User login
Mediterranean diet tied to less severe erectile dysfunction
In an observational study of 250 middle-aged men with hypertension and erectile dysfunction, those whose eating patterns more closely matched a Mediterranean diet had significantly higher testosterone levels, better exercise capacity, and better erectile performance than their peers.
In addition, more closely following a Mediterranean diet – which emphasizes eating fruit, vegetables, whole grains, and olive oil, with modest consumption of dairy products and limited red meat – was associated with better coronary blood flow and less arterial stiffness, all after adjusting for age, body mass index, type 2 diabetes, statin use, and smoking.
Athanasios Angelis, MD, First Cardiology Clinic, Hippokration Hospital, School of Medicine, University of Athens, presented the study at the annual congress of the European Society of Cardiology.
“While we did not examine mechanisms,” Dr. Angelis said in a press release from the ESC, “it seems plausible that this dietary pattern may improve fitness and erectile performance by enhancing function of the blood vessels and limiting the fall in testosterone that occurs in midlife.”
“The findings suggest that the Mediterranean diet could play a role in maintaining several parameters of vascular health and quality of life and in middle-aged men with hypertension and erectile dysfunction,” he concluded.
“A Mediterranean diet may help erectile dysfunction by improving endothelial physiology,” Dr. Angelis said in an interview. “We suggest the Mediterranean diet as a basic parameter of hypertension and erectile dysfunction treatment. We advise all our patients to be careful regarding salt consumption and to try to exercise regularly.”
“Depending on the severity of the erectile dysfunction, we may suggest only lifestyle changes (e.g., quit smoking), at least for the beginning, or combination with medication,” consisting of phosphodiesterase type 5 (PDE5) inhibitors such as Viagra.
A ‘first-choice’ diet for men with ED, low T, high CVD risk?
This research “adds to the growing evidence that a Mediterranean diet is protective against erectile dysfunction,” said Joseph Whittaker, MSc, a clinical nutritionist from the University of Worcester (England) and coauthor of a related meta-analysis about dietary fat and testosterone.
This way of eating “also improves cardiovascular health, so it could become a low-risk, first choice treatment for these three pathologies (low testosterone, erectile dysfunction, increased risk of CVD), which so commonly coexist,” he wrote in an email.
“However, most of the research to date is observational,” he cautioned, which often has a “healthy user bias,” that is, the men eating a Mediterranean diet are probably health-conscious individuals, with other healthy habits such as exercise, good sleep, low stress, etc. “So, was it the diet, the healthy habits, or both?”
Randomized studies are needed to replicate the positive results of observational studies like this one, Mr. Whittaker added. In the meantime, “a Mediterranean diet will probably improve your health anyway,” he noted, “so trying it for the purposes of erectile function (before starting drugs) is a viable option.”
Previous research has shown that dietary fat and olive oil may boost testosterone levels, Mr. Whittaker noted, and nuts have also been shown to improve erectile function.
“So, the increase in healthy fats – mono- and polyunsaturated fatty acids (MUFAs and PUFAs, respectively) – on the Mediterranean diet is probably responsible for these benefits,” he speculated.
Middle-aged hypertensive men with ED
Men with hypertension are twice as likely to have erectile dysfunction as their peers with normal blood pressure, according to background information in the ESC press release.
Erectile dysfunction is thought to be a disorder of the small arteries, which lose their ability to dilate and increase blood flow. Declining testosterone levels in middle age also contribute to weakened erectile performance.
Physical fitness is linked with longer life in men with hypertension, and the Mediterranean diet is associated with lower blood pressure and fewer heart attacks and strokes in individuals at high cardiovascular risk.
Therefore, Dr. Angelis and colleagues aimed to see if greater adherence to a Mediterranean diet was associated with better exercise capacity, testosterone levels, coronary flow reserve, and erectile performance in middle-aged hypertensive men with erectile dysfunction.
Participants were a mean age of 56. They had a treadmill test to determine their exercise capacity, expressed as metabolic equivalent of tasks (METs), and a blood test to determine testosterone levels.
They replied to two questionnaires: a food questionnaire to determine a Mediterranean Diet score (range, 0-55, where higher scores indicate greater adherence to a Mediterranean diet) and a Sexual Health Inventory for Men (SHIM) questionnaire (score range, 0-25, where higher scores indicate better erectile performance).
Researchers used echocardiography to determine participants’ coronary flow reserve, a measure of the cardiovascular system’s ability to increase blood flow when needed. They used a SphygmoCor device to determine participants’ augmentation index and central pulse pressure, measures of arterial stiffness.
The men with a higher Mediterranean diet score (>29) had better erectile performance (SHIM scores > 14), as well as higher testosterone levels, higher coronary flow reserve, and less arterial stiffness than the other men.
The fitter men with greater exercise capacity (>10 METs) were more likely to adhere to a Mediterranean diet (scores > 25), and they also had better erectile performance (SHIM scores > 12), higher testosterone levels, greater coronary flow reserve, and less arterial stiffness than the other men.
The study did not receive any funding. The study authors and Mr. Whittaker have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an observational study of 250 middle-aged men with hypertension and erectile dysfunction, those whose eating patterns more closely matched a Mediterranean diet had significantly higher testosterone levels, better exercise capacity, and better erectile performance than their peers.
In addition, more closely following a Mediterranean diet – which emphasizes eating fruit, vegetables, whole grains, and olive oil, with modest consumption of dairy products and limited red meat – was associated with better coronary blood flow and less arterial stiffness, all after adjusting for age, body mass index, type 2 diabetes, statin use, and smoking.
Athanasios Angelis, MD, First Cardiology Clinic, Hippokration Hospital, School of Medicine, University of Athens, presented the study at the annual congress of the European Society of Cardiology.
“While we did not examine mechanisms,” Dr. Angelis said in a press release from the ESC, “it seems plausible that this dietary pattern may improve fitness and erectile performance by enhancing function of the blood vessels and limiting the fall in testosterone that occurs in midlife.”
“The findings suggest that the Mediterranean diet could play a role in maintaining several parameters of vascular health and quality of life and in middle-aged men with hypertension and erectile dysfunction,” he concluded.
“A Mediterranean diet may help erectile dysfunction by improving endothelial physiology,” Dr. Angelis said in an interview. “We suggest the Mediterranean diet as a basic parameter of hypertension and erectile dysfunction treatment. We advise all our patients to be careful regarding salt consumption and to try to exercise regularly.”
“Depending on the severity of the erectile dysfunction, we may suggest only lifestyle changes (e.g., quit smoking), at least for the beginning, or combination with medication,” consisting of phosphodiesterase type 5 (PDE5) inhibitors such as Viagra.
A ‘first-choice’ diet for men with ED, low T, high CVD risk?
This research “adds to the growing evidence that a Mediterranean diet is protective against erectile dysfunction,” said Joseph Whittaker, MSc, a clinical nutritionist from the University of Worcester (England) and coauthor of a related meta-analysis about dietary fat and testosterone.
This way of eating “also improves cardiovascular health, so it could become a low-risk, first choice treatment for these three pathologies (low testosterone, erectile dysfunction, increased risk of CVD), which so commonly coexist,” he wrote in an email.
“However, most of the research to date is observational,” he cautioned, which often has a “healthy user bias,” that is, the men eating a Mediterranean diet are probably health-conscious individuals, with other healthy habits such as exercise, good sleep, low stress, etc. “So, was it the diet, the healthy habits, or both?”
Randomized studies are needed to replicate the positive results of observational studies like this one, Mr. Whittaker added. In the meantime, “a Mediterranean diet will probably improve your health anyway,” he noted, “so trying it for the purposes of erectile function (before starting drugs) is a viable option.”
Previous research has shown that dietary fat and olive oil may boost testosterone levels, Mr. Whittaker noted, and nuts have also been shown to improve erectile function.
“So, the increase in healthy fats – mono- and polyunsaturated fatty acids (MUFAs and PUFAs, respectively) – on the Mediterranean diet is probably responsible for these benefits,” he speculated.
Middle-aged hypertensive men with ED
Men with hypertension are twice as likely to have erectile dysfunction as their peers with normal blood pressure, according to background information in the ESC press release.
Erectile dysfunction is thought to be a disorder of the small arteries, which lose their ability to dilate and increase blood flow. Declining testosterone levels in middle age also contribute to weakened erectile performance.
Physical fitness is linked with longer life in men with hypertension, and the Mediterranean diet is associated with lower blood pressure and fewer heart attacks and strokes in individuals at high cardiovascular risk.
Therefore, Dr. Angelis and colleagues aimed to see if greater adherence to a Mediterranean diet was associated with better exercise capacity, testosterone levels, coronary flow reserve, and erectile performance in middle-aged hypertensive men with erectile dysfunction.
Participants were a mean age of 56. They had a treadmill test to determine their exercise capacity, expressed as metabolic equivalent of tasks (METs), and a blood test to determine testosterone levels.
They replied to two questionnaires: a food questionnaire to determine a Mediterranean Diet score (range, 0-55, where higher scores indicate greater adherence to a Mediterranean diet) and a Sexual Health Inventory for Men (SHIM) questionnaire (score range, 0-25, where higher scores indicate better erectile performance).
Researchers used echocardiography to determine participants’ coronary flow reserve, a measure of the cardiovascular system’s ability to increase blood flow when needed. They used a SphygmoCor device to determine participants’ augmentation index and central pulse pressure, measures of arterial stiffness.
The men with a higher Mediterranean diet score (>29) had better erectile performance (SHIM scores > 14), as well as higher testosterone levels, higher coronary flow reserve, and less arterial stiffness than the other men.
The fitter men with greater exercise capacity (>10 METs) were more likely to adhere to a Mediterranean diet (scores > 25), and they also had better erectile performance (SHIM scores > 12), higher testosterone levels, greater coronary flow reserve, and less arterial stiffness than the other men.
The study did not receive any funding. The study authors and Mr. Whittaker have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an observational study of 250 middle-aged men with hypertension and erectile dysfunction, those whose eating patterns more closely matched a Mediterranean diet had significantly higher testosterone levels, better exercise capacity, and better erectile performance than their peers.
In addition, more closely following a Mediterranean diet – which emphasizes eating fruit, vegetables, whole grains, and olive oil, with modest consumption of dairy products and limited red meat – was associated with better coronary blood flow and less arterial stiffness, all after adjusting for age, body mass index, type 2 diabetes, statin use, and smoking.
Athanasios Angelis, MD, First Cardiology Clinic, Hippokration Hospital, School of Medicine, University of Athens, presented the study at the annual congress of the European Society of Cardiology.
“While we did not examine mechanisms,” Dr. Angelis said in a press release from the ESC, “it seems plausible that this dietary pattern may improve fitness and erectile performance by enhancing function of the blood vessels and limiting the fall in testosterone that occurs in midlife.”
“The findings suggest that the Mediterranean diet could play a role in maintaining several parameters of vascular health and quality of life and in middle-aged men with hypertension and erectile dysfunction,” he concluded.
“A Mediterranean diet may help erectile dysfunction by improving endothelial physiology,” Dr. Angelis said in an interview. “We suggest the Mediterranean diet as a basic parameter of hypertension and erectile dysfunction treatment. We advise all our patients to be careful regarding salt consumption and to try to exercise regularly.”
“Depending on the severity of the erectile dysfunction, we may suggest only lifestyle changes (e.g., quit smoking), at least for the beginning, or combination with medication,” consisting of phosphodiesterase type 5 (PDE5) inhibitors such as Viagra.
A ‘first-choice’ diet for men with ED, low T, high CVD risk?
This research “adds to the growing evidence that a Mediterranean diet is protective against erectile dysfunction,” said Joseph Whittaker, MSc, a clinical nutritionist from the University of Worcester (England) and coauthor of a related meta-analysis about dietary fat and testosterone.
This way of eating “also improves cardiovascular health, so it could become a low-risk, first choice treatment for these three pathologies (low testosterone, erectile dysfunction, increased risk of CVD), which so commonly coexist,” he wrote in an email.
“However, most of the research to date is observational,” he cautioned, which often has a “healthy user bias,” that is, the men eating a Mediterranean diet are probably health-conscious individuals, with other healthy habits such as exercise, good sleep, low stress, etc. “So, was it the diet, the healthy habits, or both?”
Randomized studies are needed to replicate the positive results of observational studies like this one, Mr. Whittaker added. In the meantime, “a Mediterranean diet will probably improve your health anyway,” he noted, “so trying it for the purposes of erectile function (before starting drugs) is a viable option.”
Previous research has shown that dietary fat and olive oil may boost testosterone levels, Mr. Whittaker noted, and nuts have also been shown to improve erectile function.
“So, the increase in healthy fats – mono- and polyunsaturated fatty acids (MUFAs and PUFAs, respectively) – on the Mediterranean diet is probably responsible for these benefits,” he speculated.
Middle-aged hypertensive men with ED
Men with hypertension are twice as likely to have erectile dysfunction as their peers with normal blood pressure, according to background information in the ESC press release.
Erectile dysfunction is thought to be a disorder of the small arteries, which lose their ability to dilate and increase blood flow. Declining testosterone levels in middle age also contribute to weakened erectile performance.
Physical fitness is linked with longer life in men with hypertension, and the Mediterranean diet is associated with lower blood pressure and fewer heart attacks and strokes in individuals at high cardiovascular risk.
Therefore, Dr. Angelis and colleagues aimed to see if greater adherence to a Mediterranean diet was associated with better exercise capacity, testosterone levels, coronary flow reserve, and erectile performance in middle-aged hypertensive men with erectile dysfunction.
Participants were a mean age of 56. They had a treadmill test to determine their exercise capacity, expressed as metabolic equivalent of tasks (METs), and a blood test to determine testosterone levels.
They replied to two questionnaires: a food questionnaire to determine a Mediterranean Diet score (range, 0-55, where higher scores indicate greater adherence to a Mediterranean diet) and a Sexual Health Inventory for Men (SHIM) questionnaire (score range, 0-25, where higher scores indicate better erectile performance).
Researchers used echocardiography to determine participants’ coronary flow reserve, a measure of the cardiovascular system’s ability to increase blood flow when needed. They used a SphygmoCor device to determine participants’ augmentation index and central pulse pressure, measures of arterial stiffness.
The men with a higher Mediterranean diet score (>29) had better erectile performance (SHIM scores > 14), as well as higher testosterone levels, higher coronary flow reserve, and less arterial stiffness than the other men.
The fitter men with greater exercise capacity (>10 METs) were more likely to adhere to a Mediterranean diet (scores > 25), and they also had better erectile performance (SHIM scores > 12), higher testosterone levels, greater coronary flow reserve, and less arterial stiffness than the other men.
The study did not receive any funding. The study authors and Mr. Whittaker have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2021
Prevalence of high-risk HPV types dwindled since vaccine approval
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When it comes to young women, regular check-ins support ongoing PrEP use
The secret, said Gonasagrie Nair, MBChB, faculty of medicine and health sciences at Stellenbosch University, Zimbabwe, is offering intensive wraparound services to support teenagers – a lesson that may be useful as adolescent and family medicine professionals in the United States begin to roll out HIV prevention in their clinics.
This is important in the United States because cisgender Black women make up 60% of all new HIV cases in the United States while accounting for just 14% of the overall U.S. population. The Centers for Disease Control and Prevention has found that only about 1% of Black Americans who could benefit from PrEP have access to it.
“Younger women and adolescent girls in particular face a number of cultural and social challenges that impact their ability to make decisions related to their own health,” said Dr. Nair, who presented the data at the International AIDS Society (IAS) Conference 2021. “The adherence support provided by this study empowered them to make choices and stick to these choices,” she said.
In total, 247 women and girls aged 16 to 21 who were without HIV were enrolled in the Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH) trial in two sites in South Africa and one each in Uganda and Zimbabwe beginning in February 2019. One-third of the participants were minors; the average age was 18.2 years.
The women were good candidates for PrEP. More than 1 in 3 of the women started the study with a sexually transmitted infection (STI), the most prevalent of which was chlamydia. This is often a good marker for condomless sex. Of the participants, 89% had a primary sex partner; a quarter of those thought their partner was having sex with other people. Only 7% of participants reported being very worried about acquiring HIV. More than 1 in 3 (39%) weren’t worried about HIV at all. This conforms to previous data suggesting that those who could most benefit from PrEP often don’t perceive their own vulnerability.
In the study, the women were randomly assigned two groups. In one group, the participants used the dapivirine ring for 6 months; in the other, participants used oral PrEP for 6 months. The participants then swapped prevention methods and used the alternative method for 6 more months. After a year of trying both methods, the women will be asked to choose one of the two prevention method or to stop PrEP altogether. At the IAS conference, the researchers reported interim data from the first year of the study, before the girls had the opportunity to choose for themselves.
During that first year, girls received intensive adherence support, including daily or weekly text check-ins, phone check-ins, peer buddy support, additional onsite counseling visits, access to adherence support groups, participation in online support groups via apps such as WhatsApp, and in-person social events designed to empower young women and to teach them skills. Support included discussion of adherence, contraceptives, and STIs. In addition, when girls came in for study visits, staff provided feedback on how adherent the girls had been, as determined on the basis of residual levels of dapivirine in the rings or, with regard to oral pills, drug levels as determined with blood spots.
Girls were considered to have had high adherence if they were found to have oral PrEP concentrations equivalent to four or more doses per week or if residual levels of dapivirine in their rings were 0.1071 mg/d. Moderate adherence was the equivalent of one to three doses of oral PrEP a week or dapivirine levels between 0.0321 mg/d and 0.1071 mg/d.
In total, 95.6% of ring users showed some adherence to the ring. Of those, adherence was high for 50.2%; 49.8% used the ring perfectly. For oral PrEP, 98.5% showed some level of PrEP use; for 58.6%, lab results suggested adherence high enough to provide protection from HIV, and 22% took their pills at least six times a week. Between the two arms, 54.3% of all participants used the medication sufficiently to be protected from HIV.
One person acquired HIV during the study. Dr. Nair did not say which study arm that participant was in or how adherent that person has been to their prevention method.
That level of adherence is on par with studies in the United States, which have found 56% adherence to PrEP among adolescent and young men who have sex with men. But the level of adherence is far higher than has been found in other studies that tested oral PrEP among women who did not have a partner with HIV. In particular, the VOICE and FEM-PrEP trials were both stopped early for lack of adherence. In those placebo-controlled oral-PrEP trials, fewer than 25% of participants used the oral prevention pills. Although adherence to the vaginal ring was estimated to be 61% for women older than 25 in the ASPIRE trial, it was effectively zero among women aged 18 to 21 years. Adherence has been the “bugaboo of efficacy for PrEP in young women,” said Judith Auerbach, PhD, independent science and policy consultant and professor of medicine at the University of California, San Francisco. But health care professionals have a long way to go to support young people in general in using PrEP.
“Yes, this shows improvement compared to previous studies,” Dr. Auerbach told this news organization. “But is it sufficient to have an epidemiological impact at the population level?”
Medical Advocacy and Outreach (MAO) is an HIV clinic and services program in Montgomery, Alabama, that offers a clinic specifically for some of their 144 clients to receive oral PrEP. In addition to in-person testing, MAO offers home HIV testing and lab work and televisits to support the college students they serve in taking PrEP whether they’re at school or at home on break. Currently, MAO provides a series of support groups and other social support programs for their clients living with HIV, but there are none for those receiving PrEP. The organization is in the process of hiring a social worker for the PrEP side of the clinic.
Until that person is on board, “I’m their support system in an unofficial capacity,” Shericka Williams, MPH, told this news organization. She runs education programs at MAO and handles all the phone calls from PrEP clients. “My title changes a lot, but the one I like to go with most often is the PrEP navigator,” she said.
She said she was intrigued by the dapivirine ring and oral PrEP data but said that currently, the women they serve are still learning that PrEP is for them, too. The women report that all the ads and all the information they receive is aimed at gay or bisexual men or transgender women. It takes a while for them to recognize that they could benefit, so a lot of the work that Ms. Williams does is focused on explaining the benefit of PrEP.
In MAO, the number of women receiving PrEP fluctuates more than for men. Mostly, women start PrEP because of they are in a relationship with someone who receives HIV care from MAO’s other wing – women who potentially would experience less vulnerability to HIV if their partners had undetectable viral loads. The other reason women take it is because they suspect that their partner is cheating or because they are in abusive relationships in which they want their partner to use a condom but the partner won’t. As in the PrEP trials, they often see women discontinue PrEP when they leave those relationships. In part, her job is to educate women regarding all the ways PrEP could serve them.
“Most of the time, they’re just no longer in that relationship, and they’re just taking some time for themselves,” she said in an interview. “We definitely try to bring up other reasons to stay on PrEP, but we don’t want to seem like we’re bullying someone to stay on it.”
Dr. Nair, Dr. Auerbach, and Ms. Williams report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The secret, said Gonasagrie Nair, MBChB, faculty of medicine and health sciences at Stellenbosch University, Zimbabwe, is offering intensive wraparound services to support teenagers – a lesson that may be useful as adolescent and family medicine professionals in the United States begin to roll out HIV prevention in their clinics.
This is important in the United States because cisgender Black women make up 60% of all new HIV cases in the United States while accounting for just 14% of the overall U.S. population. The Centers for Disease Control and Prevention has found that only about 1% of Black Americans who could benefit from PrEP have access to it.
“Younger women and adolescent girls in particular face a number of cultural and social challenges that impact their ability to make decisions related to their own health,” said Dr. Nair, who presented the data at the International AIDS Society (IAS) Conference 2021. “The adherence support provided by this study empowered them to make choices and stick to these choices,” she said.
