User login
ObGyn malpractice liability risk: 2020 developments and probabilities
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
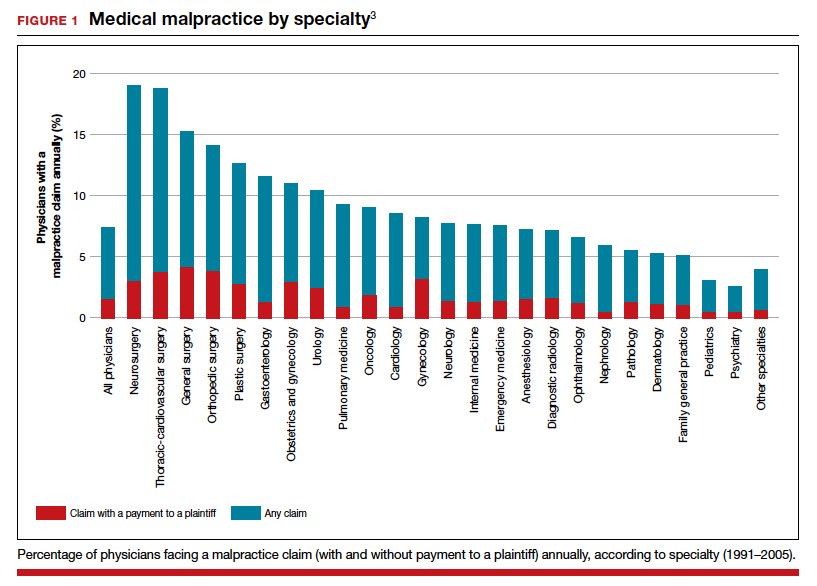
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)
Continue to: The number of ObGyn paid malpractice claims has decreased over time...
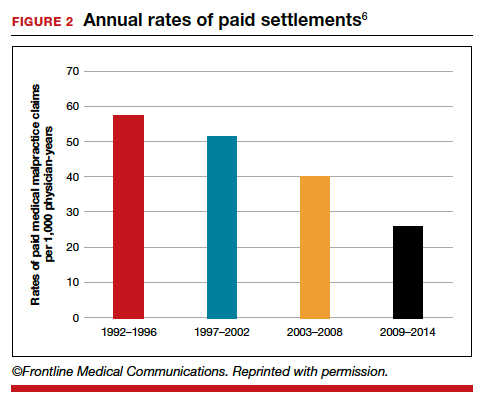
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4
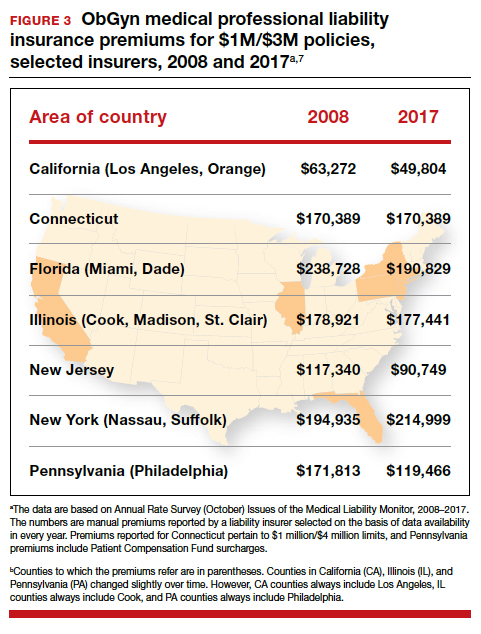
It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M
Why have malpractice payouts declined overall?
Have medical errors declined?
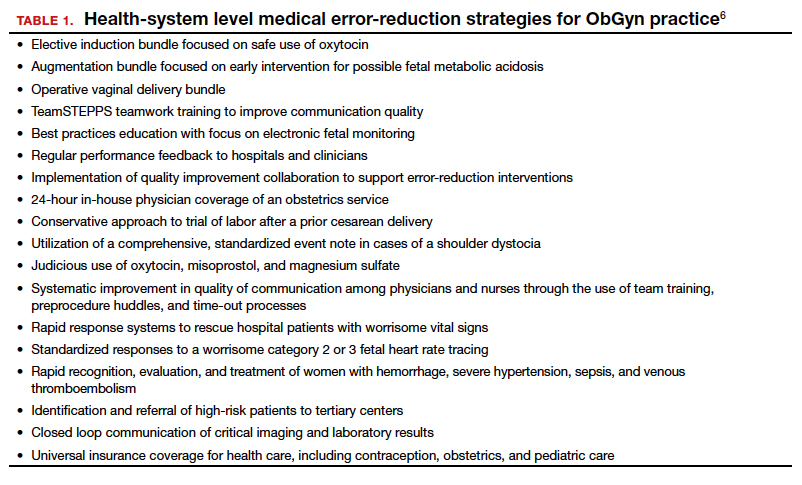
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.
The collective accountability factor
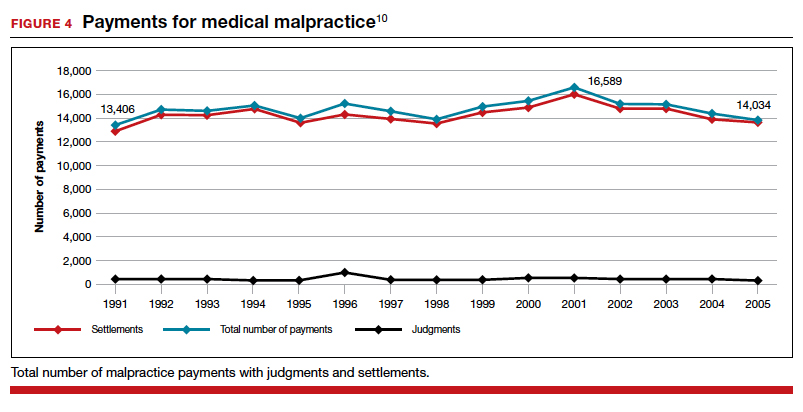
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.
Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
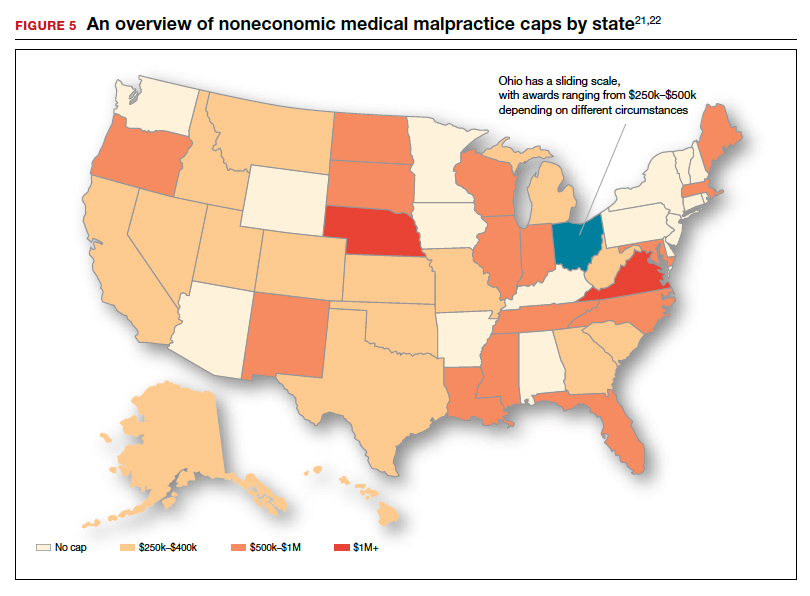
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.
Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
Racial disparities persist in preterm birth risk
Education, status are not protective for non-Hispanic black women
GRAPEVINE, TEX. – College education and high socioeconomic status do not erase U.S. racial disparities in birth outcomes, according to a new analysis of all U.S. live births from 2015-2017.
Very early preterm birth – birth before 28 weeks gestational age – was five times more likely to occur in non-Hispanic black women of high socioeconomic status as similarly situated white women, even after statistical adjustment for a host of potentially confounding factors.
Being of non-Hispanic black race was the single strongest predictor of preterm birth (PTB) at less than 28 weeks’ gestation. The adjusted odds ratio (aOR) of 4.99 surpassed an interpregnancy interval under 1 year (aOR, 4.47), chronic hypertension (aOR, 2.84), and prior history of preterm birth (aOR, 2.81).
“Even among college-educated women with private insurance who are not receiving (Women, Infants, and Children support), racial disparities in prematurity persist. These results suggest that factors other than sociodemographics are important in the underlying pathogenesis of PTB and in etiologies of racial disparities,” wrote Jasmine Johnson, MD, and her coauthors in the abstract accompanying the presentation at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The analysis that Dr. Johnson and her coinvestigators used, she explained during her plenary session presentation, still found significantly elevated risks for preterm birth for non-Hispanic black women after accounting for marital status, prior history of preterm birth, tobacco use, an interpregnancy interval of fewer than 12 months, and carrying a male fetus.
“Birth certificates do not inform the lived experiences of one’s self-identified race, and how that experience – or possibly just one’s identification with a particular racial group – may positively or negatively affect their clinical risk of preterm birth,” said Dr. Johnson. “In this study, as in others, race is a social construct. It’s a surrogate for social and societal racism that disproportionately affects birth outcomes of women of color.”
Using non-Hispanic white (NHW) women as a reference, women who described themselves as non-Hispanic black (NHB) had increased odds of preterm birth at 34 and 37 weeks gestation as well. Women identifying as both NHB and NHW had numerically elevated odds for preterm birth at all time points as well, but the odds at 37 weeks didn’t reach statistical significance.
The results were based on a retrospective population-based study of a cohort drawn from the National Vital Statistics birth certificate data of all live births in the United States between 2015 and 2017, explained Dr. Johnson, a maternal-fetal medicine fellow at the University of North Carolina, Chapel Hill. Drawing from a nationally representative sample and having a population-level design drawn were strengths of the study, she said.
Women with singleton pregnancies without anomalies who identified as NHB, NHW, or as both NHB and NHW were included if they also had high socioeconomic status. Including women who identify as both black and white was another strength of the study, Dr. Johnson added.
She explained that, for the purposes of the study, high socioeconomic status was defined as having 16 or more years of education and private insurance, and not receiving WIC benefits.
In addition to the primary outcome measure of preterm birth at fewer than 37 weeks gestation, secondary outcomes included preterm birth at fewer than 34 and fewer than 28 weeks’ gestation, as well as low birthweight (LBW) and very low birthweight (VLBW).
About 11.8 million live births occurred during the study period, and 11.3 million of those were singleton pregnancies without fetal anomaly. After excluding women who did not meet the racial self-identification or socioeconomic status inclusion criteria, the investigators arrived at the final study population of 2,170,688 individuals.
Of those, 2,017,470, or 92.9%, were non-Hispanic white, while 144,612, or 6.7%, were non-Hispanic black. The remaining 8,604 participants, or 0.4%, identified their race as non-Hispanic black and non-Hispanic white.
The groups identified in the study differed significantly in demographic characteristics, Dr. Johnson said. Women in the NHB and NHB + NHW groups were less likely to be married than NHW women – about 75% of the former two groups were married, compared with 92.5% of NHW women. This difference was statistically significant with a P value of .001, as were all the differences Dr. Johnson reported.
Pre-pregnancy body mass index (BMI) was highest in NHB women at 27.1 kg/m sq, followed by NHB = NHW women at 25.7 kg/m sq, with NHW women having the lowest BMI at 23.8 kg/m sq.
Prior preterm birth of 37 weeks’ gestation or less was more common in NHB women and NHB + NHW women, as was an interpregnancy interval of fewer than 12 months.
Chronic hypertension was more than twice as common in NHB women than in either NHB = NHW or NHW women, occurring in 3.9%, 1.8%, and 1.4% of participants, respectively.
Pregestational diabetes was about twice as common in NHB women than NHW women, occurring in 1% and 0.52% of those groups, respectively. Prevalence of pregestational diabetes was intermediate in NHB = NHW women, at 0.72%.
Tobacco use was rare overall, and less common in NHB women than NHB + NHW and NHW women.
In terms of pregnancy characteristics, though 85% of NHB women initiated prenatal care in the first trimester, they were less likely to have done so than either of the other two groups. Few women overall had no prenatal care, but 0.7% of NHB women fell into this category, more than the 0.4% and 0.3% reported for NHB + NHW and NHW women, respectively.
During their pregnancies, NHB women were more likely to develop gestational hypertension and/or pre-eclampsia as well as gestational diabetes than either of the other two groups (7.6% compared with 6% for the other two groups). Of the NHB women, 5.9% developed gestational diabetes, compared with 4.8% of NHB + NHW and 4.8% of NHW women.
Delivering a baby with a birthweight less than the 10th percentile was twice as common for NHB, compared with NHW women (7.2% versus 3.4%). The risk for NHB + NHW women was intermediate, at 4.7%.
Dr. Johnson said she and her team performed further analyses, including using initiation of prenatal care in the first trimester of pregnancy as a surrogate for high socioeconomic status; the association of race and risk for preterm birth persisted.
The study had the usual limitations of using National Vital Statistics data, such as the inability to evaluate underlying etiologies for preterm birth.
However, Dr. Johnson highlighted additional limitations that pertain to the experience of race in 21st century America. “Our definition of high socioeconomic status does not guarantee that all women in this analysis have financial stability,” she said, pointing out that the study’s definition of high socioeconomic status was insensitive to wealth accumulation. She also noted that high educational attainment does not necessarily correlate with high income. Hence, the potential burden of economic stressors could not fully be captured.
The study was supported by the National Institutes of Health. Dr. Johnson reported no conflicts of interest.
SOURCE: Johnson J et al. Obstet Gynecol. 2020 Jan;222(1):S-37-8, Abstract 44.
Education, status are not protective for non-Hispanic black women
Education, status are not protective for non-Hispanic black women
GRAPEVINE, TEX. – College education and high socioeconomic status do not erase U.S. racial disparities in birth outcomes, according to a new analysis of all U.S. live births from 2015-2017.
Very early preterm birth – birth before 28 weeks gestational age – was five times more likely to occur in non-Hispanic black women of high socioeconomic status as similarly situated white women, even after statistical adjustment for a host of potentially confounding factors.
Being of non-Hispanic black race was the single strongest predictor of preterm birth (PTB) at less than 28 weeks’ gestation. The adjusted odds ratio (aOR) of 4.99 surpassed an interpregnancy interval under 1 year (aOR, 4.47), chronic hypertension (aOR, 2.84), and prior history of preterm birth (aOR, 2.81).
“Even among college-educated women with private insurance who are not receiving (Women, Infants, and Children support), racial disparities in prematurity persist. These results suggest that factors other than sociodemographics are important in the underlying pathogenesis of PTB and in etiologies of racial disparities,” wrote Jasmine Johnson, MD, and her coauthors in the abstract accompanying the presentation at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The analysis that Dr. Johnson and her coinvestigators used, she explained during her plenary session presentation, still found significantly elevated risks for preterm birth for non-Hispanic black women after accounting for marital status, prior history of preterm birth, tobacco use, an interpregnancy interval of fewer than 12 months, and carrying a male fetus.
“Birth certificates do not inform the lived experiences of one’s self-identified race, and how that experience – or possibly just one’s identification with a particular racial group – may positively or negatively affect their clinical risk of preterm birth,” said Dr. Johnson. “In this study, as in others, race is a social construct. It’s a surrogate for social and societal racism that disproportionately affects birth outcomes of women of color.”
Using non-Hispanic white (NHW) women as a reference, women who described themselves as non-Hispanic black (NHB) had increased odds of preterm birth at 34 and 37 weeks gestation as well. Women identifying as both NHB and NHW had numerically elevated odds for preterm birth at all time points as well, but the odds at 37 weeks didn’t reach statistical significance.
The results were based on a retrospective population-based study of a cohort drawn from the National Vital Statistics birth certificate data of all live births in the United States between 2015 and 2017, explained Dr. Johnson, a maternal-fetal medicine fellow at the University of North Carolina, Chapel Hill. Drawing from a nationally representative sample and having a population-level design drawn were strengths of the study, she said.
Women with singleton pregnancies without anomalies who identified as NHB, NHW, or as both NHB and NHW were included if they also had high socioeconomic status. Including women who identify as both black and white was another strength of the study, Dr. Johnson added.
