User login
Are we shortchanging patients with obesity?
Every Wednesday evening after supper, I record in a marble notebook some anthropomorphic measurements: my weight taken first thing Monday morning and my waist circumference. I also add how I did with exercise since the previous week’s entry and some comments about sleep, energy, and nutrition.
My personal log now comprises dozens of pages. To my surprise, the first entry was 5 years ago to the month. The earlier entries were far from weekly and contained a lot of narrative on how my food-restriction scheme that month was being violated.
Looking just at the numbers, I did about as well as a control group participant in any medical study of diet modification. Until just a few months ago, there was no trend in either weight or waist circumference over those 5 years, including 2 years of retirement. But it wasn’t for lack of trying. Keeping the journal for as long as I have – and recently, as consistently as I have – suggests serious intent but inadequate execution of the same principles I offered patients, who rarely did much better. But recent studies suggest that perhaps quite a few could.
Are we underestimating our patients’ potential?
A recent abstract from the European and International Congress on Obesity suggests that the impressions clinicians get from our office encounters may leave us underestimating the potential for our patients to lose enough weight to move them from one level of risk to another.
Using a national database of primary care visits, the investigators isolated about 550,000 records. Of these, about 60,000 (11%) had records showing weight reductions of 10%-25% (mean, 13%) over at least 4 years. Weight loss was by intent rather than from illness. The remaining individuals maintained their weight within 5% of the first measurement for the duration of the study.
Participants with stable body weight were compared with the successful weight reducers. This analysis showed that the risk for type 2 diabetes, osteoarthritis, sleep apnea, hypertension, and dyslipidemia all measurably declined in weight reducers. This held true whether the patient’s baseline body mass index (BMI) showed modest or severe obesity. Patients with the highest BMI at enrollment actually reduced their risks for hypertension and dyslipidemia below population norms.
This study raises tantalizing, as yet unanswered questions: How did the successful 11% achieve their weight loss goals? Was it via a weight loss program, bariatric surgery, dietitian consult, or with no external assistance?
And of great significance to clinicians: What happened to the people who achieved 5%-10% weight reduction, as that is a more typical outcome of diabetes prevention trials or studies of weight-loss medications? Were they excluded from the study because they did not lose enough weight to achieve the unequivocal health benefit?
Because the data came from an enormous database, the weight management strategies leading to success or failure – what we really need to know to nudge our own patients into the favorable categories – remain hidden.
The Advantage of Intensive Interventions
Some answers emerged from a recently reported study in the New England Journal of Medicine comparing supervised diet and lifestyle adjustments (treatment group) with the less intense oversight typically offered by primary care clinicians (usual-care group).
The treatment group not only received the intensive lifestyle intervention, which focused on reduced caloric intake and increased physical activity, but also participated in mandated training sessions on how to best use the resources provided by the study. Much of the care was delegated by physicians to “coaches” who focused on nutrition, exercise, and behavioral health, including supermarket strategy.
Nearly a quarter of the participants in the intensive intervention group achieved the 10% weight reduction needed to change health risk in a meaningful way. A similar proportion lost less than 10% of their body weight, and about half did not have a notable weight change. Peak weight loss at 6 months averaged 17 lb, and 9.6 lb at 2 years. While this may not seem very impressive considering the extensive resources utilized, there were those who experienced an extraordinary health upgrade not otherwise available, short of bariatric surgery.
What does this mean for us?
Both studies indicate that, even under the best-controlled, resource-replete circumstances, the rate of failure to achieve desired progress is very high. But there is a success rate.
The likelihood of success is difficult to interpret from the European data, as it compared only those with major weight loss and those with weight stability, excluding patients with less robust loss or weight gain. The controlled study, however, holds forth an alluring opportunity benefiting a quarter of the targeted participants and even about 5% of the controls who realized that they were being observed.
We also learn that supervision requires a lot more than having a well-meaning but not very well-trained physician ask a patient to log measurements and food intake. Health coaches seem to make the impact.
Failure rates of 50% have a way of dampening enthusiasm, but it may be best to approach the scourge of obesity by offering treatment to everyone with the expectation that not all will experience greatly enhanced quality of life and longevity. Not everyone will benefit, but these two studies confirm that we do have an underutilized capacity to help more people benefit than we currently do.
Richard M. Plotzker, MD, is a retired endocrinologist with 40 years of experience treating patients in both the private practice and hospital settings. He has been a Medscape contributor since 2012.
A version of this article originally appeared on Medscape.com.
Every Wednesday evening after supper, I record in a marble notebook some anthropomorphic measurements: my weight taken first thing Monday morning and my waist circumference. I also add how I did with exercise since the previous week’s entry and some comments about sleep, energy, and nutrition.
My personal log now comprises dozens of pages. To my surprise, the first entry was 5 years ago to the month. The earlier entries were far from weekly and contained a lot of narrative on how my food-restriction scheme that month was being violated.
Looking just at the numbers, I did about as well as a control group participant in any medical study of diet modification. Until just a few months ago, there was no trend in either weight or waist circumference over those 5 years, including 2 years of retirement. But it wasn’t for lack of trying. Keeping the journal for as long as I have – and recently, as consistently as I have – suggests serious intent but inadequate execution of the same principles I offered patients, who rarely did much better. But recent studies suggest that perhaps quite a few could.
Are we underestimating our patients’ potential?
A recent abstract from the European and International Congress on Obesity suggests that the impressions clinicians get from our office encounters may leave us underestimating the potential for our patients to lose enough weight to move them from one level of risk to another.
Using a national database of primary care visits, the investigators isolated about 550,000 records. Of these, about 60,000 (11%) had records showing weight reductions of 10%-25% (mean, 13%) over at least 4 years. Weight loss was by intent rather than from illness. The remaining individuals maintained their weight within 5% of the first measurement for the duration of the study.
Participants with stable body weight were compared with the successful weight reducers. This analysis showed that the risk for type 2 diabetes, osteoarthritis, sleep apnea, hypertension, and dyslipidemia all measurably declined in weight reducers. This held true whether the patient’s baseline body mass index (BMI) showed modest or severe obesity. Patients with the highest BMI at enrollment actually reduced their risks for hypertension and dyslipidemia below population norms.
This study raises tantalizing, as yet unanswered questions: How did the successful 11% achieve their weight loss goals? Was it via a weight loss program, bariatric surgery, dietitian consult, or with no external assistance?
And of great significance to clinicians: What happened to the people who achieved 5%-10% weight reduction, as that is a more typical outcome of diabetes prevention trials or studies of weight-loss medications? Were they excluded from the study because they did not lose enough weight to achieve the unequivocal health benefit?
Because the data came from an enormous database, the weight management strategies leading to success or failure – what we really need to know to nudge our own patients into the favorable categories – remain hidden.
The Advantage of Intensive Interventions
Some answers emerged from a recently reported study in the New England Journal of Medicine comparing supervised diet and lifestyle adjustments (treatment group) with the less intense oversight typically offered by primary care clinicians (usual-care group).
The treatment group not only received the intensive lifestyle intervention, which focused on reduced caloric intake and increased physical activity, but also participated in mandated training sessions on how to best use the resources provided by the study. Much of the care was delegated by physicians to “coaches” who focused on nutrition, exercise, and behavioral health, including supermarket strategy.
Nearly a quarter of the participants in the intensive intervention group achieved the 10% weight reduction needed to change health risk in a meaningful way. A similar proportion lost less than 10% of their body weight, and about half did not have a notable weight change. Peak weight loss at 6 months averaged 17 lb, and 9.6 lb at 2 years. While this may not seem very impressive considering the extensive resources utilized, there were those who experienced an extraordinary health upgrade not otherwise available, short of bariatric surgery.
What does this mean for us?
Both studies indicate that, even under the best-controlled, resource-replete circumstances, the rate of failure to achieve desired progress is very high. But there is a success rate.
The likelihood of success is difficult to interpret from the European data, as it compared only those with major weight loss and those with weight stability, excluding patients with less robust loss or weight gain. The controlled study, however, holds forth an alluring opportunity benefiting a quarter of the targeted participants and even about 5% of the controls who realized that they were being observed.
We also learn that supervision requires a lot more than having a well-meaning but not very well-trained physician ask a patient to log measurements and food intake. Health coaches seem to make the impact.
Failure rates of 50% have a way of dampening enthusiasm, but it may be best to approach the scourge of obesity by offering treatment to everyone with the expectation that not all will experience greatly enhanced quality of life and longevity. Not everyone will benefit, but these two studies confirm that we do have an underutilized capacity to help more people benefit than we currently do.
Richard M. Plotzker, MD, is a retired endocrinologist with 40 years of experience treating patients in both the private practice and hospital settings. He has been a Medscape contributor since 2012.
A version of this article originally appeared on Medscape.com.
Every Wednesday evening after supper, I record in a marble notebook some anthropomorphic measurements: my weight taken first thing Monday morning and my waist circumference. I also add how I did with exercise since the previous week’s entry and some comments about sleep, energy, and nutrition.
My personal log now comprises dozens of pages. To my surprise, the first entry was 5 years ago to the month. The earlier entries were far from weekly and contained a lot of narrative on how my food-restriction scheme that month was being violated.
Looking just at the numbers, I did about as well as a control group participant in any medical study of diet modification. Until just a few months ago, there was no trend in either weight or waist circumference over those 5 years, including 2 years of retirement. But it wasn’t for lack of trying. Keeping the journal for as long as I have – and recently, as consistently as I have – suggests serious intent but inadequate execution of the same principles I offered patients, who rarely did much better. But recent studies suggest that perhaps quite a few could.
Are we underestimating our patients’ potential?
A recent abstract from the European and International Congress on Obesity suggests that the impressions clinicians get from our office encounters may leave us underestimating the potential for our patients to lose enough weight to move them from one level of risk to another.
Using a national database of primary care visits, the investigators isolated about 550,000 records. Of these, about 60,000 (11%) had records showing weight reductions of 10%-25% (mean, 13%) over at least 4 years. Weight loss was by intent rather than from illness. The remaining individuals maintained their weight within 5% of the first measurement for the duration of the study.
Participants with stable body weight were compared with the successful weight reducers. This analysis showed that the risk for type 2 diabetes, osteoarthritis, sleep apnea, hypertension, and dyslipidemia all measurably declined in weight reducers. This held true whether the patient’s baseline body mass index (BMI) showed modest or severe obesity. Patients with the highest BMI at enrollment actually reduced their risks for hypertension and dyslipidemia below population norms.
This study raises tantalizing, as yet unanswered questions: How did the successful 11% achieve their weight loss goals? Was it via a weight loss program, bariatric surgery, dietitian consult, or with no external assistance?
And of great significance to clinicians: What happened to the people who achieved 5%-10% weight reduction, as that is a more typical outcome of diabetes prevention trials or studies of weight-loss medications? Were they excluded from the study because they did not lose enough weight to achieve the unequivocal health benefit?
Because the data came from an enormous database, the weight management strategies leading to success or failure – what we really need to know to nudge our own patients into the favorable categories – remain hidden.
The Advantage of Intensive Interventions
Some answers emerged from a recently reported study in the New England Journal of Medicine comparing supervised diet and lifestyle adjustments (treatment group) with the less intense oversight typically offered by primary care clinicians (usual-care group).
The treatment group not only received the intensive lifestyle intervention, which focused on reduced caloric intake and increased physical activity, but also participated in mandated training sessions on how to best use the resources provided by the study. Much of the care was delegated by physicians to “coaches” who focused on nutrition, exercise, and behavioral health, including supermarket strategy.
Nearly a quarter of the participants in the intensive intervention group achieved the 10% weight reduction needed to change health risk in a meaningful way. A similar proportion lost less than 10% of their body weight, and about half did not have a notable weight change. Peak weight loss at 6 months averaged 17 lb, and 9.6 lb at 2 years. While this may not seem very impressive considering the extensive resources utilized, there were those who experienced an extraordinary health upgrade not otherwise available, short of bariatric surgery.
What does this mean for us?
Both studies indicate that, even under the best-controlled, resource-replete circumstances, the rate of failure to achieve desired progress is very high. But there is a success rate.
The likelihood of success is difficult to interpret from the European data, as it compared only those with major weight loss and those with weight stability, excluding patients with less robust loss or weight gain. The controlled study, however, holds forth an alluring opportunity benefiting a quarter of the targeted participants and even about 5% of the controls who realized that they were being observed.
We also learn that supervision requires a lot more than having a well-meaning but not very well-trained physician ask a patient to log measurements and food intake. Health coaches seem to make the impact.
Failure rates of 50% have a way of dampening enthusiasm, but it may be best to approach the scourge of obesity by offering treatment to everyone with the expectation that not all will experience greatly enhanced quality of life and longevity. Not everyone will benefit, but these two studies confirm that we do have an underutilized capacity to help more people benefit than we currently do.
Richard M. Plotzker, MD, is a retired endocrinologist with 40 years of experience treating patients in both the private practice and hospital settings. He has been a Medscape contributor since 2012.
A version of this article originally appeared on Medscape.com.
Diabetic retinopathy may predict greater risk of COVID-19 severity
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Noninvasive, low-cost CGM for type 2 diabetes coming in U.S. and EU
A novel lower-cost noninvasive continuous glucose monitor (CGM) combined with a digital education/guidance program is set to launch in the United States and Europe this month for use in type 2 diabetes.
With the goal of improving management, or even reversing the condition, Neumara’s SugarBEAT device is thought to be the world’s first noninvasive CGM.
Its cost is anticipated to be far lower than traditional CGM, and it’s aimed at a different patient population: those with type 2 diabetes or prediabetes who may or may not be performing fingerstick glucose monitoring, but if they are, they still aren’t using the information to guide management.
“This isn’t about handing out devices and letting patients get on about it on their own accord. This is really about supporting those individuals,” Faz Chowdhury, MD, Nemaura’s chief executive officer, said in an interview.
He pointed to studies showing improvements in glycemic control in patients with type 2 diabetes who were instructed to perform fingerstick blood glucose testing seven times a day for 3-4 days a month and given advice about how to respond to the data.
“This is well established. We’re saying we can make that process a lot more scalable and affordable and convenient for the patient. ... The behavior change side is digitized,” Dr. Chowdhury said. “We want to provide a program to help people reverse their diabetes or at least stabilize it as much as possible.”
Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, said in an interview: “It’s interesting. They’re taking a very different approach. I think there’s a lot of validity to what they’re looking at because we have great CGMs right now, but because of the price point it’s not accessible to a lot of people.
“I think they’re onto something that could prove to be useful to a larger group of patients,” he added.
Worn a few days per month and accurate despite being noninvasive
Instead of inserting a catheter under the skin with a needle, as do current CGMs, the device comprises a small rechargeable transmitter and adhesive patch with a sensor that sits on the top of the skin, typically the upper arm. Glucose molecules are drawn out of the interstitial fluid just below the skin and into a chamber where the transmitter measures the glucose level and transmits the data every 5 minutes via Bluetooth to a smartphone app.
Despite this noninvasive approach, the device appears to be about as accurate as traditional CGMs, with comparable mean absolute relative difference (MARD) from a gold standard glucose measure of about 11%-12% with once-daily calibration versus 10%-11% for the Abbott FreeStyle Libre.
Unlike traditional CGMs, SugarBEAT is meant to be worn for only 14 hours at a time during the day and for 2-4 days per month rather than every day.
It’s not aimed at patients with type 1 diabetes or those with type 2 diabetes who are at high risk for hypoglycemia. It requires once-daily fingerstick calibration and is not indicated to replace fingersticks for treatment decisions.
SugarBEAT received a CE Mark in Europe as a Class IIb medical device in May 2019. That version provides real-time glucose values visible to the wearer. In the United States the company submitted a premarketing approval application for the device to the Food and Drug Administration in July 2020, which awaits a decision.
However, FDA is allowing it to enter the U.S. market as a “wellness” device that won’t deliver real-time values for now but instead will generate retroactive reports available to the physician and the patient.
And last month, U.K.-based Neumara launched the BEATdiabetes site, which allows users to sign in and link to the device once it becomes available.
The site provides “scientifically validated, personalized coaching” based on a program developed at the Joslin Diabetes Clinic in Syracuse, N.Y., and will ultimately include monitoring of other cardiovascular risk factors with digital connectivity to a variety of wearables.
Fingerstick monitoring in type 2 diabetes is only so useful
“Fingerstick monitoring for type 2 diabetes is only so useful,” Dr. Argento said in an interview.
“It’s difficult to get people to monitor in a meaningful way.” If patients perform them only in the morning or at other sporadic times of the day, he said, “Then you get a one-dimensional picture ... and they don’t know what to do with the information anyway, so they stop doing it.”
In contrast, with SugarBEAT and BEATDiabetes, “I think it does address a need that fingerstick monitoring doesn’t.”
Dr. Argento did express a few caveats about the device, however. For one, it still requires one fingerstick a day for calibration. “If people don’t like needles, that might be a disincentive.”
Also, despite the apparently comparable mean absolute relative difference with that of conventional CGMs, that measure can still “hide” values that may be consistently either above or below target range.
“MARD is like A1c in that it’s useful but limited. ... It doesn’t tell you about variability or systemic bias,” he said.