In total, 247 women and girls aged 16 to 21 who were without HIV were enrolled in the Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH) trial in two sites in South Africa and one each in Uganda and Zimbabwe beginning in February 2019. One-third of the participants were minors; the average age was 18.2 years.
The women were good candidates for PrEP. More than 1 in 3 of the women started the study with a sexually transmitted infection (STI), the most prevalent of which was chlamydia. This is often a good marker for condomless sex. Of the participants, 89% had a primary sex partner; a quarter of those thought their partner was having sex with other people. Only 7% of participants reported being very worried about acquiring HIV. More than 1 in 3 (39%) weren’t worried about HIV at all. This conforms to previous data suggesting that those who could most benefit from PrEP often don’t perceive their own vulnerability.
In the study, the women were randomly assigned two groups. In one group, the participants used the dapivirine ring for 6 months; in the other, participants used oral PrEP for 6 months. The participants then swapped prevention methods and used the alternative method for 6 more months. After a year of trying both methods, the women will be asked to choose one of the two prevention method or to stop PrEP altogether. At the IAS conference, the researchers reported interim data from the first year of the study, before the girls had the opportunity to choose for themselves.
During that first year, girls received intensive adherence support, including daily or weekly text check-ins, phone check-ins, peer buddy support, additional onsite counseling visits, access to adherence support groups, participation in online support groups via apps such as WhatsApp, and in-person social events designed to empower young women and to teach them skills. Support included discussion of adherence, contraceptives, and STIs. In addition, when girls came in for study visits, staff provided feedback on how adherent the girls had been, as determined on the basis of residual levels of dapivirine in the rings or, with regard to oral pills, drug levels as determined with blood spots.
Girls were considered to have had high adherence if they were found to have oral PrEP concentrations equivalent to four or more doses per week or if residual levels of dapivirine in their rings were 0.1071 mg/d. Moderate adherence was the equivalent of one to three doses of oral PrEP a week or dapivirine levels between 0.0321 mg/d and 0.1071 mg/d.
In total, 95.6% of ring users showed some adherence to the ring. Of those, adherence was high for 50.2%; 49.8% used the ring perfectly. For oral PrEP, 98.5% showed some level of PrEP use; for 58.6%, lab results suggested adherence high enough to provide protection from HIV, and 22% took their pills at least six times a week. Between the two arms, 54.3% of all participants used the medication sufficiently to be protected from HIV.
One person acquired HIV during the study. Dr. Nair did not say which study arm that participant was in or how adherent that person has been to their prevention method.
That level of adherence is on par with studies in the United States, which have found 56% adherence to PrEP among adolescent and young men who have sex with men. But the level of adherence is far higher than has been found in other studies that tested oral PrEP among women who did not have a partner with HIV. In particular, the VOICE and FEM-PrEP trials were both stopped early for lack of adherence. In those placebo-controlled oral-PrEP trials, fewer than 25% of participants used the oral prevention pills. Although adherence to the vaginal ring was estimated to be 61% for women older than 25 in the ASPIRE trial, it was effectively zero among women aged 18 to 21 years. Adherence has been the “bugaboo of efficacy for PrEP in young women,” said Judith Auerbach, PhD, independent science and policy consultant and professor of medicine at the University of California, San Francisco. But health care professionals have a long way to go to support young people in general in using PrEP.
“Yes, this shows improvement compared to previous studies,” Dr. Auerbach told this news organization. “But is it sufficient to have an epidemiological impact at the population level?”
Medical Advocacy and Outreach (MAO) is an HIV clinic and services program in Montgomery, Alabama, that offers a clinic specifically for some of their 144 clients to receive oral PrEP. In addition to in-person testing, MAO offers home HIV testing and lab work and televisits to support the college students they serve in taking PrEP whether they’re at school or at home on break. Currently, MAO provides a series of support groups and other social support programs for their clients living with HIV, but there are none for those receiving PrEP. The organization is in the process of hiring a social worker for the PrEP side of the clinic.
Until that person is on board, “I’m their support system in an unofficial capacity,” Shericka Williams, MPH, told this news organization. She runs education programs at MAO and handles all the phone calls from PrEP clients. “My title changes a lot, but the one I like to go with most often is the PrEP navigator,” she said.
She said she was intrigued by the dapivirine ring and oral PrEP data but said that currently, the women they serve are still learning that PrEP is for them, too. The women report that all the ads and all the information they receive is aimed at gay or bisexual men or transgender women. It takes a while for them to recognize that they could benefit, so a lot of the work that Ms. Williams does is focused on explaining the benefit of PrEP.
In MAO, the number of women receiving PrEP fluctuates more than for men. Mostly, women start PrEP because of they are in a relationship with someone who receives HIV care from MAO’s other wing – women who potentially would experience less vulnerability to HIV if their partners had undetectable viral loads. The other reason women take it is because they suspect that their partner is cheating or because they are in abusive relationships in which they want their partner to use a condom but the partner won’t. As in the PrEP trials, they often see women discontinue PrEP when they leave those relationships. In part, her job is to educate women regarding all the ways PrEP could serve them.
“Most of the time, they’re just no longer in that relationship, and they’re just taking some time for themselves,” she said in an interview. “We definitely try to bring up other reasons to stay on PrEP, but we don’t want to seem like we’re bullying someone to stay on it.”
Dr. Nair, Dr. Auerbach, and Ms. Williams report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The secret, said Gonasagrie Nair, MBChB, faculty of medicine and health sciences at Stellenbosch University, Zimbabwe, is offering intensive wraparound services to support teenagers – a lesson that may be useful as adolescent and family medicine professionals in the United States begin to roll out HIV prevention in their clinics.
This is important in the United States because cisgender Black women make up 60% of all new HIV cases in the United States while accounting for just 14% of the overall U.S. population. The Centers for Disease Control and Prevention has found that only about 1% of Black Americans who could benefit from PrEP have access to it.
“Younger women and adolescent girls in particular face a number of cultural and social challenges that impact their ability to make decisions related to their own health,” said Dr. Nair, who presented the data at the International AIDS Society (IAS) Conference 2021. “The adherence support provided by this study empowered them to make choices and stick to these choices,” she said.
In total, 247 women and girls aged 16 to 21 who were without HIV were enrolled in the Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH) trial in two sites in South Africa and one each in Uganda and Zimbabwe beginning in February 2019. One-third of the participants were minors; the average age was 18.2 years.
The women were good candidates for PrEP. More than 1 in 3 of the women started the study with a sexually transmitted infection (STI), the most prevalent of which was chlamydia. This is often a good marker for condomless sex. Of the participants, 89% had a primary sex partner; a quarter of those thought their partner was having sex with other people. Only 7% of participants reported being very worried about acquiring HIV. More than 1 in 3 (39%) weren’t worried about HIV at all. This conforms to previous data suggesting that those who could most benefit from PrEP often don’t perceive their own vulnerability.
In the study, the women were randomly assigned two groups. In one group, the participants used the dapivirine ring for 6 months; in the other, participants used oral PrEP for 6 months. The participants then swapped prevention methods and used the alternative method for 6 more months. After a year of trying both methods, the women will be asked to choose one of the two prevention method or to stop PrEP altogether. At the IAS conference, the researchers reported interim data from the first year of the study, before the girls had the opportunity to choose for themselves.
During that first year, girls received intensive adherence support, including daily or weekly text check-ins, phone check-ins, peer buddy support, additional onsite counseling visits, access to adherence support groups, participation in online support groups via apps such as WhatsApp, and in-person social events designed to empower young women and to teach them skills. Support included discussion of adherence, contraceptives, and STIs. In addition, when girls came in for study visits, staff provided feedback on how adherent the girls had been, as determined on the basis of residual levels of dapivirine in the rings or, with regard to oral pills, drug levels as determined with blood spots.
Girls were considered to have had high adherence if they were found to have oral PrEP concentrations equivalent to four or more doses per week or if residual levels of dapivirine in their rings were 0.1071 mg/d. Moderate adherence was the equivalent of one to three doses of oral PrEP a week or dapivirine levels between 0.0321 mg/d and 0.1071 mg/d.
In total, 95.6% of ring users showed some adherence to the ring. Of those, adherence was high for 50.2%; 49.8% used the ring perfectly. For oral PrEP, 98.5% showed some level of PrEP use; for 58.6%, lab results suggested adherence high enough to provide protection from HIV, and 22% took their pills at least six times a week. Between the two arms, 54.3% of all participants used the medication sufficiently to be protected from HIV.
One person acquired HIV during the study. Dr. Nair did not say which study arm that participant was in or how adherent that person has been to their prevention method.
That level of adherence is on par with studies in the United States, which have found 56% adherence to PrEP among adolescent and young men who have sex with men. But the level of adherence is far higher than has been found in other studies that tested oral PrEP among women who did not have a partner with HIV. In particular, the VOICE and FEM-PrEP trials were both stopped early for lack of adherence. In those placebo-controlled oral-PrEP trials, fewer than 25% of participants used the oral prevention pills. Although adherence to the vaginal ring was estimated to be 61% for women older than 25 in the ASPIRE trial, it was effectively zero among women aged 18 to 21 years. Adherence has been the “bugaboo of efficacy for PrEP in young women,” said Judith Auerbach, PhD, independent science and policy consultant and professor of medicine at the University of California, San Francisco. But health care professionals have a long way to go to support young people in general in using PrEP.
“Yes, this shows improvement compared to previous studies,” Dr. Auerbach told this news organization. “But is it sufficient to have an epidemiological impact at the population level?”
Medical Advocacy and Outreach (MAO) is an HIV clinic and services program in Montgomery, Alabama, that offers a clinic specifically for some of their 144 clients to receive oral PrEP. In addition to in-person testing, MAO offers home HIV testing and lab work and televisits to support the college students they serve in taking PrEP whether they’re at school or at home on break. Currently, MAO provides a series of support groups and other social support programs for their clients living with HIV, but there are none for those receiving PrEP. The organization is in the process of hiring a social worker for the PrEP side of the clinic.
Until that person is on board, “I’m their support system in an unofficial capacity,” Shericka Williams, MPH, told this news organization. She runs education programs at MAO and handles all the phone calls from PrEP clients. “My title changes a lot, but the one I like to go with most often is the PrEP navigator,” she said.
She said she was intrigued by the dapivirine ring and oral PrEP data but said that currently, the women they serve are still learning that PrEP is for them, too. The women report that all the ads and all the information they receive is aimed at gay or bisexual men or transgender women. It takes a while for them to recognize that they could benefit, so a lot of the work that Ms. Williams does is focused on explaining the benefit of PrEP.
In MAO, the number of women receiving PrEP fluctuates more than for men. Mostly, women start PrEP because of they are in a relationship with someone who receives HIV care from MAO’s other wing – women who potentially would experience less vulnerability to HIV if their partners had undetectable viral loads. The other reason women take it is because they suspect that their partner is cheating or because they are in abusive relationships in which they want their partner to use a condom but the partner won’t. As in the PrEP trials, they often see women discontinue PrEP when they leave those relationships. In part, her job is to educate women regarding all the ways PrEP could serve them.
“Most of the time, they’re just no longer in that relationship, and they’re just taking some time for themselves,” she said in an interview. “We definitely try to bring up other reasons to stay on PrEP, but we don’t want to seem like we’re bullying someone to stay on it.”
Dr. Nair, Dr. Auerbach, and Ms. Williams report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CDC revamps STI treatment guidelines
On July 22, the Centers for Disease Control and Prevention released updated sexually transmitted infection treatment guidelines to reflect current screening, testing, and treatment recommendations. The guidelines were last updated in 2015.
The new recommendations come at a pivotal moment in the field’s history, Kimberly Workowski, MD, a medical officer at the CDC’s Division of STD Prevention, told this news organization in an email. “The COVID-19 pandemic has caused decreased clinic capacity and drug and diagnostic test kit shortages,” she says. Many of these shortages have been resolved, she added, and it is important that health care professionals use the most current evidence-based recommendations for screening and management of STIs.
Updates to these guidelines were necessary to reflect “continued advances in research in the prevention of STIs, new interventions in terms of STI prevention, and thirdly, changing epidemiology,” Jeffrey Klausner, MD, MPH, an STI specialist with the Keck School of Medicine at the University of Southern California, Los Angeles, said in an interview. “There’s been increased concern about antimicrobial resistance, and that’s really driven some of the key changes in these new STI treatment guidelines.”
Notable updates to the guidelines include the following:
- Updated treatment recommendations for gonorrhea, chlamydia, , and
- Two-step testing for diagnosing genital virus
- Expanded risk factors for testing in pregnant women
- Information on FDA-cleared rectal and oral tests to diagnose chlamydia and gonorrhea
- A recommendation that universal screening be conducted at least once in a lifetime for adults aged 18 years and older
Dr. Workowski emphasized updates to gonorrhea treatment that built on the recommendation published in December 2020 in Morbidity and Mortality Weekly Report. The CDC now recommends that gonorrhea be treated with a single 500-mg injection of ceftriaxone, and if chlamydial infection is not ruled out, treating with a regimen of 100 mg of oral doxycycline taken twice daily for 7 days. Other gonorrhea treatment recommendations include retesting patients 3 months after treatment and that a test of cure be conducted for people with pharyngeal gonorrhea 1 to 2 weeks after treatment, using either culture or nucleic-acid amplification tests.
“Effectively treating gonorrhea remains a public health priority,” Dr. Workowski said. “Gonorrhea can rapidly develop antibiotic resistance and is the second most commonly reported bacterial STI in the U.S., increasing 56% from 2015 to 2019.”
The updates to syphilis screening for pregnant women are also important, added Dr. Klausner. “We’ve seen a dramatic and shameful rise in congenital syphilis,” he said. In addition to screening all pregnant women at the first prenatal visit, the CDC recommends retesting for syphilis at 28 weeks’ gestation and at delivery if the mother lives in an area where the prevalence of syphilis is high or if she is at risk of acquiring syphilis during pregnancy. An expectant mother is at higher risk if she has multiple sex partners, has an STI during pregnancy, has a partner with an STI, has a new sex partner, or misuses drugs, the recommendations state.
Dr. Klausner also noted that the updates provide more robust guidelines for treating transgender individuals and incarcerated people.
The treatment guidelines are available online along with a wall chart and a pocket guide that summarizes these updates. The mobile app with the 2015 guidelines will be retired at the end of July 2021, Dr. Workowski said. An app with these updated treatment recommendations is in development and will be available later this year.
A version of this article first appeared on Medscape.com.
On July 22, the Centers for Disease Control and Prevention released updated sexually transmitted infection treatment guidelines to reflect current screening, testing, and treatment recommendations. The guidelines were last updated in 2015.
The new recommendations come at a pivotal moment in the field’s history, Kimberly Workowski, MD, a medical officer at the CDC’s Division of STD Prevention, told this news organization in an email. “The COVID-19 pandemic has caused decreased clinic capacity and drug and diagnostic test kit shortages,” she says. Many of these shortages have been resolved, she added, and it is important that health care professionals use the most current evidence-based recommendations for screening and management of STIs.
Updates to these guidelines were necessary to reflect “continued advances in research in the prevention of STIs, new interventions in terms of STI prevention, and thirdly, changing epidemiology,” Jeffrey Klausner, MD, MPH, an STI specialist with the Keck School of Medicine at the University of Southern California, Los Angeles, said in an interview. “There’s been increased concern about antimicrobial resistance, and that’s really driven some of the key changes in these new STI treatment guidelines.”
Notable updates to the guidelines include the following:
- Updated treatment recommendations for gonorrhea, chlamydia, , and
- Two-step testing for diagnosing genital virus
- Expanded risk factors for testing in pregnant women
- Information on FDA-cleared rectal and oral tests to diagnose chlamydia and gonorrhea
- A recommendation that universal screening be conducted at least once in a lifetime for adults aged 18 years and older
Dr. Workowski emphasized updates to gonorrhea treatment that built on the recommendation published in December 2020 in Morbidity and Mortality Weekly Report. The CDC now recommends that gonorrhea be treated with a single 500-mg injection of ceftriaxone, and if chlamydial infection is not ruled out, treating with a regimen of 100 mg of oral doxycycline taken twice daily for 7 days. Other gonorrhea treatment recommendations include retesting patients 3 months after treatment and that a test of cure be conducted for people with pharyngeal gonorrhea 1 to 2 weeks after treatment, using either culture or nucleic-acid amplification tests.
“Effectively treating gonorrhea remains a public health priority,” Dr. Workowski said. “Gonorrhea can rapidly develop antibiotic resistance and is the second most commonly reported bacterial STI in the U.S., increasing 56% from 2015 to 2019.”
The updates to syphilis screening for pregnant women are also important, added Dr. Klausner. “We’ve seen a dramatic and shameful rise in congenital syphilis,” he said. In addition to screening all pregnant women at the first prenatal visit, the CDC recommends retesting for syphilis at 28 weeks’ gestation and at delivery if the mother lives in an area where the prevalence of syphilis is high or if she is at risk of acquiring syphilis during pregnancy. An expectant mother is at higher risk if she has multiple sex partners, has an STI during pregnancy, has a partner with an STI, has a new sex partner, or misuses drugs, the recommendations state.
Dr. Klausner also noted that the updates provide more robust guidelines for treating transgender individuals and incarcerated people.
The treatment guidelines are available online along with a wall chart and a pocket guide that summarizes these updates. The mobile app with the 2015 guidelines will be retired at the end of July 2021, Dr. Workowski said. An app with these updated treatment recommendations is in development and will be available later this year.
A version of this article first appeared on Medscape.com.
On July 22, the Centers for Disease Control and Prevention released updated sexually transmitted infection treatment guidelines to reflect current screening, testing, and treatment recommendations. The guidelines were last updated in 2015.
The new recommendations come at a pivotal moment in the field’s history, Kimberly Workowski, MD, a medical officer at the CDC’s Division of STD Prevention, told this news organization in an email. “The COVID-19 pandemic has caused decreased clinic capacity and drug and diagnostic test kit shortages,” she says. Many of these shortages have been resolved, she added, and it is important that health care professionals use the most current evidence-based recommendations for screening and management of STIs.
Updates to these guidelines were necessary to reflect “continued advances in research in the prevention of STIs, new interventions in terms of STI prevention, and thirdly, changing epidemiology,” Jeffrey Klausner, MD, MPH, an STI specialist with the Keck School of Medicine at the University of Southern California, Los Angeles, said in an interview. “There’s been increased concern about antimicrobial resistance, and that’s really driven some of the key changes in these new STI treatment guidelines.”
Notable updates to the guidelines include the following:
- Updated treatment recommendations for gonorrhea, chlamydia, , and
- Two-step testing for diagnosing genital virus
- Expanded risk factors for testing in pregnant women
- Information on FDA-cleared rectal and oral tests to diagnose chlamydia and gonorrhea
- A recommendation that universal screening be conducted at least once in a lifetime for adults aged 18 years and older
Dr. Workowski emphasized updates to gonorrhea treatment that built on the recommendation published in December 2020 in Morbidity and Mortality Weekly Report. The CDC now recommends that gonorrhea be treated with a single 500-mg injection of ceftriaxone, and if chlamydial infection is not ruled out, treating with a regimen of 100 mg of oral doxycycline taken twice daily for 7 days. Other gonorrhea treatment recommendations include retesting patients 3 months after treatment and that a test of cure be conducted for people with pharyngeal gonorrhea 1 to 2 weeks after treatment, using either culture or nucleic-acid amplification tests.
“Effectively treating gonorrhea remains a public health priority,” Dr. Workowski said. “Gonorrhea can rapidly develop antibiotic resistance and is the second most commonly reported bacterial STI in the U.S., increasing 56% from 2015 to 2019.”
The updates to syphilis screening for pregnant women are also important, added Dr. Klausner. “We’ve seen a dramatic and shameful rise in congenital syphilis,” he said. In addition to screening all pregnant women at the first prenatal visit, the CDC recommends retesting for syphilis at 28 weeks’ gestation and at delivery if the mother lives in an area where the prevalence of syphilis is high or if she is at risk of acquiring syphilis during pregnancy. An expectant mother is at higher risk if she has multiple sex partners, has an STI during pregnancy, has a partner with an STI, has a new sex partner, or misuses drugs, the recommendations state.
Dr. Klausner also noted that the updates provide more robust guidelines for treating transgender individuals and incarcerated people.
The treatment guidelines are available online along with a wall chart and a pocket guide that summarizes these updates. The mobile app with the 2015 guidelines will be retired at the end of July 2021, Dr. Workowski said. An app with these updated treatment recommendations is in development and will be available later this year.
A version of this article first appeared on Medscape.com.
Left-Sided Amyand Hernia: Case Report and Review of the Literature
Left-sided Amyand hernia is a rare condition that requires a high degree of clinical suspicion to correctly diagnose.
The presence of the vermiform appendix within an inguinal hernia sac is termed an Amyand hernia. While the incidence of Amyand hernia in the general population is thought to be exceedingly rare, the presence of a left-sided Amyand hernia is even more rare due to the normal anatomical position of the appendix on the right side. Left-sided Amyand hernia presents a novel diagnosis that necessitates a high degree of clinical suspicion and special consideration during patient workup and operative treatment. We describe such a case and provide a review of all reports in the literature of this rare finding.