She explained that, for the purposes of the study, high socioeconomic status was defined as having 16 or more years of education and private insurance, and not receiving WIC benefits.
In addition to the primary outcome measure of preterm birth at fewer than 37 weeks gestation, secondary outcomes included preterm birth at fewer than 34 and fewer than 28 weeks’ gestation, as well as low birthweight (LBW) and very low birthweight (VLBW).
About 11.8 million live births occurred during the study period, and 11.3 million of those were singleton pregnancies without fetal anomaly. After excluding women who did not meet the racial self-identification or socioeconomic status inclusion criteria, the investigators arrived at the final study population of 2,170,688 individuals.
Of those, 2,017,470, or 92.9%, were non-Hispanic white, while 144,612, or 6.7%, were non-Hispanic black. The remaining 8,604 participants, or 0.4%, identified their race as non-Hispanic black and non-Hispanic white.
The groups identified in the study differed significantly in demographic characteristics, Dr. Johnson said. Women in the NHB and NHB + NHW groups were less likely to be married than NHW women – about 75% of the former two groups were married, compared with 92.5% of NHW women. This difference was statistically significant with a P value of .001, as were all the differences Dr. Johnson reported.
Pre-pregnancy body mass index (BMI) was highest in NHB women at 27.1 kg/m sq, followed by NHB = NHW women at 25.7 kg/m sq, with NHW women having the lowest BMI at 23.8 kg/m sq.
Prior preterm birth of 37 weeks’ gestation or less was more common in NHB women and NHB + NHW women, as was an interpregnancy interval of fewer than 12 months.
Chronic hypertension was more than twice as common in NHB women than in either NHB = NHW or NHW women, occurring in 3.9%, 1.8%, and 1.4% of participants, respectively.
Pregestational diabetes was about twice as common in NHB women than NHW women, occurring in 1% and 0.52% of those groups, respectively. Prevalence of pregestational diabetes was intermediate in NHB = NHW women, at 0.72%.
Tobacco use was rare overall, and less common in NHB women than NHB + NHW and NHW women.
In terms of pregnancy characteristics, though 85% of NHB women initiated prenatal care in the first trimester, they were less likely to have done so than either of the other two groups. Few women overall had no prenatal care, but 0.7% of NHB women fell into this category, more than the 0.4% and 0.3% reported for NHB + NHW and NHW women, respectively.
During their pregnancies, NHB women were more likely to develop gestational hypertension and/or pre-eclampsia as well as gestational diabetes than either of the other two groups (7.6% compared with 6% for the other two groups). Of the NHB women, 5.9% developed gestational diabetes, compared with 4.8% of NHB + NHW and 4.8% of NHW women.
Delivering a baby with a birthweight less than the 10th percentile was twice as common for NHB, compared with NHW women (7.2% versus 3.4%). The risk for NHB + NHW women was intermediate, at 4.7%.
Dr. Johnson said she and her team performed further analyses, including using initiation of prenatal care in the first trimester of pregnancy as a surrogate for high socioeconomic status; the association of race and risk for preterm birth persisted.
The study had the usual limitations of using National Vital Statistics data, such as the inability to evaluate underlying etiologies for preterm birth.
However, Dr. Johnson highlighted additional limitations that pertain to the experience of race in 21st century America. “Our definition of high socioeconomic status does not guarantee that all women in this analysis have financial stability,” she said, pointing out that the study’s definition of high socioeconomic status was insensitive to wealth accumulation. She also noted that high educational attainment does not necessarily correlate with high income. Hence, the potential burden of economic stressors could not fully be captured.
The study was supported by the National Institutes of Health. Dr. Johnson reported no conflicts of interest.
SOURCE: Johnson J et al. Obstet Gynecol. 2020 Jan;222(1):S-37-8, Abstract 44.
GRAPEVINE, TEX. – College education and high socioeconomic status do not erase U.S. racial disparities in birth outcomes, according to a new analysis of all U.S. live births from 2015-2017.
Very early preterm birth – birth before 28 weeks gestational age – was five times more likely to occur in non-Hispanic black women of high socioeconomic status as similarly situated white women, even after statistical adjustment for a host of potentially confounding factors.
Being of non-Hispanic black race was the single strongest predictor of preterm birth (PTB) at less than 28 weeks’ gestation. The adjusted odds ratio (aOR) of 4.99 surpassed an interpregnancy interval under 1 year (aOR, 4.47), chronic hypertension (aOR, 2.84), and prior history of preterm birth (aOR, 2.81).
“Even among college-educated women with private insurance who are not receiving (Women, Infants, and Children support), racial disparities in prematurity persist. These results suggest that factors other than sociodemographics are important in the underlying pathogenesis of PTB and in etiologies of racial disparities,” wrote Jasmine Johnson, MD, and her coauthors in the abstract accompanying the presentation at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The analysis that Dr. Johnson and her coinvestigators used, she explained during her plenary session presentation, still found significantly elevated risks for preterm birth for non-Hispanic black women after accounting for marital status, prior history of preterm birth, tobacco use, an interpregnancy interval of fewer than 12 months, and carrying a male fetus.
“Birth certificates do not inform the lived experiences of one’s self-identified race, and how that experience – or possibly just one’s identification with a particular racial group – may positively or negatively affect their clinical risk of preterm birth,” said Dr. Johnson. “In this study, as in others, race is a social construct. It’s a surrogate for social and societal racism that disproportionately affects birth outcomes of women of color.”
Using non-Hispanic white (NHW) women as a reference, women who described themselves as non-Hispanic black (NHB) had increased odds of preterm birth at 34 and 37 weeks gestation as well. Women identifying as both NHB and NHW had numerically elevated odds for preterm birth at all time points as well, but the odds at 37 weeks didn’t reach statistical significance.
The results were based on a retrospective population-based study of a cohort drawn from the National Vital Statistics birth certificate data of all live births in the United States between 2015 and 2017, explained Dr. Johnson, a maternal-fetal medicine fellow at the University of North Carolina, Chapel Hill. Drawing from a nationally representative sample and having a population-level design drawn were strengths of the study, she said.
Women with singleton pregnancies without anomalies who identified as NHB, NHW, or as both NHB and NHW were included if they also had high socioeconomic status. Including women who identify as both black and white was another strength of the study, Dr. Johnson added.
She explained that, for the purposes of the study, high socioeconomic status was defined as having 16 or more years of education and private insurance, and not receiving WIC benefits.
In addition to the primary outcome measure of preterm birth at fewer than 37 weeks gestation, secondary outcomes included preterm birth at fewer than 34 and fewer than 28 weeks’ gestation, as well as low birthweight (LBW) and very low birthweight (VLBW).
About 11.8 million live births occurred during the study period, and 11.3 million of those were singleton pregnancies without fetal anomaly. After excluding women who did not meet the racial self-identification or socioeconomic status inclusion criteria, the investigators arrived at the final study population of 2,170,688 individuals.
Of those, 2,017,470, or 92.9%, were non-Hispanic white, while 144,612, or 6.7%, were non-Hispanic black. The remaining 8,604 participants, or 0.4%, identified their race as non-Hispanic black and non-Hispanic white.
The groups identified in the study differed significantly in demographic characteristics, Dr. Johnson said. Women in the NHB and NHB + NHW groups were less likely to be married than NHW women – about 75% of the former two groups were married, compared with 92.5% of NHW women. This difference was statistically significant with a P value of .001, as were all the differences Dr. Johnson reported.
Pre-pregnancy body mass index (BMI) was highest in NHB women at 27.1 kg/m sq, followed by NHB = NHW women at 25.7 kg/m sq, with NHW women having the lowest BMI at 23.8 kg/m sq.
Prior preterm birth of 37 weeks’ gestation or less was more common in NHB women and NHB + NHW women, as was an interpregnancy interval of fewer than 12 months.
Chronic hypertension was more than twice as common in NHB women than in either NHB = NHW or NHW women, occurring in 3.9%, 1.8%, and 1.4% of participants, respectively.
Pregestational diabetes was about twice as common in NHB women than NHW women, occurring in 1% and 0.52% of those groups, respectively. Prevalence of pregestational diabetes was intermediate in NHB = NHW women, at 0.72%.
Tobacco use was rare overall, and less common in NHB women than NHB + NHW and NHW women.
In terms of pregnancy characteristics, though 85% of NHB women initiated prenatal care in the first trimester, they were less likely to have done so than either of the other two groups. Few women overall had no prenatal care, but 0.7% of NHB women fell into this category, more than the 0.4% and 0.3% reported for NHB + NHW and NHW women, respectively.
During their pregnancies, NHB women were more likely to develop gestational hypertension and/or pre-eclampsia as well as gestational diabetes than either of the other two groups (7.6% compared with 6% for the other two groups). Of the NHB women, 5.9% developed gestational diabetes, compared with 4.8% of NHB + NHW and 4.8% of NHW women.
Delivering a baby with a birthweight less than the 10th percentile was twice as common for NHB, compared with NHW women (7.2% versus 3.4%). The risk for NHB + NHW women was intermediate, at 4.7%.
Dr. Johnson said she and her team performed further analyses, including using initiation of prenatal care in the first trimester of pregnancy as a surrogate for high socioeconomic status; the association of race and risk for preterm birth persisted.
The study had the usual limitations of using National Vital Statistics data, such as the inability to evaluate underlying etiologies for preterm birth.
However, Dr. Johnson highlighted additional limitations that pertain to the experience of race in 21st century America. “Our definition of high socioeconomic status does not guarantee that all women in this analysis have financial stability,” she said, pointing out that the study’s definition of high socioeconomic status was insensitive to wealth accumulation. She also noted that high educational attainment does not necessarily correlate with high income. Hence, the potential burden of economic stressors could not fully be captured.
The study was supported by the National Institutes of Health. Dr. Johnson reported no conflicts of interest.
SOURCE: Johnson J et al. Obstet Gynecol. 2020 Jan;222(1):S-37-8, Abstract 44.
AT THE PREGNANCY MEETING
Blood pressure categories may signal maternal, perinatal risks
GRAPEVINE, TEX. – Blood pressure categories created by the American College of Cardiology (ACC) and American Heart Association (AHA) in 2017 identify patients with increased risk of preeclampsia, preterm birth, and perinatal death when applied to the first 20 weeks of pregnancy, according to a retrospective study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The absolute risk increases are small, and it is unknown whether treating these patients differently would be beneficial, said study author Martha Tesfalul, MD, maternal-fetal medicine clinical fellow at University of California, San Francisco. Nevertheless, , Dr. Tesfalul said.
Cutoffs with unclear implications
The ACC/AHA in November 2017 reclassified blood pressure in nonpregnant adults, but “implications of these categories in pregnancy are still unclear,” Dr. Tesfalul and colleagues said. Under the guidelines, normal blood pressure is systolic blood pressure less than 120 mm Hg and diastolic blood pressure less than 80 mm Hg. Elevated blood pressure is defined as systolic blood pressure between 120 and 129 mm Hg and diastolic blood pressure less than 80 mm Hg. Stage 1 hypertension is systolic blood pressure between 130 and 139 mm Hg or diastolic blood pressure between 80 and 89 mm Hg. And stage 2 hypertension is systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg.
For the present analysis, the researchers retrospectively compared obstetric and perinatal outcomes for approximately 6,000 pregnancies at an academic center for which they had at least one blood pressure measurement prior to 20 weeks. The highest measurement was used to identify women with normal blood pressure, elevated blood pressure, or stage 1 hypertension according to the 2017 thresholds.
The researchers included singleton pregnancies with delivery between January 2014 and October 2017. They excluded patients with a prior diagnosis of chronic hypertension, autoimmune or chronic renal disease, or fetal anomalies. They examined rates of gestational hypertension, preeclampsia, preterm birth, neonatal intensive care admission, and perinatal death.
Adjusted relative risks
Dr. Tesfalul and colleagues identified about 3,500 pregnancies with normal blood pressure, more than 1,300 pregnancies with elevated blood pressure, and nearly 1,100 pregnancies with stage 1 hypertension.
After adjusting for relevant covariates – maternal age, nulliparity, race, body mass index, in vitro fertilization, tobacco use, pregestational diabetes, and aspirin use – elevated blood pressure and stage 1 hypertension were associated with a higher risk of preeclampsia and severe preeclampsia, relative to normal blood pressure. The proportion of patients with preeclampsia was 5.7% in the normal blood pressure group, 11.7% in the elevated blood pressure group (adjusted relative risk, 1.8), and 15% in the stage 1 hypertension group (adjusted RR, 2.1). The proportion with preeclampsia with severe features was 3.1% in the normal blood pressure group, 5.7% in the elevated blood pressure group (adjusted RR, 1.6), and 6.8% in the stage 1 hypertension group (adjusted RR, 1.8).
In addition, stage 1 hypertension, compared with normal blood pressure, was associated with increased odds of preterm birth at less than 37 weeks (7.9% vs. 5.1%; adjusted RR, 1.4) and perinatal death (0.7% vs. 0.4%; adjusted RR, 2.8).
“Patients with elevated blood pressure and stage 1 hypertension prior to 20 weeks are at increased risk of adverse outcomes,” the authors concluded. “Further research [is] needed to determine optimal care of patients with elevated blood pressure and stage 1 hypertension in pregnancy.”
Dr. Tesfalul receives support from the Foundation for SMFM.
SOURCE: Tesfalul M et al. Am J Obstet Gynecol. 2020 Jan;222(1):S92-3, Abstract 119.
GRAPEVINE, TEX. – Blood pressure categories created by the American College of Cardiology (ACC) and American Heart Association (AHA) in 2017 identify patients with increased risk of preeclampsia, preterm birth, and perinatal death when applied to the first 20 weeks of pregnancy, according to a retrospective study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The absolute risk increases are small, and it is unknown whether treating these patients differently would be beneficial, said study author Martha Tesfalul, MD, maternal-fetal medicine clinical fellow at University of California, San Francisco. Nevertheless, , Dr. Tesfalul said.
Cutoffs with unclear implications
The ACC/AHA in November 2017 reclassified blood pressure in nonpregnant adults, but “implications of these categories in pregnancy are still unclear,” Dr. Tesfalul and colleagues said. Under the guidelines, normal blood pressure is systolic blood pressure less than 120 mm Hg and diastolic blood pressure less than 80 mm Hg. Elevated blood pressure is defined as systolic blood pressure between 120 and 129 mm Hg and diastolic blood pressure less than 80 mm Hg. Stage 1 hypertension is systolic blood pressure between 130 and 139 mm Hg or diastolic blood pressure between 80 and 89 mm Hg. And stage 2 hypertension is systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg.
For the present analysis, the researchers retrospectively compared obstetric and perinatal outcomes for approximately 6,000 pregnancies at an academic center for which they had at least one blood pressure measurement prior to 20 weeks. The highest measurement was used to identify women with normal blood pressure, elevated blood pressure, or stage 1 hypertension according to the 2017 thresholds.
The researchers included singleton pregnancies with delivery between January 2014 and October 2017. They excluded patients with a prior diagnosis of chronic hypertension, autoimmune or chronic renal disease, or fetal anomalies. They examined rates of gestational hypertension, preeclampsia, preterm birth, neonatal intensive care admission, and perinatal death.
Adjusted relative risks
Dr. Tesfalul and colleagues identified about 3,500 pregnancies with normal blood pressure, more than 1,300 pregnancies with elevated blood pressure, and nearly 1,100 pregnancies with stage 1 hypertension.
After adjusting for relevant covariates – maternal age, nulliparity, race, body mass index, in vitro fertilization, tobacco use, pregestational diabetes, and aspirin use – elevated blood pressure and stage 1 hypertension were associated with a higher risk of preeclampsia and severe preeclampsia, relative to normal blood pressure. The proportion of patients with preeclampsia was 5.7% in the normal blood pressure group, 11.7% in the elevated blood pressure group (adjusted relative risk, 1.8), and 15% in the stage 1 hypertension group (adjusted RR, 2.1). The proportion with preeclampsia with severe features was 3.1% in the normal blood pressure group, 5.7% in the elevated blood pressure group (adjusted RR, 1.6), and 6.8% in the stage 1 hypertension group (adjusted RR, 1.8).
In addition, stage 1 hypertension, compared with normal blood pressure, was associated with increased odds of preterm birth at less than 37 weeks (7.9% vs. 5.1%; adjusted RR, 1.4) and perinatal death (0.7% vs. 0.4%; adjusted RR, 2.8).
