User login
2020 Update on obstetrics
Attributed to the ancient Greek philosopher Heraclitus, and often quoted in contemporary times, is the expression “the only constant is change.” This sentiment rings true for the field of obstetrics this past year, as several bread-and-butter guidelines for managing common obstetric conditions were either challenged or altered.
The publication of the PROLONG trial called into question the use of intramuscular progesterone for the prevention of preterm birth. Prophylaxis guidelines for group B streptococcal disease were updated, including several significant clinical practice changes. Finally, there was a comprehensive overhaul of the guidelines for hypertensive disorders of pregnancy, which replaced a landmark Task Force document from the American College of Obstetricians and Gynecologists (ACOG) that was published only a few years ago.
Change is constant, and in obstetrics it is vital to keep up with the changing guidelines that result as new data become available for digestion and implementation into everyday clinical practice.
Results from the PROLONG trial may shake up treatment options for recurrent preterm birth
Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
The drug 17 α-hydroxyprogesterone caproate (17-OHPC, or 17P; Makena) was approved by the US Food and Drug Administration (FDA) in 2011 for the prevention of spontaneous preterm birth (PTB) in women with a singleton pregnancy and a history of singleton spontaneous PTB. The results of the trial by Meis and colleagues of 17-OHPC played a major role in achieving that approval, as it demonstrated a 34% reduction in recurrent PTB and a reduction in some neonatal morbidities.1 Following the drug's approval, both ACOG and the Society for Maternal-Fetal Medicine (SMFM) published guidelines recommending progesterone therapy, including 17-OHPC, for the prevention of recurrent spontaneous PTB.2
The FDA approval of 17-OHPC was granted under an accelerated conditional pathway that required a confirmatory trial evaluating efficacy, safety, and long-term infant follow-up to be performed by the sponsor. That trial, Progestin's Role in Optimizing Neonatal Gestation (PROLONG), was started in 2009, and its results were published on October 25, 2019.3
Continue to: Design of the trial...
Design of the trial
PROLONG was a multicenter (93 sites), randomized, placebo-controlled, double-blind study conducted in 9 countries (23% of participants were in the United States, 60% were in Russia and Ukraine). The co-primary outcome was PTB < 35 weeks and a composite neonatal morbidity and mortality index. The primary safety outcome was fetal/early infant death.
The study was designed to have 98% power to detect a 30% reduction in PTB < 35 weeks, and 90% power to detect a 35% reduction in the neonatal composite index. It included 1,708 participants (1,130 were treated with 17-OHPC, and 578 received placebo).
Trial outcomes. There was no difference in PTB < 35 weeks between the 17-OHPC and the placebo groups (11.0% vs 11.5%; relative risk [RR], 0.95; 95% confidence interval [CI], 0.71-1.26). There was no difference in PTB < 32 or < 37 weeks.
The study revealed also that there was no difference between groups in the neonatal composite index (5.6% for 17-OHPC vs 5.0% for placebo; RR, 1.12; 95% CI, 0.68-1.61). In addition, there was no difference in fetal/early infant death between the 17-OHPC and placebo groups (1.7% vs 1.9%; RR, 0.87; 95% CI, 0.4-1.81).
Conclusions. The trial investigators concluded that 17-OHPC did not demonstrate a reduction in recurrent PTB and did not decrease neonatal morbidity.
Study limitations included underpowering and selection bias
The investigators noted that the PTB rate in PROLONG was unexpectedly almost 50% lower than that in the Meis trial, and that therefore the PROLONG trial was underpowered to assess the primary outcomes.
Further, the study populations of the 2 trials were very different: The Meis trial included women at higher baseline risk for PTB (> 1 prior PTB and at least 1 other risk factor for PTB). Additionally, while the PROLONG trial included mostly white (90%), married (90%), nonsmoking women (8% smoked), the Meis trial population was 59% black and 50% married, and 20% were smokers.
The availability and common use of 17-OHPC in the United States likely led to a selection bias for the PROLONG trial population, as the highest-risk patients were most likely already receiving treatment and were therefore excluded from the PROLONG trial.
Society, and FDA, responses to the new data
The results of the PROLONG trial call into question what has become standard practice for patients with a history of spontaneous PTB in the United States. While the safety profile of 17-OHPC has not been cited as a concern, whether or not the drug should be used at all has—as has its current FDA-approved status.
In response to the publication of the PROLONG trial results, ACOG released a Practice Advisory that acknowledged the study's findings but did not alter the current recommendations to continue to offer progesterone for the prevention of preterm birth, upholding ACOG's current Practice Bulletin guidance.2,4 Additional considerations for offering 17-OHPC use include the patients' preferences, available resources, and the setting for the intervention.
SMFM's response was more specific, stating that it is reasonable to continue to use 17-OHPC in high-risk patient populations consistent with those in the Meis trial.5 In the rest of the general population at risk for recurrent PTB, SMFM recommends that, due to uncertain benefit with 17-OHPC, the high cost, patient discomfort, and increased visits should be taken into account.
Four days after the publication of the PROLONG study, the FDA Bone, Reproductive, and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17-OHPC.6 In response, SMFM released a statement supporting continued access to 17-OHPC.7 The FDA's final decision on the status of the drug is expected within the next several months from this writing.
17-OHPC continues to be considered safe and still is recommended by both ACOG and SMFM for the prevention of recurrent preterm birth in high-risk patients. The high-risk patient population who may benefit most from this therapy is still not certain, but hopefully future studies will better delineate this. The landscape for 17-OHPC use may change dramatically if FDA approval is not upheld in the future. In my current practice, I am continuing to offer 17-OHPC to patients per the current ACOG guidelines, but I am counseling patients in a shared decision-making model regarding the findings of the PROLONG trial and the potential change in FDA approval.
Continue to: ACOG updates guidance on preventing early-onset GBS disease...
ACOG updates guidance on preventing early-onset GBS disease
American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
Group B streptococcus (GBS) is the leading cause of newborn infection and is associated with maternal infections as well as preterm labor and stillbirth. Early-onset GBS disease occurs within 7 days of birth and is linked to vertical transmission via maternal colonization of the genitourinary or gastrointestinal tract and fetal/neonatal aspiration at birth.
Preventing early-onset GBS disease with maternal screening and intrapartum prophylaxis according to the Centers for Disease Control and Prevention (CDC) guidelines has reduced early-onset disease by 80% since the 1990s. By contrast, late-onset GBS infection, which occurs 7 days to 3 months after birth, usually is associated with horizontal maternal transmission or hospital or community infections, and it is not prevented by intrapartum treatment.
In 2018, the CDC transferred responsibility for GBS prophylaxis guidelines to ACOG and the American Academy of Pediatrics (AAP). In July 2019, ACOG released its Committee Opinion on preventing early-onset GBS disease in newborns.8 This guidance replaces and updates the previous guidelines, with 3 notable changes.
The screening timing has changed
In the CDC's 2010 guidelines, GBS screening was recommended to start at 35 weeks' gestation. The new guidelines recommend universal vaginal-rectal screening at 36 to 37 6/7 weeks' gestation. The new timing of culture will shift the expected 5-week window in which GBS cultures are considered valid up to at least 41 weeks' gestation. The rationale for this change is that any GBS-unknown patient who previously would have been cultured under 37 weeks' would be an automatic candidate for empiric therapy and the lower rate of birth in the 35th versus the 41st week of gestation.
Identifying candidates for intrapartum treatment
The usual indications for intrapartum antibiotic prophylaxis include a GBS-positive culture at 36 weeks or beyond, GBS bacteriuria at any point in pregnancy, a prior GBS-affected child, or unknown GBS status with any of the following: < 37 weeks, rupture of membranes ≥ 18 hours or temperature ≥ 100.4°F (38°C), and a positive rapid GBS culture in labor. In addition, antibiotics now should be considered for patients at term with unknown GBS status but with a history of GBS colonization in a prior pregnancy.
This represents a major practice change for women at ≥ 37 weeks with unknown GBS status and no other traditional risk factors. The rationale for this recommendation is that women who have been positive for GBS in a prior pregnancy have a 50% chance of being colonized in the current pregnancy, and their newborns are therefore at higher risk for early-onset GBS disease.
Managing patients with penicillin allergy
Intravenous penicillin (or ampicillin) remains the antibiotic of choice for intrapartum prophylaxis against GBS due to its efficacy and specific, narrow coverage of gram-positive organisms. The updated recommendations emphasize that it is important to carefully evaluate patients with reported penicillin allergies for several reasons: determining risk of anaphylaxis and clindamycin susceptibility testing in GBS evaluations are often overlooked by obstetric providers, the need for antibiotic stewardship to reduce the development of antibiotic resistance, and clarification of allergy status for future health care needs.
Three recommendations are made:
- Laboratory requisitions for cultures should specifically note a penicillin allergy so that clindamycin susceptibility testing can be performed.
- Penicillin allergy skin testing should be considered for patients at unknown or low risk for anaphylaxis, as it is considered safe in pregnancy and most patients (80%-90%) who report a penicillin allergy are actually penicillin tolerant.
- For patients at high risk for anaphylaxis to penicillin, the recommended vancomycin dosing has been changed from 1 g IV every 12 hours to 20 mg/kg IV every 8 hours (maximum single dose, 2 g). Renal function should be assessed prior to dosing. This weight- and renal function-based dosing increased neonatal therapeutic levels in several studies of different doses.
ACOG's key recommendations for preventing early-onset GBS disease in newborns include:
- Universal vaginal-rectal screening for GBS should be performed at 36 to 37 6/7 weeks' gestation.
- Intrapartum antibiotic prophylaxis should be considered for low-risk patients at term with unknown GBS status and a history of GBS colonization in a prior pregnancy.
- Patients with a reported penicillin allergy require careful evaluation of the nature of their allergy, including consideration of skin testing and GBS susceptibility evaluation in order to promote the best practices for antibiotic use.
- For GBS-positive patients at high risk for penicillin anaphylaxis, vancomycin 20 mg/kg IV every 8 hours (maximum single dose, 2 g) is recommended.
Continue to: Managing hypertension in pregnancy: New recommendations...
Managing hypertension in pregnancy: New recommendations
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
In 2013, ACOG released "Hypertension in pregnancy," a 99-page comprehensive document developed by their Task Force on Hypertension in Pregnancy, to summarize knowledge on the subject, provide guidelines for management, and identify needed areas of research.9 I summarized key points from that document in the 2014 "Update on Obstetrics" (OBG Manag. 2013;26[1]:28-36). Now, ACOG has released 2 Practice Bulletins—"Gestational hypertension and preeclampsia" and "Chronic hypertension in pregnancy"—that replace the 2013 document.10,11 These Practice Bulletins are quite comprehensive and warrant a thorough read. Several noteworthy changes relevant to the practicing obstetrician are summarized below.
Highlights of revised guidance
Expectant management vs early delivery in preeclampsia with fetal growth restriction. Fetal growth restriction, which was removed from the definition of preeclampsia with severe features in 2013, is no longer an indication for delivery in preeclampsia with severe features (previously, if the estimated fetal weight was < 5th percentile for gestational age, delivery after steroid administration was recommended). Rather, expectant management is reasonable if fetal antenatal testing, amniotic fluid, and Doppler ultrasound studies are reassuring. Abnormal umbilical artery Doppler studies continue to be an indication for earlier delivery.
Postpartum NSAID use in hypertension. The 2013 document cautioned against nonsteroidal anti-inflammatory drug (NSAID) use postpartum in women with hypertensive disorders of pregnancy because of concern for exacerbating hypertension. The updated Practice Bulletins recommend NSAIDs as the preferred choice over opioid analgesics as data have not shown these drugs to increase blood pressure, antihypertensive requirements, or other adverse events in postpartum patients with blood pressure issues.
More women will be diagnosed with chronic hypertension. Recently, the American College of Cardiology and the American Heart Association changed the definition of hypertension. Stage 1 hypertension is now defined as a systolic blood pressure of 130-139 mm Hg or a diastolic blood pressure of 80-89 mm Hg. Treatment of stage 1 hypertension is recommended for nonpregnant adults with risk factors for current or future cardiovascular disease. The potential impact is that more women will enter pregnancy with a diagnosis of chronic hypertension, and more may be on prepregnancy antihypertensive therapy that will need to be addressed during the pregnancy.
Blood pressure goals. The target blood pressure range for pregnant women with chronic hypertension is recommended to be ≥ 120/80 mm Hg and < 160/110 mm Hg (this represents a slight change, as previously diastolic blood pressure was to be < 105 mm Hg). Postpartum blood pressure goals of < 150/100 mm Hg remain the same.
Managing acute hypertensive emergencies. Both Practice Bulletins emphasize the importance of aggressive management of acute hypertensive emergency, with options for 3 protocols: labetalol, nifedipine, and hydralazine. The goal is to administer antihypertensive therapy within 30 to 60 minutes, but administration as soon as feasibly possible after diagnosis of severe hypertension is ideal.
Timing of delivery. Recommended delivery timing in patients with chronic hypertension was slightly altered (previous recommendations included a range of 37 to 39 6/7 weeks). The lower limit of gestational age for recommended delivery timing in chronic hypertension has not changed—it remains not before 38 weeks if no antihypertensive therapy and stable, and not before 37 weeks if antihypertensive therapy and stable.
The upper limit of 39 6/7 weeks is challenged, however, because data support that induction of labor at either 38 or 39 weeks reduces the risk of severe hypertensive complications (such as superimposed preeclampsia and eclampsia) without increasing the risk of cesarean delivery. Therefore, for patients with chronic hypertension, expectant management beyond 39 weeks is cautioned, to be done only with careful consideration of risks and with close surveillance.
As with ACOG’s original Task Force document on hypertension, clinicians should thoroughly read these 2 Practice Bulletins on hypertension in pregnancy as there are subtle changes that affect day-to-day practice, such as the definition of hypertension prior to pregnancy, treatment guidelines, and delivery timing recommendations. As always, these are guidelines, and the obstetrician’s clinical judgment and the needs of specific patient populations also must be taken into account.
- Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964-973.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
- ACOG Practice Advisory. Clinical guidance for integration of the findings of the PROLONG study: progestin’s role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing. Accessed November 10, 2019.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280-smfm-statement-use-of-17-alpha-hydroxyprogesterone-caproate-for-prevention-of-recurrent-preterm-birth. Accessed November 10, 2019.
- US Food and Drug Administration. Bone, Reproductive, and Urologic Drugs Advisory Committee Meeting, October 29, 2019. Advisory Committee Briefing Materials: Available for Public Release. https://www.fda.gov/media/132004/download. Accessed November 19, 2019.
- Society for Maternal-Fetal Medicine. SMFM responds to the FDA’s Bone, Reproductive and Urologic Advisory Committee. https://s3.amazonaws.com/cdn.smfm.org/media/2091/17P_Public_Statement.pdf. Accessed November 19, 2019.
- American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50.
Attributed to the ancient Greek philosopher Heraclitus, and often quoted in contemporary times, is the expression “the only constant is change.” This sentiment rings true for the field of obstetrics this past year, as several bread-and-butter guidelines for managing common obstetric conditions were either challenged or altered.
The publication of the PROLONG trial called into question the use of intramuscular progesterone for the prevention of preterm birth. Prophylaxis guidelines for group B streptococcal disease were updated, including several significant clinical practice changes. Finally, there was a comprehensive overhaul of the guidelines for hypertensive disorders of pregnancy, which replaced a landmark Task Force document from the American College of Obstetricians and Gynecologists (ACOG) that was published only a few years ago.
Change is constant, and in obstetrics it is vital to keep up with the changing guidelines that result as new data become available for digestion and implementation into everyday clinical practice.
Results from the PROLONG trial may shake up treatment options for recurrent preterm birth
Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
The drug 17 α-hydroxyprogesterone caproate (17-OHPC, or 17P; Makena) was approved by the US Food and Drug Administration (FDA) in 2011 for the prevention of spontaneous preterm birth (PTB) in women with a singleton pregnancy and a history of singleton spontaneous PTB. The results of the trial by Meis and colleagues of 17-OHPC played a major role in achieving that approval, as it demonstrated a 34% reduction in recurrent PTB and a reduction in some neonatal morbidities.1 Following the drug's approval, both ACOG and the Society for Maternal-Fetal Medicine (SMFM) published guidelines recommending progesterone therapy, including 17-OHPC, for the prevention of recurrent spontaneous PTB.2
The FDA approval of 17-OHPC was granted under an accelerated conditional pathway that required a confirmatory trial evaluating efficacy, safety, and long-term infant follow-up to be performed by the sponsor. That trial, Progestin's Role in Optimizing Neonatal Gestation (PROLONG), was started in 2009, and its results were published on October 25, 2019.3
Continue to: Design of the trial...
Design of the trial
PROLONG was a multicenter (93 sites), randomized, placebo-controlled, double-blind study conducted in 9 countries (23% of participants were in the United States, 60% were in Russia and Ukraine). The co-primary outcome was PTB < 35 weeks and a composite neonatal morbidity and mortality index. The primary safety outcome was fetal/early infant death.
The study was designed to have 98% power to detect a 30% reduction in PTB < 35 weeks, and 90% power to detect a 35% reduction in the neonatal composite index. It included 1,708 participants (1,130 were treated with 17-OHPC, and 578 received placebo).
Trial outcomes. There was no difference in PTB < 35 weeks between the 17-OHPC and the placebo groups (11.0% vs 11.5%; relative risk [RR], 0.95; 95% confidence interval [CI], 0.71-1.26). There was no difference in PTB < 32 or < 37 weeks.
The study revealed also that there was no difference between groups in the neonatal composite index (5.6% for 17-OHPC vs 5.0% for placebo; RR, 1.12; 95% CI, 0.68-1.61). In addition, there was no difference in fetal/early infant death between the 17-OHPC and placebo groups (1.7% vs 1.9%; RR, 0.87; 95% CI, 0.4-1.81).
Conclusions. The trial investigators concluded that 17-OHPC did not demonstrate a reduction in recurrent PTB and did not decrease neonatal morbidity.
Study limitations included underpowering and selection bias
The investigators noted that the PTB rate in PROLONG was unexpectedly almost 50% lower than that in the Meis trial, and that therefore the PROLONG trial was underpowered to assess the primary outcomes.
Further, the study populations of the 2 trials were very different: The Meis trial included women at higher baseline risk for PTB (> 1 prior PTB and at least 1 other risk factor for PTB). Additionally, while the PROLONG trial included mostly white (90%), married (90%), nonsmoking women (8% smoked), the Meis trial population was 59% black and 50% married, and 20% were smokers.
The availability and common use of 17-OHPC in the United States likely led to a selection bias for the PROLONG trial population, as the highest-risk patients were most likely already receiving treatment and were therefore excluded from the PROLONG trial.
Society, and FDA, responses to the new data
The results of the PROLONG trial call into question what has become standard practice for patients with a history of spontaneous PTB in the United States. While the safety profile of 17-OHPC has not been cited as a concern, whether or not the drug should be used at all has—as has its current FDA-approved status.
In response to the publication of the PROLONG trial results, ACOG released a Practice Advisory that acknowledged the study's findings but did not alter the current recommendations to continue to offer progesterone for the prevention of preterm birth, upholding ACOG's current Practice Bulletin guidance.2,4 Additional considerations for offering 17-OHPC use include the patients' preferences, available resources, and the setting for the intervention.
SMFM's response was more specific, stating that it is reasonable to continue to use 17-OHPC in high-risk patient populations consistent with those in the Meis trial.5 In the rest of the general population at risk for recurrent PTB, SMFM recommends that, due to uncertain benefit with 17-OHPC, the high cost, patient discomfort, and increased visits should be taken into account.
Four days after the publication of the PROLONG study, the FDA Bone, Reproductive, and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17-OHPC.6 In response, SMFM released a statement supporting continued access to 17-OHPC.7 The FDA's final decision on the status of the drug is expected within the next several months from this writing.
17-OHPC continues to be considered safe and still is recommended by both ACOG and SMFM for the prevention of recurrent preterm birth in high-risk patients. The high-risk patient population who may benefit most from this therapy is still not certain, but hopefully future studies will better delineate this. The landscape for 17-OHPC use may change dramatically if FDA approval is not upheld in the future. In my current practice, I am continuing to offer 17-OHPC to patients per the current ACOG guidelines, but I am counseling patients in a shared decision-making model regarding the findings of the PROLONG trial and the potential change in FDA approval.
Continue to: ACOG updates guidance on preventing early-onset GBS disease...
ACOG updates guidance on preventing early-onset GBS disease
American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
Group B streptococcus (GBS) is the leading cause of newborn infection and is associated with maternal infections as well as preterm labor and stillbirth. Early-onset GBS disease occurs within 7 days of birth and is linked to vertical transmission via maternal colonization of the genitourinary or gastrointestinal tract and fetal/neonatal aspiration at birth.
Preventing early-onset GBS disease with maternal screening and intrapartum prophylaxis according to the Centers for Disease Control and Prevention (CDC) guidelines has reduced early-onset disease by 80% since the 1990s. By contrast, late-onset GBS infection, which occurs 7 days to 3 months after birth, usually is associated with horizontal maternal transmission or hospital or community infections, and it is not prevented by intrapartum treatment.
In 2018, the CDC transferred responsibility for GBS prophylaxis guidelines to ACOG and the American Academy of Pediatrics (AAP). In July 2019, ACOG released its Committee Opinion on preventing early-onset GBS disease in newborns.8 This guidance replaces and updates the previous guidelines, with 3 notable changes.
The screening timing has changed
In the CDC's 2010 guidelines, GBS screening was recommended to start at 35 weeks' gestation. The new guidelines recommend universal vaginal-rectal screening at 36 to 37 6/7 weeks' gestation. The new timing of culture will shift the expected 5-week window in which GBS cultures are considered valid up to at least 41 weeks' gestation. The rationale for this change is that any GBS-unknown patient who previously would have been cultured under 37 weeks' would be an automatic candidate for empiric therapy and the lower rate of birth in the 35th versus the 41st week of gestation.
Identifying candidates for intrapartum treatment
The usual indications for intrapartum antibiotic prophylaxis include a GBS-positive culture at 36 weeks or beyond, GBS bacteriuria at any point in pregnancy, a prior GBS-affected child, or unknown GBS status with any of the following: < 37 weeks, rupture of membranes ≥ 18 hours or temperature ≥ 100.4°F (38°C), and a positive rapid GBS culture in labor. In addition, antibiotics now should be considered for patients at term with unknown GBS status but with a history of GBS colonization in a prior pregnancy.
This represents a major practice change for women at ≥ 37 weeks with unknown GBS status and no other traditional risk factors. The rationale for this recommendation is that women who have been positive for GBS in a prior pregnancy have a 50% chance of being colonized in the current pregnancy, and their newborns are therefore at higher risk for early-onset GBS disease.
