User login
Painful blue fingers
A 48-year-old woman with a history of systemic lupus erythematosus (SLE) presented to the emergency department from the rheumatology clinic for digital ischemia. The clinical manifestations of her SLE consisted predominantly of arthralgias, which had been previously well controlled on hydroxychloroquine 300 mg/d PO. On presentation, she denied oral ulcers, alopecia, shortness of breath, chest pain/pressure, and history of blood clots or miscarriages.
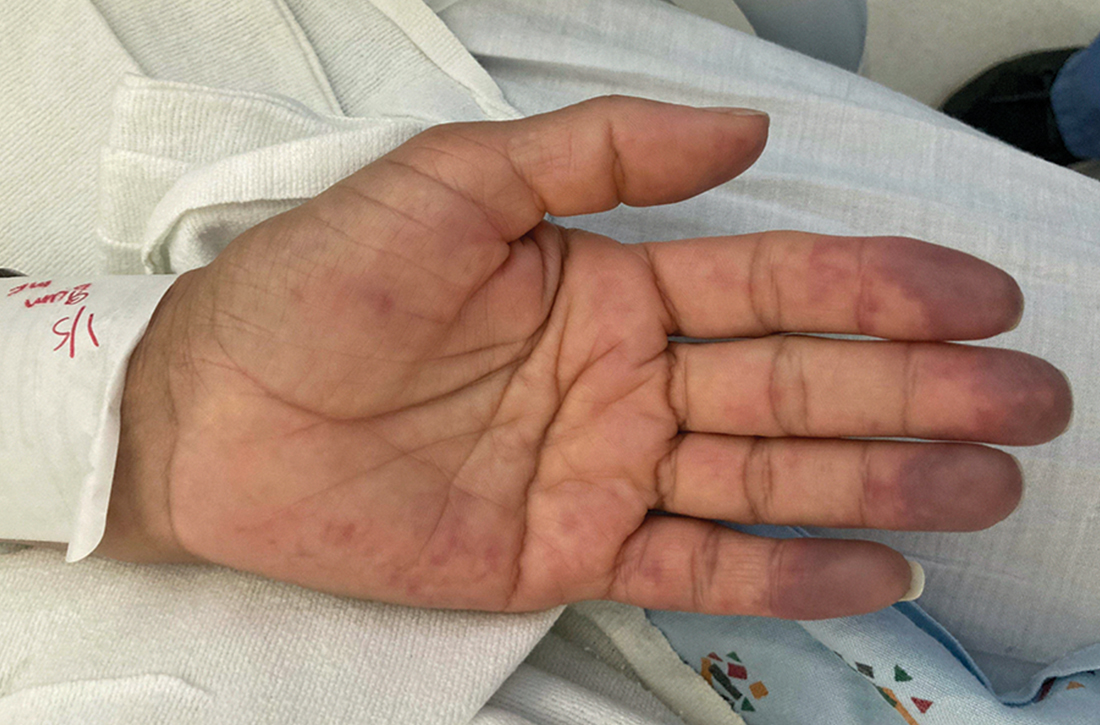
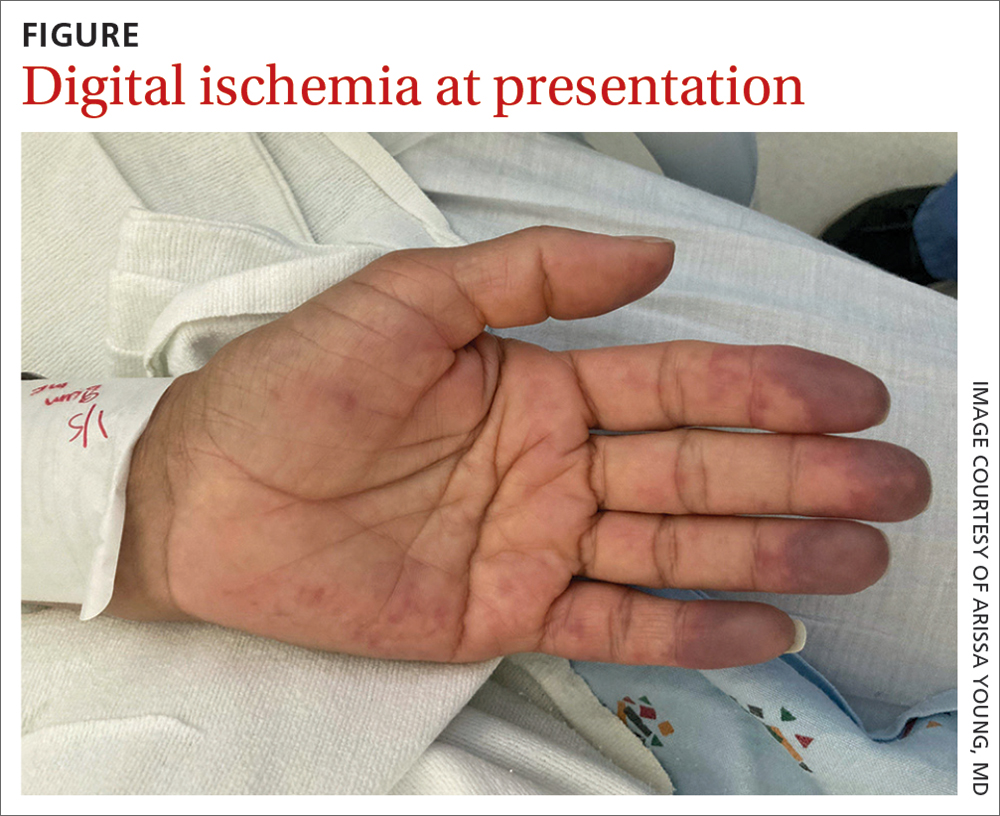
On exam, the patient was afebrile and had a heart rate of 74 bpm; blood pressure, 140/77 mm Hg; and respiratory rate, 18 breaths/min. The fingertips on her left hand were tender and cool to the touch, and the fingertips of her second through fifth digits were blue (FIGURE).
Laboratory workup was notable for the following: hemoglobin, 9.3 g/dL (normal range, 11.6-15.2 g/dL) and erythrocyte sedimentation rate, 44 mm/h (normal range, ≤ 25 mm/h). Double-stranded DNA and complement levels were normal.
Transthoracic echocardiogram did not show any valvular vegetations, and blood cultures from admission were negative. Computed tomography angiography (CTA) with contrast of her left upper extremity showed a filling defect in the origin of the left subclavian artery. Digital plethysmography showed dampened flow signals in the second through fifth digits of the left hand.
Tests for antiphospholipid antibodies were positive for lupus anticoagulant; there were also high titers of anti-β-2-glycoprotein immunoglobulin (Ig) G (58 SGU; normal, ≤ 20 SGU) and anticardiolipin IgG (242.4 CU; normal, ≤ 20 CU).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Antiphospholipid syndrome
Given our patient’s SLE, left subclavian artery thrombosis, digital ischemia, and high-titer antiphospholipid antibodies, we had significant concern for antiphospholipid syndrome (APS). The diagnosis of APS is most often based on the fulfillment of the revised Sapporo classification criteria. These criteria include both clinical criteria (vascular thrombosis or pregnancy morbidity) and laboratory criteria (the presence of antiphospholipid antibodies on at least 2 separate occasions separated by 12 weeks).1 Our patient met clinical criteria given the evidence of subclavian artery thrombosis on CTA as well as digital plethysmography findings consistent with digital emboli. To meet laboratory criteria, she would have needed to have persistent high-titer antiphospholipid antibodies 12 weeks apart.
APS is an autoimmune disease in which the presence of antiphospholipid antibodies is associated with thrombosis; it can be divided into primary and secondary APS. The estimated prevalence of APS is 50 per 100,000 people in the United States.2 Primary APS occurs in the absence of an underlying autoimmune disease, while secondary APS occurs in the presence of an underlying autoimmune disease.
The autoimmune disease most often associated with APS is SLE.3 Among patients with SLE, 15% to 34% have positive lupus anticoagulant and 12% to 30% have anticardiolipin antibodies.4-6 This is compared with young healthy subjects among whom only 1% to 4% have positive lupus anticoagulant and 1% to 5% have anticardiolipin antibodies.7 Previous studies have estimated that 30% to 50% of patients with SLE who test positive for antiphospholipid antibodies will develop thrombosis.5,7
Differential includes Raynaud phenomenon, vasculitis
The differential diagnosis for digital ischemia in a patient with SLE includes APS, Raynaud phenomenon, vasculitis, and septic emboli.
Raynaud phenomenon can manifest in patients with SLE, but the presence of thrombosis on CTA and high-titer positive antiphospholipid antibodies make the diagnosis of APS more likely. Additionally, Raynaud phenomenon is typically temperature dependent with vasospasm in the digital arteries, occurring in cold temperatures and resolving with warming.
Systemic vasculitis may develop in patients with SLE, but in our case was less likely given the patient did not have any evidence of vasculitis on CTA, such as blood vessel wall thickening and/or enhancement.8
Septic emboli from endocarditis can cause digital ischemia but is typically associated with positive blood cultures, fever, and other systemic signs of infection, and/or vegetations on an echocardiogram.
Continue to: Thrombosis determines intensity of lifelong antiocagulation Tx
Thrombosis determines intensity of lifelong anticoagulation Tx
The mainstay of therapy for patients with APS is lifelong anticoagulation with a vitamin K antagonist. The intensity of anticoagulation is determined based on the presence of venous or arterial thrombosis. In patients who present with arterial thrombosis, a higher intensity vitamin K antagonist (ie, international normalized ratio [INR] goal > 3) or the addition of low-dose aspirin should be considered.9,10
Factor Xa inhibitors are generally not recommended at this time due to the lack of evidence to support their use.10 Additionally, a randomized clinical trial comparing rivaroxaban and warfarin in patients with triple antiphospholipid antibody positivity was terminated prematurely due to increased thromboembolic events in the rivaroxaban arm.11
For patients with secondary APS in the setting of SLE, hydroxychloroquine in combination with a vitamin K antagonist has been shown to decrease the risk for recurrent thrombosis compared with treatment with a vitamin K antagonist alone.12
Our patient was started on a heparin drip and transitioned to an oral vitamin K antagonist with an INR goal of 2 to 3. Lifelong anticoagulation was planned. The pain and discoloration in her hands improved on anticoagulation and had nearly resolved by the time of discharge. Given her history of arterial thrombosis, the addition of aspirin was also considered, but this decision was ultimately deferred to her outpatient rheumatologist and hematologist.
1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
3. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. doi: 10.1056/NEJMra002974
4. Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore). 1993;72:113-124.
5. Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112:682-698. doi: 10.7326/0003-4819-112-9-682
6. Merkel PA, Chang YC, Pierangeli SS, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576-583. doi: 10.1016/s0002-9343(96)00335-x
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145-151. doi: 10.1006/jaut. 2000.0409
8. Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2—Clinical applications beyond trauma patients. AJR Am J Roentgenol. 2013;201:753-763. doi: 10.2214/AJR.13.11208
9. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;7:1487-1495. doi: 10.1002/art.23109
10. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-1304. doi: 10.1136/annrheumdis-2019-215213
Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) trial. Lupus. 2016;25:301-306. doi: 10.1177/0961203315611495
12. Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927-1929. doi: 10.1111/jth.12363
A 48-year-old woman with a history of systemic lupus erythematosus (SLE) presented to the emergency department from the rheumatology clinic for digital ischemia. The clinical manifestations of her SLE consisted predominantly of arthralgias, which had been previously well controlled on hydroxychloroquine 300 mg/d PO. On presentation, she denied oral ulcers, alopecia, shortness of breath, chest pain/pressure, and history of blood clots or miscarriages.
On exam, the patient was afebrile and had a heart rate of 74 bpm; blood pressure, 140/77 mm Hg; and respiratory rate, 18 breaths/min. The fingertips on her left hand were tender and cool to the touch, and the fingertips of her second through fifth digits were blue (FIGURE).
Laboratory workup was notable for the following: hemoglobin, 9.3 g/dL (normal range, 11.6-15.2 g/dL) and erythrocyte sedimentation rate, 44 mm/h (normal range, ≤ 25 mm/h). Double-stranded DNA and complement levels were normal.
Transthoracic echocardiogram did not show any valvular vegetations, and blood cultures from admission were negative. Computed tomography angiography (CTA) with contrast of her left upper extremity showed a filling defect in the origin of the left subclavian artery. Digital plethysmography showed dampened flow signals in the second through fifth digits of the left hand.
Tests for antiphospholipid antibodies were positive for lupus anticoagulant; there were also high titers of anti-β-2-glycoprotein immunoglobulin (Ig) G (58 SGU; normal, ≤ 20 SGU) and anticardiolipin IgG (242.4 CU; normal, ≤ 20 CU).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Antiphospholipid syndrome
Given our patient’s SLE, left subclavian artery thrombosis, digital ischemia, and high-titer antiphospholipid antibodies, we had significant concern for antiphospholipid syndrome (APS). The diagnosis of APS is most often based on the fulfillment of the revised Sapporo classification criteria. These criteria include both clinical criteria (vascular thrombosis or pregnancy morbidity) and laboratory criteria (the presence of antiphospholipid antibodies on at least 2 separate occasions separated by 12 weeks).1 Our patient met clinical criteria given the evidence of subclavian artery thrombosis on CTA as well as digital plethysmography findings consistent with digital emboli. To meet laboratory criteria, she would have needed to have persistent high-titer antiphospholipid antibodies 12 weeks apart.
APS is an autoimmune disease in which the presence of antiphospholipid antibodies is associated with thrombosis; it can be divided into primary and secondary APS. The estimated prevalence of APS is 50 per 100,000 people in the United States.2 Primary APS occurs in the absence of an underlying autoimmune disease, while secondary APS occurs in the presence of an underlying autoimmune disease.
The autoimmune disease most often associated with APS is SLE.3 Among patients with SLE, 15% to 34% have positive lupus anticoagulant and 12% to 30% have anticardiolipin antibodies.4-6 This is compared with young healthy subjects among whom only 1% to 4% have positive lupus anticoagulant and 1% to 5% have anticardiolipin antibodies.7 Previous studies have estimated that 30% to 50% of patients with SLE who test positive for antiphospholipid antibodies will develop thrombosis.5,7
Differential includes Raynaud phenomenon, vasculitis
The differential diagnosis for digital ischemia in a patient with SLE includes APS, Raynaud phenomenon, vasculitis, and septic emboli.
Raynaud phenomenon can manifest in patients with SLE, but the presence of thrombosis on CTA and high-titer positive antiphospholipid antibodies make the diagnosis of APS more likely. Additionally, Raynaud phenomenon is typically temperature dependent with vasospasm in the digital arteries, occurring in cold temperatures and resolving with warming.
Systemic vasculitis may develop in patients with SLE, but in our case was less likely given the patient did not have any evidence of vasculitis on CTA, such as blood vessel wall thickening and/or enhancement.8
Septic emboli from endocarditis can cause digital ischemia but is typically associated with positive blood cultures, fever, and other systemic signs of infection, and/or vegetations on an echocardiogram.
Continue to: Thrombosis determines intensity of lifelong antiocagulation Tx
Thrombosis determines intensity of lifelong anticoagulation Tx
The mainstay of therapy for patients with APS is lifelong anticoagulation with a vitamin K antagonist. The intensity of anticoagulation is determined based on the presence of venous or arterial thrombosis. In patients who present with arterial thrombosis, a higher intensity vitamin K antagonist (ie, international normalized ratio [INR] goal > 3) or the addition of low-dose aspirin should be considered.9,10
Factor Xa inhibitors are generally not recommended at this time due to the lack of evidence to support their use.10 Additionally, a randomized clinical trial comparing rivaroxaban and warfarin in patients with triple antiphospholipid antibody positivity was terminated prematurely due to increased thromboembolic events in the rivaroxaban arm.11
For patients with secondary APS in the setting of SLE, hydroxychloroquine in combination with a vitamin K antagonist has been shown to decrease the risk for recurrent thrombosis compared with treatment with a vitamin K antagonist alone.12
Our patient was started on a heparin drip and transitioned to an oral vitamin K antagonist with an INR goal of 2 to 3. Lifelong anticoagulation was planned. The pain and discoloration in her hands improved on anticoagulation and had nearly resolved by the time of discharge. Given her history of arterial thrombosis, the addition of aspirin was also considered, but this decision was ultimately deferred to her outpatient rheumatologist and hematologist.
A 48-year-old woman with a history of systemic lupus erythematosus (SLE) presented to the emergency department from the rheumatology clinic for digital ischemia. The clinical manifestations of her SLE consisted predominantly of arthralgias, which had been previously well controlled on hydroxychloroquine 300 mg/d PO. On presentation, she denied oral ulcers, alopecia, shortness of breath, chest pain/pressure, and history of blood clots or miscarriages.
On exam, the patient was afebrile and had a heart rate of 74 bpm; blood pressure, 140/77 mm Hg; and respiratory rate, 18 breaths/min. The fingertips on her left hand were tender and cool to the touch, and the fingertips of her second through fifth digits were blue (FIGURE).
Laboratory workup was notable for the following: hemoglobin, 9.3 g/dL (normal range, 11.6-15.2 g/dL) and erythrocyte sedimentation rate, 44 mm/h (normal range, ≤ 25 mm/h). Double-stranded DNA and complement levels were normal.
Transthoracic echocardiogram did not show any valvular vegetations, and blood cultures from admission were negative. Computed tomography angiography (CTA) with contrast of her left upper extremity showed a filling defect in the origin of the left subclavian artery. Digital plethysmography showed dampened flow signals in the second through fifth digits of the left hand.
Tests for antiphospholipid antibodies were positive for lupus anticoagulant; there were also high titers of anti-β-2-glycoprotein immunoglobulin (Ig) G (58 SGU; normal, ≤ 20 SGU) and anticardiolipin IgG (242.4 CU; normal, ≤ 20 CU).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Antiphospholipid syndrome
Given our patient’s SLE, left subclavian artery thrombosis, digital ischemia, and high-titer antiphospholipid antibodies, we had significant concern for antiphospholipid syndrome (APS). The diagnosis of APS is most often based on the fulfillment of the revised Sapporo classification criteria. These criteria include both clinical criteria (vascular thrombosis or pregnancy morbidity) and laboratory criteria (the presence of antiphospholipid antibodies on at least 2 separate occasions separated by 12 weeks).1 Our patient met clinical criteria given the evidence of subclavian artery thrombosis on CTA as well as digital plethysmography findings consistent with digital emboli. To meet laboratory criteria, she would have needed to have persistent high-titer antiphospholipid antibodies 12 weeks apart.
APS is an autoimmune disease in which the presence of antiphospholipid antibodies is associated with thrombosis; it can be divided into primary and secondary APS. The estimated prevalence of APS is 50 per 100,000 people in the United States.2 Primary APS occurs in the absence of an underlying autoimmune disease, while secondary APS occurs in the presence of an underlying autoimmune disease.
The autoimmune disease most often associated with APS is SLE.3 Among patients with SLE, 15% to 34% have positive lupus anticoagulant and 12% to 30% have anticardiolipin antibodies.4-6 This is compared with young healthy subjects among whom only 1% to 4% have positive lupus anticoagulant and 1% to 5% have anticardiolipin antibodies.7 Previous studies have estimated that 30% to 50% of patients with SLE who test positive for antiphospholipid antibodies will develop thrombosis.5,7
Differential includes Raynaud phenomenon, vasculitis
The differential diagnosis for digital ischemia in a patient with SLE includes APS, Raynaud phenomenon, vasculitis, and septic emboli.
Raynaud phenomenon can manifest in patients with SLE, but the presence of thrombosis on CTA and high-titer positive antiphospholipid antibodies make the diagnosis of APS more likely. Additionally, Raynaud phenomenon is typically temperature dependent with vasospasm in the digital arteries, occurring in cold temperatures and resolving with warming.
Systemic vasculitis may develop in patients with SLE, but in our case was less likely given the patient did not have any evidence of vasculitis on CTA, such as blood vessel wall thickening and/or enhancement.8
Septic emboli from endocarditis can cause digital ischemia but is typically associated with positive blood cultures, fever, and other systemic signs of infection, and/or vegetations on an echocardiogram.
Continue to: Thrombosis determines intensity of lifelong antiocagulation Tx
Thrombosis determines intensity of lifelong anticoagulation Tx
The mainstay of therapy for patients with APS is lifelong anticoagulation with a vitamin K antagonist. The intensity of anticoagulation is determined based on the presence of venous or arterial thrombosis. In patients who present with arterial thrombosis, a higher intensity vitamin K antagonist (ie, international normalized ratio [INR] goal > 3) or the addition of low-dose aspirin should be considered.9,10
Factor Xa inhibitors are generally not recommended at this time due to the lack of evidence to support their use.10 Additionally, a randomized clinical trial comparing rivaroxaban and warfarin in patients with triple antiphospholipid antibody positivity was terminated prematurely due to increased thromboembolic events in the rivaroxaban arm.11
For patients with secondary APS in the setting of SLE, hydroxychloroquine in combination with a vitamin K antagonist has been shown to decrease the risk for recurrent thrombosis compared with treatment with a vitamin K antagonist alone.12
Our patient was started on a heparin drip and transitioned to an oral vitamin K antagonist with an INR goal of 2 to 3. Lifelong anticoagulation was planned. The pain and discoloration in her hands improved on anticoagulation and had nearly resolved by the time of discharge. Given her history of arterial thrombosis, the addition of aspirin was also considered, but this decision was ultimately deferred to her outpatient rheumatologist and hematologist.
1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
3. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. doi: 10.1056/NEJMra002974
4. Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore). 1993;72:113-124.
5. Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112:682-698. doi: 10.7326/0003-4819-112-9-682
6. Merkel PA, Chang YC, Pierangeli SS, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576-583. doi: 10.1016/s0002-9343(96)00335-x
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145-151. doi: 10.1006/jaut. 2000.0409
8. Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2—Clinical applications beyond trauma patients. AJR Am J Roentgenol. 2013;201:753-763. doi: 10.2214/AJR.13.11208
9. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;7:1487-1495. doi: 10.1002/art.23109
10. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-1304. doi: 10.1136/annrheumdis-2019-215213
Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) trial. Lupus. 2016;25:301-306. doi: 10.1177/0961203315611495
12. Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927-1929. doi: 10.1111/jth.12363
1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
3. Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. doi: 10.1056/NEJMra002974
4. Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. Medicine (Baltimore). 1993;72:113-124.
5. Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112:682-698. doi: 10.7326/0003-4819-112-9-682
6. Merkel PA, Chang YC, Pierangeli SS, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576-583. doi: 10.1016/s0002-9343(96)00335-x
7. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145-151. doi: 10.1006/jaut. 2000.0409
8. Bozlar U, Ogur T, Khaja MS, et al. CT angiography of the upper extremity arterial system: Part 2—Clinical applications beyond trauma patients. AJR Am J Roentgenol. 2013;201:753-763. doi: 10.2214/AJR.13.11208
9. Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007;7:1487-1495. doi: 10.1002/art.23109
10. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-1304. doi: 10.1136/annrheumdis-2019-215213
Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) trial. Lupus. 2016;25:301-306. doi: 10.1177/0961203315611495
12. Schmidt-Tanguy A, Voswinkel J, Henrion D, et al. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J Thromb Haemost. 2013;11:1927-1929. doi: 10.1111/jth.12363
Should antenatal testing be performed in patients with a pre-pregnancy BMI ≥ 35?
Evidence summary
Association between higher maternal BMI and increased risk for stillbirth
The purpose of antenatal testing is to decrease the risk for stillbirth between visits. Because of the resources involved and the risk for false-positives when testing low-risk patients, antenatal testing is reserved for pregnant people with higher risk for stillbirth.
In a retrospective cohort study of more than 2.8 million singleton births including 9030 stillbirths, pregnant people with an elevated BMI had an increased risk for stillbirth compared to those with a normal BMI. The adjusted hazard ratio was 1.71 (95% CI, 1.62-1.83) for those with a BMI of 30.0 to 34.9; 2.04 (95% CI, 1.8-2.21) for those with a BMI of 35.0 to 39.9; and 2.50 (95% CI, 2.28-2.74) for those with a BMI ≥ 40.1
A meta-analysis of 38 studies, which included data on 16,274 stillbirths, found that a 5-unit increase in BMI was associated with an increased risk for stillbirth (relative risk, 1.24; 95% CI, 1.18-1.30).2
Another meta-analysis included 6 cohort studies involving more than 1 million pregnancies and 3 case-control studies involving 2530 stillbirths and 2837 controls from 1980-2005. There was an association between increasing BMI and stillbirth: the odds ratio (OR) was 1.47 (95% CI, 1.08-1.94) for those with a BMI of 25.0 to 29.9 and 2.07 (95% CI, 1.59-2.74) for those with a BMI ≥ 30.0, compared to those with a normal BMI.3
However, a retrospective cohort study of 182,362 singleton births including 442 stillbirths found no association between stillbirth and increasing BMI. The OR was 1.10 (95% CI, 0.90-1.36) for those with a BMI of 25.0 to 29.9 and 1.09 (95% CI, 0.87-1.37) for those with a BMI ≥ 30.0, compared to those with a normal BMI.4 However, this cohort study may have been underpowered to detect an association between stillbirth and BMI.
