User login
Review: Opioid prescriptions are the work of many physicians
A “broad swath” of Medicare providers wrote scripts for opioids in 2013, contradicting the idea that the overdose epidemic is mainly the work of “small groups of prolific prescribers and corrupt pill mills,” investigators wrote online in JAMA Internal Medicine.
“Contrary to the California workers’ compensation data showing a small subset of prescribers accounting for a disproportionately large percentage of opioid prescribing, Medicare opioid prescribing is distributed across many prescribers and is, if anything, less skewed than all drug prescribing,” said Dr. Jonathan H. Chen of the Veterans Affairs Palo Alto (Calif.) Health Care System, and his associates.
Their study included 808,020 prescribers and almost 1.2 billion Medicare Part D claims worth nearly $81 billion dollars. They focused on schedule II opioid prescriptions containing oxycodone, fentanyl, hydrocodone, morphine, methadone, hydromorphone, oxymorphone, meperidine, codeine, opium, or levorphanol (JAMA Intern Med. 2015 Dec 14. doi: 10.1001/jamainternmed.2015.6662).
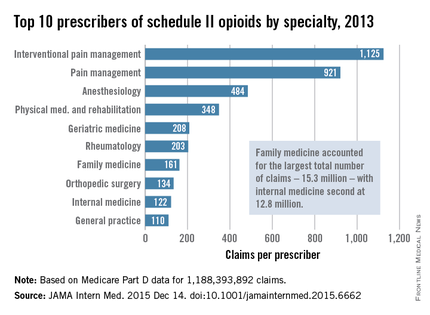
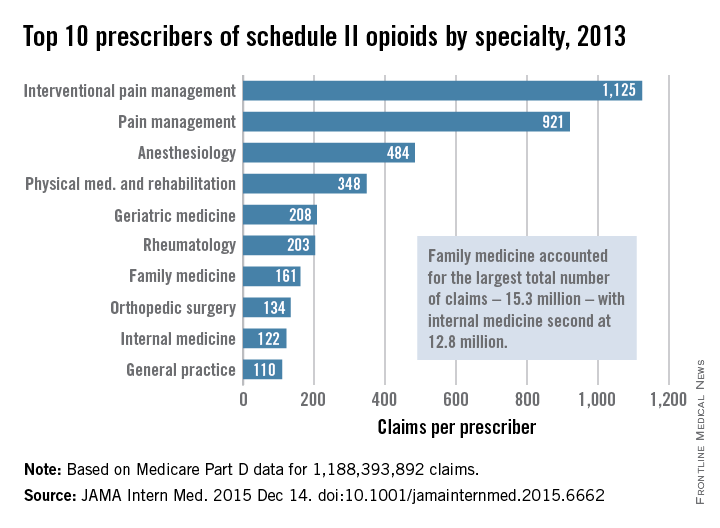
Not surprisingly, specialists in pain management, anesthesia, and physical medicine wrote the most prescriptions per provider. But family practitioners, internists, nurse practitioners, and physician assistants wrote 35,268,234 prescriptions – more than all other specialties combined. “The trends hold up across state lines, with negligible geographic variability,” the researchers said.
The findings contradict an analysis of California workers’ compensation data, in which 1% of prescribers accounted for a third of schedule II opioid prescriptions, and 10% of prescribers accounted for 80% of prescriptions, the investigators noted. Nonetheless, 10% of Medicare prescribers in Dr. Chen’s study accounted for 78% of the total cost of opioids, possibly because they were prescribing pricier formulations or higher doses.
Overall, the findings suggest that opioid prescribing is “widespread” and “relatively indifferent to individual physicians, specialty, or region” – and that efforts to stem the tide must be equally broad, the researchers concluded.
Their study was supported by the VA Office of Academic Affiliations, the VA Health Services Research and Development Service, the National Institute of General Medical Sciences, and the Peter F. McManus Charitable Trust. The researchers had no disclosures.
A “broad swath” of Medicare providers wrote scripts for opioids in 2013, contradicting the idea that the overdose epidemic is mainly the work of “small groups of prolific prescribers and corrupt pill mills,” investigators wrote online in JAMA Internal Medicine.
“Contrary to the California workers’ compensation data showing a small subset of prescribers accounting for a disproportionately large percentage of opioid prescribing, Medicare opioid prescribing is distributed across many prescribers and is, if anything, less skewed than all drug prescribing,” said Dr. Jonathan H. Chen of the Veterans Affairs Palo Alto (Calif.) Health Care System, and his associates.
Their study included 808,020 prescribers and almost 1.2 billion Medicare Part D claims worth nearly $81 billion dollars. They focused on schedule II opioid prescriptions containing oxycodone, fentanyl, hydrocodone, morphine, methadone, hydromorphone, oxymorphone, meperidine, codeine, opium, or levorphanol (JAMA Intern Med. 2015 Dec 14. doi: 10.1001/jamainternmed.2015.6662).

Not surprisingly, specialists in pain management, anesthesia, and physical medicine wrote the most prescriptions per provider. But family practitioners, internists, nurse practitioners, and physician assistants wrote 35,268,234 prescriptions – more than all other specialties combined. “The trends hold up across state lines, with negligible geographic variability,” the researchers said.
The findings contradict an analysis of California workers’ compensation data, in which 1% of prescribers accounted for a third of schedule II opioid prescriptions, and 10% of prescribers accounted for 80% of prescriptions, the investigators noted. Nonetheless, 10% of Medicare prescribers in Dr. Chen’s study accounted for 78% of the total cost of opioids, possibly because they were prescribing pricier formulations or higher doses.
Overall, the findings suggest that opioid prescribing is “widespread” and “relatively indifferent to individual physicians, specialty, or region” – and that efforts to stem the tide must be equally broad, the researchers concluded.
Their study was supported by the VA Office of Academic Affiliations, the VA Health Services Research and Development Service, the National Institute of General Medical Sciences, and the Peter F. McManus Charitable Trust. The researchers had no disclosures.
A “broad swath” of Medicare providers wrote scripts for opioids in 2013, contradicting the idea that the overdose epidemic is mainly the work of “small groups of prolific prescribers and corrupt pill mills,” investigators wrote online in JAMA Internal Medicine.
“Contrary to the California workers’ compensation data showing a small subset of prescribers accounting for a disproportionately large percentage of opioid prescribing, Medicare opioid prescribing is distributed across many prescribers and is, if anything, less skewed than all drug prescribing,” said Dr. Jonathan H. Chen of the Veterans Affairs Palo Alto (Calif.) Health Care System, and his associates.
Their study included 808,020 prescribers and almost 1.2 billion Medicare Part D claims worth nearly $81 billion dollars. They focused on schedule II opioid prescriptions containing oxycodone, fentanyl, hydrocodone, morphine, methadone, hydromorphone, oxymorphone, meperidine, codeine, opium, or levorphanol (JAMA Intern Med. 2015 Dec 14. doi: 10.1001/jamainternmed.2015.6662).

Not surprisingly, specialists in pain management, anesthesia, and physical medicine wrote the most prescriptions per provider. But family practitioners, internists, nurse practitioners, and physician assistants wrote 35,268,234 prescriptions – more than all other specialties combined. “The trends hold up across state lines, with negligible geographic variability,” the researchers said.
The findings contradict an analysis of California workers’ compensation data, in which 1% of prescribers accounted for a third of schedule II opioid prescriptions, and 10% of prescribers accounted for 80% of prescriptions, the investigators noted. Nonetheless, 10% of Medicare prescribers in Dr. Chen’s study accounted for 78% of the total cost of opioids, possibly because they were prescribing pricier formulations or higher doses.
Overall, the findings suggest that opioid prescribing is “widespread” and “relatively indifferent to individual physicians, specialty, or region” – and that efforts to stem the tide must be equally broad, the researchers concluded.
Their study was supported by the VA Office of Academic Affiliations, the VA Health Services Research and Development Service, the National Institute of General Medical Sciences, and the Peter F. McManus Charitable Trust. The researchers had no disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Many different types of general practitioners and specialists often prescribe opioids to Medicare beneficiaries.
Major finding: Family practitioners, internists, nurse practitioners, and physician assistants wrote 35,268,234 prescriptions – more than all other specialties combined.
Data source: An analysis of nearly 1.2 billion Medicare part D claims from 2013.
Disclosures: The study was supported by the VA Office of Academic Affiliations, the VA Health Services Research and Development Service, the National Institute of General Medical Sciences, and the Peter F. McManus Charitable Trust. The researchers had no disclosures.
FDA approves treatment for chemotherapy ODs, life-threatening toxicities
Uridine triacetate, a pyrimidine analogue, has been approved for the emergency treatment of fluorouracil or capecitabine overdoses in adults and children, and for patients who develop “certain severe or life-threatening toxicities within 4 days of receiving” these treatments, the Food and Drug Administration announced on Dec. 11.
“Today’s approval is a first-of-its-kind therapy that can potentially save lives following overdose or life-threatening toxicity from these chemotherapy agents,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. It will be marketed as Vistogard by Wellstat Therapeutics.
Uridine comes in an oral granule formulation that can be mixed into soft foods or, when necessary, administered via a nasogastric or gastrostomy tube, the prescribing information states. The indication is for use after an overdose “regardless of the presence of symptoms,” and for treating “early-onset, severe, or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration,” according to the prescribing information.
Uridine blocks cell damage and cell death caused by fluorouracil chemotherapy, according to the statement, which adds that it is up to the patient’s health care provider to “determine when he or she should return to the prescribed chemotherapy after treatment with Vistogard.”
Uridine was evaluated in two studies of 135 adults and children with cancer, treated with uridine for a fluorouracil or capecitabine overdose, or for early-onset, unusually severe or life-threatening toxicities within 96 hours after receiving fluorouracil (not because of an overdose). Among those treated for an overdose, 97% were alive 30 days after treatment, and among those treated for early-onset severe or life-threatening toxicity, 89% were alive 30 days after treatment. In addition, 33% of the patients resumed chemotherapy within 30 days, according to the FDA statement. Diarrhea, vomiting, and nausea were the most common adverse events associated with treatment.
Uridine was granted orphan drug, priority review, and fast track designations.
Uridine triacetate, a pyrimidine analogue, has been approved for the emergency treatment of fluorouracil or capecitabine overdoses in adults and children, and for patients who develop “certain severe or life-threatening toxicities within 4 days of receiving” these treatments, the Food and Drug Administration announced on Dec. 11.
“Today’s approval is a first-of-its-kind therapy that can potentially save lives following overdose or life-threatening toxicity from these chemotherapy agents,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. It will be marketed as Vistogard by Wellstat Therapeutics.
Uridine comes in an oral granule formulation that can be mixed into soft foods or, when necessary, administered via a nasogastric or gastrostomy tube, the prescribing information states. The indication is for use after an overdose “regardless of the presence of symptoms,” and for treating “early-onset, severe, or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration,” according to the prescribing information.
Uridine blocks cell damage and cell death caused by fluorouracil chemotherapy, according to the statement, which adds that it is up to the patient’s health care provider to “determine when he or she should return to the prescribed chemotherapy after treatment with Vistogard.”
Uridine was evaluated in two studies of 135 adults and children with cancer, treated with uridine for a fluorouracil or capecitabine overdose, or for early-onset, unusually severe or life-threatening toxicities within 96 hours after receiving fluorouracil (not because of an overdose). Among those treated for an overdose, 97% were alive 30 days after treatment, and among those treated for early-onset severe or life-threatening toxicity, 89% were alive 30 days after treatment. In addition, 33% of the patients resumed chemotherapy within 30 days, according to the FDA statement. Diarrhea, vomiting, and nausea were the most common adverse events associated with treatment.
Uridine was granted orphan drug, priority review, and fast track designations.
Uridine triacetate, a pyrimidine analogue, has been approved for the emergency treatment of fluorouracil or capecitabine overdoses in adults and children, and for patients who develop “certain severe or life-threatening toxicities within 4 days of receiving” these treatments, the Food and Drug Administration announced on Dec. 11.
“Today’s approval is a first-of-its-kind therapy that can potentially save lives following overdose or life-threatening toxicity from these chemotherapy agents,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. It will be marketed as Vistogard by Wellstat Therapeutics.
Uridine comes in an oral granule formulation that can be mixed into soft foods or, when necessary, administered via a nasogastric or gastrostomy tube, the prescribing information states. The indication is for use after an overdose “regardless of the presence of symptoms,” and for treating “early-onset, severe, or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration,” according to the prescribing information.
Uridine blocks cell damage and cell death caused by fluorouracil chemotherapy, according to the statement, which adds that it is up to the patient’s health care provider to “determine when he or she should return to the prescribed chemotherapy after treatment with Vistogard.”
Uridine was evaluated in two studies of 135 adults and children with cancer, treated with uridine for a fluorouracil or capecitabine overdose, or for early-onset, unusually severe or life-threatening toxicities within 96 hours after receiving fluorouracil (not because of an overdose). Among those treated for an overdose, 97% were alive 30 days after treatment, and among those treated for early-onset severe or life-threatening toxicity, 89% were alive 30 days after treatment. In addition, 33% of the patients resumed chemotherapy within 30 days, according to the FDA statement. Diarrhea, vomiting, and nausea were the most common adverse events associated with treatment.
Uridine was granted orphan drug, priority review, and fast track designations.
Senate calls for childproof packaging for ‘e-cig juice’
The Senate passed a bill that would require childproof packaging for liquid nicotine products.
The Child Nicotine Poisoning Prevention Act of 2015 (S. 142), also would codify Food and Drug Administration authority to regulate the packaging of liquid nicotine that is used to refill various electronic nicotine delivery systems.
S. 142 passed by unanimous consent in the Senate on Dec. 10. The House of Representatives has not taken action on the bill.
“Just a small amount of this stuff can injure or even kill a small child,” Sen. Bill Nelson (D-Fla.), the bill’s sponsor, said in a statement. “Making these bottles childproof is just common sense.”
In 2014, poison control centers received more than 3,000 calls related to e-cigarette nicotine exposure, including one toddler death, according to a statement from the American Academy of Pediatrics.
“With e-cigarettes becoming more and more common in households across the country, we cannot afford to wait another day to protect children from poisonous liquid nicotine,” AAP President Dr. Sandra Hassink said.
The Senate passed a bill that would require childproof packaging for liquid nicotine products.
The Child Nicotine Poisoning Prevention Act of 2015 (S. 142), also would codify Food and Drug Administration authority to regulate the packaging of liquid nicotine that is used to refill various electronic nicotine delivery systems.
S. 142 passed by unanimous consent in the Senate on Dec. 10. The House of Representatives has not taken action on the bill.
“Just a small amount of this stuff can injure or even kill a small child,” Sen. Bill Nelson (D-Fla.), the bill’s sponsor, said in a statement. “Making these bottles childproof is just common sense.”
In 2014, poison control centers received more than 3,000 calls related to e-cigarette nicotine exposure, including one toddler death, according to a statement from the American Academy of Pediatrics.
“With e-cigarettes becoming more and more common in households across the country, we cannot afford to wait another day to protect children from poisonous liquid nicotine,” AAP President Dr. Sandra Hassink said.
The Senate passed a bill that would require childproof packaging for liquid nicotine products.
The Child Nicotine Poisoning Prevention Act of 2015 (S. 142), also would codify Food and Drug Administration authority to regulate the packaging of liquid nicotine that is used to refill various electronic nicotine delivery systems.
S. 142 passed by unanimous consent in the Senate on Dec. 10. The House of Representatives has not taken action on the bill.
“Just a small amount of this stuff can injure or even kill a small child,” Sen. Bill Nelson (D-Fla.), the bill’s sponsor, said in a statement. “Making these bottles childproof is just common sense.”
In 2014, poison control centers received more than 3,000 calls related to e-cigarette nicotine exposure, including one toddler death, according to a statement from the American Academy of Pediatrics.
“With e-cigarettes becoming more and more common in households across the country, we cannot afford to wait another day to protect children from poisonous liquid nicotine,” AAP President Dr. Sandra Hassink said.
Naloxone to revive an addict
We are in the midst of an epidemic of heroin and prescription opioid abuse. While the two do not completely explain each other, they are tragically and irrevocably linked.
From 2001 to 2013, we have observed a threefold increase in the total number of overdose deaths from opioid pain relievers (about 16,000 in 2013) and a fivefold increase in the total number of overdose deaths from heroin (about 8,000 in 2013).
Heroin initiation is almost 20 times higher among individuals reporting nonmedical prescription pain reliever use. Among opioid-treatment seekers, the majority of individuals who initiated opioid use in the 1960s were first exposed to heroin. This is in contrast to those who initiated in the 2000s, among whom the majority were exposed to prescription opioids. For young adults, the main sources of opioids are family, friends … and clinicians.
Opioids are powerfully addictive and can be snorted, swallowed, smoked, or shot. Data from the START (Starting Treatment with Agonist Replacement Therapies) trial suggest that individuals who inject opioids are less likely to remain in treatment than noninjectors. This necessarily increases the risk for injectors to inject again and be at risk for overdose.
Opioid overdose can be reversed with the use of naloxone. But naloxone has to be immediately or quickly available for it to be effective. Take-home naloxone programs are located in 30 U.S. states and the District of Columbia. Since 1996, home naloxone programs have reported more than 26,000 drug overdose reversals with naloxone.
On Nov. 18, the Food and Drug Administration announced the approval of a naloxone nasal spray. Prior to this approval, naloxone was only available in the injectable form (syringe or auto-injector), and needle management likely posed a barrier to first responders. The nasal spray can be administered easily without medical training. Naloxone nasal spray administered in one nostril delivered approximately the same levels or higher of naloxone as a single dose of an FDA-approved naloxone intramuscular injection in approximately the same time frame.
It is one thing to save a heroin addict who has just overdosed with nasal naloxone followed by appropriate medical attention. It is entirely another to engage them in an effective drug treatment program.
If naloxone revives them, it is treatment that can save them.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
We are in the midst of an epidemic of heroin and prescription opioid abuse. While the two do not completely explain each other, they are tragically and irrevocably linked.
From 2001 to 2013, we have observed a threefold increase in the total number of overdose deaths from opioid pain relievers (about 16,000 in 2013) and a fivefold increase in the total number of overdose deaths from heroin (about 8,000 in 2013).
Heroin initiation is almost 20 times higher among individuals reporting nonmedical prescription pain reliever use. Among opioid-treatment seekers, the majority of individuals who initiated opioid use in the 1960s were first exposed to heroin. This is in contrast to those who initiated in the 2000s, among whom the majority were exposed to prescription opioids. For young adults, the main sources of opioids are family, friends … and clinicians.
Opioids are powerfully addictive and can be snorted, swallowed, smoked, or shot. Data from the START (Starting Treatment with Agonist Replacement Therapies) trial suggest that individuals who inject opioids are less likely to remain in treatment than noninjectors. This necessarily increases the risk for injectors to inject again and be at risk for overdose.
Opioid overdose can be reversed with the use of naloxone. But naloxone has to be immediately or quickly available for it to be effective. Take-home naloxone programs are located in 30 U.S. states and the District of Columbia. Since 1996, home naloxone programs have reported more than 26,000 drug overdose reversals with naloxone.
On Nov. 18, the Food and Drug Administration announced the approval of a naloxone nasal spray. Prior to this approval, naloxone was only available in the injectable form (syringe or auto-injector), and needle management likely posed a barrier to first responders. The nasal spray can be administered easily without medical training. Naloxone nasal spray administered in one nostril delivered approximately the same levels or higher of naloxone as a single dose of an FDA-approved naloxone intramuscular injection in approximately the same time frame.
It is one thing to save a heroin addict who has just overdosed with nasal naloxone followed by appropriate medical attention. It is entirely another to engage them in an effective drug treatment program.
If naloxone revives them, it is treatment that can save them.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
We are in the midst of an epidemic of heroin and prescription opioid abuse. While the two do not completely explain each other, they are tragically and irrevocably linked.
From 2001 to 2013, we have observed a threefold increase in the total number of overdose deaths from opioid pain relievers (about 16,000 in 2013) and a fivefold increase in the total number of overdose deaths from heroin (about 8,000 in 2013).
Heroin initiation is almost 20 times higher among individuals reporting nonmedical prescription pain reliever use. Among opioid-treatment seekers, the majority of individuals who initiated opioid use in the 1960s were first exposed to heroin. This is in contrast to those who initiated in the 2000s, among whom the majority were exposed to prescription opioids. For young adults, the main sources of opioids are family, friends … and clinicians.
Opioids are powerfully addictive and can be snorted, swallowed, smoked, or shot. Data from the START (Starting Treatment with Agonist Replacement Therapies) trial suggest that individuals who inject opioids are less likely to remain in treatment than noninjectors. This necessarily increases the risk for injectors to inject again and be at risk for overdose.
Opioid overdose can be reversed with the use of naloxone. But naloxone has to be immediately or quickly available for it to be effective. Take-home naloxone programs are located in 30 U.S. states and the District of Columbia. Since 1996, home naloxone programs have reported more than 26,000 drug overdose reversals with naloxone.
On Nov. 18, the Food and Drug Administration announced the approval of a naloxone nasal spray. Prior to this approval, naloxone was only available in the injectable form (syringe or auto-injector), and needle management likely posed a barrier to first responders. The nasal spray can be administered easily without medical training. Naloxone nasal spray administered in one nostril delivered approximately the same levels or higher of naloxone as a single dose of an FDA-approved naloxone intramuscular injection in approximately the same time frame.
It is one thing to save a heroin addict who has just overdosed with nasal naloxone followed by appropriate medical attention. It is entirely another to engage them in an effective drug treatment program.
If naloxone revives them, it is treatment that can save them.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
First EDition: News for and about the practice of emergency medicine
FDA approves first naloxone nasal spray for opioid overdose
BY DEEPAK CHITNIS
Frontline Medical News
The US Food and Drug Administration (FDA) has approved the first nasal spray variant of the opioid-overdose drug naloxone hydrochloride.
Marketed in the United States as Narcan (Adapt Pharma, a partner of Lightlake Therapeutics, Radnor, Pennsylvania) the nasal spray is known to stop or, in some cases, reverse the effects of opioid overdosing in patients. Narcan is the first naloxone hydrochloride nasal spray approved by the FDA.
“Combating the opioid abuse epidemic is a top priority for the FDA,” Dr Stephen Ostroff, FDA acting commissioner, said in a statement released with the November 18 approval announcement. “While naloxone will not solve the underlying problems of the opioid epidemic, we are speeding to review new formulations that will ultimately save lives that might otherwise be lost to drug addiction and overdose.”1
The nasal spray itself is available only with a prescription, and is safe for use by both adults and children, according to the FDA.
The spray delivers a dose of 4 mg naloxone in a single 0.1-mL nasal spray, which comes in a ready-to-use, needle-free device, according to Adapt Pharma. Administration of Narcan, which is sprayed into one nostril while the patient is lying on his or her back, does not require special training.
The FDA warned that body aches, diarrhea, tachycardia, fever, piloerection, nausea, nervousness, abdominal cramps, weakness, and increased blood pressure, among other conditions, are all possible side effects of Narcan.
Narcan’s approval is one step of many that must be taken to adequately address and ultimately end the problem of opioid abuse in the US, cautioned Dr Peter Friedmann, an addiction medicine specialist and chief research officer at Baystate Health in Springfield, Massachusetts. He expressed concern regarding the pricing of Narcan, noting that the drug’s affordability is crucial to its success.
