User login
Injuries/poisonings the leading cause of pediatric ED visits
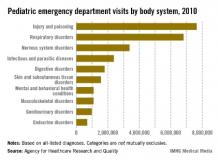
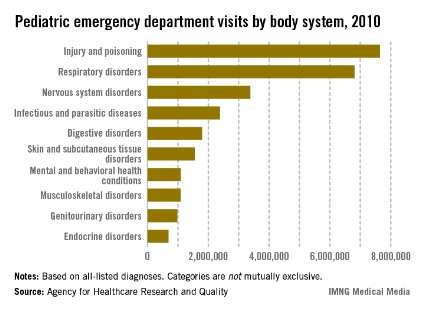
Injuries and poisonings were the most common reason for children to end up in the emergency department in 2010, accounting for over 7.6 million visits, the Agency for Healthcare Research and Quality reported.
There were a total of over 25.5 million ED visits by children under age 18 in 2010, with respiratory disorders being the second most common all-listed condition (6.8 million visits). The next most common reason was nervous system disorders (3.4 million visits), followed by infectious and parasitic diseases (2.4 million) and digestive disorders (1.8 million), according to the AHRQ.
Because the Healthcare Cost and Utilization Project – source of the AHRQ data – tracks all of a patient’s listed diagnoses, the body system categories are not mutually exclusive.
Injuries and poisonings were the most common reason for children to end up in the emergency department in 2010, accounting for over 7.6 million visits, the Agency for Healthcare Research and Quality reported.
There were a total of over 25.5 million ED visits by children under age 18 in 2010, with respiratory disorders being the second most common all-listed condition (6.8 million visits). The next most common reason was nervous system disorders (3.4 million visits), followed by infectious and parasitic diseases (2.4 million) and digestive disorders (1.8 million), according to the AHRQ.
Because the Healthcare Cost and Utilization Project – source of the AHRQ data – tracks all of a patient’s listed diagnoses, the body system categories are not mutually exclusive.
Injuries and poisonings were the most common reason for children to end up in the emergency department in 2010, accounting for over 7.6 million visits, the Agency for Healthcare Research and Quality reported.
There were a total of over 25.5 million ED visits by children under age 18 in 2010, with respiratory disorders being the second most common all-listed condition (6.8 million visits). The next most common reason was nervous system disorders (3.4 million visits), followed by infectious and parasitic diseases (2.4 million) and digestive disorders (1.8 million), according to the AHRQ.
Because the Healthcare Cost and Utilization Project – source of the AHRQ data – tracks all of a patient’s listed diagnoses, the body system categories are not mutually exclusive.
FDA issues strong warning about oral ketoconazole
Ketoconazole tablets should not be used as a first-line treatment for fungal infections because treatment has been associated with an increased risk of adrenal insufficiency, potentially fatal hepatotoxicity, and drug interactions, the Food and Drug Administration has announced.
Marketed as Nizoral, oral ketoconazole is no longer indicated for the treatment of Candida and dermatophyte infections and "should be used only for the treatment of certain life-threatening mycoses when the potential benefits outweigh the risks and alternative therapeutic options are not available or tolerated," according to the MedWatch safety alert issued on July 26.
In addition, oral ketoconazole should not be used to treat fungal infections of the skin and nails, and is only indicated for the treatment of blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis "in patients in whom other treatments have failed or who are intolerant to other therapies," according to the label, which has been modified to reflect these risks and recommendations.
There is now a Medication Guide that will be provided to patients with each filled prescription of oral ketoconazole, explaining the risks.
Because oral ketoconazole has been associated with hepatoxicity that can result in liver transplantation or death, it is now contraindicated in patients with acute or chronic liver disease. The label also now recommends that patients be assessed and monitored for liver toxicity. Monitoring of adrenal function also is now recommended in patients who take the oral formulation of the drug and have adrenal problems or "are under prolonged periods of stress such as those who have had a recent major surgery or who are under intensive care in the hospital."
In addition, coadministration of ketoconazole – a potent inhibitor of the cytochrome P450 3A4 isoenzyme (CYP3A4) – with certain drugs is either restricted or contraindicated because of the increase in drug concentrations and increased risk of QT prolongation and other serious reactions. Contraindicated drugs include dofetilide, quinidine, pimozide, and cisapride.
The FDA changes are based on risk-benefit analyses of data that include reports made to the FDA’s Adverse Events Reporting System.
On July 26, the European Medicines Agency’s Committee on Medicinal Products for Human Use (CHMP) announced that it has concluded that the risk of hepatoxicity with oral ketoconazole products was greater than the benefits in treating fungal infections and recommended that these products no longer be marketed in the European Union.
Creams, shampoos, and other topical ketoconazole formulations have not been associated with these problems, according to the FDA.
The updated label is available here. Serious adverse events associated with ketoconazole should be reported to the FDA at 800-332-1088 or MedWatch.
Ketoconazole tablets should not be used as a first-line treatment for fungal infections because treatment has been associated with an increased risk of adrenal insufficiency, potentially fatal hepatotoxicity, and drug interactions, the Food and Drug Administration has announced.
Marketed as Nizoral, oral ketoconazole is no longer indicated for the treatment of Candida and dermatophyte infections and "should be used only for the treatment of certain life-threatening mycoses when the potential benefits outweigh the risks and alternative therapeutic options are not available or tolerated," according to the MedWatch safety alert issued on July 26.
In addition, oral ketoconazole should not be used to treat fungal infections of the skin and nails, and is only indicated for the treatment of blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis "in patients in whom other treatments have failed or who are intolerant to other therapies," according to the label, which has been modified to reflect these risks and recommendations.
There is now a Medication Guide that will be provided to patients with each filled prescription of oral ketoconazole, explaining the risks.
Because oral ketoconazole has been associated with hepatoxicity that can result in liver transplantation or death, it is now contraindicated in patients with acute or chronic liver disease. The label also now recommends that patients be assessed and monitored for liver toxicity. Monitoring of adrenal function also is now recommended in patients who take the oral formulation of the drug and have adrenal problems or "are under prolonged periods of stress such as those who have had a recent major surgery or who are under intensive care in the hospital."
In addition, coadministration of ketoconazole – a potent inhibitor of the cytochrome P450 3A4 isoenzyme (CYP3A4) – with certain drugs is either restricted or contraindicated because of the increase in drug concentrations and increased risk of QT prolongation and other serious reactions. Contraindicated drugs include dofetilide, quinidine, pimozide, and cisapride.
The FDA changes are based on risk-benefit analyses of data that include reports made to the FDA’s Adverse Events Reporting System.
On July 26, the European Medicines Agency’s Committee on Medicinal Products for Human Use (CHMP) announced that it has concluded that the risk of hepatoxicity with oral ketoconazole products was greater than the benefits in treating fungal infections and recommended that these products no longer be marketed in the European Union.
Creams, shampoos, and other topical ketoconazole formulations have not been associated with these problems, according to the FDA.
The updated label is available here. Serious adverse events associated with ketoconazole should be reported to the FDA at 800-332-1088 or MedWatch.
Ketoconazole tablets should not be used as a first-line treatment for fungal infections because treatment has been associated with an increased risk of adrenal insufficiency, potentially fatal hepatotoxicity, and drug interactions, the Food and Drug Administration has announced.
Marketed as Nizoral, oral ketoconazole is no longer indicated for the treatment of Candida and dermatophyte infections and "should be used only for the treatment of certain life-threatening mycoses when the potential benefits outweigh the risks and alternative therapeutic options are not available or tolerated," according to the MedWatch safety alert issued on July 26.
In addition, oral ketoconazole should not be used to treat fungal infections of the skin and nails, and is only indicated for the treatment of blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis "in patients in whom other treatments have failed or who are intolerant to other therapies," according to the label, which has been modified to reflect these risks and recommendations.
There is now a Medication Guide that will be provided to patients with each filled prescription of oral ketoconazole, explaining the risks.
Because oral ketoconazole has been associated with hepatoxicity that can result in liver transplantation or death, it is now contraindicated in patients with acute or chronic liver disease. The label also now recommends that patients be assessed and monitored for liver toxicity. Monitoring of adrenal function also is now recommended in patients who take the oral formulation of the drug and have adrenal problems or "are under prolonged periods of stress such as those who have had a recent major surgery or who are under intensive care in the hospital."
In addition, coadministration of ketoconazole – a potent inhibitor of the cytochrome P450 3A4 isoenzyme (CYP3A4) – with certain drugs is either restricted or contraindicated because of the increase in drug concentrations and increased risk of QT prolongation and other serious reactions. Contraindicated drugs include dofetilide, quinidine, pimozide, and cisapride.
The FDA changes are based on risk-benefit analyses of data that include reports made to the FDA’s Adverse Events Reporting System.
On July 26, the European Medicines Agency’s Committee on Medicinal Products for Human Use (CHMP) announced that it has concluded that the risk of hepatoxicity with oral ketoconazole products was greater than the benefits in treating fungal infections and recommended that these products no longer be marketed in the European Union.
Creams, shampoos, and other topical ketoconazole formulations have not been associated with these problems, according to the FDA.
The updated label is available here. Serious adverse events associated with ketoconazole should be reported to the FDA at 800-332-1088 or MedWatch.
Androgen deprivation therapy linked to acute kidney injury
Androgen deprivation therapy was strongly associated with an increased risk of acute kidney injury among men with nonmetastatic prostate cancer, according to a report in the July 17 issue of JAMA.
This elevation in risk varied slightly among different types of androgen deprivation agents, and was strongest with therapies that combine gonadotropin-releasing hormone agonists with oral antiandrogens. That suggests "a possible additive effect ... on both receptor antagonism and reduction of testosterone excretion," said Francesco Lapi, Pharm.D., Ph.D., of the Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, and his associates (JAMA 2013;310:289-96).
The researchers discovered the risk elevation in what they described as the first population-based study to investigate the association between androgen deprivation therapy and acute kidney injury. They performed the study because even though the treatment traditionally has been reserved for advanced disease, it is now used increasingly in patients with earlier stages of prostate cancer.
In addition, the investigators were prompted to examine a possible link because of the high mortality (approximately 50%) associated with acute kidney injury.
"Although only one case report of flutamide-related acute kidney injury has been published to date, androgen deprivation therapy and its hypogonadal effect have well-known consequences consistent with our findings," they noted.
Dr. Lapi and his colleagues used two large databases in the United Kingdom, the Clinical Practice Research Datalink and the Hospital Episodes Statistics database, to identify 10,250 men newly diagnosed as having prostate cancer in 1998-2008 who were 40 years of age or older at diagnosis and were followed for a mean of 4 years. This yielded more than 42,000 person-years of follow-up.
A total of 232 cases of acute kidney injury occurred, for an overall incidence of 5.5/1,000 person-years, said Dr. Lapi and his associates.
These cases were matched for age, year of diagnosis, and duration of follow-up with 2,721 control subjects who did not develop acute kidney injury.
Compared with control subjects, men who were using androgen deprivation therapy had a significantly increased risk of acute kidney injury, with an odds ratio of 2.48. That association did not change when the data were adjusted to account for possible confounders, such as comorbidities known to impair kidney function, medications known to have renal toxicity, the severity of the underlying prostate cancer, and the intensity of other cancer treatments.
The investigators then analyzed the data according to type of androgen deprivation therapy, dividing the regimens into six mutually exclusive categories: gonadotropin-releasing hormone (GnRH) agonists (leuprolide, goserelin, triptorelin); oral antiandrogens (cyproterone acetate, flutamide, bicalutamide, nilutamide); combined androgen blockade (GnRH agonists plus oral antiandrogens); bilateral orchiectomy; estrogens; and combinations of those.
The odds ratios were highest for combined androgen blockade and also were significantly elevated for other combination therapies. Only the odd ratios for oral antiandrogens alone and for orchiectomy alone failed to reach statistical significance, although both were above 1.0, the investigators said.
The duration of androgen deprivation therapy was examined in a further analysis of the data. The risk of acute kidney injury was highest early in the course of treatment and decreased slightly, but it remained significantly elevated with longer duration of use.
Finally, in a sensitivity analysis that excluded the 54 cases and 842 controls who had abnormal creatinine levels at baseline, the results were consistent with those of the primary analysis.
The mechanism by which androgen deprivation therapy exerts an adverse effect on the kidney is not known, but the treatment is known to raise the risks of the metabolic syndrome and cardiovascular disease. "A similar rationale can be postulated for the risk of acute kidney injury," Dr. Lapi and his associates said.