Case Presentation
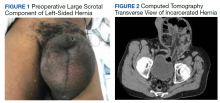
A male aged 62 years presented to the emergency department of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, in acute distress after experiencing 5 days of nausea and pain in his lower abdomen. The patient’s history was significant for cocaine abuse and a left-sided inguinal hernia that was repaired about 15 years prior to this visit. He reported having no bowel movements for the past 5 days and no other symptoms, including vomiting, hematemesis, and trauma to the abdomen. The patient’s abdominal pain was located in the suprapubic and periumbilical regions. Upon palpation of the lower abdomen, a firm, protruding mass was identified in the left lower quadrant and suspected to be a left-sided inguinal hernia.
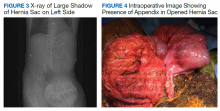
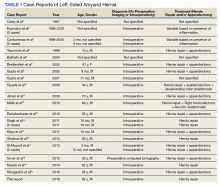
A scout film and computed tomography (CT) scan of the abdomen taken on the same day that the patient presented to the emergency department confirmed the presence of a large left-sided inguinal hernia with possible bowel strangulation involving the colon (Figures 1, 2, and 3). The patient was diagnosed with an incarcerated recurrent left inguinal hernia and was taken emergently to the operating suite. General anesthesia and an ilioinguinal nerve block were performed. An inguinal incision was made on the left side, and the large hernia sac was identified and separated from the scrotum and spermatic cord structures.
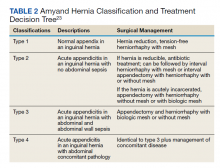
On visual inspection, the hernia was identified as both a direct and an indirect inguinal hernia, making it a pantaloon hernia. The hernia sac was opened, and contents of the herniated sac were found to include the omentum, a loop of transverse colon, as well as the entire cecum and appendix, confirming the diagnosis of an Amyand hernia (Figure 4). Though the bowel was initially dusky, all the bowel became pink and appeared to be viable after detorsion of the bowel. Diagnostic laparoscopy through a 5-mm port was performed to assess the remainder of the bowel located intra-abdominally. The remaining intra-abdominal bowel appeared healthy and without obvious signs of ischemia, twisting, or malrotation. The large hernia defect was repaired with a polypropylene mesh.
Discussion
An Amyand hernia is an inguinal hernia in which the vermiform appendix is located within the hernial sac. Named after the French surgeon Claudius Amyand who first documented such a case during an appendectomy in 1735, the Amyand hernia is rare and is thought to occur in < 1% of inguinal hernias.1 Given the normal anatomical position of the appendix on the right side of the body, most Amyand hernias occur in a right-sided inguinal hernia.
A literature review yielded 25 reported instances of a left-sided Amyand hernia (Table 1) including this report. The true age of incidence of Amyand hernia for each patient is difficult to determine, as many patients do not present until pain or discomfort reaches high levels, often many years after hernia formation. Additionally, some cases of left-sided Amyand hernia described herein, including our case, are recurrent cases of a previous hernia that have been surgically repaired.2-20
Presentation of Amyand hernia often resembles that of a complicated inguinal hernia, acute appendicitis, or both. Hence, clinicians should consider this a possibility when patients present with signs and symptoms that could otherwise be thought to be originating from an incarcerated, strangulated, or recurrent hernia. Specifically, these signs and symptoms include a tender, nonreducible mass in the inguinal region, acute lower abdominal pain, nausea, or signs of intestinal obstruction such as failure to produce bowel movements.4,17 Because of the unusual anatomy in patients presenting with left-sided Amyand hernia, tenderness at the McBurney point usually is absent and not a useful diagnostic tool to rule out acute appendicitis.
A literature review indicates that an Amyand hernia on either side tends to occur in males more often than it does in females. The rate of diagnosis of Amyand hernia also has been reported to be 3 times higher in children than it is in adults due to failure of the processus vaginalis to obliterate during development.21 Our literature review supports this finding, as 16 of the documented 25 cases of left-sided Amyand hernia were reported in males. Additionally, information regarding gender was not found in 6 cases, suggesting a potential for an even higher prevalence in males.
Explanations as to why the appendix is on the left side in these patients include developmental anomalies, such as situs inversus, intestinal rotation, mobile cecum, or an abnormally long appendix.3,8 In our case, the likely causative culprit was a mobile cecum, as there was neither indication of intestinal malformation, rotation, nor of an abnormally long appendix during surgery. Additionally, pre-operative radiologic studies, clinical evaluation, and electrocardiogram did not suggest the presence of situs inversus.
Treatment of Amyand hernia usually follows the landmark classification algorithm set forth in 2007 by Losanoff and Basson (Table 2).22 This system stratifies treatment based on intraoperative findings of the appendix and surrounding structures, ranging from type 1, which involves a normal appendix within the hernia, to type 4, which includes acute appendicitis with additional abdominal pathology. Our patient presented with a type 1 Amyand hernia and appendectomy was foregone as per the guidelines; however, there have been numerous reported cases of surgeons opting for prophylactic appendectomy in the case of a normal appearing appendix and surrounding structures. The decision to act independent of the Losanoff and Basson classification underscores the lack of true standardization, namely, when it comes to a treatment approach for type 1 Amyand hernias. Nonetheless, many contend that indiscriminately performing appendectomies in all cases of left-sided Amyand hernia is useful as a prophylactic measure, as cases of future appendicitis in these patients will have atypical presentations based on the contralateral location of the appendix.6,11,17
Others disagree, citing that prophylactic appendectomy in the case of a normal looking appendix is unnecessary and complicates an otherwise sterile surgery (clean wound classification) with the removal of an appendix containing fecal matter and gut microbiota (converted into a clean contaminated or a contaminated wound classification).17 Additionally, it is thought that in the cases of middle-aged or geriatric patients where the chances of appendicitis are far less, the risks of detriment from prophylactic appendectomy may outweigh the benefits. In these cases, a macroscopic view of the appendix based on visual examination during the operation should guide decision making.4
While the decision to remove a healthy-appearing appendix remains contentious, the decision for or against placement of a heterogenous hernia mesh has proven to be binary, with near universally accepted criteria. If signs of perforation or infection are present in the hernia sac, then surgeons will forego hernioplasty with mesh for simple herniorrhaphy. This contraindication for mesh placement is due to the increased risk of mesh infection, wound infection, and fistulae associated with the introduction of a foreign structure to an active infection site.2
While most cases of Amyand hernia are diagnosed intraoperatively, there have been documented cases of preoperative diagnosis using ultrasonography and CT imaging modalities.19,23,24 In all cases, the presence of the vermiform appendix within the hernia sac can complicate diagnosis and treatment, and preoperative knowledge of this condition may help to guide physician decision making. Identifying Amyand hernia via CT scan is not only useful for alerting physicians of a potentially inflamed appendix within the hernia sac, but also may create opportunities for the use of other treatment modalities. For example, laparoscopic Amyand hernia reduction, an approach that was performed successfully and documented for the first time by Vermillion and colleagues, was made possible by preoperative diagnosis and can potentially result in improved patient outcomes.25
Regardless, while standardization of treatment for Amyand hernia has not yet occurred, it is clear that improved preoperative diagnosis, especially in the case of an unanticipated left-sided Amyand hernia, can allow for better planning and use of a wider variety of treatment modalities. The main impediment to this approach is that suspected cases of appendicitis and inguinal hernias (the most common preoperative diagnoses of Amyand hernia) usually are diagnosed clinically without the need of additional imaging studies like CT or ultrasound. In accordance with the guiding principle of radiation safety of exposing patients to “as low as reasonably achievable” (ALARA) radiation and with consideration of expediency of care and cost efficiency, we recommend physicians continue to screen for and treat cases of potentially emergent appendicitis and/or inguinal hernia as per the conventional methodology. The best approach may involve increasing preoperative diagnoses of left-sided Amyand hernias via physician awareness of this rare finding, as well as evaluating imaging studies that have previously been obtained in order to narrow a broad differential diagnosis.
Conclusions
Left-sided Amyand hernia is an exceptionally rare condition whose preoperative diagnosis remains difficult to establish but whose treatment decision tree is significantly impacted by the condition.
1. Franko J, Raftopoulos I, Sulkowski R. A rare variation of Amyand’s hernia. Am J Gastroenterol. 2002;97(10):2684-2685. doi:10.1111/j.1572-0241.2002.06060.x
2. Carey LC. Acute appendicitis occurring in hernias: a report of 10 cases. Surgery. 1967;61(2):236-238.
3. Kaymakci A, Akillioglu I, Akkoyun I, Guven S, Ozdemir A, Gulen S. Amyand’s hernia: a series of 30 cases in children. Hernia. 2009;13(6):609-612. doi:10.1007/s10029-009-0528-8
4. Cankorkmaz L, Ozer H, Guney C, Atalar MH, Arslan MS, Koyluoglu G. Amyand’s hernia in the children: a single center experience. Surgery. 2010;147(1):140-143. doi:10.1016/j.surg.2009.09.038
5. Yasumoto R, Kawano M, Kawanishi H, et al. Left acute scrotum associated with appendicitis. Int J Urol. 1998;5(1):108-110. doi:10.1111/j.1442-2042.1998.tb00254.x
6. Bakhshi GD, Bhandarwar AH, Govila AA. Acute appendicitis in left scrotum. Indian J Gastroenterol. 2004;23(5):195.
7. Breitenstein S, Eisenbach C, Wille G, Decurtins M. Incarcerated vermiform appendix in a left-sided inguinal hernia. Hernia. 2005;9(1):100-102. doi:10.1007/s10029-004-0263-0
8. Gupta S, Sharma R, Kaushik R. Left-sided Amyand’s hernia. Singapore Med J. 2005;46(8):424-425.
9. Gupta N, Wilkinson TV, Wilkinson A, Akhtar M. Left-sided incarcerated Amyand’s hernia. Indian J Surg. 2007;69(1):17-18.
10. Tayade, MB, Bakhshi GD, Borisa AD, Deshpande G, Joshi N. A rare combination of left sided Amyand’s and Richter’s hernia. Bombay Hosp J. 2008;50(4): 644-645
11. Johari HG, Paydar S, Davani SZ, Eskandari S, Johari MG. Left-sided Amyand hernia. Ann Saudi Med. 2009;29(4):321-322. doi:10.4103/0256-4947.55305
12. Ali SM, Malik KA, Al-Qadhi H. Amyand’s Hernia: Study of four cases and literature review. Sultan Qaboos Univ Med J. 2012;12(2):232-236. doi:10.12816/0003119
13. Ravishankaran P, Mohan G, Srinivasan A, Ravindran G, Ramalingam A. Left sided amyand’s hernia, a rare occurrence: A Case Report. Indian J Surg. 2013;75(3):247-248. doi:10.1007/s12262-010-0223-0
14. Singh K, Singh RR, Kaur S. Amyand’s hernia. J Indian Assoc Pediatr Surg. 2011;16(4):171-172. doi:10.4103/0971-9261.86890
15. Khan TS, Wani ML, Bijli AH, et al. Amyand’s hernia: a rare occurrence. Ann Nigerian Med. 2011;5(2):62-64.doi:10.4103/0331-3131.92955
16. Ghafouri A, Anbara T, Foroutankia R. A rare case report of appendix and cecum in the sac of left inguinal hernia (left Amyand’s hernia). Med J Islam Repub Iran. 2012;26(2):94-95.
17. Al-Mayoof AF, Al-Ani BH. Left-sided amyand hernia: report of two cases with review of literature. European J Pediatr Surg Rep. 2014;2(1):63-66. doi:10.1055/s-0033-1347131
18. Unver M, Ozturk S, Karaman K, Turgut E. Left sided Amyand’s hernia. World J Gastrointest Surg. 2013;5(10):285-286. doi:10.4240/wjgs.v5.i10.285
19. Maeda K, Kunieda K, Kawai M, et al. Giant left-sided inguinoscrotal hernia containing the cecum and appendix (giant left-sided Amyand’s hernia). Clin Case Rep. 2014;2(6):254-257. doi:10.1002/ccr3.104
20. Mongardini M, Maturo A, De Anna L, et al. Appendiceal abscess in a giant left-sided inguinoscrotal hernia: a rare case of Amyand hernia. Springerplus. 2015;4:378. Published 2015 Jul 26. doi:10.1186/s40064-015-1162-9
21. Ivanschuk G, Cesmebasi A, Sorenson EP, Blaak C, Loukas M, Tubbs SR. Amyand’s hernia: a review. Med Sci Monit. 2014;20:140-146. Published 2014 Jan 28. doi:10.12659/MSM.889873
22. Losanoff JE, Basson MD. Amyand hernia: what lies beneath--a proposed classification scheme to determine management. Am Surg. 2007;73(12):1288-1290.
23. Coulier B, Pacary J, Broze B. Sonographic diagnosis of appendicitis within a right inguinal hernia (Amyand’s hernia). J Clin Ultrasound. 2006;34(9):454-457. doi:10.1002/jcu.20266
24. Vehbi H, Agirgun C, Agirgun F, Dogan Y. Preoperative diagnosis of Amyand’s hernia by ultrasound and computed tomography. Turk J Emerg Med. 2016;16(2):72-74. Published 2016 May 8. doi:10.1016/j.tjem.2015.11.014
25. Vermillion JM, Abernathy SW, Snyder SK. Laparoscopic reduction of Amyand’s hernia. Hernia. 1999;3:159-160. doi:10.1007/BF01195318
Left-sided Amyand hernia is a rare condition that requires a high degree of clinical suspicion to correctly diagnose.
Left-sided Amyand hernia is a rare condition that requires a high degree of clinical suspicion to correctly diagnose.
The presence of the vermiform appendix within an inguinal hernia sac is termed an Amyand hernia. While the incidence of Amyand hernia in the general population is thought to be exceedingly rare, the presence of a left-sided Amyand hernia is even more rare due to the normal anatomical position of the appendix on the right side. Left-sided Amyand hernia presents a novel diagnosis that necessitates a high degree of clinical suspicion and special consideration during patient workup and operative treatment. We describe such a case and provide a review of all reports in the literature of this rare finding.
Case Presentation
A male aged 62 years presented to the emergency department of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, in acute distress after experiencing 5 days of nausea and pain in his lower abdomen. The patient’s history was significant for cocaine abuse and a left-sided inguinal hernia that was repaired about 15 years prior to this visit. He reported having no bowel movements for the past 5 days and no other symptoms, including vomiting, hematemesis, and trauma to the abdomen. The patient’s abdominal pain was located in the suprapubic and periumbilical regions. Upon palpation of the lower abdomen, a firm, protruding mass was identified in the left lower quadrant and suspected to be a left-sided inguinal hernia.
A scout film and computed tomography (CT) scan of the abdomen taken on the same day that the patient presented to the emergency department confirmed the presence of a large left-sided inguinal hernia with possible bowel strangulation involving the colon (Figures 1, 2, and 3). The patient was diagnosed with an incarcerated recurrent left inguinal hernia and was taken emergently to the operating suite. General anesthesia and an ilioinguinal nerve block were performed. An inguinal incision was made on the left side, and the large hernia sac was identified and separated from the scrotum and spermatic cord structures.
On visual inspection, the hernia was identified as both a direct and an indirect inguinal hernia, making it a pantaloon hernia. The hernia sac was opened, and contents of the herniated sac were found to include the omentum, a loop of transverse colon, as well as the entire cecum and appendix, confirming the diagnosis of an Amyand hernia (Figure 4). Though the bowel was initially dusky, all the bowel became pink and appeared to be viable after detorsion of the bowel. Diagnostic laparoscopy through a 5-mm port was performed to assess the remainder of the bowel located intra-abdominally. The remaining intra-abdominal bowel appeared healthy and without obvious signs of ischemia, twisting, or malrotation. The large hernia defect was repaired with a polypropylene mesh.
Discussion
An Amyand hernia is an inguinal hernia in which the vermiform appendix is located within the hernial sac. Named after the French surgeon Claudius Amyand who first documented such a case during an appendectomy in 1735, the Amyand hernia is rare and is thought to occur in < 1% of inguinal hernias.1 Given the normal anatomical position of the appendix on the right side of the body, most Amyand hernias occur in a right-sided inguinal hernia.
A literature review yielded 25 reported instances of a left-sided Amyand hernia (Table 1) including this report. The true age of incidence of Amyand hernia for each patient is difficult to determine, as many patients do not present until pain or discomfort reaches high levels, often many years after hernia formation. Additionally, some cases of left-sided Amyand hernia described herein, including our case, are recurrent cases of a previous hernia that have been surgically repaired.2-20
Presentation of Amyand hernia often resembles that of a complicated inguinal hernia, acute appendicitis, or both. Hence, clinicians should consider this a possibility when patients present with signs and symptoms that could otherwise be thought to be originating from an incarcerated, strangulated, or recurrent hernia. Specifically, these signs and symptoms include a tender, nonreducible mass in the inguinal region, acute lower abdominal pain, nausea, or signs of intestinal obstruction such as failure to produce bowel movements.4,17 Because of the unusual anatomy in patients presenting with left-sided Amyand hernia, tenderness at the McBurney point usually is absent and not a useful diagnostic tool to rule out acute appendicitis.
A literature review indicates that an Amyand hernia on either side tends to occur in males more often than it does in females. The rate of diagnosis of Amyand hernia also has been reported to be 3 times higher in children than it is in adults due to failure of the processus vaginalis to obliterate during development.21 Our literature review supports this finding, as 16 of the documented 25 cases of left-sided Amyand hernia were reported in males. Additionally, information regarding gender was not found in 6 cases, suggesting a potential for an even higher prevalence in males.
Explanations as to why the appendix is on the left side in these patients include developmental anomalies, such as situs inversus, intestinal rotation, mobile cecum, or an abnormally long appendix.3,8 In our case, the likely causative culprit was a mobile cecum, as there was neither indication of intestinal malformation, rotation, nor of an abnormally long appendix during surgery. Additionally, pre-operative radiologic studies, clinical evaluation, and electrocardiogram did not suggest the presence of situs inversus.
Treatment of Amyand hernia usually follows the landmark classification algorithm set forth in 2007 by Losanoff and Basson (Table 2).22 This system stratifies treatment based on intraoperative findings of the appendix and surrounding structures, ranging from type 1, which involves a normal appendix within the hernia, to type 4, which includes acute appendicitis with additional abdominal pathology. Our patient presented with a type 1 Amyand hernia and appendectomy was foregone as per the guidelines; however, there have been numerous reported cases of surgeons opting for prophylactic appendectomy in the case of a normal appearing appendix and surrounding structures. The decision to act independent of the Losanoff and Basson classification underscores the lack of true standardization, namely, when it comes to a treatment approach for type 1 Amyand hernias. Nonetheless, many contend that indiscriminately performing appendectomies in all cases of left-sided Amyand hernia is useful as a prophylactic measure, as cases of future appendicitis in these patients will have atypical presentations based on the contralateral location of the appendix.6,11,17
Others disagree, citing that prophylactic appendectomy in the case of a normal looking appendix is unnecessary and complicates an otherwise sterile surgery (clean wound classification) with the removal of an appendix containing fecal matter and gut microbiota (converted into a clean contaminated or a contaminated wound classification).17 Additionally, it is thought that in the cases of middle-aged or geriatric patients where the chances of appendicitis are far less, the risks of detriment from prophylactic appendectomy may outweigh the benefits. In these cases, a macroscopic view of the appendix based on visual examination during the operation should guide decision making.4
While the decision to remove a healthy-appearing appendix remains contentious, the decision for or against placement of a heterogenous hernia mesh has proven to be binary, with near universally accepted criteria. If signs of perforation or infection are present in the hernia sac, then surgeons will forego hernioplasty with mesh for simple herniorrhaphy. This contraindication for mesh placement is due to the increased risk of mesh infection, wound infection, and fistulae associated with the introduction of a foreign structure to an active infection site.2
While most cases of Amyand hernia are diagnosed intraoperatively, there have been documented cases of preoperative diagnosis using ultrasonography and CT imaging modalities.19,23,24 In all cases, the presence of the vermiform appendix within the hernia sac can complicate diagnosis and treatment, and preoperative knowledge of this condition may help to guide physician decision making. Identifying Amyand hernia via CT scan is not only useful for alerting physicians of a potentially inflamed appendix within the hernia sac, but also may create opportunities for the use of other treatment modalities. For example, laparoscopic Amyand hernia reduction, an approach that was performed successfully and documented for the first time by Vermillion and colleagues, was made possible by preoperative diagnosis and can potentially result in improved patient outcomes.25
Regardless, while standardization of treatment for Amyand hernia has not yet occurred, it is clear that improved preoperative diagnosis, especially in the case of an unanticipated left-sided Amyand hernia, can allow for better planning and use of a wider variety of treatment modalities. The main impediment to this approach is that suspected cases of appendicitis and inguinal hernias (the most common preoperative diagnoses of Amyand hernia) usually are diagnosed clinically without the need of additional imaging studies like CT or ultrasound. In accordance with the guiding principle of radiation safety of exposing patients to “as low as reasonably achievable” (ALARA) radiation and with consideration of expediency of care and cost efficiency, we recommend physicians continue to screen for and treat cases of potentially emergent appendicitis and/or inguinal hernia as per the conventional methodology. The best approach may involve increasing preoperative diagnoses of left-sided Amyand hernias via physician awareness of this rare finding, as well as evaluating imaging studies that have previously been obtained in order to narrow a broad differential diagnosis.
Conclusions
Left-sided Amyand hernia is an exceptionally rare condition whose preoperative diagnosis remains difficult to establish but whose treatment decision tree is significantly impacted by the condition.
