User login
Concomitant PsA tied with higher comorbidities and low treatment persistence in psoriasis
Key clinical point: Patients with psoriasis and concomitant psoriatic arthritis (PsA) had a greater comorbidity burden compared with those with psoriasis alone, which negatively impacted treatment persistence.
Major finding: Among patients receiving ustekinumab, those with concomitant PsA vs psoriasis alone had higher comorbidity burden, including diabetes (odds ratio [OR] 1.52; 95% CI 1.16-1.97), hypertension (OR 1.55; 95% CI 1.27-1.89), and obesity (OR 1.33; 95% CI 1.1-1.61), and a shorter time to ustekinumab discontinuation (hazard ratio 1.98; P < .0001).
Study details: This was a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs.
Disclosures: This study was funded by Janssen-Cilag Ltd. W Tillett and A Ogdie declared receiving fees and grants or research support from various sources, including Janssen. A Passey and P Gorecki declared being employees of Janssen-Cilag Ltd.
Source: Tillett W et al. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open. 2023;9(1):e002533 (Jan 17). Doi: 10.1136/rmdopen-2022-002533
Key clinical point: Patients with psoriasis and concomitant psoriatic arthritis (PsA) had a greater comorbidity burden compared with those with psoriasis alone, which negatively impacted treatment persistence.
Major finding: Among patients receiving ustekinumab, those with concomitant PsA vs psoriasis alone had higher comorbidity burden, including diabetes (odds ratio [OR] 1.52; 95% CI 1.16-1.97), hypertension (OR 1.55; 95% CI 1.27-1.89), and obesity (OR 1.33; 95% CI 1.1-1.61), and a shorter time to ustekinumab discontinuation (hazard ratio 1.98; P < .0001).
Study details: This was a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs.
Disclosures: This study was funded by Janssen-Cilag Ltd. W Tillett and A Ogdie declared receiving fees and grants or research support from various sources, including Janssen. A Passey and P Gorecki declared being employees of Janssen-Cilag Ltd.
Source: Tillett W et al. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open. 2023;9(1):e002533 (Jan 17). Doi: 10.1136/rmdopen-2022-002533
Key clinical point: Patients with psoriasis and concomitant psoriatic arthritis (PsA) had a greater comorbidity burden compared with those with psoriasis alone, which negatively impacted treatment persistence.
Major finding: Among patients receiving ustekinumab, those with concomitant PsA vs psoriasis alone had higher comorbidity burden, including diabetes (odds ratio [OR] 1.52; 95% CI 1.16-1.97), hypertension (OR 1.55; 95% CI 1.27-1.89), and obesity (OR 1.33; 95% CI 1.1-1.61), and a shorter time to ustekinumab discontinuation (hazard ratio 1.98; P < .0001).
Study details: This was a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs.
Disclosures: This study was funded by Janssen-Cilag Ltd. W Tillett and A Ogdie declared receiving fees and grants or research support from various sources, including Janssen. A Passey and P Gorecki declared being employees of Janssen-Cilag Ltd.
Source: Tillett W et al. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open. 2023;9(1):e002533 (Jan 17). Doi: 10.1136/rmdopen-2022-002533
Diagnostic role of nailfold capillaroscopy for identifying PsA in psoriasis needs further investigation
Key clinical point: Nailfold capillaroscopy (NC) outcomes could not conclusively differentiate psoriasis from psoriatic arthritis (PsA).
Major finding: In addition to altered morphology, the density of capillaries at the nailfold was significantly lower in patients with psoriasis (standardized group difference [SMD] −0.91; P = .0058; area under curve [AUC] 0.740) and PsA (SMD −1.22; P = .0432; AUC, 0.806) compared with control individuals; however, no NC outcomes conclusively differentiated between psoriasis and PsA.
Study details: Findings are from a systematic review and meta-analysis of 22 studies investigating NC as a diagnostic tool for psoriasis or PsA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Lazar LT et al. Nailfold capillaroscopy as diagnostic test in patients with psoriasis and psoriatic arthritis: A systematic review. Microvasc Res. 2023;147:104476 (Jan 16). Doi: 10.1016/j.mvr.2023.104476
Key clinical point: Nailfold capillaroscopy (NC) outcomes could not conclusively differentiate psoriasis from psoriatic arthritis (PsA).
Major finding: In addition to altered morphology, the density of capillaries at the nailfold was significantly lower in patients with psoriasis (standardized group difference [SMD] −0.91; P = .0058; area under curve [AUC] 0.740) and PsA (SMD −1.22; P = .0432; AUC, 0.806) compared with control individuals; however, no NC outcomes conclusively differentiated between psoriasis and PsA.
Study details: Findings are from a systematic review and meta-analysis of 22 studies investigating NC as a diagnostic tool for psoriasis or PsA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Lazar LT et al. Nailfold capillaroscopy as diagnostic test in patients with psoriasis and psoriatic arthritis: A systematic review. Microvasc Res. 2023;147:104476 (Jan 16). Doi: 10.1016/j.mvr.2023.104476
Key clinical point: Nailfold capillaroscopy (NC) outcomes could not conclusively differentiate psoriasis from psoriatic arthritis (PsA).
Major finding: In addition to altered morphology, the density of capillaries at the nailfold was significantly lower in patients with psoriasis (standardized group difference [SMD] −0.91; P = .0058; area under curve [AUC] 0.740) and PsA (SMD −1.22; P = .0432; AUC, 0.806) compared with control individuals; however, no NC outcomes conclusively differentiated between psoriasis and PsA.
Study details: Findings are from a systematic review and meta-analysis of 22 studies investigating NC as a diagnostic tool for psoriasis or PsA.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Lazar LT et al. Nailfold capillaroscopy as diagnostic test in patients with psoriasis and psoriatic arthritis: A systematic review. Microvasc Res. 2023;147:104476 (Jan 16). Doi: 10.1016/j.mvr.2023.104476
Psoriatic arthritis: An independent risk factor for reduced bone density and fractures
Key clinical point: Regular assessment of bone mineral density and initiation of primary prevention should be considered in patients with psoriatic arthritis (PsA) as they are predisposed to falls and fractures because of reduced bone density, particularly those with late-onset psoriasis involving scalp.
Major finding: Patients with PsA were at a significantly higher risk for osteopenia or osteoporosis (odds ratio [OR] 21.9; CI 7.1-67.7) and prevalent fractures (OR 3.42; P = .002) compared with control individuals, with scalp involvement (P = .0049) and late onset of psoriasis (P = .029) being significantly associated with greater number of prevalent fractures.
Study details: Findings are from an observational cohort study including 61 patients with PsA and 69 age-matched control individuals.
Disclosures: This study did not report the source of funding. The authors reported no conflicts of interest.
Source: Halasi A et al. Psoriatic arthritis and its special features predispose not only for osteoporosis but also for fractures and falls. J Dermatol. 2023 (Jan 17). Doi: 10.1111/1346-8138.16710
Key clinical point: Regular assessment of bone mineral density and initiation of primary prevention should be considered in patients with psoriatic arthritis (PsA) as they are predisposed to falls and fractures because of reduced bone density, particularly those with late-onset psoriasis involving scalp.
Major finding: Patients with PsA were at a significantly higher risk for osteopenia or osteoporosis (odds ratio [OR] 21.9; CI 7.1-67.7) and prevalent fractures (OR 3.42; P = .002) compared with control individuals, with scalp involvement (P = .0049) and late onset of psoriasis (P = .029) being significantly associated with greater number of prevalent fractures.
Study details: Findings are from an observational cohort study including 61 patients with PsA and 69 age-matched control individuals.
Disclosures: This study did not report the source of funding. The authors reported no conflicts of interest.
Source: Halasi A et al. Psoriatic arthritis and its special features predispose not only for osteoporosis but also for fractures and falls. J Dermatol. 2023 (Jan 17). Doi: 10.1111/1346-8138.16710
Key clinical point: Regular assessment of bone mineral density and initiation of primary prevention should be considered in patients with psoriatic arthritis (PsA) as they are predisposed to falls and fractures because of reduced bone density, particularly those with late-onset psoriasis involving scalp.
Major finding: Patients with PsA were at a significantly higher risk for osteopenia or osteoporosis (odds ratio [OR] 21.9; CI 7.1-67.7) and prevalent fractures (OR 3.42; P = .002) compared with control individuals, with scalp involvement (P = .0049) and late onset of psoriasis (P = .029) being significantly associated with greater number of prevalent fractures.
Study details: Findings are from an observational cohort study including 61 patients with PsA and 69 age-matched control individuals.
Disclosures: This study did not report the source of funding. The authors reported no conflicts of interest.
Source: Halasi A et al. Psoriatic arthritis and its special features predispose not only for osteoporosis but also for fractures and falls. J Dermatol. 2023 (Jan 17). Doi: 10.1111/1346-8138.16710
PsA: Guselkumab demonstrates consistent safety profile irrespective of prior TNFi exposure
Key clinical point: A dose of 100 mg guselkumab every 4 or 8 weeks (Q4W/Q8W) demonstrated a favorable and consistent safety profile for up to 2 years in both tumor necrosis factor-α inhibitor (TNFi)-naive and TNFi-experienced patients with active psoriatic arthritis (PsA).
Major finding: In TNFi-naive vs TNFi-experienced patients receiving guselkumab, adverse events rates were consistent through 24 weeks (220.8/100 person-years [PY] vs 251.6/100 PY) and remained low through 2 years (139.69/100 PY vs 174.0/100 PY).
Study details: This pooled safety analysis of four phase 2/3 trials included 1554 TNFi-naive and TNFi-experienced patients with active PsA who were randomly assigned to receive 100 mg guselkumab Q4W or Q8W for ≤2 years or placebo with a crossover at week 24 to guselkumab Q4W or Q8W.
Disclosures: The four trials were funded by Janssen Research & Development, LLC. Seven authors declared being current or former employees of Janssen or owning stock or stock options in Johnson & Johnson. Several authors reported ties with Janssen and other sources.
Source: Rahman P et al. Safety of guselkumab with and without prior TNF-α inhibitor treatment: Pooled results across four studies in patients with psoriatic arthritis. J Rheumatol. 2023 (Jan 15). Doi: 10.3899/jrheum.220928
Key clinical point: A dose of 100 mg guselkumab every 4 or 8 weeks (Q4W/Q8W) demonstrated a favorable and consistent safety profile for up to 2 years in both tumor necrosis factor-α inhibitor (TNFi)-naive and TNFi-experienced patients with active psoriatic arthritis (PsA).
Major finding: In TNFi-naive vs TNFi-experienced patients receiving guselkumab, adverse events rates were consistent through 24 weeks (220.8/100 person-years [PY] vs 251.6/100 PY) and remained low through 2 years (139.69/100 PY vs 174.0/100 PY).
Study details: This pooled safety analysis of four phase 2/3 trials included 1554 TNFi-naive and TNFi-experienced patients with active PsA who were randomly assigned to receive 100 mg guselkumab Q4W or Q8W for ≤2 years or placebo with a crossover at week 24 to guselkumab Q4W or Q8W.
Disclosures: The four trials were funded by Janssen Research & Development, LLC. Seven authors declared being current or former employees of Janssen or owning stock or stock options in Johnson & Johnson. Several authors reported ties with Janssen and other sources.
Source: Rahman P et al. Safety of guselkumab with and without prior TNF-α inhibitor treatment: Pooled results across four studies in patients with psoriatic arthritis. J Rheumatol. 2023 (Jan 15). Doi: 10.3899/jrheum.220928
Key clinical point: A dose of 100 mg guselkumab every 4 or 8 weeks (Q4W/Q8W) demonstrated a favorable and consistent safety profile for up to 2 years in both tumor necrosis factor-α inhibitor (TNFi)-naive and TNFi-experienced patients with active psoriatic arthritis (PsA).
Major finding: In TNFi-naive vs TNFi-experienced patients receiving guselkumab, adverse events rates were consistent through 24 weeks (220.8/100 person-years [PY] vs 251.6/100 PY) and remained low through 2 years (139.69/100 PY vs 174.0/100 PY).
Study details: This pooled safety analysis of four phase 2/3 trials included 1554 TNFi-naive and TNFi-experienced patients with active PsA who were randomly assigned to receive 100 mg guselkumab Q4W or Q8W for ≤2 years or placebo with a crossover at week 24 to guselkumab Q4W or Q8W.
Disclosures: The four trials were funded by Janssen Research & Development, LLC. Seven authors declared being current or former employees of Janssen or owning stock or stock options in Johnson & Johnson. Several authors reported ties with Janssen and other sources.
Source: Rahman P et al. Safety of guselkumab with and without prior TNF-α inhibitor treatment: Pooled results across four studies in patients with psoriatic arthritis. J Rheumatol. 2023 (Jan 15). Doi: 10.3899/jrheum.220928
Effect of the COVID-19 Pandemic on Resources, Other Diseases, and Healthcare Workers’ Experience
Introduction
The COVID-19 pandemic has changed the healthcare system in a multitude of ways, affecting healthcare capacity, treatment of other illnesses, and wellness as well as professional retention of healthcare workers.1-3 During the peak of the COVID-19 pandemic, healthcare capacity was tested and resources were used up quickly.1 As the pandemic has progressed, healthcare systems have had to decide how to proceed with lessons learned, reassessing the environment of care delivery, healthcare supply chains, workforce structures, communication systems, and scientific collaboration as well as policy frameworks in healthcare.4
There have been both immediate effects and long-term consequences of the delay in care for other conditions.2,5 One stark example of this is in cancer care, where screening and procedures were postponed or canceled due to the pandemic with a resulting predicted 2% increase in cancer mortality in the next 10 years.2 The care of heart disease, chronic illnesses, and other viruses has also been similarly negatively impacted by the COVID-19 pandemic due to similar delays in diagnosis and treatment.5-7
The impact on healthcare workers has also been profound.3 Occupational stress from the pandemic has correlated with increased depression and posttraumatic stress disorder (PTSD) among other mental health diseases in healthcare workers.3 In a survey of neurosurgery residents, 26.1% of physicians reported feeling burnt out, and 65.8% were worried that they would not be able to reach surgical milestones.8,9 Among respiratory therapists, a hard hit group during this time, 79% reported burnout.10 Additionally, more healthcare workers left the field during the pandemic, with 15 million lost jobs. Future recovery of jobs looks bleak in some settings, like long-term care and among assistants and aides.11 Overall, the long-term outcomes of these resource, disease, and mental health disruptions need to be assessed and solutions created to maintain a quality and effective healthcare system, with ample resources and measures to account for disease increases and address the impact on providers.
Healthcare Capacity and Resources
With COVID-19 affecting over 100 million in the United States as of March 1, 2023, the impact on healthcare resources since the start of the pandemic has been immense.12 With 5% to 38% of hospitalized patients being admitted to the intensive care unit (ICU) and 75% to 88% of those patients requiring mechanical ventilation, a huge strain was placed on resources during and after the pandemic.1
The question of balancing resources for other hospital needs while tending to patients with COVID-19 has been an ongoing discussion at many levels.1 One core resource concern is the lack of staff. In a survey of 77 different countries, including physicians (41%), nurses (40%), respiratory therapists (11%), and advanced practice providers (8%), 15% reported insufficient intensivists and 32% reported insufficient ICU nursing staff during March and April of 2020.1 A lack of hospital and care space that led to reallocation of limited-care acute care space was a concern. Thirteen percent reported a shortage of hospital ICU beds, while others reported the conversion of postoperative recovery rooms (20%) and operating rooms (12%) for patients with COVID-19.1
Along with staff and care space concerns, hospital survey respondents reported that healthcare equipment was also challenged. Access to COVID-19 testing was one concern, with only 35% of respondents reporting availability for all patients at the beginning of the pandemic, and 56% reporting availability for only select patients based on symptom severity.1 Access to personal protective equipment (PPE) was also affected, with PPE always available according to 83% to 95% of respondents but just 35% having access to N95 masks.1 Additionally, 26% reported that there were no respirators in their hospital, and 11% reported limited ventilators.1
Although resource depletion is a problem, studies have looked at public health measures that helped to mitigate this issue. With proper public health planning and implementation, such as physical distancing, aggressive testing, contact tracing, and increased hospital capacity, by freeing up existing resources or adding additional support, public health modeling showed that resources may be able to withstand the increase.13 Development of reallocation models at local, state, national, and international levels is an important step to be able to deal with future public health crises.14
The long-term impact from the pandemic includes disruption in the physical environment of healthcare, production, supply chain, staff structure, and workforce alterations.4 For example, the physical shape of healthcare facilities is changing to accommodate increasing volumes and decrease the risk of spreading disease.4 To accommodate the burden on staffing structure and workforce alteration, telehealth gained a prominent role.4 All in all, the pandemic has changed the healthcare system; however, institutions, organizations, and policy makers need to evaluate which measures were impactful and should be considered for long-term inclusion in healthcare practice.
Impact on Other Diseases: Cancer, Heart Disease, Chronic Illnesses, and Other Viruses
The treatment of other new and existing conditions has also been affected by the pandemic. Cancer, especially, is a disease of concern. Elective surgeries and screening were halted or altered during the pandemic, which is modeled to lead to higher cancer mortality in years to come.2 The most affected cancers were breast, lung, and colorectal cancer.2 A study of colorectal cancer screening showed that colonoscopies were delayed due to COVID-19 and that gastroenterology visits declined by 49% to 61%.15 This will likely lead to delayed cancer diagnoses and possible increases in mortality.15 Breast cancer screening was also delayed and many patients continued to avoid it for various reasons such as fears of contracting COVID-19 infection in healthcare facilities, and the economic effects of the pandemic such as job loss and healthcare coverage loss.16 These delays will result in an estimated potential 0.52% overall increase in breast cancer deaths by 2030.17
A study of 368 patients from Spain showed a 56.5% decrease in hospital admissions, usually related to heart attacks, in March and April of 2020, compared to January and February 2020.18,19 For other chronic illnesses, the pandemic resulted in decreased preventative care and management.20 The care of other infections similarly suffered. The World Health Organization announced that the number of patients receiving treatment for tuberculosis (TB) dropped by 1 million, setting the disease mitigation back considerably.20 An estimated 500,000 more people died in 2020 from TB.21 The drastic shift in focus to COVID-19 care during this period will continue to have a profound impact on other diseases like these for many years post-pandemic.
Provider Experience and Mental Health Outcomes
The impact on provider experiences and mental health has been immense. One study of 510 healthcare providers (HCPs) and first responders found that occupational stress from the pandemic correlated with psychiatric symptoms, including depression, PTSD, insomnia, and generalized anxiety.3 Occupational stress also correlated with one’s likelihood to leave the medical field and trouble doing work they had once loved.3 Half of the healthcare workers surveyed indicated a decreased likelihood of staying in their current profession after the pandemic.3
Other studies have also looked at specific subspecialties and impact on trainees during the pandemic. In neurosurgery, for example, resident burnout is high, at 26.1%.9 Additionally, the lack of surgeries in the pandemic made 65.8% of neurosurgery residents anxious about meeting career milestones.9 Respiratory therapists, a highly impacted group, also experienced burnout, reporting higher levels in those who worked more in the ICU. Another study identified several themes in the concerns reported by healthcare workers during the pandemic era including “changes in personal life and enhanced negative affect,” “gaining experience, normalization, and adaptation to the pandemic,” and “mental health considerations.”22
Some studies have investigated ways to mitigate this dissatisfaction with the healthcare field post-pandemic. Intrapreneurship, reverse mentoring, and democratized learning all had a reported positive impact on employee experience and retention during this time.23 Intrapreneurship describes entrepreneurship within an existing organization, while reverse mentoring and democratized learning refer to newer employees teaching older employees and communicative learning on a breadth of topics. Other studies have examined the necessity of having mental health resources available, and that these resources need to be multi-stage and individualistic as well as specific to certain stressors HCPs faced during the pandemic.22
Conclusion and Future Directions
The COVID-19 pandemic had stark effects on the healthcare system, impacting resources and capacity, care of other diseases, and provider mental health and experiences.1-3 After the chaos of the pandemic, many questions remain. What needs to be done now by health systems and HCPs? How can we learn from the challenges and the effects on capacity to change the healthcare workflow in times of crisis and in the present? How do we mitigate the impact of the pandemic on diagnosis and management of diseases? And how do we continue to provide healthcare workers with proper mental health and professional resources now, not just in times of stress, and encourage the future generation to pursue careers in healthcare?
These are all the questions the pandemic has left us with, and more studies and initiatives are needed to investigate solutions to these issues. The COVID-19 pandemic left behind valuable lessons and changed the healthcare system, disease management, and staffing for many. Now is the time to pick up the pieces and strategize on how to make our existing system more effective for workers and patients post pandemic.
Wahlster S, Sharma M, Lewis AK, et al. The coronavirus disease 2019 pandemic's effect on critical care resources and health-care providers: a global survey. Chest. 2021;159(2):619-633. doi:10.1016/j.chest.2020.09.070
Malagón T, Yong JHE, Tope P, Miller WH Jr, Franco EL; McGill task force on the impact of COVID-19 on cancer control and care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer. 2022;150(8):1244-1254. doi:10.1002/ijc.33884
Hendrickson RC, Slevin RA, Hoerster KD, et al. The impact of the COVID-19 pandemic on mental health, occupational functioning, and professional retention among health care workers and first responders. J Gen Intern Med. 2022;37(2):397-408. doi:10.1007/s11606-021-07252-z
Davis B, Bankhead-Kendall BK, Dumas RP. A review of COVID-19's impact on modern medical systems from a health organization management perspective. Health Technol (Berl). 2022;12(4):815-824. doi:10.1007/s12553-022-00660-z
Rosenbaum L. The untold toll - the pandemic's effects on patients without COVID-19. N Engl J Med. 2020;382(24):2368-2371. doi:10.1056/NEJMms2009984
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: the impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Jalili M, Niroomand M, Hadavand F, Zeinali K, Fotouhi A. Burnout among healthcare professionals during COVID-19 pandemic: a cross-sectional study. Int Arch Occup Environ Health. 2021;94(6):1345-1352. doi:10.1007/s00420-021-01695-x
Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142. doi:10.1016/j.jocn.2020.08.012
Miller AG, Roberts KJ, Smith BJ, et al. Prevalence of burnout among respiratory therapists amidst the COVID-19 pandemic. Respir Care. 2021;respcare.09283. doi:10.4187/respcare.09283
Frogner BK, Dill JS. Tracking turnover among health care workers during the COVID-19 pandemic: a cross-sectional study. JAMA Health Forum. 2022;3(4):e220371. doi:10.1001/jamahealthforum.2022.0371
CDC COVID data tracker. Centers for Disease Control and Prevention. Accessed December 22, 2022. http://covid-data-tracker/#datatracker-home.
Barrett K, Khan YA, Mac S, Ximenes R, Naimark DMJ, Sander B. Estimation of COVID-19-induced depletion of hospital resources in Ontario, Canada. CMAJ. 2020;192(24):E640-E646. doi:10.1503/cmaj.200715
Kaul V, Chahal J, Schrarstzhaupt IN, et al. Lessons learned from a global perspective of COVID-19. Clin Chest Med. 2022 Nov. 24. [online ahead of print]. doi:10.1016/j.ccm.2022.11.020
Issaka RB, Somsouk M. Colorectal cancer screening and prevention in the COVID-19 Era. JAMA Health Forum. 2020;1(5):e200588. doi:10.1001/jamahealthforum.2020.0588
Freer PE. The impact of the COVID-19 pandemic on breast imaging. Radiol Clin North Am. 2021;59(1):1-11. doi:10.1016/j.rcl.2020.09.008
Alagoz O, Lowry KP, Kurian AW, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst. 2021;113(11):1484-1494. doi:10.1093/jnci/djab097
Jiménez-Blanco Bravo M, Cordero Pereda D, Sánchez Vega D, et al. Heart failure in the time of COVID-19. Cardiology. 2020;145(8):481-484. doi:10.1159/000509181
Frankfurter C, Buchan TA, Kobulnik J, et al. Reduced rate of hospital presentations for heart failure during the COVID-19 pandemic in Toronto, Canada. Can J Cardiol. 2020;36(10):1680-1684. doi:10.1016/j.cjca.2020.07.006
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: The impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Eftekhar Ardebili M, Naserbakht M, Bernstein C, Alazmani-Noodeh F, Hakimi H, Ranjbar H. Healthcare providers experience of working during the COVID-19 pandemic: a qualitative study. Am J Infect Control. 2021;49(5):547-554. doi:10.1016/j.ajic.2020.10.001
Jayathilake HD, Daud D, Eaw HC, Annuar N. Employee development and retention of generation-Z employees in the post-covid-19 workplace: a conceptual framework. Benchmarking: An International Journal. 2021;28(7):2343-2364. doi:10.1108/bij-06-2020-0311
Introduction
The COVID-19 pandemic has changed the healthcare system in a multitude of ways, affecting healthcare capacity, treatment of other illnesses, and wellness as well as professional retention of healthcare workers.1-3 During the peak of the COVID-19 pandemic, healthcare capacity was tested and resources were used up quickly.1 As the pandemic has progressed, healthcare systems have had to decide how to proceed with lessons learned, reassessing the environment of care delivery, healthcare supply chains, workforce structures, communication systems, and scientific collaboration as well as policy frameworks in healthcare.4
There have been both immediate effects and long-term consequences of the delay in care for other conditions.2,5 One stark example of this is in cancer care, where screening and procedures were postponed or canceled due to the pandemic with a resulting predicted 2% increase in cancer mortality in the next 10 years.2 The care of heart disease, chronic illnesses, and other viruses has also been similarly negatively impacted by the COVID-19 pandemic due to similar delays in diagnosis and treatment.5-7
The impact on healthcare workers has also been profound.3 Occupational stress from the pandemic has correlated with increased depression and posttraumatic stress disorder (PTSD) among other mental health diseases in healthcare workers.3 In a survey of neurosurgery residents, 26.1% of physicians reported feeling burnt out, and 65.8% were worried that they would not be able to reach surgical milestones.8,9 Among respiratory therapists, a hard hit group during this time, 79% reported burnout.10 Additionally, more healthcare workers left the field during the pandemic, with 15 million lost jobs. Future recovery of jobs looks bleak in some settings, like long-term care and among assistants and aides.11 Overall, the long-term outcomes of these resource, disease, and mental health disruptions need to be assessed and solutions created to maintain a quality and effective healthcare system, with ample resources and measures to account for disease increases and address the impact on providers.
Healthcare Capacity and Resources
With COVID-19 affecting over 100 million in the United States as of March 1, 2023, the impact on healthcare resources since the start of the pandemic has been immense.12 With 5% to 38% of hospitalized patients being admitted to the intensive care unit (ICU) and 75% to 88% of those patients requiring mechanical ventilation, a huge strain was placed on resources during and after the pandemic.1
The question of balancing resources for other hospital needs while tending to patients with COVID-19 has been an ongoing discussion at many levels.1 One core resource concern is the lack of staff. In a survey of 77 different countries, including physicians (41%), nurses (40%), respiratory therapists (11%), and advanced practice providers (8%), 15% reported insufficient intensivists and 32% reported insufficient ICU nursing staff during March and April of 2020.1 A lack of hospital and care space that led to reallocation of limited-care acute care space was a concern. Thirteen percent reported a shortage of hospital ICU beds, while others reported the conversion of postoperative recovery rooms (20%) and operating rooms (12%) for patients with COVID-19.1
Along with staff and care space concerns, hospital survey respondents reported that healthcare equipment was also challenged. Access to COVID-19 testing was one concern, with only 35% of respondents reporting availability for all patients at the beginning of the pandemic, and 56% reporting availability for only select patients based on symptom severity.1 Access to personal protective equipment (PPE) was also affected, with PPE always available according to 83% to 95% of respondents but just 35% having access to N95 masks.1 Additionally, 26% reported that there were no respirators in their hospital, and 11% reported limited ventilators.1
Although resource depletion is a problem, studies have looked at public health measures that helped to mitigate this issue. With proper public health planning and implementation, such as physical distancing, aggressive testing, contact tracing, and increased hospital capacity, by freeing up existing resources or adding additional support, public health modeling showed that resources may be able to withstand the increase.13 Development of reallocation models at local, state, national, and international levels is an important step to be able to deal with future public health crises.14
The long-term impact from the pandemic includes disruption in the physical environment of healthcare, production, supply chain, staff structure, and workforce alterations.4 For example, the physical shape of healthcare facilities is changing to accommodate increasing volumes and decrease the risk of spreading disease.4 To accommodate the burden on staffing structure and workforce alteration, telehealth gained a prominent role.4 All in all, the pandemic has changed the healthcare system; however, institutions, organizations, and policy makers need to evaluate which measures were impactful and should be considered for long-term inclusion in healthcare practice.
Impact on Other Diseases: Cancer, Heart Disease, Chronic Illnesses, and Other Viruses
The treatment of other new and existing conditions has also been affected by the pandemic. Cancer, especially, is a disease of concern. Elective surgeries and screening were halted or altered during the pandemic, which is modeled to lead to higher cancer mortality in years to come.2 The most affected cancers were breast, lung, and colorectal cancer.2 A study of colorectal cancer screening showed that colonoscopies were delayed due to COVID-19 and that gastroenterology visits declined by 49% to 61%.15 This will likely lead to delayed cancer diagnoses and possible increases in mortality.15 Breast cancer screening was also delayed and many patients continued to avoid it for various reasons such as fears of contracting COVID-19 infection in healthcare facilities, and the economic effects of the pandemic such as job loss and healthcare coverage loss.16 These delays will result in an estimated potential 0.52% overall increase in breast cancer deaths by 2030.17
A study of 368 patients from Spain showed a 56.5% decrease in hospital admissions, usually related to heart attacks, in March and April of 2020, compared to January and February 2020.18,19 For other chronic illnesses, the pandemic resulted in decreased preventative care and management.20 The care of other infections similarly suffered. The World Health Organization announced that the number of patients receiving treatment for tuberculosis (TB) dropped by 1 million, setting the disease mitigation back considerably.20 An estimated 500,000 more people died in 2020 from TB.21 The drastic shift in focus to COVID-19 care during this period will continue to have a profound impact on other diseases like these for many years post-pandemic.
Provider Experience and Mental Health Outcomes
The impact on provider experiences and mental health has been immense. One study of 510 healthcare providers (HCPs) and first responders found that occupational stress from the pandemic correlated with psychiatric symptoms, including depression, PTSD, insomnia, and generalized anxiety.3 Occupational stress also correlated with one’s likelihood to leave the medical field and trouble doing work they had once loved.3 Half of the healthcare workers surveyed indicated a decreased likelihood of staying in their current profession after the pandemic.3
