User login
44-year-old man • elevated total cholesterol • chest pains • ketogenic diet • Dx?
THE CASE
A 44-year-old man with a history of morbid obesity reestablished care in our clinic. He had been treated in our health care system about 5 years previously, and prior lab testing showed a total cholesterol of 203 mg/dL; triglycerides, 191 mg/dL; high-density lipoprotein (HDL), 56 mg/dL; and low-density lipoprotein (LDL), 109 mg/dL. At that time, he weighed 299 lbs (BMI, 39.4). He then started a strict ketogenic diet and a regular exercise program (running ~ 16 miles per week and lifting weights), which he maintained for several years. He had experienced remarkable weight loss; upon reestablishing care, he weighed 199 lbs (BMI, 26.33).
However, lipid testing revealed a severely elevated total cholesterol of 334 mg/dL; LDL, 248 mg/dL; HDL, 67 mg/dL; and triglycerides, 95 mg/dL. He was advised to start statin therapy and to stop his ketogenic diet, but he was hesitant to take either step. He elected to have his lab work reevaluated in 6 months.
About 4 months later, he presented with new and increasing burning pain in his mid chest and upper abdomen. He rated the pain 6/10 in severity and said it occurred during exertion or at night when lying down. Resting would relieve the pain. Reduced intake of spicy foods and caffeine had also helped. He denied dyspnea, diaphoresis, palpitations, or nausea.
The patient was a nonsmoker but did have a strong family history of cardiovascular disease. His vital signs and physical examination were unremarkable, apart from mild epigastric and periumbilical tenderness on palpation.
THE DIAGNOSIS
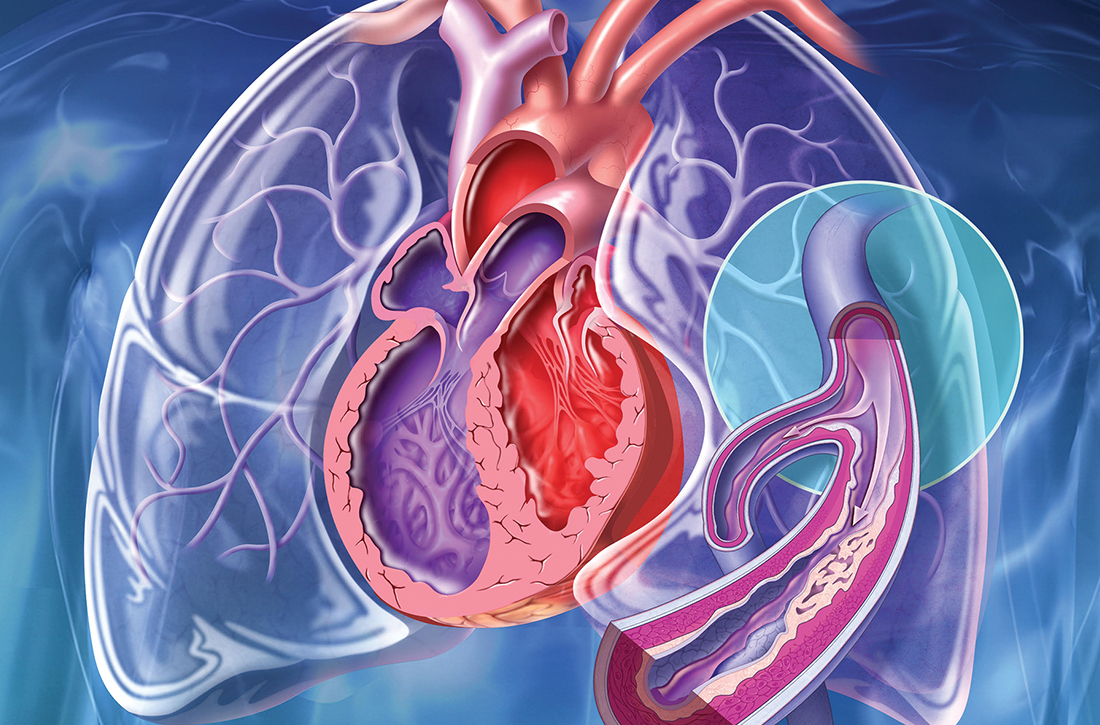
The patient’s chest pain had features of both gastroesophageal reflux disease (GERD) and coronary artery disease (CAD) with exertional angina. His high-fat diet, nightly symptoms, and the partial relief he achieved by cutting back on spicy foods and caffeine suggested GERD, but the exertional nature of the chest pain and gradual relief with rest was highly suggestive of angina, so an outpatient electrocardiogram treadmill stress test was ordered.
The stress test was markedly abnormal, showing worsening ST depressions and T-wave inversions with exertion, and he experienced chest pain during testing. An urgent left heart catheterization was performed, showing severe multivessel CAD. He subsequently underwent 3-vessel coronary artery bypass grafting. A familial hypercholesterolemia panel failed to reveal any significant variants.
As a result of these findings, the patient received a diagnosis of severe ketogenic diet–associated hypercholesterolemia and early-onset CAD.
Continue to: DISCUSSION
DISCUSSION
Low-carbohydrate (low-carb) and ketogenic diets have grown in popularity throughout the United States over the past decade, particularly for weight loss, and the diet has entered the popular consciousness with several celebrities publicly supporting it.1 Simultaneously, there also has been a growing interest in these diets for the treatment of chronic diseases, such as type 2 diabetes.2 However, the long-term cardiovascular effects of low-carb diets are not well studied, and there is significant heterogeneity among these diets.
Low-carb vs low-fat. Multiple meta-analyses comparing low-carb diets to low-fat diets have found that those following low-carb diets have significantly higher total cholesterol and LDL levels.3,4,5 The National Lipid Association’s review of evidence determined that LDL and total cholesterol responses vary in individuals following a low-carb diet, but that increasing LDL levels in particular were concerning enough to warrant lipid monitoring of patients on low-carb diets.6 Another meta-analysis evaluated the difference in estimated atherosclerotic cardiovascular disease (ASCVD) risk between low-carb and low-fat diets, finding those following a low-carb diet to have a lower estimated ASCVD risk but higher LDL levels.7
Weighing the benefits and harms. Since our patient’s dramatic weight loss and greatly increased exercise level would be expected to lower his LDL levels, the severe worsening of his LDL levels was likely related to his ketogenic diet and was a factor in the early onset of CAD. The benefits of low-carb diets for weight loss, contrasted with the consistent worsening of LDL levels, has prompted a debate about which parameters should be considered in estimating the long-term risk of these diets for patients. Diamond et al8 posit that these diets have beneficial effects on “the most reliable [cardiovascular disease] risk factors,” but long-term, patient-oriented outcome data are lacking, and these diets may not be appropriate for certain patients, as our case demonstrates.
A reasonable strategy for patients contemplating a low-carb diet specifically for weight loss would be to use such a diet for 3 to 6 months to achieve initial and rapid results, then continue with a heart-healthy diet and increased exercise levels to maintain weight loss and reduce long-term cardiovascular risk.
Our patient was started on a postoperative medication regimen of aspirin 81 mg/d, evolocumab 140 mg every 14 days, metoprolol tartrate 25 mg bid, and rosuvastatin 10 mg/d. A year later, he was able to resume a high level of physical activity (6-mile runs) without chest pain. His follow-up lipid panel showed a total cholesterol of 153 mg/dL; LDL, 53 mg/dL; HDL, 89 mg/dL; and triglycerides, 55 mg/dL. He had also switched to a regular diet and had been able to maintain his weight loss.
THE TAKEAWAY
Growing evidence suggests that low-carb diets may have a significant and detrimental effect on LDL levels. The long-term safety of these diets hasn’t been well studied, particularly regarding cardiovascular outcomes. At a minimum, patients who initiate low-carb diets should be counseled on general dietary recommendations regarding saturated fat and cholesterol intake, and they should have a follow-up lipid screening to evaluate for any significant worsening in total cholesterol and LDL levels.
CORRESPONDENCE
Samuel Dickmann, MD, 13611 NW 1st Lane, Suite 200, Newberry, FL 32669; [email protected]
1. Gorin A. What is the keto diet – and is it right for you? NBC News BETTER. February 22, 2018. Accessed February 3, 2023. www.nbcnews.com/better/health/what-keto-diet-it-right-you-ncna847256
2. Tinguely D, Gross J, Kosinski, C. Efficacy of ketogenic diets on type 2 diabetes: a systematic review. Current Diabetes Reports. 2021;21:32. doi: 10.1007/s11892-021-01399-z
3. Mansoor N, Vinknes KJ, Veierod MB, et al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466-479. doi: 10.1017/S0007114515004699
4. Bueno NB, de Melo ISV, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. doi: 10.1017/S0007114513000548
5. Chawla S, Tessarolo Silva F, Amaral Medeiros S, et al. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients. 2020;12:3774. doi: 10.3390/nu12123774
6. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13:689-711.e1. doi: 10.1016/j.jacl.2019.08.003
7. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. a meta-analysis. PLoS One. 2015;10:e0139817. doi: 10.1371/journal.pone.0139817
8. Diamond DM, O’Neill BJ, Volek JS. Low carbohydrate diet: are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr Opin Endocrinol Diabetes Obes. 2020;27:291-300. doi: 10.1097/MED.0000000000000568
THE CASE
A 44-year-old man with a history of morbid obesity reestablished care in our clinic. He had been treated in our health care system about 5 years previously, and prior lab testing showed a total cholesterol of 203 mg/dL; triglycerides, 191 mg/dL; high-density lipoprotein (HDL), 56 mg/dL; and low-density lipoprotein (LDL), 109 mg/dL. At that time, he weighed 299 lbs (BMI, 39.4). He then started a strict ketogenic diet and a regular exercise program (running ~ 16 miles per week and lifting weights), which he maintained for several years. He had experienced remarkable weight loss; upon reestablishing care, he weighed 199 lbs (BMI, 26.33).
However, lipid testing revealed a severely elevated total cholesterol of 334 mg/dL; LDL, 248 mg/dL; HDL, 67 mg/dL; and triglycerides, 95 mg/dL. He was advised to start statin therapy and to stop his ketogenic diet, but he was hesitant to take either step. He elected to have his lab work reevaluated in 6 months.
About 4 months later, he presented with new and increasing burning pain in his mid chest and upper abdomen. He rated the pain 6/10 in severity and said it occurred during exertion or at night when lying down. Resting would relieve the pain. Reduced intake of spicy foods and caffeine had also helped. He denied dyspnea, diaphoresis, palpitations, or nausea.
The patient was a nonsmoker but did have a strong family history of cardiovascular disease. His vital signs and physical examination were unremarkable, apart from mild epigastric and periumbilical tenderness on palpation.
THE DIAGNOSIS
The patient’s chest pain had features of both gastroesophageal reflux disease (GERD) and coronary artery disease (CAD) with exertional angina. His high-fat diet, nightly symptoms, and the partial relief he achieved by cutting back on spicy foods and caffeine suggested GERD, but the exertional nature of the chest pain and gradual relief with rest was highly suggestive of angina, so an outpatient electrocardiogram treadmill stress test was ordered.
The stress test was markedly abnormal, showing worsening ST depressions and T-wave inversions with exertion, and he experienced chest pain during testing. An urgent left heart catheterization was performed, showing severe multivessel CAD. He subsequently underwent 3-vessel coronary artery bypass grafting. A familial hypercholesterolemia panel failed to reveal any significant variants.
As a result of these findings, the patient received a diagnosis of severe ketogenic diet–associated hypercholesterolemia and early-onset CAD.
Continue to: DISCUSSION
DISCUSSION
Low-carbohydrate (low-carb) and ketogenic diets have grown in popularity throughout the United States over the past decade, particularly for weight loss, and the diet has entered the popular consciousness with several celebrities publicly supporting it.1 Simultaneously, there also has been a growing interest in these diets for the treatment of chronic diseases, such as type 2 diabetes.2 However, the long-term cardiovascular effects of low-carb diets are not well studied, and there is significant heterogeneity among these diets.
Low-carb vs low-fat. Multiple meta-analyses comparing low-carb diets to low-fat diets have found that those following low-carb diets have significantly higher total cholesterol and LDL levels.3,4,5 The National Lipid Association’s review of evidence determined that LDL and total cholesterol responses vary in individuals following a low-carb diet, but that increasing LDL levels in particular were concerning enough to warrant lipid monitoring of patients on low-carb diets.6 Another meta-analysis evaluated the difference in estimated atherosclerotic cardiovascular disease (ASCVD) risk between low-carb and low-fat diets, finding those following a low-carb diet to have a lower estimated ASCVD risk but higher LDL levels.7
Weighing the benefits and harms. Since our patient’s dramatic weight loss and greatly increased exercise level would be expected to lower his LDL levels, the severe worsening of his LDL levels was likely related to his ketogenic diet and was a factor in the early onset of CAD. The benefits of low-carb diets for weight loss, contrasted with the consistent worsening of LDL levels, has prompted a debate about which parameters should be considered in estimating the long-term risk of these diets for patients. Diamond et al8 posit that these diets have beneficial effects on “the most reliable [cardiovascular disease] risk factors,” but long-term, patient-oriented outcome data are lacking, and these diets may not be appropriate for certain patients, as our case demonstrates.
A reasonable strategy for patients contemplating a low-carb diet specifically for weight loss would be to use such a diet for 3 to 6 months to achieve initial and rapid results, then continue with a heart-healthy diet and increased exercise levels to maintain weight loss and reduce long-term cardiovascular risk.
Our patient was started on a postoperative medication regimen of aspirin 81 mg/d, evolocumab 140 mg every 14 days, metoprolol tartrate 25 mg bid, and rosuvastatin 10 mg/d. A year later, he was able to resume a high level of physical activity (6-mile runs) without chest pain. His follow-up lipid panel showed a total cholesterol of 153 mg/dL; LDL, 53 mg/dL; HDL, 89 mg/dL; and triglycerides, 55 mg/dL. He had also switched to a regular diet and had been able to maintain his weight loss.
THE TAKEAWAY
Growing evidence suggests that low-carb diets may have a significant and detrimental effect on LDL levels. The long-term safety of these diets hasn’t been well studied, particularly regarding cardiovascular outcomes. At a minimum, patients who initiate low-carb diets should be counseled on general dietary recommendations regarding saturated fat and cholesterol intake, and they should have a follow-up lipid screening to evaluate for any significant worsening in total cholesterol and LDL levels.
CORRESPONDENCE
Samuel Dickmann, MD, 13611 NW 1st Lane, Suite 200, Newberry, FL 32669; [email protected]
THE CASE
A 44-year-old man with a history of morbid obesity reestablished care in our clinic. He had been treated in our health care system about 5 years previously, and prior lab testing showed a total cholesterol of 203 mg/dL; triglycerides, 191 mg/dL; high-density lipoprotein (HDL), 56 mg/dL; and low-density lipoprotein (LDL), 109 mg/dL. At that time, he weighed 299 lbs (BMI, 39.4). He then started a strict ketogenic diet and a regular exercise program (running ~ 16 miles per week and lifting weights), which he maintained for several years. He had experienced remarkable weight loss; upon reestablishing care, he weighed 199 lbs (BMI, 26.33).
