User login
Considerations on the mode of delivery for pregnant women with hepatitis C infection
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
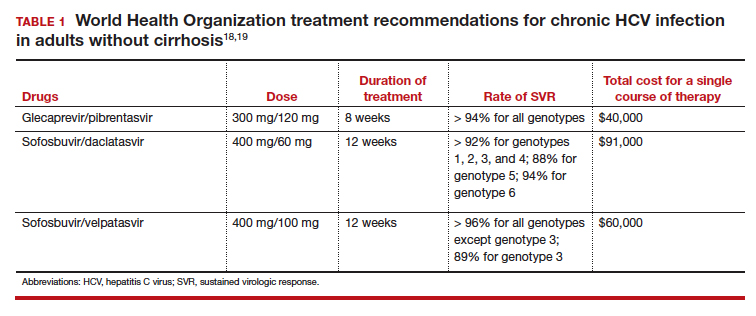
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11
Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
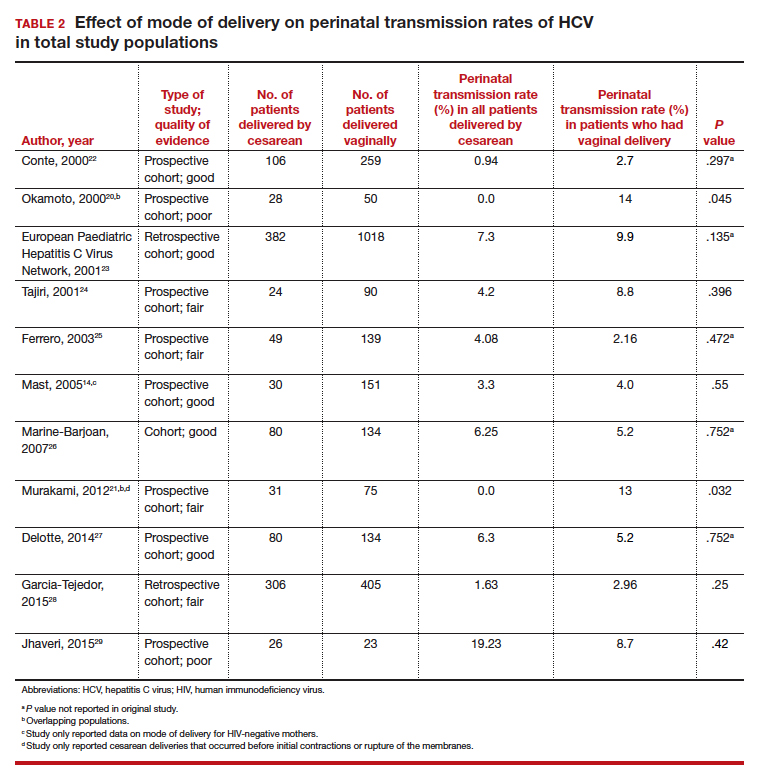
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
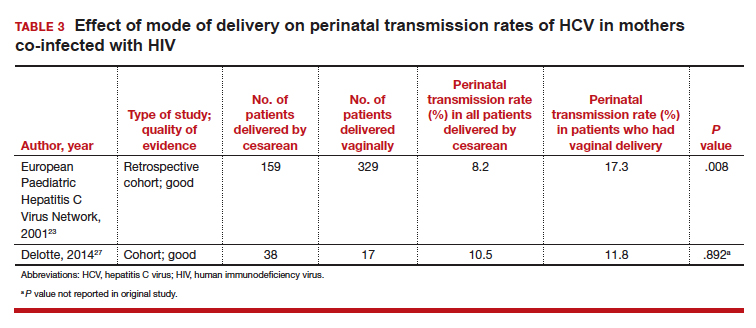
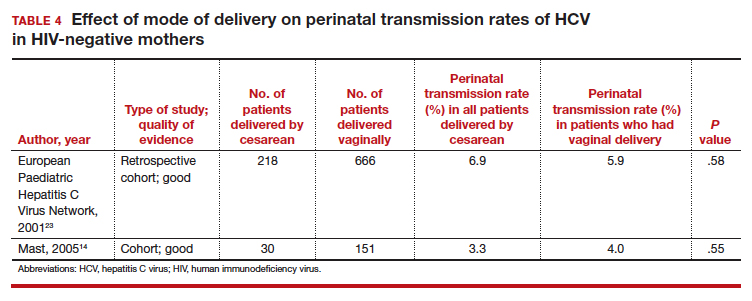
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
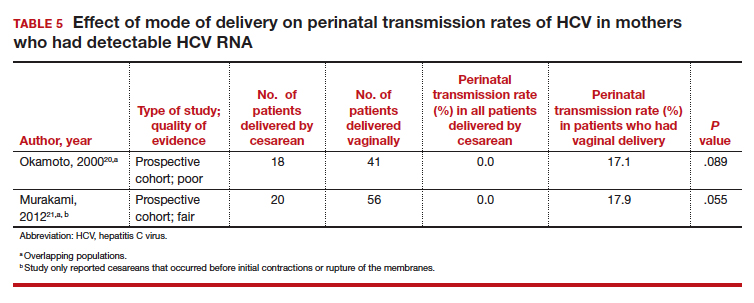
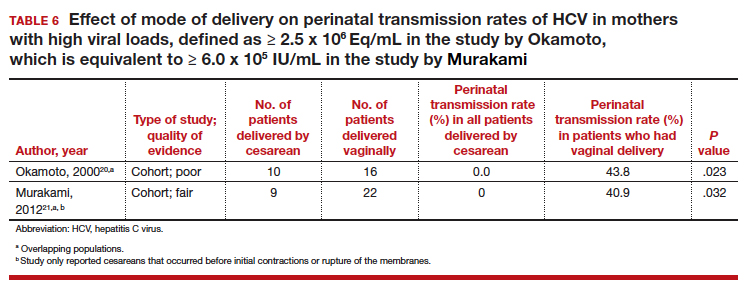
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21
Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11

Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21





Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11

Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21





Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
Women with obesity need not boost calories during pregnancy
LAS VEGAS – Contrary to current U.S. dietary recommendations for pregnancy, women with obesity should not increase their energy intake during pregnancy to achieve the current recommended level of gestational weight gain, based on findings from an intensive assessment of 54 women with obesity during weeks 13-37 of pregnancy.
To achieve the gestational weight gain of 11-20 pounds (5-9.1 kg) recommended by the Institute of Medicine, women with obesity ‒ those with a body mass index of 30 kg/m2 or greater ‒ had an average energy intake during the second and third trimesters of 125 kcal/day less than their energy expenditure, Leanne M. Redman, PhD, said at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
However, women in the study who had inadequate gestational weight gain had a daily calorie deficit that was only slightly larger, an average of 262 kcal/day below their energy expenditure. As a consequence, Dr. Redman believes the take-home message from her findings is that pregnant women with obesity should maintain their prepregnancy energy intake, though she also strongly recommended improvements in diet quality.
“Chasing a 100-kcal/day deficit in intake is extremely problematic,” Dr. Redman admitted, so she suggested that women with obesity be advised simply to not increase their calorie intake during pregnancy.
“The message is: Focus on improving diet quality rather than increasing calories,” she said in an interview. Pregnant women with obesity “do not need to increase calorie intake. They need to improve their diet quality,” with increased consumption of fruits and vegetables, said Dr. Redman, a professor and director of the Reproductive Endocrinology and Women’s Health Laboratory at Louisiana State University’s Pennington Biomedical Research Center in Baton Rouge.
The results she reported represent “the first time” researchers have examined energy expenditure and weight-gain trajectories in women with obesity throughout the second and third trimesters. Until now, dietary energy recommendations for women with obesity during pregnancy were based on observations made in women without obesity.
Those observations led the Institute of Medicine to call for a recommended pregnancy weight gain of 11-20 pounds in women with obesity, as well as gains of 25-35 pounds in women with a normal body mass index of 18.5-24.9 kg/m2 (Weight Gain During Pregnancy: Reexamining the Guidelines; May 2009). In that 2009 document, the IOM committee said that, in general, pregnant women should add 340 kcal/day to their prepregnancy intake during the second trimester and add 452 kcal/day during the third trimester without regard to their prepregnancy body mass index, a recommendation that clinicians continued to promote in subsequent years (Med Clin North Amer. 2016;100[6]:1199-215), and that was generally affirmed by the American College of Obstetricians and Gynecologists in 2013 and reaffirmed in 2018.*
The new evidence collected by Dr. Redman and associates “challenges current practice and argues that women with obesity should not be advised to consume additional energy during pregnancy as currently recommended,” they wrote in an article with their findings published a few days before Dr. Redman gave her talk (J Clin Invest. 2019;129[11]:4682-90).
The MomEE (Determinants of Gestational Weight Gain in Obese Pregnant Women) study enrolled 72 women with obesity during the first trimester of pregnancy and collected complete data through the end of the third trimester from 54 women. The researchers collected data on weight, body fat mass, and energy expenditure at multiple times during the second and third trimesters and calculated energy intake.
Based on body weights at the end of the third trimester, the researchers divided the 54 women into three subgroups: 10 women (19%) with inadequate weight gain by the IOM recommendations, 8 (15%) who had the IOM’s recommended weight gain of 11-20 pounds, and 36 women (67%; total is greater than 100% because of rounding) with excess weight gain, and within each group, they calculated the average level of energy intake relative to energy expenditure.
In addition to the daily calorie deficits associated with women who maintained recommended or inadequate weight, the researchers also found that women with excess weight gain averaged 186 more kcal/day than required to meet their daily energy expenditure.
The analyses showed that the increased energy demand of pregnancy and the fetus is compensated for by mobilization of the maternal fat mass in women with obesity, and that an imbalance between energy intake and expenditure is the main driver of weight gain during pregnancy. The results also highlighted how often pregnant women with obesity fail to follow a diet that results in the recommended weight gain of 11-20 pounds. In the MomEE cohort, two-thirds of enrolled women had excess weight gain.
The finding that women had the recommended weight gain on a diet that cut their daily calorie intake by about 100 kcal/day during the last two trimesters highlighted the nutritional challenge faced by women with obesity who are pregnant. “About three-quarters of women in the study had poor diet quality. There is an opportunity to improve diet with more fruits and vegetables to increase fullness, and [to reduce] energy-dense foods,” Dr. Redman said.
She is planning to collaborate on a study that will test the efficacy and safety of providing pregnant women with extreme obesity (class II-III) with defined meals to provide better control of energy intake and nutritional quality. Dr. Redman said she also hoped that the new findings she reported would be taken into account by the advisory committee assembled by the Department of Health & Human Services and the Department of Agriculture, which are currently preparing a revision of U.S. dietary guidelines for release in 2020.
The National Institutes of Health and the Clinical Research Cores at Pennington Biomedical Research Center funded the study. Dr. Redman had no disclosures.
SOURCE: Redman LM et al. Obesity Week 2019, Abstract T-OR-2079.
*This article was updated 2/7/2020.
The results reported by Dr. Redman from the MomEE study showed that women with obesity need not ingest surplus calories to gain weight during pregnancy. The findings indicate that pregnant women efficiently convert a portion of their accumulated fat mass to fat-free mass in the form of the fetus, uterus, blood volume, and other tissue. A deficit of about approximately 100 kcal/day effectively kept weight gain within the 11- to 20-pound target recommended by the Institute of Medicine in 2009.
But the weight gains recommended for women with obesity may be too high. The desire of the writers of the IOM recommendation to avoid negative perinatal outcomes for infants may instead lead to negative maternal outcomes, such as preeclampsia, gestational hypertension, and need for cesarean birth. Gestational weight gains below what the IOM recommended for women with obesity may be able to serve present-day standards and work better for these pregnant women by reducing their morbidity risk. Future studies should take into careful account overall nutrient values rather than just calorie intake, as well as physical activity.
The MomEE results showed that a striking two-thirds of women with obesity gained an excess of weight during pregnancy, beyond the 2009 recommendations. This finding highlights the need to identify strategies that can prevent excessive weight gain. Furthermore, results from several studies and systematic reviews suggest that the IOM recommendation for weight gain during pregnancy is too high for women with obesity, especially those with class II-III obesity, with a body mass index of 35 kg/m2 or greater. In my opinion, an appropriate weight-gain target to replace the current, blanket recommendation of 11-20 pounds gained for all women with obesity is a target of 5-15 pounds gained for women with class I obesity, less than 10 pounds for class II obesity, and no change in prepregnancy weight for women with class III obesity.
Sarah S. Comstock, PhD, is a nutrition researcher at Michigan State University, East Lansing. She is an inventor named on three patents that involve nutrition. She made these comments in an editorial that accompanied the MomEE report (J Clin Invest. 2019;129[11]:4567-9).
The results reported by Dr. Redman from the MomEE study showed that women with obesity need not ingest surplus calories to gain weight during pregnancy. The findings indicate that pregnant women efficiently convert a portion of their accumulated fat mass to fat-free mass in the form of the fetus, uterus, blood volume, and other tissue. A deficit of about approximately 100 kcal/day effectively kept weight gain within the 11- to 20-pound target recommended by the Institute of Medicine in 2009.