The dyslipidemia and hyperglycemia of the metabolic syndrome may promote tubular atrophy and interstitial fibrosis, and may impair glomerular function by expanding and thickening the membranes of the interstitial tubules. Both dyslipidemia and hyperglycemia also raise the risk of thrombosis and induce oxidative stress, which can impact renal function.
In addition, testosterone is thought to protect the kidneys by inducing vasodilation in the renal vessels and enhancing nitric oxide production. So, antagonizing testosterone could promote damage to the glomerulus. And the hypogonadism induced by androgen deprivation can also lead to estrogen deficiency, reducing estrogen’s protective effect against ischemic renal injury, the investigators said.
The study was supported by Prostate Cancer Canada, the Canadian Institutes of Health Research, and Fonds de recherche en Sant
Androgen deprivation therapy was strongly associated with an increased risk of acute kidney injury among men with nonmetastatic prostate cancer, according to a report in the July 17 issue of JAMA.
This elevation in risk varied slightly among different types of androgen deprivation agents, and was strongest with therapies that combine gonadotropin-releasing hormone agonists with oral antiandrogens. That suggests "a possible additive effect ... on both receptor antagonism and reduction of testosterone excretion," said Francesco Lapi, Pharm.D., Ph.D., of the Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, and his associates (JAMA 2013;310:289-96).
The researchers discovered the risk elevation in what they described as the first population-based study to investigate the association between androgen deprivation therapy and acute kidney injury. They performed the study because even though the treatment traditionally has been reserved for advanced disease, it is now used increasingly in patients with earlier stages of prostate cancer.
In addition, the investigators were prompted to examine a possible link because of the high mortality (approximately 50%) associated with acute kidney injury.
"Although only one case report of flutamide-related acute kidney injury has been published to date, androgen deprivation therapy and its hypogonadal effect have well-known consequences consistent with our findings," they noted.
Dr. Lapi and his colleagues used two large databases in the United Kingdom, the Clinical Practice Research Datalink and the Hospital Episodes Statistics database, to identify 10,250 men newly diagnosed as having prostate cancer in 1998-2008 who were 40 years of age or older at diagnosis and were followed for a mean of 4 years. This yielded more than 42,000 person-years of follow-up.
A total of 232 cases of acute kidney injury occurred, for an overall incidence of 5.5/1,000 person-years, said Dr. Lapi and his associates.
These cases were matched for age, year of diagnosis, and duration of follow-up with 2,721 control subjects who did not develop acute kidney injury.
Compared with control subjects, men who were using androgen deprivation therapy had a significantly increased risk of acute kidney injury, with an odds ratio of 2.48. That association did not change when the data were adjusted to account for possible confounders, such as comorbidities known to impair kidney function, medications known to have renal toxicity, the severity of the underlying prostate cancer, and the intensity of other cancer treatments.
The investigators then analyzed the data according to type of androgen deprivation therapy, dividing the regimens into six mutually exclusive categories: gonadotropin-releasing hormone (GnRH) agonists (leuprolide, goserelin, triptorelin); oral antiandrogens (cyproterone acetate, flutamide, bicalutamide, nilutamide); combined androgen blockade (GnRH agonists plus oral antiandrogens); bilateral orchiectomy; estrogens; and combinations of those.
The odds ratios were highest for combined androgen blockade and also were significantly elevated for other combination therapies. Only the odd ratios for oral antiandrogens alone and for orchiectomy alone failed to reach statistical significance, although both were above 1.0, the investigators said.
The duration of androgen deprivation therapy was examined in a further analysis of the data. The risk of acute kidney injury was highest early in the course of treatment and decreased slightly, but it remained significantly elevated with longer duration of use.
Finally, in a sensitivity analysis that excluded the 54 cases and 842 controls who had abnormal creatinine levels at baseline, the results were consistent with those of the primary analysis.
The mechanism by which androgen deprivation therapy exerts an adverse effect on the kidney is not known, but the treatment is known to raise the risks of the metabolic syndrome and cardiovascular disease. "A similar rationale can be postulated for the risk of acute kidney injury," Dr. Lapi and his associates said.
The dyslipidemia and hyperglycemia of the metabolic syndrome may promote tubular atrophy and interstitial fibrosis, and may impair glomerular function by expanding and thickening the membranes of the interstitial tubules. Both dyslipidemia and hyperglycemia also raise the risk of thrombosis and induce oxidative stress, which can impact renal function.
In addition, testosterone is thought to protect the kidneys by inducing vasodilation in the renal vessels and enhancing nitric oxide production. So, antagonizing testosterone could promote damage to the glomerulus. And the hypogonadism induced by androgen deprivation can also lead to estrogen deficiency, reducing estrogen’s protective effect against ischemic renal injury, the investigators said.
The study was supported by Prostate Cancer Canada, the Canadian Institutes of Health Research, and Fonds de recherche en Sant
Androgen deprivation therapy was strongly associated with an increased risk of acute kidney injury among men with nonmetastatic prostate cancer, according to a report in the July 17 issue of JAMA.
This elevation in risk varied slightly among different types of androgen deprivation agents, and was strongest with therapies that combine gonadotropin-releasing hormone agonists with oral antiandrogens. That suggests "a possible additive effect ... on both receptor antagonism and reduction of testosterone excretion," said Francesco Lapi, Pharm.D., Ph.D., of the Centre for Clinical Epidemiology, Jewish General Hospital, Montreal, and his associates (JAMA 2013;310:289-96).
The researchers discovered the risk elevation in what they described as the first population-based study to investigate the association between androgen deprivation therapy and acute kidney injury. They performed the study because even though the treatment traditionally has been reserved for advanced disease, it is now used increasingly in patients with earlier stages of prostate cancer.
In addition, the investigators were prompted to examine a possible link because of the high mortality (approximately 50%) associated with acute kidney injury.
"Although only one case report of flutamide-related acute kidney injury has been published to date, androgen deprivation therapy and its hypogonadal effect have well-known consequences consistent with our findings," they noted.
Dr. Lapi and his colleagues used two large databases in the United Kingdom, the Clinical Practice Research Datalink and the Hospital Episodes Statistics database, to identify 10,250 men newly diagnosed as having prostate cancer in 1998-2008 who were 40 years of age or older at diagnosis and were followed for a mean of 4 years. This yielded more than 42,000 person-years of follow-up.
A total of 232 cases of acute kidney injury occurred, for an overall incidence of 5.5/1,000 person-years, said Dr. Lapi and his associates.
These cases were matched for age, year of diagnosis, and duration of follow-up with 2,721 control subjects who did not develop acute kidney injury.
Compared with control subjects, men who were using androgen deprivation therapy had a significantly increased risk of acute kidney injury, with an odds ratio of 2.48. That association did not change when the data were adjusted to account for possible confounders, such as comorbidities known to impair kidney function, medications known to have renal toxicity, the severity of the underlying prostate cancer, and the intensity of other cancer treatments.
The investigators then analyzed the data according to type of androgen deprivation therapy, dividing the regimens into six mutually exclusive categories: gonadotropin-releasing hormone (GnRH) agonists (leuprolide, goserelin, triptorelin); oral antiandrogens (cyproterone acetate, flutamide, bicalutamide, nilutamide); combined androgen blockade (GnRH agonists plus oral antiandrogens); bilateral orchiectomy; estrogens; and combinations of those.
The odds ratios were highest for combined androgen blockade and also were significantly elevated for other combination therapies. Only the odd ratios for oral antiandrogens alone and for orchiectomy alone failed to reach statistical significance, although both were above 1.0, the investigators said.
The duration of androgen deprivation therapy was examined in a further analysis of the data. The risk of acute kidney injury was highest early in the course of treatment and decreased slightly, but it remained significantly elevated with longer duration of use.
Finally, in a sensitivity analysis that excluded the 54 cases and 842 controls who had abnormal creatinine levels at baseline, the results were consistent with those of the primary analysis.
The mechanism by which androgen deprivation therapy exerts an adverse effect on the kidney is not known, but the treatment is known to raise the risks of the metabolic syndrome and cardiovascular disease. "A similar rationale can be postulated for the risk of acute kidney injury," Dr. Lapi and his associates said.
The dyslipidemia and hyperglycemia of the metabolic syndrome may promote tubular atrophy and interstitial fibrosis, and may impair glomerular function by expanding and thickening the membranes of the interstitial tubules. Both dyslipidemia and hyperglycemia also raise the risk of thrombosis and induce oxidative stress, which can impact renal function.
In addition, testosterone is thought to protect the kidneys by inducing vasodilation in the renal vessels and enhancing nitric oxide production. So, antagonizing testosterone could promote damage to the glomerulus. And the hypogonadism induced by androgen deprivation can also lead to estrogen deficiency, reducing estrogen’s protective effect against ischemic renal injury, the investigators said.
The study was supported by Prostate Cancer Canada, the Canadian Institutes of Health Research, and Fonds de recherche en Sant
FROM JAMA
Major finding: Men with prostate cancer who used androgen deprivation therapy had a significantly increased risk of acute kidney injury, with an odds ratio of 2.48, compared with those who didn’t use the therapy.
Data Source: A population-based case-control study involving 10,250 men aged 40 years and older, newly diagnosed with nonmetastatic prostate cancer, who were followed for a mean of 4 years for the development of acute kidney injury.
Disclosures: The study was supported by Prostate Cancer Canada, the Canadian Institutes of Health Research, and Fonds de recherche en Sant
CD123 differentiates acute GVHD from infections, drug reactions
CD123 expression in the intestinal mucosa may be a useful immunohistochemical marker to differentiate acute colonic graft-versus-host disease in hematopoietic stem-cell transplant patients who develop nonspecific gastrointestinal symptoms, according to Dr. Jingmei Lin and her colleagues.
Immunostaining endoscopic biopsy samples with CD123, an interleukin-3 receptor subunit, can identify plasmacytoid dendritic cells that are critical to the development of graft-versus-host disease (GVHD) but are not known to be present in infectious processes, adverse drug reactions, or chemoradiation toxicities. CD123 immunostaining was associated with a sensitivity of 66% and a specificity of 97% for acute GVHD, the researchers said (Hum. Pathol. 2013 June 21 [doi:10.1016/j.humpath.2013.02.023]).
Gastrointestinal GVHD can present as a variety of nonspecific symptoms and can be difficult to distinguish pathologically from colonic cytomegaloivrus, a major complication following stem-cell transplantation. Gastrointestinal GVHD also can be hard to differentiate pathologically from other opportunistic infections, such as Clostridium difficile and adenovirus infections, as well as from adverse reactions to chemotherapy and radiation. Early distinction is crucial because treatment approaches differ for these three entities and because early therapy improves outcomes for acute GVHD, said Dr. Lin of the departments of pathology and laboratory medicine, Indiana University, Indianapolis, and her associates.
The researchers reviewed 38 colonic endoscopy samples from stem-cell transplant recipients known to have gastrointestinal GVHD and compared them with 14 samples from patients who had not undergone transplantation and who were known to have cytomegalovirus colitis.
The researchers also assessed 11 biopsy samples (colon, stomach, small bowel, and esophagus) from patients who had taken mycophenolate, which is used for the prophylaxis of GVHD. They additionally assessed 47 biopsies (upper and lower GI) from patients who had undergone hematopoietic stem-cell transplantation but had not developed GVHD or infection, and 5 colon biopsies from control subjects.
All of the GVHD patients had presented with nonspecific symptoms of diarrhea, abdominal pain, abdominal cramping, nausea, and/or vomiting.
Among the 38 samples from patients with acute GVHD, 25 (66%) were positive on CD123 staining, showing plasmacytoid dendritic cells in the lamina propria. This marker increased in sensitivity as lesion grade increased: 60% of grade-1 and grade-2 lesions were positive, compared with 72% of grade-3 and grade-4 lesions.
In contrast, 2 of the 14 (14%) samples from patients with CMV, none of the 47 samples from transplant patients without GVHD, none of the 11 samples from patients who took mycophenolate, and none of the 5 control samples were positive for CD123.
The investigators said that they have no funding or conflicts of interest to disclose.
CD123 expression in the intestinal mucosa may be a useful immunohistochemical marker to differentiate acute colonic graft-versus-host disease in hematopoietic stem-cell transplant patients who develop nonspecific gastrointestinal symptoms, according to Dr. Jingmei Lin and her colleagues.