The presence of the vermiform appendix within an inguinal hernia sac is termed an Amyand hernia. While the incidence of Amyand hernia in the general population is thought to be exceedingly rare, the presence of a left-sided Amyand hernia is even more rare due to the normal anatomical position of the appendix on the right side. Left-sided Amyand hernia presents a novel diagnosis that necessitates a high degree of clinical suspicion and special consideration during patient workup and operative treatment. We describe such a case and provide a review of all reports in the literature of this rare finding.
Case Presentation
A male aged 62 years presented to the emergency department of the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas, in acute distress after experiencing 5 days of nausea and pain in his lower abdomen. The patient’s history was significant for cocaine abuse and a left-sided inguinal hernia that was repaired about 15 years prior to this visit. He reported having no bowel movements for the past 5 days and no other symptoms, including vomiting, hematemesis, and trauma to the abdomen. The patient’s abdominal pain was located in the suprapubic and periumbilical regions. Upon palpation of the lower abdomen, a firm, protruding mass was identified in the left lower quadrant and suspected to be a left-sided inguinal hernia.
A scout film and computed tomography (CT) scan of the abdomen taken on the same day that the patient presented to the emergency department confirmed the presence of a large left-sided inguinal hernia with possible bowel strangulation involving the colon (Figures 1, 2, and 3). The patient was diagnosed with an incarcerated recurrent left inguinal hernia and was taken emergently to the operating suite. General anesthesia and an ilioinguinal nerve block were performed. An inguinal incision was made on the left side, and the large hernia sac was identified and separated from the scrotum and spermatic cord structures.
On visual inspection, the hernia was identified as both a direct and an indirect inguinal hernia, making it a pantaloon hernia. The hernia sac was opened, and contents of the herniated sac were found to include the omentum, a loop of transverse colon, as well as the entire cecum and appendix, confirming the diagnosis of an Amyand hernia (Figure 4). Though the bowel was initially dusky, all the bowel became pink and appeared to be viable after detorsion of the bowel. Diagnostic laparoscopy through a 5-mm port was performed to assess the remainder of the bowel located intra-abdominally. The remaining intra-abdominal bowel appeared healthy and without obvious signs of ischemia, twisting, or malrotation. The large hernia defect was repaired with a polypropylene mesh.
Discussion
An Amyand hernia is an inguinal hernia in which the vermiform appendix is located within the hernial sac. Named after the French surgeon Claudius Amyand who first documented such a case during an appendectomy in 1735, the Amyand hernia is rare and is thought to occur in < 1% of inguinal hernias.1 Given the normal anatomical position of the appendix on the right side of the body, most Amyand hernias occur in a right-sided inguinal hernia.
A literature review yielded 25 reported instances of a left-sided Amyand hernia (Table 1) including this report. The true age of incidence of Amyand hernia for each patient is difficult to determine, as many patients do not present until pain or discomfort reaches high levels, often many years after hernia formation. Additionally, some cases of left-sided Amyand hernia described herein, including our case, are recurrent cases of a previous hernia that have been surgically repaired.2-20
Presentation of Amyand hernia often resembles that of a complicated inguinal hernia, acute appendicitis, or both. Hence, clinicians should consider this a possibility when patients present with signs and symptoms that could otherwise be thought to be originating from an incarcerated, strangulated, or recurrent hernia. Specifically, these signs and symptoms include a tender, nonreducible mass in the inguinal region, acute lower abdominal pain, nausea, or signs of intestinal obstruction such as failure to produce bowel movements.4,17 Because of the unusual anatomy in patients presenting with left-sided Amyand hernia, tenderness at the McBurney point usually is absent and not a useful diagnostic tool to rule out acute appendicitis.
A literature review indicates that an Amyand hernia on either side tends to occur in males more often than it does in females. The rate of diagnosis of Amyand hernia also has been reported to be 3 times higher in children than it is in adults due to failure of the processus vaginalis to obliterate during development.21 Our literature review supports this finding, as 16 of the documented 25 cases of left-sided Amyand hernia were reported in males. Additionally, information regarding gender was not found in 6 cases, suggesting a potential for an even higher prevalence in males.
Explanations as to why the appendix is on the left side in these patients include developmental anomalies, such as situs inversus, intestinal rotation, mobile cecum, or an abnormally long appendix.3,8 In our case, the likely causative culprit was a mobile cecum, as there was neither indication of intestinal malformation, rotation, nor of an abnormally long appendix during surgery. Additionally, pre-operative radiologic studies, clinical evaluation, and electrocardiogram did not suggest the presence of situs inversus.
Treatment of Amyand hernia usually follows the landmark classification algorithm set forth in 2007 by Losanoff and Basson (Table 2).22 This system stratifies treatment based on intraoperative findings of the appendix and surrounding structures, ranging from type 1, which involves a normal appendix within the hernia, to type 4, which includes acute appendicitis with additional abdominal pathology. Our patient presented with a type 1 Amyand hernia and appendectomy was foregone as per the guidelines; however, there have been numerous reported cases of surgeons opting for prophylactic appendectomy in the case of a normal appearing appendix and surrounding structures. The decision to act independent of the Losanoff and Basson classification underscores the lack of true standardization, namely, when it comes to a treatment approach for type 1 Amyand hernias. Nonetheless, many contend that indiscriminately performing appendectomies in all cases of left-sided Amyand hernia is useful as a prophylactic measure, as cases of future appendicitis in these patients will have atypical presentations based on the contralateral location of the appendix.6,11,17
Others disagree, citing that prophylactic appendectomy in the case of a normal looking appendix is unnecessary and complicates an otherwise sterile surgery (clean wound classification) with the removal of an appendix containing fecal matter and gut microbiota (converted into a clean contaminated or a contaminated wound classification).17 Additionally, it is thought that in the cases of middle-aged or geriatric patients where the chances of appendicitis are far less, the risks of detriment from prophylactic appendectomy may outweigh the benefits. In these cases, a macroscopic view of the appendix based on visual examination during the operation should guide decision making.4
While the decision to remove a healthy-appearing appendix remains contentious, the decision for or against placement of a heterogenous hernia mesh has proven to be binary, with near universally accepted criteria. If signs of perforation or infection are present in the hernia sac, then surgeons will forego hernioplasty with mesh for simple herniorrhaphy. This contraindication for mesh placement is due to the increased risk of mesh infection, wound infection, and fistulae associated with the introduction of a foreign structure to an active infection site.2
While most cases of Amyand hernia are diagnosed intraoperatively, there have been documented cases of preoperative diagnosis using ultrasonography and CT imaging modalities.19,23,24 In all cases, the presence of the vermiform appendix within the hernia sac can complicate diagnosis and treatment, and preoperative knowledge of this condition may help to guide physician decision making. Identifying Amyand hernia via CT scan is not only useful for alerting physicians of a potentially inflamed appendix within the hernia sac, but also may create opportunities for the use of other treatment modalities. For example, laparoscopic Amyand hernia reduction, an approach that was performed successfully and documented for the first time by Vermillion and colleagues, was made possible by preoperative diagnosis and can potentially result in improved patient outcomes.25
Regardless, while standardization of treatment for Amyand hernia has not yet occurred, it is clear that improved preoperative diagnosis, especially in the case of an unanticipated left-sided Amyand hernia, can allow for better planning and use of a wider variety of treatment modalities. The main impediment to this approach is that suspected cases of appendicitis and inguinal hernias (the most common preoperative diagnoses of Amyand hernia) usually are diagnosed clinically without the need of additional imaging studies like CT or ultrasound. In accordance with the guiding principle of radiation safety of exposing patients to “as low as reasonably achievable” (ALARA) radiation and with consideration of expediency of care and cost efficiency, we recommend physicians continue to screen for and treat cases of potentially emergent appendicitis and/or inguinal hernia as per the conventional methodology. The best approach may involve increasing preoperative diagnoses of left-sided Amyand hernias via physician awareness of this rare finding, as well as evaluating imaging studies that have previously been obtained in order to narrow a broad differential diagnosis.
Conclusions
Left-sided Amyand hernia is an exceptionally rare condition whose preoperative diagnosis remains difficult to establish but whose treatment decision tree is significantly impacted by the condition.
1. Franko J, Raftopoulos I, Sulkowski R. A rare variation of Amyand’s hernia. Am J Gastroenterol. 2002;97(10):2684-2685. doi:10.1111/j.1572-0241.2002.06060.x
2. Carey LC. Acute appendicitis occurring in hernias: a report of 10 cases. Surgery. 1967;61(2):236-238.
3. Kaymakci A, Akillioglu I, Akkoyun I, Guven S, Ozdemir A, Gulen S. Amyand’s hernia: a series of 30 cases in children. Hernia. 2009;13(6):609-612. doi:10.1007/s10029-009-0528-8
4. Cankorkmaz L, Ozer H, Guney C, Atalar MH, Arslan MS, Koyluoglu G. Amyand’s hernia in the children: a single center experience. Surgery. 2010;147(1):140-143. doi:10.1016/j.surg.2009.09.038
5. Yasumoto R, Kawano M, Kawanishi H, et al. Left acute scrotum associated with appendicitis. Int J Urol. 1998;5(1):108-110. doi:10.1111/j.1442-2042.1998.tb00254.x
6. Bakhshi GD, Bhandarwar AH, Govila AA. Acute appendicitis in left scrotum. Indian J Gastroenterol. 2004;23(5):195.
7. Breitenstein S, Eisenbach C, Wille G, Decurtins M. Incarcerated vermiform appendix in a left-sided inguinal hernia. Hernia. 2005;9(1):100-102. doi:10.1007/s10029-004-0263-0
8. Gupta S, Sharma R, Kaushik R. Left-sided Amyand’s hernia. Singapore Med J. 2005;46(8):424-425.
9. Gupta N, Wilkinson TV, Wilkinson A, Akhtar M. Left-sided incarcerated Amyand’s hernia. Indian J Surg. 2007;69(1):17-18.
10. Tayade, MB, Bakhshi GD, Borisa AD, Deshpande G, Joshi N. A rare combination of left sided Amyand’s and Richter’s hernia. Bombay Hosp J. 2008;50(4): 644-645
11. Johari HG, Paydar S, Davani SZ, Eskandari S, Johari MG. Left-sided Amyand hernia. Ann Saudi Med. 2009;29(4):321-322. doi:10.4103/0256-4947.55305
12. Ali SM, Malik KA, Al-Qadhi H. Amyand’s Hernia: Study of four cases and literature review. Sultan Qaboos Univ Med J. 2012;12(2):232-236. doi:10.12816/0003119
13. Ravishankaran P, Mohan G, Srinivasan A, Ravindran G, Ramalingam A. Left sided amyand’s hernia, a rare occurrence: A Case Report. Indian J Surg. 2013;75(3):247-248. doi:10.1007/s12262-010-0223-0
14. Singh K, Singh RR, Kaur S. Amyand’s hernia. J Indian Assoc Pediatr Surg. 2011;16(4):171-172. doi:10.4103/0971-9261.86890
15. Khan TS, Wani ML, Bijli AH, et al. Amyand’s hernia: a rare occurrence. Ann Nigerian Med. 2011;5(2):62-64.doi:10.4103/0331-3131.92955
16. Ghafouri A, Anbara T, Foroutankia R. A rare case report of appendix and cecum in the sac of left inguinal hernia (left Amyand’s hernia). Med J Islam Repub Iran. 2012;26(2):94-95.
17. Al-Mayoof AF, Al-Ani BH. Left-sided amyand hernia: report of two cases with review of literature. European J Pediatr Surg Rep. 2014;2(1):63-66. doi:10.1055/s-0033-1347131
18. Unver M, Ozturk S, Karaman K, Turgut E. Left sided Amyand’s hernia. World J Gastrointest Surg. 2013;5(10):285-286. doi:10.4240/wjgs.v5.i10.285
19. Maeda K, Kunieda K, Kawai M, et al. Giant left-sided inguinoscrotal hernia containing the cecum and appendix (giant left-sided Amyand’s hernia). Clin Case Rep. 2014;2(6):254-257. doi:10.1002/ccr3.104
20. Mongardini M, Maturo A, De Anna L, et al. Appendiceal abscess in a giant left-sided inguinoscrotal hernia: a rare case of Amyand hernia. Springerplus. 2015;4:378. Published 2015 Jul 26. doi:10.1186/s40064-015-1162-9
21. Ivanschuk G, Cesmebasi A, Sorenson EP, Blaak C, Loukas M, Tubbs SR. Amyand’s hernia: a review. Med Sci Monit. 2014;20:140-146. Published 2014 Jan 28. doi:10.12659/MSM.889873
22. Losanoff JE, Basson MD. Amyand hernia: what lies beneath--a proposed classification scheme to determine management. Am Surg. 2007;73(12):1288-1290.
23. Coulier B, Pacary J, Broze B. Sonographic diagnosis of appendicitis within a right inguinal hernia (Amyand’s hernia). J Clin Ultrasound. 2006;34(9):454-457. doi:10.1002/jcu.20266
24. Vehbi H, Agirgun C, Agirgun F, Dogan Y. Preoperative diagnosis of Amyand’s hernia by ultrasound and computed tomography. Turk J Emerg Med. 2016;16(2):72-74. Published 2016 May 8. doi:10.1016/j.tjem.2015.11.014
25. Vermillion JM, Abernathy SW, Snyder SK. Laparoscopic reduction of Amyand’s hernia. Hernia. 1999;3:159-160. doi:10.1007/BF01195318
1. Franko J, Raftopoulos I, Sulkowski R. A rare variation of Amyand’s hernia. Am J Gastroenterol. 2002;97(10):2684-2685. doi:10.1111/j.1572-0241.2002.06060.x
2. Carey LC. Acute appendicitis occurring in hernias: a report of 10 cases. Surgery. 1967;61(2):236-238.
3. Kaymakci A, Akillioglu I, Akkoyun I, Guven S, Ozdemir A, Gulen S. Amyand’s hernia: a series of 30 cases in children. Hernia. 2009;13(6):609-612. doi:10.1007/s10029-009-0528-8
4. Cankorkmaz L, Ozer H, Guney C, Atalar MH, Arslan MS, Koyluoglu G. Amyand’s hernia in the children: a single center experience. Surgery. 2010;147(1):140-143. doi:10.1016/j.surg.2009.09.038
5. Yasumoto R, Kawano M, Kawanishi H, et al. Left acute scrotum associated with appendicitis. Int J Urol. 1998;5(1):108-110. doi:10.1111/j.1442-2042.1998.tb00254.x
6. Bakhshi GD, Bhandarwar AH, Govila AA. Acute appendicitis in left scrotum. Indian J Gastroenterol. 2004;23(5):195.
7. Breitenstein S, Eisenbach C, Wille G, Decurtins M. Incarcerated vermiform appendix in a left-sided inguinal hernia. Hernia. 2005;9(1):100-102. doi:10.1007/s10029-004-0263-0
8. Gupta S, Sharma R, Kaushik R. Left-sided Amyand’s hernia. Singapore Med J. 2005;46(8):424-425.
9. Gupta N, Wilkinson TV, Wilkinson A, Akhtar M. Left-sided incarcerated Amyand’s hernia. Indian J Surg. 2007;69(1):17-18.
10. Tayade, MB, Bakhshi GD, Borisa AD, Deshpande G, Joshi N. A rare combination of left sided Amyand’s and Richter’s hernia. Bombay Hosp J. 2008;50(4): 644-645
11. Johari HG, Paydar S, Davani SZ, Eskandari S, Johari MG. Left-sided Amyand hernia. Ann Saudi Med. 2009;29(4):321-322. doi:10.4103/0256-4947.55305
12. Ali SM, Malik KA, Al-Qadhi H. Amyand’s Hernia: Study of four cases and literature review. Sultan Qaboos Univ Med J. 2012;12(2):232-236. doi:10.12816/0003119
13. Ravishankaran P, Mohan G, Srinivasan A, Ravindran G, Ramalingam A. Left sided amyand’s hernia, a rare occurrence: A Case Report. Indian J Surg. 2013;75(3):247-248. doi:10.1007/s12262-010-0223-0
14. Singh K, Singh RR, Kaur S. Amyand’s hernia. J Indian Assoc Pediatr Surg. 2011;16(4):171-172. doi:10.4103/0971-9261.86890
15. Khan TS, Wani ML, Bijli AH, et al. Amyand’s hernia: a rare occurrence. Ann Nigerian Med. 2011;5(2):62-64.doi:10.4103/0331-3131.92955
16. Ghafouri A, Anbara T, Foroutankia R. A rare case report of appendix and cecum in the sac of left inguinal hernia (left Amyand’s hernia). Med J Islam Repub Iran. 2012;26(2):94-95.
17. Al-Mayoof AF, Al-Ani BH. Left-sided amyand hernia: report of two cases with review of literature. European J Pediatr Surg Rep. 2014;2(1):63-66. doi:10.1055/s-0033-1347131
18. Unver M, Ozturk S, Karaman K, Turgut E. Left sided Amyand’s hernia. World J Gastrointest Surg. 2013;5(10):285-286. doi:10.4240/wjgs.v5.i10.285
19. Maeda K, Kunieda K, Kawai M, et al. Giant left-sided inguinoscrotal hernia containing the cecum and appendix (giant left-sided Amyand’s hernia). Clin Case Rep. 2014;2(6):254-257. doi:10.1002/ccr3.104
20. Mongardini M, Maturo A, De Anna L, et al. Appendiceal abscess in a giant left-sided inguinoscrotal hernia: a rare case of Amyand hernia. Springerplus. 2015;4:378. Published 2015 Jul 26. doi:10.1186/s40064-015-1162-9
21. Ivanschuk G, Cesmebasi A, Sorenson EP, Blaak C, Loukas M, Tubbs SR. Amyand’s hernia: a review. Med Sci Monit. 2014;20:140-146. Published 2014 Jan 28. doi:10.12659/MSM.889873
22. Losanoff JE, Basson MD. Amyand hernia: what lies beneath--a proposed classification scheme to determine management. Am Surg. 2007;73(12):1288-1290.
23. Coulier B, Pacary J, Broze B. Sonographic diagnosis of appendicitis within a right inguinal hernia (Amyand’s hernia). J Clin Ultrasound. 2006;34(9):454-457. doi:10.1002/jcu.20266
24. Vehbi H, Agirgun C, Agirgun F, Dogan Y. Preoperative diagnosis of Amyand’s hernia by ultrasound and computed tomography. Turk J Emerg Med. 2016;16(2):72-74. Published 2016 May 8. doi:10.1016/j.tjem.2015.11.014
25. Vermillion JM, Abernathy SW, Snyder SK. Laparoscopic reduction of Amyand’s hernia. Hernia. 1999;3:159-160. doi:10.1007/BF01195318
Success in LGBTQ+ medicine requires awareness of risk
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
Patients who are transgender, for instance, are nine times more likely to commit suicide than the general population (2015 U.S. Transgender Survey (USTS). Inter-university Consortium for Political and Social Research. 2019 May 22. doi: 10.3886/ICPSR37229.v1), and those who are also Black have an estimated HIV prevalence of 62%, demonstrating the cumulative, negative health effects of intersectionality (www.cdc.gov/hiv/group/gender/transgender/hiv-prevalence.html).
“Experiences with marginalization and stigma directly relate to some of the poor physical and mental health outcomes that these patients experience,” Megan McNamara, MD, said during a presentation at the American College of Physicians annual Internal Medicine meeting.
Dr. McNamara, who is director of the Gender Identity Veteran’s Experience (GIVE) Clinic, Veterans Affairs Northeast Ohio Healthcare System, Cleveland, offered a brief guide to managing LGBTQ+ patients. She emphasized increased rates of psychological distress and substance abuse, and encouraged familiarity with specific risks associated with three subgroups: men who have sex with men (MSM), women who have sex with women (WSW), and those who are transgender.
Men who have sex with men
According to Dr. McNamara, preexposure prophylaxis (PrEP) should be offered based on Centers for Disease Control and Prevention eligibility criteria, which require that the patient is HIV negative, has had a male sex partner in the past 6 months, is not in a monogamous relationship, and has had anal sex or a bacterial sexually transmitted infection in the past 6 months. The two PrEP options, emtricitabine/tenofovir disoproxil fumarate and emtricitabine/tenofovir alafenamide, are equally effective and have similar safety profiles, Dr. McNamara said, but patients with impaired renal function should receive the alafenamide formulation.
Dr. McNamara also advised screening gay men for extragenital STIs, noting a 13.3% increased risk. When asked about anal Pap testing for HPV, Dr. McNamara called the subject “very controversial,” and ultimately recommended against it, citing a lack of data linking anal HPV infection and dysplasia with later development of rectal carcinoma, as well as the nonactionable impact of a positive result.
“For me, the issue is ... if [a positive anal Pap test] is not going to change my management, if I don’t know that the anal HPV that I diagnose will result in cancer, should I continue to monitor it?” Dr. McNamara said.
Women who have sex with women
Beyond higher rates of psychological distress and substance abuse among lesbian and bisexual women, Dr. McNamara described increased risks of overweight and obesity, higher rates of smoking, and lower rates of Pap testing, all of which should prompt clinicians to advise accordingly, with cervical cancer screening in alignment with guidelines. Clinicians should also discuss HPV vaccination with patients, taking care to weigh benefits and risks, as “catch-up” HPV vaccination is not unilaterally recommended for adults older than 26 years.
Transgender patients
Discussing transgender patients, Dr. McNamara focused on cross-sex hormone therapy (CSHT), first noting the significant psychological benefits, including improvements in depression, somatization, interpersonal sensitivity, hostility, anxiety, phobic anxiety/agoraphobia, and quality of life.
