User login
Acetaminophen as Renoprotective Treatment in a Patient With Severe Malaria
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
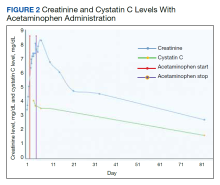
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
Real-world study compares discontinuation rates of tofacitinib and TNFi in RA
Key clinical point: The overall treatment discontinuation rate was similar among patients with rheumatoid arthritis (RA) who initiated tofacitinib vs tumor necrosis factor inhibitors (TNFi), but discontinuation because of adverse events was higher among those initiating tofacitinib.
Major finding: Patients initiating TNFi vs tofacitinib were less likely to discontinue treatment due to adverse events (hazard ratio 0.48; log-rank P = .003) but were equally likely to discontinue treatment due to any reason (log-rank P = .67) or inefficacy (log-rank P = .70). Concomitant methotrexate had no effect on treatment discontinuation.
Study details: This population-based retrospective cohort study pooled data from two registries and evaluated 1318 patients with RA who initiated tofacitinib (n = 493) or TNFi (n = 825), of which 746 patients received concomitant methotrexate.
Disclosures: This study did not receive any specific funding. Two authors declared receiving research funding, speaker honoraria or serving as consultants or advisory board members for various sources. Four authors declared being employees, faculty members, or owners of different organizations.
Source: Movahedi M et al. Discontinuation of tofacitinib and TNF inhibitors in patients with rheumatoid arthritis: Analysis of pooled data from two registries in Canada. BMJ Open. 2023;13:e063198 (Mar 6). Doi: 10.1136/bmjopen-2022-063198
Key clinical point: The overall treatment discontinuation rate was similar among patients with rheumatoid arthritis (RA) who initiated tofacitinib vs tumor necrosis factor inhibitors (TNFi), but discontinuation because of adverse events was higher among those initiating tofacitinib.
Major finding: Patients initiating TNFi vs tofacitinib were less likely to discontinue treatment due to adverse events (hazard ratio 0.48; log-rank P = .003) but were equally likely to discontinue treatment due to any reason (log-rank P = .67) or inefficacy (log-rank P = .70). Concomitant methotrexate had no effect on treatment discontinuation.
Study details: This population-based retrospective cohort study pooled data from two registries and evaluated 1318 patients with RA who initiated tofacitinib (n = 493) or TNFi (n = 825), of which 746 patients received concomitant methotrexate.
Disclosures: This study did not receive any specific funding. Two authors declared receiving research funding, speaker honoraria or serving as consultants or advisory board members for various sources. Four authors declared being employees, faculty members, or owners of different organizations.
Source: Movahedi M et al. Discontinuation of tofacitinib and TNF inhibitors in patients with rheumatoid arthritis: Analysis of pooled data from two registries in Canada. BMJ Open. 2023;13:e063198 (Mar 6). Doi: 10.1136/bmjopen-2022-063198
Key clinical point: The overall treatment discontinuation rate was similar among patients with rheumatoid arthritis (RA) who initiated tofacitinib vs tumor necrosis factor inhibitors (TNFi), but discontinuation because of adverse events was higher among those initiating tofacitinib.
Major finding: Patients initiating TNFi vs tofacitinib were less likely to discontinue treatment due to adverse events (hazard ratio 0.48; log-rank P = .003) but were equally likely to discontinue treatment due to any reason (log-rank P = .67) or inefficacy (log-rank P = .70). Concomitant methotrexate had no effect on treatment discontinuation.
Study details: This population-based retrospective cohort study pooled data from two registries and evaluated 1318 patients with RA who initiated tofacitinib (n = 493) or TNFi (n = 825), of which 746 patients received concomitant methotrexate.
Disclosures: This study did not receive any specific funding. Two authors declared receiving research funding, speaker honoraria or serving as consultants or advisory board members for various sources. Four authors declared being employees, faculty members, or owners of different organizations.
