User login
The fate of the ACA now rests with the U.S. Supreme Court
The U.S. Supreme Court has agreed to hear Texas v. California, a closely watched case that could upend the Affordable Care Act.
The justices will hear oral arguments in the case in fall 2020, with a ruling likely in 2021.
The Texas case, consolidated with a similar challenge, stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Since the Trump administration declined to defend the ACA, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. On March 2, the U.S. Supreme Court granted two petitions by the defendants requesting that the high court review the appeals court decision.
The review follows a previous look at the ACA’s mandate by the Supreme Court in 2012. In National Federation of Independent Business v. Sebelius, justices upheld the ACA’s insurance mandate as constitutional, ruling the requirement was authorized by Congress’ power to levy taxes. The vote was 5-4, with Chief Justice John G. Roberts Jr. in agreement with the court’s four more liberal members.
The U.S. Supreme Court has agreed to hear Texas v. California, a closely watched case that could upend the Affordable Care Act.
The justices will hear oral arguments in the case in fall 2020, with a ruling likely in 2021.
The Texas case, consolidated with a similar challenge, stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Since the Trump administration declined to defend the ACA, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. On March 2, the U.S. Supreme Court granted two petitions by the defendants requesting that the high court review the appeals court decision.
The review follows a previous look at the ACA’s mandate by the Supreme Court in 2012. In National Federation of Independent Business v. Sebelius, justices upheld the ACA’s insurance mandate as constitutional, ruling the requirement was authorized by Congress’ power to levy taxes. The vote was 5-4, with Chief Justice John G. Roberts Jr. in agreement with the court’s four more liberal members.
The U.S. Supreme Court has agreed to hear Texas v. California, a closely watched case that could upend the Affordable Care Act.
The justices will hear oral arguments in the case in fall 2020, with a ruling likely in 2021.
The Texas case, consolidated with a similar challenge, stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Since the Trump administration declined to defend the ACA, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. On March 2, the U.S. Supreme Court granted two petitions by the defendants requesting that the high court review the appeals court decision.
The review follows a previous look at the ACA’s mandate by the Supreme Court in 2012. In National Federation of Independent Business v. Sebelius, justices upheld the ACA’s insurance mandate as constitutional, ruling the requirement was authorized by Congress’ power to levy taxes. The vote was 5-4, with Chief Justice John G. Roberts Jr. in agreement with the court’s four more liberal members.
Handoffs in Dermatology Residency
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.

This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
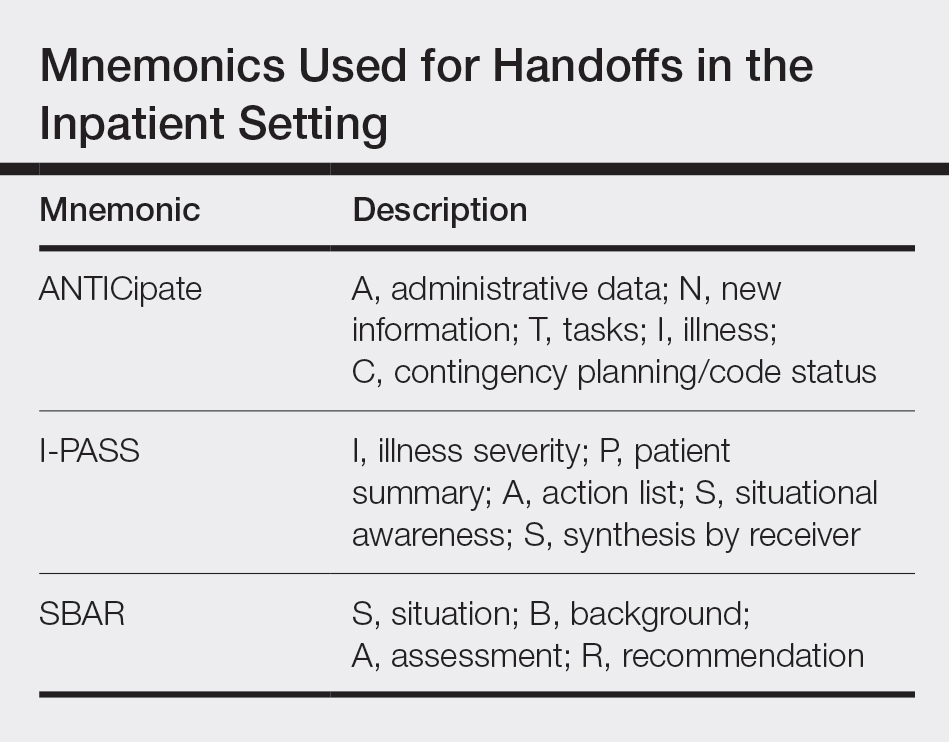
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.

This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s

Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.

This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s

Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
Resident Pearl
- For dermatology residents, ensuring organized handoff and follow-up practices is essential. Residency provides an opportunity to become familiar with different practices to take forward in one’s career.
Are patient portals living up to the hype? Ask your mother-in-law!
While preparing to write this technology column, I received a great deal of insight from the unlikeliest of sources: my mother-in-law.
Now don’t get me wrong – she’s a truly lovely, intelligent, and capable woman. I have sought her advice often on many things and have always been impressed by her wisdom and pragmatism, but I’ve just never thought of asking her for her opinion on medicine or technology, as I considered her knowledge of both subjects to be limited.
This occasion changed my opinion. In fact, I believe that, as health care IT becomes more complex, people like my mother-in-law may be exactly who we should be looking to for answers.
A few weeks ago, my mother-in-law and I were discussing her recent trip to the doctor. When she mentioned some lab tests, I suggested that we log in to her patient portal to view the results. This elicited several questions and a declaration of frustration.
“Which portal?” she asked. “I have so many and can’t keep all of the websites and passwords straight! Why can’t all of my doctors use the same portal, and why do they all have different password requirements?”
As she spoke these words, I was immediately struck with an unfortunate reality of EHRs: We have done a brilliant job creating state-of-the-art digital castles and have filled them with the data needed to revolutionize care and improve population health – but we haven’t given our patients the keys to get inside.
We must ask ourselves if, in trying to construct fortresses of information around our patients, we have lost sight of the individuals in the center. I believe that we can answer this question and improve the benefits of patient portals, but we all must agree to a few simple steps to streamline the experience for everyone.
Make it easy
A study recently published in the Journal of General Internal Medicine surveyed several hospitals on their usage of patient portals. After determining whether or not the institutions had such portals, the authors then investigated to find out what, if any, guidance was provided to patients about how to use them.
Their findings are frustrating, though not surprising. While 89% of hospitals had some form of patient portal, only 65% of those “had links that were easily found, defined as links accessible within two clicks from the home page.”
Furthermore, even in cases where portals were easily found, good instructions on how to use them were missing. Those instructions that did exist centered on rules and restrictions and laying out “terms and conditions” and informing patients on “what not to do,” rather than explaining how to make the most of the experience.
According to the authors, “this focus on curtailing behavior, and the hurdles placed on finding and understanding guidance, suggest that some hospitals may be prioritizing reducing liability over improving the patient experience with portals.”
If we want our patients to use them, portals must be easy to access and intuitive to use. They also must provide value.
Make it meaningful
Patient portals have proliferated exponentially over the last 10 years, thanks to government incentive programs. One such program, known as “meaningful use,” is primarily responsible for this, as it made implementation of a patient portal one of its core requirements.
Sadly, in spite of its oft-reviled name, the meaningful use program never defined patient-friendly standards of usability for patient portals. As a result, current portals just aren’t very good. Patients like my mother-in-law find them to be too numerous, too unfriendly to use, and too limited, so they are not being used to their full potential.
In fact, many institutions may choose not to enable all of the available features in order to limit technical issues and reduce the burden on providers. In the study referenced above, only 63% of portals offered the ability for patients to communicate directly with their physicians, and only 43% offered the ability to refill prescriptions.
When enabled, these functions improve patient engagement and efficiency. Without them, patients are less likely to log on, and physicians are forced to rely on less-efficient telephone calls or traditional letters to communicate results to their patients.
Put the patient, not the portal, at the center
History has all but forgotten the attempts by tech giants such as Google and Microsoft to create personal health records. While these initially seemed like a wonderful concept, they sadly proved to be a total flop. Some patients embraced the idea, but security concerns and the lack of buy-in from EHR vendors significantly limited their uptake.
They may simply have been ahead of their time.
A decade later, wearable technology and telemedicine are ushering in a new era of patient-centric care. Individuals have been embracing a greater share of the responsibility for their own personal health information, yet most EHRs lack the ability to easily incorporate data acquired outside physicians’ offices.
It’s time for EHR vendors to go all in and change that. Instead of enslaving patients to the tyranny of fragmented health records, they should prioritize the creation of a robust, standardized, and portable health record that travels with the patient, not the other way around.
Have any other ideas on how to improve patient engagement? We’d love to hear about them and share them in a future column.
If you want to contribute but don’t have any ideas, we have a suggestion: Ask your mother-in-law. You may be surprised at what you learn!
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Hospital–Jefferson Health. Follow him on twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health.
Reference
Lee JL et al. J Gen Intern Med. 2019 Nov 12. doi: 10.1007/s11606-019-05528-z.
While preparing to write this technology column, I received a great deal of insight from the unlikeliest of sources: my mother-in-law.
Now don’t get me wrong – she’s a truly lovely, intelligent, and capable woman. I have sought her advice often on many things and have always been impressed by her wisdom and pragmatism, but I’ve just never thought of asking her for her opinion on medicine or technology, as I considered her knowledge of both subjects to be limited.
This occasion changed my opinion. In fact, I believe that, as health care IT becomes more complex, people like my mother-in-law may be exactly who we should be looking to for answers.
A few weeks ago, my mother-in-law and I were discussing her recent trip to the doctor. When she mentioned some lab tests, I suggested that we log in to her patient portal to view the results. This elicited several questions and a declaration of frustration.
“Which portal?” she asked. “I have so many and can’t keep all of the websites and passwords straight! Why can’t all of my doctors use the same portal, and why do they all have different password requirements?”
As she spoke these words, I was immediately struck with an unfortunate reality of EHRs: We have done a brilliant job creating state-of-the-art digital castles and have filled them with the data needed to revolutionize care and improve population health – but we haven’t given our patients the keys to get inside.
We must ask ourselves if, in trying to construct fortresses of information around our patients, we have lost sight of the individuals in the center. I believe that we can answer this question and improve the benefits of patient portals, but we all must agree to a few simple steps to streamline the experience for everyone.
Make it easy
A study recently published in the Journal of General Internal Medicine surveyed several hospitals on their usage of patient portals. After determining whether or not the institutions had such portals, the authors then investigated to find out what, if any, guidance was provided to patients about how to use them.
Their findings are frustrating, though not surprising. While 89% of hospitals had some form of patient portal, only 65% of those “had links that were easily found, defined as links accessible within two clicks from the home page.”
Furthermore, even in cases where portals were easily found, good instructions on how to use them were missing. Those instructions that did exist centered on rules and restrictions and laying out “terms and conditions” and informing patients on “what not to do,” rather than explaining how to make the most of the experience.
According to the authors, “this focus on curtailing behavior, and the hurdles placed on finding and understanding guidance, suggest that some hospitals may be prioritizing reducing liability over improving the patient experience with portals.”
If we want our patients to use them, portals must be easy to access and intuitive to use. They also must provide value.
Make it meaningful
Patient portals have proliferated exponentially over the last 10 years, thanks to government incentive programs. One such program, known as “meaningful use,” is primarily responsible for this, as it made implementation of a patient portal one of its core requirements.
Sadly, in spite of its oft-reviled name, the meaningful use program never defined patient-friendly standards of usability for patient portals. As a result, current portals just aren’t very good. Patients like my mother-in-law find them to be too numerous, too unfriendly to use, and too limited, so they are not being used to their full potential.
In fact, many institutions may choose not to enable all of the available features in order to limit technical issues and reduce the burden on providers. In the study referenced above, only 63% of portals offered the ability for patients to communicate directly with their physicians, and only 43% offered the ability to refill prescriptions.
When enabled, these functions improve patient engagement and efficiency. Without them, patients are less likely to log on, and physicians are forced to rely on less-efficient telephone calls or traditional letters to communicate results to their patients.
Put the patient, not the portal, at the center
History has all but forgotten the attempts by tech giants such as Google and Microsoft to create personal health records. While these initially seemed like a wonderful concept, they sadly proved to be a total flop. Some patients embraced the idea, but security concerns and the lack of buy-in from EHR vendors significantly limited their uptake.
They may simply have been ahead of their time.
A decade later, wearable technology and telemedicine are ushering in a new era of patient-centric care. Individuals have been embracing a greater share of the responsibility for their own personal health information, yet most EHRs lack the ability to easily incorporate data acquired outside physicians’ offices.
It’s time for EHR vendors to go all in and change that. Instead of enslaving patients to the tyranny of fragmented health records, they should prioritize the creation of a robust, standardized, and portable health record that travels with the patient, not the other way around.
Have any other ideas on how to improve patient engagement? We’d love to hear about them and share them in a future column.
If you want to contribute but don’t have any ideas, we have a suggestion: Ask your mother-in-law. You may be surprised at what you learn!
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Hospital–Jefferson Health. Follow him on twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health.
Reference
Lee JL et al. J Gen Intern Med. 2019 Nov 12. doi: 10.1007/s11606-019-05528-z.
While preparing to write this technology column, I received a great deal of insight from the unlikeliest of sources: my mother-in-law.
Now don’t get me wrong – she’s a truly lovely, intelligent, and capable woman. I have sought her advice often on many things and have always been impressed by her wisdom and pragmatism, but I’ve just never thought of asking her for her opinion on medicine or technology, as I considered her knowledge of both subjects to be limited.
This occasion changed my opinion. In fact, I believe that, as health care IT becomes more complex, people like my mother-in-law may be exactly who we should be looking to for answers.
A few weeks ago, my mother-in-law and I were discussing her recent trip to the doctor. When she mentioned some lab tests, I suggested that we log in to her patient portal to view the results. This elicited several questions and a declaration of frustration.
“Which portal?” she asked. “I have so many and can’t keep all of the websites and passwords straight! Why can’t all of my doctors use the same portal, and why do they all have different password requirements?”
As she spoke these words, I was immediately struck with an unfortunate reality of EHRs: We have done a brilliant job creating state-of-the-art digital castles and have filled them with the data needed to revolutionize care and improve population health – but we haven’t given our patients the keys to get inside.
