User login
Birth method affects microbiome and vaccination response
Babies born vaginally have a different microbiome to those born by Caesarean section and have heightened responses to childhood vaccinations, according to a new study heralded as “interesting and important” by experts.
The microbiome is known to play a role in immune responses to vaccination. However, the relationship between early-life effects on intestinal microbiota composition and subsequent childhood vaccine responses had remained poorly understood. In the new study, “the findings suggest that vaginal birthing resulted in a microbiota composition associated with an increase in a specific type of antibody response to two routine childhood vaccines in healthy babies, compared with Caesarean section,” the authors said.
Researchers from the University of Edinburgh, with colleagues at Spaarne Hospital and University Medical Centre in Utrecht, and the National Institute for Public Health and the Environment in The Netherlands, tracked the development of the gut microbiome in a cohort of 120 healthy, full-term infants and assessed their antibody levels following two common childhood vaccinations.
The study, published in Nature Communications, found “a clear relationship between microbes in the gut of those babies and levels of antibodies.” Not only was vaginal birth associated with increased levels of Bifidobacterium and Escherichia coli in the gut microbiome in the first months of life but also with higher IgG antibody responses against both pneumococcal and meningococcal vaccines.
Antibody responses doubled after vaginal birth
The babies were given pneumococcal and meningococcal vaccinations at 8 and 12 weeks, and saliva was collected at follow-up visits at ages 12 and 18 months for antibody measurement. In the 101 babies tested for pneumococcal antibodies, the researchers found that antibody levels were twice as high among babies delivered naturally, compared with those delivered by C-section. High levels of two gut bacteria in particular – Bifidobacterium and E. coli – were associated with high antibody responses to the pneumococcal vaccine, showing that the microbiome mediated the link between mode of delivery and pneumococcal vaccine responses.
In 66 babies tested for anti-meningococcal antibodies, antibodies were 1.7 times higher for vaginally-born babies than those delivered via C-section, and high antibody levels were particularly associated with high levels of E. coli in the babies’ microbiome.
The results were also influenced by breast-feeding, which even among children born vaginally was linked with 3.5 times higher pneumococcal antibody levels, compared with those of formula-fed children. In contrast, levels of antibodies against meningococcus were unaffected by breast-feeding status.
Microbiome ‘sets level of infection protection’
The team said: “The baby acquires Bifidobacterium and E. coli bacteria through natural birth, and human milk is needed to provide the sugars for these bacteria to thrive on.” They explained: “The gut microbiome is seeded at birth, developing rapidly over the first few months of life, and is influenced mostly by delivery mode, breast-feeding, and antibiotic use.” The babies’ microbiome in early life contributes the immune system’s response to vaccines, they said, “and sets the level of protection against certain infections in childhood.”
Study lead Professor Debby Bogaert, chair of pediatric medicine at the University of Edinburgh, said: “I think it is especially interesting that we identified several beneficial microbes to be the link between mode of delivery and vaccine responses. In the future, we may be able to supplement those bacteria to children born by C-section shortly after birth through – for example, mother-to-baby ‘fecal transplants’ or the use of specifically designed probiotics.”
First author Dr. Emma de Koff, a microbiology trainee at the Amsterdam University Medical Center, said: “We expected to find a link between the gut microbiome and the babies’ vaccine responses, however we never thought to find the strongest effects in the first weeks of life.”
The findings “could help to inform conversations about C-sections between expectant mothers and their doctors,” commented the researchers, who said that they could also “shape the design of more tailored vaccination programs.” For example, in the future, vaccination schedules could be adjusted based on the method of delivery or analysis of the baby’s microbiome.
Potential to rectify immune system after Caesarean
Responding to the study, Professor Neil Mabbott, personal chair in immunopathology at the Roslin Institute of the University of Edinburgh, told the Science Media Centre: “This is a very interesting and important study. The authors show that infants delivered by a vaginal birth had higher responses to the two different types of vaccines against bacterial diseases, and this was associated with higher abundances of the potentially beneficial bacteria known as Bifidobacterium and E. coli in their intestines.”
He added: “This study raises the possibility that it may be possible to treat infants, especially Caesarean-delivered infants, with a bacterial supplement, or even a product produced by these beneficial bacteria, to help improve their immune systems, enhance their responses to certain vaccines and reduce their susceptibility to infections.”
The study raises important questions, he said, including whether the increased antibody levels from pneumococcal and meningococcal vaccinations following vaginal birth also leads to increased protection of the infants against infection or serious disease.
Sheena Cruickshank, immunologist and professor in biomedical sciences at the University of Manchester, England, commented: “It is now well established that the microbiome is important in immune development. In turn the mode of delivery and initial method of feeding is important in how the microbiome is first seeded in the baby.”
“However, other factors such as exposure to antibiotics and subsequent diet also play a role in how it then develops, making understanding the way the microbiome develops and changes quite complex. Microbes works as communities, and it can be difficult to determine whether changes in single species are important functionally. Breast milk also plays an important role in protecting the baby via transfer of maternal immunoglobulins, which will wane over a period of 6-12 months in the baby – thus ascertaining whether it’s the baby’s Ig is challenging.
“Given the complexity of the multitude of interactions, it is important that this is accounted for, and group sizes are large enough to ensure data is robust. Whilst this is an interesting study that adds to our knowledge of how the microbiome develops and the possible implications for immune development, it is still very preliminary, and the small group sizes warrant a need for further studies to verify this in larger groups.”
She added: “We will need to understand whether possible impacts of maternal delivery and feeding on immune development or vaccine responses can be restored by, for example, manipulating the microbiome.”
Professor Kim Barrett, vice dean for research at the University of California, Davis School of Medicine, said that, while further research was needed to uncover if and how manipulation of the human microbiome following C-section births might improve vaccine efficacy, “the work should at least lead to prompt additional consideration about an unintended consequence of the ever-increasing use of C-sections that may not be medically-necessary.”
Dr. Marie Lewis, researcher in gut microbiota at the University of Reading, England, said: “We have known for quite some time that the mode of delivery is incredibly important when it comes to the type of bacteria which colonize our guts. We also know that our gut bacteria in early life drive the development of our immune system, and natural births are linked with reduced risks of developing inflammatory conditions, such as asthma. It is therefore perhaps not really surprising that mode of delivery is also linked to responses to vaccinations.”
“The really interesting part here is the extent to which our gut microbiotas are accessible and changeable, and this important work could pave the way for administration of probiotics and prebiotics to improve vaccine responses in Caesarean-born children.”
‘Tantalizing data’
Dr. Chrissie Jones, associate professor of pediatric infectious diseases at the University of Southampton, and Southampton UK and education lead for the British Paediatric Allergy, Immunity, and Infection Group, said: “The link between method of delivery of the infant and the bacteria that live in the gut of the young infant has previously been shown. What is really interesting about this study is that, for the first time, the link between method of delivery (vaginal delivery vs. C-section), differences in bacterial communities of the gut, and differences in responses to vaccines is shown.”
“This study may give us fresh insights into the differences that we see in the amount of protective antibodies made after infant vaccination. It also gives us clues as to ways that we might be able to level the playing field for infants in the future – for instance, giving babies a safe cocktail of ‘friendly bacteria’ as a probiotic, or an additional dose of vaccine.”
“This study is the first step – it shows us a link or association but does not prove cause and effect that differences in the way babies are born alters how the immune system responds to vaccines. To prove this link we will need larger studies, but it is tantalizing data.”
The research was funded by Scotland’s Chief Scientist Office and the Netherlands Organisation for Scientific Research. DB received funding from OM pharma and Sanofi. All of the authors declared no other conflicts of interest.
A version of this article first appeared on Medscape.com.
Babies born vaginally have a different microbiome to those born by Caesarean section and have heightened responses to childhood vaccinations, according to a new study heralded as “interesting and important” by experts.
The microbiome is known to play a role in immune responses to vaccination. However, the relationship between early-life effects on intestinal microbiota composition and subsequent childhood vaccine responses had remained poorly understood. In the new study, “the findings suggest that vaginal birthing resulted in a microbiota composition associated with an increase in a specific type of antibody response to two routine childhood vaccines in healthy babies, compared with Caesarean section,” the authors said.
Researchers from the University of Edinburgh, with colleagues at Spaarne Hospital and University Medical Centre in Utrecht, and the National Institute for Public Health and the Environment in The Netherlands, tracked the development of the gut microbiome in a cohort of 120 healthy, full-term infants and assessed their antibody levels following two common childhood vaccinations.
The study, published in Nature Communications, found “a clear relationship between microbes in the gut of those babies and levels of antibodies.” Not only was vaginal birth associated with increased levels of Bifidobacterium and Escherichia coli in the gut microbiome in the first months of life but also with higher IgG antibody responses against both pneumococcal and meningococcal vaccines.
Antibody responses doubled after vaginal birth
The babies were given pneumococcal and meningococcal vaccinations at 8 and 12 weeks, and saliva was collected at follow-up visits at ages 12 and 18 months for antibody measurement. In the 101 babies tested for pneumococcal antibodies, the researchers found that antibody levels were twice as high among babies delivered naturally, compared with those delivered by C-section. High levels of two gut bacteria in particular – Bifidobacterium and E. coli – were associated with high antibody responses to the pneumococcal vaccine, showing that the microbiome mediated the link between mode of delivery and pneumococcal vaccine responses.
In 66 babies tested for anti-meningococcal antibodies, antibodies were 1.7 times higher for vaginally-born babies than those delivered via C-section, and high antibody levels were particularly associated with high levels of E. coli in the babies’ microbiome.
The results were also influenced by breast-feeding, which even among children born vaginally was linked with 3.5 times higher pneumococcal antibody levels, compared with those of formula-fed children. In contrast, levels of antibodies against meningococcus were unaffected by breast-feeding status.
Microbiome ‘sets level of infection protection’
The team said: “The baby acquires Bifidobacterium and E. coli bacteria through natural birth, and human milk is needed to provide the sugars for these bacteria to thrive on.” They explained: “The gut microbiome is seeded at birth, developing rapidly over the first few months of life, and is influenced mostly by delivery mode, breast-feeding, and antibiotic use.” The babies’ microbiome in early life contributes the immune system’s response to vaccines, they said, “and sets the level of protection against certain infections in childhood.”
Study lead Professor Debby Bogaert, chair of pediatric medicine at the University of Edinburgh, said: “I think it is especially interesting that we identified several beneficial microbes to be the link between mode of delivery and vaccine responses. In the future, we may be able to supplement those bacteria to children born by C-section shortly after birth through – for example, mother-to-baby ‘fecal transplants’ or the use of specifically designed probiotics.”
First author Dr. Emma de Koff, a microbiology trainee at the Amsterdam University Medical Center, said: “We expected to find a link between the gut microbiome and the babies’ vaccine responses, however we never thought to find the strongest effects in the first weeks of life.”
The findings “could help to inform conversations about C-sections between expectant mothers and their doctors,” commented the researchers, who said that they could also “shape the design of more tailored vaccination programs.” For example, in the future, vaccination schedules could be adjusted based on the method of delivery or analysis of the baby’s microbiome.