Source: Movahedi M et al. Discontinuation of tofacitinib and TNF inhibitors in patients with rheumatoid arthritis: Analysis of pooled data from two registries in Canada. BMJ Open. 2023;13:e063198 (Mar 6). Doi: 10.1136/bmjopen-2022-063198
Systemic immune-inflammation index: Novel biomarker to predict RA risk in adults
Key clinical point: A high level of systemic immune-inflammation index (SII) is positively correlated with an increased risk for rheumatoid arthritis (RA).
Major finding: Higher SII score was significantly associated with an increased risk of developing RA, with every unit increase in SII score increasing the risk for RA by approximately 17% (adjusted odds ratio,1.167; P = .020). The risk for RA rapidly increased when SII exceeded the cutoff value of 578.25.
Study details: This study analyzed the data of 37,604 individuals from the US National Health and Nutrition Examination Survey, a population-based cross-sectional survey, of which 2642 had RA.
Disclosures: This study did not receive any funding support. The authors declared no conflicts of interest.
Source: Liu B et al. The association between systemic immune-inflammation index and rheumatoid arthritis: Evidence from NHANES 1999–2018. Arthritis Res Ther. 2023;25:34 (Mar 4). Doi: 10.1186/s13075-023-03018-6
Key clinical point: A high level of systemic immune-inflammation index (SII) is positively correlated with an increased risk for rheumatoid arthritis (RA).
Major finding: Higher SII score was significantly associated with an increased risk of developing RA, with every unit increase in SII score increasing the risk for RA by approximately 17% (adjusted odds ratio,1.167; P = .020). The risk for RA rapidly increased when SII exceeded the cutoff value of 578.25.
Study details: This study analyzed the data of 37,604 individuals from the US National Health and Nutrition Examination Survey, a population-based cross-sectional survey, of which 2642 had RA.
Disclosures: This study did not receive any funding support. The authors declared no conflicts of interest.
Source: Liu B et al. The association between systemic immune-inflammation index and rheumatoid arthritis: Evidence from NHANES 1999–2018. Arthritis Res Ther. 2023;25:34 (Mar 4). Doi: 10.1186/s13075-023-03018-6
Key clinical point: A high level of systemic immune-inflammation index (SII) is positively correlated with an increased risk for rheumatoid arthritis (RA).
Major finding: Higher SII score was significantly associated with an increased risk of developing RA, with every unit increase in SII score increasing the risk for RA by approximately 17% (adjusted odds ratio,1.167; P = .020). The risk for RA rapidly increased when SII exceeded the cutoff value of 578.25.
Study details: This study analyzed the data of 37,604 individuals from the US National Health and Nutrition Examination Survey, a population-based cross-sectional survey, of which 2642 had RA.
Disclosures: This study did not receive any funding support. The authors declared no conflicts of interest.
Source: Liu B et al. The association between systemic immune-inflammation index and rheumatoid arthritis: Evidence from NHANES 1999–2018. Arthritis Res Ther. 2023;25:34 (Mar 4). Doi: 10.1186/s13075-023-03018-6
Joint damage associated with increased disability but not pain in early RA
Key clinical point: In early rheumatoid arthritis (RA), joint damage was significantly associated with disability but not with patient-reported pain at inclusion and during 5-year follow-up. Cartilage damage seemed to affect function in the longer run.
Major finding: The erosion score was significantly associated with Health Assessment Questionnaire score (HAQ) at inclusion (regression coefficient [β] 0.032; P = .02) and after 5 years (β 0.009; P = .02) in a cross-sectional analysis, whereas joint space narrowing score (JSNS) was associated with HAQ only at 5 years (β 0.010; 95% CI 0.004-0.016) in the longitudinal analysis. The erosion score and JSNS had no association with Visual Analogue Scale of Pain at all time points.
Study details: The data come from a longitudinal cohort study including 233 patients with early RA who had symptoms for ≤12 months and were followed for 5 years.
Disclosures: This study was supported by The Swedish Research Council and other sources. The authors declared no conflicts of interest.
