User login
Molar pregnancy: The next steps after diagnosis
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Modafinil use in pregnancy tied to congenital malformations
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
FROM JAMA
Depression after miscarriage: Follow-up care is key
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at [email protected].
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at [email protected].
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at [email protected].
Cannabis use in pregnancy and lactation: A changing landscape
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
Prenatal exposure to pollutants consumed through diet found to be associated with decreased fetal growth
Ideally, chemicals that are toxic to human health are identified and removed from use. Many such chemicals, known as persistent organic pollutants (POPs), have been studied individually for their ill effects in humans. DDT, for instance, once a widely used insecticide, is now classified as a probable human carcinogen and has not been used in the United States since the early 1970s.1 Other insecticides have similarly been banned but have long half-lives and can persist in the environment for decades. Humans are exposed to POPs mainly through diet, say Ouidir and colleagues, with exposure “nearly ubiquitous.”2
The association between POP exposure during pregnancy and birth weight has not been established, as results from previous studies have been inconsistent, according to researchers.2 In addition, birth weight does not distinguish growth-restricted fetuses from those that are constitutionally small. Therefore, Ouidir and colleagues analyzed maternal plasma levels of POPs and measures of fetal growth. Their research was published in JAMA Pediatrics.2
The investigators examined chemical class mixtures of organochlorine pesticides (OCPs), dioxin-like polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs), among other groups of POPs, as well as individual chemicals, in 2,284 nonobese low-risk pregnant women before 14 weeks of gestation. They measured 14 fetal growth biometrics using ultrasonography throughout the women’s pregnancies, with researchers focusing their main findings on head and abdominal circumference and femur length measurements.
The researchers found that the OCP mixture was negatively associated with most fetal growth measures, with a reduction of 4.7 mm (95% confidence interval [CI], -6.7 to -2.8 mm) in head circumference, 3.5 mm (95% CI, -4.7 to -2.2) in abdominal circumference, and 0.6 mm (95% CI, -1.1 to -0.2 mm) in femur length. In addition, exposure to the PCB and PBDE mixtures were associated with reduced abdominal circumference.
OCPs have been associated with adverse effects on the endocrine system, lipid metabolism, and embryonic development. They also can result in hematologic and hepatic alterations.3
The JAMA Pediatrics study authors point out that their findings may not take into account the risk pregnant women with occupational exposure to POPs, or other higher exposure situations, may have, as the POP concentrations in their sample were low compared with levels in a nationally representative sample of pregnant women. They say that their findings provide insight into the “implications of POPs for fetal growth when exposures are low and suggest that, even if exposures could be successfully minimized, these associations may persist.”2
- United States Environmental Protection Agency. DDT: A brief history and status. https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status. Accessed January 16, 2020.
- Ouidir M, Buck Louis GM, Kanner J, et al. Association of maternal exposure to persistent organic pollutants in early pregnancy with fetal growth. JAMA Pediatr. December 30, 2019. doi:10.1001/jamapediatrics.2019.5104.
- Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148.
Ideally, chemicals that are toxic to human health are identified and removed from use. Many such chemicals, known as persistent organic pollutants (POPs), have been studied individually for their ill effects in humans. DDT, for instance, once a widely used insecticide, is now classified as a probable human carcinogen and has not been used in the United States since the early 1970s.1 Other insecticides have similarly been banned but have long half-lives and can persist in the environment for decades. Humans are exposed to POPs mainly through diet, say Ouidir and colleagues, with exposure “nearly ubiquitous.”2
The association between POP exposure during pregnancy and birth weight has not been established, as results from previous studies have been inconsistent, according to researchers.2 In addition, birth weight does not distinguish growth-restricted fetuses from those that are constitutionally small. Therefore, Ouidir and colleagues analyzed maternal plasma levels of POPs and measures of fetal growth. Their research was published in JAMA Pediatrics.2
The investigators examined chemical class mixtures of organochlorine pesticides (OCPs), dioxin-like polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs), among other groups of POPs, as well as individual chemicals, in 2,284 nonobese low-risk pregnant women before 14 weeks of gestation. They measured 14 fetal growth biometrics using ultrasonography throughout the women’s pregnancies, with researchers focusing their main findings on head and abdominal circumference and femur length measurements.
The researchers found that the OCP mixture was negatively associated with most fetal growth measures, with a reduction of 4.7 mm (95% confidence interval [CI], -6.7 to -2.8 mm) in head circumference, 3.5 mm (95% CI, -4.7 to -2.2) in abdominal circumference, and 0.6 mm (95% CI, -1.1 to -0.2 mm) in femur length. In addition, exposure to the PCB and PBDE mixtures were associated with reduced abdominal circumference.
