User login
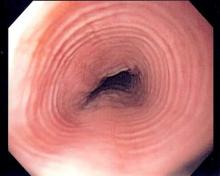
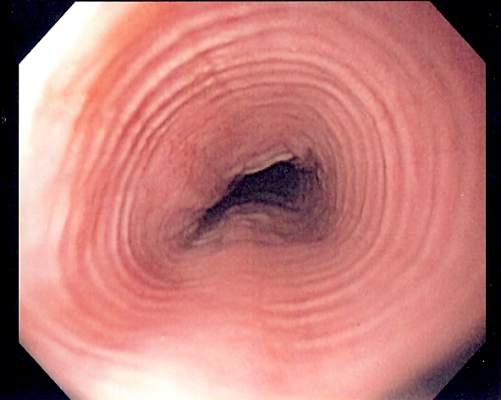
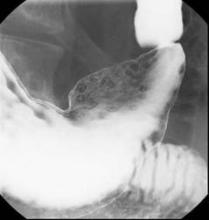
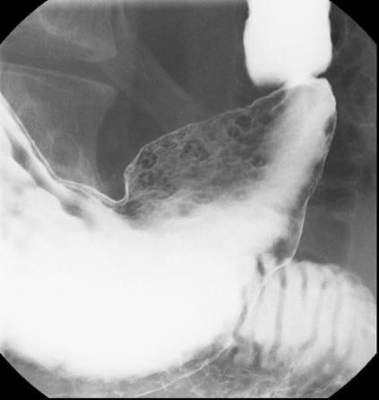
EREFS value has diagnostic utility for eosinophilic esophagitis
The Eosinophilic Esophagitis Endoscopic Reference Score, or EREFS, is not only highly predictive of eosinophilic esophagitis (EoE) but also its responsiveness to treatment, which suggests it may be used as an outcome measure, researchers say.
A prospective study of 211 adults undergoing upper endoscopy to investigate symptoms of esophageal dysfunction compared the EREFS with consensus guidelines for diagnosis of eosinophilic esophagitis.
The guidelines approach identified 67 cases of eosinophilic esophagitis and 144 control subjects without eosinophilic esophagitis. When these patients were assessed via the EREFS, researchers found multiple, highly significant differences between the cases and controls, with a mean total EREFS of 3.88 for cases and 0.42 for controls, according to a paper published online in Clinical Gastroenterology and Hepatology.
“On ROC [receiver operator characteristic] analysis, a model that contained all 5 components of the EREFS system as categorical variables had an AUC [area under the curve] of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone,” wrote Dr. Evan S. Dellon and colleagues of the University of North Carolina at Chapel Hill.
In this model, a score of 2.0 or above showed a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, negative predictive value of 94%, and accuracy of 91%.
Most of the score’s predictive ability was attributed to its inflammatory component, and less from the fibrostenotic score, which the authors suggested was due to the high prevalence of strictures in the control group.
The EREFS also improved significantly after treatment, in conjunction with endoscopic findings.
Total EREFS significantly decreased from 3.88 to 2.01, the inflammatory score decreased from 2.41 to 1.22, and the fibrostenotic score decreased from 1.46 to 0.89.
Histologic responders to treatment showed much more significant decreases in EREFS compared with nonresponders (Clin Gastro Hepatol. 2015, Sept. 12 [http://dx.doi.org/10.1016/j.cgh.2015.08.040]).
Researchers also examined the impact of weighing the various features of EREFS differently.
“The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (less than 15 eosinophil/hpf) for response,” they reported.
Based on this finding, the researchers created a set of EREFS scores using these varied weights, and showed that doubling the exudates, rings, and edema scores achieved the score’s maximum responsiveness while still keeping the weighting system simple, although these changes did not alter the score’s overall predictive ability.
The EREFS score was developed as a way to standardize the description, recognition, and reporting of eosinophilic esophagitis, but its diagnostic utility and responsiveness to treatment were unknown, the authors said.
“This prospective study found that the EREFS classification has diagnostic utility for EoE,” they wrote. “Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure.”
The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
The Eosinophilic Esophagitis Endoscopic Reference Score, or EREFS, is not only highly predictive of eosinophilic esophagitis (EoE) but also its responsiveness to treatment, which suggests it may be used as an outcome measure, researchers say.
A prospective study of 211 adults undergoing upper endoscopy to investigate symptoms of esophageal dysfunction compared the EREFS with consensus guidelines for diagnosis of eosinophilic esophagitis.
The guidelines approach identified 67 cases of eosinophilic esophagitis and 144 control subjects without eosinophilic esophagitis. When these patients were assessed via the EREFS, researchers found multiple, highly significant differences between the cases and controls, with a mean total EREFS of 3.88 for cases and 0.42 for controls, according to a paper published online in Clinical Gastroenterology and Hepatology.
“On ROC [receiver operator characteristic] analysis, a model that contained all 5 components of the EREFS system as categorical variables had an AUC [area under the curve] of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone,” wrote Dr. Evan S. Dellon and colleagues of the University of North Carolina at Chapel Hill.
In this model, a score of 2.0 or above showed a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, negative predictive value of 94%, and accuracy of 91%.
Most of the score’s predictive ability was attributed to its inflammatory component, and less from the fibrostenotic score, which the authors suggested was due to the high prevalence of strictures in the control group.
The EREFS also improved significantly after treatment, in conjunction with endoscopic findings.
Total EREFS significantly decreased from 3.88 to 2.01, the inflammatory score decreased from 2.41 to 1.22, and the fibrostenotic score decreased from 1.46 to 0.89.
Histologic responders to treatment showed much more significant decreases in EREFS compared with nonresponders (Clin Gastro Hepatol. 2015, Sept. 12 [http://dx.doi.org/10.1016/j.cgh.2015.08.040]).
Researchers also examined the impact of weighing the various features of EREFS differently.
“The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (less than 15 eosinophil/hpf) for response,” they reported.
Based on this finding, the researchers created a set of EREFS scores using these varied weights, and showed that doubling the exudates, rings, and edema scores achieved the score’s maximum responsiveness while still keeping the weighting system simple, although these changes did not alter the score’s overall predictive ability.
The EREFS score was developed as a way to standardize the description, recognition, and reporting of eosinophilic esophagitis, but its diagnostic utility and responsiveness to treatment were unknown, the authors said.
“This prospective study found that the EREFS classification has diagnostic utility for EoE,” they wrote. “Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure.”
The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
The Eosinophilic Esophagitis Endoscopic Reference Score, or EREFS, is not only highly predictive of eosinophilic esophagitis (EoE) but also its responsiveness to treatment, which suggests it may be used as an outcome measure, researchers say.
A prospective study of 211 adults undergoing upper endoscopy to investigate symptoms of esophageal dysfunction compared the EREFS with consensus guidelines for diagnosis of eosinophilic esophagitis.
The guidelines approach identified 67 cases of eosinophilic esophagitis and 144 control subjects without eosinophilic esophagitis. When these patients were assessed via the EREFS, researchers found multiple, highly significant differences between the cases and controls, with a mean total EREFS of 3.88 for cases and 0.42 for controls, according to a paper published online in Clinical Gastroenterology and Hepatology.
“On ROC [receiver operator characteristic] analysis, a model that contained all 5 components of the EREFS system as categorical variables had an AUC [area under the curve] of 0.946, indicating an excellent ability to predict EoE case status based on endoscopic findings alone,” wrote Dr. Evan S. Dellon and colleagues of the University of North Carolina at Chapel Hill.
In this model, a score of 2.0 or above showed a sensitivity of 88%, specificity of 92%, positive predictive value of 84%, negative predictive value of 94%, and accuracy of 91%.
Most of the score’s predictive ability was attributed to its inflammatory component, and less from the fibrostenotic score, which the authors suggested was due to the high prevalence of strictures in the control group.
The EREFS also improved significantly after treatment, in conjunction with endoscopic findings.
Total EREFS significantly decreased from 3.88 to 2.01, the inflammatory score decreased from 2.41 to 1.22, and the fibrostenotic score decreased from 1.46 to 0.89.
Histologic responders to treatment showed much more significant decreases in EREFS compared with nonresponders (Clin Gastro Hepatol. 2015, Sept. 12 [http://dx.doi.org/10.1016/j.cgh.2015.08.040]).
Researchers also examined the impact of weighing the various features of EREFS differently.
“The iterative analysis investigating weighing the EREFS features differently showed that increasing the weight of the exudate, rings, and edema score modestly increased the predictive power when the change in eosinophil counts was treated continuously and that increasing the weight of exudates and rings was beneficial with a threshold eosinophil count (less than 15 eosinophil/hpf) for response,” they reported.
Based on this finding, the researchers created a set of EREFS scores using these varied weights, and showed that doubling the exudates, rings, and edema scores achieved the score’s maximum responsiveness while still keeping the weighting system simple, although these changes did not alter the score’s overall predictive ability.
The EREFS score was developed as a way to standardize the description, recognition, and reporting of eosinophilic esophagitis, but its diagnostic utility and responsiveness to treatment were unknown, the authors said.
“This prospective study found that the EREFS classification has diagnostic utility for EoE,” they wrote. “Moreover, the score is responsive to treatment, decreasing significantly in histologic responders, and can be used as an outcome measure.”
The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The Eosinophilic Esophagitis Endoscopic Reference Score is highly predictive of eosinophilic esophagitis and responsiveness to treatment.
Major finding: A model containing all five components of the EREFS system as categorical variables had an AUC of 0.946.
Data source: A prospective study of 211 adults undergoing upper endoscopy to investigate esophageal dysfunction.
Disclosures: The National Institutes of Health and the University of North Carolina Center for Gastrointestinal Biology and Disease funded the study. No conflicts of interest were declared.
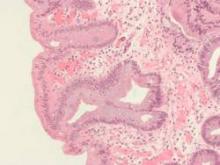
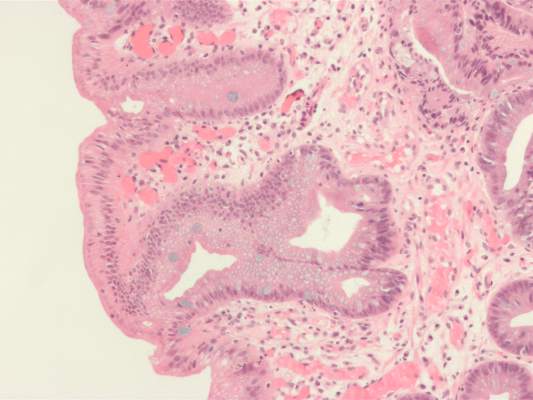
High serum leptin, insulin levels linked to Barrett’s esophagus risk
High serum insulin and leptin levels were significantly associated with Barrett’s esophagus, according to authors of a meta-analysis of nine observational studies published in the December issue of Clinical Gastroenterology and Hepatology.
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels, and were 1.74 times as likely to have hyperinsulinemia, said Dr. Apoorva Chandar of Case Western Reserve University (Cleveland) and his associates.
Central obesity was known to increase the risk of esophageal inflammation, metaplasia, and adenocarcinoma (Clin Gastroenterol Hepatol 2013 [doi: 10.1016/j.cgh.2013.05.009]), but this meta-analysis helped pinpoint the hormones that might mediate the relationship, the investigators said. However, the link between obesity and Barrett’s esophagus “is likely complex,” meriting additional longitudinal analyses, they added.
Metabolically active fat produces leptin and other adipokines. Elevated serum leptin has anti-apoptotic and angiogenic effects and also is a marker for insulin resistance, the researchers noted. “Several observational studies have examined the association of serum adipokines and insulin with Barrett’s esophagus, but evidence regarding this association remains inconclusive,” they said. Therefore, they reviewed observational studies published through April 2015 that examined relationships between Barrett’s esophagus, adipokines, and insulin. The studies included 10 separate cohorts of 1,432 patients with Barrett’s esophagus and 3,550 controls, enabling the researchers to estimate summary adjusted odds ratios (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.041]).
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels (adjusted OR, 2.23; 95% confidence interval [CI], 1.31-3.78) and 1.74 times as likely to have elevated serum insulin levels (95% CI, 1.14 to 2.65). Total serum adiponectin was not linked to risk of Barrett’s esophagus, but increased serum levels of high molecular weight (HMW) adiponectin were (aOR, 1.75; 95% CI, 1.16-2.63), and one study reported an inverse correlation between levels of low molecular weight leptin and Barrett’s esophagus risk. Low molecular weight adiponectin has anti-inflammatory effects, while HMW adiponectin is proinflammatory, the researchers noted.
“It is simplistic to assume that the effects of obesity on the development of Barrett’s esophagus are mediated by one single adipokine,” the researchers said. “Leptin and adiponectin seem to crosstalk, and both of these adipokines also affect insulin-signaling pathways.” Obesity is a chronic inflammatory state characterized by increases in other circulating cytokines, such as interleukin-6 and tumor necrosis factor–alpha, they noted. Their findings do not solely implicate leptin among the adipokines, but show that it “might be an important contributor, and support further studies on the effects of leptin on the leptin receptor in the proliferation of Barrett’s epithelium.” They also noted that although women have higher leptin levels than men, men are at much greater risk of Barrett’s esophagus, which their review could not explain. Studies to date are “not adequate” to assess gender-specific relationships between insulin, adipokines, and Barrett’s esophagus, they said.
Other evidence has linked insulin to Barrett’s esophagus, according to the researchers. Insulin and related signaling pathways are upregulated in tissue specimens of Barrett’s esophagus and esophageal adenocarcinoma, and Barrett’s esophagus is more likely to progress to esophageal adenocarcinoma in the setting of insulin resistance, they noted. “Given that recent studies have shown an association between Barrett’s esophagus and measures of central obesity and diabetes mellitus type 2, it is conceivable that hyperinsulinemia and insulin resistance, which are known consequences of central obesity, are associated with Barrett’s esophagus pathogenesis,” they said.
However, their study did not link hyperinsulinemia to Barrett’s esophagus among subjects with GERD, possibly because of confounding or overmatching, they noted. More rigorous studies would be needed to fairly evaluate any relationship between insulin resistance and risk of Barrett’s esophagus, they concluded.
The National Cancer Institute funded the study. The investigators had no conflicts of interest.
Epidemiologic studies have shown that abdominal, especially visceral as opposed to cutaneous, obesity isassociated with increased risk of Barrett’s esophagus. The precise mechanisms are unclear; however, there is increasing evidence that this association is likely mediated through both the mechanical effect of increased abdominal pressure promoting gastroesophageal reflux and the nonmechanical metabolic and inflammatory effects of abdominal obesity. Adipose tissue produces and releases a variety of proinflammatory and anti-inflammatory factors, including the adipokines leptin and adiponectin, as well as cytokines and chemokines. Leptin (higher levels in visceral fat) has proinflammatory effects that promote a low-grade inflammatory state, while adiponectin (less visceral fat) protects against the complications of obesity by exerting anti-inflammatory effects.

|
| Dr. Aaron Thrift |
Results from single-center studies examining associations of circulating adipokines, insulin, and inflammatory cytokines with Barrett’s esophagus have been conflicting, potentially due to methodologic shortcomings. In this article, Dr. Chandar and his colleagues conducted a meta-analysis and report that higher serum levels of leptin and insulin are associated with increased risk of Barrett’s esophagus, while there was no association between serum adiponectin and Barrett’s esophagus. This study highlights the complexity of these associations. For example, only leptin among the adipokines was associated with Barrett’s esophagus. Thus, additional longitudinal studies are required to further tease out these associations, and formal mediation analysis would help quantify how much of the obesity effect is through these hormones. From a clinical perspective, the importance of the findings of this paper is that these may be attractive targets for preventing Barrett’s esophagus.
Dr. Thrift is in the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. He has no conflicts of interest.
