User login
Stethoscope and Doppler may outperform newer intrapartum fetal monitoring techniques
For intrapartum fetal surveillance, the old way may be the best way, according to a meta-analysis involving more than 118,000 patients.
Intermittent auscultation with a Pinard stethoscope and handheld Doppler was associated with a significantly lower risk of emergency cesarean deliveries than newer monitoring techniques without jeopardizing maternal or neonatal outcomes, reported lead author Bassel H. Al Wattar, MD, PhD, of University of Warwick, Coventry, England, and University College London Hospitals, and colleagues.
“Over the last 50 years, several newer surveillance methods have been evaluated, with varied uptake in practice,” the investigators wrote in the Canadian Medical Association Journal, noting that cardiotocography (CTG) is the most common method for high-risk pregnancies, typically coupled with at least one other modality, such as fetal scalp pH analysis (FBS), fetal pulse oximetry (FPO), or fetal heart electrocardiogram (STAN).
“Despite extensive investment in clinical research, the overall effectiveness of such methods in improving maternal and neonatal outcomes remains debatable as stillbirth rates have plateaued worldwide, while cesarean delivery rates continue to rise,” the investigators wrote. Previous meta-analyses have relied upon head-to-head comparisons of monitoring techniques and did not take into account effects on maternal and neonatal outcomes.
To address this knowledge gap, Dr. Al Wattar and colleagues conducted the present systematic review and meta-analysis, ultimately including 33 trials with 118,863 women who underwent intrapartum fetal surveillance, dating back to 1976. Ten surveillance types were evaluated, including intermittent auscultation with Pinard stethoscope and handheld Doppler, CTG with or without computer-aided decision models (cCTG), and CTG or cCTG combined with one or two other techniques, such as FBS, FPO, and STAN.
This revealed that intermittent auscultation outperformed all other techniques in terms of emergency cesarean deliveries and emergency cesarean deliveries because of fetal distress.
Specifically, intermittent auscultation significantly reduced risk of emergency cesarean deliveries, compared with CTG (relative risk, 0.83; 95% confidence interval, 0.72-0.97), CTG-FBS (RR, 0.71; 95% CI, 0.63-0.80), CTG-lactate (RR, 0.77; 95% CI, 0.64-0.92), and FPO-CTG-FBS (RR, 0.81; 95% CI, 0.67-0.99). Conversely, compared with IA, STAN-CTG-FBS and cCTG-FBS raised risk of emergency cesarean deliveries by 17% and 21%, respectively.
Compared with other modalities, the superiority of intermittent auscultation was even more pronounced in terms of emergency cesarean deliveries because of fetal distress. Intermittent auscultation reduced risk by 43%, compared with CTG, 66% compared with CTG-FBS, 58%, compared with FPO-CTG, and 17%, compared with FPO-CTG-FBS. Conversely, compared with intermittent auscultation, STAN-CTG and cCTG-FBS increased risk of emergency cesarean deliveries because of fetal distress by 39% and 80%, respectively.
Further analysis showed that all types of surveillance had similar effects on neonatal outcomes, such as admission to neonatal unit and neonatal acidemia. Although a combination of STAN or FPO with CTG-FBS “seemed to improve the likelihood of reducing adverse neonatal outcomes,” the investigators noted that these differences were not significant in network meta-analysis.
“New fetal surveillance methods did not improve neonatal outcomes or reduce unnecessary maternal interventions,” Dr. Al Wattar and colleagues concluded. “Further evidence is needed to evaluate the effects of fetal pulse oximetry and fetal heart electrocardiography in labor.”
Courtney Rhoades, DO, MBA, FACOG, medical director of labor and delivery and assistant professor of obstetrics and gynecology at the University of Florida, Jacksonville, suggested that the meta-analysis supports the safety of intermittent auscultation, but the results may not be entirely applicable to real-world practice.
“It is hard, in practice, to draw the same conclusion that they do in the study that the newer methods may cause too many emergency C-sections because our fetal monitoring equipment, methodology for interpretation, ability to do emergency C-sections and maternal risk factors have changed in the last 50 years,” Dr. Rhoades said. “Continuous fetal monitoring gives more data points during labor, and with more data points, there are more opportunities to interpret and act – either correctly or incorrectly. As they state in the study, the decision to do a C-section is multifactorial.”
Dr. Rhoades, who recently authored a textbook chapter on intrapartum monitoring and fetal assessment, recommended that intermittent auscultation be reserved for low-risk patients.
“The American College of Obstetricians and Gynecologists has endorsed intermittent auscultation for low-risk pregnancies and this study affirms their support,” Dr. Rhoades said. “Women with a low-risk pregnancy can benefit from intermittent auscultation because it allows them more autonomy and movement during labor so it should be offered to our low-risk patients.”
Dr. Al Wattar reported a personal Academic Clinical Lectureship from the U.K. National Health Institute of Research. Dr. Khan disclosed funding from the Beatriz Galindo Program Grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Spanish Government.
For intrapartum fetal surveillance, the old way may be the best way, according to a meta-analysis involving more than 118,000 patients.
Intermittent auscultation with a Pinard stethoscope and handheld Doppler was associated with a significantly lower risk of emergency cesarean deliveries than newer monitoring techniques without jeopardizing maternal or neonatal outcomes, reported lead author Bassel H. Al Wattar, MD, PhD, of University of Warwick, Coventry, England, and University College London Hospitals, and colleagues.