Immunostaining endoscopic biopsy samples with CD123, an interleukin-3 receptor subunit, can identify plasmacytoid dendritic cells that are critical to the development of graft-versus-host disease (GVHD) but are not known to be present in infectious processes, adverse drug reactions, or chemoradiation toxicities. CD123 immunostaining was associated with a sensitivity of 66% and a specificity of 97% for acute GVHD, the researchers said (Hum. Pathol. 2013 June 21 [doi:10.1016/j.humpath.2013.02.023]).
Gastrointestinal GVHD can present as a variety of nonspecific symptoms and can be difficult to distinguish pathologically from colonic cytomegaloivrus, a major complication following stem-cell transplantation. Gastrointestinal GVHD also can be hard to differentiate pathologically from other opportunistic infections, such as Clostridium difficile and adenovirus infections, as well as from adverse reactions to chemotherapy and radiation. Early distinction is crucial because treatment approaches differ for these three entities and because early therapy improves outcomes for acute GVHD, said Dr. Lin of the departments of pathology and laboratory medicine, Indiana University, Indianapolis, and her associates.
The researchers reviewed 38 colonic endoscopy samples from stem-cell transplant recipients known to have gastrointestinal GVHD and compared them with 14 samples from patients who had not undergone transplantation and who were known to have cytomegalovirus colitis.
The researchers also assessed 11 biopsy samples (colon, stomach, small bowel, and esophagus) from patients who had taken mycophenolate, which is used for the prophylaxis of GVHD. They additionally assessed 47 biopsies (upper and lower GI) from patients who had undergone hematopoietic stem-cell transplantation but had not developed GVHD or infection, and 5 colon biopsies from control subjects.
All of the GVHD patients had presented with nonspecific symptoms of diarrhea, abdominal pain, abdominal cramping, nausea, and/or vomiting.
Among the 38 samples from patients with acute GVHD, 25 (66%) were positive on CD123 staining, showing plasmacytoid dendritic cells in the lamina propria. This marker increased in sensitivity as lesion grade increased: 60% of grade-1 and grade-2 lesions were positive, compared with 72% of grade-3 and grade-4 lesions.
In contrast, 2 of the 14 (14%) samples from patients with CMV, none of the 47 samples from transplant patients without GVHD, none of the 11 samples from patients who took mycophenolate, and none of the 5 control samples were positive for CD123.
The investigators said that they have no funding or conflicts of interest to disclose.
CD123 expression in the intestinal mucosa may be a useful immunohistochemical marker to differentiate acute colonic graft-versus-host disease in hematopoietic stem-cell transplant patients who develop nonspecific gastrointestinal symptoms, according to Dr. Jingmei Lin and her colleagues.
Immunostaining endoscopic biopsy samples with CD123, an interleukin-3 receptor subunit, can identify plasmacytoid dendritic cells that are critical to the development of graft-versus-host disease (GVHD) but are not known to be present in infectious processes, adverse drug reactions, or chemoradiation toxicities. CD123 immunostaining was associated with a sensitivity of 66% and a specificity of 97% for acute GVHD, the researchers said (Hum. Pathol. 2013 June 21 [doi:10.1016/j.humpath.2013.02.023]).
Gastrointestinal GVHD can present as a variety of nonspecific symptoms and can be difficult to distinguish pathologically from colonic cytomegaloivrus, a major complication following stem-cell transplantation. Gastrointestinal GVHD also can be hard to differentiate pathologically from other opportunistic infections, such as Clostridium difficile and adenovirus infections, as well as from adverse reactions to chemotherapy and radiation. Early distinction is crucial because treatment approaches differ for these three entities and because early therapy improves outcomes for acute GVHD, said Dr. Lin of the departments of pathology and laboratory medicine, Indiana University, Indianapolis, and her associates.
The researchers reviewed 38 colonic endoscopy samples from stem-cell transplant recipients known to have gastrointestinal GVHD and compared them with 14 samples from patients who had not undergone transplantation and who were known to have cytomegalovirus colitis.
The researchers also assessed 11 biopsy samples (colon, stomach, small bowel, and esophagus) from patients who had taken mycophenolate, which is used for the prophylaxis of GVHD. They additionally assessed 47 biopsies (upper and lower GI) from patients who had undergone hematopoietic stem-cell transplantation but had not developed GVHD or infection, and 5 colon biopsies from control subjects.
All of the GVHD patients had presented with nonspecific symptoms of diarrhea, abdominal pain, abdominal cramping, nausea, and/or vomiting.
Among the 38 samples from patients with acute GVHD, 25 (66%) were positive on CD123 staining, showing plasmacytoid dendritic cells in the lamina propria. This marker increased in sensitivity as lesion grade increased: 60% of grade-1 and grade-2 lesions were positive, compared with 72% of grade-3 and grade-4 lesions.
In contrast, 2 of the 14 (14%) samples from patients with CMV, none of the 47 samples from transplant patients without GVHD, none of the 11 samples from patients who took mycophenolate, and none of the 5 control samples were positive for CD123.
The investigators said that they have no funding or conflicts of interest to disclose.
FROM HUMAN PATHOLOGY
Major finding: Among 38 colonic endoscopy samples from patients with acute GVHD, 25 (66%) were positive on CD123 staining. The marker increased in sensitivity as lesion grade increased: 60% of grade-1 and grade-2 lesions were positive, compared with 72% of grade-3 and grade-4 lesions.
Data source: A review of endoscopic biopsy samples from stem-cell transplant recipients known to have gastrointestinal GVHD, patients known to have cytomegalovirus colitis, patients who had taken mycophenolate, patients who had hematopoietic stem cell transplants and had not developed GVHD or infection, and control subjects.
Disclosures: The investigators said that they have no funding or conflicts of interest to disclose.
Opioid overdose deaths skyrocket in women
American women are dying from prescription drug overdose at historically high rates, the Centers for Disease Control and Prevention announced July 2.
Between 1999 and 2010, the percentage increase in deaths from prescription opioid pain relievers increased more than 415% among women, compared with 265% among men, according to an analysis of national data sets.
In addition, for every woman who died of a prescription painkiller overdose, 30 went to the emergency department for painkiller misuse or abuse.
"Mothers, wives, sisters, and daughters are dying from overdoses at rates that we have never seen before," Dr. Tom Frieden, CDC director, said during a media teleconference. "The increase in opiate overdoses and opiate overdose deaths is directly proportional to the increase in prescribing of painkillers."
Prescriptions for opioid pain relievers such as hydrocodone, oxycodone, and oxymorphone "are increasing to an extent that we would not have anticipated and that could not possibly be clinically indicated," he said.
The findings underscore the importance of reserving prescriptions of opioid pain relievers for situations such as severe cancer pain, "where they can provide important and essential palliation," Dr. Frieden said. "But in many other situations, the risks far outweigh the benefits. Prescribing an opiate may condemn a patient to lifelong addiction and life-threatening complications."
For the analysis, CDC researchers used data from the 1999-2010 National Vital Statistics System and the 2004-2010 Drug Abuse Warning Network to analyze rates of fatal overdoses and ED visits related to drug use or misuse among women (MMWR 2013;62:1-6).
In 2010, 15,323 deaths among women were linked to drug overdose, for a rate of 9.8 per 100,000 population. Between 1999 and 2010, 47,935 women died of opioid pain reliever overdoses. Over that time period, the percentage increase in deaths related to opioid pain relievers was 415% for women and 265% for men. Rates for all drug overdose deaths were highest among women aged 45-54 years (a rate of 21.8 per 100,000 population).
The researchers also reported that in 2010, women made 943,365 ED visits for drug misuse or abuse, a rate of 601 per 100,000 population. The highest ED visit rates were for cocaine or heroin (147.2 per 100,000), benzodiazepines (134.6 per 100,000) and opioid pain relievers (129.6 per 100,000). ED visit rates among women for all drugs tended to be highest among those aged 25-34 years.
Compared with men, Dr. Frieden said that women "are more likely to have chronic pain, to be prescribed painkillers and other medications, to be given higher doses, and to use them for longer time periods. It may be that some of the most common forms of pain are more prevalent among women than men [such as] abdominal pain, migraines, and musculoskeletal pain."
Dr. Frieden advised prescribing clinicians to talk with patients about the risks and benefits of taking opioid pain relievers and to follow guidelines for responsible prescribing "such as screening and monitoring patients for substance abuse and for mental health problems, and [using] prescription drug monitoring programs to identify patients who may be improperly using prescription painkillers."
He also called on states to "improve and implement prescription drug monitoring programs. These programs are just getting up and running in many states."
States "need to do more to ensure that the programs are real-time, complete, and actively managed so that we identify patients who need drug treatment and doctors who need [prescribing] information and education," Dr. Frieden said.
As an example, Dr. Frieden highlighted efforts made in recent years in the state of Washington. Officials there worked with clinicians, health care insurers, and worker compensation programs to develop a consensus on how and when prescription opioids should be used, what some of the alternative treatments are, and resources for patients who are addicted.
"They enforced those guidelines through regulation and saw a more than 20% reduction in opioid deaths in about 3 years," he said.
In the MMWR article, researchers acknowledged certain limitations of the study, including the fact that vital statistics "underestimate the rates of drug involvement in deaths because the type of drug is not specified on many death certificates" and that injury mortality data "might underestimate by up to 35% the actual numbers of deaths for American Indian/Alaska natives and certain other racial/ethnic populations (e.g., Hispanics) because of the misclassification of race/ethnicity of decedents on death certificates."
The researchers had no relevant financial conflicts to disclose.
American women are dying from prescription drug overdose at historically high rates, the Centers for Disease Control and Prevention announced July 2.
Between 1999 and 2010, the percentage increase in deaths from prescription opioid pain relievers increased more than 415% among women, compared with 265% among men, according to an analysis of national data sets.
In addition, for every woman who died of a prescription painkiller overdose, 30 went to the emergency department for painkiller misuse or abuse.
"Mothers, wives, sisters, and daughters are dying from overdoses at rates that we have never seen before," Dr. Tom Frieden, CDC director, said during a media teleconference. "The increase in opiate overdoses and opiate overdose deaths is directly proportional to the increase in prescribing of painkillers."
Prescriptions for opioid pain relievers such as hydrocodone, oxycodone, and oxymorphone "are increasing to an extent that we would not have anticipated and that could not possibly be clinically indicated," he said.
The findings underscore the importance of reserving prescriptions of opioid pain relievers for situations such as severe cancer pain, "where they can provide important and essential palliation," Dr. Frieden said. "But in many other situations, the risks far outweigh the benefits. Prescribing an opiate may condemn a patient to lifelong addiction and life-threatening complications."
For the analysis, CDC researchers used data from the 1999-2010 National Vital Statistics System and the 2004-2010 Drug Abuse Warning Network to analyze rates of fatal overdoses and ED visits related to drug use or misuse among women (MMWR 2013;62:1-6).
In 2010, 15,323 deaths among women were linked to drug overdose, for a rate of 9.8 per 100,000 population. Between 1999 and 2010, 47,935 women died of opioid pain reliever overdoses. Over that time period, the percentage increase in deaths related to opioid pain relievers was 415% for women and 265% for men. Rates for all drug overdose deaths were highest among women aged 45-54 years (a rate of 21.8 per 100,000 population).
The researchers also reported that in 2010, women made 943,365 ED visits for drug misuse or abuse, a rate of 601 per 100,000 population. The highest ED visit rates were for cocaine or heroin (147.2 per 100,000), benzodiazepines (134.6 per 100,000) and opioid pain relievers (129.6 per 100,000). ED visit rates among women for all drugs tended to be highest among those aged 25-34 years.
Compared with men, Dr. Frieden said that women "are more likely to have chronic pain, to be prescribed painkillers and other medications, to be given higher doses, and to use them for longer time periods. It may be that some of the most common forms of pain are more prevalent among women than men [such as] abdominal pain, migraines, and musculoskeletal pain."
Dr. Frieden advised prescribing clinicians to talk with patients about the risks and benefits of taking opioid pain relievers and to follow guidelines for responsible prescribing "such as screening and monitoring patients for substance abuse and for mental health problems, and [using] prescription drug monitoring programs to identify patients who may be improperly using prescription painkillers."
He also called on states to "improve and implement prescription drug monitoring programs. These programs are just getting up and running in many states."
