User login
Administering concomitant methotrexate at a half vs usual dose while initiating TNFi is feasible
Key clinical point: A nearly 50% reduction in methotrexate dose at the time of initiating a tumor necrosis factor inhibitor (TNFi) is possible in patients with rheumatoid arthritis (RA) who had an inadequate response to the initial maximum tolerated dose of methotrexate.
Major finding: Reduced-dose methotrexate+adalimumab was noninferior to maximal-dose methotrexate+adalimumab in achieving Simplified Disease Activity Index-based remission at week 48 (adjusted risk difference 6.4%; 90% CI −7.0% to 19.8%) and less frequently caused adverse events after 24 weeks (20% vs 35%). No deaths were reported.
Study details: Findings are from the MIRACLE trial including 291 methotrexate-naive patients with RA and inadequate response to the maximum tolerated methotrexate dose who were randomly assigned to initiate adalimumab in combination with the maximum tolerated dose or a reduced dose of methotrexate.
Disclosures: This study was funded by Eisai. Three authors declared being employees and shareholders of Eisai. Several authors declared receiving speaker honoraria, grants, research support, consulting fees, or royalties from Eisai and other sources.
Source: Tamai H et al and MIRACLE study collaborators. Reduced versus maximum tolerated methotrexate dose concomitant with adalimumab in patients with rheumatoid arthritis (MIRACLE): A randomised, open-label, non-inferiority trial. Lancet Rheumatol. 2023;5(4):E215-E224 (Apr). Doi: 10.1016/S2665-9913(23)00070-X
Key clinical point: A nearly 50% reduction in methotrexate dose at the time of initiating a tumor necrosis factor inhibitor (TNFi) is possible in patients with rheumatoid arthritis (RA) who had an inadequate response to the initial maximum tolerated dose of methotrexate.
Major finding: Reduced-dose methotrexate+adalimumab was noninferior to maximal-dose methotrexate+adalimumab in achieving Simplified Disease Activity Index-based remission at week 48 (adjusted risk difference 6.4%; 90% CI −7.0% to 19.8%) and less frequently caused adverse events after 24 weeks (20% vs 35%). No deaths were reported.
Study details: Findings are from the MIRACLE trial including 291 methotrexate-naive patients with RA and inadequate response to the maximum tolerated methotrexate dose who were randomly assigned to initiate adalimumab in combination with the maximum tolerated dose or a reduced dose of methotrexate.
Disclosures: This study was funded by Eisai. Three authors declared being employees and shareholders of Eisai. Several authors declared receiving speaker honoraria, grants, research support, consulting fees, or royalties from Eisai and other sources.
Source: Tamai H et al and MIRACLE study collaborators. Reduced versus maximum tolerated methotrexate dose concomitant with adalimumab in patients with rheumatoid arthritis (MIRACLE): A randomised, open-label, non-inferiority trial. Lancet Rheumatol. 2023;5(4):E215-E224 (Apr). Doi: 10.1016/S2665-9913(23)00070-X
Key clinical point: A nearly 50% reduction in methotrexate dose at the time of initiating a tumor necrosis factor inhibitor (TNFi) is possible in patients with rheumatoid arthritis (RA) who had an inadequate response to the initial maximum tolerated dose of methotrexate.
Major finding: Reduced-dose methotrexate+adalimumab was noninferior to maximal-dose methotrexate+adalimumab in achieving Simplified Disease Activity Index-based remission at week 48 (adjusted risk difference 6.4%; 90% CI −7.0% to 19.8%) and less frequently caused adverse events after 24 weeks (20% vs 35%). No deaths were reported.
Study details: Findings are from the MIRACLE trial including 291 methotrexate-naive patients with RA and inadequate response to the maximum tolerated methotrexate dose who were randomly assigned to initiate adalimumab in combination with the maximum tolerated dose or a reduced dose of methotrexate.
Disclosures: This study was funded by Eisai. Three authors declared being employees and shareholders of Eisai. Several authors declared receiving speaker honoraria, grants, research support, consulting fees, or royalties from Eisai and other sources.
Source: Tamai H et al and MIRACLE study collaborators. Reduced versus maximum tolerated methotrexate dose concomitant with adalimumab in patients with rheumatoid arthritis (MIRACLE): A randomised, open-label, non-inferiority trial. Lancet Rheumatol. 2023;5(4):E215-E224 (Apr). Doi: 10.1016/S2665-9913(23)00070-X
Multi-cancer early detection liquid biopsy testing: A predictive genetic test not quite ready for prime time
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?
The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
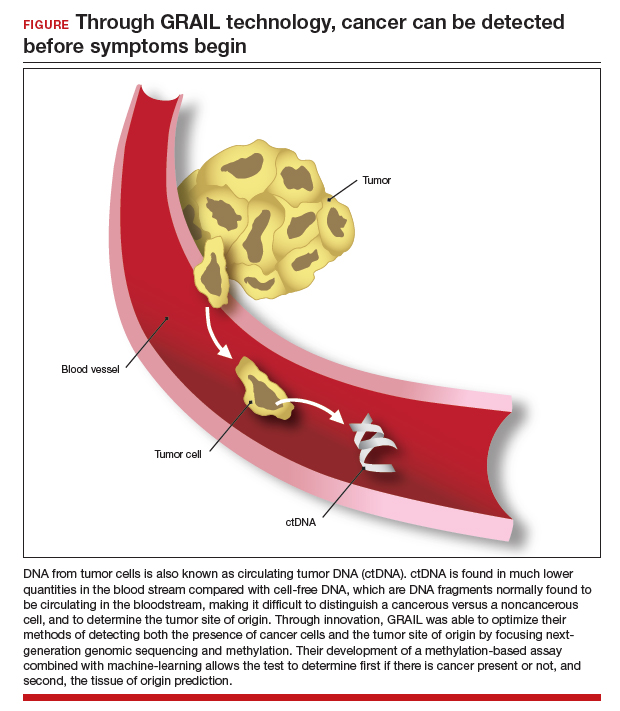
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
Product updates and reviews
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort
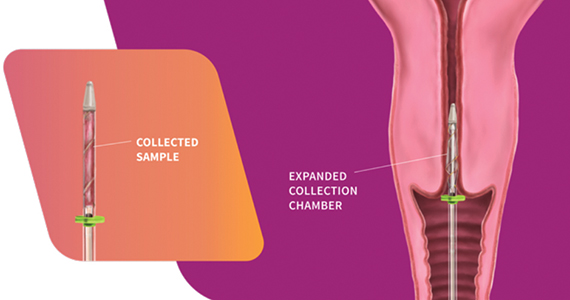
The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
REVIEW
FEMCERV®: Well-designed technology that can minimize patient discomfort

The FemCerv® Endocervical Sampler, developed by Femasys, Inc (Suwanee, Georgia) expands options for colposcopy biopsy.
Background. In the United States, approximately 3 million women per year undergo colposcopic evaluation to work-up abnormal screening cytology. While some controversy exists regarding the exact role of endocervical curettage (ECC) within each age group and clinical situation, it is nonetheless an important component of the colposcopy-biopsy examination in many cases. For over a century, the 3 mm metal endocervical curette has been the primary tool employed to obtain the tissue sample from the endocervical canal. Unfortunately, sharp curettage with a metal curette can have inadequate sampling rates as high as 14%,1 it runs the risk of ectocervical contamination, and it is painful, with almost half the participants in one study rating the procedural pain 3 ̶ 5 out of 8 on a VAS scale.2 So, maybe there is a better way.
Design/Functionality. According to Femasys, the FemCerv® Endocervical Sampler was designed to be the better way of performing an endocervical curettage. FemCerv is a single-use sterile device that comes in a standard 13 F size as well as an 11 F size for the narrower/stenotic os. In truth, at first glance it looks pretty complicated compared with a Kevorkian curette or an endocervical brush. The user end has a handle with a rotatable knob that transitions to a shaft with a flange at the end right before the sampling mechanism. To use the device, the sampling end is inserted into the endocervical canal up to the flange. The knob on the handle is then turned clockwise to open the sheath, thereby exposing sharp rigid plastic edges. The device is then rotated 360° clockwise and then 360° counterclockwise to “curette” the endocervical canal. Finally, the knob on the handle is turned counterclockwise to close the sheath and the device is removed. The specimen is then transferred to a standard vial for processing.
In my experience with its use, it actually exceeded my expectations. The device was easy to use, and the specimens were more than adequate. Truth be told, I came into the trial with a negative mindset having already convinced myself that this device was a waste of money given that doing an ECC with traditional methods is so straightforward. What I had not anticipated was the complete lack of patient discomfort when I used the FemCerv compared with a Kevorkian device.
Innovation. From an innovation standpoint, FemCerv is not super-disruptive technology, but it is well designed and pretty clever in that the opening and closing sheath prevents ectocervical cellular contamination, and the rotational sampling, rather than in-and-out sampling, does dramatically reduce the patient discomfort.
Summary. As I previously noted, before trying it, I did not anticipate liking FemCerv as much as I did. Does it add some non-reimbursable cost to a relatively low-reimbursing procedure? Absolutely. But it is not too expensive and, for me, making a painful procedure relatively painless is good value every time. I think all our patients would agree.
For more information, visit https://femasys.com/.
UPDATE
Hologic, Inc. announces that they received the following 2022 IMV ServiceTrakTM Awards for Mammography: Best Service, Best Customer Satisfaction, and Best System Performance. In addition, Hologic announces that their Affirm Contrast Biopsy software is commercially available in the United States. Tissue samples may be targeted and acquired using the Affirm Contrast Biopsy software from lesions identified using Hologic’s I-View Contrast Enhanced Mammography software. The latter software allows health care facilities an alternative to breast magnetic resonance imaging, which is used as supplemental imaging to mammography and/or ultrasonography.
For more information, visit https://www.hologic.com/
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
- Damkjaer M, Laursen JB, Petersen LK, et al. Endocervical sampling in women with suspected cervical neoplasia: a systematic review and meta-analysis of diagnostic test accuracy studies. Am J Obstet Gynecol. 2022;227:839-848.e4. doi: 10.1016/j.ajog.2022.07.036.
- Goksedef BP, Api M, Kaya O, et al. Diagnostic accuracy of two endocervical sampling method: randomized controlled trial. Arch Gynecol Obstet. 2013;287:117-22. doi: 10.1007/s00404-012-2542-9.
CarePostRoe.com: Study seeks to document poor quality medical care due to new abortion bans
In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
Implementation of a Multidisciplinary Team–Based Clinical Care Pathway Is Associated With Increased Surgery Rates for Infective Endocarditis
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
Relationships Between Residence Characteristics and Nursing Home Compare Database Quality Measures
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18
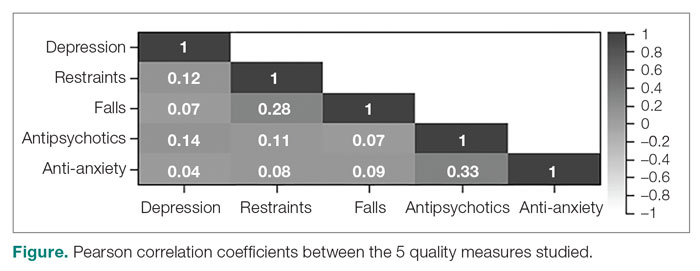
Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).
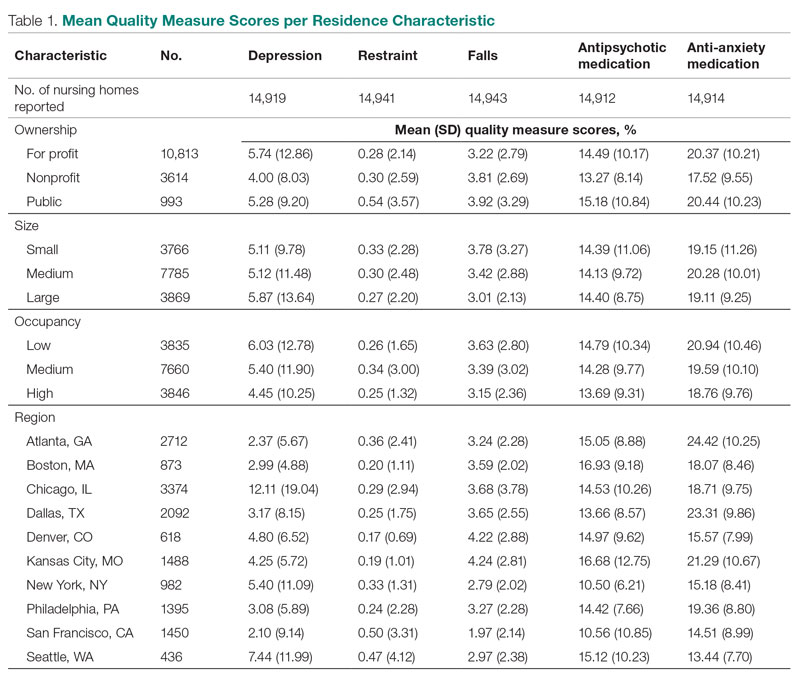
Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.
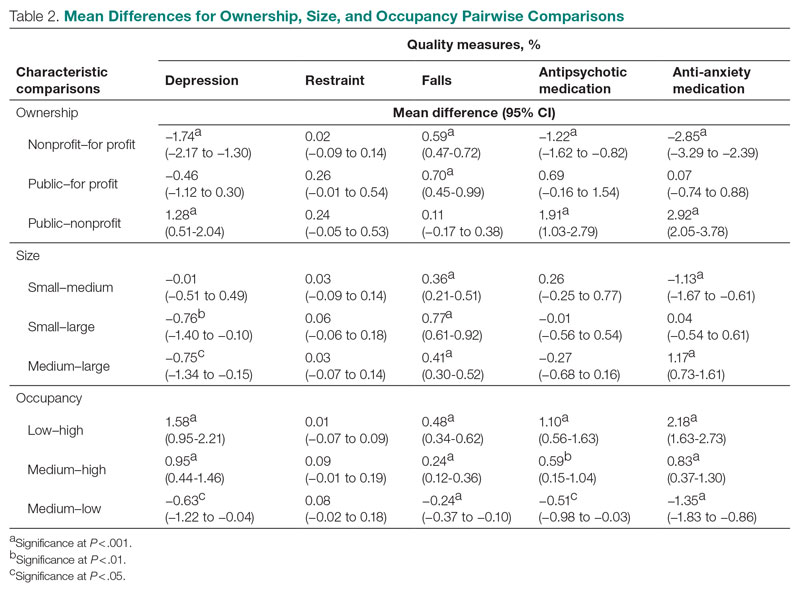
Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.
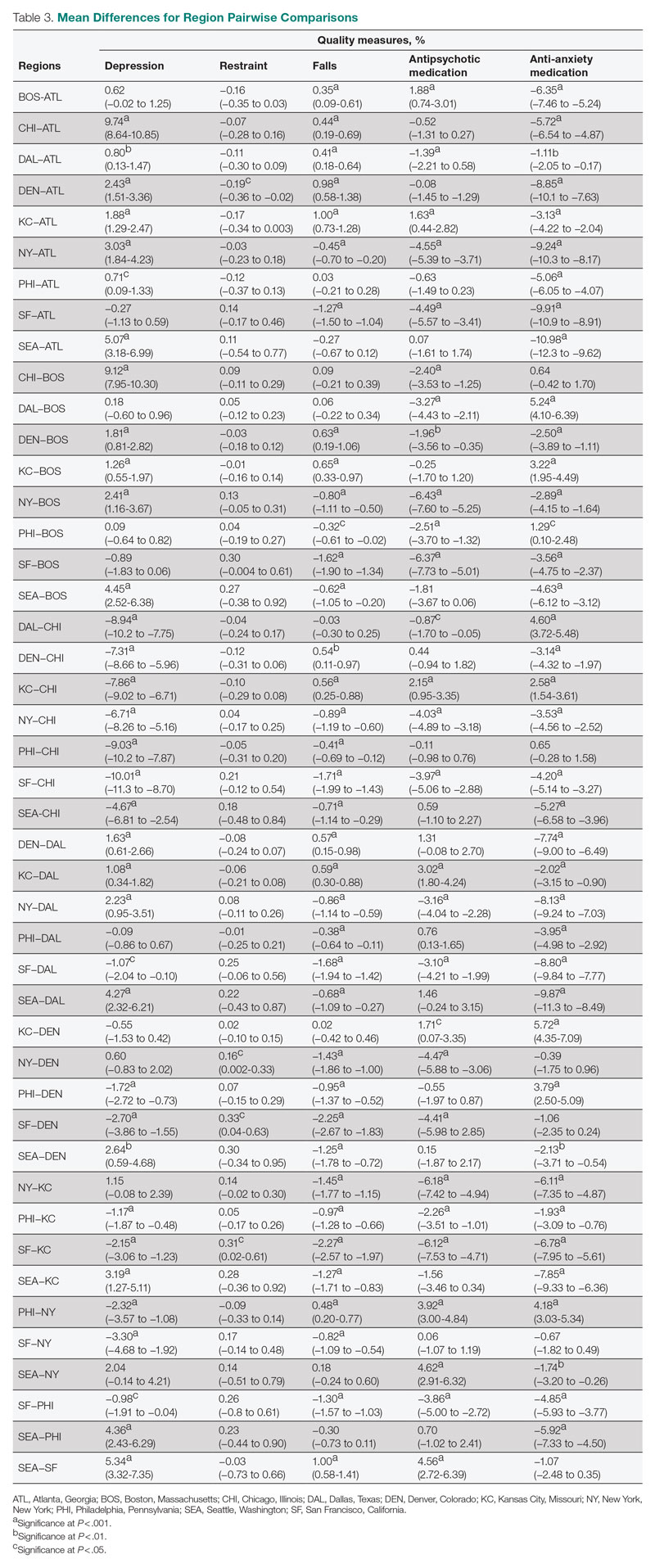
Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
Leading for High Reliability During the COVID-19 Pandemic: A Pilot Quality Improvement Initiative to Identify Challenges Faced and Lessons Learned
From the U.S. Department of Veterans Affairs (all authors), and Cognosante, LLC, Falls Church, VA (Dr. Murray, Dr. Sawyer, and Jessica Fankhauser).
Abstract
Objective: The COVID-19 pandemic posed unprecedented leadership challenges to health care organizations worldwide, especially those on the journey to high reliability. The objective of this pilot quality improvement initiative was to describe the experiences of medical center leaders continuing along the journey to high reliability during the pandemic.
Methods: A convenience sample of Veterans Health Administration medical center directors at facilities that had initiated the journey to high reliability prior to or during the COVID-19 pandemic were asked to complete a confidential survey to explore the challenges experienced and lessons learned.
Results: Of the 35 potential participants, 15 completed the confidential web-based survey. Five major themes emerged from participants’ responses: (1) managing competing priorities, (2) staying committed, (3) adapting and overcoming, (4) prioritizing competing demands, and (5) maintaining momentum.
Conclusion: This pilot quality improvement initiative provides some insight into the challenges experienced and lessons learned during the COVID-19 pandemic to help inform health care leaders’ responses during crises they may encounter along the journey to becoming a high reliability organization.
Keywords: HRO, leadership, patient safety.
