User login
Inflammatory back pain likely underrecognized in primary care
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
REPORTING FROM SPARTAN 2020
TNF inhibitors may dampen COVID-19 severity
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
REPORTING FROM SOTA 2020
Proteins in urine may predict active lupus nephritis
according to a cross-sectional study published in Nature Communications. The proteins that best differentiate active lupus nephritis from inactive systemic lupus erythematosus (SLE) vary across ethnicities, the researchers wrote.
“A longitudinal study is warranted to investigate how these molecules relate to disease pathology and progression over time,” said senior study author Chandra Mohan, MD, PhD, of the department of biomedical engineering at the University of Houston, and colleagues. In addition, researchers should investigate the roles of protein biomarkers ALCAM, PF-4, properdin, VCAM-1, and sE-selectin in mediating lupus nephritis.
Limitations of renal biopsy
About 60% of patients with SLE will develop lupus nephritis, and 10%-15% of patients who develop lupus nephritis progress to end-stage renal disease. Although renal biopsy is the gold standard for the diagnosis of renal involvement in SLE, biopsies are invasive, not serially repeatable, and may not represent the entire kidney, Dr. Mohan and colleagues wrote.
To identify potential urinary biomarkers of lupus nephritis using an unbiased, proteomic approach, the investigators screened urine samples from 23 participants – 7 with active lupus nephritis, 8 with inactive SLE, and 8 healthy controls. They used an aptamer-based screen to investigate more than 1,100 proteins. The researchers then validated biomarker candidates using enzyme-linked immunosorbent assays. Independent cross-sectional cohorts included 127 patients with inactive SLE, 107 patients with active lupus nephritis, 67 patients with active nonrenal lupus, and 74 healthy controls. The cohorts included patients who were African American, Caucasian, and Asian. The researchers excluded patients with renal failure and pediatric patients.
Of the 12 urine proteins studied, 10 outperformed traditional laboratory measures, such as C3/C4 and anti–double stranded DNA, in discriminating active lupus nephritis from inactive SLE, wrote Dr. Mohan and colleagues. A Lasso regression analysis found that the best predictive model included 8 of the 12 urine proteins as well as race. The model discriminated active lupus nephritis from inactive SLE with an area under the receiver operating characteristic curve (AUC) of 0.98.
Among African Americans, urine proteins that best distinguished active lupus nephritis from inactive disease included PF-4 (AUC, 0.88), VCAM-1 (AUC, 0.87), properdin (AUC, 0.85), and ALCAM (AUC, 0.84). Among Caucasians, they included sE-selectin (AUC, 0.87), VCAM-1 (AUC, 0.84), BFL-1 (AUC, 0.81), and hemopexin (AUC, 0.80). Among Asians, they included ALCAM (AUC, 0.93), VCAM-1 (AUC, 0.92), TFPI (AUC, 0.88), and PF-4 (AUC, 0.83).
The study is “unique in highlighting the importance of tailoring the biomarkers to patient ethnicity,” the researchers wrote.
Basing subgroups on race rather than phenotypic profiles
“This is an important study because it confirms the ability to predict active lupus nephritis from urine samples and utilized advanced technologies to find key markers for that,” said Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation in Oklahoma City. “It is unfortunate that investigators with access to such advanced technology are still using an outdated and extremely questionable method for distinguishing subgroups of patients, that of race.”
Grouping patients by phenotypic profiles that reflect current disease states “would be a more accurate method for finding optimal urinary markers for active nephritis,” and is “likely to prove more accurate for individuals in all races,” Dr. Merrill said. Certain racial subgroups may be more likely to have particular disease phenotypes, “which are usually identified based on gene pathway coexpression patterns.” Still, “people who self-identify as a given race are not genetically identical,” Dr. Merrill added. “In fact, this is a very blunt instrument, compared to phenotypic profiling now available for lupus patients.”
SLE and lupus nephritis are “heavily influenced by genetics,” and African Americans are three times more likely than Caucasians to develop SLE and are more like to develop end-stage renal disease, Dr. Mohan and colleagues wrote. Nevertheless, “influence from environmental triggers or socioeconomic factors cannot be ruled out,” they added. “Although patient demographics are widely known to affect SLE disease manifestations and outcomes, there are virtually no studies investigating this phenomenon in the context of disease biomarkers; most SLE biomarkers studies focus on one demographic group or all ethnic groups combined, which yield results that may not be equally predictive in all demographic groups of SLE patients.”
Dr. Mohan is collaborating with a biotechnology company to study drugs that may block ALCAM, according to a University of Houston news release. ALCAM is involved in immune and inflammatory responses, the researchers noted. “When all SLE patients were combined, urine ALCAM levels had the strongest bearing on disease activity status, in an unsupervised Bayesian network analysis,” they wrote. “Urine ALCAM also emerged as one of the few proteins that distinguished active [lupus nephritis] from active nonrenal lupus.”
National Institutes of Health grants supported the research. The investigators had no competing interests.
SOURCE: Stanley S et al. Nat Commun. 2020 May 4. doi: 10.1038/s41467-020-15986-3.
according to a cross-sectional study published in Nature Communications. The proteins that best differentiate active lupus nephritis from inactive systemic lupus erythematosus (SLE) vary across ethnicities, the researchers wrote.
“A longitudinal study is warranted to investigate how these molecules relate to disease pathology and progression over time,” said senior study author Chandra Mohan, MD, PhD, of the department of biomedical engineering at the University of Houston, and colleagues. In addition, researchers should investigate the roles of protein biomarkers ALCAM, PF-4, properdin, VCAM-1, and sE-selectin in mediating lupus nephritis.
Limitations of renal biopsy
About 60% of patients with SLE will develop lupus nephritis, and 10%-15% of patients who develop lupus nephritis progress to end-stage renal disease. Although renal biopsy is the gold standard for the diagnosis of renal involvement in SLE, biopsies are invasive, not serially repeatable, and may not represent the entire kidney, Dr. Mohan and colleagues wrote.
To identify potential urinary biomarkers of lupus nephritis using an unbiased, proteomic approach, the investigators screened urine samples from 23 participants – 7 with active lupus nephritis, 8 with inactive SLE, and 8 healthy controls. They used an aptamer-based screen to investigate more than 1,100 proteins. The researchers then validated biomarker candidates using enzyme-linked immunosorbent assays. Independent cross-sectional cohorts included 127 patients with inactive SLE, 107 patients with active lupus nephritis, 67 patients with active nonrenal lupus, and 74 healthy controls. The cohorts included patients who were African American, Caucasian, and Asian. The researchers excluded patients with renal failure and pediatric patients.
Of the 12 urine proteins studied, 10 outperformed traditional laboratory measures, such as C3/C4 and anti–double stranded DNA, in discriminating active lupus nephritis from inactive SLE, wrote Dr. Mohan and colleagues. A Lasso regression analysis found that the best predictive model included 8 of the 12 urine proteins as well as race. The model discriminated active lupus nephritis from inactive SLE with an area under the receiver operating characteristic curve (AUC) of 0.98.
Among African Americans, urine proteins that best distinguished active lupus nephritis from inactive disease included PF-4 (AUC, 0.88), VCAM-1 (AUC, 0.87), properdin (AUC, 0.85), and ALCAM (AUC, 0.84). Among Caucasians, they included sE-selectin (AUC, 0.87), VCAM-1 (AUC, 0.84), BFL-1 (AUC, 0.81), and hemopexin (AUC, 0.80). Among Asians, they included ALCAM (AUC, 0.93), VCAM-1 (AUC, 0.92), TFPI (AUC, 0.88), and PF-4 (AUC, 0.83).
The study is “unique in highlighting the importance of tailoring the biomarkers to patient ethnicity,” the researchers wrote.
Basing subgroups on race rather than phenotypic profiles
“This is an important study because it confirms the ability to predict active lupus nephritis from urine samples and utilized advanced technologies to find key markers for that,” said Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation in Oklahoma City. “It is unfortunate that investigators with access to such advanced technology are still using an outdated and extremely questionable method for distinguishing subgroups of patients, that of race.”
Grouping patients by phenotypic profiles that reflect current disease states “would be a more accurate method for finding optimal urinary markers for active nephritis,” and is “likely to prove more accurate for individuals in all races,” Dr. Merrill said. Certain racial subgroups may be more likely to have particular disease phenotypes, “which are usually identified based on gene pathway coexpression patterns.” Still, “people who self-identify as a given race are not genetically identical,” Dr. Merrill added. “In fact, this is a very blunt instrument, compared to phenotypic profiling now available for lupus patients.”
SLE and lupus nephritis are “heavily influenced by genetics,” and African Americans are three times more likely than Caucasians to develop SLE and are more like to develop end-stage renal disease, Dr. Mohan and colleagues wrote. Nevertheless, “influence from environmental triggers or socioeconomic factors cannot be ruled out,” they added. “Although patient demographics are widely known to affect SLE disease manifestations and outcomes, there are virtually no studies investigating this phenomenon in the context of disease biomarkers; most SLE biomarkers studies focus on one demographic group or all ethnic groups combined, which yield results that may not be equally predictive in all demographic groups of SLE patients.”
Dr. Mohan is collaborating with a biotechnology company to study drugs that may block ALCAM, according to a University of Houston news release. ALCAM is involved in immune and inflammatory responses, the researchers noted. “When all SLE patients were combined, urine ALCAM levels had the strongest bearing on disease activity status, in an unsupervised Bayesian network analysis,” they wrote. “Urine ALCAM also emerged as one of the few proteins that distinguished active [lupus nephritis] from active nonrenal lupus.”
National Institutes of Health grants supported the research. The investigators had no competing interests.
SOURCE: Stanley S et al. Nat Commun. 2020 May 4. doi: 10.1038/s41467-020-15986-3.
according to a cross-sectional study published in Nature Communications. The proteins that best differentiate active lupus nephritis from inactive systemic lupus erythematosus (SLE) vary across ethnicities, the researchers wrote.
“A longitudinal study is warranted to investigate how these molecules relate to disease pathology and progression over time,” said senior study author Chandra Mohan, MD, PhD, of the department of biomedical engineering at the University of Houston, and colleagues. In addition, researchers should investigate the roles of protein biomarkers ALCAM, PF-4, properdin, VCAM-1, and sE-selectin in mediating lupus nephritis.
Limitations of renal biopsy
About 60% of patients with SLE will develop lupus nephritis, and 10%-15% of patients who develop lupus nephritis progress to end-stage renal disease. Although renal biopsy is the gold standard for the diagnosis of renal involvement in SLE, biopsies are invasive, not serially repeatable, and may not represent the entire kidney, Dr. Mohan and colleagues wrote.
To identify potential urinary biomarkers of lupus nephritis using an unbiased, proteomic approach, the investigators screened urine samples from 23 participants – 7 with active lupus nephritis, 8 with inactive SLE, and 8 healthy controls. They used an aptamer-based screen to investigate more than 1,100 proteins. The researchers then validated biomarker candidates using enzyme-linked immunosorbent assays. Independent cross-sectional cohorts included 127 patients with inactive SLE, 107 patients with active lupus nephritis, 67 patients with active nonrenal lupus, and 74 healthy controls. The cohorts included patients who were African American, Caucasian, and Asian. The researchers excluded patients with renal failure and pediatric patients.
Of the 12 urine proteins studied, 10 outperformed traditional laboratory measures, such as C3/C4 and anti–double stranded DNA, in discriminating active lupus nephritis from inactive SLE, wrote Dr. Mohan and colleagues. A Lasso regression analysis found that the best predictive model included 8 of the 12 urine proteins as well as race. The model discriminated active lupus nephritis from inactive SLE with an area under the receiver operating characteristic curve (AUC) of 0.98.
Among African Americans, urine proteins that best distinguished active lupus nephritis from inactive disease included PF-4 (AUC, 0.88), VCAM-1 (AUC, 0.87), properdin (AUC, 0.85), and ALCAM (AUC, 0.84). Among Caucasians, they included sE-selectin (AUC, 0.87), VCAM-1 (AUC, 0.84), BFL-1 (AUC, 0.81), and hemopexin (AUC, 0.80). Among Asians, they included ALCAM (AUC, 0.93), VCAM-1 (AUC, 0.92), TFPI (AUC, 0.88), and PF-4 (AUC, 0.83).
The study is “unique in highlighting the importance of tailoring the biomarkers to patient ethnicity,” the researchers wrote.
Basing subgroups on race rather than phenotypic profiles
“This is an important study because it confirms the ability to predict active lupus nephritis from urine samples and utilized advanced technologies to find key markers for that,” said Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation in Oklahoma City. “It is unfortunate that investigators with access to such advanced technology are still using an outdated and extremely questionable method for distinguishing subgroups of patients, that of race.”
Grouping patients by phenotypic profiles that reflect current disease states “would be a more accurate method for finding optimal urinary markers for active nephritis,” and is “likely to prove more accurate for individuals in all races,” Dr. Merrill said. Certain racial subgroups may be more likely to have particular disease phenotypes, “which are usually identified based on gene pathway coexpression patterns.” Still, “people who self-identify as a given race are not genetically identical,” Dr. Merrill added. “In fact, this is a very blunt instrument, compared to phenotypic profiling now available for lupus patients.”
SLE and lupus nephritis are “heavily influenced by genetics,” and African Americans are three times more likely than Caucasians to develop SLE and are more like to develop end-stage renal disease, Dr. Mohan and colleagues wrote. Nevertheless, “influence from environmental triggers or socioeconomic factors cannot be ruled out,” they added. “Although patient demographics are widely known to affect SLE disease manifestations and outcomes, there are virtually no studies investigating this phenomenon in the context of disease biomarkers; most SLE biomarkers studies focus on one demographic group or all ethnic groups combined, which yield results that may not be equally predictive in all demographic groups of SLE patients.”
Dr. Mohan is collaborating with a biotechnology company to study drugs that may block ALCAM, according to a University of Houston news release. ALCAM is involved in immune and inflammatory responses, the researchers noted. “When all SLE patients were combined, urine ALCAM levels had the strongest bearing on disease activity status, in an unsupervised Bayesian network analysis,” they wrote. “Urine ALCAM also emerged as one of the few proteins that distinguished active [lupus nephritis] from active nonrenal lupus.”
National Institutes of Health grants supported the research. The investigators had no competing interests.
SOURCE: Stanley S et al. Nat Commun. 2020 May 4. doi: 10.1038/s41467-020-15986-3.
FROM NATURE COMMUNICATIONS
Advice on treating rheumatic diseases from a COVID-19 epicenter
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
ACR gives guidance on rheumatic disease management during pandemic
When COVID-19 is suspected or confirmed in a patient with a rheumatic disease, treatment with hydroxychloroquine may be continued, but other treatments may need to be stopped or held temporarily, according to new guidance issued by the American College of Rheumatology.
That includes disease-modifying treatment with antirheumatic drugs such as sulfasalazine, methotrexate, leflunomide, and the Janus kinase (JAK) inhibitors, as well as immunosuppressants and non-interleukin (IL)-6 biologics, and this is regardless of how severe the COVID-19 illness is. NSAIDs should also be stopped if there are respiratory symptoms.
The advice is slightly less drastic if someone with stable rheumatic disease has probably been exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are asymptomatic. In those patients, DMARDs may be continued, although there is uncertainty over whether there is a need to temporarily stop methotrexate or leflunomide. Interruption of immunosuppressive, non–IL-6, and JAK inhibitor treatment is advised pending a negative SARS-CoV-2 test result, assuming the patient’s rheumatic disease is stable.
Impetus for ACR COVID-19 guidance
“One of the earliest challenges for rheumatologists during the COVID-19 pandemic was determining how to advise our patients who were taking immunosuppressive medications and were concerned as to whether or not to discontinue their therapy,” ACR President Ellen Gravallese, MD, said in an interview about the ACR Clinical Guidance Document, which is published online in Arthritis & Rheumatology.