Source: Eberhard A et al. Radiographic damage in early rheumatoid arthritis is associated with increased disability but not with pain-a 5-year follow-up study. Arthritis Res Ther. 2023;25:29 (Feb 27). Doi: 10.1186/s13075-023-03015-9
Key clinical point: In early rheumatoid arthritis (RA), joint damage was significantly associated with disability but not with patient-reported pain at inclusion and during 5-year follow-up. Cartilage damage seemed to affect function in the longer run.
Major finding: The erosion score was significantly associated with Health Assessment Questionnaire score (HAQ) at inclusion (regression coefficient [β] 0.032; P = .02) and after 5 years (β 0.009; P = .02) in a cross-sectional analysis, whereas joint space narrowing score (JSNS) was associated with HAQ only at 5 years (β 0.010; 95% CI 0.004-0.016) in the longitudinal analysis. The erosion score and JSNS had no association with Visual Analogue Scale of Pain at all time points.
Study details: The data come from a longitudinal cohort study including 233 patients with early RA who had symptoms for ≤12 months and were followed for 5 years.
Disclosures: This study was supported by The Swedish Research Council and other sources. The authors declared no conflicts of interest.
Source: Eberhard A et al. Radiographic damage in early rheumatoid arthritis is associated with increased disability but not with pain-a 5-year follow-up study. Arthritis Res Ther. 2023;25:29 (Feb 27). Doi: 10.1186/s13075-023-03015-9
Key clinical point: In early rheumatoid arthritis (RA), joint damage was significantly associated with disability but not with patient-reported pain at inclusion and during 5-year follow-up. Cartilage damage seemed to affect function in the longer run.
Major finding: The erosion score was significantly associated with Health Assessment Questionnaire score (HAQ) at inclusion (regression coefficient [β] 0.032; P = .02) and after 5 years (β 0.009; P = .02) in a cross-sectional analysis, whereas joint space narrowing score (JSNS) was associated with HAQ only at 5 years (β 0.010; 95% CI 0.004-0.016) in the longitudinal analysis. The erosion score and JSNS had no association with Visual Analogue Scale of Pain at all time points.
Study details: The data come from a longitudinal cohort study including 233 patients with early RA who had symptoms for ≤12 months and were followed for 5 years.
Disclosures: This study was supported by The Swedish Research Council and other sources. The authors declared no conflicts of interest.
Source: Eberhard A et al. Radiographic damage in early rheumatoid arthritis is associated with increased disability but not with pain-a 5-year follow-up study. Arthritis Res Ther. 2023;25:29 (Feb 27). Doi: 10.1186/s13075-023-03015-9
Pretreatment calprotectin offers no additional variability beyond CRP in predicting response to TNFi in RA
Key clinical point: Pretreatment calprotectin (MRP8/14) levels demonstrated no additional variability in predicting treatment response to tumor necrosis factor inhibitors (TNFis) beyond that of C-reactive protein (CRP) levels alone in patients with rheumatoid arthritis (RA).
Major finding: Higher vs lower pretreatment CRP levels predicted a good/moderate European League Against Rheumatism response at 3 months (odds ratio 3.79; P < .001), but MRP8/14 levels along with CRP levels offered no significant predictive improvement (P = .62). Unlike CRP level alone, pretreatment MRP8/14 level alone did not predict response to TNFi, as determined by Clinical Disease Activity Index (P = .839).
Study details: This post hoc analysis included 470 patients with RA whose serum MRP8/14 levels were measured before initiating adalimumab (n = 196) or etanercept (n = 274) treatment and after 3 months of adalimumab treatment (n = 179).
Disclosures: This study was supported by the UK National Institute for Health Research (NIHR) and other sources. Two authors declared being NIHR Senior investigators. Several authors reported ties with various sources unrelated to this study.
Source: Smith SL et al. Pre-treatment calprotectin (MRP8/14) provides no added value to testing CRP alone in terms of predicting response to TNF inhibitors in rheumatoid arthritis in a post hoc analysis. Ann Rheum Dis. 2023 (Feb 21). Doi: 10.1136/ard-2022-222519
Key clinical point: Pretreatment calprotectin (MRP8/14) levels demonstrated no additional variability in predicting treatment response to tumor necrosis factor inhibitors (TNFis) beyond that of C-reactive protein (CRP) levels alone in patients with rheumatoid arthritis (RA).