“Patients with elevated blood pressure and stage 1 hypertension prior to 20 weeks are at increased risk of adverse outcomes,” the authors concluded. “Further research [is] needed to determine optimal care of patients with elevated blood pressure and stage 1 hypertension in pregnancy.”
Dr. Tesfalul receives support from the Foundation for SMFM.
SOURCE: Tesfalul M et al. Am J Obstet Gynecol. 2020 Jan;222(1):S92-3, Abstract 119.
GRAPEVINE, TEX. – Blood pressure categories created by the American College of Cardiology (ACC) and American Heart Association (AHA) in 2017 identify patients with increased risk of preeclampsia, preterm birth, and perinatal death when applied to the first 20 weeks of pregnancy, according to a retrospective study presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The absolute risk increases are small, and it is unknown whether treating these patients differently would be beneficial, said study author Martha Tesfalul, MD, maternal-fetal medicine clinical fellow at University of California, San Francisco. Nevertheless, , Dr. Tesfalul said.
Cutoffs with unclear implications
The ACC/AHA in November 2017 reclassified blood pressure in nonpregnant adults, but “implications of these categories in pregnancy are still unclear,” Dr. Tesfalul and colleagues said. Under the guidelines, normal blood pressure is systolic blood pressure less than 120 mm Hg and diastolic blood pressure less than 80 mm Hg. Elevated blood pressure is defined as systolic blood pressure between 120 and 129 mm Hg and diastolic blood pressure less than 80 mm Hg. Stage 1 hypertension is systolic blood pressure between 130 and 139 mm Hg or diastolic blood pressure between 80 and 89 mm Hg. And stage 2 hypertension is systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg.
For the present analysis, the researchers retrospectively compared obstetric and perinatal outcomes for approximately 6,000 pregnancies at an academic center for which they had at least one blood pressure measurement prior to 20 weeks. The highest measurement was used to identify women with normal blood pressure, elevated blood pressure, or stage 1 hypertension according to the 2017 thresholds.
The researchers included singleton pregnancies with delivery between January 2014 and October 2017. They excluded patients with a prior diagnosis of chronic hypertension, autoimmune or chronic renal disease, or fetal anomalies. They examined rates of gestational hypertension, preeclampsia, preterm birth, neonatal intensive care admission, and perinatal death.
Adjusted relative risks
Dr. Tesfalul and colleagues identified about 3,500 pregnancies with normal blood pressure, more than 1,300 pregnancies with elevated blood pressure, and nearly 1,100 pregnancies with stage 1 hypertension.
After adjusting for relevant covariates – maternal age, nulliparity, race, body mass index, in vitro fertilization, tobacco use, pregestational diabetes, and aspirin use – elevated blood pressure and stage 1 hypertension were associated with a higher risk of preeclampsia and severe preeclampsia, relative to normal blood pressure. The proportion of patients with preeclampsia was 5.7% in the normal blood pressure group, 11.7% in the elevated blood pressure group (adjusted relative risk, 1.8), and 15% in the stage 1 hypertension group (adjusted RR, 2.1). The proportion with preeclampsia with severe features was 3.1% in the normal blood pressure group, 5.7% in the elevated blood pressure group (adjusted RR, 1.6), and 6.8% in the stage 1 hypertension group (adjusted RR, 1.8).
In addition, stage 1 hypertension, compared with normal blood pressure, was associated with increased odds of preterm birth at less than 37 weeks (7.9% vs. 5.1%; adjusted RR, 1.4) and perinatal death (0.7% vs. 0.4%; adjusted RR, 2.8).
“Patients with elevated blood pressure and stage 1 hypertension prior to 20 weeks are at increased risk of adverse outcomes,” the authors concluded. “Further research [is] needed to determine optimal care of patients with elevated blood pressure and stage 1 hypertension in pregnancy.”
Dr. Tesfalul receives support from the Foundation for SMFM.
SOURCE: Tesfalul M et al. Am J Obstet Gynecol. 2020 Jan;222(1):S92-3, Abstract 119.
REPORTING FROM THE PREGNANCY MEETING
Patients remain satisfied despite reduced use of opioids post partum
GRAPEVINE, TEX. – The amount of opioids prescribed post partum may decline over time without affecting levels of pain control satisfaction, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Data from a large center indicate that trends in opioid use significantly declined from 2017 to 2019, but not at the expense of adequate pain control, said Nevert Badreldin, MD, assistant professor of obstetrics and gynecology at Northwestern University in Chicago. Patients consistently reported that they were satisfied with inpatient pain control, while opioid use per inpatient day decreased from about 30 morphine milligram equivalents (MME) to less than 20 MME during that time.
To assess trends in postpartum opioid prescribing, opioid use, and pain control satisfaction, Dr. Badreldin and colleagues evaluated data from a prospective observational study. Their analysis included data from women who used an opioid during postpartum hospitalization between May 2017 and July 2019. The researchers excluded women with NSAID or morphine allergies or recent opioid use, as well as those who received general anesthesia without concurrent neuraxial anesthesia, those who underwent peripartum hysterectomy, and women admitted to the ICU.
The investigators used nonparametric tests of trend to assess the difference over time in the proportion of patients who received an opioid prescription at discharge and in the total MME prescribed post partum.
Of 900 women with inpatient opioid use, 471 agreed to be followed after discharge. In that group, the amount of opioid use per inpatient day significantly declined. In addition, the percentage who received an opioid prescription at discharge significantly declined, as did the total MME prescribed at discharge.
“Both inpatient and outpatient satisfaction with pain control were unchanged,” the researchers reported. “In this population, both the frequency and amount of opioid use in the postpartum period declined from 2017 to 2019, without any change in satisfaction with pain control.”
The study was supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award, and a coauthor received support from the National Institute of Child Health and Human Development.
Source: Badreldin N et al. Am J Obstet Gynecol. 2020 Jan;222(1):S93, Abstract 120.
GRAPEVINE, TEX. – The amount of opioids prescribed post partum may decline over time without affecting levels of pain control satisfaction, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Data from a large center indicate that trends in opioid use significantly declined from 2017 to 2019, but not at the expense of adequate pain control, said Nevert Badreldin, MD, assistant professor of obstetrics and gynecology at Northwestern University in Chicago. Patients consistently reported that they were satisfied with inpatient pain control, while opioid use per inpatient day decreased from about 30 morphine milligram equivalents (MME) to less than 20 MME during that time.
To assess trends in postpartum opioid prescribing, opioid use, and pain control satisfaction, Dr. Badreldin and colleagues evaluated data from a prospective observational study. Their analysis included data from women who used an opioid during postpartum hospitalization between May 2017 and July 2019. The researchers excluded women with NSAID or morphine allergies or recent opioid use, as well as those who received general anesthesia without concurrent neuraxial anesthesia, those who underwent peripartum hysterectomy, and women admitted to the ICU.
The investigators used nonparametric tests of trend to assess the difference over time in the proportion of patients who received an opioid prescription at discharge and in the total MME prescribed post partum.
Of 900 women with inpatient opioid use, 471 agreed to be followed after discharge. In that group, the amount of opioid use per inpatient day significantly declined. In addition, the percentage who received an opioid prescription at discharge significantly declined, as did the total MME prescribed at discharge.
“Both inpatient and outpatient satisfaction with pain control were unchanged,” the researchers reported. “In this population, both the frequency and amount of opioid use in the postpartum period declined from 2017 to 2019, without any change in satisfaction with pain control.”
The study was supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award, and a coauthor received support from the National Institute of Child Health and Human Development.
Source: Badreldin N et al. Am J Obstet Gynecol. 2020 Jan;222(1):S93, Abstract 120.
GRAPEVINE, TEX. – The amount of opioids prescribed post partum may decline over time without affecting levels of pain control satisfaction, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Data from a large center indicate that trends in opioid use significantly declined from 2017 to 2019, but not at the expense of adequate pain control, said Nevert Badreldin, MD, assistant professor of obstetrics and gynecology at Northwestern University in Chicago. Patients consistently reported that they were satisfied with inpatient pain control, while opioid use per inpatient day decreased from about 30 morphine milligram equivalents (MME) to less than 20 MME during that time.
To assess trends in postpartum opioid prescribing, opioid use, and pain control satisfaction, Dr. Badreldin and colleagues evaluated data from a prospective observational study. Their analysis included data from women who used an opioid during postpartum hospitalization between May 2017 and July 2019. The researchers excluded women with NSAID or morphine allergies or recent opioid use, as well as those who received general anesthesia without concurrent neuraxial anesthesia, those who underwent peripartum hysterectomy, and women admitted to the ICU.
The investigators used nonparametric tests of trend to assess the difference over time in the proportion of patients who received an opioid prescription at discharge and in the total MME prescribed post partum.
Of 900 women with inpatient opioid use, 471 agreed to be followed after discharge. In that group, the amount of opioid use per inpatient day significantly declined. In addition, the percentage who received an opioid prescription at discharge significantly declined, as did the total MME prescribed at discharge.
“Both inpatient and outpatient satisfaction with pain control were unchanged,” the researchers reported. “In this population, both the frequency and amount of opioid use in the postpartum period declined from 2017 to 2019, without any change in satisfaction with pain control.”
The study was supported by the Society for Maternal-Fetal Medicine/AMAG 2017 Health Policy Award, and a coauthor received support from the National Institute of Child Health and Human Development.
Source: Badreldin N et al. Am J Obstet Gynecol. 2020 Jan;222(1):S93, Abstract 120.
REPORTING FROM THE PREGNANCY MEETING
Less gestational weight gain seen with metformin
GRAPEVINE, TEX.* – Pregnant women with type 2 diabetes or prediabetes had significantly less gestational weight gain if they had metformin exposure at any point in their pregnancies, with no differences in infant birth weight or postnatal infant hypoglycemia, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
In a retrospective single-center review of 284 women without metformin exposure and 227 with metformin exposure in pregnancy, metformin exposure at any point in pregnancy was associated with a significantly greater chance of appropriate – rather than excessive – weight gain.
The relationship held true for the 169 women who had metformin in their first trimester of pregnancy. Here, 69% of women had appropriate weight gain using Institute of Medicine and American College of Obstetricians and Gynecologists standards, compared with 54% of the 282 women who had no metformin exposure (adjusted odds ratio 1.92, P = .003). A further 22% of women receiving metformin in their first trimester of pregnancy lost weight, compared with 9% of women without metformin exposure (aOR 2.11, P = .019). There was no significant difference between the two groups in infant birth weight.
Separately, study author Jacquelyn Adams, MD, and her colleagues analyzed outcomes for the full cohort of 227 women who received metformin at any point in their pregnancy, comparing them again to the 282 women who had not received metformin. Most women (85%) were on 2 g of metformin at the time of delivery. These results again showed a greater likelihood of appropriate weight gain in the metformin group (69%; aOR 1.85; P = .002). Maternal weight loss was seen in 20% of this group (aOR 1.98, P = .018). Infant birth weights were not significantly different between these two groups.
“We found that women who had been on metformin at any point in their pregnancy had more appropriate weight gain and less excessive weight gain,” said Dr. Adams, a maternal-fetal medicine fellow at the University of Wisconsin–Madison. “Actually, some women on metformin had even had a little bit of weight loss, with no difference in their baby’s birth weight. So that’s really exciting, because our starting prepregnancy body mass index was 33-36 [kg/m2], which is considered obese,” she said in an interview.
This is an important finding, said Dr. Adams, because previous work has shown that less weight gain in pregnancy is associated with lower risk for hypertension and preeclampsia, and lower rates of fetal macrosomia.
What about infant outcomes? Dr. Adams said that there were many concerns about metformin: “Would it affect baby outcome? Were those babies more likely to be hypoglycemic? Were they more likely to be growth restricted? Were they more likely to have issues in the NICU? And the answer was really, ‘No.’ ”
“So we can both help these women have appropriate weight gain and not have any negative effects on these babies,” she added.
Specifically, Dr. Adams and her coinvestigators found no significant differences between the groups in gestational age at birth, likelihood of neonatal ICU admission, Apgar scores, neonatal hypoglycemia, respiratory distress syndrome, or fetal death. Fetal growth restriction and anomalies occurred at a low and similar rate between the groups.
Dr. Adams said that she was not surprised to see that metformin was associated with less weight gain in pregnancy, but she was surprised at how highly significant the differences were with metformin use. “Metformin is first-line for diabetes in nonpregnant individuals because it’s associated with things like weight loss, and because of ease of use and lack of hypoglycemia – so I was really hoping to see this kind of result.”
Women receiving metformin were a mean 34 years old, while those who didn’t get metformin were 32 years old, a significant difference. Prepregnancy body mass index also was higher in those receiving metformin, and they were more likely to have a type 2 diabetes diagnosis. A similar proportion of both groups – about two-thirds – were white, and about 20% were Hispanic.
The lower weight gain seen in metformin-takers also might smooth the way post partum, said Dr. Adams. “My perception is that, when these women leave us, they might not have any primary care follow-up; they might not have anybody following their diabetes; and metformin is a very viable way to help them in their life outside of pregnancy.
“Not to mention that all the weight you gain in pregnancy, you do eventually have to lose post partum,” she added, “so having less pregnancy weight gain kind of sets them up for success in their postpregnancy life as well.”
Asked whether these results inform the ongoing question of whether insulin or metformin is the most appropriate first-line treatment for gestational diabetes, Dr. Adams first noted that “there’s a lot of crossover,” pointing out that over 60% of the participants in her study eventually also required insulin.
“It’s a question I would love to address in a head-to-head trial,” she said, adding that questions about metformin’s effects on the placenta and the potential for later deleterious effects require more study.
In her practice, Dr. Adams said that patients generally are discharged with a metformin prescription, and then meet with a diabetes educator 1 week after delivery to assess blood glucose levels and adjust medical management. Following that, a warm hand-off to a primary care practice who can continue management and education is optimal, she said.
In terms of next steps, “We would really love to look at metformin in the postpartum period,” said Dr. Adams. Ideally, future work could look for outcomes that extend beyond the 6- to 8-week postpartum follow-up visit. For example, she said, there are indications that women with insulin insensitivity might benefit from metformin while breastfeeding; it’s also possible that metformin might reduce the risk of postpartum preeclampsia.
Dr. Adams reported that she had no conflicts of interest and no outside sources of funding.
SOURCE: Adams J et al. SMFM 2020, Abstract 335.
*This story was updated 2/10/2020.
GRAPEVINE, TEX.* – Pregnant women with type 2 diabetes or prediabetes had significantly less gestational weight gain if they had metformin exposure at any point in their pregnancies, with no differences in infant birth weight or postnatal infant hypoglycemia, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
In a retrospective single-center review of 284 women without metformin exposure and 227 with metformin exposure in pregnancy, metformin exposure at any point in pregnancy was associated with a significantly greater chance of appropriate – rather than excessive – weight gain.
The relationship held true for the 169 women who had metformin in their first trimester of pregnancy. Here, 69% of women had appropriate weight gain using Institute of Medicine and American College of Obstetricians and Gynecologists standards, compared with 54% of the 282 women who had no metformin exposure (adjusted odds ratio 1.92, P = .003). A further 22% of women receiving metformin in their first trimester of pregnancy lost weight, compared with 9% of women without metformin exposure (aOR 2.11, P = .019). There was no significant difference between the two groups in infant birth weight.
Separately, study author Jacquelyn Adams, MD, and her colleagues analyzed outcomes for the full cohort of 227 women who received metformin at any point in their pregnancy, comparing them again to the 282 women who had not received metformin. Most women (85%) were on 2 g of metformin at the time of delivery. These results again showed a greater likelihood of appropriate weight gain in the metformin group (69%; aOR 1.85; P = .002). Maternal weight loss was seen in 20% of this group (aOR 1.98, P = .018). Infant birth weights were not significantly different between these two groups.
“We found that women who had been on metformin at any point in their pregnancy had more appropriate weight gain and less excessive weight gain,” said Dr. Adams, a maternal-fetal medicine fellow at the University of Wisconsin–Madison. “Actually, some women on metformin had even had a little bit of weight loss, with no difference in their baby’s birth weight. So that’s really exciting, because our starting prepregnancy body mass index was 33-36 [kg/m2], which is considered obese,” she said in an interview.
