User login
Asthma exacerbation in pregnancy impacts mothers, infants
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
FROM THE EUROPEAN RESPIRATORY JOURNAL
Do ObGyns think the EMR has improved patient care?
In the roundtable article, “The electronic medical record’s role in ObGyn burnout and patient care” (October 2019), Megan L. Evans, MD, MPH; John J. Dougherty, MD, MBA; and Mark B. Woodland, MS, MD, discussed burnout’s connection with the electronic medical record (EMR) and solutions implemented at their institutions to help cope with the problem. They highlighted changes they felt their EMR systems needed to undergo. In addition, they noted as a whole that the EMR has not improved patient care.
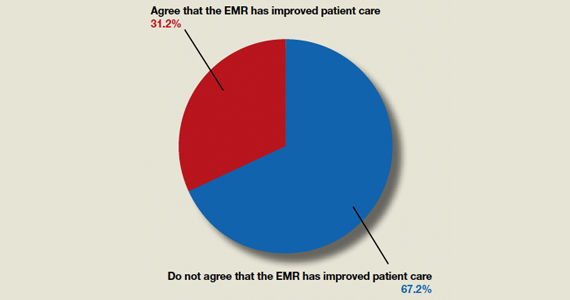
OBG Management polled readers to see their thoughts on this question: “Do you think that the EMR has improved patient care?”
A total of 123 readers cast their vote:
67.2% (84 readers)said no
31.2% (39 readers)said yes
In the roundtable article, “The electronic medical record’s role in ObGyn burnout and patient care” (October 2019), Megan L. Evans, MD, MPH; John J. Dougherty, MD, MBA; and Mark B. Woodland, MS, MD, discussed burnout’s connection with the electronic medical record (EMR) and solutions implemented at their institutions to help cope with the problem. They highlighted changes they felt their EMR systems needed to undergo. In addition, they noted as a whole that the EMR has not improved patient care.
OBG Management polled readers to see their thoughts on this question: “Do you think that the EMR has improved patient care?”
A total of 123 readers cast their vote:
67.2% (84 readers)said no
31.2% (39 readers)said yes

In the roundtable article, “The electronic medical record’s role in ObGyn burnout and patient care” (October 2019), Megan L. Evans, MD, MPH; John J. Dougherty, MD, MBA; and Mark B. Woodland, MS, MD, discussed burnout’s connection with the electronic medical record (EMR) and solutions implemented at their institutions to help cope with the problem. They highlighted changes they felt their EMR systems needed to undergo. In addition, they noted as a whole that the EMR has not improved patient care.
OBG Management polled readers to see their thoughts on this question: “Do you think that the EMR has improved patient care?”
A total of 123 readers cast their vote:
67.2% (84 readers)said no
31.2% (39 readers)said yes