Major finding: Higher vs lower pretreatment CRP levels predicted a good/moderate European League Against Rheumatism response at 3 months (odds ratio 3.79; P < .001), but MRP8/14 levels along with CRP levels offered no significant predictive improvement (P = .62). Unlike CRP level alone, pretreatment MRP8/14 level alone did not predict response to TNFi, as determined by Clinical Disease Activity Index (P = .839).
Study details: This post hoc analysis included 470 patients with RA whose serum MRP8/14 levels were measured before initiating adalimumab (n = 196) or etanercept (n = 274) treatment and after 3 months of adalimumab treatment (n = 179).
Disclosures: This study was supported by the UK National Institute for Health Research (NIHR) and other sources. Two authors declared being NIHR Senior investigators. Several authors reported ties with various sources unrelated to this study.
Source: Smith SL et al. Pre-treatment calprotectin (MRP8/14) provides no added value to testing CRP alone in terms of predicting response to TNF inhibitors in rheumatoid arthritis in a post hoc analysis. Ann Rheum Dis. 2023 (Feb 21). Doi: 10.1136/ard-2022-222519
Key clinical point: Pretreatment calprotectin (MRP8/14) levels demonstrated no additional variability in predicting treatment response to tumor necrosis factor inhibitors (TNFis) beyond that of C-reactive protein (CRP) levels alone in patients with rheumatoid arthritis (RA).
Major finding: Higher vs lower pretreatment CRP levels predicted a good/moderate European League Against Rheumatism response at 3 months (odds ratio 3.79; P < .001), but MRP8/14 levels along with CRP levels offered no significant predictive improvement (P = .62). Unlike CRP level alone, pretreatment MRP8/14 level alone did not predict response to TNFi, as determined by Clinical Disease Activity Index (P = .839).
Study details: This post hoc analysis included 470 patients with RA whose serum MRP8/14 levels were measured before initiating adalimumab (n = 196) or etanercept (n = 274) treatment and after 3 months of adalimumab treatment (n = 179).
Disclosures: This study was supported by the UK National Institute for Health Research (NIHR) and other sources. Two authors declared being NIHR Senior investigators. Several authors reported ties with various sources unrelated to this study.
Source: Smith SL et al. Pre-treatment calprotectin (MRP8/14) provides no added value to testing CRP alone in terms of predicting response to TNF inhibitors in rheumatoid arthritis in a post hoc analysis. Ann Rheum Dis. 2023 (Feb 21). Doi: 10.1136/ard-2022-222519
Real-world study: Predictors of long-term remission in rheumatoid arthritis
Key clinical point: A tight control strategy led to long-term remission in 31.5% of patients with rheumatoid arthritis (RA) in real-world practice, with certain clinical and demographic characteristics being independent predictors.
Major finding: Long-term remission was achieved by 31.5% of patients and was independently predicted by disease characteristics, such as absence of flare during disease course (odds ratio [OR] 15.12; P = .001), sustained remission at ≤6 months after starting therapy (OR 3.24; P = .001), and baseline Disease Activity Score in 28 joints of ≤5.1 (OR 2.36; P = .037). Other factors included demographic factors, such as age >60 years at disease onset (OR 2.71; P = .029), and being anticitrullinated protein antibody negative (OR 2.63; P = .008).
Study details: This longitudinal study included 499 patients with RA who were treated with a tight control strategy, including step-up combination therapy with conventional synthetic and biologic disease-modifying antirheumatic drugs.
Disclosures: This study did not report the funding source or any conflicts of interest.
Source: Khabbazi A et al. Prevalence and predictors of long-term remission in rheumatoid arthritis in real-world practice: A longitudinal study. Clin Rheumatol. 2023 (Feb 17). Doi: 10.1007/s10067-023-06548-1
Key clinical point: A tight control strategy led to long-term remission in 31.5% of patients with rheumatoid arthritis (RA) in real-world practice, with certain clinical and demographic characteristics being independent predictors.