Dr. Argento also said that he’d like to see data on the lag time between the interstitial fluid and blood glucose measures with this noninvasive method as compared with that of a subcutaneous catheter.
However, he acknowledged that these potentials for error would be less important for patients with type 2 diabetes who aren’t generally taking medications that increase their risk for hypoglycemia.
In all, he said, “stay tuned. I think this is part of a movement going away from point-in-time to looking at trends and wearables and data to enrich decision-making…There are still some unanswered questions I have but I think they’re onto a concept that’s useful for a broader population.”
Dr. Chowdhury is an employee of Neumara. Dr. Argento consults for Senseonics and Dexcom, and is also a speaker for Dexcom.
This article first appeared on Medscape.com.
A novel lower-cost noninvasive continuous glucose monitor (CGM) combined with a digital education/guidance program is set to launch in the United States and Europe this month for use in type 2 diabetes.
With the goal of improving management, or even reversing the condition, Neumara’s SugarBEAT device is thought to be the world’s first noninvasive CGM.
Its cost is anticipated to be far lower than traditional CGM, and it’s aimed at a different patient population: those with type 2 diabetes or prediabetes who may or may not be performing fingerstick glucose monitoring, but if they are, they still aren’t using the information to guide management.
“This isn’t about handing out devices and letting patients get on about it on their own accord. This is really about supporting those individuals,” Faz Chowdhury, MD, Nemaura’s chief executive officer, said in an interview.
He pointed to studies showing improvements in glycemic control in patients with type 2 diabetes who were instructed to perform fingerstick blood glucose testing seven times a day for 3-4 days a month and given advice about how to respond to the data.
“This is well established. We’re saying we can make that process a lot more scalable and affordable and convenient for the patient. ... The behavior change side is digitized,” Dr. Chowdhury said. “We want to provide a program to help people reverse their diabetes or at least stabilize it as much as possible.”
Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, said in an interview: “It’s interesting. They’re taking a very different approach. I think there’s a lot of validity to what they’re looking at because we have great CGMs right now, but because of the price point it’s not accessible to a lot of people.
“I think they’re onto something that could prove to be useful to a larger group of patients,” he added.
Worn a few days per month and accurate despite being noninvasive
Instead of inserting a catheter under the skin with a needle, as do current CGMs, the device comprises a small rechargeable transmitter and adhesive patch with a sensor that sits on the top of the skin, typically the upper arm. Glucose molecules are drawn out of the interstitial fluid just below the skin and into a chamber where the transmitter measures the glucose level and transmits the data every 5 minutes via Bluetooth to a smartphone app.
Despite this noninvasive approach, the device appears to be about as accurate as traditional CGMs, with comparable mean absolute relative difference (MARD) from a gold standard glucose measure of about 11%-12% with once-daily calibration versus 10%-11% for the Abbott FreeStyle Libre.
Unlike traditional CGMs, SugarBEAT is meant to be worn for only 14 hours at a time during the day and for 2-4 days per month rather than every day.
It’s not aimed at patients with type 1 diabetes or those with type 2 diabetes who are at high risk for hypoglycemia. It requires once-daily fingerstick calibration and is not indicated to replace fingersticks for treatment decisions.
SugarBEAT received a CE Mark in Europe as a Class IIb medical device in May 2019. That version provides real-time glucose values visible to the wearer. In the United States the company submitted a premarketing approval application for the device to the Food and Drug Administration in July 2020, which awaits a decision.
However, FDA is allowing it to enter the U.S. market as a “wellness” device that won’t deliver real-time values for now but instead will generate retroactive reports available to the physician and the patient.
And last month, U.K.-based Neumara launched the BEATdiabetes site, which allows users to sign in and link to the device once it becomes available.
The site provides “scientifically validated, personalized coaching” based on a program developed at the Joslin Diabetes Clinic in Syracuse, N.Y., and will ultimately include monitoring of other cardiovascular risk factors with digital connectivity to a variety of wearables.
Fingerstick monitoring in type 2 diabetes is only so useful
“Fingerstick monitoring for type 2 diabetes is only so useful,” Dr. Argento said in an interview.
“It’s difficult to get people to monitor in a meaningful way.” If patients perform them only in the morning or at other sporadic times of the day, he said, “Then you get a one-dimensional picture ... and they don’t know what to do with the information anyway, so they stop doing it.”
In contrast, with SugarBEAT and BEATDiabetes, “I think it does address a need that fingerstick monitoring doesn’t.”
Dr. Argento did express a few caveats about the device, however. For one, it still requires one fingerstick a day for calibration. “If people don’t like needles, that might be a disincentive.”
Also, despite the apparently comparable mean absolute relative difference with that of conventional CGMs, that measure can still “hide” values that may be consistently either above or below target range.
“MARD is like A1c in that it’s useful but limited. ... It doesn’t tell you about variability or systemic bias,” he said.
Dr. Argento also said that he’d like to see data on the lag time between the interstitial fluid and blood glucose measures with this noninvasive method as compared with that of a subcutaneous catheter.
However, he acknowledged that these potentials for error would be less important for patients with type 2 diabetes who aren’t generally taking medications that increase their risk for hypoglycemia.
In all, he said, “stay tuned. I think this is part of a movement going away from point-in-time to looking at trends and wearables and data to enrich decision-making…There are still some unanswered questions I have but I think they’re onto a concept that’s useful for a broader population.”
Dr. Chowdhury is an employee of Neumara. Dr. Argento consults for Senseonics and Dexcom, and is also a speaker for Dexcom.
This article first appeared on Medscape.com.
A novel lower-cost noninvasive continuous glucose monitor (CGM) combined with a digital education/guidance program is set to launch in the United States and Europe this month for use in type 2 diabetes.
With the goal of improving management, or even reversing the condition, Neumara’s SugarBEAT device is thought to be the world’s first noninvasive CGM.
Its cost is anticipated to be far lower than traditional CGM, and it’s aimed at a different patient population: those with type 2 diabetes or prediabetes who may or may not be performing fingerstick glucose monitoring, but if they are, they still aren’t using the information to guide management.
“This isn’t about handing out devices and letting patients get on about it on their own accord. This is really about supporting those individuals,” Faz Chowdhury, MD, Nemaura’s chief executive officer, said in an interview.
He pointed to studies showing improvements in glycemic control in patients with type 2 diabetes who were instructed to perform fingerstick blood glucose testing seven times a day for 3-4 days a month and given advice about how to respond to the data.
“This is well established. We’re saying we can make that process a lot more scalable and affordable and convenient for the patient. ... The behavior change side is digitized,” Dr. Chowdhury said. “We want to provide a program to help people reverse their diabetes or at least stabilize it as much as possible.”
Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, said in an interview: “It’s interesting. They’re taking a very different approach. I think there’s a lot of validity to what they’re looking at because we have great CGMs right now, but because of the price point it’s not accessible to a lot of people.
“I think they’re onto something that could prove to be useful to a larger group of patients,” he added.
Worn a few days per month and accurate despite being noninvasive
Instead of inserting a catheter under the skin with a needle, as do current CGMs, the device comprises a small rechargeable transmitter and adhesive patch with a sensor that sits on the top of the skin, typically the upper arm. Glucose molecules are drawn out of the interstitial fluid just below the skin and into a chamber where the transmitter measures the glucose level and transmits the data every 5 minutes via Bluetooth to a smartphone app.
Despite this noninvasive approach, the device appears to be about as accurate as traditional CGMs, with comparable mean absolute relative difference (MARD) from a gold standard glucose measure of about 11%-12% with once-daily calibration versus 10%-11% for the Abbott FreeStyle Libre.
Unlike traditional CGMs, SugarBEAT is meant to be worn for only 14 hours at a time during the day and for 2-4 days per month rather than every day.
It’s not aimed at patients with type 1 diabetes or those with type 2 diabetes who are at high risk for hypoglycemia. It requires once-daily fingerstick calibration and is not indicated to replace fingersticks for treatment decisions.
SugarBEAT received a CE Mark in Europe as a Class IIb medical device in May 2019. That version provides real-time glucose values visible to the wearer. In the United States the company submitted a premarketing approval application for the device to the Food and Drug Administration in July 2020, which awaits a decision.
However, FDA is allowing it to enter the U.S. market as a “wellness” device that won’t deliver real-time values for now but instead will generate retroactive reports available to the physician and the patient.
And last month, U.K.-based Neumara launched the BEATdiabetes site, which allows users to sign in and link to the device once it becomes available.
The site provides “scientifically validated, personalized coaching” based on a program developed at the Joslin Diabetes Clinic in Syracuse, N.Y., and will ultimately include monitoring of other cardiovascular risk factors with digital connectivity to a variety of wearables.
Fingerstick monitoring in type 2 diabetes is only so useful
“Fingerstick monitoring for type 2 diabetes is only so useful,” Dr. Argento said in an interview.
“It’s difficult to get people to monitor in a meaningful way.” If patients perform them only in the morning or at other sporadic times of the day, he said, “Then you get a one-dimensional picture ... and they don’t know what to do with the information anyway, so they stop doing it.”
In contrast, with SugarBEAT and BEATDiabetes, “I think it does address a need that fingerstick monitoring doesn’t.”
Dr. Argento did express a few caveats about the device, however. For one, it still requires one fingerstick a day for calibration. “If people don’t like needles, that might be a disincentive.”
Also, despite the apparently comparable mean absolute relative difference with that of conventional CGMs, that measure can still “hide” values that may be consistently either above or below target range.
“MARD is like A1c in that it’s useful but limited. ... It doesn’t tell you about variability or systemic bias,” he said.
Dr. Argento also said that he’d like to see data on the lag time between the interstitial fluid and blood glucose measures with this noninvasive method as compared with that of a subcutaneous catheter.
However, he acknowledged that these potentials for error would be less important for patients with type 2 diabetes who aren’t generally taking medications that increase their risk for hypoglycemia.
In all, he said, “stay tuned. I think this is part of a movement going away from point-in-time to looking at trends and wearables and data to enrich decision-making…There are still some unanswered questions I have but I think they’re onto a concept that’s useful for a broader population.”
Dr. Chowdhury is an employee of Neumara. Dr. Argento consults for Senseonics and Dexcom, and is also a speaker for Dexcom.
This article first appeared on Medscape.com.
Patient health suffers amid pandemic health care shortages
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
New study pinpoints how Mediterranean diet reduces diabetes risk
The known reduction in the risk of type 2 diabetes associated with adoption of the Mediterranean diet appears specifically attributed to its beneficial effects on some key factors, a new study published online in JAMA Network Open reveals.
While a reduction in body mass index may be somewhat obvious, other mechanisms include beneficial effects on insulin resistance, lipoprotein metabolism, and inflammation.
However, the diet’s antidiabetes effect does not appear to extend to people whose weight is considered healthy (BMI under 25 kg/m2), according to the findings.
“It is striking to see in these U.S. women how strong the long-term antidiabetic properties of a Mediterranean-type dietary pattern are,” senior author Samia Mora, MD, of the Center for Lipid Metabolomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, said in an interview.
“While it was known that the Mediterranean diet has many health benefits in particular on metabolism and inflammation, it was not previously known which of these biological pathways may be contributing to the lower risk of diabetes and to what magnitude.
“Our findings support the idea that by improving their diet, people can improve their future risk of type 2 diabetes, particularly if they are overweight or have obesity,” she added.
“And it’s important to note that many of these changes don’t happen right away. While metabolism can change over a short period of time, our study indicates that there are longer term changes happening that may provide protection over decades.”
Mediterranean diet reduced diabetes risk in those with BMI ≥ 25 kg/m2
The Mediterranean diet, with an emphasis on healthy olive oil as the predominant source of oil, favors fruits, vegetables, legumes, nuts, seeds, fish, and dairy products, while limiting intake of red and processed meats as well as sweets.
The diet has been linked to as much as a 25%-30% reduction in the risk of diabetes in previous observational studies.
To investigate the precise mechanisms that underlie the prevention of diabetes, lead author Shafqat Ahmad, PhD, also of Harvard, and colleagues examined data from 25,317 healthy women participating in the Women’s Health Study who had baseline assessments between September 1992 and May 1995. They were a mean age of 52.9 years at baseline.
Over the course of the study, 2,307 participants developed type 2 diabetes.
With a mean follow-up of 19.8 years, those who had the highest self-reported adherence to the Mediterranean diet (a score ≥ 6 on a scale of 0-6) at baseline, had as much as a 30% lower risk of developing type 2 diabetes after multivariate adjustments, compared to those with a lower Mediterranean diet score (a score ≤ 3; hazard ratio, 0.70).
The diabetes-related biomarkers that contributed the most to the reduced risk were insulin resistance, accounting for 65% of the reduction, followed by BMI (55.5%), high-density lipoprotein measures (53%), and inflammation (52.5%).
Other factors, though to a lesser degree, included branched-chain amino acids (34.5%), very low-density lipoprotein measures (32.0%), low-density lipoprotein measures (31.0%), blood pressure (29.0%), and apolipoproteins (23.5%).
Differences in hemoglobin A1c levels had a limited effect on the risk (2%).
Notably, a subgroup analysis looking at effects of the diet according to baseline BMI showed the reductions in type 2 diabetes associated with higher intake of the Mediterranean diet extended only to those with an above normal weight (BMI ≥ 25 kg/m2).
Dr. Mora noted that, as this was not a prespecified analysis, these findings should be viewed as hypothesis-generating, but are consistent with the well-known increase in diabetes risk seen with a higher BMI.
“[The finding] fits with the biology and pathogenesis of type 2 diabetes that is driven in large part by excess weight, in particular for visceral adiposity and its resulting metabolic dysregulation and inflammation,” she said.
“We know from other studies, such as the Nurses’ Health Study, that the risk for type 2 diabetes in women increases even at BMI levels below 25 kg/m2, but the risk goes up exponentially at around a BMI of 25 and higher.”
Strong role of insulin resistance a surprise
The strong role of insulin resistance was a surprise, Dr. Mora added.
“We were surprised that insulin resistance, measured by a simple blood biomarker, would have the strongest mediating effect – even stronger than BMI – for the Mediterranean diet on risk of diabetes,” she noted.
“This could represent an opportunity to intervene earlier and more intensively on improving insulin resistance through dietary approaches such as the Mediterranean diet, especially [because] insulin resistance can precede by years and decades the overt hyperglycemia and clinical diagnosis of diabetes.”
Yet another surprise was that A1c had no substantial mediating effect on the reduction of diabetes risk with the Mediterranean diet.
“This could suggest that the cat is out of the bag by the time the A1c rises,” Dr. Mora observed.
A study limitation is that the Women’s Health Study consisted of well-educated U.S. women who were health professionals and predominantly White, so the results may not be generalizable to men or individuals of other races or ethnicities.
In addition, BMI was self-reported and participants were not uniformly screened for diabetes, therefore surveillance bias could be possible.
However, the findings suggest that “even a small increase in adherence to the Mediterranean diet has substantial benefits over many years in preventing diabetes, among many other health benefits such as lowering insulin resistance and inflammation, improving lipid metabolism, and lowering blood pressure,” Mora said.
“And of course, the more the adherence, the more the benefit.”
The study received support through grants from the National Institutes of Health, the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the American Heart Association, and the Molino Family Trust. A coauthor is listed as a coinventor on patents held by Brigham and Women’s Hospital related to the use of inflammatory biomarkers in cardiovascular disease (licensed to AstraZeneca and Siemens).
A version of this article originally appeared on Medscape.com.
The known reduction in the risk of type 2 diabetes associated with adoption of the Mediterranean diet appears specifically attributed to its beneficial effects on some key factors, a new study published online in JAMA Network Open reveals.
While a reduction in body mass index may be somewhat obvious, other mechanisms include beneficial effects on insulin resistance, lipoprotein metabolism, and inflammation.
However, the diet’s antidiabetes effect does not appear to extend to people whose weight is considered healthy (BMI under 25 kg/m2), according to the findings.
“It is striking to see in these U.S. women how strong the long-term antidiabetic properties of a Mediterranean-type dietary pattern are,” senior author Samia Mora, MD, of the Center for Lipid Metabolomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, said in an interview.
“While it was known that the Mediterranean diet has many health benefits in particular on metabolism and inflammation, it was not previously known which of these biological pathways may be contributing to the lower risk of diabetes and to what magnitude.
“Our findings support the idea that by improving their diet, people can improve their future risk of type 2 diabetes, particularly if they are overweight or have obesity,” she added.
“And it’s important to note that many of these changes don’t happen right away. While metabolism can change over a short period of time, our study indicates that there are longer term changes happening that may provide protection over decades.”
Mediterranean diet reduced diabetes risk in those with BMI ≥ 25 kg/m2
The Mediterranean diet, with an emphasis on healthy olive oil as the predominant source of oil, favors fruits, vegetables, legumes, nuts, seeds, fish, and dairy products, while limiting intake of red and processed meats as well as sweets.
The diet has been linked to as much as a 25%-30% reduction in the risk of diabetes in previous observational studies.
To investigate the precise mechanisms that underlie the prevention of diabetes, lead author Shafqat Ahmad, PhD, also of Harvard, and colleagues examined data from 25,317 healthy women participating in the Women’s Health Study who had baseline assessments between September 1992 and May 1995. They were a mean age of 52.9 years at baseline.