Managing preterm birth in those at risk: Expert strategies
Obstetricians face the potential practice dilemma of having withdrawn from the market the only drug approved by the US Food and Drug Administration (FDA) for the prevention of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. In the recently published PROLONG (Progestin's Role in Optimizing Neonatal Gestation) study by Blackwell and colleagues, the trial results revealed that there were no significant differences in preterm birth between women treated with 17 α-hydroxyprogesterone caproate (17P; Makena) and those who received placebo.1 For study details and comments, see "Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?" by Michael House, MD, and Errol Norwitz, MD, PhD, MBA. Subsequently, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to recommend pursuit of approval withdrawal for 17P.
To assess how experienced obstetricians would manage women with previous preterm birth if 17P became unavailable, OBG Management conducted an informal survey. Here, 4 experts respond to the question, "What are you going to do in your practice for women with a history of a previous preterm birth if 17P is no longer an option?"
Not ready to leave behind 17P for recurrent preterm delivery
Patrick Duff, MD
Preterm delivery is arguably the most important problem in perinatal medicine. It occurs in 10% to 12% of all obstetric patients in the United States, and complications of prematurity account for the majority of neonatal deaths. A major risk factor for recurrent preterm delivery is a prior history of spontaneous preterm delivery, with or without preterm premature rupture of membranes. Clearly, prevention of recurrence is of paramount importance.
In the Maternal-Fetal Medicine Units (MFMU) Network trial, Meis and colleagues demonstrated a 34% reduction (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81) in the risk of recurrent preterm delivery in women who received weekly 250-mg injections of 17P (also called 17-OHPC). After publication of that trial, use of 17P became accepted practice in the United States.2
The PROLONG study by Blackwell and colleagues questions the value of 17P.1 In that international trial, which included 1,708 women from 41 centers in the United States and 52 outside the United States, the authors were unable to show any significant difference in the frequency of preterm delivery < 35 weeks (11.0% in the women receiving 17P and 11.5% in women receiving placebo; RR, 0.95; 95% CI, 0.71-1.26). Even when they examined the subset of women treated at US medical centers, they could not demonstrate any significant difference in treatment outcome.
At least 2 major explanations account for the discrepancy between the MFMU and the Blackwell studies. First, the participants in the PROLONG trial were clearly not at the same increased risk for recurrent preterm delivery as those in the MFMU trial. Second, in the PROLONG trial only the minority of participants were from the United States. In fact, given the relatively low rate of recurrent preterm delivery in the PROLONG trial, the study was underpowered to detect meaningful differences in maternal outcome. Therefore, I am not ready to abandon the use of progesterone supplementation in women at risk for recurrent preterm delivery.
Continue to: If the FDA removes 17P from the market...
If the FDA removes 17P from the market, my approach with at-risk patients will be as follows:
- I will encourage all at-risk women to eliminate obvious risk factors, such as smoking, illicit drug use, and excessive physical activity.
- I will encourage optimal nutrition and appropriate weight gain.
- I will test all patients for chlamydia, gonorrhea, and bacterial vaginosis and treat women who are infected.
- After the patient completes the first trimester, I will treat her with micronized progesterone, 200 mg daily, intravaginally. I will continue this medication until 36 to 37 weeks.
- I will perform an assessment of cervical length at 16, 20, and 24 weeks' gestation. In patients with demonstrable cervical shortening, I will perform a cerclage.
Rational management options for reducing risk of preterm delivery
Alex C. Vidaeff, MD, MPH
Most women who experience a spontaneous preterm delivery (sPTD) do not deliver prematurely in subsequent pregnancies.3 Two recent systematic reviews, in 2014 and 2017, found an overall risk of recurrent sPTD of 20.2% and 30%, respectively.4,5 These numbers are closer to the background event rate of 21.9% in the PROLONG trial, while only a few women have a recurrence risk of more than 50%, as in the Meis MFMU trial.1,2 A public health recommendation cannot be made for an intervention that is expected to work only in rare cases and fail in a majority of cases. Therefore, 17P is no longer a viable option for preventing recurrence in pregnant women with a history of sPTD, with only rare possible exceptions.
What evidence-based alternatives can be offered to pregnant women who had a previous sPTD?
Ultrasound assessment of cervical length has emerged as an effective prognosticator for recurrence in women with a prior sPTD, being able to predict 65.4% of sPTDs at a false-positive rate of 5%.6,7 Furthermore, sonographic cervical length measurements identify high-risk women who may not need any intervention. It has been shown that, among women with prior sPTD who maintain a normal cervical length up to 24 weeks, more than 90% will deliver at 35 weeks or after without intervention.8
In the United States, interventions to reduce sPTD, once a short cervix has been identified, include vaginal progesterone supplementation and cerclage. The benefit from vaginal progesterone has been documented by an individual patient data meta-analysis, while the benefit of cerclage has been highlighted in a Cochrane Review.9,10 The results of an adjusted indirect comparison meta-analysis suggest that both interventions are equally effective.11 Therefore, the decision on how best to minimize the risk of recurrent sPTD must be individualized based on historical and clinical circumstances, as well as the woman's informed choice.
Based on current data, the following approach appears rational to me:
- Cervical ultrasound surveillance between 16 and 24 weeks' gestation to identify the subgroup of women at significantly increased risk of sPTD recurrence.
- With cervical length ≤ 25 mm, vaginal progesterone supplementation may be considered. Preferential consideration for progesterone may be given when lower genital tract inflammation is suspected, given the possible anti-inflammatory action of progesterone.12,13
- If cervical shortening progresses to 15 to 20 mm, cerclage may be considered. Waiting for a cervix < 15 mm may be unadvisable. In conditions of a very short cervix, frequently dilated, with exposure of the fetal membranes, ascending subclinical intra-amniotic infection already may be present, reducing the efficacy of cerclage. Preferential consideration for cerclage also may be given with 2 sPTDs or mid-trimester losses or with a history of a successful cerclage.
Continue to: Screen cervical length early, and use cerclage or vaginal progesterone as appropriate...
Screen cervical length early, and use cerclage or vaginal progesterone as appropriate
Michael G. Ross, MD, MPH
In patients with a history of a previous preterm birth, if 17P is no longer an option, I would revert to screening for short cervix with transvaginal ultrasound.
Screen all high-risk patients at the first prenatal visit, so as not to miss a short cervix before 16 weeks' gestation. Then, beginning at 16 weeks, screen every 2 weeks until approximately 24 weeks.
If the cervix shortens to 25 mm or less, offer cerclage or vaginal progesterone. If the cervix shortens to 20 mm or less, I would strongly support cerclage or vaginal progesterone.
Use of 17P is still an option, for now
Errol R. Norwitz, MD, PhD, MBA
The way in which 17P was handled by the FDA is exactly the way the system is designed to work; this should be seen as a success, not a failure.
Given the urgent need for an intervention to prevent preterm birth, the lack of any alternative, and a single, well-designed randomized controlled trial that confirmed safety and suggested some benefit, the FDA approved 17P supplementation in February 2011 for a limited indication only—one or more prior unexplained sPTD—using the expedited review mechanism.2 Under this mechanism, a follow-up clinical trial is required to confirm efficacy. This was the PROLONG trial, which failed to show any significant benefit of 17P supplementation in terms of either preterm birth prevention or neonatal outcome.1
In October 2019, an FDA advisory committee met again to review these and other data. After presentations from a range of stakeholders and a robust discussion, the advisory committee voted to pursue approval withdrawal of 17P due to the lack of consistent evidence of benefit (it is important to note that this was not because of safety concerns). This is exactly the way the process is designed to work.
Where does this leave physicians and patients? It is clear that progesterone supplementation is not a panacea for preterm birth prevention and is not indicated for all women at high risk, even those with one or more prior unexplained sPTDs. Given that preterm birth is a syndrome and not a single diagnosis, it is still possible that there is a subgroup of women who may benefit from this intervention. For this reason—and because there is no clear alternative and no known downside to the administration of this drug (other than cost)—physicians still may choose to discuss this option with their patients and, after counseling, patients still may choose to accept it. If in doubt, engage the "shared decision-making model"; talk to your patients.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Iams JD, Goldenberg RL, Mercer BM, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. The Preterm Prediction Study: recurrence of spontaneous preterm birth. Am J Obstet Gynecol. 1998;178:1035-1040.
- Kazemier BM, Buijs PE, Mignini L, et al; EBM CONNECT. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: a systematic review. BJOG. 2014;121:1197-1208.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Owen J, Yost N, Berghella V, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340-1348.
- To MS, Skentou CA, Royston P, et al. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362-367.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Sakai M, Shiozaki A, Tabata M, et al. Evaluation of effectiveness of prophylactic cerclage of a short cervix according to interleukin-8 in cervical mucus. Am J Obstet Gynecol. 2006;194:14-19.
- Vidaeff AC, Ramin SM, Gilstrap LC, et al. Impact of progesterone on cytokine-stimulated nuclear factor-kappa B signaling in HeLa cells. J Matern Fetal Neonatal Med. 2007;20:23-28.
Obstetricians face the potential practice dilemma of having withdrawn from the market the only drug approved by the US Food and Drug Administration (FDA) for the prevention of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. In the recently published PROLONG (Progestin's Role in Optimizing Neonatal Gestation) study by Blackwell and colleagues, the trial results revealed that there were no significant differences in preterm birth between women treated with 17 α-hydroxyprogesterone caproate (17P; Makena) and those who received placebo.1 For study details and comments, see "Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?" by Michael House, MD, and Errol Norwitz, MD, PhD, MBA. Subsequently, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to recommend pursuit of approval withdrawal for 17P.
To assess how experienced obstetricians would manage women with previous preterm birth if 17P became unavailable, OBG Management conducted an informal survey. Here, 4 experts respond to the question, "What are you going to do in your practice for women with a history of a previous preterm birth if 17P is no longer an option?"
Not ready to leave behind 17P for recurrent preterm delivery
Patrick Duff, MD
Preterm delivery is arguably the most important problem in perinatal medicine. It occurs in 10% to 12% of all obstetric patients in the United States, and complications of prematurity account for the majority of neonatal deaths. A major risk factor for recurrent preterm delivery is a prior history of spontaneous preterm delivery, with or without preterm premature rupture of membranes. Clearly, prevention of recurrence is of paramount importance.
In the Maternal-Fetal Medicine Units (MFMU) Network trial, Meis and colleagues demonstrated a 34% reduction (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81) in the risk of recurrent preterm delivery in women who received weekly 250-mg injections of 17P (also called 17-OHPC). After publication of that trial, use of 17P became accepted practice in the United States.2
The PROLONG study by Blackwell and colleagues questions the value of 17P.1 In that international trial, which included 1,708 women from 41 centers in the United States and 52 outside the United States, the authors were unable to show any significant difference in the frequency of preterm delivery < 35 weeks (11.0% in the women receiving 17P and 11.5% in women receiving placebo; RR, 0.95; 95% CI, 0.71-1.26). Even when they examined the subset of women treated at US medical centers, they could not demonstrate any significant difference in treatment outcome.
At least 2 major explanations account for the discrepancy between the MFMU and the Blackwell studies. First, the participants in the PROLONG trial were clearly not at the same increased risk for recurrent preterm delivery as those in the MFMU trial. Second, in the PROLONG trial only the minority of participants were from the United States. In fact, given the relatively low rate of recurrent preterm delivery in the PROLONG trial, the study was underpowered to detect meaningful differences in maternal outcome. Therefore, I am not ready to abandon the use of progesterone supplementation in women at risk for recurrent preterm delivery.
Continue to: If the FDA removes 17P from the market...
If the FDA removes 17P from the market, my approach with at-risk patients will be as follows:
- I will encourage all at-risk women to eliminate obvious risk factors, such as smoking, illicit drug use, and excessive physical activity.
- I will encourage optimal nutrition and appropriate weight gain.
- I will test all patients for chlamydia, gonorrhea, and bacterial vaginosis and treat women who are infected.
- After the patient completes the first trimester, I will treat her with micronized progesterone, 200 mg daily, intravaginally. I will continue this medication until 36 to 37 weeks.
- I will perform an assessment of cervical length at 16, 20, and 24 weeks' gestation. In patients with demonstrable cervical shortening, I will perform a cerclage.
Rational management options for reducing risk of preterm delivery
Alex C. Vidaeff, MD, MPH
Most women who experience a spontaneous preterm delivery (sPTD) do not deliver prematurely in subsequent pregnancies.3 Two recent systematic reviews, in 2014 and 2017, found an overall risk of recurrent sPTD of 20.2% and 30%, respectively.4,5 These numbers are closer to the background event rate of 21.9% in the PROLONG trial, while only a few women have a recurrence risk of more than 50%, as in the Meis MFMU trial.1,2 A public health recommendation cannot be made for an intervention that is expected to work only in rare cases and fail in a majority of cases. Therefore, 17P is no longer a viable option for preventing recurrence in pregnant women with a history of sPTD, with only rare possible exceptions.
What evidence-based alternatives can be offered to pregnant women who had a previous sPTD?
Ultrasound assessment of cervical length has emerged as an effective prognosticator for recurrence in women with a prior sPTD, being able to predict 65.4% of sPTDs at a false-positive rate of 5%.6,7 Furthermore, sonographic cervical length measurements identify high-risk women who may not need any intervention. It has been shown that, among women with prior sPTD who maintain a normal cervical length up to 24 weeks, more than 90% will deliver at 35 weeks or after without intervention.8
In the United States, interventions to reduce sPTD, once a short cervix has been identified, include vaginal progesterone supplementation and cerclage. The benefit from vaginal progesterone has been documented by an individual patient data meta-analysis, while the benefit of cerclage has been highlighted in a Cochrane Review.9,10 The results of an adjusted indirect comparison meta-analysis suggest that both interventions are equally effective.11 Therefore, the decision on how best to minimize the risk of recurrent sPTD must be individualized based on historical and clinical circumstances, as well as the woman's informed choice.
Based on current data, the following approach appears rational to me:
- Cervical ultrasound surveillance between 16 and 24 weeks' gestation to identify the subgroup of women at significantly increased risk of sPTD recurrence.
- With cervical length ≤ 25 mm, vaginal progesterone supplementation may be considered. Preferential consideration for progesterone may be given when lower genital tract inflammation is suspected, given the possible anti-inflammatory action of progesterone.12,13
- If cervical shortening progresses to 15 to 20 mm, cerclage may be considered. Waiting for a cervix < 15 mm may be unadvisable. In conditions of a very short cervix, frequently dilated, with exposure of the fetal membranes, ascending subclinical intra-amniotic infection already may be present, reducing the efficacy of cerclage. Preferential consideration for cerclage also may be given with 2 sPTDs or mid-trimester losses or with a history of a successful cerclage.
Continue to: Screen cervical length early, and use cerclage or vaginal progesterone as appropriate...
Screen cervical length early, and use cerclage or vaginal progesterone as appropriate
Michael G. Ross, MD, MPH
In patients with a history of a previous preterm birth, if 17P is no longer an option, I would revert to screening for short cervix with transvaginal ultrasound.
Screen all high-risk patients at the first prenatal visit, so as not to miss a short cervix before 16 weeks' gestation. Then, beginning at 16 weeks, screen every 2 weeks until approximately 24 weeks.
If the cervix shortens to 25 mm or less, offer cerclage or vaginal progesterone. If the cervix shortens to 20 mm or less, I would strongly support cerclage or vaginal progesterone.
Use of 17P is still an option, for now
Errol R. Norwitz, MD, PhD, MBA
The way in which 17P was handled by the FDA is exactly the way the system is designed to work; this should be seen as a success, not a failure.
Given the urgent need for an intervention to prevent preterm birth, the lack of any alternative, and a single, well-designed randomized controlled trial that confirmed safety and suggested some benefit, the FDA approved 17P supplementation in February 2011 for a limited indication only—one or more prior unexplained sPTD—using the expedited review mechanism.2 Under this mechanism, a follow-up clinical trial is required to confirm efficacy. This was the PROLONG trial, which failed to show any significant benefit of 17P supplementation in terms of either preterm birth prevention or neonatal outcome.1
In October 2019, an FDA advisory committee met again to review these and other data. After presentations from a range of stakeholders and a robust discussion, the advisory committee voted to pursue approval withdrawal of 17P due to the lack of consistent evidence of benefit (it is important to note that this was not because of safety concerns). This is exactly the way the process is designed to work.
Where does this leave physicians and patients? It is clear that progesterone supplementation is not a panacea for preterm birth prevention and is not indicated for all women at high risk, even those with one or more prior unexplained sPTDs. Given that preterm birth is a syndrome and not a single diagnosis, it is still possible that there is a subgroup of women who may benefit from this intervention. For this reason—and because there is no clear alternative and no known downside to the administration of this drug (other than cost)—physicians still may choose to discuss this option with their patients and, after counseling, patients still may choose to accept it. If in doubt, engage the "shared decision-making model"; talk to your patients.
Obstetricians face the potential practice dilemma of having withdrawn from the market the only drug approved by the US Food and Drug Administration (FDA) for the prevention of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. In the recently published PROLONG (Progestin's Role in Optimizing Neonatal Gestation) study by Blackwell and colleagues, the trial results revealed that there were no significant differences in preterm birth between women treated with 17 α-hydroxyprogesterone caproate (17P; Makena) and those who received placebo.1 For study details and comments, see "Progesterone supplementation does not PROLONG pregnancy in women at risk for preterm birth: What do we do now?" by Michael House, MD, and Errol Norwitz, MD, PhD, MBA. Subsequently, the FDA's Bone, Reproductive and Urologic Drugs Advisory Committee voted 9-7 to recommend pursuit of approval withdrawal for 17P.
To assess how experienced obstetricians would manage women with previous preterm birth if 17P became unavailable, OBG Management conducted an informal survey. Here, 4 experts respond to the question, "What are you going to do in your practice for women with a history of a previous preterm birth if 17P is no longer an option?"
Not ready to leave behind 17P for recurrent preterm delivery
Patrick Duff, MD
Preterm delivery is arguably the most important problem in perinatal medicine. It occurs in 10% to 12% of all obstetric patients in the United States, and complications of prematurity account for the majority of neonatal deaths. A major risk factor for recurrent preterm delivery is a prior history of spontaneous preterm delivery, with or without preterm premature rupture of membranes. Clearly, prevention of recurrence is of paramount importance.
In the Maternal-Fetal Medicine Units (MFMU) Network trial, Meis and colleagues demonstrated a 34% reduction (relative risk [RR], 0.66; 95% confidence interval [CI], 0.54-0.81) in the risk of recurrent preterm delivery in women who received weekly 250-mg injections of 17P (also called 17-OHPC). After publication of that trial, use of 17P became accepted practice in the United States.2
The PROLONG study by Blackwell and colleagues questions the value of 17P.1 In that international trial, which included 1,708 women from 41 centers in the United States and 52 outside the United States, the authors were unable to show any significant difference in the frequency of preterm delivery < 35 weeks (11.0% in the women receiving 17P and 11.5% in women receiving placebo; RR, 0.95; 95% CI, 0.71-1.26). Even when they examined the subset of women treated at US medical centers, they could not demonstrate any significant difference in treatment outcome.
At least 2 major explanations account for the discrepancy between the MFMU and the Blackwell studies. First, the participants in the PROLONG trial were clearly not at the same increased risk for recurrent preterm delivery as those in the MFMU trial. Second, in the PROLONG trial only the minority of participants were from the United States. In fact, given the relatively low rate of recurrent preterm delivery in the PROLONG trial, the study was underpowered to detect meaningful differences in maternal outcome. Therefore, I am not ready to abandon the use of progesterone supplementation in women at risk for recurrent preterm delivery.
Continue to: If the FDA removes 17P from the market...
If the FDA removes 17P from the market, my approach with at-risk patients will be as follows:
- I will encourage all at-risk women to eliminate obvious risk factors, such as smoking, illicit drug use, and excessive physical activity.
- I will encourage optimal nutrition and appropriate weight gain.
- I will test all patients for chlamydia, gonorrhea, and bacterial vaginosis and treat women who are infected.
- After the patient completes the first trimester, I will treat her with micronized progesterone, 200 mg daily, intravaginally. I will continue this medication until 36 to 37 weeks.
- I will perform an assessment of cervical length at 16, 20, and 24 weeks' gestation. In patients with demonstrable cervical shortening, I will perform a cerclage.
Rational management options for reducing risk of preterm delivery
Alex C. Vidaeff, MD, MPH
Most women who experience a spontaneous preterm delivery (sPTD) do not deliver prematurely in subsequent pregnancies.3 Two recent systematic reviews, in 2014 and 2017, found an overall risk of recurrent sPTD of 20.2% and 30%, respectively.4,5 These numbers are closer to the background event rate of 21.9% in the PROLONG trial, while only a few women have a recurrence risk of more than 50%, as in the Meis MFMU trial.1,2 A public health recommendation cannot be made for an intervention that is expected to work only in rare cases and fail in a majority of cases. Therefore, 17P is no longer a viable option for preventing recurrence in pregnant women with a history of sPTD, with only rare possible exceptions.
What evidence-based alternatives can be offered to pregnant women who had a previous sPTD?
Ultrasound assessment of cervical length has emerged as an effective prognosticator for recurrence in women with a prior sPTD, being able to predict 65.4% of sPTDs at a false-positive rate of 5%.6,7 Furthermore, sonographic cervical length measurements identify high-risk women who may not need any intervention. It has been shown that, among women with prior sPTD who maintain a normal cervical length up to 24 weeks, more than 90% will deliver at 35 weeks or after without intervention.8
In the United States, interventions to reduce sPTD, once a short cervix has been identified, include vaginal progesterone supplementation and cerclage. The benefit from vaginal progesterone has been documented by an individual patient data meta-analysis, while the benefit of cerclage has been highlighted in a Cochrane Review.9,10 The results of an adjusted indirect comparison meta-analysis suggest that both interventions are equally effective.11 Therefore, the decision on how best to minimize the risk of recurrent sPTD must be individualized based on historical and clinical circumstances, as well as the woman's informed choice.
Based on current data, the following approach appears rational to me:
- Cervical ultrasound surveillance between 16 and 24 weeks' gestation to identify the subgroup of women at significantly increased risk of sPTD recurrence.
- With cervical length ≤ 25 mm, vaginal progesterone supplementation may be considered. Preferential consideration for progesterone may be given when lower genital tract inflammation is suspected, given the possible anti-inflammatory action of progesterone.12,13
- If cervical shortening progresses to 15 to 20 mm, cerclage may be considered. Waiting for a cervix < 15 mm may be unadvisable. In conditions of a very short cervix, frequently dilated, with exposure of the fetal membranes, ascending subclinical intra-amniotic infection already may be present, reducing the efficacy of cerclage. Preferential consideration for cerclage also may be given with 2 sPTDs or mid-trimester losses or with a history of a successful cerclage.
Continue to: Screen cervical length early, and use cerclage or vaginal progesterone as appropriate...
Screen cervical length early, and use cerclage or vaginal progesterone as appropriate
Michael G. Ross, MD, MPH
In patients with a history of a previous preterm birth, if 17P is no longer an option, I would revert to screening for short cervix with transvaginal ultrasound.
Screen all high-risk patients at the first prenatal visit, so as not to miss a short cervix before 16 weeks' gestation. Then, beginning at 16 weeks, screen every 2 weeks until approximately 24 weeks.
If the cervix shortens to 25 mm or less, offer cerclage or vaginal progesterone. If the cervix shortens to 20 mm or less, I would strongly support cerclage or vaginal progesterone.
Use of 17P is still an option, for now
Errol R. Norwitz, MD, PhD, MBA
The way in which 17P was handled by the FDA is exactly the way the system is designed to work; this should be seen as a success, not a failure.
Given the urgent need for an intervention to prevent preterm birth, the lack of any alternative, and a single, well-designed randomized controlled trial that confirmed safety and suggested some benefit, the FDA approved 17P supplementation in February 2011 for a limited indication only—one or more prior unexplained sPTD—using the expedited review mechanism.2 Under this mechanism, a follow-up clinical trial is required to confirm efficacy. This was the PROLONG trial, which failed to show any significant benefit of 17P supplementation in terms of either preterm birth prevention or neonatal outcome.1
In October 2019, an FDA advisory committee met again to review these and other data. After presentations from a range of stakeholders and a robust discussion, the advisory committee voted to pursue approval withdrawal of 17P due to the lack of consistent evidence of benefit (it is important to note that this was not because of safety concerns). This is exactly the way the process is designed to work.
Where does this leave physicians and patients? It is clear that progesterone supplementation is not a panacea for preterm birth prevention and is not indicated for all women at high risk, even those with one or more prior unexplained sPTDs. Given that preterm birth is a syndrome and not a single diagnosis, it is still possible that there is a subgroup of women who may benefit from this intervention. For this reason—and because there is no clear alternative and no known downside to the administration of this drug (other than cost)—physicians still may choose to discuss this option with their patients and, after counseling, patients still may choose to accept it. If in doubt, engage the "shared decision-making model"; talk to your patients.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Iams JD, Goldenberg RL, Mercer BM, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. The Preterm Prediction Study: recurrence of spontaneous preterm birth. Am J Obstet Gynecol. 1998;178:1035-1040.
- Kazemier BM, Buijs PE, Mignini L, et al; EBM CONNECT. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: a systematic review. BJOG. 2014;121:1197-1208.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Owen J, Yost N, Berghella V, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340-1348.
- To MS, Skentou CA, Royston P, et al. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362-367.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Sakai M, Shiozaki A, Tabata M, et al. Evaluation of effectiveness of prophylactic cerclage of a short cervix according to interleukin-8 in cervical mucus. Am J Obstet Gynecol. 2006;194:14-19.
- Vidaeff AC, Ramin SM, Gilstrap LC, et al. Impact of progesterone on cytokine-stimulated nuclear factor-kappa B signaling in HeLa cells. J Matern Fetal Neonatal Med. 2007;20:23-28.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi:10.1055/s-0039-3400227.
- Meis PJ, Klebanoff M, Thom E, et al; for the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- Iams JD, Goldenberg RL, Mercer BM, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. The Preterm Prediction Study: recurrence of spontaneous preterm birth. Am J Obstet Gynecol. 1998;178:1035-1040.
- Kazemier BM, Buijs PE, Mignini L, et al; EBM CONNECT. Impact of obstetric history on the risk of spontaneous preterm birth in singleton and multiple pregnancies: a systematic review. BJOG. 2014;121:1197-1208.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Owen J, Yost N, Berghella V, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340-1348.
- To MS, Skentou CA, Royston P, et al. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362-367.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Sakai M, Shiozaki A, Tabata M, et al. Evaluation of effectiveness of prophylactic cerclage of a short cervix according to interleukin-8 in cervical mucus. Am J Obstet Gynecol. 2006;194:14-19.
- Vidaeff AC, Ramin SM, Gilstrap LC, et al. Impact of progesterone on cytokine-stimulated nuclear factor-kappa B signaling in HeLa cells. J Matern Fetal Neonatal Med. 2007;20:23-28.
Retained placenta after vaginal birth: How long should you wait to manually remove the placenta?
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
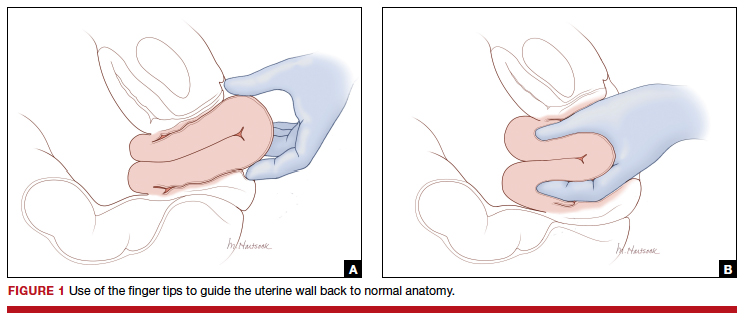
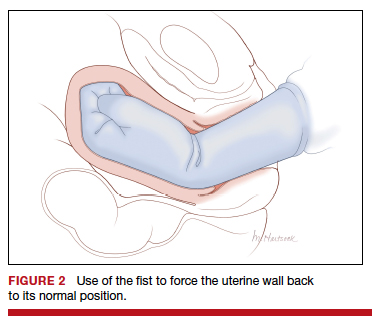
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.
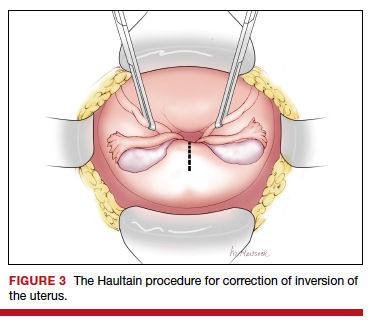
At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5
References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.


