User login
COVID-19: Frequently asked clinical questions
Question
How should patients on immunosuppressive therapy be advised during the COVID-19 pandemic?
Answer
In general, those patients who have not tested positive, have not been exposed, and are asymptomatic should continue their medications as prescribed.
The American College of Rheumatology issued a statement on April 14, recommending that stable patients continue their medications. Those with known exposure but without confirmed infection may continue hydroxychloroquine, sulfasalazine, and NSAIDs.
Immunosuppressants, non–IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily. Anti-malarial therapies (hydroxycholoroquine and chloroquine) may be continued and IL-6 inhibitors may be continued in select circumstances.1
The American Academy of Dermatology recommends that the discussion of continuation of biologics be based on a case-by-case basis, citing insufficient evidence to recommend against discontinuation at this time in those patients who have not tested positive. In patients who have tested positive for COVID-19 it is recommended that biologic therapy be suspended until symptoms have resolved.2
Question
Should I continue preventive services during peak COVID-19?
Answer
The Centers for Disease Control and Prevention recommends delaying all elective ambulatory provider visits. In general, preventative services, such as adult immunizations, lipid screening, and cancer screenings, should be delayed. Additionally, the CDC recommends reaching out to patients who are at high risk for complications from respiratory diseases to ensure medication adherence and provide resources if these patients become ill. Facilities can reduce transmission of COVID-19 by triaging and assessing patients through virtual visits through phone calls, video conferences, text-monitoring systems, and other telemedicine tools. Physicians should try to provide routine and chronic care through virtual visits when possible over in-person visits.3
Question
Should I continue to vaccinate my pediatric population during peak COVID-19?
Answer
Practices that schedule separate well visits and sick visits in different sessions or locations can continue to provide well child visits. A practice could, for example, schedule well visits in the morning and sick visits in the afternoon if a single facility is used. These practices should prioritize newborn care and vaccinations of children, especially for those under the age of 24 months.4
Question
Can physicians use telehealth (phone only or audiovisual) to conduct visits with Medicare patients even if they are new patients?
Answer
Effective March 1 through the duration of the pandemic, Medicare will pay physicians for telehealth services at the same rate as an in-office visit. On March 30th, the Centers for Medicare & Medcaid Services announced new policies for physicians and hospitals during the COVID-19 pandemic. These guidelines were updated on April 9.
Audio-only visits are now permitted and the limit on the number of these kinds of visits allowed per month has been waived. Controlled substances can be prescribed via telehealth; however, complying with each state’s individual laws is still required.
Use of any two-way, audiovisual device is permitted. The level of service billed for visits with both audio and visual components is the same as an in-office visit. Telemedicine can be used for both new and existing patients.5
A list of services that may be rendered via telehealth are available on the CMS website.6
It will be important to regularly check the references given, as information on some of these topics is updated frequently.
Dr. Chuong is a second-year resident in the family medicine residency, Dr. Flanagan is a third-year resident, and Dr. Matthews is an intern, all at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. ACR issues COVID-19 treatment guidance for rheumatic disease patients.
2. American Academy of Dermatology: Guidance on the use of biologic agents during COVID-19 outbreak.
3. Centers for Disease Control and Prevention. Actions to take in response to community transmission of COVID-19.
4. Centers for Disease Control and Prevention. Maintaining childhood immunizations during COVID19 pandemic.
5. Centers for Medicare & Medcaid Services. COVID-19 frequently asked questions (FAQs) on Medicare Fee-for-Service (FFS) billing.
6. Centers for Medicare & Medcaid Services. List of telehealth services.
Question
How should patients on immunosuppressive therapy be advised during the COVID-19 pandemic?
Answer
In general, those patients who have not tested positive, have not been exposed, and are asymptomatic should continue their medications as prescribed.
The American College of Rheumatology issued a statement on April 14, recommending that stable patients continue their medications. Those with known exposure but without confirmed infection may continue hydroxychloroquine, sulfasalazine, and NSAIDs.
Immunosuppressants, non–IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily. Anti-malarial therapies (hydroxycholoroquine and chloroquine) may be continued and IL-6 inhibitors may be continued in select circumstances.1
The American Academy of Dermatology recommends that the discussion of continuation of biologics be based on a case-by-case basis, citing insufficient evidence to recommend against discontinuation at this time in those patients who have not tested positive. In patients who have tested positive for COVID-19 it is recommended that biologic therapy be suspended until symptoms have resolved.2
Question
Should I continue preventive services during peak COVID-19?
Answer
The Centers for Disease Control and Prevention recommends delaying all elective ambulatory provider visits. In general, preventative services, such as adult immunizations, lipid screening, and cancer screenings, should be delayed. Additionally, the CDC recommends reaching out to patients who are at high risk for complications from respiratory diseases to ensure medication adherence and provide resources if these patients become ill. Facilities can reduce transmission of COVID-19 by triaging and assessing patients through virtual visits through phone calls, video conferences, text-monitoring systems, and other telemedicine tools. Physicians should try to provide routine and chronic care through virtual visits when possible over in-person visits.3
Question
Should I continue to vaccinate my pediatric population during peak COVID-19?
Answer
Practices that schedule separate well visits and sick visits in different sessions or locations can continue to provide well child visits. A practice could, for example, schedule well visits in the morning and sick visits in the afternoon if a single facility is used. These practices should prioritize newborn care and vaccinations of children, especially for those under the age of 24 months.4
Question
Can physicians use telehealth (phone only or audiovisual) to conduct visits with Medicare patients even if they are new patients?
Answer
Effective March 1 through the duration of the pandemic, Medicare will pay physicians for telehealth services at the same rate as an in-office visit. On March 30th, the Centers for Medicare & Medcaid Services announced new policies for physicians and hospitals during the COVID-19 pandemic. These guidelines were updated on April 9.
Audio-only visits are now permitted and the limit on the number of these kinds of visits allowed per month has been waived. Controlled substances can be prescribed via telehealth; however, complying with each state’s individual laws is still required.
Use of any two-way, audiovisual device is permitted. The level of service billed for visits with both audio and visual components is the same as an in-office visit. Telemedicine can be used for both new and existing patients.5
A list of services that may be rendered via telehealth are available on the CMS website.6
It will be important to regularly check the references given, as information on some of these topics is updated frequently.
Dr. Chuong is a second-year resident in the family medicine residency, Dr. Flanagan is a third-year resident, and Dr. Matthews is an intern, all at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. ACR issues COVID-19 treatment guidance for rheumatic disease patients.
2. American Academy of Dermatology: Guidance on the use of biologic agents during COVID-19 outbreak.
3. Centers for Disease Control and Prevention. Actions to take in response to community transmission of COVID-19.
4. Centers for Disease Control and Prevention. Maintaining childhood immunizations during COVID19 pandemic.
5. Centers for Medicare & Medcaid Services. COVID-19 frequently asked questions (FAQs) on Medicare Fee-for-Service (FFS) billing.
6. Centers for Medicare & Medcaid Services. List of telehealth services.
Question
How should patients on immunosuppressive therapy be advised during the COVID-19 pandemic?
Answer
In general, those patients who have not tested positive, have not been exposed, and are asymptomatic should continue their medications as prescribed.
The American College of Rheumatology issued a statement on April 14, recommending that stable patients continue their medications. Those with known exposure but without confirmed infection may continue hydroxychloroquine, sulfasalazine, and NSAIDs.
Immunosuppressants, non–IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily. Anti-malarial therapies (hydroxycholoroquine and chloroquine) may be continued and IL-6 inhibitors may be continued in select circumstances.1
The American Academy of Dermatology recommends that the discussion of continuation of biologics be based on a case-by-case basis, citing insufficient evidence to recommend against discontinuation at this time in those patients who have not tested positive. In patients who have tested positive for COVID-19 it is recommended that biologic therapy be suspended until symptoms have resolved.2
Question
Should I continue preventive services during peak COVID-19?
Answer
The Centers for Disease Control and Prevention recommends delaying all elective ambulatory provider visits. In general, preventative services, such as adult immunizations, lipid screening, and cancer screenings, should be delayed. Additionally, the CDC recommends reaching out to patients who are at high risk for complications from respiratory diseases to ensure medication adherence and provide resources if these patients become ill. Facilities can reduce transmission of COVID-19 by triaging and assessing patients through virtual visits through phone calls, video conferences, text-monitoring systems, and other telemedicine tools. Physicians should try to provide routine and chronic care through virtual visits when possible over in-person visits.3
Question
Should I continue to vaccinate my pediatric population during peak COVID-19?
Answer
Practices that schedule separate well visits and sick visits in different sessions or locations can continue to provide well child visits. A practice could, for example, schedule well visits in the morning and sick visits in the afternoon if a single facility is used. These practices should prioritize newborn care and vaccinations of children, especially for those under the age of 24 months.4
Question
Can physicians use telehealth (phone only or audiovisual) to conduct visits with Medicare patients even if they are new patients?
Answer
Effective March 1 through the duration of the pandemic, Medicare will pay physicians for telehealth services at the same rate as an in-office visit. On March 30th, the Centers for Medicare & Medcaid Services announced new policies for physicians and hospitals during the COVID-19 pandemic. These guidelines were updated on April 9.
Audio-only visits are now permitted and the limit on the number of these kinds of visits allowed per month has been waived. Controlled substances can be prescribed via telehealth; however, complying with each state’s individual laws is still required.
Use of any two-way, audiovisual device is permitted. The level of service billed for visits with both audio and visual components is the same as an in-office visit. Telemedicine can be used for both new and existing patients.5
A list of services that may be rendered via telehealth are available on the CMS website.6
It will be important to regularly check the references given, as information on some of these topics is updated frequently.
Dr. Chuong is a second-year resident in the family medicine residency, Dr. Flanagan is a third-year resident, and Dr. Matthews is an intern, all at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. ACR issues COVID-19 treatment guidance for rheumatic disease patients.
2. American Academy of Dermatology: Guidance on the use of biologic agents during COVID-19 outbreak.
3. Centers for Disease Control and Prevention. Actions to take in response to community transmission of COVID-19.
4. Centers for Disease Control and Prevention. Maintaining childhood immunizations during COVID19 pandemic.
5. Centers for Medicare & Medcaid Services. COVID-19 frequently asked questions (FAQs) on Medicare Fee-for-Service (FFS) billing.
6. Centers for Medicare & Medcaid Services. List of telehealth services.
Sacroiliac bone marrow edema on MRI may be common postpartum
A “strikingly high number” of women have bone marrow edema on MRI of the sacroiliac joints postpartum, according to a prospective study of 35 patients published in Annals of the Rheumatic Diseases. Postpartum sacroiliac bone marrow edema decreases over time. Its occurrence may be associated with a shorter duration of labor and a lack of epidural anesthesia, the researchers wrote.
Sacroiliac bone marrow edema in postpartum women “persists mainly in subjects older than 30 years,” noted Thomas Renson, MD, of Ghent (Belgium) University Hospital and colleagues. “When suspecting AxSpA [axial spondyloarthritis], our data indicate the need to wait at least 6 months to perform an MRI [of the sacroiliac joints] in postpartum women and, if positive, repeat the MRI after 12 months.”
Sacroiliac bone marrow edema is a hallmark of axSpA. However, recent studies of young, active people without the disease have found a high prevalence of bone marrow edema meeting the Assessment of SpondyloArthritis International Society (ASAS) definition of a positive MRI for sacroiliitis. Researchers have reported sacroiliac bone marrow edema in women during pregnancy and after childbirth, but prior studies have not assessed the trajectory of sacroiliac bone marrow edema after delivery, Dr. Renson and his colleagues said.
To study this question, the researchers recruited 35 subjects from the department of obstetrics at Ghent University Hospital. All participants were aged 18-45 years and had an uncomplicated, vaginal childbirth. The investigators excluded patients with a diagnosis of spondyloarthritis, inflammatory bowel disease, severe scoliosis, treatment with anti–tumor necrosis factor-alpha agents, any contraindication for MRI, childbirth through cesarean section, or pregnancy with more than one fetus.
Researchers performed a baseline MRI within 10 days of when patients gave birth and another MRI after 6 months. If the second MRI fulfilled the ASAS definition of a positive MRI for sacroiliitis, another MRI was performed at 12 months. Bone marrow edema was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) method.
In all, 77% of the patients had sacroiliac bone marrow edema on MRI an average of 5 days postpartum, and 60% fulfilled the ASAS definition of a positive MRI. After 6 months, 46% had bone marrow edema on MRI, and 15% had a positive MRI according to the ASAS definition. After 12 months, MRI was positive in 12% of the subjects. There was a high prevalence of bone marrow edema “even in subjects without back pain,” the researchers said.
“Four subjects would have fulfilled the ASAS classification criteria if there was a suspicion of axSpA: Three fulfilled the ASAS definition of a positive MRI for sacroiliitis and had inflammatory back pain, [and] one had chronic back pain, a positive MRI, and skin psoriasis,” the researchers wrote.
Misdiagnosis of axSpA based on MRI findings entails risks, the authors noted. NSAIDs may be less effective in patients who do not have axSpA, and patients may be “subsequently more likely to receive ineffective biological therapy, which has significant potential side effects and encompasses high socioeconomic costs,” the investigators said.
The study was supported by an ASAS research grant. The authors declared having no competing interests.
SOURCE: Renson T et al. Ann Rheum Dis. 2020 Apr 16. doi: 10.1136/annrheumdis-2020-217095.
A “strikingly high number” of women have bone marrow edema on MRI of the sacroiliac joints postpartum, according to a prospective study of 35 patients published in Annals of the Rheumatic Diseases. Postpartum sacroiliac bone marrow edema decreases over time. Its occurrence may be associated with a shorter duration of labor and a lack of epidural anesthesia, the researchers wrote.
Sacroiliac bone marrow edema in postpartum women “persists mainly in subjects older than 30 years,” noted Thomas Renson, MD, of Ghent (Belgium) University Hospital and colleagues. “When suspecting AxSpA [axial spondyloarthritis], our data indicate the need to wait at least 6 months to perform an MRI [of the sacroiliac joints] in postpartum women and, if positive, repeat the MRI after 12 months.”
Sacroiliac bone marrow edema is a hallmark of axSpA. However, recent studies of young, active people without the disease have found a high prevalence of bone marrow edema meeting the Assessment of SpondyloArthritis International Society (ASAS) definition of a positive MRI for sacroiliitis. Researchers have reported sacroiliac bone marrow edema in women during pregnancy and after childbirth, but prior studies have not assessed the trajectory of sacroiliac bone marrow edema after delivery, Dr. Renson and his colleagues said.
To study this question, the researchers recruited 35 subjects from the department of obstetrics at Ghent University Hospital. All participants were aged 18-45 years and had an uncomplicated, vaginal childbirth. The investigators excluded patients with a diagnosis of spondyloarthritis, inflammatory bowel disease, severe scoliosis, treatment with anti–tumor necrosis factor-alpha agents, any contraindication for MRI, childbirth through cesarean section, or pregnancy with more than one fetus.
Researchers performed a baseline MRI within 10 days of when patients gave birth and another MRI after 6 months. If the second MRI fulfilled the ASAS definition of a positive MRI for sacroiliitis, another MRI was performed at 12 months. Bone marrow edema was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) method.
In all, 77% of the patients had sacroiliac bone marrow edema on MRI an average of 5 days postpartum, and 60% fulfilled the ASAS definition of a positive MRI. After 6 months, 46% had bone marrow edema on MRI, and 15% had a positive MRI according to the ASAS definition. After 12 months, MRI was positive in 12% of the subjects. There was a high prevalence of bone marrow edema “even in subjects without back pain,” the researchers said.
“Four subjects would have fulfilled the ASAS classification criteria if there was a suspicion of axSpA: Three fulfilled the ASAS definition of a positive MRI for sacroiliitis and had inflammatory back pain, [and] one had chronic back pain, a positive MRI, and skin psoriasis,” the researchers wrote.
Misdiagnosis of axSpA based on MRI findings entails risks, the authors noted. NSAIDs may be less effective in patients who do not have axSpA, and patients may be “subsequently more likely to receive ineffective biological therapy, which has significant potential side effects and encompasses high socioeconomic costs,” the investigators said.
The study was supported by an ASAS research grant. The authors declared having no competing interests.
SOURCE: Renson T et al. Ann Rheum Dis. 2020 Apr 16. doi: 10.1136/annrheumdis-2020-217095.
A “strikingly high number” of women have bone marrow edema on MRI of the sacroiliac joints postpartum, according to a prospective study of 35 patients published in Annals of the Rheumatic Diseases. Postpartum sacroiliac bone marrow edema decreases over time. Its occurrence may be associated with a shorter duration of labor and a lack of epidural anesthesia, the researchers wrote.
Sacroiliac bone marrow edema in postpartum women “persists mainly in subjects older than 30 years,” noted Thomas Renson, MD, of Ghent (Belgium) University Hospital and colleagues. “When suspecting AxSpA [axial spondyloarthritis], our data indicate the need to wait at least 6 months to perform an MRI [of the sacroiliac joints] in postpartum women and, if positive, repeat the MRI after 12 months.”
Sacroiliac bone marrow edema is a hallmark of axSpA. However, recent studies of young, active people without the disease have found a high prevalence of bone marrow edema meeting the Assessment of SpondyloArthritis International Society (ASAS) definition of a positive MRI for sacroiliitis. Researchers have reported sacroiliac bone marrow edema in women during pregnancy and after childbirth, but prior studies have not assessed the trajectory of sacroiliac bone marrow edema after delivery, Dr. Renson and his colleagues said.
To study this question, the researchers recruited 35 subjects from the department of obstetrics at Ghent University Hospital. All participants were aged 18-45 years and had an uncomplicated, vaginal childbirth. The investigators excluded patients with a diagnosis of spondyloarthritis, inflammatory bowel disease, severe scoliosis, treatment with anti–tumor necrosis factor-alpha agents, any contraindication for MRI, childbirth through cesarean section, or pregnancy with more than one fetus.
Researchers performed a baseline MRI within 10 days of when patients gave birth and another MRI after 6 months. If the second MRI fulfilled the ASAS definition of a positive MRI for sacroiliitis, another MRI was performed at 12 months. Bone marrow edema was scored using the Spondyloarthritis Research Consortium of Canada (SPARCC) method.
In all, 77% of the patients had sacroiliac bone marrow edema on MRI an average of 5 days postpartum, and 60% fulfilled the ASAS definition of a positive MRI. After 6 months, 46% had bone marrow edema on MRI, and 15% had a positive MRI according to the ASAS definition. After 12 months, MRI was positive in 12% of the subjects. There was a high prevalence of bone marrow edema “even in subjects without back pain,” the researchers said.
“Four subjects would have fulfilled the ASAS classification criteria if there was a suspicion of axSpA: Three fulfilled the ASAS definition of a positive MRI for sacroiliitis and had inflammatory back pain, [and] one had chronic back pain, a positive MRI, and skin psoriasis,” the researchers wrote.
Misdiagnosis of axSpA based on MRI findings entails risks, the authors noted. NSAIDs may be less effective in patients who do not have axSpA, and patients may be “subsequently more likely to receive ineffective biological therapy, which has significant potential side effects and encompasses high socioeconomic costs,” the investigators said.
The study was supported by an ASAS research grant. The authors declared having no competing interests.
SOURCE: Renson T et al. Ann Rheum Dis. 2020 Apr 16. doi: 10.1136/annrheumdis-2020-217095.
