User login
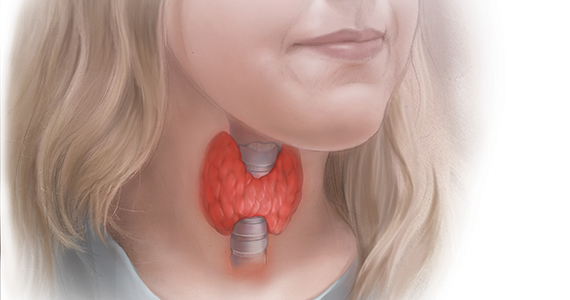
Subclinical hypothyroidism and pregnancy: Public health problem or lab finding with minimal clinical significance?
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
Fetal MRI may change pregnancy management
CHARLOTTE, N.C. – according to research presented at the annual meeting of the Child Neurology Society. This imaging technique and neurologic consultation complement the information that prenatal ultrasound and obstetric consultations provide and may influence pregnancy management and infant neurologic care significantly.
The fetal diagnosis of posterior fossa abnormalities can be challenging. The prognosis can vary greatly, depending on the diagnosis. Sarah Mulkey, MD, PhD, director of the fetal and neonatal fellowship and a fetal and neonatal neurologist at Children’s National in Washington, and colleagues conducted an analysis to evaluate whether fetal MRI and neurology consultation produce alternative diagnoses for maternal-fetal dyads who are referred to a fetal neurology program because of concern for a fetal posterior fossa anomaly. The researchers also sought to determine how often the postnatal evaluation differed from the fetal diagnosis.
Dr. Mulkey and colleagues retrospectively analyzed cases referred to the Fetal Medicine Institute at Children’s National from January 2012 to June 2018. They included the referral diagnoses of Dandy-Walker continuum, cerebellar hypoplasia, vermis hypoplasia, Blake’s pouch cyst, mega cisterna magna, and other posterior fossa anomalies in their study.
The investigators identified 188 cases that had undergone fetal MRI and neurology consultation. The average gestational age at evaluation was 25 weeks, and the average maternal age was 30 years. Approximately 43% of referrals resulted from a concern regarding Dandy-Walker malformation, and 21% of referrals resulted from a suspicion of mega cisterna magna.
Fetal MRI and neurology consultation resulted in a change from the referral diagnosis or additional information about the fetus in 124 (66%) cases. For example, after imaging and consultation, 15% of referrals were diagnosed with Dandy-Walker malformation, as opposed to the 43% who were suspected of having it. Most referrals with a diagnosis of vermis hypoplasia had a better prognosis after fetal MRI. Fetal MRI and consultation also resulted in new diagnoses of Joubert syndrome and rhombencephalosynapsis. About 19% of referrals were considered normal. “A considerable number of these referrals ended up being for conditions that would have a good outcome,” said Dr. Mulkey.
In addition, the researchers obtained the postnatal diagnosis for 60 of 138 (43%) live-born infants. The fetal diagnosis of Dandy-Walker continuum was confirmed post natally in six of six (100%) cases. Of the 13 cases of fetally diagnosed vermis hypoplasia, 7 (54%) had stable findings, 3 (23%) normalized, and diagnosis changed in 3 (23%). Of the 17 fetally diagnosed Blake’s pouch cysts, 8 (47%) remained stable, 5 (29%) normalized, and diagnosis changed in 4 (24%). Four of nine (44%) cases of fetally diagnosed mega cisterna magna remained stable, two (22%) normalized, and diagnosis changed in three (33%). Overall, prognosis did not change after postnatal imaging.
“There is a high degree of correlation between fetal and postnatal diagnoses for Dandy-Walker continuum, cerebellar hypoplasia, cyst, and ‘other’ diagnoses,” said Dr. Mulkey. “Vermis hypoplasia and Blake’s pouch cyst diagnoses were less consistent.”
The investigators reported no disclosures.
SOURCE: Schlatterer S et al. CNS 2019, Abstract 158.
CHARLOTTE, N.C. – according to research presented at the annual meeting of the Child Neurology Society. This imaging technique and neurologic consultation complement the information that prenatal ultrasound and obstetric consultations provide and may influence pregnancy management and infant neurologic care significantly.
The fetal diagnosis of posterior fossa abnormalities can be challenging. The prognosis can vary greatly, depending on the diagnosis. Sarah Mulkey, MD, PhD, director of the fetal and neonatal fellowship and a fetal and neonatal neurologist at Children’s National in Washington, and colleagues conducted an analysis to evaluate whether fetal MRI and neurology consultation produce alternative diagnoses for maternal-fetal dyads who are referred to a fetal neurology program because of concern for a fetal posterior fossa anomaly. The researchers also sought to determine how often the postnatal evaluation differed from the fetal diagnosis.
Dr. Mulkey and colleagues retrospectively analyzed cases referred to the Fetal Medicine Institute at Children’s National from January 2012 to June 2018. They included the referral diagnoses of Dandy-Walker continuum, cerebellar hypoplasia, vermis hypoplasia, Blake’s pouch cyst, mega cisterna magna, and other posterior fossa anomalies in their study.
The investigators identified 188 cases that had undergone fetal MRI and neurology consultation. The average gestational age at evaluation was 25 weeks, and the average maternal age was 30 years. Approximately 43% of referrals resulted from a concern regarding Dandy-Walker malformation, and 21% of referrals resulted from a suspicion of mega cisterna magna.
Fetal MRI and neurology consultation resulted in a change from the referral diagnosis or additional information about the fetus in 124 (66%) cases. For example, after imaging and consultation, 15% of referrals were diagnosed with Dandy-Walker malformation, as opposed to the 43% who were suspected of having it. Most referrals with a diagnosis of vermis hypoplasia had a better prognosis after fetal MRI. Fetal MRI and consultation also resulted in new diagnoses of Joubert syndrome and rhombencephalosynapsis. About 19% of referrals were considered normal. “A considerable number of these referrals ended up being for conditions that would have a good outcome,” said Dr. Mulkey.
In addition, the researchers obtained the postnatal diagnosis for 60 of 138 (43%) live-born infants. The fetal diagnosis of Dandy-Walker continuum was confirmed post natally in six of six (100%) cases. Of the 13 cases of fetally diagnosed vermis hypoplasia, 7 (54%) had stable findings, 3 (23%) normalized, and diagnosis changed in 3 (23%). Of the 17 fetally diagnosed Blake’s pouch cysts, 8 (47%) remained stable, 5 (29%) normalized, and diagnosis changed in 4 (24%). Four of nine (44%) cases of fetally diagnosed mega cisterna magna remained stable, two (22%) normalized, and diagnosis changed in three (33%). Overall, prognosis did not change after postnatal imaging.
