User login
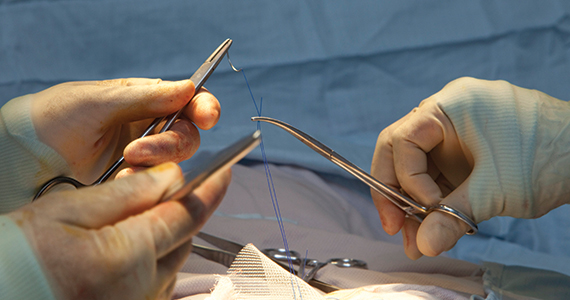
Obstetric anal sphincter injury: Prevention and repair
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
Genetic variants account for up to one-third of cases of cerebral palsy
Cerebral palsy (CP) is the most common cause of severe neurodisability in children, and it occurs in about 2 to 3 per 1,000 births worldwide.1 This nonprogressive disorder is characterized by symptoms that include spasticity, dystonia, choreoathetosis, and/or ataxia that are evident in the first few years of life. While many perinatal variables have been associated with CP, in most cases a specific cause is not identified.
Other neurodevelopmental disorders, such as intellectual disability, epilepsy, and autism spectrum disorder, are often associated with CP.2 These other neurodevelopmental disorders are often genetic, and this has raised the question as to whether CP also might have a substantial genetic component, although this has not been investigated in any significant way until recently. This topic is of great interest to the obstetric community, given that CP often is attributed to obstetric events, including mismanagement of labor and delivery.
Emerging evidence of a genetic-CP association
In an article published recently in JAMA, Moreno-De-Luca and colleagues sought to determine the diagnostic yield of exome sequencing for CP.3 This large cross-sectional study included results of exome sequencing performed in 2 settings. The first setting was a commercial laboratory in which samples were sent for analysis due to a diagnosis of CP, primarily in children (n = 1,345) with a median age of 8.8 years. A second cohort, recruited from a neurodevelopmental disorders clinic at Geisinger, included primarily adults (n = 181) with a median age of 41.9 years.
As is standard in exome sequencing, results were considered likely causative if they were classified as pathogenic or likely pathogenic based on criteria of the American College of Genetics and Genomics. In the laboratory group, 32.7% (440 of 1,345) had a genetic cause of the CP identified, while in the clinic group, 10.5% (19 of 181) had a genetic etiology found. Although most of the identified genetic variants were de novo (that is, they arose in the affected individual and were not clearly inherited), some were inherited from carrier parents.3
A number of other recent studies also have investigated genetic causes of CP and similarly have reported that a substantial number of cases are genetic. Several studies that performed chromosomal microarray analysis in individuals with CP found deleterious copy number variants in 10% to 31% of cases.4-6 Genomic variants detectable by exome sequencing have been reported in 15% to 20% of cases.3 In a recent study in Nature Genetics, researchers performed exome sequencing on 250 parent-child “trios” in which the child had CP, and they found that 14% of cases had an associated genetic variant that was thought to be causative.4 These studies all provide consistent evidence that a substantial proportion of CP cases are due to genetic causes.
Contributors to CP risk
Historically, CP was considered to occur largely as a result of perinatal anoxia. In 1862, the British orthopedic surgeon William John Little first reported an association between prematurity, asphyxia, difficult delivery, and CP in a paper presented to the Obstetrical Society of London.7 Subsequently, much effort has gone into the prevention of perinatal asphyxia and birth injury, although our ability to monitor fetal well-being remains limited. Nonreassuring fetal heart rate patterns are nonspecific and can occur for many reasons other than fetal asphyxia. Studies of electronic fetal monitoring have found that continuous monitoring primarily leads to an increase in cesarean delivery with no decrease in CP or infant mortality.8
While some have attributed this to failure to accurately interpret the fetal heart rate tracing, it also may be because a substantial number of CP cases are due to genetic and other causes, and that very few in fact result from preventable intrapartum injury.
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics agree that knowledge gaps preclude definitive determination that a given case of neonatal encephalopathy is attributable to an acute intrapartum event, and they provide criteria that must be fulfilled to establish a reasonable causal link between an intrapartum event and subsequent long-term neurologic disability.9 However, there continues to be a belief in the medical, scientific, and lay communities that birth asphyxia, secondary to adverse intrapartum events, is the leading cause of CP. A “brain-damaged infant” remains one of the most common malpractice claims, and birth injury one of the highest paid claims. Such claims generally allege that intrapartum asphyxia has caused long-term neurologic sequelae, including CP.
While it is true that prematurity, infection, hypoxia-ischemia, and pre- and perinatal stroke all have been implicated as contributing to CP risk, large population-based studies have shown that birth asphyxia accounts for less than 12% of CP cases.10 Specifically, recent data indicate that acute intrapartum hypoxia-ischemia occurs only in about 6% of CP cases. In other words, it does occur and may contribute to some cases, but this is likely a smaller percent than previously thought, and genetic factors now appear to be far more significant contributors.11
Continue to: Exploring a genetic etiology...
Exploring a genetic etiology
In considering the etiologies of CP, it is important to note that 21% to 40% of individuals with CP have an associated congenital anomaly, suggesting a genetic origin in at least some individuals. Moreover, a 40% heritability has been estimated in CP, which is comparable to the heritability rate for autism spectrum disorders.12
In the recent study by Moreno-De-Luca and colleagues, some of the gene variants detected were previously associated with other forms of neurodevelopmental disability, such as epilepsy and autism spectrum disorder.3 Many individuals in the study cohort were found to have multiple neurologic comorbidities, for example, CP as well as epilepsy, autism spectrum disorder, and/or intellectual disability. The presence of these additional comorbidities increased the likelihood of finding a genetic cause; the authors found that the diagnostic yield ranged from 11.2% with isolated CP to 32.9% with all 3 comorbidities. The yield was highest with CP and intellectual disability and CP with all 3 comorbidities. A few genes were particularly common, and some were reported previously in association with CP and/or other neurodevelopmental disorders. In some patients, variants were found in genes or gene regions associated with disorders that do not frequently include CP, such as Rett syndrome.3
Implications for ObGyns
The data from the study by Moreno-De-Luca and colleagues are interesting and relevant to pediatricians, neurologists, and geneticists, as well as obstetricians. Understanding the cause of any disease or disorder improves care, including counseling regarding the cause, the appropriate interventions or therapy, and in some families, the recurrence risk in another pregnancy. The treatment for CP has not changed significantly in many years. Increasingly, detection of an underlying genetic cause can guide precision treatments; thus, the detection of specific gene variants allows a targeted approach to therapy.
Identification of a genetic cause also can significantly impact recurrence risk counseling and prenatal diagnosis options in another pregnancy. In general, the empiric recurrence risk of CP is quoted as 1% to 2%,13 and with de novo variants this does not change. However, with inherited variants the recurrence risk in future children is substantially higher. While 72% of the genetic variants associated with CP in the Moreno-De-Luca study were de novo with a low recurrence risk, in the other 28% the mode of inheritance indicated a substantial risk of recurrence (25%–50%) in another pregnancy.3 Detecting such causative variant(s) allows not only accurate counseling about recurrence risk but also preimplantation genetic testing or prenatal diagnosis when recurrence risk is high.
In the field of obstetrics, the debate about the etiology of CP is important largely due to the medicolegal implications. Patient-oriented information on the internet often states that CP is caused by damage to the child’s brain just before, during, or soon after birth, supporting potential blame of those providing care during those times. Patient-oriented websites regarding CP do not list genetic disorders among the causes but rather include primarily environmental factors, such as prematurity, low birth weight, in utero infections, anoxia or other brain injury, or perinatal stroke. Even the Centers for Disease Control and Prevention website lists brain damage as the primary etiology of CP.14 Hopefully, these new data will increase a broader understanding of this condition.
Exome sequencing is now recommended as a first-tier test for individuals with many neurodevelopmental disorders, including epilepsy, intellectual disability, and autism spectrum disorder.15 However, comprehensive genetic testing is not typically recommended or performed in cases of CP. Based on recent data, including the report by Moreno-De-Luca and colleagues, it would seem that CP should be added to the list of disorders for which exome sequencing is ordered, given the similar prevalence and diagnostic yield. ●
- Oskoui M, Coutinho F, Dykeman J, et al. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55:509-519.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8-14.
- Moreno-De-Luca A, Millan F, Pesacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Jin SC, Lewis SA, Bakhtiari S, et al. Mutations disrupting neuritogenesis genes confer risk for cerebral palsy. Nat Genet. 2020;52:1046-1056.
- Segel R, Ben-Pazi H, Zeligson S, et al. Copy number variations in cryptogenic cerebral palsy. Neurology. 2015;84:1660-1668.
- McMichael G, Girirrajan S, Moreno-De-Luca A, et al. Rare copy number variation in cerebral palsy. Eur J Hum Genet. 2014;22:40-45.
- Little WJ. On the influence of abnormal parturition, difficult labours, premature births, and asphyxia neonatorum, on the mental and physical condition of the child, especially in relation to deformities. Trans Obstet Soc Lond. 1862;3:293-344.
- Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;5;CD006066.
- American College of Obstetricians and Gynecologists. Executive summary: neonatal encephalopathy and neurologic outcome second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896- 901.
- Ellenberg JH, Nelson KB. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210- 216.
- Himmelmann K, Uvebrant P. The panorama of cerebral palsy in Sweden part XII shows that patterns changed in the birth years 2007–2010. Acta Paediatr. 2018;107: 462-468.
- Petterson B, Stanley F, Henderson D. Cerebral palsy in multiple births in Western Australia: genetic aspects. Am J Med Genet. 1990;37:346- 351.
- Korzeniewski SJ, Slaughter J, Lenski M, et al. The complex aetiology of cerebral palsy. Nat Rev Neurol. 2018;14:528-543.
- Centers for Disease Control and Prevention. Causes and risk factors of cerebral palsy. https:// www.cdc.gov/ncbddd/cp/causes.html. Accessed March 23, 2021.
- Srivastava S, Love-Nichols JA, Dies KA, et al; NDD Exome Scoping Review Work Group. Meta-analysis and multidisciplinary consensus statement: exome sequencing is a first-tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genet Med. 2019;21:2413-2421.

Cerebral palsy (CP) is the most common cause of severe neurodisability in children, and it occurs in about 2 to 3 per 1,000 births worldwide.1 This nonprogressive disorder is characterized by symptoms that include spasticity, dystonia, choreoathetosis, and/or ataxia that are evident in the first few years of life. While many perinatal variables have been associated with CP, in most cases a specific cause is not identified.
Other neurodevelopmental disorders, such as intellectual disability, epilepsy, and autism spectrum disorder, are often associated with CP.2 These other neurodevelopmental disorders are often genetic, and this has raised the question as to whether CP also might have a substantial genetic component, although this has not been investigated in any significant way until recently. This topic is of great interest to the obstetric community, given that CP often is attributed to obstetric events, including mismanagement of labor and delivery.
Emerging evidence of a genetic-CP association
In an article published recently in JAMA, Moreno-De-Luca and colleagues sought to determine the diagnostic yield of exome sequencing for CP.3 This large cross-sectional study included results of exome sequencing performed in 2 settings. The first setting was a commercial laboratory in which samples were sent for analysis due to a diagnosis of CP, primarily in children (n = 1,345) with a median age of 8.8 years. A second cohort, recruited from a neurodevelopmental disorders clinic at Geisinger, included primarily adults (n = 181) with a median age of 41.9 years.
As is standard in exome sequencing, results were considered likely causative if they were classified as pathogenic or likely pathogenic based on criteria of the American College of Genetics and Genomics. In the laboratory group, 32.7% (440 of 1,345) had a genetic cause of the CP identified, while in the clinic group, 10.5% (19 of 181) had a genetic etiology found. Although most of the identified genetic variants were de novo (that is, they arose in the affected individual and were not clearly inherited), some were inherited from carrier parents.3
A number of other recent studies also have investigated genetic causes of CP and similarly have reported that a substantial number of cases are genetic. Several studies that performed chromosomal microarray analysis in individuals with CP found deleterious copy number variants in 10% to 31% of cases.4-6 Genomic variants detectable by exome sequencing have been reported in 15% to 20% of cases.3 In a recent study in Nature Genetics, researchers performed exome sequencing on 250 parent-child “trios” in which the child had CP, and they found that 14% of cases had an associated genetic variant that was thought to be causative.4 These studies all provide consistent evidence that a substantial proportion of CP cases are due to genetic causes.
Contributors to CP risk
Historically, CP was considered to occur largely as a result of perinatal anoxia. In 1862, the British orthopedic surgeon William John Little first reported an association between prematurity, asphyxia, difficult delivery, and CP in a paper presented to the Obstetrical Society of London.7 Subsequently, much effort has gone into the prevention of perinatal asphyxia and birth injury, although our ability to monitor fetal well-being remains limited. Nonreassuring fetal heart rate patterns are nonspecific and can occur for many reasons other than fetal asphyxia. Studies of electronic fetal monitoring have found that continuous monitoring primarily leads to an increase in cesarean delivery with no decrease in CP or infant mortality.8
While some have attributed this to failure to accurately interpret the fetal heart rate tracing, it also may be because a substantial number of CP cases are due to genetic and other causes, and that very few in fact result from preventable intrapartum injury.
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics agree that knowledge gaps preclude definitive determination that a given case of neonatal encephalopathy is attributable to an acute intrapartum event, and they provide criteria that must be fulfilled to establish a reasonable causal link between an intrapartum event and subsequent long-term neurologic disability.9 However, there continues to be a belief in the medical, scientific, and lay communities that birth asphyxia, secondary to adverse intrapartum events, is the leading cause of CP. A “brain-damaged infant” remains one of the most common malpractice claims, and birth injury one of the highest paid claims. Such claims generally allege that intrapartum asphyxia has caused long-term neurologic sequelae, including CP.
While it is true that prematurity, infection, hypoxia-ischemia, and pre- and perinatal stroke all have been implicated as contributing to CP risk, large population-based studies have shown that birth asphyxia accounts for less than 12% of CP cases.10 Specifically, recent data indicate that acute intrapartum hypoxia-ischemia occurs only in about 6% of CP cases. In other words, it does occur and may contribute to some cases, but this is likely a smaller percent than previously thought, and genetic factors now appear to be far more significant contributors.11
Continue to: Exploring a genetic etiology...
Exploring a genetic etiology
In considering the etiologies of CP, it is important to note that 21% to 40% of individuals with CP have an associated congenital anomaly, suggesting a genetic origin in at least some individuals. Moreover, a 40% heritability has been estimated in CP, which is comparable to the heritability rate for autism spectrum disorders.12
In the recent study by Moreno-De-Luca and colleagues, some of the gene variants detected were previously associated with other forms of neurodevelopmental disability, such as epilepsy and autism spectrum disorder.3 Many individuals in the study cohort were found to have multiple neurologic comorbidities, for example, CP as well as epilepsy, autism spectrum disorder, and/or intellectual disability. The presence of these additional comorbidities increased the likelihood of finding a genetic cause; the authors found that the diagnostic yield ranged from 11.2% with isolated CP to 32.9% with all 3 comorbidities. The yield was highest with CP and intellectual disability and CP with all 3 comorbidities. A few genes were particularly common, and some were reported previously in association with CP and/or other neurodevelopmental disorders. In some patients, variants were found in genes or gene regions associated with disorders that do not frequently include CP, such as Rett syndrome.3
Implications for ObGyns
The data from the study by Moreno-De-Luca and colleagues are interesting and relevant to pediatricians, neurologists, and geneticists, as well as obstetricians. Understanding the cause of any disease or disorder improves care, including counseling regarding the cause, the appropriate interventions or therapy, and in some families, the recurrence risk in another pregnancy. The treatment for CP has not changed significantly in many years. Increasingly, detection of an underlying genetic cause can guide precision treatments; thus, the detection of specific gene variants allows a targeted approach to therapy.