Health care leaders worldwide agree that the
Maintaining continuous progress toward advancing high reliability organization (HRO) principles and practices can be especially challenging during crises of unprecedented scale such as the pandemic. HROs must be continually focused on achieving safety, quality, and efficiency goals by attending to the 3 pillars of HRO: culture, leadership, and continuous process improvement. HROs promote a culture where all staff across the organization watch for and report any unsafe conditions before these conditions pose a greater risk in the workplace. Hospital leaders, from executives to frontline managers, must be cognizant of all systems and processes that have the potential to affect patient care.12 All of the principles of HROs must continue without fail to ensure patient safety; these principles include preoccupation with failure, anticipating unexpected risks, sensitivity to dynamic and ever-changing operations, avoiding oversimplifications of identified problems, fostering resilience across the organization, and deferring to those with the expertise to make the best decisions regardless of position, rank, or title.12,13 Given the demands faced by leaders during crises with unprecedented disruption to normal operating procedures, it can be especially difficult to identify systemic challenges and apply lessons learned in a timely manner. However, it is critical to identify such lessons in order to continuously improve and to increase preparedness for subsequent crises.13,14
Because of the COVID-19 pandemic’s unprecedented nature in recent history, a review of the literature produced little evidence exploring the challenges experienced and lessons learned by health care leaders, especially as it relates to implementing or sustaining HRO journeys during the COVID-19 pandemic. Related literature published to date consists of editorials on reliability, uncertainty, and the management of errors15; patient safety and high reliability preventive strategies16; and authentic leadership.17 Five viewpoints were published on HROs and maladaptive stress behaviors,18 mindful organizing and organizational reliability,19 the practical essence of HROs,20 embracing principles of HROs in crisis,8 and using observation and high reliability strategies when facing an unprecedented safety threat.21 Finally, the authors identified 2 studies that used a qualitative research approach to explore leadership functions within an HRO when managing crises22 and organizational change in response to the COVID-19 pandemic.23 Due to the paucity of available information, the authors undertook a pilot quality improvement (QI) initiative to address this knowledge gap.
The aim of this initiative was to gain a better understanding of the challenges experienced, lessons learned, and recommendations to be shared by VHA medical center directors (MCDs) of health care facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The authors hope that this information will help health care leaders across both governmental and nongovernmental organizations, nationally and globally, to prepare for future pandemics, other unanticipated crises (eg, natural disasters, terrorist attacks), and major change initiatives (eg, electronic health record modernization) that may affect the delivery of safe, high-quality, and effective patient care. The initiative is described using the SQUIRE 2.0 guidelines.24,25
Methods
Survey
We used a qualitative approach and administered a confidential web-based survey, developed by the project team, to VHA MCDs at facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The survey consisted of 8 participant characteristic questions (Table 1) and 4 open-ended questions. The open-ended questions were designed to encourage MCD participants to freely provide detailed descriptions of the challenges experienced, lessons learned, recommendations for other health care leaders, and any additional information they believed was relevant.26,27 Participants were asked to respond to the following items:
- Please describe any challenges you experienced while in the role of MCD at a facility that initiated implementation of HRO principles and practices prior to (February 2020) or during (March 2020–September 2021) the initial onset of the COVID-19 pandemic.
- What are some lessons that you learned when responding to the COVID-19 pandemic while on the journey to high reliability?
- What recommendations would you like to make to other health care leaders to enable them to respond effectively to crises while on the journey to high reliability?
- Please provide any additional information that would be of value.
An invitation to participate in this pilot QI initiative was sent via e-mail to 35 potential participants, who were all MCDs at Cohort 1 and Cohort 2 facilities. The invitation was sent on June 17, 2022, by a VHA senior High Reliability Enterprise Support government team member not directly involved with the initiative.
The invitation included the objective of the initiative, estimated time to complete the confidential web-based survey, time allotted for responses to be submitted, and a link to the survey should potential participants agree to participate. Potential participants were informed that their involvement was voluntary, based on their willingness to participate and available time to complete the survey. Finally, the invitation noted that any comments provided would remain confidential and nonattributional for the purpose of publishing and presenting. The inclusion criteria for participation were: (1) serving
Data Gathering and Analysis
To minimize bias and maintain neutrality at the organizational level, only non-VHA individuals working on the project were directly involved with participants’ data review and analysis. Participant characteristics were analyzed using descriptive statistics. Responses to the 4 open-ended questions were coded and analyzed by an experienced researcher and coauthor using NVivo 11 qualitative data analysis software.28 To ensure trustworthiness (credibility, transferability, dependability, and confirmability) in the data analysis procedure,29 inductive thematic analysis was also performed manually using the methodologies of Braun and Clarke (Table 2)30 and Erlingsson and Brysiewicz.31 The goal of inductive analysis is to allow themes to emerge from the data while minimizing preconceptions.32,33 Regular team meetings were held to discuss and review the progress of data collection and analysis. The authors agreed that the themes were representative of the participants’ responses.
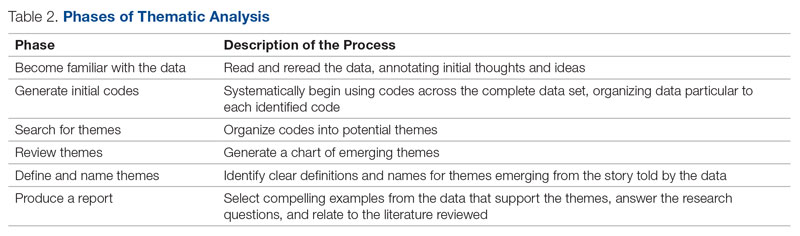
Institutional review board (IRB) review and approval were not required, as this project was a pilot QI initiative. The intent of the initiative was to explore ways to improve the quality of care delivered in the participants’ local care settings and not to generalize the findings. Under these circumstances, formal IRB review and approval of a QI initiative are not required.34 Participation in this pilot QI initiative was voluntary, and participants could withdraw at any time without consequences. Completion of the survey indicated consent. Confidentiality was ensured at all times by avoiding both the use of facility names and the collection of participant identifiers. Unique numbers were assigned to each participant. All comments provided by survey participants remained confidential and nonattributional for the purpose of publishing and presenting.
Results
Of the 35 potential participants, 15 VHA MCDs (43%) completed the confidential web-based survey. Out of the 17 potential participants in Cohort 1, 6 (35%) completed the survey. With Cohort 2, 9 (50%) of the potential 18 participants responded. Although saturation was reached at 10 responses, the additional completed surveys were included in the analysis. Saturation can be achieved with a small number of participants (n = 9–17), particularly when the potential participants are relatively homogenous and project aims are narrowly defined.35 Most participants had more than 10 years of executive-level experience and most medical centers had been on the journey to high reliability for more than 12 months at the time of the pandemic (Table 3).
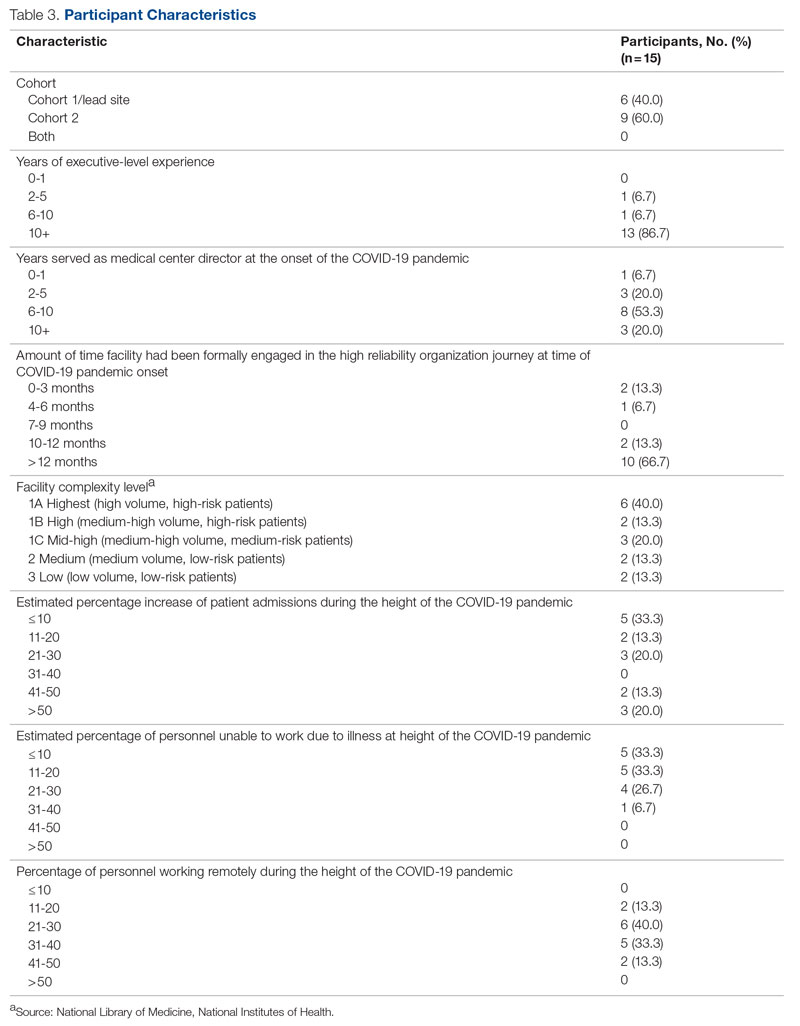
“There were too many competing priorities dealing with the pandemic and staffing crisis.” (Participant 8)
Other participants shared:
“We had our HRO mentor designated just as our first peak was descending on us. It was initially challenging to determine the proper pace of implementation when we clearly had other things going on. There was a real risk that people would say, ‘What, are you kidding?’ as we tried to roll this out.” (Participant 4)
“Prior to COVID, our main challenges were getting organized and operational rollout. During the pandemic, we had to shift our focus to COVID and the training aspects suffered. Also, many other priorities pulled us away from an HRO rollout focus.” (Participant 6)
“If you don’t need a highly reliable organization during a crisis, when do you need it? That was the message that we kicked off with. It was also VERY important to take things slowly. Education had to be done in bits, and we had a much more modest timeline than what would have been the norm for any initiative pre-COVID. The emphasis was on this being a long-term commitment, that we would be doing it the right way rather than rushing it, etc.” (Participant 4)
“Keeping HRO principles and a Just Culture on the forefront of our minds, we looked for opportunities to progress on our HRO journey, despite the challenges of the pandemic. Our monthly Town Halls became weekly events to share COVID updates and information with staff. We used the Town Halls to promote our HRO mission and to open communication lines with staff, designating 1 week each month as a ‘Safety Forum.’ The pandemic provided the springboard and backdrop for staff Safety Stories submissions, many of which were shared at our Town Halls and Safety Forums.” (Participant 7)
“We were able to utilize HRO principles in response to the COVID pandemic. Specifically standardized communication from the facility to VISN [Veterans Integrated Services Network] was initiated on a daily basis. This practice provided daily communication on key operational items and clinical items at the medical center, allowed timely feedback on actions being taken, as was instrumental in daily checks on staffing, COVID testing supplies, overall supply chain issues.” (Participant 9)
The recommendations provided by 10 participants (Cohort 1, n = 6; Cohort 2, n = 4) for other health care leaders experiencing a crisis during the journey to high reliability were insightful. The themes that frequently emerged from the responses to the survey were to adapt and overcome. Participants shared:
“Utilize the many tools you’re given, specifically your team. Try even the craziest ideas from frontline staff.” (Participant 1)
“Use your mentors for younger directors and, even if you think you know the answer, involve your staff. It makes them feel they have a voice and gives them ownership of the issues.” (Participant 5)
“Make sure that you have key leaders in place who are committed to HRO and can help the organization adjust.” (Participant 6)
“Take advantage of HRO Leader Coaching, which pairs MCDs with coaches who act as consultants for HRO leadership practices to ensure progress in reaching the next level in the journey to High Reliability.” (Participant 7)
“Meet regularly with the HRO Lead and team (more frequently during early stages of implementation) to provide support, eliminate barriers, and champion the HRO mission. It is important to include other members of the ELT [Executive Leadership Team] to ensure their involvement with the facility HRO strategic plan.” (Participant 7)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
The theme of prioritizing competing demands emerged again from 5 participants (Cohort 1, n = 3; Cohort 2, n = 2) with question 3 describing recommendations for other leaders:
“Your first priority is to the crisis. Don’t get distracted by this or any other initiative. That was not a very popular message for the people pushing HRO, but it is the reality and the necessity. However, it IS possible to move forward with HRO (or other important initiatives) during crisis times, as long as you carefully consider what you are asking of people and don’t overload/overwhelm them. It is not your ego (or that of Central Office) that needs to be stoked. If the initiative truly has value, you need to be patient to see it done properly, rather than rushed/pushed/forced. Don’t kill it by being overeager and overwhelming your already overtaxed people. That said, keep moving forward. The key is pacing—and remember that your Type A hard-driving leader types (you know who you are) will certainly fail if they push it. Or even if they go at a normal pace that would be appropriate for noncrisis times.” (Participant 4)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
“It was critical for us to always focus on the immediate workplace safety of staff (especially those on the frontlines of the pandemic response) when in the process of rolling out HRO initiatives.” (Participant 14)
Maintaining Momentum
“It seemed as though communication and education from VHA on HRO slowed down at the same time, which further slowed our progress. We are now trying to ramp our engagement up again.” (Participant 3)
“There can be synergy between crisis response and HRO implementation. As an example, one of the first steps we took was leadership rounding. That was necessary anyways for crisis management (raising the spirits on the front lines, so to speak). What we did was include scheduled time instead of (in addition to) ad hoc. And we got credit for taking an HRO step. I resisted whiteboards/visual management systems for a long time because (in my opinion) that would have been much too distracting during the crisis. Having waited for better times, I was able to move forward with that several months later and with good success.” (Participant 4)
Discussion
Health care leaders worldwide experienced an immense set of challenges because of the COVID-19 pandemic, which is a crisis of a magnitude with no parallel in modern times. Strong, adaptive leadership at all levels of health care systems was needed to effectively address the immense crisis at hand.36,37 Findings from this pilot QI initiative suggest that MCDs faced many new challenges, requiring them to perform unfamiliar tasks and manage numerous overlapping challenges (eg, staffing shortages and reassignments, safety concerns, changes to patient appointments, backlogs in essential services), all while also trying to continue with the journey to high reliability. Despite the challenges leaders faced, they recognized the need to manage competing priorities early and effectively. At times, the priority was to address the wide-ranging, urgent issues related to the pandemic. When the conditions improved, there was time to refocus efforts on important but longer-term activities related to the HRO journey. Other participants recognized that their commitment to HRO needed to remain a priority even during the periods of intense focus on COVID-19.
Some participants felt compelled to stay committed to the HRO journey despite numerous competing demands. They stayed committed to looking for opportunities to progress by implementing HRO principles and practices to achieve safety, quality, and efficiency goals. This dedication is noteworthy, especially in light of recently published research that demonstrates the vast number of patient safety issues that presented during the COVID-19 pandemic (eg, ineffective communication, poor teamwork, the absence of coordination)1 as well as perceptions that patient safety and quality of care had significantly declined as a result of the crisis.36,37
Participants also highlighted the need to be adaptive when responding to the complexity and unpredictability of the pandemic. Participants regularly sought ways to increase their knowledge, skills, and abilities by using the resources (eg, tools, experts) available to them. Research shows that in increasingly complex and ever-changing situation such as the COVID-19 pandemic, leaders must be adaptive with all levels of performance, especially when limited information is available.38,39
This is the first initiative of its kind to specifically explore the challenges experienced and lessons learned from health care leaders continuing along the journey to high reliability during the COVID-19 pandemic. Findings from this pilot QI initiative revealed that many participants recommended that leaders adapt and overcome challenges as much as possible when continuing with HRO during a crisis. These findings are echoed in the current literature suggesting that adaptive performance is a highly effective form of leadership during crises.38,40 Being able to effectively adapt during a crisis is essential for reducing further vulnerabilities across health care systems. In fact, this lesson is shared by many countries in response to the unprecedented global crisis.41A limitation of this pilot QI initiative is that the authors did not directly solicit responses from all VHA MCDs or from other health care executives (eg, Chief of Staff, Associate Director for Operations, Associate Director for Patient Care, and Nurse Executive). As such, our findings represent only a small segment of senior leadership perspectives from a large, integrated health care system. Individuals who did not respond to the survey may have had different experiences than those who did, and the authors excluded many MCDs who formally began their HRO journeys in 2022, well after the pandemic was underway. Similarly, the experiences of Veterans Affairs leaders may or may not be similar to that of other health care organizations. Although the goal of this initiative was to explore the participants’ experiences during the period of crisis, time and distance from the events at the height of the COVID-19 pandemic may have resulted in difficulty recalling information as well as making sense of the occurrence. This potential recall bias is a common occurrence in trying to explore past experiences, especially as they relate to crises. Finally, this pilot QI initiative did not explore personal challenges participants may have faced during this period of time (eg, burnout, personal or family illness), which may have also shaped their responses.
Conclusion
This initiative suggests that VHA MCDs often relied on HRO principles to guide and assist with their response to the COVID-19 pandemic, including managing periods of unprecedented crisis. The ability to adapt and prioritize was seen as an especially important lesson. Many MCDs continued their personal and organizational efforts toward high reliability even in periods of intense challenge because of the pandemic. These findings can help with future crises that may occur during an organization’s journey to high reliability. This pilot QI initiative’s findings warrant further investigation to explore the experiences of the broader range of health care leaders while responding to unplanned crises or even planned large-scale cultural change or technology modernization initiatives (eg, electronic health record modernization) to expand the state of the science of high reliability as well as inform policy and decision-making. Finally, another area for future study is examining how leadership responses vary across facilities, depending on factors such as leader roles, facility complexity level, resource availability, patient population characteristics, and organizational culture.