Major finding: Long-term remission was achieved by 31.5% of patients and was independently predicted by disease characteristics, such as absence of flare during disease course (odds ratio [OR] 15.12; P = .001), sustained remission at ≤6 months after starting therapy (OR 3.24; P = .001), and baseline Disease Activity Score in 28 joints of ≤5.1 (OR 2.36; P = .037). Other factors included demographic factors, such as age >60 years at disease onset (OR 2.71; P = .029), and being anticitrullinated protein antibody negative (OR 2.63; P = .008).
Study details: This longitudinal study included 499 patients with RA who were treated with a tight control strategy, including step-up combination therapy with conventional synthetic and biologic disease-modifying antirheumatic drugs.
Disclosures: This study did not report the funding source or any conflicts of interest.
Source: Khabbazi A et al. Prevalence and predictors of long-term remission in rheumatoid arthritis in real-world practice: A longitudinal study. Clin Rheumatol. 2023 (Feb 17). Doi: 10.1007/s10067-023-06548-1
Key clinical point: A tight control strategy led to long-term remission in 31.5% of patients with rheumatoid arthritis (RA) in real-world practice, with certain clinical and demographic characteristics being independent predictors.
Major finding: Long-term remission was achieved by 31.5% of patients and was independently predicted by disease characteristics, such as absence of flare during disease course (odds ratio [OR] 15.12; P = .001), sustained remission at ≤6 months after starting therapy (OR 3.24; P = .001), and baseline Disease Activity Score in 28 joints of ≤5.1 (OR 2.36; P = .037). Other factors included demographic factors, such as age >60 years at disease onset (OR 2.71; P = .029), and being anticitrullinated protein antibody negative (OR 2.63; P = .008).
Study details: This longitudinal study included 499 patients with RA who were treated with a tight control strategy, including step-up combination therapy with conventional synthetic and biologic disease-modifying antirheumatic drugs.
Disclosures: This study did not report the funding source or any conflicts of interest.
Source: Khabbazi A et al. Prevalence and predictors of long-term remission in rheumatoid arthritis in real-world practice: A longitudinal study. Clin Rheumatol. 2023 (Feb 17). Doi: 10.1007/s10067-023-06548-1
Early menopause worsens disease outcomes in postmenopausal women with RA
Key clinical point: Menopause at an early vs usual age (<45 vs ≥45 years) was associated with a higher disease activity and worse patient-reported outcomes in postmenopausal women with rheumatoid arthritis (RA).
Major finding: At baseline, women with early vs usual menopause had significantly higher Disease Activity Score in 28 joints (DAS28; P = .018) and Visual Analogue Scale (VAS) scores for global assessment (P = .016) and fatigue (P = .005), along with worse EuroQol-5D-VAS scores (P = .006). Early menopause was significantly associated with increased DAS28 (regression coefficient [β] 0.178; P = .013) and decreased EuroQol-5D utility values (β −0.033; P = .016) at 5-year follow-up.
Study details: This prospective observational cohort study included 2878 postmenopausal women with RA who had menopause at an early (n = 437) or usual (n = 2441) age.
Disclosures: This study was supported by Chung-Ang University research grants in 2022 and the National Research Foundation of Korea grant funded by the Korean government. The authors did not declare conflicts of interest.
Source: Park EH et al. Impact of early age at menopause on disease outcomes in postmenopausal women with rheumatoid arthritis: A large observational cohort study of Korean patients with rheumatoid arthritis. RMD Open. 2023;9:e002722 (Feb 15). Doi: 10.1136/rmdopen-2022-002722
Key clinical point: Menopause at an early vs usual age (<45 vs ≥45 years) was associated with a higher disease activity and worse patient-reported outcomes in postmenopausal women with rheumatoid arthritis (RA).