Identification of a genetic cause also can significantly impact recurrence risk counseling and prenatal diagnosis options in another pregnancy. In general, the empiric recurrence risk of CP is quoted as 1% to 2%,13 and with de novo variants this does not change. However, with inherited variants the recurrence risk in future children is substantially higher. While 72% of the genetic variants associated with CP in the Moreno-De-Luca study were de novo with a low recurrence risk, in the other 28% the mode of inheritance indicated a substantial risk of recurrence (25%–50%) in another pregnancy.3 Detecting such causative variant(s) allows not only accurate counseling about recurrence risk but also preimplantation genetic testing or prenatal diagnosis when recurrence risk is high.
In the field of obstetrics, the debate about the etiology of CP is important largely due to the medicolegal implications. Patient-oriented information on the internet often states that CP is caused by damage to the child’s brain just before, during, or soon after birth, supporting potential blame of those providing care during those times. Patient-oriented websites regarding CP do not list genetic disorders among the causes but rather include primarily environmental factors, such as prematurity, low birth weight, in utero infections, anoxia or other brain injury, or perinatal stroke. Even the Centers for Disease Control and Prevention website lists brain damage as the primary etiology of CP.14 Hopefully, these new data will increase a broader understanding of this condition.
Exome sequencing is now recommended as a first-tier test for individuals with many neurodevelopmental disorders, including epilepsy, intellectual disability, and autism spectrum disorder.15 However, comprehensive genetic testing is not typically recommended or performed in cases of CP. Based on recent data, including the report by Moreno-De-Luca and colleagues, it would seem that CP should be added to the list of disorders for which exome sequencing is ordered, given the similar prevalence and diagnostic yield. ●

Cerebral palsy (CP) is the most common cause of severe neurodisability in children, and it occurs in about 2 to 3 per 1,000 births worldwide.1 This nonprogressive disorder is characterized by symptoms that include spasticity, dystonia, choreoathetosis, and/or ataxia that are evident in the first few years of life. While many perinatal variables have been associated with CP, in most cases a specific cause is not identified.
Other neurodevelopmental disorders, such as intellectual disability, epilepsy, and autism spectrum disorder, are often associated with CP.2 These other neurodevelopmental disorders are often genetic, and this has raised the question as to whether CP also might have a substantial genetic component, although this has not been investigated in any significant way until recently. This topic is of great interest to the obstetric community, given that CP often is attributed to obstetric events, including mismanagement of labor and delivery.
Emerging evidence of a genetic-CP association
In an article published recently in JAMA, Moreno-De-Luca and colleagues sought to determine the diagnostic yield of exome sequencing for CP.3 This large cross-sectional study included results of exome sequencing performed in 2 settings. The first setting was a commercial laboratory in which samples were sent for analysis due to a diagnosis of CP, primarily in children (n = 1,345) with a median age of 8.8 years. A second cohort, recruited from a neurodevelopmental disorders clinic at Geisinger, included primarily adults (n = 181) with a median age of 41.9 years.
As is standard in exome sequencing, results were considered likely causative if they were classified as pathogenic or likely pathogenic based on criteria of the American College of Genetics and Genomics. In the laboratory group, 32.7% (440 of 1,345) had a genetic cause of the CP identified, while in the clinic group, 10.5% (19 of 181) had a genetic etiology found. Although most of the identified genetic variants were de novo (that is, they arose in the affected individual and were not clearly inherited), some were inherited from carrier parents.3
A number of other recent studies also have investigated genetic causes of CP and similarly have reported that a substantial number of cases are genetic. Several studies that performed chromosomal microarray analysis in individuals with CP found deleterious copy number variants in 10% to 31% of cases.4-6 Genomic variants detectable by exome sequencing have been reported in 15% to 20% of cases.3 In a recent study in Nature Genetics, researchers performed exome sequencing on 250 parent-child “trios” in which the child had CP, and they found that 14% of cases had an associated genetic variant that was thought to be causative.4 These studies all provide consistent evidence that a substantial proportion of CP cases are due to genetic causes.
Contributors to CP risk
Historically, CP was considered to occur largely as a result of perinatal anoxia. In 1862, the British orthopedic surgeon William John Little first reported an association between prematurity, asphyxia, difficult delivery, and CP in a paper presented to the Obstetrical Society of London.7 Subsequently, much effort has gone into the prevention of perinatal asphyxia and birth injury, although our ability to monitor fetal well-being remains limited. Nonreassuring fetal heart rate patterns are nonspecific and can occur for many reasons other than fetal asphyxia. Studies of electronic fetal monitoring have found that continuous monitoring primarily leads to an increase in cesarean delivery with no decrease in CP or infant mortality.8
While some have attributed this to failure to accurately interpret the fetal heart rate tracing, it also may be because a substantial number of CP cases are due to genetic and other causes, and that very few in fact result from preventable intrapartum injury.
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics agree that knowledge gaps preclude definitive determination that a given case of neonatal encephalopathy is attributable to an acute intrapartum event, and they provide criteria that must be fulfilled to establish a reasonable causal link between an intrapartum event and subsequent long-term neurologic disability.9 However, there continues to be a belief in the medical, scientific, and lay communities that birth asphyxia, secondary to adverse intrapartum events, is the leading cause of CP. A “brain-damaged infant” remains one of the most common malpractice claims, and birth injury one of the highest paid claims. Such claims generally allege that intrapartum asphyxia has caused long-term neurologic sequelae, including CP.
While it is true that prematurity, infection, hypoxia-ischemia, and pre- and perinatal stroke all have been implicated as contributing to CP risk, large population-based studies have shown that birth asphyxia accounts for less than 12% of CP cases.10 Specifically, recent data indicate that acute intrapartum hypoxia-ischemia occurs only in about 6% of CP cases. In other words, it does occur and may contribute to some cases, but this is likely a smaller percent than previously thought, and genetic factors now appear to be far more significant contributors.11
Continue to: Exploring a genetic etiology...
Exploring a genetic etiology
In considering the etiologies of CP, it is important to note that 21% to 40% of individuals with CP have an associated congenital anomaly, suggesting a genetic origin in at least some individuals. Moreover, a 40% heritability has been estimated in CP, which is comparable to the heritability rate for autism spectrum disorders.12
In the recent study by Moreno-De-Luca and colleagues, some of the gene variants detected were previously associated with other forms of neurodevelopmental disability, such as epilepsy and autism spectrum disorder.3 Many individuals in the study cohort were found to have multiple neurologic comorbidities, for example, CP as well as epilepsy, autism spectrum disorder, and/or intellectual disability. The presence of these additional comorbidities increased the likelihood of finding a genetic cause; the authors found that the diagnostic yield ranged from 11.2% with isolated CP to 32.9% with all 3 comorbidities. The yield was highest with CP and intellectual disability and CP with all 3 comorbidities. A few genes were particularly common, and some were reported previously in association with CP and/or other neurodevelopmental disorders. In some patients, variants were found in genes or gene regions associated with disorders that do not frequently include CP, such as Rett syndrome.3
Implications for ObGyns
The data from the study by Moreno-De-Luca and colleagues are interesting and relevant to pediatricians, neurologists, and geneticists, as well as obstetricians. Understanding the cause of any disease or disorder improves care, including counseling regarding the cause, the appropriate interventions or therapy, and in some families, the recurrence risk in another pregnancy. The treatment for CP has not changed significantly in many years. Increasingly, detection of an underlying genetic cause can guide precision treatments; thus, the detection of specific gene variants allows a targeted approach to therapy.
Identification of a genetic cause also can significantly impact recurrence risk counseling and prenatal diagnosis options in another pregnancy. In general, the empiric recurrence risk of CP is quoted as 1% to 2%,13 and with de novo variants this does not change. However, with inherited variants the recurrence risk in future children is substantially higher. While 72% of the genetic variants associated with CP in the Moreno-De-Luca study were de novo with a low recurrence risk, in the other 28% the mode of inheritance indicated a substantial risk of recurrence (25%–50%) in another pregnancy.3 Detecting such causative variant(s) allows not only accurate counseling about recurrence risk but also preimplantation genetic testing or prenatal diagnosis when recurrence risk is high.
In the field of obstetrics, the debate about the etiology of CP is important largely due to the medicolegal implications. Patient-oriented information on the internet often states that CP is caused by damage to the child’s brain just before, during, or soon after birth, supporting potential blame of those providing care during those times. Patient-oriented websites regarding CP do not list genetic disorders among the causes but rather include primarily environmental factors, such as prematurity, low birth weight, in utero infections, anoxia or other brain injury, or perinatal stroke. Even the Centers for Disease Control and Prevention website lists brain damage as the primary etiology of CP.14 Hopefully, these new data will increase a broader understanding of this condition.
Exome sequencing is now recommended as a first-tier test for individuals with many neurodevelopmental disorders, including epilepsy, intellectual disability, and autism spectrum disorder.15 However, comprehensive genetic testing is not typically recommended or performed in cases of CP. Based on recent data, including the report by Moreno-De-Luca and colleagues, it would seem that CP should be added to the list of disorders for which exome sequencing is ordered, given the similar prevalence and diagnostic yield. ●
- Oskoui M, Coutinho F, Dykeman J, et al. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55:509-519.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8-14.
- Moreno-De-Luca A, Millan F, Pesacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Jin SC, Lewis SA, Bakhtiari S, et al. Mutations disrupting neuritogenesis genes confer risk for cerebral palsy. Nat Genet. 2020;52:1046-1056.
- Segel R, Ben-Pazi H, Zeligson S, et al. Copy number variations in cryptogenic cerebral palsy. Neurology. 2015;84:1660-1668.
- McMichael G, Girirrajan S, Moreno-De-Luca A, et al. Rare copy number variation in cerebral palsy. Eur J Hum Genet. 2014;22:40-45.
- Little WJ. On the influence of abnormal parturition, difficult labours, premature births, and asphyxia neonatorum, on the mental and physical condition of the child, especially in relation to deformities. Trans Obstet Soc Lond. 1862;3:293-344.
- Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;5;CD006066.
- American College of Obstetricians and Gynecologists. Executive summary: neonatal encephalopathy and neurologic outcome second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896- 901.
- Ellenberg JH, Nelson KB. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210- 216.
- Himmelmann K, Uvebrant P. The panorama of cerebral palsy in Sweden part XII shows that patterns changed in the birth years 2007–2010. Acta Paediatr. 2018;107: 462-468.
- Petterson B, Stanley F, Henderson D. Cerebral palsy in multiple births in Western Australia: genetic aspects. Am J Med Genet. 1990;37:346- 351.
- Korzeniewski SJ, Slaughter J, Lenski M, et al. The complex aetiology of cerebral palsy. Nat Rev Neurol. 2018;14:528-543.
- Centers for Disease Control and Prevention. Causes and risk factors of cerebral palsy. https:// www.cdc.gov/ncbddd/cp/causes.html. Accessed March 23, 2021.
- Srivastava S, Love-Nichols JA, Dies KA, et al; NDD Exome Scoping Review Work Group. Meta-analysis and multidisciplinary consensus statement: exome sequencing is a first-tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genet Med. 2019;21:2413-2421.
- Oskoui M, Coutinho F, Dykeman J, et al. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55:509-519.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8-14.
- Moreno-De-Luca A, Millan F, Pesacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Jin SC, Lewis SA, Bakhtiari S, et al. Mutations disrupting neuritogenesis genes confer risk for cerebral palsy. Nat Genet. 2020;52:1046-1056.
- Segel R, Ben-Pazi H, Zeligson S, et al. Copy number variations in cryptogenic cerebral palsy. Neurology. 2015;84:1660-1668.
- McMichael G, Girirrajan S, Moreno-De-Luca A, et al. Rare copy number variation in cerebral palsy. Eur J Hum Genet. 2014;22:40-45.
- Little WJ. On the influence of abnormal parturition, difficult labours, premature births, and asphyxia neonatorum, on the mental and physical condition of the child, especially in relation to deformities. Trans Obstet Soc Lond. 1862;3:293-344.
- Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2013;5;CD006066.
- American College of Obstetricians and Gynecologists. Executive summary: neonatal encephalopathy and neurologic outcome second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896- 901.
- Ellenberg JH, Nelson KB. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210- 216.
- Himmelmann K, Uvebrant P. The panorama of cerebral palsy in Sweden part XII shows that patterns changed in the birth years 2007–2010. Acta Paediatr. 2018;107: 462-468.
- Petterson B, Stanley F, Henderson D. Cerebral palsy in multiple births in Western Australia: genetic aspects. Am J Med Genet. 1990;37:346- 351.
- Korzeniewski SJ, Slaughter J, Lenski M, et al. The complex aetiology of cerebral palsy. Nat Rev Neurol. 2018;14:528-543.
- Centers for Disease Control and Prevention. Causes and risk factors of cerebral palsy. https:// www.cdc.gov/ncbddd/cp/causes.html. Accessed March 23, 2021.
- Srivastava S, Love-Nichols JA, Dies KA, et al; NDD Exome Scoping Review Work Group. Meta-analysis and multidisciplinary consensus statement: exome sequencing is a first-tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genet Med. 2019;21:2413-2421.
More reassurance for certain antiseizure drugs in pregnancy
Further evidence supporting the safety of two antiseizure medications in pregnancy has come from a new study. Most of the women with epilepsy in the study took either lamotrigine or levetiracetam, or a combination of the two, during their pregnancy.
However, a secondary analysis suggested a possible signal of exposure-dependent effects on child outcomes – worse outcomes with higher exposure levels – with levetiracetam.
The results were presented at the American Academy of Neurology’s 2021 annual meeting.
Additional reassurance
“Our new study adds confidence to the use of lamotrigine and levetiracetam during pregnancy, adding larger numbers with a new cohort. In addition, it provides some preliminary data on some of the other new antiseizure medications, and it is the first study to address the effects of clearance in pregnancy to better assess exposure,” said lead investigator, Kimford J. Meador, MD. “Overall, I am reassured by this data, but there is still a lot that is unknown,” he added.
“Our main results show no difference in verbal index or general conceptual ability scores in children born to women with epilepsy compared to children born to healthy women. This is a big positive message,” Dr. Meador said.
In terms of secondary analysis focusing on exposure levels (dose and blood levels of antiseizure medications), there was no overall signal of harm when looking at the whole group, but when the researchers analyzed the data on individual drugs, they found a “slight signal” toward reduced verbal index scores with increasing exposure levels with levetiracetam. No differences were seen on general conceptual ability.