States "need to do more to ensure that the programs are real-time, complete, and actively managed so that we identify patients who need drug treatment and doctors who need [prescribing] information and education," Dr. Frieden said.
As an example, Dr. Frieden highlighted efforts made in recent years in the state of Washington. Officials there worked with clinicians, health care insurers, and worker compensation programs to develop a consensus on how and when prescription opioids should be used, what some of the alternative treatments are, and resources for patients who are addicted.
"They enforced those guidelines through regulation and saw a more than 20% reduction in opioid deaths in about 3 years," he said.
In the MMWR article, researchers acknowledged certain limitations of the study, including the fact that vital statistics "underestimate the rates of drug involvement in deaths because the type of drug is not specified on many death certificates" and that injury mortality data "might underestimate by up to 35% the actual numbers of deaths for American Indian/Alaska natives and certain other racial/ethnic populations (e.g., Hispanics) because of the misclassification of race/ethnicity of decedents on death certificates."
The researchers had no relevant financial conflicts to disclose.
American women are dying from prescription drug overdose at historically high rates, the Centers for Disease Control and Prevention announced July 2.
Between 1999 and 2010, the percentage increase in deaths from prescription opioid pain relievers increased more than 415% among women, compared with 265% among men, according to an analysis of national data sets.
In addition, for every woman who died of a prescription painkiller overdose, 30 went to the emergency department for painkiller misuse or abuse.
"Mothers, wives, sisters, and daughters are dying from overdoses at rates that we have never seen before," Dr. Tom Frieden, CDC director, said during a media teleconference. "The increase in opiate overdoses and opiate overdose deaths is directly proportional to the increase in prescribing of painkillers."
Prescriptions for opioid pain relievers such as hydrocodone, oxycodone, and oxymorphone "are increasing to an extent that we would not have anticipated and that could not possibly be clinically indicated," he said.
The findings underscore the importance of reserving prescriptions of opioid pain relievers for situations such as severe cancer pain, "where they can provide important and essential palliation," Dr. Frieden said. "But in many other situations, the risks far outweigh the benefits. Prescribing an opiate may condemn a patient to lifelong addiction and life-threatening complications."
For the analysis, CDC researchers used data from the 1999-2010 National Vital Statistics System and the 2004-2010 Drug Abuse Warning Network to analyze rates of fatal overdoses and ED visits related to drug use or misuse among women (MMWR 2013;62:1-6).
In 2010, 15,323 deaths among women were linked to drug overdose, for a rate of 9.8 per 100,000 population. Between 1999 and 2010, 47,935 women died of opioid pain reliever overdoses. Over that time period, the percentage increase in deaths related to opioid pain relievers was 415% for women and 265% for men. Rates for all drug overdose deaths were highest among women aged 45-54 years (a rate of 21.8 per 100,000 population).
The researchers also reported that in 2010, women made 943,365 ED visits for drug misuse or abuse, a rate of 601 per 100,000 population. The highest ED visit rates were for cocaine or heroin (147.2 per 100,000), benzodiazepines (134.6 per 100,000) and opioid pain relievers (129.6 per 100,000). ED visit rates among women for all drugs tended to be highest among those aged 25-34 years.
Compared with men, Dr. Frieden said that women "are more likely to have chronic pain, to be prescribed painkillers and other medications, to be given higher doses, and to use them for longer time periods. It may be that some of the most common forms of pain are more prevalent among women than men [such as] abdominal pain, migraines, and musculoskeletal pain."
Dr. Frieden advised prescribing clinicians to talk with patients about the risks and benefits of taking opioid pain relievers and to follow guidelines for responsible prescribing "such as screening and monitoring patients for substance abuse and for mental health problems, and [using] prescription drug monitoring programs to identify patients who may be improperly using prescription painkillers."
He also called on states to "improve and implement prescription drug monitoring programs. These programs are just getting up and running in many states."
States "need to do more to ensure that the programs are real-time, complete, and actively managed so that we identify patients who need drug treatment and doctors who need [prescribing] information and education," Dr. Frieden said.
As an example, Dr. Frieden highlighted efforts made in recent years in the state of Washington. Officials there worked with clinicians, health care insurers, and worker compensation programs to develop a consensus on how and when prescription opioids should be used, what some of the alternative treatments are, and resources for patients who are addicted.
"They enforced those guidelines through regulation and saw a more than 20% reduction in opioid deaths in about 3 years," he said.
In the MMWR article, researchers acknowledged certain limitations of the study, including the fact that vital statistics "underestimate the rates of drug involvement in deaths because the type of drug is not specified on many death certificates" and that injury mortality data "might underestimate by up to 35% the actual numbers of deaths for American Indian/Alaska natives and certain other racial/ethnic populations (e.g., Hispanics) because of the misclassification of race/ethnicity of decedents on death certificates."
The researchers had no relevant financial conflicts to disclose.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Major finding: Between 1999 and 2010, the percentage increase in deaths from overdose of opioid pain relievers rose 415% among women, compared with 265% among men.
Data source: Analysis of data from the 1999-2010 National Vital Statistics System and the 2004-2010 Drug Abuse Warning Network.
Disclosures: The researchers disclosed no relevant financial conflicts of interest.
Augmentin implicated in drug-induced liver injury
The crude incidence of drug-induced liver injury is roughly 19.1 cases per 100,000 inhabitants, with amoxicillin-clavulanate the most commonly implicated agent.
That’s according to the second published population-based cohort study of drug-induced liver injury (DILI), wrote Dr. Einar S. Björnsson. The study was published in the June issue of Gastroenterology.
Dr. Björnsson, of the University of Iceland, and colleagues looked at all patients aged older than 15 years hospitalized for liver disease with suspected DILI, plus outpatients at the National University Hospital of Iceland, and all those seen in private practice between March 1, 2010, and Feb. 29, 2012.
Source: American Gastroenterological Association
According to the authors, "In Iceland, every citizen is issued a specific personal identification number that is, among other things, connected to a nationwide pharmaceutical database on outpatient prescriptions."
Therefore, "The study examined the Icelandic Medicines Registry records of prescriptions for all drugs associated with DILI that had at least a possible causal relationship" according to the Roussel Uclaf Causality Assessment Method.
DILI was defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels greater than three times the upper limit of normal, and/or alkaline phosphatase (ALP) levels greater than two times the upper limit of normal.
Acetaminophen toxicity cases were excluded, though patients with preexisting chronic liver injury were not, if they were considered to have developed superimposed DILI on top of their baseline liver enzyme values.
The authors found that over the study period there were 96 cases eligible for inclusion, including 49 cases in the first year and 47 in the second. That translated into a crude annual incidence during the study period of 19.1 cases per 100,000 inhabitants.
Roughly half were female (56%), and the median age was 55 years (range, 16-91 years).
Looking at the clinical characteristics of the cohort, the authors calculated that only 27% of patients developed jaundice, while 10% of patients complained of rash and 6% of fever. Four of the patients had preexisting liver disease.
Overall, liver injury was judged to be due to a single prescription medication in 75% of cases, most commonly amoxicillin-clavulanate (22%), followed by diclofenac (6%), azathioprine (4%) infliximab (4%), and nitrofurantoin (4%).
By tying the injury to Iceland’s prescription drug database, that meant an incidence of DILI among outpatients of 1 per 133 filled azathioprine prescriptions and 1 in 2,350 amoxicillin-clavulanate users; among inpatients, the incidence of injury attributed to amoxicillin-clavulanate was 1 per 729 patients.
By drug classes, after antibiotics, immunosuppressants were found to be commonly associated with DILI (10%), followed by psychotropic drugs, which accounted for 7% of cases, and then nonsteroidal anti-inflammatory drugs, at 6%, "with diclofenac as the only agent."
Single-drug antineoplastic agents were the causes of DILI in 5% of the cohort, and lipid-lowering agents were the cause in just 3.1% of patients (atorvastatin, n = 2; simvastatin, n = 1).
After injuries due to a single agent, dietary supplements were assumed to be the culprit in 16% of cases, and the use of multiple agents was implicated in 9% of cases.
Looking at outcomes, the researchers reported that DILI was mild in 35 patients (36%), moderate in 55 patients (58%), and severe in 5 patients (5%); there was 1 death, in an 82-year-old patient.
Finally, the median duration from diagnosis of DILI to the normalization of liver enzymes was 64 days, and 7% still had abnormal liver tests 6 months after DILI diagnosis.
According to the authors, the only previously published population-based study, done in France, found an annual crude incidence rate of 13.9 cases per 100,000 inhabitants per year (Hepatology 2002;36:451-5).
They conceded that their rate is somewhat higher; however, "the French study provided no information about the patients at risk for DILI because information about drug consumption was not available," they wrote.
The authors stated that the study was funded by a grant from the National University Hospital of Iceland Research Fund; they disclosed no individual financial conflicts of interest.
The crude incidence of drug-induced liver injury is roughly 19.1 cases per 100,000 inhabitants, with amoxicillin-clavulanate the most commonly implicated agent.
That’s according to the second published population-based cohort study of drug-induced liver injury (DILI), wrote Dr. Einar S. Björnsson. The study was published in the June issue of Gastroenterology.
Dr. Björnsson, of the University of Iceland, and colleagues looked at all patients aged older than 15 years hospitalized for liver disease with suspected DILI, plus outpatients at the National University Hospital of Iceland, and all those seen in private practice between March 1, 2010, and Feb. 29, 2012.
Source: American Gastroenterological Association
According to the authors, "In Iceland, every citizen is issued a specific personal identification number that is, among other things, connected to a nationwide pharmaceutical database on outpatient prescriptions."
Therefore, "The study examined the Icelandic Medicines Registry records of prescriptions for all drugs associated with DILI that had at least a possible causal relationship" according to the Roussel Uclaf Causality Assessment Method.
DILI was defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels greater than three times the upper limit of normal, and/or alkaline phosphatase (ALP) levels greater than two times the upper limit of normal.
Acetaminophen toxicity cases were excluded, though patients with preexisting chronic liver injury were not, if they were considered to have developed superimposed DILI on top of their baseline liver enzyme values.
The authors found that over the study period there were 96 cases eligible for inclusion, including 49 cases in the first year and 47 in the second. That translated into a crude annual incidence during the study period of 19.1 cases per 100,000 inhabitants.
Roughly half were female (56%), and the median age was 55 years (range, 16-91 years).
Looking at the clinical characteristics of the cohort, the authors calculated that only 27% of patients developed jaundice, while 10% of patients complained of rash and 6% of fever. Four of the patients had preexisting liver disease.
Overall, liver injury was judged to be due to a single prescription medication in 75% of cases, most commonly amoxicillin-clavulanate (22%), followed by diclofenac (6%), azathioprine (4%) infliximab (4%), and nitrofurantoin (4%).
By tying the injury to Iceland’s prescription drug database, that meant an incidence of DILI among outpatients of 1 per 133 filled azathioprine prescriptions and 1 in 2,350 amoxicillin-clavulanate users; among inpatients, the incidence of injury attributed to amoxicillin-clavulanate was 1 per 729 patients.
By drug classes, after antibiotics, immunosuppressants were found to be commonly associated with DILI (10%), followed by psychotropic drugs, which accounted for 7% of cases, and then nonsteroidal anti-inflammatory drugs, at 6%, "with diclofenac as the only agent."
Single-drug antineoplastic agents were the causes of DILI in 5% of the cohort, and lipid-lowering agents were the cause in just 3.1% of patients (atorvastatin, n = 2; simvastatin, n = 1).
After injuries due to a single agent, dietary supplements were assumed to be the culprit in 16% of cases, and the use of multiple agents was implicated in 9% of cases.
Looking at outcomes, the researchers reported that DILI was mild in 35 patients (36%), moderate in 55 patients (58%), and severe in 5 patients (5%); there was 1 death, in an 82-year-old patient.
Finally, the median duration from diagnosis of DILI to the normalization of liver enzymes was 64 days, and 7% still had abnormal liver tests 6 months after DILI diagnosis.
According to the authors, the only previously published population-based study, done in France, found an annual crude incidence rate of 13.9 cases per 100,000 inhabitants per year (Hepatology 2002;36:451-5).
They conceded that their rate is somewhat higher; however, "the French study provided no information about the patients at risk for DILI because information about drug consumption was not available," they wrote.
The authors stated that the study was funded by a grant from the National University Hospital of Iceland Research Fund; they disclosed no individual financial conflicts of interest.