“Right now, nasal atomizers with syringes are used off label, and the prices have been going up with increasing demand,” he said. “But [Narcan] is a commercial product based around what is essentially a generic medication, so [I] hope it’s priced at a price point that’s accessible to the great majority of patients and their families who are facing addiction, many of whom don’t have huge means.”
Therapeutic hypothermia after nonshockable-rhythm cardiac arrest
BY MARY ANN MOON
FROM CIRCULATION
Vitals Key clinical point: Therapeutic hypothermia raises the rate of survival with a good neurologic outcome in comatose patients after a cardiac arrest with a nonshockable initial rhythm. Major finding: The rate of survival-to-hospital discharge was significantly higher with therapeutic hypothermia (29%) than without it (15%), as was the rate of survival with a favorable neurologic outcome (21% vs 10%). Data source: A retrospective cohort study involving 519 adults enrolled in a therapeutic hypothermia registry during a 3-year period. Disclosures: This study was supported by the National Institutes of Health. Dr Perman and her associates reported having no financial disclosures. |
Therapeutic hypothermia significantly raises the rate of survival with a good neurologic outcome among patients who are comatose after a cardiac arrest with a nonshockable initial rhythm, according to a report published online November 16 in Circulation.1
Many observational and retrospective cohort studies have examined the possible benefits of therapeutic hypothermia in this patient population, but they have produced conflicting results. No prospective randomized clinical trials have been published, and some clinicians insist the treatment should be reserved for patients who meet the narrow criteria for which there is good supportive evidence; others, eager for any clinical strategy that can improve the outcomes of these critically ill patients, routinely expand its use to comatose patients regardless of their initial heart rhythm or the location of the cardiac arrest, wrote Dr Sarah M. Perman of the department of emergency medicine, University of Colorado, Aurora, and her associates.
They studied the issue using data from a national registry of patients treated at 16 medical centers that sometimes use therapeutic hypothermia after cardiac arrest. They assessed the records of 519 adults who had a nontraumatic cardiac arrest and initially registered either pulseless electrical activity or asystole, then had a return of spontaneous circulation but remained comatose. Approximately half of these comatose survivors (262 patients) were treated with therapeutic hypothermia according to their hospital’s usual protocols, and the other half (257 control subjects) received standard care without therapeutic hypothermia.
In the propensity-matched cohort, the rate of survival-to-hospital discharge was significantly higher with therapeutic hypothermia (29%) than without it (15%), as was the rate of survival with a favorable neurologic outcome (21% vs 10%). And in a multivariate analysis of factors contributing to positive patient outcomes, the intervention was associated with a 3.5-fold increase in favorable neurologic outcomes. A further analysis of the data showed that therapeutic hypothermia was associated with improved survival, with an odds ratio (OR) of 2.8, the investigators said.
In addition, an analysis of outcomes across various subgroups of patients showed that regardless of the location of their cardiac arrest, patients were consistently more likely to survive to hospital discharge neurologically intact if they received therapeutic hypothermia (OR, 2.1 for out-of-hospital cardiac rest; OR, 4.2 for in-hospital cardiac arrest).
“These results lend support to a broadening of indications for therapeutic hypothermia in comatose post-arrest patients with initial nonshockable rhythms,” Dr Perman and her associates said.
Andexanet reverses anticoagulant effects of factor Xa inhibitors
BY BIANCA NOGRADY
FROM THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Andexanet reverses the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban in healthy older adults. Major finding: Andexanet achieved a 92% to 94% reduction in antifactor Xa activity, compared with an 18% to 21% reduction with placebo. Data source: A two-part randomized, placebo-controlled study in 145 healthy individuals. Disclosures: The study was supported by Portola Pharmaceuticals, Bayer, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. Several authors are employees of Portola, one with stock options and a related patent. Other authors declared grants and personal fees from the pharmaceutical industry, including the study supporters. |
Andexanet alfa has been found to reverse the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban, according to a study presented at the American Heart Association scientific sessions and published simultaneously in the November 11 issue of the New England Journal of Medicine.1
In a two-part randomized, placebo-controlled study involving 145 healthy individuals with a mean age of 58 years, patients treated first with apixaban and then given a bolus of andexanet had a 94% reduction in anti-factor Xa activity, compared with a 21% reduction with placebo. Thrombin generation was restored in 100% of patients within 2 to 5 minutes.
In the patients treated with rivaroxaban, treatment with andexanet reduced antifactor Xa activity by 92%, compared to 18% with placebo. Thrombin generation was restored in 96% of participants in the andexanet group, compared with 7% in the placebo group.
Adverse events associated with andexanet were minor, including constipation, feeling hot, or a strange taste in the mouth. The effects of the andexanet also were sustained over the course of a 2-hour infusion in addition to the bolus.1
“The rapid onset and offset of action of andexanet and the ability to administer it as a bolus or as a bolus plus an infusion may provide flexibility with regard to the restoration of hemostasis when urgent factor Xa inhibitor reversal is required,” Dr Deborah M. Siegal of McMaster University, Hamilton, Ontario, Canada and coauthors wrote.
Continuous no better than interrupted chest compressions
BY MARY ANN MOON
FROM THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Continuous chest compressions during CPR didn’t improve survival or neurologic function compared with standard compressions briefly interrupted for ventilation. Major finding: The primary outcome – the rate of survival to hospital discharge – was 9.0% for continuous chest compressions and 9.7% for interrupted compressions, a nonsignificant difference. Data source: A cluster-randomized crossover trial involving 23,711 adults treated by 114 North American EMS agencies for nontraumatic out-of-hospital cardiac arrest. Disclosures: This study was supported by the US National Heart, Lung, and Blood Institute, the US Army Medical Research and Materiel Command, the Canadian Institutes of Health Research, the Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada, the American Heart Association, and the Medic One Foundation. Dr Nichol and his associates reported ties to numerous industry sources. |
Continuous chest compressions during CPR failed to improve survival or neurologic function compared with standard chest compressions that are briefly interrupted for ventilation, based on findings in the first large randomized trial to compare the two strategies for out-of-hospital, nontraumatic cardiac arrest.
In a presentation at the American Heart Association scientific sessions, simultaneously published online November 9 in the New England Journal of Medicine, Dr Graham Nichol and his associates analyzed data from the Resuscitation Outcomes Consortium, a network of clinical centers and EMS agencies that have expertise in conducting research on out-of-hospital cardiac arrest.1
Data were analyzed for 23,711 adults treated by 114 EMS agencies affiliated with eight clinical centers across the United States and Canada. These agencies were grouped into 47 clusters that were randomly assigned to perform CPR using either continuous chest compressions (100 per minute) with asynchronous positive-pressure ventilations (10 per minute) or standard chest compressions interrupted for ventilations (at a rate of 30 compressions per two ventilations) at every response to an out-of-hospital cardiac arrest. Twice per year, each cluster crossed over to the other resuscitation strategy, said Dr Nichol of the University of Washington–Harborview Center for Prehospital Emergency Care and Clinical Trial Center in Seattle.
A total of 12,653 patients were assigned to continuous chest compressions (the intervention group) and 11,058 to interrupted chest compressions (the control group). The primary outcome—the rate of survival to hospital discharge—was 9.0% in the intervention group and 9.7% in the control group, a nonsignificant difference. Similarly, the rate of survival with favorable neurologic function did not differ significantly, at 7.0% and 7.7%, respectively, the investigators said.1
However, it is also possible that three important limitations of this trial unduly influenced the results.
First, the per-protocol analysis, which used an automated algorithm to assess adherence to the compression assignments, could not classify many patients as having received either continuous or interrupted chest compressions. Second, the quality of postresuscitation care, which certainly influences outcomes, was not monitored. And third, actual oxygenation levels were not measured, nor were minutes of ventilation delivered. Thus, “we do not know whether there were important differences in oxygenation or ventilation between the two treatment strategies,” he said.
It is not yet clear why this large randomized trial1 showed no benefit from continuous chest compressions when previous observational research showed the opposite. One possibility is that many of the previous studies assessed not just chest compressions but an entire bundle of care related to CPR, so the benefits they reported may not be attributable to chest compressions alone.
In addition, in this study the mean chest-compression fraction – the proportion of each minute during which compressions are given, an important marker of interruptions in chest compressions – was already high in the control group (0.77) and not much different from that in the intervention group (0.83). Both of these are much higher than the target recommended by both American and European guidelines, which is only 0.60.
And of course a third reason may be that the interruptions for ventilation during CPR aren’t all that critical, and may be less detrimental to survival, than is currently believed.
Dr Rudolph W. Koster is in the department of cardiology at Amsterdam Academic Medical Center. He reported having no relevant financial disclosures. Dr Koster made these remarks in an editorial accompanying Dr Nichol’s report (Koster RW. Continuous or interrupted chest compressions for cardiac arrest [published online ahead of print November 9, 2015]. N Engl J Med).
Answers elusive in quest for better chlamydia treatment
BY BRUCE JANCIN
EXPERT ANALYSIS FROM ICAAC 2015
SAN DIEGO – The hottest topic today in the treatment of sexually transmitted diseases caused by Chlamydia trachomatis is the unresolved question of whether azithromycin is still as effective as doxycycline, the other current guideline-recommended, first-line therapy, Dr Kimberly Workowski said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is important, because doxycycline is administered twice a day for 7 days, and azithromycin is given as a single pill suitable for directly observed therapy,” noted Dr Workowski, professor of medicine at Emory University in Atlanta and lead author of the 2015 Centers for Disease Control and Prevention STD treatment guidelines.1
Several recent retrospective case series have suggested azithromycin is less effective, with the biggest efficacy gap being seen in rectal C. trachomatis infections. These nonrandomized studies were further supported by an Australian meta-analysis of six randomized, controlled trials comparing the two antibiotics for the treatment of genital chlamydia. The investigators found roughly 3% greater efficacy for doxycycline, compared with azithromycin, for urogenital chlamydia, and a 7% advantage for doxycycline in treating symptomatic urethral infection in men.
However, the investigators were quick to add the caveat that “the quality of the evidence varies considerably.”2
There’s a pressing need for better data. Dr Workowski and her colleagues on the STD guidelines panel are eagerly awaiting the results of a well-structured randomized trial led by Dr William M. Geisler, professor of medicine at the University of Alabama, Birmingham. The investigators randomized more than 300 chlamydia-infected male and female inmates in youth correctional facilities to guideline-recommended azithromycin at 1 g orally in a single dose or oral doxycycline at 100 mg twice daily for 7 days. The results, which are anticipated soon, should influence clinical practice, Dr Workowski said.
Here’s what else is new in chlamydia:
Pregnancy: For treatment of chlamydia in pregnancy, amoxicillin at 500 mg orally t.i.d. for 7 days has been demoted from a first-line recommended therapy to alternative-regimen status. Now, the sole recommended first-line treatment in pregnancy is oral azithromycin at 1 g orally in a single dose.
“We did this based on in vitro studies showing Chlamydia trachomatis is not well-killed by amoxicillin. Instead, the drug induces persistent viable noninfectious forms which can sometimes reactivate,” Dr Workowski explained.
Delayed-release doxycycline: This FDA-approved drug, known as Doryx, administered as a 200-mg tablet once daily for 7 days, “might be an alternative” to the standard generic doxycycline regimen of 100 mg twice daily for 7 days, according to the current Centers for Disease Control and Prevention guidelines. In a randomized, double-blind trial, the new agent was as effective as twice-daily generic doxycycline in men and women with urogenital C. trachomatis infection, and it had fewer gastrointestinal side effects. Doryx is costlier than the twice-daily alternatives.
Lymphogranuloma venereum: The current guidelines repeat a point made in previous editions, but one Dr Workowski believes remains underappreciated and thus worthy of emphasis: Rectal exposure to C. trachomatis serovars L1, L2, and L3 in men who have sex with men or in women who have rectal sex can cause lymphogranuloma venereum, which takes the form of proctocolitis mimicking inflammatory bowel disease.
Patients suspected of having lymphogranuloma venereum should be started presumptively on the recommended regimen for this STD, which is oral doxycycline at 100 mg b.i.d. for 21 days.
“If you also see painful ulcers or, on anoscopy, mucosal ulcers, you should also treat empirically for herpes simplex until your culture results come back,” she added.
Dr Workowski reported having no financial conflicts of interest.
Catheter-directed thrombolysis trumps systemic for acute pulmonary embolism
BY MITCHEL L. ZOLER
AT CHEST 2015
Vitals Key clinical point: Catheter-directed thrombolysis was linked to reduced mortality, compared with systemic thrombolysis in patients with an acute pulmonary embolism. Major finding: In-hospital mortality in acute pulmonary embolism patients ran 10% with catheter-directed thrombolysis and 17% with systemic thrombolysis. Data source: Review of 1,521 US patients treated for acute pulmonary embolism during 2010-2012 in the National Inpatient Sample. Disclosures: Dr Saqib and Dr Muthiah had no disclosures. |
MONTREAL – Catheter-directed thrombolysis surpassed systemic thrombolysis for minimizing in-hospital mortality of patients with an acute pulmonary embolism in a review of more than 1,500 United States patients.
The review also found evidence that US pulmonary embolism (PE) patients increasingly undergo catheter-directed thrombolysis, with usage jumping by more than 50% from 2010 to 2012, although in 2012 US clinicians performed catheter-directed thrombolysis on 160 patients with an acute pulmonary embolism (PE) who were included in a national US registry of hospitalized patients, Dr Amina Saqib said at the annual meeting of the American College of Chest Physicians.
Catheter-directed thrombolysis resulted in a 9% in-hospital mortality rate and a 10% combined rate of in-hospital mortality plus intracerebral hemorrhages, rates significantly below those tallied in propensity score-matched patients who underwent systemic thrombolysis of their acute PE. The matched group with systemic thrombolysis had a 17% in-hospital mortality rate and a 17% combined mortality plus intracerebral hemorrhage rate, said Dr Saqib, a researcher at Staten Island (New York) University Hospital.
“To the best of our knowledge, this is the first, large, nationwide, observational study that compared safety and efficacy outcomes between systemic thrombolysis and catheter-directed thrombolysis in acute PE,”
Dr Saqib said.
The US data, collected during 2010-2012, also showed that, after adjustment for clinical and demographic variables, each acute PE treatment by catheter-directed thrombolysis cost an average $9,428 above the cost for systemic thrombolysis, she said.
Dr Saqib and her associates used data collected by the Federal National Inpatient Sample. Among US patients hospitalized during 2010-2012 and entered into this database, they identified 1,169 adult acute PE patients who underwent systemic thrombolysis and 352 patients who received catheter-directed thrombolysis. The patients averaged about 58 years old and just under half were men.
The propensity score-adjusted analysis also showed no statistically significant difference between the two treatment approaches for the incidence of intracerebral hemorrhage, any hemorrhages requiring a transfusion, new-onset acute renal failure, or hospital length of stay. Among the patients treated by catheter-directed thrombolysis, all the intracerebral hemorrhages occurred during 2010; during 2011 and 2012 none of the patients treated this way had an intracerebral hemorrhage, Dr Saqib noted.
Although the findings were consistent with results from prior analyses, the propensity-score adjustment used in the current study cannot fully account for all unmeasured confounding factors. The best way to compare catheter-directed thrombolysis and systemic thrombolysis for treating acute PE would be in a prospective, randomized study, Dr Saqib said.
Survivors of out-of-hospital cardiac arrest usually had intact brain function
BY AMY KARON
FROM THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, regardless of duration of CPR in the field. Major finding: Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital. Data source: A retrospective observational study of 3,814 adults who had an out-of-hospital cardiac arrest between 2005 and 2014. Disclosures: Dr Williams had no disclosures. The senior author disclosed research funding from the Medtronic Foundation. |
Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, even if cardiopulmonary resuscitation lasted longer than has been recommended, authors of a retrospective observational study reported at the American Heart Association scientific sessions.
Dr Jefferson Williams of the Wake County Department of Emergency Medical Services in Raleigh, North Carolina, and his associates studied 3,814 adults who had a cardiac arrest outside the hospital between 2005 and 2014. Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital.
Neurologically intact survival was associated with having an initial shockable rhythm, a bystander-witnessed arrest, and return of spontaneous circulation in the field rather than in the hospital. Age, basic airway management, and therapeutic hypothermia phase also predicted survival with intact brain function, but duration of CPR did not.
Procalcitonin assay detects invasive bacterial infection
BY MARY ANN MOON
FROM JAMA PEDIATRICS
Vitals Key clinical point: The procalcitonin assay was superior to three other tests at detecting invasive bacterial infection in febrile infants aged 7-91 days. Major finding: At a threshold of 0.3 ng/mL or more, procalcitonin level detected invasive bacterial infections with a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1. Data source: A multicenter prospective cohort study involving 2,047 infants treated at pediatric EDs in France during a 30-month period. Disclosures: The French Health Ministry funded the study. Dr Milcent and her associates reported having no financial disclosures. |
The procalcitonin assay was superior to C-reactive protein, neutrophil, and white blood cell measurements at identifying invasive bacterial infections in very young febrile infants, according to a study published in JAMA Pediatrics.1
Compared with other biomarker assays, procalcitonin assays allow earlier detection of certain infections in older children. A few small studies have hinted at the usefulness of procalcitonin assays in infants, but to date no large prospective studies have assessed these assays in the youngest infants. For this prospective study, researchers evaluated the diagnostic accuracy of procalcitonin and other biomarkers in a study of 2,047 febrile infants aged 7-91 days who presented to 15 pediatric emergency departments in France during a 30-month period.
“We did not include infants 6 days or younger because they are likely to have early-onset sepsis related to perinatal factors and because physiologic procalcitonin concentrations during the first [few] days of life are higher than thereafter,” said Dr Karen Milcent of Hôpital Antoine Béclère, Clamart (France), and her associates.
Serum samples were collected at the initial clinical examination, but procalcitonin assays were not performed at that time. Attending physicians diagnosed the infants as having either bacterial or nonbacterial infections without knowing the procalcitonin results. Then, procalcitonin tests were done retrospectively on frozen serum samples by lab personnel who were blinded to the infants’ clinical features. Thirteen (1.0%) infants had bacteremia and 8 (0.6%) had bacterial meningitis.
The procalcitonin assay was significantly more accurate at identifying invasive bacterial infections than was C-reactive protein level, absolute neutrophil count, or white blood cell count. At a threshold of 0.3 ng/mL or more, the procalcitonin level had a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1. In addition, the procalcitonin assay was the most accurate in a subgroup analysis restricted to patients whose fever duration was less than 6 hours and another subgroup analysis restricted to patients younger than 1 month of age, the researchers said.1
For young febrile infants, combining procalcitonin assay results with a careful case history, a thorough physical examination, and other appropriate testing offers the potential of avoiding lumbar punctures. These study findings “should encourage the development of decision-making rules that incorporate procalcitonin,” Dr Milcent and her associates said.
The findings by Milcent et al are an important step forward in managing very young febrile infants, which remains a vexing problem.
A vital next step is to find alternatives to culture-based testing of blood, urine, and CSF. Genomic technologies that reliably detect molecular signatures in small amounts of biologic samples may be one such alternative. They may offer the additional benefit of identifying the pathogen and the host’s response to the presence of the pathogen.
Dr Nathan Kuppermann is in the departments of emergency medicine and pediatrics at the University of California–Davis. Dr Prashant Mahajan is in the departments of pediatrics and emergency medicine at Children’s Hospital of Michigan and Wayne State University, Detroit. They have no relevant financial disclosures. They made these remarks in an editorial accompanying Dr Milcent’s report (Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the holy grail [published online ahead of print November 23, 2015]? JAMA Ped. doi:10.1001/jamapediatrics.2015.3267).
Out-of-hospital MI survival is best in the Midwest
BY BRUCE JANCIN
AT THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Substantial and as-yet unexplained regional differences in survival and total hospital charges following out-of-hospital cardiac arrest exist across the United States. Major finding: Mortality among adults hospitalized after experiencing out-of-hospital cardiac arrest was 14% lower in the Midwest than in the Northeast. Data source: A retrospective analysis of data from the Nationwide Inpatient Sample for 2002-2012 that included 155,592 adults with out-of-hospital cardiac arrest who survived to hospital admission. Disclosures: The presenter reported having no financial conflicts of interest. |
ORLANDO – Considerable regional variation exists across the United States in outcomes, including survival and hospital charges following out-of-hospital cardiac arrest, Dr Aiham Albaeni reported at the American Heart Association scientific sessions.1
He presented an analysis of 155,592 adults who survived at least until hospital admission following non-trauma-related out-of-hospital cardiac arrest (OHCA) during 2002-2012. The data came from the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample, the largest all-payer US inpatient database.
Mortality was lowest among patients whose OHCA occurred in the Midwest. Indeed, taking the Northeast region as the reference point in a multivariate analysis, the adjusted mortality risk was 14% lower in the Midwest and 9% lower in the South. There was no difference in survival rates between the West and Northeast in this analysis adjusted for age, gender, race, primary diagnosis, income, Charlson Comorbidity Index, primary payer, and hospital size and teaching status, reported Dr Albaeni of Johns Hopkins University, Baltimore, Maryland.
Total hospital charges for patients admitted following OHCA were far and away highest in the West, and this increased expenditure didn’t pay off in terms of a survival advantage. The Consumer Price Index–adjusted mean total hospital charges averaged $85,592 per patient in the West, $66,290 in the Northeast, $55,257 in the Midwest, and $54,878 in the South.
Outliers in terms of cost of care—that is, patients admitted with OHCA whose total hospital charges exceeded $109,000 per admission—were 85% more common in the West than the other three regions, he noted.
Hospital length of stay greater than 8 days occurred most often in the Northeast. These lengthier stays were 10% to 12% less common in the other regions.
The explanation for the marked regional differences observed in this study remains unknown.
“These findings call for more efforts to identify a high-quality model of excellence that standardizes health care delivery and improves quality of care in low-performing regions,” said Dr Albaeni.
He reported having no financial conflicts of interest regarding his study.
Modified Valsalva more than doubled conversion rate in supraventricular tachycardia
BY AMY KARON
FROM THE LANCET
Vitals Key clinical point: A modified version of the Valsalva maneuver, in which patients were immediately laid flat afterward with their legs passively raised, more than doubled the rate of conversion from acute supraventricular tachycardia to normal sinus rhythm as compared with the standard Valsalva maneuver. Major finding: The conversion rate was 43% for the modified Valsalva group and 17% for patients undergoing the standard maneuver (adjusted OR, 3.7; P < .0001). Data source: Multicenter, randomized, controlled, parallel-group trial of 428 patients presenting to emergency departments with acute SVT. Disclosures: The National Institute for Health Research funded the study. The investigators declared no competing interests. |
A modified version of the Valsalva maneuver more than doubled the rate of conversion from acute supraventricular tachycardia to normal sinus rhythm when compared with the standard maneuver, said authors of a randomized, controlled trial published in the Lancet.
In all, 93 of 214 (43%) emergency department patients with acute supraventricular tachycardia (SVT) achieved cardioversion a minute after treatment with the modified Valsalva maneuver, compared with 37 (17%) of patients treated with standard Valsalva (adjusted odds ratio, 3.7; 95% CI, 2.3-5.8; P < .0001), reported Dr Andrew Appelboam of Royal Devon and Exeter (England) Hospital NHS Foundation Trust and his associates.
