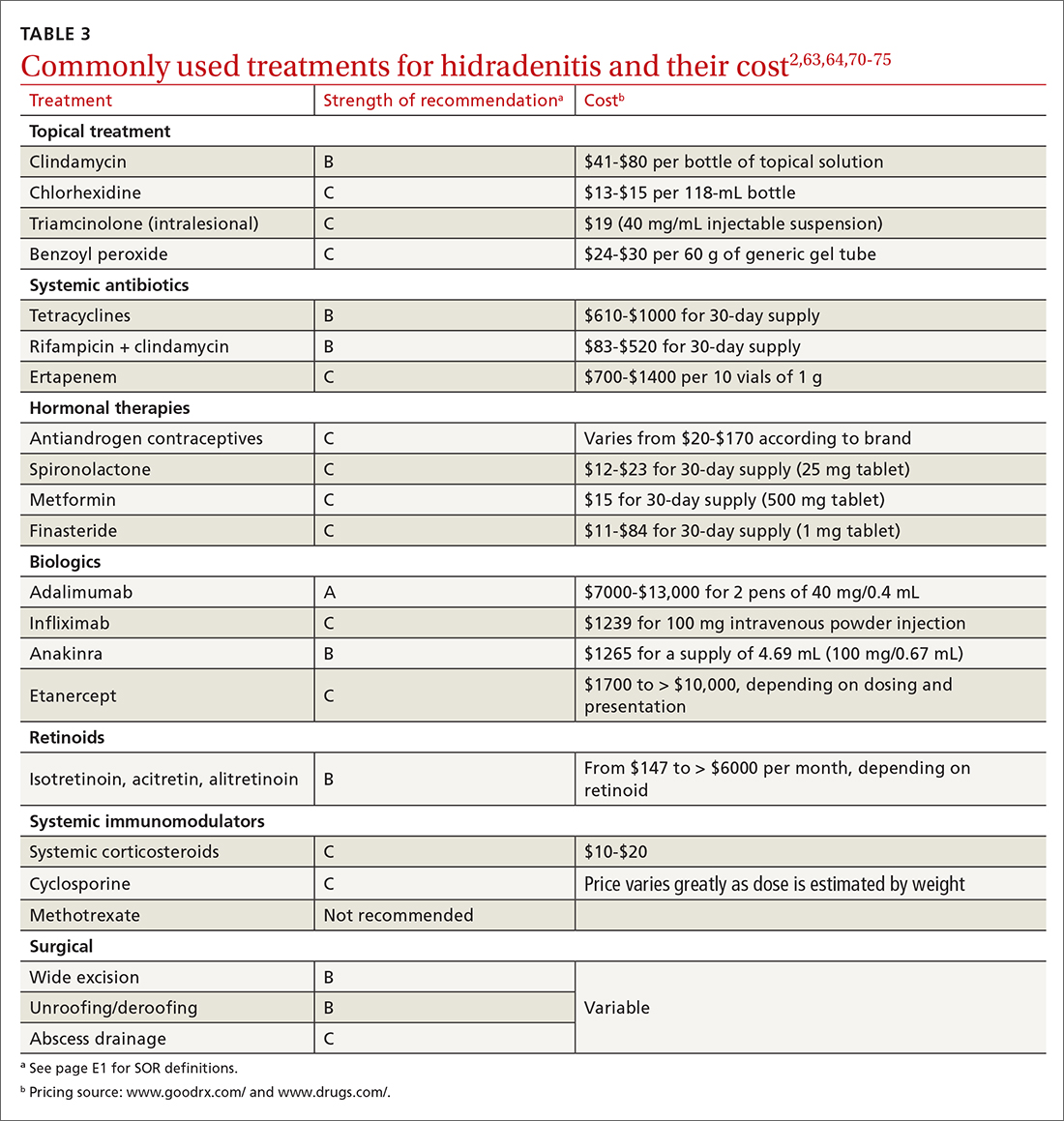
A systematic review identified more than 50 interventions used to treat HS, most based on small observational studies and randomized controlled trials (RCTs) with a high risk of bias.62 FIGURE 22,62-69 provides an evidence-based treatment algorithm for HS, and TABLE 32,63,64,70-75 summarizes the most commonly used treatments.
Biologic agents
Adalimumab (ADA) is a fully human immunoglobulin G1 monoclonal antibody that binds to TNF-alpha, neutralizes its bioactivity, and induces apoptosis of TNF-expressing mononuclear cells. It is the only medication approved by the US Food and Drug Administration for active refractory moderate and severe HS.62,65 Several double-blinded RCTs, including PIONEER I and PIONEER II, studied the effectiveness of ADA for HS and found significant clinical responses at Week 12, 50% reduction in abscess and nodule counts, no increase in abscesses or draining fistulas at Week 12, and sustained improvement in lesion counts, pain, and QOL.66,67,76
IL-1 and IL-23 inhibitors. The efficacy of etanercept and golimumab (anti-TNF), as well as anakinra (IL-1 inhibitor) and ustekinumab (IL-1/IL-23 inhibitor), continue to be investigated with variable results; they are considered second-line treatment for active refractory moderate and severe HS after ADA.65,77-80 Infliximab (IL-1 beta inhibitor) has shown no effect on reducing disease severity.70Compared to other treatments, biologic therapy is associated with higher costs (TABLE 3),2,63,64,70-75 an increased risk for reactivation of latent infections (eg, tuberculosis, herpes simplex, and hepatitis C virus [HCV], and B [HBV]), and an attenuated response to vaccines.81 Prior to starting biologic therapy, FPs should screen patients with HS for tuberculosis and HBV, consider HIV and HCV screening in at-risk patients, and optimize the immunization status of the patient.82,83 While inactivated vaccines can be administered without discontinuing biologic treatment, patients should avoid live-attenuated vaccines while taking biologics.83
Continue to: Antibiotic therapy
Antibiotic therapy
Topical antibiotics are considered first-line treatment for mild and moderate uncomplicated HS.63,64 Clindamycin 1%, the only topical antibiotic studied in a small double-blind RCT of patients with Hurley stage I and stage II HS, demonstrated significant clinical improvement after 12 weeks of treatment (twice- daily application), compared to placebo.84 Topical clindamycin is also recommended to treat flares in patients with mild disease.2,64
Oral antibiotics. Tetracycline (500 mg twice daily for 4 months) is considered a second-line treatment for patients with mild HS.64,68 Doxycycline (200 mg/d for 3 months) may also be considered as a second-line treatment in patients with mild disease.85
Combination oral clindamycin (300 mg) and rifampicin (300 mg) twice daily for 10 weeks is recommended as first-line treatment for patients with moderate HS.2,64,69 Combination rifampin (300 mg twice daily), moxifloxacin (400 mg/d), and metronidazole (500 mg three times a day) is not routinely recommended due to increased risk of toxicity.2
Ertapenem (1 g intravenously daily for 6 weeks) is supported by lower-level evidence as a third-line rescue therapy option and as a bridge to surgery; however, limitations for home infusions, costs, and concerns for antibiotic resistance limit its use.2,86
Corticosteroids and systemic immunomodulators
Intralesional triamcinolone (2-20 mg) may be beneficial in the early stages of HS, although its use is based on a small prospective open study of 33 patients.87 A recent double-blind placebo-controlled RCT comparing varying concentrations of intralesional triamcinolone (10 mg/mL and 40 mg/mL) vs normal saline showed no statistically significant difference in inflammatory clearance, pain reduction, or patient satisfaction.88
Continue to: Short-term systemic corticosteroid tapers...
Short-term systemic corticosteroid tapers (eg, prednisone, starting at 0.5-1 mg/kg) are recommended to treat flares. Long-term corticosteroids and cyclosporine are reserved for patients with severe refractory disease; however, due to safety concerns, their regular use is strongly discouraged.63,64,85 There is limited evidence to support the use of methotrexate for severe refractory disease, and its use is not recommended.63
Hormonal therapy
The use of hormonal therapy for HS is limited by the low-quality evidence (eg, anecdotal evidence, small retrospective analyses, uncontrolled trials).33,63 The only exception is a small double-blind controlled crossover trial from 1986 showing that the antiandrogen effects of combination oral contraceptives (ethinyloestradiol 50 mcg/cyproterone acetate in a reverse sequential regimen and ethinyloestradiol 50 mcg/norgestrel 500 mcg) improved HS lesions.89
Spironolactone, an antiandrogen diuretic, has been studied in small case report series with a high risk for bias. It is used mainly in female patients with mild or moderate disease, or in combination with other agents in patients with severe HS. Further research is needed to determine its utility in the treatment of HS.63,90,91
Metformin, alone or in combination with other therapies (dapsone, finasteride, liraglutide), has been analyzed in small prospective studies of primarily female patients with different severities of HS, obesity, and PCOS. These studies have shown improvement in lesions, QOL, and reduction of workdays lost.92,93
Finasteride. Studies have shown finasteride (1.25-5 mg/d) alone or in combination with other treatments (metformin, liraglutide, levonorgestrel-ethinyl estradiol, and dapsone) provided varying degrees of resolution or improvement in patients with severe and advanced HS. Finasteride has been used for 4 to 16 weeks with a good safety profile.92,94-96
Continue to: Retinoids
Retinoids
Acitretin, alitretinoin, and isotretinoin have been studied in small retrospective studies to manage HS, with variable results.97-99 Robust prospective studies are needed. Retinoids, in general, should be considered as a second- or third-line treatment for moderate to severe HS.63
Surgical intervention
Surgical interventions, which should be considered in patients with widespread mild, moderate, or severe disease, are associated with improved daily activity and work productivity.100 Incision and drainage should be avoided in patients with HS, as this technique does not remove the affected follicles and is associated with 100% recurrence.101
Wide excision is the preferred surgical technique for patients with Hurley stage II and stage III HS; it is associated with lower recurrence rates (13%) compared to local excision (22%) and deroofing (27%).102 Secondary intention healing is the most commonly chosen method, based on lower recurrence rates than primary closure.102
STEEP and laser techniques. The skin-tissue-sparing excision with electrosurgical peeling (STEEP) procedure involves successive tangential excision of affected tissue until the epithelized bottom of the sinus tracts has been reached. This allows for the removal of fibrotic tissue and the sparing of the deep subcutaneous fat. STEEP is associated with 30% of relapses after 43 months.71
Laser surgery has also been studied in patients with Hurley stage II and stage III HS. The most commonly used lasers for HS are the 1064-nm neodymium-doped yttrium aluminum garnet (Nd: YAG) and the carbon dioxide laser; they have been shown to reduce disease severity in inguinal, axillary, and inflammatory sites.72-74
Pain management: Start with lidocaine, NSAIDs
There are few studies about HS-associated pain management.103 For acute episodes, short-acting nonopioid local treatment with lidocaine, topical or oral nonsteroidal anti-inflammatory drugs, and acetaminophen are preferred. Opioids should be reserved for moderate-to-severe pain that has not responded to other analgesics. Adjuvant therapy with pregabalin, gabapentin, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors can also be considered for the comanagement of pain and depression.62,104
Consider this tool to measure treatment response
The HS clinical response (HiSCR) tool is an outcome measure used to evaluate treatment outcomes. The tool uses an HS-specific binary score with the following criteria:
- ≥ 50% reduction in the number of inflammatory nodules;
- no increase in the number of abscesses; and
- no increase in the number of draining fistulas.105
The HiSCR was developed for the PIONEER studies105,106 to assess the response to ADA treatment. It is the only HS scoring system to undergo an extensive validation process with a meaningful clinical endpoint for HS treatment evaluation that is easy to use. Compared to the HS-PGA score (clear, minimal, mild), HiSCR was more responsive to change in patients with HS.105,106
CORRESPONDENCE
Cristina Marti-Amarista, MD, 101 Nicolls Road, Stony Brook, NY, 11794-8228; [email protected]
1. Bergler-Czop B, Hadasik K, Brzezińska-Wcisło L. Acne inversa: difficulties in diagnostics and therapy. Postepy Dermatol Alergol. 2015;32:296-301. doi: 10.5114/pdia.2014.44012
2. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
3. Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol. 2010;2:9-16. doi: 10.4161/derm.2.1.12490
4. Kokolakis G, Wolk K, Schneider-Burrus S, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology. 2020;236:421-430. doi: 10.1159/000508787
5. Gulliver W, Landells IDR, Morgan D, et al. Hidradenitis suppurativa: a novel model of care and an integrative strategy to adopt an orphan disease. J Cutan Med Surg. 2018;22:71-77. doi: 10.1177/1203475417736290
6. Savage KT, Gonzalez Brant E, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi: 10.1111/jdv.16213.
7. Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000. doi: 10.12688/f1000research.26083.1
8. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965
9. Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res. 2018;7:1930. doi: 10.12688/f1000 research.17267.1
10. Sabat R, Jemec GBE, Matusiak Ł. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6:18. doi: 10.1038/s41572-020-0149-1
11. Shlyankevich J, Chen AJ, Kim GE, et al. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71:1144-1150. doi: 10.1016/j.jaad.2014.09.012
12. Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831-839. doi: 10.1111/j.1365-2133.2009.09198.x
13. von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537. doi: 10.1111/j.1600-0625.2009.00915.x
14. Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999. doi: 10.1016/s0190-9622(96)90277-7
15. Ballard K, Shuman VL. Hidradenitis suppurativa. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Updated July 15, 2022. Accessed November 28, 2022. www.ncbi.nlm.nih.gov/books/NBK534867/
16. Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi: 10.1001/jamadermatol.2017.0201
17. Phan K, Charlton O, Smith SD. Global prevalence of hidradenitis suppurativa and geographical variation—systematic review and meta-analysis. BioMed Dermatol. 2020;4. doi: 10.1186/s41702-019-0052-0
18. Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi: 10.1038/jid.2012.255
19. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi: 10.1177/1203475420972348
20. Vaidya T, Vangipuram R, Alikhan A. Examining the race-specific prevalence of hidradenitis suppurativa at a large academic center; results from a retrospective chart review. Dermatol Online J. 2017;23:13030/qt9xc0n0z1. doi: 10.5070/D3236035391
21. Price KN, Hsiao JL, Shi VY. Race and ethnicity gaps in global hidradenitis suppurativa clinical trials. Dermatology. 2021;237:97-102. doi: 10.1159/000504911
22. Schrader AM, Deckers IE, van der Zee HH, et al. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol. 2014;71:460-467. doi: 10.1016/j.jaad.2014.04.001
23. Frew JW, Vekic DA, Wood J, et al. A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol. 2017;177:987-998. doi: 10.1111/bjd.15441
24. Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol. 2020;183:999-1010. doi: 10.1111/bjd.19556
25. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi: 10.1016/j.jaad.2019.08.090
26. Sabat R, Chanwangpong A, Schneider-Burrus S, et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PloS One. 2012;7:e31810. doi: 10.1371/journal.pone.0031810
27. Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatology. 2021;237:119-124. doi: 10.1159/000501611
28. Rodríguez-Zuñiga MJM, García-Perdomo HA, Ortega-Loayza AG. Association between hidradenitis suppurativa and metabolic syndrome: a systematic review and meta-analysis. Actas Dermosifiliogr (Engl Ed). 2019;110:279-288. doi: 10.1016/j.ad.2018.10.020
29. Walker JM, Garcet S, Aleman JO, et al. Obesity and ethnicity alter gene expression in skin. Sci Rep. 2020;10:14079. doi: 10.1038/s41598-020-70244-2.
30. Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43. doi: 10.1016/j.det.2015.08.011
31. Vossen ARJV, van Straalen KR, Swolfs EFH, et al. Nicotine dependency and readiness to quit smoking among patients with hidradenitis suppurativa. Dermatology. 2021;237:383-385. doi: 10.1159/000514028
32. Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi: 10.1111/bjd.13090
33. Clark AK, Quinonez RL, Saric S, et al. Hormonal therapies for hidradenitis suppurativa: review. Dermatol Online J. 2017;23:13030/qt6383k0n4. doi: 10.5070/D32310036990
34. Saric-Bosanac S, Clark AK, Sivamani RK, et al. The role of hypothalamus-pituitary-adrenal (HPA)-like axis in inflammatory pilosebaceous disorders. Dermatol Online J. 2020;26:13030/qt8949296f. doi: 10.5070/D3262047430
35. Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa – a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
36. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270. doi: 10.1001/archdermatol.2010.328
37. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671. doi: 10.1111/j.1365-2230.2005.01897.x
38. Kirthi S, Hellen R, O’Connor R, et al. Hidradenitis suppurativa and Crohn’s disease: a case series. Ir Med J. 2017;110:618.
39. Dumont LM, Landman C, Sokol H, et al; CD-HS Study Group. Increased risk of permanent stoma in Crohn’s disease associated with hidradenitis suppurativa: a case-control study. Aliment Pharmacol Ther. 2020;52:303-310. doi: 10.1111/apt.15863
40. Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:e187. doi: 10.1097/MD.0000000000000187.
41. Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
42. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
43. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781. doi: 10.1111/bjd.16998.
44. Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221; doi: 10.1136/postgradmedj-2013-131994
45. Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol. 2014;170:884-889. doi: 10.1111/bjd.12787
46. Bassas-Vila J, González Lama Y. Hidradenitis suppurativa and perianal Crohn disease: differential diagnosis. Actas Dermosifiliogr. 2016;107(suppl 2):27-31. doi: 10.1016/S0001-7310(17) 30006-6
47. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. doi: 10.2147/IDR.S39601
48. Fuchs W, Brockmeyer NH. Sexually transmitted infections. J Dtsch Dermatol Ges. 2014;12:451-463. doi: 10.1111/ddg.12310
49. Hap W, Frejlich E, Rudno-Rudzińska J, et al. Pilonidal sinus: finding the righttrack for treatment. Pol Przegl Chir. 2017;89:68-75. doi: 10.5604/01.3001.0009.6009
50. Al-Hamdi KI, Saadoon AQ. Acne onglobate of the scalp. Int J Trichology. 2020;12:35-37. doi: 10.4103/ijt.ijt_117_19
51. Balestra A, Bytyci H, Guillod C, et al. A case of ulceroglandular tularemia presenting with lymphadenopathy and an ulcer on a linear morphoea lesion surrounded by erysipelas. Int Med Case Rep J. 2018;11:313-318. doi: 10.2147/IMCRJ.S178561
52. Ibler KS, Kromann CB. Recurrent furunculosis – challenges and management: a review. Clin Cosmet Investig Dermatol. 2014;7:59-64. doi: 10.2147/CCID.S35302
53. Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol. 2016;175:263-272. doi: 10.1111/bjd.14475
54. Zouboulis CC, Tzellos T, Kyrgidis A, et al; European Hidradenitis Suppurativa Foundation Investigator Group. Development and validation of the International Hidradenitis Suppurativa Severity Score System (I4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177:1401-1409. doi: 10.1111/bjd.15748
55. Hidradenitis Suppurativa Clinical Resource. Hidradenitis suppurativa stages: Hurley Staging System. www.hsdiseasesource.com/hs-disease-staging. Accessed October 11, 2022.
56. Ovadja ZN, Schuit MM, van der Horst CMAM, et al. Inter- and interrater reliability of Hurley staging for hidradenitis suppurativa. Br J Dermatol. 2019;181:344-349. doi: 10.1111/bjd.17588
57. Wortsman X, Jemec GBE. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg. 2007;33:1340-1342. doi: 10.1111/j.1524-4725.2007.33286.x
58. Napolitano M, Calzavara-Pinton PG, Zanca A, et al. Comparison of clinical and ultrasound scores in patients with hidradenitis suppurativa: results from an Italian ultrasound working group. J Eur Acad Dermatol Venereol. 2019;33:e84-e87. doi: 10.1111/jdv.15235
59. Bukvić Mokos Z, Miše J, Balić A, et al. Understanding the relationship between smoking and hidradenitis suppurativa. Acta Dermatovenerol Croat. 2020;28:9-13.
60. Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2015;29:371-376. doi: 10.1111/jdv.12567
61. Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life and psychosocial implications in patients with hidradenitis suppurativa. Dermatology. 2016;232:687-691. doi: 10.1159/000453355
62 Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978. doi: 10.1111/bjd.14418
63. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi: 10.1016/j.jaad.2019.02.068
64. Gulliver W, Zouboulis CC, Prens E, et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17:343-351. doi: 10.1007/s11154-016-9328-5
65. Vena GA, Cassano N. Drug focus: adalimumab in the treatment of moderate to severe psoriasis. Biologics. 2007;1:93-103.
66. Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-55. doi: 10.7326/0003-4819-157-12-201212180-00004
67. Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi: 10.1016/j.jaad.2018.05.040
68. Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi: 10.1016/s0190-9622(98)70272-5
69. Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi: 10.1159/000228334
70. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217. doi: 10.1016/j.jaad.2009.06.050
71. Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi: 10.1111/jdv.12376
72. Mahmoud BH, Tierney E, Hexsel CL, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62:637-645. doi: 10.1016/j.jaad.2009.07.048
73. Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:1188-1198. doi: 10.1111/j.1524-4725.2009.01214.x
74. Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208-213. doi: 10.1111/j.1524-4725.2009.01427.x
75. van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi: 10.1016/j.jaad.2009.12.018
76. Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi: 10.1056/NEJMoa1504370. PMID: 27518661.
77. Adams DR, Yankura JA, Fogelberg AC, et al. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146:501-504. doi: 10.1001/archdermatol.2010.72
78. Tursi A. Concomitant hidradenitis suppurativa and pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig Liver Dis. 2016;48:1511-1512. doi: 10.1016/j.dld.2016.09.010
79. Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi: 10.1001/jamadermatol.2015.3903.
80. Romaní J, Vilarrasa E, Martorell A, et al. Ustekinumab with intravenous infusion: results in hidradenitis suppurativa. Dermatology. 2020;236:21-24. doi: 10.1159/000501075
81. Kane SV. Preparing for biologic or immunosuppressant therapy. Gastroenterol Hepatol (N Y). 2011;7:544-546.
82. Davis W, Vavilin I, Malhotra N. Biologic therapy in HIV: to screen or not to screen. Cureus. 2021;13:e15941. doi: 10.7759/cureus.15941
83. Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23:50-74. doi: 10.1177/1203475418811335
84. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328. doi: 10.1111/j.1365-4362.1983.tb02150.x
85. Hunger RE, Laffitte E, Läuchli S, et al. Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatology. 2017;233:113-119. doi: 10.1159/000477459
86. Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33:19-31. doi: 10.1111/jdv.15233
87. Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi: 10.1016/j.jaad.2016.06.049
88. Fajgenbaum K, Crouse L, Dong L, et al. Intralesional triamcinolone may not be beneficial for treating acute hidradenitis suppurativa lesions: a double-blind, randomized, placebo-controlled trial. Dermatol Surg. 2020;46:685-689. doi: 10.1097/DSS.0000000000002112
89. Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268. doi: 10.1111/j.1365-2133.1986.tb05740.x
90. Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131. doi: 10.2310/7750.2007.00019
91. Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196. doi: 10.1111/ajd.12362
92. Khandalavala BN. A disease-modifying approach for advanced hidradenitis suppurativa (regimen with metformin, liraglutide, dapsone, and finasteride): a case report. Case Rep Dermatol. 2017;9:70-78. doi: 10.1159/000473873
93. Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi: 10.1111/j.1468-3083.2012.04668.x
94. Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
95. Mota F, Machado S, Selores M. Hidradenitis suppurativa in children treated with finasteride-a case series. Pediatr Dermatol. 2017;34:578-583. doi: 10.1111/pde.13216
96. Doménech C, Matarredona J, Escribano-Stablé JC, et al. Facial hidradenitis suppurativa in a 28-year-old male responding to finasteride. Dermatology. 2012;224:307-308. doi: 10.1159/000339477
97. Patel N, McKenzie SA, Harview CL, et al. Isotretinoin in the treatment of hidradenitis suppurativa: a retrospective study. J Dermatolog Treat. 2021;32:473-475. doi: 10.1080/09546634.2019.1670779
98. Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40:73-76. doi: 10.1016/s0190-9622(99) 70530-x
99. Huang CM, Kirchhof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125. doi: 10.1159/000477207
100. Prens LM, Huizinga J, Janse IC. Surgical outcomes and the impact of major surgery on quality of life, activity impairment and sexual health in hidradenitis suppurativa patients: a prospective single centre study. J Eur Acad Dermatol Venereol. 2019;33:1941-1946. doi: 10.1111/jdv.15706
101. Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164-168. doi: 10.1007/s003840050159
102. Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5 suppl 1):S70-S77. doi: 10.1016/j.jaad.2015.07.044.
103. Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444. doi: 10.1097/AJP.0b013e3181ceb80c
104. Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi: 10.1016/j.jaad.2015.07.046
105. Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994. doi: 10.1111/jdv.13216
106. Kimball AB, Jemec GB, Yang M, et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol. 2014;171:1434-1442. doi: 10.1111/bjd.13270
Hidradenitis suppurativa (HS), also known as acne inversa or Verneuil disease, is a chronic, recurrent, inflammatory occlusive disease affecting the terminal follicular epithelium in apocrine gland–bearing skin areas.1 HS manifests as painful nodules, abscesses, fistulas, and scarring and often has a severe psychological impact on the affected patient.2
When HS was first identified in the 1800s, it was believed to result from a dysfunction of the sweat glands.3 In 1939, scientists identified the true cause: follicular occlusion.3
Due to its chronic nature, heterogeneity in presentation, and apparent low prevalence,4 HS is considered an orphan disease.5 Over the past 10 years, there has been a surge in HS research—particularly in medical management—which has provided a better understanding of this condition.6,7
In this review, we discuss the most updated evidence regarding the diagnosis and treatment of HS to guide the family physician (FP)’s approach to managing this debilitating disease. But first, we offer a word about the etiology and pathophysiology of the condition.
3 events set the stage for hidradenitis suppurativa
Although the exact cause of HS is still unknown, some researchers have hypothesized that HS results from a combination of genetic predisposition and environmental and lifestyle factors.8-12 The primary mechanism of HS is the obstruction of the terminal follicular epithelium by a keratin plug.1,13,14 A systematic review of molecular inflammatory pathways involved in HS divides the pathogenesis of HS into 3 events: follicular occlusion followed by dilation, follicular rupture and inflammatory response, and chronic inflammatory state with sinus tracts.8
An underreported condition
HS is often underreported and misdiagnosed.4,15 Globally, the prevalence of HS varies from < 1% to 4%.15,16 A systematic review with meta-analysis showed a higher prevalence of HS in females compared to males in American and European populations.17 In the United States, the overall frequency of HS is 0.1%, or 98 per 100,000 persons.16 The prevalence of HS is highest among patients ages 30 to 39 years; there is decreased prevalence in patients ages 55 years and older.16,18
Who is at heightened risk?
Recent research has shown a relationship between ethnicity and HS.16,19,20 African American and biracial groups (defined as African American and White) have a 3-fold and 2-fold greater prevalence of HS, respectively, compared to White patients.16 However, the prevalence of HS in non-White ethnic groups may be underestimated in clinical trials due to a lack of representation and subgroup analyses based on ethnicity, which may affect generalizability in HS recommendations.21
Continue to: Genetic predisposition
Genetic predisposition. As many as 40% of patients with HS report having at least 1 affected family member. A positive family history of HS is associated with earlier onset, longer disease duration, and severe disease.22 HS is genetically heterogeneous, and several mutations (eg, gamma secretase, PSTPIP1, PSEN1 genes) have been identified in patients and in vitro as the cause of dysregulation of epidermal proliferation and differentiation, immune dysregulation, and promotion of amyloid formation.8,23-25
Obesity and metabolic risk factors. There is a strong relationship between HS and obesity. As many as 70% of patients with HS are obese, and 9% to 40% have metabolic syndrome.12,18,26-28 Obesity is associated with maceration and mechanical stress, increased fragility of the dermo-epidermal junction, changes in cutaneous blood flow, and subdermal fat inflammation—all of which favor the pathophysiology of HS.29,30
Smoking. Tobacco smoking is associated with severe HS and a lower chance of remission.12 Population-based studies have shown that as many as 90% of patients with HS have a history of smoking ≥ 20 packs of cigarettes per year.1,12,18,31,32 The nicotine and thousands of other chemicals present in cigarettes trigger keratinocytes and fibroblasts, resulting in epidermal hyperplasia, infundibular hyperkeratosis, excessive cornification, and dysbiosis.8,23,24
Hormones. The exact role sex hormones play in the pathogenesis of HS remains unclear.8,32 Most information is based primarily on small studies looking at antiandrogen treatments, HS activity during the menstrual cycle and pregnancy, HS exacerbation related to androgenic effects of hormonal contraception, and the association of HS with metabolic-endocrine disorders (eg, polycystic ovary syndrome [PCOS]).8,33
Androgens induce hyperkeratosis that may lead to follicular occlusion—the hallmark of HS pathology.34 A systematic review looking at the role of androgen and estrogen in HS found that while some patients with HS have elevated androgen levels, most have androgen and estrogen levels within normal range.35 Therefore, increased peripheral androgen receptor sensitivity has been hypothesized as the mechanism of action contributing to HS manifestation.34
Continue to: Host-defense defects
Host-defense defects. HS shares a similar cytokine profile with other well-established immune-mediated inflammatory diseases, including pyoderma gangrenosum (PG)36,37 and Crohn disease.38-40 HS is characterized by the expression of several immune mediators, including tumor necrosis factor-alpha (TNF-alpha), interleukin-1 alpha (IL-1 alpha), IL-1 beta, IL-8, IL-17, and the IL-23/T helper 17 pathway, all of which are upregulated in other inflammatory diseases and also result in an abnormal innate immune response.8,24 The recently described clinical triad of PG, acne, and HS (PASH) and the tetrad of pyogenic arthritis, PG, acne, and HS (PAPASH) further support the role of immune dysregulation in the pathogenesis of HS.40 Nonetheless, further studies are needed to determine the exact pathways of cytokine effect in HS.41
Use these criteria to make the diagnosis
The US and Canadian Hidradenitis Suppurativa Foundations (HSF) guidelines base the clinical diagnosis of HS on the following criteria2:
- Typical HS lesions: Erythematous skin lesions; inflamed, deep-seated painful nodules; “tombstone” double-ended comedones; sinus tracts; scarring; deformity. FIGURES 1A-1E show typical lesions seen in patients with HS.
- Typical locations: Intertriginous regions—apocrine gland–containing areas in axilla, groin, perineal region, buttocks, gluteal cleft, and mammary folds; beltline and waistband areas; areas of skin compression and friction.
- Recurrence and chronicity: Recurrent painful or suppurating lesions that appear more than twice in a 6-month period.2,41-43