“In the secondary analysis, there was a marginal signal for exposure levels with levetiracetam, with increased blood levels of the drug associated with reduced verbal index scores,” reported Dr. Meador, professor of neurology and neurological sciences at Stanford (Calif.) University. “We saw some signal in the children when they were 2 years old, and this was still there but not as striking at 3 years old.”
He said these secondary results should be interpreted with extreme caution. “We don’t want to overemphasize these secondary findings, as the primary outcome showed no difference, and there was no effect on exposure levels when looking at all the drugs together. I don’t want to oversimplify this, as I am still not sure whether this is a real association or not,” Dr. Meador commented.
He explained that conducting neurobehavioral tests on 2- and 3-year-olds was very difficult. “It is more of an art form than science, and as the children get older these signals often dissipate. We will know more by the time they are 6, when these tests become easier to conduct,” he said. He also noted that the results would need to be replicated in a different cohort.
“I don’t think these results would change how we manage women during pregnancy in terms of using levetiracetam. It is still a safe drug during pregnancy,” Dr. Meador said.
He pointed out that data on safety in pregnancy is only available for very few antiseizure drugs. “There are over 30 antiseizure medications, but we have adequate data in pregnancy on only a handful. We have data suggesting lamotrigine, levetiracetam, and carbamazepine appear to be relatively safe, and evidence showing phenobarbital and valproate are not safe.”
Antiseizure medications as a class are among the most commonly prescribed teratogenic drugs given to women of childbearing age, Dr. Meador noted. They are used not only for epilepsy but also for many other psychiatric and pain indications, so these results are applicable to quite a broad population, he added.
He pointed out that previous studies did not assess exposure using blood levels, which is important, as clearance of drug increases during pregnancy but varies across antiseizure medications and across individuals on the same drug. “Thus, it is unclear if these changes could obscure exposure-dependent effects. Our present studies assessed blood levels to better measure fetal exposure.”
Advice for pregnant patients with epilepsy
Dr. Meador explained that risk for adverse effects with antiseizure medication always needs to be balanced with risk for seizures if the medication was not used.
“In women planning a pregnancy, we recommend that they plan ahead with their physician to try and use the safest antiseizure medication and gain good control beforehand and then maintain the same blood levels of whichever drug is being used during pregnancy,” Dr. Meador said. “At present, lamotrigine and levetiracetam are the two safest drugs to use in pregnancy. They both look generally very safe compared with some other epilepsy drugs – such as valproate, which poses a serious risk to cognitive and behavioral development.”
He also advised that women should be taking folic acid regularly, as this has been shown to be related to improved cognitive and behavioral outcomes. “Since half of pregnancies are not planned, it is important to take these actions before pregnancy,” he added.
The current study involved 289 women with epilepsy and 89 women without epilepsy, all of whom enrolled in the study during pregnancy. Use of antiseizure medications was recorded. Of the women with epilepsy, 74% were on monotherapy, with 43% on lamotrigine and 37% on levetiracetam. There were 4% who took no drug and 22% took more than one drug. Of those who took more than one drug, close to half took a combination of lamotrigine and levetiracetam. Levels of medications in the blood of the women with epilepsy were measured in the third trimester.
Assessment of neurobehavioral development
For the current analysis, the children were evaluated at age 3 with a series of cognitive and developmental tests that measured vocabulary, listening comprehension, number recall, and pattern recognition, and results were adjusted for mother’s IQ, education level, age at enrollment, postbirth average BAI (Beck Anxiety Inventory score), and child’s ethnicity, sex, and breastfeeding status.
The primary outcome showed that verbal Index scores at age 3 did not differ for children of women with epilepsy versus those for children of women without epilepsy (LS mean 102.7 vs. 102.1).
Antiseizure medication exposure as evident by the maximum third trimester blood levels was not related to verbal index scores (n = 265; adjusted parameter estimate, -1.9; 95% confidence interval, -6.8 to 3.1).
General conceptual ability scores also did not differ between the two groups: 105.1 for children of women with epilepsy versus 103.5 for children of healthy women.
In terms of exposure levels, the third trimester maximum observed ratio of antiseizure medication blood levels was not significantly associated with adjusted general conceptual ability scores for children of women with epilepsy; neither were monotherapies or polytherapies evaluated separately, Dr. Meador reported.
However, when the verbal index scores for the main antiepileptic drug groups were analyzed separately, exposure level to levetiracetam was the only one that was significant, with a P value of .028. But Dr. Meador again stressed that this finding should be interpreted with caution given that it is a secondary exploratory analysis without control for multiple comparisons.
The researchers plan to assess these children at older ages where evaluations are more sensitive to ultimate outcomes.
“Information on use in pregnancy for most antiseizure medications is still unknown, so further studies to assess risks for the newer antiseizure medications are needed,” Dr. Meador added. “Further, additional research is needed on the underlying mechanisms including genetic predispositions, since teratogens act on a susceptible genotype.”
The study was supported by the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Further evidence supporting the safety of two antiseizure medications in pregnancy has come from a new study. Most of the women with epilepsy in the study took either lamotrigine or levetiracetam, or a combination of the two, during their pregnancy.
However, a secondary analysis suggested a possible signal of exposure-dependent effects on child outcomes – worse outcomes with higher exposure levels – with levetiracetam.
The results were presented at the American Academy of Neurology’s 2021 annual meeting.
Additional reassurance
“Our new study adds confidence to the use of lamotrigine and levetiracetam during pregnancy, adding larger numbers with a new cohort. In addition, it provides some preliminary data on some of the other new antiseizure medications, and it is the first study to address the effects of clearance in pregnancy to better assess exposure,” said lead investigator, Kimford J. Meador, MD. “Overall, I am reassured by this data, but there is still a lot that is unknown,” he added.
“Our main results show no difference in verbal index or general conceptual ability scores in children born to women with epilepsy compared to children born to healthy women. This is a big positive message,” Dr. Meador said.
In terms of secondary analysis focusing on exposure levels (dose and blood levels of antiseizure medications), there was no overall signal of harm when looking at the whole group, but when the researchers analyzed the data on individual drugs, they found a “slight signal” toward reduced verbal index scores with increasing exposure levels with levetiracetam. No differences were seen on general conceptual ability.
“In the secondary analysis, there was a marginal signal for exposure levels with levetiracetam, with increased blood levels of the drug associated with reduced verbal index scores,” reported Dr. Meador, professor of neurology and neurological sciences at Stanford (Calif.) University. “We saw some signal in the children when they were 2 years old, and this was still there but not as striking at 3 years old.”
He said these secondary results should be interpreted with extreme caution. “We don’t want to overemphasize these secondary findings, as the primary outcome showed no difference, and there was no effect on exposure levels when looking at all the drugs together. I don’t want to oversimplify this, as I am still not sure whether this is a real association or not,” Dr. Meador commented.
He explained that conducting neurobehavioral tests on 2- and 3-year-olds was very difficult. “It is more of an art form than science, and as the children get older these signals often dissipate. We will know more by the time they are 6, when these tests become easier to conduct,” he said. He also noted that the results would need to be replicated in a different cohort.
“I don’t think these results would change how we manage women during pregnancy in terms of using levetiracetam. It is still a safe drug during pregnancy,” Dr. Meador said.
He pointed out that data on safety in pregnancy is only available for very few antiseizure drugs. “There are over 30 antiseizure medications, but we have adequate data in pregnancy on only a handful. We have data suggesting lamotrigine, levetiracetam, and carbamazepine appear to be relatively safe, and evidence showing phenobarbital and valproate are not safe.”
Antiseizure medications as a class are among the most commonly prescribed teratogenic drugs given to women of childbearing age, Dr. Meador noted. They are used not only for epilepsy but also for many other psychiatric and pain indications, so these results are applicable to quite a broad population, he added.
He pointed out that previous studies did not assess exposure using blood levels, which is important, as clearance of drug increases during pregnancy but varies across antiseizure medications and across individuals on the same drug. “Thus, it is unclear if these changes could obscure exposure-dependent effects. Our present studies assessed blood levels to better measure fetal exposure.”
Advice for pregnant patients with epilepsy
Dr. Meador explained that risk for adverse effects with antiseizure medication always needs to be balanced with risk for seizures if the medication was not used.
“In women planning a pregnancy, we recommend that they plan ahead with their physician to try and use the safest antiseizure medication and gain good control beforehand and then maintain the same blood levels of whichever drug is being used during pregnancy,” Dr. Meador said. “At present, lamotrigine and levetiracetam are the two safest drugs to use in pregnancy. They both look generally very safe compared with some other epilepsy drugs – such as valproate, which poses a serious risk to cognitive and behavioral development.”
He also advised that women should be taking folic acid regularly, as this has been shown to be related to improved cognitive and behavioral outcomes. “Since half of pregnancies are not planned, it is important to take these actions before pregnancy,” he added.
The current study involved 289 women with epilepsy and 89 women without epilepsy, all of whom enrolled in the study during pregnancy. Use of antiseizure medications was recorded. Of the women with epilepsy, 74% were on monotherapy, with 43% on lamotrigine and 37% on levetiracetam. There were 4% who took no drug and 22% took more than one drug. Of those who took more than one drug, close to half took a combination of lamotrigine and levetiracetam. Levels of medications in the blood of the women with epilepsy were measured in the third trimester.
Assessment of neurobehavioral development
For the current analysis, the children were evaluated at age 3 with a series of cognitive and developmental tests that measured vocabulary, listening comprehension, number recall, and pattern recognition, and results were adjusted for mother’s IQ, education level, age at enrollment, postbirth average BAI (Beck Anxiety Inventory score), and child’s ethnicity, sex, and breastfeeding status.
The primary outcome showed that verbal Index scores at age 3 did not differ for children of women with epilepsy versus those for children of women without epilepsy (LS mean 102.7 vs. 102.1).
Antiseizure medication exposure as evident by the maximum third trimester blood levels was not related to verbal index scores (n = 265; adjusted parameter estimate, -1.9; 95% confidence interval, -6.8 to 3.1).
General conceptual ability scores also did not differ between the two groups: 105.1 for children of women with epilepsy versus 103.5 for children of healthy women.
In terms of exposure levels, the third trimester maximum observed ratio of antiseizure medication blood levels was not significantly associated with adjusted general conceptual ability scores for children of women with epilepsy; neither were monotherapies or polytherapies evaluated separately, Dr. Meador reported.
However, when the verbal index scores for the main antiepileptic drug groups were analyzed separately, exposure level to levetiracetam was the only one that was significant, with a P value of .028. But Dr. Meador again stressed that this finding should be interpreted with caution given that it is a secondary exploratory analysis without control for multiple comparisons.
The researchers plan to assess these children at older ages where evaluations are more sensitive to ultimate outcomes.
“Information on use in pregnancy for most antiseizure medications is still unknown, so further studies to assess risks for the newer antiseizure medications are needed,” Dr. Meador added. “Further, additional research is needed on the underlying mechanisms including genetic predispositions, since teratogens act on a susceptible genotype.”
The study was supported by the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
Further evidence supporting the safety of two antiseizure medications in pregnancy has come from a new study. Most of the women with epilepsy in the study took either lamotrigine or levetiracetam, or a combination of the two, during their pregnancy.
However, a secondary analysis suggested a possible signal of exposure-dependent effects on child outcomes – worse outcomes with higher exposure levels – with levetiracetam.
The results were presented at the American Academy of Neurology’s 2021 annual meeting.
Additional reassurance
“Our new study adds confidence to the use of lamotrigine and levetiracetam during pregnancy, adding larger numbers with a new cohort. In addition, it provides some preliminary data on some of the other new antiseizure medications, and it is the first study to address the effects of clearance in pregnancy to better assess exposure,” said lead investigator, Kimford J. Meador, MD. “Overall, I am reassured by this data, but there is still a lot that is unknown,” he added.
“Our main results show no difference in verbal index or general conceptual ability scores in children born to women with epilepsy compared to children born to healthy women. This is a big positive message,” Dr. Meador said.
In terms of secondary analysis focusing on exposure levels (dose and blood levels of antiseizure medications), there was no overall signal of harm when looking at the whole group, but when the researchers analyzed the data on individual drugs, they found a “slight signal” toward reduced verbal index scores with increasing exposure levels with levetiracetam. No differences were seen on general conceptual ability.
“In the secondary analysis, there was a marginal signal for exposure levels with levetiracetam, with increased blood levels of the drug associated with reduced verbal index scores,” reported Dr. Meador, professor of neurology and neurological sciences at Stanford (Calif.) University. “We saw some signal in the children when they were 2 years old, and this was still there but not as striking at 3 years old.”
He said these secondary results should be interpreted with extreme caution. “We don’t want to overemphasize these secondary findings, as the primary outcome showed no difference, and there was no effect on exposure levels when looking at all the drugs together. I don’t want to oversimplify this, as I am still not sure whether this is a real association or not,” Dr. Meador commented.
He explained that conducting neurobehavioral tests on 2- and 3-year-olds was very difficult. “It is more of an art form than science, and as the children get older these signals often dissipate. We will know more by the time they are 6, when these tests become easier to conduct,” he said. He also noted that the results would need to be replicated in a different cohort.
“I don’t think these results would change how we manage women during pregnancy in terms of using levetiracetam. It is still a safe drug during pregnancy,” Dr. Meador said.
He pointed out that data on safety in pregnancy is only available for very few antiseizure drugs. “There are over 30 antiseizure medications, but we have adequate data in pregnancy on only a handful. We have data suggesting lamotrigine, levetiracetam, and carbamazepine appear to be relatively safe, and evidence showing phenobarbital and valproate are not safe.”
Antiseizure medications as a class are among the most commonly prescribed teratogenic drugs given to women of childbearing age, Dr. Meador noted. They are used not only for epilepsy but also for many other psychiatric and pain indications, so these results are applicable to quite a broad population, he added.
He pointed out that previous studies did not assess exposure using blood levels, which is important, as clearance of drug increases during pregnancy but varies across antiseizure medications and across individuals on the same drug. “Thus, it is unclear if these changes could obscure exposure-dependent effects. Our present studies assessed blood levels to better measure fetal exposure.”
Advice for pregnant patients with epilepsy
Dr. Meador explained that risk for adverse effects with antiseizure medication always needs to be balanced with risk for seizures if the medication was not used.
“In women planning a pregnancy, we recommend that they plan ahead with their physician to try and use the safest antiseizure medication and gain good control beforehand and then maintain the same blood levels of whichever drug is being used during pregnancy,” Dr. Meador said. “At present, lamotrigine and levetiracetam are the two safest drugs to use in pregnancy. They both look generally very safe compared with some other epilepsy drugs – such as valproate, which poses a serious risk to cognitive and behavioral development.”
He also advised that women should be taking folic acid regularly, as this has been shown to be related to improved cognitive and behavioral outcomes. “Since half of pregnancies are not planned, it is important to take these actions before pregnancy,” he added.
The current study involved 289 women with epilepsy and 89 women without epilepsy, all of whom enrolled in the study during pregnancy. Use of antiseizure medications was recorded. Of the women with epilepsy, 74% were on monotherapy, with 43% on lamotrigine and 37% on levetiracetam. There were 4% who took no drug and 22% took more than one drug. Of those who took more than one drug, close to half took a combination of lamotrigine and levetiracetam. Levels of medications in the blood of the women with epilepsy were measured in the third trimester.
