User login
Rx: Treating chronic medical vulnerability in the mentally ill
With few exceptions, I have found that patients who have chronic moderate or severe mental illness tend to be relatively more vulnerable in terms of (1) receiving suboptimal primary medical care and (2) suffering a resulting increase in morbidity, mortality, and disability.
Across the board, I’ve found, psychiatrists are more likely to treat patients who are chronically vulnerable.
Why are they so vulnerable?
The unique vulnerability of patients with severe mental illness stems from several causative factors:
• the stigma attached to mental illness
• poor implementation of parity in reimbursement for mental health services
• a suboptimal-sized mental health workforce
• related poor patient-centered support
• most important, these patients’ lack of primary and preventive medical care.
Here are a few examples that demonstrate how dire the situation is:
Smoking cigarettes is one of the most dangerous modifiable risk factors for vascular disease and early death. People with mental illness smoke almost half (44%) of the cigarettes sold in the United States and are twice as likely to smoke than those who do not have a mental illness.1,2
HIV infection is at least 2 or 3 times more prevalent among people with severe mental illness as it is in the general population.3
Hepatitis C infection is at least twice as prevalent in people with a diagnosis of schizophrenia as it is in the general population.4
Schizophrenia. As many as 60% of premature deaths among people with schizophrenia are attributable to a medical illness.5 For example, those with schizophrenia have an increased 10-year cardiac mortality; comparatively higher rates of hypertension, diabetes, and smoking; and, on average, a lower level of high-density lipoprotein cholesterol. Nasrallah et al reported that the rate of untreated hypertension among patients with schizophrenia is 62.4%.6
Premature death. People who have a diagnosis of severe mental illness are at risk of dying prematurely by as much as 25 years.5,7-10
Who should take the lead?
How can psychiatrists address this ongoing vulnerability within the mentally ill patient population, and advocate for their patients? A comprehensive answer to this question is beyond the scope of this article, but I can offer this prescription for your consideration.
Be an advocate. You, as a psychiatrist, are well positioned to counter the mental health-related stigma and advocate for implementation of mental health parity nationwide. In addition to participating in community education and outreach, become a member of, and get involved in, established organizations, such as the American Psychiatric Association, that advocates for psychiatric patients at all levels.
Keep patients connected. Make sure your patients are connected with a primary care provider, and use your psychotherapeutic skills to help patients understand the importance of receiving primary and secondary preventive medical care.
Monitor health and disease. As a physician first and a psychiatrist second, closely monitor your patients for general medical conditions that are related to the presence and treatment of psychiatric disorders. Consider routinely reviewing pertinent lab work with patients—even results of tests ordered by a primary care provider (eg, the metabolic panel and a thyroid-stimulating hormone level in patients taking lithium).
Collaborate with your primary care colleagues; they need your help as much as you can use their help! Make sure your patients witness this collaboration, because it mirrors how you would like them to interact with their primary care provider.
Educate yourself. Education in the essentials of psychiatry-based preventive medical care is key, as we work to more effectively address the increased disability, morbidity, mortality, and overall vulnerability in our patients. Stay “current” on general medical topics by reading the “Med/Psych Update” section of Current Psychiatry and relevant articles in other clinical guides to both integrated and preventive medicine.11
1. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
2. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
3. Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev. 2005;25(4):433-457.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
6. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
7. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
8. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
9. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
10. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
11. McCarron RM, Xiong G, Keenan CR, et al. Preventive medical care in psychiatry: a practical guide for clinicians. Arlington, VA: American Psychiatric Publishing; 2014.
With few exceptions, I have found that patients who have chronic moderate or severe mental illness tend to be relatively more vulnerable in terms of (1) receiving suboptimal primary medical care and (2) suffering a resulting increase in morbidity, mortality, and disability.
Across the board, I’ve found, psychiatrists are more likely to treat patients who are chronically vulnerable.
Why are they so vulnerable?
The unique vulnerability of patients with severe mental illness stems from several causative factors:
• the stigma attached to mental illness
• poor implementation of parity in reimbursement for mental health services
• a suboptimal-sized mental health workforce
• related poor patient-centered support
• most important, these patients’ lack of primary and preventive medical care.
Here are a few examples that demonstrate how dire the situation is:
Smoking cigarettes is one of the most dangerous modifiable risk factors for vascular disease and early death. People with mental illness smoke almost half (44%) of the cigarettes sold in the United States and are twice as likely to smoke than those who do not have a mental illness.1,2
HIV infection is at least 2 or 3 times more prevalent among people with severe mental illness as it is in the general population.3
Hepatitis C infection is at least twice as prevalent in people with a diagnosis of schizophrenia as it is in the general population.4
Schizophrenia. As many as 60% of premature deaths among people with schizophrenia are attributable to a medical illness.5 For example, those with schizophrenia have an increased 10-year cardiac mortality; comparatively higher rates of hypertension, diabetes, and smoking; and, on average, a lower level of high-density lipoprotein cholesterol. Nasrallah et al reported that the rate of untreated hypertension among patients with schizophrenia is 62.4%.6
Premature death. People who have a diagnosis of severe mental illness are at risk of dying prematurely by as much as 25 years.5,7-10
Who should take the lead?
How can psychiatrists address this ongoing vulnerability within the mentally ill patient population, and advocate for their patients? A comprehensive answer to this question is beyond the scope of this article, but I can offer this prescription for your consideration.
Be an advocate. You, as a psychiatrist, are well positioned to counter the mental health-related stigma and advocate for implementation of mental health parity nationwide. In addition to participating in community education and outreach, become a member of, and get involved in, established organizations, such as the American Psychiatric Association, that advocates for psychiatric patients at all levels.
Keep patients connected. Make sure your patients are connected with a primary care provider, and use your psychotherapeutic skills to help patients understand the importance of receiving primary and secondary preventive medical care.
Monitor health and disease. As a physician first and a psychiatrist second, closely monitor your patients for general medical conditions that are related to the presence and treatment of psychiatric disorders. Consider routinely reviewing pertinent lab work with patients—even results of tests ordered by a primary care provider (eg, the metabolic panel and a thyroid-stimulating hormone level in patients taking lithium).
Collaborate with your primary care colleagues; they need your help as much as you can use their help! Make sure your patients witness this collaboration, because it mirrors how you would like them to interact with their primary care provider.
Educate yourself. Education in the essentials of psychiatry-based preventive medical care is key, as we work to more effectively address the increased disability, morbidity, mortality, and overall vulnerability in our patients. Stay “current” on general medical topics by reading the “Med/Psych Update” section of Current Psychiatry and relevant articles in other clinical guides to both integrated and preventive medicine.11
With few exceptions, I have found that patients who have chronic moderate or severe mental illness tend to be relatively more vulnerable in terms of (1) receiving suboptimal primary medical care and (2) suffering a resulting increase in morbidity, mortality, and disability.
Across the board, I’ve found, psychiatrists are more likely to treat patients who are chronically vulnerable.
Why are they so vulnerable?
The unique vulnerability of patients with severe mental illness stems from several causative factors:
• the stigma attached to mental illness
• poor implementation of parity in reimbursement for mental health services
• a suboptimal-sized mental health workforce
• related poor patient-centered support
• most important, these patients’ lack of primary and preventive medical care.
Here are a few examples that demonstrate how dire the situation is:
Smoking cigarettes is one of the most dangerous modifiable risk factors for vascular disease and early death. People with mental illness smoke almost half (44%) of the cigarettes sold in the United States and are twice as likely to smoke than those who do not have a mental illness.1,2
HIV infection is at least 2 or 3 times more prevalent among people with severe mental illness as it is in the general population.3
Hepatitis C infection is at least twice as prevalent in people with a diagnosis of schizophrenia as it is in the general population.4
Schizophrenia. As many as 60% of premature deaths among people with schizophrenia are attributable to a medical illness.5 For example, those with schizophrenia have an increased 10-year cardiac mortality; comparatively higher rates of hypertension, diabetes, and smoking; and, on average, a lower level of high-density lipoprotein cholesterol. Nasrallah et al reported that the rate of untreated hypertension among patients with schizophrenia is 62.4%.6
Premature death. People who have a diagnosis of severe mental illness are at risk of dying prematurely by as much as 25 years.5,7-10
Who should take the lead?
How can psychiatrists address this ongoing vulnerability within the mentally ill patient population, and advocate for their patients? A comprehensive answer to this question is beyond the scope of this article, but I can offer this prescription for your consideration.
Be an advocate. You, as a psychiatrist, are well positioned to counter the mental health-related stigma and advocate for implementation of mental health parity nationwide. In addition to participating in community education and outreach, become a member of, and get involved in, established organizations, such as the American Psychiatric Association, that advocates for psychiatric patients at all levels.
Keep patients connected. Make sure your patients are connected with a primary care provider, and use your psychotherapeutic skills to help patients understand the importance of receiving primary and secondary preventive medical care.
Monitor health and disease. As a physician first and a psychiatrist second, closely monitor your patients for general medical conditions that are related to the presence and treatment of psychiatric disorders. Consider routinely reviewing pertinent lab work with patients—even results of tests ordered by a primary care provider (eg, the metabolic panel and a thyroid-stimulating hormone level in patients taking lithium).
Collaborate with your primary care colleagues; they need your help as much as you can use their help! Make sure your patients witness this collaboration, because it mirrors how you would like them to interact with their primary care provider.
Educate yourself. Education in the essentials of psychiatry-based preventive medical care is key, as we work to more effectively address the increased disability, morbidity, mortality, and overall vulnerability in our patients. Stay “current” on general medical topics by reading the “Med/Psych Update” section of Current Psychiatry and relevant articles in other clinical guides to both integrated and preventive medicine.11
1. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
2. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
3. Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev. 2005;25(4):433-457.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
6. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
7. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
8. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
9. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
10. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
11. McCarron RM, Xiong G, Keenan CR, et al. Preventive medical care in psychiatry: a practical guide for clinicians. Arlington, VA: American Psychiatric Publishing; 2014.
1. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
2. Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107-1115.
3. Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev. 2005;25(4):433-457.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
6. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
7. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
8. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
9. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
10. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
11. McCarron RM, Xiong G, Keenan CR, et al. Preventive medical care in psychiatry: a practical guide for clinicians. Arlington, VA: American Psychiatric Publishing; 2014.
Reducing medical comorbidity and mortality in severe mental illness
People with serious mental illness (SMI) have a life expectancy that is 25 years less than the general population, according to the Centers for Disease Control and Prevention.1 This disparity is partially a consequence of the lack of primary and preventive medical care for those with psychiatric illness. Decades of research have shown that people with SMI experience higher medical morbidity and mortality in addition to facing the stigma of mental illness.
This article aims to advance the idea that longitudinal “cross education” between primary care providers (PCPs) and behavioral health providers (BHPs) is essential in addressing this problem. BHPs include psychiatry clinics, which often are part of a university or large health systems; county-based community mental health programs; and independent mental health clinics that contract with public and private health plans to provide mental health services.
Although suicide and injury account for 40% of the excess mortality in schizophrenia, 60% can be attributed to cardiovascular disease, diabetes, respiratory diseases, and infection.2 Patients with SMI have 2 to 3 times the risk of diabetes, dyslipidemia, hypertension, and obesity.3,4 Furthermore, those with SMI consume more than one-third of tobacco products,5 and 50% to 80% of people with SMI smoke tobacco, an important reversible risk factor for cardiovascular disease.
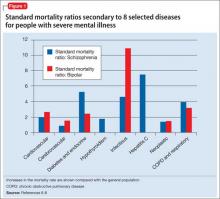
Figure 1 shows that people with SMI are at higher risk of dying from a chronic medical condition, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and hepatitis C6-8—many of which can be managed by primary and preventive medical interventions. These and other conditions often are not diagnosed or effectively managed in patients with SMI.
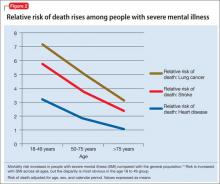
The high prevalence of metabolic syndrome and tobacco dependence among people with SMI accelerates development of cardiovascular disease, as shown by several studies. Bobes et al9 found that the prevalence of metabolic syndrome and cardiovascular risk among patients with SMI is similar to what is found in the general population at 10 to 15 years of greater age. Osborn et al10 demonstrated that people with SMI age 18 to 49 had a higher relative risk of death from coronary heart disease, stroke, and lung cancer than age-matched controls (Figure 2).
It can be said, therefore, that patients with SMI seem to “age” and die prematurely. To reduce this disparity, primary and preventive medical care—especially for cardiovascular disease—must be delivered earlier in life for those with SMI.
Iatrogenic causes of morbidity
Many psychiatric medications, especially second-generation antipsychotics (SGAs), could exacerbate cardiovascular and metabolic conditions by increasing the risk of weight gain, insulin resistance, and dyslipidemia. Antipsychotics that generally are considered to be more effective for refractory psychotic illness (eg, clozapine and olanzapine) are associated with the highest risk of metabolic syndrome. Simon et al11 found a dose-response relationship between olanzapine and clozapine serum concentrations and worsening metabolic outcomes. Valproic acid also can cause significant weight gain and could require monitoring similar to what is done with to SGAs, although there has been less clinical and research attention to this mood stabilizer.
The American Diabetes Association et al12 have published guidelines on monitoring antipsychotic-induced obesity and diabetes, but adoption of these guidelines has been slow. Mackin et al13 found that providers are slow to recognize the elevated rate of obesity and dyslipidemia among psychiatric patients, possibly because of “an alarmingly poor rate of monitoring of metabolic parameters.”
Treating adverse metabolic outcomes also seems to lag behind. The same study13 found that physical health parameters among psychiatric patients continue to become worse even when appropriate health care professionals were notified. Rates of nontreatment for diabetes, dyslipidemia, and hypertension were 30%, 60%, and 88% respectively, according to Nasrallah et al.14
Randomized controlled studies have shown that obesity and metabolic syndrome can be effectively managed using lifestyle and pharmacotherapeutic approaches,15,16 but more research is needed to test long-term outcomes and how to best incorporate these interventions. Newcomer et al17 found that gradually switching an antipsychotic with high risk of metabolic adverse effects to one with lower risk could reduce adverse metabolic outcomes; however, some patients returned to their prior antipsychotic because other medications did not effectively treat their schizophrenia symptoms. Therefore, physicians must pay careful attention to the trade-off between benefits and risks of antipsychotics and make treatment decisions on an individual basis.