Epidemiologic studies have shown that abdominal, especially visceral as opposed to cutaneous, obesity isassociated with increased risk of Barrett’s esophagus. The precise mechanisms are unclear; however, there is increasing evidence that this association is likely mediated through both the mechanical effect of increased abdominal pressure promoting gastroesophageal reflux and the nonmechanical metabolic and inflammatory effects of abdominal obesity. Adipose tissue produces and releases a variety of proinflammatory and anti-inflammatory factors, including the adipokines leptin and adiponectin, as well as cytokines and chemokines. Leptin (higher levels in visceral fat) has proinflammatory effects that promote a low-grade inflammatory state, while adiponectin (less visceral fat) protects against the complications of obesity by exerting anti-inflammatory effects.

|
| Dr. Aaron Thrift |
Results from single-center studies examining associations of circulating adipokines, insulin, and inflammatory cytokines with Barrett’s esophagus have been conflicting, potentially due to methodologic shortcomings. In this article, Dr. Chandar and his colleagues conducted a meta-analysis and report that higher serum levels of leptin and insulin are associated with increased risk of Barrett’s esophagus, while there was no association between serum adiponectin and Barrett’s esophagus. This study highlights the complexity of these associations. For example, only leptin among the adipokines was associated with Barrett’s esophagus. Thus, additional longitudinal studies are required to further tease out these associations, and formal mediation analysis would help quantify how much of the obesity effect is through these hormones. From a clinical perspective, the importance of the findings of this paper is that these may be attractive targets for preventing Barrett’s esophagus.
Dr. Thrift is in the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. He has no conflicts of interest.
Epidemiologic studies have shown that abdominal, especially visceral as opposed to cutaneous, obesity isassociated with increased risk of Barrett’s esophagus. The precise mechanisms are unclear; however, there is increasing evidence that this association is likely mediated through both the mechanical effect of increased abdominal pressure promoting gastroesophageal reflux and the nonmechanical metabolic and inflammatory effects of abdominal obesity. Adipose tissue produces and releases a variety of proinflammatory and anti-inflammatory factors, including the adipokines leptin and adiponectin, as well as cytokines and chemokines. Leptin (higher levels in visceral fat) has proinflammatory effects that promote a low-grade inflammatory state, while adiponectin (less visceral fat) protects against the complications of obesity by exerting anti-inflammatory effects.

|
| Dr. Aaron Thrift |
Results from single-center studies examining associations of circulating adipokines, insulin, and inflammatory cytokines with Barrett’s esophagus have been conflicting, potentially due to methodologic shortcomings. In this article, Dr. Chandar and his colleagues conducted a meta-analysis and report that higher serum levels of leptin and insulin are associated with increased risk of Barrett’s esophagus, while there was no association between serum adiponectin and Barrett’s esophagus. This study highlights the complexity of these associations. For example, only leptin among the adipokines was associated with Barrett’s esophagus. Thus, additional longitudinal studies are required to further tease out these associations, and formal mediation analysis would help quantify how much of the obesity effect is through these hormones. From a clinical perspective, the importance of the findings of this paper is that these may be attractive targets for preventing Barrett’s esophagus.
Dr. Thrift is in the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. He has no conflicts of interest.
High serum insulin and leptin levels were significantly associated with Barrett’s esophagus, according to authors of a meta-analysis of nine observational studies published in the December issue of Clinical Gastroenterology and Hepatology.
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels, and were 1.74 times as likely to have hyperinsulinemia, said Dr. Apoorva Chandar of Case Western Reserve University (Cleveland) and his associates.
Central obesity was known to increase the risk of esophageal inflammation, metaplasia, and adenocarcinoma (Clin Gastroenterol Hepatol 2013 [doi: 10.1016/j.cgh.2013.05.009]), but this meta-analysis helped pinpoint the hormones that might mediate the relationship, the investigators said. However, the link between obesity and Barrett’s esophagus “is likely complex,” meriting additional longitudinal analyses, they added.
Metabolically active fat produces leptin and other adipokines. Elevated serum leptin has anti-apoptotic and angiogenic effects and also is a marker for insulin resistance, the researchers noted. “Several observational studies have examined the association of serum adipokines and insulin with Barrett’s esophagus, but evidence regarding this association remains inconclusive,” they said. Therefore, they reviewed observational studies published through April 2015 that examined relationships between Barrett’s esophagus, adipokines, and insulin. The studies included 10 separate cohorts of 1,432 patients with Barrett’s esophagus and 3,550 controls, enabling the researchers to estimate summary adjusted odds ratios (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.041]).
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels (adjusted OR, 2.23; 95% confidence interval [CI], 1.31-3.78) and 1.74 times as likely to have elevated serum insulin levels (95% CI, 1.14 to 2.65). Total serum adiponectin was not linked to risk of Barrett’s esophagus, but increased serum levels of high molecular weight (HMW) adiponectin were (aOR, 1.75; 95% CI, 1.16-2.63), and one study reported an inverse correlation between levels of low molecular weight leptin and Barrett’s esophagus risk. Low molecular weight adiponectin has anti-inflammatory effects, while HMW adiponectin is proinflammatory, the researchers noted.
“It is simplistic to assume that the effects of obesity on the development of Barrett’s esophagus are mediated by one single adipokine,” the researchers said. “Leptin and adiponectin seem to crosstalk, and both of these adipokines also affect insulin-signaling pathways.” Obesity is a chronic inflammatory state characterized by increases in other circulating cytokines, such as interleukin-6 and tumor necrosis factor–alpha, they noted. Their findings do not solely implicate leptin among the adipokines, but show that it “might be an important contributor, and support further studies on the effects of leptin on the leptin receptor in the proliferation of Barrett’s epithelium.” They also noted that although women have higher leptin levels than men, men are at much greater risk of Barrett’s esophagus, which their review could not explain. Studies to date are “not adequate” to assess gender-specific relationships between insulin, adipokines, and Barrett’s esophagus, they said.
Other evidence has linked insulin to Barrett’s esophagus, according to the researchers. Insulin and related signaling pathways are upregulated in tissue specimens of Barrett’s esophagus and esophageal adenocarcinoma, and Barrett’s esophagus is more likely to progress to esophageal adenocarcinoma in the setting of insulin resistance, they noted. “Given that recent studies have shown an association between Barrett’s esophagus and measures of central obesity and diabetes mellitus type 2, it is conceivable that hyperinsulinemia and insulin resistance, which are known consequences of central obesity, are associated with Barrett’s esophagus pathogenesis,” they said.
However, their study did not link hyperinsulinemia to Barrett’s esophagus among subjects with GERD, possibly because of confounding or overmatching, they noted. More rigorous studies would be needed to fairly evaluate any relationship between insulin resistance and risk of Barrett’s esophagus, they concluded.
The National Cancer Institute funded the study. The investigators had no conflicts of interest.
High serum insulin and leptin levels were significantly associated with Barrett’s esophagus, according to authors of a meta-analysis of nine observational studies published in the December issue of Clinical Gastroenterology and Hepatology.
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels, and were 1.74 times as likely to have hyperinsulinemia, said Dr. Apoorva Chandar of Case Western Reserve University (Cleveland) and his associates.
Central obesity was known to increase the risk of esophageal inflammation, metaplasia, and adenocarcinoma (Clin Gastroenterol Hepatol 2013 [doi: 10.1016/j.cgh.2013.05.009]), but this meta-analysis helped pinpoint the hormones that might mediate the relationship, the investigators said. However, the link between obesity and Barrett’s esophagus “is likely complex,” meriting additional longitudinal analyses, they added.
Metabolically active fat produces leptin and other adipokines. Elevated serum leptin has anti-apoptotic and angiogenic effects and also is a marker for insulin resistance, the researchers noted. “Several observational studies have examined the association of serum adipokines and insulin with Barrett’s esophagus, but evidence regarding this association remains inconclusive,” they said. Therefore, they reviewed observational studies published through April 2015 that examined relationships between Barrett’s esophagus, adipokines, and insulin. The studies included 10 separate cohorts of 1,432 patients with Barrett’s esophagus and 3,550 controls, enabling the researchers to estimate summary adjusted odds ratios (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.041]).
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels (adjusted OR, 2.23; 95% confidence interval [CI], 1.31-3.78) and 1.74 times as likely to have elevated serum insulin levels (95% CI, 1.14 to 2.65). Total serum adiponectin was not linked to risk of Barrett’s esophagus, but increased serum levels of high molecular weight (HMW) adiponectin were (aOR, 1.75; 95% CI, 1.16-2.63), and one study reported an inverse correlation between levels of low molecular weight leptin and Barrett’s esophagus risk. Low molecular weight adiponectin has anti-inflammatory effects, while HMW adiponectin is proinflammatory, the researchers noted.
“It is simplistic to assume that the effects of obesity on the development of Barrett’s esophagus are mediated by one single adipokine,” the researchers said. “Leptin and adiponectin seem to crosstalk, and both of these adipokines also affect insulin-signaling pathways.” Obesity is a chronic inflammatory state characterized by increases in other circulating cytokines, such as interleukin-6 and tumor necrosis factor–alpha, they noted. Their findings do not solely implicate leptin among the adipokines, but show that it “might be an important contributor, and support further studies on the effects of leptin on the leptin receptor in the proliferation of Barrett’s epithelium.” They also noted that although women have higher leptin levels than men, men are at much greater risk of Barrett’s esophagus, which their review could not explain. Studies to date are “not adequate” to assess gender-specific relationships between insulin, adipokines, and Barrett’s esophagus, they said.
Other evidence has linked insulin to Barrett’s esophagus, according to the researchers. Insulin and related signaling pathways are upregulated in tissue specimens of Barrett’s esophagus and esophageal adenocarcinoma, and Barrett’s esophagus is more likely to progress to esophageal adenocarcinoma in the setting of insulin resistance, they noted. “Given that recent studies have shown an association between Barrett’s esophagus and measures of central obesity and diabetes mellitus type 2, it is conceivable that hyperinsulinemia and insulin resistance, which are known consequences of central obesity, are associated with Barrett’s esophagus pathogenesis,” they said.
However, their study did not link hyperinsulinemia to Barrett’s esophagus among subjects with GERD, possibly because of confounding or overmatching, they noted. More rigorous studies would be needed to fairly evaluate any relationship between insulin resistance and risk of Barrett’s esophagus, they concluded.
The National Cancer Institute funded the study. The investigators had no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: High serum levels of leptin and insulin were associated with Barrett’s esophagus in a meta-analysis.
Major finding: Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels, and were 1.74 times as likely to have hyperinsulinemia.
Data source: Meta-analysis of nine observational studies that included 1,432 Barrett’s esophagus patients and 3,550 controls.
Disclosures: The National Cancer Institute funded the study. The investigators had no conflicts of interest.
Early TIPS tied to mortality reduction in esophageal bleeds
HONOLULU – Early use of a transjugular intrahepatic portosystemic shunt (TIPS) is associated with substantial reductions in mortality, according to an analysis of a national inpatient database.
Based on this study, “early use of TIPS, together with patient and physician education on current guidelines and protocols, should continue to be a priority to improve patient outcomes” in patients with hepatic cirrhosis and risk of recurrent esophageal variceal bleeds, reported Dr. Basile Njei, a gastroenterology fellow at Yale University, New Haven, Conn.
In this study, the Nationwide Inpatient Sample database was queried by ICD-9 codes to identify patients with esophageal variceal bleeding treated between the years 2000 and 2010. The goal was to compare early use of TIPS, defined as TIPS administered within 72 hours of the bleeding, relative to rescue TIPS, defined as TIPS after two or more episodes of bleeding or one bleeding episode followed by another endoscopic intervention, such as balloon tamponade or surgery.
Over the period of study, a Poisson regression analysis used to control for multiple variables associated any TIPS utilization with an inverse association with overall mortality, producing a relative risk of 0.88 (95% confidence interval, 0.83-0.92). In the context of timing of TIPS, in-hospital mortality fell from 5.6% for those who received rescue TIPS to 1.5% in those who underwent early TIPS.
On multivariate analysis, an advantage was observed for early TIPS relative to rescue TIPS for in-hospital mortality (RR, 0.85; P less than .01), in-hospital rebleeding (RR, 0.57; P less than .01), and length of hospital stay (RR, 0.87; P less than .01). Rates of sepsis (RR, 0.83; P = .32) and hepatic encephalopathy (RR, 0.87; P = .22) were not significantly lower in the early TIPS group, but they were also not increased. For early TIPS versus no TIPS, the advantages on multivariate analysis were similar for both in-hospital deaths (RR, 0.87; P less than .01) and in-hospital rebleeding (RR, 0.57; P less than .01), but no advantage was seen for length of stay for TIPS versus no TIPS (RR, 0.99; P = .18).
Overall, there was a steady decline in mortality associated with esophageal variceal bleeding over the period of evaluation, falling incrementally over time from 656 deaths per 100,000 hospitalizations in 2000 to 412 deaths per 100,000 in 2010. This 37.2% reduction was statistically significant (P less than .01). The reduction in mortality was inversely associated with an increasing use of TIPS over the study period.
The data from this analysis are consistent with a multicenter randomized trial conducted several years ago in Europe (N Engl J Med. 2010;362:2370-9). In that study 63 patients with hepatic cirrhosis and acute variceal bleeding who had been treated with vasoactive drugs plus endoscopic therapy were randomized to early TIPS or rescue TIPS. At 1 year, 86% of those in the early TIPS group were alive versus 61% (P = .01) of those randomized to receive TIPS as a rescue strategy.
Relative to the previous study, the key finding of this study is that early TIPS “is associated with significant short-term reductions in rebleeding and mortality without a significant increase in encephalopathy in real world U.S. clinical practice,” according to Dr. Njei. It substantiates the European study and encourages a protocol that emphasizes early TIPS, particularly in those with a high risk of repeat esophageal variceal bleeding.
In the discussion that followed the presentation of these results at the annual meeting of the American College of Gastroenterology, the moderator, Dr. Paul Y. Kwo, medical director of liver transplantation, Indiana University, Indianapolis, pointed out, that some of those in the rescue TIPS group might simply have been poor candidates for this intervention. Although he praised the methodology of this study, which won the 2015 ACG Fellows-In-Training Award, he questioned whether rescue TIPS was a last resort salvage therapy in those initially considered poor risks for TIPS. Dr. Njei responded that the multivariate analysis was specifically designed to control for variables such as risk status to diminish this potential bias. Indeed, he said he believes TIPS is underemployed.
“The relatively small percentage of eligible cases receiving early TIPS suggests that there is room for further improvement in the treatment of patients with decompensated cirrhosis and esophageal variceal bleeding,” Dr. Njei concluded.
Dr. Njei reported that he had no relevant financial relationships to disclose.
HONOLULU – Early use of a transjugular intrahepatic portosystemic shunt (TIPS) is associated with substantial reductions in mortality, according to an analysis of a national inpatient database.
Based on this study, “early use of TIPS, together with patient and physician education on current guidelines and protocols, should continue to be a priority to improve patient outcomes” in patients with hepatic cirrhosis and risk of recurrent esophageal variceal bleeds, reported Dr. Basile Njei, a gastroenterology fellow at Yale University, New Haven, Conn.
In this study, the Nationwide Inpatient Sample database was queried by ICD-9 codes to identify patients with esophageal variceal bleeding treated between the years 2000 and 2010. The goal was to compare early use of TIPS, defined as TIPS administered within 72 hours of the bleeding, relative to rescue TIPS, defined as TIPS after two or more episodes of bleeding or one bleeding episode followed by another endoscopic intervention, such as balloon tamponade or surgery.
Over the period of study, a Poisson regression analysis used to control for multiple variables associated any TIPS utilization with an inverse association with overall mortality, producing a relative risk of 0.88 (95% confidence interval, 0.83-0.92). In the context of timing of TIPS, in-hospital mortality fell from 5.6% for those who received rescue TIPS to 1.5% in those who underwent early TIPS.