Other studies have also looked at specific subspecialties and impact on trainees during the pandemic. In neurosurgery, for example, resident burnout is high, at 26.1%.9 Additionally, the lack of surgeries in the pandemic made 65.8% of neurosurgery residents anxious about meeting career milestones.9 Respiratory therapists, a highly impacted group, also experienced burnout, reporting higher levels in those who worked more in the ICU. Another study identified several themes in the concerns reported by healthcare workers during the pandemic era including “changes in personal life and enhanced negative affect,” “gaining experience, normalization, and adaptation to the pandemic,” and “mental health considerations.”22
Some studies have investigated ways to mitigate this dissatisfaction with the healthcare field post-pandemic. Intrapreneurship, reverse mentoring, and democratized learning all had a reported positive impact on employee experience and retention during this time.23 Intrapreneurship describes entrepreneurship within an existing organization, while reverse mentoring and democratized learning refer to newer employees teaching older employees and communicative learning on a breadth of topics. Other studies have examined the necessity of having mental health resources available, and that these resources need to be multi-stage and individualistic as well as specific to certain stressors HCPs faced during the pandemic.22
Conclusion and Future Directions
The COVID-19 pandemic had stark effects on the healthcare system, impacting resources and capacity, care of other diseases, and provider mental health and experiences.1-3 After the chaos of the pandemic, many questions remain. What needs to be done now by health systems and HCPs? How can we learn from the challenges and the effects on capacity to change the healthcare workflow in times of crisis and in the present? How do we mitigate the impact of the pandemic on diagnosis and management of diseases? And how do we continue to provide healthcare workers with proper mental health and professional resources now, not just in times of stress, and encourage the future generation to pursue careers in healthcare?
These are all the questions the pandemic has left us with, and more studies and initiatives are needed to investigate solutions to these issues. The COVID-19 pandemic left behind valuable lessons and changed the healthcare system, disease management, and staffing for many. Now is the time to pick up the pieces and strategize on how to make our existing system more effective for workers and patients post pandemic.
Introduction
The COVID-19 pandemic has changed the healthcare system in a multitude of ways, affecting healthcare capacity, treatment of other illnesses, and wellness as well as professional retention of healthcare workers.1-3 During the peak of the COVID-19 pandemic, healthcare capacity was tested and resources were used up quickly.1 As the pandemic has progressed, healthcare systems have had to decide how to proceed with lessons learned, reassessing the environment of care delivery, healthcare supply chains, workforce structures, communication systems, and scientific collaboration as well as policy frameworks in healthcare.4
There have been both immediate effects and long-term consequences of the delay in care for other conditions.2,5 One stark example of this is in cancer care, where screening and procedures were postponed or canceled due to the pandemic with a resulting predicted 2% increase in cancer mortality in the next 10 years.2 The care of heart disease, chronic illnesses, and other viruses has also been similarly negatively impacted by the COVID-19 pandemic due to similar delays in diagnosis and treatment.5-7
The impact on healthcare workers has also been profound.3 Occupational stress from the pandemic has correlated with increased depression and posttraumatic stress disorder (PTSD) among other mental health diseases in healthcare workers.3 In a survey of neurosurgery residents, 26.1% of physicians reported feeling burnt out, and 65.8% were worried that they would not be able to reach surgical milestones.8,9 Among respiratory therapists, a hard hit group during this time, 79% reported burnout.10 Additionally, more healthcare workers left the field during the pandemic, with 15 million lost jobs. Future recovery of jobs looks bleak in some settings, like long-term care and among assistants and aides.11 Overall, the long-term outcomes of these resource, disease, and mental health disruptions need to be assessed and solutions created to maintain a quality and effective healthcare system, with ample resources and measures to account for disease increases and address the impact on providers.
Healthcare Capacity and Resources
With COVID-19 affecting over 100 million in the United States as of March 1, 2023, the impact on healthcare resources since the start of the pandemic has been immense.12 With 5% to 38% of hospitalized patients being admitted to the intensive care unit (ICU) and 75% to 88% of those patients requiring mechanical ventilation, a huge strain was placed on resources during and after the pandemic.1
The question of balancing resources for other hospital needs while tending to patients with COVID-19 has been an ongoing discussion at many levels.1 One core resource concern is the lack of staff. In a survey of 77 different countries, including physicians (41%), nurses (40%), respiratory therapists (11%), and advanced practice providers (8%), 15% reported insufficient intensivists and 32% reported insufficient ICU nursing staff during March and April of 2020.1 A lack of hospital and care space that led to reallocation of limited-care acute care space was a concern. Thirteen percent reported a shortage of hospital ICU beds, while others reported the conversion of postoperative recovery rooms (20%) and operating rooms (12%) for patients with COVID-19.1
Along with staff and care space concerns, hospital survey respondents reported that healthcare equipment was also challenged. Access to COVID-19 testing was one concern, with only 35% of respondents reporting availability for all patients at the beginning of the pandemic, and 56% reporting availability for only select patients based on symptom severity.1 Access to personal protective equipment (PPE) was also affected, with PPE always available according to 83% to 95% of respondents but just 35% having access to N95 masks.1 Additionally, 26% reported that there were no respirators in their hospital, and 11% reported limited ventilators.1
Although resource depletion is a problem, studies have looked at public health measures that helped to mitigate this issue. With proper public health planning and implementation, such as physical distancing, aggressive testing, contact tracing, and increased hospital capacity, by freeing up existing resources or adding additional support, public health modeling showed that resources may be able to withstand the increase.13 Development of reallocation models at local, state, national, and international levels is an important step to be able to deal with future public health crises.14
The long-term impact from the pandemic includes disruption in the physical environment of healthcare, production, supply chain, staff structure, and workforce alterations.4 For example, the physical shape of healthcare facilities is changing to accommodate increasing volumes and decrease the risk of spreading disease.4 To accommodate the burden on staffing structure and workforce alteration, telehealth gained a prominent role.4 All in all, the pandemic has changed the healthcare system; however, institutions, organizations, and policy makers need to evaluate which measures were impactful and should be considered for long-term inclusion in healthcare practice.
Impact on Other Diseases: Cancer, Heart Disease, Chronic Illnesses, and Other Viruses
The treatment of other new and existing conditions has also been affected by the pandemic. Cancer, especially, is a disease of concern. Elective surgeries and screening were halted or altered during the pandemic, which is modeled to lead to higher cancer mortality in years to come.2 The most affected cancers were breast, lung, and colorectal cancer.2 A study of colorectal cancer screening showed that colonoscopies were delayed due to COVID-19 and that gastroenterology visits declined by 49% to 61%.15 This will likely lead to delayed cancer diagnoses and possible increases in mortality.15 Breast cancer screening was also delayed and many patients continued to avoid it for various reasons such as fears of contracting COVID-19 infection in healthcare facilities, and the economic effects of the pandemic such as job loss and healthcare coverage loss.16 These delays will result in an estimated potential 0.52% overall increase in breast cancer deaths by 2030.17
A study of 368 patients from Spain showed a 56.5% decrease in hospital admissions, usually related to heart attacks, in March and April of 2020, compared to January and February 2020.18,19 For other chronic illnesses, the pandemic resulted in decreased preventative care and management.20 The care of other infections similarly suffered. The World Health Organization announced that the number of patients receiving treatment for tuberculosis (TB) dropped by 1 million, setting the disease mitigation back considerably.20 An estimated 500,000 more people died in 2020 from TB.21 The drastic shift in focus to COVID-19 care during this period will continue to have a profound impact on other diseases like these for many years post-pandemic.
Provider Experience and Mental Health Outcomes
The impact on provider experiences and mental health has been immense. One study of 510 healthcare providers (HCPs) and first responders found that occupational stress from the pandemic correlated with psychiatric symptoms, including depression, PTSD, insomnia, and generalized anxiety.3 Occupational stress also correlated with one’s likelihood to leave the medical field and trouble doing work they had once loved.3 Half of the healthcare workers surveyed indicated a decreased likelihood of staying in their current profession after the pandemic.3
Other studies have also looked at specific subspecialties and impact on trainees during the pandemic. In neurosurgery, for example, resident burnout is high, at 26.1%.9 Additionally, the lack of surgeries in the pandemic made 65.8% of neurosurgery residents anxious about meeting career milestones.9 Respiratory therapists, a highly impacted group, also experienced burnout, reporting higher levels in those who worked more in the ICU. Another study identified several themes in the concerns reported by healthcare workers during the pandemic era including “changes in personal life and enhanced negative affect,” “gaining experience, normalization, and adaptation to the pandemic,” and “mental health considerations.”22
Some studies have investigated ways to mitigate this dissatisfaction with the healthcare field post-pandemic. Intrapreneurship, reverse mentoring, and democratized learning all had a reported positive impact on employee experience and retention during this time.23 Intrapreneurship describes entrepreneurship within an existing organization, while reverse mentoring and democratized learning refer to newer employees teaching older employees and communicative learning on a breadth of topics. Other studies have examined the necessity of having mental health resources available, and that these resources need to be multi-stage and individualistic as well as specific to certain stressors HCPs faced during the pandemic.22
Conclusion and Future Directions
The COVID-19 pandemic had stark effects on the healthcare system, impacting resources and capacity, care of other diseases, and provider mental health and experiences.1-3 After the chaos of the pandemic, many questions remain. What needs to be done now by health systems and HCPs? How can we learn from the challenges and the effects on capacity to change the healthcare workflow in times of crisis and in the present? How do we mitigate the impact of the pandemic on diagnosis and management of diseases? And how do we continue to provide healthcare workers with proper mental health and professional resources now, not just in times of stress, and encourage the future generation to pursue careers in healthcare?
These are all the questions the pandemic has left us with, and more studies and initiatives are needed to investigate solutions to these issues. The COVID-19 pandemic left behind valuable lessons and changed the healthcare system, disease management, and staffing for many. Now is the time to pick up the pieces and strategize on how to make our existing system more effective for workers and patients post pandemic.
Wahlster S, Sharma M, Lewis AK, et al. The coronavirus disease 2019 pandemic's effect on critical care resources and health-care providers: a global survey. Chest. 2021;159(2):619-633. doi:10.1016/j.chest.2020.09.070
Malagón T, Yong JHE, Tope P, Miller WH Jr, Franco EL; McGill task force on the impact of COVID-19 on cancer control and care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer. 2022;150(8):1244-1254. doi:10.1002/ijc.33884
Hendrickson RC, Slevin RA, Hoerster KD, et al. The impact of the COVID-19 pandemic on mental health, occupational functioning, and professional retention among health care workers and first responders. J Gen Intern Med. 2022;37(2):397-408. doi:10.1007/s11606-021-07252-z
Davis B, Bankhead-Kendall BK, Dumas RP. A review of COVID-19's impact on modern medical systems from a health organization management perspective. Health Technol (Berl). 2022;12(4):815-824. doi:10.1007/s12553-022-00660-z
Rosenbaum L. The untold toll - the pandemic's effects on patients without COVID-19. N Engl J Med. 2020;382(24):2368-2371. doi:10.1056/NEJMms2009984
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: the impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Jalili M, Niroomand M, Hadavand F, Zeinali K, Fotouhi A. Burnout among healthcare professionals during COVID-19 pandemic: a cross-sectional study. Int Arch Occup Environ Health. 2021;94(6):1345-1352. doi:10.1007/s00420-021-01695-x
Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142. doi:10.1016/j.jocn.2020.08.012
Miller AG, Roberts KJ, Smith BJ, et al. Prevalence of burnout among respiratory therapists amidst the COVID-19 pandemic. Respir Care. 2021;respcare.09283. doi:10.4187/respcare.09283
Frogner BK, Dill JS. Tracking turnover among health care workers during the COVID-19 pandemic: a cross-sectional study. JAMA Health Forum. 2022;3(4):e220371. doi:10.1001/jamahealthforum.2022.0371
CDC COVID data tracker. Centers for Disease Control and Prevention. Accessed December 22, 2022. http://covid-data-tracker/#datatracker-home.
Barrett K, Khan YA, Mac S, Ximenes R, Naimark DMJ, Sander B. Estimation of COVID-19-induced depletion of hospital resources in Ontario, Canada. CMAJ. 2020;192(24):E640-E646. doi:10.1503/cmaj.200715
Kaul V, Chahal J, Schrarstzhaupt IN, et al. Lessons learned from a global perspective of COVID-19. Clin Chest Med. 2022 Nov. 24. [online ahead of print]. doi:10.1016/j.ccm.2022.11.020
Issaka RB, Somsouk M. Colorectal cancer screening and prevention in the COVID-19 Era. JAMA Health Forum. 2020;1(5):e200588. doi:10.1001/jamahealthforum.2020.0588
Freer PE. The impact of the COVID-19 pandemic on breast imaging. Radiol Clin North Am. 2021;59(1):1-11. doi:10.1016/j.rcl.2020.09.008
Alagoz O, Lowry KP, Kurian AW, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst. 2021;113(11):1484-1494. doi:10.1093/jnci/djab097
Jiménez-Blanco Bravo M, Cordero Pereda D, Sánchez Vega D, et al. Heart failure in the time of COVID-19. Cardiology. 2020;145(8):481-484. doi:10.1159/000509181
Frankfurter C, Buchan TA, Kobulnik J, et al. Reduced rate of hospital presentations for heart failure during the COVID-19 pandemic in Toronto, Canada. Can J Cardiol. 2020;36(10):1680-1684. doi:10.1016/j.cjca.2020.07.006
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: The impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Eftekhar Ardebili M, Naserbakht M, Bernstein C, Alazmani-Noodeh F, Hakimi H, Ranjbar H. Healthcare providers experience of working during the COVID-19 pandemic: a qualitative study. Am J Infect Control. 2021;49(5):547-554. doi:10.1016/j.ajic.2020.10.001
Jayathilake HD, Daud D, Eaw HC, Annuar N. Employee development and retention of generation-Z employees in the post-covid-19 workplace: a conceptual framework. Benchmarking: An International Journal. 2021;28(7):2343-2364. doi:10.1108/bij-06-2020-0311
Wahlster S, Sharma M, Lewis AK, et al. The coronavirus disease 2019 pandemic's effect on critical care resources and health-care providers: a global survey. Chest. 2021;159(2):619-633. doi:10.1016/j.chest.2020.09.070
Malagón T, Yong JHE, Tope P, Miller WH Jr, Franco EL; McGill task force on the impact of COVID-19 on cancer control and care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer. 2022;150(8):1244-1254. doi:10.1002/ijc.33884
Hendrickson RC, Slevin RA, Hoerster KD, et al. The impact of the COVID-19 pandemic on mental health, occupational functioning, and professional retention among health care workers and first responders. J Gen Intern Med. 2022;37(2):397-408. doi:10.1007/s11606-021-07252-z
Davis B, Bankhead-Kendall BK, Dumas RP. A review of COVID-19's impact on modern medical systems from a health organization management perspective. Health Technol (Berl). 2022;12(4):815-824. doi:10.1007/s12553-022-00660-z
Rosenbaum L. The untold toll - the pandemic's effects on patients without COVID-19. N Engl J Med. 2020;382(24):2368-2371. doi:10.1056/NEJMms2009984
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: the impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Jalili M, Niroomand M, Hadavand F, Zeinali K, Fotouhi A. Burnout among healthcare professionals during COVID-19 pandemic: a cross-sectional study. Int Arch Occup Environ Health. 2021;94(6):1345-1352. doi:10.1007/s00420-021-01695-x
Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142. doi:10.1016/j.jocn.2020.08.012
Miller AG, Roberts KJ, Smith BJ, et al. Prevalence of burnout among respiratory therapists amidst the COVID-19 pandemic. Respir Care. 2021;respcare.09283. doi:10.4187/respcare.09283
Frogner BK, Dill JS. Tracking turnover among health care workers during the COVID-19 pandemic: a cross-sectional study. JAMA Health Forum. 2022;3(4):e220371. doi:10.1001/jamahealthforum.2022.0371
CDC COVID data tracker. Centers for Disease Control and Prevention. Accessed December 22, 2022. http://covid-data-tracker/#datatracker-home.
Barrett K, Khan YA, Mac S, Ximenes R, Naimark DMJ, Sander B. Estimation of COVID-19-induced depletion of hospital resources in Ontario, Canada. CMAJ. 2020;192(24):E640-E646. doi:10.1503/cmaj.200715
Kaul V, Chahal J, Schrarstzhaupt IN, et al. Lessons learned from a global perspective of COVID-19. Clin Chest Med. 2022 Nov. 24. [online ahead of print]. doi:10.1016/j.ccm.2022.11.020
Issaka RB, Somsouk M. Colorectal cancer screening and prevention in the COVID-19 Era. JAMA Health Forum. 2020;1(5):e200588. doi:10.1001/jamahealthforum.2020.0588
Freer PE. The impact of the COVID-19 pandemic on breast imaging. Radiol Clin North Am. 2021;59(1):1-11. doi:10.1016/j.rcl.2020.09.008
Alagoz O, Lowry KP, Kurian AW, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst. 2021;113(11):1484-1494. doi:10.1093/jnci/djab097
Jiménez-Blanco Bravo M, Cordero Pereda D, Sánchez Vega D, et al. Heart failure in the time of COVID-19. Cardiology. 2020;145(8):481-484. doi:10.1159/000509181
Frankfurter C, Buchan TA, Kobulnik J, et al. Reduced rate of hospital presentations for heart failure during the COVID-19 pandemic in Toronto, Canada. Can J Cardiol. 2020;36(10):1680-1684. doi:10.1016/j.cjca.2020.07.006
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: The impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Eftekhar Ardebili M, Naserbakht M, Bernstein C, Alazmani-Noodeh F, Hakimi H, Ranjbar H. Healthcare providers experience of working during the COVID-19 pandemic: a qualitative study. Am J Infect Control. 2021;49(5):547-554. doi:10.1016/j.ajic.2020.10.001
Jayathilake HD, Daud D, Eaw HC, Annuar N. Employee development and retention of generation-Z employees in the post-covid-19 workplace: a conceptual framework. Benchmarking: An International Journal. 2021;28(7):2343-2364. doi:10.1108/bij-06-2020-0311
Protuberant, Pink, Irritated Growth on the Buttocks
The Diagnosis: Superficial Angiomyxoma
Superficial angiomyxoma is a rare, benign, cutaneous tumor of a myxoid matrix and blood vessels that was first described in association with Carney complex.1 Tumors may be solitary or multiple. A recent review of cases in the literature revealed a roughly equal distribution of superficial angiomyxomas in males and females occurring most frequently on the head and neck, extremities, and trunk or back. The peak incidence is between the fourth and fifth decades of life.2 Superficial angiomyxomas can occur sporadically or in association with Carney complex, an autosomal-dominant condition with germline inactivating mutations in protein kinase A, PRKAR1A. Interestingly, sporadic cases of superficial angiomyxoma also have shown loss of PRKAR1A expression on immunohistochemistry (IHC).3
Common histologic mimics of superficial angiomyxoma include aggressive angiomyxoma and angiomyofibroblastoma.4 It is thought that these 3 distinct tumor entities may arise from a common pluripotent cell of origin located near connective tissue vasculature, which may contribute to the similarities observed between them.5 For example, aggressive angiomyxomas and angiomyofibroblastomas also demonstrate a similar myxoid background and vascular proliferation that can closely mimic superficial angiomyxomas clinically. However, the vessels of superficial angiomyxomas tend to be long and thin walled, while aggressive angiomyxomas are characterized by large and thick-walled vessels and angiomyofibroblastomas by abundant smaller vessels. Additionally, unlike superficial angiomyxomas, both aggressive angiomyxomas and angiomyofibroblastomas typically occur in the genital tract of young to middle-aged women.6
Histopathologic examination is imperative for differentiating between superficial angiomyxoma and more aggressive histologic mimics. Superficial angiomyxomas typically consist of a rich myxoid stroma, thin-walled or arborizing blood vessels, and spindled to stellate fibroblastlike cells (quiz image 2).3 Although not prominent in our case, superficial angiomyxomas also frequently present with stromal neutrophils and epithelial components, including keratinous cysts, basaloid buds, and strands of squamous epithelium.7 Minimal cellular atypia, mitotic activity, and nuclear pleomorphism often are seen, with IHC negative for desmin, estrogen receptor, and progesterone receptor; positive for CD34 and smooth muscle actin; and variable for S-100 and muscle-specific actin. Although IHC has limited utility in the diagnosis of superficial angiomyxomas, it may be useful to rule out other differential diagnoses.2,3 Superficial angiomyxomas usually show fibroblastic stromal cells, proteoglycan matrix, and collagen fibers on electron microscopy.8 Importantly, histopathologic examination of aggressive angiomyxoma will comparatively present with more invasive, infiltrative, and less well-circumscribed tumors.9 Other differential diagnoses on histology may include neurofibroma, focal cutaneous mucinosis, spindle cell lipoma, and myxofibrosarcoma. Additional considerations include fibroepithelial polyp, nevus lipomatosis, angiomyxolipoma, and anetoderma.
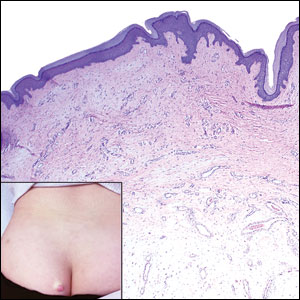
An important differential diagnosis in the evaluation of superficial angiomyxoma is neurofibroma, a benign peripheral nerve sheath tumor that presents as a smooth, flesh-colored, and painless papule or nodule commonly associated with the buttonhole sign. Histopathology of neurofibroma features elongated spindle cells with comma-shaped or buckled wavy nuclei and variably sized collagen bundles described as “shredded carrots” (Figure 1).10 Occasional mast cells also can be seen. Immunohistochemistry targeting elements of peripheral nerve sheaths may assist in the diagnosis of neurofibromas, including positive S-100 and SOX10 in Schwann cells, epithelial membrane antigen in perineural cells, and fingerprint positivity for CD34 in fibroblasts.10
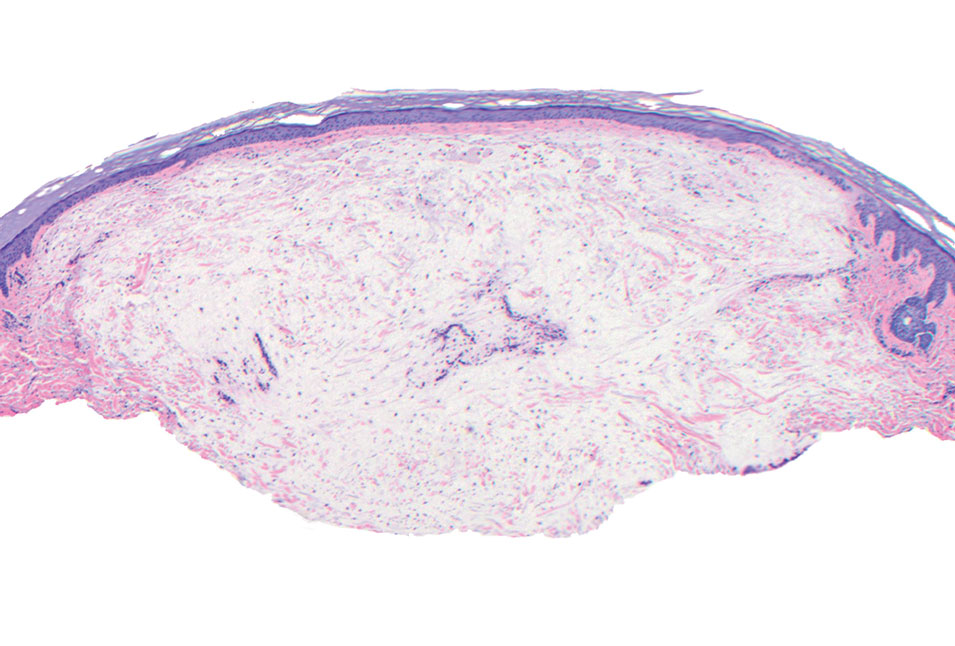
Cutaneous mucinoses encompass a diverse group of connective tissue disorders characterized by accumulation of mucin in the skin. Solitary focal cutaneous mucinoses (FCMs) are individual isolated lesions of mucin deposits that are unassociated with systemic conditions.11 Conversely, multiple FCMs presenting with multiple cutaneous lesions also have been described in association with systemic diseases such as scleroderma, systemic lupus erythematosus, and thyroid disease.12 Solitary FCM typically presents as an asymptomatic, flesh-colored papule or nodule on the extremities. It often arises in mid to late adulthood with a slightly increased frequency among males.12 Histopathology of solitary FCM commonly demonstrates a dome-shaped pool of basophilic mucin in the upper dermis sparing involvement of the underlying subcutaneous tissue (Figure 2).13 Notably, FCM often lacks the vascularity as well as stromal neutrophils and epithelial elements that are seen in superficial angiomyxomas. Although hematoxylin and eosin stains can be sufficient for diagnosis of solitary FCM, additional stains for mucin such as Alcian blue, colloidal iron, or toluidine blue also may be considered to support the diagnosis.12
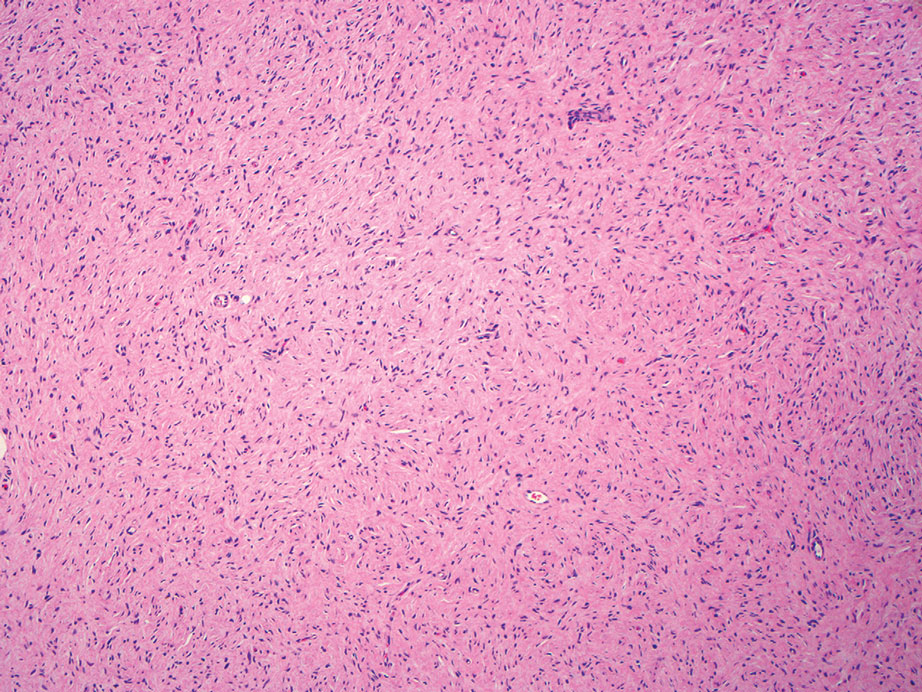
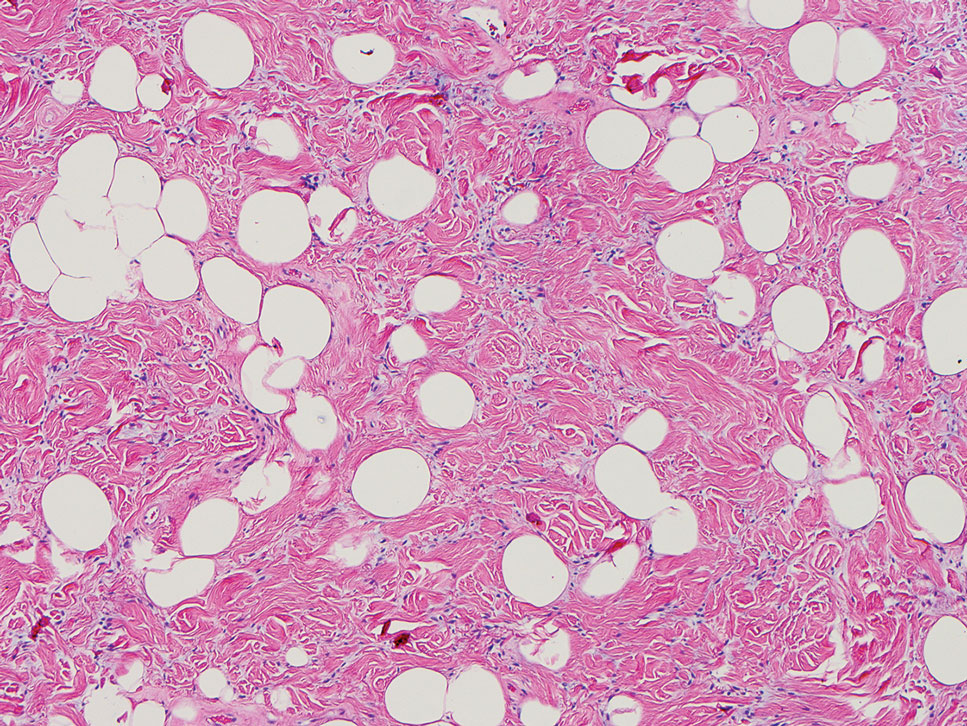
Spindle cell lipomas (SCLs) are rare, benign, subcutaneous, adipocytic tumors that arise on the upper back, posterior neck, or shoulders of middle-aged or elderly adult males.14 The clinical presentation often is an asymptomatic, well-circumscribed, mobile subcutaneous mass that is firmer than a common lipoma. Histologically, SCLs are characterized by mature adipocytes, spindle cells, and wire or ropelike collagen fibers in a myxoid background (Figure 3). The spindle cells usually are bland with a notable bipolar shape and blunted ends. Infiltrative growth patterns or mitotic figures are uncommon. Diagnosis can be supported by IHC, as SCLs stain diffusely positive for CD34 with loss of the retinoblastoma protein.7
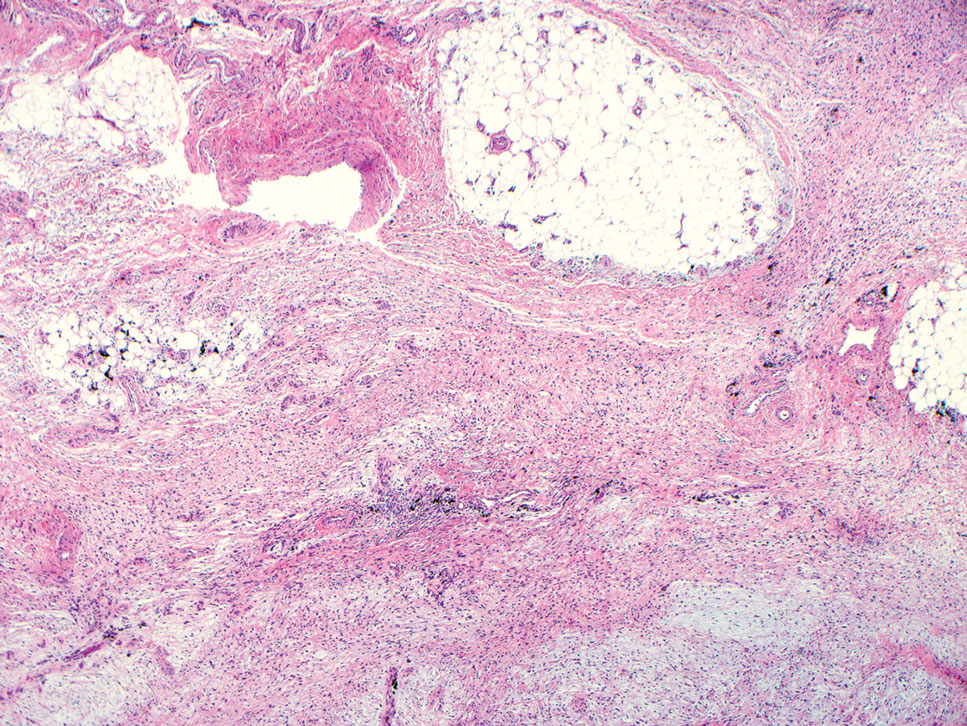
Another important differential diagnosis to consider is myxofibrosarcoma, a rare and malignant myxoid cutaneous tumor. Clinically, it presents asymptomatically as an indolent, slow-growing nodule on the limbs and limb girdles.7 Histopathologic features demonstrate a multilobular tumor composed of a mixture of hypocellular and hypercellular regions with incomplete fibrous septae (Figure 4). The presence of curvilinear vasculature is characteristic. Multinucleated giant cells and cellular atypia with nuclear pleomorphism also can be seen. Although IHC findings generally are not specific, they can be used to rule out other potential diagnoses. Myxofibrosarcomas stain positive for vimentin and occasionally smooth muscle actin, muscle-specific actin, and CD34.7
Superficial angiomyxomas are benign; however, excision is recommended to distinguish between mimics. Local recurrence after excision is common in 30% to 40% of patients.15 Mohs micrographic surgery has been considered, especially if the following are present: tumor characteristics (eg, poorly circumscribed), location (eg, head and neck or other cosmetically or functionally sensitive areas), and likelihood of recurrence (high for superficial angiomyxomas). 16 This case otherwise highlights a rare example of superficial angiomyxomas involving the buttocks.
- Allen PW, Dymock RB, MacCormac LB. Superficial angiomyxomas with and without epithelial components. report of 30 tumors in 28 patients. Am J Surg Pathol. 1988;12:519-530. doi:10.1097 /00000478-198807000-00003
- Sharma A, Khaitan N, Ko JS, et al. A clinicopathologic analysis of 54 cases of cutaneous myxoma. Hum Pathol. 2021:S0046-8177(21) 00201-X. doi:10.1016/j.humpath.2021.12.003
- Hafeez F, Krakowski AC, Lian CG, et al. Sporadic superficial angiomyxomas demonstrate loss of PRKAR1A expression [published online March 17, 2022]. Histopathology. 2022;80:1001-1003. doi:10.1111/his.14568
- Mehrotra K, Bhandari M, Khullar G, et al. Large superficial angiomyxoma of the vulva: report of two cases with varied clinical presentation. Indian Dermatol Online J. 2021;12:605-607. doi:10.4103/idoj.IDOJ_489_20
- Alameda F, Munné A, Baró T, et al. Vulvar angiomyxoma, aggressive angiomyxoma, and angiomyofibroblastoma: an immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2006;30:193-205. doi:10.1080/01913120500520911
- Haroon S, Irshad L, Zia S, et al. Aggressive angiomyxoma, angiomyofibroblastoma, and cellular angiofibroma of the lower female genital tract: related entities with different outcomes. Cureus. 2022;14:E29250. doi:10.7759/cureus.29250
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918. doi:10.1111/cup.12749
- Allen PW. Myxoma is not a single entity: a review of the concept of myxoma. Ann Diagn Pathol. 2000;4:99-123. doi:10.1016 /s1092-9134(00)90019-4
- Lee C-C, Chen Y-L, Liau J-Y, et al. Superficial angiomyxoma on the vulva of an adolescent. Taiwan J Obstet Gynecol. 2014;53:104-106. doi:10.1016/j.tjog.2013.08.001
- Magro G, Amico P, Vecchio GM, et al. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: a clinicopathologic study of 94 cases. Virchows Arch. 2010;456:71-76. doi:10.1007/s00428-009-0859-y
- Kuo KL, Lee LY, Kuo TT. Solitary cutaneous focal mucinosis: a clinicopathological study of 11 cases of soft fibroma-like cutaneous mucinous lesions. J Dermatol. 2017;44:335-338. doi:10.1111/1346-8138.13523
- Gutierrez N, Erickson C, Calame A, et al. Solitary cutaneous focal mucinosis. Cureus. 2021;13:E18618. doi:10.7759/cureus.18618
- Biondo G, Sola S, Pastorino C, et al. Clinical, dermoscopic, and histologic aspects of two cases of cutaneous focal mucinosis. An Bras Dermatol. 2019;94:334-336. doi:10.1590/abd1806-4841.20198381
- Chen S, Huang H, He S, et al. Spindle cell lipoma: clinicopathologic characterization of 40 cases. Int J Clin Exp Pathol. 2019;12:2613-2621.
- Bembem K, Jaiswal A, Singh M, et al. Cyto-histo correlation of a very rare tumor: superficial angiomyxoma. J Cytol. 2017;34:230-232. doi:10.4103/0970-9371.216119
- Aberdein G, Veitch D, Perrett C. Mohs micrographic surgery for the treatment of superficial angiomyxoma. Dermatol Surg. 2016;42: 1014-1016. doi:10.1097/DSS.0000000000000782
The Diagnosis: Superficial Angiomyxoma
Superficial angiomyxoma is a rare, benign, cutaneous tumor of a myxoid matrix and blood vessels that was first described in association with Carney complex.1 Tumors may be solitary or multiple. A recent review of cases in the literature revealed a roughly equal distribution of superficial angiomyxomas in males and females occurring most frequently on the head and neck, extremities, and trunk or back. The peak incidence is between the fourth and fifth decades of life.2 Superficial angiomyxomas can occur sporadically or in association with Carney complex, an autosomal-dominant condition with germline inactivating mutations in protein kinase A, PRKAR1A. Interestingly, sporadic cases of superficial angiomyxoma also have shown loss of PRKAR1A expression on immunohistochemistry (IHC).3
Common histologic mimics of superficial angiomyxoma include aggressive angiomyxoma and angiomyofibroblastoma.4 It is thought that these 3 distinct tumor entities may arise from a common pluripotent cell of origin located near connective tissue vasculature, which may contribute to the similarities observed between them.5 For example, aggressive angiomyxomas and angiomyofibroblastomas also demonstrate a similar myxoid background and vascular proliferation that can closely mimic superficial angiomyxomas clinically. However, the vessels of superficial angiomyxomas tend to be long and thin walled, while aggressive angiomyxomas are characterized by large and thick-walled vessels and angiomyofibroblastomas by abundant smaller vessels. Additionally, unlike superficial angiomyxomas, both aggressive angiomyxomas and angiomyofibroblastomas typically occur in the genital tract of young to middle-aged women.6
Histopathologic examination is imperative for differentiating between superficial angiomyxoma and more aggressive histologic mimics. Superficial angiomyxomas typically consist of a rich myxoid stroma, thin-walled or arborizing blood vessels, and spindled to stellate fibroblastlike cells (quiz image 2).3 Although not prominent in our case, superficial angiomyxomas also frequently present with stromal neutrophils and epithelial components, including keratinous cysts, basaloid buds, and strands of squamous epithelium.7 Minimal cellular atypia, mitotic activity, and nuclear pleomorphism often are seen, with IHC negative for desmin, estrogen receptor, and progesterone receptor; positive for CD34 and smooth muscle actin; and variable for S-100 and muscle-specific actin. Although IHC has limited utility in the diagnosis of superficial angiomyxomas, it may be useful to rule out other differential diagnoses.2,3 Superficial angiomyxomas usually show fibroblastic stromal cells, proteoglycan matrix, and collagen fibers on electron microscopy.8 Importantly, histopathologic examination of aggressive angiomyxoma will comparatively present with more invasive, infiltrative, and less well-circumscribed tumors.9 Other differential diagnoses on histology may include neurofibroma, focal cutaneous mucinosis, spindle cell lipoma, and myxofibrosarcoma. Additional considerations include fibroepithelial polyp, nevus lipomatosis, angiomyxolipoma, and anetoderma.
An important differential diagnosis in the evaluation of superficial angiomyxoma is neurofibroma, a benign peripheral nerve sheath tumor that presents as a smooth, flesh-colored, and painless papule or nodule commonly associated with the buttonhole sign. Histopathology of neurofibroma features elongated spindle cells with comma-shaped or buckled wavy nuclei and variably sized collagen bundles described as “shredded carrots” (Figure 1).10 Occasional mast cells also can be seen. Immunohistochemistry targeting elements of peripheral nerve sheaths may assist in the diagnosis of neurofibromas, including positive S-100 and SOX10 in Schwann cells, epithelial membrane antigen in perineural cells, and fingerprint positivity for CD34 in fibroblasts.10