Managing patients with penicillin allergy
Intravenous penicillin (or ampicillin) remains the antibiotic of choice for intrapartum prophylaxis against GBS due to its efficacy and specific, narrow coverage of gram-positive organisms. The updated recommendations emphasize that it is important to carefully evaluate patients with reported penicillin allergies for several reasons: determining risk of anaphylaxis and clindamycin susceptibility testing in GBS evaluations are often overlooked by obstetric providers, the need for antibiotic stewardship to reduce the development of antibiotic resistance, and clarification of allergy status for future health care needs.
Three recommendations are made:
- Laboratory requisitions for cultures should specifically note a penicillin allergy so that clindamycin susceptibility testing can be performed.
- Penicillin allergy skin testing should be considered for patients at unknown or low risk for anaphylaxis, as it is considered safe in pregnancy and most patients (80%-90%) who report a penicillin allergy are actually penicillin tolerant.
- For patients at high risk for anaphylaxis to penicillin, the recommended vancomycin dosing has been changed from 1 g IV every 12 hours to 20 mg/kg IV every 8 hours (maximum single dose, 2 g). Renal function should be assessed prior to dosing. This weight- and renal function-based dosing increased neonatal therapeutic levels in several studies of different doses.
ACOG's key recommendations for preventing early-onset GBS disease in newborns include:
- Universal vaginal-rectal screening for GBS should be performed at 36 to 37 6/7 weeks' gestation.
- Intrapartum antibiotic prophylaxis should be considered for low-risk patients at term with unknown GBS status and a history of GBS colonization in a prior pregnancy.
- Patients with a reported penicillin allergy require careful evaluation of the nature of their allergy, including consideration of skin testing and GBS susceptibility evaluation in order to promote the best practices for antibiotic use.
- For GBS-positive patients at high risk for penicillin anaphylaxis, vancomycin 20 mg/kg IV every 8 hours (maximum single dose, 2 g) is recommended.
Continue to: Managing hypertension in pregnancy: New recommendations...
Managing hypertension in pregnancy: New recommendations
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
In 2013, ACOG released "Hypertension in pregnancy," a 99-page comprehensive document developed by their Task Force on Hypertension in Pregnancy, to summarize knowledge on the subject, provide guidelines for management, and identify needed areas of research.9 I summarized key points from that document in the 2014 "Update on Obstetrics" (OBG Manag. 2013;26[1]:28-36). Now, ACOG has released 2 Practice Bulletins—"Gestational hypertension and preeclampsia" and "Chronic hypertension in pregnancy"—that replace the 2013 document.10,11 These Practice Bulletins are quite comprehensive and warrant a thorough read. Several noteworthy changes relevant to the practicing obstetrician are summarized below.
Highlights of revised guidance
Expectant management vs early delivery in preeclampsia with fetal growth restriction. Fetal growth restriction, which was removed from the definition of preeclampsia with severe features in 2013, is no longer an indication for delivery in preeclampsia with severe features (previously, if the estimated fetal weight was < 5th percentile for gestational age, delivery after steroid administration was recommended). Rather, expectant management is reasonable if fetal antenatal testing, amniotic fluid, and Doppler ultrasound studies are reassuring. Abnormal umbilical artery Doppler studies continue to be an indication for earlier delivery.
Postpartum NSAID use in hypertension. The 2013 document cautioned against nonsteroidal anti-inflammatory drug (NSAID) use postpartum in women with hypertensive disorders of pregnancy because of concern for exacerbating hypertension. The updated Practice Bulletins recommend NSAIDs as the preferred choice over opioid analgesics as data have not shown these drugs to increase blood pressure, antihypertensive requirements, or other adverse events in postpartum patients with blood pressure issues.
More women will be diagnosed with chronic hypertension. Recently, the American College of Cardiology and the American Heart Association changed the definition of hypertension. Stage 1 hypertension is now defined as a systolic blood pressure of 130-139 mm Hg or a diastolic blood pressure of 80-89 mm Hg. Treatment of stage 1 hypertension is recommended for nonpregnant adults with risk factors for current or future cardiovascular disease. The potential impact is that more women will enter pregnancy with a diagnosis of chronic hypertension, and more may be on prepregnancy antihypertensive therapy that will need to be addressed during the pregnancy.
Blood pressure goals. The target blood pressure range for pregnant women with chronic hypertension is recommended to be ≥ 120/80 mm Hg and < 160/110 mm Hg (this represents a slight change, as previously diastolic blood pressure was to be < 105 mm Hg). Postpartum blood pressure goals of < 150/100 mm Hg remain the same.
Managing acute hypertensive emergencies. Both Practice Bulletins emphasize the importance of aggressive management of acute hypertensive emergency, with options for 3 protocols: labetalol, nifedipine, and hydralazine. The goal is to administer antihypertensive therapy within 30 to 60 minutes, but administration as soon as feasibly possible after diagnosis of severe hypertension is ideal.
Timing of delivery. Recommended delivery timing in patients with chronic hypertension was slightly altered (previous recommendations included a range of 37 to 39 6/7 weeks). The lower limit of gestational age for recommended delivery timing in chronic hypertension has not changed—it remains not before 38 weeks if no antihypertensive therapy and stable, and not before 37 weeks if antihypertensive therapy and stable.
The upper limit of 39 6/7 weeks is challenged, however, because data support that induction of labor at either 38 or 39 weeks reduces the risk of severe hypertensive complications (such as superimposed preeclampsia and eclampsia) without increasing the risk of cesarean delivery. Therefore, for patients with chronic hypertension, expectant management beyond 39 weeks is cautioned, to be done only with careful consideration of risks and with close surveillance.
As with ACOG’s original Task Force document on hypertension, clinicians should thoroughly read these 2 Practice Bulletins on hypertension in pregnancy as there are subtle changes that affect day-to-day practice, such as the definition of hypertension prior to pregnancy, treatment guidelines, and delivery timing recommendations. As always, these are guidelines, and the obstetrician’s clinical judgment and the needs of specific patient populations also must be taken into account.
Attributed to the ancient Greek philosopher Heraclitus, and often quoted in contemporary times, is the expression “the only constant is change.” This sentiment rings true for the field of obstetrics this past year, as several bread-and-butter guidelines for managing common obstetric conditions were either challenged or altered.
The publication of the PROLONG trial called into question the use of intramuscular progesterone for the prevention of preterm birth. Prophylaxis guidelines for group B streptococcal disease were updated, including several significant clinical practice changes. Finally, there was a comprehensive overhaul of the guidelines for hypertensive disorders of pregnancy, which replaced a landmark Task Force document from the American College of Obstetricians and Gynecologists (ACOG) that was published only a few years ago.
Change is constant, and in obstetrics it is vital to keep up with the changing guidelines that result as new data become available for digestion and implementation into everyday clinical practice.
Results from the PROLONG trial may shake up treatment options for recurrent preterm birth
Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
The drug 17 α-hydroxyprogesterone caproate (17-OHPC, or 17P; Makena) was approved by the US Food and Drug Administration (FDA) in 2011 for the prevention of spontaneous preterm birth (PTB) in women with a singleton pregnancy and a history of singleton spontaneous PTB. The results of the trial by Meis and colleagues of 17-OHPC played a major role in achieving that approval, as it demonstrated a 34% reduction in recurrent PTB and a reduction in some neonatal morbidities.1 Following the drug's approval, both ACOG and the Society for Maternal-Fetal Medicine (SMFM) published guidelines recommending progesterone therapy, including 17-OHPC, for the prevention of recurrent spontaneous PTB.2
The FDA approval of 17-OHPC was granted under an accelerated conditional pathway that required a confirmatory trial evaluating efficacy, safety, and long-term infant follow-up to be performed by the sponsor. That trial, Progestin's Role in Optimizing Neonatal Gestation (PROLONG), was started in 2009, and its results were published on October 25, 2019.3
Continue to: Design of the trial...
Design of the trial
PROLONG was a multicenter (93 sites), randomized, placebo-controlled, double-blind study conducted in 9 countries (23% of participants were in the United States, 60% were in Russia and Ukraine). The co-primary outcome was PTB < 35 weeks and a composite neonatal morbidity and mortality index. The primary safety outcome was fetal/early infant death.
The study was designed to have 98% power to detect a 30% reduction in PTB < 35 weeks, and 90% power to detect a 35% reduction in the neonatal composite index. It included 1,708 participants (1,130 were treated with 17-OHPC, and 578 received placebo).
Trial outcomes. There was no difference in PTB < 35 weeks between the 17-OHPC and the placebo groups (11.0% vs 11.5%; relative risk [RR], 0.95; 95% confidence interval [CI], 0.71-1.26). There was no difference in PTB < 32 or < 37 weeks.
The study revealed also that there was no difference between groups in the neonatal composite index (5.6% for 17-OHPC vs 5.0% for placebo; RR, 1.12; 95% CI, 0.68-1.61). In addition, there was no difference in fetal/early infant death between the 17-OHPC and placebo groups (1.7% vs 1.9%; RR, 0.87; 95% CI, 0.4-1.81).
Conclusions. The trial investigators concluded that 17-OHPC did not demonstrate a reduction in recurrent PTB and did not decrease neonatal morbidity.
Study limitations included underpowering and selection bias
The investigators noted that the PTB rate in PROLONG was unexpectedly almost 50% lower than that in the Meis trial, and that therefore the PROLONG trial was underpowered to assess the primary outcomes.
Further, the study populations of the 2 trials were very different: The Meis trial included women at higher baseline risk for PTB (> 1 prior PTB and at least 1 other risk factor for PTB). Additionally, while the PROLONG trial included mostly white (90%), married (90%), nonsmoking women (8% smoked), the Meis trial population was 59% black and 50% married, and 20% were smokers.
The availability and common use of 17-OHPC in the United States likely led to a selection bias for the PROLONG trial population, as the highest-risk patients were most likely already receiving treatment and were therefore excluded from the PROLONG trial.
Society, and FDA, responses to the new data
The results of the PROLONG trial call into question what has become standard practice for patients with a history of spontaneous PTB in the United States. While the safety profile of 17-OHPC has not been cited as a concern, whether or not the drug should be used at all has—as has its current FDA-approved status.
In response to the publication of the PROLONG trial results, ACOG released a Practice Advisory that acknowledged the study's findings but did not alter the current recommendations to continue to offer progesterone for the prevention of preterm birth, upholding ACOG's current Practice Bulletin guidance.2,4 Additional considerations for offering 17-OHPC use include the patients' preferences, available resources, and the setting for the intervention.
SMFM's response was more specific, stating that it is reasonable to continue to use 17-OHPC in high-risk patient populations consistent with those in the Meis trial.5 In the rest of the general population at risk for recurrent PTB, SMFM recommends that, due to uncertain benefit with 17-OHPC, the high cost, patient discomfort, and increased visits should be taken into account.
Four days after the publication of the PROLONG study, the FDA Bone, Reproductive, and Urologic Drugs Advisory Committee voted 9-7 to withdraw approval for 17-OHPC.6 In response, SMFM released a statement supporting continued access to 17-OHPC.7 The FDA's final decision on the status of the drug is expected within the next several months from this writing.
17-OHPC continues to be considered safe and still is recommended by both ACOG and SMFM for the prevention of recurrent preterm birth in high-risk patients. The high-risk patient population who may benefit most from this therapy is still not certain, but hopefully future studies will better delineate this. The landscape for 17-OHPC use may change dramatically if FDA approval is not upheld in the future. In my current practice, I am continuing to offer 17-OHPC to patients per the current ACOG guidelines, but I am counseling patients in a shared decision-making model regarding the findings of the PROLONG trial and the potential change in FDA approval.
Continue to: ACOG updates guidance on preventing early-onset GBS disease...
ACOG updates guidance on preventing early-onset GBS disease
American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
Group B streptococcus (GBS) is the leading cause of newborn infection and is associated with maternal infections as well as preterm labor and stillbirth. Early-onset GBS disease occurs within 7 days of birth and is linked to vertical transmission via maternal colonization of the genitourinary or gastrointestinal tract and fetal/neonatal aspiration at birth.
Preventing early-onset GBS disease with maternal screening and intrapartum prophylaxis according to the Centers for Disease Control and Prevention (CDC) guidelines has reduced early-onset disease by 80% since the 1990s. By contrast, late-onset GBS infection, which occurs 7 days to 3 months after birth, usually is associated with horizontal maternal transmission or hospital or community infections, and it is not prevented by intrapartum treatment.
In 2018, the CDC transferred responsibility for GBS prophylaxis guidelines to ACOG and the American Academy of Pediatrics (AAP). In July 2019, ACOG released its Committee Opinion on preventing early-onset GBS disease in newborns.8 This guidance replaces and updates the previous guidelines, with 3 notable changes.
The screening timing has changed
In the CDC's 2010 guidelines, GBS screening was recommended to start at 35 weeks' gestation. The new guidelines recommend universal vaginal-rectal screening at 36 to 37 6/7 weeks' gestation. The new timing of culture will shift the expected 5-week window in which GBS cultures are considered valid up to at least 41 weeks' gestation. The rationale for this change is that any GBS-unknown patient who previously would have been cultured under 37 weeks' would be an automatic candidate for empiric therapy and the lower rate of birth in the 35th versus the 41st week of gestation.
Identifying candidates for intrapartum treatment
The usual indications for intrapartum antibiotic prophylaxis include a GBS-positive culture at 36 weeks or beyond, GBS bacteriuria at any point in pregnancy, a prior GBS-affected child, or unknown GBS status with any of the following: < 37 weeks, rupture of membranes ≥ 18 hours or temperature ≥ 100.4°F (38°C), and a positive rapid GBS culture in labor. In addition, antibiotics now should be considered for patients at term with unknown GBS status but with a history of GBS colonization in a prior pregnancy.
This represents a major practice change for women at ≥ 37 weeks with unknown GBS status and no other traditional risk factors. The rationale for this recommendation is that women who have been positive for GBS in a prior pregnancy have a 50% chance of being colonized in the current pregnancy, and their newborns are therefore at higher risk for early-onset GBS disease.
Managing patients with penicillin allergy
Intravenous penicillin (or ampicillin) remains the antibiotic of choice for intrapartum prophylaxis against GBS due to its efficacy and specific, narrow coverage of gram-positive organisms. The updated recommendations emphasize that it is important to carefully evaluate patients with reported penicillin allergies for several reasons: determining risk of anaphylaxis and clindamycin susceptibility testing in GBS evaluations are often overlooked by obstetric providers, the need for antibiotic stewardship to reduce the development of antibiotic resistance, and clarification of allergy status for future health care needs.
Three recommendations are made:
- Laboratory requisitions for cultures should specifically note a penicillin allergy so that clindamycin susceptibility testing can be performed.
- Penicillin allergy skin testing should be considered for patients at unknown or low risk for anaphylaxis, as it is considered safe in pregnancy and most patients (80%-90%) who report a penicillin allergy are actually penicillin tolerant.
- For patients at high risk for anaphylaxis to penicillin, the recommended vancomycin dosing has been changed from 1 g IV every 12 hours to 20 mg/kg IV every 8 hours (maximum single dose, 2 g). Renal function should be assessed prior to dosing. This weight- and renal function-based dosing increased neonatal therapeutic levels in several studies of different doses.
ACOG's key recommendations for preventing early-onset GBS disease in newborns include:
- Universal vaginal-rectal screening for GBS should be performed at 36 to 37 6/7 weeks' gestation.
- Intrapartum antibiotic prophylaxis should be considered for low-risk patients at term with unknown GBS status and a history of GBS colonization in a prior pregnancy.
- Patients with a reported penicillin allergy require careful evaluation of the nature of their allergy, including consideration of skin testing and GBS susceptibility evaluation in order to promote the best practices for antibiotic use.
- For GBS-positive patients at high risk for penicillin anaphylaxis, vancomycin 20 mg/kg IV every 8 hours (maximum single dose, 2 g) is recommended.
Continue to: Managing hypertension in pregnancy: New recommendations...
Managing hypertension in pregnancy: New recommendations
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202. Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
In 2013, ACOG released "Hypertension in pregnancy," a 99-page comprehensive document developed by their Task Force on Hypertension in Pregnancy, to summarize knowledge on the subject, provide guidelines for management, and identify needed areas of research.9 I summarized key points from that document in the 2014 "Update on Obstetrics" (OBG Manag. 2013;26[1]:28-36). Now, ACOG has released 2 Practice Bulletins—"Gestational hypertension and preeclampsia" and "Chronic hypertension in pregnancy"—that replace the 2013 document.10,11 These Practice Bulletins are quite comprehensive and warrant a thorough read. Several noteworthy changes relevant to the practicing obstetrician are summarized below.
Highlights of revised guidance
Expectant management vs early delivery in preeclampsia with fetal growth restriction. Fetal growth restriction, which was removed from the definition of preeclampsia with severe features in 2013, is no longer an indication for delivery in preeclampsia with severe features (previously, if the estimated fetal weight was < 5th percentile for gestational age, delivery after steroid administration was recommended). Rather, expectant management is reasonable if fetal antenatal testing, amniotic fluid, and Doppler ultrasound studies are reassuring. Abnormal umbilical artery Doppler studies continue to be an indication for earlier delivery.
Postpartum NSAID use in hypertension. The 2013 document cautioned against nonsteroidal anti-inflammatory drug (NSAID) use postpartum in women with hypertensive disorders of pregnancy because of concern for exacerbating hypertension. The updated Practice Bulletins recommend NSAIDs as the preferred choice over opioid analgesics as data have not shown these drugs to increase blood pressure, antihypertensive requirements, or other adverse events in postpartum patients with blood pressure issues.
More women will be diagnosed with chronic hypertension. Recently, the American College of Cardiology and the American Heart Association changed the definition of hypertension. Stage 1 hypertension is now defined as a systolic blood pressure of 130-139 mm Hg or a diastolic blood pressure of 80-89 mm Hg. Treatment of stage 1 hypertension is recommended for nonpregnant adults with risk factors for current or future cardiovascular disease. The potential impact is that more women will enter pregnancy with a diagnosis of chronic hypertension, and more may be on prepregnancy antihypertensive therapy that will need to be addressed during the pregnancy.
Blood pressure goals. The target blood pressure range for pregnant women with chronic hypertension is recommended to be ≥ 120/80 mm Hg and < 160/110 mm Hg (this represents a slight change, as previously diastolic blood pressure was to be < 105 mm Hg). Postpartum blood pressure goals of < 150/100 mm Hg remain the same.
Managing acute hypertensive emergencies. Both Practice Bulletins emphasize the importance of aggressive management of acute hypertensive emergency, with options for 3 protocols: labetalol, nifedipine, and hydralazine. The goal is to administer antihypertensive therapy within 30 to 60 minutes, but administration as soon as feasibly possible after diagnosis of severe hypertension is ideal.
Timing of delivery. Recommended delivery timing in patients with chronic hypertension was slightly altered (previous recommendations included a range of 37 to 39 6/7 weeks). The lower limit of gestational age for recommended delivery timing in chronic hypertension has not changed—it remains not before 38 weeks if no antihypertensive therapy and stable, and not before 37 weeks if antihypertensive therapy and stable.
The upper limit of 39 6/7 weeks is challenged, however, because data support that induction of labor at either 38 or 39 weeks reduces the risk of severe hypertensive complications (such as superimposed preeclampsia and eclampsia) without increasing the risk of cesarean delivery. Therefore, for patients with chronic hypertension, expectant management beyond 39 weeks is cautioned, to be done only with careful consideration of risks and with close surveillance.
As with ACOG’s original Task Force document on hypertension, clinicians should thoroughly read these 2 Practice Bulletins on hypertension in pregnancy as there are subtle changes that affect day-to-day practice, such as the definition of hypertension prior to pregnancy, treatment guidelines, and delivery timing recommendations. As always, these are guidelines, and the obstetrician’s clinical judgment and the needs of specific patient populations also must be taken into account.
- Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964-973.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
- ACOG Practice Advisory. Clinical guidance for integration of the findings of the PROLONG study: progestin’s role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing. Accessed November 10, 2019.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280-smfm-statement-use-of-17-alpha-hydroxyprogesterone-caproate-for-prevention-of-recurrent-preterm-birth. Accessed November 10, 2019.
- US Food and Drug Administration. Bone, Reproductive, and Urologic Drugs Advisory Committee Meeting, October 29, 2019. Advisory Committee Briefing Materials: Available for Public Release. https://www.fda.gov/media/132004/download. Accessed November 19, 2019.
- Society for Maternal-Fetal Medicine. SMFM responds to the FDA’s Bone, Reproductive and Urologic Advisory Committee. https://s3.amazonaws.com/cdn.smfm.org/media/2091/17P_Public_Statement.pdf. Accessed November 19, 2019.
- American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50.
- Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-2385.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964-973.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2019. doi: 10.1055/s-0039-3400227.
- ACOG Practice Advisory. Clinical guidance for integration of the findings of the PROLONG study: progestin’s role in optimizing neonatal gestation. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Clinical-guidance-for-integration-of-the-findings-of-The-PROLONG-study-Progestins-Role-in-Optimizing. Accessed November 10, 2019.
- Society for Maternal-Fetal Medicine Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280-smfm-statement-use-of-17-alpha-hydroxyprogesterone-caproate-for-prevention-of-recurrent-preterm-birth. Accessed November 10, 2019.
- US Food and Drug Administration. Bone, Reproductive, and Urologic Drugs Advisory Committee Meeting, October 29, 2019. Advisory Committee Briefing Materials: Available for Public Release. https://www.fda.gov/media/132004/download. Accessed November 19, 2019.
- Society for Maternal-Fetal Medicine. SMFM responds to the FDA’s Bone, Reproductive and Urologic Advisory Committee. https://s3.amazonaws.com/cdn.smfm.org/media/2091/17P_Public_Statement.pdf. Accessed November 19, 2019.
- American College of Obstetricians and Gynecologists—Committee on Obstetric Practice. ACOG committee opinion no. 782: prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19-e40.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: ACOG; November 2013.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1-e25.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50.
Eating for 2: Managing eating disorders in pregnancy
Eating disorders affect nearly 1% of US adults,1 and disordered eating, or unspecified eating disorder, affects at least 1% of all pregnancies.2 Among 739 pregnant women assessed with the Eating Disorder Diagnostic scale, 7.5% of patients met criteria for an eating disorder, with 8.8% of women reporting binge eating and 2.3% of pregnant women engaging in regular compensatory behaviors. In fact, 23.4% of the study population expressed concerns about pregnancy-related weight gain and body shape.3 Eating disorders during pregnancy are more common than previously thought, and they create unique clinical challenges for obstetric providers.
