User login
Tool May Help Prioritize High-Risk Patients for Hysteroscopy
Hysteroscopy is a crucial examination for the diagnosis of endometrial cancer. In Brazil, women with postmenopausal bleeding who need to undergo this procedure in the public health system wait in line alongside patients with less severe complaints. Until now, there has been no system to prioritize patients at high risk for cancer. But this situation may change, thanks to a Brazilian study published in February in the Journal of Clinical Medicine.
Researchers from the Municipal Hospital of Vila Santa Catarina in São Paulo, a public unit managed by Hospital Israelita Albert Einstein, have developed the Endometrial Malignancy Prediction System (EMPS), a nomogram to identify patients at high risk for endometrial cancer and prioritize them in the hysteroscopy waiting list.
Bruna Bottura, MD, a gynecologist and obstetrician at Hospital Israelita Albert Einstein and the study’s lead author, told this news organization that the idea to create the nomogram arose during the COVID-19 pandemic. “We noticed that ... when outpatient clinics resumed, we were seeing many patients for intrauterine device (IUD) removal. We thought it was unfair for a patient with postmenopausal bleeding, who has a chance of having cancer, to have to wait in the same line as a patient needing IUD removal,” she said. This realization motivated the development of the tool, which was overseen by Renato Moretti-Marques, MD, PhD.
The EMPS Score
The team conducted a retrospective case-control study involving 1945 patients with suspected endometrial cancer who had undergone diagnostic hysteroscopy at Hospital Israelita Albert Einstein between March 2019 and March 2022. Among these patients, 107 were diagnosed with precursor lesions or endometrial cancer on the basis of biopsy. The other 1838 participants, who had had cancer ruled out by biopsy, formed the control group.
Through bivariate and multivariate linear regression analysis, the authors determined that the presence or absence of hypertension, diabetes, postmenopausal bleeding, endometrial polyps, uterine volume, number of pregnancies, body mass index, age, and endometrial thickness were the main risk factors for endometrial cancer diagnosis.
On the basis of these data, the group developed the EMPS nomogram. Physicians can use it to classify the patient’s risk according to the sum of the scores assigned to each of these factors.
The Table shows the classification system. The scoring tables available in the supplemental materials of the article can be accessed here.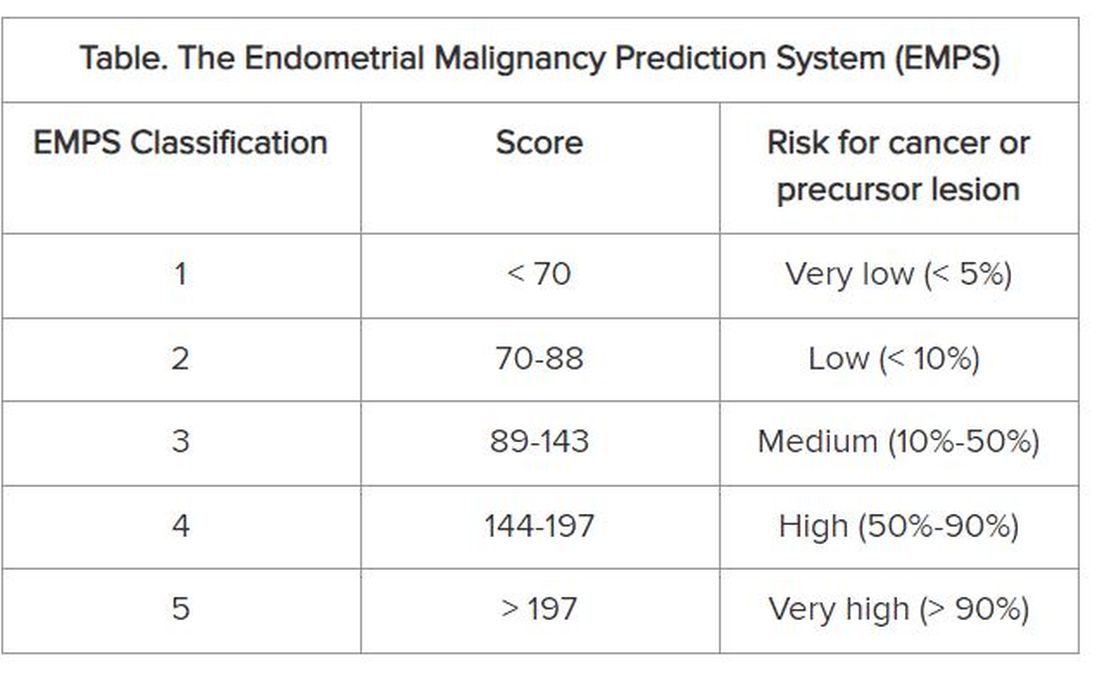
Focus on Primary Care
The goal is not to remove patients classified as low risk from the hysteroscopy waiting list, but rather to prioritize those classified as high risk to get the examination, according to Dr. Bottura.
At the Municipal Hospital of Vila Santa Catarina, the average wait time for hysteroscopy was 120 days. But because the unit is focused on oncologic patients and has a high level of organization, this time is much shorter than observed in other parts of Brazil’s National Health Service, said Dr. Bottura. “Many patients are on the hysteroscopy waiting list for 2 years. Considering patients in more advanced stages [of endometrial cancer], it makes a difference,” she said.
Although the nomogram was developed in tertiary care, it is aimed at professionals working in primary care. The reason is that physicians from primary care health units refer women with clinical indications for hysteroscopy to specialized national health services, such as the Municipal Hospital of Vila Santa Catarina. “Our goal is the primary sector, to enable the clinic to refer this high-risk patient sooner. By the time you reach the tertiary sector, where hysteroscopies are performed, all patients will undergo the procedure. Usually, it is not the hospitals that predetermine the line, but rather the health clinics,” she explained.
The researchers hope to continue the research, starting with a prospective study. “We intend to apply and evaluate the tool within our own service to observe whether any patient with a high [EMPS] score patient ended up waiting too long to be referred. In fact, this will be a system validation step,” said Dr. Bottura.
In parallel, the team has a proposal to take the tool to health clinics in the same region as the study hospital. “We know this involves changing the protocol at a national level, so it’s more challenging,” said Dr. Bottura. She added that the final goal is to create a calculator, possibly an app, that allows primary care doctors to calculate the risk score in the office. This calculator could enable risk classification to be linked to patient referrals.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Hysteroscopy is a crucial examination for the diagnosis of endometrial cancer. In Brazil, women with postmenopausal bleeding who need to undergo this procedure in the public health system wait in line alongside patients with less severe complaints. Until now, there has been no system to prioritize patients at high risk for cancer. But this situation may change, thanks to a Brazilian study published in February in the Journal of Clinical Medicine.
Researchers from the Municipal Hospital of Vila Santa Catarina in São Paulo, a public unit managed by Hospital Israelita Albert Einstein, have developed the Endometrial Malignancy Prediction System (EMPS), a nomogram to identify patients at high risk for endometrial cancer and prioritize them in the hysteroscopy waiting list.
Bruna Bottura, MD, a gynecologist and obstetrician at Hospital Israelita Albert Einstein and the study’s lead author, told this news organization that the idea to create the nomogram arose during the COVID-19 pandemic. “We noticed that ... when outpatient clinics resumed, we were seeing many patients for intrauterine device (IUD) removal. We thought it was unfair for a patient with postmenopausal bleeding, who has a chance of having cancer, to have to wait in the same line as a patient needing IUD removal,” she said. This realization motivated the development of the tool, which was overseen by Renato Moretti-Marques, MD, PhD.
The EMPS Score
The team conducted a retrospective case-control study involving 1945 patients with suspected endometrial cancer who had undergone diagnostic hysteroscopy at Hospital Israelita Albert Einstein between March 2019 and March 2022. Among these patients, 107 were diagnosed with precursor lesions or endometrial cancer on the basis of biopsy. The other 1838 participants, who had had cancer ruled out by biopsy, formed the control group.
Through bivariate and multivariate linear regression analysis, the authors determined that the presence or absence of hypertension, diabetes, postmenopausal bleeding, endometrial polyps, uterine volume, number of pregnancies, body mass index, age, and endometrial thickness were the main risk factors for endometrial cancer diagnosis.
On the basis of these data, the group developed the EMPS nomogram. Physicians can use it to classify the patient’s risk according to the sum of the scores assigned to each of these factors.
The Table shows the classification system. The scoring tables available in the supplemental materials of the article can be accessed here.
Focus on Primary Care
The goal is not to remove patients classified as low risk from the hysteroscopy waiting list, but rather to prioritize those classified as high risk to get the examination, according to Dr. Bottura.
At the Municipal Hospital of Vila Santa Catarina, the average wait time for hysteroscopy was 120 days. But because the unit is focused on oncologic patients and has a high level of organization, this time is much shorter than observed in other parts of Brazil’s National Health Service, said Dr. Bottura. “Many patients are on the hysteroscopy waiting list for 2 years. Considering patients in more advanced stages [of endometrial cancer], it makes a difference,” she said.
Although the nomogram was developed in tertiary care, it is aimed at professionals working in primary care. The reason is that physicians from primary care health units refer women with clinical indications for hysteroscopy to specialized national health services, such as the Municipal Hospital of Vila Santa Catarina. “Our goal is the primary sector, to enable the clinic to refer this high-risk patient sooner. By the time you reach the tertiary sector, where hysteroscopies are performed, all patients will undergo the procedure. Usually, it is not the hospitals that predetermine the line, but rather the health clinics,” she explained.
The researchers hope to continue the research, starting with a prospective study. “We intend to apply and evaluate the tool within our own service to observe whether any patient with a high [EMPS] score patient ended up waiting too long to be referred. In fact, this will be a system validation step,” said Dr. Bottura.
In parallel, the team has a proposal to take the tool to health clinics in the same region as the study hospital. “We know this involves changing the protocol at a national level, so it’s more challenging,” said Dr. Bottura. She added that the final goal is to create a calculator, possibly an app, that allows primary care doctors to calculate the risk score in the office. This calculator could enable risk classification to be linked to patient referrals.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Hysteroscopy is a crucial examination for the diagnosis of endometrial cancer. In Brazil, women with postmenopausal bleeding who need to undergo this procedure in the public health system wait in line alongside patients with less severe complaints. Until now, there has been no system to prioritize patients at high risk for cancer. But this situation may change, thanks to a Brazilian study published in February in the Journal of Clinical Medicine.
Researchers from the Municipal Hospital of Vila Santa Catarina in São Paulo, a public unit managed by Hospital Israelita Albert Einstein, have developed the Endometrial Malignancy Prediction System (EMPS), a nomogram to identify patients at high risk for endometrial cancer and prioritize them in the hysteroscopy waiting list.
Bruna Bottura, MD, a gynecologist and obstetrician at Hospital Israelita Albert Einstein and the study’s lead author, told this news organization that the idea to create the nomogram arose during the COVID-19 pandemic. “We noticed that ... when outpatient clinics resumed, we were seeing many patients for intrauterine device (IUD) removal. We thought it was unfair for a patient with postmenopausal bleeding, who has a chance of having cancer, to have to wait in the same line as a patient needing IUD removal,” she said. This realization motivated the development of the tool, which was overseen by Renato Moretti-Marques, MD, PhD.
The EMPS Score
The team conducted a retrospective case-control study involving 1945 patients with suspected endometrial cancer who had undergone diagnostic hysteroscopy at Hospital Israelita Albert Einstein between March 2019 and March 2022. Among these patients, 107 were diagnosed with precursor lesions or endometrial cancer on the basis of biopsy. The other 1838 participants, who had had cancer ruled out by biopsy, formed the control group.
Through bivariate and multivariate linear regression analysis, the authors determined that the presence or absence of hypertension, diabetes, postmenopausal bleeding, endometrial polyps, uterine volume, number of pregnancies, body mass index, age, and endometrial thickness were the main risk factors for endometrial cancer diagnosis.
On the basis of these data, the group developed the EMPS nomogram. Physicians can use it to classify the patient’s risk according to the sum of the scores assigned to each of these factors.
The Table shows the classification system. The scoring tables available in the supplemental materials of the article can be accessed here.
Focus on Primary Care
The goal is not to remove patients classified as low risk from the hysteroscopy waiting list, but rather to prioritize those classified as high risk to get the examination, according to Dr. Bottura.
At the Municipal Hospital of Vila Santa Catarina, the average wait time for hysteroscopy was 120 days. But because the unit is focused on oncologic patients and has a high level of organization, this time is much shorter than observed in other parts of Brazil’s National Health Service, said Dr. Bottura. “Many patients are on the hysteroscopy waiting list for 2 years. Considering patients in more advanced stages [of endometrial cancer], it makes a difference,” she said.
Although the nomogram was developed in tertiary care, it is aimed at professionals working in primary care. The reason is that physicians from primary care health units refer women with clinical indications for hysteroscopy to specialized national health services, such as the Municipal Hospital of Vila Santa Catarina. “Our goal is the primary sector, to enable the clinic to refer this high-risk patient sooner. By the time you reach the tertiary sector, where hysteroscopies are performed, all patients will undergo the procedure. Usually, it is not the hospitals that predetermine the line, but rather the health clinics,” she explained.
The researchers hope to continue the research, starting with a prospective study. “We intend to apply and evaluate the tool within our own service to observe whether any patient with a high [EMPS] score patient ended up waiting too long to be referred. In fact, this will be a system validation step,” said Dr. Bottura.
In parallel, the team has a proposal to take the tool to health clinics in the same region as the study hospital. “We know this involves changing the protocol at a national level, so it’s more challenging,” said Dr. Bottura. She added that the final goal is to create a calculator, possibly an app, that allows primary care doctors to calculate the risk score in the office. This calculator could enable risk classification to be linked to patient referrals.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Clinicians Call for Easing FDA Warnings on Low-Dose Estrogen
Charles Powell, MD, said he sometimes has a hard time persuading patients to start on low-dose vaginal estrogen, which can help prevent urinary tract infections and ease other symptoms of menopause.
Many women fear taking these vaginal products because of what Dr. Powell considers excessively strong warnings about the risk for cancer and cardiovascular disease linked to daily estrogen pills that were issued in the early 2000s.
He is advocating for the US Food and Drug Administration (FDA) to remove the boxed warning on low-dose estrogen. His efforts are separate from his roles as an associate professor of urology at the Indiana University School of Medicine, and as a member of the American Urological Association (AUA), Dr. Powell said.
In his quest to find out how to change labeling, Dr. Powell has gained a quick education about drug regulation. He has enlisted Representative Jim Baird (R-IN) and Senator Mike Braun (R-IN) to contact the FDA on his behalf, while congressional staff guided him through the hurdles of getting the warning label changed. For instance, a manufacturer of low-dose estrogen may need to become involved.
“You don’t learn this in med school,” Dr. Powell said in an interview.
With this work, Dr. Powell is wading into a long-standing argument between the FDA and some clinicians and researchers about the potential harms of low-dose estrogen.
He is doing so at a time of increased interest in understanding genitourinary syndrome of menopause (GSM), a term coined a decade ago by the International Society for the Study of Women’s Sexual Health and the North American Menopause Society to cover “a constellation of conditions” related to urogenital atrophy.
Symptoms of GSM include vaginal dryness and burning and recurrent urinary tract infections.
The federal government in 2022 began a project budgeted with nearly $1 million to review evidence on treatments, including vaginal and low-dose estrogen. The aim is to eventually help the AUA develop clinical guidelines for addressing GSM.
In addition, a bipartisan Senate bill introduced in May calls for authorizing $125 million over 5 years for the National Institutes of Health (NIH) to fund research on menopause. Senator Patty Murray (D-WA), the lead sponsor of the bill, is a longtime advocate for women’s health and serves as chairwoman for the Senate Appropriations Committee, which largely sets the NIH budget.
“The bottom line is, for too long, menopause has been overlooked, underinvested in and left behind,” Sen. Murray said during a May 2 press conference. “It is well past time to stop treating menopause like some kind of secret and start treating it like the major mainstream public health issue it is.”
Evidence Demands
Increased federal funding for menopause research could help efforts to change the warning label on low-dose estrogen, according to JoAnn Manson, MD, chief of preventive medicine at Brigham and Women’s Hospital in Boston.
Dr. Manson was a leader of the Women’s Health Initiative (WHI), a major federally funded research project launched in 1991 to investigate if hormone therapy and diet could protect older women from chronic diseases related to aging.
Before the WHI, clinicians prescribed hormones to prevent cardiovascular disease, based on evidence from earlier research.
But in 2002, a WHI trial that compared estrogen-progestin tablets with placebo was halted early because of disturbing findings, including an association with higher risk for breast cancer and cardiovascular disease.
Compared with placebo, for every 10,000 women taking estrogen plus progestin annually, incidences of cardiovascular disease, stroke, pulmonary embolism, and invasive breast cancer were seven to eight times higher.
In January 2003, the FDA announced it would put a boxed warning about cardiovascular risk and cancer risk on estrogen products, reflecting the WHI finding.
The agency at the time said clinicians should work with patients to assess risks and benefits of these products to manage the effects of menopause.
But more news on the potential harms of estrogen followed in 2004: A WHI study comparing estrogen-only pills with placebo produced signals of a small increased risk for stroke, although it also indicated no excess risk for breast cancer for at least 6.8 years of use.
Dr. Manson and the North American Menopause Society in 2016 filed a petition with the FDA to remove the boxed warning that appears on the front of low-dose estrogen products. The group wanted the information on risks moved to the usual warning section of the label.
Two years later, the FDA rejected the petition, citing the absence of “well-controlled studies,” to prove low-dose topical estrogen poses less risk to women than the high-dose pills studied in the WHI.
The FDA told this news organization that it stands by the decisions in its rejection of the petition.
Persuading the FDA to revise the labels on low-dose estrogen products likely will require evidence from randomized, large-scale studies, Dr. Manson said. The agency has not been satisfied to date with findings from other kinds of studies, including observational research.
“Once that evidence is available that the benefit-risk profile is different for different formulations and the evidence is compelling and definitive, that warning should change,” Dr. Manson told this news organization.
But the warning continues to have a chilling effect on patient willingness to use low-dose vaginal estrogen, even with the FDA’s continued endorsement of estrogen for menopause symptoms, clinicians told this news organization.
Risa Kagan, MD, a gynecologist at Sutter Health in Berkeley, California, said in many cases her patients’ partners also need to be reassured. Dr. Kagan said she still sees women who have had to discontinue sexual intercourse because of pain. In some cases, the patients will bring the medicine home only to find that the warnings frighten their spouses.
“The spouse says, ‘Oh my God, I don’t want you to get dementia, to get breast cancer, it’s not worth it, so let’s keep doing outercourse’,” meaning sexual relations without penetration, Dr. Kagan said.
Difficult Messaging
From the initial unveiling of disappointing WHI results, clinicians and researchers have stressed that women could continue using estrogen products for managing symptoms of menopause, even while advising strongly against their continued use with the intention of preventing heart disease.
Newly published findings from follow-ups of WHI participants may give clinicians and patients even more confidence for the use of estrogen products in early menopause.
According to the study, which Dr. Manson coauthored, younger women have a low risk for cardiovascular disease and other associated conditions when taking hormone therapy. Risks attributed to these drugs were less than one additional adverse event per 1000 women annually. This population may also derive significant quality-of-life benefits for symptom relief.
Dr. Manson told this news organization that estrogen in lower doses and delivered through the skin as a patch or gel may further reduce risks.
