User login
Most rheumatology drugs don’t increase COVID-19 hospitalization risk
The vast majority of patients with rheumatic and musculoskeletal diseases who contract COVID-19 recover from the virus, regardless of which medication they receive for their rheumatic condition, new international research suggests.
“These results provide, for the first time, information about the outcome of COVID-19 in patients with rheumatic and musculoskeletal diseases,” said study investigator Pedro Machado, MD, PhD, from University College London. “They should provide some reassurance to patients and healthcare providers.”
Machado and his colleagues looked at 600 COVID-19 patients from 40 countries, and found that those taking TNF inhibitors for their rheumatic disease were less likely to be hospitalized for COVID-19. However, treatment with more than 10 mg of prednisone daily — considered a moderate to high dose — was associated with a higher probability of hospitalization.
In addition, hospitalization was not associated with biologics; JAK inhibitors; conventional disease-modifying antirheumatic drugs (DMARDs), such as methotrexate; antimalarials, such as hydroxychloroquine; or nonsteroidal anti-inflammatory drugs (NSAIDs) — either alone or in combination with other biologics, such as TNF-alpha inhibitors.
The findings were presented at the virtual European League Against Rheumatism (EULAR) 2020 Congress and were published online in Annals of the Rheumatic Diseases.
“Initially, there was a huge concern that these drugs could affect the outcome of patients getting COVID-19, but what this is showing is that probably these drugs do not increase their risk of severe outcome,” Machado, who is chair of the EULAR standing committee on epidemiology and health services research, told Medscape Medical News.
As of June 1, 1061 patients from 28 participating countries had been entered into the EULAR COVID-19 database, which was launched as part of the international Global Rheumatology Alliance registry. Patient data are categorized by factors such as top rheumatology diagnosis, comorbidities, top-five COVID-19 symptoms, and DMARD therapy at the time of virus infection. Anonymized data will be shared with an international register based in the United States.
Machado’s team combined data from the EULAR and Global Rheumatology Alliance COVID-19 registries from March 24 to April 20. They looked at patient factors — such as age, sex, smoking status, rheumatic diagnosis, comorbidities, and rheumatic therapies — to examine the association of rheumatic therapies with hospitalization rates and COVID-19 disease course.
Of the 277 patients (46%) in the study cohort who required hospitalization, 55 (9%) died. But this finding shouldn’t be viewed as the true rate of hospitalization or death in patients with rheumatic disease and COVID-19, said Gerd Burmester, MD, from Charité–University Medicine Berlin.
“There’s tremendous bias in terms of more serious cases of COVID-19 being reported to the registries,” he explained, “because the mild cases won’t even show up at their rheumatologist’s office.”
“This can skew the idea that COVID-19 is much more dangerous to rheumatic patients than to the regular population,” Burmester told Medscape Medical News. “It scares the patients, obviously, but we believe this is not justified.”
It’s still unclear whether rituximab use raises the risk for severe COVID-19, he said. “It appears to be the only biologic for which the jury is still out,” he said.
“Anti-TNFs and anti-IL-6 drugs may even be beneficial, although we don’t have robust data,” he added.
The study can only highlight associations between rheumatic drugs and COVID-19 outcomes. “We cannot say there is a causal relationship between the findings,” Machado said.
Longer-term data, when available, should illuminate “more granular” aspects of COVID-19 outcomes in rheumatic patients, including their risks of requiring ventilation or developing a cytokine storm, he noted.
Burmester and Machado agree that research needs to continue as the pandemic rages on. But so far, “there are no data suggesting that, if you’re on a targeted, dedicated immunomodulator, your risk is higher to have a worse course of COVID-19 than the general population,” Burmester said.
“We simply didn’t know that when the pandemic started, and some patients even discontinued their drugs out of this fear,” he added. “It’s more reassuring than we originally thought.”
This article first appeared on Medscape.com.
The vast majority of patients with rheumatic and musculoskeletal diseases who contract COVID-19 recover from the virus, regardless of which medication they receive for their rheumatic condition, new international research suggests.
“These results provide, for the first time, information about the outcome of COVID-19 in patients with rheumatic and musculoskeletal diseases,” said study investigator Pedro Machado, MD, PhD, from University College London. “They should provide some reassurance to patients and healthcare providers.”
Machado and his colleagues looked at 600 COVID-19 patients from 40 countries, and found that those taking TNF inhibitors for their rheumatic disease were less likely to be hospitalized for COVID-19. However, treatment with more than 10 mg of prednisone daily — considered a moderate to high dose — was associated with a higher probability of hospitalization.
In addition, hospitalization was not associated with biologics; JAK inhibitors; conventional disease-modifying antirheumatic drugs (DMARDs), such as methotrexate; antimalarials, such as hydroxychloroquine; or nonsteroidal anti-inflammatory drugs (NSAIDs) — either alone or in combination with other biologics, such as TNF-alpha inhibitors.
The findings were presented at the virtual European League Against Rheumatism (EULAR) 2020 Congress and were published online in Annals of the Rheumatic Diseases.
“Initially, there was a huge concern that these drugs could affect the outcome of patients getting COVID-19, but what this is showing is that probably these drugs do not increase their risk of severe outcome,” Machado, who is chair of the EULAR standing committee on epidemiology and health services research, told Medscape Medical News.
As of June 1, 1061 patients from 28 participating countries had been entered into the EULAR COVID-19 database, which was launched as part of the international Global Rheumatology Alliance registry. Patient data are categorized by factors such as top rheumatology diagnosis, comorbidities, top-five COVID-19 symptoms, and DMARD therapy at the time of virus infection. Anonymized data will be shared with an international register based in the United States.
Machado’s team combined data from the EULAR and Global Rheumatology Alliance COVID-19 registries from March 24 to April 20. They looked at patient factors — such as age, sex, smoking status, rheumatic diagnosis, comorbidities, and rheumatic therapies — to examine the association of rheumatic therapies with hospitalization rates and COVID-19 disease course.
Of the 277 patients (46%) in the study cohort who required hospitalization, 55 (9%) died. But this finding shouldn’t be viewed as the true rate of hospitalization or death in patients with rheumatic disease and COVID-19, said Gerd Burmester, MD, from Charité–University Medicine Berlin.
“There’s tremendous bias in terms of more serious cases of COVID-19 being reported to the registries,” he explained, “because the mild cases won’t even show up at their rheumatologist’s office.”
“This can skew the idea that COVID-19 is much more dangerous to rheumatic patients than to the regular population,” Burmester told Medscape Medical News. “It scares the patients, obviously, but we believe this is not justified.”
It’s still unclear whether rituximab use raises the risk for severe COVID-19, he said. “It appears to be the only biologic for which the jury is still out,” he said.
“Anti-TNFs and anti-IL-6 drugs may even be beneficial, although we don’t have robust data,” he added.
The study can only highlight associations between rheumatic drugs and COVID-19 outcomes. “We cannot say there is a causal relationship between the findings,” Machado said.
Longer-term data, when available, should illuminate “more granular” aspects of COVID-19 outcomes in rheumatic patients, including their risks of requiring ventilation or developing a cytokine storm, he noted.
Burmester and Machado agree that research needs to continue as the pandemic rages on. But so far, “there are no data suggesting that, if you’re on a targeted, dedicated immunomodulator, your risk is higher to have a worse course of COVID-19 than the general population,” Burmester said.
“We simply didn’t know that when the pandemic started, and some patients even discontinued their drugs out of this fear,” he added. “It’s more reassuring than we originally thought.”
This article first appeared on Medscape.com.
The vast majority of patients with rheumatic and musculoskeletal diseases who contract COVID-19 recover from the virus, regardless of which medication they receive for their rheumatic condition, new international research suggests.
“These results provide, for the first time, information about the outcome of COVID-19 in patients with rheumatic and musculoskeletal diseases,” said study investigator Pedro Machado, MD, PhD, from University College London. “They should provide some reassurance to patients and healthcare providers.”
Machado and his colleagues looked at 600 COVID-19 patients from 40 countries, and found that those taking TNF inhibitors for their rheumatic disease were less likely to be hospitalized for COVID-19. However, treatment with more than 10 mg of prednisone daily — considered a moderate to high dose — was associated with a higher probability of hospitalization.
In addition, hospitalization was not associated with biologics; JAK inhibitors; conventional disease-modifying antirheumatic drugs (DMARDs), such as methotrexate; antimalarials, such as hydroxychloroquine; or nonsteroidal anti-inflammatory drugs (NSAIDs) — either alone or in combination with other biologics, such as TNF-alpha inhibitors.
The findings were presented at the virtual European League Against Rheumatism (EULAR) 2020 Congress and were published online in Annals of the Rheumatic Diseases.
“Initially, there was a huge concern that these drugs could affect the outcome of patients getting COVID-19, but what this is showing is that probably these drugs do not increase their risk of severe outcome,” Machado, who is chair of the EULAR standing committee on epidemiology and health services research, told Medscape Medical News.
As of June 1, 1061 patients from 28 participating countries had been entered into the EULAR COVID-19 database, which was launched as part of the international Global Rheumatology Alliance registry. Patient data are categorized by factors such as top rheumatology diagnosis, comorbidities, top-five COVID-19 symptoms, and DMARD therapy at the time of virus infection. Anonymized data will be shared with an international register based in the United States.
Machado’s team combined data from the EULAR and Global Rheumatology Alliance COVID-19 registries from March 24 to April 20. They looked at patient factors — such as age, sex, smoking status, rheumatic diagnosis, comorbidities, and rheumatic therapies — to examine the association of rheumatic therapies with hospitalization rates and COVID-19 disease course.
Of the 277 patients (46%) in the study cohort who required hospitalization, 55 (9%) died. But this finding shouldn’t be viewed as the true rate of hospitalization or death in patients with rheumatic disease and COVID-19, said Gerd Burmester, MD, from Charité–University Medicine Berlin.
“There’s tremendous bias in terms of more serious cases of COVID-19 being reported to the registries,” he explained, “because the mild cases won’t even show up at their rheumatologist’s office.”
“This can skew the idea that COVID-19 is much more dangerous to rheumatic patients than to the regular population,” Burmester told Medscape Medical News. “It scares the patients, obviously, but we believe this is not justified.”
It’s still unclear whether rituximab use raises the risk for severe COVID-19, he said. “It appears to be the only biologic for which the jury is still out,” he said.
“Anti-TNFs and anti-IL-6 drugs may even be beneficial, although we don’t have robust data,” he added.
The study can only highlight associations between rheumatic drugs and COVID-19 outcomes. “We cannot say there is a causal relationship between the findings,” Machado said.
Longer-term data, when available, should illuminate “more granular” aspects of COVID-19 outcomes in rheumatic patients, including their risks of requiring ventilation or developing a cytokine storm, he noted.
Burmester and Machado agree that research needs to continue as the pandemic rages on. But so far, “there are no data suggesting that, if you’re on a targeted, dedicated immunomodulator, your risk is higher to have a worse course of COVID-19 than the general population,” Burmester said.
“We simply didn’t know that when the pandemic started, and some patients even discontinued their drugs out of this fear,” he added. “It’s more reassuring than we originally thought.”
This article first appeared on Medscape.com.
RA raises cardiac risk even without CAD
In patients with rheumatoid arthritis (RA), strategies to prevent cardiovascular events, such as treating hypertension, encouraging patients to stop smoking, and reinforcing statin therapy, may be especially important, regardless of whether they have a history of coronary artery disease because their risk for adverse cardiovascular outcomes is significantly greater than for patients who have neither RA nor coronary artery disease (CAD), a large population-based study from Denmark suggests.
“Among patients with RA, risk stratification by presence or absence of documented CAD may allow for screening and personalized treatment strategies,” wrote Brian B. Løgstrup, MD, PhD, DMSc, of Aarhus (Denmark) University Hospital, and his colleagues.
The study, published in Annals of the Rheumatic Diseases, analyzed 125,331 patients with and without CAD in the Western Denmark Heart Registry who had coronary angiography from 2003 through 2016. The cohort included 671 RA patients with no confirmed CAD and 1,061 RA patients who had CAD.
The study makes a significant contribution to the literature in reporting on the additive risk of RA and CAD, said Christie M. Bartels, MD, associate professor in the division of rheumatology at the University of Wisconsin, Madison. “Even among patients with both conditions [RA and CVD], they were less likely to get statin therapy,” she said, noting that the 82.6% of study patients with both CAD and RA were on statins vs. 86.5% of those with CAD alone, while the former had significantly higher rates of hypertension – 64.3% vs. 58.8%. “We’re doing a less effective job on secondary prevention,” she said. The anti-inflammatory properties of statins can also have an additive benefit in RA, she noted.
“This study shows that the rheumatologist can play a role in reinforcing the importance of primary and secondary cardiovascular disease prevention – meaning hypertension control, counseling patients to stop smoking and following up on statin therapy in RA,” Dr. Bartels added.
The study presents two novel findings, Dr. Løgstrup and colleagues noted:
- That RA confers a statistically significant, “but numerically marginally,” heightened risk of cardiovascular events other than stroke.
- Among patients with CAD, RA confers an increased risk of cardiac and all-cause death as well as MI and major adverse cardiovascular events (MACE).
“These finding indicate that RA may have a potential impact for precipitating cardiovascular events beyond CAD and, even more importantly, that RA seems to exacerbate the clinical risk of cardiovascular events in the presence of CAD,” Dr. Løgstrup and colleagues wrote.