Types of eating disorders
There are 3 major types of eating disorders: anorexia nervosa, bulimia nervosa, and binge eating disorder, with significant fluidity existing between all 3 conditions.
Anorexia nervosa is a condition in which an individual believes he or she is significantly overweight despite being underweight. Patients with anorexia nervosa often restrict food intake and have compulsive rituals around eating and exercise, leading to weight loss and starvation.4
Bulimia nervosa is marked by intensive dieting, uncontrolled episodes of overeating, and compensatory behaviors.4 Compensatory behaviors include self-induced vomiting; excessive exercise; and misuse of laxatives, diuretics, or other medications.
Binge eating disorder is classified as recurrent episodes of uncontrolled overeating without compensatory purging behaviors, leading to excessive weight gain.4
Eating disorders and pregnancy
Pregnancy can impact the course of preexisting eating disorders, and women also can develop symptoms of eating disorders for the first time during pregnancy. This is clinically significant as there are both maternal and fetal consequences to a mother’s disordered eating.
The risks of anorexia nervosa include vitamin deficiencies (vitamin B12/folate), dehydration leading to renal injury and electrolyte imbalances, hypoglycemia, abnormal lipid profiles, cardiac arrhythmia, and even death. The mortality rate of patients with anorexia nervosa may approach 10%; however, death during pregnancy is quite rare.2 Bulimia nervosa also carries the risks of protein and vitamin deficiencies, hypoglycemia and hyperglycemia, and death, with mortality estimated at 7% for those with a 5-year history of the illness. However, death in pregnancy due to the condition is again quite rare.5
Eating disorders can cause significant maternal and fetal complications during pregnancy and postpartum.
Maternal complications. When women with eating disorders become pregnant, they have increased risks of some pregnancy complications. Approximately 10% to 25% of pregnant women with eating disorders develop hyperemesis gravidarum.6 The nausea can serve as a trigger for a woman with an eating disorder, particularly among women with a history of purging behaviors.
Cesarean delivery is more common among women with eating disorders, which may be due to preexisting fetal compromise, leading to poor tolerance of labor, or to clinicians perceiving these pregnancies as higher risk.7
It is well known that eating disorders are highly comorbid with depression and other psychiatric conditions. In fact, 30% to 40% of women with an eating disorder develop symptoms of postpartum depression.8
Continue to: Fetal risks and complications...
Fetal risks and complications. Excessive caloric restriction and dieting can lead to folate deficiency, which in turn increases the risk of neural tube defects. Such defects are more common among women with eating disorders.9 Intrauterine growth restriction also can be a concern, most likely because of maternal malnutrition and poor maternal weight gain.10 In addition, women with eating disorders are more likely to have a preterm delivery or experience perinatal mortality or stillbirth.10
Bulimia nervosa is associated with low birthweight, while anorexia nervosa is associated with the very premature birth, low birthweight, and perinatal death.11 Eating disorders during pregnancy can have long-term psychological impacts on children, including increased likelihood of childhood hyperactivity, conduct, and adjustment disorder.12
When a patient presents showing concerning signs or symptoms of an eating disorder, it is best to start by giving her a validated assessment tool. Normalize this questioning as routine amongst populations of obstetric patients. If concerning behaviors are identified, it is best to have an open and honest conversation with the patient about her history and current disordered eating behaviors, including restrictive, binging, or purging. It is also important to address concerns and fears about pregnancy and its associated triggers. If patients are willing to accept care, it is best to connect them with a multidisciplinary treatment team, including psychiatry, nutrition, obstetrics, and social work.
Assessing patients for an eating disorder
Diagnosis of eating disorders is an interview-guided process using clinical criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition.4 The Eating Disorder Examination is a semi-structured interview composed of 4 subsections (restraint, eating concern, shape concern, and weight concern). The interview’s aim is to assess the psychopathology associated with eating disorders, and it is used in research settings rather than clinically.

Clinical diagnosis. The SCOFF questionnaire is a quick, validated tool that can be used to clinically assess for an eating disorder.13 It is composed of 5 questions, with a positive test resulting from 2 yes answers:
- Do you make yourself sick because you feel uncomfortably full?
- Do you worry that you have lost control over how much you eat?
- Have you recently lost more than one stone (14 lb) in a 3-month period?
- Do you believe yourself to be fat when others say you are too thin?
- Would you say that food dominates your life?
Referral. Patients for whom you have a concern for any eating disorder should be referred to a psychiatrist for formal diagnosis. Integrated multidisciplinary care of pregnant patients with eating disorders is necessary to improve maternal and fetal outcomes. Care teams should include obstetricians or maternal-fetal medicine clinicians experienced in caring for patients with eating disorders, psychiatrists, psychologists, nutritionists, and social workers. General treatment principles require an assessment for appropriate setting of intervention, which depends on presentation severity, assessment of nutritional status, treatment of psychiatric comorbidity, and psychotherapeutic intervention.
Overall management strategy
The initial treatment strategy for pregnant women with eating disorders should involve evaluating for severe illness and life-threatening complications of the specific disorder. All patients should be screened for suicidal ideation, severe malnutrition, electrolyte abnormalities, dehydration, hemodynamic instability, and cardiac arrhythmia. Patients with any of these severe features should be admitted for medical hospitalization and psychiatric evaluation.14 Patients that are hospitalized should be watched closely for refeeding syndrome—potentially life threatening metabolic disturbances that occur when nutrition is reinstituted to patients who are severely malnourished.
Patients without severe features or acute life-threatening complications can be managed safely on an outpatient basis with close medical monitoring. Psychiatric providers should be involved to assess for treatment needs including psychotherapy and psychotropic medications. There are numerous pharmacologic options available for patients, with the use of selective serotonin reuptake inhibitors (SSRIs) most common. While SSRI use has been controversial in pregnancy in the past, the risks of untreated illness carry risk to the mother and unborn child that outweigh the small risks associated with SSRI exposure in pregnancy.15
Women should have established care with a nutritionist or dietician who can ensure adequate counseling regarding meal planning and multivitamin supplementation. The numerous food restrictions in pregnancy, such as avoidance of unpasteurized cheese or deli meats, may be triggering for many patients with a history of restrictive eating.
One of the greatest difficulties for women with disordered eating in pregnancy revolves around weight gain. Many patients find the various measurements of pregnancy (maternal weight gain, fetal weight, fetal heart rate, and fundal height) triggering, which can make appropriate maternal and fetal weight gain in pregnancy very challenging. One strategy for managing this includes using fetal weight and growth as a surrogate for appropriate maternal gestational weight gain. One other strategy involves blind weights, where the woman is turned away from the scale so her weight is not disclosed to her. Patients often will not be able to achieve the expected 28 to 40 lb of pregnancy weight gain. It is best to have an open, honest conversation in early pregnancy to discuss how she would like to address weight in her pregnancy.
A 38-year-old woman (G1) at 32 weeks' gestation presents for a routine visit. Her bulimia had been in relatively good control until the nausea of pregnancy triggered a return to purging behaviors. She reports searching her online medical record for any recording of weights, and has now started restrictive eating because a routine recent growth scan revealed the baby to be in the 80th percentile for growth. She is concerned about her mood, and thinks she may be depressed. Because her bulimia was present before pregnancy, during her pregnancy she is followed by a multidisciplinary team, including maternal-fetal medicine, perinatal psychiatry, and nutrition. At pregnancy, she elected for outpatient day program management during her pregnancy.
Continue to: Postpregnancy concerns...
Postpregnancy concerns
Patients with eating disorders are at high risk of relapse in the postpartum period, even if they are able to achieve full remission in pregnancy. Rapid postpartum weight loss may be a sign of disordered eating. Postpartum depression also is a concern, and women should be followed closely for surveillance of symptoms. Finally, postpartum contraception is extremely important. The menstrual irregularities that are common among women with eating disorders along with common misconceptions regarding fertility in the postpartum period increase the risk of unplanned pregnancy.
Remain cognizant of eating disorders
A clear surveillance plan early in the pregnancy that is developed in conjunction with the patient and her care team is crucial in improving maternal and fetal outcomes among women with an eating disorder. Clinician knowledge of complications and risks specific to disordered eating and pregnancy can affect outcomes for both mother and baby.
- Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol Psychiatry. 2018;84:345-354.
- Easter A, Bye A, Taborelli E, et al. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev. 2013;21:340-344.
- Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348-358.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). American Psychiatry Association: Arlington, VA; 2013.
- Morgan JF, Lacey JH, Sedgwick PM. Impact of pregnancy on bulimia nervosa. Br J Psychiatry. 1999;174:135-140.
- Franko DL, Spirrell EB. Detection and management of eating disorders during pregnancy. Obstet Gynecol. 2000;95:942-946.
- Bulik CM, Von Holle A, Siega-Riz AM, et al. Birth outcomes in women with eating disorders in the Norwegian Mother and Child Cohort Study. Int J Eat Disord. 2009;42:9-18.
- Mitchell-Gieleghem A, Mittelstaedt ME, Bulik CM. Eating disorders and childbearing: concealment and consequences. Birth. 2002;29:182.
- Carmichael SL, Shaw GM, Schaffer DM, et al. Dieting behaviors and risk of neural tube defects. Am J Epidemiol. 2003;158:1127-1131.
- Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190-255.
- Linna MS, Raevuori A, Haukka J. Pregnancy, obstetrics, and perinatal health outcomes in eating disorders. Am J Obstet Gynecol. 2014;211:392.e1-e8.
- Barona M, Nybo Andersen AM, Micali N. Childhood psychopathology in children of women with eating disorders. Acta Psychiatr Scand. 2016;134:295-304.
- Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319:1467.
- Andersen AE, Ryan GL. Eating disorders in the obstetric and gynecologic patient population. Obstet Gynecol. 2009;114:1353-1367.
- Weisskopf E, Fischer CJ, Bickle Graz M, et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence. Expert Opin Drug Saf. 2015;14:413-427.
Eating disorders affect nearly 1% of US adults,1 and disordered eating, or unspecified eating disorder, affects at least 1% of all pregnancies.2 Among 739 pregnant women assessed with the Eating Disorder Diagnostic scale, 7.5% of patients met criteria for an eating disorder, with 8.8% of women reporting binge eating and 2.3% of pregnant women engaging in regular compensatory behaviors. In fact, 23.4% of the study population expressed concerns about pregnancy-related weight gain and body shape.3 Eating disorders during pregnancy are more common than previously thought, and they create unique clinical challenges for obstetric providers.
Types of eating disorders
There are 3 major types of eating disorders: anorexia nervosa, bulimia nervosa, and binge eating disorder, with significant fluidity existing between all 3 conditions.
Anorexia nervosa is a condition in which an individual believes he or she is significantly overweight despite being underweight. Patients with anorexia nervosa often restrict food intake and have compulsive rituals around eating and exercise, leading to weight loss and starvation.4
Bulimia nervosa is marked by intensive dieting, uncontrolled episodes of overeating, and compensatory behaviors.4 Compensatory behaviors include self-induced vomiting; excessive exercise; and misuse of laxatives, diuretics, or other medications.
Binge eating disorder is classified as recurrent episodes of uncontrolled overeating without compensatory purging behaviors, leading to excessive weight gain.4
Eating disorders and pregnancy
Pregnancy can impact the course of preexisting eating disorders, and women also can develop symptoms of eating disorders for the first time during pregnancy. This is clinically significant as there are both maternal and fetal consequences to a mother’s disordered eating.
The risks of anorexia nervosa include vitamin deficiencies (vitamin B12/folate), dehydration leading to renal injury and electrolyte imbalances, hypoglycemia, abnormal lipid profiles, cardiac arrhythmia, and even death. The mortality rate of patients with anorexia nervosa may approach 10%; however, death during pregnancy is quite rare.2 Bulimia nervosa also carries the risks of protein and vitamin deficiencies, hypoglycemia and hyperglycemia, and death, with mortality estimated at 7% for those with a 5-year history of the illness. However, death in pregnancy due to the condition is again quite rare.5
Eating disorders can cause significant maternal and fetal complications during pregnancy and postpartum.
Maternal complications. When women with eating disorders become pregnant, they have increased risks of some pregnancy complications. Approximately 10% to 25% of pregnant women with eating disorders develop hyperemesis gravidarum.6 The nausea can serve as a trigger for a woman with an eating disorder, particularly among women with a history of purging behaviors.
Cesarean delivery is more common among women with eating disorders, which may be due to preexisting fetal compromise, leading to poor tolerance of labor, or to clinicians perceiving these pregnancies as higher risk.7
It is well known that eating disorders are highly comorbid with depression and other psychiatric conditions. In fact, 30% to 40% of women with an eating disorder develop symptoms of postpartum depression.8
Continue to: Fetal risks and complications...
Fetal risks and complications. Excessive caloric restriction and dieting can lead to folate deficiency, which in turn increases the risk of neural tube defects. Such defects are more common among women with eating disorders.9 Intrauterine growth restriction also can be a concern, most likely because of maternal malnutrition and poor maternal weight gain.10 In addition, women with eating disorders are more likely to have a preterm delivery or experience perinatal mortality or stillbirth.10
Bulimia nervosa is associated with low birthweight, while anorexia nervosa is associated with the very premature birth, low birthweight, and perinatal death.11 Eating disorders during pregnancy can have long-term psychological impacts on children, including increased likelihood of childhood hyperactivity, conduct, and adjustment disorder.12
When a patient presents showing concerning signs or symptoms of an eating disorder, it is best to start by giving her a validated assessment tool. Normalize this questioning as routine amongst populations of obstetric patients. If concerning behaviors are identified, it is best to have an open and honest conversation with the patient about her history and current disordered eating behaviors, including restrictive, binging, or purging. It is also important to address concerns and fears about pregnancy and its associated triggers. If patients are willing to accept care, it is best to connect them with a multidisciplinary treatment team, including psychiatry, nutrition, obstetrics, and social work.
Assessing patients for an eating disorder
Diagnosis of eating disorders is an interview-guided process using clinical criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition.4 The Eating Disorder Examination is a semi-structured interview composed of 4 subsections (restraint, eating concern, shape concern, and weight concern). The interview’s aim is to assess the psychopathology associated with eating disorders, and it is used in research settings rather than clinically.

Clinical diagnosis. The SCOFF questionnaire is a quick, validated tool that can be used to clinically assess for an eating disorder.13 It is composed of 5 questions, with a positive test resulting from 2 yes answers:
- Do you make yourself sick because you feel uncomfortably full?
- Do you worry that you have lost control over how much you eat?
- Have you recently lost more than one stone (14 lb) in a 3-month period?
- Do you believe yourself to be fat when others say you are too thin?
- Would you say that food dominates your life?
Referral. Patients for whom you have a concern for any eating disorder should be referred to a psychiatrist for formal diagnosis. Integrated multidisciplinary care of pregnant patients with eating disorders is necessary to improve maternal and fetal outcomes. Care teams should include obstetricians or maternal-fetal medicine clinicians experienced in caring for patients with eating disorders, psychiatrists, psychologists, nutritionists, and social workers. General treatment principles require an assessment for appropriate setting of intervention, which depends on presentation severity, assessment of nutritional status, treatment of psychiatric comorbidity, and psychotherapeutic intervention.
Overall management strategy
The initial treatment strategy for pregnant women with eating disorders should involve evaluating for severe illness and life-threatening complications of the specific disorder. All patients should be screened for suicidal ideation, severe malnutrition, electrolyte abnormalities, dehydration, hemodynamic instability, and cardiac arrhythmia. Patients with any of these severe features should be admitted for medical hospitalization and psychiatric evaluation.14 Patients that are hospitalized should be watched closely for refeeding syndrome—potentially life threatening metabolic disturbances that occur when nutrition is reinstituted to patients who are severely malnourished.
Patients without severe features or acute life-threatening complications can be managed safely on an outpatient basis with close medical monitoring. Psychiatric providers should be involved to assess for treatment needs including psychotherapy and psychotropic medications. There are numerous pharmacologic options available for patients, with the use of selective serotonin reuptake inhibitors (SSRIs) most common. While SSRI use has been controversial in pregnancy in the past, the risks of untreated illness carry risk to the mother and unborn child that outweigh the small risks associated with SSRI exposure in pregnancy.15
Women should have established care with a nutritionist or dietician who can ensure adequate counseling regarding meal planning and multivitamin supplementation. The numerous food restrictions in pregnancy, such as avoidance of unpasteurized cheese or deli meats, may be triggering for many patients with a history of restrictive eating.
One of the greatest difficulties for women with disordered eating in pregnancy revolves around weight gain. Many patients find the various measurements of pregnancy (maternal weight gain, fetal weight, fetal heart rate, and fundal height) triggering, which can make appropriate maternal and fetal weight gain in pregnancy very challenging. One strategy for managing this includes using fetal weight and growth as a surrogate for appropriate maternal gestational weight gain. One other strategy involves blind weights, where the woman is turned away from the scale so her weight is not disclosed to her. Patients often will not be able to achieve the expected 28 to 40 lb of pregnancy weight gain. It is best to have an open, honest conversation in early pregnancy to discuss how she would like to address weight in her pregnancy.
A 38-year-old woman (G1) at 32 weeks' gestation presents for a routine visit. Her bulimia had been in relatively good control until the nausea of pregnancy triggered a return to purging behaviors. She reports searching her online medical record for any recording of weights, and has now started restrictive eating because a routine recent growth scan revealed the baby to be in the 80th percentile for growth. She is concerned about her mood, and thinks she may be depressed. Because her bulimia was present before pregnancy, during her pregnancy she is followed by a multidisciplinary team, including maternal-fetal medicine, perinatal psychiatry, and nutrition. At pregnancy, she elected for outpatient day program management during her pregnancy.
Continue to: Postpregnancy concerns...
Postpregnancy concerns
Patients with eating disorders are at high risk of relapse in the postpartum period, even if they are able to achieve full remission in pregnancy. Rapid postpartum weight loss may be a sign of disordered eating. Postpartum depression also is a concern, and women should be followed closely for surveillance of symptoms. Finally, postpartum contraception is extremely important. The menstrual irregularities that are common among women with eating disorders along with common misconceptions regarding fertility in the postpartum period increase the risk of unplanned pregnancy.
Remain cognizant of eating disorders
A clear surveillance plan early in the pregnancy that is developed in conjunction with the patient and her care team is crucial in improving maternal and fetal outcomes among women with an eating disorder. Clinician knowledge of complications and risks specific to disordered eating and pregnancy can affect outcomes for both mother and baby.
Eating disorders affect nearly 1% of US adults,1 and disordered eating, or unspecified eating disorder, affects at least 1% of all pregnancies.2 Among 739 pregnant women assessed with the Eating Disorder Diagnostic scale, 7.5% of patients met criteria for an eating disorder, with 8.8% of women reporting binge eating and 2.3% of pregnant women engaging in regular compensatory behaviors. In fact, 23.4% of the study population expressed concerns about pregnancy-related weight gain and body shape.3 Eating disorders during pregnancy are more common than previously thought, and they create unique clinical challenges for obstetric providers.
Types of eating disorders
There are 3 major types of eating disorders: anorexia nervosa, bulimia nervosa, and binge eating disorder, with significant fluidity existing between all 3 conditions.
Anorexia nervosa is a condition in which an individual believes he or she is significantly overweight despite being underweight. Patients with anorexia nervosa often restrict food intake and have compulsive rituals around eating and exercise, leading to weight loss and starvation.4
Bulimia nervosa is marked by intensive dieting, uncontrolled episodes of overeating, and compensatory behaviors.4 Compensatory behaviors include self-induced vomiting; excessive exercise; and misuse of laxatives, diuretics, or other medications.
Binge eating disorder is classified as recurrent episodes of uncontrolled overeating without compensatory purging behaviors, leading to excessive weight gain.4
Eating disorders and pregnancy
Pregnancy can impact the course of preexisting eating disorders, and women also can develop symptoms of eating disorders for the first time during pregnancy. This is clinically significant as there are both maternal and fetal consequences to a mother’s disordered eating.
The risks of anorexia nervosa include vitamin deficiencies (vitamin B12/folate), dehydration leading to renal injury and electrolyte imbalances, hypoglycemia, abnormal lipid profiles, cardiac arrhythmia, and even death. The mortality rate of patients with anorexia nervosa may approach 10%; however, death during pregnancy is quite rare.2 Bulimia nervosa also carries the risks of protein and vitamin deficiencies, hypoglycemia and hyperglycemia, and death, with mortality estimated at 7% for those with a 5-year history of the illness. However, death in pregnancy due to the condition is again quite rare.5
Eating disorders can cause significant maternal and fetal complications during pregnancy and postpartum.
Maternal complications. When women with eating disorders become pregnant, they have increased risks of some pregnancy complications. Approximately 10% to 25% of pregnant women with eating disorders develop hyperemesis gravidarum.6 The nausea can serve as a trigger for a woman with an eating disorder, particularly among women with a history of purging behaviors.
Cesarean delivery is more common among women with eating disorders, which may be due to preexisting fetal compromise, leading to poor tolerance of labor, or to clinicians perceiving these pregnancies as higher risk.7
It is well known that eating disorders are highly comorbid with depression and other psychiatric conditions. In fact, 30% to 40% of women with an eating disorder develop symptoms of postpartum depression.8
Continue to: Fetal risks and complications...
Fetal risks and complications. Excessive caloric restriction and dieting can lead to folate deficiency, which in turn increases the risk of neural tube defects. Such defects are more common among women with eating disorders.9 Intrauterine growth restriction also can be a concern, most likely because of maternal malnutrition and poor maternal weight gain.10 In addition, women with eating disorders are more likely to have a preterm delivery or experience perinatal mortality or stillbirth.10
Bulimia nervosa is associated with low birthweight, while anorexia nervosa is associated with the very premature birth, low birthweight, and perinatal death.11 Eating disorders during pregnancy can have long-term psychological impacts on children, including increased likelihood of childhood hyperactivity, conduct, and adjustment disorder.12
When a patient presents showing concerning signs or symptoms of an eating disorder, it is best to start by giving her a validated assessment tool. Normalize this questioning as routine amongst populations of obstetric patients. If concerning behaviors are identified, it is best to have an open and honest conversation with the patient about her history and current disordered eating behaviors, including restrictive, binging, or purging. It is also important to address concerns and fears about pregnancy and its associated triggers. If patients are willing to accept care, it is best to connect them with a multidisciplinary treatment team, including psychiatry, nutrition, obstetrics, and social work.
Assessing patients for an eating disorder
Diagnosis of eating disorders is an interview-guided process using clinical criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition.4 The Eating Disorder Examination is a semi-structured interview composed of 4 subsections (restraint, eating concern, shape concern, and weight concern). The interview’s aim is to assess the psychopathology associated with eating disorders, and it is used in research settings rather than clinically.