“The WHI findings should never be used as a reason to deny hormone therapy to women in early menopause with bothersome menopausal symptoms,” Dr. Manson said. “Many women are good candidates for treatment and, in shared decision-making with their clinicians, should be able to receive appropriate and personalized healthcare for their needs.”
But the current FDA warning label makes it difficult to help women understand the risk and benefits of low-dose estrogen, according to Stephanie Faubion, MD, MBA, medical director at the North American Menopause Society and director of Mayo Clinic’s Center for Women’s Health in Jacksonville, Florida.
Clinicians now must set aside time to explain the warnings to women when they prescribe low-dose estrogen, Dr. Faubion said.
“The package insert is going to look scary: I prepare women for that because otherwise they often won’t even fill it or use it.”
A version of this article appeared on Medscape.com .
Charles Powell, MD, said he sometimes has a hard time persuading patients to start on low-dose vaginal estrogen, which can help prevent urinary tract infections and ease other symptoms of menopause.
Many women fear taking these vaginal products because of what Dr. Powell considers excessively strong warnings about the risk for cancer and cardiovascular disease linked to daily estrogen pills that were issued in the early 2000s.
He is advocating for the US Food and Drug Administration (FDA) to remove the boxed warning on low-dose estrogen. His efforts are separate from his roles as an associate professor of urology at the Indiana University School of Medicine, and as a member of the American Urological Association (AUA), Dr. Powell said.
In his quest to find out how to change labeling, Dr. Powell has gained a quick education about drug regulation. He has enlisted Representative Jim Baird (R-IN) and Senator Mike Braun (R-IN) to contact the FDA on his behalf, while congressional staff guided him through the hurdles of getting the warning label changed. For instance, a manufacturer of low-dose estrogen may need to become involved.
“You don’t learn this in med school,” Dr. Powell said in an interview.
With this work, Dr. Powell is wading into a long-standing argument between the FDA and some clinicians and researchers about the potential harms of low-dose estrogen.
He is doing so at a time of increased interest in understanding genitourinary syndrome of menopause (GSM), a term coined a decade ago by the International Society for the Study of Women’s Sexual Health and the North American Menopause Society to cover “a constellation of conditions” related to urogenital atrophy.
Symptoms of GSM include vaginal dryness and burning and recurrent urinary tract infections.
The federal government in 2022 began a project budgeted with nearly $1 million to review evidence on treatments, including vaginal and low-dose estrogen. The aim is to eventually help the AUA develop clinical guidelines for addressing GSM.
In addition, a bipartisan Senate bill introduced in May calls for authorizing $125 million over 5 years for the National Institutes of Health (NIH) to fund research on menopause. Senator Patty Murray (D-WA), the lead sponsor of the bill, is a longtime advocate for women’s health and serves as chairwoman for the Senate Appropriations Committee, which largely sets the NIH budget.
“The bottom line is, for too long, menopause has been overlooked, underinvested in and left behind,” Sen. Murray said during a May 2 press conference. “It is well past time to stop treating menopause like some kind of secret and start treating it like the major mainstream public health issue it is.”
Evidence Demands
Increased federal funding for menopause research could help efforts to change the warning label on low-dose estrogen, according to JoAnn Manson, MD, chief of preventive medicine at Brigham and Women’s Hospital in Boston.
Dr. Manson was a leader of the Women’s Health Initiative (WHI), a major federally funded research project launched in 1991 to investigate if hormone therapy and diet could protect older women from chronic diseases related to aging.
Before the WHI, clinicians prescribed hormones to prevent cardiovascular disease, based on evidence from earlier research.
But in 2002, a WHI trial that compared estrogen-progestin tablets with placebo was halted early because of disturbing findings, including an association with higher risk for breast cancer and cardiovascular disease.
Compared with placebo, for every 10,000 women taking estrogen plus progestin annually, incidences of cardiovascular disease, stroke, pulmonary embolism, and invasive breast cancer were seven to eight times higher.
In January 2003, the FDA announced it would put a boxed warning about cardiovascular risk and cancer risk on estrogen products, reflecting the WHI finding.
The agency at the time said clinicians should work with patients to assess risks and benefits of these products to manage the effects of menopause.
But more news on the potential harms of estrogen followed in 2004: A WHI study comparing estrogen-only pills with placebo produced signals of a small increased risk for stroke, although it also indicated no excess risk for breast cancer for at least 6.8 years of use.
Dr. Manson and the North American Menopause Society in 2016 filed a petition with the FDA to remove the boxed warning that appears on the front of low-dose estrogen products. The group wanted the information on risks moved to the usual warning section of the label.
Two years later, the FDA rejected the petition, citing the absence of “well-controlled studies,” to prove low-dose topical estrogen poses less risk to women than the high-dose pills studied in the WHI.
The FDA told this news organization that it stands by the decisions in its rejection of the petition.
Persuading the FDA to revise the labels on low-dose estrogen products likely will require evidence from randomized, large-scale studies, Dr. Manson said. The agency has not been satisfied to date with findings from other kinds of studies, including observational research.
“Once that evidence is available that the benefit-risk profile is different for different formulations and the evidence is compelling and definitive, that warning should change,” Dr. Manson told this news organization.
But the warning continues to have a chilling effect on patient willingness to use low-dose vaginal estrogen, even with the FDA’s continued endorsement of estrogen for menopause symptoms, clinicians told this news organization.
Risa Kagan, MD, a gynecologist at Sutter Health in Berkeley, California, said in many cases her patients’ partners also need to be reassured. Dr. Kagan said she still sees women who have had to discontinue sexual intercourse because of pain. In some cases, the patients will bring the medicine home only to find that the warnings frighten their spouses.
“The spouse says, ‘Oh my God, I don’t want you to get dementia, to get breast cancer, it’s not worth it, so let’s keep doing outercourse’,” meaning sexual relations without penetration, Dr. Kagan said.
Difficult Messaging
From the initial unveiling of disappointing WHI results, clinicians and researchers have stressed that women could continue using estrogen products for managing symptoms of menopause, even while advising strongly against their continued use with the intention of preventing heart disease.
Newly published findings from follow-ups of WHI participants may give clinicians and patients even more confidence for the use of estrogen products in early menopause.
According to the study, which Dr. Manson coauthored, younger women have a low risk for cardiovascular disease and other associated conditions when taking hormone therapy. Risks attributed to these drugs were less than one additional adverse event per 1000 women annually. This population may also derive significant quality-of-life benefits for symptom relief.
Dr. Manson told this news organization that estrogen in lower doses and delivered through the skin as a patch or gel may further reduce risks.
“The WHI findings should never be used as a reason to deny hormone therapy to women in early menopause with bothersome menopausal symptoms,” Dr. Manson said. “Many women are good candidates for treatment and, in shared decision-making with their clinicians, should be able to receive appropriate and personalized healthcare for their needs.”
But the current FDA warning label makes it difficult to help women understand the risk and benefits of low-dose estrogen, according to Stephanie Faubion, MD, MBA, medical director at the North American Menopause Society and director of Mayo Clinic’s Center for Women’s Health in Jacksonville, Florida.
Clinicians now must set aside time to explain the warnings to women when they prescribe low-dose estrogen, Dr. Faubion said.
“The package insert is going to look scary: I prepare women for that because otherwise they often won’t even fill it or use it.”
A version of this article appeared on Medscape.com .
Charles Powell, MD, said he sometimes has a hard time persuading patients to start on low-dose vaginal estrogen, which can help prevent urinary tract infections and ease other symptoms of menopause.
Many women fear taking these vaginal products because of what Dr. Powell considers excessively strong warnings about the risk for cancer and cardiovascular disease linked to daily estrogen pills that were issued in the early 2000s.
He is advocating for the US Food and Drug Administration (FDA) to remove the boxed warning on low-dose estrogen. His efforts are separate from his roles as an associate professor of urology at the Indiana University School of Medicine, and as a member of the American Urological Association (AUA), Dr. Powell said.
In his quest to find out how to change labeling, Dr. Powell has gained a quick education about drug regulation. He has enlisted Representative Jim Baird (R-IN) and Senator Mike Braun (R-IN) to contact the FDA on his behalf, while congressional staff guided him through the hurdles of getting the warning label changed. For instance, a manufacturer of low-dose estrogen may need to become involved.
“You don’t learn this in med school,” Dr. Powell said in an interview.
With this work, Dr. Powell is wading into a long-standing argument between the FDA and some clinicians and researchers about the potential harms of low-dose estrogen.
He is doing so at a time of increased interest in understanding genitourinary syndrome of menopause (GSM), a term coined a decade ago by the International Society for the Study of Women’s Sexual Health and the North American Menopause Society to cover “a constellation of conditions” related to urogenital atrophy.
Symptoms of GSM include vaginal dryness and burning and recurrent urinary tract infections.
The federal government in 2022 began a project budgeted with nearly $1 million to review evidence on treatments, including vaginal and low-dose estrogen. The aim is to eventually help the AUA develop clinical guidelines for addressing GSM.
In addition, a bipartisan Senate bill introduced in May calls for authorizing $125 million over 5 years for the National Institutes of Health (NIH) to fund research on menopause. Senator Patty Murray (D-WA), the lead sponsor of the bill, is a longtime advocate for women’s health and serves as chairwoman for the Senate Appropriations Committee, which largely sets the NIH budget.
“The bottom line is, for too long, menopause has been overlooked, underinvested in and left behind,” Sen. Murray said during a May 2 press conference. “It is well past time to stop treating menopause like some kind of secret and start treating it like the major mainstream public health issue it is.”
Evidence Demands
Increased federal funding for menopause research could help efforts to change the warning label on low-dose estrogen, according to JoAnn Manson, MD, chief of preventive medicine at Brigham and Women’s Hospital in Boston.
Dr. Manson was a leader of the Women’s Health Initiative (WHI), a major federally funded research project launched in 1991 to investigate if hormone therapy and diet could protect older women from chronic diseases related to aging.
Before the WHI, clinicians prescribed hormones to prevent cardiovascular disease, based on evidence from earlier research.
But in 2002, a WHI trial that compared estrogen-progestin tablets with placebo was halted early because of disturbing findings, including an association with higher risk for breast cancer and cardiovascular disease.
Compared with placebo, for every 10,000 women taking estrogen plus progestin annually, incidences of cardiovascular disease, stroke, pulmonary embolism, and invasive breast cancer were seven to eight times higher.
In January 2003, the FDA announced it would put a boxed warning about cardiovascular risk and cancer risk on estrogen products, reflecting the WHI finding.
The agency at the time said clinicians should work with patients to assess risks and benefits of these products to manage the effects of menopause.
But more news on the potential harms of estrogen followed in 2004: A WHI study comparing estrogen-only pills with placebo produced signals of a small increased risk for stroke, although it also indicated no excess risk for breast cancer for at least 6.8 years of use.
Dr. Manson and the North American Menopause Society in 2016 filed a petition with the FDA to remove the boxed warning that appears on the front of low-dose estrogen products. The group wanted the information on risks moved to the usual warning section of the label.
Two years later, the FDA rejected the petition, citing the absence of “well-controlled studies,” to prove low-dose topical estrogen poses less risk to women than the high-dose pills studied in the WHI.
The FDA told this news organization that it stands by the decisions in its rejection of the petition.
Persuading the FDA to revise the labels on low-dose estrogen products likely will require evidence from randomized, large-scale studies, Dr. Manson said. The agency has not been satisfied to date with findings from other kinds of studies, including observational research.
“Once that evidence is available that the benefit-risk profile is different for different formulations and the evidence is compelling and definitive, that warning should change,” Dr. Manson told this news organization.
But the warning continues to have a chilling effect on patient willingness to use low-dose vaginal estrogen, even with the FDA’s continued endorsement of estrogen for menopause symptoms, clinicians told this news organization.
Risa Kagan, MD, a gynecologist at Sutter Health in Berkeley, California, said in many cases her patients’ partners also need to be reassured. Dr. Kagan said she still sees women who have had to discontinue sexual intercourse because of pain. In some cases, the patients will bring the medicine home only to find that the warnings frighten their spouses.
“The spouse says, ‘Oh my God, I don’t want you to get dementia, to get breast cancer, it’s not worth it, so let’s keep doing outercourse’,” meaning sexual relations without penetration, Dr. Kagan said.
Difficult Messaging
From the initial unveiling of disappointing WHI results, clinicians and researchers have stressed that women could continue using estrogen products for managing symptoms of menopause, even while advising strongly against their continued use with the intention of preventing heart disease.
Newly published findings from follow-ups of WHI participants may give clinicians and patients even more confidence for the use of estrogen products in early menopause.
According to the study, which Dr. Manson coauthored, younger women have a low risk for cardiovascular disease and other associated conditions when taking hormone therapy. Risks attributed to these drugs were less than one additional adverse event per 1000 women annually. This population may also derive significant quality-of-life benefits for symptom relief.
Dr. Manson told this news organization that estrogen in lower doses and delivered through the skin as a patch or gel may further reduce risks.
“The WHI findings should never be used as a reason to deny hormone therapy to women in early menopause with bothersome menopausal symptoms,” Dr. Manson said. “Many women are good candidates for treatment and, in shared decision-making with their clinicians, should be able to receive appropriate and personalized healthcare for their needs.”
But the current FDA warning label makes it difficult to help women understand the risk and benefits of low-dose estrogen, according to Stephanie Faubion, MD, MBA, medical director at the North American Menopause Society and director of Mayo Clinic’s Center for Women’s Health in Jacksonville, Florida.
Clinicians now must set aside time to explain the warnings to women when they prescribe low-dose estrogen, Dr. Faubion said.
“The package insert is going to look scary: I prepare women for that because otherwise they often won’t even fill it or use it.”
A version of this article appeared on Medscape.com .
Molecular Classification of Endometrial Carcinomas
Historically, endometrial cancer has been classified as type I or type II. Type I endometrial cancers are typically estrogen driven, low grade, with endometrioid histology, and have a more favorable prognosis. In contrast, type II endometrial cancers are typically high grade, have more aggressive histologies (eg, serous or clear cell), and have a poorer prognosis.1
While this system provides a helpful schema for understanding endometrial cancers, it fails to represent the immense variation of biologic behavior and outcomes in endometrial cancers and oversimplifies what has come to be understood as a complex and molecularly diverse disease.
In 2013, The Cancer Genome Atlas (TCGA) performed genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas. They identified four categories with distinct genetic profiles corresponding to clinical outcomes: 1) DNA polymerase epsilon (POLE) mutated; 2) mismatch repair deficient; 3) copy number high/p53 abnormal; and 4) copy number low/no specific molecular profile.2 By providing both predictive and prognostic information, these molecular features may be incorporated into treatment planning decisions in the future.
The POLE-mutated subtype are endometrial cancers with recurrent mutations in the POLE gene, which is involved in DNA replication and repair. POLE mutations occur in about 5%-10% of endometrial cancers. Despite some more aggressive histopathologic findings (eg, higher grade, deeper myometrial invasion, positive lymphovascular space invasion), recurrences rarely occur, and patients with POLE mutations have the best prognosis of the four molecular subtypes, with a 5-year recurrence-free survival of 92%-100%.3
The mismatch repair–deficient (MMRd) subtype are endometrial cancers with abnormalities in any of the mismatch repair proteins (MLH1, PMS2, MSH2, MSH6). These alterations may result from hereditary or somatic mutations in any of the MMR genes or epigenetic changes in the MLH1 promoter. Germ-line mutations are associated with Lynch syndrome; thus, patients found to have a germ-line mutation in any of the MMR genes necessitate a genetics referral. The MMRd subtype accounts for about 20%-30% of endometrial cancers, and patients with MMRd tumors have an intermediate prognosis, with a 5-year recurrence-free survival of about 70%.3. These tumors are more responsive to the use of immunotherapy checkpoint inhibitors. Two recent landmark trials showed improved outcomes in patients with advanced MMRd endometrial cancer treated with immune checkpoint inhibitors in addition to standard chemotherapy.4,5
The worst prognosis belongs to the copy number high subgroup, which accounts for approximately 10% of endometrial cancers. Five-year recurrence-free survival is ~50%.3 These tumors often contain TP53 mutations and are composed of aggressive histologies, such as serous, clear cell, high-grade endometrioid, and carcinosarcomas. Recent data suggests that human epidermal growth factor receptor 2 (HER2) amplification may also be prevalent in this subgroup.6
Endometrial cancers that lack any of the above alterations fall into the no specific molecular profile (NSMP) or copy number low subgroup. Mutations in other genes, such as PTEN, PIK3CA, CTNNB1, KRAS, and ARID1A, are often present in these tumors. As the most common subtype, this heterogeneous group accounts for about 50% of all endometrial cancers. These tumors frequently comprise endometrioid histology with estrogen and progesterone receptor positivity, high rates of response to hormonal therapy, and an overall intermediate to favorable prognosis, with a 5-year recurrence-free survival of ~75%.3
The use of whole-genome sequencing in TCGA limits the clinical applicability of testing because of the cost and complex methodologies involved. Multiple algorithms have been developed in the interim that approximate TCGA subtypes using relatively less expensive and more widely available testing methods, such as immunohistochemistry and next-generation sequencing. In the ProMisE algorithm, immunohistochemistry for p53 and MMR proteins is used as a surrogate for copy number high and MMRd tumors, respectively, and targeted sequencing is used to identify POLE mutations.7
Full molecular classification of endometrial tumors provides important prognostic information and allows for incorporation into treatment planning. To this end, the new 2023 International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging incorporates an option for the addition of molecular subtype, with the stance that it allows for better prognostic prediction.8 While complete molecular classification is not required, it is encouraged. Furthermore, several clinical trials are currently investigating different treatment regimens based on these distinct molecular profiles.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15(1):10-17.
2. Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
3. León-Castillo A et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncology. 2020;38(29):3388-3397.
4. Mirza MR et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
5. Eskander RN et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170.
6. Talia KL et al. The role of HER2 as a therapeutic biomarker in gynaecological malignancy: Potential for use beyond uterine serous carcinoma. Pathology. 2023;55(1):8-18.
7. Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Annals Oncology. 2018;29(5):1180-1188.
8. Berek JS et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394.
Historically, endometrial cancer has been classified as type I or type II. Type I endometrial cancers are typically estrogen driven, low grade, with endometrioid histology, and have a more favorable prognosis. In contrast, type II endometrial cancers are typically high grade, have more aggressive histologies (eg, serous or clear cell), and have a poorer prognosis.1
While this system provides a helpful schema for understanding endometrial cancers, it fails to represent the immense variation of biologic behavior and outcomes in endometrial cancers and oversimplifies what has come to be understood as a complex and molecularly diverse disease.
In 2013, The Cancer Genome Atlas (TCGA) performed genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas. They identified four categories with distinct genetic profiles corresponding to clinical outcomes: 1) DNA polymerase epsilon (POLE) mutated; 2) mismatch repair deficient; 3) copy number high/p53 abnormal; and 4) copy number low/no specific molecular profile.2 By providing both predictive and prognostic information, these molecular features may be incorporated into treatment planning decisions in the future.