OCPs have been associated with adverse effects on the endocrine system, lipid metabolism, and embryonic development. They also can result in hematologic and hepatic alterations.3
The JAMA Pediatrics study authors point out that their findings may not take into account the risk pregnant women with occupational exposure to POPs, or other higher exposure situations, may have, as the POP concentrations in their sample were low compared with levels in a nationally representative sample of pregnant women. They say that their findings provide insight into the “implications of POPs for fetal growth when exposures are low and suggest that, even if exposures could be successfully minimized, these associations may persist.”2
Ideally, chemicals that are toxic to human health are identified and removed from use. Many such chemicals, known as persistent organic pollutants (POPs), have been studied individually for their ill effects in humans. DDT, for instance, once a widely used insecticide, is now classified as a probable human carcinogen and has not been used in the United States since the early 1970s.1 Other insecticides have similarly been banned but have long half-lives and can persist in the environment for decades. Humans are exposed to POPs mainly through diet, say Ouidir and colleagues, with exposure “nearly ubiquitous.”2
The association between POP exposure during pregnancy and birth weight has not been established, as results from previous studies have been inconsistent, according to researchers.2 In addition, birth weight does not distinguish growth-restricted fetuses from those that are constitutionally small. Therefore, Ouidir and colleagues analyzed maternal plasma levels of POPs and measures of fetal growth. Their research was published in JAMA Pediatrics.2
The investigators examined chemical class mixtures of organochlorine pesticides (OCPs), dioxin-like polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs), among other groups of POPs, as well as individual chemicals, in 2,284 nonobese low-risk pregnant women before 14 weeks of gestation. They measured 14 fetal growth biometrics using ultrasonography throughout the women’s pregnancies, with researchers focusing their main findings on head and abdominal circumference and femur length measurements.
The researchers found that the OCP mixture was negatively associated with most fetal growth measures, with a reduction of 4.7 mm (95% confidence interval [CI], -6.7 to -2.8 mm) in head circumference, 3.5 mm (95% CI, -4.7 to -2.2) in abdominal circumference, and 0.6 mm (95% CI, -1.1 to -0.2 mm) in femur length. In addition, exposure to the PCB and PBDE mixtures were associated with reduced abdominal circumference.
OCPs have been associated with adverse effects on the endocrine system, lipid metabolism, and embryonic development. They also can result in hematologic and hepatic alterations.3
The JAMA Pediatrics study authors point out that their findings may not take into account the risk pregnant women with occupational exposure to POPs, or other higher exposure situations, may have, as the POP concentrations in their sample were low compared with levels in a nationally representative sample of pregnant women. They say that their findings provide insight into the “implications of POPs for fetal growth when exposures are low and suggest that, even if exposures could be successfully minimized, these associations may persist.”2
- United States Environmental Protection Agency. DDT: A brief history and status. https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status. Accessed January 16, 2020.
- Ouidir M, Buck Louis GM, Kanner J, et al. Association of maternal exposure to persistent organic pollutants in early pregnancy with fetal growth. JAMA Pediatr. December 30, 2019. doi:10.1001/jamapediatrics.2019.5104.
- Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148.
- United States Environmental Protection Agency. DDT: A brief history and status. https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status. Accessed January 16, 2020.
- Ouidir M, Buck Louis GM, Kanner J, et al. Association of maternal exposure to persistent organic pollutants in early pregnancy with fetal growth. JAMA Pediatr. December 30, 2019. doi:10.1001/jamapediatrics.2019.5104.
- Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148.
Age, race affect preterm birth risk in women with obesity
according to new findings from an analysis that used a large, ethnically diverse population sample.
Previous study findings have demonstrated that pregnant women with obesity have a higher risk of giving birth to preterm babies, but the effect of age and race on that risk was not clear until now.
In this latest study, Wei Bao, MD, and colleagues at the University of Iowa, Iowa City, looked at records from 7.14 million live births registered in the U.S. National Vital Statistics System for 2016 and 2017, of which about 7.4% were preterm. The researchers excluded from their sample women with preexisting diabetes or hypertension.
For the cohort overall, there was a significant association between prepregnancy body mass index and preterm birth, with mothers who were overweight (adjusted odds ratio, 1.02; 95% confidence interval, 1.01–1.03) or obese (aOR, 1.18; 95%CI, 1.18–1.19), having a significantly higher risk of preterm birth, compared with healthy weight mothers. Underweight women also had a greater risk of preterm birth, compared with the healthy weight references (aOR, 1.33; 95% CI, 1.31–1.35), the researchers reported, adding that the association between maternal underweight and preterm birth was consistent across the maternal age and race/ethnicity groups.
Dr. Bao and colleagues found that, among non-Hispanic white women (who made up about half the cohort), maternal obesity was inversely associated with preterm birth when mothers were younger than 20 years (aOR, 0.92; 95% CI, 0.88-0.97), but there was a crossover effect at age 20, when maternal obesity became positively associated with preterm birth until age 39 (aOR, 1.04 at ages 20-24, to 1.40 at ages 35-39). A similar pattern was seen in Hispanic women, for whom maternal obesity was not associated with preterm birth when they were younger than 20 (aOR, 0.98; 95% CI, 0.93-1.04), but was positively associated with preterm birth after age 20 until age 39 (aOR, 1.06 at ages 20-24, to 1.38 at ages 35-39).
However, the crossover effect occurred considerably later in black women with obesity, for whom maternal obesity remained inversely associated with preterm birth until age 30 (aOR, 0.76 before age 20; 0.83 at ages 20-24; 0.98 at ages 25-29), at which point the crossover effect kicked in, and maternal obesity became positively associated with preterm birth, increasing steadily with advancing age (aOR, 1.15 at ages 30-34; 1.26 at ages 35-39; 1.29 from age 40). “Our results, which are based on a large and diverse U.S. population, provide, for the first time, a comprehensive review of the association between maternal obesity and preterm birth for women [at a] range of ages,” Dr. Bao and colleagues wrote in their analysis, which was published in Lancet Diabetes & Endocrinology.