Barriers to medical care
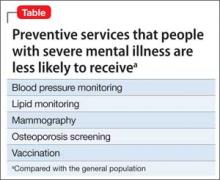
Research has demonstrated that patients with SMI receive less screening and fewer preventive medical services, especially blood pressure monitoring, vaccinations, mammography, lipid monitoring, and osteoporosis screening, compared with the general population (Table).18 Some barriers to preventive services could exist because of demographic factors and medical insurance coverage19 or medical providers’ discomfort with symptoms of SMI,20 although Mitchell et al21 found that disparities in mammography screening could not be explained by the presence of emotional distress in women with SMI.
DiMatteo et al22 reported that patients with SMI are 3 times more likely to be noncompliant with medical treatment. These patients also are less likely to receive sec ondary preventive medical care and invasive medical procedures. Those with SMI who experience acute myocardial infarction are less likely to receive drug therapy, such as a thrombolytic, aspirin, beta blocker, or angiotensin-converting enzyme inhibitor.23 They also are less likely to receive invasive cardiovascular procedures, including cardiac catheterization, angioplasty, and coronary artery bypass grafting.24
Therefore, not only are patients with SMI less likely to receive preventive care, they are also less likely to receive potentially lifesaving treatments for SMI. Because those with SMI might not be able to advocate for themselves in these matters, psychiatric clinicians can improve their patients’ lives by advocating for appropriate medical care despite multiple barriers.
Bridging the gap: Managing mental health in primary care
Research from the 1970s and 1980s demonstrated that most persons who sought help for depression or anxiety received treatment from their PCP, many of whom felt limited by their lack of behavioral health training. Moreover, many patients failed to receive a psychiatric diagnosis or adequate treatment, despite efforts to educate primary care physicians on appropriate diagnosis and treatment of mental illness.
Katon et al25 at the University of Washington developed the collaborative care model in the early 1990s to help improve treatment of depression in primary care settings. This model involved:
• case load review by psychiatrists
• use of nurses and other support staff to help monitor patients’ adherence and treatment response
• use of standardized tools such as the Patient Health Questionnaire to monitor symptoms
• enhancement of patient education with pamphlets or classes.
Studies evaluating the success of collaborative care models found overall improved outcomes, making it the only evidence-based model for integration of behavioral health and primary care.26 As a result, the collaborative care model has been implemented across the United States in primary care clinics and specialty care settings, such as obstetrics and gynecology.27
Regrettably, access to primary care has been hampered by:
• population growth
• a shortage of PCPs
• enrollment of a flood of new patients into the health care marketplace as a result of mandates of the Affordable Care Act (ACA).
In many settings, a psychiatrist might be the patient’s only consistent care provider, and could be thought of as a “primary care psychiatrist.”
To resolve this predicament, mental health professionals need to recognize the unique medical conditions faced by people with SMI, and also might need to provide treatment of common medical conditions, either directly or through collaborative arrangements. Psychiatrists who are capable of managing core medical issues likely will witness improved psychiatric and overall health outcomes in their patients. Consequently, psychiatrists and mental health professionals are increasingly called on to be advocates to improve access to medical services in patients with SMI and to participate in health systems reform.
Managing medical conditions in mental health settings
Although traditional collaborative care involves mental health providers working at primary care sites, other models have emerged that manage chronic disease in behavioral health settings. Federally funded grants for primary behavioral health care integration have allowed community mental health centers to partner with federally qualified health centers to provide on-site primary care services.28
In these models, care managers in mental health clinics:
• link patients to primary care services
• encourage lifestyle changes to improve their overall health
• identify and overcome barriers to receiving care
• track clinical outcomes in a registry format.
Currently, 126 mental health sites in the United States have received these grants and are working toward greater integration of primary care.
In addition, the ACA provided funding for “health homes” in non-primary care settings, which includes SMI. These health homes cannot provide direct primary care, but can deliver comprehensive care management, care coordination, health promotion, comprehensive transitional care services between facilities, individual and family support, and referral to community social support services. In these health homes, a PCP can act as a consultant to help establish priorities for disease management and improving health status.29 The PCP consultant also can support psychiatric staff and collaborate with providers who want to provide some direct care of medical conditions.30
Last, some behavioral health sites are choosing to apply for Federally Qualified Health Clinic status or add primary care services to their clinics, with the hope that sustainable funding will become available. Without additional funding to cover the limited reimbursement provided by public payers, such as Medicaid and Medicare, these models might be unsustainable. Current innovations in health care funding reform hopefully will offer solutions for sites to provide medical care in the natural “medical home” of the SMI population.
Bottom Line
Psychiatric providers are in a favorable position to develop and oversee a partnership with primary care physicians with the goal of addressing significant and often lethal health disparities among those with mental illness. Psychiatric providers must use evidence-based practices that include assessment and prevention of cardiopulmonary, metabolic, infectious, and oncologic disorders. True primary care–behavioral health integration must include longitudinal “cross education” and changes in health care policy, with an emphasis on decreasing morbidity and mortality in psychiatric patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
2. Parks J, Svendsen D, Singer P, et al, eds. Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
3. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
4. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trails of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
5. Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(2):212-222.
6. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
7. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
8. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based study. J Gen Intern Med. 2006;21(11):1133-1137.
9. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics; results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274.
10. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database [Erratum in: Arch Gen Psychiatry. 2007;64(6):736]. Arch Gen Psychiatry. 2007;64(2):242-249.
11. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041-1050.
12. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomized controlled trials. Br J Psychiatry. 2008; 193(2):101-107.
16. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medication used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530.
17. Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120.
18. Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32(5):519-543.
19. Xiong GL, Iosif AM, Bermudes RA, et al. Preventive medical services use among community mental health patients with severe mental illness: the influence of gender and insurance coverage. Prim Care Companion J Clin Psychiatry. 2010;12(5). doi: 10.4088/PCC.09m00927gre.
20. Daub S. Turning toward treating the seriously mentally ill in primary care. Fam Syst Health. 2014;32(1):12-13.
21. Mitchell A, Pereira IE, Yadegarfar M, et al. Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry. 2014;205(6):428-435.
22. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
23. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
24. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
25. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
26. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
27. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40.
28. Substance Abuse and Mental Health Services Administration. Request for Applications (RFA) No. SM- 09-011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
29. Parks J. Behavioral health homes. In: Integrated care: working at the interface of primary care and behavioral health. Raney LE, ed. Arlington, VA: American Psychiatric Publishing; 2015:195.
30. Raney L. Integrated care: the evolving role of psychiatry in the era of health care reform. Psychiatr Serv. 2013;64(11):1076-1078.
People with serious mental illness (SMI) have a life expectancy that is 25 years less than the general population, according to the Centers for Disease Control and Prevention.1 This disparity is partially a consequence of the lack of primary and preventive medical care for those with psychiatric illness. Decades of research have shown that people with SMI experience higher medical morbidity and mortality in addition to facing the stigma of mental illness.
This article aims to advance the idea that longitudinal “cross education” between primary care providers (PCPs) and behavioral health providers (BHPs) is essential in addressing this problem. BHPs include psychiatry clinics, which often are part of a university or large health systems; county-based community mental health programs; and independent mental health clinics that contract with public and private health plans to provide mental health services.
Although suicide and injury account for 40% of the excess mortality in schizophrenia, 60% can be attributed to cardiovascular disease, diabetes, respiratory diseases, and infection.2 Patients with SMI have 2 to 3 times the risk of diabetes, dyslipidemia, hypertension, and obesity.3,4 Furthermore, those with SMI consume more than one-third of tobacco products,5 and 50% to 80% of people with SMI smoke tobacco, an important reversible risk factor for cardiovascular disease.
Figure 1 shows that people with SMI are at higher risk of dying from a chronic medical condition, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and hepatitis C6-8—many of which can be managed by primary and preventive medical interventions. These and other conditions often are not diagnosed or effectively managed in patients with SMI.
The high prevalence of metabolic syndrome and tobacco dependence among people with SMI accelerates development of cardiovascular disease, as shown by several studies. Bobes et al9 found that the prevalence of metabolic syndrome and cardiovascular risk among patients with SMI is similar to what is found in the general population at 10 to 15 years of greater age. Osborn et al10 demonstrated that people with SMI age 18 to 49 had a higher relative risk of death from coronary heart disease, stroke, and lung cancer than age-matched controls (Figure 2).
It can be said, therefore, that patients with SMI seem to “age” and die prematurely. To reduce this disparity, primary and preventive medical care—especially for cardiovascular disease—must be delivered earlier in life for those with SMI.
Iatrogenic causes of morbidity
Many psychiatric medications, especially second-generation antipsychotics (SGAs), could exacerbate cardiovascular and metabolic conditions by increasing the risk of weight gain, insulin resistance, and dyslipidemia. Antipsychotics that generally are considered to be more effective for refractory psychotic illness (eg, clozapine and olanzapine) are associated with the highest risk of metabolic syndrome. Simon et al11 found a dose-response relationship between olanzapine and clozapine serum concentrations and worsening metabolic outcomes. Valproic acid also can cause significant weight gain and could require monitoring similar to what is done with to SGAs, although there has been less clinical and research attention to this mood stabilizer.
The American Diabetes Association et al12 have published guidelines on monitoring antipsychotic-induced obesity and diabetes, but adoption of these guidelines has been slow. Mackin et al13 found that providers are slow to recognize the elevated rate of obesity and dyslipidemia among psychiatric patients, possibly because of “an alarmingly poor rate of monitoring of metabolic parameters.”
Treating adverse metabolic outcomes also seems to lag behind. The same study13 found that physical health parameters among psychiatric patients continue to become worse even when appropriate health care professionals were notified. Rates of nontreatment for diabetes, dyslipidemia, and hypertension were 30%, 60%, and 88% respectively, according to Nasrallah et al.14
Randomized controlled studies have shown that obesity and metabolic syndrome can be effectively managed using lifestyle and pharmacotherapeutic approaches,15,16 but more research is needed to test long-term outcomes and how to best incorporate these interventions. Newcomer et al17 found that gradually switching an antipsychotic with high risk of metabolic adverse effects to one with lower risk could reduce adverse metabolic outcomes; however, some patients returned to their prior antipsychotic because other medications did not effectively treat their schizophrenia symptoms. Therefore, physicians must pay careful attention to the trade-off between benefits and risks of antipsychotics and make treatment decisions on an individual basis.
Barriers to medical care
Research has demonstrated that patients with SMI receive less screening and fewer preventive medical services, especially blood pressure monitoring, vaccinations, mammography, lipid monitoring, and osteoporosis screening, compared with the general population (Table).18 Some barriers to preventive services could exist because of demographic factors and medical insurance coverage19 or medical providers’ discomfort with symptoms of SMI,20 although Mitchell et al21 found that disparities in mammography screening could not be explained by the presence of emotional distress in women with SMI.
DiMatteo et al22 reported that patients with SMI are 3 times more likely to be noncompliant with medical treatment. These patients also are less likely to receive sec ondary preventive medical care and invasive medical procedures. Those with SMI who experience acute myocardial infarction are less likely to receive drug therapy, such as a thrombolytic, aspirin, beta blocker, or angiotensin-converting enzyme inhibitor.23 They also are less likely to receive invasive cardiovascular procedures, including cardiac catheterization, angioplasty, and coronary artery bypass grafting.24
Therefore, not only are patients with SMI less likely to receive preventive care, they are also less likely to receive potentially lifesaving treatments for SMI. Because those with SMI might not be able to advocate for themselves in these matters, psychiatric clinicians can improve their patients’ lives by advocating for appropriate medical care despite multiple barriers.
Bridging the gap: Managing mental health in primary care
Research from the 1970s and 1980s demonstrated that most persons who sought help for depression or anxiety received treatment from their PCP, many of whom felt limited by their lack of behavioral health training. Moreover, many patients failed to receive a psychiatric diagnosis or adequate treatment, despite efforts to educate primary care physicians on appropriate diagnosis and treatment of mental illness.
Katon et al25 at the University of Washington developed the collaborative care model in the early 1990s to help improve treatment of depression in primary care settings. This model involved:
• case load review by psychiatrists
• use of nurses and other support staff to help monitor patients’ adherence and treatment response
• use of standardized tools such as the Patient Health Questionnaire to monitor symptoms
• enhancement of patient education with pamphlets or classes.
Studies evaluating the success of collaborative care models found overall improved outcomes, making it the only evidence-based model for integration of behavioral health and primary care.26 As a result, the collaborative care model has been implemented across the United States in primary care clinics and specialty care settings, such as obstetrics and gynecology.27
Regrettably, access to primary care has been hampered by:
• population growth
• a shortage of PCPs
• enrollment of a flood of new patients into the health care marketplace as a result of mandates of the Affordable Care Act (ACA).
In many settings, a psychiatrist might be the patient’s only consistent care provider, and could be thought of as a “primary care psychiatrist.”
To resolve this predicament, mental health professionals need to recognize the unique medical conditions faced by people with SMI, and also might need to provide treatment of common medical conditions, either directly or through collaborative arrangements. Psychiatrists who are capable of managing core medical issues likely will witness improved psychiatric and overall health outcomes in their patients. Consequently, psychiatrists and mental health professionals are increasingly called on to be advocates to improve access to medical services in patients with SMI and to participate in health systems reform.
Managing medical conditions in mental health settings
Although traditional collaborative care involves mental health providers working at primary care sites, other models have emerged that manage chronic disease in behavioral health settings. Federally funded grants for primary behavioral health care integration have allowed community mental health centers to partner with federally qualified health centers to provide on-site primary care services.28
In these models, care managers in mental health clinics:
• link patients to primary care services
• encourage lifestyle changes to improve their overall health
• identify and overcome barriers to receiving care
• track clinical outcomes in a registry format.
Currently, 126 mental health sites in the United States have received these grants and are working toward greater integration of primary care.
In addition, the ACA provided funding for “health homes” in non-primary care settings, which includes SMI. These health homes cannot provide direct primary care, but can deliver comprehensive care management, care coordination, health promotion, comprehensive transitional care services between facilities, individual and family support, and referral to community social support services. In these health homes, a PCP can act as a consultant to help establish priorities for disease management and improving health status.29 The PCP consultant also can support psychiatric staff and collaborate with providers who want to provide some direct care of medical conditions.30
Last, some behavioral health sites are choosing to apply for Federally Qualified Health Clinic status or add primary care services to their clinics, with the hope that sustainable funding will become available. Without additional funding to cover the limited reimbursement provided by public payers, such as Medicaid and Medicare, these models might be unsustainable. Current innovations in health care funding reform hopefully will offer solutions for sites to provide medical care in the natural “medical home” of the SMI population.