Clinical diagnosis. The SCOFF questionnaire is a quick, validated tool that can be used to clinically assess for an eating disorder.13 It is composed of 5 questions, with a positive test resulting from 2 yes answers:
- Do you make yourself sick because you feel uncomfortably full?
- Do you worry that you have lost control over how much you eat?
- Have you recently lost more than one stone (14 lb) in a 3-month period?
- Do you believe yourself to be fat when others say you are too thin?
- Would you say that food dominates your life?
Referral. Patients for whom you have a concern for any eating disorder should be referred to a psychiatrist for formal diagnosis. Integrated multidisciplinary care of pregnant patients with eating disorders is necessary to improve maternal and fetal outcomes. Care teams should include obstetricians or maternal-fetal medicine clinicians experienced in caring for patients with eating disorders, psychiatrists, psychologists, nutritionists, and social workers. General treatment principles require an assessment for appropriate setting of intervention, which depends on presentation severity, assessment of nutritional status, treatment of psychiatric comorbidity, and psychotherapeutic intervention.
Overall management strategy
The initial treatment strategy for pregnant women with eating disorders should involve evaluating for severe illness and life-threatening complications of the specific disorder. All patients should be screened for suicidal ideation, severe malnutrition, electrolyte abnormalities, dehydration, hemodynamic instability, and cardiac arrhythmia. Patients with any of these severe features should be admitted for medical hospitalization and psychiatric evaluation.14 Patients that are hospitalized should be watched closely for refeeding syndrome—potentially life threatening metabolic disturbances that occur when nutrition is reinstituted to patients who are severely malnourished.
Patients without severe features or acute life-threatening complications can be managed safely on an outpatient basis with close medical monitoring. Psychiatric providers should be involved to assess for treatment needs including psychotherapy and psychotropic medications. There are numerous pharmacologic options available for patients, with the use of selective serotonin reuptake inhibitors (SSRIs) most common. While SSRI use has been controversial in pregnancy in the past, the risks of untreated illness carry risk to the mother and unborn child that outweigh the small risks associated with SSRI exposure in pregnancy.15
Women should have established care with a nutritionist or dietician who can ensure adequate counseling regarding meal planning and multivitamin supplementation. The numerous food restrictions in pregnancy, such as avoidance of unpasteurized cheese or deli meats, may be triggering for many patients with a history of restrictive eating.
One of the greatest difficulties for women with disordered eating in pregnancy revolves around weight gain. Many patients find the various measurements of pregnancy (maternal weight gain, fetal weight, fetal heart rate, and fundal height) triggering, which can make appropriate maternal and fetal weight gain in pregnancy very challenging. One strategy for managing this includes using fetal weight and growth as a surrogate for appropriate maternal gestational weight gain. One other strategy involves blind weights, where the woman is turned away from the scale so her weight is not disclosed to her. Patients often will not be able to achieve the expected 28 to 40 lb of pregnancy weight gain. It is best to have an open, honest conversation in early pregnancy to discuss how she would like to address weight in her pregnancy.
A 38-year-old woman (G1) at 32 weeks' gestation presents for a routine visit. Her bulimia had been in relatively good control until the nausea of pregnancy triggered a return to purging behaviors. She reports searching her online medical record for any recording of weights, and has now started restrictive eating because a routine recent growth scan revealed the baby to be in the 80th percentile for growth. She is concerned about her mood, and thinks she may be depressed. Because her bulimia was present before pregnancy, during her pregnancy she is followed by a multidisciplinary team, including maternal-fetal medicine, perinatal psychiatry, and nutrition. At pregnancy, she elected for outpatient day program management during her pregnancy.
Continue to: Postpregnancy concerns...
Postpregnancy concerns
Patients with eating disorders are at high risk of relapse in the postpartum period, even if they are able to achieve full remission in pregnancy. Rapid postpartum weight loss may be a sign of disordered eating. Postpartum depression also is a concern, and women should be followed closely for surveillance of symptoms. Finally, postpartum contraception is extremely important. The menstrual irregularities that are common among women with eating disorders along with common misconceptions regarding fertility in the postpartum period increase the risk of unplanned pregnancy.
Remain cognizant of eating disorders
A clear surveillance plan early in the pregnancy that is developed in conjunction with the patient and her care team is crucial in improving maternal and fetal outcomes among women with an eating disorder. Clinician knowledge of complications and risks specific to disordered eating and pregnancy can affect outcomes for both mother and baby.
- Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol Psychiatry. 2018;84:345-354.
- Easter A, Bye A, Taborelli E, et al. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev. 2013;21:340-344.
- Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348-358.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). American Psychiatry Association: Arlington, VA; 2013.
- Morgan JF, Lacey JH, Sedgwick PM. Impact of pregnancy on bulimia nervosa. Br J Psychiatry. 1999;174:135-140.
- Franko DL, Spirrell EB. Detection and management of eating disorders during pregnancy. Obstet Gynecol. 2000;95:942-946.
- Bulik CM, Von Holle A, Siega-Riz AM, et al. Birth outcomes in women with eating disorders in the Norwegian Mother and Child Cohort Study. Int J Eat Disord. 2009;42:9-18.
- Mitchell-Gieleghem A, Mittelstaedt ME, Bulik CM. Eating disorders and childbearing: concealment and consequences. Birth. 2002;29:182.
- Carmichael SL, Shaw GM, Schaffer DM, et al. Dieting behaviors and risk of neural tube defects. Am J Epidemiol. 2003;158:1127-1131.
- Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190-255.
- Linna MS, Raevuori A, Haukka J. Pregnancy, obstetrics, and perinatal health outcomes in eating disorders. Am J Obstet Gynecol. 2014;211:392.e1-e8.
- Barona M, Nybo Andersen AM, Micali N. Childhood psychopathology in children of women with eating disorders. Acta Psychiatr Scand. 2016;134:295-304.
- Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319:1467.
- Andersen AE, Ryan GL. Eating disorders in the obstetric and gynecologic patient population. Obstet Gynecol. 2009;114:1353-1367.
- Weisskopf E, Fischer CJ, Bickle Graz M, et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence. Expert Opin Drug Saf. 2015;14:413-427.
- Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol Psychiatry. 2018;84:345-354.
- Easter A, Bye A, Taborelli E, et al. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev. 2013;21:340-344.
- Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348-358.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). American Psychiatry Association: Arlington, VA; 2013.
- Morgan JF, Lacey JH, Sedgwick PM. Impact of pregnancy on bulimia nervosa. Br J Psychiatry. 1999;174:135-140.
- Franko DL, Spirrell EB. Detection and management of eating disorders during pregnancy. Obstet Gynecol. 2000;95:942-946.
- Bulik CM, Von Holle A, Siega-Riz AM, et al. Birth outcomes in women with eating disorders in the Norwegian Mother and Child Cohort Study. Int J Eat Disord. 2009;42:9-18.
- Mitchell-Gieleghem A, Mittelstaedt ME, Bulik CM. Eating disorders and childbearing: concealment and consequences. Birth. 2002;29:182.
- Carmichael SL, Shaw GM, Schaffer DM, et al. Dieting behaviors and risk of neural tube defects. Am J Epidemiol. 2003;158:1127-1131.
- Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190-255.
- Linna MS, Raevuori A, Haukka J. Pregnancy, obstetrics, and perinatal health outcomes in eating disorders. Am J Obstet Gynecol. 2014;211:392.e1-e8.
- Barona M, Nybo Andersen AM, Micali N. Childhood psychopathology in children of women with eating disorders. Acta Psychiatr Scand. 2016;134:295-304.
- Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319:1467.
- Andersen AE, Ryan GL. Eating disorders in the obstetric and gynecologic patient population. Obstet Gynecol. 2009;114:1353-1367.
- Weisskopf E, Fischer CJ, Bickle Graz M, et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence. Expert Opin Drug Saf. 2015;14:413-427.
Ideal management of RA in pregnancy improves outcomes
Women whose rheumatoid arthritis is carefully managed before and during pregnancy have a significantly lower risk of adverse pregnancy outcomes, including miscarriage or perinatal death, new research suggests.
A study published in Arthritis Care & Research presents the outcomes of a retrospective, observational study examining health care data from 443 first pregnancies in women with RA and 6,097 women without the disease.
First author Alessandra Bortoluzzi, MD, PhD, from the Rheumatology Unit at the University of Ferrara (Italy) and coauthors looked at seven diagnostic, therapeutic, and follow-up health care quality indicators during the prepregnancy and perinatal period. They included having at least one blood test in the 18 months before conception and during pregnancy, preconception musculoskeletal imaging, no exposure or wash-out from teratogenic drugs, and no exposure to biologic drugs between conception and delivery or end of pregnancy.
An ideal clinical pathway included at least one element from each of the diagnostic, therapeutic, and prenatal follow-up quality indicators.
Overall, women with RA had a significantly higher rate of thyroid diseases, adverse pregnancy outcomes, and miscarriage or perinatal death when compared with controls. However, those who followed the ideal clinical pathway for management of their disease during pregnancy had a 40% lower odds of adverse pregnancy outcomes (odds ratio, 0.60; 95% confidence interval, 0.39-0.94) and a 60% lower odds of miscarriage or perinatal death (OR, 0.40; 95% CI, 0.24-0.69) in comparison with women with RA who were not managed to the same standard. The researchers adjusted both comparisons for age, Charlson comorbidity index, and thyroid diseases.
Women with RA who met diagnostic, therapeutic, and prenatal follow-up quality indicators showed no significant differences from the general population in terms of the risk of adverse pregnancy outcomes, miscarriage, or perinatal death after adjusting for hypertension in addition to the same variables as before.
When researchers looked at some of the individual health care quality indicators, they found that testing for antiphospholipid (aPL) antibodies within 18 months of conception or pregnancy was associated with a 44% lower rate of adverse pregnancy outcomes. Similarly, antinuclear antibody or anti–extractable nuclear antigen antibody testing was associated with 36% lower odds of adverse pregnancy outcomes.
Dr. Bortoluzzi and her coauthors wrote that their findings pointed to the value of testing for aPL antibodies in women with RA who wish to get pregnant.
“In fact, despite the absence of formal recommendation or validated health care quality indicators focused on stratification of preconceptional obstetric risk in patients with RA, we started from the basic and universally accepted assumption that aPL antibodies are pathogenic autoantibodies and therefore recognized risk factors for adverse pregnancy outcome,” they wrote.
Women with RA who had either no exposure to methotrexate or leflunomide or who had a washout period from 6 months prior to conception had 72% lower odds of adverse pregnancy outcomes.
The authors also looked at the effects of drugs such as aspirin, glucocorticoids, and low-molecular-weight heparin that are used during pregnancy. They found that the relative risk of adverse pregnancy outcomes was 40% higher in women with RA who were taking glucocorticoids, compared with those with the disease but not taking that type of medication. However, low-molecular-weight heparin use was associated with an 80% lower relative risk of miscarriage or perinatal death in comparison with those not taking it. Researchers saw no significant effects of aspirin or conventional synthetic disease-modifying antirheumatic drugs on either adverse pregnancy outcomes or the risk of miscarriage or perinatal death.
“This reinforces the importance of adjustment of therapy for RA before conception and throughout pregnancy, because medication use could affect pregnancy course not only influencing maternal disease activity but also the gestational outcome,” the authors wrote. “Although this is a study conducted on administrative data, we can hypothesize that exposure to therapy represents a marker of high RA disease activity and severity. In our setting, it is possible that, the more active the disease, the greater the probability of being included in the ideal clinical pathway, but in any case, this resulted in a lower odds ratio of adverse pregnancy outcome and miscarriage/perinatal death.”
The study was supported by the Italian Society for Rheumatology. No conflicts of interest were declared.
SOURCE: Bortoluzzi A et al. Arthritis Care Res. 2020 Jan 8. doi: 10.1002/ACR.24116.
Women whose rheumatoid arthritis is carefully managed before and during pregnancy have a significantly lower risk of adverse pregnancy outcomes, including miscarriage or perinatal death, new research suggests.
A study published in Arthritis Care & Research presents the outcomes of a retrospective, observational study examining health care data from 443 first pregnancies in women with RA and 6,097 women without the disease.
First author Alessandra Bortoluzzi, MD, PhD, from the Rheumatology Unit at the University of Ferrara (Italy) and coauthors looked at seven diagnostic, therapeutic, and follow-up health care quality indicators during the prepregnancy and perinatal period. They included having at least one blood test in the 18 months before conception and during pregnancy, preconception musculoskeletal imaging, no exposure or wash-out from teratogenic drugs, and no exposure to biologic drugs between conception and delivery or end of pregnancy.
An ideal clinical pathway included at least one element from each of the diagnostic, therapeutic, and prenatal follow-up quality indicators.
Overall, women with RA had a significantly higher rate of thyroid diseases, adverse pregnancy outcomes, and miscarriage or perinatal death when compared with controls. However, those who followed the ideal clinical pathway for management of their disease during pregnancy had a 40% lower odds of adverse pregnancy outcomes (odds ratio, 0.60; 95% confidence interval, 0.39-0.94) and a 60% lower odds of miscarriage or perinatal death (OR, 0.40; 95% CI, 0.24-0.69) in comparison with women with RA who were not managed to the same standard. The researchers adjusted both comparisons for age, Charlson comorbidity index, and thyroid diseases.
Women with RA who met diagnostic, therapeutic, and prenatal follow-up quality indicators showed no significant differences from the general population in terms of the risk of adverse pregnancy outcomes, miscarriage, or perinatal death after adjusting for hypertension in addition to the same variables as before.
When researchers looked at some of the individual health care quality indicators, they found that testing for antiphospholipid (aPL) antibodies within 18 months of conception or pregnancy was associated with a 44% lower rate of adverse pregnancy outcomes. Similarly, antinuclear antibody or anti–extractable nuclear antigen antibody testing was associated with 36% lower odds of adverse pregnancy outcomes.
Dr. Bortoluzzi and her coauthors wrote that their findings pointed to the value of testing for aPL antibodies in women with RA who wish to get pregnant.
“In fact, despite the absence of formal recommendation or validated health care quality indicators focused on stratification of preconceptional obstetric risk in patients with RA, we started from the basic and universally accepted assumption that aPL antibodies are pathogenic autoantibodies and therefore recognized risk factors for adverse pregnancy outcome,” they wrote.
Women with RA who had either no exposure to methotrexate or leflunomide or who had a washout period from 6 months prior to conception had 72% lower odds of adverse pregnancy outcomes.
The authors also looked at the effects of drugs such as aspirin, glucocorticoids, and low-molecular-weight heparin that are used during pregnancy. They found that the relative risk of adverse pregnancy outcomes was 40% higher in women with RA who were taking glucocorticoids, compared with those with the disease but not taking that type of medication. However, low-molecular-weight heparin use was associated with an 80% lower relative risk of miscarriage or perinatal death in comparison with those not taking it. Researchers saw no significant effects of aspirin or conventional synthetic disease-modifying antirheumatic drugs on either adverse pregnancy outcomes or the risk of miscarriage or perinatal death.
“This reinforces the importance of adjustment of therapy for RA before conception and throughout pregnancy, because medication use could affect pregnancy course not only influencing maternal disease activity but also the gestational outcome,” the authors wrote. “Although this is a study conducted on administrative data, we can hypothesize that exposure to therapy represents a marker of high RA disease activity and severity. In our setting, it is possible that, the more active the disease, the greater the probability of being included in the ideal clinical pathway, but in any case, this resulted in a lower odds ratio of adverse pregnancy outcome and miscarriage/perinatal death.”
The study was supported by the Italian Society for Rheumatology. No conflicts of interest were declared.
SOURCE: Bortoluzzi A et al. Arthritis Care Res. 2020 Jan 8. doi: 10.1002/ACR.24116.
Women whose rheumatoid arthritis is carefully managed before and during pregnancy have a significantly lower risk of adverse pregnancy outcomes, including miscarriage or perinatal death, new research suggests.
A study published in Arthritis Care & Research presents the outcomes of a retrospective, observational study examining health care data from 443 first pregnancies in women with RA and 6,097 women without the disease.
First author Alessandra Bortoluzzi, MD, PhD, from the Rheumatology Unit at the University of Ferrara (Italy) and coauthors looked at seven diagnostic, therapeutic, and follow-up health care quality indicators during the prepregnancy and perinatal period. They included having at least one blood test in the 18 months before conception and during pregnancy, preconception musculoskeletal imaging, no exposure or wash-out from teratogenic drugs, and no exposure to biologic drugs between conception and delivery or end of pregnancy.
An ideal clinical pathway included at least one element from each of the diagnostic, therapeutic, and prenatal follow-up quality indicators.
Overall, women with RA had a significantly higher rate of thyroid diseases, adverse pregnancy outcomes, and miscarriage or perinatal death when compared with controls. However, those who followed the ideal clinical pathway for management of their disease during pregnancy had a 40% lower odds of adverse pregnancy outcomes (odds ratio, 0.60; 95% confidence interval, 0.39-0.94) and a 60% lower odds of miscarriage or perinatal death (OR, 0.40; 95% CI, 0.24-0.69) in comparison with women with RA who were not managed to the same standard. The researchers adjusted both comparisons for age, Charlson comorbidity index, and thyroid diseases.
Women with RA who met diagnostic, therapeutic, and prenatal follow-up quality indicators showed no significant differences from the general population in terms of the risk of adverse pregnancy outcomes, miscarriage, or perinatal death after adjusting for hypertension in addition to the same variables as before.
When researchers looked at some of the individual health care quality indicators, they found that testing for antiphospholipid (aPL) antibodies within 18 months of conception or pregnancy was associated with a 44% lower rate of adverse pregnancy outcomes. Similarly, antinuclear antibody or anti–extractable nuclear antigen antibody testing was associated with 36% lower odds of adverse pregnancy outcomes.
Dr. Bortoluzzi and her coauthors wrote that their findings pointed to the value of testing for aPL antibodies in women with RA who wish to get pregnant.
“In fact, despite the absence of formal recommendation or validated health care quality indicators focused on stratification of preconceptional obstetric risk in patients with RA, we started from the basic and universally accepted assumption that aPL antibodies are pathogenic autoantibodies and therefore recognized risk factors for adverse pregnancy outcome,” they wrote.
Women with RA who had either no exposure to methotrexate or leflunomide or who had a washout period from 6 months prior to conception had 72% lower odds of adverse pregnancy outcomes.
The authors also looked at the effects of drugs such as aspirin, glucocorticoids, and low-molecular-weight heparin that are used during pregnancy. They found that the relative risk of adverse pregnancy outcomes was 40% higher in women with RA who were taking glucocorticoids, compared with those with the disease but not taking that type of medication. However, low-molecular-weight heparin use was associated with an 80% lower relative risk of miscarriage or perinatal death in comparison with those not taking it. Researchers saw no significant effects of aspirin or conventional synthetic disease-modifying antirheumatic drugs on either adverse pregnancy outcomes or the risk of miscarriage or perinatal death.
“This reinforces the importance of adjustment of therapy for RA before conception and throughout pregnancy, because medication use could affect pregnancy course not only influencing maternal disease activity but also the gestational outcome,” the authors wrote. “Although this is a study conducted on administrative data, we can hypothesize that exposure to therapy represents a marker of high RA disease activity and severity. In our setting, it is possible that, the more active the disease, the greater the probability of being included in the ideal clinical pathway, but in any case, this resulted in a lower odds ratio of adverse pregnancy outcome and miscarriage/perinatal death.”
The study was supported by the Italian Society for Rheumatology. No conflicts of interest were declared.
SOURCE: Bortoluzzi A et al. Arthritis Care Res. 2020 Jan 8. doi: 10.1002/ACR.24116.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Well-managed rheumatoid arthritis during preconception and pregnancy is associated with improved pregnancy outcomes.
Major finding: Women who adhered to an ideal clinical pathway for their RA had significantly lower risk of adverse pregnancy outcomes and miscarriage and/or perinatal death.
Study details: Retrospective, observational study of 443 first pregnancies in women with RA and 6,097 women without.
Disclosures: The study was supported by the Italian Society for Rheumatology. No conflicts of interest were declared.
Source: Bortoluzzi A et al. Arthritis Care Res. 2020 Jan 8. doi: 10.1002/ACR.24116.
We can achieve opioid-free analgesia after childbirth: Stop prescribing opioids after vaginal delivery and reduce their use after cesarean
CASE New mother receives unneeded opioids after CD
A house officer wrote orders for a healthy patient who had just had an uncomplicated cesarean delivery (CD). The hospital’s tradition dictates orders for oxycodone plus acetaminophen tablets in addition to ibuprofen for all new mothers. At the time of the patient’s discharge, the same house officer prescribed 30 tablets of oxycodone plus acetaminophen “just in case,” although the patient had required only a few tablets while in the hospital on postoperative day 2 and none on the day of discharge.
Stuck in the habit
Prescribing postpartum opioids in the United States is almost habitual. Both optimizing patient satisfaction and minimizing patient phone calls may be driving this well-established pattern. Interestingly, a survey study of obstetric providers in 14 countries found that clinicians in 13 countries prescribe opioids “almost never” after vaginal delivery.1 The United States was the 1 outlier, with providers reporting prescribing opioids “on a regular basis” after vaginal birth. Similarly, providers in 10 countries reported prescribing opioids “almost never” after CD, while those in the United States reported prescribing opioids “almost always” in this context.
Moreover, mounting data suggest that many patients do not require the quantity of opioids prescribed and that our overprescribing may be causing more harm than good.
The problem of overprescribing opioids after childbirth
Opioid analgesia has long been the mainstay of treatment for postpartum pain, which when poorly controlled is associated with the development of postpartum depression and chronic pain.2 However, common adverse effects of opioids, including nausea, drowsiness, and dizziness, similarly can interfere with self-care and infant care. Of additional concern, a 2016 claims data study found that 1 of 300 opioid-naïve women who were prescribed opioids at discharge after CD used these medications persistently in the first year postpartum.3
Many women do not use the opioids that are prescribed to them at discharge, thus making tablets available for potential diversion into the community—a commonly recognized source of opioid misuse and abuse.4,5 In a 2018 Committee Opinion on postpartum pain management, the American College of Obstetricians and Gynecologists (ACOG) stated that “a stepwise, multimodal approach emphasizing nonopioid analgesia as first-line therapy is safe and effective for vaginal deliveries and cesarean deliveries.”6 The Committee Opinion also asserted that “opioid medication is an adjunct for patients with uncontrolled pain despite adequate first-line therapy.”6
Despite efforts by the Centers for Disease Control and Prevention (CDC) and ACOG to improve opioid prescribing patterns after childbirth, the vast majority of women receive opioids in the hospital and at discharge not only after CD, but after vaginal delivery as well.4,7 Why has tradition prevailed over data, and why have we not changed?
Continue to: Common misconceptions about reducing opioid use...
Common misconceptions about reducing opioid use
Two misconceptions persist regarding reducing opioid prescriptions for postpartum pain.
Misconception #1: Patients will be in pain
Randomized controlled trials that compared nonopioid with opioid regimens in the emergency room setting and opioid use after outpatient general surgery procedures have demonstrated that pain control for patients receiving opioids was equivalent to that for patients with pain managed with nonopioid regimens.8-10 In the obstetric setting, a survey study of 720 women who underwent CD found that higher quantities of opioid tablets prescribed at discharge were not associated with improved pain, higher satisfaction, or lower refill rates at 2 weeks postpartum.4 However, greater quantities of opioids prescribed at the time of discharge were associated with greater opioid consumption.
Recently, several quality improvement studies implemented various interventions and successfully decreased postpartum opioid consumption without compromising pain management. One quality improvement project eliminated the routine use of opioids after CD and decreased the proportion of patients using any opioids in the hospital from 68% to 45%, with no changes in pain scores.11 A similar study implemented an enhanced recovery after surgery (ERAS) program for women after CD; mean in-patient opioid use decreased from 10.7 to 5.4 average daily morphine equivalents, with improvement in the proportion of time that patients reported their pain as acceptable.12
Misconception #2: Clinicians will be overwhelmed with pages and phone calls
Providers commonly fear that decreasing opioid use will lead to an increased volume of pages and phone calls from patients requesting additional medication. However, data suggest otherwise. For example, a quality improvement study that eliminated the routine use of opioids after CD tracked the number of phone calls that were received requesting rescue opioid prescriptions after discharge.11 Although the percentage of women discharged with opioids decreased from 90.6% to 40.3%, the requests for rescue opioid prescriptions did not change. Of 191 women, 4 requested a rescue prescription prior to the intervention compared with no women after the intervention. At the same time, according to unpublished data (Dr. Holland), satisfaction among nurses, house staff, and faculty did not change.
Similarly, a quality improvement project that implemented shared decision-making to inform the quantity of opioids prescribed at discharge demonstrated that the number of tablets prescribed decreased from 33.2 to 26.5, and there was no change in the rate of patients requesting opioid refills.13
Success stories: Strategies for reducing opioid use after childbirth
While overall rates of opioid prescribing after vaginal delivery and CD remain high throughout the United States, various institutions have developed successful and reproducible strategies to reduce opioid use after childbirth both in the hospital and at discharge. We highlight 3 strategies below.
Strategy 1: ERAS initiatives
An integrated health care system in northern California studied the effects of an ERAS protocol for CD across 15 medical centers.12 The intervention centered on 4 pillars: multimodal pain management, early mobility, optimal nutrition, and patient engagement through education. Specifically, multimodal pain management consisted of the following:
- intrathecal opioids during CD
- scheduled intravenous acetaminophen for 24 hours followed by oral acetaminophen every 6 hours
- nonsteroidal anti-inflammatory drugs (NSAIDs) every 6 hours
- oral oxycodone for breakthrough pain
- decoupling of opioid medication from nonopioids in the post-CD order set
- decoupling of opioid and nonopioid medications in the discharge order set along with a reduction from 30 to 20 tablets as the default discharge quantity.
Continue to: Among 4,689 and 4,624 patients who underwent CD...
Among 4,689 and 4,624 patients who underwent CD before and after the intervention, the daily morphine milligram equivalents (MME) consumed in the hospital decreased from 10.7 to 5.4. The percentage of women who required no opioids while in the hospital increased from 8.3% to 21.4% after ERAS implementation, while the percentage of time that patients reported acceptable pain scores increased from 82.1% to 86.4%. The average number of opioid tablets prescribed at discharge also decreased, from 37 to 26 MME.12 (The TABLE shows oxycodone doses converted to MMEs.)
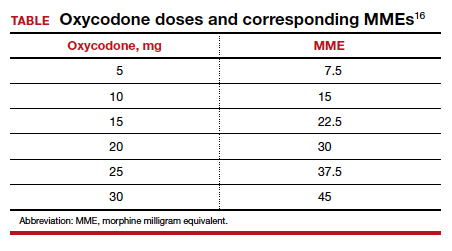
A similar initiative at a network of 5 hospitals in Texas showed that implementation of a “multimodal pain power plan” (which incorporated postpartum activity goals with standardized order sets) decreased opioid use after both vaginal delivery and CD.14
Strategy 2: Order set change to eliminate routine use of opioids
A tertiary care center in Boston, Massachusetts, implemented a quality improvement project aimed at eliminating the routine use of opioid medication after CD through an order set change.11 The intervention consisted of the following:
- intrathecal morphine
- multimodal postoperative pain management including scheduled oral acetaminophen for 72 hours followed by as-needed oral acetaminophen, scheduled NSAIDs for 72 hours followed by as-needed NSAIDs
- no postoperative order for opioids unless the patient had a contraindication to acetaminophen or NSAIDs, had a history of opioid dependence, or underwent complex surgery
- counseling patients that opioids were available for breakthrough pain if needed. In this case, nursing staff would page the responding clinician, who would order oxycodone 5 mg every 6 hours for 6 doses.
- specific criteria for discharge quantities of opioids: if the patient required no opioids in the hospital, she received no opioids at discharge; if the patient required opioids in the hospital but none at the time of discharge, she received no more than 10 tablets of oxycodone 5 mg; if the patient required opioids at the time of discharge, she received a maximum of 20 tablets of oxycodone 5 mg.
Among 191 and 181 women undergoing CD before and after the intervention, the percentage of patients who received any opioids in the hospital decreased from 68.1% to 45.3%.11 Similarly, the percentage of patients receiving a discharge prescription for opioids decreased from 90.6% to 40.3%, while patient pain scores and satisfaction with pain control remained unchanged.
Strategy 3: Shared decision-making tool
Another tertiary care center in Boston evaluated the effects of a shared decision-making tool on opioid discharge prescribing after CD.15 The intervention consisted of a 10-minute clinician-facilitated session incorporating:
- education around anticipated patterns of postoperative pain
- expected outpatient opioid use after CD
- risks and benefits of opioids and nonopioids
- education around opioid disposal and access to refills.
Among the 50 women enrolled in the study, the number of oxycodone 5-mg tablets prescribed at discharge decreased from the institutional standard of 40 to 20. Ninety percent of women reported being satisfied or very satisfied with their pain control, while only 4 of 50 women required an opioid refill. A follow-up quality improvement project, which implemented the shared decision-making model along with a standardized multimodal pain management protocol, demonstrated a similar decrease in the quantity of opioids prescribed at discharge.13
Continue to: Change is here to stay: A new culture of postpartum analgesia...
Change is here to stay: A new culture of postpartum analgesia
The CDC continues to champion responsible opioid prescribing, while ACOG advocates for a reassessment of the way that opioids are utilized postpartum. The majority of women in the United States, however, continue to receive opioids after both vaginal delivery and CD. Consciously or not, we clinicians may be contributing to an outdated tradition that is potentially harmful both to patients and society. Reproducible strategies exist to reduce opioid use without compromising pain control or overwhelming clinicians with phone calls. It is time to embrace the change.
- Wong CA, Girard T. Undertreated or overtreated? Opioids for postdelivery analgesia. Br J Anaesth. 2018;121:339-342.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87-94.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1- 353.e18.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36-41.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35-e43.
- Mills JR, Huizinga MM, Robinson SB, et al. Draft opioid prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstet Gynecol. 2019;133:81-90.
- Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318:1661-1667.
- Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206:472-479.
- Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792-3800.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97.
- Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
- Prabhu M, Dubois H, James K, et al. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstet Gynecol. 2018;132:631-636.
- Rogers RG, Nix M, Chipman Z, et al. Decreasing opioid use postpartum: a quality improvement initiative. Obstet Gynecol. 2019;134:932-940.
- Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42-46.
- Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. www.cdc.gov/ drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed December 31, 2019.
CASE New mother receives unneeded opioids after CD
A house officer wrote orders for a healthy patient who had just had an uncomplicated cesarean delivery (CD). The hospital’s tradition dictates orders for oxycodone plus acetaminophen tablets in addition to ibuprofen for all new mothers. At the time of the patient’s discharge, the same house officer prescribed 30 tablets of oxycodone plus acetaminophen “just in case,” although the patient had required only a few tablets while in the hospital on postoperative day 2 and none on the day of discharge.
Stuck in the habit
Prescribing postpartum opioids in the United States is almost habitual. Both optimizing patient satisfaction and minimizing patient phone calls may be driving this well-established pattern. Interestingly, a survey study of obstetric providers in 14 countries found that clinicians in 13 countries prescribe opioids “almost never” after vaginal delivery.1 The United States was the 1 outlier, with providers reporting prescribing opioids “on a regular basis” after vaginal birth. Similarly, providers in 10 countries reported prescribing opioids “almost never” after CD, while those in the United States reported prescribing opioids “almost always” in this context.
Moreover, mounting data suggest that many patients do not require the quantity of opioids prescribed and that our overprescribing may be causing more harm than good.
The problem of overprescribing opioids after childbirth
Opioid analgesia has long been the mainstay of treatment for postpartum pain, which when poorly controlled is associated with the development of postpartum depression and chronic pain.2 However, common adverse effects of opioids, including nausea, drowsiness, and dizziness, similarly can interfere with self-care and infant care. Of additional concern, a 2016 claims data study found that 1 of 300 opioid-naïve women who were prescribed opioids at discharge after CD used these medications persistently in the first year postpartum.3
Many women do not use the opioids that are prescribed to them at discharge, thus making tablets available for potential diversion into the community—a commonly recognized source of opioid misuse and abuse.4,5 In a 2018 Committee Opinion on postpartum pain management, the American College of Obstetricians and Gynecologists (ACOG) stated that “a stepwise, multimodal approach emphasizing nonopioid analgesia as first-line therapy is safe and effective for vaginal deliveries and cesarean deliveries.”6 The Committee Opinion also asserted that “opioid medication is an adjunct for patients with uncontrolled pain despite adequate first-line therapy.”6
Despite efforts by the Centers for Disease Control and Prevention (CDC) and ACOG to improve opioid prescribing patterns after childbirth, the vast majority of women receive opioids in the hospital and at discharge not only after CD, but after vaginal delivery as well.4,7 Why has tradition prevailed over data, and why have we not changed?
Continue to: Common misconceptions about reducing opioid use...
Common misconceptions about reducing opioid use
Two misconceptions persist regarding reducing opioid prescriptions for postpartum pain.
Misconception #1: Patients will be in pain
Randomized controlled trials that compared nonopioid with opioid regimens in the emergency room setting and opioid use after outpatient general surgery procedures have demonstrated that pain control for patients receiving opioids was equivalent to that for patients with pain managed with nonopioid regimens.8-10 In the obstetric setting, a survey study of 720 women who underwent CD found that higher quantities of opioid tablets prescribed at discharge were not associated with improved pain, higher satisfaction, or lower refill rates at 2 weeks postpartum.4 However, greater quantities of opioids prescribed at the time of discharge were associated with greater opioid consumption.
Recently, several quality improvement studies implemented various interventions and successfully decreased postpartum opioid consumption without compromising pain management. One quality improvement project eliminated the routine use of opioids after CD and decreased the proportion of patients using any opioids in the hospital from 68% to 45%, with no changes in pain scores.11 A similar study implemented an enhanced recovery after surgery (ERAS) program for women after CD; mean in-patient opioid use decreased from 10.7 to 5.4 average daily morphine equivalents, with improvement in the proportion of time that patients reported their pain as acceptable.12
Misconception #2: Clinicians will be overwhelmed with pages and phone calls
Providers commonly fear that decreasing opioid use will lead to an increased volume of pages and phone calls from patients requesting additional medication. However, data suggest otherwise. For example, a quality improvement study that eliminated the routine use of opioids after CD tracked the number of phone calls that were received requesting rescue opioid prescriptions after discharge.11 Although the percentage of women discharged with opioids decreased from 90.6% to 40.3%, the requests for rescue opioid prescriptions did not change. Of 191 women, 4 requested a rescue prescription prior to the intervention compared with no women after the intervention. At the same time, according to unpublished data (Dr. Holland), satisfaction among nurses, house staff, and faculty did not change.
Similarly, a quality improvement project that implemented shared decision-making to inform the quantity of opioids prescribed at discharge demonstrated that the number of tablets prescribed decreased from 33.2 to 26.5, and there was no change in the rate of patients requesting opioid refills.13
Success stories: Strategies for reducing opioid use after childbirth
While overall rates of opioid prescribing after vaginal delivery and CD remain high throughout the United States, various institutions have developed successful and reproducible strategies to reduce opioid use after childbirth both in the hospital and at discharge. We highlight 3 strategies below.
Strategy 1: ERAS initiatives
An integrated health care system in northern California studied the effects of an ERAS protocol for CD across 15 medical centers.12 The intervention centered on 4 pillars: multimodal pain management, early mobility, optimal nutrition, and patient engagement through education. Specifically, multimodal pain management consisted of the following:
- intrathecal opioids during CD
- scheduled intravenous acetaminophen for 24 hours followed by oral acetaminophen every 6 hours
- nonsteroidal anti-inflammatory drugs (NSAIDs) every 6 hours
- oral oxycodone for breakthrough pain
- decoupling of opioid medication from nonopioids in the post-CD order set
- decoupling of opioid and nonopioid medications in the discharge order set along with a reduction from 30 to 20 tablets as the default discharge quantity.
Continue to: Among 4,689 and 4,624 patients who underwent CD...
Among 4,689 and 4,624 patients who underwent CD before and after the intervention, the daily morphine milligram equivalents (MME) consumed in the hospital decreased from 10.7 to 5.4. The percentage of women who required no opioids while in the hospital increased from 8.3% to 21.4% after ERAS implementation, while the percentage of time that patients reported acceptable pain scores increased from 82.1% to 86.4%. The average number of opioid tablets prescribed at discharge also decreased, from 37 to 26 MME.12 (The TABLE shows oxycodone doses converted to MMEs.)

A similar initiative at a network of 5 hospitals in Texas showed that implementation of a “multimodal pain power plan” (which incorporated postpartum activity goals with standardized order sets) decreased opioid use after both vaginal delivery and CD.14
Strategy 2: Order set change to eliminate routine use of opioids
A tertiary care center in Boston, Massachusetts, implemented a quality improvement project aimed at eliminating the routine use of opioid medication after CD through an order set change.11 The intervention consisted of the following:
- intrathecal morphine
- multimodal postoperative pain management including scheduled oral acetaminophen for 72 hours followed by as-needed oral acetaminophen, scheduled NSAIDs for 72 hours followed by as-needed NSAIDs
- no postoperative order for opioids unless the patient had a contraindication to acetaminophen or NSAIDs, had a history of opioid dependence, or underwent complex surgery
- counseling patients that opioids were available for breakthrough pain if needed. In this case, nursing staff would page the responding clinician, who would order oxycodone 5 mg every 6 hours for 6 doses.
- specific criteria for discharge quantities of opioids: if the patient required no opioids in the hospital, she received no opioids at discharge; if the patient required opioids in the hospital but none at the time of discharge, she received no more than 10 tablets of oxycodone 5 mg; if the patient required opioids at the time of discharge, she received a maximum of 20 tablets of oxycodone 5 mg.
Among 191 and 181 women undergoing CD before and after the intervention, the percentage of patients who received any opioids in the hospital decreased from 68.1% to 45.3%.11 Similarly, the percentage of patients receiving a discharge prescription for opioids decreased from 90.6% to 40.3%, while patient pain scores and satisfaction with pain control remained unchanged.
Strategy 3: Shared decision-making tool
Another tertiary care center in Boston evaluated the effects of a shared decision-making tool on opioid discharge prescribing after CD.15 The intervention consisted of a 10-minute clinician-facilitated session incorporating:
- education around anticipated patterns of postoperative pain
- expected outpatient opioid use after CD
- risks and benefits of opioids and nonopioids
- education around opioid disposal and access to refills.
Among the 50 women enrolled in the study, the number of oxycodone 5-mg tablets prescribed at discharge decreased from the institutional standard of 40 to 20. Ninety percent of women reported being satisfied or very satisfied with their pain control, while only 4 of 50 women required an opioid refill. A follow-up quality improvement project, which implemented the shared decision-making model along with a standardized multimodal pain management protocol, demonstrated a similar decrease in the quantity of opioids prescribed at discharge.13
Continue to: Change is here to stay: A new culture of postpartum analgesia...
Change is here to stay: A new culture of postpartum analgesia
The CDC continues to champion responsible opioid prescribing, while ACOG advocates for a reassessment of the way that opioids are utilized postpartum. The majority of women in the United States, however, continue to receive opioids after both vaginal delivery and CD. Consciously or not, we clinicians may be contributing to an outdated tradition that is potentially harmful both to patients and society. Reproducible strategies exist to reduce opioid use without compromising pain control or overwhelming clinicians with phone calls. It is time to embrace the change.
CASE New mother receives unneeded opioids after CD
A house officer wrote orders for a healthy patient who had just had an uncomplicated cesarean delivery (CD). The hospital’s tradition dictates orders for oxycodone plus acetaminophen tablets in addition to ibuprofen for all new mothers. At the time of the patient’s discharge, the same house officer prescribed 30 tablets of oxycodone plus acetaminophen “just in case,” although the patient had required only a few tablets while in the hospital on postoperative day 2 and none on the day of discharge.
Stuck in the habit
Prescribing postpartum opioids in the United States is almost habitual. Both optimizing patient satisfaction and minimizing patient phone calls may be driving this well-established pattern. Interestingly, a survey study of obstetric providers in 14 countries found that clinicians in 13 countries prescribe opioids “almost never” after vaginal delivery.1 The United States was the 1 outlier, with providers reporting prescribing opioids “on a regular basis” after vaginal birth. Similarly, providers in 10 countries reported prescribing opioids “almost never” after CD, while those in the United States reported prescribing opioids “almost always” in this context.
Moreover, mounting data suggest that many patients do not require the quantity of opioids prescribed and that our overprescribing may be causing more harm than good.
The problem of overprescribing opioids after childbirth
Opioid analgesia has long been the mainstay of treatment for postpartum pain, which when poorly controlled is associated with the development of postpartum depression and chronic pain.2 However, common adverse effects of opioids, including nausea, drowsiness, and dizziness, similarly can interfere with self-care and infant care. Of additional concern, a 2016 claims data study found that 1 of 300 opioid-naïve women who were prescribed opioids at discharge after CD used these medications persistently in the first year postpartum.3
Many women do not use the opioids that are prescribed to them at discharge, thus making tablets available for potential diversion into the community—a commonly recognized source of opioid misuse and abuse.4,5 In a 2018 Committee Opinion on postpartum pain management, the American College of Obstetricians and Gynecologists (ACOG) stated that “a stepwise, multimodal approach emphasizing nonopioid analgesia as first-line therapy is safe and effective for vaginal deliveries and cesarean deliveries.”6 The Committee Opinion also asserted that “opioid medication is an adjunct for patients with uncontrolled pain despite adequate first-line therapy.”6
Despite efforts by the Centers for Disease Control and Prevention (CDC) and ACOG to improve opioid prescribing patterns after childbirth, the vast majority of women receive opioids in the hospital and at discharge not only after CD, but after vaginal delivery as well.4,7 Why has tradition prevailed over data, and why have we not changed?
Continue to: Common misconceptions about reducing opioid use...
Common misconceptions about reducing opioid use
Two misconceptions persist regarding reducing opioid prescriptions for postpartum pain.
Misconception #1: Patients will be in pain
Randomized controlled trials that compared nonopioid with opioid regimens in the emergency room setting and opioid use after outpatient general surgery procedures have demonstrated that pain control for patients receiving opioids was equivalent to that for patients with pain managed with nonopioid regimens.8-10 In the obstetric setting, a survey study of 720 women who underwent CD found that higher quantities of opioid tablets prescribed at discharge were not associated with improved pain, higher satisfaction, or lower refill rates at 2 weeks postpartum.4 However, greater quantities of opioids prescribed at the time of discharge were associated with greater opioid consumption.
Recently, several quality improvement studies implemented various interventions and successfully decreased postpartum opioid consumption without compromising pain management. One quality improvement project eliminated the routine use of opioids after CD and decreased the proportion of patients using any opioids in the hospital from 68% to 45%, with no changes in pain scores.11 A similar study implemented an enhanced recovery after surgery (ERAS) program for women after CD; mean in-patient opioid use decreased from 10.7 to 5.4 average daily morphine equivalents, with improvement in the proportion of time that patients reported their pain as acceptable.12
Misconception #2: Clinicians will be overwhelmed with pages and phone calls
Providers commonly fear that decreasing opioid use will lead to an increased volume of pages and phone calls from patients requesting additional medication. However, data suggest otherwise. For example, a quality improvement study that eliminated the routine use of opioids after CD tracked the number of phone calls that were received requesting rescue opioid prescriptions after discharge.11 Although the percentage of women discharged with opioids decreased from 90.6% to 40.3%, the requests for rescue opioid prescriptions did not change. Of 191 women, 4 requested a rescue prescription prior to the intervention compared with no women after the intervention. At the same time, according to unpublished data (Dr. Holland), satisfaction among nurses, house staff, and faculty did not change.
Similarly, a quality improvement project that implemented shared decision-making to inform the quantity of opioids prescribed at discharge demonstrated that the number of tablets prescribed decreased from 33.2 to 26.5, and there was no change in the rate of patients requesting opioid refills.13
Success stories: Strategies for reducing opioid use after childbirth
While overall rates of opioid prescribing after vaginal delivery and CD remain high throughout the United States, various institutions have developed successful and reproducible strategies to reduce opioid use after childbirth both in the hospital and at discharge. We highlight 3 strategies below.
Strategy 1: ERAS initiatives
An integrated health care system in northern California studied the effects of an ERAS protocol for CD across 15 medical centers.12 The intervention centered on 4 pillars: multimodal pain management, early mobility, optimal nutrition, and patient engagement through education. Specifically, multimodal pain management consisted of the following:
- intrathecal opioids during CD
- scheduled intravenous acetaminophen for 24 hours followed by oral acetaminophen every 6 hours
- nonsteroidal anti-inflammatory drugs (NSAIDs) every 6 hours
- oral oxycodone for breakthrough pain
- decoupling of opioid medication from nonopioids in the post-CD order set
- decoupling of opioid and nonopioid medications in the discharge order set along with a reduction from 30 to 20 tablets as the default discharge quantity.
Continue to: Among 4,689 and 4,624 patients who underwent CD...
Among 4,689 and 4,624 patients who underwent CD before and after the intervention, the daily morphine milligram equivalents (MME) consumed in the hospital decreased from 10.7 to 5.4. The percentage of women who required no opioids while in the hospital increased from 8.3% to 21.4% after ERAS implementation, while the percentage of time that patients reported acceptable pain scores increased from 82.1% to 86.4%. The average number of opioid tablets prescribed at discharge also decreased, from 37 to 26 MME.12 (The TABLE shows oxycodone doses converted to MMEs.)

A similar initiative at a network of 5 hospitals in Texas showed that implementation of a “multimodal pain power plan” (which incorporated postpartum activity goals with standardized order sets) decreased opioid use after both vaginal delivery and CD.14
Strategy 2: Order set change to eliminate routine use of opioids
A tertiary care center in Boston, Massachusetts, implemented a quality improvement project aimed at eliminating the routine use of opioid medication after CD through an order set change.11 The intervention consisted of the following:
- intrathecal morphine
- multimodal postoperative pain management including scheduled oral acetaminophen for 72 hours followed by as-needed oral acetaminophen, scheduled NSAIDs for 72 hours followed by as-needed NSAIDs
- no postoperative order for opioids unless the patient had a contraindication to acetaminophen or NSAIDs, had a history of opioid dependence, or underwent complex surgery
- counseling patients that opioids were available for breakthrough pain if needed. In this case, nursing staff would page the responding clinician, who would order oxycodone 5 mg every 6 hours for 6 doses.
- specific criteria for discharge quantities of opioids: if the patient required no opioids in the hospital, she received no opioids at discharge; if the patient required opioids in the hospital but none at the time of discharge, she received no more than 10 tablets of oxycodone 5 mg; if the patient required opioids at the time of discharge, she received a maximum of 20 tablets of oxycodone 5 mg.
Among 191 and 181 women undergoing CD before and after the intervention, the percentage of patients who received any opioids in the hospital decreased from 68.1% to 45.3%.11 Similarly, the percentage of patients receiving a discharge prescription for opioids decreased from 90.6% to 40.3%, while patient pain scores and satisfaction with pain control remained unchanged.
Strategy 3: Shared decision-making tool
Another tertiary care center in Boston evaluated the effects of a shared decision-making tool on opioid discharge prescribing after CD.15 The intervention consisted of a 10-minute clinician-facilitated session incorporating:
- education around anticipated patterns of postoperative pain
- expected outpatient opioid use after CD
- risks and benefits of opioids and nonopioids
- education around opioid disposal and access to refills.
Among the 50 women enrolled in the study, the number of oxycodone 5-mg tablets prescribed at discharge decreased from the institutional standard of 40 to 20. Ninety percent of women reported being satisfied or very satisfied with their pain control, while only 4 of 50 women required an opioid refill. A follow-up quality improvement project, which implemented the shared decision-making model along with a standardized multimodal pain management protocol, demonstrated a similar decrease in the quantity of opioids prescribed at discharge.13
Continue to: Change is here to stay: A new culture of postpartum analgesia...
Change is here to stay: A new culture of postpartum analgesia
The CDC continues to champion responsible opioid prescribing, while ACOG advocates for a reassessment of the way that opioids are utilized postpartum. The majority of women in the United States, however, continue to receive opioids after both vaginal delivery and CD. Consciously or not, we clinicians may be contributing to an outdated tradition that is potentially harmful both to patients and society. Reproducible strategies exist to reduce opioid use without compromising pain control or overwhelming clinicians with phone calls. It is time to embrace the change.
- Wong CA, Girard T. Undertreated or overtreated? Opioids for postdelivery analgesia. Br J Anaesth. 2018;121:339-342.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87-94.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1- 353.e18.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36-41.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35-e43.
- Mills JR, Huizinga MM, Robinson SB, et al. Draft opioid prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstet Gynecol. 2019;133:81-90.
- Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318:1661-1667.
- Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206:472-479.
- Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792-3800.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97.
- Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
- Prabhu M, Dubois H, James K, et al. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstet Gynecol. 2018;132:631-636.
- Rogers RG, Nix M, Chipman Z, et al. Decreasing opioid use postpartum: a quality improvement initiative. Obstet Gynecol. 2019;134:932-940.
- Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42-46.
- Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. www.cdc.gov/ drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed December 31, 2019.
- Wong CA, Girard T. Undertreated or overtreated? Opioids for postdelivery analgesia. Br J Anaesth. 2018;121:339-342.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87-94.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1- 353.e18.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36-41.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35-e43.
- Mills JR, Huizinga MM, Robinson SB, et al. Draft opioid prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstet Gynecol. 2019;133:81-90.
- Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318:1661-1667.
- Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206:472-479.
- Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792-3800.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97.
- Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
- Prabhu M, Dubois H, James K, et al. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstet Gynecol. 2018;132:631-636.
- Rogers RG, Nix M, Chipman Z, et al. Decreasing opioid use postpartum: a quality improvement initiative. Obstet Gynecol. 2019;134:932-940.
- Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42-46.
- Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. www.cdc.gov/ drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed December 31, 2019.
Research on statin for preeclampsia prevention advances
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
EXPERT ANALYSIS FROM THE DPSG-NA 2019
AED exposure from breastfeeding appears to be low
, according to a study published online ahead of print Dec. 30, 2019, in JAMA Neurology. The results may explain why previous research failed to find adverse neurodevelopmental effects of breastfeeding in infants whose mothers are undergoing AED treatment, said the authors.
“The results of this study add support to the general safety of breastfeeding by mothers with epilepsy who take AEDs,” wrote Angela K. Birnbaum, PhD, professor of experimental and clinical pharmacology at the University of Minnesota in Minneapolis, and colleagues.
Investigators measured infants’ blood AED concentrations
To date, medical consensus about the safety of breastfeeding while the mother is taking AEDs has been elusive. Researchers have investigated breast milk concentrations of AEDs as surrogate markers of AED concentrations in children. Breast milk concentrations, however, do not account for differences in infant pharmacokinetic processes and thus could misrepresent AED exposure in children through breastfeeding.
Dr. Birnbaum and colleagues sought to measure blood concentrations of AEDs in mothers with epilepsy and the infants that they breastfed to achieve an objective measure of AED exposure through breastfeeding. They examined data collected from December 2012 to October 2016 in the prospective Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. Eligible participants were pregnant women with epilepsy between the ages of 14 and 45 years whose pregnancies had progressed to fewer than 20 weeks’ gestational age and who had IQ scores greater than 70 points. Participants were followed up throughout pregnancy and for 9 months post partum. Children were enrolled at birth.
The investigators collected blood samples from mothers and infants who were breastfed at the same visit, which occurred at between 5 and 20 weeks after birth. The volume of ingested breast milk delivered through graduated feeding bottles each day and the total duration of all daily breastfeeding sessions were recorded. For infants, blood samples were collected from the plantar surface of the heel and stored as dried blood spots on filter paper. The study’s primary endpoint was the percentage of infant-to-mother concentration of AEDs. Concentrations of AEDs in infants at less than the lower limit of quantification were assessed as half of the lower limit.
Exposure in utero may be greater than exposure through breast milk
In all, the researchers enrolled 351 pregnant women with epilepsy into the study and collected data on 345 infants. Two hundred twenty-two (64.3%) of the infants were breastfed, and 146 (42.3%) had AED concentrations available. After excluding outliers and mothers with missing concentration data, Dr. Birnbaum and colleagues included 164 matching infant-mother concentration pairs in their analysis (i.e., of 135 mothers and 138 infants). Approximately 52% of the infants were female, and their median age at blood collection was 13 weeks. The mothers’ median age was 32 years. About 82% of mothers were receiving monotherapy. The investigators found no demographic differences between groups of mothers taking various AEDs.
Sixty-eight infants (49.3%) had AED concentrations that were less than the lower limit of quantification. AED concentration was not greater than the lower limit of quantification for any infants breastfed by mothers taking carbamazepine, oxcarbazepine, valproic acid, or topiramate. Most levetiracetam (71.4%) and zonisamide (60.0%) concentrations in infants were less than the lower limit of quantification. Most lamotrigine concentrations in infants (88.6%) were greater than the lower limit of quantification.
The median percentage of infant-to-mother concentration was 28.9% for lamotrigine, 5.3% for levetiracetam, 44.2% for zonisamide, 5.7% for carbamazepine, 5.4% for carbamazepine epoxide, 0.3% for oxcarbazepine, 17.2% for topiramate, and 21.4% for valproic acid. Multiple linear regression models indicated that maternal concentration was significantly associated with lamotrigine concentration in infants, but not levetiracetam concentration in infants.
“Prior studies at delivery demonstrated that umbilical-cord concentrations were nearly equal to maternal concentrations, suggesting extensive placental passage to the fetus,” wrote Dr. Birnbaum and colleagues. “Therefore, the amount of AED exposure via breast milk is likely substantially lower than fetal exposure during pregnancy and appears unlikely to confer any additional risks beyond those that might be associated with exposure in pregnancy, especially given prior studies showing no adverse neurodevelopmental effects of breastfeeding while taking AEDs.”
The investigators acknowledged several limitations of their research, including the observational design of the MONEAD study. The amount of AED in participants’ breast milk is unknown, and the investigators could not calculate relative infant dosages. Only one blood sample was taken per infant, thus the results may not reflect infants’ total exposure over time.
The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Development funded the research. The authors reported receiving research support from various pharmaceutical companies.
SOURCE: Birnbaum AK et al. JAMA Neurol. 2019 Dec 30. doi: 10.1001/jamaneurol.2019.4443.
, according to a study published online ahead of print Dec. 30, 2019, in JAMA Neurology. The results may explain why previous research failed to find adverse neurodevelopmental effects of breastfeeding in infants whose mothers are undergoing AED treatment, said the authors.
“The results of this study add support to the general safety of breastfeeding by mothers with epilepsy who take AEDs,” wrote Angela K. Birnbaum, PhD, professor of experimental and clinical pharmacology at the University of Minnesota in Minneapolis, and colleagues.
Investigators measured infants’ blood AED concentrations
To date, medical consensus about the safety of breastfeeding while the mother is taking AEDs has been elusive. Researchers have investigated breast milk concentrations of AEDs as surrogate markers of AED concentrations in children. Breast milk concentrations, however, do not account for differences in infant pharmacokinetic processes and thus could misrepresent AED exposure in children through breastfeeding.
Dr. Birnbaum and colleagues sought to measure blood concentrations of AEDs in mothers with epilepsy and the infants that they breastfed to achieve an objective measure of AED exposure through breastfeeding. They examined data collected from December 2012 to October 2016 in the prospective Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. Eligible participants were pregnant women with epilepsy between the ages of 14 and 45 years whose pregnancies had progressed to fewer than 20 weeks’ gestational age and who had IQ scores greater than 70 points. Participants were followed up throughout pregnancy and for 9 months post partum. Children were enrolled at birth.
The investigators collected blood samples from mothers and infants who were breastfed at the same visit, which occurred at between 5 and 20 weeks after birth. The volume of ingested breast milk delivered through graduated feeding bottles each day and the total duration of all daily breastfeeding sessions were recorded. For infants, blood samples were collected from the plantar surface of the heel and stored as dried blood spots on filter paper. The study’s primary endpoint was the percentage of infant-to-mother concentration of AEDs. Concentrations of AEDs in infants at less than the lower limit of quantification were assessed as half of the lower limit.
Exposure in utero may be greater than exposure through breast milk
In all, the researchers enrolled 351 pregnant women with epilepsy into the study and collected data on 345 infants. Two hundred twenty-two (64.3%) of the infants were breastfed, and 146 (42.3%) had AED concentrations available. After excluding outliers and mothers with missing concentration data, Dr. Birnbaum and colleagues included 164 matching infant-mother concentration pairs in their analysis (i.e., of 135 mothers and 138 infants). Approximately 52% of the infants were female, and their median age at blood collection was 13 weeks. The mothers’ median age was 32 years. About 82% of mothers were receiving monotherapy. The investigators found no demographic differences between groups of mothers taking various AEDs.
Sixty-eight infants (49.3%) had AED concentrations that were less than the lower limit of quantification. AED concentration was not greater than the lower limit of quantification for any infants breastfed by mothers taking carbamazepine, oxcarbazepine, valproic acid, or topiramate. Most levetiracetam (71.4%) and zonisamide (60.0%) concentrations in infants were less than the lower limit of quantification. Most lamotrigine concentrations in infants (88.6%) were greater than the lower limit of quantification.
The median percentage of infant-to-mother concentration was 28.9% for lamotrigine, 5.3% for levetiracetam, 44.2% for zonisamide, 5.7% for carbamazepine, 5.4% for carbamazepine epoxide, 0.3% for oxcarbazepine, 17.2% for topiramate, and 21.4% for valproic acid. Multiple linear regression models indicated that maternal concentration was significantly associated with lamotrigine concentration in infants, but not levetiracetam concentration in infants.
“Prior studies at delivery demonstrated that umbilical-cord concentrations were nearly equal to maternal concentrations, suggesting extensive placental passage to the fetus,” wrote Dr. Birnbaum and colleagues. “Therefore, the amount of AED exposure via breast milk is likely substantially lower than fetal exposure during pregnancy and appears unlikely to confer any additional risks beyond those that might be associated with exposure in pregnancy, especially given prior studies showing no adverse neurodevelopmental effects of breastfeeding while taking AEDs.”
The investigators acknowledged several limitations of their research, including the observational design of the MONEAD study. The amount of AED in participants’ breast milk is unknown, and the investigators could not calculate relative infant dosages. Only one blood sample was taken per infant, thus the results may not reflect infants’ total exposure over time.
The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Development funded the research. The authors reported receiving research support from various pharmaceutical companies.
SOURCE: Birnbaum AK et al. JAMA Neurol. 2019 Dec 30. doi: 10.1001/jamaneurol.2019.4443.
, according to a study published online ahead of print Dec. 30, 2019, in JAMA Neurology. The results may explain why previous research failed to find adverse neurodevelopmental effects of breastfeeding in infants whose mothers are undergoing AED treatment, said the authors.
“The results of this study add support to the general safety of breastfeeding by mothers with epilepsy who take AEDs,” wrote Angela K. Birnbaum, PhD, professor of experimental and clinical pharmacology at the University of Minnesota in Minneapolis, and colleagues.
Investigators measured infants’ blood AED concentrations
To date, medical consensus about the safety of breastfeeding while the mother is taking AEDs has been elusive. Researchers have investigated breast milk concentrations of AEDs as surrogate markers of AED concentrations in children. Breast milk concentrations, however, do not account for differences in infant pharmacokinetic processes and thus could misrepresent AED exposure in children through breastfeeding.
Dr. Birnbaum and colleagues sought to measure blood concentrations of AEDs in mothers with epilepsy and the infants that they breastfed to achieve an objective measure of AED exposure through breastfeeding. They examined data collected from December 2012 to October 2016 in the prospective Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. Eligible participants were pregnant women with epilepsy between the ages of 14 and 45 years whose pregnancies had progressed to fewer than 20 weeks’ gestational age and who had IQ scores greater than 70 points. Participants were followed up throughout pregnancy and for 9 months post partum. Children were enrolled at birth.
The investigators collected blood samples from mothers and infants who were breastfed at the same visit, which occurred at between 5 and 20 weeks after birth. The volume of ingested breast milk delivered through graduated feeding bottles each day and the total duration of all daily breastfeeding sessions were recorded. For infants, blood samples were collected from the plantar surface of the heel and stored as dried blood spots on filter paper. The study’s primary endpoint was the percentage of infant-to-mother concentration of AEDs. Concentrations of AEDs in infants at less than the lower limit of quantification were assessed as half of the lower limit.
Exposure in utero may be greater than exposure through breast milk
In all, the researchers enrolled 351 pregnant women with epilepsy into the study and collected data on 345 infants. Two hundred twenty-two (64.3%) of the infants were breastfed, and 146 (42.3%) had AED concentrations available. After excluding outliers and mothers with missing concentration data, Dr. Birnbaum and colleagues included 164 matching infant-mother concentration pairs in their analysis (i.e., of 135 mothers and 138 infants). Approximately 52% of the infants were female, and their median age at blood collection was 13 weeks. The mothers’ median age was 32 years. About 82% of mothers were receiving monotherapy. The investigators found no demographic differences between groups of mothers taking various AEDs.
Sixty-eight infants (49.3%) had AED concentrations that were less than the lower limit of quantification. AED concentration was not greater than the lower limit of quantification for any infants breastfed by mothers taking carbamazepine, oxcarbazepine, valproic acid, or topiramate. Most levetiracetam (71.4%) and zonisamide (60.0%) concentrations in infants were less than the lower limit of quantification. Most lamotrigine concentrations in infants (88.6%) were greater than the lower limit of quantification.
The median percentage of infant-to-mother concentration was 28.9% for lamotrigine, 5.3% for levetiracetam, 44.2% for zonisamide, 5.7% for carbamazepine, 5.4% for carbamazepine epoxide, 0.3% for oxcarbazepine, 17.2% for topiramate, and 21.4% for valproic acid. Multiple linear regression models indicated that maternal concentration was significantly associated with lamotrigine concentration in infants, but not levetiracetam concentration in infants.
“Prior studies at delivery demonstrated that umbilical-cord concentrations were nearly equal to maternal concentrations, suggesting extensive placental passage to the fetus,” wrote Dr. Birnbaum and colleagues. “Therefore, the amount of AED exposure via breast milk is likely substantially lower than fetal exposure during pregnancy and appears unlikely to confer any additional risks beyond those that might be associated with exposure in pregnancy, especially given prior studies showing no adverse neurodevelopmental effects of breastfeeding while taking AEDs.”
The investigators acknowledged several limitations of their research, including the observational design of the MONEAD study. The amount of AED in participants’ breast milk is unknown, and the investigators could not calculate relative infant dosages. Only one blood sample was taken per infant, thus the results may not reflect infants’ total exposure over time.
The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Development funded the research. The authors reported receiving research support from various pharmaceutical companies.
SOURCE: Birnbaum AK et al. JAMA Neurol. 2019 Dec 30. doi: 10.1001/jamaneurol.2019.4443.
FROM JAMA NEUROLOGY
Replacement meals boost nutrient intake by pregnant women with obesity
LAS VEGAS – Pregnant women with overweight or obesity who replaced two meals a day with bars or shakes starting at their second trimester not only had a significantly reduced rate of gestational weight gain but also benefited from significant improvements in their intake of several micronutrients, in a randomized study of 211 women who completed the regimen.
Further research needs “to examine the generalizability and effectiveness of this prenatal lifestyle modification program in improving micronutrient sufficiency in other populations and settings,” Suzanne Phelan, PhD, said at a meeting presented by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. The study she presented ran at two U.S. sites, in California and Rhode Island, and enrolled a population that was 42% Hispanic/Latina. Despite uncertainty about the applicability of the findings to other populations, the results suggested that partial meal replacement is a way to better control gestational weight gain in women with overweight or obesity while simultaneously increasing micronutrient intake, said Dr. Phelan, a clinical psychologist and professor of kinesiology and public health at the California Polytechnic State University in San Luis Obispo.
She reported data from the Healthy Beginnings/Comienzos Saludables (Preventing Excessive Gestational Weight Gain in Obese Women) study, which enrolled 257 women with overweight or obesity (body mass index of at least 25 kg/m2) at week 9-16 of pregnancy and randomized them to either a multifactorial behavioral lifestyle intervention that included two daily meal replacements, or to “enhanced” usual care. About 80% of participants in both arms, a total of 211 women, completed the study with final follow-up at 35-36 weeks’ gestational age, after enrolling at an average gestational age of just under 14 weeks. In addition to eating nutrition bars or drinking nutrition shakes as the replacement meal options, participants also ate one conventional meal daily as well as 2-4 healthy snacks. The enrolled women included 41% with overweight and 59% with obesity.
The study’s primary endpoint was the rate of gestational weight gain per week, which was 0.33 kg in the intervention group and 0.39 kg in the controls, a statistically significant difference. The proportion of women who exceeded the Institute of Medicine’s recommended maximum gestational weight gain maximum was 41% among those in the intervention group and 54% among the controls, also a statistically significant difference (Am J Clin Nutr. 2018 Feb;107[2]:183-94).
The secondary micronutrient analysis that Dr. Phelan reported documented the high prevalence of micronutrient deficiencies among the study participants at baseline. More than 90% had deficient intake of vitamin D and fiber, more than 80% had inadequate dietary levels of iron, vitamin E, and choline, and more than half had too little dietary magnesium, vitamin K, and folate. There were additional deficiencies for other micronutrients in lesser proportions of study participants.
The analysis also showed how the behavioral and diet intervention through the end of the third trimester normalized many of these deficiencies, compared with the placebo arm. For example, the prevalence of a magnesium dietary deficiency in the intervention arm dropped from 69% at baseline to 37% at follow-up, compared with hardly any change in the control arm, so that women in the intervention group had a 64% reduced rate of magnesium deficiency compared with the controls, a statistically significant difference.
Other micronutrients that had significant drops in deficiency rate included calcium, with a 63% relative reduction in the deficiency prevalence, vitamin A with a 61% cut, vitamin E with an 83% relative reduction, and vitamin K with a 51% relative drop. Other micronutrient intake levels that showed statistically significant increases during the study compared with controls included vitamin D and copper, but choline showed an inexplicable drop in consumption in the intervention group, a “potential concern,” Dr. Phelan said. The intervention also significantly reduced sodium intake. Dr. Phelan and her associates published these findings (Nutrients. 2019 May 14;11[5]:1071; doi: 10.3390/nu11051071).
“The diet quality of many of the pregnant women we have studied was poor, often eating less than half the recommended amounts of fruits and vegetables,” said Leanne M. Redman, PhD, a professor at Louisiana State University and director of the Reproductive Endocrinology and Women’s Health Laboratory at the university’s Pennington Biomedical Research Center in Baton Rouge. “Meal replacement with bars and shakes will be really important for future efforts at improving diet quality” in pregnant women with obesity, predicted Dr. Redman, who did not collaborate on the study Dr. Phelan reported.
SOURCE: Phelan S et al. Obesity Week 2019. Abstract T-OR-2081.
LAS VEGAS – Pregnant women with overweight or obesity who replaced two meals a day with bars or shakes starting at their second trimester not only had a significantly reduced rate of gestational weight gain but also benefited from significant improvements in their intake of several micronutrients, in a randomized study of 211 women who completed the regimen.
Further research needs “to examine the generalizability and effectiveness of this prenatal lifestyle modification program in improving micronutrient sufficiency in other populations and settings,” Suzanne Phelan, PhD, said at a meeting presented by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. The study she presented ran at two U.S. sites, in California and Rhode Island, and enrolled a population that was 42% Hispanic/Latina. Despite uncertainty about the applicability of the findings to other populations, the results suggested that partial meal replacement is a way to better control gestational weight gain in women with overweight or obesity while simultaneously increasing micronutrient intake, said Dr. Phelan, a clinical psychologist and professor of kinesiology and public health at the California Polytechnic State University in San Luis Obispo.