The researchers hypothesized that the inverse association between prepregnancy obesity and preterm birth in teenagers and younger women could be explained by the fact that “[healthy weight] teenagers, who are still growing and developing, might compete with the fetus for nutrients, which could subsequently affect physiological and metabolic systems involved with parturition,” whereas pregnant teenagers with obesity “might not need to compete (or compete to a lesser extent) for nutrients with their babies for their own growth.” The researchers stressed that more research was needed to understand the underlying mechanisms of the associations. The findings of a protective effect until age 30 in black women also require further study, Dr. Bao and colleagues said.
They stressed that the findings do not argue for weight gain as a preventive measure against preterm birth for normal weight young women, as “younger women, whether obese or not, have a higher risk of preterm birth than women aged 25-29 years do in Hispanic and in non-Hispanic white populations. Additionally, the adverse effects that maternal obesity has on other perinatal and neonatal outcomes should not be overlooked.”
The National Institutes of Health funded the study. The authors declared no conflicts of interest.
SOURCE: Bao et al. Lancet Diabetes Endocrinol. 2019;7:707-14.
according to new findings from an analysis that used a large, ethnically diverse population sample.
Previous study findings have demonstrated that pregnant women with obesity have a higher risk of giving birth to preterm babies, but the effect of age and race on that risk was not clear until now.
In this latest study, Wei Bao, MD, and colleagues at the University of Iowa, Iowa City, looked at records from 7.14 million live births registered in the U.S. National Vital Statistics System for 2016 and 2017, of which about 7.4% were preterm. The researchers excluded from their sample women with preexisting diabetes or hypertension.
For the cohort overall, there was a significant association between prepregnancy body mass index and preterm birth, with mothers who were overweight (adjusted odds ratio, 1.02; 95% confidence interval, 1.01–1.03) or obese (aOR, 1.18; 95%CI, 1.18–1.19), having a significantly higher risk of preterm birth, compared with healthy weight mothers. Underweight women also had a greater risk of preterm birth, compared with the healthy weight references (aOR, 1.33; 95% CI, 1.31–1.35), the researchers reported, adding that the association between maternal underweight and preterm birth was consistent across the maternal age and race/ethnicity groups.
Dr. Bao and colleagues found that, among non-Hispanic white women (who made up about half the cohort), maternal obesity was inversely associated with preterm birth when mothers were younger than 20 years (aOR, 0.92; 95% CI, 0.88-0.97), but there was a crossover effect at age 20, when maternal obesity became positively associated with preterm birth until age 39 (aOR, 1.04 at ages 20-24, to 1.40 at ages 35-39). A similar pattern was seen in Hispanic women, for whom maternal obesity was not associated with preterm birth when they were younger than 20 (aOR, 0.98; 95% CI, 0.93-1.04), but was positively associated with preterm birth after age 20 until age 39 (aOR, 1.06 at ages 20-24, to 1.38 at ages 35-39).
However, the crossover effect occurred considerably later in black women with obesity, for whom maternal obesity remained inversely associated with preterm birth until age 30 (aOR, 0.76 before age 20; 0.83 at ages 20-24; 0.98 at ages 25-29), at which point the crossover effect kicked in, and maternal obesity became positively associated with preterm birth, increasing steadily with advancing age (aOR, 1.15 at ages 30-34; 1.26 at ages 35-39; 1.29 from age 40). “Our results, which are based on a large and diverse U.S. population, provide, for the first time, a comprehensive review of the association between maternal obesity and preterm birth for women [at a] range of ages,” Dr. Bao and colleagues wrote in their analysis, which was published in Lancet Diabetes & Endocrinology.
The researchers hypothesized that the inverse association between prepregnancy obesity and preterm birth in teenagers and younger women could be explained by the fact that “[healthy weight] teenagers, who are still growing and developing, might compete with the fetus for nutrients, which could subsequently affect physiological and metabolic systems involved with parturition,” whereas pregnant teenagers with obesity “might not need to compete (or compete to a lesser extent) for nutrients with their babies for their own growth.” The researchers stressed that more research was needed to understand the underlying mechanisms of the associations. The findings of a protective effect until age 30 in black women also require further study, Dr. Bao and colleagues said.
They stressed that the findings do not argue for weight gain as a preventive measure against preterm birth for normal weight young women, as “younger women, whether obese or not, have a higher risk of preterm birth than women aged 25-29 years do in Hispanic and in non-Hispanic white populations. Additionally, the adverse effects that maternal obesity has on other perinatal and neonatal outcomes should not be overlooked.”
The National Institutes of Health funded the study. The authors declared no conflicts of interest.
SOURCE: Bao et al. Lancet Diabetes Endocrinol. 2019;7:707-14.
according to new findings from an analysis that used a large, ethnically diverse population sample.
Previous study findings have demonstrated that pregnant women with obesity have a higher risk of giving birth to preterm babies, but the effect of age and race on that risk was not clear until now.
In this latest study, Wei Bao, MD, and colleagues at the University of Iowa, Iowa City, looked at records from 7.14 million live births registered in the U.S. National Vital Statistics System for 2016 and 2017, of which about 7.4% were preterm. The researchers excluded from their sample women with preexisting diabetes or hypertension.
For the cohort overall, there was a significant association between prepregnancy body mass index and preterm birth, with mothers who were overweight (adjusted odds ratio, 1.02; 95% confidence interval, 1.01–1.03) or obese (aOR, 1.18; 95%CI, 1.18–1.19), having a significantly higher risk of preterm birth, compared with healthy weight mothers. Underweight women also had a greater risk of preterm birth, compared with the healthy weight references (aOR, 1.33; 95% CI, 1.31–1.35), the researchers reported, adding that the association between maternal underweight and preterm birth was consistent across the maternal age and race/ethnicity groups.
Dr. Bao and colleagues found that, among non-Hispanic white women (who made up about half the cohort), maternal obesity was inversely associated with preterm birth when mothers were younger than 20 years (aOR, 0.92; 95% CI, 0.88-0.97), but there was a crossover effect at age 20, when maternal obesity became positively associated with preterm birth until age 39 (aOR, 1.04 at ages 20-24, to 1.40 at ages 35-39). A similar pattern was seen in Hispanic women, for whom maternal obesity was not associated with preterm birth when they were younger than 20 (aOR, 0.98; 95% CI, 0.93-1.04), but was positively associated with preterm birth after age 20 until age 39 (aOR, 1.06 at ages 20-24, to 1.38 at ages 35-39).
However, the crossover effect occurred considerably later in black women with obesity, for whom maternal obesity remained inversely associated with preterm birth until age 30 (aOR, 0.76 before age 20; 0.83 at ages 20-24; 0.98 at ages 25-29), at which point the crossover effect kicked in, and maternal obesity became positively associated with preterm birth, increasing steadily with advancing age (aOR, 1.15 at ages 30-34; 1.26 at ages 35-39; 1.29 from age 40). “Our results, which are based on a large and diverse U.S. population, provide, for the first time, a comprehensive review of the association between maternal obesity and preterm birth for women [at a] range of ages,” Dr. Bao and colleagues wrote in their analysis, which was published in Lancet Diabetes & Endocrinology.
The researchers hypothesized that the inverse association between prepregnancy obesity and preterm birth in teenagers and younger women could be explained by the fact that “[healthy weight] teenagers, who are still growing and developing, might compete with the fetus for nutrients, which could subsequently affect physiological and metabolic systems involved with parturition,” whereas pregnant teenagers with obesity “might not need to compete (or compete to a lesser extent) for nutrients with their babies for their own growth.” The researchers stressed that more research was needed to understand the underlying mechanisms of the associations. The findings of a protective effect until age 30 in black women also require further study, Dr. Bao and colleagues said.
They stressed that the findings do not argue for weight gain as a preventive measure against preterm birth for normal weight young women, as “younger women, whether obese or not, have a higher risk of preterm birth than women aged 25-29 years do in Hispanic and in non-Hispanic white populations. Additionally, the adverse effects that maternal obesity has on other perinatal and neonatal outcomes should not be overlooked.”
The National Institutes of Health funded the study. The authors declared no conflicts of interest.
SOURCE: Bao et al. Lancet Diabetes Endocrinol. 2019;7:707-14.
FROM LANCET DIABETES & ENDOCRINOLOGY
ERAS for cesarean delivery: Antenatal and preoperative care
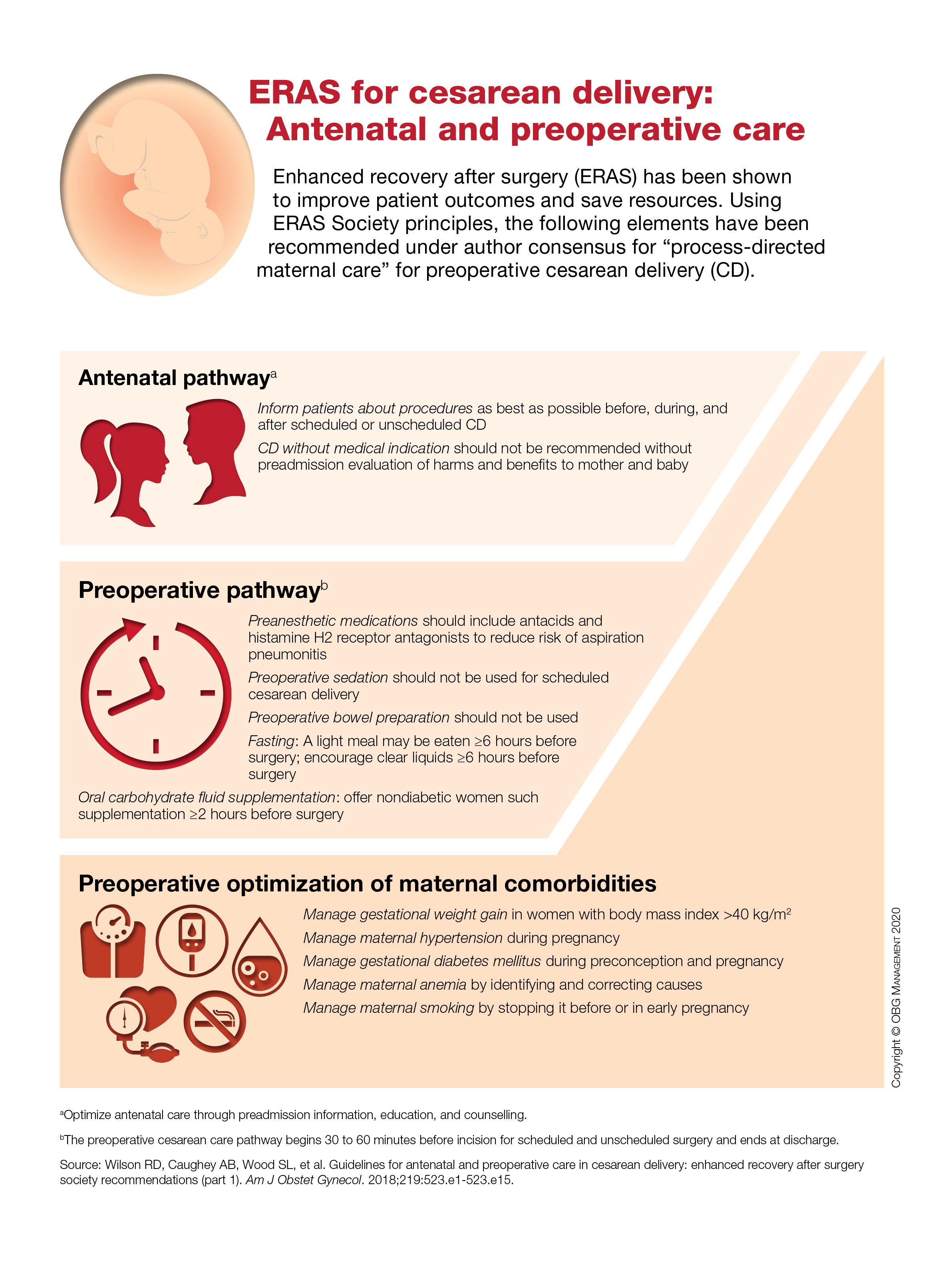


Cognitive problems after extremely preterm birth persist
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
FROM PEDIATRICS
Experts in Europe issue guidance on atopic dermatitis in pregnancy
MADRID – European atopic dermatitis experts have issued formal guidance on a seriously neglected topic: treatment of the disease during pregnancy, breastfeeding, and in men planning to father children.
The impetus for the project was clear: “,” Christian Vestergaard, MD, PhD, declared at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of the task force’s position paper on the topic, for which he served as first author. The group’s recommendations are based on expert opinion, since randomized clinical trial literature in this area is nonexistent because of ethical concerns. But the task force, comprising a who’s who in European dermatology, drew on a wealth of collective clinical experience in this area.
“We have all of Europe involved in doing this position statement. It’s meant as what we think is proper treatment and what we can say about the different drugs,” explained Dr. Vestergaard, a dermatologist at the University of Aarhus (Denmark).
Most nonobstetricians are intimidated by atopic dermatitis (AD) in pregnancy, and are concerned about the potential for treatment-related harm to the fetus. As a consequence, they are reluctant to recommend anything beyond weak class I topical corticosteroids and emollients. That’s clearly insufficient in light of the vast scope of need, he asserted. After all, AD affects 15%-20% of all children and persists or reappears in adulthood in one out of five of them. Half of those adults are women, many of whom will at some point wish to become pregnant. And many men with AD will eventually want to father children.
A key message from the task force is that untreated AD in pregnancy potentially places the mother and fetus at risk of serious complications, including Staphylococcus aureus infection and eczema herpeticum.
“If you take one thing away from our position paper, it’s that you can use class II or III topical corticosteroids in pregnant women as first-line therapy,” Dr. Vestergaard said.
This stance contradicts a longstanding widely held concern that topical steroids in pregnancy might increase the risk of facial cleft in the offspring, a worry that has been convincingly debunked in a Cochrane systematic review of 14 studies including more than 1.6 million pregnancies. The report concluded there was no association between topical corticosteroids of any potency with preterm delivery, birth defects, or low Apgar scores (Cochrane Database Syst Rev. 2015 Oct 26. doi: 10.1002/14651858.CD007346.pub3).
The task force recommends that if class II or III topical corticosteroid use in pregnancy exceeds 200 g/month, it’s worth considering add-on UV therapy, with narrow band UVB-311 nm as the regimen of choice; it can be used liberally. UV therapy with psoralens is not advised because of a theoretical risk of mutagenicity.
Product labeling for the topical calcineurin inhibitors declares that the agents should not be used during pregnancy. However, the European task force position paper takes issue with that and declares that topical tacrolimus (Protopic) can be considered an off-label first-line therapy in pregnant women with an insufficient response to liberal use of emollients. The same holds true for breastfeeding patients with AD. Just as when topical corticosteroids are used in the nipple area, topical tacrolimus should be applied after nursing, and the nipple area should be gently cleaned before nursing.
The rationale behind recommending topical tacrolimus as a first-line treatment is that systemic absorption of the drug is trivial. Plus, observational studies of oral tacrolimus in pregnant women who have received a solid organ transplant have shown no increase in congenital malformations.
The task force recommends against the use of topical pimecrolimus (Elidel) or crisaborole (Eucrisa) in pregnancy or lactation due to lack of clinical experience in these settings, Dr. Vestergaard continued.
The task force position is that chlorhexidine and other topical antiseptics – with the notable exception of triclosan – can be used in pregnancy to prevent recurrent skin infections. Aminoglycosides should be avoided, but topical fusidic acid is a reasonable antibiotic for treatment of small areas of clinically infected atopic dermatitis in pregnancy.