Bottom Line
Psychiatric providers are in a favorable position to develop and oversee a partnership with primary care physicians with the goal of addressing significant and often lethal health disparities among those with mental illness. Psychiatric providers must use evidence-based practices that include assessment and prevention of cardiopulmonary, metabolic, infectious, and oncologic disorders. True primary care–behavioral health integration must include longitudinal “cross education” and changes in health care policy, with an emphasis on decreasing morbidity and mortality in psychiatric patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
People with serious mental illness (SMI) have a life expectancy that is 25 years less than the general population, according to the Centers for Disease Control and Prevention.1 This disparity is partially a consequence of the lack of primary and preventive medical care for those with psychiatric illness. Decades of research have shown that people with SMI experience higher medical morbidity and mortality in addition to facing the stigma of mental illness.
This article aims to advance the idea that longitudinal “cross education” between primary care providers (PCPs) and behavioral health providers (BHPs) is essential in addressing this problem. BHPs include psychiatry clinics, which often are part of a university or large health systems; county-based community mental health programs; and independent mental health clinics that contract with public and private health plans to provide mental health services.
Although suicide and injury account for 40% of the excess mortality in schizophrenia, 60% can be attributed to cardiovascular disease, diabetes, respiratory diseases, and infection.2 Patients with SMI have 2 to 3 times the risk of diabetes, dyslipidemia, hypertension, and obesity.3,4 Furthermore, those with SMI consume more than one-third of tobacco products,5 and 50% to 80% of people with SMI smoke tobacco, an important reversible risk factor for cardiovascular disease.
Figure 1 shows that people with SMI are at higher risk of dying from a chronic medical condition, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and hepatitis C6-8—many of which can be managed by primary and preventive medical interventions. These and other conditions often are not diagnosed or effectively managed in patients with SMI.
The high prevalence of metabolic syndrome and tobacco dependence among people with SMI accelerates development of cardiovascular disease, as shown by several studies. Bobes et al9 found that the prevalence of metabolic syndrome and cardiovascular risk among patients with SMI is similar to what is found in the general population at 10 to 15 years of greater age. Osborn et al10 demonstrated that people with SMI age 18 to 49 had a higher relative risk of death from coronary heart disease, stroke, and lung cancer than age-matched controls (Figure 2).
It can be said, therefore, that patients with SMI seem to “age” and die prematurely. To reduce this disparity, primary and preventive medical care—especially for cardiovascular disease—must be delivered earlier in life for those with SMI.
Iatrogenic causes of morbidity
Many psychiatric medications, especially second-generation antipsychotics (SGAs), could exacerbate cardiovascular and metabolic conditions by increasing the risk of weight gain, insulin resistance, and dyslipidemia. Antipsychotics that generally are considered to be more effective for refractory psychotic illness (eg, clozapine and olanzapine) are associated with the highest risk of metabolic syndrome. Simon et al11 found a dose-response relationship between olanzapine and clozapine serum concentrations and worsening metabolic outcomes. Valproic acid also can cause significant weight gain and could require monitoring similar to what is done with to SGAs, although there has been less clinical and research attention to this mood stabilizer.
The American Diabetes Association et al12 have published guidelines on monitoring antipsychotic-induced obesity and diabetes, but adoption of these guidelines has been slow. Mackin et al13 found that providers are slow to recognize the elevated rate of obesity and dyslipidemia among psychiatric patients, possibly because of “an alarmingly poor rate of monitoring of metabolic parameters.”
Treating adverse metabolic outcomes also seems to lag behind. The same study13 found that physical health parameters among psychiatric patients continue to become worse even when appropriate health care professionals were notified. Rates of nontreatment for diabetes, dyslipidemia, and hypertension were 30%, 60%, and 88% respectively, according to Nasrallah et al.14
Randomized controlled studies have shown that obesity and metabolic syndrome can be effectively managed using lifestyle and pharmacotherapeutic approaches,15,16 but more research is needed to test long-term outcomes and how to best incorporate these interventions. Newcomer et al17 found that gradually switching an antipsychotic with high risk of metabolic adverse effects to one with lower risk could reduce adverse metabolic outcomes; however, some patients returned to their prior antipsychotic because other medications did not effectively treat their schizophrenia symptoms. Therefore, physicians must pay careful attention to the trade-off between benefits and risks of antipsychotics and make treatment decisions on an individual basis.
Barriers to medical care
Research has demonstrated that patients with SMI receive less screening and fewer preventive medical services, especially blood pressure monitoring, vaccinations, mammography, lipid monitoring, and osteoporosis screening, compared with the general population (Table).18 Some barriers to preventive services could exist because of demographic factors and medical insurance coverage19 or medical providers’ discomfort with symptoms of SMI,20 although Mitchell et al21 found that disparities in mammography screening could not be explained by the presence of emotional distress in women with SMI.
DiMatteo et al22 reported that patients with SMI are 3 times more likely to be noncompliant with medical treatment. These patients also are less likely to receive sec ondary preventive medical care and invasive medical procedures. Those with SMI who experience acute myocardial infarction are less likely to receive drug therapy, such as a thrombolytic, aspirin, beta blocker, or angiotensin-converting enzyme inhibitor.23 They also are less likely to receive invasive cardiovascular procedures, including cardiac catheterization, angioplasty, and coronary artery bypass grafting.24
Therefore, not only are patients with SMI less likely to receive preventive care, they are also less likely to receive potentially lifesaving treatments for SMI. Because those with SMI might not be able to advocate for themselves in these matters, psychiatric clinicians can improve their patients’ lives by advocating for appropriate medical care despite multiple barriers.
Bridging the gap: Managing mental health in primary care
Research from the 1970s and 1980s demonstrated that most persons who sought help for depression or anxiety received treatment from their PCP, many of whom felt limited by their lack of behavioral health training. Moreover, many patients failed to receive a psychiatric diagnosis or adequate treatment, despite efforts to educate primary care physicians on appropriate diagnosis and treatment of mental illness.
Katon et al25 at the University of Washington developed the collaborative care model in the early 1990s to help improve treatment of depression in primary care settings. This model involved:
• case load review by psychiatrists
• use of nurses and other support staff to help monitor patients’ adherence and treatment response
• use of standardized tools such as the Patient Health Questionnaire to monitor symptoms
• enhancement of patient education with pamphlets or classes.
Studies evaluating the success of collaborative care models found overall improved outcomes, making it the only evidence-based model for integration of behavioral health and primary care.26 As a result, the collaborative care model has been implemented across the United States in primary care clinics and specialty care settings, such as obstetrics and gynecology.27
Regrettably, access to primary care has been hampered by:
• population growth
• a shortage of PCPs
• enrollment of a flood of new patients into the health care marketplace as a result of mandates of the Affordable Care Act (ACA).
In many settings, a psychiatrist might be the patient’s only consistent care provider, and could be thought of as a “primary care psychiatrist.”
To resolve this predicament, mental health professionals need to recognize the unique medical conditions faced by people with SMI, and also might need to provide treatment of common medical conditions, either directly or through collaborative arrangements. Psychiatrists who are capable of managing core medical issues likely will witness improved psychiatric and overall health outcomes in their patients. Consequently, psychiatrists and mental health professionals are increasingly called on to be advocates to improve access to medical services in patients with SMI and to participate in health systems reform.
Managing medical conditions in mental health settings
Although traditional collaborative care involves mental health providers working at primary care sites, other models have emerged that manage chronic disease in behavioral health settings. Federally funded grants for primary behavioral health care integration have allowed community mental health centers to partner with federally qualified health centers to provide on-site primary care services.28
In these models, care managers in mental health clinics:
• link patients to primary care services
• encourage lifestyle changes to improve their overall health
• identify and overcome barriers to receiving care
• track clinical outcomes in a registry format.
Currently, 126 mental health sites in the United States have received these grants and are working toward greater integration of primary care.
In addition, the ACA provided funding for “health homes” in non-primary care settings, which includes SMI. These health homes cannot provide direct primary care, but can deliver comprehensive care management, care coordination, health promotion, comprehensive transitional care services between facilities, individual and family support, and referral to community social support services. In these health homes, a PCP can act as a consultant to help establish priorities for disease management and improving health status.29 The PCP consultant also can support psychiatric staff and collaborate with providers who want to provide some direct care of medical conditions.30
Last, some behavioral health sites are choosing to apply for Federally Qualified Health Clinic status or add primary care services to their clinics, with the hope that sustainable funding will become available. Without additional funding to cover the limited reimbursement provided by public payers, such as Medicaid and Medicare, these models might be unsustainable. Current innovations in health care funding reform hopefully will offer solutions for sites to provide medical care in the natural “medical home” of the SMI population.
Bottom Line
Psychiatric providers are in a favorable position to develop and oversee a partnership with primary care physicians with the goal of addressing significant and often lethal health disparities among those with mental illness. Psychiatric providers must use evidence-based practices that include assessment and prevention of cardiopulmonary, metabolic, infectious, and oncologic disorders. True primary care–behavioral health integration must include longitudinal “cross education” and changes in health care policy, with an emphasis on decreasing morbidity and mortality in psychiatric patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
2. Parks J, Svendsen D, Singer P, et al, eds. Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
3. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
4. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trails of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
5. Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(2):212-222.
6. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
7. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
8. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based study. J Gen Intern Med. 2006;21(11):1133-1137.
9. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics; results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274.
10. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database [Erratum in: Arch Gen Psychiatry. 2007;64(6):736]. Arch Gen Psychiatry. 2007;64(2):242-249.
11. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041-1050.
12. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomized controlled trials. Br J Psychiatry. 2008; 193(2):101-107.
16. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medication used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530.
17. Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120.
18. Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32(5):519-543.
19. Xiong GL, Iosif AM, Bermudes RA, et al. Preventive medical services use among community mental health patients with severe mental illness: the influence of gender and insurance coverage. Prim Care Companion J Clin Psychiatry. 2010;12(5). doi: 10.4088/PCC.09m00927gre.
20. Daub S. Turning toward treating the seriously mentally ill in primary care. Fam Syst Health. 2014;32(1):12-13.
21. Mitchell A, Pereira IE, Yadegarfar M, et al. Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry. 2014;205(6):428-435.
22. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
23. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
24. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
25. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
26. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
27. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40.
28. Substance Abuse and Mental Health Services Administration. Request for Applications (RFA) No. SM- 09-011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
29. Parks J. Behavioral health homes. In: Integrated care: working at the interface of primary care and behavioral health. Raney LE, ed. Arlington, VA: American Psychiatric Publishing; 2015:195.
30. Raney L. Integrated care: the evolving role of psychiatry in the era of health care reform. Psychiatr Serv. 2013;64(11):1076-1078.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
2. Parks J, Svendsen D, Singer P, et al, eds. Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
3. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
4. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trails of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
5. Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(2):212-222.
6. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
7. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
8. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based study. J Gen Intern Med. 2006;21(11):1133-1137.
9. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics; results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274.
10. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database [Erratum in: Arch Gen Psychiatry. 2007;64(6):736]. Arch Gen Psychiatry. 2007;64(2):242-249.
11. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041-1050.
12. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomized controlled trials. Br J Psychiatry. 2008; 193(2):101-107.
16. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medication used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530.
17. Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120.
18. Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32(5):519-543.
19. Xiong GL, Iosif AM, Bermudes RA, et al. Preventive medical services use among community mental health patients with severe mental illness: the influence of gender and insurance coverage. Prim Care Companion J Clin Psychiatry. 2010;12(5). doi: 10.4088/PCC.09m00927gre.
20. Daub S. Turning toward treating the seriously mentally ill in primary care. Fam Syst Health. 2014;32(1):12-13.
21. Mitchell A, Pereira IE, Yadegarfar M, et al. Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry. 2014;205(6):428-435.
22. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
23. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
24. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
25. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
26. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
27. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40.
28. Substance Abuse and Mental Health Services Administration. Request for Applications (RFA) No. SM- 09-011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
29. Parks J. Behavioral health homes. In: Integrated care: working at the interface of primary care and behavioral health. Raney LE, ed. Arlington, VA: American Psychiatric Publishing; 2015:195.
30. Raney L. Integrated care: the evolving role of psychiatry in the era of health care reform. Psychiatr Serv. 2013;64(11):1076-1078.
WCD: Smoking tied to worse occupational hand eczema
VANCOUVER, B.C. – Occupational hand eczema is worse and more persistent in smokers than nonsmokers, a large prospective cohort study found.
“Tobacco smoking is associated with work absenteeism and with not staying in the workforce due to occupational hand eczema. Smoking confers a worse prognosis and interferes with the outcome of prevention programs,” Dr. Richard Brans said at the World Congress of Dermatology.
Hand eczema is the most common occupational skin disease. Smoking might worsen signs and symptoms by inducing proinflammatory effects in the skin, said Dr. Brans, a dermatologist at the University of Osnabrück, Germany.
To better assess the link between smoking and hand eczema, he and his associates carried out a prospective 3-year study of 1,095 patients from throughout Germany. The patients initially had attended a 6-week residential treatment program for hand eczema that was followed by a 3-week outpatient program. Smokers comprised about half of the patients and resembled nonsmokers in terms of gender, general atopy, and degree of professional or occupational exposures, such as wetting or soiling the hands at work, Dr. Brans said. However, smokers were significantly younger than nonsmokers and were more likely to have allergic contact dermatitis, he noted.
The inpatient phase of the program markedly benefited both smokers and nonsmokers, but notably, smokers had significantly worse symptoms and signs of hand eczema at all time points assessed, Dr. Brans said. Furthermore, smokers missed an average of 37 days of work because of occupational hand eczema in the year before the program, compared with only 25 days for nonsmokers (P = .001), and smokers continued to miss more days of work because of hand eczema in the year after completing the program (P = .023), he reported. Significantly more smokers also left their professions because of their hand eczema, even after completing the prevention program (P = .021), he added.
The study found no link between number of cigarettes smoked per day and severity of hand eczema, Dr. Brans said. Smoking history was self-reported, and the study design excluded patients who changed their smoking behavior during follow-up, he noted. In addition, the researchers did not assess whether other factors associated with smoking might have confounded the association between smoking and severity of hand eczema, he said.
Dr. Brans reported no relevant disclosures.
VANCOUVER, B.C. – Occupational hand eczema is worse and more persistent in smokers than nonsmokers, a large prospective cohort study found.
