User login
Intranasal zavegepant shows potential as an effective treatment option for acute migraine
Key clinical point: Zavegepant nasal spray was effective in the acute treatment of migraine, with favorable tolerability and safety profiles.
Major finding: At 2 hours post-dose, higher proportions of patients treated with zavegepant vs placebo were free from pain (risk difference 8.8 percentage points; P < .0001) and from their most bothersome symptom (risk difference 8.7 percentage points; P = .0012). Dysgeusia (21% vs 5%), nasal discomfort (4% vs 1%), and nausea (3% vs 1%) were the most common adverse events in the zavegepant vs placebo group.
Study details: Findings are from a phase 3 trial including 1405 patients with ≥1-year history of migraine with or without aura who were randomly assigned to receive zavegepant 10 mg nasal spray (n = 703) or matching placebo (n = 702).
Disclosures: This study was funded by Biohaven Pharmaceuticals. Some authors declared being employees of and holding stocks or stock options in Biohaven Pharmaceuticals. Some others declared ties with various sources, including Biohaven Pharmaceuticals.
Source: Lipton RB et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: A phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22(3):209-217 (Mar). Doi: 10.1016/S1474-4422(22)00517-8
Key clinical point: Zavegepant nasal spray was effective in the acute treatment of migraine, with favorable tolerability and safety profiles.
Major finding: At 2 hours post-dose, higher proportions of patients treated with zavegepant vs placebo were free from pain (risk difference 8.8 percentage points; P < .0001) and from their most bothersome symptom (risk difference 8.7 percentage points; P = .0012). Dysgeusia (21% vs 5%), nasal discomfort (4% vs 1%), and nausea (3% vs 1%) were the most common adverse events in the zavegepant vs placebo group.
Study details: Findings are from a phase 3 trial including 1405 patients with ≥1-year history of migraine with or without aura who were randomly assigned to receive zavegepant 10 mg nasal spray (n = 703) or matching placebo (n = 702).
Disclosures: This study was funded by Biohaven Pharmaceuticals. Some authors declared being employees of and holding stocks or stock options in Biohaven Pharmaceuticals. Some others declared ties with various sources, including Biohaven Pharmaceuticals.
Source: Lipton RB et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: A phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22(3):209-217 (Mar). Doi: 10.1016/S1474-4422(22)00517-8
Key clinical point: Zavegepant nasal spray was effective in the acute treatment of migraine, with favorable tolerability and safety profiles.
Major finding: At 2 hours post-dose, higher proportions of patients treated with zavegepant vs placebo were free from pain (risk difference 8.8 percentage points; P < .0001) and from their most bothersome symptom (risk difference 8.7 percentage points; P = .0012). Dysgeusia (21% vs 5%), nasal discomfort (4% vs 1%), and nausea (3% vs 1%) were the most common adverse events in the zavegepant vs placebo group.
Study details: Findings are from a phase 3 trial including 1405 patients with ≥1-year history of migraine with or without aura who were randomly assigned to receive zavegepant 10 mg nasal spray (n = 703) or matching placebo (n = 702).
Disclosures: This study was funded by Biohaven Pharmaceuticals. Some authors declared being employees of and holding stocks or stock options in Biohaven Pharmaceuticals. Some others declared ties with various sources, including Biohaven Pharmaceuticals.
Source: Lipton RB et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: A phase 3, double-blind, randomised, placebo-controlled multicentre trial. Lancet Neurol. 2023;22(3):209-217 (Mar). Doi: 10.1016/S1474-4422(22)00517-8
Early treatment considerations in RA, April 2023
In evaluating the importance of early aggressive treatment of rheumatoid arthritis (RA), we often look at prognostic factors for severe disease, such as seropositivity, elevated inflammatory markers, and erosions. Eberhard and colleagues looked at the relationship between damage as seen on radiography (including erosions and joint space narrowing) and pain and disability in early RA using an inception cohort with <12 months of symptoms. Over 200 patients in Sweden were followed for 5 years with clinical, laboratory, and radiographic evaluations. Of interest, pain was associated with female sex, tender joint count, and inflammatory markers at various time points but not with radiographic damage. This may reflect that pain is related to current inflammation rather than past joint damage or that pain is related to other factors, such as central sensitization. Radiographic damage was, however, associated with disability and thus remains an important target and outcome measure for assessing treatment effectiveness.
Leon and colleagues also looked at early RA but instead, at the category of difficult-to-treat RA (D2T RA), meaning persistent RA symptoms after a trial of at least two biologic or targeted synthetic disease-modifying antirheumatic drugs. In order to gain better insight in preventing D2T RA, the authors examined its association with potentially modifiable risk factors early in the course of disease. Of the over 600 patients followed in this inception cohort, only about 6% were classified as having D2T RA. The study found that patients who had D2T RA tended to be younger, with a higher tender joint count, higher pain scores, and a higher initial level of disability. The Disease Activity Score (DAS28) itself was higher in patients with D2T RA, but the difference did not reach statistical significance. The small number of patients (35) in the D2T RA group may have affected the findings as well as their wider applicability. However, it is interesting to consider whether the associations may also reflect the impact of noninflammatory factors, as in the previous study, on the classification of D2T RA.
Park and colleagues evaluated the difference in clinical outcomes in postmenopausal patients with RA who underwent menopause at younger than 45 years or 45 years or older. Among over 2800 patients in Korea, those who underwent early menopause were more likely to be seronegative and have high disease activity and worse patient-reported outcome scores in fatigue, sleep, and health-related quality of life despite comparable treatments and prevalence of erosions. The authors suggest this may be related to lower cumulative estrogen exposure; whether this correlates to inflammatory cytokine signatures is not yet known. However, as with the prior studies, central sensitization and noninflammatory pain may also contribute and should be considered in interpreting response to therapy.
Finally, addressing the potential risk for cancer in patients with RA before or during treatment with immunosuppressive medications, Miyata and colleagues reported a study that screened nearly 2200 patients who underwent CT (from neck to pelvis) and compared them with those who underwent routine screening with physical exam plus radiography. The study found that CT screening enhanced cancer detection, with a large number of cancers detected at an earlier stage with CT screening compared with routine screening. The overall number of cancers detected was low (33), and thus routine screening with neck-to-pelvis CT for all patients with RA may not be a cost-effective practice. However, it bears further examination for potentially higher-risk populations or specific cancers.
In evaluating the importance of early aggressive treatment of rheumatoid arthritis (RA), we often look at prognostic factors for severe disease, such as seropositivity, elevated inflammatory markers, and erosions. Eberhard and colleagues looked at the relationship between damage as seen on radiography (including erosions and joint space narrowing) and pain and disability in early RA using an inception cohort with <12 months of symptoms. Over 200 patients in Sweden were followed for 5 years with clinical, laboratory, and radiographic evaluations. Of interest, pain was associated with female sex, tender joint count, and inflammatory markers at various time points but not with radiographic damage. This may reflect that pain is related to current inflammation rather than past joint damage or that pain is related to other factors, such as central sensitization. Radiographic damage was, however, associated with disability and thus remains an important target and outcome measure for assessing treatment effectiveness.
Leon and colleagues also looked at early RA but instead, at the category of difficult-to-treat RA (D2T RA), meaning persistent RA symptoms after a trial of at least two biologic or targeted synthetic disease-modifying antirheumatic drugs. In order to gain better insight in preventing D2T RA, the authors examined its association with potentially modifiable risk factors early in the course of disease. Of the over 600 patients followed in this inception cohort, only about 6% were classified as having D2T RA. The study found that patients who had D2T RA tended to be younger, with a higher tender joint count, higher pain scores, and a higher initial level of disability. The Disease Activity Score (DAS28) itself was higher in patients with D2T RA, but the difference did not reach statistical significance. The small number of patients (35) in the D2T RA group may have affected the findings as well as their wider applicability. However, it is interesting to consider whether the associations may also reflect the impact of noninflammatory factors, as in the previous study, on the classification of D2T RA.
Park and colleagues evaluated the difference in clinical outcomes in postmenopausal patients with RA who underwent menopause at younger than 45 years or 45 years or older. Among over 2800 patients in Korea, those who underwent early menopause were more likely to be seronegative and have high disease activity and worse patient-reported outcome scores in fatigue, sleep, and health-related quality of life despite comparable treatments and prevalence of erosions. The authors suggest this may be related to lower cumulative estrogen exposure; whether this correlates to inflammatory cytokine signatures is not yet known. However, as with the prior studies, central sensitization and noninflammatory pain may also contribute and should be considered in interpreting response to therapy.
Finally, addressing the potential risk for cancer in patients with RA before or during treatment with immunosuppressive medications, Miyata and colleagues reported a study that screened nearly 2200 patients who underwent CT (from neck to pelvis) and compared them with those who underwent routine screening with physical exam plus radiography. The study found that CT screening enhanced cancer detection, with a large number of cancers detected at an earlier stage with CT screening compared with routine screening. The overall number of cancers detected was low (33), and thus routine screening with neck-to-pelvis CT for all patients with RA may not be a cost-effective practice. However, it bears further examination for potentially higher-risk populations or specific cancers.
In evaluating the importance of early aggressive treatment of rheumatoid arthritis (RA), we often look at prognostic factors for severe disease, such as seropositivity, elevated inflammatory markers, and erosions. Eberhard and colleagues looked at the relationship between damage as seen on radiography (including erosions and joint space narrowing) and pain and disability in early RA using an inception cohort with <12 months of symptoms. Over 200 patients in Sweden were followed for 5 years with clinical, laboratory, and radiographic evaluations. Of interest, pain was associated with female sex, tender joint count, and inflammatory markers at various time points but not with radiographic damage. This may reflect that pain is related to current inflammation rather than past joint damage or that pain is related to other factors, such as central sensitization. Radiographic damage was, however, associated with disability and thus remains an important target and outcome measure for assessing treatment effectiveness.
Leon and colleagues also looked at early RA but instead, at the category of difficult-to-treat RA (D2T RA), meaning persistent RA symptoms after a trial of at least two biologic or targeted synthetic disease-modifying antirheumatic drugs. In order to gain better insight in preventing D2T RA, the authors examined its association with potentially modifiable risk factors early in the course of disease. Of the over 600 patients followed in this inception cohort, only about 6% were classified as having D2T RA. The study found that patients who had D2T RA tended to be younger, with a higher tender joint count, higher pain scores, and a higher initial level of disability. The Disease Activity Score (DAS28) itself was higher in patients with D2T RA, but the difference did not reach statistical significance. The small number of patients (35) in the D2T RA group may have affected the findings as well as their wider applicability. However, it is interesting to consider whether the associations may also reflect the impact of noninflammatory factors, as in the previous study, on the classification of D2T RA.
Park and colleagues evaluated the difference in clinical outcomes in postmenopausal patients with RA who underwent menopause at younger than 45 years or 45 years or older. Among over 2800 patients in Korea, those who underwent early menopause were more likely to be seronegative and have high disease activity and worse patient-reported outcome scores in fatigue, sleep, and health-related quality of life despite comparable treatments and prevalence of erosions. The authors suggest this may be related to lower cumulative estrogen exposure; whether this correlates to inflammatory cytokine signatures is not yet known. However, as with the prior studies, central sensitization and noninflammatory pain may also contribute and should be considered in interpreting response to therapy.
Finally, addressing the potential risk for cancer in patients with RA before or during treatment with immunosuppressive medications, Miyata and colleagues reported a study that screened nearly 2200 patients who underwent CT (from neck to pelvis) and compared them with those who underwent routine screening with physical exam plus radiography. The study found that CT screening enhanced cancer detection, with a large number of cancers detected at an earlier stage with CT screening compared with routine screening. The overall number of cancers detected was low (33), and thus routine screening with neck-to-pelvis CT for all patients with RA may not be a cost-effective practice. However, it bears further examination for potentially higher-risk populations or specific cancers.
Commentary: Updates on the Treatment of Mantle Cell Lymphoma, April 2023
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) that is clinically heterogeneous, ranging from indolent to aggressive in nature. As with other subtypes of NHL, the treatment landscape is rapidly evolving.
Chemoimmunotherapy remains the standard first-line therapy for younger, fit patients. Although multiple induction regimens are used in this setting, it is typical to use a cytarabine-containing approach. Recently, the long-term analysis of the MCL Younger trial continued to demonstrate improved outcomes with this strategy.1 This phase 3 study included 497 patients aged ≥ 18 to < 66 years with previously untreated MCL who were randomly assigned to R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; n = 249) or R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; n = 248). After a median follow-up of 10.6 years, the R-DHAP vs R-CHOP arm continued to have a significantly longer time to treatment failure (hazard ratio [HR] 0.59; P = .038) and overall survival (Mantle Cell Lymphoma International Prognostic Index + Ki-67–adjusted HR 0.60; P = .0066).
Following chemoimmunotherapy, treatment for this patient population typically consists of consolidation with autologous stem cell transplantation (ASCT) and maintenance rituximab.2 Recently, the role of ASCT has been called into question.3 Preliminary data from the phase 3 TRIANGLE study demonstrated improvement in outcomes when the Bruton tyrosine kinase (BTK) inhibitor ibrutinib was added to chemoimmunotherapy, regardless of whether patients received ASCT.4 Additional studies evaluating the role of transplantation, particularly among patients who are minimal residual disease negative after chemoimmunotherapy, are ongoing (NCT03267433).
Options continue to expand in the relapsed/refractory setting. The chimeric antigen receptor (CAR) T-cell therapy, brexucabtagene autoleucel (brexu-cel), was approved by the US Food and Drug Administration for relapsed/refractory MCL on the basis of the results of the ZUMA-2 study.5 Recently, a multicenter, retrospective study demonstrated promising efficacy in the real world as well (Wang et al). This study was performed across 16 medical centers and included 189 patients with relapsed/refractory MCL who underwent leukapheresis for commercial manufacturing of brexu-cel, of which 168 received brexu-cel infusion. Of all patients receiving leukapheresis, 149 (79%) would not have met the eligibility criteria for ZUMA-2. At a median follow-up of 14.3 months after infusion, the best overall and complete response rates were 90% and 82%, respectively. The 6- and 12-month progression-free survival (PFS) rates were 69% (95% CI 61%-75%) and 59% (95% CI 51%-66%), respectively. This approach, however, was associated with significant toxicity, with a nonrelapse mortality rate of 9.1% at 1 year, primarily because of infections. The grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 8% and 32%, respectively. Despite risks, this study confirms the role of CAR T-cell therapy for patients with relapsed/refractory MCL.