The POLE-mutated subtype are endometrial cancers with recurrent mutations in the POLE gene, which is involved in DNA replication and repair. POLE mutations occur in about 5%-10% of endometrial cancers. Despite some more aggressive histopathologic findings (eg, higher grade, deeper myometrial invasion, positive lymphovascular space invasion), recurrences rarely occur, and patients with POLE mutations have the best prognosis of the four molecular subtypes, with a 5-year recurrence-free survival of 92%-100%.3
The mismatch repair–deficient (MMRd) subtype are endometrial cancers with abnormalities in any of the mismatch repair proteins (MLH1, PMS2, MSH2, MSH6). These alterations may result from hereditary or somatic mutations in any of the MMR genes or epigenetic changes in the MLH1 promoter. Germ-line mutations are associated with Lynch syndrome; thus, patients found to have a germ-line mutation in any of the MMR genes necessitate a genetics referral. The MMRd subtype accounts for about 20%-30% of endometrial cancers, and patients with MMRd tumors have an intermediate prognosis, with a 5-year recurrence-free survival of about 70%.3. These tumors are more responsive to the use of immunotherapy checkpoint inhibitors. Two recent landmark trials showed improved outcomes in patients with advanced MMRd endometrial cancer treated with immune checkpoint inhibitors in addition to standard chemotherapy.4,5
The worst prognosis belongs to the copy number high subgroup, which accounts for approximately 10% of endometrial cancers. Five-year recurrence-free survival is ~50%.3 These tumors often contain TP53 mutations and are composed of aggressive histologies, such as serous, clear cell, high-grade endometrioid, and carcinosarcomas. Recent data suggests that human epidermal growth factor receptor 2 (HER2) amplification may also be prevalent in this subgroup.6
Endometrial cancers that lack any of the above alterations fall into the no specific molecular profile (NSMP) or copy number low subgroup. Mutations in other genes, such as PTEN, PIK3CA, CTNNB1, KRAS, and ARID1A, are often present in these tumors. As the most common subtype, this heterogeneous group accounts for about 50% of all endometrial cancers. These tumors frequently comprise endometrioid histology with estrogen and progesterone receptor positivity, high rates of response to hormonal therapy, and an overall intermediate to favorable prognosis, with a 5-year recurrence-free survival of ~75%.3
The use of whole-genome sequencing in TCGA limits the clinical applicability of testing because of the cost and complex methodologies involved. Multiple algorithms have been developed in the interim that approximate TCGA subtypes using relatively less expensive and more widely available testing methods, such as immunohistochemistry and next-generation sequencing. In the ProMisE algorithm, immunohistochemistry for p53 and MMR proteins is used as a surrogate for copy number high and MMRd tumors, respectively, and targeted sequencing is used to identify POLE mutations.7
Full molecular classification of endometrial tumors provides important prognostic information and allows for incorporation into treatment planning. To this end, the new 2023 International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging incorporates an option for the addition of molecular subtype, with the stance that it allows for better prognostic prediction.8 While complete molecular classification is not required, it is encouraged. Furthermore, several clinical trials are currently investigating different treatment regimens based on these distinct molecular profiles.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15(1):10-17.
2. Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
3. León-Castillo A et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncology. 2020;38(29):3388-3397.
4. Mirza MR et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
5. Eskander RN et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170.
6. Talia KL et al. The role of HER2 as a therapeutic biomarker in gynaecological malignancy: Potential for use beyond uterine serous carcinoma. Pathology. 2023;55(1):8-18.
7. Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Annals Oncology. 2018;29(5):1180-1188.
8. Berek JS et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394.
Historically, endometrial cancer has been classified as type I or type II. Type I endometrial cancers are typically estrogen driven, low grade, with endometrioid histology, and have a more favorable prognosis. In contrast, type II endometrial cancers are typically high grade, have more aggressive histologies (eg, serous or clear cell), and have a poorer prognosis.1
While this system provides a helpful schema for understanding endometrial cancers, it fails to represent the immense variation of biologic behavior and outcomes in endometrial cancers and oversimplifies what has come to be understood as a complex and molecularly diverse disease.
In 2013, The Cancer Genome Atlas (TCGA) performed genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas. They identified four categories with distinct genetic profiles corresponding to clinical outcomes: 1) DNA polymerase epsilon (POLE) mutated; 2) mismatch repair deficient; 3) copy number high/p53 abnormal; and 4) copy number low/no specific molecular profile.2 By providing both predictive and prognostic information, these molecular features may be incorporated into treatment planning decisions in the future.
The POLE-mutated subtype are endometrial cancers with recurrent mutations in the POLE gene, which is involved in DNA replication and repair. POLE mutations occur in about 5%-10% of endometrial cancers. Despite some more aggressive histopathologic findings (eg, higher grade, deeper myometrial invasion, positive lymphovascular space invasion), recurrences rarely occur, and patients with POLE mutations have the best prognosis of the four molecular subtypes, with a 5-year recurrence-free survival of 92%-100%.3
The mismatch repair–deficient (MMRd) subtype are endometrial cancers with abnormalities in any of the mismatch repair proteins (MLH1, PMS2, MSH2, MSH6). These alterations may result from hereditary or somatic mutations in any of the MMR genes or epigenetic changes in the MLH1 promoter. Germ-line mutations are associated with Lynch syndrome; thus, patients found to have a germ-line mutation in any of the MMR genes necessitate a genetics referral. The MMRd subtype accounts for about 20%-30% of endometrial cancers, and patients with MMRd tumors have an intermediate prognosis, with a 5-year recurrence-free survival of about 70%.3. These tumors are more responsive to the use of immunotherapy checkpoint inhibitors. Two recent landmark trials showed improved outcomes in patients with advanced MMRd endometrial cancer treated with immune checkpoint inhibitors in addition to standard chemotherapy.4,5
The worst prognosis belongs to the copy number high subgroup, which accounts for approximately 10% of endometrial cancers. Five-year recurrence-free survival is ~50%.3 These tumors often contain TP53 mutations and are composed of aggressive histologies, such as serous, clear cell, high-grade endometrioid, and carcinosarcomas. Recent data suggests that human epidermal growth factor receptor 2 (HER2) amplification may also be prevalent in this subgroup.6
Endometrial cancers that lack any of the above alterations fall into the no specific molecular profile (NSMP) or copy number low subgroup. Mutations in other genes, such as PTEN, PIK3CA, CTNNB1, KRAS, and ARID1A, are often present in these tumors. As the most common subtype, this heterogeneous group accounts for about 50% of all endometrial cancers. These tumors frequently comprise endometrioid histology with estrogen and progesterone receptor positivity, high rates of response to hormonal therapy, and an overall intermediate to favorable prognosis, with a 5-year recurrence-free survival of ~75%.3
The use of whole-genome sequencing in TCGA limits the clinical applicability of testing because of the cost and complex methodologies involved. Multiple algorithms have been developed in the interim that approximate TCGA subtypes using relatively less expensive and more widely available testing methods, such as immunohistochemistry and next-generation sequencing. In the ProMisE algorithm, immunohistochemistry for p53 and MMR proteins is used as a surrogate for copy number high and MMRd tumors, respectively, and targeted sequencing is used to identify POLE mutations.7
Full molecular classification of endometrial tumors provides important prognostic information and allows for incorporation into treatment planning. To this end, the new 2023 International Federation of Gynecology and Obstetrics (FIGO) endometrial cancer staging incorporates an option for the addition of molecular subtype, with the stance that it allows for better prognostic prediction.8 While complete molecular classification is not required, it is encouraged. Furthermore, several clinical trials are currently investigating different treatment regimens based on these distinct molecular profiles.
Dr. Haag is a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, University of North Carolina Hospitals, Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill. They have no conflicts of interest.
References
1. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15(1):10-17.
2. Kandoth C et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73.
3. León-Castillo A et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncology. 2020;38(29):3388-3397.
4. Mirza MR et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
5. Eskander RN et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159-2170.
6. Talia KL et al. The role of HER2 as a therapeutic biomarker in gynaecological malignancy: Potential for use beyond uterine serous carcinoma. Pathology. 2023;55(1):8-18.
7. Kommoss S et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Annals Oncology. 2018;29(5):1180-1188.
8. Berek JS et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383-394.
Hypofractionated Radiotherapy Limits Toxic Effects in Cervical Cancer
TOPLINE:
results from the phase 2 POHIM-CCRT trial suggested.
METHODOLOGY:
- To date, no studies have assessed the treatment outcomes and toxic effects of hypofractionated IMRT following radical hysterectomy in patients with cervical cancer undergoing curative radiotherapy.
- The team analyzed outcomes from 79 patients undergoing hypofractionated IMRT for cervical cancer after radical hysterectomy and pelvic lymph node dissection.
- Patients were a median age of 48; 29.5% had stage IB to IIA disease, another 29.5% had stage IIB disease, and 41% had stage III disease. Patients also had at least one of the following criteria following radical hysterectomy and pelvic lymph node dissection: lymph node metastasis (39.7%), parametrial invasion (54.4%), and positive resection margin (5.1%).
- The prescribed dose to the planning target volume was 40 Gy, delivered in 16 fractions to the whole pelvis, with any type of IMRT permitted. Overall, 71 patients also underwent concurrent weekly cisplatin (40 mg/m2 of body surface area for three cycles), and eight received fluorouracil (1000 mg/m2 on days 1-5) with cisplatin (60 mg/m2 for two cycles).
- The primary endpoint was the incidence of acute grade 3 or higher gastrointestinal tract, genitourinary, and hematologic toxic effects during radiotherapy or within 3 months of completing radiotherapy.
TAKEAWAY:
- After radiotherapy, only two patients (2.5%) experienced acute grade 3 or higher toxic effects. One was hospitalized for enterocolitis on the last day of radiotherapy and developed grade 3 anemia 3 months after completing radiotherapy; the other experienced hematologic toxic effects and also developed grade 3 anemia 3 months after completing radiotherapy.
- No patients experienced late grade 3 or higher toxic effects.
- When assessing toxic effects of any grade, acute and late gastrointestinal tract toxicities occurred in 76% and 31.6% of patients, respectively; acute and late genitourinary toxicities, all grade 1, occurred in 19% and 24.1% of patients, respectively; and hematologic toxicities occurred in 29.1% and 6.3% of patients, respectively.
- Overall, at 3 years, 79.3% of patients were disease-free and 98% were alive. After a median follow-up of 43 months, 16 patients (20.3%) experienced disease recurrence, four of whom were salvaged and three of whom died.
IN PRACTICE:
“This nonrandomized controlled trial is the first prospective trial, to our knowledge, to show acceptable acute toxic effects of hypofractionated IMRT for cervical cancer in a postoperative concurrent chemoradiotherapy setting,” the authors said, adding that the rate of grade 3 or higher acute toxic effects of 2.5% reported in this study was “substantially lower than our initial hypothesis of less than 15%.”
However , in an accompanying editorial, Mark E. Bernard, MD, of the University of Kentucky College of Medicine, Lexington, highlighted caveats to the study design and raised two core questions: “Should acute toxic effects be the primary endpoint of a single-group, phase 2 study using hypofractionation with fewer cycles of concurrent chemotherapy? Should the primary endpoint rather have been a cancer control endpoint, such as disease-free survival, overall survival, or local control?”
Still, Dr. Bernard wrote, “This trial does help lay the foundation for future pelvic hypofractionated trials with concurrent chemotherapy, especially for gynecological malignant tumors.”
SOURCE:
The research, led by Won Park, MD, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, was published in JAMA Oncology.
LIMITATIONS:
The trial is a single-arm study, with a short follow-up time. In the editorial, Bernard listed several limitations, including the fact that patients received fewer cycles of concurrent chemotherapy than what’s typically given in this population.
DISCLOSURES:
No funding or relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
TOPLINE:
results from the phase 2 POHIM-CCRT trial suggested.
METHODOLOGY:
- To date, no studies have assessed the treatment outcomes and toxic effects of hypofractionated IMRT following radical hysterectomy in patients with cervical cancer undergoing curative radiotherapy.
- The team analyzed outcomes from 79 patients undergoing hypofractionated IMRT for cervical cancer after radical hysterectomy and pelvic lymph node dissection.
- Patients were a median age of 48; 29.5% had stage IB to IIA disease, another 29.5% had stage IIB disease, and 41% had stage III disease. Patients also had at least one of the following criteria following radical hysterectomy and pelvic lymph node dissection: lymph node metastasis (39.7%), parametrial invasion (54.4%), and positive resection margin (5.1%).
- The prescribed dose to the planning target volume was 40 Gy, delivered in 16 fractions to the whole pelvis, with any type of IMRT permitted. Overall, 71 patients also underwent concurrent weekly cisplatin (40 mg/m2 of body surface area for three cycles), and eight received fluorouracil (1000 mg/m2 on days 1-5) with cisplatin (60 mg/m2 for two cycles).
- The primary endpoint was the incidence of acute grade 3 or higher gastrointestinal tract, genitourinary, and hematologic toxic effects during radiotherapy or within 3 months of completing radiotherapy.
TAKEAWAY:
- After radiotherapy, only two patients (2.5%) experienced acute grade 3 or higher toxic effects. One was hospitalized for enterocolitis on the last day of radiotherapy and developed grade 3 anemia 3 months after completing radiotherapy; the other experienced hematologic toxic effects and also developed grade 3 anemia 3 months after completing radiotherapy.
- No patients experienced late grade 3 or higher toxic effects.
- When assessing toxic effects of any grade, acute and late gastrointestinal tract toxicities occurred in 76% and 31.6% of patients, respectively; acute and late genitourinary toxicities, all grade 1, occurred in 19% and 24.1% of patients, respectively; and hematologic toxicities occurred in 29.1% and 6.3% of patients, respectively.
- Overall, at 3 years, 79.3% of patients were disease-free and 98% were alive. After a median follow-up of 43 months, 16 patients (20.3%) experienced disease recurrence, four of whom were salvaged and three of whom died.
IN PRACTICE:
“This nonrandomized controlled trial is the first prospective trial, to our knowledge, to show acceptable acute toxic effects of hypofractionated IMRT for cervical cancer in a postoperative concurrent chemoradiotherapy setting,” the authors said, adding that the rate of grade 3 or higher acute toxic effects of 2.5% reported in this study was “substantially lower than our initial hypothesis of less than 15%.”
However , in an accompanying editorial, Mark E. Bernard, MD, of the University of Kentucky College of Medicine, Lexington, highlighted caveats to the study design and raised two core questions: “Should acute toxic effects be the primary endpoint of a single-group, phase 2 study using hypofractionation with fewer cycles of concurrent chemotherapy? Should the primary endpoint rather have been a cancer control endpoint, such as disease-free survival, overall survival, or local control?”
Still, Dr. Bernard wrote, “This trial does help lay the foundation for future pelvic hypofractionated trials with concurrent chemotherapy, especially for gynecological malignant tumors.”
SOURCE:
The research, led by Won Park, MD, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, was published in JAMA Oncology.
LIMITATIONS:
The trial is a single-arm study, with a short follow-up time. In the editorial, Bernard listed several limitations, including the fact that patients received fewer cycles of concurrent chemotherapy than what’s typically given in this population.
DISCLOSURES:
No funding or relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
TOPLINE:
results from the phase 2 POHIM-CCRT trial suggested.
METHODOLOGY:
- To date, no studies have assessed the treatment outcomes and toxic effects of hypofractionated IMRT following radical hysterectomy in patients with cervical cancer undergoing curative radiotherapy.
- The team analyzed outcomes from 79 patients undergoing hypofractionated IMRT for cervical cancer after radical hysterectomy and pelvic lymph node dissection.
- Patients were a median age of 48; 29.5% had stage IB to IIA disease, another 29.5% had stage IIB disease, and 41% had stage III disease. Patients also had at least one of the following criteria following radical hysterectomy and pelvic lymph node dissection: lymph node metastasis (39.7%), parametrial invasion (54.4%), and positive resection margin (5.1%).
- The prescribed dose to the planning target volume was 40 Gy, delivered in 16 fractions to the whole pelvis, with any type of IMRT permitted. Overall, 71 patients also underwent concurrent weekly cisplatin (40 mg/m2 of body surface area for three cycles), and eight received fluorouracil (1000 mg/m2 on days 1-5) with cisplatin (60 mg/m2 for two cycles).
- The primary endpoint was the incidence of acute grade 3 or higher gastrointestinal tract, genitourinary, and hematologic toxic effects during radiotherapy or within 3 months of completing radiotherapy.
TAKEAWAY:
- After radiotherapy, only two patients (2.5%) experienced acute grade 3 or higher toxic effects. One was hospitalized for enterocolitis on the last day of radiotherapy and developed grade 3 anemia 3 months after completing radiotherapy; the other experienced hematologic toxic effects and also developed grade 3 anemia 3 months after completing radiotherapy.
- No patients experienced late grade 3 or higher toxic effects.
- When assessing toxic effects of any grade, acute and late gastrointestinal tract toxicities occurred in 76% and 31.6% of patients, respectively; acute and late genitourinary toxicities, all grade 1, occurred in 19% and 24.1% of patients, respectively; and hematologic toxicities occurred in 29.1% and 6.3% of patients, respectively.
- Overall, at 3 years, 79.3% of patients were disease-free and 98% were alive. After a median follow-up of 43 months, 16 patients (20.3%) experienced disease recurrence, four of whom were salvaged and three of whom died.
IN PRACTICE:
“This nonrandomized controlled trial is the first prospective trial, to our knowledge, to show acceptable acute toxic effects of hypofractionated IMRT for cervical cancer in a postoperative concurrent chemoradiotherapy setting,” the authors said, adding that the rate of grade 3 or higher acute toxic effects of 2.5% reported in this study was “substantially lower than our initial hypothesis of less than 15%.”
However , in an accompanying editorial, Mark E. Bernard, MD, of the University of Kentucky College of Medicine, Lexington, highlighted caveats to the study design and raised two core questions: “Should acute toxic effects be the primary endpoint of a single-group, phase 2 study using hypofractionation with fewer cycles of concurrent chemotherapy? Should the primary endpoint rather have been a cancer control endpoint, such as disease-free survival, overall survival, or local control?”
Still, Dr. Bernard wrote, “This trial does help lay the foundation for future pelvic hypofractionated trials with concurrent chemotherapy, especially for gynecological malignant tumors.”
SOURCE:
The research, led by Won Park, MD, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, was published in JAMA Oncology.
LIMITATIONS:
The trial is a single-arm study, with a short follow-up time. In the editorial, Bernard listed several limitations, including the fact that patients received fewer cycles of concurrent chemotherapy than what’s typically given in this population.
DISCLOSURES:
No funding or relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
US Researchers Call for Robust Studies Into Dequalinium, a Bacterial Vaginosis Therapy Common in Europe
Interest is growing in a standard European treatment for bacterial vaginosis (BV).
In a commentary published in JAMA Network Open, researchers and doctors from the Johns Hopkins University School of Medicine in Baltimore and the University of Maryland, Baltimore, urged clinical trials in the United States to determine if dequalinium chloride — an antiseptic that inhibits the growth of bacteria and fungi — is on par with or better than treatments currently available.
Dequalinium has been used throughout Europe for decades and is recommended as an alternative or second-line BV treatment by the World Health Organization; the International Society for the Study of Vulvovaginal Disease; and the Austrian, German, Portuguese, Spanish, and Swiss Societies of Gynecology and Obstetrics. However, the product has not been approved for clinical use in the United States, no trials studying the drug have been registered on ClinicalTrials.gov, and the US Food and Drug Administration has not received an application for approval, according to agency records.
Treatments in the United States for BV include metronidazole and clindamycin that, while effective, have a recurrence rate of up to 60%.
“Current treatments for bacterial vaginosis often fall short, primarily due to frequent recurrences after treatment,” said Rebecca M. Brotman, PhD, MPH, a professor in the Department of Epidemiology and Public Health at the Institute for Genome Sciences at the University of Maryland School of Medicine, and the corresponding author of the commentary.