Assessment of neurobehavioral development
For the current analysis, the children were evaluated at age 3 with a series of cognitive and developmental tests that measured vocabulary, listening comprehension, number recall, and pattern recognition, and results were adjusted for mother’s IQ, education level, age at enrollment, postbirth average BAI (Beck Anxiety Inventory score), and child’s ethnicity, sex, and breastfeeding status.
The primary outcome showed that verbal Index scores at age 3 did not differ for children of women with epilepsy versus those for children of women without epilepsy (LS mean 102.7 vs. 102.1).
Antiseizure medication exposure as evident by the maximum third trimester blood levels was not related to verbal index scores (n = 265; adjusted parameter estimate, -1.9; 95% confidence interval, -6.8 to 3.1).
General conceptual ability scores also did not differ between the two groups: 105.1 for children of women with epilepsy versus 103.5 for children of healthy women.
In terms of exposure levels, the third trimester maximum observed ratio of antiseizure medication blood levels was not significantly associated with adjusted general conceptual ability scores for children of women with epilepsy; neither were monotherapies or polytherapies evaluated separately, Dr. Meador reported.
However, when the verbal index scores for the main antiepileptic drug groups were analyzed separately, exposure level to levetiracetam was the only one that was significant, with a P value of .028. But Dr. Meador again stressed that this finding should be interpreted with caution given that it is a secondary exploratory analysis without control for multiple comparisons.
The researchers plan to assess these children at older ages where evaluations are more sensitive to ultimate outcomes.
“Information on use in pregnancy for most antiseizure medications is still unknown, so further studies to assess risks for the newer antiseizure medications are needed,” Dr. Meador added. “Further, additional research is needed on the underlying mechanisms including genetic predispositions, since teratogens act on a susceptible genotype.”
The study was supported by the National Institutes of Health, the National Institute of Neurological Disorders and Stroke, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A version of this article first appeared on Medscape.com.
From AAN 2021
Failure to rescue occurs more often among women of color
In the United States, the rate of mortality caused by severe maternal morbidity has improved over time, but failure to rescue is significantly more common among racial and ethnic minorities.
These failures are a “major contributing factor” to the disproportionately higher rate of maternal mortality among women of color, reported lead author Jean Guglielminotti, MD, PhD, of Columbia University, New York, and colleagues.
“Racial and ethnic disparities in severe maternal morbidity are a growing public health concern in the United States,” the investigators wrote in Obstetrics & Gynecology.
“The reported incidence of severe maternal morbidity is twofold to threefold higher among Black American women, compared with non-Hispanic White women; and although the difference is less pronounced, the incidence of severe maternal morbidity also is higher among Hispanic, Asian and Pacific Islander, and Native American women.”
The ensuant, disproportionate risk of maternal mortality may be further exacerbated by disparities in hospitals, according to the investigators. They noted that non-Hispanic White women tend to give birth in different hospitals than racial and ethnic minorities, and the hospitals serving people of color “are characterized by lower performance on maternal safety indicators.”
Even within hospitals that most often serve minorities, severe maternal morbidity is more common among women of color than women who are White, they added.
“However, the simple severe maternal morbidity rate is insufficient to assess hospital performance and should be complemented with the rate of failure to rescue,” wrote Dr. Guglielminotti and colleagues.
Measuring failure to rescue across racial and ethnic groups
According to the investigators, failure-to-rescue rate advances focus from complications themselves – which can occur when care is appropriate and may stem from patient characteristics – to a hospital’s response to such complications.
Using this metric, a 2016 study by Friedman and colleagues, which included data from 1998 to 2010, showed failure to rescue was more common among Hispanic and non-Hispanic Black women than white women.
The present study built upon these findings with data from almost 74 million delivery hospitalizations in the National Inpatient Sample (1999-2017). The population included 993,864 women with severe maternal morbidity, among whom 4,328 died.
Overall, the failure-to-rescue rate decreased over the course of the study from 13.2% in 1999-2000 to 4.5% in 2017 (P < .001).
Yet racial and ethnic inequities were apparent.
Compared with White women, non-Hispanic Black women had a significantly higher failure-to-rescue rate ratio (1.79; 95% CI, 1.77-1.81), as did Hispanic women (RR, 1.08; 95% CI, 1.06-1.09), women of other non-White racial/ethnic backgrounds (RR, 1.39; 95% CI, 1.37-1.41), and women documented without racial/ethnic designations (RR, 1.43; 95% CI, 1.42-1.45).
“Failure to rescue from severe maternal morbidity remains a major contributing factor to the excess maternal mortality in racial and ethnic minority women in the United States,” the investigators concluded. “This finding underscores the need to identify factors accounting for these disparities and develop hospital-based interventions to reduce excess maternal mortality in racial and ethnic minority women.”
Striving for progress through systemic change
According to Eve Espey, MD, MPH, of the University of New Mexico, Albuquerque, “this study adds to the literature demonstrating that structural racism and implicit bias have profound negative impacts,” which “has implications for action.”
“We must increase efforts to improve maternal safety, including the rollout of Alliance for Innovation on Maternal Health [AIM] bundles through statewide perinatal quality collaboratives,” Dr. Espey said. “AIM bundle implementation must focus on the context of health inequities related to racism and bias. Similarly, we must consider large scale public policy changes building on the Affordable Care Act, such as universal health coverage throughout the life span, [which] equitably increases access to quality health care for all.”
Constance Bohon, MD, of Sibley Memorial Hospital, Washington, offered a similar viewpoint, and suggested that further analyses could reveal the impacts of systemic changes, thereby guiding future interventions.
“It would be interesting to determine if declines in failure to rescue rates were greatest in states that implemented AIM safety bundles [in 2012] as compared with the states that did not,” Dr. Bohon said. “The same assessment could be made with a comparison between the states that did and those that did not approve the Medicaid expansion [in 2014]. Other beneficial data would be a comparison of the failure-to-rescue rates in hospitals that provide the same obstetrical level of care. Further studies need to be done in order to identify factors that have the greatest impact on the failure-to-rescue rate. Subsequently, proposals can be suggested for actions that can be taken to decrease the excess maternal mortality in racial and ethnic minorities.”
Comparing the U.S. with the rest of the world
In an accompanying editorial, Marian F. MacDorman, PhD, of the University of Maryland, College Park, and Eugene Declercq, PhD, of Boston University, put the findings in a global context.
They noted that, in the United States over the past 2 decades, the rate of maternal mortality has either remained flat or increased, depending on study methodology; however, the relative state of affairs between the United States and the rest of the world is more straightforward.
“What is clear is that U.S. maternal mortality did not decline from 2000 to 2018,” wrote Dr. MacDorman and Dr. Declercq. “This contrasts with World Health Organization data showing that maternal mortality declined by 38% worldwide and by 53% in Europe from 2000 to 2017. In fact, North America was the only world region to not show substantial declines in maternal mortality during the period, and U.S. maternal mortality rates are nearly twice those in Europe.”
Within the US, these shortcomings are felt most acutely among racial and ethnic minorities, they noted, as the present study suggests.
“The U.S. is still plagued by wide racial disparities, with similar or larger Black-White maternal mortality disparities in 2018 than existed in the 1940s,” they wrote. “Thus, any euphoria generated by the lack of increase in maternal mortality (if accurate) must be set in the context of worldwide improvements, in which the U.S. is an outlier with no improvement. The U.S. can and should do better!”
To this end, Dr. MacDorman and Dr. Declercq wrote, “additional training and vigilance among clinicians can help to avert these largely preventable deaths. In addition, applying this same rigor to preventing deaths that occur in the community before and after birth, combined with a focus on social determinants among women during the reproductive years, will be essential to lowering U.S. maternal mortality overall and eliminating longstanding racial inequities.”
The study received no external funding. The investigators reported no conflicts of interest.
In the United States, the rate of mortality caused by severe maternal morbidity has improved over time, but failure to rescue is significantly more common among racial and ethnic minorities.
These failures are a “major contributing factor” to the disproportionately higher rate of maternal mortality among women of color, reported lead author Jean Guglielminotti, MD, PhD, of Columbia University, New York, and colleagues.
“Racial and ethnic disparities in severe maternal morbidity are a growing public health concern in the United States,” the investigators wrote in Obstetrics & Gynecology.
“The reported incidence of severe maternal morbidity is twofold to threefold higher among Black American women, compared with non-Hispanic White women; and although the difference is less pronounced, the incidence of severe maternal morbidity also is higher among Hispanic, Asian and Pacific Islander, and Native American women.”
The ensuant, disproportionate risk of maternal mortality may be further exacerbated by disparities in hospitals, according to the investigators. They noted that non-Hispanic White women tend to give birth in different hospitals than racial and ethnic minorities, and the hospitals serving people of color “are characterized by lower performance on maternal safety indicators.”
Even within hospitals that most often serve minorities, severe maternal morbidity is more common among women of color than women who are White, they added.
“However, the simple severe maternal morbidity rate is insufficient to assess hospital performance and should be complemented with the rate of failure to rescue,” wrote Dr. Guglielminotti and colleagues.
Measuring failure to rescue across racial and ethnic groups
According to the investigators, failure-to-rescue rate advances focus from complications themselves – which can occur when care is appropriate and may stem from patient characteristics – to a hospital’s response to such complications.
Using this metric, a 2016 study by Friedman and colleagues, which included data from 1998 to 2010, showed failure to rescue was more common among Hispanic and non-Hispanic Black women than white women.
The present study built upon these findings with data from almost 74 million delivery hospitalizations in the National Inpatient Sample (1999-2017). The population included 993,864 women with severe maternal morbidity, among whom 4,328 died.
Overall, the failure-to-rescue rate decreased over the course of the study from 13.2% in 1999-2000 to 4.5% in 2017 (P < .001).
Yet racial and ethnic inequities were apparent.
Compared with White women, non-Hispanic Black women had a significantly higher failure-to-rescue rate ratio (1.79; 95% CI, 1.77-1.81), as did Hispanic women (RR, 1.08; 95% CI, 1.06-1.09), women of other non-White racial/ethnic backgrounds (RR, 1.39; 95% CI, 1.37-1.41), and women documented without racial/ethnic designations (RR, 1.43; 95% CI, 1.42-1.45).
“Failure to rescue from severe maternal morbidity remains a major contributing factor to the excess maternal mortality in racial and ethnic minority women in the United States,” the investigators concluded. “This finding underscores the need to identify factors accounting for these disparities and develop hospital-based interventions to reduce excess maternal mortality in racial and ethnic minority women.”
Striving for progress through systemic change
According to Eve Espey, MD, MPH, of the University of New Mexico, Albuquerque, “this study adds to the literature demonstrating that structural racism and implicit bias have profound negative impacts,” which “has implications for action.”
“We must increase efforts to improve maternal safety, including the rollout of Alliance for Innovation on Maternal Health [AIM] bundles through statewide perinatal quality collaboratives,” Dr. Espey said. “AIM bundle implementation must focus on the context of health inequities related to racism and bias. Similarly, we must consider large scale public policy changes building on the Affordable Care Act, such as universal health coverage throughout the life span, [which] equitably increases access to quality health care for all.”
Constance Bohon, MD, of Sibley Memorial Hospital, Washington, offered a similar viewpoint, and suggested that further analyses could reveal the impacts of systemic changes, thereby guiding future interventions.
“It would be interesting to determine if declines in failure to rescue rates were greatest in states that implemented AIM safety bundles [in 2012] as compared with the states that did not,” Dr. Bohon said. “The same assessment could be made with a comparison between the states that did and those that did not approve the Medicaid expansion [in 2014]. Other beneficial data would be a comparison of the failure-to-rescue rates in hospitals that provide the same obstetrical level of care. Further studies need to be done in order to identify factors that have the greatest impact on the failure-to-rescue rate. Subsequently, proposals can be suggested for actions that can be taken to decrease the excess maternal mortality in racial and ethnic minorities.”
Comparing the U.S. with the rest of the world
In an accompanying editorial, Marian F. MacDorman, PhD, of the University of Maryland, College Park, and Eugene Declercq, PhD, of Boston University, put the findings in a global context.
They noted that, in the United States over the past 2 decades, the rate of maternal mortality has either remained flat or increased, depending on study methodology; however, the relative state of affairs between the United States and the rest of the world is more straightforward.
“What is clear is that U.S. maternal mortality did not decline from 2000 to 2018,” wrote Dr. MacDorman and Dr. Declercq. “This contrasts with World Health Organization data showing that maternal mortality declined by 38% worldwide and by 53% in Europe from 2000 to 2017. In fact, North America was the only world region to not show substantial declines in maternal mortality during the period, and U.S. maternal mortality rates are nearly twice those in Europe.”
Within the US, these shortcomings are felt most acutely among racial and ethnic minorities, they noted, as the present study suggests.
“The U.S. is still plagued by wide racial disparities, with similar or larger Black-White maternal mortality disparities in 2018 than existed in the 1940s,” they wrote. “Thus, any euphoria generated by the lack of increase in maternal mortality (if accurate) must be set in the context of worldwide improvements, in which the U.S. is an outlier with no improvement. The U.S. can and should do better!”
To this end, Dr. MacDorman and Dr. Declercq wrote, “additional training and vigilance among clinicians can help to avert these largely preventable deaths. In addition, applying this same rigor to preventing deaths that occur in the community before and after birth, combined with a focus on social determinants among women during the reproductive years, will be essential to lowering U.S. maternal mortality overall and eliminating longstanding racial inequities.”
The study received no external funding. The investigators reported no conflicts of interest.
In the United States, the rate of mortality caused by severe maternal morbidity has improved over time, but failure to rescue is significantly more common among racial and ethnic minorities.
These failures are a “major contributing factor” to the disproportionately higher rate of maternal mortality among women of color, reported lead author Jean Guglielminotti, MD, PhD, of Columbia University, New York, and colleagues.
“Racial and ethnic disparities in severe maternal morbidity are a growing public health concern in the United States,” the investigators wrote in Obstetrics & Gynecology.
“The reported incidence of severe maternal morbidity is twofold to threefold higher among Black American women, compared with non-Hispanic White women; and although the difference is less pronounced, the incidence of severe maternal morbidity also is higher among Hispanic, Asian and Pacific Islander, and Native American women.”
The ensuant, disproportionate risk of maternal mortality may be further exacerbated by disparities in hospitals, according to the investigators. They noted that non-Hispanic White women tend to give birth in different hospitals than racial and ethnic minorities, and the hospitals serving people of color “are characterized by lower performance on maternal safety indicators.”
Even within hospitals that most often serve minorities, severe maternal morbidity is more common among women of color than women who are White, they added.
“However, the simple severe maternal morbidity rate is insufficient to assess hospital performance and should be complemented with the rate of failure to rescue,” wrote Dr. Guglielminotti and colleagues.
Measuring failure to rescue across racial and ethnic groups
According to the investigators, failure-to-rescue rate advances focus from complications themselves – which can occur when care is appropriate and may stem from patient characteristics – to a hospital’s response to such complications.
Using this metric, a 2016 study by Friedman and colleagues, which included data from 1998 to 2010, showed failure to rescue was more common among Hispanic and non-Hispanic Black women than white women.
The present study built upon these findings with data from almost 74 million delivery hospitalizations in the National Inpatient Sample (1999-2017). The population included 993,864 women with severe maternal morbidity, among whom 4,328 died.