We must ask ourselves if, in trying to construct fortresses of information around our patients, we have lost sight of the individuals in the center. I believe that we can answer this question and improve the benefits of patient portals, but we all must agree to a few simple steps to streamline the experience for everyone.
Make it easy
A study recently published in the Journal of General Internal Medicine surveyed several hospitals on their usage of patient portals. After determining whether or not the institutions had such portals, the authors then investigated to find out what, if any, guidance was provided to patients about how to use them.
Their findings are frustrating, though not surprising. While 89% of hospitals had some form of patient portal, only 65% of those “had links that were easily found, defined as links accessible within two clicks from the home page.”
Furthermore, even in cases where portals were easily found, good instructions on how to use them were missing. Those instructions that did exist centered on rules and restrictions and laying out “terms and conditions” and informing patients on “what not to do,” rather than explaining how to make the most of the experience.
According to the authors, “this focus on curtailing behavior, and the hurdles placed on finding and understanding guidance, suggest that some hospitals may be prioritizing reducing liability over improving the patient experience with portals.”
If we want our patients to use them, portals must be easy to access and intuitive to use. They also must provide value.
Make it meaningful
Patient portals have proliferated exponentially over the last 10 years, thanks to government incentive programs. One such program, known as “meaningful use,” is primarily responsible for this, as it made implementation of a patient portal one of its core requirements.
Sadly, in spite of its oft-reviled name, the meaningful use program never defined patient-friendly standards of usability for patient portals. As a result, current portals just aren’t very good. Patients like my mother-in-law find them to be too numerous, too unfriendly to use, and too limited, so they are not being used to their full potential.
In fact, many institutions may choose not to enable all of the available features in order to limit technical issues and reduce the burden on providers. In the study referenced above, only 63% of portals offered the ability for patients to communicate directly with their physicians, and only 43% offered the ability to refill prescriptions.
When enabled, these functions improve patient engagement and efficiency. Without them, patients are less likely to log on, and physicians are forced to rely on less-efficient telephone calls or traditional letters to communicate results to their patients.
Put the patient, not the portal, at the center
History has all but forgotten the attempts by tech giants such as Google and Microsoft to create personal health records. While these initially seemed like a wonderful concept, they sadly proved to be a total flop. Some patients embraced the idea, but security concerns and the lack of buy-in from EHR vendors significantly limited their uptake.
They may simply have been ahead of their time.
A decade later, wearable technology and telemedicine are ushering in a new era of patient-centric care. Individuals have been embracing a greater share of the responsibility for their own personal health information, yet most EHRs lack the ability to easily incorporate data acquired outside physicians’ offices.
It’s time for EHR vendors to go all in and change that. Instead of enslaving patients to the tyranny of fragmented health records, they should prioritize the creation of a robust, standardized, and portable health record that travels with the patient, not the other way around.
Have any other ideas on how to improve patient engagement? We’d love to hear about them and share them in a future column.
If you want to contribute but don’t have any ideas, we have a suggestion: Ask your mother-in-law. You may be surprised at what you learn!
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Hospital–Jefferson Health. Follow him on twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health.
Reference
Lee JL et al. J Gen Intern Med. 2019 Nov 12. doi: 10.1007/s11606-019-05528-z.
Supreme Court roundup: Latest health care decisions
The Trump administration can move forward with expanding a rule that makes it more difficult for immigrants to remain in the United States if they receive health care assistance, the U.S. Supreme Court ruled in a 5-4 vote.
The Feb. 21 order allows the administration to broaden the so-called “public charge rule” while legal challenges against the expanded regulation continue in the lower courts. The Supreme Court’s decision, which lifts a preliminary injunction against the expansion, applies to enforcement only in Illinois, where a district court blocked the revised rule from moving forward in October 2019. The Supreme Court’s measure follows another 5-4 order in January, in which justices lifted a nationwide injunction against the revised rule.
Under the long-standing public charge rule, immigration officials can refuse to admit immigrants into the United States or can deny them permanent legal status if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid, the Supplemental Nutrition Assistance Program, and several housing programs. Use of these programs for more than 12 months in the aggregate during a 36-month period may result in a “public charge” designation and lead to green card denial.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued by 14 states. At least five trial courts have since blocked the measure, while appeals courts have lifted some of the injunctions and upheld enforcement.
In its Jan. 27 order lifting the nationwide injunction, Associate Justice Neil M. Gorsuch wrote that nationwide injunctions are being overused by trial courts with negative consequences.
“The real problem here is the increasingly common practice of trial courts ordering relief that transcends the cases before them. Whether framed as injunctions of ‘nationwide,’ ‘universal,’ or ‘cosmic’ scope, these orders share the same basic flaw – they direct how the defendant must act toward persons who are not parties to the case,” he wrote. “It has become increasingly apparent that this court must, at some point, confront these important objections to this increasingly widespread practice. As the brief and furious history of the regulation before us illustrates, the routine issuance of universal injunctions is patently unworkable, sowing chaos for litigants, the government, courts, and all those affected by these conflicting decisions.”
In the court’s Feb. 21 order lifting the injunction in Illinois, justices gave no explanation for overturning the lower court’s injunction. However, Associate Justice Sonia Sotomayor issued a sharply-worded dissent, criticizing her fellow justices for allowing the rule to proceed.
“In sum, the government’s only claimed hardship is that it must enforce an existing interpretation of an immigration rule in one state – just as it has done for the past 20 years – while an updated version of the rule takes effect in the remaining 49,” she wrote. “The government has not quantified or explained any burdens that would arise from this state of the world.”
ACA cases still in limbo
Meanwhile, the Supreme Court still has not decided whether it will hear Texas v. United States, a case that could effectively dismantle the Affordable Care Act.
The high court was expected to announce whether it would take the high-profile case at a private Feb. 21 conference, but the justices have released no update. The case was relisted for consideration at the court’s Feb. 28 conference.
Texas v. United States stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Because the Trump administration declined to defend the law, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. The Democratic attorneys general and governors appealed the decision to the U.S. Supreme Court.
If the Supreme Court agrees to hear the challenge, the court could fast-track the case and schedule arguments for the current term or wait until its next term, which starts in October 2020. If justices decline to hear the case, the challenge will remain with the district court for more analysis about the law’s severability.
Another ACA-related case – Maine Community Health Options v. U.S. – also remains in limbo. Justices heard the case, which was consolidated with two similar challenges, on Dec. 10, 2019, but still have not issued a decision.
The consolidated challenges center on whether the federal government owes insurers billions based on an Affordable Care Act provision intended to help health plans mitigate risk under the law. The ACA’s risk corridor program required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged and they want the Supreme Court to force the government to reimburse them to the tune of $12 billion.
The Department of Justice counters that the government is not required to pay the insurers because of appropriations measures passed by Congress in 2014 and in later years that limited the funding available to compensate insurers for their losses.
The federal government and insurers have each experienced wins and losses at the lower court level. Most recently, the U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
A Supreme Court decision in the case could come as soon as Feb. 26.
Court to hear women’s health cases
Two closely watched reproductive health cases will go before the court this spring.
On March 4, justices will hear oral arguments in June Medical Services v. Russo, regarding the constitutionality of a Louisiana law that requires physicians performing abortions to have admitting privileges at a nearby hospital. Doctors who perform abortions without admitting privileges at a hospital within 30 miles face fines and imprisonment, according to the state law, originally passed in 2014. Clinics that employ such doctors can also have their licenses revoked.
June Medical Services LLC, a women’s health clinic, sued over the law. A district court ruled in favor of the plaintiff, but the 5th U.S. Circuit Court of Appeals reversed and upheld Louisiana’s law. The clinic appealed to the U.S. Supreme Court. Louisiana officials argue the challenge should be dismissed, and the law allowed to proceed, because the plaintiffs lack standing.
The Supreme Court in 2016 heard a similar case – Whole Woman’s Health v. Hellerstedt – concerning a comparable law in Texas. In that case, justices struck down the measure as unconstitutional.
And on April 29, justices will hear arguments in Little Sisters of the Poor v. Pennsylvania, a consolidated case about whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraceptive mandate. Entities that object to providing contraception on the basis of religious beliefs can opt out of complying with the mandate, according to the 2018 regulations. Additionally, nonprofit organizations and small businesses that have nonreligious moral convictions against the mandate can skip compliance. A number of states and entities sued over the new rules.
A federal appeals court temporarily barred the regulations from moving forward, ruling the plaintiffs were likely to succeed in proving the Trump administration did not follow appropriate procedures when it promulgated the new rules and that the regulations were not authorized under the ACA.
Justices will decide whether the parties have standing in the case, whether the Trump administration followed correct rule-making procedures, and if the regulations can stand.
The Trump administration can move forward with expanding a rule that makes it more difficult for immigrants to remain in the United States if they receive health care assistance, the U.S. Supreme Court ruled in a 5-4 vote.
The Feb. 21 order allows the administration to broaden the so-called “public charge rule” while legal challenges against the expanded regulation continue in the lower courts. The Supreme Court’s decision, which lifts a preliminary injunction against the expansion, applies to enforcement only in Illinois, where a district court blocked the revised rule from moving forward in October 2019. The Supreme Court’s measure follows another 5-4 order in January, in which justices lifted a nationwide injunction against the revised rule.
Under the long-standing public charge rule, immigration officials can refuse to admit immigrants into the United States or can deny them permanent legal status if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid, the Supplemental Nutrition Assistance Program, and several housing programs. Use of these programs for more than 12 months in the aggregate during a 36-month period may result in a “public charge” designation and lead to green card denial.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued by 14 states. At least five trial courts have since blocked the measure, while appeals courts have lifted some of the injunctions and upheld enforcement.
In its Jan. 27 order lifting the nationwide injunction, Associate Justice Neil M. Gorsuch wrote that nationwide injunctions are being overused by trial courts with negative consequences.
“The real problem here is the increasingly common practice of trial courts ordering relief that transcends the cases before them. Whether framed as injunctions of ‘nationwide,’ ‘universal,’ or ‘cosmic’ scope, these orders share the same basic flaw – they direct how the defendant must act toward persons who are not parties to the case,” he wrote. “It has become increasingly apparent that this court must, at some point, confront these important objections to this increasingly widespread practice. As the brief and furious history of the regulation before us illustrates, the routine issuance of universal injunctions is patently unworkable, sowing chaos for litigants, the government, courts, and all those affected by these conflicting decisions.”
In the court’s Feb. 21 order lifting the injunction in Illinois, justices gave no explanation for overturning the lower court’s injunction. However, Associate Justice Sonia Sotomayor issued a sharply-worded dissent, criticizing her fellow justices for allowing the rule to proceed.
“In sum, the government’s only claimed hardship is that it must enforce an existing interpretation of an immigration rule in one state – just as it has done for the past 20 years – while an updated version of the rule takes effect in the remaining 49,” she wrote. “The government has not quantified or explained any burdens that would arise from this state of the world.”
ACA cases still in limbo
Meanwhile, the Supreme Court still has not decided whether it will hear Texas v. United States, a case that could effectively dismantle the Affordable Care Act.
The high court was expected to announce whether it would take the high-profile case at a private Feb. 21 conference, but the justices have released no update. The case was relisted for consideration at the court’s Feb. 28 conference.
Texas v. United States stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Because the Trump administration declined to defend the law, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. The Democratic attorneys general and governors appealed the decision to the U.S. Supreme Court.
If the Supreme Court agrees to hear the challenge, the court could fast-track the case and schedule arguments for the current term or wait until its next term, which starts in October 2020. If justices decline to hear the case, the challenge will remain with the district court for more analysis about the law’s severability.
Another ACA-related case – Maine Community Health Options v. U.S. – also remains in limbo. Justices heard the case, which was consolidated with two similar challenges, on Dec. 10, 2019, but still have not issued a decision.
The consolidated challenges center on whether the federal government owes insurers billions based on an Affordable Care Act provision intended to help health plans mitigate risk under the law. The ACA’s risk corridor program required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged and they want the Supreme Court to force the government to reimburse them to the tune of $12 billion.
The Department of Justice counters that the government is not required to pay the insurers because of appropriations measures passed by Congress in 2014 and in later years that limited the funding available to compensate insurers for their losses.
The federal government and insurers have each experienced wins and losses at the lower court level. Most recently, the U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
A Supreme Court decision in the case could come as soon as Feb. 26.
Court to hear women’s health cases
Two closely watched reproductive health cases will go before the court this spring.
On March 4, justices will hear oral arguments in June Medical Services v. Russo, regarding the constitutionality of a Louisiana law that requires physicians performing abortions to have admitting privileges at a nearby hospital. Doctors who perform abortions without admitting privileges at a hospital within 30 miles face fines and imprisonment, according to the state law, originally passed in 2014. Clinics that employ such doctors can also have their licenses revoked.
June Medical Services LLC, a women’s health clinic, sued over the law. A district court ruled in favor of the plaintiff, but the 5th U.S. Circuit Court of Appeals reversed and upheld Louisiana’s law. The clinic appealed to the U.S. Supreme Court. Louisiana officials argue the challenge should be dismissed, and the law allowed to proceed, because the plaintiffs lack standing.
The Supreme Court in 2016 heard a similar case – Whole Woman’s Health v. Hellerstedt – concerning a comparable law in Texas. In that case, justices struck down the measure as unconstitutional.
And on April 29, justices will hear arguments in Little Sisters of the Poor v. Pennsylvania, a consolidated case about whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraceptive mandate. Entities that object to providing contraception on the basis of religious beliefs can opt out of complying with the mandate, according to the 2018 regulations. Additionally, nonprofit organizations and small businesses that have nonreligious moral convictions against the mandate can skip compliance. A number of states and entities sued over the new rules.
A federal appeals court temporarily barred the regulations from moving forward, ruling the plaintiffs were likely to succeed in proving the Trump administration did not follow appropriate procedures when it promulgated the new rules and that the regulations were not authorized under the ACA.
Justices will decide whether the parties have standing in the case, whether the Trump administration followed correct rule-making procedures, and if the regulations can stand.
The Trump administration can move forward with expanding a rule that makes it more difficult for immigrants to remain in the United States if they receive health care assistance, the U.S. Supreme Court ruled in a 5-4 vote.
The Feb. 21 order allows the administration to broaden the so-called “public charge rule” while legal challenges against the expanded regulation continue in the lower courts. The Supreme Court’s decision, which lifts a preliminary injunction against the expansion, applies to enforcement only in Illinois, where a district court blocked the revised rule from moving forward in October 2019. The Supreme Court’s measure follows another 5-4 order in January, in which justices lifted a nationwide injunction against the revised rule.