According to Dr. McNamara, CSHT is relatively simple and may be safely administered by primary care providers. For transmasculine patients, testosterone supplementation is all that is needed, whereas transfeminine patients will require spironolactone or GnRH agonists to reduce testosterone and estradiol to increase feminizing hormones to pubertal levels.
CSHT is not without risks, Dr. McNamara said, including “very high” risks of erythrocytosis among transmasculine patients and venous thromboembolic disease among transfeminine patients; but these risks need to be considered in the context of an approximate 40% suicide rate among transgender individuals.
“I can tell you in my own practice that these [suicide] data ring true,” Dr. McNamara said. “Many, many of my patients have attempted suicide, so [CSHT] is something that you really want to think about right away.”
Even when additional risk factors are present, such as preexisting cardiovascular disease, Dr. McNamara suggested that “there are very few absolute contraindications to CSHT,” and described it as a “life-sustaining treatment” that should be viewed analogously with any other long-term management strategy, such as therapy for diabetes or hypertension.
Fostering a transgender-friendly practice
In an interview, Nicole Nisly, MD, codirector of the LGBTQ+ Clinic at the University of Iowa Hospitals and Clinics, Iowa City, reflected upon Dr. McNamara’s presentation, noting that primary care providers – with a little education – are the best candidates to care for transgender patients.
“I think [primary care providers] do a better job [caring for transgender patients] than endocrinologists, honestly, because they can provide care for the whole person,” Dr. Nisly said. “They can do a Pap, they can do STI screening, they can assess mood, they can [evaluate] safety, and the whole person, as opposed to endocrinologists, who do hormone therapy, but somebody else does everything else.”
Dr. Nisly emphasized the importance of personalizing care for transgender individuals, which depends upon a welcoming practice environment, with careful attention to language.
Foremost, Dr. Nisly recommended asking patients for their preferred name, sexual orientation, and gender identity.
“One of the most difficult things [for transgender patients] is to see notes with the wrong name – the name that makes them feel uncomfortable – or the wrong pronoun,” Dr. Nisly said. “That’s very important to the community.”
Dr. Nisly also recommended an alternative term for cross-sex hormone therapy.
“I hate cross-sex hormone therapy terminology, honestly,” Dr. Nisly said. “I just think it’s so unwelcoming, and I think most of our patients don’t like the terminology, so we use ‘gender-affirming hormone therapy.’”
Dr. Nisly explained that the term “cross-sex” assumes a conventional definition of sex, which is inherently flawed.
When discussing certain medical risk factors, such as pregnancy or HIV, it is helpful to know “sex assigned at birth” for both patients and their sexual partners, Dr. Nisly said. It’s best to ask in this way, instead of using terms like “boyfriend” or “girlfriend,” as “sex assigned at birth” is “terminology the community recognizes, affirms, and feels comfortable with.”
Concerning management of medical risk factors, Dr. Nisly offered some additional perspectives.
For one, she recommended giving PrEP to any patient who has a desire to be on PrEP, noting that this desire can indicate a change in future sexual practices, which the CDC criteria do not anticipate. She also advised in-hospital self-swabbing for extragenital STIs, as this can increase patient comfort and adherence. And, in contrast with Dr. McNamara, Dr. Nisly recommended anal Pap screening for any man that has sex with men and anyone with HIV of any gender. She noted that rates of anal dysplasia are “pretty high” among men who have sex with men, and that detection may reduce cancer risk.
For clinicians who would like to learn more about caring for transgender patients, Dr. Nisly recommended that they start by reading the World Professional Association for Transgender Health guidelines.
“It’s about 300 pages,” Dr. Nisly said, “but it is great.”
Dr. McNamara and Dr. Nisly reported no conflicts of interest.
FROM INTERNAL MEDICINE 2021
HPV vaccination rates continue to climb among young adults in U.S.
Although vaccination rates against the human papillomavirus remain low for young adults across the United States, the number of self-reported HPV vaccinations among women and men aged between 18 and 21 years has markedly increased since 2010, according to new research findings.
The findings were published online April 27, 2021, as a research letter in JAMA.
In 2006, the Food and Drug Administration approved the HPV vaccine for the prevention of cervical cancer and genital warts in female patients. Three years later, the FDA approved the vaccine for the prevention of anogenital cancer and warts in male patients.
The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend two doses of the HPV vaccine for children aged 11-12 years. Adolescents and young adults may need three doses over the course of 6 months if they start their vaccine series on or following their 15th birthday.
For persons who have not previously received the HPV vaccine or who did not receive adequate doses, the HPV vaccine is recommended through age 26. Data on the rates of vaccination among young adults between 18 and 21 years of age in the United States are sparse, and it is not known how well vaccination programs are progressing in the country.
In the recently published JAMA research letter, investigators from the University of Michigan, Ann Arbor, examined data for the period 2010-2018 from the cross-sectional National Health Interview Survey. Respondents included in the analysis were aged 18-21 years. They were asked whether they had received the HPV vaccine before age 18 and at what age they had been vaccinated against the virus.
The researchers also assessed whether the respondents had received any HPV vaccine dose between the ages of 18 and 21 years. The findings were limited to self-reported vaccination status.
In total, 6,606 women and 6,038 men were included in the analysis. Approximately 42% of women and 16% of men said they had received at least one HPV vaccine dose at any age. The proportion of female patients who reported receiving an HPV vaccine dose significantly increased from 32% in 2010 to 55% in 2018 (P =.001). Similarly, among men, the percentage significantly increased from 2% in 2010 to 34% in 2018 (P <.001).
Approximately 4% of the female respondents and 3% of the male respondents reported that they had received an HPV vaccine between the ages of 18 and 21 years; 46% of women and 29% of men who received the vaccine between these ages completed the recommended vaccination series.
Findings from the study highlight the continual need for improving vaccination rates among vulnerable populations. Lead study author Michelle Chen, MD, MHS, a professor in the department of otolaryngology–head and neck surgery at the University of Michigan, explained in an interview that there are multiple barriers to HPV vaccination among young adults. “These barriers to vaccination among young adults primarily include cost, lack of knowledge and awareness, missed opportunities for vaccination, rapidly changing guidelines, and initial gender-based guidelines,” said Dr. Chen.
Clinicians play a large role in improving vaccination rates among young adults, who may lack awareness of the overall importance of inoculation against the potentially debilitating and deadly virus. Dr. Chen noted that clinicians can lead the way by increasing gender-inclusive awareness of HPV-associated diseases and HPV vaccination, by performing routine vaccine eligibility assessments for young adults regardless of sex, by developing robust reminder and recall strategies to improve series completion rates, and by offering patients resources regarding assistance programs to address cost barriers for uninsured patients.
“Young adult men are particularly vulnerable [to HPV], because they start to age out of pediatric health practices,” added Dr. Chen. “Thus, a multilevel gender-inclusive approach is needed to target clinicians, patients, parents, and community-based organizations.”
Gypsyamber D’Souza, PhD, professor of epidemiology at Johns Hopkins University, Baltimore, said in an interview that the initial uptake of HPV vaccination was slow in the United States but that progress has been made in recent years among persons in the targeted age range of 11-12 years. “However, catch-up vaccination has lagged behind, and sadly, we’re still seeing low uptake in those older ages that are still eligible and where we know there still is tremendous benefit,” she said.
Dr. D’Souza is a lead investigator in the MOUTH trial, which is currently enrolling patients. That trial will examine potential biomarkers for oropharyngeal cancer risk among people with known risk factors for HPV who came of age prior to the rollout of the vaccine.
She explained that many parents want their children to be vaccinated for HPV after they hear about the vaccine, but because the health care system in the United States is an “opt-in” system, rather than an “opt-out” one, parents need to actively seek out vaccination. Children then move toward adulthood without having received the recommended vaccine course. “There are individuals who did not get vaccinated at the ages of 11 and 12 and then forget to ask about it later, or the provider asks about it and the patients don’t have enough information,” Dr. D’Souza said.
She noted that one reason why HPV vaccination rates remain low among young adults is that the vaccine is not often kept in stock other than in pediatric clinics. “Because vaccines expire and clinics don’t have a lot of people in that age group getting vaccinated, they may not have it regularly in stock, making this one reason it might be hard for someone to get vaccinated.”
The HPV vaccine is not effective for clearing HPV once a patient acquires the infection, she added. “So young adulthood is a critical time where we have individuals who still can benefit from being vaccinated, but if we wait too long, they’ll age out of those ages where we see the highest efficacy.”
Ultimately, said Dr. D’Souza, clinicians need to catch people at multiple time points and work to remove barriers to vaccination, including letting patients know that HPV vaccination is covered by insurance. “There’s a lot of opportunity to prevent future cancers in young adults by having care providers for that age group talk about the vaccine and remember to offer it.”
A version of this article first appeared on Medscape.com.
Although vaccination rates against the human papillomavirus remain low for young adults across the United States, the number of self-reported HPV vaccinations among women and men aged between 18 and 21 years has markedly increased since 2010, according to new research findings.
The findings were published online April 27, 2021, as a research letter in JAMA.
In 2006, the Food and Drug Administration approved the HPV vaccine for the prevention of cervical cancer and genital warts in female patients. Three years later, the FDA approved the vaccine for the prevention of anogenital cancer and warts in male patients.
The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend two doses of the HPV vaccine for children aged 11-12 years. Adolescents and young adults may need three doses over the course of 6 months if they start their vaccine series on or following their 15th birthday.
For persons who have not previously received the HPV vaccine or who did not receive adequate doses, the HPV vaccine is recommended through age 26. Data on the rates of vaccination among young adults between 18 and 21 years of age in the United States are sparse, and it is not known how well vaccination programs are progressing in the country.
In the recently published JAMA research letter, investigators from the University of Michigan, Ann Arbor, examined data for the period 2010-2018 from the cross-sectional National Health Interview Survey. Respondents included in the analysis were aged 18-21 years. They were asked whether they had received the HPV vaccine before age 18 and at what age they had been vaccinated against the virus.
The researchers also assessed whether the respondents had received any HPV vaccine dose between the ages of 18 and 21 years. The findings were limited to self-reported vaccination status.
In total, 6,606 women and 6,038 men were included in the analysis. Approximately 42% of women and 16% of men said they had received at least one HPV vaccine dose at any age. The proportion of female patients who reported receiving an HPV vaccine dose significantly increased from 32% in 2010 to 55% in 2018 (P =.001). Similarly, among men, the percentage significantly increased from 2% in 2010 to 34% in 2018 (P <.001).
Approximately 4% of the female respondents and 3% of the male respondents reported that they had received an HPV vaccine between the ages of 18 and 21 years; 46% of women and 29% of men who received the vaccine between these ages completed the recommended vaccination series.
Findings from the study highlight the continual need for improving vaccination rates among vulnerable populations. Lead study author Michelle Chen, MD, MHS, a professor in the department of otolaryngology–head and neck surgery at the University of Michigan, explained in an interview that there are multiple barriers to HPV vaccination among young adults. “These barriers to vaccination among young adults primarily include cost, lack of knowledge and awareness, missed opportunities for vaccination, rapidly changing guidelines, and initial gender-based guidelines,” said Dr. Chen.
Clinicians play a large role in improving vaccination rates among young adults, who may lack awareness of the overall importance of inoculation against the potentially debilitating and deadly virus. Dr. Chen noted that clinicians can lead the way by increasing gender-inclusive awareness of HPV-associated diseases and HPV vaccination, by performing routine vaccine eligibility assessments for young adults regardless of sex, by developing robust reminder and recall strategies to improve series completion rates, and by offering patients resources regarding assistance programs to address cost barriers for uninsured patients.
“Young adult men are particularly vulnerable [to HPV], because they start to age out of pediatric health practices,” added Dr. Chen. “Thus, a multilevel gender-inclusive approach is needed to target clinicians, patients, parents, and community-based organizations.”
Gypsyamber D’Souza, PhD, professor of epidemiology at Johns Hopkins University, Baltimore, said in an interview that the initial uptake of HPV vaccination was slow in the United States but that progress has been made in recent years among persons in the targeted age range of 11-12 years. “However, catch-up vaccination has lagged behind, and sadly, we’re still seeing low uptake in those older ages that are still eligible and where we know there still is tremendous benefit,” she said.
Dr. D’Souza is a lead investigator in the MOUTH trial, which is currently enrolling patients. That trial will examine potential biomarkers for oropharyngeal cancer risk among people with known risk factors for HPV who came of age prior to the rollout of the vaccine.
She explained that many parents want their children to be vaccinated for HPV after they hear about the vaccine, but because the health care system in the United States is an “opt-in” system, rather than an “opt-out” one, parents need to actively seek out vaccination. Children then move toward adulthood without having received the recommended vaccine course. “There are individuals who did not get vaccinated at the ages of 11 and 12 and then forget to ask about it later, or the provider asks about it and the patients don’t have enough information,” Dr. D’Souza said.
She noted that one reason why HPV vaccination rates remain low among young adults is that the vaccine is not often kept in stock other than in pediatric clinics. “Because vaccines expire and clinics don’t have a lot of people in that age group getting vaccinated, they may not have it regularly in stock, making this one reason it might be hard for someone to get vaccinated.”
The HPV vaccine is not effective for clearing HPV once a patient acquires the infection, she added. “So young adulthood is a critical time where we have individuals who still can benefit from being vaccinated, but if we wait too long, they’ll age out of those ages where we see the highest efficacy.”
Ultimately, said Dr. D’Souza, clinicians need to catch people at multiple time points and work to remove barriers to vaccination, including letting patients know that HPV vaccination is covered by insurance. “There’s a lot of opportunity to prevent future cancers in young adults by having care providers for that age group talk about the vaccine and remember to offer it.”
A version of this article first appeared on Medscape.com.
Although vaccination rates against the human papillomavirus remain low for young adults across the United States, the number of self-reported HPV vaccinations among women and men aged between 18 and 21 years has markedly increased since 2010, according to new research findings.
The findings were published online April 27, 2021, as a research letter in JAMA.
In 2006, the Food and Drug Administration approved the HPV vaccine for the prevention of cervical cancer and genital warts in female patients. Three years later, the FDA approved the vaccine for the prevention of anogenital cancer and warts in male patients.
The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend two doses of the HPV vaccine for children aged 11-12 years. Adolescents and young adults may need three doses over the course of 6 months if they start their vaccine series on or following their 15th birthday.
For persons who have not previously received the HPV vaccine or who did not receive adequate doses, the HPV vaccine is recommended through age 26. Data on the rates of vaccination among young adults between 18 and 21 years of age in the United States are sparse, and it is not known how well vaccination programs are progressing in the country.
In the recently published JAMA research letter, investigators from the University of Michigan, Ann Arbor, examined data for the period 2010-2018 from the cross-sectional National Health Interview Survey. Respondents included in the analysis were aged 18-21 years. They were asked whether they had received the HPV vaccine before age 18 and at what age they had been vaccinated against the virus.
The researchers also assessed whether the respondents had received any HPV vaccine dose between the ages of 18 and 21 years. The findings were limited to self-reported vaccination status.
In total, 6,606 women and 6,038 men were included in the analysis. Approximately 42% of women and 16% of men said they had received at least one HPV vaccine dose at any age. The proportion of female patients who reported receiving an HPV vaccine dose significantly increased from 32% in 2010 to 55% in 2018 (P =.001). Similarly, among men, the percentage significantly increased from 2% in 2010 to 34% in 2018 (P <.001).
Approximately 4% of the female respondents and 3% of the male respondents reported that they had received an HPV vaccine between the ages of 18 and 21 years; 46% of women and 29% of men who received the vaccine between these ages completed the recommended vaccination series.
Findings from the study highlight the continual need for improving vaccination rates among vulnerable populations. Lead study author Michelle Chen, MD, MHS, a professor in the department of otolaryngology–head and neck surgery at the University of Michigan, explained in an interview that there are multiple barriers to HPV vaccination among young adults. “These barriers to vaccination among young adults primarily include cost, lack of knowledge and awareness, missed opportunities for vaccination, rapidly changing guidelines, and initial gender-based guidelines,” said Dr. Chen.
Clinicians play a large role in improving vaccination rates among young adults, who may lack awareness of the overall importance of inoculation against the potentially debilitating and deadly virus. Dr. Chen noted that clinicians can lead the way by increasing gender-inclusive awareness of HPV-associated diseases and HPV vaccination, by performing routine vaccine eligibility assessments for young adults regardless of sex, by developing robust reminder and recall strategies to improve series completion rates, and by offering patients resources regarding assistance programs to address cost barriers for uninsured patients.
“Young adult men are particularly vulnerable [to HPV], because they start to age out of pediatric health practices,” added Dr. Chen. “Thus, a multilevel gender-inclusive approach is needed to target clinicians, patients, parents, and community-based organizations.”
Gypsyamber D’Souza, PhD, professor of epidemiology at Johns Hopkins University, Baltimore, said in an interview that the initial uptake of HPV vaccination was slow in the United States but that progress has been made in recent years among persons in the targeted age range of 11-12 years. “However, catch-up vaccination has lagged behind, and sadly, we’re still seeing low uptake in those older ages that are still eligible and where we know there still is tremendous benefit,” she said.
Dr. D’Souza is a lead investigator in the MOUTH trial, which is currently enrolling patients. That trial will examine potential biomarkers for oropharyngeal cancer risk among people with known risk factors for HPV who came of age prior to the rollout of the vaccine.
She explained that many parents want their children to be vaccinated for HPV after they hear about the vaccine, but because the health care system in the United States is an “opt-in” system, rather than an “opt-out” one, parents need to actively seek out vaccination. Children then move toward adulthood without having received the recommended vaccine course. “There are individuals who did not get vaccinated at the ages of 11 and 12 and then forget to ask about it later, or the provider asks about it and the patients don’t have enough information,” Dr. D’Souza said.
She noted that one reason why HPV vaccination rates remain low among young adults is that the vaccine is not often kept in stock other than in pediatric clinics. “Because vaccines expire and clinics don’t have a lot of people in that age group getting vaccinated, they may not have it regularly in stock, making this one reason it might be hard for someone to get vaccinated.”
The HPV vaccine is not effective for clearing HPV once a patient acquires the infection, she added. “So young adulthood is a critical time where we have individuals who still can benefit from being vaccinated, but if we wait too long, they’ll age out of those ages where we see the highest efficacy.”
Ultimately, said Dr. D’Souza, clinicians need to catch people at multiple time points and work to remove barriers to vaccination, including letting patients know that HPV vaccination is covered by insurance. “There’s a lot of opportunity to prevent future cancers in young adults by having care providers for that age group talk about the vaccine and remember to offer it.”
A version of this article first appeared on Medscape.com.
STD Prevention: We’ve Come Far, but not Far Enough
On any given day in 2018, one in five people had a sexually transmitted infection (STI), according to the US Centers for Disease Control and Prevention’s (CDC) recently released Sexually Transmitted Disease (STD) Surveillance Report, 2018. There were nearly 68 million infections in the US—and 26 million STIs were acquired in that year.
“The CDC report is an important reminder that infectious diseases continue to do what they do best, which is to cause illness and spread from person to person,” says David Aronoff, MD, director, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center. “Sexually transmitted infections are persistent threats to human health.”
Most of the infections on the CDC’s watchlist were due to the human papillomavirus (HPV), herpes simplex virus-2 (HSV-2), and trichomoniasis. Chlamydia, gonorrhea, HIV, hepatitis B virus, and syphilis followed. Although lower on the list, gonorrhea and syphilis numbers are on the rise to a disquieting degree. Since 2014, gonorrhea cases have increased 63% and syphilis cases, 71%.
Syphilis is still treatable with penicillin. But “the tragedy of poor STI [sexually transmitted infection] control is compounded by the fact that many of the germs that cause STIs are gradually developing more and more resistance to available treatments,” Dr. Aronoff says.
The rise in gonorrhea cases is particularly concerning to many health care providers. “There’s a very limited pipeline of new antibiotics to use if we’re confronted with antibiotic-resistant STIs,” says Ina Park, MD, assistant professor at University of California San Francisco School of Medicine; medical director, California Prevention Training Center; and author of Strange Bedfellows: Adventures in the Science, History and Surprising Secrets of STDs. In the case of gonorrhea, she warns, we’re down to one class of antibiotics. When all conventional therapies fail in cases of multidrug-resistant gonorrhea, patients have to be hospitalized and treated with broad-spectrum IV antibiotics, such as ertapenem. “We really don’t want to have to resort to that for an infection as common as gonorrhea,” she says.
Syphilis’ resurgence in new populations also is a concern. In the ’80s, says Michelle Collins-Ogle, MD, there was an epidemic of syphilis in pregnant women and newborns. Then it “sort of quieted down,” she says, in part because obstetricians and gynecologists and other health care providers did a better job of screening, diagnosing, and treating in that demographic. The latest resurgence is in young men of color who have sex with men—“we didn’t see that coming.”
Women and babies are still vulnerable, though. In one year, according to the CDC, syphilis cases among women of childbearing age leaped 36%. And, alarmingly, since 2014, cases of congenital syphilis have increased 185%. Between 2017 and 2018 alone, newborn deaths due to syphilis increased 22%—a “startling” number, says Gail Bolan, MD, the CDC director of STD prevention, in a release about the surveillance report. “Too many babies are needlessly dying. Every single instance of congenital syphilis is one too many when we have the tools to prevent it.”
