User login
Plastic Surgeon Illegally Restricted Negative Reviews, Judge Rules
A plastic surgeon broke federal law when he restricted patients from posting negative reviews by requiring them to sign nondisclosure agreements before they received care, a district judge has ruled.
Seattle-based surgeon Javad Sajan, MD, ran afoul of the Consumer Review Fairness Act (CRFA) by requiring more than 10,000 patients to sign the agreements, according to a recent decision by US District Judge Ricardo S. Martinez. The law protects consumers’ rights to post truthful reviews about businesses.
Judge Martinez wrote that the terms of Dr. Sajan’s nondisclosure agreements “clearly include language prohibiting or restricting patients from posting negative reviews,” in violation of CRFA. Penalties for the offense will be determined at a September trial.
This news organization contacted Dr. Sajan’s office and his attorney for comment but did not get a response.
The decision is the latest development in an ongoing legal dispute between Dr. Sajan and the State of Washington over whether the surgeon’s efforts to limit negative online reviews were illegal.
Beginning in 2017, Dr. Sajan and his practice, Allure Esthetic, introduced agreements that “forced” patients to contact the business directly if they had concerns rather than post a negative review, according to a 2022 lawsuit against Dr. Sajan filed by Washington Attorney General Robert Ferguson.
“Online reviews are often the first stop when consumers are determining who to trust,” Mr. Ferguson said in a statement. “That’s especially critical when those services deal with a patient’s health and safety. We will take action against those who illegally stop Washingtonians from sharing reviews with the public.”
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the complaint. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In court documents, Dr. Sajan’s attorneys argued the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them, which hundreds did. The CRFA requires Mr. Ferguson to prove that consumers lacked a meaningful opportunity to negotiate the terms, attorneys for Dr. Sajan argued in court records.
But Judge Martinez wrote that the patients who declined to sign the agreements or changed the terms represented only a “tiny fraction” of the affected patients.
The agreement language restricts patients from speaking out by forcing dissatisfied patients to work with Allure until a resolution is reached, Judge Martinez noted in his decision. “At the very least, this would delay patients from posting such reviews and force patients to interact in some way with Allure, and it certainly appears to prohibit posting reviews until Allure agrees to some kind of favorable resolution.”
Surgeon Posted Fake Positive Reviews to Counteract Bad Reviews, AG Says
Employee accounts in court documents describe a physician fixated on reviews who went to great lengths to ensure positive reviews about his work outweighed the negative.
Former employees said they were instructed to track down patients who left negative reviews and either “threaten” them to take the posts down or offer them “money” or other things, according to Mr. Ferguson’s lawsuit. If patients could not be identified, the practice would file a defamation lawsuit against the anonymous person who posted the review and use litigation to subpoena the website for the reviewer’s IP address in order to identify them, according to court documents.
Employees testified they had regular meetings to review current negative reviews and discuss what steps they were taking to get them removed. At team meetings, in-house counsel would regularly present an Excel spreadsheet with updates on progress in getting patients to remove negative reviews, according to court documents.
In addition to restricting negative reviews, Mr. Ferguson accuses Dr. Sajan of posting fake positive reviews and “buying” thousands of fake followers on social media.
At Dr. Sajan’s direction, employees created Gmail accounts using stock photos for their profile pictures and used the accounts to post fake reviews of Allure Esthetic and Dr. Sajan, according to the complaint. The practice also used members of an online forum called BlackHatWorld.com to create fake email accounts and to post fake reviews, the attorney general alleges. Many of the fake positive reviews, including the fake Google reviews, still appear on online review sites today, the attorney general contends.
Dr. Sajan and his practice also allegedly manipulated social media to appear more popular. Mr. Ferguson claims that Dr. Sajan instructed his former web designer to purchase 60,000 followers through a vendor on BlackHatWorld.com. Most of Dr. Sajan’s current Instagram followers are not real, according to Mr. Ferguson.
The practice also used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media, according to court documents.
In addition, Dr. Sajan and his practice are accused of significantly altering “before and after” photos of patients and using fake email accounts to allow the clinic to take skincare rebates intended for patients.
All of these practices violated HIPAA, the state Consumer Protection Act (CPA) and the federal CRFA, according to Mr. Ferguson.
Surgeon Claims Competitor Behind Allegations
Attorneys for Dr. Sajan argue a competitor is behind the accusations and that other regulatory entities determined the practice did nothing wrong.
The competitor, a Seattle-based plastic surgeon, filed numerous complaints about Dr. Sajan to the Washington Medical Commission (WMC), according to court documents. The medical commission reviewed the third agreement and closed its investigation, finding that if the allegations were true, “no violation of law occurred,” court records show.
“Defendants relied upon this closing code from the WMC that the (non-disclosure) forms were lawful,” Dr. Sajan’s attorneys wrote in court documents.
The US Department of Health & Human Services Office for Civil Rights (OCR) also reviewed and audited Dr. Sajan’s use of the agreements, his attorneys noted. In a notice from OCR included in court exhibits, the agency wrote that all matters at issue have now been resolved through the practice’s voluntary compliance actions and that it was closing its investigation.
Attorneys for Dr. Sajan accuse Mr. Ferguson and state investigators of withholding the full extent of the competitor’s involvement in their investigation and failing to identify the competitor in written discovery or any of its initial disclosures. Dr. Sajan and his team discovered that the competitor was a source of key information through public records requests, according to court documents.
The remaining claims against Dr. Sajan will be addressed at trial, set for September 9, 2024.
A version of this article appeared on Medscape.com.
A plastic surgeon broke federal law when he restricted patients from posting negative reviews by requiring them to sign nondisclosure agreements before they received care, a district judge has ruled.
Seattle-based surgeon Javad Sajan, MD, ran afoul of the Consumer Review Fairness Act (CRFA) by requiring more than 10,000 patients to sign the agreements, according to a recent decision by US District Judge Ricardo S. Martinez. The law protects consumers’ rights to post truthful reviews about businesses.
Judge Martinez wrote that the terms of Dr. Sajan’s nondisclosure agreements “clearly include language prohibiting or restricting patients from posting negative reviews,” in violation of CRFA. Penalties for the offense will be determined at a September trial.
This news organization contacted Dr. Sajan’s office and his attorney for comment but did not get a response.
The decision is the latest development in an ongoing legal dispute between Dr. Sajan and the State of Washington over whether the surgeon’s efforts to limit negative online reviews were illegal.
Beginning in 2017, Dr. Sajan and his practice, Allure Esthetic, introduced agreements that “forced” patients to contact the business directly if they had concerns rather than post a negative review, according to a 2022 lawsuit against Dr. Sajan filed by Washington Attorney General Robert Ferguson.
“Online reviews are often the first stop when consumers are determining who to trust,” Mr. Ferguson said in a statement. “That’s especially critical when those services deal with a patient’s health and safety. We will take action against those who illegally stop Washingtonians from sharing reviews with the public.”
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the complaint. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In court documents, Dr. Sajan’s attorneys argued the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them, which hundreds did. The CRFA requires Mr. Ferguson to prove that consumers lacked a meaningful opportunity to negotiate the terms, attorneys for Dr. Sajan argued in court records.
But Judge Martinez wrote that the patients who declined to sign the agreements or changed the terms represented only a “tiny fraction” of the affected patients.
The agreement language restricts patients from speaking out by forcing dissatisfied patients to work with Allure until a resolution is reached, Judge Martinez noted in his decision. “At the very least, this would delay patients from posting such reviews and force patients to interact in some way with Allure, and it certainly appears to prohibit posting reviews until Allure agrees to some kind of favorable resolution.”
Surgeon Posted Fake Positive Reviews to Counteract Bad Reviews, AG Says
Employee accounts in court documents describe a physician fixated on reviews who went to great lengths to ensure positive reviews about his work outweighed the negative.
Former employees said they were instructed to track down patients who left negative reviews and either “threaten” them to take the posts down or offer them “money” or other things, according to Mr. Ferguson’s lawsuit. If patients could not be identified, the practice would file a defamation lawsuit against the anonymous person who posted the review and use litigation to subpoena the website for the reviewer’s IP address in order to identify them, according to court documents.
Employees testified they had regular meetings to review current negative reviews and discuss what steps they were taking to get them removed. At team meetings, in-house counsel would regularly present an Excel spreadsheet with updates on progress in getting patients to remove negative reviews, according to court documents.
In addition to restricting negative reviews, Mr. Ferguson accuses Dr. Sajan of posting fake positive reviews and “buying” thousands of fake followers on social media.
At Dr. Sajan’s direction, employees created Gmail accounts using stock photos for their profile pictures and used the accounts to post fake reviews of Allure Esthetic and Dr. Sajan, according to the complaint. The practice also used members of an online forum called BlackHatWorld.com to create fake email accounts and to post fake reviews, the attorney general alleges. Many of the fake positive reviews, including the fake Google reviews, still appear on online review sites today, the attorney general contends.
Dr. Sajan and his practice also allegedly manipulated social media to appear more popular. Mr. Ferguson claims that Dr. Sajan instructed his former web designer to purchase 60,000 followers through a vendor on BlackHatWorld.com. Most of Dr. Sajan’s current Instagram followers are not real, according to Mr. Ferguson.
The practice also used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media, according to court documents.
In addition, Dr. Sajan and his practice are accused of significantly altering “before and after” photos of patients and using fake email accounts to allow the clinic to take skincare rebates intended for patients.
All of these practices violated HIPAA, the state Consumer Protection Act (CPA) and the federal CRFA, according to Mr. Ferguson.
Surgeon Claims Competitor Behind Allegations
Attorneys for Dr. Sajan argue a competitor is behind the accusations and that other regulatory entities determined the practice did nothing wrong.
The competitor, a Seattle-based plastic surgeon, filed numerous complaints about Dr. Sajan to the Washington Medical Commission (WMC), according to court documents. The medical commission reviewed the third agreement and closed its investigation, finding that if the allegations were true, “no violation of law occurred,” court records show.
“Defendants relied upon this closing code from the WMC that the (non-disclosure) forms were lawful,” Dr. Sajan’s attorneys wrote in court documents.
The US Department of Health & Human Services Office for Civil Rights (OCR) also reviewed and audited Dr. Sajan’s use of the agreements, his attorneys noted. In a notice from OCR included in court exhibits, the agency wrote that all matters at issue have now been resolved through the practice’s voluntary compliance actions and that it was closing its investigation.
Attorneys for Dr. Sajan accuse Mr. Ferguson and state investigators of withholding the full extent of the competitor’s involvement in their investigation and failing to identify the competitor in written discovery or any of its initial disclosures. Dr. Sajan and his team discovered that the competitor was a source of key information through public records requests, according to court documents.
The remaining claims against Dr. Sajan will be addressed at trial, set for September 9, 2024.
A version of this article appeared on Medscape.com.
A plastic surgeon broke federal law when he restricted patients from posting negative reviews by requiring them to sign nondisclosure agreements before they received care, a district judge has ruled.
Seattle-based surgeon Javad Sajan, MD, ran afoul of the Consumer Review Fairness Act (CRFA) by requiring more than 10,000 patients to sign the agreements, according to a recent decision by US District Judge Ricardo S. Martinez. The law protects consumers’ rights to post truthful reviews about businesses.
Judge Martinez wrote that the terms of Dr. Sajan’s nondisclosure agreements “clearly include language prohibiting or restricting patients from posting negative reviews,” in violation of CRFA. Penalties for the offense will be determined at a September trial.
This news organization contacted Dr. Sajan’s office and his attorney for comment but did not get a response.
The decision is the latest development in an ongoing legal dispute between Dr. Sajan and the State of Washington over whether the surgeon’s efforts to limit negative online reviews were illegal.
Beginning in 2017, Dr. Sajan and his practice, Allure Esthetic, introduced agreements that “forced” patients to contact the business directly if they had concerns rather than post a negative review, according to a 2022 lawsuit against Dr. Sajan filed by Washington Attorney General Robert Ferguson.
“Online reviews are often the first stop when consumers are determining who to trust,” Mr. Ferguson said in a statement. “That’s especially critical when those services deal with a patient’s health and safety. We will take action against those who illegally stop Washingtonians from sharing reviews with the public.”
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the complaint. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In court documents, Dr. Sajan’s attorneys argued the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them, which hundreds did. The CRFA requires Mr. Ferguson to prove that consumers lacked a meaningful opportunity to negotiate the terms, attorneys for Dr. Sajan argued in court records.
But Judge Martinez wrote that the patients who declined to sign the agreements or changed the terms represented only a “tiny fraction” of the affected patients.
The agreement language restricts patients from speaking out by forcing dissatisfied patients to work with Allure until a resolution is reached, Judge Martinez noted in his decision. “At the very least, this would delay patients from posting such reviews and force patients to interact in some way with Allure, and it certainly appears to prohibit posting reviews until Allure agrees to some kind of favorable resolution.”
Surgeon Posted Fake Positive Reviews to Counteract Bad Reviews, AG Says
Employee accounts in court documents describe a physician fixated on reviews who went to great lengths to ensure positive reviews about his work outweighed the negative.
Former employees said they were instructed to track down patients who left negative reviews and either “threaten” them to take the posts down or offer them “money” or other things, according to Mr. Ferguson’s lawsuit. If patients could not be identified, the practice would file a defamation lawsuit against the anonymous person who posted the review and use litigation to subpoena the website for the reviewer’s IP address in order to identify them, according to court documents.
Employees testified they had regular meetings to review current negative reviews and discuss what steps they were taking to get them removed. At team meetings, in-house counsel would regularly present an Excel spreadsheet with updates on progress in getting patients to remove negative reviews, according to court documents.
In addition to restricting negative reviews, Mr. Ferguson accuses Dr. Sajan of posting fake positive reviews and “buying” thousands of fake followers on social media.
At Dr. Sajan’s direction, employees created Gmail accounts using stock photos for their profile pictures and used the accounts to post fake reviews of Allure Esthetic and Dr. Sajan, according to the complaint. The practice also used members of an online forum called BlackHatWorld.com to create fake email accounts and to post fake reviews, the attorney general alleges. Many of the fake positive reviews, including the fake Google reviews, still appear on online review sites today, the attorney general contends.
Dr. Sajan and his practice also allegedly manipulated social media to appear more popular. Mr. Ferguson claims that Dr. Sajan instructed his former web designer to purchase 60,000 followers through a vendor on BlackHatWorld.com. Most of Dr. Sajan’s current Instagram followers are not real, according to Mr. Ferguson.
The practice also used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media, according to court documents.
In addition, Dr. Sajan and his practice are accused of significantly altering “before and after” photos of patients and using fake email accounts to allow the clinic to take skincare rebates intended for patients.
All of these practices violated HIPAA, the state Consumer Protection Act (CPA) and the federal CRFA, according to Mr. Ferguson.
Surgeon Claims Competitor Behind Allegations
Attorneys for Dr. Sajan argue a competitor is behind the accusations and that other regulatory entities determined the practice did nothing wrong.
The competitor, a Seattle-based plastic surgeon, filed numerous complaints about Dr. Sajan to the Washington Medical Commission (WMC), according to court documents. The medical commission reviewed the third agreement and closed its investigation, finding that if the allegations were true, “no violation of law occurred,” court records show.
“Defendants relied upon this closing code from the WMC that the (non-disclosure) forms were lawful,” Dr. Sajan’s attorneys wrote in court documents.
The US Department of Health & Human Services Office for Civil Rights (OCR) also reviewed and audited Dr. Sajan’s use of the agreements, his attorneys noted. In a notice from OCR included in court exhibits, the agency wrote that all matters at issue have now been resolved through the practice’s voluntary compliance actions and that it was closing its investigation.
Attorneys for Dr. Sajan accuse Mr. Ferguson and state investigators of withholding the full extent of the competitor’s involvement in their investigation and failing to identify the competitor in written discovery or any of its initial disclosures. Dr. Sajan and his team discovered that the competitor was a source of key information through public records requests, according to court documents.
The remaining claims against Dr. Sajan will be addressed at trial, set for September 9, 2024.
A version of this article appeared on Medscape.com.
Docs Vent As Feds Investigate Private Equity, Consolidation in Medicine
As three federal agencies investigate how private equity ownership and consolidation of healthcare organizations affects patient care and costs, physicians are giving them an earful.
“Before I retired, I could already see the damage private equity was doing to hospitals and medical practices. Well-regarded physician groups were being bought and the respected doctors and staff forced out to squeeze out profit for the buyers. Hospital-based physicians were being hit especially hard,” wrote Rhonda Wright, MD, of Brookhaven, Georgia.