“There is a high degree of correlation between fetal and postnatal diagnoses for Dandy-Walker continuum, cerebellar hypoplasia, cyst, and ‘other’ diagnoses,” said Dr. Mulkey. “Vermis hypoplasia and Blake’s pouch cyst diagnoses were less consistent.”
The investigators reported no disclosures.
SOURCE: Schlatterer S et al. CNS 2019, Abstract 158.
CHARLOTTE, N.C. – according to research presented at the annual meeting of the Child Neurology Society. This imaging technique and neurologic consultation complement the information that prenatal ultrasound and obstetric consultations provide and may influence pregnancy management and infant neurologic care significantly.
The fetal diagnosis of posterior fossa abnormalities can be challenging. The prognosis can vary greatly, depending on the diagnosis. Sarah Mulkey, MD, PhD, director of the fetal and neonatal fellowship and a fetal and neonatal neurologist at Children’s National in Washington, and colleagues conducted an analysis to evaluate whether fetal MRI and neurology consultation produce alternative diagnoses for maternal-fetal dyads who are referred to a fetal neurology program because of concern for a fetal posterior fossa anomaly. The researchers also sought to determine how often the postnatal evaluation differed from the fetal diagnosis.
Dr. Mulkey and colleagues retrospectively analyzed cases referred to the Fetal Medicine Institute at Children’s National from January 2012 to June 2018. They included the referral diagnoses of Dandy-Walker continuum, cerebellar hypoplasia, vermis hypoplasia, Blake’s pouch cyst, mega cisterna magna, and other posterior fossa anomalies in their study.
The investigators identified 188 cases that had undergone fetal MRI and neurology consultation. The average gestational age at evaluation was 25 weeks, and the average maternal age was 30 years. Approximately 43% of referrals resulted from a concern regarding Dandy-Walker malformation, and 21% of referrals resulted from a suspicion of mega cisterna magna.
Fetal MRI and neurology consultation resulted in a change from the referral diagnosis or additional information about the fetus in 124 (66%) cases. For example, after imaging and consultation, 15% of referrals were diagnosed with Dandy-Walker malformation, as opposed to the 43% who were suspected of having it. Most referrals with a diagnosis of vermis hypoplasia had a better prognosis after fetal MRI. Fetal MRI and consultation also resulted in new diagnoses of Joubert syndrome and rhombencephalosynapsis. About 19% of referrals were considered normal. “A considerable number of these referrals ended up being for conditions that would have a good outcome,” said Dr. Mulkey.
In addition, the researchers obtained the postnatal diagnosis for 60 of 138 (43%) live-born infants. The fetal diagnosis of Dandy-Walker continuum was confirmed post natally in six of six (100%) cases. Of the 13 cases of fetally diagnosed vermis hypoplasia, 7 (54%) had stable findings, 3 (23%) normalized, and diagnosis changed in 3 (23%). Of the 17 fetally diagnosed Blake’s pouch cysts, 8 (47%) remained stable, 5 (29%) normalized, and diagnosis changed in 4 (24%). Four of nine (44%) cases of fetally diagnosed mega cisterna magna remained stable, two (22%) normalized, and diagnosis changed in three (33%). Overall, prognosis did not change after postnatal imaging.
“There is a high degree of correlation between fetal and postnatal diagnoses for Dandy-Walker continuum, cerebellar hypoplasia, cyst, and ‘other’ diagnoses,” said Dr. Mulkey. “Vermis hypoplasia and Blake’s pouch cyst diagnoses were less consistent.”
The investigators reported no disclosures.
SOURCE: Schlatterer S et al. CNS 2019, Abstract 158.
REPORTING FROM CNS 2019
Sleep problems in pregnancy presage postnatal depression
COPENHAGEN – Tiina Paunio, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“I think it is very important to understand that we need to screen pregnant women for sleep problems, even those without a history of depression, so we can have early treatment of insomnia – and also depression – because postnatal maternal depression is very much a risk for the child during a vulnerable period for development,” said Dr. Paunio, professor of psychiatry at the University of Helsinki.
She was a coinvestigator in a prospective study of the Finnish CHILD-SLEEP longitudinal birth cohort in which 1,398 women completed the Basic Nordic Sleep Questionnaire and the 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D) at about gestational week 32 and again around 3 months following delivery. Postnatal depressiveness as defined by a CES-D score of at least 10 points was present in 10.3% of the mothers. After adjusting for prenatal depressiveness and other potential confounders, the investigators found that tiredness during the day, poor general sleep quality, getting less than 6 hours of sleep, taking longer than 20 minutes to fall asleep, and sleep loss of 2 hours or more per night during pregnancy were each associated with clinically significant postnatal depressive symptoms, with odds ratios of 1.87-2.19.
The full details of the study have been published (Arch Womens Ment Health. 2019 Jun;22[3]:327-37).
The impetus for this study of sleep problems in pregnancy as a predictor of postnatal depressive symptoms was a body of evidence linking insomnia to depression in both men and women. But it turns out that insomnia is a significant predictor of later onset of a wide variety of psychiatric disorders, not only depression, as highlighted in a recent systematic review and meta-analysis conducted by an international team of investigators, Dr. Paunio observed.
Baseline insomnia symptoms were associated with a 183% increased risk of later onset of depression, a 223% increased risk of anxiety, a 35%greater risk of alcohol abuse, and a 28% increased risk of psychosis. However, the insomnia/psychosis link must be viewed as tentative, as it was examined in only a single published study. The investigators rated the overall risk of bias in the studies included in their meta-analysis as moderate (Sleep Med Rev. 2019 Feb;43:96-105).
For Dr. Paunio, these findings suggest that interventional studies of early and effective treatment of insomnia as a potential means of preventing psychiatric disorders are in order.
She reported receiving research funding from the Academy of Finland, the Gyllenberg Foundation, and Finska Lakaresallskapet.
COPENHAGEN – Tiina Paunio, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“I think it is very important to understand that we need to screen pregnant women for sleep problems, even those without a history of depression, so we can have early treatment of insomnia – and also depression – because postnatal maternal depression is very much a risk for the child during a vulnerable period for development,” said Dr. Paunio, professor of psychiatry at the University of Helsinki.
She was a coinvestigator in a prospective study of the Finnish CHILD-SLEEP longitudinal birth cohort in which 1,398 women completed the Basic Nordic Sleep Questionnaire and the 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D) at about gestational week 32 and again around 3 months following delivery. Postnatal depressiveness as defined by a CES-D score of at least 10 points was present in 10.3% of the mothers. After adjusting for prenatal depressiveness and other potential confounders, the investigators found that tiredness during the day, poor general sleep quality, getting less than 6 hours of sleep, taking longer than 20 minutes to fall asleep, and sleep loss of 2 hours or more per night during pregnancy were each associated with clinically significant postnatal depressive symptoms, with odds ratios of 1.87-2.19.