Other options in the relapsed setting include BTK and anti-apoptotic protein B-cell lymphoma (BCL-2) inhibitors. Although venetoclax, a BCL-2 inhibitor, has demonstrated activity in MCL in early-phase clinical trials, the role of this drug in clinical practice remains unclear.6,7 A recent multicenter, retrospective study evaluated the use of venetoclax in 81 adult patients with relapsed/refractory MCL, most of whom were heavily pretreated (median of three prior treatments) and had high-risk features, including high Ki-67 and TP53 alterations, who received venetoclax without (n = 50) or with (n = 31) other agents (Sawalha et al). In this study, venetoclax resulted in a good overall response rate (ORR) but short PFS. At a median follow-up of 16.4 months, patients had a median PFS and overall survival of 3.7 months (95% CI 2.3-5.6) and 12.5 months (95% CI 6.2-28.2), respectively, and an ORR of 40%. Studies of venetoclax in earlier lines of therapy and in combination with other agents are ongoing. There may also be a role for this treatment as a bridge to more definitive therapies, including CAR T-cell therapy or allogeneic stem cell transplantation. Other studies that are evaluating the role of bispecific antibodies and antibody drug conjugates are also underway, suggesting the potential for additional options in this patient population.
Additional References
1. Hermine O, Jiang L, Walewski J, et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41:479-484. doi: 10.1200/JCO.22.01780
2. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-1260. doi: 10.1056/NEJMoa1701769
3. Martin P, Cohen JB, Wang M, et al. Treatment outcomes and roles of transplantation and maintenance rituximab in patients with previously untreated mantle cell lymphoma: Results from large real-world cohorts. J Clin Oncol. 2023;41:541-554. doi: 10.1200/JCO.21.02698
4. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized Triangle Trial by the European MCL Network. Blood. 2022;140(Suppl 1):1-3. doi: 10.1182/blood-2022-163018
5. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342. doi: 10.1056/NEJMoa1914347
6. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826-833. doi: 10.1200/JCO.2016.70.4320
7. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-1223. doi: 10.1056/NEJMoa1715519
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) that is clinically heterogeneous, ranging from indolent to aggressive in nature. As with other subtypes of NHL, the treatment landscape is rapidly evolving.
Chemoimmunotherapy remains the standard first-line therapy for younger, fit patients. Although multiple induction regimens are used in this setting, it is typical to use a cytarabine-containing approach. Recently, the long-term analysis of the MCL Younger trial continued to demonstrate improved outcomes with this strategy.1 This phase 3 study included 497 patients aged ≥ 18 to < 66 years with previously untreated MCL who were randomly assigned to R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; n = 249) or R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; n = 248). After a median follow-up of 10.6 years, the R-DHAP vs R-CHOP arm continued to have a significantly longer time to treatment failure (hazard ratio [HR] 0.59; P = .038) and overall survival (Mantle Cell Lymphoma International Prognostic Index + Ki-67–adjusted HR 0.60; P = .0066).
Following chemoimmunotherapy, treatment for this patient population typically consists of consolidation with autologous stem cell transplantation (ASCT) and maintenance rituximab.2 Recently, the role of ASCT has been called into question.3 Preliminary data from the phase 3 TRIANGLE study demonstrated improvement in outcomes when the Bruton tyrosine kinase (BTK) inhibitor ibrutinib was added to chemoimmunotherapy, regardless of whether patients received ASCT.4 Additional studies evaluating the role of transplantation, particularly among patients who are minimal residual disease negative after chemoimmunotherapy, are ongoing (NCT03267433).
Options continue to expand in the relapsed/refractory setting. The chimeric antigen receptor (CAR) T-cell therapy, brexucabtagene autoleucel (brexu-cel), was approved by the US Food and Drug Administration for relapsed/refractory MCL on the basis of the results of the ZUMA-2 study.5 Recently, a multicenter, retrospective study demonstrated promising efficacy in the real world as well (Wang et al). This study was performed across 16 medical centers and included 189 patients with relapsed/refractory MCL who underwent leukapheresis for commercial manufacturing of brexu-cel, of which 168 received brexu-cel infusion. Of all patients receiving leukapheresis, 149 (79%) would not have met the eligibility criteria for ZUMA-2. At a median follow-up of 14.3 months after infusion, the best overall and complete response rates were 90% and 82%, respectively. The 6- and 12-month progression-free survival (PFS) rates were 69% (95% CI 61%-75%) and 59% (95% CI 51%-66%), respectively. This approach, however, was associated with significant toxicity, with a nonrelapse mortality rate of 9.1% at 1 year, primarily because of infections. The grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 8% and 32%, respectively. Despite risks, this study confirms the role of CAR T-cell therapy for patients with relapsed/refractory MCL.
Other options in the relapsed setting include BTK and anti-apoptotic protein B-cell lymphoma (BCL-2) inhibitors. Although venetoclax, a BCL-2 inhibitor, has demonstrated activity in MCL in early-phase clinical trials, the role of this drug in clinical practice remains unclear.6,7 A recent multicenter, retrospective study evaluated the use of venetoclax in 81 adult patients with relapsed/refractory MCL, most of whom were heavily pretreated (median of three prior treatments) and had high-risk features, including high Ki-67 and TP53 alterations, who received venetoclax without (n = 50) or with (n = 31) other agents (Sawalha et al). In this study, venetoclax resulted in a good overall response rate (ORR) but short PFS. At a median follow-up of 16.4 months, patients had a median PFS and overall survival of 3.7 months (95% CI 2.3-5.6) and 12.5 months (95% CI 6.2-28.2), respectively, and an ORR of 40%. Studies of venetoclax in earlier lines of therapy and in combination with other agents are ongoing. There may also be a role for this treatment as a bridge to more definitive therapies, including CAR T-cell therapy or allogeneic stem cell transplantation. Other studies that are evaluating the role of bispecific antibodies and antibody drug conjugates are also underway, suggesting the potential for additional options in this patient population.
Additional References
1. Hermine O, Jiang L, Walewski J, et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41:479-484. doi: 10.1200/JCO.22.01780
2. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-1260. doi: 10.1056/NEJMoa1701769
3. Martin P, Cohen JB, Wang M, et al. Treatment outcomes and roles of transplantation and maintenance rituximab in patients with previously untreated mantle cell lymphoma: Results from large real-world cohorts. J Clin Oncol. 2023;41:541-554. doi: 10.1200/JCO.21.02698
4. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized Triangle Trial by the European MCL Network. Blood. 2022;140(Suppl 1):1-3. doi: 10.1182/blood-2022-163018
5. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342. doi: 10.1056/NEJMoa1914347
6. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826-833. doi: 10.1200/JCO.2016.70.4320
7. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-1223. doi: 10.1056/NEJMoa1715519
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) that is clinically heterogeneous, ranging from indolent to aggressive in nature. As with other subtypes of NHL, the treatment landscape is rapidly evolving.
Chemoimmunotherapy remains the standard first-line therapy for younger, fit patients. Although multiple induction regimens are used in this setting, it is typical to use a cytarabine-containing approach. Recently, the long-term analysis of the MCL Younger trial continued to demonstrate improved outcomes with this strategy.1 This phase 3 study included 497 patients aged ≥ 18 to < 66 years with previously untreated MCL who were randomly assigned to R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; n = 249) or R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; n = 248). After a median follow-up of 10.6 years, the R-DHAP vs R-CHOP arm continued to have a significantly longer time to treatment failure (hazard ratio [HR] 0.59; P = .038) and overall survival (Mantle Cell Lymphoma International Prognostic Index + Ki-67–adjusted HR 0.60; P = .0066).
Following chemoimmunotherapy, treatment for this patient population typically consists of consolidation with autologous stem cell transplantation (ASCT) and maintenance rituximab.2 Recently, the role of ASCT has been called into question.3 Preliminary data from the phase 3 TRIANGLE study demonstrated improvement in outcomes when the Bruton tyrosine kinase (BTK) inhibitor ibrutinib was added to chemoimmunotherapy, regardless of whether patients received ASCT.4 Additional studies evaluating the role of transplantation, particularly among patients who are minimal residual disease negative after chemoimmunotherapy, are ongoing (NCT03267433).
Options continue to expand in the relapsed/refractory setting. The chimeric antigen receptor (CAR) T-cell therapy, brexucabtagene autoleucel (brexu-cel), was approved by the US Food and Drug Administration for relapsed/refractory MCL on the basis of the results of the ZUMA-2 study.5 Recently, a multicenter, retrospective study demonstrated promising efficacy in the real world as well (Wang et al). This study was performed across 16 medical centers and included 189 patients with relapsed/refractory MCL who underwent leukapheresis for commercial manufacturing of brexu-cel, of which 168 received brexu-cel infusion. Of all patients receiving leukapheresis, 149 (79%) would not have met the eligibility criteria for ZUMA-2. At a median follow-up of 14.3 months after infusion, the best overall and complete response rates were 90% and 82%, respectively. The 6- and 12-month progression-free survival (PFS) rates were 69% (95% CI 61%-75%) and 59% (95% CI 51%-66%), respectively. This approach, however, was associated with significant toxicity, with a nonrelapse mortality rate of 9.1% at 1 year, primarily because of infections. The grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 8% and 32%, respectively. Despite risks, this study confirms the role of CAR T-cell therapy for patients with relapsed/refractory MCL.
Other options in the relapsed setting include BTK and anti-apoptotic protein B-cell lymphoma (BCL-2) inhibitors. Although venetoclax, a BCL-2 inhibitor, has demonstrated activity in MCL in early-phase clinical trials, the role of this drug in clinical practice remains unclear.6,7 A recent multicenter, retrospective study evaluated the use of venetoclax in 81 adult patients with relapsed/refractory MCL, most of whom were heavily pretreated (median of three prior treatments) and had high-risk features, including high Ki-67 and TP53 alterations, who received venetoclax without (n = 50) or with (n = 31) other agents (Sawalha et al). In this study, venetoclax resulted in a good overall response rate (ORR) but short PFS. At a median follow-up of 16.4 months, patients had a median PFS and overall survival of 3.7 months (95% CI 2.3-5.6) and 12.5 months (95% CI 6.2-28.2), respectively, and an ORR of 40%. Studies of venetoclax in earlier lines of therapy and in combination with other agents are ongoing. There may also be a role for this treatment as a bridge to more definitive therapies, including CAR T-cell therapy or allogeneic stem cell transplantation. Other studies that are evaluating the role of bispecific antibodies and antibody drug conjugates are also underway, suggesting the potential for additional options in this patient population.
Additional References
1. Hermine O, Jiang L, Walewski J, et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41:479-484. doi: 10.1200/JCO.22.01780
2. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-1260. doi: 10.1056/NEJMoa1701769
3. Martin P, Cohen JB, Wang M, et al. Treatment outcomes and roles of transplantation and maintenance rituximab in patients with previously untreated mantle cell lymphoma: Results from large real-world cohorts. J Clin Oncol. 2023;41:541-554. doi: 10.1200/JCO.21.02698
4. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized Triangle Trial by the European MCL Network. Blood. 2022;140(Suppl 1):1-3. doi: 10.1182/blood-2022-163018
5. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342. doi: 10.1056/NEJMoa1914347
6. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826-833. doi: 10.1200/JCO.2016.70.4320
7. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-1223. doi: 10.1056/NEJMoa1715519
Optimal Use of CDK4/6 Inhibitors in Breast Cancer
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors have become integral to the treatment of HR+/HER2- breast cancer. Approved in 2015 for use in the metastatic setting and most recently in the adjuvant setting, CDK4/6 inhibitors have revolutionized treatment in both endocrine-sensitive and endocrine-resistant settings and in pre- and postmenopausal women.
But many questions remain regarding the optimal use of these medications in clinical practice.
In this ReCAP, Dr Virginia Kaklamani from the University of Texas Health Sciences Center in San Antonio, Texas, and Dr Harold Burstein from Dana-Farber Cancer Institute, Boston, Massachusetts, begin their discussion by examining the potential role of adjuvant CDK4/6 inhibitor therapy in early, high-risk breast cancer.
They discuss the three main studies that looked at the role of adjuvant CDK4/6 inhibitors, including the PALLAS and PENELOPE-B trials, in which palbociclib showed no benefit in invasive disease-free survival. In contrast, in the monarchE trial, abemaciclib showed a robust benefit in preventing recurrence, which was sustained after longer follow-up, as reported at the San Antonio Breast Cancer Symposium 2022.
Turning to the metastatic setting, the panelists discuss the varied side effect profiles of the three approved CDK4/6 inhibitors, palbociclib, ribociclib, and abemaciclib. They also discuss current research into the continuation of these agents beyond progression and whether sequencing of CDK4/6 inhibitors may provide benefit.
--
Virginia Kaklamani, MD, Professor of Medicine, Division of Hematology/Oncology, University of Texas Health Sciences Center; Leader, Breast Oncology Program, University of Texas Health MD Anderson Cancer Center, San Antonio, Texas
Virginia Kaklamani, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Gilead; Menarini; Pfizer; Novartis; Lilly; AstraZeneca; Genentech; Daichii; Seagen
Harold J. Burstein, MD, PhD, Professor, Department of Medicine, Harvard Medical School; Medical Oncologist, Dana-Farber Cancer Institute, Boston, Massachusetts
Harold J. Burstein, MD, PhD, has disclosed no relevant financial relationships.
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors have become integral to the treatment of HR+/HER2- breast cancer. Approved in 2015 for use in the metastatic setting and most recently in the adjuvant setting, CDK4/6 inhibitors have revolutionized treatment in both endocrine-sensitive and endocrine-resistant settings and in pre- and postmenopausal women.
But many questions remain regarding the optimal use of these medications in clinical practice.
In this ReCAP, Dr Virginia Kaklamani from the University of Texas Health Sciences Center in San Antonio, Texas, and Dr Harold Burstein from Dana-Farber Cancer Institute, Boston, Massachusetts, begin their discussion by examining the potential role of adjuvant CDK4/6 inhibitor therapy in early, high-risk breast cancer.
They discuss the three main studies that looked at the role of adjuvant CDK4/6 inhibitors, including the PALLAS and PENELOPE-B trials, in which palbociclib showed no benefit in invasive disease-free survival. In contrast, in the monarchE trial, abemaciclib showed a robust benefit in preventing recurrence, which was sustained after longer follow-up, as reported at the San Antonio Breast Cancer Symposium 2022.