FROM ANNALS OF THE RHEUMATIC DISEASES
Methotrexate adherence: It’s worse than you think
MAUI, HAWAII – Results of a carefully conducted real-world study of adherence to oral methotrexate in patients with RA were “kind of scary,” Arthur Kavanaugh, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“At 24 weeks, adherence was only 75%. And these were people who knew they were being monitored, so this is the best of the best. And yet less than 20% took the drug perfectly, meaning they took every dose as it was supposed to be,” noted Dr. Kavanaugh, professor of medicine at the University of California, San Diego, and RWCS program director.
“Adherence to methotrexate is really not very good. This is our cornerstone drug – methotrexate – and I think it certainly applies to other medications that we’re using,” he added.
He and his fellow panelist John J. Cush, MD, discussed the implications of this recent study, led by Kaleb Michaud, PhD, of the University of Nebraska Medical Center, Omaha.
The methotrexate adherence study included 60 patients with RA whose use of the disease-modifying antirheumatic drug (DMARD) over 24 weeks was monitored via the electronic Medication Event Monitoring System. These were motivated patients seen in routine clinical practice: They were participants in Forward, the National Databank for Rheumatic Diseases, and they understood that their use of methotrexate was being monitored.
Among the key findings: Patients on average took their weekly dose as directed for a total of 18 of the 24 weeks, although adherence decreased over time. Overall, 13% of participants missed 1 week, and 68% skipped 2 or more weeks. There was no significant difference in methotrexate adherence between biologic-naive and -experienced patients, nor between those on methotrexate monotherapy versus those on additional medication. Patient demographics and RA severity were similar between patients who missed taking their methotrexate for 2 weeks or more and those who missed fewer or no doses.
Higher Patient Global Assessment of Disease Activity scores were associated with correct dosing. So was being unemployed, having no prior conventional DMARD experience, and having less disability. A higher baseline score on the Beliefs about Medicines Questionnaire Specific-Necessity subscale, which indicates stronger belief in the necessity of the medication, were associated with greater likelihood of appropriate dosing, while lower scores were linked with more weeks of early dosing. However, the other elements of the Beliefs about Medicines Questionnaire weren’t significantly associated with adherence.
“This is a big problem. A lot of factors go into medication nonadherence. The solution has to begin with your relationship with the patient. If you want people to trust you, you’re going to have to work at that,” observed Dr. Cush, who is professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
Roy Fleischmann, MD, a rheumatologist and medical director of the Metroplex Clinical Research Center, Dallas, said that widespread suboptimal adherence to oral methotrexate has important implications for clinical trials. Often the placebo response rate in a randomized trial where the control group is on background methotrexate is so unexpectedly high that the potential efficacy of the active drug is masked; in such situations, it’s quite possible that patients who previously weren’t taking their methotrexate consistently start doing so when they join a closely supervised study, with a resultant inflated placebo response rate, he said.
One audience member who sees lots of medication adherence issues in his practice suggested that it might be time to become more aggressive in using intravenous infusion therapy instead of subcutaneously administered agents in patients with active RA and adherence problems.
“Maybe that’s why rituximab does so well in the clinical trials,” he said.
Dr. Cush and Dr. Kavanaugh reported receiving research funding from and/or serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Results of a carefully conducted real-world study of adherence to oral methotrexate in patients with RA were “kind of scary,” Arthur Kavanaugh, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“At 24 weeks, adherence was only 75%. And these were people who knew they were being monitored, so this is the best of the best. And yet less than 20% took the drug perfectly, meaning they took every dose as it was supposed to be,” noted Dr. Kavanaugh, professor of medicine at the University of California, San Diego, and RWCS program director.
“Adherence to methotrexate is really not very good. This is our cornerstone drug – methotrexate – and I think it certainly applies to other medications that we’re using,” he added.
He and his fellow panelist John J. Cush, MD, discussed the implications of this recent study, led by Kaleb Michaud, PhD, of the University of Nebraska Medical Center, Omaha.
The methotrexate adherence study included 60 patients with RA whose use of the disease-modifying antirheumatic drug (DMARD) over 24 weeks was monitored via the electronic Medication Event Monitoring System. These were motivated patients seen in routine clinical practice: They were participants in Forward, the National Databank for Rheumatic Diseases, and they understood that their use of methotrexate was being monitored.
Among the key findings: Patients on average took their weekly dose as directed for a total of 18 of the 24 weeks, although adherence decreased over time. Overall, 13% of participants missed 1 week, and 68% skipped 2 or more weeks. There was no significant difference in methotrexate adherence between biologic-naive and -experienced patients, nor between those on methotrexate monotherapy versus those on additional medication. Patient demographics and RA severity were similar between patients who missed taking their methotrexate for 2 weeks or more and those who missed fewer or no doses.
Higher Patient Global Assessment of Disease Activity scores were associated with correct dosing. So was being unemployed, having no prior conventional DMARD experience, and having less disability. A higher baseline score on the Beliefs about Medicines Questionnaire Specific-Necessity subscale, which indicates stronger belief in the necessity of the medication, were associated with greater likelihood of appropriate dosing, while lower scores were linked with more weeks of early dosing. However, the other elements of the Beliefs about Medicines Questionnaire weren’t significantly associated with adherence.
“This is a big problem. A lot of factors go into medication nonadherence. The solution has to begin with your relationship with the patient. If you want people to trust you, you’re going to have to work at that,” observed Dr. Cush, who is professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
Roy Fleischmann, MD, a rheumatologist and medical director of the Metroplex Clinical Research Center, Dallas, said that widespread suboptimal adherence to oral methotrexate has important implications for clinical trials. Often the placebo response rate in a randomized trial where the control group is on background methotrexate is so unexpectedly high that the potential efficacy of the active drug is masked; in such situations, it’s quite possible that patients who previously weren’t taking their methotrexate consistently start doing so when they join a closely supervised study, with a resultant inflated placebo response rate, he said.
One audience member who sees lots of medication adherence issues in his practice suggested that it might be time to become more aggressive in using intravenous infusion therapy instead of subcutaneously administered agents in patients with active RA and adherence problems.
“Maybe that’s why rituximab does so well in the clinical trials,” he said.
Dr. Cush and Dr. Kavanaugh reported receiving research funding from and/or serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Results of a carefully conducted real-world study of adherence to oral methotrexate in patients with RA were “kind of scary,” Arthur Kavanaugh, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
“At 24 weeks, adherence was only 75%. And these were people who knew they were being monitored, so this is the best of the best. And yet less than 20% took the drug perfectly, meaning they took every dose as it was supposed to be,” noted Dr. Kavanaugh, professor of medicine at the University of California, San Diego, and RWCS program director.
“Adherence to methotrexate is really not very good. This is our cornerstone drug – methotrexate – and I think it certainly applies to other medications that we’re using,” he added.
He and his fellow panelist John J. Cush, MD, discussed the implications of this recent study, led by Kaleb Michaud, PhD, of the University of Nebraska Medical Center, Omaha.
The methotrexate adherence study included 60 patients with RA whose use of the disease-modifying antirheumatic drug (DMARD) over 24 weeks was monitored via the electronic Medication Event Monitoring System. These were motivated patients seen in routine clinical practice: They were participants in Forward, the National Databank for Rheumatic Diseases, and they understood that their use of methotrexate was being monitored.
Among the key findings: Patients on average took their weekly dose as directed for a total of 18 of the 24 weeks, although adherence decreased over time. Overall, 13% of participants missed 1 week, and 68% skipped 2 or more weeks. There was no significant difference in methotrexate adherence between biologic-naive and -experienced patients, nor between those on methotrexate monotherapy versus those on additional medication. Patient demographics and RA severity were similar between patients who missed taking their methotrexate for 2 weeks or more and those who missed fewer or no doses.
Higher Patient Global Assessment of Disease Activity scores were associated with correct dosing. So was being unemployed, having no prior conventional DMARD experience, and having less disability. A higher baseline score on the Beliefs about Medicines Questionnaire Specific-Necessity subscale, which indicates stronger belief in the necessity of the medication, were associated with greater likelihood of appropriate dosing, while lower scores were linked with more weeks of early dosing. However, the other elements of the Beliefs about Medicines Questionnaire weren’t significantly associated with adherence.
“This is a big problem. A lot of factors go into medication nonadherence. The solution has to begin with your relationship with the patient. If you want people to trust you, you’re going to have to work at that,” observed Dr. Cush, who is professor of medicine and rheumatology at Baylor University Medical Center, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
Roy Fleischmann, MD, a rheumatologist and medical director of the Metroplex Clinical Research Center, Dallas, said that widespread suboptimal adherence to oral methotrexate has important implications for clinical trials. Often the placebo response rate in a randomized trial where the control group is on background methotrexate is so unexpectedly high that the potential efficacy of the active drug is masked; in such situations, it’s quite possible that patients who previously weren’t taking their methotrexate consistently start doing so when they join a closely supervised study, with a resultant inflated placebo response rate, he said.
One audience member who sees lots of medication adherence issues in his practice suggested that it might be time to become more aggressive in using intravenous infusion therapy instead of subcutaneously administered agents in patients with active RA and adherence problems.
“Maybe that’s why rituximab does so well in the clinical trials,” he said.
Dr. Cush and Dr. Kavanaugh reported receiving research funding from and/or serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2020
Latest data on COVID-19 patients with rheumatic diseases revealed in registry
An international registry of adult and pediatric rheumatology patients is beginning to identify trends in the types of patients with COVID-19 and who is recovering.
The COVID-19 Global Rheumatology Alliance (GRA) has created pediatric and adult registries for health care providers to enter information on their rheumatology patients with COVID-19. The adult registry is hosted by the University of California, San Francisco, Research Electronic Data Capture system, while the Childhood Arthritis and Rheumatology Research Alliance is supporting the pediatric registry. A separate path for data entry of both adult and pediatric cases has been established through the European League Against Rheumatism for European countries and countries with EULAR member organizations.
Prior to the creation of the registries, there were no data available to guide rheumatologists in clinical decision making for their patients, noted Jinoos Yazdany, MD, MPH, COVID-19 GRA steering committee member and chief of the division of rheumatology at Zuckerberg San Francisco General Hospital. “COVID-19 has potential to severely affect those with rheumatologic diseases or those taking immunosuppressive drugs,” she said in an interview. “The GRA registries were designed to answer critical questions that will inform the medical care of this population.”
The GRA began on Twitter, with conversations between Leonard H. Calabrese, DO, of the Cleveland Clinic; Paul Sufka, MD, of HealthPartners in St. Paul, Minn.; Philip Robinson, MBChB, PhD, of the Royal Brisbane (Australia) Hospital; and herself, Dr. Yazdany said. Dr. Robinson started work on the governance of the GRA, Dr. Yazdany designed the data infrastructure, and Dr. Sufka approached his professional networks and social media followings to promote the effort and ask for support. The COVID-19 GRA steering committee representatives include patients, private practice rheumatologists, and international investigators. Listed among official supporters of the alliance are the American College of Rheumatology and EULAR along with more than 290 medical societies, institutions, journals, and other organizations in rheumatology.
The goal of the registries is to examine the health outcomes of patients with rheumatic diseases and COVID-19 based on sociodemographic factors, comorbidities, and clinical presentations of COVID-19 as well as what role taking immunosuppressive drugs prior to a COVID-19 infection play in helping or hindering outcomes. Hydroxychloroquine, used to treat lupus and arthritis, is a potential treatment candidate for COVID-19. Biologics such as tocilizumab (Actemra) and sarilumab (Kevzara), which target interleukin-6, and anakinra (Kineret), which targets IL-1, are treatment candidates for patients who have experienced COVID-related cytokine storm syndrome, which researchers believe may contribute to worsening or fatal cases.
Dr. Yazdany, who is also vice chair of real-world data infrastructure, registry, and institutional review board/ethics for the GRA, said that there are some important high-level trends in the data thus far. “People with lupus and those taking hydroxychloroquine are becoming infected with SARS-CoV-2, which is counter to misinformation on social media. Most people with rheumatic diseases on immunosuppression are recovering, which is great news for our patients.”
One of the major strengths of the registries is that each case is entered by the rheumatologist treating the patient and contains detailed clinical information, Dr. Yazdany said. However, the registry has no control group, it is not a population surveillance study, and it may contain selection bias through rheumatologists omitting milder, undiagnosed cases.
“The Global Alliance case reporting registry represents the collective effort of hundreds of rheumatologists across the world. I have never been more inspired by the strength and collaboration of the rheumatology community,” Dr. Yazdany said.
According to a paper published in the Lancet Rheumatology, which references data on 110 cases from the combined databases up to April 1, about three-fourths of cases presented with fever (79%) and cough (77%), and about half presented with shortness of breath (50%) and myalgia (45%).
Results from the global and UCSF registries
As of April 18, 334 cases were in the global and UCSF registries, with 121 patients (36%) in the database having both COVID-19 and RA, 33 patients (10%) with psoriatic arthritis, 58 patients (17%) with systemic lupus erythematosus, 28 patients (8%) with axial spondyloarthritis, 27 patients (8%) with vasculitis, and 19 patients (6%) with Sjögren’s syndrome. There were less than five cases reported for patients with the following rheumatic diseases: inflammatory myopathy, ocular inflammation, other inflammatory arthritis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, osteoporosis, psoriasis, isolated pulmonary capillaritis, gout, and autoinflammatory disease. A majority of the patients in the registries are women (74%) aged younger than 65 years (78%) and are white (52%).
The most common comorbid conditions among patients in the registry are hypertension (33%), lung disease (18%), diabetes (11%), cardiovascular disease (10%), chronic renal insufficiency or end-stage renal disease (7%), morbid obesity (7%), and cancer (4%). Before being diagnosed with COVID-19, 219 patients (66%) in the registry were taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which included antimalarials, azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus. A total of 122 patients (37%) were taking biologic DMARDs, 101 patients were taking glucocorticoids (30%), 86 patients (26%) were taking hydroxychloroquine, 41 patients (12%) were taking NSAIDs, and 18 patients (5%) were taking a Janus kinase inhibitor.
The most recent data from the registry show that 128 patients (38%) have been hospitalized for COVID-19, and 19 patients (6%) have died. Although 104 patients (31%) resolved their infections, 177 patients (53%) have a COVID-19 infection status of “unresolved,” and 53 patients (16%) have an unknown infection status.
EULAR registry results
As of April 21, 249 cases were in the EULAR registry, including 110 hospitalizations (44%) and 37 deaths (15%). Overall, 64% of these patients were women, and they had a median age of 60 years.
The top five diagnoses of these patients were RA (39%), psoriatic arthritis (15%), spondyloarthritis (9%), systemic lupus erythematosus (9%), and gout (5%). A total of 27% had no reported comorbidities, while lung disease occurred in 26%, hypertension in 34%, diabetes in 11%, and cardiovascular disease on 11%. The registry also reported use of any DMARD in 80%, including 62% on csDMARDs, 31% on biologics, and 2% on targeted synthetic DMARDs.
Ten authors in the Lancet Rheumatology paper reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speaker’s bureau positions, personal fees, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. The other authors reported no relevant conflicts of interest.
An international registry of adult and pediatric rheumatology patients is beginning to identify trends in the types of patients with COVID-19 and who is recovering.
The COVID-19 Global Rheumatology Alliance (GRA) has created pediatric and adult registries for health care providers to enter information on their rheumatology patients with COVID-19. The adult registry is hosted by the University of California, San Francisco, Research Electronic Data Capture system, while the Childhood Arthritis and Rheumatology Research Alliance is supporting the pediatric registry. A separate path for data entry of both adult and pediatric cases has been established through the European League Against Rheumatism for European countries and countries with EULAR member organizations.
Prior to the creation of the registries, there were no data available to guide rheumatologists in clinical decision making for their patients, noted Jinoos Yazdany, MD, MPH, COVID-19 GRA steering committee member and chief of the division of rheumatology at Zuckerberg San Francisco General Hospital. “COVID-19 has potential to severely affect those with rheumatologic diseases or those taking immunosuppressive drugs,” she said in an interview. “The GRA registries were designed to answer critical questions that will inform the medical care of this population.”
The GRA began on Twitter, with conversations between Leonard H. Calabrese, DO, of the Cleveland Clinic; Paul Sufka, MD, of HealthPartners in St. Paul, Minn.; Philip Robinson, MBChB, PhD, of the Royal Brisbane (Australia) Hospital; and herself, Dr. Yazdany said. Dr. Robinson started work on the governance of the GRA, Dr. Yazdany designed the data infrastructure, and Dr. Sufka approached his professional networks and social media followings to promote the effort and ask for support. The COVID-19 GRA steering committee representatives include patients, private practice rheumatologists, and international investigators. Listed among official supporters of the alliance are the American College of Rheumatology and EULAR along with more than 290 medical societies, institutions, journals, and other organizations in rheumatology.
The goal of the registries is to examine the health outcomes of patients with rheumatic diseases and COVID-19 based on sociodemographic factors, comorbidities, and clinical presentations of COVID-19 as well as what role taking immunosuppressive drugs prior to a COVID-19 infection play in helping or hindering outcomes. Hydroxychloroquine, used to treat lupus and arthritis, is a potential treatment candidate for COVID-19. Biologics such as tocilizumab (Actemra) and sarilumab (Kevzara), which target interleukin-6, and anakinra (Kineret), which targets IL-1, are treatment candidates for patients who have experienced COVID-related cytokine storm syndrome, which researchers believe may contribute to worsening or fatal cases.
Dr. Yazdany, who is also vice chair of real-world data infrastructure, registry, and institutional review board/ethics for the GRA, said that there are some important high-level trends in the data thus far. “People with lupus and those taking hydroxychloroquine are becoming infected with SARS-CoV-2, which is counter to misinformation on social media. Most people with rheumatic diseases on immunosuppression are recovering, which is great news for our patients.”
One of the major strengths of the registries is that each case is entered by the rheumatologist treating the patient and contains detailed clinical information, Dr. Yazdany said. However, the registry has no control group, it is not a population surveillance study, and it may contain selection bias through rheumatologists omitting milder, undiagnosed cases.
“The Global Alliance case reporting registry represents the collective effort of hundreds of rheumatologists across the world. I have never been more inspired by the strength and collaboration of the rheumatology community,” Dr. Yazdany said.
According to a paper published in the Lancet Rheumatology, which references data on 110 cases from the combined databases up to April 1, about three-fourths of cases presented with fever (79%) and cough (77%), and about half presented with shortness of breath (50%) and myalgia (45%).
Results from the global and UCSF registries
As of April 18, 334 cases were in the global and UCSF registries, with 121 patients (36%) in the database having both COVID-19 and RA, 33 patients (10%) with psoriatic arthritis, 58 patients (17%) with systemic lupus erythematosus, 28 patients (8%) with axial spondyloarthritis, 27 patients (8%) with vasculitis, and 19 patients (6%) with Sjögren’s syndrome. There were less than five cases reported for patients with the following rheumatic diseases: inflammatory myopathy, ocular inflammation, other inflammatory arthritis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, osteoporosis, psoriasis, isolated pulmonary capillaritis, gout, and autoinflammatory disease. A majority of the patients in the registries are women (74%) aged younger than 65 years (78%) and are white (52%).