The study found that patients with neither RA nor CAD had the lowest 10-year rates of MI (2.7%), ischemic stroke (2.9%), all-cause death (21.6%), cardiac death (2.3%), and MACE (7.3%).
By comparison, those with RA but no CAD had 10-year rates of 3.8% for MI, 5.5% for stroke, 35.6% for all-cause death, 3% for cardiac death, and 11.5% for MACE. Rates for those outcomes for people with CAD but no RA were 9.9% for MI, 4.6% for stroke, 33.3% for all-cause death, 7% for cardiac death, and 19.1% for MACE.
For patients with both RA and CAD, 10-year rates were 12.2% for MI, 4.4% for stroke, 49% for all-cause death, 10.9% for cardiac death, and 24.3% for MACE.
The researchers also performed a risk adjustment analysis based on potential confounding variables across the different groups, such as age, gender, comorbidities including diabetes and hypertension, active smoking status, and anticoagulant, antiplatelet, and statin therapy. The adjusted analysis revealed that patients with RA alone had a 63% greater risk of MI, 68% greater risk for stroke, 42% greater risk for all-cause death, 25% greater risk for cardiac death, and 60% greater risk for MACE than did people who had neither RA nor CAD.
For people with both RA and CAD, the adjusted risks were significantly higher when compared to people with neither: more than four times greater for MI and MACE, 55% greater for stroke, almost double for all-cause death, and 3.7 times greater for cardiac death. People with CAD but no RA also had higher adjusted risk rates compared to people with neither, but had variable rates when compared to people with RA but no CAD, and significantly lower adjusted rates compared to people with both.
The nature of CAD was also a factor, Dr. Løgstrup and colleagues noted. “We found more non-obstructive CAD but no increased incidence of one-vessel, two-vessel, and three-vessel disease in patients with RA than in patients without RA,” they wrote. That’s in line with other published studies (Semin Arthritis Rheum. 2010;40[3]:215–21 and J Rheumatol. 2007;34[5]:937–42), but counter to a study that found increased plaque burden and higher rates of multivessel disease among people with RA (Ann Rheum Dis. 2014;73:1797–804). Differences in methodology, vessel disease definitions, and study population may explain these deviations.
The study authors did not declare any outside source of funding or any competing interests.
Dr. Bartels disclosed receiving institutional grant funding through Pfizer.
SOURCE: Løgstrup BB et al. Ann Rheum Dis. 2020 May 29. doi: 10.1136/annrheumdis-2020-217154.
In patients with rheumatoid arthritis (RA), strategies to prevent cardiovascular events, such as treating hypertension, encouraging patients to stop smoking, and reinforcing statin therapy, may be especially important, regardless of whether they have a history of coronary artery disease because their risk for adverse cardiovascular outcomes is significantly greater than for patients who have neither RA nor coronary artery disease (CAD), a large population-based study from Denmark suggests.
“Among patients with RA, risk stratification by presence or absence of documented CAD may allow for screening and personalized treatment strategies,” wrote Brian B. Løgstrup, MD, PhD, DMSc, of Aarhus (Denmark) University Hospital, and his colleagues.
The study, published in Annals of the Rheumatic Diseases, analyzed 125,331 patients with and without CAD in the Western Denmark Heart Registry who had coronary angiography from 2003 through 2016. The cohort included 671 RA patients with no confirmed CAD and 1,061 RA patients who had CAD.
The study makes a significant contribution to the literature in reporting on the additive risk of RA and CAD, said Christie M. Bartels, MD, associate professor in the division of rheumatology at the University of Wisconsin, Madison. “Even among patients with both conditions [RA and CVD], they were less likely to get statin therapy,” she said, noting that the 82.6% of study patients with both CAD and RA were on statins vs. 86.5% of those with CAD alone, while the former had significantly higher rates of hypertension – 64.3% vs. 58.8%. “We’re doing a less effective job on secondary prevention,” she said. The anti-inflammatory properties of statins can also have an additive benefit in RA, she noted.
“This study shows that the rheumatologist can play a role in reinforcing the importance of primary and secondary cardiovascular disease prevention – meaning hypertension control, counseling patients to stop smoking and following up on statin therapy in RA,” Dr. Bartels added.
The study presents two novel findings, Dr. Løgstrup and colleagues noted:
- That RA confers a statistically significant, “but numerically marginally,” heightened risk of cardiovascular events other than stroke.
- Among patients with CAD, RA confers an increased risk of cardiac and all-cause death as well as MI and major adverse cardiovascular events (MACE).
“These finding indicate that RA may have a potential impact for precipitating cardiovascular events beyond CAD and, even more importantly, that RA seems to exacerbate the clinical risk of cardiovascular events in the presence of CAD,” Dr. Løgstrup and colleagues wrote.
The study found that patients with neither RA nor CAD had the lowest 10-year rates of MI (2.7%), ischemic stroke (2.9%), all-cause death (21.6%), cardiac death (2.3%), and MACE (7.3%).
By comparison, those with RA but no CAD had 10-year rates of 3.8% for MI, 5.5% for stroke, 35.6% for all-cause death, 3% for cardiac death, and 11.5% for MACE. Rates for those outcomes for people with CAD but no RA were 9.9% for MI, 4.6% for stroke, 33.3% for all-cause death, 7% for cardiac death, and 19.1% for MACE.
For patients with both RA and CAD, 10-year rates were 12.2% for MI, 4.4% for stroke, 49% for all-cause death, 10.9% for cardiac death, and 24.3% for MACE.
The researchers also performed a risk adjustment analysis based on potential confounding variables across the different groups, such as age, gender, comorbidities including diabetes and hypertension, active smoking status, and anticoagulant, antiplatelet, and statin therapy. The adjusted analysis revealed that patients with RA alone had a 63% greater risk of MI, 68% greater risk for stroke, 42% greater risk for all-cause death, 25% greater risk for cardiac death, and 60% greater risk for MACE than did people who had neither RA nor CAD.
For people with both RA and CAD, the adjusted risks were significantly higher when compared to people with neither: more than four times greater for MI and MACE, 55% greater for stroke, almost double for all-cause death, and 3.7 times greater for cardiac death. People with CAD but no RA also had higher adjusted risk rates compared to people with neither, but had variable rates when compared to people with RA but no CAD, and significantly lower adjusted rates compared to people with both.
The nature of CAD was also a factor, Dr. Løgstrup and colleagues noted. “We found more non-obstructive CAD but no increased incidence of one-vessel, two-vessel, and three-vessel disease in patients with RA than in patients without RA,” they wrote. That’s in line with other published studies (Semin Arthritis Rheum. 2010;40[3]:215–21 and J Rheumatol. 2007;34[5]:937–42), but counter to a study that found increased plaque burden and higher rates of multivessel disease among people with RA (Ann Rheum Dis. 2014;73:1797–804). Differences in methodology, vessel disease definitions, and study population may explain these deviations.
The study authors did not declare any outside source of funding or any competing interests.
Dr. Bartels disclosed receiving institutional grant funding through Pfizer.
SOURCE: Løgstrup BB et al. Ann Rheum Dis. 2020 May 29. doi: 10.1136/annrheumdis-2020-217154.
In patients with rheumatoid arthritis (RA), strategies to prevent cardiovascular events, such as treating hypertension, encouraging patients to stop smoking, and reinforcing statin therapy, may be especially important, regardless of whether they have a history of coronary artery disease because their risk for adverse cardiovascular outcomes is significantly greater than for patients who have neither RA nor coronary artery disease (CAD), a large population-based study from Denmark suggests.
“Among patients with RA, risk stratification by presence or absence of documented CAD may allow for screening and personalized treatment strategies,” wrote Brian B. Løgstrup, MD, PhD, DMSc, of Aarhus (Denmark) University Hospital, and his colleagues.
The study, published in Annals of the Rheumatic Diseases, analyzed 125,331 patients with and without CAD in the Western Denmark Heart Registry who had coronary angiography from 2003 through 2016. The cohort included 671 RA patients with no confirmed CAD and 1,061 RA patients who had CAD.
The study makes a significant contribution to the literature in reporting on the additive risk of RA and CAD, said Christie M. Bartels, MD, associate professor in the division of rheumatology at the University of Wisconsin, Madison. “Even among patients with both conditions [RA and CVD], they were less likely to get statin therapy,” she said, noting that the 82.6% of study patients with both CAD and RA were on statins vs. 86.5% of those with CAD alone, while the former had significantly higher rates of hypertension – 64.3% vs. 58.8%. “We’re doing a less effective job on secondary prevention,” she said. The anti-inflammatory properties of statins can also have an additive benefit in RA, she noted.
“This study shows that the rheumatologist can play a role in reinforcing the importance of primary and secondary cardiovascular disease prevention – meaning hypertension control, counseling patients to stop smoking and following up on statin therapy in RA,” Dr. Bartels added.
The study presents two novel findings, Dr. Løgstrup and colleagues noted:
- That RA confers a statistically significant, “but numerically marginally,” heightened risk of cardiovascular events other than stroke.
- Among patients with CAD, RA confers an increased risk of cardiac and all-cause death as well as MI and major adverse cardiovascular events (MACE).
“These finding indicate that RA may have a potential impact for precipitating cardiovascular events beyond CAD and, even more importantly, that RA seems to exacerbate the clinical risk of cardiovascular events in the presence of CAD,” Dr. Løgstrup and colleagues wrote.
The study found that patients with neither RA nor CAD had the lowest 10-year rates of MI (2.7%), ischemic stroke (2.9%), all-cause death (21.6%), cardiac death (2.3%), and MACE (7.3%).
By comparison, those with RA but no CAD had 10-year rates of 3.8% for MI, 5.5% for stroke, 35.6% for all-cause death, 3% for cardiac death, and 11.5% for MACE. Rates for those outcomes for people with CAD but no RA were 9.9% for MI, 4.6% for stroke, 33.3% for all-cause death, 7% for cardiac death, and 19.1% for MACE.
For patients with both RA and CAD, 10-year rates were 12.2% for MI, 4.4% for stroke, 49% for all-cause death, 10.9% for cardiac death, and 24.3% for MACE.
The researchers also performed a risk adjustment analysis based on potential confounding variables across the different groups, such as age, gender, comorbidities including diabetes and hypertension, active smoking status, and anticoagulant, antiplatelet, and statin therapy. The adjusted analysis revealed that patients with RA alone had a 63% greater risk of MI, 68% greater risk for stroke, 42% greater risk for all-cause death, 25% greater risk for cardiac death, and 60% greater risk for MACE than did people who had neither RA nor CAD.
For people with both RA and CAD, the adjusted risks were significantly higher when compared to people with neither: more than four times greater for MI and MACE, 55% greater for stroke, almost double for all-cause death, and 3.7 times greater for cardiac death. People with CAD but no RA also had higher adjusted risk rates compared to people with neither, but had variable rates when compared to people with RA but no CAD, and significantly lower adjusted rates compared to people with both.
The nature of CAD was also a factor, Dr. Løgstrup and colleagues noted. “We found more non-obstructive CAD but no increased incidence of one-vessel, two-vessel, and three-vessel disease in patients with RA than in patients without RA,” they wrote. That’s in line with other published studies (Semin Arthritis Rheum. 2010;40[3]:215–21 and J Rheumatol. 2007;34[5]:937–42), but counter to a study that found increased plaque burden and higher rates of multivessel disease among people with RA (Ann Rheum Dis. 2014;73:1797–804). Differences in methodology, vessel disease definitions, and study population may explain these deviations.
The study authors did not declare any outside source of funding or any competing interests.
Dr. Bartels disclosed receiving institutional grant funding through Pfizer.
SOURCE: Løgstrup BB et al. Ann Rheum Dis. 2020 May 29. doi: 10.1136/annrheumdis-2020-217154.
FROM ANNALS OF THE RHEUMATIC DISEASES
Avacopan notches a win in ANCA-associated vasculitis
Avacopan, an investigational oral inhibitor of complement activation, is efficacious and safe for treating antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, based on the results of the pivotal phase 3 ADVOCATE trial.
The trial results were reported in the opening plenary abstract session at the annual European Congress of Rheumatology, held online this year because of COVID-19.
“Standard of care for induction of remission includes high-dose glucocorticoids with either cyclophosphamide or rituximab. However, glucocorticoids are the major cause of treatment-related harm,” noted lead investigator Peter A. Merkel, MD, MPH, chief of the division of rheumatology at the University of Pennsylvania, Philadelphia.
The 331 patients in the trial had active ANCA-associated vasculitis (granulomatosis with polyangiitis or microscopic polyangiitis), either new onset or relapsed, with positivity for either proteinase 3 or myeloperoxidase antibodies and moderate to high disease activity.
They were randomized evenly to double-blind avacopan 30 mg or tapering prednisone from 60 mg/day to zero over 20 weeks, each combined either with rituximab (Rituxan) or with cyclophosphamide followed by azathioprine. Avacopan (formerly called CCX168) is a selective antagonist of the complement C5a receptor that has orphan-drug designation from the Food and Drug Administration for this disease.
Trial results showed that avacopan was noninferior to prednisone with respect to the week 26 rate of remission on the Birmingham Vasculitis Activity Score, with an estimate of common difference of 3.4%. And it was superior to prednisone with respect to the week 52 rate of sustained remission, which required remission from week 26 onward, with an estimate of common difference of 12.5%.