Standard Valsalva is safe, but achieves cardioversion for only 5% to 20% of patients with acute SVT. Nonresponders usually receive intravenous adenosine, which causes transient asystole and has other side effects, including a sense of “impending doom” or imminent death, the investigators noted.1
For the modified Valsalva maneuver, patients underwent standardized strain at 40 mm Hg pressure for 15 seconds while semi-recumbent, but then immediately laid flat while a staff member raised their legs to a 45-degree angle for 15 seconds. They returned to the semi-recumbent position for another 45 seconds before their cardiac rhythm was reassessed. The control group simply remained semirecumbent for 60 seconds after 15 seconds of Valsalva strain.
The adapted technique should achieve the same rate of cardioversion in community practice, and clinicians should repeat it once if it is not initially effective, said the researchers. “As long as individuals can safely undertake a Valsalva strain and be repositioned as described, this maneuver can be used as the routine initial treatment for episodes of supraventricular tachycardia regardless of location,” they said. Patients did not experience serious adverse effects, and transient cardiac events were self-limited and affected similar proportions of both groups, they added.
The National Institute for Health Research funded the study. The researchers declared no competing interests.
Off-label prescriptions link to more adverse events
BY MARY ANN MOON
FROM JAMA INTERNAL MEDICINE
Vitals Key clinical point: Off-label prescribing in adults is common and very likely to cause adverse events. Major finding: The incidence of adverse events was 44% higher for off-label use (19.7 per 10,000 person-months) than for on-label use (12.5 per 10,000 person-months) of prescription drugs. Data source: A prospective cohort study of 46,021 adults who received 151,305 incident prescriptions from primary care clinicians in Quebec during a 5-year period. Disclosures: No sponsors were identified for this study. Dr Tewodros Eguale and his associates reported having no relevant financial disclosures. |
Off-label prescribing of drugs is common and very likely to cause adverse events, particularly when no strong scientific evidence supports the off-label use, according to a report published in JAMA Internal Medicine.
No systematic investigation of the off-label use of prescription drugs has been done to date, in part because physicians aren’t required to document intended indications. Recent innovations in electronic health records provided an opportunity to track off-label prescribing and its influence on adverse drug events for all 8.5 million residents in the Canadian province of Quebec. There, physicians must provide the indication for every new prescription, the reason for any dose changes or drug discontinuation, and the nature of any adverse events, said Dr Tewodros Eguale of the department of epidemiology, biostatistics, and occupational health at McGill University, Montreal, and his associates.
“Selected examples of adverse events associated with the most frequently used off-label drugs include akathisia resulting from the use of gabapentin for neurogenic pain; agitation associated with the use of amitriptyline hydrochloride for migraine; hallucinations with the use of trazodone hydrochloride for insomnia; QT interval prolongation with the use of quetiapine fumarate for depression; and weight gain with the use of olanzapine for depression,” the authors reported.
Prescribing information in electronic medical records of 46,021 adults (mean age 58 years) given 151,305 new prescriptions was analyzed during a 5-year period. Physicians reported off-label use in 17,847 (12%) of these prescriptions, and that off-label use lacked strong scientific evidence in 81% of cases. The median follow-up time for use of prescribed medications was 386 days (range, 1 day to 6 years).
The class of drugs with the highest rate of adverse effects was anti-infective agents (66.2 per 10,000 person-months), followed by central nervous system drugs such as antidepressants, anxiolytics, and antimigraine medicine (18.1 per 10,000); cardiovascular drugs (15.9 per 10,000); hormonal agents (12.7); autonomic drugs including albuterol and terbutaline (8.4); gastrointestinal drugs (6.1); ear, nose, and throat medications (2.8); and “other” agents such as antihistamines, blood thinners, and antineoplastics (1.3).
“Off-label use may be clinically appropriate given the complexity of the patient’s condition, the lack of alternative effective drugs, or after exhausting approved drugs.” However, previous research has shown that physicians’ lack of knowledge of approved treatment indications was one important factor contributing to off-label prescribing. And one study showed that physicians are finding it difficult to keep up with rapidly changing medication information, and this lack of knowledge is affecting treatment, Dr Eguale and his associates said.
That knowledge gap could be filled by supplying clinicians with information regarding drug approval status and the quality of supporting scientific evidence at the point of care, when they write prescriptions into patients’ electronic health records, the investigators noted. This would have the added advantage of facilitating communication among physicians, pharmacists, and patients, and could reduce medication errors such as those caused by giving drugs to the wrong patients or by giving patients sound-alike or look-alike drugs.
No sponsors were identified for this study. Dr Tewodros Eguale and his associates reported having no relevant financial disclosures.
This study, the most extensive and informative one to evaluate the safety of off-label drug use in adults to date, shows that clinicians often enter an arena of the unknown when they expand prescribing beyond the carefully devised confines of the labeled indication. It provides compelling evidence that off-label prescribing is frequently inappropriate and substantially raises the risk for an adverse event.
Even in cases in which an off-label indication has been studied, the pharmacokinetics, drug-disease interactions, drug-drug interactions, and other safety considerations weren’t studied to the degree required during the drug approval process. Moreover, how many clinicians have the time or motivation to review the evidence for those off-label indications, arriving at a balanced assessment of risks and benefits?
Dr Chester B. Good is in pharmacy benefits management services at the US Department of Veterans Affairs in Hines, Illinois, and the department of pharmacy and therapeutics at the University of Pittsburgh. Dr Good and Dr Walid F. Gellad are in the department of medicine at the University of Pittsburgh and at the Center for Health Equity Research and Promotion in the Veterans Affairs Pittsburgh Healthcare System. Dr Good and Dr Gellad reported serving as unpaid advisers to the Food and Drug Administration’s Drug Safety Oversight Board. They made these remarks in an Invited Commentary accompanying Dr Eguale’s report (Good CB, Gellad WF. Off-label drug use and adverse drug events: turning up the heat on off-label prescribing [published online ahead of print November 2, 2014]. JAMA Intern Med. doi:10.1001/jamainternmed.2015.6068).
Dr Lappin is an assistant professor and an attending physician, department of mergency medicine, New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
- FDA approves first naloxone nasal spray for opioid overdose
- FDA moves quickly to approve easy-to-use nasal spray to treat opioid overdose [press release]. Silver Spring, MD: US Food and Drug Administration; November 18, 2015. Updated November 19, 2015.
- Therapeutic hypothermia after nonshockable-rhythm cardiac arrest
- Perman SM, Grossestreuer AV, Wiee DJ, Carr BG, Abella BS, Gaieski DF. The utility of therapeutic hypothermia for post-cardiac arrest syndrome patients with an initial non-shockable rhythm [published online ahead of print November 16, 2015]. Circulation. pii:CIRCULATIONAHA.115.016317.
- Andexanet reverses anticoagulant effects of factor Xa inhibitors
- Siegal DM, Cornutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity [published online ahead of print November 11, 2015]. N Engl J Med.
- Continuous no better than interrupted chest compressions
- Nichol G, Leroux B, Wang H, et al; ROC Investigators. Trial of continuous or interrupted chest compressions during CPR [published online ahead of print November 9, 2015]. N Engl J Med.
- Answers elusive in quest for better chlamydia treatment
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Kong FY, Tabrizi SN, Law M, et al. Azithrmycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59(2):193-205.
- Procalcitonin assay detects invasive bacterial infection
- Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants [published online ahead of print November 23, 2015]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2015.3210.
- Out-of-hospital MI survival is best in the Midwest
- Shaker M, Albaeni A, Rios R. Impact of Change in Resuscitation Guidelines on National Out-of-hospital Cardiac Arrest Outcomes: Fulfilled Expectations? Paper presented at: American Heart Association 2015 Scientific Sessions; November 7-11, 2015; Orlando, Florida.
- Modified Valsalva more than doubled conversion rate in supraventricular tachycardia
- Appelboam A, Reuben A, Mann C, et al; REVERT trial collaborators. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial. Lancet. 2015;386(10005):1747-1753.
- Off-label prescriptions link to more adverse events
- Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population [published online ahead of print November 2, 2015]. JAMA Intern Med. doi: 10.1001/jamainternmed.2015.6058.
FDA approves first naloxone nasal spray for opioid overdose
BY DEEPAK CHITNIS
Frontline Medical News
The US Food and Drug Administration (FDA) has approved the first nasal spray variant of the opioid-overdose drug naloxone hydrochloride.
Marketed in the United States as Narcan (Adapt Pharma, a partner of Lightlake Therapeutics, Radnor, Pennsylvania) the nasal spray is known to stop or, in some cases, reverse the effects of opioid overdosing in patients. Narcan is the first naloxone hydrochloride nasal spray approved by the FDA.
“Combating the opioid abuse epidemic is a top priority for the FDA,” Dr Stephen Ostroff, FDA acting commissioner, said in a statement released with the November 18 approval announcement. “While naloxone will not solve the underlying problems of the opioid epidemic, we are speeding to review new formulations that will ultimately save lives that might otherwise be lost to drug addiction and overdose.”1
The nasal spray itself is available only with a prescription, and is safe for use by both adults and children, according to the FDA.
The spray delivers a dose of 4 mg naloxone in a single 0.1-mL nasal spray, which comes in a ready-to-use, needle-free device, according to Adapt Pharma. Administration of Narcan, which is sprayed into one nostril while the patient is lying on his or her back, does not require special training.
The FDA warned that body aches, diarrhea, tachycardia, fever, piloerection, nausea, nervousness, abdominal cramps, weakness, and increased blood pressure, among other conditions, are all possible side effects of Narcan.
Narcan’s approval is one step of many that must be taken to adequately address and ultimately end the problem of opioid abuse in the US, cautioned Dr Peter Friedmann, an addiction medicine specialist and chief research officer at Baystate Health in Springfield, Massachusetts. He expressed concern regarding the pricing of Narcan, noting that the drug’s affordability is crucial to its success.
“Right now, nasal atomizers with syringes are used off label, and the prices have been going up with increasing demand,” he said. “But [Narcan] is a commercial product based around what is essentially a generic medication, so [I] hope it’s priced at a price point that’s accessible to the great majority of patients and their families who are facing addiction, many of whom don’t have huge means.”
Therapeutic hypothermia after nonshockable-rhythm cardiac arrest
BY MARY ANN MOON
FROM CIRCULATION
Vitals Key clinical point: Therapeutic hypothermia raises the rate of survival with a good neurologic outcome in comatose patients after a cardiac arrest with a nonshockable initial rhythm. Major finding: The rate of survival-to-hospital discharge was significantly higher with therapeutic hypothermia (29%) than without it (15%), as was the rate of survival with a favorable neurologic outcome (21% vs 10%). Data source: A retrospective cohort study involving 519 adults enrolled in a therapeutic hypothermia registry during a 3-year period. Disclosures: This study was supported by the National Institutes of Health. Dr Perman and her associates reported having no financial disclosures. |
Therapeutic hypothermia significantly raises the rate of survival with a good neurologic outcome among patients who are comatose after a cardiac arrest with a nonshockable initial rhythm, according to a report published online November 16 in Circulation.1
Many observational and retrospective cohort studies have examined the possible benefits of therapeutic hypothermia in this patient population, but they have produced conflicting results. No prospective randomized clinical trials have been published, and some clinicians insist the treatment should be reserved for patients who meet the narrow criteria for which there is good supportive evidence; others, eager for any clinical strategy that can improve the outcomes of these critically ill patients, routinely expand its use to comatose patients regardless of their initial heart rhythm or the location of the cardiac arrest, wrote Dr Sarah M. Perman of the department of emergency medicine, University of Colorado, Aurora, and her associates.
They studied the issue using data from a national registry of patients treated at 16 medical centers that sometimes use therapeutic hypothermia after cardiac arrest. They assessed the records of 519 adults who had a nontraumatic cardiac arrest and initially registered either pulseless electrical activity or asystole, then had a return of spontaneous circulation but remained comatose. Approximately half of these comatose survivors (262 patients) were treated with therapeutic hypothermia according to their hospital’s usual protocols, and the other half (257 control subjects) received standard care without therapeutic hypothermia.
In the propensity-matched cohort, the rate of survival-to-hospital discharge was significantly higher with therapeutic hypothermia (29%) than without it (15%), as was the rate of survival with a favorable neurologic outcome (21% vs 10%). And in a multivariate analysis of factors contributing to positive patient outcomes, the intervention was associated with a 3.5-fold increase in favorable neurologic outcomes. A further analysis of the data showed that therapeutic hypothermia was associated with improved survival, with an odds ratio (OR) of 2.8, the investigators said.
In addition, an analysis of outcomes across various subgroups of patients showed that regardless of the location of their cardiac arrest, patients were consistently more likely to survive to hospital discharge neurologically intact if they received therapeutic hypothermia (OR, 2.1 for out-of-hospital cardiac rest; OR, 4.2 for in-hospital cardiac arrest).
“These results lend support to a broadening of indications for therapeutic hypothermia in comatose post-arrest patients with initial nonshockable rhythms,” Dr Perman and her associates said.
Andexanet reverses anticoagulant effects of factor Xa inhibitors
BY BIANCA NOGRADY
FROM THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Andexanet reverses the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban in healthy older adults. Major finding: Andexanet achieved a 92% to 94% reduction in antifactor Xa activity, compared with an 18% to 21% reduction with placebo. Data source: A two-part randomized, placebo-controlled study in 145 healthy individuals. Disclosures: The study was supported by Portola Pharmaceuticals, Bayer, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. Several authors are employees of Portola, one with stock options and a related patent. Other authors declared grants and personal fees from the pharmaceutical industry, including the study supporters. |
Andexanet alfa has been found to reverse the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban, according to a study presented at the American Heart Association scientific sessions and published simultaneously in the November 11 issue of the New England Journal of Medicine.1
In a two-part randomized, placebo-controlled study involving 145 healthy individuals with a mean age of 58 years, patients treated first with apixaban and then given a bolus of andexanet had a 94% reduction in anti-factor Xa activity, compared with a 21% reduction with placebo. Thrombin generation was restored in 100% of patients within 2 to 5 minutes.
In the patients treated with rivaroxaban, treatment with andexanet reduced antifactor Xa activity by 92%, compared to 18% with placebo. Thrombin generation was restored in 96% of participants in the andexanet group, compared with 7% in the placebo group.
Adverse events associated with andexanet were minor, including constipation, feeling hot, or a strange taste in the mouth. The effects of the andexanet also were sustained over the course of a 2-hour infusion in addition to the bolus.1
“The rapid onset and offset of action of andexanet and the ability to administer it as a bolus or as a bolus plus an infusion may provide flexibility with regard to the restoration of hemostasis when urgent factor Xa inhibitor reversal is required,” Dr Deborah M. Siegal of McMaster University, Hamilton, Ontario, Canada and coauthors wrote.
Continuous no better than interrupted chest compressions
BY MARY ANN MOON
FROM THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Continuous chest compressions during CPR didn’t improve survival or neurologic function compared with standard compressions briefly interrupted for ventilation. Major finding: The primary outcome – the rate of survival to hospital discharge – was 9.0% for continuous chest compressions and 9.7% for interrupted compressions, a nonsignificant difference. Data source: A cluster-randomized crossover trial involving 23,711 adults treated by 114 North American EMS agencies for nontraumatic out-of-hospital cardiac arrest. Disclosures: This study was supported by the US National Heart, Lung, and Blood Institute, the US Army Medical Research and Materiel Command, the Canadian Institutes of Health Research, the Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada, the American Heart Association, and the Medic One Foundation. Dr Nichol and his associates reported ties to numerous industry sources. |
Continuous chest compressions during CPR failed to improve survival or neurologic function compared with standard chest compressions that are briefly interrupted for ventilation, based on findings in the first large randomized trial to compare the two strategies for out-of-hospital, nontraumatic cardiac arrest.
In a presentation at the American Heart Association scientific sessions, simultaneously published online November 9 in the New England Journal of Medicine, Dr Graham Nichol and his associates analyzed data from the Resuscitation Outcomes Consortium, a network of clinical centers and EMS agencies that have expertise in conducting research on out-of-hospital cardiac arrest.1
Data were analyzed for 23,711 adults treated by 114 EMS agencies affiliated with eight clinical centers across the United States and Canada. These agencies were grouped into 47 clusters that were randomly assigned to perform CPR using either continuous chest compressions (100 per minute) with asynchronous positive-pressure ventilations (10 per minute) or standard chest compressions interrupted for ventilations (at a rate of 30 compressions per two ventilations) at every response to an out-of-hospital cardiac arrest. Twice per year, each cluster crossed over to the other resuscitation strategy, said Dr Nichol of the University of Washington–Harborview Center for Prehospital Emergency Care and Clinical Trial Center in Seattle.
A total of 12,653 patients were assigned to continuous chest compressions (the intervention group) and 11,058 to interrupted chest compressions (the control group). The primary outcome—the rate of survival to hospital discharge—was 9.0% in the intervention group and 9.7% in the control group, a nonsignificant difference. Similarly, the rate of survival with favorable neurologic function did not differ significantly, at 7.0% and 7.7%, respectively, the investigators said.1
However, it is also possible that three important limitations of this trial unduly influenced the results.
First, the per-protocol analysis, which used an automated algorithm to assess adherence to the compression assignments, could not classify many patients as having received either continuous or interrupted chest compressions. Second, the quality of postresuscitation care, which certainly influences outcomes, was not monitored. And third, actual oxygenation levels were not measured, nor were minutes of ventilation delivered. Thus, “we do not know whether there were important differences in oxygenation or ventilation between the two treatment strategies,” he said.
It is not yet clear why this large randomized trial1 showed no benefit from continuous chest compressions when previous observational research showed the opposite. One possibility is that many of the previous studies assessed not just chest compressions but an entire bundle of care related to CPR, so the benefits they reported may not be attributable to chest compressions alone.
In addition, in this study the mean chest-compression fraction – the proportion of each minute during which compressions are given, an important marker of interruptions in chest compressions – was already high in the control group (0.77) and not much different from that in the intervention group (0.83). Both of these are much higher than the target recommended by both American and European guidelines, which is only 0.60.
And of course a third reason may be that the interruptions for ventilation during CPR aren’t all that critical, and may be less detrimental to survival, than is currently believed.
Dr Rudolph W. Koster is in the department of cardiology at Amsterdam Academic Medical Center. He reported having no relevant financial disclosures. Dr Koster made these remarks in an editorial accompanying Dr Nichol’s report (Koster RW. Continuous or interrupted chest compressions for cardiac arrest [published online ahead of print November 9, 2015]. N Engl J Med).
Answers elusive in quest for better chlamydia treatment
BY BRUCE JANCIN
EXPERT ANALYSIS FROM ICAAC 2015
SAN DIEGO – The hottest topic today in the treatment of sexually transmitted diseases caused by Chlamydia trachomatis is the unresolved question of whether azithromycin is still as effective as doxycycline, the other current guideline-recommended, first-line therapy, Dr Kimberly Workowski said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is important, because doxycycline is administered twice a day for 7 days, and azithromycin is given as a single pill suitable for directly observed therapy,” noted Dr Workowski, professor of medicine at Emory University in Atlanta and lead author of the 2015 Centers for Disease Control and Prevention STD treatment guidelines.1
Several recent retrospective case series have suggested azithromycin is less effective, with the biggest efficacy gap being seen in rectal C. trachomatis infections. These nonrandomized studies were further supported by an Australian meta-analysis of six randomized, controlled trials comparing the two antibiotics for the treatment of genital chlamydia. The investigators found roughly 3% greater efficacy for doxycycline, compared with azithromycin, for urogenital chlamydia, and a 7% advantage for doxycycline in treating symptomatic urethral infection in men.
However, the investigators were quick to add the caveat that “the quality of the evidence varies considerably.”2
There’s a pressing need for better data. Dr Workowski and her colleagues on the STD guidelines panel are eagerly awaiting the results of a well-structured randomized trial led by Dr William M. Geisler, professor of medicine at the University of Alabama, Birmingham. The investigators randomized more than 300 chlamydia-infected male and female inmates in youth correctional facilities to guideline-recommended azithromycin at 1 g orally in a single dose or oral doxycycline at 100 mg twice daily for 7 days. The results, which are anticipated soon, should influence clinical practice, Dr Workowski said.
Here’s what else is new in chlamydia:
Pregnancy: For treatment of chlamydia in pregnancy, amoxicillin at 500 mg orally t.i.d. for 7 days has been demoted from a first-line recommended therapy to alternative-regimen status. Now, the sole recommended first-line treatment in pregnancy is oral azithromycin at 1 g orally in a single dose.
“We did this based on in vitro studies showing Chlamydia trachomatis is not well-killed by amoxicillin. Instead, the drug induces persistent viable noninfectious forms which can sometimes reactivate,” Dr Workowski explained.
Delayed-release doxycycline: This FDA-approved drug, known as Doryx, administered as a 200-mg tablet once daily for 7 days, “might be an alternative” to the standard generic doxycycline regimen of 100 mg twice daily for 7 days, according to the current Centers for Disease Control and Prevention guidelines. In a randomized, double-blind trial, the new agent was as effective as twice-daily generic doxycycline in men and women with urogenital C. trachomatis infection, and it had fewer gastrointestinal side effects. Doryx is costlier than the twice-daily alternatives.
Lymphogranuloma venereum: The current guidelines repeat a point made in previous editions, but one Dr Workowski believes remains underappreciated and thus worthy of emphasis: Rectal exposure to C. trachomatis serovars L1, L2, and L3 in men who have sex with men or in women who have rectal sex can cause lymphogranuloma venereum, which takes the form of proctocolitis mimicking inflammatory bowel disease.
Patients suspected of having lymphogranuloma venereum should be started presumptively on the recommended regimen for this STD, which is oral doxycycline at 100 mg b.i.d. for 21 days.
“If you also see painful ulcers or, on anoscopy, mucosal ulcers, you should also treat empirically for herpes simplex until your culture results come back,” she added.
Dr Workowski reported having no financial conflicts of interest.
Catheter-directed thrombolysis trumps systemic for acute pulmonary embolism
BY MITCHEL L. ZOLER
AT CHEST 2015
Vitals Key clinical point: Catheter-directed thrombolysis was linked to reduced mortality, compared with systemic thrombolysis in patients with an acute pulmonary embolism. Major finding: In-hospital mortality in acute pulmonary embolism patients ran 10% with catheter-directed thrombolysis and 17% with systemic thrombolysis. Data source: Review of 1,521 US patients treated for acute pulmonary embolism during 2010-2012 in the National Inpatient Sample. Disclosures: Dr Saqib and Dr Muthiah had no disclosures. |
MONTREAL – Catheter-directed thrombolysis surpassed systemic thrombolysis for minimizing in-hospital mortality of patients with an acute pulmonary embolism in a review of more than 1,500 United States patients.
The review also found evidence that US pulmonary embolism (PE) patients increasingly undergo catheter-directed thrombolysis, with usage jumping by more than 50% from 2010 to 2012, although in 2012 US clinicians performed catheter-directed thrombolysis on 160 patients with an acute pulmonary embolism (PE) who were included in a national US registry of hospitalized patients, Dr Amina Saqib said at the annual meeting of the American College of Chest Physicians.
Catheter-directed thrombolysis resulted in a 9% in-hospital mortality rate and a 10% combined rate of in-hospital mortality plus intracerebral hemorrhages, rates significantly below those tallied in propensity score-matched patients who underwent systemic thrombolysis of their acute PE. The matched group with systemic thrombolysis had a 17% in-hospital mortality rate and a 17% combined mortality plus intracerebral hemorrhage rate, said Dr Saqib, a researcher at Staten Island (New York) University Hospital.
“To the best of our knowledge, this is the first, large, nationwide, observational study that compared safety and efficacy outcomes between systemic thrombolysis and catheter-directed thrombolysis in acute PE,”
Dr Saqib said.
