User login
Cancer mortality continues to decline while cancer incidence rises in women
according to the Annual Report to the Nation on the Status of Cancer.
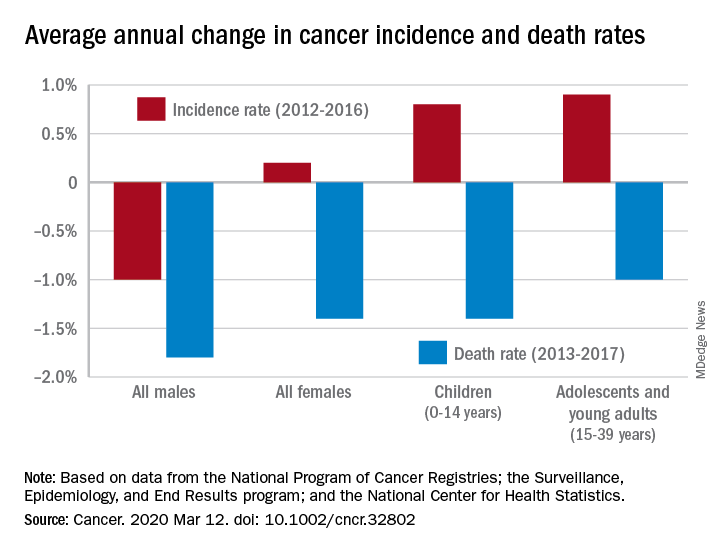
During 2013-2017, the overall age-standardized death rate for all cancers was 158.2 per 100,000 population, and the average decline over that period was 1.5% per year. The average annual change was greater for men (–1.8%) than women (–1.4%) for 2013-2017, but the death rate was higher for men (189.3 per 100,000 vs. 135.5 per 100,000) for those years, S. Jane Henley of the Centers for Disease Control and Prevention and associates reported in Cancer.
“The drops in mortality we’re seeing are real, sustained, and a strong indication of what we can do when we work to prevent and treat cancer,” William G. Cance, MD, chief medical and scientific officer of the America Cancer Society, said in a written statement accompanying the report.
Overall cancer incidence for the most recent 5-year period (2012-2016) was 447.9 per 100,000, with rates of 487.9 for men and 421.4 for women, the investigators said.
Incidence dropped by 0.6% per year overall, but that hides a major difference between men, who saw a decrease of 1.0% a year, and women, who experienced an annual increase of 0.2%.
Over those 5 years, cancer incidence also increased by 0.8% annually among children aged 0-14 years and by 0.9% in adolescents and young adults aged 15-39 years, Ms. Henley and associates said in the report, which is a collaborative effort between the CDC, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
“[W]e must not be complacent. The cancer incidence data – especially the increase in cancer among women – is a clear reminder that there is more work ahead,” Norman E. Sharpless, MD, director of the National Cancer Institute, said in the accompanying statement.
SOURCE: Henley SJ et al. Cancer. 2020 Mar 12. doi: 10.1002/cncr.32802.
according to the Annual Report to the Nation on the Status of Cancer.

During 2013-2017, the overall age-standardized death rate for all cancers was 158.2 per 100,000 population, and the average decline over that period was 1.5% per year. The average annual change was greater for men (–1.8%) than women (–1.4%) for 2013-2017, but the death rate was higher for men (189.3 per 100,000 vs. 135.5 per 100,000) for those years, S. Jane Henley of the Centers for Disease Control and Prevention and associates reported in Cancer.
“The drops in mortality we’re seeing are real, sustained, and a strong indication of what we can do when we work to prevent and treat cancer,” William G. Cance, MD, chief medical and scientific officer of the America Cancer Society, said in a written statement accompanying the report.
Overall cancer incidence for the most recent 5-year period (2012-2016) was 447.9 per 100,000, with rates of 487.9 for men and 421.4 for women, the investigators said.
Incidence dropped by 0.6% per year overall, but that hides a major difference between men, who saw a decrease of 1.0% a year, and women, who experienced an annual increase of 0.2%.
Over those 5 years, cancer incidence also increased by 0.8% annually among children aged 0-14 years and by 0.9% in adolescents and young adults aged 15-39 years, Ms. Henley and associates said in the report, which is a collaborative effort between the CDC, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
“[W]e must not be complacent. The cancer incidence data – especially the increase in cancer among women – is a clear reminder that there is more work ahead,” Norman E. Sharpless, MD, director of the National Cancer Institute, said in the accompanying statement.
SOURCE: Henley SJ et al. Cancer. 2020 Mar 12. doi: 10.1002/cncr.32802.
according to the Annual Report to the Nation on the Status of Cancer.

During 2013-2017, the overall age-standardized death rate for all cancers was 158.2 per 100,000 population, and the average decline over that period was 1.5% per year. The average annual change was greater for men (–1.8%) than women (–1.4%) for 2013-2017, but the death rate was higher for men (189.3 per 100,000 vs. 135.5 per 100,000) for those years, S. Jane Henley of the Centers for Disease Control and Prevention and associates reported in Cancer.
“The drops in mortality we’re seeing are real, sustained, and a strong indication of what we can do when we work to prevent and treat cancer,” William G. Cance, MD, chief medical and scientific officer of the America Cancer Society, said in a written statement accompanying the report.
Overall cancer incidence for the most recent 5-year period (2012-2016) was 447.9 per 100,000, with rates of 487.9 for men and 421.4 for women, the investigators said.
Incidence dropped by 0.6% per year overall, but that hides a major difference between men, who saw a decrease of 1.0% a year, and women, who experienced an annual increase of 0.2%.
Over those 5 years, cancer incidence also increased by 0.8% annually among children aged 0-14 years and by 0.9% in adolescents and young adults aged 15-39 years, Ms. Henley and associates said in the report, which is a collaborative effort between the CDC, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
“[W]e must not be complacent. The cancer incidence data – especially the increase in cancer among women – is a clear reminder that there is more work ahead,” Norman E. Sharpless, MD, director of the National Cancer Institute, said in the accompanying statement.
SOURCE: Henley SJ et al. Cancer. 2020 Mar 12. doi: 10.1002/cncr.32802.
FROM CANCER
COVID-19 update: Transmission 5% or less among close contacts
The transmission rate of coronavirus disease 2019 (COVID-19) was 1%-5% among 38,000 Chinese people in close contact with infected patients, according to the chief epidemiologist of the Chinese Centers for Disease Control and Prevention, Beijing, Zunyou Wu, MD, PhD, who gave an update on the epidemic at the Conference on Retroviruses & Opportunistic Infections.
The rate of spread to family members – the driver of the infection in China – was 10% early in the outbreak, but fell to 3% with quicker recognition and isolation. The overall numbers are lower than might have been expected, and an important insight for clinicians trying to contain the outbreak in the United States.
, but their ability to spread the infection dropped after that, Dr. Wu and others said at a special COVID-19 session at the meeting, which was scheduled to be in Boston, but was held online instead because of concerns about spreading the virus. The session has been posted.
Transmission from presymptomatic people is rare. Shedding persists to some degree for 7-12 days in mild/moderate cases, but 2 weeks or more in severe cases.
Dr. Wu said the numbers in China are moving in the right direction, which means that containment efforts there have worked.
The virus emerged in Wuhan, the capital of Hubei province in central China, in connection with a wildlife food market in December 2019. Bats are thought to be the reservoir, with perhaps an intermediate step between civet cats and raccoon dogs. Officials shut down the market.
Essentially, the entire population of China, more than a billion people, was told to stay home for 10 days to interrupt the transmission cycle after the virus spread throughout the country in a few weeks, and almost 60 million people in Hubei were put behind a cordon sanitaire, where they have been for 50 days and will remain “for a while,” Dr. Wu said.
It’s led to a steep drop in new cases and deaths in China since mid-February; both are now more common outside China than inside, and international numbers are lower than they were at the peak in China.
Meanwhile, there’s been no evidence of perinatal transmission; the virus has not been detected in amniotic fluid, cord blood, neonatal throat swabs, or breast milk. Maternal morbidity appears to be similar to uninfected women. “The data around pregnancy are reassuring,” said John Brooks, MD, chief medical officers for HIV/AIDS prevention at the Centers for Disease Control and Prevention, Atlanta, who has been involved with CDC’s containment efforts.
There’s no data yet for immunocompromised people, but for people with HIV, he said, “we think the risk of severe illness would be greater” with lower CD4 counts and unsuppressed viral loads. “People living with HIV should take precautions against this new virus,” including having at least a 30-day supply of HIV medications; keeping up flu and pneumonia vaccinations; and having a care plan if quarantined. Setting up telemedicine might be a good idea.
The usual incubation period for COVID-19 is 4-6 days but can be longer. Recovery time is about 2 weeks in mild cases and 3-6 weeks in more severe cases. People who die do so within 2 months of symptom onset.
The most common symptoms among hospitalized patients in China are fever, dry cough, fatigue, and headache. Truly asymptomatic cases are not common; most go on to develop symptoms. There have been reports of diarrhea before other symptoms by a day or two, but it’s probably a red herring. The virus has been isolated from stool, but there is no evidence of fecal-oral transmission, Dr. Wu said.
Eighty percent of COVID-19 cases are mild or moderate and most patients recover spontaneously, especially middle aged and younger people. There is no meaningful difference in distribution between the sexes.
There are limited pediatric data perhaps due to underreporting, “but we know [children] experience milder illness than adults,” the CDC’s Dr. Brooks said.
He pegged the latest case fatality estimate at 0.5% to 3.5%, which is considerably higher than seasonal flu, but might well drop as more mild cases are detected and added to the denominator, he said.
For now, death rates top 5% in adults over 60 years old and climb further with increasing age, approaching 16% in people 80 years or older. Patients with hypertension, diabetes, cardiovascular disease, and chronic respiratory illness are at increased risk. The ultimate cause of death is acute respiratory distress syndrome, said Ralph Baric, PhD, a coronavirus expert and epidemiology professor at the University of North Carolina, Chapel Hill, who also presented at the meeting.
Several drug and vaccine candidates are under study for the infection. An intriguing possibility is that angiotensin converting enzyme (ACE) inhibitors might help. Hypertension is a known risk factor for severe infection; the virus makes use of ACE receptor pathways to infect airway epithelial cells; and there have been reports of ACE inhibitors having effect against the virus that caused severe acute respiratory syndrome (SARS), another coronavirus outbreak in 2003.
“I think it’s a very good idea to go back and re-explore use of these drugs,” Dr. Baric said.
The presenters didn’t have any relevant disclosures.
The transmission rate of coronavirus disease 2019 (COVID-19) was 1%-5% among 38,000 Chinese people in close contact with infected patients, according to the chief epidemiologist of the Chinese Centers for Disease Control and Prevention, Beijing, Zunyou Wu, MD, PhD, who gave an update on the epidemic at the Conference on Retroviruses & Opportunistic Infections.
The rate of spread to family members – the driver of the infection in China – was 10% early in the outbreak, but fell to 3% with quicker recognition and isolation. The overall numbers are lower than might have been expected, and an important insight for clinicians trying to contain the outbreak in the United States.
, but their ability to spread the infection dropped after that, Dr. Wu and others said at a special COVID-19 session at the meeting, which was scheduled to be in Boston, but was held online instead because of concerns about spreading the virus. The session has been posted.
Transmission from presymptomatic people is rare. Shedding persists to some degree for 7-12 days in mild/moderate cases, but 2 weeks or more in severe cases.
Dr. Wu said the numbers in China are moving in the right direction, which means that containment efforts there have worked.
The virus emerged in Wuhan, the capital of Hubei province in central China, in connection with a wildlife food market in December 2019. Bats are thought to be the reservoir, with perhaps an intermediate step between civet cats and raccoon dogs. Officials shut down the market.
Essentially, the entire population of China, more than a billion people, was told to stay home for 10 days to interrupt the transmission cycle after the virus spread throughout the country in a few weeks, and almost 60 million people in Hubei were put behind a cordon sanitaire, where they have been for 50 days and will remain “for a while,” Dr. Wu said.
It’s led to a steep drop in new cases and deaths in China since mid-February; both are now more common outside China than inside, and international numbers are lower than they were at the peak in China.
Meanwhile, there’s been no evidence of perinatal transmission; the virus has not been detected in amniotic fluid, cord blood, neonatal throat swabs, or breast milk. Maternal morbidity appears to be similar to uninfected women. “The data around pregnancy are reassuring,” said John Brooks, MD, chief medical officers for HIV/AIDS prevention at the Centers for Disease Control and Prevention, Atlanta, who has been involved with CDC’s containment efforts.
There’s no data yet for immunocompromised people, but for people with HIV, he said, “we think the risk of severe illness would be greater” with lower CD4 counts and unsuppressed viral loads. “People living with HIV should take precautions against this new virus,” including having at least a 30-day supply of HIV medications; keeping up flu and pneumonia vaccinations; and having a care plan if quarantined. Setting up telemedicine might be a good idea.
The usual incubation period for COVID-19 is 4-6 days but can be longer. Recovery time is about 2 weeks in mild cases and 3-6 weeks in more severe cases. People who die do so within 2 months of symptom onset.
The most common symptoms among hospitalized patients in China are fever, dry cough, fatigue, and headache. Truly asymptomatic cases are not common; most go on to develop symptoms. There have been reports of diarrhea before other symptoms by a day or two, but it’s probably a red herring. The virus has been isolated from stool, but there is no evidence of fecal-oral transmission, Dr. Wu said.
Eighty percent of COVID-19 cases are mild or moderate and most patients recover spontaneously, especially middle aged and younger people. There is no meaningful difference in distribution between the sexes.
There are limited pediatric data perhaps due to underreporting, “but we know [children] experience milder illness than adults,” the CDC’s Dr. Brooks said.
He pegged the latest case fatality estimate at 0.5% to 3.5%, which is considerably higher than seasonal flu, but might well drop as more mild cases are detected and added to the denominator, he said.
For now, death rates top 5% in adults over 60 years old and climb further with increasing age, approaching 16% in people 80 years or older. Patients with hypertension, diabetes, cardiovascular disease, and chronic respiratory illness are at increased risk. The ultimate cause of death is acute respiratory distress syndrome, said Ralph Baric, PhD, a coronavirus expert and epidemiology professor at the University of North Carolina, Chapel Hill, who also presented at the meeting.
Several drug and vaccine candidates are under study for the infection. An intriguing possibility is that angiotensin converting enzyme (ACE) inhibitors might help. Hypertension is a known risk factor for severe infection; the virus makes use of ACE receptor pathways to infect airway epithelial cells; and there have been reports of ACE inhibitors having effect against the virus that caused severe acute respiratory syndrome (SARS), another coronavirus outbreak in 2003.
“I think it’s a very good idea to go back and re-explore use of these drugs,” Dr. Baric said.
The presenters didn’t have any relevant disclosures.
The transmission rate of coronavirus disease 2019 (COVID-19) was 1%-5% among 38,000 Chinese people in close contact with infected patients, according to the chief epidemiologist of the Chinese Centers for Disease Control and Prevention, Beijing, Zunyou Wu, MD, PhD, who gave an update on the epidemic at the Conference on Retroviruses & Opportunistic Infections.
The rate of spread to family members – the driver of the infection in China – was 10% early in the outbreak, but fell to 3% with quicker recognition and isolation. The overall numbers are lower than might have been expected, and an important insight for clinicians trying to contain the outbreak in the United States.
, but their ability to spread the infection dropped after that, Dr. Wu and others said at a special COVID-19 session at the meeting, which was scheduled to be in Boston, but was held online instead because of concerns about spreading the virus. The session has been posted.
Transmission from presymptomatic people is rare. Shedding persists to some degree for 7-12 days in mild/moderate cases, but 2 weeks or more in severe cases.
Dr. Wu said the numbers in China are moving in the right direction, which means that containment efforts there have worked.
The virus emerged in Wuhan, the capital of Hubei province in central China, in connection with a wildlife food market in December 2019. Bats are thought to be the reservoir, with perhaps an intermediate step between civet cats and raccoon dogs. Officials shut down the market.
Essentially, the entire population of China, more than a billion people, was told to stay home for 10 days to interrupt the transmission cycle after the virus spread throughout the country in a few weeks, and almost 60 million people in Hubei were put behind a cordon sanitaire, where they have been for 50 days and will remain “for a while,” Dr. Wu said.
It’s led to a steep drop in new cases and deaths in China since mid-February; both are now more common outside China than inside, and international numbers are lower than they were at the peak in China.
Meanwhile, there’s been no evidence of perinatal transmission; the virus has not been detected in amniotic fluid, cord blood, neonatal throat swabs, or breast milk. Maternal morbidity appears to be similar to uninfected women. “The data around pregnancy are reassuring,” said John Brooks, MD, chief medical officers for HIV/AIDS prevention at the Centers for Disease Control and Prevention, Atlanta, who has been involved with CDC’s containment efforts.
There’s no data yet for immunocompromised people, but for people with HIV, he said, “we think the risk of severe illness would be greater” with lower CD4 counts and unsuppressed viral loads. “People living with HIV should take precautions against this new virus,” including having at least a 30-day supply of HIV medications; keeping up flu and pneumonia vaccinations; and having a care plan if quarantined. Setting up telemedicine might be a good idea.
The usual incubation period for COVID-19 is 4-6 days but can be longer. Recovery time is about 2 weeks in mild cases and 3-6 weeks in more severe cases. People who die do so within 2 months of symptom onset.
The most common symptoms among hospitalized patients in China are fever, dry cough, fatigue, and headache. Truly asymptomatic cases are not common; most go on to develop symptoms. There have been reports of diarrhea before other symptoms by a day or two, but it’s probably a red herring. The virus has been isolated from stool, but there is no evidence of fecal-oral transmission, Dr. Wu said.
Eighty percent of COVID-19 cases are mild or moderate and most patients recover spontaneously, especially middle aged and younger people. There is no meaningful difference in distribution between the sexes.
There are limited pediatric data perhaps due to underreporting, “but we know [children] experience milder illness than adults,” the CDC’s Dr. Brooks said.
He pegged the latest case fatality estimate at 0.5% to 3.5%, which is considerably higher than seasonal flu, but might well drop as more mild cases are detected and added to the denominator, he said.
For now, death rates top 5% in adults over 60 years old and climb further with increasing age, approaching 16% in people 80 years or older. Patients with hypertension, diabetes, cardiovascular disease, and chronic respiratory illness are at increased risk. The ultimate cause of death is acute respiratory distress syndrome, said Ralph Baric, PhD, a coronavirus expert and epidemiology professor at the University of North Carolina, Chapel Hill, who also presented at the meeting.
Several drug and vaccine candidates are under study for the infection. An intriguing possibility is that angiotensin converting enzyme (ACE) inhibitors might help. Hypertension is a known risk factor for severe infection; the virus makes use of ACE receptor pathways to infect airway epithelial cells; and there have been reports of ACE inhibitors having effect against the virus that caused severe acute respiratory syndrome (SARS), another coronavirus outbreak in 2003.
“I think it’s a very good idea to go back and re-explore use of these drugs,” Dr. Baric said.
The presenters didn’t have any relevant disclosures.
FROM CROI 2020
Flu activity declines again but remains high
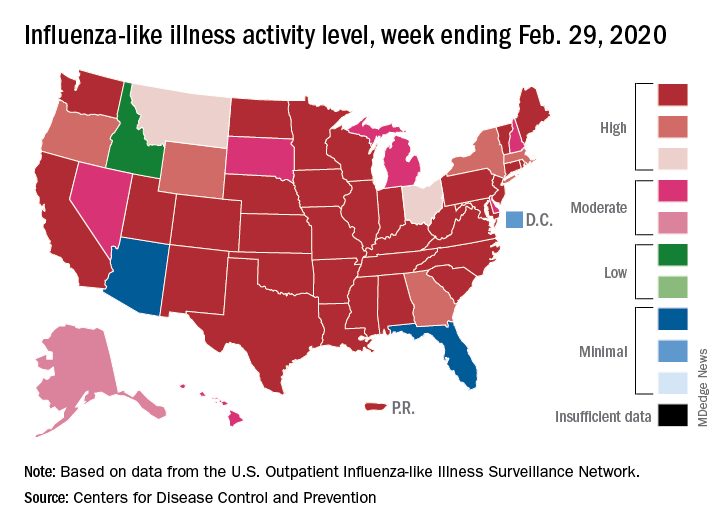
Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.

Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.

Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.
The apology in medicine—yes, no, or maybe?
This is the third and final article in a series focusing on malpractice, liability, and reform. In the first article, we looked at the background on malpractice and reasons malpractice rates have been so high—including large verdicts and lawsuit-prone physicians. In the second article we considered recent experience and developments in malpractice exposure, who is sued and why. Finally, in this third article, we focus on apologies, apology laws, and liability.
“I’m sorry”
In childhood we are all taught the basic courtesies: “please” and “thank you,” and “I’m sorry,” when harm has occurred. Should we as adult health care providers fear the consequences of apologizing? Apologies are a way for clinicians to express empathy; they also serve as a tool to reduce medical malpractice claims.1

Apologies, ethics, and care
The American Medical Association takes the position that a physician has an ethical duty to disclose a harmful error to a patient.2,3 Indeed this approach has been an impetus for states to enact apology laws, which we discuss below. As pointed out in this 2013 article title, “Dealing with a medical mistake: Should physicians apologize to patients?”,4 the legal benefits of any apology are an issue. It is a controversial area in medicine still today, including in obstetrics and gynecology.
“Ethical codes for both M.D.s and D.O.s suggest providers should display honesty and empathy following adverse events and errors.”1,3,5 In addition, the American Medical Association states, “a physician should at all times deal honestly and openly with patients.”2 Concerns about liability that may result from truthful disclosure should not affect the physician’s honesty (TABLE). Increasingly, the law has sided with that principle through apology laws.

Some patients sue to get answers to the “What happened?” and “Why did it happen?” questions.6 They also sometimes are motivated by a desire to help ensure that the same injury does not happen to others. Silence on the part of the clinician may be seen as a lack of sympathy or remorse and patients may fear that other patients will be harmed.1
The relationship between physician and patient involves vulnerability and requires trust. When an injury occurs, the relationship can be injured as well. Barriers to apology in part reflect “the culture of medicine” as well as the “inherent psychological difficulties in facing one’s mistakes and apologizing for them.” However, apology by the provider may result in “effective resolution of disputes related to medical error.”7
The patient’s perspective is critical to this type of outcome, of course. A study from the United Kingdom noted that one-third of patients who experience a medical error have a desire to receive an apology or explanation. Furthermore, patients need assurance that a plan of action to prevent such a future occurrence is in place.8 Surveys reflect that patients desire, or even expect, the physician to acknowledge an error.9 We will see that there is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 For instance, Dahan and colleagues completed a study that highlights the “act of apology,” which can be seen as a “language art.”11 Medical schools have recognized the importance of the apology and now incorporate training focused on error disclosure and provision of apologies into the curriculum.12
Continue to: Legal issues and medical apologies...
Legal issues and medical apologies
From a legal standpoint, traditionally, an apology from a physician to a patient could be used against a physician in a medical liability (malpractice) case as proof of negligence.
Statements of interest. Such out-of-court statements ordinarily would be “hearsay” and excluded from evidence; there is, however, an exception to this hearsay rule that allows “confessions” or “statements against interest” to be admissible against the party making the statement. The theory is that when a statement is harmful to the person making it, the person likely thought that it was true, and the statement should be admissible at trial. We do not generally go around confessing to things that are not true. Following an auto crash, if one driver jumps out of the car saying, “I am so sorry I hit you. I was using my cell phone and did not see you stop,” the statement is against the interest of the driver and could be used in court.
As a matter of general legal principle, the same issue can arise in medical practice. Suppose a physician says, “I am so sorry for your injury. We made a mistake in interpreting the data from the monitors.” That sounds a lot like not just an apology but a statement against interest. Malpractice cases generally are based on the claim that a “doctor failed to do what a reasonable provider in the same specialty would have done in a similar situation.”13 An apology may be little more than general sympathy (“I’m sorry to tell you that we have not cured the infection. Unfortunately, that will mean more time in the hospital.”), but it can include a confession of error (“I’m sorry we got the x-ray backward and removed the wrong kidney.”). In the latter kind of apology, courts traditionally have found a “statement against interest.”
The legal consequence of a statement against interest is that the statement may be admitted in court. Such statements do not automatically establish negligence, but they can be powerful evidence when presented to a jury.
Courts have struggled with medical apologies. General sympathy or feelings of regret or compassion do not generally rise to the level of an admission that the physician did not use reasonable care under the circumstances and ordinarily are not admissible. (For further details, we refer you to the case of Cobbs v. Grant.14 Even if a physician said to the patient that he “blamed himself for [the patient] being back in the hospital for a second time,…the statement signifies compassion, or at most, a feeling of remorse, for plaintiff’s ordeal.”) On the other hand, in cases in which a physician in an apology referred to a “careless” mistake or even a “negligent” mistake, courts have allowed it admitted at trial as a statement against interest. (A 1946 case, Woronka v. Sewall, is an example.15 In that case, the physician said to the patient, “My God, what a mess…she had a very hard delivery, and it was a burning shame to get [an injury] on top of it, and it was because of negligence when they were upstairs.”) Some of these cases come down to the provider’s use of a single word: fault, careless, or negligence.
The ambiguity over the legal place of medical apologies in medicine led attorneys to urge medical providers to avoid statements that might even remotely be taken as statements against interest, including real apologies. The confusion over the admissibility of medical apologies led state legislatures to adopt apology laws. These laws essentially limit what statements against interest may be introduced in professional liability cases when a provider has issued a responsibility or apologized.
Continue to: Apology statutes...
Apology statutes
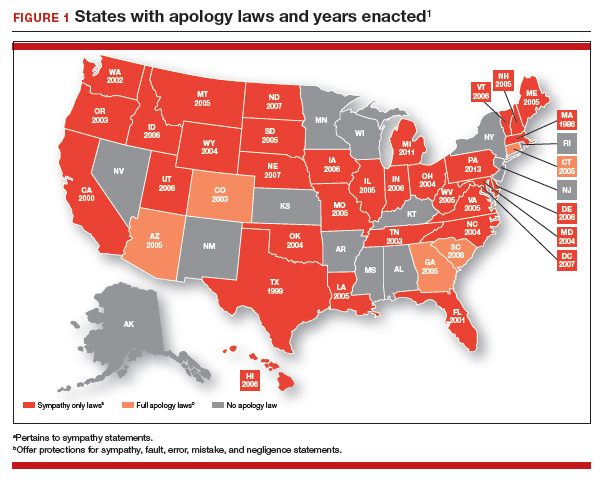
Massachusetts was the first state to enact an apology law—in 1986.1 As of 2019, a clear majority of states have some form of apology statute. “Apology laws are gaining traction,” was the first sentence in a 2012 review on the subject by Saitta and colleagues.3 Only a few (5 states) have “strong” statutes that have broad protection for statements of fault, error, and negligence, as well as sympathy. The other 33 states have statutes that only protect against statements of sympathy.4,16 FIGURE 1 is a US map showing the apology laws by state.1
Do apology statutes and apologies reduce liability?
The positive aspects of apology include personal, psychological, and emotional benefits to both the one apologizing and the one receiving the apology. It also may have financial benefits to health care providers.4 The assumption has been, and there has been some evidence for the proposition, that apologies reduce the possibility of malpractice claims. That is one of the reasons that institutions may have formal apology policies. Indeed, there is evidence that apologies reduce financial awards to patients, as manifest in the states of Pennsylvania and Kentucky.4 Apologies appear to reduce patient anger and can open the door to better communication with the provider. There is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 The conclusion from these studies might be that honest and open communication serves to decrease the incidence of medical malpractice lawsuit initiation and that honesty is the best policy.
It is important to note the difference, however, between apologies (or institutional apology policies) and apology laws. There is some evidence that apology and institutional apology policies may reduce malpractice claims or losses.17,18 On the other hand, the studies of apology laws have not found that these laws have much impact on malpractice rates. An especially good and thorough study of the effect of apology laws nationwide, using insurance claims data, essentially found little net effect of the apology laws.19,20 One other study could find no evidence that apology statutes reduce defensive medicine (so no reduction in provider concerns over liability).21
It should be noted that most studies on medical apology and its effects on malpractice claims generally have looked at the narrow or limited apology statutes (that do not cover expressions of fault or negligence). Few states have the broader statutes, and it is possible that those broader statutes would be more effective in reducing liability. Removing the disincentives to medical apologies is a good thing, but in and of itself it is probably not a liability game changer.
Continue to: Institutional policy and apology...
Institutional policy and apology
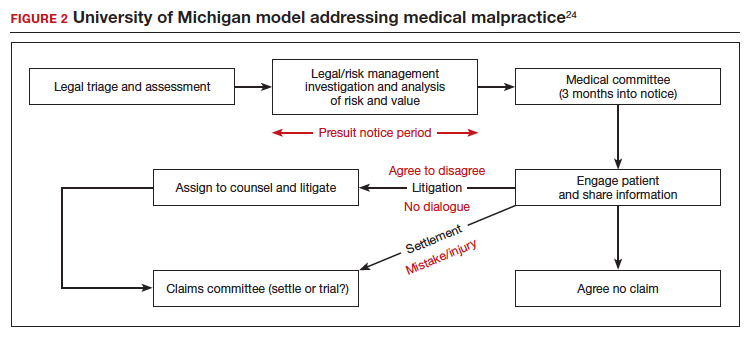
Some institutions have established an “inclusion of apology” strategy for medical errors. These policies appear to have a meaningful effect on reducing medical malpractice costs. These programs commonly include a proactive investigation, disclosure of error, and apologies. Such policies have been studied at the University of Michigan and the Veterans Affairs (VA) Hospital in Lexington, Kentucky. The University of Michigan program resulted in a 60% reduction in compensation costs for medical errors.22 It also cut litigation costs by half.23 The review of the Kentucky VA program also was positive.17 FIGURE 2 illustrates the key features of the Michigan program.24
Conclusions: Effective apologies
Our conclusions, first, are that apologies are important from all perspectives: ethical, medical, and legal. On the other hand, all of the attention given in recent years to apology statutes may have been misplaced, at least if they were intended to be malpractice reform.17
Institutional apology and response programs are likely successful because they are thoughtfully put together, generally based on the best understanding of how injured patients respond to apologies and what it takes to be sincere, and communicate that sincerity, in the apology. What is an effective apology?, “The acceptance of responsibility for having caused harm.” It may, for example, mean accepting some financial responsibility for the harm. It is also important that the apology is conveyed in such a way that it includes an element of self-critical expression.25 Although there are many formulations of the elements of an effective apology, one example is, “(1) acknowledging and accepting responsibility for the offense; (2) expressing remorse with forbearance, sincerity, and honesty; (3) explaining the understanding of the offense; and (4) willingness to make reparations.”26
At the other extreme is a medical professional, after a bad event, trying to engage in a half-hearted, awkward, or insincere apology on an ad hoc and poorly planned basis. Worse still, “when victims perceive apologies to be insincere and designed simply to cool them off, they react with more rather than less indignation.”27 Of course, the “forced apology” may be the worst of all. An instance of this was addressed in a New Zealand study in which providers were “forced” to provide a written apology to a couple (Mr. and Mrs. B) and a separate written apology to Baby B when there was failure to discuss vitamin K administration during the antenatal period when it was indicated.28 Rather than emphasizing required apology in such a case, which can seem hollow and disingenuous, emphasis was placed on the apology providing a “positive-physiological” effect for those harmed, and on strategies that “nurture the development of the moral maturity required for authentic apology.”
The great advantage of institutional or practice-wide policies is that they can be developed in the calm of planning, with good foresight and careful consideration. This is much different from having to come up with some approach in the heat of something having gone wrong. Ultimately, however, apologies are not about liability. They are about caring for, respecting, and communicating with those who are harmed. Apologizing is often the right and professional thing to do.
- Afrassiab Z. Why mediation & “sorry” make sense: apology statutes as a catalyst for change in medical malpractice. J Dispute Resolutions. 2019.
- AMA Council on Ethical and Judicial Affairs. AMA code of medical ethics’ opinions on patient safety. Virtual Mentor. 2011;13:626-628.
- Saitta N, Hodge SD. Efficacy of a physician’s words of empathy: an overview of state apology laws. J Am Osteopath Assn. 2012;112:302-306.
- Dealing with a medical mistake: Should physicians apologize to patients? Med Economics. November 10, 2013.
- AOA code of ethics. American Osteopathic Association website. http://www.osteopathic.org/inside-aoa/about /leadershipPages/aos-code-of-ethics.aspx. Accessed January 15, 2020.
- You had me at “I’m sorry”: the impact of physicians’ apologies on medical malpractice litigation. Natl Law Review. November 6, 2018. https://www.natlawreview.com /article/you-had-me-i-m-sorry-impact-physicians-apologiesmedical-malpractice-litigation. Accessed February 6, 2020.
- Robbennolt JK. Apologies and medical error. Clin Orthop Relat Res. 2009;467:376-382.
- Bismark MM. The power of apology. N Z Med J. 2009;122:96-106.
- Witman AB, Park DM, Hardin SB. How do patients want physicians to handle mistakes? A survey of internal medicine patients in an academic setting. Arch Intern Med. 1996;156:2565-2569.
- Lawthers AG, Localio AR, Laird NM, et al. Physicians’ perceptions of the risk of being sued. J Health Polit Policy Law. 1992;17:463-482.
- Dahan S, Ducard D, Caeymaex L. Apology in cases of medical error disclosure: thoughts based on a preliminary study. PLoS One. 2017;12:e0181854.
- Halbach JL, Sullivan LL. Teaching medical students about medical errors and patient safety: evaluation of a required curriculum. Acad Med. 2005;80:600-606.
- Nussbaum L. Trial and error: legislating ADR for medical malpractice reform. 2017. Scholarly Works. https://scholars .law.unlv.edu/facpub/1011. Accessed February 7, 2020.
- Cobbs v. Grant, 8 Cal. 3d 229, 104 Cal. Rptr. 505, 502 P.2d 1 (1972).
- Woronka v. Sewall, 320 Mass. 362, 69 N.E.2d 581 (1946).
- Wei M. Doctors, apologies and the law: an analysis and critique of apology law. J Health Law. 2007;40:107-159.
- Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
- Liebman CB, Hyman CS. Medical error disclosure, mediation skills, and malpractice litigation: a demonstration project in Pennsylvania. 2005. https://perma.cc/7257-99GU. Accessed February 7, 2020.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Ho B, Liu E. What’s an apology worth? Decomposing the effect of apologies on medical malpractice payments using state apology laws. J Empirical Legal Studies. 2011;8:179-199.
- McMichael BJ. The failure of sorry: an empirical evaluation of apology laws, health care, and medical malpractice. Lewis & Clark Law Rev. 2017. https://law.lclark.edu/live/files/27734- lcb224article3mcmichaelpdf. Accessed February 7, 2020.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- Boothman RC, Blackwell AC, Campbell DA Jr, et al. A better approach to medical malpractice claims? The University of Michigan experience. J Health Life Sci Law. 2009;2:125-159.
- The Michigan model: Medical malpractice and patient safety at Michigan Medicine. University of Michigan website. https:// www.uofmhealth.org/michigan-model-medical-malpracticeand-patient-safety-umhs#summary. Accessed February 7, 2020.
- Mastroianni AC, Mello MM, Sommer S, et al. The flaws in state ‘apology’ and ‘disclosure’ laws dilute their intended impact on malpractice suits. Health Aff (Millwood). 2010;29:1611-1619.
- Davis ER. I’m sorry I’m scared of litigation: evaluating the effectiveness of apology laws. Forum: Tennessee Student Legal J. 2016;3. https://trace.tennessee.edu/forum/vol3/iss1/4/. Accessed February 7, 2020.
- Miller DT. Disrespect and the experience of injustice. Annu Rev Psychol. 2001;52:527-553.
- McLennan S, Walker S, Rich LE. Should health care providers be forced to apologise after things go wrong? J Bioeth Inq. 2014;11:431-435
This is the third and final article in a series focusing on malpractice, liability, and reform. In the first article, we looked at the background on malpractice and reasons malpractice rates have been so high—including large verdicts and lawsuit-prone physicians. In the second article we considered recent experience and developments in malpractice exposure, who is sued and why. Finally, in this third article, we focus on apologies, apology laws, and liability.
“I’m sorry”
In childhood we are all taught the basic courtesies: “please” and “thank you,” and “I’m sorry,” when harm has occurred. Should we as adult health care providers fear the consequences of apologizing? Apologies are a way for clinicians to express empathy; they also serve as a tool to reduce medical malpractice claims.1