The avacopan group also had less glucocorticoid-related toxicity and, among patients with preexisting renal disease, greater improvement in renal function.
“This large, randomized trial met both of its primary endpoints. Important secondary endpoints were also achieved, with a very acceptable safety profile,” Dr. Merkel summarized.
Making sense of the results
The optimal duration of avacopan therapy is unclear, he noted. “We are still going to be learning how to use this drug, if it’s approved, in routine practice. But the data from the second 6 months – from week 26 to week 52 – implies that there is ongoing benefit to being on avacopan after remission is achieved.”
Avacopan worked similarly well regardless of disease status in ADVOCATE, according to Dr. Merkel. “We have not seen significant differences in efficacy of other drugs in our trials [by disease status], in the trials of ANCA-associated vasculitis. So I think we would treat moderate to serious disease similarly, whether it is new onset or recurrence, in terms of efficacy of the drug.”
“The topline phase 3 data from ADVOCATE sort of even exceeded my expectations in terms of the ability to show not just noninferiority, but superiority of avacopan at week 52 in maintaining sustained remission,” Lindsay S. Lally, MD, assistant professor of medicine at the Hospital for Special Surgery in New York, commented in an interview. “It’s spectacular to treat patients with this serious vasculitis without any steroids or with very minimal steroids, and see superiority at a year. That is really game changing.”
The ADVOCATE findings will likely pass muster with the FDA, according to Dr. Lally. “The bar that was set in terms of the coprimary endpoints was very stringent and in line with other registration trials, particularly the RAVE trial that led to the approval of rituximab,” she elaborated. “I don’t think there is any significant safety signal in the data related to avacopan.
“This study is going to move forward our ability to treat this disease effectively, as we have been able to do in some of our other vasculitis syndromes, by finding drugs that have significant steroid-sparing effects,” Dr. Lally predicted.
Study details
ADVOCATE results reported at the congress showed that the week 26 rate of disease remission was 72.3% with avacopan versus 70.1% with prednisone, with the difference falling within the 20% boundary for noninferiority (P < .0001) but missing the mark for superiority (P = .2387).
However, the week 52 rate of sustained disease remission was 65.7% versus 54.9%, respectively, yielding a difference in favor of avacopan that was statistically both noninferior (P < .0001) and superior (P = .0066).
At week 26, patients in the avacopan group had more favorable Glucocorticoid Toxicity Index scores for cumulative worsening (39.7 vs. 56.6; P = .0002) and for aggregate improvement (11.2 vs. 23.4; P = .008).
Among patients who had renal disease at baseline, those in the avacopan group had a greater increase in estimated glomerular filtration rate at week 52 (7.3 vs. 4.1 mL/min per 1.73 m2; P = .029).
“Particularly interesting is the fact that, even after week 26, when the patients were in remission, there was continued improvement in renal function,” Dr. Merkel noted.
Overall, avacopan had a good safety profile. “This was a sick population with many complications, but there were no important safety signals of the study medication,” he reported.
The avacopan and prednisone groups had a similar rate of severe adverse events (23.5% vs. 25.0%). But the former had lower rates of life-threatening adverse events (4.8% vs. 8.5%), adverse events potentially related to glucocorticoids (66.3% vs. 80.5%), deaths (1.2% vs. 2.4%), and deaths specifically caused by infection (0.6% vs. 1.2%).
The trial was sponsored by ChemoCentryx. Dr. Merkel disclosed receiving grant/research support from and consulting fees from ChemoCentryx, among other disclosures. Dr. Lally disclosed that she was an investigator in the trial.
SOURCE: Merkel PA et al. Ann Rheum Dis. 2020;79[suppl 1]:8, Abstract OP0011.
Avacopan, an investigational oral inhibitor of complement activation, is efficacious and safe for treating antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, based on the results of the pivotal phase 3 ADVOCATE trial.
The trial results were reported in the opening plenary abstract session at the annual European Congress of Rheumatology, held online this year because of COVID-19.
“Standard of care for induction of remission includes high-dose glucocorticoids with either cyclophosphamide or rituximab. However, glucocorticoids are the major cause of treatment-related harm,” noted lead investigator Peter A. Merkel, MD, MPH, chief of the division of rheumatology at the University of Pennsylvania, Philadelphia.
The 331 patients in the trial had active ANCA-associated vasculitis (granulomatosis with polyangiitis or microscopic polyangiitis), either new onset or relapsed, with positivity for either proteinase 3 or myeloperoxidase antibodies and moderate to high disease activity.
They were randomized evenly to double-blind avacopan 30 mg or tapering prednisone from 60 mg/day to zero over 20 weeks, each combined either with rituximab (Rituxan) or with cyclophosphamide followed by azathioprine. Avacopan (formerly called CCX168) is a selective antagonist of the complement C5a receptor that has orphan-drug designation from the Food and Drug Administration for this disease.
Trial results showed that avacopan was noninferior to prednisone with respect to the week 26 rate of remission on the Birmingham Vasculitis Activity Score, with an estimate of common difference of 3.4%. And it was superior to prednisone with respect to the week 52 rate of sustained remission, which required remission from week 26 onward, with an estimate of common difference of 12.5%.
The avacopan group also had less glucocorticoid-related toxicity and, among patients with preexisting renal disease, greater improvement in renal function.
“This large, randomized trial met both of its primary endpoints. Important secondary endpoints were also achieved, with a very acceptable safety profile,” Dr. Merkel summarized.
Making sense of the results
The optimal duration of avacopan therapy is unclear, he noted. “We are still going to be learning how to use this drug, if it’s approved, in routine practice. But the data from the second 6 months – from week 26 to week 52 – implies that there is ongoing benefit to being on avacopan after remission is achieved.”
Avacopan worked similarly well regardless of disease status in ADVOCATE, according to Dr. Merkel. “We have not seen significant differences in efficacy of other drugs in our trials [by disease status], in the trials of ANCA-associated vasculitis. So I think we would treat moderate to serious disease similarly, whether it is new onset or recurrence, in terms of efficacy of the drug.”
“The topline phase 3 data from ADVOCATE sort of even exceeded my expectations in terms of the ability to show not just noninferiority, but superiority of avacopan at week 52 in maintaining sustained remission,” Lindsay S. Lally, MD, assistant professor of medicine at the Hospital for Special Surgery in New York, commented in an interview. “It’s spectacular to treat patients with this serious vasculitis without any steroids or with very minimal steroids, and see superiority at a year. That is really game changing.”
The ADVOCATE findings will likely pass muster with the FDA, according to Dr. Lally. “The bar that was set in terms of the coprimary endpoints was very stringent and in line with other registration trials, particularly the RAVE trial that led to the approval of rituximab,” she elaborated. “I don’t think there is any significant safety signal in the data related to avacopan.
“This study is going to move forward our ability to treat this disease effectively, as we have been able to do in some of our other vasculitis syndromes, by finding drugs that have significant steroid-sparing effects,” Dr. Lally predicted.
Study details
ADVOCATE results reported at the congress showed that the week 26 rate of disease remission was 72.3% with avacopan versus 70.1% with prednisone, with the difference falling within the 20% boundary for noninferiority (P < .0001) but missing the mark for superiority (P = .2387).
However, the week 52 rate of sustained disease remission was 65.7% versus 54.9%, respectively, yielding a difference in favor of avacopan that was statistically both noninferior (P < .0001) and superior (P = .0066).
At week 26, patients in the avacopan group had more favorable Glucocorticoid Toxicity Index scores for cumulative worsening (39.7 vs. 56.6; P = .0002) and for aggregate improvement (11.2 vs. 23.4; P = .008).
Among patients who had renal disease at baseline, those in the avacopan group had a greater increase in estimated glomerular filtration rate at week 52 (7.3 vs. 4.1 mL/min per 1.73 m2; P = .029).
“Particularly interesting is the fact that, even after week 26, when the patients were in remission, there was continued improvement in renal function,” Dr. Merkel noted.
Overall, avacopan had a good safety profile. “This was a sick population with many complications, but there were no important safety signals of the study medication,” he reported.
The avacopan and prednisone groups had a similar rate of severe adverse events (23.5% vs. 25.0%). But the former had lower rates of life-threatening adverse events (4.8% vs. 8.5%), adverse events potentially related to glucocorticoids (66.3% vs. 80.5%), deaths (1.2% vs. 2.4%), and deaths specifically caused by infection (0.6% vs. 1.2%).
The trial was sponsored by ChemoCentryx. Dr. Merkel disclosed receiving grant/research support from and consulting fees from ChemoCentryx, among other disclosures. Dr. Lally disclosed that she was an investigator in the trial.
SOURCE: Merkel PA et al. Ann Rheum Dis. 2020;79[suppl 1]:8, Abstract OP0011.
Avacopan, an investigational oral inhibitor of complement activation, is efficacious and safe for treating antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, based on the results of the pivotal phase 3 ADVOCATE trial.
The trial results were reported in the opening plenary abstract session at the annual European Congress of Rheumatology, held online this year because of COVID-19.
“Standard of care for induction of remission includes high-dose glucocorticoids with either cyclophosphamide or rituximab. However, glucocorticoids are the major cause of treatment-related harm,” noted lead investigator Peter A. Merkel, MD, MPH, chief of the division of rheumatology at the University of Pennsylvania, Philadelphia.
The 331 patients in the trial had active ANCA-associated vasculitis (granulomatosis with polyangiitis or microscopic polyangiitis), either new onset or relapsed, with positivity for either proteinase 3 or myeloperoxidase antibodies and moderate to high disease activity.
They were randomized evenly to double-blind avacopan 30 mg or tapering prednisone from 60 mg/day to zero over 20 weeks, each combined either with rituximab (Rituxan) or with cyclophosphamide followed by azathioprine. Avacopan (formerly called CCX168) is a selective antagonist of the complement C5a receptor that has orphan-drug designation from the Food and Drug Administration for this disease.
Trial results showed that avacopan was noninferior to prednisone with respect to the week 26 rate of remission on the Birmingham Vasculitis Activity Score, with an estimate of common difference of 3.4%. And it was superior to prednisone with respect to the week 52 rate of sustained remission, which required remission from week 26 onward, with an estimate of common difference of 12.5%.
The avacopan group also had less glucocorticoid-related toxicity and, among patients with preexisting renal disease, greater improvement in renal function.
“This large, randomized trial met both of its primary endpoints. Important secondary endpoints were also achieved, with a very acceptable safety profile,” Dr. Merkel summarized.
Making sense of the results
The optimal duration of avacopan therapy is unclear, he noted. “We are still going to be learning how to use this drug, if it’s approved, in routine practice. But the data from the second 6 months – from week 26 to week 52 – implies that there is ongoing benefit to being on avacopan after remission is achieved.”
Avacopan worked similarly well regardless of disease status in ADVOCATE, according to Dr. Merkel. “We have not seen significant differences in efficacy of other drugs in our trials [by disease status], in the trials of ANCA-associated vasculitis. So I think we would treat moderate to serious disease similarly, whether it is new onset or recurrence, in terms of efficacy of the drug.”
“The topline phase 3 data from ADVOCATE sort of even exceeded my expectations in terms of the ability to show not just noninferiority, but superiority of avacopan at week 52 in maintaining sustained remission,” Lindsay S. Lally, MD, assistant professor of medicine at the Hospital for Special Surgery in New York, commented in an interview. “It’s spectacular to treat patients with this serious vasculitis without any steroids or with very minimal steroids, and see superiority at a year. That is really game changing.”
The ADVOCATE findings will likely pass muster with the FDA, according to Dr. Lally. “The bar that was set in terms of the coprimary endpoints was very stringent and in line with other registration trials, particularly the RAVE trial that led to the approval of rituximab,” she elaborated. “I don’t think there is any significant safety signal in the data related to avacopan.
“This study is going to move forward our ability to treat this disease effectively, as we have been able to do in some of our other vasculitis syndromes, by finding drugs that have significant steroid-sparing effects,” Dr. Lally predicted.
Study details
ADVOCATE results reported at the congress showed that the week 26 rate of disease remission was 72.3% with avacopan versus 70.1% with prednisone, with the difference falling within the 20% boundary for noninferiority (P < .0001) but missing the mark for superiority (P = .2387).
However, the week 52 rate of sustained disease remission was 65.7% versus 54.9%, respectively, yielding a difference in favor of avacopan that was statistically both noninferior (P < .0001) and superior (P = .0066).
At week 26, patients in the avacopan group had more favorable Glucocorticoid Toxicity Index scores for cumulative worsening (39.7 vs. 56.6; P = .0002) and for aggregate improvement (11.2 vs. 23.4; P = .008).
Among patients who had renal disease at baseline, those in the avacopan group had a greater increase in estimated glomerular filtration rate at week 52 (7.3 vs. 4.1 mL/min per 1.73 m2; P = .029).
“Particularly interesting is the fact that, even after week 26, when the patients were in remission, there was continued improvement in renal function,” Dr. Merkel noted.
Overall, avacopan had a good safety profile. “This was a sick population with many complications, but there were no important safety signals of the study medication,” he reported.