Systemic therapies
If disease control is insufficient with topical therapy, it’s appropriate to engage in shared decision-making with the patient regarding systemic treatment. She needs to understand up front that the worldwide overall background stillbirth rate in the general population is about 3%, and that severe congenital malformations are present in up to 6% of all live births.
“You need to inform them that they can have systemic therapy and give birth to a child with congenital defects which have nothing to do with the medication,” noted Dr. Vestergaard.
That said, the task force recommends cyclosporine as the off-label, first-line systemic therapy in pregnancy and lactation when long-term treatment is required. This guidance is based largely upon reassuring evidence in solid organ transplant recipients.
The recommended second-line therapy is systemic corticosteroids, but it’s a qualified recommendation. Dr. Vestergaard and colleagues find that systemic corticosteroid therapy is only rarely needed in pregnant AD patients, and the task force recommendation is to limit the use to less than 2-3 weeks and no more than 0.5 mg/kg per day of prednisone. Dexamethasone is not recommended.
Azathioprine should not be started in pregnancy, according to the task force, but when no other options are available, it may be continued in women already on the drug, albeit at half of the prepregnancy dose.
Dupilumab (Dupixent) is to be avoided in pregnant women with AD until more clinical experience becomes available.
Treatment of prospective fathers with AD
The European task force recommends that topical therapies can be prescribed in prospective fathers without any special concerns. The same is true for systemic corticosteroids. Methotrexate should be halted 3 months before planned pregnancy, as is the case for mycophenolate mofetil (CellCept). Azathioprine is recommended when other options have failed. Cyclosporine is deemed a reasonable option in the treatment of men with severe AD at the time of conception if other treatments have failed; of note, neither the Food and Drug Administration nor the European regulatory agency have issued contraindications for the use of the drug in men who wish to become fathers.
Mycophenolate mofetil carries a theoretical risk of teratogenicity. The European task force recommends that men should use condoms while on the drug and for at least 90 days afterward.
Unplanned pregnancy in women on systemic therapy
The recommended course of action is to immediately stop systemic therapy, intensify appropriate topical therapy in anticipation of worsening AD, and refer the patient to an obstetrician and a teratology information center for an individualized risk assessment. Methotrexate and mycophenolate mofetil are known teratogens.
The full 16-page task force position paper was published shortly before EADV 2019 (J Eur Acad Dermatol Venereol. 2019 Sep;33[9]:1644-59).
The report was developed without commercial sponsorship. Dr. Vestergaard indicated he has received research grants from and/or serves as a consultant to eight pharmaceutical companies.
Jenny Murase, MD, is with the department of dermatology, University of California, San Francisco, and is the director of medical consultative dermatology at the Palo Alto Foundation Medical Group, Mountain View, Calif. She has served on advisory boards for Dermira, Sanofi, and UCB; performed dermatologic consulting for UpToDate and Ferndale, and given nonbranded lectures for disease state management awareness for Regeneron and UCB.
Jenny Murase, MD, is with the department of dermatology, University of California, San Francisco, and is the director of medical consultative dermatology at the Palo Alto Foundation Medical Group, Mountain View, Calif. She has served on advisory boards for Dermira, Sanofi, and UCB; performed dermatologic consulting for UpToDate and Ferndale, and given nonbranded lectures for disease state management awareness for Regeneron and UCB.
Jenny Murase, MD, is with the department of dermatology, University of California, San Francisco, and is the director of medical consultative dermatology at the Palo Alto Foundation Medical Group, Mountain View, Calif. She has served on advisory boards for Dermira, Sanofi, and UCB; performed dermatologic consulting for UpToDate and Ferndale, and given nonbranded lectures for disease state management awareness for Regeneron and UCB.
MADRID – European atopic dermatitis experts have issued formal guidance on a seriously neglected topic: treatment of the disease during pregnancy, breastfeeding, and in men planning to father children.
The impetus for the project was clear: “,” Christian Vestergaard, MD, PhD, declared at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of the task force’s position paper on the topic, for which he served as first author. The group’s recommendations are based on expert opinion, since randomized clinical trial literature in this area is nonexistent because of ethical concerns. But the task force, comprising a who’s who in European dermatology, drew on a wealth of collective clinical experience in this area.
“We have all of Europe involved in doing this position statement. It’s meant as what we think is proper treatment and what we can say about the different drugs,” explained Dr. Vestergaard, a dermatologist at the University of Aarhus (Denmark).
Most nonobstetricians are intimidated by atopic dermatitis (AD) in pregnancy, and are concerned about the potential for treatment-related harm to the fetus. As a consequence, they are reluctant to recommend anything beyond weak class I topical corticosteroids and emollients. That’s clearly insufficient in light of the vast scope of need, he asserted. After all, AD affects 15%-20% of all children and persists or reappears in adulthood in one out of five of them. Half of those adults are women, many of whom will at some point wish to become pregnant. And many men with AD will eventually want to father children.
A key message from the task force is that untreated AD in pregnancy potentially places the mother and fetus at risk of serious complications, including Staphylococcus aureus infection and eczema herpeticum.
“If you take one thing away from our position paper, it’s that you can use class II or III topical corticosteroids in pregnant women as first-line therapy,” Dr. Vestergaard said.