“Over the last 50 years, several newer surveillance methods have been evaluated, with varied uptake in practice,” the investigators wrote in the Canadian Medical Association Journal, noting that cardiotocography (CTG) is the most common method for high-risk pregnancies, typically coupled with at least one other modality, such as fetal scalp pH analysis (FBS), fetal pulse oximetry (FPO), or fetal heart electrocardiogram (STAN).
“Despite extensive investment in clinical research, the overall effectiveness of such methods in improving maternal and neonatal outcomes remains debatable as stillbirth rates have plateaued worldwide, while cesarean delivery rates continue to rise,” the investigators wrote. Previous meta-analyses have relied upon head-to-head comparisons of monitoring techniques and did not take into account effects on maternal and neonatal outcomes.
To address this knowledge gap, Dr. Al Wattar and colleagues conducted the present systematic review and meta-analysis, ultimately including 33 trials with 118,863 women who underwent intrapartum fetal surveillance, dating back to 1976. Ten surveillance types were evaluated, including intermittent auscultation with Pinard stethoscope and handheld Doppler, CTG with or without computer-aided decision models (cCTG), and CTG or cCTG combined with one or two other techniques, such as FBS, FPO, and STAN.
This revealed that intermittent auscultation outperformed all other techniques in terms of emergency cesarean deliveries and emergency cesarean deliveries because of fetal distress.
Specifically, intermittent auscultation significantly reduced risk of emergency cesarean deliveries, compared with CTG (relative risk, 0.83; 95% confidence interval, 0.72-0.97), CTG-FBS (RR, 0.71; 95% CI, 0.63-0.80), CTG-lactate (RR, 0.77; 95% CI, 0.64-0.92), and FPO-CTG-FBS (RR, 0.81; 95% CI, 0.67-0.99). Conversely, compared with IA, STAN-CTG-FBS and cCTG-FBS raised risk of emergency cesarean deliveries by 17% and 21%, respectively.
Compared with other modalities, the superiority of intermittent auscultation was even more pronounced in terms of emergency cesarean deliveries because of fetal distress. Intermittent auscultation reduced risk by 43%, compared with CTG, 66% compared with CTG-FBS, 58%, compared with FPO-CTG, and 17%, compared with FPO-CTG-FBS. Conversely, compared with intermittent auscultation, STAN-CTG and cCTG-FBS increased risk of emergency cesarean deliveries because of fetal distress by 39% and 80%, respectively.
Further analysis showed that all types of surveillance had similar effects on neonatal outcomes, such as admission to neonatal unit and neonatal acidemia. Although a combination of STAN or FPO with CTG-FBS “seemed to improve the likelihood of reducing adverse neonatal outcomes,” the investigators noted that these differences were not significant in network meta-analysis.
“New fetal surveillance methods did not improve neonatal outcomes or reduce unnecessary maternal interventions,” Dr. Al Wattar and colleagues concluded. “Further evidence is needed to evaluate the effects of fetal pulse oximetry and fetal heart electrocardiography in labor.”
Courtney Rhoades, DO, MBA, FACOG, medical director of labor and delivery and assistant professor of obstetrics and gynecology at the University of Florida, Jacksonville, suggested that the meta-analysis supports the safety of intermittent auscultation, but the results may not be entirely applicable to real-world practice.
“It is hard, in practice, to draw the same conclusion that they do in the study that the newer methods may cause too many emergency C-sections because our fetal monitoring equipment, methodology for interpretation, ability to do emergency C-sections and maternal risk factors have changed in the last 50 years,” Dr. Rhoades said. “Continuous fetal monitoring gives more data points during labor, and with more data points, there are more opportunities to interpret and act – either correctly or incorrectly. As they state in the study, the decision to do a C-section is multifactorial.”
Dr. Rhoades, who recently authored a textbook chapter on intrapartum monitoring and fetal assessment, recommended that intermittent auscultation be reserved for low-risk patients.
“The American College of Obstetricians and Gynecologists has endorsed intermittent auscultation for low-risk pregnancies and this study affirms their support,” Dr. Rhoades said. “Women with a low-risk pregnancy can benefit from intermittent auscultation because it allows them more autonomy and movement during labor so it should be offered to our low-risk patients.”
Dr. Al Wattar reported a personal Academic Clinical Lectureship from the U.K. National Health Institute of Research. Dr. Khan disclosed funding from the Beatriz Galindo Program Grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Spanish Government.
For intrapartum fetal surveillance, the old way may be the best way, according to a meta-analysis involving more than 118,000 patients.
Intermittent auscultation with a Pinard stethoscope and handheld Doppler was associated with a significantly lower risk of emergency cesarean deliveries than newer monitoring techniques without jeopardizing maternal or neonatal outcomes, reported lead author Bassel H. Al Wattar, MD, PhD, of University of Warwick, Coventry, England, and University College London Hospitals, and colleagues.
“Over the last 50 years, several newer surveillance methods have been evaluated, with varied uptake in practice,” the investigators wrote in the Canadian Medical Association Journal, noting that cardiotocography (CTG) is the most common method for high-risk pregnancies, typically coupled with at least one other modality, such as fetal scalp pH analysis (FBS), fetal pulse oximetry (FPO), or fetal heart electrocardiogram (STAN).