The avacopan and prednisone groups had a similar rate of severe adverse events (23.5% vs. 25.0%). But the former had lower rates of life-threatening adverse events (4.8% vs. 8.5%), adverse events potentially related to glucocorticoids (66.3% vs. 80.5%), deaths (1.2% vs. 2.4%), and deaths specifically caused by infection (0.6% vs. 1.2%).
The trial was sponsored by ChemoCentryx. Dr. Merkel disclosed receiving grant/research support from and consulting fees from ChemoCentryx, among other disclosures. Dr. Lally disclosed that she was an investigator in the trial.
SOURCE: Merkel PA et al. Ann Rheum Dis. 2020;79[suppl 1]:8, Abstract OP0011.
FROM EULAR 2020 E-CONGRESS
Study tests a simpler low disease activity measure for lupus
An alternative disease activity index for patients with systemic lupus erythematosus called the SLE-DAS (Disease Activity Score) has shown similar results to the Lupus Low Disease Activity State (LLDAS) in classifying low disease activity but may be easier to potentially apply in daily clinical practice in treat-to-target strategies, according to research presented at the annual European Congress of Rheumatology, held online this year because of COVID-19.
A treat-to-target approach, in which therapies are adjusted and the patient monitored to achieve the desired endpoint, has been proposed for patients with SLE. Clinical remission is the ideal goal, followed by achieving low disease activity (LDA) when clinical remission is unattainable, the first author of the SLE-DAS study, Helena Assunção, MD, of the department of rheumatology at Centro Hospitalar e Universitário de Coimbra (Portugal), said in an interview prior to the presentation of the study at the e-congress.
But to conduct a treat-to-target approach in the clinical setting, clinicians must have reliable, user-friendly targets to assess a patient’s progress, she said. But that’s not available right now. Proposed definitions of LDA, such as the LLDAS, are based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K). This index doesn’t address some important manifestations of SLE and it is scored dichotomously – for example, giving a similar score for thrombocytopenia when platelet count is reduced to 100,000 or to 10,000.
To compensate for these limitations, the current LLDAS definition also requires the Physician Global Assessment and other steps, including a review of medication and changes to treatment or clinical status since the previous visit.
“It is not easy to apply,” Dr. Assunção said.
The SLE-DAS is a continuous index involving 17 parameters (4 continuous: arthritis, proteinuria, thrombocytopenia, and leukopenia), assigning higher scores when a manifestation is more severe, and has manifestation information that SLEDAI-2K lacks (cardiopulmonary involvement, lupus enteritis, and hemolytic anemia).
In contrast, the LLDAS is defined as:
- A SLEDAI-2k score of 4 or less with no major organ involvement
- No new disease activity
- A physician global assessment of the patient of 1 or less on a 0-3 scale
- Maintenance on a prednisolone dosage of 7.5 mg/day or less
- Maintenance on a standard immunosuppressive regimen
A previous study validated the SLE-DAS (Ann Rheum Dis. 2019 Mar;78[3]:365-71), and another exploratory study identified a cutoff SLE-DAS value of 3.77 or lower for LDA with SLE-DAS (Ann Rheum Dis. 2019;78:411-2).
Her group compared LDA status as measured with LLDAS versus the SLE-DAS in a cross-sectional study of 292 consecutive patients at their hospital. LDA on the SLE-DAS was defined as a score 3.77 or lower and a prednisolone dose of 7.5 mg/day or less. A total of 85% of patients were in LDA with SLE-DAS and 83.9% with LLDAS, and the agreement between LLDAS and SLE-DAS LDA was very high (Cohen’s kappa coefficient test; kappa = 0.831; P < .01). Out of 292 patients, only 13 were classified differently by the two definitions, 8 of which were classified as LDA by SLE-DAS, and 5 by LLDAS. Overall, 87% of patients were women and had a mean age of nearly 49 years, with a mean disease duration of about 14 years.
Dr. Assunção feels that the SLE-DAS LDA should be sufficient to monitor disease activity without adding the Physician Global Assessment and other steps, which would make it easier to apply than LLDAS. The fact that it is based on a continuous index is also an important difference. “Especially for low disease activity, it’s very good to be able to define it with a continuous index, because you are not that bad, but not that good, you’re in the middle,” she said.
The study should be regarded as exploratory, she said, but the results were encouraging. “We got similar results, and it’s definitely easier to apply.” She can also personally attest that the new model is easier to use, since she personally collected data for LLDAS assignment. “I had to check this, and this, and this … [SLE-DAS] is easier.”
Future work from her group will aim at deriving and validating a more robust definition of LDA, which will again be compared with the current LLDAS definition.
Her colleagues have already developed and validated a definition for clinical remission using SLE-DAS, although those results have not yet been published. They hope to define activity states using SLE-DAS, including mild, moderate, and high disease activity.
The team has produced an online SLE-DAS calculator (http://sle-das.eu/) where clinicians can score the 17 parameters. “You just input the values and it gives a number reflecting disease activity. Using this definition of SLE-DAS LDA you only need that number and to verify that the prednisolone dose is equal to or inferior to 7.5 mg/day,” said Dr. Assunção.
The study received no funding. Dr. Assunção has no financial disclosures, but one coauthor reported receiving grant/research support from Pfizer and AbbVie and serving as a consultant to Pfizer, AbbVie, Roche, Lilly, and Novartis.
SOURCE: Assunção H et al. Ann Rheum Dis 2020;79[suppl 1]:60, Abstract OP0092.
An alternative disease activity index for patients with systemic lupus erythematosus called the SLE-DAS (Disease Activity Score) has shown similar results to the Lupus Low Disease Activity State (LLDAS) in classifying low disease activity but may be easier to potentially apply in daily clinical practice in treat-to-target strategies, according to research presented at the annual European Congress of Rheumatology, held online this year because of COVID-19.
A treat-to-target approach, in which therapies are adjusted and the patient monitored to achieve the desired endpoint, has been proposed for patients with SLE. Clinical remission is the ideal goal, followed by achieving low disease activity (LDA) when clinical remission is unattainable, the first author of the SLE-DAS study, Helena Assunção, MD, of the department of rheumatology at Centro Hospitalar e Universitário de Coimbra (Portugal), said in an interview prior to the presentation of the study at the e-congress.
But to conduct a treat-to-target approach in the clinical setting, clinicians must have reliable, user-friendly targets to assess a patient’s progress, she said. But that’s not available right now. Proposed definitions of LDA, such as the LLDAS, are based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K). This index doesn’t address some important manifestations of SLE and it is scored dichotomously – for example, giving a similar score for thrombocytopenia when platelet count is reduced to 100,000 or to 10,000.
To compensate for these limitations, the current LLDAS definition also requires the Physician Global Assessment and other steps, including a review of medication and changes to treatment or clinical status since the previous visit.
“It is not easy to apply,” Dr. Assunção said.
The SLE-DAS is a continuous index involving 17 parameters (4 continuous: arthritis, proteinuria, thrombocytopenia, and leukopenia), assigning higher scores when a manifestation is more severe, and has manifestation information that SLEDAI-2K lacks (cardiopulmonary involvement, lupus enteritis, and hemolytic anemia).
In contrast, the LLDAS is defined as:
- A SLEDAI-2k score of 4 or less with no major organ involvement
- No new disease activity
- A physician global assessment of the patient of 1 or less on a 0-3 scale
- Maintenance on a prednisolone dosage of 7.5 mg/day or less
- Maintenance on a standard immunosuppressive regimen
A previous study validated the SLE-DAS (Ann Rheum Dis. 2019 Mar;78[3]:365-71), and another exploratory study identified a cutoff SLE-DAS value of 3.77 or lower for LDA with SLE-DAS (Ann Rheum Dis. 2019;78:411-2).
Her group compared LDA status as measured with LLDAS versus the SLE-DAS in a cross-sectional study of 292 consecutive patients at their hospital. LDA on the SLE-DAS was defined as a score 3.77 or lower and a prednisolone dose of 7.5 mg/day or less. A total of 85% of patients were in LDA with SLE-DAS and 83.9% with LLDAS, and the agreement between LLDAS and SLE-DAS LDA was very high (Cohen’s kappa coefficient test; kappa = 0.831; P < .01). Out of 292 patients, only 13 were classified differently by the two definitions, 8 of which were classified as LDA by SLE-DAS, and 5 by LLDAS. Overall, 87% of patients were women and had a mean age of nearly 49 years, with a mean disease duration of about 14 years.
Dr. Assunção feels that the SLE-DAS LDA should be sufficient to monitor disease activity without adding the Physician Global Assessment and other steps, which would make it easier to apply than LLDAS. The fact that it is based on a continuous index is also an important difference. “Especially for low disease activity, it’s very good to be able to define it with a continuous index, because you are not that bad, but not that good, you’re in the middle,” she said.
The study should be regarded as exploratory, she said, but the results were encouraging. “We got similar results, and it’s definitely easier to apply.” She can also personally attest that the new model is easier to use, since she personally collected data for LLDAS assignment. “I had to check this, and this, and this … [SLE-DAS] is easier.”
Future work from her group will aim at deriving and validating a more robust definition of LDA, which will again be compared with the current LLDAS definition.
Her colleagues have already developed and validated a definition for clinical remission using SLE-DAS, although those results have not yet been published. They hope to define activity states using SLE-DAS, including mild, moderate, and high disease activity.
The team has produced an online SLE-DAS calculator (http://sle-das.eu/) where clinicians can score the 17 parameters. “You just input the values and it gives a number reflecting disease activity. Using this definition of SLE-DAS LDA you only need that number and to verify that the prednisolone dose is equal to or inferior to 7.5 mg/day,” said Dr. Assunção.
The study received no funding. Dr. Assunção has no financial disclosures, but one coauthor reported receiving grant/research support from Pfizer and AbbVie and serving as a consultant to Pfizer, AbbVie, Roche, Lilly, and Novartis.
SOURCE: Assunção H et al. Ann Rheum Dis 2020;79[suppl 1]:60, Abstract OP0092.
An alternative disease activity index for patients with systemic lupus erythematosus called the SLE-DAS (Disease Activity Score) has shown similar results to the Lupus Low Disease Activity State (LLDAS) in classifying low disease activity but may be easier to potentially apply in daily clinical practice in treat-to-target strategies, according to research presented at the annual European Congress of Rheumatology, held online this year because of COVID-19.
A treat-to-target approach, in which therapies are adjusted and the patient monitored to achieve the desired endpoint, has been proposed for patients with SLE. Clinical remission is the ideal goal, followed by achieving low disease activity (LDA) when clinical remission is unattainable, the first author of the SLE-DAS study, Helena Assunção, MD, of the department of rheumatology at Centro Hospitalar e Universitário de Coimbra (Portugal), said in an interview prior to the presentation of the study at the e-congress.
But to conduct a treat-to-target approach in the clinical setting, clinicians must have reliable, user-friendly targets to assess a patient’s progress, she said. But that’s not available right now. Proposed definitions of LDA, such as the LLDAS, are based on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K). This index doesn’t address some important manifestations of SLE and it is scored dichotomously – for example, giving a similar score for thrombocytopenia when platelet count is reduced to 100,000 or to 10,000.
To compensate for these limitations, the current LLDAS definition also requires the Physician Global Assessment and other steps, including a review of medication and changes to treatment or clinical status since the previous visit.
“It is not easy to apply,” Dr. Assunção said.
The SLE-DAS is a continuous index involving 17 parameters (4 continuous: arthritis, proteinuria, thrombocytopenia, and leukopenia), assigning higher scores when a manifestation is more severe, and has manifestation information that SLEDAI-2K lacks (cardiopulmonary involvement, lupus enteritis, and hemolytic anemia).
In contrast, the LLDAS is defined as:
- A SLEDAI-2k score of 4 or less with no major organ involvement
- No new disease activity
- A physician global assessment of the patient of 1 or less on a 0-3 scale
- Maintenance on a prednisolone dosage of 7.5 mg/day or less
- Maintenance on a standard immunosuppressive regimen
A previous study validated the SLE-DAS (Ann Rheum Dis. 2019 Mar;78[3]:365-71), and another exploratory study identified a cutoff SLE-DAS value of 3.77 or lower for LDA with SLE-DAS (Ann Rheum Dis. 2019;78:411-2).
Her group compared LDA status as measured with LLDAS versus the SLE-DAS in a cross-sectional study of 292 consecutive patients at their hospital. LDA on the SLE-DAS was defined as a score 3.77 or lower and a prednisolone dose of 7.5 mg/day or less. A total of 85% of patients were in LDA with SLE-DAS and 83.9% with LLDAS, and the agreement between LLDAS and SLE-DAS LDA was very high (Cohen’s kappa coefficient test; kappa = 0.831; P < .01). Out of 292 patients, only 13 were classified differently by the two definitions, 8 of which were classified as LDA by SLE-DAS, and 5 by LLDAS. Overall, 87% of patients were women and had a mean age of nearly 49 years, with a mean disease duration of about 14 years.
Dr. Assunção feels that the SLE-DAS LDA should be sufficient to monitor disease activity without adding the Physician Global Assessment and other steps, which would make it easier to apply than LLDAS. The fact that it is based on a continuous index is also an important difference. “Especially for low disease activity, it’s very good to be able to define it with a continuous index, because you are not that bad, but not that good, you’re in the middle,” she said.