“A second challenge was keeping our patients safe from exposure to the virus, while still seeing those patients in person who required office visits,” added Dr. Gravallese, who is chief of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital in Boston.
She continued: “The ACR Clinical Guidance Document was prepared in order to assist rheumatologists with decisions as to how to handle current medications during different phases of a patient’s exposure to the SARS-CoV-2 virus.”
But with very little evidence available on how to manage COVID-19 patients generally, let alone specifically in those with rheumatic diseases, “it became evident that any recommendations made would need to be done in a thoughtful and organized manner, evaluating the evidence that was available and obtaining the advice of experts in infectious disease, epidemiology, and in the use of biologic and nonbiologic agents for rheumatic disease,” she said.
As such, the ACR convened a task force of 10 rheumatologists and 4 infectious disease specialists from North America to look at how best to manage patients with rheumatic disease during the COVID-19 pandemic.
“Our charge was to develop a guidance document for the care of adult rheumatic disease patients in the context of COVID-19 and not per se to provide guidance for the treatment of COVID-19,” explained task force member and the corresponding author for the guidance, Ted R. Mikuls, MD, MSPH, of the University of Nebraska Medical Center, Omaha.
Dr. Mikuls, who was speaking at a virtual town hall meeting hosted by the ACR on May 6, noted that the guidance was obviously based on the best consensus of the available data and as such represented a “living document” that “would change and be added to” as necessary.
General recommendations for adult rheumatic disease management
In terms of general recommendations for the management of adult rheumatic disease patients, Dr. Mikuls said that six statements had been made “specific to risk assessment, prevention of infection, and best practices related to glucocorticoid use and the use of ACE [angiotensin-converting enzyme] inhibitors and ARBs [angiotensin II receptor blockers] during the pandemic.”
For example, general advice is to counsel patients to keep up general preventive measures such as social distancing and regular hand washing, reducing the number of in-person health care visits, and undertaking other means to try to prevent potential SARS-CoV-2 exposure. As for general treatment advice, glucocorticoids should be used at their lowest doses possible and should not be abruptly stopped, and antihypertensive treatment should be used as indicated.
Additional guidance statements include those that address the treatment of patients with stable rheumatic disease in the absence of infection or known exposure to SARS-CoV-2, with guidance specific to the treatment of systemic lupus erythematosus (SLE), and those with newly diagnosed or active rheumatic disease.
SLE and inflammatory arthritis recommendations
“There are several sections within the guidance document that address the treatment of patients with systemic lupus erythematosus during this pandemic,” Dr. Gravallese pointed out. “In general, it is recommended that lupus patients who are currently taking hydroxychloroquine can remain on the therapy prior to and during infection and that newly diagnosed patients with lupus can be placed on this medication at full dose. It is recommended that pregnant patients with lupus remain on therapy with this drug.”
She also observed that, for the treatment of active inflammatory arthritis, “the recommendations were written to address specific medications that could be used in this setting. In general, the task force recommendations were guided by the importance of controlling inflammation prior to exposure to the virus, even during this pandemic.
Guidance raises questions
During the ACR’s town hall meeting, the task force answered several questions raised by the guidance, such as the reasoning behind recommending that the use of traditional DMARDs be discontinued in patients with confirmed SARS-CoV-2 infection.
Dr. Mikuls observed: “Maybe if you just read the guidance statements it isn’t terribly intuitive.” There was a lot of discussion about whether or not conventional DMARDs were immunosuppressive, and even though they may not have such effects, it was decided to err on the side of caution.
“I think the task force felt that, with a COVID-19–positive patient, there is a concern of potentially confusing adverse effects related to medicines or conflate those with problems from the infection,” he said. Although rare, examples of those issues could be drug-induced hypersensitivity, hypersensitivity pneumonitis, or gastrointestinal side effects of hepatitis, all of which have been described in COVID-19. “Not only could it cause confusion, but it could maybe worsen those sequalae of COVID-19,” he said.
“I think the other part of this answer was that the panel really felt that the risk in terms of the flaring of the underlying rheumatic disease was likely to be pretty low given the finite time frame you’d be taking about – usually a time frame of 2-3 weeks you’d be holding the agent – so I think that is really why the task force ended up with that recommendation.”
Similarly, for the JAK inhibitors, the decision was to err on the side of caution when COVID-19 was suspected or confirmed. “Not so much because of the risk of thromboembolic disease, but concerns over immunosuppression that these drugs carry with them and also the fact the JAK inhibitors are probably inhibitors of type 1 interferons, which play a significant role in viral immunity and could potentially have a negative impact,” said Stanley Cohen, MD, who practices rheumatology in the Dallas area.
“On the flipside, there is interest in some of the JAK inhibitors as a potential treatment for COVID-19,” Dr. Cohen said, referring to anecdotal evidence for baricitinib (Olumiant).
Michael Weinblatt, MD, of Brigham and Women’s Hospital, addressed the recent concern over the use of NSAIDs by the public.
“There’s been a lot in the lay press that NSAIDs – because of the effects on receptors in the lung – could lead to deleterious outcomes in patients with COVID and there’s very little data to support this.
“We did recommend that NSAIDs be held in the hospitalized patient and that wasn’t because of the COVID-19 issue, it really was just medical practice, and we didn’t want to confound the care of these really sick patients with potential toxicities from NSAIDs. But as far as routine rheumatological care in your outpatients, we did not recommend that nonsteroidals be stopped if they were tolerated.”
One part of the guidance that might already need revision is the recommendation on the continued use of hydroxychloroquine in patients who develop COVID-19.
“Our guidance document says it’s OK; we were all in very strong agreement to continue hydroxychloroquine in our patients with COVID-19 because at that point, just a couple of weeks ago, we thought it was part of the potential treatment,” Karen Costenbader, MD, MPH, of Brigham and Women’s Hospital, said during the town hall meeting.
“Now the pendulum has swung the other way, and we’re worried about maybe we shouldn’t be continuing it because COVID-19 patients will be getting many other medications,” Dr. Costenbader said, and these may affect the QT-interval. “They will not be getting azithromycin because the pendulum swung the other way on that one too, but definitely on many other medications when they are sick.”
Potentially, she added, “if the rheumatic disease is under good control the inpatient physicians could decide whether they should continue [hydroxychloroquine] or not. If the COVID-19 is a mild disease, I would say we probably could continue in accordance with what we put in the document, but we will have to revisit this as well.”
Guidance is a ‘living document’
“We will be providing updates to the Clinical Guidance Document as the need arises,” Dr. Gravallese emphasized. While the general recommendations are unlikely to change very much, “the task force will be interested in seeing the results of all new data, but the results of randomized, clinical trials will be particularly important as they become available,” she said. In particular, randomized, controlled trials of glucocorticoids and IL-6 receptor blockade for use in COVID-19 will be of great importance.
“In this initial document, we could not take on all of the medical scenarios our members will face. For example, we could not take on recommendations for the pediatric population as this group of patients has a very different response than adults to the SARS-CoV-2 virus,” Dr. Gravallese acknowledged. The plan is to provide guidance for that group of patients soon.
In addition, the ACR Executive Committee has appointed a Practice and Advocacy Task Force that will “address issues rheumatologists face on the practice side, including advice regarding how to effectively use telemedicine, address the frequency and safety of infusions, determine urgent versus nonurgent issues that would or would not require face-to-face visits, and help with financial challenges.”
The American College of Rheumatology supported the guidance-development process. Dr. Mikuls, Dr. Weinblatt, Dr. Cohen, and Dr. Costenbader each disclosed research support or consultancies with multiple pharmaceutical companies. Dr. Gravallese had no disclosures.
SOURCE: Mikuls TR et al. Arthritis Rheumatol. 2020 Apr 29. doi: 10.1002/art.41301.
When COVID-19 is suspected or confirmed in a patient with a rheumatic disease, treatment with hydroxychloroquine may be continued, but other treatments may need to be stopped or held temporarily, according to new guidance issued by the American College of Rheumatology.
That includes disease-modifying treatment with antirheumatic drugs such as sulfasalazine, methotrexate, leflunomide, and the Janus kinase (JAK) inhibitors, as well as immunosuppressants and non-interleukin (IL)-6 biologics, and this is regardless of how severe the COVID-19 illness is. NSAIDs should also be stopped if there are respiratory symptoms.
The advice is slightly less drastic if someone with stable rheumatic disease has probably been exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are asymptomatic. In those patients, DMARDs may be continued, although there is uncertainty over whether there is a need to temporarily stop methotrexate or leflunomide. Interruption of immunosuppressive, non–IL-6, and JAK inhibitor treatment is advised pending a negative SARS-CoV-2 test result, assuming the patient’s rheumatic disease is stable.
Impetus for ACR COVID-19 guidance
“One of the earliest challenges for rheumatologists during the COVID-19 pandemic was determining how to advise our patients who were taking immunosuppressive medications and were concerned as to whether or not to discontinue their therapy,” ACR President Ellen Gravallese, MD, said in an interview about the ACR Clinical Guidance Document, which is published online in Arthritis & Rheumatology.
“A second challenge was keeping our patients safe from exposure to the virus, while still seeing those patients in person who required office visits,” added Dr. Gravallese, who is chief of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital in Boston.
She continued: “The ACR Clinical Guidance Document was prepared in order to assist rheumatologists with decisions as to how to handle current medications during different phases of a patient’s exposure to the SARS-CoV-2 virus.”
But with very little evidence available on how to manage COVID-19 patients generally, let alone specifically in those with rheumatic diseases, “it became evident that any recommendations made would need to be done in a thoughtful and organized manner, evaluating the evidence that was available and obtaining the advice of experts in infectious disease, epidemiology, and in the use of biologic and nonbiologic agents for rheumatic disease,” she said.
As such, the ACR convened a task force of 10 rheumatologists and 4 infectious disease specialists from North America to look at how best to manage patients with rheumatic disease during the COVID-19 pandemic.
“Our charge was to develop a guidance document for the care of adult rheumatic disease patients in the context of COVID-19 and not per se to provide guidance for the treatment of COVID-19,” explained task force member and the corresponding author for the guidance, Ted R. Mikuls, MD, MSPH, of the University of Nebraska Medical Center, Omaha.
Dr. Mikuls, who was speaking at a virtual town hall meeting hosted by the ACR on May 6, noted that the guidance was obviously based on the best consensus of the available data and as such represented a “living document” that “would change and be added to” as necessary.
General recommendations for adult rheumatic disease management
In terms of general recommendations for the management of adult rheumatic disease patients, Dr. Mikuls said that six statements had been made “specific to risk assessment, prevention of infection, and best practices related to glucocorticoid use and the use of ACE [angiotensin-converting enzyme] inhibitors and ARBs [angiotensin II receptor blockers] during the pandemic.”
For example, general advice is to counsel patients to keep up general preventive measures such as social distancing and regular hand washing, reducing the number of in-person health care visits, and undertaking other means to try to prevent potential SARS-CoV-2 exposure. As for general treatment advice, glucocorticoids should be used at their lowest doses possible and should not be abruptly stopped, and antihypertensive treatment should be used as indicated.
Additional guidance statements include those that address the treatment of patients with stable rheumatic disease in the absence of infection or known exposure to SARS-CoV-2, with guidance specific to the treatment of systemic lupus erythematosus (SLE), and those with newly diagnosed or active rheumatic disease.
SLE and inflammatory arthritis recommendations
“There are several sections within the guidance document that address the treatment of patients with systemic lupus erythematosus during this pandemic,” Dr. Gravallese pointed out. “In general, it is recommended that lupus patients who are currently taking hydroxychloroquine can remain on the therapy prior to and during infection and that newly diagnosed patients with lupus can be placed on this medication at full dose. It is recommended that pregnant patients with lupus remain on therapy with this drug.”
She also observed that, for the treatment of active inflammatory arthritis, “the recommendations were written to address specific medications that could be used in this setting. In general, the task force recommendations were guided by the importance of controlling inflammation prior to exposure to the virus, even during this pandemic.
Guidance raises questions
During the ACR’s town hall meeting, the task force answered several questions raised by the guidance, such as the reasoning behind recommending that the use of traditional DMARDs be discontinued in patients with confirmed SARS-CoV-2 infection.
Dr. Mikuls observed: “Maybe if you just read the guidance statements it isn’t terribly intuitive.” There was a lot of discussion about whether or not conventional DMARDs were immunosuppressive, and even though they may not have such effects, it was decided to err on the side of caution.
“I think the task force felt that, with a COVID-19–positive patient, there is a concern of potentially confusing adverse effects related to medicines or conflate those with problems from the infection,” he said. Although rare, examples of those issues could be drug-induced hypersensitivity, hypersensitivity pneumonitis, or gastrointestinal side effects of hepatitis, all of which have been described in COVID-19. “Not only could it cause confusion, but it could maybe worsen those sequalae of COVID-19,” he said.
“I think the other part of this answer was that the panel really felt that the risk in terms of the flaring of the underlying rheumatic disease was likely to be pretty low given the finite time frame you’d be taking about – usually a time frame of 2-3 weeks you’d be holding the agent – so I think that is really why the task force ended up with that recommendation.”
Similarly, for the JAK inhibitors, the decision was to err on the side of caution when COVID-19 was suspected or confirmed. “Not so much because of the risk of thromboembolic disease, but concerns over immunosuppression that these drugs carry with them and also the fact the JAK inhibitors are probably inhibitors of type 1 interferons, which play a significant role in viral immunity and could potentially have a negative impact,” said Stanley Cohen, MD, who practices rheumatology in the Dallas area.
“On the flipside, there is interest in some of the JAK inhibitors as a potential treatment for COVID-19,” Dr. Cohen said, referring to anecdotal evidence for baricitinib (Olumiant).
Michael Weinblatt, MD, of Brigham and Women’s Hospital, addressed the recent concern over the use of NSAIDs by the public.
“There’s been a lot in the lay press that NSAIDs – because of the effects on receptors in the lung – could lead to deleterious outcomes in patients with COVID and there’s very little data to support this.
“We did recommend that NSAIDs be held in the hospitalized patient and that wasn’t because of the COVID-19 issue, it really was just medical practice, and we didn’t want to confound the care of these really sick patients with potential toxicities from NSAIDs. But as far as routine rheumatological care in your outpatients, we did not recommend that nonsteroidals be stopped if they were tolerated.”
One part of the guidance that might already need revision is the recommendation on the continued use of hydroxychloroquine in patients who develop COVID-19.
“Our guidance document says it’s OK; we were all in very strong agreement to continue hydroxychloroquine in our patients with COVID-19 because at that point, just a couple of weeks ago, we thought it was part of the potential treatment,” Karen Costenbader, MD, MPH, of Brigham and Women’s Hospital, said during the town hall meeting.