Overall, the failure-to-rescue rate decreased over the course of the study from 13.2% in 1999-2000 to 4.5% in 2017 (P < .001).
Yet racial and ethnic inequities were apparent.
Compared with White women, non-Hispanic Black women had a significantly higher failure-to-rescue rate ratio (1.79; 95% CI, 1.77-1.81), as did Hispanic women (RR, 1.08; 95% CI, 1.06-1.09), women of other non-White racial/ethnic backgrounds (RR, 1.39; 95% CI, 1.37-1.41), and women documented without racial/ethnic designations (RR, 1.43; 95% CI, 1.42-1.45).
“Failure to rescue from severe maternal morbidity remains a major contributing factor to the excess maternal mortality in racial and ethnic minority women in the United States,” the investigators concluded. “This finding underscores the need to identify factors accounting for these disparities and develop hospital-based interventions to reduce excess maternal mortality in racial and ethnic minority women.”
Striving for progress through systemic change
According to Eve Espey, MD, MPH, of the University of New Mexico, Albuquerque, “this study adds to the literature demonstrating that structural racism and implicit bias have profound negative impacts,” which “has implications for action.”
“We must increase efforts to improve maternal safety, including the rollout of Alliance for Innovation on Maternal Health [AIM] bundles through statewide perinatal quality collaboratives,” Dr. Espey said. “AIM bundle implementation must focus on the context of health inequities related to racism and bias. Similarly, we must consider large scale public policy changes building on the Affordable Care Act, such as universal health coverage throughout the life span, [which] equitably increases access to quality health care for all.”
Constance Bohon, MD, of Sibley Memorial Hospital, Washington, offered a similar viewpoint, and suggested that further analyses could reveal the impacts of systemic changes, thereby guiding future interventions.
“It would be interesting to determine if declines in failure to rescue rates were greatest in states that implemented AIM safety bundles [in 2012] as compared with the states that did not,” Dr. Bohon said. “The same assessment could be made with a comparison between the states that did and those that did not approve the Medicaid expansion [in 2014]. Other beneficial data would be a comparison of the failure-to-rescue rates in hospitals that provide the same obstetrical level of care. Further studies need to be done in order to identify factors that have the greatest impact on the failure-to-rescue rate. Subsequently, proposals can be suggested for actions that can be taken to decrease the excess maternal mortality in racial and ethnic minorities.”
Comparing the U.S. with the rest of the world
In an accompanying editorial, Marian F. MacDorman, PhD, of the University of Maryland, College Park, and Eugene Declercq, PhD, of Boston University, put the findings in a global context.
They noted that, in the United States over the past 2 decades, the rate of maternal mortality has either remained flat or increased, depending on study methodology; however, the relative state of affairs between the United States and the rest of the world is more straightforward.
“What is clear is that U.S. maternal mortality did not decline from 2000 to 2018,” wrote Dr. MacDorman and Dr. Declercq. “This contrasts with World Health Organization data showing that maternal mortality declined by 38% worldwide and by 53% in Europe from 2000 to 2017. In fact, North America was the only world region to not show substantial declines in maternal mortality during the period, and U.S. maternal mortality rates are nearly twice those in Europe.”
Within the US, these shortcomings are felt most acutely among racial and ethnic minorities, they noted, as the present study suggests.
“The U.S. is still plagued by wide racial disparities, with similar or larger Black-White maternal mortality disparities in 2018 than existed in the 1940s,” they wrote. “Thus, any euphoria generated by the lack of increase in maternal mortality (if accurate) must be set in the context of worldwide improvements, in which the U.S. is an outlier with no improvement. The U.S. can and should do better!”
To this end, Dr. MacDorman and Dr. Declercq wrote, “additional training and vigilance among clinicians can help to avert these largely preventable deaths. In addition, applying this same rigor to preventing deaths that occur in the community before and after birth, combined with a focus on social determinants among women during the reproductive years, will be essential to lowering U.S. maternal mortality overall and eliminating longstanding racial inequities.”
The study received no external funding. The investigators reported no conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
US methods of delivery, a snapshot
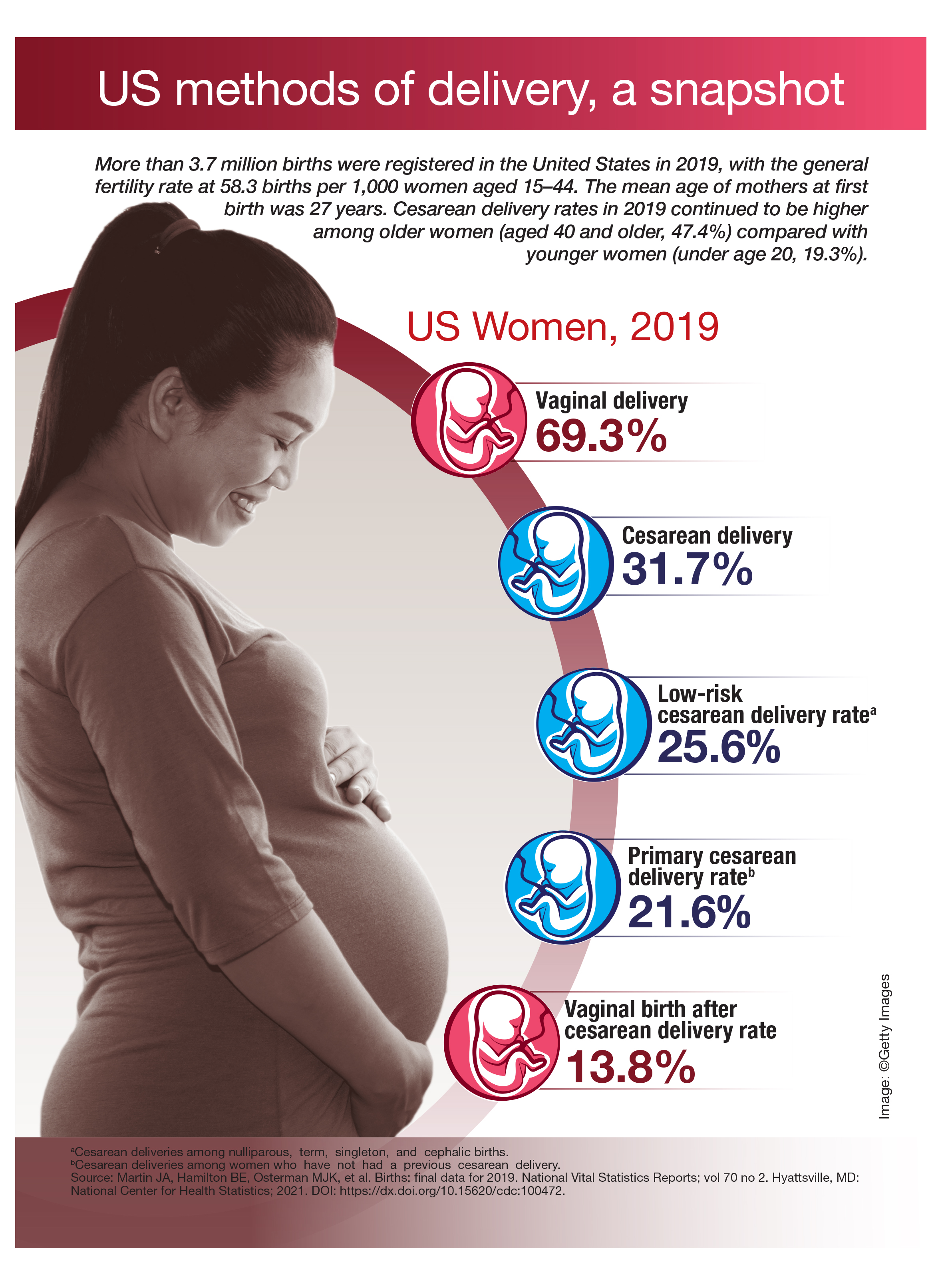


Antifungals during pregnancy and breastfeeding
There are three general classes of antifungal agents (number of agents): azole antifungals (9), echinocandins (3), and polyenes (5). The azole antifungals contain an azole ring and inhibit a wide range of fungi. Echinocandins target the fungal cell wall and the polyenes increase the fungal membrane permeability and lead to cell death.
Pregnancy
Azole antifungals inhibit the growth of fungi. Their trade names and molecular weights:
- Clotrimazole (Mycelex), an over-the-counter product, is available as a topical cream. Several studies have found no association between the drug and birth defects.
- Fluconazole (Diflucan) is a teratogen when doses of ≥400 mg/day are used during the first trimester. Smaller doses do not appear to cause embryo/fetal harm.
- Isavuconazonium (Cresemba) if used in pregnancy, exposure of the embryo/fetus would probably be low based on the >99% plasma protein binding, but the plasma half-life is 130 hours. Moreover, the drug is a potent animal teratogen and is best avoided in pregnancy.
- Itraconazole (Onmel, Sporanox, Tolsura), has a low risk, if any, of structural defects, according to what reported human experience suggests.
- Ketoconazole (Xolegel, Extina, Nizoral; 531) does not appear to adversely effect embryos and fetuses, but the human data are very limited. As with any drug, avoiding organogenesis is the best recommendation.
- Miconazole (Oravig) is usually used topically. Small amounts are absorbed from the vagina. The available evidence suggests that the drug does not increase the risk of congenital malformations.
- Posaconazole (Noxafil) does not have reported use in human pregnancy. The animal reproduction data suggest risk. Based on its molecular weight (about 701), the drug will most likely cross the placenta to the embryo/fetus. Thus, the best course is to avoid the drug during pregnancy, especially in the first trimester.
- Voriconazole (Vfend) has one human report of the drug use in pregnancy. The drug was started at about 19 weeks and continued until the woman gave birth at 35 weeks to a healthy male baby. At 6 months of age, the baby remained normal.
Echinocandin antifungals target the fungal cell wall by inhibiting its synthesis. Their trade names and molecular weights:
- Anidulafungin (Eraxis; 1,140) has no published human data. It is indicated for the treatment of candidemia and other forms of Candida infections. The animal data suggest low risk.
- Caspofungin (Cancidas; 1,213) has no published human data. It is indicated for presumed fungal infections in febrile, neutropenic patients. The animal data are suggestive of human risk, especially if exposure occurs in the first trimester. If possible, maternal treatment should be avoided in the first trimester.
- Micafungin (Mycamine; 1,292) has no published human data. It is indicated for the treatment of patients with esophageal candidiasis and for the prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation. The animal data in one species suggest high risk. If possible, maternal treatment should be avoided in the first trimester.
Polyene antifungals cause depolarization of the fungal cell membrane to increase the membrane permeability, which leads to cell death. Their trade names and molecular weights:
- Amphotericin b (Amphocin; Fungizone; 924) There are three other amphotericin agents: amphotericin b cholesteryl sulfate (Amphotec); amphotericin b lipid complex (Abelcet); amphotericin b liposomal (AmBisome). No reports linking amphotericin b with congenital defects have been found. The drug does cross the human placenta. Although there was a higher rate of spontaneous abortions in rabbits given amphotericin b, there was no fetal harm in rats and rabbits when given amphotericin b lipid complex.
- Nystatin (Bio-Statin; Mycostatin; Nilstat; 926). The drug does not appear to cause embryo-fetal harm. Based on published data, the drug can be used at any time in pregnancy.
Breastfeeding
Small amounts of all the above drugs are probably excreted into breast milk if they are used close to breastfeeding. Most can probably be used during breastfeeding, but there are no data for any of these agents. The safest decision is to not use these drugs when breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
There are three general classes of antifungal agents (number of agents): azole antifungals (9), echinocandins (3), and polyenes (5). The azole antifungals contain an azole ring and inhibit a wide range of fungi. Echinocandins target the fungal cell wall and the polyenes increase the fungal membrane permeability and lead to cell death.
Pregnancy
Azole antifungals inhibit the growth of fungi. Their trade names and molecular weights:
- Clotrimazole (Mycelex), an over-the-counter product, is available as a topical cream. Several studies have found no association between the drug and birth defects.
- Fluconazole (Diflucan) is a teratogen when doses of ≥400 mg/day are used during the first trimester. Smaller doses do not appear to cause embryo/fetal harm.
- Isavuconazonium (Cresemba) if used in pregnancy, exposure of the embryo/fetus would probably be low based on the >99% plasma protein binding, but the plasma half-life is 130 hours. Moreover, the drug is a potent animal teratogen and is best avoided in pregnancy.
- Itraconazole (Onmel, Sporanox, Tolsura), has a low risk, if any, of structural defects, according to what reported human experience suggests.
- Ketoconazole (Xolegel, Extina, Nizoral; 531) does not appear to adversely effect embryos and fetuses, but the human data are very limited. As with any drug, avoiding organogenesis is the best recommendation.
- Miconazole (Oravig) is usually used topically. Small amounts are absorbed from the vagina. The available evidence suggests that the drug does not increase the risk of congenital malformations.
- Posaconazole (Noxafil) does not have reported use in human pregnancy. The animal reproduction data suggest risk. Based on its molecular weight (about 701), the drug will most likely cross the placenta to the embryo/fetus. Thus, the best course is to avoid the drug during pregnancy, especially in the first trimester.
- Voriconazole (Vfend) has one human report of the drug use in pregnancy. The drug was started at about 19 weeks and continued until the woman gave birth at 35 weeks to a healthy male baby. At 6 months of age, the baby remained normal.
Echinocandin antifungals target the fungal cell wall by inhibiting its synthesis. Their trade names and molecular weights:
- Anidulafungin (Eraxis; 1,140) has no published human data. It is indicated for the treatment of candidemia and other forms of Candida infections. The animal data suggest low risk.
- Caspofungin (Cancidas; 1,213) has no published human data. It is indicated for presumed fungal infections in febrile, neutropenic patients. The animal data are suggestive of human risk, especially if exposure occurs in the first trimester. If possible, maternal treatment should be avoided in the first trimester.
- Micafungin (Mycamine; 1,292) has no published human data. It is indicated for the treatment of patients with esophageal candidiasis and for the prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation. The animal data in one species suggest high risk. If possible, maternal treatment should be avoided in the first trimester.
Polyene antifungals cause depolarization of the fungal cell membrane to increase the membrane permeability, which leads to cell death. Their trade names and molecular weights:
- Amphotericin b (Amphocin; Fungizone; 924) There are three other amphotericin agents: amphotericin b cholesteryl sulfate (Amphotec); amphotericin b lipid complex (Abelcet); amphotericin b liposomal (AmBisome). No reports linking amphotericin b with congenital defects have been found. The drug does cross the human placenta. Although there was a higher rate of spontaneous abortions in rabbits given amphotericin b, there was no fetal harm in rats and rabbits when given amphotericin b lipid complex.
- Nystatin (Bio-Statin; Mycostatin; Nilstat; 926). The drug does not appear to cause embryo-fetal harm. Based on published data, the drug can be used at any time in pregnancy.
Breastfeeding
Small amounts of all the above drugs are probably excreted into breast milk if they are used close to breastfeeding. Most can probably be used during breastfeeding, but there are no data for any of these agents. The safest decision is to not use these drugs when breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
There are three general classes of antifungal agents (number of agents): azole antifungals (9), echinocandins (3), and polyenes (5). The azole antifungals contain an azole ring and inhibit a wide range of fungi. Echinocandins target the fungal cell wall and the polyenes increase the fungal membrane permeability and lead to cell death.
Pregnancy
Azole antifungals inhibit the growth of fungi. Their trade names and molecular weights:
- Clotrimazole (Mycelex), an over-the-counter product, is available as a topical cream. Several studies have found no association between the drug and birth defects.
- Fluconazole (Diflucan) is a teratogen when doses of ≥400 mg/day are used during the first trimester. Smaller doses do not appear to cause embryo/fetal harm.
- Isavuconazonium (Cresemba) if used in pregnancy, exposure of the embryo/fetus would probably be low based on the >99% plasma protein binding, but the plasma half-life is 130 hours. Moreover, the drug is a potent animal teratogen and is best avoided in pregnancy.
- Itraconazole (Onmel, Sporanox, Tolsura), has a low risk, if any, of structural defects, according to what reported human experience suggests.
- Ketoconazole (Xolegel, Extina, Nizoral; 531) does not appear to adversely effect embryos and fetuses, but the human data are very limited. As with any drug, avoiding organogenesis is the best recommendation.
- Miconazole (Oravig) is usually used topically. Small amounts are absorbed from the vagina. The available evidence suggests that the drug does not increase the risk of congenital malformations.
- Posaconazole (Noxafil) does not have reported use in human pregnancy. The animal reproduction data suggest risk. Based on its molecular weight (about 701), the drug will most likely cross the placenta to the embryo/fetus. Thus, the best course is to avoid the drug during pregnancy, especially in the first trimester.
- Voriconazole (Vfend) has one human report of the drug use in pregnancy. The drug was started at about 19 weeks and continued until the woman gave birth at 35 weeks to a healthy male baby. At 6 months of age, the baby remained normal.
Echinocandin antifungals target the fungal cell wall by inhibiting its synthesis. Their trade names and molecular weights:
- Anidulafungin (Eraxis; 1,140) has no published human data. It is indicated for the treatment of candidemia and other forms of Candida infections. The animal data suggest low risk.
- Caspofungin (Cancidas; 1,213) has no published human data. It is indicated for presumed fungal infections in febrile, neutropenic patients. The animal data are suggestive of human risk, especially if exposure occurs in the first trimester. If possible, maternal treatment should be avoided in the first trimester.
- Micafungin (Mycamine; 1,292) has no published human data. It is indicated for the treatment of patients with esophageal candidiasis and for the prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation. The animal data in one species suggest high risk. If possible, maternal treatment should be avoided in the first trimester.
Polyene antifungals cause depolarization of the fungal cell membrane to increase the membrane permeability, which leads to cell death. Their trade names and molecular weights:
- Amphotericin b (Amphocin; Fungizone; 924) There are three other amphotericin agents: amphotericin b cholesteryl sulfate (Amphotec); amphotericin b lipid complex (Abelcet); amphotericin b liposomal (AmBisome). No reports linking amphotericin b with congenital defects have been found. The drug does cross the human placenta. Although there was a higher rate of spontaneous abortions in rabbits given amphotericin b, there was no fetal harm in rats and rabbits when given amphotericin b lipid complex.
- Nystatin (Bio-Statin; Mycostatin; Nilstat; 926). The drug does not appear to cause embryo-fetal harm. Based on published data, the drug can be used at any time in pregnancy.
Breastfeeding
Small amounts of all the above drugs are probably excreted into breast milk if they are used close to breastfeeding. Most can probably be used during breastfeeding, but there are no data for any of these agents. The safest decision is to not use these drugs when breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Postpartum health care needs exceed those of nonpostpartum patients
Postpartum women with commercial health insurance use substantially more inpatient and outpatient care than nonpostpartum women, particularly in the first 2 months after giving birth, according to a retrospective cohort study published in the May 2021 issue of Obstetrics & Gynecology.
“These findings are consistent with previous studies that have documented elevated postpartum ED and hospitalization rates, and we contribute new evidence that indicates postpartum health care utilization rates are significantly higher than rates of health care utilization in the general population of reproductive aged women when they are not pregnant or postpartum,” Maria W. Steenland, ScD, of Brown University, Providence, R.I., and colleagues wrote. “Most notably, postpartum women were more than three times more likely to have an ED visit and eight times more likely to be hospitalized than nonpostpartum women in the early postpartum period.”
Approximately one-third of maternal deaths occur between 1 week and 1 year post partum, the authors noted in their background information. The overall U.S. maternal mortality rate was 17.4 per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention.
“This study underscores the importance of access to health care for women, in particular in the postpartum period,” Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, said in an interview. “Maternal mortality is a public health crisis. The majority of maternal deaths are preventable and occur up to 1 year postpartum. Studies such as these are needed as the postpartum period is a critical time in the care of reproductive age women that is often overlooked.”
Using data from a large national commercial claims database, the researchers analyzed data from 149,563 women aged 18-44 years who gave birth in 2016 and from 2,048,831 women who were neither pregnant nor post partum during the same study period. In postpartum women, the researchers specifically looked at hospitalization, preventive visits, problem visits, and ED visits during three periods for postpartum women: early postpartum, defined as within 21 days of giving birth; postpartum, defined as 21-60 days after birth; and extended postpartum, defined as 61 days to 1 year after birth. These data were then compared with equivalent periods in nonpostpartum women.
“For the comparison group, we created a random start date of follow-up by generating a random variable between 0 and 365 and adding this integer to Jan. 1, 2016,” the authors explained. “These start dates correspond to the range of possible follow-up start dates among postpartum women who gave birth in 2016.”
The groups differed in age composition: 62% of the postpartum women were between 25 and 34 years old while the nonpostpartum women were more evenly distributed across the age range. A higher proportion of nonpostpartum women had a chronic disease, but median income and geographic region were similar between the groups. Just over a third of the postpartum women (36%) had a cesarean delivery, higher than the 2016 national average of 32%.
Nearly a quarter (23.7%) of postpartum women had a problem visit in the early postpartum period, compared with one in five (19.7%) nonpostpartum women in the equivalent period. This 4-point difference increased to an 4.8-point difference after adjustment for age group, chronic disease, geographic region, income, and month when follow-up began.
Postpartum women were also three times more likely to have an ED visit (3.2%) in the early postpartum period than nonpostpartum women (1%). Postpartum women’s problem visits and ED visits were most prevalent in the first 2 weeks after childbirth: 12.4% in the first week and 10.4% in the second. Complaints for these visits primarily included urinary tract infections and other genitourinary issues, hypertension, breast or breastfeeding issues, and respiratory issues, such as shortness of breath or chest pain. Hospitalization rates were also higher in the early postpartum period for postpartum women (0.8%) than nonpostpartum women (0.1%).
Problem visits showed a similar pattern during the postpartum period from 21 to 60 days after birth: 39.4% of postpartum women, compared with 30.2% of nonpostpartum women. Rate differences were narrower for ED visits (2% post partum vs. 1.9% non–post partum) and hospitalization (0.3% post partum vs. 0.2% non–post partum), but the differences remained significant, and ED visits were still 0.3 points higher after adjustment.
The biggest differences between the groups in the first 2 months occurred with preventive care. In the early postpartum period, 15% of postpartum women had a preventive visit, compared with 3.3% of nonpostpartum women. Similarly, the rates were 28.2% in postpartum women and 6.5% in nonpostpartum women in the postpartum period.
Differences between the groups evened out in the extended postpartum period, when postpartum women had slightly fewer preventive visits (42.5%) than nonpostpartum women (42.7%). ED rates (11.2% postpartum vs 11.1%) and hospitalization rates (1.4% postpartum vs. 1.6%) were similar, but postpartum women were significantly more likely to have problem visits (79.2%) in the year after childbirth than nonpostpartum women (72.8%).
“Compared with postpartum women overall, postpartum women with chronic disease, pregnancy complications, and cesarean births were more likely to receive health care of all types in the early postpartum period,” the researchers reported. “Of the three subgroups, health care use was highest among postpartum women with chronic disease, among whom 35% had at least one outpatient problem visit, 5% had an ED visit, and 1.3% were hospitalized within the first 20 days of childbirth.”
These findings as a whole point to an increased need for health care not only in the first 3 weeks after women give birth but “beyond the traditional 6-week postpartum period, which adds to the argument that the way we care for women in the postpartum period should be revised,” Dr. Krishna said.
The American College of Obstetricians and Gynecologists updated their postpartum recommendations in 2018 to advise that all postpartum women have a follow-up visit in the first 3 weeks after birth.
Dr. Krishna reiterated that postpartum patients should ideally have an initial follow-up within 3 weeks of giving birth, which the rapid expansion of telehealth has made more viable for both clinicians and mothers.
The authors similarly noted that telehealth and home visits “are promising options to promote early and consistent health care contact and reduce known barriers to postpartum care seeking such as fatigue, lack of transportation, and child care.” Predischarge guidance may also help meet postpartum patients’ health care needs.
“Health care professionals also may be able to reduce the escalation of common early postpartum problems identified in this study (e.g., respiratory problems, pain, urinary tract infections) with anticipatory postpartum counseling and care before hospital discharge such as ensuring that women have inhalers at home, developing a pain management plan, and screening for signs of urinary tract infection,” the authors wrote.
Dr. Krishna also pointed out the need to address racial inequalities in health care and material mortality.
“Black women have a maternal mortality rate two times the rate of non-Hispanic White women,” Dr. Krishna said. “One way to address health disparities between commercially insured and uninsured women is improving access to health care through Medicaid expansion for at least 1 year post partum. States that participated in the Affordable Care Acts Medicaid expansion have noted improvement in maternal mortality and a decrease in racial/ethnic inequities.”
The research was funded by the Robert Wood Johnson Foundation and the National Institutes of Health. Data was provided by Aetna, Humana, Kaiser Permanente and UnitedHealthcare. The authors and Dr. Krishna reported no disclosures.
Postpartum women with commercial health insurance use substantially more inpatient and outpatient care than nonpostpartum women, particularly in the first 2 months after giving birth, according to a retrospective cohort study published in the May 2021 issue of Obstetrics & Gynecology.
“These findings are consistent with previous studies that have documented elevated postpartum ED and hospitalization rates, and we contribute new evidence that indicates postpartum health care utilization rates are significantly higher than rates of health care utilization in the general population of reproductive aged women when they are not pregnant or postpartum,” Maria W. Steenland, ScD, of Brown University, Providence, R.I., and colleagues wrote. “Most notably, postpartum women were more than three times more likely to have an ED visit and eight times more likely to be hospitalized than nonpostpartum women in the early postpartum period.”
Approximately one-third of maternal deaths occur between 1 week and 1 year post partum, the authors noted in their background information. The overall U.S. maternal mortality rate was 17.4 per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention.
“This study underscores the importance of access to health care for women, in particular in the postpartum period,” Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, said in an interview. “Maternal mortality is a public health crisis. The majority of maternal deaths are preventable and occur up to 1 year postpartum. Studies such as these are needed as the postpartum period is a critical time in the care of reproductive age women that is often overlooked.”
Using data from a large national commercial claims database, the researchers analyzed data from 149,563 women aged 18-44 years who gave birth in 2016 and from 2,048,831 women who were neither pregnant nor post partum during the same study period. In postpartum women, the researchers specifically looked at hospitalization, preventive visits, problem visits, and ED visits during three periods for postpartum women: early postpartum, defined as within 21 days of giving birth; postpartum, defined as 21-60 days after birth; and extended postpartum, defined as 61 days to 1 year after birth. These data were then compared with equivalent periods in nonpostpartum women.
“For the comparison group, we created a random start date of follow-up by generating a random variable between 0 and 365 and adding this integer to Jan. 1, 2016,” the authors explained. “These start dates correspond to the range of possible follow-up start dates among postpartum women who gave birth in 2016.”
The groups differed in age composition: 62% of the postpartum women were between 25 and 34 years old while the nonpostpartum women were more evenly distributed across the age range. A higher proportion of nonpostpartum women had a chronic disease, but median income and geographic region were similar between the groups. Just over a third of the postpartum women (36%) had a cesarean delivery, higher than the 2016 national average of 32%.
Nearly a quarter (23.7%) of postpartum women had a problem visit in the early postpartum period, compared with one in five (19.7%) nonpostpartum women in the equivalent period. This 4-point difference increased to an 4.8-point difference after adjustment for age group, chronic disease, geographic region, income, and month when follow-up began.
Postpartum women were also three times more likely to have an ED visit (3.2%) in the early postpartum period than nonpostpartum women (1%). Postpartum women’s problem visits and ED visits were most prevalent in the first 2 weeks after childbirth: 12.4% in the first week and 10.4% in the second. Complaints for these visits primarily included urinary tract infections and other genitourinary issues, hypertension, breast or breastfeeding issues, and respiratory issues, such as shortness of breath or chest pain. Hospitalization rates were also higher in the early postpartum period for postpartum women (0.8%) than nonpostpartum women (0.1%).
Problem visits showed a similar pattern during the postpartum period from 21 to 60 days after birth: 39.4% of postpartum women, compared with 30.2% of nonpostpartum women. Rate differences were narrower for ED visits (2% post partum vs. 1.9% non–post partum) and hospitalization (0.3% post partum vs. 0.2% non–post partum), but the differences remained significant, and ED visits were still 0.3 points higher after adjustment.
The biggest differences between the groups in the first 2 months occurred with preventive care. In the early postpartum period, 15% of postpartum women had a preventive visit, compared with 3.3% of nonpostpartum women. Similarly, the rates were 28.2% in postpartum women and 6.5% in nonpostpartum women in the postpartum period.
Differences between the groups evened out in the extended postpartum period, when postpartum women had slightly fewer preventive visits (42.5%) than nonpostpartum women (42.7%). ED rates (11.2% postpartum vs 11.1%) and hospitalization rates (1.4% postpartum vs. 1.6%) were similar, but postpartum women were significantly more likely to have problem visits (79.2%) in the year after childbirth than nonpostpartum women (72.8%).
“Compared with postpartum women overall, postpartum women with chronic disease, pregnancy complications, and cesarean births were more likely to receive health care of all types in the early postpartum period,” the researchers reported. “Of the three subgroups, health care use was highest among postpartum women with chronic disease, among whom 35% had at least one outpatient problem visit, 5% had an ED visit, and 1.3% were hospitalized within the first 20 days of childbirth.”
These findings as a whole point to an increased need for health care not only in the first 3 weeks after women give birth but “beyond the traditional 6-week postpartum period, which adds to the argument that the way we care for women in the postpartum period should be revised,” Dr. Krishna said.
The American College of Obstetricians and Gynecologists updated their postpartum recommendations in 2018 to advise that all postpartum women have a follow-up visit in the first 3 weeks after birth.
Dr. Krishna reiterated that postpartum patients should ideally have an initial follow-up within 3 weeks of giving birth, which the rapid expansion of telehealth has made more viable for both clinicians and mothers.
The authors similarly noted that telehealth and home visits “are promising options to promote early and consistent health care contact and reduce known barriers to postpartum care seeking such as fatigue, lack of transportation, and child care.” Predischarge guidance may also help meet postpartum patients’ health care needs.