More than 40% of people with recurrent BV do not receive adequate treatment, according to Caroline M. Mitchell, MD, MPH, director of the Vulvovaginal Disorders Program at Massachusetts General Hospital Vincent Center for Reproductive Biology, Boston, Massachusetts.
“BV is very disruptive to people’s daily lives and causes significant distress,” said Dr. Mitchell, who was not a coauthor of the new article. “Additionally, BV is associated with higher risk for HPV [human papillomavirus] infection, progression of HPV to cervical dysplasia, as well as risk for acquisition of other sexually transmitted infections.”
Dr. Mitchell said she hopes a recent trial from Europe comparing dequalinium chloride to metronidazole spurs researchers to study its efficacy and safety among women in the United States.
“Dequalinium has some antifungal activity, so it might offer a lower chance of yeast infection after treatment, which is important because posttreatment vulvovaginal candidiasis is one of the downsides to conventional antibiotic treatments,” Dr. Mitchell said.
The recent clinical trial included 147 premenopausal women with BV who received 10 mg of dequalinium per day for 6 days or oral metronidazole (500 mg twice daily for 7 days).
Dr. Brotman said any studies in the United States would need to examine long-term recurrence of vaginosis after treatment with dequalinium chloride and its use during pregnancy.
The study was funded by various grants from the National Institutes of Health and the Gates Foundation. Various authors reported receiving royalties from UpToDate outside the submitted work or receiving a donation of sexually transmitted infection testing kits from Hologic for a study outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Interest is growing in a standard European treatment for bacterial vaginosis (BV).
In a commentary published in JAMA Network Open, researchers and doctors from the Johns Hopkins University School of Medicine in Baltimore and the University of Maryland, Baltimore, urged clinical trials in the United States to determine if dequalinium chloride — an antiseptic that inhibits the growth of bacteria and fungi — is on par with or better than treatments currently available.
Dequalinium has been used throughout Europe for decades and is recommended as an alternative or second-line BV treatment by the World Health Organization; the International Society for the Study of Vulvovaginal Disease; and the Austrian, German, Portuguese, Spanish, and Swiss Societies of Gynecology and Obstetrics. However, the product has not been approved for clinical use in the United States, no trials studying the drug have been registered on ClinicalTrials.gov, and the US Food and Drug Administration has not received an application for approval, according to agency records.
Treatments in the United States for BV include metronidazole and clindamycin that, while effective, have a recurrence rate of up to 60%.
“Current treatments for bacterial vaginosis often fall short, primarily due to frequent recurrences after treatment,” said Rebecca M. Brotman, PhD, MPH, a professor in the Department of Epidemiology and Public Health at the Institute for Genome Sciences at the University of Maryland School of Medicine, and the corresponding author of the commentary.
More than 40% of people with recurrent BV do not receive adequate treatment, according to Caroline M. Mitchell, MD, MPH, director of the Vulvovaginal Disorders Program at Massachusetts General Hospital Vincent Center for Reproductive Biology, Boston, Massachusetts.
“BV is very disruptive to people’s daily lives and causes significant distress,” said Dr. Mitchell, who was not a coauthor of the new article. “Additionally, BV is associated with higher risk for HPV [human papillomavirus] infection, progression of HPV to cervical dysplasia, as well as risk for acquisition of other sexually transmitted infections.”
Dr. Mitchell said she hopes a recent trial from Europe comparing dequalinium chloride to metronidazole spurs researchers to study its efficacy and safety among women in the United States.
“Dequalinium has some antifungal activity, so it might offer a lower chance of yeast infection after treatment, which is important because posttreatment vulvovaginal candidiasis is one of the downsides to conventional antibiotic treatments,” Dr. Mitchell said.
The recent clinical trial included 147 premenopausal women with BV who received 10 mg of dequalinium per day for 6 days or oral metronidazole (500 mg twice daily for 7 days).
Dr. Brotman said any studies in the United States would need to examine long-term recurrence of vaginosis after treatment with dequalinium chloride and its use during pregnancy.
The study was funded by various grants from the National Institutes of Health and the Gates Foundation. Various authors reported receiving royalties from UpToDate outside the submitted work or receiving a donation of sexually transmitted infection testing kits from Hologic for a study outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Interest is growing in a standard European treatment for bacterial vaginosis (BV).
In a commentary published in JAMA Network Open, researchers and doctors from the Johns Hopkins University School of Medicine in Baltimore and the University of Maryland, Baltimore, urged clinical trials in the United States to determine if dequalinium chloride — an antiseptic that inhibits the growth of bacteria and fungi — is on par with or better than treatments currently available.
Dequalinium has been used throughout Europe for decades and is recommended as an alternative or second-line BV treatment by the World Health Organization; the International Society for the Study of Vulvovaginal Disease; and the Austrian, German, Portuguese, Spanish, and Swiss Societies of Gynecology and Obstetrics. However, the product has not been approved for clinical use in the United States, no trials studying the drug have been registered on ClinicalTrials.gov, and the US Food and Drug Administration has not received an application for approval, according to agency records.
Treatments in the United States for BV include metronidazole and clindamycin that, while effective, have a recurrence rate of up to 60%.
“Current treatments for bacterial vaginosis often fall short, primarily due to frequent recurrences after treatment,” said Rebecca M. Brotman, PhD, MPH, a professor in the Department of Epidemiology and Public Health at the Institute for Genome Sciences at the University of Maryland School of Medicine, and the corresponding author of the commentary.
More than 40% of people with recurrent BV do not receive adequate treatment, according to Caroline M. Mitchell, MD, MPH, director of the Vulvovaginal Disorders Program at Massachusetts General Hospital Vincent Center for Reproductive Biology, Boston, Massachusetts.
“BV is very disruptive to people’s daily lives and causes significant distress,” said Dr. Mitchell, who was not a coauthor of the new article. “Additionally, BV is associated with higher risk for HPV [human papillomavirus] infection, progression of HPV to cervical dysplasia, as well as risk for acquisition of other sexually transmitted infections.”
Dr. Mitchell said she hopes a recent trial from Europe comparing dequalinium chloride to metronidazole spurs researchers to study its efficacy and safety among women in the United States.
“Dequalinium has some antifungal activity, so it might offer a lower chance of yeast infection after treatment, which is important because posttreatment vulvovaginal candidiasis is one of the downsides to conventional antibiotic treatments,” Dr. Mitchell said.
The recent clinical trial included 147 premenopausal women with BV who received 10 mg of dequalinium per day for 6 days or oral metronidazole (500 mg twice daily for 7 days).
Dr. Brotman said any studies in the United States would need to examine long-term recurrence of vaginosis after treatment with dequalinium chloride and its use during pregnancy.
The study was funded by various grants from the National Institutes of Health and the Gates Foundation. Various authors reported receiving royalties from UpToDate outside the submitted work or receiving a donation of sexually transmitted infection testing kits from Hologic for a study outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Starting Points if Patient Chooses Medication Abortion
An evolving legal landscape surrounding medication abortion has left physicians with as many questions as patients. A panel of clinicians at the annual meeting of the American College of Physicians offered guidance on how to help patients who choose medication abortion, which is available to women until 10 weeks of gestation.
Approved in 2000, abortion pills have become the most common method for terminating pregnancy in the United States, accounting for 63% of all abortions in 2023. The US Supreme Court is reviewing access to medication abortions, with a decision expected within months.
According to the Guttmacher Institute, 29 states as of February restrict access to medication abortion, either by limiting or banning the use of the drugs or by curtailing who can prescribe them and under what circumstances.
First, Determine Gestational Age
Cynthia Chuang, MD, MSc, an internist and professor of medicine at Penn State College of Medicine in Hershey, Pennsylvania, said in most cases, a woman can pinpoint the date of the first day of her last menstrual period, and the physician can confirm gestational age is 70 days or less. An ultrasound should be performed if the date of the last period is uncertain, she said, or if ectopic pregnancy is suspected, periods are irregular, or ultrasound is legally required in the state.
Women are not eligible for abortion pills if they have a bleeding or clotting disorder, have an intrauterine device, have adrenal insufficiency or chronic steroid use, or have porphyria or hemoglobin levels < 9 g/dL, she said.
“If the person has a known hemoglobin of less than 9, we would suggest that person have a procedural abortion,” Dr. Chuang said.
Components of Medication
Patients first receive 200 mg of oral mifepristone, which separates the pregnancy from the uterine wall. “Typically, people don’t experience side effects from the mifepristone alone,” Dr. Chaung said.
In the next 48 hours, the patient takes 800 mcg of misoprostol, either vaginally or buccally. “Patients can expect heavy cramping and bleeding 1-2 days after taking misoprostol,” she said. The bleeding should gradually subside over the following week or 2, she said. If the gestational age is 9 weeks or more, a second dose of misoprostol can be administered 3-6 hours after the first dose to improve efficacy.
If mifepristone is not available, misoprostol can be used alone in three doses spaced 3 hours apart, Dr. Chuang said.
The failure rate for mifepristone plus misoprostol has been calculated at 2% if the gestational age is less than 7 weeks. For misoprostol alone, the failure rate varies by the study, depending on gestational age and population analyzed, Dr. Chuang said. A 2023 meta-analysis in Contraception put the failure rate at 11%.
When to Call the Clinician
Dr. Chuang said clinicians should instruct women who have taken abortion pills to call the clinic if they are soaking through two sanitary pads an hour for more than 1 hour, if there is little to no bleeding, or if they think the medication isn’t working after taking the full regimen.
Mindy Sobota, MD, MS, an internist and associate professor of medicine at the Warren Alpert Medical School of Brown University in Providence, Rhode Island, recommended a continuing medical education site for evidence-based guidance on how best to talk with women about medication abortions.
She also recommended physicians and patients consult the Plan C website for guidance on telehealth services and price information on receiving abortion pills by mail in every state. “It’s a very reliable and safe clearing house,” Dr. Sobota said.
Internists should let patients know that whatever their choices are around pregnancy, they are open to discussing those choices and helping them through the process, Dr. Sobota said, adding that she makes those statements in annual visits.
Alexandra Bachorik, MD, EdM, director of education in the Women’s Health Unit and assistant professor of medicine at Boston University Chobanian & Avedisian School of Medicine, Boston, Massachusetts, said patients in restrictive states who need financial help related to obtaining abortion services in a less restricted state can visit the National Network of Abortion Funds, which can help people with travel or housing expenses.
Facilitating access to a clinic in a less restrictive state may be helpful to people approaching a gestational age limit, she said.
Adelaide McClintock, MD, an internist and assistant professor of general internal medicine at the University of Washington School of Medicine in Seattle, Washington, noted that even clinicians who cannot legally prescribe abortion pills can support their patients by providing resources and counseling on options before, during, and after pregnancy. Those who live in restrictive states also can provide support to women who have traveled to less restrictive states for an abortion if they have postabortion complications, she said.
She recommended the Reproductive Health Access Project website for a comprehensive guide for evidence-based care and counseling patients about all choices in reproductive healthcare.
Panelists urged clinicians to get familiar with abortion-access resources and keep them at their fingertips in practice. Patient requests for the services are common, she noted, as “one in four women will have an abortion by the age of 45.”
Dr. Chuang, Dr. McClintock, Dr. Sobota, and Dr. Bachorik reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
An evolving legal landscape surrounding medication abortion has left physicians with as many questions as patients. A panel of clinicians at the annual meeting of the American College of Physicians offered guidance on how to help patients who choose medication abortion, which is available to women until 10 weeks of gestation.
Approved in 2000, abortion pills have become the most common method for terminating pregnancy in the United States, accounting for 63% of all abortions in 2023. The US Supreme Court is reviewing access to medication abortions, with a decision expected within months.
According to the Guttmacher Institute, 29 states as of February restrict access to medication abortion, either by limiting or banning the use of the drugs or by curtailing who can prescribe them and under what circumstances.
First, Determine Gestational Age
Cynthia Chuang, MD, MSc, an internist and professor of medicine at Penn State College of Medicine in Hershey, Pennsylvania, said in most cases, a woman can pinpoint the date of the first day of her last menstrual period, and the physician can confirm gestational age is 70 days or less. An ultrasound should be performed if the date of the last period is uncertain, she said, or if ectopic pregnancy is suspected, periods are irregular, or ultrasound is legally required in the state.
Women are not eligible for abortion pills if they have a bleeding or clotting disorder, have an intrauterine device, have adrenal insufficiency or chronic steroid use, or have porphyria or hemoglobin levels < 9 g/dL, she said.
“If the person has a known hemoglobin of less than 9, we would suggest that person have a procedural abortion,” Dr. Chuang said.
Components of Medication
Patients first receive 200 mg of oral mifepristone, which separates the pregnancy from the uterine wall. “Typically, people don’t experience side effects from the mifepristone alone,” Dr. Chaung said.
In the next 48 hours, the patient takes 800 mcg of misoprostol, either vaginally or buccally. “Patients can expect heavy cramping and bleeding 1-2 days after taking misoprostol,” she said. The bleeding should gradually subside over the following week or 2, she said. If the gestational age is 9 weeks or more, a second dose of misoprostol can be administered 3-6 hours after the first dose to improve efficacy.
If mifepristone is not available, misoprostol can be used alone in three doses spaced 3 hours apart, Dr. Chuang said.
The failure rate for mifepristone plus misoprostol has been calculated at 2% if the gestational age is less than 7 weeks. For misoprostol alone, the failure rate varies by the study, depending on gestational age and population analyzed, Dr. Chuang said. A 2023 meta-analysis in Contraception put the failure rate at 11%.
When to Call the Clinician
Dr. Chuang said clinicians should instruct women who have taken abortion pills to call the clinic if they are soaking through two sanitary pads an hour for more than 1 hour, if there is little to no bleeding, or if they think the medication isn’t working after taking the full regimen.
Mindy Sobota, MD, MS, an internist and associate professor of medicine at the Warren Alpert Medical School of Brown University in Providence, Rhode Island, recommended a continuing medical education site for evidence-based guidance on how best to talk with women about medication abortions.
She also recommended physicians and patients consult the Plan C website for guidance on telehealth services and price information on receiving abortion pills by mail in every state. “It’s a very reliable and safe clearing house,” Dr. Sobota said.
Internists should let patients know that whatever their choices are around pregnancy, they are open to discussing those choices and helping them through the process, Dr. Sobota said, adding that she makes those statements in annual visits.
Alexandra Bachorik, MD, EdM, director of education in the Women’s Health Unit and assistant professor of medicine at Boston University Chobanian & Avedisian School of Medicine, Boston, Massachusetts, said patients in restrictive states who need financial help related to obtaining abortion services in a less restricted state can visit the National Network of Abortion Funds, which can help people with travel or housing expenses.
Facilitating access to a clinic in a less restrictive state may be helpful to people approaching a gestational age limit, she said.
Adelaide McClintock, MD, an internist and assistant professor of general internal medicine at the University of Washington School of Medicine in Seattle, Washington, noted that even clinicians who cannot legally prescribe abortion pills can support their patients by providing resources and counseling on options before, during, and after pregnancy. Those who live in restrictive states also can provide support to women who have traveled to less restrictive states for an abortion if they have postabortion complications, she said.
She recommended the Reproductive Health Access Project website for a comprehensive guide for evidence-based care and counseling patients about all choices in reproductive healthcare.
Panelists urged clinicians to get familiar with abortion-access resources and keep them at their fingertips in practice. Patient requests for the services are common, she noted, as “one in four women will have an abortion by the age of 45.”
Dr. Chuang, Dr. McClintock, Dr. Sobota, and Dr. Bachorik reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
An evolving legal landscape surrounding medication abortion has left physicians with as many questions as patients. A panel of clinicians at the annual meeting of the American College of Physicians offered guidance on how to help patients who choose medication abortion, which is available to women until 10 weeks of gestation.
Approved in 2000, abortion pills have become the most common method for terminating pregnancy in the United States, accounting for 63% of all abortions in 2023. The US Supreme Court is reviewing access to medication abortions, with a decision expected within months.
According to the Guttmacher Institute, 29 states as of February restrict access to medication abortion, either by limiting or banning the use of the drugs or by curtailing who can prescribe them and under what circumstances.
First, Determine Gestational Age
Cynthia Chuang, MD, MSc, an internist and professor of medicine at Penn State College of Medicine in Hershey, Pennsylvania, said in most cases, a woman can pinpoint the date of the first day of her last menstrual period, and the physician can confirm gestational age is 70 days or less. An ultrasound should be performed if the date of the last period is uncertain, she said, or if ectopic pregnancy is suspected, periods are irregular, or ultrasound is legally required in the state.
Women are not eligible for abortion pills if they have a bleeding or clotting disorder, have an intrauterine device, have adrenal insufficiency or chronic steroid use, or have porphyria or hemoglobin levels < 9 g/dL, she said.
“If the person has a known hemoglobin of less than 9, we would suggest that person have a procedural abortion,” Dr. Chuang said.
Components of Medication
Patients first receive 200 mg of oral mifepristone, which separates the pregnancy from the uterine wall. “Typically, people don’t experience side effects from the mifepristone alone,” Dr. Chaung said.
In the next 48 hours, the patient takes 800 mcg of misoprostol, either vaginally or buccally. “Patients can expect heavy cramping and bleeding 1-2 days after taking misoprostol,” she said. The bleeding should gradually subside over the following week or 2, she said. If the gestational age is 9 weeks or more, a second dose of misoprostol can be administered 3-6 hours after the first dose to improve efficacy.
If mifepristone is not available, misoprostol can be used alone in three doses spaced 3 hours apart, Dr. Chuang said.
The failure rate for mifepristone plus misoprostol has been calculated at 2% if the gestational age is less than 7 weeks. For misoprostol alone, the failure rate varies by the study, depending on gestational age and population analyzed, Dr. Chuang said. A 2023 meta-analysis in Contraception put the failure rate at 11%.
When to Call the Clinician
Dr. Chuang said clinicians should instruct women who have taken abortion pills to call the clinic if they are soaking through two sanitary pads an hour for more than 1 hour, if there is little to no bleeding, or if they think the medication isn’t working after taking the full regimen.
Mindy Sobota, MD, MS, an internist and associate professor of medicine at the Warren Alpert Medical School of Brown University in Providence, Rhode Island, recommended a continuing medical education site for evidence-based guidance on how best to talk with women about medication abortions.
She also recommended physicians and patients consult the Plan C website for guidance on telehealth services and price information on receiving abortion pills by mail in every state. “It’s a very reliable and safe clearing house,” Dr. Sobota said.
Internists should let patients know that whatever their choices are around pregnancy, they are open to discussing those choices and helping them through the process, Dr. Sobota said, adding that she makes those statements in annual visits.
Alexandra Bachorik, MD, EdM, director of education in the Women’s Health Unit and assistant professor of medicine at Boston University Chobanian & Avedisian School of Medicine, Boston, Massachusetts, said patients in restrictive states who need financial help related to obtaining abortion services in a less restricted state can visit the National Network of Abortion Funds, which can help people with travel or housing expenses.
Facilitating access to a clinic in a less restrictive state may be helpful to people approaching a gestational age limit, she said.
Adelaide McClintock, MD, an internist and assistant professor of general internal medicine at the University of Washington School of Medicine in Seattle, Washington, noted that even clinicians who cannot legally prescribe abortion pills can support their patients by providing resources and counseling on options before, during, and after pregnancy. Those who live in restrictive states also can provide support to women who have traveled to less restrictive states for an abortion if they have postabortion complications, she said.
She recommended the Reproductive Health Access Project website for a comprehensive guide for evidence-based care and counseling patients about all choices in reproductive healthcare.