On multivariate analysis, an advantage was observed for early TIPS relative to rescue TIPS for in-hospital mortality (RR, 0.85; P less than .01), in-hospital rebleeding (RR, 0.57; P less than .01), and length of hospital stay (RR, 0.87; P less than .01). Rates of sepsis (RR, 0.83; P = .32) and hepatic encephalopathy (RR, 0.87; P = .22) were not significantly lower in the early TIPS group, but they were also not increased. For early TIPS versus no TIPS, the advantages on multivariate analysis were similar for both in-hospital deaths (RR, 0.87; P less than .01) and in-hospital rebleeding (RR, 0.57; P less than .01), but no advantage was seen for length of stay for TIPS versus no TIPS (RR, 0.99; P = .18).
Overall, there was a steady decline in mortality associated with esophageal variceal bleeding over the period of evaluation, falling incrementally over time from 656 deaths per 100,000 hospitalizations in 2000 to 412 deaths per 100,000 in 2010. This 37.2% reduction was statistically significant (P less than .01). The reduction in mortality was inversely associated with an increasing use of TIPS over the study period.
The data from this analysis are consistent with a multicenter randomized trial conducted several years ago in Europe (N Engl J Med. 2010;362:2370-9). In that study 63 patients with hepatic cirrhosis and acute variceal bleeding who had been treated with vasoactive drugs plus endoscopic therapy were randomized to early TIPS or rescue TIPS. At 1 year, 86% of those in the early TIPS group were alive versus 61% (P = .01) of those randomized to receive TIPS as a rescue strategy.
Relative to the previous study, the key finding of this study is that early TIPS “is associated with significant short-term reductions in rebleeding and mortality without a significant increase in encephalopathy in real world U.S. clinical practice,” according to Dr. Njei. It substantiates the European study and encourages a protocol that emphasizes early TIPS, particularly in those with a high risk of repeat esophageal variceal bleeding.
In the discussion that followed the presentation of these results at the annual meeting of the American College of Gastroenterology, the moderator, Dr. Paul Y. Kwo, medical director of liver transplantation, Indiana University, Indianapolis, pointed out, that some of those in the rescue TIPS group might simply have been poor candidates for this intervention. Although he praised the methodology of this study, which won the 2015 ACG Fellows-In-Training Award, he questioned whether rescue TIPS was a last resort salvage therapy in those initially considered poor risks for TIPS. Dr. Njei responded that the multivariate analysis was specifically designed to control for variables such as risk status to diminish this potential bias. Indeed, he said he believes TIPS is underemployed.
“The relatively small percentage of eligible cases receiving early TIPS suggests that there is room for further improvement in the treatment of patients with decompensated cirrhosis and esophageal variceal bleeding,” Dr. Njei concluded.
Dr. Njei reported that he had no relevant financial relationships to disclose.
HONOLULU – Early use of a transjugular intrahepatic portosystemic shunt (TIPS) is associated with substantial reductions in mortality, according to an analysis of a national inpatient database.
Based on this study, “early use of TIPS, together with patient and physician education on current guidelines and protocols, should continue to be a priority to improve patient outcomes” in patients with hepatic cirrhosis and risk of recurrent esophageal variceal bleeds, reported Dr. Basile Njei, a gastroenterology fellow at Yale University, New Haven, Conn.
In this study, the Nationwide Inpatient Sample database was queried by ICD-9 codes to identify patients with esophageal variceal bleeding treated between the years 2000 and 2010. The goal was to compare early use of TIPS, defined as TIPS administered within 72 hours of the bleeding, relative to rescue TIPS, defined as TIPS after two or more episodes of bleeding or one bleeding episode followed by another endoscopic intervention, such as balloon tamponade or surgery.
Over the period of study, a Poisson regression analysis used to control for multiple variables associated any TIPS utilization with an inverse association with overall mortality, producing a relative risk of 0.88 (95% confidence interval, 0.83-0.92). In the context of timing of TIPS, in-hospital mortality fell from 5.6% for those who received rescue TIPS to 1.5% in those who underwent early TIPS.
On multivariate analysis, an advantage was observed for early TIPS relative to rescue TIPS for in-hospital mortality (RR, 0.85; P less than .01), in-hospital rebleeding (RR, 0.57; P less than .01), and length of hospital stay (RR, 0.87; P less than .01). Rates of sepsis (RR, 0.83; P = .32) and hepatic encephalopathy (RR, 0.87; P = .22) were not significantly lower in the early TIPS group, but they were also not increased. For early TIPS versus no TIPS, the advantages on multivariate analysis were similar for both in-hospital deaths (RR, 0.87; P less than .01) and in-hospital rebleeding (RR, 0.57; P less than .01), but no advantage was seen for length of stay for TIPS versus no TIPS (RR, 0.99; P = .18).
Overall, there was a steady decline in mortality associated with esophageal variceal bleeding over the period of evaluation, falling incrementally over time from 656 deaths per 100,000 hospitalizations in 2000 to 412 deaths per 100,000 in 2010. This 37.2% reduction was statistically significant (P less than .01). The reduction in mortality was inversely associated with an increasing use of TIPS over the study period.
The data from this analysis are consistent with a multicenter randomized trial conducted several years ago in Europe (N Engl J Med. 2010;362:2370-9). In that study 63 patients with hepatic cirrhosis and acute variceal bleeding who had been treated with vasoactive drugs plus endoscopic therapy were randomized to early TIPS or rescue TIPS. At 1 year, 86% of those in the early TIPS group were alive versus 61% (P = .01) of those randomized to receive TIPS as a rescue strategy.
Relative to the previous study, the key finding of this study is that early TIPS “is associated with significant short-term reductions in rebleeding and mortality without a significant increase in encephalopathy in real world U.S. clinical practice,” according to Dr. Njei. It substantiates the European study and encourages a protocol that emphasizes early TIPS, particularly in those with a high risk of repeat esophageal variceal bleeding.
In the discussion that followed the presentation of these results at the annual meeting of the American College of Gastroenterology, the moderator, Dr. Paul Y. Kwo, medical director of liver transplantation, Indiana University, Indianapolis, pointed out, that some of those in the rescue TIPS group might simply have been poor candidates for this intervention. Although he praised the methodology of this study, which won the 2015 ACG Fellows-In-Training Award, he questioned whether rescue TIPS was a last resort salvage therapy in those initially considered poor risks for TIPS. Dr. Njei responded that the multivariate analysis was specifically designed to control for variables such as risk status to diminish this potential bias. Indeed, he said he believes TIPS is underemployed.
“The relatively small percentage of eligible cases receiving early TIPS suggests that there is room for further improvement in the treatment of patients with decompensated cirrhosis and esophageal variceal bleeding,” Dr. Njei concluded.
Dr. Njei reported that he had no relevant financial relationships to disclose.
AT ACG 2015
Key clinical point:Early use of a transjugular intrahepatic portosystemic shunt to reduce the risk of esophageal variceal rebleeding is associated with reduced mortality.
Major finding: In those receiving early TIPS (TIPS administered within 72 hours of the bleeding) mortality was 1.5% vs. 5.6% for those receiving TIPS as rescue therapy.
Data source: A retrospective evaluation of a national inpatient database.
Disclosures: Dr. Njei reported that he had no relevant financial relationships to disclose.
Disparity found in PPI risk perception among physicians
HONOLULU – A survey of almost 500 physicians found that primary care physicians (PCPs) are far more concerned about the reported adverse effects of proton pump inhibitors (PPIs) than are gastroenterologists and use them more sparingly. The results of the survey were presented at the 2015 American College of Gastroenterology (ACG) Annual Scientific Meeting and Postgraduate Course.
“We asked physicians about a broad array of adverse effects from long-term use of PPIs and PCPs expressed greater concern for all of them,” reported Dr. Samir Kapadia, division of gastroenterology and hepatology, State University of New York at Stony Brook. “Alternatively, significantly more gastroenterologists responded that they really had no concerns for any of these adverse effects.”
The evidence may be on the side of the gastroenterologists, according to Dr. Kapadia. Although PPIs have been associated with hypomagnesemia, iron deficiency, vitamin B12 deficiency, diarrhea caused by Clostridium difficile infection, and interactions with the platelet inhibitor clopidogrel, Dr. Kapadia noted that few associations have been made on the basis of prospective trials.
“Much of the available literature is observational or based on studies that are heterogeneous and small,” Dr. Kapadia. “Confounding factors in these studies also limit interpretation.”
In this study for which surveys are still being collected, a 19-item questionnaire was distributed to 384 gastroenterologists and 88 PCPs. In addition to demographic information, the surveys were designed to capture opinions about the safety of PPIs as well as elicit information about how these agents are being used in clinical practice.
Of side effects associated with PPIs, significantly more PCPs than gastroenterologists expressed concern about hypomagnesemia (41.7% vs. 6.3%; P less than .001), iron deficiency (33.3% vs. 11.4%; P = .014) and vitamin B12 deficiency (47.6% vs. 17.3%; P = .005). From the other perspective, when asked about their concern for these and other safety issues, the answer was “none of the above” for 26.2% of PCPs and 67.1% of gastroenterologists (P less than .001).
When given specific risk scenarios, PCPs were consistently more prepared to discontinue PPI therapy than were gastroenterologists. For example, in a hypothetical 65-year-old with GERD symptoms expressing concern about risk of hip fracture, 64.5% of PCPs vs. 30.7% of gastroenterologists (P less than .001) responded that they would discontinue the PPI. In a patient of the same age about to start broad-spectrum antibiotics for cellulitis, 16.1% of PCPs, but only 4.3% of gastroenterologists (P = .001) reported that they would discontinue PPIs. Conversely, 68.5% of gastroenterologists vs. 54.2% of PCPs (P = .028) would continue therapy.
For a hypothetical 65-year-old with symptomatic gastroesophageal reflux disease (GERD) initiating clopidogrel, 50% of PCPs vs. 27.6% of gastroenterologists (P = .001) would switch to an H2-receptor antagonist. Only 27.3% of PCPs vs. 46.4% of gastroenterologists (P = .001) would continue the PPI. When the age of the hypothetical patient is raised to 75 years, PCPs, but not gastroenterologists, were even more likely to discontinue PPI therapy.
Using PPIs appropriately is an important goal, Dr. Kapadia emphasized. However, he suggested that many warnings about the risks of PPIs, including those issued by the Food and Drug Administration, are incompletely substantiated and are not being evaluated with an appropriate attention to benefit-to-risk ratio of a drug that not only controls symptoms but may also reduce risk of GI bleeding. Others share this point of view.
“The pendulum has moved too far in regard to the fear of potential side effects,” agreed Dr. Philip Katz, chairman, division of gastroenterology, Albert Einstein Medical Center, Philadelphia. First author of the 2013 ACG guidelines on GERD, which addresses the safety of PPIs (Am J Gastroenterol. 2013;108:308-28), Dr. Katz said in an interview that the data generated by this survey suggest that PCPs are misinterpreting the relative risks and need to be given more information about indications in which benefits are well established.
Making the same point, Dr. Nicholas J. Shaheen, chief, division of gastroenterology and hepatology, University of North Carolina, Chapel Hill, suggested “This may be a failure on our part [as gastroenterologists] to educate our colleagues about the role of these drugs.”
Dr. Kapadia reported no potential conflicts.
HONOLULU – A survey of almost 500 physicians found that primary care physicians (PCPs) are far more concerned about the reported adverse effects of proton pump inhibitors (PPIs) than are gastroenterologists and use them more sparingly. The results of the survey were presented at the 2015 American College of Gastroenterology (ACG) Annual Scientific Meeting and Postgraduate Course.
“We asked physicians about a broad array of adverse effects from long-term use of PPIs and PCPs expressed greater concern for all of them,” reported Dr. Samir Kapadia, division of gastroenterology and hepatology, State University of New York at Stony Brook. “Alternatively, significantly more gastroenterologists responded that they really had no concerns for any of these adverse effects.”
The evidence may be on the side of the gastroenterologists, according to Dr. Kapadia. Although PPIs have been associated with hypomagnesemia, iron deficiency, vitamin B12 deficiency, diarrhea caused by Clostridium difficile infection, and interactions with the platelet inhibitor clopidogrel, Dr. Kapadia noted that few associations have been made on the basis of prospective trials.
“Much of the available literature is observational or based on studies that are heterogeneous and small,” Dr. Kapadia. “Confounding factors in these studies also limit interpretation.”
In this study for which surveys are still being collected, a 19-item questionnaire was distributed to 384 gastroenterologists and 88 PCPs. In addition to demographic information, the surveys were designed to capture opinions about the safety of PPIs as well as elicit information about how these agents are being used in clinical practice.
Of side effects associated with PPIs, significantly more PCPs than gastroenterologists expressed concern about hypomagnesemia (41.7% vs. 6.3%; P less than .001), iron deficiency (33.3% vs. 11.4%; P = .014) and vitamin B12 deficiency (47.6% vs. 17.3%; P = .005). From the other perspective, when asked about their concern for these and other safety issues, the answer was “none of the above” for 26.2% of PCPs and 67.1% of gastroenterologists (P less than .001).
When given specific risk scenarios, PCPs were consistently more prepared to discontinue PPI therapy than were gastroenterologists. For example, in a hypothetical 65-year-old with GERD symptoms expressing concern about risk of hip fracture, 64.5% of PCPs vs. 30.7% of gastroenterologists (P less than .001) responded that they would discontinue the PPI. In a patient of the same age about to start broad-spectrum antibiotics for cellulitis, 16.1% of PCPs, but only 4.3% of gastroenterologists (P = .001) reported that they would discontinue PPIs. Conversely, 68.5% of gastroenterologists vs. 54.2% of PCPs (P = .028) would continue therapy.
For a hypothetical 65-year-old with symptomatic gastroesophageal reflux disease (GERD) initiating clopidogrel, 50% of PCPs vs. 27.6% of gastroenterologists (P = .001) would switch to an H2-receptor antagonist. Only 27.3% of PCPs vs. 46.4% of gastroenterologists (P = .001) would continue the PPI. When the age of the hypothetical patient is raised to 75 years, PCPs, but not gastroenterologists, were even more likely to discontinue PPI therapy.
Using PPIs appropriately is an important goal, Dr. Kapadia emphasized. However, he suggested that many warnings about the risks of PPIs, including those issued by the Food and Drug Administration, are incompletely substantiated and are not being evaluated with an appropriate attention to benefit-to-risk ratio of a drug that not only controls symptoms but may also reduce risk of GI bleeding. Others share this point of view.
“The pendulum has moved too far in regard to the fear of potential side effects,” agreed Dr. Philip Katz, chairman, division of gastroenterology, Albert Einstein Medical Center, Philadelphia. First author of the 2013 ACG guidelines on GERD, which addresses the safety of PPIs (Am J Gastroenterol. 2013;108:308-28), Dr. Katz said in an interview that the data generated by this survey suggest that PCPs are misinterpreting the relative risks and need to be given more information about indications in which benefits are well established.
Making the same point, Dr. Nicholas J. Shaheen, chief, division of gastroenterology and hepatology, University of North Carolina, Chapel Hill, suggested “This may be a failure on our part [as gastroenterologists] to educate our colleagues about the role of these drugs.”
Dr. Kapadia reported no potential conflicts.
HONOLULU – A survey of almost 500 physicians found that primary care physicians (PCPs) are far more concerned about the reported adverse effects of proton pump inhibitors (PPIs) than are gastroenterologists and use them more sparingly. The results of the survey were presented at the 2015 American College of Gastroenterology (ACG) Annual Scientific Meeting and Postgraduate Course.
“We asked physicians about a broad array of adverse effects from long-term use of PPIs and PCPs expressed greater concern for all of them,” reported Dr. Samir Kapadia, division of gastroenterology and hepatology, State University of New York at Stony Brook. “Alternatively, significantly more gastroenterologists responded that they really had no concerns for any of these adverse effects.”
The evidence may be on the side of the gastroenterologists, according to Dr. Kapadia. Although PPIs have been associated with hypomagnesemia, iron deficiency, vitamin B12 deficiency, diarrhea caused by Clostridium difficile infection, and interactions with the platelet inhibitor clopidogrel, Dr. Kapadia noted that few associations have been made on the basis of prospective trials.
“Much of the available literature is observational or based on studies that are heterogeneous and small,” Dr. Kapadia. “Confounding factors in these studies also limit interpretation.”
