User login
Pediatric self-administration drives cough and cold drug mishaps
BALTIMORE – The vast majority of reported U.S. episodes of cough and cold medication serious adverse event episodes in young children occurred by an accidental, self-administration overdose, according to a review of all pediatric episodes collected by the designated national surveillance system during 2008-2014.
This pattern highlights the continued need for diligent education of families about the potential danger posed by these largely OTC products as well as a possible additional need for further improvement in protective packaging, Dr. G. Sam Wang said at the annual meeting of the Pediatric Academic Societies.
Although the manufacturers of these products voluntarily changed their labeling in 2007 to say “do not use” in children younger than 4 years old, the continued vulnerability of very young children and the high rate of self-administration suggests that labeling restrictions alone are “unlikely to have a significant impact” on the problem, he said. “What could be better is storage and [packaging] engineering controls to prevent the accidental ingestions that seem to represent the majority of cases,” Dr. Wang said in an interview.
The good news was that the 4,250 reported U.S. cases during 2008-2014 in children younger than age 12 years and judged by an expert review panel to be at least potentially related to cold and cough medications represents a significant decline, compared with earlier periods, and is also “quite low” when compared with the millions of units in annual U.S. sales.
“The overall adverse event rate compared with the volume sold is in the single digits per million of products sold, and the rate has been declining,” said Dr. Wang, a pediatric toxicologist at the University of Colorado in Denver and a consultant to the Rocky Mountain Poison & Drug Center, also in Denver, the group that maintains and reviews this registry, begun in January 2008. “I think we’re making progress,” but diligent education by physicians and other health care providers about the dangers posed by these drugs must continue, he said.
The analysis also identified that two drugs were by far the top culprits in causing pediatric adverse reactions to cough and cold medications, diphenhydramine and dextromethorphan. Diphenhydramine played a role in 53% of the 4,224 nonfatal adverse reaction cases and 54% of the 26 fatal cases identified by the registry panel as at least potentially related to a cough and cold medication, while dextromethorphan was responsible for 41% of the nonfatal and 19% of the fatal cases. In a majority of cases, these drugs were in products with a single active ingredient, although products with combined ingredients also played a role for some cases. Most often these drugs were in OTC formulations and in pediatric formulations.
Dr. Wang called it unlikely that manufacturers would formulate cold and cough medications without diphenhydramine or dextromethorphan because these drugs have the antitussive and sedative properties that consumers seek from cough and cold medications. He also noted that the addition of bittering agents to formulations have not had a history of reducing accidental self-administrations by children, but added “a good taste doesn’t help.”
During 2008-2014 U.S. surveillance by the registry review panel identified a total of 5,342 unique case reports of serious adverse events in children less than 12 years old and believed related to any of eight drugs commonly found in cold and cough medications. The reports came from any of five sources: the National Poison Data System, the Food and Drug Administration’s adverse event reporting system, safety reports to manufacturers, and through surveillance of the medical literature, and the news media. The panel winnowed these down to 4,250 cases at least potentially related to these drugs.
Among the 26 fatal cases, 16 (62%) occurred in children less than 2 years old and an additional four (15%) were in children aged 2 years to less than 4 years. Nine of these cases (35%) involved parental administration, with only two cases (8%) involving self-administration. An additional nine cases (35%) had no reported source of administration, and the remaining six (23%) cases involved other sources of administration. Seven of the 26 fatalities involved confirmed overdoses, with the dose unknown for the remaining 19 cases, Dr. Wang reported.
Among the 4,224 nonfatal cases, 15% occurred in children less than 2 years, 46% in children ages 2 years to less than 4 years, 19% in children 4 years to less than 6 years and 20% in children 6 years to less than 12 years. These cases involved a confirmed overdose in 73% of cases, a therapeutic range dose in 7%, with the remainder involving a dose of unknown size. Self-administration occurred 75% of the time.
On Twitter @mitchelzoler
BALTIMORE – The vast majority of reported U.S. episodes of cough and cold medication serious adverse event episodes in young children occurred by an accidental, self-administration overdose, according to a review of all pediatric episodes collected by the designated national surveillance system during 2008-2014.
This pattern highlights the continued need for diligent education of families about the potential danger posed by these largely OTC products as well as a possible additional need for further improvement in protective packaging, Dr. G. Sam Wang said at the annual meeting of the Pediatric Academic Societies.
Although the manufacturers of these products voluntarily changed their labeling in 2007 to say “do not use” in children younger than 4 years old, the continued vulnerability of very young children and the high rate of self-administration suggests that labeling restrictions alone are “unlikely to have a significant impact” on the problem, he said. “What could be better is storage and [packaging] engineering controls to prevent the accidental ingestions that seem to represent the majority of cases,” Dr. Wang said in an interview.
The good news was that the 4,250 reported U.S. cases during 2008-2014 in children younger than age 12 years and judged by an expert review panel to be at least potentially related to cold and cough medications represents a significant decline, compared with earlier periods, and is also “quite low” when compared with the millions of units in annual U.S. sales.
“The overall adverse event rate compared with the volume sold is in the single digits per million of products sold, and the rate has been declining,” said Dr. Wang, a pediatric toxicologist at the University of Colorado in Denver and a consultant to the Rocky Mountain Poison & Drug Center, also in Denver, the group that maintains and reviews this registry, begun in January 2008. “I think we’re making progress,” but diligent education by physicians and other health care providers about the dangers posed by these drugs must continue, he said.
The analysis also identified that two drugs were by far the top culprits in causing pediatric adverse reactions to cough and cold medications, diphenhydramine and dextromethorphan. Diphenhydramine played a role in 53% of the 4,224 nonfatal adverse reaction cases and 54% of the 26 fatal cases identified by the registry panel as at least potentially related to a cough and cold medication, while dextromethorphan was responsible for 41% of the nonfatal and 19% of the fatal cases. In a majority of cases, these drugs were in products with a single active ingredient, although products with combined ingredients also played a role for some cases. Most often these drugs were in OTC formulations and in pediatric formulations.
Dr. Wang called it unlikely that manufacturers would formulate cold and cough medications without diphenhydramine or dextromethorphan because these drugs have the antitussive and sedative properties that consumers seek from cough and cold medications. He also noted that the addition of bittering agents to formulations have not had a history of reducing accidental self-administrations by children, but added “a good taste doesn’t help.”
During 2008-2014 U.S. surveillance by the registry review panel identified a total of 5,342 unique case reports of serious adverse events in children less than 12 years old and believed related to any of eight drugs commonly found in cold and cough medications. The reports came from any of five sources: the National Poison Data System, the Food and Drug Administration’s adverse event reporting system, safety reports to manufacturers, and through surveillance of the medical literature, and the news media. The panel winnowed these down to 4,250 cases at least potentially related to these drugs.
Among the 26 fatal cases, 16 (62%) occurred in children less than 2 years old and an additional four (15%) were in children aged 2 years to less than 4 years. Nine of these cases (35%) involved parental administration, with only two cases (8%) involving self-administration. An additional nine cases (35%) had no reported source of administration, and the remaining six (23%) cases involved other sources of administration. Seven of the 26 fatalities involved confirmed overdoses, with the dose unknown for the remaining 19 cases, Dr. Wang reported.
Among the 4,224 nonfatal cases, 15% occurred in children less than 2 years, 46% in children ages 2 years to less than 4 years, 19% in children 4 years to less than 6 years and 20% in children 6 years to less than 12 years. These cases involved a confirmed overdose in 73% of cases, a therapeutic range dose in 7%, with the remainder involving a dose of unknown size. Self-administration occurred 75% of the time.
On Twitter @mitchelzoler
BALTIMORE – The vast majority of reported U.S. episodes of cough and cold medication serious adverse event episodes in young children occurred by an accidental, self-administration overdose, according to a review of all pediatric episodes collected by the designated national surveillance system during 2008-2014.
This pattern highlights the continued need for diligent education of families about the potential danger posed by these largely OTC products as well as a possible additional need for further improvement in protective packaging, Dr. G. Sam Wang said at the annual meeting of the Pediatric Academic Societies.
Although the manufacturers of these products voluntarily changed their labeling in 2007 to say “do not use” in children younger than 4 years old, the continued vulnerability of very young children and the high rate of self-administration suggests that labeling restrictions alone are “unlikely to have a significant impact” on the problem, he said. “What could be better is storage and [packaging] engineering controls to prevent the accidental ingestions that seem to represent the majority of cases,” Dr. Wang said in an interview.
The good news was that the 4,250 reported U.S. cases during 2008-2014 in children younger than age 12 years and judged by an expert review panel to be at least potentially related to cold and cough medications represents a significant decline, compared with earlier periods, and is also “quite low” when compared with the millions of units in annual U.S. sales.
“The overall adverse event rate compared with the volume sold is in the single digits per million of products sold, and the rate has been declining,” said Dr. Wang, a pediatric toxicologist at the University of Colorado in Denver and a consultant to the Rocky Mountain Poison & Drug Center, also in Denver, the group that maintains and reviews this registry, begun in January 2008. “I think we’re making progress,” but diligent education by physicians and other health care providers about the dangers posed by these drugs must continue, he said.
The analysis also identified that two drugs were by far the top culprits in causing pediatric adverse reactions to cough and cold medications, diphenhydramine and dextromethorphan. Diphenhydramine played a role in 53% of the 4,224 nonfatal adverse reaction cases and 54% of the 26 fatal cases identified by the registry panel as at least potentially related to a cough and cold medication, while dextromethorphan was responsible for 41% of the nonfatal and 19% of the fatal cases. In a majority of cases, these drugs were in products with a single active ingredient, although products with combined ingredients also played a role for some cases. Most often these drugs were in OTC formulations and in pediatric formulations.
Dr. Wang called it unlikely that manufacturers would formulate cold and cough medications without diphenhydramine or dextromethorphan because these drugs have the antitussive and sedative properties that consumers seek from cough and cold medications. He also noted that the addition of bittering agents to formulations have not had a history of reducing accidental self-administrations by children, but added “a good taste doesn’t help.”
During 2008-2014 U.S. surveillance by the registry review panel identified a total of 5,342 unique case reports of serious adverse events in children less than 12 years old and believed related to any of eight drugs commonly found in cold and cough medications. The reports came from any of five sources: the National Poison Data System, the Food and Drug Administration’s adverse event reporting system, safety reports to manufacturers, and through surveillance of the medical literature, and the news media. The panel winnowed these down to 4,250 cases at least potentially related to these drugs.
Among the 26 fatal cases, 16 (62%) occurred in children less than 2 years old and an additional four (15%) were in children aged 2 years to less than 4 years. Nine of these cases (35%) involved parental administration, with only two cases (8%) involving self-administration. An additional nine cases (35%) had no reported source of administration, and the remaining six (23%) cases involved other sources of administration. Seven of the 26 fatalities involved confirmed overdoses, with the dose unknown for the remaining 19 cases, Dr. Wang reported.
Among the 4,224 nonfatal cases, 15% occurred in children less than 2 years, 46% in children ages 2 years to less than 4 years, 19% in children 4 years to less than 6 years and 20% in children 6 years to less than 12 years. These cases involved a confirmed overdose in 73% of cases, a therapeutic range dose in 7%, with the remainder involving a dose of unknown size. Self-administration occurred 75% of the time.
On Twitter @mitchelzoler
AT THE PAS ANNUAL MEETING
Key clinical point: Serious adverse events in U.S. children caused by cough and cold medications most commonly occur from self-administration in children younger than 4 years old.
Major finding: Three-quarters of serious adverse events occurred by self-administration, with 61% of episodes in children younger than 4 years old.
Data source: Review of 5,342 reported U.S. cough and cold medication serious adverse event episodes in children during 2008-2014.
Disclosures: Dr. Wang had no disclosures.
ABMS approves new addiction medicine subspecialty
Many more physicians seeking to subspecialize in addiction medicine will now have the official blessing of the American Board of Medical Specialties.
ABMS announced March 14 its approval of an addiction medicine subspecialty that the American Board of Preventive Medicine (ABPM) will sponsor.
Physicians who are certified by any of the 24 ABMS member boards can apply for the addiction medicine certification. The American Board of Psychiatry and Neurology offers certification in addiction psychiatry, but only to psychiatrists.
ABPM hasn’t set a date for the addiction medicine subspecialty’s first board certification exam, which the board will develop. ABPM will post updates on its website, www.theabpm.org.
“Increasing the number of well-trained and certified specialists in addiction medicine will significantly increase access to care for those in need of intervention and treatment,” said ABPM’s board chair, Dr. Denece O. Kesler, in a statement.
One in seven Americans older than 12 years meets medical criteria for an addiction to nicotine, alcohol, or other drugs, according to statistics from the National Center on Addiction and Substance Abuse. But only 11% of those who need treatment are able to receive it, in part because of a lack of addiction medicine providers.