“Despite extensive investment in clinical research, the overall effectiveness of such methods in improving maternal and neonatal outcomes remains debatable as stillbirth rates have plateaued worldwide, while cesarean delivery rates continue to rise,” the investigators wrote. Previous meta-analyses have relied upon head-to-head comparisons of monitoring techniques and did not take into account effects on maternal and neonatal outcomes.
To address this knowledge gap, Dr. Al Wattar and colleagues conducted the present systematic review and meta-analysis, ultimately including 33 trials with 118,863 women who underwent intrapartum fetal surveillance, dating back to 1976. Ten surveillance types were evaluated, including intermittent auscultation with Pinard stethoscope and handheld Doppler, CTG with or without computer-aided decision models (cCTG), and CTG or cCTG combined with one or two other techniques, such as FBS, FPO, and STAN.
This revealed that intermittent auscultation outperformed all other techniques in terms of emergency cesarean deliveries and emergency cesarean deliveries because of fetal distress.
Specifically, intermittent auscultation significantly reduced risk of emergency cesarean deliveries, compared with CTG (relative risk, 0.83; 95% confidence interval, 0.72-0.97), CTG-FBS (RR, 0.71; 95% CI, 0.63-0.80), CTG-lactate (RR, 0.77; 95% CI, 0.64-0.92), and FPO-CTG-FBS (RR, 0.81; 95% CI, 0.67-0.99). Conversely, compared with IA, STAN-CTG-FBS and cCTG-FBS raised risk of emergency cesarean deliveries by 17% and 21%, respectively.
Compared with other modalities, the superiority of intermittent auscultation was even more pronounced in terms of emergency cesarean deliveries because of fetal distress. Intermittent auscultation reduced risk by 43%, compared with CTG, 66% compared with CTG-FBS, 58%, compared with FPO-CTG, and 17%, compared with FPO-CTG-FBS. Conversely, compared with intermittent auscultation, STAN-CTG and cCTG-FBS increased risk of emergency cesarean deliveries because of fetal distress by 39% and 80%, respectively.
Further analysis showed that all types of surveillance had similar effects on neonatal outcomes, such as admission to neonatal unit and neonatal acidemia. Although a combination of STAN or FPO with CTG-FBS “seemed to improve the likelihood of reducing adverse neonatal outcomes,” the investigators noted that these differences were not significant in network meta-analysis.
“New fetal surveillance methods did not improve neonatal outcomes or reduce unnecessary maternal interventions,” Dr. Al Wattar and colleagues concluded. “Further evidence is needed to evaluate the effects of fetal pulse oximetry and fetal heart electrocardiography in labor.”
Courtney Rhoades, DO, MBA, FACOG, medical director of labor and delivery and assistant professor of obstetrics and gynecology at the University of Florida, Jacksonville, suggested that the meta-analysis supports the safety of intermittent auscultation, but the results may not be entirely applicable to real-world practice.
“It is hard, in practice, to draw the same conclusion that they do in the study that the newer methods may cause too many emergency C-sections because our fetal monitoring equipment, methodology for interpretation, ability to do emergency C-sections and maternal risk factors have changed in the last 50 years,” Dr. Rhoades said. “Continuous fetal monitoring gives more data points during labor, and with more data points, there are more opportunities to interpret and act – either correctly or incorrectly. As they state in the study, the decision to do a C-section is multifactorial.”
Dr. Rhoades, who recently authored a textbook chapter on intrapartum monitoring and fetal assessment, recommended that intermittent auscultation be reserved for low-risk patients.
“The American College of Obstetricians and Gynecologists has endorsed intermittent auscultation for low-risk pregnancies and this study affirms their support,” Dr. Rhoades said. “Women with a low-risk pregnancy can benefit from intermittent auscultation because it allows them more autonomy and movement during labor so it should be offered to our low-risk patients.”
Dr. Al Wattar reported a personal Academic Clinical Lectureship from the U.K. National Health Institute of Research. Dr. Khan disclosed funding from the Beatriz Galindo Program Grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Spanish Government.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Managing herpes simplex virus genital infection in pregnancy
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
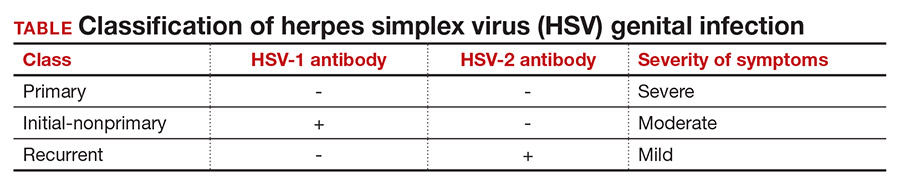
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.
Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1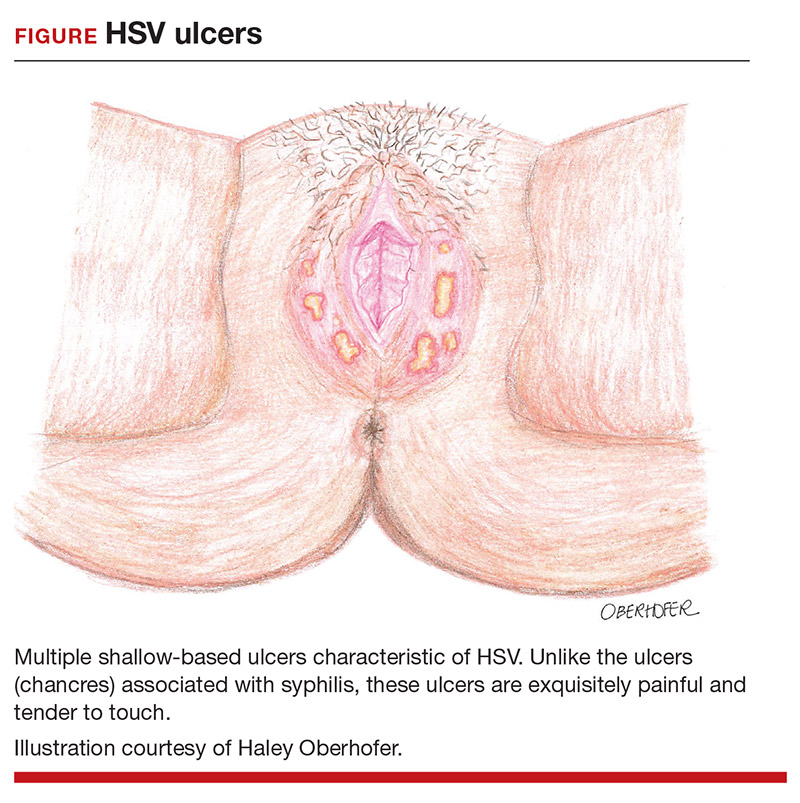
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
Managing the second stage of labor: An evidence-based approach

CASE Woman in second stage with prolonged pushing
Ms. J. is an 18-year-old woman (G1P0) at 39 weeks’ gestation whose cervix is completely dilated; she has been actively pushing for 60 minutes. The estimated fetal weight is 8 lb, and electronic fetal monitoring shows a Category I fetal heart rate (FHR) tracing. The presenting part remains at 0 station and occiput transverse despite great pushing effort.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. During the third hour of the second stage, Ms. J. is diagnosed with chorioamnionitis and the fetus remains at 0 station. She undergoes a primary cesarean delivery (CD) complicated by bilateral lower uterine extensions and postpartum hemorrhage. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 4 and 8, respectively. The umbilical cord arterial pH was 7.03.
Ms. J. and her baby were discharged home on postoperative day 4.
In 2014, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine jointly released a document, “Safe prevention of the primary cesarean delivery,” in response to the sharp rise in cesarean births from 1996 to 2011.1 It described management strategies to safely reduce the most common indications for a primary CD in nulliparous women. Specifically, it recommended that the second stage of labor—defined as the interval from complete cervical dilation through delivery of the neonate—may be prolonged, as “longer durations may be appropriate on an individualized basis (eg, with the use of epidural analgesia or with fetal malposition) as long as progress is being documented.”1
A prolonged second stage was defined as 3 hours of pushing in nulliparous women and 2 hours in multiparous women, with 1 additional hour (or longer) in those receiving epidural analgesia. Indeed, the primary CD rate decreased slightly to 21.7% in 2018, down from 21.9% in 2017.2 More recent evidence, however, has shown an increase in maternal and neonatal morbidity with prolonged second stage.3-8
Efforts to manage the second stage from an evidence-based perspective are critical to balance the desired outcome of a safe vaginal delivery against the risks of prolonged second stage and operative vaginal delivery or CD.
Perspectives on the “ideal” labor duration
It is important to consider the historical context that led to the 2014 change in recommendations for duration of the second stage.9 In 1955, Dr. Emanuel Friedman published a prospective observational study of 622 consecutive primigravid parturients at term, of which 500 were included in the analysis that led to the graphicostatistical labor curve, or the well-known “Friedman’s curve.”10 The mean duration of the second stage was 0.95 hour. The statistical maximum for “ideal labor” for the second stage was set at 2 hours, with an additional hour allotted for patients receiving epidural analgesia.
In 2010, Zhang and colleagues published contemporary labor curves using data from the Consortium on Safe Labor, a multicenter retrospective observational study of 62,415 parturients.11 Among more than 25,000 nulliparous women, the median duration (95th percentile) of the second stage in hours was 1.1 (3.6), respectively. Notably, this analysis included only women with a spontaneous vaginal delivery and normal neonatal outcome.
Prior to the publication of the “Safe prevention of primary cesarean delivery,” multiple investigations examined the relationship between the duration of the second stage and adverse maternal and neonatal outcomes, and the findings have been inconsistent.12-15
For example, Cheng and colleagues noted increased maternal complications that included postpartum hemorrhage, third- and fourth-degree perineal lacerations, and chorioamnionitis, but not neonatal morbidity, with each increasing hour within the second stage.12 By contrast, a large, population-based cohort study among low-risk women showed an increase in low 5-minute Apgar scores, admission to the neonatal intensive care unit (NICU), and composite perinatal morbidity with prolonged second stage.15 Furthermore, a secondary analysis of the Pushing Early or Pushing Late with Epidural (PEOPLE) trial showed that the chances of a vaginal delivery with a newborn without signs of asphyxia decreased significantly every hour after the first hour, and the risk of postpartum hemorrhage and intrapartum fever increased significantly after 2 hours of pushing.14
While these findings may represent the risks inherent with the intervention of operative delivery and not the duration of second stage of labor per se, one could posit that if the intervention were initiated earlier, could it prevent or at least reduce maternal and neonatal morbidity?
