User login
Pediatric obesity treatment options: Beyond lifestyle modification
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
PCOS in mothers tied to health problems in children
Children whose mothers have polycystic ovary syndrome (PCOS) have increased rates of hospitalization for various conditions, including asthma, pneumonia, and ear infection, a study of more than 1 million children shows.
The associations were not particularly strong, according to the researchers. But they raise questions about the reasons for the increased risk and whether interventions such as diet, exercise, or medications could lead to healthier outcomes for children whose mothers have PCOS.
“The findings suggest that maternal PCOS may have a negative impact on offspring development, enough to lead to a measurable increase in the risk of childhood hospitalization,” study coauthor Nathalie Auger, MD, associate professor of epidemiology at University of Montreal, and colleagues reported in Human Reproduction.
“They are minor differences, just enough that we can statistically identify them. They’re not something where everyone should be worrying at this point,” Dr. Auger told this news organization.
Still, some of the hospitalizations, such as those related to infection or allergy, could be prevented with earlier ambulatory care, so some degree of greater awareness among parents and clinicians may be warranted, she said.
Thirteen years of follow-up
PCOS – a reproductive disorder characterized by irregular periods, increased male hormones, and metabolic complications – affects some 10% of women. People with the condition are at increased risk for obesity, type 2 diabetes, and cardiovascular disease.
Although prior research has shown that maternal PCOS may be associated with higher body mass index and attention deficit disorder in children, data on long-term childhood health outcomes have been limited, Dr. Auger’s group noted.
To examine illness in children exposed to maternal PCOS, the investigators analyzed hospitalization rates for nearly 1.04 million children in Quebec between 2006 and 2020; 7,160 of the children had mothers with PCOS.
In all, 275,354 children were hospitalized during 13 years of follow-up, including 2,314 whose mothers had PCOS.
Children exposed to PCOS were hospitalized at a rate of 68.9 per 1,000 person-years – roughly 50% more often than the rate of 45.3 per 1,000 person-years for children not exposed to maternal PCOS.
In an analysis that adjusted for maternal characteristics, childhood hospitalization for any reason was 1.32 times more likely for children exposed to maternal PCOS.
Hospitalizations linked to infectious diseases – such as for bronchitis, bronchiolitis, pneumonia, nephritis, otitis media, or meningitis – were 1.31 times more likely among children exposed to PCOS. Allergy-related hospitalizations, such as for allergic asthma and anaphylaxis, were 1.47 times more likely, according to the researchers.
Metabolic hospitalizations were 1.59 times more likely. For gastrointestinal hospitalizations, the hazard ratio was 1.72. For central nervous system hospitalizations, it was 1.74.
The associations were stronger in earlier childhood, and results were similar for boys and girls, the investigators reported.
Hospitalizations for cardiovascular disease, musculoskeletal conditions, or malignancy were not increased.
‘Surprising’ links
“The findings are surprising in that some of the conditions that they showed increased risk for, like asthma and some infections, are not conditions that we think of as being typically associated with PCOS,” said Andrea E. Dunaif, MD, chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes, and Bone Disease at Mount Sinai Health System, New York, who was not part of the study team.
Earlier studies of offspring of women with PCOS have suggested that children may be at increased risk for insulin resistance and obesity.
Differences in genetics, intrauterine environments, patterns of health care use by women with PCOS, and behavioral factors, such as diet and how children are raised, are variables that could have contributed to the different hospitalization rates among children exposed to maternal PCOS, Dr. Auger said.
“Everything is interconnected,” she said.
The study was supported by a grant from the Canadian Institutes of Health Research. Dr. Auger has received a career award from Fonds de Recherche du Québec-Santé. Dr. Dunaif has consulted for Novo Nordisk and Fractyl Laboratories (now Fractyl Health).
A version of this article first appeared on Medscape.com.
Children whose mothers have polycystic ovary syndrome (PCOS) have increased rates of hospitalization for various conditions, including asthma, pneumonia, and ear infection, a study of more than 1 million children shows.
The associations were not particularly strong, according to the researchers. But they raise questions about the reasons for the increased risk and whether interventions such as diet, exercise, or medications could lead to healthier outcomes for children whose mothers have PCOS.
“The findings suggest that maternal PCOS may have a negative impact on offspring development, enough to lead to a measurable increase in the risk of childhood hospitalization,” study coauthor Nathalie Auger, MD, associate professor of epidemiology at University of Montreal, and colleagues reported in Human Reproduction.
“They are minor differences, just enough that we can statistically identify them. They’re not something where everyone should be worrying at this point,” Dr. Auger told this news organization.
Still, some of the hospitalizations, such as those related to infection or allergy, could be prevented with earlier ambulatory care, so some degree of greater awareness among parents and clinicians may be warranted, she said.
Thirteen years of follow-up
PCOS – a reproductive disorder characterized by irregular periods, increased male hormones, and metabolic complications – affects some 10% of women. People with the condition are at increased risk for obesity, type 2 diabetes, and cardiovascular disease.
Although prior research has shown that maternal PCOS may be associated with higher body mass index and attention deficit disorder in children, data on long-term childhood health outcomes have been limited, Dr. Auger’s group noted.
To examine illness in children exposed to maternal PCOS, the investigators analyzed hospitalization rates for nearly 1.04 million children in Quebec between 2006 and 2020; 7,160 of the children had mothers with PCOS.
In all, 275,354 children were hospitalized during 13 years of follow-up, including 2,314 whose mothers had PCOS.
Children exposed to PCOS were hospitalized at a rate of 68.9 per 1,000 person-years – roughly 50% more often than the rate of 45.3 per 1,000 person-years for children not exposed to maternal PCOS.
In an analysis that adjusted for maternal characteristics, childhood hospitalization for any reason was 1.32 times more likely for children exposed to maternal PCOS.
Hospitalizations linked to infectious diseases – such as for bronchitis, bronchiolitis, pneumonia, nephritis, otitis media, or meningitis – were 1.31 times more likely among children exposed to PCOS. Allergy-related hospitalizations, such as for allergic asthma and anaphylaxis, were 1.47 times more likely, according to the researchers.
Metabolic hospitalizations were 1.59 times more likely. For gastrointestinal hospitalizations, the hazard ratio was 1.72. For central nervous system hospitalizations, it was 1.74.
The associations were stronger in earlier childhood, and results were similar for boys and girls, the investigators reported.
Hospitalizations for cardiovascular disease, musculoskeletal conditions, or malignancy were not increased.
‘Surprising’ links
“The findings are surprising in that some of the conditions that they showed increased risk for, like asthma and some infections, are not conditions that we think of as being typically associated with PCOS,” said Andrea E. Dunaif, MD, chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes, and Bone Disease at Mount Sinai Health System, New York, who was not part of the study team.
Earlier studies of offspring of women with PCOS have suggested that children may be at increased risk for insulin resistance and obesity.
Differences in genetics, intrauterine environments, patterns of health care use by women with PCOS, and behavioral factors, such as diet and how children are raised, are variables that could have contributed to the different hospitalization rates among children exposed to maternal PCOS, Dr. Auger said.
“Everything is interconnected,” she said.
The study was supported by a grant from the Canadian Institutes of Health Research. Dr. Auger has received a career award from Fonds de Recherche du Québec-Santé. Dr. Dunaif has consulted for Novo Nordisk and Fractyl Laboratories (now Fractyl Health).
A version of this article first appeared on Medscape.com.
Children whose mothers have polycystic ovary syndrome (PCOS) have increased rates of hospitalization for various conditions, including asthma, pneumonia, and ear infection, a study of more than 1 million children shows.
The associations were not particularly strong, according to the researchers. But they raise questions about the reasons for the increased risk and whether interventions such as diet, exercise, or medications could lead to healthier outcomes for children whose mothers have PCOS.
“The findings suggest that maternal PCOS may have a negative impact on offspring development, enough to lead to a measurable increase in the risk of childhood hospitalization,” study coauthor Nathalie Auger, MD, associate professor of epidemiology at University of Montreal, and colleagues reported in Human Reproduction.
“They are minor differences, just enough that we can statistically identify them. They’re not something where everyone should be worrying at this point,” Dr. Auger told this news organization.
Still, some of the hospitalizations, such as those related to infection or allergy, could be prevented with earlier ambulatory care, so some degree of greater awareness among parents and clinicians may be warranted, she said.
Thirteen years of follow-up
PCOS – a reproductive disorder characterized by irregular periods, increased male hormones, and metabolic complications – affects some 10% of women. People with the condition are at increased risk for obesity, type 2 diabetes, and cardiovascular disease.
Although prior research has shown that maternal PCOS may be associated with higher body mass index and attention deficit disorder in children, data on long-term childhood health outcomes have been limited, Dr. Auger’s group noted.
To examine illness in children exposed to maternal PCOS, the investigators analyzed hospitalization rates for nearly 1.04 million children in Quebec between 2006 and 2020; 7,160 of the children had mothers with PCOS.