The American Board of Addiction Medicine (ABAM) hailed the new subspecialty. “This is a great day for addiction medicine,” Dr. Robert J. Sokol, president of ABAM and the Addiction Medicine Foundation (AMF), said in a statement. “This landmark event, more than any other, recognizes addiction as a preventable and treatable disease.”
ABAM has certified 3,902 physicians, according to the organization, which is not an ABMS member board. There are 40 AMF-sponsored fellowship training programs nationally, with a commitment to establish 125 more by 2025. AMF expects the ABMS recognition will lead to the fellowships gaining the imprimatur of the Accreditation Council on Graduate Medical Education.
“This is a positive development that has the potential to address a serious public health problem,” Dr. Daniel Lieberman, vice chairman of the psychiatry and behavioral health department at George Washington University, Washington, said in an interview. “This action will reassure doctors who are interested in addiction medicine that the time and effort they put into obtaining additional training will give them the status of a subspecialist with recognized expertise. It may also encourage young doctors to consider addiction medicine as a career path.”
Meanwhile, a package of mental health reforms moving in the U.S. Senate could improve patients’ access to addiction medicine providers. One of the bills, the TREAT Act, would increase the number of substance use detoxification patients that a qualified provider is legally allowed to treat annually, from 30 patients to 100 patients. The legislation also would allow those practitioners to request permission to annually treat unlimited numbers of patients thereafter.
On Twitter @whitneymcknight
Many more physicians seeking to subspecialize in addiction medicine will now have the official blessing of the American Board of Medical Specialties.
ABMS announced March 14 its approval of an addiction medicine subspecialty that the American Board of Preventive Medicine (ABPM) will sponsor.
Physicians who are certified by any of the 24 ABMS member boards can apply for the addiction medicine certification. The American Board of Psychiatry and Neurology offers certification in addiction psychiatry, but only to psychiatrists.
ABPM hasn’t set a date for the addiction medicine subspecialty’s first board certification exam, which the board will develop. ABPM will post updates on its website, www.theabpm.org.
“Increasing the number of well-trained and certified specialists in addiction medicine will significantly increase access to care for those in need of intervention and treatment,” said ABPM’s board chair, Dr. Denece O. Kesler, in a statement.
One in seven Americans older than 12 years meets medical criteria for an addiction to nicotine, alcohol, or other drugs, according to statistics from the National Center on Addiction and Substance Abuse. But only 11% of those who need treatment are able to receive it, in part because of a lack of addiction medicine providers.
The American Board of Addiction Medicine (ABAM) hailed the new subspecialty. “This is a great day for addiction medicine,” Dr. Robert J. Sokol, president of ABAM and the Addiction Medicine Foundation (AMF), said in a statement. “This landmark event, more than any other, recognizes addiction as a preventable and treatable disease.”
ABAM has certified 3,902 physicians, according to the organization, which is not an ABMS member board. There are 40 AMF-sponsored fellowship training programs nationally, with a commitment to establish 125 more by 2025. AMF expects the ABMS recognition will lead to the fellowships gaining the imprimatur of the Accreditation Council on Graduate Medical Education.
“This is a positive development that has the potential to address a serious public health problem,” Dr. Daniel Lieberman, vice chairman of the psychiatry and behavioral health department at George Washington University, Washington, said in an interview. “This action will reassure doctors who are interested in addiction medicine that the time and effort they put into obtaining additional training will give them the status of a subspecialist with recognized expertise. It may also encourage young doctors to consider addiction medicine as a career path.”
Meanwhile, a package of mental health reforms moving in the U.S. Senate could improve patients’ access to addiction medicine providers. One of the bills, the TREAT Act, would increase the number of substance use detoxification patients that a qualified provider is legally allowed to treat annually, from 30 patients to 100 patients. The legislation also would allow those practitioners to request permission to annually treat unlimited numbers of patients thereafter.
On Twitter @whitneymcknight
Many more physicians seeking to subspecialize in addiction medicine will now have the official blessing of the American Board of Medical Specialties.
ABMS announced March 14 its approval of an addiction medicine subspecialty that the American Board of Preventive Medicine (ABPM) will sponsor.
Physicians who are certified by any of the 24 ABMS member boards can apply for the addiction medicine certification. The American Board of Psychiatry and Neurology offers certification in addiction psychiatry, but only to psychiatrists.
ABPM hasn’t set a date for the addiction medicine subspecialty’s first board certification exam, which the board will develop. ABPM will post updates on its website, www.theabpm.org.
“Increasing the number of well-trained and certified specialists in addiction medicine will significantly increase access to care for those in need of intervention and treatment,” said ABPM’s board chair, Dr. Denece O. Kesler, in a statement.
One in seven Americans older than 12 years meets medical criteria for an addiction to nicotine, alcohol, or other drugs, according to statistics from the National Center on Addiction and Substance Abuse. But only 11% of those who need treatment are able to receive it, in part because of a lack of addiction medicine providers.
The American Board of Addiction Medicine (ABAM) hailed the new subspecialty. “This is a great day for addiction medicine,” Dr. Robert J. Sokol, president of ABAM and the Addiction Medicine Foundation (AMF), said in a statement. “This landmark event, more than any other, recognizes addiction as a preventable and treatable disease.”
ABAM has certified 3,902 physicians, according to the organization, which is not an ABMS member board. There are 40 AMF-sponsored fellowship training programs nationally, with a commitment to establish 125 more by 2025. AMF expects the ABMS recognition will lead to the fellowships gaining the imprimatur of the Accreditation Council on Graduate Medical Education.
“This is a positive development that has the potential to address a serious public health problem,” Dr. Daniel Lieberman, vice chairman of the psychiatry and behavioral health department at George Washington University, Washington, said in an interview. “This action will reassure doctors who are interested in addiction medicine that the time and effort they put into obtaining additional training will give them the status of a subspecialist with recognized expertise. It may also encourage young doctors to consider addiction medicine as a career path.”
Meanwhile, a package of mental health reforms moving in the U.S. Senate could improve patients’ access to addiction medicine providers. One of the bills, the TREAT Act, would increase the number of substance use detoxification patients that a qualified provider is legally allowed to treat annually, from 30 patients to 100 patients. The legislation also would allow those practitioners to request permission to annually treat unlimited numbers of patients thereafter.
On Twitter @whitneymcknight
New CDC opioid guideline targets overprescribing for chronic pain
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Nonopioid therapy is the preferred approach for managing chronic pain outside of active cancer, palliative, and end-of-life care, according to a new guideline released today by the Centers for Disease Control and Prevention.
The 12 recommendations included in the guideline center around this principle and two others: using the lowest possible effective dosage when opioids are used, and exercising caution and monitoring patients closely when prescribing opioids.
Specifically, the guideline states that “clinicians should consider opioid therapy only if expected benefits for both pain and function are anticipated to outweigh risks to the patient,” and that “treatment should be combined with nonpharmacologic and nonopioid therapy, as appropriate.”
The guideline also addresses steps to take before starting or continuing opioid therapy, and drug selection, dosage, duration, follow-up, and discontinuation. Recommendations for assessing risk and addressing harms of opioid use are also included.
The CDC developed the guideline as part of the U.S. government’s urgent response to the epidemic of overdose deaths, which has been fueled by a quadrupling of the prescribing and sales of opioids since 1999, according to a CDC press statement. The guideline’s purpose is to help prevent opioid misuse and overdose.
“The CDC Guideline for Prescribing Opioids for Chronic Pain, United States, 2016 will help primary care providers ensure the safest and most effective treatment for their patients,” according to the statement. The CDC’s director, Dr. Tom Frieden, noted that “overprescribing opioids – largely for chronic pain – is a key driver of America’s drug-overdose epidemic.”
In a CDC teleconference marking the release of the guideline, Dr. Frieden said it has become increasingly clear that opioids “carry substantial risks but only uncertain benefits, especially compared with other treatments for chronic pain.
“Beginning treatment with an opioid is a momentous decision, and it should only be done with full understanding by both the clinician and the patient of the substantial risks and uncertain benefits involved,” Dr. Frieden said. He added that he knows of no other medication “that’s routinely used for a nonfatal condition [and] that kills patients so frequently.
“With more than 250 million prescriptions written each year, it’s so important that doctors understand that any one of those prescriptions could potentially end a patient’s life,” he cautioned.
A 2015 study showed that 1 of every 550 patients treated with opioids for noncancer pain – and 1 of 32 who received the highest doses (more than 200 morphine milligram equivalents per day) – died within 2.5 years of the first prescription.
Dr. Frieden noted that opioids do have a place when the potential benefits outweigh the potential harms. “But for most patients – the vast majority of patients – the risks will outweigh the benefits,” he said.
The opioid epidemic is one of the most pressing public health issues in the United States today, said Sylvia M. Burwell, secretary of the Department of Health & Human Services. A year ago, she announced an HHS initiative to reduce prescription opioid and heroin-related drug overdose, death, and dependence.
“Last year, more Americans died from drug overdoses than car crashes,” Ms. Burwell said during the teleconference, noting that families across the nation and from all walks of life have been affected.
Combating the opioid epidemic is a national priority, she said, and the CDC guideline will help in that effort.
“We believe this guideline will help health care professionals provide safer and more effective care for patients dealing with chronic pain, and we also believe it will help these providers drive down the rates of opioid use disorder, overdose, and ... death,” she said.
The American Medical Association greeted the guideline with cautious support.
“While we are largely supportive of the guidelines, we remain concerned about the evidence base informing some of the recommendations,” noted Dr. Patrice A. Harris, chair-elect of the AMA board and chair of the AMA Task Force to Reduce Opioid Abuse, in a statement.
The AMA also cited potential conflicts between the guideline and product labeling and state laws, as well as obstacles such as insurance coverage limits on nonpharmacologic treatments.
“If these guidelines help reduce the deaths resulting from opioids, they will prove to be valuable,” Dr. Harris said in the statement. “If they produce unintended consequences, we will need to mitigate them.”
Of note, the guideline stresses the right of patients with chronic pain to receive safe and effective pain management, and focuses on giving primary care providers – who account for about half of all opioid prescriptions – a road map for providing such pain management by increasing the use of effective nonopioid and nonpharmacologic therapies.
It was developed through a “rigorous scientific process using the best available scientific evidence, consulting with experts, and listening to comments from the public and partner organizations,” according to the CDC statement. The organization “is dedicated to working with partners to improve the evidence base and will refine the recommendations as new research becomes available.
”In conjunction with the release of the guideline, the CDC has provided a checklist for prescribing opioids for chronic pain, and a website with additional tools for implementing the recommendations within the guideline.
The CDC's opioid recommendations
The Centers for Disease Control and Prevention’s new opioid prescription guideline includes 12 recommendations. Here they are, modified slightly for style:
1. Nonpharmacologic therapy and nonopioid pharmacologic therapy are preferred for chronic pain. Providers should only consider adding opioid therapy if expected benefits for both pain and function are anticipated to outweigh risks.
2. Before starting opioid therapy for chronic pain, providers should establish treatment goals with all patients, including realistic goals for pain and function. Providers should not initiate opioid therapy without consideration of how therapy will be discontinued if unsuccessful. Providers should continue opioid therapy only if there is clinically meaningful improvement in pain and function.
3. Before starting and periodically during opioid therapy, providers should discuss with patients known risks and realistic benefits of opioid therapy, and patient and provider responsibilities for managing therapy.
4. When starting opioid therapy for chronic pain, providers should prescribe immediate-release opioids instead of extended-release/long-acting opioids.
5. When opioids are started, providers should prescribe the lowest effective dosage. Providers should use caution when prescribing opioids at any dosage, should implement additional precautions when increasing dosage to 50 or more morphine milligram equivalents (MME) per day, and generally should avoid increasing dosage to 90 or more MME per day.
6. When opioids are used for acute pain, providers should prescribe the lowest effective dose of immediate-release opioids. Three or fewer days often will be sufficient.
7. Providers should evaluate the benefits and harms with patients within 1-4 weeks of starting opioid therapy for chronic pain or of dose escalation. They should reevaluate continued therapy’s benefits and harms every 3 months or more frequently. If continued therapy’s benefits do not outweigh harms, providers should work with patients to reduce dosages or discontinue opioids.
8. During therapy, providers should evaluate risk factors for opioid-related harm. Providers should incorporate into the management plan strategies to mitigate risk, including considering offering naloxone when factors that increase risk for opioid overdose – such as history of overdose, history of substance use disorder, or higher opioid dosage (50 MME or more) – are present.
9. Providers should review the patient’s history of controlled substance prescriptions using state prescription drug monitoring program (PDMP) data to determine whether the patient is receiving high opioid dosages or dangerous combinations that put him or her at high risk for overdose. Providers should review PDMP data when starting opioid therapy for chronic pain and periodically during opioid therapy for chronic pain, ranging from every prescription to every 3 months.
10. When prescribing opioids for chronic pain, providers should use urine drug testing before starting opioid therapy and consider urine drug testing at least annually to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs.
11. Providers should avoid concurrent prescriptions of opioid pain medication and benzodiazepines whenever possible.
12. Providers should offer or arrange evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies) for patients with opioid use disorder.