Can all STIs be prevented? Can the rising tides be turned? Dr. Aronoff says, “As with the COVID-19 pandemic, STIs provide an important opportunity for us to understand how multiple factors can contribute to their spread and difficulty controlling.” Drug use, poverty, unstable housing, and stigma can all reduce access to STD prevention and care, he says. “And, as we’ve seen with COVID-19, under-resourced public health programs can also foster epidemics and pandemics of STIs.” Moreover, he adds, many public health programs at the state and local level have been subjected to budget cuts, which translates into less control of disease.
Some STI rates have been reduced with, for instance, antiretrovirals for HIV/AIDS and the HPV vaccine. But there’s still ground to cover, and new patient groups to protect. Nearly half of all new infections in 2018 were in young people aged 15 to 24 years. Not only is it another dangerous trend, it is an expensive one. Chlamydia, gonorrhea, and syphilis combined accounted for $1.1 billion in direct medical costs in 2018, the CDC report says, and care for young people aged 15 to 24 made up about 60% of those costs.
“Low or decreasing rates of condom use among vulnerable groups, including young people and gay and bisexual men, play important roles in driving ongoing STI rates,” Aronoff says. In part, that’s due to lack of comprehensive sex education, a lack that’s taking a huge toll.
“Remember now, we basically cut out a lot of the sex education. It doesn’t exist,” says Dr. Collins-Ogle. She has run clinics for several decades, and says she continually sees young male patients who don’t know how to use a condom. We know more now, though, she points out. “Back in the ‘80s, we didn’t have a direct correlation between STIs and AIDS. Now we know that having syphilis, for example, predisposes you to HIV acquisition. We also know that having HSV2, for example, predisposes you to HIV.”
It’s an ongoing battle, though, with each new generation of pathogens—and people. And as the CDC report shows, it’s like fighting a Hydra: When one infection is wrestled to the ground, another rears its head. There’s no time to rest on laurels. “Having highly contagious infections caused by difficult or impossible-to-treat microbes,” says David Aronoff, “is not a future I would wish on anyone
On any given day in 2018, one in five people had a sexually transmitted infection (STI), according to the US Centers for Disease Control and Prevention’s (CDC) recently released Sexually Transmitted Disease (STD) Surveillance Report, 2018. There were nearly 68 million infections in the US—and 26 million STIs were acquired in that year.
“The CDC report is an important reminder that infectious diseases continue to do what they do best, which is to cause illness and spread from person to person,” says David Aronoff, MD, director, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center. “Sexually transmitted infections are persistent threats to human health.”
Most of the infections on the CDC’s watchlist were due to the human papillomavirus (HPV), herpes simplex virus-2 (HSV-2), and trichomoniasis. Chlamydia, gonorrhea, HIV, hepatitis B virus, and syphilis followed. Although lower on the list, gonorrhea and syphilis numbers are on the rise to a disquieting degree. Since 2014, gonorrhea cases have increased 63% and syphilis cases, 71%.
Syphilis is still treatable with penicillin. But “the tragedy of poor STI [sexually transmitted infection] control is compounded by the fact that many of the germs that cause STIs are gradually developing more and more resistance to available treatments,” Dr. Aronoff says.
The rise in gonorrhea cases is particularly concerning to many health care providers. “There’s a very limited pipeline of new antibiotics to use if we’re confronted with antibiotic-resistant STIs,” says Ina Park, MD, assistant professor at University of California San Francisco School of Medicine; medical director, California Prevention Training Center; and author of Strange Bedfellows: Adventures in the Science, History and Surprising Secrets of STDs. In the case of gonorrhea, she warns, we’re down to one class of antibiotics. When all conventional therapies fail in cases of multidrug-resistant gonorrhea, patients have to be hospitalized and treated with broad-spectrum IV antibiotics, such as ertapenem. “We really don’t want to have to resort to that for an infection as common as gonorrhea,” she says.
Syphilis’ resurgence in new populations also is a concern. In the ’80s, says Michelle Collins-Ogle, MD, there was an epidemic of syphilis in pregnant women and newborns. Then it “sort of quieted down,” she says, in part because obstetricians and gynecologists and other health care providers did a better job of screening, diagnosing, and treating in that demographic. The latest resurgence is in young men of color who have sex with men—“we didn’t see that coming.”
Women and babies are still vulnerable, though. In one year, according to the CDC, syphilis cases among women of childbearing age leaped 36%. And, alarmingly, since 2014, cases of congenital syphilis have increased 185%. Between 2017 and 2018 alone, newborn deaths due to syphilis increased 22%—a “startling” number, says Gail Bolan, MD, the CDC director of STD prevention, in a release about the surveillance report. “Too many babies are needlessly dying. Every single instance of congenital syphilis is one too many when we have the tools to prevent it.”
Can all STIs be prevented? Can the rising tides be turned? Dr. Aronoff says, “As with the COVID-19 pandemic, STIs provide an important opportunity for us to understand how multiple factors can contribute to their spread and difficulty controlling.” Drug use, poverty, unstable housing, and stigma can all reduce access to STD prevention and care, he says. “And, as we’ve seen with COVID-19, under-resourced public health programs can also foster epidemics and pandemics of STIs.” Moreover, he adds, many public health programs at the state and local level have been subjected to budget cuts, which translates into less control of disease.
Some STI rates have been reduced with, for instance, antiretrovirals for HIV/AIDS and the HPV vaccine. But there’s still ground to cover, and new patient groups to protect. Nearly half of all new infections in 2018 were in young people aged 15 to 24 years. Not only is it another dangerous trend, it is an expensive one. Chlamydia, gonorrhea, and syphilis combined accounted for $1.1 billion in direct medical costs in 2018, the CDC report says, and care for young people aged 15 to 24 made up about 60% of those costs.
“Low or decreasing rates of condom use among vulnerable groups, including young people and gay and bisexual men, play important roles in driving ongoing STI rates,” Aronoff says. In part, that’s due to lack of comprehensive sex education, a lack that’s taking a huge toll.
“Remember now, we basically cut out a lot of the sex education. It doesn’t exist,” says Dr. Collins-Ogle. She has run clinics for several decades, and says she continually sees young male patients who don’t know how to use a condom. We know more now, though, she points out. “Back in the ‘80s, we didn’t have a direct correlation between STIs and AIDS. Now we know that having syphilis, for example, predisposes you to HIV acquisition. We also know that having HSV2, for example, predisposes you to HIV.”
It’s an ongoing battle, though, with each new generation of pathogens—and people. And as the CDC report shows, it’s like fighting a Hydra: When one infection is wrestled to the ground, another rears its head. There’s no time to rest on laurels. “Having highly contagious infections caused by difficult or impossible-to-treat microbes,” says David Aronoff, “is not a future I would wish on anyone
On any given day in 2018, one in five people had a sexually transmitted infection (STI), according to the US Centers for Disease Control and Prevention’s (CDC) recently released Sexually Transmitted Disease (STD) Surveillance Report, 2018. There were nearly 68 million infections in the US—and 26 million STIs were acquired in that year.
“The CDC report is an important reminder that infectious diseases continue to do what they do best, which is to cause illness and spread from person to person,” says David Aronoff, MD, director, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center. “Sexually transmitted infections are persistent threats to human health.”
Most of the infections on the CDC’s watchlist were due to the human papillomavirus (HPV), herpes simplex virus-2 (HSV-2), and trichomoniasis. Chlamydia, gonorrhea, HIV, hepatitis B virus, and syphilis followed. Although lower on the list, gonorrhea and syphilis numbers are on the rise to a disquieting degree. Since 2014, gonorrhea cases have increased 63% and syphilis cases, 71%.
Syphilis is still treatable with penicillin. But “the tragedy of poor STI [sexually transmitted infection] control is compounded by the fact that many of the germs that cause STIs are gradually developing more and more resistance to available treatments,” Dr. Aronoff says.
The rise in gonorrhea cases is particularly concerning to many health care providers. “There’s a very limited pipeline of new antibiotics to use if we’re confronted with antibiotic-resistant STIs,” says Ina Park, MD, assistant professor at University of California San Francisco School of Medicine; medical director, California Prevention Training Center; and author of Strange Bedfellows: Adventures in the Science, History and Surprising Secrets of STDs. In the case of gonorrhea, she warns, we’re down to one class of antibiotics. When all conventional therapies fail in cases of multidrug-resistant gonorrhea, patients have to be hospitalized and treated with broad-spectrum IV antibiotics, such as ertapenem. “We really don’t want to have to resort to that for an infection as common as gonorrhea,” she says.
Syphilis’ resurgence in new populations also is a concern. In the ’80s, says Michelle Collins-Ogle, MD, there was an epidemic of syphilis in pregnant women and newborns. Then it “sort of quieted down,” she says, in part because obstetricians and gynecologists and other health care providers did a better job of screening, diagnosing, and treating in that demographic. The latest resurgence is in young men of color who have sex with men—“we didn’t see that coming.”
Women and babies are still vulnerable, though. In one year, according to the CDC, syphilis cases among women of childbearing age leaped 36%. And, alarmingly, since 2014, cases of congenital syphilis have increased 185%. Between 2017 and 2018 alone, newborn deaths due to syphilis increased 22%—a “startling” number, says Gail Bolan, MD, the CDC director of STD prevention, in a release about the surveillance report. “Too many babies are needlessly dying. Every single instance of congenital syphilis is one too many when we have the tools to prevent it.”
Can all STIs be prevented? Can the rising tides be turned? Dr. Aronoff says, “As with the COVID-19 pandemic, STIs provide an important opportunity for us to understand how multiple factors can contribute to their spread and difficulty controlling.” Drug use, poverty, unstable housing, and stigma can all reduce access to STD prevention and care, he says. “And, as we’ve seen with COVID-19, under-resourced public health programs can also foster epidemics and pandemics of STIs.” Moreover, he adds, many public health programs at the state and local level have been subjected to budget cuts, which translates into less control of disease.
Some STI rates have been reduced with, for instance, antiretrovirals for HIV/AIDS and the HPV vaccine. But there’s still ground to cover, and new patient groups to protect. Nearly half of all new infections in 2018 were in young people aged 15 to 24 years. Not only is it another dangerous trend, it is an expensive one. Chlamydia, gonorrhea, and syphilis combined accounted for $1.1 billion in direct medical costs in 2018, the CDC report says, and care for young people aged 15 to 24 made up about 60% of those costs.
“Low or decreasing rates of condom use among vulnerable groups, including young people and gay and bisexual men, play important roles in driving ongoing STI rates,” Aronoff says. In part, that’s due to lack of comprehensive sex education, a lack that’s taking a huge toll.
“Remember now, we basically cut out a lot of the sex education. It doesn’t exist,” says Dr. Collins-Ogle. She has run clinics for several decades, and says she continually sees young male patients who don’t know how to use a condom. We know more now, though, she points out. “Back in the ‘80s, we didn’t have a direct correlation between STIs and AIDS. Now we know that having syphilis, for example, predisposes you to HIV acquisition. We also know that having HSV2, for example, predisposes you to HIV.”
It’s an ongoing battle, though, with each new generation of pathogens—and people. And as the CDC report shows, it’s like fighting a Hydra: When one infection is wrestled to the ground, another rears its head. There’s no time to rest on laurels. “Having highly contagious infections caused by difficult or impossible-to-treat microbes,” says David Aronoff, “is not a future I would wish on anyone
Preliminary Evaluation of an Order Template to Improve Diagnosis and Testosterone Therapy of Hypogonadism in Veterans
Testosterone treatment is clinically indicated when a patient presents with symptoms and signs and biochemical evidence of testosterone deficiency, ie, male hypogonadism. Laboratory confirmation of hypogonadism requires repeatedly low serum testosterone concentrations; between 8
Recent studies have reported an increase in testosterone prescriptions and raised concerns regarding health care provider (HCP) prescribing practices despite current clinical practice guidelines from major societies, such as the Endocrine Society. In the US from 2001 to 2011, testosterone use among men aged ≥ 40 years increased more than 3-fold in all age groups.3 Subsequently in the years from 2013 to 2016, prescription rates declined perhaps due to the cardiovascular and stroke concerns.4
In the US Department of Veterans Affairs (VA), new testosterone prescriptions across VA medical centers increased from 20,437 in fiscal year (FY) 2009 to 36,394 in FY 2012. Yet only 3.1% of men who received testosterone therapy had 2 or more low morning total or free testosterone concentrations measured; LH and/or FSH levels assessed; and presence of contraindications to therapy documented. Remarkably, 16.5% of these veterans did not have a testosterone level tested prior to being prescribed testosterone. Among veterans who were prescribed testosterone, 1.4% had a diagnosis of prostate cancer, 7.6% had a diagnosis of obstructive sleep apnea (OSA), and 3.5% had elevated hematocrit at baseline.5 These findings raised concerns of whether the diagnosis and etiology of hypogonadism were appropriately established and risks were considered before testosterone treatment was initiated.5,6
To further understand VA prescribing practices of testosterone therapy, a 2018 VA Office of the Inspector General (OIG) report evaluated the initiation and follow-up of testosterone replacement therapy. The OIG randomly sampled and reviewed 1,091 male patients who filled at least 1 outpatient testosterone prescription from VA in FY 2014 and who did not have a prior testosterone prescription in FY 2013. Patients were followed through September 30, 2015. Within 1 year prior to initiating testosterone, only 1.5% had clinical signs and symptoms of testosterone deficiency documented prior to testosterone testing (76% within 18 months of starting testosterone); only 9.1% of veterans had the recommended measurements of 2 low morning testosterone levels; and only 12% had LH and FSH levels measured. Within 3 to 6 months after starting testosterone therapy, only 24% of veterans were assessed for symptom improvement, and 29% to 33% were evaluated for adverse effects, hematocrit levels and adherence to the therapy. The OIG report concluded that VA HCPs were not adhering to guidelines (referencing the Endocrine Society guidelines) when evaluating and treating veterans with testosterone deficiency.7
Considering the OIG recommendations and need to improve current practices among providers, VA Puget Sound Health Care System (VAPSHCS) in Washington established a multidisciplinary workgroup consisting of an endocrinologist, geriatrician, primary care provider (PCP), pharmacists, VA information technology (IT) specialist, and health products support (HPS) clinical team in the spring of 2019 to assess and improve testosterone prescribing practices.
Methods
A testosterone order template was developed, approved by VAPSHCS Pharmacy and Therapeutics Committee, and implemented on July 1, 2019, at VAPSHCS, a 1a medical facility caring for more than 112,000 veterans. Given its potential risks and the propensity for varied prescribing practices, testosterone was designated as a restricted drug requiring a prior authorization drug request (PADR) and required completion of the testosterone order template in the Computerized Patient Record System (CPRS).
Testosterone Order Template
The testosterone order template had 2 components. Completion of the template for new testosterone orders was required to initiate treatment unless the patient had known organic hypogonadism or was a transgender male. The template ensured documentation of defined symptoms and signs of testosterone deficiency; low serum testosterone levels on at least 2 occasions and LH and FSH concentrations; no contraindications to testosterone treatment; discussion of risks and benefits of therapy; and baseline hematocrit (Figure 1). Relevant educational content (eg, risks and benefits of testosterone) was incorporated in the template. The second template was required for the first renewal of testosterone to document adherence to or reason for discontinuation of testosterone; improvement of symptoms and signs; and confirm monitoring hematocrit and testosterone levels during treatment.
Prior to implementation, the PADR template was introduced to HCPs at 2 chief-of-medicine rounds on the diagnosis and evaluation of hypogonadism by a pharmacist and endocrinologist. These educational sessions used case examples and discussions to teach the appropriate use of testosterone therapy in men with hypogonadism. The target audience was PCPs, residents, and other specialists who might prescribe testosterone.
Retrospective Chart Review
To assess the impact of the new testosterone order template on adherence to OIG recommendations, a retrospective chart review was completed comparing the appropriateness of initiating testosterone replacement therapy pretemplate period (July 1 to December 31, 2018) vs posttemplate period (July 1 to December 31, 2019). Inclusion and exclusion criteria were modeled after the 2018 OIG report to allow for comparison with the OIG study population. Eligible veterans in each time period included males who received a new testosterone prescription without having been prescribed testosterone in the previous 12 months. Exclusion criteria included community care network prescriptions (CCNRx); current testosterone prescription from a different VA site; clinic administration of testosterone in the previous 12 months; an organic hypogonadism (ie, Klinefelter syndrome) or gender dysphoria diagnosis; and whether the testosterone prescription was never dispensed (PADR was denied or veteran never had the prescription filled). Veterans who met the inclusion criteria in CPRS were identified by an algorithm developed by the VAPSHCS pharmacoeconomist.
Determining the appropriateness of testosterone prescribing, such as symptoms and laboratory measurements to confirm the diagnosis of hypogonadism, was based on the OIG report and Endocrine Society guidelines. A chart review of the 12 months before testosterone prescribing was completed for each veteran, assessing for documentation of symptoms of testosterone deficiency and laboratory measurements of serum testosterone, LH, and FSH. Also, documentation of a discussion of risks and benefits of testosterone therapy in the 3 months before prescribing was assessed, which matched the timeframe in the VA OIG report.
Interim Analysis
After initial template implementation, the multidisciplinary workgroup reconvened for a preplanned interim analysis in November 2019. The evaluation at this meeting revealed multiple order pathways in CPRS that were not linked to the PADR testosterone order template. Testosterone could be ordered in the generic order dialog, medications by drug class, and medications by alphabet, and endocrinology specialty menus without prompting to complete the testosterone order template or redirection to the restricted drug menu (Figure 2). These alternative testosterone ordering pathways were removed in early December 2019 and additional data collection was conducted for 3 months after discontinuation of alternative order pathways, the posttemplate/no alternative ordering pathways period, from December 7, 2019 to February 29, 2020.
Exclusion of Previous Testosterone Prescriptions Predating Chart Review Period, Subgroup Analysis
In the OIG report and the initial retrospective chart review, only veterans without a testosterone prescription in the previous 12 months were evaluated. To assess whether a previous testosterone prescription influenced completion of the PADR and order template, a further subgroup analysis was conducted that excluded veterans who had a previous testosterone prescription at any time before the chart review periods. Therefore, “new testosterone prescription” refers to a veteran who never had a history of being on testosterone vs “former testosterone prescription,” meaning a patient could have had a previous testosterone prescription > 1 year prior to a new VA testosterone prescription.
Results
One hundred seventy-five veterans with a new testosterone prescription were identified in the pretemplate period; of these 80 (46%) met eligibility criteria; only 20 eligible veterans (25%) had a completed PADR (Figure 3). Ninety-one veterans with a new testosterone prescription were identified in the posttemplate period of which 41 (46%) veterans were eligible; 18 eligible veterans (44%) had a completed PADR, but only 7 (17%) had a completed testosterone order template.
After excluding veterans who had alternative ordering pathways for testosterone, 46 veterans were identified in the posttemplate/no alternative ordering pathways period of which 19 (41%) veterans were eligible. Compared with the posttemplate period, a higher proportion of eligible veterans, 68% (13) had a completed PADR, and 58% (11) had a testosterone order template during the posttemplate/no alternative ordering pathways period.
Compared with the OIG report findings, a similar percentage of veterans at VAPSHCS in the pretemplate period had documented clinical symptoms of testosterone deficiency and documented discussion of risks and benefits of testosterone therapy (Figure 4). However, a higher percentage of veterans had biochemical confirmation of testosterone deficiency with ≥ 2 low testosterone levels and evaluation of LH and FSH levels in the pretemplate period (23%) vs that in the OIG report (2%).
Compared with the pretemplate period, activation of the testosterone ordering template in the posttemplate period (Figure 4) had little effect on documented clinical symptoms and discussion of risks and benefits of testosterone treatment. However, the percentage of veterans who had ≥ 2 low testosterone levels and gonadotropins tested was higher in the posttemplate period (41%) vs both the pretemplate period and OIG report.
After removing alternative ordering pathways of testosterone, the percentages of veterans who had documented clinical symptoms, discussion of risks and benefits of testosterone, and ≥ 2 low testosterone levels and gonadotropin tests performed were similar in the posttemplate/no alternative ordering pathways vs posttemplate period.
Excluding veterans who had previously received a former testosterone prescription at any time prior to chart review periods, this subgroup analysis resulted in greater adherence to Endocrine Society guidelines for testosterone treatment with introduction of the testosterone order template, particularly after removal of alternative ordering pathway (Figure 5). With the exclusion of veterans who formerly received testosterone prescriptions, the percentages of veterans who had documented clinical symptoms, discussion of risks and benefits, and ≥ 2 low testosterone levels with gonadotropin tests were higher (100%, 57%, and 71%, respectively) in the posttemplate/no alternative ordering pathways period, compared with the pretemplate period (86%, 30%, and 32%, respectively).
Discussion
The 2018 OIG report found that VA practitioners demonstrated poor adherence to evidence-based clinical practice guidelines for testosterone treatment in men with hypogonadism. Based on OIG recommendations, we developed a PADR testosterone ordering template to help HCPs improve practice by better adherence to guidelines for the diagnosis and treatment of hypogonadism in veterans. Before implementation of the PADR template, the percentage of veterans at VAPSHCS who had biochemical confirmation of hypogonadism was higher than that in the OIG report. Activation of the PADR testosterone ordering template (with or without removal of options for alternative ordering pathways of testosterone) resulted only in an improvement of laboratory confirmation and evaluation of etiology of hypogonadism. This is when we reasoned that clinicians may have access to prior records and laboratory testing beyond just the past year, and this information may have influenced their use of the PADR template. Subsequently, with exclusion of veterans who were previously prescribed testosterone, implementation of the PADR testosterone order template improved documentation of symptoms of testosterone deficiency, discussion of risks and benefits of testosterone therapy, and biochemical diagnosis and evaluation of hypogonadism relative to the period before implementation.
