User login
Microvascular disease: An independent and exacerbating risk factor for amputation
Individuals with microvascular disease (MVD) showed a significantly increased risk of lower limb amputation in the absence of peripheral artery disease (PAD), according to the results of a large database analysis published online in Circulation.
Furthermore, those who had both MVD and PAD had a greater than 20-fold increased risk of amputation than if they had either PAD or MVD alone, according to Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn., and colleagues.
“The novelty of these findings becomes clear when put into the current framework of critical limb ischemia,” they wrote.
“In a recent state of the art review of [critical limb ischemia], MVD as a whole or its components did not receive a single mention. Our work shows that MVD helps identify a population not previously considered at particularly high risk for amputation and, when added to PAD, identify a group of patients at very high risk for amputation,” they continued.
Dr. Beckman and colleagues assessed individuals in the Veterans Aging Cohort Study (VACS), a prospective longitudinal cohort of veterans. They included all VACS participants who were alive as of April 1, 2003 with the baseline as a participant’s first clinic visit on or after this date. Participants were followed from baseline to the minimum of: date of lower extremity amputation, death, or Dec. 31, 2014.
They assessed four levels of vascular involvement: neither MVD nor PAD, MVD alone, PAD alone, and MVD plus PAD, with the primary outcome being lower limb amputation, all based on a variety of measures including appropriate ICD-9 or CPT codes.
The rate of incident amputation over a median of 9.3 years of follow-up was 1.16 per 1000 person-years. At the time of amputation, retinopathy was present in 69%, nephropathy in 67%, and neuropathy in 78% of participants.
After multivariable adjustment for 216 demographic characteristics, cardiovascular disease risk factors, and other potential confounders, they found that, compared with participants without either vascular disease, the presence of MVD alone was associated with a 3.7-fold increased risk of amputation, PAD alone conferred a 13.9-fold elevated risk of amputation, and the combination of PAD and MVD was associated with a 22.7-fold increased risk of amputation.
They also found that the location of amputation also varied depending on the type of vascular disease at the time of amputation.
Participants with MVD alone accounted for 18% of all amputations, 21% of below ankle amputations, 15% of below knee amputations, and 6% of all above knee amputations. Participants with PAD alone accounted for 22% of all amputations, 17% of below ankle, 25% of below knee, and 39% of above knee amputations. The combination of MVD and PAD accounted for 45% of all amputation and caused the most amputation at all limb levels. In addition, they found a statistically significant variation in vascular involvement and level of amputation, with MVD more likely to cause a below-ankle amputation and PAD more likely to cause below- and above-knee amputations (P less than .001)
“MVD likely participates importantly in the development of adverse limb events in PAD and suggests additional patient populations who may benefit from greater foot surveillance to minimize amputation,” the researchers concluded.
The study was supported by grants from the American Heart Association. Dr. Beckman reported consulting for multiple pharmaceutical companies and serving on the DSMC for Bayer and Novartis.
SOURCE: Beckman JA et al. Circulation. 2019. doi: 10.1161/CIRCULATIONAHA.119.040672.
Individuals with microvascular disease (MVD) showed a significantly increased risk of lower limb amputation in the absence of peripheral artery disease (PAD), according to the results of a large database analysis published online in Circulation.
Furthermore, those who had both MVD and PAD had a greater than 20-fold increased risk of amputation than if they had either PAD or MVD alone, according to Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn., and colleagues.
“The novelty of these findings becomes clear when put into the current framework of critical limb ischemia,” they wrote.
“In a recent state of the art review of [critical limb ischemia], MVD as a whole or its components did not receive a single mention. Our work shows that MVD helps identify a population not previously considered at particularly high risk for amputation and, when added to PAD, identify a group of patients at very high risk for amputation,” they continued.
Dr. Beckman and colleagues assessed individuals in the Veterans Aging Cohort Study (VACS), a prospective longitudinal cohort of veterans. They included all VACS participants who were alive as of April 1, 2003 with the baseline as a participant’s first clinic visit on or after this date. Participants were followed from baseline to the minimum of: date of lower extremity amputation, death, or Dec. 31, 2014.
They assessed four levels of vascular involvement: neither MVD nor PAD, MVD alone, PAD alone, and MVD plus PAD, with the primary outcome being lower limb amputation, all based on a variety of measures including appropriate ICD-9 or CPT codes.
The rate of incident amputation over a median of 9.3 years of follow-up was 1.16 per 1000 person-years. At the time of amputation, retinopathy was present in 69%, nephropathy in 67%, and neuropathy in 78% of participants.
After multivariable adjustment for 216 demographic characteristics, cardiovascular disease risk factors, and other potential confounders, they found that, compared with participants without either vascular disease, the presence of MVD alone was associated with a 3.7-fold increased risk of amputation, PAD alone conferred a 13.9-fold elevated risk of amputation, and the combination of PAD and MVD was associated with a 22.7-fold increased risk of amputation.
They also found that the location of amputation also varied depending on the type of vascular disease at the time of amputation.
Participants with MVD alone accounted for 18% of all amputations, 21% of below ankle amputations, 15% of below knee amputations, and 6% of all above knee amputations. Participants with PAD alone accounted for 22% of all amputations, 17% of below ankle, 25% of below knee, and 39% of above knee amputations. The combination of MVD and PAD accounted for 45% of all amputation and caused the most amputation at all limb levels. In addition, they found a statistically significant variation in vascular involvement and level of amputation, with MVD more likely to cause a below-ankle amputation and PAD more likely to cause below- and above-knee amputations (P less than .001)
“MVD likely participates importantly in the development of adverse limb events in PAD and suggests additional patient populations who may benefit from greater foot surveillance to minimize amputation,” the researchers concluded.
The study was supported by grants from the American Heart Association. Dr. Beckman reported consulting for multiple pharmaceutical companies and serving on the DSMC for Bayer and Novartis.
SOURCE: Beckman JA et al. Circulation. 2019. doi: 10.1161/CIRCULATIONAHA.119.040672.
Individuals with microvascular disease (MVD) showed a significantly increased risk of lower limb amputation in the absence of peripheral artery disease (PAD), according to the results of a large database analysis published online in Circulation.
Furthermore, those who had both MVD and PAD had a greater than 20-fold increased risk of amputation than if they had either PAD or MVD alone, according to Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn., and colleagues.
“The novelty of these findings becomes clear when put into the current framework of critical limb ischemia,” they wrote.
“In a recent state of the art review of [critical limb ischemia], MVD as a whole or its components did not receive a single mention. Our work shows that MVD helps identify a population not previously considered at particularly high risk for amputation and, when added to PAD, identify a group of patients at very high risk for amputation,” they continued.
Dr. Beckman and colleagues assessed individuals in the Veterans Aging Cohort Study (VACS), a prospective longitudinal cohort of veterans. They included all VACS participants who were alive as of April 1, 2003 with the baseline as a participant’s first clinic visit on or after this date. Participants were followed from baseline to the minimum of: date of lower extremity amputation, death, or Dec. 31, 2014.
They assessed four levels of vascular involvement: neither MVD nor PAD, MVD alone, PAD alone, and MVD plus PAD, with the primary outcome being lower limb amputation, all based on a variety of measures including appropriate ICD-9 or CPT codes.
The rate of incident amputation over a median of 9.3 years of follow-up was 1.16 per 1000 person-years. At the time of amputation, retinopathy was present in 69%, nephropathy in 67%, and neuropathy in 78% of participants.
After multivariable adjustment for 216 demographic characteristics, cardiovascular disease risk factors, and other potential confounders, they found that, compared with participants without either vascular disease, the presence of MVD alone was associated with a 3.7-fold increased risk of amputation, PAD alone conferred a 13.9-fold elevated risk of amputation, and the combination of PAD and MVD was associated with a 22.7-fold increased risk of amputation.
They also found that the location of amputation also varied depending on the type of vascular disease at the time of amputation.
Participants with MVD alone accounted for 18% of all amputations, 21% of below ankle amputations, 15% of below knee amputations, and 6% of all above knee amputations. Participants with PAD alone accounted for 22% of all amputations, 17% of below ankle, 25% of below knee, and 39% of above knee amputations. The combination of MVD and PAD accounted for 45% of all amputation and caused the most amputation at all limb levels. In addition, they found a statistically significant variation in vascular involvement and level of amputation, with MVD more likely to cause a below-ankle amputation and PAD more likely to cause below- and above-knee amputations (P less than .001)
“MVD likely participates importantly in the development of adverse limb events in PAD and suggests additional patient populations who may benefit from greater foot surveillance to minimize amputation,” the researchers concluded.
The study was supported by grants from the American Heart Association. Dr. Beckman reported consulting for multiple pharmaceutical companies and serving on the DSMC for Bayer and Novartis.
SOURCE: Beckman JA et al. Circulation. 2019. doi: 10.1161/CIRCULATIONAHA.119.040672.
FROM CIRCULATION
Key clinical point: Microvascular disease yielded a 3.7-fold increased risk of lower limb amputation.
Major finding:
Study details: Database analysis of 125,674 participants in the Veterans Aging Cohort Study from April 2003 through December 2014.
Disclosures: The study was supported by grants from the American Heart Association. Dr. Beckman reported consulting for multiple pharmaceutical companies and serving on the DSMC for Bayer and Novartis.
Source: Beckman JA et al. Circulation. 2019. doi: 10.1161/CIRCULATIONAHA.119.040672.
Mortality caused by chronic liver disease in setting of diabetes continues to rise
results from a large database analysis showed.
“While diabetes-related mortality has been reported to be decreasing due to improved awareness and management, our results highlight the need to better address NAFLD [nonalcoholic fatty liver disease] and end-stage liver disease among individuals with diabetes,” researchers led by Donghee Kim, MD, PhD, wrote in an article published in Clinical Gastroenterology and Hepatology.
In an effort to estimate the trends in chronic liver disease–related mortality among individuals with diabetes from 2007 to 2017 in the United States, Dr. Kim, of the division of gastroenterology and hepatology at Stanford (Calif.) University, and colleagues analyzed mortality records from the National Vital Statistic System database. They calculated age-specific mortality by dividing the number of deaths by the total U.S. census population for each year and standardized them according to age distribution of 2010 U.S. standard population. The researchers used joinpoint regression analysis to determine trends.
Of 2,686,590 individuals with diabetes identified, 48,761 had chronic liver disease as the underlying cause of death listed on the death certificate. Among individuals who had diabetes listed on their death certificate, the age-standardized mortality for cirrhosis and hepatocellular carcinoma as an underlying cause of death increased with an annual rate of 1.2% and 1.9%, respectively. Based on etiology, age-standardized mortality for hepatitis C and hepatitis B viral infections decreased at an annual rate of 4.4% and 5.1%, respectively. On the other hand, mortality among individuals with NAFLD and alcoholic liver disease increased at annual rates of 11.6% and 1.4%, respectively.
“When we defined chronic liver disease as an underlying or contributing cause of death among individuals with diabetes listed on the death certificate, the overall results remained similar,” the researchers wrote. They acknowledged certain limitations of the analysis, including the fact that using death certificates and ICD-10 codes “has the potential for misclassification and underestimation for diabetes and chronic liver disease–related mortality. However, the coding method has been constant over time, so it is unlikely to account for present trends. Increasing obesity and associated insulin resistance likely explain the link between diabetes and NAFLD and end-stage liver disease through hepatic inflammation and various proinflammatory cytokines.”
One of the study authors was supported by the National Institutes of Health. None of the other authors reported having relevant disclosures.
SOURCE: Kim D et al. Clin Gastroenterol Hepatol. 2019 Jun 17. doi: 10.1016/j.cgh.2019.06.011.
results from a large database analysis showed.
“While diabetes-related mortality has been reported to be decreasing due to improved awareness and management, our results highlight the need to better address NAFLD [nonalcoholic fatty liver disease] and end-stage liver disease among individuals with diabetes,” researchers led by Donghee Kim, MD, PhD, wrote in an article published in Clinical Gastroenterology and Hepatology.
In an effort to estimate the trends in chronic liver disease–related mortality among individuals with diabetes from 2007 to 2017 in the United States, Dr. Kim, of the division of gastroenterology and hepatology at Stanford (Calif.) University, and colleagues analyzed mortality records from the National Vital Statistic System database. They calculated age-specific mortality by dividing the number of deaths by the total U.S. census population for each year and standardized them according to age distribution of 2010 U.S. standard population. The researchers used joinpoint regression analysis to determine trends.
Of 2,686,590 individuals with diabetes identified, 48,761 had chronic liver disease as the underlying cause of death listed on the death certificate. Among individuals who had diabetes listed on their death certificate, the age-standardized mortality for cirrhosis and hepatocellular carcinoma as an underlying cause of death increased with an annual rate of 1.2% and 1.9%, respectively. Based on etiology, age-standardized mortality for hepatitis C and hepatitis B viral infections decreased at an annual rate of 4.4% and 5.1%, respectively. On the other hand, mortality among individuals with NAFLD and alcoholic liver disease increased at annual rates of 11.6% and 1.4%, respectively.