Potential to rectify immune system after Caesarean
Responding to the study, Professor Neil Mabbott, personal chair in immunopathology at the Roslin Institute of the University of Edinburgh, told the Science Media Centre: “This is a very interesting and important study. The authors show that infants delivered by a vaginal birth had higher responses to the two different types of vaccines against bacterial diseases, and this was associated with higher abundances of the potentially beneficial bacteria known as Bifidobacterium and E. coli in their intestines.”
He added: “This study raises the possibility that it may be possible to treat infants, especially Caesarean-delivered infants, with a bacterial supplement, or even a product produced by these beneficial bacteria, to help improve their immune systems, enhance their responses to certain vaccines and reduce their susceptibility to infections.”
The study raises important questions, he said, including whether the increased antibody levels from pneumococcal and meningococcal vaccinations following vaginal birth also leads to increased protection of the infants against infection or serious disease.
Sheena Cruickshank, immunologist and professor in biomedical sciences at the University of Manchester, England, commented: “It is now well established that the microbiome is important in immune development. In turn the mode of delivery and initial method of feeding is important in how the microbiome is first seeded in the baby.”
“However, other factors such as exposure to antibiotics and subsequent diet also play a role in how it then develops, making understanding the way the microbiome develops and changes quite complex. Microbes works as communities, and it can be difficult to determine whether changes in single species are important functionally. Breast milk also plays an important role in protecting the baby via transfer of maternal immunoglobulins, which will wane over a period of 6-12 months in the baby – thus ascertaining whether it’s the baby’s Ig is challenging.
“Given the complexity of the multitude of interactions, it is important that this is accounted for, and group sizes are large enough to ensure data is robust. Whilst this is an interesting study that adds to our knowledge of how the microbiome develops and the possible implications for immune development, it is still very preliminary, and the small group sizes warrant a need for further studies to verify this in larger groups.”
She added: “We will need to understand whether possible impacts of maternal delivery and feeding on immune development or vaccine responses can be restored by, for example, manipulating the microbiome.”
Professor Kim Barrett, vice dean for research at the University of California, Davis School of Medicine, said that, while further research was needed to uncover if and how manipulation of the human microbiome following C-section births might improve vaccine efficacy, “the work should at least lead to prompt additional consideration about an unintended consequence of the ever-increasing use of C-sections that may not be medically-necessary.”
Dr. Marie Lewis, researcher in gut microbiota at the University of Reading, England, said: “We have known for quite some time that the mode of delivery is incredibly important when it comes to the type of bacteria which colonize our guts. We also know that our gut bacteria in early life drive the development of our immune system, and natural births are linked with reduced risks of developing inflammatory conditions, such as asthma. It is therefore perhaps not really surprising that mode of delivery is also linked to responses to vaccinations.”
“The really interesting part here is the extent to which our gut microbiotas are accessible and changeable, and this important work could pave the way for administration of probiotics and prebiotics to improve vaccine responses in Caesarean-born children.”
‘Tantalizing data’
Dr. Chrissie Jones, associate professor of pediatric infectious diseases at the University of Southampton, and Southampton UK and education lead for the British Paediatric Allergy, Immunity, and Infection Group, said: “The link between method of delivery of the infant and the bacteria that live in the gut of the young infant has previously been shown. What is really interesting about this study is that, for the first time, the link between method of delivery (vaginal delivery vs. C-section), differences in bacterial communities of the gut, and differences in responses to vaccines is shown.”
“This study may give us fresh insights into the differences that we see in the amount of protective antibodies made after infant vaccination. It also gives us clues as to ways that we might be able to level the playing field for infants in the future – for instance, giving babies a safe cocktail of ‘friendly bacteria’ as a probiotic, or an additional dose of vaccine.”
“This study is the first step – it shows us a link or association but does not prove cause and effect that differences in the way babies are born alters how the immune system responds to vaccines. To prove this link we will need larger studies, but it is tantalizing data.”
The research was funded by Scotland’s Chief Scientist Office and the Netherlands Organisation for Scientific Research. DB received funding from OM pharma and Sanofi. All of the authors declared no other conflicts of interest.
A version of this article first appeared on Medscape.com.
Babies born vaginally have a different microbiome to those born by Caesarean section and have heightened responses to childhood vaccinations, according to a new study heralded as “interesting and important” by experts.
The microbiome is known to play a role in immune responses to vaccination. However, the relationship between early-life effects on intestinal microbiota composition and subsequent childhood vaccine responses had remained poorly understood. In the new study, “the findings suggest that vaginal birthing resulted in a microbiota composition associated with an increase in a specific type of antibody response to two routine childhood vaccines in healthy babies, compared with Caesarean section,” the authors said.
Researchers from the University of Edinburgh, with colleagues at Spaarne Hospital and University Medical Centre in Utrecht, and the National Institute for Public Health and the Environment in The Netherlands, tracked the development of the gut microbiome in a cohort of 120 healthy, full-term infants and assessed their antibody levels following two common childhood vaccinations.
The study, published in Nature Communications, found “a clear relationship between microbes in the gut of those babies and levels of antibodies.” Not only was vaginal birth associated with increased levels of Bifidobacterium and Escherichia coli in the gut microbiome in the first months of life but also with higher IgG antibody responses against both pneumococcal and meningococcal vaccines.
Antibody responses doubled after vaginal birth
The babies were given pneumococcal and meningococcal vaccinations at 8 and 12 weeks, and saliva was collected at follow-up visits at ages 12 and 18 months for antibody measurement. In the 101 babies tested for pneumococcal antibodies, the researchers found that antibody levels were twice as high among babies delivered naturally, compared with those delivered by C-section. High levels of two gut bacteria in particular – Bifidobacterium and E. coli – were associated with high antibody responses to the pneumococcal vaccine, showing that the microbiome mediated the link between mode of delivery and pneumococcal vaccine responses.
In 66 babies tested for anti-meningococcal antibodies, antibodies were 1.7 times higher for vaginally-born babies than those delivered via C-section, and high antibody levels were particularly associated with high levels of E. coli in the babies’ microbiome.
The results were also influenced by breast-feeding, which even among children born vaginally was linked with 3.5 times higher pneumococcal antibody levels, compared with those of formula-fed children. In contrast, levels of antibodies against meningococcus were unaffected by breast-feeding status.
Microbiome ‘sets level of infection protection’
The team said: “The baby acquires Bifidobacterium and E. coli bacteria through natural birth, and human milk is needed to provide the sugars for these bacteria to thrive on.” They explained: “The gut microbiome is seeded at birth, developing rapidly over the first few months of life, and is influenced mostly by delivery mode, breast-feeding, and antibiotic use.” The babies’ microbiome in early life contributes the immune system’s response to vaccines, they said, “and sets the level of protection against certain infections in childhood.”
Study lead Professor Debby Bogaert, chair of pediatric medicine at the University of Edinburgh, said: “I think it is especially interesting that we identified several beneficial microbes to be the link between mode of delivery and vaccine responses. In the future, we may be able to supplement those bacteria to children born by C-section shortly after birth through – for example, mother-to-baby ‘fecal transplants’ or the use of specifically designed probiotics.”
First author Dr. Emma de Koff, a microbiology trainee at the Amsterdam University Medical Center, said: “We expected to find a link between the gut microbiome and the babies’ vaccine responses, however we never thought to find the strongest effects in the first weeks of life.”
The findings “could help to inform conversations about C-sections between expectant mothers and their doctors,” commented the researchers, who said that they could also “shape the design of more tailored vaccination programs.” For example, in the future, vaccination schedules could be adjusted based on the method of delivery or analysis of the baby’s microbiome.
Potential to rectify immune system after Caesarean
Responding to the study, Professor Neil Mabbott, personal chair in immunopathology at the Roslin Institute of the University of Edinburgh, told the Science Media Centre: “This is a very interesting and important study. The authors show that infants delivered by a vaginal birth had higher responses to the two different types of vaccines against bacterial diseases, and this was associated with higher abundances of the potentially beneficial bacteria known as Bifidobacterium and E. coli in their intestines.”
He added: “This study raises the possibility that it may be possible to treat infants, especially Caesarean-delivered infants, with a bacterial supplement, or even a product produced by these beneficial bacteria, to help improve their immune systems, enhance their responses to certain vaccines and reduce their susceptibility to infections.”
The study raises important questions, he said, including whether the increased antibody levels from pneumococcal and meningococcal vaccinations following vaginal birth also leads to increased protection of the infants against infection or serious disease.
Sheena Cruickshank, immunologist and professor in biomedical sciences at the University of Manchester, England, commented: “It is now well established that the microbiome is important in immune development. In turn the mode of delivery and initial method of feeding is important in how the microbiome is first seeded in the baby.”
“However, other factors such as exposure to antibiotics and subsequent diet also play a role in how it then develops, making understanding the way the microbiome develops and changes quite complex. Microbes works as communities, and it can be difficult to determine whether changes in single species are important functionally. Breast milk also plays an important role in protecting the baby via transfer of maternal immunoglobulins, which will wane over a period of 6-12 months in the baby – thus ascertaining whether it’s the baby’s Ig is challenging.
“Given the complexity of the multitude of interactions, it is important that this is accounted for, and group sizes are large enough to ensure data is robust. Whilst this is an interesting study that adds to our knowledge of how the microbiome develops and the possible implications for immune development, it is still very preliminary, and the small group sizes warrant a need for further studies to verify this in larger groups.”
She added: “We will need to understand whether possible impacts of maternal delivery and feeding on immune development or vaccine responses can be restored by, for example, manipulating the microbiome.”
Professor Kim Barrett, vice dean for research at the University of California, Davis School of Medicine, said that, while further research was needed to uncover if and how manipulation of the human microbiome following C-section births might improve vaccine efficacy, “the work should at least lead to prompt additional consideration about an unintended consequence of the ever-increasing use of C-sections that may not be medically-necessary.”
Dr. Marie Lewis, researcher in gut microbiota at the University of Reading, England, said: “We have known for quite some time that the mode of delivery is incredibly important when it comes to the type of bacteria which colonize our guts. We also know that our gut bacteria in early life drive the development of our immune system, and natural births are linked with reduced risks of developing inflammatory conditions, such as asthma. It is therefore perhaps not really surprising that mode of delivery is also linked to responses to vaccinations.”
“The really interesting part here is the extent to which our gut microbiotas are accessible and changeable, and this important work could pave the way for administration of probiotics and prebiotics to improve vaccine responses in Caesarean-born children.”
‘Tantalizing data’
Dr. Chrissie Jones, associate professor of pediatric infectious diseases at the University of Southampton, and Southampton UK and education lead for the British Paediatric Allergy, Immunity, and Infection Group, said: “The link between method of delivery of the infant and the bacteria that live in the gut of the young infant has previously been shown. What is really interesting about this study is that, for the first time, the link between method of delivery (vaginal delivery vs. C-section), differences in bacterial communities of the gut, and differences in responses to vaccines is shown.”
“This study may give us fresh insights into the differences that we see in the amount of protective antibodies made after infant vaccination. It also gives us clues as to ways that we might be able to level the playing field for infants in the future – for instance, giving babies a safe cocktail of ‘friendly bacteria’ as a probiotic, or an additional dose of vaccine.”
“This study is the first step – it shows us a link or association but does not prove cause and effect that differences in the way babies are born alters how the immune system responds to vaccines. To prove this link we will need larger studies, but it is tantalizing data.”
The research was funded by Scotland’s Chief Scientist Office and the Netherlands Organisation for Scientific Research. DB received funding from OM pharma and Sanofi. All of the authors declared no other conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
FDA approves first-ever agent to delay type 1 diabetes onset
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
The agent, which interferes with T-cell-mediated autoimmune destruction of pancreatic beta cells, is the first disease-modifying therapy for impeding progression of type 1 diabetes. It is administered by intravenous infusion once daily for 14 consecutive days.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In type 1 diabetes staging, adopted in 2015, stage 1 is defined as the presence of beta cell autoimmunity with two or more islet autoantibodies with normoglycemia, stage 2 is beta-cell autoimmunity with dysglycemia yet asymptomatic, and stage 3 is the onset of symptomatic type 1 diabetes.
Stage 2 type 1 diabetes is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
The FDA had previously rejected teplizumab for this indication in July 2021, despite a prior endorsement from an advisory panel in May 2021.
Now, with the FDA approval, Provention Bio cofounder and CEO Ashleigh Palmer said in a statement, “This is a historic occasion for the T1D community and a paradigm shifting breakthrough ... It cannot be emphasized enough how precious a delay in the onset of stage 3 T1D can be from a patient and family perspective; more time to live without and, when necessary, prepare for the burdens, complications, and risks associated with stage 3 disease.”
T1D onset delayed by 2 years
In 2019, a pivotal phase 2, randomized, placebo-controlled trial involving 76 at-risk children and adults aged 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes compared with 32 who received placebo.
Those “game changer” data were presented at the American Diabetes Association (ADA) annual meeting in June 2019 and simultaneously published in the New England Journal of Medicine.
Three-year data were presented at the June 2020 ADA meeting and published in March 2021 in Science Translational Medicine, by Emily K. Sims, MD, department of pediatrics, Indiana University, Indianapolis, and colleagues.
At a median follow-up of 923 days, 50% of those randomly assigned to teplizumab remained diabetes free, compared with 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01). The teplizumab group had a greater average C-peptide area under the curve compared with placebo, reflecting improved beta-cell function (1.96 vs. 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“The mid-range time from randomization to stage 3 type 1 diabetes diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo. This represents a statistically significant delay in the development of stage 3 type 1 diabetes,” according to the FDA statement.
The most common side effects of Tzield include lymphopenia (73% teplizumab vs. 6% placebo), rash (36% vs. 0%), leukopenia (221% vs. 0%), and headache (11% vs. 6%). Label warnings and precautions include monitoring for cytokine release syndrome, risk for serious infections, and avoidance of live, inactivated, and mRNA vaccines.
This approval is likely to accelerate discussion about universal autoantibody screening. Currently, most individuals identified as having preclinical type 1 diabetes are first-degree relatives of people with type 1 diabetes identified through the federally funded TrialNet program. In December 2020, the type 1 diabetes research and advocacy organization JDRF began offering a $55 home blood test to screen for the antibodies, and other screening programs have been launched in the United States and Europe.
Previous studies have examined cost-effectiveness of universal screening in children and the optimal ages that such screening should take place.
In October, Provention Bio announced a co-promotion agreement with Sanofi for the U.S. launch of Tzield for delay in onset of clinical T1D in at-risk individuals. Provention Bio offers financial assistance options (e.g., copay assistance) to eligible patients for out-of-pocket costs.
A version of this article first appeared on Medscape.com.
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
The agent, which interferes with T-cell-mediated autoimmune destruction of pancreatic beta cells, is the first disease-modifying therapy for impeding progression of type 1 diabetes. It is administered by intravenous infusion once daily for 14 consecutive days.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In type 1 diabetes staging, adopted in 2015, stage 1 is defined as the presence of beta cell autoimmunity with two or more islet autoantibodies with normoglycemia, stage 2 is beta-cell autoimmunity with dysglycemia yet asymptomatic, and stage 3 is the onset of symptomatic type 1 diabetes.
Stage 2 type 1 diabetes is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
The FDA had previously rejected teplizumab for this indication in July 2021, despite a prior endorsement from an advisory panel in May 2021.
Now, with the FDA approval, Provention Bio cofounder and CEO Ashleigh Palmer said in a statement, “This is a historic occasion for the T1D community and a paradigm shifting breakthrough ... It cannot be emphasized enough how precious a delay in the onset of stage 3 T1D can be from a patient and family perspective; more time to live without and, when necessary, prepare for the burdens, complications, and risks associated with stage 3 disease.”
T1D onset delayed by 2 years
In 2019, a pivotal phase 2, randomized, placebo-controlled trial involving 76 at-risk children and adults aged 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes compared with 32 who received placebo.
Those “game changer” data were presented at the American Diabetes Association (ADA) annual meeting in June 2019 and simultaneously published in the New England Journal of Medicine.
Three-year data were presented at the June 2020 ADA meeting and published in March 2021 in Science Translational Medicine, by Emily K. Sims, MD, department of pediatrics, Indiana University, Indianapolis, and colleagues.
At a median follow-up of 923 days, 50% of those randomly assigned to teplizumab remained diabetes free, compared with 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01). The teplizumab group had a greater average C-peptide area under the curve compared with placebo, reflecting improved beta-cell function (1.96 vs. 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“The mid-range time from randomization to stage 3 type 1 diabetes diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo. This represents a statistically significant delay in the development of stage 3 type 1 diabetes,” according to the FDA statement.
The most common side effects of Tzield include lymphopenia (73% teplizumab vs. 6% placebo), rash (36% vs. 0%), leukopenia (221% vs. 0%), and headache (11% vs. 6%). Label warnings and precautions include monitoring for cytokine release syndrome, risk for serious infections, and avoidance of live, inactivated, and mRNA vaccines.
This approval is likely to accelerate discussion about universal autoantibody screening. Currently, most individuals identified as having preclinical type 1 diabetes are first-degree relatives of people with type 1 diabetes identified through the federally funded TrialNet program. In December 2020, the type 1 diabetes research and advocacy organization JDRF began offering a $55 home blood test to screen for the antibodies, and other screening programs have been launched in the United States and Europe.
Previous studies have examined cost-effectiveness of universal screening in children and the optimal ages that such screening should take place.
In October, Provention Bio announced a co-promotion agreement with Sanofi for the U.S. launch of Tzield for delay in onset of clinical T1D in at-risk individuals. Provention Bio offers financial assistance options (e.g., copay assistance) to eligible patients for out-of-pocket costs.
A version of this article first appeared on Medscape.com.
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
The agent, which interferes with T-cell-mediated autoimmune destruction of pancreatic beta cells, is the first disease-modifying therapy for impeding progression of type 1 diabetes. It is administered by intravenous infusion once daily for 14 consecutive days.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In type 1 diabetes staging, adopted in 2015, stage 1 is defined as the presence of beta cell autoimmunity with two or more islet autoantibodies with normoglycemia, stage 2 is beta-cell autoimmunity with dysglycemia yet asymptomatic, and stage 3 is the onset of symptomatic type 1 diabetes.
Stage 2 type 1 diabetes is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
The FDA had previously rejected teplizumab for this indication in July 2021, despite a prior endorsement from an advisory panel in May 2021.
Now, with the FDA approval, Provention Bio cofounder and CEO Ashleigh Palmer said in a statement, “This is a historic occasion for the T1D community and a paradigm shifting breakthrough ... It cannot be emphasized enough how precious a delay in the onset of stage 3 T1D can be from a patient and family perspective; more time to live without and, when necessary, prepare for the burdens, complications, and risks associated with stage 3 disease.”
T1D onset delayed by 2 years
In 2019, a pivotal phase 2, randomized, placebo-controlled trial involving 76 at-risk children and adults aged 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes compared with 32 who received placebo.
Those “game changer” data were presented at the American Diabetes Association (ADA) annual meeting in June 2019 and simultaneously published in the New England Journal of Medicine.
Three-year data were presented at the June 2020 ADA meeting and published in March 2021 in Science Translational Medicine, by Emily K. Sims, MD, department of pediatrics, Indiana University, Indianapolis, and colleagues.
At a median follow-up of 923 days, 50% of those randomly assigned to teplizumab remained diabetes free, compared with 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01). The teplizumab group had a greater average C-peptide area under the curve compared with placebo, reflecting improved beta-cell function (1.96 vs. 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“The mid-range time from randomization to stage 3 type 1 diabetes diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo. This represents a statistically significant delay in the development of stage 3 type 1 diabetes,” according to the FDA statement.
The most common side effects of Tzield include lymphopenia (73% teplizumab vs. 6% placebo), rash (36% vs. 0%), leukopenia (221% vs. 0%), and headache (11% vs. 6%). Label warnings and precautions include monitoring for cytokine release syndrome, risk for serious infections, and avoidance of live, inactivated, and mRNA vaccines.
This approval is likely to accelerate discussion about universal autoantibody screening. Currently, most individuals identified as having preclinical type 1 diabetes are first-degree relatives of people with type 1 diabetes identified through the federally funded TrialNet program. In December 2020, the type 1 diabetes research and advocacy organization JDRF began offering a $55 home blood test to screen for the antibodies, and other screening programs have been launched in the United States and Europe.
Previous studies have examined cost-effectiveness of universal screening in children and the optimal ages that such screening should take place.
In October, Provention Bio announced a co-promotion agreement with Sanofi for the U.S. launch of Tzield for delay in onset of clinical T1D in at-risk individuals. Provention Bio offers financial assistance options (e.g., copay assistance) to eligible patients for out-of-pocket costs.
A version of this article first appeared on Medscape.com.
HIV: Greater parental involvement needed with young men who have sex with men
“Take it from me, parents just don’t understand.”
Fresh Prince and D.J. Jazzy Jeff penned this lyric roughly 35 years ago, and coincidentally the HIV/AIDS epidemic has also been with us just as long. But the connection between the two may be highly relevant – that is, if you consider how infrequently parents appear (or have the proper tools) to engage with their gay or bisexual sons to prevent and curb HIV infections.
Currently, YMSM between the ages of 13 and 24 are among the most affected by the ongoing HIV epidemic, with CDC estimates suggesting that, in 2020, this group alone represented about 35% of new diagnoses. At the same time, about half of these HIV infections go undiagnosed. Recent data also suggest that care linkage in this group is similar to adults, but only a third of YMSM start antiretroviral therapy and are retained in care, leading to viral suppression rates as low as 12%.
With a goal to change these discouraging numbers, researchers from George Washington University, Washington, and other institutions conducted a randomized controlled pilot study targeting parents of YMSM to improve both the frequency and quality of communication around sexual health and HIV risk, prevention, and testing.
The findings, which were published online in the journal AIDS and Behavior, highlight the observation that parents could be an essential resource for combating the HIV epidemic, but they’re a resource that’s often underutilized. In fact, after participating in an online offering – PATHS (Parents and Adolescents Talking about Healthy Sexuality) – parents reported significantly greater engagement with their sons, especially around discussions focusing on HIV information and condom use.
“From what we know from the research, parents are uncomfortable talking about sex; they’re not great at talking about it. But when they do and do it effectively, those kids seem to have better health outcomes,” lead author David Huebner, PhD, MPH, associate professor of prevention and community health at George Washington University, said in an interview.
“The goal was to get parents to deliver more messages and engage in more behaviors with their sons that we think are likely to help their sons stay healthy,” he said.
For the pilot study, Huebner and his team recruited 61 parents (95% of whom were mothers) with predominantly high school-aged cisgender sons (median, 16.7-17 years) who had come out as gay or bisexual at least a month prior, whose HIV status was negative or unknown, and who were living at home.
The interventions were strictly parent focused, Dr. Huebner said, noting that the only interaction with the kids involved independent surveys at the start and end of the study that explored parental behavior and engagement.
For the study, parental participants were stratified by son’s age (13-17 or 18-22 years) and then randomly assigned to participate in a web-accessible PATHS intervention (intervention group) or view a 35-minute, documentary-style film that encouraged acceptance of lesbian, gay, or bisexual children (control group),
Parents assigned to the intervention group were asked to engage in their own time with six modules that explored the importance of communication, HIV information, using and acquiring condoms, HIV testing, and as follow-up, a “to-do” list encouraging selection of how they would follow up with their sons about the content. They were also offered the option to participate in supplemental modules on pre-exposure prophylaxis (PrEP), anal intercourse, and what to do if a child tested positive for HIV.
“The intervention ... showed strong evidence of being effective at changing the parent behaviors that we hoped to change,” Dr. Huebner explained.
“We got independent reports from parents and kids that showed the same thing: parents were more likely to communicate with their sons about HIV in the 3 months after the intervention and were more likely to help their sons get access to condoms,” he said.
Both of these findings were significant, with parents in the experimental group being almost 10 times more likely to share HIV information with their sons (odds ratio, 9.50; 95% confidence interval, 1.02-39.99; P < .05) and five times more likely to teach proper condom use (OR, 5.04; 95% CI 1.56-12.46; P < .05), compared with parents receiving the placebo.
“It’s very promising that the initial signals from their intervention do show that parents facilitating the acquisition of information for young men who have sex with men really works,” said Dalmacio Dennis Flores, PhD, ACRN, an assistant professor of nursing in family and community health at the University of Pennsylvania, Philadelphia. He was not directly involved in the study.
“On the outcomes that matter for us, such as HIV prevention or getting tested, they were able to document that parents receiving guidance on how to have these conversations does result in youth outcomes – something that has been lacking in the literature specifically for this population up until today,” Dr. Flores told this news organization.
Overall, parents engaging in the PATHS intervention showed improvements in skills, attitudes, and behavioral intention toward engagement with their sons, including assisting with HIV testing. However, what about parental involvement in these types of dialogues with children who have not yet come out to their parents?
Dr. Flores said that, although Dr. Huebner’s work is pivotal for families where the child’s sexual orientation is known to parents, there is value in inclusive sex communication for all youth, regardless of how they identify (that is, out of the closet, closeted, straight, or those who are questioning their identity), especially since younger generations of LGBTQ youth are coming out at earlier ages, compared with previous generations.
It’s not just parents. Clinicians also have critical roles to play in helping bridge the sex-talk communication gaps between parents and adolescents and young adult children.
“In my work, I’ve found that more clinicians are willing to broach this within the discussion with dyads, with parents and adolescents in the room,” said Dr. Flores.
And he added: “If clinicians signal that there’s no such thing as too early to have these conversations or that issues such as consent, safety, and sexting are all okay to talk about because these are the current realities of young people, then parents can feel that they’re empowered to broach or sustain these conversations.”
Importantly, Dr. Huebner and associates are currently recruiting larger numbers of families for a new, yearlong trial that will not only examine parental behavior changes but also whether these changes translate into improvements in their child’s sexual health and/or competency. Interested families can learn more about the study and sign up to receive updates at www.parentwithlove.org.
Dr. Huebner and Dr. Flores reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Take it from me, parents just don’t understand.”
Fresh Prince and D.J. Jazzy Jeff penned this lyric roughly 35 years ago, and coincidentally the HIV/AIDS epidemic has also been with us just as long. But the connection between the two may be highly relevant – that is, if you consider how infrequently parents appear (or have the proper tools) to engage with their gay or bisexual sons to prevent and curb HIV infections.
Currently, YMSM between the ages of 13 and 24 are among the most affected by the ongoing HIV epidemic, with CDC estimates suggesting that, in 2020, this group alone represented about 35% of new diagnoses. At the same time, about half of these HIV infections go undiagnosed. Recent data also suggest that care linkage in this group is similar to adults, but only a third of YMSM start antiretroviral therapy and are retained in care, leading to viral suppression rates as low as 12%.
With a goal to change these discouraging numbers, researchers from George Washington University, Washington, and other institutions conducted a randomized controlled pilot study targeting parents of YMSM to improve both the frequency and quality of communication around sexual health and HIV risk, prevention, and testing.
The findings, which were published online in the journal AIDS and Behavior, highlight the observation that parents could be an essential resource for combating the HIV epidemic, but they’re a resource that’s often underutilized. In fact, after participating in an online offering – PATHS (Parents and Adolescents Talking about Healthy Sexuality) – parents reported significantly greater engagement with their sons, especially around discussions focusing on HIV information and condom use.
“From what we know from the research, parents are uncomfortable talking about sex; they’re not great at talking about it. But when they do and do it effectively, those kids seem to have better health outcomes,” lead author David Huebner, PhD, MPH, associate professor of prevention and community health at George Washington University, said in an interview.
“The goal was to get parents to deliver more messages and engage in more behaviors with their sons that we think are likely to help their sons stay healthy,” he said.
For the pilot study, Huebner and his team recruited 61 parents (95% of whom were mothers) with predominantly high school-aged cisgender sons (median, 16.7-17 years) who had come out as gay or bisexual at least a month prior, whose HIV status was negative or unknown, and who were living at home.
The interventions were strictly parent focused, Dr. Huebner said, noting that the only interaction with the kids involved independent surveys at the start and end of the study that explored parental behavior and engagement.
For the study, parental participants were stratified by son’s age (13-17 or 18-22 years) and then randomly assigned to participate in a web-accessible PATHS intervention (intervention group) or view a 35-minute, documentary-style film that encouraged acceptance of lesbian, gay, or bisexual children (control group),
Parents assigned to the intervention group were asked to engage in their own time with six modules that explored the importance of communication, HIV information, using and acquiring condoms, HIV testing, and as follow-up, a “to-do” list encouraging selection of how they would follow up with their sons about the content. They were also offered the option to participate in supplemental modules on pre-exposure prophylaxis (PrEP), anal intercourse, and what to do if a child tested positive for HIV.
“The intervention ... showed strong evidence of being effective at changing the parent behaviors that we hoped to change,” Dr. Huebner explained.
“We got independent reports from parents and kids that showed the same thing: parents were more likely to communicate with their sons about HIV in the 3 months after the intervention and were more likely to help their sons get access to condoms,” he said.
Both of these findings were significant, with parents in the experimental group being almost 10 times more likely to share HIV information with their sons (odds ratio, 9.50; 95% confidence interval, 1.02-39.99; P < .05) and five times more likely to teach proper condom use (OR, 5.04; 95% CI 1.56-12.46; P < .05), compared with parents receiving the placebo.
“It’s very promising that the initial signals from their intervention do show that parents facilitating the acquisition of information for young men who have sex with men really works,” said Dalmacio Dennis Flores, PhD, ACRN, an assistant professor of nursing in family and community health at the University of Pennsylvania, Philadelphia. He was not directly involved in the study.
“On the outcomes that matter for us, such as HIV prevention or getting tested, they were able to document that parents receiving guidance on how to have these conversations does result in youth outcomes – something that has been lacking in the literature specifically for this population up until today,” Dr. Flores told this news organization.
Overall, parents engaging in the PATHS intervention showed improvements in skills, attitudes, and behavioral intention toward engagement with their sons, including assisting with HIV testing. However, what about parental involvement in these types of dialogues with children who have not yet come out to their parents?
Dr. Flores said that, although Dr. Huebner’s work is pivotal for families where the child’s sexual orientation is known to parents, there is value in inclusive sex communication for all youth, regardless of how they identify (that is, out of the closet, closeted, straight, or those who are questioning their identity), especially since younger generations of LGBTQ youth are coming out at earlier ages, compared with previous generations.
It’s not just parents. Clinicians also have critical roles to play in helping bridge the sex-talk communication gaps between parents and adolescents and young adult children.
“In my work, I’ve found that more clinicians are willing to broach this within the discussion with dyads, with parents and adolescents in the room,” said Dr. Flores.
And he added: “If clinicians signal that there’s no such thing as too early to have these conversations or that issues such as consent, safety, and sexting are all okay to talk about because these are the current realities of young people, then parents can feel that they’re empowered to broach or sustain these conversations.”
Importantly, Dr. Huebner and associates are currently recruiting larger numbers of families for a new, yearlong trial that will not only examine parental behavior changes but also whether these changes translate into improvements in their child’s sexual health and/or competency. Interested families can learn more about the study and sign up to receive updates at www.parentwithlove.org.
Dr. Huebner and Dr. Flores reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Take it from me, parents just don’t understand.”
Fresh Prince and D.J. Jazzy Jeff penned this lyric roughly 35 years ago, and coincidentally the HIV/AIDS epidemic has also been with us just as long. But the connection between the two may be highly relevant – that is, if you consider how infrequently parents appear (or have the proper tools) to engage with their gay or bisexual sons to prevent and curb HIV infections.
Currently, YMSM between the ages of 13 and 24 are among the most affected by the ongoing HIV epidemic, with CDC estimates suggesting that, in 2020, this group alone represented about 35% of new diagnoses. At the same time, about half of these HIV infections go undiagnosed. Recent data also suggest that care linkage in this group is similar to adults, but only a third of YMSM start antiretroviral therapy and are retained in care, leading to viral suppression rates as low as 12%.
With a goal to change these discouraging numbers, researchers from George Washington University, Washington, and other institutions conducted a randomized controlled pilot study targeting parents of YMSM to improve both the frequency and quality of communication around sexual health and HIV risk, prevention, and testing.
The findings, which were published online in the journal AIDS and Behavior, highlight the observation that parents could be an essential resource for combating the HIV epidemic, but they’re a resource that’s often underutilized. In fact, after participating in an online offering – PATHS (Parents and Adolescents Talking about Healthy Sexuality) – parents reported significantly greater engagement with their sons, especially around discussions focusing on HIV information and condom use.
“From what we know from the research, parents are uncomfortable talking about sex; they’re not great at talking about it. But when they do and do it effectively, those kids seem to have better health outcomes,” lead author David Huebner, PhD, MPH, associate professor of prevention and community health at George Washington University, said in an interview.
“The goal was to get parents to deliver more messages and engage in more behaviors with their sons that we think are likely to help their sons stay healthy,” he said.
For the pilot study, Huebner and his team recruited 61 parents (95% of whom were mothers) with predominantly high school-aged cisgender sons (median, 16.7-17 years) who had come out as gay or bisexual at least a month prior, whose HIV status was negative or unknown, and who were living at home.
The interventions were strictly parent focused, Dr. Huebner said, noting that the only interaction with the kids involved independent surveys at the start and end of the study that explored parental behavior and engagement.
For the study, parental participants were stratified by son’s age (13-17 or 18-22 years) and then randomly assigned to participate in a web-accessible PATHS intervention (intervention group) or view a 35-minute, documentary-style film that encouraged acceptance of lesbian, gay, or bisexual children (control group),
Parents assigned to the intervention group were asked to engage in their own time with six modules that explored the importance of communication, HIV information, using and acquiring condoms, HIV testing, and as follow-up, a “to-do” list encouraging selection of how they would follow up with their sons about the content. They were also offered the option to participate in supplemental modules on pre-exposure prophylaxis (PrEP), anal intercourse, and what to do if a child tested positive for HIV.
“The intervention ... showed strong evidence of being effective at changing the parent behaviors that we hoped to change,” Dr. Huebner explained.
“We got independent reports from parents and kids that showed the same thing: parents were more likely to communicate with their sons about HIV in the 3 months after the intervention and were more likely to help their sons get access to condoms,” he said.
Both of these findings were significant, with parents in the experimental group being almost 10 times more likely to share HIV information with their sons (odds ratio, 9.50; 95% confidence interval, 1.02-39.99; P < .05) and five times more likely to teach proper condom use (OR, 5.04; 95% CI 1.56-12.46; P < .05), compared with parents receiving the placebo.
“It’s very promising that the initial signals from their intervention do show that parents facilitating the acquisition of information for young men who have sex with men really works,” said Dalmacio Dennis Flores, PhD, ACRN, an assistant professor of nursing in family and community health at the University of Pennsylvania, Philadelphia. He was not directly involved in the study.
“On the outcomes that matter for us, such as HIV prevention or getting tested, they were able to document that parents receiving guidance on how to have these conversations does result in youth outcomes – something that has been lacking in the literature specifically for this population up until today,” Dr. Flores told this news organization.
Overall, parents engaging in the PATHS intervention showed improvements in skills, attitudes, and behavioral intention toward engagement with their sons, including assisting with HIV testing. However, what about parental involvement in these types of dialogues with children who have not yet come out to their parents?
Dr. Flores said that, although Dr. Huebner’s work is pivotal for families where the child’s sexual orientation is known to parents, there is value in inclusive sex communication for all youth, regardless of how they identify (that is, out of the closet, closeted, straight, or those who are questioning their identity), especially since younger generations of LGBTQ youth are coming out at earlier ages, compared with previous generations.
It’s not just parents. Clinicians also have critical roles to play in helping bridge the sex-talk communication gaps between parents and adolescents and young adult children.
“In my work, I’ve found that more clinicians are willing to broach this within the discussion with dyads, with parents and adolescents in the room,” said Dr. Flores.
And he added: “If clinicians signal that there’s no such thing as too early to have these conversations or that issues such as consent, safety, and sexting are all okay to talk about because these are the current realities of young people, then parents can feel that they’re empowered to broach or sustain these conversations.”
Importantly, Dr. Huebner and associates are currently recruiting larger numbers of families for a new, yearlong trial that will not only examine parental behavior changes but also whether these changes translate into improvements in their child’s sexual health and/or competency. Interested families can learn more about the study and sign up to receive updates at www.parentwithlove.org.
Dr. Huebner and Dr. Flores reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AIDS AND BEHAVIOR
A plane crash interrupts a doctor’s vacation
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
The tale of two scenarios of gender dysphoria
In a recent column, I cautiously discussed what has been called gender-affirming or transgender care.
In the days following the appearance of that Letters From Maine column on this topic, I received an unusual number of responses from readers suggesting I had touched on a topic that was on the minds of many pediatricians.
Since then, the Florida Board of Medicine and Osteopathic Medicine voted to forbid physicians from prescribing puberty blockers and hormones and/or performing surgeries in patients under age 18 who were seeking transgender care. Children already receiving treatments were exempt from the ruling. The osteopathic board added a second exception in cases where the child was a participant in a research protocol. The board of medicine inexplicably did not include this exception.
Regardless of how one feels about the ethics and the appropriateness of transgender care, it is not an issue to be decided by a politically appointed entity.
As I look back over what I have learned by watching this tragic drama play out, I am struck by a distinction that has yet to receive enough attention. When we are discussing gender dysphoria we are really talking about two different pediatric populations and scenarios. There is the child who from a very young age has consistently preferred to dress and behave in a manner that is different from the gender he or she was assigned at birth. The management of this child is a challenge that requires a careful balance of support and protection from the harsh realities of the gender-regimented world.
The second scenario stars the adolescent who has no prior history of gender dysphoria, or at least no outward manifestations. Then, faced by the challenges of puberty and adolescence, something or things happen that erupt into a full-blown gender-dysphoric storm. We currently have very little understanding of what those “things” are.
Each population can probably be further divided into subgroups – and that’s just the point. Every gender-dysphoric child, whether their dysphoria began at age 2 or 12, is an individual with a unique family dynamic and socioeconomic background. They may share some as yet unknown genetic signature, but in our current state of ignorance they deserve, as do all of our patients, to be treated as individuals by their primary care physicians and consultants who must at first do no harm. One size does not fit all and certainly their care should not be dictated by a politically influenced entity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In a recent column, I cautiously discussed what has been called gender-affirming or transgender care.
In the days following the appearance of that Letters From Maine column on this topic, I received an unusual number of responses from readers suggesting I had touched on a topic that was on the minds of many pediatricians.
Since then, the Florida Board of Medicine and Osteopathic Medicine voted to forbid physicians from prescribing puberty blockers and hormones and/or performing surgeries in patients under age 18 who were seeking transgender care. Children already receiving treatments were exempt from the ruling. The osteopathic board added a second exception in cases where the child was a participant in a research protocol. The board of medicine inexplicably did not include this exception.
Regardless of how one feels about the ethics and the appropriateness of transgender care, it is not an issue to be decided by a politically appointed entity.
As I look back over what I have learned by watching this tragic drama play out, I am struck by a distinction that has yet to receive enough attention. When we are discussing gender dysphoria we are really talking about two different pediatric populations and scenarios. There is the child who from a very young age has consistently preferred to dress and behave in a manner that is different from the gender he or she was assigned at birth. The management of this child is a challenge that requires a careful balance of support and protection from the harsh realities of the gender-regimented world.
The second scenario stars the adolescent who has no prior history of gender dysphoria, or at least no outward manifestations. Then, faced by the challenges of puberty and adolescence, something or things happen that erupt into a full-blown gender-dysphoric storm. We currently have very little understanding of what those “things” are.
Each population can probably be further divided into subgroups – and that’s just the point. Every gender-dysphoric child, whether their dysphoria began at age 2 or 12, is an individual with a unique family dynamic and socioeconomic background. They may share some as yet unknown genetic signature, but in our current state of ignorance they deserve, as do all of our patients, to be treated as individuals by their primary care physicians and consultants who must at first do no harm. One size does not fit all and certainly their care should not be dictated by a politically influenced entity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In a recent column, I cautiously discussed what has been called gender-affirming or transgender care.
In the days following the appearance of that Letters From Maine column on this topic, I received an unusual number of responses from readers suggesting I had touched on a topic that was on the minds of many pediatricians.
Since then, the Florida Board of Medicine and Osteopathic Medicine voted to forbid physicians from prescribing puberty blockers and hormones and/or performing surgeries in patients under age 18 who were seeking transgender care. Children already receiving treatments were exempt from the ruling. The osteopathic board added a second exception in cases where the child was a participant in a research protocol. The board of medicine inexplicably did not include this exception.
Regardless of how one feels about the ethics and the appropriateness of transgender care, it is not an issue to be decided by a politically appointed entity.
As I look back over what I have learned by watching this tragic drama play out, I am struck by a distinction that has yet to receive enough attention. When we are discussing gender dysphoria we are really talking about two different pediatric populations and scenarios. There is the child who from a very young age has consistently preferred to dress and behave in a manner that is different from the gender he or she was assigned at birth. The management of this child is a challenge that requires a careful balance of support and protection from the harsh realities of the gender-regimented world.
The second scenario stars the adolescent who has no prior history of gender dysphoria, or at least no outward manifestations. Then, faced by the challenges of puberty and adolescence, something or things happen that erupt into a full-blown gender-dysphoric storm. We currently have very little understanding of what those “things” are.
Each population can probably be further divided into subgroups – and that’s just the point. Every gender-dysphoric child, whether their dysphoria began at age 2 or 12, is an individual with a unique family dynamic and socioeconomic background. They may share some as yet unknown genetic signature, but in our current state of ignorance they deserve, as do all of our patients, to be treated as individuals by their primary care physicians and consultants who must at first do no harm. One size does not fit all and certainly their care should not be dictated by a politically influenced entity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Celiac disease linked to higher risk for rheumatoid arthritis, juvenile idiopathic arthritis
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Electrolyte disturbances a harbinger of eating disorders?
Electrolyte abnormalities may serve as a precursor to a future eating disorder diagnosis, a finding that may help pinpoint candidates for screening.
Researchers found that adolescents and adults with electrolyte abnormalities on routine outpatient lab work were twice as likely as those without these disturbances to be subsequently diagnosed with an eating disorder.
“These electrolyte abnormalities were in fact seen well ahead (> 1 year on average) of the time when patients were diagnosed with eating disorders,” study investigator Gregory Hundemer, MD, department of nephrology, University of Ottawa, told this news organization.
“Incidentally discovered outpatient electrolyte abnormalities may help to identify individuals who may benefit from more targeted screening into an underlying eating disorder. This, in turn, may allow for earlier diagnosis and therapeutic intervention,” Dr. Hundemer said.
The study was published online in JAMA Network Open.
Tailored screening?
Electrolyte abnormalities are often found when an individual is diagnosed with an eating disorder, but it’s largely unknown whether electrolyte abnormalities prior to the acute presentation of an eating disorder are associated with the future diagnosis of an eating disorder.
To investigate, the researchers used administrative health data to match 6,970 individuals (mean age, 28 years; 13% male) with an eating disorder diagnosis to 27,878 controls without an eating disorder diagnosis.
They found that individuals with an eating disorder were more likely to have a preceding electrolyte abnormality, compared with peers without an eating disorder (18.4% vs. 7.5%).
An outpatient electrolyte abnormality present 3 years to 30 days prior to diagnosis was associated with about a twofold higher odds for subsequent eating disorder diagnosis (adjusted odds ratio, 2.12; 95% confidence interval, 1.86-2.41).
The median time from the earliest electrolyte abnormality to eating disorder diagnosis was 386 days (range, 157-716 days).
Hypokalemia was the most common electrolyte abnormality (present in 12% of cases vs. 5% of controls), while hyponatremia, hypernatremia, hypophosphatemia, and metabolic alkalosis were the most specific for a subsequent eating disorder diagnosis.
Severe hypokalemia (serum potassium levels of 3.0 mmol/L or lower) and severe hyponatremia (serum sodium, 128 mmol/L or lower) were associated with over sevenfold and fivefold higher odds for the diagnosis of an eating disorder, respectively.
The U.S. Preventive Services Task Force issued its first-ever statement on screening for eating disorders earlier this year.
The task force concluded that there is insufficient evidence to weigh the balance of benefits and harms of screening for eating disorders in adolescents and adults with no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder.
Dr. Hundemer and colleagues believe an incidental electrolyte abnormality may identify candidates at high risk for an underlying eating disorder who many benefit from screening.