“Tobacco smoking is associated with work absenteeism and with not staying in the workforce due to occupational hand eczema. Smoking confers a worse prognosis and interferes with the outcome of prevention programs,” Dr. Richard Brans said at the World Congress of Dermatology.
Hand eczema is the most common occupational skin disease. Smoking might worsen signs and symptoms by inducing proinflammatory effects in the skin, said Dr. Brans, a dermatologist at the University of Osnabrück, Germany.
To better assess the link between smoking and hand eczema, he and his associates carried out a prospective 3-year study of 1,095 patients from throughout Germany. The patients initially had attended a 6-week residential treatment program for hand eczema that was followed by a 3-week outpatient program. Smokers comprised about half of the patients and resembled nonsmokers in terms of gender, general atopy, and degree of professional or occupational exposures, such as wetting or soiling the hands at work, Dr. Brans said. However, smokers were significantly younger than nonsmokers and were more likely to have allergic contact dermatitis, he noted.
The inpatient phase of the program markedly benefited both smokers and nonsmokers, but notably, smokers had significantly worse symptoms and signs of hand eczema at all time points assessed, Dr. Brans said. Furthermore, smokers missed an average of 37 days of work because of occupational hand eczema in the year before the program, compared with only 25 days for nonsmokers (P = .001), and smokers continued to miss more days of work because of hand eczema in the year after completing the program (P = .023), he reported. Significantly more smokers also left their professions because of their hand eczema, even after completing the prevention program (P = .021), he added.
The study found no link between number of cigarettes smoked per day and severity of hand eczema, Dr. Brans said. Smoking history was self-reported, and the study design excluded patients who changed their smoking behavior during follow-up, he noted. In addition, the researchers did not assess whether other factors associated with smoking might have confounded the association between smoking and severity of hand eczema, he said.
Dr. Brans reported no relevant disclosures.
VANCOUVER, B.C. – Occupational hand eczema is worse and more persistent in smokers than nonsmokers, a large prospective cohort study found.
“Tobacco smoking is associated with work absenteeism and with not staying in the workforce due to occupational hand eczema. Smoking confers a worse prognosis and interferes with the outcome of prevention programs,” Dr. Richard Brans said at the World Congress of Dermatology.
Hand eczema is the most common occupational skin disease. Smoking might worsen signs and symptoms by inducing proinflammatory effects in the skin, said Dr. Brans, a dermatologist at the University of Osnabrück, Germany.
To better assess the link between smoking and hand eczema, he and his associates carried out a prospective 3-year study of 1,095 patients from throughout Germany. The patients initially had attended a 6-week residential treatment program for hand eczema that was followed by a 3-week outpatient program. Smokers comprised about half of the patients and resembled nonsmokers in terms of gender, general atopy, and degree of professional or occupational exposures, such as wetting or soiling the hands at work, Dr. Brans said. However, smokers were significantly younger than nonsmokers and were more likely to have allergic contact dermatitis, he noted.
The inpatient phase of the program markedly benefited both smokers and nonsmokers, but notably, smokers had significantly worse symptoms and signs of hand eczema at all time points assessed, Dr. Brans said. Furthermore, smokers missed an average of 37 days of work because of occupational hand eczema in the year before the program, compared with only 25 days for nonsmokers (P = .001), and smokers continued to miss more days of work because of hand eczema in the year after completing the program (P = .023), he reported. Significantly more smokers also left their professions because of their hand eczema, even after completing the prevention program (P = .021), he added.
The study found no link between number of cigarettes smoked per day and severity of hand eczema, Dr. Brans said. Smoking history was self-reported, and the study design excluded patients who changed their smoking behavior during follow-up, he noted. In addition, the researchers did not assess whether other factors associated with smoking might have confounded the association between smoking and severity of hand eczema, he said.
Dr. Brans reported no relevant disclosures.
AT WDC 2015
Key clinical point: Smoking might worsen the signs and symptoms of occupational hand eczema.
Major finding: Smokers had significantly worse symptoms and signs of hand eczema at all time points assessed.
Data source: Three-year prospective study of 1,095 smokers and nonsmokers with occupational hand eczema.
Disclosures: Dr. Brans reported no relevant conflicts of interest.
DDW: New scale measures impact of IBD on sexual function in men
WASHINGTON – Almost 40% of men with inflammatory bowel disease said the disease had a negative effect on their libido and almost 30% said that the disease had prevented them from having sex, in a study that used a new scale designed to evaluate sexual dysfunction in men with IBD, Dr. Aoibhlinn O’Toole reported at the annual Digestive Disease Week.
Dr. O’Toole, an IBD fellow at Beth Israel Deaconess Medical Center, Boston, developed the IBD Sexual Dysfunction Scale (IBDSDS) with her associates at Beth Israel Deaconess and Brigham and Women’s Hospital. The results of the study were based on 175 responses to the IBDSDS questionnaire, which were sent to all the adult male patients treated at the IBD clinics at both medical centers. The IBD-specific tool is now being validated.
Because there has been no IBD-specific scale to measure sexual dysfunction in this group of patients, they developed the IBDSDS and conducted the study to evaluate the prevalence of sexual dysfunction in male patients with IBD and identify factors associated with sexual dysfunction. The process of designing the scale included a literature search of generic questionnaires to identify relevant domains of sexual function and the addition of IBD-specific questions related to the effects of symptoms, medications, and surgery on sexual function.
Despite significant issues that can clearly affect sexuality in this group of patients – including perianal disease and the effect of treatment and symptoms on body image, intimacy, and sexual function – the extent and effects of sexual dysfunction in men with IBD is not well known, Dr. O’Toole pointed out.
The 43 questions in the scale include those that pertain to libido, ejaculation, satisfaction, and body image, as well as elements of two validated screening tools, the International Index of Erectile Function (IIEF), the gold standard screening tool for erectile dysfunction; and the Patient Health Questionnaire 9 (PHQ-9), for depression.
The average age of the 175 respondents was 43 years and the average duration of IBD was 14 years; 57% had Crohn’s disease, 33% had had surgery, 6% had chronic pain, 2% used narcotics, 12% had hypertension, and 4% had diabetes. Almost 90% were in a relationship that “could involve sexual activity” and 2% had not been sexually active in the previous year. In addition, 14% said that they felt that IBD had caused a breakup in an existing relationship, and 21% said they had felt hesitant about starting a new relationship because of IBD.
As for the effects of IBD on sexual function, in the last year, 38% felt that IBD had a negative effect on their libido, 27% said IBD prevented them from having sex, and 18% said that IBD caused problems during sex. In addition, 20% said that IBD made them feel guilty about having sex and 30% said they feared having sex; 2% reported having erectile dysfunction.
The use of erectile-enhancing medications were reported by 23% (prescribed mostly by doctors other than the physician treating the IBD) and 5% were taking testosterone to enhance sexual function. While 78% said they were comfortable speaking about sexual function with their gastroenterologist, only 10% said that their gastroenterologist had initiated a discussion about this topic, Dr. O’Toole said.
Based on other analyses of the results, “we found that impaired sexual function was associated with older age, longer duration of disease, active disease, comorbid depression, presence of an ostomy, and diabetes,” she added.
While the thrombotic risks of testosterone are clear, Dr. O’Toole noted that in patients with IBD, the effects of phosphodiesterase-5 (PDE5) inhibitors like sildenafil (Viagra) on the microcirculation within the gastrointestinal tract and whether the effects of these drugs contribute to microscopic ischemia in the GI tract is unclear, she said. Dr. O’Toole had no relevant financial disclosures.
WASHINGTON – Almost 40% of men with inflammatory bowel disease said the disease had a negative effect on their libido and almost 30% said that the disease had prevented them from having sex, in a study that used a new scale designed to evaluate sexual dysfunction in men with IBD, Dr. Aoibhlinn O’Toole reported at the annual Digestive Disease Week.
Dr. O’Toole, an IBD fellow at Beth Israel Deaconess Medical Center, Boston, developed the IBD Sexual Dysfunction Scale (IBDSDS) with her associates at Beth Israel Deaconess and Brigham and Women’s Hospital. The results of the study were based on 175 responses to the IBDSDS questionnaire, which were sent to all the adult male patients treated at the IBD clinics at both medical centers. The IBD-specific tool is now being validated.
Because there has been no IBD-specific scale to measure sexual dysfunction in this group of patients, they developed the IBDSDS and conducted the study to evaluate the prevalence of sexual dysfunction in male patients with IBD and identify factors associated with sexual dysfunction. The process of designing the scale included a literature search of generic questionnaires to identify relevant domains of sexual function and the addition of IBD-specific questions related to the effects of symptoms, medications, and surgery on sexual function.
Despite significant issues that can clearly affect sexuality in this group of patients – including perianal disease and the effect of treatment and symptoms on body image, intimacy, and sexual function – the extent and effects of sexual dysfunction in men with IBD is not well known, Dr. O’Toole pointed out.
The 43 questions in the scale include those that pertain to libido, ejaculation, satisfaction, and body image, as well as elements of two validated screening tools, the International Index of Erectile Function (IIEF), the gold standard screening tool for erectile dysfunction; and the Patient Health Questionnaire 9 (PHQ-9), for depression.
The average age of the 175 respondents was 43 years and the average duration of IBD was 14 years; 57% had Crohn’s disease, 33% had had surgery, 6% had chronic pain, 2% used narcotics, 12% had hypertension, and 4% had diabetes. Almost 90% were in a relationship that “could involve sexual activity” and 2% had not been sexually active in the previous year. In addition, 14% said that they felt that IBD had caused a breakup in an existing relationship, and 21% said they had felt hesitant about starting a new relationship because of IBD.
As for the effects of IBD on sexual function, in the last year, 38% felt that IBD had a negative effect on their libido, 27% said IBD prevented them from having sex, and 18% said that IBD caused problems during sex. In addition, 20% said that IBD made them feel guilty about having sex and 30% said they feared having sex; 2% reported having erectile dysfunction.
The use of erectile-enhancing medications were reported by 23% (prescribed mostly by doctors other than the physician treating the IBD) and 5% were taking testosterone to enhance sexual function. While 78% said they were comfortable speaking about sexual function with their gastroenterologist, only 10% said that their gastroenterologist had initiated a discussion about this topic, Dr. O’Toole said.
Based on other analyses of the results, “we found that impaired sexual function was associated with older age, longer duration of disease, active disease, comorbid depression, presence of an ostomy, and diabetes,” she added.
While the thrombotic risks of testosterone are clear, Dr. O’Toole noted that in patients with IBD, the effects of phosphodiesterase-5 (PDE5) inhibitors like sildenafil (Viagra) on the microcirculation within the gastrointestinal tract and whether the effects of these drugs contribute to microscopic ischemia in the GI tract is unclear, she said. Dr. O’Toole had no relevant financial disclosures.
WASHINGTON – Almost 40% of men with inflammatory bowel disease said the disease had a negative effect on their libido and almost 30% said that the disease had prevented them from having sex, in a study that used a new scale designed to evaluate sexual dysfunction in men with IBD, Dr. Aoibhlinn O’Toole reported at the annual Digestive Disease Week.
Dr. O’Toole, an IBD fellow at Beth Israel Deaconess Medical Center, Boston, developed the IBD Sexual Dysfunction Scale (IBDSDS) with her associates at Beth Israel Deaconess and Brigham and Women’s Hospital. The results of the study were based on 175 responses to the IBDSDS questionnaire, which were sent to all the adult male patients treated at the IBD clinics at both medical centers. The IBD-specific tool is now being validated.
Because there has been no IBD-specific scale to measure sexual dysfunction in this group of patients, they developed the IBDSDS and conducted the study to evaluate the prevalence of sexual dysfunction in male patients with IBD and identify factors associated with sexual dysfunction. The process of designing the scale included a literature search of generic questionnaires to identify relevant domains of sexual function and the addition of IBD-specific questions related to the effects of symptoms, medications, and surgery on sexual function.
Despite significant issues that can clearly affect sexuality in this group of patients – including perianal disease and the effect of treatment and symptoms on body image, intimacy, and sexual function – the extent and effects of sexual dysfunction in men with IBD is not well known, Dr. O’Toole pointed out.
The 43 questions in the scale include those that pertain to libido, ejaculation, satisfaction, and body image, as well as elements of two validated screening tools, the International Index of Erectile Function (IIEF), the gold standard screening tool for erectile dysfunction; and the Patient Health Questionnaire 9 (PHQ-9), for depression.
The average age of the 175 respondents was 43 years and the average duration of IBD was 14 years; 57% had Crohn’s disease, 33% had had surgery, 6% had chronic pain, 2% used narcotics, 12% had hypertension, and 4% had diabetes. Almost 90% were in a relationship that “could involve sexual activity” and 2% had not been sexually active in the previous year. In addition, 14% said that they felt that IBD had caused a breakup in an existing relationship, and 21% said they had felt hesitant about starting a new relationship because of IBD.
As for the effects of IBD on sexual function, in the last year, 38% felt that IBD had a negative effect on their libido, 27% said IBD prevented them from having sex, and 18% said that IBD caused problems during sex. In addition, 20% said that IBD made them feel guilty about having sex and 30% said they feared having sex; 2% reported having erectile dysfunction.
The use of erectile-enhancing medications were reported by 23% (prescribed mostly by doctors other than the physician treating the IBD) and 5% were taking testosterone to enhance sexual function. While 78% said they were comfortable speaking about sexual function with their gastroenterologist, only 10% said that their gastroenterologist had initiated a discussion about this topic, Dr. O’Toole said.
Based on other analyses of the results, “we found that impaired sexual function was associated with older age, longer duration of disease, active disease, comorbid depression, presence of an ostomy, and diabetes,” she added.
While the thrombotic risks of testosterone are clear, Dr. O’Toole noted that in patients with IBD, the effects of phosphodiesterase-5 (PDE5) inhibitors like sildenafil (Viagra) on the microcirculation within the gastrointestinal tract and whether the effects of these drugs contribute to microscopic ischemia in the GI tract is unclear, she said. Dr. O’Toole had no relevant financial disclosures.
AT DDW 2015
Key clinical point: Once validated, a new scale designed to evaluate sexual dysfunction in men with inflammatory bowel disease could provide a useful tool to evaluate the impact the disease has on sexual function in male patients.
Major finding: The negative effects of IBD on sexual dysfunction in men with the disease, reflected in the responses to the IBD-specific questionnaire, included negative effects on libido in 38% and fear of having sex in 30%.