“When we defined chronic liver disease as an underlying or contributing cause of death among individuals with diabetes listed on the death certificate, the overall results remained similar,” the researchers wrote. They acknowledged certain limitations of the analysis, including the fact that using death certificates and ICD-10 codes “has the potential for misclassification and underestimation for diabetes and chronic liver disease–related mortality. However, the coding method has been constant over time, so it is unlikely to account for present trends. Increasing obesity and associated insulin resistance likely explain the link between diabetes and NAFLD and end-stage liver disease through hepatic inflammation and various proinflammatory cytokines.”
One of the study authors was supported by the National Institutes of Health. None of the other authors reported having relevant disclosures.
SOURCE: Kim D et al. Clin Gastroenterol Hepatol. 2019 Jun 17. doi: 10.1016/j.cgh.2019.06.011.
results from a large database analysis showed.
“While diabetes-related mortality has been reported to be decreasing due to improved awareness and management, our results highlight the need to better address NAFLD [nonalcoholic fatty liver disease] and end-stage liver disease among individuals with diabetes,” researchers led by Donghee Kim, MD, PhD, wrote in an article published in Clinical Gastroenterology and Hepatology.
In an effort to estimate the trends in chronic liver disease–related mortality among individuals with diabetes from 2007 to 2017 in the United States, Dr. Kim, of the division of gastroenterology and hepatology at Stanford (Calif.) University, and colleagues analyzed mortality records from the National Vital Statistic System database. They calculated age-specific mortality by dividing the number of deaths by the total U.S. census population for each year and standardized them according to age distribution of 2010 U.S. standard population. The researchers used joinpoint regression analysis to determine trends.
Of 2,686,590 individuals with diabetes identified, 48,761 had chronic liver disease as the underlying cause of death listed on the death certificate. Among individuals who had diabetes listed on their death certificate, the age-standardized mortality for cirrhosis and hepatocellular carcinoma as an underlying cause of death increased with an annual rate of 1.2% and 1.9%, respectively. Based on etiology, age-standardized mortality for hepatitis C and hepatitis B viral infections decreased at an annual rate of 4.4% and 5.1%, respectively. On the other hand, mortality among individuals with NAFLD and alcoholic liver disease increased at annual rates of 11.6% and 1.4%, respectively.
“When we defined chronic liver disease as an underlying or contributing cause of death among individuals with diabetes listed on the death certificate, the overall results remained similar,” the researchers wrote. They acknowledged certain limitations of the analysis, including the fact that using death certificates and ICD-10 codes “has the potential for misclassification and underestimation for diabetes and chronic liver disease–related mortality. However, the coding method has been constant over time, so it is unlikely to account for present trends. Increasing obesity and associated insulin resistance likely explain the link between diabetes and NAFLD and end-stage liver disease through hepatic inflammation and various proinflammatory cytokines.”
One of the study authors was supported by the National Institutes of Health. None of the other authors reported having relevant disclosures.
SOURCE: Kim D et al. Clin Gastroenterol Hepatol. 2019 Jun 17. doi: 10.1016/j.cgh.2019.06.011.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
SGLT2 inhibitors for type 1 diabetes: Doctors debate the merits
SAN FRANCISCO – At first, the diabetes professionals in the audience at the annual scientific sessions of the American Diabetes Association overwhelmingly raised their hands to say they would support using SGLT2 inhibitors as adjunctive therapy in patients with type 1 diabetes. Then two physicians debated whether the drugs were too risky – predictably, one said yes, the other said no. In the end, most of the audience was unconvinced by one of the doctors. Which one? Well, we’ll get to that.
First, let’s look at the issue that divided the two physicians: Should the sodium-glucose cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – now commonly used to treat patients with type 2 diabetes – also be prescribed for patients with type 1 diabetes?
The drugs are not cleared in the United States for use in patients with type 1 diabetes, although drug makers are seeking approval. Earlier in 2019, the Food and Drug Administration turned down a request for the approval of sotagliflozin (Zynquista), a dual SGLT1 and SGLT2 inhibitor, for adults with type 1 diabetes. However, the drug has been approved in the European Union for certain overweight patients with type 1 diabetes.
In addition, the drugs are very costly, compared with some of the other diabetes medications, and physicians say that puts them out of reach for some patients.
The case for ...
In arguing that SGLT2 inhibitors would be appropriate as a therapy for patients with type 1 diabetes, Bruce A. Perkins, MD, MPH, professor and clinician-scientist at Leadership Sinai Center for Diabetes at the University of Toronto, emphasized the need for new treatments in type 1 diabetes.
“Even today, people with type 1 tell us they feel isolated, they fear hypoglycemia, they fear complications. And they have this undue burden of self-management,” he said. “We can do much better. Insulin therapy still needs us desperately needing more.”
Dr. Perkins highlighted the drugs’ widely lauded effects on cardiac and renal health and noted that a 2019 meta-analysis of 10 trials found that, compared with placebo, the drugs were associated with mean reductions in hemoglobin A1c (–0.39%; 95% confidence interval, –0.43 to –0.36) and body weight (–3.47%; 95% CI, –3.78 to –3.16).
That analysis also showed a higher risk of genital infection (3.57; 95% CI, 2.97-4.29) and diabetic ketoacidosis (DKA; 3.11; 95% CI, 2.11-4.58) with SGLT inhibitors, but the authors concluded that, despite the adverse events, the available data suggested that adding the inhibitors to basal insulin could be beneficial in patients with type 1 diabetes (Diabetes Metab Res Rev. 2019 Apr 11. doi: 10.1002/dmrr.3169).
In reference to the findings on DKA, Dr. Perkins said recent research has suggested that the DKA risk could be lowered by decreasing the dose of the SGLT2 inhibitors. “[DKA] is a problem, there’s no question, but there’s a background population risk. Whether we introduce an SGLT2 or not, we have to deal with this issue. We can deal with and overcome the excess DKA risk.”
In the big picture, he said, “it would be a crime not to make this treatment available to some patients. Meaningful benefits far outweigh the risks.”
The case against ...
On the other side of the debate was David M. Nathan, MD, of Harvard Medical School and the Clinical Research Center and Diabetes Center at Massachusetts General Hospital, Boston, who acknowledged the benefits of the SGLT2 inhibitors in type 2 diabetes.
However, he pointed to findings from a 2015 trial of canagliflozin as an add-on in type 1 diabetes (Diabetes Care. 2015;38[12]:2258-65). In that 18-week, randomized phase 2 trial, the investigators found that patients who took the drug had significantly higher rates of serious adverse events (7.7% or 6.8%, depending on dose, vs. 0% for placebo), urinary tract infections (4.3% and 5.1% vs. 1.7%), and DKA (4.3% and 6.0% vs. 0%).
“It would have cost $400 a month for the ‘pleasure’ of those side effects,” Dr. Nathan said.
He also noted a 2015 report on a 29-day, randomized, placebo-controlled study of sotagliflozin, the dual SGLT1 and SGLT2 inhibitor drug, as an add-on treatment for type 1 diabetes, in which investigators reported two episodes of DKA (13%) in the SGLT2 group, compared with none in placebo (Diabetes Care. 2015;38[7]:1181-8).
Dr. Nathan also pointed to a recent FDA warning about cases of Fournier gangrene, a rare type of serious genital infection, in patients taking SGLT2 inhibitors.
“To me, the risk [of using SGLT2 inhibitors in type 1 diabetes] outweighs the benefit by a lot,” he said, echoing comments he made in an editorial he wrote in 2017, that “any added benefits of adjunctive therapies for type 1 diabetes must be carefully balanced against their added risk and cost. Physicians and patients should beware” (N Engl J Med. 2017; 377:2390-1).
The outcome...
The audience was not sufficiently convinced by Dr. Nathan to swing the final vote fully in his favor, but he did manage to dent the initial support for using SGLT2 inhibitors in patients with type 1 disease. Before the debate, the show of hands suggested that roughly 80% of the audience thought SGLT2 inhibitors would be an appropriate therapy option for patients with type 1 diabetes. When the moderator asked the same question again after the arguments had been presented, that initial support had been eroded to about 70%. Dr. Nathan had clearly raised some doubts among the attendees, but Dr. Perkins’ perspective prevailed.
Dr. Perkins reported speaker fees from Medtronic, Abbott, Sanofi and Lilly; advisory panel service for Abbott, Boehringer Ingelheim, and Insulet; and research support to his institution from Boehringer Ingelheim and Bank of Montreal. Dr. Nathan reports no disclosures.
SAN FRANCISCO – At first, the diabetes professionals in the audience at the annual scientific sessions of the American Diabetes Association overwhelmingly raised their hands to say they would support using SGLT2 inhibitors as adjunctive therapy in patients with type 1 diabetes. Then two physicians debated whether the drugs were too risky – predictably, one said yes, the other said no. In the end, most of the audience was unconvinced by one of the doctors. Which one? Well, we’ll get to that.
First, let’s look at the issue that divided the two physicians: Should the sodium-glucose cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – now commonly used to treat patients with type 2 diabetes – also be prescribed for patients with type 1 diabetes?
The drugs are not cleared in the United States for use in patients with type 1 diabetes, although drug makers are seeking approval. Earlier in 2019, the Food and Drug Administration turned down a request for the approval of sotagliflozin (Zynquista), a dual SGLT1 and SGLT2 inhibitor, for adults with type 1 diabetes. However, the drug has been approved in the European Union for certain overweight patients with type 1 diabetes.
In addition, the drugs are very costly, compared with some of the other diabetes medications, and physicians say that puts them out of reach for some patients.
The case for ...
In arguing that SGLT2 inhibitors would be appropriate as a therapy for patients with type 1 diabetes, Bruce A. Perkins, MD, MPH, professor and clinician-scientist at Leadership Sinai Center for Diabetes at the University of Toronto, emphasized the need for new treatments in type 1 diabetes.
“Even today, people with type 1 tell us they feel isolated, they fear hypoglycemia, they fear complications. And they have this undue burden of self-management,” he said. “We can do much better. Insulin therapy still needs us desperately needing more.”
Dr. Perkins highlighted the drugs’ widely lauded effects on cardiac and renal health and noted that a 2019 meta-analysis of 10 trials found that, compared with placebo, the drugs were associated with mean reductions in hemoglobin A1c (–0.39%; 95% confidence interval, –0.43 to –0.36) and body weight (–3.47%; 95% CI, –3.78 to –3.16).
That analysis also showed a higher risk of genital infection (3.57; 95% CI, 2.97-4.29) and diabetic ketoacidosis (DKA; 3.11; 95% CI, 2.11-4.58) with SGLT inhibitors, but the authors concluded that, despite the adverse events, the available data suggested that adding the inhibitors to basal insulin could be beneficial in patients with type 1 diabetes (Diabetes Metab Res Rev. 2019 Apr 11. doi: 10.1002/dmrr.3169).
In reference to the findings on DKA, Dr. Perkins said recent research has suggested that the DKA risk could be lowered by decreasing the dose of the SGLT2 inhibitors. “[DKA] is a problem, there’s no question, but there’s a background population risk. Whether we introduce an SGLT2 or not, we have to deal with this issue. We can deal with and overcome the excess DKA risk.”
In the big picture, he said, “it would be a crime not to make this treatment available to some patients. Meaningful benefits far outweigh the risks.”
The case against ...
On the other side of the debate was David M. Nathan, MD, of Harvard Medical School and the Clinical Research Center and Diabetes Center at Massachusetts General Hospital, Boston, who acknowledged the benefits of the SGLT2 inhibitors in type 2 diabetes.
However, he pointed to findings from a 2015 trial of canagliflozin as an add-on in type 1 diabetes (Diabetes Care. 2015;38[12]:2258-65). In that 18-week, randomized phase 2 trial, the investigators found that patients who took the drug had significantly higher rates of serious adverse events (7.7% or 6.8%, depending on dose, vs. 0% for placebo), urinary tract infections (4.3% and 5.1% vs. 1.7%), and DKA (4.3% and 6.0% vs. 0%).
“It would have cost $400 a month for the ‘pleasure’ of those side effects,” Dr. Nathan said.
He also noted a 2015 report on a 29-day, randomized, placebo-controlled study of sotagliflozin, the dual SGLT1 and SGLT2 inhibitor drug, as an add-on treatment for type 1 diabetes, in which investigators reported two episodes of DKA (13%) in the SGLT2 group, compared with none in placebo (Diabetes Care. 2015;38[7]:1181-8).
Dr. Nathan also pointed to a recent FDA warning about cases of Fournier gangrene, a rare type of serious genital infection, in patients taking SGLT2 inhibitors.
