User login
COVID-19 death rate was twice as high in cancer patients in NYC study
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
FROM CANCER DISCOVERY
Teledermatology Fast Facts
Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, many patients are working from home, which has led to a unique opportunity for dermatologists to step in and continue to care for their patients at home via telemedicine. With recent waivers and guidance from the Centers for Medicare & Medicaid Services (CMS), insurance coverage has been expanded for telehealth services, usually at the same level as an in-person visit. This editorial provides guidance for implementing telehealth services in your practice, and a tip sheet is available online for you to save and print. Please note that this information is changing on a day-to-day basis, so refer to the resources in the Table to get the latest updates.
Billing and Coding
The best reimbursements are for live telemedicine that emulates an outpatient visit and is billed using the same Current Procedural Terminology (CPT) codes (99201–99215). Previously, Medicare did not allow direct-to-patient visits to be billed, instead requiring a waiver for these services to be provided in underserved areas. During the COVID-19 pandemic, this requirement has been lifted, allowing all patients to be seen from any originating site (eg, the patient’s home).
Previously, the CMS had issued guidelines for telehealth visits that required that a physician-patient relationship be established in person prior to conducting telemedicine visits. These guidelines also have been waived for the duration of this public health emergency, allowing physicians to conduct new patient visits via telehealth and bill Medicare. Many commercial payors also are covering new patient visits via telehealth; however, it is best to check the patient’s plan first, as some plans may have different requirements or restrictions on allowable CPT codes and/or place of service. Prior requirements that physicians at a distant site (ie, the physician providing telemedicine services) be located at a site of clinical care also have been relaxed, thus allowing physicians to be located anywhere while providing services, even for those who are confined to their homes.
In general, commercial payors are covering telehealth visits at 100% of an in-person visit. Although COVID-19–related visits are covered by law, many payors including Aetna, Anthem, Blue Cross Blue Shield, Cigna, Emblem Health, Humana, and United Healthcare have indicated that they will waive all telehealth co-pays for a limited time, including visits not related to COVID-19. At the time of publication, only Aetna has issued a formal policy to this effect, so it is best to check with the insurer.1,2 However, it is important to note that regional and employer-specific plans may have different policies, so it is best to check with the insurance plans directly to confirm coverage and co-pay status.
Coding should be performed using the usual new/established patient visit codes for outpatients (99201–99215). A place of service (POS) code of 02 previously was used for all telehealth visits; however, the CMS is allowing offices to bill with their usual POS (generally POS 11) and modifier -95 in an updated rule that is active during this public health crisis. This change allows access to higher reimbursements, as POS 02 visits are paid at lower facility fee rates. Commercial insurers have varying policies on POS that are changing, so it is best to check with them individually.
In certain states, store-and-forward services may be billed using a GQ modifier for Medicaid; however, the remote check-in and telephone codes for Medicare do not reimburse well and generally are best avoided if a live telemedicine encounter is possible, as it provides better patient care and direct counseling capabilities, similar to an in-person visit. The CMS has indicated that it is now covering telephone visits (99441-99443) so that providers can contact patients through an audio-only device and bill for the encounter. Generally speaking, telephone visits reimburse the same or more than the virtual check-in codes (G2010/G2012) as long as the telephone encounter is more than 5-minutes long. Digital visits also are available (99421-99423), which include both store-and-forward photographs and a telephone call, but the reimbursements are similar to the telephone-only visit codes.3
Although the CMS has relaxed regulations for physicians to provide care across state lines, not all state licensing authorities have adopted similar measures, and the CMS waiver only applies to federally funded programs. It is important to check with state medical licensing authorities to see whether you are authorized to provide care if your patient is not located within the state where you hold your license at the time of the visit. Many states, but not all, have waived this requirement or have set up very expedient ways to apply for telemedicine licenses.
The CMS also released guidance that rules for documentation requirements have been temporarily relaxed,3 such that visits should be billed at a level of service consistent with either medical decision-making or total time spent by the provider, including face-to-face and non–face-to-face time spent on the patient. (Note: If billing by time, which usually is not advised, use the CMS definitions of time-based coding.) History and physical examination criteria do not have to be met.
Workflow
In general, it is best to maintain your current workflow as much as possible, with a live video encounter replacing only the patient interaction portion of the visit. You will need to maintain an infrastructure for scheduling visits, collecting co-pays (eg, over the telephone prior to the video visit), and documentation/billing.
It is best to have one device for conducting the actual video visit (eg, a laptop, tablet, or smartphone) and a separate device to use for documentation (eg, another device to access the electronic medical record). The CMS has advised that it will not enforce Health Insurance Portability and Accountability Act (HIPAA) rules,4 allowing physicians to use video conferencing and chat applications such as FaceTime, Skype, or Google Hangouts; however, patient safety is still an issue, and it is imperative to make sure you identify the patient correctly upon starting the visit. During the COVID-19 pandemic, numerous telehealth companies are offering temporary free video conferencing software that is HIPAA compliant, such as Doximity, VSee, Doxy.me, and Medweb. If you are able to go through one of these vendors, you will be able to continue conducting some telemedicine visits after the public health emergency, which may be helpful to your practice.
For some visits, such as acne patients on isotretinoin, you can write for a standing laboratory order that can be drawn at a laboratory center near your patient, and you can perform the counseling via telemedicine. For patients on isotretinoin, iPledge has issued a program update allowing the use of at-home pregnancy tests during the pandemic. The results must be communicated to the provider and documented with a time/date.5
Video Visit Tips and Pearls
Make sure to have well-defined parameters about what can be triaged via a single video visit. Suggestions include no total-body skin examinations and a limit of 1 rash or 2 lesions. Provide a disclaimer that it is not always possible to tell whether or not a lesion is concerning via a video visit, and the patient may have to come in for a biopsy at some point.
It is better to overcall via telemedicine than to undercall. Unless something is a very obvious seborrheic keratosis, skin tag, cherry angioma, or other benign lesion, it might be reasonable to tell a patient to come in for further evaluation of a worrisome lesion after things get back to normal. A static photograph from the patient can be helpful so it is clear what lesion is being examined during the current visit. If the patient has a skin cancer at a distant site in the future, there will be no doubt as to what lesion you examined. Having the capability to receive static images from the patient to serve as representative photographs of their chief concern is very helpful before the visit. Often, these images turn out to be better diagnostically than the live video itself, which can be compressed and show inaccurate colors. Some of the telemedicine vendors have this feature built-in, which is preferable. If you are asking patients to send you emails, it is better to have access to a HIPAA-compliant email inbox to avoid any potential issues down the line.
When scheduling a video visit, have your schedulers specifically tell patients that they should be on a high-speed Wi-Fi connection with good lighting in the room. You would be surprised that this is not intuitive for everyone!
Finally, most telemedicine visits are relatively short and to the point. In the beginning, start by scheduling patients every 15 to 20 minutes to allow for technical difficulties, but ultimately plan to be seeing patients at least every 10 minutes—it can be quite efficient!
- America’s Health Insurance Providers. Health insurance providers respond to coronavirus (COVID-19). https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/. Published April 22, 2020. Accessed April 23, 2020.
- Private payer coverage during COVID-19. American College of Physicians website. https://www.acponline.org/system/files/documents/clinical_information/resources/covid19/payer_chart_covid-19.pdf. Updated April 22, 2020. Accessed April 23, 2020.
- Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 26, 2020. Accessed April 23, 2020.
- Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed April 23, 2020.
- Program update. iPledge website. https://www.ipledgeprogram.com/iPledgeUI/home.u. Accessed April 23, 2020.
Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, many patients are working from home, which has led to a unique opportunity for dermatologists to step in and continue to care for their patients at home via telemedicine. With recent waivers and guidance from the Centers for Medicare & Medicaid Services (CMS), insurance coverage has been expanded for telehealth services, usually at the same level as an in-person visit. This editorial provides guidance for implementing telehealth services in your practice, and a tip sheet is available online for you to save and print. Please note that this information is changing on a day-to-day basis, so refer to the resources in the Table to get the latest updates.
Billing and Coding
The best reimbursements are for live telemedicine that emulates an outpatient visit and is billed using the same Current Procedural Terminology (CPT) codes (99201–99215). Previously, Medicare did not allow direct-to-patient visits to be billed, instead requiring a waiver for these services to be provided in underserved areas. During the COVID-19 pandemic, this requirement has been lifted, allowing all patients to be seen from any originating site (eg, the patient’s home).
Previously, the CMS had issued guidelines for telehealth visits that required that a physician-patient relationship be established in person prior to conducting telemedicine visits. These guidelines also have been waived for the duration of this public health emergency, allowing physicians to conduct new patient visits via telehealth and bill Medicare. Many commercial payors also are covering new patient visits via telehealth; however, it is best to check the patient’s plan first, as some plans may have different requirements or restrictions on allowable CPT codes and/or place of service. Prior requirements that physicians at a distant site (ie, the physician providing telemedicine services) be located at a site of clinical care also have been relaxed, thus allowing physicians to be located anywhere while providing services, even for those who are confined to their homes.
In general, commercial payors are covering telehealth visits at 100% of an in-person visit. Although COVID-19–related visits are covered by law, many payors including Aetna, Anthem, Blue Cross Blue Shield, Cigna, Emblem Health, Humana, and United Healthcare have indicated that they will waive all telehealth co-pays for a limited time, including visits not related to COVID-19. At the time of publication, only Aetna has issued a formal policy to this effect, so it is best to check with the insurer.1,2 However, it is important to note that regional and employer-specific plans may have different policies, so it is best to check with the insurance plans directly to confirm coverage and co-pay status.