Patients with HS usually present with painful recurrent abscesses and scarring and often report multiple visits to the emergency department for drainage or failed antibiotic treatment for abscesses.15,44
Ask patients these 2 questions. Vinding et al45 developed a survey for the diagnosis of HS using 2 simple questions based on the 3 criteria established by the HSF:
- “Have you had an outbreak of boils during the last 6 months?” and
- “Where and how many boils have you had?” (This question includes a list of the typical HS locations—eg, axilla, groin, genitals, area under breast.)
In their questionnaire, Vinding et al45 found that an affirmative answer to Question 1 and reports of > 2 boils in response to Question 2 correlated to a sensitivity of 90%, specificity of 97%, positive predictive value of 96%, and negative predictive value of 92% for the diagnosis of HS. The differential diagnosis of HS is summarized in TABLE 1.42,45-52

Continue to: These tools can help you to stage hidradenitis suppurativa
These tools can help you to stage hidradenitis suppurativa
Multiple tools are available to assess the severity of HS.53 We will describe the Hurley staging system and the International Hidradenitis Suppurativa Severity Score System (IHS4). Other diagnostic tools, such as the Sartorius score and the Hidradenitis Suppurativa Physician’s Global Assessment Scale (HS-PGA), can be time-consuming and challenging to interpret, limiting their use in the clinical setting.2,54
Hurley staging system (available at www.hsdiseasesource.com/hs-disease-staging) considers the presence of nodules, abscesses, sinus tracts, and scarring affecting an entire anatomical area.13,55 This system is most useful as a rapid classification tool for patients with HS in the clinical setting but should not be used to assess clinical response.2,13,56
The IHS4 (available at https://online library.wiley.com/doi/10.1111/bjd.15748) is a validated and easy-to-use tool for assessing HS and guiding the therapeutic strategy in clinical practice.54 With IHS4, the clinician must calculate the following:
- total number of nodules > 10 mm in diameter
- total number of abscesses multiplied by 2, and
- total number of draining tunnels (fistulae/sinuses) multiplied by 4.
Mild HS is defined as a score ≤ 3 points; moderate HS, 4 to 10 points; and severe HS, ≥ 11 points.54
No diagnostic tests, but ultrasound may be helpful
There are currently no established biological markers or specific tests for diagnosing HS.15 Ultrasound is emerging as a tool to assess dermal thickness, hair follicle morphology, and number and extent of fluid collections. Two recent studies showed that pairing clinical assessment with ultrasound findings improves accuracy of scoring in 84% of cases.57,58 For patients with severe HS, skin biopsy can be considered to rule out squamous cell carcinoma. Cultures, however, have limited utility except for suspected superimposed bacterial infection.2
Continue to: Screening for comorbidities
Screening for comorbidities
HSF recommends clinicians screen patients for comorbidities associated with HS (TABLE 2).2 Overall, screening patients for active and past history of smoking is strongly recommended, as is screening for metabolic syndrome, hyperlipidemia, type 2 diabetes (1.5- to 3-fold greater risk of type 2 diabetes in HS patients), and PCOS (3-fold greater risk).2,26,27,59 Screening patients for depression and anxiety is also routinely recommended.2 However, the authors of this article strongly recommend screening all patients with HS for psychiatric comorbidities, as research has shown a 2-fold greater risk of depression and anxiety, social isolation, and low self-esteem that severely limits quality of life (QOL) in this patient population.60,61

Management
Treat existing lesions, reduce formation of new ones
The main goals of treatment for patients with HS are to treat existing lesions and reduce associated symptoms, reduce the formation of new lesions, and minimize associated psychological morbidity.15 FPs play an important role in the early diagnosis, treatment, and comprehensive care of patients with HS. This includes monitoring patients, managing comorbidities, making appropriate referrals to dermatologists, and coordinating the multidisciplinary care that patients with HS require.

A systematic review identified more than 50 interventions used to treat HS, most based on small observational studies and randomized controlled trials (RCTs) with a high risk of bias.62 FIGURE 22,62-69 provides an evidence-based treatment algorithm for HS, and TABLE 32,63,64,70-75 summarizes the most commonly used treatments.