The crude incidence of drug-induced liver injury is roughly 19.1 cases per 100,000 inhabitants, with amoxicillin-clavulanate the most commonly implicated agent.
That’s according to the second published population-based cohort study of drug-induced liver injury (DILI), wrote Dr. Einar S. Björnsson. The study was published in the June issue of Gastroenterology.
Dr. Björnsson, of the University of Iceland, and colleagues looked at all patients aged older than 15 years hospitalized for liver disease with suspected DILI, plus outpatients at the National University Hospital of Iceland, and all those seen in private practice between March 1, 2010, and Feb. 29, 2012.
Source: American Gastroenterological Association
According to the authors, "In Iceland, every citizen is issued a specific personal identification number that is, among other things, connected to a nationwide pharmaceutical database on outpatient prescriptions."
Therefore, "The study examined the Icelandic Medicines Registry records of prescriptions for all drugs associated with DILI that had at least a possible causal relationship" according to the Roussel Uclaf Causality Assessment Method.
DILI was defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels greater than three times the upper limit of normal, and/or alkaline phosphatase (ALP) levels greater than two times the upper limit of normal.
Acetaminophen toxicity cases were excluded, though patients with preexisting chronic liver injury were not, if they were considered to have developed superimposed DILI on top of their baseline liver enzyme values.
The authors found that over the study period there were 96 cases eligible for inclusion, including 49 cases in the first year and 47 in the second. That translated into a crude annual incidence during the study period of 19.1 cases per 100,000 inhabitants.
Roughly half were female (56%), and the median age was 55 years (range, 16-91 years).
Looking at the clinical characteristics of the cohort, the authors calculated that only 27% of patients developed jaundice, while 10% of patients complained of rash and 6% of fever. Four of the patients had preexisting liver disease.
Overall, liver injury was judged to be due to a single prescription medication in 75% of cases, most commonly amoxicillin-clavulanate (22%), followed by diclofenac (6%), azathioprine (4%) infliximab (4%), and nitrofurantoin (4%).
By tying the injury to Iceland’s prescription drug database, that meant an incidence of DILI among outpatients of 1 per 133 filled azathioprine prescriptions and 1 in 2,350 amoxicillin-clavulanate users; among inpatients, the incidence of injury attributed to amoxicillin-clavulanate was 1 per 729 patients.
By drug classes, after antibiotics, immunosuppressants were found to be commonly associated with DILI (10%), followed by psychotropic drugs, which accounted for 7% of cases, and then nonsteroidal anti-inflammatory drugs, at 6%, "with diclofenac as the only agent."
Single-drug antineoplastic agents were the causes of DILI in 5% of the cohort, and lipid-lowering agents were the cause in just 3.1% of patients (atorvastatin, n = 2; simvastatin, n = 1).
After injuries due to a single agent, dietary supplements were assumed to be the culprit in 16% of cases, and the use of multiple agents was implicated in 9% of cases.
Looking at outcomes, the researchers reported that DILI was mild in 35 patients (36%), moderate in 55 patients (58%), and severe in 5 patients (5%); there was 1 death, in an 82-year-old patient.
Finally, the median duration from diagnosis of DILI to the normalization of liver enzymes was 64 days, and 7% still had abnormal liver tests 6 months after DILI diagnosis.
According to the authors, the only previously published population-based study, done in France, found an annual crude incidence rate of 13.9 cases per 100,000 inhabitants per year (Hepatology 2002;36:451-5).
They conceded that their rate is somewhat higher; however, "the French study provided no information about the patients at risk for DILI because information about drug consumption was not available," they wrote.
The authors stated that the study was funded by a grant from the National University Hospital of Iceland Research Fund; they disclosed no individual financial conflicts of interest.
FROM GASTROENTEROLOGY
Major finding: Drug-induced liver injury has an incidence of 19.1 per 100,000 persons, with the incidence per outpatient users of amoxicillin-clavulanate at 1 per 2,350.
Data source: A population-based cohort study of 251,000 Icelanders.
Disclosures: The authors stated that the study was funded by a grant from the National University Hospital of Iceland Research Fund; they disclosed no individual conflicts.
Striking rise in accidental marijuana poisonings
The number of unintentional marijuana poisonings in children rose markedly in Colorado after medical marijuana was decriminalized in 2009, with visits to one emergency department climbing from zero to 2.4% of all poisoning cases in just 2 years, according to a report published online May 27 in JAMA Pediatrics.
The toxic effects in these children were more serious than those typically reported for marijuana exposures in the past, most likely because tetrahydrocannabinol concentrations are higher in today’s medical marijuana products than in the plant parts involved in most previous exposures. So now, even when the amount ingested is small, it still produces significant adverse effects in the pediatric population, including respiratory insufficiency requiring care in the pediatric intensive care unit (PICU), said Dr. George Sam Wang of Rocky Mountain Poison and Drug Center, Denver, and his associates.
In addition, medical marijuana is sold in a variety of forms that are more palatable to children than plant parts are, such as baked goods, soft drinks, and candies. So more children are attracted to eating the drug now, and they likely eat larger amounts than in the past.
"Physicians, especially in states that have decriminalized medical marijuana, need to be cognizant of the potential for marijuana exposures and be familiar with the symptoms of marijuana ingestion," the investigators said.
After Colorado decriminalized medical marijuana, both the number of dispensaries and the number of patients authorized to use the drug increased rapidly. As of 2010, more than 300 such dispensaries were licensed in Denver alone, "roughly twice the number of the city’s public schools," Dr. Wang and his colleagues noted.
They performed the first U.S. study to asses the impact of this legislation on pediatric poisonings: a retrospective cohort study at a tertiary children’s hospital that had approximately 65,000 annual visits to its emergency department. The researchers focused on children under age 12 who were evaluated for possible toxic exposures, comparing the 790 patients seen from 2005 to Sept. 30, 2009, before legalization with the 588 seen from Oct. 1, 2009, to the end of 2011 after medical marijuana was legalized.
There were no cases of marijuana poisoning during the first time period, compared with 14 in the second time period. The proportion of such visits in relation to all pediatric ED visits for toxic exposures thus rose from 0% to 2.4% after the drug was decriminalized.
Children as young as 8 months of age were exposed to marijuana. Most presented with central nervous system (CNS) effects such as lethargy or somnolence, and some displayed rigidity, ataxia, hypoxia, or respiratory insufficiency, the researchers reported (JAMA Ped. 2013 May 27 [doi: 10.1001/jamapediatrics.2013.140]).
Only two patients were known to have been exposed to marijuana on arrival at the ED; they underwent a urine toxicology screen. The rest underwent extensive workups to determine the cause of their symptoms, which included urine toxicology screens; blood work; CT and x-ray imaging of the head, spine, and abdomen; and lumbar punctures.
Only one patient, a 13-year-old girl with minimal symptoms, was discharged after being assessed. The rest were observed in the ED (5) or admitted for hospitalization (8), including 2 children who required admission to the PICU.
This 93% rate of hospitalization reinforces the hypothesis that current marijuana exposures induce more serious effects than past exposures did, because historically only 1.3% of marijuana poisoning cases required hospitalization, Dr. Wang and his associates said.
Eight of these 14 (57%) marijuana exposures were from food products that incorporated the drug. "Currently, there are no regulations on storing medical marijuana products in child-resistant containers, including labels with warnings or precautions, or providing counseling on safe storage practices," the investigators said.
As is the case with many accidental pediatric exposures to other medications, the source of the medical marijuana in several of the cases in this study was the child’s grandparent.
Dr. Wang and his colleagues added that proponents of legalizing marijuana often claim that it is "safer" than alcohol. However, only two patients under age 12 were evaluated for alcohol exposure at this ED since 2009. One, an 11-year-old, intentionally drank alcohol and only required observation for intoxication; the other, a 2-year-old who accidentally drank a household product containing ethanol, was discharged after failing to develop any symptoms.
In comparison, the symptoms, invasive assessments, and hospitalizations described in this study can hardly be considered "safer." These findings clearly demonstrate that "the consequences of marijuana exposure in children should be part of the ongoing debate on legalizing marijuana," the researchers said.
Seventeen states and Washington, D.C., have passed laws to decriminalize medical marijuana at the state level, even though marijuana is a schedule I drug under the Controlled Substances Act. "In November 2012, Colorado and Washington [State] passed amendments legalizing the recreational use of marijuana," Dr. Wang and his associates said.
No financial conflicts of interest were reported.
Another reason for the rise in accidental marijuana poisonings is the increased potency of the drug currently available in the United States, compared with 40 years ago. THC levels have risen from 2% to nearly 8% during that time, said Dr. William Hurley and Dr. Suzan Mazor.
Physicians may now need additional training to recognize and manage toxic reactions to marijuana. Children can present with anxiety, hallucinations, panic episodes, dyspnea, chest pain, nausea, vomiting, dizziness, somnolence, CNS depression, respiratory depression, and coma, which unfortunately are the same signs and symptoms for other toxicities and disorders.
Emergency medicine, pediatric emergency medicine, and primary care pediatric providers will be the first to see patients accidentally exposed to marijuana. They should be alert to "investigating the availability of marijuana in the child’s environment" and should use a rapid urine test to confirm the diagnosis. "No antidote exists for marijuana toxic reactions, and supportive care should be provided, including control of anxiety, control of vomiting, airway control, and ventilation as needed," they said.
Dr. Hurley is at the University of Washington and the Washington Poison Center, both in Seattle. Dr. Mazor is in the division of emergency medicine at Seattle Children’s Hospital. Neither Dr. Hurley nor Dr. Mazor reported any financial conflicts of interest. These remarks were taken from their editorial accompanying Dr. Wang’s report (JAMA Ped. 2013 May 27 [doi: 10.1001/jamapediatrics.2013.2273]).
Another reason for the rise in accidental marijuana poisonings is the increased potency of the drug currently available in the United States, compared with 40 years ago. THC levels have risen from 2% to nearly 8% during that time, said Dr. William Hurley and Dr. Suzan Mazor.
Physicians may now need additional training to recognize and manage toxic reactions to marijuana. Children can present with anxiety, hallucinations, panic episodes, dyspnea, chest pain, nausea, vomiting, dizziness, somnolence, CNS depression, respiratory depression, and coma, which unfortunately are the same signs and symptoms for other toxicities and disorders.
Emergency medicine, pediatric emergency medicine, and primary care pediatric providers will be the first to see patients accidentally exposed to marijuana. They should be alert to "investigating the availability of marijuana in the child’s environment" and should use a rapid urine test to confirm the diagnosis. "No antidote exists for marijuana toxic reactions, and supportive care should be provided, including control of anxiety, control of vomiting, airway control, and ventilation as needed," they said.
Dr. Hurley is at the University of Washington and the Washington Poison Center, both in Seattle. Dr. Mazor is in the division of emergency medicine at Seattle Children’s Hospital. Neither Dr. Hurley nor Dr. Mazor reported any financial conflicts of interest. These remarks were taken from their editorial accompanying Dr. Wang’s report (JAMA Ped. 2013 May 27 [doi: 10.1001/jamapediatrics.2013.2273]).
Another reason for the rise in accidental marijuana poisonings is the increased potency of the drug currently available in the United States, compared with 40 years ago. THC levels have risen from 2% to nearly 8% during that time, said Dr. William Hurley and Dr. Suzan Mazor.
Physicians may now need additional training to recognize and manage toxic reactions to marijuana. Children can present with anxiety, hallucinations, panic episodes, dyspnea, chest pain, nausea, vomiting, dizziness, somnolence, CNS depression, respiratory depression, and coma, which unfortunately are the same signs and symptoms for other toxicities and disorders.
Emergency medicine, pediatric emergency medicine, and primary care pediatric providers will be the first to see patients accidentally exposed to marijuana. They should be alert to "investigating the availability of marijuana in the child’s environment" and should use a rapid urine test to confirm the diagnosis. "No antidote exists for marijuana toxic reactions, and supportive care should be provided, including control of anxiety, control of vomiting, airway control, and ventilation as needed," they said.
Dr. Hurley is at the University of Washington and the Washington Poison Center, both in Seattle. Dr. Mazor is in the division of emergency medicine at Seattle Children’s Hospital. Neither Dr. Hurley nor Dr. Mazor reported any financial conflicts of interest. These remarks were taken from their editorial accompanying Dr. Wang’s report (JAMA Ped. 2013 May 27 [doi: 10.1001/jamapediatrics.2013.2273]).