However, lipid testing revealed a severely elevated total cholesterol of 334 mg/dL; LDL, 248 mg/dL; HDL, 67 mg/dL; and triglycerides, 95 mg/dL. He was advised to start statin therapy and to stop his ketogenic diet, but he was hesitant to take either step. He elected to have his lab work reevaluated in 6 months.
About 4 months later, he presented with new and increasing burning pain in his mid chest and upper abdomen. He rated the pain 6/10 in severity and said it occurred during exertion or at night when lying down. Resting would relieve the pain. Reduced intake of spicy foods and caffeine had also helped. He denied dyspnea, diaphoresis, palpitations, or nausea.
The patient was a nonsmoker but did have a strong family history of cardiovascular disease. His vital signs and physical examination were unremarkable, apart from mild epigastric and periumbilical tenderness on palpation.
THE DIAGNOSIS
The patient’s chest pain had features of both gastroesophageal reflux disease (GERD) and coronary artery disease (CAD) with exertional angina. His high-fat diet, nightly symptoms, and the partial relief he achieved by cutting back on spicy foods and caffeine suggested GERD, but the exertional nature of the chest pain and gradual relief with rest was highly suggestive of angina, so an outpatient electrocardiogram treadmill stress test was ordered.
The stress test was markedly abnormal, showing worsening ST depressions and T-wave inversions with exertion, and he experienced chest pain during testing. An urgent left heart catheterization was performed, showing severe multivessel CAD. He subsequently underwent 3-vessel coronary artery bypass grafting. A familial hypercholesterolemia panel failed to reveal any significant variants.
As a result of these findings, the patient received a diagnosis of severe ketogenic diet–associated hypercholesterolemia and early-onset CAD.
Continue to: DISCUSSION
DISCUSSION
Low-carbohydrate (low-carb) and ketogenic diets have grown in popularity throughout the United States over the past decade, particularly for weight loss, and the diet has entered the popular consciousness with several celebrities publicly supporting it.1 Simultaneously, there also has been a growing interest in these diets for the treatment of chronic diseases, such as type 2 diabetes.2 However, the long-term cardiovascular effects of low-carb diets are not well studied, and there is significant heterogeneity among these diets.
Low-carb vs low-fat. Multiple meta-analyses comparing low-carb diets to low-fat diets have found that those following low-carb diets have significantly higher total cholesterol and LDL levels.3,4,5 The National Lipid Association’s review of evidence determined that LDL and total cholesterol responses vary in individuals following a low-carb diet, but that increasing LDL levels in particular were concerning enough to warrant lipid monitoring of patients on low-carb diets.6 Another meta-analysis evaluated the difference in estimated atherosclerotic cardiovascular disease (ASCVD) risk between low-carb and low-fat diets, finding those following a low-carb diet to have a lower estimated ASCVD risk but higher LDL levels.7
Weighing the benefits and harms. Since our patient’s dramatic weight loss and greatly increased exercise level would be expected to lower his LDL levels, the severe worsening of his LDL levels was likely related to his ketogenic diet and was a factor in the early onset of CAD. The benefits of low-carb diets for weight loss, contrasted with the consistent worsening of LDL levels, has prompted a debate about which parameters should be considered in estimating the long-term risk of these diets for patients. Diamond et al8 posit that these diets have beneficial effects on “the most reliable [cardiovascular disease] risk factors,” but long-term, patient-oriented outcome data are lacking, and these diets may not be appropriate for certain patients, as our case demonstrates.
A reasonable strategy for patients contemplating a low-carb diet specifically for weight loss would be to use such a diet for 3 to 6 months to achieve initial and rapid results, then continue with a heart-healthy diet and increased exercise levels to maintain weight loss and reduce long-term cardiovascular risk.
Our patient was started on a postoperative medication regimen of aspirin 81 mg/d, evolocumab 140 mg every 14 days, metoprolol tartrate 25 mg bid, and rosuvastatin 10 mg/d. A year later, he was able to resume a high level of physical activity (6-mile runs) without chest pain. His follow-up lipid panel showed a total cholesterol of 153 mg/dL; LDL, 53 mg/dL; HDL, 89 mg/dL; and triglycerides, 55 mg/dL. He had also switched to a regular diet and had been able to maintain his weight loss.
THE TAKEAWAY
Growing evidence suggests that low-carb diets may have a significant and detrimental effect on LDL levels. The long-term safety of these diets hasn’t been well studied, particularly regarding cardiovascular outcomes. At a minimum, patients who initiate low-carb diets should be counseled on general dietary recommendations regarding saturated fat and cholesterol intake, and they should have a follow-up lipid screening to evaluate for any significant worsening in total cholesterol and LDL levels.
CORRESPONDENCE
Samuel Dickmann, MD, 13611 NW 1st Lane, Suite 200, Newberry, FL 32669; [email protected]
1. Gorin A. What is the keto diet – and is it right for you? NBC News BETTER. February 22, 2018. Accessed February 3, 2023. www.nbcnews.com/better/health/what-keto-diet-it-right-you-ncna847256
2. Tinguely D, Gross J, Kosinski, C. Efficacy of ketogenic diets on type 2 diabetes: a systematic review. Current Diabetes Reports. 2021;21:32. doi: 10.1007/s11892-021-01399-z
3. Mansoor N, Vinknes KJ, Veierod MB, et al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466-479. doi: 10.1017/S0007114515004699
4. Bueno NB, de Melo ISV, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. doi: 10.1017/S0007114513000548
5. Chawla S, Tessarolo Silva F, Amaral Medeiros S, et al. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients. 2020;12:3774. doi: 10.3390/nu12123774
6. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13:689-711.e1. doi: 10.1016/j.jacl.2019.08.003
7. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. a meta-analysis. PLoS One. 2015;10:e0139817. doi: 10.1371/journal.pone.0139817
8. Diamond DM, O’Neill BJ, Volek JS. Low carbohydrate diet: are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr Opin Endocrinol Diabetes Obes. 2020;27:291-300. doi: 10.1097/MED.0000000000000568
1. Gorin A. What is the keto diet – and is it right for you? NBC News BETTER. February 22, 2018. Accessed February 3, 2023. www.nbcnews.com/better/health/what-keto-diet-it-right-you-ncna847256
2. Tinguely D, Gross J, Kosinski, C. Efficacy of ketogenic diets on type 2 diabetes: a systematic review. Current Diabetes Reports. 2021;21:32. doi: 10.1007/s11892-021-01399-z
3. Mansoor N, Vinknes KJ, Veierod MB, et al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466-479. doi: 10.1017/S0007114515004699
4. Bueno NB, de Melo ISV, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. doi: 10.1017/S0007114513000548
5. Chawla S, Tessarolo Silva F, Amaral Medeiros S, et al. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients. 2020;12:3774. doi: 10.3390/nu12123774
6. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13:689-711.e1. doi: 10.1016/j.jacl.2019.08.003
7. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. a meta-analysis. PLoS One. 2015;10:e0139817. doi: 10.1371/journal.pone.0139817
8. Diamond DM, O’Neill BJ, Volek JS. Low carbohydrate diet: are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr Opin Endocrinol Diabetes Obes. 2020;27:291-300. doi: 10.1097/MED.0000000000000568
Meaningful improvement for patients like Tante Ilse
Last year, after a long delay due to COVID, my father’s ashes were finally laid to rest at Arlington National Cemetery. Among the loved ones who came was my favorite aunt, Tante Ilse, who was suffering from dementia. While she wasn’t “following” everything that was going on, she did perk up when she heard my father’s name and would comment on how she liked him and how wonderful he had been to her.
After the ceremony, our family of about 30 gathered at a restaurant where we shared stories and old pictures. Tante Ilse seemed to relish the photos and the time with family. She was doing so well that when we went back to my mom’s home after the reception, my cousins decided to bring Tante Ilse there, too. She had a great time, as evidenced by her famous total-body laugh. In the months before her death, we all commented about that day and how happy she seemed.
My aunt’s decline comes to mind as I reflect on media reports of 2 Alzheimer drugs— aducanumab and lecanemab—that have been billed by some as “gamechangers.” These new drugs are monoclonal antibodies directed at amyloid, one of several agents thought to cause Alzheimer disease. The details of aducanumab’s approval by the US Food and Drug Administration (FDA) generated a great deal of criticism—with good reason.
Two manufacturer-sponsored studies of aducanumab were halted due to futility of finding a benefit.1 The FDA’s scientific advisory panel recommended against approval due to a lack of evidence that it did anything more than remove amyloid plaque from the brain. And yet aducanumab received accelerated approval from the FDA. (This author collaborated on an additional analysis using data presented to the FDA, after its approval, which also reported no clinically meaningful effects.2) The other agent, lecanemab, also reduces markers of amyloid and was shown to be only moderately better than placebo in decreasing the rate of decline on various measures of cognition.3 Quite notably, both aducanumab and lecanemab, which are administered parenterally, cost more than $25,000 per year4,5 and cause amyloid-related imaging abnormalities (brain edema or hemorrhage).
Expensive agents without meaningful benefit. So far, neither of these agents has shown a reduction in things that are truly important to our patients and their families/caregivers: a reduction in caregiver burden and a reduction in the need for placement in long-term care facilities.
This is in contrast to cholinesterase inhibitors, which also slow the rate of cognitive decline.6 Among the differences that exist between these agents: Cholinesterase inhibitors are taken orally and are available as generics, which cost less than a thousand dollars per year.7 Limited data also suggest that they are associated with a lower risk for nursing home placement.8,9 (A February 2023 search of clinicaltrials.gov did not reveal any completed or planned head-to-head comparisons of monoclonal antibodies and anticholinergic agents.)
Our patients, their families, and caregivers hold out hope for something that will improve the patient’s cognition and extend the meaningful time they have with their loved ones. So far, the best we have to offer falls far short of these goals. I certainly would have hoped for something better than merely clearing amyloid for my aunt.
It’s time that the FDA adopt more rigorous standards requiring new drugs to, among other things, demonstrate meaningful clinical benefits, provide real cost savings, and be safer than currently available therapies. Other nations seem to be able to do this.10,11 It is bad enough to provide “hope in a bottle”; it is worse when what is offered is false hope.
1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30
2. Ebell MH, Barry HC. Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician. 2022;105:353-354.
3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
4. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227-228. doi: 10.1038/d41586-023-00030-3
5. Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. December 20, 2021. Accessed February 20, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients
6. Takramah WK, Asem L. The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: A systematic review and meta-analysis. Health Sci Rep. 2022;5:e913. doi: 10.1002/hsr2.913
7. GoodRx. Donepezil generic Aricept. Accessed February 20, 2023. www.goodrx.com/donepezil
8. Howard R, McShane R, Lindesay J, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171-1181. doi: 10.1016/S1474-4422(15)00258-6
9. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937-944. doi: 10.1046/j.1365-2389.2003.51306.x
10. Pham C, Le K, Draves M, et al. Assessment of FDA-approved drugs not recommended for use or reimbursement in other countries, 2017-2020. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6787
11. Johnston JL, Ross JS, Ramachandran R. US Food and Drug Administration approval of drugs not meeting pivotal trial primary end points, 2018-2021. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6444
Last year, after a long delay due to COVID, my father’s ashes were finally laid to rest at Arlington National Cemetery. Among the loved ones who came was my favorite aunt, Tante Ilse, who was suffering from dementia. While she wasn’t “following” everything that was going on, she did perk up when she heard my father’s name and would comment on how she liked him and how wonderful he had been to her.
After the ceremony, our family of about 30 gathered at a restaurant where we shared stories and old pictures. Tante Ilse seemed to relish the photos and the time with family. She was doing so well that when we went back to my mom’s home after the reception, my cousins decided to bring Tante Ilse there, too. She had a great time, as evidenced by her famous total-body laugh. In the months before her death, we all commented about that day and how happy she seemed.
My aunt’s decline comes to mind as I reflect on media reports of 2 Alzheimer drugs— aducanumab and lecanemab—that have been billed by some as “gamechangers.” These new drugs are monoclonal antibodies directed at amyloid, one of several agents thought to cause Alzheimer disease. The details of aducanumab’s approval by the US Food and Drug Administration (FDA) generated a great deal of criticism—with good reason.
Two manufacturer-sponsored studies of aducanumab were halted due to futility of finding a benefit.1 The FDA’s scientific advisory panel recommended against approval due to a lack of evidence that it did anything more than remove amyloid plaque from the brain. And yet aducanumab received accelerated approval from the FDA. (This author collaborated on an additional analysis using data presented to the FDA, after its approval, which also reported no clinically meaningful effects.2) The other agent, lecanemab, also reduces markers of amyloid and was shown to be only moderately better than placebo in decreasing the rate of decline on various measures of cognition.3 Quite notably, both aducanumab and lecanemab, which are administered parenterally, cost more than $25,000 per year4,5 and cause amyloid-related imaging abnormalities (brain edema or hemorrhage).
Expensive agents without meaningful benefit. So far, neither of these agents has shown a reduction in things that are truly important to our patients and their families/caregivers: a reduction in caregiver burden and a reduction in the need for placement in long-term care facilities.
This is in contrast to cholinesterase inhibitors, which also slow the rate of cognitive decline.6 Among the differences that exist between these agents: Cholinesterase inhibitors are taken orally and are available as generics, which cost less than a thousand dollars per year.7 Limited data also suggest that they are associated with a lower risk for nursing home placement.8,9 (A February 2023 search of clinicaltrials.gov did not reveal any completed or planned head-to-head comparisons of monoclonal antibodies and anticholinergic agents.)
Our patients, their families, and caregivers hold out hope for something that will improve the patient’s cognition and extend the meaningful time they have with their loved ones. So far, the best we have to offer falls far short of these goals. I certainly would have hoped for something better than merely clearing amyloid for my aunt.
It’s time that the FDA adopt more rigorous standards requiring new drugs to, among other things, demonstrate meaningful clinical benefits, provide real cost savings, and be safer than currently available therapies. Other nations seem to be able to do this.10,11 It is bad enough to provide “hope in a bottle”; it is worse when what is offered is false hope.
Last year, after a long delay due to COVID, my father’s ashes were finally laid to rest at Arlington National Cemetery. Among the loved ones who came was my favorite aunt, Tante Ilse, who was suffering from dementia. While she wasn’t “following” everything that was going on, she did perk up when she heard my father’s name and would comment on how she liked him and how wonderful he had been to her.
After the ceremony, our family of about 30 gathered at a restaurant where we shared stories and old pictures. Tante Ilse seemed to relish the photos and the time with family. She was doing so well that when we went back to my mom’s home after the reception, my cousins decided to bring Tante Ilse there, too. She had a great time, as evidenced by her famous total-body laugh. In the months before her death, we all commented about that day and how happy she seemed.
My aunt’s decline comes to mind as I reflect on media reports of 2 Alzheimer drugs— aducanumab and lecanemab—that have been billed by some as “gamechangers.” These new drugs are monoclonal antibodies directed at amyloid, one of several agents thought to cause Alzheimer disease. The details of aducanumab’s approval by the US Food and Drug Administration (FDA) generated a great deal of criticism—with good reason.