The US data, collected during 2010-2012, also showed that, after adjustment for clinical and demographic variables, each acute PE treatment by catheter-directed thrombolysis cost an average $9,428 above the cost for systemic thrombolysis, she said.
Dr Saqib and her associates used data collected by the Federal National Inpatient Sample. Among US patients hospitalized during 2010-2012 and entered into this database, they identified 1,169 adult acute PE patients who underwent systemic thrombolysis and 352 patients who received catheter-directed thrombolysis. The patients averaged about 58 years old and just under half were men.
The propensity score-adjusted analysis also showed no statistically significant difference between the two treatment approaches for the incidence of intracerebral hemorrhage, any hemorrhages requiring a transfusion, new-onset acute renal failure, or hospital length of stay. Among the patients treated by catheter-directed thrombolysis, all the intracerebral hemorrhages occurred during 2010; during 2011 and 2012 none of the patients treated this way had an intracerebral hemorrhage, Dr Saqib noted.
Although the findings were consistent with results from prior analyses, the propensity-score adjustment used in the current study cannot fully account for all unmeasured confounding factors. The best way to compare catheter-directed thrombolysis and systemic thrombolysis for treating acute PE would be in a prospective, randomized study, Dr Saqib said.
Survivors of out-of-hospital cardiac arrest usually had intact brain function
BY AMY KARON
FROM THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, regardless of duration of CPR in the field. Major finding: Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital. Data source: A retrospective observational study of 3,814 adults who had an out-of-hospital cardiac arrest between 2005 and 2014. Disclosures: Dr Williams had no disclosures. The senior author disclosed research funding from the Medtronic Foundation. |
Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, even if cardiopulmonary resuscitation lasted longer than has been recommended, authors of a retrospective observational study reported at the American Heart Association scientific sessions.
Dr Jefferson Williams of the Wake County Department of Emergency Medical Services in Raleigh, North Carolina, and his associates studied 3,814 adults who had a cardiac arrest outside the hospital between 2005 and 2014. Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital.
Neurologically intact survival was associated with having an initial shockable rhythm, a bystander-witnessed arrest, and return of spontaneous circulation in the field rather than in the hospital. Age, basic airway management, and therapeutic hypothermia phase also predicted survival with intact brain function, but duration of CPR did not.
Procalcitonin assay detects invasive bacterial infection
BY MARY ANN MOON
FROM JAMA PEDIATRICS
Vitals Key clinical point: The procalcitonin assay was superior to three other tests at detecting invasive bacterial infection in febrile infants aged 7-91 days. Major finding: At a threshold of 0.3 ng/mL or more, procalcitonin level detected invasive bacterial infections with a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1. Data source: A multicenter prospective cohort study involving 2,047 infants treated at pediatric EDs in France during a 30-month period. Disclosures: The French Health Ministry funded the study. Dr Milcent and her associates reported having no financial disclosures. |
The procalcitonin assay was superior to C-reactive protein, neutrophil, and white blood cell measurements at identifying invasive bacterial infections in very young febrile infants, according to a study published in JAMA Pediatrics.1
Compared with other biomarker assays, procalcitonin assays allow earlier detection of certain infections in older children. A few small studies have hinted at the usefulness of procalcitonin assays in infants, but to date no large prospective studies have assessed these assays in the youngest infants. For this prospective study, researchers evaluated the diagnostic accuracy of procalcitonin and other biomarkers in a study of 2,047 febrile infants aged 7-91 days who presented to 15 pediatric emergency departments in France during a 30-month period.
“We did not include infants 6 days or younger because they are likely to have early-onset sepsis related to perinatal factors and because physiologic procalcitonin concentrations during the first [few] days of life are higher than thereafter,” said Dr Karen Milcent of Hôpital Antoine Béclère, Clamart (France), and her associates.
Serum samples were collected at the initial clinical examination, but procalcitonin assays were not performed at that time. Attending physicians diagnosed the infants as having either bacterial or nonbacterial infections without knowing the procalcitonin results. Then, procalcitonin tests were done retrospectively on frozen serum samples by lab personnel who were blinded to the infants’ clinical features. Thirteen (1.0%) infants had bacteremia and 8 (0.6%) had bacterial meningitis.
The procalcitonin assay was significantly more accurate at identifying invasive bacterial infections than was C-reactive protein level, absolute neutrophil count, or white blood cell count. At a threshold of 0.3 ng/mL or more, the procalcitonin level had a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1. In addition, the procalcitonin assay was the most accurate in a subgroup analysis restricted to patients whose fever duration was less than 6 hours and another subgroup analysis restricted to patients younger than 1 month of age, the researchers said.1
For young febrile infants, combining procalcitonin assay results with a careful case history, a thorough physical examination, and other appropriate testing offers the potential of avoiding lumbar punctures. These study findings “should encourage the development of decision-making rules that incorporate procalcitonin,” Dr Milcent and her associates said.
The findings by Milcent et al are an important step forward in managing very young febrile infants, which remains a vexing problem.
A vital next step is to find alternatives to culture-based testing of blood, urine, and CSF. Genomic technologies that reliably detect molecular signatures in small amounts of biologic samples may be one such alternative. They may offer the additional benefit of identifying the pathogen and the host’s response to the presence of the pathogen.
Dr Nathan Kuppermann is in the departments of emergency medicine and pediatrics at the University of California–Davis. Dr Prashant Mahajan is in the departments of pediatrics and emergency medicine at Children’s Hospital of Michigan and Wayne State University, Detroit. They have no relevant financial disclosures. They made these remarks in an editorial accompanying Dr Milcent’s report (Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the holy grail [published online ahead of print November 23, 2015]? JAMA Ped. doi:10.1001/jamapediatrics.2015.3267).
Out-of-hospital MI survival is best in the Midwest
BY BRUCE JANCIN
AT THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Substantial and as-yet unexplained regional differences in survival and total hospital charges following out-of-hospital cardiac arrest exist across the United States. Major finding: Mortality among adults hospitalized after experiencing out-of-hospital cardiac arrest was 14% lower in the Midwest than in the Northeast. Data source: A retrospective analysis of data from the Nationwide Inpatient Sample for 2002-2012 that included 155,592 adults with out-of-hospital cardiac arrest who survived to hospital admission. Disclosures: The presenter reported having no financial conflicts of interest. |
ORLANDO – Considerable regional variation exists across the United States in outcomes, including survival and hospital charges following out-of-hospital cardiac arrest, Dr Aiham Albaeni reported at the American Heart Association scientific sessions.1
He presented an analysis of 155,592 adults who survived at least until hospital admission following non-trauma-related out-of-hospital cardiac arrest (OHCA) during 2002-2012. The data came from the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample, the largest all-payer US inpatient database.
Mortality was lowest among patients whose OHCA occurred in the Midwest. Indeed, taking the Northeast region as the reference point in a multivariate analysis, the adjusted mortality risk was 14% lower in the Midwest and 9% lower in the South. There was no difference in survival rates between the West and Northeast in this analysis adjusted for age, gender, race, primary diagnosis, income, Charlson Comorbidity Index, primary payer, and hospital size and teaching status, reported Dr Albaeni of Johns Hopkins University, Baltimore, Maryland.
Total hospital charges for patients admitted following OHCA were far and away highest in the West, and this increased expenditure didn’t pay off in terms of a survival advantage. The Consumer Price Index–adjusted mean total hospital charges averaged $85,592 per patient in the West, $66,290 in the Northeast, $55,257 in the Midwest, and $54,878 in the South.
Outliers in terms of cost of care—that is, patients admitted with OHCA whose total hospital charges exceeded $109,000 per admission—were 85% more common in the West than the other three regions, he noted.
Hospital length of stay greater than 8 days occurred most often in the Northeast. These lengthier stays were 10% to 12% less common in the other regions.
The explanation for the marked regional differences observed in this study remains unknown.
“These findings call for more efforts to identify a high-quality model of excellence that standardizes health care delivery and improves quality of care in low-performing regions,” said Dr Albaeni.
He reported having no financial conflicts of interest regarding his study.
Modified Valsalva more than doubled conversion rate in supraventricular tachycardia
BY AMY KARON
FROM THE LANCET
Vitals Key clinical point: A modified version of the Valsalva maneuver, in which patients were immediately laid flat afterward with their legs passively raised, more than doubled the rate of conversion from acute supraventricular tachycardia to normal sinus rhythm as compared with the standard Valsalva maneuver. Major finding: The conversion rate was 43% for the modified Valsalva group and 17% for patients undergoing the standard maneuver (adjusted OR, 3.7; P < .0001). Data source: Multicenter, randomized, controlled, parallel-group trial of 428 patients presenting to emergency departments with acute SVT. Disclosures: The National Institute for Health Research funded the study. The investigators declared no competing interests. |
A modified version of the Valsalva maneuver more than doubled the rate of conversion from acute supraventricular tachycardia to normal sinus rhythm when compared with the standard maneuver, said authors of a randomized, controlled trial published in the Lancet.
In all, 93 of 214 (43%) emergency department patients with acute supraventricular tachycardia (SVT) achieved cardioversion a minute after treatment with the modified Valsalva maneuver, compared with 37 (17%) of patients treated with standard Valsalva (adjusted odds ratio, 3.7; 95% CI, 2.3-5.8; P < .0001), reported Dr Andrew Appelboam of Royal Devon and Exeter (England) Hospital NHS Foundation Trust and his associates.
Standard Valsalva is safe, but achieves cardioversion for only 5% to 20% of patients with acute SVT. Nonresponders usually receive intravenous adenosine, which causes transient asystole and has other side effects, including a sense of “impending doom” or imminent death, the investigators noted.1
For the modified Valsalva maneuver, patients underwent standardized strain at 40 mm Hg pressure for 15 seconds while semi-recumbent, but then immediately laid flat while a staff member raised their legs to a 45-degree angle for 15 seconds. They returned to the semi-recumbent position for another 45 seconds before their cardiac rhythm was reassessed. The control group simply remained semirecumbent for 60 seconds after 15 seconds of Valsalva strain.
The adapted technique should achieve the same rate of cardioversion in community practice, and clinicians should repeat it once if it is not initially effective, said the researchers. “As long as individuals can safely undertake a Valsalva strain and be repositioned as described, this maneuver can be used as the routine initial treatment for episodes of supraventricular tachycardia regardless of location,” they said. Patients did not experience serious adverse effects, and transient cardiac events were self-limited and affected similar proportions of both groups, they added.
The National Institute for Health Research funded the study. The researchers declared no competing interests.
Off-label prescriptions link to more adverse events
BY MARY ANN MOON
FROM JAMA INTERNAL MEDICINE
Vitals Key clinical point: Off-label prescribing in adults is common and very likely to cause adverse events. Major finding: The incidence of adverse events was 44% higher for off-label use (19.7 per 10,000 person-months) than for on-label use (12.5 per 10,000 person-months) of prescription drugs. Data source: A prospective cohort study of 46,021 adults who received 151,305 incident prescriptions from primary care clinicians in Quebec during a 5-year period. Disclosures: No sponsors were identified for this study. Dr Tewodros Eguale and his associates reported having no relevant financial disclosures. |
Off-label prescribing of drugs is common and very likely to cause adverse events, particularly when no strong scientific evidence supports the off-label use, according to a report published in JAMA Internal Medicine.
No systematic investigation of the off-label use of prescription drugs has been done to date, in part because physicians aren’t required to document intended indications. Recent innovations in electronic health records provided an opportunity to track off-label prescribing and its influence on adverse drug events for all 8.5 million residents in the Canadian province of Quebec. There, physicians must provide the indication for every new prescription, the reason for any dose changes or drug discontinuation, and the nature of any adverse events, said Dr Tewodros Eguale of the department of epidemiology, biostatistics, and occupational health at McGill University, Montreal, and his associates.
“Selected examples of adverse events associated with the most frequently used off-label drugs include akathisia resulting from the use of gabapentin for neurogenic pain; agitation associated with the use of amitriptyline hydrochloride for migraine; hallucinations with the use of trazodone hydrochloride for insomnia; QT interval prolongation with the use of quetiapine fumarate for depression; and weight gain with the use of olanzapine for depression,” the authors reported.
Prescribing information in electronic medical records of 46,021 adults (mean age 58 years) given 151,305 new prescriptions was analyzed during a 5-year period. Physicians reported off-label use in 17,847 (12%) of these prescriptions, and that off-label use lacked strong scientific evidence in 81% of cases. The median follow-up time for use of prescribed medications was 386 days (range, 1 day to 6 years).
The class of drugs with the highest rate of adverse effects was anti-infective agents (66.2 per 10,000 person-months), followed by central nervous system drugs such as antidepressants, anxiolytics, and antimigraine medicine (18.1 per 10,000); cardiovascular drugs (15.9 per 10,000); hormonal agents (12.7); autonomic drugs including albuterol and terbutaline (8.4); gastrointestinal drugs (6.1); ear, nose, and throat medications (2.8); and “other” agents such as antihistamines, blood thinners, and antineoplastics (1.3).
“Off-label use may be clinically appropriate given the complexity of the patient’s condition, the lack of alternative effective drugs, or after exhausting approved drugs.” However, previous research has shown that physicians’ lack of knowledge of approved treatment indications was one important factor contributing to off-label prescribing. And one study showed that physicians are finding it difficult to keep up with rapidly changing medication information, and this lack of knowledge is affecting treatment, Dr Eguale and his associates said.
That knowledge gap could be filled by supplying clinicians with information regarding drug approval status and the quality of supporting scientific evidence at the point of care, when they write prescriptions into patients’ electronic health records, the investigators noted. This would have the added advantage of facilitating communication among physicians, pharmacists, and patients, and could reduce medication errors such as those caused by giving drugs to the wrong patients or by giving patients sound-alike or look-alike drugs.
No sponsors were identified for this study. Dr Tewodros Eguale and his associates reported having no relevant financial disclosures.
This study, the most extensive and informative one to evaluate the safety of off-label drug use in adults to date, shows that clinicians often enter an arena of the unknown when they expand prescribing beyond the carefully devised confines of the labeled indication. It provides compelling evidence that off-label prescribing is frequently inappropriate and substantially raises the risk for an adverse event.
Even in cases in which an off-label indication has been studied, the pharmacokinetics, drug-disease interactions, drug-drug interactions, and other safety considerations weren’t studied to the degree required during the drug approval process. Moreover, how many clinicians have the time or motivation to review the evidence for those off-label indications, arriving at a balanced assessment of risks and benefits?
Dr Chester B. Good is in pharmacy benefits management services at the US Department of Veterans Affairs in Hines, Illinois, and the department of pharmacy and therapeutics at the University of Pittsburgh. Dr Good and Dr Walid F. Gellad are in the department of medicine at the University of Pittsburgh and at the Center for Health Equity Research and Promotion in the Veterans Affairs Pittsburgh Healthcare System. Dr Good and Dr Gellad reported serving as unpaid advisers to the Food and Drug Administration’s Drug Safety Oversight Board. They made these remarks in an Invited Commentary accompanying Dr Eguale’s report (Good CB, Gellad WF. Off-label drug use and adverse drug events: turning up the heat on off-label prescribing [published online ahead of print November 2, 2014]. JAMA Intern Med. doi:10.1001/jamainternmed.2015.6068).
Dr Lappin is an assistant professor and an attending physician, department of mergency medicine, New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
FDA approves first naloxone nasal spray for opioid overdose
BY DEEPAK CHITNIS
Frontline Medical News
The US Food and Drug Administration (FDA) has approved the first nasal spray variant of the opioid-overdose drug naloxone hydrochloride.
Marketed in the United States as Narcan (Adapt Pharma, a partner of Lightlake Therapeutics, Radnor, Pennsylvania) the nasal spray is known to stop or, in some cases, reverse the effects of opioid overdosing in patients. Narcan is the first naloxone hydrochloride nasal spray approved by the FDA.
“Combating the opioid abuse epidemic is a top priority for the FDA,” Dr Stephen Ostroff, FDA acting commissioner, said in a statement released with the November 18 approval announcement. “While naloxone will not solve the underlying problems of the opioid epidemic, we are speeding to review new formulations that will ultimately save lives that might otherwise be lost to drug addiction and overdose.”1
The nasal spray itself is available only with a prescription, and is safe for use by both adults and children, according to the FDA.
The spray delivers a dose of 4 mg naloxone in a single 0.1-mL nasal spray, which comes in a ready-to-use, needle-free device, according to Adapt Pharma. Administration of Narcan, which is sprayed into one nostril while the patient is lying on his or her back, does not require special training.
The FDA warned that body aches, diarrhea, tachycardia, fever, piloerection, nausea, nervousness, abdominal cramps, weakness, and increased blood pressure, among other conditions, are all possible side effects of Narcan.
Narcan’s approval is one step of many that must be taken to adequately address and ultimately end the problem of opioid abuse in the US, cautioned Dr Peter Friedmann, an addiction medicine specialist and chief research officer at Baystate Health in Springfield, Massachusetts. He expressed concern regarding the pricing of Narcan, noting that the drug’s affordability is crucial to its success.
“Right now, nasal atomizers with syringes are used off label, and the prices have been going up with increasing demand,” he said. “But [Narcan] is a commercial product based around what is essentially a generic medication, so [I] hope it’s priced at a price point that’s accessible to the great majority of patients and their families who are facing addiction, many of whom don’t have huge means.”
Therapeutic hypothermia after nonshockable-rhythm cardiac arrest
BY MARY ANN MOON
FROM CIRCULATION
Vitals Key clinical point: Therapeutic hypothermia raises the rate of survival with a good neurologic outcome in comatose patients after a cardiac arrest with a nonshockable initial rhythm. Major finding: The rate of survival-to-hospital discharge was significantly higher with therapeutic hypothermia (29%) than without it (15%), as was the rate of survival with a favorable neurologic outcome (21% vs 10%). Data source: A retrospective cohort study involving 519 adults enrolled in a therapeutic hypothermia registry during a 3-year period. Disclosures: This study was supported by the National Institutes of Health. Dr Perman and her associates reported having no financial disclosures. |
Therapeutic hypothermia significantly raises the rate of survival with a good neurologic outcome among patients who are comatose after a cardiac arrest with a nonshockable initial rhythm, according to a report published online November 16 in Circulation.1
Many observational and retrospective cohort studies have examined the possible benefits of therapeutic hypothermia in this patient population, but they have produced conflicting results. No prospective randomized clinical trials have been published, and some clinicians insist the treatment should be reserved for patients who meet the narrow criteria for which there is good supportive evidence; others, eager for any clinical strategy that can improve the outcomes of these critically ill patients, routinely expand its use to comatose patients regardless of their initial heart rhythm or the location of the cardiac arrest, wrote Dr Sarah M. Perman of the department of emergency medicine, University of Colorado, Aurora, and her associates.
They studied the issue using data from a national registry of patients treated at 16 medical centers that sometimes use therapeutic hypothermia after cardiac arrest. They assessed the records of 519 adults who had a nontraumatic cardiac arrest and initially registered either pulseless electrical activity or asystole, then had a return of spontaneous circulation but remained comatose. Approximately half of these comatose survivors (262 patients) were treated with therapeutic hypothermia according to their hospital’s usual protocols, and the other half (257 control subjects) received standard care without therapeutic hypothermia.
In the propensity-matched cohort, the rate of survival-to-hospital discharge was significantly higher with therapeutic hypothermia (29%) than without it (15%), as was the rate of survival with a favorable neurologic outcome (21% vs 10%). And in a multivariate analysis of factors contributing to positive patient outcomes, the intervention was associated with a 3.5-fold increase in favorable neurologic outcomes. A further analysis of the data showed that therapeutic hypothermia was associated with improved survival, with an odds ratio (OR) of 2.8, the investigators said.
In addition, an analysis of outcomes across various subgroups of patients showed that regardless of the location of their cardiac arrest, patients were consistently more likely to survive to hospital discharge neurologically intact if they received therapeutic hypothermia (OR, 2.1 for out-of-hospital cardiac rest; OR, 4.2 for in-hospital cardiac arrest).
“These results lend support to a broadening of indications for therapeutic hypothermia in comatose post-arrest patients with initial nonshockable rhythms,” Dr Perman and her associates said.
Andexanet reverses anticoagulant effects of factor Xa inhibitors
BY BIANCA NOGRADY
FROM THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Andexanet reverses the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban in healthy older adults. Major finding: Andexanet achieved a 92% to 94% reduction in antifactor Xa activity, compared with an 18% to 21% reduction with placebo. Data source: A two-part randomized, placebo-controlled study in 145 healthy individuals. Disclosures: The study was supported by Portola Pharmaceuticals, Bayer, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. Several authors are employees of Portola, one with stock options and a related patent. Other authors declared grants and personal fees from the pharmaceutical industry, including the study supporters. |
Andexanet alfa has been found to reverse the anticoagulant effects of factor Xa inhibitors rivaroxaban and apixaban, according to a study presented at the American Heart Association scientific sessions and published simultaneously in the November 11 issue of the New England Journal of Medicine.1
In a two-part randomized, placebo-controlled study involving 145 healthy individuals with a mean age of 58 years, patients treated first with apixaban and then given a bolus of andexanet had a 94% reduction in anti-factor Xa activity, compared with a 21% reduction with placebo. Thrombin generation was restored in 100% of patients within 2 to 5 minutes.
In the patients treated with rivaroxaban, treatment with andexanet reduced antifactor Xa activity by 92%, compared to 18% with placebo. Thrombin generation was restored in 96% of participants in the andexanet group, compared with 7% in the placebo group.
Adverse events associated with andexanet were minor, including constipation, feeling hot, or a strange taste in the mouth. The effects of the andexanet also were sustained over the course of a 2-hour infusion in addition to the bolus.1
“The rapid onset and offset of action of andexanet and the ability to administer it as a bolus or as a bolus plus an infusion may provide flexibility with regard to the restoration of hemostasis when urgent factor Xa inhibitor reversal is required,” Dr Deborah M. Siegal of McMaster University, Hamilton, Ontario, Canada and coauthors wrote.
Continuous no better than interrupted chest compressions
BY MARY ANN MOON
FROM THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Continuous chest compressions during CPR didn’t improve survival or neurologic function compared with standard compressions briefly interrupted for ventilation. Major finding: The primary outcome – the rate of survival to hospital discharge – was 9.0% for continuous chest compressions and 9.7% for interrupted compressions, a nonsignificant difference. Data source: A cluster-randomized crossover trial involving 23,711 adults treated by 114 North American EMS agencies for nontraumatic out-of-hospital cardiac arrest. Disclosures: This study was supported by the US National Heart, Lung, and Blood Institute, the US Army Medical Research and Materiel Command, the Canadian Institutes of Health Research, the Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada, the American Heart Association, and the Medic One Foundation. Dr Nichol and his associates reported ties to numerous industry sources. |
Continuous chest compressions during CPR failed to improve survival or neurologic function compared with standard chest compressions that are briefly interrupted for ventilation, based on findings in the first large randomized trial to compare the two strategies for out-of-hospital, nontraumatic cardiac arrest.
In a presentation at the American Heart Association scientific sessions, simultaneously published online November 9 in the New England Journal of Medicine, Dr Graham Nichol and his associates analyzed data from the Resuscitation Outcomes Consortium, a network of clinical centers and EMS agencies that have expertise in conducting research on out-of-hospital cardiac arrest.1
Data were analyzed for 23,711 adults treated by 114 EMS agencies affiliated with eight clinical centers across the United States and Canada. These agencies were grouped into 47 clusters that were randomly assigned to perform CPR using either continuous chest compressions (100 per minute) with asynchronous positive-pressure ventilations (10 per minute) or standard chest compressions interrupted for ventilations (at a rate of 30 compressions per two ventilations) at every response to an out-of-hospital cardiac arrest. Twice per year, each cluster crossed over to the other resuscitation strategy, said Dr Nichol of the University of Washington–Harborview Center for Prehospital Emergency Care and Clinical Trial Center in Seattle.