Panelists urged clinicians to get familiar with abortion-access resources and keep them at their fingertips in practice. Patient requests for the services are common, she noted, as “one in four women will have an abortion by the age of 45.”
Dr. Chuang, Dr. McClintock, Dr. Sobota, and Dr. Bachorik reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
FROM INTERNAL MEDICINE 2024
Intermittent Fasting + HIIT: Fitness Fad or Fix?
Let’s be honest: Although as physicians we have the power of the prescription pad, so much of health, in the end, comes down to lifestyle. Of course, taking a pill is often way easier than changing your longstanding habits. And what’s worse, doesn’t it always seem like the lifestyle stuff that is good for your health is unpleasant?
Two recent lifestyle interventions that I have tried and find really not enjoyable are time-restricted eating (also known as intermittent fasting) and high-intensity interval training, or HIIT. The former leaves me hangry for half the day; the latter is, well, it’s just really hard compared with my usual jog.
But given the rule of unpleasant lifestyle changes, I knew as soon as I saw this recent study what the result would be. What if we combined time-restricted eating with high-intensity interval training?
I’m referring to this study, appearing in PLOS ONE from Ranya Ameur and colleagues, which is a small trial that enrolled otherwise healthy women with a BMI > 30 and randomized them to one of three conditions.
First was time-restricted eating. Women in this group could eat whatever they wanted, but only from 8 a.m. to 4 p.m. daily.
Second: high-intensity functional training. This is a variant of high-intensity interval training which focuses a bit more on resistance exercise than on pure cardiovascular stuff but has the same vibe of doing brief bursts of intensive activity followed by a cool-down period.
Third: a combination of the two. Sounds rough to me.
The study was otherwise straightforward. At baseline, researchers collected data on body composition and dietary intake, and measured blood pressure, glucose, insulin, and lipid biomarkers. A 12-week intervention period followed, after which all of this stuff was measured again.
Now, you may have noticed that there is no control group in this study. We’ll come back to that — a few times.
Let me walk you through some of the outcomes here.
First off, body composition metrics. All three groups lost weight — on average, around 10% of body weight which, for a 12-week intervention, is fairly impressive. BMI and waist circumference went down as well, and, interestingly, much of the weight loss here was in fat mass, not fat-free mass.
Most interventions that lead to weight loss — and I’m including some of the newer drugs here — lead to both fat and muscle loss. That might not be as bad as it sounds; the truth is that muscle mass increases as fat increases because of the simple fact that if you’re carrying more weight when you walk around, your leg muscles get bigger. But to preserve muscle mass in the face of fat loss is sort of a Goldilocks finding, and, based on these results, there’s a suggestion that the high-intensity functional training helps to do just that.
The dietary intake findings were really surprising to me. Across the board, caloric intake decreased. It’s no surprise that time-restricted eating reduces calorie intake. That has been shown many times before and is probably the main reason it induces weight loss — less time to eat means you eat less.
But why would high-intensity functional training lead to lower caloric intake? Most people, myself included, get hungry after they exercise. In fact, one of the reasons it’s hard to lose weight with exercise alone is that we end up eating more calories to make up for what we lost during the exercise. This calorie reduction could be a unique effect of this type of exercise, but honestly this could also be something called the Hawthorne effect. Women in the study kept a food diary to track their intake, and the act of doing that might actually make you eat less. It makes it a little more annoying to snack a bit if you know you have to write it down. This is a situation where I would kill for a control group.
The lipid findings are also pretty striking, with around a 40% reduction in LDL across the board, and evidence of synergistic effects of combined TRE and high-intensity training on total cholesterol and triglycerides. This is like a statin level of effect — pretty impressive. Again, my heart pines for a control group, though.
Same story with glucose and insulin measures: an impressive reduction in fasting glucose and good evidence that the combination of time-restricted eating and high-intensity functional training reduces insulin levels and HOMA-IR as well.
Really the only thing that wasn’t very impressive was the change in blood pressure, with only modest decreases across the board.
Okay, so let’s take a breath after this high-intensity cerebral workout and put this all together. This was a small study, lacking a control group, but with large effect sizes in very relevant clinical areas. It confirms what we know about time-restricted eating — that it makes you eat less calories — and introduces the potential that vigorous exercise can not only magnify the benefits of time-restricted eating but maybe even mitigate some of the risks, like the risk for muscle loss. And of course, it comports with my central hypothesis, which is that the more unpleasant a lifestyle intervention is, the better it is for you. No pain, no gain, right?
Of course, I am being overly dogmatic. There are plenty of caveats. Wrestling bears is quite unpleasant and almost certainly bad for you. And there are even some pleasant things that are pretty good for you — like coffee and sex. And there are even people who find time-restricted eating and high-intensity training pleasurable. They are called masochists.
I’m joking. The truth is that Or, at least, much less painful. The trick is getting over the hump of change. If only there were a pill for that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Let’s be honest: Although as physicians we have the power of the prescription pad, so much of health, in the end, comes down to lifestyle. Of course, taking a pill is often way easier than changing your longstanding habits. And what’s worse, doesn’t it always seem like the lifestyle stuff that is good for your health is unpleasant?
Two recent lifestyle interventions that I have tried and find really not enjoyable are time-restricted eating (also known as intermittent fasting) and high-intensity interval training, or HIIT. The former leaves me hangry for half the day; the latter is, well, it’s just really hard compared with my usual jog.
But given the rule of unpleasant lifestyle changes, I knew as soon as I saw this recent study what the result would be. What if we combined time-restricted eating with high-intensity interval training?
I’m referring to this study, appearing in PLOS ONE from Ranya Ameur and colleagues, which is a small trial that enrolled otherwise healthy women with a BMI > 30 and randomized them to one of three conditions.
First was time-restricted eating. Women in this group could eat whatever they wanted, but only from 8 a.m. to 4 p.m. daily.
Second: high-intensity functional training. This is a variant of high-intensity interval training which focuses a bit more on resistance exercise than on pure cardiovascular stuff but has the same vibe of doing brief bursts of intensive activity followed by a cool-down period.
Third: a combination of the two. Sounds rough to me.
The study was otherwise straightforward. At baseline, researchers collected data on body composition and dietary intake, and measured blood pressure, glucose, insulin, and lipid biomarkers. A 12-week intervention period followed, after which all of this stuff was measured again.
Now, you may have noticed that there is no control group in this study. We’ll come back to that — a few times.
Let me walk you through some of the outcomes here.
First off, body composition metrics. All three groups lost weight — on average, around 10% of body weight which, for a 12-week intervention, is fairly impressive. BMI and waist circumference went down as well, and, interestingly, much of the weight loss here was in fat mass, not fat-free mass.
Most interventions that lead to weight loss — and I’m including some of the newer drugs here — lead to both fat and muscle loss. That might not be as bad as it sounds; the truth is that muscle mass increases as fat increases because of the simple fact that if you’re carrying more weight when you walk around, your leg muscles get bigger. But to preserve muscle mass in the face of fat loss is sort of a Goldilocks finding, and, based on these results, there’s a suggestion that the high-intensity functional training helps to do just that.
The dietary intake findings were really surprising to me. Across the board, caloric intake decreased. It’s no surprise that time-restricted eating reduces calorie intake. That has been shown many times before and is probably the main reason it induces weight loss — less time to eat means you eat less.
But why would high-intensity functional training lead to lower caloric intake? Most people, myself included, get hungry after they exercise. In fact, one of the reasons it’s hard to lose weight with exercise alone is that we end up eating more calories to make up for what we lost during the exercise. This calorie reduction could be a unique effect of this type of exercise, but honestly this could also be something called the Hawthorne effect. Women in the study kept a food diary to track their intake, and the act of doing that might actually make you eat less. It makes it a little more annoying to snack a bit if you know you have to write it down. This is a situation where I would kill for a control group.
The lipid findings are also pretty striking, with around a 40% reduction in LDL across the board, and evidence of synergistic effects of combined TRE and high-intensity training on total cholesterol and triglycerides. This is like a statin level of effect — pretty impressive. Again, my heart pines for a control group, though.
Same story with glucose and insulin measures: an impressive reduction in fasting glucose and good evidence that the combination of time-restricted eating and high-intensity functional training reduces insulin levels and HOMA-IR as well.
Really the only thing that wasn’t very impressive was the change in blood pressure, with only modest decreases across the board.
Okay, so let’s take a breath after this high-intensity cerebral workout and put this all together. This was a small study, lacking a control group, but with large effect sizes in very relevant clinical areas. It confirms what we know about time-restricted eating — that it makes you eat less calories — and introduces the potential that vigorous exercise can not only magnify the benefits of time-restricted eating but maybe even mitigate some of the risks, like the risk for muscle loss. And of course, it comports with my central hypothesis, which is that the more unpleasant a lifestyle intervention is, the better it is for you. No pain, no gain, right?
Of course, I am being overly dogmatic. There are plenty of caveats. Wrestling bears is quite unpleasant and almost certainly bad for you. And there are even some pleasant things that are pretty good for you — like coffee and sex. And there are even people who find time-restricted eating and high-intensity training pleasurable. They are called masochists.
I’m joking. The truth is that Or, at least, much less painful. The trick is getting over the hump of change. If only there were a pill for that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Let’s be honest: Although as physicians we have the power of the prescription pad, so much of health, in the end, comes down to lifestyle. Of course, taking a pill is often way easier than changing your longstanding habits. And what’s worse, doesn’t it always seem like the lifestyle stuff that is good for your health is unpleasant?
Two recent lifestyle interventions that I have tried and find really not enjoyable are time-restricted eating (also known as intermittent fasting) and high-intensity interval training, or HIIT. The former leaves me hangry for half the day; the latter is, well, it’s just really hard compared with my usual jog.
But given the rule of unpleasant lifestyle changes, I knew as soon as I saw this recent study what the result would be. What if we combined time-restricted eating with high-intensity interval training?
I’m referring to this study, appearing in PLOS ONE from Ranya Ameur and colleagues, which is a small trial that enrolled otherwise healthy women with a BMI > 30 and randomized them to one of three conditions.
First was time-restricted eating. Women in this group could eat whatever they wanted, but only from 8 a.m. to 4 p.m. daily.
Second: high-intensity functional training. This is a variant of high-intensity interval training which focuses a bit more on resistance exercise than on pure cardiovascular stuff but has the same vibe of doing brief bursts of intensive activity followed by a cool-down period.
Third: a combination of the two. Sounds rough to me.
The study was otherwise straightforward. At baseline, researchers collected data on body composition and dietary intake, and measured blood pressure, glucose, insulin, and lipid biomarkers. A 12-week intervention period followed, after which all of this stuff was measured again.
Now, you may have noticed that there is no control group in this study. We’ll come back to that — a few times.
Let me walk you through some of the outcomes here.
First off, body composition metrics. All three groups lost weight — on average, around 10% of body weight which, for a 12-week intervention, is fairly impressive. BMI and waist circumference went down as well, and, interestingly, much of the weight loss here was in fat mass, not fat-free mass.
Most interventions that lead to weight loss — and I’m including some of the newer drugs here — lead to both fat and muscle loss. That might not be as bad as it sounds; the truth is that muscle mass increases as fat increases because of the simple fact that if you’re carrying more weight when you walk around, your leg muscles get bigger. But to preserve muscle mass in the face of fat loss is sort of a Goldilocks finding, and, based on these results, there’s a suggestion that the high-intensity functional training helps to do just that.
The dietary intake findings were really surprising to me. Across the board, caloric intake decreased. It’s no surprise that time-restricted eating reduces calorie intake. That has been shown many times before and is probably the main reason it induces weight loss — less time to eat means you eat less.
But why would high-intensity functional training lead to lower caloric intake? Most people, myself included, get hungry after they exercise. In fact, one of the reasons it’s hard to lose weight with exercise alone is that we end up eating more calories to make up for what we lost during the exercise. This calorie reduction could be a unique effect of this type of exercise, but honestly this could also be something called the Hawthorne effect. Women in the study kept a food diary to track their intake, and the act of doing that might actually make you eat less. It makes it a little more annoying to snack a bit if you know you have to write it down. This is a situation where I would kill for a control group.
The lipid findings are also pretty striking, with around a 40% reduction in LDL across the board, and evidence of synergistic effects of combined TRE and high-intensity training on total cholesterol and triglycerides. This is like a statin level of effect — pretty impressive. Again, my heart pines for a control group, though.
Same story with glucose and insulin measures: an impressive reduction in fasting glucose and good evidence that the combination of time-restricted eating and high-intensity functional training reduces insulin levels and HOMA-IR as well.
Really the only thing that wasn’t very impressive was the change in blood pressure, with only modest decreases across the board.
Okay, so let’s take a breath after this high-intensity cerebral workout and put this all together. This was a small study, lacking a control group, but with large effect sizes in very relevant clinical areas. It confirms what we know about time-restricted eating — that it makes you eat less calories — and introduces the potential that vigorous exercise can not only magnify the benefits of time-restricted eating but maybe even mitigate some of the risks, like the risk for muscle loss. And of course, it comports with my central hypothesis, which is that the more unpleasant a lifestyle intervention is, the better it is for you. No pain, no gain, right?
Of course, I am being overly dogmatic. There are plenty of caveats. Wrestling bears is quite unpleasant and almost certainly bad for you. And there are even some pleasant things that are pretty good for you — like coffee and sex. And there are even people who find time-restricted eating and high-intensity training pleasurable. They are called masochists.
I’m joking. The truth is that Or, at least, much less painful. The trick is getting over the hump of change. If only there were a pill for that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
From Stigma to Support: Raising Awareness of Pelvic Organ Prolapse
Sherrie Palm, a patient advocate in Mukwonago, Wisconsin, learned in her 30s that she needed to educate herself about her own health. So when she discovered a walnut-sized lump coming out of her vagina in her mid-50s, she was stunned when her primary care provider (PCP) told her it was pelvic organ prolapse (POP), where one or more organs descend into the vaginal cavity.
“I was shocked,” Ms. Palm said. After searching online and discovering how prevalent POP was, her shock turned to anger. “I was blown away that it could be this common and I’d never heard of it,” she said. “I knew within 2 weeks that I had to do something to change the status quo.”
Ms. Palm eventually founded the nonprofit Association for Pelvic Organ Prolapse Support, or APOPS, complete with a forum where women can learn about POP and support one another. She said awareness has improved substantially since her diagnosis in 2007, but “we have a long way to go” because POP and vaginal health in general are so stigmatized.
Her website notes that about half of women with incontinence do not seek help, largely because of stigma. “The status quo is that PCPs do not POP screen,” she said. ObGyns may screen but often “because the patient has asked to be screened, they say it’s not that bad, come back and see me in a year, and do your Kegels,” Ms. Palm said.
Doctors who diagnose POP agree that the issue is often off PCPs’ radar.
“Primary care doctors are really in a time crunch, so this is one of the things that may not get addressed,” Jill Rabin, MD, vice chair of education and development in obstetrics and gynecology at Northwell Health in New York, said. Dr. Rabin is also head of urogynecology at Long Island Jewish Medical Center.
Ann Nwabuebo, PT, DPT, owner and founder of Body Connect Physical Therapy in Bethesda, Maryland, said social media has been shifting the attitude that pelvic health is a taboo subject. “It’s empowering people to seek care if they’re not finding physicians who are helping.”
But social media is also a double-edged sword, said Jenny LaCross, PT, DPT, PhD, a physical therapist at MOVE PT in Monroe, Michigan, and a postdoctoral research fellow with Michigan Medicine’s Pelvic Floor Research Group. “Pelvic health in general is talked about a lot more, but there’s also a lot more misinformation,” she said.
Part of that misinformation is the idea that pelvic prolapse is solely about weakness in the pelvic floor when it can also result from a widening of natural openings within the pelvis, Dr. LaCross said. She pointed to the two definitions of pelvic organ prolapse by the International Urogynecologic Consultation and the International Continence Society, both of which have been updated in recent years.
“This is why this is challenging for primary care providers,” Dr. LaCross said. “Even urogynecologists who are the specialists that treat prolapse and incontinence have changed how they assess it and the terminology and criteria that they use.”
What hasn’t changed is the substantial negative impact POP can have on quality of life. “This is the second most common reason that women enter nursing homes,” primarily because of urinary incontinence, Dr. Rabin said. “It’s very debilitating, but a lot of it is preventable and a lot is treatable.”
Dr. Rabin estimated that three out of every five women older than 60 and one or two out of every five women younger than 60 experience POP. Prevalence studies vary widely, from nearly a quarter of women to more than half, and racial and ethnic disparities in diagnosis further complicate the statistics.
PCPs therefore have an important role to play in screening for POP. The evidence shows that “patients want their providers to bring this up,” Dr. LaCross said. “They want to talk about it, but they want the provider to ask the questions first.”
Causes, Risk Factors, and Symptoms
Many causes contribute to POP, with gravity, aging, childbirth, and menopause at the top of the list.
“As people get older, their pelvic muscles and connective tissue get weaker, and the nerves don’t function as well,” Dr. Rabin said. Meanwhile, the body is losing estrogen, which affects how well the muscles contract and how easily the connective tissue can tear, she said.
With menopause, when baseline estrogen is lower, the tissue integrity is not as supportive as it should be and women are going to be at an increased risk of prolapse, Dr. Nwabuebo said.
POP has a range of risk factors:
- Increasing age, as muscle mass decreases and connective tissue hardens.
- Menopause.
- Vaginal delivery with complications, such as long second-stage labor, instrument-assisted delivery, multiple vaginal lacerations, and improperly repaired episiotomy.
- Multiple vaginal deliveries.
- Birthing large babies.
- Family history of pelvic organ prolapse (genetics can play a role in POP risk).
- Previous pelvic/abdominal surgery, including cesarean delivery and hysterectomy.
- Smoking (largely because of associated coughing).
- Chronic lung conditions that cause a lot of coughing.
- Chronic constipation or irritable bowel syndrome.
- Some types of high-impact activity, such as jogging or marathon running.
- Early menopause, for younger women.
- Repetitive heavy lifting in daily activities, such as occupational lifting (though not necessarily weight lifting as an exercise).
- Higher body mass index.
- Connective tissue disorders, such as joint hypermobility syndrome or Ehlers-Danlos syndrome.
Roger Dmochowski, MD, professor of urology and surgery at Vanderbilt University Medical Center, groups POP symptoms into two groups: anatomic and functional ones. A common anatomic symptom is bulging. “They’ll describe sitting on a ball, feeling like their bladder or something’s falling out, feeling a pressure or a heaviness,” Dr. Dmochowski said.
Functional symptoms can include vaginal dryness, vaginal irritation, painful intercourse, contact of the vaginal tissues with underclothes, and associated urinary symptoms, such as stress incontinence, urge incontinence, and incomplete emptying of the bladder. Dr. Dmochowski noted that women who report urinary incontinence may be at risk for being prescribed a medication without the necessary referral to a specialist for a full gynecologic evaluation.
Two other groups of functional symptoms include bowel-related disorders – primarily fecal incontinence and ongoing constipation – and pelvic pain or discomfort.
There can also be asymptomatic cases. “A lot of women have what we call silent prolapse,” Dr. Dmochowski said. That is, “they have some degree of loss of support to the bladder, vagina, or uterus, but they’re not symptomatic.” These women may be particularly good candidates for pelvic health physical therapy.
Screening and Diagnosis
Because many postmenopausal women stop seeing their ob.gyn, it’s often up to their primary care physician to determine whether their patients are experiencing POP symptoms.
