User login
How advances in genomics have informed obstetrics practice
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Genetic screening and diagnosis: Key advancements and the role of genetic counseling
Preconception and prenatal genetic screening and diagnostic testing for genetic disorders are increasingly complex, with a burgeoning number of testing options and a shift in screening from situations identified as high-risk to more universal considerations. The American College of Obstetricians and Gynecologists now recommends that all patients – regardless of age or risk for chromosomal abnormalities – be offered both screening and diagnostic tests and counseled about the relative benefits and limitations of available tests. These recommendations represent a sea change for obstetrics.
Screening options now include expanded carrier screening that evaluates an individual’s carrier status for multiple conditions at once, regardless of ethnicity, and cell-free DNA screening using fetal DNA found in the maternal circulation. Chromosomal microarray analysis from a chorionic villus sampling or amniocentesis specimen detects tiny copy number variants, and increasingly detailed ultrasound images illuminate anatomic and physiologic anomalies that could not be seen or interpreted as recently as 5 years ago.
These advancements are remarkable, but they require attentive, personalized pre- and posttest genetic counseling. Genetic counselors are critical to this process, helping women and families understand and select screening tools, interpret test results, select diagnostic panels, and make decisions about invasive testing.
Counseling is essential as we seek and utilize genetic information that is no longer binary. It used to be that predictions of normality and abnormality were made with little gray area in between. – and genetic diagnosis is increasingly a lattice of details, variable expression, and even effects timing.
Expanded carrier screening
Carrier screening to determine if one or both parents are carriers for an autosomal recessive condition has historically involved a limited number of conditions chosen based on ethnicity. However, research has demonstrated the unreliability of this approach in our multicultural, multiracial society, in which many of our patients have mixed or uncertain race and ethnicity.
Expanded carrier screening is nondirective and takes ethnic background out of the equation. ACOG has moved from advocating ethnic-based screening alone to advising that both ethnic and expanded carrier screening are acceptable strategies and that practices should choose a standard approach to offer and discuss with each patient. (Carrier screening for cystic fibrosis and spinal muscular atrophy are recommended for all patients regardless of ethnicity.)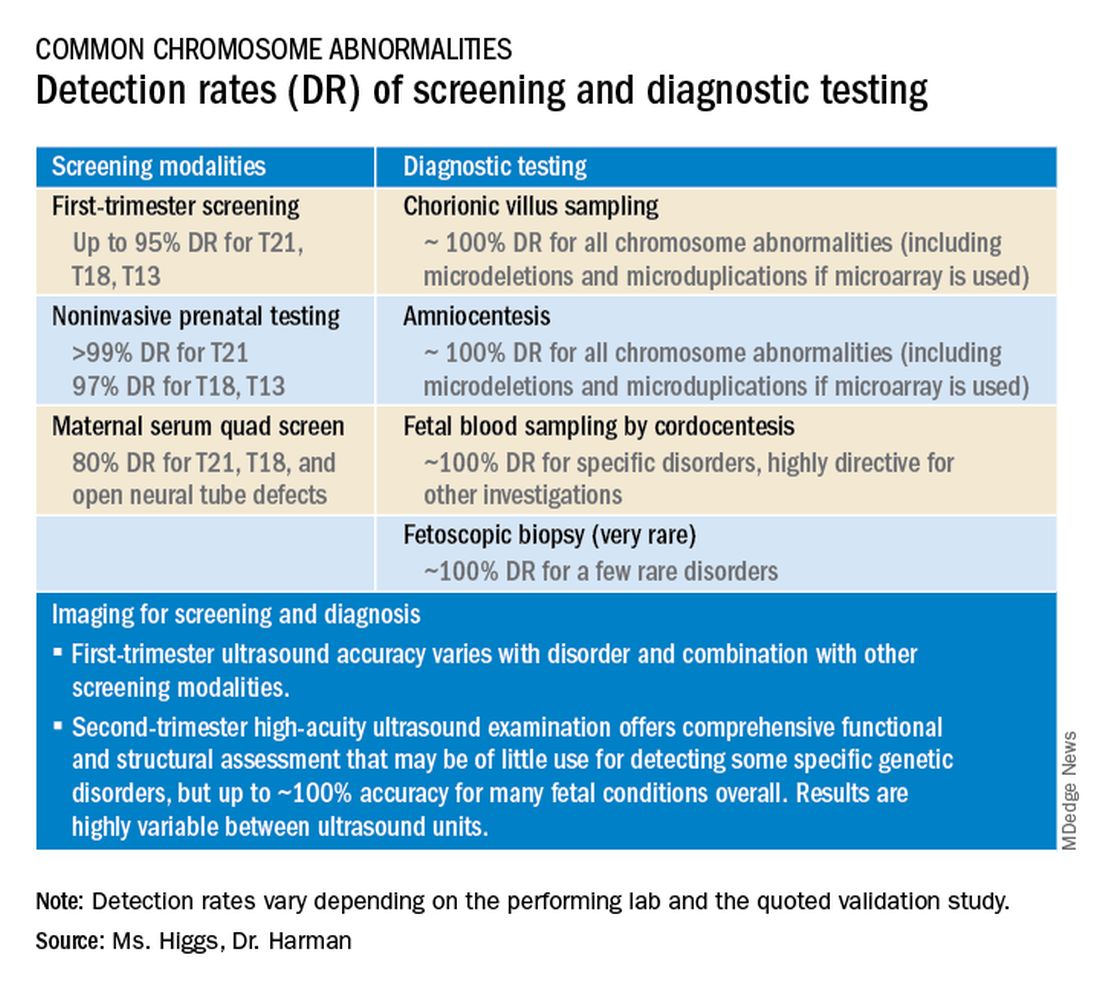
In any scenario, screening is optimally performed after counseling and prior to pregnancy when patients can fully consider their reproductive options; couples identified to be at 25% risk to have a child with a genetic condition may choose to pursue in-vitro fertilization and preimplantation genetic testing of embryos.
The expanded carrier screening panels offered by laboratories include as many as several hundred conditions, so careful scrutiny of included diseases and selection of a panel is important. We currently use an expanded panel that is restricted to conditions that limit life expectancy, have no treatment, have treatment that is most beneficial when started early, or are associated with intellectual disability.
Some panels look for mutations in genes that are quite common and often benign. Such is the case with the MTHFR gene: 40% of individuals in some populations are carriers, and offspring who inherit mutations in both gene copies are unlikely to have any medical issues at all. Yet, the lay information available on this gene can be confusing and even scary.
Laboratory methodologies should similarly be well understood. Many labs look only for a handful of common mutations in a gene, while others sequence or “read” the entire gene, looking for errors. The latter is more informative, but not all labs that purport to sequence the entire gene are actually doing so.
Patients should understand that, while a negative result significantly reduces their chance of being a carrier for a condition, it does not eliminate the risk. They should also understand that, if their partner is not available for testing or is unwilling to be tested, we will not be able to refine the risk to the pregnancy in the event they are found to be a carrier.
Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.
Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.
Advances in imaging, panel testing
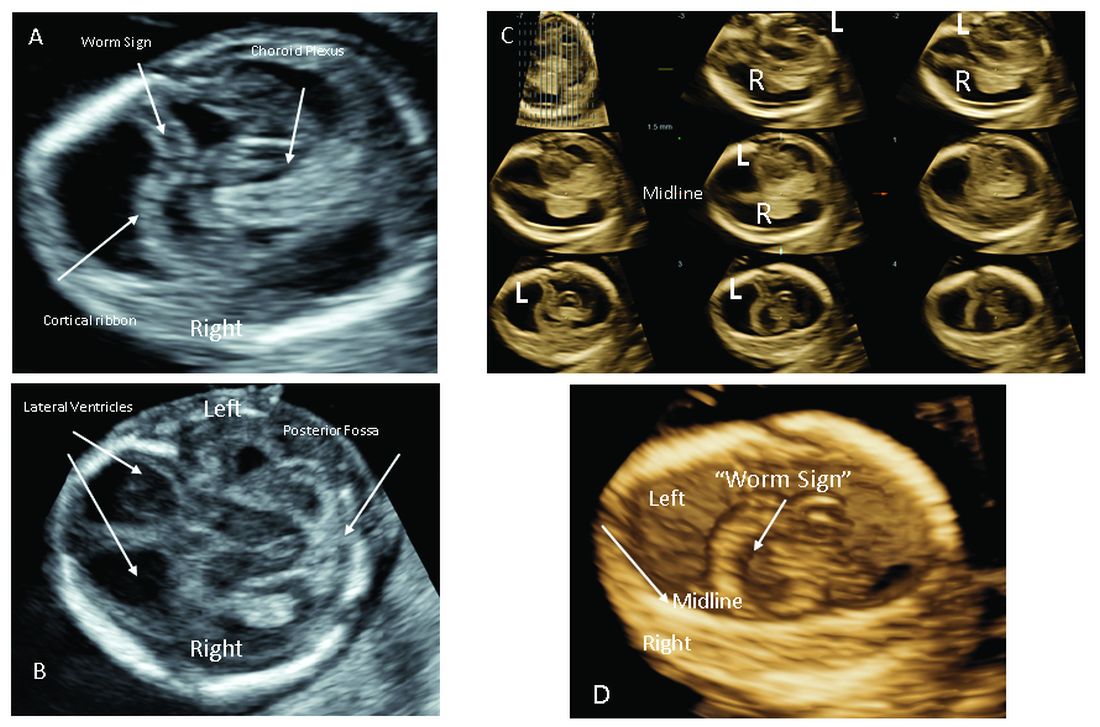
The most significant addition to the first-trimester ultrasound evaluation in recent years has been the systematic assessment of the fetal circulation and the structure of the fetal heart, with early detection of the most common forms of birth defects.
Structural assessment of the central nervous system, abdomen, and skeleton is also now possible during the first-trimester ultrasound and offers the opportunity for early genetic assessment when anomalies are detected.
Ultrasound imaging in the second and third trimesters can help refine the diagnosis of birth defects, track the evolution of suspicious findings from the first trimester, or uncover anomalies that did not present earlier. Findings may be suggestive of underlying genetic conditions and drive the use of “panel” tests, or targeted sequencing panels, to help make a diagnosis.