But the weight gains recommended for women with obesity may be too high. The desire of the writers of the IOM recommendation to avoid negative perinatal outcomes for infants may instead lead to negative maternal outcomes, such as preeclampsia, gestational hypertension, and need for cesarean birth. Gestational weight gains below what the IOM recommended for women with obesity may be able to serve present-day standards and work better for these pregnant women by reducing their morbidity risk. Future studies should take into careful account overall nutrient values rather than just calorie intake, as well as physical activity.
The MomEE results showed that a striking two-thirds of women with obesity gained an excess of weight during pregnancy, beyond the 2009 recommendations. This finding highlights the need to identify strategies that can prevent excessive weight gain. Furthermore, results from several studies and systematic reviews suggest that the IOM recommendation for weight gain during pregnancy is too high for women with obesity, especially those with class II-III obesity, with a body mass index of 35 kg/m2 or greater. In my opinion, an appropriate weight-gain target to replace the current, blanket recommendation of 11-20 pounds gained for all women with obesity is a target of 5-15 pounds gained for women with class I obesity, less than 10 pounds for class II obesity, and no change in prepregnancy weight for women with class III obesity.
Sarah S. Comstock, PhD, is a nutrition researcher at Michigan State University, East Lansing. She is an inventor named on three patents that involve nutrition. She made these comments in an editorial that accompanied the MomEE report (J Clin Invest. 2019;129[11]:4567-9).
The results reported by Dr. Redman from the MomEE study showed that women with obesity need not ingest surplus calories to gain weight during pregnancy. The findings indicate that pregnant women efficiently convert a portion of their accumulated fat mass to fat-free mass in the form of the fetus, uterus, blood volume, and other tissue. A deficit of about approximately 100 kcal/day effectively kept weight gain within the 11- to 20-pound target recommended by the Institute of Medicine in 2009.
But the weight gains recommended for women with obesity may be too high. The desire of the writers of the IOM recommendation to avoid negative perinatal outcomes for infants may instead lead to negative maternal outcomes, such as preeclampsia, gestational hypertension, and need for cesarean birth. Gestational weight gains below what the IOM recommended for women with obesity may be able to serve present-day standards and work better for these pregnant women by reducing their morbidity risk. Future studies should take into careful account overall nutrient values rather than just calorie intake, as well as physical activity.
The MomEE results showed that a striking two-thirds of women with obesity gained an excess of weight during pregnancy, beyond the 2009 recommendations. This finding highlights the need to identify strategies that can prevent excessive weight gain. Furthermore, results from several studies and systematic reviews suggest that the IOM recommendation for weight gain during pregnancy is too high for women with obesity, especially those with class II-III obesity, with a body mass index of 35 kg/m2 or greater. In my opinion, an appropriate weight-gain target to replace the current, blanket recommendation of 11-20 pounds gained for all women with obesity is a target of 5-15 pounds gained for women with class I obesity, less than 10 pounds for class II obesity, and no change in prepregnancy weight for women with class III obesity.
Sarah S. Comstock, PhD, is a nutrition researcher at Michigan State University, East Lansing. She is an inventor named on three patents that involve nutrition. She made these comments in an editorial that accompanied the MomEE report (J Clin Invest. 2019;129[11]:4567-9).
LAS VEGAS – Contrary to current U.S. dietary recommendations for pregnancy, women with obesity should not increase their energy intake during pregnancy to achieve the current recommended level of gestational weight gain, based on findings from an intensive assessment of 54 women with obesity during weeks 13-37 of pregnancy.
To achieve the gestational weight gain of 11-20 pounds (5-9.1 kg) recommended by the Institute of Medicine, women with obesity ‒ those with a body mass index of 30 kg/m2 or greater ‒ had an average energy intake during the second and third trimesters of 125 kcal/day less than their energy expenditure, Leanne M. Redman, PhD, said at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
However, women in the study who had inadequate gestational weight gain had a daily calorie deficit that was only slightly larger, an average of 262 kcal/day below their energy expenditure. As a consequence, Dr. Redman believes the take-home message from her findings is that pregnant women with obesity should maintain their prepregnancy energy intake, though she also strongly recommended improvements in diet quality.
“Chasing a 100-kcal/day deficit in intake is extremely problematic,” Dr. Redman admitted, so she suggested that women with obesity be advised simply to not increase their calorie intake during pregnancy.
“The message is: Focus on improving diet quality rather than increasing calories,” she said in an interview. Pregnant women with obesity “do not need to increase calorie intake. They need to improve their diet quality,” with increased consumption of fruits and vegetables, said Dr. Redman, a professor and director of the Reproductive Endocrinology and Women’s Health Laboratory at Louisiana State University’s Pennington Biomedical Research Center in Baton Rouge.
The results she reported represent “the first time” researchers have examined energy expenditure and weight-gain trajectories in women with obesity throughout the second and third trimesters. Until now, dietary energy recommendations for women with obesity during pregnancy were based on observations made in women without obesity.
Those observations led the Institute of Medicine to call for a recommended pregnancy weight gain of 11-20 pounds in women with obesity, as well as gains of 25-35 pounds in women with a normal body mass index of 18.5-24.9 kg/m2 (Weight Gain During Pregnancy: Reexamining the Guidelines; May 2009). In that 2009 document, the IOM committee said that, in general, pregnant women should add 340 kcal/day to their prepregnancy intake during the second trimester and add 452 kcal/day during the third trimester without regard to their prepregnancy body mass index, a recommendation that clinicians continued to promote in subsequent years (Med Clin North Amer. 2016;100[6]:1199-215), and that was generally affirmed by the American College of Obstetricians and Gynecologists in 2013 and reaffirmed in 2018.*
The new evidence collected by Dr. Redman and associates “challenges current practice and argues that women with obesity should not be advised to consume additional energy during pregnancy as currently recommended,” they wrote in an article with their findings published a few days before Dr. Redman gave her talk (J Clin Invest. 2019;129[11]:4682-90).
The MomEE (Determinants of Gestational Weight Gain in Obese Pregnant Women) study enrolled 72 women with obesity during the first trimester of pregnancy and collected complete data through the end of the third trimester from 54 women. The researchers collected data on weight, body fat mass, and energy expenditure at multiple times during the second and third trimesters and calculated energy intake.
Based on body weights at the end of the third trimester, the researchers divided the 54 women into three subgroups: 10 women (19%) with inadequate weight gain by the IOM recommendations, 8 (15%) who had the IOM’s recommended weight gain of 11-20 pounds, and 36 women (67%; total is greater than 100% because of rounding) with excess weight gain, and within each group, they calculated the average level of energy intake relative to energy expenditure.
In addition to the daily calorie deficits associated with women who maintained recommended or inadequate weight, the researchers also found that women with excess weight gain averaged 186 more kcal/day than required to meet their daily energy expenditure.
The analyses showed that the increased energy demand of pregnancy and the fetus is compensated for by mobilization of the maternal fat mass in women with obesity, and that an imbalance between energy intake and expenditure is the main driver of weight gain during pregnancy. The results also highlighted how often pregnant women with obesity fail to follow a diet that results in the recommended weight gain of 11-20 pounds. In the MomEE cohort, two-thirds of enrolled women had excess weight gain.
The finding that women had the recommended weight gain on a diet that cut their daily calorie intake by about 100 kcal/day during the last two trimesters highlighted the nutritional challenge faced by women with obesity who are pregnant. “About three-quarters of women in the study had poor diet quality. There is an opportunity to improve diet with more fruits and vegetables to increase fullness, and [to reduce] energy-dense foods,” Dr. Redman said.
She is planning to collaborate on a study that will test the efficacy and safety of providing pregnant women with extreme obesity (class II-III) with defined meals to provide better control of energy intake and nutritional quality. Dr. Redman said she also hoped that the new findings she reported would be taken into account by the advisory committee assembled by the Department of Health & Human Services and the Department of Agriculture, which are currently preparing a revision of U.S. dietary guidelines for release in 2020.
The National Institutes of Health and the Clinical Research Cores at Pennington Biomedical Research Center funded the study. Dr. Redman had no disclosures.
SOURCE: Redman LM et al. Obesity Week 2019, Abstract T-OR-2079.
*This article was updated 2/7/2020.
LAS VEGAS – Contrary to current U.S. dietary recommendations for pregnancy, women with obesity should not increase their energy intake during pregnancy to achieve the current recommended level of gestational weight gain, based on findings from an intensive assessment of 54 women with obesity during weeks 13-37 of pregnancy.
To achieve the gestational weight gain of 11-20 pounds (5-9.1 kg) recommended by the Institute of Medicine, women with obesity ‒ those with a body mass index of 30 kg/m2 or greater ‒ had an average energy intake during the second and third trimesters of 125 kcal/day less than their energy expenditure, Leanne M. Redman, PhD, said at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
However, women in the study who had inadequate gestational weight gain had a daily calorie deficit that was only slightly larger, an average of 262 kcal/day below their energy expenditure. As a consequence, Dr. Redman believes the take-home message from her findings is that pregnant women with obesity should maintain their prepregnancy energy intake, though she also strongly recommended improvements in diet quality.
“Chasing a 100-kcal/day deficit in intake is extremely problematic,” Dr. Redman admitted, so she suggested that women with obesity be advised simply to not increase their calorie intake during pregnancy.
“The message is: Focus on improving diet quality rather than increasing calories,” she said in an interview. Pregnant women with obesity “do not need to increase calorie intake. They need to improve their diet quality,” with increased consumption of fruits and vegetables, said Dr. Redman, a professor and director of the Reproductive Endocrinology and Women’s Health Laboratory at Louisiana State University’s Pennington Biomedical Research Center in Baton Rouge.
The results she reported represent “the first time” researchers have examined energy expenditure and weight-gain trajectories in women with obesity throughout the second and third trimesters. Until now, dietary energy recommendations for women with obesity during pregnancy were based on observations made in women without obesity.
Those observations led the Institute of Medicine to call for a recommended pregnancy weight gain of 11-20 pounds in women with obesity, as well as gains of 25-35 pounds in women with a normal body mass index of 18.5-24.9 kg/m2 (Weight Gain During Pregnancy: Reexamining the Guidelines; May 2009). In that 2009 document, the IOM committee said that, in general, pregnant women should add 340 kcal/day to their prepregnancy intake during the second trimester and add 452 kcal/day during the third trimester without regard to their prepregnancy body mass index, a recommendation that clinicians continued to promote in subsequent years (Med Clin North Amer. 2016;100[6]:1199-215), and that was generally affirmed by the American College of Obstetricians and Gynecologists in 2013 and reaffirmed in 2018.*
The new evidence collected by Dr. Redman and associates “challenges current practice and argues that women with obesity should not be advised to consume additional energy during pregnancy as currently recommended,” they wrote in an article with their findings published a few days before Dr. Redman gave her talk (J Clin Invest. 2019;129[11]:4682-90).
The MomEE (Determinants of Gestational Weight Gain in Obese Pregnant Women) study enrolled 72 women with obesity during the first trimester of pregnancy and collected complete data through the end of the third trimester from 54 women. The researchers collected data on weight, body fat mass, and energy expenditure at multiple times during the second and third trimesters and calculated energy intake.
Based on body weights at the end of the third trimester, the researchers divided the 54 women into three subgroups: 10 women (19%) with inadequate weight gain by the IOM recommendations, 8 (15%) who had the IOM’s recommended weight gain of 11-20 pounds, and 36 women (67%; total is greater than 100% because of rounding) with excess weight gain, and within each group, they calculated the average level of energy intake relative to energy expenditure.
In addition to the daily calorie deficits associated with women who maintained recommended or inadequate weight, the researchers also found that women with excess weight gain averaged 186 more kcal/day than required to meet their daily energy expenditure.
The analyses showed that the increased energy demand of pregnancy and the fetus is compensated for by mobilization of the maternal fat mass in women with obesity, and that an imbalance between energy intake and expenditure is the main driver of weight gain during pregnancy. The results also highlighted how often pregnant women with obesity fail to follow a diet that results in the recommended weight gain of 11-20 pounds. In the MomEE cohort, two-thirds of enrolled women had excess weight gain.
The finding that women had the recommended weight gain on a diet that cut their daily calorie intake by about 100 kcal/day during the last two trimesters highlighted the nutritional challenge faced by women with obesity who are pregnant. “About three-quarters of women in the study had poor diet quality. There is an opportunity to improve diet with more fruits and vegetables to increase fullness, and [to reduce] energy-dense foods,” Dr. Redman said.
She is planning to collaborate on a study that will test the efficacy and safety of providing pregnant women with extreme obesity (class II-III) with defined meals to provide better control of energy intake and nutritional quality. Dr. Redman said she also hoped that the new findings she reported would be taken into account by the advisory committee assembled by the Department of Health & Human Services and the Department of Agriculture, which are currently preparing a revision of U.S. dietary guidelines for release in 2020.
The National Institutes of Health and the Clinical Research Cores at Pennington Biomedical Research Center funded the study. Dr. Redman had no disclosures.
SOURCE: Redman LM et al. Obesity Week 2019, Abstract T-OR-2079.
*This article was updated 2/7/2020.
REPORTING FROM OBESITY WEEK 2019
Data build on cardiovascular disease risk after GDM, HDP
WASHINGTON – Cardiovascular risk factors may be elevated “as soon as the first postpartum year” in women who have gestational diabetes or hypertensive disorders of pregnancy, recent findings have affirmed, Deborah B. Ehrenthal, MD, MPH, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
Dr. Ehrenthal was one of several researchers who urged innovative strategies and improved care coordination to boost women’s follow-up after gestational diabetes mellitus (GDM) and other adverse pregnancy outcomes and complications. “The metabolic stress of pregnancy can uncover underlying susceptibilities,” she said. “And ”
Evidence that adverse pregnancy outcomes – including GDM and hypertensive disorders of pregnancy (HDP) – can elevate cardiovascular risk comes most recently from the Nulliparous Pregnancy Outcomes Study – Monitoring Mothers to be Heart Health Study (nuMoM2b–HHS study), a prospective observational cohort that followed 4,484 women 2-7 years after their first pregnancy. Women had a follow-up exam, with blood pressure and anthropometric measurements and clinical/biological testing, an average of 3 years post partum.
An analysis published in October 2019 in the Journal of the American Heart Association shows that women with HDP (including preeclampsia and gestational hypertension) had a relative risk of hypertension of 2.5 at follow-up, compared with women without HDP. Women who had preeclampsia specifically were 2.3 times as likely as were women who did not have preeclampsia to have incident hypertension at follow-up, said Dr. Ehrenthal, a coinvestigator of the study.
The analysis focused on incident hypertension as the primary outcome, and adjusted for age, body mass index, and other important cardiovascular disease risk factors, she noted. Researchers utilized the diagnostic threshold for hypertension extant at the time of study design: A systolic blood pressure of 140 mm Hg or greater, or a diastolic BP of 90 mm Hg or greater (J Am Heart Assoc. 2019;8:e013092).
HDP was the most common adverse pregnancy outcome in the nuMoM2b–HHS study (14%). Among all participants, 4% had GDM. Approximately 82% had neither HDP nor GDM. Other adverse pregnancy outcomes included in the analysis were preterm birth, small-for-gestational-age birth, and stillbirth.
Additional preliminary estimates presented by Dr. Ehrenthal show that, based on the new (2017) lower threshold for hypertension – 130 mg Hg systolic or 80 mm Hg diastolic – the disorder afflicted 37% of women who had experienced HDP (relative risk 2.1), and 32% of women who had GDM (RR 1.8). Prediabetes/diabetes (using a fasting blood glucose threshold of 100 mg/dL) at follow-up affected an estimated 21% of women who had HDP (RR 1.4) and 38% of women who had GDM (RR 2.5).
Notably, across the entire study cohort, 20% had hypertension at follow-up, “which is extraordinary” considering the short time frame from pregnancy and the young age of the study population – a mean maternal age of 27 years, said Dr. Ehrenthal, associate professor of population health sciences and obstetrics & gynecology at the University of Wisconsin, Madison.
Also across the cohort, 15% had prediabetes/diabetes at follow-up. “We need to think about women more generally,” she cautioned. “While we recognize the significant elevated risk of HDP and GDM [for the development of subsequent hypertension and cardiovascular risk], we will miss a lot of women [if we focus only on the history of HDP and GDM.]”
The majority of women found to have hypertension or prediabetes/diabetes at follow-up had experienced neither HDP nor GDM, but a good many of them (47% of those who had hypertension and 47% of those found to have prediabetes/diabetes) had a BMI of 30 or above, Dr. Ehrenthal said at the DPSG-NA meeting.
Nurses Health Study, hyperglycemia and adverse pregnancy outcome follow-up data
The new findings from the nuMoM2b–HHS study add to a robust and growing body of evidence that pregnancy is an important window to future health, and that follow up and screening after GDM and HDP are crucial.
Regarding GDM specifically, “there’s quite a bit of literature by now demonstrating that GDM history is a risk factor for hypertension, even 1-2 years post partum, and that the risk is elevated as well for dyslipidemia and vascular dysfunction,” Deirdre K. Tobias, D.Sc., an epidemiologist at Brigham and Women’s Hospital and assistant professor of nutrition at Harvard TH Chan School of Public Health, Boston, said at the DPSG meeting.
An analysis of the Nurses Health Study II (NHS II) cohort published in 2017 found a 40% higher relative risk of cardiovascular disease events (largely myocardial infarction) in women who had GDM, compared with women who did not have GDM over a median follow-up of 26 years. This was after adjustments were made for age, time since pregnancy, menopausal status, family history of MI or stroke, hypertension in pregnancy, white race/ethnicity, prepregnancy BMI, and other factors (JAMA Intern Med. 2017;177[12]:1735-42).
The NHS data also have shown, however, that the elevated risk for cardiovascular disease after a GDM pregnancy “can be mitigated by adopting a healthy lifestyle,” said Dr. Tobias, lead author of the 2017 NHS II analysis. Adjustments for postpregnancy weight gain and lifestyle factors attenuated the relative risk of cardiovascular disease events after a GDM pregnancy to a 30% increased risk.
Dr. Tobias and colleagues currently are looking within the NHS cohort for “metabolomic signatures” or signals – various amino acid and lipid metabolites – to identify the progression of GDM to type 2 diabetes. Metabolomics “may help further refine our understanding of the long-term links between GDM and prevention of type 2 diabetes and of cardiovascular disease in mothers,” she said.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Follow-Up Study, in the meantime, is documenting associations of maternal glucose levels during pregnancy not only with prediabetes or type 2 diabetes 10-14 years later, but also with measures of cardiovascular risk in mothers 10-14 years later.
Just as perinatal outcomes were strongly associated with glucose as a continuous variable in the original HAPO study, “it’s clear there’s a progressive increase in the risk of [later] disorders of glucose metabolism as [fasting blood glucose levels and 1- and-2-hour glucose values] in pregnancy are higher,” said Boyd E. Metzger, MD, the Tom D. Spies emeritus professor of metabolism and nutrition at Northwestern University, Chicago, and principal investigator of the original HAPO study and its follow up.
“Another message is that the more normal you are in pregnancy, the more normal you will be many years later. Good values [during pregnancy] produce good outcomes.”
Currently unpublished data from the HAPO Follow-Up Study are being analyzed, but it appears thus far that GDM is not associated with hypertension (per the old diagnostic threshold) in this cohort after adjustment for maternal age, BMI, smoking, and family history of hypertension. GDM appears to be a significant risk factor for dyslipidemia, however. HDL cholesterol at follow-up was significantly lower for mothers who had GDM compared with those without, whereas LDL cholesterol and triglycerides at follow-up were significantly higher for mothers with GDM, Dr. Metzger said.
Racial/ethnic disparities, postpartum care
Neither long-term study – the NHS II or the HAPO Follow-Up Study – has looked at racial and ethnic differences. The HAPO cohort is racially-ethnically diverse but the NHS II cohort is predominantly white women.
Research suggests that GDM is a heterogeneous condition with some unique phenotypes in subgroups that vary by race and ethnicity. And just as there appear to be racial-ethnic differences in the pathophysiology of GDM, there appear to be racial-ethnic differences in the progression to type 2 diabetes – a known risk factor for cardiovascular disease, said Monique Henderson, PhD, a research scientist at Kaiser Permanente Northern California (KPNC).
On the broadest level, while Asian Americans have the highest prevalence of GDM, African Americans have the highest rates of progressing to type 2 diabetes, Dr. Henderson said. Disparities “may [stem from] metabolic differences in terms of insulin resistance and secretion that are different between pregnancy and the postpartum period, and that might vary [across racial-ethnic subgroups],” she said. Lifestyle differences and variation in postpartum screening rates also may play a role.
At KPNC, where women with GDM receive calls and letters reminding them of the need for postpartum screening, only 48% overall completed an oral glucose tolerance test at 4-12 weeks post partum, as recommended by both the American Diabetes Association and the American College of Obstetricians and Gynecologists. Both before and after adjustment for education, attendance at a postpartum visit, and other variables, Chinese women were most likely to have screening, and black women were least likely, said Dr. Henderson, referring to ongoing research.
A study Dr. Ehrenthal led of women with GDM or HDP recruited from the postpartum service of a large community-based, academic obstetrical hospital in Delaware showed that while nearly all women attended a 6-week postpartum visit with their ob.gyns., 59% of women with GDM had not yet completed diabetes screening when they were interviewed 3 months post partum. Most women with HDP indicated they had follow-up blood pressure testing, and just over half of women with either diagnosis recalled having ever had lipid testing (J Women’s Health 2014;23[9]:760-4).
Women least likely to complete screening tests were those who had no college education, those who had less than a high school level of health literacy, and those who were not privately insured, Dr. Ehrenthal said.
A large national study of privately insured women also found low rates of follow-up testing, however. While the majority of women with GDM had a postpartum visit with an obstetrician or primary care physician within a year after delivery, only a minority of women had a glycemic screening test completed (Obstet Gynecol. 2016;128[1]:159-67).
“We can’t place the blame on women,” Dr. Ehrenthal said. “We need increased attention to screening,” including screening for cardiovascular disease risk factors, and a “deliberate hand-off to primary care.”
For follow-up cardiovascular disease risk factor assessment after HDP, ACOG recommends periodic (perhaps annually) assessment and referral for treatment as needed, and the cardiology professional organizations recommend that pregnancy history be considered when assessing risk in order to decide on lipid treatment, she noted.
Each of the speakers reported that they have no financial or other interests that pose a conflict of interest. The HAPO Follow-Up Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the nuMoM2b–HHS study has been funded by several National Institutes of Health institutes and other programs and initiatives.
WASHINGTON – Cardiovascular risk factors may be elevated “as soon as the first postpartum year” in women who have gestational diabetes or hypertensive disorders of pregnancy, recent findings have affirmed, Deborah B. Ehrenthal, MD, MPH, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
Dr. Ehrenthal was one of several researchers who urged innovative strategies and improved care coordination to boost women’s follow-up after gestational diabetes mellitus (GDM) and other adverse pregnancy outcomes and complications. “The metabolic stress of pregnancy can uncover underlying susceptibilities,” she said. “And ”
Evidence that adverse pregnancy outcomes – including GDM and hypertensive disorders of pregnancy (HDP) – can elevate cardiovascular risk comes most recently from the Nulliparous Pregnancy Outcomes Study – Monitoring Mothers to be Heart Health Study (nuMoM2b–HHS study), a prospective observational cohort that followed 4,484 women 2-7 years after their first pregnancy. Women had a follow-up exam, with blood pressure and anthropometric measurements and clinical/biological testing, an average of 3 years post partum.
An analysis published in October 2019 in the Journal of the American Heart Association shows that women with HDP (including preeclampsia and gestational hypertension) had a relative risk of hypertension of 2.5 at follow-up, compared with women without HDP. Women who had preeclampsia specifically were 2.3 times as likely as were women who did not have preeclampsia to have incident hypertension at follow-up, said Dr. Ehrenthal, a coinvestigator of the study.
The analysis focused on incident hypertension as the primary outcome, and adjusted for age, body mass index, and other important cardiovascular disease risk factors, she noted. Researchers utilized the diagnostic threshold for hypertension extant at the time of study design: A systolic blood pressure of 140 mm Hg or greater, or a diastolic BP of 90 mm Hg or greater (J Am Heart Assoc. 2019;8:e013092).
HDP was the most common adverse pregnancy outcome in the nuMoM2b–HHS study (14%). Among all participants, 4% had GDM. Approximately 82% had neither HDP nor GDM. Other adverse pregnancy outcomes included in the analysis were preterm birth, small-for-gestational-age birth, and stillbirth.
Additional preliminary estimates presented by Dr. Ehrenthal show that, based on the new (2017) lower threshold for hypertension – 130 mg Hg systolic or 80 mm Hg diastolic – the disorder afflicted 37% of women who had experienced HDP (relative risk 2.1), and 32% of women who had GDM (RR 1.8). Prediabetes/diabetes (using a fasting blood glucose threshold of 100 mg/dL) at follow-up affected an estimated 21% of women who had HDP (RR 1.4) and 38% of women who had GDM (RR 2.5).
Notably, across the entire study cohort, 20% had hypertension at follow-up, “which is extraordinary” considering the short time frame from pregnancy and the young age of the study population – a mean maternal age of 27 years, said Dr. Ehrenthal, associate professor of population health sciences and obstetrics & gynecology at the University of Wisconsin, Madison.
Also across the cohort, 15% had prediabetes/diabetes at follow-up. “We need to think about women more generally,” she cautioned. “While we recognize the significant elevated risk of HDP and GDM [for the development of subsequent hypertension and cardiovascular risk], we will miss a lot of women [if we focus only on the history of HDP and GDM.]”
The majority of women found to have hypertension or prediabetes/diabetes at follow-up had experienced neither HDP nor GDM, but a good many of them (47% of those who had hypertension and 47% of those found to have prediabetes/diabetes) had a BMI of 30 or above, Dr. Ehrenthal said at the DPSG-NA meeting.
Nurses Health Study, hyperglycemia and adverse pregnancy outcome follow-up data
The new findings from the nuMoM2b–HHS study add to a robust and growing body of evidence that pregnancy is an important window to future health, and that follow up and screening after GDM and HDP are crucial.
Regarding GDM specifically, “there’s quite a bit of literature by now demonstrating that GDM history is a risk factor for hypertension, even 1-2 years post partum, and that the risk is elevated as well for dyslipidemia and vascular dysfunction,” Deirdre K. Tobias, D.Sc., an epidemiologist at Brigham and Women’s Hospital and assistant professor of nutrition at Harvard TH Chan School of Public Health, Boston, said at the DPSG meeting.
An analysis of the Nurses Health Study II (NHS II) cohort published in 2017 found a 40% higher relative risk of cardiovascular disease events (largely myocardial infarction) in women who had GDM, compared with women who did not have GDM over a median follow-up of 26 years. This was after adjustments were made for age, time since pregnancy, menopausal status, family history of MI or stroke, hypertension in pregnancy, white race/ethnicity, prepregnancy BMI, and other factors (JAMA Intern Med. 2017;177[12]:1735-42).
The NHS data also have shown, however, that the elevated risk for cardiovascular disease after a GDM pregnancy “can be mitigated by adopting a healthy lifestyle,” said Dr. Tobias, lead author of the 2017 NHS II analysis. Adjustments for postpregnancy weight gain and lifestyle factors attenuated the relative risk of cardiovascular disease events after a GDM pregnancy to a 30% increased risk.
Dr. Tobias and colleagues currently are looking within the NHS cohort for “metabolomic signatures” or signals – various amino acid and lipid metabolites – to identify the progression of GDM to type 2 diabetes. Metabolomics “may help further refine our understanding of the long-term links between GDM and prevention of type 2 diabetes and of cardiovascular disease in mothers,” she said.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Follow-Up Study, in the meantime, is documenting associations of maternal glucose levels during pregnancy not only with prediabetes or type 2 diabetes 10-14 years later, but also with measures of cardiovascular risk in mothers 10-14 years later.
Just as perinatal outcomes were strongly associated with glucose as a continuous variable in the original HAPO study, “it’s clear there’s a progressive increase in the risk of [later] disorders of glucose metabolism as [fasting blood glucose levels and 1- and-2-hour glucose values] in pregnancy are higher,” said Boyd E. Metzger, MD, the Tom D. Spies emeritus professor of metabolism and nutrition at Northwestern University, Chicago, and principal investigator of the original HAPO study and its follow up.
“Another message is that the more normal you are in pregnancy, the more normal you will be many years later. Good values [during pregnancy] produce good outcomes.”
Currently unpublished data from the HAPO Follow-Up Study are being analyzed, but it appears thus far that GDM is not associated with hypertension (per the old diagnostic threshold) in this cohort after adjustment for maternal age, BMI, smoking, and family history of hypertension. GDM appears to be a significant risk factor for dyslipidemia, however. HDL cholesterol at follow-up was significantly lower for mothers who had GDM compared with those without, whereas LDL cholesterol and triglycerides at follow-up were significantly higher for mothers with GDM, Dr. Metzger said.
Racial/ethnic disparities, postpartum care
Neither long-term study – the NHS II or the HAPO Follow-Up Study – has looked at racial and ethnic differences. The HAPO cohort is racially-ethnically diverse but the NHS II cohort is predominantly white women.
Research suggests that GDM is a heterogeneous condition with some unique phenotypes in subgroups that vary by race and ethnicity. And just as there appear to be racial-ethnic differences in the pathophysiology of GDM, there appear to be racial-ethnic differences in the progression to type 2 diabetes – a known risk factor for cardiovascular disease, said Monique Henderson, PhD, a research scientist at Kaiser Permanente Northern California (KPNC).
On the broadest level, while Asian Americans have the highest prevalence of GDM, African Americans have the highest rates of progressing to type 2 diabetes, Dr. Henderson said. Disparities “may [stem from] metabolic differences in terms of insulin resistance and secretion that are different between pregnancy and the postpartum period, and that might vary [across racial-ethnic subgroups],” she said. Lifestyle differences and variation in postpartum screening rates also may play a role.
At KPNC, where women with GDM receive calls and letters reminding them of the need for postpartum screening, only 48% overall completed an oral glucose tolerance test at 4-12 weeks post partum, as recommended by both the American Diabetes Association and the American College of Obstetricians and Gynecologists. Both before and after adjustment for education, attendance at a postpartum visit, and other variables, Chinese women were most likely to have screening, and black women were least likely, said Dr. Henderson, referring to ongoing research.
A study Dr. Ehrenthal led of women with GDM or HDP recruited from the postpartum service of a large community-based, academic obstetrical hospital in Delaware showed that while nearly all women attended a 6-week postpartum visit with their ob.gyns., 59% of women with GDM had not yet completed diabetes screening when they were interviewed 3 months post partum. Most women with HDP indicated they had follow-up blood pressure testing, and just over half of women with either diagnosis recalled having ever had lipid testing (J Women’s Health 2014;23[9]:760-4).
Women least likely to complete screening tests were those who had no college education, those who had less than a high school level of health literacy, and those who were not privately insured, Dr. Ehrenthal said.
A large national study of privately insured women also found low rates of follow-up testing, however. While the majority of women with GDM had a postpartum visit with an obstetrician or primary care physician within a year after delivery, only a minority of women had a glycemic screening test completed (Obstet Gynecol. 2016;128[1]:159-67).
“We can’t place the blame on women,” Dr. Ehrenthal said. “We need increased attention to screening,” including screening for cardiovascular disease risk factors, and a “deliberate hand-off to primary care.”
For follow-up cardiovascular disease risk factor assessment after HDP, ACOG recommends periodic (perhaps annually) assessment and referral for treatment as needed, and the cardiology professional organizations recommend that pregnancy history be considered when assessing risk in order to decide on lipid treatment, she noted.
Each of the speakers reported that they have no financial or other interests that pose a conflict of interest. The HAPO Follow-Up Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the nuMoM2b–HHS study has been funded by several National Institutes of Health institutes and other programs and initiatives.
WASHINGTON – Cardiovascular risk factors may be elevated “as soon as the first postpartum year” in women who have gestational diabetes or hypertensive disorders of pregnancy, recent findings have affirmed, Deborah B. Ehrenthal, MD, MPH, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
Dr. Ehrenthal was one of several researchers who urged innovative strategies and improved care coordination to boost women’s follow-up after gestational diabetes mellitus (GDM) and other adverse pregnancy outcomes and complications. “The metabolic stress of pregnancy can uncover underlying susceptibilities,” she said. “And ”
Evidence that adverse pregnancy outcomes – including GDM and hypertensive disorders of pregnancy (HDP) – can elevate cardiovascular risk comes most recently from the Nulliparous Pregnancy Outcomes Study – Monitoring Mothers to be Heart Health Study (nuMoM2b–HHS study), a prospective observational cohort that followed 4,484 women 2-7 years after their first pregnancy. Women had a follow-up exam, with blood pressure and anthropometric measurements and clinical/biological testing, an average of 3 years post partum.
An analysis published in October 2019 in the Journal of the American Heart Association shows that women with HDP (including preeclampsia and gestational hypertension) had a relative risk of hypertension of 2.5 at follow-up, compared with women without HDP. Women who had preeclampsia specifically were 2.3 times as likely as were women who did not have preeclampsia to have incident hypertension at follow-up, said Dr. Ehrenthal, a coinvestigator of the study.
The analysis focused on incident hypertension as the primary outcome, and adjusted for age, body mass index, and other important cardiovascular disease risk factors, she noted. Researchers utilized the diagnostic threshold for hypertension extant at the time of study design: A systolic blood pressure of 140 mm Hg or greater, or a diastolic BP of 90 mm Hg or greater (J Am Heart Assoc. 2019;8:e013092).
HDP was the most common adverse pregnancy outcome in the nuMoM2b–HHS study (14%). Among all participants, 4% had GDM. Approximately 82% had neither HDP nor GDM. Other adverse pregnancy outcomes included in the analysis were preterm birth, small-for-gestational-age birth, and stillbirth.
Additional preliminary estimates presented by Dr. Ehrenthal show that, based on the new (2017) lower threshold for hypertension – 130 mg Hg systolic or 80 mm Hg diastolic – the disorder afflicted 37% of women who had experienced HDP (relative risk 2.1), and 32% of women who had GDM (RR 1.8). Prediabetes/diabetes (using a fasting blood glucose threshold of 100 mg/dL) at follow-up affected an estimated 21% of women who had HDP (RR 1.4) and 38% of women who had GDM (RR 2.5).
Notably, across the entire study cohort, 20% had hypertension at follow-up, “which is extraordinary” considering the short time frame from pregnancy and the young age of the study population – a mean maternal age of 27 years, said Dr. Ehrenthal, associate professor of population health sciences and obstetrics & gynecology at the University of Wisconsin, Madison.
Also across the cohort, 15% had prediabetes/diabetes at follow-up. “We need to think about women more generally,” she cautioned. “While we recognize the significant elevated risk of HDP and GDM [for the development of subsequent hypertension and cardiovascular risk], we will miss a lot of women [if we focus only on the history of HDP and GDM.]”
The majority of women found to have hypertension or prediabetes/diabetes at follow-up had experienced neither HDP nor GDM, but a good many of them (47% of those who had hypertension and 47% of those found to have prediabetes/diabetes) had a BMI of 30 or above, Dr. Ehrenthal said at the DPSG-NA meeting.
Nurses Health Study, hyperglycemia and adverse pregnancy outcome follow-up data
The new findings from the nuMoM2b–HHS study add to a robust and growing body of evidence that pregnancy is an important window to future health, and that follow up and screening after GDM and HDP are crucial.
Regarding GDM specifically, “there’s quite a bit of literature by now demonstrating that GDM history is a risk factor for hypertension, even 1-2 years post partum, and that the risk is elevated as well for dyslipidemia and vascular dysfunction,” Deirdre K. Tobias, D.Sc., an epidemiologist at Brigham and Women’s Hospital and assistant professor of nutrition at Harvard TH Chan School of Public Health, Boston, said at the DPSG meeting.
An analysis of the Nurses Health Study II (NHS II) cohort published in 2017 found a 40% higher relative risk of cardiovascular disease events (largely myocardial infarction) in women who had GDM, compared with women who did not have GDM over a median follow-up of 26 years. This was after adjustments were made for age, time since pregnancy, menopausal status, family history of MI or stroke, hypertension in pregnancy, white race/ethnicity, prepregnancy BMI, and other factors (JAMA Intern Med. 2017;177[12]:1735-42).
The NHS data also have shown, however, that the elevated risk for cardiovascular disease after a GDM pregnancy “can be mitigated by adopting a healthy lifestyle,” said Dr. Tobias, lead author of the 2017 NHS II analysis. Adjustments for postpregnancy weight gain and lifestyle factors attenuated the relative risk of cardiovascular disease events after a GDM pregnancy to a 30% increased risk.
Dr. Tobias and colleagues currently are looking within the NHS cohort for “metabolomic signatures” or signals – various amino acid and lipid metabolites – to identify the progression of GDM to type 2 diabetes. Metabolomics “may help further refine our understanding of the long-term links between GDM and prevention of type 2 diabetes and of cardiovascular disease in mothers,” she said.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Follow-Up Study, in the meantime, is documenting associations of maternal glucose levels during pregnancy not only with prediabetes or type 2 diabetes 10-14 years later, but also with measures of cardiovascular risk in mothers 10-14 years later.
Just as perinatal outcomes were strongly associated with glucose as a continuous variable in the original HAPO study, “it’s clear there’s a progressive increase in the risk of [later] disorders of glucose metabolism as [fasting blood glucose levels and 1- and-2-hour glucose values] in pregnancy are higher,” said Boyd E. Metzger, MD, the Tom D. Spies emeritus professor of metabolism and nutrition at Northwestern University, Chicago, and principal investigator of the original HAPO study and its follow up.
“Another message is that the more normal you are in pregnancy, the more normal you will be many years later. Good values [during pregnancy] produce good outcomes.”
Currently unpublished data from the HAPO Follow-Up Study are being analyzed, but it appears thus far that GDM is not associated with hypertension (per the old diagnostic threshold) in this cohort after adjustment for maternal age, BMI, smoking, and family history of hypertension. GDM appears to be a significant risk factor for dyslipidemia, however. HDL cholesterol at follow-up was significantly lower for mothers who had GDM compared with those without, whereas LDL cholesterol and triglycerides at follow-up were significantly higher for mothers with GDM, Dr. Metzger said.
Racial/ethnic disparities, postpartum care
Neither long-term study – the NHS II or the HAPO Follow-Up Study – has looked at racial and ethnic differences. The HAPO cohort is racially-ethnically diverse but the NHS II cohort is predominantly white women.
Research suggests that GDM is a heterogeneous condition with some unique phenotypes in subgroups that vary by race and ethnicity. And just as there appear to be racial-ethnic differences in the pathophysiology of GDM, there appear to be racial-ethnic differences in the progression to type 2 diabetes – a known risk factor for cardiovascular disease, said Monique Henderson, PhD, a research scientist at Kaiser Permanente Northern California (KPNC).
On the broadest level, while Asian Americans have the highest prevalence of GDM, African Americans have the highest rates of progressing to type 2 diabetes, Dr. Henderson said. Disparities “may [stem from] metabolic differences in terms of insulin resistance and secretion that are different between pregnancy and the postpartum period, and that might vary [across racial-ethnic subgroups],” she said. Lifestyle differences and variation in postpartum screening rates also may play a role.
At KPNC, where women with GDM receive calls and letters reminding them of the need for postpartum screening, only 48% overall completed an oral glucose tolerance test at 4-12 weeks post partum, as recommended by both the American Diabetes Association and the American College of Obstetricians and Gynecologists. Both before and after adjustment for education, attendance at a postpartum visit, and other variables, Chinese women were most likely to have screening, and black women were least likely, said Dr. Henderson, referring to ongoing research.
A study Dr. Ehrenthal led of women with GDM or HDP recruited from the postpartum service of a large community-based, academic obstetrical hospital in Delaware showed that while nearly all women attended a 6-week postpartum visit with their ob.gyns., 59% of women with GDM had not yet completed diabetes screening when they were interviewed 3 months post partum. Most women with HDP indicated they had follow-up blood pressure testing, and just over half of women with either diagnosis recalled having ever had lipid testing (J Women’s Health 2014;23[9]:760-4).
Women least likely to complete screening tests were those who had no college education, those who had less than a high school level of health literacy, and those who were not privately insured, Dr. Ehrenthal said.
A large national study of privately insured women also found low rates of follow-up testing, however. While the majority of women with GDM had a postpartum visit with an obstetrician or primary care physician within a year after delivery, only a minority of women had a glycemic screening test completed (Obstet Gynecol. 2016;128[1]:159-67).
“We can’t place the blame on women,” Dr. Ehrenthal said. “We need increased attention to screening,” including screening for cardiovascular disease risk factors, and a “deliberate hand-off to primary care.”
For follow-up cardiovascular disease risk factor assessment after HDP, ACOG recommends periodic (perhaps annually) assessment and referral for treatment as needed, and the cardiology professional organizations recommend that pregnancy history be considered when assessing risk in order to decide on lipid treatment, she noted.
Each of the speakers reported that they have no financial or other interests that pose a conflict of interest. The HAPO Follow-Up Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, and the nuMoM2b–HHS study has been funded by several National Institutes of Health institutes and other programs and initiatives.
REPORTING FROM THE DPSG-NA 2019
Umbilical cord management matters less for mothers than for infants
Immediate umbilical cord milking or delayed clamping of the umbilical cord had no significant impact on maternal outcomes, but infants were significantly more likely to experience severe intraventricular hemorrhage with umbilical cord milking, according to results of two studies published in JAMA.
“While the evidence for neonatal benefit with delayed cord clamping at term is strong, data related to maternal outcomes, particularly after cesarean delivery, are largely lacking,” wrote Stephanie E. Purisch, MD, of Columbia University Irving Medical Center, New York, and colleagues.
In a randomized trial of 113 women who underwent cesarean deliveries of singleton infants, the researchers hypothesized that maternal blood loss would be greater with delayed cord clamping (JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.15995).
However, maternal blood loss, based on mean hemoglobin levels 1 day after delivery, was not significantly different between the delayed group (10.1 g/dL) and the immediate group (98 g/dL). The median time to cord clamping was 63 seconds in the delayed group and 6 seconds in the immediate group.
In addition, no significant differences occurred in 15 of 19 prespecified secondary outcomes. However, for whom data were available (18.1 g/dL vs. 16.4 g/dL; P less than .001).
The results were limited by factors including lack of generalizability to other situations such as emergency or preterm deliveries and by the lack of a definition of a “clinically important postoperative hemoglobin change,” the researchers noted. However, the results show no significant impact of umbilical cord management on maternal hemoglobin in the study population.
In another study published in JAMA, Anup Katheria, MD, of Sharp Mary Birch Hospital for Women & Newborns, San Diego, and colleagues found no significant difference in rates of a composite outcome of death or severe intraventricular hemorrhage among infants randomized to umbilical cord milking (12%) vs. delayed umbilical cord clamping (8%). However, immediate umbilical cord milking was significantly associated with a higher rate of intraventricular hemorrhage alone, compared with delayed clamping (8% vs. 3%), and this signal of risk prompted the researchers to terminate the study earlier than intended.
The researchers randomized 474 infants born at less than 32 weeks’ gestation to umbilical cord milking or delayed umbilical cord clamping (JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.16004). The study was conducted at six sites in the United States and one site each in Ireland, Germany, and Canada between June 2017 and September 2018. “Because of the importance of long-term neurodevelopment, all surviving infants will be followed up to determine developmental outcomes at 22 to 26 months’ corrected gestational age,” they said.
The study was terminated early, which prevents definitive conclusions, the researchers noted, but a new study has been approved to compare umbilical cord milking with delayed umbilical cord clamping in infants of 30-32 weeks’ gestational age, they said.
“Although the safety of placental transfusion for the mother seems well established, it remains unclear which method of providing placental transfusion is best for the infant: delayed clamping and cutting the cord or milking the intact cord. The latter provides a transfusion more rapidly, which may facilitate initiation of resuscitation when needed,” Heike Rabe, MD, of the University of Sussex, Brighton, and Ola Andersson, PhD, of Lund (Sweden) University, wrote in an editorial accompanying the two studies (JAMA. 2019 Nov 19;322:1864-5. doi: 10.1001/jama.2019.16003).
The 8% incidence of severe intraventricular hemorrhage in the umbilical milking group in the study by Katheria and colleagues was higher than the 5.2% in a recent Cochrane review, but the 3% incidence of severe intraventricular hemorrhage in the delayed group was lower than the 4.5% in the Cochrane review, they said.
“Umbilical cord milking has been used in many hospitals without an increase in intraventricular hemorrhage being observed,” they noted.
“The study by Purisch et al. demonstrated the safety of delayed cord clamping for mothers delivering by cesarean at term,” the editorialists wrote. Studies are underway to identify the best techniques for cord clamping, they said.
“In the meantime, clinicians should follow the World Health Organization recommendation to delay cord clamping and cutting for 1 to 3 minutes for term infants and for at least 60 seconds for preterm infants to prevent iron deficiency and potentially enable more premature infants to survive,” they concluded.
Dr. Purisch received funding from the Maternal-Fetal Medicine Fellow Research Fund for the first study. Coauthor Cynthia Gyamfi-Bannerman, MD, reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Society for Maternal-Fetal Medicine/AMAG Pharmaceuticals, and personal fees from Sera Prognostics outside the submitted work. The second study was supported by NICHD in a grant to Dr. Katheria, who had no financial conflicts to disclose. Coauthor Gary Cutter, PhD, had numerous ties to pharmaceutical companies. The editorialists had no financial conflicts to disclose.
SOURCES: Purisch SE et al. JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.15995; Katheria A et al. JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.16004; Rabe H and Andersson O. JAMA. 2019 Nov 19; 322:1864-5.
Immediate umbilical cord milking or delayed clamping of the umbilical cord had no significant impact on maternal outcomes, but infants were significantly more likely to experience severe intraventricular hemorrhage with umbilical cord milking, according to results of two studies published in JAMA.
“While the evidence for neonatal benefit with delayed cord clamping at term is strong, data related to maternal outcomes, particularly after cesarean delivery, are largely lacking,” wrote Stephanie E. Purisch, MD, of Columbia University Irving Medical Center, New York, and colleagues.
In a randomized trial of 113 women who underwent cesarean deliveries of singleton infants, the researchers hypothesized that maternal blood loss would be greater with delayed cord clamping (JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.15995).
However, maternal blood loss, based on mean hemoglobin levels 1 day after delivery, was not significantly different between the delayed group (10.1 g/dL) and the immediate group (98 g/dL). The median time to cord clamping was 63 seconds in the delayed group and 6 seconds in the immediate group.
In addition, no significant differences occurred in 15 of 19 prespecified secondary outcomes. However, for whom data were available (18.1 g/dL vs. 16.4 g/dL; P less than .001).
The results were limited by factors including lack of generalizability to other situations such as emergency or preterm deliveries and by the lack of a definition of a “clinically important postoperative hemoglobin change,” the researchers noted. However, the results show no significant impact of umbilical cord management on maternal hemoglobin in the study population.
In another study published in JAMA, Anup Katheria, MD, of Sharp Mary Birch Hospital for Women & Newborns, San Diego, and colleagues found no significant difference in rates of a composite outcome of death or severe intraventricular hemorrhage among infants randomized to umbilical cord milking (12%) vs. delayed umbilical cord clamping (8%). However, immediate umbilical cord milking was significantly associated with a higher rate of intraventricular hemorrhage alone, compared with delayed clamping (8% vs. 3%), and this signal of risk prompted the researchers to terminate the study earlier than intended.
The researchers randomized 474 infants born at less than 32 weeks’ gestation to umbilical cord milking or delayed umbilical cord clamping (JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.16004). The study was conducted at six sites in the United States and one site each in Ireland, Germany, and Canada between June 2017 and September 2018. “Because of the importance of long-term neurodevelopment, all surviving infants will be followed up to determine developmental outcomes at 22 to 26 months’ corrected gestational age,” they said.
The study was terminated early, which prevents definitive conclusions, the researchers noted, but a new study has been approved to compare umbilical cord milking with delayed umbilical cord clamping in infants of 30-32 weeks’ gestational age, they said.
“Although the safety of placental transfusion for the mother seems well established, it remains unclear which method of providing placental transfusion is best for the infant: delayed clamping and cutting the cord or milking the intact cord. The latter provides a transfusion more rapidly, which may facilitate initiation of resuscitation when needed,” Heike Rabe, MD, of the University of Sussex, Brighton, and Ola Andersson, PhD, of Lund (Sweden) University, wrote in an editorial accompanying the two studies (JAMA. 2019 Nov 19;322:1864-5. doi: 10.1001/jama.2019.16003).
The 8% incidence of severe intraventricular hemorrhage in the umbilical milking group in the study by Katheria and colleagues was higher than the 5.2% in a recent Cochrane review, but the 3% incidence of severe intraventricular hemorrhage in the delayed group was lower than the 4.5% in the Cochrane review, they said.
“Umbilical cord milking has been used in many hospitals without an increase in intraventricular hemorrhage being observed,” they noted.
“The study by Purisch et al. demonstrated the safety of delayed cord clamping for mothers delivering by cesarean at term,” the editorialists wrote. Studies are underway to identify the best techniques for cord clamping, they said.
“In the meantime, clinicians should follow the World Health Organization recommendation to delay cord clamping and cutting for 1 to 3 minutes for term infants and for at least 60 seconds for preterm infants to prevent iron deficiency and potentially enable more premature infants to survive,” they concluded.
Dr. Purisch received funding from the Maternal-Fetal Medicine Fellow Research Fund for the first study. Coauthor Cynthia Gyamfi-Bannerman, MD, reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Society for Maternal-Fetal Medicine/AMAG Pharmaceuticals, and personal fees from Sera Prognostics outside the submitted work. The second study was supported by NICHD in a grant to Dr. Katheria, who had no financial conflicts to disclose. Coauthor Gary Cutter, PhD, had numerous ties to pharmaceutical companies. The editorialists had no financial conflicts to disclose.
SOURCES: Purisch SE et al. JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.15995; Katheria A et al. JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.16004; Rabe H and Andersson O. JAMA. 2019 Nov 19; 322:1864-5.
Immediate umbilical cord milking or delayed clamping of the umbilical cord had no significant impact on maternal outcomes, but infants were significantly more likely to experience severe intraventricular hemorrhage with umbilical cord milking, according to results of two studies published in JAMA.
“While the evidence for neonatal benefit with delayed cord clamping at term is strong, data related to maternal outcomes, particularly after cesarean delivery, are largely lacking,” wrote Stephanie E. Purisch, MD, of Columbia University Irving Medical Center, New York, and colleagues.
In a randomized trial of 113 women who underwent cesarean deliveries of singleton infants, the researchers hypothesized that maternal blood loss would be greater with delayed cord clamping (JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.15995).
However, maternal blood loss, based on mean hemoglobin levels 1 day after delivery, was not significantly different between the delayed group (10.1 g/dL) and the immediate group (98 g/dL). The median time to cord clamping was 63 seconds in the delayed group and 6 seconds in the immediate group.
In addition, no significant differences occurred in 15 of 19 prespecified secondary outcomes. However, for whom data were available (18.1 g/dL vs. 16.4 g/dL; P less than .001).
The results were limited by factors including lack of generalizability to other situations such as emergency or preterm deliveries and by the lack of a definition of a “clinically important postoperative hemoglobin change,” the researchers noted. However, the results show no significant impact of umbilical cord management on maternal hemoglobin in the study population.
In another study published in JAMA, Anup Katheria, MD, of Sharp Mary Birch Hospital for Women & Newborns, San Diego, and colleagues found no significant difference in rates of a composite outcome of death or severe intraventricular hemorrhage among infants randomized to umbilical cord milking (12%) vs. delayed umbilical cord clamping (8%). However, immediate umbilical cord milking was significantly associated with a higher rate of intraventricular hemorrhage alone, compared with delayed clamping (8% vs. 3%), and this signal of risk prompted the researchers to terminate the study earlier than intended.
The researchers randomized 474 infants born at less than 32 weeks’ gestation to umbilical cord milking or delayed umbilical cord clamping (JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.16004). The study was conducted at six sites in the United States and one site each in Ireland, Germany, and Canada between June 2017 and September 2018. “Because of the importance of long-term neurodevelopment, all surviving infants will be followed up to determine developmental outcomes at 22 to 26 months’ corrected gestational age,” they said.
The study was terminated early, which prevents definitive conclusions, the researchers noted, but a new study has been approved to compare umbilical cord milking with delayed umbilical cord clamping in infants of 30-32 weeks’ gestational age, they said.
“Although the safety of placental transfusion for the mother seems well established, it remains unclear which method of providing placental transfusion is best for the infant: delayed clamping and cutting the cord or milking the intact cord. The latter provides a transfusion more rapidly, which may facilitate initiation of resuscitation when needed,” Heike Rabe, MD, of the University of Sussex, Brighton, and Ola Andersson, PhD, of Lund (Sweden) University, wrote in an editorial accompanying the two studies (JAMA. 2019 Nov 19;322:1864-5. doi: 10.1001/jama.2019.16003).
The 8% incidence of severe intraventricular hemorrhage in the umbilical milking group in the study by Katheria and colleagues was higher than the 5.2% in a recent Cochrane review, but the 3% incidence of severe intraventricular hemorrhage in the delayed group was lower than the 4.5% in the Cochrane review, they said.
“Umbilical cord milking has been used in many hospitals without an increase in intraventricular hemorrhage being observed,” they noted.
“The study by Purisch et al. demonstrated the safety of delayed cord clamping for mothers delivering by cesarean at term,” the editorialists wrote. Studies are underway to identify the best techniques for cord clamping, they said.
“In the meantime, clinicians should follow the World Health Organization recommendation to delay cord clamping and cutting for 1 to 3 minutes for term infants and for at least 60 seconds for preterm infants to prevent iron deficiency and potentially enable more premature infants to survive,” they concluded.
Dr. Purisch received funding from the Maternal-Fetal Medicine Fellow Research Fund for the first study. Coauthor Cynthia Gyamfi-Bannerman, MD, reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Society for Maternal-Fetal Medicine/AMAG Pharmaceuticals, and personal fees from Sera Prognostics outside the submitted work. The second study was supported by NICHD in a grant to Dr. Katheria, who had no financial conflicts to disclose. Coauthor Gary Cutter, PhD, had numerous ties to pharmaceutical companies. The editorialists had no financial conflicts to disclose.
SOURCES: Purisch SE et al. JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.15995; Katheria A et al. JAMA. 2019 Nov 19. doi: 10.1001/jama.2019.16004; Rabe H and Andersson O. JAMA. 2019 Nov 19; 322:1864-5.
FROM JAMA
Multidisciplinary care could address fertility preservation in transgender youth
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASRM 2019
Ask about vaping and e-cigarette use
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
Cannabis and prenatal care
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Counseling on cannabis use in pregnancy
A flurry of research papers published this year has simultaneously documented a rise in the use of cannabis during pregnancy and offered more data about its potential harms. This confluence of findings is concerning and highlights the importance of screening our patients for cannabis use and engaging with them in a way in which we can maintain their trust and their commitment to prenatal care.
A retrospective cohort study involving 661,617 women in Ontario found a significant association between self-reported cannabis use in pregnancy and an increased risk of preterm birth (relative risk, 1.41), as well as a greater likelihood of small-for-gestational-age babies (RR, 1.53), placental abruption (RR, 1.72), and transfer to neonatal intensive care (RR, 1.40).1 The study, reported in JAMA in July 2019, carefully matched users with nonusers who had the same characteristics – for example, tobacco use or not.
This new information builds upon other meta-analyses that have demonstrated a decrease in birth weight and greater admittance to the neonatal ICU associated with cannabis use in pregnancy – and it supplements what some research suggests about long-term neurologic development and a potentially increased risk of attention and behavioral problems. Other outcomes that have been noted in long-term neurologic studies of children who were exposed to cannabis in utero include impaired visual acuity, verbal reasoning and comprehension, and short-term memory.2
Increases in use were recently documented in two studies. One, an analysis of data from the National Survey on Drug Use and Health (NSDUH) published in JAMA in June 2019, showed that, between 2002-2003 and 2016-2017, the use of cannabis “in the past month” increased from 3.4% to 7.0% among pregnant women overall, and from 6% to 12% during the first trimester.3
The use of cannabis on a daily or near-daily basis, moreover, increased from 0.9% to 3% among pregnant women overall and from 2% to 5% during the first trimester. The data were collected during face-to-face interviews and were adjusted for age, race/ethnicity, and family income.
In the second study – a cross-sectional study of 367,403 pregnancies among women who filled out a questionnaire on cannabis use during standard prenatal care at Kaiser Permanente Northern California – the adjusted prevalence of use in the year before pregnancy increased from 7% in 2009 to 13% in 2017, and the adjusted prevalence during pregnancy increased from 2% to 3%.4
As in the NSDUH analysis, daily use increased most rapidly (compared with weekly or monthly) such that, by 2017, 25% of those who reported using cannabis in the year before pregnancy – and 21% of those who used cannabis during pregnancy – were daily users. It is notable that Kaiser’s population is diverse in all respects, and that the annual relative rates of increase in cannabis use before and during pregnancy (at each level of frequency) were consistent across racial/ethnic and household income groups.
It’s also worth noting that, in earlier research covering a similar time period (2009-2016), the investigators found significant increases in use via urine toxicology testing that occurs at the first prenatal visit at Kaiser. The increase found through questionnaires, therefore, reflects more than a greater willingness to self-report.
Choosing a screening tool
Universal prenatal substance use screening is recommended by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, but we don’t have any specific recommendations on what this means. Who should be screening, and what should that screening look like? Should we use a biologic screen, a standardized screening tool, or simply ask patients whether they use illicit substances?
Screening tools seem advantageous in that they are low cost, noninvasive, potentially comprehensive, and not subject to false-positive results as biologic screens can be – but which tool or tools are best? There are several validated screening tools that can be used outside of pregnancy to determine an individual’s use of illicit substances and whether or not that use is problematic, but previous studies have not used biologic markers to validate substance use screeners in pregnancy. Nor have studies compared screeners in pregnancy.
In our prenatal population in Baltimore, we have not been getting the answers we want using various nonvalidated screening tools. Approximately 30% of patients are positive for cannabis by urine screen, but only half tell us about their use.
Through research in our two prenatal care practices (one serving mostly privately insured and the other serving primarily Medicaid-eligible patients), we assessed both the accuracy and the acceptability of three substance use screening tools that are brief and that have been validated (for the general population) by the World Health Organization for screening of multiple substances: the 4P’s Plus (Parents, Partner, Past, and Pregnancy), the National Institute on Drug Abuse Quick Screen–ASSIST (Modified Alcohol, Smoking and Substance Involvement Screening Test), and the SURP-P (Substance Use Risk Profile–Pregnancy) scale.
In one study, published in May 2019 in Obstetrics & Gynecology, we recruited 500 pregnant women and administered these three tests to each of them.5 We then compared results with those of urine and hair drug testing, and checked the test-retest reliability of each test by readministering them (albeit by telephone) a week later. Although hair testing is not an indicator of current substance use, we used it to validate the screening tools on less-recent use.
The tests with the highest sensitivity and negative predictive values – the qualities we most want for screening – were the SURP-P and the 4P’s Plus (sensitivity of 92.4% and 90.2%, respectively). Overall they were highly sensitive screening tools across all trimesters, races, and age groups, making them more ideal screening tests than the NIDA Quick Screen–ASSIST.
Of the two tests, the 4P’s Plus screening tool was the one preferred by staff from both practices. In a companion qualitative study, we conducted focus-group discussions with 40 practice staff who were responsible for administering or overseeing patient screening.6 The staff, who were unaware of the sensitivity findings, were asked what they thought about the acceptability to patients of each of the three tools and their usability in practice.
Most of the participating staff preferred the 4P’s Plus screening tool for several reasons: It is easy to understand, is brief and to the point, and it has nonjudgmental language and tone. The screener first asks the patient about her parents’ and her partner’s use of alcohol and drugs, and then asks the patient about her own use of alcohol and tobacco. Affirmative responses to these questions lead to additional questions.
The premise is that one’s genetics, history, and current exposures – as well as one’s own use of tobacco and alcohol – are significantly associated with the use of illicit substances. If the patient reports no parental history or partner usage, and has never drank or smoked before, it’s extremely unlikely that she is using other drugs. The progression of questions does indeed seem less judgmental than immediately asking: “Do you use drugs?”
For us, the insight from this staff perception study combined with the findings on accuracy mean that the 4P’s Plus may be the most useful and acceptable screening tool for routine use in prenatal care.
Talking with our patients
The increase in the use of cannabis before and after pregnancy parallels the movement toward state legalization and decriminalization. Historically, clinicians often have relied on illegality as their main focus of counseling when giving recommendations for cessation and abstinence in pregnancy.2 This approach not only leads to punitive counseling, which can fracture the doctor-patient relationship, but increasingly it is no longer valid. In our changing legal climate, we need to provide medically based counseling and be very clear with our patients that legalization does not equate to safety.
It is important that we neither minimize nor overstate the risks. The evidence base for adverse birth outcomes of cannabis use in pregnancy is quite robust, but the associations can be subtle and are moderated by other behaviors and environmental factors that continue to challenge researchers.
As with alcohol, there likely are dose-or trimester-dependent differences in perinatal outcomes, and it’s quite possible that different cannabis products and routes of consumption have different effects. At this point, however, we don’t know the full story, nor do we know the extent to which the literature is biased toward positive correlations – the reporting of adverse effects – compared with negative findings. It is our job as medical care providers to be comfortable in that gray area and to still counsel patients on what we do know, providing the best-possible medical advice based on the information available to us.
In talking with patients, I explain that cannabis may cause a spectrum of problems and that there certainly are risks. I also tell them that we’re uncertain about the conditions and magnitude of that risk and that some babies who are exposed to cannabis in utero may have no perceivable consequences. Such honesty is important for maintaining trust, especially as some patients may see friends and relatives who also are cannabis users have normal pregnancy outcomes.
Much of my concern about cannabis in pregnancy centers on its effect on the developing brain and on long-term neurologic development. I share this with patients – I tell them that cannabis crosses the placenta and may well affect their baby’s brain as it is developing. I explain that I do not know whether this effect would be big or small, but that it’s not a chance I’m willing to take for their baby.
It is also important to educate patients that cannabis products are untested and unregulated and that they may be contaminated with heavy metals, pesticides, and other toxins that may be harmful to themselves and their babies. Patients also should know that the potency of cannabis has been dramatically increasing; research shows that the tetrahydrocannabinol – the psychoactive component – concentration has tripled over the past 2 decades.7
Research tells us that women who use illicit drugs and alcohol categorically engage in some form of harm reduction once they learn they are pregnant, and the same is true for cannabis. This is seen in dramatically different rates of first- and third-trimester use in the new analysis of NSDUH data; third-trimester use is approximately halved.
Some women will not be able to discontinue use, however, or they may try to quit and fail in their attempts. As we should with substance use more broadly, we must meet patients where they are, view cannabis use as a chronic medical problem, offer our assistance in helping them reduce harms of their use, and understand that quitting is a process.
Screening for mental health disorders and trauma is, of course, especially important in patients who use cannabis and other substances recreationally. In cases of medical marijuana usage, I recommend, as ACOG and other have done, that we discuss the risks and benefits of continuing cannabis versus shifting to alternative medications if options exist.
All patients should be welcomed, congratulated on their pregnancy and on coming for prenatal care, and engaged in the overall process of optimizing their health and the health of their baby. Like any other health issue during pregnancy, cannabis use needs to be screened for and treated in an evidence-based manner, but it does not define the trajectory or success of a woman’s pregnancy or her ability to be a successful parent.
Dr. Mark is associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.
References
1. JAMA. 2019 Jul 9;322(2):145-52.
2. Preventive Medicine 2017 May 18;104:46-9.
3. JAMA. 2019 Jul 9;322(2):167-9.
4. JAMA Netw Open. 2019 Jul 3;2(7):e196471.
5. Obstet Gynecol. 2019 May;133(5):952-61.
6. J. Addict Med. 2019 May 10. doi: 10.1097/ADM.0000000000000543.
7. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
A flurry of research papers published this year has simultaneously documented a rise in the use of cannabis during pregnancy and offered more data about its potential harms. This confluence of findings is concerning and highlights the importance of screening our patients for cannabis use and engaging with them in a way in which we can maintain their trust and their commitment to prenatal care.
A retrospective cohort study involving 661,617 women in Ontario found a significant association between self-reported cannabis use in pregnancy and an increased risk of preterm birth (relative risk, 1.41), as well as a greater likelihood of small-for-gestational-age babies (RR, 1.53), placental abruption (RR, 1.72), and transfer to neonatal intensive care (RR, 1.40).1 The study, reported in JAMA in July 2019, carefully matched users with nonusers who had the same characteristics – for example, tobacco use or not.
This new information builds upon other meta-analyses that have demonstrated a decrease in birth weight and greater admittance to the neonatal ICU associated with cannabis use in pregnancy – and it supplements what some research suggests about long-term neurologic development and a potentially increased risk of attention and behavioral problems. Other outcomes that have been noted in long-term neurologic studies of children who were exposed to cannabis in utero include impaired visual acuity, verbal reasoning and comprehension, and short-term memory.2
Increases in use were recently documented in two studies. One, an analysis of data from the National Survey on Drug Use and Health (NSDUH) published in JAMA in June 2019, showed that, between 2002-2003 and 2016-2017, the use of cannabis “in the past month” increased from 3.4% to 7.0% among pregnant women overall, and from 6% to 12% during the first trimester.3
The use of cannabis on a daily or near-daily basis, moreover, increased from 0.9% to 3% among pregnant women overall and from 2% to 5% during the first trimester. The data were collected during face-to-face interviews and were adjusted for age, race/ethnicity, and family income.
In the second study – a cross-sectional study of 367,403 pregnancies among women who filled out a questionnaire on cannabis use during standard prenatal care at Kaiser Permanente Northern California – the adjusted prevalence of use in the year before pregnancy increased from 7% in 2009 to 13% in 2017, and the adjusted prevalence during pregnancy increased from 2% to 3%.4
As in the NSDUH analysis, daily use increased most rapidly (compared with weekly or monthly) such that, by 2017, 25% of those who reported using cannabis in the year before pregnancy – and 21% of those who used cannabis during pregnancy – were daily users. It is notable that Kaiser’s population is diverse in all respects, and that the annual relative rates of increase in cannabis use before and during pregnancy (at each level of frequency) were consistent across racial/ethnic and household income groups.
It’s also worth noting that, in earlier research covering a similar time period (2009-2016), the investigators found significant increases in use via urine toxicology testing that occurs at the first prenatal visit at Kaiser. The increase found through questionnaires, therefore, reflects more than a greater willingness to self-report.
Choosing a screening tool
Universal prenatal substance use screening is recommended by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, but we don’t have any specific recommendations on what this means. Who should be screening, and what should that screening look like? Should we use a biologic screen, a standardized screening tool, or simply ask patients whether they use illicit substances?
Screening tools seem advantageous in that they are low cost, noninvasive, potentially comprehensive, and not subject to false-positive results as biologic screens can be – but which tool or tools are best? There are several validated screening tools that can be used outside of pregnancy to determine an individual’s use of illicit substances and whether or not that use is problematic, but previous studies have not used biologic markers to validate substance use screeners in pregnancy. Nor have studies compared screeners in pregnancy.
In our prenatal population in Baltimore, we have not been getting the answers we want using various nonvalidated screening tools. Approximately 30% of patients are positive for cannabis by urine screen, but only half tell us about their use.
Through research in our two prenatal care practices (one serving mostly privately insured and the other serving primarily Medicaid-eligible patients), we assessed both the accuracy and the acceptability of three substance use screening tools that are brief and that have been validated (for the general population) by the World Health Organization for screening of multiple substances: the 4P’s Plus (Parents, Partner, Past, and Pregnancy), the National Institute on Drug Abuse Quick Screen–ASSIST (Modified Alcohol, Smoking and Substance Involvement Screening Test), and the SURP-P (Substance Use Risk Profile–Pregnancy) scale.
In one study, published in May 2019 in Obstetrics & Gynecology, we recruited 500 pregnant women and administered these three tests to each of them.5 We then compared results with those of urine and hair drug testing, and checked the test-retest reliability of each test by readministering them (albeit by telephone) a week later. Although hair testing is not an indicator of current substance use, we used it to validate the screening tools on less-recent use.
The tests with the highest sensitivity and negative predictive values – the qualities we most want for screening – were the SURP-P and the 4P’s Plus (sensitivity of 92.4% and 90.2%, respectively). Overall they were highly sensitive screening tools across all trimesters, races, and age groups, making them more ideal screening tests than the NIDA Quick Screen–ASSIST.
Of the two tests, the 4P’s Plus screening tool was the one preferred by staff from both practices. In a companion qualitative study, we conducted focus-group discussions with 40 practice staff who were responsible for administering or overseeing patient screening.6 The staff, who were unaware of the sensitivity findings, were asked what they thought about the acceptability to patients of each of the three tools and their usability in practice.
Most of the participating staff preferred the 4P’s Plus screening tool for several reasons: It is easy to understand, is brief and to the point, and it has nonjudgmental language and tone. The screener first asks the patient about her parents’ and her partner’s use of alcohol and drugs, and then asks the patient about her own use of alcohol and tobacco. Affirmative responses to these questions lead to additional questions.
The premise is that one’s genetics, history, and current exposures – as well as one’s own use of tobacco and alcohol – are significantly associated with the use of illicit substances. If the patient reports no parental history or partner usage, and has never drank or smoked before, it’s extremely unlikely that she is using other drugs. The progression of questions does indeed seem less judgmental than immediately asking: “Do you use drugs?”
For us, the insight from this staff perception study combined with the findings on accuracy mean that the 4P’s Plus may be the most useful and acceptable screening tool for routine use in prenatal care.
Talking with our patients
The increase in the use of cannabis before and after pregnancy parallels the movement toward state legalization and decriminalization. Historically, clinicians often have relied on illegality as their main focus of counseling when giving recommendations for cessation and abstinence in pregnancy.2 This approach not only leads to punitive counseling, which can fracture the doctor-patient relationship, but increasingly it is no longer valid. In our changing legal climate, we need to provide medically based counseling and be very clear with our patients that legalization does not equate to safety.
It is important that we neither minimize nor overstate the risks. The evidence base for adverse birth outcomes of cannabis use in pregnancy is quite robust, but the associations can be subtle and are moderated by other behaviors and environmental factors that continue to challenge researchers.
As with alcohol, there likely are dose-or trimester-dependent differences in perinatal outcomes, and it’s quite possible that different cannabis products and routes of consumption have different effects. At this point, however, we don’t know the full story, nor do we know the extent to which the literature is biased toward positive correlations – the reporting of adverse effects – compared with negative findings. It is our job as medical care providers to be comfortable in that gray area and to still counsel patients on what we do know, providing the best-possible medical advice based on the information available to us.
In talking with patients, I explain that cannabis may cause a spectrum of problems and that there certainly are risks. I also tell them that we’re uncertain about the conditions and magnitude of that risk and that some babies who are exposed to cannabis in utero may have no perceivable consequences. Such honesty is important for maintaining trust, especially as some patients may see friends and relatives who also are cannabis users have normal pregnancy outcomes.
Much of my concern about cannabis in pregnancy centers on its effect on the developing brain and on long-term neurologic development. I share this with patients – I tell them that cannabis crosses the placenta and may well affect their baby’s brain as it is developing. I explain that I do not know whether this effect would be big or small, but that it’s not a chance I’m willing to take for their baby.
It is also important to educate patients that cannabis products are untested and unregulated and that they may be contaminated with heavy metals, pesticides, and other toxins that may be harmful to themselves and their babies. Patients also should know that the potency of cannabis has been dramatically increasing; research shows that the tetrahydrocannabinol – the psychoactive component – concentration has tripled over the past 2 decades.7
Research tells us that women who use illicit drugs and alcohol categorically engage in some form of harm reduction once they learn they are pregnant, and the same is true for cannabis. This is seen in dramatically different rates of first- and third-trimester use in the new analysis of NSDUH data; third-trimester use is approximately halved.
Some women will not be able to discontinue use, however, or they may try to quit and fail in their attempts. As we should with substance use more broadly, we must meet patients where they are, view cannabis use as a chronic medical problem, offer our assistance in helping them reduce harms of their use, and understand that quitting is a process.
Screening for mental health disorders and trauma is, of course, especially important in patients who use cannabis and other substances recreationally. In cases of medical marijuana usage, I recommend, as ACOG and other have done, that we discuss the risks and benefits of continuing cannabis versus shifting to alternative medications if options exist.
All patients should be welcomed, congratulated on their pregnancy and on coming for prenatal care, and engaged in the overall process of optimizing their health and the health of their baby. Like any other health issue during pregnancy, cannabis use needs to be screened for and treated in an evidence-based manner, but it does not define the trajectory or success of a woman’s pregnancy or her ability to be a successful parent.
Dr. Mark is associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.
References
1. JAMA. 2019 Jul 9;322(2):145-52.
2. Preventive Medicine 2017 May 18;104:46-9.
3. JAMA. 2019 Jul 9;322(2):167-9.
4. JAMA Netw Open. 2019 Jul 3;2(7):e196471.
5. Obstet Gynecol. 2019 May;133(5):952-61.
6. J. Addict Med. 2019 May 10. doi: 10.1097/ADM.0000000000000543.
7. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
A flurry of research papers published this year has simultaneously documented a rise in the use of cannabis during pregnancy and offered more data about its potential harms. This confluence of findings is concerning and highlights the importance of screening our patients for cannabis use and engaging with them in a way in which we can maintain their trust and their commitment to prenatal care.
A retrospective cohort study involving 661,617 women in Ontario found a significant association between self-reported cannabis use in pregnancy and an increased risk of preterm birth (relative risk, 1.41), as well as a greater likelihood of small-for-gestational-age babies (RR, 1.53), placental abruption (RR, 1.72), and transfer to neonatal intensive care (RR, 1.40).1 The study, reported in JAMA in July 2019, carefully matched users with nonusers who had the same characteristics – for example, tobacco use or not.
This new information builds upon other meta-analyses that have demonstrated a decrease in birth weight and greater admittance to the neonatal ICU associated with cannabis use in pregnancy – and it supplements what some research suggests about long-term neurologic development and a potentially increased risk of attention and behavioral problems. Other outcomes that have been noted in long-term neurologic studies of children who were exposed to cannabis in utero include impaired visual acuity, verbal reasoning and comprehension, and short-term memory.2
Increases in use were recently documented in two studies. One, an analysis of data from the National Survey on Drug Use and Health (NSDUH) published in JAMA in June 2019, showed that, between 2002-2003 and 2016-2017, the use of cannabis “in the past month” increased from 3.4% to 7.0% among pregnant women overall, and from 6% to 12% during the first trimester.3
The use of cannabis on a daily or near-daily basis, moreover, increased from 0.9% to 3% among pregnant women overall and from 2% to 5% during the first trimester. The data were collected during face-to-face interviews and were adjusted for age, race/ethnicity, and family income.
In the second study – a cross-sectional study of 367,403 pregnancies among women who filled out a questionnaire on cannabis use during standard prenatal care at Kaiser Permanente Northern California – the adjusted prevalence of use in the year before pregnancy increased from 7% in 2009 to 13% in 2017, and the adjusted prevalence during pregnancy increased from 2% to 3%.4
As in the NSDUH analysis, daily use increased most rapidly (compared with weekly or monthly) such that, by 2017, 25% of those who reported using cannabis in the year before pregnancy – and 21% of those who used cannabis during pregnancy – were daily users. It is notable that Kaiser’s population is diverse in all respects, and that the annual relative rates of increase in cannabis use before and during pregnancy (at each level of frequency) were consistent across racial/ethnic and household income groups.
It’s also worth noting that, in earlier research covering a similar time period (2009-2016), the investigators found significant increases in use via urine toxicology testing that occurs at the first prenatal visit at Kaiser. The increase found through questionnaires, therefore, reflects more than a greater willingness to self-report.
Choosing a screening tool
Universal prenatal substance use screening is recommended by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, but we don’t have any specific recommendations on what this means. Who should be screening, and what should that screening look like? Should we use a biologic screen, a standardized screening tool, or simply ask patients whether they use illicit substances?
Screening tools seem advantageous in that they are low cost, noninvasive, potentially comprehensive, and not subject to false-positive results as biologic screens can be – but which tool or tools are best? There are several validated screening tools that can be used outside of pregnancy to determine an individual’s use of illicit substances and whether or not that use is problematic, but previous studies have not used biologic markers to validate substance use screeners in pregnancy. Nor have studies compared screeners in pregnancy.
In our prenatal population in Baltimore, we have not been getting the answers we want using various nonvalidated screening tools. Approximately 30% of patients are positive for cannabis by urine screen, but only half tell us about their use.
Through research in our two prenatal care practices (one serving mostly privately insured and the other serving primarily Medicaid-eligible patients), we assessed both the accuracy and the acceptability of three substance use screening tools that are brief and that have been validated (for the general population) by the World Health Organization for screening of multiple substances: the 4P’s Plus (Parents, Partner, Past, and Pregnancy), the National Institute on Drug Abuse Quick Screen–ASSIST (Modified Alcohol, Smoking and Substance Involvement Screening Test), and the SURP-P (Substance Use Risk Profile–Pregnancy) scale.
In one study, published in May 2019 in Obstetrics & Gynecology, we recruited 500 pregnant women and administered these three tests to each of them.5 We then compared results with those of urine and hair drug testing, and checked the test-retest reliability of each test by readministering them (albeit by telephone) a week later. Although hair testing is not an indicator of current substance use, we used it to validate the screening tools on less-recent use.
The tests with the highest sensitivity and negative predictive values – the qualities we most want for screening – were the SURP-P and the 4P’s Plus (sensitivity of 92.4% and 90.2%, respectively). Overall they were highly sensitive screening tools across all trimesters, races, and age groups, making them more ideal screening tests than the NIDA Quick Screen–ASSIST.