Two manufacturer-sponsored studies of aducanumab were halted due to futility of finding a benefit.1 The FDA’s scientific advisory panel recommended against approval due to a lack of evidence that it did anything more than remove amyloid plaque from the brain. And yet aducanumab received accelerated approval from the FDA. (This author collaborated on an additional analysis using data presented to the FDA, after its approval, which also reported no clinically meaningful effects.2) The other agent, lecanemab, also reduces markers of amyloid and was shown to be only moderately better than placebo in decreasing the rate of decline on various measures of cognition.3 Quite notably, both aducanumab and lecanemab, which are administered parenterally, cost more than $25,000 per year4,5 and cause amyloid-related imaging abnormalities (brain edema or hemorrhage).
Expensive agents without meaningful benefit. So far, neither of these agents has shown a reduction in things that are truly important to our patients and their families/caregivers: a reduction in caregiver burden and a reduction in the need for placement in long-term care facilities.
This is in contrast to cholinesterase inhibitors, which also slow the rate of cognitive decline.6 Among the differences that exist between these agents: Cholinesterase inhibitors are taken orally and are available as generics, which cost less than a thousand dollars per year.7 Limited data also suggest that they are associated with a lower risk for nursing home placement.8,9 (A February 2023 search of clinicaltrials.gov did not reveal any completed or planned head-to-head comparisons of monoclonal antibodies and anticholinergic agents.)
Our patients, their families, and caregivers hold out hope for something that will improve the patient’s cognition and extend the meaningful time they have with their loved ones. So far, the best we have to offer falls far short of these goals. I certainly would have hoped for something better than merely clearing amyloid for my aunt.
It’s time that the FDA adopt more rigorous standards requiring new drugs to, among other things, demonstrate meaningful clinical benefits, provide real cost savings, and be safer than currently available therapies. Other nations seem to be able to do this.10,11 It is bad enough to provide “hope in a bottle”; it is worse when what is offered is false hope.
1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30
2. Ebell MH, Barry HC. Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician. 2022;105:353-354.
3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
4. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227-228. doi: 10.1038/d41586-023-00030-3
5. Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. December 20, 2021. Accessed February 20, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients
6. Takramah WK, Asem L. The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: A systematic review and meta-analysis. Health Sci Rep. 2022;5:e913. doi: 10.1002/hsr2.913
7. GoodRx. Donepezil generic Aricept. Accessed February 20, 2023. www.goodrx.com/donepezil
8. Howard R, McShane R, Lindesay J, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171-1181. doi: 10.1016/S1474-4422(15)00258-6
9. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937-944. doi: 10.1046/j.1365-2389.2003.51306.x
10. Pham C, Le K, Draves M, et al. Assessment of FDA-approved drugs not recommended for use or reimbursement in other countries, 2017-2020. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6787
11. Johnston JL, Ross JS, Ramachandran R. US Food and Drug Administration approval of drugs not meeting pivotal trial primary end points, 2018-2021. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6444
1. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30
2. Ebell MH, Barry HC. Why physicians should not prescribe aducanumab for Alzheimer disease. Am Fam Physician. 2022;105:353-354.
3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948
4. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023; 613:227-228. doi: 10.1038/d41586-023-00030-3
5. Biogen announces reduced price for Aduhelm to improve access for patients with early Alzheimer’s disease. December 20, 2021. Accessed February 20, 2023. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-reduced-price-aduhelmr-improve-access-patients
6. Takramah WK, Asem L. The efficacy of pharmacological interventions to improve cognitive and behavior symptoms in people with dementia: A systematic review and meta-analysis. Health Sci Rep. 2022;5:e913. doi: 10.1002/hsr2.913
7. GoodRx. Donepezil generic Aricept. Accessed February 20, 2023. www.goodrx.com/donepezil
8. Howard R, McShane R, Lindesay J, et al. Nursing home placement in the donepezil and memantine in moderate to severe Alzheimer’s disease (DOMINO-AD) trial: secondary and post-hoc analyses. Lancet Neurol. 2015;14:1171-1181. doi: 10.1016/S1474-4422(15)00258-6
9. Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937-944. doi: 10.1046/j.1365-2389.2003.51306.x
10. Pham C, Le K, Draves M, et al. Assessment of FDA-approved drugs not recommended for use or reimbursement in other countries, 2017-2020. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6787
11. Johnston JL, Ross JS, Ramachandran R. US Food and Drug Administration approval of drugs not meeting pivotal trial primary end points, 2018-2021. JAMA Intern Med. Published online February 13, 2023. doi: 10.1001/jamainternmed.2022.6444
5 non-COVID vaccine recommendations from ACIP
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
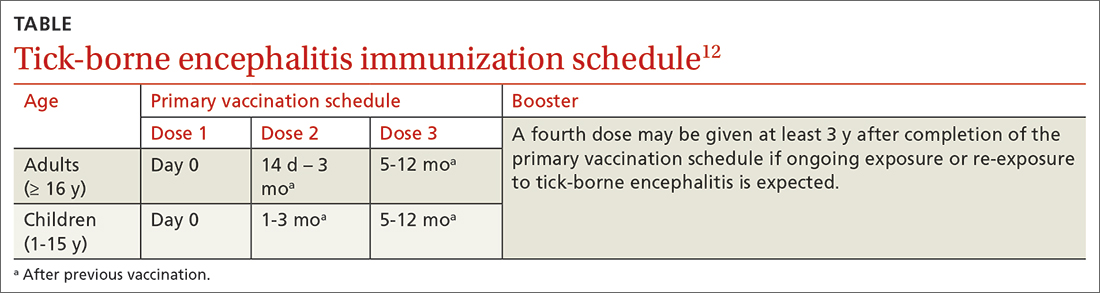
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13
Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13

Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13

Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
Pulmonary hypertension: An update of Dx and Tx guidelines
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.
Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.
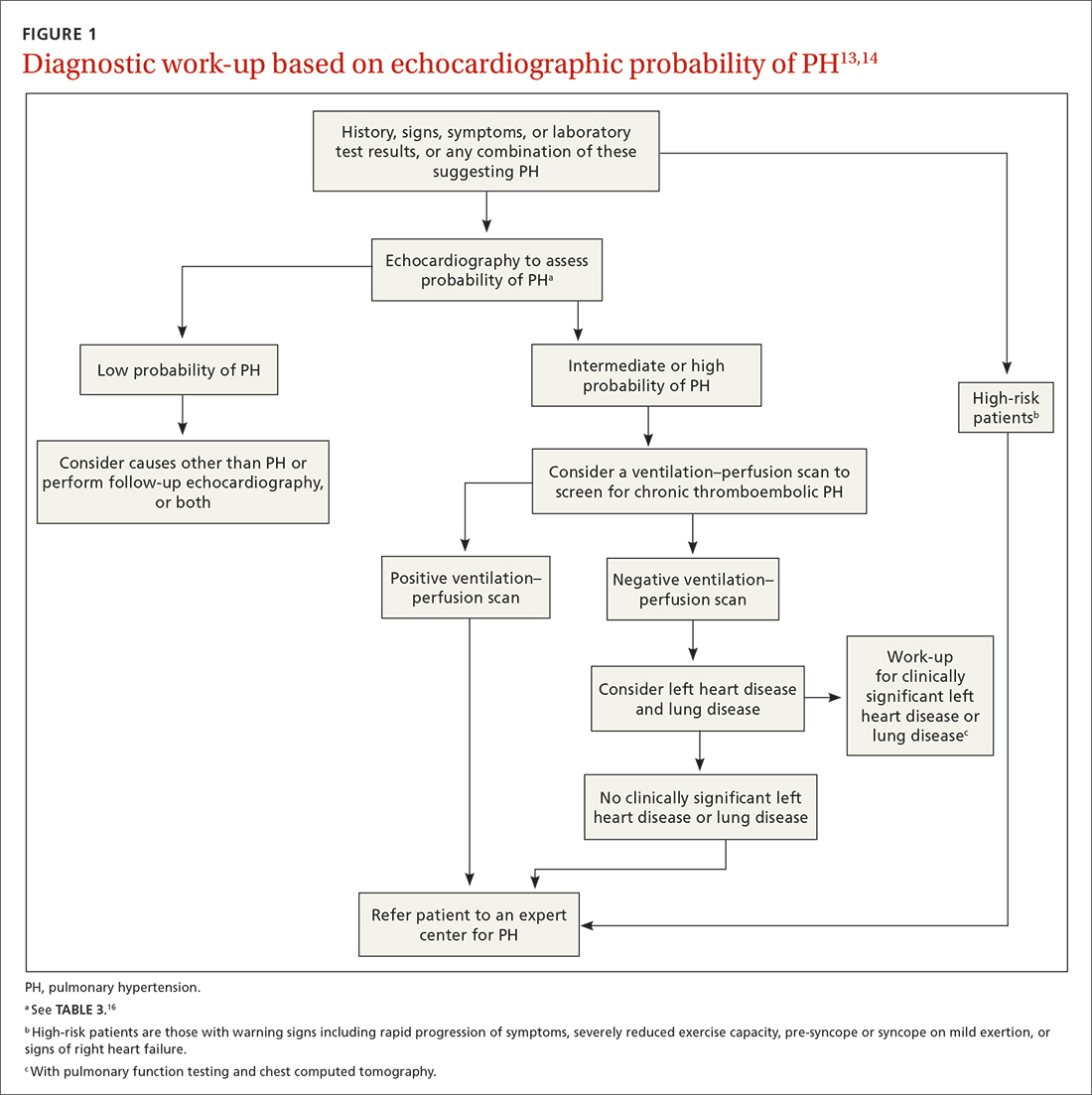
Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7
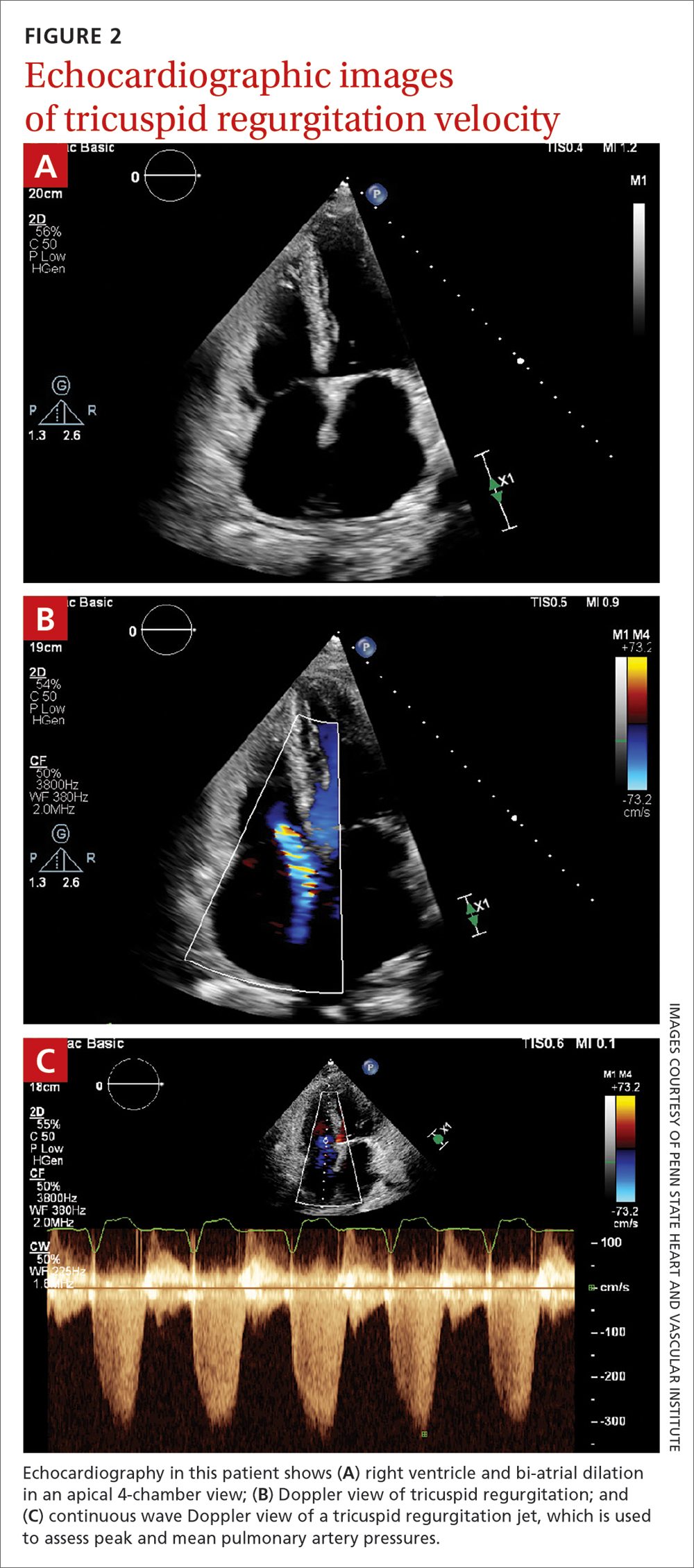
Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6
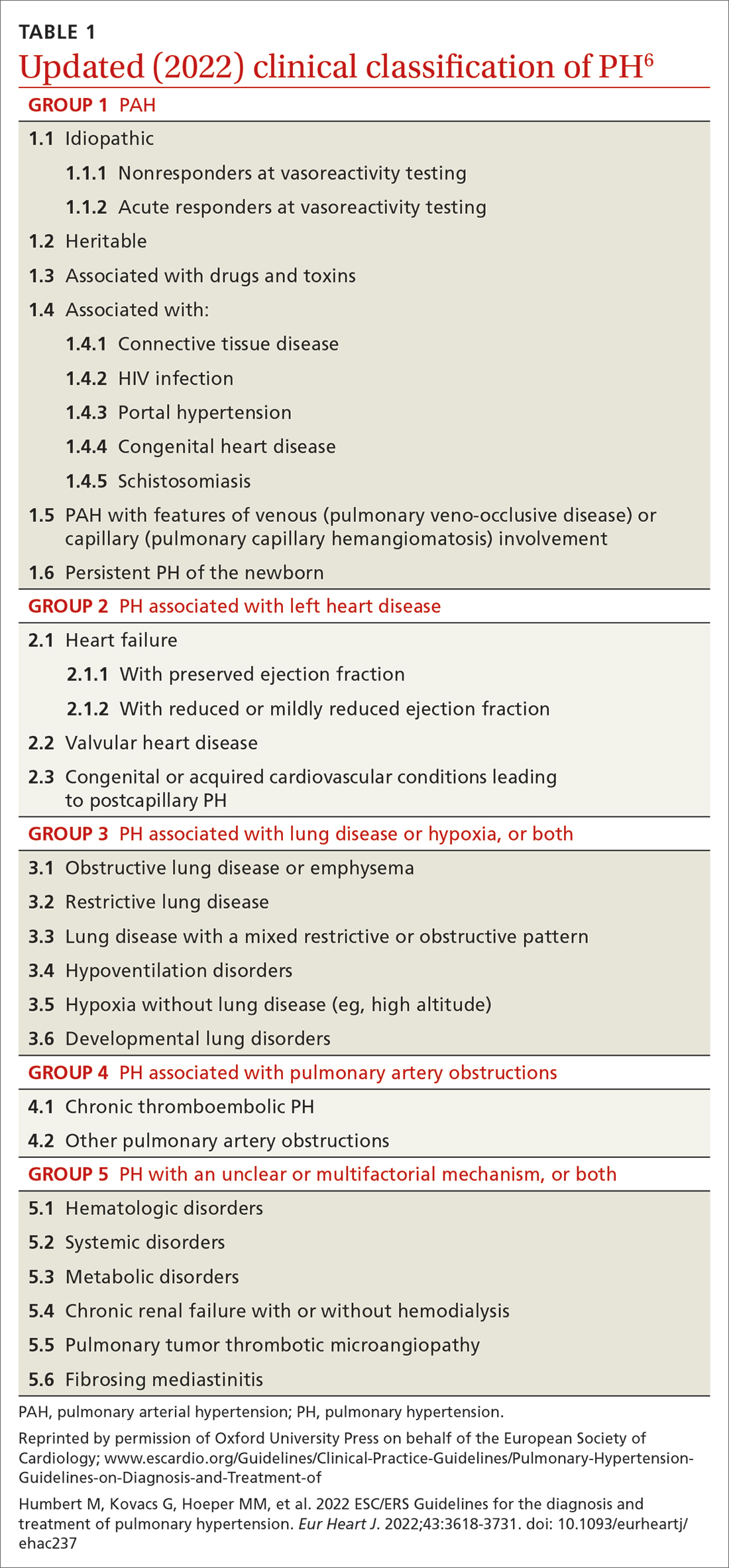
Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).