Biologic agents
Adalimumab (ADA) is a fully human immunoglobulin G1 monoclonal antibody that binds to TNF-alpha, neutralizes its bioactivity, and induces apoptosis of TNF-expressing mononuclear cells. It is the only medication approved by the US Food and Drug Administration for active refractory moderate and severe HS.62,65 Several double-blinded RCTs, including PIONEER I and PIONEER II, studied the effectiveness of ADA for HS and found significant clinical responses at Week 12, 50% reduction in abscess and nodule counts, no increase in abscesses or draining fistulas at Week 12, and sustained improvement in lesion counts, pain, and QOL.66,67,76
IL-1 and IL-23 inhibitors. The efficacy of etanercept and golimumab (anti-TNF), as well as anakinra (IL-1 inhibitor) and ustekinumab (IL-1/IL-23 inhibitor), continue to be investigated with variable results; they are considered second-line treatment for active refractory moderate and severe HS after ADA.65,77-80 Infliximab (IL-1 beta inhibitor) has shown no effect on reducing disease severity.70Compared to other treatments, biologic therapy is associated with higher costs (TABLE 3),2,63,64,70-75 an increased risk for reactivation of latent infections (eg, tuberculosis, herpes simplex, and hepatitis C virus [HCV], and B [HBV]), and an attenuated response to vaccines.81 Prior to starting biologic therapy, FPs should screen patients with HS for tuberculosis and HBV, consider HIV and HCV screening in at-risk patients, and optimize the immunization status of the patient.82,83 While inactivated vaccines can be administered without discontinuing biologic treatment, patients should avoid live-attenuated vaccines while taking biologics.83
Continue to: Antibiotic therapy
Antibiotic therapy
Topical antibiotics are considered first-line treatment for mild and moderate uncomplicated HS.63,64 Clindamycin 1%, the only topical antibiotic studied in a small double-blind RCT of patients with Hurley stage I and stage II HS, demonstrated significant clinical improvement after 12 weeks of treatment (twice- daily application), compared to placebo.84 Topical clindamycin is also recommended to treat flares in patients with mild disease.2,64
Oral antibiotics. Tetracycline (500 mg twice daily for 4 months) is considered a second-line treatment for patients with mild HS.64,68 Doxycycline (200 mg/d for 3 months) may also be considered as a second-line treatment in patients with mild disease.85
Combination oral clindamycin (300 mg) and rifampicin (300 mg) twice daily for 10 weeks is recommended as first-line treatment for patients with moderate HS.2,64,69 Combination rifampin (300 mg twice daily), moxifloxacin (400 mg/d), and metronidazole (500 mg three times a day) is not routinely recommended due to increased risk of toxicity.2
Ertapenem (1 g intravenously daily for 6 weeks) is supported by lower-level evidence as a third-line rescue therapy option and as a bridge to surgery; however, limitations for home infusions, costs, and concerns for antibiotic resistance limit its use.2,86
Corticosteroids and systemic immunomodulators
Intralesional triamcinolone (2-20 mg) may be beneficial in the early stages of HS, although its use is based on a small prospective open study of 33 patients.87 A recent double-blind placebo-controlled RCT comparing varying concentrations of intralesional triamcinolone (10 mg/mL and 40 mg/mL) vs normal saline showed no statistically significant difference in inflammatory clearance, pain reduction, or patient satisfaction.88
Continue to: Short-term systemic corticosteroid tapers...
Short-term systemic corticosteroid tapers (eg, prednisone, starting at 0.5-1 mg/kg) are recommended to treat flares. Long-term corticosteroids and cyclosporine are reserved for patients with severe refractory disease; however, due to safety concerns, their regular use is strongly discouraged.63,64,85 There is limited evidence to support the use of methotrexate for severe refractory disease, and its use is not recommended.63
Hormonal therapy
The use of hormonal therapy for HS is limited by the low-quality evidence (eg, anecdotal evidence, small retrospective analyses, uncontrolled trials).33,63 The only exception is a small double-blind controlled crossover trial from 1986 showing that the antiandrogen effects of combination oral contraceptives (ethinyloestradiol 50 mcg/cyproterone acetate in a reverse sequential regimen and ethinyloestradiol 50 mcg/norgestrel 500 mcg) improved HS lesions.89
Spironolactone, an antiandrogen diuretic, has been studied in small case report series with a high risk for bias. It is used mainly in female patients with mild or moderate disease, or in combination with other agents in patients with severe HS. Further research is needed to determine its utility in the treatment of HS.63,90,91
Metformin, alone or in combination with other therapies (dapsone, finasteride, liraglutide), has been analyzed in small prospective studies of primarily female patients with different severities of HS, obesity, and PCOS. These studies have shown improvement in lesions, QOL, and reduction of workdays lost.92,93
Finasteride. Studies have shown finasteride (1.25-5 mg/d) alone or in combination with other treatments (metformin, liraglutide, levonorgestrel-ethinyl estradiol, and dapsone) provided varying degrees of resolution or improvement in patients with severe and advanced HS. Finasteride has been used for 4 to 16 weeks with a good safety profile.92,94-96
Continue to: Retinoids
Retinoids
Acitretin, alitretinoin, and isotretinoin have been studied in small retrospective studies to manage HS, with variable results.97-99 Robust prospective studies are needed. Retinoids, in general, should be considered as a second- or third-line treatment for moderate to severe HS.63
Surgical intervention
Surgical interventions, which should be considered in patients with widespread mild, moderate, or severe disease, are associated with improved daily activity and work productivity.100 Incision and drainage should be avoided in patients with HS, as this technique does not remove the affected follicles and is associated with 100% recurrence.101
Wide excision is the preferred surgical technique for patients with Hurley stage II and stage III HS; it is associated with lower recurrence rates (13%) compared to local excision (22%) and deroofing (27%).102 Secondary intention healing is the most commonly chosen method, based on lower recurrence rates than primary closure.102
STEEP and laser techniques. The skin-tissue-sparing excision with electrosurgical peeling (STEEP) procedure involves successive tangential excision of affected tissue until the epithelized bottom of the sinus tracts has been reached. This allows for the removal of fibrotic tissue and the sparing of the deep subcutaneous fat. STEEP is associated with 30% of relapses after 43 months.71
Laser surgery has also been studied in patients with Hurley stage II and stage III HS. The most commonly used lasers for HS are the 1064-nm neodymium-doped yttrium aluminum garnet (Nd: YAG) and the carbon dioxide laser; they have been shown to reduce disease severity in inguinal, axillary, and inflammatory sites.72-74
Pain management: Start with lidocaine, NSAIDs
There are few studies about HS-associated pain management.103 For acute episodes, short-acting nonopioid local treatment with lidocaine, topical or oral nonsteroidal anti-inflammatory drugs, and acetaminophen are preferred. Opioids should be reserved for moderate-to-severe pain that has not responded to other analgesics. Adjuvant therapy with pregabalin, gabapentin, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors can also be considered for the comanagement of pain and depression.62,104
Consider this tool to measure treatment response
The HS clinical response (HiSCR) tool is an outcome measure used to evaluate treatment outcomes. The tool uses an HS-specific binary score with the following criteria:
- ≥ 50% reduction in the number of inflammatory nodules;
- no increase in the number of abscesses; and
- no increase in the number of draining fistulas.105
The HiSCR was developed for the PIONEER studies105,106 to assess the response to ADA treatment. It is the only HS scoring system to undergo an extensive validation process with a meaningful clinical endpoint for HS treatment evaluation that is easy to use. Compared to the HS-PGA score (clear, minimal, mild), HiSCR was more responsive to change in patients with HS.105,106
CORRESPONDENCE
Cristina Marti-Amarista, MD, 101 Nicolls Road, Stony Brook, NY, 11794-8228; [email protected]
Hidradenitis suppurativa (HS), also known as acne inversa or Verneuil disease, is a chronic, recurrent, inflammatory occlusive disease affecting the terminal follicular epithelium in apocrine gland–bearing skin areas.1 HS manifests as painful nodules, abscesses, fistulas, and scarring and often has a severe psychological impact on the affected patient.2
When HS was first identified in the 1800s, it was believed to result from a dysfunction of the sweat glands.3 In 1939, scientists identified the true cause: follicular occlusion.3
Due to its chronic nature, heterogeneity in presentation, and apparent low prevalence,4 HS is considered an orphan disease.5 Over the past 10 years, there has been a surge in HS research—particularly in medical management—which has provided a better understanding of this condition.6,7
In this review, we discuss the most updated evidence regarding the diagnosis and treatment of HS to guide the family physician (FP)’s approach to managing this debilitating disease. But first, we offer a word about the etiology and pathophysiology of the condition.
3 events set the stage for hidradenitis suppurativa
Although the exact cause of HS is still unknown, some researchers have hypothesized that HS results from a combination of genetic predisposition and environmental and lifestyle factors.8-12 The primary mechanism of HS is the obstruction of the terminal follicular epithelium by a keratin plug.1,13,14 A systematic review of molecular inflammatory pathways involved in HS divides the pathogenesis of HS into 3 events: follicular occlusion followed by dilation, follicular rupture and inflammatory response, and chronic inflammatory state with sinus tracts.8
An underreported condition
HS is often underreported and misdiagnosed.4,15 Globally, the prevalence of HS varies from < 1% to 4%.15,16 A systematic review with meta-analysis showed a higher prevalence of HS in females compared to males in American and European populations.17 In the United States, the overall frequency of HS is 0.1%, or 98 per 100,000 persons.16 The prevalence of HS is highest among patients ages 30 to 39 years; there is decreased prevalence in patients ages 55 years and older.16,18
Who is at heightened risk?
Recent research has shown a relationship between ethnicity and HS.16,19,20 African American and biracial groups (defined as African American and White) have a 3-fold and 2-fold greater prevalence of HS, respectively, compared to White patients.16 However, the prevalence of HS in non-White ethnic groups may be underestimated in clinical trials due to a lack of representation and subgroup analyses based on ethnicity, which may affect generalizability in HS recommendations.21
Continue to: Genetic predisposition
Genetic predisposition. As many as 40% of patients with HS report having at least 1 affected family member. A positive family history of HS is associated with earlier onset, longer disease duration, and severe disease.22 HS is genetically heterogeneous, and several mutations (eg, gamma secretase, PSTPIP1, PSEN1 genes) have been identified in patients and in vitro as the cause of dysregulation of epidermal proliferation and differentiation, immune dysregulation, and promotion of amyloid formation.8,23-25
Obesity and metabolic risk factors. There is a strong relationship between HS and obesity. As many as 70% of patients with HS are obese, and 9% to 40% have metabolic syndrome.12,18,26-28 Obesity is associated with maceration and mechanical stress, increased fragility of the dermo-epidermal junction, changes in cutaneous blood flow, and subdermal fat inflammation—all of which favor the pathophysiology of HS.29,30
Smoking. Tobacco smoking is associated with severe HS and a lower chance of remission.12 Population-based studies have shown that as many as 90% of patients with HS have a history of smoking ≥ 20 packs of cigarettes per year.1,12,18,31,32 The nicotine and thousands of other chemicals present in cigarettes trigger keratinocytes and fibroblasts, resulting in epidermal hyperplasia, infundibular hyperkeratosis, excessive cornification, and dysbiosis.8,23,24
Hormones. The exact role sex hormones play in the pathogenesis of HS remains unclear.8,32 Most information is based primarily on small studies looking at antiandrogen treatments, HS activity during the menstrual cycle and pregnancy, HS exacerbation related to androgenic effects of hormonal contraception, and the association of HS with metabolic-endocrine disorders (eg, polycystic ovary syndrome [PCOS]).8,33
Androgens induce hyperkeratosis that may lead to follicular occlusion—the hallmark of HS pathology.34 A systematic review looking at the role of androgen and estrogen in HS found that while some patients with HS have elevated androgen levels, most have androgen and estrogen levels within normal range.35 Therefore, increased peripheral androgen receptor sensitivity has been hypothesized as the mechanism of action contributing to HS manifestation.34
Continue to: Host-defense defects
Host-defense defects. HS shares a similar cytokine profile with other well-established immune-mediated inflammatory diseases, including pyoderma gangrenosum (PG)36,37 and Crohn disease.38-40 HS is characterized by the expression of several immune mediators, including tumor necrosis factor-alpha (TNF-alpha), interleukin-1 alpha (IL-1 alpha), IL-1 beta, IL-8, IL-17, and the IL-23/T helper 17 pathway, all of which are upregulated in other inflammatory diseases and also result in an abnormal innate immune response.8,24 The recently described clinical triad of PG, acne, and HS (PASH) and the tetrad of pyogenic arthritis, PG, acne, and HS (PAPASH) further support the role of immune dysregulation in the pathogenesis of HS.40 Nonetheless, further studies are needed to determine the exact pathways of cytokine effect in HS.41
Use these criteria to make the diagnosis
The US and Canadian Hidradenitis Suppurativa Foundations (HSF) guidelines base the clinical diagnosis of HS on the following criteria2:
- Typical HS lesions: Erythematous skin lesions; inflamed, deep-seated painful nodules; “tombstone” double-ended comedones; sinus tracts; scarring; deformity. FIGURES 1A-1E show typical lesions seen in patients with HS.
- Typical locations: Intertriginous regions—apocrine gland–containing areas in axilla, groin, perineal region, buttocks, gluteal cleft, and mammary folds; beltline and waistband areas; areas of skin compression and friction.
- Recurrence and chronicity: Recurrent painful or suppurating lesions that appear more than twice in a 6-month period.2,41-43

Patients with HS usually present with painful recurrent abscesses and scarring and often report multiple visits to the emergency department for drainage or failed antibiotic treatment for abscesses.15,44
Ask patients these 2 questions. Vinding et al45 developed a survey for the diagnosis of HS using 2 simple questions based on the 3 criteria established by the HSF:
- “Have you had an outbreak of boils during the last 6 months?” and
- “Where and how many boils have you had?” (This question includes a list of the typical HS locations—eg, axilla, groin, genitals, area under breast.)
In their questionnaire, Vinding et al45 found that an affirmative answer to Question 1 and reports of > 2 boils in response to Question 2 correlated to a sensitivity of 90%, specificity of 97%, positive predictive value of 96%, and negative predictive value of 92% for the diagnosis of HS. The differential diagnosis of HS is summarized in TABLE 1.42,45-52

Continue to: These tools can help you to stage hidradenitis suppurativa
These tools can help you to stage hidradenitis suppurativa
Multiple tools are available to assess the severity of HS.53 We will describe the Hurley staging system and the International Hidradenitis Suppurativa Severity Score System (IHS4). Other diagnostic tools, such as the Sartorius score and the Hidradenitis Suppurativa Physician’s Global Assessment Scale (HS-PGA), can be time-consuming and challenging to interpret, limiting their use in the clinical setting.2,54
Hurley staging system (available at www.hsdiseasesource.com/hs-disease-staging) considers the presence of nodules, abscesses, sinus tracts, and scarring affecting an entire anatomical area.13,55 This system is most useful as a rapid classification tool for patients with HS in the clinical setting but should not be used to assess clinical response.2,13,56
The IHS4 (available at https://online library.wiley.com/doi/10.1111/bjd.15748) is a validated and easy-to-use tool for assessing HS and guiding the therapeutic strategy in clinical practice.54 With IHS4, the clinician must calculate the following:
- total number of nodules > 10 mm in diameter
- total number of abscesses multiplied by 2, and
- total number of draining tunnels (fistulae/sinuses) multiplied by 4.
Mild HS is defined as a score ≤ 3 points; moderate HS, 4 to 10 points; and severe HS, ≥ 11 points.54
No diagnostic tests, but ultrasound may be helpful
There are currently no established biological markers or specific tests for diagnosing HS.15 Ultrasound is emerging as a tool to assess dermal thickness, hair follicle morphology, and number and extent of fluid collections. Two recent studies showed that pairing clinical assessment with ultrasound findings improves accuracy of scoring in 84% of cases.57,58 For patients with severe HS, skin biopsy can be considered to rule out squamous cell carcinoma. Cultures, however, have limited utility except for suspected superimposed bacterial infection.2
Continue to: Screening for comorbidities
Screening for comorbidities
HSF recommends clinicians screen patients for comorbidities associated with HS (TABLE 2).2 Overall, screening patients for active and past history of smoking is strongly recommended, as is screening for metabolic syndrome, hyperlipidemia, type 2 diabetes (1.5- to 3-fold greater risk of type 2 diabetes in HS patients), and PCOS (3-fold greater risk).2,26,27,59 Screening patients for depression and anxiety is also routinely recommended.2 However, the authors of this article strongly recommend screening all patients with HS for psychiatric comorbidities, as research has shown a 2-fold greater risk of depression and anxiety, social isolation, and low self-esteem that severely limits quality of life (QOL) in this patient population.60,61

Management
Treat existing lesions, reduce formation of new ones
The main goals of treatment for patients with HS are to treat existing lesions and reduce associated symptoms, reduce the formation of new lesions, and minimize associated psychological morbidity.15 FPs play an important role in the early diagnosis, treatment, and comprehensive care of patients with HS. This includes monitoring patients, managing comorbidities, making appropriate referrals to dermatologists, and coordinating the multidisciplinary care that patients with HS require.

A systematic review identified more than 50 interventions used to treat HS, most based on small observational studies and randomized controlled trials (RCTs) with a high risk of bias.62 FIGURE 22,62-69 provides an evidence-based treatment algorithm for HS, and TABLE 32,63,64,70-75 summarizes the most commonly used treatments.