Features of skeletal dysplasia, for instance, would lead the genetic counselor to recommend a panel of tests that target skeletal dysplasia-associated genes, looking for genetic mutations. Similarly, holoprosencephaly detected on ultrasound could prompt use of a customized gene panel to look for mutations in a series of different genes known to cause the anomaly.
Second trimester details that may guide genetic investigation are not limited to ultrasound. In certain instances, MRI has the unique capability to diagnose particular structural defects, especially brain anomalies with developmental specificity.
Commentary by Christopher R. Harman, MD
Genetic counseling is now a mandatory part of all pregnancy evaluation programs. Counselors not only explain and interpret tests and results to families but also, increasingly, guide the efforts of the obstetrics team, including the maternal-fetal medicine specialist.
The genetic counselor helps design screening for the whole patient population and focuses diagnostic testing in specific cases of screening concerns, family history, chromosomal abnormalities in prior pregnancies, and fetal abnormalities detected through ultrasonography or other prenatal surveillance. They also serve as a crucial link between the physician and the family.
The counselor also has a key role in the case of a stillbirth or other adverse pregnancy outcome in investigating possible genetic elements and working with the family on evaluation of recurrence risk and prevention of a similar outcome in future pregnancies. The details of poor outcomes hold the potential for making the next pregnancy successful.
Commentary by Amanda S. Higgs, MGC
Even in 2021, there is no “perfect baby test.” Patients can have expanded carrier screening, cell-free DNA testing, invasive testing with microarray, and all of the available imaging, with normal results, and still have a baby with a genetic disorder. Understanding the concept of residual risk is important. So is appreciation for the possibility that incidental findings – information not sought – can occur even with specific genetic testing.
Genetic counselors are there to help patients understand and assimilate information, usher them through the screening and testing process, and facilitate informed decision-making. We are nondirective in our counseling. We try to assess their values, their support systems, and their experience with disability and help them to make the best decisions for themselves regarding testing and further evaluation, as well as other reproductive decisions.
[email protected]
Preconception and prenatal genetic screening and diagnostic testing for genetic disorders are increasingly complex, with a burgeoning number of testing options and a shift in screening from situations identified as high-risk to more universal considerations. The American College of Obstetricians and Gynecologists now recommends that all patients – regardless of age or risk for chromosomal abnormalities – be offered both screening and diagnostic tests and counseled about the relative benefits and limitations of available tests. These recommendations represent a sea change for obstetrics.
Screening options now include expanded carrier screening that evaluates an individual’s carrier status for multiple conditions at once, regardless of ethnicity, and cell-free DNA screening using fetal DNA found in the maternal circulation. Chromosomal microarray analysis from a chorionic villus sampling or amniocentesis specimen detects tiny copy number variants, and increasingly detailed ultrasound images illuminate anatomic and physiologic anomalies that could not be seen or interpreted as recently as 5 years ago.
These advancements are remarkable, but they require attentive, personalized pre- and posttest genetic counseling. Genetic counselors are critical to this process, helping women and families understand and select screening tools, interpret test results, select diagnostic panels, and make decisions about invasive testing.
Counseling is essential as we seek and utilize genetic information that is no longer binary. It used to be that predictions of normality and abnormality were made with little gray area in between. – and genetic diagnosis is increasingly a lattice of details, variable expression, and even effects timing.
Expanded carrier screening
Carrier screening to determine if one or both parents are carriers for an autosomal recessive condition has historically involved a limited number of conditions chosen based on ethnicity. However, research has demonstrated the unreliability of this approach in our multicultural, multiracial society, in which many of our patients have mixed or uncertain race and ethnicity.
Expanded carrier screening is nondirective and takes ethnic background out of the equation. ACOG has moved from advocating ethnic-based screening alone to advising that both ethnic and expanded carrier screening are acceptable strategies and that practices should choose a standard approach to offer and discuss with each patient. (Carrier screening for cystic fibrosis and spinal muscular atrophy are recommended for all patients regardless of ethnicity.)
In any scenario, screening is optimally performed after counseling and prior to pregnancy when patients can fully consider their reproductive options; couples identified to be at 25% risk to have a child with a genetic condition may choose to pursue in-vitro fertilization and preimplantation genetic testing of embryos.
The expanded carrier screening panels offered by laboratories include as many as several hundred conditions, so careful scrutiny of included diseases and selection of a panel is important. We currently use an expanded panel that is restricted to conditions that limit life expectancy, have no treatment, have treatment that is most beneficial when started early, or are associated with intellectual disability.
Some panels look for mutations in genes that are quite common and often benign. Such is the case with the MTHFR gene: 40% of individuals in some populations are carriers, and offspring who inherit mutations in both gene copies are unlikely to have any medical issues at all. Yet, the lay information available on this gene can be confusing and even scary.
Laboratory methodologies should similarly be well understood. Many labs look only for a handful of common mutations in a gene, while others sequence or “read” the entire gene, looking for errors. The latter is more informative, but not all labs that purport to sequence the entire gene are actually doing so.
Patients should understand that, while a negative result significantly reduces their chance of being a carrier for a condition, it does not eliminate the risk. They should also understand that, if their partner is not available for testing or is unwilling to be tested, we will not be able to refine the risk to the pregnancy in the event they are found to be a carrier.
Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.

Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.
Advances in imaging, panel testing
The most significant addition to the first-trimester ultrasound evaluation in recent years has been the systematic assessment of the fetal circulation and the structure of the fetal heart, with early detection of the most common forms of birth defects.
Structural assessment of the central nervous system, abdomen, and skeleton is also now possible during the first-trimester ultrasound and offers the opportunity for early genetic assessment when anomalies are detected.
Ultrasound imaging in the second and third trimesters can help refine the diagnosis of birth defects, track the evolution of suspicious findings from the first trimester, or uncover anomalies that did not present earlier. Findings may be suggestive of underlying genetic conditions and drive the use of “panel” tests, or targeted sequencing panels, to help make a diagnosis.
Features of skeletal dysplasia, for instance, would lead the genetic counselor to recommend a panel of tests that target skeletal dysplasia-associated genes, looking for genetic mutations. Similarly, holoprosencephaly detected on ultrasound could prompt use of a customized gene panel to look for mutations in a series of different genes known to cause the anomaly.
Second trimester details that may guide genetic investigation are not limited to ultrasound. In certain instances, MRI has the unique capability to diagnose particular structural defects, especially brain anomalies with developmental specificity.
Commentary by Christopher R. Harman, MD
Genetic counseling is now a mandatory part of all pregnancy evaluation programs. Counselors not only explain and interpret tests and results to families but also, increasingly, guide the efforts of the obstetrics team, including the maternal-fetal medicine specialist.
The genetic counselor helps design screening for the whole patient population and focuses diagnostic testing in specific cases of screening concerns, family history, chromosomal abnormalities in prior pregnancies, and fetal abnormalities detected through ultrasonography or other prenatal surveillance. They also serve as a crucial link between the physician and the family.
The counselor also has a key role in the case of a stillbirth or other adverse pregnancy outcome in investigating possible genetic elements and working with the family on evaluation of recurrence risk and prevention of a similar outcome in future pregnancies. The details of poor outcomes hold the potential for making the next pregnancy successful.
Commentary by Amanda S. Higgs, MGC
Even in 2021, there is no “perfect baby test.” Patients can have expanded carrier screening, cell-free DNA testing, invasive testing with microarray, and all of the available imaging, with normal results, and still have a baby with a genetic disorder. Understanding the concept of residual risk is important. So is appreciation for the possibility that incidental findings – information not sought – can occur even with specific genetic testing.
Genetic counselors are there to help patients understand and assimilate information, usher them through the screening and testing process, and facilitate informed decision-making. We are nondirective in our counseling. We try to assess their values, their support systems, and their experience with disability and help them to make the best decisions for themselves regarding testing and further evaluation, as well as other reproductive decisions.
[email protected]
Preconception and prenatal genetic screening and diagnostic testing for genetic disorders are increasingly complex, with a burgeoning number of testing options and a shift in screening from situations identified as high-risk to more universal considerations. The American College of Obstetricians and Gynecologists now recommends that all patients – regardless of age or risk for chromosomal abnormalities – be offered both screening and diagnostic tests and counseled about the relative benefits and limitations of available tests. These recommendations represent a sea change for obstetrics.
Screening options now include expanded carrier screening that evaluates an individual’s carrier status for multiple conditions at once, regardless of ethnicity, and cell-free DNA screening using fetal DNA found in the maternal circulation. Chromosomal microarray analysis from a chorionic villus sampling or amniocentesis specimen detects tiny copy number variants, and increasingly detailed ultrasound images illuminate anatomic and physiologic anomalies that could not be seen or interpreted as recently as 5 years ago.
These advancements are remarkable, but they require attentive, personalized pre- and posttest genetic counseling. Genetic counselors are critical to this process, helping women and families understand and select screening tools, interpret test results, select diagnostic panels, and make decisions about invasive testing.
Counseling is essential as we seek and utilize genetic information that is no longer binary. It used to be that predictions of normality and abnormality were made with little gray area in between. – and genetic diagnosis is increasingly a lattice of details, variable expression, and even effects timing.
Expanded carrier screening
Carrier screening to determine if one or both parents are carriers for an autosomal recessive condition has historically involved a limited number of conditions chosen based on ethnicity. However, research has demonstrated the unreliability of this approach in our multicultural, multiracial society, in which many of our patients have mixed or uncertain race and ethnicity.
Expanded carrier screening is nondirective and takes ethnic background out of the equation. ACOG has moved from advocating ethnic-based screening alone to advising that both ethnic and expanded carrier screening are acceptable strategies and that practices should choose a standard approach to offer and discuss with each patient. (Carrier screening for cystic fibrosis and spinal muscular atrophy are recommended for all patients regardless of ethnicity.)
In any scenario, screening is optimally performed after counseling and prior to pregnancy when patients can fully consider their reproductive options; couples identified to be at 25% risk to have a child with a genetic condition may choose to pursue in-vitro fertilization and preimplantation genetic testing of embryos.
The expanded carrier screening panels offered by laboratories include as many as several hundred conditions, so careful scrutiny of included diseases and selection of a panel is important. We currently use an expanded panel that is restricted to conditions that limit life expectancy, have no treatment, have treatment that is most beneficial when started early, or are associated with intellectual disability.
Some panels look for mutations in genes that are quite common and often benign. Such is the case with the MTHFR gene: 40% of individuals in some populations are carriers, and offspring who inherit mutations in both gene copies are unlikely to have any medical issues at all. Yet, the lay information available on this gene can be confusing and even scary.