The study should be regarded as exploratory, she said, but the results were encouraging. “We got similar results, and it’s definitely easier to apply.” She can also personally attest that the new model is easier to use, since she personally collected data for LLDAS assignment. “I had to check this, and this, and this … [SLE-DAS] is easier.”
Future work from her group will aim at deriving and validating a more robust definition of LDA, which will again be compared with the current LLDAS definition.
Her colleagues have already developed and validated a definition for clinical remission using SLE-DAS, although those results have not yet been published. They hope to define activity states using SLE-DAS, including mild, moderate, and high disease activity.
The team has produced an online SLE-DAS calculator (http://sle-das.eu/) where clinicians can score the 17 parameters. “You just input the values and it gives a number reflecting disease activity. Using this definition of SLE-DAS LDA you only need that number and to verify that the prednisolone dose is equal to or inferior to 7.5 mg/day,” said Dr. Assunção.
The study received no funding. Dr. Assunção has no financial disclosures, but one coauthor reported receiving grant/research support from Pfizer and AbbVie and serving as a consultant to Pfizer, AbbVie, Roche, Lilly, and Novartis.
SOURCE: Assunção H et al. Ann Rheum Dis 2020;79[suppl 1]:60, Abstract OP0092.
FROM EULAR 2020 E-CONGRESS
Today’s top news highlights: COVID-19 could worsen gambling problems, food allergies less common than thought
Here are the stories our MDedge editors across specialties think you need to know about today:
Could COVID-19 worsen gambling problems?
Take isolation, add excess available time and anxiety about illness or finances and you get the potential to increase problem gambling behaviors during the COVID-19 pandemic. A call to action, recently published in the Journal of Addiction Medicine, says it’s essential to gather data and supply guidance on this issue. “People are likely to be experiencing stress at levels they haven’t experienced previously,” said coauthor Marc N. Potenza, MD, PhD, of Yale University, New Haven, Conn. While multiple factors can contribute to addictive behaviors, “with respect to the pandemic, one concern is that so-called negative reinforcement motivations – engaging in an addictive behavior to escape from depressed or negative mood states – may be a driving motivation for a significant number of people during this time,” he said. Read more.
Food allergies in children are less frequent than expected
Food allergies appear to be less common than previously reported among 6- to 10-year-olds in Europe, according to a recent study. Prevalance ranged from a low of 1.4% to a high of 3.8%, both of which are “considerably lower” than the 16% rate based on parental reports of symptoms such as rash, itching, or diarrhea, Linus Grabenhenrich, MD, MPH, and colleagues reported in Allergy. The most commonly reported allergies were to peanuts and hazelnuts, with a prevalence of just over 5% for both. Previous research on pediatric food allergy prevalence has largely consisted of single-center studies with heterogeneous designs, the researchers noted. Read more.
The grocery store hug
William G. Wilkoff, MD, grew up in a family that didn’t embrace hugging, but as a small-town pediatrician he warmed up to the concept so much that he would frequently hug a passing acquaintance at the grocery store. That’s something he misses in the current environment and that he doesn’t expect will return. “[N]early every week I encounter one or two people with whom I have a long and sometimes emotionally charged relationship,” Dr. Wilkoff wrote in a column on MDedge. “Nurses with whom I sweated over difficult delivery room resuscitations. Parents for whom their anxiety was getting in the way of their ability to parent. Parents and caregivers of complex multiply disabled children who are now adults. Peers who have lost a spouse or a child. I’m sure you have your own list of people who send off that we-need-to-hug spark.” Read more.
Identifying structural lesions of axial spondyloarthritis
What constitutes a structural lesion of the sacroiliac joints on MRI that’s indicative of axial spondyloarthritis (axSpA) has long been a matter of conjecture, but the Assessment of SpondyloArthritis International Society (ASAS) MRI Working Group has developed new definitions that show a high degree of specificity in identifying such lesions in the disease. “Previous studies have described structural lesions in different ways, precluding meaningful comparisons between studies,” Walter P. Maksymowych, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19. “The ASAS MRI group has generated updated consensus lesion definitions that describe each of the MRI lesions in the sacroiliac joint. These definitions have been validated by seven expert readers from the ASAS MRI group on MRI images from the ASAS classification cohort.” Read more.
Making the world’s skin crawl
Clinicians should be aware of the skin manifestations of COVID-19, especially when triaging patients. In a commentary published on MDedge, Kathleen M. Coerdt and Amor Khachemoune, MD, describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Could COVID-19 worsen gambling problems?
Take isolation, add excess available time and anxiety about illness or finances and you get the potential to increase problem gambling behaviors during the COVID-19 pandemic. A call to action, recently published in the Journal of Addiction Medicine, says it’s essential to gather data and supply guidance on this issue. “People are likely to be experiencing stress at levels they haven’t experienced previously,” said coauthor Marc N. Potenza, MD, PhD, of Yale University, New Haven, Conn. While multiple factors can contribute to addictive behaviors, “with respect to the pandemic, one concern is that so-called negative reinforcement motivations – engaging in an addictive behavior to escape from depressed or negative mood states – may be a driving motivation for a significant number of people during this time,” he said. Read more.
Food allergies in children are less frequent than expected
Food allergies appear to be less common than previously reported among 6- to 10-year-olds in Europe, according to a recent study. Prevalance ranged from a low of 1.4% to a high of 3.8%, both of which are “considerably lower” than the 16% rate based on parental reports of symptoms such as rash, itching, or diarrhea, Linus Grabenhenrich, MD, MPH, and colleagues reported in Allergy. The most commonly reported allergies were to peanuts and hazelnuts, with a prevalence of just over 5% for both. Previous research on pediatric food allergy prevalence has largely consisted of single-center studies with heterogeneous designs, the researchers noted. Read more.
The grocery store hug
William G. Wilkoff, MD, grew up in a family that didn’t embrace hugging, but as a small-town pediatrician he warmed up to the concept so much that he would frequently hug a passing acquaintance at the grocery store. That’s something he misses in the current environment and that he doesn’t expect will return. “[N]early every week I encounter one or two people with whom I have a long and sometimes emotionally charged relationship,” Dr. Wilkoff wrote in a column on MDedge. “Nurses with whom I sweated over difficult delivery room resuscitations. Parents for whom their anxiety was getting in the way of their ability to parent. Parents and caregivers of complex multiply disabled children who are now adults. Peers who have lost a spouse or a child. I’m sure you have your own list of people who send off that we-need-to-hug spark.” Read more.
Identifying structural lesions of axial spondyloarthritis
What constitutes a structural lesion of the sacroiliac joints on MRI that’s indicative of axial spondyloarthritis (axSpA) has long been a matter of conjecture, but the Assessment of SpondyloArthritis International Society (ASAS) MRI Working Group has developed new definitions that show a high degree of specificity in identifying such lesions in the disease. “Previous studies have described structural lesions in different ways, precluding meaningful comparisons between studies,” Walter P. Maksymowych, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19. “The ASAS MRI group has generated updated consensus lesion definitions that describe each of the MRI lesions in the sacroiliac joint. These definitions have been validated by seven expert readers from the ASAS MRI group on MRI images from the ASAS classification cohort.” Read more.
Making the world’s skin crawl
Clinicians should be aware of the skin manifestations of COVID-19, especially when triaging patients. In a commentary published on MDedge, Kathleen M. Coerdt and Amor Khachemoune, MD, describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Could COVID-19 worsen gambling problems?
Take isolation, add excess available time and anxiety about illness or finances and you get the potential to increase problem gambling behaviors during the COVID-19 pandemic. A call to action, recently published in the Journal of Addiction Medicine, says it’s essential to gather data and supply guidance on this issue. “People are likely to be experiencing stress at levels they haven’t experienced previously,” said coauthor Marc N. Potenza, MD, PhD, of Yale University, New Haven, Conn. While multiple factors can contribute to addictive behaviors, “with respect to the pandemic, one concern is that so-called negative reinforcement motivations – engaging in an addictive behavior to escape from depressed or negative mood states – may be a driving motivation for a significant number of people during this time,” he said. Read more.
Food allergies in children are less frequent than expected
Food allergies appear to be less common than previously reported among 6- to 10-year-olds in Europe, according to a recent study. Prevalance ranged from a low of 1.4% to a high of 3.8%, both of which are “considerably lower” than the 16% rate based on parental reports of symptoms such as rash, itching, or diarrhea, Linus Grabenhenrich, MD, MPH, and colleagues reported in Allergy. The most commonly reported allergies were to peanuts and hazelnuts, with a prevalence of just over 5% for both. Previous research on pediatric food allergy prevalence has largely consisted of single-center studies with heterogeneous designs, the researchers noted. Read more.
The grocery store hug
William G. Wilkoff, MD, grew up in a family that didn’t embrace hugging, but as a small-town pediatrician he warmed up to the concept so much that he would frequently hug a passing acquaintance at the grocery store. That’s something he misses in the current environment and that he doesn’t expect will return. “[N]early every week I encounter one or two people with whom I have a long and sometimes emotionally charged relationship,” Dr. Wilkoff wrote in a column on MDedge. “Nurses with whom I sweated over difficult delivery room resuscitations. Parents for whom their anxiety was getting in the way of their ability to parent. Parents and caregivers of complex multiply disabled children who are now adults. Peers who have lost a spouse or a child. I’m sure you have your own list of people who send off that we-need-to-hug spark.” Read more.
Identifying structural lesions of axial spondyloarthritis
What constitutes a structural lesion of the sacroiliac joints on MRI that’s indicative of axial spondyloarthritis (axSpA) has long been a matter of conjecture, but the Assessment of SpondyloArthritis International Society (ASAS) MRI Working Group has developed new definitions that show a high degree of specificity in identifying such lesions in the disease. “Previous studies have described structural lesions in different ways, precluding meaningful comparisons between studies,” Walter P. Maksymowych, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19. “The ASAS MRI group has generated updated consensus lesion definitions that describe each of the MRI lesions in the sacroiliac joint. These definitions have been validated by seven expert readers from the ASAS MRI group on MRI images from the ASAS classification cohort.” Read more.
Making the world’s skin crawl
Clinicians should be aware of the skin manifestations of COVID-19, especially when triaging patients. In a commentary published on MDedge, Kathleen M. Coerdt and Amor Khachemoune, MD, describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Working group proposes MRI definitions of structural lesions indicative of axial spondyloarthritis
What constitutes a structural lesion of the sacroiliac joints on MRI that’s indicative of axial spondyloarthritis (axSpA) has long been a matter of conjecture, but the Assessment of SpondyloArthritis International Society (ASAS) MRI Working Group has developed new definitions that showed a high degree of specificity in identifying such lesions in the disease.
“There is a lack of consensus as to what defines a structural lesion on MRI of the sacroiliac joint [SIJ] typical of axial spondyloarthritis. Previous studies have described structural lesions in different ways, precluding meaningful comparisons between studies. The ASAS MRI group has generated updated consensus lesion definitions that describe each of the MRI lesions in the sacroiliac joint. These definitions have been validated by seven expert readers from the ASAS MRI group on MRI images from the ASAS classification cohort,” Walter P. Maksymowych, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Making a definitive diagnosis of axSpA can be difficult because MRI can show a variety of SIJ abnormalities in healthy people as well as those with axSpA, said Dr. Maksymowych, chief medical officer of CARE Arthritis and professor in rheumatology at the University of Alberta in Edmonton, said in an interview prior to his presentation at the e-congress. “People who evaluate MRI scans are looking for clues as to what types of lesions they can be confident are indicative of axSpA.”
That started a process by the ASAS MRI group to evaluate scans from the landmark ASAS Classification Cohort study (Ann Rheum Dis. 2019;78:1550-8). “But,” said Dr. Maksymowych, “the MRI scans from that study were never evaluated.” So that work was handed off to the working group, whose 25 members included 7 expert image readers who evaluated the MRI scans.
The group adopted a standardized approach for evaluating MRIs of the SIJ in 148 cases, dividing each SIJ into quadrants and then evaluating consecutive MRI slices. The readers first documented whether they observed a definite structural lesion on the scan, which they then used as an external reference standard. They then analyzed which lesion, and in how many SIJ quadrants or slices, best reflected this external standard.
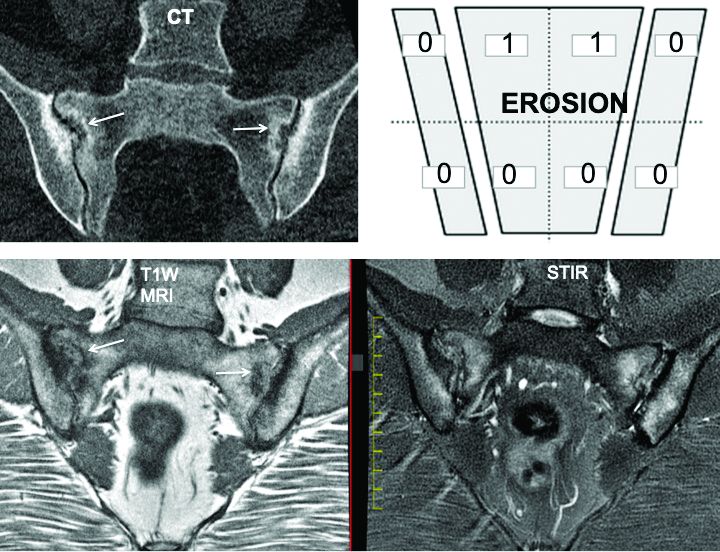
The investigators defined an erosion as “a defect in subchondral bone associated with full-thickness loss of a dark appearance of the subchondral cortex at its expected location, with loss of signal on a T1-weighted, non–fat-suppressed sequence, compared with the normal bright appearance of adjacent bone marrow.” They defined a fat lesion or fat metaplasia as a “bright signal seen on a T1-weighted, non–fat-suppressed sequence that is brighter than normal bone marrow which meets the following requirements: It is homogeneously bright, located in a typical anatomical area (specifically subchondral bone), and has a sharply defined border along its nonarticular border with normal bone marrow.”