Cutaneous mucinoses encompass a diverse group of connective tissue disorders characterized by accumulation of mucin in the skin. Solitary focal cutaneous mucinoses (FCMs) are individual isolated lesions of mucin deposits that are unassociated with systemic conditions.11 Conversely, multiple FCMs presenting with multiple cutaneous lesions also have been described in association with systemic diseases such as scleroderma, systemic lupus erythematosus, and thyroid disease.12 Solitary FCM typically presents as an asymptomatic, flesh-colored papule or nodule on the extremities. It often arises in mid to late adulthood with a slightly increased frequency among males.12 Histopathology of solitary FCM commonly demonstrates a dome-shaped pool of basophilic mucin in the upper dermis sparing involvement of the underlying subcutaneous tissue (Figure 2).13 Notably, FCM often lacks the vascularity as well as stromal neutrophils and epithelial elements that are seen in superficial angiomyxomas. Although hematoxylin and eosin stains can be sufficient for diagnosis of solitary FCM, additional stains for mucin such as Alcian blue, colloidal iron, or toluidine blue also may be considered to support the diagnosis.12

Spindle cell lipomas (SCLs) are rare, benign, subcutaneous, adipocytic tumors that arise on the upper back, posterior neck, or shoulders of middle-aged or elderly adult males.14 The clinical presentation often is an asymptomatic, well-circumscribed, mobile subcutaneous mass that is firmer than a common lipoma. Histologically, SCLs are characterized by mature adipocytes, spindle cells, and wire or ropelike collagen fibers in a myxoid background (Figure 3). The spindle cells usually are bland with a notable bipolar shape and blunted ends. Infiltrative growth patterns or mitotic figures are uncommon. Diagnosis can be supported by IHC, as SCLs stain diffusely positive for CD34 with loss of the retinoblastoma protein.7