“Women sometimes don’t bring this up with their doctor because they think there’s not enough time, or they’ll be laughed at, or their friends told them this is normal,” Dr. Rabin said. But primary care providers are really in a unique position to be able to ask the key symptom questions.
Dr. Rabin recommends a couple of questions to cover all the bases: “Do you leak urine when you cough or sneeze or on the way to the bathroom? Do you notice a bulge coming out of the vagina, or are you bothered by pelvic pressure?”
Dr. Dmochowski offered a single question that can open the conversation to more questions: “Are you bothered by any urinary or bowel or vaginal issues that we should talk about?” He also suggests asking how bothersome the symptoms are, which can help in directing treatment or prevention options. A physical exam can reveal signs of POP as well.
Diagnosis involves a detailed history, a comprehensive physical exam, and assessment with the Pelvic Organ Prolapse Quantification (POP-Q) tool. A urogynecologist can diagnose the type of POP – such as cystocele, rectocele, enterocele, uterine prolapse, or vaginal vault prolapse – and its grade (0-4).
Treatment: Physical Therapy, Pessary, and Surgery
No medications can treat prolapse, though some can treat downstream effects, such as hormonal vaginal creams for vaginal dryness and irritation, and medications for urinary incontinence. However, two mistakes PCPs can make are sending someone straight to surgery or prescribing them medication for symptoms without referring them for a diagnostic evaluation, Dr. Rabin said. “You have to have a diagnosis first to know what type of prolapse is there,” she said.
Because there can be long waiting lists for a urogynecologist or urologist, PCPs should also refer their patients to a pelvic health physical therapist (PT) who can help patients begin addressing the symptoms while they await a specialist who can diagnose them.
Though PT is often thought of as preventive, it’s also a conservative first-line intervention for prolapse, Dr. Nwabuebo said. Strong evidence shows pelvic floor muscle training from pelvic health PT can reduce symptoms of prolapse and reduce the severity by one grade in those with a grade 1 or 2 prolapse. Stage 3 is trickier, where PT may or may not be able to shift the symptom presentation, Dr. Nwabeubo said, and stage 4 is usually a surgical candidate.
“If you have a grade 4 prolapse, or the tissues are really visible outside the body, physical therapy and pelvic floor muscle training is not going to elevate that tissue back up into your body, but it can sometimes help with symptoms,” Dr. LaCross said.
The PT conducts a thorough pelvic muscle assessment, discusses lifestyle, and may teach breathing and bracing strategies for lifting, for example.
“A lot of what we’re talking about with pelvic floor therapy is lifestyle modifications,” Dr. Nwabuebo said. “If I have a patient with a history of chronic constipation, it doesn’t matter how much we do pelvic floor exercises; if we don’t manage the constipation issues by addressing their nutrition, then straining when using the bathroom will keep putting pressure on the pelvic floor.”
PTs can also recommend appropriate vaginal weights and dilators to help with pelvic floor strengthening and teach patients how to use them properly.
Even if women ultimately opt for surgery, PT prior to surgery can be beneficial. Dr. Rabin cited three reasons she recommends first-line PT: It may elevate the bladder enough to reduce stress incontinence and thicken the pelvic muscles, it can improve the effectiveness of a pessary or surgery if the woman chooses one of those options, and it can quiet bladder contractions, potentially obviating the need for pharmacologic treatment for overactive bladder.
The next nonsurgical option is a pessary, a device that fits into the vagina to provide support to the tissues displaced by prolapse. There’s a wide range of pessary types: some are short-term, worn only daily, or disposable, while others can be worn longer. Some women can self-insert and remove the pessary, and others may need a clinician to do so. Dr. Dmochowski recommends patients try a pessary to see if it benefits them. About a third of women will find them comfortable enough to wear regularly, but others will feel more sensitive to the pessary’s presence, he said.
One of the newest, most innovative pessary options for women is Gynethotics, which received Food and Drug Administration (FDA) clearance in March, as the first 3D-printed, customizable pessary capable of nearly 10 million configurations based on a person’s body.
Nearly all stage 4 prolapses and most of stage 3 prolapses can be addressed only through transvaginal or transabdominal surgery.
“We tell patients, if you can get 10 years out of your operation, you’re lucky,” Dr. Dmochowski said. A major reason for the short-lived durability is the poor quality of the tissue that needs to be pulled together. Serious complications resulting from use of polypropylene mesh during prolapse surgery led the FDA to halt sales of the devices and recommend discontinuing their use. However, one type of vaginal mesh is still considered safe to use in sacral colpopexy surgery.
Three things can shorten the durability of the surgery, Dr. Dmochowski said: heavy lifting, particularly anything over 30 pounds; chronic coughing, such as in those with chronic lung conditions; and chronic constipation.
Ms. Palm tried a pessary for her grade 3 prolapse with cystocele, rectocele, and enterocele but didn’t feel she had the time to use it regularly, so she opted for surgery. After a week on the couch recovering, she took it easy for another 12 weeks. Since then, she’s dedicated much of her time to educating and supporting women with POP and combating stigma associated with it. The APOPS website that she started has become a valuable resource for PCPs to send patients to, and the forum includes more 27,000 women from around the world.
“We encourage women to share what they’re experiencing. Tell your family, tell your friends, tell the people you work with about it,” Ms. Palm said. But many still feel uncomfortable speaking up, making PCPs’ role even more important.
*This story was updated on May 14, 2024.
Sherrie Palm, a patient advocate in Mukwonago, Wisconsin, learned in her 30s that she needed to educate herself about her own health. So when she discovered a walnut-sized lump coming out of her vagina in her mid-50s, she was stunned when her primary care provider (PCP) told her it was pelvic organ prolapse (POP), where one or more organs descend into the vaginal cavity.
“I was shocked,” Ms. Palm said. After searching online and discovering how prevalent POP was, her shock turned to anger. “I was blown away that it could be this common and I’d never heard of it,” she said. “I knew within 2 weeks that I had to do something to change the status quo.”
Ms. Palm eventually founded the nonprofit Association for Pelvic Organ Prolapse Support, or APOPS, complete with a forum where women can learn about POP and support one another. She said awareness has improved substantially since her diagnosis in 2007, but “we have a long way to go” because POP and vaginal health in general are so stigmatized.
Her website notes that about half of women with incontinence do not seek help, largely because of stigma. “The status quo is that PCPs do not POP screen,” she said. ObGyns may screen but often “because the patient has asked to be screened, they say it’s not that bad, come back and see me in a year, and do your Kegels,” Ms. Palm said.
Doctors who diagnose POP agree that the issue is often off PCPs’ radar.
“Primary care doctors are really in a time crunch, so this is one of the things that may not get addressed,” Jill Rabin, MD, vice chair of education and development in obstetrics and gynecology at Northwell Health in New York, said. Dr. Rabin is also head of urogynecology at Long Island Jewish Medical Center.
Ann Nwabuebo, PT, DPT, owner and founder of Body Connect Physical Therapy in Bethesda, Maryland, said social media has been shifting the attitude that pelvic health is a taboo subject. “It’s empowering people to seek care if they’re not finding physicians who are helping.”
But social media is also a double-edged sword, said Jenny LaCross, PT, DPT, PhD, a physical therapist at MOVE PT in Monroe, Michigan, and a postdoctoral research fellow with Michigan Medicine’s Pelvic Floor Research Group. “Pelvic health in general is talked about a lot more, but there’s also a lot more misinformation,” she said.
Part of that misinformation is the idea that pelvic prolapse is solely about weakness in the pelvic floor when it can also result from a widening of natural openings within the pelvis, Dr. LaCross said. She pointed to the two definitions of pelvic organ prolapse by the International Urogynecologic Consultation and the International Continence Society, both of which have been updated in recent years.
“This is why this is challenging for primary care providers,” Dr. LaCross said. “Even urogynecologists who are the specialists that treat prolapse and incontinence have changed how they assess it and the terminology and criteria that they use.”
What hasn’t changed is the substantial negative impact POP can have on quality of life. “This is the second most common reason that women enter nursing homes,” primarily because of urinary incontinence, Dr. Rabin said. “It’s very debilitating, but a lot of it is preventable and a lot is treatable.”
Dr. Rabin estimated that three out of every five women older than 60 and one or two out of every five women younger than 60 experience POP. Prevalence studies vary widely, from nearly a quarter of women to more than half, and racial and ethnic disparities in diagnosis further complicate the statistics.
PCPs therefore have an important role to play in screening for POP. The evidence shows that “patients want their providers to bring this up,” Dr. LaCross said. “They want to talk about it, but they want the provider to ask the questions first.”
Causes, Risk Factors, and Symptoms
Many causes contribute to POP, with gravity, aging, childbirth, and menopause at the top of the list.
“As people get older, their pelvic muscles and connective tissue get weaker, and the nerves don’t function as well,” Dr. Rabin said. Meanwhile, the body is losing estrogen, which affects how well the muscles contract and how easily the connective tissue can tear, she said.
With menopause, when baseline estrogen is lower, the tissue integrity is not as supportive as it should be and women are going to be at an increased risk of prolapse, Dr. Nwabuebo said.
POP has a range of risk factors:
- Increasing age, as muscle mass decreases and connective tissue hardens.
- Menopause.
- Vaginal delivery with complications, such as long second-stage labor, instrument-assisted delivery, multiple vaginal lacerations, and improperly repaired episiotomy.
- Multiple vaginal deliveries.
- Birthing large babies.
- Family history of pelvic organ prolapse (genetics can play a role in POP risk).
- Previous pelvic/abdominal surgery, including cesarean delivery and hysterectomy.
- Smoking (largely because of associated coughing).
- Chronic lung conditions that cause a lot of coughing.
- Chronic constipation or irritable bowel syndrome.
- Some types of high-impact activity, such as jogging or marathon running.
- Early menopause, for younger women.
- Repetitive heavy lifting in daily activities, such as occupational lifting (though not necessarily weight lifting as an exercise).
- Higher body mass index.
- Connective tissue disorders, such as joint hypermobility syndrome or Ehlers-Danlos syndrome.
Roger Dmochowski, MD, professor of urology and surgery at Vanderbilt University Medical Center, groups POP symptoms into two groups: anatomic and functional ones. A common anatomic symptom is bulging. “They’ll describe sitting on a ball, feeling like their bladder or something’s falling out, feeling a pressure or a heaviness,” Dr. Dmochowski said.
Functional symptoms can include vaginal dryness, vaginal irritation, painful intercourse, contact of the vaginal tissues with underclothes, and associated urinary symptoms, such as stress incontinence, urge incontinence, and incomplete emptying of the bladder. Dr. Dmochowski noted that women who report urinary incontinence may be at risk for being prescribed a medication without the necessary referral to a specialist for a full gynecologic evaluation.
Two other groups of functional symptoms include bowel-related disorders – primarily fecal incontinence and ongoing constipation – and pelvic pain or discomfort.
There can also be asymptomatic cases. “A lot of women have what we call silent prolapse,” Dr. Dmochowski said. That is, “they have some degree of loss of support to the bladder, vagina, or uterus, but they’re not symptomatic.” These women may be particularly good candidates for pelvic health physical therapy.
Screening and Diagnosis
Because many postmenopausal women stop seeing their ob.gyn, it’s often up to their primary care physician to determine whether their patients are experiencing POP symptoms.
“Women sometimes don’t bring this up with their doctor because they think there’s not enough time, or they’ll be laughed at, or their friends told them this is normal,” Dr. Rabin said. But primary care providers are really in a unique position to be able to ask the key symptom questions.
Dr. Rabin recommends a couple of questions to cover all the bases: “Do you leak urine when you cough or sneeze or on the way to the bathroom? Do you notice a bulge coming out of the vagina, or are you bothered by pelvic pressure?”
Dr. Dmochowski offered a single question that can open the conversation to more questions: “Are you bothered by any urinary or bowel or vaginal issues that we should talk about?” He also suggests asking how bothersome the symptoms are, which can help in directing treatment or prevention options. A physical exam can reveal signs of POP as well.
Diagnosis involves a detailed history, a comprehensive physical exam, and assessment with the Pelvic Organ Prolapse Quantification (POP-Q) tool. A urogynecologist can diagnose the type of POP – such as cystocele, rectocele, enterocele, uterine prolapse, or vaginal vault prolapse – and its grade (0-4).
Treatment: Physical Therapy, Pessary, and Surgery
No medications can treat prolapse, though some can treat downstream effects, such as hormonal vaginal creams for vaginal dryness and irritation, and medications for urinary incontinence. However, two mistakes PCPs can make are sending someone straight to surgery or prescribing them medication for symptoms without referring them for a diagnostic evaluation, Dr. Rabin said. “You have to have a diagnosis first to know what type of prolapse is there,” she said.
Because there can be long waiting lists for a urogynecologist or urologist, PCPs should also refer their patients to a pelvic health physical therapist (PT) who can help patients begin addressing the symptoms while they await a specialist who can diagnose them.
Though PT is often thought of as preventive, it’s also a conservative first-line intervention for prolapse, Dr. Nwabuebo said. Strong evidence shows pelvic floor muscle training from pelvic health PT can reduce symptoms of prolapse and reduce the severity by one grade in those with a grade 1 or 2 prolapse. Stage 3 is trickier, where PT may or may not be able to shift the symptom presentation, Dr. Nwabeubo said, and stage 4 is usually a surgical candidate.
“If you have a grade 4 prolapse, or the tissues are really visible outside the body, physical therapy and pelvic floor muscle training is not going to elevate that tissue back up into your body, but it can sometimes help with symptoms,” Dr. LaCross said.
The PT conducts a thorough pelvic muscle assessment, discusses lifestyle, and may teach breathing and bracing strategies for lifting, for example.
“A lot of what we’re talking about with pelvic floor therapy is lifestyle modifications,” Dr. Nwabuebo said. “If I have a patient with a history of chronic constipation, it doesn’t matter how much we do pelvic floor exercises; if we don’t manage the constipation issues by addressing their nutrition, then straining when using the bathroom will keep putting pressure on the pelvic floor.”
PTs can also recommend appropriate vaginal weights and dilators to help with pelvic floor strengthening and teach patients how to use them properly.
Even if women ultimately opt for surgery, PT prior to surgery can be beneficial. Dr. Rabin cited three reasons she recommends first-line PT: It may elevate the bladder enough to reduce stress incontinence and thicken the pelvic muscles, it can improve the effectiveness of a pessary or surgery if the woman chooses one of those options, and it can quiet bladder contractions, potentially obviating the need for pharmacologic treatment for overactive bladder.
The next nonsurgical option is a pessary, a device that fits into the vagina to provide support to the tissues displaced by prolapse. There’s a wide range of pessary types: some are short-term, worn only daily, or disposable, while others can be worn longer. Some women can self-insert and remove the pessary, and others may need a clinician to do so. Dr. Dmochowski recommends patients try a pessary to see if it benefits them. About a third of women will find them comfortable enough to wear regularly, but others will feel more sensitive to the pessary’s presence, he said.
One of the newest, most innovative pessary options for women is Gynethotics, which received Food and Drug Administration (FDA) clearance in March, as the first 3D-printed, customizable pessary capable of nearly 10 million configurations based on a person’s body.
Nearly all stage 4 prolapses and most of stage 3 prolapses can be addressed only through transvaginal or transabdominal surgery.
“We tell patients, if you can get 10 years out of your operation, you’re lucky,” Dr. Dmochowski said. A major reason for the short-lived durability is the poor quality of the tissue that needs to be pulled together. Serious complications resulting from use of polypropylene mesh during prolapse surgery led the FDA to halt sales of the devices and recommend discontinuing their use. However, one type of vaginal mesh is still considered safe to use in sacral colpopexy surgery.
Three things can shorten the durability of the surgery, Dr. Dmochowski said: heavy lifting, particularly anything over 30 pounds; chronic coughing, such as in those with chronic lung conditions; and chronic constipation.
Ms. Palm tried a pessary for her grade 3 prolapse with cystocele, rectocele, and enterocele but didn’t feel she had the time to use it regularly, so she opted for surgery. After a week on the couch recovering, she took it easy for another 12 weeks. Since then, she’s dedicated much of her time to educating and supporting women with POP and combating stigma associated with it. The APOPS website that she started has become a valuable resource for PCPs to send patients to, and the forum includes more 27,000 women from around the world.
“We encourage women to share what they’re experiencing. Tell your family, tell your friends, tell the people you work with about it,” Ms. Palm said. But many still feel uncomfortable speaking up, making PCPs’ role even more important.
*This story was updated on May 14, 2024.
Sherrie Palm, a patient advocate in Mukwonago, Wisconsin, learned in her 30s that she needed to educate herself about her own health. So when she discovered a walnut-sized lump coming out of her vagina in her mid-50s, she was stunned when her primary care provider (PCP) told her it was pelvic organ prolapse (POP), where one or more organs descend into the vaginal cavity.
“I was shocked,” Ms. Palm said. After searching online and discovering how prevalent POP was, her shock turned to anger. “I was blown away that it could be this common and I’d never heard of it,” she said. “I knew within 2 weeks that I had to do something to change the status quo.”
Ms. Palm eventually founded the nonprofit Association for Pelvic Organ Prolapse Support, or APOPS, complete with a forum where women can learn about POP and support one another. She said awareness has improved substantially since her diagnosis in 2007, but “we have a long way to go” because POP and vaginal health in general are so stigmatized.
Her website notes that about half of women with incontinence do not seek help, largely because of stigma. “The status quo is that PCPs do not POP screen,” she said. ObGyns may screen but often “because the patient has asked to be screened, they say it’s not that bad, come back and see me in a year, and do your Kegels,” Ms. Palm said.
Doctors who diagnose POP agree that the issue is often off PCPs’ radar.
“Primary care doctors are really in a time crunch, so this is one of the things that may not get addressed,” Jill Rabin, MD, vice chair of education and development in obstetrics and gynecology at Northwell Health in New York, said. Dr. Rabin is also head of urogynecology at Long Island Jewish Medical Center.
Ann Nwabuebo, PT, DPT, owner and founder of Body Connect Physical Therapy in Bethesda, Maryland, said social media has been shifting the attitude that pelvic health is a taboo subject. “It’s empowering people to seek care if they’re not finding physicians who are helping.”
But social media is also a double-edged sword, said Jenny LaCross, PT, DPT, PhD, a physical therapist at MOVE PT in Monroe, Michigan, and a postdoctoral research fellow with Michigan Medicine’s Pelvic Floor Research Group. “Pelvic health in general is talked about a lot more, but there’s also a lot more misinformation,” she said.
Part of that misinformation is the idea that pelvic prolapse is solely about weakness in the pelvic floor when it can also result from a widening of natural openings within the pelvis, Dr. LaCross said. She pointed to the two definitions of pelvic organ prolapse by the International Urogynecologic Consultation and the International Continence Society, both of which have been updated in recent years.
“This is why this is challenging for primary care providers,” Dr. LaCross said. “Even urogynecologists who are the specialists that treat prolapse and incontinence have changed how they assess it and the terminology and criteria that they use.”
What hasn’t changed is the substantial negative impact POP can have on quality of life. “This is the second most common reason that women enter nursing homes,” primarily because of urinary incontinence, Dr. Rabin said. “It’s very debilitating, but a lot of it is preventable and a lot is treatable.”
Dr. Rabin estimated that three out of every five women older than 60 and one or two out of every five women younger than 60 experience POP. Prevalence studies vary widely, from nearly a quarter of women to more than half, and racial and ethnic disparities in diagnosis further complicate the statistics.
PCPs therefore have an important role to play in screening for POP. The evidence shows that “patients want their providers to bring this up,” Dr. LaCross said. “They want to talk about it, but they want the provider to ask the questions first.”