“To me, the risk [of using SGLT2 inhibitors in type 1 diabetes] outweighs the benefit by a lot,” he said, echoing comments he made in an editorial he wrote in 2017, that “any added benefits of adjunctive therapies for type 1 diabetes must be carefully balanced against their added risk and cost. Physicians and patients should beware” (N Engl J Med. 2017; 377:2390-1).
The outcome...
The audience was not sufficiently convinced by Dr. Nathan to swing the final vote fully in his favor, but he did manage to dent the initial support for using SGLT2 inhibitors in patients with type 1 disease. Before the debate, the show of hands suggested that roughly 80% of the audience thought SGLT2 inhibitors would be an appropriate therapy option for patients with type 1 diabetes. When the moderator asked the same question again after the arguments had been presented, that initial support had been eroded to about 70%. Dr. Nathan had clearly raised some doubts among the attendees, but Dr. Perkins’ perspective prevailed.
Dr. Perkins reported speaker fees from Medtronic, Abbott, Sanofi and Lilly; advisory panel service for Abbott, Boehringer Ingelheim, and Insulet; and research support to his institution from Boehringer Ingelheim and Bank of Montreal. Dr. Nathan reports no disclosures.
SAN FRANCISCO – At first, the diabetes professionals in the audience at the annual scientific sessions of the American Diabetes Association overwhelmingly raised their hands to say they would support using SGLT2 inhibitors as adjunctive therapy in patients with type 1 diabetes. Then two physicians debated whether the drugs were too risky – predictably, one said yes, the other said no. In the end, most of the audience was unconvinced by one of the doctors. Which one? Well, we’ll get to that.
First, let’s look at the issue that divided the two physicians: Should the sodium-glucose cotransporter 2 (SGLT2) inhibitors canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – now commonly used to treat patients with type 2 diabetes – also be prescribed for patients with type 1 diabetes?
The drugs are not cleared in the United States for use in patients with type 1 diabetes, although drug makers are seeking approval. Earlier in 2019, the Food and Drug Administration turned down a request for the approval of sotagliflozin (Zynquista), a dual SGLT1 and SGLT2 inhibitor, for adults with type 1 diabetes. However, the drug has been approved in the European Union for certain overweight patients with type 1 diabetes.
In addition, the drugs are very costly, compared with some of the other diabetes medications, and physicians say that puts them out of reach for some patients.
The case for ...
In arguing that SGLT2 inhibitors would be appropriate as a therapy for patients with type 1 diabetes, Bruce A. Perkins, MD, MPH, professor and clinician-scientist at Leadership Sinai Center for Diabetes at the University of Toronto, emphasized the need for new treatments in type 1 diabetes.
“Even today, people with type 1 tell us they feel isolated, they fear hypoglycemia, they fear complications. And they have this undue burden of self-management,” he said. “We can do much better. Insulin therapy still needs us desperately needing more.”
Dr. Perkins highlighted the drugs’ widely lauded effects on cardiac and renal health and noted that a 2019 meta-analysis of 10 trials found that, compared with placebo, the drugs were associated with mean reductions in hemoglobin A1c (–0.39%; 95% confidence interval, –0.43 to –0.36) and body weight (–3.47%; 95% CI, –3.78 to –3.16).
That analysis also showed a higher risk of genital infection (3.57; 95% CI, 2.97-4.29) and diabetic ketoacidosis (DKA; 3.11; 95% CI, 2.11-4.58) with SGLT inhibitors, but the authors concluded that, despite the adverse events, the available data suggested that adding the inhibitors to basal insulin could be beneficial in patients with type 1 diabetes (Diabetes Metab Res Rev. 2019 Apr 11. doi: 10.1002/dmrr.3169).
In reference to the findings on DKA, Dr. Perkins said recent research has suggested that the DKA risk could be lowered by decreasing the dose of the SGLT2 inhibitors. “[DKA] is a problem, there’s no question, but there’s a background population risk. Whether we introduce an SGLT2 or not, we have to deal with this issue. We can deal with and overcome the excess DKA risk.”
In the big picture, he said, “it would be a crime not to make this treatment available to some patients. Meaningful benefits far outweigh the risks.”
The case against ...
On the other side of the debate was David M. Nathan, MD, of Harvard Medical School and the Clinical Research Center and Diabetes Center at Massachusetts General Hospital, Boston, who acknowledged the benefits of the SGLT2 inhibitors in type 2 diabetes.
However, he pointed to findings from a 2015 trial of canagliflozin as an add-on in type 1 diabetes (Diabetes Care. 2015;38[12]:2258-65). In that 18-week, randomized phase 2 trial, the investigators found that patients who took the drug had significantly higher rates of serious adverse events (7.7% or 6.8%, depending on dose, vs. 0% for placebo), urinary tract infections (4.3% and 5.1% vs. 1.7%), and DKA (4.3% and 6.0% vs. 0%).
“It would have cost $400 a month for the ‘pleasure’ of those side effects,” Dr. Nathan said.
He also noted a 2015 report on a 29-day, randomized, placebo-controlled study of sotagliflozin, the dual SGLT1 and SGLT2 inhibitor drug, as an add-on treatment for type 1 diabetes, in which investigators reported two episodes of DKA (13%) in the SGLT2 group, compared with none in placebo (Diabetes Care. 2015;38[7]:1181-8).
Dr. Nathan also pointed to a recent FDA warning about cases of Fournier gangrene, a rare type of serious genital infection, in patients taking SGLT2 inhibitors.
“To me, the risk [of using SGLT2 inhibitors in type 1 diabetes] outweighs the benefit by a lot,” he said, echoing comments he made in an editorial he wrote in 2017, that “any added benefits of adjunctive therapies for type 1 diabetes must be carefully balanced against their added risk and cost. Physicians and patients should beware” (N Engl J Med. 2017; 377:2390-1).
The outcome...
The audience was not sufficiently convinced by Dr. Nathan to swing the final vote fully in his favor, but he did manage to dent the initial support for using SGLT2 inhibitors in patients with type 1 disease. Before the debate, the show of hands suggested that roughly 80% of the audience thought SGLT2 inhibitors would be an appropriate therapy option for patients with type 1 diabetes. When the moderator asked the same question again after the arguments had been presented, that initial support had been eroded to about 70%. Dr. Nathan had clearly raised some doubts among the attendees, but Dr. Perkins’ perspective prevailed.
Dr. Perkins reported speaker fees from Medtronic, Abbott, Sanofi and Lilly; advisory panel service for Abbott, Boehringer Ingelheim, and Insulet; and research support to his institution from Boehringer Ingelheim and Bank of Montreal. Dr. Nathan reports no disclosures.
EXPERT ANALYSIS FROM ADA 2019
VOC-sniffing necklace may support early detection of hypoglycemia
SAN FRANCISCO – according to investigators from Indiana University-Purdue University, Indianapolis.
The device would detect changes in the volatile organic compounds (VOCs) that patients exhale as their plasma glucose level drops. In an insulin clamp study in 11 people with type 1 diabetes, the Indianapolis team found a marked shift in VOCs at a plasma glucose level of 90 mg/dL that persisted all the way down to a level of 50 mg/dL.
The team is now working on a sensor to detect that shift and alert patients. It’s the same trick that diabetes alert dogs do – minus the pup.
The device would be worn like a necklace, and “sense the air around your breath every 15 minutes or so,” said lead investigator Amanda P. Siegel, PhD, an analytical chemist and assistant research professor at the university.
Some continuous glucose monitors already warn of impending hypoglycemia, but the interstitial glucose levels on which they rely lag behind plasma glucose level by about 15 minutes or so. A VOC sniffer offers the hope of a real-time warning, Dr. Siegel said at the annual scientific sessions of the American Diabetes Association.
The team used gas chromatography–mass spectrometry to analyze 94 breath samples from the 11 participants, starting at a median fasting plasma glucose level of 150 mg/dL all the way down to 50 mg/dL, and back up to recovery. Samples collected at the 90-mg/dL and 80-mg/dL levels demonstrated VOC concentrations very similar to those at and below the hypoglycemia threshold of 70 mg/dL.
Even at 90 mg/dL, the VOC profile “looked like patients were already low. These volatile compounds change early” and stay on the breath as plasma glucose drops. “They are different from normal levels for the entire time, and separate out nicely,” Dr. Siegel said.
The team is not saying which volatile compounds are involved while the sensor is under development. They are looking for funding, and if all goes well, they hope to submit a device application to the Food and Drug Administration in 2020.
Other teams are also looking to VOCs to replace poor Fido, but he can alert to hyperglycemia and other problems as well, so his job is safe for now.
The work has been supported by the National Science Foundation. Dr. Siegel did not have any disclosures.
SOURCE: Siegel AP et al. ADA 2019, Abstract 968-P.
SAN FRANCISCO – according to investigators from Indiana University-Purdue University, Indianapolis.
The device would detect changes in the volatile organic compounds (VOCs) that patients exhale as their plasma glucose level drops. In an insulin clamp study in 11 people with type 1 diabetes, the Indianapolis team found a marked shift in VOCs at a plasma glucose level of 90 mg/dL that persisted all the way down to a level of 50 mg/dL.
The team is now working on a sensor to detect that shift and alert patients. It’s the same trick that diabetes alert dogs do – minus the pup.
The device would be worn like a necklace, and “sense the air around your breath every 15 minutes or so,” said lead investigator Amanda P. Siegel, PhD, an analytical chemist and assistant research professor at the university.
Some continuous glucose monitors already warn of impending hypoglycemia, but the interstitial glucose levels on which they rely lag behind plasma glucose level by about 15 minutes or so. A VOC sniffer offers the hope of a real-time warning, Dr. Siegel said at the annual scientific sessions of the American Diabetes Association.
The team used gas chromatography–mass spectrometry to analyze 94 breath samples from the 11 participants, starting at a median fasting plasma glucose level of 150 mg/dL all the way down to 50 mg/dL, and back up to recovery. Samples collected at the 90-mg/dL and 80-mg/dL levels demonstrated VOC concentrations very similar to those at and below the hypoglycemia threshold of 70 mg/dL.
Even at 90 mg/dL, the VOC profile “looked like patients were already low. These volatile compounds change early” and stay on the breath as plasma glucose drops. “They are different from normal levels for the entire time, and separate out nicely,” Dr. Siegel said.
The team is not saying which volatile compounds are involved while the sensor is under development. They are looking for funding, and if all goes well, they hope to submit a device application to the Food and Drug Administration in 2020.
Other teams are also looking to VOCs to replace poor Fido, but he can alert to hyperglycemia and other problems as well, so his job is safe for now.
The work has been supported by the National Science Foundation. Dr. Siegel did not have any disclosures.
SOURCE: Siegel AP et al. ADA 2019, Abstract 968-P.
SAN FRANCISCO – according to investigators from Indiana University-Purdue University, Indianapolis.
The device would detect changes in the volatile organic compounds (VOCs) that patients exhale as their plasma glucose level drops. In an insulin clamp study in 11 people with type 1 diabetes, the Indianapolis team found a marked shift in VOCs at a plasma glucose level of 90 mg/dL that persisted all the way down to a level of 50 mg/dL.
The team is now working on a sensor to detect that shift and alert patients. It’s the same trick that diabetes alert dogs do – minus the pup.
The device would be worn like a necklace, and “sense the air around your breath every 15 minutes or so,” said lead investigator Amanda P. Siegel, PhD, an analytical chemist and assistant research professor at the university.
Some continuous glucose monitors already warn of impending hypoglycemia, but the interstitial glucose levels on which they rely lag behind plasma glucose level by about 15 minutes or so. A VOC sniffer offers the hope of a real-time warning, Dr. Siegel said at the annual scientific sessions of the American Diabetes Association.
The team used gas chromatography–mass spectrometry to analyze 94 breath samples from the 11 participants, starting at a median fasting plasma glucose level of 150 mg/dL all the way down to 50 mg/dL, and back up to recovery. Samples collected at the 90-mg/dL and 80-mg/dL levels demonstrated VOC concentrations very similar to those at and below the hypoglycemia threshold of 70 mg/dL.
Even at 90 mg/dL, the VOC profile “looked like patients were already low. These volatile compounds change early” and stay on the breath as plasma glucose drops. “They are different from normal levels for the entire time, and separate out nicely,” Dr. Siegel said.
The team is not saying which volatile compounds are involved while the sensor is under development. They are looking for funding, and if all goes well, they hope to submit a device application to the Food and Drug Administration in 2020.
Other teams are also looking to VOCs to replace poor Fido, but he can alert to hyperglycemia and other problems as well, so his job is safe for now.
The work has been supported by the National Science Foundation. Dr. Siegel did not have any disclosures.
SOURCE: Siegel AP et al. ADA 2019, Abstract 968-P.
REPORTING FROM ADA 2019
Bariatric Surgery + Medical Therapy: Effective Tx for T2DM?

A 46-year-old woman presents with a BMI of 28, a 4-year history of type 2 diabetes mellitus (T2DM), and an A1C of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 U/d, with minimal change in A1C. Should you recommend bariatric surgery?