This is an important finding, said Dr. Adams, because previous work has shown that less weight gain in pregnancy is associated with lower risk for hypertension and preeclampsia, and lower rates of fetal macrosomia.
What about infant outcomes? Dr. Adams said that there were many concerns about metformin: “Would it affect baby outcome? Were those babies more likely to be hypoglycemic? Were they more likely to be growth restricted? Were they more likely to have issues in the NICU? And the answer was really, ‘No.’ ”
“So we can both help these women have appropriate weight gain and not have any negative effects on these babies,” she added.
Specifically, Dr. Adams and her coinvestigators found no significant differences between the groups in gestational age at birth, likelihood of neonatal ICU admission, Apgar scores, neonatal hypoglycemia, respiratory distress syndrome, or fetal death. Fetal growth restriction and anomalies occurred at a low and similar rate between the groups.
Dr. Adams said that she was not surprised to see that metformin was associated with less weight gain in pregnancy, but she was surprised at how highly significant the differences were with metformin use. “Metformin is first-line for diabetes in nonpregnant individuals because it’s associated with things like weight loss, and because of ease of use and lack of hypoglycemia – so I was really hoping to see this kind of result.”
Women receiving metformin were a mean 34 years old, while those who didn’t get metformin were 32 years old, a significant difference. Prepregnancy body mass index also was higher in those receiving metformin, and they were more likely to have a type 2 diabetes diagnosis. A similar proportion of both groups – about two-thirds – were white, and about 20% were Hispanic.
The lower weight gain seen in metformin-takers also might smooth the way post partum, said Dr. Adams. “My perception is that, when these women leave us, they might not have any primary care follow-up; they might not have anybody following their diabetes; and metformin is a very viable way to help them in their life outside of pregnancy.
“Not to mention that all the weight you gain in pregnancy, you do eventually have to lose post partum,” she added, “so having less pregnancy weight gain kind of sets them up for success in their postpregnancy life as well.”
Asked whether these results inform the ongoing question of whether insulin or metformin is the most appropriate first-line treatment for gestational diabetes, Dr. Adams first noted that “there’s a lot of crossover,” pointing out that over 60% of the participants in her study eventually also required insulin.
“It’s a question I would love to address in a head-to-head trial,” she said, adding that questions about metformin’s effects on the placenta and the potential for later deleterious effects require more study.
In her practice, Dr. Adams said that patients generally are discharged with a metformin prescription, and then meet with a diabetes educator 1 week after delivery to assess blood glucose levels and adjust medical management. Following that, a warm hand-off to a primary care practice who can continue management and education is optimal, she said.
In terms of next steps, “We would really love to look at metformin in the postpartum period,” said Dr. Adams. Ideally, future work could look for outcomes that extend beyond the 6- to 8-week postpartum follow-up visit. For example, she said, there are indications that women with insulin insensitivity might benefit from metformin while breastfeeding; it’s also possible that metformin might reduce the risk of postpartum preeclampsia.
Dr. Adams reported that she had no conflicts of interest and no outside sources of funding.
SOURCE: Adams J et al. SMFM 2020, Abstract 335.
*This story was updated 2/10/2020.
GRAPEVINE, TEX.* – Pregnant women with type 2 diabetes or prediabetes had significantly less gestational weight gain if they had metformin exposure at any point in their pregnancies, with no differences in infant birth weight or postnatal infant hypoglycemia, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
In a retrospective single-center review of 284 women without metformin exposure and 227 with metformin exposure in pregnancy, metformin exposure at any point in pregnancy was associated with a significantly greater chance of appropriate – rather than excessive – weight gain.
The relationship held true for the 169 women who had metformin in their first trimester of pregnancy. Here, 69% of women had appropriate weight gain using Institute of Medicine and American College of Obstetricians and Gynecologists standards, compared with 54% of the 282 women who had no metformin exposure (adjusted odds ratio 1.92, P = .003). A further 22% of women receiving metformin in their first trimester of pregnancy lost weight, compared with 9% of women without metformin exposure (aOR 2.11, P = .019). There was no significant difference between the two groups in infant birth weight.
Separately, study author Jacquelyn Adams, MD, and her colleagues analyzed outcomes for the full cohort of 227 women who received metformin at any point in their pregnancy, comparing them again to the 282 women who had not received metformin. Most women (85%) were on 2 g of metformin at the time of delivery. These results again showed a greater likelihood of appropriate weight gain in the metformin group (69%; aOR 1.85; P = .002). Maternal weight loss was seen in 20% of this group (aOR 1.98, P = .018). Infant birth weights were not significantly different between these two groups.
“We found that women who had been on metformin at any point in their pregnancy had more appropriate weight gain and less excessive weight gain,” said Dr. Adams, a maternal-fetal medicine fellow at the University of Wisconsin–Madison. “Actually, some women on metformin had even had a little bit of weight loss, with no difference in their baby’s birth weight. So that’s really exciting, because our starting prepregnancy body mass index was 33-36 [kg/m2], which is considered obese,” she said in an interview.
This is an important finding, said Dr. Adams, because previous work has shown that less weight gain in pregnancy is associated with lower risk for hypertension and preeclampsia, and lower rates of fetal macrosomia.
What about infant outcomes? Dr. Adams said that there were many concerns about metformin: “Would it affect baby outcome? Were those babies more likely to be hypoglycemic? Were they more likely to be growth restricted? Were they more likely to have issues in the NICU? And the answer was really, ‘No.’ ”
“So we can both help these women have appropriate weight gain and not have any negative effects on these babies,” she added.
Specifically, Dr. Adams and her coinvestigators found no significant differences between the groups in gestational age at birth, likelihood of neonatal ICU admission, Apgar scores, neonatal hypoglycemia, respiratory distress syndrome, or fetal death. Fetal growth restriction and anomalies occurred at a low and similar rate between the groups.
Dr. Adams said that she was not surprised to see that metformin was associated with less weight gain in pregnancy, but she was surprised at how highly significant the differences were with metformin use. “Metformin is first-line for diabetes in nonpregnant individuals because it’s associated with things like weight loss, and because of ease of use and lack of hypoglycemia – so I was really hoping to see this kind of result.”
Women receiving metformin were a mean 34 years old, while those who didn’t get metformin were 32 years old, a significant difference. Prepregnancy body mass index also was higher in those receiving metformin, and they were more likely to have a type 2 diabetes diagnosis. A similar proportion of both groups – about two-thirds – were white, and about 20% were Hispanic.
The lower weight gain seen in metformin-takers also might smooth the way post partum, said Dr. Adams. “My perception is that, when these women leave us, they might not have any primary care follow-up; they might not have anybody following their diabetes; and metformin is a very viable way to help them in their life outside of pregnancy.
“Not to mention that all the weight you gain in pregnancy, you do eventually have to lose post partum,” she added, “so having less pregnancy weight gain kind of sets them up for success in their postpregnancy life as well.”
Asked whether these results inform the ongoing question of whether insulin or metformin is the most appropriate first-line treatment for gestational diabetes, Dr. Adams first noted that “there’s a lot of crossover,” pointing out that over 60% of the participants in her study eventually also required insulin.
“It’s a question I would love to address in a head-to-head trial,” she said, adding that questions about metformin’s effects on the placenta and the potential for later deleterious effects require more study.
In her practice, Dr. Adams said that patients generally are discharged with a metformin prescription, and then meet with a diabetes educator 1 week after delivery to assess blood glucose levels and adjust medical management. Following that, a warm hand-off to a primary care practice who can continue management and education is optimal, she said.
In terms of next steps, “We would really love to look at metformin in the postpartum period,” said Dr. Adams. Ideally, future work could look for outcomes that extend beyond the 6- to 8-week postpartum follow-up visit. For example, she said, there are indications that women with insulin insensitivity might benefit from metformin while breastfeeding; it’s also possible that metformin might reduce the risk of postpartum preeclampsia.
Dr. Adams reported that she had no conflicts of interest and no outside sources of funding.
SOURCE: Adams J et al. SMFM 2020, Abstract 335.
*This story was updated 2/10/2020.
REPORTING FROM THE PREGNANCY MEETING
Tildrakizumab signals safe for pregnant psoriasis patients
A post hoc analysis of .
“Although contraception in female patients of childbearing age was mandatory before initiation of and during tildrakizumab therapy, some pregnancies occurred during the tildrakizumab clinical development program as protocol violations,” wrote Kathleen Haycraft, MD, of Riverside Dermatology & Spa, Hannibal, Mo., and colleagues.
Tildrakizumab (Ilumya), an interleukin-23 antagonist, was approved in 2018 by the Food and Drug Administration for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Effects on birth outcomes or on neonates exposed during pregnancy have not been studied, the researchers said.
“Tildrakizumab plasma half-life after subcutaneous administration is approximately 25 days; therefore, tildrakizumab administered even in the first trimester may cross the placental barrier,” they noted.
In a research letter published in the British Journal of Dermatology, the investigators reviewed data from nine phase 1, 2, and 3 clinical trials and identified 528 women of childbearing age who received tildrakizumab. Fourteen pregnancies were reported among these women: six from a contraceptive failure, and eight for lack of contraception use. (One of the phase 1 trials was in patients with Crohn’s disease, which included one of the pregnancies; the rest were in patients with psoriasis.)
The 14 pregnancy outcomes included 2 spontaneous abortions (14.3%), 4 elective abortions (28.6%), and 8 live births (57.1%), which included 1 premature birth, with “no identifiable congenital anomalies,” the authors wrote. The longest duration of exposure to tildrakizumab in a pregnant woman was 1,196 days; this pregnancy resulted in a premature live birth at 36 weeks with no anomalies. The spontaneous abortion rate was similar to the rate in the general population, which is 12%-15%, the authors noted.
While the study “adds to the existing evidence on the outcomes of biologic treatment of psoriasis,” the findings were limited by several factors including the small number of pregnancies, short duration of exposure to tildrakizumab, variations in dosing, and lack of controls, the researchers noted. “Additional data from a larger population following tildrakizumab exposure are required to fully evaluate the safety and tolerability of tildrakizumab treatment during pregnancy,” they said. In the meantime, they advised women of childbearing age with psoriasis to continue to avoid pregnancy and follow practice guidelines for contraceptive use while taking the biologic therapy.
The studies were supported by Merck Sharp & Dohme, a Merck & Co. subsidiary; analyses were supported by Sun Pharmaceutical Industries. Lead author Dr. Haycraft disclosed relationships with companies including Sun, Celgene, Lilly, Novartis, Ortho-Derm, and Pfizer. Other authors disclosed relationships with Novartis, Celgene, Ortho Dermatologics, Janssen, and Merck; two authors are Sun employees.
SOURCE: Haycraft K et al. Br J Dermatol. 2020 Jan 29. doi: 10.1111/bjd.18897.
A post hoc analysis of .
“Although contraception in female patients of childbearing age was mandatory before initiation of and during tildrakizumab therapy, some pregnancies occurred during the tildrakizumab clinical development program as protocol violations,” wrote Kathleen Haycraft, MD, of Riverside Dermatology & Spa, Hannibal, Mo., and colleagues.
Tildrakizumab (Ilumya), an interleukin-23 antagonist, was approved in 2018 by the Food and Drug Administration for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Effects on birth outcomes or on neonates exposed during pregnancy have not been studied, the researchers said.
“Tildrakizumab plasma half-life after subcutaneous administration is approximately 25 days; therefore, tildrakizumab administered even in the first trimester may cross the placental barrier,” they noted.
In a research letter published in the British Journal of Dermatology, the investigators reviewed data from nine phase 1, 2, and 3 clinical trials and identified 528 women of childbearing age who received tildrakizumab. Fourteen pregnancies were reported among these women: six from a contraceptive failure, and eight for lack of contraception use. (One of the phase 1 trials was in patients with Crohn’s disease, which included one of the pregnancies; the rest were in patients with psoriasis.)
The 14 pregnancy outcomes included 2 spontaneous abortions (14.3%), 4 elective abortions (28.6%), and 8 live births (57.1%), which included 1 premature birth, with “no identifiable congenital anomalies,” the authors wrote. The longest duration of exposure to tildrakizumab in a pregnant woman was 1,196 days; this pregnancy resulted in a premature live birth at 36 weeks with no anomalies. The spontaneous abortion rate was similar to the rate in the general population, which is 12%-15%, the authors noted.
While the study “adds to the existing evidence on the outcomes of biologic treatment of psoriasis,” the findings were limited by several factors including the small number of pregnancies, short duration of exposure to tildrakizumab, variations in dosing, and lack of controls, the researchers noted. “Additional data from a larger population following tildrakizumab exposure are required to fully evaluate the safety and tolerability of tildrakizumab treatment during pregnancy,” they said. In the meantime, they advised women of childbearing age with psoriasis to continue to avoid pregnancy and follow practice guidelines for contraceptive use while taking the biologic therapy.
The studies were supported by Merck Sharp & Dohme, a Merck & Co. subsidiary; analyses were supported by Sun Pharmaceutical Industries. Lead author Dr. Haycraft disclosed relationships with companies including Sun, Celgene, Lilly, Novartis, Ortho-Derm, and Pfizer. Other authors disclosed relationships with Novartis, Celgene, Ortho Dermatologics, Janssen, and Merck; two authors are Sun employees.
SOURCE: Haycraft K et al. Br J Dermatol. 2020 Jan 29. doi: 10.1111/bjd.18897.
A post hoc analysis of .
“Although contraception in female patients of childbearing age was mandatory before initiation of and during tildrakizumab therapy, some pregnancies occurred during the tildrakizumab clinical development program as protocol violations,” wrote Kathleen Haycraft, MD, of Riverside Dermatology & Spa, Hannibal, Mo., and colleagues.
Tildrakizumab (Ilumya), an interleukin-23 antagonist, was approved in 2018 by the Food and Drug Administration for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Effects on birth outcomes or on neonates exposed during pregnancy have not been studied, the researchers said.
“Tildrakizumab plasma half-life after subcutaneous administration is approximately 25 days; therefore, tildrakizumab administered even in the first trimester may cross the placental barrier,” they noted.
In a research letter published in the British Journal of Dermatology, the investigators reviewed data from nine phase 1, 2, and 3 clinical trials and identified 528 women of childbearing age who received tildrakizumab. Fourteen pregnancies were reported among these women: six from a contraceptive failure, and eight for lack of contraception use. (One of the phase 1 trials was in patients with Crohn’s disease, which included one of the pregnancies; the rest were in patients with psoriasis.)
The 14 pregnancy outcomes included 2 spontaneous abortions (14.3%), 4 elective abortions (28.6%), and 8 live births (57.1%), which included 1 premature birth, with “no identifiable congenital anomalies,” the authors wrote. The longest duration of exposure to tildrakizumab in a pregnant woman was 1,196 days; this pregnancy resulted in a premature live birth at 36 weeks with no anomalies. The spontaneous abortion rate was similar to the rate in the general population, which is 12%-15%, the authors noted.
While the study “adds to the existing evidence on the outcomes of biologic treatment of psoriasis,” the findings were limited by several factors including the small number of pregnancies, short duration of exposure to tildrakizumab, variations in dosing, and lack of controls, the researchers noted. “Additional data from a larger population following tildrakizumab exposure are required to fully evaluate the safety and tolerability of tildrakizumab treatment during pregnancy,” they said. In the meantime, they advised women of childbearing age with psoriasis to continue to avoid pregnancy and follow practice guidelines for contraceptive use while taking the biologic therapy.
The studies were supported by Merck Sharp & Dohme, a Merck & Co. subsidiary; analyses were supported by Sun Pharmaceutical Industries. Lead author Dr. Haycraft disclosed relationships with companies including Sun, Celgene, Lilly, Novartis, Ortho-Derm, and Pfizer. Other authors disclosed relationships with Novartis, Celgene, Ortho Dermatologics, Janssen, and Merck; two authors are Sun employees.
SOURCE: Haycraft K et al. Br J Dermatol. 2020 Jan 29. doi: 10.1111/bjd.18897.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Posttraumatic stress may persist up to 9 months after pregnancy loss
new research suggests.
The outcomes of a prospective cohort study involving 737 women who had experienced miscarriage or ectopic pregnancy and 171 controls with healthy pregnancies were presented in a report in the American Journal of Obstetrics & Gynecology.
One month after their pregnancy loss, 29% of these women met the criteria for posttraumatic stress, 24% reported moderate to severe anxiety, and 11% reported moderate to severe depression. In comparison, just 13% of women in the control group met the criteria for anxiety, and 2% met the criteria for depression, which meant women who had experienced early pregnancy loss had a greater than twofold odds of anxiety and nearly fourfold (odds ratio, 3.88) greater odds of depression, reported Jessica Farren, PhD, of the Queen Charlotte’s and Chelsea Hospital, London, and coauthors.
The most common posttraumatic symptom, experienced by 91% of respondents with posttraumatic stress at 1 month after the pregnancy, was reexperiencing symptoms, while 60% experienced avoidance and hyperarousal symptoms. At 3 months after the loss, 50% of those with posttraumatic stress reported an interruption of their general satisfaction with life.
While the incidence of posttraumatic stress, anxiety, and depression decreased over time in the women who had early pregnancy loss, by the third month 21% still met the criteria for posttraumatic stress, and by 9 months, 18% still were experiencing posttraumatic stress. Similarly, moderate to severe anxiety was still present in 23% of women at 3 months and 17% at 9 months, and moderate to severe depression was still experienced by 8% of women at 3 months and 6% of women at 9 months.
Dr. Farren and coauthors wrote that, given the incidence of miscarriage and ectopic pregnancy in the population, the high proportion of women still experiencing posttraumatic stress, anxiety, and depression at 9 months pointed to a significant public health issue. “It is recognized that PTSD in other contexts can have a significant impact on work, social interaction, health care utilization, and risks in future pregnancies,” they wrote. “Work is needed to evaluate strategies to effectively identify and treat affected women with these specific psychopathologies.”
The investigators also looked at the differences in outcomes in women who experienced miscarriage, compared with those who experienced ectopic pregnancy.
Of the 363 women who had a miscarriage, 30% met criteria for posttraumatic stress at 1 month, 20% at 3 months, and 17% at 9 months. Moderate to severe anxiety was reported by 25% women at 1 month, 22% at 3 months, and 17% at 9 months. Moderate to severe depression was reported by 12% at 1 month, 7% at 3 months, and 5% at 9 months.
Of the 74 women who had an ectopic pregnancy, 23% met criteria for posttraumatic stress at 1 month, 28% at 3 months, and 21% at 9 months. Moderate to severe anxiety was reported by 21% at 1 month, 30% at 3 months, and 23% at 9 months. Moderate to severe depression was reported by 7% at 1 month, 12% at 3 months, and 11% at 9 months.
The authors noted that the incidence of posttraumatic stress, anxiety, and depression decreased more strongly over time in women who had experienced miscarriage, compared with those who experienced ectopic pregnancy, although they commented that the confidence intervals were wide.
One coauthor was supported by an Imperial Health Charity grant and another by the National Institute for Health Research Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Farren J et al. Amer J Obstet Gynecol. 2019 Dec 13. doi: 10.1016/j.ajog.2019.10.102.
new research suggests.
The outcomes of a prospective cohort study involving 737 women who had experienced miscarriage or ectopic pregnancy and 171 controls with healthy pregnancies were presented in a report in the American Journal of Obstetrics & Gynecology.
One month after their pregnancy loss, 29% of these women met the criteria for posttraumatic stress, 24% reported moderate to severe anxiety, and 11% reported moderate to severe depression. In comparison, just 13% of women in the control group met the criteria for anxiety, and 2% met the criteria for depression, which meant women who had experienced early pregnancy loss had a greater than twofold odds of anxiety and nearly fourfold (odds ratio, 3.88) greater odds of depression, reported Jessica Farren, PhD, of the Queen Charlotte’s and Chelsea Hospital, London, and coauthors.
The most common posttraumatic symptom, experienced by 91% of respondents with posttraumatic stress at 1 month after the pregnancy, was reexperiencing symptoms, while 60% experienced avoidance and hyperarousal symptoms. At 3 months after the loss, 50% of those with posttraumatic stress reported an interruption of their general satisfaction with life.
While the incidence of posttraumatic stress, anxiety, and depression decreased over time in the women who had early pregnancy loss, by the third month 21% still met the criteria for posttraumatic stress, and by 9 months, 18% still were experiencing posttraumatic stress. Similarly, moderate to severe anxiety was still present in 23% of women at 3 months and 17% at 9 months, and moderate to severe depression was still experienced by 8% of women at 3 months and 6% of women at 9 months.
Dr. Farren and coauthors wrote that, given the incidence of miscarriage and ectopic pregnancy in the population, the high proportion of women still experiencing posttraumatic stress, anxiety, and depression at 9 months pointed to a significant public health issue. “It is recognized that PTSD in other contexts can have a significant impact on work, social interaction, health care utilization, and risks in future pregnancies,” they wrote. “Work is needed to evaluate strategies to effectively identify and treat affected women with these specific psychopathologies.”
The investigators also looked at the differences in outcomes in women who experienced miscarriage, compared with those who experienced ectopic pregnancy.
Of the 363 women who had a miscarriage, 30% met criteria for posttraumatic stress at 1 month, 20% at 3 months, and 17% at 9 months. Moderate to severe anxiety was reported by 25% women at 1 month, 22% at 3 months, and 17% at 9 months. Moderate to severe depression was reported by 12% at 1 month, 7% at 3 months, and 5% at 9 months.
Of the 74 women who had an ectopic pregnancy, 23% met criteria for posttraumatic stress at 1 month, 28% at 3 months, and 21% at 9 months. Moderate to severe anxiety was reported by 21% at 1 month, 30% at 3 months, and 23% at 9 months. Moderate to severe depression was reported by 7% at 1 month, 12% at 3 months, and 11% at 9 months.
The authors noted that the incidence of posttraumatic stress, anxiety, and depression decreased more strongly over time in women who had experienced miscarriage, compared with those who experienced ectopic pregnancy, although they commented that the confidence intervals were wide.
One coauthor was supported by an Imperial Health Charity grant and another by the National Institute for Health Research Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Farren J et al. Amer J Obstet Gynecol. 2019 Dec 13. doi: 10.1016/j.ajog.2019.10.102.
new research suggests.
The outcomes of a prospective cohort study involving 737 women who had experienced miscarriage or ectopic pregnancy and 171 controls with healthy pregnancies were presented in a report in the American Journal of Obstetrics & Gynecology.
One month after their pregnancy loss, 29% of these women met the criteria for posttraumatic stress, 24% reported moderate to severe anxiety, and 11% reported moderate to severe depression. In comparison, just 13% of women in the control group met the criteria for anxiety, and 2% met the criteria for depression, which meant women who had experienced early pregnancy loss had a greater than twofold odds of anxiety and nearly fourfold (odds ratio, 3.88) greater odds of depression, reported Jessica Farren, PhD, of the Queen Charlotte’s and Chelsea Hospital, London, and coauthors.
The most common posttraumatic symptom, experienced by 91% of respondents with posttraumatic stress at 1 month after the pregnancy, was reexperiencing symptoms, while 60% experienced avoidance and hyperarousal symptoms. At 3 months after the loss, 50% of those with posttraumatic stress reported an interruption of their general satisfaction with life.
While the incidence of posttraumatic stress, anxiety, and depression decreased over time in the women who had early pregnancy loss, by the third month 21% still met the criteria for posttraumatic stress, and by 9 months, 18% still were experiencing posttraumatic stress. Similarly, moderate to severe anxiety was still present in 23% of women at 3 months and 17% at 9 months, and moderate to severe depression was still experienced by 8% of women at 3 months and 6% of women at 9 months.
Dr. Farren and coauthors wrote that, given the incidence of miscarriage and ectopic pregnancy in the population, the high proportion of women still experiencing posttraumatic stress, anxiety, and depression at 9 months pointed to a significant public health issue. “It is recognized that PTSD in other contexts can have a significant impact on work, social interaction, health care utilization, and risks in future pregnancies,” they wrote. “Work is needed to evaluate strategies to effectively identify and treat affected women with these specific psychopathologies.”
The investigators also looked at the differences in outcomes in women who experienced miscarriage, compared with those who experienced ectopic pregnancy.
Of the 363 women who had a miscarriage, 30% met criteria for posttraumatic stress at 1 month, 20% at 3 months, and 17% at 9 months. Moderate to severe anxiety was reported by 25% women at 1 month, 22% at 3 months, and 17% at 9 months. Moderate to severe depression was reported by 12% at 1 month, 7% at 3 months, and 5% at 9 months.
Of the 74 women who had an ectopic pregnancy, 23% met criteria for posttraumatic stress at 1 month, 28% at 3 months, and 21% at 9 months. Moderate to severe anxiety was reported by 21% at 1 month, 30% at 3 months, and 23% at 9 months. Moderate to severe depression was reported by 7% at 1 month, 12% at 3 months, and 11% at 9 months.
The authors noted that the incidence of posttraumatic stress, anxiety, and depression decreased more strongly over time in women who had experienced miscarriage, compared with those who experienced ectopic pregnancy, although they commented that the confidence intervals were wide.
One coauthor was supported by an Imperial Health Charity grant and another by the National Institute for Health Research Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Farren J et al. Amer J Obstet Gynecol. 2019 Dec 13. doi: 10.1016/j.ajog.2019.10.102.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Hypertensive disorders of pregnancy in SLE contribute to later CV outcomes
Women with systemic lupus erythematosus (SLE) who experience hypertensive disorders of pregnancy may have a higher rate of cardiovascular outcomes after pregnancy, as well as a higher rate of hypertension later in life, than do those without maternal hypertension, according to findings from a Swedish population-based, longitudinal cohort study.
“Premature CVD [cardiovascular disease] is a well-documented complication in women with SLE, which is likely, at least in part, due to renal disease, prothrombotic [antiphospholipid antibodies], and systemic inflammation. Our data confirm that women who experience a hypertensive disorder in pregnancy [HDP] are at greater risk of developing hypertension after pregnancy, and that this association is also evident for women with SLE. Women with SLE and HDP were also at increased risk of CVD, particularly stroke, at young ages and should be monitored closely and consider treatment to attenuate risk,” wrote first author Julia F. Simard, ScD, of Stanford (Calif.) University and colleagues in Arthritis Care & Research.
To reach those conclusions, the researchers identified 3,340 women in the Swedish Medical Birth Register with their first singleton delivery during 1987-2012. They matched each of the 450 women with prevalent SLE from the Medical Birth Register to 5 women without SLE in the National Patient Register based on sex, birth year, calendar time, and county of residence.
During a median follow-up period of nearly 11 years, women with SLE had an unadjusted incidence rate of incident cardiovascular outcomes of 50 cases per 10,000 person-years versus 7.2 for women without SLE. Cardiovascular outcomes included fatal and nonfatal acute MI, fatal and nonfatal stroke, transient ischemic attacks, unstable angina, and heart failure. A history of HDP in women with SLE, including preeclampsia, was linked with about a twofold higher rate of cardiovascular outcomes regardless of multiple sensitivity analyses, both before and after adjusting for maternal age at delivery, county of birth, education, body mass index, and first-trimester smoking.
The researchers found that the hazard ratio for cardiovascular outcomes in women with SLE and HDP was about eight times higher than the hazard ratio for women without SLE but with HDP, but the relative rarity of cardiovascular events seen during the follow-up period, particularly among women without SLE, made it so that they “could not confirm established associations between HDP and CVD, possibly due to the relatively short follow-up time given that premenopausal CVD is rare among women free of SLE.”
HDP was associated with a threefold higher risk for incident hypertension later in life regardless of SLE status, even though the unadjusted incidence rate was 524 cases per 10,000 person-years among women with both SLE and HDP, compared with 177 per 10,000 person-years among women with HDP in the general population, which sensitivity analyses suggested “was not due to misclassification of antihypertensive use for renal disease in women with SLE nor antihypertensive use for possible HDP in subsequent pregnancies,” the researchers wrote.
Several authors reported research grants from the National Institutes of Health, the Karolinska Institute, the Swedish Research Council, Swedish Heart-Lung Foundation, Stockholm County Council, the King Gustaf V 80th Birthday Fund, the Swedish Rheumatism Association, and Ingegerd Johansson’s Foundation that helped to fund the study. All authors reported having no competing interests.
SOURCE: Simard JF et al. Arthritis Care Res. 2020 Jan 31. doi: 10.1002/acr.24160.
Women with systemic lupus erythematosus (SLE) who experience hypertensive disorders of pregnancy may have a higher rate of cardiovascular outcomes after pregnancy, as well as a higher rate of hypertension later in life, than do those without maternal hypertension, according to findings from a Swedish population-based, longitudinal cohort study.
“Premature CVD [cardiovascular disease] is a well-documented complication in women with SLE, which is likely, at least in part, due to renal disease, prothrombotic [antiphospholipid antibodies], and systemic inflammation. Our data confirm that women who experience a hypertensive disorder in pregnancy [HDP] are at greater risk of developing hypertension after pregnancy, and that this association is also evident for women with SLE. Women with SLE and HDP were also at increased risk of CVD, particularly stroke, at young ages and should be monitored closely and consider treatment to attenuate risk,” wrote first author Julia F. Simard, ScD, of Stanford (Calif.) University and colleagues in Arthritis Care & Research.
To reach those conclusions, the researchers identified 3,340 women in the Swedish Medical Birth Register with their first singleton delivery during 1987-2012. They matched each of the 450 women with prevalent SLE from the Medical Birth Register to 5 women without SLE in the National Patient Register based on sex, birth year, calendar time, and county of residence.
During a median follow-up period of nearly 11 years, women with SLE had an unadjusted incidence rate of incident cardiovascular outcomes of 50 cases per 10,000 person-years versus 7.2 for women without SLE. Cardiovascular outcomes included fatal and nonfatal acute MI, fatal and nonfatal stroke, transient ischemic attacks, unstable angina, and heart failure. A history of HDP in women with SLE, including preeclampsia, was linked with about a twofold higher rate of cardiovascular outcomes regardless of multiple sensitivity analyses, both before and after adjusting for maternal age at delivery, county of birth, education, body mass index, and first-trimester smoking.
The researchers found that the hazard ratio for cardiovascular outcomes in women with SLE and HDP was about eight times higher than the hazard ratio for women without SLE but with HDP, but the relative rarity of cardiovascular events seen during the follow-up period, particularly among women without SLE, made it so that they “could not confirm established associations between HDP and CVD, possibly due to the relatively short follow-up time given that premenopausal CVD is rare among women free of SLE.”
HDP was associated with a threefold higher risk for incident hypertension later in life regardless of SLE status, even though the unadjusted incidence rate was 524 cases per 10,000 person-years among women with both SLE and HDP, compared with 177 per 10,000 person-years among women with HDP in the general population, which sensitivity analyses suggested “was not due to misclassification of antihypertensive use for renal disease in women with SLE nor antihypertensive use for possible HDP in subsequent pregnancies,” the researchers wrote.
Several authors reported research grants from the National Institutes of Health, the Karolinska Institute, the Swedish Research Council, Swedish Heart-Lung Foundation, Stockholm County Council, the King Gustaf V 80th Birthday Fund, the Swedish Rheumatism Association, and Ingegerd Johansson’s Foundation that helped to fund the study. All authors reported having no competing interests.
SOURCE: Simard JF et al. Arthritis Care Res. 2020 Jan 31. doi: 10.1002/acr.24160.
Women with systemic lupus erythematosus (SLE) who experience hypertensive disorders of pregnancy may have a higher rate of cardiovascular outcomes after pregnancy, as well as a higher rate of hypertension later in life, than do those without maternal hypertension, according to findings from a Swedish population-based, longitudinal cohort study.
“Premature CVD [cardiovascular disease] is a well-documented complication in women with SLE, which is likely, at least in part, due to renal disease, prothrombotic [antiphospholipid antibodies], and systemic inflammation. Our data confirm that women who experience a hypertensive disorder in pregnancy [HDP] are at greater risk of developing hypertension after pregnancy, and that this association is also evident for women with SLE. Women with SLE and HDP were also at increased risk of CVD, particularly stroke, at young ages and should be monitored closely and consider treatment to attenuate risk,” wrote first author Julia F. Simard, ScD, of Stanford (Calif.) University and colleagues in Arthritis Care & Research.
To reach those conclusions, the researchers identified 3,340 women in the Swedish Medical Birth Register with their first singleton delivery during 1987-2012. They matched each of the 450 women with prevalent SLE from the Medical Birth Register to 5 women without SLE in the National Patient Register based on sex, birth year, calendar time, and county of residence.