Data source: Results were based on the responses to the questionnaire from 175 male patients treated at the IBD clinics at two Boston medical centers.
Disclosures: Dr. O’Toole had no relevant financial disclosures.
Rx: Preventive care
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Recognizing autophonia in patients with anorexia nervosa
Anorexia nervosa can affect a number of systems of the body, including the otolaryngologic presentation of autophonia1,2—a rare hyperperception of an abnormally intense hearing of one’s own voice and respiratory sounds.2 The most common cause of autophonia in patients with anorexia is a patulous (patent) eustachian tube, which can be caused by extreme weight loss.2,3
Significant reduction in the quantity of fat tissue at the location of the eustachian tube can cause patency.3 This creates an abnormal connection between the nasopharynx and tympanic membrane, in which sounds are transmitted directly from the oral cavity to the middle ear, causing autophonia, tinnitus, or sound distortion.4
What are the symptoms?Patients often report hearing their own voice more loudly in the affected ear. This can be distressing, and they might become preoccupied with the sound of their voice—thus affecting quality of life.2,4
The intensity of symptoms varies: from a mild sensation of a clogged ear to extremely bothersome discomfort much like a middle-ear infection.2,4 Autophonia, however, cannot be relieved by conventional therapies for those conditions.2,3
A patulous eustachian tube is difficult to detect and can be misdiagnosed as another condition. Pregnancy, stress, fatigue, radiation therapy, hormonal therapy, and dramatic weight loss also can cause a patulous eustachian tube.2
How is the diagnosis made?The diagnosis of autophonia is clinical and begins with a detailed history. Symptoms often appear within the time frame of rapid weight loss and without evidence of infection or other illness.2,3 The clinical examination is otherwise unremarkable.2,4
Is there treatment?To improve the patient’s comfort and quality of life, intervention is required, best provided by an integrated team of medical specialists. Weight gain, of course, is the treatment goal in anorexia, but this is a complex process often marked by relapse; a detailed discussion of treatment strategies is beyond the scope of this “Pearl.” Symptoms usually diminish as fatty tissue is restored upon successful treatment of anorexia, which closes the abnormal eustachian tube opening.2,3
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Olthoff A, Laskawi R, Kruse E. Successful treatment of autophonia with botulinum toxin: case report. Ann Otol Rhinol Laryngol. 2007;116(8):594-598.
2. Godbole M, Key A. Autophonia in anorexia nervosa. Int J Eat Disord. 2010;43(5):480-482.
3. Karwautz A, Hafferl A, Ungar D, et al. Patulous eustachian tube in a case of adolescent anorexia nervosa. Int J Eat Disord. 1999;25(3):353-355.
4. Dornhoffer JL, Leuwer R, Schwager K, et al. A practical guide to the eustachian tube. New York, NY: Springer; 2014:23-41.
Anorexia nervosa can affect a number of systems of the body, including the otolaryngologic presentation of autophonia1,2—a rare hyperperception of an abnormally intense hearing of one’s own voice and respiratory sounds.2 The most common cause of autophonia in patients with anorexia is a patulous (patent) eustachian tube, which can be caused by extreme weight loss.2,3
Significant reduction in the quantity of fat tissue at the location of the eustachian tube can cause patency.3 This creates an abnormal connection between the nasopharynx and tympanic membrane, in which sounds are transmitted directly from the oral cavity to the middle ear, causing autophonia, tinnitus, or sound distortion.4
What are the symptoms?Patients often report hearing their own voice more loudly in the affected ear. This can be distressing, and they might become preoccupied with the sound of their voice—thus affecting quality of life.2,4
The intensity of symptoms varies: from a mild sensation of a clogged ear to extremely bothersome discomfort much like a middle-ear infection.2,4 Autophonia, however, cannot be relieved by conventional therapies for those conditions.2,3
A patulous eustachian tube is difficult to detect and can be misdiagnosed as another condition. Pregnancy, stress, fatigue, radiation therapy, hormonal therapy, and dramatic weight loss also can cause a patulous eustachian tube.2
How is the diagnosis made?The diagnosis of autophonia is clinical and begins with a detailed history. Symptoms often appear within the time frame of rapid weight loss and without evidence of infection or other illness.2,3 The clinical examination is otherwise unremarkable.2,4
Is there treatment?To improve the patient’s comfort and quality of life, intervention is required, best provided by an integrated team of medical specialists. Weight gain, of course, is the treatment goal in anorexia, but this is a complex process often marked by relapse; a detailed discussion of treatment strategies is beyond the scope of this “Pearl.” Symptoms usually diminish as fatty tissue is restored upon successful treatment of anorexia, which closes the abnormal eustachian tube opening.2,3
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Anorexia nervosa can affect a number of systems of the body, including the otolaryngologic presentation of autophonia1,2—a rare hyperperception of an abnormally intense hearing of one’s own voice and respiratory sounds.2 The most common cause of autophonia in patients with anorexia is a patulous (patent) eustachian tube, which can be caused by extreme weight loss.2,3
Significant reduction in the quantity of fat tissue at the location of the eustachian tube can cause patency.3 This creates an abnormal connection between the nasopharynx and tympanic membrane, in which sounds are transmitted directly from the oral cavity to the middle ear, causing autophonia, tinnitus, or sound distortion.4
What are the symptoms?Patients often report hearing their own voice more loudly in the affected ear. This can be distressing, and they might become preoccupied with the sound of their voice—thus affecting quality of life.2,4
The intensity of symptoms varies: from a mild sensation of a clogged ear to extremely bothersome discomfort much like a middle-ear infection.2,4 Autophonia, however, cannot be relieved by conventional therapies for those conditions.2,3
A patulous eustachian tube is difficult to detect and can be misdiagnosed as another condition. Pregnancy, stress, fatigue, radiation therapy, hormonal therapy, and dramatic weight loss also can cause a patulous eustachian tube.2
How is the diagnosis made?The diagnosis of autophonia is clinical and begins with a detailed history. Symptoms often appear within the time frame of rapid weight loss and without evidence of infection or other illness.2,3 The clinical examination is otherwise unremarkable.2,4
Is there treatment?To improve the patient’s comfort and quality of life, intervention is required, best provided by an integrated team of medical specialists. Weight gain, of course, is the treatment goal in anorexia, but this is a complex process often marked by relapse; a detailed discussion of treatment strategies is beyond the scope of this “Pearl.” Symptoms usually diminish as fatty tissue is restored upon successful treatment of anorexia, which closes the abnormal eustachian tube opening.2,3
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Olthoff A, Laskawi R, Kruse E. Successful treatment of autophonia with botulinum toxin: case report. Ann Otol Rhinol Laryngol. 2007;116(8):594-598.
2. Godbole M, Key A. Autophonia in anorexia nervosa. Int J Eat Disord. 2010;43(5):480-482.
3. Karwautz A, Hafferl A, Ungar D, et al. Patulous eustachian tube in a case of adolescent anorexia nervosa. Int J Eat Disord. 1999;25(3):353-355.
4. Dornhoffer JL, Leuwer R, Schwager K, et al. A practical guide to the eustachian tube. New York, NY: Springer; 2014:23-41.
1. Olthoff A, Laskawi R, Kruse E. Successful treatment of autophonia with botulinum toxin: case report. Ann Otol Rhinol Laryngol. 2007;116(8):594-598.
2. Godbole M, Key A. Autophonia in anorexia nervosa. Int J Eat Disord. 2010;43(5):480-482.
3. Karwautz A, Hafferl A, Ungar D, et al. Patulous eustachian tube in a case of adolescent anorexia nervosa. Int J Eat Disord. 1999;25(3):353-355.
4. Dornhoffer JL, Leuwer R, Schwager K, et al. A practical guide to the eustachian tube. New York, NY: Springer; 2014:23-41.
A physician who feels hopeless and worthless and complains of pain
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
PSYCHIATRY UPDATE 2015
Current Psychiatry welcomed more than 650 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, April 16-18, 2015, at the Hilton Chicago in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us next year in Chicago, March 10-12, 2016.
THURSDAY, APRIL 16, 2015
MORNING SESSION
Attention-deficit/hyperactivity disorder (ADHD) is a lifespan disorder that is “everywhere,” Anthony L. Rostain, MD, MA, University of Pennsylvania Perelman School of Medicine, began—including in adults and even “seniors.” This means that the disorder “is not a diagnosis of exclusion,” and that “comorbidity is the rule,” including learning difficulties. Among adults, the focus of symptoms and management is on executive dysfunction and its characteristics: difficulty multitasking, problems keeping commitments, and excessive reliance on help from others. Inattention and disorganization are hallmarks of adult ADHD, and become worse as environmental demands (work, home) increase; hyperactivity decreases with age. Dr. Rostain recommends ruling out other causes of a patient’s symptoms when an adult self-reports ADHD, including transient stressors, medical conditions, psychiatric disorders, and malingering.
Donald W. Black, MD, University of Iowa, reviewed DSM-5 criteria for borderline personality disorder (BPD) and offered tips for avoiding misdiagnosis, including obtaining collateral information and using rating scales. Co-occuring disorders, such as depression and substance abuse, are common. Treatment for BPD patients includes psychotherapy (individual or group), medication, and lifestyle changes. Psychotropics treat symptoms of depression, anxiety, hostility, and impulsivity of BPD but not the fundamental nature of the disorder. When establishing a patient’s treatment plan, consider the stage of illness, evaluate for any co-occurring disorders, and ask the patient what he (she) wants from treatment.
Dr. Rostain began by discussing the neurobiological basis of ADHD, which guides pharmacotherapy. He reviewed the response rate of FDA-approved agents for adults with ADHD, including stimulants, atomoxetine, and alpha-adrenergic agonists. Best response is seen with stimulants, but some patients improve with bupropion and tricyclic antidepressants (TCAs). Employ a multimodal treatment approach, Dr. Rostain recommended, which should include psychoeducation and environmental restructuring, because, as he says, “Pills don’t teach skills.” He also reviewed strategies for treating ADHD in patients who have a comorbid disorder, such as bipolar disorder, major depressive disorder, or substance abuse.
Patients with psychotic depression meet criteria for major depressive disorder but also have delusions or hallucinations. Diagnostic issues include increased guilt, cognitive impairment, paranoia, and increased hopelessness. Anthony J. Rothschild, MD, University of Massachusetts Medical School, reviewed methods for differentiating psychotic depression from schizophrenia, posttraumatic stress disorder, obsessive-compulsive disorder, and body dysmorphic disorder. There are no FDA-approved medications for psychotic depression, Dr. Rothschild explained; however, evidence shows that the combination of an antidepressant and an antipsychotic is superior to monotherapy with an agent from either class. In addition, he noted, studies show a high response rate with electroconvulsive therapy (ECT).
AFTERNOON SESSION
Return of symptoms after initial remission— while the patient is still taking an antidepressant—is considered tachyphylaxis, or “poop out.” Residual depressive symptoms, when a patient meets criteria for remission but still has troubling symptoms, is a different phenomenon, although symptoms can overlap. First, Dr. Rothschild advised, ensure that patients are given an adequate trial of an antidepressant. Options are similar when tachyphylaxis or residual symptoms are present: switch drugs or add augmentation therapy, such as lithium, thyroid hormone, or an atypical antipsychotic. Data on the efficacy for bupropion and buspirone are not strong. For treatment-resistant depression when a patient does not respond to 3 adequate antidepressant trials—consider ECT or rTMS, if available, or a monoamine oxidase inhibitor or a TCA.
Dr. Black defines antisocial personality disorder (ASPD) as a disorder of lifelong serial misbehavior, one characterized by impaired relationships, aggressive behavior, non-aggressive delinquent behavior, manipulation, and a disturbing lack of conscience. There is no standard treatment for ASPD, and no FDA-approved medications; however, potential treatments have not been adequately studied, he pointed out. Cognitive-behavioral therapy might be appropriate in mild cases; some patients benefit from specific programs— for example, ones that address drug or alcohol addiction or anger, although evidence is limited. When treating ASPD patients, Dr. Black concluded, be mindful of high attrition, possible misuse of prescribed medications, and drug-drug or drug-alcohol interactions.
Bipolar disorder is associated with the highest risk of suicide and increased lethality among all psychiatric disorders. Lithium has evidence of an anti-suicidality effect and may reduce suicide by decreasing relapse, aggression, and impulsivity. An FDA advisory on increased risk of suicidality with anticonvulsants was based on data about patients with epilepsy, not bipolar disorder. Second-generation antipsychotics, including olanzapine, quetiapine, and lurasidone, have been shown to be effective for bipolar depression. Avoid antidepressants if possible, Philip G. Janicak, MD, Northwestern University Feinberg School of Medicine, advised; if you must prescribe one, reassess the need for the drug often. Several psychotherapy modalities have evidence supporting their use in bipolar disorder.
FRIDAY, APRIL 17, 2015
MORNING SESSION
Henry A. Nasrallah, MD, Saint Louis University School of Medicine, offered enlightening historical touch-points on how psychiatry’s understanding of, and its approach to, schizophrenia have changed in the past 50 years. His goal? To challenge practitioners to rethink ideas about the disorder and how they care for affected patients. From a laundry list of comparative shifts, here are a few of Dr. Nasrallah’s “then” and “now” observations:
• The old paradigm was: Clinical and functional deterioration are inevitable in schizophrenia. The new paradigm is: Complete remission and restoration of function are feasible in many patients when they are fully adherent to the treatment plan.
• The old: Long-acting injectable (LAI) antipsychotics are a last-resort treatment, to be prescribed after a patient is stabilized. The new: Use LAI antipsychotics early in the course.
• Old: Begin treatment when psychosis appears. New: Work to prevent conversion to psychosis.
• Old: The disorder is considered a consequence of neurochemical dysregulation. New: Impaired neuroplasticity is to blame.
• Old: Treatment is a matter of trial and error. New: We can apply pharmaco-genomics to predict a patient’s response to various drugs and thus increase the likelihood of therapeutic success.
In his second presentation, Dr. Nasrallah described the many pathways to psychosis and several psychotic disorders other than schizophrenia, including schizoaffective, delusional disorder, and psychotic disorder caused by a general medical condition. He listed symptom clusters in psychosis beyond positive and negative symptoms, including neuromotor symptoms, mood symptoms, and neurocognitive deficits. Development of schizophrenia is multifactorial and involves risk genes and environmental factors seen before conception, during birth, and in early childhood; good prenatal care is the best way to prevent schizophrenia, Dr. Nasrallah noted. Several general medical conditions can produce schizophrenia-like psychosis, including some CNS disorders, toxins, autoimmune diseases, infectious diseases, and chromosomal abnormalities. The session concluded with a live interview with one of Dr. Nasrallah’s patients, whose schizophrenia is in remission with clozapine.