One in 11 Americans has diabetes, and at least 95% of those have T2DM.2,3 The treatment of T2DM is generally multimodal to target the various mechanisms that cause hyperglycemia. Strategies may include making lifestyle modifications, decreasing insulin resistance, increasing insulin secretion, replacing insulin, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) recommends diet, exercise, and behavioral modifications as firstline therapy for diabetes management, but these methods are often inadequate.2 In addition to various pharmacotherapeutic strategies for some populations with T2DM, the ADA recommends bariatric surgery for those with a BMI ≥ 35 and uncontrolled hyperglycemia.2,4
However, this recommendation is based only on short-term studies. For example, in a single-center, nonblinded RCT of 60 patients with a BMI ≥ 35, the average baseline A1C levels of 8.65 ± 1.45% were reduced to 7.7 ± 0.6% in the IMT group and to 6.4 ± 1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35), gastric bypass yielded better outcomes than sleeve gastrectomy: 93% of patients in the former group and 47% of those in the latter group achieved remission of T2DM over a 12-month period.6
The current study by Schauer et al examined the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up: surgery + IMT works
This study was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were ages 20 to 60, had a BMI of 27 to 43, and had an A1C > 7%. Patients with a history of bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Patients were randomly placed in a 1:1:1 fashion into 3 groups: IMT (as defined by the ADA) only, IMT and gastric bypass, or IMT and sleeve gastrectomy. The primary outcome was the number of patients with an A1C ≤ 6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period, leaving 149. Of these, 134 completed the 5-year follow-up. Eight patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment, and 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
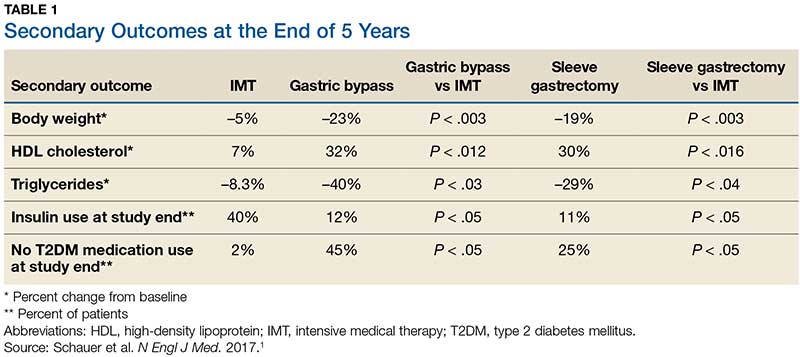
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an A1C of ≤ 6% than in the IMT group (14 of 49 gastric bypass patients, 11 of 47 sleeve gastrectomy patients, and 2 of 38 IMT patients). Compared with those in the IMT group, the patients in the 2 surgery groups showed greater reductions from baseline in body weight and triglyceride levels and greater increases from baseline in HDL cholesterol levels; they also required less antidiabetes medication for glycemic control (see Table).1
WHAT’S NEW?
Big benefits, minimal adverse effects
Prior studies evaluating the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates that bariatric surgery plus IMT has long-term benefits with minimal adverse events, compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM in patients with a BMI ≥ 27, which is below the starting BMI (35) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications—eg, gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis—in this patient population is significant.1 Other potential complications include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1C, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who underwent gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up, compared with patients who underwent sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to those of IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:102-104).
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Association. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S81-S89.
3. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: CDC, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed June 27, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011; 146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.

A 46-year-old woman presents with a BMI of 28, a 4-year history of type 2 diabetes mellitus (T2DM), and an A1C of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 U/d, with minimal change in A1C. Should you recommend bariatric surgery?
One in 11 Americans has diabetes, and at least 95% of those have T2DM.2,3 The treatment of T2DM is generally multimodal to target the various mechanisms that cause hyperglycemia. Strategies may include making lifestyle modifications, decreasing insulin resistance, increasing insulin secretion, replacing insulin, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) recommends diet, exercise, and behavioral modifications as firstline therapy for diabetes management, but these methods are often inadequate.2 In addition to various pharmacotherapeutic strategies for some populations with T2DM, the ADA recommends bariatric surgery for those with a BMI ≥ 35 and uncontrolled hyperglycemia.2,4
However, this recommendation is based only on short-term studies. For example, in a single-center, nonblinded RCT of 60 patients with a BMI ≥ 35, the average baseline A1C levels of 8.65 ± 1.45% were reduced to 7.7 ± 0.6% in the IMT group and to 6.4 ± 1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35), gastric bypass yielded better outcomes than sleeve gastrectomy: 93% of patients in the former group and 47% of those in the latter group achieved remission of T2DM over a 12-month period.6
The current study by Schauer et al examined the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up: surgery + IMT works
This study was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were ages 20 to 60, had a BMI of 27 to 43, and had an A1C > 7%. Patients with a history of bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Patients were randomly placed in a 1:1:1 fashion into 3 groups: IMT (as defined by the ADA) only, IMT and gastric bypass, or IMT and sleeve gastrectomy. The primary outcome was the number of patients with an A1C ≤ 6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period, leaving 149. Of these, 134 completed the 5-year follow-up. Eight patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment, and 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an A1C of ≤ 6% than in the IMT group (14 of 49 gastric bypass patients, 11 of 47 sleeve gastrectomy patients, and 2 of 38 IMT patients). Compared with those in the IMT group, the patients in the 2 surgery groups showed greater reductions from baseline in body weight and triglyceride levels and greater increases from baseline in HDL cholesterol levels; they also required less antidiabetes medication for glycemic control (see Table).1

WHAT’S NEW?
Big benefits, minimal adverse effects
Prior studies evaluating the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates that bariatric surgery plus IMT has long-term benefits with minimal adverse events, compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM in patients with a BMI ≥ 27, which is below the starting BMI (35) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications—eg, gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis—in this patient population is significant.1 Other potential complications include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1C, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who underwent gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up, compared with patients who underwent sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to those of IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:102-104).

A 46-year-old woman presents with a BMI of 28, a 4-year history of type 2 diabetes mellitus (T2DM), and an A1C of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 U/d, with minimal change in A1C. Should you recommend bariatric surgery?
One in 11 Americans has diabetes, and at least 95% of those have T2DM.2,3 The treatment of T2DM is generally multimodal to target the various mechanisms that cause hyperglycemia. Strategies may include making lifestyle modifications, decreasing insulin resistance, increasing insulin secretion, replacing insulin, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) recommends diet, exercise, and behavioral modifications as firstline therapy for diabetes management, but these methods are often inadequate.2 In addition to various pharmacotherapeutic strategies for some populations with T2DM, the ADA recommends bariatric surgery for those with a BMI ≥ 35 and uncontrolled hyperglycemia.2,4
However, this recommendation is based only on short-term studies. For example, in a single-center, nonblinded RCT of 60 patients with a BMI ≥ 35, the average baseline A1C levels of 8.65 ± 1.45% were reduced to 7.7 ± 0.6% in the IMT group and to 6.4 ± 1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35), gastric bypass yielded better outcomes than sleeve gastrectomy: 93% of patients in the former group and 47% of those in the latter group achieved remission of T2DM over a 12-month period.6
The current study by Schauer et al examined the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up: surgery + IMT works
This study was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were ages 20 to 60, had a BMI of 27 to 43, and had an A1C > 7%. Patients with a history of bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Patients were randomly placed in a 1:1:1 fashion into 3 groups: IMT (as defined by the ADA) only, IMT and gastric bypass, or IMT and sleeve gastrectomy. The primary outcome was the number of patients with an A1C ≤ 6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period, leaving 149. Of these, 134 completed the 5-year follow-up. Eight patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment, and 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an A1C of ≤ 6% than in the IMT group (14 of 49 gastric bypass patients, 11 of 47 sleeve gastrectomy patients, and 2 of 38 IMT patients). Compared with those in the IMT group, the patients in the 2 surgery groups showed greater reductions from baseline in body weight and triglyceride levels and greater increases from baseline in HDL cholesterol levels; they also required less antidiabetes medication for glycemic control (see Table).1

WHAT’S NEW?
Big benefits, minimal adverse effects
Prior studies evaluating the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates that bariatric surgery plus IMT has long-term benefits with minimal adverse events, compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM in patients with a BMI ≥ 27, which is below the starting BMI (35) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications—eg, gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis—in this patient population is significant.1 Other potential complications include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1C, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who underwent gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up, compared with patients who underwent sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to those of IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:102-104).
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Association. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S81-S89.
3. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: CDC, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed June 27, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011; 146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Association. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S81-S89.
3. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: CDC, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed June 27, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011; 146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
New findings cast more doubt on ‘fat-but-fit’ theory
SAN FRANCISCO – Can you be “fat but fit” if you’re obese but don’t suffer from metabolic syndrome? Some advocates have claimed you can, but new findings presented at the annual scientific sessions of the American Diabetes Association provide more evidence that those extra pounds translate to extra cardiac risk.
Fat-but-fit is a misnomer, Yvonne Commodore-Mensah, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, said in an interview. “The metabolically healthy obese are not so healthy. [We found] they had a higher risk of heart disease than people who were metabolically healthy and nonobese.”
Studies began supporting the fat-but-fit “paradox” in the late 1990s. They showed “that all-cause and CVD [cardiovascular] mortality risk in obese individuals, as defined by body mass index (BMI), body fat percentage, or waist circumference, who are fit (i.e., cardiorespiratory fitness level above the age-specific and sex-specific 20th percentile) is not significantly different from their normal-weight and fit counterparts” (Br J Sports Med. 2018;52[3]:151-3).
However, a 2017 study had found that “metabolically healthy obese individuals had a higher risk of coronary heart disease, cerebrovascular disease, and heart failure [compared with] normal weight, metabolically healthy individuals” (J Am Coll Cardiol. 2017;70[12]:1429-37). And a 2016 meta-analysis of 22 studies had produced similar results but also found that metabolically healthy obese individuals were better off, cardiac-health–wise, than those of normal weight who were metabolically unhealthy (Eur J Prev Cardiol. 2016;23[9]:956-66).
Dr. Commodore-Mensah and colleagues sought to establish through their study whether there was evidence of subclinical heart disease in people who are considered obese but metabolically healthy (Abstract 272-OR).
They tracked 11,884 participants in the Atherosclerosis Risk in Communities Study (ARIC) from 1990-1992 to 2016-2018. The study, which continues today, includes participants in suburban Minneapolis; Jackson, Miss.; Forsyth County, N.C.; and Washington County, Md.
None of the participants had previous cardiovascular disease at baseline (1990-1992). The researchers divided the participants into four groups at baseline: Nonobese (with metabolic syndrome, 20% of the total number of participants; or without metabolic syndrome, 51%) and obese (with metabolic syndrome, 20%; or without metabolic syndrome, 9%).
The average age range in the groups was 56-57 years. The percentage of women in the groups ranged from 53% to 58%, except for the obese and metabolically healthy group (73%). The percentage of black participants in the groups ranged from 17% (nonobese, metabolically unhealthy) to 45% (obese, metabolically healthy).
“People who were younger, women, and black were more likely to be classified as metabolically healthy obese,” Dr. Commodore-Mensah said.
According to one adjusted model with a median follow-up of 16 years and a total of 3,560 events, obese participants had a higher risk of incident cardiovascular disease, compared with their nonobese counterparts, regardless of whether they had metabolic syndrome.
When compared with the nonobese, metabolically healthy group, the risk grew in the nonobese, metabolically unhealthy group (hazard ratio, .24; 95% confidence interval, 1.12-1.36), as well as in the obese, metabolically healthy (HR, 1.33; 95% CI, 1.15-1.53) and the obese, metabolically unhealthy (HR, 2.11; 95% CI, 1.90-2.35) groups.
The researchers also focused on the cardiac biomarker known as high-sensitive cardiac troponin T (hs-cTnT), which indicates chronic myocardial damage. “This biomarker provides us with a window to the heart,” Dr. Commodore-Mensah said.
According to previous findings reported in 2014, ARIC participants who had hs-cTnT levels of 14 ng/L or higher were much more likely than were those with undetectable levels to suffer from heart failure, death from any cause, and coronary heart disease (JACC Heart Fail. 2014;2[6]:600-7).
Based on an analysis of the hs-cTnT levels in the present study, the researchers believe obese, metabolically healthy participants fell in the intermediate range of excess subclinical myocardial damage, between the nonobese and the obese participants who are also metabolically unhealthy.
“This group is not protected from heart disease,” Dr. Commodore-Mensah said. “They should be targeted, and they would benefit from behavioral changes, such as modifying their diet and increasing physical activity levels.”
The study is funded by the National Institutes of Health. Dr. Commodore-Mensah and six coauthors reported no relevant disclosures. Two coauthors reported various disclosures.