Recommendations from others
In 2021, ACOG suggested that weekly antenatal testing may be considered from 34w0d for pregnant people with a BMI ≥ 40.0 and from 37w0d for pregnant people with a BMI between 35.0 and 39.9.5 The 2021 ACOG Practice Bulletin on Obesity in Pregnancy rates this recommendation as Level C—based primarily on consensus and expert opinion.6
A 2018 Royal College of Obstetricians and Gynecologists Green-top Guideline recognizes “definitive recommendations for fetal surveillance are hampered by the lack of randomized controlled trials demonstrating that antepartum fetal surveillance decreases perinatal morbidity or mortality in late-term and post-term gestations…. There are no definitive studies determining the optimal type or frequency of such testing and no evidence specific for women with obesity.”7
A 2019 Society of Obstetricians and Gynecologists of Canada practice guideline states “stillbirth is more common with maternal obesity” and recommends “increased fetal surveillance … in the third trimester if reduced fetal movements are reported.” The guideline notes “the role for non-stress tests … in surveillance of well-being in this population is uncertain.” Also, for pregnant people with a BMI > 30, “assessment of fetal well-being is … recommended weekly from 37 weeks until delivery.” Finally, increased fetal surveillance is recommended in the setting of increased BMI and an abnormal pulsatility index of the umbilical artery and/or maternal uterine artery.8
Editor’s takeaway
Evidence demonstrates that increased maternal BMI is associated with increased stillbirths. However, evidence has not shown that third-trimester antenatal testing decreases this morbidity and mortality. Expert opinion varies, with ACOG recommending weekly antenatal testing from 34 and 37 weeks for pregnant people with a BMI ≥ 40 and of 35 to 39.9, respectively.
1. Yao R, Ananth C, Park B, et al; Perinatal Research Consortium. Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol. 2014;210:e1-e9. doi: 10.1016/j.ajog. 2014.01.044
2. Aune D, Saugstad O, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311:1536-1546. doi: 10.1001/jama.2014.2269
3. Chu S, Kim S, Lau J, et al. Maternal obesity and risk of stillbirth: a meta-analysis. Am J Obstet Gynecol. 2007;197:223-228. doi: 10.1016/j.ajog.2007.03.027
4. Mahomed K, Chan G, Norton M. Obesity and the risk of stillbirth—a reappraisal—a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;255:25-28. doi: 10.1016/j.ejogrb. 2020.09.044
5. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197. doi: 10.1097/AOG.0000000000004407
6. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Obesity in pregnancy: ACOG practice bulletin, number 230. Obstet Gynecol. 2021;137:e128-e144. doi: 10.1097/AOG.0000000000004395
7. Denison F, Aedla N, Keag O, et al; Royal College of Obstetricians and Gynaecologists. Care of women with obesity in pregnancy: Green-top Guideline No. 72. BJOG. 2019;126:e62-e106. doi: 10.1111/1471-0528.15386
8. Maxwell C, Gaudet L, Cassir G, et al. Guideline No. 391–Pregnancy and maternal obesity part 1: pre-conception and prenatal care. J Obstet Gynaecol Can. 2019;41:1623-1640. doi: 10.1016/j.jogc. 2019.03.026
Evidence summary
Association between higher maternal BMI and increased risk for stillbirth
The purpose of antenatal testing is to decrease the risk for stillbirth between visits. Because of the resources involved and the risk for false-positives when testing low-risk patients, antenatal testing is reserved for pregnant people with higher risk for stillbirth.
In a retrospective cohort study of more than 2.8 million singleton births including 9030 stillbirths, pregnant people with an elevated BMI had an increased risk for stillbirth compared to those with a normal BMI. The adjusted hazard ratio was 1.71 (95% CI, 1.62-1.83) for those with a BMI of 30.0 to 34.9; 2.04 (95% CI, 1.8-2.21) for those with a BMI of 35.0 to 39.9; and 2.50 (95% CI, 2.28-2.74) for those with a BMI ≥ 40.1
A meta-analysis of 38 studies, which included data on 16,274 stillbirths, found that a 5-unit increase in BMI was associated with an increased risk for stillbirth (relative risk, 1.24; 95% CI, 1.18-1.30).2
Another meta-analysis included 6 cohort studies involving more than 1 million pregnancies and 3 case-control studies involving 2530 stillbirths and 2837 controls from 1980-2005. There was an association between increasing BMI and stillbirth: the odds ratio (OR) was 1.47 (95% CI, 1.08-1.94) for those with a BMI of 25.0 to 29.9 and 2.07 (95% CI, 1.59-2.74) for those with a BMI ≥ 30.0, compared to those with a normal BMI.3
However, a retrospective cohort study of 182,362 singleton births including 442 stillbirths found no association between stillbirth and increasing BMI. The OR was 1.10 (95% CI, 0.90-1.36) for those with a BMI of 25.0 to 29.9 and 1.09 (95% CI, 0.87-1.37) for those with a BMI ≥ 30.0, compared to those with a normal BMI.4 However, this cohort study may have been underpowered to detect an association between stillbirth and BMI.
Recommendations from others
In 2021, ACOG suggested that weekly antenatal testing may be considered from 34w0d for pregnant people with a BMI ≥ 40.0 and from 37w0d for pregnant people with a BMI between 35.0 and 39.9.5 The 2021 ACOG Practice Bulletin on Obesity in Pregnancy rates this recommendation as Level C—based primarily on consensus and expert opinion.6
A 2018 Royal College of Obstetricians and Gynecologists Green-top Guideline recognizes “definitive recommendations for fetal surveillance are hampered by the lack of randomized controlled trials demonstrating that antepartum fetal surveillance decreases perinatal morbidity or mortality in late-term and post-term gestations…. There are no definitive studies determining the optimal type or frequency of such testing and no evidence specific for women with obesity.”7
A 2019 Society of Obstetricians and Gynecologists of Canada practice guideline states “stillbirth is more common with maternal obesity” and recommends “increased fetal surveillance … in the third trimester if reduced fetal movements are reported.” The guideline notes “the role for non-stress tests … in surveillance of well-being in this population is uncertain.” Also, for pregnant people with a BMI > 30, “assessment of fetal well-being is … recommended weekly from 37 weeks until delivery.” Finally, increased fetal surveillance is recommended in the setting of increased BMI and an abnormal pulsatility index of the umbilical artery and/or maternal uterine artery.8
Editor’s takeaway
Evidence demonstrates that increased maternal BMI is associated with increased stillbirths. However, evidence has not shown that third-trimester antenatal testing decreases this morbidity and mortality. Expert opinion varies, with ACOG recommending weekly antenatal testing from 34 and 37 weeks for pregnant people with a BMI ≥ 40 and of 35 to 39.9, respectively.
Evidence summary
Association between higher maternal BMI and increased risk for stillbirth
The purpose of antenatal testing is to decrease the risk for stillbirth between visits. Because of the resources involved and the risk for false-positives when testing low-risk patients, antenatal testing is reserved for pregnant people with higher risk for stillbirth.
In a retrospective cohort study of more than 2.8 million singleton births including 9030 stillbirths, pregnant people with an elevated BMI had an increased risk for stillbirth compared to those with a normal BMI. The adjusted hazard ratio was 1.71 (95% CI, 1.62-1.83) for those with a BMI of 30.0 to 34.9; 2.04 (95% CI, 1.8-2.21) for those with a BMI of 35.0 to 39.9; and 2.50 (95% CI, 2.28-2.74) for those with a BMI ≥ 40.1
A meta-analysis of 38 studies, which included data on 16,274 stillbirths, found that a 5-unit increase in BMI was associated with an increased risk for stillbirth (relative risk, 1.24; 95% CI, 1.18-1.30).2
Another meta-analysis included 6 cohort studies involving more than 1 million pregnancies and 3 case-control studies involving 2530 stillbirths and 2837 controls from 1980-2005. There was an association between increasing BMI and stillbirth: the odds ratio (OR) was 1.47 (95% CI, 1.08-1.94) for those with a BMI of 25.0 to 29.9 and 2.07 (95% CI, 1.59-2.74) for those with a BMI ≥ 30.0, compared to those with a normal BMI.3
However, a retrospective cohort study of 182,362 singleton births including 442 stillbirths found no association between stillbirth and increasing BMI. The OR was 1.10 (95% CI, 0.90-1.36) for those with a BMI of 25.0 to 29.9 and 1.09 (95% CI, 0.87-1.37) for those with a BMI ≥ 30.0, compared to those with a normal BMI.4 However, this cohort study may have been underpowered to detect an association between stillbirth and BMI.
Recommendations from others
In 2021, ACOG suggested that weekly antenatal testing may be considered from 34w0d for pregnant people with a BMI ≥ 40.0 and from 37w0d for pregnant people with a BMI between 35.0 and 39.9.5 The 2021 ACOG Practice Bulletin on Obesity in Pregnancy rates this recommendation as Level C—based primarily on consensus and expert opinion.6
A 2018 Royal College of Obstetricians and Gynecologists Green-top Guideline recognizes “definitive recommendations for fetal surveillance are hampered by the lack of randomized controlled trials demonstrating that antepartum fetal surveillance decreases perinatal morbidity or mortality in late-term and post-term gestations…. There are no definitive studies determining the optimal type or frequency of such testing and no evidence specific for women with obesity.”7
A 2019 Society of Obstetricians and Gynecologists of Canada practice guideline states “stillbirth is more common with maternal obesity” and recommends “increased fetal surveillance … in the third trimester if reduced fetal movements are reported.” The guideline notes “the role for non-stress tests … in surveillance of well-being in this population is uncertain.” Also, for pregnant people with a BMI > 30, “assessment of fetal well-being is … recommended weekly from 37 weeks until delivery.” Finally, increased fetal surveillance is recommended in the setting of increased BMI and an abnormal pulsatility index of the umbilical artery and/or maternal uterine artery.8
Editor’s takeaway
Evidence demonstrates that increased maternal BMI is associated with increased stillbirths. However, evidence has not shown that third-trimester antenatal testing decreases this morbidity and mortality. Expert opinion varies, with ACOG recommending weekly antenatal testing from 34 and 37 weeks for pregnant people with a BMI ≥ 40 and of 35 to 39.9, respectively.
1. Yao R, Ananth C, Park B, et al; Perinatal Research Consortium. Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol. 2014;210:e1-e9. doi: 10.1016/j.ajog. 2014.01.044
2. Aune D, Saugstad O, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311:1536-1546. doi: 10.1001/jama.2014.2269
3. Chu S, Kim S, Lau J, et al. Maternal obesity and risk of stillbirth: a meta-analysis. Am J Obstet Gynecol. 2007;197:223-228. doi: 10.1016/j.ajog.2007.03.027
4. Mahomed K, Chan G, Norton M. Obesity and the risk of stillbirth—a reappraisal—a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;255:25-28. doi: 10.1016/j.ejogrb. 2020.09.044
5. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197. doi: 10.1097/AOG.0000000000004407
6. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Obesity in pregnancy: ACOG practice bulletin, number 230. Obstet Gynecol. 2021;137:e128-e144. doi: 10.1097/AOG.0000000000004395
7. Denison F, Aedla N, Keag O, et al; Royal College of Obstetricians and Gynaecologists. Care of women with obesity in pregnancy: Green-top Guideline No. 72. BJOG. 2019;126:e62-e106. doi: 10.1111/1471-0528.15386
8. Maxwell C, Gaudet L, Cassir G, et al. Guideline No. 391–Pregnancy and maternal obesity part 1: pre-conception and prenatal care. J Obstet Gynaecol Can. 2019;41:1623-1640. doi: 10.1016/j.jogc. 2019.03.026
1. Yao R, Ananth C, Park B, et al; Perinatal Research Consortium. Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol. 2014;210:e1-e9. doi: 10.1016/j.ajog. 2014.01.044
2. Aune D, Saugstad O, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311:1536-1546. doi: 10.1001/jama.2014.2269
3. Chu S, Kim S, Lau J, et al. Maternal obesity and risk of stillbirth: a meta-analysis. Am J Obstet Gynecol. 2007;197:223-228. doi: 10.1016/j.ajog.2007.03.027
4. Mahomed K, Chan G, Norton M. Obesity and the risk of stillbirth—a reappraisal—a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;255:25-28. doi: 10.1016/j.ejogrb. 2020.09.044
5. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197. doi: 10.1097/AOG.0000000000004407
6. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Obesity in pregnancy: ACOG practice bulletin, number 230. Obstet Gynecol. 2021;137:e128-e144. doi: 10.1097/AOG.0000000000004395
7. Denison F, Aedla N, Keag O, et al; Royal College of Obstetricians and Gynaecologists. Care of women with obesity in pregnancy: Green-top Guideline No. 72. BJOG. 2019;126:e62-e106. doi: 10.1111/1471-0528.15386
8. Maxwell C, Gaudet L, Cassir G, et al. Guideline No. 391–Pregnancy and maternal obesity part 1: pre-conception and prenatal care. J Obstet Gynaecol Can. 2019;41:1623-1640. doi: 10.1016/j.jogc. 2019.03.026
EVIDENCE-BASED REVIEW:
Possibly. Elevated BMI is associated with an increased risk for stillbirth (strength of recommendation [SOR], B; cohort studies and meta-analysis of cohort studies). Three studies found an association between elevated BMI and stillbirth and one did not. However, no studies demonstrate that antenatal testing in pregnant people with higher BMIs decreases stillbirth rates, or that no harm is caused by unnecessary testing or resultant interventions.
Still, in 2021, the American College of Obstetricians and Gynecologists (ACOG) suggested weekly antenatal testing may be considered from 34w0d for pregnant people with a BMI ≥ 40.0 and from 37w0d for pregnant people with a BMI between 35.0 and 39.9 (SOR, C; consensus guideline). Thus, doing the antenatal testing recommended in the ACOG guideline in an attempt to prevent stillbirth is reasonable, given evidence that elevated BMI is associated with stillbirth.
Isolated third nerve palsy: Lessons from the literature and 4 case studies
Of all the cranial nerve (CN) palsies that affect the eye, the third (oculomotor) nerve palsy (TNP) requires the most urgent evaluation.1 Third nerve dysfunction may signal an underlying neurologic emergency, such as ruptured cerebral aneurysm or giant cell arteritis. Early recognition and prompt treatment choices are key to reversing clinical and visual defects. The classic presentation of isolated TNP is a “down and out eye” deviation and ptosis with or without pupillary involvement.1
Recognize varying clinical presentations. TNPs, isolated or not, may be partial or complete, congenital or acquired, pupil involving or pupil sparing. In many cases, patients may have additional constitutional, ocular, or neurologic symptoms or signs, such as ataxia or hemiplegia.2 Recognition of these clinical findings, which at times can be subtle, is crucial. Appropriate clinical diagnosis and management rely on distinguishing isolated TNP from TNP that involves other CNs.2
Further clues to underlying pathology. Disruption of the third nerve can occur anywhere along its course from the oculomotor nucleus in the brain to its terminus at the extraocular muscles in the orbit.2 TNP’s effect on the pupil can often aid in diagnosis.3 Pupil-sparing TNP is usually due to microvascular ischemia, as may occur with diabetes or hypertension. Pupil involvement, though, may be the first sign of a compressive lesion.
Influence of age. Among individuals older than 60 years, the annual incidence of isolated TNP has been shown to be 12.5 per 100,000, compared with 1.7 per 100,000 in those younger than 60 years.4 In those older than 50 years, microvascular ischemia tends to be the dominant cause.4 Other possible causes include aneurysm, trauma, and neoplasm, particularly pituitary adenoma and metastatic tumor. In childhood and young adulthood, the most common cause of TNP is trauma.5
Use of vascular imaging is influenced by an individual’s age and clinical risk for an aneurysm. Isolated partial TNP or TNP with pupil involvement suggest compression of the third nerve and the need for immediate imaging. Given the dire implications of intracranial aneurysm, most physicians will focus their initial evaluation on vascular imaging, if available.2 If clinical findings instead suggest underlying microvascular ischemia, a delay of imaging may be possible.
In the text that follows, we present 4 patient cases describing the clinical investigative process and treatment determinations based on an individual’s history, clinical presentation, and neurologic findings.
CASE 1
Herpes zoster ophthalmicus
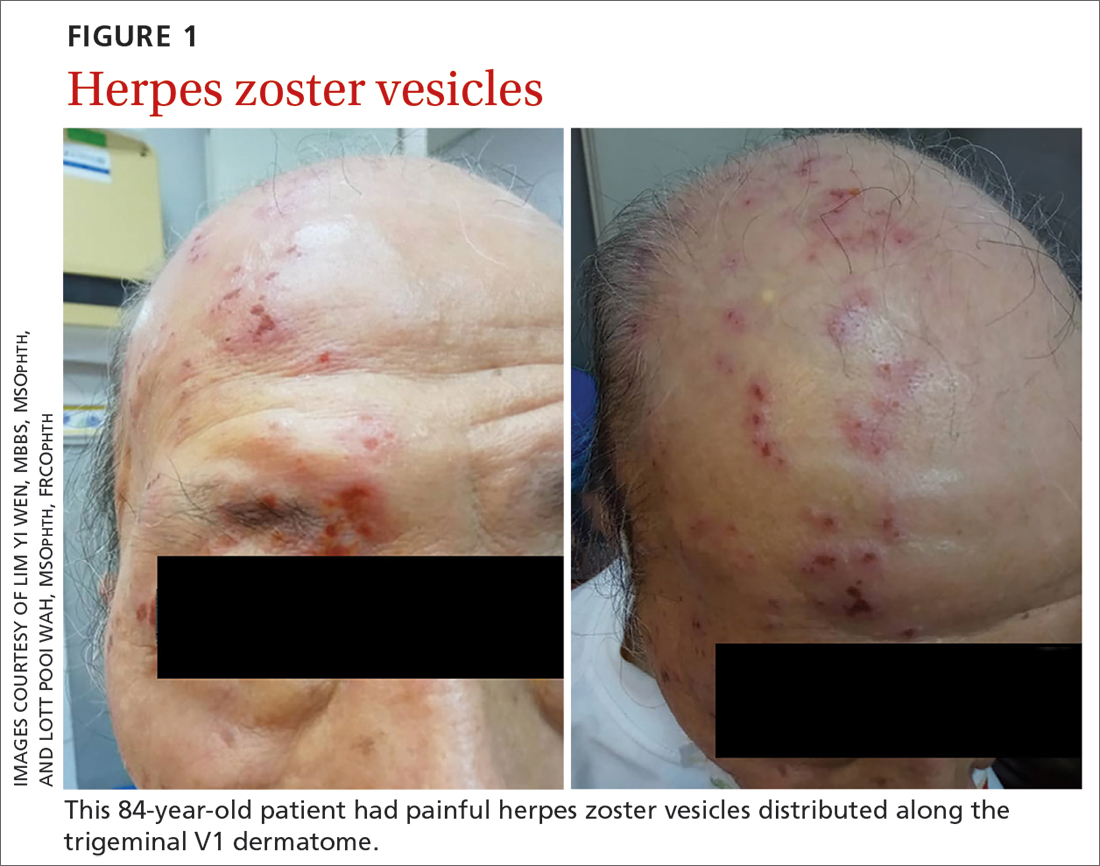
An 84-year-old man with no known medical illness presented to the emergency department (ED) with vesicular skin lesions that had appeared 4 days earlier over his scalp, right forehead, and periorbital region. The vesicles followed the distribution of the ophthalmic division of the trigeminal nerve (FIGURE 1). The patient was given a diagnosis of shingles. The only notable ocular features were the swollen right upper eyelid, injected conjunctiva, and reduced corneal sensation with otherwise normal right eye vision at 6/6. For right eye herpes zoster ophthalmicus (HZO), he was prescribed oral acyclovir 800 mg 5 times per day for 2 weeks.
Continue to: Two days later...
Two days later, he returned after experiencing a sudden onset of binocular diplopia and ptosis of the right eye. Partial ptosis was noted, with restricted adduction and elevation. Pupils were reactive and equal bilaterally. Hutchinson sign, which would indicate an impaired nasociliary nerve and increased risk for corneal and ocular sequelae,6 was absent. Relative afferent pupillary defect also was absent. All other CN functions were intact, with no systemic neurologic deficit. Contrast CT of the brain and orbit showed no radiologic evidence of meningitis, space-occupying lesion, or cerebral aneurysm.
Given the unremarkable imaging findings and lack of symptoms of meningism (eg, headache, vomiting, neck stiffness, or fever), we diagnosed right eye pupil-sparing partial TNP secondary to HZO. The patient continued taking oral acyclovir, which was tapered over 6 weeks. After 4 weeks of antiviral treatment, he recovered full extraocular movement and the ptosis subsided.
CASE 2
Posterior communicating artery aneurysm
A 71-year-old woman with hypercholesterolemia, hypertension, and ischemic heart disease presented to the ED with a 4-day history of headache, vomiting, and neck pain and a 2-day history of a drooping left eyelid. When asked if she had double vision, she said “No.” She had no other neurologic symptoms. Her blood pressure (BP) was 199/88 mm Hg. An initial plain CT of the brain ruled out ischemia, intracranial hemorrhage, and space-occupying lesion.
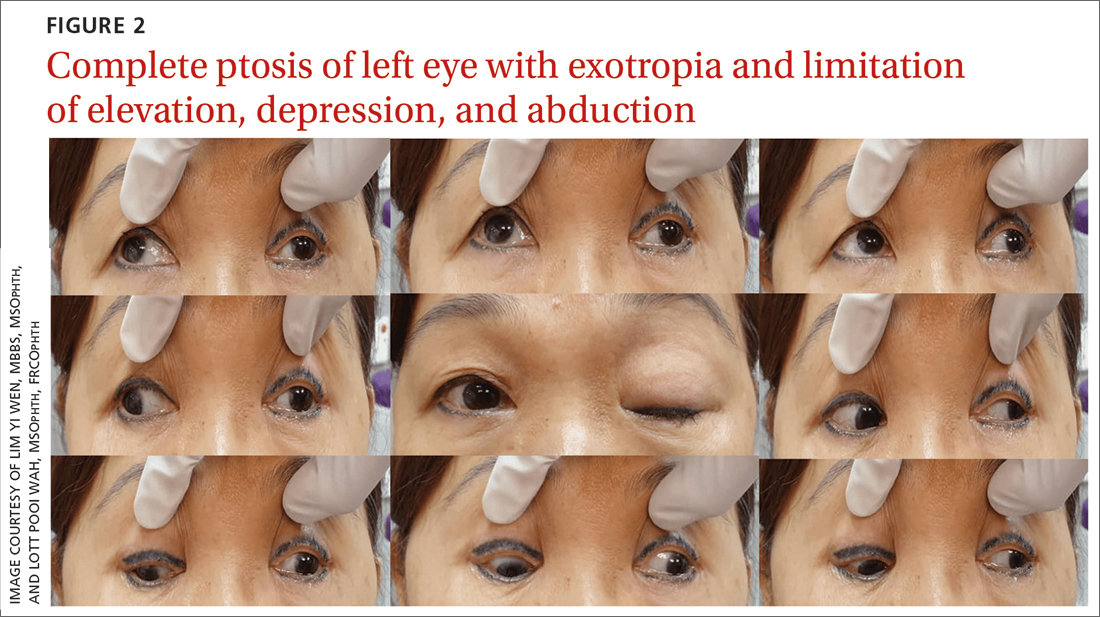
Once her BP was stabilized, she was referred to us for detailed eye assessment. Her best corrected visual acuity was 6/12 bilaterally. In contrast to her right eye pupil, which was 4 mm in diameter and reactive, her left eye pupil was 7 mm and poorly reactive to light. Optic nerve functions were preserved. There was complete ptosis of the left eye, with exotropia and total limitation of elevation, depression, and abduction (FIGURE 2). There was no proptosis; intraocular pressure was normal. Fundus examination of the left eye was unremarkable. All other CN and neurologic examinations were normal. We diagnosed left eye pupil-involving TNP.