Several screening tools of varying complexity have been developed that are validated and accurate in identifying individuals with a potential eating disorder.
They include the SCOFF questionnaire, the Eating Disorder Screen for Primary Care, the Eating Attitudes Test, and the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire.
Underdiagnosed, undertreated
Offering perspective on the findings, Kamryn T. Eddy, PhD, codirector, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, said the notion “that a physical sign may help to promote eating disorder assessment is important particularly given that early detection can improve outcomes.”
“But this finding appears in the current context of eating disorders going largely underdetected, underdiagnosed, and undertreated across medical and psychiatric settings,” said Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston.
“Indeed, eating disorders are prevalent and cut across age, sex, gender, weight, race, ethnicity, and socioeconomic strata, and still, many providers do not routinely assess for eating disorders,” Dr. Eddy said.
“I might suggest that perhaps in addition to letting electrolyte abnormalities be a cue to screen for eating disorders, an even more powerful shift toward routine screening and assessment of eating disorders by medical providers be made,” Dr. Eddy said in an interview.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Dr. Hundemer and Dr. Eddy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Electrolyte abnormalities may serve as a precursor to a future eating disorder diagnosis, a finding that may help pinpoint candidates for screening.
Researchers found that adolescents and adults with electrolyte abnormalities on routine outpatient lab work were twice as likely as those without these disturbances to be subsequently diagnosed with an eating disorder.
“These electrolyte abnormalities were in fact seen well ahead (> 1 year on average) of the time when patients were diagnosed with eating disorders,” study investigator Gregory Hundemer, MD, department of nephrology, University of Ottawa, told this news organization.
“Incidentally discovered outpatient electrolyte abnormalities may help to identify individuals who may benefit from more targeted screening into an underlying eating disorder. This, in turn, may allow for earlier diagnosis and therapeutic intervention,” Dr. Hundemer said.
The study was published online in JAMA Network Open.
Tailored screening?
Electrolyte abnormalities are often found when an individual is diagnosed with an eating disorder, but it’s largely unknown whether electrolyte abnormalities prior to the acute presentation of an eating disorder are associated with the future diagnosis of an eating disorder.
To investigate, the researchers used administrative health data to match 6,970 individuals (mean age, 28 years; 13% male) with an eating disorder diagnosis to 27,878 controls without an eating disorder diagnosis.
They found that individuals with an eating disorder were more likely to have a preceding electrolyte abnormality, compared with peers without an eating disorder (18.4% vs. 7.5%).
An outpatient electrolyte abnormality present 3 years to 30 days prior to diagnosis was associated with about a twofold higher odds for subsequent eating disorder diagnosis (adjusted odds ratio, 2.12; 95% confidence interval, 1.86-2.41).
The median time from the earliest electrolyte abnormality to eating disorder diagnosis was 386 days (range, 157-716 days).
Hypokalemia was the most common electrolyte abnormality (present in 12% of cases vs. 5% of controls), while hyponatremia, hypernatremia, hypophosphatemia, and metabolic alkalosis were the most specific for a subsequent eating disorder diagnosis.
Severe hypokalemia (serum potassium levels of 3.0 mmol/L or lower) and severe hyponatremia (serum sodium, 128 mmol/L or lower) were associated with over sevenfold and fivefold higher odds for the diagnosis of an eating disorder, respectively.
The U.S. Preventive Services Task Force issued its first-ever statement on screening for eating disorders earlier this year.
The task force concluded that there is insufficient evidence to weigh the balance of benefits and harms of screening for eating disorders in adolescents and adults with no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder.
Dr. Hundemer and colleagues believe an incidental electrolyte abnormality may identify candidates at high risk for an underlying eating disorder who many benefit from screening.
Several screening tools of varying complexity have been developed that are validated and accurate in identifying individuals with a potential eating disorder.
They include the SCOFF questionnaire, the Eating Disorder Screen for Primary Care, the Eating Attitudes Test, and the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire.
Underdiagnosed, undertreated
Offering perspective on the findings, Kamryn T. Eddy, PhD, codirector, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, said the notion “that a physical sign may help to promote eating disorder assessment is important particularly given that early detection can improve outcomes.”
“But this finding appears in the current context of eating disorders going largely underdetected, underdiagnosed, and undertreated across medical and psychiatric settings,” said Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston.
“Indeed, eating disorders are prevalent and cut across age, sex, gender, weight, race, ethnicity, and socioeconomic strata, and still, many providers do not routinely assess for eating disorders,” Dr. Eddy said.
“I might suggest that perhaps in addition to letting electrolyte abnormalities be a cue to screen for eating disorders, an even more powerful shift toward routine screening and assessment of eating disorders by medical providers be made,” Dr. Eddy said in an interview.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Dr. Hundemer and Dr. Eddy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Electrolyte abnormalities may serve as a precursor to a future eating disorder diagnosis, a finding that may help pinpoint candidates for screening.
Researchers found that adolescents and adults with electrolyte abnormalities on routine outpatient lab work were twice as likely as those without these disturbances to be subsequently diagnosed with an eating disorder.
“These electrolyte abnormalities were in fact seen well ahead (> 1 year on average) of the time when patients were diagnosed with eating disorders,” study investigator Gregory Hundemer, MD, department of nephrology, University of Ottawa, told this news organization.
“Incidentally discovered outpatient electrolyte abnormalities may help to identify individuals who may benefit from more targeted screening into an underlying eating disorder. This, in turn, may allow for earlier diagnosis and therapeutic intervention,” Dr. Hundemer said.
The study was published online in JAMA Network Open.
Tailored screening?
Electrolyte abnormalities are often found when an individual is diagnosed with an eating disorder, but it’s largely unknown whether electrolyte abnormalities prior to the acute presentation of an eating disorder are associated with the future diagnosis of an eating disorder.
To investigate, the researchers used administrative health data to match 6,970 individuals (mean age, 28 years; 13% male) with an eating disorder diagnosis to 27,878 controls without an eating disorder diagnosis.
They found that individuals with an eating disorder were more likely to have a preceding electrolyte abnormality, compared with peers without an eating disorder (18.4% vs. 7.5%).
An outpatient electrolyte abnormality present 3 years to 30 days prior to diagnosis was associated with about a twofold higher odds for subsequent eating disorder diagnosis (adjusted odds ratio, 2.12; 95% confidence interval, 1.86-2.41).
The median time from the earliest electrolyte abnormality to eating disorder diagnosis was 386 days (range, 157-716 days).
Hypokalemia was the most common electrolyte abnormality (present in 12% of cases vs. 5% of controls), while hyponatremia, hypernatremia, hypophosphatemia, and metabolic alkalosis were the most specific for a subsequent eating disorder diagnosis.
Severe hypokalemia (serum potassium levels of 3.0 mmol/L or lower) and severe hyponatremia (serum sodium, 128 mmol/L or lower) were associated with over sevenfold and fivefold higher odds for the diagnosis of an eating disorder, respectively.
The U.S. Preventive Services Task Force issued its first-ever statement on screening for eating disorders earlier this year.
The task force concluded that there is insufficient evidence to weigh the balance of benefits and harms of screening for eating disorders in adolescents and adults with no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder.
Dr. Hundemer and colleagues believe an incidental electrolyte abnormality may identify candidates at high risk for an underlying eating disorder who many benefit from screening.
Several screening tools of varying complexity have been developed that are validated and accurate in identifying individuals with a potential eating disorder.
They include the SCOFF questionnaire, the Eating Disorder Screen for Primary Care, the Eating Attitudes Test, and the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire.
Underdiagnosed, undertreated
Offering perspective on the findings, Kamryn T. Eddy, PhD, codirector, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, said the notion “that a physical sign may help to promote eating disorder assessment is important particularly given that early detection can improve outcomes.”
“But this finding appears in the current context of eating disorders going largely underdetected, underdiagnosed, and undertreated across medical and psychiatric settings,” said Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston.
“Indeed, eating disorders are prevalent and cut across age, sex, gender, weight, race, ethnicity, and socioeconomic strata, and still, many providers do not routinely assess for eating disorders,” Dr. Eddy said.
“I might suggest that perhaps in addition to letting electrolyte abnormalities be a cue to screen for eating disorders, an even more powerful shift toward routine screening and assessment of eating disorders by medical providers be made,” Dr. Eddy said in an interview.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Dr. Hundemer and Dr. Eddy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Don’t let amoxicillin shortage go to waste, antibiotic stewards say
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Doctors urge screening for autoimmune disorders for patients with celiac disease
Diagnosed at age 4, Dr. Mollo has been on a gluten-free diet for 41 years, which she says has kept her healthy and may also be why she hasn’t developed other autoimmune diseases. It’s also played a part in her thinking about screening patients with CD.
“I think [physicians] should definitely be screening people with celiac disease for autoimmune disorders, especially if they see things like anemia or if a child has dropped on the growth chart and has nutrient deficiencies,” said Dr. Mollo, whose daughter also has the disease. “I would recommend that they see someone who specializes in celiac disease so they can get monitored and have regular follow-up checks for nutrient deficiencies and other autoimmune disorders.”
Dr. Mollo’s views on screening are echoed by many CD specialists and physicians, who cite multiple studies that have found that people with the disease face higher risks for diabetes, thyroid conditions, arthritis, and other autoimmune disorders.
Gastroenterologist Alessio Fasano, MD, with Massachusetts General Hospital, Boston, said there has been a “shift in the paradigm in thinking” about cross-screening for CD and autoimmune disorders. As result, he believes the answer to the question of whether to routinely do so is a no-brainer.
“The bottom line is, if you have CD, it [should be] routine that during your annual follow-ups you check for the possibility of the onset of other autoimmune disease. And people with other autoimmune diseases, like type 1 diabetes, should also be screened for CD because of the comorbidity,” said Dr. Fasano, professor of pediatrics and gastroenterology at Harvard Medical School and professor of nutrition at the Harvard School of Public Health, both in Boston. “This is what we call good clinical practice.”
Screening, despite lack of consensus guidelines
Other CD specialists differ on the need for universal cross-screening but agree that, at least in some cases, people with one autoimmune disorder should be tested for others.
Jolanda Denham, MD, a pediatric gastroenterologist affiliated with Nemours Children’s Hospital in Orlando, routinely recommends that her patients with CD be screened for certain autoimmune disorders – such as type 1 diabetes and autoimmune thyroid and liver diseases – even though medical organizations have not developed clear consensus or standard guidelines on cross-screening.
“There currently is no evidence to support the screening of celiac patients for all autoimmune and rheumatologic disorders,” she said. “It is true that celiac disease is an autoimmune disorder, and as such, there is a definite increased risk of these disorders in patients with celiac disease and vice versa.”
Echoing Dr. Denham, New York–based gastroenterologist Benjamin Lebwohl, MD, president of the Society for the Study of Celiac Disease, urges physicians to look beyond consensus guidelines and to err on the side of caution and make the best decisions for their patients on a case-by-case basis.
“Given the increased risk of certain autoimmune conditions in people with celiac disease, it behooves physicians to have a low threshold to evaluate for these conditions if any suggestive symptoms are present,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University, New York.