In all, 275,354 children were hospitalized during 13 years of follow-up, including 2,314 whose mothers had PCOS.
Children exposed to PCOS were hospitalized at a rate of 68.9 per 1,000 person-years – roughly 50% more often than the rate of 45.3 per 1,000 person-years for children not exposed to maternal PCOS.
In an analysis that adjusted for maternal characteristics, childhood hospitalization for any reason was 1.32 times more likely for children exposed to maternal PCOS.
Hospitalizations linked to infectious diseases – such as for bronchitis, bronchiolitis, pneumonia, nephritis, otitis media, or meningitis – were 1.31 times more likely among children exposed to PCOS. Allergy-related hospitalizations, such as for allergic asthma and anaphylaxis, were 1.47 times more likely, according to the researchers.
Metabolic hospitalizations were 1.59 times more likely. For gastrointestinal hospitalizations, the hazard ratio was 1.72. For central nervous system hospitalizations, it was 1.74.
The associations were stronger in earlier childhood, and results were similar for boys and girls, the investigators reported.
Hospitalizations for cardiovascular disease, musculoskeletal conditions, or malignancy were not increased.
‘Surprising’ links
“The findings are surprising in that some of the conditions that they showed increased risk for, like asthma and some infections, are not conditions that we think of as being typically associated with PCOS,” said Andrea E. Dunaif, MD, chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes, and Bone Disease at Mount Sinai Health System, New York, who was not part of the study team.
Earlier studies of offspring of women with PCOS have suggested that children may be at increased risk for insulin resistance and obesity.
Differences in genetics, intrauterine environments, patterns of health care use by women with PCOS, and behavioral factors, such as diet and how children are raised, are variables that could have contributed to the different hospitalization rates among children exposed to maternal PCOS, Dr. Auger said.
“Everything is interconnected,” she said.
The study was supported by a grant from the Canadian Institutes of Health Research. Dr. Auger has received a career award from Fonds de Recherche du Québec-Santé. Dr. Dunaif has consulted for Novo Nordisk and Fractyl Laboratories (now Fractyl Health).
A version of this article first appeared on Medscape.com.
FROM HUMAN REPRODUCTION
Children and COVID: Does latest rise in new cases point toward stabilization?
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.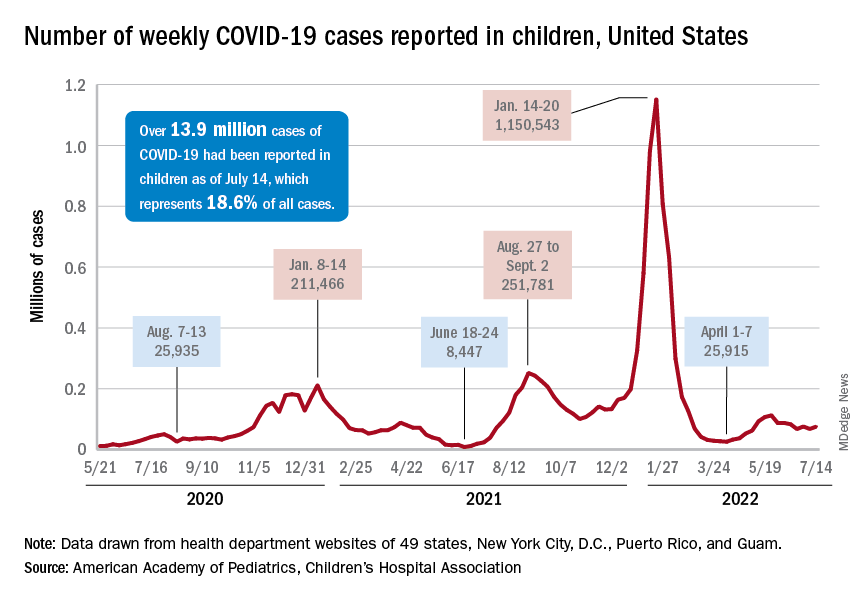
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.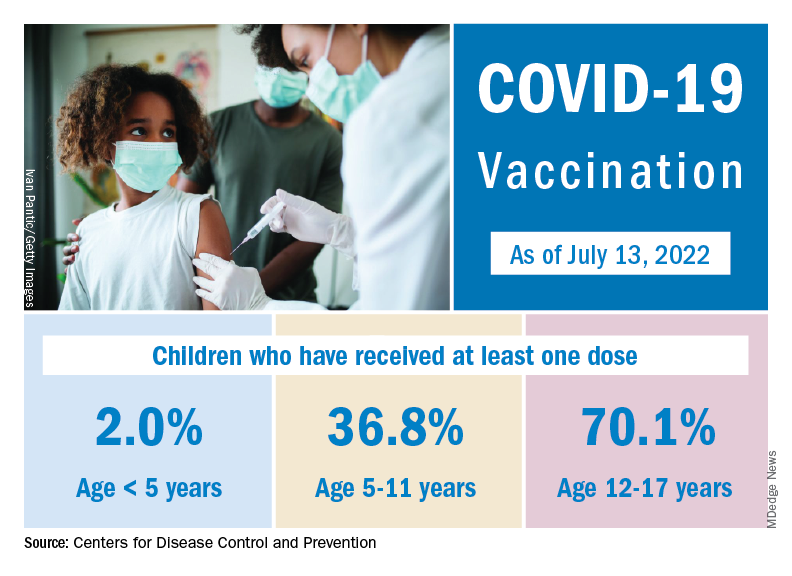
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
Anxiety spreads from mother to daughter, father to son
The new findings suggest that children learn anxious behavior from their parents, study investigator Barbara Pavlova, PhD, clinical psychologist with Nova Scotia Health Authority, told this news organization.
“This means that transmission of anxiety from parents to children may be preventable,” said Dr. Pavlova, assistant professor, department of psychiatry, Dalhousie University, Halifax, Canada.
“Treating parents’ anxiety is not just important for their own health but also for the health of their children. This may be especially true if the child and the parent are the same sex,” Dr. Pavlova added.
The study was published online in JAMA Network Open.
Parental anxiety a disruptor
Anxiety disorders run in families. Both genes and environment are thought to be at play, but there are few data on sex-specific transmission from parent to child.
To investigate, the researchers conducted a cross-sectional study of 203 girls and 195 boys and their parents. The average age of the children was 11 years, and they had a familial risk for mood disorders.
Anxiety disorder in a same-sex parent was significantly associated with anxiety disorder in offspring (odds ratio, 2.85; 95% confidence interval, 1.52-5.34; P = .001) but not in an opposite-sex parent (OR, 1.51; 95% CI, 0.81-2.81; P = .20).
Living with a same-sex parent without anxiety was associated with lower rates of offspring anxiety (OR, 0.38; 95% CI, 0.22-0.67; P = .001).
Among all 398 children, 108 (27%) had been diagnosed with one or more anxiety disorders, including generalized anxiety disorder (7.8%), social anxiety disorder (6.3%), separation anxiety disorder (8.6%), specific phobia (8%), and anxiety disorder not otherwise specified (5%).
Rates of anxiety disorders in children increased with age, from 14% in those younger than 9 years to 52% in those older than 15 years. Anxiety disorders were similarly common among boys (24%) and girls (30%).
Rates of anxiety disorders were lowest (24%) in children of two parents without anxiety disorders and highest (41%) in cases in which both parents had anxiety disorders.
The findings point to the possible role of environmental factors, “such as modeling and vicarious learning,” in the transmission of anxiety from parents to their children, the researchers note.
“A child receives [a] similar amount of genetic information from each biological parent. A strong same-sex parent effect suggests children learn resilience by modeling the behavior of their same-sex parent. A parent’s anxiety disorder may disrupt this protective learning,” said Dr. Pavlova.
Early diagnosis, treatment essential
Reached for comment, Jill Emanuele, PhD, vice president of clinical training for the Child MIND Institute, New York, said that when it comes to anxiety, it’s important to assess and treat both the parent and the child.
“We know that both environment and genetics play a role in anxiety disorders. From a clinical perspective, if we see a parent with an anxiety disorder, we know that there is a chance that that is also going to affect the child – whether or not the child has an anxiety disorder,” Dr. Emanuele said in an interview.
“Anxiety disorders are the most common psychiatric disorders diagnosed. We also know that anxiety disorders emerge earlier than mood disorders and certainly can emerge in childhood. It’s important to address anxiety early because those same problems can continue into adulthood if left untreated,” Dr. Emanuele added.
The study was supported by the Canada Research Chairs Program, the Canadian Institutes of Health Research, the Brain & Behavior Research Foundation, the Nova Scotia Health Research Foundation, and the Dalhousie Medical Research Foundation. The authors have disclosed no relevant financial relationships. Dr. Emanuele is a board member with the Anxiety and Depression Association of America.
A version of this article first appeared on Medscape.com.
The new findings suggest that children learn anxious behavior from their parents, study investigator Barbara Pavlova, PhD, clinical psychologist with Nova Scotia Health Authority, told this news organization.
“This means that transmission of anxiety from parents to children may be preventable,” said Dr. Pavlova, assistant professor, department of psychiatry, Dalhousie University, Halifax, Canada.
“Treating parents’ anxiety is not just important for their own health but also for the health of their children. This may be especially true if the child and the parent are the same sex,” Dr. Pavlova added.
The study was published online in JAMA Network Open.
Parental anxiety a disruptor
Anxiety disorders run in families. Both genes and environment are thought to be at play, but there are few data on sex-specific transmission from parent to child.
To investigate, the researchers conducted a cross-sectional study of 203 girls and 195 boys and their parents. The average age of the children was 11 years, and they had a familial risk for mood disorders.
Anxiety disorder in a same-sex parent was significantly associated with anxiety disorder in offspring (odds ratio, 2.85; 95% confidence interval, 1.52-5.34; P = .001) but not in an opposite-sex parent (OR, 1.51; 95% CI, 0.81-2.81; P = .20).
Living with a same-sex parent without anxiety was associated with lower rates of offspring anxiety (OR, 0.38; 95% CI, 0.22-0.67; P = .001).
Among all 398 children, 108 (27%) had been diagnosed with one or more anxiety disorders, including generalized anxiety disorder (7.8%), social anxiety disorder (6.3%), separation anxiety disorder (8.6%), specific phobia (8%), and anxiety disorder not otherwise specified (5%).
Rates of anxiety disorders in children increased with age, from 14% in those younger than 9 years to 52% in those older than 15 years. Anxiety disorders were similarly common among boys (24%) and girls (30%).
Rates of anxiety disorders were lowest (24%) in children of two parents without anxiety disorders and highest (41%) in cases in which both parents had anxiety disorders.
The findings point to the possible role of environmental factors, “such as modeling and vicarious learning,” in the transmission of anxiety from parents to their children, the researchers note.
“A child receives [a] similar amount of genetic information from each biological parent. A strong same-sex parent effect suggests children learn resilience by modeling the behavior of their same-sex parent. A parent’s anxiety disorder may disrupt this protective learning,” said Dr. Pavlova.
Early diagnosis, treatment essential
Reached for comment, Jill Emanuele, PhD, vice president of clinical training for the Child MIND Institute, New York, said that when it comes to anxiety, it’s important to assess and treat both the parent and the child.
“We know that both environment and genetics play a role in anxiety disorders. From a clinical perspective, if we see a parent with an anxiety disorder, we know that there is a chance that that is also going to affect the child – whether or not the child has an anxiety disorder,” Dr. Emanuele said in an interview.
“Anxiety disorders are the most common psychiatric disorders diagnosed. We also know that anxiety disorders emerge earlier than mood disorders and certainly can emerge in childhood. It’s important to address anxiety early because those same problems can continue into adulthood if left untreated,” Dr. Emanuele added.
The study was supported by the Canada Research Chairs Program, the Canadian Institutes of Health Research, the Brain & Behavior Research Foundation, the Nova Scotia Health Research Foundation, and the Dalhousie Medical Research Foundation. The authors have disclosed no relevant financial relationships. Dr. Emanuele is a board member with the Anxiety and Depression Association of America.
A version of this article first appeared on Medscape.com.
The new findings suggest that children learn anxious behavior from their parents, study investigator Barbara Pavlova, PhD, clinical psychologist with Nova Scotia Health Authority, told this news organization.
“This means that transmission of anxiety from parents to children may be preventable,” said Dr. Pavlova, assistant professor, department of psychiatry, Dalhousie University, Halifax, Canada.
“Treating parents’ anxiety is not just important for their own health but also for the health of their children. This may be especially true if the child and the parent are the same sex,” Dr. Pavlova added.
The study was published online in JAMA Network Open.
Parental anxiety a disruptor
Anxiety disorders run in families. Both genes and environment are thought to be at play, but there are few data on sex-specific transmission from parent to child.
To investigate, the researchers conducted a cross-sectional study of 203 girls and 195 boys and their parents. The average age of the children was 11 years, and they had a familial risk for mood disorders.
Anxiety disorder in a same-sex parent was significantly associated with anxiety disorder in offspring (odds ratio, 2.85; 95% confidence interval, 1.52-5.34; P = .001) but not in an opposite-sex parent (OR, 1.51; 95% CI, 0.81-2.81; P = .20).
Living with a same-sex parent without anxiety was associated with lower rates of offspring anxiety (OR, 0.38; 95% CI, 0.22-0.67; P = .001).
Among all 398 children, 108 (27%) had been diagnosed with one or more anxiety disorders, including generalized anxiety disorder (7.8%), social anxiety disorder (6.3%), separation anxiety disorder (8.6%), specific phobia (8%), and anxiety disorder not otherwise specified (5%).
Rates of anxiety disorders in children increased with age, from 14% in those younger than 9 years to 52% in those older than 15 years. Anxiety disorders were similarly common among boys (24%) and girls (30%).
Rates of anxiety disorders were lowest (24%) in children of two parents without anxiety disorders and highest (41%) in cases in which both parents had anxiety disorders.
The findings point to the possible role of environmental factors, “such as modeling and vicarious learning,” in the transmission of anxiety from parents to their children, the researchers note.
“A child receives [a] similar amount of genetic information from each biological parent. A strong same-sex parent effect suggests children learn resilience by modeling the behavior of their same-sex parent. A parent’s anxiety disorder may disrupt this protective learning,” said Dr. Pavlova.
Early diagnosis, treatment essential
Reached for comment, Jill Emanuele, PhD, vice president of clinical training for the Child MIND Institute, New York, said that when it comes to anxiety, it’s important to assess and treat both the parent and the child.
“We know that both environment and genetics play a role in anxiety disorders. From a clinical perspective, if we see a parent with an anxiety disorder, we know that there is a chance that that is also going to affect the child – whether or not the child has an anxiety disorder,” Dr. Emanuele said in an interview.
“Anxiety disorders are the most common psychiatric disorders diagnosed. We also know that anxiety disorders emerge earlier than mood disorders and certainly can emerge in childhood. It’s important to address anxiety early because those same problems can continue into adulthood if left untreated,” Dr. Emanuele added.
The study was supported by the Canada Research Chairs Program, the Canadian Institutes of Health Research, the Brain & Behavior Research Foundation, the Nova Scotia Health Research Foundation, and the Dalhousie Medical Research Foundation. The authors have disclosed no relevant financial relationships. Dr. Emanuele is a board member with the Anxiety and Depression Association of America.
A version of this article first appeared on Medscape.com.
CDC warns about potentially deadly virus in infants
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
The potentially fatal parechovirus is now circulating in multiple states, causing fevers, seizures, and sepsis-like symptoms, including confusion and extreme pain, according to the CDC.
Human parechoviruses are common in children and most have been infected before they start kindergarten, the CDC said. Between 6 months and 5 years of age, symptoms include an upper respiratory tract infection, fever, and rash.
But infants younger than 3 months may have more serious, and possibly fatal, infections. They may get “sepsis-like illness, seizures, and meningitis or meningoencephalitis, particularly in infants younger than 1 month,” the CDC said. At least one newborn has reportedly died from the infection.
Parechovirus can spread like other common germs, from feces that are later ingested – likely due to poor handwashing – and through droplets sent airborne by coughing or sneezing. It can be transmitted by people both with and without symptoms of the infection.
The microbe can reproduce for 1-3 weeks in the upper respiratory tract and up to 6 months in the gastrointestinal tract, the CDC said.
Kristina Angel Bryant, MD, a pediatric infectious disease specialist at the University of Louisville Hospital, says parechoviruses often cause rashes on the hands and feet, which some experts refer to as “mittens and booties.”
The CDC is urging doctors to test for parechovirus if they recognize these symptoms in infants if there is no other explanation for what might be distressing them.
There is no specific treatment for parechovirus. And with no standard testing system in place, experts are unsure if the number of parechovirus cases is higher in 2022 than in previous years.
The message for parents, Dr. Bryant says, is: Don’t panic. “This is not a new virus.”
“One of the most common symptoms is fever, and in some kids, that is the only symptom,” she says. “Older infants and toddlers may have only cold symptoms, and some kids have no symptoms at all.”
Parents can take the usual steps to protect their child from the viral illness, including diligent handwashing and having less contact with people who are sick, Dr. Bryant says.
A version of this article first appeared on Medscape.com.
FDA approves topical ruxolitinib for nonsegmental vitiligo
The on July 18. The treatment, which was approved for treating mild to moderate atopic dermatitis in September 2021, is a cream formulation of ruxolitinib, a Janus kinase 1 (JAK1)/JAK2 inhibitor.
Previously, no treatment was approved to repigment patients with vitiligo, says David Rosmarin, MD, vice chair for research and education in the department of dermatology at Tufts Medical Center, Boston. “It’s important to have options that we can give to patients that are both safe and effective to get them the desired results,” Dr. Rosmarin, the lead investigator of the phase 3 clinical trials of topical ruxolitinib, said in an interview. Vitiligo is “a disease that can really affect quality of life. Some people [with vitiligo] feel as if they’re being stared at or they’re being bullied; they don’t feel confident. It can affect relationships and intimacy.”