An erosion in one quadrant isn’t sufficient to define a scan as positive for a definite structural lesion, said Dr. Maksymowych; but an erosion in three quadrants or in two or more consecutive slices meets the group’s designation of a definite structural lesion. “This showed over a 95% specificity for being associated with a definite structural lesion as defined by a majority of the seven experts,” he said.
The group also determined that a fat lesion typical of axSpA has a homogeneous white appearance on T1-weighted scans with a sharply defined border. The group also determined that such a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant is strongly indicative of axSpA.
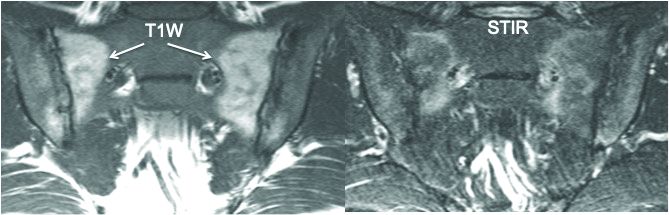
“So we now have definitions for two structural lesions, erosion and fat lesions, that reflect what a majority of experts consider to be a definite structural lesion according to at least 95% specificity,” he said. Sensitivity values were 90% for erosion in three quadrants and 83% for erosions in two or more consecutive slices. and 59% for a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant.
The second part of the analysis evaluated the predictive capacity of these lesion definitions for a rheumatologic diagnosis of axSpA at 4.4 years of follow-up. “These lesions predicted SpA with over 95% positive predictive value,” he said. “In other words, if you see them at baseline they’re going to predict SpA with high certainty at follow-up after 4.4 years.”
Three aspects of this study design are unique, Dr. Maksymowych noted. First is the high number of expert MRI readers who evaluated the scans. “There aren’t really too many studies I can think of that used more than two or three expert MRI readers,” he said.
Second is the way in which the study “very precisely and in a very standardized way” applied all the consensus-based ASAS definitions of structural SIJ lesions. “In the past, a variety of ways were used to define these lesions,” he said. “A good example would be the different ways in which erosions have been defined.”
The third novel aspect of the study is that the expert readers’ assessment of what constitutes a definite structural lesion was used as an external reference standard. For example, the study calculated sensitivity and specificity for numbers of SIJ quadrants and consecutive slices with erosion, sclerosis, and fat lesions where a majority of readers agreed on the presence of a structural lesion typical of axSpA with high confidence (3 or greater on a scale of 1-4). “The reason this was put in place is because we recognize sometimes lesions are very subtle and you can’t be certain that they’re reflecting SpA,” he said.
The investigators disclosed relationships with AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Merck, Novo Nordisk, Novartis, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB.
Maksymowych WP et al. Ann Rheum Dis, 2020;79[suppl 1]:53. Abstract OP0079.
What constitutes a structural lesion of the sacroiliac joints on MRI that’s indicative of axial spondyloarthritis (axSpA) has long been a matter of conjecture, but the Assessment of SpondyloArthritis International Society (ASAS) MRI Working Group has developed new definitions that showed a high degree of specificity in identifying such lesions in the disease.
“There is a lack of consensus as to what defines a structural lesion on MRI of the sacroiliac joint [SIJ] typical of axial spondyloarthritis. Previous studies have described structural lesions in different ways, precluding meaningful comparisons between studies. The ASAS MRI group has generated updated consensus lesion definitions that describe each of the MRI lesions in the sacroiliac joint. These definitions have been validated by seven expert readers from the ASAS MRI group on MRI images from the ASAS classification cohort,” Walter P. Maksymowych, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Making a definitive diagnosis of axSpA can be difficult because MRI can show a variety of SIJ abnormalities in healthy people as well as those with axSpA, said Dr. Maksymowych, chief medical officer of CARE Arthritis and professor in rheumatology at the University of Alberta in Edmonton, said in an interview prior to his presentation at the e-congress. “People who evaluate MRI scans are looking for clues as to what types of lesions they can be confident are indicative of axSpA.”
That started a process by the ASAS MRI group to evaluate scans from the landmark ASAS Classification Cohort study (Ann Rheum Dis. 2019;78:1550-8). “But,” said Dr. Maksymowych, “the MRI scans from that study were never evaluated.” So that work was handed off to the working group, whose 25 members included 7 expert image readers who evaluated the MRI scans.
The group adopted a standardized approach for evaluating MRIs of the SIJ in 148 cases, dividing each SIJ into quadrants and then evaluating consecutive MRI slices. The readers first documented whether they observed a definite structural lesion on the scan, which they then used as an external reference standard. They then analyzed which lesion, and in how many SIJ quadrants or slices, best reflected this external standard.

The investigators defined an erosion as “a defect in subchondral bone associated with full-thickness loss of a dark appearance of the subchondral cortex at its expected location, with loss of signal on a T1-weighted, non–fat-suppressed sequence, compared with the normal bright appearance of adjacent bone marrow.” They defined a fat lesion or fat metaplasia as a “bright signal seen on a T1-weighted, non–fat-suppressed sequence that is brighter than normal bone marrow which meets the following requirements: It is homogeneously bright, located in a typical anatomical area (specifically subchondral bone), and has a sharply defined border along its nonarticular border with normal bone marrow.”
An erosion in one quadrant isn’t sufficient to define a scan as positive for a definite structural lesion, said Dr. Maksymowych; but an erosion in three quadrants or in two or more consecutive slices meets the group’s designation of a definite structural lesion. “This showed over a 95% specificity for being associated with a definite structural lesion as defined by a majority of the seven experts,” he said.
The group also determined that a fat lesion typical of axSpA has a homogeneous white appearance on T1-weighted scans with a sharply defined border. The group also determined that such a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant is strongly indicative of axSpA.

“So we now have definitions for two structural lesions, erosion and fat lesions, that reflect what a majority of experts consider to be a definite structural lesion according to at least 95% specificity,” he said. Sensitivity values were 90% for erosion in three quadrants and 83% for erosions in two or more consecutive slices. and 59% for a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant.
The second part of the analysis evaluated the predictive capacity of these lesion definitions for a rheumatologic diagnosis of axSpA at 4.4 years of follow-up. “These lesions predicted SpA with over 95% positive predictive value,” he said. “In other words, if you see them at baseline they’re going to predict SpA with high certainty at follow-up after 4.4 years.”
Three aspects of this study design are unique, Dr. Maksymowych noted. First is the high number of expert MRI readers who evaluated the scans. “There aren’t really too many studies I can think of that used more than two or three expert MRI readers,” he said.
Second is the way in which the study “very precisely and in a very standardized way” applied all the consensus-based ASAS definitions of structural SIJ lesions. “In the past, a variety of ways were used to define these lesions,” he said. “A good example would be the different ways in which erosions have been defined.”
The third novel aspect of the study is that the expert readers’ assessment of what constitutes a definite structural lesion was used as an external reference standard. For example, the study calculated sensitivity and specificity for numbers of SIJ quadrants and consecutive slices with erosion, sclerosis, and fat lesions where a majority of readers agreed on the presence of a structural lesion typical of axSpA with high confidence (3 or greater on a scale of 1-4). “The reason this was put in place is because we recognize sometimes lesions are very subtle and you can’t be certain that they’re reflecting SpA,” he said.
The investigators disclosed relationships with AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Merck, Novo Nordisk, Novartis, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB.
Maksymowych WP et al. Ann Rheum Dis, 2020;79[suppl 1]:53. Abstract OP0079.
What constitutes a structural lesion of the sacroiliac joints on MRI that’s indicative of axial spondyloarthritis (axSpA) has long been a matter of conjecture, but the Assessment of SpondyloArthritis International Society (ASAS) MRI Working Group has developed new definitions that showed a high degree of specificity in identifying such lesions in the disease.
“There is a lack of consensus as to what defines a structural lesion on MRI of the sacroiliac joint [SIJ] typical of axial spondyloarthritis. Previous studies have described structural lesions in different ways, precluding meaningful comparisons between studies. The ASAS MRI group has generated updated consensus lesion definitions that describe each of the MRI lesions in the sacroiliac joint. These definitions have been validated by seven expert readers from the ASAS MRI group on MRI images from the ASAS classification cohort,” Walter P. Maksymowych, MD, said at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Making a definitive diagnosis of axSpA can be difficult because MRI can show a variety of SIJ abnormalities in healthy people as well as those with axSpA, said Dr. Maksymowych, chief medical officer of CARE Arthritis and professor in rheumatology at the University of Alberta in Edmonton, said in an interview prior to his presentation at the e-congress. “People who evaluate MRI scans are looking for clues as to what types of lesions they can be confident are indicative of axSpA.”
That started a process by the ASAS MRI group to evaluate scans from the landmark ASAS Classification Cohort study (Ann Rheum Dis. 2019;78:1550-8). “But,” said Dr. Maksymowych, “the MRI scans from that study were never evaluated.” So that work was handed off to the working group, whose 25 members included 7 expert image readers who evaluated the MRI scans.
The group adopted a standardized approach for evaluating MRIs of the SIJ in 148 cases, dividing each SIJ into quadrants and then evaluating consecutive MRI slices. The readers first documented whether they observed a definite structural lesion on the scan, which they then used as an external reference standard. They then analyzed which lesion, and in how many SIJ quadrants or slices, best reflected this external standard.

The investigators defined an erosion as “a defect in subchondral bone associated with full-thickness loss of a dark appearance of the subchondral cortex at its expected location, with loss of signal on a T1-weighted, non–fat-suppressed sequence, compared with the normal bright appearance of adjacent bone marrow.” They defined a fat lesion or fat metaplasia as a “bright signal seen on a T1-weighted, non–fat-suppressed sequence that is brighter than normal bone marrow which meets the following requirements: It is homogeneously bright, located in a typical anatomical area (specifically subchondral bone), and has a sharply defined border along its nonarticular border with normal bone marrow.”
An erosion in one quadrant isn’t sufficient to define a scan as positive for a definite structural lesion, said Dr. Maksymowych; but an erosion in three quadrants or in two or more consecutive slices meets the group’s designation of a definite structural lesion. “This showed over a 95% specificity for being associated with a definite structural lesion as defined by a majority of the seven experts,” he said.
The group also determined that a fat lesion typical of axSpA has a homogeneous white appearance on T1-weighted scans with a sharply defined border. The group also determined that such a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant is strongly indicative of axSpA.

“So we now have definitions for two structural lesions, erosion and fat lesions, that reflect what a majority of experts consider to be a definite structural lesion according to at least 95% specificity,” he said. Sensitivity values were 90% for erosion in three quadrants and 83% for erosions in two or more consecutive slices. and 59% for a fat lesion with at least 1-cm horizontal depth from the joint margin in at least one SIJ quadrant.
The second part of the analysis evaluated the predictive capacity of these lesion definitions for a rheumatologic diagnosis of axSpA at 4.4 years of follow-up. “These lesions predicted SpA with over 95% positive predictive value,” he said. “In other words, if you see them at baseline they’re going to predict SpA with high certainty at follow-up after 4.4 years.”
Three aspects of this study design are unique, Dr. Maksymowych noted. First is the high number of expert MRI readers who evaluated the scans. “There aren’t really too many studies I can think of that used more than two or three expert MRI readers,” he said.
Second is the way in which the study “very precisely and in a very standardized way” applied all the consensus-based ASAS definitions of structural SIJ lesions. “In the past, a variety of ways were used to define these lesions,” he said. “A good example would be the different ways in which erosions have been defined.”
The third novel aspect of the study is that the expert readers’ assessment of what constitutes a definite structural lesion was used as an external reference standard. For example, the study calculated sensitivity and specificity for numbers of SIJ quadrants and consecutive slices with erosion, sclerosis, and fat lesions where a majority of readers agreed on the presence of a structural lesion typical of axSpA with high confidence (3 or greater on a scale of 1-4). “The reason this was put in place is because we recognize sometimes lesions are very subtle and you can’t be certain that they’re reflecting SpA,” he said.
The investigators disclosed relationships with AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Merck, Novo Nordisk, Novartis, Orion, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB.
Maksymowych WP et al. Ann Rheum Dis, 2020;79[suppl 1]:53. Abstract OP0079.
FROM EULAR 2020 E-CONGRESS
FDA approves ixekizumab for nonradiographic axSpA
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
Nonpharmacologic ankylosing spondylitis recommendations not followed
Nonpharmacologic recommendations for ankylosing spondylitis aren’t often followed by rheumatologists in the Boston-based Partners Healthcare system, and probably elsewhere, according to a review presented at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
The American College of Rheumatology, Spondylitis Association of America, and SPARTAN released joint guidelines for ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis in 2016. Nonpharmacologic recommendations for AS included regular disease activity monitoring using a validated measure and C-reactive protein or erythrocyte sedimentation rate; physical therapy (PT) or home back exercises; and screening for osteoporosis with dual x-ray absorptiometry (DXA) scanning.