“Whether to screen for these conditions among people who are entirely without symptoms is less certain, and there is no consensus on that. But it is reasonable and common to include some basic tests with annual blood work, such as thyroid function and a liver profile, since both autoimmune thyroid disease and autoimmune liver disease can be silent early on and the patient would potentially benefit from identification and treatment of these conditions,” he said.
The American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes do recommend that people with diabetes be screened for CD years after diagnosis, noted Robert Rapaport, MD, a pediatric endocrinologist, with Kravis Children’s Hospital, New York. But in a study published in 2021, he and colleagues found that this wasn’t occurring, which prompted them to recommend yearly screening.
“There is a consensus that in children with type 1 diabetes, we screen them for other autoimmune disorders, specifically for thyroid disease and celiac disease,” said Dr. Rapaport, who is also Emma Elizabeth Sullivan Professor of Pediatric Endocrinology and Diabetes at Icahn School of Medicine at Mount Sinai, New York. “But there is no consensus going the other way – for patients with celiac disease, what other autoimmune conditions they should be screened for.”
This hasn’t kept some doctors from extending cross-screening efforts to their patients.
“At our center, we screen ... for thyroid disease and autoimmune liver disease as part of routine healthcare maintenance for our celiac disease patients. We discuss symptoms of diabetes and send screening with [hemoglobin] A1c for anyone who has symptoms,” said Lui Edwin, MD, a pediatric gastroenterologist with Children’s Hospital Colorado, Aurora, and director of the Colorado Center for Celiac Disease, who delivered a lecture on CD-autoimmune screening at the International Celiac Disease Symposium in October.
“It is definitely worth screening for celiac disease in [those with] other autoimmune disorders,” Dr. Edwin added.
“The symptoms can be very heterogeneous. Diagnosing and treating celiac disease can make a huge impact with respect to symptoms, quality of life, and preventing disease-related complications,” he said.
Mounting evidence linking CD to autoimmune disorders
Many studies have linked CD to a variety of other autoimmune disorders. The association could be due to common genetic factors or because CD might lead to such conditions. Researchers have found that people diagnosed with CD later in life are more likely to develop other autoimmune disorders.

Some studies have also found that people with certain autoimmune diseases are more likely to also have CD. In addition, some individuals develop what’s known as nonceliac gluten sensitivity, which is not an autoimmune disease but a gluten intolerance not unlike lactose intolerance.
In light of these coexisting conditions in many people with CD and other autoimmune disorders, as well as the fact that the prevalence of CD is on the rise, some specialists argue that the benefits of routine cross-screening outweigh the risks.
Going gluten free has preventive advantages
In a landmark 2012 study, researchers with the Celiac Disease Center at Columbia University stopped short of recommending routine screening for the general public or asymptomatic individuals in high-prevalence groups. But they concluded that more screening of symptomatic individuals – and close relatives – would speed treatment for those with more than one autoimmune disorder.
They also noted that some studies have found that a gluten-free diet might help prevent the development of other autoimmune disorders.
Marisa Gallant Stahl, MD, a gastroenterologist with Children’s Hospital Colorado, agreed that it is important that physicians keep gluten-free diets in mind when determining which patients to cross-screen.
“The literature is mixed, but some studies suggest that treating celiac disease with a gluten-free diet actually augments the treatment and control of other autoimmune disorders [and] adherence to a gluten-free diet does reduce the risk of cancer associated with celiac disease,” she said.
Dr. Denham agreed. “Strict adherence to a gluten-free diet definitely protects against the development of enteropathy-associated T-cell lymphoma but may be protective against non-Hodgkin’s lymphoma and adenocarcinoma of the small intestine as well. All three are associated with long-term nonadherence to a gluten-free diet.”
She also noted that a gluten-free diet may help people with CD manage other autoimmune disorders, which can be complicated by CD.
“Good control of celiac disease will help prevent complications that can worsen symptoms and outcomes of concomitant autoimmune and rheumatologic disorders,” she said.
Other factors to consider
Dr. Fasano added that autoimmune disorders can be complicated by CD in cases in which oral medications or healthful foods are not properly absorbed in the intestines.
“For example, with Hashimoto’s disease, if you have hormone replacement with oral treatments and your intestines are not 100% functional because you have inflammation, then you may have a problem [with] the absorption of medications like levothyroxine,” he said.
“It’s the same story with diabetes. You don’t take insulin by mouth, but glucose [control] strongly depends on several factors, mostly what comes from the diet, and if it’s erratic, that can be a problem. ... So, the treatment of autoimmune diseases can be influenced by celiac disease,” he said.
In addition, Dr. Fasano and others believe that people with CD and other autoimmune disorders should be managed by a team of experts who can personalize the care on the basis of specific needs of the individual patient. These should include specialists, dietitians, mental health counselors, and family social workers.
“It has to be a multidisciplinary approach to maintain the good health of an individual,” Dr. Fasano said. “Celiac disease is the quintessential example in which the primary care physician needs to be the quarterback of the team, the patient is active in his or her health, and [specialists] not only deliver personalized care but also preventive intervention, particularly the prevention of comorbidities.”
Financial disclosures for those quoted in this article were not available at the time of publication.
A version of this article first appeared on Medscape.com.
Diagnosed at age 4, Dr. Mollo has been on a gluten-free diet for 41 years, which she says has kept her healthy and may also be why she hasn’t developed other autoimmune diseases. It’s also played a part in her thinking about screening patients with CD.
“I think [physicians] should definitely be screening people with celiac disease for autoimmune disorders, especially if they see things like anemia or if a child has dropped on the growth chart and has nutrient deficiencies,” said Dr. Mollo, whose daughter also has the disease. “I would recommend that they see someone who specializes in celiac disease so they can get monitored and have regular follow-up checks for nutrient deficiencies and other autoimmune disorders.”
Dr. Mollo’s views on screening are echoed by many CD specialists and physicians, who cite multiple studies that have found that people with the disease face higher risks for diabetes, thyroid conditions, arthritis, and other autoimmune disorders.
Gastroenterologist Alessio Fasano, MD, with Massachusetts General Hospital, Boston, said there has been a “shift in the paradigm in thinking” about cross-screening for CD and autoimmune disorders. As result, he believes the answer to the question of whether to routinely do so is a no-brainer.
“The bottom line is, if you have CD, it [should be] routine that during your annual follow-ups you check for the possibility of the onset of other autoimmune disease. And people with other autoimmune diseases, like type 1 diabetes, should also be screened for CD because of the comorbidity,” said Dr. Fasano, professor of pediatrics and gastroenterology at Harvard Medical School and professor of nutrition at the Harvard School of Public Health, both in Boston. “This is what we call good clinical practice.”
Screening, despite lack of consensus guidelines
Other CD specialists differ on the need for universal cross-screening but agree that, at least in some cases, people with one autoimmune disorder should be tested for others.
Jolanda Denham, MD, a pediatric gastroenterologist affiliated with Nemours Children’s Hospital in Orlando, routinely recommends that her patients with CD be screened for certain autoimmune disorders – such as type 1 diabetes and autoimmune thyroid and liver diseases – even though medical organizations have not developed clear consensus or standard guidelines on cross-screening.
“There currently is no evidence to support the screening of celiac patients for all autoimmune and rheumatologic disorders,” she said. “It is true that celiac disease is an autoimmune disorder, and as such, there is a definite increased risk of these disorders in patients with celiac disease and vice versa.”
Echoing Dr. Denham, New York–based gastroenterologist Benjamin Lebwohl, MD, president of the Society for the Study of Celiac Disease, urges physicians to look beyond consensus guidelines and to err on the side of caution and make the best decisions for their patients on a case-by-case basis.
“Given the increased risk of certain autoimmune conditions in people with celiac disease, it behooves physicians to have a low threshold to evaluate for these conditions if any suggestive symptoms are present,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University, New York.
“Whether to screen for these conditions among people who are entirely without symptoms is less certain, and there is no consensus on that. But it is reasonable and common to include some basic tests with annual blood work, such as thyroid function and a liver profile, since both autoimmune thyroid disease and autoimmune liver disease can be silent early on and the patient would potentially benefit from identification and treatment of these conditions,” he said.
The American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes do recommend that people with diabetes be screened for CD years after diagnosis, noted Robert Rapaport, MD, a pediatric endocrinologist, with Kravis Children’s Hospital, New York. But in a study published in 2021, he and colleagues found that this wasn’t occurring, which prompted them to recommend yearly screening.
“There is a consensus that in children with type 1 diabetes, we screen them for other autoimmune disorders, specifically for thyroid disease and celiac disease,” said Dr. Rapaport, who is also Emma Elizabeth Sullivan Professor of Pediatric Endocrinology and Diabetes at Icahn School of Medicine at Mount Sinai, New York. “But there is no consensus going the other way – for patients with celiac disease, what other autoimmune conditions they should be screened for.”
This hasn’t kept some doctors from extending cross-screening efforts to their patients.
“At our center, we screen ... for thyroid disease and autoimmune liver disease as part of routine healthcare maintenance for our celiac disease patients. We discuss symptoms of diabetes and send screening with [hemoglobin] A1c for anyone who has symptoms,” said Lui Edwin, MD, a pediatric gastroenterologist with Children’s Hospital Colorado, Aurora, and director of the Colorado Center for Celiac Disease, who delivered a lecture on CD-autoimmune screening at the International Celiac Disease Symposium in October.
“It is definitely worth screening for celiac disease in [those with] other autoimmune disorders,” Dr. Edwin added.
“The symptoms can be very heterogeneous. Diagnosing and treating celiac disease can make a huge impact with respect to symptoms, quality of life, and preventing disease-related complications,” he said.
Mounting evidence linking CD to autoimmune disorders
Many studies have linked CD to a variety of other autoimmune disorders. The association could be due to common genetic factors or because CD might lead to such conditions. Researchers have found that people diagnosed with CD later in life are more likely to develop other autoimmune disorders.

Some studies have also found that people with certain autoimmune diseases are more likely to also have CD. In addition, some individuals develop what’s known as nonceliac gluten sensitivity, which is not an autoimmune disease but a gluten intolerance not unlike lactose intolerance.
In light of these coexisting conditions in many people with CD and other autoimmune disorders, as well as the fact that the prevalence of CD is on the rise, some specialists argue that the benefits of routine cross-screening outweigh the risks.
Going gluten free has preventive advantages
In a landmark 2012 study, researchers with the Celiac Disease Center at Columbia University stopped short of recommending routine screening for the general public or asymptomatic individuals in high-prevalence groups. But they concluded that more screening of symptomatic individuals – and close relatives – would speed treatment for those with more than one autoimmune disorder.
They also noted that some studies have found that a gluten-free diet might help prevent the development of other autoimmune disorders.
Marisa Gallant Stahl, MD, a gastroenterologist with Children’s Hospital Colorado, agreed that it is important that physicians keep gluten-free diets in mind when determining which patients to cross-screen.
“The literature is mixed, but some studies suggest that treating celiac disease with a gluten-free diet actually augments the treatment and control of other autoimmune disorders [and] adherence to a gluten-free diet does reduce the risk of cancer associated with celiac disease,” she said.
Dr. Denham agreed. “Strict adherence to a gluten-free diet definitely protects against the development of enteropathy-associated T-cell lymphoma but may be protective against non-Hodgkin’s lymphoma and adenocarcinoma of the small intestine as well. All three are associated with long-term nonadherence to a gluten-free diet.”