Acknowledgment: The authors express their sincere gratitude to the medical center directors who participated in this pilot study.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel St., Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
1. Editors: Dying in a leadership vacuum. 9.4N Engl J Med. 2020;383(15):1479-1480. doi:10.1056/NEJMe2029812
2. Geerts JM, Kinnair D, Taheri P, et al. Guidance for health care leaders during the recovery stage of the COVID-19 pandemic: a consensus statement. JAMA Netw Open. 2021;4(7):1-16. doi:10.1001/jamanetworkopen.2021.20295
3. Boiral O, Brotherton M-C, Rivaud L, et al. Organizations’ management of the COVID-19 pandemic: a scoping review of business articles. Sustainability. 2021;13:1-20. doi:10.3390/su13073993
4. Razu SR, Yasmin T, Arif TB, et al. Challenges faced by healthcare professionals during the COVID-19 pandemic: a qualitative inquiry from Bangladesh. Front Public Health. 2021;9:1-13. doi:10.3389/fpubh.2021.647315
5. Lyng HB, Ree E, Wibe T, et al. Healthcare leaders’ use of innovative solutions to ensure resilience in healthcare during the Covid-19 pandemic: a qualitative study in Norwegian nursing homes and home care services. BMC Health Serv Res. 2021;21(1):1-11. doi:1186/s12913-021-06923-1
6. Freitas J. Queiroz A, Bortotti I, et al. Nurse leaders’ challenges fighting the COVID-19 pandemic: a qualitative study. Open J Nurs. 2021;11:267-280. doi:10.4236/ojn.2021.115024
7. McGuire AL, Aulisio MP, Davis FD, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. 9.4Am J Bioeth. 2020;20(7):15-27. doi:10.1080/15265161.2020.1764138
8. Turbow RM, Scibilia JP. Embracing principles of high reliability organizations can improve patient safety during pandemic. AAP News. January 19, 2021. Accessed March 1, 2023. https://publications.aap.org/aapnews/news/8975
9. Roberts BH, Damiano LA, Graham S, et al. A case study in fostering a learning culture in the context of Covid-19. American Association for Physician Leadership. June 24, 2021. Accessed March 1, 2023. https://www.physicianleaders.org/news/a-case-study-in-fostering-a-learning-culture-in-the-context-of-covid-19
10. U.S. Department of Veterans Affairs. Department of Veterans AffairsCOVID-19 National Summary. Veterans Affairs. Accessed December 4, 2022. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
11. U.S. Department of Veterans Affairs. VA fourth mission summary. Veterans Affairs. Accessed December 4, 2022. https://www.va.gov/health/coronavirus/statesupport.asp#:~:text=As%20part%20of%20the%20Fourth,the%20facilities%20we%20are%20supporting
12. Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18(1):e320-e328. doi:10.1097/PTS.0000000000000768
13. Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. 9.4Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
14. Maison D, Jaworska D, Adamczyk D, et al. The challenges arising from the COVID-19 pandemic and the way people deal with them: a qualitative longitudinal study. PLoS One. 2021;16(10):1-17. doi:10.1371/journal.pone.0258133
15. Schulman PR. Reliability, uncertainty and the management of error: new perspectives in the COVID-19 era. J Contingencies Crisis Manag. 2022;30:92-101. doi:10.1111/1468-5973.12356
16. Adelman JS, Gandhi TK. COVID-19 and patient safety: time to tap into our investment in high reliability. J Patient Saf. 2021;17(4): 331-333. doi:10.1097/PTS.0000000000000843
17. Shingler-Nace A. COVID-19: when leadership calls. Nurs Lead. 2020;18(3):202-203. doi:10.1016/j.mnl.2020.03.017
18. Van Stralen D, Mercer TA. During pandemic COVID 19, the high reliability organization (HRO) identifies maladaptive stress behaviors: the stress-fear-threat cascade. Neonatol Tod. 2020;15(11):113-124. doi: 10.51362/neonatology.today/2020111511113124
19. Vogus TJ, Wilson AD, Randall K, et al. We’re all in this together: how COVID-19 revealed the coconstruction of mindful organising and organisational reliability. BMJ Qual Saf. 2022;31(3):230-233. doi:10.1136/bmjqs-2021-014068
20. Van Stralen D. Pragmatic high-reliability organization (HRO) during pandemic COVID-19. Neonatol Tod. 2020(4);15:109-117. doi:10.51362/neonatology.today/20208158109117
21. Thull-Freedman J, Mondoux S, Stang A, et al. Going to the COVID-19 Gemba: using observation and high reliability strategies to achieve safety in a time of crisis. CJEM. 2020;22(6):738-741. doi:10.1017/cem.2020.380
22. Sarihasan I, Dajnoki K, Oláh J, et al. The importance of the leadership functions of a high-reliability health care organization in managing the COVID-19 pandemic in Turkey. Econ Sociol. 2022;15:78-93. doi:10.14254/2071-789x.2022/15-1/5
23. Crain MA, Bush AL, Hayanga H, et al. Healthcare leadership in the COVID-19 pandemic: from innovative preparation to evolutionary transformation. J Health Leadersh. 2021;13:199-207. doi:10.2147/JHL.S319829
24. SQUIRE. Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) SQUIRE; 2020. Accessed March 1, 2023. http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&pageId=471
25. Lounsbury O. How to write a quality improvement project. Patient Safety J. 2022;4(1):65-67. doi:10.33940/culture/2022.3.6
26. Bengtsson M. How to plan and perform a qualitative study using content analysis. Nurs Plus Open. 2016;2:8-14. doi:10.1016/j.npls.2016.01.001
27. Allen M. The Sage Encyclopedia of Communication Research Methods. (Vols. 1-4). SAGE Publications, Inc; 2017
28. Unlock insights with qualitative data analysis software. Lumivero. Accessed March 2, 2023. https://lumivero.com/products/nvivo/
29. Maher C, Hadfield M, Hutchings M, et al. Ensuring rigor in qualitative data analysis: a design research approach to coding combining NVivo with traditional material methods. Int J Qual Methods. 2018;17:1-13. doi:10.1177/1609406918786362
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-101. doi:10.1191/1478088706qp063oa
31. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. doi:10.1016/j.afjem.2017.08.001
32. Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. FoHPE. 2022;23:111-127. doi:10.11157/fohpe.v23i1.544
33. Nowell LS, Norris JM, White DE, et al. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1-13. doi:10.1177/1609406917733847
34. Gautham KS, Pearlman S. Do quality improvement projects require IRB approval? J Perinatol. 2021;41:1209-1212. doi:10.1038/s41372-021-01038-1
35. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:1-10. doi:10.1016/j.socscimed.2021.114523
36. Balogun M, Dada FO, Oladimeji A, et al. Leading in a time of crisis: a qualitative study capturing experiences of health facility leaders during the early phases of the COVID-19 pandemic in Nigeria’s epicentre. Leadersh Health Serv (Bradf Engl). Published online May 12, 2022. doi:10.1108/lhs-02-2022-0017
37. Guttormson J, Calkins K, McAndrew N, et al. Critical care nurses’ experiences during the COVID-19 pandemic: a US national survey. Am J Crit Care. 2022;31:96-103. doi:10.4037/ajcc2022312
38. Bajaba A, Bajaba S, Algarni M, et al. Adaptive managers as emerging leaders during the COVID-19 crisis. Front Psychol. 2021;12:1-11. doi:10.3389/fpsyg.2021.661628
39. Ahern S, Loh E. Leadership during the COVID-19 pandemic: building and sustaining trust in times of uncertainty. BMJ Lead. 2021;59(4):266-269. doi.org/10.1136/leader-2020-000271
40. Cote R. Adaptive leadership approach with COVID 19 adaptive challenges. J Leadersh Account Ethics. 2022;19:34-44. doi:10.33423/jlae.v19i1.4992
41. Juvet TM, Corbaz-Kurth S, Roos P, et al. Adapting to the unexpected: problematic work situations and resilience strategies in healthcare institutions during the COVID-19 pandemic’s first wave. Saf Sci. 2021;139:1-9. doi:10.1016/j.ssci.2021.105277
From the U.S. Department of Veterans Affairs (all authors), and Cognosante, LLC, Falls Church, VA (Dr. Murray, Dr. Sawyer, and Jessica Fankhauser).
Abstract
Objective: The COVID-19 pandemic posed unprecedented leadership challenges to health care organizations worldwide, especially those on the journey to high reliability. The objective of this pilot quality improvement initiative was to describe the experiences of medical center leaders continuing along the journey to high reliability during the pandemic.
Methods: A convenience sample of Veterans Health Administration medical center directors at facilities that had initiated the journey to high reliability prior to or during the COVID-19 pandemic were asked to complete a confidential survey to explore the challenges experienced and lessons learned.
Results: Of the 35 potential participants, 15 completed the confidential web-based survey. Five major themes emerged from participants’ responses: (1) managing competing priorities, (2) staying committed, (3) adapting and overcoming, (4) prioritizing competing demands, and (5) maintaining momentum.
Conclusion: This pilot quality improvement initiative provides some insight into the challenges experienced and lessons learned during the COVID-19 pandemic to help inform health care leaders’ responses during crises they may encounter along the journey to becoming a high reliability organization.
Keywords: HRO, leadership, patient safety.
Health care leaders worldwide agree that the
Maintaining continuous progress toward advancing high reliability organization (HRO) principles and practices can be especially challenging during crises of unprecedented scale such as the pandemic. HROs must be continually focused on achieving safety, quality, and efficiency goals by attending to the 3 pillars of HRO: culture, leadership, and continuous process improvement. HROs promote a culture where all staff across the organization watch for and report any unsafe conditions before these conditions pose a greater risk in the workplace. Hospital leaders, from executives to frontline managers, must be cognizant of all systems and processes that have the potential to affect patient care.12 All of the principles of HROs must continue without fail to ensure patient safety; these principles include preoccupation with failure, anticipating unexpected risks, sensitivity to dynamic and ever-changing operations, avoiding oversimplifications of identified problems, fostering resilience across the organization, and deferring to those with the expertise to make the best decisions regardless of position, rank, or title.12,13 Given the demands faced by leaders during crises with unprecedented disruption to normal operating procedures, it can be especially difficult to identify systemic challenges and apply lessons learned in a timely manner. However, it is critical to identify such lessons in order to continuously improve and to increase preparedness for subsequent crises.13,14
Because of the COVID-19 pandemic’s unprecedented nature in recent history, a review of the literature produced little evidence exploring the challenges experienced and lessons learned by health care leaders, especially as it relates to implementing or sustaining HRO journeys during the COVID-19 pandemic. Related literature published to date consists of editorials on reliability, uncertainty, and the management of errors15; patient safety and high reliability preventive strategies16; and authentic leadership.17 Five viewpoints were published on HROs and maladaptive stress behaviors,18 mindful organizing and organizational reliability,19 the practical essence of HROs,20 embracing principles of HROs in crisis,8 and using observation and high reliability strategies when facing an unprecedented safety threat.21 Finally, the authors identified 2 studies that used a qualitative research approach to explore leadership functions within an HRO when managing crises22 and organizational change in response to the COVID-19 pandemic.23 Due to the paucity of available information, the authors undertook a pilot quality improvement (QI) initiative to address this knowledge gap.
The aim of this initiative was to gain a better understanding of the challenges experienced, lessons learned, and recommendations to be shared by VHA medical center directors (MCDs) of health care facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The authors hope that this information will help health care leaders across both governmental and nongovernmental organizations, nationally and globally, to prepare for future pandemics, other unanticipated crises (eg, natural disasters, terrorist attacks), and major change initiatives (eg, electronic health record modernization) that may affect the delivery of safe, high-quality, and effective patient care. The initiative is described using the SQUIRE 2.0 guidelines.24,25
Methods
Survey
We used a qualitative approach and administered a confidential web-based survey, developed by the project team, to VHA MCDs at facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The survey consisted of 8 participant characteristic questions (Table 1) and 4 open-ended questions. The open-ended questions were designed to encourage MCD participants to freely provide detailed descriptions of the challenges experienced, lessons learned, recommendations for other health care leaders, and any additional information they believed was relevant.26,27 Participants were asked to respond to the following items:
- Please describe any challenges you experienced while in the role of MCD at a facility that initiated implementation of HRO principles and practices prior to (February 2020) or during (March 2020–September 2021) the initial onset of the COVID-19 pandemic.
- What are some lessons that you learned when responding to the COVID-19 pandemic while on the journey to high reliability?
- What recommendations would you like to make to other health care leaders to enable them to respond effectively to crises while on the journey to high reliability?
- Please provide any additional information that would be of value.
An invitation to participate in this pilot QI initiative was sent via e-mail to 35 potential participants, who were all MCDs at Cohort 1 and Cohort 2 facilities. The invitation was sent on June 17, 2022, by a VHA senior High Reliability Enterprise Support government team member not directly involved with the initiative.
The invitation included the objective of the initiative, estimated time to complete the confidential web-based survey, time allotted for responses to be submitted, and a link to the survey should potential participants agree to participate. Potential participants were informed that their involvement was voluntary, based on their willingness to participate and available time to complete the survey. Finally, the invitation noted that any comments provided would remain confidential and nonattributional for the purpose of publishing and presenting. The inclusion criteria for participation were: (1) serving
Data Gathering and Analysis
To minimize bias and maintain neutrality at the organizational level, only non-VHA individuals working on the project were directly involved with participants’ data review and analysis. Participant characteristics were analyzed using descriptive statistics. Responses to the 4 open-ended questions were coded and analyzed by an experienced researcher and coauthor using NVivo 11 qualitative data analysis software.28 To ensure trustworthiness (credibility, transferability, dependability, and confirmability) in the data analysis procedure,29 inductive thematic analysis was also performed manually using the methodologies of Braun and Clarke (Table 2)30 and Erlingsson and Brysiewicz.31 The goal of inductive analysis is to allow themes to emerge from the data while minimizing preconceptions.32,33 Regular team meetings were held to discuss and review the progress of data collection and analysis. The authors agreed that the themes were representative of the participants’ responses.

Institutional review board (IRB) review and approval were not required, as this project was a pilot QI initiative. The intent of the initiative was to explore ways to improve the quality of care delivered in the participants’ local care settings and not to generalize the findings. Under these circumstances, formal IRB review and approval of a QI initiative are not required.34 Participation in this pilot QI initiative was voluntary, and participants could withdraw at any time without consequences. Completion of the survey indicated consent. Confidentiality was ensured at all times by avoiding both the use of facility names and the collection of participant identifiers. Unique numbers were assigned to each participant. All comments provided by survey participants remained confidential and nonattributional for the purpose of publishing and presenting.
Results
Of the 35 potential participants, 15 VHA MCDs (43%) completed the confidential web-based survey. Out of the 17 potential participants in Cohort 1, 6 (35%) completed the survey. With Cohort 2, 9 (50%) of the potential 18 participants responded. Although saturation was reached at 10 responses, the additional completed surveys were included in the analysis. Saturation can be achieved with a small number of participants (n = 9–17), particularly when the potential participants are relatively homogenous and project aims are narrowly defined.35 Most participants had more than 10 years of executive-level experience and most medical centers had been on the journey to high reliability for more than 12 months at the time of the pandemic (Table 3).

“There were too many competing priorities dealing with the pandemic and staffing crisis.” (Participant 8)
Other participants shared:
“We had our HRO mentor designated just as our first peak was descending on us. It was initially challenging to determine the proper pace of implementation when we clearly had other things going on. There was a real risk that people would say, ‘What, are you kidding?’ as we tried to roll this out.” (Participant 4)
“Prior to COVID, our main challenges were getting organized and operational rollout. During the pandemic, we had to shift our focus to COVID and the training aspects suffered. Also, many other priorities pulled us away from an HRO rollout focus.” (Participant 6)
“If you don’t need a highly reliable organization during a crisis, when do you need it? That was the message that we kicked off with. It was also VERY important to take things slowly. Education had to be done in bits, and we had a much more modest timeline than what would have been the norm for any initiative pre-COVID. The emphasis was on this being a long-term commitment, that we would be doing it the right way rather than rushing it, etc.” (Participant 4)
“Keeping HRO principles and a Just Culture on the forefront of our minds, we looked for opportunities to progress on our HRO journey, despite the challenges of the pandemic. Our monthly Town Halls became weekly events to share COVID updates and information with staff. We used the Town Halls to promote our HRO mission and to open communication lines with staff, designating 1 week each month as a ‘Safety Forum.’ The pandemic provided the springboard and backdrop for staff Safety Stories submissions, many of which were shared at our Town Halls and Safety Forums.” (Participant 7)
“We were able to utilize HRO principles in response to the COVID pandemic. Specifically standardized communication from the facility to VISN [Veterans Integrated Services Network] was initiated on a daily basis. This practice provided daily communication on key operational items and clinical items at the medical center, allowed timely feedback on actions being taken, as was instrumental in daily checks on staffing, COVID testing supplies, overall supply chain issues.” (Participant 9)
The recommendations provided by 10 participants (Cohort 1, n = 6; Cohort 2, n = 4) for other health care leaders experiencing a crisis during the journey to high reliability were insightful. The themes that frequently emerged from the responses to the survey were to adapt and overcome. Participants shared:
“Utilize the many tools you’re given, specifically your team. Try even the craziest ideas from frontline staff.” (Participant 1)
“Use your mentors for younger directors and, even if you think you know the answer, involve your staff. It makes them feel they have a voice and gives them ownership of the issues.” (Participant 5)
“Make sure that you have key leaders in place who are committed to HRO and can help the organization adjust.” (Participant 6)
“Take advantage of HRO Leader Coaching, which pairs MCDs with coaches who act as consultants for HRO leadership practices to ensure progress in reaching the next level in the journey to High Reliability.” (Participant 7)
“Meet regularly with the HRO Lead and team (more frequently during early stages of implementation) to provide support, eliminate barriers, and champion the HRO mission. It is important to include other members of the ELT [Executive Leadership Team] to ensure their involvement with the facility HRO strategic plan.” (Participant 7)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
The theme of prioritizing competing demands emerged again from 5 participants (Cohort 1, n = 3; Cohort 2, n = 2) with question 3 describing recommendations for other leaders:
“Your first priority is to the crisis. Don’t get distracted by this or any other initiative. That was not a very popular message for the people pushing HRO, but it is the reality and the necessity. However, it IS possible to move forward with HRO (or other important initiatives) during crisis times, as long as you carefully consider what you are asking of people and don’t overload/overwhelm them. It is not your ego (or that of Central Office) that needs to be stoked. If the initiative truly has value, you need to be patient to see it done properly, rather than rushed/pushed/forced. Don’t kill it by being overeager and overwhelming your already overtaxed people. That said, keep moving forward. The key is pacing—and remember that your Type A hard-driving leader types (you know who you are) will certainly fail if they push it. Or even if they go at a normal pace that would be appropriate for noncrisis times.” (Participant 4)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
“It was critical for us to always focus on the immediate workplace safety of staff (especially those on the frontlines of the pandemic response) when in the process of rolling out HRO initiatives.” (Participant 14)
Maintaining Momentum
“It seemed as though communication and education from VHA on HRO slowed down at the same time, which further slowed our progress. We are now trying to ramp our engagement up again.” (Participant 3)
“There can be synergy between crisis response and HRO implementation. As an example, one of the first steps we took was leadership rounding. That was necessary anyways for crisis management (raising the spirits on the front lines, so to speak). What we did was include scheduled time instead of (in addition to) ad hoc. And we got credit for taking an HRO step. I resisted whiteboards/visual management systems for a long time because (in my opinion) that would have been much too distracting during the crisis. Having waited for better times, I was able to move forward with that several months later and with good success.” (Participant 4)
Discussion
Health care leaders worldwide experienced an immense set of challenges because of the COVID-19 pandemic, which is a crisis of a magnitude with no parallel in modern times. Strong, adaptive leadership at all levels of health care systems was needed to effectively address the immense crisis at hand.36,37 Findings from this pilot QI initiative suggest that MCDs faced many new challenges, requiring them to perform unfamiliar tasks and manage numerous overlapping challenges (eg, staffing shortages and reassignments, safety concerns, changes to patient appointments, backlogs in essential services), all while also trying to continue with the journey to high reliability. Despite the challenges leaders faced, they recognized the need to manage competing priorities early and effectively. At times, the priority was to address the wide-ranging, urgent issues related to the pandemic. When the conditions improved, there was time to refocus efforts on important but longer-term activities related to the HRO journey. Other participants recognized that their commitment to HRO needed to remain a priority even during the periods of intense focus on COVID-19.
Some participants felt compelled to stay committed to the HRO journey despite numerous competing demands. They stayed committed to looking for opportunities to progress by implementing HRO principles and practices to achieve safety, quality, and efficiency goals. This dedication is noteworthy, especially in light of recently published research that demonstrates the vast number of patient safety issues that presented during the COVID-19 pandemic (eg, ineffective communication, poor teamwork, the absence of coordination)1 as well as perceptions that patient safety and quality of care had significantly declined as a result of the crisis.36,37
Participants also highlighted the need to be adaptive when responding to the complexity and unpredictability of the pandemic. Participants regularly sought ways to increase their knowledge, skills, and abilities by using the resources (eg, tools, experts) available to them. Research shows that in increasingly complex and ever-changing situation such as the COVID-19 pandemic, leaders must be adaptive with all levels of performance, especially when limited information is available.38,39
This is the first initiative of its kind to specifically explore the challenges experienced and lessons learned from health care leaders continuing along the journey to high reliability during the COVID-19 pandemic. Findings from this pilot QI initiative revealed that many participants recommended that leaders adapt and overcome challenges as much as possible when continuing with HRO during a crisis. These findings are echoed in the current literature suggesting that adaptive performance is a highly effective form of leadership during crises.38,40 Being able to effectively adapt during a crisis is essential for reducing further vulnerabilities across health care systems. In fact, this lesson is shared by many countries in response to the unprecedented global crisis.41A limitation of this pilot QI initiative is that the authors did not directly solicit responses from all VHA MCDs or from other health care executives (eg, Chief of Staff, Associate Director for Operations, Associate Director for Patient Care, and Nurse Executive). As such, our findings represent only a small segment of senior leadership perspectives from a large, integrated health care system. Individuals who did not respond to the survey may have had different experiences than those who did, and the authors excluded many MCDs who formally began their HRO journeys in 2022, well after the pandemic was underway. Similarly, the experiences of Veterans Affairs leaders may or may not be similar to that of other health care organizations. Although the goal of this initiative was to explore the participants’ experiences during the period of crisis, time and distance from the events at the height of the COVID-19 pandemic may have resulted in difficulty recalling information as well as making sense of the occurrence. This potential recall bias is a common occurrence in trying to explore past experiences, especially as they relate to crises. Finally, this pilot QI initiative did not explore personal challenges participants may have faced during this period of time (eg, burnout, personal or family illness), which may have also shaped their responses.