The lack of effects of implementing the testosterone order template on documentation of symptoms of testosterone deficiency and discussion of risks and benefits of testosterone therapy may be due to local expertise resulting in the relatively high adherence to these guideline recommendations at VAPSHCS before activation of the template vs that in the OIG report. The template improved documentation of the diagnosis and evaluation of hypogonadism for genuinely new testosterone prescriptions in veterans without a history of testosterone prescriptions; while those with a previous prescription had limited improvement. It is possible that in veterans who had testosterone prescribed previously, HCPs may have assumed or had bias that the diagnosis and evaluation of hypogonadism originally made was adequate. This finding underscores the need to develop strategies for reviewing PADR requests where there is historical testosterone use. Perhaps a clinical team member, such as a clinical pharmacist, with the background and training in guidelines for the evaluation of hypogonadism could review PADR requests in veterans with previous testosterone use.
Removal of alternative ordering pathways for testosterone increased the completion rate of PADR requests and the testosterone ordering template, although the latter was not completed in one-third of veterans. Possible reasons for HCPs’ suboptimal completion of the testosterone template despite the PADR initiation include clinicians’ lack of willingness to read the PADR completely and familiarize themselves with the clinical guidelines due to workload demands of PCPs. In addition there maybe pressure from patients to receive testosterone for age-related symptoms due to heavy marketing. In addition, there may have been pharmacists who reviewed the PADR and approved the incomplete testosterone template. At VAPSHCS there were up to 40 pharmacists during different periods reviewing the testosterone PADRs. Likely, not everyone was completely familiar with this implementation process, and a possible future consideration would be further education to staff pharmacists who are verifying these prescriptions. There were several advantages to using this new testosterone order template when HCPs attempted to order a prescription. First, they were prompted to complete the PADR. Subsequently, a pharmacist reviewed the template and approved or rejected the prescription if the template was incomplete. The completed template served as documentation in the electronic health record for the prescribing HCP. The template was constructed to populate the required laboratory tests for ease of use and documentation. In addition, educational information regarding the symptoms and signs of testosterone deficiency, laboratory tests needed to confirm and evaluate hypogonadism, contraindications to testosterone treatment, and risks and benefits of therapy were incorporated into the template to assist HCPs in understanding the requirements for a complete diagnosis and evaluation. Finally, on completion of the template, HCPs were able to order testosterone via link to various testosterone formulations.
Before its implementation, the PADR testosterone order template was introduced to PCPs and internal medicine residents at 2 case-based conferences aimed at the diagnosis and treatment of male hypogonadism. These conferences were well received and helped launch the testosterone PADR template at VAPSHCS. Similar outreach to HCPs who prescribe testosterone is highly recommended in other VA facilities before implementation of the testosterone ordering template. It is possible that more targeted education to other HCPs would have resulted in greater use of the testosterone ordering template and adherence to clinical practice guidelines.
The VAPSHCS multidisciplinary workgroup was essential for the development, implementation, evaluation, and revision of the PADR and testosterone ordering template. The workgroup met routinely to follow up on the ease of installation in CPRS and discuss technical corrections that were needed. This was an essential for quality improvement, as loopholes in CPRS were identified where the HCP could order testosterone without being prompted to use the new PADR testosterone order template (alternative ordering pathways). The workgroup swiftly informed the IT specialist and HPS team to remove alternative ordering pathways of testosterone. Continuous quality improvement evaluations are highly recommended during implementation of the template in other facilities to accommodate specific local modifications that might be needed.
After February 2020 due to the COVID-19 pandemic, the National VA Pharmacy and Medication Board halted PADR requirements. As a result, further evaluation of the New Testosterone Order template and planned initial assessment of First Renewal Testosterone Order template could not be performed. In addition, due to the COVID-19 pandemic, there was restricted in-person outpatient visits and reduced adjustments to prescribing practices. To address recommendations made in the OIG report, the VAPSHCS testosterone order template was modified into a clinical reminder dialog format by a VA National IT Specialist and HPS team, tested for usability at several VA test sites and approved by the National Clinical Template Workgroup for implementation nationally across all VAs. The National Endocrinology Ambulatory Council Workgroup will ensure that this template is adopted in a similar format when the new electronic health record system Cerner is introduced to the VA.
Conclusions
The creation and implementation of a PADR testosterone order template may be a beneficial approach to improve the diagnosis of hypogonadism and facilitate appropriate use of testosterone therapy in veterans in accordance with established clinical practice guidelines, particularly in veterans without any prior testosterone use. Key future strategies to improve testosterone prescribing should focus on identifying clinical team members, such as a local clinical pharmacist, to review and steward PADR requests to ensure that testosterone is indicated, and treatment is appropriately monitored.
1. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102(3):1067-1075. doi:10.1210/jc.2016-3580
3. Baillargeon J, Urban RJ, Kuo YF, et al. Screening and monitoring in men prescribed testosterone therapy in the US, 2001-2010. Public Health Rep. 2015;130(2):143-152. doi:10.1177/003335491513000207
4. Baillargeon J, Kuo Y, Westra JR, Urban RJ, Goodwin JS. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320(2):200-202. doi:10.1001/jama.2018.7999
5. Jasuja GK, Bhasin S, Reisman JI, Berlowitz DR, Rose AJ. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53(9):746-52. doi:10.1097/MLR.0000000000000398
6. Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):240-245. doi:10.1097/MED.0000000000000336
7. US Department of Veterans Affairs, Office of Inspector General. Office of Healthcare Inspections. Report No. 15-03215-154. Published April 11, 2018. Accessed February 24, 2021. https://www.va.gov/oig/pubs/VAOIG-15-03215-154.pdf
Testosterone treatment is clinically indicated when a patient presents with symptoms and signs and biochemical evidence of testosterone deficiency, ie, male hypogonadism. Laboratory confirmation of hypogonadism requires repeatedly low serum testosterone concentrations; between 8
Recent studies have reported an increase in testosterone prescriptions and raised concerns regarding health care provider (HCP) prescribing practices despite current clinical practice guidelines from major societies, such as the Endocrine Society. In the US from 2001 to 2011, testosterone use among men aged ≥ 40 years increased more than 3-fold in all age groups.3 Subsequently in the years from 2013 to 2016, prescription rates declined perhaps due to the cardiovascular and stroke concerns.4
In the US Department of Veterans Affairs (VA), new testosterone prescriptions across VA medical centers increased from 20,437 in fiscal year (FY) 2009 to 36,394 in FY 2012. Yet only 3.1% of men who received testosterone therapy had 2 or more low morning total or free testosterone concentrations measured; LH and/or FSH levels assessed; and presence of contraindications to therapy documented. Remarkably, 16.5% of these veterans did not have a testosterone level tested prior to being prescribed testosterone. Among veterans who were prescribed testosterone, 1.4% had a diagnosis of prostate cancer, 7.6% had a diagnosis of obstructive sleep apnea (OSA), and 3.5% had elevated hematocrit at baseline.5 These findings raised concerns of whether the diagnosis and etiology of hypogonadism were appropriately established and risks were considered before testosterone treatment was initiated.5,6
To further understand VA prescribing practices of testosterone therapy, a 2018 VA Office of the Inspector General (OIG) report evaluated the initiation and follow-up of testosterone replacement therapy. The OIG randomly sampled and reviewed 1,091 male patients who filled at least 1 outpatient testosterone prescription from VA in FY 2014 and who did not have a prior testosterone prescription in FY 2013. Patients were followed through September 30, 2015. Within 1 year prior to initiating testosterone, only 1.5% had clinical signs and symptoms of testosterone deficiency documented prior to testosterone testing (76% within 18 months of starting testosterone); only 9.1% of veterans had the recommended measurements of 2 low morning testosterone levels; and only 12% had LH and FSH levels measured. Within 3 to 6 months after starting testosterone therapy, only 24% of veterans were assessed for symptom improvement, and 29% to 33% were evaluated for adverse effects, hematocrit levels and adherence to the therapy. The OIG report concluded that VA HCPs were not adhering to guidelines (referencing the Endocrine Society guidelines) when evaluating and treating veterans with testosterone deficiency.7
Considering the OIG recommendations and need to improve current practices among providers, VA Puget Sound Health Care System (VAPSHCS) in Washington established a multidisciplinary workgroup consisting of an endocrinologist, geriatrician, primary care provider (PCP), pharmacists, VA information technology (IT) specialist, and health products support (HPS) clinical team in the spring of 2019 to assess and improve testosterone prescribing practices.
Methods
A testosterone order template was developed, approved by VAPSHCS Pharmacy and Therapeutics Committee, and implemented on July 1, 2019, at VAPSHCS, a 1a medical facility caring for more than 112,000 veterans. Given its potential risks and the propensity for varied prescribing practices, testosterone was designated as a restricted drug requiring a prior authorization drug request (PADR) and required completion of the testosterone order template in the Computerized Patient Record System (CPRS).
Testosterone Order Template
The testosterone order template had 2 components. Completion of the template for new testosterone orders was required to initiate treatment unless the patient had known organic hypogonadism or was a transgender male. The template ensured documentation of defined symptoms and signs of testosterone deficiency; low serum testosterone levels on at least 2 occasions and LH and FSH concentrations; no contraindications to testosterone treatment; discussion of risks and benefits of therapy; and baseline hematocrit (Figure 1). Relevant educational content (eg, risks and benefits of testosterone) was incorporated in the template. The second template was required for the first renewal of testosterone to document adherence to or reason for discontinuation of testosterone; improvement of symptoms and signs; and confirm monitoring hematocrit and testosterone levels during treatment.
Prior to implementation, the PADR template was introduced to HCPs at 2 chief-of-medicine rounds on the diagnosis and evaluation of hypogonadism by a pharmacist and endocrinologist. These educational sessions used case examples and discussions to teach the appropriate use of testosterone therapy in men with hypogonadism. The target audience was PCPs, residents, and other specialists who might prescribe testosterone.
Retrospective Chart Review
To assess the impact of the new testosterone order template on adherence to OIG recommendations, a retrospective chart review was completed comparing the appropriateness of initiating testosterone replacement therapy pretemplate period (July 1 to December 31, 2018) vs posttemplate period (July 1 to December 31, 2019). Inclusion and exclusion criteria were modeled after the 2018 OIG report to allow for comparison with the OIG study population. Eligible veterans in each time period included males who received a new testosterone prescription without having been prescribed testosterone in the previous 12 months. Exclusion criteria included community care network prescriptions (CCNRx); current testosterone prescription from a different VA site; clinic administration of testosterone in the previous 12 months; an organic hypogonadism (ie, Klinefelter syndrome) or gender dysphoria diagnosis; and whether the testosterone prescription was never dispensed (PADR was denied or veteran never had the prescription filled). Veterans who met the inclusion criteria in CPRS were identified by an algorithm developed by the VAPSHCS pharmacoeconomist.
Determining the appropriateness of testosterone prescribing, such as symptoms and laboratory measurements to confirm the diagnosis of hypogonadism, was based on the OIG report and Endocrine Society guidelines. A chart review of the 12 months before testosterone prescribing was completed for each veteran, assessing for documentation of symptoms of testosterone deficiency and laboratory measurements of serum testosterone, LH, and FSH. Also, documentation of a discussion of risks and benefits of testosterone therapy in the 3 months before prescribing was assessed, which matched the timeframe in the VA OIG report.
Interim Analysis
After initial template implementation, the multidisciplinary workgroup reconvened for a preplanned interim analysis in November 2019. The evaluation at this meeting revealed multiple order pathways in CPRS that were not linked to the PADR testosterone order template. Testosterone could be ordered in the generic order dialog, medications by drug class, and medications by alphabet, and endocrinology specialty menus without prompting to complete the testosterone order template or redirection to the restricted drug menu (Figure 2). These alternative testosterone ordering pathways were removed in early December 2019 and additional data collection was conducted for 3 months after discontinuation of alternative order pathways, the posttemplate/no alternative ordering pathways period, from December 7, 2019 to February 29, 2020.
Exclusion of Previous Testosterone Prescriptions Predating Chart Review Period, Subgroup Analysis
In the OIG report and the initial retrospective chart review, only veterans without a testosterone prescription in the previous 12 months were evaluated. To assess whether a previous testosterone prescription influenced completion of the PADR and order template, a further subgroup analysis was conducted that excluded veterans who had a previous testosterone prescription at any time before the chart review periods. Therefore, “new testosterone prescription” refers to a veteran who never had a history of being on testosterone vs “former testosterone prescription,” meaning a patient could have had a previous testosterone prescription > 1 year prior to a new VA testosterone prescription.
Results
One hundred seventy-five veterans with a new testosterone prescription were identified in the pretemplate period; of these 80 (46%) met eligibility criteria; only 20 eligible veterans (25%) had a completed PADR (Figure 3). Ninety-one veterans with a new testosterone prescription were identified in the posttemplate period of which 41 (46%) veterans were eligible; 18 eligible veterans (44%) had a completed PADR, but only 7 (17%) had a completed testosterone order template.
After excluding veterans who had alternative ordering pathways for testosterone, 46 veterans were identified in the posttemplate/no alternative ordering pathways period of which 19 (41%) veterans were eligible. Compared with the posttemplate period, a higher proportion of eligible veterans, 68% (13) had a completed PADR, and 58% (11) had a testosterone order template during the posttemplate/no alternative ordering pathways period.
Compared with the OIG report findings, a similar percentage of veterans at VAPSHCS in the pretemplate period had documented clinical symptoms of testosterone deficiency and documented discussion of risks and benefits of testosterone therapy (Figure 4). However, a higher percentage of veterans had biochemical confirmation of testosterone deficiency with ≥ 2 low testosterone levels and evaluation of LH and FSH levels in the pretemplate period (23%) vs that in the OIG report (2%).
Compared with the pretemplate period, activation of the testosterone ordering template in the posttemplate period (Figure 4) had little effect on documented clinical symptoms and discussion of risks and benefits of testosterone treatment. However, the percentage of veterans who had ≥ 2 low testosterone levels and gonadotropins tested was higher in the posttemplate period (41%) vs both the pretemplate period and OIG report.
After removing alternative ordering pathways of testosterone, the percentages of veterans who had documented clinical symptoms, discussion of risks and benefits of testosterone, and ≥ 2 low testosterone levels and gonadotropin tests performed were similar in the posttemplate/no alternative ordering pathways vs posttemplate period.
Excluding veterans who had previously received a former testosterone prescription at any time prior to chart review periods, this subgroup analysis resulted in greater adherence to Endocrine Society guidelines for testosterone treatment with introduction of the testosterone order template, particularly after removal of alternative ordering pathway (Figure 5). With the exclusion of veterans who formerly received testosterone prescriptions, the percentages of veterans who had documented clinical symptoms, discussion of risks and benefits, and ≥ 2 low testosterone levels with gonadotropin tests were higher (100%, 57%, and 71%, respectively) in the posttemplate/no alternative ordering pathways period, compared with the pretemplate period (86%, 30%, and 32%, respectively).
Discussion
The 2018 OIG report found that VA practitioners demonstrated poor adherence to evidence-based clinical practice guidelines for testosterone treatment in men with hypogonadism. Based on OIG recommendations, we developed a PADR testosterone ordering template to help HCPs improve practice by better adherence to guidelines for the diagnosis and treatment of hypogonadism in veterans. Before implementation of the PADR template, the percentage of veterans at VAPSHCS who had biochemical confirmation of hypogonadism was higher than that in the OIG report. Activation of the PADR testosterone ordering template (with or without removal of options for alternative ordering pathways of testosterone) resulted only in an improvement of laboratory confirmation and evaluation of etiology of hypogonadism. This is when we reasoned that clinicians may have access to prior records and laboratory testing beyond just the past year, and this information may have influenced their use of the PADR template. Subsequently, with exclusion of veterans who were previously prescribed testosterone, implementation of the PADR testosterone order template improved documentation of symptoms of testosterone deficiency, discussion of risks and benefits of testosterone therapy, and biochemical diagnosis and evaluation of hypogonadism relative to the period before implementation.
The lack of effects of implementing the testosterone order template on documentation of symptoms of testosterone deficiency and discussion of risks and benefits of testosterone therapy may be due to local expertise resulting in the relatively high adherence to these guideline recommendations at VAPSHCS before activation of the template vs that in the OIG report. The template improved documentation of the diagnosis and evaluation of hypogonadism for genuinely new testosterone prescriptions in veterans without a history of testosterone prescriptions; while those with a previous prescription had limited improvement. It is possible that in veterans who had testosterone prescribed previously, HCPs may have assumed or had bias that the diagnosis and evaluation of hypogonadism originally made was adequate. This finding underscores the need to develop strategies for reviewing PADR requests where there is historical testosterone use. Perhaps a clinical team member, such as a clinical pharmacist, with the background and training in guidelines for the evaluation of hypogonadism could review PADR requests in veterans with previous testosterone use.
Removal of alternative ordering pathways for testosterone increased the completion rate of PADR requests and the testosterone ordering template, although the latter was not completed in one-third of veterans. Possible reasons for HCPs’ suboptimal completion of the testosterone template despite the PADR initiation include clinicians’ lack of willingness to read the PADR completely and familiarize themselves with the clinical guidelines due to workload demands of PCPs. In addition there maybe pressure from patients to receive testosterone for age-related symptoms due to heavy marketing. In addition, there may have been pharmacists who reviewed the PADR and approved the incomplete testosterone template. At VAPSHCS there were up to 40 pharmacists during different periods reviewing the testosterone PADRs. Likely, not everyone was completely familiar with this implementation process, and a possible future consideration would be further education to staff pharmacists who are verifying these prescriptions. There were several advantages to using this new testosterone order template when HCPs attempted to order a prescription. First, they were prompted to complete the PADR. Subsequently, a pharmacist reviewed the template and approved or rejected the prescription if the template was incomplete. The completed template served as documentation in the electronic health record for the prescribing HCP. The template was constructed to populate the required laboratory tests for ease of use and documentation. In addition, educational information regarding the symptoms and signs of testosterone deficiency, laboratory tests needed to confirm and evaluate hypogonadism, contraindications to testosterone treatment, and risks and benefits of therapy were incorporated into the template to assist HCPs in understanding the requirements for a complete diagnosis and evaluation. Finally, on completion of the template, HCPs were able to order testosterone via link to various testosterone formulations.
Before its implementation, the PADR testosterone order template was introduced to PCPs and internal medicine residents at 2 case-based conferences aimed at the diagnosis and treatment of male hypogonadism. These conferences were well received and helped launch the testosterone PADR template at VAPSHCS. Similar outreach to HCPs who prescribe testosterone is highly recommended in other VA facilities before implementation of the testosterone ordering template. It is possible that more targeted education to other HCPs would have resulted in greater use of the testosterone ordering template and adherence to clinical practice guidelines.
The VAPSHCS multidisciplinary workgroup was essential for the development, implementation, evaluation, and revision of the PADR and testosterone ordering template. The workgroup met routinely to follow up on the ease of installation in CPRS and discuss technical corrections that were needed. This was an essential for quality improvement, as loopholes in CPRS were identified where the HCP could order testosterone without being prompted to use the new PADR testosterone order template (alternative ordering pathways). The workgroup swiftly informed the IT specialist and HPS team to remove alternative ordering pathways of testosterone. Continuous quality improvement evaluations are highly recommended during implementation of the template in other facilities to accommodate specific local modifications that might be needed.
After February 2020 due to the COVID-19 pandemic, the National VA Pharmacy and Medication Board halted PADR requirements. As a result, further evaluation of the New Testosterone Order template and planned initial assessment of First Renewal Testosterone Order template could not be performed. In addition, due to the COVID-19 pandemic, there was restricted in-person outpatient visits and reduced adjustments to prescribing practices. To address recommendations made in the OIG report, the VAPSHCS testosterone order template was modified into a clinical reminder dialog format by a VA National IT Specialist and HPS team, tested for usability at several VA test sites and approved by the National Clinical Template Workgroup for implementation nationally across all VAs. The National Endocrinology Ambulatory Council Workgroup will ensure that this template is adopted in a similar format when the new electronic health record system Cerner is introduced to the VA.
Conclusions
The creation and implementation of a PADR testosterone order template may be a beneficial approach to improve the diagnosis of hypogonadism and facilitate appropriate use of testosterone therapy in veterans in accordance with established clinical practice guidelines, particularly in veterans without any prior testosterone use. Key future strategies to improve testosterone prescribing should focus on identifying clinical team members, such as a local clinical pharmacist, to review and steward PADR requests to ensure that testosterone is indicated, and treatment is appropriately monitored.