Coding should be performed using the usual new/established patient visit codes for outpatients (99201–99215). A place of service (POS) code of 02 previously was used for all telehealth visits; however, the CMS is allowing offices to bill with their usual POS (generally POS 11) and modifier -95 in an updated rule that is active during this public health crisis. This change allows access to higher reimbursements, as POS 02 visits are paid at lower facility fee rates. Commercial insurers have varying policies on POS that are changing, so it is best to check with them individually.
In certain states, store-and-forward services may be billed using a GQ modifier for Medicaid; however, the remote check-in and telephone codes for Medicare do not reimburse well and generally are best avoided if a live telemedicine encounter is possible, as it provides better patient care and direct counseling capabilities, similar to an in-person visit. The CMS has indicated that it is now covering telephone visits (99441-99443) so that providers can contact patients through an audio-only device and bill for the encounter. Generally speaking, telephone visits reimburse the same or more than the virtual check-in codes (G2010/G2012) as long as the telephone encounter is more than 5-minutes long. Digital visits also are available (99421-99423), which include both store-and-forward photographs and a telephone call, but the reimbursements are similar to the telephone-only visit codes.3
Although the CMS has relaxed regulations for physicians to provide care across state lines, not all state licensing authorities have adopted similar measures, and the CMS waiver only applies to federally funded programs. It is important to check with state medical licensing authorities to see whether you are authorized to provide care if your patient is not located within the state where you hold your license at the time of the visit. Many states, but not all, have waived this requirement or have set up very expedient ways to apply for telemedicine licenses.
The CMS also released guidance that rules for documentation requirements have been temporarily relaxed,3 such that visits should be billed at a level of service consistent with either medical decision-making or total time spent by the provider, including face-to-face and non–face-to-face time spent on the patient. (Note: If billing by time, which usually is not advised, use the CMS definitions of time-based coding.) History and physical examination criteria do not have to be met.
Workflow
In general, it is best to maintain your current workflow as much as possible, with a live video encounter replacing only the patient interaction portion of the visit. You will need to maintain an infrastructure for scheduling visits, collecting co-pays (eg, over the telephone prior to the video visit), and documentation/billing.
It is best to have one device for conducting the actual video visit (eg, a laptop, tablet, or smartphone) and a separate device to use for documentation (eg, another device to access the electronic medical record). The CMS has advised that it will not enforce Health Insurance Portability and Accountability Act (HIPAA) rules,4 allowing physicians to use video conferencing and chat applications such as FaceTime, Skype, or Google Hangouts; however, patient safety is still an issue, and it is imperative to make sure you identify the patient correctly upon starting the visit. During the COVID-19 pandemic, numerous telehealth companies are offering temporary free video conferencing software that is HIPAA compliant, such as Doximity, VSee, Doxy.me, and Medweb. If you are able to go through one of these vendors, you will be able to continue conducting some telemedicine visits after the public health emergency, which may be helpful to your practice.
For some visits, such as acne patients on isotretinoin, you can write for a standing laboratory order that can be drawn at a laboratory center near your patient, and you can perform the counseling via telemedicine. For patients on isotretinoin, iPledge has issued a program update allowing the use of at-home pregnancy tests during the pandemic. The results must be communicated to the provider and documented with a time/date.5
Video Visit Tips and Pearls
Make sure to have well-defined parameters about what can be triaged via a single video visit. Suggestions include no total-body skin examinations and a limit of 1 rash or 2 lesions. Provide a disclaimer that it is not always possible to tell whether or not a lesion is concerning via a video visit, and the patient may have to come in for a biopsy at some point.
It is better to overcall via telemedicine than to undercall. Unless something is a very obvious seborrheic keratosis, skin tag, cherry angioma, or other benign lesion, it might be reasonable to tell a patient to come in for further evaluation of a worrisome lesion after things get back to normal. A static photograph from the patient can be helpful so it is clear what lesion is being examined during the current visit. If the patient has a skin cancer at a distant site in the future, there will be no doubt as to what lesion you examined. Having the capability to receive static images from the patient to serve as representative photographs of their chief concern is very helpful before the visit. Often, these images turn out to be better diagnostically than the live video itself, which can be compressed and show inaccurate colors. Some of the telemedicine vendors have this feature built-in, which is preferable. If you are asking patients to send you emails, it is better to have access to a HIPAA-compliant email inbox to avoid any potential issues down the line.
When scheduling a video visit, have your schedulers specifically tell patients that they should be on a high-speed Wi-Fi connection with good lighting in the room. You would be surprised that this is not intuitive for everyone!
Finally, most telemedicine visits are relatively short and to the point. In the beginning, start by scheduling patients every 15 to 20 minutes to allow for technical difficulties, but ultimately plan to be seeing patients at least every 10 minutes—it can be quite efficient!
Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, many patients are working from home, which has led to a unique opportunity for dermatologists to step in and continue to care for their patients at home via telemedicine. With recent waivers and guidance from the Centers for Medicare & Medicaid Services (CMS), insurance coverage has been expanded for telehealth services, usually at the same level as an in-person visit. This editorial provides guidance for implementing telehealth services in your practice, and a tip sheet is available online for you to save and print. Please note that this information is changing on a day-to-day basis, so refer to the resources in the Table to get the latest updates.
Billing and Coding
The best reimbursements are for live telemedicine that emulates an outpatient visit and is billed using the same Current Procedural Terminology (CPT) codes (99201–99215). Previously, Medicare did not allow direct-to-patient visits to be billed, instead requiring a waiver for these services to be provided in underserved areas. During the COVID-19 pandemic, this requirement has been lifted, allowing all patients to be seen from any originating site (eg, the patient’s home).
Previously, the CMS had issued guidelines for telehealth visits that required that a physician-patient relationship be established in person prior to conducting telemedicine visits. These guidelines also have been waived for the duration of this public health emergency, allowing physicians to conduct new patient visits via telehealth and bill Medicare. Many commercial payors also are covering new patient visits via telehealth; however, it is best to check the patient’s plan first, as some plans may have different requirements or restrictions on allowable CPT codes and/or place of service. Prior requirements that physicians at a distant site (ie, the physician providing telemedicine services) be located at a site of clinical care also have been relaxed, thus allowing physicians to be located anywhere while providing services, even for those who are confined to their homes.
In general, commercial payors are covering telehealth visits at 100% of an in-person visit. Although COVID-19–related visits are covered by law, many payors including Aetna, Anthem, Blue Cross Blue Shield, Cigna, Emblem Health, Humana, and United Healthcare have indicated that they will waive all telehealth co-pays for a limited time, including visits not related to COVID-19. At the time of publication, only Aetna has issued a formal policy to this effect, so it is best to check with the insurer.1,2 However, it is important to note that regional and employer-specific plans may have different policies, so it is best to check with the insurance plans directly to confirm coverage and co-pay status.
Coding should be performed using the usual new/established patient visit codes for outpatients (99201–99215). A place of service (POS) code of 02 previously was used for all telehealth visits; however, the CMS is allowing offices to bill with their usual POS (generally POS 11) and modifier -95 in an updated rule that is active during this public health crisis. This change allows access to higher reimbursements, as POS 02 visits are paid at lower facility fee rates. Commercial insurers have varying policies on POS that are changing, so it is best to check with them individually.
In certain states, store-and-forward services may be billed using a GQ modifier for Medicaid; however, the remote check-in and telephone codes for Medicare do not reimburse well and generally are best avoided if a live telemedicine encounter is possible, as it provides better patient care and direct counseling capabilities, similar to an in-person visit. The CMS has indicated that it is now covering telephone visits (99441-99443) so that providers can contact patients through an audio-only device and bill for the encounter. Generally speaking, telephone visits reimburse the same or more than the virtual check-in codes (G2010/G2012) as long as the telephone encounter is more than 5-minutes long. Digital visits also are available (99421-99423), which include both store-and-forward photographs and a telephone call, but the reimbursements are similar to the telephone-only visit codes.3
Although the CMS has relaxed regulations for physicians to provide care across state lines, not all state licensing authorities have adopted similar measures, and the CMS waiver only applies to federally funded programs. It is important to check with state medical licensing authorities to see whether you are authorized to provide care if your patient is not located within the state where you hold your license at the time of the visit. Many states, but not all, have waived this requirement or have set up very expedient ways to apply for telemedicine licenses.
The CMS also released guidance that rules for documentation requirements have been temporarily relaxed,3 such that visits should be billed at a level of service consistent with either medical decision-making or total time spent by the provider, including face-to-face and non–face-to-face time spent on the patient. (Note: If billing by time, which usually is not advised, use the CMS definitions of time-based coding.) History and physical examination criteria do not have to be met.
Workflow
In general, it is best to maintain your current workflow as much as possible, with a live video encounter replacing only the patient interaction portion of the visit. You will need to maintain an infrastructure for scheduling visits, collecting co-pays (eg, over the telephone prior to the video visit), and documentation/billing.
It is best to have one device for conducting the actual video visit (eg, a laptop, tablet, or smartphone) and a separate device to use for documentation (eg, another device to access the electronic medical record). The CMS has advised that it will not enforce Health Insurance Portability and Accountability Act (HIPAA) rules,4 allowing physicians to use video conferencing and chat applications such as FaceTime, Skype, or Google Hangouts; however, patient safety is still an issue, and it is imperative to make sure you identify the patient correctly upon starting the visit. During the COVID-19 pandemic, numerous telehealth companies are offering temporary free video conferencing software that is HIPAA compliant, such as Doximity, VSee, Doxy.me, and Medweb. If you are able to go through one of these vendors, you will be able to continue conducting some telemedicine visits after the public health emergency, which may be helpful to your practice.