“Now, the rot is setting in for emergency rooms. One in four ERs is now (under-)staffed by private equity firms. This is leading to longer wait times, deterioration in patient care, and higher bills,” Dr. Wright continued. “Private equity takeover of medicine must be stopped. All such deals should be strictly regulated and should be heavily scrutinized, if not barred altogether. Our health depends upon it!”
The federal government is accepting public comments like Dr. Wright’s through June 5 and has even set up a website (healthycompetition.gov) to make it easier to file complaints against health organizations possibly violating antitrust laws.
The US Department of Justice’s Antitrust Division, the Federal Trade Commission (FTC), and the Department of Health and Human Services want to hear from physicians and the public about how private equity firms’ investments in healthcare entities, such as hospitals, nursing homes, or specialty service providers, affect patients and healthcare workers. The investigation will also evaluate how market pricing, competition, and referral patterns change when practices and hospitals are acquired by health systems or insurers.
Maintaining competition in the provider and payer markets benefits healthcare workers through higher pay, while patients can access quality care at lower prices, the joint request for information said. However, consolidation and mergers — potentially driven by private equity’s entry into the market — can diminish these benefits.
Investigating private equity and consolidation in medicine is part of the Biden Administration’s focus on lowering medical and prescription drug costs and strengthening competition in healthcare. The FTC’s vote last week to ban noncompete agreements, which business groups have vowed to challenge in court, falls under the same initiative.
Alexandra Nicole Thran, MD, FACEP, president of the Vermont Chapter of the American College of Emergency Physicians, said that the private equity business model is problematic because it ties physicians’ wages to patient satisfaction and the number of patients they see per hour.
A Connecticut primary care physician expressed similar sentiments. “Physicians are being forced into a system where corporations provide financial incentives and punitive policies to direct healthcare decisions towards a profitable aim,” said Eric Schwaber, MD.
While a majority of comments criticized the role of private equity and consolidation, some reflected a more positive view.
“Private equity helps make healthcare more efficient and effective. It brings needed operational and managerial expertise to allow for better patient care,” said Reenie Abraham, MD, an associate professor in the Department of Internal Medicine at University of Texas Southwestern Medical Center, Dallas. The University of Texas is facing a lawsuit involving the liability status of its physicians who work for a private equity-backed hospital partly owned by the university.
Several public comments point to the increasing market influence UnitedHealth Group (UHG) and other payers have obtained through recent acquisitions. Retired emergency room physician Scott Davis, MD, said that the “astronomical” rate of burnout among providers has been exacerbated by “the economic takeover of the healthcare system by…United Healthcare [and] private equity groups who put profits over anything else.”
The healthcare conglomerate employs approximately 10% of active US physicians, including many through its subsidiary, Optum Health, which provides primary, urgent, and surgical care. UHG has also invested heavily in acquiring physician practices to advance its value-based care model.
“If a publicly traded private insurance or private equity company is interested in their short-term quarterly profits or stock price, there is little interest in the…effective management of chronic disease, other than that which fulfills a ‘value-based’ metric,” wrote Kenneth Dolkart, MD, FACP, clinical assistant professor at the Dartmouth Geisel School of Medicine in Hanover, New Hampshire.
Sarah Ealy, a revenue cycle professional, commented that payers like UHG have outsized bargaining power when negotiating rates with providers. “In many states, United Healthcare and its subsidiaries pay a lower reimbursement rate than state Medicaid plans — these rates are nearly 50% of the breakeven per-visit rate that practices need to keep the lights on.”
Another comment ties the recent cyberattack on UHG-owned Change Healthcare to private equity ownership and “healthcare behemoths buying up practices and data.”
“The ramrodding of consolidation and private oversight with little to no barriers to foreign intrusions…is a testament to how ill prepared [the] US market is to private equity healthcare takeovers,” said SW Dermatology Practice LLC.
The agencies request comments from all health market participants, including physicians, nurses, employers, administrators, and patients.
A version of this article first appeared on Medscape.com.
As three federal agencies investigate how private equity ownership and consolidation of healthcare organizations affects patient care and costs, physicians are giving them an earful.
“Before I retired, I could already see the damage private equity was doing to hospitals and medical practices. Well-regarded physician groups were being bought and the respected doctors and staff forced out to squeeze out profit for the buyers. Hospital-based physicians were being hit especially hard,” wrote Rhonda Wright, MD, of Brookhaven, Georgia.
“Now, the rot is setting in for emergency rooms. One in four ERs is now (under-)staffed by private equity firms. This is leading to longer wait times, deterioration in patient care, and higher bills,” Dr. Wright continued. “Private equity takeover of medicine must be stopped. All such deals should be strictly regulated and should be heavily scrutinized, if not barred altogether. Our health depends upon it!”
The federal government is accepting public comments like Dr. Wright’s through June 5 and has even set up a website (healthycompetition.gov) to make it easier to file complaints against health organizations possibly violating antitrust laws.
The US Department of Justice’s Antitrust Division, the Federal Trade Commission (FTC), and the Department of Health and Human Services want to hear from physicians and the public about how private equity firms’ investments in healthcare entities, such as hospitals, nursing homes, or specialty service providers, affect patients and healthcare workers. The investigation will also evaluate how market pricing, competition, and referral patterns change when practices and hospitals are acquired by health systems or insurers.
Maintaining competition in the provider and payer markets benefits healthcare workers through higher pay, while patients can access quality care at lower prices, the joint request for information said. However, consolidation and mergers — potentially driven by private equity’s entry into the market — can diminish these benefits.
Investigating private equity and consolidation in medicine is part of the Biden Administration’s focus on lowering medical and prescription drug costs and strengthening competition in healthcare. The FTC’s vote last week to ban noncompete agreements, which business groups have vowed to challenge in court, falls under the same initiative.
Alexandra Nicole Thran, MD, FACEP, president of the Vermont Chapter of the American College of Emergency Physicians, said that the private equity business model is problematic because it ties physicians’ wages to patient satisfaction and the number of patients they see per hour.
A Connecticut primary care physician expressed similar sentiments. “Physicians are being forced into a system where corporations provide financial incentives and punitive policies to direct healthcare decisions towards a profitable aim,” said Eric Schwaber, MD.
While a majority of comments criticized the role of private equity and consolidation, some reflected a more positive view.
“Private equity helps make healthcare more efficient and effective. It brings needed operational and managerial expertise to allow for better patient care,” said Reenie Abraham, MD, an associate professor in the Department of Internal Medicine at University of Texas Southwestern Medical Center, Dallas. The University of Texas is facing a lawsuit involving the liability status of its physicians who work for a private equity-backed hospital partly owned by the university.
Several public comments point to the increasing market influence UnitedHealth Group (UHG) and other payers have obtained through recent acquisitions. Retired emergency room physician Scott Davis, MD, said that the “astronomical” rate of burnout among providers has been exacerbated by “the economic takeover of the healthcare system by…United Healthcare [and] private equity groups who put profits over anything else.”
The healthcare conglomerate employs approximately 10% of active US physicians, including many through its subsidiary, Optum Health, which provides primary, urgent, and surgical care. UHG has also invested heavily in acquiring physician practices to advance its value-based care model.
“If a publicly traded private insurance or private equity company is interested in their short-term quarterly profits or stock price, there is little interest in the…effective management of chronic disease, other than that which fulfills a ‘value-based’ metric,” wrote Kenneth Dolkart, MD, FACP, clinical assistant professor at the Dartmouth Geisel School of Medicine in Hanover, New Hampshire.
Sarah Ealy, a revenue cycle professional, commented that payers like UHG have outsized bargaining power when negotiating rates with providers. “In many states, United Healthcare and its subsidiaries pay a lower reimbursement rate than state Medicaid plans — these rates are nearly 50% of the breakeven per-visit rate that practices need to keep the lights on.”
Another comment ties the recent cyberattack on UHG-owned Change Healthcare to private equity ownership and “healthcare behemoths buying up practices and data.”
“The ramrodding of consolidation and private oversight with little to no barriers to foreign intrusions…is a testament to how ill prepared [the] US market is to private equity healthcare takeovers,” said SW Dermatology Practice LLC.
The agencies request comments from all health market participants, including physicians, nurses, employers, administrators, and patients.
A version of this article first appeared on Medscape.com.
As three federal agencies investigate how private equity ownership and consolidation of healthcare organizations affects patient care and costs, physicians are giving them an earful.
“Before I retired, I could already see the damage private equity was doing to hospitals and medical practices. Well-regarded physician groups were being bought and the respected doctors and staff forced out to squeeze out profit for the buyers. Hospital-based physicians were being hit especially hard,” wrote Rhonda Wright, MD, of Brookhaven, Georgia.
“Now, the rot is setting in for emergency rooms. One in four ERs is now (under-)staffed by private equity firms. This is leading to longer wait times, deterioration in patient care, and higher bills,” Dr. Wright continued. “Private equity takeover of medicine must be stopped. All such deals should be strictly regulated and should be heavily scrutinized, if not barred altogether. Our health depends upon it!”
The federal government is accepting public comments like Dr. Wright’s through June 5 and has even set up a website (healthycompetition.gov) to make it easier to file complaints against health organizations possibly violating antitrust laws.
The US Department of Justice’s Antitrust Division, the Federal Trade Commission (FTC), and the Department of Health and Human Services want to hear from physicians and the public about how private equity firms’ investments in healthcare entities, such as hospitals, nursing homes, or specialty service providers, affect patients and healthcare workers. The investigation will also evaluate how market pricing, competition, and referral patterns change when practices and hospitals are acquired by health systems or insurers.
Maintaining competition in the provider and payer markets benefits healthcare workers through higher pay, while patients can access quality care at lower prices, the joint request for information said. However, consolidation and mergers — potentially driven by private equity’s entry into the market — can diminish these benefits.
Investigating private equity and consolidation in medicine is part of the Biden Administration’s focus on lowering medical and prescription drug costs and strengthening competition in healthcare. The FTC’s vote last week to ban noncompete agreements, which business groups have vowed to challenge in court, falls under the same initiative.
Alexandra Nicole Thran, MD, FACEP, president of the Vermont Chapter of the American College of Emergency Physicians, said that the private equity business model is problematic because it ties physicians’ wages to patient satisfaction and the number of patients they see per hour.
A Connecticut primary care physician expressed similar sentiments. “Physicians are being forced into a system where corporations provide financial incentives and punitive policies to direct healthcare decisions towards a profitable aim,” said Eric Schwaber, MD.
While a majority of comments criticized the role of private equity and consolidation, some reflected a more positive view.
“Private equity helps make healthcare more efficient and effective. It brings needed operational and managerial expertise to allow for better patient care,” said Reenie Abraham, MD, an associate professor in the Department of Internal Medicine at University of Texas Southwestern Medical Center, Dallas. The University of Texas is facing a lawsuit involving the liability status of its physicians who work for a private equity-backed hospital partly owned by the university.
Several public comments point to the increasing market influence UnitedHealth Group (UHG) and other payers have obtained through recent acquisitions. Retired emergency room physician Scott Davis, MD, said that the “astronomical” rate of burnout among providers has been exacerbated by “the economic takeover of the healthcare system by…United Healthcare [and] private equity groups who put profits over anything else.”
The healthcare conglomerate employs approximately 10% of active US physicians, including many through its subsidiary, Optum Health, which provides primary, urgent, and surgical care. UHG has also invested heavily in acquiring physician practices to advance its value-based care model.
“If a publicly traded private insurance or private equity company is interested in their short-term quarterly profits or stock price, there is little interest in the…effective management of chronic disease, other than that which fulfills a ‘value-based’ metric,” wrote Kenneth Dolkart, MD, FACP, clinical assistant professor at the Dartmouth Geisel School of Medicine in Hanover, New Hampshire.
Sarah Ealy, a revenue cycle professional, commented that payers like UHG have outsized bargaining power when negotiating rates with providers. “In many states, United Healthcare and its subsidiaries pay a lower reimbursement rate than state Medicaid plans — these rates are nearly 50% of the breakeven per-visit rate that practices need to keep the lights on.”
Another comment ties the recent cyberattack on UHG-owned Change Healthcare to private equity ownership and “healthcare behemoths buying up practices and data.”
“The ramrodding of consolidation and private oversight with little to no barriers to foreign intrusions…is a testament to how ill prepared [the] US market is to private equity healthcare takeovers,” said SW Dermatology Practice LLC.
The agencies request comments from all health market participants, including physicians, nurses, employers, administrators, and patients.
A version of this article first appeared on Medscape.com.
Do Health-Related Social Needs Raise Mortality Risk in Cancer Survivors?
Little is known about the specific association between health-related social needs (HRSNs) and mortality risk even though HRSNs, defined as challenges in affording food, housing, and other necessities of daily living, are potential challenges for cancer survivors, wrote Zhiyuan Zheng, PhD, of the American Cancer Society, Atlanta, and colleagues.
A 2020 study by Dr. Zheng and colleagues published in the Journal of the National Comprehensive Cancer Network (NCCN) showed that food insecurity and financial worries had a negative impact on cancer survivorship. In the new study, published in Cancer, the researchers identified cancer survivors using the 2013-2018 National Health Interview Survey (NHIS) and the NHIS Mortality File through December 31, 2019. The researchers examined mortality using the data from the Centers for Disease Control and Prevention’s National Death Index (NDI) through December 31, 2019, which links to the National Health Interview Survey Data used in the study.
Individuals’ HRSNs were categorized into three groups: severe, moderate, and minor/none. HRSNs included food insecurity and nonmedical financial concerns, such as housing costs (rent, mortgage). Medical financial hardship included material, psychological, and behavioral domains and was divided into three groups: 2-3 domains, 1 domain, or 0 domains.
What Are the Potential Financial Implications of this Research?
The high costs of cancer care often cause medical financial hardships for cancer survivors, and expenses also may cause psychological distress and nonmedical financial hardship as survivors try to make ends meet while facing medical bills, wrote Dr. Zheng and colleagues.
Policy makers are increasingly interested in adding HRSNs to insurance coverage; recent guidance from the Centers for Medicare & Medicaid Services (CMS) allows individual states to apply to provide nutrition and housing supports through state Medicaid programs, according to authors of a 2023 article published in JAMA Health Forum.
The new study adds to the understanding of how HRSNs impact people with cancer by examining the association with mortality risk, Yelak Biru, MSc, president and chief executive officer of the International Myeloma Foundation, said in an interview.
“This is a key area of study for addressing the disparities in treatments and outcomes that result in inequities,” said Mr. Biru, a patient advocate and multiple myeloma survivor who was not involved in the study.
What Does the New Study Show?
The new study characterized HRSNs in 5,855 adult cancer survivors aged 18-64 years and 5,918 aged 65-79 years. In the 18- to 64-year-old group, 25.5% reported moderate levels of HRSNs, and 18.3% reported severe HRSNs. In patients aged 65-79 years, 15.6% and 6.6% reported moderate HRSNs and severe HRSNs, respectively.
Severe HRSN was significantly associated with higher mortality risk in an adjusted analysis in patients aged 18-64 years (hazard ratio 2.00, P < .001).
Among adults aged 65-79 years, severe HRSN was not associated with higher mortality risk; however, in this older age group, those with 2-3 domains of medical financial hardship had significantly increased mortality risk compared with adults aged 65-79 years with zero domains of medical financial hardship (HR 1.58, P = .007).
Although the findings that HRSNs were associated with increased mortality risk, especially in the younger group, were not surprising, they serve as a call to action to address how HRSNs are contributing to cancer mortality, Mr. Biru said in an interview. “HRSNs, like food or housing insecurity, can lead to patients being unable to undergo the best treatment approach for their cancer,” he said.
What Are the Limitations and Research Gaps?
The study findings were limited by several factors including the use of self-reports to measure medical financial hardship, food insecurity, and nonmedical financial concerns in the NHIS, the researchers wrote in their discussion. More research with longer follow-up time beyond 1-5 years is needed, wrote Dr. Zheng and colleagues.
Studies also are needed to illustrate how patient navigation can help prevent patients from falling through the cracks with regard to social needs and financial hardships, Mr. Biru told this news organization.
Other areas for research include how addressing social needs affects health outcomes and whether programs designed to address social needs are effective, he said.
“Finally, qualitative research is needed to capture the lived experiences of cancer survivors facing these challenges. This knowledge can inform the development of more patient-centered interventions and policies that effectively address the social determinants of health and improve overall outcomes for all cancer survivors,” Mr. Biru said.
What Is the Takeaway Message for Clinicians?