A total of 12,653 patients were assigned to continuous chest compressions (the intervention group) and 11,058 to interrupted chest compressions (the control group). The primary outcome—the rate of survival to hospital discharge—was 9.0% in the intervention group and 9.7% in the control group, a nonsignificant difference. Similarly, the rate of survival with favorable neurologic function did not differ significantly, at 7.0% and 7.7%, respectively, the investigators said.1
However, it is also possible that three important limitations of this trial unduly influenced the results.
First, the per-protocol analysis, which used an automated algorithm to assess adherence to the compression assignments, could not classify many patients as having received either continuous or interrupted chest compressions. Second, the quality of postresuscitation care, which certainly influences outcomes, was not monitored. And third, actual oxygenation levels were not measured, nor were minutes of ventilation delivered. Thus, “we do not know whether there were important differences in oxygenation or ventilation between the two treatment strategies,” he said.
It is not yet clear why this large randomized trial1 showed no benefit from continuous chest compressions when previous observational research showed the opposite. One possibility is that many of the previous studies assessed not just chest compressions but an entire bundle of care related to CPR, so the benefits they reported may not be attributable to chest compressions alone.
In addition, in this study the mean chest-compression fraction – the proportion of each minute during which compressions are given, an important marker of interruptions in chest compressions – was already high in the control group (0.77) and not much different from that in the intervention group (0.83). Both of these are much higher than the target recommended by both American and European guidelines, which is only 0.60.
And of course a third reason may be that the interruptions for ventilation during CPR aren’t all that critical, and may be less detrimental to survival, than is currently believed.
Dr Rudolph W. Koster is in the department of cardiology at Amsterdam Academic Medical Center. He reported having no relevant financial disclosures. Dr Koster made these remarks in an editorial accompanying Dr Nichol’s report (Koster RW. Continuous or interrupted chest compressions for cardiac arrest [published online ahead of print November 9, 2015]. N Engl J Med).
Answers elusive in quest for better chlamydia treatment
BY BRUCE JANCIN
EXPERT ANALYSIS FROM ICAAC 2015
SAN DIEGO – The hottest topic today in the treatment of sexually transmitted diseases caused by Chlamydia trachomatis is the unresolved question of whether azithromycin is still as effective as doxycycline, the other current guideline-recommended, first-line therapy, Dr Kimberly Workowski said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is important, because doxycycline is administered twice a day for 7 days, and azithromycin is given as a single pill suitable for directly observed therapy,” noted Dr Workowski, professor of medicine at Emory University in Atlanta and lead author of the 2015 Centers for Disease Control and Prevention STD treatment guidelines.1
Several recent retrospective case series have suggested azithromycin is less effective, with the biggest efficacy gap being seen in rectal C. trachomatis infections. These nonrandomized studies were further supported by an Australian meta-analysis of six randomized, controlled trials comparing the two antibiotics for the treatment of genital chlamydia. The investigators found roughly 3% greater efficacy for doxycycline, compared with azithromycin, for urogenital chlamydia, and a 7% advantage for doxycycline in treating symptomatic urethral infection in men.
However, the investigators were quick to add the caveat that “the quality of the evidence varies considerably.”2
There’s a pressing need for better data. Dr Workowski and her colleagues on the STD guidelines panel are eagerly awaiting the results of a well-structured randomized trial led by Dr William M. Geisler, professor of medicine at the University of Alabama, Birmingham. The investigators randomized more than 300 chlamydia-infected male and female inmates in youth correctional facilities to guideline-recommended azithromycin at 1 g orally in a single dose or oral doxycycline at 100 mg twice daily for 7 days. The results, which are anticipated soon, should influence clinical practice, Dr Workowski said.
Here’s what else is new in chlamydia:
Pregnancy: For treatment of chlamydia in pregnancy, amoxicillin at 500 mg orally t.i.d. for 7 days has been demoted from a first-line recommended therapy to alternative-regimen status. Now, the sole recommended first-line treatment in pregnancy is oral azithromycin at 1 g orally in a single dose.
“We did this based on in vitro studies showing Chlamydia trachomatis is not well-killed by amoxicillin. Instead, the drug induces persistent viable noninfectious forms which can sometimes reactivate,” Dr Workowski explained.
Delayed-release doxycycline: This FDA-approved drug, known as Doryx, administered as a 200-mg tablet once daily for 7 days, “might be an alternative” to the standard generic doxycycline regimen of 100 mg twice daily for 7 days, according to the current Centers for Disease Control and Prevention guidelines. In a randomized, double-blind trial, the new agent was as effective as twice-daily generic doxycycline in men and women with urogenital C. trachomatis infection, and it had fewer gastrointestinal side effects. Doryx is costlier than the twice-daily alternatives.
Lymphogranuloma venereum: The current guidelines repeat a point made in previous editions, but one Dr Workowski believes remains underappreciated and thus worthy of emphasis: Rectal exposure to C. trachomatis serovars L1, L2, and L3 in men who have sex with men or in women who have rectal sex can cause lymphogranuloma venereum, which takes the form of proctocolitis mimicking inflammatory bowel disease.
Patients suspected of having lymphogranuloma venereum should be started presumptively on the recommended regimen for this STD, which is oral doxycycline at 100 mg b.i.d. for 21 days.
“If you also see painful ulcers or, on anoscopy, mucosal ulcers, you should also treat empirically for herpes simplex until your culture results come back,” she added.
Dr Workowski reported having no financial conflicts of interest.
Catheter-directed thrombolysis trumps systemic for acute pulmonary embolism
BY MITCHEL L. ZOLER
AT CHEST 2015
Vitals Key clinical point: Catheter-directed thrombolysis was linked to reduced mortality, compared with systemic thrombolysis in patients with an acute pulmonary embolism. Major finding: In-hospital mortality in acute pulmonary embolism patients ran 10% with catheter-directed thrombolysis and 17% with systemic thrombolysis. Data source: Review of 1,521 US patients treated for acute pulmonary embolism during 2010-2012 in the National Inpatient Sample. Disclosures: Dr Saqib and Dr Muthiah had no disclosures. |
MONTREAL – Catheter-directed thrombolysis surpassed systemic thrombolysis for minimizing in-hospital mortality of patients with an acute pulmonary embolism in a review of more than 1,500 United States patients.
The review also found evidence that US pulmonary embolism (PE) patients increasingly undergo catheter-directed thrombolysis, with usage jumping by more than 50% from 2010 to 2012, although in 2012 US clinicians performed catheter-directed thrombolysis on 160 patients with an acute pulmonary embolism (PE) who were included in a national US registry of hospitalized patients, Dr Amina Saqib said at the annual meeting of the American College of Chest Physicians.
Catheter-directed thrombolysis resulted in a 9% in-hospital mortality rate and a 10% combined rate of in-hospital mortality plus intracerebral hemorrhages, rates significantly below those tallied in propensity score-matched patients who underwent systemic thrombolysis of their acute PE. The matched group with systemic thrombolysis had a 17% in-hospital mortality rate and a 17% combined mortality plus intracerebral hemorrhage rate, said Dr Saqib, a researcher at Staten Island (New York) University Hospital.
“To the best of our knowledge, this is the first, large, nationwide, observational study that compared safety and efficacy outcomes between systemic thrombolysis and catheter-directed thrombolysis in acute PE,”
Dr Saqib said.
The US data, collected during 2010-2012, also showed that, after adjustment for clinical and demographic variables, each acute PE treatment by catheter-directed thrombolysis cost an average $9,428 above the cost for systemic thrombolysis, she said.
Dr Saqib and her associates used data collected by the Federal National Inpatient Sample. Among US patients hospitalized during 2010-2012 and entered into this database, they identified 1,169 adult acute PE patients who underwent systemic thrombolysis and 352 patients who received catheter-directed thrombolysis. The patients averaged about 58 years old and just under half were men.
The propensity score-adjusted analysis also showed no statistically significant difference between the two treatment approaches for the incidence of intracerebral hemorrhage, any hemorrhages requiring a transfusion, new-onset acute renal failure, or hospital length of stay. Among the patients treated by catheter-directed thrombolysis, all the intracerebral hemorrhages occurred during 2010; during 2011 and 2012 none of the patients treated this way had an intracerebral hemorrhage, Dr Saqib noted.
Although the findings were consistent with results from prior analyses, the propensity-score adjustment used in the current study cannot fully account for all unmeasured confounding factors. The best way to compare catheter-directed thrombolysis and systemic thrombolysis for treating acute PE would be in a prospective, randomized study, Dr Saqib said.
Survivors of out-of-hospital cardiac arrest usually had intact brain function
BY AMY KARON
FROM THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, regardless of duration of CPR in the field. Major finding: Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital. Data source: A retrospective observational study of 3,814 adults who had an out-of-hospital cardiac arrest between 2005 and 2014. Disclosures: Dr Williams had no disclosures. The senior author disclosed research funding from the Medtronic Foundation. |
Most adults who survived out-of-hospital cardiac arrests remained neurologically intact, even if cardiopulmonary resuscitation lasted longer than has been recommended, authors of a retrospective observational study reported at the American Heart Association scientific sessions.
Dr Jefferson Williams of the Wake County Department of Emergency Medical Services in Raleigh, North Carolina, and his associates studied 3,814 adults who had a cardiac arrest outside the hospital between 2005 and 2014. Only 12% of patients survived, but 84% of survivors had a cerebral performance category of 1 or 2, including 10% who underwent more than 35 minutes of CPR before reaching the hospital.
Neurologically intact survival was associated with having an initial shockable rhythm, a bystander-witnessed arrest, and return of spontaneous circulation in the field rather than in the hospital. Age, basic airway management, and therapeutic hypothermia phase also predicted survival with intact brain function, but duration of CPR did not.
Procalcitonin assay detects invasive bacterial infection
BY MARY ANN MOON
FROM JAMA PEDIATRICS
Vitals Key clinical point: The procalcitonin assay was superior to three other tests at detecting invasive bacterial infection in febrile infants aged 7-91 days. Major finding: At a threshold of 0.3 ng/mL or more, procalcitonin level detected invasive bacterial infections with a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1. Data source: A multicenter prospective cohort study involving 2,047 infants treated at pediatric EDs in France during a 30-month period. Disclosures: The French Health Ministry funded the study. Dr Milcent and her associates reported having no financial disclosures. |
The procalcitonin assay was superior to C-reactive protein, neutrophil, and white blood cell measurements at identifying invasive bacterial infections in very young febrile infants, according to a study published in JAMA Pediatrics.1
Compared with other biomarker assays, procalcitonin assays allow earlier detection of certain infections in older children. A few small studies have hinted at the usefulness of procalcitonin assays in infants, but to date no large prospective studies have assessed these assays in the youngest infants. For this prospective study, researchers evaluated the diagnostic accuracy of procalcitonin and other biomarkers in a study of 2,047 febrile infants aged 7-91 days who presented to 15 pediatric emergency departments in France during a 30-month period.
“We did not include infants 6 days or younger because they are likely to have early-onset sepsis related to perinatal factors and because physiologic procalcitonin concentrations during the first [few] days of life are higher than thereafter,” said Dr Karen Milcent of Hôpital Antoine Béclère, Clamart (France), and her associates.
Serum samples were collected at the initial clinical examination, but procalcitonin assays were not performed at that time. Attending physicians diagnosed the infants as having either bacterial or nonbacterial infections without knowing the procalcitonin results. Then, procalcitonin tests were done retrospectively on frozen serum samples by lab personnel who were blinded to the infants’ clinical features. Thirteen (1.0%) infants had bacteremia and 8 (0.6%) had bacterial meningitis.
The procalcitonin assay was significantly more accurate at identifying invasive bacterial infections than was C-reactive protein level, absolute neutrophil count, or white blood cell count. At a threshold of 0.3 ng/mL or more, the procalcitonin level had a sensitivity of 90%, a specificity of 78%, and a negative predictive value of 0.1. In addition, the procalcitonin assay was the most accurate in a subgroup analysis restricted to patients whose fever duration was less than 6 hours and another subgroup analysis restricted to patients younger than 1 month of age, the researchers said.1
For young febrile infants, combining procalcitonin assay results with a careful case history, a thorough physical examination, and other appropriate testing offers the potential of avoiding lumbar punctures. These study findings “should encourage the development of decision-making rules that incorporate procalcitonin,” Dr Milcent and her associates said.
The findings by Milcent et al are an important step forward in managing very young febrile infants, which remains a vexing problem.
A vital next step is to find alternatives to culture-based testing of blood, urine, and CSF. Genomic technologies that reliably detect molecular signatures in small amounts of biologic samples may be one such alternative. They may offer the additional benefit of identifying the pathogen and the host’s response to the presence of the pathogen.
Dr Nathan Kuppermann is in the departments of emergency medicine and pediatrics at the University of California–Davis. Dr Prashant Mahajan is in the departments of pediatrics and emergency medicine at Children’s Hospital of Michigan and Wayne State University, Detroit. They have no relevant financial disclosures. They made these remarks in an editorial accompanying Dr Milcent’s report (Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the holy grail [published online ahead of print November 23, 2015]? JAMA Ped. doi:10.1001/jamapediatrics.2015.3267).
Out-of-hospital MI survival is best in the Midwest
BY BRUCE JANCIN
AT THE AHA SCIENTIFIC SESSIONS
Vitals Key clinical point: Substantial and as-yet unexplained regional differences in survival and total hospital charges following out-of-hospital cardiac arrest exist across the United States. Major finding: Mortality among adults hospitalized after experiencing out-of-hospital cardiac arrest was 14% lower in the Midwest than in the Northeast. Data source: A retrospective analysis of data from the Nationwide Inpatient Sample for 2002-2012 that included 155,592 adults with out-of-hospital cardiac arrest who survived to hospital admission. Disclosures: The presenter reported having no financial conflicts of interest. |
ORLANDO – Considerable regional variation exists across the United States in outcomes, including survival and hospital charges following out-of-hospital cardiac arrest, Dr Aiham Albaeni reported at the American Heart Association scientific sessions.1
He presented an analysis of 155,592 adults who survived at least until hospital admission following non-trauma-related out-of-hospital cardiac arrest (OHCA) during 2002-2012. The data came from the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample, the largest all-payer US inpatient database.
Mortality was lowest among patients whose OHCA occurred in the Midwest. Indeed, taking the Northeast region as the reference point in a multivariate analysis, the adjusted mortality risk was 14% lower in the Midwest and 9% lower in the South. There was no difference in survival rates between the West and Northeast in this analysis adjusted for age, gender, race, primary diagnosis, income, Charlson Comorbidity Index, primary payer, and hospital size and teaching status, reported Dr Albaeni of Johns Hopkins University, Baltimore, Maryland.
Total hospital charges for patients admitted following OHCA were far and away highest in the West, and this increased expenditure didn’t pay off in terms of a survival advantage. The Consumer Price Index–adjusted mean total hospital charges averaged $85,592 per patient in the West, $66,290 in the Northeast, $55,257 in the Midwest, and $54,878 in the South.
Outliers in terms of cost of care—that is, patients admitted with OHCA whose total hospital charges exceeded $109,000 per admission—were 85% more common in the West than the other three regions, he noted.
Hospital length of stay greater than 8 days occurred most often in the Northeast. These lengthier stays were 10% to 12% less common in the other regions.
The explanation for the marked regional differences observed in this study remains unknown.
“These findings call for more efforts to identify a high-quality model of excellence that standardizes health care delivery and improves quality of care in low-performing regions,” said Dr Albaeni.
He reported having no financial conflicts of interest regarding his study.
Modified Valsalva more than doubled conversion rate in supraventricular tachycardia
BY AMY KARON
FROM THE LANCET
Vitals Key clinical point: A modified version of the Valsalva maneuver, in which patients were immediately laid flat afterward with their legs passively raised, more than doubled the rate of conversion from acute supraventricular tachycardia to normal sinus rhythm as compared with the standard Valsalva maneuver. Major finding: The conversion rate was 43% for the modified Valsalva group and 17% for patients undergoing the standard maneuver (adjusted OR, 3.7; P < .0001). Data source: Multicenter, randomized, controlled, parallel-group trial of 428 patients presenting to emergency departments with acute SVT. Disclosures: The National Institute for Health Research funded the study. The investigators declared no competing interests. |
A modified version of the Valsalva maneuver more than doubled the rate of conversion from acute supraventricular tachycardia to normal sinus rhythm when compared with the standard maneuver, said authors of a randomized, controlled trial published in the Lancet.
In all, 93 of 214 (43%) emergency department patients with acute supraventricular tachycardia (SVT) achieved cardioversion a minute after treatment with the modified Valsalva maneuver, compared with 37 (17%) of patients treated with standard Valsalva (adjusted odds ratio, 3.7; 95% CI, 2.3-5.8; P < .0001), reported Dr Andrew Appelboam of Royal Devon and Exeter (England) Hospital NHS Foundation Trust and his associates.
Standard Valsalva is safe, but achieves cardioversion for only 5% to 20% of patients with acute SVT. Nonresponders usually receive intravenous adenosine, which causes transient asystole and has other side effects, including a sense of “impending doom” or imminent death, the investigators noted.1
For the modified Valsalva maneuver, patients underwent standardized strain at 40 mm Hg pressure for 15 seconds while semi-recumbent, but then immediately laid flat while a staff member raised their legs to a 45-degree angle for 15 seconds. They returned to the semi-recumbent position for another 45 seconds before their cardiac rhythm was reassessed. The control group simply remained semirecumbent for 60 seconds after 15 seconds of Valsalva strain.
The adapted technique should achieve the same rate of cardioversion in community practice, and clinicians should repeat it once if it is not initially effective, said the researchers. “As long as individuals can safely undertake a Valsalva strain and be repositioned as described, this maneuver can be used as the routine initial treatment for episodes of supraventricular tachycardia regardless of location,” they said. Patients did not experience serious adverse effects, and transient cardiac events were self-limited and affected similar proportions of both groups, they added.
The National Institute for Health Research funded the study. The researchers declared no competing interests.
Off-label prescriptions link to more adverse events
BY MARY ANN MOON
FROM JAMA INTERNAL MEDICINE
Vitals Key clinical point: Off-label prescribing in adults is common and very likely to cause adverse events. Major finding: The incidence of adverse events was 44% higher for off-label use (19.7 per 10,000 person-months) than for on-label use (12.5 per 10,000 person-months) of prescription drugs. Data source: A prospective cohort study of 46,021 adults who received 151,305 incident prescriptions from primary care clinicians in Quebec during a 5-year period. Disclosures: No sponsors were identified for this study. Dr Tewodros Eguale and his associates reported having no relevant financial disclosures. |
Off-label prescribing of drugs is common and very likely to cause adverse events, particularly when no strong scientific evidence supports the off-label use, according to a report published in JAMA Internal Medicine.
No systematic investigation of the off-label use of prescription drugs has been done to date, in part because physicians aren’t required to document intended indications. Recent innovations in electronic health records provided an opportunity to track off-label prescribing and its influence on adverse drug events for all 8.5 million residents in the Canadian province of Quebec. There, physicians must provide the indication for every new prescription, the reason for any dose changes or drug discontinuation, and the nature of any adverse events, said Dr Tewodros Eguale of the department of epidemiology, biostatistics, and occupational health at McGill University, Montreal, and his associates.
“Selected examples of adverse events associated with the most frequently used off-label drugs include akathisia resulting from the use of gabapentin for neurogenic pain; agitation associated with the use of amitriptyline hydrochloride for migraine; hallucinations with the use of trazodone hydrochloride for insomnia; QT interval prolongation with the use of quetiapine fumarate for depression; and weight gain with the use of olanzapine for depression,” the authors reported.
Prescribing information in electronic medical records of 46,021 adults (mean age 58 years) given 151,305 new prescriptions was analyzed during a 5-year period. Physicians reported off-label use in 17,847 (12%) of these prescriptions, and that off-label use lacked strong scientific evidence in 81% of cases. The median follow-up time for use of prescribed medications was 386 days (range, 1 day to 6 years).
The class of drugs with the highest rate of adverse effects was anti-infective agents (66.2 per 10,000 person-months), followed by central nervous system drugs such as antidepressants, anxiolytics, and antimigraine medicine (18.1 per 10,000); cardiovascular drugs (15.9 per 10,000); hormonal agents (12.7); autonomic drugs including albuterol and terbutaline (8.4); gastrointestinal drugs (6.1); ear, nose, and throat medications (2.8); and “other” agents such as antihistamines, blood thinners, and antineoplastics (1.3).
“Off-label use may be clinically appropriate given the complexity of the patient’s condition, the lack of alternative effective drugs, or after exhausting approved drugs.” However, previous research has shown that physicians’ lack of knowledge of approved treatment indications was one important factor contributing to off-label prescribing. And one study showed that physicians are finding it difficult to keep up with rapidly changing medication information, and this lack of knowledge is affecting treatment, Dr Eguale and his associates said.
That knowledge gap could be filled by supplying clinicians with information regarding drug approval status and the quality of supporting scientific evidence at the point of care, when they write prescriptions into patients’ electronic health records, the investigators noted. This would have the added advantage of facilitating communication among physicians, pharmacists, and patients, and could reduce medication errors such as those caused by giving drugs to the wrong patients or by giving patients sound-alike or look-alike drugs.
No sponsors were identified for this study. Dr Tewodros Eguale and his associates reported having no relevant financial disclosures.
This study, the most extensive and informative one to evaluate the safety of off-label drug use in adults to date, shows that clinicians often enter an arena of the unknown when they expand prescribing beyond the carefully devised confines of the labeled indication. It provides compelling evidence that off-label prescribing is frequently inappropriate and substantially raises the risk for an adverse event.
Even in cases in which an off-label indication has been studied, the pharmacokinetics, drug-disease interactions, drug-drug interactions, and other safety considerations weren’t studied to the degree required during the drug approval process. Moreover, how many clinicians have the time or motivation to review the evidence for those off-label indications, arriving at a balanced assessment of risks and benefits?
Dr Chester B. Good is in pharmacy benefits management services at the US Department of Veterans Affairs in Hines, Illinois, and the department of pharmacy and therapeutics at the University of Pittsburgh. Dr Good and Dr Walid F. Gellad are in the department of medicine at the University of Pittsburgh and at the Center for Health Equity Research and Promotion in the Veterans Affairs Pittsburgh Healthcare System. Dr Good and Dr Gellad reported serving as unpaid advisers to the Food and Drug Administration’s Drug Safety Oversight Board. They made these remarks in an Invited Commentary accompanying Dr Eguale’s report (Good CB, Gellad WF. Off-label drug use and adverse drug events: turning up the heat on off-label prescribing [published online ahead of print November 2, 2014]. JAMA Intern Med. doi:10.1001/jamainternmed.2015.6068).
Dr Lappin is an assistant professor and an attending physician, department of mergency medicine, New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
- FDA approves first naloxone nasal spray for opioid overdose
- FDA moves quickly to approve easy-to-use nasal spray to treat opioid overdose [press release]. Silver Spring, MD: US Food and Drug Administration; November 18, 2015. Updated November 19, 2015.