The full details of the study have been published (Arch Womens Ment Health. 2019 Jun;22[3]:327-37).
The impetus for this study of sleep problems in pregnancy as a predictor of postnatal depressive symptoms was a body of evidence linking insomnia to depression in both men and women. But it turns out that insomnia is a significant predictor of later onset of a wide variety of psychiatric disorders, not only depression, as highlighted in a recent systematic review and meta-analysis conducted by an international team of investigators, Dr. Paunio observed.
Baseline insomnia symptoms were associated with a 183% increased risk of later onset of depression, a 223% increased risk of anxiety, a 35%greater risk of alcohol abuse, and a 28% increased risk of psychosis. However, the insomnia/psychosis link must be viewed as tentative, as it was examined in only a single published study. The investigators rated the overall risk of bias in the studies included in their meta-analysis as moderate (Sleep Med Rev. 2019 Feb;43:96-105).
For Dr. Paunio, these findings suggest that interventional studies of early and effective treatment of insomnia as a potential means of preventing psychiatric disorders are in order.
She reported receiving research funding from the Academy of Finland, the Gyllenberg Foundation, and Finska Lakaresallskapet.
COPENHAGEN – Tiina Paunio, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“I think it is very important to understand that we need to screen pregnant women for sleep problems, even those without a history of depression, so we can have early treatment of insomnia – and also depression – because postnatal maternal depression is very much a risk for the child during a vulnerable period for development,” said Dr. Paunio, professor of psychiatry at the University of Helsinki.
She was a coinvestigator in a prospective study of the Finnish CHILD-SLEEP longitudinal birth cohort in which 1,398 women completed the Basic Nordic Sleep Questionnaire and the 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D) at about gestational week 32 and again around 3 months following delivery. Postnatal depressiveness as defined by a CES-D score of at least 10 points was present in 10.3% of the mothers. After adjusting for prenatal depressiveness and other potential confounders, the investigators found that tiredness during the day, poor general sleep quality, getting less than 6 hours of sleep, taking longer than 20 minutes to fall asleep, and sleep loss of 2 hours or more per night during pregnancy were each associated with clinically significant postnatal depressive symptoms, with odds ratios of 1.87-2.19.
The full details of the study have been published (Arch Womens Ment Health. 2019 Jun;22[3]:327-37).
The impetus for this study of sleep problems in pregnancy as a predictor of postnatal depressive symptoms was a body of evidence linking insomnia to depression in both men and women. But it turns out that insomnia is a significant predictor of later onset of a wide variety of psychiatric disorders, not only depression, as highlighted in a recent systematic review and meta-analysis conducted by an international team of investigators, Dr. Paunio observed.
Baseline insomnia symptoms were associated with a 183% increased risk of later onset of depression, a 223% increased risk of anxiety, a 35%greater risk of alcohol abuse, and a 28% increased risk of psychosis. However, the insomnia/psychosis link must be viewed as tentative, as it was examined in only a single published study. The investigators rated the overall risk of bias in the studies included in their meta-analysis as moderate (Sleep Med Rev. 2019 Feb;43:96-105).
For Dr. Paunio, these findings suggest that interventional studies of early and effective treatment of insomnia as a potential means of preventing psychiatric disorders are in order.
She reported receiving research funding from the Academy of Finland, the Gyllenberg Foundation, and Finska Lakaresallskapet.
REPORTING FROM ECNP 2019
Preconception marijuana use by male partner raises spontaneous abortion risk
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
REPORTING FROM ASRM 2019
Lifestyle program improves chance of spontaneous conception for women with obesity
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
REPORTING FROM ASRM 2019
ACIP recommends two options for pertussis vaccination
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
Antituberculosis drugs in pregnancy and lactation
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Women with PCOS have greater risk of postpartum depression, preeclampsia, eclampsia
PHILADELPHIA – Women with polycystic ovary syndrome (PCOS) are at a higher risk for metabolic and psychiatric comorbidities prior to pregnancy, cardiometabolic complications during pregnancy, and cardiometabolic and psychiatric complications in the postpartum period, according to results from a prize paper at the annual meeting of the American Society for Reproductive Medicine.
“Our findings do support the ACOG [American College of Obstetricians and Gynecologists] recommendations that women with PCOS should be considered a high-risk group, and during the postpartum period should be screened for cardiovascular as well as psychiatric comorbidities,” Anuja Dokras, MD, PhD, director of the Penn Polycystic Ovary Syndrome Center at the University of Pennsylvania, Philadelphia, said in her presentation.
Dr. Dokras and colleagues performed a retrospective cohort study during 2000-2016 of patients aged 18-50 years, in the Optum claims database, which comprised 42,391 women with PCOS and 795,480 women without PCOS in 50 U.S. states. Women were included in the analysis if there were data available for at least 6 months to 1 year before pregnancy and between 6 weeks and 1 year after pregnancy. The researchers looked at risk factors prior to pregnancy, such as depression, hypertension, hyperlipidemia, diabetes, obesity, smoking, and use of assisted reproductive technology. During pregnancy, Dr. Dokras and colleagues analyzed complications such as preterm birth, multiple gestation, cesarean section, gestational hypertension and diabetes, preeclampsia and eclampsia, and depression in addition to outcomes in the postpartum period, such as hypertensive complications, thrombotic disease, peripartum cardiomyopathy, heart failure, arterial complications, perinatal and postpartum depression.
“Realizing that PCOS is underreported in administrative data sets, we looked at not only the diagnosis of PCOS but also tried to combine any menstrual irregularity and hirsutism occurring simultaneously, and then doing a sensitivity analysis and looking at the population,” said Dr. Dokras. “Similarly, knowing that misclassification can be an issue in these datasets, we did the same thing amongst the controls, looking for a single diagnosis of irregular menses and hirsutism.”
Prior to pregnancy, women with PCOS in the dataset tended to have a higher rate of obesity (14.7% vs. 4.7%), hyperlipidemia (11.3% vs. 5.3%), hypertension (6.2% vs. 2.5%), diabetes (5.3% vs. 1.2%), and depression (4.3% vs. 3.1%) and were also more likely to use assisted reproductive technology (5.2% vs. 1.0%) than were patients without PCOS (all P less than .001). During pregnancy, there was a higher rate of gestational diabetes (13.7% vs. 7.7%), preeclampsia (5.0% vs. 2.6%), preterm birth (16.9% vs. 12.2%), multiple gestation (6.6% vs. 2.5%), and cesarean section (45.1% vs. 32.9%) in patients with PCOS, compared with those without PCOS (all P less than .001).
For patients in the postpartum period, women with PCOS were more likely to experience postpartum thrombotic disease (adjusted odds ratio, 1.60; 95% confidence interval, 1.23-2.09; P = .001), hypertensive heart disease (aOR, 1.45; 95% CI, 1.04-2.01; P = .027), eclampsia (aOR, 1.45; 95% CI, 1.14-1.86; P = .003), heart failure (aOR, 1.33; 95% CI, 1.08-1.64; P = .007), preeclampsia (aOR, 1.30; 95% CI, 1.17-1.45; P = than .001), and peripartum cardiomyopathy (aOR, 1.26; 95% CI, 1.03-1.54; P = .027).
With regard to depression, women with PCOS also were at greater risk of developing perinatal (aOR, 1.27; 95% CI, 1.22-1.33) and postpartum (aOR, 1.46; 95% CI, 1.36-1.57) depression, compared with women without PCOS (both P less than .001).
Dr. Dokras acknowledged the limitations of administrative datasets and noted that prospective studies need to be conducted to verify their findings.
This study was funded by a grant from the National Institutes of Health. Dr. Dokras reported being a consultant for Medtronic, AbbVie, and Ferring.
SOURCE: Dokras A, et al. ASRM 2019. Abstract O-93.
PHILADELPHIA – Women with polycystic ovary syndrome (PCOS) are at a higher risk for metabolic and psychiatric comorbidities prior to pregnancy, cardiometabolic complications during pregnancy, and cardiometabolic and psychiatric complications in the postpartum period, according to results from a prize paper at the annual meeting of the American Society for Reproductive Medicine.
“Our findings do support the ACOG [American College of Obstetricians and Gynecologists] recommendations that women with PCOS should be considered a high-risk group, and during the postpartum period should be screened for cardiovascular as well as psychiatric comorbidities,” Anuja Dokras, MD, PhD, director of the Penn Polycystic Ovary Syndrome Center at the University of Pennsylvania, Philadelphia, said in her presentation.
Dr. Dokras and colleagues performed a retrospective cohort study during 2000-2016 of patients aged 18-50 years, in the Optum claims database, which comprised 42,391 women with PCOS and 795,480 women without PCOS in 50 U.S. states. Women were included in the analysis if there were data available for at least 6 months to 1 year before pregnancy and between 6 weeks and 1 year after pregnancy. The researchers looked at risk factors prior to pregnancy, such as depression, hypertension, hyperlipidemia, diabetes, obesity, smoking, and use of assisted reproductive technology. During pregnancy, Dr. Dokras and colleagues analyzed complications such as preterm birth, multiple gestation, cesarean section, gestational hypertension and diabetes, preeclampsia and eclampsia, and depression in addition to outcomes in the postpartum period, such as hypertensive complications, thrombotic disease, peripartum cardiomyopathy, heart failure, arterial complications, perinatal and postpartum depression.
“Realizing that PCOS is underreported in administrative data sets, we looked at not only the diagnosis of PCOS but also tried to combine any menstrual irregularity and hirsutism occurring simultaneously, and then doing a sensitivity analysis and looking at the population,” said Dr. Dokras. “Similarly, knowing that misclassification can be an issue in these datasets, we did the same thing amongst the controls, looking for a single diagnosis of irregular menses and hirsutism.”
Prior to pregnancy, women with PCOS in the dataset tended to have a higher rate of obesity (14.7% vs. 4.7%), hyperlipidemia (11.3% vs. 5.3%), hypertension (6.2% vs. 2.5%), diabetes (5.3% vs. 1.2%), and depression (4.3% vs. 3.1%) and were also more likely to use assisted reproductive technology (5.2% vs. 1.0%) than were patients without PCOS (all P less than .001). During pregnancy, there was a higher rate of gestational diabetes (13.7% vs. 7.7%), preeclampsia (5.0% vs. 2.6%), preterm birth (16.9% vs. 12.2%), multiple gestation (6.6% vs. 2.5%), and cesarean section (45.1% vs. 32.9%) in patients with PCOS, compared with those without PCOS (all P less than .001).
For patients in the postpartum period, women with PCOS were more likely to experience postpartum thrombotic disease (adjusted odds ratio, 1.60; 95% confidence interval, 1.23-2.09; P = .001), hypertensive heart disease (aOR, 1.45; 95% CI, 1.04-2.01; P = .027), eclampsia (aOR, 1.45; 95% CI, 1.14-1.86; P = .003), heart failure (aOR, 1.33; 95% CI, 1.08-1.64; P = .007), preeclampsia (aOR, 1.30; 95% CI, 1.17-1.45; P = than .001), and peripartum cardiomyopathy (aOR, 1.26; 95% CI, 1.03-1.54; P = .027).
With regard to depression, women with PCOS also were at greater risk of developing perinatal (aOR, 1.27; 95% CI, 1.22-1.33) and postpartum (aOR, 1.46; 95% CI, 1.36-1.57) depression, compared with women without PCOS (both P less than .001).
Dr. Dokras acknowledged the limitations of administrative datasets and noted that prospective studies need to be conducted to verify their findings.
This study was funded by a grant from the National Institutes of Health. Dr. Dokras reported being a consultant for Medtronic, AbbVie, and Ferring.
SOURCE: Dokras A, et al. ASRM 2019. Abstract O-93.
PHILADELPHIA – Women with polycystic ovary syndrome (PCOS) are at a higher risk for metabolic and psychiatric comorbidities prior to pregnancy, cardiometabolic complications during pregnancy, and cardiometabolic and psychiatric complications in the postpartum period, according to results from a prize paper at the annual meeting of the American Society for Reproductive Medicine.
“Our findings do support the ACOG [American College of Obstetricians and Gynecologists] recommendations that women with PCOS should be considered a high-risk group, and during the postpartum period should be screened for cardiovascular as well as psychiatric comorbidities,” Anuja Dokras, MD, PhD, director of the Penn Polycystic Ovary Syndrome Center at the University of Pennsylvania, Philadelphia, said in her presentation.
Dr. Dokras and colleagues performed a retrospective cohort study during 2000-2016 of patients aged 18-50 years, in the Optum claims database, which comprised 42,391 women with PCOS and 795,480 women without PCOS in 50 U.S. states. Women were included in the analysis if there were data available for at least 6 months to 1 year before pregnancy and between 6 weeks and 1 year after pregnancy. The researchers looked at risk factors prior to pregnancy, such as depression, hypertension, hyperlipidemia, diabetes, obesity, smoking, and use of assisted reproductive technology. During pregnancy, Dr. Dokras and colleagues analyzed complications such as preterm birth, multiple gestation, cesarean section, gestational hypertension and diabetes, preeclampsia and eclampsia, and depression in addition to outcomes in the postpartum period, such as hypertensive complications, thrombotic disease, peripartum cardiomyopathy, heart failure, arterial complications, perinatal and postpartum depression.
“Realizing that PCOS is underreported in administrative data sets, we looked at not only the diagnosis of PCOS but also tried to combine any menstrual irregularity and hirsutism occurring simultaneously, and then doing a sensitivity analysis and looking at the population,” said Dr. Dokras. “Similarly, knowing that misclassification can be an issue in these datasets, we did the same thing amongst the controls, looking for a single diagnosis of irregular menses and hirsutism.”
Prior to pregnancy, women with PCOS in the dataset tended to have a higher rate of obesity (14.7% vs. 4.7%), hyperlipidemia (11.3% vs. 5.3%), hypertension (6.2% vs. 2.5%), diabetes (5.3% vs. 1.2%), and depression (4.3% vs. 3.1%) and were also more likely to use assisted reproductive technology (5.2% vs. 1.0%) than were patients without PCOS (all P less than .001). During pregnancy, there was a higher rate of gestational diabetes (13.7% vs. 7.7%), preeclampsia (5.0% vs. 2.6%), preterm birth (16.9% vs. 12.2%), multiple gestation (6.6% vs. 2.5%), and cesarean section (45.1% vs. 32.9%) in patients with PCOS, compared with those without PCOS (all P less than .001).
For patients in the postpartum period, women with PCOS were more likely to experience postpartum thrombotic disease (adjusted odds ratio, 1.60; 95% confidence interval, 1.23-2.09; P = .001), hypertensive heart disease (aOR, 1.45; 95% CI, 1.04-2.01; P = .027), eclampsia (aOR, 1.45; 95% CI, 1.14-1.86; P = .003), heart failure (aOR, 1.33; 95% CI, 1.08-1.64; P = .007), preeclampsia (aOR, 1.30; 95% CI, 1.17-1.45; P = than .001), and peripartum cardiomyopathy (aOR, 1.26; 95% CI, 1.03-1.54; P = .027).
With regard to depression, women with PCOS also were at greater risk of developing perinatal (aOR, 1.27; 95% CI, 1.22-1.33) and postpartum (aOR, 1.46; 95% CI, 1.36-1.57) depression, compared with women without PCOS (both P less than .001).
Dr. Dokras acknowledged the limitations of administrative datasets and noted that prospective studies need to be conducted to verify their findings.
This study was funded by a grant from the National Institutes of Health. Dr. Dokras reported being a consultant for Medtronic, AbbVie, and Ferring.
SOURCE: Dokras A, et al. ASRM 2019. Abstract O-93.
REPORTING FROM ASRM 2019
In utero Zika exposure can have delayed consequences
WASHINGTON – Evidence continues to mount that infants born to moms infected with Zika virus during pregnancy can have neurodevelopmental abnormalities as they age even if they showed no defects at birth, based on follow-up of 890 Colombian children tracked by epidemiologists from the U.S. Centers for Disease Control and Prevention.
Among the 890 neonates born to mothers apparently infected with Zika during pregnancy and followed for up to 2 years, 40 of the 852 (5%) without a detectable birth defect at delivery went on to show some type of neurodevelopmental sequelae during up to 24 months of age, Margaret Honein, PhD, said at an annual scientific meeting on infectious diseases.
In addition, among the children without birth defects at delivery who received follow-up examinations out to about 2 years, the incidence of “alerts” for possible neurodevelopmental issues was 15%-20% for each of the four domains studied (gross motor, fine motor, hearing and language, and personal and social functions), said Dr. Honein, an epidemiologist and chief of the birth defects branch of the CDC. In contrast, 17 of the 38 children (45%) followed who had identifiable birth defects at delivery also showed neurodevelopmental abnormalities when reexamined as long as 2 years after birth. These possible neurodevelopmental abnormalities, designated as alerts, were identified in comparison with a contemporaneous cohort of children born to uninfected mothers in the same regions of Colombia and assessed by the CDC researchers.
This cohort of children born to mothers who became infected with Zika virus during the 2016 Colombian epidemic will not undergo any planned, additional follow-up beyond the initial 2 years, Dr. Honein noted.
The findings she reported were consistent with observations from a much smaller cohort of 70 infants born to Colombian mothers infected with Zika virus while pregnant who had a normal head circumference and a normal clinical examination at delivery. When assessed once or twice 4-18 months after birth, these 70 infants showed an overall greater than one standard deviation (z-score) drop in their scores on the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) metric by 12 months after birth and continuing out to 18 months, said Sarah B. Mulkey, MD, a fetal-neonatal neurologist at Children’s National Health System in Washington. These deficits were especially pronounced in the mobility and social cognition domains of the four-domain WIDEA metric. The social cognition domain is an important predictor of later problems with executive function and other neurologic disorders, Dr. Mulkey said while reporting her findings in a separate talk at the meeting. She acknowledged that the analysis was flawed by comparing the WIDEA outcomes of the Zika virus–exposed children to healthy children from either inner-city Chicago or Canada. Dr. Mulkey said that she and her associates plan to characterize a population of Zika virus–unexposed children in Colombia to use for future comparisons.
The study reported by Dr. Honein involved an enhanced surveillance program launched by the CDC in 2016 in three regions of Colombia and included 1,190 pregnancies accompanied by Zika symptoms in the mother and with a reported pregnancy outcome, including 1,185 live births. Nearly half of the Zika infections occurred during the first trimester, and 34% occurred during the second trimester. However, fewer than a third of the pregnant women underwent some type of laboratory testing to confirm their infection, either by serology or by a DNA-based assay, and of these 28% had a positive finding. Dr. Honein cautioned that many of the specimens that tested negative for Zika virus may have been false negatives.
The birth defects identified among the infants born from an apparently affected pregnancy included brain abnormalities, eye anomalies, and microcephaly, with 5% of the 1,185 live births showing one or more of these outcomes. The neurodevelopmental deficits identified during follow-up of 890 of the children out to 2 years included seizures; abnormalities of tone, movement, or swallowing; and impairments of vision or hearing.
WASHINGTON – Evidence continues to mount that infants born to moms infected with Zika virus during pregnancy can have neurodevelopmental abnormalities as they age even if they showed no defects at birth, based on follow-up of 890 Colombian children tracked by epidemiologists from the U.S. Centers for Disease Control and Prevention.
Among the 890 neonates born to mothers apparently infected with Zika during pregnancy and followed for up to 2 years, 40 of the 852 (5%) without a detectable birth defect at delivery went on to show some type of neurodevelopmental sequelae during up to 24 months of age, Margaret Honein, PhD, said at an annual scientific meeting on infectious diseases.
In addition, among the children without birth defects at delivery who received follow-up examinations out to about 2 years, the incidence of “alerts” for possible neurodevelopmental issues was 15%-20% for each of the four domains studied (gross motor, fine motor, hearing and language, and personal and social functions), said Dr. Honein, an epidemiologist and chief of the birth defects branch of the CDC. In contrast, 17 of the 38 children (45%) followed who had identifiable birth defects at delivery also showed neurodevelopmental abnormalities when reexamined as long as 2 years after birth. These possible neurodevelopmental abnormalities, designated as alerts, were identified in comparison with a contemporaneous cohort of children born to uninfected mothers in the same regions of Colombia and assessed by the CDC researchers.
This cohort of children born to mothers who became infected with Zika virus during the 2016 Colombian epidemic will not undergo any planned, additional follow-up beyond the initial 2 years, Dr. Honein noted.
The findings she reported were consistent with observations from a much smaller cohort of 70 infants born to Colombian mothers infected with Zika virus while pregnant who had a normal head circumference and a normal clinical examination at delivery. When assessed once or twice 4-18 months after birth, these 70 infants showed an overall greater than one standard deviation (z-score) drop in their scores on the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) metric by 12 months after birth and continuing out to 18 months, said Sarah B. Mulkey, MD, a fetal-neonatal neurologist at Children’s National Health System in Washington. These deficits were especially pronounced in the mobility and social cognition domains of the four-domain WIDEA metric. The social cognition domain is an important predictor of later problems with executive function and other neurologic disorders, Dr. Mulkey said while reporting her findings in a separate talk at the meeting. She acknowledged that the analysis was flawed by comparing the WIDEA outcomes of the Zika virus–exposed children to healthy children from either inner-city Chicago or Canada. Dr. Mulkey said that she and her associates plan to characterize a population of Zika virus–unexposed children in Colombia to use for future comparisons.
The study reported by Dr. Honein involved an enhanced surveillance program launched by the CDC in 2016 in three regions of Colombia and included 1,190 pregnancies accompanied by Zika symptoms in the mother and with a reported pregnancy outcome, including 1,185 live births. Nearly half of the Zika infections occurred during the first trimester, and 34% occurred during the second trimester. However, fewer than a third of the pregnant women underwent some type of laboratory testing to confirm their infection, either by serology or by a DNA-based assay, and of these 28% had a positive finding. Dr. Honein cautioned that many of the specimens that tested negative for Zika virus may have been false negatives.
The birth defects identified among the infants born from an apparently affected pregnancy included brain abnormalities, eye anomalies, and microcephaly, with 5% of the 1,185 live births showing one or more of these outcomes. The neurodevelopmental deficits identified during follow-up of 890 of the children out to 2 years included seizures; abnormalities of tone, movement, or swallowing; and impairments of vision or hearing.
WASHINGTON – Evidence continues to mount that infants born to moms infected with Zika virus during pregnancy can have neurodevelopmental abnormalities as they age even if they showed no defects at birth, based on follow-up of 890 Colombian children tracked by epidemiologists from the U.S. Centers for Disease Control and Prevention.
Among the 890 neonates born to mothers apparently infected with Zika during pregnancy and followed for up to 2 years, 40 of the 852 (5%) without a detectable birth defect at delivery went on to show some type of neurodevelopmental sequelae during up to 24 months of age, Margaret Honein, PhD, said at an annual scientific meeting on infectious diseases.
In addition, among the children without birth defects at delivery who received follow-up examinations out to about 2 years, the incidence of “alerts” for possible neurodevelopmental issues was 15%-20% for each of the four domains studied (gross motor, fine motor, hearing and language, and personal and social functions), said Dr. Honein, an epidemiologist and chief of the birth defects branch of the CDC. In contrast, 17 of the 38 children (45%) followed who had identifiable birth defects at delivery also showed neurodevelopmental abnormalities when reexamined as long as 2 years after birth. These possible neurodevelopmental abnormalities, designated as alerts, were identified in comparison with a contemporaneous cohort of children born to uninfected mothers in the same regions of Colombia and assessed by the CDC researchers.
This cohort of children born to mothers who became infected with Zika virus during the 2016 Colombian epidemic will not undergo any planned, additional follow-up beyond the initial 2 years, Dr. Honein noted.
The findings she reported were consistent with observations from a much smaller cohort of 70 infants born to Colombian mothers infected with Zika virus while pregnant who had a normal head circumference and a normal clinical examination at delivery. When assessed once or twice 4-18 months after birth, these 70 infants showed an overall greater than one standard deviation (z-score) drop in their scores on the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) metric by 12 months after birth and continuing out to 18 months, said Sarah B. Mulkey, MD, a fetal-neonatal neurologist at Children’s National Health System in Washington. These deficits were especially pronounced in the mobility and social cognition domains of the four-domain WIDEA metric. The social cognition domain is an important predictor of later problems with executive function and other neurologic disorders, Dr. Mulkey said while reporting her findings in a separate talk at the meeting. She acknowledged that the analysis was flawed by comparing the WIDEA outcomes of the Zika virus–exposed children to healthy children from either inner-city Chicago or Canada. Dr. Mulkey said that she and her associates plan to characterize a population of Zika virus–unexposed children in Colombia to use for future comparisons.
The study reported by Dr. Honein involved an enhanced surveillance program launched by the CDC in 2016 in three regions of Colombia and included 1,190 pregnancies accompanied by Zika symptoms in the mother and with a reported pregnancy outcome, including 1,185 live births. Nearly half of the Zika infections occurred during the first trimester, and 34% occurred during the second trimester. However, fewer than a third of the pregnant women underwent some type of laboratory testing to confirm their infection, either by serology or by a DNA-based assay, and of these 28% had a positive finding. Dr. Honein cautioned that many of the specimens that tested negative for Zika virus may have been false negatives.
The birth defects identified among the infants born from an apparently affected pregnancy included brain abnormalities, eye anomalies, and microcephaly, with 5% of the 1,185 live births showing one or more of these outcomes. The neurodevelopmental deficits identified during follow-up of 890 of the children out to 2 years included seizures; abnormalities of tone, movement, or swallowing; and impairments of vision or hearing.
REPORTING FROM ID WEEK 2019
Poor neonatal outcomes tied to excessive, insufficient weight gain during twin pregnancies
Lisa M. Bodnar, PhD, and colleagues determined.
The risks of cesarean section and neonatal death were elevated for those mothers who were overweight before pregnancy and then gained too much. But infants of underweight women who didn’t gain enough faced risks as well, wrote Dr. Bodnar of the University of Pittsburgh and associates in Obstetrics & Gynecology.
Among the most severely overweight women (obesity grade 2 or 3) who gained the most weight (43 kg) at 37 weeks’ gestation, there were 6 fewer small-for-gestational-age (SGA) infants per 100 births, but 14 more large-for-gestational-age (LGA) infants, 4 more cesarean deliveries, and 2 more neonatal deaths per 100 births. By contrast, among the most severely underweight women who gained the least amount of weight (9 kg), there were 18 more SGA infants, 3 fewer LGA infants, and 11 fewer cesareans, but 6 more preterm births before 32 weeks’ gestation.