The most common comorbid conditions among patients in the registry are hypertension (33%), lung disease (18%), diabetes (11%), cardiovascular disease (10%), chronic renal insufficiency or end-stage renal disease (7%), morbid obesity (7%), and cancer (4%). Before being diagnosed with COVID-19, 219 patients (66%) in the registry were taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which included antimalarials, azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus. A total of 122 patients (37%) were taking biologic DMARDs, 101 patients were taking glucocorticoids (30%), 86 patients (26%) were taking hydroxychloroquine, 41 patients (12%) were taking NSAIDs, and 18 patients (5%) were taking a Janus kinase inhibitor.
The most recent data from the registry show that 128 patients (38%) have been hospitalized for COVID-19, and 19 patients (6%) have died. Although 104 patients (31%) resolved their infections, 177 patients (53%) have a COVID-19 infection status of “unresolved,” and 53 patients (16%) have an unknown infection status.
EULAR registry results
As of April 21, 249 cases were in the EULAR registry, including 110 hospitalizations (44%) and 37 deaths (15%). Overall, 64% of these patients were women, and they had a median age of 60 years.
The top five diagnoses of these patients were RA (39%), psoriatic arthritis (15%), spondyloarthritis (9%), systemic lupus erythematosus (9%), and gout (5%). A total of 27% had no reported comorbidities, while lung disease occurred in 26%, hypertension in 34%, diabetes in 11%, and cardiovascular disease on 11%. The registry also reported use of any DMARD in 80%, including 62% on csDMARDs, 31% on biologics, and 2% on targeted synthetic DMARDs.
Ten authors in the Lancet Rheumatology paper reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speaker’s bureau positions, personal fees, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. The other authors reported no relevant conflicts of interest.
An international registry of adult and pediatric rheumatology patients is beginning to identify trends in the types of patients with COVID-19 and who is recovering.
The COVID-19 Global Rheumatology Alliance (GRA) has created pediatric and adult registries for health care providers to enter information on their rheumatology patients with COVID-19. The adult registry is hosted by the University of California, San Francisco, Research Electronic Data Capture system, while the Childhood Arthritis and Rheumatology Research Alliance is supporting the pediatric registry. A separate path for data entry of both adult and pediatric cases has been established through the European League Against Rheumatism for European countries and countries with EULAR member organizations.
Prior to the creation of the registries, there were no data available to guide rheumatologists in clinical decision making for their patients, noted Jinoos Yazdany, MD, MPH, COVID-19 GRA steering committee member and chief of the division of rheumatology at Zuckerberg San Francisco General Hospital. “COVID-19 has potential to severely affect those with rheumatologic diseases or those taking immunosuppressive drugs,” she said in an interview. “The GRA registries were designed to answer critical questions that will inform the medical care of this population.”
The GRA began on Twitter, with conversations between Leonard H. Calabrese, DO, of the Cleveland Clinic; Paul Sufka, MD, of HealthPartners in St. Paul, Minn.; Philip Robinson, MBChB, PhD, of the Royal Brisbane (Australia) Hospital; and herself, Dr. Yazdany said. Dr. Robinson started work on the governance of the GRA, Dr. Yazdany designed the data infrastructure, and Dr. Sufka approached his professional networks and social media followings to promote the effort and ask for support. The COVID-19 GRA steering committee representatives include patients, private practice rheumatologists, and international investigators. Listed among official supporters of the alliance are the American College of Rheumatology and EULAR along with more than 290 medical societies, institutions, journals, and other organizations in rheumatology.
The goal of the registries is to examine the health outcomes of patients with rheumatic diseases and COVID-19 based on sociodemographic factors, comorbidities, and clinical presentations of COVID-19 as well as what role taking immunosuppressive drugs prior to a COVID-19 infection play in helping or hindering outcomes. Hydroxychloroquine, used to treat lupus and arthritis, is a potential treatment candidate for COVID-19. Biologics such as tocilizumab (Actemra) and sarilumab (Kevzara), which target interleukin-6, and anakinra (Kineret), which targets IL-1, are treatment candidates for patients who have experienced COVID-related cytokine storm syndrome, which researchers believe may contribute to worsening or fatal cases.
Dr. Yazdany, who is also vice chair of real-world data infrastructure, registry, and institutional review board/ethics for the GRA, said that there are some important high-level trends in the data thus far. “People with lupus and those taking hydroxychloroquine are becoming infected with SARS-CoV-2, which is counter to misinformation on social media. Most people with rheumatic diseases on immunosuppression are recovering, which is great news for our patients.”
One of the major strengths of the registries is that each case is entered by the rheumatologist treating the patient and contains detailed clinical information, Dr. Yazdany said. However, the registry has no control group, it is not a population surveillance study, and it may contain selection bias through rheumatologists omitting milder, undiagnosed cases.
“The Global Alliance case reporting registry represents the collective effort of hundreds of rheumatologists across the world. I have never been more inspired by the strength and collaboration of the rheumatology community,” Dr. Yazdany said.
According to a paper published in the Lancet Rheumatology, which references data on 110 cases from the combined databases up to April 1, about three-fourths of cases presented with fever (79%) and cough (77%), and about half presented with shortness of breath (50%) and myalgia (45%).
Results from the global and UCSF registries
As of April 18, 334 cases were in the global and UCSF registries, with 121 patients (36%) in the database having both COVID-19 and RA, 33 patients (10%) with psoriatic arthritis, 58 patients (17%) with systemic lupus erythematosus, 28 patients (8%) with axial spondyloarthritis, 27 patients (8%) with vasculitis, and 19 patients (6%) with Sjögren’s syndrome. There were less than five cases reported for patients with the following rheumatic diseases: inflammatory myopathy, ocular inflammation, other inflammatory arthritis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, osteoporosis, psoriasis, isolated pulmonary capillaritis, gout, and autoinflammatory disease. A majority of the patients in the registries are women (74%) aged younger than 65 years (78%) and are white (52%).
The most common comorbid conditions among patients in the registry are hypertension (33%), lung disease (18%), diabetes (11%), cardiovascular disease (10%), chronic renal insufficiency or end-stage renal disease (7%), morbid obesity (7%), and cancer (4%). Before being diagnosed with COVID-19, 219 patients (66%) in the registry were taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which included antimalarials, azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus. A total of 122 patients (37%) were taking biologic DMARDs, 101 patients were taking glucocorticoids (30%), 86 patients (26%) were taking hydroxychloroquine, 41 patients (12%) were taking NSAIDs, and 18 patients (5%) were taking a Janus kinase inhibitor.
The most recent data from the registry show that 128 patients (38%) have been hospitalized for COVID-19, and 19 patients (6%) have died. Although 104 patients (31%) resolved their infections, 177 patients (53%) have a COVID-19 infection status of “unresolved,” and 53 patients (16%) have an unknown infection status.
EULAR registry results
As of April 21, 249 cases were in the EULAR registry, including 110 hospitalizations (44%) and 37 deaths (15%). Overall, 64% of these patients were women, and they had a median age of 60 years.
The top five diagnoses of these patients were RA (39%), psoriatic arthritis (15%), spondyloarthritis (9%), systemic lupus erythematosus (9%), and gout (5%). A total of 27% had no reported comorbidities, while lung disease occurred in 26%, hypertension in 34%, diabetes in 11%, and cardiovascular disease on 11%. The registry also reported use of any DMARD in 80%, including 62% on csDMARDs, 31% on biologics, and 2% on targeted synthetic DMARDs.
Ten authors in the Lancet Rheumatology paper reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speaker’s bureau positions, personal fees, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. The other authors reported no relevant conflicts of interest.
Proximal fractures linked to higher mortality
Bone fracture in older adults is associated with greater mortality risk, but the location of the break may be a key factor, according to a new study of outcomes in a Danish database.
Over the follow-up period, those with proximal fractures – breaks in the hip, femur, pelvis, rib, clavicle, and humerus – were more likely to be hospitalized and to die, compared with their matched controls, than were those were with distal fractures in regions like the ankle, forearm, hand, or foot, where the mortality was similar to the matched controls.
“Compared with someone with similar comorbidities without a proximal fracture, there seemed to be an increased hospitalization rate for things like diabetes, heart disease, and lung disease, and then for some of those hospitalizations, there seemed to be an increased mortality, compared with people who hadn’t fractured who were hospitalized,” said Jacqueline Center, MBBS, PhD, of the Garvan Institute of Medical Research, Sydney, in an interview. The study abstract was released online by the Endocrine Society. It had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.*
The study included 212,498 women and 95,372 men aged over 50 years who had a fragility fracture between 2001 and 2014. The researchers excluded high-trauma fractures. They matched each fracture patient with four nonfracture patients, based on sex, age, and comorbidity status. There were 30,677 deaths among women over 384,995 person-years of follow-up, and 19,519 deaths in men over 163,482 person-years of follow-up. Women were a mean age of 72 at the time of fracture, while men were a mean age of 75.
The researchers found that proximal fractures were associated with increased risk of mortality, compared with nonfractured controls, with hazard ratios ranging between 1.5 and 4.0. Distal fractures were not associated with any increased mortality risk.
Comorbidities were common in the study population, with 75% of men and 60% of women having at least one. The risk of mortality increased with increasing numbers of comorbidities in each fracture type, but only proximal fractures were associated with an independent increase in mortality risk over and above comorbidity status.
In the 2 years following fracture, compared with matched controls, proximal fractures were associated with a greater risk of major hospital admission for conditions like cardiovascular disease, cancer, stroke, diabetes, pneumonia, and pulmonary disease. There was no significant difference between controls and those with distal fractures in hospital admission rate. The 2-year mortality risk was higher among subjects with proximal fractures, compared with patients in the no-fracture control group, regardless of whether they were admitted to the hospital, but there was no significant difference in those with distal fractures.
The differing clinical trajectories between those with proximal and distal fractures is a key finding, according to Dr. Center. The cause still isn’t clear, but she suspects that, in those patients who do badly, the fractures are either a signal that something is happening with existing comorbidities of the underlying frailty or that it may exacerbate them. Comorbidity independently and additively contributes to mortality, so that someone with a hip fracture and no comorbidities might have a similar mortality risk as someone with an upper-arm fracture and a couple of comorbidities. “I think it tells us that the person has to be treated as a whole. We need to treat the fracture to treat the underlying osteoporosis, but we also need to look closely at the person with the fracture and treat their comorbidities as well, because they seem to be more vulnerable,” Dr. Center said.
Although patients and clinicians are attuned to the concerns over hip fractures, other fractures should also be noted, according to Nelson Watts, MD, who is director of osteoporosis and bone-health services at Mercy Health in Cincinnati and was not involved in the research. “I think the message for clinicians and patients is that all of these [proximal] fractures need to be taken seriously. The good news is that that we have medications that can cut the risk of further fractures by 50%-70%,” he said in an interview.
Dr. Center has been on an advisory board for Amgen. Dr. Watts has been a speaker for Amgen and Radius and has conducted numerous clinical trials of osteoporosis drugs.
In addition to a series of news conferences, the Endocrine Society is also planning to host ENDO Online 2020 during June 8-22, which will feature on-demand and live programming for clinicians and researchers.
SOURCE: Center J et al. ENDO 2020, Abstract OR13-03.
Correction, 4/21/20: An earlier version of this article misstated when the interview with Dr. Center took place.
Bone fracture in older adults is associated with greater mortality risk, but the location of the break may be a key factor, according to a new study of outcomes in a Danish database.
Over the follow-up period, those with proximal fractures – breaks in the hip, femur, pelvis, rib, clavicle, and humerus – were more likely to be hospitalized and to die, compared with their matched controls, than were those were with distal fractures in regions like the ankle, forearm, hand, or foot, where the mortality was similar to the matched controls.
“Compared with someone with similar comorbidities without a proximal fracture, there seemed to be an increased hospitalization rate for things like diabetes, heart disease, and lung disease, and then for some of those hospitalizations, there seemed to be an increased mortality, compared with people who hadn’t fractured who were hospitalized,” said Jacqueline Center, MBBS, PhD, of the Garvan Institute of Medical Research, Sydney, in an interview. The study abstract was released online by the Endocrine Society. It had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.*
The study included 212,498 women and 95,372 men aged over 50 years who had a fragility fracture between 2001 and 2014. The researchers excluded high-trauma fractures. They matched each fracture patient with four nonfracture patients, based on sex, age, and comorbidity status. There were 30,677 deaths among women over 384,995 person-years of follow-up, and 19,519 deaths in men over 163,482 person-years of follow-up. Women were a mean age of 72 at the time of fracture, while men were a mean age of 75.
The researchers found that proximal fractures were associated with increased risk of mortality, compared with nonfractured controls, with hazard ratios ranging between 1.5 and 4.0. Distal fractures were not associated with any increased mortality risk.
Comorbidities were common in the study population, with 75% of men and 60% of women having at least one. The risk of mortality increased with increasing numbers of comorbidities in each fracture type, but only proximal fractures were associated with an independent increase in mortality risk over and above comorbidity status.
In the 2 years following fracture, compared with matched controls, proximal fractures were associated with a greater risk of major hospital admission for conditions like cardiovascular disease, cancer, stroke, diabetes, pneumonia, and pulmonary disease. There was no significant difference between controls and those with distal fractures in hospital admission rate. The 2-year mortality risk was higher among subjects with proximal fractures, compared with patients in the no-fracture control group, regardless of whether they were admitted to the hospital, but there was no significant difference in those with distal fractures.
The differing clinical trajectories between those with proximal and distal fractures is a key finding, according to Dr. Center. The cause still isn’t clear, but she suspects that, in those patients who do badly, the fractures are either a signal that something is happening with existing comorbidities of the underlying frailty or that it may exacerbate them. Comorbidity independently and additively contributes to mortality, so that someone with a hip fracture and no comorbidities might have a similar mortality risk as someone with an upper-arm fracture and a couple of comorbidities. “I think it tells us that the person has to be treated as a whole. We need to treat the fracture to treat the underlying osteoporosis, but we also need to look closely at the person with the fracture and treat their comorbidities as well, because they seem to be more vulnerable,” Dr. Center said.
Although patients and clinicians are attuned to the concerns over hip fractures, other fractures should also be noted, according to Nelson Watts, MD, who is director of osteoporosis and bone-health services at Mercy Health in Cincinnati and was not involved in the research. “I think the message for clinicians and patients is that all of these [proximal] fractures need to be taken seriously. The good news is that that we have medications that can cut the risk of further fractures by 50%-70%,” he said in an interview.
Dr. Center has been on an advisory board for Amgen. Dr. Watts has been a speaker for Amgen and Radius and has conducted numerous clinical trials of osteoporosis drugs.
In addition to a series of news conferences, the Endocrine Society is also planning to host ENDO Online 2020 during June 8-22, which will feature on-demand and live programming for clinicians and researchers.
SOURCE: Center J et al. ENDO 2020, Abstract OR13-03.
Correction, 4/21/20: An earlier version of this article misstated when the interview with Dr. Center took place.
Bone fracture in older adults is associated with greater mortality risk, but the location of the break may be a key factor, according to a new study of outcomes in a Danish database.
Over the follow-up period, those with proximal fractures – breaks in the hip, femur, pelvis, rib, clavicle, and humerus – were more likely to be hospitalized and to die, compared with their matched controls, than were those were with distal fractures in regions like the ankle, forearm, hand, or foot, where the mortality was similar to the matched controls.
“Compared with someone with similar comorbidities without a proximal fracture, there seemed to be an increased hospitalization rate for things like diabetes, heart disease, and lung disease, and then for some of those hospitalizations, there seemed to be an increased mortality, compared with people who hadn’t fractured who were hospitalized,” said Jacqueline Center, MBBS, PhD, of the Garvan Institute of Medical Research, Sydney, in an interview. The study abstract was released online by the Endocrine Society. It had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.*
The study included 212,498 women and 95,372 men aged over 50 years who had a fragility fracture between 2001 and 2014. The researchers excluded high-trauma fractures. They matched each fracture patient with four nonfracture patients, based on sex, age, and comorbidity status. There were 30,677 deaths among women over 384,995 person-years of follow-up, and 19,519 deaths in men over 163,482 person-years of follow-up. Women were a mean age of 72 at the time of fracture, while men were a mean age of 75.
The researchers found that proximal fractures were associated with increased risk of mortality, compared with nonfractured controls, with hazard ratios ranging between 1.5 and 4.0. Distal fractures were not associated with any increased mortality risk.
Comorbidities were common in the study population, with 75% of men and 60% of women having at least one. The risk of mortality increased with increasing numbers of comorbidities in each fracture type, but only proximal fractures were associated with an independent increase in mortality risk over and above comorbidity status.
In the 2 years following fracture, compared with matched controls, proximal fractures were associated with a greater risk of major hospital admission for conditions like cardiovascular disease, cancer, stroke, diabetes, pneumonia, and pulmonary disease. There was no significant difference between controls and those with distal fractures in hospital admission rate. The 2-year mortality risk was higher among subjects with proximal fractures, compared with patients in the no-fracture control group, regardless of whether they were admitted to the hospital, but there was no significant difference in those with distal fractures.
The differing clinical trajectories between those with proximal and distal fractures is a key finding, according to Dr. Center. The cause still isn’t clear, but she suspects that, in those patients who do badly, the fractures are either a signal that something is happening with existing comorbidities of the underlying frailty or that it may exacerbate them. Comorbidity independently and additively contributes to mortality, so that someone with a hip fracture and no comorbidities might have a similar mortality risk as someone with an upper-arm fracture and a couple of comorbidities. “I think it tells us that the person has to be treated as a whole. We need to treat the fracture to treat the underlying osteoporosis, but we also need to look closely at the person with the fracture and treat their comorbidities as well, because they seem to be more vulnerable,” Dr. Center said.
Although patients and clinicians are attuned to the concerns over hip fractures, other fractures should also be noted, according to Nelson Watts, MD, who is director of osteoporosis and bone-health services at Mercy Health in Cincinnati and was not involved in the research. “I think the message for clinicians and patients is that all of these [proximal] fractures need to be taken seriously. The good news is that that we have medications that can cut the risk of further fractures by 50%-70%,” he said in an interview.
Dr. Center has been on an advisory board for Amgen. Dr. Watts has been a speaker for Amgen and Radius and has conducted numerous clinical trials of osteoporosis drugs.
In addition to a series of news conferences, the Endocrine Society is also planning to host ENDO Online 2020 during June 8-22, which will feature on-demand and live programming for clinicians and researchers.
SOURCE: Center J et al. ENDO 2020, Abstract OR13-03.
Correction, 4/21/20: An earlier version of this article misstated when the interview with Dr. Center took place.
FROM ENDO 2020
U.S. prevalence of antinuclear antibodies has steadily risen, study finds
Between 1988 and 2012, the prevalence of antinuclear antibodies in the United States increased from 11% to 15.9%, especially among adolescents, males, and non-Hispanic whites.
The finding comes from a retrospective, cross-sectional analysis of serum samples from individuals who participated in the U.S. National Health and Nutrition Examination Survey over three time periods: 1988-1991, 1999-2004, and 2011-2012.
“Autoimmune diseases are a diverse group of disorders characterized by damaging immune responses to self-antigens and, for the most part, are of unknown etiology,” authors led by Gregg E. Dinse, ScD, wrote in a study published in Arthritis & Rheumatology. “They are thought to impact 3%-5% of the population, with rising rates noted several decades ago. Recent studies suggest continued increases for certain autoimmune diseases, but it is unclear whether these trends are due to changes in recognition and diagnosis, or are true temporal changes in incidence.”