For some visits, such as acne patients on isotretinoin, you can write for a standing laboratory order that can be drawn at a laboratory center near your patient, and you can perform the counseling via telemedicine. For patients on isotretinoin, iPledge has issued a program update allowing the use of at-home pregnancy tests during the pandemic. The results must be communicated to the provider and documented with a time/date.5
Video Visit Tips and Pearls
Make sure to have well-defined parameters about what can be triaged via a single video visit. Suggestions include no total-body skin examinations and a limit of 1 rash or 2 lesions. Provide a disclaimer that it is not always possible to tell whether or not a lesion is concerning via a video visit, and the patient may have to come in for a biopsy at some point.
It is better to overcall via telemedicine than to undercall. Unless something is a very obvious seborrheic keratosis, skin tag, cherry angioma, or other benign lesion, it might be reasonable to tell a patient to come in for further evaluation of a worrisome lesion after things get back to normal. A static photograph from the patient can be helpful so it is clear what lesion is being examined during the current visit. If the patient has a skin cancer at a distant site in the future, there will be no doubt as to what lesion you examined. Having the capability to receive static images from the patient to serve as representative photographs of their chief concern is very helpful before the visit. Often, these images turn out to be better diagnostically than the live video itself, which can be compressed and show inaccurate colors. Some of the telemedicine vendors have this feature built-in, which is preferable. If you are asking patients to send you emails, it is better to have access to a HIPAA-compliant email inbox to avoid any potential issues down the line.
When scheduling a video visit, have your schedulers specifically tell patients that they should be on a high-speed Wi-Fi connection with good lighting in the room. You would be surprised that this is not intuitive for everyone!
Finally, most telemedicine visits are relatively short and to the point. In the beginning, start by scheduling patients every 15 to 20 minutes to allow for technical difficulties, but ultimately plan to be seeing patients at least every 10 minutes—it can be quite efficient!
- America’s Health Insurance Providers. Health insurance providers respond to coronavirus (COVID-19). https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/. Published April 22, 2020. Accessed April 23, 2020.
- Private payer coverage during COVID-19. American College of Physicians website. https://www.acponline.org/system/files/documents/clinical_information/resources/covid19/payer_chart_covid-19.pdf. Updated April 22, 2020. Accessed April 23, 2020.
- Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 26, 2020. Accessed April 23, 2020.
- Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed April 23, 2020.
- Program update. iPledge website. https://www.ipledgeprogram.com/iPledgeUI/home.u. Accessed April 23, 2020.
- America’s Health Insurance Providers. Health insurance providers respond to coronavirus (COVID-19). https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/. Published April 22, 2020. Accessed April 23, 2020.
- Private payer coverage during COVID-19. American College of Physicians website. https://www.acponline.org/system/files/documents/clinical_information/resources/covid19/payer_chart_covid-19.pdf. Updated April 22, 2020. Accessed April 23, 2020.
- Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 26, 2020. Accessed April 23, 2020.
- Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed April 23, 2020.
- Program update. iPledge website. https://www.ipledgeprogram.com/iPledgeUI/home.u. Accessed April 23, 2020.
Three months of COVID-19 may mean 80,000 missed cancer diagnoses
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.
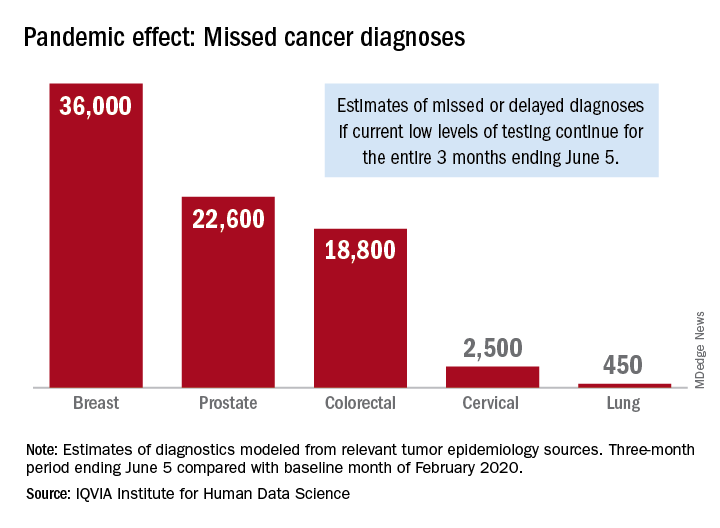
Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Cancer screening, monitoring down during pandemic
according to a report by the IQVIA Institute for Human Data Science.
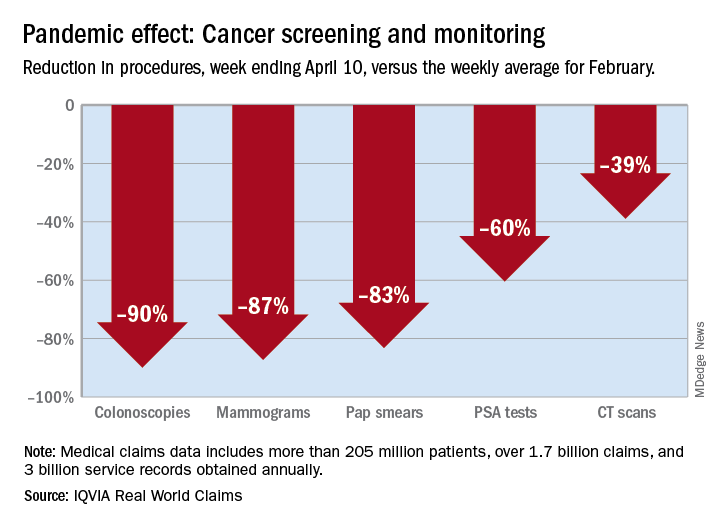
There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
FDA tightens requirements for COVID-19 antibody tests
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
Excess cancer deaths predicted as care is disrupted by COVID-19
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Medical meetings may be forever changed
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005-2015, there were more than 30,000 medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the ‘virtual meetings!’ ”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, occurred online on those days. The ACC said it would stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast-forward more than 50 years later to 2019: There were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Ky., in a regular column.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argued. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he wrote.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her health care colleagues: “... there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
A version of this article originally appeared on Medscape.com.
Editor’s note: For information on the impending virtual DDW® meeting, please go to https://ddw.org/attendee-planning/covid-19-update/.
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005-2015, there were more than 30,000 medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the ‘virtual meetings!’ ”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, occurred online on those days. The ACC said it would stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast-forward more than 50 years later to 2019: There were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Ky., in a regular column.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argued. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he wrote.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her health care colleagues: “... there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
A version of this article originally appeared on Medscape.com.
Editor’s note: For information on the impending virtual DDW® meeting, please go to https://ddw.org/attendee-planning/covid-19-update/.
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005-2015, there were more than 30,000 medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the ‘virtual meetings!’ ”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, occurred online on those days. The ACC said it would stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast-forward more than 50 years later to 2019: There were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Ky., in a regular column.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argued. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he wrote.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her health care colleagues: “... there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
A version of this article originally appeared on Medscape.com.
Editor’s note: For information on the impending virtual DDW® meeting, please go to https://ddw.org/attendee-planning/covid-19-update/.
COVID-19: Employers cut doc pay and bonuses: What’s your recourse?
Employed physicians have had to take large pay cuts, give up bonuses, go on leave, or have even been terminated. In many cases, these actions violate their contract. How can they fight them?
Michael D., MD, a colorectal surgeon employed in a large surgical practice in Georgia, is still trying to make sense of a late-night directive from the practice, received in late March.
The practice had just started seeing a steep decline in appointments because of the COVID-19 pandemic. In a hastily arranged group phone call at 11:00 p.m., the CEO told the group what would have to be done.
They would be taking a 50% reduction in salaries, their bonuses for work already done were being withheld, and they would have to use their paid time off (PTO) in order to get their full March salary.
“It’s been over 2 weeks now, and still we’ve seen nothing formalized in writing,” said Dr. D., who asked that his name not be used because he was told that, under no circumstances, should anyone talk to the media.
“They have not told us anything more since then,” he said. “There’s just been a lot of hearsay and speculation.”
Dr. D. has been in touch with employed physicians at other practices, and their experiences run the gamut. One doctor at a large multispecialty group said his salary hadn’t been reduced at all, but a cardiologist was just told he will be laid off in 60 days.
Asking for big sacrifices
As the pandemic has intensified, employed physicians have started to see massive changes in their payment arrangements. They have had to take large pay cuts, give up bonuses, go on leave, and have even been terminated.
“In my 11 years of work on physician contracts, I have never seen changes as drastic as these,” said Kyle Claussen, a physician contract attorney and CEO of Resolve, a company that advises physicians on their careers. The company is based in Columbia, Mo.
He has heard from more than 100 doctors about these proposed changes in their contracts and related matters. Even graduating residents, he said, are being told that the start dates for their new jobs will be delayed.
In many cases, these actions violate the employed physicians’ contracts, said Ericka Adler, a physician contract attorney at Roetzel & Andress in Chicago.
“Some employers are acting out of desperation and are not making legally sound decisions,” Ms. Adler said. “It’s especially upsetting when they do not try to even talk to or work with the doctor first.”
Employers making unfounded unilateral changes
Ms. Adler said some employers are simply issuing a letter to all doctors. “It goes something like, ‘Just to let you know, we are cutting compensation effective immediately,’ and this may apply across the board to all doctors,” she said.
“But the problem with letters is that this is a contractual matter,” she said. “The employer needs to renegotiate each doctor’s contract.”