Biologic agents
Adalimumab (ADA) is a fully human immunoglobulin G1 monoclonal antibody that binds to TNF-alpha, neutralizes its bioactivity, and induces apoptosis of TNF-expressing mononuclear cells. It is the only medication approved by the US Food and Drug Administration for active refractory moderate and severe HS.62,65 Several double-blinded RCTs, including PIONEER I and PIONEER II, studied the effectiveness of ADA for HS and found significant clinical responses at Week 12, 50% reduction in abscess and nodule counts, no increase in abscesses or draining fistulas at Week 12, and sustained improvement in lesion counts, pain, and QOL.66,67,76
IL-1 and IL-23 inhibitors. The efficacy of etanercept and golimumab (anti-TNF), as well as anakinra (IL-1 inhibitor) and ustekinumab (IL-1/IL-23 inhibitor), continue to be investigated with variable results; they are considered second-line treatment for active refractory moderate and severe HS after ADA.65,77-80 Infliximab (IL-1 beta inhibitor) has shown no effect on reducing disease severity.70Compared to other treatments, biologic therapy is associated with higher costs (TABLE 3),2,63,64,70-75 an increased risk for reactivation of latent infections (eg, tuberculosis, herpes simplex, and hepatitis C virus [HCV], and B [HBV]), and an attenuated response to vaccines.81 Prior to starting biologic therapy, FPs should screen patients with HS for tuberculosis and HBV, consider HIV and HCV screening in at-risk patients, and optimize the immunization status of the patient.82,83 While inactivated vaccines can be administered without discontinuing biologic treatment, patients should avoid live-attenuated vaccines while taking biologics.83
Continue to: Antibiotic therapy
Antibiotic therapy
Topical antibiotics are considered first-line treatment for mild and moderate uncomplicated HS.63,64 Clindamycin 1%, the only topical antibiotic studied in a small double-blind RCT of patients with Hurley stage I and stage II HS, demonstrated significant clinical improvement after 12 weeks of treatment (twice- daily application), compared to placebo.84 Topical clindamycin is also recommended to treat flares in patients with mild disease.2,64
Oral antibiotics. Tetracycline (500 mg twice daily for 4 months) is considered a second-line treatment for patients with mild HS.64,68 Doxycycline (200 mg/d for 3 months) may also be considered as a second-line treatment in patients with mild disease.85
Combination oral clindamycin (300 mg) and rifampicin (300 mg) twice daily for 10 weeks is recommended as first-line treatment for patients with moderate HS.2,64,69 Combination rifampin (300 mg twice daily), moxifloxacin (400 mg/d), and metronidazole (500 mg three times a day) is not routinely recommended due to increased risk of toxicity.2
Ertapenem (1 g intravenously daily for 6 weeks) is supported by lower-level evidence as a third-line rescue therapy option and as a bridge to surgery; however, limitations for home infusions, costs, and concerns for antibiotic resistance limit its use.2,86
Corticosteroids and systemic immunomodulators
Intralesional triamcinolone (2-20 mg) may be beneficial in the early stages of HS, although its use is based on a small prospective open study of 33 patients.87 A recent double-blind placebo-controlled RCT comparing varying concentrations of intralesional triamcinolone (10 mg/mL and 40 mg/mL) vs normal saline showed no statistically significant difference in inflammatory clearance, pain reduction, or patient satisfaction.88
Continue to: Short-term systemic corticosteroid tapers...
Short-term systemic corticosteroid tapers (eg, prednisone, starting at 0.5-1 mg/kg) are recommended to treat flares. Long-term corticosteroids and cyclosporine are reserved for patients with severe refractory disease; however, due to safety concerns, their regular use is strongly discouraged.63,64,85 There is limited evidence to support the use of methotrexate for severe refractory disease, and its use is not recommended.63
Hormonal therapy
The use of hormonal therapy for HS is limited by the low-quality evidence (eg, anecdotal evidence, small retrospective analyses, uncontrolled trials).33,63 The only exception is a small double-blind controlled crossover trial from 1986 showing that the antiandrogen effects of combination oral contraceptives (ethinyloestradiol 50 mcg/cyproterone acetate in a reverse sequential regimen and ethinyloestradiol 50 mcg/norgestrel 500 mcg) improved HS lesions.89
Spironolactone, an antiandrogen diuretic, has been studied in small case report series with a high risk for bias. It is used mainly in female patients with mild or moderate disease, or in combination with other agents in patients with severe HS. Further research is needed to determine its utility in the treatment of HS.63,90,91
Metformin, alone or in combination with other therapies (dapsone, finasteride, liraglutide), has been analyzed in small prospective studies of primarily female patients with different severities of HS, obesity, and PCOS. These studies have shown improvement in lesions, QOL, and reduction of workdays lost.92,93
Finasteride. Studies have shown finasteride (1.25-5 mg/d) alone or in combination with other treatments (metformin, liraglutide, levonorgestrel-ethinyl estradiol, and dapsone) provided varying degrees of resolution or improvement in patients with severe and advanced HS. Finasteride has been used for 4 to 16 weeks with a good safety profile.92,94-96
Continue to: Retinoids
Retinoids
Acitretin, alitretinoin, and isotretinoin have been studied in small retrospective studies to manage HS, with variable results.97-99 Robust prospective studies are needed. Retinoids, in general, should be considered as a second- or third-line treatment for moderate to severe HS.63
Surgical intervention
Surgical interventions, which should be considered in patients with widespread mild, moderate, or severe disease, are associated with improved daily activity and work productivity.100 Incision and drainage should be avoided in patients with HS, as this technique does not remove the affected follicles and is associated with 100% recurrence.101
Wide excision is the preferred surgical technique for patients with Hurley stage II and stage III HS; it is associated with lower recurrence rates (13%) compared to local excision (22%) and deroofing (27%).102 Secondary intention healing is the most commonly chosen method, based on lower recurrence rates than primary closure.102
STEEP and laser techniques. The skin-tissue-sparing excision with electrosurgical peeling (STEEP) procedure involves successive tangential excision of affected tissue until the epithelized bottom of the sinus tracts has been reached. This allows for the removal of fibrotic tissue and the sparing of the deep subcutaneous fat. STEEP is associated with 30% of relapses after 43 months.71
Laser surgery has also been studied in patients with Hurley stage II and stage III HS. The most commonly used lasers for HS are the 1064-nm neodymium-doped yttrium aluminum garnet (Nd: YAG) and the carbon dioxide laser; they have been shown to reduce disease severity in inguinal, axillary, and inflammatory sites.72-74
Pain management: Start with lidocaine, NSAIDs
There are few studies about HS-associated pain management.103 For acute episodes, short-acting nonopioid local treatment with lidocaine, topical or oral nonsteroidal anti-inflammatory drugs, and acetaminophen are preferred. Opioids should be reserved for moderate-to-severe pain that has not responded to other analgesics. Adjuvant therapy with pregabalin, gabapentin, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors can also be considered for the comanagement of pain and depression.62,104
Consider this tool to measure treatment response
The HS clinical response (HiSCR) tool is an outcome measure used to evaluate treatment outcomes. The tool uses an HS-specific binary score with the following criteria:
- ≥ 50% reduction in the number of inflammatory nodules;
- no increase in the number of abscesses; and
- no increase in the number of draining fistulas.105
The HiSCR was developed for the PIONEER studies105,106 to assess the response to ADA treatment. It is the only HS scoring system to undergo an extensive validation process with a meaningful clinical endpoint for HS treatment evaluation that is easy to use. Compared to the HS-PGA score (clear, minimal, mild), HiSCR was more responsive to change in patients with HS.105,106
CORRESPONDENCE
Cristina Marti-Amarista, MD, 101 Nicolls Road, Stony Brook, NY, 11794-8228; [email protected]
1. Bergler-Czop B, Hadasik K, Brzezińska-Wcisło L. Acne inversa: difficulties in diagnostics and therapy. Postepy Dermatol Alergol. 2015;32:296-301. doi: 10.5114/pdia.2014.44012
2. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
3. Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol. 2010;2:9-16. doi: 10.4161/derm.2.1.12490
4. Kokolakis G, Wolk K, Schneider-Burrus S, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology. 2020;236:421-430. doi: 10.1159/000508787
5. Gulliver W, Landells IDR, Morgan D, et al. Hidradenitis suppurativa: a novel model of care and an integrative strategy to adopt an orphan disease. J Cutan Med Surg. 2018;22:71-77. doi: 10.1177/1203475417736290
6. Savage KT, Gonzalez Brant E, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi: 10.1111/jdv.16213.
7. Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000. doi: 10.12688/f1000research.26083.1
8. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965
9. Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res. 2018;7:1930. doi: 10.12688/f1000 research.17267.1
10. Sabat R, Jemec GBE, Matusiak Ł. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6:18. doi: 10.1038/s41572-020-0149-1
11. Shlyankevich J, Chen AJ, Kim GE, et al. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71:1144-1150. doi: 10.1016/j.jaad.2014.09.012
12. Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831-839. doi: 10.1111/j.1365-2133.2009.09198.x
13. von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537. doi: 10.1111/j.1600-0625.2009.00915.x
14. Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999. doi: 10.1016/s0190-9622(96)90277-7
15. Ballard K, Shuman VL. Hidradenitis suppurativa. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Updated July 15, 2022. Accessed November 28, 2022. www.ncbi.nlm.nih.gov/books/NBK534867/
16. Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi: 10.1001/jamadermatol.2017.0201
17. Phan K, Charlton O, Smith SD. Global prevalence of hidradenitis suppurativa and geographical variation—systematic review and meta-analysis. BioMed Dermatol. 2020;4. doi: 10.1186/s41702-019-0052-0
18. Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi: 10.1038/jid.2012.255
19. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi: 10.1177/1203475420972348
20. Vaidya T, Vangipuram R, Alikhan A. Examining the race-specific prevalence of hidradenitis suppurativa at a large academic center; results from a retrospective chart review. Dermatol Online J. 2017;23:13030/qt9xc0n0z1. doi: 10.5070/D3236035391
21. Price KN, Hsiao JL, Shi VY. Race and ethnicity gaps in global hidradenitis suppurativa clinical trials. Dermatology. 2021;237:97-102. doi: 10.1159/000504911
22. Schrader AM, Deckers IE, van der Zee HH, et al. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol. 2014;71:460-467. doi: 10.1016/j.jaad.2014.04.001
23. Frew JW, Vekic DA, Wood J, et al. A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol. 2017;177:987-998. doi: 10.1111/bjd.15441
24. Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol. 2020;183:999-1010. doi: 10.1111/bjd.19556
25. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi: 10.1016/j.jaad.2019.08.090
26. Sabat R, Chanwangpong A, Schneider-Burrus S, et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PloS One. 2012;7:e31810. doi: 10.1371/journal.pone.0031810
27. Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatology. 2021;237:119-124. doi: 10.1159/000501611
28. Rodríguez-Zuñiga MJM, García-Perdomo HA, Ortega-Loayza AG. Association between hidradenitis suppurativa and metabolic syndrome: a systematic review and meta-analysis. Actas Dermosifiliogr (Engl Ed). 2019;110:279-288. doi: 10.1016/j.ad.2018.10.020
29. Walker JM, Garcet S, Aleman JO, et al. Obesity and ethnicity alter gene expression in skin. Sci Rep. 2020;10:14079. doi: 10.1038/s41598-020-70244-2.
30. Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43. doi: 10.1016/j.det.2015.08.011
31. Vossen ARJV, van Straalen KR, Swolfs EFH, et al. Nicotine dependency and readiness to quit smoking among patients with hidradenitis suppurativa. Dermatology. 2021;237:383-385. doi: 10.1159/000514028
32. Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi: 10.1111/bjd.13090
33. Clark AK, Quinonez RL, Saric S, et al. Hormonal therapies for hidradenitis suppurativa: review. Dermatol Online J. 2017;23:13030/qt6383k0n4. doi: 10.5070/D32310036990
34. Saric-Bosanac S, Clark AK, Sivamani RK, et al. The role of hypothalamus-pituitary-adrenal (HPA)-like axis in inflammatory pilosebaceous disorders. Dermatol Online J. 2020;26:13030/qt8949296f. doi: 10.5070/D3262047430
35. Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa – a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
36. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270. doi: 10.1001/archdermatol.2010.328
37. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671. doi: 10.1111/j.1365-2230.2005.01897.x
38. Kirthi S, Hellen R, O’Connor R, et al. Hidradenitis suppurativa and Crohn’s disease: a case series. Ir Med J. 2017;110:618.
39. Dumont LM, Landman C, Sokol H, et al; CD-HS Study Group. Increased risk of permanent stoma in Crohn’s disease associated with hidradenitis suppurativa: a case-control study. Aliment Pharmacol Ther. 2020;52:303-310. doi: 10.1111/apt.15863
40. Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:e187. doi: 10.1097/MD.0000000000000187.
41. Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
42. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
43. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781. doi: 10.1111/bjd.16998.
44. Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221; doi: 10.1136/postgradmedj-2013-131994
45. Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol. 2014;170:884-889. doi: 10.1111/bjd.12787
46. Bassas-Vila J, González Lama Y. Hidradenitis suppurativa and perianal Crohn disease: differential diagnosis. Actas Dermosifiliogr. 2016;107(suppl 2):27-31. doi: 10.1016/S0001-7310(17) 30006-6
47. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. doi: 10.2147/IDR.S39601
48. Fuchs W, Brockmeyer NH. Sexually transmitted infections. J Dtsch Dermatol Ges. 2014;12:451-463. doi: 10.1111/ddg.12310
49. Hap W, Frejlich E, Rudno-Rudzińska J, et al. Pilonidal sinus: finding the righttrack for treatment. Pol Przegl Chir. 2017;89:68-75. doi: 10.5604/01.3001.0009.6009
50. Al-Hamdi KI, Saadoon AQ. Acne onglobate of the scalp. Int J Trichology. 2020;12:35-37. doi: 10.4103/ijt.ijt_117_19
51. Balestra A, Bytyci H, Guillod C, et al. A case of ulceroglandular tularemia presenting with lymphadenopathy and an ulcer on a linear morphoea lesion surrounded by erysipelas. Int Med Case Rep J. 2018;11:313-318. doi: 10.2147/IMCRJ.S178561
52. Ibler KS, Kromann CB. Recurrent furunculosis – challenges and management: a review. Clin Cosmet Investig Dermatol. 2014;7:59-64. doi: 10.2147/CCID.S35302
53. Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol. 2016;175:263-272. doi: 10.1111/bjd.14475
54. Zouboulis CC, Tzellos T, Kyrgidis A, et al; European Hidradenitis Suppurativa Foundation Investigator Group. Development and validation of the International Hidradenitis Suppurativa Severity Score System (I4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177:1401-1409. doi: 10.1111/bjd.15748
55. Hidradenitis Suppurativa Clinical Resource. Hidradenitis suppurativa stages: Hurley Staging System. www.hsdiseasesource.com/hs-disease-staging. Accessed October 11, 2022.
56. Ovadja ZN, Schuit MM, van der Horst CMAM, et al. Inter- and interrater reliability of Hurley staging for hidradenitis suppurativa. Br J Dermatol. 2019;181:344-349. doi: 10.1111/bjd.17588
57. Wortsman X, Jemec GBE. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg. 2007;33:1340-1342. doi: 10.1111/j.1524-4725.2007.33286.x
58. Napolitano M, Calzavara-Pinton PG, Zanca A, et al. Comparison of clinical and ultrasound scores in patients with hidradenitis suppurativa: results from an Italian ultrasound working group. J Eur Acad Dermatol Venereol. 2019;33:e84-e87. doi: 10.1111/jdv.15235
59. Bukvić Mokos Z, Miše J, Balić A, et al. Understanding the relationship between smoking and hidradenitis suppurativa. Acta Dermatovenerol Croat. 2020;28:9-13.
60. Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2015;29:371-376. doi: 10.1111/jdv.12567
61. Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life and psychosocial implications in patients with hidradenitis suppurativa. Dermatology. 2016;232:687-691. doi: 10.1159/000453355
62 Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978. doi: 10.1111/bjd.14418
63. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi: 10.1016/j.jaad.2019.02.068
64. Gulliver W, Zouboulis CC, Prens E, et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17:343-351. doi: 10.1007/s11154-016-9328-5
65. Vena GA, Cassano N. Drug focus: adalimumab in the treatment of moderate to severe psoriasis. Biologics. 2007;1:93-103.
66. Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-55. doi: 10.7326/0003-4819-157-12-201212180-00004
67. Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi: 10.1016/j.jaad.2018.05.040
68. Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi: 10.1016/s0190-9622(98)70272-5
69. Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi: 10.1159/000228334
70. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217. doi: 10.1016/j.jaad.2009.06.050
71. Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi: 10.1111/jdv.12376
72. Mahmoud BH, Tierney E, Hexsel CL, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62:637-645. doi: 10.1016/j.jaad.2009.07.048
73. Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:1188-1198. doi: 10.1111/j.1524-4725.2009.01214.x
74. Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208-213. doi: 10.1111/j.1524-4725.2009.01427.x
75. van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi: 10.1016/j.jaad.2009.12.018
76. Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi: 10.1056/NEJMoa1504370. PMID: 27518661.
77. Adams DR, Yankura JA, Fogelberg AC, et al. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146:501-504. doi: 10.1001/archdermatol.2010.72
78. Tursi A. Concomitant hidradenitis suppurativa and pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig Liver Dis. 2016;48:1511-1512. doi: 10.1016/j.dld.2016.09.010
79. Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi: 10.1001/jamadermatol.2015.3903.
80. Romaní J, Vilarrasa E, Martorell A, et al. Ustekinumab with intravenous infusion: results in hidradenitis suppurativa. Dermatology. 2020;236:21-24. doi: 10.1159/000501075
81. Kane SV. Preparing for biologic or immunosuppressant therapy. Gastroenterol Hepatol (N Y). 2011;7:544-546.
82. Davis W, Vavilin I, Malhotra N. Biologic therapy in HIV: to screen or not to screen. Cureus. 2021;13:e15941. doi: 10.7759/cureus.15941
83. Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23:50-74. doi: 10.1177/1203475418811335
84. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328. doi: 10.1111/j.1365-4362.1983.tb02150.x
85. Hunger RE, Laffitte E, Läuchli S, et al. Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatology. 2017;233:113-119. doi: 10.1159/000477459
86. Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33:19-31. doi: 10.1111/jdv.15233
87. Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi: 10.1016/j.jaad.2016.06.049
88. Fajgenbaum K, Crouse L, Dong L, et al. Intralesional triamcinolone may not be beneficial for treating acute hidradenitis suppurativa lesions: a double-blind, randomized, placebo-controlled trial. Dermatol Surg. 2020;46:685-689. doi: 10.1097/DSS.0000000000002112
89. Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268. doi: 10.1111/j.1365-2133.1986.tb05740.x
90. Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131. doi: 10.2310/7750.2007.00019
91. Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196. doi: 10.1111/ajd.12362
92. Khandalavala BN. A disease-modifying approach for advanced hidradenitis suppurativa (regimen with metformin, liraglutide, dapsone, and finasteride): a case report. Case Rep Dermatol. 2017;9:70-78. doi: 10.1159/000473873
93. Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi: 10.1111/j.1468-3083.2012.04668.x
94. Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
95. Mota F, Machado S, Selores M. Hidradenitis suppurativa in children treated with finasteride-a case series. Pediatr Dermatol. 2017;34:578-583. doi: 10.1111/pde.13216
96. Doménech C, Matarredona J, Escribano-Stablé JC, et al. Facial hidradenitis suppurativa in a 28-year-old male responding to finasteride. Dermatology. 2012;224:307-308. doi: 10.1159/000339477
97. Patel N, McKenzie SA, Harview CL, et al. Isotretinoin in the treatment of hidradenitis suppurativa: a retrospective study. J Dermatolog Treat. 2021;32:473-475. doi: 10.1080/09546634.2019.1670779
98. Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40:73-76. doi: 10.1016/s0190-9622(99) 70530-x
99. Huang CM, Kirchhof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125. doi: 10.1159/000477207
100. Prens LM, Huizinga J, Janse IC. Surgical outcomes and the impact of major surgery on quality of life, activity impairment and sexual health in hidradenitis suppurativa patients: a prospective single centre study. J Eur Acad Dermatol Venereol. 2019;33:1941-1946. doi: 10.1111/jdv.15706
101. Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164-168. doi: 10.1007/s003840050159
102. Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5 suppl 1):S70-S77. doi: 10.1016/j.jaad.2015.07.044.
103. Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444. doi: 10.1097/AJP.0b013e3181ceb80c
104. Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi: 10.1016/j.jaad.2015.07.046
105. Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994. doi: 10.1111/jdv.13216
106. Kimball AB, Jemec GB, Yang M, et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol. 2014;171:1434-1442. doi: 10.1111/bjd.13270
1. Bergler-Czop B, Hadasik K, Brzezińska-Wcisło L. Acne inversa: difficulties in diagnostics and therapy. Postepy Dermatol Alergol. 2015;32:296-301. doi: 10.5114/pdia.2014.44012
2. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
3. Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol. 2010;2:9-16. doi: 10.4161/derm.2.1.12490
4. Kokolakis G, Wolk K, Schneider-Burrus S, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology. 2020;236:421-430. doi: 10.1159/000508787
5. Gulliver W, Landells IDR, Morgan D, et al. Hidradenitis suppurativa: a novel model of care and an integrative strategy to adopt an orphan disease. J Cutan Med Surg. 2018;22:71-77. doi: 10.1177/1203475417736290
6. Savage KT, Gonzalez Brant E, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi: 10.1111/jdv.16213.
7. Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000. doi: 10.12688/f1000research.26083.1
8. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965
9. Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res. 2018;7:1930. doi: 10.12688/f1000 research.17267.1
10. Sabat R, Jemec GBE, Matusiak Ł. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6:18. doi: 10.1038/s41572-020-0149-1
11. Shlyankevich J, Chen AJ, Kim GE, et al. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J Am Acad Dermatol. 2014;71:1144-1150. doi: 10.1016/j.jaad.2014.09.012
12. Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831-839. doi: 10.1111/j.1365-2133.2009.09198.x
13. von Laffert M, Helmbold P, Wohlrab J, et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19:533-537. doi: 10.1111/j.1600-0625.2009.00915.x
14. Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999. doi: 10.1016/s0190-9622(96)90277-7
15. Ballard K, Shuman VL. Hidradenitis suppurativa. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Updated July 15, 2022. Accessed November 28, 2022. www.ncbi.nlm.nih.gov/books/NBK534867/
16. Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi: 10.1001/jamadermatol.2017.0201
17. Phan K, Charlton O, Smith SD. Global prevalence of hidradenitis suppurativa and geographical variation—systematic review and meta-analysis. BioMed Dermatol. 2020;4. doi: 10.1186/s41702-019-0052-0
18. Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi: 10.1038/jid.2012.255
19. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi: 10.1177/1203475420972348
20. Vaidya T, Vangipuram R, Alikhan A. Examining the race-specific prevalence of hidradenitis suppurativa at a large academic center; results from a retrospective chart review. Dermatol Online J. 2017;23:13030/qt9xc0n0z1. doi: 10.5070/D3236035391
21. Price KN, Hsiao JL, Shi VY. Race and ethnicity gaps in global hidradenitis suppurativa clinical trials. Dermatology. 2021;237:97-102. doi: 10.1159/000504911
22. Schrader AM, Deckers IE, van der Zee HH, et al. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J Am Acad Dermatol. 2014;71:460-467. doi: 10.1016/j.jaad.2014.04.001
23. Frew JW, Vekic DA, Wood J, et al. A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol. 2017;177:987-998. doi: 10.1111/bjd.15441
24. Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol. 2020;183:999-1010. doi: 10.1111/bjd.19556
25. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi: 10.1016/j.jaad.2019.08.090
26. Sabat R, Chanwangpong A, Schneider-Burrus S, et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PloS One. 2012;7:e31810. doi: 10.1371/journal.pone.0031810
27. Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatology. 2021;237:119-124. doi: 10.1159/000501611
28. Rodríguez-Zuñiga MJM, García-Perdomo HA, Ortega-Loayza AG. Association between hidradenitis suppurativa and metabolic syndrome: a systematic review and meta-analysis. Actas Dermosifiliogr (Engl Ed). 2019;110:279-288. doi: 10.1016/j.ad.2018.10.020
29. Walker JM, Garcet S, Aleman JO, et al. Obesity and ethnicity alter gene expression in skin. Sci Rep. 2020;10:14079. doi: 10.1038/s41598-020-70244-2.
30. Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43. doi: 10.1016/j.det.2015.08.011
31. Vossen ARJV, van Straalen KR, Swolfs EFH, et al. Nicotine dependency and readiness to quit smoking among patients with hidradenitis suppurativa. Dermatology. 2021;237:383-385. doi: 10.1159/000514028
32. Kromann CB, Deckers IE, Esmann S, et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol. 2014;171:819-824. doi: 10.1111/bjd.13090
33. Clark AK, Quinonez RL, Saric S, et al. Hormonal therapies for hidradenitis suppurativa: review. Dermatol Online J. 2017;23:13030/qt6383k0n4. doi: 10.5070/D32310036990
34. Saric-Bosanac S, Clark AK, Sivamani RK, et al. The role of hypothalamus-pituitary-adrenal (HPA)-like axis in inflammatory pilosebaceous disorders. Dermatol Online J. 2020;26:13030/qt8949296f. doi: 10.5070/D3262047430
35. Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa – a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
36. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270. doi: 10.1001/archdermatol.2010.328
37. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671. doi: 10.1111/j.1365-2230.2005.01897.x
38. Kirthi S, Hellen R, O’Connor R, et al. Hidradenitis suppurativa and Crohn’s disease: a case series. Ir Med J. 2017;110:618.
39. Dumont LM, Landman C, Sokol H, et al; CD-HS Study Group. Increased risk of permanent stoma in Crohn’s disease associated with hidradenitis suppurativa: a case-control study. Aliment Pharmacol Ther. 2020;52:303-310. doi: 10.1111/apt.15863
40. Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:e187. doi: 10.1097/MD.0000000000000187.
41. Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
42. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
43. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781. doi: 10.1111/bjd.16998.
44. Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221; doi: 10.1136/postgradmedj-2013-131994
45. Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol. 2014;170:884-889. doi: 10.1111/bjd.12787
46. Bassas-Vila J, González Lama Y. Hidradenitis suppurativa and perianal Crohn disease: differential diagnosis. Actas Dermosifiliogr. 2016;107(suppl 2):27-31. doi: 10.1016/S0001-7310(17) 30006-6
47. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. doi: 10.2147/IDR.S39601
48. Fuchs W, Brockmeyer NH. Sexually transmitted infections. J Dtsch Dermatol Ges. 2014;12:451-463. doi: 10.1111/ddg.12310
49. Hap W, Frejlich E, Rudno-Rudzińska J, et al. Pilonidal sinus: finding the righttrack for treatment. Pol Przegl Chir. 2017;89:68-75. doi: 10.5604/01.3001.0009.6009
50. Al-Hamdi KI, Saadoon AQ. Acne onglobate of the scalp. Int J Trichology. 2020;12:35-37. doi: 10.4103/ijt.ijt_117_19
51. Balestra A, Bytyci H, Guillod C, et al. A case of ulceroglandular tularemia presenting with lymphadenopathy and an ulcer on a linear morphoea lesion surrounded by erysipelas. Int Med Case Rep J. 2018;11:313-318. doi: 10.2147/IMCRJ.S178561
52. Ibler KS, Kromann CB. Recurrent furunculosis – challenges and management: a review. Clin Cosmet Investig Dermatol. 2014;7:59-64. doi: 10.2147/CCID.S35302
53. Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol. 2016;175:263-272. doi: 10.1111/bjd.14475
54. Zouboulis CC, Tzellos T, Kyrgidis A, et al; European Hidradenitis Suppurativa Foundation Investigator Group. Development and validation of the International Hidradenitis Suppurativa Severity Score System (I4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177:1401-1409. doi: 10.1111/bjd.15748
55. Hidradenitis Suppurativa Clinical Resource. Hidradenitis suppurativa stages: Hurley Staging System. www.hsdiseasesource.com/hs-disease-staging. Accessed October 11, 2022.
56. Ovadja ZN, Schuit MM, van der Horst CMAM, et al. Inter- and interrater reliability of Hurley staging for hidradenitis suppurativa. Br J Dermatol. 2019;181:344-349. doi: 10.1111/bjd.17588
57. Wortsman X, Jemec GBE. Real-time compound imaging ultrasound of hidradenitis suppurativa. Dermatol Surg. 2007;33:1340-1342. doi: 10.1111/j.1524-4725.2007.33286.x
58. Napolitano M, Calzavara-Pinton PG, Zanca A, et al. Comparison of clinical and ultrasound scores in patients with hidradenitis suppurativa: results from an Italian ultrasound working group. J Eur Acad Dermatol Venereol. 2019;33:e84-e87. doi: 10.1111/jdv.15235
59. Bukvić Mokos Z, Miše J, Balić A, et al. Understanding the relationship between smoking and hidradenitis suppurativa. Acta Dermatovenerol Croat. 2020;28:9-13.
60. Shavit E, Dreiher J, Freud T, et al. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2015;29:371-376. doi: 10.1111/jdv.12567
61. Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life and psychosocial implications in patients with hidradenitis suppurativa. Dermatology. 2016;232:687-691. doi: 10.1159/000453355
62 Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978. doi: 10.1111/bjd.14418
63. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi: 10.1016/j.jaad.2019.02.068
64. Gulliver W, Zouboulis CC, Prens E, et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17:343-351. doi: 10.1007/s11154-016-9328-5
65. Vena GA, Cassano N. Drug focus: adalimumab in the treatment of moderate to severe psoriasis. Biologics. 2007;1:93-103.
66. Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-55. doi: 10.7326/0003-4819-157-12-201212180-00004
67. Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi: 10.1016/j.jaad.2018.05.040
68. Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971-974. doi: 10.1016/s0190-9622(98)70272-5
69. Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154. doi: 10.1159/000228334
70. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217. doi: 10.1016/j.jaad.2009.06.050
71. Blok JL, Spoo JR, Leeman FWJ, et al. Skin-tissue-sparing excision with electrosurgical peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29:379-382. doi: 10.1111/jdv.12376
72. Mahmoud BH, Tierney E, Hexsel CL, et al. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J Am Acad Dermatol. 2010;62:637-645. doi: 10.1016/j.jaad.2009.07.048
73. Tierney E, Mahmoud BH, Hexsel C, et al. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 2009;35:1188-1198. doi: 10.1111/j.1524-4725.2009.01214.x
74. Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36:208-213. doi: 10.1111/j.1524-4725.2009.01427.x
75. van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi: 10.1016/j.jaad.2009.12.018
76. Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi: 10.1056/NEJMoa1504370. PMID: 27518661.
77. Adams DR, Yankura JA, Fogelberg AC, et al. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol. 2010;146:501-504. doi: 10.1001/archdermatol.2010.72
78. Tursi A. Concomitant hidradenitis suppurativa and pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig Liver Dis. 2016;48:1511-1512. doi: 10.1016/j.dld.2016.09.010
79. Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59. doi: 10.1001/jamadermatol.2015.3903.
80. Romaní J, Vilarrasa E, Martorell A, et al. Ustekinumab with intravenous infusion: results in hidradenitis suppurativa. Dermatology. 2020;236:21-24. doi: 10.1159/000501075
81. Kane SV. Preparing for biologic or immunosuppressant therapy. Gastroenterol Hepatol (N Y). 2011;7:544-546.
82. Davis W, Vavilin I, Malhotra N. Biologic therapy in HIV: to screen or not to screen. Cureus. 2021;13:e15941. doi: 10.7759/cureus.15941
83. Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23:50-74. doi: 10.1177/1203475418811335
84. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328. doi: 10.1111/j.1365-4362.1983.tb02150.x
85. Hunger RE, Laffitte E, Läuchli S, et al. Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatology. 2017;233:113-119. doi: 10.1159/000477459
86. Zouboulis CC, Bechara FG, Dickinson-Blok JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization - systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33:19-31. doi: 10.1111/jdv.15233
87. Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155. doi: 10.1016/j.jaad.2016.06.049
88. Fajgenbaum K, Crouse L, Dong L, et al. Intralesional triamcinolone may not be beneficial for treating acute hidradenitis suppurativa lesions: a double-blind, randomized, placebo-controlled trial. Dermatol Surg. 2020;46:685-689. doi: 10.1097/DSS.0000000000002112
89. Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268. doi: 10.1111/j.1365-2133.1986.tb05740.x
90. Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131. doi: 10.2310/7750.2007.00019
91. Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196. doi: 10.1111/ajd.12362
92. Khandalavala BN. A disease-modifying approach for advanced hidradenitis suppurativa (regimen with metformin, liraglutide, dapsone, and finasteride): a case report. Case Rep Dermatol. 2017;9:70-78. doi: 10.1159/000473873
93. Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol. 2013;27:1101-1108. doi: 10.1111/j.1468-3083.2012.04668.x
94. Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
95. Mota F, Machado S, Selores M. Hidradenitis suppurativa in children treated with finasteride-a case series. Pediatr Dermatol. 2017;34:578-583. doi: 10.1111/pde.13216
96. Doménech C, Matarredona J, Escribano-Stablé JC, et al. Facial hidradenitis suppurativa in a 28-year-old male responding to finasteride. Dermatology. 2012;224:307-308. doi: 10.1159/000339477
97. Patel N, McKenzie SA, Harview CL, et al. Isotretinoin in the treatment of hidradenitis suppurativa: a retrospective study. J Dermatolog Treat. 2021;32:473-475. doi: 10.1080/09546634.2019.1670779
98. Boer J, van Gemert MJ. Long-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol. 1999;40:73-76. doi: 10.1016/s0190-9622(99) 70530-x
99. Huang CM, Kirchhof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125. doi: 10.1159/000477207
100. Prens LM, Huizinga J, Janse IC. Surgical outcomes and the impact of major surgery on quality of life, activity impairment and sexual health in hidradenitis suppurativa patients: a prospective single centre study. J Eur Acad Dermatol Venereol. 2019;33:1941-1946. doi: 10.1111/jdv.15706
101. Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164-168. doi: 10.1007/s003840050159
102. Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5 suppl 1):S70-S77. doi: 10.1016/j.jaad.2015.07.044.
103. Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444. doi: 10.1097/AJP.0b013e3181ceb80c
104. Horváth B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S47-S51. doi: 10.1016/j.jaad.2015.07.046
105. Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994. doi: 10.1111/jdv.13216
106. Kimball AB, Jemec GB, Yang M, et al. Assessing the validity, responsiveness and meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol. 2014;171:1434-1442. doi: 10.1111/bjd.13270
PRACTICE RECOMMENDATIONS
› Screen patients with hidradenitis suppurativa (HS) for depression, anxiety, history of smoking, metabolic syndrome, and type 2 diabetes. A
› Look into early surgical and dermatology referrals for patients with mild diffused, moderate, and severe disease. B
› Consider biopsy to rule out skin cancer in patients with severe and longstanding HS refractory to treatment. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A worsening abdominal rash
A 48-YEAR-OLD WOMAN presented to Dermatology for evaluation of a 6-cm abdominal lesion that had been present for 5 weeks (FIGURE 1). The lesion was originally about the size of a quarter, but it started to enlarge after treatment of an asthma exacerbation with a 4-day course of prednisone. It continued to grow after another physician, likely presuming the lesion was a corticosteroid-responsive dermatosis (eg, nummular eczema, granuloma annulare, or erythema annulare centrifugum), prescribed a 2-week trial of clobetasol ointment. Physical examination revealed a mildly pruritic, 6-cm erythematous plaque with scaly, annular, concentric rings on the left lower abdomen. The patient had no travel history.
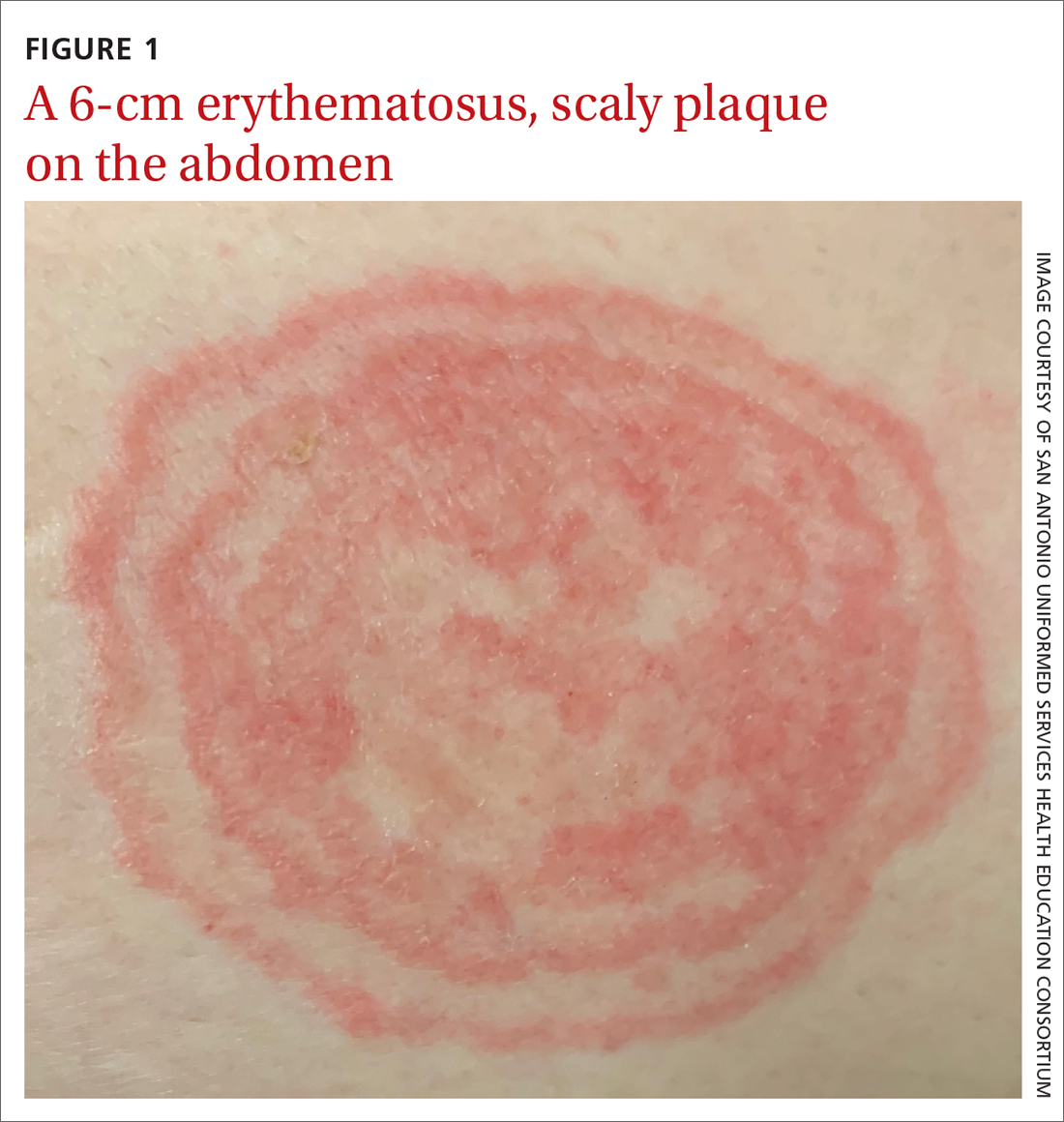
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tinea incognito
The patient was given a diagnosis of tinea incognito, a form of tinea corporis that is exacerbated by the inappropriate use of corticosteroids in the management of a cutaneous fungal infection.1 Furthermore, this patient’s case was consistent with tinea pseudoimbricata, a variant of tinea incognito. Tinea pseudoimbricata is characterized by striking concentric scaly rings that mimic tinea imbricata, a fungal infection caused by the dermatophyte Trichophyton concentricum, which is commonly found in tropical areas.2
A common infection is alteredby steroid use
Tinea corporis has a relatively high prevalence. Approximately 10% to 20% of the world population is affected by fungal skin infections.3
T rubrum is the most common cause of tinea corporis. Other causes include T tonsurans, T interdigitale, T violaceum, Microsporum canis, M gypseum, and M audouinii.
Tinea corporis can be acquired through direct contact with an infected person, animal, or fomite. It may also be acquired through autoinoculation from another area of the body containing a dermatophyte fungal infection. Tinea corporis lesions are usually pruritic, erythematous, annular plaques with overlying scale and central clearing.
How steroid use can change the picture. Treatment with corticosteroids is ineffective for fungal skin infections and causes immunosuppression, allowing the fungus to thrive. This patient had been treated with a topical steroid (clobetasol) for the abdominal lesion caused by tinea corporis, as well as an oral steroid (prednisone) for an asthma exacerbation. These steroid treatments caused the abdominal lesion to morph from the typical appearance of tinea corporis—classically an annular erythematous plaque with overlying scale and central clearing—to an erythematous plaque with striking concentric scaly rings.
Continue to: Clinical exam can provide clues; KOH examination can reveal the Dx
Clinical exam can provide clues; KOH examination can reveal the Dx
The differential diagnosis for an annular skin lesion includes not only tinea corporis, but also superficial erythema annulare centrifugum, pityriasis rosea, granuloma annulare, subacute cutaneous lupus erythematosus (SCLE), and nummular eczema.
Superficial erythema annulare centrifugum, like tinea corporis, has scale. But the location of the scale sets the 2 apart. Superficial erythema annulare centrifugum lesions have a central trailing scale, whereas tinea corporis lesions have a peripheral leading scale.4
Pityriasis rosea forms multiple lesions in a “Christmas tree” pattern on the trunk, sometimes beginning with a single herald patch. Our patient’s single lesion with concentric scaly rings was inconsistent with the distribution and quality of the lesions in pityriasis rosea.4
Granuloma annulare lesions are smooth, nonscaly plaques that are most often seen on the dorsal hands and feet. The scaly manifestation of our patient’s lesion was not consistent with this diagnosis.4
SCLE lesions are typically photodistributed on sun-exposed skin (eg, the neck, upper trunk, or arms), whereas our patient’s lesion involved a sun-protected site.4
Continue to: Nummular eczema
Nummular eczema can be differentiated from tinea corporis by potassium hydroxide (KOH) examination. Nummular eczema is characterized by a negative KOH exam and response to topical corticosteroids.4
Performing a KOH examination, using the skin scrapings from the active border of a plaque, is useful on any lesion with potential fungal etiology. If the cause is indeed a dermatophyte infection, segmented fungal hyphae will be seen under light microscopy (FIGURE 2).1 If a KOH examination is not feasible, a skin scraping can be performed with a surgical scalpel blade and collected in a sterile urine cup for stain and culture at a qualified laboratory.
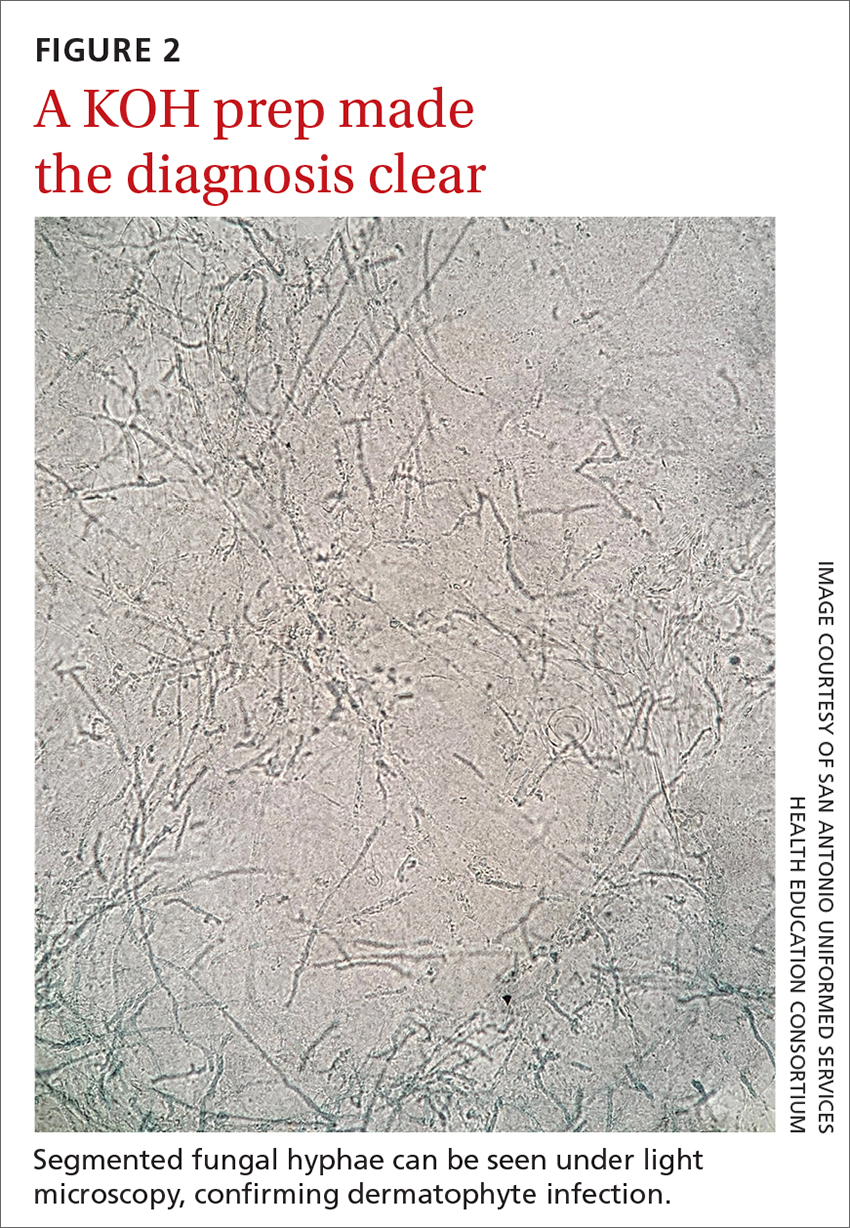
Topical and oral antifungal medications combat dermatophyte fungi
Treatments for cutaneous infections caused by dermatophyte fungi, such as tinea corporis, include topical and oral antifungals. The choice of agent depends on the extent of the disease.
Limited, localized disease can be treated topically with allylamines (terbinafine, naftifine) or imidazoles (clotrimazole). Other topical agents, such as butenafine, ciclopirox, and tolnaftate, also may be used.
Extensive disease, or tinea infection of vellus hairs, may require treatment with oral antifungal medications, such as the azoles (itraconazole, fluconazole), allylamines (terbinafine), or griseofulvin. Systemic therapy with oral antifungals has been associated with liver damage; therefore, oral therapy should not be used in patients with liver disease and liver enzymes should be monitored when appropriate.5 Nystatin is not effective in treating dermatophyte fungal infections.1
One complication of the inappropriate use of steroids on a dermatophyte infection is an increased risk of the fungus extending from the superficial skin into the hair follicles in the dermis, resulting in a condition known as Majocchi granuloma. Follicular infection is more severe and requires oral antifungal medication, such as terbinafine, itraconazole, fluconazole, or griseofulvin.1
Our patient was treated with terbinafine 250 mg/d for 4 weeks, due to the possibility of follicular infection. After the completion of 4 weeks of therapy, the patient’s cutaneous symptoms had resolved.
1. Goldstein AO, Goldstein BG. Dermatophyte (tinea) infections. In: Ofori AO, ed. UpToDate. 2022. Updated November 8, 2022. Accessed November 23, 2022. www.uptodate.com/contents/dermatophyte-tinea-infections
2. Lederman E, Craft N, Burgin S. Tinea imbricata in adult. VisualDx. Updated September 24, 2018. Accessed November 23, 2022. www.visualdx.com/visualdx/diagnosis/?moduleId=101&diagnosisId=52399
3. El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014:CD009992. doi: 10.1002/14651858.CD009992.pub2
4. Unwala R. Approach to the patient with annular skin lesions. In: Ofori AO, ed. UpToDate. 2022. Updated September 7, 2022. Accessed November 23, 2022. www.uptodate.com/contents/approach-to-the-patient-with-annular-skin-lesions
5. Wong V, High W, Burgin S. Tinea corporis in adult. VisualDx. Updated March 24, 2019. Accessed November 23, 2022. www.visualdx.com/visualdx/diagnosis/?moduleId=101&diagnosisId=52396#Therapy
A 48-YEAR-OLD WOMAN presented to Dermatology for evaluation of a 6-cm abdominal lesion that had been present for 5 weeks (FIGURE 1). The lesion was originally about the size of a quarter, but it started to enlarge after treatment of an asthma exacerbation with a 4-day course of prednisone. It continued to grow after another physician, likely presuming the lesion was a corticosteroid-responsive dermatosis (eg, nummular eczema, granuloma annulare, or erythema annulare centrifugum), prescribed a 2-week trial of clobetasol ointment. Physical examination revealed a mildly pruritic, 6-cm erythematous plaque with scaly, annular, concentric rings on the left lower abdomen. The patient had no travel history.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tinea incognito
The patient was given a diagnosis of tinea incognito, a form of tinea corporis that is exacerbated by the inappropriate use of corticosteroids in the management of a cutaneous fungal infection.1 Furthermore, this patient’s case was consistent with tinea pseudoimbricata, a variant of tinea incognito. Tinea pseudoimbricata is characterized by striking concentric scaly rings that mimic tinea imbricata, a fungal infection caused by the dermatophyte Trichophyton concentricum, which is commonly found in tropical areas.2
A common infection is alteredby steroid use
Tinea corporis has a relatively high prevalence. Approximately 10% to 20% of the world population is affected by fungal skin infections.3
T rubrum is the most common cause of tinea corporis. Other causes include T tonsurans, T interdigitale, T violaceum, Microsporum canis, M gypseum, and M audouinii.
Tinea corporis can be acquired through direct contact with an infected person, animal, or fomite. It may also be acquired through autoinoculation from another area of the body containing a dermatophyte fungal infection. Tinea corporis lesions are usually pruritic, erythematous, annular plaques with overlying scale and central clearing.
How steroid use can change the picture. Treatment with corticosteroids is ineffective for fungal skin infections and causes immunosuppression, allowing the fungus to thrive. This patient had been treated with a topical steroid (clobetasol) for the abdominal lesion caused by tinea corporis, as well as an oral steroid (prednisone) for an asthma exacerbation. These steroid treatments caused the abdominal lesion to morph from the typical appearance of tinea corporis—classically an annular erythematous plaque with overlying scale and central clearing—to an erythematous plaque with striking concentric scaly rings.
Continue to: Clinical exam can provide clues; KOH examination can reveal the Dx
Clinical exam can provide clues; KOH examination can reveal the Dx
The differential diagnosis for an annular skin lesion includes not only tinea corporis, but also superficial erythema annulare centrifugum, pityriasis rosea, granuloma annulare, subacute cutaneous lupus erythematosus (SCLE), and nummular eczema.
Superficial erythema annulare centrifugum, like tinea corporis, has scale. But the location of the scale sets the 2 apart. Superficial erythema annulare centrifugum lesions have a central trailing scale, whereas tinea corporis lesions have a peripheral leading scale.4
Pityriasis rosea forms multiple lesions in a “Christmas tree” pattern on the trunk, sometimes beginning with a single herald patch. Our patient’s single lesion with concentric scaly rings was inconsistent with the distribution and quality of the lesions in pityriasis rosea.4
Granuloma annulare lesions are smooth, nonscaly plaques that are most often seen on the dorsal hands and feet. The scaly manifestation of our patient’s lesion was not consistent with this diagnosis.4
SCLE lesions are typically photodistributed on sun-exposed skin (eg, the neck, upper trunk, or arms), whereas our patient’s lesion involved a sun-protected site.4
Continue to: Nummular eczema
Nummular eczema can be differentiated from tinea corporis by potassium hydroxide (KOH) examination. Nummular eczema is characterized by a negative KOH exam and response to topical corticosteroids.4
Performing a KOH examination, using the skin scrapings from the active border of a plaque, is useful on any lesion with potential fungal etiology. If the cause is indeed a dermatophyte infection, segmented fungal hyphae will be seen under light microscopy (FIGURE 2).1 If a KOH examination is not feasible, a skin scraping can be performed with a surgical scalpel blade and collected in a sterile urine cup for stain and culture at a qualified laboratory.