Over the course of the study, 2,307 participants developed type 2 diabetes.
With a mean follow-up of 19.8 years, those who had the highest self-reported adherence to the Mediterranean diet (a score ≥ 6 on a scale of 0-6) at baseline, had as much as a 30% lower risk of developing type 2 diabetes after multivariate adjustments, compared to those with a lower Mediterranean diet score (a score ≤ 3; hazard ratio, 0.70).
The diabetes-related biomarkers that contributed the most to the reduced risk were insulin resistance, accounting for 65% of the reduction, followed by BMI (55.5%), high-density lipoprotein measures (53%), and inflammation (52.5%).
Other factors, though to a lesser degree, included branched-chain amino acids (34.5%), very low-density lipoprotein measures (32.0%), low-density lipoprotein measures (31.0%), blood pressure (29.0%), and apolipoproteins (23.5%).
Differences in hemoglobin A1c levels had a limited effect on the risk (2%).
Notably, a subgroup analysis looking at effects of the diet according to baseline BMI showed the reductions in type 2 diabetes associated with higher intake of the Mediterranean diet extended only to those with an above normal weight (BMI ≥ 25 kg/m2).
Dr. Mora noted that, as this was not a prespecified analysis, these findings should be viewed as hypothesis-generating, but are consistent with the well-known increase in diabetes risk seen with a higher BMI.
“[The finding] fits with the biology and pathogenesis of type 2 diabetes that is driven in large part by excess weight, in particular for visceral adiposity and its resulting metabolic dysregulation and inflammation,” she said.
“We know from other studies, such as the Nurses’ Health Study, that the risk for type 2 diabetes in women increases even at BMI levels below 25 kg/m2, but the risk goes up exponentially at around a BMI of 25 and higher.”
Strong role of insulin resistance a surprise
The strong role of insulin resistance was a surprise, Dr. Mora added.
“We were surprised that insulin resistance, measured by a simple blood biomarker, would have the strongest mediating effect – even stronger than BMI – for the Mediterranean diet on risk of diabetes,” she noted.
“This could represent an opportunity to intervene earlier and more intensively on improving insulin resistance through dietary approaches such as the Mediterranean diet, especially [because] insulin resistance can precede by years and decades the overt hyperglycemia and clinical diagnosis of diabetes.”
Yet another surprise was that A1c had no substantial mediating effect on the reduction of diabetes risk with the Mediterranean diet.
“This could suggest that the cat is out of the bag by the time the A1c rises,” Dr. Mora observed.
A study limitation is that the Women’s Health Study consisted of well-educated U.S. women who were health professionals and predominantly White, so the results may not be generalizable to men or individuals of other races or ethnicities.
In addition, BMI was self-reported and participants were not uniformly screened for diabetes, therefore surveillance bias could be possible.
However, the findings suggest that “even a small increase in adherence to the Mediterranean diet has substantial benefits over many years in preventing diabetes, among many other health benefits such as lowering insulin resistance and inflammation, improving lipid metabolism, and lowering blood pressure,” Mora said.
“And of course, the more the adherence, the more the benefit.”
The study received support through grants from the National Institutes of Health, the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the American Heart Association, and the Molino Family Trust. A coauthor is listed as a coinventor on patents held by Brigham and Women’s Hospital related to the use of inflammatory biomarkers in cardiovascular disease (licensed to AstraZeneca and Siemens).
A version of this article originally appeared on Medscape.com.
The known reduction in the risk of type 2 diabetes associated with adoption of the Mediterranean diet appears specifically attributed to its beneficial effects on some key factors, a new study published online in JAMA Network Open reveals.
While a reduction in body mass index may be somewhat obvious, other mechanisms include beneficial effects on insulin resistance, lipoprotein metabolism, and inflammation.
However, the diet’s antidiabetes effect does not appear to extend to people whose weight is considered healthy (BMI under 25 kg/m2), according to the findings.
“It is striking to see in these U.S. women how strong the long-term antidiabetic properties of a Mediterranean-type dietary pattern are,” senior author Samia Mora, MD, of the Center for Lipid Metabolomics, Brigham and Women’s Hospital, Harvard Medical School, Boston, said in an interview.
“While it was known that the Mediterranean diet has many health benefits in particular on metabolism and inflammation, it was not previously known which of these biological pathways may be contributing to the lower risk of diabetes and to what magnitude.
“Our findings support the idea that by improving their diet, people can improve their future risk of type 2 diabetes, particularly if they are overweight or have obesity,” she added.
“And it’s important to note that many of these changes don’t happen right away. While metabolism can change over a short period of time, our study indicates that there are longer term changes happening that may provide protection over decades.”
Mediterranean diet reduced diabetes risk in those with BMI ≥ 25 kg/m2
The Mediterranean diet, with an emphasis on healthy olive oil as the predominant source of oil, favors fruits, vegetables, legumes, nuts, seeds, fish, and dairy products, while limiting intake of red and processed meats as well as sweets.
The diet has been linked to as much as a 25%-30% reduction in the risk of diabetes in previous observational studies.
To investigate the precise mechanisms that underlie the prevention of diabetes, lead author Shafqat Ahmad, PhD, also of Harvard, and colleagues examined data from 25,317 healthy women participating in the Women’s Health Study who had baseline assessments between September 1992 and May 1995. They were a mean age of 52.9 years at baseline.
Over the course of the study, 2,307 participants developed type 2 diabetes.
With a mean follow-up of 19.8 years, those who had the highest self-reported adherence to the Mediterranean diet (a score ≥ 6 on a scale of 0-6) at baseline, had as much as a 30% lower risk of developing type 2 diabetes after multivariate adjustments, compared to those with a lower Mediterranean diet score (a score ≤ 3; hazard ratio, 0.70).
The diabetes-related biomarkers that contributed the most to the reduced risk were insulin resistance, accounting for 65% of the reduction, followed by BMI (55.5%), high-density lipoprotein measures (53%), and inflammation (52.5%).
Other factors, though to a lesser degree, included branched-chain amino acids (34.5%), very low-density lipoprotein measures (32.0%), low-density lipoprotein measures (31.0%), blood pressure (29.0%), and apolipoproteins (23.5%).
Differences in hemoglobin A1c levels had a limited effect on the risk (2%).
Notably, a subgroup analysis looking at effects of the diet according to baseline BMI showed the reductions in type 2 diabetes associated with higher intake of the Mediterranean diet extended only to those with an above normal weight (BMI ≥ 25 kg/m2).
Dr. Mora noted that, as this was not a prespecified analysis, these findings should be viewed as hypothesis-generating, but are consistent with the well-known increase in diabetes risk seen with a higher BMI.
“[The finding] fits with the biology and pathogenesis of type 2 diabetes that is driven in large part by excess weight, in particular for visceral adiposity and its resulting metabolic dysregulation and inflammation,” she said.
“We know from other studies, such as the Nurses’ Health Study, that the risk for type 2 diabetes in women increases even at BMI levels below 25 kg/m2, but the risk goes up exponentially at around a BMI of 25 and higher.”
Strong role of insulin resistance a surprise
The strong role of insulin resistance was a surprise, Dr. Mora added.
“We were surprised that insulin resistance, measured by a simple blood biomarker, would have the strongest mediating effect – even stronger than BMI – for the Mediterranean diet on risk of diabetes,” she noted.
“This could represent an opportunity to intervene earlier and more intensively on improving insulin resistance through dietary approaches such as the Mediterranean diet, especially [because] insulin resistance can precede by years and decades the overt hyperglycemia and clinical diagnosis of diabetes.”
Yet another surprise was that A1c had no substantial mediating effect on the reduction of diabetes risk with the Mediterranean diet.
“This could suggest that the cat is out of the bag by the time the A1c rises,” Dr. Mora observed.
A study limitation is that the Women’s Health Study consisted of well-educated U.S. women who were health professionals and predominantly White, so the results may not be generalizable to men or individuals of other races or ethnicities.
In addition, BMI was self-reported and participants were not uniformly screened for diabetes, therefore surveillance bias could be possible.
However, the findings suggest that “even a small increase in adherence to the Mediterranean diet has substantial benefits over many years in preventing diabetes, among many other health benefits such as lowering insulin resistance and inflammation, improving lipid metabolism, and lowering blood pressure,” Mora said.
“And of course, the more the adherence, the more the benefit.”
The study received support through grants from the National Institutes of Health, the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the American Heart Association, and the Molino Family Trust. A coauthor is listed as a coinventor on patents held by Brigham and Women’s Hospital related to the use of inflammatory biomarkers in cardiovascular disease (licensed to AstraZeneca and Siemens).
A version of this article originally appeared on Medscape.com.
Metformin improves most outcomes for T2D during pregnancy
including reduced weight gain, reduced insulin doses, and fewer large-for-gestational-age babies, suggest the results of a randomized controlled trial.
However, the drug was associated with an increased risk of small-for-gestational-age babies, which poses the question as to risk versus benefit of metformin on the health of offspring.
“Better understanding of the short- and long-term implications of these effects on infants will be important to properly advise patients with type 2 diabetes contemplating use of metformin during pregnancy,” said lead author Denice S. Feig, MD, Mount Sinai Hospital, Toronto.
The research was presented at the Diabetes UK Professional Conference: Online Series on Nov. 17 and recently published in The Lancet Diabetes & Endocrinology.
Summing up, Dr. Feig said that, on balance, she would be inclined to give metformin to most pregnant women with type 2 diabetes, perhaps with the exception of those who may have risk factors for small-for-gestational-age babies; for example, women who’ve had intrauterine growth restriction, who are smokers, and have significant renal disease, or have a lower body mass index.
Increased prevalence of type 2 diabetes in pregnancy
Dr. Feig said that across the developed world there have been huge increases in the prevalence of type 2 diabetes in pregnancy in recent years.
Insulin is the standard treatment for the management of type 2 diabetes in pregnancy, but these women have marked insulin resistance that worsens in pregnancy, which means their insulin requirements increase, leading to weight gain, painful injections, high cost, and noncompliance.
So despite treatment with insulin, these women continue to face increased rates of adverse maternal and fetal outcomes.
And although metformin is increasingly being used in women with type 2 diabetes during pregnancy, there is a scarcity of data on the benefits and harms of metformin use on pregnancy outcomes in these women.
The MiTy trial was therefore undertaken to determine whether metformin could improve outcomes.
The team recruited 502 women from 29 sites in Canada and Australia who had type 2 diabetes prior to pregnancy or were diagnosed during pregnancy, before 20 weeks’ gestation. The women were randomized to metformin 1 g twice daily or placebo, in addition to their usual insulin regimen, at between 6 and 28 weeks’ gestation.
Type 2 diabetes was diagnosed prior to pregnancy in 83% of women in the metformin group and in 90% of those assigned to placebo. The mean hemoglobin A1c level at randomization was 47 mmol/mol (6.5%) in both groups.
The average maternal age at baseline was approximately 35 years and mean gestational age at randomization was 16 weeks. Mean prepregnancy BMI was approximately 34 kg/m2.
Of note, only 30% were of European ethnicity.
Less weight gain, lower A1c, less insulin needed with metformin
Dr. Feig reported that there was no significant difference between the treatment groups in terms of the proportion of women with the composite primary outcome of pregnancy loss, preterm birth, birth injury, respiratory distress, neonatal hypoglycemia, or admission to neonatal intensive care lasting more than 24 hours (P = 0.86).
However, women in the metformin group had significantly less overall weight gain during pregnancy than did those in the placebo group, at –1.8 kg (P < .0001).
They also had a significantly lower last A1c level in pregnancy, at 41 mmol/mol (5.9%) versus 43.2 mmol/mol (6.1%) in those given placebo (P = .015), and required fewer insulin doses, at 1.1 versus 1.5 units/kg/day (P < .0001), which translated to a reduction of almost 44 units/day.
Women given metformin were also less likely to require Cesarean section delivery, at 53.4% versus 62.7% in the placebo group (P = .03), although there was no difference between groups in terms of gestational hypertension or preeclampsia.
The most common adverse events were gastrointestinal complications, which occurred in 27.3% of women in the metformin group and 22.3% of those given placebo.
There were no significant differences between the metformin and placebo groups in rates of pregnancy loss (P = .81), preterm birth (P = .16), birth injury (P = .37), respiratory distress (P = .49), and congenital anomalies (P = .16).
Average birth weight lower with metformin
However, Dr. Feig showed that the average birth weight was lower for offspring of women given metformin than those assigned to placebo, at 3.2 kg (7.05 lb) versus 3.4 kg (7.4 lb) (P = .002).
Women given metformin were also less likely to have a baby with a birth weight of 4 kg (8.8 lb) or more, at 12.1% versus 19.2%, or a relative risk of 0.65 (P = .046), and a baby that was extremely large for gestational age, at 8.6% versus 14.8%, or a relative risk of 0.58 (P = .046).
But of concern, metformin was associated with an increased risk of small-for-gestational-age babies, at 12.9% versus 6.6% with placebo, or a relative risk of 1.96 (P = .03).
Dr. Feig suggested that this may be due to a direct effect of metformin “because as we know metformin inhibits the mTOR pathway,” which is a “primary nutrient sensor in the placenta” and could “attenuate nutrient flux and fetal growth.”
She said it is not clear whether the small-for-gestational-age babies were “healthy or unhealthy.”
To investigate further, the team has launched the MiTy Kids study, which will follow the offspring in the MiTy trial to determine whether metformin during pregnancy is associated with a reduction in adiposity and improvement in insulin resistance in the babies at 2 years of age.
Who should be given metformin?
During the discussion, Helen R. Murphy, MD, PhD, Norwich Medical School, University of East Anglia, England, asked whether Dr. Feig would recommend continuing metformin in pregnancy if it was started preconception for fertility issues rather than diabetes.
She replied: “If they don’t have diabetes and it’s simply for PCOS [polycystic ovary syndrome], then I have either stopped it as soon as they got pregnant or sometimes continued it through the first trimester, and then stopped.
“If the person has diabetes, however, I think given this work, for most people I would continue it,” she said.
The study was funded by the Canadian Institutes of Health Research, Lunenfeld-Tanenbaum Research Institute, and the University of Toronto. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
including reduced weight gain, reduced insulin doses, and fewer large-for-gestational-age babies, suggest the results of a randomized controlled trial.
However, the drug was associated with an increased risk of small-for-gestational-age babies, which poses the question as to risk versus benefit of metformin on the health of offspring.
“Better understanding of the short- and long-term implications of these effects on infants will be important to properly advise patients with type 2 diabetes contemplating use of metformin during pregnancy,” said lead author Denice S. Feig, MD, Mount Sinai Hospital, Toronto.
The research was presented at the Diabetes UK Professional Conference: Online Series on Nov. 17 and recently published in The Lancet Diabetes & Endocrinology.
Summing up, Dr. Feig said that, on balance, she would be inclined to give metformin to most pregnant women with type 2 diabetes, perhaps with the exception of those who may have risk factors for small-for-gestational-age babies; for example, women who’ve had intrauterine growth restriction, who are smokers, and have significant renal disease, or have a lower body mass index.
Increased prevalence of type 2 diabetes in pregnancy
Dr. Feig said that across the developed world there have been huge increases in the prevalence of type 2 diabetes in pregnancy in recent years.
Insulin is the standard treatment for the management of type 2 diabetes in pregnancy, but these women have marked insulin resistance that worsens in pregnancy, which means their insulin requirements increase, leading to weight gain, painful injections, high cost, and noncompliance.
So despite treatment with insulin, these women continue to face increased rates of adverse maternal and fetal outcomes.
And although metformin is increasingly being used in women with type 2 diabetes during pregnancy, there is a scarcity of data on the benefits and harms of metformin use on pregnancy outcomes in these women.
The MiTy trial was therefore undertaken to determine whether metformin could improve outcomes.
The team recruited 502 women from 29 sites in Canada and Australia who had type 2 diabetes prior to pregnancy or were diagnosed during pregnancy, before 20 weeks’ gestation. The women were randomized to metformin 1 g twice daily or placebo, in addition to their usual insulin regimen, at between 6 and 28 weeks’ gestation.
Type 2 diabetes was diagnosed prior to pregnancy in 83% of women in the metformin group and in 90% of those assigned to placebo. The mean hemoglobin A1c level at randomization was 47 mmol/mol (6.5%) in both groups.
The average maternal age at baseline was approximately 35 years and mean gestational age at randomization was 16 weeks. Mean prepregnancy BMI was approximately 34 kg/m2.
Of note, only 30% were of European ethnicity.
Less weight gain, lower A1c, less insulin needed with metformin
Dr. Feig reported that there was no significant difference between the treatment groups in terms of the proportion of women with the composite primary outcome of pregnancy loss, preterm birth, birth injury, respiratory distress, neonatal hypoglycemia, or admission to neonatal intensive care lasting more than 24 hours (P = 0.86).
However, women in the metformin group had significantly less overall weight gain during pregnancy than did those in the placebo group, at –1.8 kg (P < .0001).
They also had a significantly lower last A1c level in pregnancy, at 41 mmol/mol (5.9%) versus 43.2 mmol/mol (6.1%) in those given placebo (P = .015), and required fewer insulin doses, at 1.1 versus 1.5 units/kg/day (P < .0001), which translated to a reduction of almost 44 units/day.