At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5

References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
You have just safely delivered the baby who is quietly resting on her mother’s chest. You begin active management of the third stage of labor, administering oxytocin, performing uterine massage and applying controlled tension on the umbilical cord. There is no evidence of excess postpartum bleeding.
How long will you wait to deliver the placenta?
Active management of the third stage of labor
Most authorities recommend active management of the third stage of labor because active management reduces the risk of maternal hemorrhage >1,000 mL (relative risk [RR], 0.34), postpartum hemoglobin levels < 9 g/dL (RR, 0.50), and maternal blood transfusion (RR, 0.35) compared with expectant management.1
The most important component of active management of the third stage of labor is the administration of a uterotonic after delivery of the newborn. In the United States, oxytocin is the uterotonic most often utilized for the active management of the third stage of labor. Authors of a recent randomized clinical trial reported that intravenous oxytocin is superior to intramuscular oxytocin for reducing postpartum blood loss (385 vs 445 mL), the frequency of blood loss greater than 1,000 mL (4.6% vs 8.1%), and the rate of maternal blood transfusion (1.5% vs 4.4%).2
In addition to administering oxytocin, the active management of the third stage often involves maneuvers to accelerate placental delivery, including the Crede and Brandt-Andrews maneuvers and controlled tension on the umbilical cord. The Crede maneuver, described in 1853, involves placing a hand on the abdominal wall near the uterine fundus and squeezing the uterine fundus between the thumb and fingers.3,4
The Brandt-Andrews maneuver, described in 1933, involves placing a clamp on the umbilical cord close to the vulva.5 The clamp is used to apply judicious tension on the cord with one hand, while the other hand is placed on the mother’s abdomen with the palm and fingers overlying the junction between the uterine corpus and the lower segment. With judicious tension on the cord, the abdominal hand pushes the uterus upward toward the umbilicus. Placental separation is indicated when lengthening of the umbilical cord occurs. The Brandt-Andrews maneuver may be associated with fewer cases of uterine inversion than the Crede maneuver.5-7
Of note, umbilical cord traction has not been demonstrated to reduce the need for blood transfusion or the incidence of postpartum hemorrhage (PPH) >1,000 mL, and it is commonly utilized by obstetricians and midwives.8,9 Hence, in the third stage, the delivering clinician should routinely administer a uterotonic, but use of judicious tension on the cord can be deferred if the woman prefers a noninterventional approach to delivery.
Following a vaginal birth, when should the diagnosis of retained placenta be made?
The historic definition of retained placenta is nonexpulsion of the placenta 30 minutes after delivery of the newborn. However, many observational studies report that, when active management of the third stage is utilized, 90%, 95%, and 99% of placentas deliver by 9 minutes, 13 minutes, and 28 minutes, respectively.10 In addition, many observational studies report that the incidence of PPH increases significantly with longer intervals between birth of the newborn and delivery of the placenta. In one study the rate of blood loss >500 mL was 8.5% when the placenta delivered between 5 and 9 minutes and 35.1% when the placenta delivered ≥30 minutes following birth of the baby.10 In another observational study, compared with women delivering the placenta < 10 minutes after birth, women delivering the placenta ≥30 minutes after birth had a 3-fold increased risk of PPH.11 Similar findings have been reported in other studies.12-14
Continue to: Based on the association between a delay in delivery...
Based on the association between a delay in delivery of the placenta and an increased risk of PPH, some authorities recommend that, in term pregnancy, the diagnosis of retained placenta should be made at 20 minutes following birth and consideration should be given to removing the placenta at this time. For women with effective neuraxial anesthesia, manual removal of the placenta 20 minutes following birth may be the best decision for balancing the benefit of preventing PPH with the risk of unnecessary intervention. For women with no anesthesia, delaying manual removal of the placenta to 30 minutes or more following birth may permit more time for the placenta to deliver prior to performing an intervention that might cause pain, but the delay increases the risk of PPH.
The retained placenta may prevent the uterine muscle from effectively contracting around penetrating veins and arteries, thereby increasing the risk of postpartum hemorrhage. The placenta that has separated from the uterine wall but is trapped inside the uterine cavity can be removed easily with manual extraction. If the placenta is physiologically adherent to the uterine wall, a gentle sweeping motion with an intrauterine hand usually can separate the placenta from the uterus in preparation for manual extraction. However, if a placenta accreta spectrum disorder is contributing to a retained placenta, it may be difficult to separate the densely adherent portion of the uterus from the uterine wall. In the presence of placenta accreta spectrum disorder, vigorous attempts to remove the placenta may precipitate massive bleeding. In some cases, the acchoucheur/midwife may recognize the presence of a focal accreta and cease attempts to remove the placenta in order to organize the personnel and equipment needed to effectively treat a potential case of placenta accreta. In one study, when a placenta accreta was recognized or suspected, immediately ceasing attempts at manually removing the placenta resulted in better case outcomes than continued attempts to remove the placenta.1
Uterine inversion may occur during an attempt to manually remove the placenta. There is universal agreement that once a uterine inversion is recognized it is critically important to immediately restore normal uterine anatomy to avoid massive hemorrhage and maternal shock. The initial management of uterine inversion includes:
- stopping oxytocin infusion
- initiating high volume fluid resuscitation
- considering a dose of a uterine relaxant, such as nitroglycerin or terbutaline
- preparing for blood product replacement.
In my experience, when uterine inversion is immediately recognized and successfully treated, blood product replacement is not usually necessary. However, if uterine inversion has not been immediately recognized or treated, massive hemorrhage and shock may occur.
Two approaches to the vaginal restoration of uterine anatomy involve using the tips of the fingers and palm of the hand to guide the wall of the uterus back to its normal position (FIGURE 1) or to forcefully use a fist to force the uterine wall back to its normal position (FIGURE 2). If these maneuvers are unsuccessful, a laparotomy may be necessary.


At laparotomy, the Huntington or Haultain procedures may help restore normal uterine anatomy. The Huntington procedure involves using clamps to apply symmetrical tension to the left and right round ligaments and/or uterine serosa to sequentially tease the uterus back to normal anatomy.2,3 The Haultain procedure involves a vertical incision on the posterior wall of the uterus to release the uterine constriction ring that is preventing the return of the uterine fundus to its normal position (FIGURE 3).4,5