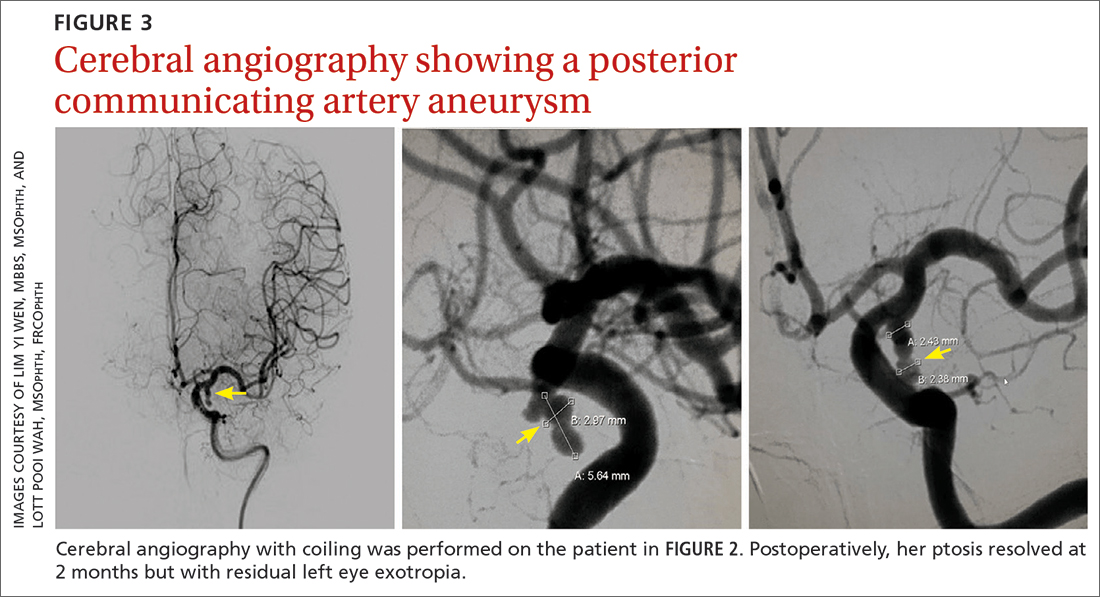
Further assessment of the brain with magnetic resonance imaging (MRI) revealed a left posterior communicating artery aneurysm. We performed cerebral angiography (FIGURE 3) with coiling. Postoperatively, her ptosis resolved at 2 months but with residual left eye exotropia.
CASE 3
Viral infection
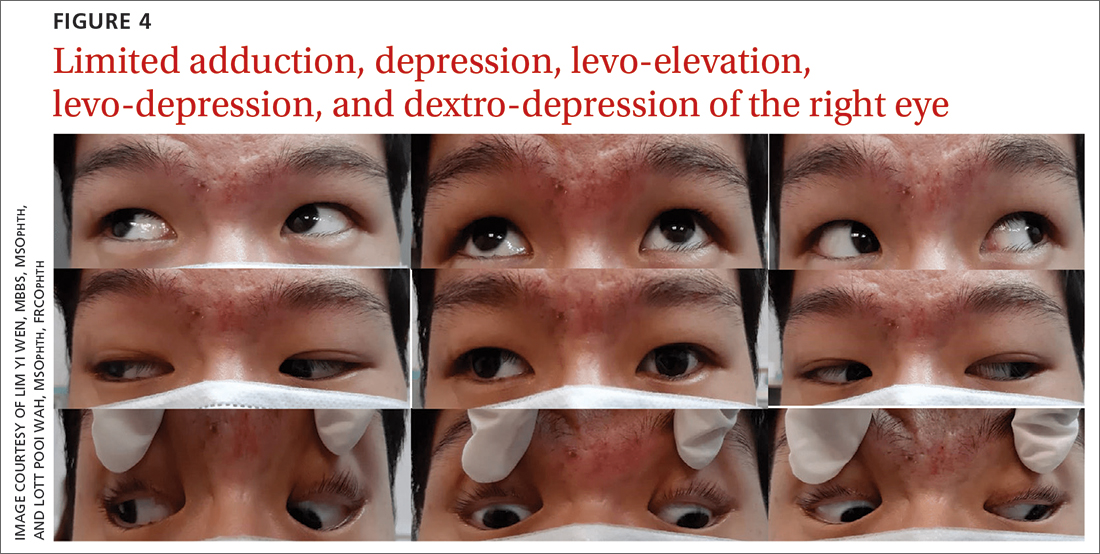
A 20-year-old male student presented to the ED for evaluation of acute-onset diplopia that was present upon awakening from sleep 4 days earlier. There was no ptosis or other neurologic symptoms. He had no history of trauma or viral illness. Examination revealed limited adduction, depression, levo-elevation, levo-depression, and dextro-depression in the right eye (FIGURE 4). Both pupils were reactive. There was no sign of aberrant third nerve regeneration. The optic nerve and other CN functions were intact. A systemic neurologic examination was unremarkable, and the fundus was normal, with no optic disc swelling. All blood work was negative for diabetes, hypercoagulability, and hyperlipidemia.
CT angiography (CTA) and MR angiography (MRA) did not reveal any vascular abnormalities such as intracranial aneurysms, arteriovenous malformations, or berry aneurysm. We treated the patient for right eye partial TNP secondary to presumed prior viral infection that led to an immune-mediated palsy of the third nerve. He was given a short course of low-dose oral prednisolone (30 mg/d for 5 days). He achieved full recovery of his ocular motility after 2 weeks.
Continue to: CASE 4
CASE 4
Trauma
A 33-year-old woman was brought to the ED after she was knocked off her motorbike by a car. A passerby found her unconscious and still wearing her helmet. En route to the hospital, the patient regained consciousness but had retrograde amnesia.
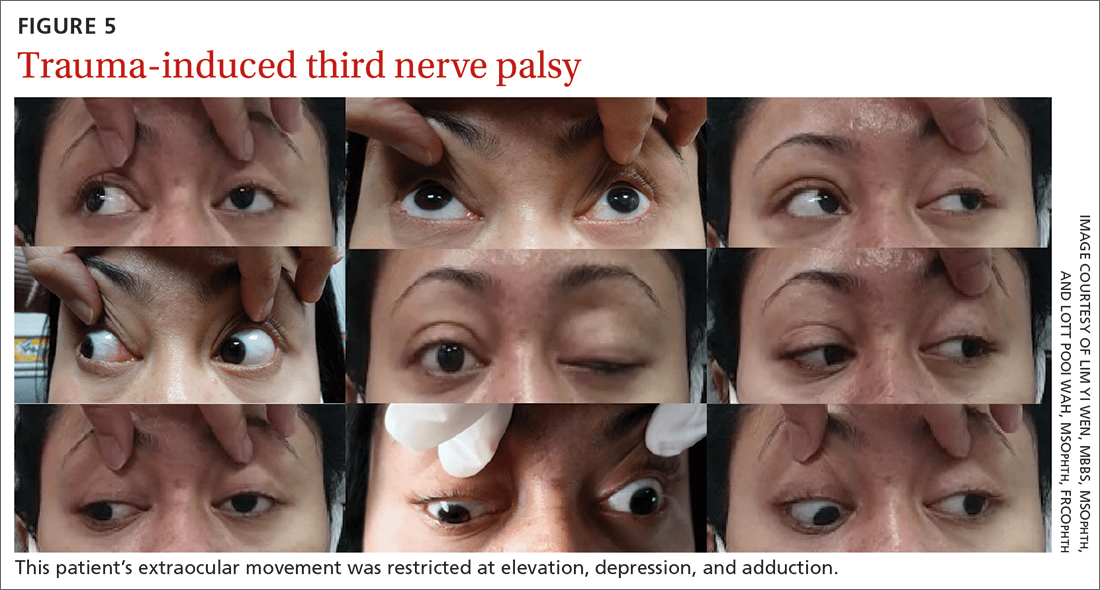
She was referred to us for evaluation of complete ptosis of her left eye. She was fully conscious during the examination. Her left eye vision was 6/9. Complete ptosis with exotropia was noted. Pupillary examination revealed a sluggish dilated left eye pupil of 7 mm with no reverse relative afferent pupillary defect. Extraocular movement was restricted at elevation, depression, and adduction with diplopia (FIGURE 5). All other CN functions were preserved.
CT of the brain and orbit revealed acute right frontotemporal subarachnoid hemorrhage (FIGURE 6). There was no radiologic evidence of orbital wall fractures or extraocular muscle entrapment. She remained stable during the first 24 hours of monitoring and was given a diagnosis of
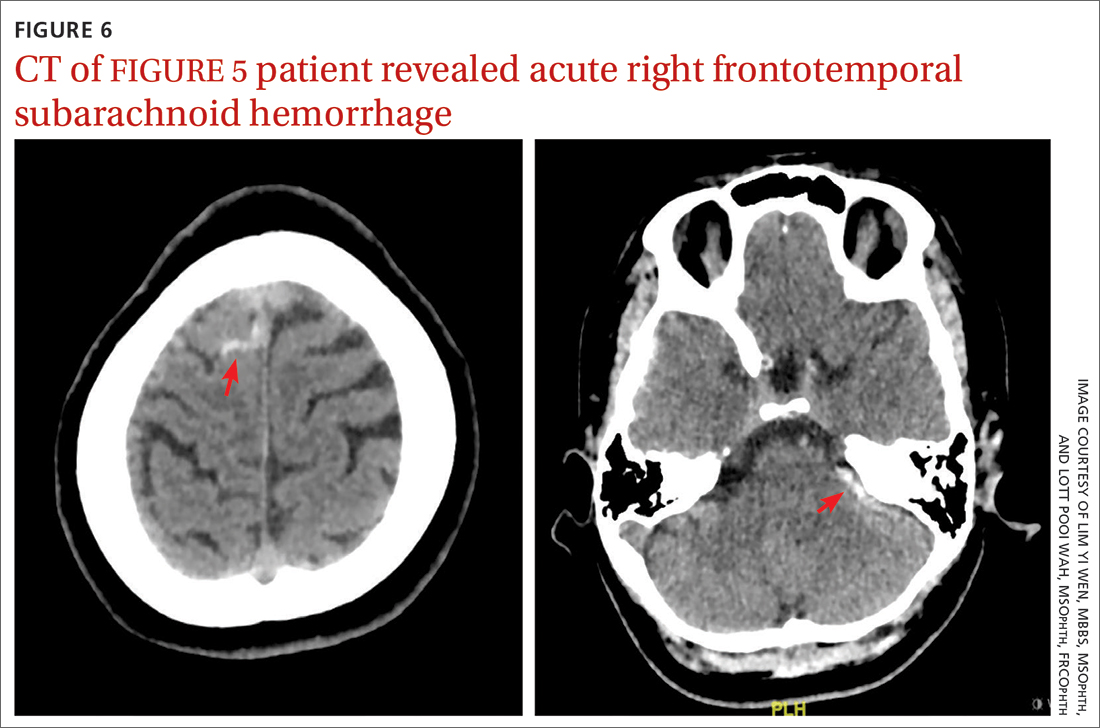
Repeat CT of the brain 5 days later revealed complete resolution of the subarachnoid hemorrhage. The patient's clinical condition improved 2 weeks later and included resolution of ptosis and recovery of ocular motility.
Key takeaways from the cases
Case 1: Herpes zoster ophthalmicus
Clinical diagnosis of HZO is straightforward, with painful vesicular lesions occurring along the trigeminal nerve (V1) dermatome, as was seen in this case. The oculomotor nerve is the CN most commonly involved; the trochlear nerve is the least-often affected.6 In a report from the Mayo Clinic, 3 of 86 patients with HZO had oculomotor nerve palsies (3.4%).7 A separate review from an eye hospital study stated that 9.8% (n = 133) of 1356 patients with HZO had extraocular muscle palsy, with TNP in 4 of the patients.8
Ocular complications such as blepharitis, keratoconjunctivits, or iritis occur in 20% to 70% of HZO cases.9 Ophthalmoplegia, which most often involves the oculomotor nerve, is seen in 7% to 31% of HZO cases (mostly in the elderly) and usually occurs within 1 to 3 weeks of the onset of rash.6 Our patient immediately underwent contrast CT of the brain to rule out meningitis and nerve compression.
Treatment with a systemic antiviral agent is crucial. Acyclovir, valaciclovir, and famciclovir are available treatment options, used for treating the skin lesions, reducing the viral load, and reducing the risk for ocular involvement or its progression. Our patient started a 2-week course of oral acyclovir 800 mg 5 times per day. Ophthalmoplegia is usually self-limiting and has a good prognosis. Time to resolution varies from 2 to 18 months. Diplopia, if present, resolves within 1 year.6 Our patient achieved full recovery of his extraocular movement after completing 4 weeks of antiviral treatment.
Continue to: Case 2
Case 2: Posterior communicating artery aneurysm
Given the patient’s high BP, ruling out a hypertensive emergency with CT was the first priority. TNP caused by microvascular ischemia is not uncommon in the elderly. However, her pupil involvement and persistent headache called for an MRI to better evaluate the soft tissues and to rule out possible vascular pathologies. Left posterior communicating artery aneurysm was discovered with MRI, and urgent cerebral angiography and coiling was performed successfully.
Incidence. One report of 1400 patients with TNP confirmed that aneurysm was the cause in 10% of cases, with posterior communicating artery aneurysm accounting for the greatest number, 119 (25.7%).10 Of these cases of posterior communicating artery aneurysm, pupillary involvement was detected in 108 (90.8%). The oculomotor nerve lies adjacent to the posterior communicating artery as it passes through the subarachnoid space of the basal cisterns, where it is susceptible to compression.3
A high index of suspicion for posterior communicating artery aneurysm is crucial for early detection and lifesaving treatment. The patient in this case did well after the coiling. Her ptosis resolved at 2 months, although she had residual left eye exotropia.
Case 3: Viral infection
We chose CTA of the brain instead of contrast CT to rule out the possibility of intracranial aneurysm. CTA has been shown to be an adequate first-line study to detect aneurysms, particularly those greater than 4 mm in diameter.2,11 One study demonstrated an 81.8% sensitivity for aneurysms smaller than 3 mm when performed on a 320-slice CT.12
Additional imaging selection. We also selected MRA to rule out berry aneurysm, which is often asymptomatic. We decided against MRI because of its higher cost and longer acquisition time. It is usually reserved for patients with a negative initial work-up with CT or cerebral angiography if suspicion of a possible aneurysm remains.11 The MRA finding in this case was negative, and we made a presumptive diagnosis of TNP secondary to viral infection.
Isolated TNP following viral infection is a clinical diagnosis of exclusion. In 1 reported case, a 39-year-old man developed a superior division palsy after a common cold without fever, underwent no serologic study, and recovered spontaneously 6 weeks later.13 A 5-year-old boy who experienced a superior division palsy immediately after a common cold with fever was found on serologic examination to have an increased titre of influenza A virus. His palsy resolved in 4 months.14
The exact mechanism of viral-induced palsy is unknown. The possibility of postinfectious cranial neuropathy has been postulated, as most reported cases following a flu-like illness resolved within a few months.15 Although the pathogenesis remains speculative, an autoimmune process might have been involved.16 Our patient recovered fully in 1 month following a short course of oral prednisolone 30 mg/d for 5 days.
Case 4: Trauma
Trauma accounts for approximately 12% of all TNP cases.17 Traumatic TNPs are usually sustained in severe, high-speed, closed-head injuries, and are often associated with other CN injuries and neurologic deficits. The damage may be caused indirectly by compression, hemorrhage, or ischemia, or directly at certain vulnerable points including the nerve’s exit from the brainstem and the point at which it crosses the petroclinoid ligament.17 In our case, despite the patient having complete TNP, there was no sign of localized orbital trauma on the CT other than the presence of subarachnoid hemorrhage at the right frontotemporal region.
In a similar reported case, the patient had a right traumatic isolated TNP and was found to have left frontal subarachnoid hemorrhage with no sign of orbital trauma.18 However, the mechanisms of isolated TNP caused by traumatic brain injury are not clear. Possible causes include rootlet avulsion, distal fascicular damage, stretching of the nerve (including the parasellar segment), and decreased blood supply.18
It has been suggested that TNP is more frequently observed in cases of frontal region injury. As orbitofrontal regions are predominantly affected by cortical contusions, the risk for ocular involvement increases.19
Keep these fundamentals in mind
The diagnosis and management of isolated TNP are guided by the patient’s age, by the degree to which each of the oculomotor nerve’s 2 major functions—pupillomotor and oculomotor—are affected, and by the circumstances preceding the onset of TNP.2 Cases 1 and 3 in our series presented with partial TNP, while Cases 2 and 4 exhibited complete TNP. Pupillary involvement was detected only in Case 2. Nevertheless, radiologic imaging was ordered for all 4 cases after the diagnosis of TNP was made, to exclude the most worrying neurologic emergencies. The choice of imaging modality depends on not only the availability of the services but also the clinical signs and symptoms and presumptive clinical diagnosis. A tailored and thoughtful approach with consideration of the anatomy and varied pathologies help clinicians to skillfully discern emergencies from nonurgent cases.
CORRESPONDENCE
Lott Pooi Wah, MSOphth, FRCOphth, Department of Ophthalmology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] Orcid no: 0000-0001-8746-1528
1. Radia M, Stahl M, Arunakirinathan M, et al. Examination of a third nerve palsy. Brit J Hosp Med. 2017;78:188-192. doi: 10.12968/hmed.2017.78.12.C188
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Motoyama Y, Nonaka J, Hironaka Y, et al. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52:202-205. doi: 10.2176/nmc.52.202
4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthal mol.2016.4456
5. Wyatt K. Three common ophthalmic emergencies. JAAPA. 2014;27:32-37. doi: 10.1097/01.JAA.0000447004.96714.34
6. Daswani M, Bhosale N, Shah VM. Rare case of herpes zoster ophthalmicus with orbital myositis, oculomotor nerve palsy and anterior uveitis. Indian J Dermatol Venereol Leprol. 2017;83:365-367. doi: 10.4103/0378-6323.199582
7. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-45. doi: 10.1001/archopht.1983.01040010044004
8. Marsh RJ, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol. 1977;61:667-682. doi: 10.1136/bjo.61.11.677
9. Lim JJ, Ong YM, Zalina MCW, et al. Herpes zoster ophthalmicus with orbital apex syndrome – difference in outcomes and literature review. Ocul Immunol Inflamm. 2017;26:187-193. doi: 10.1080/09273948.2017.1327604
10. Keane JR. Third nerve palsy: analysis of 1400 personally-examined patients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
11. Yoon NK, McNally S, Taussky P, et al. Imaging of cerebral aneurysms: a clinical perspective. Neurovasc Imaging. 2016;2:6. doi: 10.1186/s40809-016-0016-3
12. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15-20. doi: 10.1016/j.crad.2012.09.001
13. Derakhshan I. Superior branch palsy with spontaneous recovery. Ann Neurol. 1978;4:478-479. doi: 10.1002/ana.410040519
14. Engelhardt A, Credzich C, Kompf D. Isolated superior branch palsy of the oculomotor nerve in influenza A. Neuroophthalmol. 1989;9:233-235. doi: 10.3109/01658108908997359
15. Knox DL, Clark DB, Schuster FF. Benign VI nerve palsies in children. Pediatrics. 1967;40:560-564.
16. Saeki N, Yotsukura J, Adachi E, et al. Isolated superior division oculomotor palsy in a child with spontaneous recovery. J Clin Neurosci. 2000;7:62-64. doi: 10.1054/jocn.1998.0152
17. Nagendran ST, Lee V, Perry M. Traumatic orbital third nerve palsy. Brit J Oral Maxillofac Surg. 2019;57:578-581. doi: 10.1016/j.bjoms.2019.01.029
18. Kim T, Nam K, Kwon BS. Isolated oculomotor nerve palsy in mild traumatic brain injury: a literature review. Am J Phys Med Rehabil. 2020;99:430-435. doi: 10.1097/PHM.0000000000001316
19. Sharma B, Gupta R, Anand R, et al. Ocular manifestations of head injury and incidence of post-traumatic ocular motor nerve involvement in cases of head injury: a clinical review. Int Ophthalmol. 2014;34:893-900. doi: 10.1007/s10792-014-9898-8
Of all the cranial nerve (CN) palsies that affect the eye, the third (oculomotor) nerve palsy (TNP) requires the most urgent evaluation.1 Third nerve dysfunction may signal an underlying neurologic emergency, such as ruptured cerebral aneurysm or giant cell arteritis. Early recognition and prompt treatment choices are key to reversing clinical and visual defects. The classic presentation of isolated TNP is a “down and out eye” deviation and ptosis with or without pupillary involvement.1
Recognize varying clinical presentations. TNPs, isolated or not, may be partial or complete, congenital or acquired, pupil involving or pupil sparing. In many cases, patients may have additional constitutional, ocular, or neurologic symptoms or signs, such as ataxia or hemiplegia.2 Recognition of these clinical findings, which at times can be subtle, is crucial. Appropriate clinical diagnosis and management rely on distinguishing isolated TNP from TNP that involves other CNs.2
Further clues to underlying pathology. Disruption of the third nerve can occur anywhere along its course from the oculomotor nucleus in the brain to its terminus at the extraocular muscles in the orbit.2 TNP’s effect on the pupil can often aid in diagnosis.3 Pupil-sparing TNP is usually due to microvascular ischemia, as may occur with diabetes or hypertension. Pupil involvement, though, may be the first sign of a compressive lesion.
Influence of age. Among individuals older than 60 years, the annual incidence of isolated TNP has been shown to be 12.5 per 100,000, compared with 1.7 per 100,000 in those younger than 60 years.4 In those older than 50 years, microvascular ischemia tends to be the dominant cause.4 Other possible causes include aneurysm, trauma, and neoplasm, particularly pituitary adenoma and metastatic tumor. In childhood and young adulthood, the most common cause of TNP is trauma.5
Use of vascular imaging is influenced by an individual’s age and clinical risk for an aneurysm. Isolated partial TNP or TNP with pupil involvement suggest compression of the third nerve and the need for immediate imaging. Given the dire implications of intracranial aneurysm, most physicians will focus their initial evaluation on vascular imaging, if available.2 If clinical findings instead suggest underlying microvascular ischemia, a delay of imaging may be possible.
In the text that follows, we present 4 patient cases describing the clinical investigative process and treatment determinations based on an individual’s history, clinical presentation, and neurologic findings.
CASE 1
Herpes zoster ophthalmicus
An 84-year-old man with no known medical illness presented to the emergency department (ED) with vesicular skin lesions that had appeared 4 days earlier over his scalp, right forehead, and periorbital region. The vesicles followed the distribution of the ophthalmic division of the trigeminal nerve (FIGURE 1). The patient was given a diagnosis of shingles. The only notable ocular features were the swollen right upper eyelid, injected conjunctiva, and reduced corneal sensation with otherwise normal right eye vision at 6/6. For right eye herpes zoster ophthalmicus (HZO), he was prescribed oral acyclovir 800 mg 5 times per day for 2 weeks.

Continue to: Two days later...
Two days later, he returned after experiencing a sudden onset of binocular diplopia and ptosis of the right eye. Partial ptosis was noted, with restricted adduction and elevation. Pupils were reactive and equal bilaterally. Hutchinson sign, which would indicate an impaired nasociliary nerve and increased risk for corneal and ocular sequelae,6 was absent. Relative afferent pupillary defect also was absent. All other CN functions were intact, with no systemic neurologic deficit. Contrast CT of the brain and orbit showed no radiologic evidence of meningitis, space-occupying lesion, or cerebral aneurysm.
Given the unremarkable imaging findings and lack of symptoms of meningism (eg, headache, vomiting, neck stiffness, or fever), we diagnosed right eye pupil-sparing partial TNP secondary to HZO. The patient continued taking oral acyclovir, which was tapered over 6 weeks. After 4 weeks of antiviral treatment, he recovered full extraocular movement and the ptosis subsided.
CASE 2
Posterior communicating artery aneurysm
A 71-year-old woman with hypercholesterolemia, hypertension, and ischemic heart disease presented to the ED with a 4-day history of headache, vomiting, and neck pain and a 2-day history of a drooping left eyelid. When asked if she had double vision, she said “No.” She had no other neurologic symptoms. Her blood pressure (BP) was 199/88 mm Hg. An initial plain CT of the brain ruled out ischemia, intracranial hemorrhage, and space-occupying lesion.
Once her BP was stabilized, she was referred to us for detailed eye assessment. Her best corrected visual acuity was 6/12 bilaterally. In contrast to her right eye pupil, which was 4 mm in diameter and reactive, her left eye pupil was 7 mm and poorly reactive to light. Optic nerve functions were preserved. There was complete ptosis of the left eye, with exotropia and total limitation of elevation, depression, and abduction (FIGURE 2). There was no proptosis; intraocular pressure was normal. Fundus examination of the left eye was unremarkable. All other CN and neurologic examinations were normal. We diagnosed left eye pupil-involving TNP.

Further assessment of the brain with magnetic resonance imaging (MRI) revealed a left posterior communicating artery aneurysm. We performed cerebral angiography (FIGURE 3) with coiling. Postoperatively, her ptosis resolved at 2 months but with residual left eye exotropia.

CASE 3
Viral infection
A 20-year-old male student presented to the ED for evaluation of acute-onset diplopia that was present upon awakening from sleep 4 days earlier. There was no ptosis or other neurologic symptoms. He had no history of trauma or viral illness. Examination revealed limited adduction, depression, levo-elevation, levo-depression, and dextro-depression in the right eye (FIGURE 4). Both pupils were reactive. There was no sign of aberrant third nerve regeneration. The optic nerve and other CN functions were intact. A systemic neurologic examination was unremarkable, and the fundus was normal, with no optic disc swelling. All blood work was negative for diabetes, hypercoagulability, and hyperlipidemia.