Under the long-standing public charge rule, immigration officials can refuse to admit immigrants into the United States or can deny them permanent legal status if they are deemed likely to become a public charge. Previously, immigration officers considered cash aid, such as Temporary Assistance for Needy Families or long-term institutionalized care, as potential public charge reasons for denial.
The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid, the Supplemental Nutrition Assistance Program, and several housing programs. Use of these programs for more than 12 months in the aggregate during a 36-month period may result in a “public charge” designation and lead to green card denial.
Eight legal challenges were immediately filed against the rule changes, including a complaint issued by 14 states. At least five trial courts have since blocked the measure, while appeals courts have lifted some of the injunctions and upheld enforcement.
In its Jan. 27 order lifting the nationwide injunction, Associate Justice Neil M. Gorsuch wrote that nationwide injunctions are being overused by trial courts with negative consequences.
“The real problem here is the increasingly common practice of trial courts ordering relief that transcends the cases before them. Whether framed as injunctions of ‘nationwide,’ ‘universal,’ or ‘cosmic’ scope, these orders share the same basic flaw – they direct how the defendant must act toward persons who are not parties to the case,” he wrote. “It has become increasingly apparent that this court must, at some point, confront these important objections to this increasingly widespread practice. As the brief and furious history of the regulation before us illustrates, the routine issuance of universal injunctions is patently unworkable, sowing chaos for litigants, the government, courts, and all those affected by these conflicting decisions.”
In the court’s Feb. 21 order lifting the injunction in Illinois, justices gave no explanation for overturning the lower court’s injunction. However, Associate Justice Sonia Sotomayor issued a sharply-worded dissent, criticizing her fellow justices for allowing the rule to proceed.
“In sum, the government’s only claimed hardship is that it must enforce an existing interpretation of an immigration rule in one state – just as it has done for the past 20 years – while an updated version of the rule takes effect in the remaining 49,” she wrote. “The government has not quantified or explained any burdens that would arise from this state of the world.”
ACA cases still in limbo
Meanwhile, the Supreme Court still has not decided whether it will hear Texas v. United States, a case that could effectively dismantle the Affordable Care Act.
The high court was expected to announce whether it would take the high-profile case at a private Feb. 21 conference, but the justices have released no update. The case was relisted for consideration at the court’s Feb. 28 conference.
Texas v. United States stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Because the Trump administration declined to defend the law, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. The Democratic attorneys general and governors appealed the decision to the U.S. Supreme Court.
If the Supreme Court agrees to hear the challenge, the court could fast-track the case and schedule arguments for the current term or wait until its next term, which starts in October 2020. If justices decline to hear the case, the challenge will remain with the district court for more analysis about the law’s severability.
Another ACA-related case – Maine Community Health Options v. U.S. – also remains in limbo. Justices heard the case, which was consolidated with two similar challenges, on Dec. 10, 2019, but still have not issued a decision.
The consolidated challenges center on whether the federal government owes insurers billions based on an Affordable Care Act provision intended to help health plans mitigate risk under the law. The ACA’s risk corridor program required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged and they want the Supreme Court to force the government to reimburse them to the tune of $12 billion.
The Department of Justice counters that the government is not required to pay the insurers because of appropriations measures passed by Congress in 2014 and in later years that limited the funding available to compensate insurers for their losses.
The federal government and insurers have each experienced wins and losses at the lower court level. Most recently, the U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
A Supreme Court decision in the case could come as soon as Feb. 26.
Court to hear women’s health cases
Two closely watched reproductive health cases will go before the court this spring.
On March 4, justices will hear oral arguments in June Medical Services v. Russo, regarding the constitutionality of a Louisiana law that requires physicians performing abortions to have admitting privileges at a nearby hospital. Doctors who perform abortions without admitting privileges at a hospital within 30 miles face fines and imprisonment, according to the state law, originally passed in 2014. Clinics that employ such doctors can also have their licenses revoked.
June Medical Services LLC, a women’s health clinic, sued over the law. A district court ruled in favor of the plaintiff, but the 5th U.S. Circuit Court of Appeals reversed and upheld Louisiana’s law. The clinic appealed to the U.S. Supreme Court. Louisiana officials argue the challenge should be dismissed, and the law allowed to proceed, because the plaintiffs lack standing.
The Supreme Court in 2016 heard a similar case – Whole Woman’s Health v. Hellerstedt – concerning a comparable law in Texas. In that case, justices struck down the measure as unconstitutional.
And on April 29, justices will hear arguments in Little Sisters of the Poor v. Pennsylvania, a consolidated case about whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraceptive mandate. Entities that object to providing contraception on the basis of religious beliefs can opt out of complying with the mandate, according to the 2018 regulations. Additionally, nonprofit organizations and small businesses that have nonreligious moral convictions against the mandate can skip compliance. A number of states and entities sued over the new rules.
A federal appeals court temporarily barred the regulations from moving forward, ruling the plaintiffs were likely to succeed in proving the Trump administration did not follow appropriate procedures when it promulgated the new rules and that the regulations were not authorized under the ACA.
Justices will decide whether the parties have standing in the case, whether the Trump administration followed correct rule-making procedures, and if the regulations can stand.
CMS's new rule for antibiotic stewardship: The case of H. pylori
The Centers for Medicare and Medicaid Services (CMS) finalized a new regulation requiring all hospitals participating in its programs to establish antimicrobial stewardship programs by March 30, 2020 (https://federalregister.gov/d/2019-20736). This welcome action was prompted by the rise in antimicrobial resistance; recent Centers for Disease Control and Prevention estimates more than 2.8 million antibiotic-resistant infections with more than 35,000 deaths occur in the United States each year (https://www.cdc.gov/drugresistance/biggest-threats.html). CMS recommended that hospitals follow stewardship guidelines established by CDC, and other nationally recognized sources (https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf). Antibiotic stewardship includes optimization of several aspects of antimicrobial therapy including the drugs, formulations, doses, and dosing intervals. It also includes obtaining and updating local, regional, and national susceptibility data to provide regularly updated guidance regarding diagnosis and therapy.
How does this new CMS rule affect gastroenterologists and what role, if any, do we play in the epidemic of antibiotic resistance? The one infectious disease that gastroenterology effectively owns is Helicobacter pylori. Treatment of this disease has the potential to be involved in the epidemic of antimicrobial resistance. Current H. pylori therapies were largely devised without considering the principles of antibiotic stewardship. Therapies for H. pylori have largely been developed using trial and error, antimicrobial susceptibility testing is rarely available, and local and regional susceptibility are not readily available or updated. Current national treatment recommendations are most often based on comparisons of regimens grouping trials containing different drugs, doses, and durations of therapy performed in populations in whom resistance was neither assessed nor taken into account. Finally, some of the most highly recommended effective regimens inevitably contain at least one antibiotic unnecessary for the outcome, and inadvertently serve to increase population antibiotic exposure.
Clarithromycin-amoxicillin-PPI triple is still the most often used legacy therapy in the United States with an average cure rate of 70%. Recent guidelines have suggested adding a fourth drug, metronidazole to produce a quadruple therapy (concomitant therapy); the premise is that although both clarithromycin and metronidazole resistance are common, dual resistance is not. However, this benefit comes at the price of every patient receiving at least one unnecessary antibiotic, and patients with treatment failures receiving three unnecessary antibiotics. The cumulative effect given the approximately 2 million treatments annually is 10s of thousands of kilograms of inappropriate antibiotic use annually with the likely consequence of increasing resistance.
How to move forward? The CDC documents regarding antimicrobial stewardship in hospitals with limited resources (https://www.cdc.gov/antibiotic-use/core-elements/resource-limited.html) suggest creation and promotion of evidence-based treatment guidelines for common clinical syndromes, tracking of antibiotic dispensing using available data, setting of national targets for improvement, and description of resistance patterns to improve treatment guidelines and identify priority pathogens. The CDC documents require creation and promotion of evidence-and susceptibility-based treatment guidelines, tracking of antibiotic dispensing and setting targets for improvement (i.e., monitoring and reporting). It is important to note that the CMS rule focused on hospitals as hospitals have traditionally been the sites where local susceptibility data are obtained and gathered to provide the regional data and updated treatment guidelines used to treat most infectious diseases.
The Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States in 2017 had several recommendations that would effectively address the CMS rule and CDC recommendations. For example, statement 15: that empiric eradication therapy for H. pylori be based on region or population-specific antibiotic susceptibility data (Grade 1B); Statement 17: that validated diagnostic testing of stool or gastric mucosal biopsy by culture and susceptibility, or molecular analysis be universally available (Grade 1); Statement 18: that antibiotics that may be routinely evaluated for susceptibility include amoxicillin, clarithromycin, levofloxacin, metronidazole, and tetracycline. (Grade 2C); and Statement 19: that professional societies provide the research needed to support evidence-based reimbursement decisions for antibiotic susceptibility testing for H. pylori (Grade 1).
Organized gastroenterology needs to join with the infectious disease community to make H. pylori an infection of joint interest. Mass eradication of H. pylori worldwide offers the promise of elimination of gastric cancer. The CMS rule should ultimately result in significant changes in the approach of treating H. pylori infections that includes improved testing and availability and implementation of knowledge of local susceptibility and resistance patterns. Therapies that reliably cure H. pylori without unnecessary antibiotics need to be used whereas regimens that fail to reliably achieve high cure rates should be abandoned. We should consider establishing quality metrics related to appropriate diagnostic testing for both the initial infection as well as posttreatment evaluations. We must add our voice to advocate for hospitals and central laboratories to offer susceptibility testing locally or as a send out for clinicians to provide locally relevant antimicrobial therapy for H. pylori infections. The CMS rule provides both the impetus and the methods to move forward and deal with H. pylori like other infectious diseases.
Dr. Graham is a professor of medicine, Baylor College of Medicine, Houston; Dr. El-Serag is chair of the department of medicine at Baylor College of Medicine, Houston. Neither had conflicts of interest related to this comment.
The Centers for Medicare and Medicaid Services (CMS) finalized a new regulation requiring all hospitals participating in its programs to establish antimicrobial stewardship programs by March 30, 2020 (https://federalregister.gov/d/2019-20736). This welcome action was prompted by the rise in antimicrobial resistance; recent Centers for Disease Control and Prevention estimates more than 2.8 million antibiotic-resistant infections with more than 35,000 deaths occur in the United States each year (https://www.cdc.gov/drugresistance/biggest-threats.html). CMS recommended that hospitals follow stewardship guidelines established by CDC, and other nationally recognized sources (https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf). Antibiotic stewardship includes optimization of several aspects of antimicrobial therapy including the drugs, formulations, doses, and dosing intervals. It also includes obtaining and updating local, regional, and national susceptibility data to provide regularly updated guidance regarding diagnosis and therapy.
How does this new CMS rule affect gastroenterologists and what role, if any, do we play in the epidemic of antibiotic resistance? The one infectious disease that gastroenterology effectively owns is Helicobacter pylori. Treatment of this disease has the potential to be involved in the epidemic of antimicrobial resistance. Current H. pylori therapies were largely devised without considering the principles of antibiotic stewardship. Therapies for H. pylori have largely been developed using trial and error, antimicrobial susceptibility testing is rarely available, and local and regional susceptibility are not readily available or updated. Current national treatment recommendations are most often based on comparisons of regimens grouping trials containing different drugs, doses, and durations of therapy performed in populations in whom resistance was neither assessed nor taken into account. Finally, some of the most highly recommended effective regimens inevitably contain at least one antibiotic unnecessary for the outcome, and inadvertently serve to increase population antibiotic exposure.
Clarithromycin-amoxicillin-PPI triple is still the most often used legacy therapy in the United States with an average cure rate of 70%. Recent guidelines have suggested adding a fourth drug, metronidazole to produce a quadruple therapy (concomitant therapy); the premise is that although both clarithromycin and metronidazole resistance are common, dual resistance is not. However, this benefit comes at the price of every patient receiving at least one unnecessary antibiotic, and patients with treatment failures receiving three unnecessary antibiotics. The cumulative effect given the approximately 2 million treatments annually is 10s of thousands of kilograms of inappropriate antibiotic use annually with the likely consequence of increasing resistance.
How to move forward? The CDC documents regarding antimicrobial stewardship in hospitals with limited resources (https://www.cdc.gov/antibiotic-use/core-elements/resource-limited.html) suggest creation and promotion of evidence-based treatment guidelines for common clinical syndromes, tracking of antibiotic dispensing using available data, setting of national targets for improvement, and description of resistance patterns to improve treatment guidelines and identify priority pathogens. The CDC documents require creation and promotion of evidence-and susceptibility-based treatment guidelines, tracking of antibiotic dispensing and setting targets for improvement (i.e., monitoring and reporting). It is important to note that the CMS rule focused on hospitals as hospitals have traditionally been the sites where local susceptibility data are obtained and gathered to provide the regional data and updated treatment guidelines used to treat most infectious diseases.
The Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States in 2017 had several recommendations that would effectively address the CMS rule and CDC recommendations. For example, statement 15: that empiric eradication therapy for H. pylori be based on region or population-specific antibiotic susceptibility data (Grade 1B); Statement 17: that validated diagnostic testing of stool or gastric mucosal biopsy by culture and susceptibility, or molecular analysis be universally available (Grade 1); Statement 18: that antibiotics that may be routinely evaluated for susceptibility include amoxicillin, clarithromycin, levofloxacin, metronidazole, and tetracycline. (Grade 2C); and Statement 19: that professional societies provide the research needed to support evidence-based reimbursement decisions for antibiotic susceptibility testing for H. pylori (Grade 1).
Organized gastroenterology needs to join with the infectious disease community to make H. pylori an infection of joint interest. Mass eradication of H. pylori worldwide offers the promise of elimination of gastric cancer. The CMS rule should ultimately result in significant changes in the approach of treating H. pylori infections that includes improved testing and availability and implementation of knowledge of local susceptibility and resistance patterns. Therapies that reliably cure H. pylori without unnecessary antibiotics need to be used whereas regimens that fail to reliably achieve high cure rates should be abandoned. We should consider establishing quality metrics related to appropriate diagnostic testing for both the initial infection as well as posttreatment evaluations. We must add our voice to advocate for hospitals and central laboratories to offer susceptibility testing locally or as a send out for clinicians to provide locally relevant antimicrobial therapy for H. pylori infections. The CMS rule provides both the impetus and the methods to move forward and deal with H. pylori like other infectious diseases.
Dr. Graham is a professor of medicine, Baylor College of Medicine, Houston; Dr. El-Serag is chair of the department of medicine at Baylor College of Medicine, Houston. Neither had conflicts of interest related to this comment.