“Now the pendulum has swung the other way, and we’re worried about maybe we shouldn’t be continuing it because COVID-19 patients will be getting many other medications,” Dr. Costenbader said, and these may affect the QT-interval. “They will not be getting azithromycin because the pendulum swung the other way on that one too, but definitely on many other medications when they are sick.”
Potentially, she added, “if the rheumatic disease is under good control the inpatient physicians could decide whether they should continue [hydroxychloroquine] or not. If the COVID-19 is a mild disease, I would say we probably could continue in accordance with what we put in the document, but we will have to revisit this as well.”
Guidance is a ‘living document’
“We will be providing updates to the Clinical Guidance Document as the need arises,” Dr. Gravallese emphasized. While the general recommendations are unlikely to change very much, “the task force will be interested in seeing the results of all new data, but the results of randomized, clinical trials will be particularly important as they become available,” she said. In particular, randomized, controlled trials of glucocorticoids and IL-6 receptor blockade for use in COVID-19 will be of great importance.
“In this initial document, we could not take on all of the medical scenarios our members will face. For example, we could not take on recommendations for the pediatric population as this group of patients has a very different response than adults to the SARS-CoV-2 virus,” Dr. Gravallese acknowledged. The plan is to provide guidance for that group of patients soon.
In addition, the ACR Executive Committee has appointed a Practice and Advocacy Task Force that will “address issues rheumatologists face on the practice side, including advice regarding how to effectively use telemedicine, address the frequency and safety of infusions, determine urgent versus nonurgent issues that would or would not require face-to-face visits, and help with financial challenges.”
The American College of Rheumatology supported the guidance-development process. Dr. Mikuls, Dr. Weinblatt, Dr. Cohen, and Dr. Costenbader each disclosed research support or consultancies with multiple pharmaceutical companies. Dr. Gravallese had no disclosures.
SOURCE: Mikuls TR et al. Arthritis Rheumatol. 2020 Apr 29. doi: 10.1002/art.41301.
When COVID-19 is suspected or confirmed in a patient with a rheumatic disease, treatment with hydroxychloroquine may be continued, but other treatments may need to be stopped or held temporarily, according to new guidance issued by the American College of Rheumatology.
That includes disease-modifying treatment with antirheumatic drugs such as sulfasalazine, methotrexate, leflunomide, and the Janus kinase (JAK) inhibitors, as well as immunosuppressants and non-interleukin (IL)-6 biologics, and this is regardless of how severe the COVID-19 illness is. NSAIDs should also be stopped if there are respiratory symptoms.
The advice is slightly less drastic if someone with stable rheumatic disease has probably been exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are asymptomatic. In those patients, DMARDs may be continued, although there is uncertainty over whether there is a need to temporarily stop methotrexate or leflunomide. Interruption of immunosuppressive, non–IL-6, and JAK inhibitor treatment is advised pending a negative SARS-CoV-2 test result, assuming the patient’s rheumatic disease is stable.
Impetus for ACR COVID-19 guidance
“One of the earliest challenges for rheumatologists during the COVID-19 pandemic was determining how to advise our patients who were taking immunosuppressive medications and were concerned as to whether or not to discontinue their therapy,” ACR President Ellen Gravallese, MD, said in an interview about the ACR Clinical Guidance Document, which is published online in Arthritis & Rheumatology.
“A second challenge was keeping our patients safe from exposure to the virus, while still seeing those patients in person who required office visits,” added Dr. Gravallese, who is chief of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital in Boston.
She continued: “The ACR Clinical Guidance Document was prepared in order to assist rheumatologists with decisions as to how to handle current medications during different phases of a patient’s exposure to the SARS-CoV-2 virus.”
But with very little evidence available on how to manage COVID-19 patients generally, let alone specifically in those with rheumatic diseases, “it became evident that any recommendations made would need to be done in a thoughtful and organized manner, evaluating the evidence that was available and obtaining the advice of experts in infectious disease, epidemiology, and in the use of biologic and nonbiologic agents for rheumatic disease,” she said.
As such, the ACR convened a task force of 10 rheumatologists and 4 infectious disease specialists from North America to look at how best to manage patients with rheumatic disease during the COVID-19 pandemic.
“Our charge was to develop a guidance document for the care of adult rheumatic disease patients in the context of COVID-19 and not per se to provide guidance for the treatment of COVID-19,” explained task force member and the corresponding author for the guidance, Ted R. Mikuls, MD, MSPH, of the University of Nebraska Medical Center, Omaha.
Dr. Mikuls, who was speaking at a virtual town hall meeting hosted by the ACR on May 6, noted that the guidance was obviously based on the best consensus of the available data and as such represented a “living document” that “would change and be added to” as necessary.
General recommendations for adult rheumatic disease management
In terms of general recommendations for the management of adult rheumatic disease patients, Dr. Mikuls said that six statements had been made “specific to risk assessment, prevention of infection, and best practices related to glucocorticoid use and the use of ACE [angiotensin-converting enzyme] inhibitors and ARBs [angiotensin II receptor blockers] during the pandemic.”
For example, general advice is to counsel patients to keep up general preventive measures such as social distancing and regular hand washing, reducing the number of in-person health care visits, and undertaking other means to try to prevent potential SARS-CoV-2 exposure. As for general treatment advice, glucocorticoids should be used at their lowest doses possible and should not be abruptly stopped, and antihypertensive treatment should be used as indicated.
Additional guidance statements include those that address the treatment of patients with stable rheumatic disease in the absence of infection or known exposure to SARS-CoV-2, with guidance specific to the treatment of systemic lupus erythematosus (SLE), and those with newly diagnosed or active rheumatic disease.
SLE and inflammatory arthritis recommendations
“There are several sections within the guidance document that address the treatment of patients with systemic lupus erythematosus during this pandemic,” Dr. Gravallese pointed out. “In general, it is recommended that lupus patients who are currently taking hydroxychloroquine can remain on the therapy prior to and during infection and that newly diagnosed patients with lupus can be placed on this medication at full dose. It is recommended that pregnant patients with lupus remain on therapy with this drug.”
She also observed that, for the treatment of active inflammatory arthritis, “the recommendations were written to address specific medications that could be used in this setting. In general, the task force recommendations were guided by the importance of controlling inflammation prior to exposure to the virus, even during this pandemic.
Guidance raises questions
During the ACR’s town hall meeting, the task force answered several questions raised by the guidance, such as the reasoning behind recommending that the use of traditional DMARDs be discontinued in patients with confirmed SARS-CoV-2 infection.
Dr. Mikuls observed: “Maybe if you just read the guidance statements it isn’t terribly intuitive.” There was a lot of discussion about whether or not conventional DMARDs were immunosuppressive, and even though they may not have such effects, it was decided to err on the side of caution.
“I think the task force felt that, with a COVID-19–positive patient, there is a concern of potentially confusing adverse effects related to medicines or conflate those with problems from the infection,” he said. Although rare, examples of those issues could be drug-induced hypersensitivity, hypersensitivity pneumonitis, or gastrointestinal side effects of hepatitis, all of which have been described in COVID-19. “Not only could it cause confusion, but it could maybe worsen those sequalae of COVID-19,” he said.
“I think the other part of this answer was that the panel really felt that the risk in terms of the flaring of the underlying rheumatic disease was likely to be pretty low given the finite time frame you’d be taking about – usually a time frame of 2-3 weeks you’d be holding the agent – so I think that is really why the task force ended up with that recommendation.”
Similarly, for the JAK inhibitors, the decision was to err on the side of caution when COVID-19 was suspected or confirmed. “Not so much because of the risk of thromboembolic disease, but concerns over immunosuppression that these drugs carry with them and also the fact the JAK inhibitors are probably inhibitors of type 1 interferons, which play a significant role in viral immunity and could potentially have a negative impact,” said Stanley Cohen, MD, who practices rheumatology in the Dallas area.
“On the flipside, there is interest in some of the JAK inhibitors as a potential treatment for COVID-19,” Dr. Cohen said, referring to anecdotal evidence for baricitinib (Olumiant).
Michael Weinblatt, MD, of Brigham and Women’s Hospital, addressed the recent concern over the use of NSAIDs by the public.
“There’s been a lot in the lay press that NSAIDs – because of the effects on receptors in the lung – could lead to deleterious outcomes in patients with COVID and there’s very little data to support this.
“We did recommend that NSAIDs be held in the hospitalized patient and that wasn’t because of the COVID-19 issue, it really was just medical practice, and we didn’t want to confound the care of these really sick patients with potential toxicities from NSAIDs. But as far as routine rheumatological care in your outpatients, we did not recommend that nonsteroidals be stopped if they were tolerated.”
One part of the guidance that might already need revision is the recommendation on the continued use of hydroxychloroquine in patients who develop COVID-19.
“Our guidance document says it’s OK; we were all in very strong agreement to continue hydroxychloroquine in our patients with COVID-19 because at that point, just a couple of weeks ago, we thought it was part of the potential treatment,” Karen Costenbader, MD, MPH, of Brigham and Women’s Hospital, said during the town hall meeting.
“Now the pendulum has swung the other way, and we’re worried about maybe we shouldn’t be continuing it because COVID-19 patients will be getting many other medications,” Dr. Costenbader said, and these may affect the QT-interval. “They will not be getting azithromycin because the pendulum swung the other way on that one too, but definitely on many other medications when they are sick.”
Potentially, she added, “if the rheumatic disease is under good control the inpatient physicians could decide whether they should continue [hydroxychloroquine] or not. If the COVID-19 is a mild disease, I would say we probably could continue in accordance with what we put in the document, but we will have to revisit this as well.”
Guidance is a ‘living document’
“We will be providing updates to the Clinical Guidance Document as the need arises,” Dr. Gravallese emphasized. While the general recommendations are unlikely to change very much, “the task force will be interested in seeing the results of all new data, but the results of randomized, clinical trials will be particularly important as they become available,” she said. In particular, randomized, controlled trials of glucocorticoids and IL-6 receptor blockade for use in COVID-19 will be of great importance.
“In this initial document, we could not take on all of the medical scenarios our members will face. For example, we could not take on recommendations for the pediatric population as this group of patients has a very different response than adults to the SARS-CoV-2 virus,” Dr. Gravallese acknowledged. The plan is to provide guidance for that group of patients soon.
In addition, the ACR Executive Committee has appointed a Practice and Advocacy Task Force that will “address issues rheumatologists face on the practice side, including advice regarding how to effectively use telemedicine, address the frequency and safety of infusions, determine urgent versus nonurgent issues that would or would not require face-to-face visits, and help with financial challenges.”
The American College of Rheumatology supported the guidance-development process. Dr. Mikuls, Dr. Weinblatt, Dr. Cohen, and Dr. Costenbader each disclosed research support or consultancies with multiple pharmaceutical companies. Dr. Gravallese had no disclosures.
SOURCE: Mikuls TR et al. Arthritis Rheumatol. 2020 Apr 29. doi: 10.1002/art.41301.
FROM ARTHRITIS & RHEUMATOLOGY
The many variants of psoriasis
The “heartbreak of psoriasis,” coined by an advertiser in the 1960s, conveyed the notion that this disease was a cosmetic disorder mainly limited to skin involvement. John Updike’s article in the September 1985 issue of The New Yorker, “At War With My Skin,” detailed Mr. Updike’s feelings of isolation and stress related to his condition, helping to reframe the popular concept of psoriasis.1 Updike’s eloquent account describing his struggles to find effective treatment increased public awareness about psoriasis, which in fact affects other body systems as well.
The overall prevalence of psoriasis is 1.5% to 3.1% in the United States and United Kingdom.2,3 More than 6.5 million adults in the United States > 20 years of age are affected.3 The most commonly affected demographic group is non-Hispanic Caucasians.
Our expanding knowledge of pathogenesis
Studies of genetic linkage have identified genes and single nucleotide polymorphisms associated with psoriasis.4 The interaction between environmental triggers and the innate and adaptive immune systems leads to keratinocyte hyperproliferation. Tumor necrosis factor (TNF), interleukin (IL) 23, and IL-17 are important cytokines associated with psoriatic inflammation.4 There are common pathways of inflammation in both psoriasis and cardiovascular disease resulting in oxidative stress and endothelial cell dysfunction.4 Ninety percent of early-onset psoriasis is associated with human leukocyte antigen (HLA)-Cw6.4 And alterations in the microbiome of the skin may contribute, as reduced microbial diversity has been found in psoriatic lesions.5
Comorbidities are common
Psoriasis is an independent risk factor for diabetes and major adverse cardiovascular events.6 Hypertension, dyslipidemia, inflammatory bowel disease, nonalcoholic fatty liver disease, chronic kidney disease, and lymphoma (particularly cutaneous T-cell lymphoma) are also associated with psoriasis.6 Psoriatic arthritis is frequently encountered with cutaneous psoriasis; however, it is often not recognized until late in the disease course.
There also appears to be an association among psoriasis, dietary factors, and celiac disease.7-9 Positive testing for IgA anti-endomysial antibodies and IgA tissue transglutaminase antibodies should prompt consideration of starting a gluten-free diet, which has been shown to improve psoriatic
The different types of psoriasis
The classic presentation of psoriasis involves stubborn plaques with silvery scale on extensor surfaces such as the elbows and knees. The severity of the disease corresponds with the amount of body surface area affected. While plaque-type psoriasis is the most common form, other patterns exist. Individuals may exhibit 1 dominant pattern or multiple psoriatic variants simultaneously. Most types of psoriasis have 3 characteristic features: erythema, skin thickening, and scales.
Certain history and physical clues can aid in diagnosing psoriasis; these include the Koebner phenomenon, the Auspitz sign, and the Woronoff ring. The Koebner phenomenon refers to the development of psoriatic lesions in an area of trauma (FIGURE 1), frequently resulting in a linear streak-like appearance. The Auspitz sign describes the pinpoint bleeding that may be encountered with the removal of a psoriatic plaque. The Woronoff ring is a pale blanching ring that may surround a psoriatic lesion.
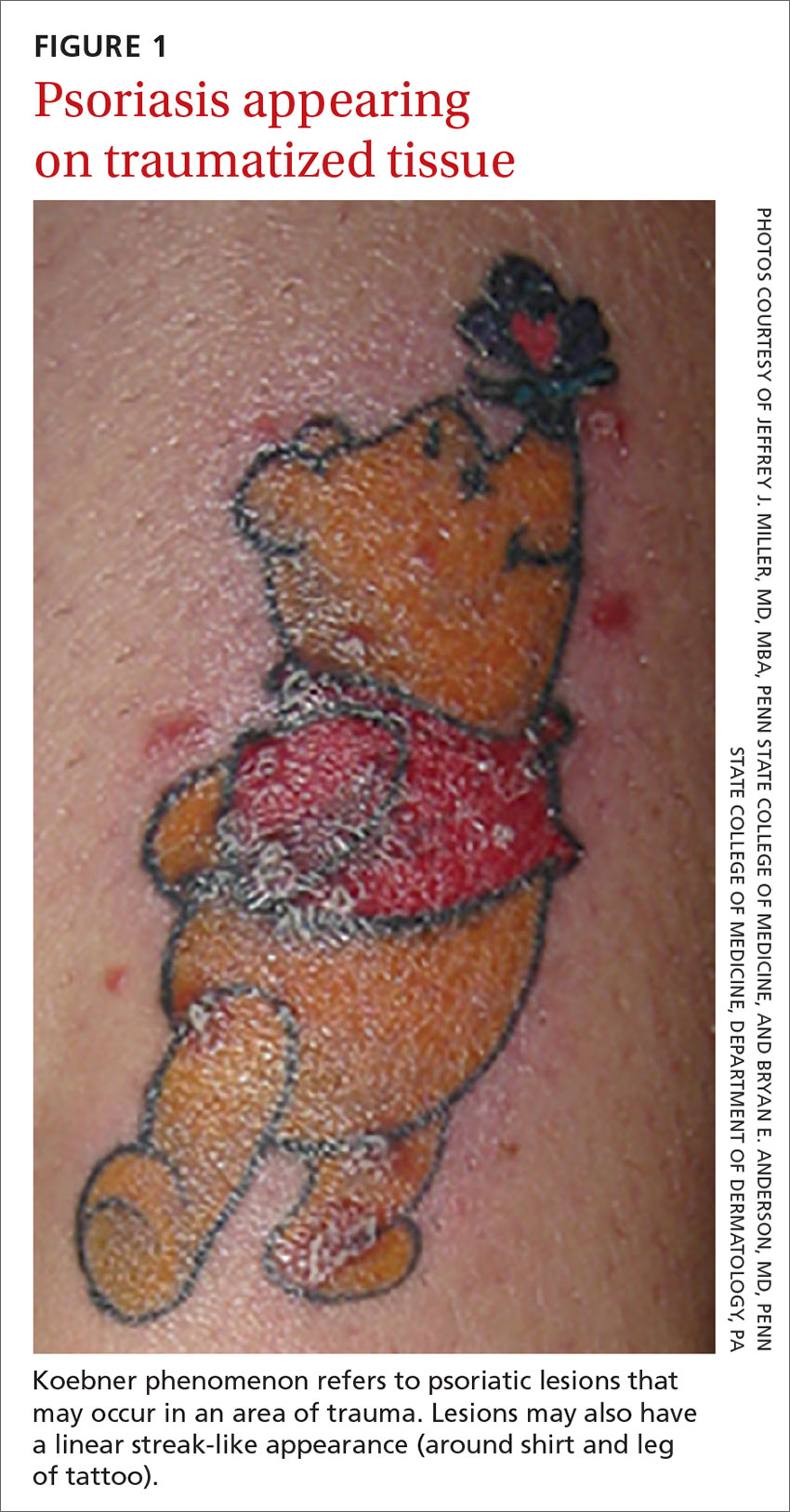
Continue to: Chronic plaque-type psoriasis
Chronic plaque-type psoriasis (Figures 2A and 2B), the most common variant, is characterized by sharply demarcated pink papules and plaques with a silvery scale in a symmetric distribution on the extensor surfaces, scalp, trunk, and lumbosacral areas.
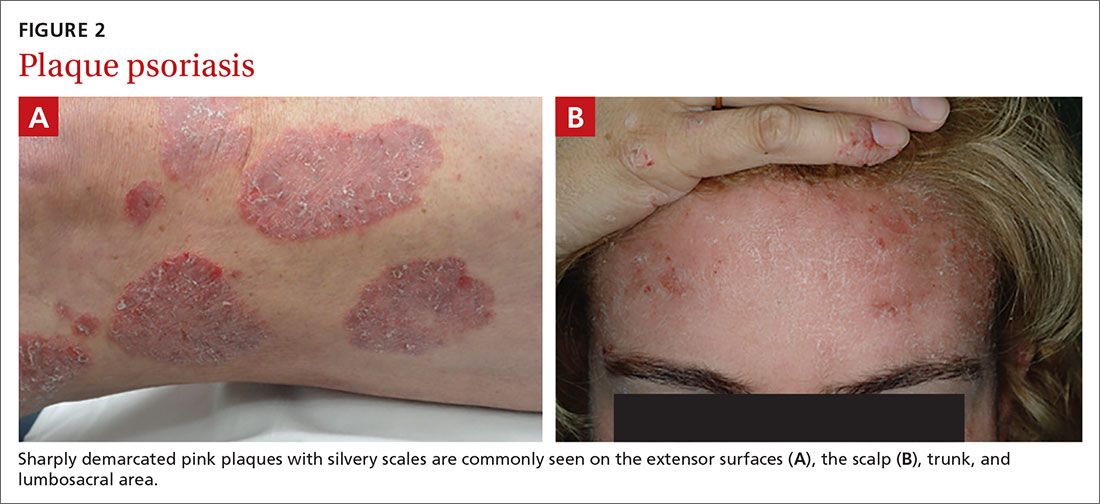
Guttate psoriasis (FIGURE 3) features small (often < 1 cm) pink scaly papules that appear suddenly. It is more commonly seen in children and is usually preceded by an upper respiratory tract infection, often with Streptococcus.10 If strep testing is positive, guttate psoriasis may improve after appropriate antibiotic treatment.
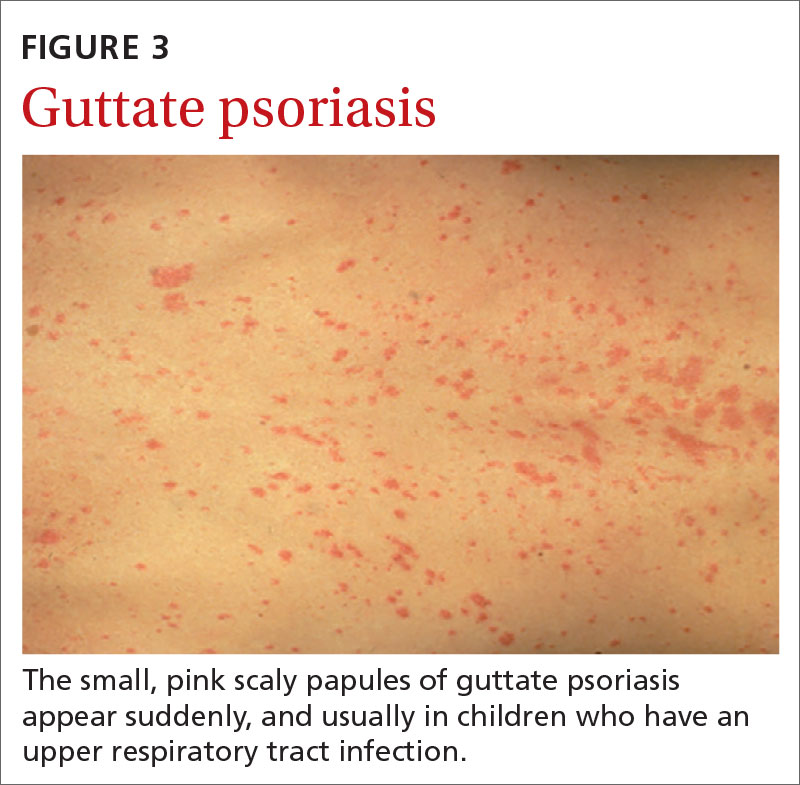
Erythrodermic psoriasis (FIGUREs 4A and 4B) involves at least 75% of the body with erythema and scaling.11 Erythroderma can be caused by many other conditions such as atopic dermatitis, a drug reaction, Sezary syndrome, seborrheic dermatitis, and pityriasis rubra pilaris. Treatments for other conditions in the differential diagnosis can potentially make psoriasis worse. Unfortunately, findings on a skin biopsy are often nonspecific, making careful clinical observation crucial to arriving at an accurate diagnosis.