CT angiography (CTA) and MR angiography (MRA) did not reveal any vascular abnormalities such as intracranial aneurysms, arteriovenous malformations, or berry aneurysm. We treated the patient for right eye partial TNP secondary to presumed prior viral infection that led to an immune-mediated palsy of the third nerve. He was given a short course of low-dose oral prednisolone (30 mg/d for 5 days). He achieved full recovery of his ocular motility after 2 weeks.
Continue to: CASE 4
CASE 4
Trauma
A 33-year-old woman was brought to the ED after she was knocked off her motorbike by a car. A passerby found her unconscious and still wearing her helmet. En route to the hospital, the patient regained consciousness but had retrograde amnesia.
She was referred to us for evaluation of complete ptosis of her left eye. She was fully conscious during the examination. Her left eye vision was 6/9. Complete ptosis with exotropia was noted. Pupillary examination revealed a sluggish dilated left eye pupil of 7 mm with no reverse relative afferent pupillary defect. Extraocular movement was restricted at elevation, depression, and adduction with diplopia (FIGURE 5). All other CN functions were preserved.

CT of the brain and orbit revealed acute right frontotemporal subarachnoid hemorrhage (FIGURE 6). There was no radiologic evidence of orbital wall fractures or extraocular muscle entrapment. She remained stable during the first 24 hours of monitoring and was given a diagnosis of

Repeat CT of the brain 5 days later revealed complete resolution of the subarachnoid hemorrhage. The patient's clinical condition improved 2 weeks later and included resolution of ptosis and recovery of ocular motility.
Key takeaways from the cases
Case 1: Herpes zoster ophthalmicus
Clinical diagnosis of HZO is straightforward, with painful vesicular lesions occurring along the trigeminal nerve (V1) dermatome, as was seen in this case. The oculomotor nerve is the CN most commonly involved; the trochlear nerve is the least-often affected.6 In a report from the Mayo Clinic, 3 of 86 patients with HZO had oculomotor nerve palsies (3.4%).7 A separate review from an eye hospital study stated that 9.8% (n = 133) of 1356 patients with HZO had extraocular muscle palsy, with TNP in 4 of the patients.8
Ocular complications such as blepharitis, keratoconjunctivits, or iritis occur in 20% to 70% of HZO cases.9 Ophthalmoplegia, which most often involves the oculomotor nerve, is seen in 7% to 31% of HZO cases (mostly in the elderly) and usually occurs within 1 to 3 weeks of the onset of rash.6 Our patient immediately underwent contrast CT of the brain to rule out meningitis and nerve compression.
Treatment with a systemic antiviral agent is crucial. Acyclovir, valaciclovir, and famciclovir are available treatment options, used for treating the skin lesions, reducing the viral load, and reducing the risk for ocular involvement or its progression. Our patient started a 2-week course of oral acyclovir 800 mg 5 times per day. Ophthalmoplegia is usually self-limiting and has a good prognosis. Time to resolution varies from 2 to 18 months. Diplopia, if present, resolves within 1 year.6 Our patient achieved full recovery of his extraocular movement after completing 4 weeks of antiviral treatment.
Continue to: Case 2
Case 2: Posterior communicating artery aneurysm
Given the patient’s high BP, ruling out a hypertensive emergency with CT was the first priority. TNP caused by microvascular ischemia is not uncommon in the elderly. However, her pupil involvement and persistent headache called for an MRI to better evaluate the soft tissues and to rule out possible vascular pathologies. Left posterior communicating artery aneurysm was discovered with MRI, and urgent cerebral angiography and coiling was performed successfully.
Incidence. One report of 1400 patients with TNP confirmed that aneurysm was the cause in 10% of cases, with posterior communicating artery aneurysm accounting for the greatest number, 119 (25.7%).10 Of these cases of posterior communicating artery aneurysm, pupillary involvement was detected in 108 (90.8%). The oculomotor nerve lies adjacent to the posterior communicating artery as it passes through the subarachnoid space of the basal cisterns, where it is susceptible to compression.3
A high index of suspicion for posterior communicating artery aneurysm is crucial for early detection and lifesaving treatment. The patient in this case did well after the coiling. Her ptosis resolved at 2 months, although she had residual left eye exotropia.
Case 3: Viral infection
We chose CTA of the brain instead of contrast CT to rule out the possibility of intracranial aneurysm. CTA has been shown to be an adequate first-line study to detect aneurysms, particularly those greater than 4 mm in diameter.2,11 One study demonstrated an 81.8% sensitivity for aneurysms smaller than 3 mm when performed on a 320-slice CT.12
Additional imaging selection. We also selected MRA to rule out berry aneurysm, which is often asymptomatic. We decided against MRI because of its higher cost and longer acquisition time. It is usually reserved for patients with a negative initial work-up with CT or cerebral angiography if suspicion of a possible aneurysm remains.11 The MRA finding in this case was negative, and we made a presumptive diagnosis of TNP secondary to viral infection.
Isolated TNP following viral infection is a clinical diagnosis of exclusion. In 1 reported case, a 39-year-old man developed a superior division palsy after a common cold without fever, underwent no serologic study, and recovered spontaneously 6 weeks later.13 A 5-year-old boy who experienced a superior division palsy immediately after a common cold with fever was found on serologic examination to have an increased titre of influenza A virus. His palsy resolved in 4 months.14
The exact mechanism of viral-induced palsy is unknown. The possibility of postinfectious cranial neuropathy has been postulated, as most reported cases following a flu-like illness resolved within a few months.15 Although the pathogenesis remains speculative, an autoimmune process might have been involved.16 Our patient recovered fully in 1 month following a short course of oral prednisolone 30 mg/d for 5 days.
Case 4: Trauma
Trauma accounts for approximately 12% of all TNP cases.17 Traumatic TNPs are usually sustained in severe, high-speed, closed-head injuries, and are often associated with other CN injuries and neurologic deficits. The damage may be caused indirectly by compression, hemorrhage, or ischemia, or directly at certain vulnerable points including the nerve’s exit from the brainstem and the point at which it crosses the petroclinoid ligament.17 In our case, despite the patient having complete TNP, there was no sign of localized orbital trauma on the CT other than the presence of subarachnoid hemorrhage at the right frontotemporal region.
In a similar reported case, the patient had a right traumatic isolated TNP and was found to have left frontal subarachnoid hemorrhage with no sign of orbital trauma.18 However, the mechanisms of isolated TNP caused by traumatic brain injury are not clear. Possible causes include rootlet avulsion, distal fascicular damage, stretching of the nerve (including the parasellar segment), and decreased blood supply.18
It has been suggested that TNP is more frequently observed in cases of frontal region injury. As orbitofrontal regions are predominantly affected by cortical contusions, the risk for ocular involvement increases.19
Keep these fundamentals in mind
The diagnosis and management of isolated TNP are guided by the patient’s age, by the degree to which each of the oculomotor nerve’s 2 major functions—pupillomotor and oculomotor—are affected, and by the circumstances preceding the onset of TNP.2 Cases 1 and 3 in our series presented with partial TNP, while Cases 2 and 4 exhibited complete TNP. Pupillary involvement was detected only in Case 2. Nevertheless, radiologic imaging was ordered for all 4 cases after the diagnosis of TNP was made, to exclude the most worrying neurologic emergencies. The choice of imaging modality depends on not only the availability of the services but also the clinical signs and symptoms and presumptive clinical diagnosis. A tailored and thoughtful approach with consideration of the anatomy and varied pathologies help clinicians to skillfully discern emergencies from nonurgent cases.
CORRESPONDENCE
Lott Pooi Wah, MSOphth, FRCOphth, Department of Ophthalmology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] Orcid no: 0000-0001-8746-1528
Of all the cranial nerve (CN) palsies that affect the eye, the third (oculomotor) nerve palsy (TNP) requires the most urgent evaluation.1 Third nerve dysfunction may signal an underlying neurologic emergency, such as ruptured cerebral aneurysm or giant cell arteritis. Early recognition and prompt treatment choices are key to reversing clinical and visual defects. The classic presentation of isolated TNP is a “down and out eye” deviation and ptosis with or without pupillary involvement.1
Recognize varying clinical presentations. TNPs, isolated or not, may be partial or complete, congenital or acquired, pupil involving or pupil sparing. In many cases, patients may have additional constitutional, ocular, or neurologic symptoms or signs, such as ataxia or hemiplegia.2 Recognition of these clinical findings, which at times can be subtle, is crucial. Appropriate clinical diagnosis and management rely on distinguishing isolated TNP from TNP that involves other CNs.2
Further clues to underlying pathology. Disruption of the third nerve can occur anywhere along its course from the oculomotor nucleus in the brain to its terminus at the extraocular muscles in the orbit.2 TNP’s effect on the pupil can often aid in diagnosis.3 Pupil-sparing TNP is usually due to microvascular ischemia, as may occur with diabetes or hypertension. Pupil involvement, though, may be the first sign of a compressive lesion.
Influence of age. Among individuals older than 60 years, the annual incidence of isolated TNP has been shown to be 12.5 per 100,000, compared with 1.7 per 100,000 in those younger than 60 years.4 In those older than 50 years, microvascular ischemia tends to be the dominant cause.4 Other possible causes include aneurysm, trauma, and neoplasm, particularly pituitary adenoma and metastatic tumor. In childhood and young adulthood, the most common cause of TNP is trauma.5
Use of vascular imaging is influenced by an individual’s age and clinical risk for an aneurysm. Isolated partial TNP or TNP with pupil involvement suggest compression of the third nerve and the need for immediate imaging. Given the dire implications of intracranial aneurysm, most physicians will focus their initial evaluation on vascular imaging, if available.2 If clinical findings instead suggest underlying microvascular ischemia, a delay of imaging may be possible.
In the text that follows, we present 4 patient cases describing the clinical investigative process and treatment determinations based on an individual’s history, clinical presentation, and neurologic findings.
CASE 1
Herpes zoster ophthalmicus
An 84-year-old man with no known medical illness presented to the emergency department (ED) with vesicular skin lesions that had appeared 4 days earlier over his scalp, right forehead, and periorbital region. The vesicles followed the distribution of the ophthalmic division of the trigeminal nerve (FIGURE 1). The patient was given a diagnosis of shingles. The only notable ocular features were the swollen right upper eyelid, injected conjunctiva, and reduced corneal sensation with otherwise normal right eye vision at 6/6. For right eye herpes zoster ophthalmicus (HZO), he was prescribed oral acyclovir 800 mg 5 times per day for 2 weeks.

Continue to: Two days later...
Two days later, he returned after experiencing a sudden onset of binocular diplopia and ptosis of the right eye. Partial ptosis was noted, with restricted adduction and elevation. Pupils were reactive and equal bilaterally. Hutchinson sign, which would indicate an impaired nasociliary nerve and increased risk for corneal and ocular sequelae,6 was absent. Relative afferent pupillary defect also was absent. All other CN functions were intact, with no systemic neurologic deficit. Contrast CT of the brain and orbit showed no radiologic evidence of meningitis, space-occupying lesion, or cerebral aneurysm.
Given the unremarkable imaging findings and lack of symptoms of meningism (eg, headache, vomiting, neck stiffness, or fever), we diagnosed right eye pupil-sparing partial TNP secondary to HZO. The patient continued taking oral acyclovir, which was tapered over 6 weeks. After 4 weeks of antiviral treatment, he recovered full extraocular movement and the ptosis subsided.
CASE 2
Posterior communicating artery aneurysm
A 71-year-old woman with hypercholesterolemia, hypertension, and ischemic heart disease presented to the ED with a 4-day history of headache, vomiting, and neck pain and a 2-day history of a drooping left eyelid. When asked if she had double vision, she said “No.” She had no other neurologic symptoms. Her blood pressure (BP) was 199/88 mm Hg. An initial plain CT of the brain ruled out ischemia, intracranial hemorrhage, and space-occupying lesion.
Once her BP was stabilized, she was referred to us for detailed eye assessment. Her best corrected visual acuity was 6/12 bilaterally. In contrast to her right eye pupil, which was 4 mm in diameter and reactive, her left eye pupil was 7 mm and poorly reactive to light. Optic nerve functions were preserved. There was complete ptosis of the left eye, with exotropia and total limitation of elevation, depression, and abduction (FIGURE 2). There was no proptosis; intraocular pressure was normal. Fundus examination of the left eye was unremarkable. All other CN and neurologic examinations were normal. We diagnosed left eye pupil-involving TNP.

Further assessment of the brain with magnetic resonance imaging (MRI) revealed a left posterior communicating artery aneurysm. We performed cerebral angiography (FIGURE 3) with coiling. Postoperatively, her ptosis resolved at 2 months but with residual left eye exotropia.

CASE 3
Viral infection
A 20-year-old male student presented to the ED for evaluation of acute-onset diplopia that was present upon awakening from sleep 4 days earlier. There was no ptosis or other neurologic symptoms. He had no history of trauma or viral illness. Examination revealed limited adduction, depression, levo-elevation, levo-depression, and dextro-depression in the right eye (FIGURE 4). Both pupils were reactive. There was no sign of aberrant third nerve regeneration. The optic nerve and other CN functions were intact. A systemic neurologic examination was unremarkable, and the fundus was normal, with no optic disc swelling. All blood work was negative for diabetes, hypercoagulability, and hyperlipidemia.

CT angiography (CTA) and MR angiography (MRA) did not reveal any vascular abnormalities such as intracranial aneurysms, arteriovenous malformations, or berry aneurysm. We treated the patient for right eye partial TNP secondary to presumed prior viral infection that led to an immune-mediated palsy of the third nerve. He was given a short course of low-dose oral prednisolone (30 mg/d for 5 days). He achieved full recovery of his ocular motility after 2 weeks.
Continue to: CASE 4
CASE 4
Trauma
A 33-year-old woman was brought to the ED after she was knocked off her motorbike by a car. A passerby found her unconscious and still wearing her helmet. En route to the hospital, the patient regained consciousness but had retrograde amnesia.
She was referred to us for evaluation of complete ptosis of her left eye. She was fully conscious during the examination. Her left eye vision was 6/9. Complete ptosis with exotropia was noted. Pupillary examination revealed a sluggish dilated left eye pupil of 7 mm with no reverse relative afferent pupillary defect. Extraocular movement was restricted at elevation, depression, and adduction with diplopia (FIGURE 5). All other CN functions were preserved.

CT of the brain and orbit revealed acute right frontotemporal subarachnoid hemorrhage (FIGURE 6). There was no radiologic evidence of orbital wall fractures or extraocular muscle entrapment. She remained stable during the first 24 hours of monitoring and was given a diagnosis of

Repeat CT of the brain 5 days later revealed complete resolution of the subarachnoid hemorrhage. The patient's clinical condition improved 2 weeks later and included resolution of ptosis and recovery of ocular motility.
Key takeaways from the cases
Case 1: Herpes zoster ophthalmicus
Clinical diagnosis of HZO is straightforward, with painful vesicular lesions occurring along the trigeminal nerve (V1) dermatome, as was seen in this case. The oculomotor nerve is the CN most commonly involved; the trochlear nerve is the least-often affected.6 In a report from the Mayo Clinic, 3 of 86 patients with HZO had oculomotor nerve palsies (3.4%).7 A separate review from an eye hospital study stated that 9.8% (n = 133) of 1356 patients with HZO had extraocular muscle palsy, with TNP in 4 of the patients.8
Ocular complications such as blepharitis, keratoconjunctivits, or iritis occur in 20% to 70% of HZO cases.9 Ophthalmoplegia, which most often involves the oculomotor nerve, is seen in 7% to 31% of HZO cases (mostly in the elderly) and usually occurs within 1 to 3 weeks of the onset of rash.6 Our patient immediately underwent contrast CT of the brain to rule out meningitis and nerve compression.
Treatment with a systemic antiviral agent is crucial. Acyclovir, valaciclovir, and famciclovir are available treatment options, used for treating the skin lesions, reducing the viral load, and reducing the risk for ocular involvement or its progression. Our patient started a 2-week course of oral acyclovir 800 mg 5 times per day. Ophthalmoplegia is usually self-limiting and has a good prognosis. Time to resolution varies from 2 to 18 months. Diplopia, if present, resolves within 1 year.6 Our patient achieved full recovery of his extraocular movement after completing 4 weeks of antiviral treatment.
Continue to: Case 2
Case 2: Posterior communicating artery aneurysm
Given the patient’s high BP, ruling out a hypertensive emergency with CT was the first priority. TNP caused by microvascular ischemia is not uncommon in the elderly. However, her pupil involvement and persistent headache called for an MRI to better evaluate the soft tissues and to rule out possible vascular pathologies. Left posterior communicating artery aneurysm was discovered with MRI, and urgent cerebral angiography and coiling was performed successfully.
Incidence. One report of 1400 patients with TNP confirmed that aneurysm was the cause in 10% of cases, with posterior communicating artery aneurysm accounting for the greatest number, 119 (25.7%).10 Of these cases of posterior communicating artery aneurysm, pupillary involvement was detected in 108 (90.8%). The oculomotor nerve lies adjacent to the posterior communicating artery as it passes through the subarachnoid space of the basal cisterns, where it is susceptible to compression.3
A high index of suspicion for posterior communicating artery aneurysm is crucial for early detection and lifesaving treatment. The patient in this case did well after the coiling. Her ptosis resolved at 2 months, although she had residual left eye exotropia.
Case 3: Viral infection
We chose CTA of the brain instead of contrast CT to rule out the possibility of intracranial aneurysm. CTA has been shown to be an adequate first-line study to detect aneurysms, particularly those greater than 4 mm in diameter.2,11 One study demonstrated an 81.8% sensitivity for aneurysms smaller than 3 mm when performed on a 320-slice CT.12
Additional imaging selection. We also selected MRA to rule out berry aneurysm, which is often asymptomatic. We decided against MRI because of its higher cost and longer acquisition time. It is usually reserved for patients with a negative initial work-up with CT or cerebral angiography if suspicion of a possible aneurysm remains.11 The MRA finding in this case was negative, and we made a presumptive diagnosis of TNP secondary to viral infection.
Isolated TNP following viral infection is a clinical diagnosis of exclusion. In 1 reported case, a 39-year-old man developed a superior division palsy after a common cold without fever, underwent no serologic study, and recovered spontaneously 6 weeks later.13 A 5-year-old boy who experienced a superior division palsy immediately after a common cold with fever was found on serologic examination to have an increased titre of influenza A virus. His palsy resolved in 4 months.14
The exact mechanism of viral-induced palsy is unknown. The possibility of postinfectious cranial neuropathy has been postulated, as most reported cases following a flu-like illness resolved within a few months.15 Although the pathogenesis remains speculative, an autoimmune process might have been involved.16 Our patient recovered fully in 1 month following a short course of oral prednisolone 30 mg/d for 5 days.
Case 4: Trauma
Trauma accounts for approximately 12% of all TNP cases.17 Traumatic TNPs are usually sustained in severe, high-speed, closed-head injuries, and are often associated with other CN injuries and neurologic deficits. The damage may be caused indirectly by compression, hemorrhage, or ischemia, or directly at certain vulnerable points including the nerve’s exit from the brainstem and the point at which it crosses the petroclinoid ligament.17 In our case, despite the patient having complete TNP, there was no sign of localized orbital trauma on the CT other than the presence of subarachnoid hemorrhage at the right frontotemporal region.
In a similar reported case, the patient had a right traumatic isolated TNP and was found to have left frontal subarachnoid hemorrhage with no sign of orbital trauma.18 However, the mechanisms of isolated TNP caused by traumatic brain injury are not clear. Possible causes include rootlet avulsion, distal fascicular damage, stretching of the nerve (including the parasellar segment), and decreased blood supply.18
It has been suggested that TNP is more frequently observed in cases of frontal region injury. As orbitofrontal regions are predominantly affected by cortical contusions, the risk for ocular involvement increases.19
Keep these fundamentals in mind
The diagnosis and management of isolated TNP are guided by the patient’s age, by the degree to which each of the oculomotor nerve’s 2 major functions—pupillomotor and oculomotor—are affected, and by the circumstances preceding the onset of TNP.2 Cases 1 and 3 in our series presented with partial TNP, while Cases 2 and 4 exhibited complete TNP. Pupillary involvement was detected only in Case 2. Nevertheless, radiologic imaging was ordered for all 4 cases after the diagnosis of TNP was made, to exclude the most worrying neurologic emergencies. The choice of imaging modality depends on not only the availability of the services but also the clinical signs and symptoms and presumptive clinical diagnosis. A tailored and thoughtful approach with consideration of the anatomy and varied pathologies help clinicians to skillfully discern emergencies from nonurgent cases.
CORRESPONDENCE
Lott Pooi Wah, MSOphth, FRCOphth, Department of Ophthalmology, Faculty of Medicine, Universiti Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] Orcid no: 0000-0001-8746-1528
1. Radia M, Stahl M, Arunakirinathan M, et al. Examination of a third nerve palsy. Brit J Hosp Med. 2017;78:188-192. doi: 10.12968/hmed.2017.78.12.C188
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Motoyama Y, Nonaka J, Hironaka Y, et al. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52:202-205. doi: 10.2176/nmc.52.202
4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthal mol.2016.4456
5. Wyatt K. Three common ophthalmic emergencies. JAAPA. 2014;27:32-37. doi: 10.1097/01.JAA.0000447004.96714.34
6. Daswani M, Bhosale N, Shah VM. Rare case of herpes zoster ophthalmicus with orbital myositis, oculomotor nerve palsy and anterior uveitis. Indian J Dermatol Venereol Leprol. 2017;83:365-367. doi: 10.4103/0378-6323.199582
7. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-45. doi: 10.1001/archopht.1983.01040010044004
8. Marsh RJ, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol. 1977;61:667-682. doi: 10.1136/bjo.61.11.677
9. Lim JJ, Ong YM, Zalina MCW, et al. Herpes zoster ophthalmicus with orbital apex syndrome – difference in outcomes and literature review. Ocul Immunol Inflamm. 2017;26:187-193. doi: 10.1080/09273948.2017.1327604
10. Keane JR. Third nerve palsy: analysis of 1400 personally-examined patients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
11. Yoon NK, McNally S, Taussky P, et al. Imaging of cerebral aneurysms: a clinical perspective. Neurovasc Imaging. 2016;2:6. doi: 10.1186/s40809-016-0016-3
12. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15-20. doi: 10.1016/j.crad.2012.09.001
13. Derakhshan I. Superior branch palsy with spontaneous recovery. Ann Neurol. 1978;4:478-479. doi: 10.1002/ana.410040519
14. Engelhardt A, Credzich C, Kompf D. Isolated superior branch palsy of the oculomotor nerve in influenza A. Neuroophthalmol. 1989;9:233-235. doi: 10.3109/01658108908997359
15. Knox DL, Clark DB, Schuster FF. Benign VI nerve palsies in children. Pediatrics. 1967;40:560-564.
16. Saeki N, Yotsukura J, Adachi E, et al. Isolated superior division oculomotor palsy in a child with spontaneous recovery. J Clin Neurosci. 2000;7:62-64. doi: 10.1054/jocn.1998.0152
17. Nagendran ST, Lee V, Perry M. Traumatic orbital third nerve palsy. Brit J Oral Maxillofac Surg. 2019;57:578-581. doi: 10.1016/j.bjoms.2019.01.029
18. Kim T, Nam K, Kwon BS. Isolated oculomotor nerve palsy in mild traumatic brain injury: a literature review. Am J Phys Med Rehabil. 2020;99:430-435. doi: 10.1097/PHM.0000000000001316
19. Sharma B, Gupta R, Anand R, et al. Ocular manifestations of head injury and incidence of post-traumatic ocular motor nerve involvement in cases of head injury: a clinical review. Int Ophthalmol. 2014;34:893-900. doi: 10.1007/s10792-014-9898-8
1. Radia M, Stahl M, Arunakirinathan M, et al. Examination of a third nerve palsy. Brit J Hosp Med. 2017;78:188-192. doi: 10.12968/hmed.2017.78.12.C188
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Motoyama Y, Nonaka J, Hironaka Y, et al. Pupil-sparing oculomotor nerve palsy caused by upward compression of a large posterior communicating artery aneurysm. Case report. Neurol Med Chir (Tokyo). 2012;52:202-205. doi: 10.2176/nmc.52.202
4. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthal mol.2016.4456
5. Wyatt K. Three common ophthalmic emergencies. JAAPA. 2014;27:32-37. doi: 10.1097/01.JAA.0000447004.96714.34
6. Daswani M, Bhosale N, Shah VM. Rare case of herpes zoster ophthalmicus with orbital myositis, oculomotor nerve palsy and anterior uveitis. Indian J Dermatol Venereol Leprol. 2017;83:365-367. doi: 10.4103/0378-6323.199582
7. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101:42-45. doi: 10.1001/archopht.1983.01040010044004
8. Marsh RJ, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol. 1977;61:667-682. doi: 10.1136/bjo.61.11.677
9. Lim JJ, Ong YM, Zalina MCW, et al. Herpes zoster ophthalmicus with orbital apex syndrome – difference in outcomes and literature review. Ocul Immunol Inflamm. 2017;26:187-193. doi: 10.1080/09273948.2017.1327604
10. Keane JR. Third nerve palsy: analysis of 1400 personally-examined patients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
11. Yoon NK, McNally S, Taussky P, et al. Imaging of cerebral aneurysms: a clinical perspective. Neurovasc Imaging. 2016;2:6. doi: 10.1186/s40809-016-0016-3
12. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol. 2013;68:e15-20. doi: 10.1016/j.crad.2012.09.001
13. Derakhshan I. Superior branch palsy with spontaneous recovery. Ann Neurol. 1978;4:478-479. doi: 10.1002/ana.410040519
14. Engelhardt A, Credzich C, Kompf D. Isolated superior branch palsy of the oculomotor nerve in influenza A. Neuroophthalmol. 1989;9:233-235. doi: 10.3109/01658108908997359
15. Knox DL, Clark DB, Schuster FF. Benign VI nerve palsies in children. Pediatrics. 1967;40:560-564.
16. Saeki N, Yotsukura J, Adachi E, et al. Isolated superior division oculomotor palsy in a child with spontaneous recovery. J Clin Neurosci. 2000;7:62-64. doi: 10.1054/jocn.1998.0152
17. Nagendran ST, Lee V, Perry M. Traumatic orbital third nerve palsy. Brit J Oral Maxillofac Surg. 2019;57:578-581. doi: 10.1016/j.bjoms.2019.01.029
18. Kim T, Nam K, Kwon BS. Isolated oculomotor nerve palsy in mild traumatic brain injury: a literature review. Am J Phys Med Rehabil. 2020;99:430-435. doi: 10.1097/PHM.0000000000001316
19. Sharma B, Gupta R, Anand R, et al. Ocular manifestations of head injury and incidence of post-traumatic ocular motor nerve involvement in cases of head injury: a clinical review. Int Ophthalmol. 2014;34:893-900. doi: 10.1007/s10792-014-9898-8
PRACTICE RECOMMENDATIONS
› Consider microvascular ischemia if third nerve palsy is pupil sparing. C
› Consider computerized tomography (CT) angiography as an alternative to plain CT for first-line study of suspected aneurysm. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Is self-administered DMPA an answer to contraception access in the post-Roe era?