Turning to the metastatic setting, the panelists discuss the varied side effect profiles of the three approved CDK4/6 inhibitors, palbociclib, ribociclib, and abemaciclib. They also discuss current research into the continuation of these agents beyond progression and whether sequencing of CDK4/6 inhibitors may provide benefit.
--
Virginia Kaklamani, MD, Professor of Medicine, Division of Hematology/Oncology, University of Texas Health Sciences Center; Leader, Breast Oncology Program, University of Texas Health MD Anderson Cancer Center, San Antonio, Texas
Virginia Kaklamani, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Gilead; Menarini; Pfizer; Novartis; Lilly; AstraZeneca; Genentech; Daichii; Seagen
Harold J. Burstein, MD, PhD, Professor, Department of Medicine, Harvard Medical School; Medical Oncologist, Dana-Farber Cancer Institute, Boston, Massachusetts
Harold J. Burstein, MD, PhD, has disclosed no relevant financial relationships.
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors have become integral to the treatment of HR+/HER2- breast cancer. Approved in 2015 for use in the metastatic setting and most recently in the adjuvant setting, CDK4/6 inhibitors have revolutionized treatment in both endocrine-sensitive and endocrine-resistant settings and in pre- and postmenopausal women.
But many questions remain regarding the optimal use of these medications in clinical practice.
In this ReCAP, Dr Virginia Kaklamani from the University of Texas Health Sciences Center in San Antonio, Texas, and Dr Harold Burstein from Dana-Farber Cancer Institute, Boston, Massachusetts, begin their discussion by examining the potential role of adjuvant CDK4/6 inhibitor therapy in early, high-risk breast cancer.
They discuss the three main studies that looked at the role of adjuvant CDK4/6 inhibitors, including the PALLAS and PENELOPE-B trials, in which palbociclib showed no benefit in invasive disease-free survival. In contrast, in the monarchE trial, abemaciclib showed a robust benefit in preventing recurrence, which was sustained after longer follow-up, as reported at the San Antonio Breast Cancer Symposium 2022.
Turning to the metastatic setting, the panelists discuss the varied side effect profiles of the three approved CDK4/6 inhibitors, palbociclib, ribociclib, and abemaciclib. They also discuss current research into the continuation of these agents beyond progression and whether sequencing of CDK4/6 inhibitors may provide benefit.
--
Virginia Kaklamani, MD, Professor of Medicine, Division of Hematology/Oncology, University of Texas Health Sciences Center; Leader, Breast Oncology Program, University of Texas Health MD Anderson Cancer Center, San Antonio, Texas
Virginia Kaklamani, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Gilead; Menarini; Pfizer; Novartis; Lilly; AstraZeneca; Genentech; Daichii; Seagen
Harold J. Burstein, MD, PhD, Professor, Department of Medicine, Harvard Medical School; Medical Oncologist, Dana-Farber Cancer Institute, Boston, Massachusetts
Harold J. Burstein, MD, PhD, has disclosed no relevant financial relationships.

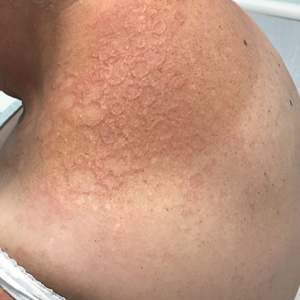
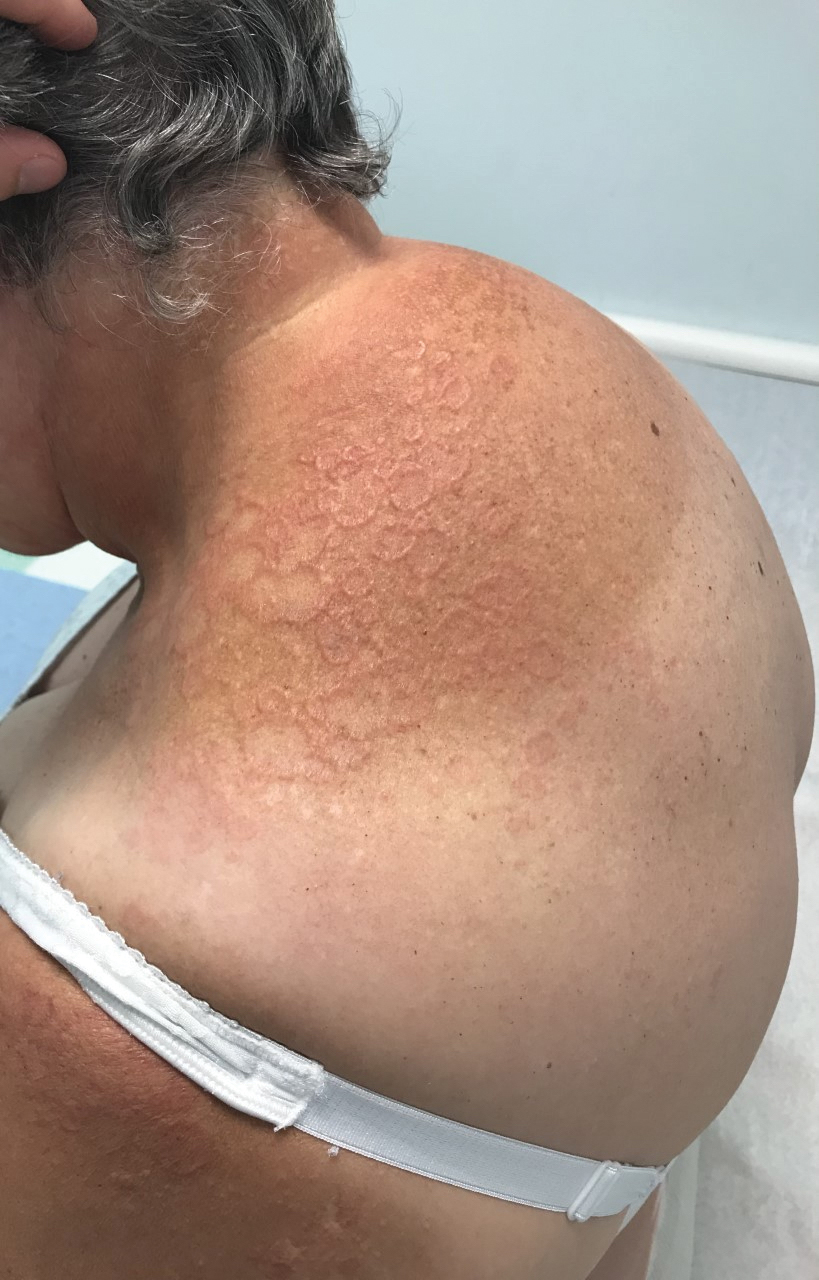
Annular Erythematous Plaques With Central Hypopigmentation on Sun-Exposed Skin
A biopsy showed a markedly elastotic dermis consisting of a palisading granulomatous inflammatory infiltrate and numerous multinucleated histiocytes (Figure). These histopathologic findings along with the clinical presentation confirmed a diagnosis of annular elastolytic granuloma (AEG). Treatment consisting of 3 months of oral minocycline, 2 months of oral doxycycline, and clobetasol ointment all failed. At that point, oral hydroxychloroquine was recommended. Our patient was lost to follow-up by dermatology, then subsequently was placed on hydroxychloroquine by rheumatology to treat both the osteoarthritis and AEG. A follow-up appointment with dermatology was planned for 3 months to monitor hydroxychloroquine treatment and monitor treatment progress; however, she did not follow-up or seek further treatment.
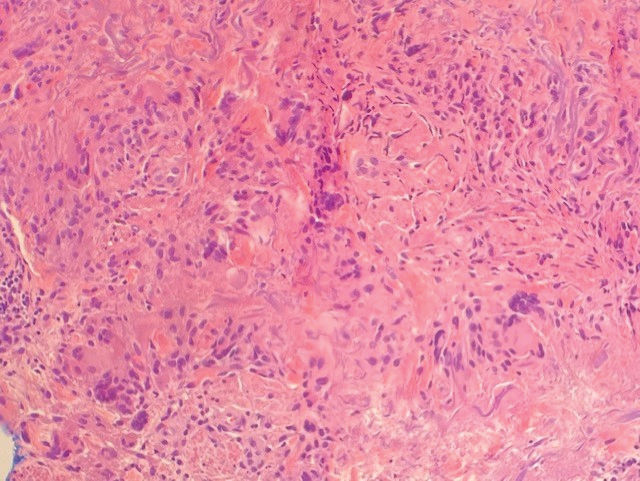
Annular elastolytic granuloma clinically is similar to granuloma annulare (GA), with both presenting as annular plaques surrounded by an elevated border.1 Although AEG clinically is distinct with hypopigmented atrophied plaque centers,2 a biopsy is required to confirm the lack of elastic tissue in zones of atrophy and the presence of multinucleated histiocytes.1,3 Lesions most commonly are seen clinically on sun-exposed areas in middle-aged White women; however, they rarely have been seen on frequently covered skin.4 Our case illustrates the striking photodistribution of AEG, especially on the posterior neck area. The clinical diagnoses of AEG, annular elastolytic giant cell granuloma, and GA in sun-exposed areas are synonymous and can be used interchangeably.5,6
Pathologies considered in the diagnosis of AEG include but are not limited to tinea corporis, annular lichen planus, erythema annulare centrifugum, and necrobiosis lipoidica. Scaling typically is absent in AEG, while tinea corporis presents with hyphae within the stratum corneum of the plaques.7 Papules along the periphery of annular lesions are more typical of annular lichen planus than AEG, and they tend to have a more purple hue.8 Erythema annulare centrifugum has annular erythematous plaques similar to those found in AEG but differs with scaling on the inner margins of these plaques. Histopathology presenting with a lymphocytic infiltrate surrounding vasculature and no indication of elastolytic degradation would further indicate a diagnosis of erythema annulare centrifugum.9 Histopathology showing necrobiosis, lipid depositions, and vascular wall thickenings is indicative of necrobiosis lipoidica.10
Similar to GA,11 the cause of AEG is idiopathic.2 Annular elastolytic granuloma and GA differ in the fact that elastin degradation is characteristic of AEG compared to collagen degradation in GA. It is suspected that elastin degradation in AEG patients is caused by an immune response triggering phagocytosis of elastin by multinucleated histiocytes.2 Actinic damage also is considered a possible cause of elastin fiber degradation in AEG.12 Granuloma annulare can be ruled out and the diagnosis of AEG confirmed with the absence of elastin fibers and mucin on pathology.13
Although there is no established first-line treatment of AEG, successful treatment has been achieved with antimalarial drugs paired with topical steroids.14 Treatment recommendations for AEG include minocycline, chloroquine, hydroxychloroquine, tranilast, and oral retinoids, as well as oral and topical steroids. In clinical cases where AEG occurs in the setting of a chronic disease such as diabetes mellitus, vascular occlusion, arthritis, or hypertension, treatment of underlying disease has been shown to resolve AEG symptoms.14
Although light therapy is not common for AEG, UV light radiation has demonstrated success in treating AEG.15,16 One study showed complete clearance of granulomatous papules after narrowband UVB treatment.15 Another study showed that 2 patients treated with psoralen plus UVA therapy reached complete clearance of AEG lasting at least 3 months after treatment.16
1. Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41:462-468. doi:10.1111/cup.12292 2. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132 3. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282. doi:10.1111/j.0309-0167.2004.01755.x 4. Revenga F, Rovira I, Pimentel J, et al. Annular elastolytic giant cell granuloma—actinic granuloma? Clin Exp Dermatol. 1996;21:51-53. 5. Hawryluk EB, Izikson L, English JC 3rd. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171-181. doi:10.2165/11530080-000000000-00000 6. Berliner JG, Haemel A, LeBoit PE, et al. The sarcoidal variant of annular elastolytic granuloma. J Cutan Pathol. 2013;40:918-920. doi:10.1111/cup.12237 7. Pflederer RT, Ahmed S, Tonkovic-Capin V, et al. Annular polycyclic plaques on the chest and upper back [published online April 24, 2018]. JAAD Case Rep. 2018;4:405-407. doi:10.1016/j.jdcr.2017.07.022 8. Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291. 9. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462. doi:10.1097/00000372-200312000-00001 10. Dowling GB, Jones EW. Atypical (annular) necrobiosis lipoidica of the face and scalp. a report of the clinical and histological features of 7 cases. Dermatologica. 1967;135:11-26. doi:10.1159/000254156 11. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015 .03.055 12. O’Brien JP, Regan W. Actinically degenerate elastic tissue is the likely antigenic basis of actinic granuloma of the skin and of temporal arteritis [published correction appears in J Am Acad Dermatol. 2000; 42(1 pt 1):148]. J Am Acad Dermatol. 1999;40(2 pt 1):214-222. doi:10.1016/s0190-9622(99)70191-x 13. Rencic A, Nousari CH. Other rheumatologic diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. 2nd ed. Elsevier Limited; 2008:600-601. 14. Burlando M, Herzum A, Cozzani E, et al. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? case report and review of the literature. Dermatol Ther. 2021;34:E14705. doi:10.1111/dth.14705 15. Takata T, Ikeda M, Kodama H, et al. Regression of papular elastolytic giant cell granuloma using narrow-band UVB irradiation. Dermatology. 2006;212:77-79. doi:10.1159/000089028 16. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralenultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266. doi:10.1111/j.1600-0781.2012.00680.x
A biopsy showed a markedly elastotic dermis consisting of a palisading granulomatous inflammatory infiltrate and numerous multinucleated histiocytes (Figure). These histopathologic findings along with the clinical presentation confirmed a diagnosis of annular elastolytic granuloma (AEG). Treatment consisting of 3 months of oral minocycline, 2 months of oral doxycycline, and clobetasol ointment all failed. At that point, oral hydroxychloroquine was recommended. Our patient was lost to follow-up by dermatology, then subsequently was placed on hydroxychloroquine by rheumatology to treat both the osteoarthritis and AEG. A follow-up appointment with dermatology was planned for 3 months to monitor hydroxychloroquine treatment and monitor treatment progress; however, she did not follow-up or seek further treatment.