M. Alexander Otto contributed to this article.
Marijuana tourists also visiting Colorado EDs
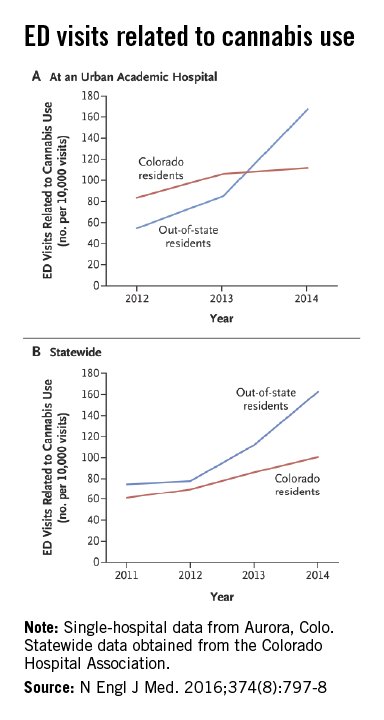
Out-of-state residents appear to be driving the recent increases in marijuana-related emergency department visits in Colorado, Dr. Howard S. Kim and his associates reported online Feb. 24 in the New England Journal of Medicine.
Using statewide data from the Colorado Hospital Association, they found that ED visits related to cannabis by out-of-state residents rose from 78 per 10,000 ED visits in 2012 to 163 per 10,000 in 2014, an increase of 109%. For Colorado residents, cannabis-related ED admissions over that same time period went up 44% – from 70 per 10,000 to 101, said Dr. Kim of Northwestern University, Chicago, and his associates (N Engl J Med. 2016 Feb 24;374[8]:797-8. doi:10.1056/NEJMc1515009).

The investigators also looked at a single urban academic hospital in Aurora, Colo., and found that cannabis-related ED visits there for out-of-state residents went from 85 per 10,000 visits in 2013 to 168 per 10,000 in 2014, compared with respective rates of 106 and 112 for Colorado residents.
“The flattening of the rates of ED visits possibly related to cannabis use among Colorado residents in an urban hospital may represent a learning curve during the period when marijuana was potentially available to Colorado residents for medical use (medical marijuana period) but was largely inaccessible to out-of-state residents,” they suggested.
Out-of-state residents appear to be driving the recent increases in marijuana-related emergency department visits in Colorado, Dr. Howard S. Kim and his associates reported online Feb. 24 in the New England Journal of Medicine.
Using statewide data from the Colorado Hospital Association, they found that ED visits related to cannabis by out-of-state residents rose from 78 per 10,000 ED visits in 2012 to 163 per 10,000 in 2014, an increase of 109%. For Colorado residents, cannabis-related ED admissions over that same time period went up 44% – from 70 per 10,000 to 101, said Dr. Kim of Northwestern University, Chicago, and his associates (N Engl J Med. 2016 Feb 24;374[8]:797-8. doi:10.1056/NEJMc1515009).

The investigators also looked at a single urban academic hospital in Aurora, Colo., and found that cannabis-related ED visits there for out-of-state residents went from 85 per 10,000 visits in 2013 to 168 per 10,000 in 2014, compared with respective rates of 106 and 112 for Colorado residents.
“The flattening of the rates of ED visits possibly related to cannabis use among Colorado residents in an urban hospital may represent a learning curve during the period when marijuana was potentially available to Colorado residents for medical use (medical marijuana period) but was largely inaccessible to out-of-state residents,” they suggested.
Out-of-state residents appear to be driving the recent increases in marijuana-related emergency department visits in Colorado, Dr. Howard S. Kim and his associates reported online Feb. 24 in the New England Journal of Medicine.
Using statewide data from the Colorado Hospital Association, they found that ED visits related to cannabis by out-of-state residents rose from 78 per 10,000 ED visits in 2012 to 163 per 10,000 in 2014, an increase of 109%. For Colorado residents, cannabis-related ED admissions over that same time period went up 44% – from 70 per 10,000 to 101, said Dr. Kim of Northwestern University, Chicago, and his associates (N Engl J Med. 2016 Feb 24;374[8]:797-8. doi:10.1056/NEJMc1515009).

The investigators also looked at a single urban academic hospital in Aurora, Colo., and found that cannabis-related ED visits there for out-of-state residents went from 85 per 10,000 visits in 2013 to 168 per 10,000 in 2014, compared with respective rates of 106 and 112 for Colorado residents.
“The flattening of the rates of ED visits possibly related to cannabis use among Colorado residents in an urban hospital may represent a learning curve during the period when marijuana was potentially available to Colorado residents for medical use (medical marijuana period) but was largely inaccessible to out-of-state residents,” they suggested.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Designer drug symptoms can mimic schizophrenia, anxiety, depression
LAS VEGAS – People who use spice, bath salts, and other so-called designer drugs may present with symptoms that resemble numerous psychiatric conditions, including schizophrenia, anxiety disorders, and depression.
“Given the recent emergence of designer drugs, the long-term consequences of their use have not been extensively studied and are relatively unknown,” Dr. William M. Sauve said at the annual psychopharmacology update held by the Nevada Psychiatric Association.
Dr. Sauve, medical director of TMS NeuroHealth Centers of Richmond and Charlottesville, both in Virginia, said designer drugs have grown in popularity in recent years because they are perceived as legal alternatives to illicit substances. In addition, their detection by standard drug toxicology screens is limited.
In October 2011, components of designer drugs, including synthetic cannabinoids and the major constituents of bath salts, were categorized as emergency Schedule I substances. In July 2012, President Obama signed the Synthetic Drug Abuse Prevention Act, which doubled the time that a substance may be temporarily assigned to Schedule I, from 18 months to 36 months.
“Under federal law, any chemical that is similar to a classified drug and is meant to be used for the same purposes is considered to be classified,” Dr. Sauve said. However, designer drugs “get labeled ‘not for human consumption’ and can be sold out in the open and camouflaged under names such as ‘stain remover,’ ‘research chemicals,’ and even ‘insect repellent.’ That’s why it’s very difficult for the law to catch up with these things. Active ingredients are also a moving target.”
He discussed three types of these designer drugs: synthetic cannabinoids, bath salts, and krokodil.
Synthetic cannabinoids mimic THC
Also known as spice, K2, and incense, these substances began to appear in the United States in 2008 and are mostly used by males. Primarily inhaled, these substances are meant to mimic the effects of tetrahydrocannabinol (THC). They work by decreasing levels of gamma-aminobutyric acid (GABA) and by increasing levels of glutamate and dopamine. “Serotonin levels can also be affected indirectly by endocannabinoid control of GABA and glutamate release,” he added.
Unlike marijuana, which is a partial agonist at the cannabinoid 1 (CB-1) receptor, synthetics are full agonists at the CB-1 receptor, “so as you use it, it will hit every receptor until you have maximal stimulation, and it may have 800 times greater affinity than THC,” he said. Signs and symptoms of acute intoxication can be wide ranging, from agitation and dysphoria to paranoia and tachycardia, and can last up to 6 hours. While commercial tests are available to detect synthetic cannabinoid metabolites, formulations change so often that “most tests quickly become obsolete,” Dr. Sauve said. He noted that intoxication with spice should be suspected in patients who present with bizarre behavior, anxiety, agitation, and/or psychosis in those with no known psychiatric history. Intravaneous benzodiazepines can be used for agitation and seizures. While knowledge of their long-term impact is lacking, synthetic cannabinoids may increase the risk of subsequent psychosis by threefold, he said, and kidney failure has been reported in several cases.
Bath salts widely available
Also labeled as “plant food,” “pond water cleaner,” “novelty collector’s items,” and “not for human consumption,” these stimulants began to be used in the United States in 2010, and are widely available online and in smoke shops. Users have a median age of 26 years, Dr. Sauve said, and are mostly male.
Bath salts may be comprised of methcathinones, especially synthetic cathinones. Natural cathinones are found in khat, a root from a shrub that is chewed upon primarily by people in North Africa. Bath salts also may contain methamphetamine analogues, which can be synthesized from ephedrine and pseudoephedrine. These include methylone (similar to MDMA, or ecstasy), mephedrone (similar to methamphetamine), and methylenedioxypyrovalerone (similar to cocaine). Bath salts can be inhaled, injected, snorted, swallowed, or inserted into the rectum or vagina, and effects occur in doses of 2-5 mg. Pharmacological effects vary and may include increased plasma norepinephrine, sympathetic effects, serotonin syndrome, and increased dopamine. He also noted that the transition from recreational to addictive use “may occur in a matter of days.”
Signs of toxicity with bath salts, Dr. Sauve continued, include the following: disorientation and agitation; dilated pupils with involuntary eye movements; lockjaw and teeth grinding; rapid, inappropriate, incoherent speech; being emotionally, verbally, or physically abusive, and having elevated liver enzymes and/or liver failure.
Treatment is primarily supportive and may include sedatives for anxiety, agitation, aggression, tremors, seizures, and psychosis. Physical restraints may be necessary.
Krokodil not seen much in U.S.
Formally known as desomorphine, this substance is synthesized from codeine and became popular in Russia after a crackdown on heroin there in 2010, Dr. Sauve said. The ingredients for krokodil synthesis include tablets containing codeine, caustic soda, gasoline, hydrochloric acid, iodine from disinfectants, and red phosphorus from matchboxes. While desomorphine is believed to be highly addictive, “all the other sequelae of krokodil are generally thought to be a result of phosphorus” and other substances. No good data exist in the prevalence of its use, he said. “We’re not really seeing this much in the United States, because it’s way too easy to get Oxycontin and heroin [here].”
Dr. Sauve reported that he is a consultant to Avanir Pharmaceuticals and Otsuka Pharmaceutical. He also reported being a member of the speakers bureau or receiving honoraria from Avanir Pharmaceuticals, Otsuka Pharmaceutical, and Sunovion Pharmaceuticals.
LAS VEGAS – People who use spice, bath salts, and other so-called designer drugs may present with symptoms that resemble numerous psychiatric conditions, including schizophrenia, anxiety disorders, and depression.
“Given the recent emergence of designer drugs, the long-term consequences of their use have not been extensively studied and are relatively unknown,” Dr. William M. Sauve said at the annual psychopharmacology update held by the Nevada Psychiatric Association.
Dr. Sauve, medical director of TMS NeuroHealth Centers of Richmond and Charlottesville, both in Virginia, said designer drugs have grown in popularity in recent years because they are perceived as legal alternatives to illicit substances. In addition, their detection by standard drug toxicology screens is limited.
In October 2011, components of designer drugs, including synthetic cannabinoids and the major constituents of bath salts, were categorized as emergency Schedule I substances. In July 2012, President Obama signed the Synthetic Drug Abuse Prevention Act, which doubled the time that a substance may be temporarily assigned to Schedule I, from 18 months to 36 months.
“Under federal law, any chemical that is similar to a classified drug and is meant to be used for the same purposes is considered to be classified,” Dr. Sauve said. However, designer drugs “get labeled ‘not for human consumption’ and can be sold out in the open and camouflaged under names such as ‘stain remover,’ ‘research chemicals,’ and even ‘insect repellent.’ That’s why it’s very difficult for the law to catch up with these things. Active ingredients are also a moving target.”
He discussed three types of these designer drugs: synthetic cannabinoids, bath salts, and krokodil.
Synthetic cannabinoids mimic THC
Also known as spice, K2, and incense, these substances began to appear in the United States in 2008 and are mostly used by males. Primarily inhaled, these substances are meant to mimic the effects of tetrahydrocannabinol (THC). They work by decreasing levels of gamma-aminobutyric acid (GABA) and by increasing levels of glutamate and dopamine. “Serotonin levels can also be affected indirectly by endocannabinoid control of GABA and glutamate release,” he added.
Unlike marijuana, which is a partial agonist at the cannabinoid 1 (CB-1) receptor, synthetics are full agonists at the CB-1 receptor, “so as you use it, it will hit every receptor until you have maximal stimulation, and it may have 800 times greater affinity than THC,” he said. Signs and symptoms of acute intoxication can be wide ranging, from agitation and dysphoria to paranoia and tachycardia, and can last up to 6 hours. While commercial tests are available to detect synthetic cannabinoid metabolites, formulations change so often that “most tests quickly become obsolete,” Dr. Sauve said. He noted that intoxication with spice should be suspected in patients who present with bizarre behavior, anxiety, agitation, and/or psychosis in those with no known psychiatric history. Intravaneous benzodiazepines can be used for agitation and seizures. While knowledge of their long-term impact is lacking, synthetic cannabinoids may increase the risk of subsequent psychosis by threefold, he said, and kidney failure has been reported in several cases.
Bath salts widely available
Also labeled as “plant food,” “pond water cleaner,” “novelty collector’s items,” and “not for human consumption,” these stimulants began to be used in the United States in 2010, and are widely available online and in smoke shops. Users have a median age of 26 years, Dr. Sauve said, and are mostly male.