In this study for which surveys are still being collected, a 19-item questionnaire was distributed to 384 gastroenterologists and 88 PCPs. In addition to demographic information, the surveys were designed to capture opinions about the safety of PPIs as well as elicit information about how these agents are being used in clinical practice.
Of side effects associated with PPIs, significantly more PCPs than gastroenterologists expressed concern about hypomagnesemia (41.7% vs. 6.3%; P less than .001), iron deficiency (33.3% vs. 11.4%; P = .014) and vitamin B12 deficiency (47.6% vs. 17.3%; P = .005). From the other perspective, when asked about their concern for these and other safety issues, the answer was “none of the above” for 26.2% of PCPs and 67.1% of gastroenterologists (P less than .001).
When given specific risk scenarios, PCPs were consistently more prepared to discontinue PPI therapy than were gastroenterologists. For example, in a hypothetical 65-year-old with GERD symptoms expressing concern about risk of hip fracture, 64.5% of PCPs vs. 30.7% of gastroenterologists (P less than .001) responded that they would discontinue the PPI. In a patient of the same age about to start broad-spectrum antibiotics for cellulitis, 16.1% of PCPs, but only 4.3% of gastroenterologists (P = .001) reported that they would discontinue PPIs. Conversely, 68.5% of gastroenterologists vs. 54.2% of PCPs (P = .028) would continue therapy.
For a hypothetical 65-year-old with symptomatic gastroesophageal reflux disease (GERD) initiating clopidogrel, 50% of PCPs vs. 27.6% of gastroenterologists (P = .001) would switch to an H2-receptor antagonist. Only 27.3% of PCPs vs. 46.4% of gastroenterologists (P = .001) would continue the PPI. When the age of the hypothetical patient is raised to 75 years, PCPs, but not gastroenterologists, were even more likely to discontinue PPI therapy.
Using PPIs appropriately is an important goal, Dr. Kapadia emphasized. However, he suggested that many warnings about the risks of PPIs, including those issued by the Food and Drug Administration, are incompletely substantiated and are not being evaluated with an appropriate attention to benefit-to-risk ratio of a drug that not only controls symptoms but may also reduce risk of GI bleeding. Others share this point of view.
“The pendulum has moved too far in regard to the fear of potential side effects,” agreed Dr. Philip Katz, chairman, division of gastroenterology, Albert Einstein Medical Center, Philadelphia. First author of the 2013 ACG guidelines on GERD, which addresses the safety of PPIs (Am J Gastroenterol. 2013;108:308-28), Dr. Katz said in an interview that the data generated by this survey suggest that PCPs are misinterpreting the relative risks and need to be given more information about indications in which benefits are well established.
Making the same point, Dr. Nicholas J. Shaheen, chief, division of gastroenterology and hepatology, University of North Carolina, Chapel Hill, suggested “This may be a failure on our part [as gastroenterologists] to educate our colleagues about the role of these drugs.”
Dr. Kapadia reported no potential conflicts.
FROM THE AMERICAN COLLEGE OF GASTROENTEROLOGY 2015 SCIENTIFIC MEETING AND POSTGRADUATE COURSE
Key clinical point: Primary care physicians used proton pump inhibitors more sparingly, were more concerned about reported adverse effects than were gastroenterologists, but are perhaps too cautious in the cost-benefit analysis.
Major finding: Primary care physicians (PCPs) are far more concerned about the reported adverse effects of proton pump inhibitors than are gastroenterologists.
Data source: A survey of nearly 500 physicians, weighted toward gastroenterologists.
Disclosures: Dr. Kapadia reported no potential conflicts of interest.
GI bleeds in obese patients more complicated but no more fatal
HONOLULU – Obese patients who develop an upper GI bleed receive more treatment, are more likely to develop hemorrhagic shock, and are more likely to require admission to an intensive care unit than those who are not obese, but they do not have higher in-hospital mortality, according to analysis of a large national database that was presented at the annual meeting of the American College of Gastroenterology.
The significantly greater odds ratio of most major complications from upper GI bleeds in patients with obesity relative to those who are not obese was expected but so was an increased rate of in-hospital mortality, according to the first author of the study, Dr. Marwan S. Abou Gergi of Catalyst Medical Consulting, Baltimore.
“In this study, patients with obesity received more frequent endoscopic interventions, which could explain why mortality rates were not significantly higher,” Dr. Abou Gergi reported.
In the analysis, characterized as the first study to evaluate the impact of obesity on outcomes in upper GI hemorrhage, data were drawn from the 2012 Nationwide Inpatient Sample database, which is considered to provide a representative sample of U.S. hospital experience. Drawn from more than 7 million hospitalizations, the study focused on patients 18 or over with a primary ICD-9 code for upper GI hemorrhage. The primary outcome was mortality. Secondary outcomes included interventions, ICU admissions, and length of stay.
Of the 132,545 discharges with upper GI hemorrhage, 11,220 (8.5%) were identified as obese. The in-hospital mortality overall was 1.97%, but the proportion of those who died was slightly lower among patients identified as obese, producing a nonsignificant adjusted odds ratio (OR) of 0.87 (P greater than .1). Yet the rates of hemorrhage shock (OR 1.31; P = .02) and admission to the ICU (OR 1.35; P less than .02) were greater in the obese. The median length of stay of 0.35 days for obese patients was also significantly longer.
One reason for the lower rate of mortality may be more aggressive treatment. In particular, repeat endoscopy therapy was more common in those who were obese (P less than .01), suggesting, “Our treatments are working,” Dr. Abou Gergi said.
The proportion of patients with a score of 3 or greater on the Charlson comorbidity index was higher in the obese than in those not identified as obese (49% vs. 39%; P less than .01). This along with previously published evidence that obese patients take more anticoagulants, take more antiplatelets, and may face delays in endoscopy due to greater difficulty in administering sedation, were among considerations predicting a higher mortality, according to Dr. Abou Gergi.
However, there are several potential explanations for these unexpected findings. One is that mortality rates overall were low, making it difficult to show differences on this outcome. In addition, ICD-9 codes may be effective for isolating a group with obesity but not in identifying a control group without obesity.
“It is likely that not all patients who are obese received this code, so we may be seeing a population of obese patients be compared to another population that includes at least some patients who also have obesity,” explained Dr. John R. Saltzman, director of endoscopy, Brigham and Women’s Hospital, Boston. A coauthor of this study, Dr. Saltzman noted that despite a comparable rate of in-hospital mortality, most of the findings in the study argued that patients who develop upper GI bleeding have a more difficult course. He noted that this is reflected in the cost of care, which was significantly higher in those who were obese.
Although he acknowledged that he was surprised that this was “essentially a negative study,” he believes that mortality may have been “too tough” as a primary endpoint for demonstrating a difference. However, he also believes that it may be appropriate to give credit for effective treatments.
“I think that may be the key. We are just getting better at taking care of these patients,” Dr. Saltzman said.
Dr. Abou Gergi reported he has no relevant financial relationships.
HONOLULU – Obese patients who develop an upper GI bleed receive more treatment, are more likely to develop hemorrhagic shock, and are more likely to require admission to an intensive care unit than those who are not obese, but they do not have higher in-hospital mortality, according to analysis of a large national database that was presented at the annual meeting of the American College of Gastroenterology.
The significantly greater odds ratio of most major complications from upper GI bleeds in patients with obesity relative to those who are not obese was expected but so was an increased rate of in-hospital mortality, according to the first author of the study, Dr. Marwan S. Abou Gergi of Catalyst Medical Consulting, Baltimore.
“In this study, patients with obesity received more frequent endoscopic interventions, which could explain why mortality rates were not significantly higher,” Dr. Abou Gergi reported.
In the analysis, characterized as the first study to evaluate the impact of obesity on outcomes in upper GI hemorrhage, data were drawn from the 2012 Nationwide Inpatient Sample database, which is considered to provide a representative sample of U.S. hospital experience. Drawn from more than 7 million hospitalizations, the study focused on patients 18 or over with a primary ICD-9 code for upper GI hemorrhage. The primary outcome was mortality. Secondary outcomes included interventions, ICU admissions, and length of stay.
Of the 132,545 discharges with upper GI hemorrhage, 11,220 (8.5%) were identified as obese. The in-hospital mortality overall was 1.97%, but the proportion of those who died was slightly lower among patients identified as obese, producing a nonsignificant adjusted odds ratio (OR) of 0.87 (P greater than .1). Yet the rates of hemorrhage shock (OR 1.31; P = .02) and admission to the ICU (OR 1.35; P less than .02) were greater in the obese. The median length of stay of 0.35 days for obese patients was also significantly longer.
One reason for the lower rate of mortality may be more aggressive treatment. In particular, repeat endoscopy therapy was more common in those who were obese (P less than .01), suggesting, “Our treatments are working,” Dr. Abou Gergi said.
The proportion of patients with a score of 3 or greater on the Charlson comorbidity index was higher in the obese than in those not identified as obese (49% vs. 39%; P less than .01). This along with previously published evidence that obese patients take more anticoagulants, take more antiplatelets, and may face delays in endoscopy due to greater difficulty in administering sedation, were among considerations predicting a higher mortality, according to Dr. Abou Gergi.
However, there are several potential explanations for these unexpected findings. One is that mortality rates overall were low, making it difficult to show differences on this outcome. In addition, ICD-9 codes may be effective for isolating a group with obesity but not in identifying a control group without obesity.
“It is likely that not all patients who are obese received this code, so we may be seeing a population of obese patients be compared to another population that includes at least some patients who also have obesity,” explained Dr. John R. Saltzman, director of endoscopy, Brigham and Women’s Hospital, Boston. A coauthor of this study, Dr. Saltzman noted that despite a comparable rate of in-hospital mortality, most of the findings in the study argued that patients who develop upper GI bleeding have a more difficult course. He noted that this is reflected in the cost of care, which was significantly higher in those who were obese.
Although he acknowledged that he was surprised that this was “essentially a negative study,” he believes that mortality may have been “too tough” as a primary endpoint for demonstrating a difference. However, he also believes that it may be appropriate to give credit for effective treatments.
“I think that may be the key. We are just getting better at taking care of these patients,” Dr. Saltzman said.
Dr. Abou Gergi reported he has no relevant financial relationships.
HONOLULU – Obese patients who develop an upper GI bleed receive more treatment, are more likely to develop hemorrhagic shock, and are more likely to require admission to an intensive care unit than those who are not obese, but they do not have higher in-hospital mortality, according to analysis of a large national database that was presented at the annual meeting of the American College of Gastroenterology.
The significantly greater odds ratio of most major complications from upper GI bleeds in patients with obesity relative to those who are not obese was expected but so was an increased rate of in-hospital mortality, according to the first author of the study, Dr. Marwan S. Abou Gergi of Catalyst Medical Consulting, Baltimore.
“In this study, patients with obesity received more frequent endoscopic interventions, which could explain why mortality rates were not significantly higher,” Dr. Abou Gergi reported.
In the analysis, characterized as the first study to evaluate the impact of obesity on outcomes in upper GI hemorrhage, data were drawn from the 2012 Nationwide Inpatient Sample database, which is considered to provide a representative sample of U.S. hospital experience. Drawn from more than 7 million hospitalizations, the study focused on patients 18 or over with a primary ICD-9 code for upper GI hemorrhage. The primary outcome was mortality. Secondary outcomes included interventions, ICU admissions, and length of stay.
Of the 132,545 discharges with upper GI hemorrhage, 11,220 (8.5%) were identified as obese. The in-hospital mortality overall was 1.97%, but the proportion of those who died was slightly lower among patients identified as obese, producing a nonsignificant adjusted odds ratio (OR) of 0.87 (P greater than .1). Yet the rates of hemorrhage shock (OR 1.31; P = .02) and admission to the ICU (OR 1.35; P less than .02) were greater in the obese. The median length of stay of 0.35 days for obese patients was also significantly longer.
One reason for the lower rate of mortality may be more aggressive treatment. In particular, repeat endoscopy therapy was more common in those who were obese (P less than .01), suggesting, “Our treatments are working,” Dr. Abou Gergi said.
The proportion of patients with a score of 3 or greater on the Charlson comorbidity index was higher in the obese than in those not identified as obese (49% vs. 39%; P less than .01). This along with previously published evidence that obese patients take more anticoagulants, take more antiplatelets, and may face delays in endoscopy due to greater difficulty in administering sedation, were among considerations predicting a higher mortality, according to Dr. Abou Gergi.
However, there are several potential explanations for these unexpected findings. One is that mortality rates overall were low, making it difficult to show differences on this outcome. In addition, ICD-9 codes may be effective for isolating a group with obesity but not in identifying a control group without obesity.
“It is likely that not all patients who are obese received this code, so we may be seeing a population of obese patients be compared to another population that includes at least some patients who also have obesity,” explained Dr. John R. Saltzman, director of endoscopy, Brigham and Women’s Hospital, Boston. A coauthor of this study, Dr. Saltzman noted that despite a comparable rate of in-hospital mortality, most of the findings in the study argued that patients who develop upper GI bleeding have a more difficult course. He noted that this is reflected in the cost of care, which was significantly higher in those who were obese.
Although he acknowledged that he was surprised that this was “essentially a negative study,” he believes that mortality may have been “too tough” as a primary endpoint for demonstrating a difference. However, he also believes that it may be appropriate to give credit for effective treatments.
“I think that may be the key. We are just getting better at taking care of these patients,” Dr. Saltzman said.
Dr. Abou Gergi reported he has no relevant financial relationships.
AT ACG 2015
Upper GI tract
This year’s session on esophagus/upper GI at the AGA Spring Postgraduate Course was packed with pragmatic, useful information for the evaluation of patients with upper GI disorders. The session began with a talk on the manifestations of extraesophageal reflux disease. The take-home message of this talk was that putative extraesophageal manifestations of gastroesophageal reflux disease rarely respond to high-dose therapy with PPIs (proton-pump inhibitors), in the absence of concurrent esophageal symptoms such as heartburn or regurgitation. In such situations, investigation of other etiologies of patients’ symptoms, including occult postnasal drainage or cough-variant asthma, may be more rewarding than escalating anti-acid therapy.
Dr. John E. Pandolfino, AGAF, of Northwestern University, Chicago, discussed the utilization of high-resolution manometry in the evaluation of dysphagia. A central focus of this discussion was the use of the Chicago classification of motility abnormalities in assessing these patients. In the future, it is likely that the care of these patients will be dictated by the type of abnormality the patient has in this classification scheme. Especially in the setting of achalasia, data are emerging that some subtypes of achalasia are less likely to respond to some therapies. For instance, type 3 achalasia is unlikely to respond to pneumatic balloon dilatation.
Dr. Rhonda Souza of the University of Texas Southwestern Medical Center, Dallas, taught us that a diagnosis of eosinophilic esophagitis requires an 8-week trial of PPI therapy with no resolution of the eosinophilia. Esophageal dilation is generally safe in the setting of a dominant stricture, but it can be delayed prior to medical therapy if the patient is tolerating oral intake well. Although the most commonly used therapies for EoE now involve either swallowed steroids or dietary elimination therapy, several new agents are on the horizon that may give us new treatment options.
Finally, Dr. Amitabh Chak of Case Western Reserve University, Cleveland, reviewed the care of patients with Barrett’s esophagus and low-grade dysplasia. This is an especially difficult group of patients to care for, due in part to the low reproducibility in the diagnosis of low-grade dysplasia, as well as the highly variable reported cancer outcomes in this patient population. Level 1 evidence now exists demonstrating that treatment with radiofrequency ablation decreases the incidence of cancer in patients with low-grade dysplasia, but most patients with this finding will not progress. Therefore, the field would benefit from better risk stratification of these patients.
Dr. Shaheen is professor of medicine and epidemiology and chief of the division of gastroenterology & hepatology, University of North Carolina School of Medicine, Chapel Hill. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2015.