Annular elastolytic granuloma clinically is similar to granuloma annulare (GA), with both presenting as annular plaques surrounded by an elevated border.1 Although AEG clinically is distinct with hypopigmented atrophied plaque centers,2 a biopsy is required to confirm the lack of elastic tissue in zones of atrophy and the presence of multinucleated histiocytes.1,3 Lesions most commonly are seen clinically on sun-exposed areas in middle-aged White women; however, they rarely have been seen on frequently covered skin.4 Our case illustrates the striking photodistribution of AEG, especially on the posterior neck area. The clinical diagnoses of AEG, annular elastolytic giant cell granuloma, and GA in sun-exposed areas are synonymous and can be used interchangeably.5,6
Pathologies considered in the diagnosis of AEG include but are not limited to tinea corporis, annular lichen planus, erythema annulare centrifugum, and necrobiosis lipoidica. Scaling typically is absent in AEG, while tinea corporis presents with hyphae within the stratum corneum of the plaques.7 Papules along the periphery of annular lesions are more typical of annular lichen planus than AEG, and they tend to have a more purple hue.8 Erythema annulare centrifugum has annular erythematous plaques similar to those found in AEG but differs with scaling on the inner margins of these plaques. Histopathology presenting with a lymphocytic infiltrate surrounding vasculature and no indication of elastolytic degradation would further indicate a diagnosis of erythema annulare centrifugum.9 Histopathology showing necrobiosis, lipid depositions, and vascular wall thickenings is indicative of necrobiosis lipoidica.10
Similar to GA,11 the cause of AEG is idiopathic.2 Annular elastolytic granuloma and GA differ in the fact that elastin degradation is characteristic of AEG compared to collagen degradation in GA. It is suspected that elastin degradation in AEG patients is caused by an immune response triggering phagocytosis of elastin by multinucleated histiocytes.2 Actinic damage also is considered a possible cause of elastin fiber degradation in AEG.12 Granuloma annulare can be ruled out and the diagnosis of AEG confirmed with the absence of elastin fibers and mucin on pathology.13
Although there is no established first-line treatment of AEG, successful treatment has been achieved with antimalarial drugs paired with topical steroids.14 Treatment recommendations for AEG include minocycline, chloroquine, hydroxychloroquine, tranilast, and oral retinoids, as well as oral and topical steroids. In clinical cases where AEG occurs in the setting of a chronic disease such as diabetes mellitus, vascular occlusion, arthritis, or hypertension, treatment of underlying disease has been shown to resolve AEG symptoms.14
Although light therapy is not common for AEG, UV light radiation has demonstrated success in treating AEG.15,16 One study showed complete clearance of granulomatous papules after narrowband UVB treatment.15 Another study showed that 2 patients treated with psoralen plus UVA therapy reached complete clearance of AEG lasting at least 3 months after treatment.16
A biopsy showed a markedly elastotic dermis consisting of a palisading granulomatous inflammatory infiltrate and numerous multinucleated histiocytes (Figure). These histopathologic findings along with the clinical presentation confirmed a diagnosis of annular elastolytic granuloma (AEG). Treatment consisting of 3 months of oral minocycline, 2 months of oral doxycycline, and clobetasol ointment all failed. At that point, oral hydroxychloroquine was recommended. Our patient was lost to follow-up by dermatology, then subsequently was placed on hydroxychloroquine by rheumatology to treat both the osteoarthritis and AEG. A follow-up appointment with dermatology was planned for 3 months to monitor hydroxychloroquine treatment and monitor treatment progress; however, she did not follow-up or seek further treatment.

Annular elastolytic granuloma clinically is similar to granuloma annulare (GA), with both presenting as annular plaques surrounded by an elevated border.1 Although AEG clinically is distinct with hypopigmented atrophied plaque centers,2 a biopsy is required to confirm the lack of elastic tissue in zones of atrophy and the presence of multinucleated histiocytes.1,3 Lesions most commonly are seen clinically on sun-exposed areas in middle-aged White women; however, they rarely have been seen on frequently covered skin.4 Our case illustrates the striking photodistribution of AEG, especially on the posterior neck area. The clinical diagnoses of AEG, annular elastolytic giant cell granuloma, and GA in sun-exposed areas are synonymous and can be used interchangeably.5,6
Pathologies considered in the diagnosis of AEG include but are not limited to tinea corporis, annular lichen planus, erythema annulare centrifugum, and necrobiosis lipoidica. Scaling typically is absent in AEG, while tinea corporis presents with hyphae within the stratum corneum of the plaques.7 Papules along the periphery of annular lesions are more typical of annular lichen planus than AEG, and they tend to have a more purple hue.8 Erythema annulare centrifugum has annular erythematous plaques similar to those found in AEG but differs with scaling on the inner margins of these plaques. Histopathology presenting with a lymphocytic infiltrate surrounding vasculature and no indication of elastolytic degradation would further indicate a diagnosis of erythema annulare centrifugum.9 Histopathology showing necrobiosis, lipid depositions, and vascular wall thickenings is indicative of necrobiosis lipoidica.10
Similar to GA,11 the cause of AEG is idiopathic.2 Annular elastolytic granuloma and GA differ in the fact that elastin degradation is characteristic of AEG compared to collagen degradation in GA. It is suspected that elastin degradation in AEG patients is caused by an immune response triggering phagocytosis of elastin by multinucleated histiocytes.2 Actinic damage also is considered a possible cause of elastin fiber degradation in AEG.12 Granuloma annulare can be ruled out and the diagnosis of AEG confirmed with the absence of elastin fibers and mucin on pathology.13
Although there is no established first-line treatment of AEG, successful treatment has been achieved with antimalarial drugs paired with topical steroids.14 Treatment recommendations for AEG include minocycline, chloroquine, hydroxychloroquine, tranilast, and oral retinoids, as well as oral and topical steroids. In clinical cases where AEG occurs in the setting of a chronic disease such as diabetes mellitus, vascular occlusion, arthritis, or hypertension, treatment of underlying disease has been shown to resolve AEG symptoms.14
Although light therapy is not common for AEG, UV light radiation has demonstrated success in treating AEG.15,16 One study showed complete clearance of granulomatous papules after narrowband UVB treatment.15 Another study showed that 2 patients treated with psoralen plus UVA therapy reached complete clearance of AEG lasting at least 3 months after treatment.16
1. Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41:462-468. doi:10.1111/cup.12292 2. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132 3. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282. doi:10.1111/j.0309-0167.2004.01755.x 4. Revenga F, Rovira I, Pimentel J, et al. Annular elastolytic giant cell granuloma—actinic granuloma? Clin Exp Dermatol. 1996;21:51-53. 5. Hawryluk EB, Izikson L, English JC 3rd. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171-181. doi:10.2165/11530080-000000000-00000 6. Berliner JG, Haemel A, LeBoit PE, et al. The sarcoidal variant of annular elastolytic granuloma. J Cutan Pathol. 2013;40:918-920. doi:10.1111/cup.12237 7. Pflederer RT, Ahmed S, Tonkovic-Capin V, et al. Annular polycyclic plaques on the chest and upper back [published online April 24, 2018]. JAAD Case Rep. 2018;4:405-407. doi:10.1016/j.jdcr.2017.07.022 8. Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291. 9. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462. doi:10.1097/00000372-200312000-00001 10. Dowling GB, Jones EW. Atypical (annular) necrobiosis lipoidica of the face and scalp. a report of the clinical and histological features of 7 cases. Dermatologica. 1967;135:11-26. doi:10.1159/000254156 11. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015 .03.055 12. O’Brien JP, Regan W. Actinically degenerate elastic tissue is the likely antigenic basis of actinic granuloma of the skin and of temporal arteritis [published correction appears in J Am Acad Dermatol. 2000; 42(1 pt 1):148]. J Am Acad Dermatol. 1999;40(2 pt 1):214-222. doi:10.1016/s0190-9622(99)70191-x 13. Rencic A, Nousari CH. Other rheumatologic diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. 2nd ed. Elsevier Limited; 2008:600-601. 14. Burlando M, Herzum A, Cozzani E, et al. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? case report and review of the literature. Dermatol Ther. 2021;34:E14705. doi:10.1111/dth.14705 15. Takata T, Ikeda M, Kodama H, et al. Regression of papular elastolytic giant cell granuloma using narrow-band UVB irradiation. Dermatology. 2006;212:77-79. doi:10.1159/000089028 16. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralenultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266. doi:10.1111/j.1600-0781.2012.00680.x
1. Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41:462-468. doi:10.1111/cup.12292 2. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132 3. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282. doi:10.1111/j.0309-0167.2004.01755.x 4. Revenga F, Rovira I, Pimentel J, et al. Annular elastolytic giant cell granuloma—actinic granuloma? Clin Exp Dermatol. 1996;21:51-53. 5. Hawryluk EB, Izikson L, English JC 3rd. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171-181. doi:10.2165/11530080-000000000-00000 6. Berliner JG, Haemel A, LeBoit PE, et al. The sarcoidal variant of annular elastolytic granuloma. J Cutan Pathol. 2013;40:918-920. doi:10.1111/cup.12237 7. Pflederer RT, Ahmed S, Tonkovic-Capin V, et al. Annular polycyclic plaques on the chest and upper back [published online April 24, 2018]. JAAD Case Rep. 2018;4:405-407. doi:10.1016/j.jdcr.2017.07.022 8. Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291. 9. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462. doi:10.1097/00000372-200312000-00001 10. Dowling GB, Jones EW. Atypical (annular) necrobiosis lipoidica of the face and scalp. a report of the clinical and histological features of 7 cases. Dermatologica. 1967;135:11-26. doi:10.1159/000254156 11. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015 .03.055 12. O’Brien JP, Regan W. Actinically degenerate elastic tissue is the likely antigenic basis of actinic granuloma of the skin and of temporal arteritis [published correction appears in J Am Acad Dermatol. 2000; 42(1 pt 1):148]. J Am Acad Dermatol. 1999;40(2 pt 1):214-222. doi:10.1016/s0190-9622(99)70191-x 13. Rencic A, Nousari CH. Other rheumatologic diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. 2nd ed. Elsevier Limited; 2008:600-601. 14. Burlando M, Herzum A, Cozzani E, et al. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? case report and review of the literature. Dermatol Ther. 2021;34:E14705. doi:10.1111/dth.14705 15. Takata T, Ikeda M, Kodama H, et al. Regression of papular elastolytic giant cell granuloma using narrow-band UVB irradiation. Dermatology. 2006;212:77-79. doi:10.1159/000089028 16. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralenultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266. doi:10.1111/j.1600-0781.2012.00680.x
A 67-year-old White woman presented to our dermatology clinic with pruritic annular erythematous plaques with central hypopigmentation on the forearms, dorsal aspect of the hands, neck, and fingers of 3 to 4 months’ duration. The patient rated the severity of pruritus an 8 on a 10-point scale. A review of symptoms was positive for fatigue, joint pain, and headache. The patient had a history of type 2 diabetes mellitus, osteoarthritis, thyroid disease, and stage 3 renal failure. A punch biopsy from the left forearm was performed.
Commentary: Alisertib, trastuzumab, and treatment timing, April 2023
HER2-positive (HER2+) BC was associated with poor outcomes compared with other BC subtypes. However, the introduction of trastuzumab has drastically changed the treatment paradigm for these patients afflicted with HER2+ BC. The pivotal trials with trastuzumab included only a few patients with lower-risk HER2+ tumors; therefore, it was not clear whether these lower-risk patients can benefit from a de-escalated adjuvant regimen. The phase 2 APT trial prospectively investigated the safety and efficacy of 12 weeks of paclitaxel with trastuzumab, followed by 9 months of trastuzumab monotherapy, in patients with small (≤ 3 cm), node-negative, HER2+ BC. After a median follow-up of 10.8 years, the 10-year invasive disease-free survival was 91.3%, the recurrence-free interval was 96.3%, the overall survival rate was 94.3%, and the BC-specific survival rate was 98.8%.
The researchers also conducted an exploratory analysis in 284 patients using the HER2DX genomic test. This is a single 27-gene expression and clinical feature-based classifier developed for early-stage, HER2+ BC. The tool identified a subset of patients with a high HER2DX score (HERDX score ≥ 32) who might harbor an increased risk for long-term recurrence.
These excellent long-term outcomes from the APT trial support the use of the currently endorsed adjuvant regimen of paclitaxel and trastuzumab in patients with stage I HER2+ BC. Furthermore, the HER2DX risk score, if validated, may provide a promising genomic tool to identify a subset of these patients who are at increased risk for recurrence and therefore may benefit from additional therapy.
Prior studies have noted worse survival outcomes with longer times from BC diagnosis to surgical treatment; however, the specific time interval that is acceptable to wait between diagnosis and surgery is still unclear. A case series study by Weiner and colleagues looked at the association between time from BC diagnosis to primary breast surgery and overall survival. The study looked at 373,334 female patients from the National Cancer Database with stage I-III BC who underwent primary breast surgery. Results showed worse overall survival outcomes when time to surgery was 9 or more weeks compared with surgery between 0 and 4 weeks (hazard ratio 1.15; P < .001). Factors associated with longer times to surgery included younger age, uninsured or Medicaid status, and lower neighborhood household income. On the basis of these findings, surgery before 8 weeks from BC diagnosis appears to be an acceptable time frame to avoid unfavorable survival outcomes and allow for appropriate multidisciplinary care. Furthermore, it is critical to identify potential barriers in a timely manner to prevent prolonged delays in care.
In hormone receptor-positive (HR+) BC, adjuvant endocrine therapy (ET) is usually delayed until after adjuvant radiotherapy, although, the optimal sequence of both therapies is still unknown. The aim of the study by Sutton and colleagues was to assess the association between time from surgery to ET initiation and cancer outcomes in high-risk HR+ patients, particularly those with residual disease after neoadjuvant chemotherapy. The study analysed 179 patients with HR+ BC from a multi-institutional database who received adjuvant radiotherapy, of which 68 patients received adjuvant ET before or during radiotherapy and 111 patients received ET after cessation of radiotherapy. Results showed that an interval of >14 weeks between surgery and the receipt of ET was independently associated with worse recurrence-free survival compared with an interval of 14 or less weeks (hazard ratio 3.20; P = .02). Of interest, the study also showed that patients receiving ET before or during radiation were more likely to experience skin and soft tissue late radiation morbidity, and this was nonsignificantly associated with worse radiation-associated complication-free survival (hazard ratio 1.87; P = .06). Although prior studies have reported that the interval from surgery to ET does not affect cancer outcomes, this was not studied in a high-risk cohort who have received neoadjuvant chemotherapy. Further studies in larger prospective cohorts are needed to validate these findings. At this time, the risks and benefits of concurrent ET with radiation need to be assessed prior to making any treatment recommendations.