During a median follow-up period of nearly 11 years, women with SLE had an unadjusted incidence rate of incident cardiovascular outcomes of 50 cases per 10,000 person-years versus 7.2 for women without SLE. Cardiovascular outcomes included fatal and nonfatal acute MI, fatal and nonfatal stroke, transient ischemic attacks, unstable angina, and heart failure. A history of HDP in women with SLE, including preeclampsia, was linked with about a twofold higher rate of cardiovascular outcomes regardless of multiple sensitivity analyses, both before and after adjusting for maternal age at delivery, county of birth, education, body mass index, and first-trimester smoking.
The researchers found that the hazard ratio for cardiovascular outcomes in women with SLE and HDP was about eight times higher than the hazard ratio for women without SLE but with HDP, but the relative rarity of cardiovascular events seen during the follow-up period, particularly among women without SLE, made it so that they “could not confirm established associations between HDP and CVD, possibly due to the relatively short follow-up time given that premenopausal CVD is rare among women free of SLE.”
HDP was associated with a threefold higher risk for incident hypertension later in life regardless of SLE status, even though the unadjusted incidence rate was 524 cases per 10,000 person-years among women with both SLE and HDP, compared with 177 per 10,000 person-years among women with HDP in the general population, which sensitivity analyses suggested “was not due to misclassification of antihypertensive use for renal disease in women with SLE nor antihypertensive use for possible HDP in subsequent pregnancies,” the researchers wrote.
Several authors reported research grants from the National Institutes of Health, the Karolinska Institute, the Swedish Research Council, Swedish Heart-Lung Foundation, Stockholm County Council, the King Gustaf V 80th Birthday Fund, the Swedish Rheumatism Association, and Ingegerd Johansson’s Foundation that helped to fund the study. All authors reported having no competing interests.
SOURCE: Simard JF et al. Arthritis Care Res. 2020 Jan 31. doi: 10.1002/acr.24160.
FROM ARTHRITIS CARE & RESEARCH
Gestational diabetes: Treatment controversy rages on
WASHINGTON – Pharmacologic treatment of gestational diabetes remains controversial, with the American College of Obstetricians and Gynecologists and the American Diabetes Association firmly recommending insulin as the preferred first-line pharmacologic therapy, and the Society of Maternal-Fetal Medicine more accepting of metformin as a “reasonable and safe first-line” alternative to insulin and stating that there are no strong data supporting metformin over the sulfonylurea glyburide.
If there’s one main take-away, Mark B. Landon, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America, it was that “the primary concern” about the use of oral agents for treating gestational diabetes mellitus (GDM) is that there is limited long-term follow-up of exposed offspring.
“The claim that long-term safety data are not available for any oral agent is probably the most valid warning [of any of the concerns voiced by professional organizations],” said Dr. Landon, Richard L. Meiling professor and chair of the department of obstetrics and gynecology at The Ohio State University Wexner Medical Center, Columbus.
Otherwise, he said, there are not enough data to firmly prioritize the drugs most commonly used for GDM, and “the superiority of insulin over oral agents simply remains questionable.”
ACOG’s 2017 level A recommendation for insulin as the first-line option when pharmacologic treatment is needed for treating GDM (Obstet Gynecol. 2017;130[1]:e17-37) was followed in 2018 by another updated practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64) that considered several meta-analyses published in 2017 and reiterated a preference for insulin.
Those recent meta-analyses of pharmacologic treatment of GDM show that the available literature is generally of “poor trial quality,” and that studies are small and not designed to assess equivalence or noninferiority, Mark Turrentine, MD, chair of ACOG’s committee on practice bulletins, said in an interview. “Taking that into account and [considering] that oral antidiabetic medications are not approved by the Food and Drug Administration [for the treatment of GDM], that they cross the placenta, and that we currently lack long-term neonatal safety data ... we felt that insulin is the preferred treatment.”
In its 2017 and 2018 bulletins, ACOG said that metformin is a “reasonable alternative choice” for women who decline insulin therapy or who may be unable to safely administer it (a level B recommendation). The 2018 practice bulletin mentions one additional factor: affordability. “Insurance companies aren’t always covering [insulin],” said Dr. Turrentine, of the department of obstetrics and gynecology, Baylor College of Medicine, Houston. “It’s a challenge – no question.”
ACOG says glyburide should not be recommended as a first-line pharmacologic treatment, “because, in most studies, it does not yield outcomes equivalent to insulin or metformin,” Dr. Turrentine emphasized.
Glyburide’s role
Dr. Landon took issue with ACOG’s stance on the sulfonylurea. “Frankly, I think this [conclusion] is debatable,” he said. The trend in the United States – “at least after the 2017 ACOG document came out”– has been toward use of metformin over glyburide when an oral agent is [used], but “I think glyburide has been unfairly trashed. It probably still has a place.”
As Dr. Landon sees it, research published in 2015 put a damper on the use of glyburide, which “had become the number one agent” after an earlier, seminal trial, led by Oded Langer, MD, had shown equivalent glycemic control in about 400 women with GDM who were randomized to receive either insulin or glyburide (N Engl J Med. 2000;343;1134-8). The trial was not powered to evaluate other outcomes, but there were no significant differences in neonatal complications, Dr. Landon said.
One of the 2015 studies – a large, retrospective, population-based study of more than 9,000 women with GDM treated with glyburide or insulin – showed a higher risk of admission to the neonatal intensive care unit (relative risk, 1.41), hypoglycemia in the newborn (RR, 1.40), and large-for-gestational age (RR, 1.43) with glyburide, compared with insulin (JAMA Pediatr. 2015;169[5]:452-8).
A meta-analysis of glyburide, metformin, and insulin showed significant differences between glyburide and insulin in birth weight, macrosomia (RR, 2.62), and neonatal hypoglycemia (RR, 2.04; BMJ. 2015;350;h102). However, “this was basically a conglomeration of studies with about 50 [individuals] in each arm, and in which entry criteria for the diagnosis of GDM were rather heterogeneous,” said Dr. Landon. “There are real problems with this and other meta-analyses.”
The authors of a 2018 multicenter, noninferiority, randomized, controlled trial of about 900 women concluded that their study failed to show that the use of glyburide, compared with insulin, does not result in a greater frequency of perinatal complications. The authors also wrote, however, that the “increase in perinatal complications [with glyburide] may be no more than 10.5%, compared with insulin” (JAMA. 2018;319[17]:1773-80).
That increase, Dr. Landon said, was “not an absolute 10%, but 10% of the complication rate, which probably translates to about 2%.” The only component of a composite outcome (including macrosomia, hypoglycemia, and hyperbilirubinemia) that was significantly different, he noted, was hypoglycemia, which affected 12.2% of neonates in the glyburide group and 7.2% in the insulin group.
Glyburide’s role may well be substantiated in the future, Dr. Landon said during a discussion period at the meeting, through research underway at the University of Pittsburgh aimed at tailoring treatment to the underlying pathophysiology of a patient’s GDM.
The MATCh-GDM study (Metabolic Analysis for Treatment Choice in GDM) is randomizing women to receive usual, unmatched treatment or treatment matched to GDM mechanism – metformin for predominant insulin resistance, glyburide or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. The study’s principal investigator, Maisa Feghali, MD, of the department of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh, stressed in a presentation on the study that GDM is a heterogeneous condition and that research is needed to understand the impact of GDM subtypes on treatment response.
Metformin outcomes
Concerns about the impact of metformin on short-term perinatal outcomes focus on preterm birth, Dr. Landon said. The only study to date that has shown an increased rate of prematurity, however, is the “seminal” Metformin in Gestational Diabetes (MiG) trial led by Janet A. Rowan, MBChB, that randomized 751 women with GDM in Australia and New Zealand to treatment with metformin or insulin. The researchers found no significant differences between a composite of neonatal complications but did establish that severe hypoglycemia was less common in the metformin group and preterm birth was more common (N Engl J Med. 2008;358:2003-15).
A 2016 systematic review and meta-analysis of short- and long-term outcomes of metformin, compared with insulin, found that metformin did not increase preterm delivery (Diabet Med. 2017;34[1]:27-36). And while the 2015 BMJ meta-analysis found that metformin was associated with higher rates of preterm birth (RR, 1.50), the increased risk “was all driven by the Rowan study,” Dr. Landon said. The 2015 meta-analysis also found that metformin was associated with less maternal weight gain and fewer infants who were large for gestational age.
Metformin is also tainted by high rates of failure in GDM. In the 2008 Rowan study, 46% of patients on metformin failed to achieve glycemic control. “But this is a classic half-full, half-empty [phenomena],” Dr. Landon said. “Some people say this isn’t good, but on the other hand, 54% avoided insulin.”
Indeed, the Society of Maternal-Fetal Medicine (SMFM), in its 2018 statement on the pharmacologic treatment of GDM, said that oral hypoglycemic agents that are used as monotherapy work in “more than half” of GDM pregnancies. The need for adjunctive insulin to achieve glycemic control ranges between 26% and 46% for women using metformin, and 4% and 16% for women using glyburide, it says.
In the society’s view, recent meta-analyses and systemic reviews “support the efficacy and safety of oral agents,” and “although concerns have been raised for more frequent adverse neonatal outcomes with glyburide, including macrosomia and hypoglycemia, the evidence of benefit of one oral agent over the other remains limited.”
The society says that the difference between its statement and the ACOG recommendations is “based on the values placed by different experts and providers on the available evidence,” and it adds that more long-term data are needed.
But as Dr. Landon said, the SMFM is “a little more forgiving” in its interpretation of a limited body of literature. And clinicians, in the meantime, have to navigate the controversy. “The professional organizations don’t make it easy for [us],” he said. At this point, “insulin does not cross the placenta, and the oral agents do cross it. Informed consent is absolutely necessary when choosing oral agents for treating GDM.”
Offspring well-being
Of greater concern than neonatal outcomes are the potential long-term issues for offspring, Dr. Landon said. On the one hand, it is theorized that metformin may protect beta-cell function in offspring and thereby reduce the cross-generational effects of obesity and type 2 diabetes. On the other hand, it is theorized that the drug may cause a decrease in cell-cycle proliferation, which could have “unknown fetal programming effects,” and it may inhibit the mTOR signaling pathway, thus restricting the transport of glucose and amino acids across the placenta, he said. (Findings from in vitro research have suggested that glyburide treatment in GDM might be associated with enhanced transport across the placenta, he noted.)
Long-term follow-up studies of offspring are “clearly needed,” Dr. Landon said. At this point, in regard to long-term safety, he and other experts are concerned primarily about the potential for obesity and metabolic dysfunction in offspring who are exposed to metformin in utero. They are watching follow-up from Dr. Rowan’s MiG trial, as well as elsewhere in the literature, on metformin-exposed offspring from mothers with polycystic ovary syndrome.
A follow-up analysis of offspring from the MiG trial found that children of women with GDM who were exposed to metformin had larger measures of subcutaneous fat at age 2 years, compared with children of mothers treated with insulin alone, but that overall body fat was the same, Dr. Landon noted. The investigators postulated that these children may have less visceral fat and a more favorable pattern of fat distribution (Diab Care. 2011;34:2279-84).
A recently published follow-up analysis of two randomized, controlled trials of women with polycystic ovary syndrome is cause for more concern, he said. That analysis showed that offspring exposed to metformin in utero had a higher body mass index and an increased prevalence of obesity or overweight at age 4 years, compared with placebo groups (J Clin Endocrinol Metab. 2018;103[4]:1612-21).
That analysis of metformin-exposed offspring in the context of polycystic ovary syndrome was published after the SMFM statement, as was another follow-up analysis of MiG trial offspring – this one, at ages 7-9 years – that showed an increase in weight, size, and fat mass in one of two subsets analyzed, despite no difference in large-for-gestational age rates between the metformin- and insulin-exposed offspring (BMJ Open Diabetes Res Care. 2018;6[1]: e000456).
In 2018, a group of 17 prominent diabetes and maternal-fetal medicine researchers cited these findings in a response to the SMFM statement and cautioned against the widespread adoption of metformin use during pregnancy, writing that, based on “both pharmacologic and randomized trial evidence that metformin may create an atypical intrauterine environment ... we believe it is premature to embrace metformin as equivalent to insulin or as superior to glyburide, and that patients should be counseled on the limited long-term safety data and potential for adverse childhood metabolic effects” (Am J Obstet Gynecol. 2018;219[4]:367.e1-7).
WASHINGTON – Pharmacologic treatment of gestational diabetes remains controversial, with the American College of Obstetricians and Gynecologists and the American Diabetes Association firmly recommending insulin as the preferred first-line pharmacologic therapy, and the Society of Maternal-Fetal Medicine more accepting of metformin as a “reasonable and safe first-line” alternative to insulin and stating that there are no strong data supporting metformin over the sulfonylurea glyburide.
If there’s one main take-away, Mark B. Landon, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America, it was that “the primary concern” about the use of oral agents for treating gestational diabetes mellitus (GDM) is that there is limited long-term follow-up of exposed offspring.
“The claim that long-term safety data are not available for any oral agent is probably the most valid warning [of any of the concerns voiced by professional organizations],” said Dr. Landon, Richard L. Meiling professor and chair of the department of obstetrics and gynecology at The Ohio State University Wexner Medical Center, Columbus.
Otherwise, he said, there are not enough data to firmly prioritize the drugs most commonly used for GDM, and “the superiority of insulin over oral agents simply remains questionable.”
ACOG’s 2017 level A recommendation for insulin as the first-line option when pharmacologic treatment is needed for treating GDM (Obstet Gynecol. 2017;130[1]:e17-37) was followed in 2018 by another updated practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64) that considered several meta-analyses published in 2017 and reiterated a preference for insulin.
Those recent meta-analyses of pharmacologic treatment of GDM show that the available literature is generally of “poor trial quality,” and that studies are small and not designed to assess equivalence or noninferiority, Mark Turrentine, MD, chair of ACOG’s committee on practice bulletins, said in an interview. “Taking that into account and [considering] that oral antidiabetic medications are not approved by the Food and Drug Administration [for the treatment of GDM], that they cross the placenta, and that we currently lack long-term neonatal safety data ... we felt that insulin is the preferred treatment.”
In its 2017 and 2018 bulletins, ACOG said that metformin is a “reasonable alternative choice” for women who decline insulin therapy or who may be unable to safely administer it (a level B recommendation). The 2018 practice bulletin mentions one additional factor: affordability. “Insurance companies aren’t always covering [insulin],” said Dr. Turrentine, of the department of obstetrics and gynecology, Baylor College of Medicine, Houston. “It’s a challenge – no question.”
ACOG says glyburide should not be recommended as a first-line pharmacologic treatment, “because, in most studies, it does not yield outcomes equivalent to insulin or metformin,” Dr. Turrentine emphasized.
Glyburide’s role
Dr. Landon took issue with ACOG’s stance on the sulfonylurea. “Frankly, I think this [conclusion] is debatable,” he said. The trend in the United States – “at least after the 2017 ACOG document came out”– has been toward use of metformin over glyburide when an oral agent is [used], but “I think glyburide has been unfairly trashed. It probably still has a place.”
As Dr. Landon sees it, research published in 2015 put a damper on the use of glyburide, which “had become the number one agent” after an earlier, seminal trial, led by Oded Langer, MD, had shown equivalent glycemic control in about 400 women with GDM who were randomized to receive either insulin or glyburide (N Engl J Med. 2000;343;1134-8). The trial was not powered to evaluate other outcomes, but there were no significant differences in neonatal complications, Dr. Landon said.
One of the 2015 studies – a large, retrospective, population-based study of more than 9,000 women with GDM treated with glyburide or insulin – showed a higher risk of admission to the neonatal intensive care unit (relative risk, 1.41), hypoglycemia in the newborn (RR, 1.40), and large-for-gestational age (RR, 1.43) with glyburide, compared with insulin (JAMA Pediatr. 2015;169[5]:452-8).
A meta-analysis of glyburide, metformin, and insulin showed significant differences between glyburide and insulin in birth weight, macrosomia (RR, 2.62), and neonatal hypoglycemia (RR, 2.04; BMJ. 2015;350;h102). However, “this was basically a conglomeration of studies with about 50 [individuals] in each arm, and in which entry criteria for the diagnosis of GDM were rather heterogeneous,” said Dr. Landon. “There are real problems with this and other meta-analyses.”