Women given metformin were also less likely to require Cesarean section delivery, at 53.4% versus 62.7% in the placebo group (P = .03), although there was no difference between groups in terms of gestational hypertension or preeclampsia.
The most common adverse events were gastrointestinal complications, which occurred in 27.3% of women in the metformin group and 22.3% of those given placebo.
There were no significant differences between the metformin and placebo groups in rates of pregnancy loss (P = .81), preterm birth (P = .16), birth injury (P = .37), respiratory distress (P = .49), and congenital anomalies (P = .16).
Average birth weight lower with metformin
However, Dr. Feig showed that the average birth weight was lower for offspring of women given metformin than those assigned to placebo, at 3.2 kg (7.05 lb) versus 3.4 kg (7.4 lb) (P = .002).
Women given metformin were also less likely to have a baby with a birth weight of 4 kg (8.8 lb) or more, at 12.1% versus 19.2%, or a relative risk of 0.65 (P = .046), and a baby that was extremely large for gestational age, at 8.6% versus 14.8%, or a relative risk of 0.58 (P = .046).
But of concern, metformin was associated with an increased risk of small-for-gestational-age babies, at 12.9% versus 6.6% with placebo, or a relative risk of 1.96 (P = .03).
Dr. Feig suggested that this may be due to a direct effect of metformin “because as we know metformin inhibits the mTOR pathway,” which is a “primary nutrient sensor in the placenta” and could “attenuate nutrient flux and fetal growth.”
She said it is not clear whether the small-for-gestational-age babies were “healthy or unhealthy.”
To investigate further, the team has launched the MiTy Kids study, which will follow the offspring in the MiTy trial to determine whether metformin during pregnancy is associated with a reduction in adiposity and improvement in insulin resistance in the babies at 2 years of age.
Who should be given metformin?
During the discussion, Helen R. Murphy, MD, PhD, Norwich Medical School, University of East Anglia, England, asked whether Dr. Feig would recommend continuing metformin in pregnancy if it was started preconception for fertility issues rather than diabetes.
She replied: “If they don’t have diabetes and it’s simply for PCOS [polycystic ovary syndrome], then I have either stopped it as soon as they got pregnant or sometimes continued it through the first trimester, and then stopped.
“If the person has diabetes, however, I think given this work, for most people I would continue it,” she said.
The study was funded by the Canadian Institutes of Health Research, Lunenfeld-Tanenbaum Research Institute, and the University of Toronto. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
including reduced weight gain, reduced insulin doses, and fewer large-for-gestational-age babies, suggest the results of a randomized controlled trial.
However, the drug was associated with an increased risk of small-for-gestational-age babies, which poses the question as to risk versus benefit of metformin on the health of offspring.
“Better understanding of the short- and long-term implications of these effects on infants will be important to properly advise patients with type 2 diabetes contemplating use of metformin during pregnancy,” said lead author Denice S. Feig, MD, Mount Sinai Hospital, Toronto.
The research was presented at the Diabetes UK Professional Conference: Online Series on Nov. 17 and recently published in The Lancet Diabetes & Endocrinology.
Summing up, Dr. Feig said that, on balance, she would be inclined to give metformin to most pregnant women with type 2 diabetes, perhaps with the exception of those who may have risk factors for small-for-gestational-age babies; for example, women who’ve had intrauterine growth restriction, who are smokers, and have significant renal disease, or have a lower body mass index.
Increased prevalence of type 2 diabetes in pregnancy
Dr. Feig said that across the developed world there have been huge increases in the prevalence of type 2 diabetes in pregnancy in recent years.
Insulin is the standard treatment for the management of type 2 diabetes in pregnancy, but these women have marked insulin resistance that worsens in pregnancy, which means their insulin requirements increase, leading to weight gain, painful injections, high cost, and noncompliance.
So despite treatment with insulin, these women continue to face increased rates of adverse maternal and fetal outcomes.
And although metformin is increasingly being used in women with type 2 diabetes during pregnancy, there is a scarcity of data on the benefits and harms of metformin use on pregnancy outcomes in these women.
The MiTy trial was therefore undertaken to determine whether metformin could improve outcomes.
The team recruited 502 women from 29 sites in Canada and Australia who had type 2 diabetes prior to pregnancy or were diagnosed during pregnancy, before 20 weeks’ gestation. The women were randomized to metformin 1 g twice daily or placebo, in addition to their usual insulin regimen, at between 6 and 28 weeks’ gestation.
Type 2 diabetes was diagnosed prior to pregnancy in 83% of women in the metformin group and in 90% of those assigned to placebo. The mean hemoglobin A1c level at randomization was 47 mmol/mol (6.5%) in both groups.
The average maternal age at baseline was approximately 35 years and mean gestational age at randomization was 16 weeks. Mean prepregnancy BMI was approximately 34 kg/m2.
Of note, only 30% were of European ethnicity.
Less weight gain, lower A1c, less insulin needed with metformin
Dr. Feig reported that there was no significant difference between the treatment groups in terms of the proportion of women with the composite primary outcome of pregnancy loss, preterm birth, birth injury, respiratory distress, neonatal hypoglycemia, or admission to neonatal intensive care lasting more than 24 hours (P = 0.86).
However, women in the metformin group had significantly less overall weight gain during pregnancy than did those in the placebo group, at –1.8 kg (P < .0001).
They also had a significantly lower last A1c level in pregnancy, at 41 mmol/mol (5.9%) versus 43.2 mmol/mol (6.1%) in those given placebo (P = .015), and required fewer insulin doses, at 1.1 versus 1.5 units/kg/day (P < .0001), which translated to a reduction of almost 44 units/day.
Women given metformin were also less likely to require Cesarean section delivery, at 53.4% versus 62.7% in the placebo group (P = .03), although there was no difference between groups in terms of gestational hypertension or preeclampsia.
The most common adverse events were gastrointestinal complications, which occurred in 27.3% of women in the metformin group and 22.3% of those given placebo.
There were no significant differences between the metformin and placebo groups in rates of pregnancy loss (P = .81), preterm birth (P = .16), birth injury (P = .37), respiratory distress (P = .49), and congenital anomalies (P = .16).
Average birth weight lower with metformin
However, Dr. Feig showed that the average birth weight was lower for offspring of women given metformin than those assigned to placebo, at 3.2 kg (7.05 lb) versus 3.4 kg (7.4 lb) (P = .002).
Women given metformin were also less likely to have a baby with a birth weight of 4 kg (8.8 lb) or more, at 12.1% versus 19.2%, or a relative risk of 0.65 (P = .046), and a baby that was extremely large for gestational age, at 8.6% versus 14.8%, or a relative risk of 0.58 (P = .046).
But of concern, metformin was associated with an increased risk of small-for-gestational-age babies, at 12.9% versus 6.6% with placebo, or a relative risk of 1.96 (P = .03).
Dr. Feig suggested that this may be due to a direct effect of metformin “because as we know metformin inhibits the mTOR pathway,” which is a “primary nutrient sensor in the placenta” and could “attenuate nutrient flux and fetal growth.”
She said it is not clear whether the small-for-gestational-age babies were “healthy or unhealthy.”
To investigate further, the team has launched the MiTy Kids study, which will follow the offspring in the MiTy trial to determine whether metformin during pregnancy is associated with a reduction in adiposity and improvement in insulin resistance in the babies at 2 years of age.
Who should be given metformin?
During the discussion, Helen R. Murphy, MD, PhD, Norwich Medical School, University of East Anglia, England, asked whether Dr. Feig would recommend continuing metformin in pregnancy if it was started preconception for fertility issues rather than diabetes.
She replied: “If they don’t have diabetes and it’s simply for PCOS [polycystic ovary syndrome], then I have either stopped it as soon as they got pregnant or sometimes continued it through the first trimester, and then stopped.
“If the person has diabetes, however, I think given this work, for most people I would continue it,” she said.
The study was funded by the Canadian Institutes of Health Research, Lunenfeld-Tanenbaum Research Institute, and the University of Toronto. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Golimumab preserves insulin production in type 1 diabetes
The human monoclonal antibody golimumab (Simponi) preserved endogenous insulin secretion in patients with new-onset type 1 diabetes and reduced their exogenous insulin requirements at 1 year, newly published phase 2 data indicate.
Results from the multicenter, double-blind, placebo-controlled trial were first reported as a poster at the virtual American Diabetes Association 80th Scientific Sessions in June. They were published online Nov. 18 in the New England Journal of Medicine.
In the 52-week study of 84 children and adults with new-onset type 1 diabetes, those given golimumab injections every 2 weeks had significantly higher levels of C-peptide, a marker of insulin secretion, and required less injected or infused insulin than did those who received placebo injections. There were no treatment-associated serious adverse events.
Golimumab is a human monoclonal antibody specific for tumor necrosis factor–alpha. It is approved for the treatment of several autoimmune diseases, including rheumatoid arthritis and ulcerative colitis, in the United States, Europe, and elsewhere.
An intermediate step toward a cure
Although none of the patients were able to stop taking insulin entirely, the results have important clinical implications, lead author Teresa Quattrin, MD, said in an interview.
“People want a cure, but the fact is, a cure is not available yet. So, this is an intermediate step towards a cure.... There are advantages to being on a small insulin dose,” including lower rates of hypoglycemia and maintenance of intraportal insulin, said Dr. Quattrin, of the State University of New York at Buffalo.
But in an accompanying editorial, Domenico Accili, MD, points to potential risks from immunotherapy and from attempting additional interventions at an “emotionally fraught” time when patients and families are coping with the new diabetes diagnosis.
He said of golimumab, “the effect is actually very small. ... There’s nothing wrong in and of itself with improving those outcomes. I just wouldn’t assign them as game changers.”
If this or a similar immunotherapeutic intervention were approved for this indication, “I would tell patients it exists and let them make the decision whether they want to try it. I wouldn’t say you must try it,” said Dr. Accili, of the Columbia University Diabetes and Endocrinology Research Center, New York.
With golimumab, higher C-peptide, lower insulin requirement
Of the 84 patients, who ranged in age from 6 to 21 years, 56 were randomly assigned within 100 days of being diagnosed with type 1 diabetes to receive golimumab, and 28 were assigned to receive placebo injections, given every 2 weeks.
The drug resulted in lower insulin use (0.51U/Kg per day vs. 0.69 U/kg per day), and the increase in insulin use over 52 weeks was less with golimumab than with placebo (0.07 vs. 0.24 U/kg per day; P = .001).
The mean percent decrease of C-peptide production from baseline was 12% with golimumab versus 56% with placebo.
Although the mean number of overall hypoglycemic events was similar, the mean number of level 2 hypoglycemic events (<54 mg/dL) was 36% lower with golimumab (11.5 vs. 17.6). There were no severe cases of hypoglycemia in either group.
No severe or serious infections occurred in either group, although mild to moderate infections were reported in 71% with golimumab versus 61% with placebo. More patients in the golimumab group experienced a decrease in neutrophils (29% vs. 19%).
Immunotherapy: Which one, and when should it start?
These findings come on the heels of the 2019 landmark results with another monoclonal antibody, the investigational anti-CD3 teplizumab (PRV-031). Among patients at risk, a diagnosis of type 1 diabetes was delayed by 2 years, and continued benefit was seen at 3 years.
However, Dr. Quattrin said teplizumab is limited by the fact that it must be administered via a 14-day infusion, whereas golimumab can be injected by patients themselves at home.
Moreover, the phase 2 teplizumab study was conducted in people who had antibodies that placed them at high risk for type 1 diabetes, but those patients did not yet have the condition. They were identified because they had close relatives with type 1 diabetes and were enrolled in the federally funded TrialNet screening program.
Dr. Quattrin is now participating in an ongoing phase 3 study of teplizumab that involves patients newly diagnosed with type 1 diabetes.
A Janssen spokesperson said in an interview that the company isn’t planning to further develop golimumab for use in type 1 diabetes.
“Our focus is to apply insights from the phase 2 ... proof-of-concept study to progress what we believe are novel, immunologically targeted pipeline candidates in stage 2 disease or presymptomatic stages of type 1 diabetes, which is consistent with our mission to intercept and prevent type 1 diabetes,” the spokesperson said.
To identify more individuals at risk for type 1 diabetes beyond the close relatives of those who already have it, so as to be able to intervene at a presymptomatic stage, Janssen is organizing a public-private effort to advocate for routine population screening for type 1 diabetes–related autoantibodies.
Dr. Quattrin said: “Preserving some insulin is key. Having somebody with beta cell functioning still is an intermediate step to a cure and will make their life easier, and that’s what people should care about.”
Dr. Accili, who cofounded and leads a company working on a novel approach to type 1 diabetes treatment, writes in his editorial: “We should also be mindful that this treatment debate is first world–centric.
“Current treatments for type 1 diabetes require resources not readily available in most parts of the world, where something as simple as refrigeration of insulin can become a logistic nightmare. While combinations of [approaches] tailored to individual risk and potential benefits are likely to make inroads in clinical practice, the need for a simpler, safer, and equally effective alternative to insulin remains,” he wrote.
Dr. Quattrin is a researcher and consultant for Janssen and conducts clinical trials for Provention Bio, Opko, and Ascendis. Dr. Accili is founder and director of Forkhead Therapeutics.
A version of this article originally appeared on Medscape.com.
The human monoclonal antibody golimumab (Simponi) preserved endogenous insulin secretion in patients with new-onset type 1 diabetes and reduced their exogenous insulin requirements at 1 year, newly published phase 2 data indicate.
Results from the multicenter, double-blind, placebo-controlled trial were first reported as a poster at the virtual American Diabetes Association 80th Scientific Sessions in June. They were published online Nov. 18 in the New England Journal of Medicine.
In the 52-week study of 84 children and adults with new-onset type 1 diabetes, those given golimumab injections every 2 weeks had significantly higher levels of C-peptide, a marker of insulin secretion, and required less injected or infused insulin than did those who received placebo injections. There were no treatment-associated serious adverse events.
Golimumab is a human monoclonal antibody specific for tumor necrosis factor–alpha. It is approved for the treatment of several autoimmune diseases, including rheumatoid arthritis and ulcerative colitis, in the United States, Europe, and elsewhere.
An intermediate step toward a cure
Although none of the patients were able to stop taking insulin entirely, the results have important clinical implications, lead author Teresa Quattrin, MD, said in an interview.
“People want a cure, but the fact is, a cure is not available yet. So, this is an intermediate step towards a cure.... There are advantages to being on a small insulin dose,” including lower rates of hypoglycemia and maintenance of intraportal insulin, said Dr. Quattrin, of the State University of New York at Buffalo.
But in an accompanying editorial, Domenico Accili, MD, points to potential risks from immunotherapy and from attempting additional interventions at an “emotionally fraught” time when patients and families are coping with the new diabetes diagnosis.
He said of golimumab, “the effect is actually very small. ... There’s nothing wrong in and of itself with improving those outcomes. I just wouldn’t assign them as game changers.”
If this or a similar immunotherapeutic intervention were approved for this indication, “I would tell patients it exists and let them make the decision whether they want to try it. I wouldn’t say you must try it,” said Dr. Accili, of the Columbia University Diabetes and Endocrinology Research Center, New York.
With golimumab, higher C-peptide, lower insulin requirement
Of the 84 patients, who ranged in age from 6 to 21 years, 56 were randomly assigned within 100 days of being diagnosed with type 1 diabetes to receive golimumab, and 28 were assigned to receive placebo injections, given every 2 weeks.
The drug resulted in lower insulin use (0.51U/Kg per day vs. 0.69 U/kg per day), and the increase in insulin use over 52 weeks was less with golimumab than with placebo (0.07 vs. 0.24 U/kg per day; P = .001).
The mean percent decrease of C-peptide production from baseline was 12% with golimumab versus 56% with placebo.
Although the mean number of overall hypoglycemic events was similar, the mean number of level 2 hypoglycemic events (<54 mg/dL) was 36% lower with golimumab (11.5 vs. 17.6). There were no severe cases of hypoglycemia in either group.
No severe or serious infections occurred in either group, although mild to moderate infections were reported in 71% with golimumab versus 61% with placebo. More patients in the golimumab group experienced a decrease in neutrophils (29% vs. 19%).
Immunotherapy: Which one, and when should it start?
These findings come on the heels of the 2019 landmark results with another monoclonal antibody, the investigational anti-CD3 teplizumab (PRV-031). Among patients at risk, a diagnosis of type 1 diabetes was delayed by 2 years, and continued benefit was seen at 3 years.
However, Dr. Quattrin said teplizumab is limited by the fact that it must be administered via a 14-day infusion, whereas golimumab can be injected by patients themselves at home.
Moreover, the phase 2 teplizumab study was conducted in people who had antibodies that placed them at high risk for type 1 diabetes, but those patients did not yet have the condition. They were identified because they had close relatives with type 1 diabetes and were enrolled in the federally funded TrialNet screening program.
Dr. Quattrin is now participating in an ongoing phase 3 study of teplizumab that involves patients newly diagnosed with type 1 diabetes.