HER2-positive (HER2+) BC was associated with poor outcomes compared with other BC subtypes. However, the introduction of trastuzumab has drastically changed the treatment paradigm for these patients afflicted with HER2+ BC. The pivotal trials with trastuzumab included only a few patients with lower-risk HER2+ tumors; therefore, it was not clear whether these lower-risk patients can benefit from a de-escalated adjuvant regimen. The phase 2 APT trial prospectively investigated the safety and efficacy of 12 weeks of paclitaxel with trastuzumab, followed by 9 months of trastuzumab monotherapy, in patients with small (≤ 3 cm), node-negative, HER2+ BC. After a median follow-up of 10.8 years, the 10-year invasive disease-free survival was 91.3%, the recurrence-free interval was 96.3%, the overall survival rate was 94.3%, and the BC-specific survival rate was 98.8%.
The researchers also conducted an exploratory analysis in 284 patients using the HER2DX genomic test. This is a single 27-gene expression and clinical feature-based classifier developed for early-stage, HER2+ BC. The tool identified a subset of patients with a high HER2DX score (HERDX score ≥ 32) who might harbor an increased risk for long-term recurrence.
These excellent long-term outcomes from the APT trial support the use of the currently endorsed adjuvant regimen of paclitaxel and trastuzumab in patients with stage I HER2+ BC. Furthermore, the HER2DX risk score, if validated, may provide a promising genomic tool to identify a subset of these patients who are at increased risk for recurrence and therefore may benefit from additional therapy.
Prior studies have noted worse survival outcomes with longer times from BC diagnosis to surgical treatment; however, the specific time interval that is acceptable to wait between diagnosis and surgery is still unclear. A case series study by Weiner and colleagues looked at the association between time from BC diagnosis to primary breast surgery and overall survival. The study looked at 373,334 female patients from the National Cancer Database with stage I-III BC who underwent primary breast surgery. Results showed worse overall survival outcomes when time to surgery was 9 or more weeks compared with surgery between 0 and 4 weeks (hazard ratio 1.15; P < .001). Factors associated with longer times to surgery included younger age, uninsured or Medicaid status, and lower neighborhood household income. On the basis of these findings, surgery before 8 weeks from BC diagnosis appears to be an acceptable time frame to avoid unfavorable survival outcomes and allow for appropriate multidisciplinary care. Furthermore, it is critical to identify potential barriers in a timely manner to prevent prolonged delays in care.
In hormone receptor-positive (HR+) BC, adjuvant endocrine therapy (ET) is usually delayed until after adjuvant radiotherapy, although, the optimal sequence of both therapies is still unknown. The aim of the study by Sutton and colleagues was to assess the association between time from surgery to ET initiation and cancer outcomes in high-risk HR+ patients, particularly those with residual disease after neoadjuvant chemotherapy. The study analysed 179 patients with HR+ BC from a multi-institutional database who received adjuvant radiotherapy, of which 68 patients received adjuvant ET before or during radiotherapy and 111 patients received ET after cessation of radiotherapy. Results showed that an interval of >14 weeks between surgery and the receipt of ET was independently associated with worse recurrence-free survival compared with an interval of 14 or less weeks (hazard ratio 3.20; P = .02). Of interest, the study also showed that patients receiving ET before or during radiation were more likely to experience skin and soft tissue late radiation morbidity, and this was nonsignificantly associated with worse radiation-associated complication-free survival (hazard ratio 1.87; P = .06). Although prior studies have reported that the interval from surgery to ET does not affect cancer outcomes, this was not studied in a high-risk cohort who have received neoadjuvant chemotherapy. Further studies in larger prospective cohorts are needed to validate these findings. At this time, the risks and benefits of concurrent ET with radiation need to be assessed prior to making any treatment recommendations.
HER2-positive (HER2+) BC was associated with poor outcomes compared with other BC subtypes. However, the introduction of trastuzumab has drastically changed the treatment paradigm for these patients afflicted with HER2+ BC. The pivotal trials with trastuzumab included only a few patients with lower-risk HER2+ tumors; therefore, it was not clear whether these lower-risk patients can benefit from a de-escalated adjuvant regimen. The phase 2 APT trial prospectively investigated the safety and efficacy of 12 weeks of paclitaxel with trastuzumab, followed by 9 months of trastuzumab monotherapy, in patients with small (≤ 3 cm), node-negative, HER2+ BC. After a median follow-up of 10.8 years, the 10-year invasive disease-free survival was 91.3%, the recurrence-free interval was 96.3%, the overall survival rate was 94.3%, and the BC-specific survival rate was 98.8%.
The researchers also conducted an exploratory analysis in 284 patients using the HER2DX genomic test. This is a single 27-gene expression and clinical feature-based classifier developed for early-stage, HER2+ BC. The tool identified a subset of patients with a high HER2DX score (HERDX score ≥ 32) who might harbor an increased risk for long-term recurrence.
These excellent long-term outcomes from the APT trial support the use of the currently endorsed adjuvant regimen of paclitaxel and trastuzumab in patients with stage I HER2+ BC. Furthermore, the HER2DX risk score, if validated, may provide a promising genomic tool to identify a subset of these patients who are at increased risk for recurrence and therefore may benefit from additional therapy.
Prior studies have noted worse survival outcomes with longer times from BC diagnosis to surgical treatment; however, the specific time interval that is acceptable to wait between diagnosis and surgery is still unclear. A case series study by Weiner and colleagues looked at the association between time from BC diagnosis to primary breast surgery and overall survival. The study looked at 373,334 female patients from the National Cancer Database with stage I-III BC who underwent primary breast surgery. Results showed worse overall survival outcomes when time to surgery was 9 or more weeks compared with surgery between 0 and 4 weeks (hazard ratio 1.15; P < .001). Factors associated with longer times to surgery included younger age, uninsured or Medicaid status, and lower neighborhood household income. On the basis of these findings, surgery before 8 weeks from BC diagnosis appears to be an acceptable time frame to avoid unfavorable survival outcomes and allow for appropriate multidisciplinary care. Furthermore, it is critical to identify potential barriers in a timely manner to prevent prolonged delays in care.
In hormone receptor-positive (HR+) BC, adjuvant endocrine therapy (ET) is usually delayed until after adjuvant radiotherapy, although, the optimal sequence of both therapies is still unknown. The aim of the study by Sutton and colleagues was to assess the association between time from surgery to ET initiation and cancer outcomes in high-risk HR+ patients, particularly those with residual disease after neoadjuvant chemotherapy. The study analysed 179 patients with HR+ BC from a multi-institutional database who received adjuvant radiotherapy, of which 68 patients received adjuvant ET before or during radiotherapy and 111 patients received ET after cessation of radiotherapy. Results showed that an interval of >14 weeks between surgery and the receipt of ET was independently associated with worse recurrence-free survival compared with an interval of 14 or less weeks (hazard ratio 3.20; P = .02). Of interest, the study also showed that patients receiving ET before or during radiation were more likely to experience skin and soft tissue late radiation morbidity, and this was nonsignificantly associated with worse radiation-associated complication-free survival (hazard ratio 1.87; P = .06). Although prior studies have reported that the interval from surgery to ET does not affect cancer outcomes, this was not studied in a high-risk cohort who have received neoadjuvant chemotherapy. Further studies in larger prospective cohorts are needed to validate these findings. At this time, the risks and benefits of concurrent ET with radiation need to be assessed prior to making any treatment recommendations.
Commentary: Chemotherapies and gynecologic surgeries relative to breast cancer, April 2023
However, a combined analysis of two other trials (PlanB and SUCCESS C) did not show a benefit with the addition of anthracycline for most patients with human epidermal growth factor receptor 2 (HER2)–negative early breast cancer.2 Roy and colleagues performed a retrospective study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, including 1106 women ≥ 66 years of age with node-positive TNBC, of whom 69.3% received adjuvant chemotherapy (N = 767). The use of chemotherapy led to a statistically significant improvement in survival outcomes (3-year cancer-specific survival [CSS] 81.8% vs 71.4%; overall survival 70.7% vs 51.3%). Although the anthracycline/taxane–based therapy did not improve CSS in the overall population vs taxane-based (hazard ratio [HR] 0.94; P = .79), among patients aged ≥ 76 years with four or more positive nodes, there was improvement in CSS with anthracycline/taxane therapy (HR 0.09; P = .02). These data further support the benefits of adjuvant chemotherapy in older patients when indicated; stimulate consideration of nonanthracycline combinations, particularly now with the use of immunotherapy for early TNBC; and highlight the need for inclusion of older individuals in clinical trials.
Treatment strategies to improve efficacy and minimize toxicity are highly desired for patients with early breast cancer (EBC). As an example, for small, node-negative, HER2-positive tumors, adjuvant systemic therapy with 12 weeks of paclitaxel/trastuzumab followed by continuation of trastuzumab to complete 1 full year has demonstrated excellent survival outcomes at over 10 years of follow-up.3 In the WSG-ADAPT-TP phase 2 trial, 375 patients with hormone receptor–positive , HER2-positive EBC were randomized to receive neoadjuvant T-DM1 (trastuzumab emtansine) with or without endocrine therapy or trastuzumab plus endocrine therapy. Similar 5-year invasive disease-free and overall survival rates were seen between the three arms. Patients who achieved a pathologic complete response (pCR) vs non-pCR had improved 5-year invasive disease-free survival (iDFS) rates (92.7% vs 82.7%; unadjusted HR 0.40). Furthermore, among the 117 patients who achieved pCR, the omission of adjuvant chemotherapy did not compromise survival outcomes (5-year iDFS 93% vs 92.1% for those who had vs those who did not have chemotherapy, respectively; unadjusted HR 1.15) (Harbeck et al). De-escalation approaches should ideally focus on the identification of biomarkers of response and resistance, as well as tools that can help predict patient outcomes and allow modification of therapy in real time. An example of this latter concept is the use of 18F-FDG-PET to identify patients with HER2-positive EBC who were likely to benefit from a chemotherapy-free dual HER2 blockade (trastuzumab/pertuzumab) treatment approach.4
Gynecologic surgery has been shown to reduce the risk for breast cancer,5 although the specific type of surgery and the impact of hormone replacement therapy add complexity to understanding the risks and outcomes for women. A prospective cohort, the Sister Study, included 50,701 women without a prior diagnosis of breast cancer but with a biological sister who had breast cancer; of these, 13.8% reported having hysterectomy only and 18.1% reported having bilateral oophorectomy with or without hysterectomy. Bilateral oophorectomy was inversely associated with breast cancer incidence (HR 0.91; 95% CI 0.83-1.00), with comparable results for women receiving estrogen only or combination estrogen plus progestin hormone replacement therapy. Contrary to these findings, having a hysterectomy only showed a positive association with breast cancer incidence (HR 1.12; 95% CI 1.02-1.23), with the strongest association among women who used combination estrogen and progestin therapy (HR 1.25; 95% CI 1.01-1.55) (Lovett et al). The impact of other gynecologic surgeries (such as salpingectomy)6 and the timing of the initiation of hormone replacement therapy, as well as the duration, should be investigated in future research.
Additional References
1. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: The ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG oncology). J Clin Oncol. 2017;35:2647e55. doi: 10.1200/JCO.2016.71.4147
2. de Gregorio A, Janni W, Friedl TW, et al. The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer-a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer. 2022;126:1715-1724. doi: 10.1038/s41416-021-01690-6
3. Tolaney SM, Tarantino P, Graham N, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273-285. doi: 10.1016/S1470-2045(23)00051-7
4. Pérez-García JM, Gebhart G, Ruiz Borrego M, et al; on behalf of PHERGain steering committee and trial investigators. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22:858-871. doi: 10.1016/S1470-2045(21)00122-4
5. Chow S, Raine-Bennett T, Samant ND, Postlethwaite DA, Holzapfel M. Breast cancer risk after hysterectomy with and without salpingo-oophorectomy for benign indications. Am J Obstet Gynecol. 2020;223:900.e1-900.e7. doi: 10.1016/j.ajog.2020.06.040
6. ACOG Committee Opinion No. 774: Opportunistic salpingectomy as a strategy for epithelial ovarian cancer prevention. Obstet Gynecol. 2019;133:e279-e284. doi: 10.1097/AOG.0000000000003164
However, a combined analysis of two other trials (PlanB and SUCCESS C) did not show a benefit with the addition of anthracycline for most patients with human epidermal growth factor receptor 2 (HER2)–negative early breast cancer.2 Roy and colleagues performed a retrospective study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, including 1106 women ≥ 66 years of age with node-positive TNBC, of whom 69.3% received adjuvant chemotherapy (N = 767). The use of chemotherapy led to a statistically significant improvement in survival outcomes (3-year cancer-specific survival [CSS] 81.8% vs 71.4%; overall survival 70.7% vs 51.3%). Although the anthracycline/taxane–based therapy did not improve CSS in the overall population vs taxane-based (hazard ratio [HR] 0.94; P = .79), among patients aged ≥ 76 years with four or more positive nodes, there was improvement in CSS with anthracycline/taxane therapy (HR 0.09; P = .02). These data further support the benefits of adjuvant chemotherapy in older patients when indicated; stimulate consideration of nonanthracycline combinations, particularly now with the use of immunotherapy for early TNBC; and highlight the need for inclusion of older individuals in clinical trials.
Treatment strategies to improve efficacy and minimize toxicity are highly desired for patients with early breast cancer (EBC). As an example, for small, node-negative, HER2-positive tumors, adjuvant systemic therapy with 12 weeks of paclitaxel/trastuzumab followed by continuation of trastuzumab to complete 1 full year has demonstrated excellent survival outcomes at over 10 years of follow-up.3 In the WSG-ADAPT-TP phase 2 trial, 375 patients with hormone receptor–positive , HER2-positive EBC were randomized to receive neoadjuvant T-DM1 (trastuzumab emtansine) with or without endocrine therapy or trastuzumab plus endocrine therapy. Similar 5-year invasive disease-free and overall survival rates were seen between the three arms. Patients who achieved a pathologic complete response (pCR) vs non-pCR had improved 5-year invasive disease-free survival (iDFS) rates (92.7% vs 82.7%; unadjusted HR 0.40). Furthermore, among the 117 patients who achieved pCR, the omission of adjuvant chemotherapy did not compromise survival outcomes (5-year iDFS 93% vs 92.1% for those who had vs those who did not have chemotherapy, respectively; unadjusted HR 1.15) (Harbeck et al). De-escalation approaches should ideally focus on the identification of biomarkers of response and resistance, as well as tools that can help predict patient outcomes and allow modification of therapy in real time. An example of this latter concept is the use of 18F-FDG-PET to identify patients with HER2-positive EBC who were likely to benefit from a chemotherapy-free dual HER2 blockade (trastuzumab/pertuzumab) treatment approach.4
Gynecologic surgery has been shown to reduce the risk for breast cancer,5 although the specific type of surgery and the impact of hormone replacement therapy add complexity to understanding the risks and outcomes for women. A prospective cohort, the Sister Study, included 50,701 women without a prior diagnosis of breast cancer but with a biological sister who had breast cancer; of these, 13.8% reported having hysterectomy only and 18.1% reported having bilateral oophorectomy with or without hysterectomy. Bilateral oophorectomy was inversely associated with breast cancer incidence (HR 0.91; 95% CI 0.83-1.00), with comparable results for women receiving estrogen only or combination estrogen plus progestin hormone replacement therapy. Contrary to these findings, having a hysterectomy only showed a positive association with breast cancer incidence (HR 1.12; 95% CI 1.02-1.23), with the strongest association among women who used combination estrogen and progestin therapy (HR 1.25; 95% CI 1.01-1.55) (Lovett et al). The impact of other gynecologic surgeries (such as salpingectomy)6 and the timing of the initiation of hormone replacement therapy, as well as the duration, should be investigated in future research.