Another important differential diagnosis to consider is myxofibrosarcoma, a rare and malignant myxoid cutaneous tumor. Clinically, it presents asymptomatically as an indolent, slow-growing nodule on the limbs and limb girdles.7 Histopathologic features demonstrate a multilobular tumor composed of a mixture of hypocellular and hypercellular regions with incomplete fibrous septae (Figure 4). The presence of curvilinear vasculature is characteristic. Multinucleated giant cells and cellular atypia with nuclear pleomorphism also can be seen. Although IHC findings generally are not specific, they can be used to rule out other potential diagnoses. Myxofibrosarcomas stain positive for vimentin and occasionally smooth muscle actin, muscle-specific actin, and CD34.7

Superficial angiomyxomas are benign; however, excision is recommended to distinguish between mimics. Local recurrence after excision is common in 30% to 40% of patients.15 Mohs micrographic surgery has been considered, especially if the following are present: tumor characteristics (eg, poorly circumscribed), location (eg, head and neck or other cosmetically or functionally sensitive areas), and likelihood of recurrence (high for superficial angiomyxomas). 16 This case otherwise highlights a rare example of superficial angiomyxomas involving the buttocks.
The Diagnosis: Superficial Angiomyxoma
Superficial angiomyxoma is a rare, benign, cutaneous tumor of a myxoid matrix and blood vessels that was first described in association with Carney complex.1 Tumors may be solitary or multiple. A recent review of cases in the literature revealed a roughly equal distribution of superficial angiomyxomas in males and females occurring most frequently on the head and neck, extremities, and trunk or back. The peak incidence is between the fourth and fifth decades of life.2 Superficial angiomyxomas can occur sporadically or in association with Carney complex, an autosomal-dominant condition with germline inactivating mutations in protein kinase A, PRKAR1A. Interestingly, sporadic cases of superficial angiomyxoma also have shown loss of PRKAR1A expression on immunohistochemistry (IHC).3
Common histologic mimics of superficial angiomyxoma include aggressive angiomyxoma and angiomyofibroblastoma.4 It is thought that these 3 distinct tumor entities may arise from a common pluripotent cell of origin located near connective tissue vasculature, which may contribute to the similarities observed between them.5 For example, aggressive angiomyxomas and angiomyofibroblastomas also demonstrate a similar myxoid background and vascular proliferation that can closely mimic superficial angiomyxomas clinically. However, the vessels of superficial angiomyxomas tend to be long and thin walled, while aggressive angiomyxomas are characterized by large and thick-walled vessels and angiomyofibroblastomas by abundant smaller vessels. Additionally, unlike superficial angiomyxomas, both aggressive angiomyxomas and angiomyofibroblastomas typically occur in the genital tract of young to middle-aged women.6
Histopathologic examination is imperative for differentiating between superficial angiomyxoma and more aggressive histologic mimics. Superficial angiomyxomas typically consist of a rich myxoid stroma, thin-walled or arborizing blood vessels, and spindled to stellate fibroblastlike cells (quiz image 2).3 Although not prominent in our case, superficial angiomyxomas also frequently present with stromal neutrophils and epithelial components, including keratinous cysts, basaloid buds, and strands of squamous epithelium.7 Minimal cellular atypia, mitotic activity, and nuclear pleomorphism often are seen, with IHC negative for desmin, estrogen receptor, and progesterone receptor; positive for CD34 and smooth muscle actin; and variable for S-100 and muscle-specific actin. Although IHC has limited utility in the diagnosis of superficial angiomyxomas, it may be useful to rule out other differential diagnoses.2,3 Superficial angiomyxomas usually show fibroblastic stromal cells, proteoglycan matrix, and collagen fibers on electron microscopy.8 Importantly, histopathologic examination of aggressive angiomyxoma will comparatively present with more invasive, infiltrative, and less well-circumscribed tumors.9 Other differential diagnoses on histology may include neurofibroma, focal cutaneous mucinosis, spindle cell lipoma, and myxofibrosarcoma. Additional considerations include fibroepithelial polyp, nevus lipomatosis, angiomyxolipoma, and anetoderma.
An important differential diagnosis in the evaluation of superficial angiomyxoma is neurofibroma, a benign peripheral nerve sheath tumor that presents as a smooth, flesh-colored, and painless papule or nodule commonly associated with the buttonhole sign. Histopathology of neurofibroma features elongated spindle cells with comma-shaped or buckled wavy nuclei and variably sized collagen bundles described as “shredded carrots” (Figure 1).10 Occasional mast cells also can be seen. Immunohistochemistry targeting elements of peripheral nerve sheaths may assist in the diagnosis of neurofibromas, including positive S-100 and SOX10 in Schwann cells, epithelial membrane antigen in perineural cells, and fingerprint positivity for CD34 in fibroblasts.10

Cutaneous mucinoses encompass a diverse group of connective tissue disorders characterized by accumulation of mucin in the skin. Solitary focal cutaneous mucinoses (FCMs) are individual isolated lesions of mucin deposits that are unassociated with systemic conditions.11 Conversely, multiple FCMs presenting with multiple cutaneous lesions also have been described in association with systemic diseases such as scleroderma, systemic lupus erythematosus, and thyroid disease.12 Solitary FCM typically presents as an asymptomatic, flesh-colored papule or nodule on the extremities. It often arises in mid to late adulthood with a slightly increased frequency among males.12 Histopathology of solitary FCM commonly demonstrates a dome-shaped pool of basophilic mucin in the upper dermis sparing involvement of the underlying subcutaneous tissue (Figure 2).13 Notably, FCM often lacks the vascularity as well as stromal neutrophils and epithelial elements that are seen in superficial angiomyxomas. Although hematoxylin and eosin stains can be sufficient for diagnosis of solitary FCM, additional stains for mucin such as Alcian blue, colloidal iron, or toluidine blue also may be considered to support the diagnosis.12

Spindle cell lipomas (SCLs) are rare, benign, subcutaneous, adipocytic tumors that arise on the upper back, posterior neck, or shoulders of middle-aged or elderly adult males.14 The clinical presentation often is an asymptomatic, well-circumscribed, mobile subcutaneous mass that is firmer than a common lipoma. Histologically, SCLs are characterized by mature adipocytes, spindle cells, and wire or ropelike collagen fibers in a myxoid background (Figure 3). The spindle cells usually are bland with a notable bipolar shape and blunted ends. Infiltrative growth patterns or mitotic figures are uncommon. Diagnosis can be supported by IHC, as SCLs stain diffusely positive for CD34 with loss of the retinoblastoma protein.7

Another important differential diagnosis to consider is myxofibrosarcoma, a rare and malignant myxoid cutaneous tumor. Clinically, it presents asymptomatically as an indolent, slow-growing nodule on the limbs and limb girdles.7 Histopathologic features demonstrate a multilobular tumor composed of a mixture of hypocellular and hypercellular regions with incomplete fibrous septae (Figure 4). The presence of curvilinear vasculature is characteristic. Multinucleated giant cells and cellular atypia with nuclear pleomorphism also can be seen. Although IHC findings generally are not specific, they can be used to rule out other potential diagnoses. Myxofibrosarcomas stain positive for vimentin and occasionally smooth muscle actin, muscle-specific actin, and CD34.7