Pustular psoriasis is characterized by bright erythema and sterile pustules. Pustular psoriasis can be triggered by pregnancy, sudden tapering of corticosteroids, hypocalcemia, and infection. Involvement of the palms and soles with severe desquamation can drastically impact daily functioning and quality of life.
Inverse or flexural psoriasis (FIGUREs 5A and 5B) is characterized by shiny, pink-to-red sharply demarcated plaques involving intertriginous areas, typically the groin, inguinal crease, axilla, inframammary regions, and intergluteal cleft.
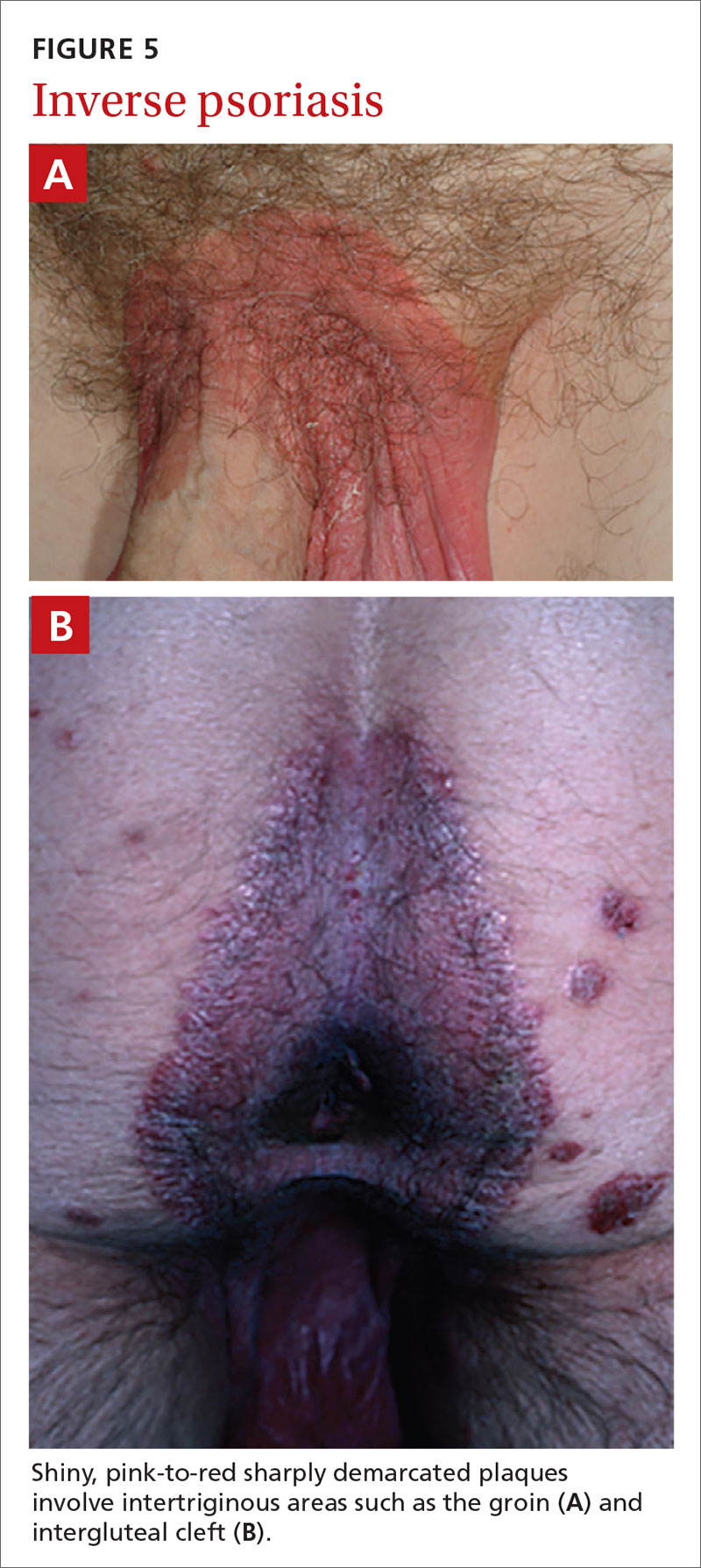
Continue to: Geographic tongue
Geographic tongue describes psoriasis of the tongue. The mucosa of the tongue has white plaques with a geographic border. Instead of scale, the moisture on the tongue causes areas of hyperkeratosis that appear white.
Nail psoriasis can manifest as nail pitting (FIGURE 6), oil staining, onycholysis (distal lifting of the nail), and subungual hyperkeratosis. Nail psoriasis is often quite distressing for patients and can be difficult to treat.
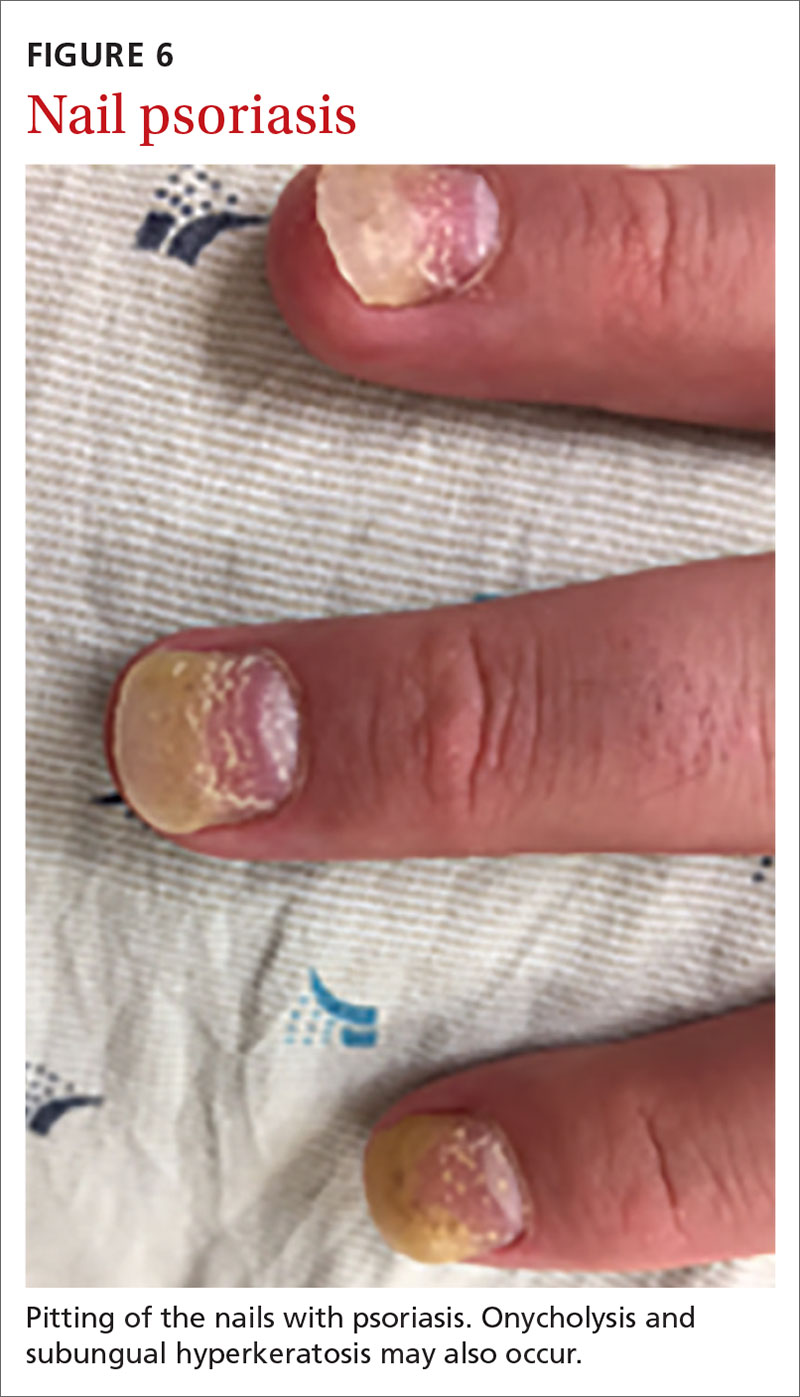
Palmoplantar psoriasis (FIGUREs 7A and 7B) can be painful due to the involvement of the palms of the hands and soles of the feet. Lesions will either be similar to other psoriatic plaques with well-demarcated erythematous scaling lesions or involve thickening and scale without associated erythema.
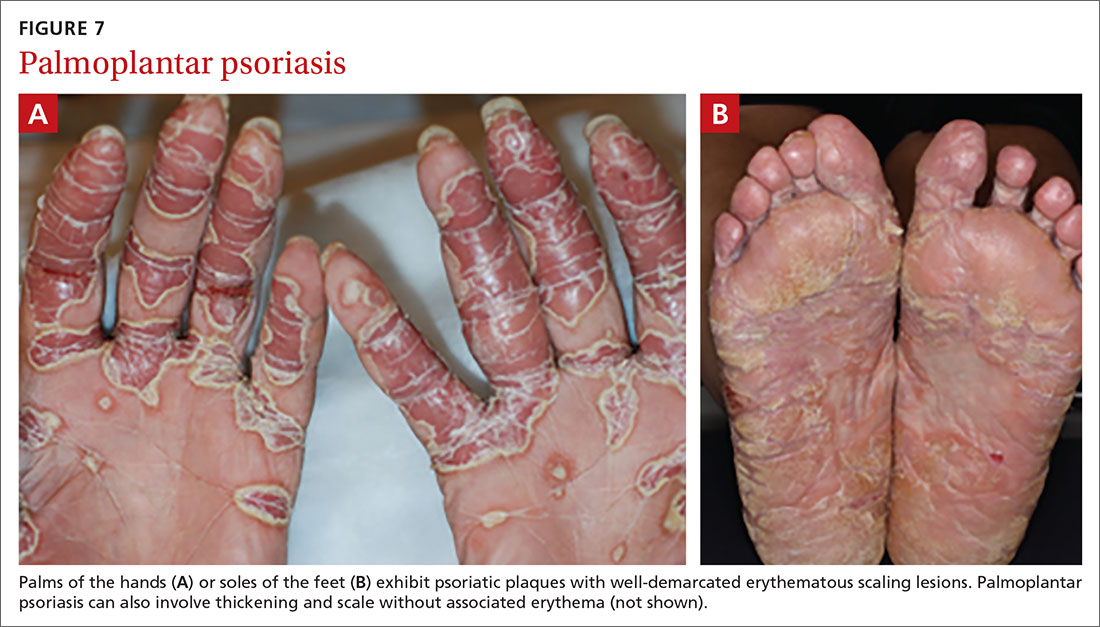
Psoriatic arthritis can cause significant joint damage and disability. Most affected individuals with psoriatic arthritis have a history of preceding skin disease.12 There are no specific lab tests for psoriasis; radiologic studies can show bulky syndesmophytes, central and marginal erosions, and periostitis. Patterns of joint involvement are variable. Psoriatic arthritis is more likely to affect the distal interphalangeal joints than rheumatoid arthritis and is more likely to affect the metacarpophalangeal joints than osteoarthritis.13
Psoriatic arthritis often progresses insidiously and is commonly described as causing discomfort rather than acute pain. Enthesitis, inflammation at the site where tendons or ligaments insert into the bone, is often present. Joint destruction may lead to the telescoping “opera glass” digit (FIGURE 8).
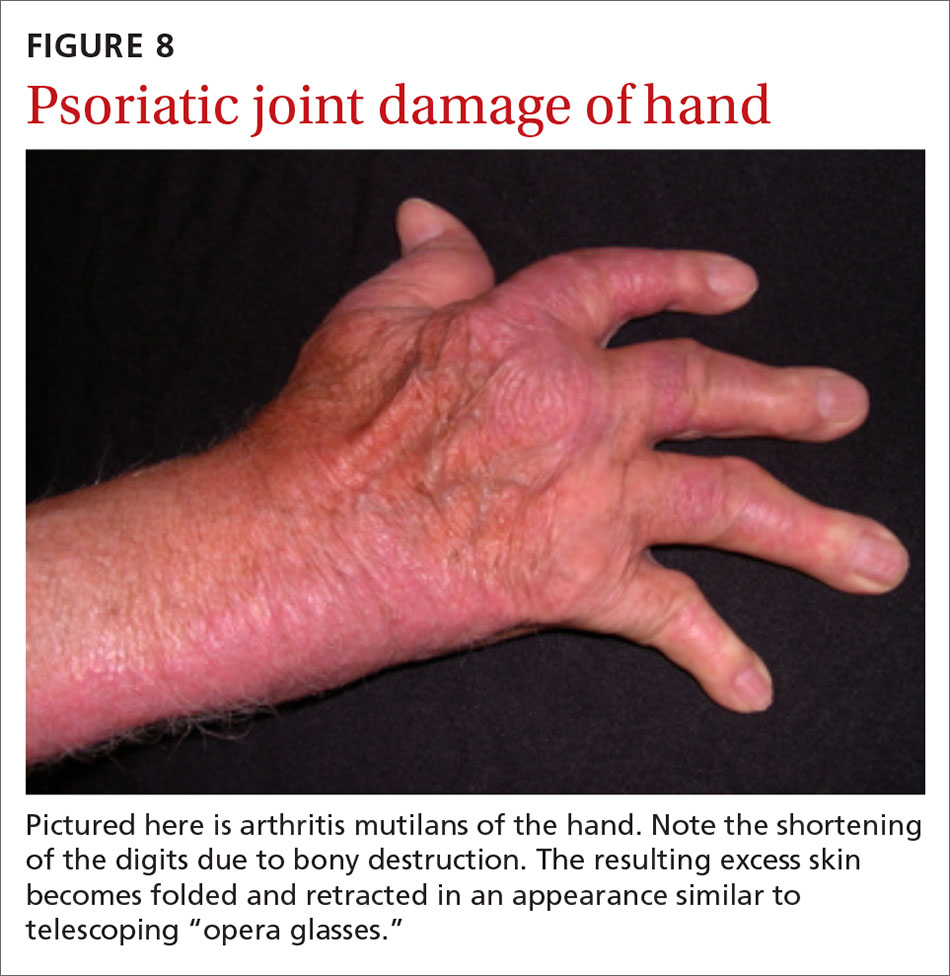
Continue to: Drug-provoked psoriasis
Drug-provoked psoriasis is divided into 2 groups: drug-induced and drug-aggravated. Drug-induced psoriasis will improve after discontinuation of the causative drug and tends to occur in patients without a personal or family history of psoriasis. Drug-aggravated psoriasis continues to progress after the discontinuation of the offending drug and is more often seen in patients with a history of psoriasis.14 Drugs that most commonly provoke psoriasis are beta-blockers, lithium, and antimalarials.10 Other potentially aggravating agents include antibiotics, digoxin, and nonsteroidal anti-inflammatory drugs.10
Consider these skin disorders in the differential diagnosis
The diagnosis of psoriasis is usually clinical, and a skin biopsy is rarely needed. However, a range of other skin disorders should be kept in mind when considering the differential diagnosis.
Mycosis fungoides is a type of cutaneous T-cell lymphoma that forms erythematous plaques that may show wrinkling and epidermal atrophy in sun-protected sites. Onset usually occurs among the elderly.
Pityriasis rubra pilaris is characterized by salmon-colored patches that may have small areas of normal skin (“islands of sparing”), hyperkeratotic follicular papules, and hyperkeratosis of the palms and soles.
Seborrheic dermatitis, dandruff of the skin, usually involves the scalp and nasolabial areas and the T-zone of the face.
Continue to: Lichen planus
Lichen planus usually appears slightly more purple than psoriasis and typically involves the mouth, flexural surfaces of the wrists, genitals, and ankles.
Other conditions in the differential include pityriasis lichenoides chronica, which may be identified on skin biopsy. Inverse psoriasis can be difficult to differentiate from candida intertrigo, erythrasma, or tinea cruris.
A potassium hydroxide (KOH) preparation can help differentiate psoriasis from candida or tinea. In psoriasis, a KOH test will be negative for fungal elements. Mycology culture on skin scrapings may be performed to rule out fungal infection. Erythrasma may exhibit a coral red appearance under Wood lamp examination.
If a lesion fails to respond to appropriate treatment, a careful drug history and biopsy can help clarify the diagnosis.
Document disease
It’s important to thoroughly document the extent and severity of the psoriasis and to monitor the impact of treatment. The Psoriasis Area and Severity Index is a commonly used method that calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions (head, upper extremities, trunk, and lower extremities), as well as the degree of erythema, induration, and scaling of lesions. The average redness, thickness, and scaling are graded on a scale of 0 to 4 and the extent of involvement is calculated to form a total numerical score ranging from 0 (no disease) to 72 (maximal disease).
Continue to: Many options in the treatment arsenal
Many options in the treatment arsenal
Many treatments can improve psoriasis.9,15-19 Most affected individuals discover that emollients and exposure to natural sunlight can be effective, as are soothing baths (balneotherapy) or topical coal tar application. More persistent disease requires prescription therapy. Individualize therapy according to the severity of disease, location of the lesions, involvement of joints, and comorbidities (FIGURE 9).15
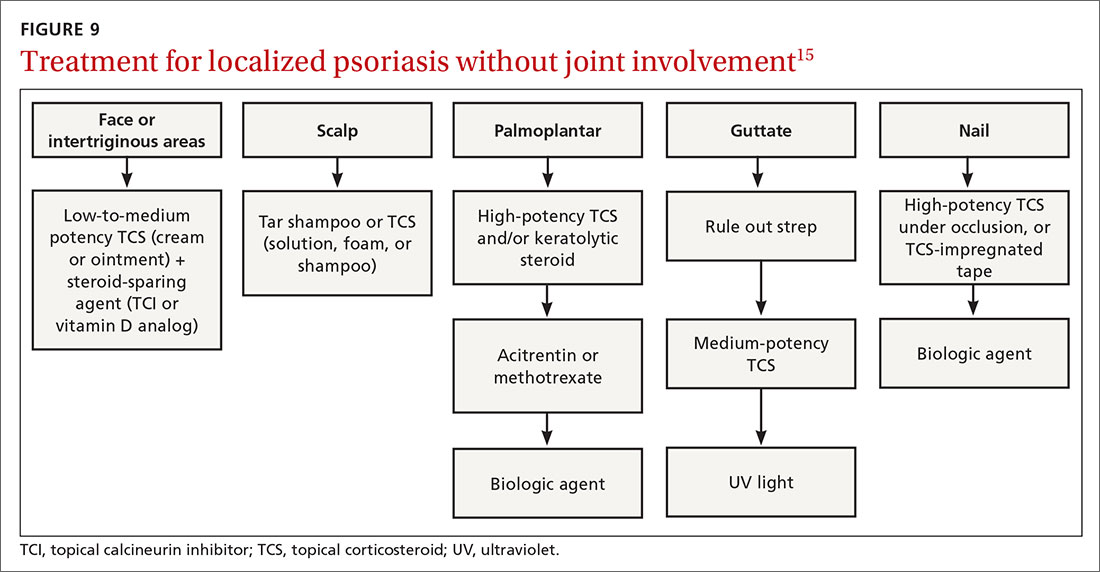
If ≤ 10% of the body surface area is involved, treatment options generally are explored in a stepwise progression from safest and most affordable to more involved therapies as needed: moisturization and avoidance of repetitive trauma, topical corticosteroids (TCS), vitamin D analogs, topical calcineurin inhibitors, and vitamin A creams. Recalcitrant disease will likely require ultraviolet (UV) light treatment or a systemic agent.15
If > 10% of the body surface area is involved, but joints are not involved, consider UV light treatment or a combination of alcitretin and TCS. If the joints are involved, likely initial options would be methotrexate, cyclosporine, or TNF-α inhibitor. Additional options to consider are anti-IL-17 or anti-IL-23 agents.15
If there’s joint involvement. In individuals with mild peripheral arthritis involving fewer than 4 joints without evidence of joint damage on imaging, nonsteroidal anti-inflammatory drugs are the mainstay of treatment. If the peripheral arthritis persists, or if it is associated with moderate-to-severe erosions or with substantial functional limitations, initiate treatment with a conventional disease-modifying antirheumatic drug. If the disease remains active, consider biologic agents.
Case studies
Mild-to-moderate psoriasis
Patient A is a 19-year-old woman presenting for evaluation of a persistently dry, flaking scalp. She has had the itchy scalp for years, as well as several small “patches” across her elbows, legs, knees, and abdomen. Over-the-counter emollients have not helped. The patient also says she has had brittle nails on several of her fingers, which she keeps covered with thick polish.
Continue to: The condition exemplified...
The condition exemplified by Patient A can typically be managed with topical products.
Topical steroids may be classified by different delivery vehicles, active ingredients, and potencies. The National Psoriasis Foundation's Topical Steroids Potency Chart can provide guidance (visit www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart and scroll down). Prescribing an appropriate amount is important; the standard 30-g prescription tube is generally required to cover the entire skin surface. Ointments have a greasy consistency (typically a petroleum base), which enhances potency and hydrates the skin. Creams and lotions are easier to rub on and spread. Gels are alcohol based and readily absorbed.16 Solutions, foams, and shampoos are particularly useful to treat psoriasis in hairy areas such as the scalp.
Corticosteroid potency ranges from Class I to Class VII, with the former being the most potent. While TCS products are typically effective with minimal systemic absorption, it is important to counsel patients on the risk of skin atrophy, impaired wound healing, and skin pigmentation changes with chronic use. With nail psoriasis, a potent topical steroid (including flurandrenolide [Cordran] tape) applied to the proximal nail fold has shown benefit.20
Topical calcineurin inhibitors (TCIs; eg, tacrolimus ointment and pimecrolimus cream) are anti-inflammatory agents often used in conjunction with topical steroids to minimize steroid use and associated adverse effects.15 A possible steroid-sparing regimen includes using a TCI Monday through Friday and a topical steroid on the weekend.
Topical vitamin D analogs (calcipotriene, calcipotriol, calcitriol) inhibit proliferation of keratinocytes and decrease the production of inflammatory mediators.15,17-19,21 Application of a vitamin D analog in combination with a high-potency TCS, systemic treatment, or phototherapy can provide greater efficacy, a more rapid onset of action, and less irritation than can the vitamin D analog used alone.21 If used in combination with UV light, apply topical vitamin D after the light therapy to prevent degradation.
Continue to: UV light therapy
UV light therapy is often used in cases refractory to topical therapy. Patients are typically prescribed 2 to 3 treatments per week with narrowband UVB (311-313 nm), the excimer laser (308 nm), or, less commonly, PUVA (UV treatment with psoralens). Treatment begins with a minimal erythema dose—the lowest dose to achieve minimal erythema of the skin before burning. When that is determined, exposure is increased as needed—depending on the response. If this is impractical or too time-consuming for the patient, an alternative recommendation would be increased exposure to natural sunlight or even use of a tanning booth. However, patients must then be cautioned about the increased risk of skin cancer.
Refractory/severe psoriasis
Patient B is a 35-year-old man with a longstanding history of psoriasis affecting his scalp and nails. Over the past 10 years, psoriatic lesions have also appeared and grown across his lower back, gluteal fold, legs, abdomen, and arms. He is now being evaluated by a rheumatologist for worsening symmetric joint pain that includes his lower back.
Methotrexate has been used to treat psoriasis and psoriatic arthritis since the 1950s. Methotrexate is a competitive inhibitor of dihydrofolate reductase and is typically given as an oral medication dosed once weekly with folic acid supplementation on the other 6 days.17 The most common adverse effects encountered with methotrexate are gastrointestinal upset and oral ulcers; however, routine monitoring for myelosuppression and hepatotoxicity is required.
Biologic therapy. When conventional therapies fail, immune-targeted treatment with “biologics” may be initiated. As knowledge of signaling pathways and the immunopathogenesis of psoriasis has increased, so has the number of biologic agents, which are generally well tolerated and effective in managing plaque psoriasis and psoriatic arthritis. Although their use, which requires monitoring, is handled primarily by specialists, familiarizing yourself with available agents can be helpful (TABLE).22
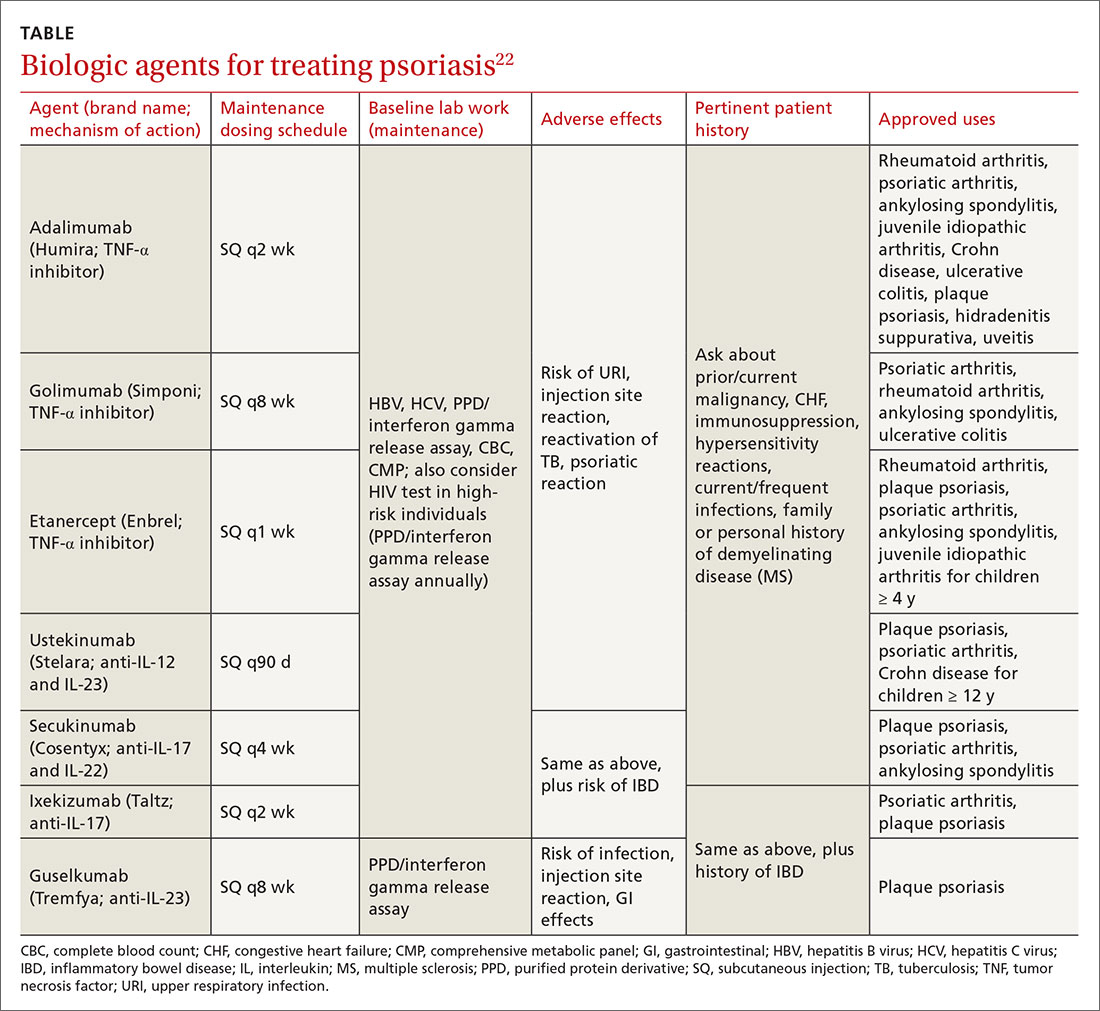
Nutritional modification and supplementation in treating skin disease still requires further investigation. Fish oil has shown benefit for cutaneous psoriasis in randomized controlled trials.7,8 Oral vitamin D supplementation requires further study, whereas selenium and B12 supplementation have not conferred consistent benefit.7 Given that several studies have demonstrated a relationship between body mass index and psoriatic disease severity, weight loss may be helpful in the management of psoriasis as well as psoriatic arthritis.8
Continue to: Other systemic agents
Other systemic agents—for individuals who cannot tolerate the biologic agents—include acitretin, azathioprine, mycophenolate mofetil, and cyclosporine.15,17
Paradoxical psoriatic reactions
When a psoriatic condition develops during biologic drug therapy, it is known as a paradoxical psoriatic reaction. The onset of de novo psoriasis has been documented during TNF-α inhibitor therapy for individuals with underlying rheumatoid arthritis.23 Skin biopsy reveals the same findings as common plaque psoriasis.
Using immunosuppressive Tx? Screen for tuberculosis
Testing to exclude a diagnosis of latent or undiagnosed tuberculosis must be performed prior to initiating immunosuppressive therapy with methotrexate or a biologic agent. Tuberculin skin testing, QuantiFERON-TB gold test, and the T-SPOT.TB test are accepted screening modalities. Discordance between tuberculin skin tests and the interferon gamma release assays in latent TB highlights the need for further study using the available QuantiFERON-TB gold test and the T-SPOT.TB test.24
CORRESPONDENCE
Karl T. Clebak, MD, FAAFP, Penn State Health Milton S. Hershey Medical Center, Department of Family and Community Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
1. Jackson R. John Updike on psoriasis. At war with my skin, from the journal of a leper. J Cutan Med Surg. 2000;4:113-115.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Alexander H, Nestle FO. Pathogenesis and immunotherapy in cutaneous psoriasis: what can rheumatologists learn? Curr Opin Rheumatol. 2017;29:71-78.
5. Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
7. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
8. Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
9. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
10. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
11. Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Aukl). 2016;6:93-104.
12. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748.
13. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29-38.
14. Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32-38.
15. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2013;26:787-801.
16. Helm MF, Farah JB, Carvalho M, et al. Compounded topical medications for diseases of the skin: a long tradition still relevant today. N Am J Med Sci. 2017;10:116-118.
17. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
18. Helfrich YR, Sachs DL, Kang S. Topical vitamin D3. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia, PA: Saunders; 2007:691-695.
19. Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35:268-269.
20. Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76:675-705.
21. Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
22. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:e2297.
23. Toussirot É, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biologic agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
24. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624.
The “heartbreak of psoriasis,” coined by an advertiser in the 1960s, conveyed the notion that this disease was a cosmetic disorder mainly limited to skin involvement. John Updike’s article in the September 1985 issue of The New Yorker, “At War With My Skin,” detailed Mr. Updike’s feelings of isolation and stress related to his condition, helping to reframe the popular concept of psoriasis.1 Updike’s eloquent account describing his struggles to find effective treatment increased public awareness about psoriasis, which in fact affects other body systems as well.
The overall prevalence of psoriasis is 1.5% to 3.1% in the United States and United Kingdom.2,3 More than 6.5 million adults in the United States > 20 years of age are affected.3 The most commonly affected demographic group is non-Hispanic Caucasians.
Our expanding knowledge of pathogenesis
Studies of genetic linkage have identified genes and single nucleotide polymorphisms associated with psoriasis.4 The interaction between environmental triggers and the innate and adaptive immune systems leads to keratinocyte hyperproliferation. Tumor necrosis factor (TNF), interleukin (IL) 23, and IL-17 are important cytokines associated with psoriatic inflammation.4 There are common pathways of inflammation in both psoriasis and cardiovascular disease resulting in oxidative stress and endothelial cell dysfunction.4 Ninety percent of early-onset psoriasis is associated with human leukocyte antigen (HLA)-Cw6.4 And alterations in the microbiome of the skin may contribute, as reduced microbial diversity has been found in psoriatic lesions.5
Comorbidities are common
Psoriasis is an independent risk factor for diabetes and major adverse cardiovascular events.6 Hypertension, dyslipidemia, inflammatory bowel disease, nonalcoholic fatty liver disease, chronic kidney disease, and lymphoma (particularly cutaneous T-cell lymphoma) are also associated with psoriasis.6 Psoriatic arthritis is frequently encountered with cutaneous psoriasis; however, it is often not recognized until late in the disease course.
There also appears to be an association among psoriasis, dietary factors, and celiac disease.7-9 Positive testing for IgA anti-endomysial antibodies and IgA tissue transglutaminase antibodies should prompt consideration of starting a gluten-free diet, which has been shown to improve psoriatic
The different types of psoriasis
The classic presentation of psoriasis involves stubborn plaques with silvery scale on extensor surfaces such as the elbows and knees. The severity of the disease corresponds with the amount of body surface area affected. While plaque-type psoriasis is the most common form, other patterns exist. Individuals may exhibit 1 dominant pattern or multiple psoriatic variants simultaneously. Most types of psoriasis have 3 characteristic features: erythema, skin thickening, and scales.
Certain history and physical clues can aid in diagnosing psoriasis; these include the Koebner phenomenon, the Auspitz sign, and the Woronoff ring. The Koebner phenomenon refers to the development of psoriatic lesions in an area of trauma (FIGURE 1), frequently resulting in a linear streak-like appearance. The Auspitz sign describes the pinpoint bleeding that may be encountered with the removal of a psoriatic plaque. The Woronoff ring is a pale blanching ring that may surround a psoriatic lesion.