SAN FRANCISCO – Can you be “fat but fit” if you’re obese but don’t suffer from metabolic syndrome? Some advocates have claimed you can, but new findings presented at the annual scientific sessions of the American Diabetes Association provide more evidence that those extra pounds translate to extra cardiac risk.
Fat-but-fit is a misnomer, Yvonne Commodore-Mensah, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, said in an interview. “The metabolically healthy obese are not so healthy. [We found] they had a higher risk of heart disease than people who were metabolically healthy and nonobese.”
Studies began supporting the fat-but-fit “paradox” in the late 1990s. They showed “that all-cause and CVD [cardiovascular] mortality risk in obese individuals, as defined by body mass index (BMI), body fat percentage, or waist circumference, who are fit (i.e., cardiorespiratory fitness level above the age-specific and sex-specific 20th percentile) is not significantly different from their normal-weight and fit counterparts” (Br J Sports Med. 2018;52[3]:151-3).
However, a 2017 study had found that “metabolically healthy obese individuals had a higher risk of coronary heart disease, cerebrovascular disease, and heart failure [compared with] normal weight, metabolically healthy individuals” (J Am Coll Cardiol. 2017;70[12]:1429-37). And a 2016 meta-analysis of 22 studies had produced similar results but also found that metabolically healthy obese individuals were better off, cardiac-health–wise, than those of normal weight who were metabolically unhealthy (Eur J Prev Cardiol. 2016;23[9]:956-66).
Dr. Commodore-Mensah and colleagues sought to establish through their study whether there was evidence of subclinical heart disease in people who are considered obese but metabolically healthy (Abstract 272-OR).
They tracked 11,884 participants in the Atherosclerosis Risk in Communities Study (ARIC) from 1990-1992 to 2016-2018. The study, which continues today, includes participants in suburban Minneapolis; Jackson, Miss.; Forsyth County, N.C.; and Washington County, Md.
None of the participants had previous cardiovascular disease at baseline (1990-1992). The researchers divided the participants into four groups at baseline: Nonobese (with metabolic syndrome, 20% of the total number of participants; or without metabolic syndrome, 51%) and obese (with metabolic syndrome, 20%; or without metabolic syndrome, 9%).
The average age range in the groups was 56-57 years. The percentage of women in the groups ranged from 53% to 58%, except for the obese and metabolically healthy group (73%). The percentage of black participants in the groups ranged from 17% (nonobese, metabolically unhealthy) to 45% (obese, metabolically healthy).
“People who were younger, women, and black were more likely to be classified as metabolically healthy obese,” Dr. Commodore-Mensah said.
According to one adjusted model with a median follow-up of 16 years and a total of 3,560 events, obese participants had a higher risk of incident cardiovascular disease, compared with their nonobese counterparts, regardless of whether they had metabolic syndrome.
When compared with the nonobese, metabolically healthy group, the risk grew in the nonobese, metabolically unhealthy group (hazard ratio, .24; 95% confidence interval, 1.12-1.36), as well as in the obese, metabolically healthy (HR, 1.33; 95% CI, 1.15-1.53) and the obese, metabolically unhealthy (HR, 2.11; 95% CI, 1.90-2.35) groups.
The researchers also focused on the cardiac biomarker known as high-sensitive cardiac troponin T (hs-cTnT), which indicates chronic myocardial damage. “This biomarker provides us with a window to the heart,” Dr. Commodore-Mensah said.
According to previous findings reported in 2014, ARIC participants who had hs-cTnT levels of 14 ng/L or higher were much more likely than were those with undetectable levels to suffer from heart failure, death from any cause, and coronary heart disease (JACC Heart Fail. 2014;2[6]:600-7).
Based on an analysis of the hs-cTnT levels in the present study, the researchers believe obese, metabolically healthy participants fell in the intermediate range of excess subclinical myocardial damage, between the nonobese and the obese participants who are also metabolically unhealthy.
“This group is not protected from heart disease,” Dr. Commodore-Mensah said. “They should be targeted, and they would benefit from behavioral changes, such as modifying their diet and increasing physical activity levels.”
The study is funded by the National Institutes of Health. Dr. Commodore-Mensah and six coauthors reported no relevant disclosures. Two coauthors reported various disclosures.
SAN FRANCISCO – Can you be “fat but fit” if you’re obese but don’t suffer from metabolic syndrome? Some advocates have claimed you can, but new findings presented at the annual scientific sessions of the American Diabetes Association provide more evidence that those extra pounds translate to extra cardiac risk.
Fat-but-fit is a misnomer, Yvonne Commodore-Mensah, PhD, RN, assistant professor at Johns Hopkins School of Nursing, Baltimore, said in an interview. “The metabolically healthy obese are not so healthy. [We found] they had a higher risk of heart disease than people who were metabolically healthy and nonobese.”
Studies began supporting the fat-but-fit “paradox” in the late 1990s. They showed “that all-cause and CVD [cardiovascular] mortality risk in obese individuals, as defined by body mass index (BMI), body fat percentage, or waist circumference, who are fit (i.e., cardiorespiratory fitness level above the age-specific and sex-specific 20th percentile) is not significantly different from their normal-weight and fit counterparts” (Br J Sports Med. 2018;52[3]:151-3).
However, a 2017 study had found that “metabolically healthy obese individuals had a higher risk of coronary heart disease, cerebrovascular disease, and heart failure [compared with] normal weight, metabolically healthy individuals” (J Am Coll Cardiol. 2017;70[12]:1429-37). And a 2016 meta-analysis of 22 studies had produced similar results but also found that metabolically healthy obese individuals were better off, cardiac-health–wise, than those of normal weight who were metabolically unhealthy (Eur J Prev Cardiol. 2016;23[9]:956-66).
Dr. Commodore-Mensah and colleagues sought to establish through their study whether there was evidence of subclinical heart disease in people who are considered obese but metabolically healthy (Abstract 272-OR).
They tracked 11,884 participants in the Atherosclerosis Risk in Communities Study (ARIC) from 1990-1992 to 2016-2018. The study, which continues today, includes participants in suburban Minneapolis; Jackson, Miss.; Forsyth County, N.C.; and Washington County, Md.
None of the participants had previous cardiovascular disease at baseline (1990-1992). The researchers divided the participants into four groups at baseline: Nonobese (with metabolic syndrome, 20% of the total number of participants; or without metabolic syndrome, 51%) and obese (with metabolic syndrome, 20%; or without metabolic syndrome, 9%).
The average age range in the groups was 56-57 years. The percentage of women in the groups ranged from 53% to 58%, except for the obese and metabolically healthy group (73%). The percentage of black participants in the groups ranged from 17% (nonobese, metabolically unhealthy) to 45% (obese, metabolically healthy).
“People who were younger, women, and black were more likely to be classified as metabolically healthy obese,” Dr. Commodore-Mensah said.
According to one adjusted model with a median follow-up of 16 years and a total of 3,560 events, obese participants had a higher risk of incident cardiovascular disease, compared with their nonobese counterparts, regardless of whether they had metabolic syndrome.
When compared with the nonobese, metabolically healthy group, the risk grew in the nonobese, metabolically unhealthy group (hazard ratio, .24; 95% confidence interval, 1.12-1.36), as well as in the obese, metabolically healthy (HR, 1.33; 95% CI, 1.15-1.53) and the obese, metabolically unhealthy (HR, 2.11; 95% CI, 1.90-2.35) groups.
The researchers also focused on the cardiac biomarker known as high-sensitive cardiac troponin T (hs-cTnT), which indicates chronic myocardial damage. “This biomarker provides us with a window to the heart,” Dr. Commodore-Mensah said.
According to previous findings reported in 2014, ARIC participants who had hs-cTnT levels of 14 ng/L or higher were much more likely than were those with undetectable levels to suffer from heart failure, death from any cause, and coronary heart disease (JACC Heart Fail. 2014;2[6]:600-7).
Based on an analysis of the hs-cTnT levels in the present study, the researchers believe obese, metabolically healthy participants fell in the intermediate range of excess subclinical myocardial damage, between the nonobese and the obese participants who are also metabolically unhealthy.
“This group is not protected from heart disease,” Dr. Commodore-Mensah said. “They should be targeted, and they would benefit from behavioral changes, such as modifying their diet and increasing physical activity levels.”
The study is funded by the National Institutes of Health. Dr. Commodore-Mensah and six coauthors reported no relevant disclosures. Two coauthors reported various disclosures.
REPORTING FROM ADA 2019
CAROLINA findings reaffirm linagliptin’s safety, free glimepiride of CV-risk stigma
SAN FRANCISCO – The sulfonylurea glimepiride (Amaryl) did not increase the risk of cardiovascular events in patients with type 2 diabetes and cardiovascular risk in a head-to-head comparison with the dipeptidyl peptidase–4 (DPP-4) inhibitor linagliptin (Tradjenta), a drug with proven cardiovascular safety, according to findings from the CAROLINA study presented at the scientific sessions of the American Diabetes Association.
Linagliptin’s cardiovascular safety, compared with placebo, was demonstrated in the CARMELINA study (JAMA. 2019;321[1]:69-79), but CAROLINA pitted the DPP-4 inhibitor against an active comparator, glimepiride, which along with other sulfonylureas, carries a warning for increased risk of cardiovascular mortality. The latest findings about the comparator were not expected, but seem to set aside the lingering doubts about cardiovascular safety in at least the modern-day sulfonylureas.
“The stigma that sulfonylureas have had for more than 50 years” has been lifted. “We take a lot of pride in this study,” said principal investigator and endocrinologist Julio Rosenstock, MD, a clinical professor of medicine at the University of Texas Southwestern Medical Center, Dallas.
In 1970, the University Group Diabetes Program trial reported 26 cardiovascular deaths in 204 patients who received with tolbutamide – a first-generation sulfonylurea no longer in common use – compared with 10 deaths in 205 patients who received placebo (Diabetes. 1970;19[Suppl]:789-830). The finding led to a warning of increased risk of cardiovascular mortality that still appears on sulfonylurea labels today.
The results were never confirmed by subsequent studies, and debate about the cardiovascular safety of sulfonylureas continued, with many physicians over the years calling for a large, rigorous trial to resolve the issue once and for all.
]And that’s what the CAROLINA findings delivered – a resolution to the “decades-long debate” about cardiovascular safety with glimepiride and likely other modern sulfonylureas, according to coinvestigator and cardiologist Nikolaus Marx, MD, a professor of medicine at Aachen (Germany) University.
The CAROLINA investigators randomized 1:1 more than 6,000 patients with type 2 diabetes at 607 sites in 43 countries to receive either linagliptin 5 mg daily or glimepiride 1-4 mg daily on a background therapy, in most cases, of metformin. The median diabetes duration was 6.3 years, and baseline hemoglobin A1c was 7.15%. In all, 42% of the patients had established cardiovascular disease, and 37% had two or more risk factors that were managed by standard care.
After a median follow-up of 6.3 years, there was no difference between linagliptin and glimepiride in the primary composite outcome of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke (11.8% and 12%, respectively; P = .76), nor were there any differences in the individual components. Likewise, there were no differences between the two drugs in hospitalization for heart failure (3.7% and 3.1%, P = .18) or all-cause mortality (10.2% and 11.2%; P = .23), and no difference in glucose control – a drop of about 0.3% in the HbA1c level at 1 year, then a slow creep back to baseline at around 4 years.
“We believe cardiovascular safety should no longer be a consideration in [deciding] between these two agents,” said Dr. Rosenstock, who cochaired the session with Dr. Marx.
However, there was a modest weight gain with glimepiride, and a marked increase in the risk of moderate to severe hypoglycemia, compared with linagliptin (30.9% and 6.5%, respectively; P less than .0001). Both weight gain and hypoglycemia are recognized side effects of sulfonylureas.
Sulfonylureas are far less expensive than the DPP-4 inhibitors are, so “other than cost consideration ... [the findings] support use of DPP-4 inhibitors before sulfonylureas if hypoglycemia and weight gain are also considerations,” Dr. Rosenstock said.
Robert H. Eckel, MD, an endocrinologist, said he has been “taking people off sulfonylureas for years because I [wasn’t] sure they were safe. This study has helped me know that I can continue [them] safely.”
However, even with the new data, “the only reason I would [use a sulfonylurea] is cost. Cost is the bottom line in the clinic,” added Dr. Eckel, a professor of medicine at the University of Colorado, Aurora.
The trial findings pose no challenge to current guidelines for type 2 diabetes and the recommendation to opt first for a second-line medication with proven cardiovascular benefit, such as a sodium-glucose transporter 2 inhibitor or glucagonlike peptide–1 receptor agonist, in at-risk patients.
Boehringer Ingelheim, the maker of linagliptin, funded the study in collaboration with comarketer, Eli Lilly. Dr. Rosenstock and Dr. Marx disclosed numerous ties to Boehringer Ingelheim and Eli Lilly, and other companies not associated with the study. Dr. Eckel disclosed ties with companies not associated with the study.