Additional References
1. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: The ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG oncology). J Clin Oncol. 2017;35:2647e55. doi: 10.1200/JCO.2016.71.4147
2. de Gregorio A, Janni W, Friedl TW, et al. The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer-a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer. 2022;126:1715-1724. doi: 10.1038/s41416-021-01690-6
3. Tolaney SM, Tarantino P, Graham N, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273-285. doi: 10.1016/S1470-2045(23)00051-7
4. Pérez-García JM, Gebhart G, Ruiz Borrego M, et al; on behalf of PHERGain steering committee and trial investigators. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22:858-871. doi: 10.1016/S1470-2045(21)00122-4
5. Chow S, Raine-Bennett T, Samant ND, Postlethwaite DA, Holzapfel M. Breast cancer risk after hysterectomy with and without salpingo-oophorectomy for benign indications. Am J Obstet Gynecol. 2020;223:900.e1-900.e7. doi: 10.1016/j.ajog.2020.06.040
6. ACOG Committee Opinion No. 774: Opportunistic salpingectomy as a strategy for epithelial ovarian cancer prevention. Obstet Gynecol. 2019;133:e279-e284. doi: 10.1097/AOG.0000000000003164
However, a combined analysis of two other trials (PlanB and SUCCESS C) did not show a benefit with the addition of anthracycline for most patients with human epidermal growth factor receptor 2 (HER2)–negative early breast cancer.2 Roy and colleagues performed a retrospective study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, including 1106 women ≥ 66 years of age with node-positive TNBC, of whom 69.3% received adjuvant chemotherapy (N = 767). The use of chemotherapy led to a statistically significant improvement in survival outcomes (3-year cancer-specific survival [CSS] 81.8% vs 71.4%; overall survival 70.7% vs 51.3%). Although the anthracycline/taxane–based therapy did not improve CSS in the overall population vs taxane-based (hazard ratio [HR] 0.94; P = .79), among patients aged ≥ 76 years with four or more positive nodes, there was improvement in CSS with anthracycline/taxane therapy (HR 0.09; P = .02). These data further support the benefits of adjuvant chemotherapy in older patients when indicated; stimulate consideration of nonanthracycline combinations, particularly now with the use of immunotherapy for early TNBC; and highlight the need for inclusion of older individuals in clinical trials.
Treatment strategies to improve efficacy and minimize toxicity are highly desired for patients with early breast cancer (EBC). As an example, for small, node-negative, HER2-positive tumors, adjuvant systemic therapy with 12 weeks of paclitaxel/trastuzumab followed by continuation of trastuzumab to complete 1 full year has demonstrated excellent survival outcomes at over 10 years of follow-up.3 In the WSG-ADAPT-TP phase 2 trial, 375 patients with hormone receptor–positive , HER2-positive EBC were randomized to receive neoadjuvant T-DM1 (trastuzumab emtansine) with or without endocrine therapy or trastuzumab plus endocrine therapy. Similar 5-year invasive disease-free and overall survival rates were seen between the three arms. Patients who achieved a pathologic complete response (pCR) vs non-pCR had improved 5-year invasive disease-free survival (iDFS) rates (92.7% vs 82.7%; unadjusted HR 0.40). Furthermore, among the 117 patients who achieved pCR, the omission of adjuvant chemotherapy did not compromise survival outcomes (5-year iDFS 93% vs 92.1% for those who had vs those who did not have chemotherapy, respectively; unadjusted HR 1.15) (Harbeck et al). De-escalation approaches should ideally focus on the identification of biomarkers of response and resistance, as well as tools that can help predict patient outcomes and allow modification of therapy in real time. An example of this latter concept is the use of 18F-FDG-PET to identify patients with HER2-positive EBC who were likely to benefit from a chemotherapy-free dual HER2 blockade (trastuzumab/pertuzumab) treatment approach.4
Gynecologic surgery has been shown to reduce the risk for breast cancer,5 although the specific type of surgery and the impact of hormone replacement therapy add complexity to understanding the risks and outcomes for women. A prospective cohort, the Sister Study, included 50,701 women without a prior diagnosis of breast cancer but with a biological sister who had breast cancer; of these, 13.8% reported having hysterectomy only and 18.1% reported having bilateral oophorectomy with or without hysterectomy. Bilateral oophorectomy was inversely associated with breast cancer incidence (HR 0.91; 95% CI 0.83-1.00), with comparable results for women receiving estrogen only or combination estrogen plus progestin hormone replacement therapy. Contrary to these findings, having a hysterectomy only showed a positive association with breast cancer incidence (HR 1.12; 95% CI 1.02-1.23), with the strongest association among women who used combination estrogen and progestin therapy (HR 1.25; 95% CI 1.01-1.55) (Lovett et al). The impact of other gynecologic surgeries (such as salpingectomy)6 and the timing of the initiation of hormone replacement therapy, as well as the duration, should be investigated in future research.
Additional References
1. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: The ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG oncology). J Clin Oncol. 2017;35:2647e55. doi: 10.1200/JCO.2016.71.4147
2. de Gregorio A, Janni W, Friedl TW, et al. The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer-a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer. 2022;126:1715-1724. doi: 10.1038/s41416-021-01690-6
3. Tolaney SM, Tarantino P, Graham N, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273-285. doi: 10.1016/S1470-2045(23)00051-7
4. Pérez-García JM, Gebhart G, Ruiz Borrego M, et al; on behalf of PHERGain steering committee and trial investigators. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22:858-871. doi: 10.1016/S1470-2045(21)00122-4
5. Chow S, Raine-Bennett T, Samant ND, Postlethwaite DA, Holzapfel M. Breast cancer risk after hysterectomy with and without salpingo-oophorectomy for benign indications. Am J Obstet Gynecol. 2020;223:900.e1-900.e7. doi: 10.1016/j.ajog.2020.06.040
6. ACOG Committee Opinion No. 774: Opportunistic salpingectomy as a strategy for epithelial ovarian cancer prevention. Obstet Gynecol. 2019;133:e279-e284. doi: 10.1097/AOG.0000000000003164
Commentary: IL-31 inhibitor, e-cigarettes, and upadacitinib in AD, April 2023
Good news! There's not a lot to say about this. Dupilumab is so easy. No blood work, no immunosuppression. Dupilumab is highly effective and very safe. It's safe enough for children as young as 6 months! It's so effective that if it is not working, I question my diagnosis (Could it be contact dermatitis or mycosis fungoides instead?) and whether the patient is taking the medication properly.
Boesjes and colleagues describe in Acta Dermato-Venereologica the Dutch experience with upadacitinib in patients who have not been successfully treated with dupilumab or baricitinib. Presumably, such patients, because treatment with dupilumab or baricitinib or both was unsuccessful, have very resistant atopic dermatitis (either due to strong genetic propensity or perhaps because they don't take their medications). Despite having such refractory disease, most patients did well on the treatment with rapid disease improvement. Upadacitinib didn't work for everyone, though. About 30% of the patients discontinued upadacitinib treatment due to ineffectiveness, adverse events, or both (8.5%, 14.9%, and 6.4%, respectively).
How much of that ineffectiveness was due to poor adherence to taking the treatment was not assessed. Upadacitinib is extraordinarily effective for atopic dermatitis. I didn't think I would ever see a drug more effective than dupilumab for atopic dermatitis, but a low dose of upadacitinib (15 mg/day) seems about twice as effective as dupilumab for complete clearing of atopic dermatitis. The higher dose of 30 mg may be 3.5 times as effective as dupiliumab at getting atopic dermatitis completely clear.1
I dislike the word significant. Significant is ambiguous. It could mean that an observed association would not be likely to occur by chance, or it could mean that an observed association is clinically meaningful. Smith and colleagues in "Association between electronic cigarette use and atopic dermatitis among United States adults" reported finding a "significant" association between e-cigarette use and atopic dermatitis. A total of 23% of 2119 e-cigarette users had atopic dermatitis vs 17.1% of 26,444 nonusers. Clearly, the observed association was statistically significant (the 6% difference was not likely to occur due to chance alone). Is the finding clinically meaningful? I don't think it would affect our practice in any way.
The authors made the point that the study doesn't tell us whether e-cigarette use causes atopic dermatitis or if atopic dermatitis causes people to smoke. I wonder if just being younger (or some other factor) might make people more likely to use e-cigarettes and more likely to have atopic dermatitis (assuming atopic dermatitis gradually subsides over time, a dogma that may not be true).
Kabashima and colleagues report on the efficacy of the interleukin (IL)–31 antagonist nemolizumab. IL-31 mediates itch and having a new drug to block IL-31 may be a great treatment for our itchy patients. In this study, patients who had greater itch reduction had greater improvement in eczema and in quality of life. I'm quite sure that reducing itch improves patients' quality of life. But when it comes to the itch and the inflammation, I'm not sure which comes first. Does controlling the itch make the inflammation better? Maybe. Does controlling inflammation make itch better? Certainly.
For atopic patients with inflammation, controlling that inflammation seems to me to be the best approach, and we don't need more new treatments to accomplish that. For those patients who have a lot of itch and little inflammation, an IL-31 antagonist may be a revolutionary addition to our treatment options.
Additional References
1. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. doi: 10.1001/jamadermatol.2021.3023. Erratum in: JAMA Dermatol. 2022;158:219. doi: 10.1001/jamadermatol.2021.5451
Good news! There's not a lot to say about this. Dupilumab is so easy. No blood work, no immunosuppression. Dupilumab is highly effective and very safe. It's safe enough for children as young as 6 months! It's so effective that if it is not working, I question my diagnosis (Could it be contact dermatitis or mycosis fungoides instead?) and whether the patient is taking the medication properly.
Boesjes and colleagues describe in Acta Dermato-Venereologica the Dutch experience with upadacitinib in patients who have not been successfully treated with dupilumab or baricitinib. Presumably, such patients, because treatment with dupilumab or baricitinib or both was unsuccessful, have very resistant atopic dermatitis (either due to strong genetic propensity or perhaps because they don't take their medications). Despite having such refractory disease, most patients did well on the treatment with rapid disease improvement. Upadacitinib didn't work for everyone, though. About 30% of the patients discontinued upadacitinib treatment due to ineffectiveness, adverse events, or both (8.5%, 14.9%, and 6.4%, respectively).
How much of that ineffectiveness was due to poor adherence to taking the treatment was not assessed. Upadacitinib is extraordinarily effective for atopic dermatitis. I didn't think I would ever see a drug more effective than dupilumab for atopic dermatitis, but a low dose of upadacitinib (15 mg/day) seems about twice as effective as dupilumab for complete clearing of atopic dermatitis. The higher dose of 30 mg may be 3.5 times as effective as dupiliumab at getting atopic dermatitis completely clear.1
I dislike the word significant. Significant is ambiguous. It could mean that an observed association would not be likely to occur by chance, or it could mean that an observed association is clinically meaningful. Smith and colleagues in "Association between electronic cigarette use and atopic dermatitis among United States adults" reported finding a "significant" association between e-cigarette use and atopic dermatitis. A total of 23% of 2119 e-cigarette users had atopic dermatitis vs 17.1% of 26,444 nonusers. Clearly, the observed association was statistically significant (the 6% difference was not likely to occur due to chance alone). Is the finding clinically meaningful? I don't think it would affect our practice in any way.
The authors made the point that the study doesn't tell us whether e-cigarette use causes atopic dermatitis or if atopic dermatitis causes people to smoke. I wonder if just being younger (or some other factor) might make people more likely to use e-cigarettes and more likely to have atopic dermatitis (assuming atopic dermatitis gradually subsides over time, a dogma that may not be true).
Kabashima and colleagues report on the efficacy of the interleukin (IL)–31 antagonist nemolizumab. IL-31 mediates itch and having a new drug to block IL-31 may be a great treatment for our itchy patients. In this study, patients who had greater itch reduction had greater improvement in eczema and in quality of life. I'm quite sure that reducing itch improves patients' quality of life. But when it comes to the itch and the inflammation, I'm not sure which comes first. Does controlling the itch make the inflammation better? Maybe. Does controlling inflammation make itch better? Certainly.
For atopic patients with inflammation, controlling that inflammation seems to me to be the best approach, and we don't need more new treatments to accomplish that. For those patients who have a lot of itch and little inflammation, an IL-31 antagonist may be a revolutionary addition to our treatment options.
Additional References
1. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. doi: 10.1001/jamadermatol.2021.3023. Erratum in: JAMA Dermatol. 2022;158:219. doi: 10.1001/jamadermatol.2021.5451
Good news! There's not a lot to say about this. Dupilumab is so easy. No blood work, no immunosuppression. Dupilumab is highly effective and very safe. It's safe enough for children as young as 6 months! It's so effective that if it is not working, I question my diagnosis (Could it be contact dermatitis or mycosis fungoides instead?) and whether the patient is taking the medication properly.
Boesjes and colleagues describe in Acta Dermato-Venereologica the Dutch experience with upadacitinib in patients who have not been successfully treated with dupilumab or baricitinib. Presumably, such patients, because treatment with dupilumab or baricitinib or both was unsuccessful, have very resistant atopic dermatitis (either due to strong genetic propensity or perhaps because they don't take their medications). Despite having such refractory disease, most patients did well on the treatment with rapid disease improvement. Upadacitinib didn't work for everyone, though. About 30% of the patients discontinued upadacitinib treatment due to ineffectiveness, adverse events, or both (8.5%, 14.9%, and 6.4%, respectively).