References
- Kayem G, Anselem O, Schmitz T, et al. Conservative versus radical management in cases of placenta accreta: a historical study. J Gynecol Obstet Biol Reprod (Paris). 2007;36:680-687.
- Huntington JL. Acute inversion of the uterus. Boston Med Surg J. 1921;184:376-378.
- Huntington JL, Irving FC, Kellogg FS. Abdominal reposition in acute inversion of the puerperal uterus. Am J Obstet Gynecol. 1928;15:34-40.
- Haultain FW. Abdominal hysterotomy for chronic uterine inversion: a record of 3 cases. Proc Roy Soc Med. 1908;1:528-535.
- Easterday CL, Reid DE. Inversion of the puerperal uterus managed by the Haultain technique; A case report. Am J Obstet Gynecol. 1959;78:1224-1226.
Manual extraction of the placenta
Prior to performing manual extraction of the placenta, a decision should be made regarding the approach to anesthesia and perioperative antibiotics. Manual extraction of the placenta is performed by placing one hand on the uterine fundus to stabilize the uterus and using the other hand to follow the umbilical cord into the uterine cavity. The intrauterine hand is used to separate the uterine-placental interface with a gentle sweeping motion. The placental mass is grasped and gently teased through the cervix and vagina. Inspection of the placenta to ensure complete removal is necessary.
An alternative to manual extraction of the placenta is the use of Bierer forceps and ultrasound guidance to tease the placenta through the cervical os. This technique involves the following steps15:
1. use ultrasound to locate the placenta
2. place a ring forceps on the anterior lip of the cervix
3. introduce the Bierer forcep into the uterus
4. use the forceps to grasp the placenta and pull it toward the vagina
5. stop frequently to re-grasp placental tissue that is deeper in the uterine cavity
6. once the placenta is extracted, examine the placenta to ensure complete removal.
Of note when manual extraction is used to deliver a retained placenta, randomized clinical trials report no benefit for the following interventions:
- perioperative antibiotics16
- nitroglycerin to relax the uterus17
- ultrasound to detect retained placental tissue.18
Best timing for manual extraction of the placenta
The timing for the diagnosis of retained placenta, and the risks and benefits of manual extraction would be best evaluated in a large, randomized clinical trial. However, based on observational studies, in a term pregnancy, the diagnosis of retained placenta is best made using a 20-minute interval. In women with effective neuraxial anesthesia, consideration should be given to manual removal of the placenta at that time.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labor. Cochrane Database Syst Rev. 2019;2:CD007412.
- Adnan N, Conlan-Trant R, McCormick C, et al. Intramuscular versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery: randomized controlled trial. BMJ. 2018;362:k3546.
- Gülmezoglu AM, Souza JP. The evolving management of the third stage of labour. BJOG. 2009;116(suppl 1):26-28.
- Ebert AD, David M. Meilensteine der Praventionsmedizin. Carl Siegmund Franz Credé (1819-1882), der Credesche Handgriff und die Credesche Augenprophylaxe. Geburtshilfe Frauenheilkd. 2016;76:675-678.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol. 1933;25:662-667.
- Kimbell N. Brandt-Andrews technique of delivery of the placenta. Br Med J. 1958;1:203-204.
- De Lee JB, Greenhill JP. Principles and Practice of Obstetrics. 9th ed. Philadelphia, PA: Saunders; 1947:275.
- Du Y, Ye M, Zheng F. Active management of the third stage of labor with and without controlled cord traction: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand. 2014;93:626-633.
- Hofmeyr GJ, Mshweshwe NT, Gülmezoglu AM. Controlled cord traction for the third stage of labor. Cochrane Database Syst Rev. 2015;1:CD008020.
- Frolova AI, Stout MJ, Tuuli MG, et al. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016;127:951-956.
- Shinar S, Schwartz A, Maslovitz S, et al. How long is safe? Setting the cutoff for uncomplicated third stage length: a retrospective case-control study. Birth. 2016;43:36-41.
- Magann EF, Evans S, Chauhan SP, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290-293.
- Cummings K, Doherty DA, Magann EF, et al. Timing of manual placenta removal to prevent postpartum hemorrhage: is it time to act? J Matern Fetal Neonatal Med. 2016;29:3930-3933.
- Rabie NZ, Ounpraseuth S, Hughes D, et al. Association of the length of the third stage of labor and blood loss following vaginal delivery. South Med J. 2018;111:178-182.
- Rosenstein MG, Vargas JE, Drey EA. Ultrasound-guided instrumental removal of the retained placenta after vaginal delivery. Am J Obstet Gynecol. 2014;211:180.e1-e3.
- Chibueze EC, Parsons AJ, Ota E, et al. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;15:313.
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;(11):CD007708.
- Weissback T, Haikin-Herzberger E, Bacci-Hugger K, et al. Immediate postpartum ultrasound evaluation for suspected retained placental tissue in patients undergoing manual removal of placenta. Eur J Obstet Gynecol Reprod Biol. 2015;192:37-40.
Preventing early-onset group B streptococcal disease in newborns
In 1992, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) published their first joint guidelines on the prevention of early-onset neonatal group B streptococcal (GBS) infection.1 In this initial statement, the organizations recommended universal culturing of obstetric patients at 28 weeks’ gestation and treatment of colonized women during labor if they had a recognized risk factor for neonatal GBS infection.
In 1996, the Centers for Disease Control and Prevention (CDC) published its first set of official guidelines on the topic and suggested that both universal screening and a risk-factor–based approach were reasonable options.2 The 2002 update of the CDC guidelines strongly recommended universal screening of all pregnant women at 35 to 37 weeks’ gestation and intrapartum prophylaxis for all colonized women regardless of risk factors.3
The third set of CDC guidelines was published in 2010.4 The key features of this version were the elimination of erythromycin as an alternative to penicillin in patients who are allergic to beta-lactam antibiotics and the establishment of 4 hours as the critical interval for administration of prophylaxis prior to delivery. The 2010 publication was the last such report from the CDC. Since then ACOG and AAP have been tasked with providing updated practice guidelines. To that end, ACOG recently issued a new Committee Opinion on “Prevention of Group B Streptococcal Early-Onset Disease in Newborns.”5 Here we will highlight the key features of our current strategy for preventing neonatal GBS infection.
CASE Pregnant patient presents with many questions about GBS
A 26-year-old primigravid woman presents for her first prenatal appointment at 9 weeks’ gestation. Her older sister recently delivered a term infant that died in the first week of life from GBS sepsis. Understandably, she has many questions.
1. Your patient first wants to know, “What is this streptococcal organism and how likely am I to have this infection?”
Streptococcus agalactiae, also known as GBS, is a gram-positive encapsulated bacterium that produces beta hemolysis when grown on blood agar. Approximately 25% of pregnant women harbor this organism in the lower genital tract and/or rectum.6
GBS is one of the most important causes of neonatal infection, particularly in preterm infants. The frequency of infection is now 0.23 per 1,000 live births in the US.5
Neonatal infection can be divided into early-onset infection (occurring within the first 7 days of life) and late-onset infection (occurring from after the first week until the third month of life). Approximately 80% to 85% of cases of neonatal GBS infections are early in onset. Virtually all of the early-onset infections result from vertical transmission during delivery from a colonized mother to her infant.5-7
2. “How dangerous is this infection to my baby and me? Are there certain factors that increase the risk of my baby becoming infected?”
GBS is responsible for approximately 2% to 3% of cases of either asymptomatic bacteriuria or acute cystitis. Women with urinary tract infections caused by GBS are at increased risk for preterm premature rupture of membranes and preterm delivery. Genital tract colonization also increases a woman’s risk for chorioamnionitis and endometritis, particularly after cesarean delivery (CD). In addition, GBS can be part of the polymicrobial flora in women who have a wound (incisional site) infection following CD.6,7
Continue to: In colonized women, several risk factors...
In colonized women, several risk factors have been identified that increase the probability of early-onset neonatal GBS infection. These factors include: preterm labor, especially when complicated by premature rupture of membranes; intrapartum maternal fever (usually due to chorioamnionitis); rupture of membranes greater than 18 hours before delivery; previous delivery of an infected infant; young age; and black or Hispanic ethnicity. Approximately 25% of colonized women will have one of these risk factors.5-7
These risk factors have a profound impact on neonatal attack rates and mortality. Without the interventions outlined below, the neonatal infection rate is 40% to 50% in the presence of a risk factor and less than 5% in the absence of a risk factor. In infected infants, neonatal mortality approaches 30% to 35% when a maternal risk factor is present, but is less than 5% when risk factors are absent.5-7
3. “What will you do to determine if I am colonized with this organism?”
The current guidelines set forth in the ACOG Committee Opinion recommend that selected high-risk patients (patients with preterm labor or preterm premature rupture of membranes) be tested for GBS at the time of initial presentation. All other women should be tested for GBS during the interval 36 0/7 to 37 6/7 weeks’ gestation.5 Testing at this point in pregnancy is almost 90% sensitive for identifying patients who will be colonized at the time of admission for labor if no more than 5 weeks elapse between the time the culture is obtained and labor begins. The positive predictive value of this test is 87%, and the negative predictive value is 96%.8
ACOG’s previous guidelines provided for testing at 35 rather than 36 weeks. The change in the recommendations was based on 2 factors. First, all women with unknown GBS status who may deliver before 37 weeks already should be targeted for prophylaxis. Second, the new 5-week window now will include women who deliver up to 41 weeks’ gestation. Given current obstetric practice in the US, delivery beyond 41 weeks is unlikely.5
At the present time, the best test for identification of GBS colonization is bacteriologic culture. A cotton swab is placed into the lower third of the vagina, streaked along the perineum, and then placed into the rectum. The swab is withdrawn, placed in a culturette tube, and transported to the laboratory. In the laboratory, the swab is cultured for approximately 24 hours in a nutrient broth and then subcultured on a selective blood agar plate. Failure to sample both the vagina and rectum or failure to use selective broth and selective blood agar will reduce the yield of positive cultures by approximately 50%.5-7
In recent years, researchers have become interested in the use of rapid nucleic acid amplification tests for the identification of GBS. These tests perform well if the test protocol provides for an 18- to 24-hour incubation in nutrient broth prior to application of the nucleic acid probe. When the tests are performed without this enrichment phase, sensitivities are inferior to those associated with bacteriologic culture. In addition, because the rapid tests do not isolate the organisms, they do not allow for antibiotic sensitivity testing.5-7
Continue to: “If I test positive for GBS, how and when will you treat me?”...
4. “If I test positive for GBS, how and when will you treat me?”
The current ACOG guidelines recommend that all colonized women be treated intrapartum with prophylactic antibiotics regardless of whether risk factors are present. Treatment should be started at the time of admission and continued until the infant is delivered.5
The drugs of choice for intrapartum prophylaxis are intravenous penicillin or ampicillin. If the patient has a mild allergy to penicillin, cefazolin is the appropriate alternative. If the patient has a severe allergy to penicillin, the 2 options are vancomycin or clindamycin. If the latter drug is used, the laboratory must perform sensitivity testing because 13% to 20% of strains of GBS may be resistant to clindamycin. The frequency of resistance to erythromycin now ranges from 25% to 32%. Thus, erythromycin is no longer used for intrapartum prophylaxis.5-7,9
The appropriate intravenous dosages of these antibiotics are listed in the TABLE.5 The new ACOG guidelines have revised the previous recommendations for dosing of penicillin, eliminating the 2.5 million-unit dose. They also have revised the dosing recommendations for vancomyin, eliminating the previous recommendation of 1 g every 12 hours.5 The new recommendations regarding vancomycin are particularly important and are based, at least in part, on an interesting report from Onwuchuruba and colleagues.10 These authors studied maternal and cord blood concentrations of vancomycin in mother-infant dyads receiving either the original recommended dosage of vancomycin (1 g every 12 hours) or a dosage of 15 to 20 mg/kg every 8 hours. With standard dosing, only 9% of neonates had therapeutic vancomycin serum concentrations at delivery. With the 20 mg/kg dose of vancomycin, the percent of neonates with therapeutic serum concentrations of vancomycin increased to 80%.
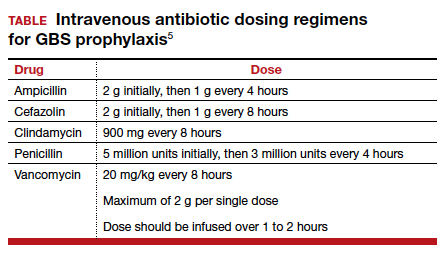
5. “For how long must I be treated in labor before my baby will be protected by the antibiotics?”
The current ACOG Committee Opinion stresses the importance of treating the colonized mother for at least 4 hours prior to delivery.5 This recommendation is based primarily on the landmark report by De Cueto and colleagues.11 These authors evaluated colonized women who received intrapartum prophylaxis at varying times prior to delivery. Their primary endpoint was the percentage of newborns who were colonized with GBS. If the mothers had received antibiotics for less than 1 hour prior to delivery, 46% of neonates were colonized. This figure was equal to the rate of colonization in neonates whose mothers received no antibiotics. When the interval was 1 to 2 hours, the percentage was 29%. When mothers had received antibiotics for 2 to 4 hours, the neonatal colonization rate fell to 2.9%. When antibiotics had been administered for greater than 4 hours, the rate of neonatal colonization was only 1.2%.
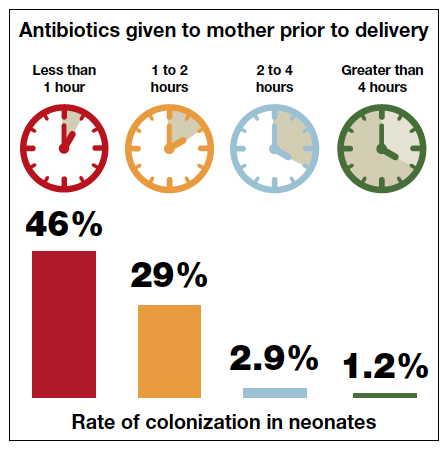
Fairlie and colleagues recently reported the results of another interesting investigation comparing the effectiveness of prophylaxis based on duration of treatment and choice of individual antibiotics.12 Prophylaxis with penicillin or ampicillin for 4 hours or more was 91% effective in preventing early-onset neonatal infection in term infants and 86% effective in preventing infection in preterm infants. These outcomes were superior to the outcomes in both term and preterm infants who received penicillin or ampicillin for less than 4 hours.
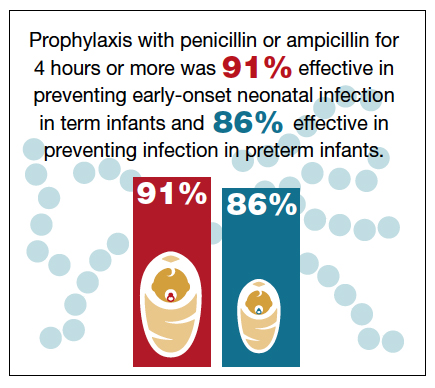
These observations agree with the findings of McNanley and colleagues who evaluated vaginal colony counts of GBS following different periods of antibiotic administration.13 These authors noted that mean colony counts decreased 5-fold within 2 hours of penicillin administration, 50-fold within 4 hours, and 1,000-fold within 6 hours.
Despite these compelling findings, the ACOG Committee Opinion stresses that obstetric interventions such as amniotomy and oxytocin augmentation should not be delayed simply to permit a certain time period of antibiotic administration.5
Continue to: “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”...
6. “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”
If a mother is scheduled to have a CD, for example because of a prior cesarean or because of a persistent fetal malpresentation, she should still have a GBS culture at 36 0/7 to 37 6/7 weeks’ gestation. The information obtained from this culture may be of value to both the obstetrician and pediatrician if the patient experiences labor or rupture of membranes prior to her scheduled surgery. If she does not experience spontaneous labor prior to her scheduled date of surgery, she does not require specific GBS prophylaxis at the time of her operation.5 Rather, she should receive prophylactic antibiotics to prevent post–cesarean infection, ideally, the combination of cefazolin (2 g IV) plus azithromycin (500 mg IV).14 Cefazolin, of course, provides excellent coverage of GBS.
7. “If I am colonized with GBS and I receive treatment during labor, will my baby be safe after delivery?”
The interventions outlined above will prevent almost 90% of early-onset GBS infections, but they are not foolproof.5-7,15,16 Successful management of the neonate is dependent upon several factors, including:5-7
- gestational age
- presence of maternal chorioamnionitis
- presence or absence of risk factors for early-onset infection
- duration (adequacy) of maternal treatment during labor
- presence of immediate clinical signs of infection in the neonate (such as fever, lethargy, hemodynamic instability, respiratory distress, or elevated or decreased white blood cell count).
If the mother is at term and receives intrapartum prophylaxis for at least 4 hours prior to delivery, the neonate usually will not require any special tests and simply will be observed for 24 to 48 hours for signs of infection.
If the mother delivers preterm and receives appropriate intrapartum prophylaxis, the pediatricians typically will obtain a complete blood count (CBC) and treat with prophylactic antibiotics (ampicillin plus gentamicin) for 48 hours if abnormalities are noted on the CBC or the baby exhibits signs of infection. If the CBC is normal and the baby shows no signs of infection, no treatment is indicated.
Regardless of gestational age, if the mother does not receive prophylaxis for at least 4 hours before delivery, the pediatricians usually will obtain a CBC and closely observe the baby in the hospital for signs of infection. If such signs develop or the CBC is abnormal, blood and cerebrospinal fluid cultures will be obtained. Antibiotic therapy (usually ampicillin plus gentamicin) is then initiated, and the drugs are continued until cultures return with no growth. If either culture is positive, antibiotics will then be continued for 7 to 10 days.
If the mother has documented chorioamnionitis and receives treatment intrapartum with appropriate antibiotics (usually ampicillin plus gentamicin), the pediatricians usually will obtain a CBC, C-reactive protein (CRP) level, and blood cultures and then start the infant on antibiotics, pending the result of the laboratory tests. If the CBC and CRP are reassuring, the cultures are negative after 48 hours, and the infant demonstrates no signs of clinical infection, many pediatricians will then discontinue antibiotics. Others may still continue the antibiotics for 7 to 10 days.
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992;90:775-778.
- CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24.
- Schrag S, Gorwitz R, Fultz-Butts K, et al. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010;59:1-36.
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134:206-210.
- Duff P, Birsner M. Maternal and perinatal infection in pregnancy: bacteria. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics. Normal and Problem Pregnancies. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019.
- Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
- Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:540-544.
- Onwuchuruba CN, Towers CV, Howard BC, et al. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210:352.e1-352.e4.
- de Cueto M, Sanchez MJ, Sampedro A, et al. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol. 1998;91:112-114.
- Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013;121:570-577.
- McNanley AR, Glantz JC, Hardy DJ, et al. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am J Obstet Gynecol. 2007;197:583.e1-583.e4.
- Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Brozanski BS, Jones JG, Krohn MA, et al. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol. 2000;95:496-501.
- Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The National Group B Streptococcal Disease Study Group. Obstet Gynecol. 1997;90:901-906.
In 1992, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) published their first joint guidelines on the prevention of early-onset neonatal group B streptococcal (GBS) infection.1 In this initial statement, the organizations recommended universal culturing of obstetric patients at 28 weeks’ gestation and treatment of colonized women during labor if they had a recognized risk factor for neonatal GBS infection.
In 1996, the Centers for Disease Control and Prevention (CDC) published its first set of official guidelines on the topic and suggested that both universal screening and a risk-factor–based approach were reasonable options.2 The 2002 update of the CDC guidelines strongly recommended universal screening of all pregnant women at 35 to 37 weeks’ gestation and intrapartum prophylaxis for all colonized women regardless of risk factors.3
The third set of CDC guidelines was published in 2010.4 The key features of this version were the elimination of erythromycin as an alternative to penicillin in patients who are allergic to beta-lactam antibiotics and the establishment of 4 hours as the critical interval for administration of prophylaxis prior to delivery. The 2010 publication was the last such report from the CDC. Since then ACOG and AAP have been tasked with providing updated practice guidelines. To that end, ACOG recently issued a new Committee Opinion on “Prevention of Group B Streptococcal Early-Onset Disease in Newborns.”5 Here we will highlight the key features of our current strategy for preventing neonatal GBS infection.
CASE Pregnant patient presents with many questions about GBS
A 26-year-old primigravid woman presents for her first prenatal appointment at 9 weeks’ gestation. Her older sister recently delivered a term infant that died in the first week of life from GBS sepsis. Understandably, she has many questions.
1. Your patient first wants to know, “What is this streptococcal organism and how likely am I to have this infection?”
Streptococcus agalactiae, also known as GBS, is a gram-positive encapsulated bacterium that produces beta hemolysis when grown on blood agar. Approximately 25% of pregnant women harbor this organism in the lower genital tract and/or rectum.6
GBS is one of the most important causes of neonatal infection, particularly in preterm infants. The frequency of infection is now 0.23 per 1,000 live births in the US.5
Neonatal infection can be divided into early-onset infection (occurring within the first 7 days of life) and late-onset infection (occurring from after the first week until the third month of life). Approximately 80% to 85% of cases of neonatal GBS infections are early in onset. Virtually all of the early-onset infections result from vertical transmission during delivery from a colonized mother to her infant.5-7
2. “How dangerous is this infection to my baby and me? Are there certain factors that increase the risk of my baby becoming infected?”
GBS is responsible for approximately 2% to 3% of cases of either asymptomatic bacteriuria or acute cystitis. Women with urinary tract infections caused by GBS are at increased risk for preterm premature rupture of membranes and preterm delivery. Genital tract colonization also increases a woman’s risk for chorioamnionitis and endometritis, particularly after cesarean delivery (CD). In addition, GBS can be part of the polymicrobial flora in women who have a wound (incisional site) infection following CD.6,7
Continue to: In colonized women, several risk factors...
In colonized women, several risk factors have been identified that increase the probability of early-onset neonatal GBS infection. These factors include: preterm labor, especially when complicated by premature rupture of membranes; intrapartum maternal fever (usually due to chorioamnionitis); rupture of membranes greater than 18 hours before delivery; previous delivery of an infected infant; young age; and black or Hispanic ethnicity. Approximately 25% of colonized women will have one of these risk factors.5-7
These risk factors have a profound impact on neonatal attack rates and mortality. Without the interventions outlined below, the neonatal infection rate is 40% to 50% in the presence of a risk factor and less than 5% in the absence of a risk factor. In infected infants, neonatal mortality approaches 30% to 35% when a maternal risk factor is present, but is less than 5% when risk factors are absent.5-7
3. “What will you do to determine if I am colonized with this organism?”
The current guidelines set forth in the ACOG Committee Opinion recommend that selected high-risk patients (patients with preterm labor or preterm premature rupture of membranes) be tested for GBS at the time of initial presentation. All other women should be tested for GBS during the interval 36 0/7 to 37 6/7 weeks’ gestation.5 Testing at this point in pregnancy is almost 90% sensitive for identifying patients who will be colonized at the time of admission for labor if no more than 5 weeks elapse between the time the culture is obtained and labor begins. The positive predictive value of this test is 87%, and the negative predictive value is 96%.8
ACOG’s previous guidelines provided for testing at 35 rather than 36 weeks. The change in the recommendations was based on 2 factors. First, all women with unknown GBS status who may deliver before 37 weeks already should be targeted for prophylaxis. Second, the new 5-week window now will include women who deliver up to 41 weeks’ gestation. Given current obstetric practice in the US, delivery beyond 41 weeks is unlikely.5
At the present time, the best test for identification of GBS colonization is bacteriologic culture. A cotton swab is placed into the lower third of the vagina, streaked along the perineum, and then placed into the rectum. The swab is withdrawn, placed in a culturette tube, and transported to the laboratory. In the laboratory, the swab is cultured for approximately 24 hours in a nutrient broth and then subcultured on a selective blood agar plate. Failure to sample both the vagina and rectum or failure to use selective broth and selective blood agar will reduce the yield of positive cultures by approximately 50%.5-7
In recent years, researchers have become interested in the use of rapid nucleic acid amplification tests for the identification of GBS. These tests perform well if the test protocol provides for an 18- to 24-hour incubation in nutrient broth prior to application of the nucleic acid probe. When the tests are performed without this enrichment phase, sensitivities are inferior to those associated with bacteriologic culture. In addition, because the rapid tests do not isolate the organisms, they do not allow for antibiotic sensitivity testing.5-7
Continue to: “If I test positive for GBS, how and when will you treat me?”...
4. “If I test positive for GBS, how and when will you treat me?”
The current ACOG guidelines recommend that all colonized women be treated intrapartum with prophylactic antibiotics regardless of whether risk factors are present. Treatment should be started at the time of admission and continued until the infant is delivered.5
The drugs of choice for intrapartum prophylaxis are intravenous penicillin or ampicillin. If the patient has a mild allergy to penicillin, cefazolin is the appropriate alternative. If the patient has a severe allergy to penicillin, the 2 options are vancomycin or clindamycin. If the latter drug is used, the laboratory must perform sensitivity testing because 13% to 20% of strains of GBS may be resistant to clindamycin. The frequency of resistance to erythromycin now ranges from 25% to 32%. Thus, erythromycin is no longer used for intrapartum prophylaxis.5-7,9
The appropriate intravenous dosages of these antibiotics are listed in the TABLE.5 The new ACOG guidelines have revised the previous recommendations for dosing of penicillin, eliminating the 2.5 million-unit dose. They also have revised the dosing recommendations for vancomyin, eliminating the previous recommendation of 1 g every 12 hours.5 The new recommendations regarding vancomycin are particularly important and are based, at least in part, on an interesting report from Onwuchuruba and colleagues.10 These authors studied maternal and cord blood concentrations of vancomycin in mother-infant dyads receiving either the original recommended dosage of vancomycin (1 g every 12 hours) or a dosage of 15 to 20 mg/kg every 8 hours. With standard dosing, only 9% of neonates had therapeutic vancomycin serum concentrations at delivery. With the 20 mg/kg dose of vancomycin, the percent of neonates with therapeutic serum concentrations of vancomycin increased to 80%.

5. “For how long must I be treated in labor before my baby will be protected by the antibiotics?”
The current ACOG Committee Opinion stresses the importance of treating the colonized mother for at least 4 hours prior to delivery.5 This recommendation is based primarily on the landmark report by De Cueto and colleagues.11 These authors evaluated colonized women who received intrapartum prophylaxis at varying times prior to delivery. Their primary endpoint was the percentage of newborns who were colonized with GBS. If the mothers had received antibiotics for less than 1 hour prior to delivery, 46% of neonates were colonized. This figure was equal to the rate of colonization in neonates whose mothers received no antibiotics. When the interval was 1 to 2 hours, the percentage was 29%. When mothers had received antibiotics for 2 to 4 hours, the neonatal colonization rate fell to 2.9%. When antibiotics had been administered for greater than 4 hours, the rate of neonatal colonization was only 1.2%.

Fairlie and colleagues recently reported the results of another interesting investigation comparing the effectiveness of prophylaxis based on duration of treatment and choice of individual antibiotics.12 Prophylaxis with penicillin or ampicillin for 4 hours or more was 91% effective in preventing early-onset neonatal infection in term infants and 86% effective in preventing infection in preterm infants. These outcomes were superior to the outcomes in both term and preterm infants who received penicillin or ampicillin for less than 4 hours.

These observations agree with the findings of McNanley and colleagues who evaluated vaginal colony counts of GBS following different periods of antibiotic administration.13 These authors noted that mean colony counts decreased 5-fold within 2 hours of penicillin administration, 50-fold within 4 hours, and 1,000-fold within 6 hours.
Despite these compelling findings, the ACOG Committee Opinion stresses that obstetric interventions such as amniotomy and oxytocin augmentation should not be delayed simply to permit a certain time period of antibiotic administration.5
Continue to: “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”...
6. “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”
If a mother is scheduled to have a CD, for example because of a prior cesarean or because of a persistent fetal malpresentation, she should still have a GBS culture at 36 0/7 to 37 6/7 weeks’ gestation. The information obtained from this culture may be of value to both the obstetrician and pediatrician if the patient experiences labor or rupture of membranes prior to her scheduled surgery. If she does not experience spontaneous labor prior to her scheduled date of surgery, she does not require specific GBS prophylaxis at the time of her operation.5 Rather, she should receive prophylactic antibiotics to prevent post–cesarean infection, ideally, the combination of cefazolin (2 g IV) plus azithromycin (500 mg IV).14 Cefazolin, of course, provides excellent coverage of GBS.
7. “If I am colonized with GBS and I receive treatment during labor, will my baby be safe after delivery?”
The interventions outlined above will prevent almost 90% of early-onset GBS infections, but they are not foolproof.5-7,15,16 Successful management of the neonate is dependent upon several factors, including:5-7
- gestational age
- presence of maternal chorioamnionitis
- presence or absence of risk factors for early-onset infection
- duration (adequacy) of maternal treatment during labor
- presence of immediate clinical signs of infection in the neonate (such as fever, lethargy, hemodynamic instability, respiratory distress, or elevated or decreased white blood cell count).
If the mother is at term and receives intrapartum prophylaxis for at least 4 hours prior to delivery, the neonate usually will not require any special tests and simply will be observed for 24 to 48 hours for signs of infection.
If the mother delivers preterm and receives appropriate intrapartum prophylaxis, the pediatricians typically will obtain a complete blood count (CBC) and treat with prophylactic antibiotics (ampicillin plus gentamicin) for 48 hours if abnormalities are noted on the CBC or the baby exhibits signs of infection. If the CBC is normal and the baby shows no signs of infection, no treatment is indicated.
Regardless of gestational age, if the mother does not receive prophylaxis for at least 4 hours before delivery, the pediatricians usually will obtain a CBC and closely observe the baby in the hospital for signs of infection. If such signs develop or the CBC is abnormal, blood and cerebrospinal fluid cultures will be obtained. Antibiotic therapy (usually ampicillin plus gentamicin) is then initiated, and the drugs are continued until cultures return with no growth. If either culture is positive, antibiotics will then be continued for 7 to 10 days.
If the mother has documented chorioamnionitis and receives treatment intrapartum with appropriate antibiotics (usually ampicillin plus gentamicin), the pediatricians usually will obtain a CBC, C-reactive protein (CRP) level, and blood cultures and then start the infant on antibiotics, pending the result of the laboratory tests. If the CBC and CRP are reassuring, the cultures are negative after 48 hours, and the infant demonstrates no signs of clinical infection, many pediatricians will then discontinue antibiotics. Others may still continue the antibiotics for 7 to 10 days.
In 1992, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) published their first joint guidelines on the prevention of early-onset neonatal group B streptococcal (GBS) infection.1 In this initial statement, the organizations recommended universal culturing of obstetric patients at 28 weeks’ gestation and treatment of colonized women during labor if they had a recognized risk factor for neonatal GBS infection.
In 1996, the Centers for Disease Control and Prevention (CDC) published its first set of official guidelines on the topic and suggested that both universal screening and a risk-factor–based approach were reasonable options.2 The 2002 update of the CDC guidelines strongly recommended universal screening of all pregnant women at 35 to 37 weeks’ gestation and intrapartum prophylaxis for all colonized women regardless of risk factors.3
The third set of CDC guidelines was published in 2010.4 The key features of this version were the elimination of erythromycin as an alternative to penicillin in patients who are allergic to beta-lactam antibiotics and the establishment of 4 hours as the critical interval for administration of prophylaxis prior to delivery. The 2010 publication was the last such report from the CDC. Since then ACOG and AAP have been tasked with providing updated practice guidelines. To that end, ACOG recently issued a new Committee Opinion on “Prevention of Group B Streptococcal Early-Onset Disease in Newborns.”5 Here we will highlight the key features of our current strategy for preventing neonatal GBS infection.
CASE Pregnant patient presents with many questions about GBS
A 26-year-old primigravid woman presents for her first prenatal appointment at 9 weeks’ gestation. Her older sister recently delivered a term infant that died in the first week of life from GBS sepsis. Understandably, she has many questions.
1. Your patient first wants to know, “What is this streptococcal organism and how likely am I to have this infection?”
Streptococcus agalactiae, also known as GBS, is a gram-positive encapsulated bacterium that produces beta hemolysis when grown on blood agar. Approximately 25% of pregnant women harbor this organism in the lower genital tract and/or rectum.6
GBS is one of the most important causes of neonatal infection, particularly in preterm infants. The frequency of infection is now 0.23 per 1,000 live births in the US.5
Neonatal infection can be divided into early-onset infection (occurring within the first 7 days of life) and late-onset infection (occurring from after the first week until the third month of life). Approximately 80% to 85% of cases of neonatal GBS infections are early in onset. Virtually all of the early-onset infections result from vertical transmission during delivery from a colonized mother to her infant.5-7
2. “How dangerous is this infection to my baby and me? Are there certain factors that increase the risk of my baby becoming infected?”
GBS is responsible for approximately 2% to 3% of cases of either asymptomatic bacteriuria or acute cystitis. Women with urinary tract infections caused by GBS are at increased risk for preterm premature rupture of membranes and preterm delivery. Genital tract colonization also increases a woman’s risk for chorioamnionitis and endometritis, particularly after cesarean delivery (CD). In addition, GBS can be part of the polymicrobial flora in women who have a wound (incisional site) infection following CD.6,7
Continue to: In colonized women, several risk factors...
In colonized women, several risk factors have been identified that increase the probability of early-onset neonatal GBS infection. These factors include: preterm labor, especially when complicated by premature rupture of membranes; intrapartum maternal fever (usually due to chorioamnionitis); rupture of membranes greater than 18 hours before delivery; previous delivery of an infected infant; young age; and black or Hispanic ethnicity. Approximately 25% of colonized women will have one of these risk factors.5-7
These risk factors have a profound impact on neonatal attack rates and mortality. Without the interventions outlined below, the neonatal infection rate is 40% to 50% in the presence of a risk factor and less than 5% in the absence of a risk factor. In infected infants, neonatal mortality approaches 30% to 35% when a maternal risk factor is present, but is less than 5% when risk factors are absent.5-7
3. “What will you do to determine if I am colonized with this organism?”
The current guidelines set forth in the ACOG Committee Opinion recommend that selected high-risk patients (patients with preterm labor or preterm premature rupture of membranes) be tested for GBS at the time of initial presentation. All other women should be tested for GBS during the interval 36 0/7 to 37 6/7 weeks’ gestation.5 Testing at this point in pregnancy is almost 90% sensitive for identifying patients who will be colonized at the time of admission for labor if no more than 5 weeks elapse between the time the culture is obtained and labor begins. The positive predictive value of this test is 87%, and the negative predictive value is 96%.8
ACOG’s previous guidelines provided for testing at 35 rather than 36 weeks. The change in the recommendations was based on 2 factors. First, all women with unknown GBS status who may deliver before 37 weeks already should be targeted for prophylaxis. Second, the new 5-week window now will include women who deliver up to 41 weeks’ gestation. Given current obstetric practice in the US, delivery beyond 41 weeks is unlikely.5
At the present time, the best test for identification of GBS colonization is bacteriologic culture. A cotton swab is placed into the lower third of the vagina, streaked along the perineum, and then placed into the rectum. The swab is withdrawn, placed in a culturette tube, and transported to the laboratory. In the laboratory, the swab is cultured for approximately 24 hours in a nutrient broth and then subcultured on a selective blood agar plate. Failure to sample both the vagina and rectum or failure to use selective broth and selective blood agar will reduce the yield of positive cultures by approximately 50%.5-7
In recent years, researchers have become interested in the use of rapid nucleic acid amplification tests for the identification of GBS. These tests perform well if the test protocol provides for an 18- to 24-hour incubation in nutrient broth prior to application of the nucleic acid probe. When the tests are performed without this enrichment phase, sensitivities are inferior to those associated with bacteriologic culture. In addition, because the rapid tests do not isolate the organisms, they do not allow for antibiotic sensitivity testing.5-7
Continue to: “If I test positive for GBS, how and when will you treat me?”...
4. “If I test positive for GBS, how and when will you treat me?”
The current ACOG guidelines recommend that all colonized women be treated intrapartum with prophylactic antibiotics regardless of whether risk factors are present. Treatment should be started at the time of admission and continued until the infant is delivered.5
The drugs of choice for intrapartum prophylaxis are intravenous penicillin or ampicillin. If the patient has a mild allergy to penicillin, cefazolin is the appropriate alternative. If the patient has a severe allergy to penicillin, the 2 options are vancomycin or clindamycin. If the latter drug is used, the laboratory must perform sensitivity testing because 13% to 20% of strains of GBS may be resistant to clindamycin. The frequency of resistance to erythromycin now ranges from 25% to 32%. Thus, erythromycin is no longer used for intrapartum prophylaxis.5-7,9
The appropriate intravenous dosages of these antibiotics are listed in the TABLE.5 The new ACOG guidelines have revised the previous recommendations for dosing of penicillin, eliminating the 2.5 million-unit dose. They also have revised the dosing recommendations for vancomyin, eliminating the previous recommendation of 1 g every 12 hours.5 The new recommendations regarding vancomycin are particularly important and are based, at least in part, on an interesting report from Onwuchuruba and colleagues.10 These authors studied maternal and cord blood concentrations of vancomycin in mother-infant dyads receiving either the original recommended dosage of vancomycin (1 g every 12 hours) or a dosage of 15 to 20 mg/kg every 8 hours. With standard dosing, only 9% of neonates had therapeutic vancomycin serum concentrations at delivery. With the 20 mg/kg dose of vancomycin, the percent of neonates with therapeutic serum concentrations of vancomycin increased to 80%.

5. “For how long must I be treated in labor before my baby will be protected by the antibiotics?”
The current ACOG Committee Opinion stresses the importance of treating the colonized mother for at least 4 hours prior to delivery.5 This recommendation is based primarily on the landmark report by De Cueto and colleagues.11 These authors evaluated colonized women who received intrapartum prophylaxis at varying times prior to delivery. Their primary endpoint was the percentage of newborns who were colonized with GBS. If the mothers had received antibiotics for less than 1 hour prior to delivery, 46% of neonates were colonized. This figure was equal to the rate of colonization in neonates whose mothers received no antibiotics. When the interval was 1 to 2 hours, the percentage was 29%. When mothers had received antibiotics for 2 to 4 hours, the neonatal colonization rate fell to 2.9%. When antibiotics had been administered for greater than 4 hours, the rate of neonatal colonization was only 1.2%.

Fairlie and colleagues recently reported the results of another interesting investigation comparing the effectiveness of prophylaxis based on duration of treatment and choice of individual antibiotics.12 Prophylaxis with penicillin or ampicillin for 4 hours or more was 91% effective in preventing early-onset neonatal infection in term infants and 86% effective in preventing infection in preterm infants. These outcomes were superior to the outcomes in both term and preterm infants who received penicillin or ampicillin for less than 4 hours.