A Janssen spokesperson said in an interview that the company isn’t planning to further develop golimumab for use in type 1 diabetes.
“Our focus is to apply insights from the phase 2 ... proof-of-concept study to progress what we believe are novel, immunologically targeted pipeline candidates in stage 2 disease or presymptomatic stages of type 1 diabetes, which is consistent with our mission to intercept and prevent type 1 diabetes,” the spokesperson said.
To identify more individuals at risk for type 1 diabetes beyond the close relatives of those who already have it, so as to be able to intervene at a presymptomatic stage, Janssen is organizing a public-private effort to advocate for routine population screening for type 1 diabetes–related autoantibodies.
Dr. Quattrin said: “Preserving some insulin is key. Having somebody with beta cell functioning still is an intermediate step to a cure and will make their life easier, and that’s what people should care about.”
Dr. Accili, who cofounded and leads a company working on a novel approach to type 1 diabetes treatment, writes in his editorial: “We should also be mindful that this treatment debate is first world–centric.
“Current treatments for type 1 diabetes require resources not readily available in most parts of the world, where something as simple as refrigeration of insulin can become a logistic nightmare. While combinations of [approaches] tailored to individual risk and potential benefits are likely to make inroads in clinical practice, the need for a simpler, safer, and equally effective alternative to insulin remains,” he wrote.
Dr. Quattrin is a researcher and consultant for Janssen and conducts clinical trials for Provention Bio, Opko, and Ascendis. Dr. Accili is founder and director of Forkhead Therapeutics.
A version of this article originally appeared on Medscape.com.
The human monoclonal antibody golimumab (Simponi) preserved endogenous insulin secretion in patients with new-onset type 1 diabetes and reduced their exogenous insulin requirements at 1 year, newly published phase 2 data indicate.
Results from the multicenter, double-blind, placebo-controlled trial were first reported as a poster at the virtual American Diabetes Association 80th Scientific Sessions in June. They were published online Nov. 18 in the New England Journal of Medicine.
In the 52-week study of 84 children and adults with new-onset type 1 diabetes, those given golimumab injections every 2 weeks had significantly higher levels of C-peptide, a marker of insulin secretion, and required less injected or infused insulin than did those who received placebo injections. There were no treatment-associated serious adverse events.
Golimumab is a human monoclonal antibody specific for tumor necrosis factor–alpha. It is approved for the treatment of several autoimmune diseases, including rheumatoid arthritis and ulcerative colitis, in the United States, Europe, and elsewhere.
An intermediate step toward a cure
Although none of the patients were able to stop taking insulin entirely, the results have important clinical implications, lead author Teresa Quattrin, MD, said in an interview.
“People want a cure, but the fact is, a cure is not available yet. So, this is an intermediate step towards a cure.... There are advantages to being on a small insulin dose,” including lower rates of hypoglycemia and maintenance of intraportal insulin, said Dr. Quattrin, of the State University of New York at Buffalo.
But in an accompanying editorial, Domenico Accili, MD, points to potential risks from immunotherapy and from attempting additional interventions at an “emotionally fraught” time when patients and families are coping with the new diabetes diagnosis.
He said of golimumab, “the effect is actually very small. ... There’s nothing wrong in and of itself with improving those outcomes. I just wouldn’t assign them as game changers.”
If this or a similar immunotherapeutic intervention were approved for this indication, “I would tell patients it exists and let them make the decision whether they want to try it. I wouldn’t say you must try it,” said Dr. Accili, of the Columbia University Diabetes and Endocrinology Research Center, New York.
With golimumab, higher C-peptide, lower insulin requirement
Of the 84 patients, who ranged in age from 6 to 21 years, 56 were randomly assigned within 100 days of being diagnosed with type 1 diabetes to receive golimumab, and 28 were assigned to receive placebo injections, given every 2 weeks.
The drug resulted in lower insulin use (0.51U/Kg per day vs. 0.69 U/kg per day), and the increase in insulin use over 52 weeks was less with golimumab than with placebo (0.07 vs. 0.24 U/kg per day; P = .001).
The mean percent decrease of C-peptide production from baseline was 12% with golimumab versus 56% with placebo.
Although the mean number of overall hypoglycemic events was similar, the mean number of level 2 hypoglycemic events (<54 mg/dL) was 36% lower with golimumab (11.5 vs. 17.6). There were no severe cases of hypoglycemia in either group.
No severe or serious infections occurred in either group, although mild to moderate infections were reported in 71% with golimumab versus 61% with placebo. More patients in the golimumab group experienced a decrease in neutrophils (29% vs. 19%).
Immunotherapy: Which one, and when should it start?
These findings come on the heels of the 2019 landmark results with another monoclonal antibody, the investigational anti-CD3 teplizumab (PRV-031). Among patients at risk, a diagnosis of type 1 diabetes was delayed by 2 years, and continued benefit was seen at 3 years.
However, Dr. Quattrin said teplizumab is limited by the fact that it must be administered via a 14-day infusion, whereas golimumab can be injected by patients themselves at home.
Moreover, the phase 2 teplizumab study was conducted in people who had antibodies that placed them at high risk for type 1 diabetes, but those patients did not yet have the condition. They were identified because they had close relatives with type 1 diabetes and were enrolled in the federally funded TrialNet screening program.
Dr. Quattrin is now participating in an ongoing phase 3 study of teplizumab that involves patients newly diagnosed with type 1 diabetes.
A Janssen spokesperson said in an interview that the company isn’t planning to further develop golimumab for use in type 1 diabetes.
“Our focus is to apply insights from the phase 2 ... proof-of-concept study to progress what we believe are novel, immunologically targeted pipeline candidates in stage 2 disease or presymptomatic stages of type 1 diabetes, which is consistent with our mission to intercept and prevent type 1 diabetes,” the spokesperson said.
To identify more individuals at risk for type 1 diabetes beyond the close relatives of those who already have it, so as to be able to intervene at a presymptomatic stage, Janssen is organizing a public-private effort to advocate for routine population screening for type 1 diabetes–related autoantibodies.
Dr. Quattrin said: “Preserving some insulin is key. Having somebody with beta cell functioning still is an intermediate step to a cure and will make their life easier, and that’s what people should care about.”
Dr. Accili, who cofounded and leads a company working on a novel approach to type 1 diabetes treatment, writes in his editorial: “We should also be mindful that this treatment debate is first world–centric.
“Current treatments for type 1 diabetes require resources not readily available in most parts of the world, where something as simple as refrigeration of insulin can become a logistic nightmare. While combinations of [approaches] tailored to individual risk and potential benefits are likely to make inroads in clinical practice, the need for a simpler, safer, and equally effective alternative to insulin remains,” he wrote.
Dr. Quattrin is a researcher and consultant for Janssen and conducts clinical trials for Provention Bio, Opko, and Ascendis. Dr. Accili is founder and director of Forkhead Therapeutics.
A version of this article originally appeared on Medscape.com.
Finerenone’s heart benefits hold up in T2D patients without CVD
Finerenone, the first nonsteroidal mineralocorticoid receptor antagonist to complete a phase 3 trial, showed cardiovascular benefits in patients with type 2 diabetes and chronic kidney disease, regardless of whether they entered the study with a history of cardiovascular disease, in follow-up analyses of the FIDELIO-DKD trial, which included 5,674 patients.
“Finerenone demonstrated benefits for primary and secondary cardiovascular disease protection,” said Gerasimos Filippatos, MD, at the American Heart Association scientific sessions. Finerenone treatment cut the rate of cardiovascular death, nonfatal MI or stroke, or heart failure hospitalization, when compared with placebo, by a relative 15% among patients with a history of cardiovascular disease (CVD), and by a relative 14% in patients without this history, differences that met a statistical test for consistency. But the absolute, drug-associated increments in benefit over placebo differed between the two CVD subgroups because of a sharp underlying difference in event rates.
In contrast, the analyses reported by Dr. Filippatos and associates from the FIDELIO-DKD study showed significant heterogeneity based on the presence or absence of CVD for the study’s primary endpoint, a composite renal metric that tallied the combined rate of death from renal causes, renal failure, or a sustained drop in estimated glomerular filtration rate of at least 40%. Researchers enrolled patients into FIDELIO-DKD based on having type 2 diabetes (T2D) and chronic kidney disease (CKD). The prevalence of a history of CVD was 46%.
Among patients with a history of CVD, the composite adverse CVD outcome occurred at a rate of 8.5/100 patient-years in patients on placebo and in 7.18/100 patients years among those on finerenone during a median of 2.6 years of follow-up, a 1.32/100–patient-year absolute between-group difference. Among patients in a primary prevention setting, incident CVD event rates during follow-up were roughly half that in the secondary prevention patients. The upshot was that, in the placebo group, the rate was 3.92/100 patient- years, and in those on finerenone was 3.43/100 patient-years, a 0.49/100–patient-year absolute difference.
CVD history produced heterogeneity for the primary endpoint
In the analysis that focused on the study’s primary, renal endpoint, among patients identified as having CVD at study entry, the outcome occurred at a rate of 9.06/100 patient-years in the placebo subgroup and at a rate of 6.6/100 patient years in those who received finerenone, a significant 30% relative risk reduction and an absolute between-group difference of 2.46/100 patient-years.
In contrast, among patients without a CVD history, the composite renal endpoint occurred at a rate of 9.1/100 patient-years in the placebo patients and 8.42/100 patient-years in those on finerenone, a 6% relative risk reduction that was not significant, and a 0.68/100–patient-year absolute difference. This disparity in the primary event rate between the two treatment arms reached statistical significance (P = .016), the investigators reported in the published version of the report in Circulation that simultaneously appeared online.
“The totality of evidence suggests that finerenone could be used in patients with T2D with or without a history of CVD,” explained Dr. Filippatos in an interview. “The P-interaction for the composite kidney outcome is significant, but it is not corrected for multiple testing; therefore, it might be a false-chance finding and must be interpreted cautiously.
Furthermore, in another prespecified kidney composite outcome the results were consistent in patients with and without a history of CVD. In sum, all the FIDELIO-DKD analyses so far are “suggestive of a beneficial effect in patients without a history of CVD.”
Despite these patients receiving guideline directed therapies, “there remains a high unmet medical need in patients with T2D and CKD,” added Dr. Filippatos, professor of cardiology at the University of Athens. “We use multiple treatments for patients with heart failure, and we should use the same mindset for treating patients with T2D and CKD. The costs of dialysis and kidney transplant are very high, so it is important to consider options that slow progression of CKD in these patients.”
In FIDELIO-DKD, virtually all patients were on background therapy with a renin-angiotensin-system (RAS) inhibitor, so the trial’s results suggest that treatment should at least involve dual therapy with finerenone and a RAS inhibitor. Fewer than 5% were on background therapy with a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a drug class recently established as another key agent for treating CKD in patients with T2D, setting up the prospect for triple therapy, although this approach has not yet undergone prospective testing.
Combining RAS inhibition, finerenone, and an SGLT2 inhibitor is “potentially a marriage made in diabetes heaven,” commented Deepak L. Bhatt, MD, a professor of medicine at Harvard Medical School, Boston, who has not participated in finerenone studies.
Finerenone looks better for safety
Regardless of subgroup analyses based on history of CVD, the findings from all patients enrolled in FIDELIO-DKD were positive for the both the primary renal outcome and key secondary outcome of composite CVD events. In the total randomized cohort, treatment with finerenone on top of optimized treatment with an ACE inhibitor or angiotensin receptor blocker (RAS inhibition) led to a significant 18% relative risk reduction, compared with placebo, for the primary renal endpoint, and a significant 14% relative drop in the key secondary CVD outcome. Those results were published in October in the New England Journal of Medicine.
For treating patients with T2D and CKD ,finerenone overall “looks like a major advance,” Dr. Bhatt said in an interview.
In addition to the positive efficacy results, several experts also focused on what they saw as superior safety of finerenone in the trial, compared with the historical safety of the steroidal mineralocorticoid receptor antagonists (MRAs) now in use: spironolactone and eplerenone.
“I’m a big believer in spironolactone, but it has issues with side effects, and eplerenone never seemed to catch on,” said Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
“A lot of physicians like these MRAs, but acknowledge that side effects have kept these drugs from being used to the extent they should.” The existing MRAs, especially spironolactone, have become a key drug class for treating heart failure with reduced ejection fraction (and, some claim, for also treating heart failure with preserved ejection fraction), as well as treatment-resistant hypertension and primary aldosteronism. By design, FIDELIO-DKD did not enroll patients with heart failure because treatment with an MRA is indicated for those with heart failure with reduced ejection fraction.
The spironolactone adverse effect that generates the greatest concern is hyperkalemia. During his discussion of FIDELIO-DKD as designated discussant, Christoph Wanner, MD, noted a recent study in which the incidence of hyperkalemia severe enough to cause study discontinuation was 23% among patients treated with spironolactone for heart failure, which contrasts with the 2.3% rate in FIDELIO-DKD among finerenone recipients. This hyperkalemia incidence from finerenone also improved on the historical performance of other drugs, like aliskiren (Tekturna), said Dr. Wanner, professor and head of nephrology at the University of Würzburg (Germany).
The FIDELIO-DKD results place finerenone alongside the RAS- and SGLT2-inhibitor drug classes as appropriate treatments for most patients with T2D and CKD. “We have entered a new era of effective treatment for diabetic kidney disease,” Dr. Wanner declared.
“The overall safety profile of finerenone looked better, including hyperkalemia,” said Dr. Bhatt. “Hyperkalemia with spironolactone is not necessarily as bad as the perception. With careful monitoring of spironolactone, the hyperkalemia is manageable. But the perception is that it’s bad, and along with gynecomastia it’s a real killer.”
While some dismiss gynecomastia as a major concern (for men) with spironolactone treatment, “if medical students learn one thing about spironolactone, it’s that it can cause gynecomastia,” adding to the negative image that the approved MRAs carry, Dr. Bhatt said.
“The hyperkalemia was manageable. This is very important because of past problems with potassium when using spironolactone,” Dr. Filippatos said. Finerenone also looks “more cardiorenal protective” than the steroidal MRAs, exerting renal benefits in FIDELIO-DKD never previously described for a steroidal MRA.
Some of the uncertainty about the efficacy of finerenone in patients with a history of cardiovascular disease will lift when results are available in about another year from the FIGARO-DKD pivotal trial of finerenone, which enrolled more than 7,000 patients with T2D and CKD (entry criteria very similar to FIDELIO-CKD). A big difference is that FIGARO-DKD has a composite CVD event metric as its primary endpoint, and includes hospitalization for heart failure as one facet of the composite.
FIDELIO-DKD was sponsored by Bayer. Dr. Filippatos has been a lecturer on behalf of, served as a researcher for, or both for Bayer and also for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Bayer and also from several other companies, and he also is an adviser to several companies. Dr. Wanner has received honoraria from Bayer, and also from AstraZeneca, Boehringer Ingelheim, FMC, Gilead, GlaxoSmithKline, Lilly, and Merck.
Finerenone, the first nonsteroidal mineralocorticoid receptor antagonist to complete a phase 3 trial, showed cardiovascular benefits in patients with type 2 diabetes and chronic kidney disease, regardless of whether they entered the study with a history of cardiovascular disease, in follow-up analyses of the FIDELIO-DKD trial, which included 5,674 patients.
“Finerenone demonstrated benefits for primary and secondary cardiovascular disease protection,” said Gerasimos Filippatos, MD, at the American Heart Association scientific sessions. Finerenone treatment cut the rate of cardiovascular death, nonfatal MI or stroke, or heart failure hospitalization, when compared with placebo, by a relative 15% among patients with a history of cardiovascular disease (CVD), and by a relative 14% in patients without this history, differences that met a statistical test for consistency. But the absolute, drug-associated increments in benefit over placebo differed between the two CVD subgroups because of a sharp underlying difference in event rates.
In contrast, the analyses reported by Dr. Filippatos and associates from the FIDELIO-DKD study showed significant heterogeneity based on the presence or absence of CVD for the study’s primary endpoint, a composite renal metric that tallied the combined rate of death from renal causes, renal failure, or a sustained drop in estimated glomerular filtration rate of at least 40%. Researchers enrolled patients into FIDELIO-DKD based on having type 2 diabetes (T2D) and chronic kidney disease (CKD). The prevalence of a history of CVD was 46%.
Among patients with a history of CVD, the composite adverse CVD outcome occurred at a rate of 8.5/100 patient-years in patients on placebo and in 7.18/100 patients years among those on finerenone during a median of 2.6 years of follow-up, a 1.32/100–patient-year absolute between-group difference. Among patients in a primary prevention setting, incident CVD event rates during follow-up were roughly half that in the secondary prevention patients. The upshot was that, in the placebo group, the rate was 3.92/100 patient- years, and in those on finerenone was 3.43/100 patient-years, a 0.49/100–patient-year absolute difference.
CVD history produced heterogeneity for the primary endpoint
In the analysis that focused on the study’s primary, renal endpoint, among patients identified as having CVD at study entry, the outcome occurred at a rate of 9.06/100 patient-years in the placebo subgroup and at a rate of 6.6/100 patient years in those who received finerenone, a significant 30% relative risk reduction and an absolute between-group difference of 2.46/100 patient-years.