Major finding: At baseline, women with early vs usual menopause had significantly higher Disease Activity Score in 28 joints (DAS28; P = .018) and Visual Analogue Scale (VAS) scores for global assessment (P = .016) and fatigue (P = .005), along with worse EuroQol-5D-VAS scores (P = .006). Early menopause was significantly associated with increased DAS28 (regression coefficient [β] 0.178; P = .013) and decreased EuroQol-5D utility values (β −0.033; P = .016) at 5-year follow-up.
Study details: This prospective observational cohort study included 2878 postmenopausal women with RA who had menopause at an early (n = 437) or usual (n = 2441) age.
Disclosures: This study was supported by Chung-Ang University research grants in 2022 and the National Research Foundation of Korea grant funded by the Korean government. The authors did not declare conflicts of interest.
Source: Park EH et al. Impact of early age at menopause on disease outcomes in postmenopausal women with rheumatoid arthritis: A large observational cohort study of Korean patients with rheumatoid arthritis. RMD Open. 2023;9:e002722 (Feb 15). Doi: 10.1136/rmdopen-2022-002722
Key clinical point: Menopause at an early vs usual age (<45 vs ≥45 years) was associated with a higher disease activity and worse patient-reported outcomes in postmenopausal women with rheumatoid arthritis (RA).
Major finding: At baseline, women with early vs usual menopause had significantly higher Disease Activity Score in 28 joints (DAS28; P = .018) and Visual Analogue Scale (VAS) scores for global assessment (P = .016) and fatigue (P = .005), along with worse EuroQol-5D-VAS scores (P = .006). Early menopause was significantly associated with increased DAS28 (regression coefficient [β] 0.178; P = .013) and decreased EuroQol-5D utility values (β −0.033; P = .016) at 5-year follow-up.
Study details: This prospective observational cohort study included 2878 postmenopausal women with RA who had menopause at an early (n = 437) or usual (n = 2441) age.
Disclosures: This study was supported by Chung-Ang University research grants in 2022 and the National Research Foundation of Korea grant funded by the Korean government. The authors did not declare conflicts of interest.
Source: Park EH et al. Impact of early age at menopause on disease outcomes in postmenopausal women with rheumatoid arthritis: A large observational cohort study of Korean patients with rheumatoid arthritis. RMD Open. 2023;9:e002722 (Feb 15). Doi: 10.1136/rmdopen-2022-002722
CT-based screening for malignancies may benefit patients with RA who initiated b/tsDMARD
Key clinical point: Computed tomography (CT) screening before initiating biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) in patients with rheumatoid arthritis (RA) may help in early detection and treatment of malignancies, resulting in a safer and more stable RA treatment course.
Major finding: Malignancy was confirmed in 33 patients; however, only 7 vs 33 cases were detected with regular vs CT screening, respectively. Overall, 6 of 7 cases detected by regular screening were progressed stage malignancies, whereas 19 of 33 cases detected by CT screening were early stage malignancies; 80% of patients diagnosed with early stage malignancy achieved low-disease activity after 1 year of RA treatment.
Study details: This study evaluated 2192 patients with RA who were screened for malignancy using regular physical examination followed by CT before initiating b/tsDMARD.
Disclosures: This study was partly supported by the University of Occupational and Environmental Health, Japan. Five authors declared receiving research grants, consulting fees, lecture fees, speaking fees, or honoraria from various sources.
Source: Miyata H et al. Computed tomography for malignancy screening in patients with rheumatoid arthritis before initiation of disease modifying antirheumatic drug. Rheumatology (Oxford). 2023 (Feb 14). Doi: 10.1093/rheumatology/kead075
Key clinical point: Computed tomography (CT) screening before initiating biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) in patients with rheumatoid arthritis (RA) may help in early detection and treatment of malignancies, resulting in a safer and more stable RA treatment course.
Major finding: Malignancy was confirmed in 33 patients; however, only 7 vs 33 cases were detected with regular vs CT screening, respectively. Overall, 6 of 7 cases detected by regular screening were progressed stage malignancies, whereas 19 of 33 cases detected by CT screening were early stage malignancies; 80% of patients diagnosed with early stage malignancy achieved low-disease activity after 1 year of RA treatment.