ILLUSTRATIVE CASE
A 32-year-old woman with a history of migraine with aura presents to your office for contraception management. She works full-time, has 2 children, and has transportation barriers. She previously used injectable DMPA (administered every 3 months at a health care facility) and would like to restart it. However, because she had to reschedule her last appointment due to a lack of transportation, she missed her injection window and subsequently became pregnant with her second child. She would still prefer injectable DMPA over the other contraceptive options offered—etonogestrel implant, oral contraceptive, or intrauterine device (IUD)—given her migraine history. However, she’s concerned she may have difficulty coming to the office every 3 months for her injection. What alternative injectable option can you offer?
When not pregnant or seeking to become pregnant, women may spend a significant amount of their lives trying to avoid pregnancy, and almost all women use contraception at some point.2 During the childbearing years of 15 to 49, 65% of women report using contraception.2 Although DMPA is a safe and effective option, only 2% of women report using it for contraception.2
For patients who have migraine with aura, there are fewer contraception options because their risk for ischemic stroke is increased 2- to 4-fold if they use combined hormonal contraceptives in pill, patch, or vaginal ring form.3 Safe options for these patients include the copper IUD, levonorgestrel-releasing intrauterine system, progestin implant, and DMPA injection.3
DMPA is a progestogen-only contraceptive approved by the US Food and Drug Administration to prevent pregnancy. It is available in an intramuscular formulation (DMPA-IM; 150 mg/mL every 13 weeks) and a subcutaneous formulation (DMPA-SC; 104 mg/0.65 mL every 12-14 weeks). DMPA-IM is administered by a health care provider and thus requires patients to present every 3 months for an injection. About 6% of DMPA-IM users have an unintended pregnancy in the first year due to inconsistent or incorrect use or late receipt of injection.4 DMPA-SC is produced as a prefilled needle that can be self-injected by patients.
Barriers to access are a growing concern. During the COVID-19 pandemic, one-third of women surveyed by the Guttmacher Institute (n = 2009) reported delaying or canceling a health care visit or having difficulty obtaining their contraception. Barriers to health care and contraception access were more common among Black and Hispanic women (vs White women), queer women (vs straight women), and low-income women (vs higher-income women).5
Following the overturning of Roe v Wade in June 2022, abortion access is now limited in parts of the United States. Given this significant policy change, physicians have an increasingly important role in providing contraception care and reducing barriers to contraception access. Since the SC forms of injectable contraception can be administered at home rather than in the health care setting, both the World Health Organization and the Centers for Disease Control and Prevention have recommended that self-administered injectable contraception be made widely available to expand access to contraception.6,7
STUDY SUMMARY
Higher contraceptive continuation rates with comparable safety and efficacy
This 2019 systematic review and meta-analysis evaluated the outcomes associated with use of self-administered DMPA-SC vs provider-administered DMPA in 5 countries.1 The authors searched several electronic databases for peer-reviewed studies of women who chose the option to self-administer DMPA-SC vs those who received DMPA injections from a health care provider.
Continue to: Outcomes included pregnancy
Outcomes included pregnancy; adverse effects or events (bleeding, injection site reactions, mental health concerns); initial use of injectable contraception (contraception uptake); and continuation rate of injectable contraception. Two reviewers extracted the data and assessed trials for bias. The authors used random-effects models to calculate pooled relative risk (RR) for studies with the same outcomes.
The analysis included a total of 6 trials (N = 3851): 3 RCTs (n = 1263) and 3 controlled cohort studies (n = 2588), conducted in the United States (2 trials), Malawi, Scotland, Uganda, and Senegal. All studies compared 12-month continuation rates of self-injected DMPA-SC vs provider-administered DMPA-SC or DMPA-IM every 3 months (12-13 weeks, with a window for early and late injections). Participants were at least 15 years of age (mean range, 26 to 29 years). In some studies, reminders (eg, texts, emails, calendar notifications) were provided to either the self-injection cohort only or to both cohorts of the trial. The RCTs were generally graded as having a low risk for bias, except for nonblinding of participants and personnel, given the nature of the interventions. The authors reported no evidence of significant heterogeneity in the studies.
The meta-analysis found higher continuation rates at 12 months with self-administrated DMPA compared with provider administration in the RCTs (RR = 1.27; 95% CI, 1.16-1.39) and in the observational cohort studies (RR = 1.18; 95% CI, 1.10-1.26). Pregnancy outcomes were reported in 4 studies, with the meta-analysis finding no significant difference in pregnancy rates in 2 RCTs (RR = 0.58; 95% CI, 0.15-2.22) or 2 observational cohort studies (RR = 1.1; 95% CI, 0.23-5.26).
Adverse effects or events were reported in 4 studies: 2 cohort studies reported increased injection site reactions with self-administration, and 1 RCT reported increased injection site pain or irritation with self-administration at 3 and 9 months. No other reported adverse effects occurred at higher rates with self-administration vs provider administration.
WHAT’S NEW
Demonstrated effectiveness of self-administered formulation
This systematic review and meta-analysis demonstrated that self-administration of DMPA-SC leads to higher contraception continuation rates at 12 months, without notable increased pregnancy rates or adverse effects, when compared with provider-administered DMPA.
Continue to: CAVEATS
CAVEATS
Outcome data limited to 12 months
Although self-administered DMPA-SC has the theoretical risk for user error and incorrect administration, this study did not find increased rates of pregnancy despite administration outside a health care center. However, the total number of pregnancies in each of the 4 studies measuring this outcome was low (< 5), and thus the authors noted that the effect size estimates may not be accurate.
Currently, there are no data on long-term outcomes beyond 12 months. Additionally, the health care visits for provider-administered DMPA every 3 months may afford other benefits, such as regular discussion of reproductive health concerns or testing for sexually transmitted infections, which must be weighed against the benefit of increased contraception access with self-administration. However, using the DMPA-SC self-administered formulation at home would not inhibit women from making separate health care visits as needed.
CHALLENGES TO IMPLEMENTATION
Limited resources to teach patients how to self-inject
Barriers to implementation include limited experience with prescribing DMPA-SC and changing practice culture to offer it to patients. Additionally, successful implementation of self-administered DMPA-SC is reliant on providing patients with appropriate information and training on self-injection, which requires knowledge, time, and other resources that may be limited in practices. Another potential barrier is product access, as not all insurers cover DMPA-SC and some pharmacies do not carry it.
1. Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
2. Daniels K, Abma J. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;(388):1-8.
3. Paradise SL, Landis CA, Klein DA. Evidence-based contraception: common questions and answers. Am Fam Physician. 2022;106:251-259.
4. Marx M. Evidence‐based guidance for self‐administration of injectable contraception. J Midwifery Womens Health. 2021;66:108-112. doi: 10.1111/jmwh.13190
5. Lindberg LD, VandeVusse A, Mueller J, et al. Early Impacts of the COVID-19 Pandemic: Findings from the 2020 Guttmacher Survey of Reproductive Health Experiences. Guttmacher Institute; 2020. Accessed October 25, 2022. www.guttmacher.org/report/early-impacts-covid-19-pandemic-findings-2020-guttmacher-survey-reproductive-health
6. World Health Organization. WHO consolidated guidance on self-care interventions for health: sexual and reproductive health and rights. Published 2019. Accessed February 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf
7. Curtis KM, Nguyen A, Reeves JA, et al. Update to US selected practice recommendations for contraceptive use: self-administration of subcutaneous depot medroxyprogesterone acetate. MMWR Morb Mortal Wkly Rep. 2021;70:739-743. doi: 10.15585/mmwr.mm7020a2
ILLUSTRATIVE CASE
A 32-year-old woman with a history of migraine with aura presents to your office for contraception management. She works full-time, has 2 children, and has transportation barriers. She previously used injectable DMPA (administered every 3 months at a health care facility) and would like to restart it. However, because she had to reschedule her last appointment due to a lack of transportation, she missed her injection window and subsequently became pregnant with her second child. She would still prefer injectable DMPA over the other contraceptive options offered—etonogestrel implant, oral contraceptive, or intrauterine device (IUD)—given her migraine history. However, she’s concerned she may have difficulty coming to the office every 3 months for her injection. What alternative injectable option can you offer?
When not pregnant or seeking to become pregnant, women may spend a significant amount of their lives trying to avoid pregnancy, and almost all women use contraception at some point.2 During the childbearing years of 15 to 49, 65% of women report using contraception.2 Although DMPA is a safe and effective option, only 2% of women report using it for contraception.2
For patients who have migraine with aura, there are fewer contraception options because their risk for ischemic stroke is increased 2- to 4-fold if they use combined hormonal contraceptives in pill, patch, or vaginal ring form.3 Safe options for these patients include the copper IUD, levonorgestrel-releasing intrauterine system, progestin implant, and DMPA injection.3
DMPA is a progestogen-only contraceptive approved by the US Food and Drug Administration to prevent pregnancy. It is available in an intramuscular formulation (DMPA-IM; 150 mg/mL every 13 weeks) and a subcutaneous formulation (DMPA-SC; 104 mg/0.65 mL every 12-14 weeks). DMPA-IM is administered by a health care provider and thus requires patients to present every 3 months for an injection. About 6% of DMPA-IM users have an unintended pregnancy in the first year due to inconsistent or incorrect use or late receipt of injection.4 DMPA-SC is produced as a prefilled needle that can be self-injected by patients.
Barriers to access are a growing concern. During the COVID-19 pandemic, one-third of women surveyed by the Guttmacher Institute (n = 2009) reported delaying or canceling a health care visit or having difficulty obtaining their contraception. Barriers to health care and contraception access were more common among Black and Hispanic women (vs White women), queer women (vs straight women), and low-income women (vs higher-income women).5
Following the overturning of Roe v Wade in June 2022, abortion access is now limited in parts of the United States. Given this significant policy change, physicians have an increasingly important role in providing contraception care and reducing barriers to contraception access. Since the SC forms of injectable contraception can be administered at home rather than in the health care setting, both the World Health Organization and the Centers for Disease Control and Prevention have recommended that self-administered injectable contraception be made widely available to expand access to contraception.6,7
STUDY SUMMARY
Higher contraceptive continuation rates with comparable safety and efficacy
This 2019 systematic review and meta-analysis evaluated the outcomes associated with use of self-administered DMPA-SC vs provider-administered DMPA in 5 countries.1 The authors searched several electronic databases for peer-reviewed studies of women who chose the option to self-administer DMPA-SC vs those who received DMPA injections from a health care provider.
Continue to: Outcomes included pregnancy
Outcomes included pregnancy; adverse effects or events (bleeding, injection site reactions, mental health concerns); initial use of injectable contraception (contraception uptake); and continuation rate of injectable contraception. Two reviewers extracted the data and assessed trials for bias. The authors used random-effects models to calculate pooled relative risk (RR) for studies with the same outcomes.
The analysis included a total of 6 trials (N = 3851): 3 RCTs (n = 1263) and 3 controlled cohort studies (n = 2588), conducted in the United States (2 trials), Malawi, Scotland, Uganda, and Senegal. All studies compared 12-month continuation rates of self-injected DMPA-SC vs provider-administered DMPA-SC or DMPA-IM every 3 months (12-13 weeks, with a window for early and late injections). Participants were at least 15 years of age (mean range, 26 to 29 years). In some studies, reminders (eg, texts, emails, calendar notifications) were provided to either the self-injection cohort only or to both cohorts of the trial. The RCTs were generally graded as having a low risk for bias, except for nonblinding of participants and personnel, given the nature of the interventions. The authors reported no evidence of significant heterogeneity in the studies.
The meta-analysis found higher continuation rates at 12 months with self-administrated DMPA compared with provider administration in the RCTs (RR = 1.27; 95% CI, 1.16-1.39) and in the observational cohort studies (RR = 1.18; 95% CI, 1.10-1.26). Pregnancy outcomes were reported in 4 studies, with the meta-analysis finding no significant difference in pregnancy rates in 2 RCTs (RR = 0.58; 95% CI, 0.15-2.22) or 2 observational cohort studies (RR = 1.1; 95% CI, 0.23-5.26).
Adverse effects or events were reported in 4 studies: 2 cohort studies reported increased injection site reactions with self-administration, and 1 RCT reported increased injection site pain or irritation with self-administration at 3 and 9 months. No other reported adverse effects occurred at higher rates with self-administration vs provider administration.
WHAT’S NEW
Demonstrated effectiveness of self-administered formulation
This systematic review and meta-analysis demonstrated that self-administration of DMPA-SC leads to higher contraception continuation rates at 12 months, without notable increased pregnancy rates or adverse effects, when compared with provider-administered DMPA.
Continue to: CAVEATS
CAVEATS
Outcome data limited to 12 months
Although self-administered DMPA-SC has the theoretical risk for user error and incorrect administration, this study did not find increased rates of pregnancy despite administration outside a health care center. However, the total number of pregnancies in each of the 4 studies measuring this outcome was low (< 5), and thus the authors noted that the effect size estimates may not be accurate.
Currently, there are no data on long-term outcomes beyond 12 months. Additionally, the health care visits for provider-administered DMPA every 3 months may afford other benefits, such as regular discussion of reproductive health concerns or testing for sexually transmitted infections, which must be weighed against the benefit of increased contraception access with self-administration. However, using the DMPA-SC self-administered formulation at home would not inhibit women from making separate health care visits as needed.
CHALLENGES TO IMPLEMENTATION
Limited resources to teach patients how to self-inject
Barriers to implementation include limited experience with prescribing DMPA-SC and changing practice culture to offer it to patients. Additionally, successful implementation of self-administered DMPA-SC is reliant on providing patients with appropriate information and training on self-injection, which requires knowledge, time, and other resources that may be limited in practices. Another potential barrier is product access, as not all insurers cover DMPA-SC and some pharmacies do not carry it.
ILLUSTRATIVE CASE
A 32-year-old woman with a history of migraine with aura presents to your office for contraception management. She works full-time, has 2 children, and has transportation barriers. She previously used injectable DMPA (administered every 3 months at a health care facility) and would like to restart it. However, because she had to reschedule her last appointment due to a lack of transportation, she missed her injection window and subsequently became pregnant with her second child. She would still prefer injectable DMPA over the other contraceptive options offered—etonogestrel implant, oral contraceptive, or intrauterine device (IUD)—given her migraine history. However, she’s concerned she may have difficulty coming to the office every 3 months for her injection. What alternative injectable option can you offer?
When not pregnant or seeking to become pregnant, women may spend a significant amount of their lives trying to avoid pregnancy, and almost all women use contraception at some point.2 During the childbearing years of 15 to 49, 65% of women report using contraception.2 Although DMPA is a safe and effective option, only 2% of women report using it for contraception.2
For patients who have migraine with aura, there are fewer contraception options because their risk for ischemic stroke is increased 2- to 4-fold if they use combined hormonal contraceptives in pill, patch, or vaginal ring form.3 Safe options for these patients include the copper IUD, levonorgestrel-releasing intrauterine system, progestin implant, and DMPA injection.3
DMPA is a progestogen-only contraceptive approved by the US Food and Drug Administration to prevent pregnancy. It is available in an intramuscular formulation (DMPA-IM; 150 mg/mL every 13 weeks) and a subcutaneous formulation (DMPA-SC; 104 mg/0.65 mL every 12-14 weeks). DMPA-IM is administered by a health care provider and thus requires patients to present every 3 months for an injection. About 6% of DMPA-IM users have an unintended pregnancy in the first year due to inconsistent or incorrect use or late receipt of injection.4 DMPA-SC is produced as a prefilled needle that can be self-injected by patients.
Barriers to access are a growing concern. During the COVID-19 pandemic, one-third of women surveyed by the Guttmacher Institute (n = 2009) reported delaying or canceling a health care visit or having difficulty obtaining their contraception. Barriers to health care and contraception access were more common among Black and Hispanic women (vs White women), queer women (vs straight women), and low-income women (vs higher-income women).5
Following the overturning of Roe v Wade in June 2022, abortion access is now limited in parts of the United States. Given this significant policy change, physicians have an increasingly important role in providing contraception care and reducing barriers to contraception access. Since the SC forms of injectable contraception can be administered at home rather than in the health care setting, both the World Health Organization and the Centers for Disease Control and Prevention have recommended that self-administered injectable contraception be made widely available to expand access to contraception.6,7
STUDY SUMMARY
Higher contraceptive continuation rates with comparable safety and efficacy
This 2019 systematic review and meta-analysis evaluated the outcomes associated with use of self-administered DMPA-SC vs provider-administered DMPA in 5 countries.1 The authors searched several electronic databases for peer-reviewed studies of women who chose the option to self-administer DMPA-SC vs those who received DMPA injections from a health care provider.
Continue to: Outcomes included pregnancy
Outcomes included pregnancy; adverse effects or events (bleeding, injection site reactions, mental health concerns); initial use of injectable contraception (contraception uptake); and continuation rate of injectable contraception. Two reviewers extracted the data and assessed trials for bias. The authors used random-effects models to calculate pooled relative risk (RR) for studies with the same outcomes.
The analysis included a total of 6 trials (N = 3851): 3 RCTs (n = 1263) and 3 controlled cohort studies (n = 2588), conducted in the United States (2 trials), Malawi, Scotland, Uganda, and Senegal. All studies compared 12-month continuation rates of self-injected DMPA-SC vs provider-administered DMPA-SC or DMPA-IM every 3 months (12-13 weeks, with a window for early and late injections). Participants were at least 15 years of age (mean range, 26 to 29 years). In some studies, reminders (eg, texts, emails, calendar notifications) were provided to either the self-injection cohort only or to both cohorts of the trial. The RCTs were generally graded as having a low risk for bias, except for nonblinding of participants and personnel, given the nature of the interventions. The authors reported no evidence of significant heterogeneity in the studies.
The meta-analysis found higher continuation rates at 12 months with self-administrated DMPA compared with provider administration in the RCTs (RR = 1.27; 95% CI, 1.16-1.39) and in the observational cohort studies (RR = 1.18; 95% CI, 1.10-1.26). Pregnancy outcomes were reported in 4 studies, with the meta-analysis finding no significant difference in pregnancy rates in 2 RCTs (RR = 0.58; 95% CI, 0.15-2.22) or 2 observational cohort studies (RR = 1.1; 95% CI, 0.23-5.26).
Adverse effects or events were reported in 4 studies: 2 cohort studies reported increased injection site reactions with self-administration, and 1 RCT reported increased injection site pain or irritation with self-administration at 3 and 9 months. No other reported adverse effects occurred at higher rates with self-administration vs provider administration.
WHAT’S NEW
Demonstrated effectiveness of self-administered formulation
This systematic review and meta-analysis demonstrated that self-administration of DMPA-SC leads to higher contraception continuation rates at 12 months, without notable increased pregnancy rates or adverse effects, when compared with provider-administered DMPA.
Continue to: CAVEATS
CAVEATS
Outcome data limited to 12 months
Although self-administered DMPA-SC has the theoretical risk for user error and incorrect administration, this study did not find increased rates of pregnancy despite administration outside a health care center. However, the total number of pregnancies in each of the 4 studies measuring this outcome was low (< 5), and thus the authors noted that the effect size estimates may not be accurate.
Currently, there are no data on long-term outcomes beyond 12 months. Additionally, the health care visits for provider-administered DMPA every 3 months may afford other benefits, such as regular discussion of reproductive health concerns or testing for sexually transmitted infections, which must be weighed against the benefit of increased contraception access with self-administration. However, using the DMPA-SC self-administered formulation at home would not inhibit women from making separate health care visits as needed.
CHALLENGES TO IMPLEMENTATION
Limited resources to teach patients how to self-inject
Barriers to implementation include limited experience with prescribing DMPA-SC and changing practice culture to offer it to patients. Additionally, successful implementation of self-administered DMPA-SC is reliant on providing patients with appropriate information and training on self-injection, which requires knowledge, time, and other resources that may be limited in practices. Another potential barrier is product access, as not all insurers cover DMPA-SC and some pharmacies do not carry it.
1. Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
2. Daniels K, Abma J. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;(388):1-8.
3. Paradise SL, Landis CA, Klein DA. Evidence-based contraception: common questions and answers. Am Fam Physician. 2022;106:251-259.
4. Marx M. Evidence‐based guidance for self‐administration of injectable contraception. J Midwifery Womens Health. 2021;66:108-112. doi: 10.1111/jmwh.13190
5. Lindberg LD, VandeVusse A, Mueller J, et al. Early Impacts of the COVID-19 Pandemic: Findings from the 2020 Guttmacher Survey of Reproductive Health Experiences. Guttmacher Institute; 2020. Accessed October 25, 2022. www.guttmacher.org/report/early-impacts-covid-19-pandemic-findings-2020-guttmacher-survey-reproductive-health
6. World Health Organization. WHO consolidated guidance on self-care interventions for health: sexual and reproductive health and rights. Published 2019. Accessed February 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf
7. Curtis KM, Nguyen A, Reeves JA, et al. Update to US selected practice recommendations for contraceptive use: self-administration of subcutaneous depot medroxyprogesterone acetate. MMWR Morb Mortal Wkly Rep. 2021;70:739-743. doi: 10.15585/mmwr.mm7020a2
1. Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
2. Daniels K, Abma J. Current contraceptive status among women aged 15-49: United States, 2017-2019. NCHS Data Brief. 2020;(388):1-8.
3. Paradise SL, Landis CA, Klein DA. Evidence-based contraception: common questions and answers. Am Fam Physician. 2022;106:251-259.
4. Marx M. Evidence‐based guidance for self‐administration of injectable contraception. J Midwifery Womens Health. 2021;66:108-112. doi: 10.1111/jmwh.13190
5. Lindberg LD, VandeVusse A, Mueller J, et al. Early Impacts of the COVID-19 Pandemic: Findings from the 2020 Guttmacher Survey of Reproductive Health Experiences. Guttmacher Institute; 2020. Accessed October 25, 2022. www.guttmacher.org/report/early-impacts-covid-19-pandemic-findings-2020-guttmacher-survey-reproductive-health
6. World Health Organization. WHO consolidated guidance on self-care interventions for health: sexual and reproductive health and rights. Published 2019. Accessed February 14, 2023. https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf
7. Curtis KM, Nguyen A, Reeves JA, et al. Update to US selected practice recommendations for contraceptive use: self-administration of subcutaneous depot medroxyprogesterone acetate. MMWR Morb Mortal Wkly Rep. 2021;70:739-743. doi: 10.15585/mmwr.mm7020a2
PRACTICE CHANGER
Consider prescribing self-administered subcutaneous depot medroxyprogesterone acetate (DMPA) for contraception instead of provider-administered DMPA. Self-administration improves contraception continuation rates without notable increases in pregnancy or adverse effects.