Topical and oral antifungal medications combat dermatophyte fungi
Treatments for cutaneous infections caused by dermatophyte fungi, such as tinea corporis, include topical and oral antifungals. The choice of agent depends on the extent of the disease.
Limited, localized disease can be treated topically with allylamines (terbinafine, naftifine) or imidazoles (clotrimazole). Other topical agents, such as butenafine, ciclopirox, and tolnaftate, also may be used.
Extensive disease, or tinea infection of vellus hairs, may require treatment with oral antifungal medications, such as the azoles (itraconazole, fluconazole), allylamines (terbinafine), or griseofulvin. Systemic therapy with oral antifungals has been associated with liver damage; therefore, oral therapy should not be used in patients with liver disease and liver enzymes should be monitored when appropriate.5 Nystatin is not effective in treating dermatophyte fungal infections.1
One complication of the inappropriate use of steroids on a dermatophyte infection is an increased risk of the fungus extending from the superficial skin into the hair follicles in the dermis, resulting in a condition known as Majocchi granuloma. Follicular infection is more severe and requires oral antifungal medication, such as terbinafine, itraconazole, fluconazole, or griseofulvin.1
Our patient was treated with terbinafine 250 mg/d for 4 weeks, due to the possibility of follicular infection. After the completion of 4 weeks of therapy, the patient’s cutaneous symptoms had resolved.
A 48-YEAR-OLD WOMAN presented to Dermatology for evaluation of a 6-cm abdominal lesion that had been present for 5 weeks (FIGURE 1). The lesion was originally about the size of a quarter, but it started to enlarge after treatment of an asthma exacerbation with a 4-day course of prednisone. It continued to grow after another physician, likely presuming the lesion was a corticosteroid-responsive dermatosis (eg, nummular eczema, granuloma annulare, or erythema annulare centrifugum), prescribed a 2-week trial of clobetasol ointment. Physical examination revealed a mildly pruritic, 6-cm erythematous plaque with scaly, annular, concentric rings on the left lower abdomen. The patient had no travel history.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tinea incognito
The patient was given a diagnosis of tinea incognito, a form of tinea corporis that is exacerbated by the inappropriate use of corticosteroids in the management of a cutaneous fungal infection.1 Furthermore, this patient’s case was consistent with tinea pseudoimbricata, a variant of tinea incognito. Tinea pseudoimbricata is characterized by striking concentric scaly rings that mimic tinea imbricata, a fungal infection caused by the dermatophyte Trichophyton concentricum, which is commonly found in tropical areas.2
A common infection is alteredby steroid use
Tinea corporis has a relatively high prevalence. Approximately 10% to 20% of the world population is affected by fungal skin infections.3
T rubrum is the most common cause of tinea corporis. Other causes include T tonsurans, T interdigitale, T violaceum, Microsporum canis, M gypseum, and M audouinii.
Tinea corporis can be acquired through direct contact with an infected person, animal, or fomite. It may also be acquired through autoinoculation from another area of the body containing a dermatophyte fungal infection. Tinea corporis lesions are usually pruritic, erythematous, annular plaques with overlying scale and central clearing.
How steroid use can change the picture. Treatment with corticosteroids is ineffective for fungal skin infections and causes immunosuppression, allowing the fungus to thrive. This patient had been treated with a topical steroid (clobetasol) for the abdominal lesion caused by tinea corporis, as well as an oral steroid (prednisone) for an asthma exacerbation. These steroid treatments caused the abdominal lesion to morph from the typical appearance of tinea corporis—classically an annular erythematous plaque with overlying scale and central clearing—to an erythematous plaque with striking concentric scaly rings.
Continue to: Clinical exam can provide clues; KOH examination can reveal the Dx
Clinical exam can provide clues; KOH examination can reveal the Dx
The differential diagnosis for an annular skin lesion includes not only tinea corporis, but also superficial erythema annulare centrifugum, pityriasis rosea, granuloma annulare, subacute cutaneous lupus erythematosus (SCLE), and nummular eczema.
Superficial erythema annulare centrifugum, like tinea corporis, has scale. But the location of the scale sets the 2 apart. Superficial erythema annulare centrifugum lesions have a central trailing scale, whereas tinea corporis lesions have a peripheral leading scale.4
Pityriasis rosea forms multiple lesions in a “Christmas tree” pattern on the trunk, sometimes beginning with a single herald patch. Our patient’s single lesion with concentric scaly rings was inconsistent with the distribution and quality of the lesions in pityriasis rosea.4
Granuloma annulare lesions are smooth, nonscaly plaques that are most often seen on the dorsal hands and feet. The scaly manifestation of our patient’s lesion was not consistent with this diagnosis.4
SCLE lesions are typically photodistributed on sun-exposed skin (eg, the neck, upper trunk, or arms), whereas our patient’s lesion involved a sun-protected site.4
Continue to: Nummular eczema
Nummular eczema can be differentiated from tinea corporis by potassium hydroxide (KOH) examination. Nummular eczema is characterized by a negative KOH exam and response to topical corticosteroids.4
Performing a KOH examination, using the skin scrapings from the active border of a plaque, is useful on any lesion with potential fungal etiology. If the cause is indeed a dermatophyte infection, segmented fungal hyphae will be seen under light microscopy (FIGURE 2).1 If a KOH examination is not feasible, a skin scraping can be performed with a surgical scalpel blade and collected in a sterile urine cup for stain and culture at a qualified laboratory.