Dr. Dinse, of the National Institute of Environmental Health Sciences in Research Triangle Park, N.C., and his colleagues evaluated sera samples of 14,211 survey participants aged 12 years and older at 1:80 dilution for antinuclear antibodies (ANA) using a standard indirect immunofluorescence assay (HEp-2 assay). The samples that received a grade of 3 or 4 on a 0-4 scale (compared with standard references, with values of 1-4 indicating positivity) underwent additional assessment by sequential ANA titers up to 1:1,280 dilution. To estimate changes in ANA prevalence over the time periods, they used logistic regression adjusted for age, sex, race/ethnicity, and survey design variables.
The researchers observed an ANA prevalence of 11% in 1988-1991, 11.5% in 1999-2004, and 15.9% in 2011-2012. This corresponds to 22, 27, and 41 million affected individuals, respectively. Females were more likely than males to have ANA (odds ratios of 2.53, 2.97, and 1.94 in 1988-1991, 1999-2004, and 2011-2012, respectively; P less than .0001), as were older adults relative to adolescents (ORs of 3.63, 1.80, and 1.71; P less than .002). Among adolescents, the prevalence of ANA rose steeply, with odds ratios of 2.02 in 1999-2004 and 2.88 in 2011-2012 in the second and third time periods relative to the first (trend P less than .0001). The researchers also found that, compared with non-Hispanic whites, the odds of having ANA were higher for non-Hispanic blacks (OR, 1.75) and Mexican-Americans (OR, 1.87) in 1988-1991, but racial/ethnic differences diminished in 1999-2004 and 2011-2012.
After adjustment for covariates, the researchers found that the estimated odds ratios for the second and third time periods relative to the first were 1.02 and 1.47, respectively, reflecting an overall ANA time trend (P less than .0001). Increases in ANA prevalence among cohorts did not correlate with contemporaneous trends in body mass index, smoking, or alcohol consumption.
Dr. Dinse and his colleagues acknowledged certain limitations of the study, including the fact that associations were based on cross-sectional data rather than repeated measures, and that some variables were self-reported, including the limited questionnaire data on autoimmune diseases.
In an interview, David S. Pisetsky, MD, professor of medicine/rheumatology and immunology at Duke University, Durham, N.C., characterized the study findings as “hypothesis generating” and said that he would like to know if the researchers would find the same results if they used a different ANA assay. “There’s a lot of variability from ANA kit to ANA kit – much greater than what was thought,” said Dr. Pisetsky, who is an authority on the topic. “One thing that needs to be done is to find out what the frequency is with other tests. One should recognize that the actual frequency is going to vary by the assay used. In another test format, the frequency may have been lower; it could have been higher.”
He added that the precise reasons why the prevalence of ANAs are rising in the general population remains elusive. “We know the target antigens in people with autoantibody-associated rheumatic disease,” Dr. Pisetsky said. “But what we don’t know a lot of times is, what are the target antigens in the otherwise healthy population? There has only been one antibody system that people have felt is associated with the otherwise healthy population. Those are called anti-DFS-70 antibodies, but there is even uncertainty about those. If you know what the antigens recognized were, then I think you could begin to speculate more about what’s going on in the population that’s increasing the frequency [of ANAs].”
In an accompanying editorial, Richard J. Bucala, MD, chief of rheumatology, allergy, and immunology at Yale University, New Haven, Conn., noted that the origins of autoantibodies in different rheumatic diseases and the steps that lead to disease progression remain elusive. “Modern societies experience an ever increasing variety of exposures due to travel and population migration, an increase in both the internationalization of agriculture and the industrialization of food production, a higher environmental burden of synthetic chemicals, emerging pathogens, and the inexorable effects of climate change,” Dr. Bucala wrote. “The speed and intensity of these influences is arguably unprecedented in human history and clearly outpace the possibility of protective genetic mechanisms to evolve and adapt.” He went on to note that the study’s findings “give impetus to multidisciplinary efforts aimed at preventative strategies, identifying environmental hazards, defining high-risk individuals, and preventing disease development in susceptible populations.”
The study was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. The authors reported having no disclosures.
SOURCE: Dinse G et al. Arthritis Rheumatol. 2020 April 7. doi: 10.1002/ART.41214.
Between 1988 and 2012, the prevalence of antinuclear antibodies in the United States increased from 11% to 15.9%, especially among adolescents, males, and non-Hispanic whites.
The finding comes from a retrospective, cross-sectional analysis of serum samples from individuals who participated in the U.S. National Health and Nutrition Examination Survey over three time periods: 1988-1991, 1999-2004, and 2011-2012.
“Autoimmune diseases are a diverse group of disorders characterized by damaging immune responses to self-antigens and, for the most part, are of unknown etiology,” authors led by Gregg E. Dinse, ScD, wrote in a study published in Arthritis & Rheumatology. “They are thought to impact 3%-5% of the population, with rising rates noted several decades ago. Recent studies suggest continued increases for certain autoimmune diseases, but it is unclear whether these trends are due to changes in recognition and diagnosis, or are true temporal changes in incidence.”
Dr. Dinse, of the National Institute of Environmental Health Sciences in Research Triangle Park, N.C., and his colleagues evaluated sera samples of 14,211 survey participants aged 12 years and older at 1:80 dilution for antinuclear antibodies (ANA) using a standard indirect immunofluorescence assay (HEp-2 assay). The samples that received a grade of 3 or 4 on a 0-4 scale (compared with standard references, with values of 1-4 indicating positivity) underwent additional assessment by sequential ANA titers up to 1:1,280 dilution. To estimate changes in ANA prevalence over the time periods, they used logistic regression adjusted for age, sex, race/ethnicity, and survey design variables.
The researchers observed an ANA prevalence of 11% in 1988-1991, 11.5% in 1999-2004, and 15.9% in 2011-2012. This corresponds to 22, 27, and 41 million affected individuals, respectively. Females were more likely than males to have ANA (odds ratios of 2.53, 2.97, and 1.94 in 1988-1991, 1999-2004, and 2011-2012, respectively; P less than .0001), as were older adults relative to adolescents (ORs of 3.63, 1.80, and 1.71; P less than .002). Among adolescents, the prevalence of ANA rose steeply, with odds ratios of 2.02 in 1999-2004 and 2.88 in 2011-2012 in the second and third time periods relative to the first (trend P less than .0001). The researchers also found that, compared with non-Hispanic whites, the odds of having ANA were higher for non-Hispanic blacks (OR, 1.75) and Mexican-Americans (OR, 1.87) in 1988-1991, but racial/ethnic differences diminished in 1999-2004 and 2011-2012.
After adjustment for covariates, the researchers found that the estimated odds ratios for the second and third time periods relative to the first were 1.02 and 1.47, respectively, reflecting an overall ANA time trend (P less than .0001). Increases in ANA prevalence among cohorts did not correlate with contemporaneous trends in body mass index, smoking, or alcohol consumption.
Dr. Dinse and his colleagues acknowledged certain limitations of the study, including the fact that associations were based on cross-sectional data rather than repeated measures, and that some variables were self-reported, including the limited questionnaire data on autoimmune diseases.
In an interview, David S. Pisetsky, MD, professor of medicine/rheumatology and immunology at Duke University, Durham, N.C., characterized the study findings as “hypothesis generating” and said that he would like to know if the researchers would find the same results if they used a different ANA assay. “There’s a lot of variability from ANA kit to ANA kit – much greater than what was thought,” said Dr. Pisetsky, who is an authority on the topic. “One thing that needs to be done is to find out what the frequency is with other tests. One should recognize that the actual frequency is going to vary by the assay used. In another test format, the frequency may have been lower; it could have been higher.”
He added that the precise reasons why the prevalence of ANAs are rising in the general population remains elusive. “We know the target antigens in people with autoantibody-associated rheumatic disease,” Dr. Pisetsky said. “But what we don’t know a lot of times is, what are the target antigens in the otherwise healthy population? There has only been one antibody system that people have felt is associated with the otherwise healthy population. Those are called anti-DFS-70 antibodies, but there is even uncertainty about those. If you know what the antigens recognized were, then I think you could begin to speculate more about what’s going on in the population that’s increasing the frequency [of ANAs].”
In an accompanying editorial, Richard J. Bucala, MD, chief of rheumatology, allergy, and immunology at Yale University, New Haven, Conn., noted that the origins of autoantibodies in different rheumatic diseases and the steps that lead to disease progression remain elusive. “Modern societies experience an ever increasing variety of exposures due to travel and population migration, an increase in both the internationalization of agriculture and the industrialization of food production, a higher environmental burden of synthetic chemicals, emerging pathogens, and the inexorable effects of climate change,” Dr. Bucala wrote. “The speed and intensity of these influences is arguably unprecedented in human history and clearly outpace the possibility of protective genetic mechanisms to evolve and adapt.” He went on to note that the study’s findings “give impetus to multidisciplinary efforts aimed at preventative strategies, identifying environmental hazards, defining high-risk individuals, and preventing disease development in susceptible populations.”
The study was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. The authors reported having no disclosures.
SOURCE: Dinse G et al. Arthritis Rheumatol. 2020 April 7. doi: 10.1002/ART.41214.
Between 1988 and 2012, the prevalence of antinuclear antibodies in the United States increased from 11% to 15.9%, especially among adolescents, males, and non-Hispanic whites.
The finding comes from a retrospective, cross-sectional analysis of serum samples from individuals who participated in the U.S. National Health and Nutrition Examination Survey over three time periods: 1988-1991, 1999-2004, and 2011-2012.
“Autoimmune diseases are a diverse group of disorders characterized by damaging immune responses to self-antigens and, for the most part, are of unknown etiology,” authors led by Gregg E. Dinse, ScD, wrote in a study published in Arthritis & Rheumatology. “They are thought to impact 3%-5% of the population, with rising rates noted several decades ago. Recent studies suggest continued increases for certain autoimmune diseases, but it is unclear whether these trends are due to changes in recognition and diagnosis, or are true temporal changes in incidence.”
Dr. Dinse, of the National Institute of Environmental Health Sciences in Research Triangle Park, N.C., and his colleagues evaluated sera samples of 14,211 survey participants aged 12 years and older at 1:80 dilution for antinuclear antibodies (ANA) using a standard indirect immunofluorescence assay (HEp-2 assay). The samples that received a grade of 3 or 4 on a 0-4 scale (compared with standard references, with values of 1-4 indicating positivity) underwent additional assessment by sequential ANA titers up to 1:1,280 dilution. To estimate changes in ANA prevalence over the time periods, they used logistic regression adjusted for age, sex, race/ethnicity, and survey design variables.
The researchers observed an ANA prevalence of 11% in 1988-1991, 11.5% in 1999-2004, and 15.9% in 2011-2012. This corresponds to 22, 27, and 41 million affected individuals, respectively. Females were more likely than males to have ANA (odds ratios of 2.53, 2.97, and 1.94 in 1988-1991, 1999-2004, and 2011-2012, respectively; P less than .0001), as were older adults relative to adolescents (ORs of 3.63, 1.80, and 1.71; P less than .002). Among adolescents, the prevalence of ANA rose steeply, with odds ratios of 2.02 in 1999-2004 and 2.88 in 2011-2012 in the second and third time periods relative to the first (trend P less than .0001). The researchers also found that, compared with non-Hispanic whites, the odds of having ANA were higher for non-Hispanic blacks (OR, 1.75) and Mexican-Americans (OR, 1.87) in 1988-1991, but racial/ethnic differences diminished in 1999-2004 and 2011-2012.
After adjustment for covariates, the researchers found that the estimated odds ratios for the second and third time periods relative to the first were 1.02 and 1.47, respectively, reflecting an overall ANA time trend (P less than .0001). Increases in ANA prevalence among cohorts did not correlate with contemporaneous trends in body mass index, smoking, or alcohol consumption.
Dr. Dinse and his colleagues acknowledged certain limitations of the study, including the fact that associations were based on cross-sectional data rather than repeated measures, and that some variables were self-reported, including the limited questionnaire data on autoimmune diseases.
In an interview, David S. Pisetsky, MD, professor of medicine/rheumatology and immunology at Duke University, Durham, N.C., characterized the study findings as “hypothesis generating” and said that he would like to know if the researchers would find the same results if they used a different ANA assay. “There’s a lot of variability from ANA kit to ANA kit – much greater than what was thought,” said Dr. Pisetsky, who is an authority on the topic. “One thing that needs to be done is to find out what the frequency is with other tests. One should recognize that the actual frequency is going to vary by the assay used. In another test format, the frequency may have been lower; it could have been higher.”
He added that the precise reasons why the prevalence of ANAs are rising in the general population remains elusive. “We know the target antigens in people with autoantibody-associated rheumatic disease,” Dr. Pisetsky said. “But what we don’t know a lot of times is, what are the target antigens in the otherwise healthy population? There has only been one antibody system that people have felt is associated with the otherwise healthy population. Those are called anti-DFS-70 antibodies, but there is even uncertainty about those. If you know what the antigens recognized were, then I think you could begin to speculate more about what’s going on in the population that’s increasing the frequency [of ANAs].”
In an accompanying editorial, Richard J. Bucala, MD, chief of rheumatology, allergy, and immunology at Yale University, New Haven, Conn., noted that the origins of autoantibodies in different rheumatic diseases and the steps that lead to disease progression remain elusive. “Modern societies experience an ever increasing variety of exposures due to travel and population migration, an increase in both the internationalization of agriculture and the industrialization of food production, a higher environmental burden of synthetic chemicals, emerging pathogens, and the inexorable effects of climate change,” Dr. Bucala wrote. “The speed and intensity of these influences is arguably unprecedented in human history and clearly outpace the possibility of protective genetic mechanisms to evolve and adapt.” He went on to note that the study’s findings “give impetus to multidisciplinary efforts aimed at preventative strategies, identifying environmental hazards, defining high-risk individuals, and preventing disease development in susceptible populations.”
The study was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. The authors reported having no disclosures.
SOURCE: Dinse G et al. Arthritis Rheumatol. 2020 April 7. doi: 10.1002/ART.41214.
FROM ARTHRITIS & RHEUMATOLOGY
Financial incentives affect the adoption of biosimilars
during the same time period in 2015-2019, according to an analysis published in Arthritis and Rheumatology.
The use of the biosimilars also was associated with cost savings at the VAMC, but not at the academic medical center, which illustrates that insufficient financial incentives can delay the adoption of biosimilars and the health care system’s realization of cost savings, according to the authors.
Medicare, which is not allowed to negotiate drug prices, is one of the largest payers for infused therapies. Medicare reimbursement for infused therapies is based on the latter’s average selling price (ASP) during the previous quarter. Institutions may negotiate purchase prices with drug manufacturers and receive Medicare reimbursement. Biosimilars generally have lower ASPs than their corresponding reference therapies, and biosimilar manufacturers may have less room to negotiate prices than reference therapy manufacturers. Consequently, a given institution might have a greater incentive to use reference products than to use biosimilars.
An examination of pharmacy data
The VA negotiates drug prices for all of its medical centers and has mandated that clinicians prefer biosimilars to their corresponding reference therapies, so Joshua F. Baker, MD, of the University of Pennsylvania and the Corporal Michael J. Crescenz VAMC, both in Philadelphia, and his colleagues hypothesized that the adoption of biosimilars had proceeded more quickly at a VAMC than at a nearby academic medical center.
The investigators examined pharmacy data from the University of Pennsylvania Health System (UPHS) electronic medical record and the Corporal Michael J. Crescenz VAMC to compare the frequency of prescribing biosimilars at these sites between Jan. 1, 2015, and May 31, 2019. Dr. Baker and his associates focused specifically on reference infliximab (Remicade) and the reference noninfusion therapies filgrastim (Neupogen) and pegfilgrastim (Neulasta) and on biosimilars of these therapies (infliximab-dyyb [Inflectra], infliximab-abda [Renflexis], filgrastim-sndz [Zarxio], and pegfilgrastim-jmdb [Fulphila]).
Because Medicare was the predominant payer, the researchers estimated reimbursement for reference and biosimilar infliximabs according to the Medicare Part B reimbursement policy. They defined an institution’s incentive to use a given therapy as the difference between the reimbursement and acquisition cost for that therapy. Dr. Baker and colleagues compared the incentives for UPHS with those for the VAMC.
VAMC saved 81% of reference product cost
The researchers identified 15,761 infusions of infliximab at UPHS and 446 at the VAMC during the study period. The proportion of infusions that used the reference product was 99% at UPHS and 62% at the VAMC. ASPs for biosimilar infliximab have been consistently lower than those for the reference product since July 2017. In December 2017, the VAMC switched to the biosimilar infliximab.
Institutional incentives based on Medicare Part B reimbursement and acquisitions costs for reference and biosimilar infliximab have been similar since 2018. In 2019, the institutional incentive favored the reference product by $49-$64 per 100-mg vial. But at the VAMC, the cost per 100-mg vial was $623.48 for the reference product and $115.58 for the biosimilar Renflexis. Purchasing the biosimilar thus yielded a savings of 81%. The current costs for the therapies are $546 and $116, respectively.
In addition, Dr. Baker and colleagues identified 46,683 orders for filgrastim or pegfilgrastim at UPHS. Approximately 90% of the orders were for either of the two reference products despite the ASP of biosimilar filgrastim being approximately 40% lower than that of its reference product. At the VAMC, about 88% of orders were for the reference products. Biosimilars became available in 2016. UPHS began using them at a modest rate, but their adoption was greater at the VAMC, which designated them as preferred products.
Tendering and a nationwide policy mandating use of biosimilars have resulted in financial savings for the VAMC, wrote Dr. Baker and colleagues. “These data suggest that, with current Medicare Part B reimbursement policy, the absence of financial incentives to encourage use of infliximab biosimilars has resulted in slower uptake of biosimilar use at institutions outside of the VA system. The implications of this are a slower reduction in costs to the health care system, since decreases in ASP over time are predicated on negotiations at the institutional level, which have been gradual and stepwise. ...
“Although some of our results may not be applicable to other geographical regions of the U.S., the comparison of two affiliated institutions in geographical proximity and with shared health care providers is a strength,” they continued. “Our findings should be replicated using national VAMC data or data from other health care systems.”
The researchers said that their findings may not apply to noninfused therapies, which are not covered under Medicare Part B, and they did not directly study the impact of pharmacy benefit managers. However, they noted that their data on filgrastim and pegfilgrastim support the hypothesis that pharmacy benefit managers receive “incentives that continue to promote the use of reference products that have higher manufacturer’s list prices, which likely will limit the uptake of both infused and injectable biosimilar therapies over time.” They said that “this finding has important implications for when noninfused biosimilars (e.g. etanercept and adalimumab) are eventually introduced to the U.S. market.”
European governments incentivize use of biosimilars
Government and institutional incentives have increased the adoption of biosimilars in Europe, wrote Guro Lovik Goll, MD, and Tore Kristian Kvien, MD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, in an accompanying editorial. Norway and Denmark have annual national tender systems in which biosimilars and reference products compete. The price of infliximab biosimilar was 39% lower than the reference product in 2014 and 69% lower in 2015. “Competition has caused dramatically lower prices both for biosimilars and also for the originator drugs competing with them,” wrote the authors.
In 2015, the government of Denmark mandated that patients on infliximab be switched to a biosimilar, and patients in Norway also have been switched to biosimilars. The use of etanercept in Norway increased by 40% from 2016 to 2019, and the use of infliximab has increased by more than threefold since 2015. “In Norway, the consequence of competition, national tenders, and availability of biosimilars have led to better access to therapy for more people in need of biologic drugs, while at the same time showing a total cost reduction of biologics for use in rheumatology, gastroenterology, and dermatology,” wrote the authors.