The same U-shaped pattern also occurred within the individual weight categories. For example, compared with the outcomes among the most underweight women who gained least, among underweight women who gained the most (37 kg), there were eight fewer SGA infants, but four more LGA infants, 16 excess preterm births, and 9 excess infant deaths.
“If the associations we observed are even partially reflective of causality, targeted modification of pregnancy weight gain in women carrying twins might improve pregnancy outcomes,” wrote Dr. Bodnar and her team. “Data on a wide range of short- and long-term outcomes and information on the relative seriousness of these outcomes are needed to determine optimal gestational weight gain ranges for twin pregnancies.”
The cohort comprised 54,836 live-born twins from 27,723 twin pregnancies who were included in the MOMs database maintained by the University of Pennsylvania, Philadelphia. The population-based study tracks maternal obesity, gestational weight gain, and adverse birth outcomes. The information came from infant birth and death vital statistics records from 2003 to 2013.
However, this very source puts the findings in some degree of uncertainty, Ozhan Turan, MD, said in an interview.
“It’s a very nice study, and the statistics are very well done,” said Dr. Turan, who is the director of fetal therapy and complex obstetric surgery at the University of Maryland School of Medicine. “But that kind of data has pitfalls that are unavoidable. For example, they don’t have access to maternal medical comorbidities which are mostly related to the outcome, particularly gestational diabetes and preeclampsia. They also don’t have the information on chorionicity – and we know that monochorionic twins face much greater risk for these outcomes than dichorionic twins.”
The investigators calculated total gestational weight gain by subtracting prepregnancy weight from maternal weight at delivery. The analysis controlled for race and ethnicity, education, neonatal care, level of birth facility, parity, payment at delivery, smoking during pregnancy, marital status, year of birth, height, maternal age, preexisting diabetes or hypertension, infertility treatment, neonatal sex, and racial composition of neighborhood, as a proxy of neighborhood-level socioeconomic status. Approximately 16% of mothers received infertility treatment.
Of the cohort, 3% were underweight, 48% were normal weight, 24% were overweight, 13% were grade 1 obese, 7% grade 2 obese, and 5% grade 3 obese.
“Pregnancy weight gain was negatively associated with SGA and positively associated with LGA and cesarean delivery in all [body mass index] groups. For example, among normal-weight women, compared with a pregnancy weight gain equivalent to 20 kg at 37 weeks’ of gestation, a weight gain of 27 kg at 37 weeks’ of gestation was associated with 2.2 fewer cases of SGA but 2.9 more cases of LGA and 3.7 more cases of cesarean delivery,” Dr. Bodnar and associates wrote.
The investigators found that “weight gains well above or well below the [Institute of Medicine] provisional guidelines (less than 14 kg or more than 27 kg in underweight or normal-weight women, less than 11 kg or more than 28 kg in overweight women, and less than 6.4 kg or more than 26 kg in women with obesity) were associated with the highest risk of adverse outcomes.”
“I would not say this is practice-changing information,” said Dr. Turan. “We already know all this. What would be very helpful is an algorithm to tell us, if a patient is pregnant with twins, this is the amount of weight you have to gain.”
For overweight patients, Dr. Turan tries to impart the key message of moderate or slight weight gain, according to prepregnancy body mass index. For underweight patients, the picture is a bit more complex.
“There are not that many who are underweight before pregnancy, so first thing I look for is the reason a woman is underweight. Is she just not eating properly? Is there a drug dependence issue, alcohol dependence, HIV? Is there smoking? A gut problem that causes malnutrition. You can’t just say ‘eat more.’ That does not solve the problem. We need to find out why she is underweight and fix that first,” said Dr. Turan.
Neither Dr. Bodnar nor Dr. Turan had any relevant financial disclosures. One coauthor disclosed her institution received funds from the University of Pittsburgh. The study was funded by National Institutes of Health grants.
SOURCE: Bodnar LM et al. Obstet Gynecol. 2019;134:1075-86.
Lisa M. Bodnar, PhD, and colleagues determined.
The risks of cesarean section and neonatal death were elevated for those mothers who were overweight before pregnancy and then gained too much. But infants of underweight women who didn’t gain enough faced risks as well, wrote Dr. Bodnar of the University of Pittsburgh and associates in Obstetrics & Gynecology.
Among the most severely overweight women (obesity grade 2 or 3) who gained the most weight (43 kg) at 37 weeks’ gestation, there were 6 fewer small-for-gestational-age (SGA) infants per 100 births, but 14 more large-for-gestational-age (LGA) infants, 4 more cesarean deliveries, and 2 more neonatal deaths per 100 births. By contrast, among the most severely underweight women who gained the least amount of weight (9 kg), there were 18 more SGA infants, 3 fewer LGA infants, and 11 fewer cesareans, but 6 more preterm births before 32 weeks’ gestation.
The same U-shaped pattern also occurred within the individual weight categories. For example, compared with the outcomes among the most underweight women who gained least, among underweight women who gained the most (37 kg), there were eight fewer SGA infants, but four more LGA infants, 16 excess preterm births, and 9 excess infant deaths.
“If the associations we observed are even partially reflective of causality, targeted modification of pregnancy weight gain in women carrying twins might improve pregnancy outcomes,” wrote Dr. Bodnar and her team. “Data on a wide range of short- and long-term outcomes and information on the relative seriousness of these outcomes are needed to determine optimal gestational weight gain ranges for twin pregnancies.”
The cohort comprised 54,836 live-born twins from 27,723 twin pregnancies who were included in the MOMs database maintained by the University of Pennsylvania, Philadelphia. The population-based study tracks maternal obesity, gestational weight gain, and adverse birth outcomes. The information came from infant birth and death vital statistics records from 2003 to 2013.
However, this very source puts the findings in some degree of uncertainty, Ozhan Turan, MD, said in an interview.
“It’s a very nice study, and the statistics are very well done,” said Dr. Turan, who is the director of fetal therapy and complex obstetric surgery at the University of Maryland School of Medicine. “But that kind of data has pitfalls that are unavoidable. For example, they don’t have access to maternal medical comorbidities which are mostly related to the outcome, particularly gestational diabetes and preeclampsia. They also don’t have the information on chorionicity – and we know that monochorionic twins face much greater risk for these outcomes than dichorionic twins.”
The investigators calculated total gestational weight gain by subtracting prepregnancy weight from maternal weight at delivery. The analysis controlled for race and ethnicity, education, neonatal care, level of birth facility, parity, payment at delivery, smoking during pregnancy, marital status, year of birth, height, maternal age, preexisting diabetes or hypertension, infertility treatment, neonatal sex, and racial composition of neighborhood, as a proxy of neighborhood-level socioeconomic status. Approximately 16% of mothers received infertility treatment.