HRSNs and financial hardship are significantly associated with increased risk of mortality in adult cancer survivors, Dr. Zheng and colleagues concluded. Looking ahead, comprehensive assessment of HRSNs and financial hardship may help clinicians connect patients with relevant services to mitigate the social and financial impacts of cancer, they wrote.
“The takeaway message for oncologists in practice is that addressing [HRSNs] and financial hardship is crucial for providing comprehensive and equitable cancer care,” Mr. Biru said during his interview.
“The impact of social determinants of health on cancer outcomes cannot be ignored, and oncologists play a vital role in identifying and addressing these needs,” he said. Sensitive, discussion-based screenings are needed to identify core needs such as food and transportation, but clinicians also can consider broader social factors and work with a team to connect patients to appropriate resources, he added.
“By recognizing the importance of HRSN screening and taking proactive steps to address these needs, oncologists can contribute to improving health outcomes, reducing healthcare disparities, and providing more equitable cancer care for their patients,” he said.
What Other Guidance Is Available?
“High-quality cancer care requires treating the whole person; measuring and addressing anything in their life that could result in poorer health outcomes is a key component of comprehensive care,” Mr. Biru emphasized.
In September 2023, the National Comprehensive Cancer Network (NCCN) convened a working group cochaired by Mr. Biru that developed recommendations for how oncology practices should routinely measure HRSNs (NCCN.org/social-needs).
“The working group proposed that every cancer patient be assessed for food, transportation access, and financial and housing security at least once a year, and be reassessed at every care transition point as well,” Mr. Biru said. Such screenings should include follow-up to connect patients with services to address any HRSNs they are experiencing, he added.
Lead author Dr. Zheng is employed by the American Cancer Society, which as a nonprofit receives funds from the public through fundraising and contributions, as well as some support from corporations and industry to support its mission programs and services. Mr. Biru had no financial conflicts to disclose.
Little is known about the specific association between health-related social needs (HRSNs) and mortality risk even though HRSNs, defined as challenges in affording food, housing, and other necessities of daily living, are potential challenges for cancer survivors, wrote Zhiyuan Zheng, PhD, of the American Cancer Society, Atlanta, and colleagues.
A 2020 study by Dr. Zheng and colleagues published in the Journal of the National Comprehensive Cancer Network (NCCN) showed that food insecurity and financial worries had a negative impact on cancer survivorship. In the new study, published in Cancer, the researchers identified cancer survivors using the 2013-2018 National Health Interview Survey (NHIS) and the NHIS Mortality File through December 31, 2019. The researchers examined mortality using the data from the Centers for Disease Control and Prevention’s National Death Index (NDI) through December 31, 2019, which links to the National Health Interview Survey Data used in the study.
Individuals’ HRSNs were categorized into three groups: severe, moderate, and minor/none. HRSNs included food insecurity and nonmedical financial concerns, such as housing costs (rent, mortgage). Medical financial hardship included material, psychological, and behavioral domains and was divided into three groups: 2-3 domains, 1 domain, or 0 domains.
What Are the Potential Financial Implications of this Research?
The high costs of cancer care often cause medical financial hardships for cancer survivors, and expenses also may cause psychological distress and nonmedical financial hardship as survivors try to make ends meet while facing medical bills, wrote Dr. Zheng and colleagues.
Policy makers are increasingly interested in adding HRSNs to insurance coverage; recent guidance from the Centers for Medicare & Medicaid Services (CMS) allows individual states to apply to provide nutrition and housing supports through state Medicaid programs, according to authors of a 2023 article published in JAMA Health Forum.
The new study adds to the understanding of how HRSNs impact people with cancer by examining the association with mortality risk, Yelak Biru, MSc, president and chief executive officer of the International Myeloma Foundation, said in an interview.
“This is a key area of study for addressing the disparities in treatments and outcomes that result in inequities,” said Mr. Biru, a patient advocate and multiple myeloma survivor who was not involved in the study.
What Does the New Study Show?
The new study characterized HRSNs in 5,855 adult cancer survivors aged 18-64 years and 5,918 aged 65-79 years. In the 18- to 64-year-old group, 25.5% reported moderate levels of HRSNs, and 18.3% reported severe HRSNs. In patients aged 65-79 years, 15.6% and 6.6% reported moderate HRSNs and severe HRSNs, respectively.
Severe HRSN was significantly associated with higher mortality risk in an adjusted analysis in patients aged 18-64 years (hazard ratio 2.00, P < .001).
Among adults aged 65-79 years, severe HRSN was not associated with higher mortality risk; however, in this older age group, those with 2-3 domains of medical financial hardship had significantly increased mortality risk compared with adults aged 65-79 years with zero domains of medical financial hardship (HR 1.58, P = .007).
Although the findings that HRSNs were associated with increased mortality risk, especially in the younger group, were not surprising, they serve as a call to action to address how HRSNs are contributing to cancer mortality, Mr. Biru said in an interview. “HRSNs, like food or housing insecurity, can lead to patients being unable to undergo the best treatment approach for their cancer,” he said.
What Are the Limitations and Research Gaps?
The study findings were limited by several factors including the use of self-reports to measure medical financial hardship, food insecurity, and nonmedical financial concerns in the NHIS, the researchers wrote in their discussion. More research with longer follow-up time beyond 1-5 years is needed, wrote Dr. Zheng and colleagues.
Studies also are needed to illustrate how patient navigation can help prevent patients from falling through the cracks with regard to social needs and financial hardships, Mr. Biru told this news organization.
Other areas for research include how addressing social needs affects health outcomes and whether programs designed to address social needs are effective, he said.
“Finally, qualitative research is needed to capture the lived experiences of cancer survivors facing these challenges. This knowledge can inform the development of more patient-centered interventions and policies that effectively address the social determinants of health and improve overall outcomes for all cancer survivors,” Mr. Biru said.
What Is the Takeaway Message for Clinicians?
HRSNs and financial hardship are significantly associated with increased risk of mortality in adult cancer survivors, Dr. Zheng and colleagues concluded. Looking ahead, comprehensive assessment of HRSNs and financial hardship may help clinicians connect patients with relevant services to mitigate the social and financial impacts of cancer, they wrote.
“The takeaway message for oncologists in practice is that addressing [HRSNs] and financial hardship is crucial for providing comprehensive and equitable cancer care,” Mr. Biru said during his interview.
“The impact of social determinants of health on cancer outcomes cannot be ignored, and oncologists play a vital role in identifying and addressing these needs,” he said. Sensitive, discussion-based screenings are needed to identify core needs such as food and transportation, but clinicians also can consider broader social factors and work with a team to connect patients to appropriate resources, he added.
“By recognizing the importance of HRSN screening and taking proactive steps to address these needs, oncologists can contribute to improving health outcomes, reducing healthcare disparities, and providing more equitable cancer care for their patients,” he said.
What Other Guidance Is Available?
“High-quality cancer care requires treating the whole person; measuring and addressing anything in their life that could result in poorer health outcomes is a key component of comprehensive care,” Mr. Biru emphasized.
In September 2023, the National Comprehensive Cancer Network (NCCN) convened a working group cochaired by Mr. Biru that developed recommendations for how oncology practices should routinely measure HRSNs (NCCN.org/social-needs).
“The working group proposed that every cancer patient be assessed for food, transportation access, and financial and housing security at least once a year, and be reassessed at every care transition point as well,” Mr. Biru said. Such screenings should include follow-up to connect patients with services to address any HRSNs they are experiencing, he added.
Lead author Dr. Zheng is employed by the American Cancer Society, which as a nonprofit receives funds from the public through fundraising and contributions, as well as some support from corporations and industry to support its mission programs and services. Mr. Biru had no financial conflicts to disclose.
Little is known about the specific association between health-related social needs (HRSNs) and mortality risk even though HRSNs, defined as challenges in affording food, housing, and other necessities of daily living, are potential challenges for cancer survivors, wrote Zhiyuan Zheng, PhD, of the American Cancer Society, Atlanta, and colleagues.
A 2020 study by Dr. Zheng and colleagues published in the Journal of the National Comprehensive Cancer Network (NCCN) showed that food insecurity and financial worries had a negative impact on cancer survivorship. In the new study, published in Cancer, the researchers identified cancer survivors using the 2013-2018 National Health Interview Survey (NHIS) and the NHIS Mortality File through December 31, 2019. The researchers examined mortality using the data from the Centers for Disease Control and Prevention’s National Death Index (NDI) through December 31, 2019, which links to the National Health Interview Survey Data used in the study.
Individuals’ HRSNs were categorized into three groups: severe, moderate, and minor/none. HRSNs included food insecurity and nonmedical financial concerns, such as housing costs (rent, mortgage). Medical financial hardship included material, psychological, and behavioral domains and was divided into three groups: 2-3 domains, 1 domain, or 0 domains.
What Are the Potential Financial Implications of this Research?
The high costs of cancer care often cause medical financial hardships for cancer survivors, and expenses also may cause psychological distress and nonmedical financial hardship as survivors try to make ends meet while facing medical bills, wrote Dr. Zheng and colleagues.
Policy makers are increasingly interested in adding HRSNs to insurance coverage; recent guidance from the Centers for Medicare & Medicaid Services (CMS) allows individual states to apply to provide nutrition and housing supports through state Medicaid programs, according to authors of a 2023 article published in JAMA Health Forum.
The new study adds to the understanding of how HRSNs impact people with cancer by examining the association with mortality risk, Yelak Biru, MSc, president and chief executive officer of the International Myeloma Foundation, said in an interview.
“This is a key area of study for addressing the disparities in treatments and outcomes that result in inequities,” said Mr. Biru, a patient advocate and multiple myeloma survivor who was not involved in the study.
What Does the New Study Show?
The new study characterized HRSNs in 5,855 adult cancer survivors aged 18-64 years and 5,918 aged 65-79 years. In the 18- to 64-year-old group, 25.5% reported moderate levels of HRSNs, and 18.3% reported severe HRSNs. In patients aged 65-79 years, 15.6% and 6.6% reported moderate HRSNs and severe HRSNs, respectively.
Severe HRSN was significantly associated with higher mortality risk in an adjusted analysis in patients aged 18-64 years (hazard ratio 2.00, P < .001).
Among adults aged 65-79 years, severe HRSN was not associated with higher mortality risk; however, in this older age group, those with 2-3 domains of medical financial hardship had significantly increased mortality risk compared with adults aged 65-79 years with zero domains of medical financial hardship (HR 1.58, P = .007).
Although the findings that HRSNs were associated with increased mortality risk, especially in the younger group, were not surprising, they serve as a call to action to address how HRSNs are contributing to cancer mortality, Mr. Biru said in an interview. “HRSNs, like food or housing insecurity, can lead to patients being unable to undergo the best treatment approach for their cancer,” he said.
What Are the Limitations and Research Gaps?
The study findings were limited by several factors including the use of self-reports to measure medical financial hardship, food insecurity, and nonmedical financial concerns in the NHIS, the researchers wrote in their discussion. More research with longer follow-up time beyond 1-5 years is needed, wrote Dr. Zheng and colleagues.
Studies also are needed to illustrate how patient navigation can help prevent patients from falling through the cracks with regard to social needs and financial hardships, Mr. Biru told this news organization.
Other areas for research include how addressing social needs affects health outcomes and whether programs designed to address social needs are effective, he said.
“Finally, qualitative research is needed to capture the lived experiences of cancer survivors facing these challenges. This knowledge can inform the development of more patient-centered interventions and policies that effectively address the social determinants of health and improve overall outcomes for all cancer survivors,” Mr. Biru said.
What Is the Takeaway Message for Clinicians?
HRSNs and financial hardship are significantly associated with increased risk of mortality in adult cancer survivors, Dr. Zheng and colleagues concluded. Looking ahead, comprehensive assessment of HRSNs and financial hardship may help clinicians connect patients with relevant services to mitigate the social and financial impacts of cancer, they wrote.
“The takeaway message for oncologists in practice is that addressing [HRSNs] and financial hardship is crucial for providing comprehensive and equitable cancer care,” Mr. Biru said during his interview.
“The impact of social determinants of health on cancer outcomes cannot be ignored, and oncologists play a vital role in identifying and addressing these needs,” he said. Sensitive, discussion-based screenings are needed to identify core needs such as food and transportation, but clinicians also can consider broader social factors and work with a team to connect patients to appropriate resources, he added.
“By recognizing the importance of HRSN screening and taking proactive steps to address these needs, oncologists can contribute to improving health outcomes, reducing healthcare disparities, and providing more equitable cancer care for their patients,” he said.
What Other Guidance Is Available?
“High-quality cancer care requires treating the whole person; measuring and addressing anything in their life that could result in poorer health outcomes is a key component of comprehensive care,” Mr. Biru emphasized.
In September 2023, the National Comprehensive Cancer Network (NCCN) convened a working group cochaired by Mr. Biru that developed recommendations for how oncology practices should routinely measure HRSNs (NCCN.org/social-needs).
“The working group proposed that every cancer patient be assessed for food, transportation access, and financial and housing security at least once a year, and be reassessed at every care transition point as well,” Mr. Biru said. Such screenings should include follow-up to connect patients with services to address any HRSNs they are experiencing, he added.
Lead author Dr. Zheng is employed by the American Cancer Society, which as a nonprofit receives funds from the public through fundraising and contributions, as well as some support from corporations and industry to support its mission programs and services. Mr. Biru had no financial conflicts to disclose.
FROM CANCER
Terminal Cancer: What Matters to Patients and Caregivers
New research found that patients and caregivers both tend to prioritize symptom control over life extension but often preferring a balance. Patients and caregivers, however, are less aligned on decisions about cost containment, with patients more likely to prioritize cost containment.
“Our research has revealed that patients and caregivers generally share similar end-of-life goals,” with a “notable exception” when it comes to costs, first author Semra Ozdemir, PhD, with the Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore, told this news organization.
However, when patients and caregivers have a better understanding of the patient’s prognosis, both may be more inclined to avoid costly life-extending treatments and prioritize symptom management.
In other words, the survey suggests that “knowing the prognosis helps patients and their families set realistic expectations for care and adequately prepare for end-of-life decisions,” said Dr. Ozdemir.
This study was published online in JAMA Network Open.
Patients with advanced cancer often face difficult decisions: Do they opt for treatments that may — or may not — extend life or do they focus more on symptom control?
Family caregivers, who also play an important role in this decision-making process, may have different care goals. Some research suggests that caregivers tend to prioritize treatments that could extend life, whereas patients prioritize symptom management, but it’s less clear how these priorities may change over time and how patients and caregivers may influence each other.
In the current study, the researchers examined goals of care among patients with stage IV solid tumors and caregivers during the last 2 years of life, focusing on life extension vs symptom management and cost containment, as well as how these goals changed over time.
The survey included 210 patient-caregiver pairs, recruited from outpatient clinics at two major cancer centers in Singapore. Patients had a mean age of 63 years, and about half were men. The caregivers had a mean age of 49 years, and almost two third (63%) were women.
Overall, 34% patients and 29% caregivers prioritized symptom management over life extension, whereas 24% patients and 19% caregivers prioritized life extension. Most patients and caregivers preferred balancing the two, with 34%-47% patients and 37%-69% caregivers supporting this approach.
When balancing cost and treatment decisions, however, patients were more likely to prioritize containing costs — 28% vs 17% for caregivers — over extending life — 26% of patients vs 35% of caregivers.
Cost containment tended to be more of a priority for older patients, those with a higher symptom burden, and those with less family caregiver support. For caregivers, cost containment was more of a priority for those who reported that caregiving had a big impact on their finances, those with worse self-esteem related to their caregiving abilities, as well as those caring for older patients.
To better align cost containment priorities between patients and caregivers, it’s essential for families to engage in open and thorough discussions about the allocation of resources, Dr. Ozdemir said.
Although “patients, families, and physicians often avoid discussions about prognosis,” such conversations are essential for setting realistic expectations for care and adequately preparing for end-of-life decisions, Dr. Ozdemir told this news organization.
“These conversations should aim to balance competing interests and create care plans that are mutually acceptable to both patients and caregivers,” she said, adding that “this approach will help in minimizing any potential conflicts and ensure that both parties feel respected and understood in their decision-making process.”
Managing Unrealistic Expectations
As patients approached the end of life, neither patients nor caregivers shifted their priorities from life extension to symptom management.
This finding raises concerns because it suggests that many patients hold unrealistic expectations regarding their care and “underscores the need for continuous dialogue and reassessment of care goals throughout the progression of illness,” Dr. Ozdemir said.