Employers might insist that the unilateral changes are based on terms in the contract, but this is usually unfounded, both lawyers said. A “force majeure” clause in contracts would allow the employer to set aside terms under certain specified emergencies, and the pandemic might be one of them. But Mr. Claussen said force majeure clauses are rare in physician contracts, and Ms. Adler said she has never seen one.
Lacking a force majeure clause, employers may try to turn to a common law doctrine that allows employers to set aside a contract when it is impossible to perform its terms, owing, for example, to “an unexpected intervening event.” But this tactic is also questionable, says Ms. Adler, who also represents the employer’s side of the contract. “This is a very high standard and unlikely to be satisfied,” she said.
Employers are desperate to amend contracts
Lacking a cause to take unilateral action, many employers are desperately trying to amend their physician contracts – the subject of the plaintive emails that employed physicians started receiving in mid-March.
“Doctors are trying to decide how they will react to these documents,” Mr. Claussen said. “If they don’t sign, they run the risk of being terminated.” He is expecting termination letters for some of these doctors to start coming in 2-3 weeks.
In response to these amendments, “doctors want to reach out to their employers and see if something can be negotiated,” he said. “Some employers have been amenable, but others have so far not been.”
Ms. Adler said the amendments typically offer open-ended arrangements favoring the employer. “The document might call for a temporary pay cut until the employer thinks they can restore the old salary, but it is up to the employer to decide when that would be,” she said.
Also, when the employer owes the physician for services already performed, the amendments don’t promise to pay them the full amount owed, she said.
Ms. Adler advises doctors to ask for a provision that restoration of their original salary will occur at a definite point in time, such as 30 days after the organization is back at previous volume. And if the doctor is owed money, the doctor should ask for full payment – and to sweeten that offer, allow the employer to pay the doctor back over a period, she says.
Just in the past month, employers have been pushing for several specific changes in doctors’ employment status. Here are some changes that Mr. Claussen and Ms. Adler have been seeing.
Withholding quarterly bonuses
In March, just before quarterly bonus payments were due, employed physicians started getting notices that they would not get the bonus, Mr. Claussen said. This covered work already done, and it amounted to a lot of money because practices were busy then.
“Not paying bonuses is a very big deal because they can make up to 50% of a physician’s total compensation,” Mr. Claussen said. He added that unilaterally withholding those funds, without a change in the contract, is legally questionable.
In addition to these changes on past bonuses, he said employers are now trying to temporarily end bonuses going forward through the contract amendments. “It’s not a good idea to sign this,” he said.
Doctors paid on pure production are left in the lurch
As volume falls, some hospitals and practices are shutting down doctors’ offices for all but emergencies, leaving their employed doctors with practically nothing to do. Doctors paid purely on their productivity are devastated by this change because their income virtually goes to zero, Mr. Claussen said.
He said office shutdowns are particularly common for specialists because hospitals have been stopping elective procedures during the pandemic, but they can also happen in primary care, which has seen steep declines in patient volume, too.
Having doctors on pure production means that employers can keep doctors hired without having to pay them, Mr. Claussen said. He has seen some employers try to shift more doctors into pure production through the amended contracts.
But Ms. Adler said having doctors on pure production is actually disadvantageous for employers in the current climate. The employer may end up being owed money because of advance payments they have already made to these doctors, she said.
In any case, both lawyers agreed that doctors on a pure production model are in an untenable situation right now. Ms. Adler said they are not earning money but are still technically at work, so they cannot collect unemployment compensation, which would give them some income.
Forcing doctors to use paid time off
To provide some pay for doctors who have no volume, many employers are forcing these doctors to use up their PTO days, which typically amount to about 4 weeks, Mr. Claussen said. “These doctors have no choice in the matter,” he said.
Furthermore, while on PTO, they are being required to take call. Employers are still obligated to cover call, and there may not be enough doctors still working to fill the call schedule. But making doctors do this work on their time off may be a violation of the contract, Mr. Claussen said.
Terminating physicians
Doctors who have little to do are often put on furlough. This means they don’t get paid but they keep their benefits, Ms. Adler said. The next step, she said, is to lay them off, with the stated intention of rehiring them.
Once laid off, she said, they can get unemployment payments. “Unemployment payments may not be anywhere near what they were earning before, but they are better than not earning anything,” Ms. Adler said.
In some cases, employers are just terminating them and are offering no prospect of rehiring them, she said. Ms. Adler said terminations can be a big problem for doctors. Physicians might have to repay a signing bonus or they might lose their malpractice coverage, forcing them to buy a tail. They could also be subject to a noncompete clause, which would not allow them to practice in the area, she said.
Terminating without cause typically requires 60-90 days’ notice, which both sides might use to negotiate some changes in the contract. But Ms. Adler said some employers are firing doctors with cause, and are using legally questionable reasons to do so.
“In most cases, these employers are grasping at straws,” she said. As a result, she expects many fired doctors will file wrongful termination lawsuits. She thinks employers are better advised to negotiate with the physicians.
Delaying start dates for new physicians
Typically, graduating residents and fellows signed with their new employers months ago and are ready to start working on July 1. But some employers are pushing back the start date for several months, Mr. Claussen said.
Mr. Claussen has been helping several clients in this situation. He said these delays are often a clear violation of the employment contract. Most contracts require an amendment to change the start date, he said.
Mr. Claussen said some employers have agreed to a new start date in an amended contract, giving the new physicians a solid date to work with. Not having work can be a real problem for graduating residents, who typically have to start paying off loans.
Now physicians won’t become a new partner
Mr. Claussen said physicians who are up for becoming partner are now being told that the deal is off. With less money coming in, existing partners are not willing to share it with a new partner, and there is no work for a new partner.
“The promise to make them partner is usually a verbal promise, so it is much less likely to be a breach of contract,” he said. “It is frustrating for physicians who were expecting to become partner.”
What can physicians do?
When employers present changes to them, physicians often feel their hands are tied, Ms. Adler said. In these dangerous times, they are expected to make sacrifices to keep the organization from going out of business.
Even if they wanted to file suits against their employer, “they can’t go to court right now because the courts are closed,” Ms. Adler said. “Employers are banking on doctors not doing anything.”
In most cases, however, doctors don’t have to act right away, she said. “Just because you have not reacted to the new situation does not mean you accepted it,” she said. “You can wait months, even years to file a lawsuit, depending on the state and the cause of action.”
Ms. Adler recommended that doctors make it clear that they don’t agree with the changes. An attorney experienced in physician contracts can review the changes being made and the amended contracts being offered.
Thanks to recent federal changes, employers have to have some ways to continue paying physicians, Ms. Adler said. Medical practices with fewer than 500 employees can get loans from the federal government that would not have to be repaid if they met certain stipulations, such as hiring back all the employees they terminate, she said.
Mr. Claussen said physicians should resist the obvious dangers, such as a shift to a pure production salary, denying bonus payments for work already done, and forcing physicians you use up PTO days.
He also suggested persuading employers to postpone rather than eliminate payments. “Some employers have agreed to postpone payments until a date later in 2020 rather than eliminate them,” he said. “The aim is that the organization will be back on its feet at that time.”
Mr. Claussen said he is trying to limit the contract amendments to 1 or 2 months. Because the situation caused by the pandemic is so fluid, “this allows for flexibility,” he said. “We can revisit the situation and come up with different changes.”
Ms. Adler doubts employers would accept short-term changes with a definite end date because such changes would not be in the employer’s interest. But Mr. Claussen said one employer has agreed to reevaluate its contracts in June.
Both lawyers agreed that many employers are trying to work with their physicians. “In 90% of the cases I have seen, both sides cooperate,” Ms. Adler said. “Because of the situation, people are being much more conciliatory than they would have been.”
A version of this article originally appeared on Medscape.com.
Employed physicians have had to take large pay cuts, give up bonuses, go on leave, or have even been terminated. In many cases, these actions violate their contract. How can they fight them?
Michael D., MD, a colorectal surgeon employed in a large surgical practice in Georgia, is still trying to make sense of a late-night directive from the practice, received in late March.
The practice had just started seeing a steep decline in appointments because of the COVID-19 pandemic. In a hastily arranged group phone call at 11:00 p.m., the CEO told the group what would have to be done.
They would be taking a 50% reduction in salaries, their bonuses for work already done were being withheld, and they would have to use their paid time off (PTO) in order to get their full March salary.
“It’s been over 2 weeks now, and still we’ve seen nothing formalized in writing,” said Dr. D., who asked that his name not be used because he was told that, under no circumstances, should anyone talk to the media.
“They have not told us anything more since then,” he said. “There’s just been a lot of hearsay and speculation.”
Dr. D. has been in touch with employed physicians at other practices, and their experiences run the gamut. One doctor at a large multispecialty group said his salary hadn’t been reduced at all, but a cardiologist was just told he will be laid off in 60 days.
Asking for big sacrifices
As the pandemic has intensified, employed physicians have started to see massive changes in their payment arrangements. They have had to take large pay cuts, give up bonuses, go on leave, and have even been terminated.
“In my 11 years of work on physician contracts, I have never seen changes as drastic as these,” said Kyle Claussen, a physician contract attorney and CEO of Resolve, a company that advises physicians on their careers. The company is based in Columbia, Mo.
He has heard from more than 100 doctors about these proposed changes in their contracts and related matters. Even graduating residents, he said, are being told that the start dates for their new jobs will be delayed.
In many cases, these actions violate the employed physicians’ contracts, said Ericka Adler, a physician contract attorney at Roetzel & Andress in Chicago.
“Some employers are acting out of desperation and are not making legally sound decisions,” Ms. Adler said. “It’s especially upsetting when they do not try to even talk to or work with the doctor first.”