Conclusion
This initiative suggests that VHA MCDs often relied on HRO principles to guide and assist with their response to the COVID-19 pandemic, including managing periods of unprecedented crisis. The ability to adapt and prioritize was seen as an especially important lesson. Many MCDs continued their personal and organizational efforts toward high reliability even in periods of intense challenge because of the pandemic. These findings can help with future crises that may occur during an organization’s journey to high reliability. This pilot QI initiative’s findings warrant further investigation to explore the experiences of the broader range of health care leaders while responding to unplanned crises or even planned large-scale cultural change or technology modernization initiatives (eg, electronic health record modernization) to expand the state of the science of high reliability as well as inform policy and decision-making. Finally, another area for future study is examining how leadership responses vary across facilities, depending on factors such as leader roles, facility complexity level, resource availability, patient population characteristics, and organizational culture.
Acknowledgment: The authors express their sincere gratitude to the medical center directors who participated in this pilot study.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel St., Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
From the U.S. Department of Veterans Affairs (all authors), and Cognosante, LLC, Falls Church, VA (Dr. Murray, Dr. Sawyer, and Jessica Fankhauser).
Abstract
Objective: The COVID-19 pandemic posed unprecedented leadership challenges to health care organizations worldwide, especially those on the journey to high reliability. The objective of this pilot quality improvement initiative was to describe the experiences of medical center leaders continuing along the journey to high reliability during the pandemic.
Methods: A convenience sample of Veterans Health Administration medical center directors at facilities that had initiated the journey to high reliability prior to or during the COVID-19 pandemic were asked to complete a confidential survey to explore the challenges experienced and lessons learned.
Results: Of the 35 potential participants, 15 completed the confidential web-based survey. Five major themes emerged from participants’ responses: (1) managing competing priorities, (2) staying committed, (3) adapting and overcoming, (4) prioritizing competing demands, and (5) maintaining momentum.
Conclusion: This pilot quality improvement initiative provides some insight into the challenges experienced and lessons learned during the COVID-19 pandemic to help inform health care leaders’ responses during crises they may encounter along the journey to becoming a high reliability organization.
Keywords: HRO, leadership, patient safety.
Health care leaders worldwide agree that the
Maintaining continuous progress toward advancing high reliability organization (HRO) principles and practices can be especially challenging during crises of unprecedented scale such as the pandemic. HROs must be continually focused on achieving safety, quality, and efficiency goals by attending to the 3 pillars of HRO: culture, leadership, and continuous process improvement. HROs promote a culture where all staff across the organization watch for and report any unsafe conditions before these conditions pose a greater risk in the workplace. Hospital leaders, from executives to frontline managers, must be cognizant of all systems and processes that have the potential to affect patient care.12 All of the principles of HROs must continue without fail to ensure patient safety; these principles include preoccupation with failure, anticipating unexpected risks, sensitivity to dynamic and ever-changing operations, avoiding oversimplifications of identified problems, fostering resilience across the organization, and deferring to those with the expertise to make the best decisions regardless of position, rank, or title.12,13 Given the demands faced by leaders during crises with unprecedented disruption to normal operating procedures, it can be especially difficult to identify systemic challenges and apply lessons learned in a timely manner. However, it is critical to identify such lessons in order to continuously improve and to increase preparedness for subsequent crises.13,14
Because of the COVID-19 pandemic’s unprecedented nature in recent history, a review of the literature produced little evidence exploring the challenges experienced and lessons learned by health care leaders, especially as it relates to implementing or sustaining HRO journeys during the COVID-19 pandemic. Related literature published to date consists of editorials on reliability, uncertainty, and the management of errors15; patient safety and high reliability preventive strategies16; and authentic leadership.17 Five viewpoints were published on HROs and maladaptive stress behaviors,18 mindful organizing and organizational reliability,19 the practical essence of HROs,20 embracing principles of HROs in crisis,8 and using observation and high reliability strategies when facing an unprecedented safety threat.21 Finally, the authors identified 2 studies that used a qualitative research approach to explore leadership functions within an HRO when managing crises22 and organizational change in response to the COVID-19 pandemic.23 Due to the paucity of available information, the authors undertook a pilot quality improvement (QI) initiative to address this knowledge gap.
The aim of this initiative was to gain a better understanding of the challenges experienced, lessons learned, and recommendations to be shared by VHA medical center directors (MCDs) of health care facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The authors hope that this information will help health care leaders across both governmental and nongovernmental organizations, nationally and globally, to prepare for future pandemics, other unanticipated crises (eg, natural disasters, terrorist attacks), and major change initiatives (eg, electronic health record modernization) that may affect the delivery of safe, high-quality, and effective patient care. The initiative is described using the SQUIRE 2.0 guidelines.24,25
Methods
Survey
We used a qualitative approach and administered a confidential web-based survey, developed by the project team, to VHA MCDs at facilities that had initiated the journey to high reliability before or during the COVID-19 pandemic. The survey consisted of 8 participant characteristic questions (Table 1) and 4 open-ended questions. The open-ended questions were designed to encourage MCD participants to freely provide detailed descriptions of the challenges experienced, lessons learned, recommendations for other health care leaders, and any additional information they believed was relevant.26,27 Participants were asked to respond to the following items:
- Please describe any challenges you experienced while in the role of MCD at a facility that initiated implementation of HRO principles and practices prior to (February 2020) or during (March 2020–September 2021) the initial onset of the COVID-19 pandemic.
- What are some lessons that you learned when responding to the COVID-19 pandemic while on the journey to high reliability?
- What recommendations would you like to make to other health care leaders to enable them to respond effectively to crises while on the journey to high reliability?
- Please provide any additional information that would be of value.
An invitation to participate in this pilot QI initiative was sent via e-mail to 35 potential participants, who were all MCDs at Cohort 1 and Cohort 2 facilities. The invitation was sent on June 17, 2022, by a VHA senior High Reliability Enterprise Support government team member not directly involved with the initiative.
The invitation included the objective of the initiative, estimated time to complete the confidential web-based survey, time allotted for responses to be submitted, and a link to the survey should potential participants agree to participate. Potential participants were informed that their involvement was voluntary, based on their willingness to participate and available time to complete the survey. Finally, the invitation noted that any comments provided would remain confidential and nonattributional for the purpose of publishing and presenting. The inclusion criteria for participation were: (1) serving
Data Gathering and Analysis
To minimize bias and maintain neutrality at the organizational level, only non-VHA individuals working on the project were directly involved with participants’ data review and analysis. Participant characteristics were analyzed using descriptive statistics. Responses to the 4 open-ended questions were coded and analyzed by an experienced researcher and coauthor using NVivo 11 qualitative data analysis software.28 To ensure trustworthiness (credibility, transferability, dependability, and confirmability) in the data analysis procedure,29 inductive thematic analysis was also performed manually using the methodologies of Braun and Clarke (Table 2)30 and Erlingsson and Brysiewicz.31 The goal of inductive analysis is to allow themes to emerge from the data while minimizing preconceptions.32,33 Regular team meetings were held to discuss and review the progress of data collection and analysis. The authors agreed that the themes were representative of the participants’ responses.

Institutional review board (IRB) review and approval were not required, as this project was a pilot QI initiative. The intent of the initiative was to explore ways to improve the quality of care delivered in the participants’ local care settings and not to generalize the findings. Under these circumstances, formal IRB review and approval of a QI initiative are not required.34 Participation in this pilot QI initiative was voluntary, and participants could withdraw at any time without consequences. Completion of the survey indicated consent. Confidentiality was ensured at all times by avoiding both the use of facility names and the collection of participant identifiers. Unique numbers were assigned to each participant. All comments provided by survey participants remained confidential and nonattributional for the purpose of publishing and presenting.
Results
Of the 35 potential participants, 15 VHA MCDs (43%) completed the confidential web-based survey. Out of the 17 potential participants in Cohort 1, 6 (35%) completed the survey. With Cohort 2, 9 (50%) of the potential 18 participants responded. Although saturation was reached at 10 responses, the additional completed surveys were included in the analysis. Saturation can be achieved with a small number of participants (n = 9–17), particularly when the potential participants are relatively homogenous and project aims are narrowly defined.35 Most participants had more than 10 years of executive-level experience and most medical centers had been on the journey to high reliability for more than 12 months at the time of the pandemic (Table 3).

“There were too many competing priorities dealing with the pandemic and staffing crisis.” (Participant 8)
Other participants shared:
“We had our HRO mentor designated just as our first peak was descending on us. It was initially challenging to determine the proper pace of implementation when we clearly had other things going on. There was a real risk that people would say, ‘What, are you kidding?’ as we tried to roll this out.” (Participant 4)
“Prior to COVID, our main challenges were getting organized and operational rollout. During the pandemic, we had to shift our focus to COVID and the training aspects suffered. Also, many other priorities pulled us away from an HRO rollout focus.” (Participant 6)
“If you don’t need a highly reliable organization during a crisis, when do you need it? That was the message that we kicked off with. It was also VERY important to take things slowly. Education had to be done in bits, and we had a much more modest timeline than what would have been the norm for any initiative pre-COVID. The emphasis was on this being a long-term commitment, that we would be doing it the right way rather than rushing it, etc.” (Participant 4)
“Keeping HRO principles and a Just Culture on the forefront of our minds, we looked for opportunities to progress on our HRO journey, despite the challenges of the pandemic. Our monthly Town Halls became weekly events to share COVID updates and information with staff. We used the Town Halls to promote our HRO mission and to open communication lines with staff, designating 1 week each month as a ‘Safety Forum.’ The pandemic provided the springboard and backdrop for staff Safety Stories submissions, many of which were shared at our Town Halls and Safety Forums.” (Participant 7)
“We were able to utilize HRO principles in response to the COVID pandemic. Specifically standardized communication from the facility to VISN [Veterans Integrated Services Network] was initiated on a daily basis. This practice provided daily communication on key operational items and clinical items at the medical center, allowed timely feedback on actions being taken, as was instrumental in daily checks on staffing, COVID testing supplies, overall supply chain issues.” (Participant 9)
The recommendations provided by 10 participants (Cohort 1, n = 6; Cohort 2, n = 4) for other health care leaders experiencing a crisis during the journey to high reliability were insightful. The themes that frequently emerged from the responses to the survey were to adapt and overcome. Participants shared:
“Utilize the many tools you’re given, specifically your team. Try even the craziest ideas from frontline staff.” (Participant 1)
“Use your mentors for younger directors and, even if you think you know the answer, involve your staff. It makes them feel they have a voice and gives them ownership of the issues.” (Participant 5)
“Make sure that you have key leaders in place who are committed to HRO and can help the organization adjust.” (Participant 6)
“Take advantage of HRO Leader Coaching, which pairs MCDs with coaches who act as consultants for HRO leadership practices to ensure progress in reaching the next level in the journey to High Reliability.” (Participant 7)
“Meet regularly with the HRO Lead and team (more frequently during early stages of implementation) to provide support, eliminate barriers, and champion the HRO mission. It is important to include other members of the ELT [Executive Leadership Team] to ensure their involvement with the facility HRO strategic plan.” (Participant 7)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
The theme of prioritizing competing demands emerged again from 5 participants (Cohort 1, n = 3; Cohort 2, n = 2) with question 3 describing recommendations for other leaders:
“Your first priority is to the crisis. Don’t get distracted by this or any other initiative. That was not a very popular message for the people pushing HRO, but it is the reality and the necessity. However, it IS possible to move forward with HRO (or other important initiatives) during crisis times, as long as you carefully consider what you are asking of people and don’t overload/overwhelm them. It is not your ego (or that of Central Office) that needs to be stoked. If the initiative truly has value, you need to be patient to see it done properly, rather than rushed/pushed/forced. Don’t kill it by being overeager and overwhelming your already overtaxed people. That said, keep moving forward. The key is pacing—and remember that your Type A hard-driving leader types (you know who you are) will certainly fail if they push it. Or even if they go at a normal pace that would be appropriate for noncrisis times.” (Participant 4)
“Prioritize and understand that not everything is priority #1. Continue what you can with HRO, incorporate high reliability principles into the work being done during a crisis, but understand you may need to modify rollout schedules.” (Participant 8)
“It was critical for us to always focus on the immediate workplace safety of staff (especially those on the frontlines of the pandemic response) when in the process of rolling out HRO initiatives.” (Participant 14)
Maintaining Momentum
“It seemed as though communication and education from VHA on HRO slowed down at the same time, which further slowed our progress. We are now trying to ramp our engagement up again.” (Participant 3)
“There can be synergy between crisis response and HRO implementation. As an example, one of the first steps we took was leadership rounding. That was necessary anyways for crisis management (raising the spirits on the front lines, so to speak). What we did was include scheduled time instead of (in addition to) ad hoc. And we got credit for taking an HRO step. I resisted whiteboards/visual management systems for a long time because (in my opinion) that would have been much too distracting during the crisis. Having waited for better times, I was able to move forward with that several months later and with good success.” (Participant 4)
Discussion
Health care leaders worldwide experienced an immense set of challenges because of the COVID-19 pandemic, which is a crisis of a magnitude with no parallel in modern times. Strong, adaptive leadership at all levels of health care systems was needed to effectively address the immense crisis at hand.36,37 Findings from this pilot QI initiative suggest that MCDs faced many new challenges, requiring them to perform unfamiliar tasks and manage numerous overlapping challenges (eg, staffing shortages and reassignments, safety concerns, changes to patient appointments, backlogs in essential services), all while also trying to continue with the journey to high reliability. Despite the challenges leaders faced, they recognized the need to manage competing priorities early and effectively. At times, the priority was to address the wide-ranging, urgent issues related to the pandemic. When the conditions improved, there was time to refocus efforts on important but longer-term activities related to the HRO journey. Other participants recognized that their commitment to HRO needed to remain a priority even during the periods of intense focus on COVID-19.
Some participants felt compelled to stay committed to the HRO journey despite numerous competing demands. They stayed committed to looking for opportunities to progress by implementing HRO principles and practices to achieve safety, quality, and efficiency goals. This dedication is noteworthy, especially in light of recently published research that demonstrates the vast number of patient safety issues that presented during the COVID-19 pandemic (eg, ineffective communication, poor teamwork, the absence of coordination)1 as well as perceptions that patient safety and quality of care had significantly declined as a result of the crisis.36,37
Participants also highlighted the need to be adaptive when responding to the complexity and unpredictability of the pandemic. Participants regularly sought ways to increase their knowledge, skills, and abilities by using the resources (eg, tools, experts) available to them. Research shows that in increasingly complex and ever-changing situation such as the COVID-19 pandemic, leaders must be adaptive with all levels of performance, especially when limited information is available.38,39
This is the first initiative of its kind to specifically explore the challenges experienced and lessons learned from health care leaders continuing along the journey to high reliability during the COVID-19 pandemic. Findings from this pilot QI initiative revealed that many participants recommended that leaders adapt and overcome challenges as much as possible when continuing with HRO during a crisis. These findings are echoed in the current literature suggesting that adaptive performance is a highly effective form of leadership during crises.38,40 Being able to effectively adapt during a crisis is essential for reducing further vulnerabilities across health care systems. In fact, this lesson is shared by many countries in response to the unprecedented global crisis.41A limitation of this pilot QI initiative is that the authors did not directly solicit responses from all VHA MCDs or from other health care executives (eg, Chief of Staff, Associate Director for Operations, Associate Director for Patient Care, and Nurse Executive). As such, our findings represent only a small segment of senior leadership perspectives from a large, integrated health care system. Individuals who did not respond to the survey may have had different experiences than those who did, and the authors excluded many MCDs who formally began their HRO journeys in 2022, well after the pandemic was underway. Similarly, the experiences of Veterans Affairs leaders may or may not be similar to that of other health care organizations. Although the goal of this initiative was to explore the participants’ experiences during the period of crisis, time and distance from the events at the height of the COVID-19 pandemic may have resulted in difficulty recalling information as well as making sense of the occurrence. This potential recall bias is a common occurrence in trying to explore past experiences, especially as they relate to crises. Finally, this pilot QI initiative did not explore personal challenges participants may have faced during this period of time (eg, burnout, personal or family illness), which may have also shaped their responses.
Conclusion
This initiative suggests that VHA MCDs often relied on HRO principles to guide and assist with their response to the COVID-19 pandemic, including managing periods of unprecedented crisis. The ability to adapt and prioritize was seen as an especially important lesson. Many MCDs continued their personal and organizational efforts toward high reliability even in periods of intense challenge because of the pandemic. These findings can help with future crises that may occur during an organization’s journey to high reliability. This pilot QI initiative’s findings warrant further investigation to explore the experiences of the broader range of health care leaders while responding to unplanned crises or even planned large-scale cultural change or technology modernization initiatives (eg, electronic health record modernization) to expand the state of the science of high reliability as well as inform policy and decision-making. Finally, another area for future study is examining how leadership responses vary across facilities, depending on factors such as leader roles, facility complexity level, resource availability, patient population characteristics, and organizational culture.