How much of that ineffectiveness was due to poor adherence to taking the treatment was not assessed. Upadacitinib is extraordinarily effective for atopic dermatitis. I didn't think I would ever see a drug more effective than dupilumab for atopic dermatitis, but a low dose of upadacitinib (15 mg/day) seems about twice as effective as dupilumab for complete clearing of atopic dermatitis. The higher dose of 30 mg may be 3.5 times as effective as dupiliumab at getting atopic dermatitis completely clear.1
I dislike the word significant. Significant is ambiguous. It could mean that an observed association would not be likely to occur by chance, or it could mean that an observed association is clinically meaningful. Smith and colleagues in "Association between electronic cigarette use and atopic dermatitis among United States adults" reported finding a "significant" association between e-cigarette use and atopic dermatitis. A total of 23% of 2119 e-cigarette users had atopic dermatitis vs 17.1% of 26,444 nonusers. Clearly, the observed association was statistically significant (the 6% difference was not likely to occur due to chance alone). Is the finding clinically meaningful? I don't think it would affect our practice in any way.
The authors made the point that the study doesn't tell us whether e-cigarette use causes atopic dermatitis or if atopic dermatitis causes people to smoke. I wonder if just being younger (or some other factor) might make people more likely to use e-cigarettes and more likely to have atopic dermatitis (assuming atopic dermatitis gradually subsides over time, a dogma that may not be true).
Kabashima and colleagues report on the efficacy of the interleukin (IL)–31 antagonist nemolizumab. IL-31 mediates itch and having a new drug to block IL-31 may be a great treatment for our itchy patients. In this study, patients who had greater itch reduction had greater improvement in eczema and in quality of life. I'm quite sure that reducing itch improves patients' quality of life. But when it comes to the itch and the inflammation, I'm not sure which comes first. Does controlling the itch make the inflammation better? Maybe. Does controlling inflammation make itch better? Certainly.
For atopic patients with inflammation, controlling that inflammation seems to me to be the best approach, and we don't need more new treatments to accomplish that. For those patients who have a lot of itch and little inflammation, an IL-31 antagonist may be a revolutionary addition to our treatment options.
Additional References
1. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. doi: 10.1001/jamadermatol.2021.3023. Erratum in: JAMA Dermatol. 2022;158:219. doi: 10.1001/jamadermatol.2021.5451
Folic acid: A recommendation worth making
The US Preventive Services Task Force (USPSTF) recently published a draft recommendation on the use of folic acid before and during pregnancy to prevent fetal neural tube defects.1 This reaffirmation of the 2017 recommendation states that all persons planning to or who could become pregnant should take a daily supplement of folic acid.1,2 This is an “A” recommendation.
Neural tube defects are caused by a failure of the embryonic neural tube to close completely, which should occur in the first 28 days following fertilization. This is why folic acid is most effective if started at least 1 month before conception and continued for the first 2 to 3 months of pregnancy.
An estimated 3000 neural tube defects occur each year in the United States. Spina bifida, anencephaly, and encephalocele occur at respective rates of 3.9, 2.5, and 1.0 in 10,000 live births in the United States, which totals 7.4/10,000.3
Folic acid, if taken before and during pregnancy, can prevent about half of neural tube defects; if taken only during pregnancy, it prevents about one-third. If 50% of neural tube defects could be prevented with folic acid supplements, the number needed to treat (NNT) to prevent 1 case is about 3000.4
The case for supplementation. The recommended daily dose of folic acid is between 0.4 mg (400 μg) and 0.8 mg (800 μg), which is contained in many multivitamin products. Certain enriched cereal grain products in the United States have been fortified with folic acid for more than 2 decades, but it is unknown whether women in the United States are ingesting enough of these fortified foods to provide maximum prevention of neural tube defects. There are no known harms to mother or fetus from folic acid supplementation at recommended levels.
Room for improvement. Only 20% to 40% of people who are pregnant or trying to get pregnant, and 5% to 10% of people with an unplanned pregnancy, take folic acid supplements. Half of all pregnancies in the United States are unplanned.4 This leaves a lot of room for improvement in the prevention of neural tube defects.
An important recommendation, even if you don’t see the results. The NNT to prevent a case of neural tube defect is high; most family physicians providing perinatal care will not prevent a case during their career. And, as with most preventive interventions, we do not see the cases prevented. However, on a population-wide basis, if all women took folic acid as recommended, the number of severe birth defects prevented would be significant—making this simple recommendation worth mentioning to those of reproductive age.
1. USPSTF. Folic acid supplementation to prevent neural tube defects. Published February 21, 2023. Accessed March 22, 2023. https://uspreventiveservicestaskforce.org/home/getfilebytoken/sX6CTKHncTJT2nzmu7yLHh
2. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Published January 10, 2017. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
3. Mai CT, Isenburg JL, Canfield MA, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
4. Viswanathan M, Urrutia RP, Hudson KN, et al. Folic acid supplementation to prevent neural tube defects: a limited systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Published February 2023. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/home/getfilebytoken/AjUYoBvpfUBDAFjHeCcfPz
The US Preventive Services Task Force (USPSTF) recently published a draft recommendation on the use of folic acid before and during pregnancy to prevent fetal neural tube defects.1 This reaffirmation of the 2017 recommendation states that all persons planning to or who could become pregnant should take a daily supplement of folic acid.1,2 This is an “A” recommendation.
Neural tube defects are caused by a failure of the embryonic neural tube to close completely, which should occur in the first 28 days following fertilization. This is why folic acid is most effective if started at least 1 month before conception and continued for the first 2 to 3 months of pregnancy.
An estimated 3000 neural tube defects occur each year in the United States. Spina bifida, anencephaly, and encephalocele occur at respective rates of 3.9, 2.5, and 1.0 in 10,000 live births in the United States, which totals 7.4/10,000.3
Folic acid, if taken before and during pregnancy, can prevent about half of neural tube defects; if taken only during pregnancy, it prevents about one-third. If 50% of neural tube defects could be prevented with folic acid supplements, the number needed to treat (NNT) to prevent 1 case is about 3000.4
The case for supplementation. The recommended daily dose of folic acid is between 0.4 mg (400 μg) and 0.8 mg (800 μg), which is contained in many multivitamin products. Certain enriched cereal grain products in the United States have been fortified with folic acid for more than 2 decades, but it is unknown whether women in the United States are ingesting enough of these fortified foods to provide maximum prevention of neural tube defects. There are no known harms to mother or fetus from folic acid supplementation at recommended levels.
Room for improvement. Only 20% to 40% of people who are pregnant or trying to get pregnant, and 5% to 10% of people with an unplanned pregnancy, take folic acid supplements. Half of all pregnancies in the United States are unplanned.4 This leaves a lot of room for improvement in the prevention of neural tube defects.
An important recommendation, even if you don’t see the results. The NNT to prevent a case of neural tube defect is high; most family physicians providing perinatal care will not prevent a case during their career. And, as with most preventive interventions, we do not see the cases prevented. However, on a population-wide basis, if all women took folic acid as recommended, the number of severe birth defects prevented would be significant—making this simple recommendation worth mentioning to those of reproductive age.
The US Preventive Services Task Force (USPSTF) recently published a draft recommendation on the use of folic acid before and during pregnancy to prevent fetal neural tube defects.1 This reaffirmation of the 2017 recommendation states that all persons planning to or who could become pregnant should take a daily supplement of folic acid.1,2 This is an “A” recommendation.
Neural tube defects are caused by a failure of the embryonic neural tube to close completely, which should occur in the first 28 days following fertilization. This is why folic acid is most effective if started at least 1 month before conception and continued for the first 2 to 3 months of pregnancy.
An estimated 3000 neural tube defects occur each year in the United States. Spina bifida, anencephaly, and encephalocele occur at respective rates of 3.9, 2.5, and 1.0 in 10,000 live births in the United States, which totals 7.4/10,000.3
Folic acid, if taken before and during pregnancy, can prevent about half of neural tube defects; if taken only during pregnancy, it prevents about one-third. If 50% of neural tube defects could be prevented with folic acid supplements, the number needed to treat (NNT) to prevent 1 case is about 3000.4
The case for supplementation. The recommended daily dose of folic acid is between 0.4 mg (400 μg) and 0.8 mg (800 μg), which is contained in many multivitamin products. Certain enriched cereal grain products in the United States have been fortified with folic acid for more than 2 decades, but it is unknown whether women in the United States are ingesting enough of these fortified foods to provide maximum prevention of neural tube defects. There are no known harms to mother or fetus from folic acid supplementation at recommended levels.
Room for improvement. Only 20% to 40% of people who are pregnant or trying to get pregnant, and 5% to 10% of people with an unplanned pregnancy, take folic acid supplements. Half of all pregnancies in the United States are unplanned.4 This leaves a lot of room for improvement in the prevention of neural tube defects.
An important recommendation, even if you don’t see the results. The NNT to prevent a case of neural tube defect is high; most family physicians providing perinatal care will not prevent a case during their career. And, as with most preventive interventions, we do not see the cases prevented. However, on a population-wide basis, if all women took folic acid as recommended, the number of severe birth defects prevented would be significant—making this simple recommendation worth mentioning to those of reproductive age.
1. USPSTF. Folic acid supplementation to prevent neural tube defects. Published February 21, 2023. Accessed March 22, 2023. https://uspreventiveservicestaskforce.org/home/getfilebytoken/sX6CTKHncTJT2nzmu7yLHh
2. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Published January 10, 2017. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
3. Mai CT, Isenburg JL, Canfield MA, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
4. Viswanathan M, Urrutia RP, Hudson KN, et al. Folic acid supplementation to prevent neural tube defects: a limited systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Published February 2023. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/home/getfilebytoken/AjUYoBvpfUBDAFjHeCcfPz
1. USPSTF. Folic acid supplementation to prevent neural tube defects. Published February 21, 2023. Accessed March 22, 2023. https://uspreventiveservicestaskforce.org/home/getfilebytoken/sX6CTKHncTJT2nzmu7yLHh
2. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Published January 10, 2017. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
3. Mai CT, Isenburg JL, Canfield MA, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
4. Viswanathan M, Urrutia RP, Hudson KN, et al. Folic acid supplementation to prevent neural tube defects: a limited systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Published February 2023. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/home/getfilebytoken/AjUYoBvpfUBDAFjHeCcfPz
Advances in the treatment of fetal demise in the second and third trimester
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.
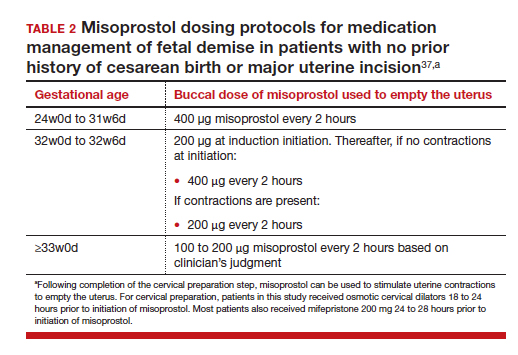

For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
Clinical care for fetal demise is complex and multidimensional, including empathic emotional support for the patient and family members who are experiencing a tragedy, investigation of the cause of the demise, and a plan for emptying the uterus. This editorial narrowly focuses on the options for treatment of fetal demise with the goal of emptying the uterus while minimizing complications.
When planning treatment of fetal demise, focus on fetal size and gestational age
Most guidelines for the treatment of fetal demise use gestational age to guide selection of a treatment.1,2 I believe that fetal size is as important as gestational age for selecting a treatment plan. When considering treatment, there are 2 reasons why fetal size is as important as gestational age:
- The physiologic processes that caused fetal demise may have caused fetal growth restriction, resulting in a fetal size that is 2 or more weeks below expected fetal size for gestational age.
- Fetal demise may have occurred weeks before the diagnosis was made, resulting in gestational age being greater than fetal size. This editorial will use ultrasonography estimate of fetal size in gestational weeks to guide treatment recommendations. When discussing fetal size, we will use the convention of weeks-days (w-d). Twenty-five weeks and zero days gestation is represented as 25w0d.
Treatment in the second and third trimester is a 2-step process
Step 1: Cervical preparation
In most cases of first trimester fetal demise, no cervical preparation is necessary. Cervical dilation with metal dilators followed by uterine evacuation with an appropriately sized vacuum catheter is a highly successful treatment.3 However for second and third trimester fetal demise, it is best to use a 2-step process, beginning with cervical preparation followed by emptying the uterus. For example, at a fetal size of 13w0d to 16w0d, cervical preparation can be achieved by administering a single buccal dose of misoprostol 400 µg 3 to 4 hours prior to uterine evacuation or by inserting a Dilapan-S (Medicem Inc) osmotic cervical dilator 3 to 6 hours prior to uterine evacuation.4-7 At a fetal size of 16w0d to 19w6d, cervical preparation can be achieved by placing osmotic cervical dilators 4 to 6 hours before surgical evacuation and administering buccal misoprostol 400 µg 3 hours before surgical evacuation.8
Alternatively, from 16w0d to 25w0d osmotic cervical dilators can be placed on day 1 of a 2-day process, and the patient can return on day 2 to have the cervical dilators removed followed by surgical evacuation of the uterus. Mifepristone 200 mg oral dose can be administered on day 1 to facilitate cervical preparation. In my practice, I use mifepristone 200 mg on day 1 when the fetal size is ≥20w0d gestation. Options for cervical preparation include use of osmotic dilators, cervical balloons, misoprostol, and/or mifepristone. These options are discussed below. With fetal demise, natural physiologic processes often have caused sufficient cervical softening and dilation that no cervical preparation is necessary and immediate uterine surgical evacuation or induction of labor can be initiated.
Step 2: Emptying the uterus
In the second and third trimesters, the approach to uterine evacuation is based on fetal size. At fetal sizes <25w0d, options for emptying the uterus include surgical evacuation with a vacuum catheter and grasping forceps or induction of labor with misoprostol followed by vaginal birth and expulsion of the placenta. At fetal sizes ˃25w0d gestation, following completion of cervical preparation, the most common approaches to uterine evacuation are induction of labor with misoprostol or oxytocin. Rarely, with a stillbirth at term, some clinicians will select hysterotomy to empty the uterus, avoiding uterine rupture during labor induction for patients at the highest risk, including those with a prior classical cesarean birth or more than 2 prior cesarean births with a low-transverse uterine incision.