Drug abuse can mask signs and symptoms of bipolar disorder, which can delay diagnosis. Commonly abused substances are nicotine, alcohol, Cannabis, and cocaine; polysubstance abuse is the rule. Bipolar disorder and substance abuse share common mechanisms: impulsivity, poor modulation of motivation and response to reward, and behavioral sensitization. Treatment approaches should be flexible. Dr. Janicak reviewed the evidence for using anticonvulsants, antipsychotics, and bupropion for alcohol, Cannabis, and cocaine abuse; there are no data on treating opioid abuse. He also discussed the evidence for using naltrexone, acamprosate, disulfiram, and varenicline, as well as psychotherapeutic options, to treat substance abuse. Dr. Janicak encouraged clinicians in the audience to treat substance abuse in bipolar disorder patients themselves, instead of referring them to a subspecialist.
Untreated psychiatric disorders increase obstetrical complications, possibly through decreased self-care or increased stress. For mild or moderate depression, psychotherapy might be sufficient treatment; but for severe cases, medication is the first-line approach. In her presentation on mood disorders during pregnancy, Marlene P. Freeman, MD, Massachusetts General Hospital, advises that clinicians select medications based on known safety information, patient preference, and the previous course of illness. Results of studies that lasted 4 to 5 years do not show major long-term adverse effects of antidepressant exposure on neurodevelopment or neurobehavior. When treating patients for bipolar disorder, valproate is associated with an increased risk of adverse cognitive and neurodevelopmental effects in infants compared with other anticonvulsants; evidence suggests that lamotrigine is a safer option. The research does not show an increased risk of major malformations with second-generation antipsychotics.
AFTERNOON SESSION
Most women have premenstrual symptoms; a minority have a full-blown syndrome, now known as premenstrual dysphoric disorder (PMDD). This is not an existing mood disorder that becomes worse premenstrually. Clinician and patients should track the temporal relationship of symptoms on a calendar for a few months. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine have been well studied and are effective compared with placebo, but don’t help all patients with PMDD. Consider flexible dosing strategies with SSRIs—perhaps daily use, a higher dosage premenstrually, and as-needed administration. Start with an oral contraceptive or SSRI; if symptoms don’t respond, add the other. Serotonergic antidepressants have been shown helpful for hot flashes and depressive symptoms in perimenopause. Dr. Freeman reviewed the evidence for using complementary and alternative therapies for menopausal symptoms and hot flushes.
Smoking contributes to excess mortality in seriously mentally ill patients as a result of such tobacco-related illnesses as heart disease, lung disease, and cancer. Overall improvement in mental health as well as physical health is seen when a patient stops smoking. All nicotine replacement products are effective, but patients often don’t use them long enough or correctly. Robert M. Anthenelli, MD, University of California, San Diego, said to begin sustained-release bupropion 1 or 2 weeks before quit date; maintain the dosage for 1 to 12 weeks after quit date and consider maintenance therapy for as long as 6 months. Varenicline is superior to placebo and bupropion, but is known to have gastrointestinal (GI) and sleep disturbance adverse effects. Quitting smoking can increase the blood level of some psychotropics, meaning that you might need to reduce their dosage. It is best to begin smoking cessation when patients are mentally stable, when motivated, and stable on their medications.
In discussing trends in substance abuse, Dr. Anthenelli
faddish. Fentanyl and fentanyl analogues are 100 times more powerful than morphine; ingestion of even a minuscule dose can be fatal. Synthetic cannabinoids primarily are a problem among adolescents; they are more dangerous than marijuana and are associated with aggressive and suicidal behaviors. A standard toxicology screen will not detect synthetic cannabinoids.
E-cigarettes are considered by users to be safer than tobacco cigarettes—and probably are—but they still put patients at risk of nicotine addiction. There are no safety data on e-cigarettes; the devices might contain potentially harmful chemicals and potentially toxic nicotine levels. Dr. Anthenelli reported that topiramate is “the best medication I’ve used” for alcohol abuse disorder. The drug is not FDA-approved for this use, but has been used in a number of studies with positive outcomes.
SATURDAY, APRIL 18, 2015
MORNING SESSION
Psychiatrists are well positioned to help patients with mental illness lose weight because of their psychotherapeutic background. Best treatment strategy is diet plus exercise plus behavioral modification. Robert M. McCarron, DO, University of California, Davis, recommends keeping it simple and telling patients to only consider calories of foods, and not to worry about sodium or fat content. Ask patients “How many minutes a day of exercise can you do?” but recommend that patients walk for 30 minutes a day at 4 mph, 5 days per week, which will help patients lose 1% to 3% of body weight. For treatment-refractory obese patients, consider medications such as bupropion, orlistat, lorcaserin, topiramate, or metformin; for those with a BMI ≥40, recommend bariatric surgery.
George T. Grossberg, MD, Saint Louis University School of Medicine, reviewed the evidence for anxiety disorders in older adults, including generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder. Older patients with cardiovascular disease, cancer, Parkinson’s disease, diabetes, GI disorders, or chronic obstructive pulmonary disease are at high risk of anxiety symptoms. In a study of centenarians, predictors of anxiety are worse health perception, financial concerns related to medical expenses, higher number of medical conditions, and loneliness. Secondary anxiety is prevalent in Alzheimer’s disease; the condition can present as fidgeting, pacing, anger, or agitation, and can be prompted by a change in routine. Acute, new-onset anxiety symptoms should trigger a complete medical evaluation, including a review of medications, supplements, and substance use. In geriatric patients, minimize use of benzodiazepines and avoid anticholinergics.
Overall, psychiatry patients do not receive optimal preventive and primary medical care, leading to decreased life expectancy, often as a result of cardiovascular disease. Psychiatric patients have a high rate of dyslipidemia, hypertension, smoking, and obesity. Psychiatrists often don’t treat these conditions, but they need to be aware of changing standard practices in preventive medicine; be able to recognize a potential problem; and make referrals when appropriate. Dr. McCarron reviewed age-based screening recommendations for hypertension, dyslipidemia, and diabetes from the book Preventive Medical Care in Psychiatry, which he co-edited. He recommends using online cardiovascular risk calculators to determine which patients need to be screened.
AFTERNOON SESSION
Some older patients who abuse substances took drugs as young adults and never gave them up; others have rediscovered drugs in later life. Potential indicators of alcohol abuse in older patients are changes in cognition, mood, memory, hygiene, or sleep. Substance abuse in older adults frequently is comorbid with depression or bereavement, anxiety, and adjustment disorders. Dr. Grossberg recommends addressing the topic directly with patients. Although there are few data to guide treatment, prompt detection and appropriate treatment can improve the quality of life of older adults and their family.
SPONSORS AND SUPPORTERS
• American Professional Agency
• American Psychiatric Publishing
• Arbor Pharmaceuticals
• AstraZeneca
• Banner Health
• Bassett Healthcare Network
• Ministry Health Care
• Pine Rest Christian Mental Health Services
• PRMS
• Sinai Health System
• Sunovion
• Takeda Pharmaceuticals
• U.S. Army Healthcare
• Wexford Health Sources
• Wolters Kluwer Health
The meeting organizers acknowledge the support provided by the sponsors. Determination of educational content for this program and the selection of speakers are responsibilities of the program director and co-directors. Sponsors and supporters did not have input in these areas.
personality disorder, DSM-5, adults with ADHD, residual depressive symptoms, treatment-resistant depression,antisocial personality disorder, bipolar disorder, schizophrenia, psychotic disorder, clozapine, bipolar disorder and substance abuse, mood disorders during pregnancy, premenstrual dysphoric disorder, depressive symptoms in perimenopause, smoking and the mentally ill, help patients with mental illness lose weight, substance abuse in older adults
Current Psychiatry welcomed more than 650 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, April 16-18, 2015, at the Hilton Chicago in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us next year in Chicago, March 10-12, 2016.
THURSDAY, APRIL 16, 2015
MORNING SESSION
Attention-deficit/hyperactivity disorder (ADHD) is a lifespan disorder that is “everywhere,” Anthony L. Rostain, MD, MA, University of Pennsylvania Perelman School of Medicine, began—including in adults and even “seniors.” This means that the disorder “is not a diagnosis of exclusion,” and that “comorbidity is the rule,” including learning difficulties. Among adults, the focus of symptoms and management is on executive dysfunction and its characteristics: difficulty multitasking, problems keeping commitments, and excessive reliance on help from others. Inattention and disorganization are hallmarks of adult ADHD, and become worse as environmental demands (work, home) increase; hyperactivity decreases with age. Dr. Rostain recommends ruling out other causes of a patient’s symptoms when an adult self-reports ADHD, including transient stressors, medical conditions, psychiatric disorders, and malingering.
Donald W. Black, MD, University of Iowa, reviewed DSM-5 criteria for borderline personality disorder (BPD) and offered tips for avoiding misdiagnosis, including obtaining collateral information and using rating scales. Co-occuring disorders, such as depression and substance abuse, are common. Treatment for BPD patients includes psychotherapy (individual or group), medication, and lifestyle changes. Psychotropics treat symptoms of depression, anxiety, hostility, and impulsivity of BPD but not the fundamental nature of the disorder. When establishing a patient’s treatment plan, consider the stage of illness, evaluate for any co-occurring disorders, and ask the patient what he (she) wants from treatment.
Dr. Rostain began by discussing the neurobiological basis of ADHD, which guides pharmacotherapy. He reviewed the response rate of FDA-approved agents for adults with ADHD, including stimulants, atomoxetine, and alpha-adrenergic agonists. Best response is seen with stimulants, but some patients improve with bupropion and tricyclic antidepressants (TCAs). Employ a multimodal treatment approach, Dr. Rostain recommended, which should include psychoeducation and environmental restructuring, because, as he says, “Pills don’t teach skills.” He also reviewed strategies for treating ADHD in patients who have a comorbid disorder, such as bipolar disorder, major depressive disorder, or substance abuse.
Patients with psychotic depression meet criteria for major depressive disorder but also have delusions or hallucinations. Diagnostic issues include increased guilt, cognitive impairment, paranoia, and increased hopelessness. Anthony J. Rothschild, MD, University of Massachusetts Medical School, reviewed methods for differentiating psychotic depression from schizophrenia, posttraumatic stress disorder, obsessive-compulsive disorder, and body dysmorphic disorder. There are no FDA-approved medications for psychotic depression, Dr. Rothschild explained; however, evidence shows that the combination of an antidepressant and an antipsychotic is superior to monotherapy with an agent from either class. In addition, he noted, studies show a high response rate with electroconvulsive therapy (ECT).
AFTERNOON SESSION
Return of symptoms after initial remission— while the patient is still taking an antidepressant—is considered tachyphylaxis, or “poop out.” Residual depressive symptoms, when a patient meets criteria for remission but still has troubling symptoms, is a different phenomenon, although symptoms can overlap. First, Dr. Rothschild advised, ensure that patients are given an adequate trial of an antidepressant. Options are similar when tachyphylaxis or residual symptoms are present: switch drugs or add augmentation therapy, such as lithium, thyroid hormone, or an atypical antipsychotic. Data on the efficacy for bupropion and buspirone are not strong. For treatment-resistant depression when a patient does not respond to 3 adequate antidepressant trials—consider ECT or rTMS, if available, or a monoamine oxidase inhibitor or a TCA.
Dr. Black defines antisocial personality disorder (ASPD) as a disorder of lifelong serial misbehavior, one characterized by impaired relationships, aggressive behavior, non-aggressive delinquent behavior, manipulation, and a disturbing lack of conscience. There is no standard treatment for ASPD, and no FDA-approved medications; however, potential treatments have not been adequately studied, he pointed out. Cognitive-behavioral therapy might be appropriate in mild cases; some patients benefit from specific programs— for example, ones that address drug or alcohol addiction or anger, although evidence is limited. When treating ASPD patients, Dr. Black concluded, be mindful of high attrition, possible misuse of prescribed medications, and drug-drug or drug-alcohol interactions.
Bipolar disorder is associated with the highest risk of suicide and increased lethality among all psychiatric disorders. Lithium has evidence of an anti-suicidality effect and may reduce suicide by decreasing relapse, aggression, and impulsivity. An FDA advisory on increased risk of suicidality with anticonvulsants was based on data about patients with epilepsy, not bipolar disorder. Second-generation antipsychotics, including olanzapine, quetiapine, and lurasidone, have been shown to be effective for bipolar depression. Avoid antidepressants if possible, Philip G. Janicak, MD, Northwestern University Feinberg School of Medicine, advised; if you must prescribe one, reassess the need for the drug often. Several psychotherapy modalities have evidence supporting their use in bipolar disorder.
FRIDAY, APRIL 17, 2015
MORNING SESSION
Henry A. Nasrallah, MD, Saint Louis University School of Medicine, offered enlightening historical touch-points on how psychiatry’s understanding of, and its approach to, schizophrenia have changed in the past 50 years. His goal? To challenge practitioners to rethink ideas about the disorder and how they care for affected patients. From a laundry list of comparative shifts, here are a few of Dr. Nasrallah’s “then” and “now” observations:
• The old paradigm was: Clinical and functional deterioration are inevitable in schizophrenia. The new paradigm is: Complete remission and restoration of function are feasible in many patients when they are fully adherent to the treatment plan.
• The old: Long-acting injectable (LAI) antipsychotics are a last-resort treatment, to be prescribed after a patient is stabilized. The new: Use LAI antipsychotics early in the course.
• Old: Begin treatment when psychosis appears. New: Work to prevent conversion to psychosis.
• Old: The disorder is considered a consequence of neurochemical dysregulation. New: Impaired neuroplasticity is to blame.
• Old: Treatment is a matter of trial and error. New: We can apply pharmaco-genomics to predict a patient’s response to various drugs and thus increase the likelihood of therapeutic success.
In his second presentation, Dr. Nasrallah described the many pathways to psychosis and several psychotic disorders other than schizophrenia, including schizoaffective, delusional disorder, and psychotic disorder caused by a general medical condition. He listed symptom clusters in psychosis beyond positive and negative symptoms, including neuromotor symptoms, mood symptoms, and neurocognitive deficits. Development of schizophrenia is multifactorial and involves risk genes and environmental factors seen before conception, during birth, and in early childhood; good prenatal care is the best way to prevent schizophrenia, Dr. Nasrallah noted. Several general medical conditions can produce schizophrenia-like psychosis, including some CNS disorders, toxins, autoimmune diseases, infectious diseases, and chromosomal abnormalities. The session concluded with a live interview with one of Dr. Nasrallah’s patients, whose schizophrenia is in remission with clozapine.