In contrast, among patients without a CVD history, the composite renal endpoint occurred at a rate of 9.1/100 patient-years in the placebo patients and 8.42/100 patient-years in those on finerenone, a 6% relative risk reduction that was not significant, and a 0.68/100–patient-year absolute difference. This disparity in the primary event rate between the two treatment arms reached statistical significance (P = .016), the investigators reported in the published version of the report in Circulation that simultaneously appeared online.
“The totality of evidence suggests that finerenone could be used in patients with T2D with or without a history of CVD,” explained Dr. Filippatos in an interview. “The P-interaction for the composite kidney outcome is significant, but it is not corrected for multiple testing; therefore, it might be a false-chance finding and must be interpreted cautiously.
Furthermore, in another prespecified kidney composite outcome the results were consistent in patients with and without a history of CVD. In sum, all the FIDELIO-DKD analyses so far are “suggestive of a beneficial effect in patients without a history of CVD.”
Despite these patients receiving guideline directed therapies, “there remains a high unmet medical need in patients with T2D and CKD,” added Dr. Filippatos, professor of cardiology at the University of Athens. “We use multiple treatments for patients with heart failure, and we should use the same mindset for treating patients with T2D and CKD. The costs of dialysis and kidney transplant are very high, so it is important to consider options that slow progression of CKD in these patients.”
In FIDELIO-DKD, virtually all patients were on background therapy with a renin-angiotensin-system (RAS) inhibitor, so the trial’s results suggest that treatment should at least involve dual therapy with finerenone and a RAS inhibitor. Fewer than 5% were on background therapy with a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a drug class recently established as another key agent for treating CKD in patients with T2D, setting up the prospect for triple therapy, although this approach has not yet undergone prospective testing.
Combining RAS inhibition, finerenone, and an SGLT2 inhibitor is “potentially a marriage made in diabetes heaven,” commented Deepak L. Bhatt, MD, a professor of medicine at Harvard Medical School, Boston, who has not participated in finerenone studies.
Finerenone looks better for safety
Regardless of subgroup analyses based on history of CVD, the findings from all patients enrolled in FIDELIO-DKD were positive for the both the primary renal outcome and key secondary outcome of composite CVD events. In the total randomized cohort, treatment with finerenone on top of optimized treatment with an ACE inhibitor or angiotensin receptor blocker (RAS inhibition) led to a significant 18% relative risk reduction, compared with placebo, for the primary renal endpoint, and a significant 14% relative drop in the key secondary CVD outcome. Those results were published in October in the New England Journal of Medicine.
For treating patients with T2D and CKD ,finerenone overall “looks like a major advance,” Dr. Bhatt said in an interview.
In addition to the positive efficacy results, several experts also focused on what they saw as superior safety of finerenone in the trial, compared with the historical safety of the steroidal mineralocorticoid receptor antagonists (MRAs) now in use: spironolactone and eplerenone.
“I’m a big believer in spironolactone, but it has issues with side effects, and eplerenone never seemed to catch on,” said Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
“A lot of physicians like these MRAs, but acknowledge that side effects have kept these drugs from being used to the extent they should.” The existing MRAs, especially spironolactone, have become a key drug class for treating heart failure with reduced ejection fraction (and, some claim, for also treating heart failure with preserved ejection fraction), as well as treatment-resistant hypertension and primary aldosteronism. By design, FIDELIO-DKD did not enroll patients with heart failure because treatment with an MRA is indicated for those with heart failure with reduced ejection fraction.
The spironolactone adverse effect that generates the greatest concern is hyperkalemia. During his discussion of FIDELIO-DKD as designated discussant, Christoph Wanner, MD, noted a recent study in which the incidence of hyperkalemia severe enough to cause study discontinuation was 23% among patients treated with spironolactone for heart failure, which contrasts with the 2.3% rate in FIDELIO-DKD among finerenone recipients. This hyperkalemia incidence from finerenone also improved on the historical performance of other drugs, like aliskiren (Tekturna), said Dr. Wanner, professor and head of nephrology at the University of Würzburg (Germany).
The FIDELIO-DKD results place finerenone alongside the RAS- and SGLT2-inhibitor drug classes as appropriate treatments for most patients with T2D and CKD. “We have entered a new era of effective treatment for diabetic kidney disease,” Dr. Wanner declared.
“The overall safety profile of finerenone looked better, including hyperkalemia,” said Dr. Bhatt. “Hyperkalemia with spironolactone is not necessarily as bad as the perception. With careful monitoring of spironolactone, the hyperkalemia is manageable. But the perception is that it’s bad, and along with gynecomastia it’s a real killer.”
While some dismiss gynecomastia as a major concern (for men) with spironolactone treatment, “if medical students learn one thing about spironolactone, it’s that it can cause gynecomastia,” adding to the negative image that the approved MRAs carry, Dr. Bhatt said.
“The hyperkalemia was manageable. This is very important because of past problems with potassium when using spironolactone,” Dr. Filippatos said. Finerenone also looks “more cardiorenal protective” than the steroidal MRAs, exerting renal benefits in FIDELIO-DKD never previously described for a steroidal MRA.
Some of the uncertainty about the efficacy of finerenone in patients with a history of cardiovascular disease will lift when results are available in about another year from the FIGARO-DKD pivotal trial of finerenone, which enrolled more than 7,000 patients with T2D and CKD (entry criteria very similar to FIDELIO-CKD). A big difference is that FIGARO-DKD has a composite CVD event metric as its primary endpoint, and includes hospitalization for heart failure as one facet of the composite.
FIDELIO-DKD was sponsored by Bayer. Dr. Filippatos has been a lecturer on behalf of, served as a researcher for, or both for Bayer and also for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Bayer and also from several other companies, and he also is an adviser to several companies. Dr. Wanner has received honoraria from Bayer, and also from AstraZeneca, Boehringer Ingelheim, FMC, Gilead, GlaxoSmithKline, Lilly, and Merck.
Finerenone, the first nonsteroidal mineralocorticoid receptor antagonist to complete a phase 3 trial, showed cardiovascular benefits in patients with type 2 diabetes and chronic kidney disease, regardless of whether they entered the study with a history of cardiovascular disease, in follow-up analyses of the FIDELIO-DKD trial, which included 5,674 patients.
“Finerenone demonstrated benefits for primary and secondary cardiovascular disease protection,” said Gerasimos Filippatos, MD, at the American Heart Association scientific sessions. Finerenone treatment cut the rate of cardiovascular death, nonfatal MI or stroke, or heart failure hospitalization, when compared with placebo, by a relative 15% among patients with a history of cardiovascular disease (CVD), and by a relative 14% in patients without this history, differences that met a statistical test for consistency. But the absolute, drug-associated increments in benefit over placebo differed between the two CVD subgroups because of a sharp underlying difference in event rates.
In contrast, the analyses reported by Dr. Filippatos and associates from the FIDELIO-DKD study showed significant heterogeneity based on the presence or absence of CVD for the study’s primary endpoint, a composite renal metric that tallied the combined rate of death from renal causes, renal failure, or a sustained drop in estimated glomerular filtration rate of at least 40%. Researchers enrolled patients into FIDELIO-DKD based on having type 2 diabetes (T2D) and chronic kidney disease (CKD). The prevalence of a history of CVD was 46%.
Among patients with a history of CVD, the composite adverse CVD outcome occurred at a rate of 8.5/100 patient-years in patients on placebo and in 7.18/100 patients years among those on finerenone during a median of 2.6 years of follow-up, a 1.32/100–patient-year absolute between-group difference. Among patients in a primary prevention setting, incident CVD event rates during follow-up were roughly half that in the secondary prevention patients. The upshot was that, in the placebo group, the rate was 3.92/100 patient- years, and in those on finerenone was 3.43/100 patient-years, a 0.49/100–patient-year absolute difference.
CVD history produced heterogeneity for the primary endpoint
In the analysis that focused on the study’s primary, renal endpoint, among patients identified as having CVD at study entry, the outcome occurred at a rate of 9.06/100 patient-years in the placebo subgroup and at a rate of 6.6/100 patient years in those who received finerenone, a significant 30% relative risk reduction and an absolute between-group difference of 2.46/100 patient-years.
In contrast, among patients without a CVD history, the composite renal endpoint occurred at a rate of 9.1/100 patient-years in the placebo patients and 8.42/100 patient-years in those on finerenone, a 6% relative risk reduction that was not significant, and a 0.68/100–patient-year absolute difference. This disparity in the primary event rate between the two treatment arms reached statistical significance (P = .016), the investigators reported in the published version of the report in Circulation that simultaneously appeared online.
“The totality of evidence suggests that finerenone could be used in patients with T2D with or without a history of CVD,” explained Dr. Filippatos in an interview. “The P-interaction for the composite kidney outcome is significant, but it is not corrected for multiple testing; therefore, it might be a false-chance finding and must be interpreted cautiously.
Furthermore, in another prespecified kidney composite outcome the results were consistent in patients with and without a history of CVD. In sum, all the FIDELIO-DKD analyses so far are “suggestive of a beneficial effect in patients without a history of CVD.”
Despite these patients receiving guideline directed therapies, “there remains a high unmet medical need in patients with T2D and CKD,” added Dr. Filippatos, professor of cardiology at the University of Athens. “We use multiple treatments for patients with heart failure, and we should use the same mindset for treating patients with T2D and CKD. The costs of dialysis and kidney transplant are very high, so it is important to consider options that slow progression of CKD in these patients.”
In FIDELIO-DKD, virtually all patients were on background therapy with a renin-angiotensin-system (RAS) inhibitor, so the trial’s results suggest that treatment should at least involve dual therapy with finerenone and a RAS inhibitor. Fewer than 5% were on background therapy with a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a drug class recently established as another key agent for treating CKD in patients with T2D, setting up the prospect for triple therapy, although this approach has not yet undergone prospective testing.
Combining RAS inhibition, finerenone, and an SGLT2 inhibitor is “potentially a marriage made in diabetes heaven,” commented Deepak L. Bhatt, MD, a professor of medicine at Harvard Medical School, Boston, who has not participated in finerenone studies.
Finerenone looks better for safety
Regardless of subgroup analyses based on history of CVD, the findings from all patients enrolled in FIDELIO-DKD were positive for the both the primary renal outcome and key secondary outcome of composite CVD events. In the total randomized cohort, treatment with finerenone on top of optimized treatment with an ACE inhibitor or angiotensin receptor blocker (RAS inhibition) led to a significant 18% relative risk reduction, compared with placebo, for the primary renal endpoint, and a significant 14% relative drop in the key secondary CVD outcome. Those results were published in October in the New England Journal of Medicine.
For treating patients with T2D and CKD ,finerenone overall “looks like a major advance,” Dr. Bhatt said in an interview.
In addition to the positive efficacy results, several experts also focused on what they saw as superior safety of finerenone in the trial, compared with the historical safety of the steroidal mineralocorticoid receptor antagonists (MRAs) now in use: spironolactone and eplerenone.
“I’m a big believer in spironolactone, but it has issues with side effects, and eplerenone never seemed to catch on,” said Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
“A lot of physicians like these MRAs, but acknowledge that side effects have kept these drugs from being used to the extent they should.” The existing MRAs, especially spironolactone, have become a key drug class for treating heart failure with reduced ejection fraction (and, some claim, for also treating heart failure with preserved ejection fraction), as well as treatment-resistant hypertension and primary aldosteronism. By design, FIDELIO-DKD did not enroll patients with heart failure because treatment with an MRA is indicated for those with heart failure with reduced ejection fraction.
The spironolactone adverse effect that generates the greatest concern is hyperkalemia. During his discussion of FIDELIO-DKD as designated discussant, Christoph Wanner, MD, noted a recent study in which the incidence of hyperkalemia severe enough to cause study discontinuation was 23% among patients treated with spironolactone for heart failure, which contrasts with the 2.3% rate in FIDELIO-DKD among finerenone recipients. This hyperkalemia incidence from finerenone also improved on the historical performance of other drugs, like aliskiren (Tekturna), said Dr. Wanner, professor and head of nephrology at the University of Würzburg (Germany).
The FIDELIO-DKD results place finerenone alongside the RAS- and SGLT2-inhibitor drug classes as appropriate treatments for most patients with T2D and CKD. “We have entered a new era of effective treatment for diabetic kidney disease,” Dr. Wanner declared.
“The overall safety profile of finerenone looked better, including hyperkalemia,” said Dr. Bhatt. “Hyperkalemia with spironolactone is not necessarily as bad as the perception. With careful monitoring of spironolactone, the hyperkalemia is manageable. But the perception is that it’s bad, and along with gynecomastia it’s a real killer.”
While some dismiss gynecomastia as a major concern (for men) with spironolactone treatment, “if medical students learn one thing about spironolactone, it’s that it can cause gynecomastia,” adding to the negative image that the approved MRAs carry, Dr. Bhatt said.
“The hyperkalemia was manageable. This is very important because of past problems with potassium when using spironolactone,” Dr. Filippatos said. Finerenone also looks “more cardiorenal protective” than the steroidal MRAs, exerting renal benefits in FIDELIO-DKD never previously described for a steroidal MRA.
Some of the uncertainty about the efficacy of finerenone in patients with a history of cardiovascular disease will lift when results are available in about another year from the FIGARO-DKD pivotal trial of finerenone, which enrolled more than 7,000 patients with T2D and CKD (entry criteria very similar to FIDELIO-CKD). A big difference is that FIGARO-DKD has a composite CVD event metric as its primary endpoint, and includes hospitalization for heart failure as one facet of the composite.
FIDELIO-DKD was sponsored by Bayer. Dr. Filippatos has been a lecturer on behalf of, served as a researcher for, or both for Bayer and also for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Bayer and also from several other companies, and he also is an adviser to several companies. Dr. Wanner has received honoraria from Bayer, and also from AstraZeneca, Boehringer Ingelheim, FMC, Gilead, GlaxoSmithKline, Lilly, and Merck.
FROM AHA 2020
Equitable Post-COVID-19 Care: A Practical Framework to Integrate Health Equity in Diabetes Management
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.
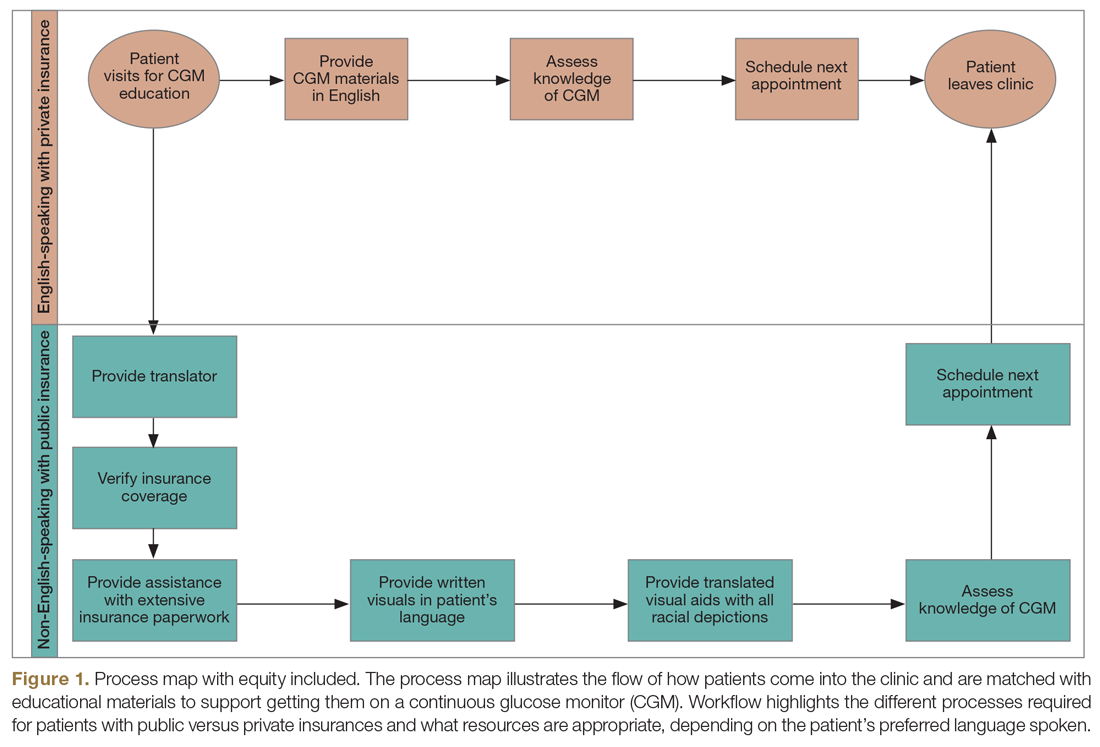
Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.