Laboratory methodologies should similarly be well understood. Many labs look only for a handful of common mutations in a gene, while others sequence or “read” the entire gene, looking for errors. The latter is more informative, but not all labs that purport to sequence the entire gene are actually doing so.
Patients should understand that, while a negative result significantly reduces their chance of being a carrier for a condition, it does not eliminate the risk. They should also understand that, if their partner is not available for testing or is unwilling to be tested, we will not be able to refine the risk to the pregnancy in the event they are found to be a carrier.
Noninvasive prenatal screening
Cell-free DNA testing, or noninvasive prenatal testing (NIPT), is a powerful noninvasive screening technology for aneuploidy that analyzes fetal DNA floating freely in maternal blood starting at about 9-10 weeks of pregnancy. However, it is not a substitute for invasive testing and is not diagnostic.
Patients we see are commonly misinformed that a negative cell-free DNA testing result means their baby is without doubt unaffected by a chromosomal abnormality. NIPT is the most sensitive and specific screening test for the common fetal aneuploidies (trisomies 13, 18, and 21), with a significantly better positive predictive value than previous noninvasive chromosome screening. However, NIPT findings still include false-negative results and some false-positive results. Patients must be counseled that NIPT does not offer absolute findings.

Laboratories are adding screening tests for additional aneuploidies, microdeletions, and other disorders and variants. However, as ACOG and other professional colleges advise, the reliability of these tests (e.g.. their screening accuracy with respect to detection and false-positive rates) is not yet established, and these newer tests are not ready for routine adoption in practice.
Microarray analysis, variants of unknown significance (VUS)
Chromosomal microarray analysis of DNA from a chorionic villus sampling or amniocentesis specimen enables prenatal detection of exceptionally small genomic deletions and duplications – tiny chunks of DNA – that cannot be seen with standard karyotype testing.
That microdeletions and microduplications can produce abnormalities and conditions that can be significantly more severe than the absence or addition of entire chromosomes is not necessarily intuitive. It is as if the entire plot of a book is revealed in just one page.
For instance, Turner syndrome results when one of the X chromosomes is entirely missing. (Occasionally, there is a large, partial absence.) The absence can cause a variety of symptoms, including failure of the ovaries to develop and heart defects, but most affected individuals can lead healthy and independent lives with the only features being short stature and a wide neck.
Angelman syndrome, in contrast, is most often caused by a microdeletion of genetic material from chromosome 15 – a tiny snip of the chromosome – but results in ataxia, severe intellectual disability, lifelong seizures, and severe lifelong speech impairment.
In our program, we counsel patients before testing that results may come back one of three ways: completely normal, definitely abnormal, or with a VUS.
A VUS is a challenging finding because it represents a loss or gain of a small portion of a chromosome with unclear clinical significance. In some cases, the uncertainty stems from the microdeletion or duplication not having been seen before — or not seen enough to be accurately characterized as benign or pathogenic. In other cases, the uncertainty stems from an associated phenotype that is highly variable. Either way, a VUS often makes the investigation for genetic conditions and subsequent decision-making more difficult, and a genetic counselor’s expertise and guidance is needed.
Advances in imaging, panel testing
The most significant addition to the first-trimester ultrasound evaluation in recent years has been the systematic assessment of the fetal circulation and the structure of the fetal heart, with early detection of the most common forms of birth defects.
Structural assessment of the central nervous system, abdomen, and skeleton is also now possible during the first-trimester ultrasound and offers the opportunity for early genetic assessment when anomalies are detected.
Ultrasound imaging in the second and third trimesters can help refine the diagnosis of birth defects, track the evolution of suspicious findings from the first trimester, or uncover anomalies that did not present earlier. Findings may be suggestive of underlying genetic conditions and drive the use of “panel” tests, or targeted sequencing panels, to help make a diagnosis.
Features of skeletal dysplasia, for instance, would lead the genetic counselor to recommend a panel of tests that target skeletal dysplasia-associated genes, looking for genetic mutations. Similarly, holoprosencephaly detected on ultrasound could prompt use of a customized gene panel to look for mutations in a series of different genes known to cause the anomaly.
Second trimester details that may guide genetic investigation are not limited to ultrasound. In certain instances, MRI has the unique capability to diagnose particular structural defects, especially brain anomalies with developmental specificity.
Commentary by Christopher R. Harman, MD
Genetic counseling is now a mandatory part of all pregnancy evaluation programs. Counselors not only explain and interpret tests and results to families but also, increasingly, guide the efforts of the obstetrics team, including the maternal-fetal medicine specialist.
The genetic counselor helps design screening for the whole patient population and focuses diagnostic testing in specific cases of screening concerns, family history, chromosomal abnormalities in prior pregnancies, and fetal abnormalities detected through ultrasonography or other prenatal surveillance. They also serve as a crucial link between the physician and the family.
The counselor also has a key role in the case of a stillbirth or other adverse pregnancy outcome in investigating possible genetic elements and working with the family on evaluation of recurrence risk and prevention of a similar outcome in future pregnancies. The details of poor outcomes hold the potential for making the next pregnancy successful.
Commentary by Amanda S. Higgs, MGC
Even in 2021, there is no “perfect baby test.” Patients can have expanded carrier screening, cell-free DNA testing, invasive testing with microarray, and all of the available imaging, with normal results, and still have a baby with a genetic disorder. Understanding the concept of residual risk is important. So is appreciation for the possibility that incidental findings – information not sought – can occur even with specific genetic testing.
Genetic counselors are there to help patients understand and assimilate information, usher them through the screening and testing process, and facilitate informed decision-making. We are nondirective in our counseling. We try to assess their values, their support systems, and their experience with disability and help them to make the best decisions for themselves regarding testing and further evaluation, as well as other reproductive decisions.
[email protected]
Mother-to-infant COVID-19 transmission is unlikely
Mothers with a history of COVID-19 exposure during pregnancy are not likely to transmit the infection to their newborns, based on data from more than 2,000 women.
“Uncertainty at the onset of the COVID-19 pandemic led to varying postnatal care recommendations for newborns exposed to SARS-CoV-2 in utero,” said Margaret H. Kyle, of Columbia University, New York, and colleagues.
The Columbia University Irving Medical Center, an early epicenter of the pandemic, allowed rooming-in and encouraged direct breastfeeding between infected mothers and their newborns while adopting extensive safety measures, the researchers said.
In a study presented at the virtual meeting of the Pediatric Academic Societies (Poster 141), the researchers conducted a retrospective chart review of all newborns born at the medical center from March 22, 2020, through August 7, 2020. The study was part of Columbia University’s ongoing COVID-19 Mother Baby Outcomes (COMBO) initiative to “describe the health and well-being of mother-infant dyads with and without prenatal SARS-CoV-2 infections,” according to the researchers.
During the study period, the researchers identified newborns of 327 women who tested positive for COVID-19 at any point during pregnancy and compared them to newborns of 2,125 unexposed women. Demographics were similar between the groups.
Overall, the total test positivity was 0.7% for exposed newborns; 1.0% tested positive on an initial test, and 0% were positive on retest. During the newborn hospital stay and a 2-week follow-up, 0% of all newborns showed clinical evidence of infection.
No significant differences were noted between exposed and unexposed newborns in clinical outcomes including gestational age, mode of delivery, 5-minute Apgar score, heart rate, respiratory rate, or temperature. Although more infants of COVID-19–exposed mothers compared with unexposed mothers had an emergency department visit within the first 14 days of life (6% vs. 3%, P = .002), none of the infants was diagnosed with COVID-19 during these visits. Cough, fever, congestion, or bilirubin were more frequent reasons for emergency department visits in the exposed infants compared with unexposed infants, but these differences were not significant.
The study findings were limited by several factors, including the retrospective design and the limited follow-up period to only the first 2 weeks of life, the researchers noted. In addition, perinatal transmission rates were available only for the 202 newborns who were followed up in the hospital system, they said. However, the results suggest that the risk of mother-to-newborn vertical transmission of COVID-19 remains low, even when mothers are breastfeeding and infants are rooming in, they concluded.
Study supports safety of rooming in
The study is important because of the value of mother and infant bonding, Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview. “We know maternal and infant bonding and breastfeeding are extremely important in the first few days of life,” she said. “Initially, COVID-positive moms were separated from their babies during this important time.” Dr. Kinsella said she was not surprised by the study findings, as they reflect other research that newborns have not been getting infected with COVID-19 from their mothers.
Consequently, the take-home message is that newborns can room in with their mothers in the hospital setting, and they are at low risk for COVID-19 regardless of the mother’s exposure history, said Dr. Kinsella. Looking ahead, future areas of research could include examining SARS-CoV-2 antibodies in newborns, she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Mothers with a history of COVID-19 exposure during pregnancy are not likely to transmit the infection to their newborns, based on data from more than 2,000 women.
“Uncertainty at the onset of the COVID-19 pandemic led to varying postnatal care recommendations for newborns exposed to SARS-CoV-2 in utero,” said Margaret H. Kyle, of Columbia University, New York, and colleagues.
The Columbia University Irving Medical Center, an early epicenter of the pandemic, allowed rooming-in and encouraged direct breastfeeding between infected mothers and their newborns while adopting extensive safety measures, the researchers said.
In a study presented at the virtual meeting of the Pediatric Academic Societies (Poster 141), the researchers conducted a retrospective chart review of all newborns born at the medical center from March 22, 2020, through August 7, 2020. The study was part of Columbia University’s ongoing COVID-19 Mother Baby Outcomes (COMBO) initiative to “describe the health and well-being of mother-infant dyads with and without prenatal SARS-CoV-2 infections,” according to the researchers.
During the study period, the researchers identified newborns of 327 women who tested positive for COVID-19 at any point during pregnancy and compared them to newborns of 2,125 unexposed women. Demographics were similar between the groups.
Overall, the total test positivity was 0.7% for exposed newborns; 1.0% tested positive on an initial test, and 0% were positive on retest. During the newborn hospital stay and a 2-week follow-up, 0% of all newborns showed clinical evidence of infection.