Drug abuse can mask signs and symptoms of bipolar disorder, which can delay diagnosis. Commonly abused substances are nicotine, alcohol, Cannabis, and cocaine; polysubstance abuse is the rule. Bipolar disorder and substance abuse share common mechanisms: impulsivity, poor modulation of motivation and response to reward, and behavioral sensitization. Treatment approaches should be flexible. Dr. Janicak reviewed the evidence for using anticonvulsants, antipsychotics, and bupropion for alcohol, Cannabis, and cocaine abuse; there are no data on treating opioid abuse. He also discussed the evidence for using naltrexone, acamprosate, disulfiram, and varenicline, as well as psychotherapeutic options, to treat substance abuse. Dr. Janicak encouraged clinicians in the audience to treat substance abuse in bipolar disorder patients themselves, instead of referring them to a subspecialist.
Untreated psychiatric disorders increase obstetrical complications, possibly through decreased self-care or increased stress. For mild or moderate depression, psychotherapy might be sufficient treatment; but for severe cases, medication is the first-line approach. In her presentation on mood disorders during pregnancy, Marlene P. Freeman, MD, Massachusetts General Hospital, advises that clinicians select medications based on known safety information, patient preference, and the previous course of illness. Results of studies that lasted 4 to 5 years do not show major long-term adverse effects of antidepressant exposure on neurodevelopment or neurobehavior. When treating patients for bipolar disorder, valproate is associated with an increased risk of adverse cognitive and neurodevelopmental effects in infants compared with other anticonvulsants; evidence suggests that lamotrigine is a safer option. The research does not show an increased risk of major malformations with second-generation antipsychotics.
AFTERNOON SESSION
Most women have premenstrual symptoms; a minority have a full-blown syndrome, now known as premenstrual dysphoric disorder (PMDD). This is not an existing mood disorder that becomes worse premenstrually. Clinician and patients should track the temporal relationship of symptoms on a calendar for a few months. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine have been well studied and are effective compared with placebo, but don’t help all patients with PMDD. Consider flexible dosing strategies with SSRIs—perhaps daily use, a higher dosage premenstrually, and as-needed administration. Start with an oral contraceptive or SSRI; if symptoms don’t respond, add the other. Serotonergic antidepressants have been shown helpful for hot flashes and depressive symptoms in perimenopause. Dr. Freeman reviewed the evidence for using complementary and alternative therapies for menopausal symptoms and hot flushes.
Smoking contributes to excess mortality in seriously mentally ill patients as a result of such tobacco-related illnesses as heart disease, lung disease, and cancer. Overall improvement in mental health as well as physical health is seen when a patient stops smoking. All nicotine replacement products are effective, but patients often don’t use them long enough or correctly. Robert M. Anthenelli, MD, University of California, San Diego, said to begin sustained-release bupropion 1 or 2 weeks before quit date; maintain the dosage for 1 to 12 weeks after quit date and consider maintenance therapy for as long as 6 months. Varenicline is superior to placebo and bupropion, but is known to have gastrointestinal (GI) and sleep disturbance adverse effects. Quitting smoking can increase the blood level of some psychotropics, meaning that you might need to reduce their dosage. It is best to begin smoking cessation when patients are mentally stable, when motivated, and stable on their medications.
In discussing trends in substance abuse, Dr. Anthenelli
faddish. Fentanyl and fentanyl analogues are 100 times more powerful than morphine; ingestion of even a minuscule dose can be fatal. Synthetic cannabinoids primarily are a problem among adolescents; they are more dangerous than marijuana and are associated with aggressive and suicidal behaviors. A standard toxicology screen will not detect synthetic cannabinoids.
E-cigarettes are considered by users to be safer than tobacco cigarettes—and probably are—but they still put patients at risk of nicotine addiction. There are no safety data on e-cigarettes; the devices might contain potentially harmful chemicals and potentially toxic nicotine levels. Dr. Anthenelli reported that topiramate is “the best medication I’ve used” for alcohol abuse disorder. The drug is not FDA-approved for this use, but has been used in a number of studies with positive outcomes.
SATURDAY, APRIL 18, 2015
MORNING SESSION
Psychiatrists are well positioned to help patients with mental illness lose weight because of their psychotherapeutic background. Best treatment strategy is diet plus exercise plus behavioral modification. Robert M. McCarron, DO, University of California, Davis, recommends keeping it simple and telling patients to only consider calories of foods, and not to worry about sodium or fat content. Ask patients “How many minutes a day of exercise can you do?” but recommend that patients walk for 30 minutes a day at 4 mph, 5 days per week, which will help patients lose 1% to 3% of body weight. For treatment-refractory obese patients, consider medications such as bupropion, orlistat, lorcaserin, topiramate, or metformin; for those with a BMI ≥40, recommend bariatric surgery.
George T. Grossberg, MD, Saint Louis University School of Medicine, reviewed the evidence for anxiety disorders in older adults, including generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder. Older patients with cardiovascular disease, cancer, Parkinson’s disease, diabetes, GI disorders, or chronic obstructive pulmonary disease are at high risk of anxiety symptoms. In a study of centenarians, predictors of anxiety are worse health perception, financial concerns related to medical expenses, higher number of medical conditions, and loneliness. Secondary anxiety is prevalent in Alzheimer’s disease; the condition can present as fidgeting, pacing, anger, or agitation, and can be prompted by a change in routine. Acute, new-onset anxiety symptoms should trigger a complete medical evaluation, including a review of medications, supplements, and substance use. In geriatric patients, minimize use of benzodiazepines and avoid anticholinergics.
Overall, psychiatry patients do not receive optimal preventive and primary medical care, leading to decreased life expectancy, often as a result of cardiovascular disease. Psychiatric patients have a high rate of dyslipidemia, hypertension, smoking, and obesity. Psychiatrists often don’t treat these conditions, but they need to be aware of changing standard practices in preventive medicine; be able to recognize a potential problem; and make referrals when appropriate. Dr. McCarron reviewed age-based screening recommendations for hypertension, dyslipidemia, and diabetes from the book Preventive Medical Care in Psychiatry, which he co-edited. He recommends using online cardiovascular risk calculators to determine which patients need to be screened.
AFTERNOON SESSION
Some older patients who abuse substances took drugs as young adults and never gave them up; others have rediscovered drugs in later life. Potential indicators of alcohol abuse in older patients are changes in cognition, mood, memory, hygiene, or sleep. Substance abuse in older adults frequently is comorbid with depression or bereavement, anxiety, and adjustment disorders. Dr. Grossberg recommends addressing the topic directly with patients. Although there are few data to guide treatment, prompt detection and appropriate treatment can improve the quality of life of older adults and their family.
SPONSORS AND SUPPORTERS
• American Professional Agency
• American Psychiatric Publishing
• Arbor Pharmaceuticals
• AstraZeneca
• Banner Health
• Bassett Healthcare Network
• Ministry Health Care
• Pine Rest Christian Mental Health Services
• PRMS
• Sinai Health System
• Sunovion
• Takeda Pharmaceuticals
• U.S. Army Healthcare
• Wexford Health Sources
• Wolters Kluwer Health
The meeting organizers acknowledge the support provided by the sponsors. Determination of educational content for this program and the selection of speakers are responsibilities of the program director and co-directors. Sponsors and supporters did not have input in these areas.
Current Psychiatry welcomed more than 650 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, April 16-18, 2015, at the Hilton Chicago in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us next year in Chicago, March 10-12, 2016.
THURSDAY, APRIL 16, 2015
MORNING SESSION
Attention-deficit/hyperactivity disorder (ADHD) is a lifespan disorder that is “everywhere,” Anthony L. Rostain, MD, MA, University of Pennsylvania Perelman School of Medicine, began—including in adults and even “seniors.” This means that the disorder “is not a diagnosis of exclusion,” and that “comorbidity is the rule,” including learning difficulties. Among adults, the focus of symptoms and management is on executive dysfunction and its characteristics: difficulty multitasking, problems keeping commitments, and excessive reliance on help from others. Inattention and disorganization are hallmarks of adult ADHD, and become worse as environmental demands (work, home) increase; hyperactivity decreases with age. Dr. Rostain recommends ruling out other causes of a patient’s symptoms when an adult self-reports ADHD, including transient stressors, medical conditions, psychiatric disorders, and malingering.
Donald W. Black, MD, University of Iowa, reviewed DSM-5 criteria for borderline personality disorder (BPD) and offered tips for avoiding misdiagnosis, including obtaining collateral information and using rating scales. Co-occuring disorders, such as depression and substance abuse, are common. Treatment for BPD patients includes psychotherapy (individual or group), medication, and lifestyle changes. Psychotropics treat symptoms of depression, anxiety, hostility, and impulsivity of BPD but not the fundamental nature of the disorder. When establishing a patient’s treatment plan, consider the stage of illness, evaluate for any co-occurring disorders, and ask the patient what he (she) wants from treatment.
Dr. Rostain began by discussing the neurobiological basis of ADHD, which guides pharmacotherapy. He reviewed the response rate of FDA-approved agents for adults with ADHD, including stimulants, atomoxetine, and alpha-adrenergic agonists. Best response is seen with stimulants, but some patients improve with bupropion and tricyclic antidepressants (TCAs). Employ a multimodal treatment approach, Dr. Rostain recommended, which should include psychoeducation and environmental restructuring, because, as he says, “Pills don’t teach skills.” He also reviewed strategies for treating ADHD in patients who have a comorbid disorder, such as bipolar disorder, major depressive disorder, or substance abuse.
Patients with psychotic depression meet criteria for major depressive disorder but also have delusions or hallucinations. Diagnostic issues include increased guilt, cognitive impairment, paranoia, and increased hopelessness. Anthony J. Rothschild, MD, University of Massachusetts Medical School, reviewed methods for differentiating psychotic depression from schizophrenia, posttraumatic stress disorder, obsessive-compulsive disorder, and body dysmorphic disorder. There are no FDA-approved medications for psychotic depression, Dr. Rothschild explained; however, evidence shows that the combination of an antidepressant and an antipsychotic is superior to monotherapy with an agent from either class. In addition, he noted, studies show a high response rate with electroconvulsive therapy (ECT).
AFTERNOON SESSION
Return of symptoms after initial remission— while the patient is still taking an antidepressant—is considered tachyphylaxis, or “poop out.” Residual depressive symptoms, when a patient meets criteria for remission but still has troubling symptoms, is a different phenomenon, although symptoms can overlap. First, Dr. Rothschild advised, ensure that patients are given an adequate trial of an antidepressant. Options are similar when tachyphylaxis or residual symptoms are present: switch drugs or add augmentation therapy, such as lithium, thyroid hormone, or an atypical antipsychotic. Data on the efficacy for bupropion and buspirone are not strong. For treatment-resistant depression when a patient does not respond to 3 adequate antidepressant trials—consider ECT or rTMS, if available, or a monoamine oxidase inhibitor or a TCA.
Dr. Black defines antisocial personality disorder (ASPD) as a disorder of lifelong serial misbehavior, one characterized by impaired relationships, aggressive behavior, non-aggressive delinquent behavior, manipulation, and a disturbing lack of conscience. There is no standard treatment for ASPD, and no FDA-approved medications; however, potential treatments have not been adequately studied, he pointed out. Cognitive-behavioral therapy might be appropriate in mild cases; some patients benefit from specific programs— for example, ones that address drug or alcohol addiction or anger, although evidence is limited. When treating ASPD patients, Dr. Black concluded, be mindful of high attrition, possible misuse of prescribed medications, and drug-drug or drug-alcohol interactions.
Bipolar disorder is associated with the highest risk of suicide and increased lethality among all psychiatric disorders. Lithium has evidence of an anti-suicidality effect and may reduce suicide by decreasing relapse, aggression, and impulsivity. An FDA advisory on increased risk of suicidality with anticonvulsants was based on data about patients with epilepsy, not bipolar disorder. Second-generation antipsychotics, including olanzapine, quetiapine, and lurasidone, have been shown to be effective for bipolar depression. Avoid antidepressants if possible, Philip G. Janicak, MD, Northwestern University Feinberg School of Medicine, advised; if you must prescribe one, reassess the need for the drug often. Several psychotherapy modalities have evidence supporting their use in bipolar disorder.
FRIDAY, APRIL 17, 2015
MORNING SESSION
Henry A. Nasrallah, MD, Saint Louis University School of Medicine, offered enlightening historical touch-points on how psychiatry’s understanding of, and its approach to, schizophrenia have changed in the past 50 years. His goal? To challenge practitioners to rethink ideas about the disorder and how they care for affected patients. From a laundry list of comparative shifts, here are a few of Dr. Nasrallah’s “then” and “now” observations:
• The old paradigm was: Clinical and functional deterioration are inevitable in schizophrenia. The new paradigm is: Complete remission and restoration of function are feasible in many patients when they are fully adherent to the treatment plan.
• The old: Long-acting injectable (LAI) antipsychotics are a last-resort treatment, to be prescribed after a patient is stabilized. The new: Use LAI antipsychotics early in the course.
• Old: Begin treatment when psychosis appears. New: Work to prevent conversion to psychosis.
• Old: The disorder is considered a consequence of neurochemical dysregulation. New: Impaired neuroplasticity is to blame.
• Old: Treatment is a matter of trial and error. New: We can apply pharmaco-genomics to predict a patient’s response to various drugs and thus increase the likelihood of therapeutic success.
In his second presentation, Dr. Nasrallah described the many pathways to psychosis and several psychotic disorders other than schizophrenia, including schizoaffective, delusional disorder, and psychotic disorder caused by a general medical condition. He listed symptom clusters in psychosis beyond positive and negative symptoms, including neuromotor symptoms, mood symptoms, and neurocognitive deficits. Development of schizophrenia is multifactorial and involves risk genes and environmental factors seen before conception, during birth, and in early childhood; good prenatal care is the best way to prevent schizophrenia, Dr. Nasrallah noted. Several general medical conditions can produce schizophrenia-like psychosis, including some CNS disorders, toxins, autoimmune diseases, infectious diseases, and chromosomal abnormalities. The session concluded with a live interview with one of Dr. Nasrallah’s patients, whose schizophrenia is in remission with clozapine.