The Centers for Medicare and Medicaid Services (CMS) finalized a new regulation requiring all hospitals participating in its programs to establish antimicrobial stewardship programs by March 30, 2020 (https://federalregister.gov/d/2019-20736). This welcome action was prompted by the rise in antimicrobial resistance; recent Centers for Disease Control and Prevention estimates more than 2.8 million antibiotic-resistant infections with more than 35,000 deaths occur in the United States each year (https://www.cdc.gov/drugresistance/biggest-threats.html). CMS recommended that hospitals follow stewardship guidelines established by CDC, and other nationally recognized sources (https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf). Antibiotic stewardship includes optimization of several aspects of antimicrobial therapy including the drugs, formulations, doses, and dosing intervals. It also includes obtaining and updating local, regional, and national susceptibility data to provide regularly updated guidance regarding diagnosis and therapy.
How does this new CMS rule affect gastroenterologists and what role, if any, do we play in the epidemic of antibiotic resistance? The one infectious disease that gastroenterology effectively owns is Helicobacter pylori. Treatment of this disease has the potential to be involved in the epidemic of antimicrobial resistance. Current H. pylori therapies were largely devised without considering the principles of antibiotic stewardship. Therapies for H. pylori have largely been developed using trial and error, antimicrobial susceptibility testing is rarely available, and local and regional susceptibility are not readily available or updated. Current national treatment recommendations are most often based on comparisons of regimens grouping trials containing different drugs, doses, and durations of therapy performed in populations in whom resistance was neither assessed nor taken into account. Finally, some of the most highly recommended effective regimens inevitably contain at least one antibiotic unnecessary for the outcome, and inadvertently serve to increase population antibiotic exposure.
Clarithromycin-amoxicillin-PPI triple is still the most often used legacy therapy in the United States with an average cure rate of 70%. Recent guidelines have suggested adding a fourth drug, metronidazole to produce a quadruple therapy (concomitant therapy); the premise is that although both clarithromycin and metronidazole resistance are common, dual resistance is not. However, this benefit comes at the price of every patient receiving at least one unnecessary antibiotic, and patients with treatment failures receiving three unnecessary antibiotics. The cumulative effect given the approximately 2 million treatments annually is 10s of thousands of kilograms of inappropriate antibiotic use annually with the likely consequence of increasing resistance.
How to move forward? The CDC documents regarding antimicrobial stewardship in hospitals with limited resources (https://www.cdc.gov/antibiotic-use/core-elements/resource-limited.html) suggest creation and promotion of evidence-based treatment guidelines for common clinical syndromes, tracking of antibiotic dispensing using available data, setting of national targets for improvement, and description of resistance patterns to improve treatment guidelines and identify priority pathogens. The CDC documents require creation and promotion of evidence-and susceptibility-based treatment guidelines, tracking of antibiotic dispensing and setting targets for improvement (i.e., monitoring and reporting). It is important to note that the CMS rule focused on hospitals as hospitals have traditionally been the sites where local susceptibility data are obtained and gathered to provide the regional data and updated treatment guidelines used to treat most infectious diseases.
The Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States in 2017 had several recommendations that would effectively address the CMS rule and CDC recommendations. For example, statement 15: that empiric eradication therapy for H. pylori be based on region or population-specific antibiotic susceptibility data (Grade 1B); Statement 17: that validated diagnostic testing of stool or gastric mucosal biopsy by culture and susceptibility, or molecular analysis be universally available (Grade 1); Statement 18: that antibiotics that may be routinely evaluated for susceptibility include amoxicillin, clarithromycin, levofloxacin, metronidazole, and tetracycline. (Grade 2C); and Statement 19: that professional societies provide the research needed to support evidence-based reimbursement decisions for antibiotic susceptibility testing for H. pylori (Grade 1).
Organized gastroenterology needs to join with the infectious disease community to make H. pylori an infection of joint interest. Mass eradication of H. pylori worldwide offers the promise of elimination of gastric cancer. The CMS rule should ultimately result in significant changes in the approach of treating H. pylori infections that includes improved testing and availability and implementation of knowledge of local susceptibility and resistance patterns. Therapies that reliably cure H. pylori without unnecessary antibiotics need to be used whereas regimens that fail to reliably achieve high cure rates should be abandoned. We should consider establishing quality metrics related to appropriate diagnostic testing for both the initial infection as well as posttreatment evaluations. We must add our voice to advocate for hospitals and central laboratories to offer susceptibility testing locally or as a send out for clinicians to provide locally relevant antimicrobial therapy for H. pylori infections. The CMS rule provides both the impetus and the methods to move forward and deal with H. pylori like other infectious diseases.
Dr. Graham is a professor of medicine, Baylor College of Medicine, Houston; Dr. El-Serag is chair of the department of medicine at Baylor College of Medicine, Houston. Neither had conflicts of interest related to this comment.
Prepare for major changes to E/M coding starting in 2021
Evaluation and Management (E/M) coding and guidelines are about to undergo the most significant changes since their implementation in the 1990s. For now, the changes are limited to new and established outpatient visits (CPT codes 99202-99205, 99211-99215) and will take place as of Jan. 1, 2021. Changes to all E/M codes are anticipated in the coming years.
The changes to the new and established office/outpatient codes will impact everyone in health care who assigns codes, manages health information, or pays claims including physicians and qualified health professionals, coders, health information managers, payers, health systems, and hospitals. The American Medical Association (AMA) has already released a preview of the CPT 2021 changes as well as free E/M education modules. They are planning to release more educational resources in the near future.
Why were changes needed?
The AMA developed the 2021 E/M changes in response to interest from the Centers for Medicare & Medicaid Services (CMS) in reducing physician burden, simplifying documentation requirements, and making changes to payments for the E/M codes. CMS’s initial proposal was to collapse office visit E/M levels 2-5 to a single payment. While the new rates would have provided a modest increase for level 2 and 3 E/M codes, they would have cut reimbursement for the top-level codes by more than 50%. There was concern that these changes would adversely affect physicians caring for complex patients across medical specialties. There was an outcry from the physician community opposing CMS’s proposal, and the agency agreed to get more input from the public before moving forward.
The AMA worked with stakeholders, including the AGA and our sister GI societies, to create E/M guidelines that decrease documentation requirements while also continuing to differentiate payment based on complexity of care. CMS announced in the 2020 Medicare Physician Fee Schedule (MPFS) final rule that it would adopt the AMA’s proposal as well as their recommended relative values for 2021 CPT E/M codes. Of note, there will be modest payment increases for most office E/M codes beginning Jan. 1, 2021, which may benefit those who manage patients with complex conditions.
In sum, what are the 2021 E/M changes
While there will be many changes to office/outpatient E/M visits, the most significant are deletion of code 99201 (Level 1 new patient visit), addition of a 15-minute prolonged services code that can be reported with 99205 and 99215, and the following restructuring of office visit code selection:
1. Elimination of history and physical as elements for code selection: While obtaining a pertinent history and performing a relevant physical exam are clinically necessary and contribute to both time and medical decision making, these elements will not factor in to code selection. Instead, the code level will be determined solely by medical decision making or time.
2. Choice of using medical decision making (MDM) or total time as the basis of E/M level documentation:
- MDM. While there will still be three MDM subcomponents (number/complexity of problems, data, and risk), extensive edits were made to the ways in which these elements are defined and tallied.
- Time. The definition of time is now minimum time, not typical time or “face-to-face” time. Minimum time represents total physician/qualified health care professional time on the date of service. This redefinition of time allows Medicare to better recognize the work involved in non–face-to-face services like care coordination and record review. Of note, these definitions only apply when code selection is based on time and not MDM.
3. Modification of the criteria for MDM: The current CMS Table of Risk was used as a foundation for designing the revised required elements for MDM.
- Terms. Removed ambiguous terms (e.g., “mild”) and defined previously ambiguous concepts (e.g., “acute or chronic illness with systemic symptoms”).
- Definitions. Defined important terms, such as “independent historian.”
- Data elements. Re-defined the data elements to move away from simply adding up tasks to focusing on how those tasks affect the management of the patient (e.g., independent interpretation of a test performed by another provider and/or discussion of test interpretation with another physician).
CMS also plans to add a new Healthcare Common Procedure Coding System (HCPCS) add-on code as of Jan. 1, 2021, that can be used to recognize additional resource costs that are inherent in treating complex patients.
- GPCX1 - Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex chronic condition. (Add-on code, list separately in addition to office/outpatient evaluation and management visit, new or established.).
GPC1X can be reported with all levels of E/M office/outpatient codes in which care of a patient’s single, serious, or complex chronic condition is the focus. CMS plans to reimburse GPC1X at 0.33 RVUs (about $12).
Who do these changes apply to?
The changes to the E/M office/outpatient CPT codes and guidelines for new and established patients apply to all traditional Medicare and Medicare Advantage plans, Medicaid, and all commercial payers. E/M HCPCS codes apply to Medicare, Medicare Advantage plans, and Medicaid only; commercial payers are not required to accept HCPCS codes.
What should you do?
Visit the AMA E/M Microsite; there you will find the AMA’s early release of the 2021 E/M coding and guideline changes, the AMA E/M learning module and future resources on the use of time and MDM that are expected to be released in March.
AMA E/M Microsite: https://www.ama-assn.org/practice-management/cpt/cpt-evaluation-and-management
2021 E/M changes: https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
AMA E/M learning module: https://edhub.ama-assn.org/interactive/18057429
AMA MDM table: https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf
Connect with your coders and/or medical billing company to create a plan for training physicians and staff to ensure a smooth transition on Jan. 1, 2021.
Contact your Electronic Health Records (EHR) vendor to confirm the system your practice uses will be ready to implement the new E/M coding and guidelines changes on Jan. 1, 2021.
Run an analysis using the new E/M office/outpatient payment rates recommended by the AMA for 2021 (https://www.ama-assn.org/about/rvs-update-committee-ruc/ruc-recommendations-minutes-voting) for each of your practice’s contracted payers to determine if your practice will benefit from the new rates. While CMS has proposed to accept the AMA recommended rates, this will not be finalized until CMS publishes the 2021 proposed rule in early July 2020.
Once CMS confirms its decision, reach out to your payers to negotiate implementing the new E/M rates starting in 2021.
With changes this big, we encourage you to prepare early. Watch for more information on the 2021 E/M changes in Washington Insider and AGA eDigest.
Dr. Kuo is the AGA’s Advisor to the AMA CPT Editorial Panel and a member of the AGA Practice Management and Economics Committee’s (PMEC) Coverage and Reimbursement Subcommittee (CRS) and assistant professor of medicine and gastroenterology, Harvard Medical School and Massachusetts General Hospital, Boston; Dr. Losurdo is the AGA’s Alternate Advisor to the AMA CPT Editorial Panel, a member of the AGA PMEC’s CRS, and Managing Partner and medical director of Illinois Gastroenterology Group, Elgin, Ill.; Dr. Mehta is the AGA’s advisor to the AMA RVS Update Committee (RUC), a member of the AGA PMEC’s CRS, and assistant professor of medicine at the University of Pennsylvania, Philadelphia; and Dr. Garcia is the AGA’s Alternate Advisor to the AMA RUC, a member of the AGA PMEC’s CRS, and assistant professor of medicine and gastroenterology at Stanford (Calif.) University. There were no conflicts of interest.
Evaluation and Management (E/M) coding and guidelines are about to undergo the most significant changes since their implementation in the 1990s. For now, the changes are limited to new and established outpatient visits (CPT codes 99202-99205, 99211-99215) and will take place as of Jan. 1, 2021. Changes to all E/M codes are anticipated in the coming years.
The changes to the new and established office/outpatient codes will impact everyone in health care who assigns codes, manages health information, or pays claims including physicians and qualified health professionals, coders, health information managers, payers, health systems, and hospitals. The American Medical Association (AMA) has already released a preview of the CPT 2021 changes as well as free E/M education modules. They are planning to release more educational resources in the near future.
Why were changes needed?
The AMA developed the 2021 E/M changes in response to interest from the Centers for Medicare & Medicaid Services (CMS) in reducing physician burden, simplifying documentation requirements, and making changes to payments for the E/M codes. CMS’s initial proposal was to collapse office visit E/M levels 2-5 to a single payment. While the new rates would have provided a modest increase for level 2 and 3 E/M codes, they would have cut reimbursement for the top-level codes by more than 50%. There was concern that these changes would adversely affect physicians caring for complex patients across medical specialties. There was an outcry from the physician community opposing CMS’s proposal, and the agency agreed to get more input from the public before moving forward.
The AMA worked with stakeholders, including the AGA and our sister GI societies, to create E/M guidelines that decrease documentation requirements while also continuing to differentiate payment based on complexity of care. CMS announced in the 2020 Medicare Physician Fee Schedule (MPFS) final rule that it would adopt the AMA’s proposal as well as their recommended relative values for 2021 CPT E/M codes. Of note, there will be modest payment increases for most office E/M codes beginning Jan. 1, 2021, which may benefit those who manage patients with complex conditions.
In sum, what are the 2021 E/M changes
While there will be many changes to office/outpatient E/M visits, the most significant are deletion of code 99201 (Level 1 new patient visit), addition of a 15-minute prolonged services code that can be reported with 99205 and 99215, and the following restructuring of office visit code selection:
1. Elimination of history and physical as elements for code selection: While obtaining a pertinent history and performing a relevant physical exam are clinically necessary and contribute to both time and medical decision making, these elements will not factor in to code selection. Instead, the code level will be determined solely by medical decision making or time.
2. Choice of using medical decision making (MDM) or total time as the basis of E/M level documentation:
- MDM. While there will still be three MDM subcomponents (number/complexity of problems, data, and risk), extensive edits were made to the ways in which these elements are defined and tallied.
- Time. The definition of time is now minimum time, not typical time or “face-to-face” time. Minimum time represents total physician/qualified health care professional time on the date of service. This redefinition of time allows Medicare to better recognize the work involved in non–face-to-face services like care coordination and record review. Of note, these definitions only apply when code selection is based on time and not MDM.
3. Modification of the criteria for MDM: The current CMS Table of Risk was used as a foundation for designing the revised required elements for MDM.
- Terms. Removed ambiguous terms (e.g., “mild”) and defined previously ambiguous concepts (e.g., “acute or chronic illness with systemic symptoms”).
- Definitions. Defined important terms, such as “independent historian.”
- Data elements. Re-defined the data elements to move away from simply adding up tasks to focusing on how those tasks affect the management of the patient (e.g., independent interpretation of a test performed by another provider and/or discussion of test interpretation with another physician).
CMS also plans to add a new Healthcare Common Procedure Coding System (HCPCS) add-on code as of Jan. 1, 2021, that can be used to recognize additional resource costs that are inherent in treating complex patients.