Testosterone treatment is clinically indicated when a patient presents with symptoms and signs and biochemical evidence of testosterone deficiency, ie, male hypogonadism. Laboratory confirmation of hypogonadism requires repeatedly low serum testosterone concentrations; between 8
Recent studies have reported an increase in testosterone prescriptions and raised concerns regarding health care provider (HCP) prescribing practices despite current clinical practice guidelines from major societies, such as the Endocrine Society. In the US from 2001 to 2011, testosterone use among men aged ≥ 40 years increased more than 3-fold in all age groups.3 Subsequently in the years from 2013 to 2016, prescription rates declined perhaps due to the cardiovascular and stroke concerns.4
In the US Department of Veterans Affairs (VA), new testosterone prescriptions across VA medical centers increased from 20,437 in fiscal year (FY) 2009 to 36,394 in FY 2012. Yet only 3.1% of men who received testosterone therapy had 2 or more low morning total or free testosterone concentrations measured; LH and/or FSH levels assessed; and presence of contraindications to therapy documented. Remarkably, 16.5% of these veterans did not have a testosterone level tested prior to being prescribed testosterone. Among veterans who were prescribed testosterone, 1.4% had a diagnosis of prostate cancer, 7.6% had a diagnosis of obstructive sleep apnea (OSA), and 3.5% had elevated hematocrit at baseline.5 These findings raised concerns of whether the diagnosis and etiology of hypogonadism were appropriately established and risks were considered before testosterone treatment was initiated.5,6
To further understand VA prescribing practices of testosterone therapy, a 2018 VA Office of the Inspector General (OIG) report evaluated the initiation and follow-up of testosterone replacement therapy. The OIG randomly sampled and reviewed 1,091 male patients who filled at least 1 outpatient testosterone prescription from VA in FY 2014 and who did not have a prior testosterone prescription in FY 2013. Patients were followed through September 30, 2015. Within 1 year prior to initiating testosterone, only 1.5% had clinical signs and symptoms of testosterone deficiency documented prior to testosterone testing (76% within 18 months of starting testosterone); only 9.1% of veterans had the recommended measurements of 2 low morning testosterone levels; and only 12% had LH and FSH levels measured. Within 3 to 6 months after starting testosterone therapy, only 24% of veterans were assessed for symptom improvement, and 29% to 33% were evaluated for adverse effects, hematocrit levels and adherence to the therapy. The OIG report concluded that VA HCPs were not adhering to guidelines (referencing the Endocrine Society guidelines) when evaluating and treating veterans with testosterone deficiency.7
Considering the OIG recommendations and need to improve current practices among providers, VA Puget Sound Health Care System (VAPSHCS) in Washington established a multidisciplinary workgroup consisting of an endocrinologist, geriatrician, primary care provider (PCP), pharmacists, VA information technology (IT) specialist, and health products support (HPS) clinical team in the spring of 2019 to assess and improve testosterone prescribing practices.
Methods
A testosterone order template was developed, approved by VAPSHCS Pharmacy and Therapeutics Committee, and implemented on July 1, 2019, at VAPSHCS, a 1a medical facility caring for more than 112,000 veterans. Given its potential risks and the propensity for varied prescribing practices, testosterone was designated as a restricted drug requiring a prior authorization drug request (PADR) and required completion of the testosterone order template in the Computerized Patient Record System (CPRS).
Testosterone Order Template
The testosterone order template had 2 components. Completion of the template for new testosterone orders was required to initiate treatment unless the patient had known organic hypogonadism or was a transgender male. The template ensured documentation of defined symptoms and signs of testosterone deficiency; low serum testosterone levels on at least 2 occasions and LH and FSH concentrations; no contraindications to testosterone treatment; discussion of risks and benefits of therapy; and baseline hematocrit (Figure 1). Relevant educational content (eg, risks and benefits of testosterone) was incorporated in the template. The second template was required for the first renewal of testosterone to document adherence to or reason for discontinuation of testosterone; improvement of symptoms and signs; and confirm monitoring hematocrit and testosterone levels during treatment.
Prior to implementation, the PADR template was introduced to HCPs at 2 chief-of-medicine rounds on the diagnosis and evaluation of hypogonadism by a pharmacist and endocrinologist. These educational sessions used case examples and discussions to teach the appropriate use of testosterone therapy in men with hypogonadism. The target audience was PCPs, residents, and other specialists who might prescribe testosterone.
Retrospective Chart Review
To assess the impact of the new testosterone order template on adherence to OIG recommendations, a retrospective chart review was completed comparing the appropriateness of initiating testosterone replacement therapy pretemplate period (July 1 to December 31, 2018) vs posttemplate period (July 1 to December 31, 2019). Inclusion and exclusion criteria were modeled after the 2018 OIG report to allow for comparison with the OIG study population. Eligible veterans in each time period included males who received a new testosterone prescription without having been prescribed testosterone in the previous 12 months. Exclusion criteria included community care network prescriptions (CCNRx); current testosterone prescription from a different VA site; clinic administration of testosterone in the previous 12 months; an organic hypogonadism (ie, Klinefelter syndrome) or gender dysphoria diagnosis; and whether the testosterone prescription was never dispensed (PADR was denied or veteran never had the prescription filled). Veterans who met the inclusion criteria in CPRS were identified by an algorithm developed by the VAPSHCS pharmacoeconomist.
Determining the appropriateness of testosterone prescribing, such as symptoms and laboratory measurements to confirm the diagnosis of hypogonadism, was based on the OIG report and Endocrine Society guidelines. A chart review of the 12 months before testosterone prescribing was completed for each veteran, assessing for documentation of symptoms of testosterone deficiency and laboratory measurements of serum testosterone, LH, and FSH. Also, documentation of a discussion of risks and benefits of testosterone therapy in the 3 months before prescribing was assessed, which matched the timeframe in the VA OIG report.
Interim Analysis
After initial template implementation, the multidisciplinary workgroup reconvened for a preplanned interim analysis in November 2019. The evaluation at this meeting revealed multiple order pathways in CPRS that were not linked to the PADR testosterone order template. Testosterone could be ordered in the generic order dialog, medications by drug class, and medications by alphabet, and endocrinology specialty menus without prompting to complete the testosterone order template or redirection to the restricted drug menu (Figure 2). These alternative testosterone ordering pathways were removed in early December 2019 and additional data collection was conducted for 3 months after discontinuation of alternative order pathways, the posttemplate/no alternative ordering pathways period, from December 7, 2019 to February 29, 2020.
Exclusion of Previous Testosterone Prescriptions Predating Chart Review Period, Subgroup Analysis
In the OIG report and the initial retrospective chart review, only veterans without a testosterone prescription in the previous 12 months were evaluated. To assess whether a previous testosterone prescription influenced completion of the PADR and order template, a further subgroup analysis was conducted that excluded veterans who had a previous testosterone prescription at any time before the chart review periods. Therefore, “new testosterone prescription” refers to a veteran who never had a history of being on testosterone vs “former testosterone prescription,” meaning a patient could have had a previous testosterone prescription > 1 year prior to a new VA testosterone prescription.
Results
One hundred seventy-five veterans with a new testosterone prescription were identified in the pretemplate period; of these 80 (46%) met eligibility criteria; only 20 eligible veterans (25%) had a completed PADR (Figure 3). Ninety-one veterans with a new testosterone prescription were identified in the posttemplate period of which 41 (46%) veterans were eligible; 18 eligible veterans (44%) had a completed PADR, but only 7 (17%) had a completed testosterone order template.
After excluding veterans who had alternative ordering pathways for testosterone, 46 veterans were identified in the posttemplate/no alternative ordering pathways period of which 19 (41%) veterans were eligible. Compared with the posttemplate period, a higher proportion of eligible veterans, 68% (13) had a completed PADR, and 58% (11) had a testosterone order template during the posttemplate/no alternative ordering pathways period.
Compared with the OIG report findings, a similar percentage of veterans at VAPSHCS in the pretemplate period had documented clinical symptoms of testosterone deficiency and documented discussion of risks and benefits of testosterone therapy (Figure 4). However, a higher percentage of veterans had biochemical confirmation of testosterone deficiency with ≥ 2 low testosterone levels and evaluation of LH and FSH levels in the pretemplate period (23%) vs that in the OIG report (2%).
Compared with the pretemplate period, activation of the testosterone ordering template in the posttemplate period (Figure 4) had little effect on documented clinical symptoms and discussion of risks and benefits of testosterone treatment. However, the percentage of veterans who had ≥ 2 low testosterone levels and gonadotropins tested was higher in the posttemplate period (41%) vs both the pretemplate period and OIG report.
After removing alternative ordering pathways of testosterone, the percentages of veterans who had documented clinical symptoms, discussion of risks and benefits of testosterone, and ≥ 2 low testosterone levels and gonadotropin tests performed were similar in the posttemplate/no alternative ordering pathways vs posttemplate period.
Excluding veterans who had previously received a former testosterone prescription at any time prior to chart review periods, this subgroup analysis resulted in greater adherence to Endocrine Society guidelines for testosterone treatment with introduction of the testosterone order template, particularly after removal of alternative ordering pathway (Figure 5). With the exclusion of veterans who formerly received testosterone prescriptions, the percentages of veterans who had documented clinical symptoms, discussion of risks and benefits, and ≥ 2 low testosterone levels with gonadotropin tests were higher (100%, 57%, and 71%, respectively) in the posttemplate/no alternative ordering pathways period, compared with the pretemplate period (86%, 30%, and 32%, respectively).
Discussion
The 2018 OIG report found that VA practitioners demonstrated poor adherence to evidence-based clinical practice guidelines for testosterone treatment in men with hypogonadism. Based on OIG recommendations, we developed a PADR testosterone ordering template to help HCPs improve practice by better adherence to guidelines for the diagnosis and treatment of hypogonadism in veterans. Before implementation of the PADR template, the percentage of veterans at VAPSHCS who had biochemical confirmation of hypogonadism was higher than that in the OIG report. Activation of the PADR testosterone ordering template (with or without removal of options for alternative ordering pathways of testosterone) resulted only in an improvement of laboratory confirmation and evaluation of etiology of hypogonadism. This is when we reasoned that clinicians may have access to prior records and laboratory testing beyond just the past year, and this information may have influenced their use of the PADR template. Subsequently, with exclusion of veterans who were previously prescribed testosterone, implementation of the PADR testosterone order template improved documentation of symptoms of testosterone deficiency, discussion of risks and benefits of testosterone therapy, and biochemical diagnosis and evaluation of hypogonadism relative to the period before implementation.
The lack of effects of implementing the testosterone order template on documentation of symptoms of testosterone deficiency and discussion of risks and benefits of testosterone therapy may be due to local expertise resulting in the relatively high adherence to these guideline recommendations at VAPSHCS before activation of the template vs that in the OIG report. The template improved documentation of the diagnosis and evaluation of hypogonadism for genuinely new testosterone prescriptions in veterans without a history of testosterone prescriptions; while those with a previous prescription had limited improvement. It is possible that in veterans who had testosterone prescribed previously, HCPs may have assumed or had bias that the diagnosis and evaluation of hypogonadism originally made was adequate. This finding underscores the need to develop strategies for reviewing PADR requests where there is historical testosterone use. Perhaps a clinical team member, such as a clinical pharmacist, with the background and training in guidelines for the evaluation of hypogonadism could review PADR requests in veterans with previous testosterone use.
Removal of alternative ordering pathways for testosterone increased the completion rate of PADR requests and the testosterone ordering template, although the latter was not completed in one-third of veterans. Possible reasons for HCPs’ suboptimal completion of the testosterone template despite the PADR initiation include clinicians’ lack of willingness to read the PADR completely and familiarize themselves with the clinical guidelines due to workload demands of PCPs. In addition there maybe pressure from patients to receive testosterone for age-related symptoms due to heavy marketing. In addition, there may have been pharmacists who reviewed the PADR and approved the incomplete testosterone template. At VAPSHCS there were up to 40 pharmacists during different periods reviewing the testosterone PADRs. Likely, not everyone was completely familiar with this implementation process, and a possible future consideration would be further education to staff pharmacists who are verifying these prescriptions. There were several advantages to using this new testosterone order template when HCPs attempted to order a prescription. First, they were prompted to complete the PADR. Subsequently, a pharmacist reviewed the template and approved or rejected the prescription if the template was incomplete. The completed template served as documentation in the electronic health record for the prescribing HCP. The template was constructed to populate the required laboratory tests for ease of use and documentation. In addition, educational information regarding the symptoms and signs of testosterone deficiency, laboratory tests needed to confirm and evaluate hypogonadism, contraindications to testosterone treatment, and risks and benefits of therapy were incorporated into the template to assist HCPs in understanding the requirements for a complete diagnosis and evaluation. Finally, on completion of the template, HCPs were able to order testosterone via link to various testosterone formulations.
Before its implementation, the PADR testosterone order template was introduced to PCPs and internal medicine residents at 2 case-based conferences aimed at the diagnosis and treatment of male hypogonadism. These conferences were well received and helped launch the testosterone PADR template at VAPSHCS. Similar outreach to HCPs who prescribe testosterone is highly recommended in other VA facilities before implementation of the testosterone ordering template. It is possible that more targeted education to other HCPs would have resulted in greater use of the testosterone ordering template and adherence to clinical practice guidelines.
The VAPSHCS multidisciplinary workgroup was essential for the development, implementation, evaluation, and revision of the PADR and testosterone ordering template. The workgroup met routinely to follow up on the ease of installation in CPRS and discuss technical corrections that were needed. This was an essential for quality improvement, as loopholes in CPRS were identified where the HCP could order testosterone without being prompted to use the new PADR testosterone order template (alternative ordering pathways). The workgroup swiftly informed the IT specialist and HPS team to remove alternative ordering pathways of testosterone. Continuous quality improvement evaluations are highly recommended during implementation of the template in other facilities to accommodate specific local modifications that might be needed.
After February 2020 due to the COVID-19 pandemic, the National VA Pharmacy and Medication Board halted PADR requirements. As a result, further evaluation of the New Testosterone Order template and planned initial assessment of First Renewal Testosterone Order template could not be performed. In addition, due to the COVID-19 pandemic, there was restricted in-person outpatient visits and reduced adjustments to prescribing practices. To address recommendations made in the OIG report, the VAPSHCS testosterone order template was modified into a clinical reminder dialog format by a VA National IT Specialist and HPS team, tested for usability at several VA test sites and approved by the National Clinical Template Workgroup for implementation nationally across all VAs. The National Endocrinology Ambulatory Council Workgroup will ensure that this template is adopted in a similar format when the new electronic health record system Cerner is introduced to the VA.
Conclusions
The creation and implementation of a PADR testosterone order template may be a beneficial approach to improve the diagnosis of hypogonadism and facilitate appropriate use of testosterone therapy in veterans in accordance with established clinical practice guidelines, particularly in veterans without any prior testosterone use. Key future strategies to improve testosterone prescribing should focus on identifying clinical team members, such as a local clinical pharmacist, to review and steward PADR requests to ensure that testosterone is indicated, and treatment is appropriately monitored.
1. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102(3):1067-1075. doi:10.1210/jc.2016-3580
3. Baillargeon J, Urban RJ, Kuo YF, et al. Screening and monitoring in men prescribed testosterone therapy in the US, 2001-2010. Public Health Rep. 2015;130(2):143-152. doi:10.1177/003335491513000207
4. Baillargeon J, Kuo Y, Westra JR, Urban RJ, Goodwin JS. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320(2):200-202. doi:10.1001/jama.2018.7999
5. Jasuja GK, Bhasin S, Reisman JI, Berlowitz DR, Rose AJ. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53(9):746-52. doi:10.1097/MLR.0000000000000398
6. Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):240-245. doi:10.1097/MED.0000000000000336
7. US Department of Veterans Affairs, Office of Inspector General. Office of Healthcare Inspections. Report No. 15-03215-154. Published April 11, 2018. Accessed February 24, 2021. https://www.va.gov/oig/pubs/VAOIG-15-03215-154.pdf
1. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102(3):1067-1075. doi:10.1210/jc.2016-3580
3. Baillargeon J, Urban RJ, Kuo YF, et al. Screening and monitoring in men prescribed testosterone therapy in the US, 2001-2010. Public Health Rep. 2015;130(2):143-152. doi:10.1177/003335491513000207
4. Baillargeon J, Kuo Y, Westra JR, Urban RJ, Goodwin JS. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320(2):200-202. doi:10.1001/jama.2018.7999
5. Jasuja GK, Bhasin S, Reisman JI, Berlowitz DR, Rose AJ. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53(9):746-52. doi:10.1097/MLR.0000000000000398
6. Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):240-245. doi:10.1097/MED.0000000000000336
7. US Department of Veterans Affairs, Office of Inspector General. Office of Healthcare Inspections. Report No. 15-03215-154. Published April 11, 2018. Accessed February 24, 2021. https://www.va.gov/oig/pubs/VAOIG-15-03215-154.pdf
Pityriasis rosea carries few risks for pregnant women
according to a review of 33 patients.
“Though generally considered benign, PR may be associated with an increased risk of birth complications if acquired during pregnancy,” and previous studies have shown increased rates of complications including miscarriage and neonatal hypotonia in these patients, wrote Julian Stashower of the University of Virginia, Charlottesville, and colleagues.
In a retrospective study published in the Journal of the American Academy of Dermatology, the researchers assessed pregnancy outcomes in women who developed PR during pregnancy. They were identified from medical records at three institutions between September 2010 and June 2020. Diagnosis of PR, a papulosquamous skin eruption associated with human herpesvirus (HHV)–6/7 reactivation, was based on history and physical examination.
Overall, 8 of the 33 women (24%) had birth complications; the rates of preterm delivery, spontaneous pregnancy loss in clinically detectable pregnancies, and oligohydramnios were 6%, 0%, and 3%, respectively. The average onset of PR during pregnancy was earlier among women with complications, compared with those without complications (10.75 weeks’ gestation vs. 15.21 weeks’ gestation), but the difference was not statistically significant.
The researchers noted that their findings differed from the most recent study of PR in pregnancy, which included 60 patients and found a notably higher incidence of overall birth complications (50%), as well as higher incidence of neonatal hypotonia (25%), and miscarriage (13%).
The previous study also showed an increased risk of birth complications when PR onset occurred prior to 15 weeks’ gestation, but the current study did not reflect that finding, they wrote.
The current study findings were limited by several factors including the small sample size, retrospective design, and lack of confirmation of PR with HHV-6/7 testing, as well as lack of exclusion of atypical PR cases, the researchers noted. However, the results suggest that birth complications associated with PR may be lower than previously reported. “Further research is needed to guide future care and fully elucidate this possible association, which has important implications for both pregnant women with PR and their providers.”
The study received no outside funding. The researchers had no financial conflict to disclose.
according to a review of 33 patients.
“Though generally considered benign, PR may be associated with an increased risk of birth complications if acquired during pregnancy,” and previous studies have shown increased rates of complications including miscarriage and neonatal hypotonia in these patients, wrote Julian Stashower of the University of Virginia, Charlottesville, and colleagues.
In a retrospective study published in the Journal of the American Academy of Dermatology, the researchers assessed pregnancy outcomes in women who developed PR during pregnancy. They were identified from medical records at three institutions between September 2010 and June 2020. Diagnosis of PR, a papulosquamous skin eruption associated with human herpesvirus (HHV)–6/7 reactivation, was based on history and physical examination.
Overall, 8 of the 33 women (24%) had birth complications; the rates of preterm delivery, spontaneous pregnancy loss in clinically detectable pregnancies, and oligohydramnios were 6%, 0%, and 3%, respectively. The average onset of PR during pregnancy was earlier among women with complications, compared with those without complications (10.75 weeks’ gestation vs. 15.21 weeks’ gestation), but the difference was not statistically significant.
The researchers noted that their findings differed from the most recent study of PR in pregnancy, which included 60 patients and found a notably higher incidence of overall birth complications (50%), as well as higher incidence of neonatal hypotonia (25%), and miscarriage (13%).
The previous study also showed an increased risk of birth complications when PR onset occurred prior to 15 weeks’ gestation, but the current study did not reflect that finding, they wrote.
The current study findings were limited by several factors including the small sample size, retrospective design, and lack of confirmation of PR with HHV-6/7 testing, as well as lack of exclusion of atypical PR cases, the researchers noted. However, the results suggest that birth complications associated with PR may be lower than previously reported. “Further research is needed to guide future care and fully elucidate this possible association, which has important implications for both pregnant women with PR and their providers.”
The study received no outside funding. The researchers had no financial conflict to disclose.
according to a review of 33 patients.
“Though generally considered benign, PR may be associated with an increased risk of birth complications if acquired during pregnancy,” and previous studies have shown increased rates of complications including miscarriage and neonatal hypotonia in these patients, wrote Julian Stashower of the University of Virginia, Charlottesville, and colleagues.
In a retrospective study published in the Journal of the American Academy of Dermatology, the researchers assessed pregnancy outcomes in women who developed PR during pregnancy. They were identified from medical records at three institutions between September 2010 and June 2020. Diagnosis of PR, a papulosquamous skin eruption associated with human herpesvirus (HHV)–6/7 reactivation, was based on history and physical examination.
Overall, 8 of the 33 women (24%) had birth complications; the rates of preterm delivery, spontaneous pregnancy loss in clinically detectable pregnancies, and oligohydramnios were 6%, 0%, and 3%, respectively. The average onset of PR during pregnancy was earlier among women with complications, compared with those without complications (10.75 weeks’ gestation vs. 15.21 weeks’ gestation), but the difference was not statistically significant.
The researchers noted that their findings differed from the most recent study of PR in pregnancy, which included 60 patients and found a notably higher incidence of overall birth complications (50%), as well as higher incidence of neonatal hypotonia (25%), and miscarriage (13%).
The previous study also showed an increased risk of birth complications when PR onset occurred prior to 15 weeks’ gestation, but the current study did not reflect that finding, they wrote.
The current study findings were limited by several factors including the small sample size, retrospective design, and lack of confirmation of PR with HHV-6/7 testing, as well as lack of exclusion of atypical PR cases, the researchers noted. However, the results suggest that birth complications associated with PR may be lower than previously reported. “Further research is needed to guide future care and fully elucidate this possible association, which has important implications for both pregnant women with PR and their providers.”
The study received no outside funding. The researchers had no financial conflict to disclose.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY