User login
T3 levels are higher in combatants with PTSD
CHICAGO – Higher levels of triiodothyronine (T3) were seen in combatants with PTSD, compared with patients whose PTSD arose from other adverse experiences, according to findings from a systematic review and meta-analysis.
who had experienced childhood or sexual abuse or were a wartime refugee without PTSD (FT3, 0.36 pg/mL higher, and total T3, 31.6 ng/mL higher, respectively; P = .0004 and P less than .00001).
“We found statistically higher free T3 and total T3 levels in patients with [combat-related] PTSD, compared with controls,” said Freddy J.K. Toloza, MD, in an interview during a poster session of the annual meeting of the American Thyroid Association.
However, he noted that there were no overall differences in thyroid-stimulating hormone, free tetraiodothyronine (T4), and total T4 levels between individuals with PTSD and the non-PTSD control participants. In addition, though free and total T3 levels were significantly higher for the overall PTSD cohort than for control participants, the differences were driven by the studies that included combat-exposed individuals.
Dr. Toloza and colleagues included 10 observational studies in their final review and meta-analysis. Five studies looked at war veterans; the others examined individuals who had experienced child abuse or sexual abuse, who were refugees, or who were from the general population.
For inclusion, the studies had to report both mean values and standard deviations for standard thyroid-hormone test values in patients with PTSD, compared with a non-PTSD control group. These included 373 patients with PTSD and 301 control participants. Just under half (47%) were women. None of the studies, wrote the investigators, “compared rates of overt/subclinical thyroid disease between groups.”
There are known links between many endocrine disorders and psychiatric conditions, said Dr. Toloza, but the interplay between disordered thyroid function and neuropsychiatric problems is still being examined. Looking at PTSD is important because it’s estimated that 6%-9% of the U.S. adult population has experienced PTSD over the course of a lifetime.
Levels of thyroid hormones in the systematic review and meta-analysis were still within normal range for the participants with PTSD, acknowledged Dr. Toloza, a research fellow in the division of endocrinology and metabolism at University of Arkansas for Medical Sciences, Little Rock.
However, even though there was no sign of frank thyroid disease in the PTSD population, the elevated T3 levels seen in the analysis are consistent with other studies showing a correlation between higher T3 levels and more-severe PTSD.
It is not known exactly why significant increases in the levels of total and free T3 were seen only in the combat-exposed PTSD population, Dr. Toloza said. “The type of trauma trigger may influence the adaptive responses to stress and might result in diverse thyroid alterations.”
Elevated catecholamine levels, seen in individuals with PTSD, can increase peripheral conversion of T4 to T3, explained Dr. Toloza. Ongoing catecholamine elevation may account for the isolated elevation in T3 levels in the PTSD population. Beta1-adrenergic blockade is an accepted pharmacologic strategy to help alleviate PTSD symptoms.
Dr. Toloza and coinvestigators did not have access to data that would have allowed them to ascertain what types of injuries were sustained by individuals with combat-related PTSD, but he noted in response to a question, that it would be worthwhile to see whether combatants who were blast exposed had different thyroid hormone values than those who were not, because hypothalamic injury is common in blast. This is a future direction Dr. Toloza wishes to pursue.
“Our findings add to the growing literature suggesting that thyroid function changes may be associated with PTSD,” the investigators wrote, but “further research is needed to ascertain the role of thyroid function alterations in PTSD.”
Dr. Toloza reported no financial disclosures or conflicts of interest.
CHICAGO – Higher levels of triiodothyronine (T3) were seen in combatants with PTSD, compared with patients whose PTSD arose from other adverse experiences, according to findings from a systematic review and meta-analysis.
who had experienced childhood or sexual abuse or were a wartime refugee without PTSD (FT3, 0.36 pg/mL higher, and total T3, 31.6 ng/mL higher, respectively; P = .0004 and P less than .00001).
“We found statistically higher free T3 and total T3 levels in patients with [combat-related] PTSD, compared with controls,” said Freddy J.K. Toloza, MD, in an interview during a poster session of the annual meeting of the American Thyroid Association.
However, he noted that there were no overall differences in thyroid-stimulating hormone, free tetraiodothyronine (T4), and total T4 levels between individuals with PTSD and the non-PTSD control participants. In addition, though free and total T3 levels were significantly higher for the overall PTSD cohort than for control participants, the differences were driven by the studies that included combat-exposed individuals.
Dr. Toloza and colleagues included 10 observational studies in their final review and meta-analysis. Five studies looked at war veterans; the others examined individuals who had experienced child abuse or sexual abuse, who were refugees, or who were from the general population.
For inclusion, the studies had to report both mean values and standard deviations for standard thyroid-hormone test values in patients with PTSD, compared with a non-PTSD control group. These included 373 patients with PTSD and 301 control participants. Just under half (47%) were women. None of the studies, wrote the investigators, “compared rates of overt/subclinical thyroid disease between groups.”
There are known links between many endocrine disorders and psychiatric conditions, said Dr. Toloza, but the interplay between disordered thyroid function and neuropsychiatric problems is still being examined. Looking at PTSD is important because it’s estimated that 6%-9% of the U.S. adult population has experienced PTSD over the course of a lifetime.
Levels of thyroid hormones in the systematic review and meta-analysis were still within normal range for the participants with PTSD, acknowledged Dr. Toloza, a research fellow in the division of endocrinology and metabolism at University of Arkansas for Medical Sciences, Little Rock.
However, even though there was no sign of frank thyroid disease in the PTSD population, the elevated T3 levels seen in the analysis are consistent with other studies showing a correlation between higher T3 levels and more-severe PTSD.
It is not known exactly why significant increases in the levels of total and free T3 were seen only in the combat-exposed PTSD population, Dr. Toloza said. “The type of trauma trigger may influence the adaptive responses to stress and might result in diverse thyroid alterations.”
Elevated catecholamine levels, seen in individuals with PTSD, can increase peripheral conversion of T4 to T3, explained Dr. Toloza. Ongoing catecholamine elevation may account for the isolated elevation in T3 levels in the PTSD population. Beta1-adrenergic blockade is an accepted pharmacologic strategy to help alleviate PTSD symptoms.
Dr. Toloza and coinvestigators did not have access to data that would have allowed them to ascertain what types of injuries were sustained by individuals with combat-related PTSD, but he noted in response to a question, that it would be worthwhile to see whether combatants who were blast exposed had different thyroid hormone values than those who were not, because hypothalamic injury is common in blast. This is a future direction Dr. Toloza wishes to pursue.
“Our findings add to the growing literature suggesting that thyroid function changes may be associated with PTSD,” the investigators wrote, but “further research is needed to ascertain the role of thyroid function alterations in PTSD.”
Dr. Toloza reported no financial disclosures or conflicts of interest.
CHICAGO – Higher levels of triiodothyronine (T3) were seen in combatants with PTSD, compared with patients whose PTSD arose from other adverse experiences, according to findings from a systematic review and meta-analysis.
who had experienced childhood or sexual abuse or were a wartime refugee without PTSD (FT3, 0.36 pg/mL higher, and total T3, 31.6 ng/mL higher, respectively; P = .0004 and P less than .00001).
“We found statistically higher free T3 and total T3 levels in patients with [combat-related] PTSD, compared with controls,” said Freddy J.K. Toloza, MD, in an interview during a poster session of the annual meeting of the American Thyroid Association.
However, he noted that there were no overall differences in thyroid-stimulating hormone, free tetraiodothyronine (T4), and total T4 levels between individuals with PTSD and the non-PTSD control participants. In addition, though free and total T3 levels were significantly higher for the overall PTSD cohort than for control participants, the differences were driven by the studies that included combat-exposed individuals.
Dr. Toloza and colleagues included 10 observational studies in their final review and meta-analysis. Five studies looked at war veterans; the others examined individuals who had experienced child abuse or sexual abuse, who were refugees, or who were from the general population.
For inclusion, the studies had to report both mean values and standard deviations for standard thyroid-hormone test values in patients with PTSD, compared with a non-PTSD control group. These included 373 patients with PTSD and 301 control participants. Just under half (47%) were women. None of the studies, wrote the investigators, “compared rates of overt/subclinical thyroid disease between groups.”
There are known links between many endocrine disorders and psychiatric conditions, said Dr. Toloza, but the interplay between disordered thyroid function and neuropsychiatric problems is still being examined. Looking at PTSD is important because it’s estimated that 6%-9% of the U.S. adult population has experienced PTSD over the course of a lifetime.
Levels of thyroid hormones in the systematic review and meta-analysis were still within normal range for the participants with PTSD, acknowledged Dr. Toloza, a research fellow in the division of endocrinology and metabolism at University of Arkansas for Medical Sciences, Little Rock.
However, even though there was no sign of frank thyroid disease in the PTSD population, the elevated T3 levels seen in the analysis are consistent with other studies showing a correlation between higher T3 levels and more-severe PTSD.
It is not known exactly why significant increases in the levels of total and free T3 were seen only in the combat-exposed PTSD population, Dr. Toloza said. “The type of trauma trigger may influence the adaptive responses to stress and might result in diverse thyroid alterations.”
Elevated catecholamine levels, seen in individuals with PTSD, can increase peripheral conversion of T4 to T3, explained Dr. Toloza. Ongoing catecholamine elevation may account for the isolated elevation in T3 levels in the PTSD population. Beta1-adrenergic blockade is an accepted pharmacologic strategy to help alleviate PTSD symptoms.
Dr. Toloza and coinvestigators did not have access to data that would have allowed them to ascertain what types of injuries were sustained by individuals with combat-related PTSD, but he noted in response to a question, that it would be worthwhile to see whether combatants who were blast exposed had different thyroid hormone values than those who were not, because hypothalamic injury is common in blast. This is a future direction Dr. Toloza wishes to pursue.
“Our findings add to the growing literature suggesting that thyroid function changes may be associated with PTSD,” the investigators wrote, but “further research is needed to ascertain the role of thyroid function alterations in PTSD.”
Dr. Toloza reported no financial disclosures or conflicts of interest.
REPORTING FROM ATA 2019
Hypothyroidism may have more impact on cardiac health than hyperthyroidism
ORLANDO – Thyroid disorders can have significant effects on the heart and the cardiovascular system, Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, said at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
Even subclinical hypothyroidism “can be really impactful,” said Ms. Kessler, the founder of Metabolic Medicine Associates in King George, Va.
Thyroid function should be evaluated in patients with a fast resting heart fate, new-onset atrial fibrillation (AFib), idiopathic heart failure, bradycardia, using amiodarone, resistant hypertension, pericardial effusion, and statin-resistant hyperlipidemia, said Ms. Kessler, who also is a nurse practitioner and researcher. Other patients who should be evaluated: those older than 60 years, with a family history of autoimmune disease, fertility issues, new-onset anxiety/depression, and patients with high-risk pregnancy. Hypothyroidism may have more impact on cardiac health than does hyperthyroidism.
Levels of thyroid-stimulating hormone (TSH), triiodothyronine (T3), and free thyroxine (FT4) are typically used to evaluate thyroid function. High TSH levels are usually indicative of hypothyroidism if FT4 and T3 are low; hyperthyroidism is likely the diagnosis if TSH is low while FT4 and T3 are high. Subclinical hypothyroidism is characterized by high TSH and normal FT4 and T3 levels; subclinical hyperthyroidism is associated with low TSH with normal FT4 and T3 levels.*
Hypothyroidism can cause increased diastolic hypertension and systemic vascular resistance, elevated levels of C-reactive protein (CRP) and homocysteine, decreased myocardial contractility, decreased cardiac output, reduced heart rate, and liver function abnormalities. Most commonly, it is caused by the autoimmune disease Hashimoto’s hypothyroidism but also can result from radiation, thyroidectomy, nontoxic multinodular goiter, and drugs with antithyroid activity such as birth control medications that contain estrogen. Hypothyroidism also raises the risk of coronary artery disease through lipid aberrations such as increased LDL level and decreased number of LDL cholesterol receptors. Myocardial contractility from hypothyroidism also increases the risk of mortality in patients with heart disease and heart failure. Clinicians also should take special precautions with narcotics and anesthesia when caring for patients with hypothyroidism because of the risk of bradycardia, Ms. Kessler said.
In subclinical hypothyroidism, clinicians should treat with half the recommended dose of levothyroxine in patients aged 45-65 years and who have high levels of lipids and CRP. “At the age of 70, I don’t worry if you’re subclinical unless [the patient is] profoundly, profoundly symptomatic,” she said. In overt hypotension, the main concern is an increased risk of ischemic heart disease that can result from overtreatment, and these patients usually are started on a low dose of levothyroxine with escalated doses until the patient achieves a euthyroid state.
Hyperthyroidism is most commonly caused by Grave’s disease, with 85% of those affected younger than age 40 years. Other causes include toxic multinodular goiter, toxic adenoma, TSH-producing pituitary adenomas, resistance to thyroid hormone, thyroiditis, excessive ingestion of iodine, shrinking of the thyroid gland, and a human chorionic gonadotropin–producing tumor such as struma ovarii. Common cardiac complications of hyperthyroidism are increased heart rate and blood pressure, reduced systemic vascular resistance, and increased cardiovascular disease and mortality in addition to increased cardiac hypertrophy, pulmonary hypertension, and heart failure. Heart rhythm, atrial fibrillation, atrial flutter, increased sinus tachycardia, and increased angina are all possible complications of hyperthyroidism.
Treatment priorities for hyperthyroidism are the immediately relieving any symptoms, reducing thyroid hormone production, and blocking the conversion of T4 to T3. For symptomatic patients, beta-blockers will help relieve symptoms and block the T4/T3 conversion. Follow-up treatment should include antithyroid drugs such as methimazole or propylthiouracil.
Patients who have subclinical hyperthyroidism are usually asymptomatic and may not always require treatment.
“In subclinical hypothyroidism, keep your mitts off the older patients. They’re usually going to do better, and you don’t want to throw them into hyperthyroidism,” she said. “If they’re [experiencing] subclinical hyperthyroidism, you’re going to treat them, because if not, they’re going to go into AFib, cardiovascular death, [and] they’re going to have osteoporosis. It’s not a good thing.”
Ms. Kessler reports being on the speakers bureau and an adviser for Novo Nordisk on obesity, and an adviser for them on type 2 diabetes, as well. She also reports being the study chairperson for the Florajen Patient Trial Program. The Cardiovascular & Respiratory Summit is part of Global Academy for Medical Education. Global Academy for Medical Education and this news organization are owned by the same parent company.
Correction, 7/31/19: An earlier version of this article misstated the definition of subclinical hypothyroidism.
ORLANDO – Thyroid disorders can have significant effects on the heart and the cardiovascular system, Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, said at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
Even subclinical hypothyroidism “can be really impactful,” said Ms. Kessler, the founder of Metabolic Medicine Associates in King George, Va.
Thyroid function should be evaluated in patients with a fast resting heart fate, new-onset atrial fibrillation (AFib), idiopathic heart failure, bradycardia, using amiodarone, resistant hypertension, pericardial effusion, and statin-resistant hyperlipidemia, said Ms. Kessler, who also is a nurse practitioner and researcher. Other patients who should be evaluated: those older than 60 years, with a family history of autoimmune disease, fertility issues, new-onset anxiety/depression, and patients with high-risk pregnancy. Hypothyroidism may have more impact on cardiac health than does hyperthyroidism.
Levels of thyroid-stimulating hormone (TSH), triiodothyronine (T3), and free thyroxine (FT4) are typically used to evaluate thyroid function. High TSH levels are usually indicative of hypothyroidism if FT4 and T3 are low; hyperthyroidism is likely the diagnosis if TSH is low while FT4 and T3 are high. Subclinical hypothyroidism is characterized by high TSH and normal FT4 and T3 levels; subclinical hyperthyroidism is associated with low TSH with normal FT4 and T3 levels.*
Hypothyroidism can cause increased diastolic hypertension and systemic vascular resistance, elevated levels of C-reactive protein (CRP) and homocysteine, decreased myocardial contractility, decreased cardiac output, reduced heart rate, and liver function abnormalities. Most commonly, it is caused by the autoimmune disease Hashimoto’s hypothyroidism but also can result from radiation, thyroidectomy, nontoxic multinodular goiter, and drugs with antithyroid activity such as birth control medications that contain estrogen. Hypothyroidism also raises the risk of coronary artery disease through lipid aberrations such as increased LDL level and decreased number of LDL cholesterol receptors. Myocardial contractility from hypothyroidism also increases the risk of mortality in patients with heart disease and heart failure. Clinicians also should take special precautions with narcotics and anesthesia when caring for patients with hypothyroidism because of the risk of bradycardia, Ms. Kessler said.
In subclinical hypothyroidism, clinicians should treat with half the recommended dose of levothyroxine in patients aged 45-65 years and who have high levels of lipids and CRP. “At the age of 70, I don’t worry if you’re subclinical unless [the patient is] profoundly, profoundly symptomatic,” she said. In overt hypotension, the main concern is an increased risk of ischemic heart disease that can result from overtreatment, and these patients usually are started on a low dose of levothyroxine with escalated doses until the patient achieves a euthyroid state.
Hyperthyroidism is most commonly caused by Grave’s disease, with 85% of those affected younger than age 40 years. Other causes include toxic multinodular goiter, toxic adenoma, TSH-producing pituitary adenomas, resistance to thyroid hormone, thyroiditis, excessive ingestion of iodine, shrinking of the thyroid gland, and a human chorionic gonadotropin–producing tumor such as struma ovarii. Common cardiac complications of hyperthyroidism are increased heart rate and blood pressure, reduced systemic vascular resistance, and increased cardiovascular disease and mortality in addition to increased cardiac hypertrophy, pulmonary hypertension, and heart failure. Heart rhythm, atrial fibrillation, atrial flutter, increased sinus tachycardia, and increased angina are all possible complications of hyperthyroidism.
Treatment priorities for hyperthyroidism are the immediately relieving any symptoms, reducing thyroid hormone production, and blocking the conversion of T4 to T3. For symptomatic patients, beta-blockers will help relieve symptoms and block the T4/T3 conversion. Follow-up treatment should include antithyroid drugs such as methimazole or propylthiouracil.
Patients who have subclinical hyperthyroidism are usually asymptomatic and may not always require treatment.
“In subclinical hypothyroidism, keep your mitts off the older patients. They’re usually going to do better, and you don’t want to throw them into hyperthyroidism,” she said. “If they’re [experiencing] subclinical hyperthyroidism, you’re going to treat them, because if not, they’re going to go into AFib, cardiovascular death, [and] they’re going to have osteoporosis. It’s not a good thing.”
Ms. Kessler reports being on the speakers bureau and an adviser for Novo Nordisk on obesity, and an adviser for them on type 2 diabetes, as well. She also reports being the study chairperson for the Florajen Patient Trial Program. The Cardiovascular & Respiratory Summit is part of Global Academy for Medical Education. Global Academy for Medical Education and this news organization are owned by the same parent company.
Correction, 7/31/19: An earlier version of this article misstated the definition of subclinical hypothyroidism.
ORLANDO – Thyroid disorders can have significant effects on the heart and the cardiovascular system, Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, said at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
Even subclinical hypothyroidism “can be really impactful,” said Ms. Kessler, the founder of Metabolic Medicine Associates in King George, Va.
Thyroid function should be evaluated in patients with a fast resting heart fate, new-onset atrial fibrillation (AFib), idiopathic heart failure, bradycardia, using amiodarone, resistant hypertension, pericardial effusion, and statin-resistant hyperlipidemia, said Ms. Kessler, who also is a nurse practitioner and researcher. Other patients who should be evaluated: those older than 60 years, with a family history of autoimmune disease, fertility issues, new-onset anxiety/depression, and patients with high-risk pregnancy. Hypothyroidism may have more impact on cardiac health than does hyperthyroidism.
Levels of thyroid-stimulating hormone (TSH), triiodothyronine (T3), and free thyroxine (FT4) are typically used to evaluate thyroid function. High TSH levels are usually indicative of hypothyroidism if FT4 and T3 are low; hyperthyroidism is likely the diagnosis if TSH is low while FT4 and T3 are high. Subclinical hypothyroidism is characterized by high TSH and normal FT4 and T3 levels; subclinical hyperthyroidism is associated with low TSH with normal FT4 and T3 levels.*
Hypothyroidism can cause increased diastolic hypertension and systemic vascular resistance, elevated levels of C-reactive protein (CRP) and homocysteine, decreased myocardial contractility, decreased cardiac output, reduced heart rate, and liver function abnormalities. Most commonly, it is caused by the autoimmune disease Hashimoto’s hypothyroidism but also can result from radiation, thyroidectomy, nontoxic multinodular goiter, and drugs with antithyroid activity such as birth control medications that contain estrogen. Hypothyroidism also raises the risk of coronary artery disease through lipid aberrations such as increased LDL level and decreased number of LDL cholesterol receptors. Myocardial contractility from hypothyroidism also increases the risk of mortality in patients with heart disease and heart failure. Clinicians also should take special precautions with narcotics and anesthesia when caring for patients with hypothyroidism because of the risk of bradycardia, Ms. Kessler said.
In subclinical hypothyroidism, clinicians should treat with half the recommended dose of levothyroxine in patients aged 45-65 years and who have high levels of lipids and CRP. “At the age of 70, I don’t worry if you’re subclinical unless [the patient is] profoundly, profoundly symptomatic,” she said. In overt hypotension, the main concern is an increased risk of ischemic heart disease that can result from overtreatment, and these patients usually are started on a low dose of levothyroxine with escalated doses until the patient achieves a euthyroid state.
Hyperthyroidism is most commonly caused by Grave’s disease, with 85% of those affected younger than age 40 years. Other causes include toxic multinodular goiter, toxic adenoma, TSH-producing pituitary adenomas, resistance to thyroid hormone, thyroiditis, excessive ingestion of iodine, shrinking of the thyroid gland, and a human chorionic gonadotropin–producing tumor such as struma ovarii. Common cardiac complications of hyperthyroidism are increased heart rate and blood pressure, reduced systemic vascular resistance, and increased cardiovascular disease and mortality in addition to increased cardiac hypertrophy, pulmonary hypertension, and heart failure. Heart rhythm, atrial fibrillation, atrial flutter, increased sinus tachycardia, and increased angina are all possible complications of hyperthyroidism.
Treatment priorities for hyperthyroidism are the immediately relieving any symptoms, reducing thyroid hormone production, and blocking the conversion of T4 to T3. For symptomatic patients, beta-blockers will help relieve symptoms and block the T4/T3 conversion. Follow-up treatment should include antithyroid drugs such as methimazole or propylthiouracil.
Patients who have subclinical hyperthyroidism are usually asymptomatic and may not always require treatment.
“In subclinical hypothyroidism, keep your mitts off the older patients. They’re usually going to do better, and you don’t want to throw them into hyperthyroidism,” she said. “If they’re [experiencing] subclinical hyperthyroidism, you’re going to treat them, because if not, they’re going to go into AFib, cardiovascular death, [and] they’re going to have osteoporosis. It’s not a good thing.”
Ms. Kessler reports being on the speakers bureau and an adviser for Novo Nordisk on obesity, and an adviser for them on type 2 diabetes, as well. She also reports being the study chairperson for the Florajen Patient Trial Program. The Cardiovascular & Respiratory Summit is part of Global Academy for Medical Education. Global Academy for Medical Education and this news organization are owned by the same parent company.
Correction, 7/31/19: An earlier version of this article misstated the definition of subclinical hypothyroidism.
EXPERT ANALYSIS FROM CARPS 2019
The costs and benefits of SGLT2 inhibitors & GLP-1 RAs
The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
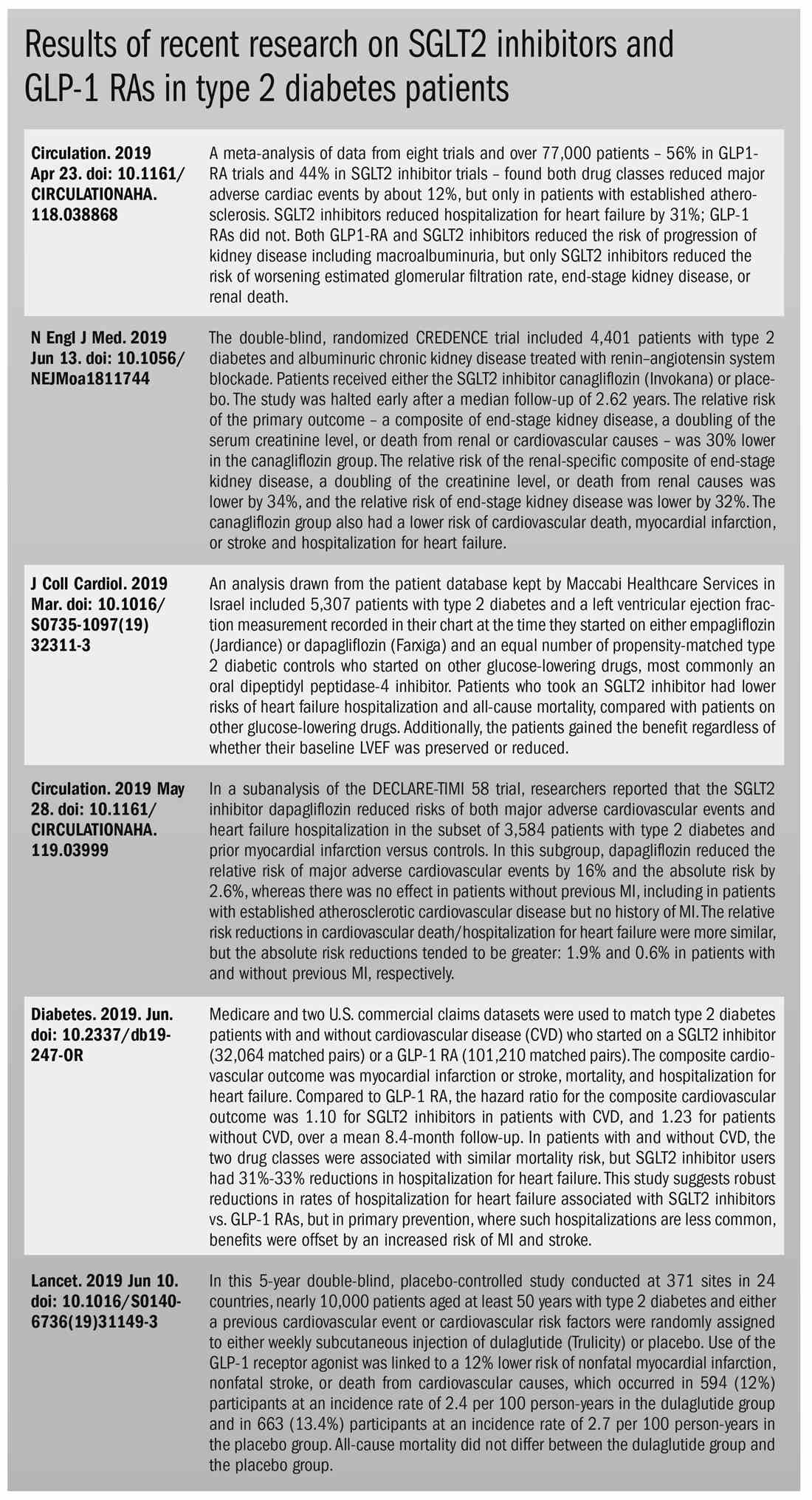
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.
The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.

The options for treating type 2 diabetes without insulin have grown beyond metformin to include a long list of sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists that can be taken with or without metformin. These new drugs have cardiovascular and kidney benefits and help with weight loss, but they also carry risks and, according to some experts, their costs can be prohibitively expensive.
Given the medical community’s long-term experience with treating patients with metformin, and metformin’s lower cost, most of the physicians interviewed for this article advise using SGLT2 inhibitors and GLP-1 receptor agonists as second-line treatments. Others said that they would prefer to use the newer drugs as first-line therapies in select high-risk patients, but prior authorization hurdles created by insurance companies make that approach too burdensome.
“The economics of U.S. health care is stacked against many of our patients with diabetes in the current era,” Robert H. Hopkins Jr., MD, said in an interview.
Even when their insurance approves the drugs, patients still may not be able to afford the copay, explained Dr. Hopkins, professor of internal medicine and pediatrics and director of the division of general internal medicine at the University of Arkansas for Medical Sciences, Little Rock. “Sometimes patients can purchase drugs at a lower cost than the copay to purchase with the ‘drug coverage’ in their insurance plan – unfortunately, this is not the case with the newer diabetes medications we are discussing here.”
“SGLT2 inhibitors and GLP-1 agonists can cost several hundred dollars a month, and insurers often balk at paying for them. They’ll say, ‘Have you tried metformin?’ ” explained endocrinologist Victor Lawrence Roberts, MD, in a interview. “We have to work with insurance companies the best we can in a stepwise fashion.”
According to Dr. Roberts, 80% of his patients with diabetes struggle with the cost of medicine in general. “They’re either underinsured or not insured or their formulary is limited.
Douglas S. Paauw, MD, agreed in an interview that the newer drugs can be problematic on the insurance front.
“For some patients they aren’t affordable, especially for the uninsured if you can’t get them on an assistance program,” said Dr. Paauw, who is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the university.
Dr. Hopkins, who is on the Internal Medicine News board, noted that “unfortunately, the treatment of type 2 diabetes in patients who cannot achieve control with metformin, diet, weight control, and exercise is a story of the ‘haves’ and the ‘have nots.’ The ‘haves’ are those who have pharmacy benefits which make access to newer agents like SGLT2 inhibitors and GLP-1 agonists a possibility.”
“I have had very few of the ‘have nots’ who have been able to even consider these newer agents, which carry price tags of $600-$1,300 a month even with the availability of discounting coupons in the marketplace,” he added. “Most of these patients end up requiring a sulfonylurea or TZD [thiazolidinedione] as a second agent to achieve glycemic control. This makes it very difficult to achieve sufficient weight and metabolic control to avoid an eventual switch to insulin.”
Fatima Z. Syed, MD, an endocrine-trained general internist at DukeHealth in Durham, N.C., said she prescribes SGLT2 inhibitors and GLP-1 receptor agonists in combination with metformin. “I prescribe them frequently, but they are not first-line treatments,” she explained.
“Nothing replaces diet and exercise” as therapy for patients with type 2 diabetes, she added.
Neil S. Skolnik, MD, said that insurance companies were not preventing patients from using these drugs in his experience. He also provided an optimistic take on the accessibility of these drugs in the near future.
“Most insurance companies are now covering select SGLT2 inhibitors and GLP-1 receptor agonists for appropriate patients and those companies that currently do not will soon have to,” said Dr. Skolnik, who is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
“The outcomes [associated with use of the new drugs] are robust, the benefits are large, and are well worth the cost,” he added.
The side effects
While others praised these drugs for their beneficial effects, they also noted that the side effects of these drugs are serious and must be discussed with patients.
GLP-1 receptor agonists are linked to gastrointestinal symptoms, especially nausea, while SGLT2 inhibitors have been linked to kidney failure, ketoacidosis, and more. The Food and Drug Administration warned in 2018 that the SGLT2 inhibitors can cause a rare serious infection known as Fournier’s gangrene – necrotizing fasciitis of the perineum.
“We have to tell our patients to let us know right away if they get pain or swelling in the genital area,” Dr. Paauw, who is on the Internal Medicine News board, noted. “The chance that an infection could explode quickly is higher in those who take these drugs.”
Amputation risks also are associated with taking the SGLT2 inhibitor canagliflozin (Invokana). The FDA requires the manufacturer of this drug to include a black-box warning about the risk of “lower-limb amputations, most frequently of the toe and midfoot,” but also the leg. In approval trials, the risk doubled versus placebo.
These amputation risks “put a damper on some of the enthusiasm on behalf of physicians and patients ... for taking this drug,” noted Dr. Roberts, who is a professor of internal medicine at the University of Central Florida, Orlando.
While a manufacturer-funded study released last year found no link to amputations, the results weren’t powerful enough to rule out a moderately increased risk.
“[If] you are at high risk for having an amputation, we really have to take this risk very seriously,” said John B. Buse, MD, chief of the division of endocrinology at the University of North Carolina at Chapel Hill, in a presentation about the study at the 2018 annual scientific sessions of the American Diabetes Association.
The benefits
Despite these risks of adverse events, most interviewed agreed that the many benefits observed in those taking SGLT2 inhibitors or GLP-1 receptor agonists make them worth prescribing, at least to those who are able to afford them.
Both SGLT2 inhibitors and GLP-1 receptor agonists appear to have significant cardiovascular benefits. A 2019 meta-analysis and systematic review found that both drugs reduced major adverse cardiac events by about 12% (Circulation. 2019 Apr 23;139[17]:2022-31).
“They don’t cause hypoglycemia, they lower blood pressure, they don’t cause weight gain, and they might promote weight loss,” noted Dr. Paauw.
SGLT2 inhibitors also have shown signs of kidney benefits. The CREDENCE trial linked canagliflozin to a lowering of kidney disorders versus placebo (N Engl J Med. 2019 Jun 13;380[24]:2295-306). “The relative risk of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes was lower by 34% (hazard ratio, 0.66; 95% confidence interval, 0.53-0.81; P less than .001), and the relative risk of end-stage kidney disease was lower by 32% (HR, 0.68; 95% CI, 0.54-0.86; P = .002),” the trial investigators wrote.
“They showed very nicely that the drug improved the kidney function of those patients and reduced the kidney deterioration,” said Yehuda Handelsman, MD, an endocrinologist in Tarzana, Calif., who chaired the 2011 and 2015 American Association of Clinical Endocrinologists’ Comprehensive Diabetes Guidelines. The study was especially impressive, he added, because it included patients with low kidney function.
SGLT2 inhibitors’ “diuretic mechanism explains why there is a substantial reduction in heart failure hospitalizations in patients who take these drugs,” said cardiologist Marc E. Goldschmidt, MD, director of the Heart Success Program at Atlantic Health System’s Morristown (N.J.) Medical Center, in an interview. “Both the EMPA-REG Outcome and the CREDENCE trials demonstrated substantial benefit of this class of medications by showing a lower risk of cardiovascular death as well as death from any cause and a lower risk of hospitalization for heart failure."
Overall, the SGLT2 trial data have been very consistent with a benefit for cardiovascular risk reduction, particularly in regard to heart failure hospitalizations and even in potentially preventing heart failure in diabetics,” he added.
Dr. Skolnik, a columnist for Family Practice News, cited SGLT2 inhibitors and GLP-1 receptor agonists’ ability to slow renal disease progression, promote weight loss, and prevent poor cardiac outcomes.“These drugs should be used, in addition to metformin, in all patients with diabetes and vascular disease. These proven outcomes are far better than we ever were able to achieve previously and the strength of the evidence at this point is very strong,” said Dr. Skolnik. “In addition to the benefits of decreasing the development of cardiovascular disease, serious heart failure, and slowing progression of renal disease, these two classes of medication have additional benefits. Both classes help patients lose weight, which is very different from what was found with either sulfonylureas or insulin, which cause patients to gain weight. Also both the SGLT2 inhibitors and the GLP-1 RAs [receptor agonists] have a low incidence of hypoglycemia. For all these reasons, these have become important medications for us to use in primary care.”
Other recent trials offer “very powerful data” about SGLT2 inhibitors, Dr. Roberts said. That’s good news, since “our approach needs to be toward cardiovascular protection and preservation as well as managing blood sugar.”An Israeli trial, whose results were released in May 2019 at the annual meeting of the American College of Cardiology, found that, compared with other glucose-lowering drugs, taking an SGLT2 inhibitor was associated with lower risks of heart failure hospitalization and all-cause mortality (HR, 0.54; 95% CI, 0.44-0.65; P less than .001). This trial also offered a new detail: The patients gained the benefit regardless of whether their baseline left ventricular ejection fraction was preserved or reduced (J Coll Cardiol. 2019 Mar;73[9]:suppl 1). The SGLT2 inhibitors used in this trial included dapagliflozin (Farxiga) and empagliflozin (Jardiance).
In another study released this year, a subanalysis of the DECLARE-TIMI 58 trial, researchers reported that the SGLT2 inhibitor dapagliflozin reduced risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes and prior myocardial infarction versus controls (Circulation. 2019 May 28;139[22]:2516-27). The absolute risk reduction for major adverse cardiovascular events was 1.9% (HR, 0.81; 95% CI, 0.65-1.00; P = .046), while it was 0.6% for heart failure hospitalization (HR, 0.85; 95% CI, 0.72-1.00; P = .055).
These and other studies “speak volumes about the efficacy of managing blood sugar and addressing our biggest nemesis, which is cardiovascular disease,” Dr. Roberts said. “It’s irrefutable. The data [are] very good.”
Dr. Paauw said an SGLT2 inhibitor or GLP-1 receptor agonist is best reserved for use in select patients with cardiovascular risks and type 2 diabetes that need management beyond metformin.
For example, they might fit a 70-year-old with persistent hypertension who’s already taking a couple of blood pressure medications. “If they have another cardiovascular risk factor, the cardiovascular protection piece will be a bigger deal,” he said. Also, “it will probably help lower their blood pressure so they can avoid taking another blood pressure medicine.”
Trials of both GLP-1 receptor agonists and SGLT2 inhibitors have shown benefits “in improving [major adverse cardiac events], with the SGLT2 class showing substantial benefit in improving both heart failure and renal outcomes as well,” noted Dr. Skolnik. “It is in this context that one must address the question of whether the price of the medications are worthwhile. With such substantial benefit, there is no question in my mind that – for patients who have underlying cardiovascular illness, which includes patients with existent coronary disease, history of stroke, transient ischemic attack, or peripheral vascular disease – it is far and away worth it to prescribe these classes of medications.”
Indeed, the American Diabetes Association and the European Association for the Study of Diabetes’ most recent guidelines now call for a GLP-1 receptor agonist – instead of insulin – to be the first injectable used to treat type 2 diabetes (Diabetes Care 2018 Dec; 41[12]:2669-701).
“For the relatively small number of my patients who have been able to access and use these medications for months or longer, more have tolerated the GLP-1 agonists than SGLT2 inhibitors primarily due to urinary issues,” noted Dr. Hopkins.
Dipeptidyl peptidase–4 inhibitors are another option in patients with type 2 diabetes, but research suggests they may not be a top option for patients with cardiovascular risk. A 2018 review noted that cardiovascular outcome trials for alogliptin (Nesina), saxagliptin (Onglyza), and sitagliptin (Januvia) showed noninferiority but failed to demonstrate any superiority, compared with placebo in patients with type 2 diabetes mellitus and high cardiovascular risk (Circ Res. 2018 May 11;122[10]:1439-59).
The combination therapies
Many of the newer drugs are available as combinations with other types of diabetes drugs. In some cases, physicians create their own form of combination therapy by separately prescribing two or more diabetes drugs. Earlier this year, a study suggested the benefits of this kind of add-on therapy: Diabetes outcomes improved in patients who took the GLP-1 receptor agonist semaglutide and an SGLT2 inhibitor (Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587[19]30066-X).
Dr. Roberts suggested caution, however, when prescribing combination therapies. “My recommendation is always to begin with the individual medications to see if the patient tolerates the drugs and then decide which component needs to be titrated. It’s hard to titrate a combination drug, and it doesn’t leave a lot of flexibility. You never know which drug is doing what.
Dr. Handelsman said some patients may need to take three medications such as metformin, an SGLT2 inhibitor, and a GLP-1 receptor agonist.
“I don’t recommend using the combinations if you’re not familiar with the drugs ... These are relatively new pharmaceuticals, and most of us are on a learning curve as to how they fit into the armamentarium. If a drug is tolerated with a good response, you can certainly consider going to the combination tablets,” he added.
There is at least one drug that combines these three classes: The newly FDA-approved Qternmet XR, which combines dapagliflozin (an SGLT2 inhibitor), saxagliptin (a GLP-1 receptor agonist), and metformin. As of mid-June 2019, it was not yet available in the United States. Its sister drug Qtern, which combines dapagliflozin and saxagliptin, costs more than $500 a month with a free coupon, according to goodrx.com. In contrast, metformin is extremely inexpensive, costing just a few dollars a month for a common starting dose.
What about adding insulin?
“Both [SGLT2 inhibitors and GLP-1 receptor agonists] work very well with insulin,” Dr. Handelsman said. “There is a nice additive effect on the reduction of [hemoglobin] A1c. The only caution is that, although neither SGLT2 inhibitors nor GLP-1 receptor agonists cause hypoglycemia, in combination with insulin they do increase the risk of hypoglycemia. You may have to adjust the dose of insulin.”
Dr. Hopkins warned that cost becomes an even bigger issue when you add insulin into the mix.
“When insulin comes into the discussion, we are again stuck with astronomical costs which many struggle to afford,” he explained.
Indeed, the price tag on these drugs seems to be the biggest problem physicians have with them.
“The challenges in managing patients with diabetes aren’t the risks associated with the drugs. It’s dealing with their insurers,” noted Dr. Roberts.
Dr. Hopkins, Dr. Paauw, Dr. Roberts, and Dr. Syed reported no disclosures. Dr. Buse is an investigator for Johnson and Johnson. Dr. Goldschmidt is paid to speak by Novartis. Dr. Handelsman reported research grants, consulting work, and speaker honoraria from Amgen, Gilead, Lilly, Merck, Novo Nordisk, and others. Dr Skolnik reported nonfinancial support from AstraZeneca, Boehringer Ingelheim, Sanofi, and GlaxoSmithKline and personal fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly. He also serves on the advisory boards of AstraZeneca, Boehringer Ingelheim, Teva Pharmaceutical, Eli Lilly, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline.
Dr. Paauw and Dr. Skolnik are columnists for Family Practice News and Internal Medicine News.
M. Alexander Otto contributed to this report.

Hyperthyroid? She sure doesn’t look like it
A 50-year-old woman returns for routine follow-up. She has a 15-year history of hypothyroidism, pernicious anemia, and celiac disease. She has had some recent abdominal pain, but no changes in her bowel patterns, and she has not experienced any problems with chest pain, palpitations, or weakness recently. The only medication she is taking is levothyroxine 125 mcg. She has reported no recent weight loss, her blood pressure is 100/60 mm Hg, pulse is 66 beats per minute, temperature is 36.8 degrees Celsius, body mass index is 20, and she does not have a neck goiter. Her cardiac exam was normal and her neurological exam revealed no tremor. Her lab for thyroid-stimulating hormone (TSH) was less than 0.03, and her lab for free thyroxine (FT4) was 2.2, while her TSH level had been 1.4 a year ago. Her levothyroxine dose was decreased to 100 mcg/day, and her repeat lab for TSH, which occurred 12 weeks later, was still less than 0.03. What is the best explanation for why this patient’s labs look like hyperthyroidism, but this patient clinically does not appear to have hyperthyroidism?
A) She was initially given too much levothyroxine; her TSH response is lagging to dose reduction.
B) She has Graves’ disease.
C) She has acute thyroiditis.
D) She is taking extra thyroid hormone.
E) She is taking biotin.
This patient has a history that includes multiple autoimmune diseases including hypothyroidism. It would be extremely unlikely that she would develop Graves' disease or develop acute thyroiditis in the setting of a gland that has been underfunctioning for years. She has no symptoms suggesting that she has hyperthyroidism, which makes taking more thyroid hormone than she is reporting less likely, although this could be possible. The TSH response can lag after dose adjustments of thyroid, but usually a 6-week interval is adequate. This patient’s testing was done 12 weeks after dose reduction making this very unlikely.
The cause for the labs that look like hyperthyroidism in this patient who appears clinically euthyroid is that she is taking biotin. Biotin (vitamin B7) has become a very popular supplement in the past few years for thin hair, brittle nails, and fatigue. The RDA for biotin is 30 mcg. It is widely available in high doses – 5,000-10,000 mcg – which are common doses for supplements.
Biotin has been used extensively as a key component of immunoassays. Streptavidin, a protein produced by the bacteria Streptomyces avidinii, binds biotin with an extremely high affinity, and this binding is utilized in a number of immunoassays, including the assays for thyroid hormone and TSH.1
High serum levels of biotin can make the assays inaccurate, with lower-than-actual TSH and higher-than-actual thyroid hormone levels. Multiple case reports have documented this happening clinically.1-3 I personally saw a case of this recently in my practice. Katzman and colleagues looked at the prevalence of biotin use in outpatients.4 They found that 7.7% were taking supplemental biotin, while 7.4% had levels of biotin in serum samples that were at a level that could interfere with biotin-based serum assays.
Theoretically, biotin can affect multiple other assays that use the streptavidin-biotin assay. The most concerning of these potential problems is with troponin assays. Biotin can falsely lower troponin assays and this can lead to missing the diagnosis of cardiac injury. The Food and Drug Administration released a warning about this and other biotin lab interactions in November 2017.5 Several studies have demonstrated that this effect can occur at serum levels achievable with available over-the-counter doses of biotin.6,7
Not all troponin assays are affected by high serum levels of biotin: The Gen 5 cTnT assay is the only troponin assay affected.7 I could not find any case reports that have been published where biotin had caused a clinical missed diagnosis with troponins.
Pearls
Consider biotin supplement use when you have patients whose labs look like hyperthyroidism, but clinically do not appear to be hyperthyroid.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Charles S, Agrawal N, and Blum M. Erroneous thyroid diagnosis due to over-the-counter biotin. Nutrition 2019;57:257-8.
2. Elston MS et al. Factitious Graves’ disease due to a biotin immunoassay interference – a case and review of the literature. J Clin Endocrinol Metab 2016;101:3251-5.
3. Barbesino G. Misdiagnosis of Graves ’disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid 2016;26(6):860-3.
4. Katzman et al. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin Biochem. 2018 Sep;60:11-16.
5. “The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication,” Nov. 28, 2017.
6. Trambas et al. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem. 2018 Mar;55(2):205-15.
7. Frame IJ et al. Susceptibility of cardiac troponin assays to biotin interference. Am J Clin Pathol. 2019 Apr 2;151(5):486-93.
A 50-year-old woman returns for routine follow-up. She has a 15-year history of hypothyroidism, pernicious anemia, and celiac disease. She has had some recent abdominal pain, but no changes in her bowel patterns, and she has not experienced any problems with chest pain, palpitations, or weakness recently. The only medication she is taking is levothyroxine 125 mcg. She has reported no recent weight loss, her blood pressure is 100/60 mm Hg, pulse is 66 beats per minute, temperature is 36.8 degrees Celsius, body mass index is 20, and she does not have a neck goiter. Her cardiac exam was normal and her neurological exam revealed no tremor. Her lab for thyroid-stimulating hormone (TSH) was less than 0.03, and her lab for free thyroxine (FT4) was 2.2, while her TSH level had been 1.4 a year ago. Her levothyroxine dose was decreased to 100 mcg/day, and her repeat lab for TSH, which occurred 12 weeks later, was still less than 0.03. What is the best explanation for why this patient’s labs look like hyperthyroidism, but this patient clinically does not appear to have hyperthyroidism?
A) She was initially given too much levothyroxine; her TSH response is lagging to dose reduction.
B) She has Graves’ disease.
C) She has acute thyroiditis.
D) She is taking extra thyroid hormone.
E) She is taking biotin.
This patient has a history that includes multiple autoimmune diseases including hypothyroidism. It would be extremely unlikely that she would develop Graves' disease or develop acute thyroiditis in the setting of a gland that has been underfunctioning for years. She has no symptoms suggesting that she has hyperthyroidism, which makes taking more thyroid hormone than she is reporting less likely, although this could be possible. The TSH response can lag after dose adjustments of thyroid, but usually a 6-week interval is adequate. This patient’s testing was done 12 weeks after dose reduction making this very unlikely.
The cause for the labs that look like hyperthyroidism in this patient who appears clinically euthyroid is that she is taking biotin. Biotin (vitamin B7) has become a very popular supplement in the past few years for thin hair, brittle nails, and fatigue. The RDA for biotin is 30 mcg. It is widely available in high doses – 5,000-10,000 mcg – which are common doses for supplements.
Biotin has been used extensively as a key component of immunoassays. Streptavidin, a protein produced by the bacteria Streptomyces avidinii, binds biotin with an extremely high affinity, and this binding is utilized in a number of immunoassays, including the assays for thyroid hormone and TSH.1
High serum levels of biotin can make the assays inaccurate, with lower-than-actual TSH and higher-than-actual thyroid hormone levels. Multiple case reports have documented this happening clinically.1-3 I personally saw a case of this recently in my practice. Katzman and colleagues looked at the prevalence of biotin use in outpatients.4 They found that 7.7% were taking supplemental biotin, while 7.4% had levels of biotin in serum samples that were at a level that could interfere with biotin-based serum assays.
Theoretically, biotin can affect multiple other assays that use the streptavidin-biotin assay. The most concerning of these potential problems is with troponin assays. Biotin can falsely lower troponin assays and this can lead to missing the diagnosis of cardiac injury. The Food and Drug Administration released a warning about this and other biotin lab interactions in November 2017.5 Several studies have demonstrated that this effect can occur at serum levels achievable with available over-the-counter doses of biotin.6,7
Not all troponin assays are affected by high serum levels of biotin: The Gen 5 cTnT assay is the only troponin assay affected.7 I could not find any case reports that have been published where biotin had caused a clinical missed diagnosis with troponins.
Pearls
Consider biotin supplement use when you have patients whose labs look like hyperthyroidism, but clinically do not appear to be hyperthyroid.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Charles S, Agrawal N, and Blum M. Erroneous thyroid diagnosis due to over-the-counter biotin. Nutrition 2019;57:257-8.
2. Elston MS et al. Factitious Graves’ disease due to a biotin immunoassay interference – a case and review of the literature. J Clin Endocrinol Metab 2016;101:3251-5.
3. Barbesino G. Misdiagnosis of Graves ’disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid 2016;26(6):860-3.
4. Katzman et al. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin Biochem. 2018 Sep;60:11-16.
5. “The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication,” Nov. 28, 2017.
6. Trambas et al. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem. 2018 Mar;55(2):205-15.
7. Frame IJ et al. Susceptibility of cardiac troponin assays to biotin interference. Am J Clin Pathol. 2019 Apr 2;151(5):486-93.
A 50-year-old woman returns for routine follow-up. She has a 15-year history of hypothyroidism, pernicious anemia, and celiac disease. She has had some recent abdominal pain, but no changes in her bowel patterns, and she has not experienced any problems with chest pain, palpitations, or weakness recently. The only medication she is taking is levothyroxine 125 mcg. She has reported no recent weight loss, her blood pressure is 100/60 mm Hg, pulse is 66 beats per minute, temperature is 36.8 degrees Celsius, body mass index is 20, and she does not have a neck goiter. Her cardiac exam was normal and her neurological exam revealed no tremor. Her lab for thyroid-stimulating hormone (TSH) was less than 0.03, and her lab for free thyroxine (FT4) was 2.2, while her TSH level had been 1.4 a year ago. Her levothyroxine dose was decreased to 100 mcg/day, and her repeat lab for TSH, which occurred 12 weeks later, was still less than 0.03. What is the best explanation for why this patient’s labs look like hyperthyroidism, but this patient clinically does not appear to have hyperthyroidism?
A) She was initially given too much levothyroxine; her TSH response is lagging to dose reduction.
B) She has Graves’ disease.
C) She has acute thyroiditis.
D) She is taking extra thyroid hormone.
E) She is taking biotin.
This patient has a history that includes multiple autoimmune diseases including hypothyroidism. It would be extremely unlikely that she would develop Graves' disease or develop acute thyroiditis in the setting of a gland that has been underfunctioning for years. She has no symptoms suggesting that she has hyperthyroidism, which makes taking more thyroid hormone than she is reporting less likely, although this could be possible. The TSH response can lag after dose adjustments of thyroid, but usually a 6-week interval is adequate. This patient’s testing was done 12 weeks after dose reduction making this very unlikely.
The cause for the labs that look like hyperthyroidism in this patient who appears clinically euthyroid is that she is taking biotin. Biotin (vitamin B7) has become a very popular supplement in the past few years for thin hair, brittle nails, and fatigue. The RDA for biotin is 30 mcg. It is widely available in high doses – 5,000-10,000 mcg – which are common doses for supplements.
Biotin has been used extensively as a key component of immunoassays. Streptavidin, a protein produced by the bacteria Streptomyces avidinii, binds biotin with an extremely high affinity, and this binding is utilized in a number of immunoassays, including the assays for thyroid hormone and TSH.1
High serum levels of biotin can make the assays inaccurate, with lower-than-actual TSH and higher-than-actual thyroid hormone levels. Multiple case reports have documented this happening clinically.1-3 I personally saw a case of this recently in my practice. Katzman and colleagues looked at the prevalence of biotin use in outpatients.4 They found that 7.7% were taking supplemental biotin, while 7.4% had levels of biotin in serum samples that were at a level that could interfere with biotin-based serum assays.
Theoretically, biotin can affect multiple other assays that use the streptavidin-biotin assay. The most concerning of these potential problems is with troponin assays. Biotin can falsely lower troponin assays and this can lead to missing the diagnosis of cardiac injury. The Food and Drug Administration released a warning about this and other biotin lab interactions in November 2017.5 Several studies have demonstrated that this effect can occur at serum levels achievable with available over-the-counter doses of biotin.6,7
Not all troponin assays are affected by high serum levels of biotin: The Gen 5 cTnT assay is the only troponin assay affected.7 I could not find any case reports that have been published where biotin had caused a clinical missed diagnosis with troponins.
Pearls
Consider biotin supplement use when you have patients whose labs look like hyperthyroidism, but clinically do not appear to be hyperthyroid.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Charles S, Agrawal N, and Blum M. Erroneous thyroid diagnosis due to over-the-counter biotin. Nutrition 2019;57:257-8.
2. Elston MS et al. Factitious Graves’ disease due to a biotin immunoassay interference – a case and review of the literature. J Clin Endocrinol Metab 2016;101:3251-5.
3. Barbesino G. Misdiagnosis of Graves ’disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid 2016;26(6):860-3.
4. Katzman et al. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin Biochem. 2018 Sep;60:11-16.
5. “The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication,” Nov. 28, 2017.
6. Trambas et al. Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem. 2018 Mar;55(2):205-15.
7. Frame IJ et al. Susceptibility of cardiac troponin assays to biotin interference. Am J Clin Pathol. 2019 Apr 2;151(5):486-93.
Levothyroxine did not reduce fatigue in older patients with hypothyroidism
WASHINGTON – according to a new study.
The double-blind, randomized, placebo-controlled, parallel-group trial used subsets of data from the TRUST study. This new research, which was presented at the Annual Meeting of the Society of General Internal Medicine, provides further evidence that prescribing levothyroxine to older patients with subclinical hypothyroidism will not reduce fatigue.
Like the findings in the TRUST study, the new study, “Effect of Thyroid Hormone Replacement on Fatigability in Older Adults with Subclinical Hypothyroidism: A Randomized Placebo Controlled Trial,” showed that the use of levothyroxine in subclinical hypothyroidism was not effective in changing physical and mental fatigability in older adults.
“This study shows that levothyroxine treatment may not be necessary and results in high costs and side effects,” noted Mirah Stuber, MD, during her presentation of the findings at the meeting.
The new study examined the effect of levothyroxine on the tiredness of older adults but with a more detailed analysis of fatigue than the full TRUST study provided. In this new study, investigators used a new assessment tool, called the Pittsburgh Fatigability Scale (PFS), a 10-item self-administered questionnaire that can measure both physical and mental fatigability. Perceived fatigability anchors tiredness to a set of activities and has been shown to be a more sensitive measure than global fatigue, explained Dr. Stuber of the University of Chicago.
The scores range from 0-50, with higher scores indicating higher fatigability. The scales were divided between mental and physical activities and measured fatigue in relation to a defined activity of a specific duration and intensity. These activities included a leisurely 30-minute walk and participation in a 1-hour social activity.
This study involved 230 participants from Switzerland and Ireland, with a mean age of 73.4 years, who had persistent hypothyroidism. The population was randomized to 119 patients who were administered levothyroxine and 111 patients who received a placebo.
At baseline, the levothyroxine group had a mean physical PFS score of 14.7 ± 9.3, and the placebo group had a score of 11.1 ± 9.1. The baseline mean mental PFS score for the levothyroxine group was 7.4 ± 8.0, while it was 5.1 ± 6.9 for the placebo group.
After 12 months of the participants’ use of levothyroxine or placebo, the physical PFS scores increased for both the treatment and placebo groups. For the levothyroxine group, the mean physical PFS score was 14.8 ± 9.6, while it was 12.4 ± 9.3 for the placebo group (P = 0.88). The investigators found no significant differences between these scores for the levothyroxine and placebo groups.
The mean mental PFS score slightly decreased to 6.0 ± 7.8 for the levothyroxine group, while it slightly increased to 6.0 ± 8.0 for the placebo group (P = 0.26) at 12 months. The difference between the mental fatigability scores for the levothyroxine and placebo arms at 12 months was also not significant.
The physical fatigability between-group difference was 0.2 (95% confidence interval, –1.8 to 2.1; P = 0.88), while the mental fatigability difference was –1.0 (95% CI, –2.8 to 0.8; P = 0.26).
The study was funded by the European Union FP7 and the Swiss National Science Foundation. Merck KGaA, Darmstadt (Germany) provided levothyroxine and the placebo. Dr. Stuber has no conflicts of interest.
WASHINGTON – according to a new study.
The double-blind, randomized, placebo-controlled, parallel-group trial used subsets of data from the TRUST study. This new research, which was presented at the Annual Meeting of the Society of General Internal Medicine, provides further evidence that prescribing levothyroxine to older patients with subclinical hypothyroidism will not reduce fatigue.
Like the findings in the TRUST study, the new study, “Effect of Thyroid Hormone Replacement on Fatigability in Older Adults with Subclinical Hypothyroidism: A Randomized Placebo Controlled Trial,” showed that the use of levothyroxine in subclinical hypothyroidism was not effective in changing physical and mental fatigability in older adults.
“This study shows that levothyroxine treatment may not be necessary and results in high costs and side effects,” noted Mirah Stuber, MD, during her presentation of the findings at the meeting.
The new study examined the effect of levothyroxine on the tiredness of older adults but with a more detailed analysis of fatigue than the full TRUST study provided. In this new study, investigators used a new assessment tool, called the Pittsburgh Fatigability Scale (PFS), a 10-item self-administered questionnaire that can measure both physical and mental fatigability. Perceived fatigability anchors tiredness to a set of activities and has been shown to be a more sensitive measure than global fatigue, explained Dr. Stuber of the University of Chicago.
The scores range from 0-50, with higher scores indicating higher fatigability. The scales were divided between mental and physical activities and measured fatigue in relation to a defined activity of a specific duration and intensity. These activities included a leisurely 30-minute walk and participation in a 1-hour social activity.
This study involved 230 participants from Switzerland and Ireland, with a mean age of 73.4 years, who had persistent hypothyroidism. The population was randomized to 119 patients who were administered levothyroxine and 111 patients who received a placebo.
At baseline, the levothyroxine group had a mean physical PFS score of 14.7 ± 9.3, and the placebo group had a score of 11.1 ± 9.1. The baseline mean mental PFS score for the levothyroxine group was 7.4 ± 8.0, while it was 5.1 ± 6.9 for the placebo group.
After 12 months of the participants’ use of levothyroxine or placebo, the physical PFS scores increased for both the treatment and placebo groups. For the levothyroxine group, the mean physical PFS score was 14.8 ± 9.6, while it was 12.4 ± 9.3 for the placebo group (P = 0.88). The investigators found no significant differences between these scores for the levothyroxine and placebo groups.
The mean mental PFS score slightly decreased to 6.0 ± 7.8 for the levothyroxine group, while it slightly increased to 6.0 ± 8.0 for the placebo group (P = 0.26) at 12 months. The difference between the mental fatigability scores for the levothyroxine and placebo arms at 12 months was also not significant.
The physical fatigability between-group difference was 0.2 (95% confidence interval, –1.8 to 2.1; P = 0.88), while the mental fatigability difference was –1.0 (95% CI, –2.8 to 0.8; P = 0.26).
The study was funded by the European Union FP7 and the Swiss National Science Foundation. Merck KGaA, Darmstadt (Germany) provided levothyroxine and the placebo. Dr. Stuber has no conflicts of interest.
WASHINGTON – according to a new study.
The double-blind, randomized, placebo-controlled, parallel-group trial used subsets of data from the TRUST study. This new research, which was presented at the Annual Meeting of the Society of General Internal Medicine, provides further evidence that prescribing levothyroxine to older patients with subclinical hypothyroidism will not reduce fatigue.
Like the findings in the TRUST study, the new study, “Effect of Thyroid Hormone Replacement on Fatigability in Older Adults with Subclinical Hypothyroidism: A Randomized Placebo Controlled Trial,” showed that the use of levothyroxine in subclinical hypothyroidism was not effective in changing physical and mental fatigability in older adults.
“This study shows that levothyroxine treatment may not be necessary and results in high costs and side effects,” noted Mirah Stuber, MD, during her presentation of the findings at the meeting.
The new study examined the effect of levothyroxine on the tiredness of older adults but with a more detailed analysis of fatigue than the full TRUST study provided. In this new study, investigators used a new assessment tool, called the Pittsburgh Fatigability Scale (PFS), a 10-item self-administered questionnaire that can measure both physical and mental fatigability. Perceived fatigability anchors tiredness to a set of activities and has been shown to be a more sensitive measure than global fatigue, explained Dr. Stuber of the University of Chicago.
The scores range from 0-50, with higher scores indicating higher fatigability. The scales were divided between mental and physical activities and measured fatigue in relation to a defined activity of a specific duration and intensity. These activities included a leisurely 30-minute walk and participation in a 1-hour social activity.
This study involved 230 participants from Switzerland and Ireland, with a mean age of 73.4 years, who had persistent hypothyroidism. The population was randomized to 119 patients who were administered levothyroxine and 111 patients who received a placebo.
At baseline, the levothyroxine group had a mean physical PFS score of 14.7 ± 9.3, and the placebo group had a score of 11.1 ± 9.1. The baseline mean mental PFS score for the levothyroxine group was 7.4 ± 8.0, while it was 5.1 ± 6.9 for the placebo group.
After 12 months of the participants’ use of levothyroxine or placebo, the physical PFS scores increased for both the treatment and placebo groups. For the levothyroxine group, the mean physical PFS score was 14.8 ± 9.6, while it was 12.4 ± 9.3 for the placebo group (P = 0.88). The investigators found no significant differences between these scores for the levothyroxine and placebo groups.
The mean mental PFS score slightly decreased to 6.0 ± 7.8 for the levothyroxine group, while it slightly increased to 6.0 ± 8.0 for the placebo group (P = 0.26) at 12 months. The difference between the mental fatigability scores for the levothyroxine and placebo arms at 12 months was also not significant.
The physical fatigability between-group difference was 0.2 (95% confidence interval, –1.8 to 2.1; P = 0.88), while the mental fatigability difference was –1.0 (95% CI, –2.8 to 0.8; P = 0.26).
The study was funded by the European Union FP7 and the Swiss National Science Foundation. Merck KGaA, Darmstadt (Germany) provided levothyroxine and the placebo. Dr. Stuber has no conflicts of interest.
REPORTING FROM SGIM 2019
New insights, advances offer better perspective on AGHD
LOS ANGELES – Kevin C.J. Yuen, MD, FRCP(UK), FACE, told colleagues at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists (AACE).
“The data show that growth hormone replacement is safe and may improve survival,” said Dr Yuen, professor of medicine and medical director at Barrow Neurological Institute Pituitary Center in Phoenix.
Dr. Yuen is chair of the AACE’s growth hormone task force and coauthored both the 2009 and soon-to-be-published 2019 AACE guidelines for the treatment of adult growth hormone deficiency (AGHD).
Updated AGHD guidelines were needed for a variety of reasons, including a greater awareness of the benefits of hormone replacement in these patients and new developments in areas such as testing, he said. The guidelines also are also necessary because of the skepticism about the cost and benefits of AGHD therapy, concerns about the safety of long-term therapy, and the misuse of treatment in certain patients, he added.
On the treatment front, Dr. Yuen said it has become more clear over recent years that patients with AGHD benefit from hormone replacement. Findings from two studies have linked treatment to improvements in exercise capacity (Clin Endocrinol [Oxf]. 2016;85[4]:660-8) and patient quality of life (Eur J Endocrinol. 2017;176:99-109). “Even just after 6 months there’s an improvement in aerobic power,” he said.
And, he continued, other findings have suggested that treatment could lower mortality in men and reduce the number of deaths from malignant neoplasms in all patients (Eur J Endocrinol. 2017;176[1]:67-75).
However, Dr. Yuen cautioned that the confirmation of a survival benefit from hormone replacement will be speculative as long as there are no prospective data available.
He offered five tips about diagnosing and treating AGHD:
First, be aware that a number of conditions other than AGHD can cause low levels of insulin-like growth factor 1, including malnutrition, diabetes, untreated hypothyroidism, liver disease, and kidney failure.
Second, follow recommended algorithms for testing adult patients and transition those pediatric patients who seem to be at risk of having the condition. (Those moving from pediatric to adult care are known as transition patients.) The algorithms suggest that three diagnostic tests can be helpful, depending on the situation: the macimorelin test, the insulin tolerance test, and the glucagon stimulation test.
In 2017, the Food and Drug Administration approved the macimorelin (Macrilen) test, which requires the administration of an oral medication before a blood test. Dr. Yuen cited a phase 3 study that demonstrated that the test was “highly reproducible” and has a “good safety profile” (J Clin Endocrinol Metab. 2018;103[8]:3083-93). Dr. Yuen believes the test will become the preferred alternative to the insulin tolerance test.
Third, transition patients require special care as they move from pediatric care. “The handover is still very challenging,” Dr. Yuen said. “There’s still much to be done to improve the quality of treatment for these patients.” Challenges during the transition can include the patient’s reluctance to continue taking hormones in adulthood, he said. “Encourage pediatricians to start educating patients from early on that they’ll need to remain on hormones,” he advised, and help patients take accountability for their health in areas such as self-injection.
Patients may suffer from “injection fatigue,” they may be concerned about the side effects of the therapy, and/or they may be overwhelmed by the cost of care, he said. They may not understand how to manage their care and lack insight into the consequences of treatment cessation.
He advised endocrinologists to monitor transition patients who aren’t growth-hormone deficient because their status may change in the future.
Fourth, start treatment in adults with recommended doses of growth hormone. For those younger than 30 years, use 0.4-0.5 mg/day (higher for transition patients); for those aged between 30 and 60 years, use 0.2-0.3 mg/day; and for those who are older than 60 years, use 0.1-0.2 mg/day. Doses should be adjusted to 0.1-0.2 mg/day in patients with diabetes, obesity, and/or previous gestational diabetes.
Fifth, treat patients with growth hormone indefinitely if benefits are seen, but consider stopping treatment after a year if there doesn’t seem to be a benefit, Dr. Yuen advised. Follow up at 6 months, he recommended.
Dr. Yuen disclosed receiving research grants from and consulting for Pfizer, Novo Nordisk, and Aeterna Zentaris. He has also consulted for Strongbridge.
LOS ANGELES – Kevin C.J. Yuen, MD, FRCP(UK), FACE, told colleagues at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists (AACE).
“The data show that growth hormone replacement is safe and may improve survival,” said Dr Yuen, professor of medicine and medical director at Barrow Neurological Institute Pituitary Center in Phoenix.
Dr. Yuen is chair of the AACE’s growth hormone task force and coauthored both the 2009 and soon-to-be-published 2019 AACE guidelines for the treatment of adult growth hormone deficiency (AGHD).
Updated AGHD guidelines were needed for a variety of reasons, including a greater awareness of the benefits of hormone replacement in these patients and new developments in areas such as testing, he said. The guidelines also are also necessary because of the skepticism about the cost and benefits of AGHD therapy, concerns about the safety of long-term therapy, and the misuse of treatment in certain patients, he added.
On the treatment front, Dr. Yuen said it has become more clear over recent years that patients with AGHD benefit from hormone replacement. Findings from two studies have linked treatment to improvements in exercise capacity (Clin Endocrinol [Oxf]. 2016;85[4]:660-8) and patient quality of life (Eur J Endocrinol. 2017;176:99-109). “Even just after 6 months there’s an improvement in aerobic power,” he said.
And, he continued, other findings have suggested that treatment could lower mortality in men and reduce the number of deaths from malignant neoplasms in all patients (Eur J Endocrinol. 2017;176[1]:67-75).
However, Dr. Yuen cautioned that the confirmation of a survival benefit from hormone replacement will be speculative as long as there are no prospective data available.
He offered five tips about diagnosing and treating AGHD:
First, be aware that a number of conditions other than AGHD can cause low levels of insulin-like growth factor 1, including malnutrition, diabetes, untreated hypothyroidism, liver disease, and kidney failure.
Second, follow recommended algorithms for testing adult patients and transition those pediatric patients who seem to be at risk of having the condition. (Those moving from pediatric to adult care are known as transition patients.) The algorithms suggest that three diagnostic tests can be helpful, depending on the situation: the macimorelin test, the insulin tolerance test, and the glucagon stimulation test.
In 2017, the Food and Drug Administration approved the macimorelin (Macrilen) test, which requires the administration of an oral medication before a blood test. Dr. Yuen cited a phase 3 study that demonstrated that the test was “highly reproducible” and has a “good safety profile” (J Clin Endocrinol Metab. 2018;103[8]:3083-93). Dr. Yuen believes the test will become the preferred alternative to the insulin tolerance test.
Third, transition patients require special care as they move from pediatric care. “The handover is still very challenging,” Dr. Yuen said. “There’s still much to be done to improve the quality of treatment for these patients.” Challenges during the transition can include the patient’s reluctance to continue taking hormones in adulthood, he said. “Encourage pediatricians to start educating patients from early on that they’ll need to remain on hormones,” he advised, and help patients take accountability for their health in areas such as self-injection.
Patients may suffer from “injection fatigue,” they may be concerned about the side effects of the therapy, and/or they may be overwhelmed by the cost of care, he said. They may not understand how to manage their care and lack insight into the consequences of treatment cessation.
He advised endocrinologists to monitor transition patients who aren’t growth-hormone deficient because their status may change in the future.
Fourth, start treatment in adults with recommended doses of growth hormone. For those younger than 30 years, use 0.4-0.5 mg/day (higher for transition patients); for those aged between 30 and 60 years, use 0.2-0.3 mg/day; and for those who are older than 60 years, use 0.1-0.2 mg/day. Doses should be adjusted to 0.1-0.2 mg/day in patients with diabetes, obesity, and/or previous gestational diabetes.
Fifth, treat patients with growth hormone indefinitely if benefits are seen, but consider stopping treatment after a year if there doesn’t seem to be a benefit, Dr. Yuen advised. Follow up at 6 months, he recommended.
Dr. Yuen disclosed receiving research grants from and consulting for Pfizer, Novo Nordisk, and Aeterna Zentaris. He has also consulted for Strongbridge.
LOS ANGELES – Kevin C.J. Yuen, MD, FRCP(UK), FACE, told colleagues at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists (AACE).
“The data show that growth hormone replacement is safe and may improve survival,” said Dr Yuen, professor of medicine and medical director at Barrow Neurological Institute Pituitary Center in Phoenix.
Dr. Yuen is chair of the AACE’s growth hormone task force and coauthored both the 2009 and soon-to-be-published 2019 AACE guidelines for the treatment of adult growth hormone deficiency (AGHD).
Updated AGHD guidelines were needed for a variety of reasons, including a greater awareness of the benefits of hormone replacement in these patients and new developments in areas such as testing, he said. The guidelines also are also necessary because of the skepticism about the cost and benefits of AGHD therapy, concerns about the safety of long-term therapy, and the misuse of treatment in certain patients, he added.
On the treatment front, Dr. Yuen said it has become more clear over recent years that patients with AGHD benefit from hormone replacement. Findings from two studies have linked treatment to improvements in exercise capacity (Clin Endocrinol [Oxf]. 2016;85[4]:660-8) and patient quality of life (Eur J Endocrinol. 2017;176:99-109). “Even just after 6 months there’s an improvement in aerobic power,” he said.
And, he continued, other findings have suggested that treatment could lower mortality in men and reduce the number of deaths from malignant neoplasms in all patients (Eur J Endocrinol. 2017;176[1]:67-75).
However, Dr. Yuen cautioned that the confirmation of a survival benefit from hormone replacement will be speculative as long as there are no prospective data available.
He offered five tips about diagnosing and treating AGHD:
First, be aware that a number of conditions other than AGHD can cause low levels of insulin-like growth factor 1, including malnutrition, diabetes, untreated hypothyroidism, liver disease, and kidney failure.
Second, follow recommended algorithms for testing adult patients and transition those pediatric patients who seem to be at risk of having the condition. (Those moving from pediatric to adult care are known as transition patients.) The algorithms suggest that three diagnostic tests can be helpful, depending on the situation: the macimorelin test, the insulin tolerance test, and the glucagon stimulation test.
In 2017, the Food and Drug Administration approved the macimorelin (Macrilen) test, which requires the administration of an oral medication before a blood test. Dr. Yuen cited a phase 3 study that demonstrated that the test was “highly reproducible” and has a “good safety profile” (J Clin Endocrinol Metab. 2018;103[8]:3083-93). Dr. Yuen believes the test will become the preferred alternative to the insulin tolerance test.
Third, transition patients require special care as they move from pediatric care. “The handover is still very challenging,” Dr. Yuen said. “There’s still much to be done to improve the quality of treatment for these patients.” Challenges during the transition can include the patient’s reluctance to continue taking hormones in adulthood, he said. “Encourage pediatricians to start educating patients from early on that they’ll need to remain on hormones,” he advised, and help patients take accountability for their health in areas such as self-injection.
Patients may suffer from “injection fatigue,” they may be concerned about the side effects of the therapy, and/or they may be overwhelmed by the cost of care, he said. They may not understand how to manage their care and lack insight into the consequences of treatment cessation.
He advised endocrinologists to monitor transition patients who aren’t growth-hormone deficient because their status may change in the future.
Fourth, start treatment in adults with recommended doses of growth hormone. For those younger than 30 years, use 0.4-0.5 mg/day (higher for transition patients); for those aged between 30 and 60 years, use 0.2-0.3 mg/day; and for those who are older than 60 years, use 0.1-0.2 mg/day. Doses should be adjusted to 0.1-0.2 mg/day in patients with diabetes, obesity, and/or previous gestational diabetes.
Fifth, treat patients with growth hormone indefinitely if benefits are seen, but consider stopping treatment after a year if there doesn’t seem to be a benefit, Dr. Yuen advised. Follow up at 6 months, he recommended.
Dr. Yuen disclosed receiving research grants from and consulting for Pfizer, Novo Nordisk, and Aeterna Zentaris. He has also consulted for Strongbridge.
REPORTING FROM AACE 2019
Experimental drug holds promise for the treatment of thyroid eye disease
LOS ANGELES – researchers reported at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
“For the first time, there appears to be a medicine that can be given during the active phase of the disease and can actually reverse not just the eyelid swelling and the clinical activity score, but also reduce the eye bulging and double vision and [improve] the [patient’s] quality of life. It could be a watershed moment in the treatment of the disease,” said ophthalmologist Raymond Douglas, MD, PhD, professor of surgery at Cedar-Sinai Medical Center Los Angeles and the study’s coprincipal investigator, in an interview at the meeting.
According to Dr. Douglas, thyroid eye disease, which is also known as Graves’ eye disease, is a severely disabling condition that causes swelling, pain, discomfort, and blindness. “The burden really is quite significant,” he said, with an impact that’s been compared with that of breast cancer in quality-of-life studies.
“Current treatments for thyroid eye disease are rather limited,” he said. “They really encompass just reducing the swelling and the short-term manifestations of the disease. Treatments such as IV steroids and radiation have been shown to not have any effect on long-term manifestations such as eye bulging and double vision.”
Teprotumumab is a fully human monoclonal antibody that targets the insulinlike growth factor I receptor (IGF-IR). It seems to downregulate thyroid eye disease, Dr. Douglas said.
Researchers studied the drug in a randomized, placebo-controlled study: 41 patients were designated to receive eight intravenous infusions of the drug over 21 weeks (10 mg/kg for the first infusion, then 20 mg/kg thereafter). At week 24, 83% of the study group (34 of 41 patients) reached the endpoint of a reduction of eye bulging by at least 2 mm, compared with 10% of patients (4 of 42) in the placebo group, which received infusions of saline solution.
Two millimeters is significant, Dr. Douglas said. “If you noticed someone’s eye was bulging 2 millimeters, you’d say, ‘Hey, I think something is wrong with your eye.’ ”
Initial study results were released in February 2019. New data about secondary endpoints were released at the AACE meeting: Researchers reported that the average reduction in proptosis (eye bulging) was 2.82 mm in the study group, compared with 0.54 mm in the placebo group (P less than .001).
Dr. Douglas said he can “achieve 3 mm of reduction through surgery to drill out the bone between the eye and the brain. [The patients in the study group] were able to achieve almost 3 millimeters of reduction by the drug alone. It’s a rather significant improvement.”
The new data also provided some insight into the timing of clinical improvements. According to Dr. Douglas, “most of the endpoints were met as early as 6 weeks or [after] two infusions of this drug.”
The adverse effects were relatively mild and included muscle spasms, said Dr. Douglas. The side effects seem to be “well tolerated,” he noted, and none led to cessation of therapy.
Participants with potential for motherhood or fatherhood during the trial had to agree to take precautions to avoid becoming pregnant or impregnating a partner.
Dr. Douglas didn’t provide cost information about the drug. However, it seems likely to be expensive. A writer with Seeking Alpha, a stock market analysis site, estimated that the cost could reach “$250,000 or $300,000 per patient per year, which translates to a $3.7 billion to $6 billion market in the United States alone (based on the estimated patient population in the 15,000-20,000 range).”
The drug’s manufacturer, Horizon Therapeutics, expects to apply to the Food and Drug Administration later this year for approval of the drug.
If it is approved, endocrinologists will have an opportunity to partner with eye surgeons to treat thyroid eye disease, Dr. Douglas said. “The crux will be the comanagement with the endocrinologist helping to control the thyroid function and manage some of the side effects of this medication, and the oculoplastic surgeon [working on] diagnosis, appropriate use, and management.”
Horizon Therapeutics funded the study. Dr. Douglas disclosed that he is a consultant with the company.
LOS ANGELES – researchers reported at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
“For the first time, there appears to be a medicine that can be given during the active phase of the disease and can actually reverse not just the eyelid swelling and the clinical activity score, but also reduce the eye bulging and double vision and [improve] the [patient’s] quality of life. It could be a watershed moment in the treatment of the disease,” said ophthalmologist Raymond Douglas, MD, PhD, professor of surgery at Cedar-Sinai Medical Center Los Angeles and the study’s coprincipal investigator, in an interview at the meeting.
According to Dr. Douglas, thyroid eye disease, which is also known as Graves’ eye disease, is a severely disabling condition that causes swelling, pain, discomfort, and blindness. “The burden really is quite significant,” he said, with an impact that’s been compared with that of breast cancer in quality-of-life studies.
“Current treatments for thyroid eye disease are rather limited,” he said. “They really encompass just reducing the swelling and the short-term manifestations of the disease. Treatments such as IV steroids and radiation have been shown to not have any effect on long-term manifestations such as eye bulging and double vision.”
Teprotumumab is a fully human monoclonal antibody that targets the insulinlike growth factor I receptor (IGF-IR). It seems to downregulate thyroid eye disease, Dr. Douglas said.
Researchers studied the drug in a randomized, placebo-controlled study: 41 patients were designated to receive eight intravenous infusions of the drug over 21 weeks (10 mg/kg for the first infusion, then 20 mg/kg thereafter). At week 24, 83% of the study group (34 of 41 patients) reached the endpoint of a reduction of eye bulging by at least 2 mm, compared with 10% of patients (4 of 42) in the placebo group, which received infusions of saline solution.
Two millimeters is significant, Dr. Douglas said. “If you noticed someone’s eye was bulging 2 millimeters, you’d say, ‘Hey, I think something is wrong with your eye.’ ”
Initial study results were released in February 2019. New data about secondary endpoints were released at the AACE meeting: Researchers reported that the average reduction in proptosis (eye bulging) was 2.82 mm in the study group, compared with 0.54 mm in the placebo group (P less than .001).
Dr. Douglas said he can “achieve 3 mm of reduction through surgery to drill out the bone between the eye and the brain. [The patients in the study group] were able to achieve almost 3 millimeters of reduction by the drug alone. It’s a rather significant improvement.”
The new data also provided some insight into the timing of clinical improvements. According to Dr. Douglas, “most of the endpoints were met as early as 6 weeks or [after] two infusions of this drug.”
The adverse effects were relatively mild and included muscle spasms, said Dr. Douglas. The side effects seem to be “well tolerated,” he noted, and none led to cessation of therapy.
Participants with potential for motherhood or fatherhood during the trial had to agree to take precautions to avoid becoming pregnant or impregnating a partner.
Dr. Douglas didn’t provide cost information about the drug. However, it seems likely to be expensive. A writer with Seeking Alpha, a stock market analysis site, estimated that the cost could reach “$250,000 or $300,000 per patient per year, which translates to a $3.7 billion to $6 billion market in the United States alone (based on the estimated patient population in the 15,000-20,000 range).”
The drug’s manufacturer, Horizon Therapeutics, expects to apply to the Food and Drug Administration later this year for approval of the drug.
If it is approved, endocrinologists will have an opportunity to partner with eye surgeons to treat thyroid eye disease, Dr. Douglas said. “The crux will be the comanagement with the endocrinologist helping to control the thyroid function and manage some of the side effects of this medication, and the oculoplastic surgeon [working on] diagnosis, appropriate use, and management.”
Horizon Therapeutics funded the study. Dr. Douglas disclosed that he is a consultant with the company.
LOS ANGELES – researchers reported at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
“For the first time, there appears to be a medicine that can be given during the active phase of the disease and can actually reverse not just the eyelid swelling and the clinical activity score, but also reduce the eye bulging and double vision and [improve] the [patient’s] quality of life. It could be a watershed moment in the treatment of the disease,” said ophthalmologist Raymond Douglas, MD, PhD, professor of surgery at Cedar-Sinai Medical Center Los Angeles and the study’s coprincipal investigator, in an interview at the meeting.
According to Dr. Douglas, thyroid eye disease, which is also known as Graves’ eye disease, is a severely disabling condition that causes swelling, pain, discomfort, and blindness. “The burden really is quite significant,” he said, with an impact that’s been compared with that of breast cancer in quality-of-life studies.
“Current treatments for thyroid eye disease are rather limited,” he said. “They really encompass just reducing the swelling and the short-term manifestations of the disease. Treatments such as IV steroids and radiation have been shown to not have any effect on long-term manifestations such as eye bulging and double vision.”
Teprotumumab is a fully human monoclonal antibody that targets the insulinlike growth factor I receptor (IGF-IR). It seems to downregulate thyroid eye disease, Dr. Douglas said.
Researchers studied the drug in a randomized, placebo-controlled study: 41 patients were designated to receive eight intravenous infusions of the drug over 21 weeks (10 mg/kg for the first infusion, then 20 mg/kg thereafter). At week 24, 83% of the study group (34 of 41 patients) reached the endpoint of a reduction of eye bulging by at least 2 mm, compared with 10% of patients (4 of 42) in the placebo group, which received infusions of saline solution.
Two millimeters is significant, Dr. Douglas said. “If you noticed someone’s eye was bulging 2 millimeters, you’d say, ‘Hey, I think something is wrong with your eye.’ ”
Initial study results were released in February 2019. New data about secondary endpoints were released at the AACE meeting: Researchers reported that the average reduction in proptosis (eye bulging) was 2.82 mm in the study group, compared with 0.54 mm in the placebo group (P less than .001).
Dr. Douglas said he can “achieve 3 mm of reduction through surgery to drill out the bone between the eye and the brain. [The patients in the study group] were able to achieve almost 3 millimeters of reduction by the drug alone. It’s a rather significant improvement.”
The new data also provided some insight into the timing of clinical improvements. According to Dr. Douglas, “most of the endpoints were met as early as 6 weeks or [after] two infusions of this drug.”
The adverse effects were relatively mild and included muscle spasms, said Dr. Douglas. The side effects seem to be “well tolerated,” he noted, and none led to cessation of therapy.
Participants with potential for motherhood or fatherhood during the trial had to agree to take precautions to avoid becoming pregnant or impregnating a partner.
Dr. Douglas didn’t provide cost information about the drug. However, it seems likely to be expensive. A writer with Seeking Alpha, a stock market analysis site, estimated that the cost could reach “$250,000 or $300,000 per patient per year, which translates to a $3.7 billion to $6 billion market in the United States alone (based on the estimated patient population in the 15,000-20,000 range).”
The drug’s manufacturer, Horizon Therapeutics, expects to apply to the Food and Drug Administration later this year for approval of the drug.
If it is approved, endocrinologists will have an opportunity to partner with eye surgeons to treat thyroid eye disease, Dr. Douglas said. “The crux will be the comanagement with the endocrinologist helping to control the thyroid function and manage some of the side effects of this medication, and the oculoplastic surgeon [working on] diagnosis, appropriate use, and management.”
Horizon Therapeutics funded the study. Dr. Douglas disclosed that he is a consultant with the company.
REPORTING FROM AACE 2019
Subclinical hypothyroidism may be associated with increased cancer risks
LOS ANGELES – There is no consistent evidence to suggest that subclinical hypothyroidism is linked to an increased risk of incident breast, prostate, or colon cancer. The condition, however, may be linked to an increased risk of thyroid malignancy and to an increased risk of overall cancer mortality.
These findings come from the first systematic review to examine the effect of subclinical hypothyroidism on the risk of incident cancer and cancer mortality.
“Subclinical hypothyroidism is very prevalent,” lead study author Oriana Yu, MD, MSc, said in an interview at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “It affects up to 10% of people, yet at this time we are uncertain as to whether or not [we should] treat them. Most studies have focused on cardiovascular outcomes, because we know that thyroid hormone is very important in lipid metabolism.”
Dr. Yu, an endocrinologist at McGill University, Montreal, and her colleagues searched the Ovid MEDLINE database for articles published from the date of its inception until Nov. 13, 2017, and combined words related to thyroid function and cancer in their search. They limited the analysis to randomized clinical trials and cohort and case-control studies in which the thyroid dysfunction chronologically preceded the cancer incidence or mortality by at least one year.
Of the 180 records screened, 51 full-text articles were assessed for eligibility. Of those, nine met the criteria for systematic review – seven related to cancer risk and two to cancer mortality. The studies were deemed to be of good to medium quality, but six of the nine were cohort studies, the rest case-control studies. “We had hoped to do a systematic review and meta-analysis, but there was high heterogeneity in the studies, especially in terms of different risk measures and outcomes reported,” Dr. Yu said. “As a result, we were not able to perform a meta-analysis.”
The researchers found that the studies had inconsistent findings when it came to the impact of subclinical hypothyroidism on the risk of breast, prostate, and colon cancer. For example, one prospective cohort study of 2,738 patients found no association between subclinical hypothyroidism and the risk of breast cancer (odds ratio, 1.9; 95% confidence interval, 0.8-4.9; Thyroid. 2005;15[11]:1253-9). Women with breast cancer, however, were more likely to have thyroid autoantibodies.
Meanwhile, a case-control study of 1,201 men found that those with elevated TSH levels had a decreased risk of prostate cancer (OR, 0.71; 95% CI, 0.47-1.06; PLoS One. 2012 Oct 30. doi: 10.1371/journal.pone.0047730). “That was a bit surprising to us,” Dr. Yu said. In addition, a nested case-control study of 103,044 patients found that subclinical hypothyroidism was linked to an increased risk for colon cancer (OR, 1.16; 95% CI, 1.08-1.24; J Natl Cancer Inst. 2015 Apr 8. doi: 10.1093/jnci/djv084).
Of the two studies that focused on cancer mortality, one retrospective cohort analysis of 4,735 patients showed that treatment of subclinical hypothyroidism was associated with a decreased risk of cancer mortality in those aged 40-70 years (hazard ratio, 0.59; 95% CI, 0.21-0.99; Arch Intern Med. 2012;172[10]:811-7). The other study, a retrospective cohort analysis of 115,746 patients, found that subclinical hypothyroidism was associated with an increased risk of cancer mortality (relative risk, 1.51; 95% CI, 1.06-2.15; PLoS One. 2015 Apr 1. doi: 10.1371/journal.pone.0122955).
“We need to interpret the cancer-related mortality findings with caution,” Dr. Yu said. “There’s concern about whether patients who are treated might be [generally] healthier or were less frail, compared with those who were not treated. Although these studies adjusted for a number of confounders, it may be difficult to measure and adjust for [those two] factors. That might explain the findings in the two studies on cancer-related mortality.”
Dr. Yu and two coauthors have received salary awards from the Fonds de recherche du Québec–Santé.
LOS ANGELES – There is no consistent evidence to suggest that subclinical hypothyroidism is linked to an increased risk of incident breast, prostate, or colon cancer. The condition, however, may be linked to an increased risk of thyroid malignancy and to an increased risk of overall cancer mortality.
These findings come from the first systematic review to examine the effect of subclinical hypothyroidism on the risk of incident cancer and cancer mortality.
“Subclinical hypothyroidism is very prevalent,” lead study author Oriana Yu, MD, MSc, said in an interview at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “It affects up to 10% of people, yet at this time we are uncertain as to whether or not [we should] treat them. Most studies have focused on cardiovascular outcomes, because we know that thyroid hormone is very important in lipid metabolism.”
Dr. Yu, an endocrinologist at McGill University, Montreal, and her colleagues searched the Ovid MEDLINE database for articles published from the date of its inception until Nov. 13, 2017, and combined words related to thyroid function and cancer in their search. They limited the analysis to randomized clinical trials and cohort and case-control studies in which the thyroid dysfunction chronologically preceded the cancer incidence or mortality by at least one year.
Of the 180 records screened, 51 full-text articles were assessed for eligibility. Of those, nine met the criteria for systematic review – seven related to cancer risk and two to cancer mortality. The studies were deemed to be of good to medium quality, but six of the nine were cohort studies, the rest case-control studies. “We had hoped to do a systematic review and meta-analysis, but there was high heterogeneity in the studies, especially in terms of different risk measures and outcomes reported,” Dr. Yu said. “As a result, we were not able to perform a meta-analysis.”
The researchers found that the studies had inconsistent findings when it came to the impact of subclinical hypothyroidism on the risk of breast, prostate, and colon cancer. For example, one prospective cohort study of 2,738 patients found no association between subclinical hypothyroidism and the risk of breast cancer (odds ratio, 1.9; 95% confidence interval, 0.8-4.9; Thyroid. 2005;15[11]:1253-9). Women with breast cancer, however, were more likely to have thyroid autoantibodies.
Meanwhile, a case-control study of 1,201 men found that those with elevated TSH levels had a decreased risk of prostate cancer (OR, 0.71; 95% CI, 0.47-1.06; PLoS One. 2012 Oct 30. doi: 10.1371/journal.pone.0047730). “That was a bit surprising to us,” Dr. Yu said. In addition, a nested case-control study of 103,044 patients found that subclinical hypothyroidism was linked to an increased risk for colon cancer (OR, 1.16; 95% CI, 1.08-1.24; J Natl Cancer Inst. 2015 Apr 8. doi: 10.1093/jnci/djv084).
Of the two studies that focused on cancer mortality, one retrospective cohort analysis of 4,735 patients showed that treatment of subclinical hypothyroidism was associated with a decreased risk of cancer mortality in those aged 40-70 years (hazard ratio, 0.59; 95% CI, 0.21-0.99; Arch Intern Med. 2012;172[10]:811-7). The other study, a retrospective cohort analysis of 115,746 patients, found that subclinical hypothyroidism was associated with an increased risk of cancer mortality (relative risk, 1.51; 95% CI, 1.06-2.15; PLoS One. 2015 Apr 1. doi: 10.1371/journal.pone.0122955).
“We need to interpret the cancer-related mortality findings with caution,” Dr. Yu said. “There’s concern about whether patients who are treated might be [generally] healthier or were less frail, compared with those who were not treated. Although these studies adjusted for a number of confounders, it may be difficult to measure and adjust for [those two] factors. That might explain the findings in the two studies on cancer-related mortality.”
Dr. Yu and two coauthors have received salary awards from the Fonds de recherche du Québec–Santé.
LOS ANGELES – There is no consistent evidence to suggest that subclinical hypothyroidism is linked to an increased risk of incident breast, prostate, or colon cancer. The condition, however, may be linked to an increased risk of thyroid malignancy and to an increased risk of overall cancer mortality.
These findings come from the first systematic review to examine the effect of subclinical hypothyroidism on the risk of incident cancer and cancer mortality.
“Subclinical hypothyroidism is very prevalent,” lead study author Oriana Yu, MD, MSc, said in an interview at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “It affects up to 10% of people, yet at this time we are uncertain as to whether or not [we should] treat them. Most studies have focused on cardiovascular outcomes, because we know that thyroid hormone is very important in lipid metabolism.”
Dr. Yu, an endocrinologist at McGill University, Montreal, and her colleagues searched the Ovid MEDLINE database for articles published from the date of its inception until Nov. 13, 2017, and combined words related to thyroid function and cancer in their search. They limited the analysis to randomized clinical trials and cohort and case-control studies in which the thyroid dysfunction chronologically preceded the cancer incidence or mortality by at least one year.
Of the 180 records screened, 51 full-text articles were assessed for eligibility. Of those, nine met the criteria for systematic review – seven related to cancer risk and two to cancer mortality. The studies were deemed to be of good to medium quality, but six of the nine were cohort studies, the rest case-control studies. “We had hoped to do a systematic review and meta-analysis, but there was high heterogeneity in the studies, especially in terms of different risk measures and outcomes reported,” Dr. Yu said. “As a result, we were not able to perform a meta-analysis.”
The researchers found that the studies had inconsistent findings when it came to the impact of subclinical hypothyroidism on the risk of breast, prostate, and colon cancer. For example, one prospective cohort study of 2,738 patients found no association between subclinical hypothyroidism and the risk of breast cancer (odds ratio, 1.9; 95% confidence interval, 0.8-4.9; Thyroid. 2005;15[11]:1253-9). Women with breast cancer, however, were more likely to have thyroid autoantibodies.
Meanwhile, a case-control study of 1,201 men found that those with elevated TSH levels had a decreased risk of prostate cancer (OR, 0.71; 95% CI, 0.47-1.06; PLoS One. 2012 Oct 30. doi: 10.1371/journal.pone.0047730). “That was a bit surprising to us,” Dr. Yu said. In addition, a nested case-control study of 103,044 patients found that subclinical hypothyroidism was linked to an increased risk for colon cancer (OR, 1.16; 95% CI, 1.08-1.24; J Natl Cancer Inst. 2015 Apr 8. doi: 10.1093/jnci/djv084).
Of the two studies that focused on cancer mortality, one retrospective cohort analysis of 4,735 patients showed that treatment of subclinical hypothyroidism was associated with a decreased risk of cancer mortality in those aged 40-70 years (hazard ratio, 0.59; 95% CI, 0.21-0.99; Arch Intern Med. 2012;172[10]:811-7). The other study, a retrospective cohort analysis of 115,746 patients, found that subclinical hypothyroidism was associated with an increased risk of cancer mortality (relative risk, 1.51; 95% CI, 1.06-2.15; PLoS One. 2015 Apr 1. doi: 10.1371/journal.pone.0122955).
“We need to interpret the cancer-related mortality findings with caution,” Dr. Yu said. “There’s concern about whether patients who are treated might be [generally] healthier or were less frail, compared with those who were not treated. Although these studies adjusted for a number of confounders, it may be difficult to measure and adjust for [those two] factors. That might explain the findings in the two studies on cancer-related mortality.”
Dr. Yu and two coauthors have received salary awards from the Fonds de recherche du Québec–Santé.
REPORTING FROM AACE 2019
Key clinical point: Further studies are needed to assess the association between untreated subclinical hypothyroidism and the risks of different types of cancer.
Major finding:
Study details: A systematic review of nine studies.
Disclosures: Dr. Yu and two coauthors have received salary awards from the Fonds de recherche du Québec–Santé.
New adventures of an old device: Clinic delivers cortisol via the insulin pump
LOS ANGELES – The venerable insulin pump is being repurposed: A Detroit-area endocrinology
“We’ve seen amazing results,” said endocrinologist Opada Alzohaili, MD, MBA, of Wayne State University, Detroit, coauthor of a study released at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Alzohaili said he and his colleagues developed the approach to manage patients who are “so sick that they go to the hospital 10-12 times a year.” Oral medication just did not control their disorder.
“Most of the time, we end up way overdosing them [on oral medication] just to prevent them from going to the hospital in adrenal crisis,” he said in an interview.
Dr. Alzohaili said his clinic has tested delivering hydrocortisone via insulin pump in about 20 patients. The report presented at the conference focused on six patients who had failed oral hydrocortisone treatment for adrenal insufficiency. Testing showed that all had malabsorption of the drug.
The patients underwent training in how to use and adjust the pump, which allows dosing adjustments in increments of 1 mg. They learned how to adjust their doses based on their situation, Dr. Alzohaili said.
According to the report, the average number of adrenal crises in the patients over a 6-month period fell from a mean of 2.3 before baseline to 0.5 after treatment began. The maximum dose of hydrocortisone dose fell by 38%, while the average mean weight of patients rose from 182 pounds to 199 pounds.
In addition, the mean dose of hydrocortisone decreased with the use of the pump delivery system, from 85.8 mg with oral treatment to 32.4 mg on pump therapy, and the mean level of cortisol increased from 11.8 mcg/dL with oral treatment to 12.3 mcg/dL on pump therapy.
The researchers said that the pump provides better delivery of the medication compared with the oral route, and that the patients experienced fewer interactions with other medications.
Some patients developed skin reactions to the pump, but those adverse events were resolved by changing the pump’s location on the body and by using hypoallergenic needles, Dr. Alzohaili said.
There were fewer cases of clogging with the pumps than is normally seen when they’re used with insulin, he added.
As for expense, Dr. Alzohaili said the pumps cost thousands of dollars and supplies can cost between $100 and $150 a month. In the first couple of cases, patients paid for the treatment themselves, he said, but in later cases, insurers were willing to pay for the treatment once they learned about the results.
Other researchers have successfully used insulin pumps to deliver hydrocortisone to small numbers of patients with adrenal insufficiency, including British and U.S. teams that reported positive results in 2015 and 2018, respectively.
The next step, Dr. Alzohaili said, is to attract the interest of insulin pump manufacturers by using the treatment in more patients. “I’ve spoken to CEOs, but none of them is interested in using cortisol in their pumps,” he said. “If you don’t have the company supporting the research, it becomes difficult for it to become standard of care. So I’m trying to build awareness [of its use] and the number of patients [who use the pump].”
Dr. Alzohaili reported no financial conflicts of interest or disclosures.
LOS ANGELES – The venerable insulin pump is being repurposed: A Detroit-area endocrinology
“We’ve seen amazing results,” said endocrinologist Opada Alzohaili, MD, MBA, of Wayne State University, Detroit, coauthor of a study released at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Alzohaili said he and his colleagues developed the approach to manage patients who are “so sick that they go to the hospital 10-12 times a year.” Oral medication just did not control their disorder.
“Most of the time, we end up way overdosing them [on oral medication] just to prevent them from going to the hospital in adrenal crisis,” he said in an interview.
Dr. Alzohaili said his clinic has tested delivering hydrocortisone via insulin pump in about 20 patients. The report presented at the conference focused on six patients who had failed oral hydrocortisone treatment for adrenal insufficiency. Testing showed that all had malabsorption of the drug.
The patients underwent training in how to use and adjust the pump, which allows dosing adjustments in increments of 1 mg. They learned how to adjust their doses based on their situation, Dr. Alzohaili said.
According to the report, the average number of adrenal crises in the patients over a 6-month period fell from a mean of 2.3 before baseline to 0.5 after treatment began. The maximum dose of hydrocortisone dose fell by 38%, while the average mean weight of patients rose from 182 pounds to 199 pounds.
In addition, the mean dose of hydrocortisone decreased with the use of the pump delivery system, from 85.8 mg with oral treatment to 32.4 mg on pump therapy, and the mean level of cortisol increased from 11.8 mcg/dL with oral treatment to 12.3 mcg/dL on pump therapy.
The researchers said that the pump provides better delivery of the medication compared with the oral route, and that the patients experienced fewer interactions with other medications.
Some patients developed skin reactions to the pump, but those adverse events were resolved by changing the pump’s location on the body and by using hypoallergenic needles, Dr. Alzohaili said.
There were fewer cases of clogging with the pumps than is normally seen when they’re used with insulin, he added.
As for expense, Dr. Alzohaili said the pumps cost thousands of dollars and supplies can cost between $100 and $150 a month. In the first couple of cases, patients paid for the treatment themselves, he said, but in later cases, insurers were willing to pay for the treatment once they learned about the results.
Other researchers have successfully used insulin pumps to deliver hydrocortisone to small numbers of patients with adrenal insufficiency, including British and U.S. teams that reported positive results in 2015 and 2018, respectively.
The next step, Dr. Alzohaili said, is to attract the interest of insulin pump manufacturers by using the treatment in more patients. “I’ve spoken to CEOs, but none of them is interested in using cortisol in their pumps,” he said. “If you don’t have the company supporting the research, it becomes difficult for it to become standard of care. So I’m trying to build awareness [of its use] and the number of patients [who use the pump].”
Dr. Alzohaili reported no financial conflicts of interest or disclosures.
LOS ANGELES – The venerable insulin pump is being repurposed: A Detroit-area endocrinology
“We’ve seen amazing results,” said endocrinologist Opada Alzohaili, MD, MBA, of Wayne State University, Detroit, coauthor of a study released at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Alzohaili said he and his colleagues developed the approach to manage patients who are “so sick that they go to the hospital 10-12 times a year.” Oral medication just did not control their disorder.
“Most of the time, we end up way overdosing them [on oral medication] just to prevent them from going to the hospital in adrenal crisis,” he said in an interview.
Dr. Alzohaili said his clinic has tested delivering hydrocortisone via insulin pump in about 20 patients. The report presented at the conference focused on six patients who had failed oral hydrocortisone treatment for adrenal insufficiency. Testing showed that all had malabsorption of the drug.
The patients underwent training in how to use and adjust the pump, which allows dosing adjustments in increments of 1 mg. They learned how to adjust their doses based on their situation, Dr. Alzohaili said.
According to the report, the average number of adrenal crises in the patients over a 6-month period fell from a mean of 2.3 before baseline to 0.5 after treatment began. The maximum dose of hydrocortisone dose fell by 38%, while the average mean weight of patients rose from 182 pounds to 199 pounds.
In addition, the mean dose of hydrocortisone decreased with the use of the pump delivery system, from 85.8 mg with oral treatment to 32.4 mg on pump therapy, and the mean level of cortisol increased from 11.8 mcg/dL with oral treatment to 12.3 mcg/dL on pump therapy.
The researchers said that the pump provides better delivery of the medication compared with the oral route, and that the patients experienced fewer interactions with other medications.
Some patients developed skin reactions to the pump, but those adverse events were resolved by changing the pump’s location on the body and by using hypoallergenic needles, Dr. Alzohaili said.
There were fewer cases of clogging with the pumps than is normally seen when they’re used with insulin, he added.
As for expense, Dr. Alzohaili said the pumps cost thousands of dollars and supplies can cost between $100 and $150 a month. In the first couple of cases, patients paid for the treatment themselves, he said, but in later cases, insurers were willing to pay for the treatment once they learned about the results.
Other researchers have successfully used insulin pumps to deliver hydrocortisone to small numbers of patients with adrenal insufficiency, including British and U.S. teams that reported positive results in 2015 and 2018, respectively.
The next step, Dr. Alzohaili said, is to attract the interest of insulin pump manufacturers by using the treatment in more patients. “I’ve spoken to CEOs, but none of them is interested in using cortisol in their pumps,” he said. “If you don’t have the company supporting the research, it becomes difficult for it to become standard of care. So I’m trying to build awareness [of its use] and the number of patients [who use the pump].”
Dr. Alzohaili reported no financial conflicts of interest or disclosures.
REPORTING FROM AACE 2019
Anxiety, not depression, commonly afflicts euthyroid patients with thyroid disease
LOS ANGELES – results from a cross-sectional study have shown.
“Thyroid disease is often associated with impaired quality of life and psychological well-being,” lead study author Anette Merke, MD, MS, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “In daily practice, anxiety and depression complaints are common in patients with thyroid dysfunctions. While depressive symptoms are often associated with hypothyroidism, anxiety is claimed to be mainly linked to hyperthyroidism. Data on euthyroid patients with thyroid disease are controversial. Some studies point out that autoimmunity itself contributes to psychosomatic malfunctions. Overall, the mechanisms underlying the interaction between thyroid dysfunction and neuropsychiatric processes are still unknown.”
Dr. Merke, of the Thyroid Center Bergstrasse in Bensheim, Germany, and her husband/coauthor Jüergen Merke, MD, PhD, used the self-administered German version of the validated Hospital Anxiety and Depression Scale (HADS-D) to perform a cross-sectional study of 215 euthyroid adults with thyroid disease between January and February of 2019. Of the 14 items on the measure, half relate to anxiety and the other half to depression. Each item on the HADS-D is scored from 0-3, and a score of 10 or higher is considered a positive case of anxiety or depression. Patients completed the HADS-D within 3 months of routine lab testing, and the researchers collected the patients’ demographic data after they had assessed the individual scores.
Of the 215 study participants, most (89%) were women, the mean age was 47 years, and the mean anxiety and depression scores were 6.68 and 4.68, respectively (P = .0001). There was no significant difference in severity with respect to anxiety or depression. Of the 70 patients (33%) with antibody-positive Hashimoto’s thyroiditis, the mean anxiety and depression scores were 7.26 and 4.17.
In patients with HADS-D scores of 10 or greater, 50 (23%) had prominent anxiety scores (mean, 12.4), whereas 22 (10%) had prominent depression scores (mean, 13.18). Among the subset of Hashimoto’s thyroiditis patients, 18 (26%) had a mean anxiety score of 12.6 and 8 (11%) had a mean depression score of 13.18. Overall, significantly more cases were found in those who met criteria for anxiety, compared with those who met criteria for depression (P = .0001), with no significant difference in severity of either condition.
Depressive symptoms are usually more closely associated with thyroid disease, and there are more studies that have examined that relationship, so “we were surprised to find no significant difference in depressive symptoms between our study cohort and the German general population,” Dr. Merke said. “We were also surprised that anxiety had a significantly higher incidence in the cohort.”
The findings suggest that clinicians should focus on signs of anxiety symptoms when dealing with euthyroid patients with thyroid disease who report psychosomatic impairments, she continued, especially when patients complain of not being able to relax and release somatic tension.
“According to HADS, fears and worries are an expression of anxiety, and not of depression,” Dr. Merke said. “With this in mind, doctors [should] actively ask [about] the above-mentioned symptoms and advise patients to learn relaxation techniques to improve their quality of life. Interdisciplinary collaboration is needed between clinicians and psychotherapeutic professionals for the sake of the patients and to evaluate a cause-and-effect relationship and potential risk factors for development of psychosomatic cofactors in thyroid and other chronic somatic diseases. We should all be aware that misinterpretation or even denial of psychosomatic complaints may lead to complications and even a higher mortality of somatic diseases, as shown for chronic heart failure. This could also be true for thyroid disease.”
Dr Merke acknowledged certain limitations of the study, including the fact that neither the duration of thyroid disease nor the use of specific thyroid medications was assessed. In addition, “the definition of euthyroidism in our study is somewhat broad, especially compared with the recommendations of the [American Association of Clinical Endocrinologists],” she said. “Division of the respective thyroid hormone status into quartiles may be helpful to indicate the critical thyroid hormone serum concentration, which may be associated with clinically relevant anxiety symptoms in euthyroid patients.”
Dr. Merke reported having no financial disclosures or conflict of interest.
LOS ANGELES – results from a cross-sectional study have shown.
“Thyroid disease is often associated with impaired quality of life and psychological well-being,” lead study author Anette Merke, MD, MS, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “In daily practice, anxiety and depression complaints are common in patients with thyroid dysfunctions. While depressive symptoms are often associated with hypothyroidism, anxiety is claimed to be mainly linked to hyperthyroidism. Data on euthyroid patients with thyroid disease are controversial. Some studies point out that autoimmunity itself contributes to psychosomatic malfunctions. Overall, the mechanisms underlying the interaction between thyroid dysfunction and neuropsychiatric processes are still unknown.”
Dr. Merke, of the Thyroid Center Bergstrasse in Bensheim, Germany, and her husband/coauthor Jüergen Merke, MD, PhD, used the self-administered German version of the validated Hospital Anxiety and Depression Scale (HADS-D) to perform a cross-sectional study of 215 euthyroid adults with thyroid disease between January and February of 2019. Of the 14 items on the measure, half relate to anxiety and the other half to depression. Each item on the HADS-D is scored from 0-3, and a score of 10 or higher is considered a positive case of anxiety or depression. Patients completed the HADS-D within 3 months of routine lab testing, and the researchers collected the patients’ demographic data after they had assessed the individual scores.
Of the 215 study participants, most (89%) were women, the mean age was 47 years, and the mean anxiety and depression scores were 6.68 and 4.68, respectively (P = .0001). There was no significant difference in severity with respect to anxiety or depression. Of the 70 patients (33%) with antibody-positive Hashimoto’s thyroiditis, the mean anxiety and depression scores were 7.26 and 4.17.
In patients with HADS-D scores of 10 or greater, 50 (23%) had prominent anxiety scores (mean, 12.4), whereas 22 (10%) had prominent depression scores (mean, 13.18). Among the subset of Hashimoto’s thyroiditis patients, 18 (26%) had a mean anxiety score of 12.6 and 8 (11%) had a mean depression score of 13.18. Overall, significantly more cases were found in those who met criteria for anxiety, compared with those who met criteria for depression (P = .0001), with no significant difference in severity of either condition.
Depressive symptoms are usually more closely associated with thyroid disease, and there are more studies that have examined that relationship, so “we were surprised to find no significant difference in depressive symptoms between our study cohort and the German general population,” Dr. Merke said. “We were also surprised that anxiety had a significantly higher incidence in the cohort.”
The findings suggest that clinicians should focus on signs of anxiety symptoms when dealing with euthyroid patients with thyroid disease who report psychosomatic impairments, she continued, especially when patients complain of not being able to relax and release somatic tension.
“According to HADS, fears and worries are an expression of anxiety, and not of depression,” Dr. Merke said. “With this in mind, doctors [should] actively ask [about] the above-mentioned symptoms and advise patients to learn relaxation techniques to improve their quality of life. Interdisciplinary collaboration is needed between clinicians and psychotherapeutic professionals for the sake of the patients and to evaluate a cause-and-effect relationship and potential risk factors for development of psychosomatic cofactors in thyroid and other chronic somatic diseases. We should all be aware that misinterpretation or even denial of psychosomatic complaints may lead to complications and even a higher mortality of somatic diseases, as shown for chronic heart failure. This could also be true for thyroid disease.”
Dr Merke acknowledged certain limitations of the study, including the fact that neither the duration of thyroid disease nor the use of specific thyroid medications was assessed. In addition, “the definition of euthyroidism in our study is somewhat broad, especially compared with the recommendations of the [American Association of Clinical Endocrinologists],” she said. “Division of the respective thyroid hormone status into quartiles may be helpful to indicate the critical thyroid hormone serum concentration, which may be associated with clinically relevant anxiety symptoms in euthyroid patients.”
Dr. Merke reported having no financial disclosures or conflict of interest.
LOS ANGELES – results from a cross-sectional study have shown.
“Thyroid disease is often associated with impaired quality of life and psychological well-being,” lead study author Anette Merke, MD, MS, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “In daily practice, anxiety and depression complaints are common in patients with thyroid dysfunctions. While depressive symptoms are often associated with hypothyroidism, anxiety is claimed to be mainly linked to hyperthyroidism. Data on euthyroid patients with thyroid disease are controversial. Some studies point out that autoimmunity itself contributes to psychosomatic malfunctions. Overall, the mechanisms underlying the interaction between thyroid dysfunction and neuropsychiatric processes are still unknown.”
Dr. Merke, of the Thyroid Center Bergstrasse in Bensheim, Germany, and her husband/coauthor Jüergen Merke, MD, PhD, used the self-administered German version of the validated Hospital Anxiety and Depression Scale (HADS-D) to perform a cross-sectional study of 215 euthyroid adults with thyroid disease between January and February of 2019. Of the 14 items on the measure, half relate to anxiety and the other half to depression. Each item on the HADS-D is scored from 0-3, and a score of 10 or higher is considered a positive case of anxiety or depression. Patients completed the HADS-D within 3 months of routine lab testing, and the researchers collected the patients’ demographic data after they had assessed the individual scores.
Of the 215 study participants, most (89%) were women, the mean age was 47 years, and the mean anxiety and depression scores were 6.68 and 4.68, respectively (P = .0001). There was no significant difference in severity with respect to anxiety or depression. Of the 70 patients (33%) with antibody-positive Hashimoto’s thyroiditis, the mean anxiety and depression scores were 7.26 and 4.17.
In patients with HADS-D scores of 10 or greater, 50 (23%) had prominent anxiety scores (mean, 12.4), whereas 22 (10%) had prominent depression scores (mean, 13.18). Among the subset of Hashimoto’s thyroiditis patients, 18 (26%) had a mean anxiety score of 12.6 and 8 (11%) had a mean depression score of 13.18. Overall, significantly more cases were found in those who met criteria for anxiety, compared with those who met criteria for depression (P = .0001), with no significant difference in severity of either condition.
Depressive symptoms are usually more closely associated with thyroid disease, and there are more studies that have examined that relationship, so “we were surprised to find no significant difference in depressive symptoms between our study cohort and the German general population,” Dr. Merke said. “We were also surprised that anxiety had a significantly higher incidence in the cohort.”
The findings suggest that clinicians should focus on signs of anxiety symptoms when dealing with euthyroid patients with thyroid disease who report psychosomatic impairments, she continued, especially when patients complain of not being able to relax and release somatic tension.
“According to HADS, fears and worries are an expression of anxiety, and not of depression,” Dr. Merke said. “With this in mind, doctors [should] actively ask [about] the above-mentioned symptoms and advise patients to learn relaxation techniques to improve their quality of life. Interdisciplinary collaboration is needed between clinicians and psychotherapeutic professionals for the sake of the patients and to evaluate a cause-and-effect relationship and potential risk factors for development of psychosomatic cofactors in thyroid and other chronic somatic diseases. We should all be aware that misinterpretation or even denial of psychosomatic complaints may lead to complications and even a higher mortality of somatic diseases, as shown for chronic heart failure. This could also be true for thyroid disease.”
Dr Merke acknowledged certain limitations of the study, including the fact that neither the duration of thyroid disease nor the use of specific thyroid medications was assessed. In addition, “the definition of euthyroidism in our study is somewhat broad, especially compared with the recommendations of the [American Association of Clinical Endocrinologists],” she said. “Division of the respective thyroid hormone status into quartiles may be helpful to indicate the critical thyroid hormone serum concentration, which may be associated with clinically relevant anxiety symptoms in euthyroid patients.”
Dr. Merke reported having no financial disclosures or conflict of interest.
REPORTING FROM AACE 2019
Key clinical point: Anxiety in patients with thyroid disease might have a pathological pathway different from depressive disorders.
Major finding: In patients with HADS-D scores of 10 or greater, 50 (23%) had prominent anxiety scores (mean, 12.4) and 22 (10%) had prominent depression scores (mean, 13.18).
Study details: A cross-sectional study of 215 euthyroid patients with thyroid disease.
Disclosures: Dr. Merke reported having no financial disclosures or conflicts of interest.