She reported data from the Healthy Beginnings/Comienzos Saludables (Preventing Excessive Gestational Weight Gain in Obese Women) study, which enrolled 257 women with overweight or obesity (body mass index of at least 25 kg/m2) at week 9-16 of pregnancy and randomized them to either a multifactorial behavioral lifestyle intervention that included two daily meal replacements, or to “enhanced” usual care. About 80% of participants in both arms, a total of 211 women, completed the study with final follow-up at 35-36 weeks’ gestational age, after enrolling at an average gestational age of just under 14 weeks. In addition to eating nutrition bars or drinking nutrition shakes as the replacement meal options, participants also ate one conventional meal daily as well as 2-4 healthy snacks. The enrolled women included 41% with overweight and 59% with obesity.
The study’s primary endpoint was the rate of gestational weight gain per week, which was 0.33 kg in the intervention group and 0.39 kg in the controls, a statistically significant difference. The proportion of women who exceeded the Institute of Medicine’s recommended maximum gestational weight gain maximum was 41% among those in the intervention group and 54% among the controls, also a statistically significant difference (Am J Clin Nutr. 2018 Feb;107[2]:183-94).
The secondary micronutrient analysis that Dr. Phelan reported documented the high prevalence of micronutrient deficiencies among the study participants at baseline. More than 90% had deficient intake of vitamin D and fiber, more than 80% had inadequate dietary levels of iron, vitamin E, and choline, and more than half had too little dietary magnesium, vitamin K, and folate. There were additional deficiencies for other micronutrients in lesser proportions of study participants.
The analysis also showed how the behavioral and diet intervention through the end of the third trimester normalized many of these deficiencies, compared with the placebo arm. For example, the prevalence of a magnesium dietary deficiency in the intervention arm dropped from 69% at baseline to 37% at follow-up, compared with hardly any change in the control arm, so that women in the intervention group had a 64% reduced rate of magnesium deficiency compared with the controls, a statistically significant difference.
Other micronutrients that had significant drops in deficiency rate included calcium, with a 63% relative reduction in the deficiency prevalence, vitamin A with a 61% cut, vitamin E with an 83% relative reduction, and vitamin K with a 51% relative drop. Other micronutrient intake levels that showed statistically significant increases during the study compared with controls included vitamin D and copper, but choline showed an inexplicable drop in consumption in the intervention group, a “potential concern,” Dr. Phelan said. The intervention also significantly reduced sodium intake. Dr. Phelan and her associates published these findings (Nutrients. 2019 May 14;11[5]:1071; doi: 10.3390/nu11051071).
“The diet quality of many of the pregnant women we have studied was poor, often eating less than half the recommended amounts of fruits and vegetables,” said Leanne M. Redman, PhD, a professor at Louisiana State University and director of the Reproductive Endocrinology and Women’s Health Laboratory at the university’s Pennington Biomedical Research Center in Baton Rouge. “Meal replacement with bars and shakes will be really important for future efforts at improving diet quality” in pregnant women with obesity, predicted Dr. Redman, who did not collaborate on the study Dr. Phelan reported.
SOURCE: Phelan S et al. Obesity Week 2019. Abstract T-OR-2081.
LAS VEGAS – Pregnant women with overweight or obesity who replaced two meals a day with bars or shakes starting at their second trimester not only had a significantly reduced rate of gestational weight gain but also benefited from significant improvements in their intake of several micronutrients, in a randomized study of 211 women who completed the regimen.
Further research needs “to examine the generalizability and effectiveness of this prenatal lifestyle modification program in improving micronutrient sufficiency in other populations and settings,” Suzanne Phelan, PhD, said at a meeting presented by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. The study she presented ran at two U.S. sites, in California and Rhode Island, and enrolled a population that was 42% Hispanic/Latina. Despite uncertainty about the applicability of the findings to other populations, the results suggested that partial meal replacement is a way to better control gestational weight gain in women with overweight or obesity while simultaneously increasing micronutrient intake, said Dr. Phelan, a clinical psychologist and professor of kinesiology and public health at the California Polytechnic State University in San Luis Obispo.
She reported data from the Healthy Beginnings/Comienzos Saludables (Preventing Excessive Gestational Weight Gain in Obese Women) study, which enrolled 257 women with overweight or obesity (body mass index of at least 25 kg/m2) at week 9-16 of pregnancy and randomized them to either a multifactorial behavioral lifestyle intervention that included two daily meal replacements, or to “enhanced” usual care. About 80% of participants in both arms, a total of 211 women, completed the study with final follow-up at 35-36 weeks’ gestational age, after enrolling at an average gestational age of just under 14 weeks. In addition to eating nutrition bars or drinking nutrition shakes as the replacement meal options, participants also ate one conventional meal daily as well as 2-4 healthy snacks. The enrolled women included 41% with overweight and 59% with obesity.
The study’s primary endpoint was the rate of gestational weight gain per week, which was 0.33 kg in the intervention group and 0.39 kg in the controls, a statistically significant difference. The proportion of women who exceeded the Institute of Medicine’s recommended maximum gestational weight gain maximum was 41% among those in the intervention group and 54% among the controls, also a statistically significant difference (Am J Clin Nutr. 2018 Feb;107[2]:183-94).
The secondary micronutrient analysis that Dr. Phelan reported documented the high prevalence of micronutrient deficiencies among the study participants at baseline. More than 90% had deficient intake of vitamin D and fiber, more than 80% had inadequate dietary levels of iron, vitamin E, and choline, and more than half had too little dietary magnesium, vitamin K, and folate. There were additional deficiencies for other micronutrients in lesser proportions of study participants.
The analysis also showed how the behavioral and diet intervention through the end of the third trimester normalized many of these deficiencies, compared with the placebo arm. For example, the prevalence of a magnesium dietary deficiency in the intervention arm dropped from 69% at baseline to 37% at follow-up, compared with hardly any change in the control arm, so that women in the intervention group had a 64% reduced rate of magnesium deficiency compared with the controls, a statistically significant difference.
Other micronutrients that had significant drops in deficiency rate included calcium, with a 63% relative reduction in the deficiency prevalence, vitamin A with a 61% cut, vitamin E with an 83% relative reduction, and vitamin K with a 51% relative drop. Other micronutrient intake levels that showed statistically significant increases during the study compared with controls included vitamin D and copper, but choline showed an inexplicable drop in consumption in the intervention group, a “potential concern,” Dr. Phelan said. The intervention also significantly reduced sodium intake. Dr. Phelan and her associates published these findings (Nutrients. 2019 May 14;11[5]:1071; doi: 10.3390/nu11051071).
“The diet quality of many of the pregnant women we have studied was poor, often eating less than half the recommended amounts of fruits and vegetables,” said Leanne M. Redman, PhD, a professor at Louisiana State University and director of the Reproductive Endocrinology and Women’s Health Laboratory at the university’s Pennington Biomedical Research Center in Baton Rouge. “Meal replacement with bars and shakes will be really important for future efforts at improving diet quality” in pregnant women with obesity, predicted Dr. Redman, who did not collaborate on the study Dr. Phelan reported.
SOURCE: Phelan S et al. Obesity Week 2019. Abstract T-OR-2081.
REPORTING FROM OBESITY WEEK 2019
Can insulin plus metformin improve pregnancy outcomes in women with type 2 diabetes?
WASHINGTON – Insulin is the preferred agent for type 2 diabetes in pregnant women, yet about a third of pregnancies still have an adverse outcome, according Kim Boggess, MD, who spoke at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“We are not where we need to be,” said Dr. Boggess, who is leading a trial that brings metformin, the first-line agent for type 2 diabetes outside of pregnancy, back into the picture for pregnant women – as an add-on to insulin.
It is an interesting twist, because pregnant women taking metformin for preexisting type 2 or gestational diabetes have been shown in some studies to require supplemental insulin, more than occasionally, to achieve target glycemic control.
This was the case in a small, randomized, controlled trial at Dr. Boggess’ institution, the University of North Carolina at Chapel Hill, in which 43% of pregnant women with type 2 diabetes who were assigned to metformin required supplemental insulin (Am J Perinatol. 2013;30[6]:483-90). (0% vs. 36%, respectively) and fewer reports of glucose values less than 60 mg/dL (7.1% vs. 50%).
“I don’t consider this [need for supplemental insulin] ‘metformin failure,’ because studies that use metformin as monotherapy and that [show some patients] ultimately requiring insulin support ... also show that these women need less insulin,” she said. “What’s the risk of insulin alone? Hypoglycemia. So using less insulin could be a good thing.”
Other research suggests there may be less maternal weight gain, less neonatal hypoglycemia, fewer neonatal complications, and improved maternal glycemic control in patients treated with metformin, alone or with add-on insulin, than with insulin alone. “We’re starting to get a sense in the literature that, at least in the [pregnant] population with type 2 diabetes, there may be a role for metformin,” said Dr. Boggess, professor and program director for maternal-fetal medicine at the university.
Currently, the multisite MOMPOD trial (Medical Optimization of Management of T2DM Complicating Pregnancy) is randomizing 950 women to insulin plus 1,000 mg metformin twice daily or insulin plus placebo. The primary outcome of the trial is a composite of pregnancy loss, preterm birth, birth injury, neonatal hypoglycemia, or hyperbilirubinemia. Infant fat mass (within 72 hours of birth) is a secondary outcome, along with maternal safety and maternal side effects.
The MiTy (Metformin in Women with T2DM in Pregnancy) trial in Canada, with similar randomization arms and outcomes measures, is completed and undergoing analysis. “Hopefully we’ll [soon] be able to say whether the addition of adjuvant metformin to insulin to treat type 2 diabetes brings the perinatal adverse outcome rate down from 30%,” said Dr. Boggess.
Metformin is the recommended first-line agent for type 2 diabetes in nonpregnant adults. But during pregnancy, insulin, which does not cross the placenta, is the preferred agent, according to recommendations of the American Diabetes Association and the American College of Obstetricians and Gynecologists, she noted. Lingering in the background is the fact that the long-term effects of in utero metformin exposure on offspring – and of exposure to any oral hypoglycemic agent – are unknown, she said*
A majority of the adverse pregnancy outcomes that occur in the context of type 2 diabetes involve macrosomia. “It’s a big deal,” Dr. Boggess said, that results in numerous maternal and infant risks and complications. “We also know that the in utero environment that contributes to, or causes, macrosomia predisposes to childhood obesity and obesity later on.”
Diabetes is the “leading risk factor” for adverse pregnancy outcomes today, said E. Albert Reece, MD, PhD, MBA, executive vice president for medical affairs at the University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers distinguished professor and dean of the University of Maryland School of Medicine. In the United States, 11% of women aged 20 years and older have diabetes, and the disease affects more than 1% of all pregnancies, he said.
The MOMPOD trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Boggess reported no conflicts of interest.
* This article was updated 1/2/2020.
WASHINGTON – Insulin is the preferred agent for type 2 diabetes in pregnant women, yet about a third of pregnancies still have an adverse outcome, according Kim Boggess, MD, who spoke at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“We are not where we need to be,” said Dr. Boggess, who is leading a trial that brings metformin, the first-line agent for type 2 diabetes outside of pregnancy, back into the picture for pregnant women – as an add-on to insulin.
It is an interesting twist, because pregnant women taking metformin for preexisting type 2 or gestational diabetes have been shown in some studies to require supplemental insulin, more than occasionally, to achieve target glycemic control.
This was the case in a small, randomized, controlled trial at Dr. Boggess’ institution, the University of North Carolina at Chapel Hill, in which 43% of pregnant women with type 2 diabetes who were assigned to metformin required supplemental insulin (Am J Perinatol. 2013;30[6]:483-90). (0% vs. 36%, respectively) and fewer reports of glucose values less than 60 mg/dL (7.1% vs. 50%).
“I don’t consider this [need for supplemental insulin] ‘metformin failure,’ because studies that use metformin as monotherapy and that [show some patients] ultimately requiring insulin support ... also show that these women need less insulin,” she said. “What’s the risk of insulin alone? Hypoglycemia. So using less insulin could be a good thing.”
Other research suggests there may be less maternal weight gain, less neonatal hypoglycemia, fewer neonatal complications, and improved maternal glycemic control in patients treated with metformin, alone or with add-on insulin, than with insulin alone. “We’re starting to get a sense in the literature that, at least in the [pregnant] population with type 2 diabetes, there may be a role for metformin,” said Dr. Boggess, professor and program director for maternal-fetal medicine at the university.
Currently, the multisite MOMPOD trial (Medical Optimization of Management of T2DM Complicating Pregnancy) is randomizing 950 women to insulin plus 1,000 mg metformin twice daily or insulin plus placebo. The primary outcome of the trial is a composite of pregnancy loss, preterm birth, birth injury, neonatal hypoglycemia, or hyperbilirubinemia. Infant fat mass (within 72 hours of birth) is a secondary outcome, along with maternal safety and maternal side effects.
The MiTy (Metformin in Women with T2DM in Pregnancy) trial in Canada, with similar randomization arms and outcomes measures, is completed and undergoing analysis. “Hopefully we’ll [soon] be able to say whether the addition of adjuvant metformin to insulin to treat type 2 diabetes brings the perinatal adverse outcome rate down from 30%,” said Dr. Boggess.
Metformin is the recommended first-line agent for type 2 diabetes in nonpregnant adults. But during pregnancy, insulin, which does not cross the placenta, is the preferred agent, according to recommendations of the American Diabetes Association and the American College of Obstetricians and Gynecologists, she noted. Lingering in the background is the fact that the long-term effects of in utero metformin exposure on offspring – and of exposure to any oral hypoglycemic agent – are unknown, she said*
A majority of the adverse pregnancy outcomes that occur in the context of type 2 diabetes involve macrosomia. “It’s a big deal,” Dr. Boggess said, that results in numerous maternal and infant risks and complications. “We also know that the in utero environment that contributes to, or causes, macrosomia predisposes to childhood obesity and obesity later on.”
Diabetes is the “leading risk factor” for adverse pregnancy outcomes today, said E. Albert Reece, MD, PhD, MBA, executive vice president for medical affairs at the University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers distinguished professor and dean of the University of Maryland School of Medicine. In the United States, 11% of women aged 20 years and older have diabetes, and the disease affects more than 1% of all pregnancies, he said.
The MOMPOD trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Boggess reported no conflicts of interest.
* This article was updated 1/2/2020.
WASHINGTON – Insulin is the preferred agent for type 2 diabetes in pregnant women, yet about a third of pregnancies still have an adverse outcome, according Kim Boggess, MD, who spoke at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“We are not where we need to be,” said Dr. Boggess, who is leading a trial that brings metformin, the first-line agent for type 2 diabetes outside of pregnancy, back into the picture for pregnant women – as an add-on to insulin.
It is an interesting twist, because pregnant women taking metformin for preexisting type 2 or gestational diabetes have been shown in some studies to require supplemental insulin, more than occasionally, to achieve target glycemic control.
This was the case in a small, randomized, controlled trial at Dr. Boggess’ institution, the University of North Carolina at Chapel Hill, in which 43% of pregnant women with type 2 diabetes who were assigned to metformin required supplemental insulin (Am J Perinatol. 2013;30[6]:483-90). (0% vs. 36%, respectively) and fewer reports of glucose values less than 60 mg/dL (7.1% vs. 50%).
“I don’t consider this [need for supplemental insulin] ‘metformin failure,’ because studies that use metformin as monotherapy and that [show some patients] ultimately requiring insulin support ... also show that these women need less insulin,” she said. “What’s the risk of insulin alone? Hypoglycemia. So using less insulin could be a good thing.”
Other research suggests there may be less maternal weight gain, less neonatal hypoglycemia, fewer neonatal complications, and improved maternal glycemic control in patients treated with metformin, alone or with add-on insulin, than with insulin alone. “We’re starting to get a sense in the literature that, at least in the [pregnant] population with type 2 diabetes, there may be a role for metformin,” said Dr. Boggess, professor and program director for maternal-fetal medicine at the university.
Currently, the multisite MOMPOD trial (Medical Optimization of Management of T2DM Complicating Pregnancy) is randomizing 950 women to insulin plus 1,000 mg metformin twice daily or insulin plus placebo. The primary outcome of the trial is a composite of pregnancy loss, preterm birth, birth injury, neonatal hypoglycemia, or hyperbilirubinemia. Infant fat mass (within 72 hours of birth) is a secondary outcome, along with maternal safety and maternal side effects.
The MiTy (Metformin in Women with T2DM in Pregnancy) trial in Canada, with similar randomization arms and outcomes measures, is completed and undergoing analysis. “Hopefully we’ll [soon] be able to say whether the addition of adjuvant metformin to insulin to treat type 2 diabetes brings the perinatal adverse outcome rate down from 30%,” said Dr. Boggess.
Metformin is the recommended first-line agent for type 2 diabetes in nonpregnant adults. But during pregnancy, insulin, which does not cross the placenta, is the preferred agent, according to recommendations of the American Diabetes Association and the American College of Obstetricians and Gynecologists, she noted. Lingering in the background is the fact that the long-term effects of in utero metformin exposure on offspring – and of exposure to any oral hypoglycemic agent – are unknown, she said*
A majority of the adverse pregnancy outcomes that occur in the context of type 2 diabetes involve macrosomia. “It’s a big deal,” Dr. Boggess said, that results in numerous maternal and infant risks and complications. “We also know that the in utero environment that contributes to, or causes, macrosomia predisposes to childhood obesity and obesity later on.”
Diabetes is the “leading risk factor” for adverse pregnancy outcomes today, said E. Albert Reece, MD, PhD, MBA, executive vice president for medical affairs at the University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers distinguished professor and dean of the University of Maryland School of Medicine. In the United States, 11% of women aged 20 years and older have diabetes, and the disease affects more than 1% of all pregnancies, he said.
The MOMPOD trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Boggess reported no conflicts of interest.
* This article was updated 1/2/2020.
REPORTING FROM DPSG-NA 2019
Hydroxychloroquine prevents congenital heart block recurrence in anti-Ro pregnancies
ATLANTA – Hydroxychloroquine (Plaquenil) 400 mg/day starting by pregnancy week 10 reduces recurrence of congenital heart block in infants born to women with anti-Ro antibodies, according to an open-label, prospective study presented at the annual meeting of the American College of Rheumatology.
Among antibody-positive women who had a previous pregnancy complicated by congenital heart block (CHB), the regimen reduced recurrence in a subsequent pregnancy from the expected historical rate of 18% to 7.4%, a more than 50% drop. “Given the potential benefit of hydroxychloroquine” (HCQ) and its relative safety during pregnancy, “testing all pregnancies for anti-Ro antibodies, regardless of maternal health, should be considered,” concluded investigators led by rheumatologist Peter Izmirly, MD, associate professor of medicine at New York (N.Y.) University.
About 40% of women with systemic lupus erythematosus and nearly 100% of women with Sjögren’s syndrome, as well as about 1% of women in the general population, have anti-Ro antibodies. They can be present in completely asymptomatic women, which is why the authors called for general screening. Indeed, half of the women in the trial had no or only mild, undifferentiated rheumatic symptoms. Often, “women who carry anti-Ro antibodies have no idea they have them” until they have a child with CHB and are tested, Dr. Izmirly said.
The antibodies cross the placenta and interfere with the normal development of the AV node; about 18% of infants die and most of the rest require lifelong pacing. The risk of CHB in antibody-positive women is about 2%, but once a child is born with the condition, the risk climbs to about 18% in subsequent pregnancies.
Years ago, Dr. Izmirly and his colleagues had a hunch that HCQ might help because it disrupts the toll-like receptor signaling involved in the disease process. A database review he led added weight to the idea, finding that among 257 anti-Ro positive pregnancies, the rate of CHB was 7.5% among the 40 women who happened to take HCQ, versus 21.2% among the 217 who did not. “We wanted to see if we could replicate that prospectively,” he said.
The Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) trial enrolled 54 antibody positive women with a previous CHB pregnancy. They were started on 400 mg/day HCQ by gestation week 10.
There were four cases of second- or third-degree CHB among the women (7.4%, P = 0.02), all detected by fetal echocardiogram around week 20.
Nine of the women were treated with IVIG and/or dexamethasone for lupus flares or fetal heart issues other than advanced block, which confounded the results. To analyze the effect in a purely HCQ cohort, the team recruited an additional nine women not treated with any other medication during pregnancy, one of whose fetus developed third-degree heart block.
In total, 5 of 63 pregnancies (7.9%) resulted in advanced block. Among the 54 women exposed only to HCQ, the rate of second- or third-degree block was again 7.4% (4 of 54, P = .02). HCQ compliance, assessed by maternal blood levels above 200 ng/mL at least once, was 98%, and cord blood confirmed fetal exposure to HCQ.
Once detected, CHB was treated with dexamethasone or IVIG. One case progressed to cardiomyopathy, and the pregnancy was terminated. Another child required pacing after birth. Other children reverted to normal sinus rhythm but had intermittent second-degree block at age 2.
Overall, “the safety in this study was excellent,” said rheumatologist and senior investigator Jill Buyon, MD, director of the division of rheumatology at New York University.
The complications – nine births before 37 weeks, one infant small for gestational age – were not unexpected in a rheumatic population. “We were very nervous about Plaquenil cardiomyopathy” in the pregnancy that was terminated, but there was no evidence of it on histology.
The children will have ocular optical coherence tomography at age 5 to check for retinal toxicity; the 12 who have been tested so far show no obvious signs. Dr. Izmirly said he doesn’t expect to see any problems. “We are just being super cautious.”
The audience had questions about why the trial didn’t have a placebo arm. He explained that CHB is a rare event – one in 15,000 pregnancies – and it took 8 years just to adequately power the single-arm study; recruiting more than 100 additional women for a placebo-controlled trial wasn’t practical.
Also, “there was no way” women were going to be randomized to placebo when HCQ seemed so promising; 35% of the enrollees had already lost a child to CHB. “Everyone wanted the drug,” Dr. Izmirly said.
The majority of women were white, and about half met criteria for lupus and/or Sjögren’s. Anti-Ro levels remained above 1,000 EU throughout pregnancy. Women were excluded if they were taking high-dose prednisone or any dose of fluorinated corticosteroids at baseline.
The National Institutes of Health funded the work. The investigators had no relevant disclosures.
SOURCE: Izmirly P et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1761.
ATLANTA – Hydroxychloroquine (Plaquenil) 400 mg/day starting by pregnancy week 10 reduces recurrence of congenital heart block in infants born to women with anti-Ro antibodies, according to an open-label, prospective study presented at the annual meeting of the American College of Rheumatology.