Topical and oral antifungal medications combat dermatophyte fungi
Treatments for cutaneous infections caused by dermatophyte fungi, such as tinea corporis, include topical and oral antifungals. The choice of agent depends on the extent of the disease.
Limited, localized disease can be treated topically with allylamines (terbinafine, naftifine) or imidazoles (clotrimazole). Other topical agents, such as butenafine, ciclopirox, and tolnaftate, also may be used.
Extensive disease, or tinea infection of vellus hairs, may require treatment with oral antifungal medications, such as the azoles (itraconazole, fluconazole), allylamines (terbinafine), or griseofulvin. Systemic therapy with oral antifungals has been associated with liver damage; therefore, oral therapy should not be used in patients with liver disease and liver enzymes should be monitored when appropriate.5 Nystatin is not effective in treating dermatophyte fungal infections.1
One complication of the inappropriate use of steroids on a dermatophyte infection is an increased risk of the fungus extending from the superficial skin into the hair follicles in the dermis, resulting in a condition known as Majocchi granuloma. Follicular infection is more severe and requires oral antifungal medication, such as terbinafine, itraconazole, fluconazole, or griseofulvin.1
Our patient was treated with terbinafine 250 mg/d for 4 weeks, due to the possibility of follicular infection. After the completion of 4 weeks of therapy, the patient’s cutaneous symptoms had resolved.
1. Goldstein AO, Goldstein BG. Dermatophyte (tinea) infections. In: Ofori AO, ed. UpToDate. 2022. Updated November 8, 2022. Accessed November 23, 2022. www.uptodate.com/contents/dermatophyte-tinea-infections
2. Lederman E, Craft N, Burgin S. Tinea imbricata in adult. VisualDx. Updated September 24, 2018. Accessed November 23, 2022. www.visualdx.com/visualdx/diagnosis/?moduleId=101&diagnosisId=52399
3. El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014:CD009992. doi: 10.1002/14651858.CD009992.pub2
4. Unwala R. Approach to the patient with annular skin lesions. In: Ofori AO, ed. UpToDate. 2022. Updated September 7, 2022. Accessed November 23, 2022. www.uptodate.com/contents/approach-to-the-patient-with-annular-skin-lesions
5. Wong V, High W, Burgin S. Tinea corporis in adult. VisualDx. Updated March 24, 2019. Accessed November 23, 2022. www.visualdx.com/visualdx/diagnosis/?moduleId=101&diagnosisId=52396#Therapy
1. Goldstein AO, Goldstein BG. Dermatophyte (tinea) infections. In: Ofori AO, ed. UpToDate. 2022. Updated November 8, 2022. Accessed November 23, 2022. www.uptodate.com/contents/dermatophyte-tinea-infections
2. Lederman E, Craft N, Burgin S. Tinea imbricata in adult. VisualDx. Updated September 24, 2018. Accessed November 23, 2022. www.visualdx.com/visualdx/diagnosis/?moduleId=101&diagnosisId=52399
3. El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014:CD009992. doi: 10.1002/14651858.CD009992.pub2
4. Unwala R. Approach to the patient with annular skin lesions. In: Ofori AO, ed. UpToDate. 2022. Updated September 7, 2022. Accessed November 23, 2022. www.uptodate.com/contents/approach-to-the-patient-with-annular-skin-lesions
5. Wong V, High W, Burgin S. Tinea corporis in adult. VisualDx. Updated March 24, 2019. Accessed November 23, 2022. www.visualdx.com/visualdx/diagnosis/?moduleId=101&diagnosisId=52396#Therapy
The family physician’s role in long COVID management
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
Several years into the pandemic, COVID-19 continues to deeply impact our society; at the time of publication of this review, 98.8 million cases in the United States have been reported to the Centers for Disease Control and Prevention (CDC).1 Although many people recover well from infection, there is mounting concern regarding long-term sequelae of COVID-19. These long-term symptoms have been termed long COVID, among other names.
What exactly is long COVID?
The CDC and National Institutes of Health define long COVID as new or ongoing health problems experienced ≥ 4 weeks after initial infection.2 Evidence suggests that even people who have mild initial COVID-19 symptoms are at risk for long COVID.
Available data about long COVID are imperfect, however; much about the condition remains poorly understood. For example, there is little evidence regarding the effect of vaccination and viral variants on the prevalence of long COVID. A recent study of more than 13 million people from the US Department of Veterans Affairs database did demonstrate that vaccination against SARS-CoV-2 lowered the risk for long COVID by only about 15%.3
Persistent symptoms associated with long COVID often lead to disability and decreased quality of life. Furthermore, long COVID is a challenge to treat because there is a paucity of evidence to guide COVID-19 treatment beyond initial infection.
Because many patients who have ongoing COVID-19 symptoms will be seen in primary care, it is important to understand how to manage and support them. In this article, we discuss current understanding of long COVID epidemiology, symptoms that can persist 4 weeks after initial infection, and potential treatment options.
Prevalence and diagnosis
The prevalence of long COVID is not well defined because many epidemiologic studies rely on self-reporting. The CDC reports that 20% to 25% of COVID-19 survivors experience a new condition that might be attributable to their initial infection.4 Other studies variously cite 5% to 85% of people who have had a diagnosis of COVID-19 as experiencing long COVID, although that rate more consistently appears to be 10% to 30%.5