Acknowledgment: The authors express their sincere gratitude to the medical center directors who participated in this pilot study.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel St., Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
1. Editors: Dying in a leadership vacuum. 9.4N Engl J Med. 2020;383(15):1479-1480. doi:10.1056/NEJMe2029812
2. Geerts JM, Kinnair D, Taheri P, et al. Guidance for health care leaders during the recovery stage of the COVID-19 pandemic: a consensus statement. JAMA Netw Open. 2021;4(7):1-16. doi:10.1001/jamanetworkopen.2021.20295
3. Boiral O, Brotherton M-C, Rivaud L, et al. Organizations’ management of the COVID-19 pandemic: a scoping review of business articles. Sustainability. 2021;13:1-20. doi:10.3390/su13073993
4. Razu SR, Yasmin T, Arif TB, et al. Challenges faced by healthcare professionals during the COVID-19 pandemic: a qualitative inquiry from Bangladesh. Front Public Health. 2021;9:1-13. doi:10.3389/fpubh.2021.647315
5. Lyng HB, Ree E, Wibe T, et al. Healthcare leaders’ use of innovative solutions to ensure resilience in healthcare during the Covid-19 pandemic: a qualitative study in Norwegian nursing homes and home care services. BMC Health Serv Res. 2021;21(1):1-11. doi:1186/s12913-021-06923-1
6. Freitas J. Queiroz A, Bortotti I, et al. Nurse leaders’ challenges fighting the COVID-19 pandemic: a qualitative study. Open J Nurs. 2021;11:267-280. doi:10.4236/ojn.2021.115024
7. McGuire AL, Aulisio MP, Davis FD, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. 9.4Am J Bioeth. 2020;20(7):15-27. doi:10.1080/15265161.2020.1764138
8. Turbow RM, Scibilia JP. Embracing principles of high reliability organizations can improve patient safety during pandemic. AAP News. January 19, 2021. Accessed March 1, 2023. https://publications.aap.org/aapnews/news/8975
9. Roberts BH, Damiano LA, Graham S, et al. A case study in fostering a learning culture in the context of Covid-19. American Association for Physician Leadership. June 24, 2021. Accessed March 1, 2023. https://www.physicianleaders.org/news/a-case-study-in-fostering-a-learning-culture-in-the-context-of-covid-19
10. U.S. Department of Veterans Affairs. Department of Veterans AffairsCOVID-19 National Summary. Veterans Affairs. Accessed December 4, 2022. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
11. U.S. Department of Veterans Affairs. VA fourth mission summary. Veterans Affairs. Accessed December 4, 2022. https://www.va.gov/health/coronavirus/statesupport.asp#:~:text=As%20part%20of%20the%20Fourth,the%20facilities%20we%20are%20supporting
12. Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18(1):e320-e328. doi:10.1097/PTS.0000000000000768
13. Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. 9.4Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
14. Maison D, Jaworska D, Adamczyk D, et al. The challenges arising from the COVID-19 pandemic and the way people deal with them: a qualitative longitudinal study. PLoS One. 2021;16(10):1-17. doi:10.1371/journal.pone.0258133
15. Schulman PR. Reliability, uncertainty and the management of error: new perspectives in the COVID-19 era. J Contingencies Crisis Manag. 2022;30:92-101. doi:10.1111/1468-5973.12356
16. Adelman JS, Gandhi TK. COVID-19 and patient safety: time to tap into our investment in high reliability. J Patient Saf. 2021;17(4): 331-333. doi:10.1097/PTS.0000000000000843
17. Shingler-Nace A. COVID-19: when leadership calls. Nurs Lead. 2020;18(3):202-203. doi:10.1016/j.mnl.2020.03.017
18. Van Stralen D, Mercer TA. During pandemic COVID 19, the high reliability organization (HRO) identifies maladaptive stress behaviors: the stress-fear-threat cascade. Neonatol Tod. 2020;15(11):113-124. doi: 10.51362/neonatology.today/2020111511113124
19. Vogus TJ, Wilson AD, Randall K, et al. We’re all in this together: how COVID-19 revealed the coconstruction of mindful organising and organisational reliability. BMJ Qual Saf. 2022;31(3):230-233. doi:10.1136/bmjqs-2021-014068
20. Van Stralen D. Pragmatic high-reliability organization (HRO) during pandemic COVID-19. Neonatol Tod. 2020(4);15:109-117. doi:10.51362/neonatology.today/20208158109117
21. Thull-Freedman J, Mondoux S, Stang A, et al. Going to the COVID-19 Gemba: using observation and high reliability strategies to achieve safety in a time of crisis. CJEM. 2020;22(6):738-741. doi:10.1017/cem.2020.380
22. Sarihasan I, Dajnoki K, Oláh J, et al. The importance of the leadership functions of a high-reliability health care organization in managing the COVID-19 pandemic in Turkey. Econ Sociol. 2022;15:78-93. doi:10.14254/2071-789x.2022/15-1/5
23. Crain MA, Bush AL, Hayanga H, et al. Healthcare leadership in the COVID-19 pandemic: from innovative preparation to evolutionary transformation. J Health Leadersh. 2021;13:199-207. doi:10.2147/JHL.S319829
24. SQUIRE. Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) SQUIRE; 2020. Accessed March 1, 2023. http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&pageId=471
25. Lounsbury O. How to write a quality improvement project. Patient Safety J. 2022;4(1):65-67. doi:10.33940/culture/2022.3.6
26. Bengtsson M. How to plan and perform a qualitative study using content analysis. Nurs Plus Open. 2016;2:8-14. doi:10.1016/j.npls.2016.01.001
27. Allen M. The Sage Encyclopedia of Communication Research Methods. (Vols. 1-4). SAGE Publications, Inc; 2017
28. Unlock insights with qualitative data analysis software. Lumivero. Accessed March 2, 2023. https://lumivero.com/products/nvivo/
29. Maher C, Hadfield M, Hutchings M, et al. Ensuring rigor in qualitative data analysis: a design research approach to coding combining NVivo with traditional material methods. Int J Qual Methods. 2018;17:1-13. doi:10.1177/1609406918786362
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-101. doi:10.1191/1478088706qp063oa
31. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. doi:10.1016/j.afjem.2017.08.001
32. Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. FoHPE. 2022;23:111-127. doi:10.11157/fohpe.v23i1.544
33. Nowell LS, Norris JM, White DE, et al. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1-13. doi:10.1177/1609406917733847
34. Gautham KS, Pearlman S. Do quality improvement projects require IRB approval? J Perinatol. 2021;41:1209-1212. doi:10.1038/s41372-021-01038-1
35. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:1-10. doi:10.1016/j.socscimed.2021.114523
36. Balogun M, Dada FO, Oladimeji A, et al. Leading in a time of crisis: a qualitative study capturing experiences of health facility leaders during the early phases of the COVID-19 pandemic in Nigeria’s epicentre. Leadersh Health Serv (Bradf Engl). Published online May 12, 2022. doi:10.1108/lhs-02-2022-0017
37. Guttormson J, Calkins K, McAndrew N, et al. Critical care nurses’ experiences during the COVID-19 pandemic: a US national survey. Am J Crit Care. 2022;31:96-103. doi:10.4037/ajcc2022312
38. Bajaba A, Bajaba S, Algarni M, et al. Adaptive managers as emerging leaders during the COVID-19 crisis. Front Psychol. 2021;12:1-11. doi:10.3389/fpsyg.2021.661628
39. Ahern S, Loh E. Leadership during the COVID-19 pandemic: building and sustaining trust in times of uncertainty. BMJ Lead. 2021;59(4):266-269. doi.org/10.1136/leader-2020-000271
40. Cote R. Adaptive leadership approach with COVID 19 adaptive challenges. J Leadersh Account Ethics. 2022;19:34-44. doi:10.33423/jlae.v19i1.4992
41. Juvet TM, Corbaz-Kurth S, Roos P, et al. Adapting to the unexpected: problematic work situations and resilience strategies in healthcare institutions during the COVID-19 pandemic’s first wave. Saf Sci. 2021;139:1-9. doi:10.1016/j.ssci.2021.105277
1. Editors: Dying in a leadership vacuum. 9.4N Engl J Med. 2020;383(15):1479-1480. doi:10.1056/NEJMe2029812
2. Geerts JM, Kinnair D, Taheri P, et al. Guidance for health care leaders during the recovery stage of the COVID-19 pandemic: a consensus statement. JAMA Netw Open. 2021;4(7):1-16. doi:10.1001/jamanetworkopen.2021.20295
3. Boiral O, Brotherton M-C, Rivaud L, et al. Organizations’ management of the COVID-19 pandemic: a scoping review of business articles. Sustainability. 2021;13:1-20. doi:10.3390/su13073993
4. Razu SR, Yasmin T, Arif TB, et al. Challenges faced by healthcare professionals during the COVID-19 pandemic: a qualitative inquiry from Bangladesh. Front Public Health. 2021;9:1-13. doi:10.3389/fpubh.2021.647315
5. Lyng HB, Ree E, Wibe T, et al. Healthcare leaders’ use of innovative solutions to ensure resilience in healthcare during the Covid-19 pandemic: a qualitative study in Norwegian nursing homes and home care services. BMC Health Serv Res. 2021;21(1):1-11. doi:1186/s12913-021-06923-1
6. Freitas J. Queiroz A, Bortotti I, et al. Nurse leaders’ challenges fighting the COVID-19 pandemic: a qualitative study. Open J Nurs. 2021;11:267-280. doi:10.4236/ojn.2021.115024
7. McGuire AL, Aulisio MP, Davis FD, et al. Ethical challenges arising in the COVID-19 pandemic: an overview from the Association of Bioethics Program Directors (ABPD) Task Force. 9.4Am J Bioeth. 2020;20(7):15-27. doi:10.1080/15265161.2020.1764138
8. Turbow RM, Scibilia JP. Embracing principles of high reliability organizations can improve patient safety during pandemic. AAP News. January 19, 2021. Accessed March 1, 2023. https://publications.aap.org/aapnews/news/8975
9. Roberts BH, Damiano LA, Graham S, et al. A case study in fostering a learning culture in the context of Covid-19. American Association for Physician Leadership. June 24, 2021. Accessed March 1, 2023. https://www.physicianleaders.org/news/a-case-study-in-fostering-a-learning-culture-in-the-context-of-covid-19
10. U.S. Department of Veterans Affairs. Department of Veterans AffairsCOVID-19 National Summary. Veterans Affairs. Accessed December 4, 2022. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary
11. U.S. Department of Veterans Affairs. VA fourth mission summary. Veterans Affairs. Accessed December 4, 2022. https://www.va.gov/health/coronavirus/statesupport.asp#:~:text=As%20part%20of%20the%20Fourth,the%20facilities%20we%20are%20supporting
12. Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18(1):e320-e328. doi:10.1097/PTS.0000000000000768
13. Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. 9.4Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
14. Maison D, Jaworska D, Adamczyk D, et al. The challenges arising from the COVID-19 pandemic and the way people deal with them: a qualitative longitudinal study. PLoS One. 2021;16(10):1-17. doi:10.1371/journal.pone.0258133
15. Schulman PR. Reliability, uncertainty and the management of error: new perspectives in the COVID-19 era. J Contingencies Crisis Manag. 2022;30:92-101. doi:10.1111/1468-5973.12356
16. Adelman JS, Gandhi TK. COVID-19 and patient safety: time to tap into our investment in high reliability. J Patient Saf. 2021;17(4): 331-333. doi:10.1097/PTS.0000000000000843
17. Shingler-Nace A. COVID-19: when leadership calls. Nurs Lead. 2020;18(3):202-203. doi:10.1016/j.mnl.2020.03.017
18. Van Stralen D, Mercer TA. During pandemic COVID 19, the high reliability organization (HRO) identifies maladaptive stress behaviors: the stress-fear-threat cascade. Neonatol Tod. 2020;15(11):113-124. doi: 10.51362/neonatology.today/2020111511113124
19. Vogus TJ, Wilson AD, Randall K, et al. We’re all in this together: how COVID-19 revealed the coconstruction of mindful organising and organisational reliability. BMJ Qual Saf. 2022;31(3):230-233. doi:10.1136/bmjqs-2021-014068
20. Van Stralen D. Pragmatic high-reliability organization (HRO) during pandemic COVID-19. Neonatol Tod. 2020(4);15:109-117. doi:10.51362/neonatology.today/20208158109117
21. Thull-Freedman J, Mondoux S, Stang A, et al. Going to the COVID-19 Gemba: using observation and high reliability strategies to achieve safety in a time of crisis. CJEM. 2020;22(6):738-741. doi:10.1017/cem.2020.380
22. Sarihasan I, Dajnoki K, Oláh J, et al. The importance of the leadership functions of a high-reliability health care organization in managing the COVID-19 pandemic in Turkey. Econ Sociol. 2022;15:78-93. doi:10.14254/2071-789x.2022/15-1/5
23. Crain MA, Bush AL, Hayanga H, et al. Healthcare leadership in the COVID-19 pandemic: from innovative preparation to evolutionary transformation. J Health Leadersh. 2021;13:199-207. doi:10.2147/JHL.S319829
24. SQUIRE. Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) SQUIRE; 2020. Accessed March 1, 2023. http://www.squire-statement.org/index.cfm?fuseaction=Page.ViewPage&pageId=471
25. Lounsbury O. How to write a quality improvement project. Patient Safety J. 2022;4(1):65-67. doi:10.33940/culture/2022.3.6
26. Bengtsson M. How to plan and perform a qualitative study using content analysis. Nurs Plus Open. 2016;2:8-14. doi:10.1016/j.npls.2016.01.001
27. Allen M. The Sage Encyclopedia of Communication Research Methods. (Vols. 1-4). SAGE Publications, Inc; 2017
28. Unlock insights with qualitative data analysis software. Lumivero. Accessed March 2, 2023. https://lumivero.com/products/nvivo/
29. Maher C, Hadfield M, Hutchings M, et al. Ensuring rigor in qualitative data analysis: a design research approach to coding combining NVivo with traditional material methods. Int J Qual Methods. 2018;17:1-13. doi:10.1177/1609406918786362
30. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77-101. doi:10.1191/1478088706qp063oa
31. Erlingsson C, Brysiewicz P. A hands-on guide to doing content analysis. Afr J Emerg Med. 2017;7:93-99. doi:10.1016/j.afjem.2017.08.001
32. Vears DF, Gillam L. Inductive content analysis: a guide for beginning qualitative researchers. FoHPE. 2022;23:111-127. doi:10.11157/fohpe.v23i1.544
33. Nowell LS, Norris JM, White DE, et al. Thematic analysis: striving to meet the trustworthiness criteria. Int J Qual Methods. 2017;16:1-13. doi:10.1177/1609406917733847
34. Gautham KS, Pearlman S. Do quality improvement projects require IRB approval? J Perinatol. 2021;41:1209-1212. doi:10.1038/s41372-021-01038-1
35. Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:1-10. doi:10.1016/j.socscimed.2021.114523
36. Balogun M, Dada FO, Oladimeji A, et al. Leading in a time of crisis: a qualitative study capturing experiences of health facility leaders during the early phases of the COVID-19 pandemic in Nigeria’s epicentre. Leadersh Health Serv (Bradf Engl). Published online May 12, 2022. doi:10.1108/lhs-02-2022-0017
37. Guttormson J, Calkins K, McAndrew N, et al. Critical care nurses’ experiences during the COVID-19 pandemic: a US national survey. Am J Crit Care. 2022;31:96-103. doi:10.4037/ajcc2022312
38. Bajaba A, Bajaba S, Algarni M, et al. Adaptive managers as emerging leaders during the COVID-19 crisis. Front Psychol. 2021;12:1-11. doi:10.3389/fpsyg.2021.661628
39. Ahern S, Loh E. Leadership during the COVID-19 pandemic: building and sustaining trust in times of uncertainty. BMJ Lead. 2021;59(4):266-269. doi.org/10.1136/leader-2020-000271
40. Cote R. Adaptive leadership approach with COVID 19 adaptive challenges. J Leadersh Account Ethics. 2022;19:34-44. doi:10.33423/jlae.v19i1.4992
41. Juvet TM, Corbaz-Kurth S, Roos P, et al. Adapting to the unexpected: problematic work situations and resilience strategies in healthcare institutions during the COVID-19 pandemic’s first wave. Saf Sci. 2021;139:1-9. doi:10.1016/j.ssci.2021.105277
JCOM: 30 Years of Advancing Quality Improvement and Innovation in Care Delivery
This year marks the publication of the 30th volume of the Journal of Clinical Outcomes Management (JCOM). As we celebrate JCOM’s 30th year, we look forward to the future and continuing the journey to inform quality improvement leaders and practitioners about advances in the field and share experiences. The path forward on this journey involves collaboration across stakeholders, the application of innovative improvement methods, and a commitment to achieving health equity. Health care quality improvement plans must prioritize patient-centered care, promote evidence-based practices and continuous learning, and establish clear metrics to measure progress and success. Furthermore, engagement with patients and communities must be at the forefront of any quality improvement plan, as their perspectives and experiences are essential to understanding and addressing the root causes of disparities in health care delivery. Additionally, effective communication and coordination among health care providers, administrators, policymakers, and other stakeholders are crucial to achieving sustainable improvements in health care quality.
JCOM’s mission is to serve as a platform for sharing knowledge, experiences, and best practices to improve patient outcomes and promote health equity. The vision encompasses a world where all individuals have access to high-quality, patient-centered health care that is free of disparities and achieves optimal health outcomes. JCOM’s strategy is to publish articles that showcase innovative quality improvement initiatives, share evidence-based practices and research findings, highlight successful collaborations, and provide practical guidance for health care professionals to implement quality improvement initiatives in their organizations.
We believe that by sharing these insights and experiences, we can accelerate progress toward achieving equitable and high-quality health care for all individuals and communities, regardless of their socioeconomic status, race/ethnicity, gender identity, or any other factor that may impact their access to care and health outcomes. We continue to welcome submissions from all health care professionals, researchers, and other stakeholders involved in quality improvement initiatives. Together, we can work toward a future where every individual has access to the highest quality of health care and experiences equitable health outcomes.
A comprehensive and collaborative approach to health care quality improvement, which is led by a peer review process and scientific publication of the progress, is a necessary part of ensuring that all patients receive high-quality care that is equitable and patient-centered. The future of health care quality will require further research and scholarly work in the areas of training and development, data infrastructure and analytics, as well as technology-enabled solutions that support continuous improvement and innovation. Health care organizations can build a culture of quality improvement that drives meaningful progress toward achieving health equity and improving health care delivery for all by sharing the output from their research.
Thank you for joining us in this mission to improve health care quality, promote optimal health care delivery methods, and create a world where health care is not only accessible, but also equitable and of the highest standards. Let us continue to work toward building a health care system that prioritizes patient-centered care. Together, we can make a difference and ensure that every individual receives the care they need and deserve.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
This year marks the publication of the 30th volume of the Journal of Clinical Outcomes Management (JCOM). As we celebrate JCOM’s 30th year, we look forward to the future and continuing the journey to inform quality improvement leaders and practitioners about advances in the field and share experiences. The path forward on this journey involves collaboration across stakeholders, the application of innovative improvement methods, and a commitment to achieving health equity. Health care quality improvement plans must prioritize patient-centered care, promote evidence-based practices and continuous learning, and establish clear metrics to measure progress and success. Furthermore, engagement with patients and communities must be at the forefront of any quality improvement plan, as their perspectives and experiences are essential to understanding and addressing the root causes of disparities in health care delivery. Additionally, effective communication and coordination among health care providers, administrators, policymakers, and other stakeholders are crucial to achieving sustainable improvements in health care quality.
JCOM’s mission is to serve as a platform for sharing knowledge, experiences, and best practices to improve patient outcomes and promote health equity. The vision encompasses a world where all individuals have access to high-quality, patient-centered health care that is free of disparities and achieves optimal health outcomes. JCOM’s strategy is to publish articles that showcase innovative quality improvement initiatives, share evidence-based practices and research findings, highlight successful collaborations, and provide practical guidance for health care professionals to implement quality improvement initiatives in their organizations.
We believe that by sharing these insights and experiences, we can accelerate progress toward achieving equitable and high-quality health care for all individuals and communities, regardless of their socioeconomic status, race/ethnicity, gender identity, or any other factor that may impact their access to care and health outcomes. We continue to welcome submissions from all health care professionals, researchers, and other stakeholders involved in quality improvement initiatives. Together, we can work toward a future where every individual has access to the highest quality of health care and experiences equitable health outcomes.
A comprehensive and collaborative approach to health care quality improvement, which is led by a peer review process and scientific publication of the progress, is a necessary part of ensuring that all patients receive high-quality care that is equitable and patient-centered. The future of health care quality will require further research and scholarly work in the areas of training and development, data infrastructure and analytics, as well as technology-enabled solutions that support continuous improvement and innovation. Health care organizations can build a culture of quality improvement that drives meaningful progress toward achieving health equity and improving health care delivery for all by sharing the output from their research.