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials (RCTs) and cohort studies.1
Kennedy CE, Yeh PT, Gaffield ML, et al. Self-administration of injectable contraception: a systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001350. doi: 10.1136/bmjgh-2018-001350
Tips for treating patients with late-life depression
Late-life depression is the onset of a major depressive disorder in an individual ≥ 60 years of age. Depressive illness compromises quality of life and is especially troublesome for older people. The prevalence of depression among individuals > 65 years of age is about 4% in women and 3% in men.1 The estimated lifetime prevalence is approximately 24% for women and 10% for men.2 Three factors account for this disparity: women exhibit greater susceptibility to depression; the illness persists longer in women than it does in men; and the probability of death related to depression is lower in women.2
Beyond its direct mental and emotional impacts, depression takes a financial toll; health care costs are higher for those with depression than for those without depression.3 Unpaid caregiver expense is the largest indirect financial burden with late-life depression.4 Additional indirect costs include less work productivity, early retirement, and diminished financial security.4
Many individuals with depression never receive treatment. Fortunately, there are many interventions in the primary care arsenal that can be used to treat older patients with depression and dramatically improve mood, comfort, and function.
The interactions of emotional and physical health
The pathophysiology of depression remains unclear. However, numerous factors are known to contribute to, exacerbate, or prolong depression among elderly populations. Insufficient social engagement and support is strongly associated with depressive mood.5 The loss of independence in giving up automobile driving can compromise self-confidence.6 Sleep difficulties predispose to, and predict, the emergence of a mood disorder, independent of other symptoms.7 Age-related hearing deficits also are associated with depression.8
There is a close relationship between emotional and physical health.9 Depression adds to the likelihood of medical illness, and somatic pathology increases the risk for mood disorders.9 Depression has been linked with obesity, frailty, diabetes, cognitive impairment, and terminal illness.9
Inflammatory markers and depression may also be related. Plasma levels of interleukin-6 and C-reactive protein were measured in a longitudinal aging study.14 A high level of interleukin-6, but not C-reactive protein, correlated with an increased prevalence of depression in older people.
Chronic cerebral ischemia can result in a “vascular depression”13 in which disruption of prefrontal systems by ischemic lesions is hypothesized to be an important factor in developing despair. Psychomotor retardation, executive dysfunction, severe disability, and a heightened risk for relapse are common features of vascular depression.15 Poststroke depression often follows a cerebrovascular episode16; the exact pathogenic mechanism is unknown.17
Continue to: A summation of common risk factors
A summation of common risk factors. A personal or family history of depression increases the risk for late-life depression. Other risk factors are female gender, bereavement, sleep disturbance, and disability.18 Poor general health, chronic pain, cognitive impairment, poor social support, and medical comorbidities with impaired functioning increase the likelihood of resultant mood disorders.18
Somatic complaints may overshadow diagnostic symptoms
Manifestations of depression include disturbed sleep and reductions in appetite, concentration, activity, and energy for daily function.19 These features, of course, may accompany medical disorders and some normal physiologic changes among elderly people. We find that while older individuals may report a sad mood, disturbed sleep, or other dysfunctions, they frequently emphasize their somatic complaints much more prominently than their emotions. This can make it difficult to recognize clinical depression.
For a diagnosis of major depression, 5 of the following 9 symptoms must be present for most of the day or nearly every day over a period of at least 2 weeks19: depressed mood; diminished interest in most activities; significant weight loss or decreased appetite; insomnia or hypersomnia; agitation or retardation; fatigue or loss of energy; feelings of worthlessness or guilt; diminished concentration; and recurrent thoughts of death or suicide.19
Planning difficulties, apathy, disability, and anhedonia frequently occur. Executive dysfunction and inefficacy of antidepressant pharmacotherapy are related to compromised frontal-striatal-limbic pathways.20 Since difficulties with planning and organization are associated with suboptimal response to antidepressant medications, a psychotherapeutic focus on these executive functions can augment drug-induced benefit.
Rule out these alternative diagnoses
Dementias can manifest as depression. Other brain pathologies, particularly Parkinson disease or stroke, also should be ruled out. Overmedication can simulate depression, so be sure to review the prescription and over-the-counter agents a patient is taking. Some medications can occasionally precipitate a clinical depression; these include stimulants, steroids, methyldopa, triptans, chemotherapeutic agents, and immunologic drugs, to name a few.19
Continue to: Pharmacotherapy, Yes, but first, consider these factors
Pharmacotherapy, Yes, but first, consider these factors
Maintaining a close patient–doctor relationship augments all therapeutic interventions. Good eye contact when listening to and counseling patients is key, as is providing close follow-up appointments.
Encourage social interactions with family and friends, which can be particularly productive. Encouraging spiritual endeavors, such as attendance at religious services, can be beneficial.21
Recommend exercise. Physical exercise yields positive outcomes22; it can enhance mood, improve sleep, and help to diminish anxiety. Encourage patients with depression to take a daily walk during the day; doing so can enhance emotional outlook, health, and even socialization.
What treatment will best serve your patient?
It’s important when caring for patients with depression to assess and address suicidal ideation. Depression with a previous suicide attempt is a strong risk factor for suicide. Inquire about suicidal intent or death wishes, access to guns, and other life-ending behaviors. Whenever suicide is an active issue, immediate crisis management is required. Psychiatric referral is an option, and hospitalization may be indicated. Advise family members to remove firearms or restrict access, be with the patient as much as possible, and assist at intervention planning and implementation.
It is worth mentioning, here, the connection between chronic pain and suicidal ideation. Pain management reduces suicidal ideation, regardless of depression severity.23
Continue to: Psychotherapy and pharmacotherapies...
Psychotherapy and pharmacotherapies offered for the treatment of depression in geriatric practices are both effective, without much difference seen in efficacy.24 Psychotherapy might include direct physician and family support to the patient or referral to a mental health professional. Base treatment choices on clinical access, patient preference, and medical contraindications and other illnesses.
Pros and cons of various pharmacotherapies
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed first for elderly patients with depression.25 Escitalopram is often better tolerated than paroxetine, which exhibits muscarinic antagonism and enzyme inhibition of cytochrome P450-2D6.26 Escitalopram also has fewer pharmaceutical interactions compared with sertraline.26
Generally, when prescribing an antidepressant drug, stay with the initial choice, gradually increasing the dose as clinically needed to its maximum limit. Suicidal ideation may be worsened by too quickly switching from one antidepressant to another or by co-prescribing anxiolytic or hypnotic medicines. Benzodiazepines have addictive and disinhibiting properties and should be avoided, if possible.27 For patients with insomnia, consider initially selecting a sedating antidepressant medication such as paroxetine or mirtazapine to augment sleep.
Alternatives to SSRIs. Nonselective serotonin reuptake inhibitors have similar efficacy as SSRIs. However, escitalopram is as effective as venlafaxine (a selective serotonin and norepinephrine reuptake inhibitor [SSNRI]) and is better tolerated.28 Duloxetine, another SSNRI, improves mood and often diminishes chronic pain.29 Mirtazapine, an alpha-2 antagonist, might cause fewer drug-drug interactions and is effective, well tolerated, and especially helpful for patients with anxiety or insomnia.30 Dry mouth, sedation, and weight gain are common adverse effects of mirtazapine. Obesity precautions are often necessary during mirtazapine therapy; this includes monitoring body weight and metabolic profiles, instituting dietary changes, and recommending an exercise regimen. In contrast to SSRIs, mirtazapine might induce less sexual dysfunction.31
Tricyclic antidepressant drugs can also be effective but may worsen cardiac conduction abnormalities, prostatic hypertrophy, or narrow angle glaucoma. Tricyclic antidepressants may be useful in patients without cardiac disease who have not responded to an SSRI or an SSNRI.
Continue to: The role of aripiprazole
The role of aripiprazole. Elderly patients not achieving remission from depression with antidepressant agents alone may benefit from co-prescribing aripiprazole.32 As an adjunct, aripiprazole is effective in achieving and sustaining remission
Minimize risks and maximize benefits of antidepressants by following these recommendations:
- Ascertain whether any antidepressant treatments have worked well in the past.
- Start with an SSRI if no other antidepressant treatment has worked in the past.
- Counsel patients about the need for treatment adherence. Antidepressants may take 2 weeks to 2 months to provide noticeable improvement.
- Prescribe up to the maximum drug dose if needed to enhance benefit.
- Use a mood measurement tool (eg, the Patient Health Questionnaire-9) to help evaluate treatment response.
Try a different class of drugs for patients who do not respond to treatment. For patients who have a partial response, augment with bupropion XL, mirtazapine, aripiprazole, or quetiapine.33 Sertraline and nortriptyline are similarly effective on a population-wide basis, with sertraline having less-problematic adverse effects.34 Trial-and-error treatments in practice may find one patient responding only to sertraline and another patient only to nortriptyline.
Combinations of different drug classes may provide benefit for patients not responding to a single antidepressant. In geriatric patients, combined treatment with methylphenidate and citalopram enhances mood and well-being.35 Compared with either drug alone, the combination yielded an augmented clinical response profile and a higher rate of remission. Cognitive functioning, energy, and mood improve even with methylphenidate alone, especially when fatigue is an issue. However, addictive properties limit its use to cases in which conventional antidepressant medications are not effective or indicated, and only when drug refills are closely monitored.
The challenges of advancing age. Antidepressant treatment needs increase with advanced age.36 As mentioned earlier, elderly people often have medical illnesses complicating their depression and frequently are dealing with pain from the medical illness. When dementia coexists with depression, the efficacy of pharmacotherapies is compromised.
Continue to: When drug-related interventions fail
When drug-related interventions fail, therapy ought to be more psychologically focused.37 Psychotherapy is usually helpful and is particularly indicated when recovery is suboptimal. Counseling might come from the treating physician or referral to a psychotherapist.
Nasal esketamine can be efficacious when supplementing antidepressant pharmacotherapy among older patients with treatment-resistant depression.38 Elderly individuals responding to antidepressants do not benefit from adjunctive donepezil to correct mild cognitive impairment.39 There is no advantage to off-label cholinesterase inhibitor prescribing for patients with both depression and dementia.
Other options. Electroconvulsive therapy (ECT) does not cause long-term cognitive problems and is reserved for treatment-resistant cases.40 Patients with depression who also have had previous cognitive impairment often improve in mental ability following ECT.41
A promising new option. Transcranial magnetic stimulation (TMS) is a promising, relatively new therapeutic option for treating refractory cases of depressive mood disorders. In TMS, an electromagnetic coil that creates a magnetic field is placed over the left dorsolateral prefrontal cortex (which is responsible for mood regulation). Referral for TMS administration may offer new hope for older patients with treatment-resistant depression.42
Keep comorbidities in mind as you address depression
Coexisting psychiatric illnesses worsen emotions. Geriatric patients are susceptible to psychiatric comorbidities that include substance abuse, obsessive-compulsive characteristics, dysfunctional eating, and panic disorder.19 Myocardial and cerebral infarctions are detrimental to mental health, especially soon after such events.43 Poststroke depression magnifies the risk for disability and mortality,16,17 yet antidepressant pharmacotherapy often enhances prognoses. Along with early intervention algorithm-based plans and inclusion of a depression care manager, antidepressants often diminish poststroke depression severity.44 Even when cancer is present, depression care reduces mortality.44 So with this in mind, persist with antidepressant treatment, which will often benefit an elderly individual with depression.
Continue to: When possible, get ahead of depression before it sets in
When possible, get ahead of depression before it sets in
Social participation and employment help to sustain an optimistic, euthymic mood.45 Maintaining good physical health, in part through consistent activity levels (including exercise), can help prevent depression. Since persistent sleep disturbance predicts depression among those with a depression history, optimizing sleep among geriatric adults can avoid or alleviate depression.46
Sleep hygiene education for patients is also helpful. A regular waking time often promotes a better sleeping schedule. Restful sleep also is more likely when an individual avoids excess caffeine, exercises during the day, and uses the bed only for sleeping (not for listening to music or watching television).
Because inflammation may precede depression, anti-inflammatory medications have been proposed as potential treatment, but such pharmacotherapies are often ineffective. Older adults generally do not benefit from low-dose aspirin administration to prevent depression.47 Low vitamin D levels can contribute to depression, yet vitamin D supplementation may not improve mood.48
Offering hope. Tell your patients that if they are feeling depressed, they should make an appointment with you, their primary care physician, because there are medications they can take and counseling they can avail themselves of that could help.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville-Psychiatry, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psych. 2000;57:601-607. doi: 10.1001/ archpsyc.57.6.601
2. Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psych. 2008;65:172-178. doi: 10.1001/archgenpsychiatry.2007.17
3. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psych. 2003;60:897-903. doi: 10.1001/archpsyc.60.9.897
4. Snow CE, Abrams RC. The indirect costs of late-life depression in the United States: a literature review and perspective. Geriatrics. 2016;1,30. doi.org/10.3390/geriatrics/1040030
5. George LK, Blazer DG, Hughes D, et al. Social support and the outcome of major depression. Br J Psych. 1989;154:478-485. doi: 10.1192/bjp.154.4.478
6. Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol Psychol Soc Sci. 2001;56:S343-S351. doi: 10.1093/geronb/56.6.s343
7. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community dwelling older adults—a prospective study. Am J Psych. 2008;165:1543-1550. doi: 10.1176/appi.ajp.2008.07121882
8. Golub JS, Brewster KK, Brickman AM, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. 2020;28:545-556. doi: 10.1016/j.jagp.2019.12.008
9. Alexopoulos GS. Mechanisms and treatment of late-life depression. Focus (Am Psychiatr Publ). 2021;19:340-354. doi: 10.1176/appi.focus.19304
10. Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Disord. 1990;178:27-31. doi: 10.1097/00005053-199001000-00005
11. Gilman SE, Abraham HE. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alco Depend. 2001;63:277-286. doi: 10.1016/s0376-8716(00)00216-7
12. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15-P21. doi: 10.1093/geronj/46.1.p15
13. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psych. 1997;54:915-922. doi: 10.1001/archpsyc.1997.01830220033006
14. Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249-255. doi: 10.1016/j.jad.2007.07.002
15. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963-974. doi: 10.1038/mp.2013.20
16. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psych. 2016;173:221-231. doi: 10.1176/appi.ajp.2015.15030363
17. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. doi: 10.1016/ j.arr.2019.01.013
18. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psych. 2003;160:1147-1156. doi: 10.1176/appi.ajp.160.6.1147
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013:160-168.
20. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psych. 2016;24:31-34. doi: 10.1016/j.jagp.2015.05.010
21. Koenig HG, Cohen HJ, Blazer DG, et al. Religious coping and depression in elderly hospitalized medically ill men. Am J Psychiatry. 1992;149:1693-1700. doi: 10.1176/ajp.149.12.1693
22. Blake H, Mo P, Malik S, et al. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;10:873-887. doi: 10.1177/0269215509337449
23. Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081-1091. doi: 10.1001/jama.291.9.1081
24. Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493-1501. doi: 10.1176/ajp.2006.163.9.1493
25. Solai LK, Mulsant BH, Pollack BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355-368. doi: 10.2165/00002512-200118050-00006
26. Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline. Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. doi: 10.1097/YIC.0000000000000023
27. Hedna K, Sundell KA, Hamidi A, et al. Antidepressants and suicidal behaviour in late life: a prospective population-based study of use patterns in new users aged 75 and above. Eur J Clin Pharmacol. 2018;74:201-208. doi: 10.1007/s00228-017-2360-x
28. Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190-1196. doi: 10.4088/jcp.v65n0906
29. Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34-45. doi: 10.1016/ j.jagp.2013.01.019
30. Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. doi: 10.2165/00003495-199957040-00010
31. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x
32. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet. 2015;386:2404-2412. doi: 10.1016/S0140-6736(15)00308-6
33. Lenze EJ, Oughli HA. Antidepressant treatment for late-life depression: considering risks and benefits. J Am Geriatr Soc. 2019;67:1555-1556. doi: 10.1111/jgs.15964
34. Bondareff W, Alpert M, Friedhoff AJ, et al: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729-736. doi: 10.1176/appi.ajp.157.5.729
35. Lavretsky H, Reinlieb M, St Cyr N. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo controlled trial. Am J Psych. 2015;72:561-569. doi: 10.1176/appi.ajp.2014.14070889
36. Arthur A, Savva GM, Barnes LE, et al. Changing prevalence and treatment of depression among older people over two decades. Br J Psychiatry. 2020;21:49-54. doi: 10.1192/bjp.2019.193
37. Zuidersma M, Chua K-C, Hellier J, et al. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019;27:920-931. doi: 10.1016/ j.jagp.2019.03.021
38. Ochs-Ross R, Wajs E, Daly EJ, et al. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30:541-556. doi: 10.1016/j.jagp.2021.09.014
39. Devanand DP, Pelton GH, D’Antonio K, et al. Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am J Geriatr Psychiatry. 2018;26:1050-1060. doi: 10.1016/ j.jagp.2018.05.008
40. Obbels J, Vansteelandt K, Verwijk E, et al. MMSE changes during and after ECT in late life depression: a prospective study. Am J Geriatr Psychiatry. 2019;27:934-944. doi: 10.1016/ j.jagp.2019.04.006
41. Wagenmakers MJ, Vansteelandt K, van Exel E, et al. Transient cognitive impairment and white matter hyperintensities in severely depressed older patients treated with electroconvulsive therapy. Am J Geriatr Psychiatry. 2021:29:1117-1128. doi: 10.1016/j.jagp.2020.12.028
42. Trevizol AP, Goldberger KW, Mulsant BH, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Ger Psychiatry. 2019;34:822-827. doi: 10.1002/gps.5091
43. Aben I, Verhey F, Stik J, et al. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581-585. doi: 10.1136/jnnp.74.5.581
44. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698. doi: 10.7326/0003-4819-146-10-200705150-00002
45. Lee J, Jang SN, Cho SL. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int Psychogeriatr. 2017;29:1495-1505. doi: 10.1017/S1041610217000709
46. Lee E, Cho HJ, Olmstead R, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685-1691. doi: 10.5665/sleep.3128
47. Berk M, Woods RL, Nelson MR, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. J Am Med A Psych. 2020;77:1012-1020. doi: 10.1001/jamapsychiatry.2020.1214
48. Okereke OI, Reynolds CF, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. doi: 10.1001/jama.2020.10224
Late-life depression is the onset of a major depressive disorder in an individual ≥ 60 years of age. Depressive illness compromises quality of life and is especially troublesome for older people. The prevalence of depression among individuals > 65 years of age is about 4% in women and 3% in men.1 The estimated lifetime prevalence is approximately 24% for women and 10% for men.2 Three factors account for this disparity: women exhibit greater susceptibility to depression; the illness persists longer in women than it does in men; and the probability of death related to depression is lower in women.2
Beyond its direct mental and emotional impacts, depression takes a financial toll; health care costs are higher for those with depression than for those without depression.3 Unpaid caregiver expense is the largest indirect financial burden with late-life depression.4 Additional indirect costs include less work productivity, early retirement, and diminished financial security.4
Many individuals with depression never receive treatment. Fortunately, there are many interventions in the primary care arsenal that can be used to treat older patients with depression and dramatically improve mood, comfort, and function.
The interactions of emotional and physical health
The pathophysiology of depression remains unclear. However, numerous factors are known to contribute to, exacerbate, or prolong depression among elderly populations. Insufficient social engagement and support is strongly associated with depressive mood.5 The loss of independence in giving up automobile driving can compromise self-confidence.6 Sleep difficulties predispose to, and predict, the emergence of a mood disorder, independent of other symptoms.7 Age-related hearing deficits also are associated with depression.8
There is a close relationship between emotional and physical health.9 Depression adds to the likelihood of medical illness, and somatic pathology increases the risk for mood disorders.9 Depression has been linked with obesity, frailty, diabetes, cognitive impairment, and terminal illness.9
Inflammatory markers and depression may also be related. Plasma levels of interleukin-6 and C-reactive protein were measured in a longitudinal aging study.14 A high level of interleukin-6, but not C-reactive protein, correlated with an increased prevalence of depression in older people.
Chronic cerebral ischemia can result in a “vascular depression”13 in which disruption of prefrontal systems by ischemic lesions is hypothesized to be an important factor in developing despair. Psychomotor retardation, executive dysfunction, severe disability, and a heightened risk for relapse are common features of vascular depression.15 Poststroke depression often follows a cerebrovascular episode16; the exact pathogenic mechanism is unknown.17
Continue to: A summation of common risk factors
A summation of common risk factors. A personal or family history of depression increases the risk for late-life depression. Other risk factors are female gender, bereavement, sleep disturbance, and disability.18 Poor general health, chronic pain, cognitive impairment, poor social support, and medical comorbidities with impaired functioning increase the likelihood of resultant mood disorders.18
Somatic complaints may overshadow diagnostic symptoms
Manifestations of depression include disturbed sleep and reductions in appetite, concentration, activity, and energy for daily function.19 These features, of course, may accompany medical disorders and some normal physiologic changes among elderly people. We find that while older individuals may report a sad mood, disturbed sleep, or other dysfunctions, they frequently emphasize their somatic complaints much more prominently than their emotions. This can make it difficult to recognize clinical depression.
For a diagnosis of major depression, 5 of the following 9 symptoms must be present for most of the day or nearly every day over a period of at least 2 weeks19: depressed mood; diminished interest in most activities; significant weight loss or decreased appetite; insomnia or hypersomnia; agitation or retardation; fatigue or loss of energy; feelings of worthlessness or guilt; diminished concentration; and recurrent thoughts of death or suicide.19
Planning difficulties, apathy, disability, and anhedonia frequently occur. Executive dysfunction and inefficacy of antidepressant pharmacotherapy are related to compromised frontal-striatal-limbic pathways.20 Since difficulties with planning and organization are associated with suboptimal response to antidepressant medications, a psychotherapeutic focus on these executive functions can augment drug-induced benefit.
Rule out these alternative diagnoses
Dementias can manifest as depression. Other brain pathologies, particularly Parkinson disease or stroke, also should be ruled out. Overmedication can simulate depression, so be sure to review the prescription and over-the-counter agents a patient is taking. Some medications can occasionally precipitate a clinical depression; these include stimulants, steroids, methyldopa, triptans, chemotherapeutic agents, and immunologic drugs, to name a few.19
Continue to: Pharmacotherapy, Yes, but first, consider these factors
Pharmacotherapy, Yes, but first, consider these factors
Maintaining a close patient–doctor relationship augments all therapeutic interventions. Good eye contact when listening to and counseling patients is key, as is providing close follow-up appointments.
Encourage social interactions with family and friends, which can be particularly productive. Encouraging spiritual endeavors, such as attendance at religious services, can be beneficial.21
Recommend exercise. Physical exercise yields positive outcomes22; it can enhance mood, improve sleep, and help to diminish anxiety. Encourage patients with depression to take a daily walk during the day; doing so can enhance emotional outlook, health, and even socialization.
What treatment will best serve your patient?
It’s important when caring for patients with depression to assess and address suicidal ideation. Depression with a previous suicide attempt is a strong risk factor for suicide. Inquire about suicidal intent or death wishes, access to guns, and other life-ending behaviors. Whenever suicide is an active issue, immediate crisis management is required. Psychiatric referral is an option, and hospitalization may be indicated. Advise family members to remove firearms or restrict access, be with the patient as much as possible, and assist at intervention planning and implementation.
It is worth mentioning, here, the connection between chronic pain and suicidal ideation. Pain management reduces suicidal ideation, regardless of depression severity.23
Continue to: Psychotherapy and pharmacotherapies...
Psychotherapy and pharmacotherapies offered for the treatment of depression in geriatric practices are both effective, without much difference seen in efficacy.24 Psychotherapy might include direct physician and family support to the patient or referral to a mental health professional. Base treatment choices on clinical access, patient preference, and medical contraindications and other illnesses.
Pros and cons of various pharmacotherapies
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed first for elderly patients with depression.25 Escitalopram is often better tolerated than paroxetine, which exhibits muscarinic antagonism and enzyme inhibition of cytochrome P450-2D6.26 Escitalopram also has fewer pharmaceutical interactions compared with sertraline.26
Generally, when prescribing an antidepressant drug, stay with the initial choice, gradually increasing the dose as clinically needed to its maximum limit. Suicidal ideation may be worsened by too quickly switching from one antidepressant to another or by co-prescribing anxiolytic or hypnotic medicines. Benzodiazepines have addictive and disinhibiting properties and should be avoided, if possible.27 For patients with insomnia, consider initially selecting a sedating antidepressant medication such as paroxetine or mirtazapine to augment sleep.