Bath salts may be comprised of methcathinones, especially synthetic cathinones. Natural cathinones are found in khat, a root from a shrub that is chewed upon primarily by people in North Africa. Bath salts also may contain methamphetamine analogues, which can be synthesized from ephedrine and pseudoephedrine. These include methylone (similar to MDMA, or ecstasy), mephedrone (similar to methamphetamine), and methylenedioxypyrovalerone (similar to cocaine). Bath salts can be inhaled, injected, snorted, swallowed, or inserted into the rectum or vagina, and effects occur in doses of 2-5 mg. Pharmacological effects vary and may include increased plasma norepinephrine, sympathetic effects, serotonin syndrome, and increased dopamine. He also noted that the transition from recreational to addictive use “may occur in a matter of days.”
Signs of toxicity with bath salts, Dr. Sauve continued, include the following: disorientation and agitation; dilated pupils with involuntary eye movements; lockjaw and teeth grinding; rapid, inappropriate, incoherent speech; being emotionally, verbally, or physically abusive, and having elevated liver enzymes and/or liver failure.
Treatment is primarily supportive and may include sedatives for anxiety, agitation, aggression, tremors, seizures, and psychosis. Physical restraints may be necessary.
Krokodil not seen much in U.S.
Formally known as desomorphine, this substance is synthesized from codeine and became popular in Russia after a crackdown on heroin there in 2010, Dr. Sauve said. The ingredients for krokodil synthesis include tablets containing codeine, caustic soda, gasoline, hydrochloric acid, iodine from disinfectants, and red phosphorus from matchboxes. While desomorphine is believed to be highly addictive, “all the other sequelae of krokodil are generally thought to be a result of phosphorus” and other substances. No good data exist in the prevalence of its use, he said. “We’re not really seeing this much in the United States, because it’s way too easy to get Oxycontin and heroin [here].”
Dr. Sauve reported that he is a consultant to Avanir Pharmaceuticals and Otsuka Pharmaceutical. He also reported being a member of the speakers bureau or receiving honoraria from Avanir Pharmaceuticals, Otsuka Pharmaceutical, and Sunovion Pharmaceuticals.
LAS VEGAS – People who use spice, bath salts, and other so-called designer drugs may present with symptoms that resemble numerous psychiatric conditions, including schizophrenia, anxiety disorders, and depression.
“Given the recent emergence of designer drugs, the long-term consequences of their use have not been extensively studied and are relatively unknown,” Dr. William M. Sauve said at the annual psychopharmacology update held by the Nevada Psychiatric Association.
Dr. Sauve, medical director of TMS NeuroHealth Centers of Richmond and Charlottesville, both in Virginia, said designer drugs have grown in popularity in recent years because they are perceived as legal alternatives to illicit substances. In addition, their detection by standard drug toxicology screens is limited.
In October 2011, components of designer drugs, including synthetic cannabinoids and the major constituents of bath salts, were categorized as emergency Schedule I substances. In July 2012, President Obama signed the Synthetic Drug Abuse Prevention Act, which doubled the time that a substance may be temporarily assigned to Schedule I, from 18 months to 36 months.
“Under federal law, any chemical that is similar to a classified drug and is meant to be used for the same purposes is considered to be classified,” Dr. Sauve said. However, designer drugs “get labeled ‘not for human consumption’ and can be sold out in the open and camouflaged under names such as ‘stain remover,’ ‘research chemicals,’ and even ‘insect repellent.’ That’s why it’s very difficult for the law to catch up with these things. Active ingredients are also a moving target.”
He discussed three types of these designer drugs: synthetic cannabinoids, bath salts, and krokodil.
Synthetic cannabinoids mimic THC
Also known as spice, K2, and incense, these substances began to appear in the United States in 2008 and are mostly used by males. Primarily inhaled, these substances are meant to mimic the effects of tetrahydrocannabinol (THC). They work by decreasing levels of gamma-aminobutyric acid (GABA) and by increasing levels of glutamate and dopamine. “Serotonin levels can also be affected indirectly by endocannabinoid control of GABA and glutamate release,” he added.
Unlike marijuana, which is a partial agonist at the cannabinoid 1 (CB-1) receptor, synthetics are full agonists at the CB-1 receptor, “so as you use it, it will hit every receptor until you have maximal stimulation, and it may have 800 times greater affinity than THC,” he said. Signs and symptoms of acute intoxication can be wide ranging, from agitation and dysphoria to paranoia and tachycardia, and can last up to 6 hours. While commercial tests are available to detect synthetic cannabinoid metabolites, formulations change so often that “most tests quickly become obsolete,” Dr. Sauve said. He noted that intoxication with spice should be suspected in patients who present with bizarre behavior, anxiety, agitation, and/or psychosis in those with no known psychiatric history. Intravaneous benzodiazepines can be used for agitation and seizures. While knowledge of their long-term impact is lacking, synthetic cannabinoids may increase the risk of subsequent psychosis by threefold, he said, and kidney failure has been reported in several cases.
Bath salts widely available
Also labeled as “plant food,” “pond water cleaner,” “novelty collector’s items,” and “not for human consumption,” these stimulants began to be used in the United States in 2010, and are widely available online and in smoke shops. Users have a median age of 26 years, Dr. Sauve said, and are mostly male.
Bath salts may be comprised of methcathinones, especially synthetic cathinones. Natural cathinones are found in khat, a root from a shrub that is chewed upon primarily by people in North Africa. Bath salts also may contain methamphetamine analogues, which can be synthesized from ephedrine and pseudoephedrine. These include methylone (similar to MDMA, or ecstasy), mephedrone (similar to methamphetamine), and methylenedioxypyrovalerone (similar to cocaine). Bath salts can be inhaled, injected, snorted, swallowed, or inserted into the rectum or vagina, and effects occur in doses of 2-5 mg. Pharmacological effects vary and may include increased plasma norepinephrine, sympathetic effects, serotonin syndrome, and increased dopamine. He also noted that the transition from recreational to addictive use “may occur in a matter of days.”
Signs of toxicity with bath salts, Dr. Sauve continued, include the following: disorientation and agitation; dilated pupils with involuntary eye movements; lockjaw and teeth grinding; rapid, inappropriate, incoherent speech; being emotionally, verbally, or physically abusive, and having elevated liver enzymes and/or liver failure.
Treatment is primarily supportive and may include sedatives for anxiety, agitation, aggression, tremors, seizures, and psychosis. Physical restraints may be necessary.
Krokodil not seen much in U.S.
Formally known as desomorphine, this substance is synthesized from codeine and became popular in Russia after a crackdown on heroin there in 2010, Dr. Sauve said. The ingredients for krokodil synthesis include tablets containing codeine, caustic soda, gasoline, hydrochloric acid, iodine from disinfectants, and red phosphorus from matchboxes. While desomorphine is believed to be highly addictive, “all the other sequelae of krokodil are generally thought to be a result of phosphorus” and other substances. No good data exist in the prevalence of its use, he said. “We’re not really seeing this much in the United States, because it’s way too easy to get Oxycontin and heroin [here].”
Dr. Sauve reported that he is a consultant to Avanir Pharmaceuticals and Otsuka Pharmaceutical. He also reported being a member of the speakers bureau or receiving honoraria from Avanir Pharmaceuticals, Otsuka Pharmaceutical, and Sunovion Pharmaceuticals.
EXPERT ANALYSIS AT THE NPA PSYCHOPHARMACOLOGY UPDATE
Case Studies In Toxicology: Withdrawal: Another Danger of Diversion
Case
A 34-year-old man with a history of polysubstance abuse presented to the ED after he had a seizure during his regular methadone-treatment program meeting. While at the clinic, attendees witnessed the patient experience a loss of consciousness accompanied by generalized shaking movements of his extremities, which lasted for several minutes.
Upon arrival in the ED, the patient stated that he had a mild headache; he was otherwise asymptomatic. Initial vital signs were: blood pressure, 126/80 mm Hg; heart rate, 82 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97.3°F. Oxygen saturation was 98% on room air, and a finger-stick glucose test was 140 mg/dL.
Physical examination revealed a small right-sided parietal hematoma. The patient had no tremors and his neurological examination, including mental status, was normal. When reviewing the patient’s medical history and medications in the health record, it was noted that the patient had a prescription for alprazolam for an anxiety disorder. On further questioning, the patient admitted that he had sold his last alprazolam prescription and had not been taking the drug for the past week.
What characterizes the benzodiazepine withdrawal syndrome?
Although introduced into clinical practice in the 1960s, the potential for dependence and a withdrawal syndrome was not appreciated until the early 1980s. This clinical syndrome can manifest with a wide variety of findings, most commonly with what are termed “rebound effects” or “rebound hyperexcitability.” These effects include anxiety, insomnia or sleep disturbance, tremulousness, irritability, sweating, psychomotor agitation, difficulty in concentration, nausea, weight loss, palpitations, headache, muscular pain and stiffness, or generalized weakness.2 More severe manifestations include delirium, seizures, or psychosis. Often, these symptoms and signs may be confused with the very manifestations that prompted the initial use of the BZD, a reemergence of which can exacerbate the withdrawal syndrome.
When does benzodiazepine withdrawal occur?
The exact time course of BZD withdrawal can vary considerably and, unlike alcohol withdrawal (which occurs from a single compound, ethanol), can be difficult to characterize. The onset of withdrawal symptoms is dependent on a number of factors, including the half-life of the BZD involved. For example, delayed onset withdrawal symptoms of up to 3 weeks after cessation of the medication are described with long-acting BZDs such as chlordiazepoxide and diazepam. Conversely, symptoms may present as early as 24 to 48 hours after abrupt termination of BZDs with shorter half-lives, alprazolam and lorazepam. This variable time of onset differs considerably from other withdrawal syndromes, notably ethanol withdrawal. While both syndromes correlate to the individual patient’s severity of dependence, alcohol withdrawal follows a more predictable time course.
Some authors distinguish a rebound syndrome from a true withdrawal syndrome, the former of which is self-limited in nature and the result of cessation of treatment for the primary disease process. In this model, rebound symptoms begin 1 to 4 days after the abrupt cessation or dose reduction of the BZD, and are relatively short-lived, lasting 2 to 3 days.2
What is the appropriate treatment for benzodiazepine withdrawal?
The standard therapy for almost all withdrawal syndromes is reinstitution of the causal agent. A number of non-BZD-based treatment strategies have been investigated, and all have met with limited success. Of these, anticonvulsant drugs such as carbamazepine and valproic acid were initially considered promising based on case reports and small case series.4 These medications ultimately proved ineffective in randomized, placebo-controlled studies.5 β-Adrenergic antagonists, such as propranolol, have been studied as a method to normalize a patient’s vital signs but also proved nonbeneficial in managing withdrawal.5,6
The safest and most effective management approach for patients with BZD withdrawal is reinstitution of the BZD followed by a prolonged and gradual tapering until cessation, if that is desired.1,2,5,6 While all BZDs share structural and mechanistic similarities, there are subtle variations within this class that can affect their pharmacologic effects. These structural differences may result in incomplete cross-tolerance, which may lead to inadequate mitigation of the withdrawal syndrome. For example, previous reports suggest that alprazolam and clonazepam are structurally unique and bind to the BZD receptor with higher affinity than other BZDs. Therefore, while in general any BZD can be used to treat withdrawal from another BZD, it is recommended to treat withdrawal from these two agents with the implicated BZD.
There are, however, limitations to this approach. Namely, some BZDs are only available in oral formulations (eg, alprazolam and clonazepam) or the BZD of choice may not be readily available or on formulary within a given institution. In a patient with a severe withdrawal syndrome where it is not feasible or potentially harmful to administer an oral medication, it is reasonable to provide parenteral (preferably intravenous [IV]) BZD therapy. The optimal approach is to start with a small “standard” dose and titrate to effect while monitoring for adverse effects (eg, oversedation, ventilatory depression). Redosing should be triggered by symptoms or signs, and not performed in a timed or standing-order fashion. If this approach proves ineffective and withdrawal symptoms persist despite adequate BZD therapy, a direct GABA agonist such as propofol is a sensible alternative or adjuvant treatment. This may sound similar to the management of patients with ethanol withdrawal; indeed, this approach is essentially the same, with the exception of the more drawn-out time course.
Case Conclusion
After arrival in the ED, the patient received diazepam 10 mg IV and was subsequently admitted to the hospital for further evaluation. During his hospitalization, the patient was re-started on his usual dose of oral alprazolam. No further withdrawal syndrome was observed, and he was discharged on hospital day 2 with a plan to slowly taper his alprazolam dose with his outpatient psychiatrist.
Dr Repplinger is a senior medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Withdrawal: Another Danger of Diversion
- Marriott S, Tyrer P. Benzodiazepine dependence. Avoidance and withdrawal. Drug Saf. 1993;9(2):93-103.
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
- Authier N, Balayssac D, Sautereau M, et al. Benzodiazepine dependence: focus on withdrawal syndrome. Ann Pharm Fr. 2009;67(6):408-413.