The authors of a 2018 multicenter, noninferiority, randomized, controlled trial of about 900 women concluded that their study failed to show that the use of glyburide, compared with insulin, does not result in a greater frequency of perinatal complications. The authors also wrote, however, that the “increase in perinatal complications [with glyburide] may be no more than 10.5%, compared with insulin” (JAMA. 2018;319[17]:1773-80).
That increase, Dr. Landon said, was “not an absolute 10%, but 10% of the complication rate, which probably translates to about 2%.” The only component of a composite outcome (including macrosomia, hypoglycemia, and hyperbilirubinemia) that was significantly different, he noted, was hypoglycemia, which affected 12.2% of neonates in the glyburide group and 7.2% in the insulin group.
Glyburide’s role may well be substantiated in the future, Dr. Landon said during a discussion period at the meeting, through research underway at the University of Pittsburgh aimed at tailoring treatment to the underlying pathophysiology of a patient’s GDM.
The MATCh-GDM study (Metabolic Analysis for Treatment Choice in GDM) is randomizing women to receive usual, unmatched treatment or treatment matched to GDM mechanism – metformin for predominant insulin resistance, glyburide or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. The study’s principal investigator, Maisa Feghali, MD, of the department of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh, stressed in a presentation on the study that GDM is a heterogeneous condition and that research is needed to understand the impact of GDM subtypes on treatment response.
Metformin outcomes
Concerns about the impact of metformin on short-term perinatal outcomes focus on preterm birth, Dr. Landon said. The only study to date that has shown an increased rate of prematurity, however, is the “seminal” Metformin in Gestational Diabetes (MiG) trial led by Janet A. Rowan, MBChB, that randomized 751 women with GDM in Australia and New Zealand to treatment with metformin or insulin. The researchers found no significant differences between a composite of neonatal complications but did establish that severe hypoglycemia was less common in the metformin group and preterm birth was more common (N Engl J Med. 2008;358:2003-15).
A 2016 systematic review and meta-analysis of short- and long-term outcomes of metformin, compared with insulin, found that metformin did not increase preterm delivery (Diabet Med. 2017;34[1]:27-36). And while the 2015 BMJ meta-analysis found that metformin was associated with higher rates of preterm birth (RR, 1.50), the increased risk “was all driven by the Rowan study,” Dr. Landon said. The 2015 meta-analysis also found that metformin was associated with less maternal weight gain and fewer infants who were large for gestational age.
Metformin is also tainted by high rates of failure in GDM. In the 2008 Rowan study, 46% of patients on metformin failed to achieve glycemic control. “But this is a classic half-full, half-empty [phenomena],” Dr. Landon said. “Some people say this isn’t good, but on the other hand, 54% avoided insulin.”
Indeed, the Society of Maternal-Fetal Medicine (SMFM), in its 2018 statement on the pharmacologic treatment of GDM, said that oral hypoglycemic agents that are used as monotherapy work in “more than half” of GDM pregnancies. The need for adjunctive insulin to achieve glycemic control ranges between 26% and 46% for women using metformin, and 4% and 16% for women using glyburide, it says.
In the society’s view, recent meta-analyses and systemic reviews “support the efficacy and safety of oral agents,” and “although concerns have been raised for more frequent adverse neonatal outcomes with glyburide, including macrosomia and hypoglycemia, the evidence of benefit of one oral agent over the other remains limited.”
The society says that the difference between its statement and the ACOG recommendations is “based on the values placed by different experts and providers on the available evidence,” and it adds that more long-term data are needed.
But as Dr. Landon said, the SMFM is “a little more forgiving” in its interpretation of a limited body of literature. And clinicians, in the meantime, have to navigate the controversy. “The professional organizations don’t make it easy for [us],” he said. At this point, “insulin does not cross the placenta, and the oral agents do cross it. Informed consent is absolutely necessary when choosing oral agents for treating GDM.”
Offspring well-being
Of greater concern than neonatal outcomes are the potential long-term issues for offspring, Dr. Landon said. On the one hand, it is theorized that metformin may protect beta-cell function in offspring and thereby reduce the cross-generational effects of obesity and type 2 diabetes. On the other hand, it is theorized that the drug may cause a decrease in cell-cycle proliferation, which could have “unknown fetal programming effects,” and it may inhibit the mTOR signaling pathway, thus restricting the transport of glucose and amino acids across the placenta, he said. (Findings from in vitro research have suggested that glyburide treatment in GDM might be associated with enhanced transport across the placenta, he noted.)
Long-term follow-up studies of offspring are “clearly needed,” Dr. Landon said. At this point, in regard to long-term safety, he and other experts are concerned primarily about the potential for obesity and metabolic dysfunction in offspring who are exposed to metformin in utero. They are watching follow-up from Dr. Rowan’s MiG trial, as well as elsewhere in the literature, on metformin-exposed offspring from mothers with polycystic ovary syndrome.
A follow-up analysis of offspring from the MiG trial found that children of women with GDM who were exposed to metformin had larger measures of subcutaneous fat at age 2 years, compared with children of mothers treated with insulin alone, but that overall body fat was the same, Dr. Landon noted. The investigators postulated that these children may have less visceral fat and a more favorable pattern of fat distribution (Diab Care. 2011;34:2279-84).
A recently published follow-up analysis of two randomized, controlled trials of women with polycystic ovary syndrome is cause for more concern, he said. That analysis showed that offspring exposed to metformin in utero had a higher body mass index and an increased prevalence of obesity or overweight at age 4 years, compared with placebo groups (J Clin Endocrinol Metab. 2018;103[4]:1612-21).
That analysis of metformin-exposed offspring in the context of polycystic ovary syndrome was published after the SMFM statement, as was another follow-up analysis of MiG trial offspring – this one, at ages 7-9 years – that showed an increase in weight, size, and fat mass in one of two subsets analyzed, despite no difference in large-for-gestational age rates between the metformin- and insulin-exposed offspring (BMJ Open Diabetes Res Care. 2018;6[1]: e000456).
In 2018, a group of 17 prominent diabetes and maternal-fetal medicine researchers cited these findings in a response to the SMFM statement and cautioned against the widespread adoption of metformin use during pregnancy, writing that, based on “both pharmacologic and randomized trial evidence that metformin may create an atypical intrauterine environment ... we believe it is premature to embrace metformin as equivalent to insulin or as superior to glyburide, and that patients should be counseled on the limited long-term safety data and potential for adverse childhood metabolic effects” (Am J Obstet Gynecol. 2018;219[4]:367.e1-7).
WASHINGTON – Pharmacologic treatment of gestational diabetes remains controversial, with the American College of Obstetricians and Gynecologists and the American Diabetes Association firmly recommending insulin as the preferred first-line pharmacologic therapy, and the Society of Maternal-Fetal Medicine more accepting of metformin as a “reasonable and safe first-line” alternative to insulin and stating that there are no strong data supporting metformin over the sulfonylurea glyburide.
If there’s one main take-away, Mark B. Landon, MD, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America, it was that “the primary concern” about the use of oral agents for treating gestational diabetes mellitus (GDM) is that there is limited long-term follow-up of exposed offspring.
“The claim that long-term safety data are not available for any oral agent is probably the most valid warning [of any of the concerns voiced by professional organizations],” said Dr. Landon, Richard L. Meiling professor and chair of the department of obstetrics and gynecology at The Ohio State University Wexner Medical Center, Columbus.
Otherwise, he said, there are not enough data to firmly prioritize the drugs most commonly used for GDM, and “the superiority of insulin over oral agents simply remains questionable.”
ACOG’s 2017 level A recommendation for insulin as the first-line option when pharmacologic treatment is needed for treating GDM (Obstet Gynecol. 2017;130[1]:e17-37) was followed in 2018 by another updated practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64) that considered several meta-analyses published in 2017 and reiterated a preference for insulin.
Those recent meta-analyses of pharmacologic treatment of GDM show that the available literature is generally of “poor trial quality,” and that studies are small and not designed to assess equivalence or noninferiority, Mark Turrentine, MD, chair of ACOG’s committee on practice bulletins, said in an interview. “Taking that into account and [considering] that oral antidiabetic medications are not approved by the Food and Drug Administration [for the treatment of GDM], that they cross the placenta, and that we currently lack long-term neonatal safety data ... we felt that insulin is the preferred treatment.”
In its 2017 and 2018 bulletins, ACOG said that metformin is a “reasonable alternative choice” for women who decline insulin therapy or who may be unable to safely administer it (a level B recommendation). The 2018 practice bulletin mentions one additional factor: affordability. “Insurance companies aren’t always covering [insulin],” said Dr. Turrentine, of the department of obstetrics and gynecology, Baylor College of Medicine, Houston. “It’s a challenge – no question.”
ACOG says glyburide should not be recommended as a first-line pharmacologic treatment, “because, in most studies, it does not yield outcomes equivalent to insulin or metformin,” Dr. Turrentine emphasized.
Glyburide’s role
Dr. Landon took issue with ACOG’s stance on the sulfonylurea. “Frankly, I think this [conclusion] is debatable,” he said. The trend in the United States – “at least after the 2017 ACOG document came out”– has been toward use of metformin over glyburide when an oral agent is [used], but “I think glyburide has been unfairly trashed. It probably still has a place.”
As Dr. Landon sees it, research published in 2015 put a damper on the use of glyburide, which “had become the number one agent” after an earlier, seminal trial, led by Oded Langer, MD, had shown equivalent glycemic control in about 400 women with GDM who were randomized to receive either insulin or glyburide (N Engl J Med. 2000;343;1134-8). The trial was not powered to evaluate other outcomes, but there were no significant differences in neonatal complications, Dr. Landon said.
One of the 2015 studies – a large, retrospective, population-based study of more than 9,000 women with GDM treated with glyburide or insulin – showed a higher risk of admission to the neonatal intensive care unit (relative risk, 1.41), hypoglycemia in the newborn (RR, 1.40), and large-for-gestational age (RR, 1.43) with glyburide, compared with insulin (JAMA Pediatr. 2015;169[5]:452-8).
A meta-analysis of glyburide, metformin, and insulin showed significant differences between glyburide and insulin in birth weight, macrosomia (RR, 2.62), and neonatal hypoglycemia (RR, 2.04; BMJ. 2015;350;h102). However, “this was basically a conglomeration of studies with about 50 [individuals] in each arm, and in which entry criteria for the diagnosis of GDM were rather heterogeneous,” said Dr. Landon. “There are real problems with this and other meta-analyses.”
The authors of a 2018 multicenter, noninferiority, randomized, controlled trial of about 900 women concluded that their study failed to show that the use of glyburide, compared with insulin, does not result in a greater frequency of perinatal complications. The authors also wrote, however, that the “increase in perinatal complications [with glyburide] may be no more than 10.5%, compared with insulin” (JAMA. 2018;319[17]:1773-80).
That increase, Dr. Landon said, was “not an absolute 10%, but 10% of the complication rate, which probably translates to about 2%.” The only component of a composite outcome (including macrosomia, hypoglycemia, and hyperbilirubinemia) that was significantly different, he noted, was hypoglycemia, which affected 12.2% of neonates in the glyburide group and 7.2% in the insulin group.
Glyburide’s role may well be substantiated in the future, Dr. Landon said during a discussion period at the meeting, through research underway at the University of Pittsburgh aimed at tailoring treatment to the underlying pathophysiology of a patient’s GDM.
The MATCh-GDM study (Metabolic Analysis for Treatment Choice in GDM) is randomizing women to receive usual, unmatched treatment or treatment matched to GDM mechanism – metformin for predominant insulin resistance, glyburide or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. The study’s principal investigator, Maisa Feghali, MD, of the department of obstetrics, gynecology, and reproductive sciences at the University of Pittsburgh, stressed in a presentation on the study that GDM is a heterogeneous condition and that research is needed to understand the impact of GDM subtypes on treatment response.
Metformin outcomes
Concerns about the impact of metformin on short-term perinatal outcomes focus on preterm birth, Dr. Landon said. The only study to date that has shown an increased rate of prematurity, however, is the “seminal” Metformin in Gestational Diabetes (MiG) trial led by Janet A. Rowan, MBChB, that randomized 751 women with GDM in Australia and New Zealand to treatment with metformin or insulin. The researchers found no significant differences between a composite of neonatal complications but did establish that severe hypoglycemia was less common in the metformin group and preterm birth was more common (N Engl J Med. 2008;358:2003-15).
A 2016 systematic review and meta-analysis of short- and long-term outcomes of metformin, compared with insulin, found that metformin did not increase preterm delivery (Diabet Med. 2017;34[1]:27-36). And while the 2015 BMJ meta-analysis found that metformin was associated with higher rates of preterm birth (RR, 1.50), the increased risk “was all driven by the Rowan study,” Dr. Landon said. The 2015 meta-analysis also found that metformin was associated with less maternal weight gain and fewer infants who were large for gestational age.
Metformin is also tainted by high rates of failure in GDM. In the 2008 Rowan study, 46% of patients on metformin failed to achieve glycemic control. “But this is a classic half-full, half-empty [phenomena],” Dr. Landon said. “Some people say this isn’t good, but on the other hand, 54% avoided insulin.”
Indeed, the Society of Maternal-Fetal Medicine (SMFM), in its 2018 statement on the pharmacologic treatment of GDM, said that oral hypoglycemic agents that are used as monotherapy work in “more than half” of GDM pregnancies. The need for adjunctive insulin to achieve glycemic control ranges between 26% and 46% for women using metformin, and 4% and 16% for women using glyburide, it says.
In the society’s view, recent meta-analyses and systemic reviews “support the efficacy and safety of oral agents,” and “although concerns have been raised for more frequent adverse neonatal outcomes with glyburide, including macrosomia and hypoglycemia, the evidence of benefit of one oral agent over the other remains limited.”
The society says that the difference between its statement and the ACOG recommendations is “based on the values placed by different experts and providers on the available evidence,” and it adds that more long-term data are needed.
But as Dr. Landon said, the SMFM is “a little more forgiving” in its interpretation of a limited body of literature. And clinicians, in the meantime, have to navigate the controversy. “The professional organizations don’t make it easy for [us],” he said. At this point, “insulin does not cross the placenta, and the oral agents do cross it. Informed consent is absolutely necessary when choosing oral agents for treating GDM.”
Offspring well-being
Of greater concern than neonatal outcomes are the potential long-term issues for offspring, Dr. Landon said. On the one hand, it is theorized that metformin may protect beta-cell function in offspring and thereby reduce the cross-generational effects of obesity and type 2 diabetes. On the other hand, it is theorized that the drug may cause a decrease in cell-cycle proliferation, which could have “unknown fetal programming effects,” and it may inhibit the mTOR signaling pathway, thus restricting the transport of glucose and amino acids across the placenta, he said. (Findings from in vitro research have suggested that glyburide treatment in GDM might be associated with enhanced transport across the placenta, he noted.)
Long-term follow-up studies of offspring are “clearly needed,” Dr. Landon said. At this point, in regard to long-term safety, he and other experts are concerned primarily about the potential for obesity and metabolic dysfunction in offspring who are exposed to metformin in utero. They are watching follow-up from Dr. Rowan’s MiG trial, as well as elsewhere in the literature, on metformin-exposed offspring from mothers with polycystic ovary syndrome.
A follow-up analysis of offspring from the MiG trial found that children of women with GDM who were exposed to metformin had larger measures of subcutaneous fat at age 2 years, compared with children of mothers treated with insulin alone, but that overall body fat was the same, Dr. Landon noted. The investigators postulated that these children may have less visceral fat and a more favorable pattern of fat distribution (Diab Care. 2011;34:2279-84).
A recently published follow-up analysis of two randomized, controlled trials of women with polycystic ovary syndrome is cause for more concern, he said. That analysis showed that offspring exposed to metformin in utero had a higher body mass index and an increased prevalence of obesity or overweight at age 4 years, compared with placebo groups (J Clin Endocrinol Metab. 2018;103[4]:1612-21).
That analysis of metformin-exposed offspring in the context of polycystic ovary syndrome was published after the SMFM statement, as was another follow-up analysis of MiG trial offspring – this one, at ages 7-9 years – that showed an increase in weight, size, and fat mass in one of two subsets analyzed, despite no difference in large-for-gestational age rates between the metformin- and insulin-exposed offspring (BMJ Open Diabetes Res Care. 2018;6[1]: e000456).