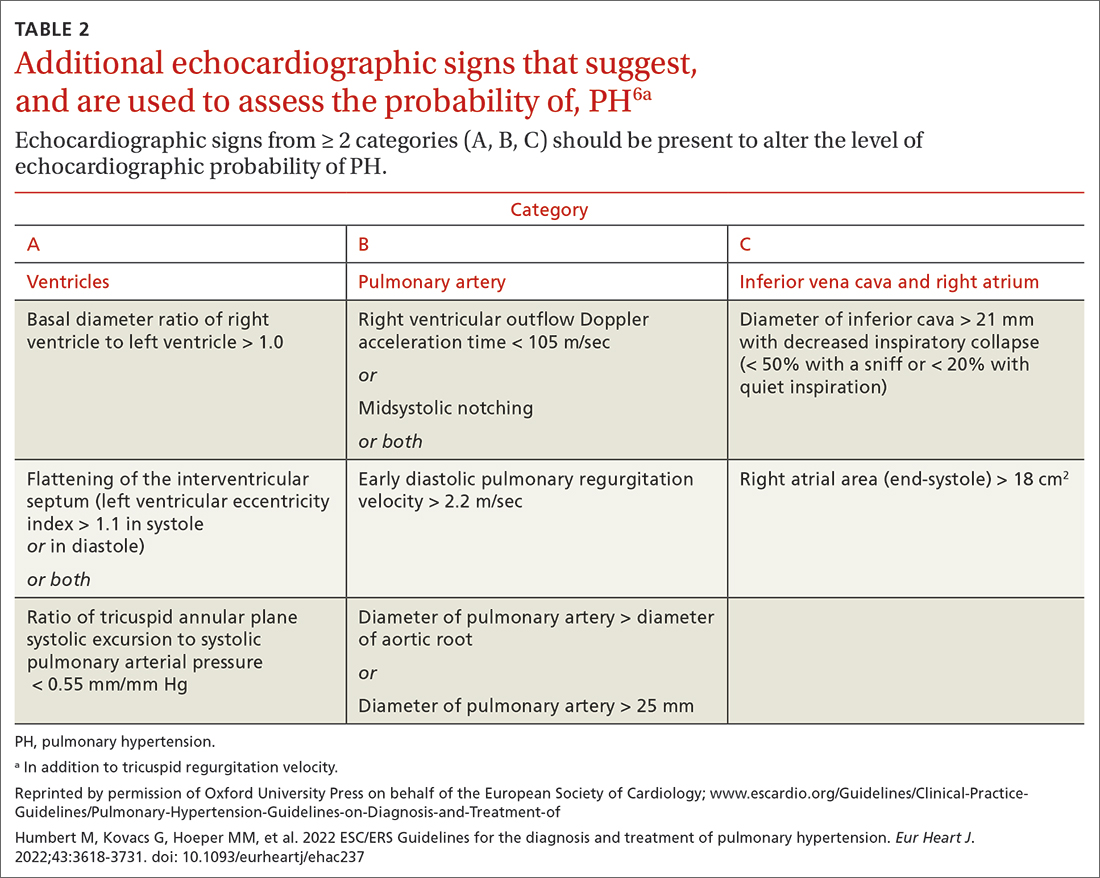
Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18
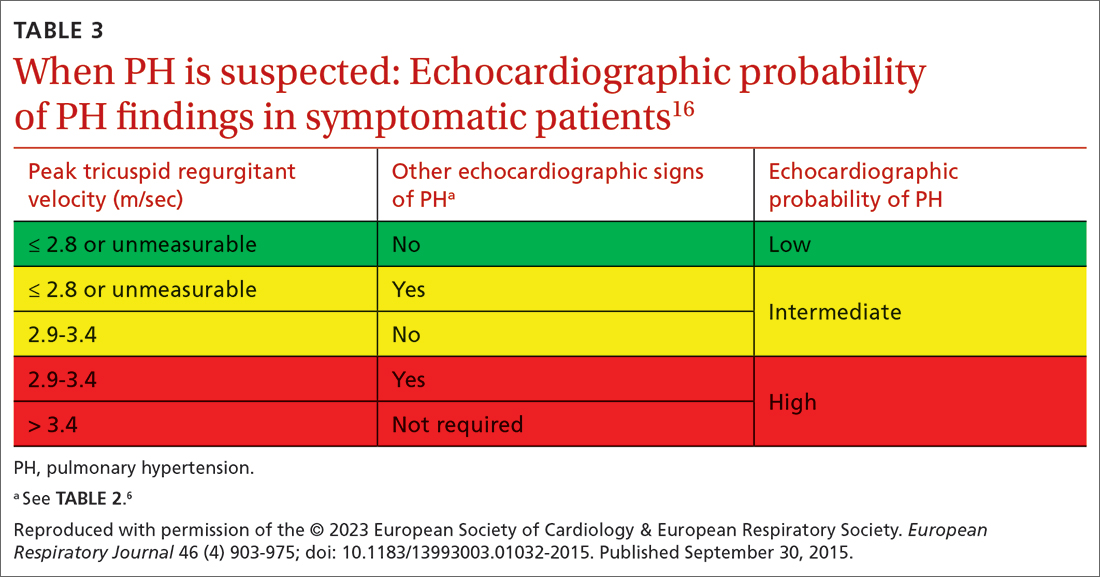
TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
PRACTICE RECOMMENDATIONS
› Employ echocardiography as the first-line diagnostic test when pulmonary hypertension (PH) is suspected. C
› Order a ventilation– perfusion scan in patients with unexplained PH to exclude chronic thromboembolic PH. C
› Order lung function testing with diffusion capacity for carbon monoxide as part of the initial evaluation of PH. C
› Use right heart catheterization to confirm the diagnosis of pulmonary arterial hypertension. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Widespread flaky red skin
This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
Botanical Briefs: Primula obconica Dermatitis
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
Practice Points
- Primula obconica is a household plant that can cause contact dermatitis (CD). Spent blossoms must be pinched off to keep the plant blooming, resulting in fingertip dermatitis.
- In the United States, P obconica is not a component of routine patch testing; therefore, it might be missed as the cause of an allergic reaction.
- Primin and miconidin are the principal allergens known to be responsible for causing P obconica dermatitis.
- Treatment of this condition is similar to the usual treatment of plant-induced CD: avoiding exposure to the plant and applying a topical steroid.
How to Advise Medical Students Interested in Dermatology: A Survey of Academic Dermatology Mentors
Dermatology remains one of the most competitive specialties in medicine. In 2022, there were 851 applicants (613 doctor of medicine seniors, 85 doctor of osteopathic medicine seniors) for 492 postgraduate year (PGY) 2 positions.1 During the 2022 application season, the average matched dermatology candidate had 7.2 research experiences; 20.9 abstracts, presentations, or publications; 11 volunteer experiences; and a US Medical Licensing Examination (USMLE) Step 2 Clinical Knowledge score of 257.1 With hopes of matching into such a competitive field, students often seek advice from academic dermatology mentors. Such advice may substantially differ based on each mentor and may or may not be evidence based.
We sought to analyze the range of advice given to medical students applying to dermatology residency programs via a survey to members of the Association of Professors of Dermatology (APD) with the intent to help applicants and mentors understand how letters of intent, letters of recommendation (LORs), and Electronic Residency Application Service (ERAS) supplemental applications are used by dermatology programs nationwide.
Methods
The study was reviewed by The Ohio State University institutional review board and was deemed exempt. A branching-logic survey with common questions from medical students while applying to dermatology residency programs (Table) was sent to all members of APD through the email listserve. Study data were collected and managed using REDCap electronic data capture tools hosted at The Ohio State University (Columbus, Ohio) to ensure data security.

The survey was distributed from August 28, 2022, to September 12, 2022. A total of 101 surveys were returned from 646 listserve members (15.6%). Given the branching-logic questions, differing numbers of responses were collected for each question. Descriptive statistics were utilized to analyze and report the results.
Results
Residency Program Number—Members of the APD were asked if they recommend students apply to a certain number of programs, and if so, how many programs. Of members who responded, 62.2% (61/98) either always (22.4% [22/98]) or sometimes (40.2% [39/97]) suggested students apply to a certain number of programs. When mentors made a recommendation, 54.1% (33/61) recommended applying to 59 or fewer programs, with only 9.8% (6/61) recommending students apply to 80 or more programs.
Gap Year—We queried mentors about their recommendations for a research gap year and asked which applicants should pursue this extra year. Our survey found that 74.5% of mentors (73/98) almost always (4.1% [4/98]) or sometimes (70.4% [69/98]) recommended a research gap year, most commonly for those applicants with a strong research interest (71.8% [51/71]). Other reasons mentors recommended a dedicated research year during medical school included low USMLE Step scores (50.7% [36/71]), low grades (45.1% [32/71]), little research (46.5% [33/71]), and no home program (43.7% [31/71]).
Internship Choices—Our survey results indicated that nearly two-thirds (63.3% [62/98]) of mentors did not give applicants a recommendation on type of internship (PGY-1). If a recommendation was given, academic dermatologists more commonly recommended an internal medicine preliminary year (29.6% [29/98]) over a transitional year (7.1% [7/98]).
Communication of Interest Via a Letter of Intent—We asked mentors if they recommended applicants send a letter of intent and conversely if receiving a letter of intent impacted their rank list. Nearly half (48.5% [47/97]) of mentors indicated they did not recommend sending a letter of intent, with only 15.5% (15/97) of mentors regularly recommending this practice. Additionally, 75.8% of mentors indicated that a letter of intent never (42.1% [40/95]) or rarely (33.7% [32/95]) impacted their rank list.
Rotation Choices—We queried mentors if they recommended students complete away rotations, and if so, how many rotations did they recommend. We found that 85.9% (85/99) of mentors recommended students complete an away rotation; 63.1% (53/84) of them recommended performing 2 away rotations, and 14.3% (12/84) of respondents recommended students complete 3 away rotations. More than a quarter of mentors (27.1% [23/85]) indicated their home medical schools limited the number of away rotations a medical student could complete in any 1 specialty, and 42.4% (36/85) of respondents were unsure if such a limitation existed.
Letters of Recommendation—Our survey asked respondents to rank various factors on a 5-point scale (1=not important; 5=very important) when deciding who should write the students’ LORs. Mentors indicated that the most important factor for letter-writer selection was how well the letter writer knows the applicant, with 90.8% (89/98) of mentors rating the importance of this quality as a 4 or 5 (Figure). More than half of respondents rated the name recognition of the letter writer and program director letter as a 4 or 5 in importance (54.1% [53/98] and 58.2% [57/98], respectively). Type of letter (standardized vs nonstandardized), title of letter writer, letters from an away rotation, and chair letter scored lower, with fewer than half of mentors rating these as a 4 or 5 in importance.
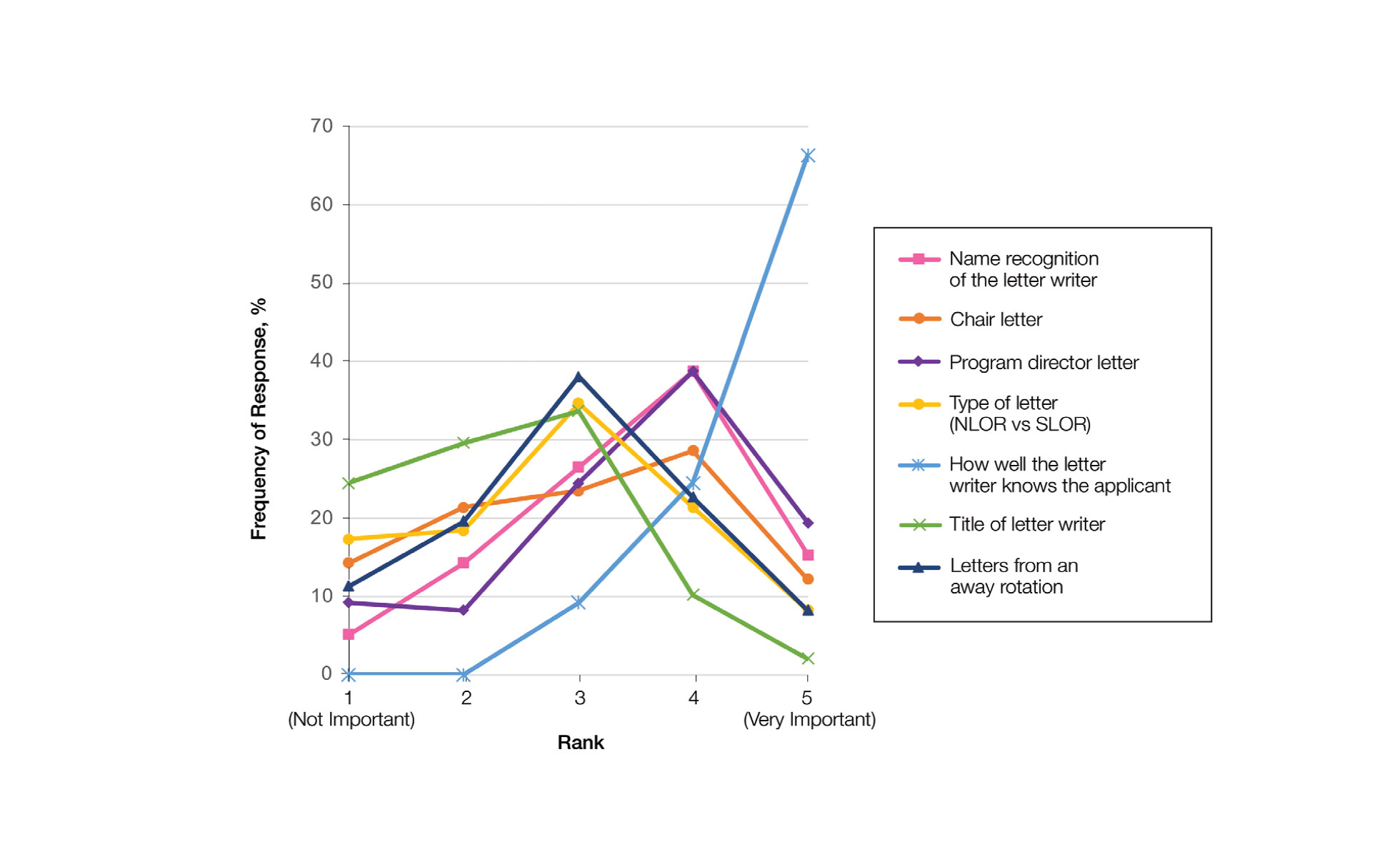
Supplemental Application—When asked about the 2022 application cycle, respondents of our survey reported that the supplemental application was overall more important in deciding which applicants to interview vs which to rank highly. Prior experiences were important (ranked 4 or 5) for 58.8% (57/97) of respondents in choosing applicants to interview, and 49.4% (48/97) of respondents thought prior experiences were important for ranking. Similarly, 34.0% (33/97) of mentors indicated geographic preference was important (ranked 4 or 5) for interview compared with only 23.8% (23/97) for ranking. Finally, 57.7% (56/97) of our survey respondents denoted that program signals were important or very important in choosing which applicants to interview, while 32.0% (31/97) indicated that program signals were important in ranking applicants.