Health care costs $10,000 per capita in the United States, compared with $5,300 for other wealthy countries in the Organization for Economic Cooperation and Development. Low life expectancy and high infant mortality in the U.S. indicate that high costs are not associated with better outcomes. “As Americans seem to lose out on the cost-cutting potential of biosimilars, this missed opportunity is set to get even more expensive,” the authors concluded.
The U.S. Department of Veterans Affairs, the National Institutes of Health, and the American Diabetes Association contributed funding for the study. Dr. Baker reported receiving consulting fees from Bristol-Myers Squibb and Gilead, and another author reported receiving research support paid to his institution by Pfizer and UCB, as well as receiving consulting fees from nine pharmaceutical companies. Dr. Goll and Dr. Kvien both reported receiving fees for speaking and/or consulting from numerous pharmaceutical companies, including Pfizer.
SOURCES: Baker JF et al. Arthritis Rheumatol. 2020 Apr 6. doi: 10.1002/art.41277.
during the same time period in 2015-2019, according to an analysis published in Arthritis and Rheumatology.
The use of the biosimilars also was associated with cost savings at the VAMC, but not at the academic medical center, which illustrates that insufficient financial incentives can delay the adoption of biosimilars and the health care system’s realization of cost savings, according to the authors.
Medicare, which is not allowed to negotiate drug prices, is one of the largest payers for infused therapies. Medicare reimbursement for infused therapies is based on the latter’s average selling price (ASP) during the previous quarter. Institutions may negotiate purchase prices with drug manufacturers and receive Medicare reimbursement. Biosimilars generally have lower ASPs than their corresponding reference therapies, and biosimilar manufacturers may have less room to negotiate prices than reference therapy manufacturers. Consequently, a given institution might have a greater incentive to use reference products than to use biosimilars.
An examination of pharmacy data
The VA negotiates drug prices for all of its medical centers and has mandated that clinicians prefer biosimilars to their corresponding reference therapies, so Joshua F. Baker, MD, of the University of Pennsylvania and the Corporal Michael J. Crescenz VAMC, both in Philadelphia, and his colleagues hypothesized that the adoption of biosimilars had proceeded more quickly at a VAMC than at a nearby academic medical center.
The investigators examined pharmacy data from the University of Pennsylvania Health System (UPHS) electronic medical record and the Corporal Michael J. Crescenz VAMC to compare the frequency of prescribing biosimilars at these sites between Jan. 1, 2015, and May 31, 2019. Dr. Baker and his associates focused specifically on reference infliximab (Remicade) and the reference noninfusion therapies filgrastim (Neupogen) and pegfilgrastim (Neulasta) and on biosimilars of these therapies (infliximab-dyyb [Inflectra], infliximab-abda [Renflexis], filgrastim-sndz [Zarxio], and pegfilgrastim-jmdb [Fulphila]).
Because Medicare was the predominant payer, the researchers estimated reimbursement for reference and biosimilar infliximabs according to the Medicare Part B reimbursement policy. They defined an institution’s incentive to use a given therapy as the difference between the reimbursement and acquisition cost for that therapy. Dr. Baker and colleagues compared the incentives for UPHS with those for the VAMC.
VAMC saved 81% of reference product cost
The researchers identified 15,761 infusions of infliximab at UPHS and 446 at the VAMC during the study period. The proportion of infusions that used the reference product was 99% at UPHS and 62% at the VAMC. ASPs for biosimilar infliximab have been consistently lower than those for the reference product since July 2017. In December 2017, the VAMC switched to the biosimilar infliximab.
Institutional incentives based on Medicare Part B reimbursement and acquisitions costs for reference and biosimilar infliximab have been similar since 2018. In 2019, the institutional incentive favored the reference product by $49-$64 per 100-mg vial. But at the VAMC, the cost per 100-mg vial was $623.48 for the reference product and $115.58 for the biosimilar Renflexis. Purchasing the biosimilar thus yielded a savings of 81%. The current costs for the therapies are $546 and $116, respectively.
In addition, Dr. Baker and colleagues identified 46,683 orders for filgrastim or pegfilgrastim at UPHS. Approximately 90% of the orders were for either of the two reference products despite the ASP of biosimilar filgrastim being approximately 40% lower than that of its reference product. At the VAMC, about 88% of orders were for the reference products. Biosimilars became available in 2016. UPHS began using them at a modest rate, but their adoption was greater at the VAMC, which designated them as preferred products.
Tendering and a nationwide policy mandating use of biosimilars have resulted in financial savings for the VAMC, wrote Dr. Baker and colleagues. “These data suggest that, with current Medicare Part B reimbursement policy, the absence of financial incentives to encourage use of infliximab biosimilars has resulted in slower uptake of biosimilar use at institutions outside of the VA system. The implications of this are a slower reduction in costs to the health care system, since decreases in ASP over time are predicated on negotiations at the institutional level, which have been gradual and stepwise. ...
“Although some of our results may not be applicable to other geographical regions of the U.S., the comparison of two affiliated institutions in geographical proximity and with shared health care providers is a strength,” they continued. “Our findings should be replicated using national VAMC data or data from other health care systems.”
The researchers said that their findings may not apply to noninfused therapies, which are not covered under Medicare Part B, and they did not directly study the impact of pharmacy benefit managers. However, they noted that their data on filgrastim and pegfilgrastim support the hypothesis that pharmacy benefit managers receive “incentives that continue to promote the use of reference products that have higher manufacturer’s list prices, which likely will limit the uptake of both infused and injectable biosimilar therapies over time.” They said that “this finding has important implications for when noninfused biosimilars (e.g. etanercept and adalimumab) are eventually introduced to the U.S. market.”
European governments incentivize use of biosimilars
Government and institutional incentives have increased the adoption of biosimilars in Europe, wrote Guro Lovik Goll, MD, and Tore Kristian Kvien, MD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, in an accompanying editorial. Norway and Denmark have annual national tender systems in which biosimilars and reference products compete. The price of infliximab biosimilar was 39% lower than the reference product in 2014 and 69% lower in 2015. “Competition has caused dramatically lower prices both for biosimilars and also for the originator drugs competing with them,” wrote the authors.
In 2015, the government of Denmark mandated that patients on infliximab be switched to a biosimilar, and patients in Norway also have been switched to biosimilars. The use of etanercept in Norway increased by 40% from 2016 to 2019, and the use of infliximab has increased by more than threefold since 2015. “In Norway, the consequence of competition, national tenders, and availability of biosimilars have led to better access to therapy for more people in need of biologic drugs, while at the same time showing a total cost reduction of biologics for use in rheumatology, gastroenterology, and dermatology,” wrote the authors.
Health care costs $10,000 per capita in the United States, compared with $5,300 for other wealthy countries in the Organization for Economic Cooperation and Development. Low life expectancy and high infant mortality in the U.S. indicate that high costs are not associated with better outcomes. “As Americans seem to lose out on the cost-cutting potential of biosimilars, this missed opportunity is set to get even more expensive,” the authors concluded.
The U.S. Department of Veterans Affairs, the National Institutes of Health, and the American Diabetes Association contributed funding for the study. Dr. Baker reported receiving consulting fees from Bristol-Myers Squibb and Gilead, and another author reported receiving research support paid to his institution by Pfizer and UCB, as well as receiving consulting fees from nine pharmaceutical companies. Dr. Goll and Dr. Kvien both reported receiving fees for speaking and/or consulting from numerous pharmaceutical companies, including Pfizer.
SOURCES: Baker JF et al. Arthritis Rheumatol. 2020 Apr 6. doi: 10.1002/art.41277.
during the same time period in 2015-2019, according to an analysis published in Arthritis and Rheumatology.
The use of the biosimilars also was associated with cost savings at the VAMC, but not at the academic medical center, which illustrates that insufficient financial incentives can delay the adoption of biosimilars and the health care system’s realization of cost savings, according to the authors.
Medicare, which is not allowed to negotiate drug prices, is one of the largest payers for infused therapies. Medicare reimbursement for infused therapies is based on the latter’s average selling price (ASP) during the previous quarter. Institutions may negotiate purchase prices with drug manufacturers and receive Medicare reimbursement. Biosimilars generally have lower ASPs than their corresponding reference therapies, and biosimilar manufacturers may have less room to negotiate prices than reference therapy manufacturers. Consequently, a given institution might have a greater incentive to use reference products than to use biosimilars.
An examination of pharmacy data
The VA negotiates drug prices for all of its medical centers and has mandated that clinicians prefer biosimilars to their corresponding reference therapies, so Joshua F. Baker, MD, of the University of Pennsylvania and the Corporal Michael J. Crescenz VAMC, both in Philadelphia, and his colleagues hypothesized that the adoption of biosimilars had proceeded more quickly at a VAMC than at a nearby academic medical center.
The investigators examined pharmacy data from the University of Pennsylvania Health System (UPHS) electronic medical record and the Corporal Michael J. Crescenz VAMC to compare the frequency of prescribing biosimilars at these sites between Jan. 1, 2015, and May 31, 2019. Dr. Baker and his associates focused specifically on reference infliximab (Remicade) and the reference noninfusion therapies filgrastim (Neupogen) and pegfilgrastim (Neulasta) and on biosimilars of these therapies (infliximab-dyyb [Inflectra], infliximab-abda [Renflexis], filgrastim-sndz [Zarxio], and pegfilgrastim-jmdb [Fulphila]).
Because Medicare was the predominant payer, the researchers estimated reimbursement for reference and biosimilar infliximabs according to the Medicare Part B reimbursement policy. They defined an institution’s incentive to use a given therapy as the difference between the reimbursement and acquisition cost for that therapy. Dr. Baker and colleagues compared the incentives for UPHS with those for the VAMC.
VAMC saved 81% of reference product cost
The researchers identified 15,761 infusions of infliximab at UPHS and 446 at the VAMC during the study period. The proportion of infusions that used the reference product was 99% at UPHS and 62% at the VAMC. ASPs for biosimilar infliximab have been consistently lower than those for the reference product since July 2017. In December 2017, the VAMC switched to the biosimilar infliximab.
Institutional incentives based on Medicare Part B reimbursement and acquisitions costs for reference and biosimilar infliximab have been similar since 2018. In 2019, the institutional incentive favored the reference product by $49-$64 per 100-mg vial. But at the VAMC, the cost per 100-mg vial was $623.48 for the reference product and $115.58 for the biosimilar Renflexis. Purchasing the biosimilar thus yielded a savings of 81%. The current costs for the therapies are $546 and $116, respectively.
In addition, Dr. Baker and colleagues identified 46,683 orders for filgrastim or pegfilgrastim at UPHS. Approximately 90% of the orders were for either of the two reference products despite the ASP of biosimilar filgrastim being approximately 40% lower than that of its reference product. At the VAMC, about 88% of orders were for the reference products. Biosimilars became available in 2016. UPHS began using them at a modest rate, but their adoption was greater at the VAMC, which designated them as preferred products.
Tendering and a nationwide policy mandating use of biosimilars have resulted in financial savings for the VAMC, wrote Dr. Baker and colleagues. “These data suggest that, with current Medicare Part B reimbursement policy, the absence of financial incentives to encourage use of infliximab biosimilars has resulted in slower uptake of biosimilar use at institutions outside of the VA system. The implications of this are a slower reduction in costs to the health care system, since decreases in ASP over time are predicated on negotiations at the institutional level, which have been gradual and stepwise. ...
“Although some of our results may not be applicable to other geographical regions of the U.S., the comparison of two affiliated institutions in geographical proximity and with shared health care providers is a strength,” they continued. “Our findings should be replicated using national VAMC data or data from other health care systems.”
The researchers said that their findings may not apply to noninfused therapies, which are not covered under Medicare Part B, and they did not directly study the impact of pharmacy benefit managers. However, they noted that their data on filgrastim and pegfilgrastim support the hypothesis that pharmacy benefit managers receive “incentives that continue to promote the use of reference products that have higher manufacturer’s list prices, which likely will limit the uptake of both infused and injectable biosimilar therapies over time.” They said that “this finding has important implications for when noninfused biosimilars (e.g. etanercept and adalimumab) are eventually introduced to the U.S. market.”
European governments incentivize use of biosimilars
Government and institutional incentives have increased the adoption of biosimilars in Europe, wrote Guro Lovik Goll, MD, and Tore Kristian Kvien, MD, of the department of rheumatology at Diakonhjemmet Hospital in Oslo, in an accompanying editorial. Norway and Denmark have annual national tender systems in which biosimilars and reference products compete. The price of infliximab biosimilar was 39% lower than the reference product in 2014 and 69% lower in 2015. “Competition has caused dramatically lower prices both for biosimilars and also for the originator drugs competing with them,” wrote the authors.
In 2015, the government of Denmark mandated that patients on infliximab be switched to a biosimilar, and patients in Norway also have been switched to biosimilars. The use of etanercept in Norway increased by 40% from 2016 to 2019, and the use of infliximab has increased by more than threefold since 2015. “In Norway, the consequence of competition, national tenders, and availability of biosimilars have led to better access to therapy for more people in need of biologic drugs, while at the same time showing a total cost reduction of biologics for use in rheumatology, gastroenterology, and dermatology,” wrote the authors.
Health care costs $10,000 per capita in the United States, compared with $5,300 for other wealthy countries in the Organization for Economic Cooperation and Development. Low life expectancy and high infant mortality in the U.S. indicate that high costs are not associated with better outcomes. “As Americans seem to lose out on the cost-cutting potential of biosimilars, this missed opportunity is set to get even more expensive,” the authors concluded.
The U.S. Department of Veterans Affairs, the National Institutes of Health, and the American Diabetes Association contributed funding for the study. Dr. Baker reported receiving consulting fees from Bristol-Myers Squibb and Gilead, and another author reported receiving research support paid to his institution by Pfizer and UCB, as well as receiving consulting fees from nine pharmaceutical companies. Dr. Goll and Dr. Kvien both reported receiving fees for speaking and/or consulting from numerous pharmaceutical companies, including Pfizer.
SOURCES: Baker JF et al. Arthritis Rheumatol. 2020 Apr 6. doi: 10.1002/art.41277.
FROM ARTHRITIS & RHEUMATOLOGY
Most e-consults not followed by specialist visit
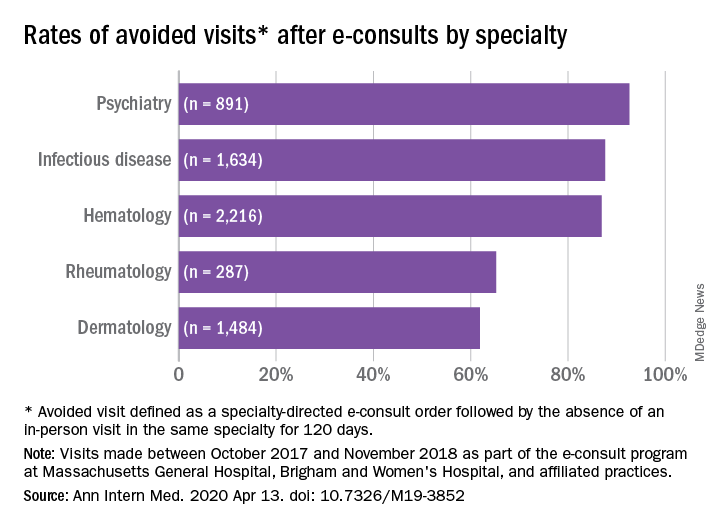
Studies have shown that e-consults increase access to specialist care and primary care physician (PCP) education, according to research published in the Annals of Internal Medicine (2020. Apr 14. doi: 10.7326/M19-3852) by Salman Ahmed, MD, and colleagues.
These resources are already being frequently used by physicians, but more often by general internists and hospitalists than by subspecialists, according to a recent survey by the American College of Physicians. That survey found that 42% of its respondents are using e-consults and that subspecialists’ use is less common primarily because of the lack of access to e-consult technology.
What hasn’t been widely researched are the effects of large-scale e-consult programs, said Dr. Ahmed, who is associate physician in the renal division at Brigham and Women’s Hospital, Boston, in an interview.
For frontline providers such as PCPs, e-consults are a way to quickly seek out answers to clinical questions from specialists. In turn, the specialist can help a wider pool of participants, he noted.
The findings of Dr. Ahmed’s study, which included several academic centers and hospitals affiliated with Partners HealthCare System, a nonprofit network in eastern Massachusetts that includes Brigham and Women’s Hospital, used several metrics to analyze the appropriateness and utility of e-consults across a range of specialties. An e-consult was considered useful if it resulted in the avoidance of a visit to a specialist, which was defined as the absence of an in-person visit to the type of specialist consulted electronically for 120 days. An e-consult was considered appropriate if it met the following four criteria.
- It could not be answered by referring to society guidelines or widely available, evidence-based summary sources.
- It did not seek logistic information, such as where to have a specific laboratory test done.
- It did not include a question of high urgency.
- The medical complexity of the clinical situation was not substantial enough to warrant an in-person consultation.
The investigators examined e-consult inquiries to mostly physician health care providers in five specialties – hematology, infectious disease, dermatology, rheumatology, and psychiatry – over a year.
High rates of appropriateness
The search spanned 6,512 eligible e-consults from 1,096 referring providers to 121 specialist consultants. Narrowing their search to 741 records with complete data, the investigators found that 70.2% of these consults met the criteria for appropriateness. In an analysis of four reviewers blinded to each other’s results, raters agreed on the appropriateness of 94% of e-consults.
Across specialties, more than 81% of e-consults were associated with avoided in-person visits.
The reasons for most e-consults were to seek answers to questions about diagnosis, therapeutics, or patient inquiries, or to request further education by PCPs.
“Across all specialties, the most common reasons an e-consult was not considered appropriate were failing the point-of-care resource test and asking a question of inappropriately high complexity,” the authors summarized.
Physicians and PCPs from tertiary care practices made up the majority of referring providers, with turnaround time for consults averaging 24 hours across specialties.
Rates of appropriateness, content, patient demographics, and timeliness of e-consult responses varied among the four specialties. Those with high avoidance of visits rates tended to have high appropriateness rates, indicating that some specialties may be more conducive to e-consults than others, the authors noted. Psychiatry and hematology had the highest proportion of appropriate e-consults (77.9% and 73.3% respectively). Rheumatology had the lowest proportion of appropriate e-consults and one of the lowest rates of avoided in-person visits, and dermatology had the lowest rate of avoided in-person visits, at 61.9%.
The majority (93%) of e-consults sought in psychiatry were therapy related, whereas 88.4% of the e-consult questions in rheumatology related to diagnosis.
“Questions about diagnosis were less likely to be answerable via e-consult, which suggests that to provide diagnoses, consultants may wish to engage with the patient directly,” Dr. Ahmed said in an interview.
Infectious disease specialists seemed to be the fastest responders, with nearly 90% of their consultations having been answered within a day. Dermatology specialists had the distinction of having the youngest e-consult patients (mean age, 38.6 years).
PCPs weigh in on results
Physicians said in interviews that the study data reflects their own positive experiences with e-consults.
“Although I don’t always think [an e-consult] is able to fully prevent the specialist visit, it does allow the specialist to provide recommendations for work-up that can be done prior to the specialist visit,” said Santina Wheat MD, a family physician at Erie Family Health Center in Chicago. This reduces the time in which the consult is placed to when effective treatment can take place.
Patients who may have to wait months or even years to see a specialty doctor, benefit from e-consults, said Dr. Wheat, who is also a member of the editorial advisory board of Family Practice News. “As part of an organization that does e-consults to another hospital with a different electronic medical record, the e-consult increases the likelihood that all of the clinical information reaches the specialists and prevents tests from being repeated.”