Thank you for joining us in this mission to improve health care quality, promote optimal health care delivery methods, and create a world where health care is not only accessible, but also equitable and of the highest standards. Let us continue to work toward building a health care system that prioritizes patient-centered care. Together, we can make a difference and ensure that every individual receives the care they need and deserve.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
This year marks the publication of the 30th volume of the Journal of Clinical Outcomes Management (JCOM). As we celebrate JCOM’s 30th year, we look forward to the future and continuing the journey to inform quality improvement leaders and practitioners about advances in the field and share experiences. The path forward on this journey involves collaboration across stakeholders, the application of innovative improvement methods, and a commitment to achieving health equity. Health care quality improvement plans must prioritize patient-centered care, promote evidence-based practices and continuous learning, and establish clear metrics to measure progress and success. Furthermore, engagement with patients and communities must be at the forefront of any quality improvement plan, as their perspectives and experiences are essential to understanding and addressing the root causes of disparities in health care delivery. Additionally, effective communication and coordination among health care providers, administrators, policymakers, and other stakeholders are crucial to achieving sustainable improvements in health care quality.
JCOM’s mission is to serve as a platform for sharing knowledge, experiences, and best practices to improve patient outcomes and promote health equity. The vision encompasses a world where all individuals have access to high-quality, patient-centered health care that is free of disparities and achieves optimal health outcomes. JCOM’s strategy is to publish articles that showcase innovative quality improvement initiatives, share evidence-based practices and research findings, highlight successful collaborations, and provide practical guidance for health care professionals to implement quality improvement initiatives in their organizations.
We believe that by sharing these insights and experiences, we can accelerate progress toward achieving equitable and high-quality health care for all individuals and communities, regardless of their socioeconomic status, race/ethnicity, gender identity, or any other factor that may impact their access to care and health outcomes. We continue to welcome submissions from all health care professionals, researchers, and other stakeholders involved in quality improvement initiatives. Together, we can work toward a future where every individual has access to the highest quality of health care and experiences equitable health outcomes.
A comprehensive and collaborative approach to health care quality improvement, which is led by a peer review process and scientific publication of the progress, is a necessary part of ensuring that all patients receive high-quality care that is equitable and patient-centered. The future of health care quality will require further research and scholarly work in the areas of training and development, data infrastructure and analytics, as well as technology-enabled solutions that support continuous improvement and innovation. Health care organizations can build a culture of quality improvement that drives meaningful progress toward achieving health equity and improving health care delivery for all by sharing the output from their research.
Thank you for joining us in this mission to improve health care quality, promote optimal health care delivery methods, and create a world where health care is not only accessible, but also equitable and of the highest standards. Let us continue to work toward building a health care system that prioritizes patient-centered care. Together, we can make a difference and ensure that every individual receives the care they need and deserve.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
The Shifting Landscape of Thrombolytic Therapy for Acute Ischemic Stroke
Study 1 Overview (Menon et al)
Objective: To determine whether a 0.25 mg/kg dose of intravenous tenecteplase is noninferior to intravenous alteplase 0.9 mg/kg for patients with acute ischemic stroke eligible for thrombolytic therapy.
Design: Multicenter, parallel-group, open-label randomized controlled trial.
Setting and participants: The trial was conducted at 22 primary and comprehensive stroke centers across Canada. A primary stroke center was defined as a hospital capable of offering intravenous thrombolysis to patients with acute ischemic stroke, while a comprehensive stroke center was able to offer thrombectomy services in addition. The involved centers also participated in Canadian quality improvement registries (either Quality Improvement and Clinical Research [QuiCR] or Optimizing Patient Treatment in Major Ischemic Stroke with EVT [OPTIMISE]) that track patient outcomes. Patients were eligible for inclusion if they were aged 18 years or older, had a diagnosis of acute ischemic stroke, presented within 4.5 hours of symptom onset, and were eligible for thrombolysis according to Canadian guidelines.
Patients were randomized in a 1:1 fashion to either intravenous tenecteplase (0.25 mg/kg single dose, maximum of 25 mg) or intravenous alteplase (0.9 mg/kg total dose to a maximum of 90 mg, delivered as a bolus followed by a continuous infusion). A total of 1600 patients were enrolled, with 816 randomly assigned to the tenecteplase arm and 784 to the alteplase arm; 1577 patients were included in the intention-to-treat (ITT) analysis (n = 806 tenecteplase; n = 771 alteplase). The median age of enrollees was 74 years, and 52.1% of the ITT population were men.
Main outcome measures: In the ITT population, the primary outcome measure was a modified Rankin score (mRS) of 0 or 1 at 90 to 120 days post treatment. Safety outcomes included symptomatic intracerebral hemorrhage, orolingual angioedema, extracranial bleeding that required blood transfusion (all within 24 hours of thrombolytic administration), and all-cause mortality at 90 days. The noninferiority threshold for intravenous tenecteplase was set as the lower 95% CI of the difference between the tenecteplase and alteplase groups in the proportion of patients who met the primary outcome exceeding –5%.
Main results: The primary outcome of mRS of either 0 or 1 at 90 to 120 days of treatment occurred in 296 (36.9%) of the 802 patients assigned to tenecteplase and 266 (34.8%) of the 765 patients assigned to alteplase (unadjusted risk difference, 2.1%; 95% CI, –2.6 to 6.9). The prespecified noninferiority threshold was met. There were no significant differences between the groups in rates of intracerebral hemorrhage at 24 hours or 90-day all-cause mortality.
Conclusion: Intravenous tenecteplase is a reasonable alternative to alteplase for patients eligible for thrombolytic therapy.
Study 2 Overview (Wang et al)
Objective: To determine whether tenecteplase (dose 0.25 mg/kg) is noninferior to alteplase in patients with acute ischemic stroke who are within 4.5 hours of symptom onset and eligible for thrombolytic therapy but either refused or were ineligible for endovascular thrombectomy.
Design: Multicenter, prospective, open-label, randomized, controlled noninferiority trial.
Setting and participants: This trial was conducted at 53 centers across China and included patients 18 years of age or older who were within 4.5 hours of symptom onset and were thrombolytic eligible, had a mRS ≤ 1 at enrollment, and had a National Institutes of Health Stroke Scale score between 5 and 25. Eligible participants were randomized 1:1 to either tenecteplase 0.25 mg/kg (maximum dose 25 mg) or alteplase 0.9 mg/kg (maximum dose 90 mg, administered as a bolus followed by infusion). During the enrollment period (June 12, 2021, to May 29, 2022), a total of 1430 participants were enrolled, and, of those, 716 were randomly assigned to tenecteplase and 714 to alteplase. Six patients assigned to tenecteplase and 7 assigned to alteplase did not receive drugs. At 90 days, 5 in the tenecteplase group and 11 in the alteplase group were lost to follow up.
Main outcome measures: The primary efficacy outcome was a mRS of 0 or 1 at 90 days. The primary safety outcome was intracranial hemorrhage within 36 hours. Safety outcomes included parenchymal hematoma 2, as defined by the European Cooperative Acute Stroke Study III; any intracranial or significant hemorrhage, as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria; and death from all causes at 90 days. Noninferiority for tenecteplase would be declared if the lower 97.5% 1-sided CI for the relative risk (RR) for the primary outcome did not cross 0.937.
Main results: In the modified ITT population, the primary outcome occurred in 439 (62%) of the tenecteplase group and 405 (68%) of the alteplase group (RR, 1.07; 95% CI, 0.98-1.16). This met the prespecified margin for noninferiority. Intracranial hemorrhage within 36 hours was experienced by 15 (2%) patients in the tenecteplase group and 13 (2%) in the alteplase group (RR, 1.18; 95% CI, 0.56-2.50). Death at 90 days occurred in 46 (7%) patients in the tenecteplase group and 35 (5%) in the alteplase group (RR, 1.31; 95% CI, 0.86-2.01).
Conclusion: Tenecteplase was noninferior to alteplase in patients with acute ischemic stroke who met criteria for thrombolysis and either refused or were ineligible for endovascular thrombectomy.
Commentary
Alteplase has been FDA-approved for managing acute ischemic stroke since 1996 and has demonstrated positive effects on functional outcomes. Drawbacks of alteplase therapy, however, include bleeding risk as well as cumbersome administration of a bolus dose followed by a 60-minute infusion. In recent years, the question of whether or not tenecteplase could replace alteplase as the preferred thrombolytic for acute ischemic stroke has garnered much attention. Several features of tenecteplase make it an attractive option, including increased fibrin specificity, a longer half-life, and ease of administration as a single, rapid bolus dose. In phase 2 trials that compared tenecteplase 0.25 mg/kg with alteplase, findings suggested the potential for early neurological improvement as well as improved outcomes at 90 days. While the role of tenecteplase in acute myocardial infarction has been well established due to ease of use and a favorable adverse-effect profile,1 there is much less evidence from phase 3 randomized controlled clinical trials to secure the role of tenecteplase in acute ischemic stroke.2
Menon et al attempted to close this gap in the literature by conducting a randomized controlled clinical trial (AcT) comparing tenecteplase to alteplase in a Canadian patient population. The trial's patient population mirrors that of real-world data from global registries in terms of age, sex, and baseline stroke severity. In addition, the eligibility window of 4.5 hours from symptom onset as well as the inclusion and exclusion criteria for therapy are common to those utilized in other countries, making the findings generalizable. There were some limitations to the study, however, including the impact of COVID-19 on recruitment efforts as well as limitations of research infrastructure and staffing, which may have limited enrollment efforts at primary stroke centers. Nonetheless, the authors concluded that their results provide evidence that tenecteplase is comparable to alteplase, with similar functional and safety outcomes.
TRACE-2 focused on an Asian patient population and provided follow up to the dose-ranging TRACE-1 phase 2 trial. TRACE-1 showed that tenecteplase 0.25 mg/kg had a similar safety profile to alteplase 0.9 mg/kg in Chinese patients presenting with acute ischemic stroke. TRACE-2 sought to establish noninferiority of tenecteplase and excluded patients who were ineligible for or refused thrombectomy. Interestingly, the tenecteplase arm, as the authors point out, had numerically greater mortality as well as intracranial hemorrhage, but these differences were not statistically significant between the treatment groups at 90 days. The TRACE-2 results parallel those of AcT, and although there were differences in ethnicity between the 2 trials, the authors cite this as evidence that the results are consistent and provide evidence for the role of tenecteplase in the management of acute ischemic stroke. Limitations of this trial include potential bias from its open-label design, as well as exclusion of patients with more severe strokes eligible for thrombectomy, which may limit generalizability to patients with more disabling strokes who could have a higher risk of intracranial hemorrhage.
Application for Clinical Practice and System Implementation
Across the country, many organizations have adopted the off-label use of tenecteplase for managing fibrinolytic-eligible acute ischemic stroke patients. In most cases, the impetus for change is the ease of dosing and administration of tenecteplase compared to alteplase, while the inclusion and exclusion criteria and overall management remain the same. Timely administration of therapy in stroke is critical. This, along with other time constraints in stroke workflows, the weight-based calculation of alteplase doses, and alteplase’s administration method may lead to medication errors when using this agent to treat patients with acute stroke. The rapid, single-dose administration of tenecteplase removes many barriers that hospitals face when patients may need to be treated and then transferred to another site for further care. Without the worry to “drip and ship,” the completion of administration may allow for timely patient transfer and eliminate the need for monitoring of an infusion during transfer. For some organizations, there may be a potential for drug cost-savings as well as improved metrics, such as door-to-needle time, but the overall effects of switching from alteplase to tenecteplase remain to be seen. Currently, tenecteplase is included in stroke guidelines as a “reasonable choice,” though with a low level of evidence.3 However, these 2 studies support the role of tenecteplase in acute ischemic stroke treatment and may provide a foundation for further studies to establish the role of tenecteplase in the acute ischemic stroke population.
Practice Points
- Tenecteplase may be considered as an alternative to alteplase for acute ischemic stroke for patients who meet eligibility criteria for thrombolytics; this recommendation is included in the most recent stroke guidelines, although tenecteplase has not been demonstrated to be superior to alteplase.
- The ease of administration of tenecteplase as a single intravenous bolus dose represents a benefit compared to alteplase; it is an off-label use, however, and further studies are needed to establish the superiority of tenecteplase in terms of functional and safety outcomes.
– Carol Heunisch, PharmD, BCPS, BCCP
Pharmacy Department, NorthShore–Edward-Elmhurst Health, Evanston, IL
1. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators; F Van De Werf, J Adgey, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354(9180):716-722. doi:10.1016/s0140-6736(99)07403-6
2. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischaemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
Study 1 Overview (Menon et al)
Objective: To determine whether a 0.25 mg/kg dose of intravenous tenecteplase is noninferior to intravenous alteplase 0.9 mg/kg for patients with acute ischemic stroke eligible for thrombolytic therapy.
Design: Multicenter, parallel-group, open-label randomized controlled trial.
Setting and participants: The trial was conducted at 22 primary and comprehensive stroke centers across Canada. A primary stroke center was defined as a hospital capable of offering intravenous thrombolysis to patients with acute ischemic stroke, while a comprehensive stroke center was able to offer thrombectomy services in addition. The involved centers also participated in Canadian quality improvement registries (either Quality Improvement and Clinical Research [QuiCR] or Optimizing Patient Treatment in Major Ischemic Stroke with EVT [OPTIMISE]) that track patient outcomes. Patients were eligible for inclusion if they were aged 18 years or older, had a diagnosis of acute ischemic stroke, presented within 4.5 hours of symptom onset, and were eligible for thrombolysis according to Canadian guidelines.
Patients were randomized in a 1:1 fashion to either intravenous tenecteplase (0.25 mg/kg single dose, maximum of 25 mg) or intravenous alteplase (0.9 mg/kg total dose to a maximum of 90 mg, delivered as a bolus followed by a continuous infusion). A total of 1600 patients were enrolled, with 816 randomly assigned to the tenecteplase arm and 784 to the alteplase arm; 1577 patients were included in the intention-to-treat (ITT) analysis (n = 806 tenecteplase; n = 771 alteplase). The median age of enrollees was 74 years, and 52.1% of the ITT population were men.
Main outcome measures: In the ITT population, the primary outcome measure was a modified Rankin score (mRS) of 0 or 1 at 90 to 120 days post treatment. Safety outcomes included symptomatic intracerebral hemorrhage, orolingual angioedema, extracranial bleeding that required blood transfusion (all within 24 hours of thrombolytic administration), and all-cause mortality at 90 days. The noninferiority threshold for intravenous tenecteplase was set as the lower 95% CI of the difference between the tenecteplase and alteplase groups in the proportion of patients who met the primary outcome exceeding –5%.
Main results: The primary outcome of mRS of either 0 or 1 at 90 to 120 days of treatment occurred in 296 (36.9%) of the 802 patients assigned to tenecteplase and 266 (34.8%) of the 765 patients assigned to alteplase (unadjusted risk difference, 2.1%; 95% CI, –2.6 to 6.9). The prespecified noninferiority threshold was met. There were no significant differences between the groups in rates of intracerebral hemorrhage at 24 hours or 90-day all-cause mortality.
Conclusion: Intravenous tenecteplase is a reasonable alternative to alteplase for patients eligible for thrombolytic therapy.
Study 2 Overview (Wang et al)
Objective: To determine whether tenecteplase (dose 0.25 mg/kg) is noninferior to alteplase in patients with acute ischemic stroke who are within 4.5 hours of symptom onset and eligible for thrombolytic therapy but either refused or were ineligible for endovascular thrombectomy.
Design: Multicenter, prospective, open-label, randomized, controlled noninferiority trial.
Setting and participants: This trial was conducted at 53 centers across China and included patients 18 years of age or older who were within 4.5 hours of symptom onset and were thrombolytic eligible, had a mRS ≤ 1 at enrollment, and had a National Institutes of Health Stroke Scale score between 5 and 25. Eligible participants were randomized 1:1 to either tenecteplase 0.25 mg/kg (maximum dose 25 mg) or alteplase 0.9 mg/kg (maximum dose 90 mg, administered as a bolus followed by infusion). During the enrollment period (June 12, 2021, to May 29, 2022), a total of 1430 participants were enrolled, and, of those, 716 were randomly assigned to tenecteplase and 714 to alteplase. Six patients assigned to tenecteplase and 7 assigned to alteplase did not receive drugs. At 90 days, 5 in the tenecteplase group and 11 in the alteplase group were lost to follow up.
Main outcome measures: The primary efficacy outcome was a mRS of 0 or 1 at 90 days. The primary safety outcome was intracranial hemorrhage within 36 hours. Safety outcomes included parenchymal hematoma 2, as defined by the European Cooperative Acute Stroke Study III; any intracranial or significant hemorrhage, as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria; and death from all causes at 90 days. Noninferiority for tenecteplase would be declared if the lower 97.5% 1-sided CI for the relative risk (RR) for the primary outcome did not cross 0.937.
Main results: In the modified ITT population, the primary outcome occurred in 439 (62%) of the tenecteplase group and 405 (68%) of the alteplase group (RR, 1.07; 95% CI, 0.98-1.16). This met the prespecified margin for noninferiority. Intracranial hemorrhage within 36 hours was experienced by 15 (2%) patients in the tenecteplase group and 13 (2%) in the alteplase group (RR, 1.18; 95% CI, 0.56-2.50). Death at 90 days occurred in 46 (7%) patients in the tenecteplase group and 35 (5%) in the alteplase group (RR, 1.31; 95% CI, 0.86-2.01).
Conclusion: Tenecteplase was noninferior to alteplase in patients with acute ischemic stroke who met criteria for thrombolysis and either refused or were ineligible for endovascular thrombectomy.
Commentary
Alteplase has been FDA-approved for managing acute ischemic stroke since 1996 and has demonstrated positive effects on functional outcomes. Drawbacks of alteplase therapy, however, include bleeding risk as well as cumbersome administration of a bolus dose followed by a 60-minute infusion. In recent years, the question of whether or not tenecteplase could replace alteplase as the preferred thrombolytic for acute ischemic stroke has garnered much attention. Several features of tenecteplase make it an attractive option, including increased fibrin specificity, a longer half-life, and ease of administration as a single, rapid bolus dose. In phase 2 trials that compared tenecteplase 0.25 mg/kg with alteplase, findings suggested the potential for early neurological improvement as well as improved outcomes at 90 days. While the role of tenecteplase in acute myocardial infarction has been well established due to ease of use and a favorable adverse-effect profile,1 there is much less evidence from phase 3 randomized controlled clinical trials to secure the role of tenecteplase in acute ischemic stroke.2
Menon et al attempted to close this gap in the literature by conducting a randomized controlled clinical trial (AcT) comparing tenecteplase to alteplase in a Canadian patient population. The trial's patient population mirrors that of real-world data from global registries in terms of age, sex, and baseline stroke severity. In addition, the eligibility window of 4.5 hours from symptom onset as well as the inclusion and exclusion criteria for therapy are common to those utilized in other countries, making the findings generalizable. There were some limitations to the study, however, including the impact of COVID-19 on recruitment efforts as well as limitations of research infrastructure and staffing, which may have limited enrollment efforts at primary stroke centers. Nonetheless, the authors concluded that their results provide evidence that tenecteplase is comparable to alteplase, with similar functional and safety outcomes.
TRACE-2 focused on an Asian patient population and provided follow up to the dose-ranging TRACE-1 phase 2 trial. TRACE-1 showed that tenecteplase 0.25 mg/kg had a similar safety profile to alteplase 0.9 mg/kg in Chinese patients presenting with acute ischemic stroke. TRACE-2 sought to establish noninferiority of tenecteplase and excluded patients who were ineligible for or refused thrombectomy. Interestingly, the tenecteplase arm, as the authors point out, had numerically greater mortality as well as intracranial hemorrhage, but these differences were not statistically significant between the treatment groups at 90 days. The TRACE-2 results parallel those of AcT, and although there were differences in ethnicity between the 2 trials, the authors cite this as evidence that the results are consistent and provide evidence for the role of tenecteplase in the management of acute ischemic stroke. Limitations of this trial include potential bias from its open-label design, as well as exclusion of patients with more severe strokes eligible for thrombectomy, which may limit generalizability to patients with more disabling strokes who could have a higher risk of intracranial hemorrhage.