Employers making unfounded unilateral changes
Ms. Adler said some employers are simply issuing a letter to all doctors. “It goes something like, ‘Just to let you know, we are cutting compensation effective immediately,’ and this may apply across the board to all doctors,” she said.
“But the problem with letters is that this is a contractual matter,” she said. “The employer needs to renegotiate each doctor’s contract.”
Employers might insist that the unilateral changes are based on terms in the contract, but this is usually unfounded, both lawyers said. A “force majeure” clause in contracts would allow the employer to set aside terms under certain specified emergencies, and the pandemic might be one of them. But Mr. Claussen said force majeure clauses are rare in physician contracts, and Ms. Adler said she has never seen one.
Lacking a force majeure clause, employers may try to turn to a common law doctrine that allows employers to set aside a contract when it is impossible to perform its terms, owing, for example, to “an unexpected intervening event.” But this tactic is also questionable, says Ms. Adler, who also represents the employer’s side of the contract. “This is a very high standard and unlikely to be satisfied,” she said.
Employers are desperate to amend contracts
Lacking a cause to take unilateral action, many employers are desperately trying to amend their physician contracts – the subject of the plaintive emails that employed physicians started receiving in mid-March.
“Doctors are trying to decide how they will react to these documents,” Mr. Claussen said. “If they don’t sign, they run the risk of being terminated.” He is expecting termination letters for some of these doctors to start coming in 2-3 weeks.
In response to these amendments, “doctors want to reach out to their employers and see if something can be negotiated,” he said. “Some employers have been amenable, but others have so far not been.”
Ms. Adler said the amendments typically offer open-ended arrangements favoring the employer. “The document might call for a temporary pay cut until the employer thinks they can restore the old salary, but it is up to the employer to decide when that would be,” she said.
Also, when the employer owes the physician for services already performed, the amendments don’t promise to pay them the full amount owed, she said.
Ms. Adler advises doctors to ask for a provision that restoration of their original salary will occur at a definite point in time, such as 30 days after the organization is back at previous volume. And if the doctor is owed money, the doctor should ask for full payment – and to sweeten that offer, allow the employer to pay the doctor back over a period, she says.
Just in the past month, employers have been pushing for several specific changes in doctors’ employment status. Here are some changes that Mr. Claussen and Ms. Adler have been seeing.
Withholding quarterly bonuses
In March, just before quarterly bonus payments were due, employed physicians started getting notices that they would not get the bonus, Mr. Claussen said. This covered work already done, and it amounted to a lot of money because practices were busy then.
“Not paying bonuses is a very big deal because they can make up to 50% of a physician’s total compensation,” Mr. Claussen said. He added that unilaterally withholding those funds, without a change in the contract, is legally questionable.
In addition to these changes on past bonuses, he said employers are now trying to temporarily end bonuses going forward through the contract amendments. “It’s not a good idea to sign this,” he said.
Doctors paid on pure production are left in the lurch
As volume falls, some hospitals and practices are shutting down doctors’ offices for all but emergencies, leaving their employed doctors with practically nothing to do. Doctors paid purely on their productivity are devastated by this change because their income virtually goes to zero, Mr. Claussen said.
He said office shutdowns are particularly common for specialists because hospitals have been stopping elective procedures during the pandemic, but they can also happen in primary care, which has seen steep declines in patient volume, too.
Having doctors on pure production means that employers can keep doctors hired without having to pay them, Mr. Claussen said. He has seen some employers try to shift more doctors into pure production through the amended contracts.
But Ms. Adler said having doctors on pure production is actually disadvantageous for employers in the current climate. The employer may end up being owed money because of advance payments they have already made to these doctors, she said.
In any case, both lawyers agreed that doctors on a pure production model are in an untenable situation right now. Ms. Adler said they are not earning money but are still technically at work, so they cannot collect unemployment compensation, which would give them some income.
Forcing doctors to use paid time off
To provide some pay for doctors who have no volume, many employers are forcing these doctors to use up their PTO days, which typically amount to about 4 weeks, Mr. Claussen said. “These doctors have no choice in the matter,” he said.
Furthermore, while on PTO, they are being required to take call. Employers are still obligated to cover call, and there may not be enough doctors still working to fill the call schedule. But making doctors do this work on their time off may be a violation of the contract, Mr. Claussen said.
Terminating physicians
Doctors who have little to do are often put on furlough. This means they don’t get paid but they keep their benefits, Ms. Adler said. The next step, she said, is to lay them off, with the stated intention of rehiring them.
Once laid off, she said, they can get unemployment payments. “Unemployment payments may not be anywhere near what they were earning before, but they are better than not earning anything,” Ms. Adler said.
In some cases, employers are just terminating them and are offering no prospect of rehiring them, she said. Ms. Adler said terminations can be a big problem for doctors. Physicians might have to repay a signing bonus or they might lose their malpractice coverage, forcing them to buy a tail. They could also be subject to a noncompete clause, which would not allow them to practice in the area, she said.
Terminating without cause typically requires 60-90 days’ notice, which both sides might use to negotiate some changes in the contract. But Ms. Adler said some employers are firing doctors with cause, and are using legally questionable reasons to do so.
“In most cases, these employers are grasping at straws,” she said. As a result, she expects many fired doctors will file wrongful termination lawsuits. She thinks employers are better advised to negotiate with the physicians.
Delaying start dates for new physicians
Typically, graduating residents and fellows signed with their new employers months ago and are ready to start working on July 1. But some employers are pushing back the start date for several months, Mr. Claussen said.
Mr. Claussen has been helping several clients in this situation. He said these delays are often a clear violation of the employment contract. Most contracts require an amendment to change the start date, he said.
Mr. Claussen said some employers have agreed to a new start date in an amended contract, giving the new physicians a solid date to work with. Not having work can be a real problem for graduating residents, who typically have to start paying off loans.
Now physicians won’t become a new partner
Mr. Claussen said physicians who are up for becoming partner are now being told that the deal is off. With less money coming in, existing partners are not willing to share it with a new partner, and there is no work for a new partner.
“The promise to make them partner is usually a verbal promise, so it is much less likely to be a breach of contract,” he said. “It is frustrating for physicians who were expecting to become partner.”
What can physicians do?
When employers present changes to them, physicians often feel their hands are tied, Ms. Adler said. In these dangerous times, they are expected to make sacrifices to keep the organization from going out of business.
Even if they wanted to file suits against their employer, “they can’t go to court right now because the courts are closed,” Ms. Adler said. “Employers are banking on doctors not doing anything.”
In most cases, however, doctors don’t have to act right away, she said. “Just because you have not reacted to the new situation does not mean you accepted it,” she said. “You can wait months, even years to file a lawsuit, depending on the state and the cause of action.”
Ms. Adler recommended that doctors make it clear that they don’t agree with the changes. An attorney experienced in physician contracts can review the changes being made and the amended contracts being offered.
Thanks to recent federal changes, employers have to have some ways to continue paying physicians, Ms. Adler said. Medical practices with fewer than 500 employees can get loans from the federal government that would not have to be repaid if they met certain stipulations, such as hiring back all the employees they terminate, she said.
Mr. Claussen said physicians should resist the obvious dangers, such as a shift to a pure production salary, denying bonus payments for work already done, and forcing physicians you use up PTO days.
He also suggested persuading employers to postpone rather than eliminate payments. “Some employers have agreed to postpone payments until a date later in 2020 rather than eliminate them,” he said. “The aim is that the organization will be back on its feet at that time.”
Mr. Claussen said he is trying to limit the contract amendments to 1 or 2 months. Because the situation caused by the pandemic is so fluid, “this allows for flexibility,” he said. “We can revisit the situation and come up with different changes.”
Ms. Adler doubts employers would accept short-term changes with a definite end date because such changes would not be in the employer’s interest. But Mr. Claussen said one employer has agreed to reevaluate its contracts in June.
Both lawyers agreed that many employers are trying to work with their physicians. “In 90% of the cases I have seen, both sides cooperate,” Ms. Adler said. “Because of the situation, people are being much more conciliatory than they would have been.”
A version of this article originally appeared on Medscape.com.
Employed physicians have had to take large pay cuts, give up bonuses, go on leave, or have even been terminated. In many cases, these actions violate their contract. How can they fight them?
Michael D., MD, a colorectal surgeon employed in a large surgical practice in Georgia, is still trying to make sense of a late-night directive from the practice, received in late March.
The practice had just started seeing a steep decline in appointments because of the COVID-19 pandemic. In a hastily arranged group phone call at 11:00 p.m., the CEO told the group what would have to be done.
They would be taking a 50% reduction in salaries, their bonuses for work already done were being withheld, and they would have to use their paid time off (PTO) in order to get their full March salary.
“It’s been over 2 weeks now, and still we’ve seen nothing formalized in writing,” said Dr. D., who asked that his name not be used because he was told that, under no circumstances, should anyone talk to the media.
“They have not told us anything more since then,” he said. “There’s just been a lot of hearsay and speculation.”
Dr. D. has been in touch with employed physicians at other practices, and their experiences run the gamut. One doctor at a large multispecialty group said his salary hadn’t been reduced at all, but a cardiologist was just told he will be laid off in 60 days.
Asking for big sacrifices
As the pandemic has intensified, employed physicians have started to see massive changes in their payment arrangements. They have had to take large pay cuts, give up bonuses, go on leave, and have even been terminated.
“In my 11 years of work on physician contracts, I have never seen changes as drastic as these,” said Kyle Claussen, a physician contract attorney and CEO of Resolve, a company that advises physicians on their careers. The company is based in Columbia, Mo.