Apologies, ethics, and care
The American Medical Association takes the position that a physician has an ethical duty to disclose a harmful error to a patient.2,3 Indeed this approach has been an impetus for states to enact apology laws, which we discuss below. As pointed out in this 2013 article title, “Dealing with a medical mistake: Should physicians apologize to patients?”,4 the legal benefits of any apology are an issue. It is a controversial area in medicine still today, including in obstetrics and gynecology.
“Ethical codes for both M.D.s and D.O.s suggest providers should display honesty and empathy following adverse events and errors.”1,3,5 In addition, the American Medical Association states, “a physician should at all times deal honestly and openly with patients.”2 Concerns about liability that may result from truthful disclosure should not affect the physician’s honesty (TABLE). Increasingly, the law has sided with that principle through apology laws.

Some patients sue to get answers to the “What happened?” and “Why did it happen?” questions.6 They also sometimes are motivated by a desire to help ensure that the same injury does not happen to others. Silence on the part of the clinician may be seen as a lack of sympathy or remorse and patients may fear that other patients will be harmed.1
The relationship between physician and patient involves vulnerability and requires trust. When an injury occurs, the relationship can be injured as well. Barriers to apology in part reflect “the culture of medicine” as well as the “inherent psychological difficulties in facing one’s mistakes and apologizing for them.” However, apology by the provider may result in “effective resolution of disputes related to medical error.”7
The patient’s perspective is critical to this type of outcome, of course. A study from the United Kingdom noted that one-third of patients who experience a medical error have a desire to receive an apology or explanation. Furthermore, patients need assurance that a plan of action to prevent such a future occurrence is in place.8 Surveys reflect that patients desire, or even expect, the physician to acknowledge an error.9 We will see that there is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 For instance, Dahan and colleagues completed a study that highlights the “act of apology,” which can be seen as a “language art.”11 Medical schools have recognized the importance of the apology and now incorporate training focused on error disclosure and provision of apologies into the curriculum.12
Continue to: Legal issues and medical apologies...
Legal issues and medical apologies
From a legal standpoint, traditionally, an apology from a physician to a patient could be used against a physician in a medical liability (malpractice) case as proof of negligence.
Statements of interest. Such out-of-court statements ordinarily would be “hearsay” and excluded from evidence; there is, however, an exception to this hearsay rule that allows “confessions” or “statements against interest” to be admissible against the party making the statement. The theory is that when a statement is harmful to the person making it, the person likely thought that it was true, and the statement should be admissible at trial. We do not generally go around confessing to things that are not true. Following an auto crash, if one driver jumps out of the car saying, “I am so sorry I hit you. I was using my cell phone and did not see you stop,” the statement is against the interest of the driver and could be used in court.
As a matter of general legal principle, the same issue can arise in medical practice. Suppose a physician says, “I am so sorry for your injury. We made a mistake in interpreting the data from the monitors.” That sounds a lot like not just an apology but a statement against interest. Malpractice cases generally are based on the claim that a “doctor failed to do what a reasonable provider in the same specialty would have done in a similar situation.”13 An apology may be little more than general sympathy (“I’m sorry to tell you that we have not cured the infection. Unfortunately, that will mean more time in the hospital.”), but it can include a confession of error (“I’m sorry we got the x-ray backward and removed the wrong kidney.”). In the latter kind of apology, courts traditionally have found a “statement against interest.”
The legal consequence of a statement against interest is that the statement may be admitted in court. Such statements do not automatically establish negligence, but they can be powerful evidence when presented to a jury.
Courts have struggled with medical apologies. General sympathy or feelings of regret or compassion do not generally rise to the level of an admission that the physician did not use reasonable care under the circumstances and ordinarily are not admissible. (For further details, we refer you to the case of Cobbs v. Grant.14 Even if a physician said to the patient that he “blamed himself for [the patient] being back in the hospital for a second time,…the statement signifies compassion, or at most, a feeling of remorse, for plaintiff’s ordeal.”) On the other hand, in cases in which a physician in an apology referred to a “careless” mistake or even a “negligent” mistake, courts have allowed it admitted at trial as a statement against interest. (A 1946 case, Woronka v. Sewall, is an example.15 In that case, the physician said to the patient, “My God, what a mess…she had a very hard delivery, and it was a burning shame to get [an injury] on top of it, and it was because of negligence when they were upstairs.”) Some of these cases come down to the provider’s use of a single word: fault, careless, or negligence.
The ambiguity over the legal place of medical apologies in medicine led attorneys to urge medical providers to avoid statements that might even remotely be taken as statements against interest, including real apologies. The confusion over the admissibility of medical apologies led state legislatures to adopt apology laws. These laws essentially limit what statements against interest may be introduced in professional liability cases when a provider has issued a responsibility or apologized.
Continue to: Apology statutes...
Apology statutes
Massachusetts was the first state to enact an apology law—in 1986.1 As of 2019, a clear majority of states have some form of apology statute. “Apology laws are gaining traction,” was the first sentence in a 2012 review on the subject by Saitta and colleagues.3 Only a few (5 states) have “strong” statutes that have broad protection for statements of fault, error, and negligence, as well as sympathy. The other 33 states have statutes that only protect against statements of sympathy.4,16 FIGURE 1 is a US map showing the apology laws by state.1

Do apology statutes and apologies reduce liability?
The positive aspects of apology include personal, psychological, and emotional benefits to both the one apologizing and the one receiving the apology. It also may have financial benefits to health care providers.4 The assumption has been, and there has been some evidence for the proposition, that apologies reduce the possibility of malpractice claims. That is one of the reasons that institutions may have formal apology policies. Indeed, there is evidence that apologies reduce financial awards to patients, as manifest in the states of Pennsylvania and Kentucky.4 Apologies appear to reduce patient anger and can open the door to better communication with the provider. There is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 The conclusion from these studies might be that honest and open communication serves to decrease the incidence of medical malpractice lawsuit initiation and that honesty is the best policy.
It is important to note the difference, however, between apologies (or institutional apology policies) and apology laws. There is some evidence that apology and institutional apology policies may reduce malpractice claims or losses.17,18 On the other hand, the studies of apology laws have not found that these laws have much impact on malpractice rates. An especially good and thorough study of the effect of apology laws nationwide, using insurance claims data, essentially found little net effect of the apology laws.19,20 One other study could find no evidence that apology statutes reduce defensive medicine (so no reduction in provider concerns over liability).21
It should be noted that most studies on medical apology and its effects on malpractice claims generally have looked at the narrow or limited apology statutes (that do not cover expressions of fault or negligence). Few states have the broader statutes, and it is possible that those broader statutes would be more effective in reducing liability. Removing the disincentives to medical apologies is a good thing, but in and of itself it is probably not a liability game changer.
Continue to: Institutional policy and apology...
Institutional policy and apology
Some institutions have established an “inclusion of apology” strategy for medical errors. These policies appear to have a meaningful effect on reducing medical malpractice costs. These programs commonly include a proactive investigation, disclosure of error, and apologies. Such policies have been studied at the University of Michigan and the Veterans Affairs (VA) Hospital in Lexington, Kentucky. The University of Michigan program resulted in a 60% reduction in compensation costs for medical errors.22 It also cut litigation costs by half.23 The review of the Kentucky VA program also was positive.17 FIGURE 2 illustrates the key features of the Michigan program.24

Conclusions: Effective apologies
Our conclusions, first, are that apologies are important from all perspectives: ethical, medical, and legal. On the other hand, all of the attention given in recent years to apology statutes may have been misplaced, at least if they were intended to be malpractice reform.17
Institutional apology and response programs are likely successful because they are thoughtfully put together, generally based on the best understanding of how injured patients respond to apologies and what it takes to be sincere, and communicate that sincerity, in the apology. What is an effective apology?, “The acceptance of responsibility for having caused harm.” It may, for example, mean accepting some financial responsibility for the harm. It is also important that the apology is conveyed in such a way that it includes an element of self-critical expression.25 Although there are many formulations of the elements of an effective apology, one example is, “(1) acknowledging and accepting responsibility for the offense; (2) expressing remorse with forbearance, sincerity, and honesty; (3) explaining the understanding of the offense; and (4) willingness to make reparations.”26
At the other extreme is a medical professional, after a bad event, trying to engage in a half-hearted, awkward, or insincere apology on an ad hoc and poorly planned basis. Worse still, “when victims perceive apologies to be insincere and designed simply to cool them off, they react with more rather than less indignation.”27 Of course, the “forced apology” may be the worst of all. An instance of this was addressed in a New Zealand study in which providers were “forced” to provide a written apology to a couple (Mr. and Mrs. B) and a separate written apology to Baby B when there was failure to discuss vitamin K administration during the antenatal period when it was indicated.28 Rather than emphasizing required apology in such a case, which can seem hollow and disingenuous, emphasis was placed on the apology providing a “positive-physiological” effect for those harmed, and on strategies that “nurture the development of the moral maturity required for authentic apology.”
The great advantage of institutional or practice-wide policies is that they can be developed in the calm of planning, with good foresight and careful consideration. This is much different from having to come up with some approach in the heat of something having gone wrong. Ultimately, however, apologies are not about liability. They are about caring for, respecting, and communicating with those who are harmed. Apologizing is often the right and professional thing to do.
This is the third and final article in a series focusing on malpractice, liability, and reform. In the first article, we looked at the background on malpractice and reasons malpractice rates have been so high—including large verdicts and lawsuit-prone physicians. In the second article we considered recent experience and developments in malpractice exposure, who is sued and why. Finally, in this third article, we focus on apologies, apology laws, and liability.
“I’m sorry”
In childhood we are all taught the basic courtesies: “please” and “thank you,” and “I’m sorry,” when harm has occurred. Should we as adult health care providers fear the consequences of apologizing? Apologies are a way for clinicians to express empathy; they also serve as a tool to reduce medical malpractice claims.1

Apologies, ethics, and care
The American Medical Association takes the position that a physician has an ethical duty to disclose a harmful error to a patient.2,3 Indeed this approach has been an impetus for states to enact apology laws, which we discuss below. As pointed out in this 2013 article title, “Dealing with a medical mistake: Should physicians apologize to patients?”,4 the legal benefits of any apology are an issue. It is a controversial area in medicine still today, including in obstetrics and gynecology.
“Ethical codes for both M.D.s and D.O.s suggest providers should display honesty and empathy following adverse events and errors.”1,3,5 In addition, the American Medical Association states, “a physician should at all times deal honestly and openly with patients.”2 Concerns about liability that may result from truthful disclosure should not affect the physician’s honesty (TABLE). Increasingly, the law has sided with that principle through apology laws.