Continue to: Chronic plaque-type psoriasis
Chronic plaque-type psoriasis (Figures 2A and 2B), the most common variant, is characterized by sharply demarcated pink papules and plaques with a silvery scale in a symmetric distribution on the extensor surfaces, scalp, trunk, and lumbosacral areas.

Guttate psoriasis (FIGURE 3) features small (often < 1 cm) pink scaly papules that appear suddenly. It is more commonly seen in children and is usually preceded by an upper respiratory tract infection, often with Streptococcus.10 If strep testing is positive, guttate psoriasis may improve after appropriate antibiotic treatment.

Erythrodermic psoriasis (FIGUREs 4A and 4B) involves at least 75% of the body with erythema and scaling.11 Erythroderma can be caused by many other conditions such as atopic dermatitis, a drug reaction, Sezary syndrome, seborrheic dermatitis, and pityriasis rubra pilaris. Treatments for other conditions in the differential diagnosis can potentially make psoriasis worse. Unfortunately, findings on a skin biopsy are often nonspecific, making careful clinical observation crucial to arriving at an accurate diagnosis.

Pustular psoriasis is characterized by bright erythema and sterile pustules. Pustular psoriasis can be triggered by pregnancy, sudden tapering of corticosteroids, hypocalcemia, and infection. Involvement of the palms and soles with severe desquamation can drastically impact daily functioning and quality of life.
Inverse or flexural psoriasis (FIGUREs 5A and 5B) is characterized by shiny, pink-to-red sharply demarcated plaques involving intertriginous areas, typically the groin, inguinal crease, axilla, inframammary regions, and intergluteal cleft.

Continue to: Geographic tongue
Geographic tongue describes psoriasis of the tongue. The mucosa of the tongue has white plaques with a geographic border. Instead of scale, the moisture on the tongue causes areas of hyperkeratosis that appear white.
Nail psoriasis can manifest as nail pitting (FIGURE 6), oil staining, onycholysis (distal lifting of the nail), and subungual hyperkeratosis. Nail psoriasis is often quite distressing for patients and can be difficult to treat.

Palmoplantar psoriasis (FIGUREs 7A and 7B) can be painful due to the involvement of the palms of the hands and soles of the feet. Lesions will either be similar to other psoriatic plaques with well-demarcated erythematous scaling lesions or involve thickening and scale without associated erythema.

Psoriatic arthritis can cause significant joint damage and disability. Most affected individuals with psoriatic arthritis have a history of preceding skin disease.12 There are no specific lab tests for psoriasis; radiologic studies can show bulky syndesmophytes, central and marginal erosions, and periostitis. Patterns of joint involvement are variable. Psoriatic arthritis is more likely to affect the distal interphalangeal joints than rheumatoid arthritis and is more likely to affect the metacarpophalangeal joints than osteoarthritis.13
Psoriatic arthritis often progresses insidiously and is commonly described as causing discomfort rather than acute pain. Enthesitis, inflammation at the site where tendons or ligaments insert into the bone, is often present. Joint destruction may lead to the telescoping “opera glass” digit (FIGURE 8).

Continue to: Drug-provoked psoriasis
Drug-provoked psoriasis is divided into 2 groups: drug-induced and drug-aggravated. Drug-induced psoriasis will improve after discontinuation of the causative drug and tends to occur in patients without a personal or family history of psoriasis. Drug-aggravated psoriasis continues to progress after the discontinuation of the offending drug and is more often seen in patients with a history of psoriasis.14 Drugs that most commonly provoke psoriasis are beta-blockers, lithium, and antimalarials.10 Other potentially aggravating agents include antibiotics, digoxin, and nonsteroidal anti-inflammatory drugs.10
Consider these skin disorders in the differential diagnosis
The diagnosis of psoriasis is usually clinical, and a skin biopsy is rarely needed. However, a range of other skin disorders should be kept in mind when considering the differential diagnosis.
Mycosis fungoides is a type of cutaneous T-cell lymphoma that forms erythematous plaques that may show wrinkling and epidermal atrophy in sun-protected sites. Onset usually occurs among the elderly.
Pityriasis rubra pilaris is characterized by salmon-colored patches that may have small areas of normal skin (“islands of sparing”), hyperkeratotic follicular papules, and hyperkeratosis of the palms and soles.
Seborrheic dermatitis, dandruff of the skin, usually involves the scalp and nasolabial areas and the T-zone of the face.
Continue to: Lichen planus
Lichen planus usually appears slightly more purple than psoriasis and typically involves the mouth, flexural surfaces of the wrists, genitals, and ankles.
Other conditions in the differential include pityriasis lichenoides chronica, which may be identified on skin biopsy. Inverse psoriasis can be difficult to differentiate from candida intertrigo, erythrasma, or tinea cruris.
A potassium hydroxide (KOH) preparation can help differentiate psoriasis from candida or tinea. In psoriasis, a KOH test will be negative for fungal elements. Mycology culture on skin scrapings may be performed to rule out fungal infection. Erythrasma may exhibit a coral red appearance under Wood lamp examination.
If a lesion fails to respond to appropriate treatment, a careful drug history and biopsy can help clarify the diagnosis.
Document disease
It’s important to thoroughly document the extent and severity of the psoriasis and to monitor the impact of treatment. The Psoriasis Area and Severity Index is a commonly used method that calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions (head, upper extremities, trunk, and lower extremities), as well as the degree of erythema, induration, and scaling of lesions. The average redness, thickness, and scaling are graded on a scale of 0 to 4 and the extent of involvement is calculated to form a total numerical score ranging from 0 (no disease) to 72 (maximal disease).
Continue to: Many options in the treatment arsenal
Many options in the treatment arsenal
Many treatments can improve psoriasis.9,15-19 Most affected individuals discover that emollients and exposure to natural sunlight can be effective, as are soothing baths (balneotherapy) or topical coal tar application. More persistent disease requires prescription therapy. Individualize therapy according to the severity of disease, location of the lesions, involvement of joints, and comorbidities (FIGURE 9).15

If ≤ 10% of the body surface area is involved, treatment options generally are explored in a stepwise progression from safest and most affordable to more involved therapies as needed: moisturization and avoidance of repetitive trauma, topical corticosteroids (TCS), vitamin D analogs, topical calcineurin inhibitors, and vitamin A creams. Recalcitrant disease will likely require ultraviolet (UV) light treatment or a systemic agent.15
If > 10% of the body surface area is involved, but joints are not involved, consider UV light treatment or a combination of alcitretin and TCS. If the joints are involved, likely initial options would be methotrexate, cyclosporine, or TNF-α inhibitor. Additional options to consider are anti-IL-17 or anti-IL-23 agents.15
If there’s joint involvement. In individuals with mild peripheral arthritis involving fewer than 4 joints without evidence of joint damage on imaging, nonsteroidal anti-inflammatory drugs are the mainstay of treatment. If the peripheral arthritis persists, or if it is associated with moderate-to-severe erosions or with substantial functional limitations, initiate treatment with a conventional disease-modifying antirheumatic drug. If the disease remains active, consider biologic agents.
Case studies
Mild-to-moderate psoriasis
Patient A is a 19-year-old woman presenting for evaluation of a persistently dry, flaking scalp. She has had the itchy scalp for years, as well as several small “patches” across her elbows, legs, knees, and abdomen. Over-the-counter emollients have not helped. The patient also says she has had brittle nails on several of her fingers, which she keeps covered with thick polish.
Continue to: The condition exemplified...
The condition exemplified by Patient A can typically be managed with topical products.
Topical steroids may be classified by different delivery vehicles, active ingredients, and potencies. The National Psoriasis Foundation's Topical Steroids Potency Chart can provide guidance (visit www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart and scroll down). Prescribing an appropriate amount is important; the standard 30-g prescription tube is generally required to cover the entire skin surface. Ointments have a greasy consistency (typically a petroleum base), which enhances potency and hydrates the skin. Creams and lotions are easier to rub on and spread. Gels are alcohol based and readily absorbed.16 Solutions, foams, and shampoos are particularly useful to treat psoriasis in hairy areas such as the scalp.
Corticosteroid potency ranges from Class I to Class VII, with the former being the most potent. While TCS products are typically effective with minimal systemic absorption, it is important to counsel patients on the risk of skin atrophy, impaired wound healing, and skin pigmentation changes with chronic use. With nail psoriasis, a potent topical steroid (including flurandrenolide [Cordran] tape) applied to the proximal nail fold has shown benefit.20
Topical calcineurin inhibitors (TCIs; eg, tacrolimus ointment and pimecrolimus cream) are anti-inflammatory agents often used in conjunction with topical steroids to minimize steroid use and associated adverse effects.15 A possible steroid-sparing regimen includes using a TCI Monday through Friday and a topical steroid on the weekend.
Topical vitamin D analogs (calcipotriene, calcipotriol, calcitriol) inhibit proliferation of keratinocytes and decrease the production of inflammatory mediators.15,17-19,21 Application of a vitamin D analog in combination with a high-potency TCS, systemic treatment, or phototherapy can provide greater efficacy, a more rapid onset of action, and less irritation than can the vitamin D analog used alone.21 If used in combination with UV light, apply topical vitamin D after the light therapy to prevent degradation.
Continue to: UV light therapy
UV light therapy is often used in cases refractory to topical therapy. Patients are typically prescribed 2 to 3 treatments per week with narrowband UVB (311-313 nm), the excimer laser (308 nm), or, less commonly, PUVA (UV treatment with psoralens). Treatment begins with a minimal erythema dose—the lowest dose to achieve minimal erythema of the skin before burning. When that is determined, exposure is increased as needed—depending on the response. If this is impractical or too time-consuming for the patient, an alternative recommendation would be increased exposure to natural sunlight or even use of a tanning booth. However, patients must then be cautioned about the increased risk of skin cancer.
Refractory/severe psoriasis
Patient B is a 35-year-old man with a longstanding history of psoriasis affecting his scalp and nails. Over the past 10 years, psoriatic lesions have also appeared and grown across his lower back, gluteal fold, legs, abdomen, and arms. He is now being evaluated by a rheumatologist for worsening symmetric joint pain that includes his lower back.
Methotrexate has been used to treat psoriasis and psoriatic arthritis since the 1950s. Methotrexate is a competitive inhibitor of dihydrofolate reductase and is typically given as an oral medication dosed once weekly with folic acid supplementation on the other 6 days.17 The most common adverse effects encountered with methotrexate are gastrointestinal upset and oral ulcers; however, routine monitoring for myelosuppression and hepatotoxicity is required.
Biologic therapy. When conventional therapies fail, immune-targeted treatment with “biologics” may be initiated. As knowledge of signaling pathways and the immunopathogenesis of psoriasis has increased, so has the number of biologic agents, which are generally well tolerated and effective in managing plaque psoriasis and psoriatic arthritis. Although their use, which requires monitoring, is handled primarily by specialists, familiarizing yourself with available agents can be helpful (TABLE).22