However, “the extent to which these recommendations are followed in clinical practice is unknown,” said lead investigator Akash Patel, of the Brigham and Women’s Hospital Division of Rheumatology, Immunology, and Allergy, in Boston.
To find out, the team reviewed electronic records for 304 AS patients who had 564 rheumatology clinic visits with Brigham and Women’s and other Partners Healthcare physicians from July 1, 2016, to June 30, 2019.
Records documented DXA scans in less than 20% of visits. PT was documented in only 9% of visits, and home back exercise in just 7%. Inflammatory marker measurement was documented in about half of visits, and disease activity was measured in only 17%.
Comparing the first year of the study – right after the recommendations came out – to the third year, the team found just an 8% increase in disease activity documentation, and about a 3% increase in documentation of PT and back exercises.
In short, the recommendations “were performed at low frequencies in this study population,” Mr. Patel said at the meeting, which was held online this year because of the COVID-19 pandemic.
It’s unclear what’s going on. Perhaps some physicians disagree with the 2016 advice – the regular monitoring of disease activity, after all, was a conditional recommendation based on low-quality evidence. Other times, physicians might not have had enough time to talk about exercise or draw blood for AS biomarkers. Maybe they didn’t bring up PT when they knew their patients couldn’t afford the out-of-pocket cost.
Whatever the case, future iterations of the guidelines should include advice on how to implement them. “We believe that including some sort of strategy for rheumatologists may help increase compliance,” Mr. Patel said.
A member of the online viewing audience suggested that the problem may be widespread in rheumatology. "I think if we did this at my institution,” for example, “it would also look abysmal. I think we all just suck at this,” the attendee said.*
Mr. Patel and his team presented the results to Brigham and Women’s rheumatologists in February 2020, but it’s too early to tell if it made a difference.
It was a typical AS cohort. Almost three-quarters of the subjects were men; the average age was 50 years old; and the diagnosis was made by imaging. The majority of patients were HLA-B27 positive, and over one-third had a history of uveitis.
The study’s funding source and disclosures – if any – weren’t reported.
*Correction, 6/3/2020: A previous version of this story misattributed this quote.
SOURCE: Patel A et al. SPARTAN 2020 abstract session May 15.
Nonpharmacologic recommendations for ankylosing spondylitis aren’t often followed by rheumatologists in the Boston-based Partners Healthcare system, and probably elsewhere, according to a review presented at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
The American College of Rheumatology, Spondylitis Association of America, and SPARTAN released joint guidelines for ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis in 2016. Nonpharmacologic recommendations for AS included regular disease activity monitoring using a validated measure and C-reactive protein or erythrocyte sedimentation rate; physical therapy (PT) or home back exercises; and screening for osteoporosis with dual x-ray absorptiometry (DXA) scanning.
However, “the extent to which these recommendations are followed in clinical practice is unknown,” said lead investigator Akash Patel, of the Brigham and Women’s Hospital Division of Rheumatology, Immunology, and Allergy, in Boston.
To find out, the team reviewed electronic records for 304 AS patients who had 564 rheumatology clinic visits with Brigham and Women’s and other Partners Healthcare physicians from July 1, 2016, to June 30, 2019.
Records documented DXA scans in less than 20% of visits. PT was documented in only 9% of visits, and home back exercise in just 7%. Inflammatory marker measurement was documented in about half of visits, and disease activity was measured in only 17%.
Comparing the first year of the study – right after the recommendations came out – to the third year, the team found just an 8% increase in disease activity documentation, and about a 3% increase in documentation of PT and back exercises.
In short, the recommendations “were performed at low frequencies in this study population,” Mr. Patel said at the meeting, which was held online this year because of the COVID-19 pandemic.
It’s unclear what’s going on. Perhaps some physicians disagree with the 2016 advice – the regular monitoring of disease activity, after all, was a conditional recommendation based on low-quality evidence. Other times, physicians might not have had enough time to talk about exercise or draw blood for AS biomarkers. Maybe they didn’t bring up PT when they knew their patients couldn’t afford the out-of-pocket cost.
Whatever the case, future iterations of the guidelines should include advice on how to implement them. “We believe that including some sort of strategy for rheumatologists may help increase compliance,” Mr. Patel said.
A member of the online viewing audience suggested that the problem may be widespread in rheumatology. "I think if we did this at my institution,” for example, “it would also look abysmal. I think we all just suck at this,” the attendee said.*
Mr. Patel and his team presented the results to Brigham and Women’s rheumatologists in February 2020, but it’s too early to tell if it made a difference.
It was a typical AS cohort. Almost three-quarters of the subjects were men; the average age was 50 years old; and the diagnosis was made by imaging. The majority of patients were HLA-B27 positive, and over one-third had a history of uveitis.
The study’s funding source and disclosures – if any – weren’t reported.
*Correction, 6/3/2020: A previous version of this story misattributed this quote.
SOURCE: Patel A et al. SPARTAN 2020 abstract session May 15.
Nonpharmacologic recommendations for ankylosing spondylitis aren’t often followed by rheumatologists in the Boston-based Partners Healthcare system, and probably elsewhere, according to a review presented at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
The American College of Rheumatology, Spondylitis Association of America, and SPARTAN released joint guidelines for ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis in 2016. Nonpharmacologic recommendations for AS included regular disease activity monitoring using a validated measure and C-reactive protein or erythrocyte sedimentation rate; physical therapy (PT) or home back exercises; and screening for osteoporosis with dual x-ray absorptiometry (DXA) scanning.
However, “the extent to which these recommendations are followed in clinical practice is unknown,” said lead investigator Akash Patel, of the Brigham and Women’s Hospital Division of Rheumatology, Immunology, and Allergy, in Boston.
To find out, the team reviewed electronic records for 304 AS patients who had 564 rheumatology clinic visits with Brigham and Women’s and other Partners Healthcare physicians from July 1, 2016, to June 30, 2019.
Records documented DXA scans in less than 20% of visits. PT was documented in only 9% of visits, and home back exercise in just 7%. Inflammatory marker measurement was documented in about half of visits, and disease activity was measured in only 17%.
Comparing the first year of the study – right after the recommendations came out – to the third year, the team found just an 8% increase in disease activity documentation, and about a 3% increase in documentation of PT and back exercises.
In short, the recommendations “were performed at low frequencies in this study population,” Mr. Patel said at the meeting, which was held online this year because of the COVID-19 pandemic.
It’s unclear what’s going on. Perhaps some physicians disagree with the 2016 advice – the regular monitoring of disease activity, after all, was a conditional recommendation based on low-quality evidence. Other times, physicians might not have had enough time to talk about exercise or draw blood for AS biomarkers. Maybe they didn’t bring up PT when they knew their patients couldn’t afford the out-of-pocket cost.
Whatever the case, future iterations of the guidelines should include advice on how to implement them. “We believe that including some sort of strategy for rheumatologists may help increase compliance,” Mr. Patel said.
A member of the online viewing audience suggested that the problem may be widespread in rheumatology. "I think if we did this at my institution,” for example, “it would also look abysmal. I think we all just suck at this,” the attendee said.*
Mr. Patel and his team presented the results to Brigham and Women’s rheumatologists in February 2020, but it’s too early to tell if it made a difference.
It was a typical AS cohort. Almost three-quarters of the subjects were men; the average age was 50 years old; and the diagnosis was made by imaging. The majority of patients were HLA-B27 positive, and over one-third had a history of uveitis.
The study’s funding source and disclosures – if any – weren’t reported.
*Correction, 6/3/2020: A previous version of this story misattributed this quote.
SOURCE: Patel A et al. SPARTAN 2020 abstract session May 15.
FROM SPARTAN 2020
Unacceptable RA pain may drop with TNFi treatment but still lingers in many patients
according to findings from 21 months of follow-up in a post hoc analysis of data from the randomized, controlled Swedish Farmacotherapy (SWEFOT) trial.
Although RA patients who took biologic combination therapy had 32% lower risk for unacceptable pain (rated at >40 mm on a 0- to 100-mm visual analog scale) at 21 months, they still had no difference from patients taking triple therapy in the rate of pain described as refractory, or unacceptable despite inflammation control (C-reactive protein <10 mg/L).
While these results lend “some support to a better effect on long-term pain for the biological treatment, compared with triple therapy ... our findings are also in line with insufficient effects of current treatment strategies to prevent development of inflammation-independent pain components, warranting early alternative treatment approaches in affected patients,” Tor Olofsson, MD, PhD, of Lund (Sweden) University, and colleagues wrote in Arthritis Care & Research.
The pain outcomes analyzed in this post hoc study were all secondary outcomes of the original open-label SWEFOT trial, which during 2002-2005 enrolled 258 RA patients with less than a year of symptoms who did not reach low disease activity (28-joint Disease Activity Score ≤3.2) after 3 months of methotrexate and randomized them to an addition of either infliximab (3 mg/kg rounded up to nearest 100-mg increment) or sulfasalazine 1,000 mg twice daily plus hydroxychloroquine 400 mg once daily.
Overall, 90 of 128 patients in the infliximab group and 74 of 130 in the triple-therapy group continued the protocol until the 21-month follow-up. Patients in the infliximab group had a significantly lower area under the curve for visual analog scale for pain, most of which was accounted for during months 9-21. The percentage of patients in the infliximab group with unacceptable pain also dropped significantly from 57% at randomization to 32% at 21 months, while no difference was seen for triple therapy patients, of whom 45% had unacceptable pain at 21 months.
While patients in the infliximab group had a significantly lower risk of unacceptable pain without inflammatory control at 21 months, neither treatment arm showed a within-group difference in refractory pain from randomization to the 21-month follow-up.
Nearly one-third of patients overall still reported unacceptable pain 21 months after addition of either infliximab or sulfasalazine plus hydroxychloroquine. And at that time point, refractory pain constituted 82% of all unacceptable pain. “Notably, this pattern – with a domination of refractory pain – was evident already 3 months after starting combination therapy,” Dr. Olofsson and colleagues wrote.
The original SWEFOT study was supported in part by a grant from the Swedish Rheumatism Association, and in part by an annual unrestricted grant from Schering-Plough Sweden (now Merck Sharp & Dohme). The post hoc analysis was supported by Lund University and the Kockska Foundation, the Swedish Research Council, and the Stockholm County Council. Two authors disclosed financial relationships with multiple pharmaceutical companies.
SOURCE: Olofsson T et al. Arthritis Care Res. 2020 May 20. doi: 10.1002/acr.24264.
according to findings from 21 months of follow-up in a post hoc analysis of data from the randomized, controlled Swedish Farmacotherapy (SWEFOT) trial.
Although RA patients who took biologic combination therapy had 32% lower risk for unacceptable pain (rated at >40 mm on a 0- to 100-mm visual analog scale) at 21 months, they still had no difference from patients taking triple therapy in the rate of pain described as refractory, or unacceptable despite inflammation control (C-reactive protein <10 mg/L).
While these results lend “some support to a better effect on long-term pain for the biological treatment, compared with triple therapy ... our findings are also in line with insufficient effects of current treatment strategies to prevent development of inflammation-independent pain components, warranting early alternative treatment approaches in affected patients,” Tor Olofsson, MD, PhD, of Lund (Sweden) University, and colleagues wrote in Arthritis Care & Research.
The pain outcomes analyzed in this post hoc study were all secondary outcomes of the original open-label SWEFOT trial, which during 2002-2005 enrolled 258 RA patients with less than a year of symptoms who did not reach low disease activity (28-joint Disease Activity Score ≤3.2) after 3 months of methotrexate and randomized them to an addition of either infliximab (3 mg/kg rounded up to nearest 100-mg increment) or sulfasalazine 1,000 mg twice daily plus hydroxychloroquine 400 mg once daily.
Overall, 90 of 128 patients in the infliximab group and 74 of 130 in the triple-therapy group continued the protocol until the 21-month follow-up. Patients in the infliximab group had a significantly lower area under the curve for visual analog scale for pain, most of which was accounted for during months 9-21. The percentage of patients in the infliximab group with unacceptable pain also dropped significantly from 57% at randomization to 32% at 21 months, while no difference was seen for triple therapy patients, of whom 45% had unacceptable pain at 21 months.
While patients in the infliximab group had a significantly lower risk of unacceptable pain without inflammatory control at 21 months, neither treatment arm showed a within-group difference in refractory pain from randomization to the 21-month follow-up.
Nearly one-third of patients overall still reported unacceptable pain 21 months after addition of either infliximab or sulfasalazine plus hydroxychloroquine. And at that time point, refractory pain constituted 82% of all unacceptable pain. “Notably, this pattern – with a domination of refractory pain – was evident already 3 months after starting combination therapy,” Dr. Olofsson and colleagues wrote.
The original SWEFOT study was supported in part by a grant from the Swedish Rheumatism Association, and in part by an annual unrestricted grant from Schering-Plough Sweden (now Merck Sharp & Dohme). The post hoc analysis was supported by Lund University and the Kockska Foundation, the Swedish Research Council, and the Stockholm County Council. Two authors disclosed financial relationships with multiple pharmaceutical companies.