Comment
Residency Programs: Which Ones, and How Many?—The number of applications for dermatology residency programs has increased 33.9% from 2010 to 2019.2 The American Association of Medical Colleges Apply Smart data from 2013 to 2017 indicate that dermatology applicants arrive at a point of diminishing return between 37 and 62 applications, with variation within that range based on USMLE Step 1 score,3 and our data support this with nearly two-thirds of dermatology advisors recommending students apply within this range. Despite this data, dermatology residency applicants applied to more programs over the last decade (64.8 vs 77.0),2 likely to maximize their chance of matching.
Research Gap Years During Medical School—Prior research has shown that nearly half of faculty indicated that a research year during medical school can distinguish similar applicants, and close to 25% of applicants completed a research gap year.4,5 However, available data indicate that taking a research gap year has no effect on match rate or number of interview invites but does correlate with match rates at the highest ranked dermatology residency programs.6-8
Our data indicate that the most commonly recommended reason for a research gap year was an applicants’ strong interest in research. However, nearly half of dermatology mentors recommended research years during medical school for reasons other than an interest in research. As research gap years increase in popularity, future research is needed to confirm the consequence of this additional year and which applicants, if any, will benefit from such a year.
Preferences for Intern Year—Prior research suggests that dermatology residency program directors favor PGY-1 preliminary medicine internships because of the rigor of training.9,10 Our data continue to show a preference for internal medicine preliminary years over transitional years. However, given nearly two-thirds of dermatology mentors do not give applicants any recommendations on PGY-1 year, this preference may be fading.
Letters of Intent Not Recommended—Research in 2022 found that 78.8% of dermatology applicants sent a letter of intent communicating a plan to rank that program number 1, with nearly 13% sending such a letter to more than 1 program.11 With nearly half of mentors in our survey actively discouraging this process and more than 75% of mentors not utilizing this letter, the APD issued a brief statement on the 2022-2023 application cycle stating, “Post-interview communication of preference—including ‘letters of intent’ and thank you letters—should not be sent to programs. These types of communication are typically not used by residency programs in decision-making and lead to downstream pressures on applicants.”12
Away Rotations—Prior to the COVID-19 pandemic, data demonstrated that nearly one-third of dermatology applicants (29%) matched at their home institution, and nearly one-fifth (18%) matched where they completed an away rotation.13 In-person away rotations were eliminated in 2020 and restricted to 1 away rotation in 2021. Restrictions regarding away rotations were removed in 2022. Our data indicate that dermatology mentors strongly supported an away rotation, with more than half of them recommending at least 2 away rotations.
Further research is needed to determine the effect numerous away rotations have on minimizing students’ exposure to other specialties outside their chosen field. Additionally, further studies are needed to determine the impact away rotations have on economically disadvantaged students, students without home programs, and students with families. In an effort to standardize the number of away rotations, the APD issued a statement for the 2023-2024 application cycle indicating that dermatology applicants should limit away rotations to 2 in-person electives. Students without a home dermatology program could consider completing up to 3 electives.14
Who Should Write LORs?—Research in 2014 demonstrated that LORs were very important in determining applicants to interview, with a strong preference for LORs from academic dermatologists and colleagues.15 Our data strongly indicated applicants should predominantly ask for letters from writers who know them well. The majority of mentors did not give value to the rank of the letter writer (eg, assistant professor, associate professor, professor), type of letter, chair letters, or letters from an away rotation. These data may help alleviate stress many students feel as they search for letter writers.
How is the Supplemental Application Used?—In 2022, the ERAS supplemental application was introduced, which allowed applicants to detail 5 meaningful experiences, describe impactful life challenges, and indicate preferences for geographic region. Dermatology residency applicants also were able to choose 3 residency programs to signal interest in that program. Our data found that the supplemental application was utilized predominantly to select applicants to interview, which is in line with the Association of American Medical Colleges’ and APD guidelines indicating that this tool is solely meant to assist with application review.16 Further research and data will hopefully inform approaches to best utilize the ERAS supplemental application data.
Limitations—Our data were limited by response rate and sample size, as only academic dermatologists belonging to the APD were queried. Additionally, we did not track personal information of the mentors, so more than 1 mentor may have responded from a single institution, making it possible that our data may not be broadly applicable to all institutions.
Conclusion
Although there is no algorithmic method of advising medical students who are interested in dermatology, our survey data help to describe the range of advice currently given to students, which can improve and guide future recommendations. Additionally, some of our data demonstrate a discrepancy between mentor advice and current medical student practice for the number of applications and use of a letter of intent. We hope our data will assist academic dermatology mentors in the provision of advice to mentees as well as inform organizations seeking to create standards and official recommendations regarding aspects of the application process.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. May 2022. Accessed February 21, 2023. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Secrest AM, Coman GC, Swink JM, et al. Limiting residency applications to dermatology benefits nearly everyone. J Clin Aesthet Dermatol. 2021;14:30-32.
- Apply smart for residency. Association of American Medical Colleges website. Accessed February 21, 2023. https://students-residents.aamc.org/apply-smart-residency
- Shamloul N, Grandhi R, Hossler E. Perceived importance of dermatology research fellowships. Presented at: Dermatology Teachers Exchange Group; October 3, 2020.
- Runge M, Jairath NK, Renati S, et al. Pursuit of a research year or dual degree by dermatology residency applicants: a cross-sectional study. Cutis. 2022;109:E12-E13.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the Match. Dermatol Online J. 2020;26:13030/qt4604h1w4.
- Hopkins C, Jalali O, Guffey D, et al. A survey of dermatology residents and program directors assessing the transition to dermatology residency. Proc (Bayl Univ Med Center). 2021;34:59-62.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Brumfiel CM, Jefferson IS, Rinderknecht FA, et al. Current perspectives of and potential reforms to the dermatology residency application process: a nationwide survey of program directors and applicants. Clin Dermatol. 2022;40:595-601.
- Association of Professors of Dermatology. Residency Program Directors Section. Updated Information Regarding the 2022-2023 Application Cycle. Updated October 18, 2022. Accessed February 24, 2023. https://www.dermatologyprofessors.org/files/APD%20statement%20on%202022-2023%20application%20cycle_updated%20Oct.pdf
- Narang J, Morgan F, Eversman A, et al. Trends in geographic and home program preferences in the dermatology residency match: a retrospective cohort analysis. J Am Acad Dermatol. 2022;86:645-647.
- Association of Professors of Dermatology Residency Program Directors Section. Recommendations Regarding Away Electives. Updated December 14, 2022. Accessed February 24, 2022. https://www.dermatologyprofessors.org/files/APD%20recommendations%20on%20away%20rotations%202023-2024.pdf
- Kaffenberger BH, Kaffenberger JA, Zirwas MJ. Academic dermatologists’ views on the value of residency letters of recommendation. J Am Acad Dermatol. 2014;71:395-396.
- Supplemental ERAS Application: Guide for Residency Program. Association of American Medical Colleges; June 2022.
Dermatology remains one of the most competitive specialties in medicine. In 2022, there were 851 applicants (613 doctor of medicine seniors, 85 doctor of osteopathic medicine seniors) for 492 postgraduate year (PGY) 2 positions.1 During the 2022 application season, the average matched dermatology candidate had 7.2 research experiences; 20.9 abstracts, presentations, or publications; 11 volunteer experiences; and a US Medical Licensing Examination (USMLE) Step 2 Clinical Knowledge score of 257.1 With hopes of matching into such a competitive field, students often seek advice from academic dermatology mentors. Such advice may substantially differ based on each mentor and may or may not be evidence based.
We sought to analyze the range of advice given to medical students applying to dermatology residency programs via a survey to members of the Association of Professors of Dermatology (APD) with the intent to help applicants and mentors understand how letters of intent, letters of recommendation (LORs), and Electronic Residency Application Service (ERAS) supplemental applications are used by dermatology programs nationwide.
Methods
The study was reviewed by The Ohio State University institutional review board and was deemed exempt. A branching-logic survey with common questions from medical students while applying to dermatology residency programs (Table) was sent to all members of APD through the email listserve. Study data were collected and managed using REDCap electronic data capture tools hosted at The Ohio State University (Columbus, Ohio) to ensure data security.

The survey was distributed from August 28, 2022, to September 12, 2022. A total of 101 surveys were returned from 646 listserve members (15.6%). Given the branching-logic questions, differing numbers of responses were collected for each question. Descriptive statistics were utilized to analyze and report the results.
Results
Residency Program Number—Members of the APD were asked if they recommend students apply to a certain number of programs, and if so, how many programs. Of members who responded, 62.2% (61/98) either always (22.4% [22/98]) or sometimes (40.2% [39/97]) suggested students apply to a certain number of programs. When mentors made a recommendation, 54.1% (33/61) recommended applying to 59 or fewer programs, with only 9.8% (6/61) recommending students apply to 80 or more programs.
Gap Year—We queried mentors about their recommendations for a research gap year and asked which applicants should pursue this extra year. Our survey found that 74.5% of mentors (73/98) almost always (4.1% [4/98]) or sometimes (70.4% [69/98]) recommended a research gap year, most commonly for those applicants with a strong research interest (71.8% [51/71]). Other reasons mentors recommended a dedicated research year during medical school included low USMLE Step scores (50.7% [36/71]), low grades (45.1% [32/71]), little research (46.5% [33/71]), and no home program (43.7% [31/71]).
Internship Choices—Our survey results indicated that nearly two-thirds (63.3% [62/98]) of mentors did not give applicants a recommendation on type of internship (PGY-1). If a recommendation was given, academic dermatologists more commonly recommended an internal medicine preliminary year (29.6% [29/98]) over a transitional year (7.1% [7/98]).
Communication of Interest Via a Letter of Intent—We asked mentors if they recommended applicants send a letter of intent and conversely if receiving a letter of intent impacted their rank list. Nearly half (48.5% [47/97]) of mentors indicated they did not recommend sending a letter of intent, with only 15.5% (15/97) of mentors regularly recommending this practice. Additionally, 75.8% of mentors indicated that a letter of intent never (42.1% [40/95]) or rarely (33.7% [32/95]) impacted their rank list.
Rotation Choices—We queried mentors if they recommended students complete away rotations, and if so, how many rotations did they recommend. We found that 85.9% (85/99) of mentors recommended students complete an away rotation; 63.1% (53/84) of them recommended performing 2 away rotations, and 14.3% (12/84) of respondents recommended students complete 3 away rotations. More than a quarter of mentors (27.1% [23/85]) indicated their home medical schools limited the number of away rotations a medical student could complete in any 1 specialty, and 42.4% (36/85) of respondents were unsure if such a limitation existed.
Letters of Recommendation—Our survey asked respondents to rank various factors on a 5-point scale (1=not important; 5=very important) when deciding who should write the students’ LORs. Mentors indicated that the most important factor for letter-writer selection was how well the letter writer knows the applicant, with 90.8% (89/98) of mentors rating the importance of this quality as a 4 or 5 (Figure). More than half of respondents rated the name recognition of the letter writer and program director letter as a 4 or 5 in importance (54.1% [53/98] and 58.2% [57/98], respectively). Type of letter (standardized vs nonstandardized), title of letter writer, letters from an away rotation, and chair letter scored lower, with fewer than half of mentors rating these as a 4 or 5 in importance.

Supplemental Application—When asked about the 2022 application cycle, respondents of our survey reported that the supplemental application was overall more important in deciding which applicants to interview vs which to rank highly. Prior experiences were important (ranked 4 or 5) for 58.8% (57/97) of respondents in choosing applicants to interview, and 49.4% (48/97) of respondents thought prior experiences were important for ranking. Similarly, 34.0% (33/97) of mentors indicated geographic preference was important (ranked 4 or 5) for interview compared with only 23.8% (23/97) for ranking. Finally, 57.7% (56/97) of our survey respondents denoted that program signals were important or very important in choosing which applicants to interview, while 32.0% (31/97) indicated that program signals were important in ranking applicants.
Comment
Residency Programs: Which Ones, and How Many?—The number of applications for dermatology residency programs has increased 33.9% from 2010 to 2019.2 The American Association of Medical Colleges Apply Smart data from 2013 to 2017 indicate that dermatology applicants arrive at a point of diminishing return between 37 and 62 applications, with variation within that range based on USMLE Step 1 score,3 and our data support this with nearly two-thirds of dermatology advisors recommending students apply within this range. Despite this data, dermatology residency applicants applied to more programs over the last decade (64.8 vs 77.0),2 likely to maximize their chance of matching.
Research Gap Years During Medical School—Prior research has shown that nearly half of faculty indicated that a research year during medical school can distinguish similar applicants, and close to 25% of applicants completed a research gap year.4,5 However, available data indicate that taking a research gap year has no effect on match rate or number of interview invites but does correlate with match rates at the highest ranked dermatology residency programs.6-8
Our data indicate that the most commonly recommended reason for a research gap year was an applicants’ strong interest in research. However, nearly half of dermatology mentors recommended research years during medical school for reasons other than an interest in research. As research gap years increase in popularity, future research is needed to confirm the consequence of this additional year and which applicants, if any, will benefit from such a year.
Preferences for Intern Year—Prior research suggests that dermatology residency program directors favor PGY-1 preliminary medicine internships because of the rigor of training.9,10 Our data continue to show a preference for internal medicine preliminary years over transitional years. However, given nearly two-thirds of dermatology mentors do not give applicants any recommendations on PGY-1 year, this preference may be fading.
Letters of Intent Not Recommended—Research in 2022 found that 78.8% of dermatology applicants sent a letter of intent communicating a plan to rank that program number 1, with nearly 13% sending such a letter to more than 1 program.11 With nearly half of mentors in our survey actively discouraging this process and more than 75% of mentors not utilizing this letter, the APD issued a brief statement on the 2022-2023 application cycle stating, “Post-interview communication of preference—including ‘letters of intent’ and thank you letters—should not be sent to programs. These types of communication are typically not used by residency programs in decision-making and lead to downstream pressures on applicants.”12
Away Rotations—Prior to the COVID-19 pandemic, data demonstrated that nearly one-third of dermatology applicants (29%) matched at their home institution, and nearly one-fifth (18%) matched where they completed an away rotation.13 In-person away rotations were eliminated in 2020 and restricted to 1 away rotation in 2021. Restrictions regarding away rotations were removed in 2022. Our data indicate that dermatology mentors strongly supported an away rotation, with more than half of them recommending at least 2 away rotations.
Further research is needed to determine the effect numerous away rotations have on minimizing students’ exposure to other specialties outside their chosen field. Additionally, further studies are needed to determine the impact away rotations have on economically disadvantaged students, students without home programs, and students with families. In an effort to standardize the number of away rotations, the APD issued a statement for the 2023-2024 application cycle indicating that dermatology applicants should limit away rotations to 2 in-person electives. Students without a home dermatology program could consider completing up to 3 electives.14
Who Should Write LORs?—Research in 2014 demonstrated that LORs were very important in determining applicants to interview, with a strong preference for LORs from academic dermatologists and colleagues.15 Our data strongly indicated applicants should predominantly ask for letters from writers who know them well. The majority of mentors did not give value to the rank of the letter writer (eg, assistant professor, associate professor, professor), type of letter, chair letters, or letters from an away rotation. These data may help alleviate stress many students feel as they search for letter writers.
How is the Supplemental Application Used?—In 2022, the ERAS supplemental application was introduced, which allowed applicants to detail 5 meaningful experiences, describe impactful life challenges, and indicate preferences for geographic region. Dermatology residency applicants also were able to choose 3 residency programs to signal interest in that program. Our data found that the supplemental application was utilized predominantly to select applicants to interview, which is in line with the Association of American Medical Colleges’ and APD guidelines indicating that this tool is solely meant to assist with application review.16 Further research and data will hopefully inform approaches to best utilize the ERAS supplemental application data.
Limitations—Our data were limited by response rate and sample size, as only academic dermatologists belonging to the APD were queried. Additionally, we did not track personal information of the mentors, so more than 1 mentor may have responded from a single institution, making it possible that our data may not be broadly applicable to all institutions.
Conclusion
Although there is no algorithmic method of advising medical students who are interested in dermatology, our survey data help to describe the range of advice currently given to students, which can improve and guide future recommendations. Additionally, some of our data demonstrate a discrepancy between mentor advice and current medical student practice for the number of applications and use of a letter of intent. We hope our data will assist academic dermatology mentors in the provision of advice to mentees as well as inform organizations seeking to create standards and official recommendations regarding aspects of the application process.
Dermatology remains one of the most competitive specialties in medicine. In 2022, there were 851 applicants (613 doctor of medicine seniors, 85 doctor of osteopathic medicine seniors) for 492 postgraduate year (PGY) 2 positions.1 During the 2022 application season, the average matched dermatology candidate had 7.2 research experiences; 20.9 abstracts, presentations, or publications; 11 volunteer experiences; and a US Medical Licensing Examination (USMLE) Step 2 Clinical Knowledge score of 257.1 With hopes of matching into such a competitive field, students often seek advice from academic dermatology mentors. Such advice may substantially differ based on each mentor and may or may not be evidence based.
We sought to analyze the range of advice given to medical students applying to dermatology residency programs via a survey to members of the Association of Professors of Dermatology (APD) with the intent to help applicants and mentors understand how letters of intent, letters of recommendation (LORs), and Electronic Residency Application Service (ERAS) supplemental applications are used by dermatology programs nationwide.
Methods
The study was reviewed by The Ohio State University institutional review board and was deemed exempt. A branching-logic survey with common questions from medical students while applying to dermatology residency programs (Table) was sent to all members of APD through the email listserve. Study data were collected and managed using REDCap electronic data capture tools hosted at The Ohio State University (Columbus, Ohio) to ensure data security.

The survey was distributed from August 28, 2022, to September 12, 2022. A total of 101 surveys were returned from 646 listserve members (15.6%). Given the branching-logic questions, differing numbers of responses were collected for each question. Descriptive statistics were utilized to analyze and report the results.
Results
Residency Program Number—Members of the APD were asked if they recommend students apply to a certain number of programs, and if so, how many programs. Of members who responded, 62.2% (61/98) either always (22.4% [22/98]) or sometimes (40.2% [39/97]) suggested students apply to a certain number of programs. When mentors made a recommendation, 54.1% (33/61) recommended applying to 59 or fewer programs, with only 9.8% (6/61) recommending students apply to 80 or more programs.
Gap Year—We queried mentors about their recommendations for a research gap year and asked which applicants should pursue this extra year. Our survey found that 74.5% of mentors (73/98) almost always (4.1% [4/98]) or sometimes (70.4% [69/98]) recommended a research gap year, most commonly for those applicants with a strong research interest (71.8% [51/71]). Other reasons mentors recommended a dedicated research year during medical school included low USMLE Step scores (50.7% [36/71]), low grades (45.1% [32/71]), little research (46.5% [33/71]), and no home program (43.7% [31/71]).
Internship Choices—Our survey results indicated that nearly two-thirds (63.3% [62/98]) of mentors did not give applicants a recommendation on type of internship (PGY-1). If a recommendation was given, academic dermatologists more commonly recommended an internal medicine preliminary year (29.6% [29/98]) over a transitional year (7.1% [7/98]).
Communication of Interest Via a Letter of Intent—We asked mentors if they recommended applicants send a letter of intent and conversely if receiving a letter of intent impacted their rank list. Nearly half (48.5% [47/97]) of mentors indicated they did not recommend sending a letter of intent, with only 15.5% (15/97) of mentors regularly recommending this practice. Additionally, 75.8% of mentors indicated that a letter of intent never (42.1% [40/95]) or rarely (33.7% [32/95]) impacted their rank list.
Rotation Choices—We queried mentors if they recommended students complete away rotations, and if so, how many rotations did they recommend. We found that 85.9% (85/99) of mentors recommended students complete an away rotation; 63.1% (53/84) of them recommended performing 2 away rotations, and 14.3% (12/84) of respondents recommended students complete 3 away rotations. More than a quarter of mentors (27.1% [23/85]) indicated their home medical schools limited the number of away rotations a medical student could complete in any 1 specialty, and 42.4% (36/85) of respondents were unsure if such a limitation existed.
Letters of Recommendation—Our survey asked respondents to rank various factors on a 5-point scale (1=not important; 5=very important) when deciding who should write the students’ LORs. Mentors indicated that the most important factor for letter-writer selection was how well the letter writer knows the applicant, with 90.8% (89/98) of mentors rating the importance of this quality as a 4 or 5 (Figure). More than half of respondents rated the name recognition of the letter writer and program director letter as a 4 or 5 in importance (54.1% [53/98] and 58.2% [57/98], respectively). Type of letter (standardized vs nonstandardized), title of letter writer, letters from an away rotation, and chair letter scored lower, with fewer than half of mentors rating these as a 4 or 5 in importance.

Supplemental Application—When asked about the 2022 application cycle, respondents of our survey reported that the supplemental application was overall more important in deciding which applicants to interview vs which to rank highly. Prior experiences were important (ranked 4 or 5) for 58.8% (57/97) of respondents in choosing applicants to interview, and 49.4% (48/97) of respondents thought prior experiences were important for ranking. Similarly, 34.0% (33/97) of mentors indicated geographic preference was important (ranked 4 or 5) for interview compared with only 23.8% (23/97) for ranking. Finally, 57.7% (56/97) of our survey respondents denoted that program signals were important or very important in choosing which applicants to interview, while 32.0% (31/97) indicated that program signals were important in ranking applicants.
Comment
Residency Programs: Which Ones, and How Many?—The number of applications for dermatology residency programs has increased 33.9% from 2010 to 2019.2 The American Association of Medical Colleges Apply Smart data from 2013 to 2017 indicate that dermatology applicants arrive at a point of diminishing return between 37 and 62 applications, with variation within that range based on USMLE Step 1 score,3 and our data support this with nearly two-thirds of dermatology advisors recommending students apply within this range. Despite this data, dermatology residency applicants applied to more programs over the last decade (64.8 vs 77.0),2 likely to maximize their chance of matching.
Research Gap Years During Medical School—Prior research has shown that nearly half of faculty indicated that a research year during medical school can distinguish similar applicants, and close to 25% of applicants completed a research gap year.4,5 However, available data indicate that taking a research gap year has no effect on match rate or number of interview invites but does correlate with match rates at the highest ranked dermatology residency programs.6-8
Our data indicate that the most commonly recommended reason for a research gap year was an applicants’ strong interest in research. However, nearly half of dermatology mentors recommended research years during medical school for reasons other than an interest in research. As research gap years increase in popularity, future research is needed to confirm the consequence of this additional year and which applicants, if any, will benefit from such a year.
Preferences for Intern Year—Prior research suggests that dermatology residency program directors favor PGY-1 preliminary medicine internships because of the rigor of training.9,10 Our data continue to show a preference for internal medicine preliminary years over transitional years. However, given nearly two-thirds of dermatology mentors do not give applicants any recommendations on PGY-1 year, this preference may be fading.
Letters of Intent Not Recommended—Research in 2022 found that 78.8% of dermatology applicants sent a letter of intent communicating a plan to rank that program number 1, with nearly 13% sending such a letter to more than 1 program.11 With nearly half of mentors in our survey actively discouraging this process and more than 75% of mentors not utilizing this letter, the APD issued a brief statement on the 2022-2023 application cycle stating, “Post-interview communication of preference—including ‘letters of intent’ and thank you letters—should not be sent to programs. These types of communication are typically not used by residency programs in decision-making and lead to downstream pressures on applicants.”12
Away Rotations—Prior to the COVID-19 pandemic, data demonstrated that nearly one-third of dermatology applicants (29%) matched at their home institution, and nearly one-fifth (18%) matched where they completed an away rotation.13 In-person away rotations were eliminated in 2020 and restricted to 1 away rotation in 2021. Restrictions regarding away rotations were removed in 2022. Our data indicate that dermatology mentors strongly supported an away rotation, with more than half of them recommending at least 2 away rotations.
Further research is needed to determine the effect numerous away rotations have on minimizing students’ exposure to other specialties outside their chosen field. Additionally, further studies are needed to determine the impact away rotations have on economically disadvantaged students, students without home programs, and students with families. In an effort to standardize the number of away rotations, the APD issued a statement for the 2023-2024 application cycle indicating that dermatology applicants should limit away rotations to 2 in-person electives. Students without a home dermatology program could consider completing up to 3 electives.14
Who Should Write LORs?—Research in 2014 demonstrated that LORs were very important in determining applicants to interview, with a strong preference for LORs from academic dermatologists and colleagues.15 Our data strongly indicated applicants should predominantly ask for letters from writers who know them well. The majority of mentors did not give value to the rank of the letter writer (eg, assistant professor, associate professor, professor), type of letter, chair letters, or letters from an away rotation. These data may help alleviate stress many students feel as they search for letter writers.
How is the Supplemental Application Used?—In 2022, the ERAS supplemental application was introduced, which allowed applicants to detail 5 meaningful experiences, describe impactful life challenges, and indicate preferences for geographic region. Dermatology residency applicants also were able to choose 3 residency programs to signal interest in that program. Our data found that the supplemental application was utilized predominantly to select applicants to interview, which is in line with the Association of American Medical Colleges’ and APD guidelines indicating that this tool is solely meant to assist with application review.16 Further research and data will hopefully inform approaches to best utilize the ERAS supplemental application data.
Limitations—Our data were limited by response rate and sample size, as only academic dermatologists belonging to the APD were queried. Additionally, we did not track personal information of the mentors, so more than 1 mentor may have responded from a single institution, making it possible that our data may not be broadly applicable to all institutions.
Conclusion
Although there is no algorithmic method of advising medical students who are interested in dermatology, our survey data help to describe the range of advice currently given to students, which can improve and guide future recommendations. Additionally, some of our data demonstrate a discrepancy between mentor advice and current medical student practice for the number of applications and use of a letter of intent. We hope our data will assist academic dermatology mentors in the provision of advice to mentees as well as inform organizations seeking to create standards and official recommendations regarding aspects of the application process.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. May 2022. Accessed February 21, 2023. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Secrest AM, Coman GC, Swink JM, et al. Limiting residency applications to dermatology benefits nearly everyone. J Clin Aesthet Dermatol. 2021;14:30-32.
- Apply smart for residency. Association of American Medical Colleges website. Accessed February 21, 2023. https://students-residents.aamc.org/apply-smart-residency
- Shamloul N, Grandhi R, Hossler E. Perceived importance of dermatology research fellowships. Presented at: Dermatology Teachers Exchange Group; October 3, 2020.
- Runge M, Jairath NK, Renati S, et al. Pursuit of a research year or dual degree by dermatology residency applicants: a cross-sectional study. Cutis. 2022;109:E12-E13.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the Match. Dermatol Online J. 2020;26:13030/qt4604h1w4.
- Hopkins C, Jalali O, Guffey D, et al. A survey of dermatology residents and program directors assessing the transition to dermatology residency. Proc (Bayl Univ Med Center). 2021;34:59-62.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Brumfiel CM, Jefferson IS, Rinderknecht FA, et al. Current perspectives of and potential reforms to the dermatology residency application process: a nationwide survey of program directors and applicants. Clin Dermatol. 2022;40:595-601.
- Association of Professors of Dermatology. Residency Program Directors Section. Updated Information Regarding the 2022-2023 Application Cycle. Updated October 18, 2022. Accessed February 24, 2023. https://www.dermatologyprofessors.org/files/APD%20statement%20on%202022-2023%20application%20cycle_updated%20Oct.pdf
- Narang J, Morgan F, Eversman A, et al. Trends in geographic and home program preferences in the dermatology residency match: a retrospective cohort analysis. J Am Acad Dermatol. 2022;86:645-647.
- Association of Professors of Dermatology Residency Program Directors Section. Recommendations Regarding Away Electives. Updated December 14, 2022. Accessed February 24, 2022. https://www.dermatologyprofessors.org/files/APD%20recommendations%20on%20away%20rotations%202023-2024.pdf
- Kaffenberger BH, Kaffenberger JA, Zirwas MJ. Academic dermatologists’ views on the value of residency letters of recommendation. J Am Acad Dermatol. 2014;71:395-396.
- Supplemental ERAS Application: Guide for Residency Program. Association of American Medical Colleges; June 2022.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. May 2022. Accessed February 21, 2023. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Secrest AM, Coman GC, Swink JM, et al. Limiting residency applications to dermatology benefits nearly everyone. J Clin Aesthet Dermatol. 2021;14:30-32.
- Apply smart for residency. Association of American Medical Colleges website. Accessed February 21, 2023. https://students-residents.aamc.org/apply-smart-residency
- Shamloul N, Grandhi R, Hossler E. Perceived importance of dermatology research fellowships. Presented at: Dermatology Teachers Exchange Group; October 3, 2020.
- Runge M, Jairath NK, Renati S, et al. Pursuit of a research year or dual degree by dermatology residency applicants: a cross-sectional study. Cutis. 2022;109:E12-E13.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the Match. Dermatol Online J. 2020;26:13030/qt4604h1w4.
- Hopkins C, Jalali O, Guffey D, et al. A survey of dermatology residents and program directors assessing the transition to dermatology residency. Proc (Bayl Univ Med Center). 2021;34:59-62.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Brumfiel CM, Jefferson IS, Rinderknecht FA, et al. Current perspectives of and potential reforms to the dermatology residency application process: a nationwide survey of program directors and applicants. Clin Dermatol. 2022;40:595-601.
- Association of Professors of Dermatology. Residency Program Directors Section. Updated Information Regarding the 2022-2023 Application Cycle. Updated October 18, 2022. Accessed February 24, 2023. https://www.dermatologyprofessors.org/files/APD%20statement%20on%202022-2023%20application%20cycle_updated%20Oct.pdf
- Narang J, Morgan F, Eversman A, et al. Trends in geographic and home program preferences in the dermatology residency match: a retrospective cohort analysis. J Am Acad Dermatol. 2022;86:645-647.
- Association of Professors of Dermatology Residency Program Directors Section. Recommendations Regarding Away Electives. Updated December 14, 2022. Accessed February 24, 2022. https://www.dermatologyprofessors.org/files/APD%20recommendations%20on%20away%20rotations%202023-2024.pdf
- Kaffenberger BH, Kaffenberger JA, Zirwas MJ. Academic dermatologists’ views on the value of residency letters of recommendation. J Am Acad Dermatol. 2014;71:395-396.
- Supplemental ERAS Application: Guide for Residency Program. Association of American Medical Colleges; June 2022.
Practice Points
- Dermatology mentors recommend students apply to 60 or fewer programs, with only a small percentage of faculty routinely recommending students apply to more than 80 programs.
- Dermatology mentors strongly recommend that students should not send a letter of intent to programs, as it rarely is used in the ranking process.
- Dermatology mentors encourage students to ask for letters of recommendation from writers who know them the best, irrespective of the letter writer’s rank or title. The type of letter (standardized vs nonstandardized), chair letter, or letters from an away rotation do not hold as much importance.
Inequity, Bias, Racism, and Physician Burnout: Staying Connected to Purpose and Identity as an Antidote
“Where are you really from?”
When I tell patients I am from Casper, Wyoming—wh ere I have lived the majority of my life—it’smet with disbelief. The subtext: YOU can’t be from THERE.
I didn’t used to think much of comments like this, but as I have continued to hear them, I find myself feeling tired—tired of explaining myself, tired of being treated differently than my colleagues, and tired of justifying myself. My experiences as a woman of color sadly are not uncommon in medicine.
Sara Martinez-Garcia, BA
Racial bias and racism are steeped in the culture of medicine—from the medical school admissions process1,2 to the medical training itself.3 More than half of medical students who identify as underrepresented in medicine (UIM) experience microaggressions.4 Experiencing racism and sexism in the learning environment can lead to burnout, and microaggressions promote feelings of self-doubt and isolation. Medical students who experience microaggressions are more likely to report feelings of burnout and impaired learning.4 These experiences can leave one feeling as if “You do not belong” and “You are unworthy of being in this position.”
Addressing physician burnout already is complex, and addressing burnout caused by inequity, bias, and racism is even more so. In an ideal world, we would eliminate inequity, bias, and racism in medicine through institutional and individual actions. There has been movement to do so. For example, the Accreditation Council for Graduate Medical Education (ACGME), which oversees standards for US resident and fellow training, launched ACGME Equity Matters (https://www.acgme.org/what-we-do/diversity-equity-and-inclusion/ACGME-Equity-Matters/), an initiative aimed to improve diversity, equity, and antiracism practices within graduate medical eduation. However, we know that education alone isn’t enough to fix this monumental problem. Traditional diversity training as we have known it has never been demonstrated to contribute to lasting changes in behavior; it takes much more extensive and complex interventions to meaningfully reduce bias.5 In the meantime, we need action. As a medical community, we need to be better about not turning the other way when we see these things happening in our classrooms and in our hospitals. As individuals, we must self-reflect on the role that we each play in contributing to or combatting injustices and seek out bystander training to empower us to speak out against acts of bias such as sexism or racism. Whether it is supporting a fellow colleague or speaking out against an inappropriate interaction, we can all do our part. A very brief list of actions and resources to support our UIM students and colleagues are listed in the Table; those interested in more in-depth resources are encouraged to explore the Association of American Medical Colleges Diversity and Inclusion Toolkit (https://www.aamc.org/professional-development/affinity-groups/cfas/diversity-inclusion-toolkit/resources).

We can’t change the culture of medicine quickly or even in our lifetime. In the meantime, those who are UIM will continue to experience these events that erode our well-being. They will continue to need support. Discussing mental health has long been stigmatized, and physicians are no exception. Many physicians are hesitant to discuss mental health issues out of fear of judgement and perceived or even real repercussions on their careers.10 However, times are changing and evolving with the current generation of medical students. It’s no secret that medicine is stressful. Most medical schools provide free counseling services, which lowers the barrier for discussions of mental health from the beginning. Making talk about mental health just as normal as talking about other aspects of health takes away the fear that “something is wrong with me” if someone seeks out counseling and mental health services. Faculty should actively check in and maintain open lines of communication, which can be invaluable for UIM students and their training experience. Creating an environment where trainees can be real and honest about the struggles they face in and out of the classroom can make everyone feel like they are not alone.
Addressing burnout in medicine is going to require an all-hands-on-deck approach. At an institutional level, there is a lot of room for improvement—improving systems for physicians so they are able to operate at their highest level (eg, addressing the burdens of prior authorizations and the electronic medical record), setting reasonable expectations around productivity, and creating work structures that respect work-life balance.11 But what can we do for ourselves? We believe that one of the most important ways to protect ourselves from burnout is to remember why. As a medical student, there is enormous pressure—pressure to learn an enormous volume of information, pass examinations, get involved in extracurricular activities, make connections, and seek research opportunities, while also cooking healthy food, grocery shopping, maintaining relationships with loved ones, and generally taking care of oneself. At times it can feel as if our lives outside of medical school are not important enough or valuable enough to make time for, but the pieces of our identity outside of medicine are what shape us into who we are today and are the roots of our purpose in medicine. Sometimes you can feel the most motivated, valued, and supported when you make time to have dinner with friends, call a family member, or simply spend time alone in the outdoors. Who you are and how you got to this point in your life are your identity. Reminding yourself of that can help when experiencing microaggressions or when that voice tries to tell you that you are not worthy. As you progress further in your career, maintaining that relationship with who you are outside of medicine can be your armor against burnout.
- Capers Q IV, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369.
- Lucey CR, Saguil A. The consequences of structural racism on MCAT scores and medical school admissions: the past is prologue. Acad Med. 2020;95:351-356.
- Nguemeni Tiako MJ, South EC, Ray V. Medical schools as racialized organizations: a primer. Ann Intern Med. 2021;174:1143-1144.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2021;113:310-314.
- Dobbin F, Kalev A. Why doesn’t diversity training work? the challenge for industry and academia. Anthropology Now. 2018;10:48-55.
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Int J Womens Dermatol. 2020;6:57-60.
- Hackworth JM, Kotagal M, Bignall ONR, et al. Microaggressions: privileged observers’ duty to act and what they can do [published online December 1, 2021]. Pediatrics. doi:10.1542/peds.2021-052758.
- Wheeler DJ, Zapata J, Davis D, et al. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2019;41:1112-1117.
- Scott K. Just Work: How to Root Out Bias, Prejudice, and Bullying to Build a Kick-Ass Culture of Inclusivity. St. Martin’s Press; 2021.
- Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289:3161-3166.
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
“Where are you really from?”
When I tell patients I am from Casper, Wyoming—wh ere I have lived the majority of my life—it’smet with disbelief. The subtext: YOU can’t be from THERE.
I didn’t used to think much of comments like this, but as I have continued to hear them, I find myself feeling tired—tired of explaining myself, tired of being treated differently than my colleagues, and tired of justifying myself. My experiences as a woman of color sadly are not uncommon in medicine.
Sara Martinez-Garcia, BA
Racial bias and racism are steeped in the culture of medicine—from the medical school admissions process1,2 to the medical training itself.3 More than half of medical students who identify as underrepresented in medicine (UIM) experience microaggressions.4 Experiencing racism and sexism in the learning environment can lead to burnout, and microaggressions promote feelings of self-doubt and isolation. Medical students who experience microaggressions are more likely to report feelings of burnout and impaired learning.4 These experiences can leave one feeling as if “You do not belong” and “You are unworthy of being in this position.”
Addressing physician burnout already is complex, and addressing burnout caused by inequity, bias, and racism is even more so. In an ideal world, we would eliminate inequity, bias, and racism in medicine through institutional and individual actions. There has been movement to do so. For example, the Accreditation Council for Graduate Medical Education (ACGME), which oversees standards for US resident and fellow training, launched ACGME Equity Matters (https://www.acgme.org/what-we-do/diversity-equity-and-inclusion/ACGME-Equity-Matters/), an initiative aimed to improve diversity, equity, and antiracism practices within graduate medical eduation. However, we know that education alone isn’t enough to fix this monumental problem. Traditional diversity training as we have known it has never been demonstrated to contribute to lasting changes in behavior; it takes much more extensive and complex interventions to meaningfully reduce bias.5 In the meantime, we need action. As a medical community, we need to be better about not turning the other way when we see these things happening in our classrooms and in our hospitals. As individuals, we must self-reflect on the role that we each play in contributing to or combatting injustices and seek out bystander training to empower us to speak out against acts of bias such as sexism or racism. Whether it is supporting a fellow colleague or speaking out against an inappropriate interaction, we can all do our part. A very brief list of actions and resources to support our UIM students and colleagues are listed in the Table; those interested in more in-depth resources are encouraged to explore the Association of American Medical Colleges Diversity and Inclusion Toolkit (https://www.aamc.org/professional-development/affinity-groups/cfas/diversity-inclusion-toolkit/resources).

We can’t change the culture of medicine quickly or even in our lifetime. In the meantime, those who are UIM will continue to experience these events that erode our well-being. They will continue to need support. Discussing mental health has long been stigmatized, and physicians are no exception. Many physicians are hesitant to discuss mental health issues out of fear of judgement and perceived or even real repercussions on their careers.10 However, times are changing and evolving with the current generation of medical students. It’s no secret that medicine is stressful. Most medical schools provide free counseling services, which lowers the barrier for discussions of mental health from the beginning. Making talk about mental health just as normal as talking about other aspects of health takes away the fear that “something is wrong with me” if someone seeks out counseling and mental health services. Faculty should actively check in and maintain open lines of communication, which can be invaluable for UIM students and their training experience. Creating an environment where trainees can be real and honest about the struggles they face in and out of the classroom can make everyone feel like they are not alone.
Addressing burnout in medicine is going to require an all-hands-on-deck approach. At an institutional level, there is a lot of room for improvement—improving systems for physicians so they are able to operate at their highest level (eg, addressing the burdens of prior authorizations and the electronic medical record), setting reasonable expectations around productivity, and creating work structures that respect work-life balance.11 But what can we do for ourselves? We believe that one of the most important ways to protect ourselves from burnout is to remember why. As a medical student, there is enormous pressure—pressure to learn an enormous volume of information, pass examinations, get involved in extracurricular activities, make connections, and seek research opportunities, while also cooking healthy food, grocery shopping, maintaining relationships with loved ones, and generally taking care of oneself. At times it can feel as if our lives outside of medical school are not important enough or valuable enough to make time for, but the pieces of our identity outside of medicine are what shape us into who we are today and are the roots of our purpose in medicine. Sometimes you can feel the most motivated, valued, and supported when you make time to have dinner with friends, call a family member, or simply spend time alone in the outdoors. Who you are and how you got to this point in your life are your identity. Reminding yourself of that can help when experiencing microaggressions or when that voice tries to tell you that you are not worthy. As you progress further in your career, maintaining that relationship with who you are outside of medicine can be your armor against burnout.
“Where are you really from?”
When I tell patients I am from Casper, Wyoming—wh ere I have lived the majority of my life—it’smet with disbelief. The subtext: YOU can’t be from THERE.
I didn’t used to think much of comments like this, but as I have continued to hear them, I find myself feeling tired—tired of explaining myself, tired of being treated differently than my colleagues, and tired of justifying myself. My experiences as a woman of color sadly are not uncommon in medicine.
Sara Martinez-Garcia, BA
Racial bias and racism are steeped in the culture of medicine—from the medical school admissions process1,2 to the medical training itself.3 More than half of medical students who identify as underrepresented in medicine (UIM) experience microaggressions.4 Experiencing racism and sexism in the learning environment can lead to burnout, and microaggressions promote feelings of self-doubt and isolation. Medical students who experience microaggressions are more likely to report feelings of burnout and impaired learning.4 These experiences can leave one feeling as if “You do not belong” and “You are unworthy of being in this position.”
Addressing physician burnout already is complex, and addressing burnout caused by inequity, bias, and racism is even more so. In an ideal world, we would eliminate inequity, bias, and racism in medicine through institutional and individual actions. There has been movement to do so. For example, the Accreditation Council for Graduate Medical Education (ACGME), which oversees standards for US resident and fellow training, launched ACGME Equity Matters (https://www.acgme.org/what-we-do/diversity-equity-and-inclusion/ACGME-Equity-Matters/), an initiative aimed to improve diversity, equity, and antiracism practices within graduate medical eduation. However, we know that education alone isn’t enough to fix this monumental problem. Traditional diversity training as we have known it has never been demonstrated to contribute to lasting changes in behavior; it takes much more extensive and complex interventions to meaningfully reduce bias.5 In the meantime, we need action. As a medical community, we need to be better about not turning the other way when we see these things happening in our classrooms and in our hospitals. As individuals, we must self-reflect on the role that we each play in contributing to or combatting injustices and seek out bystander training to empower us to speak out against acts of bias such as sexism or racism. Whether it is supporting a fellow colleague or speaking out against an inappropriate interaction, we can all do our part. A very brief list of actions and resources to support our UIM students and colleagues are listed in the Table; those interested in more in-depth resources are encouraged to explore the Association of American Medical Colleges Diversity and Inclusion Toolkit (https://www.aamc.org/professional-development/affinity-groups/cfas/diversity-inclusion-toolkit/resources).