Causes, Risk Factors, and Symptoms
Many causes contribute to POP, with gravity, aging, childbirth, and menopause at the top of the list.
“As people get older, their pelvic muscles and connective tissue get weaker, and the nerves don’t function as well,” Dr. Rabin said. Meanwhile, the body is losing estrogen, which affects how well the muscles contract and how easily the connective tissue can tear, she said.
With menopause, when baseline estrogen is lower, the tissue integrity is not as supportive as it should be and women are going to be at an increased risk of prolapse, Dr. Nwabuebo said.
POP has a range of risk factors:
- Increasing age, as muscle mass decreases and connective tissue hardens.
- Menopause.
- Vaginal delivery with complications, such as long second-stage labor, instrument-assisted delivery, multiple vaginal lacerations, and improperly repaired episiotomy.
- Multiple vaginal deliveries.
- Birthing large babies.
- Family history of pelvic organ prolapse (genetics can play a role in POP risk).
- Previous pelvic/abdominal surgery, including cesarean delivery and hysterectomy.
- Smoking (largely because of associated coughing).
- Chronic lung conditions that cause a lot of coughing.
- Chronic constipation or irritable bowel syndrome.
- Some types of high-impact activity, such as jogging or marathon running.
- Early menopause, for younger women.
- Repetitive heavy lifting in daily activities, such as occupational lifting (though not necessarily weight lifting as an exercise).
- Higher body mass index.
- Connective tissue disorders, such as joint hypermobility syndrome or Ehlers-Danlos syndrome.
Roger Dmochowski, MD, professor of urology and surgery at Vanderbilt University Medical Center, groups POP symptoms into two groups: anatomic and functional ones. A common anatomic symptom is bulging. “They’ll describe sitting on a ball, feeling like their bladder or something’s falling out, feeling a pressure or a heaviness,” Dr. Dmochowski said.
Functional symptoms can include vaginal dryness, vaginal irritation, painful intercourse, contact of the vaginal tissues with underclothes, and associated urinary symptoms, such as stress incontinence, urge incontinence, and incomplete emptying of the bladder. Dr. Dmochowski noted that women who report urinary incontinence may be at risk for being prescribed a medication without the necessary referral to a specialist for a full gynecologic evaluation.
Two other groups of functional symptoms include bowel-related disorders – primarily fecal incontinence and ongoing constipation – and pelvic pain or discomfort.
There can also be asymptomatic cases. “A lot of women have what we call silent prolapse,” Dr. Dmochowski said. That is, “they have some degree of loss of support to the bladder, vagina, or uterus, but they’re not symptomatic.” These women may be particularly good candidates for pelvic health physical therapy.
Screening and Diagnosis
Because many postmenopausal women stop seeing their ob.gyn, it’s often up to their primary care physician to determine whether their patients are experiencing POP symptoms.
“Women sometimes don’t bring this up with their doctor because they think there’s not enough time, or they’ll be laughed at, or their friends told them this is normal,” Dr. Rabin said. But primary care providers are really in a unique position to be able to ask the key symptom questions.
Dr. Rabin recommends a couple of questions to cover all the bases: “Do you leak urine when you cough or sneeze or on the way to the bathroom? Do you notice a bulge coming out of the vagina, or are you bothered by pelvic pressure?”
Dr. Dmochowski offered a single question that can open the conversation to more questions: “Are you bothered by any urinary or bowel or vaginal issues that we should talk about?” He also suggests asking how bothersome the symptoms are, which can help in directing treatment or prevention options. A physical exam can reveal signs of POP as well.
Diagnosis involves a detailed history, a comprehensive physical exam, and assessment with the Pelvic Organ Prolapse Quantification (POP-Q) tool. A urogynecologist can diagnose the type of POP – such as cystocele, rectocele, enterocele, uterine prolapse, or vaginal vault prolapse – and its grade (0-4).
Treatment: Physical Therapy, Pessary, and Surgery
No medications can treat prolapse, though some can treat downstream effects, such as hormonal vaginal creams for vaginal dryness and irritation, and medications for urinary incontinence. However, two mistakes PCPs can make are sending someone straight to surgery or prescribing them medication for symptoms without referring them for a diagnostic evaluation, Dr. Rabin said. “You have to have a diagnosis first to know what type of prolapse is there,” she said.
Because there can be long waiting lists for a urogynecologist or urologist, PCPs should also refer their patients to a pelvic health physical therapist (PT) who can help patients begin addressing the symptoms while they await a specialist who can diagnose them.
Though PT is often thought of as preventive, it’s also a conservative first-line intervention for prolapse, Dr. Nwabuebo said. Strong evidence shows pelvic floor muscle training from pelvic health PT can reduce symptoms of prolapse and reduce the severity by one grade in those with a grade 1 or 2 prolapse. Stage 3 is trickier, where PT may or may not be able to shift the symptom presentation, Dr. Nwabeubo said, and stage 4 is usually a surgical candidate.
“If you have a grade 4 prolapse, or the tissues are really visible outside the body, physical therapy and pelvic floor muscle training is not going to elevate that tissue back up into your body, but it can sometimes help with symptoms,” Dr. LaCross said.
The PT conducts a thorough pelvic muscle assessment, discusses lifestyle, and may teach breathing and bracing strategies for lifting, for example.
“A lot of what we’re talking about with pelvic floor therapy is lifestyle modifications,” Dr. Nwabuebo said. “If I have a patient with a history of chronic constipation, it doesn’t matter how much we do pelvic floor exercises; if we don’t manage the constipation issues by addressing their nutrition, then straining when using the bathroom will keep putting pressure on the pelvic floor.”
PTs can also recommend appropriate vaginal weights and dilators to help with pelvic floor strengthening and teach patients how to use them properly.
Even if women ultimately opt for surgery, PT prior to surgery can be beneficial. Dr. Rabin cited three reasons she recommends first-line PT: It may elevate the bladder enough to reduce stress incontinence and thicken the pelvic muscles, it can improve the effectiveness of a pessary or surgery if the woman chooses one of those options, and it can quiet bladder contractions, potentially obviating the need for pharmacologic treatment for overactive bladder.
The next nonsurgical option is a pessary, a device that fits into the vagina to provide support to the tissues displaced by prolapse. There’s a wide range of pessary types: some are short-term, worn only daily, or disposable, while others can be worn longer. Some women can self-insert and remove the pessary, and others may need a clinician to do so. Dr. Dmochowski recommends patients try a pessary to see if it benefits them. About a third of women will find them comfortable enough to wear regularly, but others will feel more sensitive to the pessary’s presence, he said.
One of the newest, most innovative pessary options for women is Gynethotics, which received Food and Drug Administration (FDA) clearance in March, as the first 3D-printed, customizable pessary capable of nearly 10 million configurations based on a person’s body.
Nearly all stage 4 prolapses and most of stage 3 prolapses can be addressed only through transvaginal or transabdominal surgery.
“We tell patients, if you can get 10 years out of your operation, you’re lucky,” Dr. Dmochowski said. A major reason for the short-lived durability is the poor quality of the tissue that needs to be pulled together. Serious complications resulting from use of polypropylene mesh during prolapse surgery led the FDA to halt sales of the devices and recommend discontinuing their use. However, one type of vaginal mesh is still considered safe to use in sacral colpopexy surgery.
Three things can shorten the durability of the surgery, Dr. Dmochowski said: heavy lifting, particularly anything over 30 pounds; chronic coughing, such as in those with chronic lung conditions; and chronic constipation.
Ms. Palm tried a pessary for her grade 3 prolapse with cystocele, rectocele, and enterocele but didn’t feel she had the time to use it regularly, so she opted for surgery. After a week on the couch recovering, she took it easy for another 12 weeks. Since then, she’s dedicated much of her time to educating and supporting women with POP and combating stigma associated with it. The APOPS website that she started has become a valuable resource for PCPs to send patients to, and the forum includes more 27,000 women from around the world.
“We encourage women to share what they’re experiencing. Tell your family, tell your friends, tell the people you work with about it,” Ms. Palm said. But many still feel uncomfortable speaking up, making PCPs’ role even more important.
*This story was updated on May 14, 2024.
Cervical Cancer Screening: US Clinicians Unclear About Best Practices
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines, proposing two major changes: start cervical cancer screening at age 25, rather than 21, and perform primary human papillomavirus (HPV) testing, instead of a Pap test.
First, healthcare providers in the US may be unsure how to reconcile conflicting cervical cancer screening guidelines from another major organization — the US Preventive Services Task Force (USPSTF), which published guidelines in 2018.
Although the ACS guidelines are based on an analysis of the latest evidence,
the recommendations challenge those from the USPSTF, which dictates insurance coverage in the US. Last year, the American College of Obstetricians and Gynecologists (ACOG) aligned its guidelines with those from the USPSTF.
The USPSTF recommends average-risk individuals start Pap, not HPV, testing at age 21, and broadens the options to primary HPV testing, Pap testing, or both together starting at age 30. The ACS, on the other hand, says primary HPV testing is the preferred screening approach from the start, which should be age 25.
Because the ACS guidelines marked a notable departure from prevailing practice, a team of researchers from five US universities decided to find out if anyone was following them.
The results, published in the journal Cancer in March, revealed that most healthcare providers had not changed practice.
Lead author Rebecca Perkins, MD, MSc, and colleagues found that, among the 70 respondents, few were starting screening at age 25, and none had switched to primary HPV testing.
The survey then probed clinicians’ willingness to adopt the ACS guidelines as well as their reservations and barriers to doing so.
Notably, more than half of the survey participants said they would be willing to adopt the ACS guidelines if the best evidence supported the changes and other professional medical organizations endorsed them.
On the age change, participants highlighted a range of benefits to moving to a later screening age, including that earlier screening may not be valuable and delaying screening could reduce overtreatment.
One participant noted: “We know that cervical cancer is usually a slow‐growing, long‐term progressive disease that does not typically show up that early in life, and we also know that, if infected, oftentimes their immune system can fight off the virus. So, it sounds reasonable at first glance [to delay screening to age 25 years].”
Others, however, brought up barriers to initiating screening at age 25. Some mentioned that later screening may not work for high‐risk populations and others voiced concerns about missing high‐grade precancer or cancer. “It’s not unusual for us to see women in their early 20s that have already had 10 or 15 partners. … a lot of them smoke too … they just have a lot of bad habits that put them at more risk,” one respondent noted.
On the HPV vs Pap testing front, many participants described a growing confidence in HPV tests after trying co-testing. One participant said, “Honestly, I do look more at the HPV results than the cytology. I put more faith in knowing what their HPV status is than anything.”
The main barriers to primary HPV testing, however, included lack of autonomy when working in a large health system, concerns about the efficacy of HPV testing, and a belief that cytology was valuable.
Some clinicians were worried about missing high-grade lesions or cancer. One healthcare provider said, “My only concern with primary HPV screening is occasionally you will pick up endometrial abnormalities on a Pap that you’re not going to pick up with HPV screening.”
Logistics and finances also played a role in clinicians’ hesitancy to switch to the ACS recommendation. Labs that could handle primary HPV tests were not available to some participants, and lack of insurance coverage was a barrier for others. One respondent noted, for instance, that his institution has a “cytology infrastructure that already exists in the lab and I can’t really see them switching.”
Many survey respondents also said they were waiting for endorsement from organizations, such as ACOG and USPSTF. “We run by the USPSTF and … ACOG. We don’t run by the ACS guidelines,” one person said.
Finally, some participants were not aware of the ACS recommendations at all or the data behind them but said they would be willing to change to primary HPV testing in the future.
Overall, Dr. Perkins said she was happy to see that more than half of the respondents would be willing to shift to the ACS screening guidelines, but noted that many remain reluctant to do so until the USPSTF and ACOG change their guidelines.
“It’s really just a matter of the USPSTF and ACOG endorsing” the ACS guidelines, said Dr. Perkins, professor of obstetrics and gynecology at Boston University.
The USPSTF is currently updating its cervical screening guidelines, which could potentially help reconcile this discord between the guidelines and close the gaps in practice patterns.
The USPSTF’s review of the evidence, which led to the 2018 guidelines, did highlight the effectiveness of HPV testing. The review authors concluded that “the evidence was consistent across trials” that primary, high-risk HPV screening increased detection of grade 3 or worse cervical intraepithelial neoplasia in the initial round of screening “by as much as 2 to 3 times when compared with cytology.”
However, Joy Melnikow, MD, MPH, first author on the USPSTF evidence review, explained that the reviewers factored in access to HPV testing when making their final recommendations.
“The consideration was making sure that a recommendation could be inclusive of all providers and all populations and not restricting access for clinics that couldn’t afford or didn’t have the machine to do [HPV testing],” Dr. Melnikow, director of the Center for Healthcare Policy and Research and professor of family and community medicine at the University of California Davis, told this news organization.
The ACS, however, did not consider potential access problems in its analysis of the evidence.
Although the ACS evidence is “excellent,” Dr. Perkins said, “it’s really just a matter of the USPSTF and ACOG endorsing that, and then it seems like a lot of people are willing to make the change.”
Dr. Perkins reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines, proposing two major changes: start cervical cancer screening at age 25, rather than 21, and perform primary human papillomavirus (HPV) testing, instead of a Pap test.
First, healthcare providers in the US may be unsure how to reconcile conflicting cervical cancer screening guidelines from another major organization — the US Preventive Services Task Force (USPSTF), which published guidelines in 2018.
Although the ACS guidelines are based on an analysis of the latest evidence,
the recommendations challenge those from the USPSTF, which dictates insurance coverage in the US. Last year, the American College of Obstetricians and Gynecologists (ACOG) aligned its guidelines with those from the USPSTF.
The USPSTF recommends average-risk individuals start Pap, not HPV, testing at age 21, and broadens the options to primary HPV testing, Pap testing, or both together starting at age 30. The ACS, on the other hand, says primary HPV testing is the preferred screening approach from the start, which should be age 25.
Because the ACS guidelines marked a notable departure from prevailing practice, a team of researchers from five US universities decided to find out if anyone was following them.
The results, published in the journal Cancer in March, revealed that most healthcare providers had not changed practice.
Lead author Rebecca Perkins, MD, MSc, and colleagues found that, among the 70 respondents, few were starting screening at age 25, and none had switched to primary HPV testing.
The survey then probed clinicians’ willingness to adopt the ACS guidelines as well as their reservations and barriers to doing so.
Notably, more than half of the survey participants said they would be willing to adopt the ACS guidelines if the best evidence supported the changes and other professional medical organizations endorsed them.
On the age change, participants highlighted a range of benefits to moving to a later screening age, including that earlier screening may not be valuable and delaying screening could reduce overtreatment.
One participant noted: “We know that cervical cancer is usually a slow‐growing, long‐term progressive disease that does not typically show up that early in life, and we also know that, if infected, oftentimes their immune system can fight off the virus. So, it sounds reasonable at first glance [to delay screening to age 25 years].”
Others, however, brought up barriers to initiating screening at age 25. Some mentioned that later screening may not work for high‐risk populations and others voiced concerns about missing high‐grade precancer or cancer. “It’s not unusual for us to see women in their early 20s that have already had 10 or 15 partners. … a lot of them smoke too … they just have a lot of bad habits that put them at more risk,” one respondent noted.
On the HPV vs Pap testing front, many participants described a growing confidence in HPV tests after trying co-testing. One participant said, “Honestly, I do look more at the HPV results than the cytology. I put more faith in knowing what their HPV status is than anything.”
The main barriers to primary HPV testing, however, included lack of autonomy when working in a large health system, concerns about the efficacy of HPV testing, and a belief that cytology was valuable.
Some clinicians were worried about missing high-grade lesions or cancer. One healthcare provider said, “My only concern with primary HPV screening is occasionally you will pick up endometrial abnormalities on a Pap that you’re not going to pick up with HPV screening.”
Logistics and finances also played a role in clinicians’ hesitancy to switch to the ACS recommendation. Labs that could handle primary HPV tests were not available to some participants, and lack of insurance coverage was a barrier for others. One respondent noted, for instance, that his institution has a “cytology infrastructure that already exists in the lab and I can’t really see them switching.”
Many survey respondents also said they were waiting for endorsement from organizations, such as ACOG and USPSTF. “We run by the USPSTF and … ACOG. We don’t run by the ACS guidelines,” one person said.
Finally, some participants were not aware of the ACS recommendations at all or the data behind them but said they would be willing to change to primary HPV testing in the future.
Overall, Dr. Perkins said she was happy to see that more than half of the respondents would be willing to shift to the ACS screening guidelines, but noted that many remain reluctant to do so until the USPSTF and ACOG change their guidelines.
“It’s really just a matter of the USPSTF and ACOG endorsing” the ACS guidelines, said Dr. Perkins, professor of obstetrics and gynecology at Boston University.
The USPSTF is currently updating its cervical screening guidelines, which could potentially help reconcile this discord between the guidelines and close the gaps in practice patterns.
The USPSTF’s review of the evidence, which led to the 2018 guidelines, did highlight the effectiveness of HPV testing. The review authors concluded that “the evidence was consistent across trials” that primary, high-risk HPV screening increased detection of grade 3 or worse cervical intraepithelial neoplasia in the initial round of screening “by as much as 2 to 3 times when compared with cytology.”
However, Joy Melnikow, MD, MPH, first author on the USPSTF evidence review, explained that the reviewers factored in access to HPV testing when making their final recommendations.
“The consideration was making sure that a recommendation could be inclusive of all providers and all populations and not restricting access for clinics that couldn’t afford or didn’t have the machine to do [HPV testing],” Dr. Melnikow, director of the Center for Healthcare Policy and Research and professor of family and community medicine at the University of California Davis, told this news organization.
The ACS, however, did not consider potential access problems in its analysis of the evidence.
Although the ACS evidence is “excellent,” Dr. Perkins said, “it’s really just a matter of the USPSTF and ACOG endorsing that, and then it seems like a lot of people are willing to make the change.”
Dr. Perkins reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines, proposing two major changes: start cervical cancer screening at age 25, rather than 21, and perform primary human papillomavirus (HPV) testing, instead of a Pap test.
First, healthcare providers in the US may be unsure how to reconcile conflicting cervical cancer screening guidelines from another major organization — the US Preventive Services Task Force (USPSTF), which published guidelines in 2018.
Although the ACS guidelines are based on an analysis of the latest evidence,
the recommendations challenge those from the USPSTF, which dictates insurance coverage in the US. Last year, the American College of Obstetricians and Gynecologists (ACOG) aligned its guidelines with those from the USPSTF.
The USPSTF recommends average-risk individuals start Pap, not HPV, testing at age 21, and broadens the options to primary HPV testing, Pap testing, or both together starting at age 30. The ACS, on the other hand, says primary HPV testing is the preferred screening approach from the start, which should be age 25.
Because the ACS guidelines marked a notable departure from prevailing practice, a team of researchers from five US universities decided to find out if anyone was following them.
The results, published in the journal Cancer in March, revealed that most healthcare providers had not changed practice.
Lead author Rebecca Perkins, MD, MSc, and colleagues found that, among the 70 respondents, few were starting screening at age 25, and none had switched to primary HPV testing.
The survey then probed clinicians’ willingness to adopt the ACS guidelines as well as their reservations and barriers to doing so.
Notably, more than half of the survey participants said they would be willing to adopt the ACS guidelines if the best evidence supported the changes and other professional medical organizations endorsed them.
On the age change, participants highlighted a range of benefits to moving to a later screening age, including that earlier screening may not be valuable and delaying screening could reduce overtreatment.