“This stability in preferences over time suggests that initial care decisions are deeply ingrained or that there may be a lack of ongoing communication about evolving care needs and possibilities as conditions change,” Ozdemir said.
Yet, it can be hard to define what unrealistic expectations mean, said Olivia Seecof, MD, who wasn’t involved in the study.
“I think people are hopeful that a devastating diagnosis won’t lead to the end of their life and that there will be a treatment or something that will change [their prognosis], and they’ll get better,” said Dr. Seecof, palliative care expert with the Supportive Oncology Program at NYU Langone Health’s Perlmutter Cancer Center in New York City.
Giving patients and caregivers a realistic understanding of the prognosis is important, but “there’s more to it than just telling the patient their diagnosis,” she said.
“We have to plan for end of life, what it can look like,” said Dr. Seecof, adding that “often we don’t do a very good job of talking about that early on in an illness course.”
Overall, though, Dr. Seecof stressed that no two patients or situations are the same, and it’s important to understand what’s important in each scenario. End-of-life care requires “an individual approach because every patient is different, even if they have the same diagnosis as someone else,” she said.
This work was supported by funding from the Singapore Millennium Foundation and the Lien Centre for Palliative Care. Dr. Ozdemir and Dr. Seecof had no relevant disclosures.
A version of this article appeared on Medscape.com.
New research found that patients and caregivers both tend to prioritize symptom control over life extension but often preferring a balance. Patients and caregivers, however, are less aligned on decisions about cost containment, with patients more likely to prioritize cost containment.
“Our research has revealed that patients and caregivers generally share similar end-of-life goals,” with a “notable exception” when it comes to costs, first author Semra Ozdemir, PhD, with the Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore, told this news organization.
However, when patients and caregivers have a better understanding of the patient’s prognosis, both may be more inclined to avoid costly life-extending treatments and prioritize symptom management.
In other words, the survey suggests that “knowing the prognosis helps patients and their families set realistic expectations for care and adequately prepare for end-of-life decisions,” said Dr. Ozdemir.
This study was published online in JAMA Network Open.
Patients with advanced cancer often face difficult decisions: Do they opt for treatments that may — or may not — extend life or do they focus more on symptom control?
Family caregivers, who also play an important role in this decision-making process, may have different care goals. Some research suggests that caregivers tend to prioritize treatments that could extend life, whereas patients prioritize symptom management, but it’s less clear how these priorities may change over time and how patients and caregivers may influence each other.
In the current study, the researchers examined goals of care among patients with stage IV solid tumors and caregivers during the last 2 years of life, focusing on life extension vs symptom management and cost containment, as well as how these goals changed over time.
The survey included 210 patient-caregiver pairs, recruited from outpatient clinics at two major cancer centers in Singapore. Patients had a mean age of 63 years, and about half were men. The caregivers had a mean age of 49 years, and almost two third (63%) were women.
Overall, 34% patients and 29% caregivers prioritized symptom management over life extension, whereas 24% patients and 19% caregivers prioritized life extension. Most patients and caregivers preferred balancing the two, with 34%-47% patients and 37%-69% caregivers supporting this approach.
When balancing cost and treatment decisions, however, patients were more likely to prioritize containing costs — 28% vs 17% for caregivers — over extending life — 26% of patients vs 35% of caregivers.
Cost containment tended to be more of a priority for older patients, those with a higher symptom burden, and those with less family caregiver support. For caregivers, cost containment was more of a priority for those who reported that caregiving had a big impact on their finances, those with worse self-esteem related to their caregiving abilities, as well as those caring for older patients.
To better align cost containment priorities between patients and caregivers, it’s essential for families to engage in open and thorough discussions about the allocation of resources, Dr. Ozdemir said.
Although “patients, families, and physicians often avoid discussions about prognosis,” such conversations are essential for setting realistic expectations for care and adequately preparing for end-of-life decisions, Dr. Ozdemir told this news organization.
“These conversations should aim to balance competing interests and create care plans that are mutually acceptable to both patients and caregivers,” she said, adding that “this approach will help in minimizing any potential conflicts and ensure that both parties feel respected and understood in their decision-making process.”
Managing Unrealistic Expectations
As patients approached the end of life, neither patients nor caregivers shifted their priorities from life extension to symptom management.
This finding raises concerns because it suggests that many patients hold unrealistic expectations regarding their care and “underscores the need for continuous dialogue and reassessment of care goals throughout the progression of illness,” Dr. Ozdemir said.
“This stability in preferences over time suggests that initial care decisions are deeply ingrained or that there may be a lack of ongoing communication about evolving care needs and possibilities as conditions change,” Ozdemir said.
Yet, it can be hard to define what unrealistic expectations mean, said Olivia Seecof, MD, who wasn’t involved in the study.
“I think people are hopeful that a devastating diagnosis won’t lead to the end of their life and that there will be a treatment or something that will change [their prognosis], and they’ll get better,” said Dr. Seecof, palliative care expert with the Supportive Oncology Program at NYU Langone Health’s Perlmutter Cancer Center in New York City.
Giving patients and caregivers a realistic understanding of the prognosis is important, but “there’s more to it than just telling the patient their diagnosis,” she said.
“We have to plan for end of life, what it can look like,” said Dr. Seecof, adding that “often we don’t do a very good job of talking about that early on in an illness course.”
Overall, though, Dr. Seecof stressed that no two patients or situations are the same, and it’s important to understand what’s important in each scenario. End-of-life care requires “an individual approach because every patient is different, even if they have the same diagnosis as someone else,” she said.
This work was supported by funding from the Singapore Millennium Foundation and the Lien Centre for Palliative Care. Dr. Ozdemir and Dr. Seecof had no relevant disclosures.
A version of this article appeared on Medscape.com.
New research found that patients and caregivers both tend to prioritize symptom control over life extension but often preferring a balance. Patients and caregivers, however, are less aligned on decisions about cost containment, with patients more likely to prioritize cost containment.
“Our research has revealed that patients and caregivers generally share similar end-of-life goals,” with a “notable exception” when it comes to costs, first author Semra Ozdemir, PhD, with the Lien Centre for Palliative Care, Duke-NUS Medical School, Singapore, told this news organization.
However, when patients and caregivers have a better understanding of the patient’s prognosis, both may be more inclined to avoid costly life-extending treatments and prioritize symptom management.
In other words, the survey suggests that “knowing the prognosis helps patients and their families set realistic expectations for care and adequately prepare for end-of-life decisions,” said Dr. Ozdemir.
This study was published online in JAMA Network Open.
Patients with advanced cancer often face difficult decisions: Do they opt for treatments that may — or may not — extend life or do they focus more on symptom control?
Family caregivers, who also play an important role in this decision-making process, may have different care goals. Some research suggests that caregivers tend to prioritize treatments that could extend life, whereas patients prioritize symptom management, but it’s less clear how these priorities may change over time and how patients and caregivers may influence each other.
In the current study, the researchers examined goals of care among patients with stage IV solid tumors and caregivers during the last 2 years of life, focusing on life extension vs symptom management and cost containment, as well as how these goals changed over time.
The survey included 210 patient-caregiver pairs, recruited from outpatient clinics at two major cancer centers in Singapore. Patients had a mean age of 63 years, and about half were men. The caregivers had a mean age of 49 years, and almost two third (63%) were women.
Overall, 34% patients and 29% caregivers prioritized symptom management over life extension, whereas 24% patients and 19% caregivers prioritized life extension. Most patients and caregivers preferred balancing the two, with 34%-47% patients and 37%-69% caregivers supporting this approach.
When balancing cost and treatment decisions, however, patients were more likely to prioritize containing costs — 28% vs 17% for caregivers — over extending life — 26% of patients vs 35% of caregivers.
Cost containment tended to be more of a priority for older patients, those with a higher symptom burden, and those with less family caregiver support. For caregivers, cost containment was more of a priority for those who reported that caregiving had a big impact on their finances, those with worse self-esteem related to their caregiving abilities, as well as those caring for older patients.
To better align cost containment priorities between patients and caregivers, it’s essential for families to engage in open and thorough discussions about the allocation of resources, Dr. Ozdemir said.
Although “patients, families, and physicians often avoid discussions about prognosis,” such conversations are essential for setting realistic expectations for care and adequately preparing for end-of-life decisions, Dr. Ozdemir told this news organization.
“These conversations should aim to balance competing interests and create care plans that are mutually acceptable to both patients and caregivers,” she said, adding that “this approach will help in minimizing any potential conflicts and ensure that both parties feel respected and understood in their decision-making process.”
Managing Unrealistic Expectations
As patients approached the end of life, neither patients nor caregivers shifted their priorities from life extension to symptom management.
This finding raises concerns because it suggests that many patients hold unrealistic expectations regarding their care and “underscores the need for continuous dialogue and reassessment of care goals throughout the progression of illness,” Dr. Ozdemir said.
“This stability in preferences over time suggests that initial care decisions are deeply ingrained or that there may be a lack of ongoing communication about evolving care needs and possibilities as conditions change,” Ozdemir said.
Yet, it can be hard to define what unrealistic expectations mean, said Olivia Seecof, MD, who wasn’t involved in the study.
“I think people are hopeful that a devastating diagnosis won’t lead to the end of their life and that there will be a treatment or something that will change [their prognosis], and they’ll get better,” said Dr. Seecof, palliative care expert with the Supportive Oncology Program at NYU Langone Health’s Perlmutter Cancer Center in New York City.
Giving patients and caregivers a realistic understanding of the prognosis is important, but “there’s more to it than just telling the patient their diagnosis,” she said.
“We have to plan for end of life, what it can look like,” said Dr. Seecof, adding that “often we don’t do a very good job of talking about that early on in an illness course.”
Overall, though, Dr. Seecof stressed that no two patients or situations are the same, and it’s important to understand what’s important in each scenario. End-of-life care requires “an individual approach because every patient is different, even if they have the same diagnosis as someone else,” she said.
This work was supported by funding from the Singapore Millennium Foundation and the Lien Centre for Palliative Care. Dr. Ozdemir and Dr. Seecof had no relevant disclosures.
A version of this article appeared on Medscape.com.
Understanding the Evaluation and Management Add-on Complexity Code
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
On January 1, 2024, a new add-on complexity code, G2211, was implemented to the documentation of evaluation and management (E/M) visits.1 Created by the Centers for Medicare & Medicaid Services (CMS), G2211 is defined as “visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex condition.”2 It is an add-on code, meaning that it must be listed with either a new or established outpatient E/M visit.
G2211 originally was introduced in the 2021 Proposed Rule but was delayed via a congressional mandate for 3 years.1 It originally was estimated that this code would be billed with 90% of all office visit claims, accounting for an approximately $3.3 billion increase in physician fee schedule spending; however, this estimate was revised with its reintroduction in the 2024 Final Rule, and it currently is estimated that it will be billed with 38% of all office visit claims.3,4
This add-on code was created to capture the inherent complexity of an E/M visit that is derived from the longitudinal nature of the physician-patient relationship and to better account for the additional resources of these outpatient E/M visits.5 Although these criteria often are met in the setting of an E/M visit within a primary care specialty (eg, family practice, internal medicine, obstetrics/gynecology, pediatrics), this code is not restricted to medical professionals based on specialties. The CMS noted that “the most important information used to determine whether the add-on code could be billed is the relationship between the practitioner and the patient,” specifically if they are fulfilling one of the following roles: “the continuing focal point for all needed health care services” or “ongoing care related to a patient's single, serious and complex condition.”6
Of note, further definitions regarding what constitutes a single, serious or complex condition have not yet been provided by CMS. The code should not be utilized when the relationship with the patient is of a discrete, routine, or time-limited nature. The resulting care should be personalized and should result in a comprehensive, longitudinal, and continuous relationship with the patient and should involve delivery of team-based care that is accessible, coordinated with other practitioners and providers, and integrated with the broader health care landscape.6
Herein, 5 examples are provided of scenarios when G2211 might be utilized as well as when it would not be appropriate to bill for this code.
Example 1
A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. The dermatologist has been managing both conditions for 3 years with methotrexate. The patient’s disease is well controlled at the current visit, and he presents for follow-up of disease activity and laboratory monitoring every 3 months. The dermatologist continues the patient on methotrexate after reviewing the risks, benefits, and adverse effects and orders a complete blood cell count and comprehensive metabolic panel.
Would use of G2211 be appropriate for this visit?—Yes, in this case it would be appropriate to bill for G2211. In this example, the physician is providing longitudinal ongoing medical care related to a patient’s single, serious or complex condition—specifically psoriasis and psoriatic arthritis—via managing methotrexate therapy.
Example 2
Let’s alter the previous example slightly: A 48-year-old man (an established patient) with a history of psoriasis and psoriatic arthritis presents to a dermatologist for follow-up. He is being followed by both a dermatologist and a rheumatologist. The patient is on methotrexate, which was prescribed by the rheumatologist, who also conducts the appropriate laboratory monitoring. The patient’s skin disease currently is well controlled, and the dermatologist discusses this with the patient and advises that he continue to follow up with rheumatology.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to utilize G2211. In this example, the dermatologist is providing longitudinal ongoing medical care; however, unlike in the first example, much of the ongoing medical care—in particular the management of the patient’s methotrexate therapy—is being performed by the rheumatologist. Therefore, although these conditions are serious or complex, the dermatologist is not the primary manager of treatment, and it would not be appropriate to bill for G2211.
Example 3
A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions that are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to continue infliximab therapy and schedule a deroofing procedure of the persistent areas.
Would use of G2211 be appropriate for this visit?—Yes, in this example it would be appropriate to utilize G2211. The patient has hidradenitis suppurativa, which would be considered a single, serious or complex condition. Additionally, the dermatologist is the primary manager of this condition by prescribing infliximab as well as counseling the patient on the appropriateness of procedural interventions and scheduling for these procedures; the dermatologist also is providing ongoing longitudinal care.
Example 4
Let’s alter the previous example slightly: A 35-year-old woman (an established patient) presents to a dermatologist for follow-up of hidradenitis suppurativa. She currently is receiving infliximab infusions, which are managed by the dermatologist. At the current presentation, physical examination reveals several persistent active lesions. After discussing possible treatment options, the dermatologist elects to perform intralesional triamcinolone injections to active areas during the current visit.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. Similar to Example 3, the dermatologist is treating a single, serious and complex condition and is primarily managing the disease and providing longitudinal care; however, in this case the dermatologist also is performing a minor procedure during the visit: injection of intralesional triamcinolone.
Importantly, G2211 cannot be utilized when modifier -25 is being appended to an outpatient E/M visit. Modifier -25 is defined as a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of the procedure or other service.”7 Modifier -25 is utilized when a minor procedure is performed by a qualified health care professional on the same day (generally during the same visit) as an E/M visit. Therefore, G2211 cannot be utilized when a minor procedure (eg, a tangential biopsy, punch biopsy, destruction or intralesional injection into skin) is performed during a visit.
Example 5
A 6-year-old girl presents to a dermatologist for a new rash on the trunk that started 5 days after an upper respiratory infection. The dermatologist evaluates the patient and identifies a blanchable macular eruption on the trunk; the patient is diagnosed with a viral exanthem. Because the patient reported associated pruritus, topical triamcinolone is prescribed.
Would use of G2211 be appropriate for this visit?—No, in this case it would not be appropriate to bill for G2211. A viral exanthem would not be considered an ongoing single, serious or complex condition and would be more consistent with a discrete condition; therefore, even though the dermatologist is primarily managing the disease process, it still would not fulfill the criteria necessary to bill for G2211.