Superficial angiomyxomas are benign; however, excision is recommended to distinguish between mimics. Local recurrence after excision is common in 30% to 40% of patients.15 Mohs micrographic surgery has been considered, especially if the following are present: tumor characteristics (eg, poorly circumscribed), location (eg, head and neck or other cosmetically or functionally sensitive areas), and likelihood of recurrence (high for superficial angiomyxomas). 16 This case otherwise highlights a rare example of superficial angiomyxomas involving the buttocks.
- Allen PW, Dymock RB, MacCormac LB. Superficial angiomyxomas with and without epithelial components. report of 30 tumors in 28 patients. Am J Surg Pathol. 1988;12:519-530. doi:10.1097 /00000478-198807000-00003
- Sharma A, Khaitan N, Ko JS, et al. A clinicopathologic analysis of 54 cases of cutaneous myxoma. Hum Pathol. 2021:S0046-8177(21) 00201-X. doi:10.1016/j.humpath.2021.12.003
- Hafeez F, Krakowski AC, Lian CG, et al. Sporadic superficial angiomyxomas demonstrate loss of PRKAR1A expression [published online March 17, 2022]. Histopathology. 2022;80:1001-1003. doi:10.1111/his.14568
- Mehrotra K, Bhandari M, Khullar G, et al. Large superficial angiomyxoma of the vulva: report of two cases with varied clinical presentation. Indian Dermatol Online J. 2021;12:605-607. doi:10.4103/idoj.IDOJ_489_20
- Alameda F, Munné A, Baró T, et al. Vulvar angiomyxoma, aggressive angiomyxoma, and angiomyofibroblastoma: an immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2006;30:193-205. doi:10.1080/01913120500520911
- Haroon S, Irshad L, Zia S, et al. Aggressive angiomyxoma, angiomyofibroblastoma, and cellular angiofibroma of the lower female genital tract: related entities with different outcomes. Cureus. 2022;14:E29250. doi:10.7759/cureus.29250
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918. doi:10.1111/cup.12749
- Allen PW. Myxoma is not a single entity: a review of the concept of myxoma. Ann Diagn Pathol. 2000;4:99-123. doi:10.1016 /s1092-9134(00)90019-4
- Lee C-C, Chen Y-L, Liau J-Y, et al. Superficial angiomyxoma on the vulva of an adolescent. Taiwan J Obstet Gynecol. 2014;53:104-106. doi:10.1016/j.tjog.2013.08.001
- Magro G, Amico P, Vecchio GM, et al. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: a clinicopathologic study of 94 cases. Virchows Arch. 2010;456:71-76. doi:10.1007/s00428-009-0859-y
- Kuo KL, Lee LY, Kuo TT. Solitary cutaneous focal mucinosis: a clinicopathological study of 11 cases of soft fibroma-like cutaneous mucinous lesions. J Dermatol. 2017;44:335-338. doi:10.1111/1346-8138.13523
- Gutierrez N, Erickson C, Calame A, et al. Solitary cutaneous focal mucinosis. Cureus. 2021;13:E18618. doi:10.7759/cureus.18618
- Biondo G, Sola S, Pastorino C, et al. Clinical, dermoscopic, and histologic aspects of two cases of cutaneous focal mucinosis. An Bras Dermatol. 2019;94:334-336. doi:10.1590/abd1806-4841.20198381
- Chen S, Huang H, He S, et al. Spindle cell lipoma: clinicopathologic characterization of 40 cases. Int J Clin Exp Pathol. 2019;12:2613-2621.
- Bembem K, Jaiswal A, Singh M, et al. Cyto-histo correlation of a very rare tumor: superficial angiomyxoma. J Cytol. 2017;34:230-232. doi:10.4103/0970-9371.216119
- Aberdein G, Veitch D, Perrett C. Mohs micrographic surgery for the treatment of superficial angiomyxoma. Dermatol Surg. 2016;42: 1014-1016. doi:10.1097/DSS.0000000000000782
- Allen PW, Dymock RB, MacCormac LB. Superficial angiomyxomas with and without epithelial components. report of 30 tumors in 28 patients. Am J Surg Pathol. 1988;12:519-530. doi:10.1097 /00000478-198807000-00003
- Sharma A, Khaitan N, Ko JS, et al. A clinicopathologic analysis of 54 cases of cutaneous myxoma. Hum Pathol. 2021:S0046-8177(21) 00201-X. doi:10.1016/j.humpath.2021.12.003
- Hafeez F, Krakowski AC, Lian CG, et al. Sporadic superficial angiomyxomas demonstrate loss of PRKAR1A expression [published online March 17, 2022]. Histopathology. 2022;80:1001-1003. doi:10.1111/his.14568
- Mehrotra K, Bhandari M, Khullar G, et al. Large superficial angiomyxoma of the vulva: report of two cases with varied clinical presentation. Indian Dermatol Online J. 2021;12:605-607. doi:10.4103/idoj.IDOJ_489_20
- Alameda F, Munné A, Baró T, et al. Vulvar angiomyxoma, aggressive angiomyxoma, and angiomyofibroblastoma: an immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2006;30:193-205. doi:10.1080/01913120500520911
- Haroon S, Irshad L, Zia S, et al. Aggressive angiomyxoma, angiomyofibroblastoma, and cellular angiofibroma of the lower female genital tract: related entities with different outcomes. Cureus. 2022;14:E29250. doi:10.7759/cureus.29250
- Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-918. doi:10.1111/cup.12749
- Allen PW. Myxoma is not a single entity: a review of the concept of myxoma. Ann Diagn Pathol. 2000;4:99-123. doi:10.1016 /s1092-9134(00)90019-4
- Lee C-C, Chen Y-L, Liau J-Y, et al. Superficial angiomyxoma on the vulva of an adolescent. Taiwan J Obstet Gynecol. 2014;53:104-106. doi:10.1016/j.tjog.2013.08.001
- Magro G, Amico P, Vecchio GM, et al. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: a clinicopathologic study of 94 cases. Virchows Arch. 2010;456:71-76. doi:10.1007/s00428-009-0859-y
- Kuo KL, Lee LY, Kuo TT. Solitary cutaneous focal mucinosis: a clinicopathological study of 11 cases of soft fibroma-like cutaneous mucinous lesions. J Dermatol. 2017;44:335-338. doi:10.1111/1346-8138.13523
- Gutierrez N, Erickson C, Calame A, et al. Solitary cutaneous focal mucinosis. Cureus. 2021;13:E18618. doi:10.7759/cureus.18618
- Biondo G, Sola S, Pastorino C, et al. Clinical, dermoscopic, and histologic aspects of two cases of cutaneous focal mucinosis. An Bras Dermatol. 2019;94:334-336. doi:10.1590/abd1806-4841.20198381
- Chen S, Huang H, He S, et al. Spindle cell lipoma: clinicopathologic characterization of 40 cases. Int J Clin Exp Pathol. 2019;12:2613-2621.
- Bembem K, Jaiswal A, Singh M, et al. Cyto-histo correlation of a very rare tumor: superficial angiomyxoma. J Cytol. 2017;34:230-232. doi:10.4103/0970-9371.216119
- Aberdein G, Veitch D, Perrett C. Mohs micrographic surgery for the treatment of superficial angiomyxoma. Dermatol Surg. 2016;42: 1014-1016. doi:10.1097/DSS.0000000000000782
A 25-year-old woman presented with an irritated growth on the left buttock of 6 months’ duration. The lesion had grown slowly over time and became irritated because of the constant rubbing on her clothing due to its location. Physical examination revealed a 1-cm, pink, protuberant, soft, dome-shaped nodule on the left upper medial buttock (inset). A biopsy was performed for diagnostic purposes.
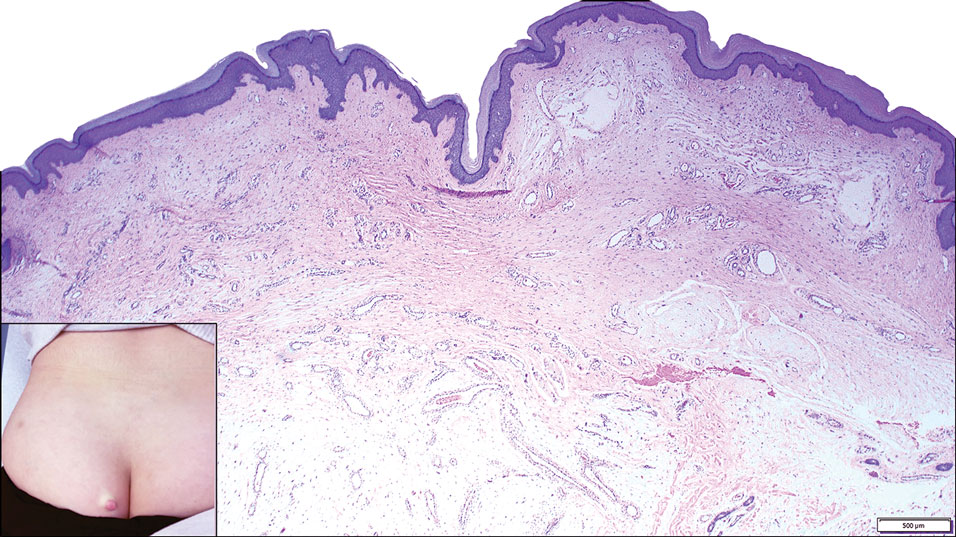
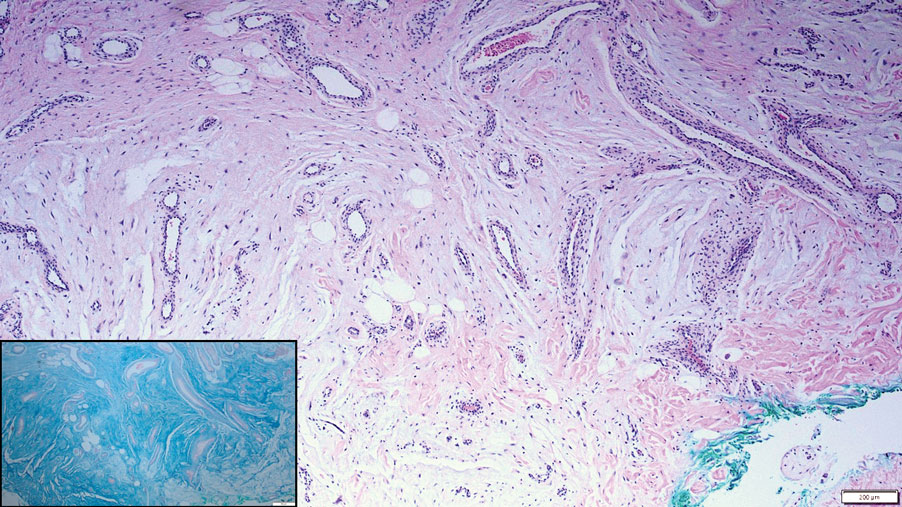
Dermatologic Implications of Sleep Deprivation in the US Military
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Practice Points
- Sleep deprivation may have negative effects on skin function and worsen dermatologic conditions.
- Proposed mechanisms of action for these negative effects include dysregulation of the hypothalamic-pituitary-adrenal axis, impairment of cutaneous barrier function, and alteration of cutaneous immune function.
- Members of the US Military are at an increased risk for sleep deprivation, especially during training and overseas deployments.
Severe Esophageal Lichen Planus Treated With Tofacitinib
To reach early diagnoses and improve outcomes in cases of mucosal and esophageal lichen planus (ELP), patient education along with a multidisciplinary approach centered on collaboration among dermatologists, gastroenterologists, gynecologists, and dental practitioners should be a priority. Tofacitinib therapy should be considered in the treatment of patients presenting with cutaneous lichen planus (CLP), mucosal lichen planus, and ELP.
Lichen planus is a papulosquamous disease of the skin and mucous membranes that is most common on the skin and oral mucosa. Typical lesions of CLP present as purple, pruritic, polygonal papules and plaques on the flexural surfaces of the wrists and ankles as well as areas of friction or trauma due to scratching such as the shins and lower back. Various subtypes of lichen planus can present simultaneously, resulting in extensive involvement that worsens through koebnerization and affects the oral cavity, esophagus, larynx, sclera, genitalia, scalp, and nails.1,2
Esophageal lichen planus can develop with or without the presence of CLP, oral lichen planus (OLP), or genital lichen planus.3 It typically affects women older than 50 years and is linked to OLP and vulvar lichen planus, with 1 study reporting that 87% (63/72) of ELP patients were women with a median age of 61.9 years at the time of diagnosis (range, 22–85 years). Almost all ELP patients in the study had lichen planus symptoms in other locations; 89% (64/72) had OLP, and 42% (30/72) had vulvar lichen planus.4 Consequently, a diagnosis of ELP should be followed by a thorough full-body examination to check for lichen planus at other sites. Studies that examined lichen planus patients for ELP found that 25% to 50% of patients diagnosed with orocutaneous lichen planus also had ELP, with ELP frequently presenting without symptoms.3,5 These findings indicate that ELP likely is underdiagnosed and often misdiagnosed, resulting in an underestimation of its prevalence.
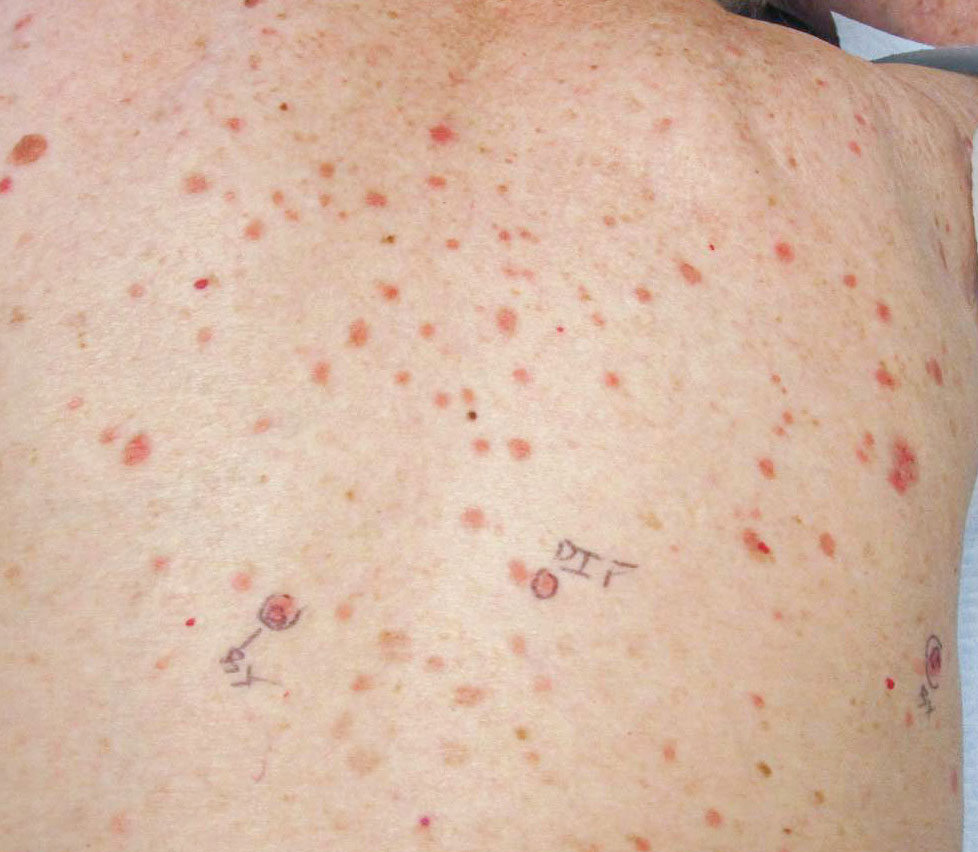
Our case highlights a frequently misdiagnosed condition and underscores the importance of close examination of patients presenting with CLP and OLP for signs and symptoms of ELP. Furthermore, we discuss the importance of patient education and collaboration among different specialties in attaining an early diagnosis to improve patient outcomes. Finally, we review the clinical presentation, diagnosis, and treatment of CLP, OLP, and ELP, as well as the utility of tofacitinib for ELP.
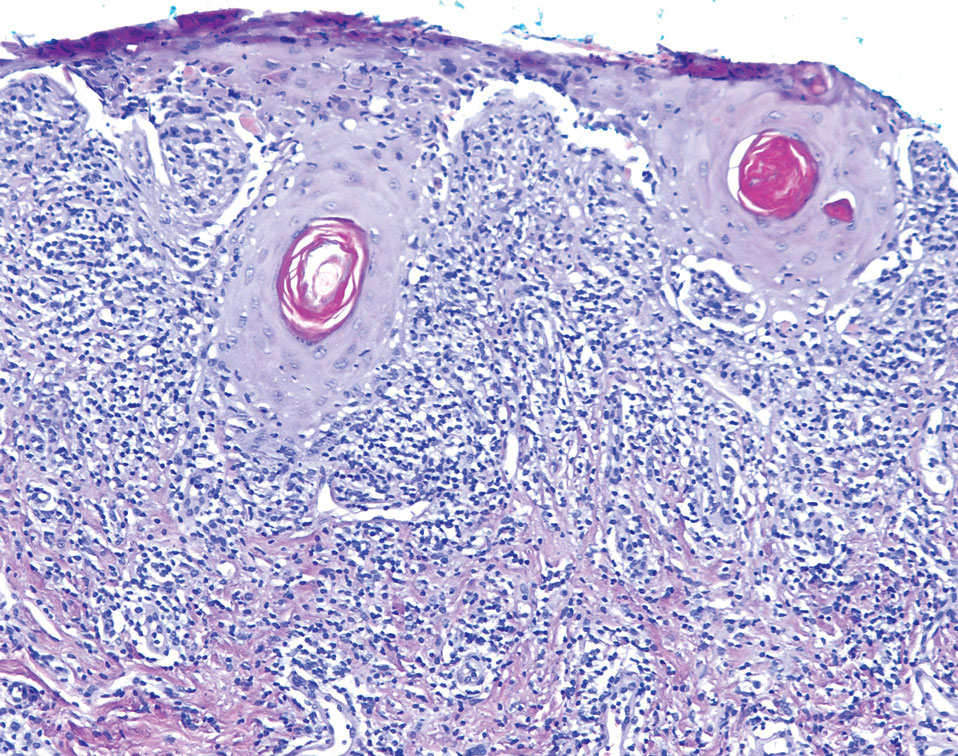
Case Report
An emaciated 89-year-old woman with an 11-year history of CLP, OLP, and genital lichen planus that had been successfully treated with topicals presented with an OLP recurrence alongside difficulties eating and swallowing. Her symptoms lasted 1 year and would recur when treatment was paused. Her medical history included rheumatoid arthritis, hypothyroidism, and hypertension, and she was taking levothyroxine, olmesartan, and vitamin D supplements. Dentures and olmesartan previously were ruled out as potential triggers following a 2-month elimination. None of her remaining natural teeth had fillings. She also reported that neither she nor her partner had ever smoked or chewed tobacco.
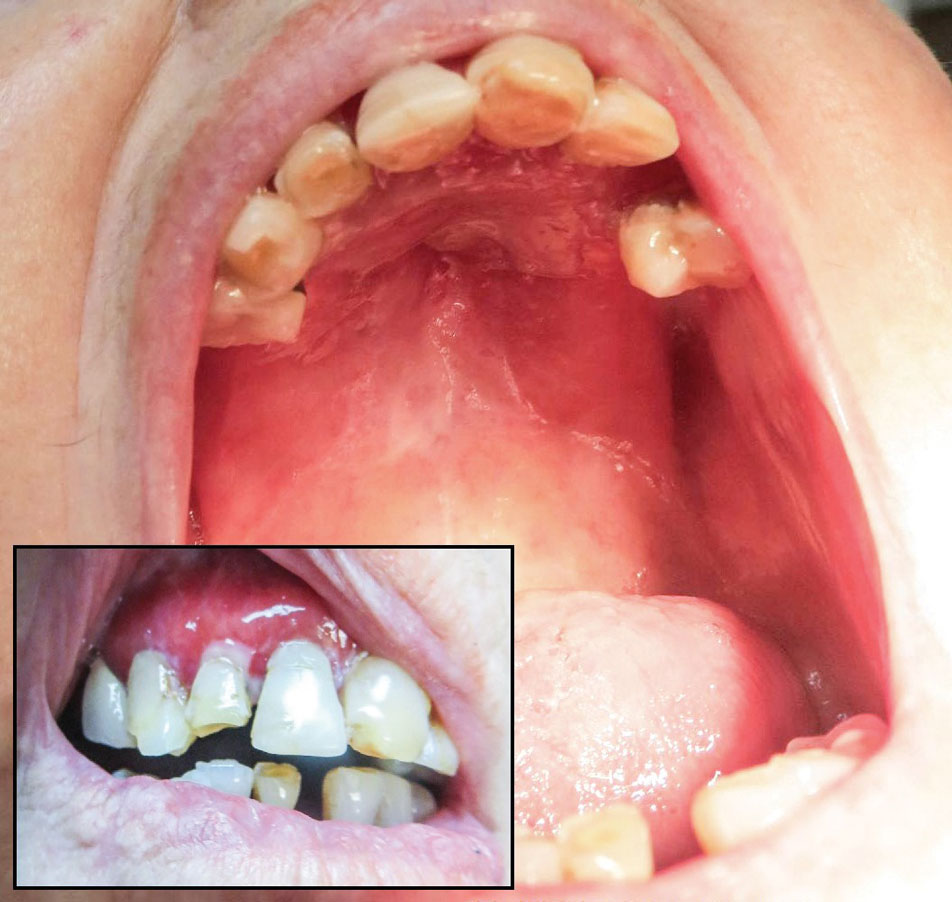
The patient’s lichen planus involvement first manifested as red, itchy, polygonal, lichenoid papules on the superior and inferior mid back 11 years prior to the current presentation (Figure 1). Further examination noted erosions on the genitalia, and a subsequent biopsy of the vulva confirmed a diagnosis of lichen planus (Figure 2). Treatment with halobetasol propionate ointment and tacrolimus ointment 0.1% twice daily (BID) resulted in remission of the CLP and vulvar lichen planus. She presented a year later with oral involvement revealing Wickham striae on the buccal mucosa and erosions on the upper palate that resolved after 2 months of treatment with cyclosporine oral solution mixed with a 5-times-daily nystatin swish-and-spit (Figure 3). The CLP did not recur but OLP was punctuated by remissions and recurrences on a yearly basis, often related to the cessation of mouthwash and topical creams. The OLP and vulvar lichen planus were successfully treated with as-needed use of a cyclosporine mouthwash swish-and-spit 3 times daily as well as halobetasol ointment 0.05% 3 times daily, respectively. Six years later, the patient was hospitalized for unrelated causes and was lost to follow-up for 2 years.
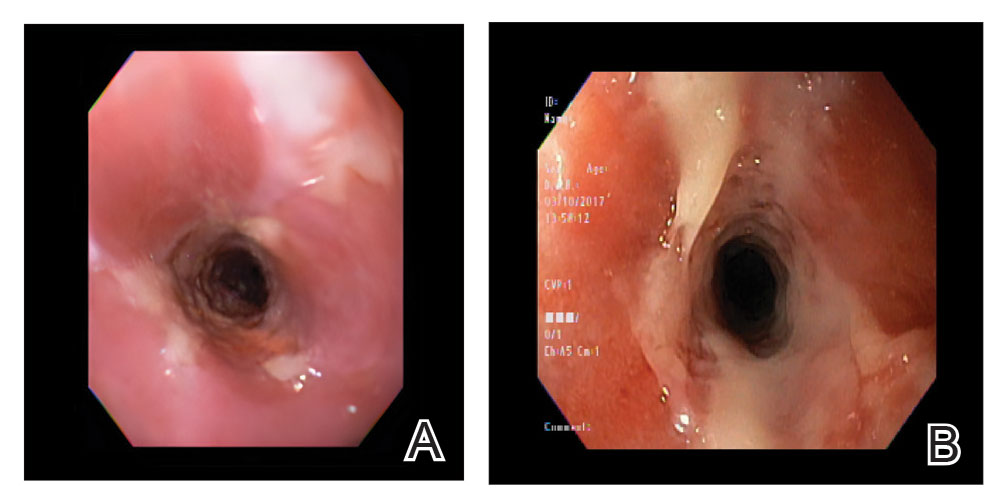
The patient experienced worsening dysphagia and odynophagia over a period of 2 years (mild dysphagia was first recorded 7 years prior to the initial presentation) and reported an unintentional weight loss of 20 pounds. An endoscopy was performed 3 years after the initial report of dysphagia and noted esophageal erosions (Figure 4A) and a stricture (Figure 4B), but all abnormal involvement was attributed to active gastroesophageal reflux disease. She underwent 8 esophageal dilations to treat the stricture but noted that the duration of symptomatic relief decreased with every subsequent dilation. An esophageal stent was placed 4 years after the initial concern of dysphagia, but it was not well tolerated and had to be removed soon thereafter. A year later, the patient underwent an esophageal bypass with a substernal gastric conduit that provided relief for 2 months but failed to permanently resolve the condition. In fact, her condition worsened over the next 1.5 years when she presented with extreme emaciation attributed to a low appetite and pain while eating. A review of the slides from a prior hospital esophageal biopsy revealed lichen planus (Figure 5). She was prescribed tofacitinib 5 mg BID as a dual-purpose treatment for the rheumatoid arthritis and OLP/ELP. At 1-month follow-up she noted that she had only taken one 5-mg pill daily without notable improvement, and after the visit she started the initial recommendation of 5 mg BID. Over the next several months, her condition continued to consistently improve; the odynophagia resolved, and she regained the majority of her lost weight. Tofacitinib was well tolerated across the course of treatment, and no adverse side effects were noted. Furthermore, the patient regained a full range of motion in the previously immobile arthritic right shoulder. She has experienced no recurrence of the genital lichen planus, OLP, or CLP since starting tofacitinib. To date, the patient is still taking only tofacitinib 5 mg BID with no recurrence of the cutaneous, mucosal, or esophageal lichen planus and has experienced no adverse events from the medication.
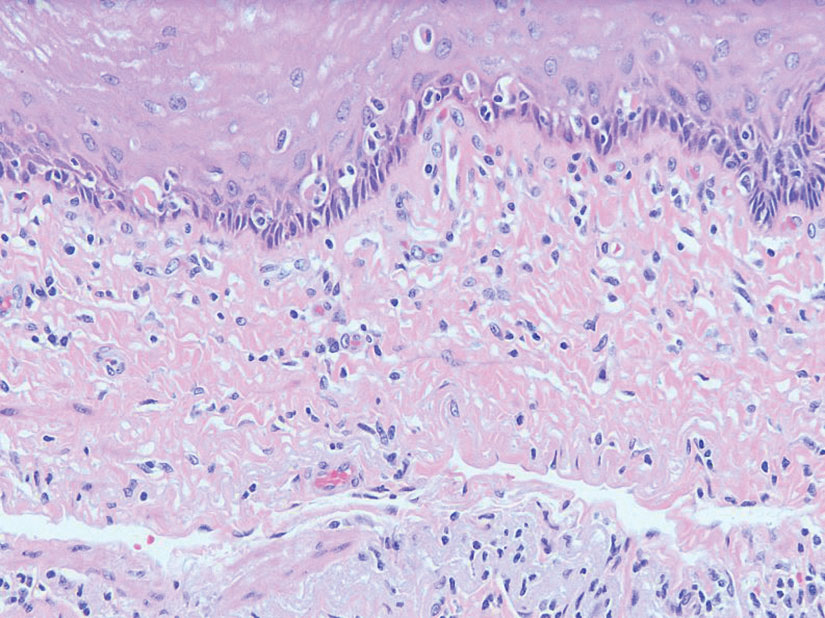
Comment
Clinical Presentation—Lichen planus—CLP and OLP—most frequently presents between the ages of 40 and 60 years, with a slight female predilection.1,2 The lesions typically present with the 5 P’s—purple, pruritic, polygonal papules and plaques—with some lesions revealing white lacy lines overlying them called Wickham striae.6 The lesions may be red at first before turning purple. They often present on the flexural surfaces of the wrists and ankles as well as the shins and back but rarely affect the face, perhaps because of increased chronic sun exposure.2,6 Less common locations include the scalp, nails, and mucosal areas (eg, oral, vulvar, conjunctival, laryngeal, esophageal, anal).1
If CLP is diagnosed, the patient likely will also have oral lesions, which occur in 50% of patients.2 Once any form of lichen planus is found, it is important to examine all of the most frequently involved locations—mucocutaneous and cutaneous as well as the nails and scalp. Special care should be taken when examining OLP and genital lichen planus, as long-standing lesions have a 2% to 5% chance of transforming into squamous cell carcinoma.2
Although cases of traditional OLP and CLP are ubiquitous in the literature, ELP rarely is documented because of frequent misdiagnoses. Esophageal lichen planus has a closer histopathologic resemblance to OLP compared to CLP, and its highly variable presentation often results in an inconclusive diagnosis.3 A review of 27 patients with lichen planus highlighted the difficult nature of diagnosing ELP; ELP manifested up to 20 years after initial lichen planus diagnosis, and patients underwent an average of 2.5 dilations prior to the successful diagnosis of ELP. Interestingly, 2 patients in the study presented with ELP in isolation, which emphasizes the importance of secondary examination for lichen planus in the presence of esophageal strictures.7 The eTable provides common patient demographics and symptoms to more effectively identify ELP.Differential Diagnosis—Because lichen planus can present anywhere on the body, it may be difficult to differentiate it from other skin conditions. Clinical appearance alone often is insufficient for diagnosing lichen planus, and a punch biopsy often is needed.2,20 Cutaneous lichen planus may resemble eczema, lichen simplex chronicus, pityriasis rosea, prurigo nodularis, and psoriasis, while OLP may resemble bite trauma, leukoplakia, pemphigus, and thrush.20 Dermoscopy of the tissue makes Wickham striae easier to visualize and assists in the diagnosis of lichen planus. Furthermore, thickening of the stratum granulosum, a prevalence of lymphocytes in the dermoepidermal junction, and vacuolar alteration of the stratum basale help to distinguish between lichen planus and other inflammatory dermatoses.20 A diagnosis of lichen planus merits a full-body skin examination—hair, nails, eyes, oral mucosa, and genitalia—to rule out additional involvement.
Esophageal lichen planus most frequently presents as dysphagia, odynophagia, and weight loss, but other symptoms including heartburn, hoarseness, choking, and epigastric pain may suggest esophageal involvement.4 Typically, ELP presents in the proximal and/or central esophagus, assisting in the differentiation between ELP and other esophageal conditions.3 Special consideration should be taken when both ELP and gastroesophageal reflux disease are considered in a differential diagnosis, and it is recommended to pair an upper endoscopy with pH monitoring to avoid misdiagnosis.8 Screening endoscopies also are helpful, as they assist in identifying the characteristic white webs, skin peeling, skin surface erosion, and strictures of ELP.4 Taken together, dermatologists should encourage patients with cutaneous or mucocutaneous lichen planus to undergo an esophagogastroduodenoscopy, especially in the presence of any of ELP’s common symptoms (eTable).
Etiology—Although the exact etiology of lichen planus is not well established, there are several known correlative factors, including hepatitis C; increased stress; dental materials; oral medications, most frequently antihypertensives and nonsteroidal anti-inflammatory drugs; systemic diseases; and tobacco usage.6,21
Dental materials used in oral treatments such as silver amalgam, gold, cobalt, palladium, chromium, epoxy resins, and dentures can trigger or exacerbate OLP, and patch testing of a patient’s dental materials can help determine if the reaction was caused by the materials.6,22 The removal of material contributing to lesions often will cause OLP to resolve.22
It also has been suggested that the presence of thyroid disorders, autoimmune disease, various cancers, hypertension, type 2 diabetes mellitus, hyperlipidemia, oral sedative usage, and/or vitamin D deficiency may be associated with OLP.21,23 Although OLP patients who were initially deficient in vitamin D demonstrated marked improvement with supplementation, it is unlikely that vitamin D supplements impacted our patient’s presentation of OLP, as she had been consistently taking them for more than 5 years with no change in OLP presentation.24
Pathogenesis—Lichen planus is thought to be a cytotoxic CD8+ T cell–mediated autoimmune disease to a virally modified epidermal self-antigen on keratinocytes. The cytotoxic T cells target the modified self-antigens on basal keratinocytes and induce apoptosis.25 The cytokine-mediated lymphocyte homing mechanism is human leukocyte antigen dependent and involves tumor necrosis factor α as well as IFN-γ and IL-1. The latter cytokines lead to upregulation of vascular adhesion molecules on endothelial vessels of subepithelial vascular plexus as well as a cascade of nonspecific mechanisms such as mast cell degranulation and matrix metalloproteinase activation, resulting in increased basement membrane disruption.6
Shao et al19 underscored the role of IFN-γ in CD8+ T cell–mediated cytotoxic cellular responses, noting that the Janus kinase (JAK)–signal transducer and activator of transcription pathway may play a key role in the pathogenesis of lichen planus. They proposed using JAK inhibitors for the treatment of lichen planus, specifically tofacitinib, a JAK1/JAK3 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor, as top therapeutic agents for lichen planus (eTable).19 Tofacitinib has been reported to successfully treat conditions such as psoriasis, psoriatic arthritis, alopecia areata, vitiligo, atopic dermatitis, sarcoidosis, pyoderma gangrenosum, and lichen planopilaris.26 Additionally, the efficacy of tofacitinib has been established in patients with erosive lichen planus; tofacitinib resulted in marked improvement while prednisone, acitretin, methotrexate, mycophenolate mofetil, and cyclosporine treatment failed.27 Although more studies on tofacitinib’s long-term efficacy, cost, and safety are necessary, tofacitinib may soon play an integral role in the battle against inflammatory dermatoses.
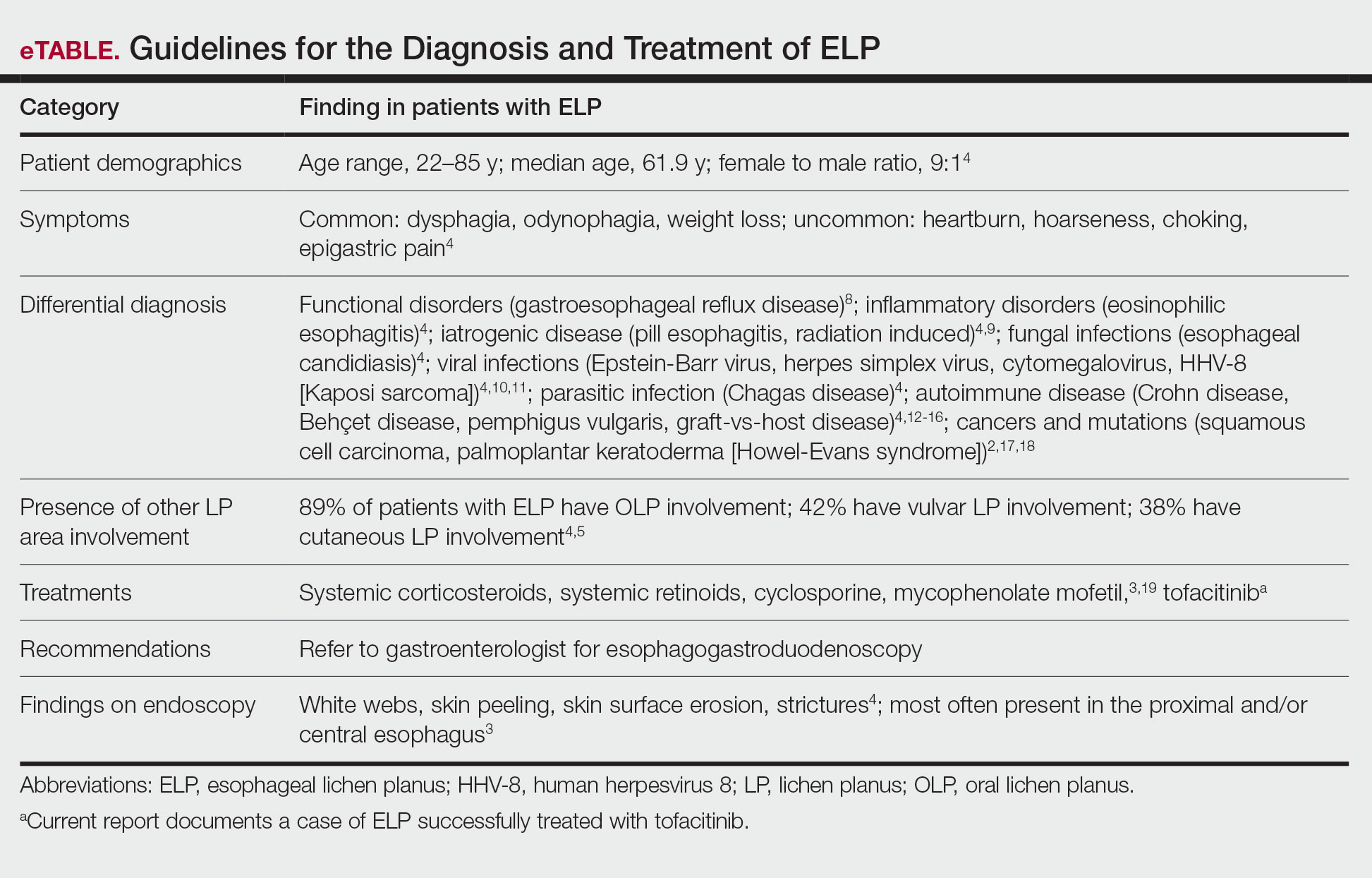
Conclusion
Esophageal lichen planus is an underreported form of lichen planus that often is misdiagnosed. It frequently causes dysphagia and odynophagia, resulting in a major decrease in a patient’s quality of life. We present the case of an 89-year-old woman who underwent procedures to dilate her esophagus that worsened her condition. We emphasize the importance of considering ELP in the differential diagnosis of patients presenting with lichen planus in another region. In our patient, tofacitinib 5 mg BID resolved her condition without any adverse effects.
- Le Cleach L, Chosidow O. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/nejmcp1103641
- Heath L, Matin R. Lichen planus. InnovAiT. 2017;10:133-138. doi:10.1177/1755738016686804
- Oliveira JP, Uribe NC, Abulafia LA, et al. Esophageal lichenplanus. An Bras Dermatol. 2015;90:394-396. doi:10.1590/abd1806-4841.20153255
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. doi:10.1016/j.jaad.2010.03.029
- Quispel R, van Boxel O, Schipper M, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. doi:10.1055/s-0028-1119590
- Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222-229. doi:10.4103/0019-5154.156315
- Katzka DA, Smyrk TC, Bruce AJ, et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. doi:10.1016/j.cgh.2010.04.024
- Abraham SC, Ravich WJ, Anhalt GJ, et al. Esophageal lichen planus. Am J Surg Pathol. 2000;24:1678-1682. doi:10.1097/00000478-200012000-00014
- Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827-830. doi:10.5858/arpa.2014-0111-RS
- Wilcox CM. Infectious esophagitis. Gastroenterol Hepatol (N Y). 2006;2:567-568.
- Cancio A, Cruz C. A case of Kaposi’s sarcoma of the esophagus presenting with odynophagia. Am J Gastroenterol. 2018;113:S995-S996.
- Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behçet’s disease. Patholog Res Int. 2012;2012:690390. doi:10.1155/2012/690390
- Madhusudhan KS, Sharma R. Esophageal lichen planus: a case report and review of literature. Indian J Dermatol. 2008;53:26-27. doi:10.4103/0019-5154.39738
- Bottomley WW, Dakkak M, Walton S, et al. Esophageal involvement in Behçet’s disease. is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. doi:10.1007/BF01307585
- McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-921.
- Trabulo D, Ferreira S, Lage P, et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-vs-host disease. World J Gastroenterol. 2015;21:9217-9222. doi:10.3748/wjg.v21.i30.9217
- Abbas H, Ghazanfar H, Ul Hussain AN, et al. Atypical presentation of esophageal squamous cell carcinoma masquerading as diffuse severe esophagitis. Case Rep Gastroenterol. 2021;15:533-538. doi:10.1159/000517129
- Ellis A, Risk JM, Maruthappu T, et al. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi:10.1186/s13023-015-0346-2
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11:eaav7561. doi:10.1126/scitranslmed.aav7561
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Dave A, Shariff J, Philipone E. Association between oral lichen planus and systemic conditions and medications: case-control study. Oral Dis. 2020;27:515-524. doi:10.1111/odi.13572
- Krupaa RJ, Sankari SL, Masthan KM, et al. Oral lichen planus: an overview. J Pharm Bioallied Sci. 2015;7(suppl 1):S158-S161. doi:10.4103/0975-7406.155873
- Tak MM, Chalkoo AH. Vitamin D deficiency—a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evolution Med Dent Sci. 2017;6:4769-4772. doi:10.14260/jemds/2017/1033
- Gupta J, Aggarwal A, Asadullah M, et al. Vitamin D in thetreatment of oral lichen planus: a pilot clinical study. J Indian Acad Oral Med Radiol. 2019;31:222-227. doi:10.4103/jiaomr.jiaomr_97_19
- Shiohara T, Moriya N, Mochizuki T, et al. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987;89:8-14.
- Sonthalia S, Aggarwal P. Oral tofacitinib: contemporary appraisal of its role in dermatology. Indian Dermatol Online J. 2019;10:503-518. doi:10.4103/idoj.idoj_474_18
- Damsky W, Wang A, Olamiju B, et al. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710.e2. doi:10.1016/j.jaci.2020.01.031
To reach early diagnoses and improve outcomes in cases of mucosal and esophageal lichen planus (ELP), patient education along with a multidisciplinary approach centered on collaboration among dermatologists, gastroenterologists, gynecologists, and dental practitioners should be a priority. Tofacitinib therapy should be considered in the treatment of patients presenting with cutaneous lichen planus (CLP), mucosal lichen planus, and ELP.
Lichen planus is a papulosquamous disease of the skin and mucous membranes that is most common on the skin and oral mucosa. Typical lesions of CLP present as purple, pruritic, polygonal papules and plaques on the flexural surfaces of the wrists and ankles as well as areas of friction or trauma due to scratching such as the shins and lower back. Various subtypes of lichen planus can present simultaneously, resulting in extensive involvement that worsens through koebnerization and affects the oral cavity, esophagus, larynx, sclera, genitalia, scalp, and nails.1,2
Esophageal lichen planus can develop with or without the presence of CLP, oral lichen planus (OLP), or genital lichen planus.3 It typically affects women older than 50 years and is linked to OLP and vulvar lichen planus, with 1 study reporting that 87% (63/72) of ELP patients were women with a median age of 61.9 years at the time of diagnosis (range, 22–85 years). Almost all ELP patients in the study had lichen planus symptoms in other locations; 89% (64/72) had OLP, and 42% (30/72) had vulvar lichen planus.4 Consequently, a diagnosis of ELP should be followed by a thorough full-body examination to check for lichen planus at other sites. Studies that examined lichen planus patients for ELP found that 25% to 50% of patients diagnosed with orocutaneous lichen planus also had ELP, with ELP frequently presenting without symptoms.3,5 These findings indicate that ELP likely is underdiagnosed and often misdiagnosed, resulting in an underestimation of its prevalence.