This stance contradicts a longstanding widely held concern that topical steroids in pregnancy might increase the risk of facial cleft in the offspring, a worry that has been convincingly debunked in a Cochrane systematic review of 14 studies including more than 1.6 million pregnancies. The report concluded there was no association between topical corticosteroids of any potency with preterm delivery, birth defects, or low Apgar scores (Cochrane Database Syst Rev. 2015 Oct 26. doi: 10.1002/14651858.CD007346.pub3).
The task force recommends that if class II or III topical corticosteroid use in pregnancy exceeds 200 g/month, it’s worth considering add-on UV therapy, with narrow band UVB-311 nm as the regimen of choice; it can be used liberally. UV therapy with psoralens is not advised because of a theoretical risk of mutagenicity.
Product labeling for the topical calcineurin inhibitors declares that the agents should not be used during pregnancy. However, the European task force position paper takes issue with that and declares that topical tacrolimus (Protopic) can be considered an off-label first-line therapy in pregnant women with an insufficient response to liberal use of emollients. The same holds true for breastfeeding patients with AD. Just as when topical corticosteroids are used in the nipple area, topical tacrolimus should be applied after nursing, and the nipple area should be gently cleaned before nursing.
The rationale behind recommending topical tacrolimus as a first-line treatment is that systemic absorption of the drug is trivial. Plus, observational studies of oral tacrolimus in pregnant women who have received a solid organ transplant have shown no increase in congenital malformations.
The task force recommends against the use of topical pimecrolimus (Elidel) or crisaborole (Eucrisa) in pregnancy or lactation due to lack of clinical experience in these settings, Dr. Vestergaard continued.
The task force position is that chlorhexidine and other topical antiseptics – with the notable exception of triclosan – can be used in pregnancy to prevent recurrent skin infections. Aminoglycosides should be avoided, but topical fusidic acid is a reasonable antibiotic for treatment of small areas of clinically infected atopic dermatitis in pregnancy.
Systemic therapies
If disease control is insufficient with topical therapy, it’s appropriate to engage in shared decision-making with the patient regarding systemic treatment. She needs to understand up front that the worldwide overall background stillbirth rate in the general population is about 3%, and that severe congenital malformations are present in up to 6% of all live births.
“You need to inform them that they can have systemic therapy and give birth to a child with congenital defects which have nothing to do with the medication,” noted Dr. Vestergaard.
That said, the task force recommends cyclosporine as the off-label, first-line systemic therapy in pregnancy and lactation when long-term treatment is required. This guidance is based largely upon reassuring evidence in solid organ transplant recipients.
The recommended second-line therapy is systemic corticosteroids, but it’s a qualified recommendation. Dr. Vestergaard and colleagues find that systemic corticosteroid therapy is only rarely needed in pregnant AD patients, and the task force recommendation is to limit the use to less than 2-3 weeks and no more than 0.5 mg/kg per day of prednisone. Dexamethasone is not recommended.
Azathioprine should not be started in pregnancy, according to the task force, but when no other options are available, it may be continued in women already on the drug, albeit at half of the prepregnancy dose.
Dupilumab (Dupixent) is to be avoided in pregnant women with AD until more clinical experience becomes available.
Treatment of prospective fathers with AD
The European task force recommends that topical therapies can be prescribed in prospective fathers without any special concerns. The same is true for systemic corticosteroids. Methotrexate should be halted 3 months before planned pregnancy, as is the case for mycophenolate mofetil (CellCept). Azathioprine is recommended when other options have failed. Cyclosporine is deemed a reasonable option in the treatment of men with severe AD at the time of conception if other treatments have failed; of note, neither the Food and Drug Administration nor the European regulatory agency have issued contraindications for the use of the drug in men who wish to become fathers.
Mycophenolate mofetil carries a theoretical risk of teratogenicity. The European task force recommends that men should use condoms while on the drug and for at least 90 days afterward.
Unplanned pregnancy in women on systemic therapy
The recommended course of action is to immediately stop systemic therapy, intensify appropriate topical therapy in anticipation of worsening AD, and refer the patient to an obstetrician and a teratology information center for an individualized risk assessment. Methotrexate and mycophenolate mofetil are known teratogens.
The full 16-page task force position paper was published shortly before EADV 2019 (J Eur Acad Dermatol Venereol. 2019 Sep;33[9]:1644-59).
The report was developed without commercial sponsorship. Dr. Vestergaard indicated he has received research grants from and/or serves as a consultant to eight pharmaceutical companies.
MADRID – European atopic dermatitis experts have issued formal guidance on a seriously neglected topic: treatment of the disease during pregnancy, breastfeeding, and in men planning to father children.
The impetus for the project was clear: “,” Christian Vestergaard, MD, PhD, declared at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of the task force’s position paper on the topic, for which he served as first author. The group’s recommendations are based on expert opinion, since randomized clinical trial literature in this area is nonexistent because of ethical concerns. But the task force, comprising a who’s who in European dermatology, drew on a wealth of collective clinical experience in this area.
“We have all of Europe involved in doing this position statement. It’s meant as what we think is proper treatment and what we can say about the different drugs,” explained Dr. Vestergaard, a dermatologist at the University of Aarhus (Denmark).