These observations agree with the findings of McNanley and colleagues who evaluated vaginal colony counts of GBS following different periods of antibiotic administration.13 These authors noted that mean colony counts decreased 5-fold within 2 hours of penicillin administration, 50-fold within 4 hours, and 1,000-fold within 6 hours.
Despite these compelling findings, the ACOG Committee Opinion stresses that obstetric interventions such as amniotomy and oxytocin augmentation should not be delayed simply to permit a certain time period of antibiotic administration.5
Continue to: “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”...
6. “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”
If a mother is scheduled to have a CD, for example because of a prior cesarean or because of a persistent fetal malpresentation, she should still have a GBS culture at 36 0/7 to 37 6/7 weeks’ gestation. The information obtained from this culture may be of value to both the obstetrician and pediatrician if the patient experiences labor or rupture of membranes prior to her scheduled surgery. If she does not experience spontaneous labor prior to her scheduled date of surgery, she does not require specific GBS prophylaxis at the time of her operation.5 Rather, she should receive prophylactic antibiotics to prevent post–cesarean infection, ideally, the combination of cefazolin (2 g IV) plus azithromycin (500 mg IV).14 Cefazolin, of course, provides excellent coverage of GBS.
7. “If I am colonized with GBS and I receive treatment during labor, will my baby be safe after delivery?”
The interventions outlined above will prevent almost 90% of early-onset GBS infections, but they are not foolproof.5-7,15,16 Successful management of the neonate is dependent upon several factors, including:5-7
- gestational age
- presence of maternal chorioamnionitis
- presence or absence of risk factors for early-onset infection
- duration (adequacy) of maternal treatment during labor
- presence of immediate clinical signs of infection in the neonate (such as fever, lethargy, hemodynamic instability, respiratory distress, or elevated or decreased white blood cell count).
If the mother is at term and receives intrapartum prophylaxis for at least 4 hours prior to delivery, the neonate usually will not require any special tests and simply will be observed for 24 to 48 hours for signs of infection.
If the mother delivers preterm and receives appropriate intrapartum prophylaxis, the pediatricians typically will obtain a complete blood count (CBC) and treat with prophylactic antibiotics (ampicillin plus gentamicin) for 48 hours if abnormalities are noted on the CBC or the baby exhibits signs of infection. If the CBC is normal and the baby shows no signs of infection, no treatment is indicated.
Regardless of gestational age, if the mother does not receive prophylaxis for at least 4 hours before delivery, the pediatricians usually will obtain a CBC and closely observe the baby in the hospital for signs of infection. If such signs develop or the CBC is abnormal, blood and cerebrospinal fluid cultures will be obtained. Antibiotic therapy (usually ampicillin plus gentamicin) is then initiated, and the drugs are continued until cultures return with no growth. If either culture is positive, antibiotics will then be continued for 7 to 10 days.
If the mother has documented chorioamnionitis and receives treatment intrapartum with appropriate antibiotics (usually ampicillin plus gentamicin), the pediatricians usually will obtain a CBC, C-reactive protein (CRP) level, and blood cultures and then start the infant on antibiotics, pending the result of the laboratory tests. If the CBC and CRP are reassuring, the cultures are negative after 48 hours, and the infant demonstrates no signs of clinical infection, many pediatricians will then discontinue antibiotics. Others may still continue the antibiotics for 7 to 10 days.
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992;90:775-778.
- CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24.
- Schrag S, Gorwitz R, Fultz-Butts K, et al. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010;59:1-36.
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134:206-210.
- Duff P, Birsner M. Maternal and perinatal infection in pregnancy: bacteria. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics. Normal and Problem Pregnancies. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019.
- Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
- Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:540-544.
- Onwuchuruba CN, Towers CV, Howard BC, et al. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210:352.e1-352.e4.
- de Cueto M, Sanchez MJ, Sampedro A, et al. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol. 1998;91:112-114.
- Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013;121:570-577.
- McNanley AR, Glantz JC, Hardy DJ, et al. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am J Obstet Gynecol. 2007;197:583.e1-583.e4.
- Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Brozanski BS, Jones JG, Krohn MA, et al. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol. 2000;95:496-501.
- Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The National Group B Streptococcal Disease Study Group. Obstet Gynecol. 1997;90:901-906.
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992;90:775-778.
- CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24.
- Schrag S, Gorwitz R, Fultz-Butts K, et al. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010;59:1-36.
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134:206-210.
- Duff P, Birsner M. Maternal and perinatal infection in pregnancy: bacteria. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics. Normal and Problem Pregnancies. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019.
- Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
- Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:540-544.
- Onwuchuruba CN, Towers CV, Howard BC, et al. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210:352.e1-352.e4.
- de Cueto M, Sanchez MJ, Sampedro A, et al. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol. 1998;91:112-114.
- Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013;121:570-577.
- McNanley AR, Glantz JC, Hardy DJ, et al. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am J Obstet Gynecol. 2007;197:583.e1-583.e4.
- Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Brozanski BS, Jones JG, Krohn MA, et al. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol. 2000;95:496-501.
- Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The National Group B Streptococcal Disease Study Group. Obstet Gynecol. 1997;90:901-906.
The One Step test: The better diagnostic approach for gestational diabetes mellitus
Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19
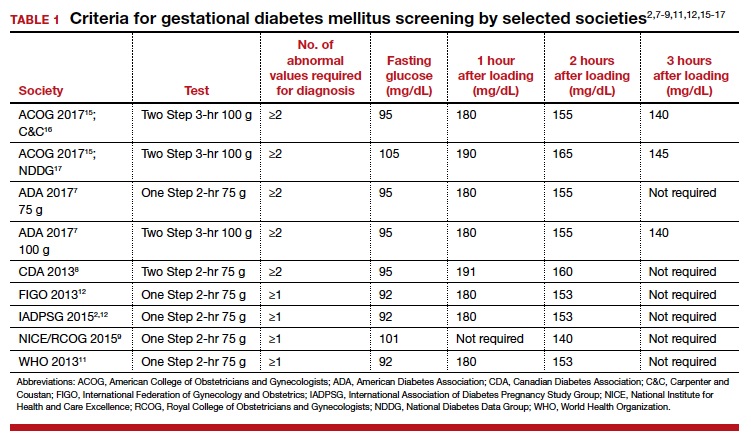
Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19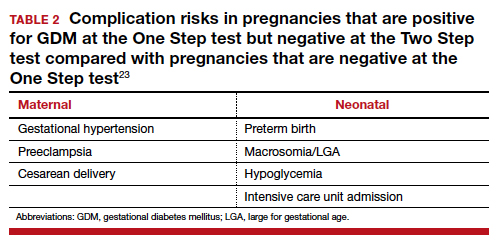
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.
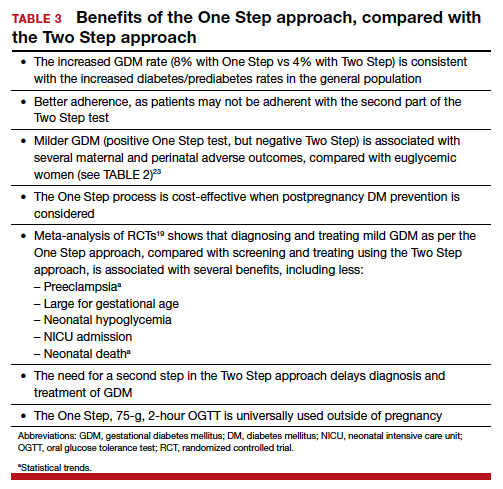
- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19

Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.

Gestational diabetes mellitus (GDM) generally is defined as any degree of glucose intolerance with onset or first recognition during pregnancy.1-14 The best approach and exact criteria to use for GDM screening and diagnosis are under worldwide debate. In TABLE 1 we present just some of the many differing suggestions by varying organizations.2,7-9,11,12,15-17 The American College of Obstetricians and Gynecologists, for instance, suggests a Two Step approach to diagnosis.15 We will make the argument in this article, however, that diagnosis should be defined universally as an abnormal result with the One Step 75-g glucose testing, as adopted by the World Health Organization, International Federation of Gynecology and Obstetrics, and others. Approximately 8% of all pregnancies are complicated by GDM by the One Step test in the United States.18-22 The prevalence may range from 1% to 14% of all pregnancies, depending on the population studied and the diagnostic tests employed.1,19

Diagnostic options
Different methods for screening and diagnosis of GDM have been proposed by international societies; there is controversy regarding the diagnosis of GDM by either the One Step or the Two Step approach.6
The One Step approach includes an oral glucose tolerance test with a 75-g glucose load with measurement of plasma glucose concentration at fasting state and 1 hour and 2 hours post–glucose administration. A positive result for the One Step approach is defined as at least 1 measurement higher than 92, 180, or 153 mg/dL at fasting, 1 hour, or 2 hours, respectively.
The Two Step approach includes a nonfasting oral 50-g glucose load, with a glucose blood measurement 1 hour later. A positive screening, defined often as a blood glucose value higher than 135 mg/dL (range, 130 to 140 mg/dL), is followed by a diagnostic test with a 100-g glucose load with measurements at fasting and 1, 2, and 3 hours post–glucose administration. A positive diagnostic test is defined as 2 measurements higher than the target value.
Why we support the One Step test
There are several reasons to prefer the One Step approach for the diagnosis of GDM, compared with the Two Step approach.
Women testing negative for GDM with Two Step still experience complications pregnancy. Women who test positive for GDM with the One Step test, but negative with the Two Step test, despite having therefore a milder degree of glucose intolerance, do have a higher risk of experiencing several complications.23 For the mother, these complications include gestational hypertension, preeclampsia, and cesarean delivery. The baby also can experience problems at birth (TABLE 2).23 Therefore, women who test positive for GDM with the One Step test deserve to be diagnosed with and treated for the condition, as not only are they at risk for these complications but also treatment of the GDM decreases the incidence of these complications.18,19
There is indeed an increased GDM diagnosis rate with the One Step (about 8%) compared with the Two Step test (about 4%). Nonetheless, this increase is mild and nonsignificant in the meta-analysis of randomized controlled trials (RCTs),18,19 is less than the 18% difference in diagnosis rate previously hypothesized, is consistent with the increased diabetes/prediabetes rates in the general population, and is linked to the increasing incidence of obesity and insulin resistance.
Overall test adherence is better. Five percent to 15% of patients, depending on the study, are not adherent with taking the second part of the Two Step test. Women indeed prefer the One Step approach; the second step in the Two Step approach may be a burden.
Less costly. The One Step process is cost-effective when postpregnancy diabetes mellitus prevention is considered.
Better maternal and perinatal outcomes. Probably the most important and convincing reason the One Step test should be used is that meta-analysis of the 4 RCTs comparing the approaches (including 2 US trials) shows that diagnosing and treating mild GDM as per the One Step approach, compared with screening and treating using the Two Step approach, is associated with increased incidence of GDM (8% vs 4%) and with better maternal and perinatal outcomes.13,18,19 In fact, the One Step approach is associated with significant reductions in: large for gestational age (56%), admission to neonatal intensive care unit (51%), and neonatal hypoglycemia (48%). Tests of heterogeneity in the meta-analysis and of quality all pointed to better outcomes in the One Step test group.13,19
The need for a second step in the Two Step approach delays diagnosis and treatment. The One Step approach is associated with an increase in GDM test adherence and earlier diagnosis,13 which is another reason for better outcomes with the One Step approach. In the presence of risk factors, such as prior GDM, prior macrosomia, advanced maternal age, multiple gestations, and others, the One Step test should be done at the first prenatal visit.
Continue to: US guidelines should be reconsidered...
US guidelines should be reconsidered
The One Step, 75-g, 2-hour oral glucose tolerance test is universally used to diagnose diabetes mellitus outside of pregnancy. Given our many noted reasons (TABLE 3), we recommend universal screening of GDM by using the One Step approach. It is time, indeed, for the United States to reconsider its guidelines for screening for GDM.

- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
- Kampmann U, Madsen LR, Skajaa GO, et al. Gestational diabetes: a clinical update. World J Diabetes. 2015;6:1065-1072.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Meltzer SJ, Snyder J, Penrod JR, et al. Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG. 2010;117:407-415.
- Sevket O, Ates S, Uysal O, et al. To evaluate the prevalence and clinical outcomes using a one-step method versus a two-step method to screen gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:36-41.
- Scifres CM, Abebe KZ, Jones KA, et al. Gestational diabetes diagnostic methods (GD2M) pilot randomized trial. Matern Child Health J. 2015;19:1472-1480.
- Farrar D, Duley L, Medley N, et al. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015;1:CD007122.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11-S24.
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Diabetes and Pregnancy. Can J Diabetes. 2013;37(suppl 1):S168-S183.
- NICE guideline. Diabetes in pregnancy: management from preconception to the postnatal period. February 2015. https://www.nice.org.uk/guidance/ng3/. Last updated August 2015. Accessed November 18, 2019.
- WHO 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. From http://apps.who.int/iris/bitstream/10665/66040/1/WHO
_NCD_NCS_99.2.pdf. Accessed November 18, 2019. - World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013. http://apps.who.int/iris/bitstream/10665/85975/1/WHO_
NMH_MND_13.2_eng.pdf. Accessed November 18, 2019. - Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(suppl 3):S173-S211.
- Berghella V, Caissutti C, Saccone G, et al. The One Step approach for diagnosing gestational diabetes is associated with better perinatal outcomes than using the Two Step approach: evidence of randomized clinical trials. Am J Obstet Gynecol. 2019;220:562-564.
- Berghella V, Caissutti C, Saccone G, et al. One-Step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2019;133:383.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 180: gestational diabetes mellitus. Obstet Gynecol. 2017;130:e17-e31.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039-1057.
- Saccone G, Khalifeh A, Al-Kouatly HB, et al. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J Matern Fetal Neonatal Med. 2018:1-9.
- Saccone G, Caissutti C, Khalifeh A, et al. One step versus two step approach for gestational diabetes screening: systematic review and meta-analysis of the randomized trials. J Matern Fetal Neonatal Med. 2019;32:1547-1555.
- Khalifeh A, Eckler R, Felder L, et al. One-step versus two-step diagnostic testing for gestational diabetes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2018:1-6.
- Caissutti C, Saccone G, Khalifeh A, et al. Which criteria should be used for starting pharmacologic therapy for management of gestational diabetes in pregnancy? Evidence from randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2905-2914.
- Caissutti C, Saccone G, Ciardulli A, et al. Very tight vs. tight control: which should be the criteria for pharmacologic therapy dose adjustment in diabetes in pregnancy? Evidence from randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:235-247.
- Caissutti C, Khalifeh A, Saccone G, et al. Are women positive for the One Step but negative for the Two Step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018;97:122-134.
Does planned early delivery make sense in women with preterm preeclampsia?
Chappell LC, Brocklehurst P, Green ME, et al; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181-1190.
EXPERT COMMENTARY
Preeclampsia is a common hypertensive disorder of pregnancy. Among women who develop the disease at late preterm gestation, the question remains, “What is the optimal timing for delivery?” The American College of Obstetricians and Gynecologists (ACOG) categorizes preeclampsia as “with and without severe features.”1 Delivery is recommended for women with preeclampsia with severe features at or beyond 34 weeks’ gestation, and for women with preeclampsia without severe features at or beyond 37 weeks’ gestation.1 For patients with fetal growth restriction and preeclampsia, ACOG also recommends delivery between 34 and 37 weeks’ gestation.
Details of the study
Chappell and colleagues conducted a randomized controlled trial among women with singleton or dichorionic diamniotic twin pregnancy between 34 and 36.6 weeks’ gestation. Women were assigned to either planned delivery within 48 hours of randomization or expectant management until 37 weeks or earlier with clinical deterioration.
Among the 901 women included in the study, 450 were allocated to planned delivery and 451 to expectant management.
Study outcomes. The co-primary short-term maternal outcome was a composite of maternal morbidity with the addition of recorded systolic blood pressure of at least 160 mm Hg postrandomization (on any occasion). The co-primary short-term perinatal outcome was a composite of neonatal deaths within 7 days of delivery and perinatal deaths or neonatal unit admissions.
Participant details. At baseline, the average gestational age at randomization was 35.6 weeks, with equal distribution through the 3 weeks (34 through 36 weeks). About 37% of the women had severe hypertension (≥ 160 mm Hg) in the previous 48 hours prior to randomization, and approximately 22% had fetal growth restriction. The authors did not categorize the women based on severe features of preeclampsia.
Results. The investigators found that the proportion of women with the maternal co-primary outcome was significantly lower in the planned delivery group compared with the expectant management group (65% vs 75%), and the proportion of infants with the perinatal co-primary outcome was significantly higher in the planned delivery group compared with the expectant management group (42% vs 34%). The fact that early delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay, is very reassuring.
Study strengths and limitations
This is the largest study of women in this group allocated, randomized, and multicenter investigation addressing a very important clinical question. The patient population was mostly white, with only 13% black women, and had an average body mass index of 29 kg/m2 (which is low compared with many practices in the United States). The average difference between the 2 study groups was the additional prolongation of pregnancy from enrollment to delivery of only 3 days, which may not be clinically relevant. More than half of the women in the expectant management group had medically indicated delivery before 37 weeks’ gestation.
Continue to: A limitation of this study...
A limitation of this study is that all women with preeclampsia were considered the same—that is, no distinction was made between severe and nonsevere preeclampsia, and a significant proportion of women had severe hypertension at enrollment, which would make them ineligible for expectant management anyway.
The maternal composite outcome was driven mostly by severe hypertension and progression to severe preeclampsia (likely driven by severe hypertension). All other maternal outcomes were very rare or did not happen; however, the incidence of delivery indications for various preeclampsia-related complications was higher in the expectant management group.
The takeaway
In the absence of biomarkers for risk stratification and treatment of preeclampsia, delivering women who have a diagnosis of preeclampsia at or beyond 34 weeks’ gestation may be a viable option for preventing maternal complications.
In the United States, preeclampsia is categorized as severe or nonsevere, and gestational age at delivery depends on the type of preeclampsia. Clinicians should discuss expectant management after 34 weeks with patients who have preeclampsia without severe features, noting that this may decrease the chances for adverse maternal outcomes (mostly severe hypertension) at the cost of neonatal intensive care unit admission, which may depend on local practices. Attention also should be paid to particular patient populations (such as obese and African American women) who are at higher risk for developing adverse maternal outcomes. This may be particularly relevant in a smaller hospital setting in which patient follow-up may not be universal or access to a maternal-fetal medicine specialist may not be available to discuss management plans.
My personal take: I work in a large tertiary medical center. I worry about added prematurity, especially among women with superimposed preeclampsia where the diagnosis may be unclear. In my practice, we monitor patients with preeclampsia very closely, and with any signs of severe features we deliver them after 34 weeks. We follow ACOG guidelines for managing preeclampsia based on severity of disease and gestational age. I am not planning to immediately change my practice based on this study by Chappell and colleagues, and I will wait for results of long-term effects on neonatal outcomes, studies using biomarkers for risk assessment of women at risk for adverse outcomes, and opinions from ACOG and the Society for Maternal-Fetal Medicine about this management plan.
SAROSH RANA, MD, MPH
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
Chappell LC, Brocklehurst P, Green ME, et al; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181-1190.
EXPERT COMMENTARY
Preeclampsia is a common hypertensive disorder of pregnancy. Among women who develop the disease at late preterm gestation, the question remains, “What is the optimal timing for delivery?” The American College of Obstetricians and Gynecologists (ACOG) categorizes preeclampsia as “with and without severe features.”1 Delivery is recommended for women with preeclampsia with severe features at or beyond 34 weeks’ gestation, and for women with preeclampsia without severe features at or beyond 37 weeks’ gestation.1 For patients with fetal growth restriction and preeclampsia, ACOG also recommends delivery between 34 and 37 weeks’ gestation.
Details of the study
Chappell and colleagues conducted a randomized controlled trial among women with singleton or dichorionic diamniotic twin pregnancy between 34 and 36.6 weeks’ gestation. Women were assigned to either planned delivery within 48 hours of randomization or expectant management until 37 weeks or earlier with clinical deterioration.
Among the 901 women included in the study, 450 were allocated to planned delivery and 451 to expectant management.
Study outcomes. The co-primary short-term maternal outcome was a composite of maternal morbidity with the addition of recorded systolic blood pressure of at least 160 mm Hg postrandomization (on any occasion). The co-primary short-term perinatal outcome was a composite of neonatal deaths within 7 days of delivery and perinatal deaths or neonatal unit admissions.
Participant details. At baseline, the average gestational age at randomization was 35.6 weeks, with equal distribution through the 3 weeks (34 through 36 weeks). About 37% of the women had severe hypertension (≥ 160 mm Hg) in the previous 48 hours prior to randomization, and approximately 22% had fetal growth restriction. The authors did not categorize the women based on severe features of preeclampsia.
Results. The investigators found that the proportion of women with the maternal co-primary outcome was significantly lower in the planned delivery group compared with the expectant management group (65% vs 75%), and the proportion of infants with the perinatal co-primary outcome was significantly higher in the planned delivery group compared with the expectant management group (42% vs 34%). The fact that early delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay, is very reassuring.
Study strengths and limitations
This is the largest study of women in this group allocated, randomized, and multicenter investigation addressing a very important clinical question. The patient population was mostly white, with only 13% black women, and had an average body mass index of 29 kg/m2 (which is low compared with many practices in the United States). The average difference between the 2 study groups was the additional prolongation of pregnancy from enrollment to delivery of only 3 days, which may not be clinically relevant. More than half of the women in the expectant management group had medically indicated delivery before 37 weeks’ gestation.
Continue to: A limitation of this study...
A limitation of this study is that all women with preeclampsia were considered the same—that is, no distinction was made between severe and nonsevere preeclampsia, and a significant proportion of women had severe hypertension at enrollment, which would make them ineligible for expectant management anyway.
The maternal composite outcome was driven mostly by severe hypertension and progression to severe preeclampsia (likely driven by severe hypertension). All other maternal outcomes were very rare or did not happen; however, the incidence of delivery indications for various preeclampsia-related complications was higher in the expectant management group.
The takeaway
In the absence of biomarkers for risk stratification and treatment of preeclampsia, delivering women who have a diagnosis of preeclampsia at or beyond 34 weeks’ gestation may be a viable option for preventing maternal complications.
In the United States, preeclampsia is categorized as severe or nonsevere, and gestational age at delivery depends on the type of preeclampsia. Clinicians should discuss expectant management after 34 weeks with patients who have preeclampsia without severe features, noting that this may decrease the chances for adverse maternal outcomes (mostly severe hypertension) at the cost of neonatal intensive care unit admission, which may depend on local practices. Attention also should be paid to particular patient populations (such as obese and African American women) who are at higher risk for developing adverse maternal outcomes. This may be particularly relevant in a smaller hospital setting in which patient follow-up may not be universal or access to a maternal-fetal medicine specialist may not be available to discuss management plans.
My personal take: I work in a large tertiary medical center. I worry about added prematurity, especially among women with superimposed preeclampsia where the diagnosis may be unclear. In my practice, we monitor patients with preeclampsia very closely, and with any signs of severe features we deliver them after 34 weeks. We follow ACOG guidelines for managing preeclampsia based on severity of disease and gestational age. I am not planning to immediately change my practice based on this study by Chappell and colleagues, and I will wait for results of long-term effects on neonatal outcomes, studies using biomarkers for risk assessment of women at risk for adverse outcomes, and opinions from ACOG and the Society for Maternal-Fetal Medicine about this management plan.
SAROSH RANA, MD, MPH
Chappell LC, Brocklehurst P, Green ME, et al; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet. 2019;394:1181-1190.
EXPERT COMMENTARY
Preeclampsia is a common hypertensive disorder of pregnancy. Among women who develop the disease at late preterm gestation, the question remains, “What is the optimal timing for delivery?” The American College of Obstetricians and Gynecologists (ACOG) categorizes preeclampsia as “with and without severe features.”1 Delivery is recommended for women with preeclampsia with severe features at or beyond 34 weeks’ gestation, and for women with preeclampsia without severe features at or beyond 37 weeks’ gestation.1 For patients with fetal growth restriction and preeclampsia, ACOG also recommends delivery between 34 and 37 weeks’ gestation.
Details of the study
Chappell and colleagues conducted a randomized controlled trial among women with singleton or dichorionic diamniotic twin pregnancy between 34 and 36.6 weeks’ gestation. Women were assigned to either planned delivery within 48 hours of randomization or expectant management until 37 weeks or earlier with clinical deterioration.
Among the 901 women included in the study, 450 were allocated to planned delivery and 451 to expectant management.
Study outcomes. The co-primary short-term maternal outcome was a composite of maternal morbidity with the addition of recorded systolic blood pressure of at least 160 mm Hg postrandomization (on any occasion). The co-primary short-term perinatal outcome was a composite of neonatal deaths within 7 days of delivery and perinatal deaths or neonatal unit admissions.
Participant details. At baseline, the average gestational age at randomization was 35.6 weeks, with equal distribution through the 3 weeks (34 through 36 weeks). About 37% of the women had severe hypertension (≥ 160 mm Hg) in the previous 48 hours prior to randomization, and approximately 22% had fetal growth restriction. The authors did not categorize the women based on severe features of preeclampsia.
Results. The investigators found that the proportion of women with the maternal co-primary outcome was significantly lower in the planned delivery group compared with the expectant management group (65% vs 75%), and the proportion of infants with the perinatal co-primary outcome was significantly higher in the planned delivery group compared with the expectant management group (42% vs 34%). The fact that early delivery led to more neonatal unit admissions for the infant, principally for a listed indication of prematurity and without an excess of respiratory or other morbidity, intensity of care, or length of stay, is very reassuring.
Study strengths and limitations
This is the largest study of women in this group allocated, randomized, and multicenter investigation addressing a very important clinical question. The patient population was mostly white, with only 13% black women, and had an average body mass index of 29 kg/m2 (which is low compared with many practices in the United States). The average difference between the 2 study groups was the additional prolongation of pregnancy from enrollment to delivery of only 3 days, which may not be clinically relevant. More than half of the women in the expectant management group had medically indicated delivery before 37 weeks’ gestation.
Continue to: A limitation of this study...
A limitation of this study is that all women with preeclampsia were considered the same—that is, no distinction was made between severe and nonsevere preeclampsia, and a significant proportion of women had severe hypertension at enrollment, which would make them ineligible for expectant management anyway.
The maternal composite outcome was driven mostly by severe hypertension and progression to severe preeclampsia (likely driven by severe hypertension). All other maternal outcomes were very rare or did not happen; however, the incidence of delivery indications for various preeclampsia-related complications was higher in the expectant management group.
The takeaway
In the absence of biomarkers for risk stratification and treatment of preeclampsia, delivering women who have a diagnosis of preeclampsia at or beyond 34 weeks’ gestation may be a viable option for preventing maternal complications.
In the United States, preeclampsia is categorized as severe or nonsevere, and gestational age at delivery depends on the type of preeclampsia. Clinicians should discuss expectant management after 34 weeks with patients who have preeclampsia without severe features, noting that this may decrease the chances for adverse maternal outcomes (mostly severe hypertension) at the cost of neonatal intensive care unit admission, which may depend on local practices. Attention also should be paid to particular patient populations (such as obese and African American women) who are at higher risk for developing adverse maternal outcomes. This may be particularly relevant in a smaller hospital setting in which patient follow-up may not be universal or access to a maternal-fetal medicine specialist may not be available to discuss management plans.
My personal take: I work in a large tertiary medical center. I worry about added prematurity, especially among women with superimposed preeclampsia where the diagnosis may be unclear. In my practice, we monitor patients with preeclampsia very closely, and with any signs of severe features we deliver them after 34 weeks. We follow ACOG guidelines for managing preeclampsia based on severity of disease and gestational age. I am not planning to immediately change my practice based on this study by Chappell and colleagues, and I will wait for results of long-term effects on neonatal outcomes, studies using biomarkers for risk assessment of women at risk for adverse outcomes, and opinions from ACOG and the Society for Maternal-Fetal Medicine about this management plan.
SAROSH RANA, MD, MPH
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
Click for Credit: PPI use & dementia; Weight loss after gastroplasty; more
Here are 5 articles from the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty
To take the posttest, go to: https://bit.ly/37lteRX
Expires May 16, 2020
2. PT beats steroid injections for knee OA
To take the posttest, go to: https://bit.ly/2KIWKY6
Expires May 17, 2020
3. Better screening needed to reduce pregnancy-related overdose, death
To take the posttest, go to: https://bit.ly/2XEZyuG
Expires May 17, 2020
4. Meta-analysis finds no link between PPI use and risk of dementia
To take the posttest, go to: https://bit.ly/2Xzs7JM
Expires June 3, 2020
5. Study: Cardiac biomarkers predicted CV events in CAP
To take the posttest, go to: https://bit.ly/33bAH2u
Expires August 13, 2020
Here are 5 articles from the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty
To take the posttest, go to: https://bit.ly/37lteRX
Expires May 16, 2020
2. PT beats steroid injections for knee OA
To take the posttest, go to: https://bit.ly/2KIWKY6
Expires May 17, 2020
3. Better screening needed to reduce pregnancy-related overdose, death
To take the posttest, go to: https://bit.ly/2XEZyuG
Expires May 17, 2020
4. Meta-analysis finds no link between PPI use and risk of dementia
To take the posttest, go to: https://bit.ly/2Xzs7JM
Expires June 3, 2020
5. Study: Cardiac biomarkers predicted CV events in CAP
To take the posttest, go to: https://bit.ly/33bAH2u
Expires August 13, 2020
Here are 5 articles from the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty
To take the posttest, go to: https://bit.ly/37lteRX
Expires May 16, 2020
2. PT beats steroid injections for knee OA
To take the posttest, go to: https://bit.ly/2KIWKY6
Expires May 17, 2020
3. Better screening needed to reduce pregnancy-related overdose, death
To take the posttest, go to: https://bit.ly/2XEZyuG
Expires May 17, 2020
4. Meta-analysis finds no link between PPI use and risk of dementia
To take the posttest, go to: https://bit.ly/2Xzs7JM
Expires June 3, 2020