He has heard from more than 100 doctors about these proposed changes in their contracts and related matters. Even graduating residents, he said, are being told that the start dates for their new jobs will be delayed.
In many cases, these actions violate the employed physicians’ contracts, said Ericka Adler, a physician contract attorney at Roetzel & Andress in Chicago.
“Some employers are acting out of desperation and are not making legally sound decisions,” Ms. Adler said. “It’s especially upsetting when they do not try to even talk to or work with the doctor first.”
Employers making unfounded unilateral changes
Ms. Adler said some employers are simply issuing a letter to all doctors. “It goes something like, ‘Just to let you know, we are cutting compensation effective immediately,’ and this may apply across the board to all doctors,” she said.
“But the problem with letters is that this is a contractual matter,” she said. “The employer needs to renegotiate each doctor’s contract.”
Employers might insist that the unilateral changes are based on terms in the contract, but this is usually unfounded, both lawyers said. A “force majeure” clause in contracts would allow the employer to set aside terms under certain specified emergencies, and the pandemic might be one of them. But Mr. Claussen said force majeure clauses are rare in physician contracts, and Ms. Adler said she has never seen one.
Lacking a force majeure clause, employers may try to turn to a common law doctrine that allows employers to set aside a contract when it is impossible to perform its terms, owing, for example, to “an unexpected intervening event.” But this tactic is also questionable, says Ms. Adler, who also represents the employer’s side of the contract. “This is a very high standard and unlikely to be satisfied,” she said.
Employers are desperate to amend contracts
Lacking a cause to take unilateral action, many employers are desperately trying to amend their physician contracts – the subject of the plaintive emails that employed physicians started receiving in mid-March.
“Doctors are trying to decide how they will react to these documents,” Mr. Claussen said. “If they don’t sign, they run the risk of being terminated.” He is expecting termination letters for some of these doctors to start coming in 2-3 weeks.
In response to these amendments, “doctors want to reach out to their employers and see if something can be negotiated,” he said. “Some employers have been amenable, but others have so far not been.”
Ms. Adler said the amendments typically offer open-ended arrangements favoring the employer. “The document might call for a temporary pay cut until the employer thinks they can restore the old salary, but it is up to the employer to decide when that would be,” she said.
Also, when the employer owes the physician for services already performed, the amendments don’t promise to pay them the full amount owed, she said.
Ms. Adler advises doctors to ask for a provision that restoration of their original salary will occur at a definite point in time, such as 30 days after the organization is back at previous volume. And if the doctor is owed money, the doctor should ask for full payment – and to sweeten that offer, allow the employer to pay the doctor back over a period, she says.
Just in the past month, employers have been pushing for several specific changes in doctors’ employment status. Here are some changes that Mr. Claussen and Ms. Adler have been seeing.
Withholding quarterly bonuses
In March, just before quarterly bonus payments were due, employed physicians started getting notices that they would not get the bonus, Mr. Claussen said. This covered work already done, and it amounted to a lot of money because practices were busy then.
“Not paying bonuses is a very big deal because they can make up to 50% of a physician’s total compensation,” Mr. Claussen said. He added that unilaterally withholding those funds, without a change in the contract, is legally questionable.
In addition to these changes on past bonuses, he said employers are now trying to temporarily end bonuses going forward through the contract amendments. “It’s not a good idea to sign this,” he said.
Doctors paid on pure production are left in the lurch
As volume falls, some hospitals and practices are shutting down doctors’ offices for all but emergencies, leaving their employed doctors with practically nothing to do. Doctors paid purely on their productivity are devastated by this change because their income virtually goes to zero, Mr. Claussen said.
He said office shutdowns are particularly common for specialists because hospitals have been stopping elective procedures during the pandemic, but they can also happen in primary care, which has seen steep declines in patient volume, too.
Having doctors on pure production means that employers can keep doctors hired without having to pay them, Mr. Claussen said. He has seen some employers try to shift more doctors into pure production through the amended contracts.
But Ms. Adler said having doctors on pure production is actually disadvantageous for employers in the current climate. The employer may end up being owed money because of advance payments they have already made to these doctors, she said.
In any case, both lawyers agreed that doctors on a pure production model are in an untenable situation right now. Ms. Adler said they are not earning money but are still technically at work, so they cannot collect unemployment compensation, which would give them some income.
Forcing doctors to use paid time off
To provide some pay for doctors who have no volume, many employers are forcing these doctors to use up their PTO days, which typically amount to about 4 weeks, Mr. Claussen said. “These doctors have no choice in the matter,” he said.
Furthermore, while on PTO, they are being required to take call. Employers are still obligated to cover call, and there may not be enough doctors still working to fill the call schedule. But making doctors do this work on their time off may be a violation of the contract, Mr. Claussen said.
Terminating physicians
Doctors who have little to do are often put on furlough. This means they don’t get paid but they keep their benefits, Ms. Adler said. The next step, she said, is to lay them off, with the stated intention of rehiring them.
Once laid off, she said, they can get unemployment payments. “Unemployment payments may not be anywhere near what they were earning before, but they are better than not earning anything,” Ms. Adler said.
In some cases, employers are just terminating them and are offering no prospect of rehiring them, she said. Ms. Adler said terminations can be a big problem for doctors. Physicians might have to repay a signing bonus or they might lose their malpractice coverage, forcing them to buy a tail. They could also be subject to a noncompete clause, which would not allow them to practice in the area, she said.
Terminating without cause typically requires 60-90 days’ notice, which both sides might use to negotiate some changes in the contract. But Ms. Adler said some employers are firing doctors with cause, and are using legally questionable reasons to do so.
“In most cases, these employers are grasping at straws,” she said. As a result, she expects many fired doctors will file wrongful termination lawsuits. She thinks employers are better advised to negotiate with the physicians.
Delaying start dates for new physicians
Typically, graduating residents and fellows signed with their new employers months ago and are ready to start working on July 1. But some employers are pushing back the start date for several months, Mr. Claussen said.
Mr. Claussen has been helping several clients in this situation. He said these delays are often a clear violation of the employment contract. Most contracts require an amendment to change the start date, he said.
Mr. Claussen said some employers have agreed to a new start date in an amended contract, giving the new physicians a solid date to work with. Not having work can be a real problem for graduating residents, who typically have to start paying off loans.
Now physicians won’t become a new partner
Mr. Claussen said physicians who are up for becoming partner are now being told that the deal is off. With less money coming in, existing partners are not willing to share it with a new partner, and there is no work for a new partner.
“The promise to make them partner is usually a verbal promise, so it is much less likely to be a breach of contract,” he said. “It is frustrating for physicians who were expecting to become partner.”
What can physicians do?
When employers present changes to them, physicians often feel their hands are tied, Ms. Adler said. In these dangerous times, they are expected to make sacrifices to keep the organization from going out of business.
Even if they wanted to file suits against their employer, “they can’t go to court right now because the courts are closed,” Ms. Adler said. “Employers are banking on doctors not doing anything.”
In most cases, however, doctors don’t have to act right away, she said. “Just because you have not reacted to the new situation does not mean you accepted it,” she said. “You can wait months, even years to file a lawsuit, depending on the state and the cause of action.”
Ms. Adler recommended that doctors make it clear that they don’t agree with the changes. An attorney experienced in physician contracts can review the changes being made and the amended contracts being offered.
Thanks to recent federal changes, employers have to have some ways to continue paying physicians, Ms. Adler said. Medical practices with fewer than 500 employees can get loans from the federal government that would not have to be repaid if they met certain stipulations, such as hiring back all the employees they terminate, she said.
Mr. Claussen said physicians should resist the obvious dangers, such as a shift to a pure production salary, denying bonus payments for work already done, and forcing physicians you use up PTO days.
He also suggested persuading employers to postpone rather than eliminate payments. “Some employers have agreed to postpone payments until a date later in 2020 rather than eliminate them,” he said. “The aim is that the organization will be back on its feet at that time.”
Mr. Claussen said he is trying to limit the contract amendments to 1 or 2 months. Because the situation caused by the pandemic is so fluid, “this allows for flexibility,” he said. “We can revisit the situation and come up with different changes.”
Ms. Adler doubts employers would accept short-term changes with a definite end date because such changes would not be in the employer’s interest. But Mr. Claussen said one employer has agreed to reevaluate its contracts in June.
Both lawyers agreed that many employers are trying to work with their physicians. “In 90% of the cases I have seen, both sides cooperate,” Ms. Adler said. “Because of the situation, people are being much more conciliatory than they would have been.”
A version of this article originally appeared on Medscape.com.
Supreme Court: Government owes more than $12 billion to health plans
The federal government owes billions of dollars to health insurers under an Affordable Care Act provision intended to help insurers mitigate risk, the U.S. Supreme Court has ruled.
In an 8-to-1 vote announced April 27, justices sided with the plaintiff health plans in Maine Community Health Options v. United States, ruling that the risk corridors statute created a government obligation to pay insurers the full amount originally calculated and that appropriation measures later passed by Congress did not repeal this obligation.
“In establishing the temporary risk corridors program, Congress created a rare money-mandating obligation requiring the federal government to make payments under [Section 1342 of the Affordable Care Act’s] formula,” Associate Justice Sonia Sotomayor wrote in the majority opinion. “Lacking other statutory paths to relief ... petitioners may seek to collect payment through a damages action in the Court of Federal Claims.”
Maine Community Health Options v. United States, which consolidates several lawsuits against the government, centers on the ACA’s risk corridor program, which required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged by more than $12 billion all together.
The U.S. Department of Justice countered that the government is not required to pay the plans because of measures passed by Congress in 2014 and later years that limited the funding available to compensate insurers for their losses.