- GPCX1 - Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex chronic condition. (Add-on code, list separately in addition to office/outpatient evaluation and management visit, new or established.).
GPC1X can be reported with all levels of E/M office/outpatient codes in which care of a patient’s single, serious, or complex chronic condition is the focus. CMS plans to reimburse GPC1X at 0.33 RVUs (about $12).
Who do these changes apply to?
The changes to the E/M office/outpatient CPT codes and guidelines for new and established patients apply to all traditional Medicare and Medicare Advantage plans, Medicaid, and all commercial payers. E/M HCPCS codes apply to Medicare, Medicare Advantage plans, and Medicaid only; commercial payers are not required to accept HCPCS codes.
What should you do?
Visit the AMA E/M Microsite; there you will find the AMA’s early release of the 2021 E/M coding and guideline changes, the AMA E/M learning module and future resources on the use of time and MDM that are expected to be released in March.
AMA E/M Microsite: https://www.ama-assn.org/practice-management/cpt/cpt-evaluation-and-management
2021 E/M changes: https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
AMA E/M learning module: https://edhub.ama-assn.org/interactive/18057429
AMA MDM table: https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf
Connect with your coders and/or medical billing company to create a plan for training physicians and staff to ensure a smooth transition on Jan. 1, 2021.
Contact your Electronic Health Records (EHR) vendor to confirm the system your practice uses will be ready to implement the new E/M coding and guidelines changes on Jan. 1, 2021.
Run an analysis using the new E/M office/outpatient payment rates recommended by the AMA for 2021 (https://www.ama-assn.org/about/rvs-update-committee-ruc/ruc-recommendations-minutes-voting) for each of your practice’s contracted payers to determine if your practice will benefit from the new rates. While CMS has proposed to accept the AMA recommended rates, this will not be finalized until CMS publishes the 2021 proposed rule in early July 2020.
Once CMS confirms its decision, reach out to your payers to negotiate implementing the new E/M rates starting in 2021.
With changes this big, we encourage you to prepare early. Watch for more information on the 2021 E/M changes in Washington Insider and AGA eDigest.
Dr. Kuo is the AGA’s Advisor to the AMA CPT Editorial Panel and a member of the AGA Practice Management and Economics Committee’s (PMEC) Coverage and Reimbursement Subcommittee (CRS) and assistant professor of medicine and gastroenterology, Harvard Medical School and Massachusetts General Hospital, Boston; Dr. Losurdo is the AGA’s Alternate Advisor to the AMA CPT Editorial Panel, a member of the AGA PMEC’s CRS, and Managing Partner and medical director of Illinois Gastroenterology Group, Elgin, Ill.; Dr. Mehta is the AGA’s advisor to the AMA RVS Update Committee (RUC), a member of the AGA PMEC’s CRS, and assistant professor of medicine at the University of Pennsylvania, Philadelphia; and Dr. Garcia is the AGA’s Alternate Advisor to the AMA RUC, a member of the AGA PMEC’s CRS, and assistant professor of medicine and gastroenterology at Stanford (Calif.) University. There were no conflicts of interest.
Evaluation and Management (E/M) coding and guidelines are about to undergo the most significant changes since their implementation in the 1990s. For now, the changes are limited to new and established outpatient visits (CPT codes 99202-99205, 99211-99215) and will take place as of Jan. 1, 2021. Changes to all E/M codes are anticipated in the coming years.
The changes to the new and established office/outpatient codes will impact everyone in health care who assigns codes, manages health information, or pays claims including physicians and qualified health professionals, coders, health information managers, payers, health systems, and hospitals. The American Medical Association (AMA) has already released a preview of the CPT 2021 changes as well as free E/M education modules. They are planning to release more educational resources in the near future.
Why were changes needed?
The AMA developed the 2021 E/M changes in response to interest from the Centers for Medicare & Medicaid Services (CMS) in reducing physician burden, simplifying documentation requirements, and making changes to payments for the E/M codes. CMS’s initial proposal was to collapse office visit E/M levels 2-5 to a single payment. While the new rates would have provided a modest increase for level 2 and 3 E/M codes, they would have cut reimbursement for the top-level codes by more than 50%. There was concern that these changes would adversely affect physicians caring for complex patients across medical specialties. There was an outcry from the physician community opposing CMS’s proposal, and the agency agreed to get more input from the public before moving forward.
The AMA worked with stakeholders, including the AGA and our sister GI societies, to create E/M guidelines that decrease documentation requirements while also continuing to differentiate payment based on complexity of care. CMS announced in the 2020 Medicare Physician Fee Schedule (MPFS) final rule that it would adopt the AMA’s proposal as well as their recommended relative values for 2021 CPT E/M codes. Of note, there will be modest payment increases for most office E/M codes beginning Jan. 1, 2021, which may benefit those who manage patients with complex conditions.
In sum, what are the 2021 E/M changes
While there will be many changes to office/outpatient E/M visits, the most significant are deletion of code 99201 (Level 1 new patient visit), addition of a 15-minute prolonged services code that can be reported with 99205 and 99215, and the following restructuring of office visit code selection:
1. Elimination of history and physical as elements for code selection: While obtaining a pertinent history and performing a relevant physical exam are clinically necessary and contribute to both time and medical decision making, these elements will not factor in to code selection. Instead, the code level will be determined solely by medical decision making or time.
2. Choice of using medical decision making (MDM) or total time as the basis of E/M level documentation:
- MDM. While there will still be three MDM subcomponents (number/complexity of problems, data, and risk), extensive edits were made to the ways in which these elements are defined and tallied.
- Time. The definition of time is now minimum time, not typical time or “face-to-face” time. Minimum time represents total physician/qualified health care professional time on the date of service. This redefinition of time allows Medicare to better recognize the work involved in non–face-to-face services like care coordination and record review. Of note, these definitions only apply when code selection is based on time and not MDM.
3. Modification of the criteria for MDM: The current CMS Table of Risk was used as a foundation for designing the revised required elements for MDM.
- Terms. Removed ambiguous terms (e.g., “mild”) and defined previously ambiguous concepts (e.g., “acute or chronic illness with systemic symptoms”).
- Definitions. Defined important terms, such as “independent historian.”
- Data elements. Re-defined the data elements to move away from simply adding up tasks to focusing on how those tasks affect the management of the patient (e.g., independent interpretation of a test performed by another provider and/or discussion of test interpretation with another physician).
CMS also plans to add a new Healthcare Common Procedure Coding System (HCPCS) add-on code as of Jan. 1, 2021, that can be used to recognize additional resource costs that are inherent in treating complex patients.
- GPCX1 - Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex chronic condition. (Add-on code, list separately in addition to office/outpatient evaluation and management visit, new or established.).
GPC1X can be reported with all levels of E/M office/outpatient codes in which care of a patient’s single, serious, or complex chronic condition is the focus. CMS plans to reimburse GPC1X at 0.33 RVUs (about $12).
Who do these changes apply to?
The changes to the E/M office/outpatient CPT codes and guidelines for new and established patients apply to all traditional Medicare and Medicare Advantage plans, Medicaid, and all commercial payers. E/M HCPCS codes apply to Medicare, Medicare Advantage plans, and Medicaid only; commercial payers are not required to accept HCPCS codes.
What should you do?
Visit the AMA E/M Microsite; there you will find the AMA’s early release of the 2021 E/M coding and guideline changes, the AMA E/M learning module and future resources on the use of time and MDM that are expected to be released in March.
AMA E/M Microsite: https://www.ama-assn.org/practice-management/cpt/cpt-evaluation-and-management
2021 E/M changes: https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
AMA E/M learning module: https://edhub.ama-assn.org/interactive/18057429
AMA MDM table: https://www.ama-assn.org/system/files/2019-06/cpt-revised-mdm-grid.pdf
Connect with your coders and/or medical billing company to create a plan for training physicians and staff to ensure a smooth transition on Jan. 1, 2021.
Contact your Electronic Health Records (EHR) vendor to confirm the system your practice uses will be ready to implement the new E/M coding and guidelines changes on Jan. 1, 2021.
Run an analysis using the new E/M office/outpatient payment rates recommended by the AMA for 2021 (https://www.ama-assn.org/about/rvs-update-committee-ruc/ruc-recommendations-minutes-voting) for each of your practice’s contracted payers to determine if your practice will benefit from the new rates. While CMS has proposed to accept the AMA recommended rates, this will not be finalized until CMS publishes the 2021 proposed rule in early July 2020.
Once CMS confirms its decision, reach out to your payers to negotiate implementing the new E/M rates starting in 2021.
With changes this big, we encourage you to prepare early. Watch for more information on the 2021 E/M changes in Washington Insider and AGA eDigest.
Dr. Kuo is the AGA’s Advisor to the AMA CPT Editorial Panel and a member of the AGA Practice Management and Economics Committee’s (PMEC) Coverage and Reimbursement Subcommittee (CRS) and assistant professor of medicine and gastroenterology, Harvard Medical School and Massachusetts General Hospital, Boston; Dr. Losurdo is the AGA’s Alternate Advisor to the AMA CPT Editorial Panel, a member of the AGA PMEC’s CRS, and Managing Partner and medical director of Illinois Gastroenterology Group, Elgin, Ill.; Dr. Mehta is the AGA’s advisor to the AMA RVS Update Committee (RUC), a member of the AGA PMEC’s CRS, and assistant professor of medicine at the University of Pennsylvania, Philadelphia; and Dr. Garcia is the AGA’s Alternate Advisor to the AMA RUC, a member of the AGA PMEC’s CRS, and assistant professor of medicine and gastroenterology at Stanford (Calif.) University. There were no conflicts of interest.
Medicaid expansion linked to more early cancer diagnoses
Cancer patients in states that opted to expand Medicaid insurance coverage under the Affordable Care Act saw a slightly better rate of early diagnosis, compared with patients in states that refused expansion, according to a new study. However, time to treatment was similar in states that opted for expansion and states that did not.
Samuel U. Takvorian, MD, of the University of Pennsylvania, Philadelphia, and colleagues reported these results in JAMA Network Open.
The researchers used the National Cancer Database to examine the changes in health insurance coverage and cancer health outcomes in nonelderly patients following implementation of the Affordable Care Act in January 2014. The investigators identified records for 925,543 patients who had new-onset breast (59%), colon (15%), or non–small cell lung (27%) cancer between 2011 and 2016. The patients’ mean age was 55 years (range, 40-64 years), 79% were women, 14% were black, and 6% were Hispanic.
The researchers looked at insurance status, cancer stage at diagnosis, and treatment initiation within 30 and 90 days of diagnosis. The cohort was equally divided between residents of Medicaid expansion states (48%) and nonexpansion states (52%).
Using a statistical technique that mimics a controlled experiment, the investigators found the percentage of uninsured patients decreased more in the expansion states (adjusted difference-in-differences, −0.7 percentage points; 95% confidence interval, −1.2 to −0.3; P = .001), compared with nonexpansion states. Expansion states also had a greater increase in early-stage cancer diagnoses (adjusted DID, 0.8; 95% CI 0.3-1.2; P = .001) and a greater decrease in advanced-stage cancer diagnoses (adjusted DID, −0.5; 95% CI, −0.9 to −0.2; P = .003).
Among the 848,329 patients who underwent cancer treatment within a year of diagnosis, the percentage initiating treatment within 30 days declined from 52.7% before to 48% after Medicaid expansion in states opting in (unadjusted DID, −4.7; percentage points, 95% CI; −5.1 to −4.5). States that did not expand their Medicaid programs, meanwhile, saw the share decline from 56.9% to 51.5% in the same time period (adjusted DID, −5.4; 95% CI, −5.6 to −5.1). There was no statistically significant difference in timely treatment associated with Medicaid expansion (adjusted DID, 0.6; 95% CI, −0.2 to 1.4; P = .14).
The researchers speculated that the lack of significant between-group differences in time to treatment, despite an improvement in early-stage diagnoses associated with Medicaid expansion, could reflect a cancer care system strained by a surge in insured patients, overall increases in cancer prevalence and complexity of care, a shortage of workers, or a mixture of factors.
In a related editorial, Sue Fu, MD, of Stanford (Calif.) University, and colleagues wrote that, while the findings of increased early diagnosis seen in the study are promising, the time to treatment results are “puzzling” and deserve further consideration.
Time to treatment is important in cancer, as longer times are associated with increased mortality, Dr. Fu and colleagues noted. Slowing times to cancer treatment is a systemic problem in the United States that has been documented since the mid-2000s. Paradoxically, expanded insurance coverage could contribute to increasing time to treatment even after timely diagnosis by adding administrative burdens leading to longer wait times. “Newly insured and underinsured individuals may be particularly vulnerable to this,” the editorialists wrote.
Dr. Takvorian and colleagues noted as weaknesses of their study its observational design, a limited range of ages and cancers included, and an inability to adjust for state-level effects.
This study was funded by the National Cancer Institute and the Agency for Health Research and Quality. The authors of the study and the editorial disclosed no relevant conflicts of interest.
SOURCES: Takvorian SU et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921653; Fu S et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921690.
Cancer patients in states that opted to expand Medicaid insurance coverage under the Affordable Care Act saw a slightly better rate of early diagnosis, compared with patients in states that refused expansion, according to a new study. However, time to treatment was similar in states that opted for expansion and states that did not.
Samuel U. Takvorian, MD, of the University of Pennsylvania, Philadelphia, and colleagues reported these results in JAMA Network Open.
The researchers used the National Cancer Database to examine the changes in health insurance coverage and cancer health outcomes in nonelderly patients following implementation of the Affordable Care Act in January 2014. The investigators identified records for 925,543 patients who had new-onset breast (59%), colon (15%), or non–small cell lung (27%) cancer between 2011 and 2016. The patients’ mean age was 55 years (range, 40-64 years), 79% were women, 14% were black, and 6% were Hispanic.
The researchers looked at insurance status, cancer stage at diagnosis, and treatment initiation within 30 and 90 days of diagnosis. The cohort was equally divided between residents of Medicaid expansion states (48%) and nonexpansion states (52%).
Using a statistical technique that mimics a controlled experiment, the investigators found the percentage of uninsured patients decreased more in the expansion states (adjusted difference-in-differences, −0.7 percentage points; 95% confidence interval, −1.2 to −0.3; P = .001), compared with nonexpansion states. Expansion states also had a greater increase in early-stage cancer diagnoses (adjusted DID, 0.8; 95% CI 0.3-1.2; P = .001) and a greater decrease in advanced-stage cancer diagnoses (adjusted DID, −0.5; 95% CI, −0.9 to −0.2; P = .003).