The number of unintentional marijuana poisonings in children rose markedly in Colorado after medical marijuana was decriminalized in 2009, with visits to one emergency department climbing from zero to 2.4% of all poisoning cases in just 2 years, according to a report published online May 27 in JAMA Pediatrics.
The toxic effects in these children were more serious than those typically reported for marijuana exposures in the past, most likely because tetrahydrocannabinol concentrations are higher in today’s medical marijuana products than in the plant parts involved in most previous exposures. So now, even when the amount ingested is small, it still produces significant adverse effects in the pediatric population, including respiratory insufficiency requiring care in the pediatric intensive care unit (PICU), said Dr. George Sam Wang of Rocky Mountain Poison and Drug Center, Denver, and his associates.
In addition, medical marijuana is sold in a variety of forms that are more palatable to children than plant parts are, such as baked goods, soft drinks, and candies. So more children are attracted to eating the drug now, and they likely eat larger amounts than in the past.
"Physicians, especially in states that have decriminalized medical marijuana, need to be cognizant of the potential for marijuana exposures and be familiar with the symptoms of marijuana ingestion," the investigators said.
After Colorado decriminalized medical marijuana, both the number of dispensaries and the number of patients authorized to use the drug increased rapidly. As of 2010, more than 300 such dispensaries were licensed in Denver alone, "roughly twice the number of the city’s public schools," Dr. Wang and his colleagues noted.
They performed the first U.S. study to asses the impact of this legislation on pediatric poisonings: a retrospective cohort study at a tertiary children’s hospital that had approximately 65,000 annual visits to its emergency department. The researchers focused on children under age 12 who were evaluated for possible toxic exposures, comparing the 790 patients seen from 2005 to Sept. 30, 2009, before legalization with the 588 seen from Oct. 1, 2009, to the end of 2011 after medical marijuana was legalized.
There were no cases of marijuana poisoning during the first time period, compared with 14 in the second time period. The proportion of such visits in relation to all pediatric ED visits for toxic exposures thus rose from 0% to 2.4% after the drug was decriminalized.
Children as young as 8 months of age were exposed to marijuana. Most presented with central nervous system (CNS) effects such as lethargy or somnolence, and some displayed rigidity, ataxia, hypoxia, or respiratory insufficiency, the researchers reported (JAMA Ped. 2013 May 27 [doi: 10.1001/jamapediatrics.2013.140]).
Only two patients were known to have been exposed to marijuana on arrival at the ED; they underwent a urine toxicology screen. The rest underwent extensive workups to determine the cause of their symptoms, which included urine toxicology screens; blood work; CT and x-ray imaging of the head, spine, and abdomen; and lumbar punctures.
Only one patient, a 13-year-old girl with minimal symptoms, was discharged after being assessed. The rest were observed in the ED (5) or admitted for hospitalization (8), including 2 children who required admission to the PICU.
This 93% rate of hospitalization reinforces the hypothesis that current marijuana exposures induce more serious effects than past exposures did, because historically only 1.3% of marijuana poisoning cases required hospitalization, Dr. Wang and his associates said.
Eight of these 14 (57%) marijuana exposures were from food products that incorporated the drug. "Currently, there are no regulations on storing medical marijuana products in child-resistant containers, including labels with warnings or precautions, or providing counseling on safe storage practices," the investigators said.
As is the case with many accidental pediatric exposures to other medications, the source of the medical marijuana in several of the cases in this study was the child’s grandparent.
Dr. Wang and his colleagues added that proponents of legalizing marijuana often claim that it is "safer" than alcohol. However, only two patients under age 12 were evaluated for alcohol exposure at this ED since 2009. One, an 11-year-old, intentionally drank alcohol and only required observation for intoxication; the other, a 2-year-old who accidentally drank a household product containing ethanol, was discharged after failing to develop any symptoms.
In comparison, the symptoms, invasive assessments, and hospitalizations described in this study can hardly be considered "safer." These findings clearly demonstrate that "the consequences of marijuana exposure in children should be part of the ongoing debate on legalizing marijuana," the researchers said.
Seventeen states and Washington, D.C., have passed laws to decriminalize medical marijuana at the state level, even though marijuana is a schedule I drug under the Controlled Substances Act. "In November 2012, Colorado and Washington [State] passed amendments legalizing the recreational use of marijuana," Dr. Wang and his associates said.
No financial conflicts of interest were reported.
The number of unintentional marijuana poisonings in children rose markedly in Colorado after medical marijuana was decriminalized in 2009, with visits to one emergency department climbing from zero to 2.4% of all poisoning cases in just 2 years, according to a report published online May 27 in JAMA Pediatrics.
The toxic effects in these children were more serious than those typically reported for marijuana exposures in the past, most likely because tetrahydrocannabinol concentrations are higher in today’s medical marijuana products than in the plant parts involved in most previous exposures. So now, even when the amount ingested is small, it still produces significant adverse effects in the pediatric population, including respiratory insufficiency requiring care in the pediatric intensive care unit (PICU), said Dr. George Sam Wang of Rocky Mountain Poison and Drug Center, Denver, and his associates.
In addition, medical marijuana is sold in a variety of forms that are more palatable to children than plant parts are, such as baked goods, soft drinks, and candies. So more children are attracted to eating the drug now, and they likely eat larger amounts than in the past.
"Physicians, especially in states that have decriminalized medical marijuana, need to be cognizant of the potential for marijuana exposures and be familiar with the symptoms of marijuana ingestion," the investigators said.
After Colorado decriminalized medical marijuana, both the number of dispensaries and the number of patients authorized to use the drug increased rapidly. As of 2010, more than 300 such dispensaries were licensed in Denver alone, "roughly twice the number of the city’s public schools," Dr. Wang and his colleagues noted.
They performed the first U.S. study to asses the impact of this legislation on pediatric poisonings: a retrospective cohort study at a tertiary children’s hospital that had approximately 65,000 annual visits to its emergency department. The researchers focused on children under age 12 who were evaluated for possible toxic exposures, comparing the 790 patients seen from 2005 to Sept. 30, 2009, before legalization with the 588 seen from Oct. 1, 2009, to the end of 2011 after medical marijuana was legalized.
There were no cases of marijuana poisoning during the first time period, compared with 14 in the second time period. The proportion of such visits in relation to all pediatric ED visits for toxic exposures thus rose from 0% to 2.4% after the drug was decriminalized.
Children as young as 8 months of age were exposed to marijuana. Most presented with central nervous system (CNS) effects such as lethargy or somnolence, and some displayed rigidity, ataxia, hypoxia, or respiratory insufficiency, the researchers reported (JAMA Ped. 2013 May 27 [doi: 10.1001/jamapediatrics.2013.140]).
Only two patients were known to have been exposed to marijuana on arrival at the ED; they underwent a urine toxicology screen. The rest underwent extensive workups to determine the cause of their symptoms, which included urine toxicology screens; blood work; CT and x-ray imaging of the head, spine, and abdomen; and lumbar punctures.
Only one patient, a 13-year-old girl with minimal symptoms, was discharged after being assessed. The rest were observed in the ED (5) or admitted for hospitalization (8), including 2 children who required admission to the PICU.
This 93% rate of hospitalization reinforces the hypothesis that current marijuana exposures induce more serious effects than past exposures did, because historically only 1.3% of marijuana poisoning cases required hospitalization, Dr. Wang and his associates said.
Eight of these 14 (57%) marijuana exposures were from food products that incorporated the drug. "Currently, there are no regulations on storing medical marijuana products in child-resistant containers, including labels with warnings or precautions, or providing counseling on safe storage practices," the investigators said.
As is the case with many accidental pediatric exposures to other medications, the source of the medical marijuana in several of the cases in this study was the child’s grandparent.
Dr. Wang and his colleagues added that proponents of legalizing marijuana often claim that it is "safer" than alcohol. However, only two patients under age 12 were evaluated for alcohol exposure at this ED since 2009. One, an 11-year-old, intentionally drank alcohol and only required observation for intoxication; the other, a 2-year-old who accidentally drank a household product containing ethanol, was discharged after failing to develop any symptoms.
In comparison, the symptoms, invasive assessments, and hospitalizations described in this study can hardly be considered "safer." These findings clearly demonstrate that "the consequences of marijuana exposure in children should be part of the ongoing debate on legalizing marijuana," the researchers said.
Seventeen states and Washington, D.C., have passed laws to decriminalize medical marijuana at the state level, even though marijuana is a schedule I drug under the Controlled Substances Act. "In November 2012, Colorado and Washington [State] passed amendments legalizing the recreational use of marijuana," Dr. Wang and his associates said.
No financial conflicts of interest were reported.
FROM JAMA PEDIATRICS
Major finding: There were no cases of marijuana poisoning among children during the 5 years before medical marijuana was decriminalized, compared with 14 during the 2 years afterward.
Data source: A retrospective cohort study comparing cases of unintentional marijuana poisoning before and after the decriminalization of medical marijuana in Colorado.
Disclosures: No financial conflicts of interest were reported.
Consider steroids in anti-TNF liver injury
Patients with liver injury secondary to anti–tumor necrosis factor–alpha agents often exhibit histological changes similar to those seen in spontaneous autoimmune hepatitis, wrote Dr. Marwan Ghabril and his colleagues. The report was published in the May issue of Clinical Gastroenterology and Hepatology.
The finding comes from a review seeking to characterize the presentation, injury pattern, course of illness, and treatment of anti-TNF agent–induced liver injury, which Dr. Ghabril, of Indiana University in Indianapolis, said "may be severe and prolonged."
To that end, he and his colleagues studied six patients from the U.S. Drug-Induced Liver Injury Network (DILIN) database who had liver damage that was likely secondary to anti-TNFs, according to DILIN criteria.
Additionally, 28 cases were culled from a literature review of the PubMed database using the search terms "hepatotoxicity," "liver injury," "tumor necrosis factor," and the generic names of all TNF-alpha antagonists.
Among all patients, infliximab was most commonly the offending agent, followed by etanercept and adalimumab; no cases were attributed to natalizumab, golimumab, or certolizumab.
Diseases for which the anti-TNFs were prescribed included ankylosing spondylitis, Crohn’s disease, chronic ulcerative colitis, juvenile inflammatory arthritis, psoriatic arthritis, psoriasis, and rheumatoid arthritis.
First, Dr. Ghabril looked at the six patients from the DILIN database. Among these patients, the median duration of drug use before onset of liver injury was 16 weeks (range, 2-52 weeks).
"At presentation, half had jaundice, half had nausea, but only one had fever and none had immuno-allergic features of skin rash or eosinophilia," wrote the authors.
Only one of these patients developed significantly impaired coagulation, with an INR of 3.5, and none of the DILIN patients developed ascites or other signs of hepatic failure, they added.
The peak ALT ranged from 384 to 1,687 U/L, and the peak bilirubin from 1.5 to 27.7 mg/dL.
"Five of the six patients were treated with corticosteroids. One patient had a protracted illness, but all ultimately recovered and could be withdrawn from corticosteroid therapy without recurrence," they wrote.
Next, the authors turned their attention to the PubMed cases.
"Peak serum ALT ranged from 140 to 2,250 U/L and bilirubin from normal to 27.7 mg/dL," the authors reported.
Most patients (n = 22) had autoimmune serological markers and/or histological features at some point during the clinical course, they found.
Twelve patients stopped the drug and initiated corticosteroid therapy, oral or parenteral; all recovered. The rest improved after discontinuation of the anti-TNF, without steroid treatment, except for one patient who had underlying cirrhosis and required liver transplantation.
Notably, several patients were able to continue anti-TNF treatment with another agent without further incident, the authors wrote. Indeed, "three tolerated treatment with etanercept without recurrence of liver injury after cessation of infliximab or adalimumab. Two did well with adalimumab after [drug-induced liver injury] associated with infliximab, and one was successfully switched from adalimumab to abatacept."
Finally, the investigators contrasted patients with serological or histological autoimmune features with patients who lacked any autoimmune characteristics.
The autoimmune patients tended to have a longer latency period. The median period after the drug was initiated but before liver injury was noted was 16 weeks for the autoimmune patients versus 10 weeks for the nonautoimmune patients (P = .17). The autoimmune patients also had a higher peak ALT (median 784 vs. 528; P = .03).
"The mechanism by which the TNF-alpha antagonists lead to drug-induced liver injury is unknown," the authors wrote.