Some patients sue to get answers to the “What happened?” and “Why did it happen?” questions.6 They also sometimes are motivated by a desire to help ensure that the same injury does not happen to others. Silence on the part of the clinician may be seen as a lack of sympathy or remorse and patients may fear that other patients will be harmed.1
The relationship between physician and patient involves vulnerability and requires trust. When an injury occurs, the relationship can be injured as well. Barriers to apology in part reflect “the culture of medicine” as well as the “inherent psychological difficulties in facing one’s mistakes and apologizing for them.” However, apology by the provider may result in “effective resolution of disputes related to medical error.”7
The patient’s perspective is critical to this type of outcome, of course. A study from the United Kingdom noted that one-third of patients who experience a medical error have a desire to receive an apology or explanation. Furthermore, patients need assurance that a plan of action to prevent such a future occurrence is in place.8 Surveys reflect that patients desire, or even expect, the physician to acknowledge an error.9 We will see that there is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 For instance, Dahan and colleagues completed a study that highlights the “act of apology,” which can be seen as a “language art.”11 Medical schools have recognized the importance of the apology and now incorporate training focused on error disclosure and provision of apologies into the curriculum.12
Continue to: Legal issues and medical apologies...
Legal issues and medical apologies
From a legal standpoint, traditionally, an apology from a physician to a patient could be used against a physician in a medical liability (malpractice) case as proof of negligence.
Statements of interest. Such out-of-court statements ordinarily would be “hearsay” and excluded from evidence; there is, however, an exception to this hearsay rule that allows “confessions” or “statements against interest” to be admissible against the party making the statement. The theory is that when a statement is harmful to the person making it, the person likely thought that it was true, and the statement should be admissible at trial. We do not generally go around confessing to things that are not true. Following an auto crash, if one driver jumps out of the car saying, “I am so sorry I hit you. I was using my cell phone and did not see you stop,” the statement is against the interest of the driver and could be used in court.
As a matter of general legal principle, the same issue can arise in medical practice. Suppose a physician says, “I am so sorry for your injury. We made a mistake in interpreting the data from the monitors.” That sounds a lot like not just an apology but a statement against interest. Malpractice cases generally are based on the claim that a “doctor failed to do what a reasonable provider in the same specialty would have done in a similar situation.”13 An apology may be little more than general sympathy (“I’m sorry to tell you that we have not cured the infection. Unfortunately, that will mean more time in the hospital.”), but it can include a confession of error (“I’m sorry we got the x-ray backward and removed the wrong kidney.”). In the latter kind of apology, courts traditionally have found a “statement against interest.”
The legal consequence of a statement against interest is that the statement may be admitted in court. Such statements do not automatically establish negligence, but they can be powerful evidence when presented to a jury.
Courts have struggled with medical apologies. General sympathy or feelings of regret or compassion do not generally rise to the level of an admission that the physician did not use reasonable care under the circumstances and ordinarily are not admissible. (For further details, we refer you to the case of Cobbs v. Grant.14 Even if a physician said to the patient that he “blamed himself for [the patient] being back in the hospital for a second time,…the statement signifies compassion, or at most, a feeling of remorse, for plaintiff’s ordeal.”) On the other hand, in cases in which a physician in an apology referred to a “careless” mistake or even a “negligent” mistake, courts have allowed it admitted at trial as a statement against interest. (A 1946 case, Woronka v. Sewall, is an example.15 In that case, the physician said to the patient, “My God, what a mess…she had a very hard delivery, and it was a burning shame to get [an injury] on top of it, and it was because of negligence when they were upstairs.”) Some of these cases come down to the provider’s use of a single word: fault, careless, or negligence.
The ambiguity over the legal place of medical apologies in medicine led attorneys to urge medical providers to avoid statements that might even remotely be taken as statements against interest, including real apologies. The confusion over the admissibility of medical apologies led state legislatures to adopt apology laws. These laws essentially limit what statements against interest may be introduced in professional liability cases when a provider has issued a responsibility or apologized.
Continue to: Apology statutes...
Apology statutes
Massachusetts was the first state to enact an apology law—in 1986.1 As of 2019, a clear majority of states have some form of apology statute. “Apology laws are gaining traction,” was the first sentence in a 2012 review on the subject by Saitta and colleagues.3 Only a few (5 states) have “strong” statutes that have broad protection for statements of fault, error, and negligence, as well as sympathy. The other 33 states have statutes that only protect against statements of sympathy.4,16 FIGURE 1 is a US map showing the apology laws by state.1

Do apology statutes and apologies reduce liability?
The positive aspects of apology include personal, psychological, and emotional benefits to both the one apologizing and the one receiving the apology. It also may have financial benefits to health care providers.4 The assumption has been, and there has been some evidence for the proposition, that apologies reduce the possibility of malpractice claims. That is one of the reasons that institutions may have formal apology policies. Indeed, there is evidence that apologies reduce financial awards to patients, as manifest in the states of Pennsylvania and Kentucky.4 Apologies appear to reduce patient anger and can open the door to better communication with the provider. There is evidence that some kinds of apologies tend to diminish blame and make the injured patient less likely to pursue litigation.10 The conclusion from these studies might be that honest and open communication serves to decrease the incidence of medical malpractice lawsuit initiation and that honesty is the best policy.
It is important to note the difference, however, between apologies (or institutional apology policies) and apology laws. There is some evidence that apology and institutional apology policies may reduce malpractice claims or losses.17,18 On the other hand, the studies of apology laws have not found that these laws have much impact on malpractice rates. An especially good and thorough study of the effect of apology laws nationwide, using insurance claims data, essentially found little net effect of the apology laws.19,20 One other study could find no evidence that apology statutes reduce defensive medicine (so no reduction in provider concerns over liability).21
It should be noted that most studies on medical apology and its effects on malpractice claims generally have looked at the narrow or limited apology statutes (that do not cover expressions of fault or negligence). Few states have the broader statutes, and it is possible that those broader statutes would be more effective in reducing liability. Removing the disincentives to medical apologies is a good thing, but in and of itself it is probably not a liability game changer.
Continue to: Institutional policy and apology...
Institutional policy and apology
Some institutions have established an “inclusion of apology” strategy for medical errors. These policies appear to have a meaningful effect on reducing medical malpractice costs. These programs commonly include a proactive investigation, disclosure of error, and apologies. Such policies have been studied at the University of Michigan and the Veterans Affairs (VA) Hospital in Lexington, Kentucky. The University of Michigan program resulted in a 60% reduction in compensation costs for medical errors.22 It also cut litigation costs by half.23 The review of the Kentucky VA program also was positive.17 FIGURE 2 illustrates the key features of the Michigan program.24