Application for Clinical Practice and System Implementation
Across the country, many organizations have adopted the off-label use of tenecteplase for managing fibrinolytic-eligible acute ischemic stroke patients. In most cases, the impetus for change is the ease of dosing and administration of tenecteplase compared to alteplase, while the inclusion and exclusion criteria and overall management remain the same. Timely administration of therapy in stroke is critical. This, along with other time constraints in stroke workflows, the weight-based calculation of alteplase doses, and alteplase’s administration method may lead to medication errors when using this agent to treat patients with acute stroke. The rapid, single-dose administration of tenecteplase removes many barriers that hospitals face when patients may need to be treated and then transferred to another site for further care. Without the worry to “drip and ship,” the completion of administration may allow for timely patient transfer and eliminate the need for monitoring of an infusion during transfer. For some organizations, there may be a potential for drug cost-savings as well as improved metrics, such as door-to-needle time, but the overall effects of switching from alteplase to tenecteplase remain to be seen. Currently, tenecteplase is included in stroke guidelines as a “reasonable choice,” though with a low level of evidence.3 However, these 2 studies support the role of tenecteplase in acute ischemic stroke treatment and may provide a foundation for further studies to establish the role of tenecteplase in the acute ischemic stroke population.
Practice Points
- Tenecteplase may be considered as an alternative to alteplase for acute ischemic stroke for patients who meet eligibility criteria for thrombolytics; this recommendation is included in the most recent stroke guidelines, although tenecteplase has not been demonstrated to be superior to alteplase.
- The ease of administration of tenecteplase as a single intravenous bolus dose represents a benefit compared to alteplase; it is an off-label use, however, and further studies are needed to establish the superiority of tenecteplase in terms of functional and safety outcomes.
– Carol Heunisch, PharmD, BCPS, BCCP
Pharmacy Department, NorthShore–Edward-Elmhurst Health, Evanston, IL
Study 1 Overview (Menon et al)
Objective: To determine whether a 0.25 mg/kg dose of intravenous tenecteplase is noninferior to intravenous alteplase 0.9 mg/kg for patients with acute ischemic stroke eligible for thrombolytic therapy.
Design: Multicenter, parallel-group, open-label randomized controlled trial.
Setting and participants: The trial was conducted at 22 primary and comprehensive stroke centers across Canada. A primary stroke center was defined as a hospital capable of offering intravenous thrombolysis to patients with acute ischemic stroke, while a comprehensive stroke center was able to offer thrombectomy services in addition. The involved centers also participated in Canadian quality improvement registries (either Quality Improvement and Clinical Research [QuiCR] or Optimizing Patient Treatment in Major Ischemic Stroke with EVT [OPTIMISE]) that track patient outcomes. Patients were eligible for inclusion if they were aged 18 years or older, had a diagnosis of acute ischemic stroke, presented within 4.5 hours of symptom onset, and were eligible for thrombolysis according to Canadian guidelines.
Patients were randomized in a 1:1 fashion to either intravenous tenecteplase (0.25 mg/kg single dose, maximum of 25 mg) or intravenous alteplase (0.9 mg/kg total dose to a maximum of 90 mg, delivered as a bolus followed by a continuous infusion). A total of 1600 patients were enrolled, with 816 randomly assigned to the tenecteplase arm and 784 to the alteplase arm; 1577 patients were included in the intention-to-treat (ITT) analysis (n = 806 tenecteplase; n = 771 alteplase). The median age of enrollees was 74 years, and 52.1% of the ITT population were men.
Main outcome measures: In the ITT population, the primary outcome measure was a modified Rankin score (mRS) of 0 or 1 at 90 to 120 days post treatment. Safety outcomes included symptomatic intracerebral hemorrhage, orolingual angioedema, extracranial bleeding that required blood transfusion (all within 24 hours of thrombolytic administration), and all-cause mortality at 90 days. The noninferiority threshold for intravenous tenecteplase was set as the lower 95% CI of the difference between the tenecteplase and alteplase groups in the proportion of patients who met the primary outcome exceeding –5%.
Main results: The primary outcome of mRS of either 0 or 1 at 90 to 120 days of treatment occurred in 296 (36.9%) of the 802 patients assigned to tenecteplase and 266 (34.8%) of the 765 patients assigned to alteplase (unadjusted risk difference, 2.1%; 95% CI, –2.6 to 6.9). The prespecified noninferiority threshold was met. There were no significant differences between the groups in rates of intracerebral hemorrhage at 24 hours or 90-day all-cause mortality.
Conclusion: Intravenous tenecteplase is a reasonable alternative to alteplase for patients eligible for thrombolytic therapy.
Study 2 Overview (Wang et al)
Objective: To determine whether tenecteplase (dose 0.25 mg/kg) is noninferior to alteplase in patients with acute ischemic stroke who are within 4.5 hours of symptom onset and eligible for thrombolytic therapy but either refused or were ineligible for endovascular thrombectomy.
Design: Multicenter, prospective, open-label, randomized, controlled noninferiority trial.
Setting and participants: This trial was conducted at 53 centers across China and included patients 18 years of age or older who were within 4.5 hours of symptom onset and were thrombolytic eligible, had a mRS ≤ 1 at enrollment, and had a National Institutes of Health Stroke Scale score between 5 and 25. Eligible participants were randomized 1:1 to either tenecteplase 0.25 mg/kg (maximum dose 25 mg) or alteplase 0.9 mg/kg (maximum dose 90 mg, administered as a bolus followed by infusion). During the enrollment period (June 12, 2021, to May 29, 2022), a total of 1430 participants were enrolled, and, of those, 716 were randomly assigned to tenecteplase and 714 to alteplase. Six patients assigned to tenecteplase and 7 assigned to alteplase did not receive drugs. At 90 days, 5 in the tenecteplase group and 11 in the alteplase group were lost to follow up.
Main outcome measures: The primary efficacy outcome was a mRS of 0 or 1 at 90 days. The primary safety outcome was intracranial hemorrhage within 36 hours. Safety outcomes included parenchymal hematoma 2, as defined by the European Cooperative Acute Stroke Study III; any intracranial or significant hemorrhage, as defined by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria; and death from all causes at 90 days. Noninferiority for tenecteplase would be declared if the lower 97.5% 1-sided CI for the relative risk (RR) for the primary outcome did not cross 0.937.
Main results: In the modified ITT population, the primary outcome occurred in 439 (62%) of the tenecteplase group and 405 (68%) of the alteplase group (RR, 1.07; 95% CI, 0.98-1.16). This met the prespecified margin for noninferiority. Intracranial hemorrhage within 36 hours was experienced by 15 (2%) patients in the tenecteplase group and 13 (2%) in the alteplase group (RR, 1.18; 95% CI, 0.56-2.50). Death at 90 days occurred in 46 (7%) patients in the tenecteplase group and 35 (5%) in the alteplase group (RR, 1.31; 95% CI, 0.86-2.01).
Conclusion: Tenecteplase was noninferior to alteplase in patients with acute ischemic stroke who met criteria for thrombolysis and either refused or were ineligible for endovascular thrombectomy.
Commentary
Alteplase has been FDA-approved for managing acute ischemic stroke since 1996 and has demonstrated positive effects on functional outcomes. Drawbacks of alteplase therapy, however, include bleeding risk as well as cumbersome administration of a bolus dose followed by a 60-minute infusion. In recent years, the question of whether or not tenecteplase could replace alteplase as the preferred thrombolytic for acute ischemic stroke has garnered much attention. Several features of tenecteplase make it an attractive option, including increased fibrin specificity, a longer half-life, and ease of administration as a single, rapid bolus dose. In phase 2 trials that compared tenecteplase 0.25 mg/kg with alteplase, findings suggested the potential for early neurological improvement as well as improved outcomes at 90 days. While the role of tenecteplase in acute myocardial infarction has been well established due to ease of use and a favorable adverse-effect profile,1 there is much less evidence from phase 3 randomized controlled clinical trials to secure the role of tenecteplase in acute ischemic stroke.2
Menon et al attempted to close this gap in the literature by conducting a randomized controlled clinical trial (AcT) comparing tenecteplase to alteplase in a Canadian patient population. The trial's patient population mirrors that of real-world data from global registries in terms of age, sex, and baseline stroke severity. In addition, the eligibility window of 4.5 hours from symptom onset as well as the inclusion and exclusion criteria for therapy are common to those utilized in other countries, making the findings generalizable. There were some limitations to the study, however, including the impact of COVID-19 on recruitment efforts as well as limitations of research infrastructure and staffing, which may have limited enrollment efforts at primary stroke centers. Nonetheless, the authors concluded that their results provide evidence that tenecteplase is comparable to alteplase, with similar functional and safety outcomes.
TRACE-2 focused on an Asian patient population and provided follow up to the dose-ranging TRACE-1 phase 2 trial. TRACE-1 showed that tenecteplase 0.25 mg/kg had a similar safety profile to alteplase 0.9 mg/kg in Chinese patients presenting with acute ischemic stroke. TRACE-2 sought to establish noninferiority of tenecteplase and excluded patients who were ineligible for or refused thrombectomy. Interestingly, the tenecteplase arm, as the authors point out, had numerically greater mortality as well as intracranial hemorrhage, but these differences were not statistically significant between the treatment groups at 90 days. The TRACE-2 results parallel those of AcT, and although there were differences in ethnicity between the 2 trials, the authors cite this as evidence that the results are consistent and provide evidence for the role of tenecteplase in the management of acute ischemic stroke. Limitations of this trial include potential bias from its open-label design, as well as exclusion of patients with more severe strokes eligible for thrombectomy, which may limit generalizability to patients with more disabling strokes who could have a higher risk of intracranial hemorrhage.
Application for Clinical Practice and System Implementation
Across the country, many organizations have adopted the off-label use of tenecteplase for managing fibrinolytic-eligible acute ischemic stroke patients. In most cases, the impetus for change is the ease of dosing and administration of tenecteplase compared to alteplase, while the inclusion and exclusion criteria and overall management remain the same. Timely administration of therapy in stroke is critical. This, along with other time constraints in stroke workflows, the weight-based calculation of alteplase doses, and alteplase’s administration method may lead to medication errors when using this agent to treat patients with acute stroke. The rapid, single-dose administration of tenecteplase removes many barriers that hospitals face when patients may need to be treated and then transferred to another site for further care. Without the worry to “drip and ship,” the completion of administration may allow for timely patient transfer and eliminate the need for monitoring of an infusion during transfer. For some organizations, there may be a potential for drug cost-savings as well as improved metrics, such as door-to-needle time, but the overall effects of switching from alteplase to tenecteplase remain to be seen. Currently, tenecteplase is included in stroke guidelines as a “reasonable choice,” though with a low level of evidence.3 However, these 2 studies support the role of tenecteplase in acute ischemic stroke treatment and may provide a foundation for further studies to establish the role of tenecteplase in the acute ischemic stroke population.
Practice Points
- Tenecteplase may be considered as an alternative to alteplase for acute ischemic stroke for patients who meet eligibility criteria for thrombolytics; this recommendation is included in the most recent stroke guidelines, although tenecteplase has not been demonstrated to be superior to alteplase.
- The ease of administration of tenecteplase as a single intravenous bolus dose represents a benefit compared to alteplase; it is an off-label use, however, and further studies are needed to establish the superiority of tenecteplase in terms of functional and safety outcomes.
– Carol Heunisch, PharmD, BCPS, BCCP
Pharmacy Department, NorthShore–Edward-Elmhurst Health, Evanston, IL
1. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators; F Van De Werf, J Adgey, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354(9180):716-722. doi:10.1016/s0140-6736(99)07403-6
2. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischaemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
1. Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators; F Van De Werf, J Adgey, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354(9180):716-722. doi:10.1016/s0140-6736(99)07403-6
2. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischaemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
Upfront Transplants in Patients With Mantle Cell Lymphoma
What is your outlook on the role of upfront autologous stem cell transplant (ASCT) for patients with mantle cell lymphoma (MCL)?
Dr. Barrientos: Most of the data that we have for upfront ASCT for young patients in frontline therapy come from the era when we did not use rituximab, and the data have not kept up with the pace of all the recent advances. Rituximab has changed the way we approach maintenance therapy after induction therapy. No randomized controlled trial data (in regimens that incorporate rituximab and cytarabine) have demonstrated a benefit in overall survival (OS) with ASCT in the modern era.
There is a lot to consider for every patient with MCL before we start therapy or discuss upfront transplant. MCL is one of these non-Hodgkin lymphomas that unfortunately can be aggressive in some patients depending on their prognostic markers and particular clinical features of the disease. Some patients have a more indolent form, whereas others have a more aggressive presentation at the time of diagnosis. The disease is heterogeneous and will respond differently to certain regimens. For example, patients with MCL who have a high proliferation rate, blastoid morphology, multiple chromosomal aberrations, complex karyotype, and/or the presence of tumor suppressor protein P53 (TP53) mutation will likely have a more aggressive course. Fitness for transplant is also an important consideration regardless of age; that is, a patient with comorbid end-stage chronic kidney or liver disease will not be able to tolerate a transplant.
Even with optimal therapy that incorporates rituximab and cytarabine, pursuing a transplant does not necessarily benefit survival in patients with a known TP53 mutation, as these patients typically experience increased toxicity without improved OS. We know they will not respond well, and we should discuss the available data so that the patients can make a sound decision and consider participation in a clinical trial that incorporates novel agents. Another type of mutation—cyclin-dependent kinase inhibitor 2A (CDKN2A)—also has lower OS. Concurrent deletion of CDKN2A and TP53 aberration (deletion and/or mutation) are known to be associated with lower OS given their chemoresistant nature. Patients with these genetic mutations should not be offered standard ASCT, but rather they should be identified early on and prioritized to participate in clinical trials.
Importantly, the role of upfront ASCT is changing right now, based on a recent trial that was presented at the latest American Society of Hematology meeting in 2022. The TRIANGLE trial demonstrated the addition of ibrutinib (a first-generation Bruton tyrosine kinase [BTK] inhibitor) to standard chemoimmunotherapy induction and 2 years of ibrutinib maintenance can improve outcomes vs standard chemoimmunotherapy induction and ASCT alone for younger patients with MCL. However, longer follow-up is needed to fully elucidate the role of ASCT in the era of BTK inhibitors when incorporated early on into the treatment paradigm.
The TRIANGLE trial was an international, randomized 3-arm phase 3 trial (EudraCT-no. 2014-001363-12) for young (up to 65 years) fit patients with histologically confirmed, untreated, advanced stage II-IV MCL. In the control arm A, patients received an alternating R-CHOP/R-DHAP induction followed by myeloablative consolidation (ASCT). In arm A+I, ibrutinib was added to the R-CHOP cycles (560 mg day 1-19) and was applied as maintenance (continuous dosing) for 2 years. In arm I, the same induction and maintenance was applied but high-dose consolidation and ASCT was skipped. A rituximab maintenance (single doses every 2 months for up to 3 years) was allowed to be added in all study arms according to national clinical routine.
The study showed that failure-free survival at 3 years was 72% with chemotherapy alone, 86% with ibrutinib alone, and 88% with ibrutinib plus ASCT. However, the ibrutinib plus ASCT group seemed to have much more toxicity, comorbidities, and other complications from the transplant. The OS data are not mature yet, but looking at the available data, ibrutinib alone might be more beneficial to our patients— not only in terms of efficacy, but also in tolerability and response, with less toxicity over time.
To put things in perspective, we did not have good salvage therapies a decade ago. At the time ASCT was incorporated, it was a good option that allowed numerous patients to achieve a deep response with durable remission duration. Before ibrutinib was approved, the overall response rate for the best salvage therapies was not as encouraging as the initial therapy and, with each relapse, the duration of response shortened. When ibrutinib came along, the overall response rate improved significantly. But again, these patients had relapsed/refractory disease. Researchers have been investigating what would happen if we used such a drug in earlier lines of therapy. Can we get better outcomes? Can we get patients in remission longer, similar to what we have seen with ASCT, but without the ASCT?
There has never been a single modern trial that has demonstrated that transplant improves survival. Transplantation can improve progression-free survival, but not OS. For a disease for which we do not have a cure, if we can keep patients in remission with a good salvage therapy and give them a better quality of life, without subjecting them to an ASCT, then I might choose that. New targeted agents and novel therapies are in clinical development all the time, so the future is bright for patients with this diagnosis. Given the novel salvage therapies in the pipeline, we may be able to no longer recommend ASCT upfront for most patients soon.
Can you share more about the potential benefits of using salvage therapies over ASCT, and particularly any promising newer agents in the salvage therapy setting?
Dr. Barrientos: Recently we had the FDA approval of pirtobrutinib—a noncovalently bound BTK inhibitor—for patients with relapsed/refractory MCL in whom at least 2 lines of systemic therapy had failed, including another BTK inhibitor. In the trial that led to the accelerated approval, pirtobrutinib-treated patients showed an overall response rate of 50% in those who received the drug at 200 mg daily (n = 120); most of the responses were partial responses. The efficacy of other novel drugs are being studied in patients with MCL. For example, ROR1 (receptor tyrosine kinase–like orphan receptor 1) inhibitors and BTK degraders are currently in clinical trials. Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 has been approved for the treatment of adult patients with relapsed or refractory MCL, and this may be an option for some patients.
Multiple novel agents might be able to salvage our patients without subjecting them to an upfront transplant. My hope is to get away from using the intense chemotherapy regimens that might cause myelosuppression, infection risk, or other toxicities, and try to stay with the novel agents. We need to do better for our patients.
Based on the data we now have, until there is a trial that demonstrates a higher OS rate with ASCT, it is hard for me to tell a patient to blindly pursue ASCT without learning more about all the available options. If you have access to a good salvage therapy, especially with all these new promising agents, a patient might be able to stay in remission without having ASCT, which can still have an increased risk of morbidity.
Are there certain patient groups that should never be considered for ASCT?
Dr. Barrientos: Younger patients with the CDKN2A gene—which represents about 22% of patients—and those who have a TP53 mutation should not be considered for a standard transplant because they have a worse outcome independent of the treatment. I would also include complex karyotype patients because of the same nature of the chromosomal aberrations. The more genetic aberrations that a patient has, the more likelihood that any chemotherapy will damage the DNA further and create a more aggressive clone. Instead, I would recommend that young patients in this category participate in a clinical trial with novel agents.
With novel therapies in the pipeline, the availability of CAR T, and now the bispecific antibodies such as blinatumomab and HexAbs coming along, the number of patients who may opt out of ASCT may increase. I have a long discussion with my patients. The more educated they are, the better it is for the patient. At the end of the day, the most important thing for me, with any therapy, is: how does the patient feel? Because if we cannot cure a patient or provide a survival advantage, I do not want to give that patient something that will decrease their quality of life. I would rather keep the patient in some sort of stable disease remission, comfortable, and having a good quality of life. That is my goal for anyone who cannot be cured. Now if it is a curable disease, like a diffuse large cell lymphoma or a Burkitt’s lymphoma, then it is a different story. But for people with MCL, a disease that you cannot cure, or chronic lymphocytic leukemia or follicular lymphoma, then it becomes a different discussion. Undetectable minimal residual disease correlates with longer remission durations, but sometimes trying to achieve that, you can actually do a lot of harm to some patients.