Nutritional modification and supplementation in treating skin disease still requires further investigation. Fish oil has shown benefit for cutaneous psoriasis in randomized controlled trials.7,8 Oral vitamin D supplementation requires further study, whereas selenium and B12 supplementation have not conferred consistent benefit.7 Given that several studies have demonstrated a relationship between body mass index and psoriatic disease severity, weight loss may be helpful in the management of psoriasis as well as psoriatic arthritis.8
Continue to: Other systemic agents
Other systemic agents—for individuals who cannot tolerate the biologic agents—include acitretin, azathioprine, mycophenolate mofetil, and cyclosporine.15,17
Paradoxical psoriatic reactions
When a psoriatic condition develops during biologic drug therapy, it is known as a paradoxical psoriatic reaction. The onset of de novo psoriasis has been documented during TNF-α inhibitor therapy for individuals with underlying rheumatoid arthritis.23 Skin biopsy reveals the same findings as common plaque psoriasis.
Using immunosuppressive Tx? Screen for tuberculosis
Testing to exclude a diagnosis of latent or undiagnosed tuberculosis must be performed prior to initiating immunosuppressive therapy with methotrexate or a biologic agent. Tuberculin skin testing, QuantiFERON-TB gold test, and the T-SPOT.TB test are accepted screening modalities. Discordance between tuberculin skin tests and the interferon gamma release assays in latent TB highlights the need for further study using the available QuantiFERON-TB gold test and the T-SPOT.TB test.24
CORRESPONDENCE
Karl T. Clebak, MD, FAAFP, Penn State Health Milton S. Hershey Medical Center, Department of Family and Community Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
The “heartbreak of psoriasis,” coined by an advertiser in the 1960s, conveyed the notion that this disease was a cosmetic disorder mainly limited to skin involvement. John Updike’s article in the September 1985 issue of The New Yorker, “At War With My Skin,” detailed Mr. Updike’s feelings of isolation and stress related to his condition, helping to reframe the popular concept of psoriasis.1 Updike’s eloquent account describing his struggles to find effective treatment increased public awareness about psoriasis, which in fact affects other body systems as well.
The overall prevalence of psoriasis is 1.5% to 3.1% in the United States and United Kingdom.2,3 More than 6.5 million adults in the United States > 20 years of age are affected.3 The most commonly affected demographic group is non-Hispanic Caucasians.
Our expanding knowledge of pathogenesis
Studies of genetic linkage have identified genes and single nucleotide polymorphisms associated with psoriasis.4 The interaction between environmental triggers and the innate and adaptive immune systems leads to keratinocyte hyperproliferation. Tumor necrosis factor (TNF), interleukin (IL) 23, and IL-17 are important cytokines associated with psoriatic inflammation.4 There are common pathways of inflammation in both psoriasis and cardiovascular disease resulting in oxidative stress and endothelial cell dysfunction.4 Ninety percent of early-onset psoriasis is associated with human leukocyte antigen (HLA)-Cw6.4 And alterations in the microbiome of the skin may contribute, as reduced microbial diversity has been found in psoriatic lesions.5
Comorbidities are common
Psoriasis is an independent risk factor for diabetes and major adverse cardiovascular events.6 Hypertension, dyslipidemia, inflammatory bowel disease, nonalcoholic fatty liver disease, chronic kidney disease, and lymphoma (particularly cutaneous T-cell lymphoma) are also associated with psoriasis.6 Psoriatic arthritis is frequently encountered with cutaneous psoriasis; however, it is often not recognized until late in the disease course.
There also appears to be an association among psoriasis, dietary factors, and celiac disease.7-9 Positive testing for IgA anti-endomysial antibodies and IgA tissue transglutaminase antibodies should prompt consideration of starting a gluten-free diet, which has been shown to improve psoriatic
The different types of psoriasis
The classic presentation of psoriasis involves stubborn plaques with silvery scale on extensor surfaces such as the elbows and knees. The severity of the disease corresponds with the amount of body surface area affected. While plaque-type psoriasis is the most common form, other patterns exist. Individuals may exhibit 1 dominant pattern or multiple psoriatic variants simultaneously. Most types of psoriasis have 3 characteristic features: erythema, skin thickening, and scales.
Certain history and physical clues can aid in diagnosing psoriasis; these include the Koebner phenomenon, the Auspitz sign, and the Woronoff ring. The Koebner phenomenon refers to the development of psoriatic lesions in an area of trauma (FIGURE 1), frequently resulting in a linear streak-like appearance. The Auspitz sign describes the pinpoint bleeding that may be encountered with the removal of a psoriatic plaque. The Woronoff ring is a pale blanching ring that may surround a psoriatic lesion.

Continue to: Chronic plaque-type psoriasis
Chronic plaque-type psoriasis (Figures 2A and 2B), the most common variant, is characterized by sharply demarcated pink papules and plaques with a silvery scale in a symmetric distribution on the extensor surfaces, scalp, trunk, and lumbosacral areas.

Guttate psoriasis (FIGURE 3) features small (often < 1 cm) pink scaly papules that appear suddenly. It is more commonly seen in children and is usually preceded by an upper respiratory tract infection, often with Streptococcus.10 If strep testing is positive, guttate psoriasis may improve after appropriate antibiotic treatment.

Erythrodermic psoriasis (FIGUREs 4A and 4B) involves at least 75% of the body with erythema and scaling.11 Erythroderma can be caused by many other conditions such as atopic dermatitis, a drug reaction, Sezary syndrome, seborrheic dermatitis, and pityriasis rubra pilaris. Treatments for other conditions in the differential diagnosis can potentially make psoriasis worse. Unfortunately, findings on a skin biopsy are often nonspecific, making careful clinical observation crucial to arriving at an accurate diagnosis.

Pustular psoriasis is characterized by bright erythema and sterile pustules. Pustular psoriasis can be triggered by pregnancy, sudden tapering of corticosteroids, hypocalcemia, and infection. Involvement of the palms and soles with severe desquamation can drastically impact daily functioning and quality of life.
Inverse or flexural psoriasis (FIGUREs 5A and 5B) is characterized by shiny, pink-to-red sharply demarcated plaques involving intertriginous areas, typically the groin, inguinal crease, axilla, inframammary regions, and intergluteal cleft.

Continue to: Geographic tongue
Geographic tongue describes psoriasis of the tongue. The mucosa of the tongue has white plaques with a geographic border. Instead of scale, the moisture on the tongue causes areas of hyperkeratosis that appear white.
Nail psoriasis can manifest as nail pitting (FIGURE 6), oil staining, onycholysis (distal lifting of the nail), and subungual hyperkeratosis. Nail psoriasis is often quite distressing for patients and can be difficult to treat.

Palmoplantar psoriasis (FIGUREs 7A and 7B) can be painful due to the involvement of the palms of the hands and soles of the feet. Lesions will either be similar to other psoriatic plaques with well-demarcated erythematous scaling lesions or involve thickening and scale without associated erythema.

Psoriatic arthritis can cause significant joint damage and disability. Most affected individuals with psoriatic arthritis have a history of preceding skin disease.12 There are no specific lab tests for psoriasis; radiologic studies can show bulky syndesmophytes, central and marginal erosions, and periostitis. Patterns of joint involvement are variable. Psoriatic arthritis is more likely to affect the distal interphalangeal joints than rheumatoid arthritis and is more likely to affect the metacarpophalangeal joints than osteoarthritis.13
Psoriatic arthritis often progresses insidiously and is commonly described as causing discomfort rather than acute pain. Enthesitis, inflammation at the site where tendons or ligaments insert into the bone, is often present. Joint destruction may lead to the telescoping “opera glass” digit (FIGURE 8).

Continue to: Drug-provoked psoriasis
Drug-provoked psoriasis is divided into 2 groups: drug-induced and drug-aggravated. Drug-induced psoriasis will improve after discontinuation of the causative drug and tends to occur in patients without a personal or family history of psoriasis. Drug-aggravated psoriasis continues to progress after the discontinuation of the offending drug and is more often seen in patients with a history of psoriasis.14 Drugs that most commonly provoke psoriasis are beta-blockers, lithium, and antimalarials.10 Other potentially aggravating agents include antibiotics, digoxin, and nonsteroidal anti-inflammatory drugs.10
Consider these skin disorders in the differential diagnosis
The diagnosis of psoriasis is usually clinical, and a skin biopsy is rarely needed. However, a range of other skin disorders should be kept in mind when considering the differential diagnosis.
Mycosis fungoides is a type of cutaneous T-cell lymphoma that forms erythematous plaques that may show wrinkling and epidermal atrophy in sun-protected sites. Onset usually occurs among the elderly.
Pityriasis rubra pilaris is characterized by salmon-colored patches that may have small areas of normal skin (“islands of sparing”), hyperkeratotic follicular papules, and hyperkeratosis of the palms and soles.
Seborrheic dermatitis, dandruff of the skin, usually involves the scalp and nasolabial areas and the T-zone of the face.
Continue to: Lichen planus
Lichen planus usually appears slightly more purple than psoriasis and typically involves the mouth, flexural surfaces of the wrists, genitals, and ankles.
Other conditions in the differential include pityriasis lichenoides chronica, which may be identified on skin biopsy. Inverse psoriasis can be difficult to differentiate from candida intertrigo, erythrasma, or tinea cruris.
A potassium hydroxide (KOH) preparation can help differentiate psoriasis from candida or tinea. In psoriasis, a KOH test will be negative for fungal elements. Mycology culture on skin scrapings may be performed to rule out fungal infection. Erythrasma may exhibit a coral red appearance under Wood lamp examination.
If a lesion fails to respond to appropriate treatment, a careful drug history and biopsy can help clarify the diagnosis.
Document disease
It’s important to thoroughly document the extent and severity of the psoriasis and to monitor the impact of treatment. The Psoriasis Area and Severity Index is a commonly used method that calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions (head, upper extremities, trunk, and lower extremities), as well as the degree of erythema, induration, and scaling of lesions. The average redness, thickness, and scaling are graded on a scale of 0 to 4 and the extent of involvement is calculated to form a total numerical score ranging from 0 (no disease) to 72 (maximal disease).
Continue to: Many options in the treatment arsenal
Many options in the treatment arsenal
Many treatments can improve psoriasis.9,15-19 Most affected individuals discover that emollients and exposure to natural sunlight can be effective, as are soothing baths (balneotherapy) or topical coal tar application. More persistent disease requires prescription therapy. Individualize therapy according to the severity of disease, location of the lesions, involvement of joints, and comorbidities (FIGURE 9).15

If ≤ 10% of the body surface area is involved, treatment options generally are explored in a stepwise progression from safest and most affordable to more involved therapies as needed: moisturization and avoidance of repetitive trauma, topical corticosteroids (TCS), vitamin D analogs, topical calcineurin inhibitors, and vitamin A creams. Recalcitrant disease will likely require ultraviolet (UV) light treatment or a systemic agent.15
If > 10% of the body surface area is involved, but joints are not involved, consider UV light treatment or a combination of alcitretin and TCS. If the joints are involved, likely initial options would be methotrexate, cyclosporine, or TNF-α inhibitor. Additional options to consider are anti-IL-17 or anti-IL-23 agents.15
If there’s joint involvement. In individuals with mild peripheral arthritis involving fewer than 4 joints without evidence of joint damage on imaging, nonsteroidal anti-inflammatory drugs are the mainstay of treatment. If the peripheral arthritis persists, or if it is associated with moderate-to-severe erosions or with substantial functional limitations, initiate treatment with a conventional disease-modifying antirheumatic drug. If the disease remains active, consider biologic agents.
Case studies
Mild-to-moderate psoriasis
Patient A is a 19-year-old woman presenting for evaluation of a persistently dry, flaking scalp. She has had the itchy scalp for years, as well as several small “patches” across her elbows, legs, knees, and abdomen. Over-the-counter emollients have not helped. The patient also says she has had brittle nails on several of her fingers, which she keeps covered with thick polish.
Continue to: The condition exemplified...
The condition exemplified by Patient A can typically be managed with topical products.
Topical steroids may be classified by different delivery vehicles, active ingredients, and potencies. The National Psoriasis Foundation's Topical Steroids Potency Chart can provide guidance (visit www.psoriasis.org/about-psoriasis/treatments/topicals/steroids/potency-chart and scroll down). Prescribing an appropriate amount is important; the standard 30-g prescription tube is generally required to cover the entire skin surface. Ointments have a greasy consistency (typically a petroleum base), which enhances potency and hydrates the skin. Creams and lotions are easier to rub on and spread. Gels are alcohol based and readily absorbed.16 Solutions, foams, and shampoos are particularly useful to treat psoriasis in hairy areas such as the scalp.
Corticosteroid potency ranges from Class I to Class VII, with the former being the most potent. While TCS products are typically effective with minimal systemic absorption, it is important to counsel patients on the risk of skin atrophy, impaired wound healing, and skin pigmentation changes with chronic use. With nail psoriasis, a potent topical steroid (including flurandrenolide [Cordran] tape) applied to the proximal nail fold has shown benefit.20
Topical calcineurin inhibitors (TCIs; eg, tacrolimus ointment and pimecrolimus cream) are anti-inflammatory agents often used in conjunction with topical steroids to minimize steroid use and associated adverse effects.15 A possible steroid-sparing regimen includes using a TCI Monday through Friday and a topical steroid on the weekend.
Topical vitamin D analogs (calcipotriene, calcipotriol, calcitriol) inhibit proliferation of keratinocytes and decrease the production of inflammatory mediators.15,17-19,21 Application of a vitamin D analog in combination with a high-potency TCS, systemic treatment, or phototherapy can provide greater efficacy, a more rapid onset of action, and less irritation than can the vitamin D analog used alone.21 If used in combination with UV light, apply topical vitamin D after the light therapy to prevent degradation.
Continue to: UV light therapy
UV light therapy is often used in cases refractory to topical therapy. Patients are typically prescribed 2 to 3 treatments per week with narrowband UVB (311-313 nm), the excimer laser (308 nm), or, less commonly, PUVA (UV treatment with psoralens). Treatment begins with a minimal erythema dose—the lowest dose to achieve minimal erythema of the skin before burning. When that is determined, exposure is increased as needed—depending on the response. If this is impractical or too time-consuming for the patient, an alternative recommendation would be increased exposure to natural sunlight or even use of a tanning booth. However, patients must then be cautioned about the increased risk of skin cancer.
Refractory/severe psoriasis
Patient B is a 35-year-old man with a longstanding history of psoriasis affecting his scalp and nails. Over the past 10 years, psoriatic lesions have also appeared and grown across his lower back, gluteal fold, legs, abdomen, and arms. He is now being evaluated by a rheumatologist for worsening symmetric joint pain that includes his lower back.
Methotrexate has been used to treat psoriasis and psoriatic arthritis since the 1950s. Methotrexate is a competitive inhibitor of dihydrofolate reductase and is typically given as an oral medication dosed once weekly with folic acid supplementation on the other 6 days.17 The most common adverse effects encountered with methotrexate are gastrointestinal upset and oral ulcers; however, routine monitoring for myelosuppression and hepatotoxicity is required.
Biologic therapy. When conventional therapies fail, immune-targeted treatment with “biologics” may be initiated. As knowledge of signaling pathways and the immunopathogenesis of psoriasis has increased, so has the number of biologic agents, which are generally well tolerated and effective in managing plaque psoriasis and psoriatic arthritis. Although their use, which requires monitoring, is handled primarily by specialists, familiarizing yourself with available agents can be helpful (TABLE).22