Our case highlights a frequently misdiagnosed condition and underscores the importance of close examination of patients presenting with CLP and OLP for signs and symptoms of ELP. Furthermore, we discuss the importance of patient education and collaboration among different specialties in attaining an early diagnosis to improve patient outcomes. Finally, we review the clinical presentation, diagnosis, and treatment of CLP, OLP, and ELP, as well as the utility of tofacitinib for ELP.

Case Report
An emaciated 89-year-old woman with an 11-year history of CLP, OLP, and genital lichen planus that had been successfully treated with topicals presented with an OLP recurrence alongside difficulties eating and swallowing. Her symptoms lasted 1 year and would recur when treatment was paused. Her medical history included rheumatoid arthritis, hypothyroidism, and hypertension, and she was taking levothyroxine, olmesartan, and vitamin D supplements. Dentures and olmesartan previously were ruled out as potential triggers following a 2-month elimination. None of her remaining natural teeth had fillings. She also reported that neither she nor her partner had ever smoked or chewed tobacco.

The patient’s lichen planus involvement first manifested as red, itchy, polygonal, lichenoid papules on the superior and inferior mid back 11 years prior to the current presentation (Figure 1). Further examination noted erosions on the genitalia, and a subsequent biopsy of the vulva confirmed a diagnosis of lichen planus (Figure 2). Treatment with halobetasol propionate ointment and tacrolimus ointment 0.1% twice daily (BID) resulted in remission of the CLP and vulvar lichen planus. She presented a year later with oral involvement revealing Wickham striae on the buccal mucosa and erosions on the upper palate that resolved after 2 months of treatment with cyclosporine oral solution mixed with a 5-times-daily nystatin swish-and-spit (Figure 3). The CLP did not recur but OLP was punctuated by remissions and recurrences on a yearly basis, often related to the cessation of mouthwash and topical creams. The OLP and vulvar lichen planus were successfully treated with as-needed use of a cyclosporine mouthwash swish-and-spit 3 times daily as well as halobetasol ointment 0.05% 3 times daily, respectively. Six years later, the patient was hospitalized for unrelated causes and was lost to follow-up for 2 years.

The patient experienced worsening dysphagia and odynophagia over a period of 2 years (mild dysphagia was first recorded 7 years prior to the initial presentation) and reported an unintentional weight loss of 20 pounds. An endoscopy was performed 3 years after the initial report of dysphagia and noted esophageal erosions (Figure 4A) and a stricture (Figure 4B), but all abnormal involvement was attributed to active gastroesophageal reflux disease. She underwent 8 esophageal dilations to treat the stricture but noted that the duration of symptomatic relief decreased with every subsequent dilation. An esophageal stent was placed 4 years after the initial concern of dysphagia, but it was not well tolerated and had to be removed soon thereafter. A year later, the patient underwent an esophageal bypass with a substernal gastric conduit that provided relief for 2 months but failed to permanently resolve the condition. In fact, her condition worsened over the next 1.5 years when she presented with extreme emaciation attributed to a low appetite and pain while eating. A review of the slides from a prior hospital esophageal biopsy revealed lichen planus (Figure 5). She was prescribed tofacitinib 5 mg BID as a dual-purpose treatment for the rheumatoid arthritis and OLP/ELP. At 1-month follow-up she noted that she had only taken one 5-mg pill daily without notable improvement, and after the visit she started the initial recommendation of 5 mg BID. Over the next several months, her condition continued to consistently improve; the odynophagia resolved, and she regained the majority of her lost weight. Tofacitinib was well tolerated across the course of treatment, and no adverse side effects were noted. Furthermore, the patient regained a full range of motion in the previously immobile arthritic right shoulder. She has experienced no recurrence of the genital lichen planus, OLP, or CLP since starting tofacitinib. To date, the patient is still taking only tofacitinib 5 mg BID with no recurrence of the cutaneous, mucosal, or esophageal lichen planus and has experienced no adverse events from the medication.

Comment
Clinical Presentation—Lichen planus—CLP and OLP—most frequently presents between the ages of 40 and 60 years, with a slight female predilection.1,2 The lesions typically present with the 5 P’s—purple, pruritic, polygonal papules and plaques—with some lesions revealing white lacy lines overlying them called Wickham striae.6 The lesions may be red at first before turning purple. They often present on the flexural surfaces of the wrists and ankles as well as the shins and back but rarely affect the face, perhaps because of increased chronic sun exposure.2,6 Less common locations include the scalp, nails, and mucosal areas (eg, oral, vulvar, conjunctival, laryngeal, esophageal, anal).1
If CLP is diagnosed, the patient likely will also have oral lesions, which occur in 50% of patients.2 Once any form of lichen planus is found, it is important to examine all of the most frequently involved locations—mucocutaneous and cutaneous as well as the nails and scalp. Special care should be taken when examining OLP and genital lichen planus, as long-standing lesions have a 2% to 5% chance of transforming into squamous cell carcinoma.2
Although cases of traditional OLP and CLP are ubiquitous in the literature, ELP rarely is documented because of frequent misdiagnoses. Esophageal lichen planus has a closer histopathologic resemblance to OLP compared to CLP, and its highly variable presentation often results in an inconclusive diagnosis.3 A review of 27 patients with lichen planus highlighted the difficult nature of diagnosing ELP; ELP manifested up to 20 years after initial lichen planus diagnosis, and patients underwent an average of 2.5 dilations prior to the successful diagnosis of ELP. Interestingly, 2 patients in the study presented with ELP in isolation, which emphasizes the importance of secondary examination for lichen planus in the presence of esophageal strictures.7 The eTable provides common patient demographics and symptoms to more effectively identify ELP.Differential Diagnosis—Because lichen planus can present anywhere on the body, it may be difficult to differentiate it from other skin conditions. Clinical appearance alone often is insufficient for diagnosing lichen planus, and a punch biopsy often is needed.2,20 Cutaneous lichen planus may resemble eczema, lichen simplex chronicus, pityriasis rosea, prurigo nodularis, and psoriasis, while OLP may resemble bite trauma, leukoplakia, pemphigus, and thrush.20 Dermoscopy of the tissue makes Wickham striae easier to visualize and assists in the diagnosis of lichen planus. Furthermore, thickening of the stratum granulosum, a prevalence of lymphocytes in the dermoepidermal junction, and vacuolar alteration of the stratum basale help to distinguish between lichen planus and other inflammatory dermatoses.20 A diagnosis of lichen planus merits a full-body skin examination—hair, nails, eyes, oral mucosa, and genitalia—to rule out additional involvement.
Esophageal lichen planus most frequently presents as dysphagia, odynophagia, and weight loss, but other symptoms including heartburn, hoarseness, choking, and epigastric pain may suggest esophageal involvement.4 Typically, ELP presents in the proximal and/or central esophagus, assisting in the differentiation between ELP and other esophageal conditions.3 Special consideration should be taken when both ELP and gastroesophageal reflux disease are considered in a differential diagnosis, and it is recommended to pair an upper endoscopy with pH monitoring to avoid misdiagnosis.8 Screening endoscopies also are helpful, as they assist in identifying the characteristic white webs, skin peeling, skin surface erosion, and strictures of ELP.4 Taken together, dermatologists should encourage patients with cutaneous or mucocutaneous lichen planus to undergo an esophagogastroduodenoscopy, especially in the presence of any of ELP’s common symptoms (eTable).
Etiology—Although the exact etiology of lichen planus is not well established, there are several known correlative factors, including hepatitis C; increased stress; dental materials; oral medications, most frequently antihypertensives and nonsteroidal anti-inflammatory drugs; systemic diseases; and tobacco usage.6,21
Dental materials used in oral treatments such as silver amalgam, gold, cobalt, palladium, chromium, epoxy resins, and dentures can trigger or exacerbate OLP, and patch testing of a patient’s dental materials can help determine if the reaction was caused by the materials.6,22 The removal of material contributing to lesions often will cause OLP to resolve.22
It also has been suggested that the presence of thyroid disorders, autoimmune disease, various cancers, hypertension, type 2 diabetes mellitus, hyperlipidemia, oral sedative usage, and/or vitamin D deficiency may be associated with OLP.21,23 Although OLP patients who were initially deficient in vitamin D demonstrated marked improvement with supplementation, it is unlikely that vitamin D supplements impacted our patient’s presentation of OLP, as she had been consistently taking them for more than 5 years with no change in OLP presentation.24
Pathogenesis—Lichen planus is thought to be a cytotoxic CD8+ T cell–mediated autoimmune disease to a virally modified epidermal self-antigen on keratinocytes. The cytotoxic T cells target the modified self-antigens on basal keratinocytes and induce apoptosis.25 The cytokine-mediated lymphocyte homing mechanism is human leukocyte antigen dependent and involves tumor necrosis factor α as well as IFN-γ and IL-1. The latter cytokines lead to upregulation of vascular adhesion molecules on endothelial vessels of subepithelial vascular plexus as well as a cascade of nonspecific mechanisms such as mast cell degranulation and matrix metalloproteinase activation, resulting in increased basement membrane disruption.6
Shao et al19 underscored the role of IFN-γ in CD8+ T cell–mediated cytotoxic cellular responses, noting that the Janus kinase (JAK)–signal transducer and activator of transcription pathway may play a key role in the pathogenesis of lichen planus. They proposed using JAK inhibitors for the treatment of lichen planus, specifically tofacitinib, a JAK1/JAK3 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor, as top therapeutic agents for lichen planus (eTable).19 Tofacitinib has been reported to successfully treat conditions such as psoriasis, psoriatic arthritis, alopecia areata, vitiligo, atopic dermatitis, sarcoidosis, pyoderma gangrenosum, and lichen planopilaris.26 Additionally, the efficacy of tofacitinib has been established in patients with erosive lichen planus; tofacitinib resulted in marked improvement while prednisone, acitretin, methotrexate, mycophenolate mofetil, and cyclosporine treatment failed.27 Although more studies on tofacitinib’s long-term efficacy, cost, and safety are necessary, tofacitinib may soon play an integral role in the battle against inflammatory dermatoses.

Conclusion
Esophageal lichen planus is an underreported form of lichen planus that often is misdiagnosed. It frequently causes dysphagia and odynophagia, resulting in a major decrease in a patient’s quality of life. We present the case of an 89-year-old woman who underwent procedures to dilate her esophagus that worsened her condition. We emphasize the importance of considering ELP in the differential diagnosis of patients presenting with lichen planus in another region. In our patient, tofacitinib 5 mg BID resolved her condition without any adverse effects.
To reach early diagnoses and improve outcomes in cases of mucosal and esophageal lichen planus (ELP), patient education along with a multidisciplinary approach centered on collaboration among dermatologists, gastroenterologists, gynecologists, and dental practitioners should be a priority. Tofacitinib therapy should be considered in the treatment of patients presenting with cutaneous lichen planus (CLP), mucosal lichen planus, and ELP.
Lichen planus is a papulosquamous disease of the skin and mucous membranes that is most common on the skin and oral mucosa. Typical lesions of CLP present as purple, pruritic, polygonal papules and plaques on the flexural surfaces of the wrists and ankles as well as areas of friction or trauma due to scratching such as the shins and lower back. Various subtypes of lichen planus can present simultaneously, resulting in extensive involvement that worsens through koebnerization and affects the oral cavity, esophagus, larynx, sclera, genitalia, scalp, and nails.1,2
Esophageal lichen planus can develop with or without the presence of CLP, oral lichen planus (OLP), or genital lichen planus.3 It typically affects women older than 50 years and is linked to OLP and vulvar lichen planus, with 1 study reporting that 87% (63/72) of ELP patients were women with a median age of 61.9 years at the time of diagnosis (range, 22–85 years). Almost all ELP patients in the study had lichen planus symptoms in other locations; 89% (64/72) had OLP, and 42% (30/72) had vulvar lichen planus.4 Consequently, a diagnosis of ELP should be followed by a thorough full-body examination to check for lichen planus at other sites. Studies that examined lichen planus patients for ELP found that 25% to 50% of patients diagnosed with orocutaneous lichen planus also had ELP, with ELP frequently presenting without symptoms.3,5 These findings indicate that ELP likely is underdiagnosed and often misdiagnosed, resulting in an underestimation of its prevalence.