SOURCE: Olofsson T et al. Arthritis Care Res. 2020 May 20. doi: 10.1002/acr.24264.
according to findings from 21 months of follow-up in a post hoc analysis of data from the randomized, controlled Swedish Farmacotherapy (SWEFOT) trial.
Although RA patients who took biologic combination therapy had 32% lower risk for unacceptable pain (rated at >40 mm on a 0- to 100-mm visual analog scale) at 21 months, they still had no difference from patients taking triple therapy in the rate of pain described as refractory, or unacceptable despite inflammation control (C-reactive protein <10 mg/L).
While these results lend “some support to a better effect on long-term pain for the biological treatment, compared with triple therapy ... our findings are also in line with insufficient effects of current treatment strategies to prevent development of inflammation-independent pain components, warranting early alternative treatment approaches in affected patients,” Tor Olofsson, MD, PhD, of Lund (Sweden) University, and colleagues wrote in Arthritis Care & Research.
The pain outcomes analyzed in this post hoc study were all secondary outcomes of the original open-label SWEFOT trial, which during 2002-2005 enrolled 258 RA patients with less than a year of symptoms who did not reach low disease activity (28-joint Disease Activity Score ≤3.2) after 3 months of methotrexate and randomized them to an addition of either infliximab (3 mg/kg rounded up to nearest 100-mg increment) or sulfasalazine 1,000 mg twice daily plus hydroxychloroquine 400 mg once daily.
Overall, 90 of 128 patients in the infliximab group and 74 of 130 in the triple-therapy group continued the protocol until the 21-month follow-up. Patients in the infliximab group had a significantly lower area under the curve for visual analog scale for pain, most of which was accounted for during months 9-21. The percentage of patients in the infliximab group with unacceptable pain also dropped significantly from 57% at randomization to 32% at 21 months, while no difference was seen for triple therapy patients, of whom 45% had unacceptable pain at 21 months.
While patients in the infliximab group had a significantly lower risk of unacceptable pain without inflammatory control at 21 months, neither treatment arm showed a within-group difference in refractory pain from randomization to the 21-month follow-up.
Nearly one-third of patients overall still reported unacceptable pain 21 months after addition of either infliximab or sulfasalazine plus hydroxychloroquine. And at that time point, refractory pain constituted 82% of all unacceptable pain. “Notably, this pattern – with a domination of refractory pain – was evident already 3 months after starting combination therapy,” Dr. Olofsson and colleagues wrote.
The original SWEFOT study was supported in part by a grant from the Swedish Rheumatism Association, and in part by an annual unrestricted grant from Schering-Plough Sweden (now Merck Sharp & Dohme). The post hoc analysis was supported by Lund University and the Kockska Foundation, the Swedish Research Council, and the Stockholm County Council. Two authors disclosed financial relationships with multiple pharmaceutical companies.
SOURCE: Olofsson T et al. Arthritis Care Res. 2020 May 20. doi: 10.1002/acr.24264.
FROM ARTHRITIS CARE & RESEARCH
COVID-19 vaccine won’t be a slam dunk
A successful vaccine for prevention of SARS-CoV-2 infection will probably need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against infection. In mouse studies, T-cell immunity has protected against reinfection with the novel coronaviruses. And in some but not all studies of patients infected with the SARS virus, which shares 80% genetic overlap with the SARS-CoV-2 virus responsible for the COVID-19 pandemic, neutralizing antibodies have waned over time.
“In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco.
Dr. Matloubian is among those who are convinced that the ongoing massive global accelerated effort to develop a safe and effective vaccine affords the best opportunity to gain the upper hand in the COVID-19 pandemic. A large array of vaccines are in development.
A key safety concern to watch for in the coming months is whether a vaccine candidate is able to sidestep the issue of antibody-dependent enhancement, whereby prior infection with a non-SARS coronavirus, such as those that cause the common cold, might result in creation of rogue subneutralizing coronavirus antibodies in response to vaccination. There is concern that these nonneutralizing antibodies could facilitate entry of the virus into monocytes and other cells lacking the ACE2 receptor, its usual portal of entry. This in turn could trigger expanded viral replication, a hyperinflammatory response, and viral spread to sites beyond the lung, such as the heart or kidneys.
Little optimism about antivirals’ impact
Dr. Matloubian predicted that antiviral medications, including the much-ballyhooed remdesivir, are unlikely to be a game changer in the COVID-19 pandemic. That’s because most patients who become symptomatic don’t do so until at least 2 days post infection. By that point, their viral load has already peaked and is waning and the B- and T-cell immune responses are starting to gear up.
“Timing seems to be everything when it comes to treatment with antivirals,” he observed. “The virus titer is usually declining by the time people present with severe COVID-19, suggesting that at this time antiviral therapy might be of little use to change the course of the disease, especially if it’s mainly immune-mediated by then. Even with influenza virus, there’s a really short window where Tamiflu [oseltamivir] is effective. It’s going to be the same case for antivirals used for treatment of COVID-19.”
He noted that in a placebo-controlled, randomized trial of remdesivir in 236 Chinese patients with severe COVID-19, intravenous remdesivir wasn’t associated with a significantly shorter time to clinical improvement, although there was a trend in that direction in the subgroup with symptom duration of 10 days or less at initiation of treatment.
A National Institutes of Health press release announcing that remdesivir had a positive impact on duration of hospitalization in a separate randomized trial drew enormous attention from a public desperate for good news. However, the full study has yet to be published, and it’s unclear when during the disease course the antiviral agent was started.
“We need a blockbuster antiviral that’s oral, highly effective, and doesn’t have any side effects to be used in prophylaxis of health care workers and for people who are exposed by family members being infected. And so far there is no such thing, even on the horizon,” according to the rheumatologist.
Fellow panelist Jinoos Yazdany, MD, concurred.
“As we talk to experts around the country, it seems like there isn’t very much optimism about such a blockbuster drug. Most people are actually putting their hope in a vaccine,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
Another research priority is identification of biomarkers in blood or bronchoalveolar lavage fluid to identify early on the subgroup of infected patients who are likely to crash and develop severe disease. That would permit a targeted approach to inhibition of the inflammatory pathways contributing to development of acute respiratory distress syndrome before this full-blown cytokine storm-like syndrome can occur. There is great interest in trying to achieve this by repurposing many biologic agents widely used by rheumatologists, including the interleukin-1 blocker anakinra (Kineret) and the IL-6 blocker tocilizumab (Actemra).
Dr. Matloubian reported having no financial conflicts of interest regarding his presentation.
A successful vaccine for prevention of SARS-CoV-2 infection will probably need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against infection. In mouse studies, T-cell immunity has protected against reinfection with the novel coronaviruses. And in some but not all studies of patients infected with the SARS virus, which shares 80% genetic overlap with the SARS-CoV-2 virus responsible for the COVID-19 pandemic, neutralizing antibodies have waned over time.
“In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco.
Dr. Matloubian is among those who are convinced that the ongoing massive global accelerated effort to develop a safe and effective vaccine affords the best opportunity to gain the upper hand in the COVID-19 pandemic. A large array of vaccines are in development.
A key safety concern to watch for in the coming months is whether a vaccine candidate is able to sidestep the issue of antibody-dependent enhancement, whereby prior infection with a non-SARS coronavirus, such as those that cause the common cold, might result in creation of rogue subneutralizing coronavirus antibodies in response to vaccination. There is concern that these nonneutralizing antibodies could facilitate entry of the virus into monocytes and other cells lacking the ACE2 receptor, its usual portal of entry. This in turn could trigger expanded viral replication, a hyperinflammatory response, and viral spread to sites beyond the lung, such as the heart or kidneys.
Little optimism about antivirals’ impact
Dr. Matloubian predicted that antiviral medications, including the much-ballyhooed remdesivir, are unlikely to be a game changer in the COVID-19 pandemic. That’s because most patients who become symptomatic don’t do so until at least 2 days post infection. By that point, their viral load has already peaked and is waning and the B- and T-cell immune responses are starting to gear up.
“Timing seems to be everything when it comes to treatment with antivirals,” he observed. “The virus titer is usually declining by the time people present with severe COVID-19, suggesting that at this time antiviral therapy might be of little use to change the course of the disease, especially if it’s mainly immune-mediated by then. Even with influenza virus, there’s a really short window where Tamiflu [oseltamivir] is effective. It’s going to be the same case for antivirals used for treatment of COVID-19.”
He noted that in a placebo-controlled, randomized trial of remdesivir in 236 Chinese patients with severe COVID-19, intravenous remdesivir wasn’t associated with a significantly shorter time to clinical improvement, although there was a trend in that direction in the subgroup with symptom duration of 10 days or less at initiation of treatment.
A National Institutes of Health press release announcing that remdesivir had a positive impact on duration of hospitalization in a separate randomized trial drew enormous attention from a public desperate for good news. However, the full study has yet to be published, and it’s unclear when during the disease course the antiviral agent was started.
“We need a blockbuster antiviral that’s oral, highly effective, and doesn’t have any side effects to be used in prophylaxis of health care workers and for people who are exposed by family members being infected. And so far there is no such thing, even on the horizon,” according to the rheumatologist.
Fellow panelist Jinoos Yazdany, MD, concurred.
“As we talk to experts around the country, it seems like there isn’t very much optimism about such a blockbuster drug. Most people are actually putting their hope in a vaccine,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
Another research priority is identification of biomarkers in blood or bronchoalveolar lavage fluid to identify early on the subgroup of infected patients who are likely to crash and develop severe disease. That would permit a targeted approach to inhibition of the inflammatory pathways contributing to development of acute respiratory distress syndrome before this full-blown cytokine storm-like syndrome can occur. There is great interest in trying to achieve this by repurposing many biologic agents widely used by rheumatologists, including the interleukin-1 blocker anakinra (Kineret) and the IL-6 blocker tocilizumab (Actemra).
Dr. Matloubian reported having no financial conflicts of interest regarding his presentation.
A successful vaccine for prevention of SARS-CoV-2 infection will probably need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against infection. In mouse studies, T-cell immunity has protected against reinfection with the novel coronaviruses. And in some but not all studies of patients infected with the SARS virus, which shares 80% genetic overlap with the SARS-CoV-2 virus responsible for the COVID-19 pandemic, neutralizing antibodies have waned over time.
“In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco.
Dr. Matloubian is among those who are convinced that the ongoing massive global accelerated effort to develop a safe and effective vaccine affords the best opportunity to gain the upper hand in the COVID-19 pandemic. A large array of vaccines are in development.
A key safety concern to watch for in the coming months is whether a vaccine candidate is able to sidestep the issue of antibody-dependent enhancement, whereby prior infection with a non-SARS coronavirus, such as those that cause the common cold, might result in creation of rogue subneutralizing coronavirus antibodies in response to vaccination. There is concern that these nonneutralizing antibodies could facilitate entry of the virus into monocytes and other cells lacking the ACE2 receptor, its usual portal of entry. This in turn could trigger expanded viral replication, a hyperinflammatory response, and viral spread to sites beyond the lung, such as the heart or kidneys.
Little optimism about antivirals’ impact
Dr. Matloubian predicted that antiviral medications, including the much-ballyhooed remdesivir, are unlikely to be a game changer in the COVID-19 pandemic. That’s because most patients who become symptomatic don’t do so until at least 2 days post infection. By that point, their viral load has already peaked and is waning and the B- and T-cell immune responses are starting to gear up.
“Timing seems to be everything when it comes to treatment with antivirals,” he observed. “The virus titer is usually declining by the time people present with severe COVID-19, suggesting that at this time antiviral therapy might be of little use to change the course of the disease, especially if it’s mainly immune-mediated by then. Even with influenza virus, there’s a really short window where Tamiflu [oseltamivir] is effective. It’s going to be the same case for antivirals used for treatment of COVID-19.”
He noted that in a placebo-controlled, randomized trial of remdesivir in 236 Chinese patients with severe COVID-19, intravenous remdesivir wasn’t associated with a significantly shorter time to clinical improvement, although there was a trend in that direction in the subgroup with symptom duration of 10 days or less at initiation of treatment.
A National Institutes of Health press release announcing that remdesivir had a positive impact on duration of hospitalization in a separate randomized trial drew enormous attention from a public desperate for good news. However, the full study has yet to be published, and it’s unclear when during the disease course the antiviral agent was started.
“We need a blockbuster antiviral that’s oral, highly effective, and doesn’t have any side effects to be used in prophylaxis of health care workers and for people who are exposed by family members being infected. And so far there is no such thing, even on the horizon,” according to the rheumatologist.
Fellow panelist Jinoos Yazdany, MD, concurred.
“As we talk to experts around the country, it seems like there isn’t very much optimism about such a blockbuster drug. Most people are actually putting their hope in a vaccine,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
Another research priority is identification of biomarkers in blood or bronchoalveolar lavage fluid to identify early on the subgroup of infected patients who are likely to crash and develop severe disease. That would permit a targeted approach to inhibition of the inflammatory pathways contributing to development of acute respiratory distress syndrome before this full-blown cytokine storm-like syndrome can occur. There is great interest in trying to achieve this by repurposing many biologic agents widely used by rheumatologists, including the interleukin-1 blocker anakinra (Kineret) and the IL-6 blocker tocilizumab (Actemra).
Dr. Matloubian reported having no financial conflicts of interest regarding his presentation.
FROM SOTA 2020