The U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
The U.S. Supreme Court disagreed. Justices noted that even after Congress enacted the first rider, HHS and the Centers for Medicare & Medicaid Services reiterated that the ACA requires the Secretary to make full payments to issuers and that “HHS [would] record risk corridors payments due as an obligation of the United States government for which full payment is required,” according to the Supreme Court opinion.
“They understood that profitable insurers’ payments to the government would not dispel the Secretary’s obligation to pay unprofitable insurers, even ‘in the event of a shortfall,’ ” Justice Sotomayor wrote in the majority opinion.
Associate Justice Samuel Alito Jr. however, took issue with his fellow justices’ decision. In his dissenting opinion, Justice Alito wrote that under the ruling, billions of taxpayer dollars will be turned over to insurance companies that bet unsuccessfully on the success of the program in question.
“This money will have to be paid even though Congress has pointedly declined to appropriate money for that purpose,” he wrote. “Not only will today’s decision have a massive immediate impact, its potential consequences go much further.”
The high court remanded the consolidated cases to the lower court for further proceedings on details of the disbursement.
The federal government owes billions of dollars to health insurers under an Affordable Care Act provision intended to help insurers mitigate risk, the U.S. Supreme Court has ruled.
In an 8-to-1 vote announced April 27, justices sided with the plaintiff health plans in Maine Community Health Options v. United States, ruling that the risk corridors statute created a government obligation to pay insurers the full amount originally calculated and that appropriation measures later passed by Congress did not repeal this obligation.
“In establishing the temporary risk corridors program, Congress created a rare money-mandating obligation requiring the federal government to make payments under [Section 1342 of the Affordable Care Act’s] formula,” Associate Justice Sonia Sotomayor wrote in the majority opinion. “Lacking other statutory paths to relief ... petitioners may seek to collect payment through a damages action in the Court of Federal Claims.”
Maine Community Health Options v. United States, which consolidates several lawsuits against the government, centers on the ACA’s risk corridor program, which required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged by more than $12 billion all together.
The U.S. Department of Justice countered that the government is not required to pay the plans because of measures passed by Congress in 2014 and later years that limited the funding available to compensate insurers for their losses.
The U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
The U.S. Supreme Court disagreed. Justices noted that even after Congress enacted the first rider, HHS and the Centers for Medicare & Medicaid Services reiterated that the ACA requires the Secretary to make full payments to issuers and that “HHS [would] record risk corridors payments due as an obligation of the United States government for which full payment is required,” according to the Supreme Court opinion.
“They understood that profitable insurers’ payments to the government would not dispel the Secretary’s obligation to pay unprofitable insurers, even ‘in the event of a shortfall,’ ” Justice Sotomayor wrote in the majority opinion.
Associate Justice Samuel Alito Jr. however, took issue with his fellow justices’ decision. In his dissenting opinion, Justice Alito wrote that under the ruling, billions of taxpayer dollars will be turned over to insurance companies that bet unsuccessfully on the success of the program in question.
“This money will have to be paid even though Congress has pointedly declined to appropriate money for that purpose,” he wrote. “Not only will today’s decision have a massive immediate impact, its potential consequences go much further.”
The high court remanded the consolidated cases to the lower court for further proceedings on details of the disbursement.
The federal government owes billions of dollars to health insurers under an Affordable Care Act provision intended to help insurers mitigate risk, the U.S. Supreme Court has ruled.
In an 8-to-1 vote announced April 27, justices sided with the plaintiff health plans in Maine Community Health Options v. United States, ruling that the risk corridors statute created a government obligation to pay insurers the full amount originally calculated and that appropriation measures later passed by Congress did not repeal this obligation.
“In establishing the temporary risk corridors program, Congress created a rare money-mandating obligation requiring the federal government to make payments under [Section 1342 of the Affordable Care Act’s] formula,” Associate Justice Sonia Sotomayor wrote in the majority opinion. “Lacking other statutory paths to relief ... petitioners may seek to collect payment through a damages action in the Court of Federal Claims.”
Maine Community Health Options v. United States, which consolidates several lawsuits against the government, centers on the ACA’s risk corridor program, which required the U.S. Department of Health & Human Services to collect funds from profitable insurers that offered qualified health plans under the exchanges and distribute the funds to insurers with excessive losses. Collections from profitable insurers under the program fell short in 2014, 2015, and 2016, while losses steadily grew, resulting in the HHS paying about 12 cents on the dollar in payments to insurers. More than 150 insurers now allege they were shortchanged by more than $12 billion all together.
The U.S. Department of Justice countered that the government is not required to pay the plans because of measures passed by Congress in 2014 and later years that limited the funding available to compensate insurers for their losses.
The U.S. Court of Appeals for the Federal Circuit decided in favor of the government, ruling that while the ACA required the government to compensate the insurers for their losses, the appropriations measures repealed or suspended that requirement.
The U.S. Supreme Court disagreed. Justices noted that even after Congress enacted the first rider, HHS and the Centers for Medicare & Medicaid Services reiterated that the ACA requires the Secretary to make full payments to issuers and that “HHS [would] record risk corridors payments due as an obligation of the United States government for which full payment is required,” according to the Supreme Court opinion.
“They understood that profitable insurers’ payments to the government would not dispel the Secretary’s obligation to pay unprofitable insurers, even ‘in the event of a shortfall,’ ” Justice Sotomayor wrote in the majority opinion.
Associate Justice Samuel Alito Jr. however, took issue with his fellow justices’ decision. In his dissenting opinion, Justice Alito wrote that under the ruling, billions of taxpayer dollars will be turned over to insurance companies that bet unsuccessfully on the success of the program in question.
“This money will have to be paid even though Congress has pointedly declined to appropriate money for that purpose,” he wrote. “Not only will today’s decision have a massive immediate impact, its potential consequences go much further.”
The high court remanded the consolidated cases to the lower court for further proceedings on details of the disbursement.
More doctors used digital tools in 2019
The use of digital tools among physicians has markedly risen since 2016, with telehealth visits and remote patient monitoring making the greatest strides in usage, an American Medical Association report shows.
In 2019, 28% of physicians used televisits/virtual visits, up from 14% in 2016, while remote monitoring and management for improved care rose to 22% in 2019, an increase from 13% in 2016, according to the AMA report, released in February 2020. The report, which surveyed 1,359 doctors, includes responses from 672 primary care physicians and 687 specialists.
Remote monitoring for efficiency, meanwhile, grew to 16% in 2019 from 12% in 2016. Remote monitoring for efficiency pertains to smart versions of common clinical devices such as thermometers, blood pressure cuffs, and scales that automatically enter readings in the record. Remote monitoring for improved care refers to mobile applications and devices used for daily measurement of vital signs such as weight, blood pressure, blood glucose.
Adoption of other digital tools by physicians have also grown, including clinical decision support, which climbed to 37% in 2019 from 28% in 2016 and patient engagement tools, which rose to 33% in 2019, up from 26% in 2016. Clinical decision support tools pertain to modules used in conjunction with the electronic health record (EHR), or mobile applications integrated with an EHR that can signify changes in patient data, such as weight gain/loss, or change in blood chemistry. Patient engagement tools, meanwhile, refer to solutions that promote patient wellness and active patient participation in their care.
Tools that encompass use of point of care/workflow enhancement increased to 47% in 2019, from 42% in 2016. This area includes communication and sharing of electronic clinical data to consult with specialists, make referrals and/or transitions of care. Tools that address consumer access to their clinical data, meanwhile, rose to 58% in 2019 from 53% in 2016, the highest adoption rate among the digital health tool categories.
Overall, more physicians see an advantage to digital health solutions than did 3 years ago. More primary care physicians and specialists in 2019 reported a “definite advantage” to digital tools enhancing care of patients than in 2016. Doctors who see no advantage to such tools are trending downward and are concentrated to those age 50 and older, according to the report.
Solo-practice physicians are slowly increasing their use of digital health tools. In 2016, solo physicians reported using an average of 1.5 digital tools, which in 2019 increased to an average of 2.2 digital tools. Small practices with between one and three doctors used an average of 1.4 tools in 2016, which rose to an average of 2.2 tools in 2019, the report found. PCPs used slightly more digital tools, compared with specialists, in both 2016 and 2019.
Female doctors are slightly ahead of their male counterparts when it comes to digital health tools. In 2019, female physicians used an average of 2.6 digital tools, up from 1.9 in 2016. Male doctors used an average of 2.4 tools in 2019, compared with 1.9 tools in 2016.
For the physicians surveyed, the most important factor associated with usage was that digital tools were covered by malpractice insurance, followed by the importance of data privacy/security ensured by the EHR vendor, and that the tools were well integrated with the EHR. Other important factors included that data security was ensured by the practice or hospital, that doctors were reimbursed for their time spent using digital tools, and that the tools were supported by the EHR vendor.
Regarding the top motivator for doctors to use digital tools, 51% of physicians in 2019 said improved efficiency was “very important,” up from 48% in 2016. Other top motivators included that digital tools increased safety, improved diagnostic ability, and addressed physician burnout.
In 2019, the demonstration of safety and efficacy in peer-reviewed publications as it relates to digital tools also grew in importance. Of the physicians surveyed, 36% reported that safety and efficacy demonstrated in peer-reviewed publications was “very important,” an increase from 32% in 2016. Other “very important” factors for physicians are that digital tools used are proven to be as good/superior to traditional care, that they are intuitive/require no special training, that they align with the standard of care, and that their safety and efficacy is validated by the Food and Drug Administration.