This year’s session on esophagus/upper GI at the AGA Spring Postgraduate Course was packed with pragmatic, useful information for the evaluation of patients with upper GI disorders. The session began with a talk on the manifestations of extraesophageal reflux disease. The take-home message of this talk was that putative extraesophageal manifestations of gastroesophageal reflux disease rarely respond to high-dose therapy with PPIs (proton-pump inhibitors), in the absence of concurrent esophageal symptoms such as heartburn or regurgitation. In such situations, investigation of other etiologies of patients’ symptoms, including occult postnasal drainage or cough-variant asthma, may be more rewarding than escalating anti-acid therapy.
Dr. John E. Pandolfino, AGAF, of Northwestern University, Chicago, discussed the utilization of high-resolution manometry in the evaluation of dysphagia. A central focus of this discussion was the use of the Chicago classification of motility abnormalities in assessing these patients. In the future, it is likely that the care of these patients will be dictated by the type of abnormality the patient has in this classification scheme. Especially in the setting of achalasia, data are emerging that some subtypes of achalasia are less likely to respond to some therapies. For instance, type 3 achalasia is unlikely to respond to pneumatic balloon dilatation.
Dr. Rhonda Souza of the University of Texas Southwestern Medical Center, Dallas, taught us that a diagnosis of eosinophilic esophagitis requires an 8-week trial of PPI therapy with no resolution of the eosinophilia. Esophageal dilation is generally safe in the setting of a dominant stricture, but it can be delayed prior to medical therapy if the patient is tolerating oral intake well. Although the most commonly used therapies for EoE now involve either swallowed steroids or dietary elimination therapy, several new agents are on the horizon that may give us new treatment options.
Finally, Dr. Amitabh Chak of Case Western Reserve University, Cleveland, reviewed the care of patients with Barrett’s esophagus and low-grade dysplasia. This is an especially difficult group of patients to care for, due in part to the low reproducibility in the diagnosis of low-grade dysplasia, as well as the highly variable reported cancer outcomes in this patient population. Level 1 evidence now exists demonstrating that treatment with radiofrequency ablation decreases the incidence of cancer in patients with low-grade dysplasia, but most patients with this finding will not progress. Therefore, the field would benefit from better risk stratification of these patients.
Dr. Shaheen is professor of medicine and epidemiology and chief of the division of gastroenterology & hepatology, University of North Carolina School of Medicine, Chapel Hill. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2015.
This year’s session on esophagus/upper GI at the AGA Spring Postgraduate Course was packed with pragmatic, useful information for the evaluation of patients with upper GI disorders. The session began with a talk on the manifestations of extraesophageal reflux disease. The take-home message of this talk was that putative extraesophageal manifestations of gastroesophageal reflux disease rarely respond to high-dose therapy with PPIs (proton-pump inhibitors), in the absence of concurrent esophageal symptoms such as heartburn or regurgitation. In such situations, investigation of other etiologies of patients’ symptoms, including occult postnasal drainage or cough-variant asthma, may be more rewarding than escalating anti-acid therapy.
Dr. John E. Pandolfino, AGAF, of Northwestern University, Chicago, discussed the utilization of high-resolution manometry in the evaluation of dysphagia. A central focus of this discussion was the use of the Chicago classification of motility abnormalities in assessing these patients. In the future, it is likely that the care of these patients will be dictated by the type of abnormality the patient has in this classification scheme. Especially in the setting of achalasia, data are emerging that some subtypes of achalasia are less likely to respond to some therapies. For instance, type 3 achalasia is unlikely to respond to pneumatic balloon dilatation.
Dr. Rhonda Souza of the University of Texas Southwestern Medical Center, Dallas, taught us that a diagnosis of eosinophilic esophagitis requires an 8-week trial of PPI therapy with no resolution of the eosinophilia. Esophageal dilation is generally safe in the setting of a dominant stricture, but it can be delayed prior to medical therapy if the patient is tolerating oral intake well. Although the most commonly used therapies for EoE now involve either swallowed steroids or dietary elimination therapy, several new agents are on the horizon that may give us new treatment options.
Finally, Dr. Amitabh Chak of Case Western Reserve University, Cleveland, reviewed the care of patients with Barrett’s esophagus and low-grade dysplasia. This is an especially difficult group of patients to care for, due in part to the low reproducibility in the diagnosis of low-grade dysplasia, as well as the highly variable reported cancer outcomes in this patient population. Level 1 evidence now exists demonstrating that treatment with radiofrequency ablation decreases the incidence of cancer in patients with low-grade dysplasia, but most patients with this finding will not progress. Therefore, the field would benefit from better risk stratification of these patients.
Dr. Shaheen is professor of medicine and epidemiology and chief of the division of gastroenterology & hepatology, University of North Carolina School of Medicine, Chapel Hill. This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2015.
ESC: Celecoxib safety study may soothe cardio concerns
LONDON – Celecoxib was associated with very low cardiovascular event rates, and its use posed no more risk than other painkillers commonly used to treat elderly individuals with arthritic conditions but no heart disease in a large, pragmatic, family practice–based study.
Results of the Standard Care Versus Celecoxib Outcome Trial (SCOT) reported at the annual congress of the European Society of Cardiology also showed that celecoxib was no more likely than nonselective nonsteroidal anti-inflammatory drugs (nsNSAIDs) to cause ulcer-related upper gastrointestinal (GI) tract complications.
In fact, the rates of both cardiovascular and GI events were so low overall that it made the trial difficult to complete, said study investigator Dr. Tom MacDonald, professor of clinical pharmacology and pharmacoepidemiology at the University of Dundee (Scotland), which sponsored the study.
The on-treatment and intention-to-treat (ITT) cardiovascular event rates were 0.9% and 1.1% per 100 patient-years, he observed, adding that he would have expected the event rate to be around 2%-3% in the population studied. GI complication rates were even lower, with just 12 on-treatment and 15 ITT events reported during the entire follow-up period, which was a maximum of 6.3 years and mean of about 3 years.
“You may remember the brouhaha surrounding the use of rofecoxib and other [cyclo-oxygenase-2 inhibitors],” said Dr. MacDonald. Both coxibs and nsNSAIDs have been associated with adverse cardiovascular outcomes such as myocardial infarction (BMJ 2005;330:1366), and rofecoxib was voluntarily withdrawn in 2004 by its manufacturer from the U.S. market. A recent meta-analysis (Lancet 2013;382:769-79) has suggested that coxibs increase the risk of major cardiovascular events by about 37%.
The SCOT study (BMJ Open 2013;3:e002295) was designed to assess if celecoxib was better, worse, or the same as the other available NSAIDs in terms of its cardiovascular and gastrointestinal safety. It was originally set up because of a requirement by the European Medicines Agency, Dr. McDonald explained.
More than 9,400 patients aged 60 years or older with osteoarthritis or rheumatoid arthritis who were prescribed chronic NSAID therapy and had no existing cardiovascular disease were screened at 706 family practices in Scotland, England, Denmark, and the Netherlands. A total of 7,297 patients were included in the prospective study and were randomized to switch to treatment with celecoxib or to continue their current nsNSAID.
General practice records were linked to hospital and mortality databases to derive the primary composite endpoint of the first occurrence of hospitalization for nonfatal MI, nonfatal stroke, or cardiovascular death, as well as secondary endpoints such as time to first hospitalization or death from upper GI complications and all-cause mortality.
Randomized patients were about 68 years old, and about 40% of patients were male. Dr. MacDonald noted that, although there was no known existing cardiovascular disease at enrollment, the baseline characteristics showed that around 44% of patients had high blood pressure; a third of patients had high cholesterol; and 20%, 12%, and 38% were taking a statin, aspirin, or ulcer-healing treatments, respectively. The most common nsNSAIDs being used were diclofenac (38.7%) and ibuprofen (31%).
There was no significant difference between celecoxib or nsNSAIDs for any of the cardiovascular endpoints studied, with hazard ratios (HR) for the primary composite cardiovascular endpoints of 1.12 (95% confidence interval, 0.81-1.55; P = .5) while on celecoxib treatment and 1.04 (95% CI, 0.81-1.33; P = .75) in the ITT analysis. Similar results were obtained for all-cause mortality (HR, 1.2 and 0.92, respectively).
Dr. MacDonald reported that 50% of patients randomized to celecoxib and 30% randomized to continue nsNSAIDs withdrew from the study. The main reasons for stopping celecoxib were a lack of efficacy (11.2% vs. 2% for nsNSAIDs), adverse events (8.3% vs. 4.4%), patient request (6% vs. 2.3%), not tolerated (3.9% vs. 1.2%), or a serious adverse event (2.6% vs. 1.9%). There was, however, a lot of adverse publicity about the coxibs, he noted, and patients who had been happy on an nsNSAID might not have been happy with the switch.
The rates of serious cardiovascular adverse events (31.7% vs. 32.4%) or reactions (5.2% vs. 5.8%) were similar with celecoxib and nsNSAIDs, but there were significantly fewer serious GI adverse reactions with celecoxib than with nsNSAIDs (38 vs. 66; P = .007). Overall, the adverse reaction rate was 22% vs. 16.1%, respectively (P <.001).
“In the study population, nsNSAIDs and celecoxib both appeared acceptably safe,” Dr. MacDonald concluded. “In patients who get significant symptomatic relief from these medicines, the benefit/risk balance appears positive.”
Although the findings are perhaps reassuring, they are unlikely to change clinical practice, observed Dr. José López-Sendon, who was invited to comment on the study results after their presentation at the conference.
The study findings suggest that celecoxib may continue to be safe to use in patients without existing cardiac disease, noted Dr. López-Sendon of Hospital Universitario La Paz in Madrid, but he would not modify the guidelines that advise that the lowest effective dose be used for the shortest duration of time in low-risk patients.
The study was sponsored by the University of Dundee and funded by an investigator-initiated research grant from Pfizer. The university’s Medicines Monitoring Unit also holds research grants from Amgen, Menarini, and Novartis. Dr. MacDonald has consulted on the use of NSAIDs for AstraZeneca, NiCox, Novartis, and Pfizer. Dr. López-Sendon did not have any disclosures relevant to his comments.
LONDON – Celecoxib was associated with very low cardiovascular event rates, and its use posed no more risk than other painkillers commonly used to treat elderly individuals with arthritic conditions but no heart disease in a large, pragmatic, family practice–based study.
Results of the Standard Care Versus Celecoxib Outcome Trial (SCOT) reported at the annual congress of the European Society of Cardiology also showed that celecoxib was no more likely than nonselective nonsteroidal anti-inflammatory drugs (nsNSAIDs) to cause ulcer-related upper gastrointestinal (GI) tract complications.
In fact, the rates of both cardiovascular and GI events were so low overall that it made the trial difficult to complete, said study investigator Dr. Tom MacDonald, professor of clinical pharmacology and pharmacoepidemiology at the University of Dundee (Scotland), which sponsored the study.
The on-treatment and intention-to-treat (ITT) cardiovascular event rates were 0.9% and 1.1% per 100 patient-years, he observed, adding that he would have expected the event rate to be around 2%-3% in the population studied. GI complication rates were even lower, with just 12 on-treatment and 15 ITT events reported during the entire follow-up period, which was a maximum of 6.3 years and mean of about 3 years.
“You may remember the brouhaha surrounding the use of rofecoxib and other [cyclo-oxygenase-2 inhibitors],” said Dr. MacDonald. Both coxibs and nsNSAIDs have been associated with adverse cardiovascular outcomes such as myocardial infarction (BMJ 2005;330:1366), and rofecoxib was voluntarily withdrawn in 2004 by its manufacturer from the U.S. market. A recent meta-analysis (Lancet 2013;382:769-79) has suggested that coxibs increase the risk of major cardiovascular events by about 37%.
The SCOT study (BMJ Open 2013;3:e002295) was designed to assess if celecoxib was better, worse, or the same as the other available NSAIDs in terms of its cardiovascular and gastrointestinal safety. It was originally set up because of a requirement by the European Medicines Agency, Dr. McDonald explained.
More than 9,400 patients aged 60 years or older with osteoarthritis or rheumatoid arthritis who were prescribed chronic NSAID therapy and had no existing cardiovascular disease were screened at 706 family practices in Scotland, England, Denmark, and the Netherlands. A total of 7,297 patients were included in the prospective study and were randomized to switch to treatment with celecoxib or to continue their current nsNSAID.
General practice records were linked to hospital and mortality databases to derive the primary composite endpoint of the first occurrence of hospitalization for nonfatal MI, nonfatal stroke, or cardiovascular death, as well as secondary endpoints such as time to first hospitalization or death from upper GI complications and all-cause mortality.
Randomized patients were about 68 years old, and about 40% of patients were male. Dr. MacDonald noted that, although there was no known existing cardiovascular disease at enrollment, the baseline characteristics showed that around 44% of patients had high blood pressure; a third of patients had high cholesterol; and 20%, 12%, and 38% were taking a statin, aspirin, or ulcer-healing treatments, respectively. The most common nsNSAIDs being used were diclofenac (38.7%) and ibuprofen (31%).
There was no significant difference between celecoxib or nsNSAIDs for any of the cardiovascular endpoints studied, with hazard ratios (HR) for the primary composite cardiovascular endpoints of 1.12 (95% confidence interval, 0.81-1.55; P = .5) while on celecoxib treatment and 1.04 (95% CI, 0.81-1.33; P = .75) in the ITT analysis. Similar results were obtained for all-cause mortality (HR, 1.2 and 0.92, respectively).
Dr. MacDonald reported that 50% of patients randomized to celecoxib and 30% randomized to continue nsNSAIDs withdrew from the study. The main reasons for stopping celecoxib were a lack of efficacy (11.2% vs. 2% for nsNSAIDs), adverse events (8.3% vs. 4.4%), patient request (6% vs. 2.3%), not tolerated (3.9% vs. 1.2%), or a serious adverse event (2.6% vs. 1.9%). There was, however, a lot of adverse publicity about the coxibs, he noted, and patients who had been happy on an nsNSAID might not have been happy with the switch.
The rates of serious cardiovascular adverse events (31.7% vs. 32.4%) or reactions (5.2% vs. 5.8%) were similar with celecoxib and nsNSAIDs, but there were significantly fewer serious GI adverse reactions with celecoxib than with nsNSAIDs (38 vs. 66; P = .007). Overall, the adverse reaction rate was 22% vs. 16.1%, respectively (P <.001).
“In the study population, nsNSAIDs and celecoxib both appeared acceptably safe,” Dr. MacDonald concluded. “In patients who get significant symptomatic relief from these medicines, the benefit/risk balance appears positive.”
Although the findings are perhaps reassuring, they are unlikely to change clinical practice, observed Dr. José López-Sendon, who was invited to comment on the study results after their presentation at the conference.
The study findings suggest that celecoxib may continue to be safe to use in patients without existing cardiac disease, noted Dr. López-Sendon of Hospital Universitario La Paz in Madrid, but he would not modify the guidelines that advise that the lowest effective dose be used for the shortest duration of time in low-risk patients.
The study was sponsored by the University of Dundee and funded by an investigator-initiated research grant from Pfizer. The university’s Medicines Monitoring Unit also holds research grants from Amgen, Menarini, and Novartis. Dr. MacDonald has consulted on the use of NSAIDs for AstraZeneca, NiCox, Novartis, and Pfizer. Dr. López-Sendon did not have any disclosures relevant to his comments.
LONDON – Celecoxib was associated with very low cardiovascular event rates, and its use posed no more risk than other painkillers commonly used to treat elderly individuals with arthritic conditions but no heart disease in a large, pragmatic, family practice–based study.
Results of the Standard Care Versus Celecoxib Outcome Trial (SCOT) reported at the annual congress of the European Society of Cardiology also showed that celecoxib was no more likely than nonselective nonsteroidal anti-inflammatory drugs (nsNSAIDs) to cause ulcer-related upper gastrointestinal (GI) tract complications.
In fact, the rates of both cardiovascular and GI events were so low overall that it made the trial difficult to complete, said study investigator Dr. Tom MacDonald, professor of clinical pharmacology and pharmacoepidemiology at the University of Dundee (Scotland), which sponsored the study.