Continue to: Factors to assess and monitor in the second stage...
Factors to assess and monitor in the second stage
When assessing progress in the second stage of labor, consider:
- maternal factors
- fetal/neonatal factors, and
- modifiable factors.
Maternal factors that influence the second stage of labor include parity, body mass index (BMI), age, and clinical pelvimetry.11,16-19 Fetal/neonatal factors that impact the second stage include the estimated fetal weight, fetal presentation (cephalic, face, and so on), position, and station, as well as the FHR Category.20, 21 Factors that can be modified in the second stage include the effect of epidural analgesia (turning it down to reduce motor blockade while maintaining sensory pain relief so that patients feel the “urge” to push), maternal pushing position and technique, the presence of maternal support person(s), manual rotation for a fetal position that is not optimal, immediate versus delayed pushing, and prevention of perineal tears.22-32 Interestingly, epidural analgesia, parity, birth weight, and station at complete dilation predicted second stage duration but accounted for only 25% of the variability in second stage length, leaving 75% of the variance unexplained.16
A specific absolute maximum length of time spent in the second stage of labor beyond which all women should undergo operative delivery has not been identified.1 Therefore, maternal, fetal/neonatal, and modifiable factors need to be critically assessed and continually monitored to determine whether a prolonged second stage or an operative delivery is warranted to prevent or minimize adverse maternal and neonatal outcomes.
Maternal factors
Maternal age correlates directly with the length of the second stage. That is, the length of the second stage increases with increasing age.17
Multiparous women have a shorter length of the second stage, regardless of epidural analgesia, compared with nulliparous women.11 In the Consortium for Safe Labor, multiparous women had a significantly shorter median second stage compared with nulliparous women.11
In adjusted analyses, maternal obesity was associated with an increased risk for CD, with the risk of CD more than 3 times greater in women with a BMI higher than 40 kg/m2 compared with those who had a BMI less than 25 kg/m2.18 There were no significant differences in the length of the second stage of labor by BMI catgeories.19
Fetal factors
Birth weight greater than 4,000 g was associated with an increased risk for arrest of descent during the second stage.33
Persistent fetal occiput posterior or transverse position may impact the duration of the second stage. A retrospective cohort study in women who underwent a trial of manual rotation compared with expectant management during the second stage of labor with the fetus in occiput posterior or occiput transverse position found that women with manual rotation were less likely to have a CD, severe perineal laceration, postpartum hemorrhage, and chorioamnionitis. However, an increased risk of cervical laceration was associated with manual rotation.20
Regarding FHR status, FHR abnormalities occurred in 91% of second stage labor patterns, with Category II being the most common.21 The fetal status should remain reassuring to allow for continuation of the second stage.
Continue to: Epidural analgesia...
Epidural analgesia
About 60% of women receive neuraxial analgesia in the United States,22 although rates vary widely across different populations. A Cochrane review showed no difference in the duration of the second stage among women who had early versus late initiation of epidural analgesia in labor.23 Epidural analgesia has no impact on the risk of CD; however, women with epidural analgesia experienced more hypotension, motor blockade, fever, and urinary retention.24
One management practice has been to discontinue epidural analgesia to allow resumption of sensory and motor nerve function. Another Cochrane systematic review found no difference in mode of delivery or neonatal outcomes.25 Rather than discontinuing epidural analgesia, which results in a profound increase in inadequate pain relief, one may consider titrating the dose with joint patient decision-making to allow for greater motor capability while maintaining adequate analgesia.34
Immediate vs delayed pushing
The 2 most common approaches to managing the second stage were either to initiate pushing with contractions once complete dilation occurred (immediate pushing) or to allow for a rest period in which the fetus passively rotated and descended while conserving a woman’s energy for pushing efforts (delayed pushing, laboring down, or passive descent). Since the publication of “Safe prevention of primary cesarean delivery,” however, studies have shown a concerning association between maternal and neonatal complications and prolonged second stage (which may occur with delayed pushing).3-8,35 An observational study of nearly 44,000 nulliparous women without epidural analgesia found that prolonged second stage was associated with increased chorioamnionitis, third- and fourth-degree lacerations, neonatal sepsis, neonatal asphyxia, and perinatal mortality.35
A pragmatic multicenter randomized clinical trial on the optimal management of second stage of labor across the United States recently was conducted.7 More than 2,000 nulliparous women at term in spontaneous or induced labor with epidural analgesia were randomly assigned at complete dilation to immediate pushing or delayed pushing (1 hour after complete dilation). There was no difference in the rate of vaginal delivery. The rate of postpartum hemorrhage was significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (2.3% vs 4.0%, respectively; relative risk [RR], 0.6; 95% confidence interval [CI], 0.3–0.9; P = .03). Furthermore, rates of chorioamnionitis were significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (6.7% vs 9.1%, respectively; RR, 0.70; 95% CI, 0.66–0.90; P = .005). No significant difference occurred in the composite outcome of neonatal morbidity between the groups. However neonatal acidemia (umbilical cord arterial pH <7.1) and confirmed or suspected sepsis were significantly increased in the delayed-pushing group.