Among antibody-positive women who had a previous pregnancy complicated by congenital heart block (CHB), the regimen reduced recurrence in a subsequent pregnancy from the expected historical rate of 18% to 7.4%, a more than 50% drop. “Given the potential benefit of hydroxychloroquine” (HCQ) and its relative safety during pregnancy, “testing all pregnancies for anti-Ro antibodies, regardless of maternal health, should be considered,” concluded investigators led by rheumatologist Peter Izmirly, MD, associate professor of medicine at New York (N.Y.) University.
About 40% of women with systemic lupus erythematosus and nearly 100% of women with Sjögren’s syndrome, as well as about 1% of women in the general population, have anti-Ro antibodies. They can be present in completely asymptomatic women, which is why the authors called for general screening. Indeed, half of the women in the trial had no or only mild, undifferentiated rheumatic symptoms. Often, “women who carry anti-Ro antibodies have no idea they have them” until they have a child with CHB and are tested, Dr. Izmirly said.
The antibodies cross the placenta and interfere with the normal development of the AV node; about 18% of infants die and most of the rest require lifelong pacing. The risk of CHB in antibody-positive women is about 2%, but once a child is born with the condition, the risk climbs to about 18% in subsequent pregnancies.
Years ago, Dr. Izmirly and his colleagues had a hunch that HCQ might help because it disrupts the toll-like receptor signaling involved in the disease process. A database review he led added weight to the idea, finding that among 257 anti-Ro positive pregnancies, the rate of CHB was 7.5% among the 40 women who happened to take HCQ, versus 21.2% among the 217 who did not. “We wanted to see if we could replicate that prospectively,” he said.
The Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) trial enrolled 54 antibody positive women with a previous CHB pregnancy. They were started on 400 mg/day HCQ by gestation week 10.
There were four cases of second- or third-degree CHB among the women (7.4%, P = 0.02), all detected by fetal echocardiogram around week 20.
Nine of the women were treated with IVIG and/or dexamethasone for lupus flares or fetal heart issues other than advanced block, which confounded the results. To analyze the effect in a purely HCQ cohort, the team recruited an additional nine women not treated with any other medication during pregnancy, one of whose fetus developed third-degree heart block.
In total, 5 of 63 pregnancies (7.9%) resulted in advanced block. Among the 54 women exposed only to HCQ, the rate of second- or third-degree block was again 7.4% (4 of 54, P = .02). HCQ compliance, assessed by maternal blood levels above 200 ng/mL at least once, was 98%, and cord blood confirmed fetal exposure to HCQ.
Once detected, CHB was treated with dexamethasone or IVIG. One case progressed to cardiomyopathy, and the pregnancy was terminated. Another child required pacing after birth. Other children reverted to normal sinus rhythm but had intermittent second-degree block at age 2.
Overall, “the safety in this study was excellent,” said rheumatologist and senior investigator Jill Buyon, MD, director of the division of rheumatology at New York University.
The complications – nine births before 37 weeks, one infant small for gestational age – were not unexpected in a rheumatic population. “We were very nervous about Plaquenil cardiomyopathy” in the pregnancy that was terminated, but there was no evidence of it on histology.
The children will have ocular optical coherence tomography at age 5 to check for retinal toxicity; the 12 who have been tested so far show no obvious signs. Dr. Izmirly said he doesn’t expect to see any problems. “We are just being super cautious.”
The audience had questions about why the trial didn’t have a placebo arm. He explained that CHB is a rare event – one in 15,000 pregnancies – and it took 8 years just to adequately power the single-arm study; recruiting more than 100 additional women for a placebo-controlled trial wasn’t practical.
Also, “there was no way” women were going to be randomized to placebo when HCQ seemed so promising; 35% of the enrollees had already lost a child to CHB. “Everyone wanted the drug,” Dr. Izmirly said.
The majority of women were white, and about half met criteria for lupus and/or Sjögren’s. Anti-Ro levels remained above 1,000 EU throughout pregnancy. Women were excluded if they were taking high-dose prednisone or any dose of fluorinated corticosteroids at baseline.
The National Institutes of Health funded the work. The investigators had no relevant disclosures.
SOURCE: Izmirly P et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1761.
ATLANTA – Hydroxychloroquine (Plaquenil) 400 mg/day starting by pregnancy week 10 reduces recurrence of congenital heart block in infants born to women with anti-Ro antibodies, according to an open-label, prospective study presented at the annual meeting of the American College of Rheumatology.
Among antibody-positive women who had a previous pregnancy complicated by congenital heart block (CHB), the regimen reduced recurrence in a subsequent pregnancy from the expected historical rate of 18% to 7.4%, a more than 50% drop. “Given the potential benefit of hydroxychloroquine” (HCQ) and its relative safety during pregnancy, “testing all pregnancies for anti-Ro antibodies, regardless of maternal health, should be considered,” concluded investigators led by rheumatologist Peter Izmirly, MD, associate professor of medicine at New York (N.Y.) University.
About 40% of women with systemic lupus erythematosus and nearly 100% of women with Sjögren’s syndrome, as well as about 1% of women in the general population, have anti-Ro antibodies. They can be present in completely asymptomatic women, which is why the authors called for general screening. Indeed, half of the women in the trial had no or only mild, undifferentiated rheumatic symptoms. Often, “women who carry anti-Ro antibodies have no idea they have them” until they have a child with CHB and are tested, Dr. Izmirly said.
The antibodies cross the placenta and interfere with the normal development of the AV node; about 18% of infants die and most of the rest require lifelong pacing. The risk of CHB in antibody-positive women is about 2%, but once a child is born with the condition, the risk climbs to about 18% in subsequent pregnancies.
Years ago, Dr. Izmirly and his colleagues had a hunch that HCQ might help because it disrupts the toll-like receptor signaling involved in the disease process. A database review he led added weight to the idea, finding that among 257 anti-Ro positive pregnancies, the rate of CHB was 7.5% among the 40 women who happened to take HCQ, versus 21.2% among the 217 who did not. “We wanted to see if we could replicate that prospectively,” he said.
The Preventive Approach to Congenital Heart Block with Hydroxychloroquine (PATCH) trial enrolled 54 antibody positive women with a previous CHB pregnancy. They were started on 400 mg/day HCQ by gestation week 10.
There were four cases of second- or third-degree CHB among the women (7.4%, P = 0.02), all detected by fetal echocardiogram around week 20.
Nine of the women were treated with IVIG and/or dexamethasone for lupus flares or fetal heart issues other than advanced block, which confounded the results. To analyze the effect in a purely HCQ cohort, the team recruited an additional nine women not treated with any other medication during pregnancy, one of whose fetus developed third-degree heart block.
In total, 5 of 63 pregnancies (7.9%) resulted in advanced block. Among the 54 women exposed only to HCQ, the rate of second- or third-degree block was again 7.4% (4 of 54, P = .02). HCQ compliance, assessed by maternal blood levels above 200 ng/mL at least once, was 98%, and cord blood confirmed fetal exposure to HCQ.
Once detected, CHB was treated with dexamethasone or IVIG. One case progressed to cardiomyopathy, and the pregnancy was terminated. Another child required pacing after birth. Other children reverted to normal sinus rhythm but had intermittent second-degree block at age 2.
Overall, “the safety in this study was excellent,” said rheumatologist and senior investigator Jill Buyon, MD, director of the division of rheumatology at New York University.
The complications – nine births before 37 weeks, one infant small for gestational age – were not unexpected in a rheumatic population. “We were very nervous about Plaquenil cardiomyopathy” in the pregnancy that was terminated, but there was no evidence of it on histology.
The children will have ocular optical coherence tomography at age 5 to check for retinal toxicity; the 12 who have been tested so far show no obvious signs. Dr. Izmirly said he doesn’t expect to see any problems. “We are just being super cautious.”
The audience had questions about why the trial didn’t have a placebo arm. He explained that CHB is a rare event – one in 15,000 pregnancies – and it took 8 years just to adequately power the single-arm study; recruiting more than 100 additional women for a placebo-controlled trial wasn’t practical.
Also, “there was no way” women were going to be randomized to placebo when HCQ seemed so promising; 35% of the enrollees had already lost a child to CHB. “Everyone wanted the drug,” Dr. Izmirly said.
The majority of women were white, and about half met criteria for lupus and/or Sjögren’s. Anti-Ro levels remained above 1,000 EU throughout pregnancy. Women were excluded if they were taking high-dose prednisone or any dose of fluorinated corticosteroids at baseline.
The National Institutes of Health funded the work. The investigators had no relevant disclosures.
SOURCE: Izmirly P et al. Arthritis Rheumatol. 2019;71(suppl 10). Abstract 1761.
REPORTING FROM ACR 2019
Obstetrical care in crisis
For the last 25 years I have had the privilege of caring for a rural community, practicing full-scope family medicine including obstetrics with cesarean sections. I have had a deeply rewarding career, and delivering babies and watching them grow up has been one of the most gratifying parts of my work.
My concern is that, as the number of family physicians who practice maternity care has decreased, the infant and maternal mortality rate in the United States has increased, especially in rural and minority populations. Currently, 5 million women of reproductive age have no access to maternity care.
At the same time 23% of incoming family medicine residents would like to offer maternity care and are trained to do so, but few are able to find a job where this is possible.1 This is unfortunate because family physicians have the training and expertise to provide comprehensive maternity care. Although they have lower rates of cesarean section than ob-gyns, with similar outcomes, family physicians do have surgical skills, including providing cesarean sections, that are often necessary for safe delivery.2,3
In addition, family physicians have the internal medicine and behavioral health background to care for postpartum complications, as well as substance use disorders. Because they also care for children, they see postpartum women when they come in with their children for well-child checks. These visits offer an excellent opportunity to also check on the mother for postpartum depression and other signs of postpartum illness.
Centers for Disease Control and Prevention data reveal that maternal mortality can be divided into three nearly equal parts: pregnancy, delivery, and post partum. They define delivery as the week of delivery. The 48 hours post delivery accounted for only 12% of overall mortality. This means that even if women travel to metropolitan areas, they are likely to be home when they have fatal complications. The lack of trained and experienced physicians in the communities where women live increases their risks should they have complications. Most maternal fatalities occur when conditions are not recognized in a timely fashion. Some responses require procedural skills such as dilation and curettage (D&C).
As a member of the National Advisory Committee on Rural Health and Human Services, I visited several states to evaluate their rural health systems. We looked at infant mortality by county and found an enormous disparity between counties, largely caused by lack of prenatal services and obstetrical services.
These disparities between counties are getting worse. The United States is losing critical access hospitals at a rapid pace. We have lost 117 critical access hospitals in the last 10 years, with 40 in the last year alone. According to the National Rural Health Association, 4,673 additional facilities – representing more than one-third of rural hospitals in the United States – are vulnerable and could close. The reasons are multiple, but the result has been an erosion of the rural safety net, especially with regard to maternity care.
These hospital closures force women to travel farther distances for maternity care, including cesarean sections, and this contributes to increased maternal and infant mortality.5 In a study from Canada, the complication rates increased substantially as distances increased. Women are more likely to have premature deliveries, deliver on the side of the road, or end up in inappropriate facilities.
The distance from delivery is directly related to outcomes. A study from the early 1990s showed that women did better if they received maternity care from local hospitals and physicians.6 From a family medicine perspective, this makes sense because traveling to a metropolitan area means isolation from family and social networks. Stress increases because pregnant women also are often the primary caregiver of other children and the primary wage earner of the family. Although we are unsure what impact stress has on pregnancy, we do know it does have an effect on greater risk of prematurity and poor outcomes.
Obstetricians provide excellent care, but they are not a panacea. Only half of U.S. counties have adequate ob.gyn. coverage. Moreover, in many of those counties, the ob.gyns. subspecialize in gynecologic surgery and infertility, but don’t provide obstetrical care. Another challenge: ob.gyns. cannot survive financially in smaller communities; our policies must include incentives to recruit and retain them in underserved areas.
Certified nurse midwives also provide excellent care and are an invaluable member of the patient-care team, but again, they cannot be the only solution. Obstetrical emergencies do occur, and mothers need a physician trained in providing on-site medical or surgical care. They also need a hospital with adequate staff to care for emergencies.
In communities large enough to support a multispecialty group, certified medical technicians, family physicians, and ob.gyns. would ideally work alongside each other. In small communities four family physicians can provide a high level of maternity care including surgical deliveries, while supporting themselves with caring for children and elders in clinics, hospitals, and EDs.
It is unconscionable that a country as wealthy as ours would accept rates of maternal and infant mortality that rival and are often worse than developing countries. Although the reasons are many, there is no excuse. Family physicians are an essential part of reversing this trend. We need policies that enable family physicians to help resolve the shortage of maternity care for underserved communities, to address the maternal and infant mortality rate, and to provide maternity care that is part of family medicine’s full scope of practice.
Dr. Cullen is board chair of the American Academy of Family Physicians and a practicing family physician in Valdez, Alaska.
References
1. Am Board Fam Med. 2017 Jul-Aug;30(4):405-6.
2. CMAJ. 2015 Oct 27;187:1125-32.
3. J Am Board Fam Med. 2013 Jul-Aug;26(4):366-72.
4. NRHA Save Rural Hospitals Action Center. www.ruralhealthweb.org/advocate/save-rural-hospitals.
5. BMC Health Serv Res. 2011 Jun 10;11:147.
6. Am J Public Health. 1990 Jul;80(7):814-8.
For the last 25 years I have had the privilege of caring for a rural community, practicing full-scope family medicine including obstetrics with cesarean sections. I have had a deeply rewarding career, and delivering babies and watching them grow up has been one of the most gratifying parts of my work.
My concern is that, as the number of family physicians who practice maternity care has decreased, the infant and maternal mortality rate in the United States has increased, especially in rural and minority populations. Currently, 5 million women of reproductive age have no access to maternity care.
At the same time 23% of incoming family medicine residents would like to offer maternity care and are trained to do so, but few are able to find a job where this is possible.1 This is unfortunate because family physicians have the training and expertise to provide comprehensive maternity care. Although they have lower rates of cesarean section than ob-gyns, with similar outcomes, family physicians do have surgical skills, including providing cesarean sections, that are often necessary for safe delivery.2,3
In addition, family physicians have the internal medicine and behavioral health background to care for postpartum complications, as well as substance use disorders. Because they also care for children, they see postpartum women when they come in with their children for well-child checks. These visits offer an excellent opportunity to also check on the mother for postpartum depression and other signs of postpartum illness.
Centers for Disease Control and Prevention data reveal that maternal mortality can be divided into three nearly equal parts: pregnancy, delivery, and post partum. They define delivery as the week of delivery. The 48 hours post delivery accounted for only 12% of overall mortality. This means that even if women travel to metropolitan areas, they are likely to be home when they have fatal complications. The lack of trained and experienced physicians in the communities where women live increases their risks should they have complications. Most maternal fatalities occur when conditions are not recognized in a timely fashion. Some responses require procedural skills such as dilation and curettage (D&C).
As a member of the National Advisory Committee on Rural Health and Human Services, I visited several states to evaluate their rural health systems. We looked at infant mortality by county and found an enormous disparity between counties, largely caused by lack of prenatal services and obstetrical services.
These disparities between counties are getting worse. The United States is losing critical access hospitals at a rapid pace. We have lost 117 critical access hospitals in the last 10 years, with 40 in the last year alone. According to the National Rural Health Association, 4,673 additional facilities – representing more than one-third of rural hospitals in the United States – are vulnerable and could close. The reasons are multiple, but the result has been an erosion of the rural safety net, especially with regard to maternity care.
These hospital closures force women to travel farther distances for maternity care, including cesarean sections, and this contributes to increased maternal and infant mortality.5 In a study from Canada, the complication rates increased substantially as distances increased. Women are more likely to have premature deliveries, deliver on the side of the road, or end up in inappropriate facilities.
The distance from delivery is directly related to outcomes. A study from the early 1990s showed that women did better if they received maternity care from local hospitals and physicians.6 From a family medicine perspective, this makes sense because traveling to a metropolitan area means isolation from family and social networks. Stress increases because pregnant women also are often the primary caregiver of other children and the primary wage earner of the family. Although we are unsure what impact stress has on pregnancy, we do know it does have an effect on greater risk of prematurity and poor outcomes.
Obstetricians provide excellent care, but they are not a panacea. Only half of U.S. counties have adequate ob.gyn. coverage. Moreover, in many of those counties, the ob.gyns. subspecialize in gynecologic surgery and infertility, but don’t provide obstetrical care. Another challenge: ob.gyns. cannot survive financially in smaller communities; our policies must include incentives to recruit and retain them in underserved areas.
Certified nurse midwives also provide excellent care and are an invaluable member of the patient-care team, but again, they cannot be the only solution. Obstetrical emergencies do occur, and mothers need a physician trained in providing on-site medical or surgical care. They also need a hospital with adequate staff to care for emergencies.
In communities large enough to support a multispecialty group, certified medical technicians, family physicians, and ob.gyns. would ideally work alongside each other. In small communities four family physicians can provide a high level of maternity care including surgical deliveries, while supporting themselves with caring for children and elders in clinics, hospitals, and EDs.
It is unconscionable that a country as wealthy as ours would accept rates of maternal and infant mortality that rival and are often worse than developing countries. Although the reasons are many, there is no excuse. Family physicians are an essential part of reversing this trend. We need policies that enable family physicians to help resolve the shortage of maternity care for underserved communities, to address the maternal and infant mortality rate, and to provide maternity care that is part of family medicine’s full scope of practice.
Dr. Cullen is board chair of the American Academy of Family Physicians and a practicing family physician in Valdez, Alaska.
References
1. Am Board Fam Med. 2017 Jul-Aug;30(4):405-6.
2. CMAJ. 2015 Oct 27;187:1125-32.
3. J Am Board Fam Med. 2013 Jul-Aug;26(4):366-72.
4. NRHA Save Rural Hospitals Action Center. www.ruralhealthweb.org/advocate/save-rural-hospitals.
5. BMC Health Serv Res. 2011 Jun 10;11:147.
6. Am J Public Health. 1990 Jul;80(7):814-8.
For the last 25 years I have had the privilege of caring for a rural community, practicing full-scope family medicine including obstetrics with cesarean sections. I have had a deeply rewarding career, and delivering babies and watching them grow up has been one of the most gratifying parts of my work.
My concern is that, as the number of family physicians who practice maternity care has decreased, the infant and maternal mortality rate in the United States has increased, especially in rural and minority populations. Currently, 5 million women of reproductive age have no access to maternity care.
At the same time 23% of incoming family medicine residents would like to offer maternity care and are trained to do so, but few are able to find a job where this is possible.1 This is unfortunate because family physicians have the training and expertise to provide comprehensive maternity care. Although they have lower rates of cesarean section than ob-gyns, with similar outcomes, family physicians do have surgical skills, including providing cesarean sections, that are often necessary for safe delivery.2,3
In addition, family physicians have the internal medicine and behavioral health background to care for postpartum complications, as well as substance use disorders. Because they also care for children, they see postpartum women when they come in with their children for well-child checks. These visits offer an excellent opportunity to also check on the mother for postpartum depression and other signs of postpartum illness.
Centers for Disease Control and Prevention data reveal that maternal mortality can be divided into three nearly equal parts: pregnancy, delivery, and post partum. They define delivery as the week of delivery. The 48 hours post delivery accounted for only 12% of overall mortality. This means that even if women travel to metropolitan areas, they are likely to be home when they have fatal complications. The lack of trained and experienced physicians in the communities where women live increases their risks should they have complications. Most maternal fatalities occur when conditions are not recognized in a timely fashion. Some responses require procedural skills such as dilation and curettage (D&C).
As a member of the National Advisory Committee on Rural Health and Human Services, I visited several states to evaluate their rural health systems. We looked at infant mortality by county and found an enormous disparity between counties, largely caused by lack of prenatal services and obstetrical services.
These disparities between counties are getting worse. The United States is losing critical access hospitals at a rapid pace. We have lost 117 critical access hospitals in the last 10 years, with 40 in the last year alone. According to the National Rural Health Association, 4,673 additional facilities – representing more than one-third of rural hospitals in the United States – are vulnerable and could close. The reasons are multiple, but the result has been an erosion of the rural safety net, especially with regard to maternity care.
These hospital closures force women to travel farther distances for maternity care, including cesarean sections, and this contributes to increased maternal and infant mortality.5 In a study from Canada, the complication rates increased substantially as distances increased. Women are more likely to have premature deliveries, deliver on the side of the road, or end up in inappropriate facilities.
The distance from delivery is directly related to outcomes. A study from the early 1990s showed that women did better if they received maternity care from local hospitals and physicians.6 From a family medicine perspective, this makes sense because traveling to a metropolitan area means isolation from family and social networks. Stress increases because pregnant women also are often the primary caregiver of other children and the primary wage earner of the family. Although we are unsure what impact stress has on pregnancy, we do know it does have an effect on greater risk of prematurity and poor outcomes.
Obstetricians provide excellent care, but they are not a panacea. Only half of U.S. counties have adequate ob.gyn. coverage. Moreover, in many of those counties, the ob.gyns. subspecialize in gynecologic surgery and infertility, but don’t provide obstetrical care. Another challenge: ob.gyns. cannot survive financially in smaller communities; our policies must include incentives to recruit and retain them in underserved areas.
Certified nurse midwives also provide excellent care and are an invaluable member of the patient-care team, but again, they cannot be the only solution. Obstetrical emergencies do occur, and mothers need a physician trained in providing on-site medical or surgical care. They also need a hospital with adequate staff to care for emergencies.
In communities large enough to support a multispecialty group, certified medical technicians, family physicians, and ob.gyns. would ideally work alongside each other. In small communities four family physicians can provide a high level of maternity care including surgical deliveries, while supporting themselves with caring for children and elders in clinics, hospitals, and EDs.
It is unconscionable that a country as wealthy as ours would accept rates of maternal and infant mortality that rival and are often worse than developing countries. Although the reasons are many, there is no excuse. Family physicians are an essential part of reversing this trend. We need policies that enable family physicians to help resolve the shortage of maternity care for underserved communities, to address the maternal and infant mortality rate, and to provide maternity care that is part of family medicine’s full scope of practice.
Dr. Cullen is board chair of the American Academy of Family Physicians and a practicing family physician in Valdez, Alaska.
References
1. Am Board Fam Med. 2017 Jul-Aug;30(4):405-6.
2. CMAJ. 2015 Oct 27;187:1125-32.
3. J Am Board Fam Med. 2013 Jul-Aug;26(4):366-72.
4. NRHA Save Rural Hospitals Action Center. www.ruralhealthweb.org/advocate/save-rural-hospitals.
5. BMC Health Serv Res. 2011 Jun 10;11:147.
6. Am J Public Health. 1990 Jul;80(7):814-8.