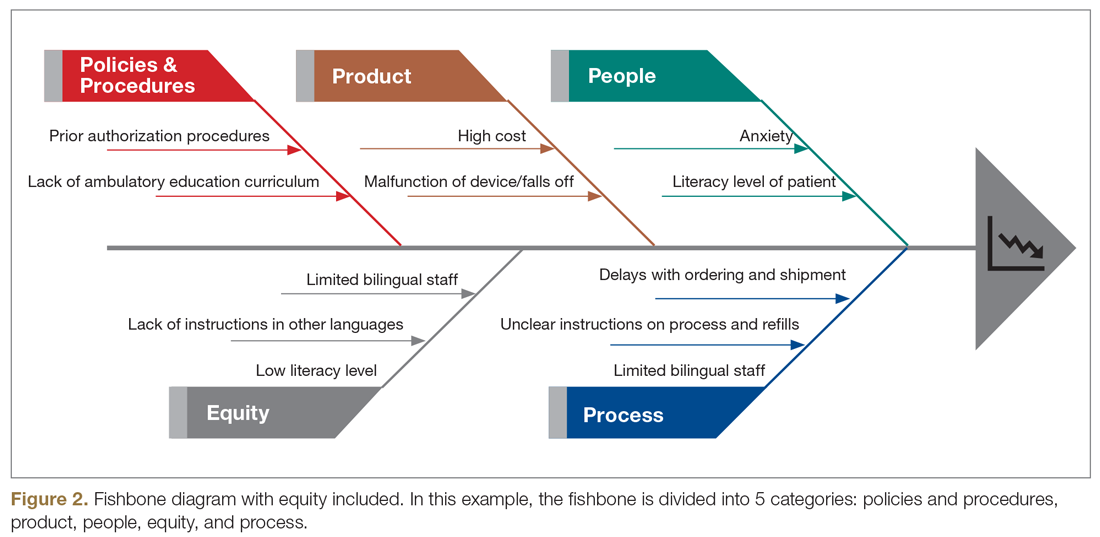
Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.

Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.

Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.

Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.

Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
Dapagliflozin Reduces Adverse Renal and Cardiovascular Events in Patients With Chronic Kidney Disease
Study Overview
Objective. To assess whether dapagliflozin added to guideline-recommended therapies is effective and safe over the long-term to reduce the rate of renal and cardiovascular events in patients across multiple chronic kidney disease (CKD) stages, with and without type 2 diabetes.
Design. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) was a randomized, double-blind, parallel-group, placebo-controlled, multicenter event-driven, clinical trial sponsored by Astra-Zeneca. It was conducted at 386 sites in 21 countries from February 2, 2017, to June 12, 2020. A recruitment period of 24 months and a total study duration of 45 months were initially planned. The primary efficacy analysis was based on the intention-to-treat population. This was the first randomized controlled trial designed to assess the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal and cardiovascular outcomes in patients with CKD.
Setting and participants. This trial randomly assigned 4304 adult participants with CKD stages 2 to 4 (an estimated glomerular filtration rate [GFR] of 25 to 75 mL/min/1.73 m2 of body-surface area) and elevated urinary albumin excretion (urinary albumin-to-creatinine ratio of 200 to 5000, measured in mg of albumin per g of creatinine) to receive dapagliflozin (10 mg once daily) or placebo. Exclusion criteria included type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, recent immunosuppressive therapy for primary or secondary kidney disease, New York Heart Association class IV congestive heart failure, myocardial infarction, unstable angina, stroke or transient ischemic attacks, or recent coronary revascularization or valvular repair/replacement. All participants received a stable dose of renin–angiotensin system inhibitor for 4 weeks prior to screening, and the vast majority received a maximum tolerated dose at enrollment. Randomization was monitored to ensure that at least 30% of participants recruited did not have diabetes and that no more than 10% had stage 2 CKD. Participants were randomly assigned to receive dapagliflozin (n = 2152) or matching placebo (n = 2152) to ensure a 1:1 ratio of the 2 regimens. Dapagliflozin and placebo had identical appearance and administration schedules. All participants and trial personnel (except members of the independent data monitoring committee) were unaware of the trial-group assignments. After randomization, in-person study visits were conducted at 2 weeks, at 2, 4, and 8 months, and at 4-month intervals thereafter.
Main outcome measures. The primary outcome was a composite of the first occurrence of either a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Secondary outcomes, in hierarchical order, were: (1) the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes; (2) a composite cardiovascular outcome defined as hospitalization for heart failure or death from cardiovascular causes; and (3) death from any cause. All outcomes were assessed by time-to-event analyses.
Given the extensive prior experience with dapagliflozin, only selected adverse events were recorded. These included serious adverse events, adverse events resulting in the discontinuation of dapagliflozin or placebo, and adverse events of interest to dapagliflozin (eg, volume depletion symptoms, renal events, major hypoglycemia, fractures, diabetic ketoacidosis, events leading to higher risk of lower limb amputation, and lower limb amputations).
Main results. On March 26, 2020, the independent data monitoring committee recommended stopping the trial because of clear efficacy on the basis of 408 primary outcome events. The participants were 61.8 ± 12.1 years of age, and 1425 participants (33.1%) were female. The baseline mean estimated GFR was 43.1 ± 12.4 mL/min/1.73 m2, the median urinary albumin-to-creatinine ratio was 949, and 2906 participants (67.5%) had type 2 diabetes. Over a median of 2.4 years, a primary outcome event occurred in 197 participants (9.2%) in the dapagliflozin group and 312 (14.5%) in the placebo group (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51-0.72; P < 0.001). The number of participants who needed to be treated during the trial period to prevent 1 primary outcome event was 19 (95% CI, 15-27). The beneficial effect of dapagliflozin compared with placebo was consistent across all 8 prespecified subgroups (ie, age, sex, race, geographic region, type 2 diabetes, estimated GFR, urinary albumin-to-creatinine ratio, and systolic blood pressure) for the primary outcome. The effects of dapagliflozin were similar in participants with type 2 diabetes and in those without type 2 diabetes.
The incidence of each secondary outcome was similarly lower in the dapagliflozin-treated group than in the placebo group. The HR for the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56 (95% CI, 0.45-0.68; P < 0.001), and the HR for the composite cardiovascular outcome of hospitalization for heart failure or death from cardiovascular causes was 0.71 (95% CI, 0.55-0.92; P = 0.009). Death occurred in 101 participants (4.7%) in the dapagliflozin group and 146 participants (6.8%) in the placebo group (HR, 0.69; 95% CI, 0.53-0.88; P = 0.004). The known safety profile of dapagliflozin was confirmed by the similar overall incidences of adverse events and serious adverse events in the dapagliflozin and placebo groups.
Conclusion. In patients with CKD, with or without type 2 diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes was significantly lowered by dapagliflozin treatment.
Commentary
Although SGLT2 inhibitors were designed to reduce plasma glucose and hemoglobin A1c (HbA1c) by increasing urinary glucose excretion in a non-insulin-dependent fashion, an increasing number of clinical trials have demonstrated their possible cardiovascular and renal benefits that extend beyond glycemic control. In 2008, the US Food and Drug Administration (FDA) issued a guidance recommending the evaluation of long-term cardiovascular outcomes prior to approval and commercialization of new antidiabetic therapies to ensure minimum cardiovascular risks following the discovery of cardiovascular safety issues associated with antidiabetic compounds, including rosiglitazone, after drug approval. No one foresaw that this recommendation would lead to the discovery of new classes of antidiabetic drugs (glucagon-like peptide 1 [GLP1] and SGLT2 inhibitors) that improve cardiovascular outcomes. A series of clinical trials of SGLT2 inhibitors, including empagliflozin,1 canagliflozin,2 and dapagliflozin,3 showed a reduction in cardiovascular death and hospitalization due to heart failure among patients with type 2 diabetes. Furthermore, a meta-analysis from 2019 found that SGLT2 inhibitors reduced the risk of a composite of cardiovascular death or hospitalization for heart failure by 23% and the risk of progression of kidney failure by 45% in patients with diabetes.4 Thus, the strong and consistent evidence from these large and well-designed outcome trials led the American Diabetes Association in its most recent guidelines to recommend adding SGLT2 inhibitors to metformin for the treatment of patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease, heart failure, or CKD, regardless of baseline HbA1c levels or HbA1c target.5 As a result of the compelling effects of SGLT2 inhibitors on cardiovascular outcomes in diabetic patients, as well as increasing evidence that these clinical effects were independent of glycemic control, several subsequent trials were conducted to evaluate whether this new class of drugs may improve clinical outcomes in nondiabetic patients.
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) was the first clinical trial to investigate the effect of SGLT2 inhibitors on cardiovascular disease in nondiabetic patients. Findings from DAPA-HF showed that dapagliflozin reduced the risk of worsening heart failure or death from cardiovascular causes, independent of the presence of underlying diabetes. This initial finding resonates with a growing body of evidence6,7 that supports the use of SGLT2 inhibitors as an adjunctive therapy for heart failure in the absence of diabetes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that long-term administration of canagliflozin conferred cardiovascular, as well as renal, protection in patients with type 2 diabetes with CKD.8 Similar to the protective effects on heart failure, the renal benefits of SGLT2 inhibitors appeared to be independent of their blood glucose-lowering effects. Thus, these recent discoveries led to the design of the DAPA-CKD trial to further assess the long-term efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with CKD precipitated by causes other than type 2 diabetes. Although diabetes is the most common cause for CKD, it nonetheless only accounts for 40% of all CKD etiologies. To date, the only classes of medication that have been shown to slow a decline in kidney function in patients with diabetes are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Given that CKD is an important contributor to illness, is associated with diminished quality of life and reduced life expectancy, and increases health care costs, the findings of the DAPA-CKD trial are particularly significant as they show a renal benefit of dapagliflozin treatment across CKD stages that is independent of underlying diabetes. Therefore, SGLT2 inhibitors may offer a new and unique treatment option for millions of patients with CKD worldwide for whom ACE inhibitors and ARBs were otherwise the only treatments to prevent kidney failure. Moreover, with a number-needed-to-treat of 19 to prevent 1 composite renal vascular event over a period of 2.4 years, dapagliflozin requires a much lower number needed to treat compared to ACE inhibitors and ARBs in similar patients.
The trial has several limitations in study design. For example, the management of diabetes and hypertension were left to the discretion of each trial site, in keeping with local clinical practice and guidelines. It is unknown whether this variability in the management of comorbidities that impact kidney function had an effect on the study’s results. In addition, the trial was stopped early as a result of recommendations from an independent committee due to the demonstrated efficacy of dapagliflozin. This may have reduced the statistical power to assess some of the secondary outcomes. Finally, the authors discussed an initial dip in the estimated GFR after initiation of dapagliflozin treatment, similar to that observed in other SGLT2 inhibitor clinical trials. However, they were unable to ascertain the reversibility of this effect after the discontinuation of dapagliflozin because assessment of GFR was not completed after trial closure. Nonetheless, the authors specified that the reversibility of this initial estimated GFR dip had been assessed and observed in other clinical trials involving dapagliflozin.
The nonglycemic benefits of SGLT2 inhibitors, including improvement in renal outcomes, have strong implications for the future management of patients with CKD. If this indication is approved by the FDA and recommended by clinical guidelines, the ease of SGLT2 inhibitor prescription (eg, minimal drug-drug interaction, no titration), treatment administration (orally once daily), and safety profile may lead to wide use of SGLT2 inhibitors by generalists, nephrologists, and endocrinologists in preserving or improving renal outcomes in patients at risk for end-stage kidney disease. Given that SGLT2 inhibitors are a new class of pharmacologic therapeutics, patient education should include a discussion of the possible side effects, such as euglycemic ketoacidosis, genital and urinary tract infection, and foot and leg amputation. Finally, as Strandberg and colleagues reported in a recent commentary,9 the safety of SGLT2 inhibitors in older adults with multimorbidity, frailty, and polypharmacy remains unclear. Thus, future studies of SGLT2 inhibitors are needed to better evaluate their clinical effects in older adults.
Applications for Clinical Practice
This trial enrolled a dedicated patient population with CKD and demonstrated a benefit of dapagliflozin in reducing renal and cardiovascular outcomes, regardless of baseline diabetes status. These drugs (dapagliflozin as well as other SGLT2 inhibitors) will likely have a prominent role in future CKD management guidelines. Until then, several barriers remain before SGLT2 inhibitors can be widely used in clinical practice. Among these barriers are FDA approval for their use in patients with and without diabetes with an estimated GFR < 30 mL/min/1.73 m2 and lowering the costs of this class of drugs.
—Rachel Litke, MD, PhD
Icahn School of Medicine at Mount Sinai
Fred Ko, MD, MS
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
4. Zelniker TA, Wiviott SD, Raz I, Sabatine MS. SGLT-2 inhibitors for people with type 2 diabetes - Authors’ reply. Lancet. 2019;394:560-561.
5. American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111-S34.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
7. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829.
8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
9. Strandberg TE, Petrovic M, Benetos A. SGLT-2 inhibitors for people with type 2 diabetes. Lancet. 2019;394:560.
Study Overview
Objective. To assess whether dapagliflozin added to guideline-recommended therapies is effective and safe over the long-term to reduce the rate of renal and cardiovascular events in patients across multiple chronic kidney disease (CKD) stages, with and without type 2 diabetes.
Design. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) was a randomized, double-blind, parallel-group, placebo-controlled, multicenter event-driven, clinical trial sponsored by Astra-Zeneca. It was conducted at 386 sites in 21 countries from February 2, 2017, to June 12, 2020. A recruitment period of 24 months and a total study duration of 45 months were initially planned. The primary efficacy analysis was based on the intention-to-treat population. This was the first randomized controlled trial designed to assess the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal and cardiovascular outcomes in patients with CKD.
Setting and participants. This trial randomly assigned 4304 adult participants with CKD stages 2 to 4 (an estimated glomerular filtration rate [GFR] of 25 to 75 mL/min/1.73 m2 of body-surface area) and elevated urinary albumin excretion (urinary albumin-to-creatinine ratio of 200 to 5000, measured in mg of albumin per g of creatinine) to receive dapagliflozin (10 mg once daily) or placebo. Exclusion criteria included type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, recent immunosuppressive therapy for primary or secondary kidney disease, New York Heart Association class IV congestive heart failure, myocardial infarction, unstable angina, stroke or transient ischemic attacks, or recent coronary revascularization or valvular repair/replacement. All participants received a stable dose of renin–angiotensin system inhibitor for 4 weeks prior to screening, and the vast majority received a maximum tolerated dose at enrollment. Randomization was monitored to ensure that at least 30% of participants recruited did not have diabetes and that no more than 10% had stage 2 CKD. Participants were randomly assigned to receive dapagliflozin (n = 2152) or matching placebo (n = 2152) to ensure a 1:1 ratio of the 2 regimens. Dapagliflozin and placebo had identical appearance and administration schedules. All participants and trial personnel (except members of the independent data monitoring committee) were unaware of the trial-group assignments. After randomization, in-person study visits were conducted at 2 weeks, at 2, 4, and 8 months, and at 4-month intervals thereafter.
Main outcome measures. The primary outcome was a composite of the first occurrence of either a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Secondary outcomes, in hierarchical order, were: (1) the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes; (2) a composite cardiovascular outcome defined as hospitalization for heart failure or death from cardiovascular causes; and (3) death from any cause. All outcomes were assessed by time-to-event analyses.
Given the extensive prior experience with dapagliflozin, only selected adverse events were recorded. These included serious adverse events, adverse events resulting in the discontinuation of dapagliflozin or placebo, and adverse events of interest to dapagliflozin (eg, volume depletion symptoms, renal events, major hypoglycemia, fractures, diabetic ketoacidosis, events leading to higher risk of lower limb amputation, and lower limb amputations).
Main results. On March 26, 2020, the independent data monitoring committee recommended stopping the trial because of clear efficacy on the basis of 408 primary outcome events. The participants were 61.8 ± 12.1 years of age, and 1425 participants (33.1%) were female. The baseline mean estimated GFR was 43.1 ± 12.4 mL/min/1.73 m2, the median urinary albumin-to-creatinine ratio was 949, and 2906 participants (67.5%) had type 2 diabetes. Over a median of 2.4 years, a primary outcome event occurred in 197 participants (9.2%) in the dapagliflozin group and 312 (14.5%) in the placebo group (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51-0.72; P < 0.001). The number of participants who needed to be treated during the trial period to prevent 1 primary outcome event was 19 (95% CI, 15-27). The beneficial effect of dapagliflozin compared with placebo was consistent across all 8 prespecified subgroups (ie, age, sex, race, geographic region, type 2 diabetes, estimated GFR, urinary albumin-to-creatinine ratio, and systolic blood pressure) for the primary outcome. The effects of dapagliflozin were similar in participants with type 2 diabetes and in those without type 2 diabetes.
The incidence of each secondary outcome was similarly lower in the dapagliflozin-treated group than in the placebo group. The HR for the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56 (95% CI, 0.45-0.68; P < 0.001), and the HR for the composite cardiovascular outcome of hospitalization for heart failure or death from cardiovascular causes was 0.71 (95% CI, 0.55-0.92; P = 0.009). Death occurred in 101 participants (4.7%) in the dapagliflozin group and 146 participants (6.8%) in the placebo group (HR, 0.69; 95% CI, 0.53-0.88; P = 0.004). The known safety profile of dapagliflozin was confirmed by the similar overall incidences of adverse events and serious adverse events in the dapagliflozin and placebo groups.
Conclusion. In patients with CKD, with or without type 2 diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes was significantly lowered by dapagliflozin treatment.