Are there any other conversations you have with your patients in day-to-day practice?
Dr. Barrientos: I always tell my patients to be on top of the age-appropriate cancer screening recommendations. For example, they should see a dermatologist once a year. Men should make sure that their prostate is checked. I recommend women get breast mammograms, Pap smears, and most importantly to avoid smoking—and that includes vaping. It is important to lead a healthy life to minimize the risk of secondary malignancies.
For risk of infections, I recommend to all my patients to be up to date on their vaccinations, such as pneumonia if they are older than 65, Shingrix for prevention of reactivation of varicella or chickenpox, and the flu shot once a year. I also recommend the COVID-19 vaccine even now, as our patients with blood disorders might have a harder time fighting COVID-19 infection. I always tell my patients to please reach out to us because we can discuss the use of antivirals such as Paxlovid (nirmatrelvir/ritonavir), and if they are sick, then they can get remdesivir in the hospital.
I want to touch on health literacy and disparities for a moment. I have some younger patients who are Latin or Black with uncontrolled hypertension or diabetes, even at a young age, and do not realize that I can treat their cancer into remission, but if their blood glucose is in the 500 range, they could die from their diabetes. So talking with patients about their overall health is important. Survivorship issues are important, especially if patients are diagnosed at a young age. We have known for a long time that chemotherapy can create cardiac events, arrhythmias, and heart disease. Therefore, I always tell patients with metabolic syndrome to try to exercise and eat healthy. Patients should get an electrocardiogram and see an internist at least once a year to make sure their cholesterol is well controlled. I think now we are being more cognizant that many complications can happen even 10 years after cancer treatment.
What is your outlook on the role of upfront autologous stem cell transplant (ASCT) for patients with mantle cell lymphoma (MCL)?
Dr. Barrientos: Most of the data that we have for upfront ASCT for young patients in frontline therapy come from the era when we did not use rituximab, and the data have not kept up with the pace of all the recent advances. Rituximab has changed the way we approach maintenance therapy after induction therapy. No randomized controlled trial data (in regimens that incorporate rituximab and cytarabine) have demonstrated a benefit in overall survival (OS) with ASCT in the modern era.
There is a lot to consider for every patient with MCL before we start therapy or discuss upfront transplant. MCL is one of these non-Hodgkin lymphomas that unfortunately can be aggressive in some patients depending on their prognostic markers and particular clinical features of the disease. Some patients have a more indolent form, whereas others have a more aggressive presentation at the time of diagnosis. The disease is heterogeneous and will respond differently to certain regimens. For example, patients with MCL who have a high proliferation rate, blastoid morphology, multiple chromosomal aberrations, complex karyotype, and/or the presence of tumor suppressor protein P53 (TP53) mutation will likely have a more aggressive course. Fitness for transplant is also an important consideration regardless of age; that is, a patient with comorbid end-stage chronic kidney or liver disease will not be able to tolerate a transplant.
Even with optimal therapy that incorporates rituximab and cytarabine, pursuing a transplant does not necessarily benefit survival in patients with a known TP53 mutation, as these patients typically experience increased toxicity without improved OS. We know they will not respond well, and we should discuss the available data so that the patients can make a sound decision and consider participation in a clinical trial that incorporates novel agents. Another type of mutation—cyclin-dependent kinase inhibitor 2A (CDKN2A)—also has lower OS. Concurrent deletion of CDKN2A and TP53 aberration (deletion and/or mutation) are known to be associated with lower OS given their chemoresistant nature. Patients with these genetic mutations should not be offered standard ASCT, but rather they should be identified early on and prioritized to participate in clinical trials.
Importantly, the role of upfront ASCT is changing right now, based on a recent trial that was presented at the latest American Society of Hematology meeting in 2022. The TRIANGLE trial demonstrated the addition of ibrutinib (a first-generation Bruton tyrosine kinase [BTK] inhibitor) to standard chemoimmunotherapy induction and 2 years of ibrutinib maintenance can improve outcomes vs standard chemoimmunotherapy induction and ASCT alone for younger patients with MCL. However, longer follow-up is needed to fully elucidate the role of ASCT in the era of BTK inhibitors when incorporated early on into the treatment paradigm.
The TRIANGLE trial was an international, randomized 3-arm phase 3 trial (EudraCT-no. 2014-001363-12) for young (up to 65 years) fit patients with histologically confirmed, untreated, advanced stage II-IV MCL. In the control arm A, patients received an alternating R-CHOP/R-DHAP induction followed by myeloablative consolidation (ASCT). In arm A+I, ibrutinib was added to the R-CHOP cycles (560 mg day 1-19) and was applied as maintenance (continuous dosing) for 2 years. In arm I, the same induction and maintenance was applied but high-dose consolidation and ASCT was skipped. A rituximab maintenance (single doses every 2 months for up to 3 years) was allowed to be added in all study arms according to national clinical routine.
The study showed that failure-free survival at 3 years was 72% with chemotherapy alone, 86% with ibrutinib alone, and 88% with ibrutinib plus ASCT. However, the ibrutinib plus ASCT group seemed to have much more toxicity, comorbidities, and other complications from the transplant. The OS data are not mature yet, but looking at the available data, ibrutinib alone might be more beneficial to our patients— not only in terms of efficacy, but also in tolerability and response, with less toxicity over time.
To put things in perspective, we did not have good salvage therapies a decade ago. At the time ASCT was incorporated, it was a good option that allowed numerous patients to achieve a deep response with durable remission duration. Before ibrutinib was approved, the overall response rate for the best salvage therapies was not as encouraging as the initial therapy and, with each relapse, the duration of response shortened. When ibrutinib came along, the overall response rate improved significantly. But again, these patients had relapsed/refractory disease. Researchers have been investigating what would happen if we used such a drug in earlier lines of therapy. Can we get better outcomes? Can we get patients in remission longer, similar to what we have seen with ASCT, but without the ASCT?
There has never been a single modern trial that has demonstrated that transplant improves survival. Transplantation can improve progression-free survival, but not OS. For a disease for which we do not have a cure, if we can keep patients in remission with a good salvage therapy and give them a better quality of life, without subjecting them to an ASCT, then I might choose that. New targeted agents and novel therapies are in clinical development all the time, so the future is bright for patients with this diagnosis. Given the novel salvage therapies in the pipeline, we may be able to no longer recommend ASCT upfront for most patients soon.
Can you share more about the potential benefits of using salvage therapies over ASCT, and particularly any promising newer agents in the salvage therapy setting?
Dr. Barrientos: Recently we had the FDA approval of pirtobrutinib—a noncovalently bound BTK inhibitor—for patients with relapsed/refractory MCL in whom at least 2 lines of systemic therapy had failed, including another BTK inhibitor. In the trial that led to the accelerated approval, pirtobrutinib-treated patients showed an overall response rate of 50% in those who received the drug at 200 mg daily (n = 120); most of the responses were partial responses. The efficacy of other novel drugs are being studied in patients with MCL. For example, ROR1 (receptor tyrosine kinase–like orphan receptor 1) inhibitors and BTK degraders are currently in clinical trials. Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 has been approved for the treatment of adult patients with relapsed or refractory MCL, and this may be an option for some patients.
Multiple novel agents might be able to salvage our patients without subjecting them to an upfront transplant. My hope is to get away from using the intense chemotherapy regimens that might cause myelosuppression, infection risk, or other toxicities, and try to stay with the novel agents. We need to do better for our patients.
Based on the data we now have, until there is a trial that demonstrates a higher OS rate with ASCT, it is hard for me to tell a patient to blindly pursue ASCT without learning more about all the available options. If you have access to a good salvage therapy, especially with all these new promising agents, a patient might be able to stay in remission without having ASCT, which can still have an increased risk of morbidity.
Are there certain patient groups that should never be considered for ASCT?
Dr. Barrientos: Younger patients with the CDKN2A gene—which represents about 22% of patients—and those who have a TP53 mutation should not be considered for a standard transplant because they have a worse outcome independent of the treatment. I would also include complex karyotype patients because of the same nature of the chromosomal aberrations. The more genetic aberrations that a patient has, the more likelihood that any chemotherapy will damage the DNA further and create a more aggressive clone. Instead, I would recommend that young patients in this category participate in a clinical trial with novel agents.
With novel therapies in the pipeline, the availability of CAR T, and now the bispecific antibodies such as blinatumomab and HexAbs coming along, the number of patients who may opt out of ASCT may increase. I have a long discussion with my patients. The more educated they are, the better it is for the patient. At the end of the day, the most important thing for me, with any therapy, is: how does the patient feel? Because if we cannot cure a patient or provide a survival advantage, I do not want to give that patient something that will decrease their quality of life. I would rather keep the patient in some sort of stable disease remission, comfortable, and having a good quality of life. That is my goal for anyone who cannot be cured. Now if it is a curable disease, like a diffuse large cell lymphoma or a Burkitt’s lymphoma, then it is a different story. But for people with MCL, a disease that you cannot cure, or chronic lymphocytic leukemia or follicular lymphoma, then it becomes a different discussion. Undetectable minimal residual disease correlates with longer remission durations, but sometimes trying to achieve that, you can actually do a lot of harm to some patients.
Are there any other conversations you have with your patients in day-to-day practice?
Dr. Barrientos: I always tell my patients to be on top of the age-appropriate cancer screening recommendations. For example, they should see a dermatologist once a year. Men should make sure that their prostate is checked. I recommend women get breast mammograms, Pap smears, and most importantly to avoid smoking—and that includes vaping. It is important to lead a healthy life to minimize the risk of secondary malignancies.
For risk of infections, I recommend to all my patients to be up to date on their vaccinations, such as pneumonia if they are older than 65, Shingrix for prevention of reactivation of varicella or chickenpox, and the flu shot once a year. I also recommend the COVID-19 vaccine even now, as our patients with blood disorders might have a harder time fighting COVID-19 infection. I always tell my patients to please reach out to us because we can discuss the use of antivirals such as Paxlovid (nirmatrelvir/ritonavir), and if they are sick, then they can get remdesivir in the hospital.
I want to touch on health literacy and disparities for a moment. I have some younger patients who are Latin or Black with uncontrolled hypertension or diabetes, even at a young age, and do not realize that I can treat their cancer into remission, but if their blood glucose is in the 500 range, they could die from their diabetes. So talking with patients about their overall health is important. Survivorship issues are important, especially if patients are diagnosed at a young age. We have known for a long time that chemotherapy can create cardiac events, arrhythmias, and heart disease. Therefore, I always tell patients with metabolic syndrome to try to exercise and eat healthy. Patients should get an electrocardiogram and see an internist at least once a year to make sure their cholesterol is well controlled. I think now we are being more cognizant that many complications can happen even 10 years after cancer treatment.
What is your outlook on the role of upfront autologous stem cell transplant (ASCT) for patients with mantle cell lymphoma (MCL)?
Dr. Barrientos: Most of the data that we have for upfront ASCT for young patients in frontline therapy come from the era when we did not use rituximab, and the data have not kept up with the pace of all the recent advances. Rituximab has changed the way we approach maintenance therapy after induction therapy. No randomized controlled trial data (in regimens that incorporate rituximab and cytarabine) have demonstrated a benefit in overall survival (OS) with ASCT in the modern era.
There is a lot to consider for every patient with MCL before we start therapy or discuss upfront transplant. MCL is one of these non-Hodgkin lymphomas that unfortunately can be aggressive in some patients depending on their prognostic markers and particular clinical features of the disease. Some patients have a more indolent form, whereas others have a more aggressive presentation at the time of diagnosis. The disease is heterogeneous and will respond differently to certain regimens. For example, patients with MCL who have a high proliferation rate, blastoid morphology, multiple chromosomal aberrations, complex karyotype, and/or the presence of tumor suppressor protein P53 (TP53) mutation will likely have a more aggressive course. Fitness for transplant is also an important consideration regardless of age; that is, a patient with comorbid end-stage chronic kidney or liver disease will not be able to tolerate a transplant.
Even with optimal therapy that incorporates rituximab and cytarabine, pursuing a transplant does not necessarily benefit survival in patients with a known TP53 mutation, as these patients typically experience increased toxicity without improved OS. We know they will not respond well, and we should discuss the available data so that the patients can make a sound decision and consider participation in a clinical trial that incorporates novel agents. Another type of mutation—cyclin-dependent kinase inhibitor 2A (CDKN2A)—also has lower OS. Concurrent deletion of CDKN2A and TP53 aberration (deletion and/or mutation) are known to be associated with lower OS given their chemoresistant nature. Patients with these genetic mutations should not be offered standard ASCT, but rather they should be identified early on and prioritized to participate in clinical trials.
Importantly, the role of upfront ASCT is changing right now, based on a recent trial that was presented at the latest American Society of Hematology meeting in 2022. The TRIANGLE trial demonstrated the addition of ibrutinib (a first-generation Bruton tyrosine kinase [BTK] inhibitor) to standard chemoimmunotherapy induction and 2 years of ibrutinib maintenance can improve outcomes vs standard chemoimmunotherapy induction and ASCT alone for younger patients with MCL. However, longer follow-up is needed to fully elucidate the role of ASCT in the era of BTK inhibitors when incorporated early on into the treatment paradigm.
The TRIANGLE trial was an international, randomized 3-arm phase 3 trial (EudraCT-no. 2014-001363-12) for young (up to 65 years) fit patients with histologically confirmed, untreated, advanced stage II-IV MCL. In the control arm A, patients received an alternating R-CHOP/R-DHAP induction followed by myeloablative consolidation (ASCT). In arm A+I, ibrutinib was added to the R-CHOP cycles (560 mg day 1-19) and was applied as maintenance (continuous dosing) for 2 years. In arm I, the same induction and maintenance was applied but high-dose consolidation and ASCT was skipped. A rituximab maintenance (single doses every 2 months for up to 3 years) was allowed to be added in all study arms according to national clinical routine.
The study showed that failure-free survival at 3 years was 72% with chemotherapy alone, 86% with ibrutinib alone, and 88% with ibrutinib plus ASCT. However, the ibrutinib plus ASCT group seemed to have much more toxicity, comorbidities, and other complications from the transplant. The OS data are not mature yet, but looking at the available data, ibrutinib alone might be more beneficial to our patients— not only in terms of efficacy, but also in tolerability and response, with less toxicity over time.
To put things in perspective, we did not have good salvage therapies a decade ago. At the time ASCT was incorporated, it was a good option that allowed numerous patients to achieve a deep response with durable remission duration. Before ibrutinib was approved, the overall response rate for the best salvage therapies was not as encouraging as the initial therapy and, with each relapse, the duration of response shortened. When ibrutinib came along, the overall response rate improved significantly. But again, these patients had relapsed/refractory disease. Researchers have been investigating what would happen if we used such a drug in earlier lines of therapy. Can we get better outcomes? Can we get patients in remission longer, similar to what we have seen with ASCT, but without the ASCT?
There has never been a single modern trial that has demonstrated that transplant improves survival. Transplantation can improve progression-free survival, but not OS. For a disease for which we do not have a cure, if we can keep patients in remission with a good salvage therapy and give them a better quality of life, without subjecting them to an ASCT, then I might choose that. New targeted agents and novel therapies are in clinical development all the time, so the future is bright for patients with this diagnosis. Given the novel salvage therapies in the pipeline, we may be able to no longer recommend ASCT upfront for most patients soon.
Can you share more about the potential benefits of using salvage therapies over ASCT, and particularly any promising newer agents in the salvage therapy setting?
Dr. Barrientos: Recently we had the FDA approval of pirtobrutinib—a noncovalently bound BTK inhibitor—for patients with relapsed/refractory MCL in whom at least 2 lines of systemic therapy had failed, including another BTK inhibitor. In the trial that led to the accelerated approval, pirtobrutinib-treated patients showed an overall response rate of 50% in those who received the drug at 200 mg daily (n = 120); most of the responses were partial responses. The efficacy of other novel drugs are being studied in patients with MCL. For example, ROR1 (receptor tyrosine kinase–like orphan receptor 1) inhibitors and BTK degraders are currently in clinical trials. Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 has been approved for the treatment of adult patients with relapsed or refractory MCL, and this may be an option for some patients.
Multiple novel agents might be able to salvage our patients without subjecting them to an upfront transplant. My hope is to get away from using the intense chemotherapy regimens that might cause myelosuppression, infection risk, or other toxicities, and try to stay with the novel agents. We need to do better for our patients.
Based on the data we now have, until there is a trial that demonstrates a higher OS rate with ASCT, it is hard for me to tell a patient to blindly pursue ASCT without learning more about all the available options. If you have access to a good salvage therapy, especially with all these new promising agents, a patient might be able to stay in remission without having ASCT, which can still have an increased risk of morbidity.
Are there certain patient groups that should never be considered for ASCT?
Dr. Barrientos: Younger patients with the CDKN2A gene—which represents about 22% of patients—and those who have a TP53 mutation should not be considered for a standard transplant because they have a worse outcome independent of the treatment. I would also include complex karyotype patients because of the same nature of the chromosomal aberrations. The more genetic aberrations that a patient has, the more likelihood that any chemotherapy will damage the DNA further and create a more aggressive clone. Instead, I would recommend that young patients in this category participate in a clinical trial with novel agents.
With novel therapies in the pipeline, the availability of CAR T, and now the bispecific antibodies such as blinatumomab and HexAbs coming along, the number of patients who may opt out of ASCT may increase. I have a long discussion with my patients. The more educated they are, the better it is for the patient. At the end of the day, the most important thing for me, with any therapy, is: how does the patient feel? Because if we cannot cure a patient or provide a survival advantage, I do not want to give that patient something that will decrease their quality of life. I would rather keep the patient in some sort of stable disease remission, comfortable, and having a good quality of life. That is my goal for anyone who cannot be cured. Now if it is a curable disease, like a diffuse large cell lymphoma or a Burkitt’s lymphoma, then it is a different story. But for people with MCL, a disease that you cannot cure, or chronic lymphocytic leukemia or follicular lymphoma, then it becomes a different discussion. Undetectable minimal residual disease correlates with longer remission durations, but sometimes trying to achieve that, you can actually do a lot of harm to some patients.
Are there any other conversations you have with your patients in day-to-day practice?
Dr. Barrientos: I always tell my patients to be on top of the age-appropriate cancer screening recommendations. For example, they should see a dermatologist once a year. Men should make sure that their prostate is checked. I recommend women get breast mammograms, Pap smears, and most importantly to avoid smoking—and that includes vaping. It is important to lead a healthy life to minimize the risk of secondary malignancies.
For risk of infections, I recommend to all my patients to be up to date on their vaccinations, such as pneumonia if they are older than 65, Shingrix for prevention of reactivation of varicella or chickenpox, and the flu shot once a year. I also recommend the COVID-19 vaccine even now, as our patients with blood disorders might have a harder time fighting COVID-19 infection. I always tell my patients to please reach out to us because we can discuss the use of antivirals such as Paxlovid (nirmatrelvir/ritonavir), and if they are sick, then they can get remdesivir in the hospital.
I want to touch on health literacy and disparities for a moment. I have some younger patients who are Latin or Black with uncontrolled hypertension or diabetes, even at a young age, and do not realize that I can treat their cancer into remission, but if their blood glucose is in the 500 range, they could die from their diabetes. So talking with patients about their overall health is important. Survivorship issues are important, especially if patients are diagnosed at a young age. We have known for a long time that chemotherapy can create cardiac events, arrhythmias, and heart disease. Therefore, I always tell patients with metabolic syndrome to try to exercise and eat healthy. Patients should get an electrocardiogram and see an internist at least once a year to make sure their cholesterol is well controlled. I think now we are being more cognizant that many complications can happen even 10 years after cancer treatment.