Nutritional modification and supplementation in treating skin disease still requires further investigation. Fish oil has shown benefit for cutaneous psoriasis in randomized controlled trials.7,8 Oral vitamin D supplementation requires further study, whereas selenium and B12 supplementation have not conferred consistent benefit.7 Given that several studies have demonstrated a relationship between body mass index and psoriatic disease severity, weight loss may be helpful in the management of psoriasis as well as psoriatic arthritis.8
Continue to: Other systemic agents
Other systemic agents—for individuals who cannot tolerate the biologic agents—include acitretin, azathioprine, mycophenolate mofetil, and cyclosporine.15,17
Paradoxical psoriatic reactions
When a psoriatic condition develops during biologic drug therapy, it is known as a paradoxical psoriatic reaction. The onset of de novo psoriasis has been documented during TNF-α inhibitor therapy for individuals with underlying rheumatoid arthritis.23 Skin biopsy reveals the same findings as common plaque psoriasis.
Using immunosuppressive Tx? Screen for tuberculosis
Testing to exclude a diagnosis of latent or undiagnosed tuberculosis must be performed prior to initiating immunosuppressive therapy with methotrexate or a biologic agent. Tuberculin skin testing, QuantiFERON-TB gold test, and the T-SPOT.TB test are accepted screening modalities. Discordance between tuberculin skin tests and the interferon gamma release assays in latent TB highlights the need for further study using the available QuantiFERON-TB gold test and the T-SPOT.TB test.24
CORRESPONDENCE
Karl T. Clebak, MD, FAAFP, Penn State Health Milton S. Hershey Medical Center, Department of Family and Community Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
1. Jackson R. John Updike on psoriasis. At war with my skin, from the journal of a leper. J Cutan Med Surg. 2000;4:113-115.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Alexander H, Nestle FO. Pathogenesis and immunotherapy in cutaneous psoriasis: what can rheumatologists learn? Curr Opin Rheumatol. 2017;29:71-78.
5. Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
7. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
8. Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
9. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
10. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
11. Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Aukl). 2016;6:93-104.
12. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748.
13. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29-38.
14. Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32-38.
15. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2013;26:787-801.
16. Helm MF, Farah JB, Carvalho M, et al. Compounded topical medications for diseases of the skin: a long tradition still relevant today. N Am J Med Sci. 2017;10:116-118.
17. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
18. Helfrich YR, Sachs DL, Kang S. Topical vitamin D3. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia, PA: Saunders; 2007:691-695.
19. Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35:268-269.
20. Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76:675-705.
21. Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
22. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:e2297.
23. Toussirot É, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biologic agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
24. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624.
1. Jackson R. John Updike on psoriasis. At war with my skin, from the journal of a leper. J Cutan Med Surg. 2000;4:113-115.
2. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
3. Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
4. Alexander H, Nestle FO. Pathogenesis and immunotherapy in cutaneous psoriasis: what can rheumatologists learn? Curr Opin Rheumatol. 2017;29:71-78.
5. Fahlén A, Engstrand L, Baker BS, et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15-22.
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
7. Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
8. Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
9. Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
10. Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
11. Singh RK, Lee KM, Ucmak D, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Aukl). 2016;6:93-104.
12. Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63:733-748.
13. McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology. 2015;54:29-38.
14. Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32-38.
15. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidence-based guide for primary care. J Am Board Fam Med. 2013;26:787-801.
16. Helm MF, Farah JB, Carvalho M, et al. Compounded topical medications for diseases of the skin: a long tradition still relevant today. N Am J Med Sci. 2017;10:116-118.
17. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
18. Helfrich YR, Sachs DL, Kang S. Topical vitamin D3. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 2nd ed. Philadelphia, PA: Saunders; 2007:691-695.
19. Lebwohl M, Siskin SB, Epinette W, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35:268-269.
20. Pasch MC. Nail psoriasis: a review of treatment options. Drugs. 2016;76:675-705.
21. Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
22. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18:e2297.
23. Toussirot É, Aubin F. Paradoxical reactions under TNF-alpha blocking agents and other biologic agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
24. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3:e2624.
PRACTICE RECOMMENDATIONS
› Consider guttate psoriasis if small (often < 1 cm) pink scaly papules appear suddenly, particularly in a child who has an upper respiratory tract infection. C
› Document extent of disease using a tool such as the Psoriasis Area and Severity Index, which calculates a score based on the area (extent) of involvement surrounding 4 major anatomical regions. C
› Consider prescribing UV light treatment or a combination of alcitretin and topical corticosteroid if > 10% of the body surface area is involved but joints are not affected. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Biologics better solo than with methotrexate in psoriatic arthritis
Ustekinumab or a tumor necrosis factor inhibitor (TNFi) are better used alone than with methotrexate in the treatment of psoriatic arthritis suggest the results of PsABio (A Study on Assessment of STELARA and Tumor Necrosis Factor Alpha Inhibitor Therapies in Participants With Psoriatic Arthritis), a large, ongoing, prospective observational study.
The percentages of patients achieving multiple psoriatic arthritis disease activity outcome measures at 6 months were higher if biologic monotherapy was used rather than a biologic in combination with methotrexate.
For example, minimal disease activity (MDA) was achieved by 27.5% of patients taking ustekinumab as monotherapy and by 32.1% of those taking a TNFi alone. When methotrexate was used in combination, the respective percentages of patients achieving MDA were 23.7% and 27.8%.
A similar pattern was seen for very-low disease activity (VLDA), with 9.8% of patients in the ustekinumab monotherapy arm and 12% of those in the TNFi monotherapy arm achieving this target, compared with 5.7% and 5.4% when these drugs were combined with methotrexate.
MDA is defined as meeting five or more cutoffs for seven domains of disease activity, and VLDA for all seven: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area Severity Index 1 or less or body surface area involved 3% or less, 0-1 tender entheseal points, Health Assessment Questionnaire score of 0.5 or less, patient global disease activity visual analog scale score of 20 or lower, and patient pain visual analog scale score of 15 or lower.
Other outcome measures used that showed no advantage of adding methotrexate to these biologics were the Clinical Disease Activity in Psoriatic Arthritis low disease activity and remission scores, the patient acceptable symptoms rate of the 12-item Psoriatic Arthritis Impact of Disease Questionnaire, and improvement in skin involvement.
“Patients were no more likely to achieve lower disease activity or a remission target having received methotrexate than they did just on the biologic drug on its own,” Stefan Siebert, MBBCh, PhD, one of the PsABio investigators, said in an interview.
Dr. Siebert, who is clinical senior lecturer in inflammation and rheumatology at the University of Glasgow (Scotland), was scheduled to present the findings at the British Society for Rheumatology annual conference. The meeting was canceled because of the ongoing COVID-19 crisis. Abstracts and ePosters from the meeting have since been released in a supplement to Rheumatology and via the BSR’s conference app.
First data for ustekinumab
“There certainly doesn’t appear to be any added benefit from using methotrexate on a group level in patients getting ustekinumab and TNF inhibitors,” Dr. Siebert said. “We’ve looked at everything,” he emphasized, and “none of the single domains or composite measures were improved by the addition of methotrexate. I think we knew that for TNF inhibitors, but the key thing is we’ve never known that for ustekinumab, and this is the first study to show that.”
Indeed, the findings match up with those from the SEAM-PsA (Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) study in which patients who were treated with the TNFi etanercept as monotherapy did much better than those given the TNFi in combination with methotrexate or methotrexate alone. While not a randomized trial, PsABio now shows that the same is true for ustekinumab.
Obviously, there are some clear differences between a clinical trial and an observational study such as PsABio. For one thing, there was no randomization and patients taking methotrexate were presumably doing so for good reason, Dr. Siebert said. Secondly, there was no methotrexate-only arm.
PsABio recruited patients who were starting treatment with either ustekinumab or a new TNFi as first-, second-, or third-line biologic disease-modifying antirheumatic therapy (DMARD). “These are all people starting on a biologic, so they’ve already got severe disease and have failed methotrexate on some level. So everything we’ve done is biologic without methotrexate or biologic with methotrexate,” Dr. Siebert explained. Patients may not have been taking methotrexate for a variety of reasons, such as inefficacy or side effects, so PsABio “doesn’t tell us anything about methotrexate on its own.”
Time to rethink ingrained methotrexate use
The rationale for using methotrexate in combination with biologics in psoriatic arthritis comes from its long-standing use in rheumatoid arthritis. Much of what is advocated in guidelines comes from experience in RA, Dr. Siebert said.
“In rheumatoid arthritis, we know that the TNF inhibitors work much better if you use methotrexate, that’s a given,” he noted. “We’ve been trained that you have to have methotrexate to have a biologic. However, PsABio, together with other studies, show that you don’t have to, and you should have a good reason to add methotrexate.”
Individual patients may still benefit from methotrexate use, but the decision to treat all patients the same is not supported by the current evidence. “It’s good that it shows that, actually, once you get someone on a decent biologic, it’s working: It’s doing what it ‘says on the tin’ for a lot of patients. I really think that is the key message, here, that you don’t have to; this reassures clinicians and actually makes them think ‘should this patient be on methotrexate?’ ” Dr. Siebert said.
The PsABio study was funded by Janssen. Dr. Siebert has acted as a consultant to and received research funding from Janssen, UCB, Pfizer, Boehringer Ingelheim, Novartis, and Celgene. He has also acted as a consultant for AbbVie and received research support from Bristol-Myers Squibb.
SOURCE: Siebert S et al. Rheumatology. 2020;59(Suppl 2). doi: 10.1093/rheumatology/keaa110.023, Abstract O24.
Ustekinumab or a tumor necrosis factor inhibitor (TNFi) are better used alone than with methotrexate in the treatment of psoriatic arthritis suggest the results of PsABio (A Study on Assessment of STELARA and Tumor Necrosis Factor Alpha Inhibitor Therapies in Participants With Psoriatic Arthritis), a large, ongoing, prospective observational study.
The percentages of patients achieving multiple psoriatic arthritis disease activity outcome measures at 6 months were higher if biologic monotherapy was used rather than a biologic in combination with methotrexate.
For example, minimal disease activity (MDA) was achieved by 27.5% of patients taking ustekinumab as monotherapy and by 32.1% of those taking a TNFi alone. When methotrexate was used in combination, the respective percentages of patients achieving MDA were 23.7% and 27.8%.
A similar pattern was seen for very-low disease activity (VLDA), with 9.8% of patients in the ustekinumab monotherapy arm and 12% of those in the TNFi monotherapy arm achieving this target, compared with 5.7% and 5.4% when these drugs were combined with methotrexate.
MDA is defined as meeting five or more cutoffs for seven domains of disease activity, and VLDA for all seven: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area Severity Index 1 or less or body surface area involved 3% or less, 0-1 tender entheseal points, Health Assessment Questionnaire score of 0.5 or less, patient global disease activity visual analog scale score of 20 or lower, and patient pain visual analog scale score of 15 or lower.
Other outcome measures used that showed no advantage of adding methotrexate to these biologics were the Clinical Disease Activity in Psoriatic Arthritis low disease activity and remission scores, the patient acceptable symptoms rate of the 12-item Psoriatic Arthritis Impact of Disease Questionnaire, and improvement in skin involvement.
“Patients were no more likely to achieve lower disease activity or a remission target having received methotrexate than they did just on the biologic drug on its own,” Stefan Siebert, MBBCh, PhD, one of the PsABio investigators, said in an interview.
Dr. Siebert, who is clinical senior lecturer in inflammation and rheumatology at the University of Glasgow (Scotland), was scheduled to present the findings at the British Society for Rheumatology annual conference. The meeting was canceled because of the ongoing COVID-19 crisis. Abstracts and ePosters from the meeting have since been released in a supplement to Rheumatology and via the BSR’s conference app.
First data for ustekinumab
“There certainly doesn’t appear to be any added benefit from using methotrexate on a group level in patients getting ustekinumab and TNF inhibitors,” Dr. Siebert said. “We’ve looked at everything,” he emphasized, and “none of the single domains or composite measures were improved by the addition of methotrexate. I think we knew that for TNF inhibitors, but the key thing is we’ve never known that for ustekinumab, and this is the first study to show that.”
Indeed, the findings match up with those from the SEAM-PsA (Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) study in which patients who were treated with the TNFi etanercept as monotherapy did much better than those given the TNFi in combination with methotrexate or methotrexate alone. While not a randomized trial, PsABio now shows that the same is true for ustekinumab.
Obviously, there are some clear differences between a clinical trial and an observational study such as PsABio. For one thing, there was no randomization and patients taking methotrexate were presumably doing so for good reason, Dr. Siebert said. Secondly, there was no methotrexate-only arm.
PsABio recruited patients who were starting treatment with either ustekinumab or a new TNFi as first-, second-, or third-line biologic disease-modifying antirheumatic therapy (DMARD). “These are all people starting on a biologic, so they’ve already got severe disease and have failed methotrexate on some level. So everything we’ve done is biologic without methotrexate or biologic with methotrexate,” Dr. Siebert explained. Patients may not have been taking methotrexate for a variety of reasons, such as inefficacy or side effects, so PsABio “doesn’t tell us anything about methotrexate on its own.”
Time to rethink ingrained methotrexate use
The rationale for using methotrexate in combination with biologics in psoriatic arthritis comes from its long-standing use in rheumatoid arthritis. Much of what is advocated in guidelines comes from experience in RA, Dr. Siebert said.
“In rheumatoid arthritis, we know that the TNF inhibitors work much better if you use methotrexate, that’s a given,” he noted. “We’ve been trained that you have to have methotrexate to have a biologic. However, PsABio, together with other studies, show that you don’t have to, and you should have a good reason to add methotrexate.”
Individual patients may still benefit from methotrexate use, but the decision to treat all patients the same is not supported by the current evidence. “It’s good that it shows that, actually, once you get someone on a decent biologic, it’s working: It’s doing what it ‘says on the tin’ for a lot of patients. I really think that is the key message, here, that you don’t have to; this reassures clinicians and actually makes them think ‘should this patient be on methotrexate?’ ” Dr. Siebert said.
The PsABio study was funded by Janssen. Dr. Siebert has acted as a consultant to and received research funding from Janssen, UCB, Pfizer, Boehringer Ingelheim, Novartis, and Celgene. He has also acted as a consultant for AbbVie and received research support from Bristol-Myers Squibb.
SOURCE: Siebert S et al. Rheumatology. 2020;59(Suppl 2). doi: 10.1093/rheumatology/keaa110.023, Abstract O24.
Ustekinumab or a tumor necrosis factor inhibitor (TNFi) are better used alone than with methotrexate in the treatment of psoriatic arthritis suggest the results of PsABio (A Study on Assessment of STELARA and Tumor Necrosis Factor Alpha Inhibitor Therapies in Participants With Psoriatic Arthritis), a large, ongoing, prospective observational study.
The percentages of patients achieving multiple psoriatic arthritis disease activity outcome measures at 6 months were higher if biologic monotherapy was used rather than a biologic in combination with methotrexate.
For example, minimal disease activity (MDA) was achieved by 27.5% of patients taking ustekinumab as monotherapy and by 32.1% of those taking a TNFi alone. When methotrexate was used in combination, the respective percentages of patients achieving MDA were 23.7% and 27.8%.
A similar pattern was seen for very-low disease activity (VLDA), with 9.8% of patients in the ustekinumab monotherapy arm and 12% of those in the TNFi monotherapy arm achieving this target, compared with 5.7% and 5.4% when these drugs were combined with methotrexate.
MDA is defined as meeting five or more cutoffs for seven domains of disease activity, and VLDA for all seven: 0-1 tender joints, 0-1 swollen joints, Psoriasis Area Severity Index 1 or less or body surface area involved 3% or less, 0-1 tender entheseal points, Health Assessment Questionnaire score of 0.5 or less, patient global disease activity visual analog scale score of 20 or lower, and patient pain visual analog scale score of 15 or lower.
Other outcome measures used that showed no advantage of adding methotrexate to these biologics were the Clinical Disease Activity in Psoriatic Arthritis low disease activity and remission scores, the patient acceptable symptoms rate of the 12-item Psoriatic Arthritis Impact of Disease Questionnaire, and improvement in skin involvement.
“Patients were no more likely to achieve lower disease activity or a remission target having received methotrexate than they did just on the biologic drug on its own,” Stefan Siebert, MBBCh, PhD, one of the PsABio investigators, said in an interview.
Dr. Siebert, who is clinical senior lecturer in inflammation and rheumatology at the University of Glasgow (Scotland), was scheduled to present the findings at the British Society for Rheumatology annual conference. The meeting was canceled because of the ongoing COVID-19 crisis. Abstracts and ePosters from the meeting have since been released in a supplement to Rheumatology and via the BSR’s conference app.
First data for ustekinumab
“There certainly doesn’t appear to be any added benefit from using methotrexate on a group level in patients getting ustekinumab and TNF inhibitors,” Dr. Siebert said. “We’ve looked at everything,” he emphasized, and “none of the single domains or composite measures were improved by the addition of methotrexate. I think we knew that for TNF inhibitors, but the key thing is we’ve never known that for ustekinumab, and this is the first study to show that.”
Indeed, the findings match up with those from the SEAM-PsA (Etanercept and Methotrexate in Subjects with Psoriatic Arthritis) study in which patients who were treated with the TNFi etanercept as monotherapy did much better than those given the TNFi in combination with methotrexate or methotrexate alone. While not a randomized trial, PsABio now shows that the same is true for ustekinumab.
Obviously, there are some clear differences between a clinical trial and an observational study such as PsABio. For one thing, there was no randomization and patients taking methotrexate were presumably doing so for good reason, Dr. Siebert said. Secondly, there was no methotrexate-only arm.
PsABio recruited patients who were starting treatment with either ustekinumab or a new TNFi as first-, second-, or third-line biologic disease-modifying antirheumatic therapy (DMARD). “These are all people starting on a biologic, so they’ve already got severe disease and have failed methotrexate on some level. So everything we’ve done is biologic without methotrexate or biologic with methotrexate,” Dr. Siebert explained. Patients may not have been taking methotrexate for a variety of reasons, such as inefficacy or side effects, so PsABio “doesn’t tell us anything about methotrexate on its own.”
Time to rethink ingrained methotrexate use
The rationale for using methotrexate in combination with biologics in psoriatic arthritis comes from its long-standing use in rheumatoid arthritis. Much of what is advocated in guidelines comes from experience in RA, Dr. Siebert said.
“In rheumatoid arthritis, we know that the TNF inhibitors work much better if you use methotrexate, that’s a given,” he noted. “We’ve been trained that you have to have methotrexate to have a biologic. However, PsABio, together with other studies, show that you don’t have to, and you should have a good reason to add methotrexate.”
Individual patients may still benefit from methotrexate use, but the decision to treat all patients the same is not supported by the current evidence. “It’s good that it shows that, actually, once you get someone on a decent biologic, it’s working: It’s doing what it ‘says on the tin’ for a lot of patients. I really think that is the key message, here, that you don’t have to; this reassures clinicians and actually makes them think ‘should this patient be on methotrexate?’ ” Dr. Siebert said.
The PsABio study was funded by Janssen. Dr. Siebert has acted as a consultant to and received research funding from Janssen, UCB, Pfizer, Boehringer Ingelheim, Novartis, and Celgene. He has also acted as a consultant for AbbVie and received research support from Bristol-Myers Squibb.
SOURCE: Siebert S et al. Rheumatology. 2020;59(Suppl 2). doi: 10.1093/rheumatology/keaa110.023, Abstract O24.
FROM BSR 2020
Case series suggests biologics, JAK inhibitors safe during pandemic
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
Use of biologics and Janus kinase (JAK) inhibitors was not associated with worse outcomes in 86 people with inflammatory diseases who contracted COVID-19, according to a case series from New York University Langone Health.
“We are not seeing worse outcomes with overall use of either. It’s reassuring” that the data support continued use during the pandemic, said rheumatologist and senior investigator Jose Scher, MD, an associate professor at New York University.
There have been concerns among rheumatologists, gastroenterologists, and dermatologists that underlying inflammatory diseases and the agents used to treat them would impact outcomes in COVID-19.
Dr. Scher and colleagues, including lead author and rheumatologist Rebecca Haberman, MD, wanted to address the issue, so they reviewed the experience in their own health system of patients with inflammatory diseases – most commonly psoriatic arthritis, RA, and Crohn’s disease – who were assessed for COVID-19 from March 3 to April 3.
Fever, cough, and shortness of breath were the most common symptoms. The infection was confirmed by polymerase chain reaction in 59 (69%) and highly suspected in 27.
A total of 62 patients (72%) were on JAK inhibitors or biologics at baseline, including 38 (44%) on tumor necrosis factor inhibitors.
Overall, 14 patients (16%) were hospitalized with COVID-19, which is consistent the 26% hospitalization rate among the general population in New York City.
Baseline biologic and JAK inhibitor use was actually lower among hospitalized patients than among those who weren’t hospitalized (50% vs. 76%), and the hospitalization rate was only 11% among 62 subjects who had been on the agents long term, more than a year among most.