Study details: This study evaluated 2192 patients with RA who were screened for malignancy using regular physical examination followed by CT before initiating b/tsDMARD.
Disclosures: This study was partly supported by the University of Occupational and Environmental Health, Japan. Five authors declared receiving research grants, consulting fees, lecture fees, speaking fees, or honoraria from various sources.
Source: Miyata H et al. Computed tomography for malignancy screening in patients with rheumatoid arthritis before initiation of disease modifying antirheumatic drug. Rheumatology (Oxford). 2023 (Feb 14). Doi: 10.1093/rheumatology/kead075
Key clinical point: Computed tomography (CT) screening before initiating biologic/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) in patients with rheumatoid arthritis (RA) may help in early detection and treatment of malignancies, resulting in a safer and more stable RA treatment course.
Major finding: Malignancy was confirmed in 33 patients; however, only 7 vs 33 cases were detected with regular vs CT screening, respectively. Overall, 6 of 7 cases detected by regular screening were progressed stage malignancies, whereas 19 of 33 cases detected by CT screening were early stage malignancies; 80% of patients diagnosed with early stage malignancy achieved low-disease activity after 1 year of RA treatment.
Study details: This study evaluated 2192 patients with RA who were screened for malignancy using regular physical examination followed by CT before initiating b/tsDMARD.
Disclosures: This study was partly supported by the University of Occupational and Environmental Health, Japan. Five authors declared receiving research grants, consulting fees, lecture fees, speaking fees, or honoraria from various sources.
Source: Miyata H et al. Computed tomography for malignancy screening in patients with rheumatoid arthritis before initiation of disease modifying antirheumatic drug. Rheumatology (Oxford). 2023 (Feb 14). Doi: 10.1093/rheumatology/kead075
RA raises risk for long-term MACE in patients undergoing percutaneous coronary intervention
Key clinical point: Rheumatoid arthritis (RA) reduced survival rates and increased the risk for long-term major adverse cardiovascular events (MACE) in patients with ischemic heart disease who underwent percutaneous coronary intervention (PCI); however, RA had no influence over short-term MACE.
Major finding: Patients with vs without RA who underwent PCI had lower survival rates (log-rank P < .001) and were at a significantly higher risk for long-term MACE (adjusted hazard ratio 1.07; P < .001), although the risk for short-term MACE was not significantly different between both the cohorts (P = .222).
Study details: This retrospective cohort study included 236,134 patients who underwent PCI, of which 34,493 patients had RA.
Disclosures: This study was supported by the Medical Research Promotion Program of Gangneung Asan Hospital, funded by the Asan Foundation, South Korea. The authors declared no conflicts of interest.
Source: Ha SJ et al. Clinical outcomes of patients with rheumatoid arthritis who underwent percutaneous coronary intervention: A Korean nationwide cohort study. PLoS One. 2023;18(2):e0281067 (Feb 14). Doi: 10.1371/journal.pone.0281067
Key clinical point: Rheumatoid arthritis (RA) reduced survival rates and increased the risk for long-term major adverse cardiovascular events (MACE) in patients with ischemic heart disease who underwent percutaneous coronary intervention (PCI); however, RA had no influence over short-term MACE.
Major finding: Patients with vs without RA who underwent PCI had lower survival rates (log-rank P < .001) and were at a significantly higher risk for long-term MACE (adjusted hazard ratio 1.07; P < .001), although the risk for short-term MACE was not significantly different between both the cohorts (P = .222).
Study details: This retrospective cohort study included 236,134 patients who underwent PCI, of which 34,493 patients had RA.
Disclosures: This study was supported by the Medical Research Promotion Program of Gangneung Asan Hospital, funded by the Asan Foundation, South Korea. The authors declared no conflicts of interest.
Source: Ha SJ et al. Clinical outcomes of patients with rheumatoid arthritis who underwent percutaneous coronary intervention: A Korean nationwide cohort study. PLoS One. 2023;18(2):e0281067 (Feb 14). Doi: 10.1371/journal.pone.0281067
Key clinical point: Rheumatoid arthritis (RA) reduced survival rates and increased the risk for long-term major adverse cardiovascular events (MACE) in patients with ischemic heart disease who underwent percutaneous coronary intervention (PCI); however, RA had no influence over short-term MACE.