The evidence supports active pushing at the start of the second stage. Women who consider delayed pushing should be informed that delayed pushing has not been shown to increase the likelihood of vaginal birth and that it is associated with increased risks of infection, hemorrhage, and neonatal acidemia.36
Maternal pushing position and technique
Spontaneous pushing (in which women are free to follow their instincts and generally push 3 to 5 times per contraction) versus directed pushing (women are encouraged to take a deep breath at the beginning of a contraction then hold it and bear down throughout the contraction) demonstrated no clear difference in duration of the second stage, perineal laceration, episiotomy, time spent pushing, or number of women with spontaneous vaginal birth. There was no difference in 5-minute Apgar score less than 7 or admission to the NICU.26
With regard to maternal positioning during the second stage, a Cochrane systematic review found benefits for upright posture, including a very small reduction in the duration of the second stage, reduction in episiotomy rates, and reduction in assisted deliveries.37 There was an increased risk of blood loss greater than 500 mL and possibly an increased risk in second-degree tears.37 Compared with women allocated to lying down, women in the upright position during the second stage with epidural analgesia had significantly fewer spontaneous vaginal births. There was no difference in operative vaginal delivery, obstetric anal sphincter injury (OASI), infant Apgar score of less than 4 at 5 minutes, and maternal fecal incontinence at 1 year.28
Continue to: Maternal support person...
Maternal support person
Continuous support during labor may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labor, and decreased cesarean birth.29 In a randomized trial of 412 healthy nulliparous women, women in labor were assigned to either a support group that received continuous support from a doula or an observed group that was monitored by an inconspicuous observer. Continuous labor support significantly reduced the rate of CDs and forceps deliveries.30,36 Indeed, during the COVID-19 pandemic, doulas have found innovative ways to continue to provide this essential support through virtual health.38
Prevention of perineal tears
Evidence suggests that warm compresses, and massage, may reduce third- and fourth-degree perineal tears.31 A meta-analysis of observational studies showed a significant reduction in the risk of OASI.32
Second stage steps: Recap
Throughout the second stage of labor, the decision to continue with expectant management or intervene with either an operative vaginal delivery or a CD is complex and requires consistent assessment and integration of multiple factors. An evidence-based approach to second stage labor management includes active pushing that is either Valsalva pushing or spontaneous, coached or uncoached, but most importantly, at the start of the second stage when a patient reaches complete dilation. Reassessment should occur at regular intervals to determine progress, after ensuring maternal and fetal well-being.
If there has been no advancement in station, an attempt at manual rotation or titration of epidural analgesia should be considered. Importantly, fetal descent with adequate pushing should be demonstrated throughout the second stage.
Additional considerations that improve outcomes include warm compresses or perineal massage to prevent third- and fourth-degree tears and the presence of a continuous support person to reduce the risk for an operative delivery.
Delivery should be expected within 2 hours for multiparous women and 3 hours for nulliparous women in the second stage. Prolonging the second stage beyond these thresholds should be individualized and occur only in the setting of assured maternal and fetal well-being.

CASE An alternative management strategy
Despite Ms. J.’s great active pushing effort for 60 minutes, the presenting part remains at 0 station and occiput transverse. Ms. J. is counseled regarding the risks and benefits of an attempt at manual rotation of the fetal head, and she wishes to proceed. The fetal position remains occiput transverse.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. At this time, Ms. J. is informed that there has been no descent, and she is counseled on the risks and benefits of continued pushing versus CD. Through shared decision-making, she consents to a CD. She undergoes a primary CD without complication. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 8 and 9, respectively. The umbilical cord arterial pH was 7.13.
Ms. J. and her baby were discharged home on postoperative day 4. ●
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine; Caughey AB, Cahill AG, Guise JM, et al. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179-193. doi:10.1016/j.ajog.2014.01.026.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1-47.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127:667-673. doi:10.1097/aog.0000000000001354.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-6. doi:10.1016/j.ajog.2015.12.042.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8. doi:10.1016/j.ajog.2017.10.007.
- Grantz KL, Sundaram R, Ma L, et al. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet Gynecol. 2018;131:345-353. doi:10.1097/aog.0000000000002431.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454. doi:10.1001/jama.2018.13986.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019;220:191.e1-191.e7. doi:10.1016/j.ajog.2018.10.028.
- Leveno KJ, Nelson DB, McIntire DD. Second-stage labor: how long is too long? Am J Obstet Gynecol. 2016;214:484-489. doi:10.1016/j.ajog.2015.10.926.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567-589. doi:10.1097/00006250-195512000-00001.
- Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281-1287. doi:10.1097/AOG.0b013e3181fdef6e.
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol. 2004;191:933-938. doi:10.1016/j.ajog.2004.05.044.
- Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1-7. doi:10.1016/j.ajog.2009.08.003.
- Le Ray C, Audibert F, Goffinet F, et al. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361.e1-7. doi:10.1016/j.ajog.2009.08.002.
- Allen VM, Baskett TF, O’Connell CM, et al. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113:1248-1258. doi:10.1097/AOG.0b013e3181a722d6.
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. Am J Obstet Gynecol. 1991;165(4 pt 1):976-979. doi:10.1016/0002-9378(91)90452-w.
- Zaki MN, Hibbard JU, Kominiarek MA. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018-1024. doi:10.1097/AOG.0b013e3182a9c92c.