Our case highlights a frequently misdiagnosed condition and underscores the importance of close examination of patients presenting with CLP and OLP for signs and symptoms of ELP. Furthermore, we discuss the importance of patient education and collaboration among different specialties in attaining an early diagnosis to improve patient outcomes. Finally, we review the clinical presentation, diagnosis, and treatment of CLP, OLP, and ELP, as well as the utility of tofacitinib for ELP.

Case Report
An emaciated 89-year-old woman with an 11-year history of CLP, OLP, and genital lichen planus that had been successfully treated with topicals presented with an OLP recurrence alongside difficulties eating and swallowing. Her symptoms lasted 1 year and would recur when treatment was paused. Her medical history included rheumatoid arthritis, hypothyroidism, and hypertension, and she was taking levothyroxine, olmesartan, and vitamin D supplements. Dentures and olmesartan previously were ruled out as potential triggers following a 2-month elimination. None of her remaining natural teeth had fillings. She also reported that neither she nor her partner had ever smoked or chewed tobacco.

The patient’s lichen planus involvement first manifested as red, itchy, polygonal, lichenoid papules on the superior and inferior mid back 11 years prior to the current presentation (Figure 1). Further examination noted erosions on the genitalia, and a subsequent biopsy of the vulva confirmed a diagnosis of lichen planus (Figure 2). Treatment with halobetasol propionate ointment and tacrolimus ointment 0.1% twice daily (BID) resulted in remission of the CLP and vulvar lichen planus. She presented a year later with oral involvement revealing Wickham striae on the buccal mucosa and erosions on the upper palate that resolved after 2 months of treatment with cyclosporine oral solution mixed with a 5-times-daily nystatin swish-and-spit (Figure 3). The CLP did not recur but OLP was punctuated by remissions and recurrences on a yearly basis, often related to the cessation of mouthwash and topical creams. The OLP and vulvar lichen planus were successfully treated with as-needed use of a cyclosporine mouthwash swish-and-spit 3 times daily as well as halobetasol ointment 0.05% 3 times daily, respectively. Six years later, the patient was hospitalized for unrelated causes and was lost to follow-up for 2 years.

The patient experienced worsening dysphagia and odynophagia over a period of 2 years (mild dysphagia was first recorded 7 years prior to the initial presentation) and reported an unintentional weight loss of 20 pounds. An endoscopy was performed 3 years after the initial report of dysphagia and noted esophageal erosions (Figure 4A) and a stricture (Figure 4B), but all abnormal involvement was attributed to active gastroesophageal reflux disease. She underwent 8 esophageal dilations to treat the stricture but noted that the duration of symptomatic relief decreased with every subsequent dilation. An esophageal stent was placed 4 years after the initial concern of dysphagia, but it was not well tolerated and had to be removed soon thereafter. A year later, the patient underwent an esophageal bypass with a substernal gastric conduit that provided relief for 2 months but failed to permanently resolve the condition. In fact, her condition worsened over the next 1.5 years when she presented with extreme emaciation attributed to a low appetite and pain while eating. A review of the slides from a prior hospital esophageal biopsy revealed lichen planus (Figure 5). She was prescribed tofacitinib 5 mg BID as a dual-purpose treatment for the rheumatoid arthritis and OLP/ELP. At 1-month follow-up she noted that she had only taken one 5-mg pill daily without notable improvement, and after the visit she started the initial recommendation of 5 mg BID. Over the next several months, her condition continued to consistently improve; the odynophagia resolved, and she regained the majority of her lost weight. Tofacitinib was well tolerated across the course of treatment, and no adverse side effects were noted. Furthermore, the patient regained a full range of motion in the previously immobile arthritic right shoulder. She has experienced no recurrence of the genital lichen planus, OLP, or CLP since starting tofacitinib. To date, the patient is still taking only tofacitinib 5 mg BID with no recurrence of the cutaneous, mucosal, or esophageal lichen planus and has experienced no adverse events from the medication.

Comment
Clinical Presentation—Lichen planus—CLP and OLP—most frequently presents between the ages of 40 and 60 years, with a slight female predilection.1,2 The lesions typically present with the 5 P’s—purple, pruritic, polygonal papules and plaques—with some lesions revealing white lacy lines overlying them called Wickham striae.6 The lesions may be red at first before turning purple. They often present on the flexural surfaces of the wrists and ankles as well as the shins and back but rarely affect the face, perhaps because of increased chronic sun exposure.2,6 Less common locations include the scalp, nails, and mucosal areas (eg, oral, vulvar, conjunctival, laryngeal, esophageal, anal).1
If CLP is diagnosed, the patient likely will also have oral lesions, which occur in 50% of patients.2 Once any form of lichen planus is found, it is important to examine all of the most frequently involved locations—mucocutaneous and cutaneous as well as the nails and scalp. Special care should be taken when examining OLP and genital lichen planus, as long-standing lesions have a 2% to 5% chance of transforming into squamous cell carcinoma.2
Although cases of traditional OLP and CLP are ubiquitous in the literature, ELP rarely is documented because of frequent misdiagnoses. Esophageal lichen planus has a closer histopathologic resemblance to OLP compared to CLP, and its highly variable presentation often results in an inconclusive diagnosis.3 A review of 27 patients with lichen planus highlighted the difficult nature of diagnosing ELP; ELP manifested up to 20 years after initial lichen planus diagnosis, and patients underwent an average of 2.5 dilations prior to the successful diagnosis of ELP. Interestingly, 2 patients in the study presented with ELP in isolation, which emphasizes the importance of secondary examination for lichen planus in the presence of esophageal strictures.7 The eTable provides common patient demographics and symptoms to more effectively identify ELP.Differential Diagnosis—Because lichen planus can present anywhere on the body, it may be difficult to differentiate it from other skin conditions. Clinical appearance alone often is insufficient for diagnosing lichen planus, and a punch biopsy often is needed.2,20 Cutaneous lichen planus may resemble eczema, lichen simplex chronicus, pityriasis rosea, prurigo nodularis, and psoriasis, while OLP may resemble bite trauma, leukoplakia, pemphigus, and thrush.20 Dermoscopy of the tissue makes Wickham striae easier to visualize and assists in the diagnosis of lichen planus. Furthermore, thickening of the stratum granulosum, a prevalence of lymphocytes in the dermoepidermal junction, and vacuolar alteration of the stratum basale help to distinguish between lichen planus and other inflammatory dermatoses.20 A diagnosis of lichen planus merits a full-body skin examination—hair, nails, eyes, oral mucosa, and genitalia—to rule out additional involvement.
Esophageal lichen planus most frequently presents as dysphagia, odynophagia, and weight loss, but other symptoms including heartburn, hoarseness, choking, and epigastric pain may suggest esophageal involvement.4 Typically, ELP presents in the proximal and/or central esophagus, assisting in the differentiation between ELP and other esophageal conditions.3 Special consideration should be taken when both ELP and gastroesophageal reflux disease are considered in a differential diagnosis, and it is recommended to pair an upper endoscopy with pH monitoring to avoid misdiagnosis.8 Screening endoscopies also are helpful, as they assist in identifying the characteristic white webs, skin peeling, skin surface erosion, and strictures of ELP.4 Taken together, dermatologists should encourage patients with cutaneous or mucocutaneous lichen planus to undergo an esophagogastroduodenoscopy, especially in the presence of any of ELP’s common symptoms (eTable).
Etiology—Although the exact etiology of lichen planus is not well established, there are several known correlative factors, including hepatitis C; increased stress; dental materials; oral medications, most frequently antihypertensives and nonsteroidal anti-inflammatory drugs; systemic diseases; and tobacco usage.6,21
Dental materials used in oral treatments such as silver amalgam, gold, cobalt, palladium, chromium, epoxy resins, and dentures can trigger or exacerbate OLP, and patch testing of a patient’s dental materials can help determine if the reaction was caused by the materials.6,22 The removal of material contributing to lesions often will cause OLP to resolve.22
It also has been suggested that the presence of thyroid disorders, autoimmune disease, various cancers, hypertension, type 2 diabetes mellitus, hyperlipidemia, oral sedative usage, and/or vitamin D deficiency may be associated with OLP.21,23 Although OLP patients who were initially deficient in vitamin D demonstrated marked improvement with supplementation, it is unlikely that vitamin D supplements impacted our patient’s presentation of OLP, as she had been consistently taking them for more than 5 years with no change in OLP presentation.24
Pathogenesis—Lichen planus is thought to be a cytotoxic CD8+ T cell–mediated autoimmune disease to a virally modified epidermal self-antigen on keratinocytes. The cytotoxic T cells target the modified self-antigens on basal keratinocytes and induce apoptosis.25 The cytokine-mediated lymphocyte homing mechanism is human leukocyte antigen dependent and involves tumor necrosis factor α as well as IFN-γ and IL-1. The latter cytokines lead to upregulation of vascular adhesion molecules on endothelial vessels of subepithelial vascular plexus as well as a cascade of nonspecific mechanisms such as mast cell degranulation and matrix metalloproteinase activation, resulting in increased basement membrane disruption.6
Shao et al19 underscored the role of IFN-γ in CD8+ T cell–mediated cytotoxic cellular responses, noting that the Janus kinase (JAK)–signal transducer and activator of transcription pathway may play a key role in the pathogenesis of lichen planus. They proposed using JAK inhibitors for the treatment of lichen planus, specifically tofacitinib, a JAK1/JAK3 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor, as top therapeutic agents for lichen planus (eTable).19 Tofacitinib has been reported to successfully treat conditions such as psoriasis, psoriatic arthritis, alopecia areata, vitiligo, atopic dermatitis, sarcoidosis, pyoderma gangrenosum, and lichen planopilaris.26 Additionally, the efficacy of tofacitinib has been established in patients with erosive lichen planus; tofacitinib resulted in marked improvement while prednisone, acitretin, methotrexate, mycophenolate mofetil, and cyclosporine treatment failed.27 Although more studies on tofacitinib’s long-term efficacy, cost, and safety are necessary, tofacitinib may soon play an integral role in the battle against inflammatory dermatoses.

Conclusion
Esophageal lichen planus is an underreported form of lichen planus that often is misdiagnosed. It frequently causes dysphagia and odynophagia, resulting in a major decrease in a patient’s quality of life. We present the case of an 89-year-old woman who underwent procedures to dilate her esophagus that worsened her condition. We emphasize the importance of considering ELP in the differential diagnosis of patients presenting with lichen planus in another region. In our patient, tofacitinib 5 mg BID resolved her condition without any adverse effects.
- Le Cleach L, Chosidow O. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/nejmcp1103641
- Heath L, Matin R. Lichen planus. InnovAiT. 2017;10:133-138. doi:10.1177/1755738016686804
- Oliveira JP, Uribe NC, Abulafia LA, et al. Esophageal lichenplanus. An Bras Dermatol. 2015;90:394-396. doi:10.1590/abd1806-4841.20153255
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. doi:10.1016/j.jaad.2010.03.029
- Quispel R, van Boxel O, Schipper M, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. doi:10.1055/s-0028-1119590
- Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222-229. doi:10.4103/0019-5154.156315
- Katzka DA, Smyrk TC, Bruce AJ, et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. doi:10.1016/j.cgh.2010.04.024
- Abraham SC, Ravich WJ, Anhalt GJ, et al. Esophageal lichen planus. Am J Surg Pathol. 2000;24:1678-1682. doi:10.1097/00000478-200012000-00014
- Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827-830. doi:10.5858/arpa.2014-0111-RS
- Wilcox CM. Infectious esophagitis. Gastroenterol Hepatol (N Y). 2006;2:567-568.
- Cancio A, Cruz C. A case of Kaposi’s sarcoma of the esophagus presenting with odynophagia. Am J Gastroenterol. 2018;113:S995-S996.
- Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behçet’s disease. Patholog Res Int. 2012;2012:690390. doi:10.1155/2012/690390
- Madhusudhan KS, Sharma R. Esophageal lichen planus: a case report and review of literature. Indian J Dermatol. 2008;53:26-27. doi:10.4103/0019-5154.39738
- Bottomley WW, Dakkak M, Walton S, et al. Esophageal involvement in Behçet’s disease. is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. doi:10.1007/BF01307585
- McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-921.
- Trabulo D, Ferreira S, Lage P, et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-vs-host disease. World J Gastroenterol. 2015;21:9217-9222. doi:10.3748/wjg.v21.i30.9217
- Abbas H, Ghazanfar H, Ul Hussain AN, et al. Atypical presentation of esophageal squamous cell carcinoma masquerading as diffuse severe esophagitis. Case Rep Gastroenterol. 2021;15:533-538. doi:10.1159/000517129
- Ellis A, Risk JM, Maruthappu T, et al. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi:10.1186/s13023-015-0346-2
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11:eaav7561. doi:10.1126/scitranslmed.aav7561
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Dave A, Shariff J, Philipone E. Association between oral lichen planus and systemic conditions and medications: case-control study. Oral Dis. 2020;27:515-524. doi:10.1111/odi.13572
- Krupaa RJ, Sankari SL, Masthan KM, et al. Oral lichen planus: an overview. J Pharm Bioallied Sci. 2015;7(suppl 1):S158-S161. doi:10.4103/0975-7406.155873
- Tak MM, Chalkoo AH. Vitamin D deficiency—a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evolution Med Dent Sci. 2017;6:4769-4772. doi:10.14260/jemds/2017/1033
- Gupta J, Aggarwal A, Asadullah M, et al. Vitamin D in thetreatment of oral lichen planus: a pilot clinical study. J Indian Acad Oral Med Radiol. 2019;31:222-227. doi:10.4103/jiaomr.jiaomr_97_19
- Shiohara T, Moriya N, Mochizuki T, et al. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987;89:8-14.
- Sonthalia S, Aggarwal P. Oral tofacitinib: contemporary appraisal of its role in dermatology. Indian Dermatol Online J. 2019;10:503-518. doi:10.4103/idoj.idoj_474_18
- Damsky W, Wang A, Olamiju B, et al. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710.e2. doi:10.1016/j.jaci.2020.01.031
- Le Cleach L, Chosidow O. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/nejmcp1103641
- Heath L, Matin R. Lichen planus. InnovAiT. 2017;10:133-138. doi:10.1177/1755738016686804
- Oliveira JP, Uribe NC, Abulafia LA, et al. Esophageal lichenplanus. An Bras Dermatol. 2015;90:394-396. doi:10.1590/abd1806-4841.20153255
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. doi:10.1016/j.jaad.2010.03.029
- Quispel R, van Boxel O, Schipper M, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. doi:10.1055/s-0028-1119590
- Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222-229. doi:10.4103/0019-5154.156315
- Katzka DA, Smyrk TC, Bruce AJ, et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. doi:10.1016/j.cgh.2010.04.024
- Abraham SC, Ravich WJ, Anhalt GJ, et al. Esophageal lichen planus. Am J Surg Pathol. 2000;24:1678-1682. doi:10.1097/00000478-200012000-00014
- Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827-830. doi:10.5858/arpa.2014-0111-RS
- Wilcox CM. Infectious esophagitis. Gastroenterol Hepatol (N Y). 2006;2:567-568.
- Cancio A, Cruz C. A case of Kaposi’s sarcoma of the esophagus presenting with odynophagia. Am J Gastroenterol. 2018;113:S995-S996.
- Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behçet’s disease. Patholog Res Int. 2012;2012:690390. doi:10.1155/2012/690390
- Madhusudhan KS, Sharma R. Esophageal lichen planus: a case report and review of literature. Indian J Dermatol. 2008;53:26-27. doi:10.4103/0019-5154.39738
- Bottomley WW, Dakkak M, Walton S, et al. Esophageal involvement in Behçet’s disease. is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. doi:10.1007/BF01307585
- McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-921.
- Trabulo D, Ferreira S, Lage P, et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-vs-host disease. World J Gastroenterol. 2015;21:9217-9222. doi:10.3748/wjg.v21.i30.9217
- Abbas H, Ghazanfar H, Ul Hussain AN, et al. Atypical presentation of esophageal squamous cell carcinoma masquerading as diffuse severe esophagitis. Case Rep Gastroenterol. 2021;15:533-538. doi:10.1159/000517129
- Ellis A, Risk JM, Maruthappu T, et al. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi:10.1186/s13023-015-0346-2
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11:eaav7561. doi:10.1126/scitranslmed.aav7561
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Dave A, Shariff J, Philipone E. Association between oral lichen planus and systemic conditions and medications: case-control study. Oral Dis. 2020;27:515-524. doi:10.1111/odi.13572
- Krupaa RJ, Sankari SL, Masthan KM, et al. Oral lichen planus: an overview. J Pharm Bioallied Sci. 2015;7(suppl 1):S158-S161. doi:10.4103/0975-7406.155873
- Tak MM, Chalkoo AH. Vitamin D deficiency—a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evolution Med Dent Sci. 2017;6:4769-4772. doi:10.14260/jemds/2017/1033
- Gupta J, Aggarwal A, Asadullah M, et al. Vitamin D in thetreatment of oral lichen planus: a pilot clinical study. J Indian Acad Oral Med Radiol. 2019;31:222-227. doi:10.4103/jiaomr.jiaomr_97_19
- Shiohara T, Moriya N, Mochizuki T, et al. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987;89:8-14.
- Sonthalia S, Aggarwal P. Oral tofacitinib: contemporary appraisal of its role in dermatology. Indian Dermatol Online J. 2019;10:503-518. doi:10.4103/idoj.idoj_474_18
- Damsky W, Wang A, Olamiju B, et al. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710.e2. doi:10.1016/j.jaci.2020.01.031
Practice Points
- Patients diagnosed with lichen planus should be informed about the signs of esophageal lichen planus (ELP).
- Twenty-five percent to 50% of patients with oral lichen planus (OLP) have been shown to have concomitant ELP.
- Esophageal lichen planus may be asymptomatic and often is misdiagnosed.
- Tofacitinib should be considered for the treatment of ELP, OLP, and cutaneous lichen planus.
Characterization of Blood-borne Pathogen Exposures During Dermatologic Procedures: The Mayo Clinic Experience
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).
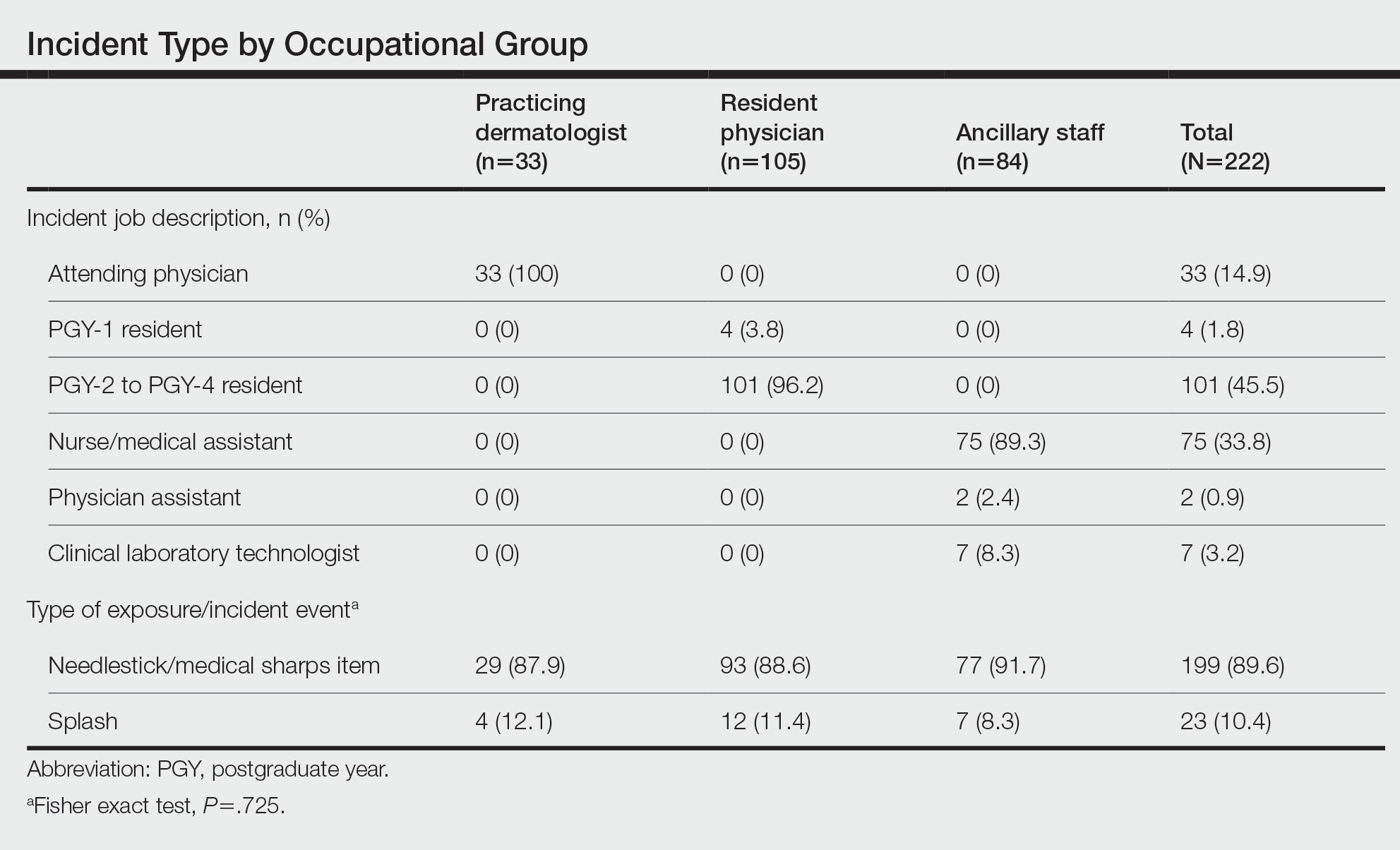
Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.
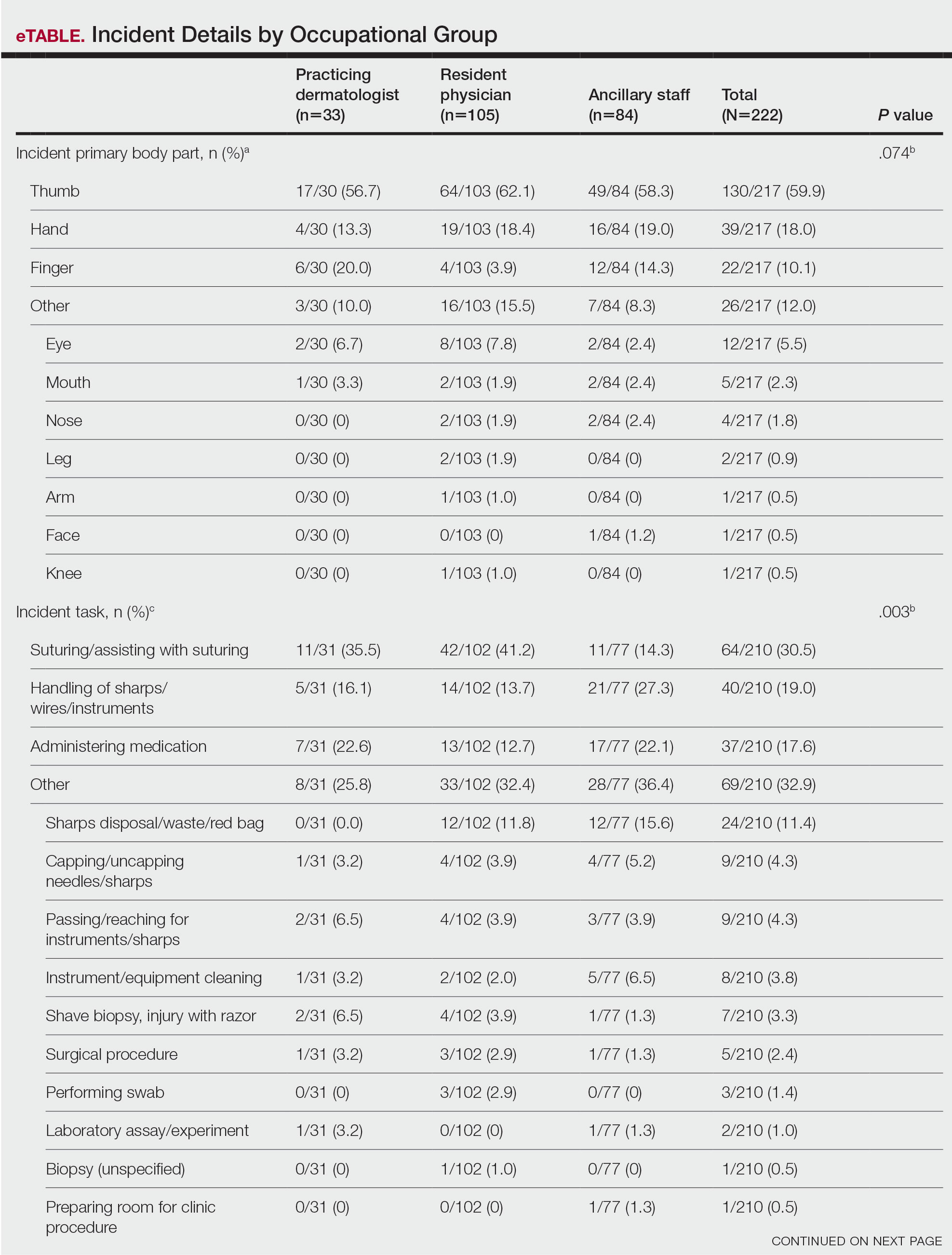
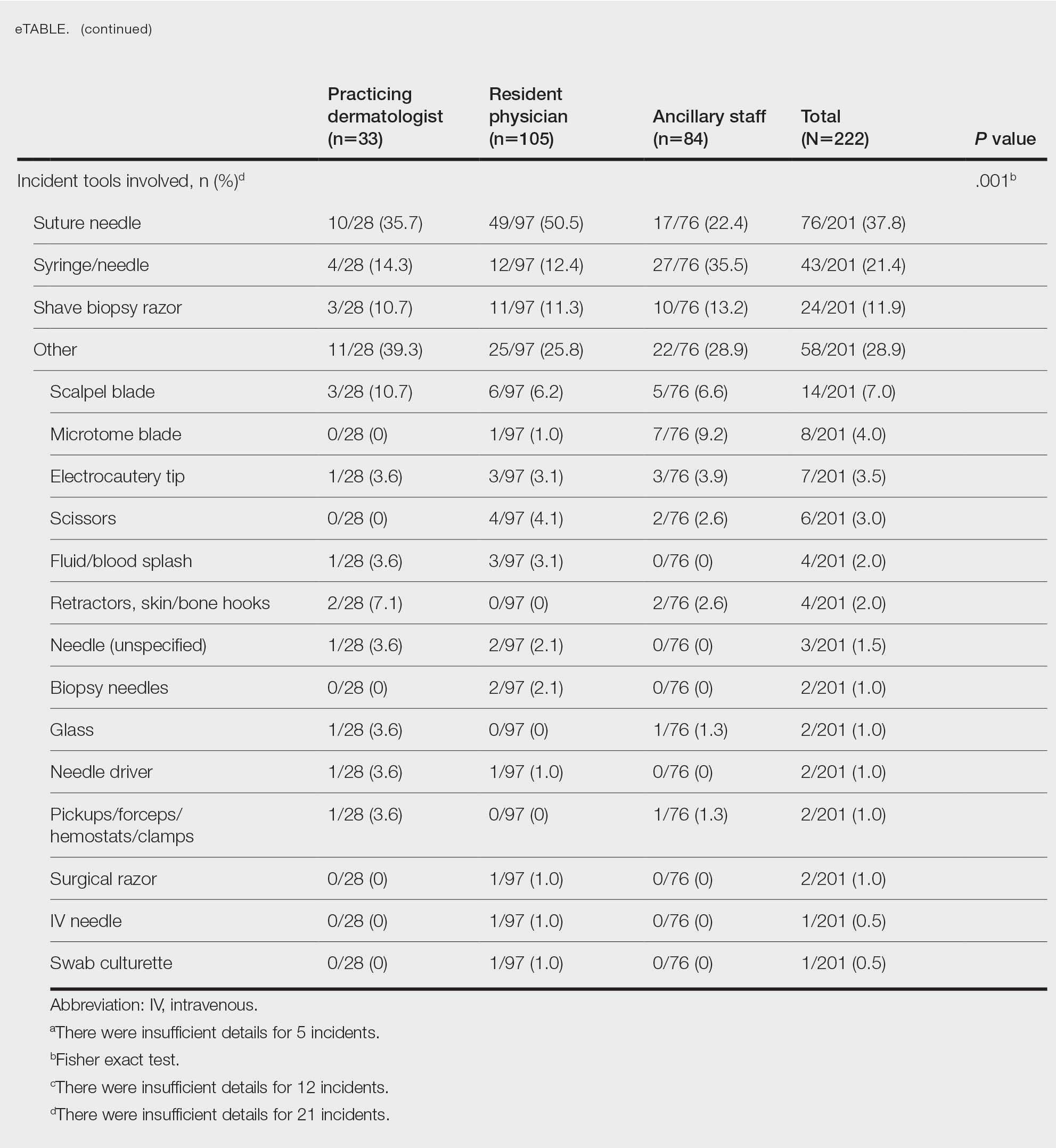
Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).

Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.


Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).

Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.


Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
Practice Points
- Most blood-borne pathogen (BBP) exposures in dermatologic staff occur due to medical sharps as opposed to splash incidents.
- The most common implicated task in resident physicians and practicing dermatologists is suturing or assisting with suturing, and the most commonly associated instrument is the suture needle. In contrast, ancillary staff experience most BBP exposures during handling of sharps, wires, or instruments, and the injection syringe/needle is the most common instrument of injury.
- Quality improvement measures are needed in prevention of BBP exposures and should focus on identified risk factors among occupational groups in the workplace.
Catheterized urine color change
Physical examination revealed an older man whose vital signs were normal and who had a regular heart rate and rhythm. He denied any pain, and his abdomen was soft and nontender with normal bowel sounds. There was no suprapubic or costovertebral angle tenderness, and his urinary catheter was correctly placed. His urine output was within normal limits, but the urine in the catheter and collection bag was purple.
The patient’s medical history was remarkable for mild cognitive impairment, BPH, and hypertension. A urine culture was significant for > 100,000 CFU/mL pan-sensitive Pseudomonas aeruginosa.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Purple urine bag syndrome
The diagnosis of purple urine bag syndrome (PUBS) was made based on the patient’s clinical presentation and medical history. PUBS is generally a benign condition that can occur in patients who have urinary catheters for prolonged periods of time and urinary tract infections (UTIs), often with constipation.1
PUBS was first described in the literature in 1978.2 Its prevalence has been estimated to be 9.8% in long-term wards and higher in patients with chronic catheters.3-5 PUBS is reported more often in institutionalized older women, although it has been documented in men as well.1 Risk factors include having a chronic indwelling urinary catheter; alkaline urine; the use of plastic, polyvinylchloride urine bags3; chronic constipation6; renal failure4,5; and dementia.1 In many cases, patients with PUBS have been found to have stable vitals and lack systemic symptoms, such as fever, that could indicate an infection.1,5
The pathogenesis of PUBS has been associated with tryptophan.3 Gut bacteria metabolize tryptophan to indole, which is converted to indoxyl sulfate in the liver.3,7 Then certain bacteria associated with UTIs, including Pseudomonas, Escherichia coli, Proteus mirabilis, Providencia spp, Enterococcus faecalis, and Klebsiella,5-7 which contain indoxyl phosphatase and sulfatase enzymes, can convert indoxyl sulfate into indirubin (red) and indigo (blue) compounds; this results in a purple hue in the urine seen in a Foley catheter and bag.
Differential is generally limited to medication and food consumption
Clinical presentation and a detailed history and review of medication and/or food ingestion may distinguish PUBS from other conditions.
Medications and foods, such as rifampicin or beets, may discolor urine and need to be ruled out as a cause with a thorough history.3
Cyanide toxicity in those taking vitamin B12 can result in purple-tinged urine.8 Signs and symptoms can also include reddening of the skin, dyspnea, nausea, headache, erythema at the injection site, and a modest increase in blood pressure.8
Identify the infection and treat as needed
There have been some case reports regarding the progression of PUBS to Fournier gangrene,4 but such cases are rare and associated with immunocompromised patients.9 PUBS is generally a benign condition associated with UTIs. Management involves identifying the underlying infection, treating with antibiotics if indicated (ie, patient is symptomatic or immunocompromised),3 providing proper treatment of constipation if needed, and replacing the Foley catheter.4 Some studies suggest that simply exchanging the catheter may resolve PUBS, particularly in asymptomatic patients.5
In light of his complicated urologic history, our patient was treated with a 10-day course of renally dosed intravenous cefepime (500 mg every 24 hours based on calculated creatine clearance of 21 mL/min) and Foley exchange. The patient’s urine color returned to normal after Foley exchange and 24 hours of antibiotics. His kidney function continued to improve and normalized by the time he was discharged from the facility approximately 2 weeks later.
1. Goyal A, Vikas G, Jindal J. Purple urine bag syndrome: series of nine cases and review of literature. J Clin Diagn Res. 2018;12:PR01-PR03. doi: 10.7860/JCDR/2018/34951.12202
2. Barlow GB, Dickson JAS. Purple urine bags. Lancet. 1978;28:220-221. doi: 10.1016/S0140-6736(78)90667-0
3. Richardson-May J. Single case of purple urine bag syndrome in an elderly woman with stroke. BMJ Case Rep. 2016;2016:bcr2016215465. doi: 10.1136/bcr-2016-215465
4. Khan F, Chaudhry MA, Qureshi N, et al. Purple urine bag syndrome: an alarming hue? A brief review of the literature. Int J Nephrol. 2011;2011:419213. doi: 10.4061/2011/419213
5. Ben-Chetrit E, Munter G. Purple urine. JAMA. 2012;307:193-194. doi: 10.1001/jama.2011.1997
6. Al Montasir A, Al Mustaque A. Purple urine bag syndrome. J Family Med Prim Care. 2013;2:104-105. doi: 10.4103/2249-4863.109970
7. Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol. 1988;26:2152-2156. doi: 10.1128/jcm.26.10.2152-2156.1988
8. Hudson M, Cashin BV, Matlock AG, et al. A man with purple urine. Hydroxocobalamin-induced chromaturia. Clin Toxicol (Phila). 2012;50:77. doi: 10.3109/15563650.2011.626782
9. Tasi Y-M, Huang M-S, Yang C-J, et al. Purple urine bag syndrome, not always a benign process. Am J Emerg Med. 2009;27:895-897. doi: 10.1016/j.ajem.2009.01.030
Physical examination revealed an older man whose vital signs were normal and who had a regular heart rate and rhythm. He denied any pain, and his abdomen was soft and nontender with normal bowel sounds. There was no suprapubic or costovertebral angle tenderness, and his urinary catheter was correctly placed. His urine output was within normal limits, but the urine in the catheter and collection bag was purple.
The patient’s medical history was remarkable for mild cognitive impairment, BPH, and hypertension. A urine culture was significant for > 100,000 CFU/mL pan-sensitive Pseudomonas aeruginosa.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Purple urine bag syndrome
The diagnosis of purple urine bag syndrome (PUBS) was made based on the patient’s clinical presentation and medical history. PUBS is generally a benign condition that can occur in patients who have urinary catheters for prolonged periods of time and urinary tract infections (UTIs), often with constipation.1
PUBS was first described in the literature in 1978.2 Its prevalence has been estimated to be 9.8% in long-term wards and higher in patients with chronic catheters.3-5 PUBS is reported more often in institutionalized older women, although it has been documented in men as well.1 Risk factors include having a chronic indwelling urinary catheter; alkaline urine; the use of plastic, polyvinylchloride urine bags3; chronic constipation6; renal failure4,5; and dementia.1 In many cases, patients with PUBS have been found to have stable vitals and lack systemic symptoms, such as fever, that could indicate an infection.1,5
The pathogenesis of PUBS has been associated with tryptophan.3 Gut bacteria metabolize tryptophan to indole, which is converted to indoxyl sulfate in the liver.3,7 Then certain bacteria associated with UTIs, including Pseudomonas, Escherichia coli, Proteus mirabilis, Providencia spp, Enterococcus faecalis, and Klebsiella,5-7 which contain indoxyl phosphatase and sulfatase enzymes, can convert indoxyl sulfate into indirubin (red) and indigo (blue) compounds; this results in a purple hue in the urine seen in a Foley catheter and bag.
Differential is generally limited to medication and food consumption
Clinical presentation and a detailed history and review of medication and/or food ingestion may distinguish PUBS from other conditions.
Medications and foods, such as rifampicin or beets, may discolor urine and need to be ruled out as a cause with a thorough history.3
Cyanide toxicity in those taking vitamin B12 can result in purple-tinged urine.8 Signs and symptoms can also include reddening of the skin, dyspnea, nausea, headache, erythema at the injection site, and a modest increase in blood pressure.8
Identify the infection and treat as needed
There have been some case reports regarding the progression of PUBS to Fournier gangrene,4 but such cases are rare and associated with immunocompromised patients.9 PUBS is generally a benign condition associated with UTIs. Management involves identifying the underlying infection, treating with antibiotics if indicated (ie, patient is symptomatic or immunocompromised),3 providing proper treatment of constipation if needed, and replacing the Foley catheter.4 Some studies suggest that simply exchanging the catheter may resolve PUBS, particularly in asymptomatic patients.5
In light of his complicated urologic history, our patient was treated with a 10-day course of renally dosed intravenous cefepime (500 mg every 24 hours based on calculated creatine clearance of 21 mL/min) and Foley exchange. The patient’s urine color returned to normal after Foley exchange and 24 hours of antibiotics. His kidney function continued to improve and normalized by the time he was discharged from the facility approximately 2 weeks later.
Physical examination revealed an older man whose vital signs were normal and who had a regular heart rate and rhythm. He denied any pain, and his abdomen was soft and nontender with normal bowel sounds. There was no suprapubic or costovertebral angle tenderness, and his urinary catheter was correctly placed. His urine output was within normal limits, but the urine in the catheter and collection bag was purple.
The patient’s medical history was remarkable for mild cognitive impairment, BPH, and hypertension. A urine culture was significant for > 100,000 CFU/mL pan-sensitive Pseudomonas aeruginosa.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Purple urine bag syndrome
The diagnosis of purple urine bag syndrome (PUBS) was made based on the patient’s clinical presentation and medical history. PUBS is generally a benign condition that can occur in patients who have urinary catheters for prolonged periods of time and urinary tract infections (UTIs), often with constipation.1
PUBS was first described in the literature in 1978.2 Its prevalence has been estimated to be 9.8% in long-term wards and higher in patients with chronic catheters.3-5 PUBS is reported more often in institutionalized older women, although it has been documented in men as well.1 Risk factors include having a chronic indwelling urinary catheter; alkaline urine; the use of plastic, polyvinylchloride urine bags3; chronic constipation6; renal failure4,5; and dementia.1 In many cases, patients with PUBS have been found to have stable vitals and lack systemic symptoms, such as fever, that could indicate an infection.1,5
The pathogenesis of PUBS has been associated with tryptophan.3 Gut bacteria metabolize tryptophan to indole, which is converted to indoxyl sulfate in the liver.3,7 Then certain bacteria associated with UTIs, including Pseudomonas, Escherichia coli, Proteus mirabilis, Providencia spp, Enterococcus faecalis, and Klebsiella,5-7 which contain indoxyl phosphatase and sulfatase enzymes, can convert indoxyl sulfate into indirubin (red) and indigo (blue) compounds; this results in a purple hue in the urine seen in a Foley catheter and bag.
Differential is generally limited to medication and food consumption
Clinical presentation and a detailed history and review of medication and/or food ingestion may distinguish PUBS from other conditions.
Medications and foods, such as rifampicin or beets, may discolor urine and need to be ruled out as a cause with a thorough history.3
Cyanide toxicity in those taking vitamin B12 can result in purple-tinged urine.8 Signs and symptoms can also include reddening of the skin, dyspnea, nausea, headache, erythema at the injection site, and a modest increase in blood pressure.8
Identify the infection and treat as needed
There have been some case reports regarding the progression of PUBS to Fournier gangrene,4 but such cases are rare and associated with immunocompromised patients.9 PUBS is generally a benign condition associated with UTIs. Management involves identifying the underlying infection, treating with antibiotics if indicated (ie, patient is symptomatic or immunocompromised),3 providing proper treatment of constipation if needed, and replacing the Foley catheter.4 Some studies suggest that simply exchanging the catheter may resolve PUBS, particularly in asymptomatic patients.5
In light of his complicated urologic history, our patient was treated with a 10-day course of renally dosed intravenous cefepime (500 mg every 24 hours based on calculated creatine clearance of 21 mL/min) and Foley exchange. The patient’s urine color returned to normal after Foley exchange and 24 hours of antibiotics. His kidney function continued to improve and normalized by the time he was discharged from the facility approximately 2 weeks later.
1. Goyal A, Vikas G, Jindal J. Purple urine bag syndrome: series of nine cases and review of literature. J Clin Diagn Res. 2018;12:PR01-PR03. doi: 10.7860/JCDR/2018/34951.12202
2. Barlow GB, Dickson JAS. Purple urine bags. Lancet. 1978;28:220-221. doi: 10.1016/S0140-6736(78)90667-0
3. Richardson-May J. Single case of purple urine bag syndrome in an elderly woman with stroke. BMJ Case Rep. 2016;2016:bcr2016215465. doi: 10.1136/bcr-2016-215465
4. Khan F, Chaudhry MA, Qureshi N, et al. Purple urine bag syndrome: an alarming hue? A brief review of the literature. Int J Nephrol. 2011;2011:419213. doi: 10.4061/2011/419213
5. Ben-Chetrit E, Munter G. Purple urine. JAMA. 2012;307:193-194. doi: 10.1001/jama.2011.1997
6. Al Montasir A, Al Mustaque A. Purple urine bag syndrome. J Family Med Prim Care. 2013;2:104-105. doi: 10.4103/2249-4863.109970
7. Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol. 1988;26:2152-2156. doi: 10.1128/jcm.26.10.2152-2156.1988
8. Hudson M, Cashin BV, Matlock AG, et al. A man with purple urine. Hydroxocobalamin-induced chromaturia. Clin Toxicol (Phila). 2012;50:77. doi: 10.3109/15563650.2011.626782
9. Tasi Y-M, Huang M-S, Yang C-J, et al. Purple urine bag syndrome, not always a benign process. Am J Emerg Med. 2009;27:895-897. doi: 10.1016/j.ajem.2009.01.030
1. Goyal A, Vikas G, Jindal J. Purple urine bag syndrome: series of nine cases and review of literature. J Clin Diagn Res. 2018;12:PR01-PR03. doi: 10.7860/JCDR/2018/34951.12202
2. Barlow GB, Dickson JAS. Purple urine bags. Lancet. 1978;28:220-221. doi: 10.1016/S0140-6736(78)90667-0
3. Richardson-May J. Single case of purple urine bag syndrome in an elderly woman with stroke. BMJ Case Rep. 2016;2016:bcr2016215465. doi: 10.1136/bcr-2016-215465
4. Khan F, Chaudhry MA, Qureshi N, et al. Purple urine bag syndrome: an alarming hue? A brief review of the literature. Int J Nephrol. 2011;2011:419213. doi: 10.4061/2011/419213
5. Ben-Chetrit E, Munter G. Purple urine. JAMA. 2012;307:193-194. doi: 10.1001/jama.2011.1997
6. Al Montasir A, Al Mustaque A. Purple urine bag syndrome. J Family Med Prim Care. 2013;2:104-105. doi: 10.4103/2249-4863.109970
7. Dealler SF, Hawkey PM, Millar MR. Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumoniae causes the purple urine bag syndrome. J Clin Microbiol. 1988;26:2152-2156. doi: 10.1128/jcm.26.10.2152-2156.1988
8. Hudson M, Cashin BV, Matlock AG, et al. A man with purple urine. Hydroxocobalamin-induced chromaturia. Clin Toxicol (Phila). 2012;50:77. doi: 10.3109/15563650.2011.626782
9. Tasi Y-M, Huang M-S, Yang C-J, et al. Purple urine bag syndrome, not always a benign process. Am J Emerg Med. 2009;27:895-897. doi: 10.1016/j.ajem.2009.01.030