Among the 848,329 patients who underwent cancer treatment within a year of diagnosis, the percentage initiating treatment within 30 days declined from 52.7% before to 48% after Medicaid expansion in states opting in (unadjusted DID, −4.7; percentage points, 95% CI; −5.1 to −4.5). States that did not expand their Medicaid programs, meanwhile, saw the share decline from 56.9% to 51.5% in the same time period (adjusted DID, −5.4; 95% CI, −5.6 to −5.1). There was no statistically significant difference in timely treatment associated with Medicaid expansion (adjusted DID, 0.6; 95% CI, −0.2 to 1.4; P = .14).
The researchers speculated that the lack of significant between-group differences in time to treatment, despite an improvement in early-stage diagnoses associated with Medicaid expansion, could reflect a cancer care system strained by a surge in insured patients, overall increases in cancer prevalence and complexity of care, a shortage of workers, or a mixture of factors.
In a related editorial, Sue Fu, MD, of Stanford (Calif.) University, and colleagues wrote that, while the findings of increased early diagnosis seen in the study are promising, the time to treatment results are “puzzling” and deserve further consideration.
Time to treatment is important in cancer, as longer times are associated with increased mortality, Dr. Fu and colleagues noted. Slowing times to cancer treatment is a systemic problem in the United States that has been documented since the mid-2000s. Paradoxically, expanded insurance coverage could contribute to increasing time to treatment even after timely diagnosis by adding administrative burdens leading to longer wait times. “Newly insured and underinsured individuals may be particularly vulnerable to this,” the editorialists wrote.
Dr. Takvorian and colleagues noted as weaknesses of their study its observational design, a limited range of ages and cancers included, and an inability to adjust for state-level effects.
This study was funded by the National Cancer Institute and the Agency for Health Research and Quality. The authors of the study and the editorial disclosed no relevant conflicts of interest.
SOURCES: Takvorian SU et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921653; Fu S et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921690.
Cancer patients in states that opted to expand Medicaid insurance coverage under the Affordable Care Act saw a slightly better rate of early diagnosis, compared with patients in states that refused expansion, according to a new study. However, time to treatment was similar in states that opted for expansion and states that did not.
Samuel U. Takvorian, MD, of the University of Pennsylvania, Philadelphia, and colleagues reported these results in JAMA Network Open.
The researchers used the National Cancer Database to examine the changes in health insurance coverage and cancer health outcomes in nonelderly patients following implementation of the Affordable Care Act in January 2014. The investigators identified records for 925,543 patients who had new-onset breast (59%), colon (15%), or non–small cell lung (27%) cancer between 2011 and 2016. The patients’ mean age was 55 years (range, 40-64 years), 79% were women, 14% were black, and 6% were Hispanic.
The researchers looked at insurance status, cancer stage at diagnosis, and treatment initiation within 30 and 90 days of diagnosis. The cohort was equally divided between residents of Medicaid expansion states (48%) and nonexpansion states (52%).
Using a statistical technique that mimics a controlled experiment, the investigators found the percentage of uninsured patients decreased more in the expansion states (adjusted difference-in-differences, −0.7 percentage points; 95% confidence interval, −1.2 to −0.3; P = .001), compared with nonexpansion states. Expansion states also had a greater increase in early-stage cancer diagnoses (adjusted DID, 0.8; 95% CI 0.3-1.2; P = .001) and a greater decrease in advanced-stage cancer diagnoses (adjusted DID, −0.5; 95% CI, −0.9 to −0.2; P = .003).
Among the 848,329 patients who underwent cancer treatment within a year of diagnosis, the percentage initiating treatment within 30 days declined from 52.7% before to 48% after Medicaid expansion in states opting in (unadjusted DID, −4.7; percentage points, 95% CI; −5.1 to −4.5). States that did not expand their Medicaid programs, meanwhile, saw the share decline from 56.9% to 51.5% in the same time period (adjusted DID, −5.4; 95% CI, −5.6 to −5.1). There was no statistically significant difference in timely treatment associated with Medicaid expansion (adjusted DID, 0.6; 95% CI, −0.2 to 1.4; P = .14).
The researchers speculated that the lack of significant between-group differences in time to treatment, despite an improvement in early-stage diagnoses associated with Medicaid expansion, could reflect a cancer care system strained by a surge in insured patients, overall increases in cancer prevalence and complexity of care, a shortage of workers, or a mixture of factors.
In a related editorial, Sue Fu, MD, of Stanford (Calif.) University, and colleagues wrote that, while the findings of increased early diagnosis seen in the study are promising, the time to treatment results are “puzzling” and deserve further consideration.
Time to treatment is important in cancer, as longer times are associated with increased mortality, Dr. Fu and colleagues noted. Slowing times to cancer treatment is a systemic problem in the United States that has been documented since the mid-2000s. Paradoxically, expanded insurance coverage could contribute to increasing time to treatment even after timely diagnosis by adding administrative burdens leading to longer wait times. “Newly insured and underinsured individuals may be particularly vulnerable to this,” the editorialists wrote.
Dr. Takvorian and colleagues noted as weaknesses of their study its observational design, a limited range of ages and cancers included, and an inability to adjust for state-level effects.
This study was funded by the National Cancer Institute and the Agency for Health Research and Quality. The authors of the study and the editorial disclosed no relevant conflicts of interest.
SOURCES: Takvorian SU et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921653; Fu S et al. JAMA Netw Open. 2020 Feb 5;3(2):e1921690.
FROM JAMA NETWORK OPEN
June Medical Services v. Russo: Understanding this high-stakes abortion case
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
Guidelines for today and tomorrow
In this edition of “How I Will Treat My Next Patient,” I review “guidelines for today” and speculate about “guidelines for tomorrow,” highlighting recommendations from the American Society of Clinical Oncology about hereditary cancer testing in epithelial ovarian cancer (OC) and data that support a reexamination of the age at which screening for colorectal cancer (CRC) should begin.
ASCO guidelines on genetic testing in epithelial ovarian cancer
After reviewing 19 studies, including 6 meta-analyses; 11 randomized, controlled trials; and 2 observational studies, an ASCO panel recommended germline genetic testing for BRCA1, BRCA2, and other ovarian cancer susceptibility genes for all women with newly diagnosed epithelial OC, regardless of family history (J Clin Oncol. 2020 Jan 27. doi: 10.1200/JCO.19.02960).
For OC patients with a germline mutation, cascade testing of first- and second-degree relatives was strongly urged. For patients without a germline mutation, the guidelines recommended offering somatic tumor testing for BRCA1/2 pathogenic or likely pathogenic variants at disease recurrence or after initial therapy and for mismatch repair deficiency (MMRD) in patients with clear cell, endometrioid, or mucinous and potentially other histologic types of OC. The authors cautioned that the discussion of testing results should involve professionals with expertise in the surveillance and management of hereditary cancer syndromes.
The panel said the discovery of germline or somatic pathogenic or likely pathogenic BRCA1/2 variants should lead to considering treatment with Food and Drug Administration–approved poly (ADP-ribose) polymerase inhibitors, including niraparib, olaparib, and rucaparib. Identification of MMRD in a patient with recurrent OC should trigger consideration of treatment with pembrolizumab, consistent with its labeled indications, and surveillance for other malignancies.
The guidelines cautioned that, when patients have variants of uncertain significance on germline testing, “clinical features and family history should inform clinical decision making.” Similarly, the panel made no recommendation regarding testing for or making treatment decisions based on tests for homologous recombination deficiency.
How these results influence practice
Every oncologist recognizes that better understanding of cancer biology can guide personalized diagnostic, predictive, prognostic, and therapeutic strategies for patients and their family members.
It is estimated that approximately 25% of all OC is caused by a heritable genetic condition. Germline mutations in BRCA1 and BRCA2 are identified in 13%-15% of patients with OC, and somatic mutations are found in an additional 7%. Perhaps 6% of all ovarian/fallopian tube/peritoneal cancers are caused by mutations in genes other than BRCA1/2. For that reason, germline sequencing should be performed via multigene panels that assess BRCA1/2 and other relevant mutations.
MMDR has been found in 10%-12% of unselected epithelial OC, with increased representation in nonserous histologies. That frequency is high enough to justify testing for it routinely.
Unfortunately, only about 30% of women undergo genetic testing. Given the frequency of molecular abnormalities in OC, this is problematic in every conceivable domain of clinical care for patients and family members. ASCO’s comprehensive, educational guidelines provide a template for shared decision making and utilize resources that are available in almost all clinical settings. For those clinicians who have recommended genetic testing for all epithelial OC patients, these guidelines are practice reaffirming. For the rest of us, they are practice changing.
Colorectal cancer cases spike after start of routine screening
Instead of examining CRC incidence by the usual 5- or 10-year age ranges, a group of researchers looked at CRC incidence in 1-year intervals for adults aged 30-60 years in the SEER-18 registry from 2000 to 2015 (JAMA Network Open. 2020 Jan 31. doi: 10.1001/jamanetworkopen.2019.20407). The researchers focused their attention on the transition between age 49 and 50 years, which is when routine screening generally begins and case-finding based on symptoms and signs of CRC alone ideally ends.
The group’s hypothesis was that steep increases in CRC incidence between ages 49 and 50 would be consistent with a high, undetected preclinical case burden in patients aged younger than 50 years and that this “real-world” registry data could help estimate outcomes of screening at younger ages. The researchers found that CRC incidence increased by 46.1% in the transition period from age 49 to 50 years. A majority (93%) of these cases were invasive and, therefore, likely to be clinically relevant. The increase in cancer rates occurred across geographical regions, gender, and race, and likely reflected the impact of screening. The states with the steepest increases in CRC between ages 49 and 50 (Connecticut and Utah) were the states with the first and third highest CRC screening rates for individuals 50 years of age and older.
Stage stratification showed steep increases in incidence in the target age range for localized and regional CRC and for colon and rectal tumors. In the transition between age 49 and 50, the researchers found a significant increase in 5-year relative survival (6.9% absolute increase, 10% relative increase), suggesting that earlier screening had a survival impact, apart from the effects of treatment in cases diagnosed after symptoms occurred.
The authors concluded that their analysis of the transition from age 49 to 50 years provides registry-based data regarding CRC risk among individuals younger than 50, which can add to existing modeling studies to help inform guidelines about the age at which to initiate screening.
How these results influence practice
Early-onset CRC (EOCRC) incidence is increasing, with controversy regarding whether average-risk screening should begin before age 50 years. The justification for starting screening at age 50 is that there is a near doubling of incidence from patients aged 45-49 years (34 per 100,000) to those aged 50-54 years (60.2 per 100,000).
However, the increase in CRC incidence beyond age 50 may not be because rates are truly lower among younger individuals but rather because of uneven screening between the two populations. Doubling times for CRCs have been estimated to be perhaps as long as 1,000 days. Because many CRCs are asymptomatic, observed incidence rates of EOCRC in SEER registries do not reflect preclinical CRC case burdens in younger patients.
The current interrogation of SEER-18 data to identify preexisting CRC that was clinically silent in the 1-year interval between age 49 and 50 is highly supportive of a large undiagnosed number of EOCRC cases. In SEER-18, CRC rates increased 46.1% in this 1-year age transition, more than in earlier 1-year age transitions. With almost 93% of cases being invasive, these data suggest a high case burden of preclinical, undetected, clinically relevant EOCRC in younger patients that is not reflected in observed SEER incidence rates examining wider age group intervals.
The dual goals of screening for CRC are to prevent malignant neoplasms by the removal of precancerous polyps and improve cancer-specific survival. The data presented suggest that, by starting average-risk screening at age 50 years, we may be “missing the window.” The 6.9% absolute and 10.1% relative survival increase in the target transition period suggest the authors’ hypothesis is correct.
As in any real-world database survey, the analysis is limited by a lack of specific outcomes data, the inability to determine when the cancers developed, and how long they germinated. Because of those limitations and others, more detailed studies are needed to determine the ideal age at which to begin CRC screening.
Modeling studies incorporating the steep incidence inflection point at 49-50 years can be conducted to estimate the incidence rate increase at, for example, 45 years; the cost-benefit ratio; quality-adjusted life-years gained; and other important endpoints. However, this review of over 170,000 cases of CRC, with a data-completeness rate of over 98%, over the 15-year time frame when CRC screening became common, supports a fresh look at whether it is within our power to improve outcomes for EOCRC patients by using existing technology but applying it earlier.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I review “guidelines for today” and speculate about “guidelines for tomorrow,” highlighting recommendations from the American Society of Clinical Oncology about hereditary cancer testing in epithelial ovarian cancer (OC) and data that support a reexamination of the age at which screening for colorectal cancer (CRC) should begin.
ASCO guidelines on genetic testing in epithelial ovarian cancer
After reviewing 19 studies, including 6 meta-analyses; 11 randomized, controlled trials; and 2 observational studies, an ASCO panel recommended germline genetic testing for BRCA1, BRCA2, and other ovarian cancer susceptibility genes for all women with newly diagnosed epithelial OC, regardless of family history (J Clin Oncol. 2020 Jan 27. doi: 10.1200/JCO.19.02960).
For OC patients with a germline mutation, cascade testing of first- and second-degree relatives was strongly urged. For patients without a germline mutation, the guidelines recommended offering somatic tumor testing for BRCA1/2 pathogenic or likely pathogenic variants at disease recurrence or after initial therapy and for mismatch repair deficiency (MMRD) in patients with clear cell, endometrioid, or mucinous and potentially other histologic types of OC. The authors cautioned that the discussion of testing results should involve professionals with expertise in the surveillance and management of hereditary cancer syndromes.
The panel said the discovery of germline or somatic pathogenic or likely pathogenic BRCA1/2 variants should lead to considering treatment with Food and Drug Administration–approved poly (ADP-ribose) polymerase inhibitors, including niraparib, olaparib, and rucaparib. Identification of MMRD in a patient with recurrent OC should trigger consideration of treatment with pembrolizumab, consistent with its labeled indications, and surveillance for other malignancies.
The guidelines cautioned that, when patients have variants of uncertain significance on germline testing, “clinical features and family history should inform clinical decision making.” Similarly, the panel made no recommendation regarding testing for or making treatment decisions based on tests for homologous recombination deficiency.
How these results influence practice
Every oncologist recognizes that better understanding of cancer biology can guide personalized diagnostic, predictive, prognostic, and therapeutic strategies for patients and their family members.