SOURCE: Rosenstock J et al. ADA 2019.
SAN FRANCISCO – The sulfonylurea glimepiride (Amaryl) did not increase the risk of cardiovascular events in patients with type 2 diabetes and cardiovascular risk in a head-to-head comparison with the dipeptidyl peptidase–4 (DPP-4) inhibitor linagliptin (Tradjenta), a drug with proven cardiovascular safety, according to findings from the CAROLINA study presented at the scientific sessions of the American Diabetes Association.
Linagliptin’s cardiovascular safety, compared with placebo, was demonstrated in the CARMELINA study (JAMA. 2019;321[1]:69-79), but CAROLINA pitted the DPP-4 inhibitor against an active comparator, glimepiride, which along with other sulfonylureas, carries a warning for increased risk of cardiovascular mortality. The latest findings about the comparator were not expected, but seem to set aside the lingering doubts about cardiovascular safety in at least the modern-day sulfonylureas.
“The stigma that sulfonylureas have had for more than 50 years” has been lifted. “We take a lot of pride in this study,” said principal investigator and endocrinologist Julio Rosenstock, MD, a clinical professor of medicine at the University of Texas Southwestern Medical Center, Dallas.
In 1970, the University Group Diabetes Program trial reported 26 cardiovascular deaths in 204 patients who received with tolbutamide – a first-generation sulfonylurea no longer in common use – compared with 10 deaths in 205 patients who received placebo (Diabetes. 1970;19[Suppl]:789-830). The finding led to a warning of increased risk of cardiovascular mortality that still appears on sulfonylurea labels today.
The results were never confirmed by subsequent studies, and debate about the cardiovascular safety of sulfonylureas continued, with many physicians over the years calling for a large, rigorous trial to resolve the issue once and for all.
]And that’s what the CAROLINA findings delivered – a resolution to the “decades-long debate” about cardiovascular safety with glimepiride and likely other modern sulfonylureas, according to coinvestigator and cardiologist Nikolaus Marx, MD, a professor of medicine at Aachen (Germany) University.
The CAROLINA investigators randomized 1:1 more than 6,000 patients with type 2 diabetes at 607 sites in 43 countries to receive either linagliptin 5 mg daily or glimepiride 1-4 mg daily on a background therapy, in most cases, of metformin. The median diabetes duration was 6.3 years, and baseline hemoglobin A1c was 7.15%. In all, 42% of the patients had established cardiovascular disease, and 37% had two or more risk factors that were managed by standard care.
After a median follow-up of 6.3 years, there was no difference between linagliptin and glimepiride in the primary composite outcome of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke (11.8% and 12%, respectively; P = .76), nor were there any differences in the individual components. Likewise, there were no differences between the two drugs in hospitalization for heart failure (3.7% and 3.1%, P = .18) or all-cause mortality (10.2% and 11.2%; P = .23), and no difference in glucose control – a drop of about 0.3% in the HbA1c level at 1 year, then a slow creep back to baseline at around 4 years.
“We believe cardiovascular safety should no longer be a consideration in [deciding] between these two agents,” said Dr. Rosenstock, who cochaired the session with Dr. Marx.
However, there was a modest weight gain with glimepiride, and a marked increase in the risk of moderate to severe hypoglycemia, compared with linagliptin (30.9% and 6.5%, respectively; P less than .0001). Both weight gain and hypoglycemia are recognized side effects of sulfonylureas.
Sulfonylureas are far less expensive than the DPP-4 inhibitors are, so “other than cost consideration ... [the findings] support use of DPP-4 inhibitors before sulfonylureas if hypoglycemia and weight gain are also considerations,” Dr. Rosenstock said.
Robert H. Eckel, MD, an endocrinologist, said he has been “taking people off sulfonylureas for years because I [wasn’t] sure they were safe. This study has helped me know that I can continue [them] safely.”
However, even with the new data, “the only reason I would [use a sulfonylurea] is cost. Cost is the bottom line in the clinic,” added Dr. Eckel, a professor of medicine at the University of Colorado, Aurora.
The trial findings pose no challenge to current guidelines for type 2 diabetes and the recommendation to opt first for a second-line medication with proven cardiovascular benefit, such as a sodium-glucose transporter 2 inhibitor or glucagonlike peptide–1 receptor agonist, in at-risk patients.
Boehringer Ingelheim, the maker of linagliptin, funded the study in collaboration with comarketer, Eli Lilly. Dr. Rosenstock and Dr. Marx disclosed numerous ties to Boehringer Ingelheim and Eli Lilly, and other companies not associated with the study. Dr. Eckel disclosed ties with companies not associated with the study.
SOURCE: Rosenstock J et al. ADA 2019.
SAN FRANCISCO – The sulfonylurea glimepiride (Amaryl) did not increase the risk of cardiovascular events in patients with type 2 diabetes and cardiovascular risk in a head-to-head comparison with the dipeptidyl peptidase–4 (DPP-4) inhibitor linagliptin (Tradjenta), a drug with proven cardiovascular safety, according to findings from the CAROLINA study presented at the scientific sessions of the American Diabetes Association.
Linagliptin’s cardiovascular safety, compared with placebo, was demonstrated in the CARMELINA study (JAMA. 2019;321[1]:69-79), but CAROLINA pitted the DPP-4 inhibitor against an active comparator, glimepiride, which along with other sulfonylureas, carries a warning for increased risk of cardiovascular mortality. The latest findings about the comparator were not expected, but seem to set aside the lingering doubts about cardiovascular safety in at least the modern-day sulfonylureas.
“The stigma that sulfonylureas have had for more than 50 years” has been lifted. “We take a lot of pride in this study,” said principal investigator and endocrinologist Julio Rosenstock, MD, a clinical professor of medicine at the University of Texas Southwestern Medical Center, Dallas.
In 1970, the University Group Diabetes Program trial reported 26 cardiovascular deaths in 204 patients who received with tolbutamide – a first-generation sulfonylurea no longer in common use – compared with 10 deaths in 205 patients who received placebo (Diabetes. 1970;19[Suppl]:789-830). The finding led to a warning of increased risk of cardiovascular mortality that still appears on sulfonylurea labels today.
The results were never confirmed by subsequent studies, and debate about the cardiovascular safety of sulfonylureas continued, with many physicians over the years calling for a large, rigorous trial to resolve the issue once and for all.
]And that’s what the CAROLINA findings delivered – a resolution to the “decades-long debate” about cardiovascular safety with glimepiride and likely other modern sulfonylureas, according to coinvestigator and cardiologist Nikolaus Marx, MD, a professor of medicine at Aachen (Germany) University.
The CAROLINA investigators randomized 1:1 more than 6,000 patients with type 2 diabetes at 607 sites in 43 countries to receive either linagliptin 5 mg daily or glimepiride 1-4 mg daily on a background therapy, in most cases, of metformin. The median diabetes duration was 6.3 years, and baseline hemoglobin A1c was 7.15%. In all, 42% of the patients had established cardiovascular disease, and 37% had two or more risk factors that were managed by standard care.
After a median follow-up of 6.3 years, there was no difference between linagliptin and glimepiride in the primary composite outcome of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke (11.8% and 12%, respectively; P = .76), nor were there any differences in the individual components. Likewise, there were no differences between the two drugs in hospitalization for heart failure (3.7% and 3.1%, P = .18) or all-cause mortality (10.2% and 11.2%; P = .23), and no difference in glucose control – a drop of about 0.3% in the HbA1c level at 1 year, then a slow creep back to baseline at around 4 years.
“We believe cardiovascular safety should no longer be a consideration in [deciding] between these two agents,” said Dr. Rosenstock, who cochaired the session with Dr. Marx.
However, there was a modest weight gain with glimepiride, and a marked increase in the risk of moderate to severe hypoglycemia, compared with linagliptin (30.9% and 6.5%, respectively; P less than .0001). Both weight gain and hypoglycemia are recognized side effects of sulfonylureas.
Sulfonylureas are far less expensive than the DPP-4 inhibitors are, so “other than cost consideration ... [the findings] support use of DPP-4 inhibitors before sulfonylureas if hypoglycemia and weight gain are also considerations,” Dr. Rosenstock said.
Robert H. Eckel, MD, an endocrinologist, said he has been “taking people off sulfonylureas for years because I [wasn’t] sure they were safe. This study has helped me know that I can continue [them] safely.”
However, even with the new data, “the only reason I would [use a sulfonylurea] is cost. Cost is the bottom line in the clinic,” added Dr. Eckel, a professor of medicine at the University of Colorado, Aurora.
The trial findings pose no challenge to current guidelines for type 2 diabetes and the recommendation to opt first for a second-line medication with proven cardiovascular benefit, such as a sodium-glucose transporter 2 inhibitor or glucagonlike peptide–1 receptor agonist, in at-risk patients.
Boehringer Ingelheim, the maker of linagliptin, funded the study in collaboration with comarketer, Eli Lilly. Dr. Rosenstock and Dr. Marx disclosed numerous ties to Boehringer Ingelheim and Eli Lilly, and other companies not associated with the study. Dr. Eckel disclosed ties with companies not associated with the study.
SOURCE: Rosenstock J et al. ADA 2019.
REPORTING FROM ADA 2019
To help patients stay on diabetes regimens: Communicate, educate, and use technology
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
SAN FRANCISCO – Are you having trouble helping patients take their diabetes medications as directed? Try installing 32-inch screens in the examination rooms for a lab result show-and-tell. Keep pharmaceutical marketers out of your hair (and office).
Those are among the suggestions offered by two physicians during a symposium on drug adherence at the annual scientific sessions of the American Diabetes Association.
“Nonadherence is not a case of patients being bad,” said internist and researcher Niteesh K. Choudhry, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital, Boston. “When half of your patients are nonadherent, I can guarantee you [they] aren’t trying to hurt themselves.”
According to Dr. Choudhry’s own research published in 2011 and based on 2008 data, about 25% of patients do not fill prescriptions after leaving their doctors’ offices. That level for diabetes medications – 42% of patients – is especially high (Am J Med. 2011;124[11]:1081.e9-22).
Other findings, he said, have suggested that half of patients fail to adhere to evidence-based prescribed regimens over the long term. And three groups have especially low levels of adherence: people of color, women, and patients who are caregivers (possibly because they are too busy caring for others to care for themselves).
Various factors affect adherence, including forgetfulness, drug interactions or side effects, and the different colors and shapes of pills. The latter can confuse patients because colors and shapes may be different from prescription to prescription even for the same medication, he said.
Dr. Choudhry added that there’s another factor: multiple prescriptions from multiple physicians that require multiple pharmacy visits. His findings suggest that adherence improves when prescriptions are consolidated to limit the need to visit the drugstore. “The chaos of our health care system leads to nonadherence,” he said (Arch Intern Med. 2011;171[9]:814-22).
Internist Lawrence Garber, MD, of Reliant Medical Group in Worcester, Mass., offered these tips about boosting drug adherence:
- Develop trust with patients. “They need to trust that I’m their advocate, and that they’re my No. 1 reason for prescribing the medication, and not making myself more money,” he said.
- Provide educational resources. “We give them resources online. If their EHR [electronic health record] identifies that they’re diabetic, then they get information about diabetes printed out.”
- Use technology to promote messages about diabetes. Dr. Garber said his clinic has installed screens in the examination rooms so that he can show patients their data. “It [makes it] very clear for them to see why what they’re doing now is not working,’’ and why there is a need to change to a different regimen. In addition, screens in the waiting room can display educational slides about diabetes.
- Set up clinic-wide medication protocols. “We’ve set up protocols and pathways for diabetes, hypertension, and high cholesterol to make it easy to prescribe medications that are lower cost and to make sure we’re following the same path,” Dr. Garber said.
- Stay independent. “I haven’t seen a drug rep in decades. It’s an organizational policy that we don’t see them, so we’re less likely to be biased.”
- Make it easier for patients to take medications. Dr. Garber urged colleagues to talk to their patients about using strategies such as printed pill schedules, weekly pill organizers, auto refills, and smartphone alarm reminders to facilitate adherence.
And, he said, you may wish to make it clear that you will check on whether prescriptions are filled. That way, “the patients know that you’re looking,” and it can actually lead to improved adherence.
Dr. Choudhry reported that his research has been funded by unrestricted grants to his institution from insurers, government funders, nonprofit foundations, pharmaceutical companies (including Merck, Sanofi, and Astra Zeneca), and device makers (including Medisafe). Dr. Garber reported no relevant disclosures.
REPORTING FROM ADA 2019
Type 2 diabetes: Evolving concepts and treatment
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1
PROFILES OF DIABETES DRUGS
The sections below highlight some of the recent data on the profiles of most of the currently available agents.
Metformin: Still the first-line treatment
Current guidelines from the ACP, ADA, and AACE keep metformin14 as the backbone of treatment, although debate continues as to whether newer agents such as GLP-1 receptor agonists are superior for first-line therapy.