Most nonobstetricians are intimidated by atopic dermatitis (AD) in pregnancy, and are concerned about the potential for treatment-related harm to the fetus. As a consequence, they are reluctant to recommend anything beyond weak class I topical corticosteroids and emollients. That’s clearly insufficient in light of the vast scope of need, he asserted. After all, AD affects 15%-20% of all children and persists or reappears in adulthood in one out of five of them. Half of those adults are women, many of whom will at some point wish to become pregnant. And many men with AD will eventually want to father children.
A key message from the task force is that untreated AD in pregnancy potentially places the mother and fetus at risk of serious complications, including Staphylococcus aureus infection and eczema herpeticum.
“If you take one thing away from our position paper, it’s that you can use class II or III topical corticosteroids in pregnant women as first-line therapy,” Dr. Vestergaard said.
This stance contradicts a longstanding widely held concern that topical steroids in pregnancy might increase the risk of facial cleft in the offspring, a worry that has been convincingly debunked in a Cochrane systematic review of 14 studies including more than 1.6 million pregnancies. The report concluded there was no association between topical corticosteroids of any potency with preterm delivery, birth defects, or low Apgar scores (Cochrane Database Syst Rev. 2015 Oct 26. doi: 10.1002/14651858.CD007346.pub3).
The task force recommends that if class II or III topical corticosteroid use in pregnancy exceeds 200 g/month, it’s worth considering add-on UV therapy, with narrow band UVB-311 nm as the regimen of choice; it can be used liberally. UV therapy with psoralens is not advised because of a theoretical risk of mutagenicity.
Product labeling for the topical calcineurin inhibitors declares that the agents should not be used during pregnancy. However, the European task force position paper takes issue with that and declares that topical tacrolimus (Protopic) can be considered an off-label first-line therapy in pregnant women with an insufficient response to liberal use of emollients. The same holds true for breastfeeding patients with AD. Just as when topical corticosteroids are used in the nipple area, topical tacrolimus should be applied after nursing, and the nipple area should be gently cleaned before nursing.
The rationale behind recommending topical tacrolimus as a first-line treatment is that systemic absorption of the drug is trivial. Plus, observational studies of oral tacrolimus in pregnant women who have received a solid organ transplant have shown no increase in congenital malformations.
The task force recommends against the use of topical pimecrolimus (Elidel) or crisaborole (Eucrisa) in pregnancy or lactation due to lack of clinical experience in these settings, Dr. Vestergaard continued.
The task force position is that chlorhexidine and other topical antiseptics – with the notable exception of triclosan – can be used in pregnancy to prevent recurrent skin infections. Aminoglycosides should be avoided, but topical fusidic acid is a reasonable antibiotic for treatment of small areas of clinically infected atopic dermatitis in pregnancy.
Systemic therapies
If disease control is insufficient with topical therapy, it’s appropriate to engage in shared decision-making with the patient regarding systemic treatment. She needs to understand up front that the worldwide overall background stillbirth rate in the general population is about 3%, and that severe congenital malformations are present in up to 6% of all live births.
“You need to inform them that they can have systemic therapy and give birth to a child with congenital defects which have nothing to do with the medication,” noted Dr. Vestergaard.
That said, the task force recommends cyclosporine as the off-label, first-line systemic therapy in pregnancy and lactation when long-term treatment is required. This guidance is based largely upon reassuring evidence in solid organ transplant recipients.
The recommended second-line therapy is systemic corticosteroids, but it’s a qualified recommendation. Dr. Vestergaard and colleagues find that systemic corticosteroid therapy is only rarely needed in pregnant AD patients, and the task force recommendation is to limit the use to less than 2-3 weeks and no more than 0.5 mg/kg per day of prednisone. Dexamethasone is not recommended.
Azathioprine should not be started in pregnancy, according to the task force, but when no other options are available, it may be continued in women already on the drug, albeit at half of the prepregnancy dose.
Dupilumab (Dupixent) is to be avoided in pregnant women with AD until more clinical experience becomes available.
Treatment of prospective fathers with AD
The European task force recommends that topical therapies can be prescribed in prospective fathers without any special concerns. The same is true for systemic corticosteroids. Methotrexate should be halted 3 months before planned pregnancy, as is the case for mycophenolate mofetil (CellCept). Azathioprine is recommended when other options have failed. Cyclosporine is deemed a reasonable option in the treatment of men with severe AD at the time of conception if other treatments have failed; of note, neither the Food and Drug Administration nor the European regulatory agency have issued contraindications for the use of the drug in men who wish to become fathers.
Mycophenolate mofetil carries a theoretical risk of teratogenicity. The European task force recommends that men should use condoms while on the drug and for at least 90 days afterward.
Unplanned pregnancy in women on systemic therapy
The recommended course of action is to immediately stop systemic therapy, intensify appropriate topical therapy in anticipation of worsening AD, and refer the patient to an obstetrician and a teratology information center for an individualized risk assessment. Methotrexate and mycophenolate mofetil are known teratogens.
The full 16-page task force position paper was published shortly before EADV 2019 (J Eur Acad Dermatol Venereol. 2019 Sep;33[9]:1644-59).
The report was developed without commercial sponsorship. Dr. Vestergaard indicated he has received research grants from and/or serves as a consultant to eight pharmaceutical companies.
REPORTING FROM THE EADV CONGRESS
Product update: Neuromodulation device, cystoscopy simplified, hysteroscopy seal, next immunization frontier
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/