It is estimated that approximately 25% of all OC is caused by a heritable genetic condition. Germline mutations in BRCA1 and BRCA2 are identified in 13%-15% of patients with OC, and somatic mutations are found in an additional 7%. Perhaps 6% of all ovarian/fallopian tube/peritoneal cancers are caused by mutations in genes other than BRCA1/2. For that reason, germline sequencing should be performed via multigene panels that assess BRCA1/2 and other relevant mutations.
MMDR has been found in 10%-12% of unselected epithelial OC, with increased representation in nonserous histologies. That frequency is high enough to justify testing for it routinely.
Unfortunately, only about 30% of women undergo genetic testing. Given the frequency of molecular abnormalities in OC, this is problematic in every conceivable domain of clinical care for patients and family members. ASCO’s comprehensive, educational guidelines provide a template for shared decision making and utilize resources that are available in almost all clinical settings. For those clinicians who have recommended genetic testing for all epithelial OC patients, these guidelines are practice reaffirming. For the rest of us, they are practice changing.
Colorectal cancer cases spike after start of routine screening
Instead of examining CRC incidence by the usual 5- or 10-year age ranges, a group of researchers looked at CRC incidence in 1-year intervals for adults aged 30-60 years in the SEER-18 registry from 2000 to 2015 (JAMA Network Open. 2020 Jan 31. doi: 10.1001/jamanetworkopen.2019.20407). The researchers focused their attention on the transition between age 49 and 50 years, which is when routine screening generally begins and case-finding based on symptoms and signs of CRC alone ideally ends.
The group’s hypothesis was that steep increases in CRC incidence between ages 49 and 50 would be consistent with a high, undetected preclinical case burden in patients aged younger than 50 years and that this “real-world” registry data could help estimate outcomes of screening at younger ages. The researchers found that CRC incidence increased by 46.1% in the transition period from age 49 to 50 years. A majority (93%) of these cases were invasive and, therefore, likely to be clinically relevant. The increase in cancer rates occurred across geographical regions, gender, and race, and likely reflected the impact of screening. The states with the steepest increases in CRC between ages 49 and 50 (Connecticut and Utah) were the states with the first and third highest CRC screening rates for individuals 50 years of age and older.
Stage stratification showed steep increases in incidence in the target age range for localized and regional CRC and for colon and rectal tumors. In the transition between age 49 and 50, the researchers found a significant increase in 5-year relative survival (6.9% absolute increase, 10% relative increase), suggesting that earlier screening had a survival impact, apart from the effects of treatment in cases diagnosed after symptoms occurred.
The authors concluded that their analysis of the transition from age 49 to 50 years provides registry-based data regarding CRC risk among individuals younger than 50, which can add to existing modeling studies to help inform guidelines about the age at which to initiate screening.
How these results influence practice
Early-onset CRC (EOCRC) incidence is increasing, with controversy regarding whether average-risk screening should begin before age 50 years. The justification for starting screening at age 50 is that there is a near doubling of incidence from patients aged 45-49 years (34 per 100,000) to those aged 50-54 years (60.2 per 100,000).
However, the increase in CRC incidence beyond age 50 may not be because rates are truly lower among younger individuals but rather because of uneven screening between the two populations. Doubling times for CRCs have been estimated to be perhaps as long as 1,000 days. Because many CRCs are asymptomatic, observed incidence rates of EOCRC in SEER registries do not reflect preclinical CRC case burdens in younger patients.
The current interrogation of SEER-18 data to identify preexisting CRC that was clinically silent in the 1-year interval between age 49 and 50 is highly supportive of a large undiagnosed number of EOCRC cases. In SEER-18, CRC rates increased 46.1% in this 1-year age transition, more than in earlier 1-year age transitions. With almost 93% of cases being invasive, these data suggest a high case burden of preclinical, undetected, clinically relevant EOCRC in younger patients that is not reflected in observed SEER incidence rates examining wider age group intervals.
The dual goals of screening for CRC are to prevent malignant neoplasms by the removal of precancerous polyps and improve cancer-specific survival. The data presented suggest that, by starting average-risk screening at age 50 years, we may be “missing the window.” The 6.9% absolute and 10.1% relative survival increase in the target transition period suggest the authors’ hypothesis is correct.
As in any real-world database survey, the analysis is limited by a lack of specific outcomes data, the inability to determine when the cancers developed, and how long they germinated. Because of those limitations and others, more detailed studies are needed to determine the ideal age at which to begin CRC screening.
Modeling studies incorporating the steep incidence inflection point at 49-50 years can be conducted to estimate the incidence rate increase at, for example, 45 years; the cost-benefit ratio; quality-adjusted life-years gained; and other important endpoints. However, this review of over 170,000 cases of CRC, with a data-completeness rate of over 98%, over the 15-year time frame when CRC screening became common, supports a fresh look at whether it is within our power to improve outcomes for EOCRC patients by using existing technology but applying it earlier.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I review “guidelines for today” and speculate about “guidelines for tomorrow,” highlighting recommendations from the American Society of Clinical Oncology about hereditary cancer testing in epithelial ovarian cancer (OC) and data that support a reexamination of the age at which screening for colorectal cancer (CRC) should begin.
ASCO guidelines on genetic testing in epithelial ovarian cancer
After reviewing 19 studies, including 6 meta-analyses; 11 randomized, controlled trials; and 2 observational studies, an ASCO panel recommended germline genetic testing for BRCA1, BRCA2, and other ovarian cancer susceptibility genes for all women with newly diagnosed epithelial OC, regardless of family history (J Clin Oncol. 2020 Jan 27. doi: 10.1200/JCO.19.02960).
For OC patients with a germline mutation, cascade testing of first- and second-degree relatives was strongly urged. For patients without a germline mutation, the guidelines recommended offering somatic tumor testing for BRCA1/2 pathogenic or likely pathogenic variants at disease recurrence or after initial therapy and for mismatch repair deficiency (MMRD) in patients with clear cell, endometrioid, or mucinous and potentially other histologic types of OC. The authors cautioned that the discussion of testing results should involve professionals with expertise in the surveillance and management of hereditary cancer syndromes.
The panel said the discovery of germline or somatic pathogenic or likely pathogenic BRCA1/2 variants should lead to considering treatment with Food and Drug Administration–approved poly (ADP-ribose) polymerase inhibitors, including niraparib, olaparib, and rucaparib. Identification of MMRD in a patient with recurrent OC should trigger consideration of treatment with pembrolizumab, consistent with its labeled indications, and surveillance for other malignancies.
The guidelines cautioned that, when patients have variants of uncertain significance on germline testing, “clinical features and family history should inform clinical decision making.” Similarly, the panel made no recommendation regarding testing for or making treatment decisions based on tests for homologous recombination deficiency.
How these results influence practice
Every oncologist recognizes that better understanding of cancer biology can guide personalized diagnostic, predictive, prognostic, and therapeutic strategies for patients and their family members.
It is estimated that approximately 25% of all OC is caused by a heritable genetic condition. Germline mutations in BRCA1 and BRCA2 are identified in 13%-15% of patients with OC, and somatic mutations are found in an additional 7%. Perhaps 6% of all ovarian/fallopian tube/peritoneal cancers are caused by mutations in genes other than BRCA1/2. For that reason, germline sequencing should be performed via multigene panels that assess BRCA1/2 and other relevant mutations.
MMDR has been found in 10%-12% of unselected epithelial OC, with increased representation in nonserous histologies. That frequency is high enough to justify testing for it routinely.
Unfortunately, only about 30% of women undergo genetic testing. Given the frequency of molecular abnormalities in OC, this is problematic in every conceivable domain of clinical care for patients and family members. ASCO’s comprehensive, educational guidelines provide a template for shared decision making and utilize resources that are available in almost all clinical settings. For those clinicians who have recommended genetic testing for all epithelial OC patients, these guidelines are practice reaffirming. For the rest of us, they are practice changing.
Colorectal cancer cases spike after start of routine screening
Instead of examining CRC incidence by the usual 5- or 10-year age ranges, a group of researchers looked at CRC incidence in 1-year intervals for adults aged 30-60 years in the SEER-18 registry from 2000 to 2015 (JAMA Network Open. 2020 Jan 31. doi: 10.1001/jamanetworkopen.2019.20407). The researchers focused their attention on the transition between age 49 and 50 years, which is when routine screening generally begins and case-finding based on symptoms and signs of CRC alone ideally ends.
The group’s hypothesis was that steep increases in CRC incidence between ages 49 and 50 would be consistent with a high, undetected preclinical case burden in patients aged younger than 50 years and that this “real-world” registry data could help estimate outcomes of screening at younger ages. The researchers found that CRC incidence increased by 46.1% in the transition period from age 49 to 50 years. A majority (93%) of these cases were invasive and, therefore, likely to be clinically relevant. The increase in cancer rates occurred across geographical regions, gender, and race, and likely reflected the impact of screening. The states with the steepest increases in CRC between ages 49 and 50 (Connecticut and Utah) were the states with the first and third highest CRC screening rates for individuals 50 years of age and older.
Stage stratification showed steep increases in incidence in the target age range for localized and regional CRC and for colon and rectal tumors. In the transition between age 49 and 50, the researchers found a significant increase in 5-year relative survival (6.9% absolute increase, 10% relative increase), suggesting that earlier screening had a survival impact, apart from the effects of treatment in cases diagnosed after symptoms occurred.
The authors concluded that their analysis of the transition from age 49 to 50 years provides registry-based data regarding CRC risk among individuals younger than 50, which can add to existing modeling studies to help inform guidelines about the age at which to initiate screening.
How these results influence practice
Early-onset CRC (EOCRC) incidence is increasing, with controversy regarding whether average-risk screening should begin before age 50 years. The justification for starting screening at age 50 is that there is a near doubling of incidence from patients aged 45-49 years (34 per 100,000) to those aged 50-54 years (60.2 per 100,000).
However, the increase in CRC incidence beyond age 50 may not be because rates are truly lower among younger individuals but rather because of uneven screening between the two populations. Doubling times for CRCs have been estimated to be perhaps as long as 1,000 days. Because many CRCs are asymptomatic, observed incidence rates of EOCRC in SEER registries do not reflect preclinical CRC case burdens in younger patients.
The current interrogation of SEER-18 data to identify preexisting CRC that was clinically silent in the 1-year interval between age 49 and 50 is highly supportive of a large undiagnosed number of EOCRC cases. In SEER-18, CRC rates increased 46.1% in this 1-year age transition, more than in earlier 1-year age transitions. With almost 93% of cases being invasive, these data suggest a high case burden of preclinical, undetected, clinically relevant EOCRC in younger patients that is not reflected in observed SEER incidence rates examining wider age group intervals.
The dual goals of screening for CRC are to prevent malignant neoplasms by the removal of precancerous polyps and improve cancer-specific survival. The data presented suggest that, by starting average-risk screening at age 50 years, we may be “missing the window.” The 6.9% absolute and 10.1% relative survival increase in the target transition period suggest the authors’ hypothesis is correct.
As in any real-world database survey, the analysis is limited by a lack of specific outcomes data, the inability to determine when the cancers developed, and how long they germinated. Because of those limitations and others, more detailed studies are needed to determine the ideal age at which to begin CRC screening.
Modeling studies incorporating the steep incidence inflection point at 49-50 years can be conducted to estimate the incidence rate increase at, for example, 45 years; the cost-benefit ratio; quality-adjusted life-years gained; and other important endpoints. However, this review of over 170,000 cases of CRC, with a data-completeness rate of over 98%, over the 15-year time frame when CRC screening became common, supports a fresh look at whether it is within our power to improve outcomes for EOCRC patients by using existing technology but applying it earlier.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
‘Momentous’ USMLE change: New pass/fail format stuns medicine
News that the United States Medical Licensing Examination (USMLE) program will change its Step 1 scoring from a 3-digit number to pass/fail starting Jan. 1, 2022, has set off a flurry of shocked responses from students and physicians.
J. Bryan Carmody, MD, MPH, an assistant professor at Eastern Virginia Medical School in Norfolk, said in an interview that he was “stunned” when he heard the news on Wednesday and said the switch presents “the single biggest opportunity for medical school education reform since the Flexner Report,” which in 1910 established standards for modern medical education.
Numbers will continue for some tests
The USMLE cosponsors – the Federation of State Medical Boards (FSMB) and the National Board of Medical Examiners (NBME) – said that the Step 2 Clinical Knowledge (CK) exam and Step 3 will continue to be scored numerically. Step 2 Clinical Skills (CS) will continue its pass/fail system.
The change was made after Step 1 had been roundly criticized as playing too big a role in the process of becoming a physician and for causing students to study for the test instead of engaging fully in their medical education.
Ramie Fathy, a third-year medical student at the University of Pennsylvania, Philadelphia, currently studying for Step 1, said in an interview that it would have been nice personally to have the pass/fail choice, but he predicts both good and unintended consequences in the change.
The positive news, Mr. Fathy said, is that less emphasis will be put on the Step 1 test, which includes memorizing basic science details that may or not be relevant depending on later specialty choice.
“It’s not necessarily measuring what the test makers intended, which was whether or not a student can understand and apply basic science concepts to the practice of medicine,” he said.
“The current system encourages students to get as high a score as possible, which – after a certain point – translates to memorizing many little details that become increasingly less practically relevant,” Mr. Fathy said.
Pressure may move elsewhere?
However, Mr. Fathy worries that, without a scoring system to help decide who stands out in Step 1, residency program directors will depend more on the reputation of candidates’ medical school and the clout of the person writing a letter of recommendation – factors that are often influenced by family resources and social standing. That could wedge a further economic divide into the path to becoming a physician.
Mr. Fathy said he and fellow students are watching for information on what the passing bar will be and what happens with Step 2 Clinical Knowledge exam. USMLE has promised more information as soon as it is available.
“The question is whether that test will replace Step 1 as the standardized metric of student competency,” Mr. Fathy said, which would put more pressure on students further down the medical path.
Will Step 2 anxiety increase?
Dr. Carmody agreed that there is the danger that students now will spend their time studying for Step 2 CK at the expense of other parts of their education.
Meaningful reform will depend on the pass/fail move being coupled with other reforms, most importantly application caps, said Dr. Carmody, who teaches preclinical medical students and works with the residency program.
He has been blogging about Step 1 pass/fail for the past year.
Currently students can apply for as many residencies as they can pay for and Carmody said the number of applications per student has been rising over the past decade.
“That puts program directors under an impossible burden,” he said. “With our Step 1-based system, there’s significant inequality in the number of interviews people get. Programs end up overinviting the same group of people who look good on paper.”
People outside that group respond by sending more applications than they need to just to get a few interviews, Dr. Carmody added.
With caps, students would have an incentive to apply to only those programs in which they had a sincere interest, he said. Program directors also would then be better able to evaluate each application.