Starting an e-consult may also increase the likelihood that the patient quickly sees a specialist at the contracted hospital, she added.
Sarah G. Candler, MD, said in an interview that she also sees e-consults as an essential tool. “When patients present with rare, complex, or atypical pictures, I find it helpful to have specialists weigh in. The e-consult helps me ensure that I work to the top of my abilities as an internist,” said Dr. Candler, who is practice medical director and physician director of academic relations at Iora Primary Care, Northside Clinic, Houston. However, she did not agree with the study’s avoided in-person visits metric for assessing utility.
“In some cases, the end result of an e-consult is a referral for an in-person evaluation, and the role of the e-consult is to ensure that I have done my due diligence as a primary care doctor asking the correct questions, getting the appropriate work-up completed, and referring to the appropriate specialty for next steps, when necessary,” noted Dr. Candler, who also serves on the editorial advisory board of Internal Medicine News.
Financial considerations
The study’s authors suggested taking a closer look at standardizing payment for the use of e-consults and developing appropriateness criteria for them.
Health systems could use such criteria to study what makes an e-consult useful and how to best utilize this tool, Dr. Ahmed said in an interview.
“Compensation models that promote high-quality, effective, and efficient e-consults are needed to reinforce the ability of health systems to optimize the mix of e-consults and in-person visits,” Dr. Ahmed and colleagues suggested.
Because not all patient care requires e-consults, the model makes the most sense in practices that already participate in value-based payment programs. In these types of programs, the cost can be shared according to the variable risk and patient need for the service, Dr. Candler explained.
“I have been fortunate to work in two different systems that function in this way, which means that e-consults have been readily available and encouraged-both to improve patient care and decrease overall cost by decreasing unnecessary testing or specialist referral,” she said.
Dr. Wheat said that the managed care organization affiliated with her practice seems to be saving money with e-consults, as it decreases the need to pay for specialist visits in some instances and for repeated work-ups.
Future studies
The study’s cohort represented just one large health care system with a shared electronic health record. “Single-system descriptive studies, such as that of Ahmed and colleagues, are particularly useful for local evaluation and quality improvement efforts,” Varsha G. Vimalananda, MD, and B. Graeme Fincke, MD, both of the Center for Healthcare Organization and Implementation Research at Bedford (Mass.) Veterans Affairs Hospital, wrote in a related editorial.
“However, we need innovative approaches to evaluation that estimate the effect of e-consults on quality and cost of care across health care systems and over time. Implementation studies can help to identify key contributors to success,” the editorialists wrote.
One of the study authors, reported receiving personal fees from Bayer outside the submitted work. The other authors of the paper and the authors of the editorial reported no conflicts of interest. Dr. Candler said her employer contracts with an e-consult service, but that she is not compensated for use of the service. She is also a coeditor of Annals of Internal Medicine’s blog, “Fresh Look.”
SOURCE: Ahmed S et al. Ann Intern Med. 2020 Apr 14. doi: 10.7326/M19-3852.

Studies have shown that e-consults increase access to specialist care and primary care physician (PCP) education, according to research published in the Annals of Internal Medicine (2020. Apr 14. doi: 10.7326/M19-3852) by Salman Ahmed, MD, and colleagues.
These resources are already being frequently used by physicians, but more often by general internists and hospitalists than by subspecialists, according to a recent survey by the American College of Physicians. That survey found that 42% of its respondents are using e-consults and that subspecialists’ use is less common primarily because of the lack of access to e-consult technology.
What hasn’t been widely researched are the effects of large-scale e-consult programs, said Dr. Ahmed, who is associate physician in the renal division at Brigham and Women’s Hospital, Boston, in an interview.
For frontline providers such as PCPs, e-consults are a way to quickly seek out answers to clinical questions from specialists. In turn, the specialist can help a wider pool of participants, he noted.
The findings of Dr. Ahmed’s study, which included several academic centers and hospitals affiliated with Partners HealthCare System, a nonprofit network in eastern Massachusetts that includes Brigham and Women’s Hospital, used several metrics to analyze the appropriateness and utility of e-consults across a range of specialties. An e-consult was considered useful if it resulted in the avoidance of a visit to a specialist, which was defined as the absence of an in-person visit to the type of specialist consulted electronically for 120 days. An e-consult was considered appropriate if it met the following four criteria.
- It could not be answered by referring to society guidelines or widely available, evidence-based summary sources.
- It did not seek logistic information, such as where to have a specific laboratory test done.
- It did not include a question of high urgency.
- The medical complexity of the clinical situation was not substantial enough to warrant an in-person consultation.
The investigators examined e-consult inquiries to mostly physician health care providers in five specialties – hematology, infectious disease, dermatology, rheumatology, and psychiatry – over a year.
High rates of appropriateness
The search spanned 6,512 eligible e-consults from 1,096 referring providers to 121 specialist consultants. Narrowing their search to 741 records with complete data, the investigators found that 70.2% of these consults met the criteria for appropriateness. In an analysis of four reviewers blinded to each other’s results, raters agreed on the appropriateness of 94% of e-consults.
Across specialties, more than 81% of e-consults were associated with avoided in-person visits.
The reasons for most e-consults were to seek answers to questions about diagnosis, therapeutics, or patient inquiries, or to request further education by PCPs.
“Across all specialties, the most common reasons an e-consult was not considered appropriate were failing the point-of-care resource test and asking a question of inappropriately high complexity,” the authors summarized.
Physicians and PCPs from tertiary care practices made up the majority of referring providers, with turnaround time for consults averaging 24 hours across specialties.
Rates of appropriateness, content, patient demographics, and timeliness of e-consult responses varied among the four specialties. Those with high avoidance of visits rates tended to have high appropriateness rates, indicating that some specialties may be more conducive to e-consults than others, the authors noted. Psychiatry and hematology had the highest proportion of appropriate e-consults (77.9% and 73.3% respectively). Rheumatology had the lowest proportion of appropriate e-consults and one of the lowest rates of avoided in-person visits, and dermatology had the lowest rate of avoided in-person visits, at 61.9%.
The majority (93%) of e-consults sought in psychiatry were therapy related, whereas 88.4% of the e-consult questions in rheumatology related to diagnosis.
“Questions about diagnosis were less likely to be answerable via e-consult, which suggests that to provide diagnoses, consultants may wish to engage with the patient directly,” Dr. Ahmed said in an interview.
Infectious disease specialists seemed to be the fastest responders, with nearly 90% of their consultations having been answered within a day. Dermatology specialists had the distinction of having the youngest e-consult patients (mean age, 38.6 years).
PCPs weigh in on results
Physicians said in interviews that the study data reflects their own positive experiences with e-consults.
“Although I don’t always think [an e-consult] is able to fully prevent the specialist visit, it does allow the specialist to provide recommendations for work-up that can be done prior to the specialist visit,” said Santina Wheat MD, a family physician at Erie Family Health Center in Chicago. This reduces the time in which the consult is placed to when effective treatment can take place.
Patients who may have to wait months or even years to see a specialty doctor, benefit from e-consults, said Dr. Wheat, who is also a member of the editorial advisory board of Family Practice News. “As part of an organization that does e-consults to another hospital with a different electronic medical record, the e-consult increases the likelihood that all of the clinical information reaches the specialists and prevents tests from being repeated.”
Starting an e-consult may also increase the likelihood that the patient quickly sees a specialist at the contracted hospital, she added.
Sarah G. Candler, MD, said in an interview that she also sees e-consults as an essential tool. “When patients present with rare, complex, or atypical pictures, I find it helpful to have specialists weigh in. The e-consult helps me ensure that I work to the top of my abilities as an internist,” said Dr. Candler, who is practice medical director and physician director of academic relations at Iora Primary Care, Northside Clinic, Houston. However, she did not agree with the study’s avoided in-person visits metric for assessing utility.
“In some cases, the end result of an e-consult is a referral for an in-person evaluation, and the role of the e-consult is to ensure that I have done my due diligence as a primary care doctor asking the correct questions, getting the appropriate work-up completed, and referring to the appropriate specialty for next steps, when necessary,” noted Dr. Candler, who also serves on the editorial advisory board of Internal Medicine News.
Financial considerations
The study’s authors suggested taking a closer look at standardizing payment for the use of e-consults and developing appropriateness criteria for them.
Health systems could use such criteria to study what makes an e-consult useful and how to best utilize this tool, Dr. Ahmed said in an interview.
“Compensation models that promote high-quality, effective, and efficient e-consults are needed to reinforce the ability of health systems to optimize the mix of e-consults and in-person visits,” Dr. Ahmed and colleagues suggested.
Because not all patient care requires e-consults, the model makes the most sense in practices that already participate in value-based payment programs. In these types of programs, the cost can be shared according to the variable risk and patient need for the service, Dr. Candler explained.
“I have been fortunate to work in two different systems that function in this way, which means that e-consults have been readily available and encouraged-both to improve patient care and decrease overall cost by decreasing unnecessary testing or specialist referral,” she said.
Dr. Wheat said that the managed care organization affiliated with her practice seems to be saving money with e-consults, as it decreases the need to pay for specialist visits in some instances and for repeated work-ups.
Future studies
The study’s cohort represented just one large health care system with a shared electronic health record. “Single-system descriptive studies, such as that of Ahmed and colleagues, are particularly useful for local evaluation and quality improvement efforts,” Varsha G. Vimalananda, MD, and B. Graeme Fincke, MD, both of the Center for Healthcare Organization and Implementation Research at Bedford (Mass.) Veterans Affairs Hospital, wrote in a related editorial.
“However, we need innovative approaches to evaluation that estimate the effect of e-consults on quality and cost of care across health care systems and over time. Implementation studies can help to identify key contributors to success,” the editorialists wrote.
One of the study authors, reported receiving personal fees from Bayer outside the submitted work. The other authors of the paper and the authors of the editorial reported no conflicts of interest. Dr. Candler said her employer contracts with an e-consult service, but that she is not compensated for use of the service. She is also a coeditor of Annals of Internal Medicine’s blog, “Fresh Look.”
SOURCE: Ahmed S et al. Ann Intern Med. 2020 Apr 14. doi: 10.7326/M19-3852.

Studies have shown that e-consults increase access to specialist care and primary care physician (PCP) education, according to research published in the Annals of Internal Medicine (2020. Apr 14. doi: 10.7326/M19-3852) by Salman Ahmed, MD, and colleagues.
These resources are already being frequently used by physicians, but more often by general internists and hospitalists than by subspecialists, according to a recent survey by the American College of Physicians. That survey found that 42% of its respondents are using e-consults and that subspecialists’ use is less common primarily because of the lack of access to e-consult technology.
What hasn’t been widely researched are the effects of large-scale e-consult programs, said Dr. Ahmed, who is associate physician in the renal division at Brigham and Women’s Hospital, Boston, in an interview.
For frontline providers such as PCPs, e-consults are a way to quickly seek out answers to clinical questions from specialists. In turn, the specialist can help a wider pool of participants, he noted.
The findings of Dr. Ahmed’s study, which included several academic centers and hospitals affiliated with Partners HealthCare System, a nonprofit network in eastern Massachusetts that includes Brigham and Women’s Hospital, used several metrics to analyze the appropriateness and utility of e-consults across a range of specialties. An e-consult was considered useful if it resulted in the avoidance of a visit to a specialist, which was defined as the absence of an in-person visit to the type of specialist consulted electronically for 120 days. An e-consult was considered appropriate if it met the following four criteria.
- It could not be answered by referring to society guidelines or widely available, evidence-based summary sources.
- It did not seek logistic information, such as where to have a specific laboratory test done.
- It did not include a question of high urgency.
- The medical complexity of the clinical situation was not substantial enough to warrant an in-person consultation.
The investigators examined e-consult inquiries to mostly physician health care providers in five specialties – hematology, infectious disease, dermatology, rheumatology, and psychiatry – over a year.
High rates of appropriateness
The search spanned 6,512 eligible e-consults from 1,096 referring providers to 121 specialist consultants. Narrowing their search to 741 records with complete data, the investigators found that 70.2% of these consults met the criteria for appropriateness. In an analysis of four reviewers blinded to each other’s results, raters agreed on the appropriateness of 94% of e-consults.
Across specialties, more than 81% of e-consults were associated with avoided in-person visits.
The reasons for most e-consults were to seek answers to questions about diagnosis, therapeutics, or patient inquiries, or to request further education by PCPs.
“Across all specialties, the most common reasons an e-consult was not considered appropriate were failing the point-of-care resource test and asking a question of inappropriately high complexity,” the authors summarized.
Physicians and PCPs from tertiary care practices made up the majority of referring providers, with turnaround time for consults averaging 24 hours across specialties.
Rates of appropriateness, content, patient demographics, and timeliness of e-consult responses varied among the four specialties. Those with high avoidance of visits rates tended to have high appropriateness rates, indicating that some specialties may be more conducive to e-consults than others, the authors noted. Psychiatry and hematology had the highest proportion of appropriate e-consults (77.9% and 73.3% respectively). Rheumatology had the lowest proportion of appropriate e-consults and one of the lowest rates of avoided in-person visits, and dermatology had the lowest rate of avoided in-person visits, at 61.9%.
The majority (93%) of e-consults sought in psychiatry were therapy related, whereas 88.4% of the e-consult questions in rheumatology related to diagnosis.
“Questions about diagnosis were less likely to be answerable via e-consult, which suggests that to provide diagnoses, consultants may wish to engage with the patient directly,” Dr. Ahmed said in an interview.
Infectious disease specialists seemed to be the fastest responders, with nearly 90% of their consultations having been answered within a day. Dermatology specialists had the distinction of having the youngest e-consult patients (mean age, 38.6 years).
PCPs weigh in on results
Physicians said in interviews that the study data reflects their own positive experiences with e-consults.
“Although I don’t always think [an e-consult] is able to fully prevent the specialist visit, it does allow the specialist to provide recommendations for work-up that can be done prior to the specialist visit,” said Santina Wheat MD, a family physician at Erie Family Health Center in Chicago. This reduces the time in which the consult is placed to when effective treatment can take place.
Patients who may have to wait months or even years to see a specialty doctor, benefit from e-consults, said Dr. Wheat, who is also a member of the editorial advisory board of Family Practice News. “As part of an organization that does e-consults to another hospital with a different electronic medical record, the e-consult increases the likelihood that all of the clinical information reaches the specialists and prevents tests from being repeated.”
Starting an e-consult may also increase the likelihood that the patient quickly sees a specialist at the contracted hospital, she added.
Sarah G. Candler, MD, said in an interview that she also sees e-consults as an essential tool. “When patients present with rare, complex, or atypical pictures, I find it helpful to have specialists weigh in. The e-consult helps me ensure that I work to the top of my abilities as an internist,” said Dr. Candler, who is practice medical director and physician director of academic relations at Iora Primary Care, Northside Clinic, Houston. However, she did not agree with the study’s avoided in-person visits metric for assessing utility.
“In some cases, the end result of an e-consult is a referral for an in-person evaluation, and the role of the e-consult is to ensure that I have done my due diligence as a primary care doctor asking the correct questions, getting the appropriate work-up completed, and referring to the appropriate specialty for next steps, when necessary,” noted Dr. Candler, who also serves on the editorial advisory board of Internal Medicine News.
Financial considerations
The study’s authors suggested taking a closer look at standardizing payment for the use of e-consults and developing appropriateness criteria for them.
Health systems could use such criteria to study what makes an e-consult useful and how to best utilize this tool, Dr. Ahmed said in an interview.
“Compensation models that promote high-quality, effective, and efficient e-consults are needed to reinforce the ability of health systems to optimize the mix of e-consults and in-person visits,” Dr. Ahmed and colleagues suggested.
Because not all patient care requires e-consults, the model makes the most sense in practices that already participate in value-based payment programs. In these types of programs, the cost can be shared according to the variable risk and patient need for the service, Dr. Candler explained.
“I have been fortunate to work in two different systems that function in this way, which means that e-consults have been readily available and encouraged-both to improve patient care and decrease overall cost by decreasing unnecessary testing or specialist referral,” she said.
Dr. Wheat said that the managed care organization affiliated with her practice seems to be saving money with e-consults, as it decreases the need to pay for specialist visits in some instances and for repeated work-ups.
Future studies
The study’s cohort represented just one large health care system with a shared electronic health record. “Single-system descriptive studies, such as that of Ahmed and colleagues, are particularly useful for local evaluation and quality improvement efforts,” Varsha G. Vimalananda, MD, and B. Graeme Fincke, MD, both of the Center for Healthcare Organization and Implementation Research at Bedford (Mass.) Veterans Affairs Hospital, wrote in a related editorial.
“However, we need innovative approaches to evaluation that estimate the effect of e-consults on quality and cost of care across health care systems and over time. Implementation studies can help to identify key contributors to success,” the editorialists wrote.
One of the study authors, reported receiving personal fees from Bayer outside the submitted work. The other authors of the paper and the authors of the editorial reported no conflicts of interest. Dr. Candler said her employer contracts with an e-consult service, but that she is not compensated for use of the service. She is also a coeditor of Annals of Internal Medicine’s blog, “Fresh Look.”
SOURCE: Ahmed S et al. Ann Intern Med. 2020 Apr 14. doi: 10.7326/M19-3852.
FROM ANNALS OF INTERNAL MEDICINE
Experts review recent winners and losers in the RA pipeline
MAUI, HAWAII – Filgotinib, the oral Janus kinase (JAK) inhibitor now under Food and Drug Administration review for the treatment of RA, has a better safety profile than some of the approved oral JAK inhibitors, but that’s unlikely to save it from being saddled with a black-box safety warning label, experts agreed at the 2020 Rheumatology Winter Clinical Symposium.
“There’s probably a class label out there,” according to Mark C. Genovese, MD, professor of medicine and clinical chief of the division of immunology and rheumatology at Stanford (Calif.) University.
He cited the example of upadacitinib (Rinvoq), approved last year as the third oral JAK inhibitor for RA. Even though venous thromboembolic (VTE) events weren’t seen with any significantly increased frequency, compared with placebo, in the upadacitinib development program – unlike for the earlier-approved tofacitinib (Xeljanz) and baricitinib (Olumiant) –the FDA nevertheless required that upadacitinib’s product labeling include this black-box warning: “Thrombosis, including deep vein thrombosis, pulmonary embolism, and arterial thrombosis, have occurred in patients treated with Janus kinase inhibitors used to treat inflammatory conditions.”
“I would fully expect that there’ll be a similar label in the filgotinib package insert saying that VTEs have been seen in other members of the class,” he predicted.
His copanelist Roy Fleischmann, MD, noted that filgotinib displayed a clean long-term safety profile in a pooled analysis of the 24-week results in the 2,088 filgotinib-treated participants in all phase 3 clinical trials for RA. For example, the incidence of herpes zoster in that large treated population was a mere 0.1%.
“Herpes zoster is almost nonexistent across the program,” observed Dr. Fleischmann, a rheumatologist at the University of Texas and medical director of the Metroplex Clinical Research Center, both in Dallas.
That’s consistent with what he’s heard from Japanese investigators about their experience. They tell him that in their studies the incidence of herpes zoster with filgotinib is five times less than with other JAK inhibitors.
The long-term pooled phase 3 filgotinib safety data also show less than a 0.1% incidence of adjudicated VTE/pulmonary embolism through 24 weeks. That’s a substantially lower rate than with tofacitinib or baricitinib, he noted.