Commentary
Although SGLT2 inhibitors were designed to reduce plasma glucose and hemoglobin A1c (HbA1c) by increasing urinary glucose excretion in a non-insulin-dependent fashion, an increasing number of clinical trials have demonstrated their possible cardiovascular and renal benefits that extend beyond glycemic control. In 2008, the US Food and Drug Administration (FDA) issued a guidance recommending the evaluation of long-term cardiovascular outcomes prior to approval and commercialization of new antidiabetic therapies to ensure minimum cardiovascular risks following the discovery of cardiovascular safety issues associated with antidiabetic compounds, including rosiglitazone, after drug approval. No one foresaw that this recommendation would lead to the discovery of new classes of antidiabetic drugs (glucagon-like peptide 1 [GLP1] and SGLT2 inhibitors) that improve cardiovascular outcomes. A series of clinical trials of SGLT2 inhibitors, including empagliflozin,1 canagliflozin,2 and dapagliflozin,3 showed a reduction in cardiovascular death and hospitalization due to heart failure among patients with type 2 diabetes. Furthermore, a meta-analysis from 2019 found that SGLT2 inhibitors reduced the risk of a composite of cardiovascular death or hospitalization for heart failure by 23% and the risk of progression of kidney failure by 45% in patients with diabetes.4 Thus, the strong and consistent evidence from these large and well-designed outcome trials led the American Diabetes Association in its most recent guidelines to recommend adding SGLT2 inhibitors to metformin for the treatment of patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease, heart failure, or CKD, regardless of baseline HbA1c levels or HbA1c target.5 As a result of the compelling effects of SGLT2 inhibitors on cardiovascular outcomes in diabetic patients, as well as increasing evidence that these clinical effects were independent of glycemic control, several subsequent trials were conducted to evaluate whether this new class of drugs may improve clinical outcomes in nondiabetic patients.
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) was the first clinical trial to investigate the effect of SGLT2 inhibitors on cardiovascular disease in nondiabetic patients. Findings from DAPA-HF showed that dapagliflozin reduced the risk of worsening heart failure or death from cardiovascular causes, independent of the presence of underlying diabetes. This initial finding resonates with a growing body of evidence6,7 that supports the use of SGLT2 inhibitors as an adjunctive therapy for heart failure in the absence of diabetes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that long-term administration of canagliflozin conferred cardiovascular, as well as renal, protection in patients with type 2 diabetes with CKD.8 Similar to the protective effects on heart failure, the renal benefits of SGLT2 inhibitors appeared to be independent of their blood glucose-lowering effects. Thus, these recent discoveries led to the design of the DAPA-CKD trial to further assess the long-term efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with CKD precipitated by causes other than type 2 diabetes. Although diabetes is the most common cause for CKD, it nonetheless only accounts for 40% of all CKD etiologies. To date, the only classes of medication that have been shown to slow a decline in kidney function in patients with diabetes are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Given that CKD is an important contributor to illness, is associated with diminished quality of life and reduced life expectancy, and increases health care costs, the findings of the DAPA-CKD trial are particularly significant as they show a renal benefit of dapagliflozin treatment across CKD stages that is independent of underlying diabetes. Therefore, SGLT2 inhibitors may offer a new and unique treatment option for millions of patients with CKD worldwide for whom ACE inhibitors and ARBs were otherwise the only treatments to prevent kidney failure. Moreover, with a number-needed-to-treat of 19 to prevent 1 composite renal vascular event over a period of 2.4 years, dapagliflozin requires a much lower number needed to treat compared to ACE inhibitors and ARBs in similar patients.
The trial has several limitations in study design. For example, the management of diabetes and hypertension were left to the discretion of each trial site, in keeping with local clinical practice and guidelines. It is unknown whether this variability in the management of comorbidities that impact kidney function had an effect on the study’s results. In addition, the trial was stopped early as a result of recommendations from an independent committee due to the demonstrated efficacy of dapagliflozin. This may have reduced the statistical power to assess some of the secondary outcomes. Finally, the authors discussed an initial dip in the estimated GFR after initiation of dapagliflozin treatment, similar to that observed in other SGLT2 inhibitor clinical trials. However, they were unable to ascertain the reversibility of this effect after the discontinuation of dapagliflozin because assessment of GFR was not completed after trial closure. Nonetheless, the authors specified that the reversibility of this initial estimated GFR dip had been assessed and observed in other clinical trials involving dapagliflozin.
The nonglycemic benefits of SGLT2 inhibitors, including improvement in renal outcomes, have strong implications for the future management of patients with CKD. If this indication is approved by the FDA and recommended by clinical guidelines, the ease of SGLT2 inhibitor prescription (eg, minimal drug-drug interaction, no titration), treatment administration (orally once daily), and safety profile may lead to wide use of SGLT2 inhibitors by generalists, nephrologists, and endocrinologists in preserving or improving renal outcomes in patients at risk for end-stage kidney disease. Given that SGLT2 inhibitors are a new class of pharmacologic therapeutics, patient education should include a discussion of the possible side effects, such as euglycemic ketoacidosis, genital and urinary tract infection, and foot and leg amputation. Finally, as Strandberg and colleagues reported in a recent commentary,9 the safety of SGLT2 inhibitors in older adults with multimorbidity, frailty, and polypharmacy remains unclear. Thus, future studies of SGLT2 inhibitors are needed to better evaluate their clinical effects in older adults.
Applications for Clinical Practice
This trial enrolled a dedicated patient population with CKD and demonstrated a benefit of dapagliflozin in reducing renal and cardiovascular outcomes, regardless of baseline diabetes status. These drugs (dapagliflozin as well as other SGLT2 inhibitors) will likely have a prominent role in future CKD management guidelines. Until then, several barriers remain before SGLT2 inhibitors can be widely used in clinical practice. Among these barriers are FDA approval for their use in patients with and without diabetes with an estimated GFR < 30 mL/min/1.73 m2 and lowering the costs of this class of drugs.
—Rachel Litke, MD, PhD
Icahn School of Medicine at Mount Sinai
Fred Ko, MD, MS
Study Overview
Objective. To assess whether dapagliflozin added to guideline-recommended therapies is effective and safe over the long-term to reduce the rate of renal and cardiovascular events in patients across multiple chronic kidney disease (CKD) stages, with and without type 2 diabetes.
Design. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) was a randomized, double-blind, parallel-group, placebo-controlled, multicenter event-driven, clinical trial sponsored by Astra-Zeneca. It was conducted at 386 sites in 21 countries from February 2, 2017, to June 12, 2020. A recruitment period of 24 months and a total study duration of 45 months were initially planned. The primary efficacy analysis was based on the intention-to-treat population. This was the first randomized controlled trial designed to assess the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal and cardiovascular outcomes in patients with CKD.
Setting and participants. This trial randomly assigned 4304 adult participants with CKD stages 2 to 4 (an estimated glomerular filtration rate [GFR] of 25 to 75 mL/min/1.73 m2 of body-surface area) and elevated urinary albumin excretion (urinary albumin-to-creatinine ratio of 200 to 5000, measured in mg of albumin per g of creatinine) to receive dapagliflozin (10 mg once daily) or placebo. Exclusion criteria included type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, recent immunosuppressive therapy for primary or secondary kidney disease, New York Heart Association class IV congestive heart failure, myocardial infarction, unstable angina, stroke or transient ischemic attacks, or recent coronary revascularization or valvular repair/replacement. All participants received a stable dose of renin–angiotensin system inhibitor for 4 weeks prior to screening, and the vast majority received a maximum tolerated dose at enrollment. Randomization was monitored to ensure that at least 30% of participants recruited did not have diabetes and that no more than 10% had stage 2 CKD. Participants were randomly assigned to receive dapagliflozin (n = 2152) or matching placebo (n = 2152) to ensure a 1:1 ratio of the 2 regimens. Dapagliflozin and placebo had identical appearance and administration schedules. All participants and trial personnel (except members of the independent data monitoring committee) were unaware of the trial-group assignments. After randomization, in-person study visits were conducted at 2 weeks, at 2, 4, and 8 months, and at 4-month intervals thereafter.
Main outcome measures. The primary outcome was a composite of the first occurrence of either a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Secondary outcomes, in hierarchical order, were: (1) the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes; (2) a composite cardiovascular outcome defined as hospitalization for heart failure or death from cardiovascular causes; and (3) death from any cause. All outcomes were assessed by time-to-event analyses.
Given the extensive prior experience with dapagliflozin, only selected adverse events were recorded. These included serious adverse events, adverse events resulting in the discontinuation of dapagliflozin or placebo, and adverse events of interest to dapagliflozin (eg, volume depletion symptoms, renal events, major hypoglycemia, fractures, diabetic ketoacidosis, events leading to higher risk of lower limb amputation, and lower limb amputations).
Main results. On March 26, 2020, the independent data monitoring committee recommended stopping the trial because of clear efficacy on the basis of 408 primary outcome events. The participants were 61.8 ± 12.1 years of age, and 1425 participants (33.1%) were female. The baseline mean estimated GFR was 43.1 ± 12.4 mL/min/1.73 m2, the median urinary albumin-to-creatinine ratio was 949, and 2906 participants (67.5%) had type 2 diabetes. Over a median of 2.4 years, a primary outcome event occurred in 197 participants (9.2%) in the dapagliflozin group and 312 (14.5%) in the placebo group (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51-0.72; P < 0.001). The number of participants who needed to be treated during the trial period to prevent 1 primary outcome event was 19 (95% CI, 15-27). The beneficial effect of dapagliflozin compared with placebo was consistent across all 8 prespecified subgroups (ie, age, sex, race, geographic region, type 2 diabetes, estimated GFR, urinary albumin-to-creatinine ratio, and systolic blood pressure) for the primary outcome. The effects of dapagliflozin were similar in participants with type 2 diabetes and in those without type 2 diabetes.
The incidence of each secondary outcome was similarly lower in the dapagliflozin-treated group than in the placebo group. The HR for the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56 (95% CI, 0.45-0.68; P < 0.001), and the HR for the composite cardiovascular outcome of hospitalization for heart failure or death from cardiovascular causes was 0.71 (95% CI, 0.55-0.92; P = 0.009). Death occurred in 101 participants (4.7%) in the dapagliflozin group and 146 participants (6.8%) in the placebo group (HR, 0.69; 95% CI, 0.53-0.88; P = 0.004). The known safety profile of dapagliflozin was confirmed by the similar overall incidences of adverse events and serious adverse events in the dapagliflozin and placebo groups.
Conclusion. In patients with CKD, with or without type 2 diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes was significantly lowered by dapagliflozin treatment.
Commentary
Although SGLT2 inhibitors were designed to reduce plasma glucose and hemoglobin A1c (HbA1c) by increasing urinary glucose excretion in a non-insulin-dependent fashion, an increasing number of clinical trials have demonstrated their possible cardiovascular and renal benefits that extend beyond glycemic control. In 2008, the US Food and Drug Administration (FDA) issued a guidance recommending the evaluation of long-term cardiovascular outcomes prior to approval and commercialization of new antidiabetic therapies to ensure minimum cardiovascular risks following the discovery of cardiovascular safety issues associated with antidiabetic compounds, including rosiglitazone, after drug approval. No one foresaw that this recommendation would lead to the discovery of new classes of antidiabetic drugs (glucagon-like peptide 1 [GLP1] and SGLT2 inhibitors) that improve cardiovascular outcomes. A series of clinical trials of SGLT2 inhibitors, including empagliflozin,1 canagliflozin,2 and dapagliflozin,3 showed a reduction in cardiovascular death and hospitalization due to heart failure among patients with type 2 diabetes. Furthermore, a meta-analysis from 2019 found that SGLT2 inhibitors reduced the risk of a composite of cardiovascular death or hospitalization for heart failure by 23% and the risk of progression of kidney failure by 45% in patients with diabetes.4 Thus, the strong and consistent evidence from these large and well-designed outcome trials led the American Diabetes Association in its most recent guidelines to recommend adding SGLT2 inhibitors to metformin for the treatment of patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease, heart failure, or CKD, regardless of baseline HbA1c levels or HbA1c target.5 As a result of the compelling effects of SGLT2 inhibitors on cardiovascular outcomes in diabetic patients, as well as increasing evidence that these clinical effects were independent of glycemic control, several subsequent trials were conducted to evaluate whether this new class of drugs may improve clinical outcomes in nondiabetic patients.
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) was the first clinical trial to investigate the effect of SGLT2 inhibitors on cardiovascular disease in nondiabetic patients. Findings from DAPA-HF showed that dapagliflozin reduced the risk of worsening heart failure or death from cardiovascular causes, independent of the presence of underlying diabetes. This initial finding resonates with a growing body of evidence6,7 that supports the use of SGLT2 inhibitors as an adjunctive therapy for heart failure in the absence of diabetes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that long-term administration of canagliflozin conferred cardiovascular, as well as renal, protection in patients with type 2 diabetes with CKD.8 Similar to the protective effects on heart failure, the renal benefits of SGLT2 inhibitors appeared to be independent of their blood glucose-lowering effects. Thus, these recent discoveries led to the design of the DAPA-CKD trial to further assess the long-term efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with CKD precipitated by causes other than type 2 diabetes. Although diabetes is the most common cause for CKD, it nonetheless only accounts for 40% of all CKD etiologies. To date, the only classes of medication that have been shown to slow a decline in kidney function in patients with diabetes are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Given that CKD is an important contributor to illness, is associated with diminished quality of life and reduced life expectancy, and increases health care costs, the findings of the DAPA-CKD trial are particularly significant as they show a renal benefit of dapagliflozin treatment across CKD stages that is independent of underlying diabetes. Therefore, SGLT2 inhibitors may offer a new and unique treatment option for millions of patients with CKD worldwide for whom ACE inhibitors and ARBs were otherwise the only treatments to prevent kidney failure. Moreover, with a number-needed-to-treat of 19 to prevent 1 composite renal vascular event over a period of 2.4 years, dapagliflozin requires a much lower number needed to treat compared to ACE inhibitors and ARBs in similar patients.
The trial has several limitations in study design. For example, the management of diabetes and hypertension were left to the discretion of each trial site, in keeping with local clinical practice and guidelines. It is unknown whether this variability in the management of comorbidities that impact kidney function had an effect on the study’s results. In addition, the trial was stopped early as a result of recommendations from an independent committee due to the demonstrated efficacy of dapagliflozin. This may have reduced the statistical power to assess some of the secondary outcomes. Finally, the authors discussed an initial dip in the estimated GFR after initiation of dapagliflozin treatment, similar to that observed in other SGLT2 inhibitor clinical trials. However, they were unable to ascertain the reversibility of this effect after the discontinuation of dapagliflozin because assessment of GFR was not completed after trial closure. Nonetheless, the authors specified that the reversibility of this initial estimated GFR dip had been assessed and observed in other clinical trials involving dapagliflozin.
The nonglycemic benefits of SGLT2 inhibitors, including improvement in renal outcomes, have strong implications for the future management of patients with CKD. If this indication is approved by the FDA and recommended by clinical guidelines, the ease of SGLT2 inhibitor prescription (eg, minimal drug-drug interaction, no titration), treatment administration (orally once daily), and safety profile may lead to wide use of SGLT2 inhibitors by generalists, nephrologists, and endocrinologists in preserving or improving renal outcomes in patients at risk for end-stage kidney disease. Given that SGLT2 inhibitors are a new class of pharmacologic therapeutics, patient education should include a discussion of the possible side effects, such as euglycemic ketoacidosis, genital and urinary tract infection, and foot and leg amputation. Finally, as Strandberg and colleagues reported in a recent commentary,9 the safety of SGLT2 inhibitors in older adults with multimorbidity, frailty, and polypharmacy remains unclear. Thus, future studies of SGLT2 inhibitors are needed to better evaluate their clinical effects in older adults.
Applications for Clinical Practice
This trial enrolled a dedicated patient population with CKD and demonstrated a benefit of dapagliflozin in reducing renal and cardiovascular outcomes, regardless of baseline diabetes status. These drugs (dapagliflozin as well as other SGLT2 inhibitors) will likely have a prominent role in future CKD management guidelines. Until then, several barriers remain before SGLT2 inhibitors can be widely used in clinical practice. Among these barriers are FDA approval for their use in patients with and without diabetes with an estimated GFR < 30 mL/min/1.73 m2 and lowering the costs of this class of drugs.
—Rachel Litke, MD, PhD
Icahn School of Medicine at Mount Sinai
Fred Ko, MD, MS
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
4. Zelniker TA, Wiviott SD, Raz I, Sabatine MS. SGLT-2 inhibitors for people with type 2 diabetes - Authors’ reply. Lancet. 2019;394:560-561.
5. American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111-S34.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
7. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829.
8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
9. Strandberg TE, Petrovic M, Benetos A. SGLT-2 inhibitors for people with type 2 diabetes. Lancet. 2019;394:560.
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
4. Zelniker TA, Wiviott SD, Raz I, Sabatine MS. SGLT-2 inhibitors for people with type 2 diabetes - Authors’ reply. Lancet. 2019;394:560-561.
5. American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111-S34.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
7. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829.
8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
9. Strandberg TE, Petrovic M, Benetos A. SGLT-2 inhibitors for people with type 2 diabetes. Lancet. 2019;394:560.