Pathways affected. Metformin improves insulin resistance in the liver, increases endogenous GLP-1 levels via the gut, and appears to modulate gut flora composition, which is increasingly suspected to contribute to dysmetabolism.
Advantages, benefits. Metformin is easy to use and does not cause hypoglycemia. It was found to modestly reduce the number of cardiovascular events and deaths in a number of clinical outcome studies.15–19
Disadvantages, adverse effects. In some patients, tolerability restricts the use of this drug at higher doses. The most common adverse effects of metformin are gastrointestinal symptoms (diarrhea, nausea, vomiting, flatulence); other risks include lactic acidosis in patients with impaired kidney function, heart failure, hypoxemia, alcoholism, cirrhosis, contrast exposure, sepsis, and shock.
GLP-1 receptor agonists
GLP-1 receptor agonists20–25 are injectable medications approved for adults with type 2 diabetes. Exenatide and liraglutide lower hemoglobin A1c by 1 to 1.5 absolute percentage points and reduce body weight; these effects persist over the long term.26 Newer once-weekly GLP-1 receptor agonists (albiglutide,20 dulaglutide,21 and semaglutide25) have similar benefits. In 2019, new drug applications were submitted to the FDA for the first-in-kind oral GLP-1 receptor agonists, which would improve convenience and adherence and make this class even more attractive.
Pathways affected. GLP-1 receptor agonists address multiple pathways of hyperglycemia. They increase insulin production and release, promote weight loss, and reduce insulin resistance, glucagon secretion, and inflammation. They also increase amylin, help overcome GLP-1 resistance, slow gastric emptying, and favorably modify gut flora.27
Advantages, benefits. The cardioprotective actions of GLP-1 receptor agonists include reducing inflammation and dysfunction in endothelial and myocardial cells; slowing atherosclerosis; reducing oxidative stress-induced injury and scavenging of reactive oxygen species in coronary endothelial, smooth muscle, and other cells; and enhancing endogenous antioxidant defenses.27 GLP-1 receptor agonism has also been found to inhibit apoptosis in cardiomyocytes, as well as in beta cells.
Several large-scale studies have shown improved outcomes with GLP-1 receptor agonists. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial26 found that liraglutide reduced major adverse cardiovascular events by 13% and myocardial infarctions by 22% in more than 9,000 adults with type 2 diabetes who were at high risk of major adverse cardiovascular events compared with placebo. Rates of microvascular outcomes were also reduced.
A retrospective database analysis of 39,275 patients with type 2 diabetes who were treated with exenatide reported a lower incidence of cardiovascular events than in patients not treated with exenatide.28
However, no effect on cardiovascular outcomes was found with a third GLP-1 agent, lixisenatide, in a large-scale trial in high-risk patients with diabetes.29
The most recently evaluated GLP-1 receptor agonist is semaglutide. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) demonstrated a reduced risk of major adverse cardiovascular events.30
Disadvantages, adverse effects. The most common adverse effects in this class include nausea, hypoglycemia, diarrhea, constipation, vomiting, headache, decreased appetite, dyspepsia, fatigue, dizziness, abdominal pain, and increased lipase. The nausea can be mitigated by advising patients to stop eating at first sensation of stomach fullness.
DPP-4 inhibitors
Dipeptidyl peptidase 4 (DPP-4) is a ubiquitous enzyme that rapidly degrades GLP-1 and other endogenous peptides.31 Saxagliptin,32 sitagliptin,33 linagliptin,34 and alogliptin35 are approved for use in the United States, and vildagliptin36 is available in Europe.
Pathways affected. These agents modify 3 pathways of hyperglycemia: they increase insulin secretion, decrease glucagon levels, and help overcome GLP-1 resistance.
Advantages, benefits. DPP-4 inhibitors have been used safely and effectively in clinically challenging populations of patients with long-standing type 2 diabetes (> 10 years).
Disadvantages, adverse effects. As this class increases GLP-1 levels only 2- to 4-fold, their efficacy is more modest than that of GLP-1 receptor agonists (hemoglobin A1c reductions of 0.5% to 1%; neutral effects on weight).37
Outcome trials have largely been neutral. Saxagliptin has been associated with an increase in admissions for heart failure. There have been a very small but statistically significant number of drug-related cases of acute pancreatitis.38
The most common adverse effects with this class include headache, nasopharyngitis, urinary tract infection, upper respiratory tract infection, and elevated liver enzymes.
SGLT2 inhibitors
Drugs of this class currently available in the United States are canagliflozin,39 dapagliflozin,40 empagliflozin,41 and ertugliflozin.42
Pathways affected. SGLT2 inhibitors lower the glucose reabsorption threshold in the kidney so that more glucose is excreted in the urine; they also decrease insulin resistance in muscle, liver, and fat cells (via weight loss) and possibly preserve beta-cell function by reducing glucotoxicity. A nonrenal mechanism—delayed gut absorption reducing postprandial glucose excursion—has been proposed to contribute to the glucose-lowering effects of canagliflozin.43
Advantages, benefits. These agents reduce hemoglobin A1c by about 0.5% to 1.0% from a baseline of about 8%. Because their action is independent of insulin, they can be used at any stage of type 2 diabetes, even after insulin secretion has significantly waned. Additional potential advantages include weight loss (up to 3.5% of body mass index) and lowering of systolic blood pressure (2–4 mm Hg) and diastolic blood pressure (1–2 mm Hg).39–42
Canagliflozin was shown in the Canagliflozin Cardiovascular Assessment Study (CANVAS)44 to significantly reduce the overall risk of cardiovascular disease by 14% and risk of heart failure hospitalization by 33% while significantly slowing the progression of renal disease.
In the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),45 empagliflozin reduced heart failure hospitalizations by 35%, cardiovascular deaths by 38%, and all-cause mortality by about 32%. These benefits are thought to be due to less arterial stiffness, lower sympathetic tone, and decreased arrhythmias. Notably, these dramatic benefits accrued in only about 3 years with use of add-on therapy, even though the reduction in hemoglobin A1c was modest (0.6%), suggesting that pleiotropic effects are at work.
Disadvantages, adverse effects. The most common adverse effects of this class include urinary tract infections, yeast infections, dehydration, and hypovolemic symptoms; these can often be prevented. A trend toward increased incidence of amputations in earlier studies was not borne out in a 2018 meta-analysis of 4 observational databases.46
Thiazolidinediones
There are currently 2 approved thiazolidinediones in the United States, pioglitazone47 and rosiglitazone.48 Only pioglitazone is in common use, as rosiglitazone is associated with safety issues.49
Pathways affected. Pioglitazone reduces insulin resistance in muscle, liver, and adipose tissue.
Advantages, benefits. Decreased levels of low-density lipoprotein cholesterol and triglycerides and increased high-density lipoprotein cholesterol levels49 could plausibly account for the cardiovascular benefits reported in the Prospective Pioglitazone Clinical Trial in Macrovascular Events.50 Pioglitazone has also been found to improve insulin secretion, endothelial function, and diastolic dysfunction; reduce inflammation; decrease plasminogen activator inhibitor 1; reverse lipotoxicity; and help correct nonalcoholic fatty liver disease and steatohepatitis.
Pioglitazone has also been found to reduce plaque in carotid and coronary arteries51; improve outcomes in patients with heart failure and myocardial infarction compared with insulin-sensitizing drugs52; and reduce stroke and myocardial infarction in patients with insulin resistance (but not diabetes) and a recent history of ischemic stroke or transient ischemic attack (in the Insulin Resistance Intervention After Stroke trial).53 It may also help maintain beta-cell function; the Actos Now for the Prevention of Diabetes Study found that pioglitazone reduced the risk of conversion of impaired glucose tolerance to frank diabetes by 72%.54
Disadvantages, adverse effects. The most common adverse effects seen with this class include weight gain and salt retention, swelling, edema,55 and related cardiovascular consequences in certain patients. While this may be mitigatable with lifestyle changes or use in combination with a GLP-1 receptor agonist or SGLT2 inhibitor,56 pioglitazone is contraindicated in patients with heart failure, hemodynamic instability, or hepatic dysfunction.
Concerns that pioglitazone might increase the risk of bladder cancer seem to have been put to rest when a study in nearly 200,000 patients found no statistically significant association,57 but the warning remains in the US label.
Long-term use of this class of drugs has been associated with an increased risk of bone fractures,58 which warrants a risk-benefit assessment in each patient.
Injected insulin: Less safe than thought
Recent research suggests that injected insulin has a less favorable safety profile than previously thought.15–19,59 Studies of the long-term safety of insulin therapy have had inconsistent results but suggest that injected insulin is associated with poorer cardiovascular and renal outcomes (in some of the same studies that showed metformin or other agents to improve outcomes),17–19 and the association was dose-dependent. Several studies attempted to cancel out the poorer outcomes by adjusting for hemoglobin A1c levels, stage of disease,17–19,26,27 or severe hypoglycemic episodes.60 However, it may be inappropriate to reduce the impact of these variables, as these may themselves be the mediators of any deleterious effects of exogenous insulin.
When exogenous insulin is introduced into the peripheral circulation it causes a state of persistent iatrogenic hyperinsulinemia, which leads to insulin resistance and also appears to compromise the cardiovascular system. In contrast, endogenous insulin is released into the portal system in tightly controlled amounts.5,61 This suggests that the same insulin peptide may not be equivalently beneficial when introduced in an artificial manner.
Before starting insulin therapy, consider its side effects such as weight gain and hypoglycemia. Most (about 85%) episodes of hypoglycemia occur with basal-bolus insulin regimens.62 Moreover, iatrogenic hyperinsulinemia can damage the vascular system.63,64
We recommend. Insulin therapy is used early in the course of the disease as a short-term intervention for glucolipotoxicity. However, this can be accomplished without attendant risks of hypoglycemia and weight gain by using agents such as SGLT2 inhibitors and incretins. When insulin therapy is necessary, using it as add-on therapy might be considered instead of drug-switching. We have found alternate pharmacologic approaches successful in avoiding or delaying bolus insulin therapy. And in some patients taking insulin, we have had success in progressively introducing a noninsulin agent and were ultimately able to eliminate insulin altogether.
Bromocriptine-QR
Bromocriptine-QR (quick release)65 is a short-acting dopamine agonist that mimics the morning dopamine surge in the suprachiasmatic nucleus—the biologic clock.
Pathways affected. Bromocriptine addresses part of the brain contribution to hyperglycemia, with resultant reductions in both peripheral insulin resistance and sympathetic tone. This reduces muscle, liver, and adipose insulin resistance. It is moderately effective in glucose-lowering, especially in patients with significant insulin resistance.66
Advantages, benefits. A 1-year clinical trial reported that bromocriptine reduced cardiovascular adverse outcomes by 39%, and the composite end point of myocardial infarction, stroke, and cardiovascular death by 52% compared with placebo.67
Disadvantages, adverse effects. The most common adverse effects are nausea, rhinitis, headache, asthenia, dizziness, constipation, and sinusitis.
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors (acarbose,68 miglitol69) work by decreasing the rate of absorption of glucose from the gastrointestinal tract.
Advantages, benefits. These drugs decrease hemoglobin A1c by 0.5% to 0.8%.70 They are weight-neutral and do not pose a risk of hypoglycemia. Clinical studies suggest that they may delay or prevent diabetes progression. They were also found to reduce cardiovascular events, acute myocardial infarction, and the onset of hypertension.69
Disadvantages, adverse effects. Their use remains limited due to gastrointestinal adverse effects. They may be contraindicated in patients with inflammatory bowel disease, partial bowel obstruction, or severe renal or hepatic disease.
Pramlintide
Pramlintide71 is an injectable amylin analogue. It is used as monotherapy or in combination with a sulfonylurea, metformin, or insulin glargine.
Pathways affected. Pramlintide decreases appetite, reduces glucagon levels, and minimizes absorption of glucose in the gut.
Disadvantages, adverse effects. Common side effects include mild to moderate hypoglycemia and nausea. Nausea may help explain the ability of pramlintide to confer weight loss when used in combination with insulin.
Sulfonylureas and meglitinides
These classes are still widely used in the treatment of type 2 diabetes, although the AACE6 and ADA72 guidelines de-emphasize their use based on associated risks of hypoglycemia, weight gain, morbidity, mortality, and loss of effect over time.
Pathways affected. Sulfonylureas stimulate insulin secretion from beta cells.
Disadvantages, adverse effects. Sulfonylureas and glinides are associated with poorer outcomes than newer agents in clinical trials15–19,59,60 and may be generally less beta-cell friendly.73 Their harmful effects are difficult to measure in vivo, but these drugs sometimes appear to be associated with more rapid beta-cell failure and progression to insulin dependence compared with newer ones. Several large-scale registry studies have found sulfonylureas and glinides to be associated with poorer outcomes (reviewed by Herman et al).74
Adverse effects include asthenia, headache, dizziness, nausea, diarrhea, epigastric fullness, and heartburn. Although they are often selected based on their low cost, other factors may offset their cost-effectiveness, such as need for glucose monitoring and hospital charges due to sulfonylurea-induced hypoglycemia. Their utility is also limited by dependence on beta-cell function.