- Pages KP, Ries RK. Use of anticonvulsants in benzodiazepine withdrawal. Am J Addict. 1998;7(3):198-204.
- Ashton H. The treatment of benzodiazepine dependence. Addiction. 1994;89(11):1535-1541.
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13-24.
Case
A 34-year-old man with a history of polysubstance abuse presented to the ED after he had a seizure during his regular methadone-treatment program meeting. While at the clinic, attendees witnessed the patient experience a loss of consciousness accompanied by generalized shaking movements of his extremities, which lasted for several minutes.
Upon arrival in the ED, the patient stated that he had a mild headache; he was otherwise asymptomatic. Initial vital signs were: blood pressure, 126/80 mm Hg; heart rate, 82 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97.3°F. Oxygen saturation was 98% on room air, and a finger-stick glucose test was 140 mg/dL.
Physical examination revealed a small right-sided parietal hematoma. The patient had no tremors and his neurological examination, including mental status, was normal. When reviewing the patient’s medical history and medications in the health record, it was noted that the patient had a prescription for alprazolam for an anxiety disorder. On further questioning, the patient admitted that he had sold his last alprazolam prescription and had not been taking the drug for the past week.
What characterizes the benzodiazepine withdrawal syndrome?
Although introduced into clinical practice in the 1960s, the potential for dependence and a withdrawal syndrome was not appreciated until the early 1980s. This clinical syndrome can manifest with a wide variety of findings, most commonly with what are termed “rebound effects” or “rebound hyperexcitability.” These effects include anxiety, insomnia or sleep disturbance, tremulousness, irritability, sweating, psychomotor agitation, difficulty in concentration, nausea, weight loss, palpitations, headache, muscular pain and stiffness, or generalized weakness.2 More severe manifestations include delirium, seizures, or psychosis. Often, these symptoms and signs may be confused with the very manifestations that prompted the initial use of the BZD, a reemergence of which can exacerbate the withdrawal syndrome.
When does benzodiazepine withdrawal occur?
The exact time course of BZD withdrawal can vary considerably and, unlike alcohol withdrawal (which occurs from a single compound, ethanol), can be difficult to characterize. The onset of withdrawal symptoms is dependent on a number of factors, including the half-life of the BZD involved. For example, delayed onset withdrawal symptoms of up to 3 weeks after cessation of the medication are described with long-acting BZDs such as chlordiazepoxide and diazepam. Conversely, symptoms may present as early as 24 to 48 hours after abrupt termination of BZDs with shorter half-lives, alprazolam and lorazepam. This variable time of onset differs considerably from other withdrawal syndromes, notably ethanol withdrawal. While both syndromes correlate to the individual patient’s severity of dependence, alcohol withdrawal follows a more predictable time course.
Some authors distinguish a rebound syndrome from a true withdrawal syndrome, the former of which is self-limited in nature and the result of cessation of treatment for the primary disease process. In this model, rebound symptoms begin 1 to 4 days after the abrupt cessation or dose reduction of the BZD, and are relatively short-lived, lasting 2 to 3 days.2
What is the appropriate treatment for benzodiazepine withdrawal?
The standard therapy for almost all withdrawal syndromes is reinstitution of the causal agent. A number of non-BZD-based treatment strategies have been investigated, and all have met with limited success. Of these, anticonvulsant drugs such as carbamazepine and valproic acid were initially considered promising based on case reports and small case series.4 These medications ultimately proved ineffective in randomized, placebo-controlled studies.5 β-Adrenergic antagonists, such as propranolol, have been studied as a method to normalize a patient’s vital signs but also proved nonbeneficial in managing withdrawal.5,6
The safest and most effective management approach for patients with BZD withdrawal is reinstitution of the BZD followed by a prolonged and gradual tapering until cessation, if that is desired.1,2,5,6 While all BZDs share structural and mechanistic similarities, there are subtle variations within this class that can affect their pharmacologic effects. These structural differences may result in incomplete cross-tolerance, which may lead to inadequate mitigation of the withdrawal syndrome. For example, previous reports suggest that alprazolam and clonazepam are structurally unique and bind to the BZD receptor with higher affinity than other BZDs. Therefore, while in general any BZD can be used to treat withdrawal from another BZD, it is recommended to treat withdrawal from these two agents with the implicated BZD.
There are, however, limitations to this approach. Namely, some BZDs are only available in oral formulations (eg, alprazolam and clonazepam) or the BZD of choice may not be readily available or on formulary within a given institution. In a patient with a severe withdrawal syndrome where it is not feasible or potentially harmful to administer an oral medication, it is reasonable to provide parenteral (preferably intravenous [IV]) BZD therapy. The optimal approach is to start with a small “standard” dose and titrate to effect while monitoring for adverse effects (eg, oversedation, ventilatory depression). Redosing should be triggered by symptoms or signs, and not performed in a timed or standing-order fashion. If this approach proves ineffective and withdrawal symptoms persist despite adequate BZD therapy, a direct GABA agonist such as propofol is a sensible alternative or adjuvant treatment. This may sound similar to the management of patients with ethanol withdrawal; indeed, this approach is essentially the same, with the exception of the more drawn-out time course.
Case Conclusion
After arrival in the ED, the patient received diazepam 10 mg IV and was subsequently admitted to the hospital for further evaluation. During his hospitalization, the patient was re-started on his usual dose of oral alprazolam. No further withdrawal syndrome was observed, and he was discharged on hospital day 2 with a plan to slowly taper his alprazolam dose with his outpatient psychiatrist.
Dr Repplinger is a senior medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 34-year-old man with a history of polysubstance abuse presented to the ED after he had a seizure during his regular methadone-treatment program meeting. While at the clinic, attendees witnessed the patient experience a loss of consciousness accompanied by generalized shaking movements of his extremities, which lasted for several minutes.
Upon arrival in the ED, the patient stated that he had a mild headache; he was otherwise asymptomatic. Initial vital signs were: blood pressure, 126/80 mm Hg; heart rate, 82 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97.3°F. Oxygen saturation was 98% on room air, and a finger-stick glucose test was 140 mg/dL.
Physical examination revealed a small right-sided parietal hematoma. The patient had no tremors and his neurological examination, including mental status, was normal. When reviewing the patient’s medical history and medications in the health record, it was noted that the patient had a prescription for alprazolam for an anxiety disorder. On further questioning, the patient admitted that he had sold his last alprazolam prescription and had not been taking the drug for the past week.
What characterizes the benzodiazepine withdrawal syndrome?
Although introduced into clinical practice in the 1960s, the potential for dependence and a withdrawal syndrome was not appreciated until the early 1980s. This clinical syndrome can manifest with a wide variety of findings, most commonly with what are termed “rebound effects” or “rebound hyperexcitability.” These effects include anxiety, insomnia or sleep disturbance, tremulousness, irritability, sweating, psychomotor agitation, difficulty in concentration, nausea, weight loss, palpitations, headache, muscular pain and stiffness, or generalized weakness.2 More severe manifestations include delirium, seizures, or psychosis. Often, these symptoms and signs may be confused with the very manifestations that prompted the initial use of the BZD, a reemergence of which can exacerbate the withdrawal syndrome.
When does benzodiazepine withdrawal occur?
The exact time course of BZD withdrawal can vary considerably and, unlike alcohol withdrawal (which occurs from a single compound, ethanol), can be difficult to characterize. The onset of withdrawal symptoms is dependent on a number of factors, including the half-life of the BZD involved. For example, delayed onset withdrawal symptoms of up to 3 weeks after cessation of the medication are described with long-acting BZDs such as chlordiazepoxide and diazepam. Conversely, symptoms may present as early as 24 to 48 hours after abrupt termination of BZDs with shorter half-lives, alprazolam and lorazepam. This variable time of onset differs considerably from other withdrawal syndromes, notably ethanol withdrawal. While both syndromes correlate to the individual patient’s severity of dependence, alcohol withdrawal follows a more predictable time course.
Some authors distinguish a rebound syndrome from a true withdrawal syndrome, the former of which is self-limited in nature and the result of cessation of treatment for the primary disease process. In this model, rebound symptoms begin 1 to 4 days after the abrupt cessation or dose reduction of the BZD, and are relatively short-lived, lasting 2 to 3 days.2
What is the appropriate treatment for benzodiazepine withdrawal?
The standard therapy for almost all withdrawal syndromes is reinstitution of the causal agent. A number of non-BZD-based treatment strategies have been investigated, and all have met with limited success. Of these, anticonvulsant drugs such as carbamazepine and valproic acid were initially considered promising based on case reports and small case series.4 These medications ultimately proved ineffective in randomized, placebo-controlled studies.5 β-Adrenergic antagonists, such as propranolol, have been studied as a method to normalize a patient’s vital signs but also proved nonbeneficial in managing withdrawal.5,6
The safest and most effective management approach for patients with BZD withdrawal is reinstitution of the BZD followed by a prolonged and gradual tapering until cessation, if that is desired.1,2,5,6 While all BZDs share structural and mechanistic similarities, there are subtle variations within this class that can affect their pharmacologic effects. These structural differences may result in incomplete cross-tolerance, which may lead to inadequate mitigation of the withdrawal syndrome. For example, previous reports suggest that alprazolam and clonazepam are structurally unique and bind to the BZD receptor with higher affinity than other BZDs. Therefore, while in general any BZD can be used to treat withdrawal from another BZD, it is recommended to treat withdrawal from these two agents with the implicated BZD.
There are, however, limitations to this approach. Namely, some BZDs are only available in oral formulations (eg, alprazolam and clonazepam) or the BZD of choice may not be readily available or on formulary within a given institution. In a patient with a severe withdrawal syndrome where it is not feasible or potentially harmful to administer an oral medication, it is reasonable to provide parenteral (preferably intravenous [IV]) BZD therapy. The optimal approach is to start with a small “standard” dose and titrate to effect while monitoring for adverse effects (eg, oversedation, ventilatory depression). Redosing should be triggered by symptoms or signs, and not performed in a timed or standing-order fashion. If this approach proves ineffective and withdrawal symptoms persist despite adequate BZD therapy, a direct GABA agonist such as propofol is a sensible alternative or adjuvant treatment. This may sound similar to the management of patients with ethanol withdrawal; indeed, this approach is essentially the same, with the exception of the more drawn-out time course.
Case Conclusion
After arrival in the ED, the patient received diazepam 10 mg IV and was subsequently admitted to the hospital for further evaluation. During his hospitalization, the patient was re-started on his usual dose of oral alprazolam. No further withdrawal syndrome was observed, and he was discharged on hospital day 2 with a plan to slowly taper his alprazolam dose with his outpatient psychiatrist.
Dr Repplinger is a senior medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Withdrawal: Another Danger of Diversion
- Marriott S, Tyrer P. Benzodiazepine dependence. Avoidance and withdrawal. Drug Saf. 1993;9(2):93-103.
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
- Authier N, Balayssac D, Sautereau M, et al. Benzodiazepine dependence: focus on withdrawal syndrome. Ann Pharm Fr. 2009;67(6):408-413.
- Pages KP, Ries RK. Use of anticonvulsants in benzodiazepine withdrawal. Am J Addict. 1998;7(3):198-204.
- Ashton H. The treatment of benzodiazepine dependence. Addiction. 1994;89(11):1535-1541.
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13-24.
- Withdrawal: Another Danger of Diversion
- Marriott S, Tyrer P. Benzodiazepine dependence. Avoidance and withdrawal. Drug Saf. 1993;9(2):93-103.
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
- Authier N, Balayssac D, Sautereau M, et al. Benzodiazepine dependence: focus on withdrawal syndrome. Ann Pharm Fr. 2009;67(6):408-413.
- Pages KP, Ries RK. Use of anticonvulsants in benzodiazepine withdrawal. Am J Addict. 1998;7(3):198-204.
- Ashton H. The treatment of benzodiazepine dependence. Addiction. 1994;89(11):1535-1541.
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13-24.
Increased heroin use may not be linked to rise in prescription opioid use
The increase in nonmedical prescription opioid use in the United States does not appear to be strongly related to the concurrent increase in heroin use, according to a review by Dr. Wilson Compton of the National Institute on Drug Abuse, Bethesda, Md., and his associates.
While heroin users are 3.9 times more likely to have used nonmedical prescription opioids than are those who haven’t used heroin, heroin use only occurs in a small number of nonmedical prescription opioid users. The researchers cited studies showing that 3.6% of opioid users began using heroin within 5 years of beginning opioid use, and 4.2% of opioid users reported also using heroin in the past year.
A more likely driver for the increased use of heroin and heroin death rate is decreased cost and increased availability, the investigators wrote. For every $100 decrease in price per gram of heroin, hospitalizations for heroin overdose increase by 2.9%. In addition, heroin use has grown significantly in areas of the United States that were not typically centers for heroin distribution, the researchers reported.
“Fundamentally, prescription opioids and heroin are each elements of a larger epidemic of opioid-related disorders and death. Viewing them from a unified perspective is essential to improving public health. The perniciousness of this epidemic requires a multipronged interventional approach that engages all sectors of society,” the investigators wrote.