Final Thoughts
G2211 is an add-on code created by the CMS that can be utilized in conjunction with an outpatient E/M visit when certain requirements are fulfilled. Specifically, this code can be utilized when the dermatologist is the primary provider of care for a patient’s ongoing single, serious or complex condition or serves as the continuing focal point for all of the patient’s health care needs. Understanding the nuances associated with this code are critical for correct billing.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare physician fee schedule final rule. Published November 2, 2023. Accessed April 15, 2024. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-final-rule
- Centers for Medicare & Medicaid Services. Fact Sheet—Physician Fee Schedule (PFS) payment for office/outpatient evaluation and management (E/M) visits. Published January 11, 2021. Accessed April 15, 2024. https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf
- American Society of Anesthesiologists. Broken Medicare system results in CMS proposing reduced physician payments in 2024. Published July 13, 2023. Accessed April 15, 2024. https://www.asahq.org/advocacy-and-asapac/fda-and-washington-alerts/washington-alerts/2023/07/broken-medicare-system-results-in-cms-proposing-reduced-physician-payments-in-2024
- American Medical Association. CY 2024 Medicare physician payment schedule and quality payment program (QPP) final rule summary. Accessed April 15, 2024. https://www.ama-assn.org/system/files/ama-summary-2024-mfs-proposed-rule.pdf
- Centers for Medicare & Medicaid Services. How to use the office & outpatient evaluation and management visit complexity add-on code G2211. MM13473. MLN Matters. Updated January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/mm13473-how-use-office-and-outpatient-evaluation-and-management-visit-complexity-add-code-g2211.pdf
- Centers for Medicare & Medicaid Services. CMS manual system. Published January 18, 2024. Accessed April 15, 2024. https://www.cms.gov/files/document/r12461cp.pdf
- American Medical Association. Reporting CPT Modifier 25. CPT Assistant (Online). 2023;33:1-12. Accessed April 15, 2024. https://www.ama-assn.org/system/files/reporting-CPT-modifier-25.pdf
PRACTICE POINTS
- The add-on code G2211 went into effect on January 1, 2024, and can be applied to outpatient evaluation and management visits that fulfill certain criteria.
- This code should be utilized when one is serving as the continuing focal point for all of the patient's health care needs or providing ongoing medical care related to a patient’s single, serious or complex condition.
Cervical Cancer Screening: US Clinicians Unclear About Best Practices
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines, proposing two major changes: start cervical cancer screening at age 25, rather than 21, and perform primary human papillomavirus (HPV) testing, instead of a Pap test.
First, healthcare providers in the US may be unsure how to reconcile conflicting cervical cancer screening guidelines from another major organization — the US Preventive Services Task Force (USPSTF), which published guidelines in 2018.
Although the ACS guidelines are based on an analysis of the latest evidence,
the recommendations challenge those from the USPSTF, which dictates insurance coverage in the US. Last year, the American College of Obstetricians and Gynecologists (ACOG) aligned its guidelines with those from the USPSTF.
The USPSTF recommends average-risk individuals start Pap, not HPV, testing at age 21, and broadens the options to primary HPV testing, Pap testing, or both together starting at age 30. The ACS, on the other hand, says primary HPV testing is the preferred screening approach from the start, which should be age 25.
Because the ACS guidelines marked a notable departure from prevailing practice, a team of researchers from five US universities decided to find out if anyone was following them.
The results, published in the journal Cancer in March, revealed that most healthcare providers had not changed practice.
Lead author Rebecca Perkins, MD, MSc, and colleagues found that, among the 70 respondents, few were starting screening at age 25, and none had switched to primary HPV testing.
The survey then probed clinicians’ willingness to adopt the ACS guidelines as well as their reservations and barriers to doing so.
Notably, more than half of the survey participants said they would be willing to adopt the ACS guidelines if the best evidence supported the changes and other professional medical organizations endorsed them.
On the age change, participants highlighted a range of benefits to moving to a later screening age, including that earlier screening may not be valuable and delaying screening could reduce overtreatment.
One participant noted: “We know that cervical cancer is usually a slow‐growing, long‐term progressive disease that does not typically show up that early in life, and we also know that, if infected, oftentimes their immune system can fight off the virus. So, it sounds reasonable at first glance [to delay screening to age 25 years].”
Others, however, brought up barriers to initiating screening at age 25. Some mentioned that later screening may not work for high‐risk populations and others voiced concerns about missing high‐grade precancer or cancer. “It’s not unusual for us to see women in their early 20s that have already had 10 or 15 partners. … a lot of them smoke too … they just have a lot of bad habits that put them at more risk,” one respondent noted.
On the HPV vs Pap testing front, many participants described a growing confidence in HPV tests after trying co-testing. One participant said, “Honestly, I do look more at the HPV results than the cytology. I put more faith in knowing what their HPV status is than anything.”
The main barriers to primary HPV testing, however, included lack of autonomy when working in a large health system, concerns about the efficacy of HPV testing, and a belief that cytology was valuable.
Some clinicians were worried about missing high-grade lesions or cancer. One healthcare provider said, “My only concern with primary HPV screening is occasionally you will pick up endometrial abnormalities on a Pap that you’re not going to pick up with HPV screening.”
Logistics and finances also played a role in clinicians’ hesitancy to switch to the ACS recommendation. Labs that could handle primary HPV tests were not available to some participants, and lack of insurance coverage was a barrier for others. One respondent noted, for instance, that his institution has a “cytology infrastructure that already exists in the lab and I can’t really see them switching.”
Many survey respondents also said they were waiting for endorsement from organizations, such as ACOG and USPSTF. “We run by the USPSTF and … ACOG. We don’t run by the ACS guidelines,” one person said.
Finally, some participants were not aware of the ACS recommendations at all or the data behind them but said they would be willing to change to primary HPV testing in the future.
Overall, Dr. Perkins said she was happy to see that more than half of the respondents would be willing to shift to the ACS screening guidelines, but noted that many remain reluctant to do so until the USPSTF and ACOG change their guidelines.
“It’s really just a matter of the USPSTF and ACOG endorsing” the ACS guidelines, said Dr. Perkins, professor of obstetrics and gynecology at Boston University.
The USPSTF is currently updating its cervical screening guidelines, which could potentially help reconcile this discord between the guidelines and close the gaps in practice patterns.
The USPSTF’s review of the evidence, which led to the 2018 guidelines, did highlight the effectiveness of HPV testing. The review authors concluded that “the evidence was consistent across trials” that primary, high-risk HPV screening increased detection of grade 3 or worse cervical intraepithelial neoplasia in the initial round of screening “by as much as 2 to 3 times when compared with cytology.”
However, Joy Melnikow, MD, MPH, first author on the USPSTF evidence review, explained that the reviewers factored in access to HPV testing when making their final recommendations.
“The consideration was making sure that a recommendation could be inclusive of all providers and all populations and not restricting access for clinics that couldn’t afford or didn’t have the machine to do [HPV testing],” Dr. Melnikow, director of the Center for Healthcare Policy and Research and professor of family and community medicine at the University of California Davis, told this news organization.
The ACS, however, did not consider potential access problems in its analysis of the evidence.
Although the ACS evidence is “excellent,” Dr. Perkins said, “it’s really just a matter of the USPSTF and ACOG endorsing that, and then it seems like a lot of people are willing to make the change.”
Dr. Perkins reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines, proposing two major changes: start cervical cancer screening at age 25, rather than 21, and perform primary human papillomavirus (HPV) testing, instead of a Pap test.
First, healthcare providers in the US may be unsure how to reconcile conflicting cervical cancer screening guidelines from another major organization — the US Preventive Services Task Force (USPSTF), which published guidelines in 2018.
Although the ACS guidelines are based on an analysis of the latest evidence,
the recommendations challenge those from the USPSTF, which dictates insurance coverage in the US. Last year, the American College of Obstetricians and Gynecologists (ACOG) aligned its guidelines with those from the USPSTF.
The USPSTF recommends average-risk individuals start Pap, not HPV, testing at age 21, and broadens the options to primary HPV testing, Pap testing, or both together starting at age 30. The ACS, on the other hand, says primary HPV testing is the preferred screening approach from the start, which should be age 25.
Because the ACS guidelines marked a notable departure from prevailing practice, a team of researchers from five US universities decided to find out if anyone was following them.
The results, published in the journal Cancer in March, revealed that most healthcare providers had not changed practice.
Lead author Rebecca Perkins, MD, MSc, and colleagues found that, among the 70 respondents, few were starting screening at age 25, and none had switched to primary HPV testing.
The survey then probed clinicians’ willingness to adopt the ACS guidelines as well as their reservations and barriers to doing so.
Notably, more than half of the survey participants said they would be willing to adopt the ACS guidelines if the best evidence supported the changes and other professional medical organizations endorsed them.
On the age change, participants highlighted a range of benefits to moving to a later screening age, including that earlier screening may not be valuable and delaying screening could reduce overtreatment.
One participant noted: “We know that cervical cancer is usually a slow‐growing, long‐term progressive disease that does not typically show up that early in life, and we also know that, if infected, oftentimes their immune system can fight off the virus. So, it sounds reasonable at first glance [to delay screening to age 25 years].”
Others, however, brought up barriers to initiating screening at age 25. Some mentioned that later screening may not work for high‐risk populations and others voiced concerns about missing high‐grade precancer or cancer. “It’s not unusual for us to see women in their early 20s that have already had 10 or 15 partners. … a lot of them smoke too … they just have a lot of bad habits that put them at more risk,” one respondent noted.
On the HPV vs Pap testing front, many participants described a growing confidence in HPV tests after trying co-testing. One participant said, “Honestly, I do look more at the HPV results than the cytology. I put more faith in knowing what their HPV status is than anything.”
The main barriers to primary HPV testing, however, included lack of autonomy when working in a large health system, concerns about the efficacy of HPV testing, and a belief that cytology was valuable.
Some clinicians were worried about missing high-grade lesions or cancer. One healthcare provider said, “My only concern with primary HPV screening is occasionally you will pick up endometrial abnormalities on a Pap that you’re not going to pick up with HPV screening.”
Logistics and finances also played a role in clinicians’ hesitancy to switch to the ACS recommendation. Labs that could handle primary HPV tests were not available to some participants, and lack of insurance coverage was a barrier for others. One respondent noted, for instance, that his institution has a “cytology infrastructure that already exists in the lab and I can’t really see them switching.”
Many survey respondents also said they were waiting for endorsement from organizations, such as ACOG and USPSTF. “We run by the USPSTF and … ACOG. We don’t run by the ACS guidelines,” one person said.
Finally, some participants were not aware of the ACS recommendations at all or the data behind them but said they would be willing to change to primary HPV testing in the future.
Overall, Dr. Perkins said she was happy to see that more than half of the respondents would be willing to shift to the ACS screening guidelines, but noted that many remain reluctant to do so until the USPSTF and ACOG change their guidelines.
“It’s really just a matter of the USPSTF and ACOG endorsing” the ACS guidelines, said Dr. Perkins, professor of obstetrics and gynecology at Boston University.
The USPSTF is currently updating its cervical screening guidelines, which could potentially help reconcile this discord between the guidelines and close the gaps in practice patterns.
The USPSTF’s review of the evidence, which led to the 2018 guidelines, did highlight the effectiveness of HPV testing. The review authors concluded that “the evidence was consistent across trials” that primary, high-risk HPV screening increased detection of grade 3 or worse cervical intraepithelial neoplasia in the initial round of screening “by as much as 2 to 3 times when compared with cytology.”
However, Joy Melnikow, MD, MPH, first author on the USPSTF evidence review, explained that the reviewers factored in access to HPV testing when making their final recommendations.
“The consideration was making sure that a recommendation could be inclusive of all providers and all populations and not restricting access for clinics that couldn’t afford or didn’t have the machine to do [HPV testing],” Dr. Melnikow, director of the Center for Healthcare Policy and Research and professor of family and community medicine at the University of California Davis, told this news organization.
The ACS, however, did not consider potential access problems in its analysis of the evidence.
Although the ACS evidence is “excellent,” Dr. Perkins said, “it’s really just a matter of the USPSTF and ACOG endorsing that, and then it seems like a lot of people are willing to make the change.”
Dr. Perkins reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines, proposing two major changes: start cervical cancer screening at age 25, rather than 21, and perform primary human papillomavirus (HPV) testing, instead of a Pap test.
First, healthcare providers in the US may be unsure how to reconcile conflicting cervical cancer screening guidelines from another major organization — the US Preventive Services Task Force (USPSTF), which published guidelines in 2018.
Although the ACS guidelines are based on an analysis of the latest evidence,
the recommendations challenge those from the USPSTF, which dictates insurance coverage in the US. Last year, the American College of Obstetricians and Gynecologists (ACOG) aligned its guidelines with those from the USPSTF.
The USPSTF recommends average-risk individuals start Pap, not HPV, testing at age 21, and broadens the options to primary HPV testing, Pap testing, or both together starting at age 30. The ACS, on the other hand, says primary HPV testing is the preferred screening approach from the start, which should be age 25.
Because the ACS guidelines marked a notable departure from prevailing practice, a team of researchers from five US universities decided to find out if anyone was following them.
The results, published in the journal Cancer in March, revealed that most healthcare providers had not changed practice.
Lead author Rebecca Perkins, MD, MSc, and colleagues found that, among the 70 respondents, few were starting screening at age 25, and none had switched to primary HPV testing.
The survey then probed clinicians’ willingness to adopt the ACS guidelines as well as their reservations and barriers to doing so.
Notably, more than half of the survey participants said they would be willing to adopt the ACS guidelines if the best evidence supported the changes and other professional medical organizations endorsed them.
On the age change, participants highlighted a range of benefits to moving to a later screening age, including that earlier screening may not be valuable and delaying screening could reduce overtreatment.
One participant noted: “We know that cervical cancer is usually a slow‐growing, long‐term progressive disease that does not typically show up that early in life, and we also know that, if infected, oftentimes their immune system can fight off the virus. So, it sounds reasonable at first glance [to delay screening to age 25 years].”
Others, however, brought up barriers to initiating screening at age 25. Some mentioned that later screening may not work for high‐risk populations and others voiced concerns about missing high‐grade precancer or cancer. “It’s not unusual for us to see women in their early 20s that have already had 10 or 15 partners. … a lot of them smoke too … they just have a lot of bad habits that put them at more risk,” one respondent noted.
On the HPV vs Pap testing front, many participants described a growing confidence in HPV tests after trying co-testing. One participant said, “Honestly, I do look more at the HPV results than the cytology. I put more faith in knowing what their HPV status is than anything.”
The main barriers to primary HPV testing, however, included lack of autonomy when working in a large health system, concerns about the efficacy of HPV testing, and a belief that cytology was valuable.
Some clinicians were worried about missing high-grade lesions or cancer. One healthcare provider said, “My only concern with primary HPV screening is occasionally you will pick up endometrial abnormalities on a Pap that you’re not going to pick up with HPV screening.”
Logistics and finances also played a role in clinicians’ hesitancy to switch to the ACS recommendation. Labs that could handle primary HPV tests were not available to some participants, and lack of insurance coverage was a barrier for others. One respondent noted, for instance, that his institution has a “cytology infrastructure that already exists in the lab and I can’t really see them switching.”
Many survey respondents also said they were waiting for endorsement from organizations, such as ACOG and USPSTF. “We run by the USPSTF and … ACOG. We don’t run by the ACS guidelines,” one person said.
Finally, some participants were not aware of the ACS recommendations at all or the data behind them but said they would be willing to change to primary HPV testing in the future.
Overall, Dr. Perkins said she was happy to see that more than half of the respondents would be willing to shift to the ACS screening guidelines, but noted that many remain reluctant to do so until the USPSTF and ACOG change their guidelines.
“It’s really just a matter of the USPSTF and ACOG endorsing” the ACS guidelines, said Dr. Perkins, professor of obstetrics and gynecology at Boston University.
The USPSTF is currently updating its cervical screening guidelines, which could potentially help reconcile this discord between the guidelines and close the gaps in practice patterns.
The USPSTF’s review of the evidence, which led to the 2018 guidelines, did highlight the effectiveness of HPV testing. The review authors concluded that “the evidence was consistent across trials” that primary, high-risk HPV screening increased detection of grade 3 or worse cervical intraepithelial neoplasia in the initial round of screening “by as much as 2 to 3 times when compared with cytology.”
However, Joy Melnikow, MD, MPH, first author on the USPSTF evidence review, explained that the reviewers factored in access to HPV testing when making their final recommendations.
“The consideration was making sure that a recommendation could be inclusive of all providers and all populations and not restricting access for clinics that couldn’t afford or didn’t have the machine to do [HPV testing],” Dr. Melnikow, director of the Center for Healthcare Policy and Research and professor of family and community medicine at the University of California Davis, told this news organization.
The ACS, however, did not consider potential access problems in its analysis of the evidence.
Although the ACS evidence is “excellent,” Dr. Perkins said, “it’s really just a matter of the USPSTF and ACOG endorsing that, and then it seems like a lot of people are willing to make the change.”
Dr. Perkins reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
Prospect of Better Hours, Less Burnout Fuels Locum Tenens
Insane hours and work-driven burnout are increasingly pernicious forces in medical workplaces. They apparently also are helping steer more physicians toward locum tenens, or temporary, assignments.
In its “2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals,” Coppell, Texas–based staffing firm AMN Healthcare asked doctors, nurse practitioners, and physician assistants why they chose locum tenens work.