“Health care professionals also may be able to reduce the escalation of common early postpartum problems identified in this study (e.g., respiratory problems, pain, urinary tract infections) with anticipatory postpartum counseling and care before hospital discharge such as ensuring that women have inhalers at home, developing a pain management plan, and screening for signs of urinary tract infection,” the authors wrote.
Dr. Krishna also pointed out the need to address racial inequalities in health care and material mortality.
“Black women have a maternal mortality rate two times the rate of non-Hispanic White women,” Dr. Krishna said. “One way to address health disparities between commercially insured and uninsured women is improving access to health care through Medicaid expansion for at least 1 year post partum. States that participated in the Affordable Care Acts Medicaid expansion have noted improvement in maternal mortality and a decrease in racial/ethnic inequities.”
The research was funded by the Robert Wood Johnson Foundation and the National Institutes of Health. Data was provided by Aetna, Humana, Kaiser Permanente and UnitedHealthcare. The authors and Dr. Krishna reported no disclosures.
Postpartum women with commercial health insurance use substantially more inpatient and outpatient care than nonpostpartum women, particularly in the first 2 months after giving birth, according to a retrospective cohort study published in the May 2021 issue of Obstetrics & Gynecology.
“These findings are consistent with previous studies that have documented elevated postpartum ED and hospitalization rates, and we contribute new evidence that indicates postpartum health care utilization rates are significantly higher than rates of health care utilization in the general population of reproductive aged women when they are not pregnant or postpartum,” Maria W. Steenland, ScD, of Brown University, Providence, R.I., and colleagues wrote. “Most notably, postpartum women were more than three times more likely to have an ED visit and eight times more likely to be hospitalized than nonpostpartum women in the early postpartum period.”
Approximately one-third of maternal deaths occur between 1 week and 1 year post partum, the authors noted in their background information. The overall U.S. maternal mortality rate was 17.4 per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention.
“This study underscores the importance of access to health care for women, in particular in the postpartum period,” Iris Krishna, MD, MPH, an assistant professor of maternal-fetal medicine at Emory University, Atlanta, said in an interview. “Maternal mortality is a public health crisis. The majority of maternal deaths are preventable and occur up to 1 year postpartum. Studies such as these are needed as the postpartum period is a critical time in the care of reproductive age women that is often overlooked.”
Using data from a large national commercial claims database, the researchers analyzed data from 149,563 women aged 18-44 years who gave birth in 2016 and from 2,048,831 women who were neither pregnant nor post partum during the same study period. In postpartum women, the researchers specifically looked at hospitalization, preventive visits, problem visits, and ED visits during three periods for postpartum women: early postpartum, defined as within 21 days of giving birth; postpartum, defined as 21-60 days after birth; and extended postpartum, defined as 61 days to 1 year after birth. These data were then compared with equivalent periods in nonpostpartum women.
“For the comparison group, we created a random start date of follow-up by generating a random variable between 0 and 365 and adding this integer to Jan. 1, 2016,” the authors explained. “These start dates correspond to the range of possible follow-up start dates among postpartum women who gave birth in 2016.”
The groups differed in age composition: 62% of the postpartum women were between 25 and 34 years old while the nonpostpartum women were more evenly distributed across the age range. A higher proportion of nonpostpartum women had a chronic disease, but median income and geographic region were similar between the groups. Just over a third of the postpartum women (36%) had a cesarean delivery, higher than the 2016 national average of 32%.
Nearly a quarter (23.7%) of postpartum women had a problem visit in the early postpartum period, compared with one in five (19.7%) nonpostpartum women in the equivalent period. This 4-point difference increased to an 4.8-point difference after adjustment for age group, chronic disease, geographic region, income, and month when follow-up began.
Postpartum women were also three times more likely to have an ED visit (3.2%) in the early postpartum period than nonpostpartum women (1%). Postpartum women’s problem visits and ED visits were most prevalent in the first 2 weeks after childbirth: 12.4% in the first week and 10.4% in the second. Complaints for these visits primarily included urinary tract infections and other genitourinary issues, hypertension, breast or breastfeeding issues, and respiratory issues, such as shortness of breath or chest pain. Hospitalization rates were also higher in the early postpartum period for postpartum women (0.8%) than nonpostpartum women (0.1%).
Problem visits showed a similar pattern during the postpartum period from 21 to 60 days after birth: 39.4% of postpartum women, compared with 30.2% of nonpostpartum women. Rate differences were narrower for ED visits (2% post partum vs. 1.9% non–post partum) and hospitalization (0.3% post partum vs. 0.2% non–post partum), but the differences remained significant, and ED visits were still 0.3 points higher after adjustment.
The biggest differences between the groups in the first 2 months occurred with preventive care. In the early postpartum period, 15% of postpartum women had a preventive visit, compared with 3.3% of nonpostpartum women. Similarly, the rates were 28.2% in postpartum women and 6.5% in nonpostpartum women in the postpartum period.
Differences between the groups evened out in the extended postpartum period, when postpartum women had slightly fewer preventive visits (42.5%) than nonpostpartum women (42.7%). ED rates (11.2% postpartum vs 11.1%) and hospitalization rates (1.4% postpartum vs. 1.6%) were similar, but postpartum women were significantly more likely to have problem visits (79.2%) in the year after childbirth than nonpostpartum women (72.8%).
“Compared with postpartum women overall, postpartum women with chronic disease, pregnancy complications, and cesarean births were more likely to receive health care of all types in the early postpartum period,” the researchers reported. “Of the three subgroups, health care use was highest among postpartum women with chronic disease, among whom 35% had at least one outpatient problem visit, 5% had an ED visit, and 1.3% were hospitalized within the first 20 days of childbirth.”
These findings as a whole point to an increased need for health care not only in the first 3 weeks after women give birth but “beyond the traditional 6-week postpartum period, which adds to the argument that the way we care for women in the postpartum period should be revised,” Dr. Krishna said.
The American College of Obstetricians and Gynecologists updated their postpartum recommendations in 2018 to advise that all postpartum women have a follow-up visit in the first 3 weeks after birth.
Dr. Krishna reiterated that postpartum patients should ideally have an initial follow-up within 3 weeks of giving birth, which the rapid expansion of telehealth has made more viable for both clinicians and mothers.
The authors similarly noted that telehealth and home visits “are promising options to promote early and consistent health care contact and reduce known barriers to postpartum care seeking such as fatigue, lack of transportation, and child care.” Predischarge guidance may also help meet postpartum patients’ health care needs.
“Health care professionals also may be able to reduce the escalation of common early postpartum problems identified in this study (e.g., respiratory problems, pain, urinary tract infections) with anticipatory postpartum counseling and care before hospital discharge such as ensuring that women have inhalers at home, developing a pain management plan, and screening for signs of urinary tract infection,” the authors wrote.
Dr. Krishna also pointed out the need to address racial inequalities in health care and material mortality.
“Black women have a maternal mortality rate two times the rate of non-Hispanic White women,” Dr. Krishna said. “One way to address health disparities between commercially insured and uninsured women is improving access to health care through Medicaid expansion for at least 1 year post partum. States that participated in the Affordable Care Acts Medicaid expansion have noted improvement in maternal mortality and a decrease in racial/ethnic inequities.”
The research was funded by the Robert Wood Johnson Foundation and the National Institutes of Health. Data was provided by Aetna, Humana, Kaiser Permanente and UnitedHealthcare. The authors and Dr. Krishna reported no disclosures.
FROM OBSTETRICS & GYNECOLOGY
Quicker fertility rebound in young women with breast cancer
Researchers found that omitting cyclophosphamide from a regimen of epirubicin and paclitaxel increased the likelihood of an early return of menses, and there was a trend toward improved disease-free survival.
The phase 3 SPECTRUM trial involved 521 women with estrogen receptor–positive, HER2-negative breast cancer who had undergone definitive surgery at one of eight institutions in China. The average age of the patients was 34 years.
Cyclophosphamide is a standard component of adjuvant chemotherapy, but it’s strongly associated with premature ovarian failure and infertility.
“For the first time, we demonstrate that a cyclophosphamide-free regimen [can] increase the rate of menses recovery without compromising survival,” said the researchers, led by Ke-Da Yu, MD, PhD, of the Fudan University Shanghai (China) Cancer Center.
They also reported that, among the women who tried to conceive at a later date, there was a higher pregnancy success rate among those who did not take cyclophosphamide.
“Our findings can be extrapolated to patients with other subtypes of breast cancer, such as triple-negative or HER2-enriched, because the effect of paclitaxel and cyclophosphamide on menstrual resumption is not subtype specific,” the investigators commented.
The results were published online in the Journal of the National Cancer Institute.
This is the first prospective trial specifically designed to find an adjuvant breast cancer regimen less toxic to the ovaries. The “investigators ... should be applauded,” wrote Matteo Lambertini, MD, PhD, of the University of Genova (Italy), and Ann Partridge, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
Although promising, there are a few caveats, the editorialists wrote. In a past trial of doxorubicin and docetaxel in lieu of a cyclophosphamide regimen, disease outcomes were inferior. There is also a question as to whether the SPECTRUM results apply to non-Asian women.
The editorialists also noted that enrollment in this trial ended in 2016, before it was recommended that ovarian suppression be used in conjunction with adjuvant chemotherapy to prevent premature menopause.
“[It’s] notable that the absolute benefit in reducing [premature ovarian insufficiency] rates with the use of a cyclophosphamide-free regimen is similar to the effect demonstrated with the administration of a gonadotropin-releasing hormone agonist during cyclophosphamide-based chemotherapy,” they commented. It’s possible that combining the two approaches might have an additive effect, but for now the possibility remains unknown.
In addition, the SPECTRUM trial predates the widespread use of genetic testing to guide treatment, the editorialists pointed out.
“Therefore, caution should be taken in adopting wholesale such regimens,” Dr. Lambertini and Dr. Partridge said.
Switch to paclitaxel
The research team was inspired by previous reports that swapping out cyclophosphamide for paclitaxel did not reduce adjuvant efficacy in the general breast cancer population.
The SPECTRUM trial randomly assigned 260 women to receive a cyclophosphamide-free regimen of epirubicin (75 mg/m2) and paclitaxel (175 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks.
Another 261 women were randomly assigned to receive cyclophosphamide (600 mg/m2) and epirubicin (75 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks. These patients constituted the control group.
A year after completing chemotherapy, 63.1% of the cyclophosphamide-free arm versus 48.3% of the control group, had resumed menses, defined as having two consecutive menstrual cycles or one cycle but with premenopausal levels of estradiol and follicle-stimulating hormone (P < .001).
Another caveat of the study is that assessments of women who resumed menses were conducted at the 1-year point; rates may have been higher in the cyclophosphamide arm had the investigators conducted the assessments at 2 years, the editorialists said.
The 5-year disease-free survival was 84.7% in the cyclophosphamide-free arm versus 78.3% in the control group, an absolute difference of 14.8% (P = .07).
Patients with node-positive disease appeared to benefit the most from cyclophosphamide sparing.
There were no statistically significant differences in overall or distant disease-free survival.
Higher pregnancy rates
Almost 18% of women in the experimental arm reported trying to conceive, and 9.6% of them did so. About 10% of women in the cyclophosphamide arm tried to conceive, and 2.7% did so (P = .03).
The median interval between randomization and pregnancy was 42 months.
For all of the women who became pregnant, endocrine therapy was interrupted. “Women who temporarily interrupt endocrine therapy due to pregnancy should be reminded to resume endocrine therapy following attempted or successful pregnancy,” the investigators wrote.
The patients were taking tamoxifen at least 5 years after receiving chemotherapy, most often as monotherapy. About 5% of the patients underwent up-front ovarian suppression with an aromatase inhibitor, which is a current standard option.
The study was supported by the National Natural Science Foundation of China and other organizations. The investigators and Dr. Partridge disclosed no relevant financial relationships. Dr. Lambertini has consulted for and/or has received speakers fees from Roche, AstraZeneca, Lilly, Novartis, and other companies.
A version of this article first appeared on Medscape.com.
Researchers found that omitting cyclophosphamide from a regimen of epirubicin and paclitaxel increased the likelihood of an early return of menses, and there was a trend toward improved disease-free survival.
The phase 3 SPECTRUM trial involved 521 women with estrogen receptor–positive, HER2-negative breast cancer who had undergone definitive surgery at one of eight institutions in China. The average age of the patients was 34 years.
Cyclophosphamide is a standard component of adjuvant chemotherapy, but it’s strongly associated with premature ovarian failure and infertility.
“For the first time, we demonstrate that a cyclophosphamide-free regimen [can] increase the rate of menses recovery without compromising survival,” said the researchers, led by Ke-Da Yu, MD, PhD, of the Fudan University Shanghai (China) Cancer Center.
They also reported that, among the women who tried to conceive at a later date, there was a higher pregnancy success rate among those who did not take cyclophosphamide.
“Our findings can be extrapolated to patients with other subtypes of breast cancer, such as triple-negative or HER2-enriched, because the effect of paclitaxel and cyclophosphamide on menstrual resumption is not subtype specific,” the investigators commented.
The results were published online in the Journal of the National Cancer Institute.
This is the first prospective trial specifically designed to find an adjuvant breast cancer regimen less toxic to the ovaries. The “investigators ... should be applauded,” wrote Matteo Lambertini, MD, PhD, of the University of Genova (Italy), and Ann Partridge, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
Although promising, there are a few caveats, the editorialists wrote. In a past trial of doxorubicin and docetaxel in lieu of a cyclophosphamide regimen, disease outcomes were inferior. There is also a question as to whether the SPECTRUM results apply to non-Asian women.
The editorialists also noted that enrollment in this trial ended in 2016, before it was recommended that ovarian suppression be used in conjunction with adjuvant chemotherapy to prevent premature menopause.
“[It’s] notable that the absolute benefit in reducing [premature ovarian insufficiency] rates with the use of a cyclophosphamide-free regimen is similar to the effect demonstrated with the administration of a gonadotropin-releasing hormone agonist during cyclophosphamide-based chemotherapy,” they commented. It’s possible that combining the two approaches might have an additive effect, but for now the possibility remains unknown.
In addition, the SPECTRUM trial predates the widespread use of genetic testing to guide treatment, the editorialists pointed out.
“Therefore, caution should be taken in adopting wholesale such regimens,” Dr. Lambertini and Dr. Partridge said.
Switch to paclitaxel
The research team was inspired by previous reports that swapping out cyclophosphamide for paclitaxel did not reduce adjuvant efficacy in the general breast cancer population.
The SPECTRUM trial randomly assigned 260 women to receive a cyclophosphamide-free regimen of epirubicin (75 mg/m2) and paclitaxel (175 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks.
Another 261 women were randomly assigned to receive cyclophosphamide (600 mg/m2) and epirubicin (75 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks. These patients constituted the control group.
A year after completing chemotherapy, 63.1% of the cyclophosphamide-free arm versus 48.3% of the control group, had resumed menses, defined as having two consecutive menstrual cycles or one cycle but with premenopausal levels of estradiol and follicle-stimulating hormone (P < .001).
Another caveat of the study is that assessments of women who resumed menses were conducted at the 1-year point; rates may have been higher in the cyclophosphamide arm had the investigators conducted the assessments at 2 years, the editorialists said.