No significant differences were noted between exposed and unexposed newborns in clinical outcomes including gestational age, mode of delivery, 5-minute Apgar score, heart rate, respiratory rate, or temperature. Although more infants of COVID-19–exposed mothers compared with unexposed mothers had an emergency department visit within the first 14 days of life (6% vs. 3%, P = .002), none of the infants was diagnosed with COVID-19 during these visits. Cough, fever, congestion, or bilirubin were more frequent reasons for emergency department visits in the exposed infants compared with unexposed infants, but these differences were not significant.
The study findings were limited by several factors, including the retrospective design and the limited follow-up period to only the first 2 weeks of life, the researchers noted. In addition, perinatal transmission rates were available only for the 202 newborns who were followed up in the hospital system, they said. However, the results suggest that the risk of mother-to-newborn vertical transmission of COVID-19 remains low, even when mothers are breastfeeding and infants are rooming in, they concluded.
Study supports safety of rooming in
The study is important because of the value of mother and infant bonding, Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview. “We know maternal and infant bonding and breastfeeding are extremely important in the first few days of life,” she said. “Initially, COVID-positive moms were separated from their babies during this important time.” Dr. Kinsella said she was not surprised by the study findings, as they reflect other research that newborns have not been getting infected with COVID-19 from their mothers.
Consequently, the take-home message is that newborns can room in with their mothers in the hospital setting, and they are at low risk for COVID-19 regardless of the mother’s exposure history, said Dr. Kinsella. Looking ahead, future areas of research could include examining SARS-CoV-2 antibodies in newborns, she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Mothers with a history of COVID-19 exposure during pregnancy are not likely to transmit the infection to their newborns, based on data from more than 2,000 women.
“Uncertainty at the onset of the COVID-19 pandemic led to varying postnatal care recommendations for newborns exposed to SARS-CoV-2 in utero,” said Margaret H. Kyle, of Columbia University, New York, and colleagues.
The Columbia University Irving Medical Center, an early epicenter of the pandemic, allowed rooming-in and encouraged direct breastfeeding between infected mothers and their newborns while adopting extensive safety measures, the researchers said.
In a study presented at the virtual meeting of the Pediatric Academic Societies (Poster 141), the researchers conducted a retrospective chart review of all newborns born at the medical center from March 22, 2020, through August 7, 2020. The study was part of Columbia University’s ongoing COVID-19 Mother Baby Outcomes (COMBO) initiative to “describe the health and well-being of mother-infant dyads with and without prenatal SARS-CoV-2 infections,” according to the researchers.
During the study period, the researchers identified newborns of 327 women who tested positive for COVID-19 at any point during pregnancy and compared them to newborns of 2,125 unexposed women. Demographics were similar between the groups.
Overall, the total test positivity was 0.7% for exposed newborns; 1.0% tested positive on an initial test, and 0% were positive on retest. During the newborn hospital stay and a 2-week follow-up, 0% of all newborns showed clinical evidence of infection.
No significant differences were noted between exposed and unexposed newborns in clinical outcomes including gestational age, mode of delivery, 5-minute Apgar score, heart rate, respiratory rate, or temperature. Although more infants of COVID-19–exposed mothers compared with unexposed mothers had an emergency department visit within the first 14 days of life (6% vs. 3%, P = .002), none of the infants was diagnosed with COVID-19 during these visits. Cough, fever, congestion, or bilirubin were more frequent reasons for emergency department visits in the exposed infants compared with unexposed infants, but these differences were not significant.
The study findings were limited by several factors, including the retrospective design and the limited follow-up period to only the first 2 weeks of life, the researchers noted. In addition, perinatal transmission rates were available only for the 202 newborns who were followed up in the hospital system, they said. However, the results suggest that the risk of mother-to-newborn vertical transmission of COVID-19 remains low, even when mothers are breastfeeding and infants are rooming in, they concluded.
Study supports safety of rooming in
The study is important because of the value of mother and infant bonding, Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview. “We know maternal and infant bonding and breastfeeding are extremely important in the first few days of life,” she said. “Initially, COVID-positive moms were separated from their babies during this important time.” Dr. Kinsella said she was not surprised by the study findings, as they reflect other research that newborns have not been getting infected with COVID-19 from their mothers.
Consequently, the take-home message is that newborns can room in with their mothers in the hospital setting, and they are at low risk for COVID-19 regardless of the mother’s exposure history, said Dr. Kinsella. Looking ahead, future areas of research could include examining SARS-CoV-2 antibodies in newborns, she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
FROM PAS 2021
Addressing today’s racial health inequities requires understanding their roots
The health disparities seen in today’s high rates of Black infant and maternal morbidity and mortality are rooted in health inequities and generational stress dating back centuries in the United States, but today’s obstetricians can make changes in their own practices to address this inequity, according to Haywood L. Brown, MD, professor of ob.gyn. and associate dean of diversity at the Morsani College of Medicine and vice president of institutional equity at the University of South Florida, Tampa.
Dr. Brown delivered his remarks during the Benson and Pamela Harer Seminar on History at the annual meeting of the American College of Obstetricians and Gynecologists on May 2. His talk focused on the origins of perinatal and maternal health inequities and how those original factors play out today in increased maternal and neonatal morbidity and mortality among Black women and their babies.
“Racial and ethnic disparities and inequity in maternal and child health are prevalent and persistent. We have to move beyond the documentation,” Dr. Brown told attendees. “We have to adopt uniform care standards, recognizing our own biases and understanding that the contribution of social determinants of health are important in the care and outcome of women. And we have to work on decreasing the stress of women who give birth.”
Evelyn Nicole Mitchell, MD, faculty chair of the ob.gyn. diversity and inclusion committee at the University of Southern California, found Dr. Brown’s talk compelling and hopes it opens the eyes of others who attended.
“You really have to understand the why behind the problems we have, and it really goes back to slavery and this historical distrust that’s been here from the beginning,” Dr. Mitchell said in an interview. “I hope this allows people to open their eyes and think about this situation from their patients’ shoes, to really put their guard down and explore, ‘how can I contribute to fixing this system that has been here from the beginning?’ I think a lot of people get defensive and think: ‘Oh, I’m not a racist. I just don’t want to talk about this,’ but it’s about a system being racist.” The question then, Dr. Mitchell said, is: “So how do I contribute to that system?”
Dr. Brown frequently returned to the theme of high stress levels in Black mothers contributing to poorer outcomes, such as preterm birth. That stress arises originally from the generational stress brought on by racism and oppression over the centuries but has been compounded by poverty, racial injustice, lack of access to adequate nutrition, lower education levels, environmental factors, and other determinants of health.
“The bottom line, as Dr. Brown said, is that we need to decrease the stress level of Black mothers giving birth,” Dr. Mitchell said. “How can I, as a provider, decrease the stress level of my patients? Well, No. 1, I can identify and eliminate implicit bias that I may harbor.”
Slavery husbandry laid the groundwork for today
The most surprising aspect of Dr. Brown’s lecture for Dr. Mitchell was the fact that enslaved women received a measure of protection that other enslaved people did not to “ensure that they were healthy and that they were able to reproduce in the future,” Dr. Mitchell said. “It was for the wrong reasons – to keep slavery going – but in a sense they were prioritizing Black women to take advantage of their reproductive capacity, compared to nowadays where Black women are facing severe disparities.”
To safeguard enslaved women’s fecundity, plantation owners attempted to reduce stressors in the women’s lives, such as allowing them to cohabitate with a husband and nuclear family, though sexual assault and abuse still occurred. The owners also tracked the enslaved girls’ menstrual cycles after menarche to maximize their “breeding” potential, especially between the ages of 15 and 24. Slave owners delegated older enslaved women as maternity caregivers and midwives, leading to the passing down of midwifery skills through generations of Black American women.
“Pregnant women received the best medical care on the plantation because of the premium placed on reproduction,” Dr. Brown said. Wealthier planters called in doctors for complicated deliveries, which provided J. Marian Sims the ability conduct surgical experiments on Betsey, Lucy, and Anarcha to treat vesicovaginal fistula since fistula “limited her ability to do the maximum work she could in the house or on the plantation,” Dr. Brown said.
After slavery ended, health care access did not improve for Black people. In 1920, there was approximately 1 Black physician for every 3,000 Black people, compared with 1 in 500 for the White population, and grannie midwives continued to be the primary birthing attendants for Black women. Over the next several decades, however, both maternal and infant mortality across all races began steeply dropping. Reasons for the drop included the incorporation of the American Board of Obstetrics and Gynecology in 1930, a shift from home births to hospital births, and the legalization of abortion, which led to an 89% decline in deaths from septic illegal abortions from 1950 to 1973.
Still, Black maternal and infant mortality remained higher than White, and the poverty gap further exacerbated outcomes.
“Substandard maternity care really is the origin of many of the Black maternal and infant morbidity and mortality” complications, such as low birth weight, small for gestational age, growth restriction, and intrauterine starvation, “which we now believe are the origin of things like hypertension, diabetes, and obesity,” Dr. Brown said.
Today, inequities persist because of the systemic racism throughout this history.
“As we talk about health disparities, prematurity, growth restriction, and maternal morbidity, the fetal origins for adult disease in diabetes and hypertension and obesity have generational implications over the last 400 years,” Dr. Brown said. “Generational stress and stresses in lack women from slavery to present times are some of the origins of the things that we see today, including segregation, economic inequities, eugenic sterilizations, the quality of education, and of course, systemic racism on health care access and quality.”
It is this long arc of history that Dr. Mitchell hopes attendees will begin to grasp.
“If you don’t understand all that and have that depth, there’s no way for you to truly understand the problems that are going on and how to solve them,” Dr. Mitchell said. She hopes that especially those who have been more “resistant to accepting these truths” can start to see the big picture. “Hopefully, they can look at it as a systemic problem and then focus on how they can change the system.”
Dr Brown is a contributor to UpToDate and the Merck Manual and serves on the advisory boards of Merck for Mothers Global Women’s Health and BabyScripts. Dr. Mitchell has no disclosures.
The health disparities seen in today’s high rates of Black infant and maternal morbidity and mortality are rooted in health inequities and generational stress dating back centuries in the United States, but today’s obstetricians can make changes in their own practices to address this inequity, according to Haywood L. Brown, MD, professor of ob.gyn. and associate dean of diversity at the Morsani College of Medicine and vice president of institutional equity at the University of South Florida, Tampa.