The reason chosen most often is improving work hours. Eighty-six percent of respondents said that was the “most important” or a “moderately important” factor. Next was addressing work burnout (80% of respondents), followed by unhappiness with compensation (75%), and dissatisfaction with being a full-time employee (71%).
“During the COVID pandemic, healthcare professionals began to rethink how, when, and where they work,” said Jeff Decker, president of AMN Healthcare’s physician solutions division, adding that he estimates about 52,000 US physicians now work on a locum tenens basis.
“Locum tenens offers relief from the long, inflexible work hours and onerous bureaucratic duties that often cause dissatisfaction and burnout among physicians and other healthcare providers.”
These feelings of dissatisfaction dovetail with findings in recent reports by this news organization based on surveys of physicians about burnout and employment. For example:
- Forty-nine percent of physicians acknowledged feeling burned out, up from 42% 6 years earlier.
- Eighty-three percent of doctors attributed their burnout and/or depression to the job entirely or most of the time.
- Flexibility in work schedules was one of the improvements chosen most often as a potential aid to burnout.
- The leading reasons cited for burnout were the number of bureaucratic tasks and too many hours at work.
Trying Locum Tenens Early in Career
According to AMN Healthcare, 81% of the physicians and APPs in its latest survey said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. Only 19% waited until after retiring from medicine compared with 36% in AMN Healthcare’s 2016 survey.
In the 2024 report, a strong plurality of respondents (47%) said they found locum tenens work more satisfying than permanent healthcare employment. Twelve percent said the opposite, and 30% found the choices about equal.
Even so, it doesn’t appear that locum tenens represents a permanent career path for many. About as many (45%) of physicians and APPs said they would return to full-time employment if progress were made with conditions like hours and burnout, as said they would not (43%).
“Many physicians and other healthcare professionals feel they are being pushed from permanent positions by unsatisfactory work conditions,” Mr. Decker said. “To get them back, employers should offer practice conditions that appeal to today’s providers.”
A version of this article appeared on Medscape.com.
Insane hours and work-driven burnout are increasingly pernicious forces in medical workplaces. They apparently also are helping steer more physicians toward locum tenens, or temporary, assignments.
In its “2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals,” Coppell, Texas–based staffing firm AMN Healthcare asked doctors, nurse practitioners, and physician assistants why they chose locum tenens work.
The reason chosen most often is improving work hours. Eighty-six percent of respondents said that was the “most important” or a “moderately important” factor. Next was addressing work burnout (80% of respondents), followed by unhappiness with compensation (75%), and dissatisfaction with being a full-time employee (71%).
“During the COVID pandemic, healthcare professionals began to rethink how, when, and where they work,” said Jeff Decker, president of AMN Healthcare’s physician solutions division, adding that he estimates about 52,000 US physicians now work on a locum tenens basis.
“Locum tenens offers relief from the long, inflexible work hours and onerous bureaucratic duties that often cause dissatisfaction and burnout among physicians and other healthcare providers.”
These feelings of dissatisfaction dovetail with findings in recent reports by this news organization based on surveys of physicians about burnout and employment. For example:
- Forty-nine percent of physicians acknowledged feeling burned out, up from 42% 6 years earlier.
- Eighty-three percent of doctors attributed their burnout and/or depression to the job entirely or most of the time.
- Flexibility in work schedules was one of the improvements chosen most often as a potential aid to burnout.
- The leading reasons cited for burnout were the number of bureaucratic tasks and too many hours at work.
Trying Locum Tenens Early in Career
According to AMN Healthcare, 81% of the physicians and APPs in its latest survey said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. Only 19% waited until after retiring from medicine compared with 36% in AMN Healthcare’s 2016 survey.
In the 2024 report, a strong plurality of respondents (47%) said they found locum tenens work more satisfying than permanent healthcare employment. Twelve percent said the opposite, and 30% found the choices about equal.
Even so, it doesn’t appear that locum tenens represents a permanent career path for many. About as many (45%) of physicians and APPs said they would return to full-time employment if progress were made with conditions like hours and burnout, as said they would not (43%).
“Many physicians and other healthcare professionals feel they are being pushed from permanent positions by unsatisfactory work conditions,” Mr. Decker said. “To get them back, employers should offer practice conditions that appeal to today’s providers.”
A version of this article appeared on Medscape.com.
Insane hours and work-driven burnout are increasingly pernicious forces in medical workplaces. They apparently also are helping steer more physicians toward locum tenens, or temporary, assignments.
In its “2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals,” Coppell, Texas–based staffing firm AMN Healthcare asked doctors, nurse practitioners, and physician assistants why they chose locum tenens work.
The reason chosen most often is improving work hours. Eighty-six percent of respondents said that was the “most important” or a “moderately important” factor. Next was addressing work burnout (80% of respondents), followed by unhappiness with compensation (75%), and dissatisfaction with being a full-time employee (71%).
“During the COVID pandemic, healthcare professionals began to rethink how, when, and where they work,” said Jeff Decker, president of AMN Healthcare’s physician solutions division, adding that he estimates about 52,000 US physicians now work on a locum tenens basis.
“Locum tenens offers relief from the long, inflexible work hours and onerous bureaucratic duties that often cause dissatisfaction and burnout among physicians and other healthcare providers.”
These feelings of dissatisfaction dovetail with findings in recent reports by this news organization based on surveys of physicians about burnout and employment. For example:
- Forty-nine percent of physicians acknowledged feeling burned out, up from 42% 6 years earlier.
- Eighty-three percent of doctors attributed their burnout and/or depression to the job entirely or most of the time.
- Flexibility in work schedules was one of the improvements chosen most often as a potential aid to burnout.
- The leading reasons cited for burnout were the number of bureaucratic tasks and too many hours at work.
Trying Locum Tenens Early in Career
According to AMN Healthcare, 81% of the physicians and APPs in its latest survey said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. Only 19% waited until after retiring from medicine compared with 36% in AMN Healthcare’s 2016 survey.
In the 2024 report, a strong plurality of respondents (47%) said they found locum tenens work more satisfying than permanent healthcare employment. Twelve percent said the opposite, and 30% found the choices about equal.
Even so, it doesn’t appear that locum tenens represents a permanent career path for many. About as many (45%) of physicians and APPs said they would return to full-time employment if progress were made with conditions like hours and burnout, as said they would not (43%).
“Many physicians and other healthcare professionals feel they are being pushed from permanent positions by unsatisfactory work conditions,” Mr. Decker said. “To get them back, employers should offer practice conditions that appeal to today’s providers.”
A version of this article appeared on Medscape.com.
This Tech Will Change Your Practice Sooner Than You Think
Medical innovations don’t happen overnight — but in today’s digital world, they happen pretty fast. Some are advancing faster than you think.
1. Artificial Intelligence (AI) Medical Scribes
You may already be using this or, at the very least, have heard about it.
Physician burnout is a growing problem, with many doctors spending 2 hours on paperwork for every hour with patients. But some doctors, such as Gregory Ator, MD, chief medical informatics officer at the University of Kansas Medical Center, Kansas City, Kansas, have found a better way.
“I have been using it for 9 months now, and it truly is a life changer,” Dr. Ator said of Abridge, an AI helper that transcribes and summarizes his conversations with patients. “Now, I go into the room, place my phone just about anywhere, and I can just listen.” He estimated that the tech saves him between 3 and 10 minutes per patient. “At 20 patients a day, that saves me around 2 hours,” he said.
Bonus: Patients “get a doctor’s full attention instead of just looking at the top of his head while they play with the computer,” Dr. Ator said. “I have yet to have a patient who didn’t think that was a positive thing.”
Several companies are already selling these AI devices, including Ambience Healthcare, Augmedix, Nuance, and Suki, and they offer more than just transcriptions, said John D. Halamka, MD, president of Mayo Clinic Platform, who oversees Mayo’s adoption of AI. They also generate notes for treatment and billing and update data in the electronic health record.
“It’s preparation of documentation based on ambient listening of doctor-patient conversations,” Dr. Halamka explained. “I’m very optimistic about the use of emerging AI technologies to enable every clinician to practice at the top of their license.”
Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford Health Care, has spent much of the last year co-running the medical center’s pilot program for AI scribes, and she’s so impressed with the technology that she “expects it’ll become more widely available as an option for any clinician that wants to use it in the next 12-18 months.”
2. Three-Dimensional (3D) Printing
Although 3D-printed organs may not happen anytime soon, the future is here for some 3D-printed prosthetics and implants — everything from dentures to spinal implants to prosthetic fingers and noses.
“In the next few years, I see rapid growth in the use of 3D printing technology across orthopedic surgery,” said Rishin J. Kadakia, MD, an orthopedic surgeon in Atlanta. “It’s becoming more common not just at large academic institutions. More and more providers will turn to using 3D printing technology to help tackle challenging cases that previously did not have good solutions.”
Dr. Kadakia has experienced this firsthand with his patients at the Emory Orthopaedics & Spine Center. One female patient developed talar avascular necrosis due to a bone break she’d sustained in a serious car crash. An ankle and subtalar joint fusion would repair the damage but limit her mobility and change her gait. So instead, in August of 2021, Dr. Kadakia and fellow orthopedic surgeon Jason Bariteau, MD, created for her a 3D-printed cobalt chrome talus implant.
“It provided an opportunity for her to keep her ankle’s range of motion, and also mobilize faster than with a subtalar and ankle joint fusion,” said Dr. Kadakia.
The technology is also playing a role in customized medical devices — patient-specific tools for greater precision — and 3D-printed anatomical models, built to the exact specifications of individual patients. Mayo Clinic already has 3D modeling units in three states, and other hospitals are following suit. The models not only help doctors prepare for complicated surgeries but also can dramatically cut down on costs. A 2021 study from Durham University reported that 3D models helped reduce surgery time by between 1.5 and 2.5 hours in lengthy procedures.
3. Drones
For patients who can’t make it to a pharmacy to pick up their prescriptions, either because of distance or lack of transportation, drones — which can deliver medications onto a customer’s back yard or front porch — offer a compelling solution.
Several companies and hospitals are already experimenting with drones, like WellSpan Health in Pennsylvania, Amazon Pharmacy, and the Cleveland Clinic, which announced a partnership with drone delivery company Zipline and plans to begin prescription deliveries across Northeast Ohio by 2025.
Healthcare systems are just beginning to explore the potential of drone deliveries, for everything from lab samples to medical and surgical supplies — even defibrillators that could arrive at an ailing patient’s front door before an emergency medical technician arrives.
“For many providers, when you take a sample from a patient, that sample waits around for hours until a courier picks up all of the facility’s samples and drives them to an outside facility for processing,” said Hillary Brendzel, head of Zipline’s US Healthcare Practice.
According to a 2022 survey from American Nurse Journal, 71% of nurses said that medical courier delays and errors negatively affected their ability to provide patient care. But with drone delivery, “lab samples can be sent for processing immediately, on-demand, resulting in faster diagnosis, treatment, and ultimately better outcomes,” said Ms. Brendzel.
4. Portable Ultrasound
Within the next 2 years, portable ultrasound — pocket-sized devices that connect to a smartphone or tablet — will become the “21st-century stethoscope,” said Abhilash Hareendranathan, PhD, assistant professor in the Department of Radiology and Diagnostic Imaging at the University of Alberta, in Edmonton, Alberta, Canada.
AI can make these devices easy to use, allowing clinicians with minimal imaging training to capture clear images and understand the results. Dr. Hareendranathan developed the Ultrasound Arm Injury Detection tool, a portable ultrasound that uses AI to detect fracture.
“We plan to introduce this technology in emergency departments, where it could be used by triage nurses to perform quick examinations to detect fractures of the wrist, elbow, or shoulder,” he said.
More pocket-sized scanners like these could “reshape the way diagnostic care is provided in rural and remote communities,” Dr. Hareendranathan said, and will “reduce wait times in crowded emergency departments.” Bill Gates believes enough in portable ultrasound that last September, the Bill & Melinda Gates Foundation granted $44 million to GE HealthCare to develop the technology for under-resourced communities.
5. Virtual Reality (VR)
When RelieVRx became the first US Food and Drug Administration (FDA)–approved VR therapy for chronic back pain in 2021, the technology was used in just a handful of Veterans Affairs (VA) facilities. But today, thousands of VR headsets have been deployed to more than 160 VA medical centers and clinics across the country.
“The VR experiences encompass pain neuroscience education, mindfulness, pleasant and relaxing distraction, and key skills to calm the nervous system,” said Beth Darnall, PhD, director of the Stanford Pain Relief Innovations Lab, who helped design the RelieVRx. She expects VR to go mainstream soon, not just because of increasing evidence that it works but also thanks to the Centers for Medicare & Medicaid Services, which recently issued a Healthcare Common Procedure Coding System code for VR. “This billing infrastructure will encourage adoption and uptake,” she said.
Hundreds of hospitals across the United States have already adopted the technology, for everything from childbirth pain to wound debridement, said Josh Sackman, the president and cofounder of AppliedVR, the company that developed RelieVRx.
“Over the next few years, we may see hundreds more deploy unique applications [for VR] that can handle multiple clinical indications,” he said. “Given the modality’s ability to scale and reduce reliance on pharmacological interventions, it has the power to improve the cost and quality of care.”
Hospital systems like Geisinger and Cedars-Sinai are already finding unique ways to implement the technology, he said, like using VR to reduce “scanxiety” during imaging service.
Other VR innovations are already being introduced, from the Smileyscope, a VR device for children that’s been proven to lessen the pain of a blood draw or intravenous insertion (it was cleared by the FDA last November) to several VR platforms launched by Cedars-Sinai in recent months, for applications that range from gastrointestinal issues to mental health therapy. “There may already be a thousand hospitals using VR in some capacity,” said Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai.
A version of this article appeared on Medscape.com.
Medical innovations don’t happen overnight — but in today’s digital world, they happen pretty fast. Some are advancing faster than you think.
1. Artificial Intelligence (AI) Medical Scribes
You may already be using this or, at the very least, have heard about it.
Physician burnout is a growing problem, with many doctors spending 2 hours on paperwork for every hour with patients. But some doctors, such as Gregory Ator, MD, chief medical informatics officer at the University of Kansas Medical Center, Kansas City, Kansas, have found a better way.
“I have been using it for 9 months now, and it truly is a life changer,” Dr. Ator said of Abridge, an AI helper that transcribes and summarizes his conversations with patients. “Now, I go into the room, place my phone just about anywhere, and I can just listen.” He estimated that the tech saves him between 3 and 10 minutes per patient. “At 20 patients a day, that saves me around 2 hours,” he said.
Bonus: Patients “get a doctor’s full attention instead of just looking at the top of his head while they play with the computer,” Dr. Ator said. “I have yet to have a patient who didn’t think that was a positive thing.”
Several companies are already selling these AI devices, including Ambience Healthcare, Augmedix, Nuance, and Suki, and they offer more than just transcriptions, said John D. Halamka, MD, president of Mayo Clinic Platform, who oversees Mayo’s adoption of AI. They also generate notes for treatment and billing and update data in the electronic health record.
“It’s preparation of documentation based on ambient listening of doctor-patient conversations,” Dr. Halamka explained. “I’m very optimistic about the use of emerging AI technologies to enable every clinician to practice at the top of their license.”
Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford Health Care, has spent much of the last year co-running the medical center’s pilot program for AI scribes, and she’s so impressed with the technology that she “expects it’ll become more widely available as an option for any clinician that wants to use it in the next 12-18 months.”
2. Three-Dimensional (3D) Printing
Although 3D-printed organs may not happen anytime soon, the future is here for some 3D-printed prosthetics and implants — everything from dentures to spinal implants to prosthetic fingers and noses.
“In the next few years, I see rapid growth in the use of 3D printing technology across orthopedic surgery,” said Rishin J. Kadakia, MD, an orthopedic surgeon in Atlanta. “It’s becoming more common not just at large academic institutions. More and more providers will turn to using 3D printing technology to help tackle challenging cases that previously did not have good solutions.”
Dr. Kadakia has experienced this firsthand with his patients at the Emory Orthopaedics & Spine Center. One female patient developed talar avascular necrosis due to a bone break she’d sustained in a serious car crash. An ankle and subtalar joint fusion would repair the damage but limit her mobility and change her gait. So instead, in August of 2021, Dr. Kadakia and fellow orthopedic surgeon Jason Bariteau, MD, created for her a 3D-printed cobalt chrome talus implant.
“It provided an opportunity for her to keep her ankle’s range of motion, and also mobilize faster than with a subtalar and ankle joint fusion,” said Dr. Kadakia.