Hospitalized patients tended to be slightly older (mean, 50 vs. 46 years) with a higher prevalence of hypertension, diabetes, and chronic obstructive pulmonary disease. They also had a higher prevalence of RA (43% vs. 19%), methotrexate use (43% vs. 15%), and use of hydroxychloroquine (21% vs. 7%) and oral glucocorticoids (29% vs. 6%).
It’s unknown what to make of those findings for now, Dr. Scher said. The study didn’t address differences in the severity of the underlying inflammatory illness, but a new and significantly larger case series is in the works that will analyze that and other potential confounders.
Dr. Scher noted that he’s particularly interested in drilling down further on the higher prevalence of RA and methotrexate in hospitalized patients. “We want to understand those signals better. All of this needs further validation,” he said.
Of the 14 hospitalized patients, 11 (79%) were discharged after a mean of 5.6 days. One died in the ED, and two remained hospitalized as of April 3, including one in the ICU.
The investigators are contributing to COVID-19 registries for inflammatory disease patients. The registries are tending to report higher hospitalization rates, but Dr. Scher noted they might be biased towards more severe cases, among other issues.
As for the current situation in New York City, he said that the “last week in March and first 3 in April were indescribable in terms of admissions, intubations, and deaths. Over the last week or so, it has calmed down significantly.”
There was no external funding. Dr. Haberman reported ties to Janssen, and Dr. Scher reported ties to Janssen, Novartis, Pfizer, and other companies.
SOURCE: Haberman R et al. N Engl J Med. 2020 Apr 29. doi: 10.1056/NEJMc2009567.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Adalimumab serum levels and anti-drug antibodies fail to predict responses to other TNFi
Both antiadalimumab (Humira) antibodies and adalimumab serum levels fell short on predicting drug responses in rheumatoid arthritis patients who failed initial adalimumab therapy, based on a retrospective cohort study of 137 adults.
Biologic disease-modifying antirheumatic drugs (bDMARDs), notably adalimumab, are often prescribed for RA, but “approximately 41% of RA patients do not achieve good response after 6 months of treatment with adalimumab,” wrote Evy Ulijn of Sint Maartenskliniek, Nijmegen, the Netherlands, and colleagues. Preliminary studies have suggested that antiadalimumab antibodies (antidrug antibodies, ADA) and adalimumab serum levels (ADL) may predict the response to a second bDMARD in patients who fail initial adalimumab treatment, they said.
In a study published in Annals of the Rheumatic Diseases, the researchers examined data from 137 adult RA patients seen at the clinic during Jan. 2012–Jan. 2018 who failed to respond to adalimumab after at least 3 months of treatment and started another bDMARD. The average age of the patients was 64 years, and approximately 69% were women.
Overall, the presence of ADA was not a significant predictor of a European League Against Rheumatism good response in patients who switched to another TNFi (etanercept, golimumab, infliximab, or certolizumab pegol), with sensitivity of 18% and specificity of 75%. ADA also was not predictive of response to a non-TNFi bDMARD (rituximab, tocilizumab, or abatacept), with sensitivity and specificity of 33% and 70%, respectively.
Similarly, ADL levels in patients who switched to a TNFi or non-TNFi were not significant predictors of treatment response, with sensitivities and specificities of 50% and 52%, respectively, for TNFi and 32% and 69%, respectively, for non-TNFi.
The findings that neither ADA nor ADL showed predictive values contrast with previous studies, the researchers said.
“Not only did the results of this study show no predictive values, in some analyses a prediction is found in the opposite direction of what was expected,” they wrote.
The study findings were limited by several factors, including the random timing of sample collection, retrospective study design, and potential for misclassification of responders or nonresponders, the researchers noted.
However, the results were strengthened by the blinded choice of treatment and outcome assessment, larger sample size than previous studies, and focus on adalimumab in particular, they said.
“While counterintuitive, it is hard to find an explanation for the lack of a positive finding,” they concluded.
More research is needed to confirm the predictive value of ADA and ADL, they said. In the meantime, rheumatologists should base decisions to switch patients to TNFi or non-TNFi treatment after adalimumab failure based on factors including side effects, local protocol, economical aspects, and patient preferences, they said.
The study received no external funding. The researchers had no financial conflicts to disclose.
SOURCE: Ulijn E et al. Ann Rheum Dis. 2020 Apr 21. doi: 10.1136/annrheumdis-2020-216996.
Both antiadalimumab (Humira) antibodies and adalimumab serum levels fell short on predicting drug responses in rheumatoid arthritis patients who failed initial adalimumab therapy, based on a retrospective cohort study of 137 adults.
Biologic disease-modifying antirheumatic drugs (bDMARDs), notably adalimumab, are often prescribed for RA, but “approximately 41% of RA patients do not achieve good response after 6 months of treatment with adalimumab,” wrote Evy Ulijn of Sint Maartenskliniek, Nijmegen, the Netherlands, and colleagues. Preliminary studies have suggested that antiadalimumab antibodies (antidrug antibodies, ADA) and adalimumab serum levels (ADL) may predict the response to a second bDMARD in patients who fail initial adalimumab treatment, they said.
In a study published in Annals of the Rheumatic Diseases, the researchers examined data from 137 adult RA patients seen at the clinic during Jan. 2012–Jan. 2018 who failed to respond to adalimumab after at least 3 months of treatment and started another bDMARD. The average age of the patients was 64 years, and approximately 69% were women.
Overall, the presence of ADA was not a significant predictor of a European League Against Rheumatism good response in patients who switched to another TNFi (etanercept, golimumab, infliximab, or certolizumab pegol), with sensitivity of 18% and specificity of 75%. ADA also was not predictive of response to a non-TNFi bDMARD (rituximab, tocilizumab, or abatacept), with sensitivity and specificity of 33% and 70%, respectively.
Similarly, ADL levels in patients who switched to a TNFi or non-TNFi were not significant predictors of treatment response, with sensitivities and specificities of 50% and 52%, respectively, for TNFi and 32% and 69%, respectively, for non-TNFi.
The findings that neither ADA nor ADL showed predictive values contrast with previous studies, the researchers said.
“Not only did the results of this study show no predictive values, in some analyses a prediction is found in the opposite direction of what was expected,” they wrote.
The study findings were limited by several factors, including the random timing of sample collection, retrospective study design, and potential for misclassification of responders or nonresponders, the researchers noted.
However, the results were strengthened by the blinded choice of treatment and outcome assessment, larger sample size than previous studies, and focus on adalimumab in particular, they said.
“While counterintuitive, it is hard to find an explanation for the lack of a positive finding,” they concluded.
More research is needed to confirm the predictive value of ADA and ADL, they said. In the meantime, rheumatologists should base decisions to switch patients to TNFi or non-TNFi treatment after adalimumab failure based on factors including side effects, local protocol, economical aspects, and patient preferences, they said.
The study received no external funding. The researchers had no financial conflicts to disclose.
SOURCE: Ulijn E et al. Ann Rheum Dis. 2020 Apr 21. doi: 10.1136/annrheumdis-2020-216996.
Both antiadalimumab (Humira) antibodies and adalimumab serum levels fell short on predicting drug responses in rheumatoid arthritis patients who failed initial adalimumab therapy, based on a retrospective cohort study of 137 adults.
Biologic disease-modifying antirheumatic drugs (bDMARDs), notably adalimumab, are often prescribed for RA, but “approximately 41% of RA patients do not achieve good response after 6 months of treatment with adalimumab,” wrote Evy Ulijn of Sint Maartenskliniek, Nijmegen, the Netherlands, and colleagues. Preliminary studies have suggested that antiadalimumab antibodies (antidrug antibodies, ADA) and adalimumab serum levels (ADL) may predict the response to a second bDMARD in patients who fail initial adalimumab treatment, they said.
In a study published in Annals of the Rheumatic Diseases, the researchers examined data from 137 adult RA patients seen at the clinic during Jan. 2012–Jan. 2018 who failed to respond to adalimumab after at least 3 months of treatment and started another bDMARD. The average age of the patients was 64 years, and approximately 69% were women.
Overall, the presence of ADA was not a significant predictor of a European League Against Rheumatism good response in patients who switched to another TNFi (etanercept, golimumab, infliximab, or certolizumab pegol), with sensitivity of 18% and specificity of 75%. ADA also was not predictive of response to a non-TNFi bDMARD (rituximab, tocilizumab, or abatacept), with sensitivity and specificity of 33% and 70%, respectively.
Similarly, ADL levels in patients who switched to a TNFi or non-TNFi were not significant predictors of treatment response, with sensitivities and specificities of 50% and 52%, respectively, for TNFi and 32% and 69%, respectively, for non-TNFi.
The findings that neither ADA nor ADL showed predictive values contrast with previous studies, the researchers said.
“Not only did the results of this study show no predictive values, in some analyses a prediction is found in the opposite direction of what was expected,” they wrote.
The study findings were limited by several factors, including the random timing of sample collection, retrospective study design, and potential for misclassification of responders or nonresponders, the researchers noted.
However, the results were strengthened by the blinded choice of treatment and outcome assessment, larger sample size than previous studies, and focus on adalimumab in particular, they said.
“While counterintuitive, it is hard to find an explanation for the lack of a positive finding,” they concluded.
More research is needed to confirm the predictive value of ADA and ADL, they said. In the meantime, rheumatologists should base decisions to switch patients to TNFi or non-TNFi treatment after adalimumab failure based on factors including side effects, local protocol, economical aspects, and patient preferences, they said.
The study received no external funding. The researchers had no financial conflicts to disclose.
SOURCE: Ulijn E et al. Ann Rheum Dis. 2020 Apr 21. doi: 10.1136/annrheumdis-2020-216996.
FROM ANNALS OF THE RHEUMATIC DISEASES
Price increases for RA biologics keep out-of-pocket costs high for Medicare patients
Although the 2010 Patient Protection and Affordable Care Act attempted to close the coverage gap for prescription drugs, a new study has found that yearly price increases for expensive treatments like rheumatoid arthritis biologics have kept out-of-pocket spending high for patients enrolled in Medicare Part D.
“As the coverage gap is now considered closed, our results suggest a need for out-of-pocket maximums in the catastrophic phase to limit older Americans’ yearly financial burden and allow them to better estimate their annual drug costs,” wrote coauthors Alexandra Erath and Stacie B. Dusetzina, PhD, of Vanderbilt University in Nashville, Tenn. The study was published in JAMA Network Open.
To determine if closing the Medicare Part D coverage gap lowered out-of-pocket costs as anticipated, the researchers embarked on a cross-sectional study of Medicare data from the first quarter of each calendar year from 2010 to 2019. They analyzed the costs of 17 RA biologic drug and strength combinations, calculating the median point-of-sale price per fill for each drug and adjusting for medical inflation.
From 2010 to 2019, the median price per fill increased for all 17 drugs studied. With the exception of the 100-mg/1-mL golimumab (Simponi) autoinjector, every drug that had been on the market for 5 years or longer had a price increase of more than 20%. For the six drugs that have been on the market since 2010 – 200 mg of certolizumab pegol (Cimzia), 25 mg of etanercept (Enbrel), 50 mg of etanercept, 20 mg/0.4 mL of adalimumab (Humira), 40 mg/0.8 mL of adalimumab, and 50 mg/0.5 mL of golimumab – the median list price increased by a mean of 160% (standard deviation [SD], 17%; range, 136%-180%).
Mean (SD) annual out-of-pocket spending for those six drugs did decrease from $6,108 (SD, $234; range, $5,647-6,282) in 2010 to $4,801 (SD, $620; range, $3,594-$5,196) in 2019. However, the most significant decrease actually occurred between 2010 and 2011, when out-of-pocket spending dropped to $4,026 because the Affordable Care Act’s 50% manufacturer rebate for brand-name drugs filled in the coverage gap. This meant there was actually a mean increase of 19% in out-of-pocket costs from 2011 to 2019.
“This is the same story as the EpiPen,” said Maria Greenwald, MD, of Desert Medical Advances in Palm Desert, Calif., in an interview. “Patients have to have it, so you’re going to pay $600 even if you used to pay $50. Why do pharmaceutical companies keep raising their prices? Because they can. There’s no cap on list prices. And these drugs are miracles. They’re the difference between a high quality of life and being bound to a wheelchair. These patients don’t sleep without them. They’ll do whatever they can to pay for them, and so the prices continue to go up.”
This study reinforces the need for physicians to advocate for affordable biologics across the board, wrote Joel Lexchin, MD, of York University in Toronto in an accompanying editorial (doi: 10.1001/jamanetworkopen.2020.4753).
“Price increases for biologics do not only affect patients with rheumatologic conditions,” Dr. Lexchin noted, citing how the cost of multiple sclerosis therapies increased by thousands of dollars from the mid-1990s to 2013. In addition, although biologics make up a single-digit percentage of prescriptions in the United States, he highlighted that “they are responsible for $120 billion or 37% of net drug spending and, since 2014, for 93% of the overall growth in total spending.”
When it comes to potential solutions, he said that Medicare should negotiate directly with drug companies, that substitutions with biosimilars should become mandatory whenever possible, and that pharmaceutical companies should publicly validate their alleged research and development expenses. “Biologics can be transformational treatments,” he wrote, “but only if they are affordable at both the individual and societal levels.”
The authors shared their study’s potential limitations, including relying on list prices that do not factor in rebates and focusing on a single biologic filled every month rather than all treatments filled under Medicare, which could “result in our underestimating out-of-pocket spending by patients.” In addition, although a growing RA biosimilar market could increase price competition and lower costs, they added that progress in that area is limited by “aggressive litigation by the biologic manufacturers and an insufficient number of competitors to markedly affect price.”
The study was supported by the Commonwealth Fund and the Leukemia and Lymphoma Society. Dr. Dusetzina reported receiving grants from Arnold Ventures and personal fees from the Institute for Clinical and Economic Review.
SOURCE: Erath A et al. JAMA Netw Open. 2020 Apr 27. doi: 10.1001/jamanetworkopen.2020.3969.
Although the 2010 Patient Protection and Affordable Care Act attempted to close the coverage gap for prescription drugs, a new study has found that yearly price increases for expensive treatments like rheumatoid arthritis biologics have kept out-of-pocket spending high for patients enrolled in Medicare Part D.
“As the coverage gap is now considered closed, our results suggest a need for out-of-pocket maximums in the catastrophic phase to limit older Americans’ yearly financial burden and allow them to better estimate their annual drug costs,” wrote coauthors Alexandra Erath and Stacie B. Dusetzina, PhD, of Vanderbilt University in Nashville, Tenn. The study was published in JAMA Network Open.
To determine if closing the Medicare Part D coverage gap lowered out-of-pocket costs as anticipated, the researchers embarked on a cross-sectional study of Medicare data from the first quarter of each calendar year from 2010 to 2019. They analyzed the costs of 17 RA biologic drug and strength combinations, calculating the median point-of-sale price per fill for each drug and adjusting for medical inflation.
From 2010 to 2019, the median price per fill increased for all 17 drugs studied. With the exception of the 100-mg/1-mL golimumab (Simponi) autoinjector, every drug that had been on the market for 5 years or longer had a price increase of more than 20%. For the six drugs that have been on the market since 2010 – 200 mg of certolizumab pegol (Cimzia), 25 mg of etanercept (Enbrel), 50 mg of etanercept, 20 mg/0.4 mL of adalimumab (Humira), 40 mg/0.8 mL of adalimumab, and 50 mg/0.5 mL of golimumab – the median list price increased by a mean of 160% (standard deviation [SD], 17%; range, 136%-180%).
Mean (SD) annual out-of-pocket spending for those six drugs did decrease from $6,108 (SD, $234; range, $5,647-6,282) in 2010 to $4,801 (SD, $620; range, $3,594-$5,196) in 2019. However, the most significant decrease actually occurred between 2010 and 2011, when out-of-pocket spending dropped to $4,026 because the Affordable Care Act’s 50% manufacturer rebate for brand-name drugs filled in the coverage gap. This meant there was actually a mean increase of 19% in out-of-pocket costs from 2011 to 2019.
“This is the same story as the EpiPen,” said Maria Greenwald, MD, of Desert Medical Advances in Palm Desert, Calif., in an interview. “Patients have to have it, so you’re going to pay $600 even if you used to pay $50. Why do pharmaceutical companies keep raising their prices? Because they can. There’s no cap on list prices. And these drugs are miracles. They’re the difference between a high quality of life and being bound to a wheelchair. These patients don’t sleep without them. They’ll do whatever they can to pay for them, and so the prices continue to go up.”
This study reinforces the need for physicians to advocate for affordable biologics across the board, wrote Joel Lexchin, MD, of York University in Toronto in an accompanying editorial (doi: 10.1001/jamanetworkopen.2020.4753).
“Price increases for biologics do not only affect patients with rheumatologic conditions,” Dr. Lexchin noted, citing how the cost of multiple sclerosis therapies increased by thousands of dollars from the mid-1990s to 2013. In addition, although biologics make up a single-digit percentage of prescriptions in the United States, he highlighted that “they are responsible for $120 billion or 37% of net drug spending and, since 2014, for 93% of the overall growth in total spending.”
When it comes to potential solutions, he said that Medicare should negotiate directly with drug companies, that substitutions with biosimilars should become mandatory whenever possible, and that pharmaceutical companies should publicly validate their alleged research and development expenses. “Biologics can be transformational treatments,” he wrote, “but only if they are affordable at both the individual and societal levels.”
The authors shared their study’s potential limitations, including relying on list prices that do not factor in rebates and focusing on a single biologic filled every month rather than all treatments filled under Medicare, which could “result in our underestimating out-of-pocket spending by patients.” In addition, although a growing RA biosimilar market could increase price competition and lower costs, they added that progress in that area is limited by “aggressive litigation by the biologic manufacturers and an insufficient number of competitors to markedly affect price.”
The study was supported by the Commonwealth Fund and the Leukemia and Lymphoma Society. Dr. Dusetzina reported receiving grants from Arnold Ventures and personal fees from the Institute for Clinical and Economic Review.
SOURCE: Erath A et al. JAMA Netw Open. 2020 Apr 27. doi: 10.1001/jamanetworkopen.2020.3969.
Although the 2010 Patient Protection and Affordable Care Act attempted to close the coverage gap for prescription drugs, a new study has found that yearly price increases for expensive treatments like rheumatoid arthritis biologics have kept out-of-pocket spending high for patients enrolled in Medicare Part D.
“As the coverage gap is now considered closed, our results suggest a need for out-of-pocket maximums in the catastrophic phase to limit older Americans’ yearly financial burden and allow them to better estimate their annual drug costs,” wrote coauthors Alexandra Erath and Stacie B. Dusetzina, PhD, of Vanderbilt University in Nashville, Tenn. The study was published in JAMA Network Open.
To determine if closing the Medicare Part D coverage gap lowered out-of-pocket costs as anticipated, the researchers embarked on a cross-sectional study of Medicare data from the first quarter of each calendar year from 2010 to 2019. They analyzed the costs of 17 RA biologic drug and strength combinations, calculating the median point-of-sale price per fill for each drug and adjusting for medical inflation.
From 2010 to 2019, the median price per fill increased for all 17 drugs studied. With the exception of the 100-mg/1-mL golimumab (Simponi) autoinjector, every drug that had been on the market for 5 years or longer had a price increase of more than 20%. For the six drugs that have been on the market since 2010 – 200 mg of certolizumab pegol (Cimzia), 25 mg of etanercept (Enbrel), 50 mg of etanercept, 20 mg/0.4 mL of adalimumab (Humira), 40 mg/0.8 mL of adalimumab, and 50 mg/0.5 mL of golimumab – the median list price increased by a mean of 160% (standard deviation [SD], 17%; range, 136%-180%).
Mean (SD) annual out-of-pocket spending for those six drugs did decrease from $6,108 (SD, $234; range, $5,647-6,282) in 2010 to $4,801 (SD, $620; range, $3,594-$5,196) in 2019. However, the most significant decrease actually occurred between 2010 and 2011, when out-of-pocket spending dropped to $4,026 because the Affordable Care Act’s 50% manufacturer rebate for brand-name drugs filled in the coverage gap. This meant there was actually a mean increase of 19% in out-of-pocket costs from 2011 to 2019.
“This is the same story as the EpiPen,” said Maria Greenwald, MD, of Desert Medical Advances in Palm Desert, Calif., in an interview. “Patients have to have it, so you’re going to pay $600 even if you used to pay $50. Why do pharmaceutical companies keep raising their prices? Because they can. There’s no cap on list prices. And these drugs are miracles. They’re the difference between a high quality of life and being bound to a wheelchair. These patients don’t sleep without them. They’ll do whatever they can to pay for them, and so the prices continue to go up.”
This study reinforces the need for physicians to advocate for affordable biologics across the board, wrote Joel Lexchin, MD, of York University in Toronto in an accompanying editorial (doi: 10.1001/jamanetworkopen.2020.4753).
“Price increases for biologics do not only affect patients with rheumatologic conditions,” Dr. Lexchin noted, citing how the cost of multiple sclerosis therapies increased by thousands of dollars from the mid-1990s to 2013. In addition, although biologics make up a single-digit percentage of prescriptions in the United States, he highlighted that “they are responsible for $120 billion or 37% of net drug spending and, since 2014, for 93% of the overall growth in total spending.”
When it comes to potential solutions, he said that Medicare should negotiate directly with drug companies, that substitutions with biosimilars should become mandatory whenever possible, and that pharmaceutical companies should publicly validate their alleged research and development expenses. “Biologics can be transformational treatments,” he wrote, “but only if they are affordable at both the individual and societal levels.”
The authors shared their study’s potential limitations, including relying on list prices that do not factor in rebates and focusing on a single biologic filled every month rather than all treatments filled under Medicare, which could “result in our underestimating out-of-pocket spending by patients.” In addition, although a growing RA biosimilar market could increase price competition and lower costs, they added that progress in that area is limited by “aggressive litigation by the biologic manufacturers and an insufficient number of competitors to markedly affect price.”
The study was supported by the Commonwealth Fund and the Leukemia and Lymphoma Society. Dr. Dusetzina reported receiving grants from Arnold Ventures and personal fees from the Institute for Clinical and Economic Review.
SOURCE: Erath A et al. JAMA Netw Open. 2020 Apr 27. doi: 10.1001/jamanetworkopen.2020.3969.
FROM JAMA NETWORK OPEN