"Because the injury can occur after only one infusion, dose-dependent toxicity is unlikely. Unpredictable, idiosyncratic drug-induced liver injury seems most likely, as in this series no patients had clinical evidence of a rash or eosinophilia, and only one presented with fever."
"Further studies are needed to ascertain whether genetic or other markers of the hepatotoxicity associated with TNF-alpha antagonists can be identified," they said.
The DILIN database is supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors reported having no conflicts of interest.
Patients with liver injury secondary to anti–tumor necrosis factor–alpha agents often exhibit histological changes similar to those seen in spontaneous autoimmune hepatitis, wrote Dr. Marwan Ghabril and his colleagues. The report was published in the May issue of Clinical Gastroenterology and Hepatology.
The finding comes from a review seeking to characterize the presentation, injury pattern, course of illness, and treatment of anti-TNF agent–induced liver injury, which Dr. Ghabril, of Indiana University in Indianapolis, said "may be severe and prolonged."
To that end, he and his colleagues studied six patients from the U.S. Drug-Induced Liver Injury Network (DILIN) database who had liver damage that was likely secondary to anti-TNFs, according to DILIN criteria.
Additionally, 28 cases were culled from a literature review of the PubMed database using the search terms "hepatotoxicity," "liver injury," "tumor necrosis factor," and the generic names of all TNF-alpha antagonists.
Among all patients, infliximab was most commonly the offending agent, followed by etanercept and adalimumab; no cases were attributed to natalizumab, golimumab, or certolizumab.
Diseases for which the anti-TNFs were prescribed included ankylosing spondylitis, Crohn’s disease, chronic ulcerative colitis, juvenile inflammatory arthritis, psoriatic arthritis, psoriasis, and rheumatoid arthritis.
First, Dr. Ghabril looked at the six patients from the DILIN database. Among these patients, the median duration of drug use before onset of liver injury was 16 weeks (range, 2-52 weeks).
"At presentation, half had jaundice, half had nausea, but only one had fever and none had immuno-allergic features of skin rash or eosinophilia," wrote the authors.
Only one of these patients developed significantly impaired coagulation, with an INR of 3.5, and none of the DILIN patients developed ascites or other signs of hepatic failure, they added.
The peak ALT ranged from 384 to 1,687 U/L, and the peak bilirubin from 1.5 to 27.7 mg/dL.
"Five of the six patients were treated with corticosteroids. One patient had a protracted illness, but all ultimately recovered and could be withdrawn from corticosteroid therapy without recurrence," they wrote.
Next, the authors turned their attention to the PubMed cases.
"Peak serum ALT ranged from 140 to 2,250 U/L and bilirubin from normal to 27.7 mg/dL," the authors reported.
Most patients (n = 22) had autoimmune serological markers and/or histological features at some point during the clinical course, they found.
Twelve patients stopped the drug and initiated corticosteroid therapy, oral or parenteral; all recovered. The rest improved after discontinuation of the anti-TNF, without steroid treatment, except for one patient who had underlying cirrhosis and required liver transplantation.
Notably, several patients were able to continue anti-TNF treatment with another agent without further incident, the authors wrote. Indeed, "three tolerated treatment with etanercept without recurrence of liver injury after cessation of infliximab or adalimumab. Two did well with adalimumab after [drug-induced liver injury] associated with infliximab, and one was successfully switched from adalimumab to abatacept."
Finally, the investigators contrasted patients with serological or histological autoimmune features with patients who lacked any autoimmune characteristics.
The autoimmune patients tended to have a longer latency period. The median period after the drug was initiated but before liver injury was noted was 16 weeks for the autoimmune patients versus 10 weeks for the nonautoimmune patients (P = .17). The autoimmune patients also had a higher peak ALT (median 784 vs. 528; P = .03).
"The mechanism by which the TNF-alpha antagonists lead to drug-induced liver injury is unknown," the authors wrote.
"Because the injury can occur after only one infusion, dose-dependent toxicity is unlikely. Unpredictable, idiosyncratic drug-induced liver injury seems most likely, as in this series no patients had clinical evidence of a rash or eosinophilia, and only one presented with fever."
"Further studies are needed to ascertain whether genetic or other markers of the hepatotoxicity associated with TNF-alpha antagonists can be identified," they said.
The DILIN database is supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors reported having no conflicts of interest.
Patients with liver injury secondary to anti–tumor necrosis factor–alpha agents often exhibit histological changes similar to those seen in spontaneous autoimmune hepatitis, wrote Dr. Marwan Ghabril and his colleagues. The report was published in the May issue of Clinical Gastroenterology and Hepatology.
The finding comes from a review seeking to characterize the presentation, injury pattern, course of illness, and treatment of anti-TNF agent–induced liver injury, which Dr. Ghabril, of Indiana University in Indianapolis, said "may be severe and prolonged."
To that end, he and his colleagues studied six patients from the U.S. Drug-Induced Liver Injury Network (DILIN) database who had liver damage that was likely secondary to anti-TNFs, according to DILIN criteria.
Additionally, 28 cases were culled from a literature review of the PubMed database using the search terms "hepatotoxicity," "liver injury," "tumor necrosis factor," and the generic names of all TNF-alpha antagonists.
Among all patients, infliximab was most commonly the offending agent, followed by etanercept and adalimumab; no cases were attributed to natalizumab, golimumab, or certolizumab.
Diseases for which the anti-TNFs were prescribed included ankylosing spondylitis, Crohn’s disease, chronic ulcerative colitis, juvenile inflammatory arthritis, psoriatic arthritis, psoriasis, and rheumatoid arthritis.
First, Dr. Ghabril looked at the six patients from the DILIN database. Among these patients, the median duration of drug use before onset of liver injury was 16 weeks (range, 2-52 weeks).
"At presentation, half had jaundice, half had nausea, but only one had fever and none had immuno-allergic features of skin rash or eosinophilia," wrote the authors.
Only one of these patients developed significantly impaired coagulation, with an INR of 3.5, and none of the DILIN patients developed ascites or other signs of hepatic failure, they added.
The peak ALT ranged from 384 to 1,687 U/L, and the peak bilirubin from 1.5 to 27.7 mg/dL.
"Five of the six patients were treated with corticosteroids. One patient had a protracted illness, but all ultimately recovered and could be withdrawn from corticosteroid therapy without recurrence," they wrote.
Next, the authors turned their attention to the PubMed cases.
"Peak serum ALT ranged from 140 to 2,250 U/L and bilirubin from normal to 27.7 mg/dL," the authors reported.
Most patients (n = 22) had autoimmune serological markers and/or histological features at some point during the clinical course, they found.
Twelve patients stopped the drug and initiated corticosteroid therapy, oral or parenteral; all recovered. The rest improved after discontinuation of the anti-TNF, without steroid treatment, except for one patient who had underlying cirrhosis and required liver transplantation.
Notably, several patients were able to continue anti-TNF treatment with another agent without further incident, the authors wrote. Indeed, "three tolerated treatment with etanercept without recurrence of liver injury after cessation of infliximab or adalimumab. Two did well with adalimumab after [drug-induced liver injury] associated with infliximab, and one was successfully switched from adalimumab to abatacept."
Finally, the investigators contrasted patients with serological or histological autoimmune features with patients who lacked any autoimmune characteristics.
The autoimmune patients tended to have a longer latency period. The median period after the drug was initiated but before liver injury was noted was 16 weeks for the autoimmune patients versus 10 weeks for the nonautoimmune patients (P = .17). The autoimmune patients also had a higher peak ALT (median 784 vs. 528; P = .03).
"The mechanism by which the TNF-alpha antagonists lead to drug-induced liver injury is unknown," the authors wrote.
"Because the injury can occur after only one infusion, dose-dependent toxicity is unlikely. Unpredictable, idiosyncratic drug-induced liver injury seems most likely, as in this series no patients had clinical evidence of a rash or eosinophilia, and only one presented with fever."
"Further studies are needed to ascertain whether genetic or other markers of the hepatotoxicity associated with TNF-alpha antagonists can be identified," they said.
The DILIN database is supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Major finding: Peak ALT levels ranged from 140 to 2,250 U/L among patients with liver injury secondary to tumor necrosis factor–alpha antagonists.
Data source: An analysis of 34 cases of liver injury from the literature and a liver-injury database.
Disclosures: The DILIN database is supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The authors reported having no conflicts of interest.
DEA bans 'bath salt' chemical
One of the main chemicals in synthetic stimulants sold as "bath salts" or "plant food" is now a Schedule I drug, the Drug Enforcement Administration has announced.
The ruling permanently places methylone (3,4-methylenedioxy-N-methylcathinone) in the most restrictive category created by the Controlled Substance Act; Schedule I is reserved for unsafe and highly abused substances with no accepted medical use.
The agency first put methylone on emergency scheduling in 2011 while studying whether the chemical should be permanently controlled. In a related effort, the agency also issued a Notice of Intent to temporarily control three synthetic cannabinoids (UR-144, XLR11, and AKB48) that are falsely marketed as "herbal incense."
"This action will become effective upon publishing a Final Order to temporarily control these substances as Schedule I substances for up to 2 years, with the possibility of a 1-year extension," according to a DEA news release.
Designer drugs like bath salts have become a public health concern in recent years. The substances are popular among some young adults and teens, especially since they are obtained easily in certain stores and hookah bars.
Officials first became aware of the new intoxicants when poison control centers began receiving calls from emergency departments and physicians who could not identify the substance affecting their paranoid, and sometimes violent, patients.
The effects of bath salts are similar to those of cocaine, LSD, and methamphetamine – increased heart rate and blood pressure, as well as hallucinations. Users also can experience extreme paranoia and violent behavior leading to self-harm or harm of others, according to the Office of National Drug Policy Control (ONDCP).
When it comes to synthetic marijuana, the effects include agitation, extreme nervousness, nausea, vomiting, tachycardia, elevated blood pressure, tremors and seizures, hallucinations, and dilated pupils, according to ONDCP.
Although there’s no antidote to these designer drugs, physicians recommend screening for them. The long-term effects of the drugs are not known but are potentially severe. Use of these drugs has led to dozens of deaths.
In 2010, poison control centers received 2,906 calls relating to human exposure to synthetic marijuana, according to the American Association of Poison Control Centers. That number more than doubled to 6,959 in 2011 but then dropped to 2,655 in 2012, partly due to the emergency ban that the DEA put on the substances in late 2011. As of the end of this March, there had been 254 calls to poison control centers.
On Twitter @NaseemSMiller
One of the main chemicals in synthetic stimulants sold as "bath salts" or "plant food" is now a Schedule I drug, the Drug Enforcement Administration has announced.
The ruling permanently places methylone (3,4-methylenedioxy-N-methylcathinone) in the most restrictive category created by the Controlled Substance Act; Schedule I is reserved for unsafe and highly abused substances with no accepted medical use.
The agency first put methylone on emergency scheduling in 2011 while studying whether the chemical should be permanently controlled. In a related effort, the agency also issued a Notice of Intent to temporarily control three synthetic cannabinoids (UR-144, XLR11, and AKB48) that are falsely marketed as "herbal incense."
"This action will become effective upon publishing a Final Order to temporarily control these substances as Schedule I substances for up to 2 years, with the possibility of a 1-year extension," according to a DEA news release.
Designer drugs like bath salts have become a public health concern in recent years. The substances are popular among some young adults and teens, especially since they are obtained easily in certain stores and hookah bars.
Officials first became aware of the new intoxicants when poison control centers began receiving calls from emergency departments and physicians who could not identify the substance affecting their paranoid, and sometimes violent, patients.
The effects of bath salts are similar to those of cocaine, LSD, and methamphetamine – increased heart rate and blood pressure, as well as hallucinations. Users also can experience extreme paranoia and violent behavior leading to self-harm or harm of others, according to the Office of National Drug Policy Control (ONDCP).
When it comes to synthetic marijuana, the effects include agitation, extreme nervousness, nausea, vomiting, tachycardia, elevated blood pressure, tremors and seizures, hallucinations, and dilated pupils, according to ONDCP.
Although there’s no antidote to these designer drugs, physicians recommend screening for them. The long-term effects of the drugs are not known but are potentially severe. Use of these drugs has led to dozens of deaths.
In 2010, poison control centers received 2,906 calls relating to human exposure to synthetic marijuana, according to the American Association of Poison Control Centers. That number more than doubled to 6,959 in 2011 but then dropped to 2,655 in 2012, partly due to the emergency ban that the DEA put on the substances in late 2011. As of the end of this March, there had been 254 calls to poison control centers.