Major finding: Patients with vs without RA who underwent PCI had lower survival rates (log-rank P < .001) and were at a significantly higher risk for long-term MACE (adjusted hazard ratio 1.07; P < .001), although the risk for short-term MACE was not significantly different between both the cohorts (P = .222).
Study details: This retrospective cohort study included 236,134 patients who underwent PCI, of which 34,493 patients had RA.
Disclosures: This study was supported by the Medical Research Promotion Program of Gangneung Asan Hospital, funded by the Asan Foundation, South Korea. The authors declared no conflicts of interest.
Source: Ha SJ et al. Clinical outcomes of patients with rheumatoid arthritis who underwent percutaneous coronary intervention: A Korean nationwide cohort study. PLoS One. 2023;18(2):e0281067 (Feb 14). Doi: 10.1371/journal.pone.0281067
Higher disability at early stages raises risk for progression to difficult-to-treat RA
Key clinical point: Younger patients and patients with higher disability scores at early stages were more likely to develop difficult-to-treat rheumatoid arthritis (RA). Thus focusing on severe disability during initial stages may alter disease course.
Major finding: Elevated initial disability score (odds ratio [OR] 1.89; P = .01) and a younger age at baseline (OR 0.95; P = .01) were associated with an increased risk for difficult-to-treat RA, whereas initial disease activity failed to show any influence.
Study details: The data come from a longitudinal, prospective cohort study including 631 patients with newly diagnosed RA, of which 35 patients developed difficult-to-treat RA.
Disclosures: This study was supported by the Instituto de Salud Carlos III, Spain, and other sources. The authors did not declare any conflicts of interest.
Source: Leon L et al. Difficult-to-treat rheumatoid arthritis (D2T RA): Clinical issues at early stages of disease. RMD Open. 2023;9:e002842 (Mar 8). Doi: 10.1136/rmdopen-2022-002842
Key clinical point: Younger patients and patients with higher disability scores at early stages were more likely to develop difficult-to-treat rheumatoid arthritis (RA). Thus focusing on severe disability during initial stages may alter disease course.
Major finding: Elevated initial disability score (odds ratio [OR] 1.89; P = .01) and a younger age at baseline (OR 0.95; P = .01) were associated with an increased risk for difficult-to-treat RA, whereas initial disease activity failed to show any influence.
Study details: The data come from a longitudinal, prospective cohort study including 631 patients with newly diagnosed RA, of which 35 patients developed difficult-to-treat RA.
Disclosures: This study was supported by the Instituto de Salud Carlos III, Spain, and other sources. The authors did not declare any conflicts of interest.
Source: Leon L et al. Difficult-to-treat rheumatoid arthritis (D2T RA): Clinical issues at early stages of disease. RMD Open. 2023;9:e002842 (Mar 8). Doi: 10.1136/rmdopen-2022-002842
Key clinical point: Younger patients and patients with higher disability scores at early stages were more likely to develop difficult-to-treat rheumatoid arthritis (RA). Thus focusing on severe disability during initial stages may alter disease course.
Major finding: Elevated initial disability score (odds ratio [OR] 1.89; P = .01) and a younger age at baseline (OR 0.95; P = .01) were associated with an increased risk for difficult-to-treat RA, whereas initial disease activity failed to show any influence.
Study details: The data come from a longitudinal, prospective cohort study including 631 patients with newly diagnosed RA, of which 35 patients developed difficult-to-treat RA.
Disclosures: This study was supported by the Instituto de Salud Carlos III, Spain, and other sources. The authors did not declare any conflicts of interest.
Source: Leon L et al. Difficult-to-treat rheumatoid arthritis (D2T RA): Clinical issues at early stages of disease. RMD Open. 2023;9:e002842 (Mar 8). Doi: 10.1136/rmdopen-2022-002842