The two rheumatologists, long-time observers of the FDA regulatory scene, stressed that they have no inside information regarding what the agency will do about filgotinib. It seems beyond doubt that the JAK inhibitor will be approved. But an open question of practical importance to clinicians is whether the agency will approve only the 100-mg dose or the 200-mg dose as well. The panelists agreed that having access to both would be advantageous since the clinical trials data indicate the higher dose is more effective and this greater efficacy doesn’t come at a cost of additional safety issues.
“If the 100 mg is sufficient, that’s great, but the reality is if you want to push to low disease activity or remission, the 200 mg seems to work better, particularly in patients who’ve already failed TNF [tumor necrosis factor] inhibitors or other biologics,” Dr. Genovese said. “If you’re not having additional safety concerns and you can get additional efficacy, I love the idea of having flexibility.”
Dr. Fleischmann is skeptical that the regulators will see things that way.
“There is a real risk that the FDA will do what it’s done before and say: ‘Well, the 200 works and the 100 works, so we’re going to approve the lower dose.’ But there doesn’t appear to be a big safety difference between 100 and 200. So I can see why they would approve the two doses, but I think that’d be unusual,” according to the rheumatologist.
The RA pipeline
The two speakers also highlighted several agents with novel mechanisms of action moving through the RA developmental pipeline, including olokizumab, otilimab, fenebrutinib, and a promising oral selective interleukin-1 receptor–associated kinase 4 inhibitor (IRAK4).
Olokizumab: This humanized monoclonal antibody targets IL-6. It has a different mechanism of action than the two commercially available IL-6 inhibitors approved for RA, tocilizumab (Actemra) and sarilumab (Kevzara), in that olokizumab uniquely targets the IL-6 ligand.
At the 2019 annual meeting of the American College of Rheumatology, Dr. Genovese presented the positive findings of a double-blind, placebo-controlled, randomized, phase 3 clinical trial of olokizumab in 428 RA patients with an inadequate response to methotrexate. The primary outcome, an ACR 20 response at 12 weeks, occurred in 25.9% of patients on placebo, 63.6% with 64 mg of olokizumab given subcutaneously every 2 weeks, and 70.4% with 64 mg every 4 weeks, with all participants on background methotrexate. An ACR 50 response at week 24 occurred in 7.7%, 42.7%, and 48.6%, respectively, with an acceptable side effect profile.
This was the first phase 3 trial to be presented from a large, ongoing phase 3 olokizumab developmental program for a variety of diseases.
“The results certainly support the idea that a 4-week regimen would probably be quite useful with this medication, although we’ll have to see what happens with the remaining phase 3 trials,” Dr. Genovese said.
Dr. Fleischmann posed a question: Do we really need a third IL-6 inhibitor?
That would make for a crowded field, Dr. Genovese agreed, adding that grabbing a reasonable market share for olokizumab may come down to cost, formulary access, and the convenience factor of once-monthly dosing. Whether the biologic’s unique mechanism of action in blocking the IL-6 ligand makes any practical difference in outcomes is unknown.
IRAK4 inhibitor: PF-06650833 is an oral selective reversible inhibitor of IRAK4, a key signaling kinase for IL-1 and toll-like receptors.
“This should be a really good drug for IL-1-mediated diseases,” according to Dr. Fleischmann.
In a phase 2b, double-blind, randomized, placebo-controlled, 12-week study featuring tofacitinib at 5 mg twice daily as an active comparator, the IRAK4 inhibitor exhibited dose-dependent efficacy for the primary endpoint of improvement from baseline in Simple Disease Activity Index score, compared with placebo. The same was true for the secondary endpoint, change over time in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP). The trial results were also presented at the 2019 ACR annual meeting.
“This is a drug they should probably take forward and see how far it goes in RA,” he said.
Dr. Genovese concurred.
“We’re still trying to figure out how we can put together rational combinations in RA, and this might be something that could be considered as a combination play. In fact, Pfizer has already teed up a study looking at a JAK inhibitor/IRAK4 combination. The question will be whether this is a standalone or has an opportunity to be part of a combination approach,” the rheumatologist said.
Otilimab: This monoclonal antibody is a granulocyte-macrophage colony-stimulating factor inhibitor. In a secondary analysis of the BAROQUE trial, a phase 2b, 50-week study in RA inadequately responsive to methotrexate, otilimab demonstrated an impressive effect in terms of pain reduction. This new analysis, which was first presented at the 2019 ACR annual meeting, showed that, at week 12, a minimum clinically important difference in pain was achieved in 29% of placebo-treated controls, compared with 65%-75% of patients on low to higher doses of otilimab.
“The question is: Is this pain effect unique to this molecule, this pathway, or is it a simple reflection of the treated patient population?” Dr. Genovese commented. “It’s an interesting molecule. It’s being developed in RA, and it might have unique benefits on the pain side.”
A tale of two BTK inhibitors: Bruton tyrosine kinase (BTK) is an intracellular kinase viewed as a promising target in autoimmune disease. Fenebrutinib is an oral, noncovalent, reversible, and highly selective BTK inhibitor that performed well in a phase 2, randomized, double-blind, placebo-controlled clinical trial with adalimumab as an active comparator in 480 patients with an inadequate response to methotrexate in one branch, and in 98 patients with an inadequate response to TNF inhibitor therapy in the other. All subjects were on background methotrexate. (The study results were published April 9 in Arthritis & Rheumatology.)
In the group with a prior inadequate response to TNF inhibitors, the ACR 50 response rate at 12 weeks was 25% in the group on fenebrutinib at 200 mg twice daily, significantly better than the 12% rate in placebo. And there were favorable effects on biomarkers: The reduction in erythrocyte sedimentation rate from baseline was nearly twice as great with fenebrutinib, the drop in CRP was nearly three times greater than with placebo, and there was also a significantly greater decrease over time in DAS28-CRP.
In the methotrexate-inadequate responders, the ACR50 rates were 28% and 35% with the BTK inhibitor at 150 and 200 mg once daily, respectively, compared with 15% in controls.
The safety picture was encouraging, with similarly low adverse event rates across all treatment arms.
In contrast to the fenebrutinib experience, Dr. Genovese was lead investigator in a 250-patient, phase 2 study of another oral BTK inhibitor, poseltinib, which differs from fenebrutinib in that it is an irreversible covalent inhibitor. It was a failed study, with no significant difference between poseltinib and placebo in ACR 20 response at 12 weeks. It’s unclear whether the problem was insufficient dosing or that poseltinib is a failed molecule, perhaps because of its irreversible covalent binding to BTK, he said.
Other notable failures
The spleen tyrosine kinase (Syk) inhibitor known as GS-9876 showed no clinical efficacy in a phase 2, double-blind, randomized trial in 83 RA patients with an inadequate response to methotrexate or a biologic DMARD.
“This is like the fourth Syk inhibitor that’s failed. Syk inhibition is not sick, Syk is dead,” Dr. Fleischmann declared.
Cadherin-11 is an inflammatory cytokine expressed on fibroblasts in RA joints. In a phase 2, double-blind, randomized trial in 109 patients with RA inadequately responsive to TNF inhibitors, RG6125, a humanized monoclonal antibody directed against cadherin-11, failed to outperform placebo.
“It should have worked. It didn’t. So the question is whether this pathway is not an appropriate pathway, or the molecule was not quite the right molecule. I have a feeling it was possibly not the right molecule and the pathway may be viable,” according to Dr. Fleischmann.
He reported receiving clinical trial research grants from and serving as a consultant to more than a dozen pharmaceutical companies. Dr. Genovese also reported financial relationships with more than a dozen pharmaceutical companies.
MAUI, HAWAII – Filgotinib, the oral Janus kinase (JAK) inhibitor now under Food and Drug Administration review for the treatment of RA, has a better safety profile than some of the approved oral JAK inhibitors, but that’s unlikely to save it from being saddled with a black-box safety warning label, experts agreed at the 2020 Rheumatology Winter Clinical Symposium.
“There’s probably a class label out there,” according to Mark C. Genovese, MD, professor of medicine and clinical chief of the division of immunology and rheumatology at Stanford (Calif.) University.
He cited the example of upadacitinib (Rinvoq), approved last year as the third oral JAK inhibitor for RA. Even though venous thromboembolic (VTE) events weren’t seen with any significantly increased frequency, compared with placebo, in the upadacitinib development program – unlike for the earlier-approved tofacitinib (Xeljanz) and baricitinib (Olumiant) –the FDA nevertheless required that upadacitinib’s product labeling include this black-box warning: “Thrombosis, including deep vein thrombosis, pulmonary embolism, and arterial thrombosis, have occurred in patients treated with Janus kinase inhibitors used to treat inflammatory conditions.”
“I would fully expect that there’ll be a similar label in the filgotinib package insert saying that VTEs have been seen in other members of the class,” he predicted.
His copanelist Roy Fleischmann, MD, noted that filgotinib displayed a clean long-term safety profile in a pooled analysis of the 24-week results in the 2,088 filgotinib-treated participants in all phase 3 clinical trials for RA. For example, the incidence of herpes zoster in that large treated population was a mere 0.1%.
“Herpes zoster is almost nonexistent across the program,” observed Dr. Fleischmann, a rheumatologist at the University of Texas and medical director of the Metroplex Clinical Research Center, both in Dallas.
That’s consistent with what he’s heard from Japanese investigators about their experience. They tell him that in their studies the incidence of herpes zoster with filgotinib is five times less than with other JAK inhibitors.
The long-term pooled phase 3 filgotinib safety data also show less than a 0.1% incidence of adjudicated VTE/pulmonary embolism through 24 weeks. That’s a substantially lower rate than with tofacitinib or baricitinib, he noted.
The two rheumatologists, long-time observers of the FDA regulatory scene, stressed that they have no inside information regarding what the agency will do about filgotinib. It seems beyond doubt that the JAK inhibitor will be approved. But an open question of practical importance to clinicians is whether the agency will approve only the 100-mg dose or the 200-mg dose as well. The panelists agreed that having access to both would be advantageous since the clinical trials data indicate the higher dose is more effective and this greater efficacy doesn’t come at a cost of additional safety issues.
“If the 100 mg is sufficient, that’s great, but the reality is if you want to push to low disease activity or remission, the 200 mg seems to work better, particularly in patients who’ve already failed TNF [tumor necrosis factor] inhibitors or other biologics,” Dr. Genovese said. “If you’re not having additional safety concerns and you can get additional efficacy, I love the idea of having flexibility.”
Dr. Fleischmann is skeptical that the regulators will see things that way.
“There is a real risk that the FDA will do what it’s done before and say: ‘Well, the 200 works and the 100 works, so we’re going to approve the lower dose.’ But there doesn’t appear to be a big safety difference between 100 and 200. So I can see why they would approve the two doses, but I think that’d be unusual,” according to the rheumatologist.
The RA pipeline
The two speakers also highlighted several agents with novel mechanisms of action moving through the RA developmental pipeline, including olokizumab, otilimab, fenebrutinib, and a promising oral selective interleukin-1 receptor–associated kinase 4 inhibitor (IRAK4).
Olokizumab: This humanized monoclonal antibody targets IL-6. It has a different mechanism of action than the two commercially available IL-6 inhibitors approved for RA, tocilizumab (Actemra) and sarilumab (Kevzara), in that olokizumab uniquely targets the IL-6 ligand.
At the 2019 annual meeting of the American College of Rheumatology, Dr. Genovese presented the positive findings of a double-blind, placebo-controlled, randomized, phase 3 clinical trial of olokizumab in 428 RA patients with an inadequate response to methotrexate. The primary outcome, an ACR 20 response at 12 weeks, occurred in 25.9% of patients on placebo, 63.6% with 64 mg of olokizumab given subcutaneously every 2 weeks, and 70.4% with 64 mg every 4 weeks, with all participants on background methotrexate. An ACR 50 response at week 24 occurred in 7.7%, 42.7%, and 48.6%, respectively, with an acceptable side effect profile.
This was the first phase 3 trial to be presented from a large, ongoing phase 3 olokizumab developmental program for a variety of diseases.
“The results certainly support the idea that a 4-week regimen would probably be quite useful with this medication, although we’ll have to see what happens with the remaining phase 3 trials,” Dr. Genovese said.
Dr. Fleischmann posed a question: Do we really need a third IL-6 inhibitor?
That would make for a crowded field, Dr. Genovese agreed, adding that grabbing a reasonable market share for olokizumab may come down to cost, formulary access, and the convenience factor of once-monthly dosing. Whether the biologic’s unique mechanism of action in blocking the IL-6 ligand makes any practical difference in outcomes is unknown.
IRAK4 inhibitor: PF-06650833 is an oral selective reversible inhibitor of IRAK4, a key signaling kinase for IL-1 and toll-like receptors.
“This should be a really good drug for IL-1-mediated diseases,” according to Dr. Fleischmann.
In a phase 2b, double-blind, randomized, placebo-controlled, 12-week study featuring tofacitinib at 5 mg twice daily as an active comparator, the IRAK4 inhibitor exhibited dose-dependent efficacy for the primary endpoint of improvement from baseline in Simple Disease Activity Index score, compared with placebo. The same was true for the secondary endpoint, change over time in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP). The trial results were also presented at the 2019 ACR annual meeting.
“This is a drug they should probably take forward and see how far it goes in RA,” he said.
Dr. Genovese concurred.
“We’re still trying to figure out how we can put together rational combinations in RA, and this might be something that could be considered as a combination play. In fact, Pfizer has already teed up a study looking at a JAK inhibitor/IRAK4 combination. The question will be whether this is a standalone or has an opportunity to be part of a combination approach,” the rheumatologist said.
Otilimab: This monoclonal antibody is a granulocyte-macrophage colony-stimulating factor inhibitor. In a secondary analysis of the BAROQUE trial, a phase 2b, 50-week study in RA inadequately responsive to methotrexate, otilimab demonstrated an impressive effect in terms of pain reduction. This new analysis, which was first presented at the 2019 ACR annual meeting, showed that, at week 12, a minimum clinically important difference in pain was achieved in 29% of placebo-treated controls, compared with 65%-75% of patients on low to higher doses of otilimab.
“The question is: Is this pain effect unique to this molecule, this pathway, or is it a simple reflection of the treated patient population?” Dr. Genovese commented. “It’s an interesting molecule. It’s being developed in RA, and it might have unique benefits on the pain side.”
A tale of two BTK inhibitors: Bruton tyrosine kinase (BTK) is an intracellular kinase viewed as a promising target in autoimmune disease. Fenebrutinib is an oral, noncovalent, reversible, and highly selective BTK inhibitor that performed well in a phase 2, randomized, double-blind, placebo-controlled clinical trial with adalimumab as an active comparator in 480 patients with an inadequate response to methotrexate in one branch, and in 98 patients with an inadequate response to TNF inhibitor therapy in the other. All subjects were on background methotrexate. (The study results were published April 9 in Arthritis & Rheumatology.)
In the group with a prior inadequate response to TNF inhibitors, the ACR 50 response rate at 12 weeks was 25% in the group on fenebrutinib at 200 mg twice daily, significantly better than the 12% rate in placebo. And there were favorable effects on biomarkers: The reduction in erythrocyte sedimentation rate from baseline was nearly twice as great with fenebrutinib, the drop in CRP was nearly three times greater than with placebo, and there was also a significantly greater decrease over time in DAS28-CRP.
In the methotrexate-inadequate responders, the ACR50 rates were 28% and 35% with the BTK inhibitor at 150 and 200 mg once daily, respectively, compared with 15% in controls.
The safety picture was encouraging, with similarly low adverse event rates across all treatment arms.
In contrast to the fenebrutinib experience, Dr. Genovese was lead investigator in a 250-patient, phase 2 study of another oral BTK inhibitor, poseltinib, which differs from fenebrutinib in that it is an irreversible covalent inhibitor. It was a failed study, with no significant difference between poseltinib and placebo in ACR 20 response at 12 weeks. It’s unclear whether the problem was insufficient dosing or that poseltinib is a failed molecule, perhaps because of its irreversible covalent binding to BTK, he said.
Other notable failures
The spleen tyrosine kinase (Syk) inhibitor known as GS-9876 showed no clinical efficacy in a phase 2, double-blind, randomized trial in 83 RA patients with an inadequate response to methotrexate or a biologic DMARD.
“This is like the fourth Syk inhibitor that’s failed. Syk inhibition is not sick, Syk is dead,” Dr. Fleischmann declared.
Cadherin-11 is an inflammatory cytokine expressed on fibroblasts in RA joints. In a phase 2, double-blind, randomized trial in 109 patients with RA inadequately responsive to TNF inhibitors, RG6125, a humanized monoclonal antibody directed against cadherin-11, failed to outperform placebo.
“It should have worked. It didn’t. So the question is whether this pathway is not an appropriate pathway, or the molecule was not quite the right molecule. I have a feeling it was possibly not the right molecule and the pathway may be viable,” according to Dr. Fleischmann.
He reported receiving clinical trial research grants from and serving as a consultant to more than a dozen pharmaceutical companies. Dr. Genovese also reported financial relationships with more than a dozen pharmaceutical companies.
MAUI, HAWAII – Filgotinib, the oral Janus kinase (JAK) inhibitor now under Food and Drug Administration review for the treatment of RA, has a better safety profile than some of the approved oral JAK inhibitors, but that’s unlikely to save it from being saddled with a black-box safety warning label, experts agreed at the 2020 Rheumatology Winter Clinical Symposium.
“There’s probably a class label out there,” according to Mark C. Genovese, MD, professor of medicine and clinical chief of the division of immunology and rheumatology at Stanford (Calif.) University.
He cited the example of upadacitinib (Rinvoq), approved last year as the third oral JAK inhibitor for RA. Even though venous thromboembolic (VTE) events weren’t seen with any significantly increased frequency, compared with placebo, in the upadacitinib development program – unlike for the earlier-approved tofacitinib (Xeljanz) and baricitinib (Olumiant) –the FDA nevertheless required that upadacitinib’s product labeling include this black-box warning: “Thrombosis, including deep vein thrombosis, pulmonary embolism, and arterial thrombosis, have occurred in patients treated with Janus kinase inhibitors used to treat inflammatory conditions.”
“I would fully expect that there’ll be a similar label in the filgotinib package insert saying that VTEs have been seen in other members of the class,” he predicted.
His copanelist Roy Fleischmann, MD, noted that filgotinib displayed a clean long-term safety profile in a pooled analysis of the 24-week results in the 2,088 filgotinib-treated participants in all phase 3 clinical trials for RA. For example, the incidence of herpes zoster in that large treated population was a mere 0.1%.
“Herpes zoster is almost nonexistent across the program,” observed Dr. Fleischmann, a rheumatologist at the University of Texas and medical director of the Metroplex Clinical Research Center, both in Dallas.
That’s consistent with what he’s heard from Japanese investigators about their experience. They tell him that in their studies the incidence of herpes zoster with filgotinib is five times less than with other JAK inhibitors.
The long-term pooled phase 3 filgotinib safety data also show less than a 0.1% incidence of adjudicated VTE/pulmonary embolism through 24 weeks. That’s a substantially lower rate than with tofacitinib or baricitinib, he noted.
The two rheumatologists, long-time observers of the FDA regulatory scene, stressed that they have no inside information regarding what the agency will do about filgotinib. It seems beyond doubt that the JAK inhibitor will be approved. But an open question of practical importance to clinicians is whether the agency will approve only the 100-mg dose or the 200-mg dose as well. The panelists agreed that having access to both would be advantageous since the clinical trials data indicate the higher dose is more effective and this greater efficacy doesn’t come at a cost of additional safety issues.