A study of adult patients in France found that self-reported symptoms of long COVID, 10 to 12 months after the first wave of the pandemic (May through November 2020), were associated with the belief of having had COVID-19 but not necessarily with having tested positive for anti-SARS-CoV-2 antibodies,6 which indicates prior COVID-19. This complicates research on long COVID because, first, there is no specific test to confirm a diagnosis of long COVID and, second, studies often rely on self-reporting of earlier COVID-19.
Continue to: As such, long COVID...
As such, long COVID is diagnosed primarily through a medical history and physical examination. The medical history provides a guide as to whether additional testing is warranted to evaluate for known complications of COVID-19, such as deep vein thrombosis, pulmonary embolism, myocarditis, and pulmonary fibrosis. As of October 1, 2021, a new International Classification of Disease (10th Revision) code went into effect for post COVID condition, unspecified (U09.9).7
The prevalence of long COVID symptoms appears to increase with age. Among patients whose disease was diagnosed using code U09.9, most were 36 to 64 years of age; children and adults ages 22 years or younger constituted only 10.5% of diagnoses.7 Long COVID symptoms might also be more prevalent among women and in people with a preexisting chronic comorbidity.2,7
Symptoms can be numerous, severe or mild, and lasting
Initially, there was no widely accepted definition of long COVID; follow-up in early studies ranged from 21 days to 2 years after initial infection (or from discharge, for hospitalized patients).8 Differences in descriptions that have been used on surveys to self-report symptoms make it a challenge to clearly summarize the frequency of each aspect of long COVID.
Long COVID can be mild or debilitating; severity can fluctuate. Common symptoms include fatigue, dyspnea or other breathing difficulties, headache, and cognitive dysfunction, but as many as 203 lasting symptoms have been reported.2,8-12 From October 1, 2021, through January 31, 2022, the most common accompanying manifestations of long COVID were difficulty breathing, cough, and fatigue.7 Long COVID can affect multiple organ systems,13,14 with symptoms varying by organ system affected. Regardless of the need for hospitalization initially, having had COVID-19 significantly increases the risk for subsequent death at 30 days and at 6 months after initial infection.15
Symptoms of long COVID have been reported as long as 2 years after initial infection.8 When Davis and colleagues studied the onset and progression of reported symptoms of long COVID,9 they determined that, among patients who reported recovery from COVID-19 in < 90 days, symptoms peaked at approximately Week 2 of infection. In comparison, patients who reported not having recovered in < 90 days had (1) symptoms that peaked later (2 months) and (2) on average, more symptoms (mean, 17 reported symptoms, compared to 11 in recovered patients).9
Continue to: Fatigue
Fatigue, including postexertion malaise and impaired daily function and mobility, is the most common symptom of long COVID,8-10,14 reported in 28% to 98%14 of patients after initial COVID-19. This fatigue is more than simply being tired: Patients describe profound exhaustion, in which fatigue is out of proportion to exertion. Fatigue and myalgia are commonly reported among patients with impaired hepatic and pulmonary function as a consequence of long COVID.13 Patients often report that even minor activities result in decreased attention, focus, and energy, for many hours or days afterward. Fatigue has been reported to persist from 2.5 months to as long as 6 months after initial infection or hospitalization.9,16
Postviral fatigue has been seen in other viral outbreaks and seems to share characteristics with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, which itself has historically been stigmatized and poorly understood.17 Long COVID fatigue might be more common among women and patients who have an existing diagnosis of depression and antidepressant use,10,11,16,18 although the mechanism of this relationship is unclear. Potential mechanisms include damage from systemic inflammation to metabolism in the frontal lobe and cerebellum19 and direct infection by SARS-CoV-2 in skeletal muscle.20 Townsend and colleagues16 found no relationship between long COVID fatigue and markers of inflammation (leukocyte, neutrophil, and lymphocyte counts; the neutrophil-to-lymphocyte ratio; lactate dehydrogenase; C-reactive protein; serum interleukin-6; and soluble CD25).
Neuropsychiatric symptoms are also common in long COVID and can have a significant impact on patients’ quality of life. Studies have reported poor sleep quality or insomnia (38% to 90%), headache (17% to 91.2%), speech and language problems (48% to 50%), confusion (20%), dementia (28.6%), difficulty concentrating (1.9% to 27%), and memory loss or cognitive impairment (5.4% to 73%).9,10,14,15 For some patients, these symptoms persisted for ≥ 6 months, making it difficult for those affected to return to work.9
Isolation and loneliness, a common situation for patients with COVID-19, can have long-term effects on mental health.21 The COVID-19 pandemic itself has had a negative effect on behavioral health, including depression (4.3% to 25% of patients), anxiety (1.9% to 46%), obsessive compulsive disorder (4.9% to 20%), and posttraumatic stress disorder (29%).22 The persistence of symptoms of long COVID has resulted in a great deal of frustration, fear, and confusion for those affected—some of whom report a loss of trust in their community health care providers to address their ongoing struggles.23 Such loss can be accompanied by a reported increase in feelings of anxiety and changes to perceptions of self (ie, “how I used to be” in contrast to “how I am now”).23 These neuropsychiatric symptoms, including mental health conditions, appear to be more common among older adults.4
Other neurologic deficits found in long COVID include olfactory disorders (9% to 27% of patients), altered taste (5% to 18%), numbness or tingling sensations (6%), blurred vision (17.1%), and tinnitus (16.%).14 Dizziness (2.6% to 6%) and lightheadedness or presyncope (7%) have also been reported, although these symptoms appear to be less common than other neurocognitive effects.14
Continue to: The mechanism of action...
The mechanism of action of damage to the nervous system in long COVID is likely multifactorial. COVID-19 can directly infect the central nervous system through a hematogenous route, which can result in direct cytolytic damage to neurons. Infection can also affect the blood–brain barrier.24 Additionally, COVID-19 can invade the central nervous system through peripheral nerves, including the olfactory and vagus nerves.25 Many human respiratory viruses, including SARS-CoV-2, result in an increase in pro-inflammatory and anti-inflammatory cytokines; this so-called cytokine storm is an exaggerated response to infection and can trigger neurodegenerative and psychiatric syndromes.26 It is unclear whether the cytokine storm is different for people with COVID-19, compared to other respiratory viruses.
Respiratory symptoms are very common after COVID-1915: In studies, as many as 87.1% of patients continued to have shortness of breath ≥ 140 days after initial symptom onset, including breathlessness (48% to 60%), wheezing (5.3%), cough (10.5% to 46%), and congestion (32%),14,18 any of which can persist for as long as 6 months.9 Among a sample of previously hospitalized COVID-19 patients in Wuhan, China, 22% to 56% displayed a pulmonary diffusion abnormality 6 months later, with those who required supplemental oxygen during initial COVID-19 having a greater risk for these abnormalities at follow-up, compared to those who did not require supplemental oxygen (odds ratio = 2.42; 95% CI, 1.15-5.08).11
Cardiovascular symptoms. New-onset autonomic dysfunction has been described in multiple case reports and in some larger cohort studies of patients post COVID-19.27 Many common long COVID symptoms, including fatigue and orthostatic intolerance, are commonly seen in postural orthostatic tachycardia syndrome. Emerging evidence indicates that there are likely similar underlying mechanisms and a significant amount of overlap between long COVID and postural orthostatic tachycardia syndrome.27
A study of patients within the US Department of Veterans Affairs population found that, regardless of disease severity, patients who had a positive COVID-19 test had a higher rate of cardiac disease 30 days after diagnosis,28 including stroke, transient ischemic attack, dysrhythmia, inflammatory heart disease, acute coronary disease, myocardial infarction, ischemic cardiopathy, angina, heart failure, nonischemic cardiomyopathy, and cardiac arrest. Patients with COVID-19 were at increased risk for major adverse cardiovascular events (myocardial infarction, stroke, and all-cause mortality).28 Demographics of the VA population (ie, most are White men) might limit the generalizability of these data, but similar findings have been found elsewhere.5,10,15Given that, in general, chest pain is common after the acute phase of an infection and the causes of chest pain are broad, the high rate of cardiac complications post COVID-19 nevertheless highlights the importance of a thorough evaluation and work-up of chest pain in patients who have had COVID-19.
Other symptoms. Body aches and generalized joint pain are another common symptom group of long COVID.9 These include body aches (20%), joint pain (78%), and muscle aches (87.7%).14,18
Continue to: Commonly reported...
Commonly reported gastrointestinal symptoms include diarrhea, loss of appetite, nausea, and abdominal pain.9,15
Other symptoms reported less commonly include dermatologic conditions, such as pruritus and rash; reproductive and endocrine symptoms, including extreme thirst, irregular menstruation, and sexual dysfunction; and new or exacerbated allergic response.9
Does severity of initial disease play a role?
Keep in mind that long COVID is not specific to patients who were hospitalized or had severe initial infection. In fact, 75% of patients who have a diagnosis of a post–COVID-19 condition were not hospitalized for their initial infection.7 However, the severity of initial COVID-19 infection might contribute to the presence or severity of long COVID symptoms2—although findings in current literature are mixed. For example:
- In reporting from Wuhan, China, higher position on a disease severity scale during a hospital stay for COVID-19 was associated with:
- greater likelihood of reporting ≥ 1 symptoms at a 6-month follow-up
- increased risk for pulmonary diffusion abnormalities, fatigue, and mood disorders.11
- After 2 years’ follow-up of the same cohort, 55% of patients continued to report ≥ 1 symptoms of long COVID, and those who had been hospitalized with COVID-19 continued to report reduced health-related quality of life, compared to the control group.8
- Similarly, patients initially hospitalized with COVID-19 were more likely to experience impairment of ≥ 2 organs—in particular, the liver and pancreas—compared to nonhospitalized patients after a median 5 months post initial infection, among a sample in the United Kingdom.13
- In an international cohort, patients who reported a greater number of symptoms during initial COVID-19 were more likely to experience long COVID.12
- Last, long COVID fatigue did not vary by severity of initial COVID-19 infection among a sample of hospitalized and nonhospitalized participants in Dublin, Ireland.16
No specific treatments yet available
There are no specific treatments for long COVID; overall, the emphasis is on providing supportive care and managing preexisting chronic conditions.5 This is where expertise in primary care, relationships with patients and the community, and psychosocial knowledge can help patients recover from ongoing COVID-19 symptoms.
Clinicians should continue to perform a thorough physical assessment of patients with previous or ongoing COVID-19 to identify and monitor new or recurring symptoms after hospital discharge or initial resolution of symptoms.29 This approach includes developing an individualized plan for care and rehabilitation that is specific to presenting symptoms, including psychological support. We encourage family physicians to familiarize themselves with the work of Vance and colleagues,30 who have created a comprehensive tablea to guide treatment and referral for the gamut of long COVID symptoms, including cardiovascular issues (eg, palpitations, edema), chronic cough, headache, pain, and insomnia.
Continue to: This new clinical entity is a formidable challenge
This new clinical entity is a formidable challenge
Long COVID is a new condition that requires comprehensive evaluation to understand the full, often long-term, effects of COVID-19. Our review of this condition substantiated that symptoms of long COVID often affect a variety of organs13,14 and have been observed to persist for ≥ 2 years.8
Some studies that have examined the long-term effects of COVID-19 included only participants who were not hospitalized; others include hospitalized patients exclusively. The literature is mixed in regard to including severity of initial infection as it relates to long COVID. Available research demonstrates that it is common for people with COVID-19 to experience persistent symptoms that can significantly impact daily life and well-being.
Likely, it will be several years before we even begin to understand the full extent of COVID-19. Until research elucidates the relationship between the disease and short- and long-term health outcomes, clinicians should:
- acknowledge and address the reality of long COVID when meeting with persistently symptomatic patients,
- provide support, therapeutic listening, and referral to rehabilitation as appropriate, and
- offer information on the potential for long-term effects of COVID-19 to vaccine-hesitant patients.
a “Systems, symptoms, and treatments for post-COVID patients,” pages 1231-1234 in the source article (www.jabfm.org/content/jabfp/34/6/1229.full.pdf).30
CORRESPONDENCE
Nicole Mayo, PhD, 46 Prince Street, Rochester, NY 14607; [email protected]
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
1. Centers for Disease Control and Prevention. COVID data tracker. December 6, 2022. Accessed December 7, 2022. https://covid.cdc.gov/covid-data-tracker
2. Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated September 1, 2021. Accessed November 17, 2022. www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461-1467. doi: 10.1038/s41591-022-01840-0
4. Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. doi: 10.15585/mmwr.mm7121e1
5. Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026
6. Matta J, Wiernik E, Robineau O, et al; . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19-25. doi: 10.1001/jamainternmed.2021.6454
7. FAIR Health. Patients diagnosed with post-COVID conditions: an analysis of private healthcare claims using the official ICD-10 diagnostic code. May 18, 2022. Accessed October 15, 2022. https://s3.amazonaws.com/media2.fairhealth.org/whitepaper/asset/Patients%20Diagnosed%20with%20Post-COVID%20Con ditions%20-%20A%20FAIR%20Health%20White%20Paper.pdf
8. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10:863-876. doi: 10.1016/S2213-2600(22)00126-6
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019
10. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8
11. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. doi: 10.1016/S0140-6736(20)32656-8
12. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. doi: 10.1038/s41591-021-01292-y
13. Dennis A, Wamil M, Alberts J, et al; . Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391
14. Crook H, Raza S, Nowell J, et al.. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648
15. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi: 10.1038/s41586-021-03553-9
16. Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784
17. Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—a systematic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas). 2021;57:418. doi: 10.3390/ medicina57050418
18. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113-119. doi: 10.1007/s00408-021-00423-z
19. Guedj E, Million M, Dudouet P, et al. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Euro J Nucl Med Mol Imaging. 2021;48:592-595. doi: 10.1007/s00259-020-04973-x
20. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985). 2020;129:864-867. doi: 10.1152/japplphysiol.00321.2020
21. Leigh-Hunt N, Bagguley D, Bash K, et al. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public health. 2017;152:157-171.
22. Kathirvel N. Post COVID-19 pandemic mental health challenges. Asian J Psychiatr. 2020;53:102430. doi: 10.1016/j.ajp.2020.102430
23. Macpherson K, Cooper K, Harbour J, et al. Experiences of living with long COVID and of accessing healthcare services: a qualitative systematic review. BMJ Open. 2022;12:e050979. doi: 10.1136/bmjopen-2021-050979
24. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neuro Sci. 2020;41:2657-2669. doi: 10.1007/s10072-020-04575-3
25. Gialluisi A, de Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41:1349-1350. doi: 10.1007/s10072-020-04444-z
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39. doi: 10.1016/j.bbi.2020.04.027
27. Bisaccia G, Ricci F, Recce V, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156
28. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi: 10.1038/s41591-022-01689-3
29. Gorna R, MacDermott N, Rayner C, et al. Long COVID guidelines need to reflect lived experience. Lancet. 2021;397:455-457. doi: 10.1016/S0140-6736(20)32705-7
30. Vance H, Maslach A, Stoneman E, et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34:1229-1242. doi: 10.3122/jabfm.2021.06.210254
PRACTICE RECOMMENDATIONS
› Acknowledge and address the persistence of COVID-19 symptoms when meeting with patients. C
› Continue to monitor persistent, fluctuating symptoms of COVID-19 well after hospital discharge or apparent resolution of initial symptoms. C
› Provide psychological support and resources for mental health care to patients regarding their ongoing fears and frustrations with persistent COVID-19 symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Incorporating medication abortion into your ObGyn practice: Why and how
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.
Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
Knee lesion that bleeds
This combination of vascular features with excess keratin fit perfectly with the name of the diagnosis: angiokeratoma. The dark color of the lesion on magnification, or in this case with dermoscopy, showed the lacunar pattern of dilated vessels. The overlying keratin was likely accentuated because it was on an extensor surface; the rim of hyperpigmentation is common for these lesions.
Angiokeratomas result from dilation of the blood vessels underneath the epidermis. There are different inciting events that lead to the 5 different types of angiokeratomas. The overlying epidermal changes are secondary to the underlying process of capillary ectasia.1 This lesion was not part of a cluster, so it was characterized as a solitary angiokeratoma. Smaller lesions are usually less keratinized and are commonly seen on the scrotum and vulva, where there are usually multiple lesions (referred to as angiokeratoma of Fordyce).
Zaballos2 studied the dermoscopic characteristics of 32 solitary angiokeratomas and reported 6 findings in at least half of the solitary lesions. The most common features were dark lacunae in 94% of the lesions, white veil in 91%, and erythema in 69%. Peripheral erythema, red lacunae, and hemorrhagic crusts were all seen at a rate of 53%. The most common location was the lower extremities.
This patient’s previous pathology report from a shave biopsy was found, confirming that the original diagnosis was angiokeratoma. Since the patient’s lesion had not resolved and was symptomatic from minor trauma, he was scheduled to come back in for an elliptical excision to remove the lesion.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282. doi: 10.1159/000246270
2. Zaballos P, Daufí C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318–325. doi:10.1001/archderm.143.3.318