- Therapeutic hypothermia after nonshockable-rhythm cardiac arrest
- Perman SM, Grossestreuer AV, Wiee DJ, Carr BG, Abella BS, Gaieski DF. The utility of therapeutic hypothermia for post-cardiac arrest syndrome patients with an initial non-shockable rhythm [published online ahead of print November 16, 2015]. Circulation. pii:CIRCULATIONAHA.115.016317.
- Andexanet reverses anticoagulant effects of factor Xa inhibitors
- Siegal DM, Cornutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity [published online ahead of print November 11, 2015]. N Engl J Med.
- Continuous no better than interrupted chest compressions
- Nichol G, Leroux B, Wang H, et al; ROC Investigators. Trial of continuous or interrupted chest compressions during CPR [published online ahead of print November 9, 2015]. N Engl J Med.
- Answers elusive in quest for better chlamydia treatment
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Kong FY, Tabrizi SN, Law M, et al. Azithrmycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59(2):193-205.
- Procalcitonin assay detects invasive bacterial infection
- Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants [published online ahead of print November 23, 2015]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2015.3210.
- Out-of-hospital MI survival is best in the Midwest
- Shaker M, Albaeni A, Rios R. Impact of Change in Resuscitation Guidelines on National Out-of-hospital Cardiac Arrest Outcomes: Fulfilled Expectations? Paper presented at: American Heart Association 2015 Scientific Sessions; November 7-11, 2015; Orlando, Florida.
- Modified Valsalva more than doubled conversion rate in supraventricular tachycardia
- Appelboam A, Reuben A, Mann C, et al; REVERT trial collaborators. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial. Lancet. 2015;386(10005):1747-1753.
- Off-label prescriptions link to more adverse events
- Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population [published online ahead of print November 2, 2015]. JAMA Intern Med. doi: 10.1001/jamainternmed.2015.6058.
- FDA approves first naloxone nasal spray for opioid overdose
- FDA moves quickly to approve easy-to-use nasal spray to treat opioid overdose [press release]. Silver Spring, MD: US Food and Drug Administration; November 18, 2015. Updated November 19, 2015.
- Therapeutic hypothermia after nonshockable-rhythm cardiac arrest
- Perman SM, Grossestreuer AV, Wiee DJ, Carr BG, Abella BS, Gaieski DF. The utility of therapeutic hypothermia for post-cardiac arrest syndrome patients with an initial non-shockable rhythm [published online ahead of print November 16, 2015]. Circulation. pii:CIRCULATIONAHA.115.016317.
- Andexanet reverses anticoagulant effects of factor Xa inhibitors
- Siegal DM, Cornutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity [published online ahead of print November 11, 2015]. N Engl J Med.
- Continuous no better than interrupted chest compressions
- Nichol G, Leroux B, Wang H, et al; ROC Investigators. Trial of continuous or interrupted chest compressions during CPR [published online ahead of print November 9, 2015]. N Engl J Med.
- Answers elusive in quest for better chlamydia treatment
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Kong FY, Tabrizi SN, Law M, et al. Azithrmycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59(2):193-205.
- Procalcitonin assay detects invasive bacterial infection
- Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants [published online ahead of print November 23, 2015]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2015.3210.
- Out-of-hospital MI survival is best in the Midwest
- Shaker M, Albaeni A, Rios R. Impact of Change in Resuscitation Guidelines on National Out-of-hospital Cardiac Arrest Outcomes: Fulfilled Expectations? Paper presented at: American Heart Association 2015 Scientific Sessions; November 7-11, 2015; Orlando, Florida.
- Modified Valsalva more than doubled conversion rate in supraventricular tachycardia
- Appelboam A, Reuben A, Mann C, et al; REVERT trial collaborators. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial. Lancet. 2015;386(10005):1747-1753.
- Off-label prescriptions link to more adverse events
- Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population [published online ahead of print November 2, 2015]. JAMA Intern Med. doi: 10.1001/jamainternmed.2015.6058.
Case Studies in Toxicology: The Perilous Pursuit of Perfection
Case
A 37-year-old woman had undergone outpatient tumescent liposuction of her abdominal adipose tissue. Upon initiation of the procedure, she developed acute confusion followed by agitation and hallucinations and was transported by emergency medical services to the nearest hospital.
Upon arrival to the ED, the patient was mildly agitated. Her initial vital signs were: blood pressure, 122/79 mm Hg; heart rate, 57 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 96.8°F. Oxygen saturation was 100% on room air.
On examination, the patient was awake and oriented, but was restless and perseverating. She described the sensation of “dying and coming back to life.” The patient’s pupils were equal, round, and normally reactive; her skin was neither diaphoretic nor dry; her heart rate and rhythm were normal; and her lungs were clear to auscultation bilaterally. The skin of the patient’s abdomen was pale, cool, and clammy, but otherwise soft with normoactive bowel sounds. An electrocardiogram revealed sinus rhythm with normal QRS and QTc intervals.
What is tumescent liposuction?
The tumescent technique (the word tumescent derived from the Latin word meaning “to swell”) involves the infiltration of a large volume of a dilute solution of lidocaine and epinephrine into the subcutaneous fat until it becomes distended and tense. The administration of this solution allows for the removal of significant adipose tissue with minimal blood loss and without general anesthesia. Advocates of tumescent liposuction argue that this dilution and subcutaneous infiltration alter the pharmacokinetics of lidocaine, providing a safe delivery of large doses of the drug. In addition, a substantial quantity of the infiltrated lidocaine is suctioned out with the fat removal. Klein3 specifically recommends administering a dilution of 1 g lidocaine in 1,000 mL of saline (0.1%) to the patient in aliquots of up to 2 g lidocaine. Such a high dose may approach or surpass 35 mg/kg, far exceeding the recommended lidocaine infiltration dose of 7 mg/kg when mixed with epinephrine.
Several studies have investigated the safety of the tumescent technique and the risk of lidocaine toxicity. In a review of pharmacokinetic studies, patients received between 10.5 and 67.7 mg/kg of lidocaine with a reported maximum serum lidocaine concentration in all patients of only 2.93 mcg/mL (therapeutic, 1-5 mcg/mL).4 However, peak serum lidocaine concentrations may not occur for up to 28 hours following tumescent infiltration.4 Despite the purported safety findings of this and other studies, there are also reports of tumescent anesthesia-associated toxicity and fatalities.5,6
Case Continuation
The patient’s mental status slowly improved throughout her hospital stay and she was reportedly at baseline by the following morning. Her serum lidocaine concentration drawn at presentation to the ED was 9.7 mcg/mL.
What are the clinical effects of lidocaine toxicity?
The clinical effects of lidocaine overdose are dependent on both the magnitude of the exposure and the rate at which it occurs. The central nervous system (CNS) and heart are the organ systems primarily affected by lidocaine. As a local anesthetic, lidocaine inhibits the action potential formation in electrically excitable cells. This reversible inhibition of the voltage-gated sodium channels prevents the influx of positively charged sodium ions and the resultant depolarization of the cell. Lidocaine, initially or at low concentrations, has a quiescent effect on neurons, which explains its therapeutic use as a local anesthetic.
Similarly, lidocaine initially reduces impulse propagation through the cardiac conduction system and can be used to treat rhythm disturbances. When used for the suppression of cardiac dysrhythmias, the therapeutic serum concentration of lidocaine is 1 to 5 mcg/mL. Subjective symptoms of lidocaine toxicity arising from therapeutic use (primarily when it is used to manage dysrhythmia) include light-headedness, disorientation, confusion, and psychosis, and are associated with serum concentrations of 3 to 6 mcg/mL. As the concentration increases, the clinical effects of lidocaine appear to shift from inhibitory to excitatory. At serum concentrations of 5 to 9 mcg/mL, objective symptoms, such as excitation, tremor, and seizure predominate. As the concentration continues to rise, coma, respiratory depression, cardiovascular (CV) collapse, and death may occur.7
The exact mechanism of this toxicity transition from inhibitory effect of sodium channel blockade to excitatory effect is not well understood. Some suggest a preferential inhibition of inhibitory interneurons in the CNS is responsible.8 Another potential mechanism is a concentration-dependent inhibition of the potassium rectifier channel.9 Inhibition of the efflux of positively charged potassium ions would result in slowing cellular repolarization, leaving the cells in a relatively excitable state. In the CNS, this produces seizures; in the heart, it may result in dysrhythmia. Cardiovascular collapse may occur with very high serum concentrations of lidocaine or following a rapid serum increase—eg, after a large intravenous (IV) bolus dose,7 which can potentially result from an unintentional intravascular injection during tumescent liposuction.
What is the treatment for lidocaine toxicity?
The first step in the treatment of lidocaine-associated CNS toxicity is the discontinuation of the drug. Failure to appropriately recognize the symptoms of early lidocaine toxicity may result in the progression to severe CNS effects and eventual CV collapse. Benzodiazepines should be used as needed for mild symptoms. Seizures should be treated aggressively with benzodiazepines or barbiturates, while ensuring maintenance of oxygenation, ventilation, and perfusion.7
In cases of lidocaine-associated CV toxicity, treatment begins with airway management, oxygen administration, and life support. Potential antidotal treatment of severe local anesthetic-associated CV toxicity involves the rapid administration of IV fat emulsion, or “lipid rescue.” Although best studied for bupivacaine toxicity, the exact mechanism of IV fat emulsion as an antidote is not completely understood. However, in the treatment of local anesthetic toxicity, lipid rescue is believed to offer a “sink” to remove the lipid-soluble anesthetics from their site of action and trap them within the vascular space. Suggested dosing of 20% lipid solution is a bolus of 1.5 mL/kg over a 1-minute period, followed by 0.25mL/kg per minute or 15 mL/kg per hour to run over 30 to 60 minutes.10
Case Conclusion
The patient made a full recovery and was discharged home in normal condition. Her healthcare provider was informed about the complication of the procedure.
Dr Hines is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Lozinski A, Huq NS. Tumescent liposuction. Clin Plastic Surg. 2013;40(4):593-613.
- Klein JA. Tumescent technique chronicles: local anesthesia, liposuction, and beyond. Dermatol Surg. 1995;21(5):449-457.
- Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248-263.
- Conroy PH, O’Rourke J. Tumescent anesthesia. The Surgeon. 2012;210-201.
- Rao RR, Fly SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(19):1471-1475.
- Martinez MA, Ballesteros S, Segura LJ, Garcia M. Reporting a fatality during tumescent liposuction. Forensic Sci Int. 2008;178(1):e11-e-16.
- Schwartz DR, Kaufman B. Local anesthestics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:921-930.
- Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidoaine. Nature. 1966;209(5019):207-208.
- Friederich P, Benzenberg D, Urban BW. Bupivacaine inhibits human neuronal Kv3 channels in SH-SY5Y human neuroblastoma cells. Br J Anaesth. 2002;88(6):864-866.
- Bania TC. Antidotes in depth, intravenous fat emulsions. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:931-937.
Case
A 37-year-old woman had undergone outpatient tumescent liposuction of her abdominal adipose tissue. Upon initiation of the procedure, she developed acute confusion followed by agitation and hallucinations and was transported by emergency medical services to the nearest hospital.
Upon arrival to the ED, the patient was mildly agitated. Her initial vital signs were: blood pressure, 122/79 mm Hg; heart rate, 57 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 96.8°F. Oxygen saturation was 100% on room air.
On examination, the patient was awake and oriented, but was restless and perseverating. She described the sensation of “dying and coming back to life.” The patient’s pupils were equal, round, and normally reactive; her skin was neither diaphoretic nor dry; her heart rate and rhythm were normal; and her lungs were clear to auscultation bilaterally. The skin of the patient’s abdomen was pale, cool, and clammy, but otherwise soft with normoactive bowel sounds. An electrocardiogram revealed sinus rhythm with normal QRS and QTc intervals.
What is tumescent liposuction?
The tumescent technique (the word tumescent derived from the Latin word meaning “to swell”) involves the infiltration of a large volume of a dilute solution of lidocaine and epinephrine into the subcutaneous fat until it becomes distended and tense. The administration of this solution allows for the removal of significant adipose tissue with minimal blood loss and without general anesthesia. Advocates of tumescent liposuction argue that this dilution and subcutaneous infiltration alter the pharmacokinetics of lidocaine, providing a safe delivery of large doses of the drug. In addition, a substantial quantity of the infiltrated lidocaine is suctioned out with the fat removal. Klein3 specifically recommends administering a dilution of 1 g lidocaine in 1,000 mL of saline (0.1%) to the patient in aliquots of up to 2 g lidocaine. Such a high dose may approach or surpass 35 mg/kg, far exceeding the recommended lidocaine infiltration dose of 7 mg/kg when mixed with epinephrine.
Several studies have investigated the safety of the tumescent technique and the risk of lidocaine toxicity. In a review of pharmacokinetic studies, patients received between 10.5 and 67.7 mg/kg of lidocaine with a reported maximum serum lidocaine concentration in all patients of only 2.93 mcg/mL (therapeutic, 1-5 mcg/mL).4 However, peak serum lidocaine concentrations may not occur for up to 28 hours following tumescent infiltration.4 Despite the purported safety findings of this and other studies, there are also reports of tumescent anesthesia-associated toxicity and fatalities.5,6
Case Continuation
The patient’s mental status slowly improved throughout her hospital stay and she was reportedly at baseline by the following morning. Her serum lidocaine concentration drawn at presentation to the ED was 9.7 mcg/mL.
What are the clinical effects of lidocaine toxicity?
The clinical effects of lidocaine overdose are dependent on both the magnitude of the exposure and the rate at which it occurs. The central nervous system (CNS) and heart are the organ systems primarily affected by lidocaine. As a local anesthetic, lidocaine inhibits the action potential formation in electrically excitable cells. This reversible inhibition of the voltage-gated sodium channels prevents the influx of positively charged sodium ions and the resultant depolarization of the cell. Lidocaine, initially or at low concentrations, has a quiescent effect on neurons, which explains its therapeutic use as a local anesthetic.
Similarly, lidocaine initially reduces impulse propagation through the cardiac conduction system and can be used to treat rhythm disturbances. When used for the suppression of cardiac dysrhythmias, the therapeutic serum concentration of lidocaine is 1 to 5 mcg/mL. Subjective symptoms of lidocaine toxicity arising from therapeutic use (primarily when it is used to manage dysrhythmia) include light-headedness, disorientation, confusion, and psychosis, and are associated with serum concentrations of 3 to 6 mcg/mL. As the concentration increases, the clinical effects of lidocaine appear to shift from inhibitory to excitatory. At serum concentrations of 5 to 9 mcg/mL, objective symptoms, such as excitation, tremor, and seizure predominate. As the concentration continues to rise, coma, respiratory depression, cardiovascular (CV) collapse, and death may occur.7
The exact mechanism of this toxicity transition from inhibitory effect of sodium channel blockade to excitatory effect is not well understood. Some suggest a preferential inhibition of inhibitory interneurons in the CNS is responsible.8 Another potential mechanism is a concentration-dependent inhibition of the potassium rectifier channel.9 Inhibition of the efflux of positively charged potassium ions would result in slowing cellular repolarization, leaving the cells in a relatively excitable state. In the CNS, this produces seizures; in the heart, it may result in dysrhythmia. Cardiovascular collapse may occur with very high serum concentrations of lidocaine or following a rapid serum increase—eg, after a large intravenous (IV) bolus dose,7 which can potentially result from an unintentional intravascular injection during tumescent liposuction.
What is the treatment for lidocaine toxicity?
The first step in the treatment of lidocaine-associated CNS toxicity is the discontinuation of the drug. Failure to appropriately recognize the symptoms of early lidocaine toxicity may result in the progression to severe CNS effects and eventual CV collapse. Benzodiazepines should be used as needed for mild symptoms. Seizures should be treated aggressively with benzodiazepines or barbiturates, while ensuring maintenance of oxygenation, ventilation, and perfusion.7
In cases of lidocaine-associated CV toxicity, treatment begins with airway management, oxygen administration, and life support. Potential antidotal treatment of severe local anesthetic-associated CV toxicity involves the rapid administration of IV fat emulsion, or “lipid rescue.” Although best studied for bupivacaine toxicity, the exact mechanism of IV fat emulsion as an antidote is not completely understood. However, in the treatment of local anesthetic toxicity, lipid rescue is believed to offer a “sink” to remove the lipid-soluble anesthetics from their site of action and trap them within the vascular space. Suggested dosing of 20% lipid solution is a bolus of 1.5 mL/kg over a 1-minute period, followed by 0.25mL/kg per minute or 15 mL/kg per hour to run over 30 to 60 minutes.10
Case Conclusion
The patient made a full recovery and was discharged home in normal condition. Her healthcare provider was informed about the complication of the procedure.
Dr Hines is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 37-year-old woman had undergone outpatient tumescent liposuction of her abdominal adipose tissue. Upon initiation of the procedure, she developed acute confusion followed by agitation and hallucinations and was transported by emergency medical services to the nearest hospital.
Upon arrival to the ED, the patient was mildly agitated. Her initial vital signs were: blood pressure, 122/79 mm Hg; heart rate, 57 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 96.8°F. Oxygen saturation was 100% on room air.
On examination, the patient was awake and oriented, but was restless and perseverating. She described the sensation of “dying and coming back to life.” The patient’s pupils were equal, round, and normally reactive; her skin was neither diaphoretic nor dry; her heart rate and rhythm were normal; and her lungs were clear to auscultation bilaterally. The skin of the patient’s abdomen was pale, cool, and clammy, but otherwise soft with normoactive bowel sounds. An electrocardiogram revealed sinus rhythm with normal QRS and QTc intervals.
What is tumescent liposuction?
The tumescent technique (the word tumescent derived from the Latin word meaning “to swell”) involves the infiltration of a large volume of a dilute solution of lidocaine and epinephrine into the subcutaneous fat until it becomes distended and tense. The administration of this solution allows for the removal of significant adipose tissue with minimal blood loss and without general anesthesia. Advocates of tumescent liposuction argue that this dilution and subcutaneous infiltration alter the pharmacokinetics of lidocaine, providing a safe delivery of large doses of the drug. In addition, a substantial quantity of the infiltrated lidocaine is suctioned out with the fat removal. Klein3 specifically recommends administering a dilution of 1 g lidocaine in 1,000 mL of saline (0.1%) to the patient in aliquots of up to 2 g lidocaine. Such a high dose may approach or surpass 35 mg/kg, far exceeding the recommended lidocaine infiltration dose of 7 mg/kg when mixed with epinephrine.
Several studies have investigated the safety of the tumescent technique and the risk of lidocaine toxicity. In a review of pharmacokinetic studies, patients received between 10.5 and 67.7 mg/kg of lidocaine with a reported maximum serum lidocaine concentration in all patients of only 2.93 mcg/mL (therapeutic, 1-5 mcg/mL).4 However, peak serum lidocaine concentrations may not occur for up to 28 hours following tumescent infiltration.4 Despite the purported safety findings of this and other studies, there are also reports of tumescent anesthesia-associated toxicity and fatalities.5,6
Case Continuation
The patient’s mental status slowly improved throughout her hospital stay and she was reportedly at baseline by the following morning. Her serum lidocaine concentration drawn at presentation to the ED was 9.7 mcg/mL.
What are the clinical effects of lidocaine toxicity?
The clinical effects of lidocaine overdose are dependent on both the magnitude of the exposure and the rate at which it occurs. The central nervous system (CNS) and heart are the organ systems primarily affected by lidocaine. As a local anesthetic, lidocaine inhibits the action potential formation in electrically excitable cells. This reversible inhibition of the voltage-gated sodium channels prevents the influx of positively charged sodium ions and the resultant depolarization of the cell. Lidocaine, initially or at low concentrations, has a quiescent effect on neurons, which explains its therapeutic use as a local anesthetic.
Similarly, lidocaine initially reduces impulse propagation through the cardiac conduction system and can be used to treat rhythm disturbances. When used for the suppression of cardiac dysrhythmias, the therapeutic serum concentration of lidocaine is 1 to 5 mcg/mL. Subjective symptoms of lidocaine toxicity arising from therapeutic use (primarily when it is used to manage dysrhythmia) include light-headedness, disorientation, confusion, and psychosis, and are associated with serum concentrations of 3 to 6 mcg/mL. As the concentration increases, the clinical effects of lidocaine appear to shift from inhibitory to excitatory. At serum concentrations of 5 to 9 mcg/mL, objective symptoms, such as excitation, tremor, and seizure predominate. As the concentration continues to rise, coma, respiratory depression, cardiovascular (CV) collapse, and death may occur.7
The exact mechanism of this toxicity transition from inhibitory effect of sodium channel blockade to excitatory effect is not well understood. Some suggest a preferential inhibition of inhibitory interneurons in the CNS is responsible.8 Another potential mechanism is a concentration-dependent inhibition of the potassium rectifier channel.9 Inhibition of the efflux of positively charged potassium ions would result in slowing cellular repolarization, leaving the cells in a relatively excitable state. In the CNS, this produces seizures; in the heart, it may result in dysrhythmia. Cardiovascular collapse may occur with very high serum concentrations of lidocaine or following a rapid serum increase—eg, after a large intravenous (IV) bolus dose,7 which can potentially result from an unintentional intravascular injection during tumescent liposuction.
What is the treatment for lidocaine toxicity?
The first step in the treatment of lidocaine-associated CNS toxicity is the discontinuation of the drug. Failure to appropriately recognize the symptoms of early lidocaine toxicity may result in the progression to severe CNS effects and eventual CV collapse. Benzodiazepines should be used as needed for mild symptoms. Seizures should be treated aggressively with benzodiazepines or barbiturates, while ensuring maintenance of oxygenation, ventilation, and perfusion.7
In cases of lidocaine-associated CV toxicity, treatment begins with airway management, oxygen administration, and life support. Potential antidotal treatment of severe local anesthetic-associated CV toxicity involves the rapid administration of IV fat emulsion, or “lipid rescue.” Although best studied for bupivacaine toxicity, the exact mechanism of IV fat emulsion as an antidote is not completely understood. However, in the treatment of local anesthetic toxicity, lipid rescue is believed to offer a “sink” to remove the lipid-soluble anesthetics from their site of action and trap them within the vascular space. Suggested dosing of 20% lipid solution is a bolus of 1.5 mL/kg over a 1-minute period, followed by 0.25mL/kg per minute or 15 mL/kg per hour to run over 30 to 60 minutes.10
Case Conclusion
The patient made a full recovery and was discharged home in normal condition. Her healthcare provider was informed about the complication of the procedure.
Dr Hines is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Lozinski A, Huq NS. Tumescent liposuction. Clin Plastic Surg. 2013;40(4):593-613.
- Klein JA. Tumescent technique chronicles: local anesthesia, liposuction, and beyond. Dermatol Surg. 1995;21(5):449-457.
- Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248-263.
- Conroy PH, O’Rourke J. Tumescent anesthesia. The Surgeon. 2012;210-201.
- Rao RR, Fly SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(19):1471-1475.
- Martinez MA, Ballesteros S, Segura LJ, Garcia M. Reporting a fatality during tumescent liposuction. Forensic Sci Int. 2008;178(1):e11-e-16.
- Schwartz DR, Kaufman B. Local anesthestics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:921-930.
- Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidoaine. Nature. 1966;209(5019):207-208.
- Friederich P, Benzenberg D, Urban BW. Bupivacaine inhibits human neuronal Kv3 channels in SH-SY5Y human neuroblastoma cells. Br J Anaesth. 2002;88(6):864-866.
- Bania TC. Antidotes in depth, intravenous fat emulsions. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:931-937.