Of the two tests, the 4P’s Plus screening tool was the one preferred by staff from both practices. In a companion qualitative study, we conducted focus-group discussions with 40 practice staff who were responsible for administering or overseeing patient screening.6 The staff, who were unaware of the sensitivity findings, were asked what they thought about the acceptability to patients of each of the three tools and their usability in practice.
Most of the participating staff preferred the 4P’s Plus screening tool for several reasons: It is easy to understand, is brief and to the point, and it has nonjudgmental language and tone. The screener first asks the patient about her parents’ and her partner’s use of alcohol and drugs, and then asks the patient about her own use of alcohol and tobacco. Affirmative responses to these questions lead to additional questions.
The premise is that one’s genetics, history, and current exposures – as well as one’s own use of tobacco and alcohol – are significantly associated with the use of illicit substances. If the patient reports no parental history or partner usage, and has never drank or smoked before, it’s extremely unlikely that she is using other drugs. The progression of questions does indeed seem less judgmental than immediately asking: “Do you use drugs?”
For us, the insight from this staff perception study combined with the findings on accuracy mean that the 4P’s Plus may be the most useful and acceptable screening tool for routine use in prenatal care.
Talking with our patients
The increase in the use of cannabis before and after pregnancy parallels the movement toward state legalization and decriminalization. Historically, clinicians often have relied on illegality as their main focus of counseling when giving recommendations for cessation and abstinence in pregnancy.2 This approach not only leads to punitive counseling, which can fracture the doctor-patient relationship, but increasingly it is no longer valid. In our changing legal climate, we need to provide medically based counseling and be very clear with our patients that legalization does not equate to safety.
It is important that we neither minimize nor overstate the risks. The evidence base for adverse birth outcomes of cannabis use in pregnancy is quite robust, but the associations can be subtle and are moderated by other behaviors and environmental factors that continue to challenge researchers.
As with alcohol, there likely are dose-or trimester-dependent differences in perinatal outcomes, and it’s quite possible that different cannabis products and routes of consumption have different effects. At this point, however, we don’t know the full story, nor do we know the extent to which the literature is biased toward positive correlations – the reporting of adverse effects – compared with negative findings. It is our job as medical care providers to be comfortable in that gray area and to still counsel patients on what we do know, providing the best-possible medical advice based on the information available to us.
In talking with patients, I explain that cannabis may cause a spectrum of problems and that there certainly are risks. I also tell them that we’re uncertain about the conditions and magnitude of that risk and that some babies who are exposed to cannabis in utero may have no perceivable consequences. Such honesty is important for maintaining trust, especially as some patients may see friends and relatives who also are cannabis users have normal pregnancy outcomes.
Much of my concern about cannabis in pregnancy centers on its effect on the developing brain and on long-term neurologic development. I share this with patients – I tell them that cannabis crosses the placenta and may well affect their baby’s brain as it is developing. I explain that I do not know whether this effect would be big or small, but that it’s not a chance I’m willing to take for their baby.
It is also important to educate patients that cannabis products are untested and unregulated and that they may be contaminated with heavy metals, pesticides, and other toxins that may be harmful to themselves and their babies. Patients also should know that the potency of cannabis has been dramatically increasing; research shows that the tetrahydrocannabinol – the psychoactive component – concentration has tripled over the past 2 decades.7
Research tells us that women who use illicit drugs and alcohol categorically engage in some form of harm reduction once they learn they are pregnant, and the same is true for cannabis. This is seen in dramatically different rates of first- and third-trimester use in the new analysis of NSDUH data; third-trimester use is approximately halved.
Some women will not be able to discontinue use, however, or they may try to quit and fail in their attempts. As we should with substance use more broadly, we must meet patients where they are, view cannabis use as a chronic medical problem, offer our assistance in helping them reduce harms of their use, and understand that quitting is a process.
Screening for mental health disorders and trauma is, of course, especially important in patients who use cannabis and other substances recreationally. In cases of medical marijuana usage, I recommend, as ACOG and other have done, that we discuss the risks and benefits of continuing cannabis versus shifting to alternative medications if options exist.
All patients should be welcomed, congratulated on their pregnancy and on coming for prenatal care, and engaged in the overall process of optimizing their health and the health of their baby. Like any other health issue during pregnancy, cannabis use needs to be screened for and treated in an evidence-based manner, but it does not define the trajectory or success of a woman’s pregnancy or her ability to be a successful parent.
Dr. Mark is associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.
References
1. JAMA. 2019 Jul 9;322(2):145-52.
2. Preventive Medicine 2017 May 18;104:46-9.
3. JAMA. 2019 Jul 9;322(2):167-9.
4. JAMA Netw Open. 2019 Jul 3;2(7):e196471.
5. Obstet Gynecol. 2019 May;133(5):952-61.
6. J. Addict Med. 2019 May 10. doi: 10.1097/ADM.0000000000000543.
7. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
Product Update: Addyi alcohol ban lifted, fezolinetant trial, outcomes tracker, comfort gown
FDA REMOVES ALCOHOL BAN WITH ADDYI
Sprout Pharmaceuticals announced that the US Food and Drug Administration (FDA) has removed their contraindication on alcohol use with Addyi® (flibanserin). Addyi was approved in 2015 and is an oral nonhormonal pill for acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women. Patients are advised to discontinue drinking alcohol at least 2 hours before taking Addyi at bedtime or skip the Addyi dose that evening.
The FDA also removed the requirement, under its Risk Evaluation and Mitigation Strategy (REMS) program, for health care practitioners or pharmacies to be certified to prescribe or dispense Addyi. Sprout says that to make all labeling elements consistent with the FDA’s findings the boxed warning will change and the medication guide will be updated and included under the REMS.
The most commonly reported adverse events among patients taking Addyi are dizziness, sleepiness, nausea, fatigue, insomnia, and dry mouth. Addyi is contraindicated in patients taking moderate or strong cytochrome P450 3A4 (CYP3A4) inhibitors and in those with hepatic impairment.
FOR MORE INFORMATION AND THE FULL PRESCRIBING INFORMATION AND MEDICATION GUIDE, VISIT: www.addyi.com
FEZOLINETANT FOR VMS
FOR MORE INFORMATION, VISIT: http://www.clinicaltrials.gov, TRIAL IDENTIFIERS NCT04003155, NCT04003142, AND NCT04003389
SOLUTIONS FOR OUTCOME TRACKING
DrChrono and OutcomeMD announce a partnership to track and analyze patient outcome data and confounding factors. DrChrono is an electronic health record (EHR) system, and OutcomeMD is a software solution that uses literature-validated patient-reported outcome instruments to score and track a patient’s symptom severity and inform treatment decisions for users.
Via a HIPAA compliant process, patients answer a list of questions that are accessed through a web link on their mobile or desktop devices. OutcomeMD summarizes the symptoms into a score that displays to both the physician and patient. Patients’ answers and scores are pushed to the clinician’s DrChrono EHR medical note.
FOR MORE INFORMATION, VISIT: www.outcomemd.com
Continue to: NEW MATERNITY GOWN...
NEW MATERNITY GOWN
ImageFIRST launched a new maternity gown for expecting mothers. The Comfort Care® Maternity Gown is a lightweight, premium polyester/nylon fabric that front snaps to allow for skin-to-skin access and optional breastfeeding. The gown also includes shoulder snaps and a full cut for extra coverage and to accommodate a variety of body types, says ImageFIRST.
ImageFIRST is a national linen rental provider. It developed the Comfort Care® Maternity Gown with input from labor and delivery departments to best meet the needs of expecting mothers. It also says that a portion of the proceeds from each gown rental will be donated to the National Pediatric Cancer Foundation.
FOR MORE INFORMATION, VISIT: www.imagefirst.com
FDA REMOVES ALCOHOL BAN WITH ADDYI
Sprout Pharmaceuticals announced that the US Food and Drug Administration (FDA) has removed their contraindication on alcohol use with Addyi® (flibanserin). Addyi was approved in 2015 and is an oral nonhormonal pill for acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women. Patients are advised to discontinue drinking alcohol at least 2 hours before taking Addyi at bedtime or skip the Addyi dose that evening.
The FDA also removed the requirement, under its Risk Evaluation and Mitigation Strategy (REMS) program, for health care practitioners or pharmacies to be certified to prescribe or dispense Addyi. Sprout says that to make all labeling elements consistent with the FDA’s findings the boxed warning will change and the medication guide will be updated and included under the REMS.
The most commonly reported adverse events among patients taking Addyi are dizziness, sleepiness, nausea, fatigue, insomnia, and dry mouth. Addyi is contraindicated in patients taking moderate or strong cytochrome P450 3A4 (CYP3A4) inhibitors and in those with hepatic impairment.
FOR MORE INFORMATION AND THE FULL PRESCRIBING INFORMATION AND MEDICATION GUIDE, VISIT: www.addyi.com
FEZOLINETANT FOR VMS
FOR MORE INFORMATION, VISIT: http://www.clinicaltrials.gov, TRIAL IDENTIFIERS NCT04003155, NCT04003142, AND NCT04003389
SOLUTIONS FOR OUTCOME TRACKING
DrChrono and OutcomeMD announce a partnership to track and analyze patient outcome data and confounding factors. DrChrono is an electronic health record (EHR) system, and OutcomeMD is a software solution that uses literature-validated patient-reported outcome instruments to score and track a patient’s symptom severity and inform treatment decisions for users.
Via a HIPAA compliant process, patients answer a list of questions that are accessed through a web link on their mobile or desktop devices. OutcomeMD summarizes the symptoms into a score that displays to both the physician and patient. Patients’ answers and scores are pushed to the clinician’s DrChrono EHR medical note.
FOR MORE INFORMATION, VISIT: www.outcomemd.com
Continue to: NEW MATERNITY GOWN...
NEW MATERNITY GOWN
ImageFIRST launched a new maternity gown for expecting mothers. The Comfort Care® Maternity Gown is a lightweight, premium polyester/nylon fabric that front snaps to allow for skin-to-skin access and optional breastfeeding. The gown also includes shoulder snaps and a full cut for extra coverage and to accommodate a variety of body types, says ImageFIRST.
ImageFIRST is a national linen rental provider. It developed the Comfort Care® Maternity Gown with input from labor and delivery departments to best meet the needs of expecting mothers. It also says that a portion of the proceeds from each gown rental will be donated to the National Pediatric Cancer Foundation.
FOR MORE INFORMATION, VISIT: www.imagefirst.com
FDA REMOVES ALCOHOL BAN WITH ADDYI
Sprout Pharmaceuticals announced that the US Food and Drug Administration (FDA) has removed their contraindication on alcohol use with Addyi® (flibanserin). Addyi was approved in 2015 and is an oral nonhormonal pill for acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women. Patients are advised to discontinue drinking alcohol at least 2 hours before taking Addyi at bedtime or skip the Addyi dose that evening.
The FDA also removed the requirement, under its Risk Evaluation and Mitigation Strategy (REMS) program, for health care practitioners or pharmacies to be certified to prescribe or dispense Addyi. Sprout says that to make all labeling elements consistent with the FDA’s findings the boxed warning will change and the medication guide will be updated and included under the REMS.
The most commonly reported adverse events among patients taking Addyi are dizziness, sleepiness, nausea, fatigue, insomnia, and dry mouth. Addyi is contraindicated in patients taking moderate or strong cytochrome P450 3A4 (CYP3A4) inhibitors and in those with hepatic impairment.
FOR MORE INFORMATION AND THE FULL PRESCRIBING INFORMATION AND MEDICATION GUIDE, VISIT: www.addyi.com
FEZOLINETANT FOR VMS
FOR MORE INFORMATION, VISIT: http://www.clinicaltrials.gov, TRIAL IDENTIFIERS NCT04003155, NCT04003142, AND NCT04003389
SOLUTIONS FOR OUTCOME TRACKING
DrChrono and OutcomeMD announce a partnership to track and analyze patient outcome data and confounding factors. DrChrono is an electronic health record (EHR) system, and OutcomeMD is a software solution that uses literature-validated patient-reported outcome instruments to score and track a patient’s symptom severity and inform treatment decisions for users.
Via a HIPAA compliant process, patients answer a list of questions that are accessed through a web link on their mobile or desktop devices. OutcomeMD summarizes the symptoms into a score that displays to both the physician and patient. Patients’ answers and scores are pushed to the clinician’s DrChrono EHR medical note.
FOR MORE INFORMATION, VISIT: www.outcomemd.com
Continue to: NEW MATERNITY GOWN...
NEW MATERNITY GOWN
ImageFIRST launched a new maternity gown for expecting mothers. The Comfort Care® Maternity Gown is a lightweight, premium polyester/nylon fabric that front snaps to allow for skin-to-skin access and optional breastfeeding. The gown also includes shoulder snaps and a full cut for extra coverage and to accommodate a variety of body types, says ImageFIRST.
ImageFIRST is a national linen rental provider. It developed the Comfort Care® Maternity Gown with input from labor and delivery departments to best meet the needs of expecting mothers. It also says that a portion of the proceeds from each gown rental will be donated to the National Pediatric Cancer Foundation.
FOR MORE INFORMATION, VISIT: www.imagefirst.com
What every ObGyn should know about Supreme Court rulings in the recent term
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.