The on-treatment and intention-to-treat (ITT) cardiovascular event rates were 0.9% and 1.1% per 100 patient-years, he observed, adding that he would have expected the event rate to be around 2%-3% in the population studied. GI complication rates were even lower, with just 12 on-treatment and 15 ITT events reported during the entire follow-up period, which was a maximum of 6.3 years and mean of about 3 years.
“You may remember the brouhaha surrounding the use of rofecoxib and other [cyclo-oxygenase-2 inhibitors],” said Dr. MacDonald. Both coxibs and nsNSAIDs have been associated with adverse cardiovascular outcomes such as myocardial infarction (BMJ 2005;330:1366), and rofecoxib was voluntarily withdrawn in 2004 by its manufacturer from the U.S. market. A recent meta-analysis (Lancet 2013;382:769-79) has suggested that coxibs increase the risk of major cardiovascular events by about 37%.
The SCOT study (BMJ Open 2013;3:e002295) was designed to assess if celecoxib was better, worse, or the same as the other available NSAIDs in terms of its cardiovascular and gastrointestinal safety. It was originally set up because of a requirement by the European Medicines Agency, Dr. McDonald explained.
More than 9,400 patients aged 60 years or older with osteoarthritis or rheumatoid arthritis who were prescribed chronic NSAID therapy and had no existing cardiovascular disease were screened at 706 family practices in Scotland, England, Denmark, and the Netherlands. A total of 7,297 patients were included in the prospective study and were randomized to switch to treatment with celecoxib or to continue their current nsNSAID.
General practice records were linked to hospital and mortality databases to derive the primary composite endpoint of the first occurrence of hospitalization for nonfatal MI, nonfatal stroke, or cardiovascular death, as well as secondary endpoints such as time to first hospitalization or death from upper GI complications and all-cause mortality.
Randomized patients were about 68 years old, and about 40% of patients were male. Dr. MacDonald noted that, although there was no known existing cardiovascular disease at enrollment, the baseline characteristics showed that around 44% of patients had high blood pressure; a third of patients had high cholesterol; and 20%, 12%, and 38% were taking a statin, aspirin, or ulcer-healing treatments, respectively. The most common nsNSAIDs being used were diclofenac (38.7%) and ibuprofen (31%).
There was no significant difference between celecoxib or nsNSAIDs for any of the cardiovascular endpoints studied, with hazard ratios (HR) for the primary composite cardiovascular endpoints of 1.12 (95% confidence interval, 0.81-1.55; P = .5) while on celecoxib treatment and 1.04 (95% CI, 0.81-1.33; P = .75) in the ITT analysis. Similar results were obtained for all-cause mortality (HR, 1.2 and 0.92, respectively).
Dr. MacDonald reported that 50% of patients randomized to celecoxib and 30% randomized to continue nsNSAIDs withdrew from the study. The main reasons for stopping celecoxib were a lack of efficacy (11.2% vs. 2% for nsNSAIDs), adverse events (8.3% vs. 4.4%), patient request (6% vs. 2.3%), not tolerated (3.9% vs. 1.2%), or a serious adverse event (2.6% vs. 1.9%). There was, however, a lot of adverse publicity about the coxibs, he noted, and patients who had been happy on an nsNSAID might not have been happy with the switch.
The rates of serious cardiovascular adverse events (31.7% vs. 32.4%) or reactions (5.2% vs. 5.8%) were similar with celecoxib and nsNSAIDs, but there were significantly fewer serious GI adverse reactions with celecoxib than with nsNSAIDs (38 vs. 66; P = .007). Overall, the adverse reaction rate was 22% vs. 16.1%, respectively (P <.001).
“In the study population, nsNSAIDs and celecoxib both appeared acceptably safe,” Dr. MacDonald concluded. “In patients who get significant symptomatic relief from these medicines, the benefit/risk balance appears positive.”
Although the findings are perhaps reassuring, they are unlikely to change clinical practice, observed Dr. José López-Sendon, who was invited to comment on the study results after their presentation at the conference.
The study findings suggest that celecoxib may continue to be safe to use in patients without existing cardiac disease, noted Dr. López-Sendon of Hospital Universitario La Paz in Madrid, but he would not modify the guidelines that advise that the lowest effective dose be used for the shortest duration of time in low-risk patients.
The study was sponsored by the University of Dundee and funded by an investigator-initiated research grant from Pfizer. The university’s Medicines Monitoring Unit also holds research grants from Amgen, Menarini, and Novartis. Dr. MacDonald has consulted on the use of NSAIDs for AstraZeneca, NiCox, Novartis, and Pfizer. Dr. López-Sendon did not have any disclosures relevant to his comments.
AT THE ESC CONGRESS 2015
Key clinical point: Celecoxib and nonselective NSAIDs were “acceptably safe” in a population without confirmed cardiovascular disease.
Major finding: The hazard ratio for the primary composite cardiovascular endpoint with celecoxib use was 1.12 (95% confidence interval, 0.81-1.55; P = .5), compared with NSAIDs.
Data source: The Standard Care Versus Celecoxib Outcome Trial (SCOT) of more than 7,200 elderly patients with osteoarthritis or rheumatoid arthritis and no confirmed cardiovascular disease.
Disclosures: The study was sponsored by the University of Dundee and funded by an investigator-initiated research grant from Pfizer. The university’s Medicines Monitoring Unit also holds research grants from Amgen, Menarini, and Novartis. Dr. MacDonald has consulted on the use of NSAIDs for AstraZeneca, NiCox, Novartis, and Pfizer. Dr. López-Sendon did not have any disclosures relevant to his comments.
H. pylori resistance highlights need for guided therapy
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Because H. pylori showed high rates of resistance to clarithromycin, metronidazole, and levofloxacin, they should be excluded from triple therapy regimens for helicobacteriosis.
Major finding: Half of strains were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin, 20% were resistant to metronidazole, 16% were resistant to clarithromycin, 0.8% were resistant to tetracycline, and none were resistant to amoxicillin.
Data source: Analysis of gastric biopsies from 656 U.S. veterans who underwent esophagogastroduodenoscopy in Texas between 2009 and 2013.
Disclosures: The National Institutes of Health and the VA Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Assessing progression, impact of radiofrequency ablation in Barrett’s esophagus
Patients with Barrett’s esophagus have about a 0.2% annual chance of developing esophageal adenocarcinoma in the 5 years after initial diagnosis, but the likelihood then rises so that about 9% of all patients will develop cancer by 20 years out, according to a study in the September issue of Gastroenterology.
The modeled rates of progression for the early years after diagnosis are substantially lower than are those reported by prospective studies, which involve more intensive surveillance and therefore suffer from detection bias, said Dr. Sonja Kroep of Erasmus Medical Center, Rotterdam, the Netherlands, and her associates. “Clinicians informing their patients about their cancer risk can best use this clinical progression rate, which is not influenced by surveillance-detected cancers,” they wrote.
Past analyses have yielded varying results for the rate at which Barrett’s esophagus with low-grade dysplasia progresses to high-grade dysplasia and esophageal carcinoma. For their study, Dr. Kroep and her associates calibrated a model based on the annual rate of 0.18% reported by population-level studies, and used it to simulate prospective studies and to predict results from both population-based and prospective studies for various follow-up periods (Gastroenterology 2015 Apr 29. pii: S0016-5085(15)00601-0).
For the first 5 years of follow-up, the model predicted a 0.19% annual rate of transformation to esophageal adenocarcinoma for population-based studies and a 0.36% annual rate for prospective studies, the researchers reported. At 20 years, these rates rose to 0.63% and 0.65% annually, for a cumulative incidence rate of 9.1% to 9.5%. Between the 5-year and 20-year thresholds, the gap between rates of progression for the two types of studies narrowed from 91% to 5%. Taken together, the findings suggest that for the first 5 years after a diagnosis of Barrett’s esophagus, rates of progression to esophageal adenocarcinoma reflect those from population-level studies instead of surveillance-based prospective studies, the investigators said. “Clinicians should use this information to explain to patients their short-term and long-term risks if no action is taken, and then discuss the risks and benefits of surveillance,” they added.
In a separate retrospective study, radiofrequency ablation of low-grade esophageal dysplasia was linked to substantially lower rates of progression compared with watchful waiting in the form of endoscopic surveillance, said Dr. Aaron Small of the University of Pennsylvania, Philadelphia, and his associates. Their study included 125 patients with Barrett’s esophagus and low-grade dysplasia who underwent surveillance only, and 45 patients who underwent radiofrequency ablation at three university medical centers.
Over median follow-up periods of more than 2 years, the risk of progression with radiofrequency ablation was significantly lower than with endoscopic surveillance only, even after the researchers controlled for year of diagnosis (adjusted hazard ratio, 0.06; 95% confidence interval, 0.008-0.48; P = .008). The ablation group also had fewer visible macroscopic lesions, although the difference was not significant. “We estimate that for every three patients treated with radiofrequency ablation, one additional patient with low-grade dysplasia will avoid progression to high-grade dysplasia or esophageal adenocarcinoma within 3 years,” the researchers wrote. “Although selection bias cannot be excluded, these findings provide additional evidence for the use of endoscopic ablation therapy for low-grade dysplasia” (Gastroenterology 2015 Apr 24. pii: S0016-5085(15)00569-7).
The study by Dr. Kroep and her associates was funded by grant U01 CA152926, and the investigators reported having no conflicts of interest. The study by Dr. Small and his associates was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases and by institutional funds. Dr. Small reported no conflicts of interest, but seven coauthors reported ties with a number of pharmaceutical companies.
These two studies highlight two different hot topics in the management of patients with a Barrett’s esophagus. The first is the low rate of neoplastic progression in patients undergoing surveillance for nondysplastic BE. The second relates to the management of patients with low-grade dysplasia (LG

|
| Dr. Jacques Bergman |
Population-based BE surveillance studies have shown lower progression rates than have prospective surveillance studies. The biggest difference between these two is that not all patients in population-based studies actually undergo subsequent surveillance endoscopies and/or surveillance is carried out less rigorously than in prospective surveillance studies. Patients who have undergone a baseline endoscopy showing no neoplasia first need to develop early neoplasia (which is generally asymptomatic) that then needs to progress to a symptomatic stage before they are diagnosed. During this interval they may die from other causes or may be lost to follow-up. Patients in strict surveillance programs will be diagnosed at an earlier stage and at a higher rate. This is especially true in the first years of follow-up, when the initial screening endoscopy has its largest effect. Over time, the difference then fades away as suggested by the 9% progression rate of both types of studies at 20 years of follow-up. Both perspectives are relevant for patients. For elderly patients with significant comorbidity, the 5-year data from population-based studies reassure them not to undergo surveillance endoscopies because even when an early cancer develops it is unlikely to bear any clinical relevance, whereas for patients with a long life expectancy, the 9% cancer risk at 20 years and the dismal prognosis of a symptomatic Barrett’s cancer may be strong arguments for participating in a surveillance program.
For patients with LGD, the situation is different: The rate of progression is much higher than that reported for nondysplastic BE, and with radiofrequency ablation (RFA), an effective and safe tool is at hand to significantly reduce this rate of neoplastic progression. Small et al. reported that only three patients need to be treated with RFA to prevent one patient from progressing to high-grade dysplasia or cancer. These data are in agreement with data from a prospective randomized study on the use of RFA for patients with a confirmed diagnosis of LGD. Most societies therefore consider a confirmed histologic diagnosis of LGD a justified indication for prophylactic ablation with RFA.
However, this does not imply that all patients with LGD should be ablated. First, only patients in whom the histologic diagnosis of LGD is confirmed by an expert BE pathologist should be considered for RFA. In approximately 75% of patients, the LGD diagnosis will be downstaged to nondysplastic BE upon expert review. Second, the lessons learned from the Kroep study also apply here: For an elderly LGD patient with or without significant comorbidity, the decision to proceed to RFA is different from the decision for patients with a longer life expectancy, especially if an intermediate solution – to continue endoscopic surveillance and proceed to endoscopic management in case neoplasia is diagnosed – is also considered.
Jacques Bergman, M.D., Ph.D., is professor of gastrointestinal endoscopy, director of endoscopy, at the Academic Medical Center, Amsterdam. He received research support for clinical studies and consulted for Covidien/Medtronic GI solutions.
These two studies highlight two different hot topics in the management of patients with a Barrett’s esophagus. The first is the low rate of neoplastic progression in patients undergoing surveillance for nondysplastic BE. The second relates to the management of patients with low-grade dysplasia (LG

|
| Dr. Jacques Bergman |
Population-based BE surveillance studies have shown lower progression rates than have prospective surveillance studies. The biggest difference between these two is that not all patients in population-based studies actually undergo subsequent surveillance endoscopies and/or surveillance is carried out less rigorously than in prospective surveillance studies. Patients who have undergone a baseline endoscopy showing no neoplasia first need to develop early neoplasia (which is generally asymptomatic) that then needs to progress to a symptomatic stage before they are diagnosed. During this interval they may die from other causes or may be lost to follow-up. Patients in strict surveillance programs will be diagnosed at an earlier stage and at a higher rate. This is especially true in the first years of follow-up, when the initial screening endoscopy has its largest effect. Over time, the difference then fades away as suggested by the 9% progression rate of both types of studies at 20 years of follow-up. Both perspectives are relevant for patients. For elderly patients with significant comorbidity, the 5-year data from population-based studies reassure them not to undergo surveillance endoscopies because even when an early cancer develops it is unlikely to bear any clinical relevance, whereas for patients with a long life expectancy, the 9% cancer risk at 20 years and the dismal prognosis of a symptomatic Barrett’s cancer may be strong arguments for participating in a surveillance program.
For patients with LGD, the situation is different: The rate of progression is much higher than that reported for nondysplastic BE, and with radiofrequency ablation (RFA), an effective and safe tool is at hand to significantly reduce this rate of neoplastic progression. Small et al. reported that only three patients need to be treated with RFA to prevent one patient from progressing to high-grade dysplasia or cancer. These data are in agreement with data from a prospective randomized study on the use of RFA for patients with a confirmed diagnosis of LGD. Most societies therefore consider a confirmed histologic diagnosis of LGD a justified indication for prophylactic ablation with RFA.
However, this does not imply that all patients with LGD should be ablated. First, only patients in whom the histologic diagnosis of LGD is confirmed by an expert BE pathologist should be considered for RFA. In approximately 75% of patients, the LGD diagnosis will be downstaged to nondysplastic BE upon expert review. Second, the lessons learned from the Kroep study also apply here: For an elderly LGD patient with or without significant comorbidity, the decision to proceed to RFA is different from the decision for patients with a longer life expectancy, especially if an intermediate solution – to continue endoscopic surveillance and proceed to endoscopic management in case neoplasia is diagnosed – is also considered.
Jacques Bergman, M.D., Ph.D., is professor of gastrointestinal endoscopy, director of endoscopy, at the Academic Medical Center, Amsterdam. He received research support for clinical studies and consulted for Covidien/Medtronic GI solutions.
These two studies highlight two different hot topics in the management of patients with a Barrett’s esophagus. The first is the low rate of neoplastic progression in patients undergoing surveillance for nondysplastic BE. The second relates to the management of patients with low-grade dysplasia (LG

|
| Dr. Jacques Bergman |
Population-based BE surveillance studies have shown lower progression rates than have prospective surveillance studies. The biggest difference between these two is that not all patients in population-based studies actually undergo subsequent surveillance endoscopies and/or surveillance is carried out less rigorously than in prospective surveillance studies. Patients who have undergone a baseline endoscopy showing no neoplasia first need to develop early neoplasia (which is generally asymptomatic) that then needs to progress to a symptomatic stage before they are diagnosed. During this interval they may die from other causes or may be lost to follow-up. Patients in strict surveillance programs will be diagnosed at an earlier stage and at a higher rate. This is especially true in the first years of follow-up, when the initial screening endoscopy has its largest effect. Over time, the difference then fades away as suggested by the 9% progression rate of both types of studies at 20 years of follow-up. Both perspectives are relevant for patients. For elderly patients with significant comorbidity, the 5-year data from population-based studies reassure them not to undergo surveillance endoscopies because even when an early cancer develops it is unlikely to bear any clinical relevance, whereas for patients with a long life expectancy, the 9% cancer risk at 20 years and the dismal prognosis of a symptomatic Barrett’s cancer may be strong arguments for participating in a surveillance program.