- Kominiarek MA, Vanveldhuisen P, Hibbard J, et al; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264.e1-7. doi:10.1016/j.ajog.2010.06.024.
- Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244.e1-8. doi:10.1016/j.ajog.2011.06.014.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72. doi:10.3109/14767051003710276.
- Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. V. Fetal heart rate patterns in the second stage of labor. Am J Obstet Gynecol. 1981;140:435-439. doi:10.1016/0002-9378(81)90041-7.
- Grant EN, Tao W, Craig M, et al. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG. 2015;122:288-293. doi:10.1111/1471-0528.12966.
- Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation of epidural analgesia for labour. Cochrane Database Syst Rev. 2014;(10):CD007238. doi:10.1002/14651858.CD007238.pub2.
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi:10.1002/14651858.CD000331.pub4.
- Torvaldsen S, Roberts CL, Bell JC, et al. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;(4):CD004457. doi:10.1002/14651858.CD004457.pub2.
- Lemos A, Amorim MM, Dornelas de Andrade A, et al. Pushing/bearing down methods for the second stage of labour. Cochrane Database Syst Rev. 2017;3(3):CD009124. doi:10.1002/14651858.CD009124.pub3.
- Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG. 2011;118:662-670. doi:10.1111/j.1471-0528.2011 .02910.x.
- Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ. 2017;359:j4471. doi:10.1136/bmj.j4471.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7(7):CD003766. doi:10.1002/14651858.CD003766.pub6.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197-2201.
- Aasheim V, Nilsen AB, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6(6):CD006672. doi:10.1002/14651858.CD006672.pub3.
- Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG. 2015;122:1157-1165. doi:10.1111/1471-0528.13431.
- Feinstein U, Sheiner E, Levy A, et al. Risk factors for arrest of descent during the second stage of labor. Int J Gynaecol Obstet. 2002;77:7-14. doi:10.1016/s0020-7292(02)00007-3.
- Cheng YW, Caughey AB. Defining and managing normal and abnormal second stage of labor. Obstet Gynecol Clin North Am. 2017;44:547-566. doi:10.1016/j.ogc.2017.08.009.
- Laughon SK, Berghella V, Reddy UM, et al. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124:57-67. doi:10.1097/aog.0000000000000278.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 766: approaches to limit intervention during labor and birth. Obstet Gynecol. 2019;133:e164-e173. doi:10.1097/aog.0000000000003074.
- Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2017;5(5):CD002006. doi:10.1002/14651858.CD002006.pub4.
- Castaneda AN, Searcy JJ. Practising intimate labour: birth doulas respond during COVID-19. Anthropol Action. 2021;28:21-24. https://www.berghahnjournals.com/view/ journals/aia/28/1/aia280104.xml. Accessed February 8, 2021.

CASE Woman in second stage with prolonged pushing
Ms. J. is an 18-year-old woman (G1P0) at 39 weeks’ gestation whose cervix is completely dilated; she has been actively pushing for 60 minutes. The estimated fetal weight is 8 lb, and electronic fetal monitoring shows a Category I fetal heart rate (FHR) tracing. The presenting part remains at 0 station and occiput transverse despite great pushing effort.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. During the third hour of the second stage, Ms. J. is diagnosed with chorioamnionitis and the fetus remains at 0 station. She undergoes a primary cesarean delivery (CD) complicated by bilateral lower uterine extensions and postpartum hemorrhage. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 4 and 8, respectively. The umbilical cord arterial pH was 7.03.
Ms. J. and her baby were discharged home on postoperative day 4.
In 2014, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine jointly released a document, “Safe prevention of the primary cesarean delivery,” in response to the sharp rise in cesarean births from 1996 to 2011.1 It described management strategies to safely reduce the most common indications for a primary CD in nulliparous women. Specifically, it recommended that the second stage of labor—defined as the interval from complete cervical dilation through delivery of the neonate—may be prolonged, as “longer durations may be appropriate on an individualized basis (eg, with the use of epidural analgesia or with fetal malposition) as long as progress is being documented.”1
A prolonged second stage was defined as 3 hours of pushing in nulliparous women and 2 hours in multiparous women, with 1 additional hour (or longer) in those receiving epidural analgesia. Indeed, the primary CD rate decreased slightly to 21.7% in 2018, down from 21.9% in 2017.2 More recent evidence, however, has shown an increase in maternal and neonatal morbidity with prolonged second stage.3-8
Efforts to manage the second stage from an evidence-based perspective are critical to balance the desired outcome of a safe vaginal delivery against the risks of prolonged second stage and operative vaginal delivery or CD.
Perspectives on the “ideal” labor duration
It is important to consider the historical context that led to the 2014 change in recommendations for duration of the second stage.9 In 1955, Dr. Emanuel Friedman published a prospective observational study of 622 consecutive primigravid parturients at term, of which 500 were included in the analysis that led to the graphicostatistical labor curve, or the well-known “Friedman’s curve.”10 The mean duration of the second stage was 0.95 hour. The statistical maximum for “ideal labor” for the second stage was set at 2 hours, with an additional hour allotted for patients receiving epidural analgesia.