- Lozinski A, Huq NS. Tumescent liposuction. Clin Plastic Surg. 2013;40(4):593-613.
- Klein JA. Tumescent technique chronicles: local anesthesia, liposuction, and beyond. Dermatol Surg. 1995;21(5):449-457.
- Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248-263.
- Conroy PH, O’Rourke J. Tumescent anesthesia. The Surgeon. 2012;210-201.
- Rao RR, Fly SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(19):1471-1475.
- Martinez MA, Ballesteros S, Segura LJ, Garcia M. Reporting a fatality during tumescent liposuction. Forensic Sci Int. 2008;178(1):e11-e-16.
- Schwartz DR, Kaufman B. Local anesthestics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:921-930.
- Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidoaine. Nature. 1966;209(5019):207-208.
- Friederich P, Benzenberg D, Urban BW. Bupivacaine inhibits human neuronal Kv3 channels in SH-SY5Y human neuroblastoma cells. Br J Anaesth. 2002;88(6):864-866.
- Bania TC. Antidotes in depth, intravenous fat emulsions. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:931-937.
FDA approves first naloxone nasal spray for opioid overdose
The Food and Drug Administration has approved the first nasal spray variant of the opioid-overdose drug naloxone hydrochloride.
Marketed in the United States as Narcan by Adapt Pharma, a partner of Lightlake Therapeutics, the nasal spray is known to stop or, in some cases, reverse the effects of opioid overdosing in patients. Narcan is the first naloxone hydrochloride nasal spray approved by the FDA.
“Combating the opioid abuse epidemic is a top priority for the FDA,” Dr. Stephen Ostroff, FDA acting commissioner, said in a statement released with the Nov. 18 approval announcement. “We cannot stand by while Americans are dying. While naloxone will not solve the underlying problems of the opioid epidemic, we are speeding to review new formulations that will ultimately save lives that might otherwise be lost to drug addiction and overdose.”
The nasal spray itself is available only with a prescription, and is safe for use by both adults and children, according to the FDA.
The spray delivers a dose of 4 mg naloxone in a single 0.1-mL nasal spray, which comes in a ready-to-use, needle-free device, according to Adapt Pharma. Dosage varies for each individual and should be determined by physicians.
Administration of Narcan, which is sprayed into one nostril while the patient is lying on his or her back, does not require special training and can be performed by anyone.
Narcan’s approval comes less than 4 months after the FDA granted the medication a fast-track designation and priority review status, both of which are meant to expedite the review and approval processes for drugs that “demonstrate the potential to address an unmet medical need” and “offer a significant improvement in the safety or effectiveness of the treatment, prevention, or diagnosis of a serious condition,” according to the FDA’s approval statement.
Adverse events associated with opiate withdrawal have been noted in Narcan patients. Specifically, the FDA warned that body aches, diarrhea, tachycardia, fever, piloerection, nausea, nervousness, abdominal cramps, weakness, and increased blood pressure, among other conditions, are all possible side effects of Narcan.
“Opioid overdose is responsible for the deaths of thousands of Americans in communities throughout the country, leaving a trail of devastation for friends and families,” Seamus Mulligan, Adapt Pharma’s chairman and CEO, said in a statement. “This new device makes naloxone readily available for emergency use by a friend, family member, or caregiver, as well as offering an alternative treatment option for first responders and health care providers.”
Narcan’s approval is one step of many that must be taken to adequately address and ultimately end the problem of opioid abuse in the United States, cautioned Dr. Peter Friedmann, chief research officer at Baystate Health in Springfield, Mass.
“[Narcan] is just addressing overdose; we need more and better medications for treating addiction, we need more physicians and clinicians who are skilled in using these medications,” said Dr. Friedmann. “Given the ongoing crisis of deaths from opioid overuse, this expands the options that physicians, pharmacies, and community distribution programs can use to reduce these deaths.”
Dr. Friedmann also voiced his concern regarding the pricing of Narcan. Making the drug affordable is crucial to its success at successfully treating opioid overuse, he said.
“Right now, nasal atomizers with syringes are used off label, and the prices have been going up with increasing demand,” he said. “But [Narcan] is a commercial product based around what is essentially a generic medication, so [I] hope it’s priced at a price point that’s accessible to the great majority of patients and their families who are facing addiction, many of whom don’t have huge means.”
Dr. Friedmann said that he hopes addiction medicine becomes a more attractive field for medical students and residents. It’s important for future physicians to know how to properly treat patients of addiction and administer drugs safely and effectively, he said.
“Addiction medicine is on the cusp of full recognition as a medical specialty, and we need people to go into that field to teach patients, medical students, and residents how to treat people with addiction,” Dr. Friedmann said. At the current rate, “we’re never going to have enough, so we need generalists to take this on.”
The Food and Drug Administration has approved the first nasal spray variant of the opioid-overdose drug naloxone hydrochloride.
Marketed in the United States as Narcan by Adapt Pharma, a partner of Lightlake Therapeutics, the nasal spray is known to stop or, in some cases, reverse the effects of opioid overdosing in patients. Narcan is the first naloxone hydrochloride nasal spray approved by the FDA.
“Combating the opioid abuse epidemic is a top priority for the FDA,” Dr. Stephen Ostroff, FDA acting commissioner, said in a statement released with the Nov. 18 approval announcement. “We cannot stand by while Americans are dying. While naloxone will not solve the underlying problems of the opioid epidemic, we are speeding to review new formulations that will ultimately save lives that might otherwise be lost to drug addiction and overdose.”
The nasal spray itself is available only with a prescription, and is safe for use by both adults and children, according to the FDA.
The spray delivers a dose of 4 mg naloxone in a single 0.1-mL nasal spray, which comes in a ready-to-use, needle-free device, according to Adapt Pharma. Dosage varies for each individual and should be determined by physicians.
Administration of Narcan, which is sprayed into one nostril while the patient is lying on his or her back, does not require special training and can be performed by anyone.
Narcan’s approval comes less than 4 months after the FDA granted the medication a fast-track designation and priority review status, both of which are meant to expedite the review and approval processes for drugs that “demonstrate the potential to address an unmet medical need” and “offer a significant improvement in the safety or effectiveness of the treatment, prevention, or diagnosis of a serious condition,” according to the FDA’s approval statement.
Adverse events associated with opiate withdrawal have been noted in Narcan patients. Specifically, the FDA warned that body aches, diarrhea, tachycardia, fever, piloerection, nausea, nervousness, abdominal cramps, weakness, and increased blood pressure, among other conditions, are all possible side effects of Narcan.
“Opioid overdose is responsible for the deaths of thousands of Americans in communities throughout the country, leaving a trail of devastation for friends and families,” Seamus Mulligan, Adapt Pharma’s chairman and CEO, said in a statement. “This new device makes naloxone readily available for emergency use by a friend, family member, or caregiver, as well as offering an alternative treatment option for first responders and health care providers.”
Narcan’s approval is one step of many that must be taken to adequately address and ultimately end the problem of opioid abuse in the United States, cautioned Dr. Peter Friedmann, chief research officer at Baystate Health in Springfield, Mass.
“[Narcan] is just addressing overdose; we need more and better medications for treating addiction, we need more physicians and clinicians who are skilled in using these medications,” said Dr. Friedmann. “Given the ongoing crisis of deaths from opioid overuse, this expands the options that physicians, pharmacies, and community distribution programs can use to reduce these deaths.”
Dr. Friedmann also voiced his concern regarding the pricing of Narcan. Making the drug affordable is crucial to its success at successfully treating opioid overuse, he said.
“Right now, nasal atomizers with syringes are used off label, and the prices have been going up with increasing demand,” he said. “But [Narcan] is a commercial product based around what is essentially a generic medication, so [I] hope it’s priced at a price point that’s accessible to the great majority of patients and their families who are facing addiction, many of whom don’t have huge means.”
Dr. Friedmann said that he hopes addiction medicine becomes a more attractive field for medical students and residents. It’s important for future physicians to know how to properly treat patients of addiction and administer drugs safely and effectively, he said.
“Addiction medicine is on the cusp of full recognition as a medical specialty, and we need people to go into that field to teach patients, medical students, and residents how to treat people with addiction,” Dr. Friedmann said. At the current rate, “we’re never going to have enough, so we need generalists to take this on.”
The Food and Drug Administration has approved the first nasal spray variant of the opioid-overdose drug naloxone hydrochloride.
Marketed in the United States as Narcan by Adapt Pharma, a partner of Lightlake Therapeutics, the nasal spray is known to stop or, in some cases, reverse the effects of opioid overdosing in patients. Narcan is the first naloxone hydrochloride nasal spray approved by the FDA.
“Combating the opioid abuse epidemic is a top priority for the FDA,” Dr. Stephen Ostroff, FDA acting commissioner, said in a statement released with the Nov. 18 approval announcement. “We cannot stand by while Americans are dying. While naloxone will not solve the underlying problems of the opioid epidemic, we are speeding to review new formulations that will ultimately save lives that might otherwise be lost to drug addiction and overdose.”
The nasal spray itself is available only with a prescription, and is safe for use by both adults and children, according to the FDA.
The spray delivers a dose of 4 mg naloxone in a single 0.1-mL nasal spray, which comes in a ready-to-use, needle-free device, according to Adapt Pharma. Dosage varies for each individual and should be determined by physicians.
Administration of Narcan, which is sprayed into one nostril while the patient is lying on his or her back, does not require special training and can be performed by anyone.
Narcan’s approval comes less than 4 months after the FDA granted the medication a fast-track designation and priority review status, both of which are meant to expedite the review and approval processes for drugs that “demonstrate the potential to address an unmet medical need” and “offer a significant improvement in the safety or effectiveness of the treatment, prevention, or diagnosis of a serious condition,” according to the FDA’s approval statement.
Adverse events associated with opiate withdrawal have been noted in Narcan patients. Specifically, the FDA warned that body aches, diarrhea, tachycardia, fever, piloerection, nausea, nervousness, abdominal cramps, weakness, and increased blood pressure, among other conditions, are all possible side effects of Narcan.
“Opioid overdose is responsible for the deaths of thousands of Americans in communities throughout the country, leaving a trail of devastation for friends and families,” Seamus Mulligan, Adapt Pharma’s chairman and CEO, said in a statement. “This new device makes naloxone readily available for emergency use by a friend, family member, or caregiver, as well as offering an alternative treatment option for first responders and health care providers.”
Narcan’s approval is one step of many that must be taken to adequately address and ultimately end the problem of opioid abuse in the United States, cautioned Dr. Peter Friedmann, chief research officer at Baystate Health in Springfield, Mass.
“[Narcan] is just addressing overdose; we need more and better medications for treating addiction, we need more physicians and clinicians who are skilled in using these medications,” said Dr. Friedmann. “Given the ongoing crisis of deaths from opioid overuse, this expands the options that physicians, pharmacies, and community distribution programs can use to reduce these deaths.”
Dr. Friedmann also voiced his concern regarding the pricing of Narcan. Making the drug affordable is crucial to its success at successfully treating opioid overuse, he said.
“Right now, nasal atomizers with syringes are used off label, and the prices have been going up with increasing demand,” he said. “But [Narcan] is a commercial product based around what is essentially a generic medication, so [I] hope it’s priced at a price point that’s accessible to the great majority of patients and their families who are facing addiction, many of whom don’t have huge means.”
Dr. Friedmann said that he hopes addiction medicine becomes a more attractive field for medical students and residents. It’s important for future physicians to know how to properly treat patients of addiction and administer drugs safely and effectively, he said.
“Addiction medicine is on the cusp of full recognition as a medical specialty, and we need people to go into that field to teach patients, medical students, and residents how to treat people with addiction,” Dr. Friedmann said. At the current rate, “we’re never going to have enough, so we need generalists to take this on.”
Case reports: Ingestion of aripiprazole precedes false positives for amphetamine use
Two children who had ingested aripiprazole but not amphetamines tested positive for amphetamine use in urine drug screens (UDSs) performed within 24 hours of their drug use, according to two case reports by Justin Kaplan, Pharm.D., of Hackensack (N.J.) University Medical Center and his colleagues.
In both cases, aripiprazole had been prescribed to the father of the child and had been taken by the child without the knowledge or auspices of a parent. Both of the children were admitted to hospitals where their urine was screened for drugs.
“To our knowledge this case series is the first to document potential false-positive UDSs after accidental ingestion of aripiprazole,” said the researchers. “In both cases, the presentation of drowsiness, lethargy, and ataxia were more consistent with ingestion of an atypical antipsychotic than with amphetamines.”
In one of the cases, a 2-year-old girl was found holding an open bottle of aripiprazole 15-mg tablets by her parents. The parents’ report of the incident suggested that the child had ingested three such tablets. The child’s urine was screened for amphetamines twice, in the hospital where she was admitted; the first screen, which was performed the morning after the child had been taken to a hospital, revealed an amphetamine concentration of 1,048 ng/mL. The child’s second UDS, which was performed the following day, indicated a 949 ng/mL concentration of amphetamines. Outside of the hospital, laboratory tests were performed on the child’s blood and urine samples from the day following her admittance to the hospital. Both of these additional tests were negative for amphetamines, suggesting that the results of the in-hospital UDSs had been false positives.
The other case involved a 20-month-old girl, whose father found her with pills scattered around her crib. The drugs were part of a 1-week supply of drugs of the father. The medications included alprazolam 2.5 mg, fluvoxamine 2,100 mg, clonazepam 17.5 mg, buspirone 420 mg, and aripiprazole 35 mg. This child’s urine was also screened for drugs twice at the hospital where she was admitted; this child only tested positive for amphetamine in the first assessment, with a 311 ng/mL concentration of amphetamines having been found in that UDS. As with the first case, this child’s urine and blood samples were subjected to off-site laboratory tests, which found no presence of amphetamines.
“There are several limitations to UDS immunoassays. Most important, poor specificity is associated with a risk of false-positive testing. A negative result does not exclude the possibility that the substance is present if it is below the lower threshold of detection. Additionally, there is no way to quantitatively correlate a positive result with the extent of immunoassays. Therefore immunoassays are the first step in a two-step system, in which all positive results must be confirmed by more reliable methods such as [gas chromatography mass spectrometry],” the researchers said.
Read the full study in Pediatrics. doi: 10:1542/peds.2014-3333.
Two children who had ingested aripiprazole but not amphetamines tested positive for amphetamine use in urine drug screens (UDSs) performed within 24 hours of their drug use, according to two case reports by Justin Kaplan, Pharm.D., of Hackensack (N.J.) University Medical Center and his colleagues.
In both cases, aripiprazole had been prescribed to the father of the child and had been taken by the child without the knowledge or auspices of a parent. Both of the children were admitted to hospitals where their urine was screened for drugs.
“To our knowledge this case series is the first to document potential false-positive UDSs after accidental ingestion of aripiprazole,” said the researchers. “In both cases, the presentation of drowsiness, lethargy, and ataxia were more consistent with ingestion of an atypical antipsychotic than with amphetamines.”
In one of the cases, a 2-year-old girl was found holding an open bottle of aripiprazole 15-mg tablets by her parents. The parents’ report of the incident suggested that the child had ingested three such tablets. The child’s urine was screened for amphetamines twice, in the hospital where she was admitted; the first screen, which was performed the morning after the child had been taken to a hospital, revealed an amphetamine concentration of 1,048 ng/mL. The child’s second UDS, which was performed the following day, indicated a 949 ng/mL concentration of amphetamines. Outside of the hospital, laboratory tests were performed on the child’s blood and urine samples from the day following her admittance to the hospital. Both of these additional tests were negative for amphetamines, suggesting that the results of the in-hospital UDSs had been false positives.
The other case involved a 20-month-old girl, whose father found her with pills scattered around her crib. The drugs were part of a 1-week supply of drugs of the father. The medications included alprazolam 2.5 mg, fluvoxamine 2,100 mg, clonazepam 17.5 mg, buspirone 420 mg, and aripiprazole 35 mg. This child’s urine was also screened for drugs twice at the hospital where she was admitted; this child only tested positive for amphetamine in the first assessment, with a 311 ng/mL concentration of amphetamines having been found in that UDS. As with the first case, this child’s urine and blood samples were subjected to off-site laboratory tests, which found no presence of amphetamines.
“There are several limitations to UDS immunoassays. Most important, poor specificity is associated with a risk of false-positive testing. A negative result does not exclude the possibility that the substance is present if it is below the lower threshold of detection. Additionally, there is no way to quantitatively correlate a positive result with the extent of immunoassays. Therefore immunoassays are the first step in a two-step system, in which all positive results must be confirmed by more reliable methods such as [gas chromatography mass spectrometry],” the researchers said.
Read the full study in Pediatrics. doi: 10:1542/peds.2014-3333.
Two children who had ingested aripiprazole but not amphetamines tested positive for amphetamine use in urine drug screens (UDSs) performed within 24 hours of their drug use, according to two case reports by Justin Kaplan, Pharm.D., of Hackensack (N.J.) University Medical Center and his colleagues.
In both cases, aripiprazole had been prescribed to the father of the child and had been taken by the child without the knowledge or auspices of a parent. Both of the children were admitted to hospitals where their urine was screened for drugs.
“To our knowledge this case series is the first to document potential false-positive UDSs after accidental ingestion of aripiprazole,” said the researchers. “In both cases, the presentation of drowsiness, lethargy, and ataxia were more consistent with ingestion of an atypical antipsychotic than with amphetamines.”
In one of the cases, a 2-year-old girl was found holding an open bottle of aripiprazole 15-mg tablets by her parents. The parents’ report of the incident suggested that the child had ingested three such tablets. The child’s urine was screened for amphetamines twice, in the hospital where she was admitted; the first screen, which was performed the morning after the child had been taken to a hospital, revealed an amphetamine concentration of 1,048 ng/mL. The child’s second UDS, which was performed the following day, indicated a 949 ng/mL concentration of amphetamines. Outside of the hospital, laboratory tests were performed on the child’s blood and urine samples from the day following her admittance to the hospital. Both of these additional tests were negative for amphetamines, suggesting that the results of the in-hospital UDSs had been false positives.
The other case involved a 20-month-old girl, whose father found her with pills scattered around her crib. The drugs were part of a 1-week supply of drugs of the father. The medications included alprazolam 2.5 mg, fluvoxamine 2,100 mg, clonazepam 17.5 mg, buspirone 420 mg, and aripiprazole 35 mg. This child’s urine was also screened for drugs twice at the hospital where she was admitted; this child only tested positive for amphetamine in the first assessment, with a 311 ng/mL concentration of amphetamines having been found in that UDS. As with the first case, this child’s urine and blood samples were subjected to off-site laboratory tests, which found no presence of amphetamines.
“There are several limitations to UDS immunoassays. Most important, poor specificity is associated with a risk of false-positive testing. A negative result does not exclude the possibility that the substance is present if it is below the lower threshold of detection. Additionally, there is no way to quantitatively correlate a positive result with the extent of immunoassays. Therefore immunoassays are the first step in a two-step system, in which all positive results must be confirmed by more reliable methods such as [gas chromatography mass spectrometry],” the researchers said.
Read the full study in Pediatrics. doi: 10:1542/peds.2014-3333.
FROM PEDIATRICS
Complex picture emerges of prescription opioid abuse
The percentage of the nonmedical use of prescription opioids decreased among U.S. adults over the last decade, but the prevalence of opioid use disorders, the frequency of opioid abuse, and related mortality all increased, according to a report published online Oct. 13 in JAMA.
These findings, from an analysis of two large nationally representative data sets, paint a picture that is complex and more nuanced than that suggested by some recent reports. For example, a study of the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System found that the abuse and diversion of prescription opioids plateaued or decreased in recent years. “The nationally representative results in our study may be especially important in providing an accurate picture of the current status of the epidemic,” said Dr. Beth Han of the Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, Md., and her associates.
The nonmedical use of prescription opioids is an acknowledged epidemic, but that epidemic’s changing pattern over time needed to be updated. The investigators assessed the changes in use during the most recent decade for which data are available (2003-2013) using annual surveys conducted by SAMHSA and cause of death files from the National Vital Statistics System.
Based on responses from 472,200 people aged 18-64 years, the 1-year prevalence of nonmedical use of prescription opioids decreased from 5.4% to 4.9% during the study period. However, the 1-year prevalence of use disorders rose from 0.6% to 0.9%, the 1-year prevalence of high-frequency use (200 days or more per year) increased from 0.3% to 0.4%, and the rate of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000. In addition, the mean number of days of opioid abuse increased from 2.1 to 2.6 per year in the general population and from 40.0 to 54.2 days per year among acknowledged opioid users, the investigators said (JAMA. 2015 Oct 13;314[14]:1468-1478. doi:10.1001/jama.2015.11859).
Compared with white users of prescription opioids, both black and Hispanic users had a lower prevalence of use disorders. The prevalence of use disorders was higher among less-educated than more-educated adults, among those with no health insurance or Medicaid as opposed to private health insurance, and among smokers than nonsmokers, Dr. Han and her associates added.
Previous research has shown that most adults who abuse prescription opioids neither receive treatment nor perceive that they need treatment. Clinicians can help by using prescription-drug monitoring programs to identify inappropriate receipt of prescription opioids, then offering treatments, which are highly effective, for patients who need them, the investigators noted.
The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
The slight decline (approximately 0.4% over 10 years) in opioid initiation reported by Han et al. may be encouraging, but their other findings suggest that more patients are experiencing an inexorable progression from initial opioid use to frequent use to highly frequent use to a use disorder.
The source of most opioid abuse is often a seemingly legitimate prescription, and the key to addressing the opioid-abuse epidemic is to keep opioid-naive patients opioid naive. It is still unclear why clinicians continue to prescribe opioids, despite recommendations to the contrary and the fact that these agents provide little or no long-term benefit for most types of chronic pain.
Lewis S. Nelson, M.D., is in the Ronald O. Perelman department of emergency medicine at New York University. He and his associates made these remarks in an editorial accompanying Dr. Han’s report (JAMA. 2015 Oct 13;314[14]:1453-1454. doi:10.1001/jama.2015.12397).
The slight decline (approximately 0.4% over 10 years) in opioid initiation reported by Han et al. may be encouraging, but their other findings suggest that more patients are experiencing an inexorable progression from initial opioid use to frequent use to highly frequent use to a use disorder.
The source of most opioid abuse is often a seemingly legitimate prescription, and the key to addressing the opioid-abuse epidemic is to keep opioid-naive patients opioid naive. It is still unclear why clinicians continue to prescribe opioids, despite recommendations to the contrary and the fact that these agents provide little or no long-term benefit for most types of chronic pain.
Lewis S. Nelson, M.D., is in the Ronald O. Perelman department of emergency medicine at New York University. He and his associates made these remarks in an editorial accompanying Dr. Han’s report (JAMA. 2015 Oct 13;314[14]:1453-1454. doi:10.1001/jama.2015.12397).
The slight decline (approximately 0.4% over 10 years) in opioid initiation reported by Han et al. may be encouraging, but their other findings suggest that more patients are experiencing an inexorable progression from initial opioid use to frequent use to highly frequent use to a use disorder.
The source of most opioid abuse is often a seemingly legitimate prescription, and the key to addressing the opioid-abuse epidemic is to keep opioid-naive patients opioid naive. It is still unclear why clinicians continue to prescribe opioids, despite recommendations to the contrary and the fact that these agents provide little or no long-term benefit for most types of chronic pain.