Dr. Compton has ties with General Electric, 3M, and Pfizer. No other conflicts were reported. Find the study in the New England Journal of Medicine (doi: 10.1056/NEJMra1508490).
The increase in nonmedical prescription opioid use in the United States does not appear to be strongly related to the concurrent increase in heroin use, according to a review by Dr. Wilson Compton of the National Institute on Drug Abuse, Bethesda, Md., and his associates.
While heroin users are 3.9 times more likely to have used nonmedical prescription opioids than are those who haven’t used heroin, heroin use only occurs in a small number of nonmedical prescription opioid users. The researchers cited studies showing that 3.6% of opioid users began using heroin within 5 years of beginning opioid use, and 4.2% of opioid users reported also using heroin in the past year.
A more likely driver for the increased use of heroin and heroin death rate is decreased cost and increased availability, the investigators wrote. For every $100 decrease in price per gram of heroin, hospitalizations for heroin overdose increase by 2.9%. In addition, heroin use has grown significantly in areas of the United States that were not typically centers for heroin distribution, the researchers reported.
“Fundamentally, prescription opioids and heroin are each elements of a larger epidemic of opioid-related disorders and death. Viewing them from a unified perspective is essential to improving public health. The perniciousness of this epidemic requires a multipronged interventional approach that engages all sectors of society,” the investigators wrote.
Dr. Compton has ties with General Electric, 3M, and Pfizer. No other conflicts were reported. Find the study in the New England Journal of Medicine (doi: 10.1056/NEJMra1508490).
The increase in nonmedical prescription opioid use in the United States does not appear to be strongly related to the concurrent increase in heroin use, according to a review by Dr. Wilson Compton of the National Institute on Drug Abuse, Bethesda, Md., and his associates.
While heroin users are 3.9 times more likely to have used nonmedical prescription opioids than are those who haven’t used heroin, heroin use only occurs in a small number of nonmedical prescription opioid users. The researchers cited studies showing that 3.6% of opioid users began using heroin within 5 years of beginning opioid use, and 4.2% of opioid users reported also using heroin in the past year.
A more likely driver for the increased use of heroin and heroin death rate is decreased cost and increased availability, the investigators wrote. For every $100 decrease in price per gram of heroin, hospitalizations for heroin overdose increase by 2.9%. In addition, heroin use has grown significantly in areas of the United States that were not typically centers for heroin distribution, the researchers reported.
“Fundamentally, prescription opioids and heroin are each elements of a larger epidemic of opioid-related disorders and death. Viewing them from a unified perspective is essential to improving public health. The perniciousness of this epidemic requires a multipronged interventional approach that engages all sectors of society,” the investigators wrote.
Dr. Compton has ties with General Electric, 3M, and Pfizer. No other conflicts were reported. Find the study in the New England Journal of Medicine (doi: 10.1056/NEJMra1508490).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Malpractice Counsel: Allergic Reaction Versus Anaphylaxis
Allergic Reaction Versus Anaphylaxis
A 34-year-old woman presented to ED with complaints of an allergic reaction, the onset of which began approximately 1 hour prior. The patient did not know what might have caused her symptoms. She complained of hives and itching all over; she denied difficulty swallowing, wheezing, and shortness of breath. Her medical history was unremarkable. She was on no medications, and she denied any alcohol or tobacco use. She had no known medication or food allergies.
Physical examination revealed a woman in mild discomfort, secondary to generalized itching. Her vital signs, including pulse oximetry, were normal. There was no swelling of the face, lips, or oropharynx. The lungs were clear to auscultation bilaterally. The heart and abdominal examinations were normal. Examination of the skin revealed diffuse urticaria without petechiae or purpura.
The emergency physician (EP) ordered 125 mg of methylprednisolone sodium succinate and 25 mg of diphenhydramine intravenously (IV). After approximately 1 hour, the hives and itching decreased and the patient felt improved. She was diagnosed with an allergic reaction and discharged home with a prescription for diphenhydramine and a methylprednisolone dose pack.
The following day, the patient collapsed at home and emergency medical services was called. Unfortunately, the patient could not be resuscitated and was pronounced dead at the scene. An autopsy revealed the patient had died from anaphylaxis and laryngeal edema, with an extremely elevated tryptase level of 200 ng/mL (normal, <11.5 ng/mL).
The patient’s family sued the EP for failure to diagnose and treat anaphylaxis, failure to treat with epinephrine, and failure to admit the patient to the hospital. The defense claimed the patient did not present with anaphylaxis, but rather simply a worsening of the hives and angioedema, and that the treatment provided was appropriate. The jury found in favor of the defendants.
Discussion
It does not appear the patient presented with anaphylaxis on the first visit, but may have had it on the second visit. In 2004, the National Institutes of Allergy and Infectious Disease (NIAID) panel and the Food Allergy and Anaphylaxis Network (FAAN) developed criteria for the diagnosis of anaphylaxis.1 According to the criteria, anaphylaxis is likely when any one of the following three criteria are present: (1) acute onset of symptoms involving the skin or mucosa (eg, pruritus, hives, angioedema), and either respiratory compromise (eg, dyspnea, wheezing, stridor, hypoxia) or hypotension/end-organ dysfunction (eg, syncope, incontinence); (2) two or more symptoms (eg, respiratory compromise, hypotension/end-organ dysfunction, persistent gastrointestinal [GI] symptoms such as vomiting, diarrhea, or crampy abdominal pain) that occur rapidly after exposure involving the skin or mucosa; or (3) hypotension from a known allergen to the patient. The accuracy of these criteria has been retrospectively evaluated in an ED study, and found to have a 97% sensitivity and an 82% specificity.2 The negative predictive value was good at 98%, but the positive predictive value was only 69%.2
When a patient presents with minimal or subtle symptoms, anaphylaxis can be a very difficult diagnosis to make in the ED early on in the process. While no EP will miss the diagnosis in a patient with hives, hypotension, and wheezing, it can be easy to miss when the predominant symptoms are GI, such as nausea, vomiting, or diarrhea. In addition, the differential diagnosis for the presentation of anaphylaxis in the ED can be extremely broad and include vasovagal reaction, asthma attack, myocardial infarction, gastroenteritis, panic attack, or airway obstruction.
Due to the nature of emergency medicine, EPs must consider multiple etiologies before determining an evaluation and management plan. While recognizing there are limitations to the NIAID/FAAN criteria, EPs should be aware of them. We are very good at treating these types of symptoms with antihistamines and steroids; however, we frequently fail to give epinephrine when indicated. It is important to remember that epinephrine is the first-line treatment for anaphylaxis—not corticosteroids or antihistamines.3
Reasons for not administering epinephrine are multiple. First, as discussed above, if the diagnosis of anaphylaxis is not considered, the EP is not going to administer the drug of choice. Secondly, EPs have been taught to have a healthy respect for epinephrine and its effects, especially in older patients. Due to this cautious approach, epinephrine is frequently not given to patients with mild symptoms or to those who present early in the course of disease.
Emergency physicians have experience giving epinephrine subcutaneously, but not nearly as much with the intramuscular (IM) route. This is important, because an IM injection in the anterolateral thigh is the recommended location for the treatment of anaphylaxis. The dose should be weight based (0.01 mg/kg) to a maximum of 0.5 mg. This dose can be given every 5 to 15 minutes as necessary to control symptoms.3 The dosing is important to remember, since many EDs stock only autoinjectable epinephrine devices for use in anaphylaxis. These autoinjectors only contain 0.3 mg of epinephrine, so some patients may be underdosed if used.
In the management of allergic reactions and anaphylaxis, EPs frequently administer antihistamines and corticosteroids. While there is no direct evidence to support their use in the management of anaphylaxis, theoretical benefits do exist.3 This, combined with the excellent medication safety profile and lack of serious side effects, make these two medication classes appropriate for use in the ED.
- Allergic Reaction Versus Anaphylaxis
- Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3-5.
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
- Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608.
Allergic Reaction Versus Anaphylaxis
A 34-year-old woman presented to ED with complaints of an allergic reaction, the onset of which began approximately 1 hour prior. The patient did not know what might have caused her symptoms. She complained of hives and itching all over; she denied difficulty swallowing, wheezing, and shortness of breath. Her medical history was unremarkable. She was on no medications, and she denied any alcohol or tobacco use. She had no known medication or food allergies.
Physical examination revealed a woman in mild discomfort, secondary to generalized itching. Her vital signs, including pulse oximetry, were normal. There was no swelling of the face, lips, or oropharynx. The lungs were clear to auscultation bilaterally. The heart and abdominal examinations were normal. Examination of the skin revealed diffuse urticaria without petechiae or purpura.
The emergency physician (EP) ordered 125 mg of methylprednisolone sodium succinate and 25 mg of diphenhydramine intravenously (IV). After approximately 1 hour, the hives and itching decreased and the patient felt improved. She was diagnosed with an allergic reaction and discharged home with a prescription for diphenhydramine and a methylprednisolone dose pack.
The following day, the patient collapsed at home and emergency medical services was called. Unfortunately, the patient could not be resuscitated and was pronounced dead at the scene. An autopsy revealed the patient had died from anaphylaxis and laryngeal edema, with an extremely elevated tryptase level of 200 ng/mL (normal, <11.5 ng/mL).
The patient’s family sued the EP for failure to diagnose and treat anaphylaxis, failure to treat with epinephrine, and failure to admit the patient to the hospital. The defense claimed the patient did not present with anaphylaxis, but rather simply a worsening of the hives and angioedema, and that the treatment provided was appropriate. The jury found in favor of the defendants.
Discussion
It does not appear the patient presented with anaphylaxis on the first visit, but may have had it on the second visit. In 2004, the National Institutes of Allergy and Infectious Disease (NIAID) panel and the Food Allergy and Anaphylaxis Network (FAAN) developed criteria for the diagnosis of anaphylaxis.1 According to the criteria, anaphylaxis is likely when any one of the following three criteria are present: (1) acute onset of symptoms involving the skin or mucosa (eg, pruritus, hives, angioedema), and either respiratory compromise (eg, dyspnea, wheezing, stridor, hypoxia) or hypotension/end-organ dysfunction (eg, syncope, incontinence); (2) two or more symptoms (eg, respiratory compromise, hypotension/end-organ dysfunction, persistent gastrointestinal [GI] symptoms such as vomiting, diarrhea, or crampy abdominal pain) that occur rapidly after exposure involving the skin or mucosa; or (3) hypotension from a known allergen to the patient. The accuracy of these criteria has been retrospectively evaluated in an ED study, and found to have a 97% sensitivity and an 82% specificity.2 The negative predictive value was good at 98%, but the positive predictive value was only 69%.2
When a patient presents with minimal or subtle symptoms, anaphylaxis can be a very difficult diagnosis to make in the ED early on in the process. While no EP will miss the diagnosis in a patient with hives, hypotension, and wheezing, it can be easy to miss when the predominant symptoms are GI, such as nausea, vomiting, or diarrhea. In addition, the differential diagnosis for the presentation of anaphylaxis in the ED can be extremely broad and include vasovagal reaction, asthma attack, myocardial infarction, gastroenteritis, panic attack, or airway obstruction.
Due to the nature of emergency medicine, EPs must consider multiple etiologies before determining an evaluation and management plan. While recognizing there are limitations to the NIAID/FAAN criteria, EPs should be aware of them. We are very good at treating these types of symptoms with antihistamines and steroids; however, we frequently fail to give epinephrine when indicated. It is important to remember that epinephrine is the first-line treatment for anaphylaxis—not corticosteroids or antihistamines.3
Reasons for not administering epinephrine are multiple. First, as discussed above, if the diagnosis of anaphylaxis is not considered, the EP is not going to administer the drug of choice. Secondly, EPs have been taught to have a healthy respect for epinephrine and its effects, especially in older patients. Due to this cautious approach, epinephrine is frequently not given to patients with mild symptoms or to those who present early in the course of disease.
Emergency physicians have experience giving epinephrine subcutaneously, but not nearly as much with the intramuscular (IM) route. This is important, because an IM injection in the anterolateral thigh is the recommended location for the treatment of anaphylaxis. The dose should be weight based (0.01 mg/kg) to a maximum of 0.5 mg. This dose can be given every 5 to 15 minutes as necessary to control symptoms.3 The dosing is important to remember, since many EDs stock only autoinjectable epinephrine devices for use in anaphylaxis. These autoinjectors only contain 0.3 mg of epinephrine, so some patients may be underdosed if used.
In the management of allergic reactions and anaphylaxis, EPs frequently administer antihistamines and corticosteroids. While there is no direct evidence to support their use in the management of anaphylaxis, theoretical benefits do exist.3 This, combined with the excellent medication safety profile and lack of serious side effects, make these two medication classes appropriate for use in the ED.