On Twitter @NaseemSMiller
One of the main chemicals in synthetic stimulants sold as "bath salts" or "plant food" is now a Schedule I drug, the Drug Enforcement Administration has announced.
The ruling permanently places methylone (3,4-methylenedioxy-N-methylcathinone) in the most restrictive category created by the Controlled Substance Act; Schedule I is reserved for unsafe and highly abused substances with no accepted medical use.
The agency first put methylone on emergency scheduling in 2011 while studying whether the chemical should be permanently controlled. In a related effort, the agency also issued a Notice of Intent to temporarily control three synthetic cannabinoids (UR-144, XLR11, and AKB48) that are falsely marketed as "herbal incense."
"This action will become effective upon publishing a Final Order to temporarily control these substances as Schedule I substances for up to 2 years, with the possibility of a 1-year extension," according to a DEA news release.
Designer drugs like bath salts have become a public health concern in recent years. The substances are popular among some young adults and teens, especially since they are obtained easily in certain stores and hookah bars.
Officials first became aware of the new intoxicants when poison control centers began receiving calls from emergency departments and physicians who could not identify the substance affecting their paranoid, and sometimes violent, patients.
The effects of bath salts are similar to those of cocaine, LSD, and methamphetamine – increased heart rate and blood pressure, as well as hallucinations. Users also can experience extreme paranoia and violent behavior leading to self-harm or harm of others, according to the Office of National Drug Policy Control (ONDCP).
When it comes to synthetic marijuana, the effects include agitation, extreme nervousness, nausea, vomiting, tachycardia, elevated blood pressure, tremors and seizures, hallucinations, and dilated pupils, according to ONDCP.
Although there’s no antidote to these designer drugs, physicians recommend screening for them. The long-term effects of the drugs are not known but are potentially severe. Use of these drugs has led to dozens of deaths.
In 2010, poison control centers received 2,906 calls relating to human exposure to synthetic marijuana, according to the American Association of Poison Control Centers. That number more than doubled to 6,959 in 2011 but then dropped to 2,655 in 2012, partly due to the emergency ban that the DEA put on the substances in late 2011. As of the end of this March, there had been 254 calls to poison control centers.
On Twitter @NaseemSMiller
Methylisothiazolinone named contact allergen of the year
The increasing use of methylisothiazolinone by itself in cosmetic and toiletry products has earned the preservative the title of contact allergen of the year from the American Contact Dermatitis Society.
Methylisothiazolinone (MI), a moderate-strong sensitizer, is important to know about, because a few years ago it was introduced into the market as a stand-alone preservative at up to 100 parts per million, Dr. Donald V. Belsito, professor of clinical dermatology at Columbia University, N.Y., said at the society’s annual meeting.
Previously, MI was used only in combination with methylchloroisothiazolinone (MCI), and the concentration limit of MI in that combination was 3.75 ppm for rinse-off products, and 1.8 ppm for leave-on products. Even at those lower limits, the MCI/MI combination in 2009-2010 was considered the fifth most common cause of preservative allergy.
The new 100-ppm limit (instituted based on the belief that MI was a weaker sensitizer than MCI) represents a 25-fold increase in the permitted concentration in cosmetics, Dr. Belsito noted.
At least one study, however, has shown that the eliciting concentration for MI reactivity can be as low as 5 ppm, Dr. Mari Paz Castanedo-Tardana of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Kathryn A. Zug of Geisel School of Medicine at Dartmouth, Hanover, N.H., noted in an article in the January/February issue of Dermatitis, which granted MI its dubious 2013 title.
Dr. Castanedo-Tardana and Dr. Zug noted that in 2010, data suggested that more than 2,400 U.S. cosmetic products contained MI, which represents a doubling since 2007. The preservative is also used in industrial products without limit, thus providing other opportunities for exposure, such as occupational exposure (Dermatitis 2013;24:1-6).
An important problem is that few data exist concerning the cross-reaction patterns of MCI and MI, the authors noted.
"Bruze et al. showed that a small proportion of patch test subjects sensitized to MCI/MI also reacted to MI. Isaksson et al. also suggested that patients with high patch test reactivity to MCI may also react to high concentrations of MI (1,000 ppm). The percentage of concomitant reactions between MCI/MI and MI in the patch test population is relevant with regard to the diagnosis of MI contact allergy," according to the authors. They also noted that in a Danish study, a Finnish study, and a German study, only 40%, 66%, and 67% of patients with a positive reaction to MI, respectively, also had a positive reaction to MCI/MI.
The limited experience with MI-only patch tests concentrations also helped the allergen gain its newly bestowed title.
Concentrations of 300 ppm (0.03% aq), 500 ppm (0.05% aq), 1,000 ppm (0.1% aq), and 2,000 ppm (0.2% aq) have been tested, and all have yielded relatively similar percentages of positive test reactions.
"As with any other allergen, the ideal patch test concentration should be able to detect as many cases of contact allergy as possible without causing irritant reactions or active sensitization," the authors said, noting that preliminary results from Denmark suggest that 2,000 ppm (0.2% aq) is an appropriate concentration.
For now, however, it is likely that since MI is not routinely tested in any standard screening series, allergy to this preservative is being widely overlooked.
In fact, reports from Europe show that the frequency of allergy to MI is already at the same level as other preservatives that have been available for many years.
"Methylisothiazolinone should be considered as a potential suspect allergen among patients with suspected cosmetic dermatitis, facial dermatitis, and sunscreen allergy," the authors said, concluding that the addition of MI to an allergen screening series "will likely uncover otherwise undiagnosed cases of preservative contact allergy."
The presenter and authors reported having no disclosures.
The increasing use of methylisothiazolinone by itself in cosmetic and toiletry products has earned the preservative the title of contact allergen of the year from the American Contact Dermatitis Society.
Methylisothiazolinone (MI), a moderate-strong sensitizer, is important to know about, because a few years ago it was introduced into the market as a stand-alone preservative at up to 100 parts per million, Dr. Donald V. Belsito, professor of clinical dermatology at Columbia University, N.Y., said at the society’s annual meeting.
Previously, MI was used only in combination with methylchloroisothiazolinone (MCI), and the concentration limit of MI in that combination was 3.75 ppm for rinse-off products, and 1.8 ppm for leave-on products. Even at those lower limits, the MCI/MI combination in 2009-2010 was considered the fifth most common cause of preservative allergy.
The new 100-ppm limit (instituted based on the belief that MI was a weaker sensitizer than MCI) represents a 25-fold increase in the permitted concentration in cosmetics, Dr. Belsito noted.
At least one study, however, has shown that the eliciting concentration for MI reactivity can be as low as 5 ppm, Dr. Mari Paz Castanedo-Tardana of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Kathryn A. Zug of Geisel School of Medicine at Dartmouth, Hanover, N.H., noted in an article in the January/February issue of Dermatitis, which granted MI its dubious 2013 title.
Dr. Castanedo-Tardana and Dr. Zug noted that in 2010, data suggested that more than 2,400 U.S. cosmetic products contained MI, which represents a doubling since 2007. The preservative is also used in industrial products without limit, thus providing other opportunities for exposure, such as occupational exposure (Dermatitis 2013;24:1-6).
An important problem is that few data exist concerning the cross-reaction patterns of MCI and MI, the authors noted.
"Bruze et al. showed that a small proportion of patch test subjects sensitized to MCI/MI also reacted to MI. Isaksson et al. also suggested that patients with high patch test reactivity to MCI may also react to high concentrations of MI (1,000 ppm). The percentage of concomitant reactions between MCI/MI and MI in the patch test population is relevant with regard to the diagnosis of MI contact allergy," according to the authors. They also noted that in a Danish study, a Finnish study, and a German study, only 40%, 66%, and 67% of patients with a positive reaction to MI, respectively, also had a positive reaction to MCI/MI.
The limited experience with MI-only patch tests concentrations also helped the allergen gain its newly bestowed title.
Concentrations of 300 ppm (0.03% aq), 500 ppm (0.05% aq), 1,000 ppm (0.1% aq), and 2,000 ppm (0.2% aq) have been tested, and all have yielded relatively similar percentages of positive test reactions.
"As with any other allergen, the ideal patch test concentration should be able to detect as many cases of contact allergy as possible without causing irritant reactions or active sensitization," the authors said, noting that preliminary results from Denmark suggest that 2,000 ppm (0.2% aq) is an appropriate concentration.
For now, however, it is likely that since MI is not routinely tested in any standard screening series, allergy to this preservative is being widely overlooked.
In fact, reports from Europe show that the frequency of allergy to MI is already at the same level as other preservatives that have been available for many years.
"Methylisothiazolinone should be considered as a potential suspect allergen among patients with suspected cosmetic dermatitis, facial dermatitis, and sunscreen allergy," the authors said, concluding that the addition of MI to an allergen screening series "will likely uncover otherwise undiagnosed cases of preservative contact allergy."
The presenter and authors reported having no disclosures.
The increasing use of methylisothiazolinone by itself in cosmetic and toiletry products has earned the preservative the title of contact allergen of the year from the American Contact Dermatitis Society.
Methylisothiazolinone (MI), a moderate-strong sensitizer, is important to know about, because a few years ago it was introduced into the market as a stand-alone preservative at up to 100 parts per million, Dr. Donald V. Belsito, professor of clinical dermatology at Columbia University, N.Y., said at the society’s annual meeting.
Previously, MI was used only in combination with methylchloroisothiazolinone (MCI), and the concentration limit of MI in that combination was 3.75 ppm for rinse-off products, and 1.8 ppm for leave-on products. Even at those lower limits, the MCI/MI combination in 2009-2010 was considered the fifth most common cause of preservative allergy.
The new 100-ppm limit (instituted based on the belief that MI was a weaker sensitizer than MCI) represents a 25-fold increase in the permitted concentration in cosmetics, Dr. Belsito noted.
At least one study, however, has shown that the eliciting concentration for MI reactivity can be as low as 5 ppm, Dr. Mari Paz Castanedo-Tardana of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Kathryn A. Zug of Geisel School of Medicine at Dartmouth, Hanover, N.H., noted in an article in the January/February issue of Dermatitis, which granted MI its dubious 2013 title.
Dr. Castanedo-Tardana and Dr. Zug noted that in 2010, data suggested that more than 2,400 U.S. cosmetic products contained MI, which represents a doubling since 2007. The preservative is also used in industrial products without limit, thus providing other opportunities for exposure, such as occupational exposure (Dermatitis 2013;24:1-6).
An important problem is that few data exist concerning the cross-reaction patterns of MCI and MI, the authors noted.
"Bruze et al. showed that a small proportion of patch test subjects sensitized to MCI/MI also reacted to MI. Isaksson et al. also suggested that patients with high patch test reactivity to MCI may also react to high concentrations of MI (1,000 ppm). The percentage of concomitant reactions between MCI/MI and MI in the patch test population is relevant with regard to the diagnosis of MI contact allergy," according to the authors. They also noted that in a Danish study, a Finnish study, and a German study, only 40%, 66%, and 67% of patients with a positive reaction to MI, respectively, also had a positive reaction to MCI/MI.
The limited experience with MI-only patch tests concentrations also helped the allergen gain its newly bestowed title.
Concentrations of 300 ppm (0.03% aq), 500 ppm (0.05% aq), 1,000 ppm (0.1% aq), and 2,000 ppm (0.2% aq) have been tested, and all have yielded relatively similar percentages of positive test reactions.
"As with any other allergen, the ideal patch test concentration should be able to detect as many cases of contact allergy as possible without causing irritant reactions or active sensitization," the authors said, noting that preliminary results from Denmark suggest that 2,000 ppm (0.2% aq) is an appropriate concentration.
For now, however, it is likely that since MI is not routinely tested in any standard screening series, allergy to this preservative is being widely overlooked.
In fact, reports from Europe show that the frequency of allergy to MI is already at the same level as other preservatives that have been available for many years.
"Methylisothiazolinone should be considered as a potential suspect allergen among patients with suspected cosmetic dermatitis, facial dermatitis, and sunscreen allergy," the authors said, concluding that the addition of MI to an allergen screening series "will likely uncover otherwise undiagnosed cases of preservative contact allergy."
The presenter and authors reported having no disclosures.
AT THE ACDS ANNUAL MEETING