Dr. Brown delivered his remarks during the Benson and Pamela Harer Seminar on History at the annual meeting of the American College of Obstetricians and Gynecologists on May 2. His talk focused on the origins of perinatal and maternal health inequities and how those original factors play out today in increased maternal and neonatal morbidity and mortality among Black women and their babies.
“Racial and ethnic disparities and inequity in maternal and child health are prevalent and persistent. We have to move beyond the documentation,” Dr. Brown told attendees. “We have to adopt uniform care standards, recognizing our own biases and understanding that the contribution of social determinants of health are important in the care and outcome of women. And we have to work on decreasing the stress of women who give birth.”
Evelyn Nicole Mitchell, MD, faculty chair of the ob.gyn. diversity and inclusion committee at the University of Southern California, found Dr. Brown’s talk compelling and hopes it opens the eyes of others who attended.
“You really have to understand the why behind the problems we have, and it really goes back to slavery and this historical distrust that’s been here from the beginning,” Dr. Mitchell said in an interview. “I hope this allows people to open their eyes and think about this situation from their patients’ shoes, to really put their guard down and explore, ‘how can I contribute to fixing this system that has been here from the beginning?’ I think a lot of people get defensive and think: ‘Oh, I’m not a racist. I just don’t want to talk about this,’ but it’s about a system being racist.” The question then, Dr. Mitchell said, is: “So how do I contribute to that system?”
Dr. Brown frequently returned to the theme of high stress levels in Black mothers contributing to poorer outcomes, such as preterm birth. That stress arises originally from the generational stress brought on by racism and oppression over the centuries but has been compounded by poverty, racial injustice, lack of access to adequate nutrition, lower education levels, environmental factors, and other determinants of health.
“The bottom line, as Dr. Brown said, is that we need to decrease the stress level of Black mothers giving birth,” Dr. Mitchell said. “How can I, as a provider, decrease the stress level of my patients? Well, No. 1, I can identify and eliminate implicit bias that I may harbor.”
Slavery husbandry laid the groundwork for today
The most surprising aspect of Dr. Brown’s lecture for Dr. Mitchell was the fact that enslaved women received a measure of protection that other enslaved people did not to “ensure that they were healthy and that they were able to reproduce in the future,” Dr. Mitchell said. “It was for the wrong reasons – to keep slavery going – but in a sense they were prioritizing Black women to take advantage of their reproductive capacity, compared to nowadays where Black women are facing severe disparities.”
To safeguard enslaved women’s fecundity, plantation owners attempted to reduce stressors in the women’s lives, such as allowing them to cohabitate with a husband and nuclear family, though sexual assault and abuse still occurred. The owners also tracked the enslaved girls’ menstrual cycles after menarche to maximize their “breeding” potential, especially between the ages of 15 and 24. Slave owners delegated older enslaved women as maternity caregivers and midwives, leading to the passing down of midwifery skills through generations of Black American women.
“Pregnant women received the best medical care on the plantation because of the premium placed on reproduction,” Dr. Brown said. Wealthier planters called in doctors for complicated deliveries, which provided J. Marian Sims the ability conduct surgical experiments on Betsey, Lucy, and Anarcha to treat vesicovaginal fistula since fistula “limited her ability to do the maximum work she could in the house or on the plantation,” Dr. Brown said.
After slavery ended, health care access did not improve for Black people. In 1920, there was approximately 1 Black physician for every 3,000 Black people, compared with 1 in 500 for the White population, and grannie midwives continued to be the primary birthing attendants for Black women. Over the next several decades, however, both maternal and infant mortality across all races began steeply dropping. Reasons for the drop included the incorporation of the American Board of Obstetrics and Gynecology in 1930, a shift from home births to hospital births, and the legalization of abortion, which led to an 89% decline in deaths from septic illegal abortions from 1950 to 1973.
Still, Black maternal and infant mortality remained higher than White, and the poverty gap further exacerbated outcomes.
“Substandard maternity care really is the origin of many of the Black maternal and infant morbidity and mortality” complications, such as low birth weight, small for gestational age, growth restriction, and intrauterine starvation, “which we now believe are the origin of things like hypertension, diabetes, and obesity,” Dr. Brown said.
Today, inequities persist because of the systemic racism throughout this history.
“As we talk about health disparities, prematurity, growth restriction, and maternal morbidity, the fetal origins for adult disease in diabetes and hypertension and obesity have generational implications over the last 400 years,” Dr. Brown said. “Generational stress and stresses in lack women from slavery to present times are some of the origins of the things that we see today, including segregation, economic inequities, eugenic sterilizations, the quality of education, and of course, systemic racism on health care access and quality.”
It is this long arc of history that Dr. Mitchell hopes attendees will begin to grasp.
“If you don’t understand all that and have that depth, there’s no way for you to truly understand the problems that are going on and how to solve them,” Dr. Mitchell said. She hopes that especially those who have been more “resistant to accepting these truths” can start to see the big picture. “Hopefully, they can look at it as a systemic problem and then focus on how they can change the system.”
Dr Brown is a contributor to UpToDate and the Merck Manual and serves on the advisory boards of Merck for Mothers Global Women’s Health and BabyScripts. Dr. Mitchell has no disclosures.
The health disparities seen in today’s high rates of Black infant and maternal morbidity and mortality are rooted in health inequities and generational stress dating back centuries in the United States, but today’s obstetricians can make changes in their own practices to address this inequity, according to Haywood L. Brown, MD, professor of ob.gyn. and associate dean of diversity at the Morsani College of Medicine and vice president of institutional equity at the University of South Florida, Tampa.
Dr. Brown delivered his remarks during the Benson and Pamela Harer Seminar on History at the annual meeting of the American College of Obstetricians and Gynecologists on May 2. His talk focused on the origins of perinatal and maternal health inequities and how those original factors play out today in increased maternal and neonatal morbidity and mortality among Black women and their babies.
“Racial and ethnic disparities and inequity in maternal and child health are prevalent and persistent. We have to move beyond the documentation,” Dr. Brown told attendees. “We have to adopt uniform care standards, recognizing our own biases and understanding that the contribution of social determinants of health are important in the care and outcome of women. And we have to work on decreasing the stress of women who give birth.”
Evelyn Nicole Mitchell, MD, faculty chair of the ob.gyn. diversity and inclusion committee at the University of Southern California, found Dr. Brown’s talk compelling and hopes it opens the eyes of others who attended.
“You really have to understand the why behind the problems we have, and it really goes back to slavery and this historical distrust that’s been here from the beginning,” Dr. Mitchell said in an interview. “I hope this allows people to open their eyes and think about this situation from their patients’ shoes, to really put their guard down and explore, ‘how can I contribute to fixing this system that has been here from the beginning?’ I think a lot of people get defensive and think: ‘Oh, I’m not a racist. I just don’t want to talk about this,’ but it’s about a system being racist.” The question then, Dr. Mitchell said, is: “So how do I contribute to that system?”
Dr. Brown frequently returned to the theme of high stress levels in Black mothers contributing to poorer outcomes, such as preterm birth. That stress arises originally from the generational stress brought on by racism and oppression over the centuries but has been compounded by poverty, racial injustice, lack of access to adequate nutrition, lower education levels, environmental factors, and other determinants of health.
“The bottom line, as Dr. Brown said, is that we need to decrease the stress level of Black mothers giving birth,” Dr. Mitchell said. “How can I, as a provider, decrease the stress level of my patients? Well, No. 1, I can identify and eliminate implicit bias that I may harbor.”
Slavery husbandry laid the groundwork for today
The most surprising aspect of Dr. Brown’s lecture for Dr. Mitchell was the fact that enslaved women received a measure of protection that other enslaved people did not to “ensure that they were healthy and that they were able to reproduce in the future,” Dr. Mitchell said. “It was for the wrong reasons – to keep slavery going – but in a sense they were prioritizing Black women to take advantage of their reproductive capacity, compared to nowadays where Black women are facing severe disparities.”
To safeguard enslaved women’s fecundity, plantation owners attempted to reduce stressors in the women’s lives, such as allowing them to cohabitate with a husband and nuclear family, though sexual assault and abuse still occurred. The owners also tracked the enslaved girls’ menstrual cycles after menarche to maximize their “breeding” potential, especially between the ages of 15 and 24. Slave owners delegated older enslaved women as maternity caregivers and midwives, leading to the passing down of midwifery skills through generations of Black American women.
“Pregnant women received the best medical care on the plantation because of the premium placed on reproduction,” Dr. Brown said. Wealthier planters called in doctors for complicated deliveries, which provided J. Marian Sims the ability conduct surgical experiments on Betsey, Lucy, and Anarcha to treat vesicovaginal fistula since fistula “limited her ability to do the maximum work she could in the house or on the plantation,” Dr. Brown said.
After slavery ended, health care access did not improve for Black people. In 1920, there was approximately 1 Black physician for every 3,000 Black people, compared with 1 in 500 for the White population, and grannie midwives continued to be the primary birthing attendants for Black women. Over the next several decades, however, both maternal and infant mortality across all races began steeply dropping. Reasons for the drop included the incorporation of the American Board of Obstetrics and Gynecology in 1930, a shift from home births to hospital births, and the legalization of abortion, which led to an 89% decline in deaths from septic illegal abortions from 1950 to 1973.
Still, Black maternal and infant mortality remained higher than White, and the poverty gap further exacerbated outcomes.
“Substandard maternity care really is the origin of many of the Black maternal and infant morbidity and mortality” complications, such as low birth weight, small for gestational age, growth restriction, and intrauterine starvation, “which we now believe are the origin of things like hypertension, diabetes, and obesity,” Dr. Brown said.
Today, inequities persist because of the systemic racism throughout this history.
“As we talk about health disparities, prematurity, growth restriction, and maternal morbidity, the fetal origins for adult disease in diabetes and hypertension and obesity have generational implications over the last 400 years,” Dr. Brown said. “Generational stress and stresses in lack women from slavery to present times are some of the origins of the things that we see today, including segregation, economic inequities, eugenic sterilizations, the quality of education, and of course, systemic racism on health care access and quality.”
It is this long arc of history that Dr. Mitchell hopes attendees will begin to grasp.
“If you don’t understand all that and have that depth, there’s no way for you to truly understand the problems that are going on and how to solve them,” Dr. Mitchell said. She hopes that especially those who have been more “resistant to accepting these truths” can start to see the big picture. “Hopefully, they can look at it as a systemic problem and then focus on how they can change the system.”