This combination of vascular features with excess keratin fit perfectly with the name of the diagnosis: angiokeratoma. The dark color of the lesion on magnification, or in this case with dermoscopy, showed the lacunar pattern of dilated vessels. The overlying keratin was likely accentuated because it was on an extensor surface; the rim of hyperpigmentation is common for these lesions.
Angiokeratomas result from dilation of the blood vessels underneath the epidermis. There are different inciting events that lead to the 5 different types of angiokeratomas. The overlying epidermal changes are secondary to the underlying process of capillary ectasia.1 This lesion was not part of a cluster, so it was characterized as a solitary angiokeratoma. Smaller lesions are usually less keratinized and are commonly seen on the scrotum and vulva, where there are usually multiple lesions (referred to as angiokeratoma of Fordyce).
Zaballos2 studied the dermoscopic characteristics of 32 solitary angiokeratomas and reported 6 findings in at least half of the solitary lesions. The most common features were dark lacunae in 94% of the lesions, white veil in 91%, and erythema in 69%. Peripheral erythema, red lacunae, and hemorrhagic crusts were all seen at a rate of 53%. The most common location was the lower extremities.
This patient’s previous pathology report from a shave biopsy was found, confirming that the original diagnosis was angiokeratoma. Since the patient’s lesion had not resolved and was symptomatic from minor trauma, he was scheduled to come back in for an elliptical excision to remove the lesion.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This combination of vascular features with excess keratin fit perfectly with the name of the diagnosis: angiokeratoma. The dark color of the lesion on magnification, or in this case with dermoscopy, showed the lacunar pattern of dilated vessels. The overlying keratin was likely accentuated because it was on an extensor surface; the rim of hyperpigmentation is common for these lesions.
Angiokeratomas result from dilation of the blood vessels underneath the epidermis. There are different inciting events that lead to the 5 different types of angiokeratomas. The overlying epidermal changes are secondary to the underlying process of capillary ectasia.1 This lesion was not part of a cluster, so it was characterized as a solitary angiokeratoma. Smaller lesions are usually less keratinized and are commonly seen on the scrotum and vulva, where there are usually multiple lesions (referred to as angiokeratoma of Fordyce).
Zaballos2 studied the dermoscopic characteristics of 32 solitary angiokeratomas and reported 6 findings in at least half of the solitary lesions. The most common features were dark lacunae in 94% of the lesions, white veil in 91%, and erythema in 69%. Peripheral erythema, red lacunae, and hemorrhagic crusts were all seen at a rate of 53%. The most common location was the lower extremities.
This patient’s previous pathology report from a shave biopsy was found, confirming that the original diagnosis was angiokeratoma. Since the patient’s lesion had not resolved and was symptomatic from minor trauma, he was scheduled to come back in for an elliptical excision to remove the lesion.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282. doi: 10.1159/000246270
2. Zaballos P, Daufí C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318–325. doi:10.1001/archderm.143.3.318
1. Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282. doi: 10.1159/000246270
2. Zaballos P, Daufí C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318–325. doi:10.1001/archderm.143.3.318
Erythrasma
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.
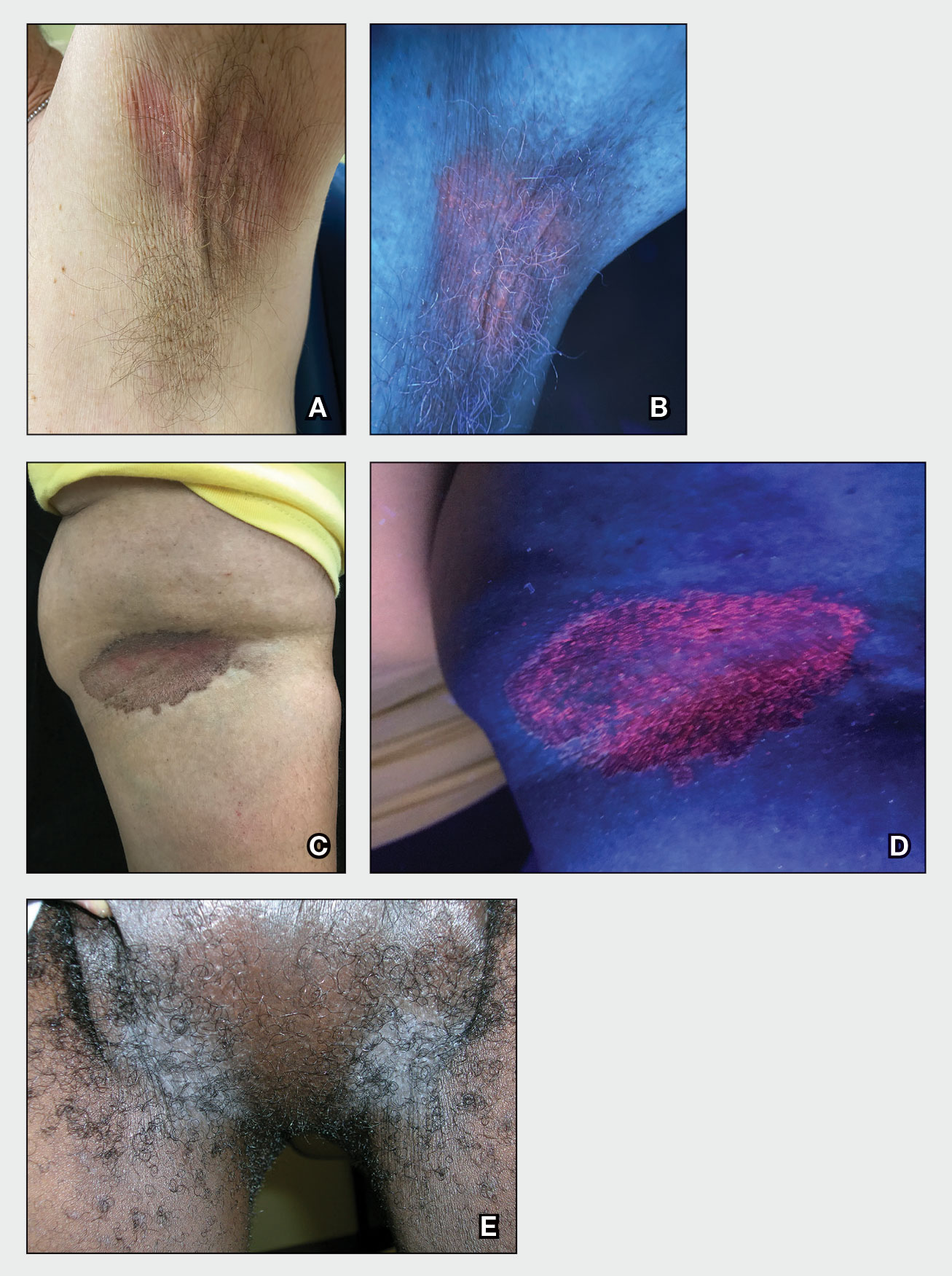
Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
Mobile Enlarging Scalp Nodule
The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6
Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9
Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7
Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15
Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
A 50-year-old man presented with a 2.5-cm, subcutaneous, freely mobile nodule on the occipital scalp that first appeared 35 years prior but recently had started enlarging. Histologically the lesion was well circumscribed. Immunohistochemical staining was positive for SRY-box transcription factor 10 in some of the spindle cells, and staining for epithelial membrane antigen was positive in a separate population of intermixed spindle cells.
Advances in Lupus From ACR 2022
Dr Anca Askanase, director of the Lupus Center at Columbia University Medical Center, highlights the latest research on systemic lupus erythematosus (SLE) and lupus nephritis from the American College of Rheumatology (ACR) 2022.
Dr Askanase first discusses a small study using autologous chimeric antigen receptor T-cell (CAR-T) therapy, which is approved for use in several blood cancers, as an alternative for patients with refractory SLE. Five patients received CAR-T cells, and all achieved sustained, drug-free remission.
Next, Dr Askanase highlights a phase 2B study evaluating the tyrosine kinase 2 inhibitor deucravacitinib in patients with SLE. In early results, patients taking deucravacitinib showed a statistically meaningful response in disease activity compared with placebo.
She then summarizes her presentation on oral cenerimod. The phase 2, 12-week study demonstrated that cenerimod reduced total lymphocyte count compared with placebo in SLE patients.
Next, Dr Askanase details the long-term extension of the TULIP trials. Researchers found that anifrolumab has a favorable benefit-risk profile when compared with placebo and is therefore a possible long-term treatment option for patients with moderate to severe SLE.
Finally, Dr Askanase discusses the positive findings from the phase 3 AURORA 1 and AURORA 2 studies, which sought to determine whether the addition of voclosporin to mycophenolate mofetil and low-dose steroids could maintain the reduction in proteinuria in patients with lupus nephritis.
--
Anca Askanase, MD, MPH, Professor of Medicine, Director, Lupus Center, Department of Rheumatology, Columbia University Medical Center, New York, New York
Anca Askanase, MD, MPH, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Received income in an amount equal to or greater than $250 from: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Dr Anca Askanase, director of the Lupus Center at Columbia University Medical Center, highlights the latest research on systemic lupus erythematosus (SLE) and lupus nephritis from the American College of Rheumatology (ACR) 2022.
Dr Askanase first discusses a small study using autologous chimeric antigen receptor T-cell (CAR-T) therapy, which is approved for use in several blood cancers, as an alternative for patients with refractory SLE. Five patients received CAR-T cells, and all achieved sustained, drug-free remission.
Next, Dr Askanase highlights a phase 2B study evaluating the tyrosine kinase 2 inhibitor deucravacitinib in patients with SLE. In early results, patients taking deucravacitinib showed a statistically meaningful response in disease activity compared with placebo.
She then summarizes her presentation on oral cenerimod. The phase 2, 12-week study demonstrated that cenerimod reduced total lymphocyte count compared with placebo in SLE patients.
Next, Dr Askanase details the long-term extension of the TULIP trials. Researchers found that anifrolumab has a favorable benefit-risk profile when compared with placebo and is therefore a possible long-term treatment option for patients with moderate to severe SLE.
Finally, Dr Askanase discusses the positive findings from the phase 3 AURORA 1 and AURORA 2 studies, which sought to determine whether the addition of voclosporin to mycophenolate mofetil and low-dose steroids could maintain the reduction in proteinuria in patients with lupus nephritis.
--
Anca Askanase, MD, MPH, Professor of Medicine, Director, Lupus Center, Department of Rheumatology, Columbia University Medical Center, New York, New York
Anca Askanase, MD, MPH, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Received income in an amount equal to or greater than $250 from: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Dr Anca Askanase, director of the Lupus Center at Columbia University Medical Center, highlights the latest research on systemic lupus erythematosus (SLE) and lupus nephritis from the American College of Rheumatology (ACR) 2022.
Dr Askanase first discusses a small study using autologous chimeric antigen receptor T-cell (CAR-T) therapy, which is approved for use in several blood cancers, as an alternative for patients with refractory SLE. Five patients received CAR-T cells, and all achieved sustained, drug-free remission.
Next, Dr Askanase highlights a phase 2B study evaluating the tyrosine kinase 2 inhibitor deucravacitinib in patients with SLE. In early results, patients taking deucravacitinib showed a statistically meaningful response in disease activity compared with placebo.
She then summarizes her presentation on oral cenerimod. The phase 2, 12-week study demonstrated that cenerimod reduced total lymphocyte count compared with placebo in SLE patients.
Next, Dr Askanase details the long-term extension of the TULIP trials. Researchers found that anifrolumab has a favorable benefit-risk profile when compared with placebo and is therefore a possible long-term treatment option for patients with moderate to severe SLE.
Finally, Dr Askanase discusses the positive findings from the phase 3 AURORA 1 and AURORA 2 studies, which sought to determine whether the addition of voclosporin to mycophenolate mofetil and low-dose steroids could maintain the reduction in proteinuria in patients with lupus nephritis.
--
Anca Askanase, MD, MPH, Professor of Medicine, Director, Lupus Center, Department of Rheumatology, Columbia University Medical Center, New York, New York
Anca Askanase, MD, MPH, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Received income in an amount equal to or greater than $250 from: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer

Janus Kinase Inhibitors in the Treatment of Atopic Dermatitis: Military Considerations
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
Practice Points
- Oral Janus kinase (JAK) inhibitors are novel therapies available for the treatment of atopic dermatitis (AD), with multiple recently approved agents within the class.
- Recommended laboratory monitoring during treatment with oral JAK inhibitors may limit the use of these medications in the active-duty military population or in those with special-duty assignments.
- The oral and topical bioavailability of these medications makes them a more feasible option for deploying service members or for those requiring flexible dosing.
- The rapid improvement in AD seen in multiple trials of oral JAK inhibitors suggests these agents could prove useful in management of acute AD flares, especially in military environments, where injectable agents are either unavailable or unsupported.