Lewis S. Nelson, M.D., is in the Ronald O. Perelman department of emergency medicine at New York University. He and his associates made these remarks in an editorial accompanying Dr. Han’s report (JAMA. 2015 Oct 13;314[14]:1453-1454. doi:10.1001/jama.2015.12397).
The percentage of the nonmedical use of prescription opioids decreased among U.S. adults over the last decade, but the prevalence of opioid use disorders, the frequency of opioid abuse, and related mortality all increased, according to a report published online Oct. 13 in JAMA.
These findings, from an analysis of two large nationally representative data sets, paint a picture that is complex and more nuanced than that suggested by some recent reports. For example, a study of the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System found that the abuse and diversion of prescription opioids plateaued or decreased in recent years. “The nationally representative results in our study may be especially important in providing an accurate picture of the current status of the epidemic,” said Dr. Beth Han of the Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, Md., and her associates.
The nonmedical use of prescription opioids is an acknowledged epidemic, but that epidemic’s changing pattern over time needed to be updated. The investigators assessed the changes in use during the most recent decade for which data are available (2003-2013) using annual surveys conducted by SAMHSA and cause of death files from the National Vital Statistics System.
Based on responses from 472,200 people aged 18-64 years, the 1-year prevalence of nonmedical use of prescription opioids decreased from 5.4% to 4.9% during the study period. However, the 1-year prevalence of use disorders rose from 0.6% to 0.9%, the 1-year prevalence of high-frequency use (200 days or more per year) increased from 0.3% to 0.4%, and the rate of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000. In addition, the mean number of days of opioid abuse increased from 2.1 to 2.6 per year in the general population and from 40.0 to 54.2 days per year among acknowledged opioid users, the investigators said (JAMA. 2015 Oct 13;314[14]:1468-1478. doi:10.1001/jama.2015.11859).
Compared with white users of prescription opioids, both black and Hispanic users had a lower prevalence of use disorders. The prevalence of use disorders was higher among less-educated than more-educated adults, among those with no health insurance or Medicaid as opposed to private health insurance, and among smokers than nonsmokers, Dr. Han and her associates added.
Previous research has shown that most adults who abuse prescription opioids neither receive treatment nor perceive that they need treatment. Clinicians can help by using prescription-drug monitoring programs to identify inappropriate receipt of prescription opioids, then offering treatments, which are highly effective, for patients who need them, the investigators noted.
The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
The percentage of the nonmedical use of prescription opioids decreased among U.S. adults over the last decade, but the prevalence of opioid use disorders, the frequency of opioid abuse, and related mortality all increased, according to a report published online Oct. 13 in JAMA.
These findings, from an analysis of two large nationally representative data sets, paint a picture that is complex and more nuanced than that suggested by some recent reports. For example, a study of the Researched Abuse, Diversion, and Addiction-Related Surveillance (RADARS) System found that the abuse and diversion of prescription opioids plateaued or decreased in recent years. “The nationally representative results in our study may be especially important in providing an accurate picture of the current status of the epidemic,” said Dr. Beth Han of the Substance Abuse and Mental Health Services Administration (SAMHSA), Rockville, Md., and her associates.
The nonmedical use of prescription opioids is an acknowledged epidemic, but that epidemic’s changing pattern over time needed to be updated. The investigators assessed the changes in use during the most recent decade for which data are available (2003-2013) using annual surveys conducted by SAMHSA and cause of death files from the National Vital Statistics System.
Based on responses from 472,200 people aged 18-64 years, the 1-year prevalence of nonmedical use of prescription opioids decreased from 5.4% to 4.9% during the study period. However, the 1-year prevalence of use disorders rose from 0.6% to 0.9%, the 1-year prevalence of high-frequency use (200 days or more per year) increased from 0.3% to 0.4%, and the rate of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000. In addition, the mean number of days of opioid abuse increased from 2.1 to 2.6 per year in the general population and from 40.0 to 54.2 days per year among acknowledged opioid users, the investigators said (JAMA. 2015 Oct 13;314[14]:1468-1478. doi:10.1001/jama.2015.11859).
Compared with white users of prescription opioids, both black and Hispanic users had a lower prevalence of use disorders. The prevalence of use disorders was higher among less-educated than more-educated adults, among those with no health insurance or Medicaid as opposed to private health insurance, and among smokers than nonsmokers, Dr. Han and her associates added.
Previous research has shown that most adults who abuse prescription opioids neither receive treatment nor perceive that they need treatment. Clinicians can help by using prescription-drug monitoring programs to identify inappropriate receipt of prescription opioids, then offering treatments, which are highly effective, for patients who need them, the investigators noted.
The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
FROM JAMA
Key clinical point: The percentage of nonmedical use of prescription opioids declined during the last decade, but the prevalence of use disorders, the frequency of abuse, and related mortality all increased.
Major finding: The 1-year prevalence of opioid use disorders rose from 0.6% to 0.9%, that of high-frequency use increased from 0.3% to 0.4%, and that of opioid-related deaths increased from 4.5 per 100,000 to 7.8 per 100,000.
Data source: An analysis of time trends in prescription opioid use, based on two nationally representative data sets involving 472,200 adults.
Disclosures: The Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse, and the Food and Drug Administration sponsored the study. Dr. Han reported having no relevant disclosures; an associate reported owning stock in General Electric, 3M Company, and Pfizer.
Case Studies in Toxicology: One Last Kick—Transverse Myelitis After an Overdose of Heroin via Insufflation
Case
A 17-year-old adolescent girl with a history of depression and opioid dependence, for which she was taking buprenorphine until 2 weeks earlier, presented to the ED via emergency medical services (EMS) after her father found her lying on the couch unresponsive and with shallow respirations. Naloxone was administered by EMS and her mental status improved.
At presentation, the patient admitted to insufflation of an unknown amount of heroin and ingestion of 2 mg of alprazolam earlier in the day. She denied any past or current use of intravenous (IV) drugs. During monitoring, she began to complain of numbness in her legs and an inability to urinate. Examination revealed paralysis and decreased sensation of her bilateral lower extremities to the midthigh, with decreased rectal tone. Because of the patient’s history of drug use and temporal association with the heroin overdose, both neurosurgery and toxicology services were consulted.
What can cause lower extremity paralysis in a drug user?
The differential diagnosis for the patient at this point included toxin-induced myelopathy, Guillain-Barré syndrome, hypokalemic periodic paralysis, spinal compression, epidural abscess, cerebrovascular accident, spinal lesion, and spinal artery dissection or infarction.
Although Guillain-Barré syndrome presents with ascending paralysis, there is usually an antecedent respiratory or gastrointestinal infection. While epidural abscess with spinal compression is associated with IV drug use and can present similarly, the patient in this case denied IV use. In the absence of any risk factors, cerebrovascular accident and spinal artery dissection were also unlikely.
Case Continuation
A bladder catheter was placed due to the patient’s inability to urinate, and approximately 1 L of urine output was retrieved. Immediate magnetic resonance imaging (MRI) demonstrated increased T2 signal intensity and expansion of the distal thoracic cord and conus without mass lesion, consistent with transverse myelitis (TM).
What is transverse myelitis and why does it occur?
Transverse myelitis is an inflammatory demyelinating disorder that focally affects the spinal cord, resulting in a specific pattern of motor, sensory, and autonomic dysfunction.1 Signs and symptoms include paresthesia, paralysis of the extremities, and loss of bladder and bowel control. The level of the spinal cord affected determines the clinical effects. Demyelination typically occurs at the thoracic segment, producing findings in the legs, as well as bladder and bowel dysfunction.
The exact cause of TM is unknown, but the inflammation may result from a viral complication or an abnormal immune response. Infectious viral agents suspected of causing TM include varicella zoster, herpes simplex, cytomegalovirus, Epstein-Barr, influenza, human immunodeficiency virus, hepatitis A, and rubella. It has also been postulated that an autoimmune reaction is responsible for the condition.
In some individuals, TM represents the first manifestation of an underlying demyelinating disorder such as multiple sclerosis or neuromyelitis optica. A diagnosis of TM is made through patient history, physical examination, and characteristic findings on neuroimaging, specifically MRI.
Heroin use has long been associated with the development of TM, and is usually associated with IV administration of the drug after a period of abstinence.2 This association strengthens the basis for an immunologic etiology—an initial sensitization and subsequent reexposure causing the effects of TM. There have also been cases of TM coexisting with rhabdomyolysis due to the patient being found in a contorted position.3 Another theory of the etiology of heroin-associated TM is a reaction to a possible adulterant or contaminant in the heroin.4
What is the treatment and prognosis of transverse myelitis?
Since there is no cure for TM, treatment is directed at reducing inflammation in the spinal cord. Initial therapy generally includes corticosteroids. In patients with a minimal response to corticosteroids, plasma exchange can be attempted. There are also limited data to suggest a beneficial role for the use of IV immunoglobulin.5 In addition to treatment, general supportive care must also be optimized, such as the use of prophylaxis for thrombophlebitis due to immobility and physical therapy, if possible.
The prognosis of patients with TM is variable, and up to two thirds of patients will have moderate-to-severe residual neurological disability.6 Recovery is slow, with most patients beginning to show improvement within the first 2 to 12 weeks from treatment and supportive care. The recovery process can continue for 2 years. However, if no improvement is made within the first 3 to 6 months, recovery is unlikely.7 Cases of heroin-associated TM may have a more favorable prognosis.8
A majority of individuals will only experience this clinical entity once, but there are rare causes of recurrent or relapsing TM.7 In these situations, a search for underlying demyelinating diseases should be performed.
Case Conclusion
The patient was immediately started on IV corticosteroids, but as there was no improvement after 5 days, plasmapheresis was performed. She received 5 cycles of plasmapheresis and a 5-day course of IV immunoglobulin but still without any improvement. A repeat MRI of the thoracic spine was performed and raised the possibility of cord infarct, but infectious or inflammatory myelitis remained within differential consideration. The patient continued to make minimal improvement with physical therapy and, after a 3-week hospital course, she was transferred to inpatient rehabilitation for further care. Over the next 2 months, the loss of sensation and motor ability of her legs did not improve, but she did regain control of her bowels and bladder.
Dr Regina is a medical toxicology fellow in the department of emergency medicine at North Shore Long Island Jewish Health System, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Pandit L. Transverse myelitis spectrum disorders. Neurol India. 2009;57(2):126-133.
- Richter RW, Rosenberg RN. Transverse myelitis associated with heroin addiction. JAMA. 1968;206(6):1255-1257.
- Sahni V, Garg D, Garg S, Agarwal SK, Singh NP. Unusual complications of heroin abuse: transverse myelitis, rhabdomyolysis, compartment syndrome, and ARF. Clin Toxicol (Phila). 2008;46(2):153-155.
- Schein PS, Yessayan L, Mayman CI. Acute transverse myelitis associated with intravenous opium. Neurology. 1971;21(1):101-102.
- Absoud M, Gadian J, Hellier J, et al. Protocol for a multicentre randomiSed controlled TRial of IntraVEnous immunoglobulin versus standard therapy for the treatment of transverse myelitis in adults and children (STRIVE). BMJ Open. 2015;5(5):e008312.
- West TW. Transverse myelitis--a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16(88):167-177.
- Transverse myelitis fact sheet. National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders/transversemyelitis/detail_transversemyelitis.htm. Updated June 24, 2015. Accessed September 2, 2015.
- McGuire JL, Beslow LA, Finkel RS, Zimmerman RA, Henretig FM. A teenager with focal weakness. Pediatr Emerg Care. 2008;24(12):875-879.
Case
A 17-year-old adolescent girl with a history of depression and opioid dependence, for which she was taking buprenorphine until 2 weeks earlier, presented to the ED via emergency medical services (EMS) after her father found her lying on the couch unresponsive and with shallow respirations. Naloxone was administered by EMS and her mental status improved.
At presentation, the patient admitted to insufflation of an unknown amount of heroin and ingestion of 2 mg of alprazolam earlier in the day. She denied any past or current use of intravenous (IV) drugs. During monitoring, she began to complain of numbness in her legs and an inability to urinate. Examination revealed paralysis and decreased sensation of her bilateral lower extremities to the midthigh, with decreased rectal tone. Because of the patient’s history of drug use and temporal association with the heroin overdose, both neurosurgery and toxicology services were consulted.
What can cause lower extremity paralysis in a drug user?
The differential diagnosis for the patient at this point included toxin-induced myelopathy, Guillain-Barré syndrome, hypokalemic periodic paralysis, spinal compression, epidural abscess, cerebrovascular accident, spinal lesion, and spinal artery dissection or infarction.
Although Guillain-Barré syndrome presents with ascending paralysis, there is usually an antecedent respiratory or gastrointestinal infection. While epidural abscess with spinal compression is associated with IV drug use and can present similarly, the patient in this case denied IV use. In the absence of any risk factors, cerebrovascular accident and spinal artery dissection were also unlikely.
Case Continuation
A bladder catheter was placed due to the patient’s inability to urinate, and approximately 1 L of urine output was retrieved. Immediate magnetic resonance imaging (MRI) demonstrated increased T2 signal intensity and expansion of the distal thoracic cord and conus without mass lesion, consistent with transverse myelitis (TM).
What is transverse myelitis and why does it occur?
Transverse myelitis is an inflammatory demyelinating disorder that focally affects the spinal cord, resulting in a specific pattern of motor, sensory, and autonomic dysfunction.1 Signs and symptoms include paresthesia, paralysis of the extremities, and loss of bladder and bowel control. The level of the spinal cord affected determines the clinical effects. Demyelination typically occurs at the thoracic segment, producing findings in the legs, as well as bladder and bowel dysfunction.
The exact cause of TM is unknown, but the inflammation may result from a viral complication or an abnormal immune response. Infectious viral agents suspected of causing TM include varicella zoster, herpes simplex, cytomegalovirus, Epstein-Barr, influenza, human immunodeficiency virus, hepatitis A, and rubella. It has also been postulated that an autoimmune reaction is responsible for the condition.
In some individuals, TM represents the first manifestation of an underlying demyelinating disorder such as multiple sclerosis or neuromyelitis optica. A diagnosis of TM is made through patient history, physical examination, and characteristic findings on neuroimaging, specifically MRI.
Heroin use has long been associated with the development of TM, and is usually associated with IV administration of the drug after a period of abstinence.2 This association strengthens the basis for an immunologic etiology—an initial sensitization and subsequent reexposure causing the effects of TM. There have also been cases of TM coexisting with rhabdomyolysis due to the patient being found in a contorted position.3 Another theory of the etiology of heroin-associated TM is a reaction to a possible adulterant or contaminant in the heroin.4
What is the treatment and prognosis of transverse myelitis?
Since there is no cure for TM, treatment is directed at reducing inflammation in the spinal cord. Initial therapy generally includes corticosteroids. In patients with a minimal response to corticosteroids, plasma exchange can be attempted. There are also limited data to suggest a beneficial role for the use of IV immunoglobulin.5 In addition to treatment, general supportive care must also be optimized, such as the use of prophylaxis for thrombophlebitis due to immobility and physical therapy, if possible.
The prognosis of patients with TM is variable, and up to two thirds of patients will have moderate-to-severe residual neurological disability.6 Recovery is slow, with most patients beginning to show improvement within the first 2 to 12 weeks from treatment and supportive care. The recovery process can continue for 2 years. However, if no improvement is made within the first 3 to 6 months, recovery is unlikely.7 Cases of heroin-associated TM may have a more favorable prognosis.8
A majority of individuals will only experience this clinical entity once, but there are rare causes of recurrent or relapsing TM.7 In these situations, a search for underlying demyelinating diseases should be performed.
Case Conclusion
The patient was immediately started on IV corticosteroids, but as there was no improvement after 5 days, plasmapheresis was performed. She received 5 cycles of plasmapheresis and a 5-day course of IV immunoglobulin but still without any improvement. A repeat MRI of the thoracic spine was performed and raised the possibility of cord infarct, but infectious or inflammatory myelitis remained within differential consideration. The patient continued to make minimal improvement with physical therapy and, after a 3-week hospital course, she was transferred to inpatient rehabilitation for further care. Over the next 2 months, the loss of sensation and motor ability of her legs did not improve, but she did regain control of her bowels and bladder.
Dr Regina is a medical toxicology fellow in the department of emergency medicine at North Shore Long Island Jewish Health System, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 17-year-old adolescent girl with a history of depression and opioid dependence, for which she was taking buprenorphine until 2 weeks earlier, presented to the ED via emergency medical services (EMS) after her father found her lying on the couch unresponsive and with shallow respirations. Naloxone was administered by EMS and her mental status improved.
At presentation, the patient admitted to insufflation of an unknown amount of heroin and ingestion of 2 mg of alprazolam earlier in the day. She denied any past or current use of intravenous (IV) drugs. During monitoring, she began to complain of numbness in her legs and an inability to urinate. Examination revealed paralysis and decreased sensation of her bilateral lower extremities to the midthigh, with decreased rectal tone. Because of the patient’s history of drug use and temporal association with the heroin overdose, both neurosurgery and toxicology services were consulted.
What can cause lower extremity paralysis in a drug user?
The differential diagnosis for the patient at this point included toxin-induced myelopathy, Guillain-Barré syndrome, hypokalemic periodic paralysis, spinal compression, epidural abscess, cerebrovascular accident, spinal lesion, and spinal artery dissection or infarction.
Although Guillain-Barré syndrome presents with ascending paralysis, there is usually an antecedent respiratory or gastrointestinal infection. While epidural abscess with spinal compression is associated with IV drug use and can present similarly, the patient in this case denied IV use. In the absence of any risk factors, cerebrovascular accident and spinal artery dissection were also unlikely.
Case Continuation
A bladder catheter was placed due to the patient’s inability to urinate, and approximately 1 L of urine output was retrieved. Immediate magnetic resonance imaging (MRI) demonstrated increased T2 signal intensity and expansion of the distal thoracic cord and conus without mass lesion, consistent with transverse myelitis (TM).
What is transverse myelitis and why does it occur?
Transverse myelitis is an inflammatory demyelinating disorder that focally affects the spinal cord, resulting in a specific pattern of motor, sensory, and autonomic dysfunction.1 Signs and symptoms include paresthesia, paralysis of the extremities, and loss of bladder and bowel control. The level of the spinal cord affected determines the clinical effects. Demyelination typically occurs at the thoracic segment, producing findings in the legs, as well as bladder and bowel dysfunction.
The exact cause of TM is unknown, but the inflammation may result from a viral complication or an abnormal immune response. Infectious viral agents suspected of causing TM include varicella zoster, herpes simplex, cytomegalovirus, Epstein-Barr, influenza, human immunodeficiency virus, hepatitis A, and rubella. It has also been postulated that an autoimmune reaction is responsible for the condition.
In some individuals, TM represents the first manifestation of an underlying demyelinating disorder such as multiple sclerosis or neuromyelitis optica. A diagnosis of TM is made through patient history, physical examination, and characteristic findings on neuroimaging, specifically MRI.
Heroin use has long been associated with the development of TM, and is usually associated with IV administration of the drug after a period of abstinence.2 This association strengthens the basis for an immunologic etiology—an initial sensitization and subsequent reexposure causing the effects of TM. There have also been cases of TM coexisting with rhabdomyolysis due to the patient being found in a contorted position.3 Another theory of the etiology of heroin-associated TM is a reaction to a possible adulterant or contaminant in the heroin.4
What is the treatment and prognosis of transverse myelitis?
Since there is no cure for TM, treatment is directed at reducing inflammation in the spinal cord. Initial therapy generally includes corticosteroids. In patients with a minimal response to corticosteroids, plasma exchange can be attempted. There are also limited data to suggest a beneficial role for the use of IV immunoglobulin.5 In addition to treatment, general supportive care must also be optimized, such as the use of prophylaxis for thrombophlebitis due to immobility and physical therapy, if possible.
The prognosis of patients with TM is variable, and up to two thirds of patients will have moderate-to-severe residual neurological disability.6 Recovery is slow, with most patients beginning to show improvement within the first 2 to 12 weeks from treatment and supportive care. The recovery process can continue for 2 years. However, if no improvement is made within the first 3 to 6 months, recovery is unlikely.7 Cases of heroin-associated TM may have a more favorable prognosis.8
A majority of individuals will only experience this clinical entity once, but there are rare causes of recurrent or relapsing TM.7 In these situations, a search for underlying demyelinating diseases should be performed.
Case Conclusion
The patient was immediately started on IV corticosteroids, but as there was no improvement after 5 days, plasmapheresis was performed. She received 5 cycles of plasmapheresis and a 5-day course of IV immunoglobulin but still without any improvement. A repeat MRI of the thoracic spine was performed and raised the possibility of cord infarct, but infectious or inflammatory myelitis remained within differential consideration. The patient continued to make minimal improvement with physical therapy and, after a 3-week hospital course, she was transferred to inpatient rehabilitation for further care. Over the next 2 months, the loss of sensation and motor ability of her legs did not improve, but she did regain control of her bowels and bladder.
Dr Regina is a medical toxicology fellow in the department of emergency medicine at North Shore Long Island Jewish Health System, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Pandit L. Transverse myelitis spectrum disorders. Neurol India. 2009;57(2):126-133.
- Richter RW, Rosenberg RN. Transverse myelitis associated with heroin addiction. JAMA. 1968;206(6):1255-1257.
- Sahni V, Garg D, Garg S, Agarwal SK, Singh NP. Unusual complications of heroin abuse: transverse myelitis, rhabdomyolysis, compartment syndrome, and ARF. Clin Toxicol (Phila). 2008;46(2):153-155.
- Schein PS, Yessayan L, Mayman CI. Acute transverse myelitis associated with intravenous opium. Neurology. 1971;21(1):101-102.
- Absoud M, Gadian J, Hellier J, et al. Protocol for a multicentre randomiSed controlled TRial of IntraVEnous immunoglobulin versus standard therapy for the treatment of transverse myelitis in adults and children (STRIVE). BMJ Open. 2015;5(5):e008312.
- West TW. Transverse myelitis--a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16(88):167-177.
- Transverse myelitis fact sheet. National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders/transversemyelitis/detail_transversemyelitis.htm. Updated June 24, 2015. Accessed September 2, 2015.
- McGuire JL, Beslow LA, Finkel RS, Zimmerman RA, Henretig FM. A teenager with focal weakness. Pediatr Emerg Care. 2008;24(12):875-879.
- Pandit L. Transverse myelitis spectrum disorders. Neurol India. 2009;57(2):126-133.
- Richter RW, Rosenberg RN. Transverse myelitis associated with heroin addiction. JAMA. 1968;206(6):1255-1257.
- Sahni V, Garg D, Garg S, Agarwal SK, Singh NP. Unusual complications of heroin abuse: transverse myelitis, rhabdomyolysis, compartment syndrome, and ARF. Clin Toxicol (Phila). 2008;46(2):153-155.
- Schein PS, Yessayan L, Mayman CI. Acute transverse myelitis associated with intravenous opium. Neurology. 1971;21(1):101-102.
- Absoud M, Gadian J, Hellier J, et al. Protocol for a multicentre randomiSed controlled TRial of IntraVEnous immunoglobulin versus standard therapy for the treatment of transverse myelitis in adults and children (STRIVE). BMJ Open. 2015;5(5):e008312.
- West TW. Transverse myelitis--a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16(88):167-177.
- Transverse myelitis fact sheet. National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders/transversemyelitis/detail_transversemyelitis.htm. Updated June 24, 2015. Accessed September 2, 2015.
- McGuire JL, Beslow LA, Finkel RS, Zimmerman RA, Henretig FM. A teenager with focal weakness. Pediatr Emerg Care. 2008;24(12):875-879.