Drug abuse can mask signs and symptoms of bipolar disorder, which can delay diagnosis. Commonly abused substances are nicotine, alcohol, Cannabis, and cocaine; polysubstance abuse is the rule. Bipolar disorder and substance abuse share common mechanisms: impulsivity, poor modulation of motivation and response to reward, and behavioral sensitization. Treatment approaches should be flexible. Dr. Janicak reviewed the evidence for using anticonvulsants, antipsychotics, and bupropion for alcohol, Cannabis, and cocaine abuse; there are no data on treating opioid abuse. He also discussed the evidence for using naltrexone, acamprosate, disulfiram, and varenicline, as well as psychotherapeutic options, to treat substance abuse. Dr. Janicak encouraged clinicians in the audience to treat substance abuse in bipolar disorder patients themselves, instead of referring them to a subspecialist.
Untreated psychiatric disorders increase obstetrical complications, possibly through decreased self-care or increased stress. For mild or moderate depression, psychotherapy might be sufficient treatment; but for severe cases, medication is the first-line approach. In her presentation on mood disorders during pregnancy, Marlene P. Freeman, MD, Massachusetts General Hospital, advises that clinicians select medications based on known safety information, patient preference, and the previous course of illness. Results of studies that lasted 4 to 5 years do not show major long-term adverse effects of antidepressant exposure on neurodevelopment or neurobehavior. When treating patients for bipolar disorder, valproate is associated with an increased risk of adverse cognitive and neurodevelopmental effects in infants compared with other anticonvulsants; evidence suggests that lamotrigine is a safer option. The research does not show an increased risk of major malformations with second-generation antipsychotics.
AFTERNOON SESSION
Most women have premenstrual symptoms; a minority have a full-blown syndrome, now known as premenstrual dysphoric disorder (PMDD). This is not an existing mood disorder that becomes worse premenstrually. Clinician and patients should track the temporal relationship of symptoms on a calendar for a few months. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine have been well studied and are effective compared with placebo, but don’t help all patients with PMDD. Consider flexible dosing strategies with SSRIs—perhaps daily use, a higher dosage premenstrually, and as-needed administration. Start with an oral contraceptive or SSRI; if symptoms don’t respond, add the other. Serotonergic antidepressants have been shown helpful for hot flashes and depressive symptoms in perimenopause. Dr. Freeman reviewed the evidence for using complementary and alternative therapies for menopausal symptoms and hot flushes.
Smoking contributes to excess mortality in seriously mentally ill patients as a result of such tobacco-related illnesses as heart disease, lung disease, and cancer. Overall improvement in mental health as well as physical health is seen when a patient stops smoking. All nicotine replacement products are effective, but patients often don’t use them long enough or correctly. Robert M. Anthenelli, MD, University of California, San Diego, said to begin sustained-release bupropion 1 or 2 weeks before quit date; maintain the dosage for 1 to 12 weeks after quit date and consider maintenance therapy for as long as 6 months. Varenicline is superior to placebo and bupropion, but is known to have gastrointestinal (GI) and sleep disturbance adverse effects. Quitting smoking can increase the blood level of some psychotropics, meaning that you might need to reduce their dosage. It is best to begin smoking cessation when patients are mentally stable, when motivated, and stable on their medications.
In discussing trends in substance abuse, Dr. Anthenelli
faddish. Fentanyl and fentanyl analogues are 100 times more powerful than morphine; ingestion of even a minuscule dose can be fatal. Synthetic cannabinoids primarily are a problem among adolescents; they are more dangerous than marijuana and are associated with aggressive and suicidal behaviors. A standard toxicology screen will not detect synthetic cannabinoids.
E-cigarettes are considered by users to be safer than tobacco cigarettes—and probably are—but they still put patients at risk of nicotine addiction. There are no safety data on e-cigarettes; the devices might contain potentially harmful chemicals and potentially toxic nicotine levels. Dr. Anthenelli reported that topiramate is “the best medication I’ve used” for alcohol abuse disorder. The drug is not FDA-approved for this use, but has been used in a number of studies with positive outcomes.
SATURDAY, APRIL 18, 2015
MORNING SESSION
Psychiatrists are well positioned to help patients with mental illness lose weight because of their psychotherapeutic background. Best treatment strategy is diet plus exercise plus behavioral modification. Robert M. McCarron, DO, University of California, Davis, recommends keeping it simple and telling patients to only consider calories of foods, and not to worry about sodium or fat content. Ask patients “How many minutes a day of exercise can you do?” but recommend that patients walk for 30 minutes a day at 4 mph, 5 days per week, which will help patients lose 1% to 3% of body weight. For treatment-refractory obese patients, consider medications such as bupropion, orlistat, lorcaserin, topiramate, or metformin; for those with a BMI ≥40, recommend bariatric surgery.
George T. Grossberg, MD, Saint Louis University School of Medicine, reviewed the evidence for anxiety disorders in older adults, including generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder. Older patients with cardiovascular disease, cancer, Parkinson’s disease, diabetes, GI disorders, or chronic obstructive pulmonary disease are at high risk of anxiety symptoms. In a study of centenarians, predictors of anxiety are worse health perception, financial concerns related to medical expenses, higher number of medical conditions, and loneliness. Secondary anxiety is prevalent in Alzheimer’s disease; the condition can present as fidgeting, pacing, anger, or agitation, and can be prompted by a change in routine. Acute, new-onset anxiety symptoms should trigger a complete medical evaluation, including a review of medications, supplements, and substance use. In geriatric patients, minimize use of benzodiazepines and avoid anticholinergics.
Overall, psychiatry patients do not receive optimal preventive and primary medical care, leading to decreased life expectancy, often as a result of cardiovascular disease. Psychiatric patients have a high rate of dyslipidemia, hypertension, smoking, and obesity. Psychiatrists often don’t treat these conditions, but they need to be aware of changing standard practices in preventive medicine; be able to recognize a potential problem; and make referrals when appropriate. Dr. McCarron reviewed age-based screening recommendations for hypertension, dyslipidemia, and diabetes from the book Preventive Medical Care in Psychiatry, which he co-edited. He recommends using online cardiovascular risk calculators to determine which patients need to be screened.
AFTERNOON SESSION
Some older patients who abuse substances took drugs as young adults and never gave them up; others have rediscovered drugs in later life. Potential indicators of alcohol abuse in older patients are changes in cognition, mood, memory, hygiene, or sleep. Substance abuse in older adults frequently is comorbid with depression or bereavement, anxiety, and adjustment disorders. Dr. Grossberg recommends addressing the topic directly with patients. Although there are few data to guide treatment, prompt detection and appropriate treatment can improve the quality of life of older adults and their family.
SPONSORS AND SUPPORTERS
• American Professional Agency
• American Psychiatric Publishing
• Arbor Pharmaceuticals
• AstraZeneca
• Banner Health
• Bassett Healthcare Network
• Ministry Health Care
• Pine Rest Christian Mental Health Services
• PRMS
• Sinai Health System
• Sunovion
• Takeda Pharmaceuticals
• U.S. Army Healthcare
• Wexford Health Sources
• Wolters Kluwer Health
The meeting organizers acknowledge the support provided by the sponsors. Determination of educational content for this program and the selection of speakers are responsibilities of the program director and co-directors. Sponsors and supporters did not have input in these areas.
personality disorder, DSM-5, adults with ADHD, residual depressive symptoms, treatment-resistant depression,antisocial personality disorder, bipolar disorder, schizophrenia, psychotic disorder, clozapine, bipolar disorder and substance abuse, mood disorders during pregnancy, premenstrual dysphoric disorder, depressive symptoms in perimenopause, smoking and the mentally ill, help patients with mental illness lose weight, substance abuse in older adults
personality disorder, DSM-5, adults with ADHD, residual depressive symptoms, treatment-resistant depression,antisocial personality disorder, bipolar disorder, schizophrenia, psychotic disorder, clozapine, bipolar disorder and substance abuse, mood disorders during pregnancy, premenstrual dysphoric disorder, depressive symptoms in perimenopause, smoking and the mentally ill, help patients with mental illness lose weight, substance abuse in older adults
Slower teen reaction times may increase anxiety and depression risk later in life
Adolescents with slower processing speeds and longer reaction times were at a greater risk of anxiety and depression later in life, according to Catharine R. Gale, Ph.D., of the University of Southampton (England) and her associates.
In this 20-year study of 705 males and females, longer reaction time at 16 years indicated a small but significant association with poorer mental health at age 36.
Adjusting for sex, parental social class, General Health Questionnaire (GHQ) score at age 16 years, health behaviors at age 36 years, and allostatic load had little effect on the association between reaction time and the GHQ score, but the association was weakened with Hospital Anxiety and Depression Scale (HADS) scores for both anxiety and depression. Smoking had a mediating effect on the HADS anxiety score, but not on the depression subscale.
“Further prospective studies of the relation between reaction time and mental health outcomes in other samples are needed to gauge whether reaction time is a true risk factor for mental disorders and to confirm the mediating roles played by smoking and allostatic load,” the investigators noted.
Find the full study in Psychosomatic Medicine (doi:10.1097/PSY.0000000000000189).
Adolescents with slower processing speeds and longer reaction times were at a greater risk of anxiety and depression later in life, according to Catharine R. Gale, Ph.D., of the University of Southampton (England) and her associates.
In this 20-year study of 705 males and females, longer reaction time at 16 years indicated a small but significant association with poorer mental health at age 36.
Adjusting for sex, parental social class, General Health Questionnaire (GHQ) score at age 16 years, health behaviors at age 36 years, and allostatic load had little effect on the association between reaction time and the GHQ score, but the association was weakened with Hospital Anxiety and Depression Scale (HADS) scores for both anxiety and depression. Smoking had a mediating effect on the HADS anxiety score, but not on the depression subscale.
“Further prospective studies of the relation between reaction time and mental health outcomes in other samples are needed to gauge whether reaction time is a true risk factor for mental disorders and to confirm the mediating roles played by smoking and allostatic load,” the investigators noted.
Find the full study in Psychosomatic Medicine (doi:10.1097/PSY.0000000000000189).
Adolescents with slower processing speeds and longer reaction times were at a greater risk of anxiety and depression later in life, according to Catharine R. Gale, Ph.D., of the University of Southampton (England) and her associates.
In this 20-year study of 705 males and females, longer reaction time at 16 years indicated a small but significant association with poorer mental health at age 36.
Adjusting for sex, parental social class, General Health Questionnaire (GHQ) score at age 16 years, health behaviors at age 36 years, and allostatic load had little effect on the association between reaction time and the GHQ score, but the association was weakened with Hospital Anxiety and Depression Scale (HADS) scores for both anxiety and depression. Smoking had a mediating effect on the HADS anxiety score, but not on the depression subscale.
“Further prospective studies of the relation between reaction time and mental health outcomes in other samples are needed to gauge whether reaction time is a true risk factor for mental disorders and to confirm the mediating roles played by smoking and allostatic load,” the investigators noted.
Find the full study in Psychosomatic Medicine (doi:10.1097/PSY.0000000000000189).
VIDEO: Is it IBS? Blood test may offer conclusive answer
WASHINGTON – A new blood test could conclusively determine if a patient with chronic diarrhea has diarrhea-predominant irritable bowel syndrome (D-IBS).
The IBSchek blood test detects the presence of antibodies to cytolethal distending toxin B and vinculin. In a study presented at the annual Digestive Disease Week and published in PLoS ONE, the positive predictive value for D-IBS of just one of the antibodies was greater than 98%, explained study lead author Dr. Mark Pimentel of Cedars-Sinai Medical Center, Los Angeles. If the test is positive for both antibodies, “the post-test probability is 95% that you have IBS.”
In a video interview, Dr. Pimentel discussed the study’s findings and the potential impact for physicians and patients. The search for diagnostic answers leads to “a lot of doctor-shopping, certainly a lot of colonoscopies and unnecessary testing that are always negative with these patients,” he noted. “Maybe this will put an end to that.
“People used to think this is all psychological,” Dr. Pimentel added. “Now we can say, No, it’s organic. There’s something real going on; I’ve got a test that proves that.”
Dr. Pimentel has received consulting fees from Commonwealth Laboratories, which makes the IBSchek blood test.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – A new blood test could conclusively determine if a patient with chronic diarrhea has diarrhea-predominant irritable bowel syndrome (D-IBS).
The IBSchek blood test detects the presence of antibodies to cytolethal distending toxin B and vinculin. In a study presented at the annual Digestive Disease Week and published in PLoS ONE, the positive predictive value for D-IBS of just one of the antibodies was greater than 98%, explained study lead author Dr. Mark Pimentel of Cedars-Sinai Medical Center, Los Angeles. If the test is positive for both antibodies, “the post-test probability is 95% that you have IBS.”
In a video interview, Dr. Pimentel discussed the study’s findings and the potential impact for physicians and patients. The search for diagnostic answers leads to “a lot of doctor-shopping, certainly a lot of colonoscopies and unnecessary testing that are always negative with these patients,” he noted. “Maybe this will put an end to that.
“People used to think this is all psychological,” Dr. Pimentel added. “Now we can say, No, it’s organic. There’s something real going on; I’ve got a test that proves that.”
Dr. Pimentel has received consulting fees from Commonwealth Laboratories, which makes the IBSchek blood test.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – A new blood test could conclusively determine if a patient with chronic diarrhea has diarrhea-predominant irritable bowel syndrome (D-IBS).
The IBSchek blood test detects the presence of antibodies to cytolethal distending toxin B and vinculin. In a study presented at the annual Digestive Disease Week and published in PLoS ONE, the positive predictive value for D-IBS of just one of the antibodies was greater than 98%, explained study lead author Dr. Mark Pimentel of Cedars-Sinai Medical Center, Los Angeles. If the test is positive for both antibodies, “the post-test probability is 95% that you have IBS.”
In a video interview, Dr. Pimentel discussed the study’s findings and the potential impact for physicians and patients. The search for diagnostic answers leads to “a lot of doctor-shopping, certainly a lot of colonoscopies and unnecessary testing that are always negative with these patients,” he noted. “Maybe this will put an end to that.
“People used to think this is all psychological,” Dr. Pimentel added. “Now we can say, No, it’s organic. There’s something real going on; I’ve got a test that proves that.”
Dr. Pimentel has received consulting fees from Commonwealth Laboratories, which makes the IBSchek blood test.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2015