Alternatives to SSRIs. Nonselective serotonin reuptake inhibitors have similar efficacy as SSRIs. However, escitalopram is as effective as venlafaxine (a selective serotonin and norepinephrine reuptake inhibitor [SSNRI]) and is better tolerated.28 Duloxetine, another SSNRI, improves mood and often diminishes chronic pain.29 Mirtazapine, an alpha-2 antagonist, might cause fewer drug-drug interactions and is effective, well tolerated, and especially helpful for patients with anxiety or insomnia.30 Dry mouth, sedation, and weight gain are common adverse effects of mirtazapine. Obesity precautions are often necessary during mirtazapine therapy; this includes monitoring body weight and metabolic profiles, instituting dietary changes, and recommending an exercise regimen. In contrast to SSRIs, mirtazapine might induce less sexual dysfunction.31
Tricyclic antidepressant drugs can also be effective but may worsen cardiac conduction abnormalities, prostatic hypertrophy, or narrow angle glaucoma. Tricyclic antidepressants may be useful in patients without cardiac disease who have not responded to an SSRI or an SSNRI.
Continue to: The role of aripiprazole
The role of aripiprazole. Elderly patients not achieving remission from depression with antidepressant agents alone may benefit from co-prescribing aripiprazole.32 As an adjunct, aripiprazole is effective in achieving and sustaining remission
Minimize risks and maximize benefits of antidepressants by following these recommendations:
- Ascertain whether any antidepressant treatments have worked well in the past.
- Start with an SSRI if no other antidepressant treatment has worked in the past.
- Counsel patients about the need for treatment adherence. Antidepressants may take 2 weeks to 2 months to provide noticeable improvement.
- Prescribe up to the maximum drug dose if needed to enhance benefit.
- Use a mood measurement tool (eg, the Patient Health Questionnaire-9) to help evaluate treatment response.
Try a different class of drugs for patients who do not respond to treatment. For patients who have a partial response, augment with bupropion XL, mirtazapine, aripiprazole, or quetiapine.33 Sertraline and nortriptyline are similarly effective on a population-wide basis, with sertraline having less-problematic adverse effects.34 Trial-and-error treatments in practice may find one patient responding only to sertraline and another patient only to nortriptyline.
Combinations of different drug classes may provide benefit for patients not responding to a single antidepressant. In geriatric patients, combined treatment with methylphenidate and citalopram enhances mood and well-being.35 Compared with either drug alone, the combination yielded an augmented clinical response profile and a higher rate of remission. Cognitive functioning, energy, and mood improve even with methylphenidate alone, especially when fatigue is an issue. However, addictive properties limit its use to cases in which conventional antidepressant medications are not effective or indicated, and only when drug refills are closely monitored.
The challenges of advancing age. Antidepressant treatment needs increase with advanced age.36 As mentioned earlier, elderly people often have medical illnesses complicating their depression and frequently are dealing with pain from the medical illness. When dementia coexists with depression, the efficacy of pharmacotherapies is compromised.
Continue to: When drug-related interventions fail
When drug-related interventions fail, therapy ought to be more psychologically focused.37 Psychotherapy is usually helpful and is particularly indicated when recovery is suboptimal. Counseling might come from the treating physician or referral to a psychotherapist.
Nasal esketamine can be efficacious when supplementing antidepressant pharmacotherapy among older patients with treatment-resistant depression.38 Elderly individuals responding to antidepressants do not benefit from adjunctive donepezil to correct mild cognitive impairment.39 There is no advantage to off-label cholinesterase inhibitor prescribing for patients with both depression and dementia.
Other options. Electroconvulsive therapy (ECT) does not cause long-term cognitive problems and is reserved for treatment-resistant cases.40 Patients with depression who also have had previous cognitive impairment often improve in mental ability following ECT.41
A promising new option. Transcranial magnetic stimulation (TMS) is a promising, relatively new therapeutic option for treating refractory cases of depressive mood disorders. In TMS, an electromagnetic coil that creates a magnetic field is placed over the left dorsolateral prefrontal cortex (which is responsible for mood regulation). Referral for TMS administration may offer new hope for older patients with treatment-resistant depression.42
Keep comorbidities in mind as you address depression
Coexisting psychiatric illnesses worsen emotions. Geriatric patients are susceptible to psychiatric comorbidities that include substance abuse, obsessive-compulsive characteristics, dysfunctional eating, and panic disorder.19 Myocardial and cerebral infarctions are detrimental to mental health, especially soon after such events.43 Poststroke depression magnifies the risk for disability and mortality,16,17 yet antidepressant pharmacotherapy often enhances prognoses. Along with early intervention algorithm-based plans and inclusion of a depression care manager, antidepressants often diminish poststroke depression severity.44 Even when cancer is present, depression care reduces mortality.44 So with this in mind, persist with antidepressant treatment, which will often benefit an elderly individual with depression.
Continue to: When possible, get ahead of depression before it sets in
When possible, get ahead of depression before it sets in
Social participation and employment help to sustain an optimistic, euthymic mood.45 Maintaining good physical health, in part through consistent activity levels (including exercise), can help prevent depression. Since persistent sleep disturbance predicts depression among those with a depression history, optimizing sleep among geriatric adults can avoid or alleviate depression.46
Sleep hygiene education for patients is also helpful. A regular waking time often promotes a better sleeping schedule. Restful sleep also is more likely when an individual avoids excess caffeine, exercises during the day, and uses the bed only for sleeping (not for listening to music or watching television).
Because inflammation may precede depression, anti-inflammatory medications have been proposed as potential treatment, but such pharmacotherapies are often ineffective. Older adults generally do not benefit from low-dose aspirin administration to prevent depression.47 Low vitamin D levels can contribute to depression, yet vitamin D supplementation may not improve mood.48
Offering hope. Tell your patients that if they are feeling depressed, they should make an appointment with you, their primary care physician, because there are medications they can take and counseling they can avail themselves of that could help.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville-Psychiatry, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
Late-life depression is the onset of a major depressive disorder in an individual ≥ 60 years of age. Depressive illness compromises quality of life and is especially troublesome for older people. The prevalence of depression among individuals > 65 years of age is about 4% in women and 3% in men.1 The estimated lifetime prevalence is approximately 24% for women and 10% for men.2 Three factors account for this disparity: women exhibit greater susceptibility to depression; the illness persists longer in women than it does in men; and the probability of death related to depression is lower in women.2
Beyond its direct mental and emotional impacts, depression takes a financial toll; health care costs are higher for those with depression than for those without depression.3 Unpaid caregiver expense is the largest indirect financial burden with late-life depression.4 Additional indirect costs include less work productivity, early retirement, and diminished financial security.4
Many individuals with depression never receive treatment. Fortunately, there are many interventions in the primary care arsenal that can be used to treat older patients with depression and dramatically improve mood, comfort, and function.
The interactions of emotional and physical health
The pathophysiology of depression remains unclear. However, numerous factors are known to contribute to, exacerbate, or prolong depression among elderly populations. Insufficient social engagement and support is strongly associated with depressive mood.5 The loss of independence in giving up automobile driving can compromise self-confidence.6 Sleep difficulties predispose to, and predict, the emergence of a mood disorder, independent of other symptoms.7 Age-related hearing deficits also are associated with depression.8
There is a close relationship between emotional and physical health.9 Depression adds to the likelihood of medical illness, and somatic pathology increases the risk for mood disorders.9 Depression has been linked with obesity, frailty, diabetes, cognitive impairment, and terminal illness.9
Inflammatory markers and depression may also be related. Plasma levels of interleukin-6 and C-reactive protein were measured in a longitudinal aging study.14 A high level of interleukin-6, but not C-reactive protein, correlated with an increased prevalence of depression in older people.
Chronic cerebral ischemia can result in a “vascular depression”13 in which disruption of prefrontal systems by ischemic lesions is hypothesized to be an important factor in developing despair. Psychomotor retardation, executive dysfunction, severe disability, and a heightened risk for relapse are common features of vascular depression.15 Poststroke depression often follows a cerebrovascular episode16; the exact pathogenic mechanism is unknown.17
Continue to: A summation of common risk factors
A summation of common risk factors. A personal or family history of depression increases the risk for late-life depression. Other risk factors are female gender, bereavement, sleep disturbance, and disability.18 Poor general health, chronic pain, cognitive impairment, poor social support, and medical comorbidities with impaired functioning increase the likelihood of resultant mood disorders.18
Somatic complaints may overshadow diagnostic symptoms
Manifestations of depression include disturbed sleep and reductions in appetite, concentration, activity, and energy for daily function.19 These features, of course, may accompany medical disorders and some normal physiologic changes among elderly people. We find that while older individuals may report a sad mood, disturbed sleep, or other dysfunctions, they frequently emphasize their somatic complaints much more prominently than their emotions. This can make it difficult to recognize clinical depression.
For a diagnosis of major depression, 5 of the following 9 symptoms must be present for most of the day or nearly every day over a period of at least 2 weeks19: depressed mood; diminished interest in most activities; significant weight loss or decreased appetite; insomnia or hypersomnia; agitation or retardation; fatigue or loss of energy; feelings of worthlessness or guilt; diminished concentration; and recurrent thoughts of death or suicide.19
Planning difficulties, apathy, disability, and anhedonia frequently occur. Executive dysfunction and inefficacy of antidepressant pharmacotherapy are related to compromised frontal-striatal-limbic pathways.20 Since difficulties with planning and organization are associated with suboptimal response to antidepressant medications, a psychotherapeutic focus on these executive functions can augment drug-induced benefit.
Rule out these alternative diagnoses
Dementias can manifest as depression. Other brain pathologies, particularly Parkinson disease or stroke, also should be ruled out. Overmedication can simulate depression, so be sure to review the prescription and over-the-counter agents a patient is taking. Some medications can occasionally precipitate a clinical depression; these include stimulants, steroids, methyldopa, triptans, chemotherapeutic agents, and immunologic drugs, to name a few.19
Continue to: Pharmacotherapy, Yes, but first, consider these factors
Pharmacotherapy, Yes, but first, consider these factors
Maintaining a close patient–doctor relationship augments all therapeutic interventions. Good eye contact when listening to and counseling patients is key, as is providing close follow-up appointments.
Encourage social interactions with family and friends, which can be particularly productive. Encouraging spiritual endeavors, such as attendance at religious services, can be beneficial.21
Recommend exercise. Physical exercise yields positive outcomes22; it can enhance mood, improve sleep, and help to diminish anxiety. Encourage patients with depression to take a daily walk during the day; doing so can enhance emotional outlook, health, and even socialization.
What treatment will best serve your patient?
It’s important when caring for patients with depression to assess and address suicidal ideation. Depression with a previous suicide attempt is a strong risk factor for suicide. Inquire about suicidal intent or death wishes, access to guns, and other life-ending behaviors. Whenever suicide is an active issue, immediate crisis management is required. Psychiatric referral is an option, and hospitalization may be indicated. Advise family members to remove firearms or restrict access, be with the patient as much as possible, and assist at intervention planning and implementation.
It is worth mentioning, here, the connection between chronic pain and suicidal ideation. Pain management reduces suicidal ideation, regardless of depression severity.23
Continue to: Psychotherapy and pharmacotherapies...
Psychotherapy and pharmacotherapies offered for the treatment of depression in geriatric practices are both effective, without much difference seen in efficacy.24 Psychotherapy might include direct physician and family support to the patient or referral to a mental health professional. Base treatment choices on clinical access, patient preference, and medical contraindications and other illnesses.
Pros and cons of various pharmacotherapies
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed first for elderly patients with depression.25 Escitalopram is often better tolerated than paroxetine, which exhibits muscarinic antagonism and enzyme inhibition of cytochrome P450-2D6.26 Escitalopram also has fewer pharmaceutical interactions compared with sertraline.26
Generally, when prescribing an antidepressant drug, stay with the initial choice, gradually increasing the dose as clinically needed to its maximum limit. Suicidal ideation may be worsened by too quickly switching from one antidepressant to another or by co-prescribing anxiolytic or hypnotic medicines. Benzodiazepines have addictive and disinhibiting properties and should be avoided, if possible.27 For patients with insomnia, consider initially selecting a sedating antidepressant medication such as paroxetine or mirtazapine to augment sleep.
Alternatives to SSRIs. Nonselective serotonin reuptake inhibitors have similar efficacy as SSRIs. However, escitalopram is as effective as venlafaxine (a selective serotonin and norepinephrine reuptake inhibitor [SSNRI]) and is better tolerated.28 Duloxetine, another SSNRI, improves mood and often diminishes chronic pain.29 Mirtazapine, an alpha-2 antagonist, might cause fewer drug-drug interactions and is effective, well tolerated, and especially helpful for patients with anxiety or insomnia.30 Dry mouth, sedation, and weight gain are common adverse effects of mirtazapine. Obesity precautions are often necessary during mirtazapine therapy; this includes monitoring body weight and metabolic profiles, instituting dietary changes, and recommending an exercise regimen. In contrast to SSRIs, mirtazapine might induce less sexual dysfunction.31
Tricyclic antidepressant drugs can also be effective but may worsen cardiac conduction abnormalities, prostatic hypertrophy, or narrow angle glaucoma. Tricyclic antidepressants may be useful in patients without cardiac disease who have not responded to an SSRI or an SSNRI.
Continue to: The role of aripiprazole
The role of aripiprazole. Elderly patients not achieving remission from depression with antidepressant agents alone may benefit from co-prescribing aripiprazole.32 As an adjunct, aripiprazole is effective in achieving and sustaining remission
Minimize risks and maximize benefits of antidepressants by following these recommendations:
- Ascertain whether any antidepressant treatments have worked well in the past.
- Start with an SSRI if no other antidepressant treatment has worked in the past.
- Counsel patients about the need for treatment adherence. Antidepressants may take 2 weeks to 2 months to provide noticeable improvement.
- Prescribe up to the maximum drug dose if needed to enhance benefit.
- Use a mood measurement tool (eg, the Patient Health Questionnaire-9) to help evaluate treatment response.
Try a different class of drugs for patients who do not respond to treatment. For patients who have a partial response, augment with bupropion XL, mirtazapine, aripiprazole, or quetiapine.33 Sertraline and nortriptyline are similarly effective on a population-wide basis, with sertraline having less-problematic adverse effects.34 Trial-and-error treatments in practice may find one patient responding only to sertraline and another patient only to nortriptyline.
Combinations of different drug classes may provide benefit for patients not responding to a single antidepressant. In geriatric patients, combined treatment with methylphenidate and citalopram enhances mood and well-being.35 Compared with either drug alone, the combination yielded an augmented clinical response profile and a higher rate of remission. Cognitive functioning, energy, and mood improve even with methylphenidate alone, especially when fatigue is an issue. However, addictive properties limit its use to cases in which conventional antidepressant medications are not effective or indicated, and only when drug refills are closely monitored.
The challenges of advancing age. Antidepressant treatment needs increase with advanced age.36 As mentioned earlier, elderly people often have medical illnesses complicating their depression and frequently are dealing with pain from the medical illness. When dementia coexists with depression, the efficacy of pharmacotherapies is compromised.
Continue to: When drug-related interventions fail
When drug-related interventions fail, therapy ought to be more psychologically focused.37 Psychotherapy is usually helpful and is particularly indicated when recovery is suboptimal. Counseling might come from the treating physician or referral to a psychotherapist.
Nasal esketamine can be efficacious when supplementing antidepressant pharmacotherapy among older patients with treatment-resistant depression.38 Elderly individuals responding to antidepressants do not benefit from adjunctive donepezil to correct mild cognitive impairment.39 There is no advantage to off-label cholinesterase inhibitor prescribing for patients with both depression and dementia.
Other options. Electroconvulsive therapy (ECT) does not cause long-term cognitive problems and is reserved for treatment-resistant cases.40 Patients with depression who also have had previous cognitive impairment often improve in mental ability following ECT.41
A promising new option. Transcranial magnetic stimulation (TMS) is a promising, relatively new therapeutic option for treating refractory cases of depressive mood disorders. In TMS, an electromagnetic coil that creates a magnetic field is placed over the left dorsolateral prefrontal cortex (which is responsible for mood regulation). Referral for TMS administration may offer new hope for older patients with treatment-resistant depression.42
Keep comorbidities in mind as you address depression
Coexisting psychiatric illnesses worsen emotions. Geriatric patients are susceptible to psychiatric comorbidities that include substance abuse, obsessive-compulsive characteristics, dysfunctional eating, and panic disorder.19 Myocardial and cerebral infarctions are detrimental to mental health, especially soon after such events.43 Poststroke depression magnifies the risk for disability and mortality,16,17 yet antidepressant pharmacotherapy often enhances prognoses. Along with early intervention algorithm-based plans and inclusion of a depression care manager, antidepressants often diminish poststroke depression severity.44 Even when cancer is present, depression care reduces mortality.44 So with this in mind, persist with antidepressant treatment, which will often benefit an elderly individual with depression.
Continue to: When possible, get ahead of depression before it sets in
When possible, get ahead of depression before it sets in
Social participation and employment help to sustain an optimistic, euthymic mood.45 Maintaining good physical health, in part through consistent activity levels (including exercise), can help prevent depression. Since persistent sleep disturbance predicts depression among those with a depression history, optimizing sleep among geriatric adults can avoid or alleviate depression.46
Sleep hygiene education for patients is also helpful. A regular waking time often promotes a better sleeping schedule. Restful sleep also is more likely when an individual avoids excess caffeine, exercises during the day, and uses the bed only for sleeping (not for listening to music or watching television).
Because inflammation may precede depression, anti-inflammatory medications have been proposed as potential treatment, but such pharmacotherapies are often ineffective. Older adults generally do not benefit from low-dose aspirin administration to prevent depression.47 Low vitamin D levels can contribute to depression, yet vitamin D supplementation may not improve mood.48
Offering hope. Tell your patients that if they are feeling depressed, they should make an appointment with you, their primary care physician, because there are medications they can take and counseling they can avail themselves of that could help.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville-Psychiatry, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psych. 2000;57:601-607. doi: 10.1001/ archpsyc.57.6.601
2. Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psych. 2008;65:172-178. doi: 10.1001/archgenpsychiatry.2007.17
3. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psych. 2003;60:897-903. doi: 10.1001/archpsyc.60.9.897
4. Snow CE, Abrams RC. The indirect costs of late-life depression in the United States: a literature review and perspective. Geriatrics. 2016;1,30. doi.org/10.3390/geriatrics/1040030
5. George LK, Blazer DG, Hughes D, et al. Social support and the outcome of major depression. Br J Psych. 1989;154:478-485. doi: 10.1192/bjp.154.4.478
6. Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol Psychol Soc Sci. 2001;56:S343-S351. doi: 10.1093/geronb/56.6.s343
7. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community dwelling older adults—a prospective study. Am J Psych. 2008;165:1543-1550. doi: 10.1176/appi.ajp.2008.07121882
8. Golub JS, Brewster KK, Brickman AM, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. 2020;28:545-556. doi: 10.1016/j.jagp.2019.12.008
9. Alexopoulos GS. Mechanisms and treatment of late-life depression. Focus (Am Psychiatr Publ). 2021;19:340-354. doi: 10.1176/appi.focus.19304
10. Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Disord. 1990;178:27-31. doi: 10.1097/00005053-199001000-00005
11. Gilman SE, Abraham HE. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alco Depend. 2001;63:277-286. doi: 10.1016/s0376-8716(00)00216-7
12. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15-P21. doi: 10.1093/geronj/46.1.p15
13. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psych. 1997;54:915-922. doi: 10.1001/archpsyc.1997.01830220033006
14. Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249-255. doi: 10.1016/j.jad.2007.07.002
15. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963-974. doi: 10.1038/mp.2013.20
16. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psych. 2016;173:221-231. doi: 10.1176/appi.ajp.2015.15030363
17. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. doi: 10.1016/ j.arr.2019.01.013
18. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psych. 2003;160:1147-1156. doi: 10.1176/appi.ajp.160.6.1147
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013:160-168.
20. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psych. 2016;24:31-34. doi: 10.1016/j.jagp.2015.05.010
21. Koenig HG, Cohen HJ, Blazer DG, et al. Religious coping and depression in elderly hospitalized medically ill men. Am J Psychiatry. 1992;149:1693-1700. doi: 10.1176/ajp.149.12.1693
22. Blake H, Mo P, Malik S, et al. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;10:873-887. doi: 10.1177/0269215509337449
23. Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081-1091. doi: 10.1001/jama.291.9.1081
24. Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493-1501. doi: 10.1176/ajp.2006.163.9.1493
25. Solai LK, Mulsant BH, Pollack BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355-368. doi: 10.2165/00002512-200118050-00006
26. Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline. Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. doi: 10.1097/YIC.0000000000000023
27. Hedna K, Sundell KA, Hamidi A, et al. Antidepressants and suicidal behaviour in late life: a prospective population-based study of use patterns in new users aged 75 and above. Eur J Clin Pharmacol. 2018;74:201-208. doi: 10.1007/s00228-017-2360-x
28. Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190-1196. doi: 10.4088/jcp.v65n0906
29. Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34-45. doi: 10.1016/ j.jagp.2013.01.019
30. Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. doi: 10.2165/00003495-199957040-00010
31. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x
32. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet. 2015;386:2404-2412. doi: 10.1016/S0140-6736(15)00308-6
33. Lenze EJ, Oughli HA. Antidepressant treatment for late-life depression: considering risks and benefits. J Am Geriatr Soc. 2019;67:1555-1556. doi: 10.1111/jgs.15964
34. Bondareff W, Alpert M, Friedhoff AJ, et al: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729-736. doi: 10.1176/appi.ajp.157.5.729
35. Lavretsky H, Reinlieb M, St Cyr N. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo controlled trial. Am J Psych. 2015;72:561-569. doi: 10.1176/appi.ajp.2014.14070889
36. Arthur A, Savva GM, Barnes LE, et al. Changing prevalence and treatment of depression among older people over two decades. Br J Psychiatry. 2020;21:49-54. doi: 10.1192/bjp.2019.193
37. Zuidersma M, Chua K-C, Hellier J, et al. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019;27:920-931. doi: 10.1016/ j.jagp.2019.03.021
38. Ochs-Ross R, Wajs E, Daly EJ, et al. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30:541-556. doi: 10.1016/j.jagp.2021.09.014
39. Devanand DP, Pelton GH, D’Antonio K, et al. Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am J Geriatr Psychiatry. 2018;26:1050-1060. doi: 10.1016/ j.jagp.2018.05.008
40. Obbels J, Vansteelandt K, Verwijk E, et al. MMSE changes during and after ECT in late life depression: a prospective study. Am J Geriatr Psychiatry. 2019;27:934-944. doi: 10.1016/ j.jagp.2019.04.006
41. Wagenmakers MJ, Vansteelandt K, van Exel E, et al. Transient cognitive impairment and white matter hyperintensities in severely depressed older patients treated with electroconvulsive therapy. Am J Geriatr Psychiatry. 2021:29:1117-1128. doi: 10.1016/j.jagp.2020.12.028
42. Trevizol AP, Goldberger KW, Mulsant BH, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Ger Psychiatry. 2019;34:822-827. doi: 10.1002/gps.5091
43. Aben I, Verhey F, Stik J, et al. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581-585. doi: 10.1136/jnnp.74.5.581
44. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698. doi: 10.7326/0003-4819-146-10-200705150-00002
45. Lee J, Jang SN, Cho SL. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int Psychogeriatr. 2017;29:1495-1505. doi: 10.1017/S1041610217000709
46. Lee E, Cho HJ, Olmstead R, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685-1691. doi: 10.5665/sleep.3128
47. Berk M, Woods RL, Nelson MR, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. J Am Med A Psych. 2020;77:1012-1020. doi: 10.1001/jamapsychiatry.2020.1214
48. Okereke OI, Reynolds CF, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. doi: 10.1001/jama.2020.10224
1. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psych. 2000;57:601-607. doi: 10.1001/ archpsyc.57.6.601
2. Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psych. 2008;65:172-178. doi: 10.1001/archgenpsychiatry.2007.17
3. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psych. 2003;60:897-903. doi: 10.1001/archpsyc.60.9.897
4. Snow CE, Abrams RC. The indirect costs of late-life depression in the United States: a literature review and perspective. Geriatrics. 2016;1,30. doi.org/10.3390/geriatrics/1040030
5. George LK, Blazer DG, Hughes D, et al. Social support and the outcome of major depression. Br J Psych. 1989;154:478-485. doi: 10.1192/bjp.154.4.478
6. Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol Psychol Soc Sci. 2001;56:S343-S351. doi: 10.1093/geronb/56.6.s343
7. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community dwelling older adults—a prospective study. Am J Psych. 2008;165:1543-1550. doi: 10.1176/appi.ajp.2008.07121882
8. Golub JS, Brewster KK, Brickman AM, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. 2020;28:545-556. doi: 10.1016/j.jagp.2019.12.008
9. Alexopoulos GS. Mechanisms and treatment of late-life depression. Focus (Am Psychiatr Publ). 2021;19:340-354. doi: 10.1176/appi.focus.19304
10. Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Disord. 1990;178:27-31. doi: 10.1097/00005053-199001000-00005
11. Gilman SE, Abraham HE. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alco Depend. 2001;63:277-286. doi: 10.1016/s0376-8716(00)00216-7
12. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15-P21. doi: 10.1093/geronj/46.1.p15
13. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psych. 1997;54:915-922. doi: 10.1001/archpsyc.1997.01830220033006
14. Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249-255. doi: 10.1016/j.jad.2007.07.002
15. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963-974. doi: 10.1038/mp.2013.20
16. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psych. 2016;173:221-231. doi: 10.1176/appi.ajp.2015.15030363
17. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. doi: 10.1016/ j.arr.2019.01.013
18. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psych. 2003;160:1147-1156. doi: 10.1176/appi.ajp.160.6.1147
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013:160-168.
20. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psych. 2016;24:31-34. doi: 10.1016/j.jagp.2015.05.010
21. Koenig HG, Cohen HJ, Blazer DG, et al. Religious coping and depression in elderly hospitalized medically ill men. Am J Psychiatry. 1992;149:1693-1700. doi: 10.1176/ajp.149.12.1693
22. Blake H, Mo P, Malik S, et al. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;10:873-887. doi: 10.1177/0269215509337449
23. Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081-1091. doi: 10.1001/jama.291.9.1081
24. Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493-1501. doi: 10.1176/ajp.2006.163.9.1493
25. Solai LK, Mulsant BH, Pollack BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355-368. doi: 10.2165/00002512-200118050-00006
26. Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline. Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. doi: 10.1097/YIC.0000000000000023
27. Hedna K, Sundell KA, Hamidi A, et al. Antidepressants and suicidal behaviour in late life: a prospective population-based study of use patterns in new users aged 75 and above. Eur J Clin Pharmacol. 2018;74:201-208. doi: 10.1007/s00228-017-2360-x
28. Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190-1196. doi: 10.4088/jcp.v65n0906
29. Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34-45. doi: 10.1016/ j.jagp.2013.01.019
30. Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. doi: 10.2165/00003495-199957040-00010
31. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x
32. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet. 2015;386:2404-2412. doi: 10.1016/S0140-6736(15)00308-6
33. Lenze EJ, Oughli HA. Antidepressant treatment for late-life depression: considering risks and benefits. J Am Geriatr Soc. 2019;67:1555-1556. doi: 10.1111/jgs.15964
34. Bondareff W, Alpert M, Friedhoff AJ, et al: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729-736. doi: 10.1176/appi.ajp.157.5.729
35. Lavretsky H, Reinlieb M, St Cyr N. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo controlled trial. Am J Psych. 2015;72:561-569. doi: 10.1176/appi.ajp.2014.14070889
36. Arthur A, Savva GM, Barnes LE, et al. Changing prevalence and treatment of depression among older people over two decades. Br J Psychiatry. 2020;21:49-54. doi: 10.1192/bjp.2019.193
37. Zuidersma M, Chua K-C, Hellier J, et al. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019;27:920-931. doi: 10.1016/ j.jagp.2019.03.021
38. Ochs-Ross R, Wajs E, Daly EJ, et al. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30:541-556. doi: 10.1016/j.jagp.2021.09.014
39. Devanand DP, Pelton GH, D’Antonio K, et al. Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am J Geriatr Psychiatry. 2018;26:1050-1060. doi: 10.1016/ j.jagp.2018.05.008
40. Obbels J, Vansteelandt K, Verwijk E, et al. MMSE changes during and after ECT in late life depression: a prospective study. Am J Geriatr Psychiatry. 2019;27:934-944. doi: 10.1016/ j.jagp.2019.04.006
41. Wagenmakers MJ, Vansteelandt K, van Exel E, et al. Transient cognitive impairment and white matter hyperintensities in severely depressed older patients treated with electroconvulsive therapy. Am J Geriatr Psychiatry. 2021:29:1117-1128. doi: 10.1016/j.jagp.2020.12.028
42. Trevizol AP, Goldberger KW, Mulsant BH, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Ger Psychiatry. 2019;34:822-827. doi: 10.1002/gps.5091
43. Aben I, Verhey F, Stik J, et al. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581-585. doi: 10.1136/jnnp.74.5.581
44. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698. doi: 10.7326/0003-4819-146-10-200705150-00002
45. Lee J, Jang SN, Cho SL. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int Psychogeriatr. 2017;29:1495-1505. doi: 10.1017/S1041610217000709
46. Lee E, Cho HJ, Olmstead R, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685-1691. doi: 10.5665/sleep.3128
47. Berk M, Woods RL, Nelson MR, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. J Am Med A Psych. 2020;77:1012-1020. doi: 10.1001/jamapsychiatry.2020.1214
48. Okereke OI, Reynolds CF, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. doi: 10.1001/jama.2020.10224
PRACTICE RECOMMENDATIONS
› Begin treatment with a selective serotonin reuptake inhibitor (SSRI) unless another antidepressant has worked well in the past. A
› Consider augmenting therapy with bupropion XL, mirtazapine, aripiprazole, or quetiapine for any patient who responds only partially to an SSRI. C
› Add psychotherapy to antidepressant pharmacotherapy, particularly for patients who have difficulties with executive functions such as planning and organization. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Commentary: Concerning PsA treatments and comorbidities, March 2023
With regard to advanced targeted therapies, there is concern about the side effects of Janus kinase (JAK) inhibitors, especially in patients with comorbidities. To address safety concerns with upadacitinib, a selective JAK1 inhibitor, Burmester and colleagues conducted an integrated safety analysis of 12 phase 3 trials that included 6991 patients (PsA n = 907; rheumatoid arthritis [RA] n = 3209; ankylosing spondylitis n = 182; and atopic dermatitis n = 2693) who received upadacitinib (15 or 30 mg once daily). Some trials included active comparators; therefore, safety among 1008 patients (RA n = 579; PsA n = 429) who received 40-mg adalimumab every other week and 314 patients with RA who received methotrexate were compared with those treated with upadacitinib. Overall, patients with PsA receiving 15-mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and death (0.8/100 PY). Patients with PsA treated with upadacitinib had higher rates of herpes zoster, nonmelanoma skin cancer, and elevations in creatine phosphokinase when compared with patients treated with adalimumab. Although these results are reassuring to clinicians treating PsA, continued surveillance regarding the risks for venous thrombosis, cardiovascular events, and cancer are required.
In a post hoc analysis of 10 clinical trials that included patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib, Kristensen and colleagues reported that the risk for major adverse cardiac events was higher among patients with PsA and a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk vs patients with a low ASCVD risk. The incidence of cancer was highest in patients with PsA and an intermediate 10-year ASCVD risk. Although these studies are reassuring, the assessment and risk stratification of adverse events with JAK inhibitors and therapies in PsA will require longer-term comparative clinical trials as well as an evaluation of observational data from disease registries.
Comorbidities also have an impact on treatment persistence in PsA. Tillett and colleagues conducted a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs. They demonstrated that among patients receiving ustekinumab, those with concomitant PsA had a higher comorbidity burden, including diabetes, hypertension, and obesity, and a shorter time to ustekinumab discontinuation when compared with those with psoriasis alone. Secondary failure of advanced therapies is increasingly noted in the management of psoriatic disease. Female sex, depression, previous exposure to biologics, and the presence of comorbidities are important risk factors. Comprehensive management of psoriatic disease should include appropriate management of comorbidities for better long-term treatment persistence and outcomes.
With regard to advanced targeted therapies, there is concern about the side effects of Janus kinase (JAK) inhibitors, especially in patients with comorbidities. To address safety concerns with upadacitinib, a selective JAK1 inhibitor, Burmester and colleagues conducted an integrated safety analysis of 12 phase 3 trials that included 6991 patients (PsA n = 907; rheumatoid arthritis [RA] n = 3209; ankylosing spondylitis n = 182; and atopic dermatitis n = 2693) who received upadacitinib (15 or 30 mg once daily). Some trials included active comparators; therefore, safety among 1008 patients (RA n = 579; PsA n = 429) who received 40-mg adalimumab every other week and 314 patients with RA who received methotrexate were compared with those treated with upadacitinib. Overall, patients with PsA receiving 15-mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and death (0.8/100 PY). Patients with PsA treated with upadacitinib had higher rates of herpes zoster, nonmelanoma skin cancer, and elevations in creatine phosphokinase when compared with patients treated with adalimumab. Although these results are reassuring to clinicians treating PsA, continued surveillance regarding the risks for venous thrombosis, cardiovascular events, and cancer are required.
In a post hoc analysis of 10 clinical trials that included patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib, Kristensen and colleagues reported that the risk for major adverse cardiac events was higher among patients with PsA and a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk vs patients with a low ASCVD risk. The incidence of cancer was highest in patients with PsA and an intermediate 10-year ASCVD risk. Although these studies are reassuring, the assessment and risk stratification of adverse events with JAK inhibitors and therapies in PsA will require longer-term comparative clinical trials as well as an evaluation of observational data from disease registries.
Comorbidities also have an impact on treatment persistence in PsA. Tillett and colleagues conducted a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs. They demonstrated that among patients receiving ustekinumab, those with concomitant PsA had a higher comorbidity burden, including diabetes, hypertension, and obesity, and a shorter time to ustekinumab discontinuation when compared with those with psoriasis alone. Secondary failure of advanced therapies is increasingly noted in the management of psoriatic disease. Female sex, depression, previous exposure to biologics, and the presence of comorbidities are important risk factors. Comprehensive management of psoriatic disease should include appropriate management of comorbidities for better long-term treatment persistence and outcomes.
With regard to advanced targeted therapies, there is concern about the side effects of Janus kinase (JAK) inhibitors, especially in patients with comorbidities. To address safety concerns with upadacitinib, a selective JAK1 inhibitor, Burmester and colleagues conducted an integrated safety analysis of 12 phase 3 trials that included 6991 patients (PsA n = 907; rheumatoid arthritis [RA] n = 3209; ankylosing spondylitis n = 182; and atopic dermatitis n = 2693) who received upadacitinib (15 or 30 mg once daily). Some trials included active comparators; therefore, safety among 1008 patients (RA n = 579; PsA n = 429) who received 40-mg adalimumab every other week and 314 patients with RA who received methotrexate were compared with those treated with upadacitinib. Overall, patients with PsA receiving 15-mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and death (0.8/100 PY). Patients with PsA treated with upadacitinib had higher rates of herpes zoster, nonmelanoma skin cancer, and elevations in creatine phosphokinase when compared with patients treated with adalimumab. Although these results are reassuring to clinicians treating PsA, continued surveillance regarding the risks for venous thrombosis, cardiovascular events, and cancer are required.
In a post hoc analysis of 10 clinical trials that included patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib, Kristensen and colleagues reported that the risk for major adverse cardiac events was higher among patients with PsA and a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk vs patients with a low ASCVD risk. The incidence of cancer was highest in patients with PsA and an intermediate 10-year ASCVD risk. Although these studies are reassuring, the assessment and risk stratification of adverse events with JAK inhibitors and therapies in PsA will require longer-term comparative clinical trials as well as an evaluation of observational data from disease registries.
Comorbidities also have an impact on treatment persistence in PsA. Tillett and colleagues conducted a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs. They demonstrated that among patients receiving ustekinumab, those with concomitant PsA had a higher comorbidity burden, including diabetes, hypertension, and obesity, and a shorter time to ustekinumab discontinuation when compared with those with psoriasis alone. Secondary failure of advanced therapies is increasingly noted in the management of psoriatic disease. Female sex, depression, previous exposure to biologics, and the presence of comorbidities are important risk factors. Comprehensive management of psoriatic disease should include appropriate management of comorbidities for better long-term treatment persistence and outcomes.
PsA: Baseline disease activity predicts DAPSA response in patients treated with apremilast
Key clinical point: Nearly half of the patients with psoriatic arthritis (PsA) treated with apremilast achieved disease activity index for psoriatic arthritis (DAPSA) low disease activity/remission at 6 or 12 months, with lower baseline disease activity being the only factor associated with the achievement of low disease activity or remission.
Major finding: Overall, 42.7% and 54.9% of patients achieved DAPSA low disease activity or remission at 6 and 12 months, respectively. Baseline DAPSA was inversely associated with the odds of achieving low disease activity or remission at 6 months (odds ratio [OR] 0.84) and 12 months (OR 0.91; both P < .01).
Study details: Findings are from a retrospective study including 293 patients with PsA who were treated with apremilast.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Becciolini A et al. Predictors of DAPSA response in psoriatic arthritis patients treated with apremilast in a retrospective observational multi-centric study. Biomedicines. 2023;11(2):433 (Feb 2). Doi: 10.3390/biomedicines11020433
Key clinical point: Nearly half of the patients with psoriatic arthritis (PsA) treated with apremilast achieved disease activity index for psoriatic arthritis (DAPSA) low disease activity/remission at 6 or 12 months, with lower baseline disease activity being the only factor associated with the achievement of low disease activity or remission.
Major finding: Overall, 42.7% and 54.9% of patients achieved DAPSA low disease activity or remission at 6 and 12 months, respectively. Baseline DAPSA was inversely associated with the odds of achieving low disease activity or remission at 6 months (odds ratio [OR] 0.84) and 12 months (OR 0.91; both P < .01).
Study details: Findings are from a retrospective study including 293 patients with PsA who were treated with apremilast.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Becciolini A et al. Predictors of DAPSA response in psoriatic arthritis patients treated with apremilast in a retrospective observational multi-centric study. Biomedicines. 2023;11(2):433 (Feb 2). Doi: 10.3390/biomedicines11020433
Key clinical point: Nearly half of the patients with psoriatic arthritis (PsA) treated with apremilast achieved disease activity index for psoriatic arthritis (DAPSA) low disease activity/remission at 6 or 12 months, with lower baseline disease activity being the only factor associated with the achievement of low disease activity or remission.
Major finding: Overall, 42.7% and 54.9% of patients achieved DAPSA low disease activity or remission at 6 and 12 months, respectively. Baseline DAPSA was inversely associated with the odds of achieving low disease activity or remission at 6 months (odds ratio [OR] 0.84) and 12 months (OR 0.91; both P < .01).
Study details: Findings are from a retrospective study including 293 patients with PsA who were treated with apremilast.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Becciolini A et al. Predictors of DAPSA response in psoriatic arthritis patients treated with apremilast in a retrospective observational multi-centric study. Biomedicines. 2023;11(2):433 (Feb 2). Doi: 10.3390/biomedicines11020433
Long-term safety and tolerability of upadacitinib in PsA
Key clinical point: Upadacitinib demonstrated an acceptable long-term safety profile and was generally well tolerated with no new safety signals in patients with psoriatic arthritis (PsA).
Major finding: Overall, patients with PsA receiving 15 mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and deaths (0.8/100 PY).
Study details: This integrated safety analysis of 12 phase 3 trials included 6991 patients with PsA (n = 907), rheumatoid arthritis (n = 3,209), ankylosing spondylitis (n = 182), and atopic dermatitis (n = 2693) who received upadacitinib (15 or 30 mg once daily); 1008 patients with RA (n = 579) and PsA (n = 429) who received 40 mg adalimumab every other week; and 314 patients with RA who received methotrexate.
Disclosures: This study was funded by AbbVie. Five authors declared being full-time employees of AbbVie or Mount Sinai or holding stock or stock options in AbbVie. Several authors reported ties with various sources, including AbbVie.
Source: Burmester GR et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735;15(Feb 8). Doi: 10.1136/rmdopen-2022-002735
Key clinical point: Upadacitinib demonstrated an acceptable long-term safety profile and was generally well tolerated with no new safety signals in patients with psoriatic arthritis (PsA).
Major finding: Overall, patients with PsA receiving 15 mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and deaths (0.8/100 PY).
Study details: This integrated safety analysis of 12 phase 3 trials included 6991 patients with PsA (n = 907), rheumatoid arthritis (n = 3,209), ankylosing spondylitis (n = 182), and atopic dermatitis (n = 2693) who received upadacitinib (15 or 30 mg once daily); 1008 patients with RA (n = 579) and PsA (n = 429) who received 40 mg adalimumab every other week; and 314 patients with RA who received methotrexate.
Disclosures: This study was funded by AbbVie. Five authors declared being full-time employees of AbbVie or Mount Sinai or holding stock or stock options in AbbVie. Several authors reported ties with various sources, including AbbVie.
Source: Burmester GR et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735;15(Feb 8). Doi: 10.1136/rmdopen-2022-002735
Key clinical point: Upadacitinib demonstrated an acceptable long-term safety profile and was generally well tolerated with no new safety signals in patients with psoriatic arthritis (PsA).
Major finding: Overall, patients with PsA receiving 15 mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and deaths (0.8/100 PY).
Study details: This integrated safety analysis of 12 phase 3 trials included 6991 patients with PsA (n = 907), rheumatoid arthritis (n = 3,209), ankylosing spondylitis (n = 182), and atopic dermatitis (n = 2693) who received upadacitinib (15 or 30 mg once daily); 1008 patients with RA (n = 579) and PsA (n = 429) who received 40 mg adalimumab every other week; and 314 patients with RA who received methotrexate.
Disclosures: This study was funded by AbbVie. Five authors declared being full-time employees of AbbVie or Mount Sinai or holding stock or stock options in AbbVie. Several authors reported ties with various sources, including AbbVie.
Source: Burmester GR et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735;15(Feb 8). Doi: 10.1136/rmdopen-2022-002735
Baseline cardiovascular risk may influence MACE and malignancy incidences in tofacitinib-treated PsA patients
Key clinical point: Preventive monitoring for cardiovascular risk is suggested in tofacitinib-treated patients with psoriatic arthritis (PsA) as higher atherosclerotic cardiovascular disease (ASCVD) risk appears to be associated with a higher incidence of major cardiovascular events (MACE) and malignancies.
Major finding: The risk for MACE appeared to be higher among patients with PsA and high vs low 10-year ASCVD risk (incidence rate [IR] 1.37 [95% CI 0.03-7.63] vs 0.08 [95% CI 0.0-0.42]), with the incidence of malignancies being the highest in patients with PsA and an intermediate 10-year ASCVD risk (IR 2.56, 95% CI 1.11-5.05).
Study details: Findings are from a post hoc analysis of 10 clinical trials including patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib.
Disclosures: This study was sponsored by Pfizer. Some authors declared receiving speaker fees or grant or research support or serving as consultants for various sources, including Pfizer. Some authors declared being employees and shareholders of Pfizer or Syneos Health, a paid contractor to Pfizer.
Source: Kristensen LE et al. Association between baseline cardiovascular risk and incidence rates of major adverse cardiovascular events and malignancies in patients with psoriatic arthritis and psoriasis receiving tofacitinib. Ther Adv Musculoskelet Dis. 2023 (Feb 7). Doi: 10.1177/1759720X221149965
Key clinical point: Preventive monitoring for cardiovascular risk is suggested in tofacitinib-treated patients with psoriatic arthritis (PsA) as higher atherosclerotic cardiovascular disease (ASCVD) risk appears to be associated with a higher incidence of major cardiovascular events (MACE) and malignancies.
Major finding: The risk for MACE appeared to be higher among patients with PsA and high vs low 10-year ASCVD risk (incidence rate [IR] 1.37 [95% CI 0.03-7.63] vs 0.08 [95% CI 0.0-0.42]), with the incidence of malignancies being the highest in patients with PsA and an intermediate 10-year ASCVD risk (IR 2.56, 95% CI 1.11-5.05).
Study details: Findings are from a post hoc analysis of 10 clinical trials including patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib.
Disclosures: This study was sponsored by Pfizer. Some authors declared receiving speaker fees or grant or research support or serving as consultants for various sources, including Pfizer. Some authors declared being employees and shareholders of Pfizer or Syneos Health, a paid contractor to Pfizer.
Source: Kristensen LE et al. Association between baseline cardiovascular risk and incidence rates of major adverse cardiovascular events and malignancies in patients with psoriatic arthritis and psoriasis receiving tofacitinib. Ther Adv Musculoskelet Dis. 2023 (Feb 7). Doi: 10.1177/1759720X221149965
Key clinical point: Preventive monitoring for cardiovascular risk is suggested in tofacitinib-treated patients with psoriatic arthritis (PsA) as higher atherosclerotic cardiovascular disease (ASCVD) risk appears to be associated with a higher incidence of major cardiovascular events (MACE) and malignancies.
Major finding: The risk for MACE appeared to be higher among patients with PsA and high vs low 10-year ASCVD risk (incidence rate [IR] 1.37 [95% CI 0.03-7.63] vs 0.08 [95% CI 0.0-0.42]), with the incidence of malignancies being the highest in patients with PsA and an intermediate 10-year ASCVD risk (IR 2.56, 95% CI 1.11-5.05).
Study details: Findings are from a post hoc analysis of 10 clinical trials including patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib.
Disclosures: This study was sponsored by Pfizer. Some authors declared receiving speaker fees or grant or research support or serving as consultants for various sources, including Pfizer. Some authors declared being employees and shareholders of Pfizer or Syneos Health, a paid contractor to Pfizer.
Source: Kristensen LE et al. Association between baseline cardiovascular risk and incidence rates of major adverse cardiovascular events and malignancies in patients with psoriatic arthritis and psoriasis receiving tofacitinib. Ther Adv Musculoskelet Dis. 2023 (Feb 7). Doi: 10.1177/1759720X221149965
Circulating microRNA can differentiate between psoriasis and psoriatic arthritis
Key clinical point: Signatures of circulating microRNA in patients with psoriatic arthritis (PsA) and patients with psoriasis were significantly different from those in control individuals, with some even being differentially regulated between PsA and psoriasis.
Major finding: Overall, 9 microRNA best differentiated patients with PsA and psoriasis from control individuals (area under the curve [AUC] ≥0.70; all P < .05) and 4 microRNA best differentiated patients with PsA from patients with psoriasis (all P < .05). A combination of 4 microRNA (miR-19b-3p, miR-21-5p, miR-92a-3p, and let-7b-5p) vs miR-92a-3p alone could better differentiate between patients with PsA and psoriasis (AUC 0.92 vs 0.82).
Study details: This cross-sectional study included 51 patients with PsA, 40 patients with psoriasis, and 50 control individuals.
Disclosures: This study did not receive any specific funding. Two authors declared being employees or shareholders of TAmiRNA GmbH or holding intellectual property for the diagnostic use of microRNA in bone diseases.
Source: Haschka J et al. Identification of circulating microRNA patterns in patients in psoriasis and psoriatic arthritis. Rheumatology (Oxford). 2023 (Feb 3). Doi: 10.1093/rheumatology/kead059
Key clinical point: Signatures of circulating microRNA in patients with psoriatic arthritis (PsA) and patients with psoriasis were significantly different from those in control individuals, with some even being differentially regulated between PsA and psoriasis.
Major finding: Overall, 9 microRNA best differentiated patients with PsA and psoriasis from control individuals (area under the curve [AUC] ≥0.70; all P < .05) and 4 microRNA best differentiated patients with PsA from patients with psoriasis (all P < .05). A combination of 4 microRNA (miR-19b-3p, miR-21-5p, miR-92a-3p, and let-7b-5p) vs miR-92a-3p alone could better differentiate between patients with PsA and psoriasis (AUC 0.92 vs 0.82).
Study details: This cross-sectional study included 51 patients with PsA, 40 patients with psoriasis, and 50 control individuals.
Disclosures: This study did not receive any specific funding. Two authors declared being employees or shareholders of TAmiRNA GmbH or holding intellectual property for the diagnostic use of microRNA in bone diseases.
Source: Haschka J et al. Identification of circulating microRNA patterns in patients in psoriasis and psoriatic arthritis. Rheumatology (Oxford). 2023 (Feb 3). Doi: 10.1093/rheumatology/kead059
Key clinical point: Signatures of circulating microRNA in patients with psoriatic arthritis (PsA) and patients with psoriasis were significantly different from those in control individuals, with some even being differentially regulated between PsA and psoriasis.
Major finding: Overall, 9 microRNA best differentiated patients with PsA and psoriasis from control individuals (area under the curve [AUC] ≥0.70; all P < .05) and 4 microRNA best differentiated patients with PsA from patients with psoriasis (all P < .05). A combination of 4 microRNA (miR-19b-3p, miR-21-5p, miR-92a-3p, and let-7b-5p) vs miR-92a-3p alone could better differentiate between patients with PsA and psoriasis (AUC 0.92 vs 0.82).
Study details: This cross-sectional study included 51 patients with PsA, 40 patients with psoriasis, and 50 control individuals.
Disclosures: This study did not receive any specific funding. Two authors declared being employees or shareholders of TAmiRNA GmbH or holding intellectual property for the diagnostic use of microRNA in bone diseases.
Source: Haschka J et al. Identification of circulating microRNA patterns in patients in psoriasis and psoriatic arthritis. Rheumatology (Oxford). 2023 (Feb 3). Doi: 10.1093/rheumatology/kead059