“The rise of the digital-native physician will have a profound impact on health care and patient outcomes, and will place digital health technologies under pressure to perform according to higher expectations,” AMA board chair Jesse M. Ehrenfeld, MD, PhD, said in a statement. “The AMA survey provides deep insight into the emerging requirements that physicians expect from digital technologies and sets an industry guidepost for understanding what a growing number of physicians require to adopt new technology.”
The survey was derived from the same physician panel used in 2016, provided by WebMD. For the 2019 survey, the basic 2016 survey was followed in wording and question order, with a few variations to remove some questions no longer relevant. The 2019 sample used careful quotas to ensure a sample composition similar to that of 2016, according to the report.
SOURCE: AMA Digital Health Research: Physicians’ motivations and requirements for adopting digital health – Adoption and attitudinal shifts from 2016 to 2019. American Medical Association. February 2020.
The use of digital tools among physicians has markedly risen since 2016, with telehealth visits and remote patient monitoring making the greatest strides in usage, an American Medical Association report shows.
In 2019, 28% of physicians used televisits/virtual visits, up from 14% in 2016, while remote monitoring and management for improved care rose to 22% in 2019, an increase from 13% in 2016, according to the AMA report, released in February 2020. The report, which surveyed 1,359 doctors, includes responses from 672 primary care physicians and 687 specialists.
Remote monitoring for efficiency, meanwhile, grew to 16% in 2019 from 12% in 2016. Remote monitoring for efficiency pertains to smart versions of common clinical devices such as thermometers, blood pressure cuffs, and scales that automatically enter readings in the record. Remote monitoring for improved care refers to mobile applications and devices used for daily measurement of vital signs such as weight, blood pressure, blood glucose.
Adoption of other digital tools by physicians have also grown, including clinical decision support, which climbed to 37% in 2019 from 28% in 2016 and patient engagement tools, which rose to 33% in 2019, up from 26% in 2016. Clinical decision support tools pertain to modules used in conjunction with the electronic health record (EHR), or mobile applications integrated with an EHR that can signify changes in patient data, such as weight gain/loss, or change in blood chemistry. Patient engagement tools, meanwhile, refer to solutions that promote patient wellness and active patient participation in their care.
Tools that encompass use of point of care/workflow enhancement increased to 47% in 2019, from 42% in 2016. This area includes communication and sharing of electronic clinical data to consult with specialists, make referrals and/or transitions of care. Tools that address consumer access to their clinical data, meanwhile, rose to 58% in 2019 from 53% in 2016, the highest adoption rate among the digital health tool categories.
Overall, more physicians see an advantage to digital health solutions than did 3 years ago. More primary care physicians and specialists in 2019 reported a “definite advantage” to digital tools enhancing care of patients than in 2016. Doctors who see no advantage to such tools are trending downward and are concentrated to those age 50 and older, according to the report.
Solo-practice physicians are slowly increasing their use of digital health tools. In 2016, solo physicians reported using an average of 1.5 digital tools, which in 2019 increased to an average of 2.2 digital tools. Small practices with between one and three doctors used an average of 1.4 tools in 2016, which rose to an average of 2.2 tools in 2019, the report found. PCPs used slightly more digital tools, compared with specialists, in both 2016 and 2019.
Female doctors are slightly ahead of their male counterparts when it comes to digital health tools. In 2019, female physicians used an average of 2.6 digital tools, up from 1.9 in 2016. Male doctors used an average of 2.4 tools in 2019, compared with 1.9 tools in 2016.
For the physicians surveyed, the most important factor associated with usage was that digital tools were covered by malpractice insurance, followed by the importance of data privacy/security ensured by the EHR vendor, and that the tools were well integrated with the EHR. Other important factors included that data security was ensured by the practice or hospital, that doctors were reimbursed for their time spent using digital tools, and that the tools were supported by the EHR vendor.
Regarding the top motivator for doctors to use digital tools, 51% of physicians in 2019 said improved efficiency was “very important,” up from 48% in 2016. Other top motivators included that digital tools increased safety, improved diagnostic ability, and addressed physician burnout.
In 2019, the demonstration of safety and efficacy in peer-reviewed publications as it relates to digital tools also grew in importance. Of the physicians surveyed, 36% reported that safety and efficacy demonstrated in peer-reviewed publications was “very important,” an increase from 32% in 2016. Other “very important” factors for physicians are that digital tools used are proven to be as good/superior to traditional care, that they are intuitive/require no special training, that they align with the standard of care, and that their safety and efficacy is validated by the Food and Drug Administration.
“The rise of the digital-native physician will have a profound impact on health care and patient outcomes, and will place digital health technologies under pressure to perform according to higher expectations,” AMA board chair Jesse M. Ehrenfeld, MD, PhD, said in a statement. “The AMA survey provides deep insight into the emerging requirements that physicians expect from digital technologies and sets an industry guidepost for understanding what a growing number of physicians require to adopt new technology.”
The survey was derived from the same physician panel used in 2016, provided by WebMD. For the 2019 survey, the basic 2016 survey was followed in wording and question order, with a few variations to remove some questions no longer relevant. The 2019 sample used careful quotas to ensure a sample composition similar to that of 2016, according to the report.
SOURCE: AMA Digital Health Research: Physicians’ motivations and requirements for adopting digital health – Adoption and attitudinal shifts from 2016 to 2019. American Medical Association. February 2020.
The use of digital tools among physicians has markedly risen since 2016, with telehealth visits and remote patient monitoring making the greatest strides in usage, an American Medical Association report shows.
In 2019, 28% of physicians used televisits/virtual visits, up from 14% in 2016, while remote monitoring and management for improved care rose to 22% in 2019, an increase from 13% in 2016, according to the AMA report, released in February 2020. The report, which surveyed 1,359 doctors, includes responses from 672 primary care physicians and 687 specialists.
Remote monitoring for efficiency, meanwhile, grew to 16% in 2019 from 12% in 2016. Remote monitoring for efficiency pertains to smart versions of common clinical devices such as thermometers, blood pressure cuffs, and scales that automatically enter readings in the record. Remote monitoring for improved care refers to mobile applications and devices used for daily measurement of vital signs such as weight, blood pressure, blood glucose.
Adoption of other digital tools by physicians have also grown, including clinical decision support, which climbed to 37% in 2019 from 28% in 2016 and patient engagement tools, which rose to 33% in 2019, up from 26% in 2016. Clinical decision support tools pertain to modules used in conjunction with the electronic health record (EHR), or mobile applications integrated with an EHR that can signify changes in patient data, such as weight gain/loss, or change in blood chemistry. Patient engagement tools, meanwhile, refer to solutions that promote patient wellness and active patient participation in their care.
Tools that encompass use of point of care/workflow enhancement increased to 47% in 2019, from 42% in 2016. This area includes communication and sharing of electronic clinical data to consult with specialists, make referrals and/or transitions of care. Tools that address consumer access to their clinical data, meanwhile, rose to 58% in 2019 from 53% in 2016, the highest adoption rate among the digital health tool categories.
Overall, more physicians see an advantage to digital health solutions than did 3 years ago. More primary care physicians and specialists in 2019 reported a “definite advantage” to digital tools enhancing care of patients than in 2016. Doctors who see no advantage to such tools are trending downward and are concentrated to those age 50 and older, according to the report.
Solo-practice physicians are slowly increasing their use of digital health tools. In 2016, solo physicians reported using an average of 1.5 digital tools, which in 2019 increased to an average of 2.2 digital tools. Small practices with between one and three doctors used an average of 1.4 tools in 2016, which rose to an average of 2.2 tools in 2019, the report found. PCPs used slightly more digital tools, compared with specialists, in both 2016 and 2019.
Female doctors are slightly ahead of their male counterparts when it comes to digital health tools. In 2019, female physicians used an average of 2.6 digital tools, up from 1.9 in 2016. Male doctors used an average of 2.4 tools in 2019, compared with 1.9 tools in 2016.
For the physicians surveyed, the most important factor associated with usage was that digital tools were covered by malpractice insurance, followed by the importance of data privacy/security ensured by the EHR vendor, and that the tools were well integrated with the EHR. Other important factors included that data security was ensured by the practice or hospital, that doctors were reimbursed for their time spent using digital tools, and that the tools were supported by the EHR vendor.
Regarding the top motivator for doctors to use digital tools, 51% of physicians in 2019 said improved efficiency was “very important,” up from 48% in 2016. Other top motivators included that digital tools increased safety, improved diagnostic ability, and addressed physician burnout.
In 2019, the demonstration of safety and efficacy in peer-reviewed publications as it relates to digital tools also grew in importance. Of the physicians surveyed, 36% reported that safety and efficacy demonstrated in peer-reviewed publications was “very important,” an increase from 32% in 2016. Other “very important” factors for physicians are that digital tools used are proven to be as good/superior to traditional care, that they are intuitive/require no special training, that they align with the standard of care, and that their safety and efficacy is validated by the Food and Drug Administration.
“The rise of the digital-native physician will have a profound impact on health care and patient outcomes, and will place digital health technologies under pressure to perform according to higher expectations,” AMA board chair Jesse M. Ehrenfeld, MD, PhD, said in a statement. “The AMA survey provides deep insight into the emerging requirements that physicians expect from digital technologies and sets an industry guidepost for understanding what a growing number of physicians require to adopt new technology.”
The survey was derived from the same physician panel used in 2016, provided by WebMD. For the 2019 survey, the basic 2016 survey was followed in wording and question order, with a few variations to remove some questions no longer relevant. The 2019 sample used careful quotas to ensure a sample composition similar to that of 2016, according to the report.
SOURCE: AMA Digital Health Research: Physicians’ motivations and requirements for adopting digital health – Adoption and attitudinal shifts from 2016 to 2019. American Medical Association. February 2020.