Dr Brown is a contributor to UpToDate and the Merck Manual and serves on the advisory boards of Merck for Mothers Global Women’s Health and BabyScripts. Dr. Mitchell has no disclosures.
FROM ACOG 2021
Heavy cannabis use in pregnancy correlates with risks to infant
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Cannabis use that interferes with a woman’s ability to function during pregnancy is a risk factor for severe health problems in the child, new research indicates.
Pregnant women with cannabis use disorder are more likely to have children with low birth weights and children who die within 1 year of birth, compared with matched controls, according to a study published online in Addiction.
The death rate among infants exposed to prenatal cannabis use disorder was 0.98%, compared with 0.75% among infants whose mothers did not have this diagnosis.
Cannabis use disorder during pregnancy “has increased dramatically in the past two decades,” but few studies have examined the health impacts on offspring, study author Yuyan Shi, PhD, said in an interview. “It is particularly concerning in states with cannabis legalization where cannabis is increasingly available.”
Dr. Shi, a researcher at the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, and colleagues analyzed data from more than 4.8 million mothers who delivered a live singleton birth in California between 2001 and 2012 and their infants. They focused on 20,237 mothers who had a diagnosis of cannabis use disorder at delivery. The disorder is defined by continued use of the drug despite impairments in physical, psychological, and social functioning.
The researchers matched mothers with cannabis use disorder 1:2 to mothers who did not have this diagnosis. They aimed to balance factors such as maternal age, educational attainment, health insurance, physical and mental health conditions, prenatal care, and alcohol and opioid use disorder.
An increasingly common diagnosis
Over the study period, the rate of cannabis use disorder increased from 2.8 cases per 1,000 deliveries in 2001 to 6.9 cases per 1,000 deliveries in 2012.
Cannabis use disorder was associated with increased odds of preterm birth (odds ratio, 1.06), small for gestational age (OR, 1.13), low birth weight (OR, 1.13), and death within 1 year of birth (OR, 1.35), according to the researchers’ estimates. Cannabis use disorder was associated with lower odds of hospitalization within 1 year of birth, however (OR, 0.91).
“The most notable observation is that exposed infants were 35% more likely to die within 1 year of birth than unexposed infants,” Dr. Shi and colleagues wrote. More research is needed to understand the causes of death at different stages of infancy, they said.
The results “imply that cannabis use disorder screening as well as appropriate education, counseling, or referral to substance abuse treatment services should be encouraged among pregnant women,” Dr. Shi said.
The study does not establish that cannabis use disorder causes adverse effects, and it is not clear how the results might apply to mothers who use cannabis but do not meet diagnostic criteria for the disorder, the authors noted.
“Presumably the health consequences of mothers who use cannabis but do not meet the criteria ... are less severe than mothers with cannabis use disorder,” Dr. Shi said. “Unfortunately, no research has been conducted to test this hypothesis.”
Enough data to recommend abstaining
Many clinicians may not feel equipped to make a diagnosis of cannabis use disorder, said Jamie Lo, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University in Portland.
Although many clinicians ask patients about substance use in general, specifically screening for cannabis use is not necessarily routine practice. “I think people are starting to adopt that, but it probably will take a little bit of time,” Dr. Lo said.
Dr. Lo, who was not involved in the study, researches the effects of marijuana during pregnancy.
Confounding factors such as frequent co-use of tobacco have so far made it “difficult to suss out” whether observed effects are directly from cannabis use, other substances or exposures, or a combination, said Dr. Lo. The possibility that stigma may lead to inaccurate self-reporting poses another challenge. And the range of cannabis delivery devices further complicates matters.
“It is hard to compare smoking a bowl versus a joint versus using the oils or CBD or edibles,” Dr. Lo said. The data regarding cigarettes and alcohol are cleaner and more precise, in comparison.
Still, federal agencies and professional societies agree that “what we do know is enough to recommend that pregnant women abstain from using cannabis during pregnancy,” Dr. Lo said.
The National Institute on Drug Abuse, which funded the study, said the results add to the evidence that prenatal exposure to cannabis may be associated with poor birth outcomes and infant health.
“While we cannot establish that cannabis use caused negative outcomes in this study, these data reinforce the case for caution around using cannabis during pregnancy,” Nora D. Volkow, MD, the director of the agency, said in a news release.
“Careful analysis of data like these is one way we can responsibly study how cannabis use affects the developing child, all while a natural experiment is playing out across our country in places where cannabis is becoming widely available to pregnant consumers.”
The study authors and Dr. Lo had no disclosures.
Addressing an uncharted front in the war on COVID-19: Vaccination during pregnancy
In December 2020, the US Food and Drug Administration’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.1 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.2 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.3 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.4,5
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- The presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes.
- Potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- The ability to take precautions (social distancing, wearing a mask, etc)
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
In December 2020, the US Food and Drug Administration’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.1 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.2 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.3 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.4,5
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- The presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes.
- Potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- The ability to take precautions (social distancing, wearing a mask, etc)
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
In December 2020, the US Food and Drug Administration’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.1 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.2 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.3 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.4,5
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- The presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes.
- Potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- The ability to take precautions (social distancing, wearing a mask, etc)
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
Self-harm is a leading cause of death for new moms
Death by self-harm through suicide or overdose is a leading cause of death for women in the first year post partum, data indicate. Many of these deaths may be preventable, said Adrienne Griffen, MPP, executive director of the Maternal Mental Health Leadership Alliance.
Ms. Griffen discussed these findings and ways clinicians may be able to help at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Women “visit a health care provider an average of 25 times during a healthy pregnancy and first year of baby’s life,” she said. “Obstetric and primary care providers who serve pregnant and postpartum women are uniquely positioned to intervene effectively to screen and assess women for mental health disorders.”
To that end, clinicians should discuss mental health “early and often,” Ms. Griffen said.
“Asking about mental health issues and suicide will not cause women to think these thoughts,” she said. “We cannot wait for women to raise their hand and ask for help because by the time they do that, they needed help many weeks ago.”
For example, a doctor might tell a patient: “Your mental health is just as important as your physical health, and anxiety and depression are the most common complications of pregnancy and childbirth,” Ms. Griffen suggested. “Every time I see you, I’m going to ask you how you are doing, and we’ll do a formal screening assessment periodically over the course of the pregnancy. … Your job is to answer us honestly so that we can connect you with resources as soon as possible to minimize the impact on you and your baby.”
Although the obstetric provider should introduce this topic, a nurse, lactation consultant, or social worker may conduct screenings and help patients who are experiencing distress, she said.
During the past decade, several medical associations have issued new guidance around screening new mothers for anxiety and depression. One recent ACOG committee opinion recommends screening for depression at least once during pregnancy and once post partum, and encourages doctors to initiate medical therapy if possible and provide resources and referrals.
Another committee opinion suggests that doctors should have contact with a patient between 2 and 3 weeks post partum, primarily to assess for mental health.
Limited data
In discussing maternal suicide statistics, Ms. Griffen focused on data from Maternal Mortality Review Committees (MMRCs).
Two other sources of data about maternal mortality – the National Vital Statistics System and the Pregnancy Mortality Surveillance System – do not include information about suicide, which may be a reason this cause of death is not discussed more often, Ms. Griffen noted.
MMRCs, on the other hand, include information about suicide and self-harm. About half of the states in the United States have these multidisciplinary committees. Committee members review deaths of all women during pregnancy or within 1 year of pregnancy. Members consider a range of clinical and nonclinical data, including reports from social services and police, to try to understand the circumstances of each death.
A report that examined pregnancy-related deaths using data from 14 U.S. MMRCs between 2008 and 2017 showed that mental health conditions were the leading cause of death for non-Hispanic White women. In all, 34% of pregnancy-related suicide deaths had a documented prior suicide attempt, and the majority of suicides happened in the late postpartum time frame (43-365 days post partum).
Some physicians cite a lack of education, time, reimbursement, or referral resources as barriers to maternal mental health screening and treatment, but there may be useful options available, Ms. Griffen said. Postpartum Support International provides resources for physicians, as well as mothers. The National Curriculum in Reproductive Psychiatry and the Seleni Institute also have educational resources.
Some states have psychiatry access programs, where psychiatrists educate obstetricians, family physicians, and pediatricians about how to assess for and treat maternal mental health issues, Ms. Griffen noted.
Self care, social support, and talk therapy may help patients. “Sometimes medication is needed, but a combination of all of these things … can help women recover from maternal mental health conditions,” Ms. Griffen said.
Need to intervene
Although medical societies have emphasized the importance of maternal mental health screening and treatment in recent years, the risk of self-harm has been a concern for obstetricians and gynecologists long before then, said Marc Alan Landsberg, MD, a member of the meeting’s scientific committee who moderated the session.
“We have been talking about this at ACOG for a long time,” Dr. Landsberg said in an interview.
The presentation highlighted why obstetricians, gynecologists, and other doctors who deliver babies and care for women post partum “have got to screen these people,” he said. The finding that 34% of pregnancy-related suicide deaths had a prior suicide attempt indicates that clinicians may be able to identify these patients, Dr. Landsberg said. Suicide and overdose are leading causes of death in the first year post partum and “probably 100% of these are preventable,” he said.
As a first step, screening may be relatively simple. The Edinburgh Postnatal Depression Scale, highlighted during the talk, is an easy and quick tool to use, Dr. Landsberg said. It contains 10 items and assesses for anxiety and depression. It also specifically asks about suicide.
Ms. Griffen and Dr. Landsberg had no conflicts of interest.
Death by self-harm through suicide or overdose is a leading cause of death for women in the first year post partum, data indicate. Many of these deaths may be preventable, said Adrienne Griffen, MPP, executive director of the Maternal Mental Health Leadership Alliance.
Ms. Griffen discussed these findings and ways clinicians may be able to help at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Women “visit a health care provider an average of 25 times during a healthy pregnancy and first year of baby’s life,” she said. “Obstetric and primary care providers who serve pregnant and postpartum women are uniquely positioned to intervene effectively to screen and assess women for mental health disorders.”
To that end, clinicians should discuss mental health “early and often,” Ms. Griffen said.
“Asking about mental health issues and suicide will not cause women to think these thoughts,” she said. “We cannot wait for women to raise their hand and ask for help because by the time they do that, they needed help many weeks ago.”