“If the 100 mg is sufficient, that’s great, but the reality is if you want to push to low disease activity or remission, the 200 mg seems to work better, particularly in patients who’ve already failed TNF [tumor necrosis factor] inhibitors or other biologics,” Dr. Genovese said. “If you’re not having additional safety concerns and you can get additional efficacy, I love the idea of having flexibility.”
Dr. Fleischmann is skeptical that the regulators will see things that way.
“There is a real risk that the FDA will do what it’s done before and say: ‘Well, the 200 works and the 100 works, so we’re going to approve the lower dose.’ But there doesn’t appear to be a big safety difference between 100 and 200. So I can see why they would approve the two doses, but I think that’d be unusual,” according to the rheumatologist.
The RA pipeline
The two speakers also highlighted several agents with novel mechanisms of action moving through the RA developmental pipeline, including olokizumab, otilimab, fenebrutinib, and a promising oral selective interleukin-1 receptor–associated kinase 4 inhibitor (IRAK4).
Olokizumab: This humanized monoclonal antibody targets IL-6. It has a different mechanism of action than the two commercially available IL-6 inhibitors approved for RA, tocilizumab (Actemra) and sarilumab (Kevzara), in that olokizumab uniquely targets the IL-6 ligand.
At the 2019 annual meeting of the American College of Rheumatology, Dr. Genovese presented the positive findings of a double-blind, placebo-controlled, randomized, phase 3 clinical trial of olokizumab in 428 RA patients with an inadequate response to methotrexate. The primary outcome, an ACR 20 response at 12 weeks, occurred in 25.9% of patients on placebo, 63.6% with 64 mg of olokizumab given subcutaneously every 2 weeks, and 70.4% with 64 mg every 4 weeks, with all participants on background methotrexate. An ACR 50 response at week 24 occurred in 7.7%, 42.7%, and 48.6%, respectively, with an acceptable side effect profile.
This was the first phase 3 trial to be presented from a large, ongoing phase 3 olokizumab developmental program for a variety of diseases.
“The results certainly support the idea that a 4-week regimen would probably be quite useful with this medication, although we’ll have to see what happens with the remaining phase 3 trials,” Dr. Genovese said.
Dr. Fleischmann posed a question: Do we really need a third IL-6 inhibitor?
That would make for a crowded field, Dr. Genovese agreed, adding that grabbing a reasonable market share for olokizumab may come down to cost, formulary access, and the convenience factor of once-monthly dosing. Whether the biologic’s unique mechanism of action in blocking the IL-6 ligand makes any practical difference in outcomes is unknown.
IRAK4 inhibitor: PF-06650833 is an oral selective reversible inhibitor of IRAK4, a key signaling kinase for IL-1 and toll-like receptors.
“This should be a really good drug for IL-1-mediated diseases,” according to Dr. Fleischmann.
In a phase 2b, double-blind, randomized, placebo-controlled, 12-week study featuring tofacitinib at 5 mg twice daily as an active comparator, the IRAK4 inhibitor exhibited dose-dependent efficacy for the primary endpoint of improvement from baseline in Simple Disease Activity Index score, compared with placebo. The same was true for the secondary endpoint, change over time in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP). The trial results were also presented at the 2019 ACR annual meeting.
“This is a drug they should probably take forward and see how far it goes in RA,” he said.
Dr. Genovese concurred.
“We’re still trying to figure out how we can put together rational combinations in RA, and this might be something that could be considered as a combination play. In fact, Pfizer has already teed up a study looking at a JAK inhibitor/IRAK4 combination. The question will be whether this is a standalone or has an opportunity to be part of a combination approach,” the rheumatologist said.
Otilimab: This monoclonal antibody is a granulocyte-macrophage colony-stimulating factor inhibitor. In a secondary analysis of the BAROQUE trial, a phase 2b, 50-week study in RA inadequately responsive to methotrexate, otilimab demonstrated an impressive effect in terms of pain reduction. This new analysis, which was first presented at the 2019 ACR annual meeting, showed that, at week 12, a minimum clinically important difference in pain was achieved in 29% of placebo-treated controls, compared with 65%-75% of patients on low to higher doses of otilimab.
“The question is: Is this pain effect unique to this molecule, this pathway, or is it a simple reflection of the treated patient population?” Dr. Genovese commented. “It’s an interesting molecule. It’s being developed in RA, and it might have unique benefits on the pain side.”
A tale of two BTK inhibitors: Bruton tyrosine kinase (BTK) is an intracellular kinase viewed as a promising target in autoimmune disease. Fenebrutinib is an oral, noncovalent, reversible, and highly selective BTK inhibitor that performed well in a phase 2, randomized, double-blind, placebo-controlled clinical trial with adalimumab as an active comparator in 480 patients with an inadequate response to methotrexate in one branch, and in 98 patients with an inadequate response to TNF inhibitor therapy in the other. All subjects were on background methotrexate. (The study results were published April 9 in Arthritis & Rheumatology.)
In the group with a prior inadequate response to TNF inhibitors, the ACR 50 response rate at 12 weeks was 25% in the group on fenebrutinib at 200 mg twice daily, significantly better than the 12% rate in placebo. And there were favorable effects on biomarkers: The reduction in erythrocyte sedimentation rate from baseline was nearly twice as great with fenebrutinib, the drop in CRP was nearly three times greater than with placebo, and there was also a significantly greater decrease over time in DAS28-CRP.
In the methotrexate-inadequate responders, the ACR50 rates were 28% and 35% with the BTK inhibitor at 150 and 200 mg once daily, respectively, compared with 15% in controls.
The safety picture was encouraging, with similarly low adverse event rates across all treatment arms.
In contrast to the fenebrutinib experience, Dr. Genovese was lead investigator in a 250-patient, phase 2 study of another oral BTK inhibitor, poseltinib, which differs from fenebrutinib in that it is an irreversible covalent inhibitor. It was a failed study, with no significant difference between poseltinib and placebo in ACR 20 response at 12 weeks. It’s unclear whether the problem was insufficient dosing or that poseltinib is a failed molecule, perhaps because of its irreversible covalent binding to BTK, he said.
Other notable failures
The spleen tyrosine kinase (Syk) inhibitor known as GS-9876 showed no clinical efficacy in a phase 2, double-blind, randomized trial in 83 RA patients with an inadequate response to methotrexate or a biologic DMARD.
“This is like the fourth Syk inhibitor that’s failed. Syk inhibition is not sick, Syk is dead,” Dr. Fleischmann declared.
Cadherin-11 is an inflammatory cytokine expressed on fibroblasts in RA joints. In a phase 2, double-blind, randomized trial in 109 patients with RA inadequately responsive to TNF inhibitors, RG6125, a humanized monoclonal antibody directed against cadherin-11, failed to outperform placebo.
“It should have worked. It didn’t. So the question is whether this pathway is not an appropriate pathway, or the molecule was not quite the right molecule. I have a feeling it was possibly not the right molecule and the pathway may be viable,” according to Dr. Fleischmann.
He reported receiving clinical trial research grants from and serving as a consultant to more than a dozen pharmaceutical companies. Dr. Genovese also reported financial relationships with more than a dozen pharmaceutical companies.
REPORTING FROM RWCS 2020
AI could identify fracture risk
A natural language processing algorithm, designed to scour emergency department records for fracture cases, has the potential to improve treatment of osteoporosis and prevent future, more severe fractures.
The approach led to a notable increase in referrals to the osteoporosis refracture prevention service at the Prince of Wales Hospital in Sydney, where the work was done.
The strongest predictor of a future fracture is a recent previous fracture, said Christopher White, MBBS, the hospital’s director of research, who presented results of an analysis at a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“We have really effective therapies that can reduce the risk of [future] fractures by 50%, and yet 80% of osteoporotic patients leave the hospital untreated after fracture,” said Dr. White.
That, he explained, is because of a fundamental disconnect in fracture care – emergency department physicians tackle the immediate aftermath of a broken bone, but they are not tasked with treating the underlying condition. As a result, many patients who would be candidates for follow-up care are not referred.
The current work grew out of Dr. White’s frustration with not being able to recruit patients for osteoporosis clinical trials. In fact, he got so annoyed trying to recruit and not getting patients referred to him – even though he’d find they were actually in the hospital – that he decided “to start an AI [artificial intelligence] program that would read the radiology report and bypass the referrer,” he said.
To that end, with the help of an industry partner, he developed a software program called XRAIT (X-Ray Artificial Intelligence Tool), which analyzed the reports and, with Dr. White’s iterated guidance, learned to identify fractures.
The system performed a little too well. “You have to be careful what you wish for, because suddenly I went from 70 referrals to 339,” he said.
That influx is a potential downside, however, according to Angela Cheung, MD, PhD, director of the Centre of Excellence in Skeletal Health Assessment and Osteoporosis Program at the University of Toronto’s University Health Network. Natural language processing can help identify patients that a human reviewer would miss, because reviewers tend to focus on cases in which the fracture was the reason for the hospital visit, rather than on incidental findings. But not all incidental findings are clinically important. “A pneumonia patient might have had the fracture 30 years ago, falling off a tree as a college student. It may not pick up the highest-risk group in terms of fractures, because we know that recency of fractures matters,” Dr. Cheung, who was not associated with the research, said in an interview.
“It means the fracture liaison coordinator would need to review [more] numbers in trying to figure out whether the patient should get attention and whether they should be treated as well,” said Dr. Cheung, adding that more studies would need to be done to determine if the approach would be cost effective.
The researchers performed a technical evaluation of 2,445 nonfracture and 433 fracture reports, in which the tool performed with more than 99% sensitivity and specificity.
In a clinical validation, a fracture clinician and XRAIT reviewed 5,089 x-ray and computed tomography reports from ED patients who were older than 50 years. The ED referred 70 cases, leading to identification of 65 fractures. The combination of ED referral and a fracture clinician’s review of 224 cases revealed 98 fracture cases. By contrast, XRAIT nearly instantaneously analyzed 5,089 reports from 3,217 patients, and identified fractures in 349 patients – a nearly fivefold higher number than the manual case finding of 70. Of those 349 patients, results for 10 were false positives, leading to a total find of 339 patients.
In all, 57 cases were found both by XRAIT and the ED referral/fracture clinician, resulting in 282 unique cases identified by XRAIT alone. That translated to a 3.5-fold increase in cases that were identifiable using XRAIT.
In an external validation, the researchers tested the system on 327 reports from a subset of the Dubbo Osteoporosis Epidemiology Study, based in the city of Dubbo in New South Wales, Australia. In that cohort, XRAIT identified 97 positive cases, of which 87 were true fractures (10 false positives). Of 230 cases that it considered not to be fractures, there were 38 false negatives. Those numbers translated to a sensitivity of 69.6% and a specificity of 95.0%.
All of those hits have the potential to overwhelm osteoporosis services. “I now have to adjust to that, and further development will be to link the AI with clinical risk factors and treatment data to assist my fracture coordinators to target the right patients. We’ll increase the number of patients with osteoporosis on treatment, improve productivity and safety, and reduce the burden of care,” said Dr. White.
The study was funded by The Sydney Partnership for Health, Education, Research and Enterprise and the Musculoskeletal Consumer Advisory Group. The researchers reported no financial conflicts of interest, as did Dr. Cheung.
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
A natural language processing algorithm, designed to scour emergency department records for fracture cases, has the potential to improve treatment of osteoporosis and prevent future, more severe fractures.
The approach led to a notable increase in referrals to the osteoporosis refracture prevention service at the Prince of Wales Hospital in Sydney, where the work was done.
The strongest predictor of a future fracture is a recent previous fracture, said Christopher White, MBBS, the hospital’s director of research, who presented results of an analysis at a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“We have really effective therapies that can reduce the risk of [future] fractures by 50%, and yet 80% of osteoporotic patients leave the hospital untreated after fracture,” said Dr. White.
That, he explained, is because of a fundamental disconnect in fracture care – emergency department physicians tackle the immediate aftermath of a broken bone, but they are not tasked with treating the underlying condition. As a result, many patients who would be candidates for follow-up care are not referred.
The current work grew out of Dr. White’s frustration with not being able to recruit patients for osteoporosis clinical trials. In fact, he got so annoyed trying to recruit and not getting patients referred to him – even though he’d find they were actually in the hospital – that he decided “to start an AI [artificial intelligence] program that would read the radiology report and bypass the referrer,” he said.
To that end, with the help of an industry partner, he developed a software program called XRAIT (X-Ray Artificial Intelligence Tool), which analyzed the reports and, with Dr. White’s iterated guidance, learned to identify fractures.
The system performed a little too well. “You have to be careful what you wish for, because suddenly I went from 70 referrals to 339,” he said.
That influx is a potential downside, however, according to Angela Cheung, MD, PhD, director of the Centre of Excellence in Skeletal Health Assessment and Osteoporosis Program at the University of Toronto’s University Health Network. Natural language processing can help identify patients that a human reviewer would miss, because reviewers tend to focus on cases in which the fracture was the reason for the hospital visit, rather than on incidental findings. But not all incidental findings are clinically important. “A pneumonia patient might have had the fracture 30 years ago, falling off a tree as a college student. It may not pick up the highest-risk group in terms of fractures, because we know that recency of fractures matters,” Dr. Cheung, who was not associated with the research, said in an interview.
“It means the fracture liaison coordinator would need to review [more] numbers in trying to figure out whether the patient should get attention and whether they should be treated as well,” said Dr. Cheung, adding that more studies would need to be done to determine if the approach would be cost effective.
The researchers performed a technical evaluation of 2,445 nonfracture and 433 fracture reports, in which the tool performed with more than 99% sensitivity and specificity.
In a clinical validation, a fracture clinician and XRAIT reviewed 5,089 x-ray and computed tomography reports from ED patients who were older than 50 years. The ED referred 70 cases, leading to identification of 65 fractures. The combination of ED referral and a fracture clinician’s review of 224 cases revealed 98 fracture cases. By contrast, XRAIT nearly instantaneously analyzed 5,089 reports from 3,217 patients, and identified fractures in 349 patients – a nearly fivefold higher number than the manual case finding of 70. Of those 349 patients, results for 10 were false positives, leading to a total find of 339 patients.
In all, 57 cases were found both by XRAIT and the ED referral/fracture clinician, resulting in 282 unique cases identified by XRAIT alone. That translated to a 3.5-fold increase in cases that were identifiable using XRAIT.
In an external validation, the researchers tested the system on 327 reports from a subset of the Dubbo Osteoporosis Epidemiology Study, based in the city of Dubbo in New South Wales, Australia. In that cohort, XRAIT identified 97 positive cases, of which 87 were true fractures (10 false positives). Of 230 cases that it considered not to be fractures, there were 38 false negatives. Those numbers translated to a sensitivity of 69.6% and a specificity of 95.0%.
All of those hits have the potential to overwhelm osteoporosis services. “I now have to adjust to that, and further development will be to link the AI with clinical risk factors and treatment data to assist my fracture coordinators to target the right patients. We’ll increase the number of patients with osteoporosis on treatment, improve productivity and safety, and reduce the burden of care,” said Dr. White.
The study was funded by The Sydney Partnership for Health, Education, Research and Enterprise and the Musculoskeletal Consumer Advisory Group. The researchers reported no financial conflicts of interest, as did Dr. Cheung.
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
A natural language processing algorithm, designed to scour emergency department records for fracture cases, has the potential to improve treatment of osteoporosis and prevent future, more severe fractures.
The approach led to a notable increase in referrals to the osteoporosis refracture prevention service at the Prince of Wales Hospital in Sydney, where the work was done.
The strongest predictor of a future fracture is a recent previous fracture, said Christopher White, MBBS, the hospital’s director of research, who presented results of an analysis at a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“We have really effective therapies that can reduce the risk of [future] fractures by 50%, and yet 80% of osteoporotic patients leave the hospital untreated after fracture,” said Dr. White.
That, he explained, is because of a fundamental disconnect in fracture care – emergency department physicians tackle the immediate aftermath of a broken bone, but they are not tasked with treating the underlying condition. As a result, many patients who would be candidates for follow-up care are not referred.
The current work grew out of Dr. White’s frustration with not being able to recruit patients for osteoporosis clinical trials. In fact, he got so annoyed trying to recruit and not getting patients referred to him – even though he’d find they were actually in the hospital – that he decided “to start an AI [artificial intelligence] program that would read the radiology report and bypass the referrer,” he said.
To that end, with the help of an industry partner, he developed a software program called XRAIT (X-Ray Artificial Intelligence Tool), which analyzed the reports and, with Dr. White’s iterated guidance, learned to identify fractures.
The system performed a little too well. “You have to be careful what you wish for, because suddenly I went from 70 referrals to 339,” he said.
That influx is a potential downside, however, according to Angela Cheung, MD, PhD, director of the Centre of Excellence in Skeletal Health Assessment and Osteoporosis Program at the University of Toronto’s University Health Network. Natural language processing can help identify patients that a human reviewer would miss, because reviewers tend to focus on cases in which the fracture was the reason for the hospital visit, rather than on incidental findings. But not all incidental findings are clinically important. “A pneumonia patient might have had the fracture 30 years ago, falling off a tree as a college student. It may not pick up the highest-risk group in terms of fractures, because we know that recency of fractures matters,” Dr. Cheung, who was not associated with the research, said in an interview.
“It means the fracture liaison coordinator would need to review [more] numbers in trying to figure out whether the patient should get attention and whether they should be treated as well,” said Dr. Cheung, adding that more studies would need to be done to determine if the approach would be cost effective.
The researchers performed a technical evaluation of 2,445 nonfracture and 433 fracture reports, in which the tool performed with more than 99% sensitivity and specificity.
In a clinical validation, a fracture clinician and XRAIT reviewed 5,089 x-ray and computed tomography reports from ED patients who were older than 50 years. The ED referred 70 cases, leading to identification of 65 fractures. The combination of ED referral and a fracture clinician’s review of 224 cases revealed 98 fracture cases. By contrast, XRAIT nearly instantaneously analyzed 5,089 reports from 3,217 patients, and identified fractures in 349 patients – a nearly fivefold higher number than the manual case finding of 70. Of those 349 patients, results for 10 were false positives, leading to a total find of 339 patients.
In all, 57 cases were found both by XRAIT and the ED referral/fracture clinician, resulting in 282 unique cases identified by XRAIT alone. That translated to a 3.5-fold increase in cases that were identifiable using XRAIT.
In an external validation, the researchers tested the system on 327 reports from a subset of the Dubbo Osteoporosis Epidemiology Study, based in the city of Dubbo in New South Wales, Australia. In that cohort, XRAIT identified 97 positive cases, of which 87 were true fractures (10 false positives). Of 230 cases that it considered not to be fractures, there were 38 false negatives. Those numbers translated to a sensitivity of 69.6% and a specificity of 95.0%.
All of those hits have the potential to overwhelm osteoporosis services. “I now have to adjust to that, and further development will be to link the AI with clinical risk factors and treatment data to assist my fracture coordinators to target the right patients. We’ll increase the number of patients with osteoporosis on treatment, improve productivity and safety, and reduce the burden of care,” said Dr. White.
The study was funded by The Sydney Partnership for Health, Education, Research and Enterprise and the Musculoskeletal Consumer Advisory Group. The researchers reported no financial conflicts of interest, as did Dr. Cheung.
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
FROM ENDO 2020