Approval was based on the results of two phase 3 trials (TruE-V1 and TruE-V2) in 674 patients with nonsegmental vitiligo aged 12 years or older. At 24 weeks, about 30% of the patients on treatment, applied twice a day, achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 8% and 13% among those in the vehicle groups in the two trials.
At 52 weeks, about 50% of the patients treated with topical ruxolitinib achieved F-VASI75.
Also, using self-reporting as measured by the Vitiligo Noticeability Scale, about 30%-40% of patients described their vitiligo as being “a lot less noticeable” or “no longer noticeable” at week 52. Dr. Rosmarin reported the 52-week results at the 2022 annual meeting of the American Academy of Dermatology.
The trial group used 1.5% ruxolitinib cream twice daily for the full year. The vehicle group began using ruxolitinib halfway through the trial. In this group, 26.8% and 29.6% achieved F-VASI 75 at 52 weeks in the two trials.
For treating vitiligo, patients are advised to apply a thin layer of topical ruxolitinib to affected areas twice a day, “up to 10% body surface area,” according to the prescribing information, which adds: “Satisfactory patient response may require treatment … for more than 24 weeks. If the patient does not find the repigmentation meaningful by 24 weeks, the patient should be reevaluated by the health care provider.”
The most common side effects during the vehicle-controlled part of the trials were development of acne and pruritus at the application site, headache, urinary tract infections, erythema at the application site, and pyrexia, according to the company.
The approved label for topical ruxolitinib includes a boxed warning about serious infections, mortality, cancer, major adverse cardiovascular events, and thrombosis – which, the warning notes, is based on reports in patients treated with oral JAK inhibitors for inflammatory conditions.
Dr. Rosmarin believes that using this drug with other therapies, like light treatment, might yield even better responses. The available data are in patients treated with ruxolitinib as monotherapy, without complementary therapies.
William Damsky, MD, PhD, professor of dermatology and dermatopathology at Yale University, New Haven, who was not involved in the trials, said what is most exciting about this drug is its novelty. Although some topical steroids are used off-label to treat vitiligo, their efficacy is far from what’s been observed in these trials of topical ruxolitinib, he told this news organization. “It’s huge for a number of reasons. … One very big reason is it just provides some hope” for the many patients with vitiligo who, over the years, have been told “that there’s nothing that could be done for their disease, and this really changes that.”
Dr. Rosmarin reports financial relationships with over 20 pharmaceutical companies. Dr. Damsky disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The on July 18. The treatment, which was approved for treating mild to moderate atopic dermatitis in September 2021, is a cream formulation of ruxolitinib, a Janus kinase 1 (JAK1)/JAK2 inhibitor.
Previously, no treatment was approved to repigment patients with vitiligo, says David Rosmarin, MD, vice chair for research and education in the department of dermatology at Tufts Medical Center, Boston. “It’s important to have options that we can give to patients that are both safe and effective to get them the desired results,” Dr. Rosmarin, the lead investigator of the phase 3 clinical trials of topical ruxolitinib, said in an interview. Vitiligo is “a disease that can really affect quality of life. Some people [with vitiligo] feel as if they’re being stared at or they’re being bullied; they don’t feel confident. It can affect relationships and intimacy.”
Approval was based on the results of two phase 3 trials (TruE-V1 and TruE-V2) in 674 patients with nonsegmental vitiligo aged 12 years or older. At 24 weeks, about 30% of the patients on treatment, applied twice a day, achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 8% and 13% among those in the vehicle groups in the two trials.
At 52 weeks, about 50% of the patients treated with topical ruxolitinib achieved F-VASI75.
Also, using self-reporting as measured by the Vitiligo Noticeability Scale, about 30%-40% of patients described their vitiligo as being “a lot less noticeable” or “no longer noticeable” at week 52. Dr. Rosmarin reported the 52-week results at the 2022 annual meeting of the American Academy of Dermatology.
The trial group used 1.5% ruxolitinib cream twice daily for the full year. The vehicle group began using ruxolitinib halfway through the trial. In this group, 26.8% and 29.6% achieved F-VASI 75 at 52 weeks in the two trials.
For treating vitiligo, patients are advised to apply a thin layer of topical ruxolitinib to affected areas twice a day, “up to 10% body surface area,” according to the prescribing information, which adds: “Satisfactory patient response may require treatment … for more than 24 weeks. If the patient does not find the repigmentation meaningful by 24 weeks, the patient should be reevaluated by the health care provider.”
The most common side effects during the vehicle-controlled part of the trials were development of acne and pruritus at the application site, headache, urinary tract infections, erythema at the application site, and pyrexia, according to the company.
The approved label for topical ruxolitinib includes a boxed warning about serious infections, mortality, cancer, major adverse cardiovascular events, and thrombosis – which, the warning notes, is based on reports in patients treated with oral JAK inhibitors for inflammatory conditions.
Dr. Rosmarin believes that using this drug with other therapies, like light treatment, might yield even better responses. The available data are in patients treated with ruxolitinib as monotherapy, without complementary therapies.
William Damsky, MD, PhD, professor of dermatology and dermatopathology at Yale University, New Haven, who was not involved in the trials, said what is most exciting about this drug is its novelty. Although some topical steroids are used off-label to treat vitiligo, their efficacy is far from what’s been observed in these trials of topical ruxolitinib, he told this news organization. “It’s huge for a number of reasons. … One very big reason is it just provides some hope” for the many patients with vitiligo who, over the years, have been told “that there’s nothing that could be done for their disease, and this really changes that.”
Dr. Rosmarin reports financial relationships with over 20 pharmaceutical companies. Dr. Damsky disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The on July 18. The treatment, which was approved for treating mild to moderate atopic dermatitis in September 2021, is a cream formulation of ruxolitinib, a Janus kinase 1 (JAK1)/JAK2 inhibitor.
Previously, no treatment was approved to repigment patients with vitiligo, says David Rosmarin, MD, vice chair for research and education in the department of dermatology at Tufts Medical Center, Boston. “It’s important to have options that we can give to patients that are both safe and effective to get them the desired results,” Dr. Rosmarin, the lead investigator of the phase 3 clinical trials of topical ruxolitinib, said in an interview. Vitiligo is “a disease that can really affect quality of life. Some people [with vitiligo] feel as if they’re being stared at or they’re being bullied; they don’t feel confident. It can affect relationships and intimacy.”
Approval was based on the results of two phase 3 trials (TruE-V1 and TruE-V2) in 674 patients with nonsegmental vitiligo aged 12 years or older. At 24 weeks, about 30% of the patients on treatment, applied twice a day, achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 8% and 13% among those in the vehicle groups in the two trials.
At 52 weeks, about 50% of the patients treated with topical ruxolitinib achieved F-VASI75.
Also, using self-reporting as measured by the Vitiligo Noticeability Scale, about 30%-40% of patients described their vitiligo as being “a lot less noticeable” or “no longer noticeable” at week 52. Dr. Rosmarin reported the 52-week results at the 2022 annual meeting of the American Academy of Dermatology.
The trial group used 1.5% ruxolitinib cream twice daily for the full year. The vehicle group began using ruxolitinib halfway through the trial. In this group, 26.8% and 29.6% achieved F-VASI 75 at 52 weeks in the two trials.
For treating vitiligo, patients are advised to apply a thin layer of topical ruxolitinib to affected areas twice a day, “up to 10% body surface area,” according to the prescribing information, which adds: “Satisfactory patient response may require treatment … for more than 24 weeks. If the patient does not find the repigmentation meaningful by 24 weeks, the patient should be reevaluated by the health care provider.”
The most common side effects during the vehicle-controlled part of the trials were development of acne and pruritus at the application site, headache, urinary tract infections, erythema at the application site, and pyrexia, according to the company.
The approved label for topical ruxolitinib includes a boxed warning about serious infections, mortality, cancer, major adverse cardiovascular events, and thrombosis – which, the warning notes, is based on reports in patients treated with oral JAK inhibitors for inflammatory conditions.
Dr. Rosmarin believes that using this drug with other therapies, like light treatment, might yield even better responses. The available data are in patients treated with ruxolitinib as monotherapy, without complementary therapies.
William Damsky, MD, PhD, professor of dermatology and dermatopathology at Yale University, New Haven, who was not involved in the trials, said what is most exciting about this drug is its novelty. Although some topical steroids are used off-label to treat vitiligo, their efficacy is far from what’s been observed in these trials of topical ruxolitinib, he told this news organization. “It’s huge for a number of reasons. … One very big reason is it just provides some hope” for the many patients with vitiligo who, over the years, have been told “that there’s nothing that could be done for their disease, and this really changes that.”
Dr. Rosmarin reports financial relationships with over 20 pharmaceutical companies. Dr. Damsky disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Commentary: Reversal of Roe v. Wade affects adolescents
The Supreme Court decision to strike down the 50-year ruling on Roe v. Wade, which allowed legal abortion, will affect all patients and families seeking care in pediatric and adolescent medicine clinics. Regardless of how you view abortion, the reality is your adolescent female patients and their parents will seek your counsel.
The overturning of Roe has resulted in much confusion for both patients and providers. The overall effect of this decision in Wisconsin is yet to be known but currently we have had to create road maps to direct adolescent patients who experience an unplanned pregnancy and wish to abort. Unfortunately, these road maps include only resources out of state or online. Providing adolescents confidential care may be challenged as the teens may need to disclose the unplanned pregnancy to an adult to access resources.
Providers remain unsettled regarding their risk of assisting an adolescent who discloses an unplanned pregnancy. Recently, many questions arose regarding dispensing Plan B and the risk to prescribers. Communication was needed to assure providers that Plan B is contraception and at this time contraception remains legal in our state.
Daily I educate adolescent females on the risks of unplanned pregnancy and what the Supreme Court decision will mean to them if they become pregnant. Unfortunately, many teens do not understand the ruling and how this decision affects them personally. Education is needed today more than ever regarding pregnancy prevention.
The recent AAP policy statement reaffirms its position that the rights of adolescents to seek confidential care when considering abortion must be protected.1 It further reaffirms access to safe and legal abortion is a core tenant of sexual and reproductive health care.
A recent article published in AAP News by Elise D. Berlan, MD, “AAP’s teen reproductive health policies reaffirm right to comprehensive care,” further advises on the role of the pediatric provider.2 Pediatric providers should continue offering option counseling for pregnant adolescents, be prepared to provide accurate information regarding these options with awareness that some options such as the IUD may no longer be available, remain supportive of the decision they choose, and encourage discussion with a family member to support their decisions. It is imperative that we familiarize ourselves with the abortion policies in our states, advocate to prevent government interference with the patient-doctor relationship, and recognize the impact restrictive abortion has regarding marginalized individuals, she stated. Finally we must recognize our own bias regarding option counseling and refer appropriately to another professional if we are unable to confidently offer guidance.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee.
References
1. AAP Committee on Adolescence. Pediatrics. 2022. doi: 10.1542/peds.2022-058780.
2. Berlan ED. AAP’s teen reproductive health policies reaffirm right to comprehensive care. 2022. AAP News.
The Supreme Court decision to strike down the 50-year ruling on Roe v. Wade, which allowed legal abortion, will affect all patients and families seeking care in pediatric and adolescent medicine clinics. Regardless of how you view abortion, the reality is your adolescent female patients and their parents will seek your counsel.
The overturning of Roe has resulted in much confusion for both patients and providers. The overall effect of this decision in Wisconsin is yet to be known but currently we have had to create road maps to direct adolescent patients who experience an unplanned pregnancy and wish to abort. Unfortunately, these road maps include only resources out of state or online. Providing adolescents confidential care may be challenged as the teens may need to disclose the unplanned pregnancy to an adult to access resources.
Providers remain unsettled regarding their risk of assisting an adolescent who discloses an unplanned pregnancy. Recently, many questions arose regarding dispensing Plan B and the risk to prescribers. Communication was needed to assure providers that Plan B is contraception and at this time contraception remains legal in our state.
Daily I educate adolescent females on the risks of unplanned pregnancy and what the Supreme Court decision will mean to them if they become pregnant. Unfortunately, many teens do not understand the ruling and how this decision affects them personally. Education is needed today more than ever regarding pregnancy prevention.
The recent AAP policy statement reaffirms its position that the rights of adolescents to seek confidential care when considering abortion must be protected.1 It further reaffirms access to safe and legal abortion is a core tenant of sexual and reproductive health care.
A recent article published in AAP News by Elise D. Berlan, MD, “AAP’s teen reproductive health policies reaffirm right to comprehensive care,” further advises on the role of the pediatric provider.2 Pediatric providers should continue offering option counseling for pregnant adolescents, be prepared to provide accurate information regarding these options with awareness that some options such as the IUD may no longer be available, remain supportive of the decision they choose, and encourage discussion with a family member to support their decisions. It is imperative that we familiarize ourselves with the abortion policies in our states, advocate to prevent government interference with the patient-doctor relationship, and recognize the impact restrictive abortion has regarding marginalized individuals, she stated. Finally we must recognize our own bias regarding option counseling and refer appropriately to another professional if we are unable to confidently offer guidance.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee.
References
1. AAP Committee on Adolescence. Pediatrics. 2022. doi: 10.1542/peds.2022-058780.
2. Berlan ED. AAP’s teen reproductive health policies reaffirm right to comprehensive care. 2022. AAP News.
The Supreme Court decision to strike down the 50-year ruling on Roe v. Wade, which allowed legal abortion, will affect all patients and families seeking care in pediatric and adolescent medicine clinics. Regardless of how you view abortion, the reality is your adolescent female patients and their parents will seek your counsel.
The overturning of Roe has resulted in much confusion for both patients and providers. The overall effect of this decision in Wisconsin is yet to be known but currently we have had to create road maps to direct adolescent patients who experience an unplanned pregnancy and wish to abort. Unfortunately, these road maps include only resources out of state or online. Providing adolescents confidential care may be challenged as the teens may need to disclose the unplanned pregnancy to an adult to access resources.
Providers remain unsettled regarding their risk of assisting an adolescent who discloses an unplanned pregnancy. Recently, many questions arose regarding dispensing Plan B and the risk to prescribers. Communication was needed to assure providers that Plan B is contraception and at this time contraception remains legal in our state.
Daily I educate adolescent females on the risks of unplanned pregnancy and what the Supreme Court decision will mean to them if they become pregnant. Unfortunately, many teens do not understand the ruling and how this decision affects them personally. Education is needed today more than ever regarding pregnancy prevention.
The recent AAP policy statement reaffirms its position that the rights of adolescents to seek confidential care when considering abortion must be protected.1 It further reaffirms access to safe and legal abortion is a core tenant of sexual and reproductive health care.
A recent article published in AAP News by Elise D. Berlan, MD, “AAP’s teen reproductive health policies reaffirm right to comprehensive care,” further advises on the role of the pediatric provider.2 Pediatric providers should continue offering option counseling for pregnant adolescents, be prepared to provide accurate information regarding these options with awareness that some options such as the IUD may no longer be available, remain supportive of the decision they choose, and encourage discussion with a family member to support their decisions. It is imperative that we familiarize ourselves with the abortion policies in our states, advocate to prevent government interference with the patient-doctor relationship, and recognize the impact restrictive abortion has regarding marginalized individuals, she stated. Finally we must recognize our own bias regarding option counseling and refer appropriately to another professional if we are unable to confidently offer guidance.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee.
References
1. AAP Committee on Adolescence. Pediatrics. 2022. doi: 10.1542/peds.2022-058780.
2. Berlan ED. AAP’s teen reproductive health policies reaffirm right to comprehensive care. 2022. AAP News.
Think of pediatric morphea as a systemic, chronic disease, expert advises
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
AT SPD 2022
Berdazimer gel beats vehicle for molluscum contagiosum in phase 3 study
Treatment with .
Molluscum contagiosum (MC) remains a common infection that, despite being self-limiting, may persist for months or years, and is associated with quality of life concerns and the need for ongoing therapy, wrote John C. Browning, MD, of Texas Dermatology and Laser Specialists, San Antonio, and colleagues, who conducted the phase 3 randomized study.
The infection is most common in children aged 1-14 years, and treatment may be needed in part to avoid infecting peers and family members, they said. No treatments for molluscum are currently approved by the Food and Drug Administration.
In the study, which was published in JAMA Dermatology, the researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years (range was 0.9-49 years), and about 85% were White. Participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment. At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
Most adverse events were mild or moderate, and rates of adverse events resulting in treatment discontinuation were low overall for both groups; the most common adverse events were application-site pain and erythema, which were mostly mild. Overall, 4.1% of berdazimer-treated patients and 0.7% of placebo patients discontinued the study because of adverse events.
The study findings were limited by several factors, including the small number of patients in subgroups for race, ethnicity, and age; and the lack of data on patients with sexually transmitted MC and on concomitant use with other topical MC therapies, the researchers noted.
However, the results represent the largest randomized clinical trial of berdazimer 10.3% to date, and support its potential as a first-line therapy for MC patients aged 6 months and older, according to the authors. “Berdazimer is under consideration as a first in-class therapeutic agent for MC and may provide a topical prescription alternative to other therapies used for this highly contagious and psychosocially challenging skin condition,” they said.
Having a reliable, steroid-free, safe, and efficacious medication to treat molluscum in the pediatric population, as early as age 6 months, that can be used at home would “change the whole therapeutic paradigm,” one of the study authors, Adelaide Hebert, MD, said in an interview at the Society for Pediatric Dermatology annual meeting in July, where she presented phase 2 data on berdazimer gel. “This is a common problem and the rate of infections among siblings if it goes untreated is 41%. Affected kids have a sense of isolation; they don’t get invited to swimming parties.”
The lack of a safe and effective topical therapy “has been challenging,” added Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. She noted that treatments that have been used but have not been successful include imiquimod. “I’m not impressed with tretinoin,” although it is prescribed for MC, and the most common treatment prescribed by pediatricians for molluscum – mupirocin – is “usually not effective,” she said.
Another MC treatment in trials
Another investigative treatment for molluscum contagiosum, VP-102, a drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, has been evaluated in phase 3 studies of patients with MC aged 2 years and older. The results of two phase 3 studies were published in 2020.
In May 2022, Verrica Pharmaceuticals, which is developing VP-102, announced that Food and Drug Administration approval had been delayed because of deficiencies identified at a contract manufacturing organization, and that the company was working with the agency to bring VP-102 to the market as soon as possible.
A step in the right direction
Although MC is self-resolving, cases last an average of 13.5 months, and “many families look to fast-forward their child’s experience with the infection,” Vikash S. Oza, MD, a pediatric dermatologist at New York University, New York, wrote in an editorial that accompanied the berdazimer study.
“To truly create a paradigm shift in the decision to treat MC, a therapeutic treatment would need to be developed that would lead to resolution of the infection over a short time frame (ideally, weeks) with minimal discomfort,” Dr. Oza noted. “Both VP-102 and berdazimer gel, 10.3%, have the potential to be the first-ever MC therapies approved by the U.S. Food and Drug Administration,” and families seeking to reduce MC in visible areas would welcome this option for a home therapy, he said.
However, Dr. Oza emphasized that potential barriers to widespread use of these therapies include whether the efficacy can be maintained in patients who fail to comply with daily application, and the ongoing need for office-based therapy to manage sexually transmitted MC in adults and periocular and perianal MC in children. The study was funded by Novan. Lead author Dr. Browning disclosed grants from Novan during the conduct of the study; Dr. Hebert reported grants from the University of Texas Health Science Center McGovern Medical School-Houston during the conduct of the study. Disclosures of other authors included having reported equity in Novan during the conduct of the study and receiving a grant from Novan. Dr. Oza had no financial conflicts to disclose.
Treatment with .
Molluscum contagiosum (MC) remains a common infection that, despite being self-limiting, may persist for months or years, and is associated with quality of life concerns and the need for ongoing therapy, wrote John C. Browning, MD, of Texas Dermatology and Laser Specialists, San Antonio, and colleagues, who conducted the phase 3 randomized study.
The infection is most common in children aged 1-14 years, and treatment may be needed in part to avoid infecting peers and family members, they said. No treatments for molluscum are currently approved by the Food and Drug Administration.
In the study, which was published in JAMA Dermatology, the researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years (range was 0.9-49 years), and about 85% were White. Participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment. At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
Most adverse events were mild or moderate, and rates of adverse events resulting in treatment discontinuation were low overall for both groups; the most common adverse events were application-site pain and erythema, which were mostly mild. Overall, 4.1% of berdazimer-treated patients and 0.7% of placebo patients discontinued the study because of adverse events.
The study findings were limited by several factors, including the small number of patients in subgroups for race, ethnicity, and age; and the lack of data on patients with sexually transmitted MC and on concomitant use with other topical MC therapies, the researchers noted.
However, the results represent the largest randomized clinical trial of berdazimer 10.3% to date, and support its potential as a first-line therapy for MC patients aged 6 months and older, according to the authors. “Berdazimer is under consideration as a first in-class therapeutic agent for MC and may provide a topical prescription alternative to other therapies used for this highly contagious and psychosocially challenging skin condition,” they said.
Having a reliable, steroid-free, safe, and efficacious medication to treat molluscum in the pediatric population, as early as age 6 months, that can be used at home would “change the whole therapeutic paradigm,” one of the study authors, Adelaide Hebert, MD, said in an interview at the Society for Pediatric Dermatology annual meeting in July, where she presented phase 2 data on berdazimer gel. “This is a common problem and the rate of infections among siblings if it goes untreated is 41%. Affected kids have a sense of isolation; they don’t get invited to swimming parties.”
The lack of a safe and effective topical therapy “has been challenging,” added Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. She noted that treatments that have been used but have not been successful include imiquimod. “I’m not impressed with tretinoin,” although it is prescribed for MC, and the most common treatment prescribed by pediatricians for molluscum – mupirocin – is “usually not effective,” she said.
Another MC treatment in trials
Another investigative treatment for molluscum contagiosum, VP-102, a drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, has been evaluated in phase 3 studies of patients with MC aged 2 years and older. The results of two phase 3 studies were published in 2020.
In May 2022, Verrica Pharmaceuticals, which is developing VP-102, announced that Food and Drug Administration approval had been delayed because of deficiencies identified at a contract manufacturing organization, and that the company was working with the agency to bring VP-102 to the market as soon as possible.
A step in the right direction
Although MC is self-resolving, cases last an average of 13.5 months, and “many families look to fast-forward their child’s experience with the infection,” Vikash S. Oza, MD, a pediatric dermatologist at New York University, New York, wrote in an editorial that accompanied the berdazimer study.
“To truly create a paradigm shift in the decision to treat MC, a therapeutic treatment would need to be developed that would lead to resolution of the infection over a short time frame (ideally, weeks) with minimal discomfort,” Dr. Oza noted. “Both VP-102 and berdazimer gel, 10.3%, have the potential to be the first-ever MC therapies approved by the U.S. Food and Drug Administration,” and families seeking to reduce MC in visible areas would welcome this option for a home therapy, he said.
However, Dr. Oza emphasized that potential barriers to widespread use of these therapies include whether the efficacy can be maintained in patients who fail to comply with daily application, and the ongoing need for office-based therapy to manage sexually transmitted MC in adults and periocular and perianal MC in children. The study was funded by Novan. Lead author Dr. Browning disclosed grants from Novan during the conduct of the study; Dr. Hebert reported grants from the University of Texas Health Science Center McGovern Medical School-Houston during the conduct of the study. Disclosures of other authors included having reported equity in Novan during the conduct of the study and receiving a grant from Novan. Dr. Oza had no financial conflicts to disclose.
Treatment with .
Molluscum contagiosum (MC) remains a common infection that, despite being self-limiting, may persist for months or years, and is associated with quality of life concerns and the need for ongoing therapy, wrote John C. Browning, MD, of Texas Dermatology and Laser Specialists, San Antonio, and colleagues, who conducted the phase 3 randomized study.
The infection is most common in children aged 1-14 years, and treatment may be needed in part to avoid infecting peers and family members, they said. No treatments for molluscum are currently approved by the Food and Drug Administration.
In the study, which was published in JAMA Dermatology, the researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years (range was 0.9-49 years), and about 85% were White. Participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment. At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
Most adverse events were mild or moderate, and rates of adverse events resulting in treatment discontinuation were low overall for both groups; the most common adverse events were application-site pain and erythema, which were mostly mild. Overall, 4.1% of berdazimer-treated patients and 0.7% of placebo patients discontinued the study because of adverse events.
The study findings were limited by several factors, including the small number of patients in subgroups for race, ethnicity, and age; and the lack of data on patients with sexually transmitted MC and on concomitant use with other topical MC therapies, the researchers noted.
However, the results represent the largest randomized clinical trial of berdazimer 10.3% to date, and support its potential as a first-line therapy for MC patients aged 6 months and older, according to the authors. “Berdazimer is under consideration as a first in-class therapeutic agent for MC and may provide a topical prescription alternative to other therapies used for this highly contagious and psychosocially challenging skin condition,” they said.
Having a reliable, steroid-free, safe, and efficacious medication to treat molluscum in the pediatric population, as early as age 6 months, that can be used at home would “change the whole therapeutic paradigm,” one of the study authors, Adelaide Hebert, MD, said in an interview at the Society for Pediatric Dermatology annual meeting in July, where she presented phase 2 data on berdazimer gel. “This is a common problem and the rate of infections among siblings if it goes untreated is 41%. Affected kids have a sense of isolation; they don’t get invited to swimming parties.”
The lack of a safe and effective topical therapy “has been challenging,” added Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. She noted that treatments that have been used but have not been successful include imiquimod. “I’m not impressed with tretinoin,” although it is prescribed for MC, and the most common treatment prescribed by pediatricians for molluscum – mupirocin – is “usually not effective,” she said.
Another MC treatment in trials
Another investigative treatment for molluscum contagiosum, VP-102, a drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, has been evaluated in phase 3 studies of patients with MC aged 2 years and older. The results of two phase 3 studies were published in 2020.
In May 2022, Verrica Pharmaceuticals, which is developing VP-102, announced that Food and Drug Administration approval had been delayed because of deficiencies identified at a contract manufacturing organization, and that the company was working with the agency to bring VP-102 to the market as soon as possible.
A step in the right direction
Although MC is self-resolving, cases last an average of 13.5 months, and “many families look to fast-forward their child’s experience with the infection,” Vikash S. Oza, MD, a pediatric dermatologist at New York University, New York, wrote in an editorial that accompanied the berdazimer study.
“To truly create a paradigm shift in the decision to treat MC, a therapeutic treatment would need to be developed that would lead to resolution of the infection over a short time frame (ideally, weeks) with minimal discomfort,” Dr. Oza noted. “Both VP-102 and berdazimer gel, 10.3%, have the potential to be the first-ever MC therapies approved by the U.S. Food and Drug Administration,” and families seeking to reduce MC in visible areas would welcome this option for a home therapy, he said.
However, Dr. Oza emphasized that potential barriers to widespread use of these therapies include whether the efficacy can be maintained in patients who fail to comply with daily application, and the ongoing need for office-based therapy to manage sexually transmitted MC in adults and periocular and perianal MC in children. The study was funded by Novan. Lead author Dr. Browning disclosed grants from Novan during the conduct of the study; Dr. Hebert reported grants from the University of Texas Health Science Center McGovern Medical School-Houston during the conduct of the study. Disclosures of other authors included having reported equity in Novan during the conduct of the study and receiving a grant from Novan. Dr. Oza had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
LGBTQ+ Youth Consult: Let’s talk about PrEP!
As pediatricians, almost all of our clinic visits include some anticipatory guidance and recommendations on ways to promote well-being and prevent illness and injury for our patients. Because of minority stress, discrimination, and increased exposure to adverse childhood experiences, LGBTQ+ patients are disproportionately affected by certain health conditions including depression, anxiety, substance use, homelessness, as well as HIV and other sexually transmitted infections (STIs).1 While LGBTQ+ youth could benefit from additional guidance, counseling, and interventions related to these health disparities and have expressed interest in talking about these topics with their providers, sexual and gender minority youth also stress that they want to be treated as any other youth.2 Extending counseling for preventive care measures such as preexposure prophylaxis (PrEP) for HIV to all sexually active youth could help to destigmatize LGBTQ+ youth as being “different” from other youth and also help to increase overall access to HIV prevention services.3
Described by some as the “birth control” for HIV infection, PrEP is taken on an ongoing basis by those who are HIV negative before potential exposures to HIV in order to prevent new HIV infections. PrEP was first approved as a daily pill for adults in 2015 by the Food and Drug Administration with extension in 2018 to all individuals at risk for HIV weighing at least 35 kg after safety and efficacy data showed it could be used routinely for adolescents.4 When taken daily, oral PrEP can decrease the risk of HIV from sexual contact by more than 90% and from injection drug use by around 70%. As PrEP is highly effective with low risk for side effects, the U.S. Preventive Services Task Force (USPSTF) gave PrEP a “Grade A” recommendation for use in those at high risk for HIV infection in 2019.5 Since efficacy is closely tied to adherence, the first injectable PrEP (given at 0, 1, and 2 months with dosing then every 2 months) was also recently FDA approved in late 2021.6
Since HIV infection disproportionately affects LBGTQ+ individuals, and particularly LBGTQ+ youth of color, counseling related to PrEP has been largely targeted to these groups.7 Insurance and financial barriers to PrEP have been greatly reduced over the past several years through changes in insurance coverage (strengthened by the USPSTF recommendation), supplemental insurance programs, and pharmaceutical copay programs. Many states (but not all) also include HIV in the definition of STIs and allow minors to consent to PrEP services without a parent or guardian. Unfortunately, despite the high efficacy of PrEP and efforts to decrease barriers, rates of PrEP use continue to be extremely low, especially in youth, with only 15.6% of those aged 16-24 who are at risk for HIV in the United States actually taking PrEP in 2019.8 Many barriers to PrEP continue to exist including lack of awareness of PrEP, stigma surrounding HIV and PrEP, and lack of PrEP providers.
In order to address these low rates of PrEP uptake, the Centers for Disease Control and Prevention now recommends that medical providers discuss PrEP with all sexually active patients.6 PrEP should not be seen or discussed as something only relevant to LBGTQ+ populations, but rather as another tool in everyone’s “sexual health toolbox” that can allow us to experience human connection and pleasure through sexual activity while also having more control over what happens to our bodies. Not only will this allow more patients to access PrEP directly, it will also decrease the stigma of talking about HIV and PrEP and strengthen youths’ sense of autonomy and control over their own sexual health.
Since PrEP is a relatively new medical service, many providers will need to learn more about PrEP to at least have initial discussions with patients and to feel comfortable prescribing this themselves (See Resources). Below are also some suggestions to incorporate into your practice in order to advocate for the health and well-being of all your patients, including LGBTQ+ youth.
- Once your patients are 13 years and older, spend time with them alone to confidentially discuss more sensitive topics such as sexual health, mental health, and substance use.
- For all patients who are sexually active or considering sexual activity in the near future, discuss topics to help them control what happens to their bodies including consent, condoms, birth control, PrEP, and routine STI screening.
- Recommend PrEP to anyone who is sexually active and may be at increased risk for HIV infection or who is interested in taking PrEP for HIV prevention.
- Learn more about PrEP and start prescribing it to your own patients or become familiar with providers in your area to whom you could refer patients who are interested. While no certification is needed to prescribe PrEP, programs exist to help providers become more familiar with how to prescribe PrEP.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, HIV prevention for adolescents and young adults, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is an assistant professor of clinical pediatrics and a University of Southern California faculty member.
Resources
CDC PrEP resources for clinicians: www.cdc.gov/hiv/clinicians/prevention/prep.html.Health HIV’s HIV Prevention Certified Provider Certification Program: https://healthhiv.org/programs/hpcp/.PrEP providers in the United States: https://preplocator.org/.Adolescent Health Working Group’s Sexual and Reproductive Health Toolkit for Adolescent Providers: https://ahwg.org/download/sexual-and-reproductive-health-toolkit-for-adolescent-providers/.
References
1. Lund EM and Burgess CM. Prim Care Clin Office Pract. 2021:48:179-89.
2. Hoffman ND et al. J Adolesc Health. 2009;45:222-9.
3. Mayer KH et al. Adv Ther. 2020;37:1778-811.
4. Hosek SG et al. JAMA Pediatr. 2017;171(11):1063-71.
5. U.S. Preventive Services Task Force; Owens DK et al. JAMA. 2019;321(22):2203-13.
6. Centers for Disease Control and Prevention: U.S. Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update: A Clinical Practice Guideline. Published 2021. Accessed July 10, 2022.
7. Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2015-2019. HIV Surveillance Supplemental Report. 2021;26(1). Published May 2021. Accessed July 10, 2022.
8. Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data–United States and 6 Dependent Areas, 2020. HIV Surveillance Supplemental Report. 2022;27(3).
As pediatricians, almost all of our clinic visits include some anticipatory guidance and recommendations on ways to promote well-being and prevent illness and injury for our patients. Because of minority stress, discrimination, and increased exposure to adverse childhood experiences, LGBTQ+ patients are disproportionately affected by certain health conditions including depression, anxiety, substance use, homelessness, as well as HIV and other sexually transmitted infections (STIs).1 While LGBTQ+ youth could benefit from additional guidance, counseling, and interventions related to these health disparities and have expressed interest in talking about these topics with their providers, sexual and gender minority youth also stress that they want to be treated as any other youth.2 Extending counseling for preventive care measures such as preexposure prophylaxis (PrEP) for HIV to all sexually active youth could help to destigmatize LGBTQ+ youth as being “different” from other youth and also help to increase overall access to HIV prevention services.3
Described by some as the “birth control” for HIV infection, PrEP is taken on an ongoing basis by those who are HIV negative before potential exposures to HIV in order to prevent new HIV infections. PrEP was first approved as a daily pill for adults in 2015 by the Food and Drug Administration with extension in 2018 to all individuals at risk for HIV weighing at least 35 kg after safety and efficacy data showed it could be used routinely for adolescents.4 When taken daily, oral PrEP can decrease the risk of HIV from sexual contact by more than 90% and from injection drug use by around 70%. As PrEP is highly effective with low risk for side effects, the U.S. Preventive Services Task Force (USPSTF) gave PrEP a “Grade A” recommendation for use in those at high risk for HIV infection in 2019.5 Since efficacy is closely tied to adherence, the first injectable PrEP (given at 0, 1, and 2 months with dosing then every 2 months) was also recently FDA approved in late 2021.6
Since HIV infection disproportionately affects LBGTQ+ individuals, and particularly LBGTQ+ youth of color, counseling related to PrEP has been largely targeted to these groups.7 Insurance and financial barriers to PrEP have been greatly reduced over the past several years through changes in insurance coverage (strengthened by the USPSTF recommendation), supplemental insurance programs, and pharmaceutical copay programs. Many states (but not all) also include HIV in the definition of STIs and allow minors to consent to PrEP services without a parent or guardian. Unfortunately, despite the high efficacy of PrEP and efforts to decrease barriers, rates of PrEP use continue to be extremely low, especially in youth, with only 15.6% of those aged 16-24 who are at risk for HIV in the United States actually taking PrEP in 2019.8 Many barriers to PrEP continue to exist including lack of awareness of PrEP, stigma surrounding HIV and PrEP, and lack of PrEP providers.