Osmotic cervical dilators
The 2 most used cervical dilators are Dilapan-S, a polyacrylate-based hydrogel rod, and laminaria, dried compressed seaweed stipe (stalk) from Laminaria japonica or Laminaria digitata. Dilapan-S rods are available in diameters of 3 mm and 4 mm and rod lengths of 55 mm and 65 mm. Laminaria dilators are available in diameters of 2, 3, 4, 5, 6, 8 and 10 mm and rod length of 60 and 70 mm. Dilapan-S dilators reach near-maximal dilation in approximately 4 to 6 hours but continue to expand over the following 18 hours to achieve a maximum dilation of 3.3 to 3.6 times their dry diameter.9 Laminaria dilators expand to 2.7 to 2.9 times their dry diameter over 24 hours.9
A general rule is that as many dilators as possible should be placed until significant resistance to the placement of additional dilators is encountered.10 In my practice, for fetal size ≥20 weeks’ gestation, I place 2 Dilapan-S rods, 4 mm in diameter, 55 mm in length, and then encircle the Dilapan-S with laminaria rods that are 4 mm in diameter and 60 mm in length. Once cervical resistance to the placement of the 4 mm laminaria rods is observed, I encircle those laminaria with laminaria 2 mm in diameter, filling in the interstices between the 4 mm laminaria. The next day, cervical dilation is routinely ≥3 cm.
In a retrospective study of 491 patients undergoing pregnancy termination after 14 weeks’ gestation, with a mean gestational age of 24 weeks, compared with no osmotic cervical dilators, inserting osmotic cervical dilators the day before initiating misoprostol for induction of labor resulted in a decrease in time to delivery (428 min vs 640 min; P<.001) and a decrease in total misoprostol dose (990 µg vs 1,449 µg; P<.0001).11
Cervical balloons
All clinicians know that a Foley catheter or a Cook cervical ripening balloon can be used for cervical preparation in the third trimester.12,13 The Foley catheter also has been reported to be useful for cervical preparation in the second trimester. In one study of 43 patients 17 to 24 weeks’ gestation scheduled for a second-trimester dilation and evacuation, an intracervical Foley catheter was placed the evening before evacuation, and the balloon was inflated with 30 mL to 50 mL of saline. At the same time, mifepristone 200 mg was administered to the patients.14 The following day, dilation and evacuation was performed. In 72% of cases no additional cervical dilation was required on the day of evacuation. The investigators concluded that if osmotic cervical dilators are not available, the placement of an intracervical Foley catheter plus administration of mifepristone facilitates performance of an evacuation on the following day. If the patient prefers a 1-day procedure, the Foley can be inserted in the morning to facilitate cervical preparation, and the uterus can be evacuated in the afternoon.
Continue to: Misoprostol...
Misoprostol
Misoprostol, a derivative of prostaglandin E1, is useful for both cervical preparation and induction of labor. The dose of misoprostol and the route of administration are major determinants of uterine response.15-19 When administered by an oral route, misoprostol has fast onset and offset of action and often does not cause sustained uterine contractions. Hence, oral misoprostol, at a low dose is useful for cervical ripening, but not as useful for stimulation of sustained uterine contractions for induction of labor. When administered by a buccal or vaginal route, misoprostol has prolonged activity and often results in sustained uterine contractions. At any given dose of misoprostol, buccal and vaginal misoprostol administration are more effective than oral administration in inducing sustained uterine contractions sufficient to empty the uterus.15-19
Mifepristone
Mifepristone, an anti-progestin, is useful for cervical preparation and sensitizing myocytes to the action of uterotonics. Progesterone reduces cell-to-cell communication among uterine myocytes, facilitating uterine quiescence by suppressing connexin 43 and other proteins. Mifepristone blocks the effect of progesterone, inducing the production of myocyte connexin 43, enhancing efficient cell-to-cell communication, permitting uterine myoctes to contract in unison, creating the potential for powerful and sustained contractions.20-23 Randomized clinical trials report that administration of mifepristone 200 mg prior to misoprostol induced labor results in more rapid emptying of the uterus.24-27
It takes time for mifepristone to have its full effect on uterine myocytes. Hence, most protocols recommend waiting 24 hours following mifepristone administration before initiating treatment with an agent to stimulate uterine contractions such as misoprostol or oxytocin. However, preliminary data suggest that partial benefit of mifepristone can be obtained when initiating misoprostol 3 to 5 hours after mifepristone administration.28 In a study of 481 patients undergoing induction of labor in the second or third trimester, the time from initiation of misoprostol to vaginal birth was 15 hours with no mifepristone pretreatment, 13.2 hours if mifepristone was administered 3 to 5 hours before initiating misoprostol, 9.3 hours if mifepristone was administered 24 hours before initiating misoprostol, and 10.5 hours if mifepristone was administered 48 hours before initiating misoprostol.28
Fetal size <25w0d gestation: Cervical preparation and surgical evacuation
For fetal demise at a fetal size less than 25w0d, if clinical experts are available, the best treatment option is cervical preparation followed by surgical evacuation of the uterus using a vacuum catheter and grasping forceps to empty the uterus.29,30 A disadvantage of surgical evacuation of the uterus is that an intact fetus is not available for the patient to hold and mourn, and pathologic examination of an intact fetus is not possible. An alternative approach is cervical preparation followed by induction of labor using misoprostol with the goal of delivering an intact fetus. Although no prospective clinical trials are available comparing these 2 options, retrospective studies have reported that, at fetal size <25w0d gestation, compared with induction of labor, surgical evacuation of the uterus results in fewer complications,30 including fewer cases of retained placenta requiring an unplanned procedure and fewer presumed uterine infections.29
For surgical evacuation of fetal demise with a fetal size of <25w0d gestation, the first step on day 1 is placement of osmotic cervical dilators. In addition to osmotic cervical dilators, if the gestational age or fetal size is ≥19 weeks’ gestation an oral dose of mifepristone 200 mg to facilitate cervical preparation may be considered. On day 2, the osmotic dilators are removed and surgical evacuation is performed. In one randomized study, for pregnancies at 19 to 24 weeks’ gestation, compared with osmotic dilators alone, administration of mifepristone 200 mg at the time of placement of osmotic dilators resulted in fewer procedures that were difficult to complete.31 In some cases, 2 consecutive days of cervical preparation with osmotic dilators may be needed to properly prepare the cervix for uterine evacuation. For example, the cervix of a nulliparous teenage patient may require 2 days of cervical preparation with osmotic dilators to facilitate uterine evacuation. In some cases of fetal demise, the cervix is already dilated to ≥3 cm and surgical evacuation of the uterus or induction of labor can be initiated without the need for cervical preparation.
Continue to: Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor...
Fetal size 14w0d to 28w6d gestation: Cervical preparation and induction of labor
Treatment of fetal demise at 14w0d to 28w6d gestation with the goal of the vaginal birth of an intact fetus is optimized by the administration of mifepristone for cervical preparation followed by induction of labor with misoprostol.26,27
In one clinical trial, 66 patients with fetal demise between 14w0d and 28w6d gestation were randomly assigned to receive mifepristone 200 mg or placebo followed 24 to 48 hours later with initiation of misoprostol induction of labor.26 Among the patients from 14w0d to 24 weeks’ gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24w0d to 28 weeks’ gestation, the misoprostol dose was 200 µg vaginally every 4 hours. At 24 hours, a consultant obstetrician determined if additional misoprostol should be given. The median time from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups was 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P= .002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage, 4 in the placebo group and 1 in the mifepristone group.26
In a second clinical trial, 105 patients with fetal demise after 20 weeks of gestation were randomly assigned to receive mifepristone 200 mg or placebo.27 In this study, 86% of the patients were ≥26w0d gestation, with a mean gestational age of approximately 32w2d. Thirty-six to 48 hours later, misoprostol induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostoldose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients from ≥26 weeks’ gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours, respectively (P=.001). Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg; P<.001). Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.27
Fetal size ≥29w0d gestation
At a fetal size ≥29w0d gestation, if the cervix is ripe with a Bishop score of ≥7, oxytocin induction of labor is often used as a first-line treatment. If the cervix is not ripe, misoprostol induction of labor may be considered at doses less than those used in the second trimester of pregnancy.32 TABLES 1,1, 26, 33–36 2,37 and 337 summarize regimens proposed for fetal size ≥29w0d. One regimen begins with an initial misoprostol dose of 50 µg. If adequate uterine contractions occur, the 50 µg dose is repeated every 4 hours up to 6 total doses. If contractions are inadequate, the dose can be increased to 100 µg every 4 hours for 5 additional doses.



For fetal demise after 28w0d gestation, the
A multidisciplinary approach can optimize compassionate care
There are many gaps in the holistic care of patients and partners experiencing fetal demise. Patients with fetal demise often report that they did not receive sufficient information about the cause of the demise and wanted more opportunity to be involved in decision making about their care.39 The patient’s partner often reports feeling unacknowledged as a grieving parent.40 Fetal demise is experienced by many patients as a tragedy, triggering feelings of grief, anger, denial, anxiety and depression, sometimes resulting in isolation and substance misuse.
Using a 5-round Delphi process, experts identified 8 core goals in the care of patients with fetal demise:
- reduce stigma
- provide respectful care
- involve patients in care planning
- attempt to provide an explanation for the demise1
- acknowledge the depth of the grief response and provide emotional support
- offer information about ongoing psychological support
- provide information about future pregnancy planning
- provide opportunities for specialized training and support for care providers.41
Management of stillbirth is optimized by a multidisciplinary approach that includes the expert care of obstetrician-gynecologists, obstetric nurses, anesthesiologists, and expert consultation from social work, chaplaincy, and pathology. A heart-to-heart connection between clinician and patient is a key component of stillbirth care. ●
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.
- American College of Obstetricians and Gynecologists. Management of stillbirth. ACOG Obstetric Care Consensus. No. 10. Obstet Gynecol. 2020;135:e110-132.
- Tsakiridis I, Giouleka S, Mamopoulos A, et al. Investigation and management of stillbirths: a descriptive review of major guidelines. J Perinat Med. 2022;50:796-813.
- Spingler T, Sonek J, Hoopman M, et al. Complication rate after termination of pregnancy due to fetal defects. Ultrasound Obstet Gynecol. 2023;Epub January 7.
- Goldberg AB, Drey EA, Whitaker AK, et al. Misoprostol compared with laminaria before early second-trimester surgical abortion: a randomized trial. Obstet Gynecol. 2005;106:234-241.
- Meirik O, My Huong NT, Piaggio G, et al. WHOR-GoP-MoF Regulation. Complications of first trimester abortion by vacuum aspiration after cervical preparation with and without misoprostol: a multicentre randomised trial. Lancet. 2012;379(9829):1817-1824.
- Bartz D, Maurer R, Allen RH, et al. Buccal misoprostol compared with synthetic osmotic cervical dilator before surgical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122:57-63.
- Ngo LL, Mokashi M, Janiak E, et al. Acute complications with same-day versus overnight cervical preparation before dilation and evacuation at 14 to 16 weeks. Contraception. 2023;117:61-66.
- Kim CS, Dragoman M, Prosch L, et al. Same-day compared with overnight cervical preparation before dilation and evacuation between 16 and 19 6/7 weeks of gestation: a randomized controlled trial. Obstet Gynecol. 2022;139:1141-1144.
- Drunecky T, Reidingerova M, Plisova M, et al. Experimental comparison of properties of natural and synthetic osmotic dilators. Arch Gynecol Obstet. 2015;292:349-354.
- Hern WM. Laminaria versus Dilapan osmotic cervical dilators for outpatient dilation and evacuation abortion: randomized cohort comparison of 1001 patients. Am J Obstet Gynecol. 1994;171:1324-1328.
- Berthold C, Gomes David M, Gabriel P, et al. Effect of the addition of osmotic dilators to medical induction of labor abortion: a before-and-after study. Eur J Obstet Gynecol. 2020;244:185-189.
- Kemper JI, Li W, Goni S, et al. Foley catheter vs oral misoprostol for induction of labor: individual participant data meta-analysis. Ultrasound Obstet Gynecol. 2021;57:215-223.
- Attalli E, Kern Guy, Fouks Y, et al. Labor induction in third trimester non-viable fetus. J Matern Fetal Neonatal Med. 2022;Epub October 1.
- Fessehaye Sium A, Prager S, Wolderufael M, et al. Foley catheter for cervical preparation prior to second trimester dilation and evacuation: a supply-based alternative for surgical abortion: a case series. Contracept X. 2022;4:100085.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590.
- Barbieri RL. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022;34:8-10, 12.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290.
- Barbieri RL. Mifepristone for the treatment of miscarriage and fetal demise. OBG Manag. 2022;34:811, 15.
- Kapp N, Borgatta L, Stubblefield P, et al. Mifepristone in second-trimester medical abortion. Obstet Gynecol. 2007;110:1304-1310.
- Ngoc NTN, Shochet T, Raghavan S, et al. Mifepristone and misoprostol compared with misoprostol alone for second trimester abortion: a randomized controlled trial. Obstet Gynecol. 2011;118:601608.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890.
- Prodan N, Breisch J, Hoopman M, et al. Dosing interval between mifepristone and misoprostol in second and third trimester termination. Arch Gynecol Obstet. 2019;299:675-679.
- Edlow AG, Hou MY, Maurer R, et al. Uterine evacuation for second trimester fetal death and maternal morbidity. Obstet Gynecol. 2011;117:1-10.
- Bryan AG, Grimes DA, Garrett JM, et al. Second-trimester abortion for fetal anomalies or fetal death. Obstet Gynecol. 2011;117:788-792.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol or mifepristone compared with overnight osmotic dilators alone. Obstet Gynecol. 2015;126:599-609.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Gomez-Ponce de Leon R, Wing D, Fiala C. Misoprostol for intrauterine fetal death. Int J Gynaecol Obstet. 2007;99(suppl 2):S190-S193.
- American College of Obstetricians and Gynecologists. Second-trimester abortion. Practice Bulletin No. 135. Obstet Gynecol. 2013;121:1394-1406.
- Wingo E, Raifman S, Landau C, et al. Mifepristone-misoprostol versus misoprostol-alone regimen for medication abortion at ≥ 24 weeks gestation. Contraception. Appendix 1. 2020;102:99-103.
- American College of Obstetricians and Gynecologists. Vaginal birth after cesarean delivery. ACOG Practice Bulletin No. 205. Obstet Gynecol. 2019;133:e110-e127.
- Atkins B, Blencowe H, Boyle FM, et al. Is care of stillborn babies and their parents respectful? Results from an international online survey. BJOG. 2022;129:1731-1739.
- Haezell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387(10018):604-616.
- Shakespeare C, Merriel A, Bakhbakhi D, et al. The RESPECT Study for consensus on global bereavement care after stillbirth. Int J Gynaecol Obstet. 2020;149:137-147.