Conclusions: Effective apologies
Our conclusions, first, are that apologies are important from all perspectives: ethical, medical, and legal. On the other hand, all of the attention given in recent years to apology statutes may have been misplaced, at least if they were intended to be malpractice reform.17
Institutional apology and response programs are likely successful because they are thoughtfully put together, generally based on the best understanding of how injured patients respond to apologies and what it takes to be sincere, and communicate that sincerity, in the apology. What is an effective apology?, “The acceptance of responsibility for having caused harm.” It may, for example, mean accepting some financial responsibility for the harm. It is also important that the apology is conveyed in such a way that it includes an element of self-critical expression.25 Although there are many formulations of the elements of an effective apology, one example is, “(1) acknowledging and accepting responsibility for the offense; (2) expressing remorse with forbearance, sincerity, and honesty; (3) explaining the understanding of the offense; and (4) willingness to make reparations.”26
At the other extreme is a medical professional, after a bad event, trying to engage in a half-hearted, awkward, or insincere apology on an ad hoc and poorly planned basis. Worse still, “when victims perceive apologies to be insincere and designed simply to cool them off, they react with more rather than less indignation.”27 Of course, the “forced apology” may be the worst of all. An instance of this was addressed in a New Zealand study in which providers were “forced” to provide a written apology to a couple (Mr. and Mrs. B) and a separate written apology to Baby B when there was failure to discuss vitamin K administration during the antenatal period when it was indicated.28 Rather than emphasizing required apology in such a case, which can seem hollow and disingenuous, emphasis was placed on the apology providing a “positive-physiological” effect for those harmed, and on strategies that “nurture the development of the moral maturity required for authentic apology.”
The great advantage of institutional or practice-wide policies is that they can be developed in the calm of planning, with good foresight and careful consideration. This is much different from having to come up with some approach in the heat of something having gone wrong. Ultimately, however, apologies are not about liability. They are about caring for, respecting, and communicating with those who are harmed. Apologizing is often the right and professional thing to do.
- Afrassiab Z. Why mediation & “sorry” make sense: apology statutes as a catalyst for change in medical malpractice. J Dispute Resolutions. 2019.
- AMA Council on Ethical and Judicial Affairs. AMA code of medical ethics’ opinions on patient safety. Virtual Mentor. 2011;13:626-628.
- Saitta N, Hodge SD. Efficacy of a physician’s words of empathy: an overview of state apology laws. J Am Osteopath Assn. 2012;112:302-306.
- Dealing with a medical mistake: Should physicians apologize to patients? Med Economics. November 10, 2013.
- AOA code of ethics. American Osteopathic Association website. http://www.osteopathic.org/inside-aoa/about /leadershipPages/aos-code-of-ethics.aspx. Accessed January 15, 2020.
- You had me at “I’m sorry”: the impact of physicians’ apologies on medical malpractice litigation. Natl Law Review. November 6, 2018. https://www.natlawreview.com /article/you-had-me-i-m-sorry-impact-physicians-apologiesmedical-malpractice-litigation. Accessed February 6, 2020.
- Robbennolt JK. Apologies and medical error. Clin Orthop Relat Res. 2009;467:376-382.
- Bismark MM. The power of apology. N Z Med J. 2009;122:96-106.
- Witman AB, Park DM, Hardin SB. How do patients want physicians to handle mistakes? A survey of internal medicine patients in an academic setting. Arch Intern Med. 1996;156:2565-2569.
- Lawthers AG, Localio AR, Laird NM, et al. Physicians’ perceptions of the risk of being sued. J Health Polit Policy Law. 1992;17:463-482.
- Dahan S, Ducard D, Caeymaex L. Apology in cases of medical error disclosure: thoughts based on a preliminary study. PLoS One. 2017;12:e0181854.
- Halbach JL, Sullivan LL. Teaching medical students about medical errors and patient safety: evaluation of a required curriculum. Acad Med. 2005;80:600-606.
- Nussbaum L. Trial and error: legislating ADR for medical malpractice reform. 2017. Scholarly Works. https://scholars .law.unlv.edu/facpub/1011. Accessed February 7, 2020.
- Cobbs v. Grant, 8 Cal. 3d 229, 104 Cal. Rptr. 505, 502 P.2d 1 (1972).
- Woronka v. Sewall, 320 Mass. 362, 69 N.E.2d 581 (1946).
- Wei M. Doctors, apologies and the law: an analysis and critique of apology law. J Health Law. 2007;40:107-159.
- Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
- Liebman CB, Hyman CS. Medical error disclosure, mediation skills, and malpractice litigation: a demonstration project in Pennsylvania. 2005. https://perma.cc/7257-99GU. Accessed February 7, 2020.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Ho B, Liu E. What’s an apology worth? Decomposing the effect of apologies on medical malpractice payments using state apology laws. J Empirical Legal Studies. 2011;8:179-199.
- McMichael BJ. The failure of sorry: an empirical evaluation of apology laws, health care, and medical malpractice. Lewis & Clark Law Rev. 2017. https://law.lclark.edu/live/files/27734- lcb224article3mcmichaelpdf. Accessed February 7, 2020.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- Boothman RC, Blackwell AC, Campbell DA Jr, et al. A better approach to medical malpractice claims? The University of Michigan experience. J Health Life Sci Law. 2009;2:125-159.
- The Michigan model: Medical malpractice and patient safety at Michigan Medicine. University of Michigan website. https:// www.uofmhealth.org/michigan-model-medical-malpracticeand-patient-safety-umhs#summary. Accessed February 7, 2020.
- Mastroianni AC, Mello MM, Sommer S, et al. The flaws in state ‘apology’ and ‘disclosure’ laws dilute their intended impact on malpractice suits. Health Aff (Millwood). 2010;29:1611-1619.
- Davis ER. I’m sorry I’m scared of litigation: evaluating the effectiveness of apology laws. Forum: Tennessee Student Legal J. 2016;3. https://trace.tennessee.edu/forum/vol3/iss1/4/. Accessed February 7, 2020.
- Miller DT. Disrespect and the experience of injustice. Annu Rev Psychol. 2001;52:527-553.
- McLennan S, Walker S, Rich LE. Should health care providers be forced to apologise after things go wrong? J Bioeth Inq. 2014;11:431-435
- Afrassiab Z. Why mediation & “sorry” make sense: apology statutes as a catalyst for change in medical malpractice. J Dispute Resolutions. 2019.
- AMA Council on Ethical and Judicial Affairs. AMA code of medical ethics’ opinions on patient safety. Virtual Mentor. 2011;13:626-628.
- Saitta N, Hodge SD. Efficacy of a physician’s words of empathy: an overview of state apology laws. J Am Osteopath Assn. 2012;112:302-306.
- Dealing with a medical mistake: Should physicians apologize to patients? Med Economics. November 10, 2013.
- AOA code of ethics. American Osteopathic Association website. http://www.osteopathic.org/inside-aoa/about /leadershipPages/aos-code-of-ethics.aspx. Accessed January 15, 2020.
- You had me at “I’m sorry”: the impact of physicians’ apologies on medical malpractice litigation. Natl Law Review. November 6, 2018. https://www.natlawreview.com /article/you-had-me-i-m-sorry-impact-physicians-apologiesmedical-malpractice-litigation. Accessed February 6, 2020.
- Robbennolt JK. Apologies and medical error. Clin Orthop Relat Res. 2009;467:376-382.
- Bismark MM. The power of apology. N Z Med J. 2009;122:96-106.
- Witman AB, Park DM, Hardin SB. How do patients want physicians to handle mistakes? A survey of internal medicine patients in an academic setting. Arch Intern Med. 1996;156:2565-2569.
- Lawthers AG, Localio AR, Laird NM, et al. Physicians’ perceptions of the risk of being sued. J Health Polit Policy Law. 1992;17:463-482.
- Dahan S, Ducard D, Caeymaex L. Apology in cases of medical error disclosure: thoughts based on a preliminary study. PLoS One. 2017;12:e0181854.
- Halbach JL, Sullivan LL. Teaching medical students about medical errors and patient safety: evaluation of a required curriculum. Acad Med. 2005;80:600-606.
- Nussbaum L. Trial and error: legislating ADR for medical malpractice reform. 2017. Scholarly Works. https://scholars .law.unlv.edu/facpub/1011. Accessed February 7, 2020.
- Cobbs v. Grant, 8 Cal. 3d 229, 104 Cal. Rptr. 505, 502 P.2d 1 (1972).
- Woronka v. Sewall, 320 Mass. 362, 69 N.E.2d 581 (1946).
- Wei M. Doctors, apologies and the law: an analysis and critique of apology law. J Health Law. 2007;40:107-159.
- Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
- Liebman CB, Hyman CS. Medical error disclosure, mediation skills, and malpractice litigation: a demonstration project in Pennsylvania. 2005. https://perma.cc/7257-99GU. Accessed February 7, 2020.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Ho B, Liu E. What’s an apology worth? Decomposing the effect of apologies on medical malpractice payments using state apology laws. J Empirical Legal Studies. 2011;8:179-199.
- McMichael BJ. The failure of sorry: an empirical evaluation of apology laws, health care, and medical malpractice. Lewis & Clark Law Rev. 2017. https://law.lclark.edu/live/files/27734- lcb224article3mcmichaelpdf. Accessed February 7, 2020.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- Boothman RC, Blackwell AC, Campbell DA Jr, et al. A better approach to medical malpractice claims? The University of Michigan experience. J Health Life Sci Law. 2009;2:125-159.
- The Michigan model: Medical malpractice and patient safety at Michigan Medicine. University of Michigan website. https:// www.uofmhealth.org/michigan-model-medical-malpracticeand-patient-safety-umhs#summary. Accessed February 7, 2020.
- Mastroianni AC, Mello MM, Sommer S, et al. The flaws in state ‘apology’ and ‘disclosure’ laws dilute their intended impact on malpractice suits. Health Aff (Millwood). 2010;29:1611-1619.
- Davis ER. I’m sorry I’m scared of litigation: evaluating the effectiveness of apology laws. Forum: Tennessee Student Legal J. 2016;3. https://trace.tennessee.edu/forum/vol3/iss1/4/. Accessed February 7, 2020.
- Miller DT. Disrespect and the experience of injustice. Annu Rev Psychol. 2001;52:527-553.
- McLennan S, Walker S, Rich LE. Should health care providers be forced to apologise after things go wrong? J Bioeth Inq. 2014;11:431-435
CMS issues guidance on containing spread of coronavirus
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
The first guidance document, “Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge,” issued March 4, provides some basic guidance, including identifying which patients are at risk, how facilities should screen for COVID-19, how facilities should monitor or restrict health care facility staff, and other recommendations for infection prevention and control.
“Hospitals should identify visitors and patients at risk for having COVID-19 infection before or immediately upon arrival to the healthcare facility,” the guidance document notes. “For patients, implement respiratory hygiene and cough etiquette (i.e., placing a face mask over the patient’s nose and mouth if that has not already been done) and isolate the patient in an examination room with the door closed. If the patient cannot be immediately moved to an examination room, ensure they are not allowed to wait among other patients seeking care.”
The document offers further information regarding the care of patients and provides numerous links to existing guidance from the Centers for Disease Control and Prevention.
The second document, “Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in Nursing Homes,” issued the same day, provides information on how to limit and monitor visitors as well as monitor and restrict health staff. It details when to transfer residents with suspected or confirmed coronavirus infection, and when a nursing home should accept a resident diagnosed with COVID-19.
Facilities “should contact their local health department if they have questions or suspect a resident of a nursing home has COVID-19,” the document states. “Per CDC, prompt detection, triage and isolation of potentially infectious patients are essential to prevent unnecessary exposure among patients, healthcare personnel, and visitors at the facility.”
The CMS also announced that it is suspending all nonemergency survey activity.
“CMS is suspending nonemergency inspections across the country, allowing inspectors to turn their focus on the most serious health and safety threats like infectious diseases and abuse,” the agency stated in a March 4 memo. “This shift in approach will also allow inspectors to focus on addressing the spread of ... COVID-19. CMS is issuing this memorandum to State Survey Agencies to provide important guidelines for the inspection process in situations in which a COVID-19 is suspected.”
In a statement, CMS Administrator Seema Verma said these actions “represent a call to action across the health care system. All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention.”
Residency Training During the #MeToo Movement
The #MeToo movement that took hold in the wake of the Harvey Weinstein allegations in 2017 likely will be considered one of the major cultural touchpoints of the 2010s. Although activism within the entertainment industry initially drew attention to this movement, it is understood that virtually no workplace is immune to sexual misconduct. Many medical professionals acknowledge #MeToo as a catchy hashtag summarizing a problem that has long been recognized in the field of medicine but often has been inadequately addressed.1 As dermatology residency program directors (PDs) at the University of Southern California (USC) Keck School of Medicine (Los Angeles, California), we have seen the considerable impact that recent high-profile allegations of sexual assault have had at our institution, leading us to take part in institutional and departmental initiatives and reflections that we believe have strengthened the culture within our residency program and positioned us to be proactive in addressing this critical issue.
Before we discuss the efforts to combat sexual misconduct and gender inequality at USC and within our dermatology department, it is worth reflecting on where we stand as a specialty with regard to gender representation. A recent JAMA Dermatology article reported that in 1970 only 10.8% of dermatology academic faculty were women but by 2018 that number had skyrocketed to 51.2%; however, in contrast to this overall increase, only 19.4% of dermatology department chairs in 2018 were women.2 Although we have made large strides as a field, this discrepancy indicates that we still have a long way to go to achieve gender equality.
Although dermatology as a specialty is working toward gender equality, we believe it is crucial to consider this issue in the context of the entire field of medicine, particularly because academic physicians and trainees often interface with a myriad of specialties. It is well known that women in medicine are more likely to be victims of sexual harassment or assault in the workplace and that subsequent issues with imposter syndrome and/or depression are more prevalent in female physicians.3,4 Gender inequality and sexism, among other factors, can make it difficult for women to obtain and maintain leadership positions and can negatively impact the culture of an academic institution in numerous downstream ways.
We also know that academic environments in medicine have a higher prevalence of gender equality issues than in private practice or in settings where medicine is practiced without trainees due to the hierarchical nature of training and the necessary differences in experience between trainees and faculty.3 Furthermore, because trainees form and solidify their professional identities during graduate medical education (GME) training, it is a prime time to emphasize the importance of gender equality and establish zero tolerance policies for workplace abuse and transgressions.5
The data and our personal experiences delineate a clear need for continued vigilance regarding gender equality issues both in dermatology as a specialty and in medicine in general. As PDs, we feel fortunate to have worked in conjunction with our GME committee and our dermatology department to solidify and create policies that work to promote a culture of gender equality. Herein, we will outline some of these efforts with the hope that other academic institutions may consider implementing these programs to protect members of their community from harassment, sexual violence, and gender discrimination.
Create a SAFE Committee
At the institutional level, our GME committee has created the SAFE (Safety, Fairness & Equity) committee under the leadership of Lawrence Opas, MD. The SAFE committee is headed by a female faculty physician and includes members of the medical community who have the influence to affect change and a commitment to protect vulnerable populations. Members include the Chief Medical Officer, the Designated Institutional Officer, the Director of Resident Wellness, and the Dean of the Keck School of Medicine at USC. The SAFE committee serves as a 24/7 reporting resource whereby trainees can report any issues relating to harassment in the workplace via a telephone hotline or online platform. Issues brought to this committee are immediately dealt with and reviewed at monthly GME meetings to keep institutional PDs up-to-date on issues pertaining to sexual harassment and assault within our workplace. The SAFE committee also has departmental resident liaisons who bring information to residents and help guide them to appropriate resources.
Emphasize Resident Wellness
Along with the development of robust reporting resources, our institution has continued to build upon a culture that places a strong emphasis on resident wellness. One of the most meaningful efforts over the last 5 years has included recruitment of a clinical psychologist, Tobi Fishel, PhD, to serve as our institution’s Director of Wellness. She is available to meet confidentially with our residents and helps to serve as a link between trainees and the GME committee.
Our dermatology department takes a tremendous amount of pride in its culture. We are fortunate to have David Peng, MD, MPH, Chair, and Stefani Takahashi, MD, Vice Chair of Education, working daily to create an environment that values teamwork, selflessness, and wellness. We have been continuously grateful for their leadership and guidance in addressing the allegations of sexual assault and harassment that arose at USC over the past several years. Our department has a zero tolerance policy for sexual harassment or harassment of any kind, and we have taken important steps to ensure and promote a safe environment for our trainees, many of which are focused on communication. We try to avoid assumptions and encourage both residents and faculty to explicitly state their experiences and opinions in general but also in relation to instances of potential misconduct.
Encourage Communication
When allegations of sexual misconduct in the workplace were made at our institution, we prioritized immediate in-person communication with our residents to reinforce our zero tolerance policy and to remind them that we are available should any similar issues arise in our department. It was of equal value to remind our trainees of potential resources, such as the SAFE committee, to whom they could bring their concerns if they were not comfortable communicating directly with us. Although we hoped that our trainees understood that we would not be tolerant of any form of harassment based on our past actions and communications, we felt that it was helpful to explicitly delineate this by laying out other avenues of support on a regular basis with them. By ensuring there is a space for a dialogue with others, if needed, our institution and department have provided an extra layer of security for our trainees. Multiple channels of support are crucial to ensure trainee safety.
Dr. Peng also created a workplace safety committee that includes several female faculty members. The committee regularly shares and highlights institutional and departmental resources as they pertain to gender equality and safety within the workplace and also has considerable faculty overlap with our departmental diversity committee. Together, these committees work toward the common goal of fostering an environment in which all members of our department feel comfortable voicing concerns, and we are best able to recruit and retain a diverse faculty.
As PDs, we work to reinforce departmental and institutional messages in our daily communication with residents. We have found that ensuring frequent and varied interactions—quarterly meetings, biannual evaluations, faculty-led didactics 2 half-days per week, and weekly clinical interactions—with our trainees can help to create a culture where they feel comfortable bringing up issues, be they routine clinical operations questions or issues relating to their professional identity. We hope it also has created the space for them to approach us with any issues pertaining to harassment should they ever arise, and we are grateful to know that even if this comfort does not exist, our institution and department have other resources for them.
Final Thoughts
Although some of the measures discussed here were reactionary, many predated the recent institutional concerns and allegations at USC. We hope and believe that the culture we foster within our department has helped our trainees feel safe and cared for during a time of institutional turbulence. We also believe that taking similar proactive measures may benefit the overall culture and foster the development of diverse physicians and leadership at other institutions. In conjunction with reworking legislation and implementing institutional safeguards, the long-term goals of taking these proactive measures are to promote gender equality and workplace safety and to cultivate and retain effective female leadership in medical institutions and training programs.