In order to address these low rates of PrEP uptake, the Centers for Disease Control and Prevention now recommends that medical providers discuss PrEP with all sexually active patients.6 PrEP should not be seen or discussed as something only relevant to LBGTQ+ populations, but rather as another tool in everyone’s “sexual health toolbox” that can allow us to experience human connection and pleasure through sexual activity while also having more control over what happens to our bodies. Not only will this allow more patients to access PrEP directly, it will also decrease the stigma of talking about HIV and PrEP and strengthen youths’ sense of autonomy and control over their own sexual health.
Since PrEP is a relatively new medical service, many providers will need to learn more about PrEP to at least have initial discussions with patients and to feel comfortable prescribing this themselves (See Resources). Below are also some suggestions to incorporate into your practice in order to advocate for the health and well-being of all your patients, including LGBTQ+ youth.
- Once your patients are 13 years and older, spend time with them alone to confidentially discuss more sensitive topics such as sexual health, mental health, and substance use.
- For all patients who are sexually active or considering sexual activity in the near future, discuss topics to help them control what happens to their bodies including consent, condoms, birth control, PrEP, and routine STI screening.
- Recommend PrEP to anyone who is sexually active and may be at increased risk for HIV infection or who is interested in taking PrEP for HIV prevention.
- Learn more about PrEP and start prescribing it to your own patients or become familiar with providers in your area to whom you could refer patients who are interested. While no certification is needed to prescribe PrEP, programs exist to help providers become more familiar with how to prescribe PrEP.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, HIV prevention for adolescents and young adults, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is an assistant professor of clinical pediatrics and a University of Southern California faculty member.
Resources
CDC PrEP resources for clinicians: www.cdc.gov/hiv/clinicians/prevention/prep.html.Health HIV’s HIV Prevention Certified Provider Certification Program: https://healthhiv.org/programs/hpcp/.PrEP providers in the United States: https://preplocator.org/.Adolescent Health Working Group’s Sexual and Reproductive Health Toolkit for Adolescent Providers: https://ahwg.org/download/sexual-and-reproductive-health-toolkit-for-adolescent-providers/.
References
1. Lund EM and Burgess CM. Prim Care Clin Office Pract. 2021:48:179-89.
2. Hoffman ND et al. J Adolesc Health. 2009;45:222-9.
3. Mayer KH et al. Adv Ther. 2020;37:1778-811.
4. Hosek SG et al. JAMA Pediatr. 2017;171(11):1063-71.
5. U.S. Preventive Services Task Force; Owens DK et al. JAMA. 2019;321(22):2203-13.
6. Centers for Disease Control and Prevention: U.S. Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update: A Clinical Practice Guideline. Published 2021. Accessed July 10, 2022.
7. Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2015-2019. HIV Surveillance Supplemental Report. 2021;26(1). Published May 2021. Accessed July 10, 2022.
8. Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data–United States and 6 Dependent Areas, 2020. HIV Surveillance Supplemental Report. 2022;27(3).
As pediatricians, almost all of our clinic visits include some anticipatory guidance and recommendations on ways to promote well-being and prevent illness and injury for our patients. Because of minority stress, discrimination, and increased exposure to adverse childhood experiences, LGBTQ+ patients are disproportionately affected by certain health conditions including depression, anxiety, substance use, homelessness, as well as HIV and other sexually transmitted infections (STIs).1 While LGBTQ+ youth could benefit from additional guidance, counseling, and interventions related to these health disparities and have expressed interest in talking about these topics with their providers, sexual and gender minority youth also stress that they want to be treated as any other youth.2 Extending counseling for preventive care measures such as preexposure prophylaxis (PrEP) for HIV to all sexually active youth could help to destigmatize LGBTQ+ youth as being “different” from other youth and also help to increase overall access to HIV prevention services.3
Described by some as the “birth control” for HIV infection, PrEP is taken on an ongoing basis by those who are HIV negative before potential exposures to HIV in order to prevent new HIV infections. PrEP was first approved as a daily pill for adults in 2015 by the Food and Drug Administration with extension in 2018 to all individuals at risk for HIV weighing at least 35 kg after safety and efficacy data showed it could be used routinely for adolescents.4 When taken daily, oral PrEP can decrease the risk of HIV from sexual contact by more than 90% and from injection drug use by around 70%. As PrEP is highly effective with low risk for side effects, the U.S. Preventive Services Task Force (USPSTF) gave PrEP a “Grade A” recommendation for use in those at high risk for HIV infection in 2019.5 Since efficacy is closely tied to adherence, the first injectable PrEP (given at 0, 1, and 2 months with dosing then every 2 months) was also recently FDA approved in late 2021.6
Since HIV infection disproportionately affects LBGTQ+ individuals, and particularly LBGTQ+ youth of color, counseling related to PrEP has been largely targeted to these groups.7 Insurance and financial barriers to PrEP have been greatly reduced over the past several years through changes in insurance coverage (strengthened by the USPSTF recommendation), supplemental insurance programs, and pharmaceutical copay programs. Many states (but not all) also include HIV in the definition of STIs and allow minors to consent to PrEP services without a parent or guardian. Unfortunately, despite the high efficacy of PrEP and efforts to decrease barriers, rates of PrEP use continue to be extremely low, especially in youth, with only 15.6% of those aged 16-24 who are at risk for HIV in the United States actually taking PrEP in 2019.8 Many barriers to PrEP continue to exist including lack of awareness of PrEP, stigma surrounding HIV and PrEP, and lack of PrEP providers.
In order to address these low rates of PrEP uptake, the Centers for Disease Control and Prevention now recommends that medical providers discuss PrEP with all sexually active patients.6 PrEP should not be seen or discussed as something only relevant to LBGTQ+ populations, but rather as another tool in everyone’s “sexual health toolbox” that can allow us to experience human connection and pleasure through sexual activity while also having more control over what happens to our bodies. Not only will this allow more patients to access PrEP directly, it will also decrease the stigma of talking about HIV and PrEP and strengthen youths’ sense of autonomy and control over their own sexual health.
Since PrEP is a relatively new medical service, many providers will need to learn more about PrEP to at least have initial discussions with patients and to feel comfortable prescribing this themselves (See Resources). Below are also some suggestions to incorporate into your practice in order to advocate for the health and well-being of all your patients, including LGBTQ+ youth.
- Once your patients are 13 years and older, spend time with them alone to confidentially discuss more sensitive topics such as sexual health, mental health, and substance use.
- For all patients who are sexually active or considering sexual activity in the near future, discuss topics to help them control what happens to their bodies including consent, condoms, birth control, PrEP, and routine STI screening.
- Recommend PrEP to anyone who is sexually active and may be at increased risk for HIV infection or who is interested in taking PrEP for HIV prevention.
- Learn more about PrEP and start prescribing it to your own patients or become familiar with providers in your area to whom you could refer patients who are interested. While no certification is needed to prescribe PrEP, programs exist to help providers become more familiar with how to prescribe PrEP.
Dr. Warus is an adolescent medicine physician who specializes in care for transgender and gender-nonconforming youth, HIV prevention for adolescents and young adults, and LGBTQ health for youth at Children’s Hospital of Los Angeles. He is an assistant professor of clinical pediatrics and a University of Southern California faculty member.
Resources
CDC PrEP resources for clinicians: www.cdc.gov/hiv/clinicians/prevention/prep.html.Health HIV’s HIV Prevention Certified Provider Certification Program: https://healthhiv.org/programs/hpcp/.PrEP providers in the United States: https://preplocator.org/.Adolescent Health Working Group’s Sexual and Reproductive Health Toolkit for Adolescent Providers: https://ahwg.org/download/sexual-and-reproductive-health-toolkit-for-adolescent-providers/.
References
1. Lund EM and Burgess CM. Prim Care Clin Office Pract. 2021:48:179-89.
2. Hoffman ND et al. J Adolesc Health. 2009;45:222-9.
3. Mayer KH et al. Adv Ther. 2020;37:1778-811.
4. Hosek SG et al. JAMA Pediatr. 2017;171(11):1063-71.
5. U.S. Preventive Services Task Force; Owens DK et al. JAMA. 2019;321(22):2203-13.
6. Centers for Disease Control and Prevention: U.S. Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update: A Clinical Practice Guideline. Published 2021. Accessed July 10, 2022.
7. Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2015-2019. HIV Surveillance Supplemental Report. 2021;26(1). Published May 2021. Accessed July 10, 2022.
8. Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data–United States and 6 Dependent Areas, 2020. HIV Surveillance Supplemental Report. 2022;27(3).