Allergic Reaction Versus Anaphylaxis
A 34-year-old woman presented to ED with complaints of an allergic reaction, the onset of which began approximately 1 hour prior. The patient did not know what might have caused her symptoms. She complained of hives and itching all over; she denied difficulty swallowing, wheezing, and shortness of breath. Her medical history was unremarkable. She was on no medications, and she denied any alcohol or tobacco use. She had no known medication or food allergies.
Physical examination revealed a woman in mild discomfort, secondary to generalized itching. Her vital signs, including pulse oximetry, were normal. There was no swelling of the face, lips, or oropharynx. The lungs were clear to auscultation bilaterally. The heart and abdominal examinations were normal. Examination of the skin revealed diffuse urticaria without petechiae or purpura.
The emergency physician (EP) ordered 125 mg of methylprednisolone sodium succinate and 25 mg of diphenhydramine intravenously (IV). After approximately 1 hour, the hives and itching decreased and the patient felt improved. She was diagnosed with an allergic reaction and discharged home with a prescription for diphenhydramine and a methylprednisolone dose pack.
The following day, the patient collapsed at home and emergency medical services was called. Unfortunately, the patient could not be resuscitated and was pronounced dead at the scene. An autopsy revealed the patient had died from anaphylaxis and laryngeal edema, with an extremely elevated tryptase level of 200 ng/mL (normal, <11.5 ng/mL).
The patient’s family sued the EP for failure to diagnose and treat anaphylaxis, failure to treat with epinephrine, and failure to admit the patient to the hospital. The defense claimed the patient did not present with anaphylaxis, but rather simply a worsening of the hives and angioedema, and that the treatment provided was appropriate. The jury found in favor of the defendants.
Discussion
It does not appear the patient presented with anaphylaxis on the first visit, but may have had it on the second visit. In 2004, the National Institutes of Allergy and Infectious Disease (NIAID) panel and the Food Allergy and Anaphylaxis Network (FAAN) developed criteria for the diagnosis of anaphylaxis.1 According to the criteria, anaphylaxis is likely when any one of the following three criteria are present: (1) acute onset of symptoms involving the skin or mucosa (eg, pruritus, hives, angioedema), and either respiratory compromise (eg, dyspnea, wheezing, stridor, hypoxia) or hypotension/end-organ dysfunction (eg, syncope, incontinence); (2) two or more symptoms (eg, respiratory compromise, hypotension/end-organ dysfunction, persistent gastrointestinal [GI] symptoms such as vomiting, diarrhea, or crampy abdominal pain) that occur rapidly after exposure involving the skin or mucosa; or (3) hypotension from a known allergen to the patient. The accuracy of these criteria has been retrospectively evaluated in an ED study, and found to have a 97% sensitivity and an 82% specificity.2 The negative predictive value was good at 98%, but the positive predictive value was only 69%.2
When a patient presents with minimal or subtle symptoms, anaphylaxis can be a very difficult diagnosis to make in the ED early on in the process. While no EP will miss the diagnosis in a patient with hives, hypotension, and wheezing, it can be easy to miss when the predominant symptoms are GI, such as nausea, vomiting, or diarrhea. In addition, the differential diagnosis for the presentation of anaphylaxis in the ED can be extremely broad and include vasovagal reaction, asthma attack, myocardial infarction, gastroenteritis, panic attack, or airway obstruction.
Due to the nature of emergency medicine, EPs must consider multiple etiologies before determining an evaluation and management plan. While recognizing there are limitations to the NIAID/FAAN criteria, EPs should be aware of them. We are very good at treating these types of symptoms with antihistamines and steroids; however, we frequently fail to give epinephrine when indicated. It is important to remember that epinephrine is the first-line treatment for anaphylaxis—not corticosteroids or antihistamines.3
Reasons for not administering epinephrine are multiple. First, as discussed above, if the diagnosis of anaphylaxis is not considered, the EP is not going to administer the drug of choice. Secondly, EPs have been taught to have a healthy respect for epinephrine and its effects, especially in older patients. Due to this cautious approach, epinephrine is frequently not given to patients with mild symptoms or to those who present early in the course of disease.
Emergency physicians have experience giving epinephrine subcutaneously, but not nearly as much with the intramuscular (IM) route. This is important, because an IM injection in the anterolateral thigh is the recommended location for the treatment of anaphylaxis. The dose should be weight based (0.01 mg/kg) to a maximum of 0.5 mg. This dose can be given every 5 to 15 minutes as necessary to control symptoms.3 The dosing is important to remember, since many EDs stock only autoinjectable epinephrine devices for use in anaphylaxis. These autoinjectors only contain 0.3 mg of epinephrine, so some patients may be underdosed if used.
In the management of allergic reactions and anaphylaxis, EPs frequently administer antihistamines and corticosteroids. While there is no direct evidence to support their use in the management of anaphylaxis, theoretical benefits do exist.3 This, combined with the excellent medication safety profile and lack of serious side effects, make these two medication classes appropriate for use in the ED.
- Allergic Reaction Versus Anaphylaxis
- Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3-5.
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
- Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608.
- Allergic Reaction Versus Anaphylaxis
- Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3-5.
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
- Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608.
91% who overdose on opioids continue to receive opioid prescriptions
Almost all patients who had nonfatal overdoses while taking long-term opioids for noncancer pain continued to receive opioid prescriptions, usually from the same physicians, in a nationwide cohort study published online Dec. 28 in Annals of Internal Medicine.
Clinical guidelines specify that adverse events related to the misuse of opioids are clear indications to discontinue long-term opioid therapy. But patterns of prescribing after opioid overdoses are not monitored. To examine prescribing trends following nonfatal opioid overdoses, researchers analyzed information in a database of inpatient, outpatient, and pharmacy claims from a large U.S. health insurer covering all 50 states.
They focused on 2,848 insured adults enrolled in 2000-2012 who received hospital or ED treatment for a prescription opioid overdose and were followed in the database for a median of 15 months. The prescribed drugs included codeine, dihydrocodeine, meperidine, morphine, oxycodone, hydrocodone, hydromorphone, fentanyl, oxymorphone, propoxyphene, methadone, tramadol, and levorphanol, said Dr. Marc R. Larochelle of Boston Medical Center and his associates.
A total of 2,597 of these patients (91%) continued to receive opioid prescriptions after their overdose. The primary prescriber was the same person before and after the overdose in 1,198 cases (61%). Two hundred twelve of these patients (7%) had another opioid overdose during follow-up. The likelihood of a second overdose was much higher for patients taking the highest doses of opioids (100 mg or more morphine-equivalent dosage per day), with hazard ratios of 1.13 for patients taking low doses of opioids, 1.89 for those taking mid-range doses, and 2.57 for those taking high doses.
“We could not determine the reason for the treatment patterns after the overdose; however, some prescribers may have been unaware that the opioid overdose had occurred” because there are no procedures in place to ensure provider notification in such cases. Newly introduced prescription monitoring programs may facilitate such communication, but a more rigorous approach would mandate that all overdoses be reported to public health departments, which would then notify providers and pharmacies, and perhaps secure patient referral to substance abuse treatment programs, the investigators said (Ann Intern Med. 2015 Dec 28. doi: 10.7326/M15-0038). It is possible that some overdoses stemmed from therapeutic error rather than opioid misuse, and that providers felt the risk-benefit ratio justified continued opioid treatment. But it also is likely that many providers simply did not have the knowledge and skills to identify and treat opioid misuse, they added.
“Simply eliminating opioid prescribing for patients who had an overdose is not sufficient. … because some [patients] may turn to diverted or illicit opioids. Rather, efforts to identify and treat substance use disorders in these patients are needed,” Dr. Larochelle and his associates said.
Overall, the study findings indicate that nonfatal overdoses provide a meaningful opportunity to improve the safety of opioid prescribing, but that most prescribers at present are missing this opportunity.
It’s tempting to attribute these astonishing findings to poor medical care, bad medical decisions, or sloppy prescribing, but the problem extends well beyond individual prescribers’ practices. These prescribing behaviors take place within a context in which substantial, even deadly, mistakes are inevitable.
Clinicians must be notified when their patients overdose and must know how to act on that notification. They must be taught to recognize pain and addiction as chronic diseases that require team approaches. They must learn how to taper opioid dosages appropriately, how to use buprenorphine, and what other resources in their communities are reliable. Health systems must give physicians the tools and the time they need to identify and coordinate care for affected patients, and would do well to connect overdose patients directly to addiction services at hospital discharge.
This approach would turn an opioid overdose from a devastating event into an opportunity for hope.
Dr. Jessica Gregg is at Central City Concern, a nonprofit agency serving adults and families impacted by homelessness, poverty, and addiction in Portland, Ore. She reported having no relevant financial conflicts of interest. Dr. Gregg made these remarks in an editorial accompanying Dr. Larochelle’s report (Ann Intern Med. 2015 Dec 28. doi: 10.7326/M152687).
It’s tempting to attribute these astonishing findings to poor medical care, bad medical decisions, or sloppy prescribing, but the problem extends well beyond individual prescribers’ practices. These prescribing behaviors take place within a context in which substantial, even deadly, mistakes are inevitable.
Clinicians must be notified when their patients overdose and must know how to act on that notification. They must be taught to recognize pain and addiction as chronic diseases that require team approaches. They must learn how to taper opioid dosages appropriately, how to use buprenorphine, and what other resources in their communities are reliable. Health systems must give physicians the tools and the time they need to identify and coordinate care for affected patients, and would do well to connect overdose patients directly to addiction services at hospital discharge.
This approach would turn an opioid overdose from a devastating event into an opportunity for hope.
Dr. Jessica Gregg is at Central City Concern, a nonprofit agency serving adults and families impacted by homelessness, poverty, and addiction in Portland, Ore. She reported having no relevant financial conflicts of interest. Dr. Gregg made these remarks in an editorial accompanying Dr. Larochelle’s report (Ann Intern Med. 2015 Dec 28. doi: 10.7326/M152687).
It’s tempting to attribute these astonishing findings to poor medical care, bad medical decisions, or sloppy prescribing, but the problem extends well beyond individual prescribers’ practices. These prescribing behaviors take place within a context in which substantial, even deadly, mistakes are inevitable.
Clinicians must be notified when their patients overdose and must know how to act on that notification. They must be taught to recognize pain and addiction as chronic diseases that require team approaches. They must learn how to taper opioid dosages appropriately, how to use buprenorphine, and what other resources in their communities are reliable. Health systems must give physicians the tools and the time they need to identify and coordinate care for affected patients, and would do well to connect overdose patients directly to addiction services at hospital discharge.
This approach would turn an opioid overdose from a devastating event into an opportunity for hope.
Dr. Jessica Gregg is at Central City Concern, a nonprofit agency serving adults and families impacted by homelessness, poverty, and addiction in Portland, Ore. She reported having no relevant financial conflicts of interest. Dr. Gregg made these remarks in an editorial accompanying Dr. Larochelle’s report (Ann Intern Med. 2015 Dec 28. doi: 10.7326/M152687).
Almost all patients who had nonfatal overdoses while taking long-term opioids for noncancer pain continued to receive opioid prescriptions, usually from the same physicians, in a nationwide cohort study published online Dec. 28 in Annals of Internal Medicine.
Clinical guidelines specify that adverse events related to the misuse of opioids are clear indications to discontinue long-term opioid therapy. But patterns of prescribing after opioid overdoses are not monitored. To examine prescribing trends following nonfatal opioid overdoses, researchers analyzed information in a database of inpatient, outpatient, and pharmacy claims from a large U.S. health insurer covering all 50 states.
They focused on 2,848 insured adults enrolled in 2000-2012 who received hospital or ED treatment for a prescription opioid overdose and were followed in the database for a median of 15 months. The prescribed drugs included codeine, dihydrocodeine, meperidine, morphine, oxycodone, hydrocodone, hydromorphone, fentanyl, oxymorphone, propoxyphene, methadone, tramadol, and levorphanol, said Dr. Marc R. Larochelle of Boston Medical Center and his associates.
A total of 2,597 of these patients (91%) continued to receive opioid prescriptions after their overdose. The primary prescriber was the same person before and after the overdose in 1,198 cases (61%). Two hundred twelve of these patients (7%) had another opioid overdose during follow-up. The likelihood of a second overdose was much higher for patients taking the highest doses of opioids (100 mg or more morphine-equivalent dosage per day), with hazard ratios of 1.13 for patients taking low doses of opioids, 1.89 for those taking mid-range doses, and 2.57 for those taking high doses.
“We could not determine the reason for the treatment patterns after the overdose; however, some prescribers may have been unaware that the opioid overdose had occurred” because there are no procedures in place to ensure provider notification in such cases. Newly introduced prescription monitoring programs may facilitate such communication, but a more rigorous approach would mandate that all overdoses be reported to public health departments, which would then notify providers and pharmacies, and perhaps secure patient referral to substance abuse treatment programs, the investigators said (Ann Intern Med. 2015 Dec 28. doi: 10.7326/M15-0038). It is possible that some overdoses stemmed from therapeutic error rather than opioid misuse, and that providers felt the risk-benefit ratio justified continued opioid treatment. But it also is likely that many providers simply did not have the knowledge and skills to identify and treat opioid misuse, they added.