One participant noted: “We know that cervical cancer is usually a slow‐growing, long‐term progressive disease that does not typically show up that early in life, and we also know that, if infected, oftentimes their immune system can fight off the virus. So, it sounds reasonable at first glance [to delay screening to age 25 years].”
Others, however, brought up barriers to initiating screening at age 25. Some mentioned that later screening may not work for high‐risk populations and others voiced concerns about missing high‐grade precancer or cancer. “It’s not unusual for us to see women in their early 20s that have already had 10 or 15 partners. … a lot of them smoke too … they just have a lot of bad habits that put them at more risk,” one respondent noted.
On the HPV vs Pap testing front, many participants described a growing confidence in HPV tests after trying co-testing. One participant said, “Honestly, I do look more at the HPV results than the cytology. I put more faith in knowing what their HPV status is than anything.”
The main barriers to primary HPV testing, however, included lack of autonomy when working in a large health system, concerns about the efficacy of HPV testing, and a belief that cytology was valuable.
Some clinicians were worried about missing high-grade lesions or cancer. One healthcare provider said, “My only concern with primary HPV screening is occasionally you will pick up endometrial abnormalities on a Pap that you’re not going to pick up with HPV screening.”
Logistics and finances also played a role in clinicians’ hesitancy to switch to the ACS recommendation. Labs that could handle primary HPV tests were not available to some participants, and lack of insurance coverage was a barrier for others. One respondent noted, for instance, that his institution has a “cytology infrastructure that already exists in the lab and I can’t really see them switching.”
Many survey respondents also said they were waiting for endorsement from organizations, such as ACOG and USPSTF. “We run by the USPSTF and … ACOG. We don’t run by the ACS guidelines,” one person said.
Finally, some participants were not aware of the ACS recommendations at all or the data behind them but said they would be willing to change to primary HPV testing in the future.
Overall, Dr. Perkins said she was happy to see that more than half of the respondents would be willing to shift to the ACS screening guidelines, but noted that many remain reluctant to do so until the USPSTF and ACOG change their guidelines.
“It’s really just a matter of the USPSTF and ACOG endorsing” the ACS guidelines, said Dr. Perkins, professor of obstetrics and gynecology at Boston University.
The USPSTF is currently updating its cervical screening guidelines, which could potentially help reconcile this discord between the guidelines and close the gaps in practice patterns.
The USPSTF’s review of the evidence, which led to the 2018 guidelines, did highlight the effectiveness of HPV testing. The review authors concluded that “the evidence was consistent across trials” that primary, high-risk HPV screening increased detection of grade 3 or worse cervical intraepithelial neoplasia in the initial round of screening “by as much as 2 to 3 times when compared with cytology.”
However, Joy Melnikow, MD, MPH, first author on the USPSTF evidence review, explained that the reviewers factored in access to HPV testing when making their final recommendations.
“The consideration was making sure that a recommendation could be inclusive of all providers and all populations and not restricting access for clinics that couldn’t afford or didn’t have the machine to do [HPV testing],” Dr. Melnikow, director of the Center for Healthcare Policy and Research and professor of family and community medicine at the University of California Davis, told this news organization.
The ACS, however, did not consider potential access problems in its analysis of the evidence.
Although the ACS evidence is “excellent,” Dr. Perkins said, “it’s really just a matter of the USPSTF and ACOG endorsing that, and then it seems like a lot of people are willing to make the change.”
Dr. Perkins reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
Routine Breast Cancer Screening Should Start at Age 40: USPSTF
In its updated recommendations published April 30 in JAMA, the USPSTF also made an urgent call to address reasons why Black women are more likely to die from breast cancer than are White women and pressed for more research to address persisting questions about how best to screen for cancer in dense breasts, which about 40% of women have. The USPSTF highlighted evidence gaps on the benefits and harms of continuing mammography after age 75 years as well.
The updated USPSTF recommendations were first unveiled last year in a draft version.
In 2016, the task force recommended biennial mammograms for women starting 10 years later, at age 50 years, while stressing a need for clinicians and patients to weigh the risks and benefits of screening for those in their 40s.
The shift to a general recommendation to start at age 40 years is based on a broad review of available data on mammography, including modeling from Cancer Intervention and Surveillance Modeling Network (CISNET).
Alongside the USPSTF report, JAMA published three separate editorials — a reflection of the controversy that these breast cancer screening recommendations often generate.
In one editorial, published in JAMA Network Open, Lydia E. Pace, MD, MPH, and Nancy L. Keating, MD, MPH, highlighted that though screening earlier will prevent more deaths from breast cancer, it will also lead to more false positive findings and increase rates of overdiagnosis.
Dr. Pace and Dr. Keating explained that the modeling study commissioned by the USPSTF estimated that screening every 2 years starting at age 40 years would avoid an additional 1.3 breast cancer deaths compared with screening at age 50 years. Among Black women, screening every 2 years starting at age 40 years would avert an extra 1.8 breast cancer deaths per 1000 people screened.
However, the model also found that screening every 2 years starting at age 40 years would lead to more false positive tests — a rate of about 8.5% vs 7.8% for those starting at age 50.
“Given mammography screening’s modest benefits, we feel that all women — and particularly those aged 40 to 49 years —should be counseled about the benefits and harms of mammography and supported in deciding whether the balance of benefits to harms fits with their priorities and values,” wrote Dr. Pace and Dr. Keating, who specialize in internal medicine.
In a second editorial, in JAMA, Joann G. Elmore, MD, MPH, of UCLA, and Christoph I. Lee, MD, MS, of the University of Washington, Seattle, noted that the revised recommendations “shed light on 2 major issues that demand greater attention: addressing health inequities related to breast cancer outcomes and ensuring benefits for all women amid rapid screening technological advancements.”
The USPSTF’s decision to recommend an earlier start age for routine mammography was partly intended to begin to address the fact that Black women are about 40% more likely to die from breast cancer than are White women.
“Despite greater absolute benefits of screening for Black women, the modeling study and systematic review underscore that mammography’s benefits (ie, breast cancer deaths averted) are modest for both Black women and the general population,” wrote Dr. Elmore and Dr. Lee.
The editorialists also cautioned against adopting artificial intelligence (AI) support tools too rapidly, criticizing the USPSTF for overlooking this “pressing issue.”
“While AI algorithms show promise for enhancing cancer detection, their impact on patient outcomes and the balance between benefit and harms remain uncertain,” the editorialists wrote.
In a third editorial, in JAMA Oncology, Wendie A. Berg, MD, PhD, a radiologist at the University of Pittsburgh, argued that though the updated recommendations are “an important step forward,” they don’t go far enough.
Dr. Berg, for instance, noted her surprise “ to see the USPSTF recommendation only for biennial, rather than annual, screening among women aged 40 to 74 years.”
Compared with no screening, annual screening would reduce rates of breast cancer mortality (35.2%) more than biennial (28.4%) screening does among women aged 40-74 years, according to the CISNET modeling that informed the USPSTF’s decision.
Plus, Dr. Berg noted, regular risk assessments should begin at age 25 years “to identify women at high risk who should start annual MRI screenings.”
The American College of Radiology (ACR) offered similar views in a statement, saying the recommendations “do not go far enough to save more women’s lives.” It urged a more aggressive screening schedule, which starts at age 40 years but occurs annually vs biennially and continues past age 74 years. Like Dr. Berg, the ACR advocated for breast cancer risk assessments to begin at age 25 years.
The American Cancer Society also recommended annual mammography screening, starting as early as age 40 years in average-risk women, with high-risk women receiving a breast MRI and a mammogram every year starting at age 30 years.
Ongoing Uncertainties
The USPSTF’s 2024 update highlighted persistent evidence gaps in several key areas.
The USPSTF, for instance, highlighted insufficient evidence on the benefits and harms of continuing to screen women who are 75 years or older as well as the benefits and harms of supplemental screening with breast ultrasonography or MRI in women with dense breasts who had a negative screening mammogram.
In the update, USPSTF noted that it’s still clear what proportion of ductal carcinoma in situ involves lesions detected by screening would not have ultimately caused harm.
For women with dense breasts, the USPSTF said that “research is needed to help clinicians and patients understand the best strategy for breast cancer screening in women found to have dense breasts,” which includes supplemental screening.
Women with dense breasts should still get mammograms, but there is not enough evidence for a blanket statement about which benefit they might get from additional screening, Carol Mangione, MD, past chair of USPSTF, told this news organization.
“We don’t want to send a message that the mammogram doesn’t have value in that group, because it does have high value,” said Dr. Mangione, chief of the division of general internal medicine and health services research at UCLA Health.
Women with dense breasts should work with primary care clinicians who can take a holistic view of their preferences and needs, allowing them to make an informed choice about additional screening, she said.
“But we can’t make a global population choice because we don’t have the studies to do that,” Dr. Mangione said.
A version of this article appeared on Medscape.com.
In its updated recommendations published April 30 in JAMA, the USPSTF also made an urgent call to address reasons why Black women are more likely to die from breast cancer than are White women and pressed for more research to address persisting questions about how best to screen for cancer in dense breasts, which about 40% of women have. The USPSTF highlighted evidence gaps on the benefits and harms of continuing mammography after age 75 years as well.
The updated USPSTF recommendations were first unveiled last year in a draft version.
In 2016, the task force recommended biennial mammograms for women starting 10 years later, at age 50 years, while stressing a need for clinicians and patients to weigh the risks and benefits of screening for those in their 40s.
The shift to a general recommendation to start at age 40 years is based on a broad review of available data on mammography, including modeling from Cancer Intervention and Surveillance Modeling Network (CISNET).
Alongside the USPSTF report, JAMA published three separate editorials — a reflection of the controversy that these breast cancer screening recommendations often generate.
In one editorial, published in JAMA Network Open, Lydia E. Pace, MD, MPH, and Nancy L. Keating, MD, MPH, highlighted that though screening earlier will prevent more deaths from breast cancer, it will also lead to more false positive findings and increase rates of overdiagnosis.
Dr. Pace and Dr. Keating explained that the modeling study commissioned by the USPSTF estimated that screening every 2 years starting at age 40 years would avoid an additional 1.3 breast cancer deaths compared with screening at age 50 years. Among Black women, screening every 2 years starting at age 40 years would avert an extra 1.8 breast cancer deaths per 1000 people screened.
However, the model also found that screening every 2 years starting at age 40 years would lead to more false positive tests — a rate of about 8.5% vs 7.8% for those starting at age 50.
“Given mammography screening’s modest benefits, we feel that all women — and particularly those aged 40 to 49 years —should be counseled about the benefits and harms of mammography and supported in deciding whether the balance of benefits to harms fits with their priorities and values,” wrote Dr. Pace and Dr. Keating, who specialize in internal medicine.
In a second editorial, in JAMA, Joann G. Elmore, MD, MPH, of UCLA, and Christoph I. Lee, MD, MS, of the University of Washington, Seattle, noted that the revised recommendations “shed light on 2 major issues that demand greater attention: addressing health inequities related to breast cancer outcomes and ensuring benefits for all women amid rapid screening technological advancements.”
The USPSTF’s decision to recommend an earlier start age for routine mammography was partly intended to begin to address the fact that Black women are about 40% more likely to die from breast cancer than are White women.
“Despite greater absolute benefits of screening for Black women, the modeling study and systematic review underscore that mammography’s benefits (ie, breast cancer deaths averted) are modest for both Black women and the general population,” wrote Dr. Elmore and Dr. Lee.
The editorialists also cautioned against adopting artificial intelligence (AI) support tools too rapidly, criticizing the USPSTF for overlooking this “pressing issue.”
“While AI algorithms show promise for enhancing cancer detection, their impact on patient outcomes and the balance between benefit and harms remain uncertain,” the editorialists wrote.
In a third editorial, in JAMA Oncology, Wendie A. Berg, MD, PhD, a radiologist at the University of Pittsburgh, argued that though the updated recommendations are “an important step forward,” they don’t go far enough.
Dr. Berg, for instance, noted her surprise “ to see the USPSTF recommendation only for biennial, rather than annual, screening among women aged 40 to 74 years.”
Compared with no screening, annual screening would reduce rates of breast cancer mortality (35.2%) more than biennial (28.4%) screening does among women aged 40-74 years, according to the CISNET modeling that informed the USPSTF’s decision.
Plus, Dr. Berg noted, regular risk assessments should begin at age 25 years “to identify women at high risk who should start annual MRI screenings.”
The American College of Radiology (ACR) offered similar views in a statement, saying the recommendations “do not go far enough to save more women’s lives.” It urged a more aggressive screening schedule, which starts at age 40 years but occurs annually vs biennially and continues past age 74 years. Like Dr. Berg, the ACR advocated for breast cancer risk assessments to begin at age 25 years.
The American Cancer Society also recommended annual mammography screening, starting as early as age 40 years in average-risk women, with high-risk women receiving a breast MRI and a mammogram every year starting at age 30 years.
Ongoing Uncertainties
The USPSTF’s 2024 update highlighted persistent evidence gaps in several key areas.
The USPSTF, for instance, highlighted insufficient evidence on the benefits and harms of continuing to screen women who are 75 years or older as well as the benefits and harms of supplemental screening with breast ultrasonography or MRI in women with dense breasts who had a negative screening mammogram.
In the update, USPSTF noted that it’s still clear what proportion of ductal carcinoma in situ involves lesions detected by screening would not have ultimately caused harm.
For women with dense breasts, the USPSTF said that “research is needed to help clinicians and patients understand the best strategy for breast cancer screening in women found to have dense breasts,” which includes supplemental screening.
Women with dense breasts should still get mammograms, but there is not enough evidence for a blanket statement about which benefit they might get from additional screening, Carol Mangione, MD, past chair of USPSTF, told this news organization.
“We don’t want to send a message that the mammogram doesn’t have value in that group, because it does have high value,” said Dr. Mangione, chief of the division of general internal medicine and health services research at UCLA Health.
Women with dense breasts should work with primary care clinicians who can take a holistic view of their preferences and needs, allowing them to make an informed choice about additional screening, she said.
“But we can’t make a global population choice because we don’t have the studies to do that,” Dr. Mangione said.
A version of this article appeared on Medscape.com.
In its updated recommendations published April 30 in JAMA, the USPSTF also made an urgent call to address reasons why Black women are more likely to die from breast cancer than are White women and pressed for more research to address persisting questions about how best to screen for cancer in dense breasts, which about 40% of women have. The USPSTF highlighted evidence gaps on the benefits and harms of continuing mammography after age 75 years as well.
The updated USPSTF recommendations were first unveiled last year in a draft version.
In 2016, the task force recommended biennial mammograms for women starting 10 years later, at age 50 years, while stressing a need for clinicians and patients to weigh the risks and benefits of screening for those in their 40s.
The shift to a general recommendation to start at age 40 years is based on a broad review of available data on mammography, including modeling from Cancer Intervention and Surveillance Modeling Network (CISNET).
Alongside the USPSTF report, JAMA published three separate editorials — a reflection of the controversy that these breast cancer screening recommendations often generate.
In one editorial, published in JAMA Network Open, Lydia E. Pace, MD, MPH, and Nancy L. Keating, MD, MPH, highlighted that though screening earlier will prevent more deaths from breast cancer, it will also lead to more false positive findings and increase rates of overdiagnosis.
Dr. Pace and Dr. Keating explained that the modeling study commissioned by the USPSTF estimated that screening every 2 years starting at age 40 years would avoid an additional 1.3 breast cancer deaths compared with screening at age 50 years. Among Black women, screening every 2 years starting at age 40 years would avert an extra 1.8 breast cancer deaths per 1000 people screened.
However, the model also found that screening every 2 years starting at age 40 years would lead to more false positive tests — a rate of about 8.5% vs 7.8% for those starting at age 50.
“Given mammography screening’s modest benefits, we feel that all women — and particularly those aged 40 to 49 years —should be counseled about the benefits and harms of mammography and supported in deciding whether the balance of benefits to harms fits with their priorities and values,” wrote Dr. Pace and Dr. Keating, who specialize in internal medicine.
In a second editorial, in JAMA, Joann G. Elmore, MD, MPH, of UCLA, and Christoph I. Lee, MD, MS, of the University of Washington, Seattle, noted that the revised recommendations “shed light on 2 major issues that demand greater attention: addressing health inequities related to breast cancer outcomes and ensuring benefits for all women amid rapid screening technological advancements.”
The USPSTF’s decision to recommend an earlier start age for routine mammography was partly intended to begin to address the fact that Black women are about 40% more likely to die from breast cancer than are White women.
“Despite greater absolute benefits of screening for Black women, the modeling study and systematic review underscore that mammography’s benefits (ie, breast cancer deaths averted) are modest for both Black women and the general population,” wrote Dr. Elmore and Dr. Lee.
The editorialists also cautioned against adopting artificial intelligence (AI) support tools too rapidly, criticizing the USPSTF for overlooking this “pressing issue.”
“While AI algorithms show promise for enhancing cancer detection, their impact on patient outcomes and the balance between benefit and harms remain uncertain,” the editorialists wrote.
In a third editorial, in JAMA Oncology, Wendie A. Berg, MD, PhD, a radiologist at the University of Pittsburgh, argued that though the updated recommendations are “an important step forward,” they don’t go far enough.
Dr. Berg, for instance, noted her surprise “ to see the USPSTF recommendation only for biennial, rather than annual, screening among women aged 40 to 74 years.”
Compared with no screening, annual screening would reduce rates of breast cancer mortality (35.2%) more than biennial (28.4%) screening does among women aged 40-74 years, according to the CISNET modeling that informed the USPSTF’s decision.
Plus, Dr. Berg noted, regular risk assessments should begin at age 25 years “to identify women at high risk who should start annual MRI screenings.”
The American College of Radiology (ACR) offered similar views in a statement, saying the recommendations “do not go far enough to save more women’s lives.” It urged a more aggressive screening schedule, which starts at age 40 years but occurs annually vs biennially and continues past age 74 years. Like Dr. Berg, the ACR advocated for breast cancer risk assessments to begin at age 25 years.
The American Cancer Society also recommended annual mammography screening, starting as early as age 40 years in average-risk women, with high-risk women receiving a breast MRI and a mammogram every year starting at age 30 years.
Ongoing Uncertainties
The USPSTF’s 2024 update highlighted persistent evidence gaps in several key areas.
The USPSTF, for instance, highlighted insufficient evidence on the benefits and harms of continuing to screen women who are 75 years or older as well as the benefits and harms of supplemental screening with breast ultrasonography or MRI in women with dense breasts who had a negative screening mammogram.
In the update, USPSTF noted that it’s still clear what proportion of ductal carcinoma in situ involves lesions detected by screening would not have ultimately caused harm.
For women with dense breasts, the USPSTF said that “research is needed to help clinicians and patients understand the best strategy for breast cancer screening in women found to have dense breasts,” which includes supplemental screening.
Women with dense breasts should still get mammograms, but there is not enough evidence for a blanket statement about which benefit they might get from additional screening, Carol Mangione, MD, past chair of USPSTF, told this news organization.
“We don’t want to send a message that the mammogram doesn’t have value in that group, because it does have high value,” said Dr. Mangione, chief of the division of general internal medicine and health services research at UCLA Health.
Women with dense breasts should work with primary care clinicians who can take a holistic view of their preferences and needs, allowing them to make an informed choice about additional screening, she said.
“But we can’t make a global population choice because we don’t have the studies to do that,” Dr. Mangione said.
A version of this article appeared on Medscape.com.