5. Study: Cardiac biomarkers predicted CV events in CAP
To take the posttest, go to: https://bit.ly/33bAH2u
Expires August 13, 2020
ART treatment at birth found to benefit neonates with HIV
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Antiretroviral treatment initiation immediately after birth reduced HIV-1 viral reservoir size and alters innate immune responses in neonates.
Major finding: Very-early ART intervention in neonates infected with HIV limited the number of virally infected cells and improves immune response.
Study details: A cohort study of 10 infants infected with HIV who were born in Botswana, Africa.
Disclosures: The Early Infant Treatment study is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
Source: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Metformin after GDM: Lessons from landmark diabetes prevention trial
WASHINGTON – Metformin’s role in preventing or delaying the onset of type 2 diabetes in women with a history of gestational diabetes mellitus has been firmly established by the Diabetes Prevention Program (DPP) trial – most recently, by 15-year follow-up data reported this year – and the drug should be front and center for clinicians who hope to stave off the “remarkable” incidence of type 2 diabetes after GDM, Robert E. Ratner, MD, maintained at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
The DPP included “the single largest population of women with a history of GDM that’s been looked at in a randomized controlled trial,” and considering its multiethnic population, the trial offers a reliable representative sample to ponder today when evaluating long-term use of metformin after GDM, said Dr. Ratner, a principal investigator of the National Institutes of Health–sponsored DPP and the DPP Outcomes Study and a former chief scientific & medical officer for the American Diabetes Association.
The drug stacked up equally to lifestyle interventions among DPP participants who had a history of GDM, but it’s important to appreciate that these interventions were intensive and that metformin is inexpensive, well tolerated, and “has a long safety record,” he said.
Results of follow-up out to 15 years
Of the more than 3,000 men and women enrolled in the landmark DPP, conducted during 1996-2001, 350 were women with a documented history of GDM and over 1,400 were women who had deliveries but no history of GDM. All participants had impaired glucose tolerance – defined for the trial as having both a fasting plasma glucose value of 95-125 mg/dL and a 2-hour value of 140-199 mg/dL after a 75-g glucose load – and were randomized to placebo, metformin, or intensive lifestyle intervention.
Metformin therapy reduced the incidence of diabetes by approximately 50% in women with a history of GDM, compared with the placebo group – as did lifestyle – over 3 years. The number needed to treat to prevent one case of diabetes was five. Women without a history of GDM, on the other hand, saw only a 14% reduction with metformin when compared with placebo (and a 49% reduction with lifestyle).
“In women with a history of GDM ... one pill twice a day for $4 a month worked as well as intensive lifestyle [change],” Dr. Ratner said, referring to the initial GDM-specific analysis of DPP data published in 2008 (J Clin Endocrinol Metab. 2008;93[12]:4774-9).
In a 10-year postrandomization follow-up, published in 2015, both metformin and lifestyle continued to be equally effective for the GDM group, reducing the progression to diabetes by 40% and 35%, respectively (J Clin Endocrinol Metab. 2015;100:1646-53). The number needed to treat to prevent one case of diabetes was seven. (Among women without a history of GDM, metformin did not reduce progression to diabetes.)
A recent DPP Outcomes Study analysis of metformin’s impact on diabetes prevention at 15 years, moreover, showed a 41% risk reduction among women with a history of GDM (Diabetes Care. 2019;42[4]:601-8).
Advice on prescribing metformin prophylactically
Asked after his presentation whether women with a history of GDM and either an elevated fasting plasma glucose value or an elevated 2-hour oral glucose tolerance test (GTT) value – or neither of the two – would benefit from taking metformin, Dr. Ratner said that “we’re stuck with inclusion criteria of the DPP, in which they had to meet both criteria ... What I’d say, though, is that not everyone with a history of GDM needs to be on metformin prophylactically. But [for women who have] prediabetes as defined by the ADA, the cost-benefit analysis points toward metformin.”
And with respect to early initiation and long-term use of the drug, “I would have absolutely no qualms about medicating a 25-year-old who had developed GDM and who in the postpartum period has prediabetes,” Dr. Ratner said during an open discussion. “She’s actually at the highest risk for developing type 2 very early.”
Kim Boggess, MD, who also presented on long-term use of metformin after GDM, said in the discussion period that she is often quick to recommend metformin therapy to her patients who have an elevated fasting plasma glucose value in the postpartum period, even when a 75-g oral GTT has not yet been performed. (The ADA and the American College of Obstetricians and Gynecologists recommend completion of an oral GTT at 4-12 weeks postpartum after GDM.)
“I start them [on metformin] especially if they’ve had a cesarean section. Even 2, 3, 4 weeks of profound hyperglycemia could have potentially deleterious effects,” said Dr. Boggess, professor and maternal-fetal medicine program director at the University of North Carolina, Chapel Hill. “If someone comes in [shortly after] and looks like they have pristine control, then it might be worth stopping the metformin for 3-5 days (and retesting).”
Dr. Ratner said that, in this clinical scenario, he would first ensure that the fasting glucose value “is a true fasting glucose” and “if it’s substantially elevated – I’m talking 100, 105, 110 mg/dL – I’d start metformin, and I’m not even sure I’d do the GTT.” But, he advised, “if you’re going to do the GTT, I’d stop the metformin the day before.”
In her presentation, Dr. Boggess pointed out that metformin wasn’t shown to be superior to lifestyle interventions in the DPP for preventing progression to type 2 DM, and that some women are more motivated for intensive lifestyle change than others. The ADA recommends, in fact, that either metformin or lifestyle interventions be prescribed to women with a history of GDM who are found to have prediabetes.
There are no data to support the use of metformin either during or after pregnancy to improve weight loss or reduce weight retention following pregnancy, but at least several studies have shown that lifestyle interventions are effective, she noted.
What is needed, Dr. Boggess said, are more data on the effects of metformin on cardiovascular disease risk, as well as larger studies of metformin in the postpartum period “to help us determine the best dose.” Some research on metformin use in the postpartum period has reported gastrointestinal side effects and dissatisfaction, she noted.
Dr. Ratner said that metformin’s main drawback is the need for occasional testing of B12 levels. Regarding weight loss and what was observed in the DPP, he said, women with a history of GDM who were randomized to intensive lifestyle interventions did not lose as much weight as women without a history of GDM.
Women who entered the DPP with a GDM history, he noted in his presentation, were essentially a “cohort of survivors.” They had an average age of 43 (compared with 52 years in the parous women without GDM) and a mean interval from the index GDM pregnancy of 11 years, which means that women with the highest risk of diabetes conversion were excluded, Dr. Ratner said.
Age was the only significantly different baseline characteristic between parous women with and without GDM, he noted. Women with a history of GDM who were randomized to placebo had a 71% higher incidence of diabetes than women without such a history – a striking natural history, Dr. Ratner said.
He and Dr. Boggess each reported that they have no financial or other interests that pose a conflict of interest.
WASHINGTON – Metformin’s role in preventing or delaying the onset of type 2 diabetes in women with a history of gestational diabetes mellitus has been firmly established by the Diabetes Prevention Program (DPP) trial – most recently, by 15-year follow-up data reported this year – and the drug should be front and center for clinicians who hope to stave off the “remarkable” incidence of type 2 diabetes after GDM, Robert E. Ratner, MD, maintained at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
The DPP included “the single largest population of women with a history of GDM that’s been looked at in a randomized controlled trial,” and considering its multiethnic population, the trial offers a reliable representative sample to ponder today when evaluating long-term use of metformin after GDM, said Dr. Ratner, a principal investigator of the National Institutes of Health–sponsored DPP and the DPP Outcomes Study and a former chief scientific & medical officer for the American Diabetes Association.
The drug stacked up equally to lifestyle interventions among DPP participants who had a history of GDM, but it’s important to appreciate that these interventions were intensive and that metformin is inexpensive, well tolerated, and “has a long safety record,” he said.
Results of follow-up out to 15 years
Of the more than 3,000 men and women enrolled in the landmark DPP, conducted during 1996-2001, 350 were women with a documented history of GDM and over 1,400 were women who had deliveries but no history of GDM. All participants had impaired glucose tolerance – defined for the trial as having both a fasting plasma glucose value of 95-125 mg/dL and a 2-hour value of 140-199 mg/dL after a 75-g glucose load – and were randomized to placebo, metformin, or intensive lifestyle intervention.
Metformin therapy reduced the incidence of diabetes by approximately 50% in women with a history of GDM, compared with the placebo group – as did lifestyle – over 3 years. The number needed to treat to prevent one case of diabetes was five. Women without a history of GDM, on the other hand, saw only a 14% reduction with metformin when compared with placebo (and a 49% reduction with lifestyle).
“In women with a history of GDM ... one pill twice a day for $4 a month worked as well as intensive lifestyle [change],” Dr. Ratner said, referring to the initial GDM-specific analysis of DPP data published in 2008 (J Clin Endocrinol Metab. 2008;93[12]:4774-9).
In a 10-year postrandomization follow-up, published in 2015, both metformin and lifestyle continued to be equally effective for the GDM group, reducing the progression to diabetes by 40% and 35%, respectively (J Clin Endocrinol Metab. 2015;100:1646-53). The number needed to treat to prevent one case of diabetes was seven. (Among women without a history of GDM, metformin did not reduce progression to diabetes.)
A recent DPP Outcomes Study analysis of metformin’s impact on diabetes prevention at 15 years, moreover, showed a 41% risk reduction among women with a history of GDM (Diabetes Care. 2019;42[4]:601-8).
Advice on prescribing metformin prophylactically
Asked after his presentation whether women with a history of GDM and either an elevated fasting plasma glucose value or an elevated 2-hour oral glucose tolerance test (GTT) value – or neither of the two – would benefit from taking metformin, Dr. Ratner said that “we’re stuck with inclusion criteria of the DPP, in which they had to meet both criteria ... What I’d say, though, is that not everyone with a history of GDM needs to be on metformin prophylactically. But [for women who have] prediabetes as defined by the ADA, the cost-benefit analysis points toward metformin.”
And with respect to early initiation and long-term use of the drug, “I would have absolutely no qualms about medicating a 25-year-old who had developed GDM and who in the postpartum period has prediabetes,” Dr. Ratner said during an open discussion. “She’s actually at the highest risk for developing type 2 very early.”
Kim Boggess, MD, who also presented on long-term use of metformin after GDM, said in the discussion period that she is often quick to recommend metformin therapy to her patients who have an elevated fasting plasma glucose value in the postpartum period, even when a 75-g oral GTT has not yet been performed. (The ADA and the American College of Obstetricians and Gynecologists recommend completion of an oral GTT at 4-12 weeks postpartum after GDM.)
“I start them [on metformin] especially if they’ve had a cesarean section. Even 2, 3, 4 weeks of profound hyperglycemia could have potentially deleterious effects,” said Dr. Boggess, professor and maternal-fetal medicine program director at the University of North Carolina, Chapel Hill. “If someone comes in [shortly after] and looks like they have pristine control, then it might be worth stopping the metformin for 3-5 days (and retesting).”
Dr. Ratner said that, in this clinical scenario, he would first ensure that the fasting glucose value “is a true fasting glucose” and “if it’s substantially elevated – I’m talking 100, 105, 110 mg/dL – I’d start metformin, and I’m not even sure I’d do the GTT.” But, he advised, “if you’re going to do the GTT, I’d stop the metformin the day before.”
In her presentation, Dr. Boggess pointed out that metformin wasn’t shown to be superior to lifestyle interventions in the DPP for preventing progression to type 2 DM, and that some women are more motivated for intensive lifestyle change than others. The ADA recommends, in fact, that either metformin or lifestyle interventions be prescribed to women with a history of GDM who are found to have prediabetes.
There are no data to support the use of metformin either during or after pregnancy to improve weight loss or reduce weight retention following pregnancy, but at least several studies have shown that lifestyle interventions are effective, she noted.
What is needed, Dr. Boggess said, are more data on the effects of metformin on cardiovascular disease risk, as well as larger studies of metformin in the postpartum period “to help us determine the best dose.” Some research on metformin use in the postpartum period has reported gastrointestinal side effects and dissatisfaction, she noted.
Dr. Ratner said that metformin’s main drawback is the need for occasional testing of B12 levels. Regarding weight loss and what was observed in the DPP, he said, women with a history of GDM who were randomized to intensive lifestyle interventions did not lose as much weight as women without a history of GDM.
Women who entered the DPP with a GDM history, he noted in his presentation, were essentially a “cohort of survivors.” They had an average age of 43 (compared with 52 years in the parous women without GDM) and a mean interval from the index GDM pregnancy of 11 years, which means that women with the highest risk of diabetes conversion were excluded, Dr. Ratner said.
Age was the only significantly different baseline characteristic between parous women with and without GDM, he noted. Women with a history of GDM who were randomized to placebo had a 71% higher incidence of diabetes than women without such a history – a striking natural history, Dr. Ratner said.
He and Dr. Boggess each reported that they have no financial or other interests that pose a conflict of interest.
WASHINGTON – Metformin’s role in preventing or delaying the onset of type 2 diabetes in women with a history of gestational diabetes mellitus has been firmly established by the Diabetes Prevention Program (DPP) trial – most recently, by 15-year follow-up data reported this year – and the drug should be front and center for clinicians who hope to stave off the “remarkable” incidence of type 2 diabetes after GDM, Robert E. Ratner, MD, maintained at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
The DPP included “the single largest population of women with a history of GDM that’s been looked at in a randomized controlled trial,” and considering its multiethnic population, the trial offers a reliable representative sample to ponder today when evaluating long-term use of metformin after GDM, said Dr. Ratner, a principal investigator of the National Institutes of Health–sponsored DPP and the DPP Outcomes Study and a former chief scientific & medical officer for the American Diabetes Association.
The drug stacked up equally to lifestyle interventions among DPP participants who had a history of GDM, but it’s important to appreciate that these interventions were intensive and that metformin is inexpensive, well tolerated, and “has a long safety record,” he said.
Results of follow-up out to 15 years
Of the more than 3,000 men and women enrolled in the landmark DPP, conducted during 1996-2001, 350 were women with a documented history of GDM and over 1,400 were women who had deliveries but no history of GDM. All participants had impaired glucose tolerance – defined for the trial as having both a fasting plasma glucose value of 95-125 mg/dL and a 2-hour value of 140-199 mg/dL after a 75-g glucose load – and were randomized to placebo, metformin, or intensive lifestyle intervention.
Metformin therapy reduced the incidence of diabetes by approximately 50% in women with a history of GDM, compared with the placebo group – as did lifestyle – over 3 years. The number needed to treat to prevent one case of diabetes was five. Women without a history of GDM, on the other hand, saw only a 14% reduction with metformin when compared with placebo (and a 49% reduction with lifestyle).
“In women with a history of GDM ... one pill twice a day for $4 a month worked as well as intensive lifestyle [change],” Dr. Ratner said, referring to the initial GDM-specific analysis of DPP data published in 2008 (J Clin Endocrinol Metab. 2008;93[12]:4774-9).
In a 10-year postrandomization follow-up, published in 2015, both metformin and lifestyle continued to be equally effective for the GDM group, reducing the progression to diabetes by 40% and 35%, respectively (J Clin Endocrinol Metab. 2015;100:1646-53). The number needed to treat to prevent one case of diabetes was seven. (Among women without a history of GDM, metformin did not reduce progression to diabetes.)
A recent DPP Outcomes Study analysis of metformin’s impact on diabetes prevention at 15 years, moreover, showed a 41% risk reduction among women with a history of GDM (Diabetes Care. 2019;42[4]:601-8).
Advice on prescribing metformin prophylactically
Asked after his presentation whether women with a history of GDM and either an elevated fasting plasma glucose value or an elevated 2-hour oral glucose tolerance test (GTT) value – or neither of the two – would benefit from taking metformin, Dr. Ratner said that “we’re stuck with inclusion criteria of the DPP, in which they had to meet both criteria ... What I’d say, though, is that not everyone with a history of GDM needs to be on metformin prophylactically. But [for women who have] prediabetes as defined by the ADA, the cost-benefit analysis points toward metformin.”
And with respect to early initiation and long-term use of the drug, “I would have absolutely no qualms about medicating a 25-year-old who had developed GDM and who in the postpartum period has prediabetes,” Dr. Ratner said during an open discussion. “She’s actually at the highest risk for developing type 2 very early.”
Kim Boggess, MD, who also presented on long-term use of metformin after GDM, said in the discussion period that she is often quick to recommend metformin therapy to her patients who have an elevated fasting plasma glucose value in the postpartum period, even when a 75-g oral GTT has not yet been performed. (The ADA and the American College of Obstetricians and Gynecologists recommend completion of an oral GTT at 4-12 weeks postpartum after GDM.)
“I start them [on metformin] especially if they’ve had a cesarean section. Even 2, 3, 4 weeks of profound hyperglycemia could have potentially deleterious effects,” said Dr. Boggess, professor and maternal-fetal medicine program director at the University of North Carolina, Chapel Hill. “If someone comes in [shortly after] and looks like they have pristine control, then it might be worth stopping the metformin for 3-5 days (and retesting).”
Dr. Ratner said that, in this clinical scenario, he would first ensure that the fasting glucose value “is a true fasting glucose” and “if it’s substantially elevated – I’m talking 100, 105, 110 mg/dL – I’d start metformin, and I’m not even sure I’d do the GTT.” But, he advised, “if you’re going to do the GTT, I’d stop the metformin the day before.”
In her presentation, Dr. Boggess pointed out that metformin wasn’t shown to be superior to lifestyle interventions in the DPP for preventing progression to type 2 DM, and that some women are more motivated for intensive lifestyle change than others. The ADA recommends, in fact, that either metformin or lifestyle interventions be prescribed to women with a history of GDM who are found to have prediabetes.
There are no data to support the use of metformin either during or after pregnancy to improve weight loss or reduce weight retention following pregnancy, but at least several studies have shown that lifestyle interventions are effective, she noted.
What is needed, Dr. Boggess said, are more data on the effects of metformin on cardiovascular disease risk, as well as larger studies of metformin in the postpartum period “to help us determine the best dose.” Some research on metformin use in the postpartum period has reported gastrointestinal side effects and dissatisfaction, she noted.
Dr. Ratner said that metformin’s main drawback is the need for occasional testing of B12 levels. Regarding weight loss and what was observed in the DPP, he said, women with a history of GDM who were randomized to intensive lifestyle interventions did not lose as much weight as women without a history of GDM.
Women who entered the DPP with a GDM history, he noted in his presentation, were essentially a “cohort of survivors.” They had an average age of 43 (compared with 52 years in the parous women without GDM) and a mean interval from the index GDM pregnancy of 11 years, which means that women with the highest risk of diabetes conversion were excluded, Dr. Ratner said.
Age was the only significantly different baseline characteristic between parous women with and without GDM, he noted. Women with a history of GDM who were randomized to placebo had a 71% higher incidence of diabetes than women without such a history – a striking natural history, Dr. Ratner said.
He and Dr. Boggess each reported that they have no financial or other interests that pose a conflict of interest.
REPORTING FROM THE DPSG-NA 2019