Colesevelam
Colesevelam75 is a bile acid sequestrant and low-density lipoprotein cholesterol-reducing agent that has been approved for use in diabetes. The mode of action of colesevelam in this capacity is under investigation. Its effect on hemoglobin A1c is modest. It is associated with gastrointestinal adverse effects, particularly constipation.
Ranolazine
Ranolazine76 is an antianginal drug that also lowers glucose by increasing insulin release. It also possesses cardioprotective properties. In patients with diabetes and non-ST-segment elevation acute coronary syndromes, ranolazine reduced hemoglobin A1c by 1.2% and appeared to be weight-neutral.76 Ranolazine is under clinical development for use in diabetes. Adverse effects include dizziness, headache, constipation, and nausea.
Rational combinations of agents
The ideal strategy would use combinations of agents that mechanistically complement one another and address each path of hyperglycemia present in a patient. This approach should supplant the former approaches of adding-on agents only after treatment failure or sequentially trying first-, second-, and third-line treatments.
Examples of synergistic combinations include those that target fasting plasma glucose and postprandial glucose, reduce reliance on insulin with add-on therapies, or manage hyperglycemia in specific patient groups, such as renal-impaired patients.
Large-scale long-term clinical studies are needed to determine the safety, efficacy, and outcomes of various combinations and whether they confer additive benefits. Some studies have begun to explore possible combinations.
Combined metformin, pioglitazone, and exenatide was reported to delay progression of diabetes in early dysglycemia.77,78 Notably, this combination addresses multiple mediating pathways of hyperglycemia (Table 1).
A GLP-1 receptor agonist with an SGLT2 inhibitor would be another intriguing combination, as the mechanisms of action of these 2 classes complement one another. In limited clinical trials—the DURATION-8 study (lasting 26 weeks),79 the Canagliflozin Cardiovascular Assessment Study (18 weeks),80 and a 24-week study in nondiabetic obese patients81—additive benefits were also seen in systolic blood pressure, body weight, and cardiac risk factors by adding an SGLT2 inhibitor to a GLP-1 receptor agonist, compared with either agent alone. In theory, these improvements might slow or reverse cardiorenal compromise. Lower doses of 1 or more may be possible, and the regimen could prove cost-effective and life-sparing should it slow the progression of the disease and the onset of its complications. A clinical study of this combination is under way (Ralph DeFronzo, personal communication, July 2018). Similarly, the combination of metformin, saxagliptin and dapagliflozin has been shown to be effective.82
CONCLUSION
Care for diabetes mellitus can be particularly challenging for the primary care physician. The progressive nature of diabetes, with worsening hyperglycemia over the course of the disease, further complicates disease management.
Best practices for care nonetheless need to evolve with well-evidenced data, and without years of delay for “trickle-down” education from the specialties to primary care. We have arrived at a juncture to leverage therapies that address the 11 mediating pathways of hyperglycemia, optimally protect beta cells, minimize hypoglycemia, manage risk factors associated with diabetes, and improve diabetes-related outcomes.
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017; 66(2):241–255. doi:10.2337/db16-0806
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12):2669–2701. doi:10.2337/dci18-0033
- Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The time is right for a new classification system for diabetes mellitus: rationale and implications of the beta-cell centric classification schema. Diabetes Care 2016; 39(2):179–186. doi:10.2337/dc15-1585
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 2016; 118(11):1808–1829. doi:10.1161/CIRCRESAHA.116.306923
- Schwartz SS, Jellinger PS, Herman ME. Obviating much of the need for insulin therapy in type 2 diabetes mellitus: a re-assessment of insulin therapy’s safety profile. Postgrad Med 2016; 128(6):609–619. doi:10.1080/00325481.2016.1191955
- Garber AJ, Abrahamson MJ, Barzilay JE, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract 2019; 25(1):69–100. doi:10.4158/CS-2018-0535
- Sniderman AD, LaChapelle KJ, Rachon NA , Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc 2013; 88(10):1108–1114. doi:10.1016/j.mayocp.2013.07.012
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168(8):569–576. doi:10.7326/M17-0939
- Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011; 34(suppl 2):S132–S137. doi:10.2337/dc11-s220
- Chico A, Vidal-Ríos P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003; 26(4):1153–1157. pmid:12663589
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007; 115(8):491–494. doi:10.1055/s-2007-984452
- American Diabetes Association (ADA). The American Diabetes Association, the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators and the Endocrine Society strongly disagree with the American College of Physicians’ guidance for higher blood glucose targets for people with type 2 diabetes www.diabetes.org/newsroom/press-releases/2018/joint-acp-guidance-response.html. Accessed June 6, 2019.
- Freed S; Diabetes in Control. American College of Physicians recommending controversial increase in A1c of 7% to 8%. www.diabetesincontrol.com/american-college-of-physicians-recommending-controversial-increase-in-a1c-of-7-to-8. Accessed June 6, 2019.
- Glucophage XR (metformin hydrochloride) extended release tablets prescribing information. Princeton, NJ, Bristol-Myers Squibb Company, 2009.
- Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators. The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 2008; 29(2):166–176. doi:10.1093/eurheartj/ehm518
- Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L; Euro Heart Survey Investigators. Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 2008; 29(2):177–184. doi:10.1093/eurheartj/ehm519
- Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J 2005; 149(1):168–174. doi:10.1016/j.ahj.2004.07.005
- Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011; 34(1):77–83. doi:10.2337/dc10-1318
- Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab 2015; 17(4):350–362. doi:10.1111/dom.12412
- Tanzeum (albiglutide) prescribing information. Wilmington, DE, GlaxoSmithKline LLC, 2014.
- Trulicity (dulaglutide) prescribing information. Indianapolis, IN, Eli Lilly and Company, 2014.
- Byetta (exenatide) prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Victoza (liraglutide injection) prescribing information. Plainsboro, NJ, Novo Nordisk Inc, 2013.
- Adlyxin (lixisenatide injection) prescribing information. Bridgewater, NJ, Sanofi, 2016.
- Ozempic (semaglutide) prescribing information. Plainsboro, NJ, Novo Nordisk, 2017.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Chang G, Zhang D, Yu H, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med 2013; 32(5):1011–1020. doi:10.3892/ijmm.2013.1475
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care; 34(1):90–95. doi:10.2337/dc10-1393
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375(19):1834–1844. doi:10.1056/NEJMoa1607141
- Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab 2009; 23(4):443–452. doi:10.1016/j.beem.2009.03.005
- Onglyza (saxagliptin) tablets prescribing information. Wilmington, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Januvia (sitagliptin) tablets prescribing information. Whitehouse Station, NJ, Merck & Co., Inc, 2014.
- Tradjenta (linagliptin) tablets prescribing information. Ingelheim, Germany, Boehringer Ingelheim International GmbH, 2014.
- Nesina (alogliptin) tablets prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Galvus (vildagliptin) prescribing information. North Ryde, Australia, Novartis Pharmaceuticals, 2014.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696–1705. doi:10.1016/S0140-6736(06)69705-5
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14):1317–1326. doi:10.1056/NEJMoa1307684
- Invokana (canagliflozin) tablets prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2013.
- Farxiga (dapagliflozin) prescribing information. Princeton, NJ, Bristol-Myers Squibb, 2014.
- Jardiance (empagliflozin) prescribing information. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals, Inc, 2014.
- Steglatro (ertugliflozin) prescribing information. Whitehouse Station, NJ, Merck, Sharp & Dohme Corp, 2017.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018; 20(11):2485–2597. doi:10.1111/dom.13424
- Actos (pioglitazone) tablets for oral use prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc, 2013.
- Avandia (rosiglitazone maleate tablets) prescribing information. Research Triangle Park, NC, GlaxoSmithKline, 1999.
- Goldberg RB, Kendall DK, Deeg MA, et al; GLAI Study Investigators. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2005; 28(7):1547–1554. pmid:15983299
- Dormandy JA, Charbonnel B, Eckland DJ, et al; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone clinical trial in macroVascular Events): a randomised controlled trial. Lancet 2005; 366:1279–1289. doi:10.1016/S0140-6736(05)67528-9
- Nissen SE, Nicholls SJ, Wolski K, et al; PERISCOPE Investigators. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes the PERISCOPE randomized controlled trial. JAMA 2008; 299(13):1561–1573. doi:10.1001/jama.299.13.1561
- Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005; 111(5):583–590. doi:10.1161/01.CIR.0000154542.13412.B1
- Kernan WN, Viscoli CM, Furie KL, et al; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374(14):1321–1331. doi:10.1056/NEJMoa1506930
- DeFronzo RA, Tripathy D, Schwenke DC, et al; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364(12):1104–1115. doi:10.1056/NEJMoa1010949
- Nesto RW, Bell D, Bonow RO, et al; American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108(23):2941–2948. doi:10.1161/01.CIR.0000103683.99399.7E
- Kushner RF, Sujak M. Prevention of weight gain in adult patients with type 2 diabetes treated with pioglitazone. Obesity (Silver Spring) 2009; 17(5):1017–1022. doi:10.1038/oby.2008.651
- Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015; 314(3):265–277. doi:10.1001/jama.2015.7996
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med 2008; 168(8):820–825. doi:10.1001/archinte.168.8.820
- Gamble JM, Chibrikov E, Twells LK, et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol 2017; 5(1):43–52. doi:10.1016/S2213-8587(16)30316-3
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Wang X, Yu C, Zhang B, Wang Y. The injurious effects of hyperinsulinism on blood vessels. Cell Biochem Biophys 2014; 69(2):213–218. doi:10.1007/s12013-013-9810-6
- Garber AJ, King AB, Del Prato S, et al; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012; 379(9825):1498–1507. doi:10.1016/S0140-6736(12)60205-0
- Hanefeld M, Monnier L, Schnell O, Owens D. Early treatment with basal insulin glargine in people with type 2 diabetes: lessons from ORIGIN and other cardiovascular trials. Diabetes Ther 2016; 7(2):187–201. doi:10.1007/s13300-016-0153-3
- Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013; 1(1):9–10. doi:10.1016/S2213-8587(13)70027-5
- Cycloset (bromocriptine mesylate) tablets prescribing information. Tiverton, RI, VeroScience LLC, 2019.
- Schwartz S, Zangeneh F. Evidence-based practice use of quick-release bromocriptine across the natural history of type 2 diabetes mellitus. Postgrad Med 2016; 128(8):828–838. doi:10.1080/00325481.2016.1214059
- Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc 2012; 1(5):e002279. doi:10.1161/JAHA.112.002279
- Precose (acarbose) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals Inc, 2011.
- Glyset (miglitol) tablets prescribing information. Germany, Bayer HealthCare Pharmaceuticals, Inc, 2012.
- Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (2):CD003639. doi:10.1002/14651858.CD003639.pub2
- Symlin (pramlintide acetate) injection for subcutaneous use prescribing information. Wilmongton, DE, AstraZeneca Pharmaceuticals LP, 2014.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58(3):429–442. doi:10.1007/s00125-014-3460-0
- Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28(2):187–218. doi:10.1210/10.1210/er.2006-0038
- Herman ME, O’Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis 2017; 60(3):422–434. doi:10.1016/j.pcad.2017.09.001
- Welchol (colesevelam hydrochloride) prescribing information. Parsippany, NJ, Daiichi Sankyo Inc, 2014.
- Ranexa (ranolazine) prescribing information. Foster City, CA: Gilead Sciences, Inc, 2016.
- Armato J, DeFronzo R, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and beta-cell dysfunction. Endocr Pract 2012; 18(3):342–350. doi:10.4158/EP11194.OR
- Abdul-Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the efficacy and durability of initial combination therapy for type 2 diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015; 17(3):268–275. doi:10.1111/dom.12417
- Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4(12):1004–1016. doi:10.1016/S2213-8587(16)30267-4
- Fulcher G, Matthews DR, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18(1):82–91. doi:10.1111/dom.12589
- Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab 2017; 19(1):49–60. doi:10.1111/dom.12779
- Del Prato S, Rosenstock J, Garcia-Sanchez R, et al. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab 2018; 20(6):1542–1546. doi:10.1111/dom.13258
KEY POINTS
- At least 11 pathways lead to hyperglycemia; of these, beta-cell dysfunction is central.
- As different classes of diabetes drugs act on different pathways, we can target the pathways contributing to hyperglycemia in the individual patient, using fewer agents and lessening the risk of hypoglycemic episodes.
- In selecting treatment, we should favor drugs that are “gentle” on beta cells, do not cause dangerous hypoglycemia, and improve long-term outcomes as shown in randomized clinical trials.
Click for Credit: Roux-en-Y for diabetes; Exercise & fall prevention; more
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020