We feel incredibly fortunate to be part of a specialty in which gender equality has long been considered and sought after. We also are proud to be members of the Association of Professors of Dermatology, which has addressed issues such as diversity and gender equality in a transparent and head-on manner and continues to do so. As a specialty, we hope we can support our trainees in their professional growth and help to cultivate sensitive physicians who will care for an increasingly diverse population and better support each other in their own career development.
- Ladika S. Sexual harassment: health care, it is #youtoo. Manag Care. 2018;27:14-17.
- Xierali IM, Nivet MA, Pandya AG. US dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970 to 2018 [published online January 8, 2020]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.4297.
- Minkina N. Can #MeToo abolish sexual harassment and discrimination in medicine? Lancet. 2019;394:383-384.
- Dzau VJ, Johnson PA. Ending sexual harassment in academic medicine. N Engl J Med. 2018;379:1589-1591.
- Nothnagle M, Reis S, Goldman RE, et al. Fostering professional formation in residency: development and evaluation of the “forum” seminar series. Teach Learn Med. 2014;26:230-238.
The #MeToo movement that took hold in the wake of the Harvey Weinstein allegations in 2017 likely will be considered one of the major cultural touchpoints of the 2010s. Although activism within the entertainment industry initially drew attention to this movement, it is understood that virtually no workplace is immune to sexual misconduct. Many medical professionals acknowledge #MeToo as a catchy hashtag summarizing a problem that has long been recognized in the field of medicine but often has been inadequately addressed.1 As dermatology residency program directors (PDs) at the University of Southern California (USC) Keck School of Medicine (Los Angeles, California), we have seen the considerable impact that recent high-profile allegations of sexual assault have had at our institution, leading us to take part in institutional and departmental initiatives and reflections that we believe have strengthened the culture within our residency program and positioned us to be proactive in addressing this critical issue.
Before we discuss the efforts to combat sexual misconduct and gender inequality at USC and within our dermatology department, it is worth reflecting on where we stand as a specialty with regard to gender representation. A recent JAMA Dermatology article reported that in 1970 only 10.8% of dermatology academic faculty were women but by 2018 that number had skyrocketed to 51.2%; however, in contrast to this overall increase, only 19.4% of dermatology department chairs in 2018 were women.2 Although we have made large strides as a field, this discrepancy indicates that we still have a long way to go to achieve gender equality.
Although dermatology as a specialty is working toward gender equality, we believe it is crucial to consider this issue in the context of the entire field of medicine, particularly because academic physicians and trainees often interface with a myriad of specialties. It is well known that women in medicine are more likely to be victims of sexual harassment or assault in the workplace and that subsequent issues with imposter syndrome and/or depression are more prevalent in female physicians.3,4 Gender inequality and sexism, among other factors, can make it difficult for women to obtain and maintain leadership positions and can negatively impact the culture of an academic institution in numerous downstream ways.
We also know that academic environments in medicine have a higher prevalence of gender equality issues than in private practice or in settings where medicine is practiced without trainees due to the hierarchical nature of training and the necessary differences in experience between trainees and faculty.3 Furthermore, because trainees form and solidify their professional identities during graduate medical education (GME) training, it is a prime time to emphasize the importance of gender equality and establish zero tolerance policies for workplace abuse and transgressions.5
The data and our personal experiences delineate a clear need for continued vigilance regarding gender equality issues both in dermatology as a specialty and in medicine in general. As PDs, we feel fortunate to have worked in conjunction with our GME committee and our dermatology department to solidify and create policies that work to promote a culture of gender equality. Herein, we will outline some of these efforts with the hope that other academic institutions may consider implementing these programs to protect members of their community from harassment, sexual violence, and gender discrimination.
Create a SAFE Committee
At the institutional level, our GME committee has created the SAFE (Safety, Fairness & Equity) committee under the leadership of Lawrence Opas, MD. The SAFE committee is headed by a female faculty physician and includes members of the medical community who have the influence to affect change and a commitment to protect vulnerable populations. Members include the Chief Medical Officer, the Designated Institutional Officer, the Director of Resident Wellness, and the Dean of the Keck School of Medicine at USC. The SAFE committee serves as a 24/7 reporting resource whereby trainees can report any issues relating to harassment in the workplace via a telephone hotline or online platform. Issues brought to this committee are immediately dealt with and reviewed at monthly GME meetings to keep institutional PDs up-to-date on issues pertaining to sexual harassment and assault within our workplace. The SAFE committee also has departmental resident liaisons who bring information to residents and help guide them to appropriate resources.
Emphasize Resident Wellness
Along with the development of robust reporting resources, our institution has continued to build upon a culture that places a strong emphasis on resident wellness. One of the most meaningful efforts over the last 5 years has included recruitment of a clinical psychologist, Tobi Fishel, PhD, to serve as our institution’s Director of Wellness. She is available to meet confidentially with our residents and helps to serve as a link between trainees and the GME committee.
Our dermatology department takes a tremendous amount of pride in its culture. We are fortunate to have David Peng, MD, MPH, Chair, and Stefani Takahashi, MD, Vice Chair of Education, working daily to create an environment that values teamwork, selflessness, and wellness. We have been continuously grateful for their leadership and guidance in addressing the allegations of sexual assault and harassment that arose at USC over the past several years. Our department has a zero tolerance policy for sexual harassment or harassment of any kind, and we have taken important steps to ensure and promote a safe environment for our trainees, many of which are focused on communication. We try to avoid assumptions and encourage both residents and faculty to explicitly state their experiences and opinions in general but also in relation to instances of potential misconduct.
Encourage Communication
When allegations of sexual misconduct in the workplace were made at our institution, we prioritized immediate in-person communication with our residents to reinforce our zero tolerance policy and to remind them that we are available should any similar issues arise in our department. It was of equal value to remind our trainees of potential resources, such as the SAFE committee, to whom they could bring their concerns if they were not comfortable communicating directly with us. Although we hoped that our trainees understood that we would not be tolerant of any form of harassment based on our past actions and communications, we felt that it was helpful to explicitly delineate this by laying out other avenues of support on a regular basis with them. By ensuring there is a space for a dialogue with others, if needed, our institution and department have provided an extra layer of security for our trainees. Multiple channels of support are crucial to ensure trainee safety.
Dr. Peng also created a workplace safety committee that includes several female faculty members. The committee regularly shares and highlights institutional and departmental resources as they pertain to gender equality and safety within the workplace and also has considerable faculty overlap with our departmental diversity committee. Together, these committees work toward the common goal of fostering an environment in which all members of our department feel comfortable voicing concerns, and we are best able to recruit and retain a diverse faculty.
As PDs, we work to reinforce departmental and institutional messages in our daily communication with residents. We have found that ensuring frequent and varied interactions—quarterly meetings, biannual evaluations, faculty-led didactics 2 half-days per week, and weekly clinical interactions—with our trainees can help to create a culture where they feel comfortable bringing up issues, be they routine clinical operations questions or issues relating to their professional identity. We hope it also has created the space for them to approach us with any issues pertaining to harassment should they ever arise, and we are grateful to know that even if this comfort does not exist, our institution and department have other resources for them.
Final Thoughts
Although some of the measures discussed here were reactionary, many predated the recent institutional concerns and allegations at USC. We hope and believe that the culture we foster within our department has helped our trainees feel safe and cared for during a time of institutional turbulence. We also believe that taking similar proactive measures may benefit the overall culture and foster the development of diverse physicians and leadership at other institutions. In conjunction with reworking legislation and implementing institutional safeguards, the long-term goals of taking these proactive measures are to promote gender equality and workplace safety and to cultivate and retain effective female leadership in medical institutions and training programs.
We feel incredibly fortunate to be part of a specialty in which gender equality has long been considered and sought after. We also are proud to be members of the Association of Professors of Dermatology, which has addressed issues such as diversity and gender equality in a transparent and head-on manner and continues to do so. As a specialty, we hope we can support our trainees in their professional growth and help to cultivate sensitive physicians who will care for an increasingly diverse population and better support each other in their own career development.
The #MeToo movement that took hold in the wake of the Harvey Weinstein allegations in 2017 likely will be considered one of the major cultural touchpoints of the 2010s. Although activism within the entertainment industry initially drew attention to this movement, it is understood that virtually no workplace is immune to sexual misconduct. Many medical professionals acknowledge #MeToo as a catchy hashtag summarizing a problem that has long been recognized in the field of medicine but often has been inadequately addressed.1 As dermatology residency program directors (PDs) at the University of Southern California (USC) Keck School of Medicine (Los Angeles, California), we have seen the considerable impact that recent high-profile allegations of sexual assault have had at our institution, leading us to take part in institutional and departmental initiatives and reflections that we believe have strengthened the culture within our residency program and positioned us to be proactive in addressing this critical issue.
Before we discuss the efforts to combat sexual misconduct and gender inequality at USC and within our dermatology department, it is worth reflecting on where we stand as a specialty with regard to gender representation. A recent JAMA Dermatology article reported that in 1970 only 10.8% of dermatology academic faculty were women but by 2018 that number had skyrocketed to 51.2%; however, in contrast to this overall increase, only 19.4% of dermatology department chairs in 2018 were women.2 Although we have made large strides as a field, this discrepancy indicates that we still have a long way to go to achieve gender equality.
Although dermatology as a specialty is working toward gender equality, we believe it is crucial to consider this issue in the context of the entire field of medicine, particularly because academic physicians and trainees often interface with a myriad of specialties. It is well known that women in medicine are more likely to be victims of sexual harassment or assault in the workplace and that subsequent issues with imposter syndrome and/or depression are more prevalent in female physicians.3,4 Gender inequality and sexism, among other factors, can make it difficult for women to obtain and maintain leadership positions and can negatively impact the culture of an academic institution in numerous downstream ways.
We also know that academic environments in medicine have a higher prevalence of gender equality issues than in private practice or in settings where medicine is practiced without trainees due to the hierarchical nature of training and the necessary differences in experience between trainees and faculty.3 Furthermore, because trainees form and solidify their professional identities during graduate medical education (GME) training, it is a prime time to emphasize the importance of gender equality and establish zero tolerance policies for workplace abuse and transgressions.5
The data and our personal experiences delineate a clear need for continued vigilance regarding gender equality issues both in dermatology as a specialty and in medicine in general. As PDs, we feel fortunate to have worked in conjunction with our GME committee and our dermatology department to solidify and create policies that work to promote a culture of gender equality. Herein, we will outline some of these efforts with the hope that other academic institutions may consider implementing these programs to protect members of their community from harassment, sexual violence, and gender discrimination.
Create a SAFE Committee
At the institutional level, our GME committee has created the SAFE (Safety, Fairness & Equity) committee under the leadership of Lawrence Opas, MD. The SAFE committee is headed by a female faculty physician and includes members of the medical community who have the influence to affect change and a commitment to protect vulnerable populations. Members include the Chief Medical Officer, the Designated Institutional Officer, the Director of Resident Wellness, and the Dean of the Keck School of Medicine at USC. The SAFE committee serves as a 24/7 reporting resource whereby trainees can report any issues relating to harassment in the workplace via a telephone hotline or online platform. Issues brought to this committee are immediately dealt with and reviewed at monthly GME meetings to keep institutional PDs up-to-date on issues pertaining to sexual harassment and assault within our workplace. The SAFE committee also has departmental resident liaisons who bring information to residents and help guide them to appropriate resources.
Emphasize Resident Wellness
Along with the development of robust reporting resources, our institution has continued to build upon a culture that places a strong emphasis on resident wellness. One of the most meaningful efforts over the last 5 years has included recruitment of a clinical psychologist, Tobi Fishel, PhD, to serve as our institution’s Director of Wellness. She is available to meet confidentially with our residents and helps to serve as a link between trainees and the GME committee.
Our dermatology department takes a tremendous amount of pride in its culture. We are fortunate to have David Peng, MD, MPH, Chair, and Stefani Takahashi, MD, Vice Chair of Education, working daily to create an environment that values teamwork, selflessness, and wellness. We have been continuously grateful for their leadership and guidance in addressing the allegations of sexual assault and harassment that arose at USC over the past several years. Our department has a zero tolerance policy for sexual harassment or harassment of any kind, and we have taken important steps to ensure and promote a safe environment for our trainees, many of which are focused on communication. We try to avoid assumptions and encourage both residents and faculty to explicitly state their experiences and opinions in general but also in relation to instances of potential misconduct.
Encourage Communication
When allegations of sexual misconduct in the workplace were made at our institution, we prioritized immediate in-person communication with our residents to reinforce our zero tolerance policy and to remind them that we are available should any similar issues arise in our department. It was of equal value to remind our trainees of potential resources, such as the SAFE committee, to whom they could bring their concerns if they were not comfortable communicating directly with us. Although we hoped that our trainees understood that we would not be tolerant of any form of harassment based on our past actions and communications, we felt that it was helpful to explicitly delineate this by laying out other avenues of support on a regular basis with them. By ensuring there is a space for a dialogue with others, if needed, our institution and department have provided an extra layer of security for our trainees. Multiple channels of support are crucial to ensure trainee safety.
Dr. Peng also created a workplace safety committee that includes several female faculty members. The committee regularly shares and highlights institutional and departmental resources as they pertain to gender equality and safety within the workplace and also has considerable faculty overlap with our departmental diversity committee. Together, these committees work toward the common goal of fostering an environment in which all members of our department feel comfortable voicing concerns, and we are best able to recruit and retain a diverse faculty.
As PDs, we work to reinforce departmental and institutional messages in our daily communication with residents. We have found that ensuring frequent and varied interactions—quarterly meetings, biannual evaluations, faculty-led didactics 2 half-days per week, and weekly clinical interactions—with our trainees can help to create a culture where they feel comfortable bringing up issues, be they routine clinical operations questions or issues relating to their professional identity. We hope it also has created the space for them to approach us with any issues pertaining to harassment should they ever arise, and we are grateful to know that even if this comfort does not exist, our institution and department have other resources for them.
Final Thoughts
Although some of the measures discussed here were reactionary, many predated the recent institutional concerns and allegations at USC. We hope and believe that the culture we foster within our department has helped our trainees feel safe and cared for during a time of institutional turbulence. We also believe that taking similar proactive measures may benefit the overall culture and foster the development of diverse physicians and leadership at other institutions. In conjunction with reworking legislation and implementing institutional safeguards, the long-term goals of taking these proactive measures are to promote gender equality and workplace safety and to cultivate and retain effective female leadership in medical institutions and training programs.
We feel incredibly fortunate to be part of a specialty in which gender equality has long been considered and sought after. We also are proud to be members of the Association of Professors of Dermatology, which has addressed issues such as diversity and gender equality in a transparent and head-on manner and continues to do so. As a specialty, we hope we can support our trainees in their professional growth and help to cultivate sensitive physicians who will care for an increasingly diverse population and better support each other in their own career development.
- Ladika S. Sexual harassment: health care, it is #youtoo. Manag Care. 2018;27:14-17.
- Xierali IM, Nivet MA, Pandya AG. US dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970 to 2018 [published online January 8, 2020]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.4297.
- Minkina N. Can #MeToo abolish sexual harassment and discrimination in medicine? Lancet. 2019;394:383-384.
- Dzau VJ, Johnson PA. Ending sexual harassment in academic medicine. N Engl J Med. 2018;379:1589-1591.
- Nothnagle M, Reis S, Goldman RE, et al. Fostering professional formation in residency: development and evaluation of the “forum” seminar series. Teach Learn Med. 2014;26:230-238.
- Ladika S. Sexual harassment: health care, it is #youtoo. Manag Care. 2018;27:14-17.
- Xierali IM, Nivet MA, Pandya AG. US dermatology department faculty diversity trends by sex and underrepresented-in-medicine status, 1970 to 2018 [published online January 8, 2020]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.4297.
- Minkina N. Can #MeToo abolish sexual harassment and discrimination in medicine? Lancet. 2019;394:383-384.
- Dzau VJ, Johnson PA. Ending sexual harassment in academic medicine. N Engl J Med. 2018;379:1589-1591.
- Nothnagle M, Reis S, Goldman RE, et al. Fostering professional formation in residency: development and evaluation of the “forum” seminar series. Teach Learn Med. 2014;26:230-238.
Survey: 2020 will see more attacks on ACA
When physicians gaze into their crystal balls to predict what’s coming in 2020, they see continued efforts to defund the Affordable Care Act – meaning the ACA will still be around to be defunded – but they don’t see a lot of support for universal health care, according to health care market research company InCrowd.