For patients with LGD, the situation is different: The rate of progression is much higher than that reported for nondysplastic BE, and with radiofrequency ablation (RFA), an effective and safe tool is at hand to significantly reduce this rate of neoplastic progression. Small et al. reported that only three patients need to be treated with RFA to prevent one patient from progressing to high-grade dysplasia or cancer. These data are in agreement with data from a prospective randomized study on the use of RFA for patients with a confirmed diagnosis of LGD. Most societies therefore consider a confirmed histologic diagnosis of LGD a justified indication for prophylactic ablation with RFA.
However, this does not imply that all patients with LGD should be ablated. First, only patients in whom the histologic diagnosis of LGD is confirmed by an expert BE pathologist should be considered for RFA. In approximately 75% of patients, the LGD diagnosis will be downstaged to nondysplastic BE upon expert review. Second, the lessons learned from the Kroep study also apply here: For an elderly LGD patient with or without significant comorbidity, the decision to proceed to RFA is different from the decision for patients with a longer life expectancy, especially if an intermediate solution – to continue endoscopic surveillance and proceed to endoscopic management in case neoplasia is diagnosed – is also considered.
Jacques Bergman, M.D., Ph.D., is professor of gastrointestinal endoscopy, director of endoscopy, at the Academic Medical Center, Amsterdam. He received research support for clinical studies and consulted for Covidien/Medtronic GI solutions.
Patients with Barrett’s esophagus have about a 0.2% annual chance of developing esophageal adenocarcinoma in the 5 years after initial diagnosis, but the likelihood then rises so that about 9% of all patients will develop cancer by 20 years out, according to a study in the September issue of Gastroenterology.
The modeled rates of progression for the early years after diagnosis are substantially lower than are those reported by prospective studies, which involve more intensive surveillance and therefore suffer from detection bias, said Dr. Sonja Kroep of Erasmus Medical Center, Rotterdam, the Netherlands, and her associates. “Clinicians informing their patients about their cancer risk can best use this clinical progression rate, which is not influenced by surveillance-detected cancers,” they wrote.
Past analyses have yielded varying results for the rate at which Barrett’s esophagus with low-grade dysplasia progresses to high-grade dysplasia and esophageal carcinoma. For their study, Dr. Kroep and her associates calibrated a model based on the annual rate of 0.18% reported by population-level studies, and used it to simulate prospective studies and to predict results from both population-based and prospective studies for various follow-up periods (Gastroenterology 2015 Apr 29. pii: S0016-5085(15)00601-0).
For the first 5 years of follow-up, the model predicted a 0.19% annual rate of transformation to esophageal adenocarcinoma for population-based studies and a 0.36% annual rate for prospective studies, the researchers reported. At 20 years, these rates rose to 0.63% and 0.65% annually, for a cumulative incidence rate of 9.1% to 9.5%. Between the 5-year and 20-year thresholds, the gap between rates of progression for the two types of studies narrowed from 91% to 5%. Taken together, the findings suggest that for the first 5 years after a diagnosis of Barrett’s esophagus, rates of progression to esophageal adenocarcinoma reflect those from population-level studies instead of surveillance-based prospective studies, the investigators said. “Clinicians should use this information to explain to patients their short-term and long-term risks if no action is taken, and then discuss the risks and benefits of surveillance,” they added.
In a separate retrospective study, radiofrequency ablation of low-grade esophageal dysplasia was linked to substantially lower rates of progression compared with watchful waiting in the form of endoscopic surveillance, said Dr. Aaron Small of the University of Pennsylvania, Philadelphia, and his associates. Their study included 125 patients with Barrett’s esophagus and low-grade dysplasia who underwent surveillance only, and 45 patients who underwent radiofrequency ablation at three university medical centers.
Over median follow-up periods of more than 2 years, the risk of progression with radiofrequency ablation was significantly lower than with endoscopic surveillance only, even after the researchers controlled for year of diagnosis (adjusted hazard ratio, 0.06; 95% confidence interval, 0.008-0.48; P = .008). The ablation group also had fewer visible macroscopic lesions, although the difference was not significant. “We estimate that for every three patients treated with radiofrequency ablation, one additional patient with low-grade dysplasia will avoid progression to high-grade dysplasia or esophageal adenocarcinoma within 3 years,” the researchers wrote. “Although selection bias cannot be excluded, these findings provide additional evidence for the use of endoscopic ablation therapy for low-grade dysplasia” (Gastroenterology 2015 Apr 24. pii: S0016-5085(15)00569-7).
The study by Dr. Kroep and her associates was funded by grant U01 CA152926, and the investigators reported having no conflicts of interest. The study by Dr. Small and his associates was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases and by institutional funds. Dr. Small reported no conflicts of interest, but seven coauthors reported ties with a number of pharmaceutical companies.
Patients with Barrett’s esophagus have about a 0.2% annual chance of developing esophageal adenocarcinoma in the 5 years after initial diagnosis, but the likelihood then rises so that about 9% of all patients will develop cancer by 20 years out, according to a study in the September issue of Gastroenterology.
The modeled rates of progression for the early years after diagnosis are substantially lower than are those reported by prospective studies, which involve more intensive surveillance and therefore suffer from detection bias, said Dr. Sonja Kroep of Erasmus Medical Center, Rotterdam, the Netherlands, and her associates. “Clinicians informing their patients about their cancer risk can best use this clinical progression rate, which is not influenced by surveillance-detected cancers,” they wrote.
Past analyses have yielded varying results for the rate at which Barrett’s esophagus with low-grade dysplasia progresses to high-grade dysplasia and esophageal carcinoma. For their study, Dr. Kroep and her associates calibrated a model based on the annual rate of 0.18% reported by population-level studies, and used it to simulate prospective studies and to predict results from both population-based and prospective studies for various follow-up periods (Gastroenterology 2015 Apr 29. pii: S0016-5085(15)00601-0).
For the first 5 years of follow-up, the model predicted a 0.19% annual rate of transformation to esophageal adenocarcinoma for population-based studies and a 0.36% annual rate for prospective studies, the researchers reported. At 20 years, these rates rose to 0.63% and 0.65% annually, for a cumulative incidence rate of 9.1% to 9.5%. Between the 5-year and 20-year thresholds, the gap between rates of progression for the two types of studies narrowed from 91% to 5%. Taken together, the findings suggest that for the first 5 years after a diagnosis of Barrett’s esophagus, rates of progression to esophageal adenocarcinoma reflect those from population-level studies instead of surveillance-based prospective studies, the investigators said. “Clinicians should use this information to explain to patients their short-term and long-term risks if no action is taken, and then discuss the risks and benefits of surveillance,” they added.
In a separate retrospective study, radiofrequency ablation of low-grade esophageal dysplasia was linked to substantially lower rates of progression compared with watchful waiting in the form of endoscopic surveillance, said Dr. Aaron Small of the University of Pennsylvania, Philadelphia, and his associates. Their study included 125 patients with Barrett’s esophagus and low-grade dysplasia who underwent surveillance only, and 45 patients who underwent radiofrequency ablation at three university medical centers.
Over median follow-up periods of more than 2 years, the risk of progression with radiofrequency ablation was significantly lower than with endoscopic surveillance only, even after the researchers controlled for year of diagnosis (adjusted hazard ratio, 0.06; 95% confidence interval, 0.008-0.48; P = .008). The ablation group also had fewer visible macroscopic lesions, although the difference was not significant. “We estimate that for every three patients treated with radiofrequency ablation, one additional patient with low-grade dysplasia will avoid progression to high-grade dysplasia or esophageal adenocarcinoma within 3 years,” the researchers wrote. “Although selection bias cannot be excluded, these findings provide additional evidence for the use of endoscopic ablation therapy for low-grade dysplasia” (Gastroenterology 2015 Apr 24. pii: S0016-5085(15)00569-7).
The study by Dr. Kroep and her associates was funded by grant U01 CA152926, and the investigators reported having no conflicts of interest. The study by Dr. Small and his associates was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases and by institutional funds. Dr. Small reported no conflicts of interest, but seven coauthors reported ties with a number of pharmaceutical companies.
FROM GASTROENTEROLOGY
Key clinical point: Barrett’s esophagus with low-grade dysplasia had a lower rate of progression to cancer than that suggested by prospective surveillance studies, but radiofrequency ablation might further cut the risk.
Major finding: About 0.2% of cases progress during the 5 years after diagnosis, and RFA might significantly decrease risk of progression (adjusted hazard ratio, 0.06).
Data source: A model of rates of progression based on population-level studies, and a multicenter retrospective study of 170 patients with Barrett’s esophagus and low-grade dysplasia.
Disclosures: The study by Dr. Small and associates was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases and by institutional funds. Dr. Small reported no conflicts of interest; seven coauthors reported ties with a number of pharmaceutical companies. The study by Dr. Kroep and her associates was funded by grant U01 CA152926, and the investigators reported having no conflicts of interest.
DDW: LINX device beneficial, safe for GERD
WASHINGTON – Five-year follow-up data on the magnetic device approved for treating gastroesophageal reflux disease confirm its long-term safety and efficacy, Dr. Robert A. Ganz reported at the annual Digestive Disease Week.
Five years after device implantation, the proportion of patients experiencing moderate to severe regurgitation had dropped to about 1%, from almost 60% at baseline, and two-thirds of patients were not taking any proton pump inhibitors (PPIs), said Dr. Ganz, chief of gastroenterology at Abbott Northwestern Hospital, Minneapolis, and one of the study investigators. These were among the results of the study that evaluated the device, the LINX Reflux Management System. The device was approved by the Food and Drug Administration FDA) in 2012 and is for the treatment of people with GERD as defined by abnormal pH testing, who continue to have chronic GERD symptoms that persist despite maximum medical therapy for the treatment of reflux.
“Magnetic sphincter augmentation should be considered first-line surgical therapy for those with gastroesophageal reflux disease, based on the results of this study,” he said.
The 2-year results of the prospective, multicenter study were the basis of the FDA approval of the device, described by the manufacturer, Torax Medical, as a “small implant [composed] of interlinked titanium beads with magnetic cores,” implanted during standard laparoscopy. The magnetic attraction between the beads augments the existing esophageal sphincter’s barrier function to prevent reflux,” according to the company.
The study enrolled 100 patients with reflux disease with a median age of 53 years, who had experienced typical heartburn for at least 6 months with or without regurgitation and were taking PPIs daily for at least 3 months (median use 5 years). Patients had GERD for a median of 10 years (range: 1-40 years). People who had any type of previous gastric or esophageal surgery, Barrett’s esophagus, a hiatal hernia greater than 3 cm, a body mass index over 35 kg/m2, or grade C or D esophagitis were excluded.
The device was implanted in all patients, who served as their own controls; 85 patients were followed through 5 years (6 were lost to follow-up, the device was explanted in 6 patients, 2 patients did not consent to extended follow-up, and 1 patient died of an unrelated cancer). The median procedure time was 36 minutes with a range of 7-125 minutes); all procedures were successfully completed with no intraoperative complications and all patients were discharged within 24 hours on an unrestricted diet.
The median total Gastroesophageal Reflux Disease–Health-Related Quality of Life (GERD-HRQL) score at baseline was 27 points among those not on PPIs and 11 points on PPIs, dropping to 4 points at 5 years off PPIs. At baseline, 95% of patients expressed dissatisfaction related to reflux, which dropped to 7% at year 5. Moderate to severe heartburn was reported by 89% at baseline, dropping to about 12% at year 5. The proportion of patients experiencing moderate to severe regurgitation dropped from 57% at baseline to about 1% at 5 years, Dr. Ganz said.
At baseline, 100% were taking PPIs every day, compared with 15% at 5 years. (At 5 years, 75% had discontinued PPIs, and about 9% reported PRN use only). Grade A and B esophagitis decreased from 40% at baseline to 16% at 5 years, at which point most cases were grade A, and there were no patients with grade C or D esophagitis, he said. In addition, at 5 years, 100% of patients “reported the ability to belch, and those needing to vomit – about 16% – reported the ability to vomit,” demonstrating that normal physiology was preserved with the device.
At 5 years, there were no device erosions or migrations, or any significant adverse events other than dysphagia, which “was typically mild and not associated with weight loss and tended to resolve over time,” from about 70% in the first few weeks after surgery to 11% at 1 year and 7% at 5 years, Dr. Ganz said.
In seven cases, the device was removed laparoscopically, with no complications and gastric anatomy was preserved for future treatments. All removals were elective. The device was removed in four patients because of dysphagia, which completely resolved in those patients. One patient had the device removed because of vomiting of unknown cause that persisted after removal. Another two patients who “had the device removed for disease management” continued to experience reflux and had “uneventful” Nissen fundoplication,” he said.
“Five years after magnetic augmentation, we have demonstrated objective evidence of reduction in acid exposure and in the majority of patients, normalized pH [and] we demonstrated significant and durable improvement in all group parameters measured, with preservation of fundic anatomy and normal physiology, with the ability to belch and vomit,” Dr. Ganz concluded. The results also show that the “procedure is reproducible, safe and reversible if necessary,” he added, noting that one of the limitations of the study was that subjects served as their own controls. During the discussion period, he was asked about hiatal hernia repairs, an apparent trend to “decay” from years 1 to 5 in some parameters measured, and dysphagia after the procedure.
About 40% of the patients in the study had a hiatal hernia, and about one-third of these patients had a hernia repair. A subgroup analysis of the data is being performed to evaluate the impact of hernia repair, Dr. Ganz said.
PPI use increased from 8% in year 4, to 15% in year 5. The reason for this s difficult to determine but “even though there is a bit of a decay, patients are still quite satisfied at 5 years,” Dr. Ganz remarked, also referring to the marked impact on regurgitation. Many U.S. patients use PPIs for reasons other than reflux, and studies show that many patients are on PPIs after the Nissen procedure in the absence of pathologic pH scores, he pointed out.
Compared with the type of dysphagia patients experience after the Nissen procedure, which is immediate and improves with time, Dr. Ganz said that the dysphagia associated with the device “seemed to peak around 2 weeks and then it slowly improved with time, so this may be more of a scar tissue–associated dysphagia than an edema dysphagia, but … it does improve with time.
Three-year results of the study were published in 2013 (N. Engl. J. Med. 2013;368:719-72), Dr. Ganz was the lead author.
The study was funded by Torax Medical. Dr. Ganz had no disclosures related to the topic of this presentation.
*This story was updated 7/9/2015.
At DDW this year, Dr. Ganz reported on the 5-year follow-up of the original LINX data that was published in the New England Journal of Medicine in 2013 (368:2039-40). The original study enrolled and followed 100 reflux patients for 3 years after implantation of the magnetic sphincter augmentation device, and it appears that the successful outcomes are sustained over the 5-year period. Most notable are the lasting improvement in regurgitation and the dramatic reduction in requirement for maintenance PPI therapy. These findings led the investigators to suggest that this should be considered a first-line surgical therapy for GERD. Overall, this is not an unreasonable statement when one considers the current model wherein antireflux surgery fits in the treatment of GERD. Medical therapy with proton pump inhibitors is extremely safe and effective for a substantial number of patients with GERD and based on this risk/benefit profile should be the first line therapy (Am. J. Gastroenterol. 2013;108:308-28; quiz 329). However, this treatment is not perfect and there are many patients who continue to have persistent symptoms despite PPI therapy (Clin. Gastroenterol. Hepatol. 2012;10:612-9). Although the majority of PPI nonresponders have a functional etiology, there is a distinct population that continue to have refractory reflux-related symptoms, such as regurgitation, that escape the therapeutic target of PPIs. These patients will require an augmentation of the antireflux barrier and the LINX approach appears to be as effective as fundoplication in this regard (J. Am. Coll. Surg. 2015;221:123-8). The question is whether the side effect profile and durability of LINX is better than fundoplication. The answer here is not clear and I would carefully state that LINX and fundoplication can be considered first-line surgical therapies for GERD patients who have documented pathologic acid gastroesophageal reflux and are intolerant to PPIs or not responding to PPIs.