The technology is also playing a role in customized medical devices — patient-specific tools for greater precision — and 3D-printed anatomical models, built to the exact specifications of individual patients. Mayo Clinic already has 3D modeling units in three states, and other hospitals are following suit. The models not only help doctors prepare for complicated surgeries but also can dramatically cut down on costs. A 2021 study from Durham University reported that 3D models helped reduce surgery time by between 1.5 and 2.5 hours in lengthy procedures.
3. Drones
For patients who can’t make it to a pharmacy to pick up their prescriptions, either because of distance or lack of transportation, drones — which can deliver medications onto a customer’s back yard or front porch — offer a compelling solution.
Several companies and hospitals are already experimenting with drones, like WellSpan Health in Pennsylvania, Amazon Pharmacy, and the Cleveland Clinic, which announced a partnership with drone delivery company Zipline and plans to begin prescription deliveries across Northeast Ohio by 2025.
Healthcare systems are just beginning to explore the potential of drone deliveries, for everything from lab samples to medical and surgical supplies — even defibrillators that could arrive at an ailing patient’s front door before an emergency medical technician arrives.
“For many providers, when you take a sample from a patient, that sample waits around for hours until a courier picks up all of the facility’s samples and drives them to an outside facility for processing,” said Hillary Brendzel, head of Zipline’s US Healthcare Practice.
According to a 2022 survey from American Nurse Journal, 71% of nurses said that medical courier delays and errors negatively affected their ability to provide patient care. But with drone delivery, “lab samples can be sent for processing immediately, on-demand, resulting in faster diagnosis, treatment, and ultimately better outcomes,” said Ms. Brendzel.
4. Portable Ultrasound
Within the next 2 years, portable ultrasound — pocket-sized devices that connect to a smartphone or tablet — will become the “21st-century stethoscope,” said Abhilash Hareendranathan, PhD, assistant professor in the Department of Radiology and Diagnostic Imaging at the University of Alberta, in Edmonton, Alberta, Canada.
AI can make these devices easy to use, allowing clinicians with minimal imaging training to capture clear images and understand the results. Dr. Hareendranathan developed the Ultrasound Arm Injury Detection tool, a portable ultrasound that uses AI to detect fracture.
“We plan to introduce this technology in emergency departments, where it could be used by triage nurses to perform quick examinations to detect fractures of the wrist, elbow, or shoulder,” he said.
More pocket-sized scanners like these could “reshape the way diagnostic care is provided in rural and remote communities,” Dr. Hareendranathan said, and will “reduce wait times in crowded emergency departments.” Bill Gates believes enough in portable ultrasound that last September, the Bill & Melinda Gates Foundation granted $44 million to GE HealthCare to develop the technology for under-resourced communities.
5. Virtual Reality (VR)
When RelieVRx became the first US Food and Drug Administration (FDA)–approved VR therapy for chronic back pain in 2021, the technology was used in just a handful of Veterans Affairs (VA) facilities. But today, thousands of VR headsets have been deployed to more than 160 VA medical centers and clinics across the country.
“The VR experiences encompass pain neuroscience education, mindfulness, pleasant and relaxing distraction, and key skills to calm the nervous system,” said Beth Darnall, PhD, director of the Stanford Pain Relief Innovations Lab, who helped design the RelieVRx. She expects VR to go mainstream soon, not just because of increasing evidence that it works but also thanks to the Centers for Medicare & Medicaid Services, which recently issued a Healthcare Common Procedure Coding System code for VR. “This billing infrastructure will encourage adoption and uptake,” she said.
Hundreds of hospitals across the United States have already adopted the technology, for everything from childbirth pain to wound debridement, said Josh Sackman, the president and cofounder of AppliedVR, the company that developed RelieVRx.
“Over the next few years, we may see hundreds more deploy unique applications [for VR] that can handle multiple clinical indications,” he said. “Given the modality’s ability to scale and reduce reliance on pharmacological interventions, it has the power to improve the cost and quality of care.”
Hospital systems like Geisinger and Cedars-Sinai are already finding unique ways to implement the technology, he said, like using VR to reduce “scanxiety” during imaging service.
Other VR innovations are already being introduced, from the Smileyscope, a VR device for children that’s been proven to lessen the pain of a blood draw or intravenous insertion (it was cleared by the FDA last November) to several VR platforms launched by Cedars-Sinai in recent months, for applications that range from gastrointestinal issues to mental health therapy. “There may already be a thousand hospitals using VR in some capacity,” said Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai.
A version of this article appeared on Medscape.com.
Medical innovations don’t happen overnight — but in today’s digital world, they happen pretty fast. Some are advancing faster than you think.
1. Artificial Intelligence (AI) Medical Scribes
You may already be using this or, at the very least, have heard about it.
Physician burnout is a growing problem, with many doctors spending 2 hours on paperwork for every hour with patients. But some doctors, such as Gregory Ator, MD, chief medical informatics officer at the University of Kansas Medical Center, Kansas City, Kansas, have found a better way.
“I have been using it for 9 months now, and it truly is a life changer,” Dr. Ator said of Abridge, an AI helper that transcribes and summarizes his conversations with patients. “Now, I go into the room, place my phone just about anywhere, and I can just listen.” He estimated that the tech saves him between 3 and 10 minutes per patient. “At 20 patients a day, that saves me around 2 hours,” he said.
Bonus: Patients “get a doctor’s full attention instead of just looking at the top of his head while they play with the computer,” Dr. Ator said. “I have yet to have a patient who didn’t think that was a positive thing.”
Several companies are already selling these AI devices, including Ambience Healthcare, Augmedix, Nuance, and Suki, and they offer more than just transcriptions, said John D. Halamka, MD, president of Mayo Clinic Platform, who oversees Mayo’s adoption of AI. They also generate notes for treatment and billing and update data in the electronic health record.
“It’s preparation of documentation based on ambient listening of doctor-patient conversations,” Dr. Halamka explained. “I’m very optimistic about the use of emerging AI technologies to enable every clinician to practice at the top of their license.”
Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford Health Care, has spent much of the last year co-running the medical center’s pilot program for AI scribes, and she’s so impressed with the technology that she “expects it’ll become more widely available as an option for any clinician that wants to use it in the next 12-18 months.”
2. Three-Dimensional (3D) Printing
Although 3D-printed organs may not happen anytime soon, the future is here for some 3D-printed prosthetics and implants — everything from dentures to spinal implants to prosthetic fingers and noses.
“In the next few years, I see rapid growth in the use of 3D printing technology across orthopedic surgery,” said Rishin J. Kadakia, MD, an orthopedic surgeon in Atlanta. “It’s becoming more common not just at large academic institutions. More and more providers will turn to using 3D printing technology to help tackle challenging cases that previously did not have good solutions.”
Dr. Kadakia has experienced this firsthand with his patients at the Emory Orthopaedics & Spine Center. One female patient developed talar avascular necrosis due to a bone break she’d sustained in a serious car crash. An ankle and subtalar joint fusion would repair the damage but limit her mobility and change her gait. So instead, in August of 2021, Dr. Kadakia and fellow orthopedic surgeon Jason Bariteau, MD, created for her a 3D-printed cobalt chrome talus implant.
“It provided an opportunity for her to keep her ankle’s range of motion, and also mobilize faster than with a subtalar and ankle joint fusion,” said Dr. Kadakia.
The technology is also playing a role in customized medical devices — patient-specific tools for greater precision — and 3D-printed anatomical models, built to the exact specifications of individual patients. Mayo Clinic already has 3D modeling units in three states, and other hospitals are following suit. The models not only help doctors prepare for complicated surgeries but also can dramatically cut down on costs. A 2021 study from Durham University reported that 3D models helped reduce surgery time by between 1.5 and 2.5 hours in lengthy procedures.
3. Drones
For patients who can’t make it to a pharmacy to pick up their prescriptions, either because of distance or lack of transportation, drones — which can deliver medications onto a customer’s back yard or front porch — offer a compelling solution.
Several companies and hospitals are already experimenting with drones, like WellSpan Health in Pennsylvania, Amazon Pharmacy, and the Cleveland Clinic, which announced a partnership with drone delivery company Zipline and plans to begin prescription deliveries across Northeast Ohio by 2025.
Healthcare systems are just beginning to explore the potential of drone deliveries, for everything from lab samples to medical and surgical supplies — even defibrillators that could arrive at an ailing patient’s front door before an emergency medical technician arrives.
“For many providers, when you take a sample from a patient, that sample waits around for hours until a courier picks up all of the facility’s samples and drives them to an outside facility for processing,” said Hillary Brendzel, head of Zipline’s US Healthcare Practice.
According to a 2022 survey from American Nurse Journal, 71% of nurses said that medical courier delays and errors negatively affected their ability to provide patient care. But with drone delivery, “lab samples can be sent for processing immediately, on-demand, resulting in faster diagnosis, treatment, and ultimately better outcomes,” said Ms. Brendzel.
4. Portable Ultrasound
Within the next 2 years, portable ultrasound — pocket-sized devices that connect to a smartphone or tablet — will become the “21st-century stethoscope,” said Abhilash Hareendranathan, PhD, assistant professor in the Department of Radiology and Diagnostic Imaging at the University of Alberta, in Edmonton, Alberta, Canada.
AI can make these devices easy to use, allowing clinicians with minimal imaging training to capture clear images and understand the results. Dr. Hareendranathan developed the Ultrasound Arm Injury Detection tool, a portable ultrasound that uses AI to detect fracture.
“We plan to introduce this technology in emergency departments, where it could be used by triage nurses to perform quick examinations to detect fractures of the wrist, elbow, or shoulder,” he said.
More pocket-sized scanners like these could “reshape the way diagnostic care is provided in rural and remote communities,” Dr. Hareendranathan said, and will “reduce wait times in crowded emergency departments.” Bill Gates believes enough in portable ultrasound that last September, the Bill & Melinda Gates Foundation granted $44 million to GE HealthCare to develop the technology for under-resourced communities.
5. Virtual Reality (VR)
When RelieVRx became the first US Food and Drug Administration (FDA)–approved VR therapy for chronic back pain in 2021, the technology was used in just a handful of Veterans Affairs (VA) facilities. But today, thousands of VR headsets have been deployed to more than 160 VA medical centers and clinics across the country.
“The VR experiences encompass pain neuroscience education, mindfulness, pleasant and relaxing distraction, and key skills to calm the nervous system,” said Beth Darnall, PhD, director of the Stanford Pain Relief Innovations Lab, who helped design the RelieVRx. She expects VR to go mainstream soon, not just because of increasing evidence that it works but also thanks to the Centers for Medicare & Medicaid Services, which recently issued a Healthcare Common Procedure Coding System code for VR. “This billing infrastructure will encourage adoption and uptake,” she said.
Hundreds of hospitals across the United States have already adopted the technology, for everything from childbirth pain to wound debridement, said Josh Sackman, the president and cofounder of AppliedVR, the company that developed RelieVRx.
“Over the next few years, we may see hundreds more deploy unique applications [for VR] that can handle multiple clinical indications,” he said. “Given the modality’s ability to scale and reduce reliance on pharmacological interventions, it has the power to improve the cost and quality of care.”
Hospital systems like Geisinger and Cedars-Sinai are already finding unique ways to implement the technology, he said, like using VR to reduce “scanxiety” during imaging service.
Other VR innovations are already being introduced, from the Smileyscope, a VR device for children that’s been proven to lessen the pain of a blood draw or intravenous insertion (it was cleared by the FDA last November) to several VR platforms launched by Cedars-Sinai in recent months, for applications that range from gastrointestinal issues to mental health therapy. “There may already be a thousand hospitals using VR in some capacity,” said Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai.
A version of this article appeared on Medscape.com.
The AGA Future Leaders Program: A Mentee-Mentor Triad Perspective
Two of us (Parakkal Deepak and Edward L. Barnes) were part of the American Gastroenterological Association’s (AGA) Future Leaders Program (FLP) class of 2022-2023, and our mentor was Aasma Shaukat. We were invited to share our experiences as participants in the FLP and its impact in our careers.
Why Was the Future Leaders Program Conceived?
To understand this, one must first understand that the AGA, like all other GI professional organizations, relies on volunteer leaders to develop its long-term vision and execute this through strategic initiatives and programs. and understand the governance structure of the AGA to help lead it to face these challenges effectively.
The AGA FLP was thus conceived and launched in 2014-2015 by the founding chairs, Byron Cryer, MD, who is a professor of medicine and associate dean for faculty diversity at University of Texas Southwestern Medical School and Suzanne Rose, MD, MSEd, AGAF, who is a professor of medicine and senior vice dean for medical education at Perelman School of Medicine at the University of Pennsylvania. They envisioned a leadership pathway that would position early career GIs on a track to positively affect the AGA and the field of GI.
How Does One Apply for the Program?
Our FLP cohort applications were invited in October of 2021 and mentees accepted into the program in November 2021. The application process is competitive – applicants are encouraged to detail why they feel they would benefit from the FLP, what existing skillsets they have that can be further enhanced through the program, and what their long-term vision is for their growth as leaders, both within their institution and within the AGA. This is further accompanied by letters of support from their divisional chiefs and other key supervisors within the division who are intimately aware of their leadership potential and career trajectory. This process identified 18 future leaders for our class of 2022-2023.
What Is Involved?
Following acceptance into the AGA Future Leaders Program, we embarked on a series of virtual and in-person meetings with our mentorship triads (one mentor and two mentees) and other mentorship teams over the 18-month program (see Figure). These meetings covered highly focused topics ranging from the role of advocacy in leadership to negotiation and developing a business plan, with ample opportunities for individually tailored mentorship within the mentorship triads.
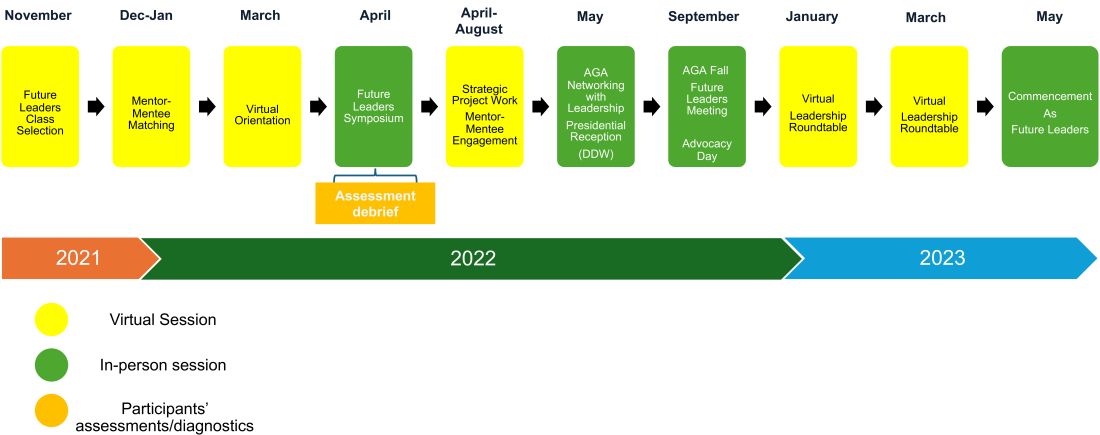
We also completed personality assessments that helped us understand our strengths and areas of improvement, and ways to use the information to hone our leadership styles.
A large portion of programming and the mentorship experience during the AGA Future Leaders Program is focused on a leadership project that is aimed at addressing a societal driver of interest for the AGA. Examples of these societal drivers of interest include maximizing the role of women in gastroenterology, the role of artificial intelligence in gastroenterology, burnout, and the impact of climate change on gastroenterology. Mentorship triads propose novel methods for addressing these critical issues, outlining the roles that the AGA and other stakeholders may embrace to address these anticipated growing challenges head on.
Our mentorship triad was asked to address the issue of ending disparities within gastroenterology. Given our research and clinical interest in inflammatory bowel disease (IBD), we immediately recognized an opportunity to evaluate and potentially offer solutions for the geographic disparities that exist in the field of IBD. These disparities affect access to care for patients with Crohn’s disease and ulcerative colitis, leading to delays in diagnosis and ultimately effective therapy decisions.
In addition to developing a proposal for the AGA to expand access to care to major IBD centers in rural areas where these disparities exist, we also initiated an examination of geographic disparities in our own multidisciplinary IBD centers (abstract accepted for presentation at Digestive Diseases Week 2024). This allowed us to expand our respective research footprints at our institutions, utilizing new methods of geocoding to directly measure factors affecting clinical outcomes in IBD. Given our in-depth evaluation of this topic as part of our Future Leaders Program training, at the suggestion of our mentor, our mentorship triad also published a commentary on geographic disparities in the Diversity, Equity, and Inclusion sections of Gastroenterology and Clinical Gastroenterology and Hepatology.1, 2
Impact on the Field and Our Careers
Our mentorship triad had the unique experience of having a mentor who had previously participated in the Future Leaders Program as a mentee. As the Future Leaders Program has now enrolled 72 participants, these occasions will likely become more frequent, given the opportunities for career development and growth within the AGA (and our field) that are available after participating in the Future Leaders Program.
To have a mentor with this insight of having been a mentee in the program was invaluable, given her direct experience and understanding of the growth opportunities available, and opportunities to maximize participation in the Future Leaders Program. Additionally, as evidenced by Dr. Shaukat’s recommendations to grow our initial assignment into published commentaries, need statements for our field, and ultimately growing research projects, her keen insights as a mentor were a critical component of our individual growth in the program and the success of our mentorship triad. We benefited from networking with peers and learning about their work, which can lead to future collaborations. We had access to the highly accomplished mentors from diverse settings and learned models of leadership, while developing skills to foster our own leadership style.
In terms of programmatic impact, more than 90% of FLP alumni are serving in AGA leadership on committees, task forces, editorial boards, and councils. What is also important is the impact of content developed by mentee-mentor triads during the FLP cohorts over time. More than 700 GIs have benefited from online leadership development content created by the FLP. Based on our experience, we highly recommend all early career GI physicians to apply!
Dr. Parakkal (@P_DeepakIBDMD) is based in the division of gastroenterology, Washington University in St. Louis (Mo.) School of Medicine. He is supported by a Junior Faculty Development Award from the American College of Gastroenterology and IBD Plexus of the Crohn’s & Colitis Foundation. He has received research support under a sponsored research agreement unrelated to the data in the paper from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Prometheus Biosciences, Takeda Pharmaceuticals, Roche-Genentech, and CorEvitas LLC. He has served as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Scipher Medicine, Fresenius Kabi, Roche-Genentech, and CorEvitas LLC. Dr. Barnes (@EdBarnesMD) is based in the division of gastroenterology and hepatology, University of North Carolina at Chapel Hill. He is supported by National Institutes of Health K23DK127157-01, and has served as a consultant for Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Dr. Shaukat (@AasmaShaukatMD) is based in the division of gastroenterology, New York University, New York. She has served as a consultant for Iterative health, Motus, Freenome, and Geneoscopy. Research support by the Steve and Alex Cohen Foundation.
References
1. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 July. doi: 10.1053/j.gastro.2023.05.017.
2. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Clin Gastroenterol Hepatol. 2023 July. doi: 10.1016/j.cgh.2023.04.006.
Two of us (Parakkal Deepak and Edward L. Barnes) were part of the American Gastroenterological Association’s (AGA) Future Leaders Program (FLP) class of 2022-2023, and our mentor was Aasma Shaukat. We were invited to share our experiences as participants in the FLP and its impact in our careers.
Why Was the Future Leaders Program Conceived?
To understand this, one must first understand that the AGA, like all other GI professional organizations, relies on volunteer leaders to develop its long-term vision and execute this through strategic initiatives and programs. and understand the governance structure of the AGA to help lead it to face these challenges effectively.
The AGA FLP was thus conceived and launched in 2014-2015 by the founding chairs, Byron Cryer, MD, who is a professor of medicine and associate dean for faculty diversity at University of Texas Southwestern Medical School and Suzanne Rose, MD, MSEd, AGAF, who is a professor of medicine and senior vice dean for medical education at Perelman School of Medicine at the University of Pennsylvania. They envisioned a leadership pathway that would position early career GIs on a track to positively affect the AGA and the field of GI.
How Does One Apply for the Program?
Our FLP cohort applications were invited in October of 2021 and mentees accepted into the program in November 2021. The application process is competitive – applicants are encouraged to detail why they feel they would benefit from the FLP, what existing skillsets they have that can be further enhanced through the program, and what their long-term vision is for their growth as leaders, both within their institution and within the AGA. This is further accompanied by letters of support from their divisional chiefs and other key supervisors within the division who are intimately aware of their leadership potential and career trajectory. This process identified 18 future leaders for our class of 2022-2023.
What Is Involved?
Following acceptance into the AGA Future Leaders Program, we embarked on a series of virtual and in-person meetings with our mentorship triads (one mentor and two mentees) and other mentorship teams over the 18-month program (see Figure). These meetings covered highly focused topics ranging from the role of advocacy in leadership to negotiation and developing a business plan, with ample opportunities for individually tailored mentorship within the mentorship triads.

We also completed personality assessments that helped us understand our strengths and areas of improvement, and ways to use the information to hone our leadership styles.
A large portion of programming and the mentorship experience during the AGA Future Leaders Program is focused on a leadership project that is aimed at addressing a societal driver of interest for the AGA. Examples of these societal drivers of interest include maximizing the role of women in gastroenterology, the role of artificial intelligence in gastroenterology, burnout, and the impact of climate change on gastroenterology. Mentorship triads propose novel methods for addressing these critical issues, outlining the roles that the AGA and other stakeholders may embrace to address these anticipated growing challenges head on.
Our mentorship triad was asked to address the issue of ending disparities within gastroenterology. Given our research and clinical interest in inflammatory bowel disease (IBD), we immediately recognized an opportunity to evaluate and potentially offer solutions for the geographic disparities that exist in the field of IBD. These disparities affect access to care for patients with Crohn’s disease and ulcerative colitis, leading to delays in diagnosis and ultimately effective therapy decisions.
In addition to developing a proposal for the AGA to expand access to care to major IBD centers in rural areas where these disparities exist, we also initiated an examination of geographic disparities in our own multidisciplinary IBD centers (abstract accepted for presentation at Digestive Diseases Week 2024). This allowed us to expand our respective research footprints at our institutions, utilizing new methods of geocoding to directly measure factors affecting clinical outcomes in IBD. Given our in-depth evaluation of this topic as part of our Future Leaders Program training, at the suggestion of our mentor, our mentorship triad also published a commentary on geographic disparities in the Diversity, Equity, and Inclusion sections of Gastroenterology and Clinical Gastroenterology and Hepatology.1, 2
Impact on the Field and Our Careers
Our mentorship triad had the unique experience of having a mentor who had previously participated in the Future Leaders Program as a mentee. As the Future Leaders Program has now enrolled 72 participants, these occasions will likely become more frequent, given the opportunities for career development and growth within the AGA (and our field) that are available after participating in the Future Leaders Program.
To have a mentor with this insight of having been a mentee in the program was invaluable, given her direct experience and understanding of the growth opportunities available, and opportunities to maximize participation in the Future Leaders Program. Additionally, as evidenced by Dr. Shaukat’s recommendations to grow our initial assignment into published commentaries, need statements for our field, and ultimately growing research projects, her keen insights as a mentor were a critical component of our individual growth in the program and the success of our mentorship triad. We benefited from networking with peers and learning about their work, which can lead to future collaborations. We had access to the highly accomplished mentors from diverse settings and learned models of leadership, while developing skills to foster our own leadership style.
In terms of programmatic impact, more than 90% of FLP alumni are serving in AGA leadership on committees, task forces, editorial boards, and councils. What is also important is the impact of content developed by mentee-mentor triads during the FLP cohorts over time. More than 700 GIs have benefited from online leadership development content created by the FLP. Based on our experience, we highly recommend all early career GI physicians to apply!
Dr. Parakkal (@P_DeepakIBDMD) is based in the division of gastroenterology, Washington University in St. Louis (Mo.) School of Medicine. He is supported by a Junior Faculty Development Award from the American College of Gastroenterology and IBD Plexus of the Crohn’s & Colitis Foundation. He has received research support under a sponsored research agreement unrelated to the data in the paper from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Prometheus Biosciences, Takeda Pharmaceuticals, Roche-Genentech, and CorEvitas LLC. He has served as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Scipher Medicine, Fresenius Kabi, Roche-Genentech, and CorEvitas LLC. Dr. Barnes (@EdBarnesMD) is based in the division of gastroenterology and hepatology, University of North Carolina at Chapel Hill. He is supported by National Institutes of Health K23DK127157-01, and has served as a consultant for Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Dr. Shaukat (@AasmaShaukatMD) is based in the division of gastroenterology, New York University, New York. She has served as a consultant for Iterative health, Motus, Freenome, and Geneoscopy. Research support by the Steve and Alex Cohen Foundation.
References
1. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 July. doi: 10.1053/j.gastro.2023.05.017.
2. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Clin Gastroenterol Hepatol. 2023 July. doi: 10.1016/j.cgh.2023.04.006.
Two of us (Parakkal Deepak and Edward L. Barnes) were part of the American Gastroenterological Association’s (AGA) Future Leaders Program (FLP) class of 2022-2023, and our mentor was Aasma Shaukat. We were invited to share our experiences as participants in the FLP and its impact in our careers.
Why Was the Future Leaders Program Conceived?
To understand this, one must first understand that the AGA, like all other GI professional organizations, relies on volunteer leaders to develop its long-term vision and execute this through strategic initiatives and programs. and understand the governance structure of the AGA to help lead it to face these challenges effectively.
The AGA FLP was thus conceived and launched in 2014-2015 by the founding chairs, Byron Cryer, MD, who is a professor of medicine and associate dean for faculty diversity at University of Texas Southwestern Medical School and Suzanne Rose, MD, MSEd, AGAF, who is a professor of medicine and senior vice dean for medical education at Perelman School of Medicine at the University of Pennsylvania. They envisioned a leadership pathway that would position early career GIs on a track to positively affect the AGA and the field of GI.
How Does One Apply for the Program?
Our FLP cohort applications were invited in October of 2021 and mentees accepted into the program in November 2021. The application process is competitive – applicants are encouraged to detail why they feel they would benefit from the FLP, what existing skillsets they have that can be further enhanced through the program, and what their long-term vision is for their growth as leaders, both within their institution and within the AGA. This is further accompanied by letters of support from their divisional chiefs and other key supervisors within the division who are intimately aware of their leadership potential and career trajectory. This process identified 18 future leaders for our class of 2022-2023.
What Is Involved?
Following acceptance into the AGA Future Leaders Program, we embarked on a series of virtual and in-person meetings with our mentorship triads (one mentor and two mentees) and other mentorship teams over the 18-month program (see Figure). These meetings covered highly focused topics ranging from the role of advocacy in leadership to negotiation and developing a business plan, with ample opportunities for individually tailored mentorship within the mentorship triads.

We also completed personality assessments that helped us understand our strengths and areas of improvement, and ways to use the information to hone our leadership styles.
A large portion of programming and the mentorship experience during the AGA Future Leaders Program is focused on a leadership project that is aimed at addressing a societal driver of interest for the AGA. Examples of these societal drivers of interest include maximizing the role of women in gastroenterology, the role of artificial intelligence in gastroenterology, burnout, and the impact of climate change on gastroenterology. Mentorship triads propose novel methods for addressing these critical issues, outlining the roles that the AGA and other stakeholders may embrace to address these anticipated growing challenges head on.
Our mentorship triad was asked to address the issue of ending disparities within gastroenterology. Given our research and clinical interest in inflammatory bowel disease (IBD), we immediately recognized an opportunity to evaluate and potentially offer solutions for the geographic disparities that exist in the field of IBD. These disparities affect access to care for patients with Crohn’s disease and ulcerative colitis, leading to delays in diagnosis and ultimately effective therapy decisions.
In addition to developing a proposal for the AGA to expand access to care to major IBD centers in rural areas where these disparities exist, we also initiated an examination of geographic disparities in our own multidisciplinary IBD centers (abstract accepted for presentation at Digestive Diseases Week 2024). This allowed us to expand our respective research footprints at our institutions, utilizing new methods of geocoding to directly measure factors affecting clinical outcomes in IBD. Given our in-depth evaluation of this topic as part of our Future Leaders Program training, at the suggestion of our mentor, our mentorship triad also published a commentary on geographic disparities in the Diversity, Equity, and Inclusion sections of Gastroenterology and Clinical Gastroenterology and Hepatology.1, 2
Impact on the Field and Our Careers
Our mentorship triad had the unique experience of having a mentor who had previously participated in the Future Leaders Program as a mentee. As the Future Leaders Program has now enrolled 72 participants, these occasions will likely become more frequent, given the opportunities for career development and growth within the AGA (and our field) that are available after participating in the Future Leaders Program.
To have a mentor with this insight of having been a mentee in the program was invaluable, given her direct experience and understanding of the growth opportunities available, and opportunities to maximize participation in the Future Leaders Program. Additionally, as evidenced by Dr. Shaukat’s recommendations to grow our initial assignment into published commentaries, need statements for our field, and ultimately growing research projects, her keen insights as a mentor were a critical component of our individual growth in the program and the success of our mentorship triad. We benefited from networking with peers and learning about their work, which can lead to future collaborations. We had access to the highly accomplished mentors from diverse settings and learned models of leadership, while developing skills to foster our own leadership style.
In terms of programmatic impact, more than 90% of FLP alumni are serving in AGA leadership on committees, task forces, editorial boards, and councils. What is also important is the impact of content developed by mentee-mentor triads during the FLP cohorts over time. More than 700 GIs have benefited from online leadership development content created by the FLP. Based on our experience, we highly recommend all early career GI physicians to apply!
Dr. Parakkal (@P_DeepakIBDMD) is based in the division of gastroenterology, Washington University in St. Louis (Mo.) School of Medicine. He is supported by a Junior Faculty Development Award from the American College of Gastroenterology and IBD Plexus of the Crohn’s & Colitis Foundation. He has received research support under a sponsored research agreement unrelated to the data in the paper from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Prometheus Biosciences, Takeda Pharmaceuticals, Roche-Genentech, and CorEvitas LLC. He has served as a consultant for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Scipher Medicine, Fresenius Kabi, Roche-Genentech, and CorEvitas LLC. Dr. Barnes (@EdBarnesMD) is based in the division of gastroenterology and hepatology, University of North Carolina at Chapel Hill. He is supported by National Institutes of Health K23DK127157-01, and has served as a consultant for Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Dr. Shaukat (@AasmaShaukatMD) is based in the division of gastroenterology, New York University, New York. She has served as a consultant for Iterative health, Motus, Freenome, and Geneoscopy. Research support by the Steve and Alex Cohen Foundation.
References
1. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Gastroenterology. 2023 July. doi: 10.1053/j.gastro.2023.05.017.
2. Deepak P, Barnes EL, Shaukat A. Health Disparities in Inflammatory Bowel Disease Care Driven by Rural Versus Urban Residence: Challenges and Potential Solutions. Clin Gastroenterol Hepatol. 2023 July. doi: 10.1016/j.cgh.2023.04.006.
Navigating the Search for a Financial Adviser
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?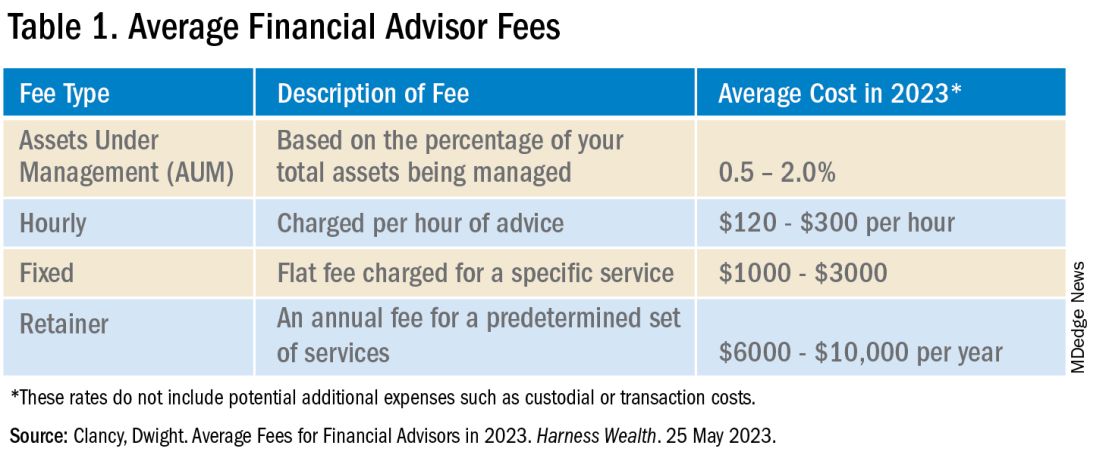
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.