Expectations for universal health care came in at 18% of the 100 generalists and 101 specialists who responded to InCrowd’s fifth annual health care predictions survey, which left 82% who thought that “election outcomes will result in universal healthcare support” was somewhat or very unlikely in 2020.
One respondent, a specialist from California, commented that “the global data on universal healthcare for all shows that it results in overall improved population health. Unfortunately, we are so polarized in the US against universal healthcare driven by bias from health insurance companies and decision makers that are quick to ignore scientific data.”
This was the first time InCrowd asked physicians about universal health care, but ACA-related predictions have been included before, and all three scenarios presented were deemed to be increasingly likely, compared with 2019.
Respondents thought that federal government defunding was more likely to occur in 2020 (80%) than in 2019 (73%), but increased majorities also said that preexisting conditions coverage would continue (78% in 2020 vs. 70% in 2019) and that the ACA would remain in place (74% in 2020 vs. 60% in 2019), InCrowd reported after the survey, which was conducted from Dec. 30, 2019, to Jan. 2, 2020.
A respondent who thought the ACA will be eliminated said, “I have as many uninsured today as before the ACA. They are just different. Mainly younger patients who spend less in a year on healthcare than one month’s premium.” Another suggested that eliminateing it “will limit access to care and overload [emergency departments]. More people will die.”
Cost was addressed in a separate survey question that asked how physicians could help to reduce health care spending in 2020.
The leading answer, given by 37% of respondents, was for physicians to “inform themselves of costs and adapt cost-saving prescription practices.” Next came “limit use of expensive tests and scans” with 21%, followed by “prescribe generics when possible” at 20%, which was a substantial drop from the 38% it garnered in 2019, InCrowd noted.
“Participation in [shared savings] programs and risk-based incentive programs and pay-for-performance programs” would provide “better stewardship of resources,” a primary care physician from Michigan wrote.
When the survey turned to pharmaceutical industry predictions for 2020, cost was the major issue.
“What’s interesting about this year’s data is that we’re seeing less emphasis on the importance of bringing innovative, new therapies to market faster … versus expanding affordability, which was nearly a unanimous top priority for respondents,” Daniel S. Fitzgerald, InCrowd’s CEO and president, said in a separate statement.
When physicians gaze into their crystal balls to predict what’s coming in 2020, they see continued efforts to defund the Affordable Care Act – meaning the ACA will still be around to be defunded – but they don’t see a lot of support for universal health care, according to health care market research company InCrowd.

Expectations for universal health care came in at 18% of the 100 generalists and 101 specialists who responded to InCrowd’s fifth annual health care predictions survey, which left 82% who thought that “election outcomes will result in universal healthcare support” was somewhat or very unlikely in 2020.
One respondent, a specialist from California, commented that “the global data on universal healthcare for all shows that it results in overall improved population health. Unfortunately, we are so polarized in the US against universal healthcare driven by bias from health insurance companies and decision makers that are quick to ignore scientific data.”
This was the first time InCrowd asked physicians about universal health care, but ACA-related predictions have been included before, and all three scenarios presented were deemed to be increasingly likely, compared with 2019.
Respondents thought that federal government defunding was more likely to occur in 2020 (80%) than in 2019 (73%), but increased majorities also said that preexisting conditions coverage would continue (78% in 2020 vs. 70% in 2019) and that the ACA would remain in place (74% in 2020 vs. 60% in 2019), InCrowd reported after the survey, which was conducted from Dec. 30, 2019, to Jan. 2, 2020.
A respondent who thought the ACA will be eliminated said, “I have as many uninsured today as before the ACA. They are just different. Mainly younger patients who spend less in a year on healthcare than one month’s premium.” Another suggested that eliminateing it “will limit access to care and overload [emergency departments]. More people will die.”
Cost was addressed in a separate survey question that asked how physicians could help to reduce health care spending in 2020.
The leading answer, given by 37% of respondents, was for physicians to “inform themselves of costs and adapt cost-saving prescription practices.” Next came “limit use of expensive tests and scans” with 21%, followed by “prescribe generics when possible” at 20%, which was a substantial drop from the 38% it garnered in 2019, InCrowd noted.
“Participation in [shared savings] programs and risk-based incentive programs and pay-for-performance programs” would provide “better stewardship of resources,” a primary care physician from Michigan wrote.
When the survey turned to pharmaceutical industry predictions for 2020, cost was the major issue.
“What’s interesting about this year’s data is that we’re seeing less emphasis on the importance of bringing innovative, new therapies to market faster … versus expanding affordability, which was nearly a unanimous top priority for respondents,” Daniel S. Fitzgerald, InCrowd’s CEO and president, said in a separate statement.
When physicians gaze into their crystal balls to predict what’s coming in 2020, they see continued efforts to defund the Affordable Care Act – meaning the ACA will still be around to be defunded – but they don’t see a lot of support for universal health care, according to health care market research company InCrowd.