For example, a doctor might tell a patient: “Your mental health is just as important as your physical health, and anxiety and depression are the most common complications of pregnancy and childbirth,” Ms. Griffen suggested. “Every time I see you, I’m going to ask you how you are doing, and we’ll do a formal screening assessment periodically over the course of the pregnancy. … Your job is to answer us honestly so that we can connect you with resources as soon as possible to minimize the impact on you and your baby.”
Although the obstetric provider should introduce this topic, a nurse, lactation consultant, or social worker may conduct screenings and help patients who are experiencing distress, she said.
During the past decade, several medical associations have issued new guidance around screening new mothers for anxiety and depression. One recent ACOG committee opinion recommends screening for depression at least once during pregnancy and once post partum, and encourages doctors to initiate medical therapy if possible and provide resources and referrals.
Another committee opinion suggests that doctors should have contact with a patient between 2 and 3 weeks post partum, primarily to assess for mental health.
Limited data
In discussing maternal suicide statistics, Ms. Griffen focused on data from Maternal Mortality Review Committees (MMRCs).
Two other sources of data about maternal mortality – the National Vital Statistics System and the Pregnancy Mortality Surveillance System – do not include information about suicide, which may be a reason this cause of death is not discussed more often, Ms. Griffen noted.
MMRCs, on the other hand, include information about suicide and self-harm. About half of the states in the United States have these multidisciplinary committees. Committee members review deaths of all women during pregnancy or within 1 year of pregnancy. Members consider a range of clinical and nonclinical data, including reports from social services and police, to try to understand the circumstances of each death.
A report that examined pregnancy-related deaths using data from 14 U.S. MMRCs between 2008 and 2017 showed that mental health conditions were the leading cause of death for non-Hispanic White women. In all, 34% of pregnancy-related suicide deaths had a documented prior suicide attempt, and the majority of suicides happened in the late postpartum time frame (43-365 days post partum).
Some physicians cite a lack of education, time, reimbursement, or referral resources as barriers to maternal mental health screening and treatment, but there may be useful options available, Ms. Griffen said. Postpartum Support International provides resources for physicians, as well as mothers. The National Curriculum in Reproductive Psychiatry and the Seleni Institute also have educational resources.
Some states have psychiatry access programs, where psychiatrists educate obstetricians, family physicians, and pediatricians about how to assess for and treat maternal mental health issues, Ms. Griffen noted.
Self care, social support, and talk therapy may help patients. “Sometimes medication is needed, but a combination of all of these things … can help women recover from maternal mental health conditions,” Ms. Griffen said.
Need to intervene
Although medical societies have emphasized the importance of maternal mental health screening and treatment in recent years, the risk of self-harm has been a concern for obstetricians and gynecologists long before then, said Marc Alan Landsberg, MD, a member of the meeting’s scientific committee who moderated the session.
“We have been talking about this at ACOG for a long time,” Dr. Landsberg said in an interview.
The presentation highlighted why obstetricians, gynecologists, and other doctors who deliver babies and care for women post partum “have got to screen these people,” he said. The finding that 34% of pregnancy-related suicide deaths had a prior suicide attempt indicates that clinicians may be able to identify these patients, Dr. Landsberg said. Suicide and overdose are leading causes of death in the first year post partum and “probably 100% of these are preventable,” he said.
As a first step, screening may be relatively simple. The Edinburgh Postnatal Depression Scale, highlighted during the talk, is an easy and quick tool to use, Dr. Landsberg said. It contains 10 items and assesses for anxiety and depression. It also specifically asks about suicide.
Ms. Griffen and Dr. Landsberg had no conflicts of interest.
Death by self-harm through suicide or overdose is a leading cause of death for women in the first year post partum, data indicate. Many of these deaths may be preventable, said Adrienne Griffen, MPP, executive director of the Maternal Mental Health Leadership Alliance.
Ms. Griffen discussed these findings and ways clinicians may be able to help at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Women “visit a health care provider an average of 25 times during a healthy pregnancy and first year of baby’s life,” she said. “Obstetric and primary care providers who serve pregnant and postpartum women are uniquely positioned to intervene effectively to screen and assess women for mental health disorders.”
To that end, clinicians should discuss mental health “early and often,” Ms. Griffen said.
“Asking about mental health issues and suicide will not cause women to think these thoughts,” she said. “We cannot wait for women to raise their hand and ask for help because by the time they do that, they needed help many weeks ago.”
For example, a doctor might tell a patient: “Your mental health is just as important as your physical health, and anxiety and depression are the most common complications of pregnancy and childbirth,” Ms. Griffen suggested. “Every time I see you, I’m going to ask you how you are doing, and we’ll do a formal screening assessment periodically over the course of the pregnancy. … Your job is to answer us honestly so that we can connect you with resources as soon as possible to minimize the impact on you and your baby.”
Although the obstetric provider should introduce this topic, a nurse, lactation consultant, or social worker may conduct screenings and help patients who are experiencing distress, she said.
During the past decade, several medical associations have issued new guidance around screening new mothers for anxiety and depression. One recent ACOG committee opinion recommends screening for depression at least once during pregnancy and once post partum, and encourages doctors to initiate medical therapy if possible and provide resources and referrals.
Another committee opinion suggests that doctors should have contact with a patient between 2 and 3 weeks post partum, primarily to assess for mental health.
Limited data
In discussing maternal suicide statistics, Ms. Griffen focused on data from Maternal Mortality Review Committees (MMRCs).
Two other sources of data about maternal mortality – the National Vital Statistics System and the Pregnancy Mortality Surveillance System – do not include information about suicide, which may be a reason this cause of death is not discussed more often, Ms. Griffen noted.
MMRCs, on the other hand, include information about suicide and self-harm. About half of the states in the United States have these multidisciplinary committees. Committee members review deaths of all women during pregnancy or within 1 year of pregnancy. Members consider a range of clinical and nonclinical data, including reports from social services and police, to try to understand the circumstances of each death.
A report that examined pregnancy-related deaths using data from 14 U.S. MMRCs between 2008 and 2017 showed that mental health conditions were the leading cause of death for non-Hispanic White women. In all, 34% of pregnancy-related suicide deaths had a documented prior suicide attempt, and the majority of suicides happened in the late postpartum time frame (43-365 days post partum).
Some physicians cite a lack of education, time, reimbursement, or referral resources as barriers to maternal mental health screening and treatment, but there may be useful options available, Ms. Griffen said. Postpartum Support International provides resources for physicians, as well as mothers. The National Curriculum in Reproductive Psychiatry and the Seleni Institute also have educational resources.
Some states have psychiatry access programs, where psychiatrists educate obstetricians, family physicians, and pediatricians about how to assess for and treat maternal mental health issues, Ms. Griffen noted.
Self care, social support, and talk therapy may help patients. “Sometimes medication is needed, but a combination of all of these things … can help women recover from maternal mental health conditions,” Ms. Griffen said.
Need to intervene
Although medical societies have emphasized the importance of maternal mental health screening and treatment in recent years, the risk of self-harm has been a concern for obstetricians and gynecologists long before then, said Marc Alan Landsberg, MD, a member of the meeting’s scientific committee who moderated the session.
“We have been talking about this at ACOG for a long time,” Dr. Landsberg said in an interview.
The presentation highlighted why obstetricians, gynecologists, and other doctors who deliver babies and care for women post partum “have got to screen these people,” he said. The finding that 34% of pregnancy-related suicide deaths had a prior suicide attempt indicates that clinicians may be able to identify these patients, Dr. Landsberg said. Suicide and overdose are leading causes of death in the first year post partum and “probably 100% of these are preventable,” he said.
As a first step, screening may be relatively simple. The Edinburgh Postnatal Depression Scale, highlighted during the talk, is an easy and quick tool to use, Dr. Landsberg said. It contains 10 items and assesses for anxiety and depression. It also specifically asks about suicide.
Ms. Griffen and Dr. Landsberg had no conflicts of interest.
FROM ACOG 2021
Genetic testing and the future of cerebral palsy malpractice cases
CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.) strong="">
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.
A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7
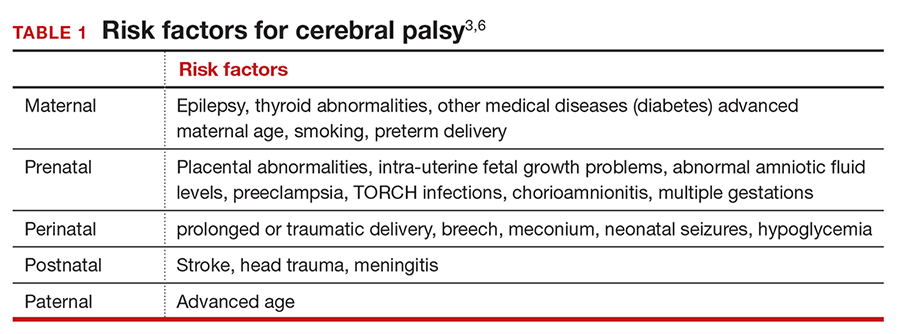
DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).
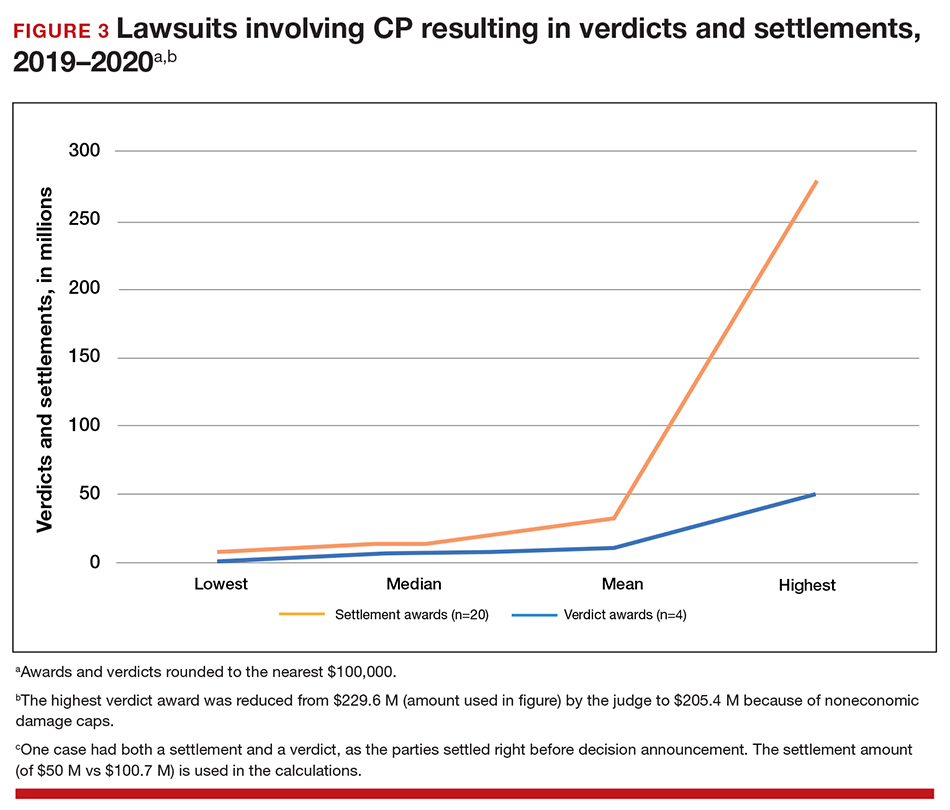
These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.

CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.) strong="">
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.

A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7

DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).

These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.

CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.) strong="">
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.

A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7

DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).

These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.
Stop checking routine lipid panels every year
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
Are pregnant and lactating women and their infants protected with the COVID-19 mRNA vaccines?
Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023
EXPERT COMMENTARY
Pregnant women are among those at highest risk for severe disease and death from SARS-CoV-2 infection. However, exclusion of pregnant and lactating women from the initial COVID-19 vaccine trials has made counseling these patients challenging due to both the novelty of the vaccines themselves and the general lack of data in this vulnerable population. Data for the efficacy and risks of vaccination are needed to inform shared decision making between clinician and patient.
Details of the study
Gray and colleagues conducted a prospective cohort study of 84 pregnant, 31 lactating, and 16 nonpregnant women who received 1 of the 2 COVID-19 mRNA vaccines approved by the US Food and Drug Administration for emergency use authorization (BNT162b2 Pfizer/BioNTech or mRNA-1273 Moderna). The study’s primary objective was to evaluate the humoral immune response (antibody quantification) and adverse effects of these vaccines in the pregnant and lactating women compared with both nonpregnant women and a cohort of 37 women who had natural COVID-19 infection during pregnancy.
Antibody quantification from blood and breast milk was performed at 4 time points: V0, the first vaccine dose; V1, the second vaccine dose; V2, 2 to 6 weeks after the second vaccine dose; and at delivery. Umbilical cord blood also was collected from the subset of delivered patients (n = 13).
Results. The ultimately IgG-dominated antibody response to the vaccine in pregnant and lactating women was comparable to that in nonpregnant women, and all vaccine antibody responses were significantly higher than that in response to natural SARS-CoV-2 infection. IgG antibodies also were found in umbilical cord blood and breast milk, supporting the transfer of immunity to both the fetus and infant. There were no significant differences in adverse effects between pregnant and nonpregnant women.
Study strengths and limitations
This study’s main strength is that it demonstrated a similar increase in humoral immune response to the COVID-19 mRNA vaccines in a previously unstudied population of pregnant and lactating women, supporting the potential efficacy of the vaccines in this group at high risk for complications from SARS-CoV-2. Other data to support this include the increased vaccine antibody response compared with the antibody response after SARS-CoV-2 infection in pregnant women as well as the presence of maternal-infant transfer of antibodies via cord blood and breast milk. All of these are important data to inform patients and practitioners who are trying to make shared, informed decisions about a novel vaccine during a global pandemic.
The main limitation of this study is a limited patient population of mostly White, non-Hispanic, health care workers with few comorbidities and only 13 delivered vaccinated patients within the study period. Long-term immunity, immune responses other than antibody titers, and potential fetal effects also were not explored in this study. ●
The study by Gray and colleagues provides some of the first data supporting the potential efficacy of the novel mRNA vaccines in pregnant and lactating women, as the antibody-mediated response is similar in this population to that in the nonpregnant population. Moreover, it provides reassurance that the antibodies are getting to the fetus and the infant via the umbilical cord blood and breast milk and that the adverse effect profile is similar. Clinicians can use these data to help their patients make more informed decisions about COVID-19 vaccination. Future studies still are needed for long-term data on immunity and safety for the fetus.
JAIMEY M. PAULI, MD
Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023
EXPERT COMMENTARY
Pregnant women are among those at highest risk for severe disease and death from SARS-CoV-2 infection. However, exclusion of pregnant and lactating women from the initial COVID-19 vaccine trials has made counseling these patients challenging due to both the novelty of the vaccines themselves and the general lack of data in this vulnerable population. Data for the efficacy and risks of vaccination are needed to inform shared decision making between clinician and patient.
Details of the study
Gray and colleagues conducted a prospective cohort study of 84 pregnant, 31 lactating, and 16 nonpregnant women who received 1 of the 2 COVID-19 mRNA vaccines approved by the US Food and Drug Administration for emergency use authorization (BNT162b2 Pfizer/BioNTech or mRNA-1273 Moderna). The study’s primary objective was to evaluate the humoral immune response (antibody quantification) and adverse effects of these vaccines in the pregnant and lactating women compared with both nonpregnant women and a cohort of 37 women who had natural COVID-19 infection during pregnancy.
Antibody quantification from blood and breast milk was performed at 4 time points: V0, the first vaccine dose; V1, the second vaccine dose; V2, 2 to 6 weeks after the second vaccine dose; and at delivery. Umbilical cord blood also was collected from the subset of delivered patients (n = 13).
Results. The ultimately IgG-dominated antibody response to the vaccine in pregnant and lactating women was comparable to that in nonpregnant women, and all vaccine antibody responses were significantly higher than that in response to natural SARS-CoV-2 infection. IgG antibodies also were found in umbilical cord blood and breast milk, supporting the transfer of immunity to both the fetus and infant. There were no significant differences in adverse effects between pregnant and nonpregnant women.
Study strengths and limitations
This study’s main strength is that it demonstrated a similar increase in humoral immune response to the COVID-19 mRNA vaccines in a previously unstudied population of pregnant and lactating women, supporting the potential efficacy of the vaccines in this group at high risk for complications from SARS-CoV-2. Other data to support this include the increased vaccine antibody response compared with the antibody response after SARS-CoV-2 infection in pregnant women as well as the presence of maternal-infant transfer of antibodies via cord blood and breast milk. All of these are important data to inform patients and practitioners who are trying to make shared, informed decisions about a novel vaccine during a global pandemic.
The main limitation of this study is a limited patient population of mostly White, non-Hispanic, health care workers with few comorbidities and only 13 delivered vaccinated patients within the study period. Long-term immunity, immune responses other than antibody titers, and potential fetal effects also were not explored in this study. ●
The study by Gray and colleagues provides some of the first data supporting the potential efficacy of the novel mRNA vaccines in pregnant and lactating women, as the antibody-mediated response is similar in this population to that in the nonpregnant population. Moreover, it provides reassurance that the antibodies are getting to the fetus and the infant via the umbilical cord blood and breast milk and that the adverse effect profile is similar. Clinicians can use these data to help their patients make more informed decisions about COVID-19 vaccination. Future studies still are needed for long-term data on immunity and safety for the fetus.
JAIMEY M. PAULI, MD
Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002-9378(21)00187-3. doi: 10.1016/j.ajog.2021.03.023
EXPERT COMMENTARY
Pregnant women are among those at highest risk for severe disease and death from SARS-CoV-2 infection. However, exclusion of pregnant and lactating women from the initial COVID-19 vaccine trials has made counseling these patients challenging due to both the novelty of the vaccines themselves and the general lack of data in this vulnerable population. Data for the efficacy and risks of vaccination are needed to inform shared decision making between clinician and patient.
Details of the study
Gray and colleagues conducted a prospective cohort study of 84 pregnant, 31 lactating, and 16 nonpregnant women who received 1 of the 2 COVID-19 mRNA vaccines approved by the US Food and Drug Administration for emergency use authorization (BNT162b2 Pfizer/BioNTech or mRNA-1273 Moderna). The study’s primary objective was to evaluate the humoral immune response (antibody quantification) and adverse effects of these vaccines in the pregnant and lactating women compared with both nonpregnant women and a cohort of 37 women who had natural COVID-19 infection during pregnancy.
Antibody quantification from blood and breast milk was performed at 4 time points: V0, the first vaccine dose; V1, the second vaccine dose; V2, 2 to 6 weeks after the second vaccine dose; and at delivery. Umbilical cord blood also was collected from the subset of delivered patients (n = 13).
Results. The ultimately IgG-dominated antibody response to the vaccine in pregnant and lactating women was comparable to that in nonpregnant women, and all vaccine antibody responses were significantly higher than that in response to natural SARS-CoV-2 infection. IgG antibodies also were found in umbilical cord blood and breast milk, supporting the transfer of immunity to both the fetus and infant. There were no significant differences in adverse effects between pregnant and nonpregnant women.
Study strengths and limitations
This study’s main strength is that it demonstrated a similar increase in humoral immune response to the COVID-19 mRNA vaccines in a previously unstudied population of pregnant and lactating women, supporting the potential efficacy of the vaccines in this group at high risk for complications from SARS-CoV-2. Other data to support this include the increased vaccine antibody response compared with the antibody response after SARS-CoV-2 infection in pregnant women as well as the presence of maternal-infant transfer of antibodies via cord blood and breast milk. All of these are important data to inform patients and practitioners who are trying to make shared, informed decisions about a novel vaccine during a global pandemic.
The main limitation of this study is a limited patient population of mostly White, non-Hispanic, health care workers with few comorbidities and only 13 delivered vaccinated patients within the study period. Long-term immunity, immune responses other than antibody titers, and potential fetal effects also were not explored in this study. ●
The study by Gray and colleagues provides some of the first data supporting the potential efficacy of the novel mRNA vaccines in pregnant and lactating women, as the antibody-mediated response is similar in this population to that in the nonpregnant population. Moreover, it provides reassurance that the antibodies are getting to the fetus and the infant via the umbilical cord blood and breast milk and that the adverse effect profile is similar. Clinicians can use these data to help their patients make more informed decisions about COVID-19 vaccination. Future studies still are needed for long-term data on immunity and safety for the fetus.
JAIMEY M. PAULI, MD