She also noted that a gluten-free diet may help people with CD manage other autoimmune disorders, which can be complicated by CD.
“Good control of celiac disease will help prevent complications that can worsen symptoms and outcomes of concomitant autoimmune and rheumatologic disorders,” she said.
Other factors to consider
Dr. Fasano added that autoimmune disorders can be complicated by CD in cases in which oral medications or healthful foods are not properly absorbed in the intestines.
“For example, with Hashimoto’s disease, if you have hormone replacement with oral treatments and your intestines are not 100% functional because you have inflammation, then you may have a problem [with] the absorption of medications like levothyroxine,” he said.
“It’s the same story with diabetes. You don’t take insulin by mouth, but glucose [control] strongly depends on several factors, mostly what comes from the diet, and if it’s erratic, that can be a problem. ... So, the treatment of autoimmune diseases can be influenced by celiac disease,” he said.
In addition, Dr. Fasano and others believe that people with CD and other autoimmune disorders should be managed by a team of experts who can personalize the care on the basis of specific needs of the individual patient. These should include specialists, dietitians, mental health counselors, and family social workers.
“It has to be a multidisciplinary approach to maintain the good health of an individual,” Dr. Fasano said. “Celiac disease is the quintessential example in which the primary care physician needs to be the quarterback of the team, the patient is active in his or her health, and [specialists] not only deliver personalized care but also preventive intervention, particularly the prevention of comorbidities.”
Financial disclosures for those quoted in this article were not available at the time of publication.
A version of this article first appeared on Medscape.com.
Diagnosed at age 4, Dr. Mollo has been on a gluten-free diet for 41 years, which she says has kept her healthy and may also be why she hasn’t developed other autoimmune diseases. It’s also played a part in her thinking about screening patients with CD.
“I think [physicians] should definitely be screening people with celiac disease for autoimmune disorders, especially if they see things like anemia or if a child has dropped on the growth chart and has nutrient deficiencies,” said Dr. Mollo, whose daughter also has the disease. “I would recommend that they see someone who specializes in celiac disease so they can get monitored and have regular follow-up checks for nutrient deficiencies and other autoimmune disorders.”
Dr. Mollo’s views on screening are echoed by many CD specialists and physicians, who cite multiple studies that have found that people with the disease face higher risks for diabetes, thyroid conditions, arthritis, and other autoimmune disorders.
Gastroenterologist Alessio Fasano, MD, with Massachusetts General Hospital, Boston, said there has been a “shift in the paradigm in thinking” about cross-screening for CD and autoimmune disorders. As result, he believes the answer to the question of whether to routinely do so is a no-brainer.
“The bottom line is, if you have CD, it [should be] routine that during your annual follow-ups you check for the possibility of the onset of other autoimmune disease. And people with other autoimmune diseases, like type 1 diabetes, should also be screened for CD because of the comorbidity,” said Dr. Fasano, professor of pediatrics and gastroenterology at Harvard Medical School and professor of nutrition at the Harvard School of Public Health, both in Boston. “This is what we call good clinical practice.”
Screening, despite lack of consensus guidelines
Other CD specialists differ on the need for universal cross-screening but agree that, at least in some cases, people with one autoimmune disorder should be tested for others.
Jolanda Denham, MD, a pediatric gastroenterologist affiliated with Nemours Children’s Hospital in Orlando, routinely recommends that her patients with CD be screened for certain autoimmune disorders – such as type 1 diabetes and autoimmune thyroid and liver diseases – even though medical organizations have not developed clear consensus or standard guidelines on cross-screening.
“There currently is no evidence to support the screening of celiac patients for all autoimmune and rheumatologic disorders,” she said. “It is true that celiac disease is an autoimmune disorder, and as such, there is a definite increased risk of these disorders in patients with celiac disease and vice versa.”
Echoing Dr. Denham, New York–based gastroenterologist Benjamin Lebwohl, MD, president of the Society for the Study of Celiac Disease, urges physicians to look beyond consensus guidelines and to err on the side of caution and make the best decisions for their patients on a case-by-case basis.
“Given the increased risk of certain autoimmune conditions in people with celiac disease, it behooves physicians to have a low threshold to evaluate for these conditions if any suggestive symptoms are present,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University, New York.
“Whether to screen for these conditions among people who are entirely without symptoms is less certain, and there is no consensus on that. But it is reasonable and common to include some basic tests with annual blood work, such as thyroid function and a liver profile, since both autoimmune thyroid disease and autoimmune liver disease can be silent early on and the patient would potentially benefit from identification and treatment of these conditions,” he said.
The American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes do recommend that people with diabetes be screened for CD years after diagnosis, noted Robert Rapaport, MD, a pediatric endocrinologist, with Kravis Children’s Hospital, New York. But in a study published in 2021, he and colleagues found that this wasn’t occurring, which prompted them to recommend yearly screening.
“There is a consensus that in children with type 1 diabetes, we screen them for other autoimmune disorders, specifically for thyroid disease and celiac disease,” said Dr. Rapaport, who is also Emma Elizabeth Sullivan Professor of Pediatric Endocrinology and Diabetes at Icahn School of Medicine at Mount Sinai, New York. “But there is no consensus going the other way – for patients with celiac disease, what other autoimmune conditions they should be screened for.”
This hasn’t kept some doctors from extending cross-screening efforts to their patients.
“At our center, we screen ... for thyroid disease and autoimmune liver disease as part of routine healthcare maintenance for our celiac disease patients. We discuss symptoms of diabetes and send screening with [hemoglobin] A1c for anyone who has symptoms,” said Lui Edwin, MD, a pediatric gastroenterologist with Children’s Hospital Colorado, Aurora, and director of the Colorado Center for Celiac Disease, who delivered a lecture on CD-autoimmune screening at the International Celiac Disease Symposium in October.
“It is definitely worth screening for celiac disease in [those with] other autoimmune disorders,” Dr. Edwin added.
“The symptoms can be very heterogeneous. Diagnosing and treating celiac disease can make a huge impact with respect to symptoms, quality of life, and preventing disease-related complications,” he said.
Mounting evidence linking CD to autoimmune disorders
Many studies have linked CD to a variety of other autoimmune disorders. The association could be due to common genetic factors or because CD might lead to such conditions. Researchers have found that people diagnosed with CD later in life are more likely to develop other autoimmune disorders.

Some studies have also found that people with certain autoimmune diseases are more likely to also have CD. In addition, some individuals develop what’s known as nonceliac gluten sensitivity, which is not an autoimmune disease but a gluten intolerance not unlike lactose intolerance.
In light of these coexisting conditions in many people with CD and other autoimmune disorders, as well as the fact that the prevalence of CD is on the rise, some specialists argue that the benefits of routine cross-screening outweigh the risks.
Going gluten free has preventive advantages
In a landmark 2012 study, researchers with the Celiac Disease Center at Columbia University stopped short of recommending routine screening for the general public or asymptomatic individuals in high-prevalence groups. But they concluded that more screening of symptomatic individuals – and close relatives – would speed treatment for those with more than one autoimmune disorder.
They also noted that some studies have found that a gluten-free diet might help prevent the development of other autoimmune disorders.
Marisa Gallant Stahl, MD, a gastroenterologist with Children’s Hospital Colorado, agreed that it is important that physicians keep gluten-free diets in mind when determining which patients to cross-screen.
“The literature is mixed, but some studies suggest that treating celiac disease with a gluten-free diet actually augments the treatment and control of other autoimmune disorders [and] adherence to a gluten-free diet does reduce the risk of cancer associated with celiac disease,” she said.
Dr. Denham agreed. “Strict adherence to a gluten-free diet definitely protects against the development of enteropathy-associated T-cell lymphoma but may be protective against non-Hodgkin’s lymphoma and adenocarcinoma of the small intestine as well. All three are associated with long-term nonadherence to a gluten-free diet.”
She also noted that a gluten-free diet may help people with CD manage other autoimmune disorders, which can be complicated by CD.
“Good control of celiac disease will help prevent complications that can worsen symptoms and outcomes of concomitant autoimmune and rheumatologic disorders,” she said.
Other factors to consider
Dr. Fasano added that autoimmune disorders can be complicated by CD in cases in which oral medications or healthful foods are not properly absorbed in the intestines.
“For example, with Hashimoto’s disease, if you have hormone replacement with oral treatments and your intestines are not 100% functional because you have inflammation, then you may have a problem [with] the absorption of medications like levothyroxine,” he said.
“It’s the same story with diabetes. You don’t take insulin by mouth, but glucose [control] strongly depends on several factors, mostly what comes from the diet, and if it’s erratic, that can be a problem. ... So, the treatment of autoimmune diseases can be influenced by celiac disease,” he said.
In addition, Dr. Fasano and others believe that people with CD and other autoimmune disorders should be managed by a team of experts who can personalize the care on the basis of specific needs of the individual patient. These should include specialists, dietitians, mental health counselors, and family social workers.
“It has to be a multidisciplinary approach to maintain the good health of an individual,” Dr. Fasano said. “Celiac disease is the quintessential example in which the primary care physician needs to be the quarterback of the team, the patient is active in his or her health, and [specialists] not only deliver personalized care but also preventive intervention, particularly the prevention of comorbidities.”
Financial disclosures for those quoted in this article were not available at the time of publication.
A version of this article first appeared on Medscape.com.
Severe pediatric oral mucositis
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).
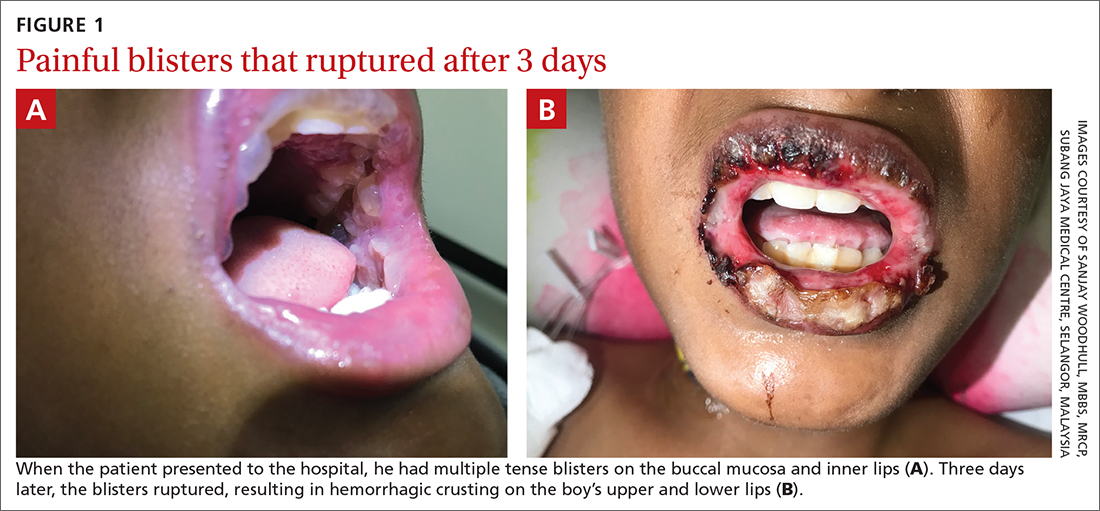
The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444