Expectations for universal health care came in at 18% of the 100 generalists and 101 specialists who responded to InCrowd’s fifth annual health care predictions survey, which left 82% who thought that “election outcomes will result in universal healthcare support” was somewhat or very unlikely in 2020.
One respondent, a specialist from California, commented that “the global data on universal healthcare for all shows that it results in overall improved population health. Unfortunately, we are so polarized in the US against universal healthcare driven by bias from health insurance companies and decision makers that are quick to ignore scientific data.”
This was the first time InCrowd asked physicians about universal health care, but ACA-related predictions have been included before, and all three scenarios presented were deemed to be increasingly likely, compared with 2019.
Respondents thought that federal government defunding was more likely to occur in 2020 (80%) than in 2019 (73%), but increased majorities also said that preexisting conditions coverage would continue (78% in 2020 vs. 70% in 2019) and that the ACA would remain in place (74% in 2020 vs. 60% in 2019), InCrowd reported after the survey, which was conducted from Dec. 30, 2019, to Jan. 2, 2020.
A respondent who thought the ACA will be eliminated said, “I have as many uninsured today as before the ACA. They are just different. Mainly younger patients who spend less in a year on healthcare than one month’s premium.” Another suggested that eliminateing it “will limit access to care and overload [emergency departments]. More people will die.”
Cost was addressed in a separate survey question that asked how physicians could help to reduce health care spending in 2020.
The leading answer, given by 37% of respondents, was for physicians to “inform themselves of costs and adapt cost-saving prescription practices.” Next came “limit use of expensive tests and scans” with 21%, followed by “prescribe generics when possible” at 20%, which was a substantial drop from the 38% it garnered in 2019, InCrowd noted.
“Participation in [shared savings] programs and risk-based incentive programs and pay-for-performance programs” would provide “better stewardship of resources,” a primary care physician from Michigan wrote.
When the survey turned to pharmaceutical industry predictions for 2020, cost was the major issue.
“What’s interesting about this year’s data is that we’re seeing less emphasis on the importance of bringing innovative, new therapies to market faster … versus expanding affordability, which was nearly a unanimous top priority for respondents,” Daniel S. Fitzgerald, InCrowd’s CEO and president, said in a separate statement.
Are computers and AI prompting us to think less?
The collection of vast amounts of data and the use of more sophisticated algorithms seem beneficial in all fields. However, I have deep concerns about the “other side of the coin” when it comes to artificial intelligence (AI) as discussed in “An FP’s guide to AI-enabled clinical decision support” (J Fam Pract. 2019;68:486-492).
Years ago, when I worked in urgent care, one of my colleagues would log in to her favorite Web site to search for the appropriate diagnosis for almost all of her patients. Surely this physician was able to memorize and regurgitate enough information to get through medical school and pass the boards, but was she able to think, in the sense of using/applying the information she stored away? My answer is, “No!”
Certainly, having a computer helps one to get through medical school. However, while we use terms such as “AI,” I would argue that none of these machines do more than duplicate the algorithmic functioning of the brain. Which leads me to the other side of the coin: Are computers, of which we ask questions and expect legitimate answers in return, helping us to think? Or are they leading us to think less?
In other words, are we inadvertently “dumbing down” as physicians (and as a species)? And do we want a physician who seems less capable of actually processing the sum total of a patient’s complaints, symptoms, and findings in trying to understand the patient’s problem?
While we cannot go back and disconnect from computers, we can make sure that we do not become totally dependent on them. We need to acknowledge this possible blind spot in the evolution of technology (particularly AI)—the potential to reinforce “not thinking”—especially within the medical school environment. There needs to be an awareness of, and a conscious effort to counter, an overreliance on computers thinking for us.
As individual physicians, we owe it to our patients and ourselves, each and every working day, to use our brains to apply our education, training, and accumulated data to help diagnose and treat our patients effectively.
Barry Marged, DO, ABD, MA
Mansfield, OH
The collection of vast amounts of data and the use of more sophisticated algorithms seem beneficial in all fields. However, I have deep concerns about the “other side of the coin” when it comes to artificial intelligence (AI) as discussed in “An FP’s guide to AI-enabled clinical decision support” (J Fam Pract. 2019;68:486-492).
Years ago, when I worked in urgent care, one of my colleagues would log in to her favorite Web site to search for the appropriate diagnosis for almost all of her patients. Surely this physician was able to memorize and regurgitate enough information to get through medical school and pass the boards, but was she able to think, in the sense of using/applying the information she stored away? My answer is, “No!”
Certainly, having a computer helps one to get through medical school. However, while we use terms such as “AI,” I would argue that none of these machines do more than duplicate the algorithmic functioning of the brain. Which leads me to the other side of the coin: Are computers, of which we ask questions and expect legitimate answers in return, helping us to think? Or are they leading us to think less?
In other words, are we inadvertently “dumbing down” as physicians (and as a species)? And do we want a physician who seems less capable of actually processing the sum total of a patient’s complaints, symptoms, and findings in trying to understand the patient’s problem?
While we cannot go back and disconnect from computers, we can make sure that we do not become totally dependent on them. We need to acknowledge this possible blind spot in the evolution of technology (particularly AI)—the potential to reinforce “not thinking”—especially within the medical school environment. There needs to be an awareness of, and a conscious effort to counter, an overreliance on computers thinking for us.
As individual physicians, we owe it to our patients and ourselves, each and every working day, to use our brains to apply our education, training, and accumulated data to help diagnose and treat our patients effectively.
Barry Marged, DO, ABD, MA
Mansfield, OH
The collection of vast amounts of data and the use of more sophisticated algorithms seem beneficial in all fields. However, I have deep concerns about the “other side of the coin” when it comes to artificial intelligence (AI) as discussed in “An FP’s guide to AI-enabled clinical decision support” (J Fam Pract. 2019;68:486-492).
Years ago, when I worked in urgent care, one of my colleagues would log in to her favorite Web site to search for the appropriate diagnosis for almost all of her patients. Surely this physician was able to memorize and regurgitate enough information to get through medical school and pass the boards, but was she able to think, in the sense of using/applying the information she stored away? My answer is, “No!”
Certainly, having a computer helps one to get through medical school. However, while we use terms such as “AI,” I would argue that none of these machines do more than duplicate the algorithmic functioning of the brain. Which leads me to the other side of the coin: Are computers, of which we ask questions and expect legitimate answers in return, helping us to think? Or are they leading us to think less?
In other words, are we inadvertently “dumbing down” as physicians (and as a species)? And do we want a physician who seems less capable of actually processing the sum total of a patient’s complaints, symptoms, and findings in trying to understand the patient’s problem?
While we cannot go back and disconnect from computers, we can make sure that we do not become totally dependent on them. We need to acknowledge this possible blind spot in the evolution of technology (particularly AI)—the potential to reinforce “not thinking”—especially within the medical school environment. There needs to be an awareness of, and a conscious effort to counter, an overreliance on computers thinking for us.
As individual physicians, we owe it to our patients and ourselves, each and every working day, to use our brains to apply our education, training, and accumulated data to help diagnose and treat our patients effectively.
Barry Marged, DO, ABD, MA
Mansfield, OH
What medical conferences are being canceled by coronavirus?
In a typical year, March marks the start of conference season, made all the more attractive by collegial gatherings and travel to warmer climes. But 2020 has already proven anything but typical as the number of novel coronavirus cases continues to increase around the globe. As a potential pandemic looms, these meetings – full of handshakes and crowded lecture halls – are also nirvana for opportunistic viruses. As are the airports, airplanes, and cabs required to get there.
So, as COVID-19 continues to spread, medical and scientific societies must make some difficult decisions. In Europe, at least a few societies have already suspended their upcoming meetings, while France has temporarily banned all gatherings over 5000 people.
In the United States, however, most medical conferences are moving forward as planned – at least for now. But one conference of 10,000 attendees, the American Physical Society annual meeting, which was scheduled for March 2-6 in Denver, was canceled the day before the meeting started. Although it’s not a medical conference, it speaks to the “rapidly escalating health concerns” that all conference organizers must grapple with.
APS Physics Meetings
@APSMeetings
Due to rapidly escalating health concerns relating to the spread of the coronavirus disease (COVID-19), the 2020 APS March Meeting in Denver, CO, has been canceled. Please do not travel to Denver to attend the March Meeting. More information will follow shortly. #apsmarch
734 9:59 PM - Feb 29, 2020
Just one smaller medical meeting, the Ataxia Conference, which was scheduled for March 6-7 in Denver, has been canceled.
Most societies hosting these meetings have put out statements to their attendees saying that they’re monitoring the situation and will adapt as necessary. The United States and Canadian Academy of Pathology, which is holding its annual meeting in Los Angeles this week, sent out an email beforehand asking international travelers to consider staying home. The Healthcare Information and Management Systems Society (HIMSS) Global Health Conference, which is slated to have about 50,000 attendees from around the world, has declared itself a “handshake-free” conference but otherwise intends to move ahead as planned.
All of these conferences will be pushing forward without at least one prominent group of attendees. New York University’s Langone Health has removed its employees from the decision-making process and instead is taking a proactive stance: The health system just declared a 60-day (minimum) ban preventing employees from attending any meetings or conferences and from all domestic and international work-related travel.
Here’s what some of the societies have said to attendees about their intent to proceed or modify their plans:
- Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3/8/20 - 3/11/20: Monitoring the situation and seeking input from local, state, and federal infectious-disease and public-health experts. Final decision expected by the evening of March 3.
- American Academy of Allergy, Asthma & Immunology (AAAAI), Philadelphia, 3/13/20 - 3/16/20: Monitoring developments but no plans to cancel or postpone at this time.
- American Academy of Orthopedic Surgeons (AAOS), Orlando, 3/24/20 - 3/28/20: Proceeding as planned.
- American Academy of Dermatology (AAD), Denver, 3/20/20 - 3/24/20: The AAD’s 2020 Annual Meeting is scheduled to take place as planned. The organization will increase the number of hand-sanitizing stations throughout the convention center, and it is adding a nursing station specifically designated for anyone with flu-like symptoms.
- American College of Cardiology (ACC), Chicago, 3/28/20 - 3/30/20: The organization is working with attendees, faculty, exhibitors, and other stakeholders in affected countries to ensure access to research and education from the meeting, but is otherwise proceeding as planned.
- Endocrine Society (ENDO), San Francisco, 3/28/20 - 3/31/20: ENDO 2020 will take place as scheduled, but this is an evolving situation worldwide. The society will continue to monitor and provide updates on its FAQ page.
- American College of Physicians Internal Medicine (ACP IM), Los Angeles, 4/23/20 - 4/25/20: ACP leadership is closely monitoring the COVID-19 situation and is actively working with the Centers for Disease Control and Prevention (CDC) to ensure authoritative communication of safety updates and recommendations as the situation evolves.
- American Association for Cancer Research (AACR), San Diego, 4/24/20 - 4/29/20: At this time, there is no plan to cancel or postpone any scheduled AACR meetings. The organization is tracking all travel restrictions as well as information and guidance from the CDC and World Health Organization.
- American Academy of Neurology (AAN), Toronto, 4/25/20 - 5/1/20: The group is continuing to closely monitor the situation in Toronto and will provide updates as the situation warrants.
This article originally appeared on Medscape.com.
In a typical year, March marks the start of conference season, made all the more attractive by collegial gatherings and travel to warmer climes. But 2020 has already proven anything but typical as the number of novel coronavirus cases continues to increase around the globe. As a potential pandemic looms, these meetings – full of handshakes and crowded lecture halls – are also nirvana for opportunistic viruses. As are the airports, airplanes, and cabs required to get there.
So, as COVID-19 continues to spread, medical and scientific societies must make some difficult decisions. In Europe, at least a few societies have already suspended their upcoming meetings, while France has temporarily banned all gatherings over 5000 people.
In the United States, however, most medical conferences are moving forward as planned – at least for now. But one conference of 10,000 attendees, the American Physical Society annual meeting, which was scheduled for March 2-6 in Denver, was canceled the day before the meeting started. Although it’s not a medical conference, it speaks to the “rapidly escalating health concerns” that all conference organizers must grapple with.
APS Physics Meetings
@APSMeetings
Due to rapidly escalating health concerns relating to the spread of the coronavirus disease (COVID-19), the 2020 APS March Meeting in Denver, CO, has been canceled. Please do not travel to Denver to attend the March Meeting. More information will follow shortly. #apsmarch
734 9:59 PM - Feb 29, 2020
Just one smaller medical meeting, the Ataxia Conference, which was scheduled for March 6-7 in Denver, has been canceled.
Most societies hosting these meetings have put out statements to their attendees saying that they’re monitoring the situation and will adapt as necessary. The United States and Canadian Academy of Pathology, which is holding its annual meeting in Los Angeles this week, sent out an email beforehand asking international travelers to consider staying home. The Healthcare Information and Management Systems Society (HIMSS) Global Health Conference, which is slated to have about 50,000 attendees from around the world, has declared itself a “handshake-free” conference but otherwise intends to move ahead as planned.
All of these conferences will be pushing forward without at least one prominent group of attendees. New York University’s Langone Health has removed its employees from the decision-making process and instead is taking a proactive stance: The health system just declared a 60-day (minimum) ban preventing employees from attending any meetings or conferences and from all domestic and international work-related travel.
Here’s what some of the societies have said to attendees about their intent to proceed or modify their plans:
- Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3/8/20 - 3/11/20: Monitoring the situation and seeking input from local, state, and federal infectious-disease and public-health experts. Final decision expected by the evening of March 3.
- American Academy of Allergy, Asthma & Immunology (AAAAI), Philadelphia, 3/13/20 - 3/16/20: Monitoring developments but no plans to cancel or postpone at this time.
- American Academy of Orthopedic Surgeons (AAOS), Orlando, 3/24/20 - 3/28/20: Proceeding as planned.
- American Academy of Dermatology (AAD), Denver, 3/20/20 - 3/24/20: The AAD’s 2020 Annual Meeting is scheduled to take place as planned. The organization will increase the number of hand-sanitizing stations throughout the convention center, and it is adding a nursing station specifically designated for anyone with flu-like symptoms.
- American College of Cardiology (ACC), Chicago, 3/28/20 - 3/30/20: The organization is working with attendees, faculty, exhibitors, and other stakeholders in affected countries to ensure access to research and education from the meeting, but is otherwise proceeding as planned.
- Endocrine Society (ENDO), San Francisco, 3/28/20 - 3/31/20: ENDO 2020 will take place as scheduled, but this is an evolving situation worldwide. The society will continue to monitor and provide updates on its FAQ page.
- American College of Physicians Internal Medicine (ACP IM), Los Angeles, 4/23/20 - 4/25/20: ACP leadership is closely monitoring the COVID-19 situation and is actively working with the Centers for Disease Control and Prevention (CDC) to ensure authoritative communication of safety updates and recommendations as the situation evolves.
- American Association for Cancer Research (AACR), San Diego, 4/24/20 - 4/29/20: At this time, there is no plan to cancel or postpone any scheduled AACR meetings. The organization is tracking all travel restrictions as well as information and guidance from the CDC and World Health Organization.
- American Academy of Neurology (AAN), Toronto, 4/25/20 - 5/1/20: The group is continuing to closely monitor the situation in Toronto and will provide updates as the situation warrants.
This article originally appeared on Medscape.com.
In a typical year, March marks the start of conference season, made all the more attractive by collegial gatherings and travel to warmer climes. But 2020 has already proven anything but typical as the number of novel coronavirus cases continues to increase around the globe. As a potential pandemic looms, these meetings – full of handshakes and crowded lecture halls – are also nirvana for opportunistic viruses. As are the airports, airplanes, and cabs required to get there.
So, as COVID-19 continues to spread, medical and scientific societies must make some difficult decisions. In Europe, at least a few societies have already suspended their upcoming meetings, while France has temporarily banned all gatherings over 5000 people.
In the United States, however, most medical conferences are moving forward as planned – at least for now. But one conference of 10,000 attendees, the American Physical Society annual meeting, which was scheduled for March 2-6 in Denver, was canceled the day before the meeting started. Although it’s not a medical conference, it speaks to the “rapidly escalating health concerns” that all conference organizers must grapple with.
APS Physics Meetings
@APSMeetings
Due to rapidly escalating health concerns relating to the spread of the coronavirus disease (COVID-19), the 2020 APS March Meeting in Denver, CO, has been canceled. Please do not travel to Denver to attend the March Meeting. More information will follow shortly. #apsmarch
734 9:59 PM - Feb 29, 2020
Just one smaller medical meeting, the Ataxia Conference, which was scheduled for March 6-7 in Denver, has been canceled.
Most societies hosting these meetings have put out statements to their attendees saying that they’re monitoring the situation and will adapt as necessary. The United States and Canadian Academy of Pathology, which is holding its annual meeting in Los Angeles this week, sent out an email beforehand asking international travelers to consider staying home. The Healthcare Information and Management Systems Society (HIMSS) Global Health Conference, which is slated to have about 50,000 attendees from around the world, has declared itself a “handshake-free” conference but otherwise intends to move ahead as planned.
All of these conferences will be pushing forward without at least one prominent group of attendees. New York University’s Langone Health has removed its employees from the decision-making process and instead is taking a proactive stance: The health system just declared a 60-day (minimum) ban preventing employees from attending any meetings or conferences and from all domestic and international work-related travel.
Here’s what some of the societies have said to attendees about their intent to proceed or modify their plans:
- Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3/8/20 - 3/11/20: Monitoring the situation and seeking input from local, state, and federal infectious-disease and public-health experts. Final decision expected by the evening of March 3.
- American Academy of Allergy, Asthma & Immunology (AAAAI), Philadelphia, 3/13/20 - 3/16/20: Monitoring developments but no plans to cancel or postpone at this time.
- American Academy of Orthopedic Surgeons (AAOS), Orlando, 3/24/20 - 3/28/20: Proceeding as planned.
- American Academy of Dermatology (AAD), Denver, 3/20/20 - 3/24/20: The AAD’s 2020 Annual Meeting is scheduled to take place as planned. The organization will increase the number of hand-sanitizing stations throughout the convention center, and it is adding a nursing station specifically designated for anyone with flu-like symptoms.
- American College of Cardiology (ACC), Chicago, 3/28/20 - 3/30/20: The organization is working with attendees, faculty, exhibitors, and other stakeholders in affected countries to ensure access to research and education from the meeting, but is otherwise proceeding as planned.
- Endocrine Society (ENDO), San Francisco, 3/28/20 - 3/31/20: ENDO 2020 will take place as scheduled, but this is an evolving situation worldwide. The society will continue to monitor and provide updates on its FAQ page.
- American College of Physicians Internal Medicine (ACP IM), Los Angeles, 4/23/20 - 4/25/20: ACP leadership is closely monitoring the COVID-19 situation and is actively working with the Centers for Disease Control and Prevention (CDC) to ensure authoritative communication of safety updates and recommendations as the situation evolves.
- American Association for Cancer Research (AACR), San Diego, 4/24/20 - 4/29/20: At this time, there is no plan to cancel or postpone any scheduled AACR meetings. The organization is tracking all travel restrictions as well as information and guidance from the CDC and World Health Organization.
- American Academy of Neurology (AAN), Toronto, 4/25/20 - 5/1/20: The group is continuing to closely monitor the situation in Toronto and will provide updates as the situation warrants.
This article originally appeared on Medscape.com.
Bad behavior by medical trainees target of new proposal
Some instances of unprofessional behavior by medical trainees are universally deemed egregious and worthy of discipline — for example, looking up a friend’s medical data after HIPAA training.
Conversely, some professionalism lapses may be widely thought of as a teaching and consoling moment, such as the human error involved in forgetting a scheduled repositioning of a patient.
But between the extremes is a vast gray area. To deal with those cases appropriately, Jason Wasserman, PhD, and colleagues propose a new framework by which to judge each infraction.
The framework draws from “just culture” concepts used to evaluate medical errors, Wasserman, associate professor of biomedical science at Oakland University William Beaumont School of Medicine in Rochester, Michigan, told Medscape Medical News. Such an approach takes into account the environment in which the error was made, the knowledge and intent of the person making the error, and the severity and consequences of the infraction so that trainees and institutions can learn from mistakes.
“Trainees by definition are not going to fully get it,” he explained. “By definition they’re not going to fully achieve professional expectations. So how can we respond to the things we need to respond to, but do it in a way that’s educational?”
Wasserman and coauthors’ framework for remediation, which they published February 20 in The New England Journal of Medicine, takes into account several questions: Was the expectation clear? Were there factors beyond the trainees› control? What were the trainees› intentions and did they understand the consequences? Did the person genuinely believe the action was inconsequential?
An example requiring discipline, the authors say, would be using a crib sheet during an exam. In that case the intent is clear, there is no defensible belief that the action is inconsequential, and there is a clear understanding the action is wrong.
But a response of “affirm, support, and advise” is more appropriate, for example, when a student’s alarm doesn’t go off after a power outage and they miss a mandatory meeting.
Wasserman points out that this framework won’t cover all situations.
“This is not an algorithm for answering your questions about what to do,” he said. “It’s an architecture for clarifying the discussion about that. It can really tease out all the threads that need to be considered to best respond to and correct the professionalism lapse, but do it in a way that is developmentally appropriate.”
A Core Competency
For two decades, professionalism has been considered a core competency of medical education. In 1999, the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties formalized it as such. In 2013, the Association of American Medical Colleges formally required related professionalism competencies.
However, identifying lapses has operated largely on an “I-know-it-when-I-see-it” basis, leading to widely varying remediation practices judged by a small number of faculty members or administrators.
The ideas outlined by Wasserman and colleagues are “a terrific application of the ‘just-culture’ framework,” according to Nicole Treadway, MD, a first-year primary care resident at Emory School of Medicine in Atlanta, Georgia.
At Emory, discussions of professionalism start from day 1 of medical school and the subject is revisited throughout training in small groups, Treadway told Medscape Medical News.
But, she said, as the authors point out, definitions of unprofessionalism are not always clear and the examples the authors put forward help put lapses in context.
The framework also allows for looking at mistakes in light of the stress trainees encounter and the greater chance of making a professionalism error in those situations, she noted.
In her own work, she says, because she is juggling both inpatient and outpatient care, she is finding it is easy to get behind on correspondence or communicating lab results or having follow-up conversations.
Those delays could be seen as lapses in professionalism, but under this framework, there may be system solutions or training opportunities to consider.
“We do need this organizational architecture, and I think it could serve us well in really helping us identify and appropriately respond to what we see regarding professionalism,” she said.
Framework Helps Standardize Thinking
She said having a universal framework also helps because while standards of professionalism are easier to monitor in a single medical school, when students scatter to other hospitals for clinical training, those hospitals may have different professionalism standards.
Wasserman agrees, saying, “This could be easily adopted in any environment where people deal with professionalism lapses. I don’t even think it’s necessarily relegated to trainees. It’s a great way to think about any kind of lapses, just as hospitals think about medical errors.”
He said the next step is presenting the framework at various medical schools for feedback and research to see whether the framework improves processes.
Potential criticism, he said, might come from those who say such a construct avoids punishing students who make errors.
“There will always be people who say we’re pandering to medical students whenever we worry about the learning environment,” he said. “There are old-school purists who say when people screw up you should punish them.”
But he adds healthcare broadly has moved past that thinking.
“People recognized 20 years ago or more from the standpoint of improving healthcare systems and safety that is a bad strategy. You’ll never get error-free humans working in your system, and what you have to do is consider how the system is functioning and think about ways to optimize the system so people can be their best within it.”
Wasserman and Treadway have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Some instances of unprofessional behavior by medical trainees are universally deemed egregious and worthy of discipline — for example, looking up a friend’s medical data after HIPAA training.
Conversely, some professionalism lapses may be widely thought of as a teaching and consoling moment, such as the human error involved in forgetting a scheduled repositioning of a patient.
But between the extremes is a vast gray area. To deal with those cases appropriately, Jason Wasserman, PhD, and colleagues propose a new framework by which to judge each infraction.
The framework draws from “just culture” concepts used to evaluate medical errors, Wasserman, associate professor of biomedical science at Oakland University William Beaumont School of Medicine in Rochester, Michigan, told Medscape Medical News. Such an approach takes into account the environment in which the error was made, the knowledge and intent of the person making the error, and the severity and consequences of the infraction so that trainees and institutions can learn from mistakes.
“Trainees by definition are not going to fully get it,” he explained. “By definition they’re not going to fully achieve professional expectations. So how can we respond to the things we need to respond to, but do it in a way that’s educational?”
Wasserman and coauthors’ framework for remediation, which they published February 20 in The New England Journal of Medicine, takes into account several questions: Was the expectation clear? Were there factors beyond the trainees› control? What were the trainees› intentions and did they understand the consequences? Did the person genuinely believe the action was inconsequential?
An example requiring discipline, the authors say, would be using a crib sheet during an exam. In that case the intent is clear, there is no defensible belief that the action is inconsequential, and there is a clear understanding the action is wrong.
But a response of “affirm, support, and advise” is more appropriate, for example, when a student’s alarm doesn’t go off after a power outage and they miss a mandatory meeting.
Wasserman points out that this framework won’t cover all situations.
“This is not an algorithm for answering your questions about what to do,” he said. “It’s an architecture for clarifying the discussion about that. It can really tease out all the threads that need to be considered to best respond to and correct the professionalism lapse, but do it in a way that is developmentally appropriate.”
A Core Competency
For two decades, professionalism has been considered a core competency of medical education. In 1999, the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties formalized it as such. In 2013, the Association of American Medical Colleges formally required related professionalism competencies.
However, identifying lapses has operated largely on an “I-know-it-when-I-see-it” basis, leading to widely varying remediation practices judged by a small number of faculty members or administrators.
The ideas outlined by Wasserman and colleagues are “a terrific application of the ‘just-culture’ framework,” according to Nicole Treadway, MD, a first-year primary care resident at Emory School of Medicine in Atlanta, Georgia.
At Emory, discussions of professionalism start from day 1 of medical school and the subject is revisited throughout training in small groups, Treadway told Medscape Medical News.
But, she said, as the authors point out, definitions of unprofessionalism are not always clear and the examples the authors put forward help put lapses in context.
The framework also allows for looking at mistakes in light of the stress trainees encounter and the greater chance of making a professionalism error in those situations, she noted.
In her own work, she says, because she is juggling both inpatient and outpatient care, she is finding it is easy to get behind on correspondence or communicating lab results or having follow-up conversations.
Those delays could be seen as lapses in professionalism, but under this framework, there may be system solutions or training opportunities to consider.
“We do need this organizational architecture, and I think it could serve us well in really helping us identify and appropriately respond to what we see regarding professionalism,” she said.
Framework Helps Standardize Thinking
She said having a universal framework also helps because while standards of professionalism are easier to monitor in a single medical school, when students scatter to other hospitals for clinical training, those hospitals may have different professionalism standards.
Wasserman agrees, saying, “This could be easily adopted in any environment where people deal with professionalism lapses. I don’t even think it’s necessarily relegated to trainees. It’s a great way to think about any kind of lapses, just as hospitals think about medical errors.”
He said the next step is presenting the framework at various medical schools for feedback and research to see whether the framework improves processes.
Potential criticism, he said, might come from those who say such a construct avoids punishing students who make errors.
“There will always be people who say we’re pandering to medical students whenever we worry about the learning environment,” he said. “There are old-school purists who say when people screw up you should punish them.”
But he adds healthcare broadly has moved past that thinking.
“People recognized 20 years ago or more from the standpoint of improving healthcare systems and safety that is a bad strategy. You’ll never get error-free humans working in your system, and what you have to do is consider how the system is functioning and think about ways to optimize the system so people can be their best within it.”
Wasserman and Treadway have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Some instances of unprofessional behavior by medical trainees are universally deemed egregious and worthy of discipline — for example, looking up a friend’s medical data after HIPAA training.
Conversely, some professionalism lapses may be widely thought of as a teaching and consoling moment, such as the human error involved in forgetting a scheduled repositioning of a patient.
But between the extremes is a vast gray area. To deal with those cases appropriately, Jason Wasserman, PhD, and colleagues propose a new framework by which to judge each infraction.
The framework draws from “just culture” concepts used to evaluate medical errors, Wasserman, associate professor of biomedical science at Oakland University William Beaumont School of Medicine in Rochester, Michigan, told Medscape Medical News. Such an approach takes into account the environment in which the error was made, the knowledge and intent of the person making the error, and the severity and consequences of the infraction so that trainees and institutions can learn from mistakes.
“Trainees by definition are not going to fully get it,” he explained. “By definition they’re not going to fully achieve professional expectations. So how can we respond to the things we need to respond to, but do it in a way that’s educational?”
Wasserman and coauthors’ framework for remediation, which they published February 20 in The New England Journal of Medicine, takes into account several questions: Was the expectation clear? Were there factors beyond the trainees› control? What were the trainees› intentions and did they understand the consequences? Did the person genuinely believe the action was inconsequential?
An example requiring discipline, the authors say, would be using a crib sheet during an exam. In that case the intent is clear, there is no defensible belief that the action is inconsequential, and there is a clear understanding the action is wrong.
But a response of “affirm, support, and advise” is more appropriate, for example, when a student’s alarm doesn’t go off after a power outage and they miss a mandatory meeting.
Wasserman points out that this framework won’t cover all situations.
“This is not an algorithm for answering your questions about what to do,” he said. “It’s an architecture for clarifying the discussion about that. It can really tease out all the threads that need to be considered to best respond to and correct the professionalism lapse, but do it in a way that is developmentally appropriate.”
A Core Competency
For two decades, professionalism has been considered a core competency of medical education. In 1999, the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties formalized it as such. In 2013, the Association of American Medical Colleges formally required related professionalism competencies.
However, identifying lapses has operated largely on an “I-know-it-when-I-see-it” basis, leading to widely varying remediation practices judged by a small number of faculty members or administrators.
The ideas outlined by Wasserman and colleagues are “a terrific application of the ‘just-culture’ framework,” according to Nicole Treadway, MD, a first-year primary care resident at Emory School of Medicine in Atlanta, Georgia.
At Emory, discussions of professionalism start from day 1 of medical school and the subject is revisited throughout training in small groups, Treadway told Medscape Medical News.
But, she said, as the authors point out, definitions of unprofessionalism are not always clear and the examples the authors put forward help put lapses in context.
The framework also allows for looking at mistakes in light of the stress trainees encounter and the greater chance of making a professionalism error in those situations, she noted.
In her own work, she says, because she is juggling both inpatient and outpatient care, she is finding it is easy to get behind on correspondence or communicating lab results or having follow-up conversations.
Those delays could be seen as lapses in professionalism, but under this framework, there may be system solutions or training opportunities to consider.
“We do need this organizational architecture, and I think it could serve us well in really helping us identify and appropriately respond to what we see regarding professionalism,” she said.
Framework Helps Standardize Thinking
She said having a universal framework also helps because while standards of professionalism are easier to monitor in a single medical school, when students scatter to other hospitals for clinical training, those hospitals may have different professionalism standards.
Wasserman agrees, saying, “This could be easily adopted in any environment where people deal with professionalism lapses. I don’t even think it’s necessarily relegated to trainees. It’s a great way to think about any kind of lapses, just as hospitals think about medical errors.”
He said the next step is presenting the framework at various medical schools for feedback and research to see whether the framework improves processes.
Potential criticism, he said, might come from those who say such a construct avoids punishing students who make errors.
“There will always be people who say we’re pandering to medical students whenever we worry about the learning environment,” he said. “There are old-school purists who say when people screw up you should punish them.”
But he adds healthcare broadly has moved past that thinking.
“People recognized 20 years ago or more from the standpoint of improving healthcare systems and safety that is a bad strategy. You’ll never get error-free humans working in your system, and what you have to do is consider how the system is functioning and think about ways to optimize the system so people can be their best within it.”
Wasserman and Treadway have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.