We can’t change the culture of medicine quickly or even in our lifetime. In the meantime, those who are UIM will continue to experience these events that erode our well-being. They will continue to need support. Discussing mental health has long been stigmatized, and physicians are no exception. Many physicians are hesitant to discuss mental health issues out of fear of judgement and perceived or even real repercussions on their careers.10 However, times are changing and evolving with the current generation of medical students. It’s no secret that medicine is stressful. Most medical schools provide free counseling services, which lowers the barrier for discussions of mental health from the beginning. Making talk about mental health just as normal as talking about other aspects of health takes away the fear that “something is wrong with me” if someone seeks out counseling and mental health services. Faculty should actively check in and maintain open lines of communication, which can be invaluable for UIM students and their training experience. Creating an environment where trainees can be real and honest about the struggles they face in and out of the classroom can make everyone feel like they are not alone.
Addressing burnout in medicine is going to require an all-hands-on-deck approach. At an institutional level, there is a lot of room for improvement—improving systems for physicians so they are able to operate at their highest level (eg, addressing the burdens of prior authorizations and the electronic medical record), setting reasonable expectations around productivity, and creating work structures that respect work-life balance.11 But what can we do for ourselves? We believe that one of the most important ways to protect ourselves from burnout is to remember why. As a medical student, there is enormous pressure—pressure to learn an enormous volume of information, pass examinations, get involved in extracurricular activities, make connections, and seek research opportunities, while also cooking healthy food, grocery shopping, maintaining relationships with loved ones, and generally taking care of oneself. At times it can feel as if our lives outside of medical school are not important enough or valuable enough to make time for, but the pieces of our identity outside of medicine are what shape us into who we are today and are the roots of our purpose in medicine. Sometimes you can feel the most motivated, valued, and supported when you make time to have dinner with friends, call a family member, or simply spend time alone in the outdoors. Who you are and how you got to this point in your life are your identity. Reminding yourself of that can help when experiencing microaggressions or when that voice tries to tell you that you are not worthy. As you progress further in your career, maintaining that relationship with who you are outside of medicine can be your armor against burnout.
- Capers Q IV, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369.
- Lucey CR, Saguil A. The consequences of structural racism on MCAT scores and medical school admissions: the past is prologue. Acad Med. 2020;95:351-356.
- Nguemeni Tiako MJ, South EC, Ray V. Medical schools as racialized organizations: a primer. Ann Intern Med. 2021;174:1143-1144.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2021;113:310-314.
- Dobbin F, Kalev A. Why doesn’t diversity training work? the challenge for industry and academia. Anthropology Now. 2018;10:48-55.
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Int J Womens Dermatol. 2020;6:57-60.
- Hackworth JM, Kotagal M, Bignall ONR, et al. Microaggressions: privileged observers’ duty to act and what they can do [published online December 1, 2021]. Pediatrics. doi:10.1542/peds.2021-052758.
- Wheeler DJ, Zapata J, Davis D, et al. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2019;41:1112-1117.
- Scott K. Just Work: How to Root Out Bias, Prejudice, and Bullying to Build a Kick-Ass Culture of Inclusivity. St. Martin’s Press; 2021.
- Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289:3161-3166.
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
- Capers Q IV, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369.
- Lucey CR, Saguil A. The consequences of structural racism on MCAT scores and medical school admissions: the past is prologue. Acad Med. 2020;95:351-356.
- Nguemeni Tiako MJ, South EC, Ray V. Medical schools as racialized organizations: a primer. Ann Intern Med. 2021;174:1143-1144.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2021;113:310-314.
- Dobbin F, Kalev A. Why doesn’t diversity training work? the challenge for industry and academia. Anthropology Now. 2018;10:48-55.
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Int J Womens Dermatol. 2020;6:57-60.
- Hackworth JM, Kotagal M, Bignall ONR, et al. Microaggressions: privileged observers’ duty to act and what they can do [published online December 1, 2021]. Pediatrics. doi:10.1542/peds.2021-052758.
- Wheeler DJ, Zapata J, Davis D, et al. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2019;41:1112-1117.
- Scott K. Just Work: How to Root Out Bias, Prejudice, and Bullying to Build a Kick-Ass Culture of Inclusivity. St. Martin’s Press; 2021.
- Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289:3161-3166.
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
Bridging the Digital Divide in Teledermatology Usage: A Retrospective Review of Patient Visits
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).
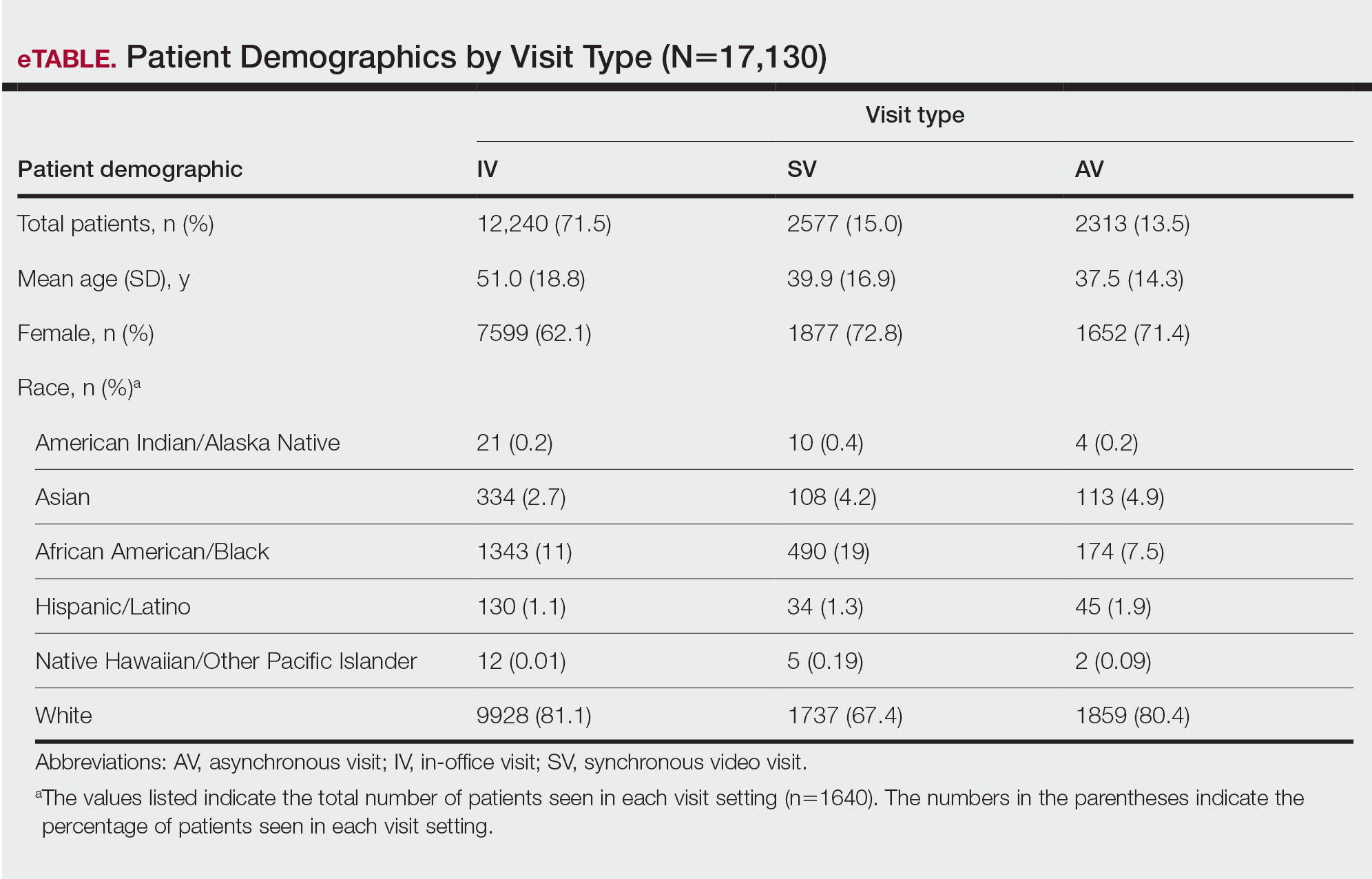
Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.
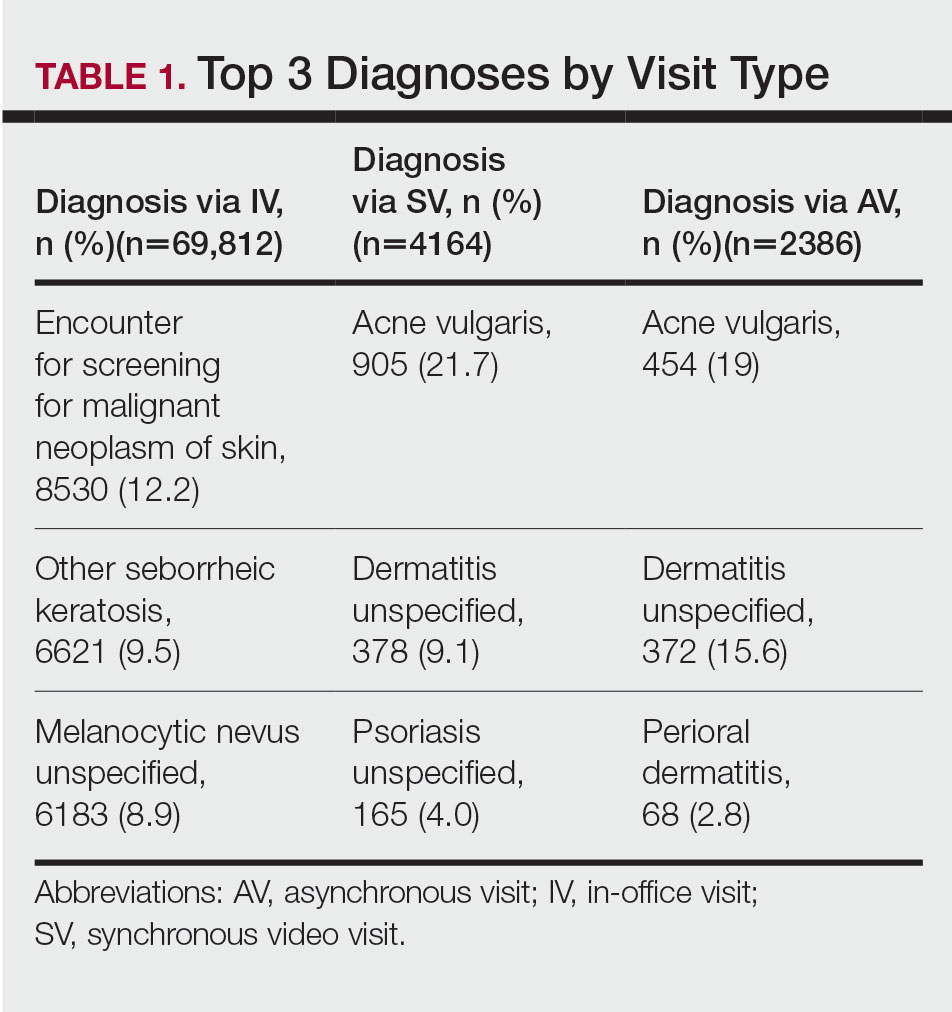
Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.
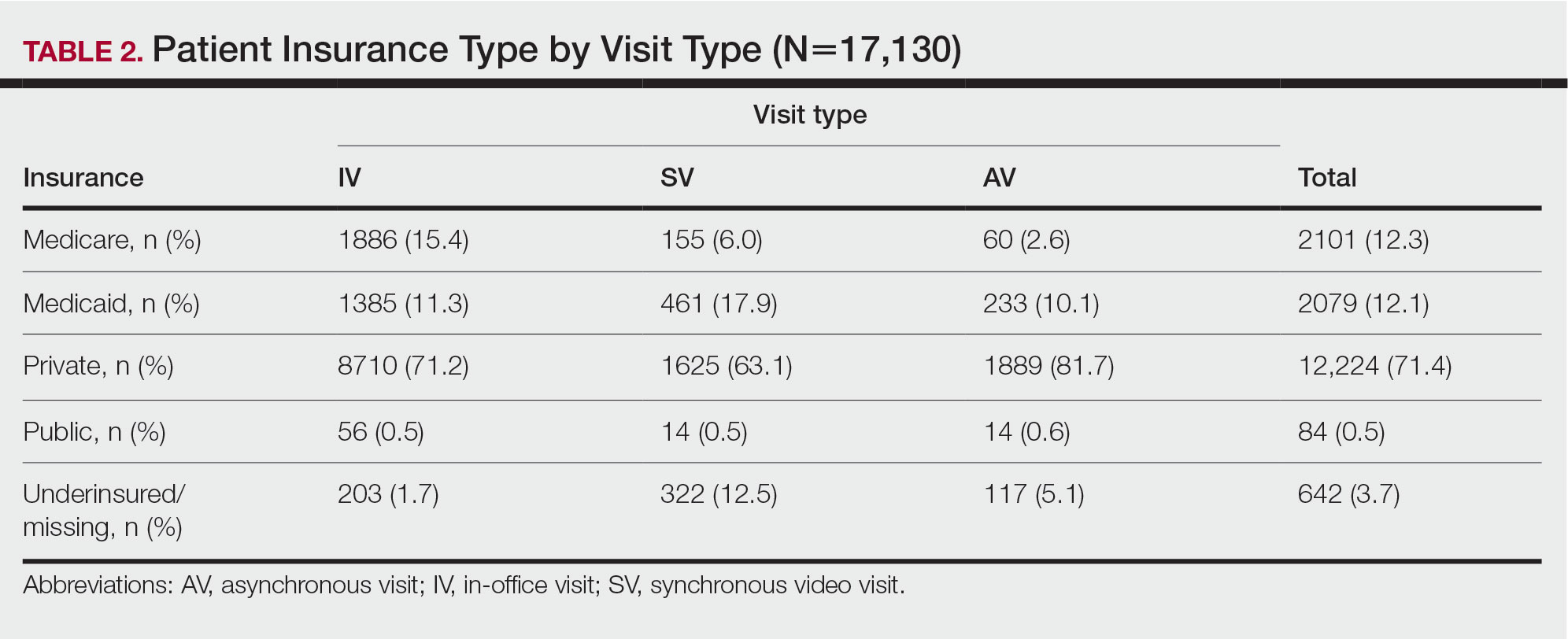
Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Practice Points
- There is increased use of synchronous video visits (SVs) among Black patients, patients with Medicaid, and patients who are underinsured.
- Synchronous video visits may increase dermatologic care utilization for medically marginalized groups.
- Efforts are needed to increase engagement with dermatologic care for Hispanic and male patients.
A “Solution” for Patients Unable to Swallow a Pill: Crushed Terbinafine Mixed With Syrup
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x