Dr. John E. Pandolfino is professor of medicine and chief of the division of gastroenterology and hepatology at Northwestern University, Chicago. He is a speaker for Astra Zeneca/Takeda and a consultant for EndoGastric Solutions.
At DDW this year, Dr. Ganz reported on the 5-year follow-up of the original LINX data that was published in the New England Journal of Medicine in 2013 (368:2039-40). The original study enrolled and followed 100 reflux patients for 3 years after implantation of the magnetic sphincter augmentation device, and it appears that the successful outcomes are sustained over the 5-year period. Most notable are the lasting improvement in regurgitation and the dramatic reduction in requirement for maintenance PPI therapy. These findings led the investigators to suggest that this should be considered a first-line surgical therapy for GERD. Overall, this is not an unreasonable statement when one considers the current model wherein antireflux surgery fits in the treatment of GERD. Medical therapy with proton pump inhibitors is extremely safe and effective for a substantial number of patients with GERD and based on this risk/benefit profile should be the first line therapy (Am. J. Gastroenterol. 2013;108:308-28; quiz 329). However, this treatment is not perfect and there are many patients who continue to have persistent symptoms despite PPI therapy (Clin. Gastroenterol. Hepatol. 2012;10:612-9). Although the majority of PPI nonresponders have a functional etiology, there is a distinct population that continue to have refractory reflux-related symptoms, such as regurgitation, that escape the therapeutic target of PPIs. These patients will require an augmentation of the antireflux barrier and the LINX approach appears to be as effective as fundoplication in this regard (J. Am. Coll. Surg. 2015;221:123-8). The question is whether the side effect profile and durability of LINX is better than fundoplication. The answer here is not clear and I would carefully state that LINX and fundoplication can be considered first-line surgical therapies for GERD patients who have documented pathologic acid gastroesophageal reflux and are intolerant to PPIs or not responding to PPIs.
Dr. John E. Pandolfino is professor of medicine and chief of the division of gastroenterology and hepatology at Northwestern University, Chicago. He is a speaker for Astra Zeneca/Takeda and a consultant for EndoGastric Solutions.
At DDW this year, Dr. Ganz reported on the 5-year follow-up of the original LINX data that was published in the New England Journal of Medicine in 2013 (368:2039-40). The original study enrolled and followed 100 reflux patients for 3 years after implantation of the magnetic sphincter augmentation device, and it appears that the successful outcomes are sustained over the 5-year period. Most notable are the lasting improvement in regurgitation and the dramatic reduction in requirement for maintenance PPI therapy. These findings led the investigators to suggest that this should be considered a first-line surgical therapy for GERD. Overall, this is not an unreasonable statement when one considers the current model wherein antireflux surgery fits in the treatment of GERD. Medical therapy with proton pump inhibitors is extremely safe and effective for a substantial number of patients with GERD and based on this risk/benefit profile should be the first line therapy (Am. J. Gastroenterol. 2013;108:308-28; quiz 329). However, this treatment is not perfect and there are many patients who continue to have persistent symptoms despite PPI therapy (Clin. Gastroenterol. Hepatol. 2012;10:612-9). Although the majority of PPI nonresponders have a functional etiology, there is a distinct population that continue to have refractory reflux-related symptoms, such as regurgitation, that escape the therapeutic target of PPIs. These patients will require an augmentation of the antireflux barrier and the LINX approach appears to be as effective as fundoplication in this regard (J. Am. Coll. Surg. 2015;221:123-8). The question is whether the side effect profile and durability of LINX is better than fundoplication. The answer here is not clear and I would carefully state that LINX and fundoplication can be considered first-line surgical therapies for GERD patients who have documented pathologic acid gastroesophageal reflux and are intolerant to PPIs or not responding to PPIs.
Dr. John E. Pandolfino is professor of medicine and chief of the division of gastroenterology and hepatology at Northwestern University, Chicago. He is a speaker for Astra Zeneca/Takeda and a consultant for EndoGastric Solutions.
WASHINGTON – Five-year follow-up data on the magnetic device approved for treating gastroesophageal reflux disease confirm its long-term safety and efficacy, Dr. Robert A. Ganz reported at the annual Digestive Disease Week.
Five years after device implantation, the proportion of patients experiencing moderate to severe regurgitation had dropped to about 1%, from almost 60% at baseline, and two-thirds of patients were not taking any proton pump inhibitors (PPIs), said Dr. Ganz, chief of gastroenterology at Abbott Northwestern Hospital, Minneapolis, and one of the study investigators. These were among the results of the study that evaluated the device, the LINX Reflux Management System. The device was approved by the Food and Drug Administration FDA) in 2012 and is for the treatment of people with GERD as defined by abnormal pH testing, who continue to have chronic GERD symptoms that persist despite maximum medical therapy for the treatment of reflux.
“Magnetic sphincter augmentation should be considered first-line surgical therapy for those with gastroesophageal reflux disease, based on the results of this study,” he said.
The 2-year results of the prospective, multicenter study were the basis of the FDA approval of the device, described by the manufacturer, Torax Medical, as a “small implant [composed] of interlinked titanium beads with magnetic cores,” implanted during standard laparoscopy. The magnetic attraction between the beads augments the existing esophageal sphincter’s barrier function to prevent reflux,” according to the company.
The study enrolled 100 patients with reflux disease with a median age of 53 years, who had experienced typical heartburn for at least 6 months with or without regurgitation and were taking PPIs daily for at least 3 months (median use 5 years). Patients had GERD for a median of 10 years (range: 1-40 years). People who had any type of previous gastric or esophageal surgery, Barrett’s esophagus, a hiatal hernia greater than 3 cm, a body mass index over 35 kg/m2, or grade C or D esophagitis were excluded.
The device was implanted in all patients, who served as their own controls; 85 patients were followed through 5 years (6 were lost to follow-up, the device was explanted in 6 patients, 2 patients did not consent to extended follow-up, and 1 patient died of an unrelated cancer). The median procedure time was 36 minutes with a range of 7-125 minutes); all procedures were successfully completed with no intraoperative complications and all patients were discharged within 24 hours on an unrestricted diet.
The median total Gastroesophageal Reflux Disease–Health-Related Quality of Life (GERD-HRQL) score at baseline was 27 points among those not on PPIs and 11 points on PPIs, dropping to 4 points at 5 years off PPIs. At baseline, 95% of patients expressed dissatisfaction related to reflux, which dropped to 7% at year 5. Moderate to severe heartburn was reported by 89% at baseline, dropping to about 12% at year 5. The proportion of patients experiencing moderate to severe regurgitation dropped from 57% at baseline to about 1% at 5 years, Dr. Ganz said.
At baseline, 100% were taking PPIs every day, compared with 15% at 5 years. (At 5 years, 75% had discontinued PPIs, and about 9% reported PRN use only). Grade A and B esophagitis decreased from 40% at baseline to 16% at 5 years, at which point most cases were grade A, and there were no patients with grade C or D esophagitis, he said. In addition, at 5 years, 100% of patients “reported the ability to belch, and those needing to vomit – about 16% – reported the ability to vomit,” demonstrating that normal physiology was preserved with the device.
At 5 years, there were no device erosions or migrations, or any significant adverse events other than dysphagia, which “was typically mild and not associated with weight loss and tended to resolve over time,” from about 70% in the first few weeks after surgery to 11% at 1 year and 7% at 5 years, Dr. Ganz said.
In seven cases, the device was removed laparoscopically, with no complications and gastric anatomy was preserved for future treatments. All removals were elective. The device was removed in four patients because of dysphagia, which completely resolved in those patients. One patient had the device removed because of vomiting of unknown cause that persisted after removal. Another two patients who “had the device removed for disease management” continued to experience reflux and had “uneventful” Nissen fundoplication,” he said.
“Five years after magnetic augmentation, we have demonstrated objective evidence of reduction in acid exposure and in the majority of patients, normalized pH [and] we demonstrated significant and durable improvement in all group parameters measured, with preservation of fundic anatomy and normal physiology, with the ability to belch and vomit,” Dr. Ganz concluded. The results also show that the “procedure is reproducible, safe and reversible if necessary,” he added, noting that one of the limitations of the study was that subjects served as their own controls. During the discussion period, he was asked about hiatal hernia repairs, an apparent trend to “decay” from years 1 to 5 in some parameters measured, and dysphagia after the procedure.
About 40% of the patients in the study had a hiatal hernia, and about one-third of these patients had a hernia repair. A subgroup analysis of the data is being performed to evaluate the impact of hernia repair, Dr. Ganz said.
PPI use increased from 8% in year 4, to 15% in year 5. The reason for this s difficult to determine but “even though there is a bit of a decay, patients are still quite satisfied at 5 years,” Dr. Ganz remarked, also referring to the marked impact on regurgitation. Many U.S. patients use PPIs for reasons other than reflux, and studies show that many patients are on PPIs after the Nissen procedure in the absence of pathologic pH scores, he pointed out.
Compared with the type of dysphagia patients experience after the Nissen procedure, which is immediate and improves with time, Dr. Ganz said that the dysphagia associated with the device “seemed to peak around 2 weeks and then it slowly improved with time, so this may be more of a scar tissue–associated dysphagia than an edema dysphagia, but … it does improve with time.
Three-year results of the study were published in 2013 (N. Engl. J. Med. 2013;368:719-72), Dr. Ganz was the lead author.
The study was funded by Torax Medical. Dr. Ganz had no disclosures related to the topic of this presentation.
*This story was updated 7/9/2015.
WASHINGTON – Five-year follow-up data on the magnetic device approved for treating gastroesophageal reflux disease confirm its long-term safety and efficacy, Dr. Robert A. Ganz reported at the annual Digestive Disease Week.
Five years after device implantation, the proportion of patients experiencing moderate to severe regurgitation had dropped to about 1%, from almost 60% at baseline, and two-thirds of patients were not taking any proton pump inhibitors (PPIs), said Dr. Ganz, chief of gastroenterology at Abbott Northwestern Hospital, Minneapolis, and one of the study investigators. These were among the results of the study that evaluated the device, the LINX Reflux Management System. The device was approved by the Food and Drug Administration FDA) in 2012 and is for the treatment of people with GERD as defined by abnormal pH testing, who continue to have chronic GERD symptoms that persist despite maximum medical therapy for the treatment of reflux.
“Magnetic sphincter augmentation should be considered first-line surgical therapy for those with gastroesophageal reflux disease, based on the results of this study,” he said.
The 2-year results of the prospective, multicenter study were the basis of the FDA approval of the device, described by the manufacturer, Torax Medical, as a “small implant [composed] of interlinked titanium beads with magnetic cores,” implanted during standard laparoscopy. The magnetic attraction between the beads augments the existing esophageal sphincter’s barrier function to prevent reflux,” according to the company.
The study enrolled 100 patients with reflux disease with a median age of 53 years, who had experienced typical heartburn for at least 6 months with or without regurgitation and were taking PPIs daily for at least 3 months (median use 5 years). Patients had GERD for a median of 10 years (range: 1-40 years). People who had any type of previous gastric or esophageal surgery, Barrett’s esophagus, a hiatal hernia greater than 3 cm, a body mass index over 35 kg/m2, or grade C or D esophagitis were excluded.
The device was implanted in all patients, who served as their own controls; 85 patients were followed through 5 years (6 were lost to follow-up, the device was explanted in 6 patients, 2 patients did not consent to extended follow-up, and 1 patient died of an unrelated cancer). The median procedure time was 36 minutes with a range of 7-125 minutes); all procedures were successfully completed with no intraoperative complications and all patients were discharged within 24 hours on an unrestricted diet.
The median total Gastroesophageal Reflux Disease–Health-Related Quality of Life (GERD-HRQL) score at baseline was 27 points among those not on PPIs and 11 points on PPIs, dropping to 4 points at 5 years off PPIs. At baseline, 95% of patients expressed dissatisfaction related to reflux, which dropped to 7% at year 5. Moderate to severe heartburn was reported by 89% at baseline, dropping to about 12% at year 5. The proportion of patients experiencing moderate to severe regurgitation dropped from 57% at baseline to about 1% at 5 years, Dr. Ganz said.
At baseline, 100% were taking PPIs every day, compared with 15% at 5 years. (At 5 years, 75% had discontinued PPIs, and about 9% reported PRN use only). Grade A and B esophagitis decreased from 40% at baseline to 16% at 5 years, at which point most cases were grade A, and there were no patients with grade C or D esophagitis, he said. In addition, at 5 years, 100% of patients “reported the ability to belch, and those needing to vomit – about 16% – reported the ability to vomit,” demonstrating that normal physiology was preserved with the device.
At 5 years, there were no device erosions or migrations, or any significant adverse events other than dysphagia, which “was typically mild and not associated with weight loss and tended to resolve over time,” from about 70% in the first few weeks after surgery to 11% at 1 year and 7% at 5 years, Dr. Ganz said.
In seven cases, the device was removed laparoscopically, with no complications and gastric anatomy was preserved for future treatments. All removals were elective. The device was removed in four patients because of dysphagia, which completely resolved in those patients. One patient had the device removed because of vomiting of unknown cause that persisted after removal. Another two patients who “had the device removed for disease management” continued to experience reflux and had “uneventful” Nissen fundoplication,” he said.
“Five years after magnetic augmentation, we have demonstrated objective evidence of reduction in acid exposure and in the majority of patients, normalized pH [and] we demonstrated significant and durable improvement in all group parameters measured, with preservation of fundic anatomy and normal physiology, with the ability to belch and vomit,” Dr. Ganz concluded. The results also show that the “procedure is reproducible, safe and reversible if necessary,” he added, noting that one of the limitations of the study was that subjects served as their own controls. During the discussion period, he was asked about hiatal hernia repairs, an apparent trend to “decay” from years 1 to 5 in some parameters measured, and dysphagia after the procedure.
About 40% of the patients in the study had a hiatal hernia, and about one-third of these patients had a hernia repair. A subgroup analysis of the data is being performed to evaluate the impact of hernia repair, Dr. Ganz said.
PPI use increased from 8% in year 4, to 15% in year 5. The reason for this s difficult to determine but “even though there is a bit of a decay, patients are still quite satisfied at 5 years,” Dr. Ganz remarked, also referring to the marked impact on regurgitation. Many U.S. patients use PPIs for reasons other than reflux, and studies show that many patients are on PPIs after the Nissen procedure in the absence of pathologic pH scores, he pointed out.
Compared with the type of dysphagia patients experience after the Nissen procedure, which is immediate and improves with time, Dr. Ganz said that the dysphagia associated with the device “seemed to peak around 2 weeks and then it slowly improved with time, so this may be more of a scar tissue–associated dysphagia than an edema dysphagia, but … it does improve with time.
Three-year results of the study were published in 2013 (N. Engl. J. Med. 2013;368:719-72), Dr. Ganz was the lead author.
The study was funded by Torax Medical. Dr. Ganz had no disclosures related to the topic of this presentation.
*This story was updated 7/9/2015.
AT DDW 2015
Key clinical point: A magnetic device designed to augment the lower esophageal sphincter is a surgical option that can be expected to provide long-term control of reflux symptoms in patients with GERD.
Major finding: Improvements 5 years after treatment with the Linx Reflux Management System include a drop from 60% experiencing regurgitation to 1%, and two-third of patients no longer taking PPIs.
Data source: The multicenter, prospective study evaluated the long-term safety and efficacy of the device over 5 years in 100 patients with GERD, who served as their own controls; 85 were included in the 5-year follow-up.
Disclosures: The study was funded by the device manufacturer, Torax Medical. Dr. Ganz had no disclosures related to the topic of this presentation.