“Simply eliminating opioid prescribing for patients who had an overdose is not sufficient. … because some [patients] may turn to diverted or illicit opioids. Rather, efforts to identify and treat substance use disorders in these patients are needed,” Dr. Larochelle and his associates said.
Overall, the study findings indicate that nonfatal overdoses provide a meaningful opportunity to improve the safety of opioid prescribing, but that most prescribers at present are missing this opportunity.
Almost all patients who had nonfatal overdoses while taking long-term opioids for noncancer pain continued to receive opioid prescriptions, usually from the same physicians, in a nationwide cohort study published online Dec. 28 in Annals of Internal Medicine.
Clinical guidelines specify that adverse events related to the misuse of opioids are clear indications to discontinue long-term opioid therapy. But patterns of prescribing after opioid overdoses are not monitored. To examine prescribing trends following nonfatal opioid overdoses, researchers analyzed information in a database of inpatient, outpatient, and pharmacy claims from a large U.S. health insurer covering all 50 states.
They focused on 2,848 insured adults enrolled in 2000-2012 who received hospital or ED treatment for a prescription opioid overdose and were followed in the database for a median of 15 months. The prescribed drugs included codeine, dihydrocodeine, meperidine, morphine, oxycodone, hydrocodone, hydromorphone, fentanyl, oxymorphone, propoxyphene, methadone, tramadol, and levorphanol, said Dr. Marc R. Larochelle of Boston Medical Center and his associates.
A total of 2,597 of these patients (91%) continued to receive opioid prescriptions after their overdose. The primary prescriber was the same person before and after the overdose in 1,198 cases (61%). Two hundred twelve of these patients (7%) had another opioid overdose during follow-up. The likelihood of a second overdose was much higher for patients taking the highest doses of opioids (100 mg or more morphine-equivalent dosage per day), with hazard ratios of 1.13 for patients taking low doses of opioids, 1.89 for those taking mid-range doses, and 2.57 for those taking high doses.
“We could not determine the reason for the treatment patterns after the overdose; however, some prescribers may have been unaware that the opioid overdose had occurred” because there are no procedures in place to ensure provider notification in such cases. Newly introduced prescription monitoring programs may facilitate such communication, but a more rigorous approach would mandate that all overdoses be reported to public health departments, which would then notify providers and pharmacies, and perhaps secure patient referral to substance abuse treatment programs, the investigators said (Ann Intern Med. 2015 Dec 28. doi: 10.7326/M15-0038). It is possible that some overdoses stemmed from therapeutic error rather than opioid misuse, and that providers felt the risk-benefit ratio justified continued opioid treatment. But it also is likely that many providers simply did not have the knowledge and skills to identify and treat opioid misuse, they added.
“Simply eliminating opioid prescribing for patients who had an overdose is not sufficient. … because some [patients] may turn to diverted or illicit opioids. Rather, efforts to identify and treat substance use disorders in these patients are needed,” Dr. Larochelle and his associates said.
Overall, the study findings indicate that nonfatal overdoses provide a meaningful opportunity to improve the safety of opioid prescribing, but that most prescribers at present are missing this opportunity.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Almost all patients treated at EDs for nonfatal opioid overdose continue to receive opioid prescriptions.
Major finding: 2,597 patients (91%) continued to receive opioid prescriptions after their overdose; the primary prescriber was the same person before and after the overdose in 1,198 cases (61%).
Data source: A retrospective cohort study involving 2,848 adults taking opioids for noncancer pain who overdosed and were followed for up to 2 years.
Disclosures: This study was funded by the U.S. Health Resources and Services Administration, which had no role in the design or conduct of the study. Dr. Larochelle reported also receiving support from the Ryoichi Sasakawa Fellowship Fund and Harvard Pilgrim Health Care Institute. His associates reported having no relevant financial disclosures.
2014 sets U.S. record for drug overdose deaths
In 2014, 47,055 people in the United States died from drug overdoses – more deaths than attributed to this cause in any previous year on record, according to data from the National Vital Statistics System.
Opioids, primarily prescription pain relievers and heroin, were the main drugs associated with overdose deaths. In 2014, opioids were involved in 28,647 deaths, or 61% of all drug overdose deaths, Rose A. Rudd of the Centers for Disease Control and Prevention and her colleagues wrote (MMWR. 2015 Dec 18;64[Early release]:1-5).
The rate of opioid overdoses has tripled since 2000; the 15-year trend data implicate prescription opioid pain relievers and a recent surge in illicit opioid overdose deaths, driven largely by heroin.
From 2013 to 2014, synthetic opioids other than methadone (e.g., fentanyl and tramadol) drove the largest increase in the rate of drug overdose deaths. The rate nearly doubled from 1 per 100,000 persons to 1.8 per 100,000 persons. In 2014, the rate of drug overdose deaths involving natural and semisynthetic opioids (for example, morphine, oxycodone, and hydrocodone) was 3.8 per 100,000. The rate of drug overdose deaths involving methadone, a synthetic opioid classified separately from other synthetic opioids, was similar in 2013 and 2014.
The five states with the highest rates of drug overdose deaths in 2014 were West Virginia (35.5 deaths per 100,000), New Mexico (27.3), New Hampshire (26.2), Kentucky (24.7), and Ohio (24.6).
States with statistically significant increases in the rate of drug overdose deaths from 2013 to 2014 included Alabama, Georgia, Illinois, Indiana, Maine, Maryland, Massachusetts, Michigan, New Hampshire, New Mexico, North Dakota, Ohio, Pennsylvania, and Virginia.
The rates were noted in all adult age groups. From 2013 to 2014, statistically significant increases in drug overdose death rates were seen for both males and females, persons aged 25-34 years, 35-44 years, 55-64 years, and 65 years and older. Based on ethnicity, increases were seen in non-Hispanic whites and non-Hispanic blacks. Based on residency, increases were most common in the Northeast, Midwest, and South.
The authors noted three limitations of the data: First, the substances tested for and circumstances under which toxicologic tests are performed vary by jurisdiction; in 2013 and 2014, 22% and 19% of drug overdose deaths, respectively, did not include information on the death certificate about the specific types of drugs involved, and the percent of overdose deaths with specific drugs identified on the death certificate varies widely by state. Second, an increase from 2013 to 2014 in reporting of specific drugs involved in drug overdose deaths might have contributed to some of the observed increases in drug overdose death rates involving different types of opioids. Finally, some heroin deaths might be misclassified or underreported because morphine and heroin are similarly metabolized.
Efforts to encourage safer prescribing of opioid pain relievers should be strengthened, according to the authors. CDC has developed a draft guideline for the prescribing of opioids for chronic pain to address this need. The guideline is available at www.cdc.gov/drugoverdose/prescribing/guideline.html.
On Twitter @maryjodales
In 2014, 47,055 people in the United States died from drug overdoses – more deaths than attributed to this cause in any previous year on record, according to data from the National Vital Statistics System.
Opioids, primarily prescription pain relievers and heroin, were the main drugs associated with overdose deaths. In 2014, opioids were involved in 28,647 deaths, or 61% of all drug overdose deaths, Rose A. Rudd of the Centers for Disease Control and Prevention and her colleagues wrote (MMWR. 2015 Dec 18;64[Early release]:1-5).
The rate of opioid overdoses has tripled since 2000; the 15-year trend data implicate prescription opioid pain relievers and a recent surge in illicit opioid overdose deaths, driven largely by heroin.
From 2013 to 2014, synthetic opioids other than methadone (e.g., fentanyl and tramadol) drove the largest increase in the rate of drug overdose deaths. The rate nearly doubled from 1 per 100,000 persons to 1.8 per 100,000 persons. In 2014, the rate of drug overdose deaths involving natural and semisynthetic opioids (for example, morphine, oxycodone, and hydrocodone) was 3.8 per 100,000. The rate of drug overdose deaths involving methadone, a synthetic opioid classified separately from other synthetic opioids, was similar in 2013 and 2014.
The five states with the highest rates of drug overdose deaths in 2014 were West Virginia (35.5 deaths per 100,000), New Mexico (27.3), New Hampshire (26.2), Kentucky (24.7), and Ohio (24.6).
States with statistically significant increases in the rate of drug overdose deaths from 2013 to 2014 included Alabama, Georgia, Illinois, Indiana, Maine, Maryland, Massachusetts, Michigan, New Hampshire, New Mexico, North Dakota, Ohio, Pennsylvania, and Virginia.
The rates were noted in all adult age groups. From 2013 to 2014, statistically significant increases in drug overdose death rates were seen for both males and females, persons aged 25-34 years, 35-44 years, 55-64 years, and 65 years and older. Based on ethnicity, increases were seen in non-Hispanic whites and non-Hispanic blacks. Based on residency, increases were most common in the Northeast, Midwest, and South.
The authors noted three limitations of the data: First, the substances tested for and circumstances under which toxicologic tests are performed vary by jurisdiction; in 2013 and 2014, 22% and 19% of drug overdose deaths, respectively, did not include information on the death certificate about the specific types of drugs involved, and the percent of overdose deaths with specific drugs identified on the death certificate varies widely by state. Second, an increase from 2013 to 2014 in reporting of specific drugs involved in drug overdose deaths might have contributed to some of the observed increases in drug overdose death rates involving different types of opioids. Finally, some heroin deaths might be misclassified or underreported because morphine and heroin are similarly metabolized.
Efforts to encourage safer prescribing of opioid pain relievers should be strengthened, according to the authors. CDC has developed a draft guideline for the prescribing of opioids for chronic pain to address this need. The guideline is available at www.cdc.gov/drugoverdose/prescribing/guideline.html.
On Twitter @maryjodales
In 2014, 47,055 people in the United States died from drug overdoses – more deaths than attributed to this cause in any previous year on record, according to data from the National Vital Statistics System.
Opioids, primarily prescription pain relievers and heroin, were the main drugs associated with overdose deaths. In 2014, opioids were involved in 28,647 deaths, or 61% of all drug overdose deaths, Rose A. Rudd of the Centers for Disease Control and Prevention and her colleagues wrote (MMWR. 2015 Dec 18;64[Early release]:1-5).
The rate of opioid overdoses has tripled since 2000; the 15-year trend data implicate prescription opioid pain relievers and a recent surge in illicit opioid overdose deaths, driven largely by heroin.
From 2013 to 2014, synthetic opioids other than methadone (e.g., fentanyl and tramadol) drove the largest increase in the rate of drug overdose deaths. The rate nearly doubled from 1 per 100,000 persons to 1.8 per 100,000 persons. In 2014, the rate of drug overdose deaths involving natural and semisynthetic opioids (for example, morphine, oxycodone, and hydrocodone) was 3.8 per 100,000. The rate of drug overdose deaths involving methadone, a synthetic opioid classified separately from other synthetic opioids, was similar in 2013 and 2014.
The five states with the highest rates of drug overdose deaths in 2014 were West Virginia (35.5 deaths per 100,000), New Mexico (27.3), New Hampshire (26.2), Kentucky (24.7), and Ohio (24.6).
States with statistically significant increases in the rate of drug overdose deaths from 2013 to 2014 included Alabama, Georgia, Illinois, Indiana, Maine, Maryland, Massachusetts, Michigan, New Hampshire, New Mexico, North Dakota, Ohio, Pennsylvania, and Virginia.
The rates were noted in all adult age groups. From 2013 to 2014, statistically significant increases in drug overdose death rates were seen for both males and females, persons aged 25-34 years, 35-44 years, 55-64 years, and 65 years and older. Based on ethnicity, increases were seen in non-Hispanic whites and non-Hispanic blacks. Based on residency, increases were most common in the Northeast, Midwest, and South.
The authors noted three limitations of the data: First, the substances tested for and circumstances under which toxicologic tests are performed vary by jurisdiction; in 2013 and 2014, 22% and 19% of drug overdose deaths, respectively, did not include information on the death certificate about the specific types of drugs involved, and the percent of overdose deaths with specific drugs identified on the death certificate varies widely by state. Second, an increase from 2013 to 2014 in reporting of specific drugs involved in drug overdose deaths might have contributed to some of the observed increases in drug overdose death rates involving different types of opioids. Finally, some heroin deaths might be misclassified or underreported because morphine and heroin are similarly metabolized.
Efforts to encourage safer prescribing of opioid pain relievers should be strengthened, according to the authors. CDC has developed a draft guideline for the prescribing of opioids for chronic pain to address this need. The guideline is available at www.cdc.gov/drugoverdose/prescribing/guideline.html.
On Twitter @maryjodales
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Key clinical point: Efforts to encourage safer prescribing of opioid pain relievers should be strengthened.
Major finding: In 2014, opioids were involved in 28,647 deaths, or 61% of all drug overdose deaths.
Data source: The National Vital Statistics System multiple cause-of-death mortality files.
Disclosures: The authors had no relevant financial disclosures.