The 5-year disease-free survival was 84.7% in the cyclophosphamide-free arm versus 78.3% in the control group, an absolute difference of 14.8% (P = .07).
Patients with node-positive disease appeared to benefit the most from cyclophosphamide sparing.
There were no statistically significant differences in overall or distant disease-free survival.
Higher pregnancy rates
Almost 18% of women in the experimental arm reported trying to conceive, and 9.6% of them did so. About 10% of women in the cyclophosphamide arm tried to conceive, and 2.7% did so (P = .03).
The median interval between randomization and pregnancy was 42 months.
For all of the women who became pregnant, endocrine therapy was interrupted. “Women who temporarily interrupt endocrine therapy due to pregnancy should be reminded to resume endocrine therapy following attempted or successful pregnancy,” the investigators wrote.
The patients were taking tamoxifen at least 5 years after receiving chemotherapy, most often as monotherapy. About 5% of the patients underwent up-front ovarian suppression with an aromatase inhibitor, which is a current standard option.
The study was supported by the National Natural Science Foundation of China and other organizations. The investigators and Dr. Partridge disclosed no relevant financial relationships. Dr. Lambertini has consulted for and/or has received speakers fees from Roche, AstraZeneca, Lilly, Novartis, and other companies.
A version of this article first appeared on Medscape.com.
Researchers found that omitting cyclophosphamide from a regimen of epirubicin and paclitaxel increased the likelihood of an early return of menses, and there was a trend toward improved disease-free survival.
The phase 3 SPECTRUM trial involved 521 women with estrogen receptor–positive, HER2-negative breast cancer who had undergone definitive surgery at one of eight institutions in China. The average age of the patients was 34 years.
Cyclophosphamide is a standard component of adjuvant chemotherapy, but it’s strongly associated with premature ovarian failure and infertility.
“For the first time, we demonstrate that a cyclophosphamide-free regimen [can] increase the rate of menses recovery without compromising survival,” said the researchers, led by Ke-Da Yu, MD, PhD, of the Fudan University Shanghai (China) Cancer Center.
They also reported that, among the women who tried to conceive at a later date, there was a higher pregnancy success rate among those who did not take cyclophosphamide.
“Our findings can be extrapolated to patients with other subtypes of breast cancer, such as triple-negative or HER2-enriched, because the effect of paclitaxel and cyclophosphamide on menstrual resumption is not subtype specific,” the investigators commented.
The results were published online in the Journal of the National Cancer Institute.
This is the first prospective trial specifically designed to find an adjuvant breast cancer regimen less toxic to the ovaries. The “investigators ... should be applauded,” wrote Matteo Lambertini, MD, PhD, of the University of Genova (Italy), and Ann Partridge, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
Although promising, there are a few caveats, the editorialists wrote. In a past trial of doxorubicin and docetaxel in lieu of a cyclophosphamide regimen, disease outcomes were inferior. There is also a question as to whether the SPECTRUM results apply to non-Asian women.
The editorialists also noted that enrollment in this trial ended in 2016, before it was recommended that ovarian suppression be used in conjunction with adjuvant chemotherapy to prevent premature menopause.
“[It’s] notable that the absolute benefit in reducing [premature ovarian insufficiency] rates with the use of a cyclophosphamide-free regimen is similar to the effect demonstrated with the administration of a gonadotropin-releasing hormone agonist during cyclophosphamide-based chemotherapy,” they commented. It’s possible that combining the two approaches might have an additive effect, but for now the possibility remains unknown.
In addition, the SPECTRUM trial predates the widespread use of genetic testing to guide treatment, the editorialists pointed out.
“Therefore, caution should be taken in adopting wholesale such regimens,” Dr. Lambertini and Dr. Partridge said.
Switch to paclitaxel
The research team was inspired by previous reports that swapping out cyclophosphamide for paclitaxel did not reduce adjuvant efficacy in the general breast cancer population.
The SPECTRUM trial randomly assigned 260 women to receive a cyclophosphamide-free regimen of epirubicin (75 mg/m2) and paclitaxel (175 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks.
Another 261 women were randomly assigned to receive cyclophosphamide (600 mg/m2) and epirubicin (75 mg/m2) every 3 weeks for four cycles followed by weekly paclitaxel (80 mg/m2) for 12 weeks. These patients constituted the control group.
A year after completing chemotherapy, 63.1% of the cyclophosphamide-free arm versus 48.3% of the control group, had resumed menses, defined as having two consecutive menstrual cycles or one cycle but with premenopausal levels of estradiol and follicle-stimulating hormone (P < .001).
Another caveat of the study is that assessments of women who resumed menses were conducted at the 1-year point; rates may have been higher in the cyclophosphamide arm had the investigators conducted the assessments at 2 years, the editorialists said.
The 5-year disease-free survival was 84.7% in the cyclophosphamide-free arm versus 78.3% in the control group, an absolute difference of 14.8% (P = .07).
Patients with node-positive disease appeared to benefit the most from cyclophosphamide sparing.
There were no statistically significant differences in overall or distant disease-free survival.
Higher pregnancy rates
Almost 18% of women in the experimental arm reported trying to conceive, and 9.6% of them did so. About 10% of women in the cyclophosphamide arm tried to conceive, and 2.7% did so (P = .03).
The median interval between randomization and pregnancy was 42 months.
For all of the women who became pregnant, endocrine therapy was interrupted. “Women who temporarily interrupt endocrine therapy due to pregnancy should be reminded to resume endocrine therapy following attempted or successful pregnancy,” the investigators wrote.
The patients were taking tamoxifen at least 5 years after receiving chemotherapy, most often as monotherapy. About 5% of the patients underwent up-front ovarian suppression with an aromatase inhibitor, which is a current standard option.
The study was supported by the National Natural Science Foundation of China and other organizations. The investigators and Dr. Partridge disclosed no relevant financial relationships. Dr. Lambertini has consulted for and/or has received speakers fees from Roche, AstraZeneca, Lilly, Novartis, and other companies.
A version of this article first appeared on Medscape.com.
HHS proposes overturning Title X ‘gag’ rule
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
Feds let Illinois extend postpartum Medicaid coverage: HHS encourages other states to follow suit
The federal government has approved a request by Illinois to extend postpartum Medicaid coverage to a full year from the current standard of 60 days.
Health & Human Services Secretary Xavier Becerra announced the approval at a press briefing on April 12, noting that it was occurring during Black Maternal Health Week. The coverage extension is aimed at decreasing maternal morbidity and mortality, particularly among women of color.
Black women are two times more likely to die from a pregnancy-related cause than White women, according to HHS. Mr. Becerra noted that, in the United States, 52% of pregnancy-related deaths take place up to 1 year post partum, and that in Illinois the figure is 80%.
“The continuity of coverage available through this action will help new mothers manage chronic conditions like hypertension and diabetes, and it will provide access to behavioral health and other mental health care services,” he said.
Continuing Medicaid coverage for new mothers has been backed by the American Medical Association, is a priority of the American College of Obstetricians and Gynecologists, and has been promoted by Republicans and Democrats in Congress and state legislatures.
Illinois is the first state to seek and win approval to extend its Medicaid coverage from the current 60-days postbirth requirement. The program was granted through an existing section 1115 waiver program. It begins today and is authorized through Dec. 31, 2025. The state estimates that some 2,500 women with incomes up to 208% of the federal poverty level will receive the year of continuous Medicaid coverage. Illinois will evaluate whether the extension improves women’s health and if it benefits the Medicaid program overall.
However, the recently passed coronavirus rescue package creates a new process that lets states more easily expand postpartum coverage, but they must act by April 2022. Mr. Becerra said the federal government is encouraging more states to follow Illinois’ lead in extending postpartum eligibility by taking advantage of the new process.
States won’t get extra money – they will receive the regular per capita–based federal match if they extend Medicaid coverage through this pathway. Even so, Mr. Becerra said there has been much interest.
“I hope that we begin to see states not only express interest but actually submit their proposals on how they would do this,” Mr. Becerra said.
Medicaid has become one of the key providers of maternal health care in the United States, as it covers 4 in 10 births, according to the Kaiser Family Foundation. But postpartum coverage after the 60-day federal requirement is a patchwork. In 38 states (plus Washington, D.C.) that have expanded Medicaid under the Affordable Care Act, mothers who earn up to 138% of the federal poverty level can continue on Medicaid; for those who earn more than that, they can get coverage through the ACA.
In the 12 states that did not expand Medicaid, new mothers have to seek Medicaid coverage after 60 days as parents, and the income limits are strict. In Texas, for example, a married mother with a newborn loses Medicaid coverage 2 months after giving birth if she and her partner have an annual income above $3,733, reports the Kaiser Family Foundation.
Coverage disruptions are harmful to mothers, said Mr. Becerra. HHS data shows that more than half of pregnant women in Medicaid experienced a coverage gap in the first 6 months postpartum and that disruptions in coverage often lead to delayed care and less preventive care.
Mr. Becerra also announced that the Health Resources and Services Administration will make $12 million available over 4 years for the Rural Maternity and Obstetrics Management Strategies program. Applicants for the new funds will be required to focus on populations that have historically suffered from poorer health outcomes, health disparities, and other inequities.
A version of this article first appeared on Medscape.com.
The federal government has approved a request by Illinois to extend postpartum Medicaid coverage to a full year from the current standard of 60 days.
Health & Human Services Secretary Xavier Becerra announced the approval at a press briefing on April 12, noting that it was occurring during Black Maternal Health Week. The coverage extension is aimed at decreasing maternal morbidity and mortality, particularly among women of color.
Black women are two times more likely to die from a pregnancy-related cause than White women, according to HHS. Mr. Becerra noted that, in the United States, 52% of pregnancy-related deaths take place up to 1 year post partum, and that in Illinois the figure is 80%.
“The continuity of coverage available through this action will help new mothers manage chronic conditions like hypertension and diabetes, and it will provide access to behavioral health and other mental health care services,” he said.
Continuing Medicaid coverage for new mothers has been backed by the American Medical Association, is a priority of the American College of Obstetricians and Gynecologists, and has been promoted by Republicans and Democrats in Congress and state legislatures.
Illinois is the first state to seek and win approval to extend its Medicaid coverage from the current 60-days postbirth requirement. The program was granted through an existing section 1115 waiver program. It begins today and is authorized through Dec. 31, 2025. The state estimates that some 2,500 women with incomes up to 208% of the federal poverty level will receive the year of continuous Medicaid coverage. Illinois will evaluate whether the extension improves women’s health and if it benefits the Medicaid program overall.
However, the recently passed coronavirus rescue package creates a new process that lets states more easily expand postpartum coverage, but they must act by April 2022. Mr. Becerra said the federal government is encouraging more states to follow Illinois’ lead in extending postpartum eligibility by taking advantage of the new process.
States won’t get extra money – they will receive the regular per capita–based federal match if they extend Medicaid coverage through this pathway. Even so, Mr. Becerra said there has been much interest.
“I hope that we begin to see states not only express interest but actually submit their proposals on how they would do this,” Mr. Becerra said.
Medicaid has become one of the key providers of maternal health care in the United States, as it covers 4 in 10 births, according to the Kaiser Family Foundation. But postpartum coverage after the 60-day federal requirement is a patchwork. In 38 states (plus Washington, D.C.) that have expanded Medicaid under the Affordable Care Act, mothers who earn up to 138% of the federal poverty level can continue on Medicaid; for those who earn more than that, they can get coverage through the ACA.
In the 12 states that did not expand Medicaid, new mothers have to seek Medicaid coverage after 60 days as parents, and the income limits are strict. In Texas, for example, a married mother with a newborn loses Medicaid coverage 2 months after giving birth if she and her partner have an annual income above $3,733, reports the Kaiser Family Foundation.
Coverage disruptions are harmful to mothers, said Mr. Becerra. HHS data shows that more than half of pregnant women in Medicaid experienced a coverage gap in the first 6 months postpartum and that disruptions in coverage often lead to delayed care and less preventive care.
Mr. Becerra also announced that the Health Resources and Services Administration will make $12 million available over 4 years for the Rural Maternity and Obstetrics Management Strategies program. Applicants for the new funds will be required to focus on populations that have historically suffered from poorer health outcomes, health disparities, and other inequities.
A version of this article first appeared on Medscape.com.
The federal government has approved a request by Illinois to extend postpartum Medicaid coverage to a full year from the current standard of 60 days.
Health & Human Services Secretary Xavier Becerra announced the approval at a press briefing on April 12, noting that it was occurring during Black Maternal Health Week. The coverage extension is aimed at decreasing maternal morbidity and mortality, particularly among women of color.
Black women are two times more likely to die from a pregnancy-related cause than White women, according to HHS. Mr. Becerra noted that, in the United States, 52% of pregnancy-related deaths take place up to 1 year post partum, and that in Illinois the figure is 80%.
“The continuity of coverage available through this action will help new mothers manage chronic conditions like hypertension and diabetes, and it will provide access to behavioral health and other mental health care services,” he said.
Continuing Medicaid coverage for new mothers has been backed by the American Medical Association, is a priority of the American College of Obstetricians and Gynecologists, and has been promoted by Republicans and Democrats in Congress and state legislatures.
Illinois is the first state to seek and win approval to extend its Medicaid coverage from the current 60-days postbirth requirement. The program was granted through an existing section 1115 waiver program. It begins today and is authorized through Dec. 31, 2025. The state estimates that some 2,500 women with incomes up to 208% of the federal poverty level will receive the year of continuous Medicaid coverage. Illinois will evaluate whether the extension improves women’s health and if it benefits the Medicaid program overall.
However, the recently passed coronavirus rescue package creates a new process that lets states more easily expand postpartum coverage, but they must act by April 2022. Mr. Becerra said the federal government is encouraging more states to follow Illinois’ lead in extending postpartum eligibility by taking advantage of the new process.
States won’t get extra money – they will receive the regular per capita–based federal match if they extend Medicaid coverage through this pathway. Even so, Mr. Becerra said there has been much interest.
“I hope that we begin to see states not only express interest but actually submit their proposals on how they would do this,” Mr. Becerra said.
Medicaid has become one of the key providers of maternal health care in the United States, as it covers 4 in 10 births, according to the Kaiser Family Foundation. But postpartum coverage after the 60-day federal requirement is a patchwork. In 38 states (plus Washington, D.C.) that have expanded Medicaid under the Affordable Care Act, mothers who earn up to 138% of the federal poverty level can continue on Medicaid; for those who earn more than that, they can get coverage through the ACA.
In the 12 states that did not expand Medicaid, new mothers have to seek Medicaid coverage after 60 days as parents, and the income limits are strict. In Texas, for example, a married mother with a newborn loses Medicaid coverage 2 months after giving birth if she and her partner have an annual income above $3,733, reports the Kaiser Family Foundation.
Coverage disruptions are harmful to mothers, said Mr. Becerra. HHS data shows that more than half of pregnant women in Medicaid experienced a coverage gap in the first 6 months postpartum and that disruptions in coverage often lead to delayed care and less preventive care.
Mr. Becerra also announced that the Health Resources and Services Administration will make $12 million available over 4 years for the Rural Maternity and Obstetrics Management Strategies program. Applicants for the new funds will be required to focus on populations that have historically suffered from poorer health outcomes, health disparities, and other inequities.
A version of this article first appeared on Medscape.com.