In 2018, a group of 17 prominent diabetes and maternal-fetal medicine researchers cited these findings in a response to the SMFM statement and cautioned against the widespread adoption of metformin use during pregnancy, writing that, based on “both pharmacologic and randomized trial evidence that metformin may create an atypical intrauterine environment ... we believe it is premature to embrace metformin as equivalent to insulin or as superior to glyburide, and that patients should be counseled on the limited long-term safety data and potential for adverse childhood metabolic effects” (Am J Obstet Gynecol. 2018;219[4]:367.e1-7).
EXPERT ANALYSIS FROM DPSG-NA 2019
Understanding postpartum psychosis: From course to treatment
Although the last decade has brought appropriate increased interest in the diagnosis and treatment of postpartum depression, with screening initiatives across more than 40 states in place and even new medications being brought to market for treatment, far less attention has been given to diagnosis and treatment of a particularly serious psychiatric illness: postpartum psychosis.
Clinically, women can experience rapid mood changes, most often with the presentation that is consistent with a manic-like psychosis, with associated symptoms of delusional thinking, hallucinations, paranoia and either depression or elation, or an amalgam of these so-called “mixed symptoms.” Onset of symptoms typically is early, within 72 hours as is classically described, but may have a somewhat later time of onset in some women.
Many investigators have studied risk factors for postpartum psychosis, and it has been well established that a history of mood disorder, particularly bipolar disorder, is one of the strongest predictors of risk for postpartum psychosis. Women with histories of postpartum psychosis are at very high risk of recurrence, with as many as 70%-90% of women experiencing recurrence if not prophylaxed with an appropriate agent. From a clinical point of view, women with postpartum psychosis typically are hospitalized, given that this is both a psychiatric and potential obstetrical emergency. In fact, the data would suggest that although postpartum suicide and infanticide are not common, they can be a tragic concomitant of postpartum psychosis (Am J Psychiatry. 2016 Dec 1;173[12]:1179-88).
A great amount of interest has been placed on the etiology of postpartum psychosis, as it’s a dramatic presentation with very rapid onset in the acute postpartum period. A rich evidence base with respect to an algorithm of treatment that maximizes likelihood of full recovery or sustaining of euthymia after recovery is limited. Few studies have looked systematically at the optimum way to treat postpartum psychosis. Clinical wisdom has dictated that, given the dramatic symptoms with which these patients present, most patients are treated with lithium and an antipsychotic medication as if they have a manic-like psychosis. It may take brief or extended periods of time for patients to stabilize. Once they are stabilized, one of the most challenging questions for clinicians is how long to treat. Again, an evidence base clearly informing this question is lacking.
Over the years, many clinicians have treated patients with postpartum psychosis as if they have bipolar disorder, given the index presentation of the illness, so some of these patients are treated with antimanic drugs indefinitely. However, clinical experience from several centers that treat women with postpartum psychosis suggests that in remitted patients, a proportion of them may be able to taper and discontinue treatment, then sustain well-being for protracted periods.
One obstacle with respect to treatment of postpartum psychosis derives from the short length of stay after delivery for many women. Some women who present with symptoms of postpartum psychosis in the first 24-48 hours frequently are managed with direct admission to an inpatient psychiatric service. But others may not develop symptoms until they are home, which may place both mother and newborn at risk.
Given that the risk for recurrent postpartum psychosis is so great (70%-90%), women with histories of postpartum psychosis invariably are prophylaxed with mood stabilizer prior to delivery in a subsequent pregnancy. In our own center, we have published on the value of such prophylactic intervention, not just in women with postpartum psychosis, but in women with bipolar disorder, who are, as noted, at great risk for developing postpartum psychotic symptoms (Am J Psychiatry. 1995 Nov;152[11]:1641-5.)
Although postpartum psychosis may be rare, over the last 3 decades we have seen a substantial number of women with postpartum psychosis and have been fascinated with the spectrum of symptoms with which some women with postpartum psychotic illness present. We also have been impressed with the time required for some women to recompensate from their illness and the course of their disorder after they have seemingly remitted. Some women appear to be able to discontinue treatment as noted above; others, particularly if there is any history of bipolar disorder, need to be maintained on treatment with mood stabilizer indefinitely.
To better understand the phenomenology of postpartum psychosis, as well as the longitudinal course of the illness, in 2019, the Mass General Hospital Postpartum Psychosis Project (MGHP3) was established. The project is conducted as a hospital-based registry where women with histories of postpartum psychosis over the last decade are invited to participate in an in-depth interview to understand both symptoms and course of underlying illness. This is complemented by obtaining a sample of saliva, which is used for genetic testing to try to identify a genetic underpinning associated with postpartum psychosis, as the question of genetic etiology of postpartum psychosis is still an open one.
As part of the MGHP3 project, clinicians across the country are able to contact perinatal psychiatrists in our center with expertise in the treatment of postpartum psychosis. Our psychiatrists also can counsel clinicians on issues regarding long-term management of postpartum psychosis because for many, knowledge of precisely how to manage this disorder or the follow-up treatment may be incomplete.
From a clinical point of view, the relevant questions really include not only acute treatment, which has already been outlined, but also the issue of duration of treatment. While some patients may be able to taper and discontinue treatment after, for example, a year of being totally well, to date we are unable to know who those patients are. We tend to be more conservative in our own center and treat patients with puerperal psychosis for a more protracted period of time, usually over several years. We also ask women about their family history of bipolar disorder or postpartum psychosis. Depending on the clinical course (if the patient really has sustained euthymia), we consider slow taper and ultimate discontinuation. As always, treatment decisions are tailored to individual clinical history, course, and patient wishes.
Postpartum psychosis remains one of the most serious illnesses that we find in reproductive psychiatry, and incomplete attention has been given to this devastating illness, which we read about periodically in newspapers and magazines. Greater understanding of postpartum psychosis will lead to a more precision-like psychiatric approach, tailoring treatment to the invariable heterogeneity of this illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Although the last decade has brought appropriate increased interest in the diagnosis and treatment of postpartum depression, with screening initiatives across more than 40 states in place and even new medications being brought to market for treatment, far less attention has been given to diagnosis and treatment of a particularly serious psychiatric illness: postpartum psychosis.
Clinically, women can experience rapid mood changes, most often with the presentation that is consistent with a manic-like psychosis, with associated symptoms of delusional thinking, hallucinations, paranoia and either depression or elation, or an amalgam of these so-called “mixed symptoms.” Onset of symptoms typically is early, within 72 hours as is classically described, but may have a somewhat later time of onset in some women.
Many investigators have studied risk factors for postpartum psychosis, and it has been well established that a history of mood disorder, particularly bipolar disorder, is one of the strongest predictors of risk for postpartum psychosis. Women with histories of postpartum psychosis are at very high risk of recurrence, with as many as 70%-90% of women experiencing recurrence if not prophylaxed with an appropriate agent. From a clinical point of view, women with postpartum psychosis typically are hospitalized, given that this is both a psychiatric and potential obstetrical emergency. In fact, the data would suggest that although postpartum suicide and infanticide are not common, they can be a tragic concomitant of postpartum psychosis (Am J Psychiatry. 2016 Dec 1;173[12]:1179-88).
A great amount of interest has been placed on the etiology of postpartum psychosis, as it’s a dramatic presentation with very rapid onset in the acute postpartum period. A rich evidence base with respect to an algorithm of treatment that maximizes likelihood of full recovery or sustaining of euthymia after recovery is limited. Few studies have looked systematically at the optimum way to treat postpartum psychosis. Clinical wisdom has dictated that, given the dramatic symptoms with which these patients present, most patients are treated with lithium and an antipsychotic medication as if they have a manic-like psychosis. It may take brief or extended periods of time for patients to stabilize. Once they are stabilized, one of the most challenging questions for clinicians is how long to treat. Again, an evidence base clearly informing this question is lacking.
Over the years, many clinicians have treated patients with postpartum psychosis as if they have bipolar disorder, given the index presentation of the illness, so some of these patients are treated with antimanic drugs indefinitely. However, clinical experience from several centers that treat women with postpartum psychosis suggests that in remitted patients, a proportion of them may be able to taper and discontinue treatment, then sustain well-being for protracted periods.
One obstacle with respect to treatment of postpartum psychosis derives from the short length of stay after delivery for many women. Some women who present with symptoms of postpartum psychosis in the first 24-48 hours frequently are managed with direct admission to an inpatient psychiatric service. But others may not develop symptoms until they are home, which may place both mother and newborn at risk.
Given that the risk for recurrent postpartum psychosis is so great (70%-90%), women with histories of postpartum psychosis invariably are prophylaxed with mood stabilizer prior to delivery in a subsequent pregnancy. In our own center, we have published on the value of such prophylactic intervention, not just in women with postpartum psychosis, but in women with bipolar disorder, who are, as noted, at great risk for developing postpartum psychotic symptoms (Am J Psychiatry. 1995 Nov;152[11]:1641-5.)
Although postpartum psychosis may be rare, over the last 3 decades we have seen a substantial number of women with postpartum psychosis and have been fascinated with the spectrum of symptoms with which some women with postpartum psychotic illness present. We also have been impressed with the time required for some women to recompensate from their illness and the course of their disorder after they have seemingly remitted. Some women appear to be able to discontinue treatment as noted above; others, particularly if there is any history of bipolar disorder, need to be maintained on treatment with mood stabilizer indefinitely.
To better understand the phenomenology of postpartum psychosis, as well as the longitudinal course of the illness, in 2019, the Mass General Hospital Postpartum Psychosis Project (MGHP3) was established. The project is conducted as a hospital-based registry where women with histories of postpartum psychosis over the last decade are invited to participate in an in-depth interview to understand both symptoms and course of underlying illness. This is complemented by obtaining a sample of saliva, which is used for genetic testing to try to identify a genetic underpinning associated with postpartum psychosis, as the question of genetic etiology of postpartum psychosis is still an open one.
As part of the MGHP3 project, clinicians across the country are able to contact perinatal psychiatrists in our center with expertise in the treatment of postpartum psychosis. Our psychiatrists also can counsel clinicians on issues regarding long-term management of postpartum psychosis because for many, knowledge of precisely how to manage this disorder or the follow-up treatment may be incomplete.
From a clinical point of view, the relevant questions really include not only acute treatment, which has already been outlined, but also the issue of duration of treatment. While some patients may be able to taper and discontinue treatment after, for example, a year of being totally well, to date we are unable to know who those patients are. We tend to be more conservative in our own center and treat patients with puerperal psychosis for a more protracted period of time, usually over several years. We also ask women about their family history of bipolar disorder or postpartum psychosis. Depending on the clinical course (if the patient really has sustained euthymia), we consider slow taper and ultimate discontinuation. As always, treatment decisions are tailored to individual clinical history, course, and patient wishes.
Postpartum psychosis remains one of the most serious illnesses that we find in reproductive psychiatry, and incomplete attention has been given to this devastating illness, which we read about periodically in newspapers and magazines. Greater understanding of postpartum psychosis will lead to a more precision-like psychiatric approach, tailoring treatment to the invariable heterogeneity of this illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Although the last decade has brought appropriate increased interest in the diagnosis and treatment of postpartum depression, with screening initiatives across more than 40 states in place and even new medications being brought to market for treatment, far less attention has been given to diagnosis and treatment of a particularly serious psychiatric illness: postpartum psychosis.
Clinically, women can experience rapid mood changes, most often with the presentation that is consistent with a manic-like psychosis, with associated symptoms of delusional thinking, hallucinations, paranoia and either depression or elation, or an amalgam of these so-called “mixed symptoms.” Onset of symptoms typically is early, within 72 hours as is classically described, but may have a somewhat later time of onset in some women.
Many investigators have studied risk factors for postpartum psychosis, and it has been well established that a history of mood disorder, particularly bipolar disorder, is one of the strongest predictors of risk for postpartum psychosis. Women with histories of postpartum psychosis are at very high risk of recurrence, with as many as 70%-90% of women experiencing recurrence if not prophylaxed with an appropriate agent. From a clinical point of view, women with postpartum psychosis typically are hospitalized, given that this is both a psychiatric and potential obstetrical emergency. In fact, the data would suggest that although postpartum suicide and infanticide are not common, they can be a tragic concomitant of postpartum psychosis (Am J Psychiatry. 2016 Dec 1;173[12]:1179-88).
A great amount of interest has been placed on the etiology of postpartum psychosis, as it’s a dramatic presentation with very rapid onset in the acute postpartum period. A rich evidence base with respect to an algorithm of treatment that maximizes likelihood of full recovery or sustaining of euthymia after recovery is limited. Few studies have looked systematically at the optimum way to treat postpartum psychosis. Clinical wisdom has dictated that, given the dramatic symptoms with which these patients present, most patients are treated with lithium and an antipsychotic medication as if they have a manic-like psychosis. It may take brief or extended periods of time for patients to stabilize. Once they are stabilized, one of the most challenging questions for clinicians is how long to treat. Again, an evidence base clearly informing this question is lacking.
Over the years, many clinicians have treated patients with postpartum psychosis as if they have bipolar disorder, given the index presentation of the illness, so some of these patients are treated with antimanic drugs indefinitely. However, clinical experience from several centers that treat women with postpartum psychosis suggests that in remitted patients, a proportion of them may be able to taper and discontinue treatment, then sustain well-being for protracted periods.
One obstacle with respect to treatment of postpartum psychosis derives from the short length of stay after delivery for many women. Some women who present with symptoms of postpartum psychosis in the first 24-48 hours frequently are managed with direct admission to an inpatient psychiatric service. But others may not develop symptoms until they are home, which may place both mother and newborn at risk.
Given that the risk for recurrent postpartum psychosis is so great (70%-90%), women with histories of postpartum psychosis invariably are prophylaxed with mood stabilizer prior to delivery in a subsequent pregnancy. In our own center, we have published on the value of such prophylactic intervention, not just in women with postpartum psychosis, but in women with bipolar disorder, who are, as noted, at great risk for developing postpartum psychotic symptoms (Am J Psychiatry. 1995 Nov;152[11]:1641-5.)
Although postpartum psychosis may be rare, over the last 3 decades we have seen a substantial number of women with postpartum psychosis and have been fascinated with the spectrum of symptoms with which some women with postpartum psychotic illness present. We also have been impressed with the time required for some women to recompensate from their illness and the course of their disorder after they have seemingly remitted. Some women appear to be able to discontinue treatment as noted above; others, particularly if there is any history of bipolar disorder, need to be maintained on treatment with mood stabilizer indefinitely.
To better understand the phenomenology of postpartum psychosis, as well as the longitudinal course of the illness, in 2019, the Mass General Hospital Postpartum Psychosis Project (MGHP3) was established. The project is conducted as a hospital-based registry where women with histories of postpartum psychosis over the last decade are invited to participate in an in-depth interview to understand both symptoms and course of underlying illness. This is complemented by obtaining a sample of saliva, which is used for genetic testing to try to identify a genetic underpinning associated with postpartum psychosis, as the question of genetic etiology of postpartum psychosis is still an open one.
As part of the MGHP3 project, clinicians across the country are able to contact perinatal psychiatrists in our center with expertise in the treatment of postpartum psychosis. Our psychiatrists also can counsel clinicians on issues regarding long-term management of postpartum psychosis because for many, knowledge of precisely how to manage this disorder or the follow-up treatment may be incomplete.
From a clinical point of view, the relevant questions really include not only acute treatment, which has already been outlined, but also the issue of duration of treatment. While some patients may be able to taper and discontinue treatment after, for example, a year of being totally well, to date we are unable to know who those patients are. We tend to be more conservative in our own center and treat patients with puerperal psychosis for a more protracted period of time, usually over several years. We also ask women about their family history of bipolar disorder or postpartum psychosis. Depending on the clinical course (if the patient really has sustained euthymia), we consider slow taper and ultimate discontinuation. As always, treatment decisions are tailored to individual clinical history, course, and patient wishes.
Postpartum psychosis remains one of the most serious illnesses that we find in reproductive psychiatry, and incomplete attention has been given to this devastating illness, which we read about periodically in newspapers and magazines. Greater understanding of postpartum psychosis will lead to a more precision-like psychiatric approach, tailoring treatment to the invariable heterogeneity of this illness.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].