Of the cohort, 3% were underweight, 48% were normal weight, 24% were overweight, 13% were grade 1 obese, 7% grade 2 obese, and 5% grade 3 obese.
“Pregnancy weight gain was negatively associated with SGA and positively associated with LGA and cesarean delivery in all [body mass index] groups. For example, among normal-weight women, compared with a pregnancy weight gain equivalent to 20 kg at 37 weeks’ of gestation, a weight gain of 27 kg at 37 weeks’ of gestation was associated with 2.2 fewer cases of SGA but 2.9 more cases of LGA and 3.7 more cases of cesarean delivery,” Dr. Bodnar and associates wrote.
The investigators found that “weight gains well above or well below the [Institute of Medicine] provisional guidelines (less than 14 kg or more than 27 kg in underweight or normal-weight women, less than 11 kg or more than 28 kg in overweight women, and less than 6.4 kg or more than 26 kg in women with obesity) were associated with the highest risk of adverse outcomes.”
“I would not say this is practice-changing information,” said Dr. Turan. “We already know all this. What would be very helpful is an algorithm to tell us, if a patient is pregnant with twins, this is the amount of weight you have to gain.”
For overweight patients, Dr. Turan tries to impart the key message of moderate or slight weight gain, according to prepregnancy body mass index. For underweight patients, the picture is a bit more complex.
“There are not that many who are underweight before pregnancy, so first thing I look for is the reason a woman is underweight. Is she just not eating properly? Is there a drug dependence issue, alcohol dependence, HIV? Is there smoking? A gut problem that causes malnutrition. You can’t just say ‘eat more.’ That does not solve the problem. We need to find out why she is underweight and fix that first,” said Dr. Turan.
Neither Dr. Bodnar nor Dr. Turan had any relevant financial disclosures. One coauthor disclosed her institution received funds from the University of Pittsburgh. The study was funded by National Institutes of Health grants.
SOURCE: Bodnar LM et al. Obstet Gynecol. 2019;134:1075-86.
Lisa M. Bodnar, PhD, and colleagues determined.
The risks of cesarean section and neonatal death were elevated for those mothers who were overweight before pregnancy and then gained too much. But infants of underweight women who didn’t gain enough faced risks as well, wrote Dr. Bodnar of the University of Pittsburgh and associates in Obstetrics & Gynecology.
Among the most severely overweight women (obesity grade 2 or 3) who gained the most weight (43 kg) at 37 weeks’ gestation, there were 6 fewer small-for-gestational-age (SGA) infants per 100 births, but 14 more large-for-gestational-age (LGA) infants, 4 more cesarean deliveries, and 2 more neonatal deaths per 100 births. By contrast, among the most severely underweight women who gained the least amount of weight (9 kg), there were 18 more SGA infants, 3 fewer LGA infants, and 11 fewer cesareans, but 6 more preterm births before 32 weeks’ gestation.
The same U-shaped pattern also occurred within the individual weight categories. For example, compared with the outcomes among the most underweight women who gained least, among underweight women who gained the most (37 kg), there were eight fewer SGA infants, but four more LGA infants, 16 excess preterm births, and 9 excess infant deaths.
“If the associations we observed are even partially reflective of causality, targeted modification of pregnancy weight gain in women carrying twins might improve pregnancy outcomes,” wrote Dr. Bodnar and her team. “Data on a wide range of short- and long-term outcomes and information on the relative seriousness of these outcomes are needed to determine optimal gestational weight gain ranges for twin pregnancies.”
The cohort comprised 54,836 live-born twins from 27,723 twin pregnancies who were included in the MOMs database maintained by the University of Pennsylvania, Philadelphia. The population-based study tracks maternal obesity, gestational weight gain, and adverse birth outcomes. The information came from infant birth and death vital statistics records from 2003 to 2013.
However, this very source puts the findings in some degree of uncertainty, Ozhan Turan, MD, said in an interview.
“It’s a very nice study, and the statistics are very well done,” said Dr. Turan, who is the director of fetal therapy and complex obstetric surgery at the University of Maryland School of Medicine. “But that kind of data has pitfalls that are unavoidable. For example, they don’t have access to maternal medical comorbidities which are mostly related to the outcome, particularly gestational diabetes and preeclampsia. They also don’t have the information on chorionicity – and we know that monochorionic twins face much greater risk for these outcomes than dichorionic twins.”
The investigators calculated total gestational weight gain by subtracting prepregnancy weight from maternal weight at delivery. The analysis controlled for race and ethnicity, education, neonatal care, level of birth facility, parity, payment at delivery, smoking during pregnancy, marital status, year of birth, height, maternal age, preexisting diabetes or hypertension, infertility treatment, neonatal sex, and racial composition of neighborhood, as a proxy of neighborhood-level socioeconomic status. Approximately 16% of mothers received infertility treatment.
Of the cohort, 3% were underweight, 48% were normal weight, 24% were overweight, 13% were grade 1 obese, 7% grade 2 obese, and 5% grade 3 obese.
“Pregnancy weight gain was negatively associated with SGA and positively associated with LGA and cesarean delivery in all [body mass index] groups. For example, among normal-weight women, compared with a pregnancy weight gain equivalent to 20 kg at 37 weeks’ of gestation, a weight gain of 27 kg at 37 weeks’ of gestation was associated with 2.2 fewer cases of SGA but 2.9 more cases of LGA and 3.7 more cases of cesarean delivery,” Dr. Bodnar and associates wrote.
The investigators found that “weight gains well above or well below the [Institute of Medicine] provisional guidelines (less than 14 kg or more than 27 kg in underweight or normal-weight women, less than 11 kg or more than 28 kg in overweight women, and less than 6.4 kg or more than 26 kg in women with obesity) were associated with the highest risk of adverse outcomes.”
“I would not say this is practice-changing information,” said Dr. Turan. “We already know all this. What would be very helpful is an algorithm to tell us, if a patient is pregnant with twins, this is the amount of weight you have to gain.”
For overweight patients, Dr. Turan tries to impart the key message of moderate or slight weight gain, according to prepregnancy body mass index. For underweight patients, the picture is a bit more complex.
“There are not that many who are underweight before pregnancy, so first thing I look for is the reason a woman is underweight. Is she just not eating properly? Is there a drug dependence issue, alcohol dependence, HIV? Is there smoking? A gut problem that causes malnutrition. You can’t just say ‘eat more.’ That does not solve the problem. We need to find out why she is underweight and fix that first,” said Dr. Turan.
Neither Dr. Bodnar nor Dr. Turan had any relevant financial disclosures. One coauthor disclosed her institution received funds from the University of Pittsburgh. The study was funded by National Institutes of Health grants.
SOURCE: Bodnar LM et al. Obstet Gynecol. 2019;134:1075-86.
FROM OBSTETRICS & GYNECOLOGY