Switching Step 1 to pass/fail may have some effect on medical school burnout, Dr. Carmody said.
“It’s one thing to work hard when you’re on call and your patients depend on it,” he said. “But I would have a hard time staying up late every night studying something that I know in my heart is not going to help my patients, but I have to do it because I have to do better than the person who’s studying in the apartment next to me.”
Test has strayed from original purpose
Joseph Safdieh, MD, an assistant dean for clinical curriculum and director of the medical student neurology clerkship for the Weill Cornell Medicine, New York, sees the move as positive overall.
“We should not be using any single metric to define or describe our students’ overall profile,” he said in an interview.
“This has been a very significant anxiety point for our medical students for quite a number of years,” Dr. Safdieh said. “They were frustrated that their entire 4 years of medical school seemingly came down to one number.”
The test was created originally as one of three parts of licensure, he pointed out.
“Over the past 10 or 15 years, the exam has morphed to become a litmus test for very specific residency programs,” he said.
However, Dr. Safdieh has concerns that Step 2 will cultivate the same anxiety and may get too big a spotlight without the Step 1 metric, “although one could argue that test does more accurately reflect clinical material,” he said.
He also worries that students who have selected a specialty by the time they take Step 2 may find late in the game that they are less competitive in their field than they thought they were and may have to make a last-minute switch.
Dr. Safdieh said he thinks Step 2 will be next to go the pass/fail route. In reading between the lines of the announcement, he believes the test cosponsors didn’t make both pass/fail at once because it would have been “a nuclear bomb to the system.”
He credited the cosponsors with making what he called a “bold and momentous decision to initiate radical change in the overall transition between undergraduate and graduate medical education.”
Dr. Safdieh added that few in medicine were expecting Wednesday’s announcement.
“I think many of us were expecting them to go to quartile grading, not to go this far,” he said.
Dr. Safdieh suggested that, among those who may see downstream effects from the pass/fail move are offshore schools, such as those in the Caribbean. “Those schools rely on Step 1 to demonstrate that their students are meeting the rigor,” he said. But he hopes that this will lead to more holistic review.
“We’re hoping that this will force change in the system so that residency directors will look at more than just test-taking ability. They’ll look at publications and scholarship, community service and advocacy and performance in medical school,” Dr. Safdieh said.
Alison J. Whelan, MD, chief medical education officer of the Association of American Medical Colleges said in a statement, “The transition from medical school to residency training is a matter of great concern throughout academic medicine.
“The decision by the NBME and FSMB to change USMLE Step 1 score reporting to pass/fail was very carefully considered to balance student learning and student well-being,” she said. “The medical education community must now work together to identify and implement additional changes to improve the overall UME-GME [undergraduate and graduate medical education] transition system for all stakeholders and the AAMC is committed to helping lead this work.”
Dr. Fathy, Dr. Carmody, and Dr. Safdieh have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
News that the United States Medical Licensing Examination (USMLE) program will change its Step 1 scoring from a 3-digit number to pass/fail starting Jan. 1, 2022, has set off a flurry of shocked responses from students and physicians.
J. Bryan Carmody, MD, MPH, an assistant professor at Eastern Virginia Medical School in Norfolk, said in an interview that he was “stunned” when he heard the news on Wednesday and said the switch presents “the single biggest opportunity for medical school education reform since the Flexner Report,” which in 1910 established standards for modern medical education.
Numbers will continue for some tests
The USMLE cosponsors – the Federation of State Medical Boards (FSMB) and the National Board of Medical Examiners (NBME) – said that the Step 2 Clinical Knowledge (CK) exam and Step 3 will continue to be scored numerically. Step 2 Clinical Skills (CS) will continue its pass/fail system.
The change was made after Step 1 had been roundly criticized as playing too big a role in the process of becoming a physician and for causing students to study for the test instead of engaging fully in their medical education.
Ramie Fathy, a third-year medical student at the University of Pennsylvania, Philadelphia, currently studying for Step 1, said in an interview that it would have been nice personally to have the pass/fail choice, but he predicts both good and unintended consequences in the change.
The positive news, Mr. Fathy said, is that less emphasis will be put on the Step 1 test, which includes memorizing basic science details that may or not be relevant depending on later specialty choice.
“It’s not necessarily measuring what the test makers intended, which was whether or not a student can understand and apply basic science concepts to the practice of medicine,” he said.
“The current system encourages students to get as high a score as possible, which – after a certain point – translates to memorizing many little details that become increasingly less practically relevant,” Mr. Fathy said.
Pressure may move elsewhere?
However, Mr. Fathy worries that, without a scoring system to help decide who stands out in Step 1, residency program directors will depend more on the reputation of candidates’ medical school and the clout of the person writing a letter of recommendation – factors that are often influenced by family resources and social standing. That could wedge a further economic divide into the path to becoming a physician.
Mr. Fathy said he and fellow students are watching for information on what the passing bar will be and what happens with Step 2 Clinical Knowledge exam. USMLE has promised more information as soon as it is available.
“The question is whether that test will replace Step 1 as the standardized metric of student competency,” Mr. Fathy said, which would put more pressure on students further down the medical path.
Will Step 2 anxiety increase?
Dr. Carmody agreed that there is the danger that students now will spend their time studying for Step 2 CK at the expense of other parts of their education.
Meaningful reform will depend on the pass/fail move being coupled with other reforms, most importantly application caps, said Dr. Carmody, who teaches preclinical medical students and works with the residency program.
He has been blogging about Step 1 pass/fail for the past year.
Currently students can apply for as many residencies as they can pay for and Carmody said the number of applications per student has been rising over the past decade.
“That puts program directors under an impossible burden,” he said. “With our Step 1-based system, there’s significant inequality in the number of interviews people get. Programs end up overinviting the same group of people who look good on paper.”
People outside that group respond by sending more applications than they need to just to get a few interviews, Dr. Carmody added.
With caps, students would have an incentive to apply to only those programs in which they had a sincere interest, he said. Program directors also would then be better able to evaluate each application.
Switching Step 1 to pass/fail may have some effect on medical school burnout, Dr. Carmody said.
“It’s one thing to work hard when you’re on call and your patients depend on it,” he said. “But I would have a hard time staying up late every night studying something that I know in my heart is not going to help my patients, but I have to do it because I have to do better than the person who’s studying in the apartment next to me.”
Test has strayed from original purpose
Joseph Safdieh, MD, an assistant dean for clinical curriculum and director of the medical student neurology clerkship for the Weill Cornell Medicine, New York, sees the move as positive overall.
“We should not be using any single metric to define or describe our students’ overall profile,” he said in an interview.
“This has been a very significant anxiety point for our medical students for quite a number of years,” Dr. Safdieh said. “They were frustrated that their entire 4 years of medical school seemingly came down to one number.”
The test was created originally as one of three parts of licensure, he pointed out.
“Over the past 10 or 15 years, the exam has morphed to become a litmus test for very specific residency programs,” he said.
However, Dr. Safdieh has concerns that Step 2 will cultivate the same anxiety and may get too big a spotlight without the Step 1 metric, “although one could argue that test does more accurately reflect clinical material,” he said.
He also worries that students who have selected a specialty by the time they take Step 2 may find late in the game that they are less competitive in their field than they thought they were and may have to make a last-minute switch.
Dr. Safdieh said he thinks Step 2 will be next to go the pass/fail route. In reading between the lines of the announcement, he believes the test cosponsors didn’t make both pass/fail at once because it would have been “a nuclear bomb to the system.”
He credited the cosponsors with making what he called a “bold and momentous decision to initiate radical change in the overall transition between undergraduate and graduate medical education.”
Dr. Safdieh added that few in medicine were expecting Wednesday’s announcement.
“I think many of us were expecting them to go to quartile grading, not to go this far,” he said.
Dr. Safdieh suggested that, among those who may see downstream effects from the pass/fail move are offshore schools, such as those in the Caribbean. “Those schools rely on Step 1 to demonstrate that their students are meeting the rigor,” he said. But he hopes that this will lead to more holistic review.
“We’re hoping that this will force change in the system so that residency directors will look at more than just test-taking ability. They’ll look at publications and scholarship, community service and advocacy and performance in medical school,” Dr. Safdieh said.
Alison J. Whelan, MD, chief medical education officer of the Association of American Medical Colleges said in a statement, “The transition from medical school to residency training is a matter of great concern throughout academic medicine.
“The decision by the NBME and FSMB to change USMLE Step 1 score reporting to pass/fail was very carefully considered to balance student learning and student well-being,” she said. “The medical education community must now work together to identify and implement additional changes to improve the overall UME-GME [undergraduate and graduate medical education] transition system for all stakeholders and the AAMC is committed to helping lead this work.”
Dr. Fathy, Dr. Carmody, and Dr. Safdieh have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
News that the United States Medical Licensing Examination (USMLE) program will change its Step 1 scoring from a 3-digit number to pass/fail starting Jan. 1, 2022, has set off a flurry of shocked responses from students and physicians.
J. Bryan Carmody, MD, MPH, an assistant professor at Eastern Virginia Medical School in Norfolk, said in an interview that he was “stunned” when he heard the news on Wednesday and said the switch presents “the single biggest opportunity for medical school education reform since the Flexner Report,” which in 1910 established standards for modern medical education.
Numbers will continue for some tests
The USMLE cosponsors – the Federation of State Medical Boards (FSMB) and the National Board of Medical Examiners (NBME) – said that the Step 2 Clinical Knowledge (CK) exam and Step 3 will continue to be scored numerically. Step 2 Clinical Skills (CS) will continue its pass/fail system.
The change was made after Step 1 had been roundly criticized as playing too big a role in the process of becoming a physician and for causing students to study for the test instead of engaging fully in their medical education.
Ramie Fathy, a third-year medical student at the University of Pennsylvania, Philadelphia, currently studying for Step 1, said in an interview that it would have been nice personally to have the pass/fail choice, but he predicts both good and unintended consequences in the change.
The positive news, Mr. Fathy said, is that less emphasis will be put on the Step 1 test, which includes memorizing basic science details that may or not be relevant depending on later specialty choice.
“It’s not necessarily measuring what the test makers intended, which was whether or not a student can understand and apply basic science concepts to the practice of medicine,” he said.
“The current system encourages students to get as high a score as possible, which – after a certain point – translates to memorizing many little details that become increasingly less practically relevant,” Mr. Fathy said.
Pressure may move elsewhere?
However, Mr. Fathy worries that, without a scoring system to help decide who stands out in Step 1, residency program directors will depend more on the reputation of candidates’ medical school and the clout of the person writing a letter of recommendation – factors that are often influenced by family resources and social standing. That could wedge a further economic divide into the path to becoming a physician.
Mr. Fathy said he and fellow students are watching for information on what the passing bar will be and what happens with Step 2 Clinical Knowledge exam. USMLE has promised more information as soon as it is available.
“The question is whether that test will replace Step 1 as the standardized metric of student competency,” Mr. Fathy said, which would put more pressure on students further down the medical path.
Will Step 2 anxiety increase?
Dr. Carmody agreed that there is the danger that students now will spend their time studying for Step 2 CK at the expense of other parts of their education.
Meaningful reform will depend on the pass/fail move being coupled with other reforms, most importantly application caps, said Dr. Carmody, who teaches preclinical medical students and works with the residency program.
He has been blogging about Step 1 pass/fail for the past year.
Currently students can apply for as many residencies as they can pay for and Carmody said the number of applications per student has been rising over the past decade.
“That puts program directors under an impossible burden,” he said. “With our Step 1-based system, there’s significant inequality in the number of interviews people get. Programs end up overinviting the same group of people who look good on paper.”
People outside that group respond by sending more applications than they need to just to get a few interviews, Dr. Carmody added.
With caps, students would have an incentive to apply to only those programs in which they had a sincere interest, he said. Program directors also would then be better able to evaluate each application.
Switching Step 1 to pass/fail may have some effect on medical school burnout, Dr. Carmody said.
“It’s one thing to work hard when you’re on call and your patients depend on it,” he said. “But I would have a hard time staying up late every night studying something that I know in my heart is not going to help my patients, but I have to do it because I have to do better than the person who’s studying in the apartment next to me.”
Test has strayed from original purpose
Joseph Safdieh, MD, an assistant dean for clinical curriculum and director of the medical student neurology clerkship for the Weill Cornell Medicine, New York, sees the move as positive overall.
“We should not be using any single metric to define or describe our students’ overall profile,” he said in an interview.
“This has been a very significant anxiety point for our medical students for quite a number of years,” Dr. Safdieh said. “They were frustrated that their entire 4 years of medical school seemingly came down to one number.”
The test was created originally as one of three parts of licensure, he pointed out.
“Over the past 10 or 15 years, the exam has morphed to become a litmus test for very specific residency programs,” he said.
However, Dr. Safdieh has concerns that Step 2 will cultivate the same anxiety and may get too big a spotlight without the Step 1 metric, “although one could argue that test does more accurately reflect clinical material,” he said.
He also worries that students who have selected a specialty by the time they take Step 2 may find late in the game that they are less competitive in their field than they thought they were and may have to make a last-minute switch.
Dr. Safdieh said he thinks Step 2 will be next to go the pass/fail route. In reading between the lines of the announcement, he believes the test cosponsors didn’t make both pass/fail at once because it would have been “a nuclear bomb to the system.”
He credited the cosponsors with making what he called a “bold and momentous decision to initiate radical change in the overall transition between undergraduate and graduate medical education.”
Dr. Safdieh added that few in medicine were expecting Wednesday’s announcement.
“I think many of us were expecting them to go to quartile grading, not to go this far,” he said.
Dr. Safdieh suggested that, among those who may see downstream effects from the pass/fail move are offshore schools, such as those in the Caribbean. “Those schools rely on Step 1 to demonstrate that their students are meeting the rigor,” he said. But he hopes that this will lead to more holistic review.
“We’re hoping that this will force change in the system so that residency directors will look at more than just test-taking ability. They’ll look at publications and scholarship, community service and advocacy and performance in medical school,” Dr. Safdieh said.
Alison J. Whelan, MD, chief medical education officer of the Association of American Medical Colleges said in a statement, “The transition from medical school to residency training is a matter of great concern throughout academic medicine.
“The decision by the NBME and FSMB to change USMLE Step 1 score reporting to pass/fail was very carefully considered to balance student learning and student well-being,” she said. “The medical education community must now work together to identify and implement additional changes to improve the overall UME-GME [undergraduate and graduate medical education] transition system for all stakeholders and the AAMC is committed to helping lead this work.”
Dr. Fathy, Dr. Carmody, and Dr. Safdieh have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.