In 2010, Zhang and colleagues published contemporary labor curves using data from the Consortium on Safe Labor, a multicenter retrospective observational study of 62,415 parturients.11 Among more than 25,000 nulliparous women, the median duration (95th percentile) of the second stage in hours was 1.1 (3.6), respectively. Notably, this analysis included only women with a spontaneous vaginal delivery and normal neonatal outcome.
Prior to the publication of the “Safe prevention of primary cesarean delivery,” multiple investigations examined the relationship between the duration of the second stage and adverse maternal and neonatal outcomes, and the findings have been inconsistent.12-15
For example, Cheng and colleagues noted increased maternal complications that included postpartum hemorrhage, third- and fourth-degree perineal lacerations, and chorioamnionitis, but not neonatal morbidity, with each increasing hour within the second stage.12 By contrast, a large, population-based cohort study among low-risk women showed an increase in low 5-minute Apgar scores, admission to the neonatal intensive care unit (NICU), and composite perinatal morbidity with prolonged second stage.15 Furthermore, a secondary analysis of the Pushing Early or Pushing Late with Epidural (PEOPLE) trial showed that the chances of a vaginal delivery with a newborn without signs of asphyxia decreased significantly every hour after the first hour, and the risk of postpartum hemorrhage and intrapartum fever increased significantly after 2 hours of pushing.14
While these findings may represent the risks inherent with the intervention of operative delivery and not the duration of second stage of labor per se, one could posit that if the intervention were initiated earlier, could it prevent or at least reduce maternal and neonatal morbidity?
Continue to: Factors to assess and monitor in the second stage...
Factors to assess and monitor in the second stage
When assessing progress in the second stage of labor, consider:
- maternal factors
- fetal/neonatal factors, and
- modifiable factors.
Maternal factors that influence the second stage of labor include parity, body mass index (BMI), age, and clinical pelvimetry.11,16-19 Fetal/neonatal factors that impact the second stage include the estimated fetal weight, fetal presentation (cephalic, face, and so on), position, and station, as well as the FHR Category.20, 21 Factors that can be modified in the second stage include the effect of epidural analgesia (turning it down to reduce motor blockade while maintaining sensory pain relief so that patients feel the “urge” to push), maternal pushing position and technique, the presence of maternal support person(s), manual rotation for a fetal position that is not optimal, immediate versus delayed pushing, and prevention of perineal tears.22-32 Interestingly, epidural analgesia, parity, birth weight, and station at complete dilation predicted second stage duration but accounted for only 25% of the variability in second stage length, leaving 75% of the variance unexplained.16
A specific absolute maximum length of time spent in the second stage of labor beyond which all women should undergo operative delivery has not been identified.1 Therefore, maternal, fetal/neonatal, and modifiable factors need to be critically assessed and continually monitored to determine whether a prolonged second stage or an operative delivery is warranted to prevent or minimize adverse maternal and neonatal outcomes.
Maternal factors
Maternal age correlates directly with the length of the second stage. That is, the length of the second stage increases with increasing age.17
Multiparous women have a shorter length of the second stage, regardless of epidural analgesia, compared with nulliparous women.11 In the Consortium for Safe Labor, multiparous women had a significantly shorter median second stage compared with nulliparous women.11
In adjusted analyses, maternal obesity was associated with an increased risk for CD, with the risk of CD more than 3 times greater in women with a BMI higher than 40 kg/m2 compared with those who had a BMI less than 25 kg/m2.18 There were no significant differences in the length of the second stage of labor by BMI catgeories.19
Fetal factors
Birth weight greater than 4,000 g was associated with an increased risk for arrest of descent during the second stage.33
Persistent fetal occiput posterior or transverse position may impact the duration of the second stage. A retrospective cohort study in women who underwent a trial of manual rotation compared with expectant management during the second stage of labor with the fetus in occiput posterior or occiput transverse position found that women with manual rotation were less likely to have a CD, severe perineal laceration, postpartum hemorrhage, and chorioamnionitis. However, an increased risk of cervical laceration was associated with manual rotation.20
Regarding FHR status, FHR abnormalities occurred in 91% of second stage labor patterns, with Category II being the most common.21 The fetal status should remain reassuring to allow for continuation of the second stage.
Continue to: Epidural analgesia...
Epidural analgesia
About 60% of women receive neuraxial analgesia in the United States,22 although rates vary widely across different populations. A Cochrane review showed no difference in the duration of the second stage among women who had early versus late initiation of epidural analgesia in labor.23 Epidural analgesia has no impact on the risk of CD; however, women with epidural analgesia experienced more hypotension, motor blockade, fever, and urinary retention.24
One management practice has been to discontinue epidural analgesia to allow resumption of sensory and motor nerve function. Another Cochrane systematic review found no difference in mode of delivery or neonatal outcomes.25 Rather than discontinuing epidural analgesia, which results in a profound increase in inadequate pain relief, one may consider titrating the dose with joint patient decision-making to allow for greater motor capability while maintaining adequate analgesia.34
Immediate vs delayed pushing
The 2 most common approaches to managing the second stage were either to initiate pushing with contractions once complete dilation occurred (immediate pushing) or to allow for a rest period in which the fetus passively rotated and descended while conserving a woman’s energy for pushing efforts (delayed pushing, laboring down, or passive descent). Since the publication of “Safe prevention of primary cesarean delivery,” however, studies have shown a concerning association between maternal and neonatal complications and prolonged second stage (which may occur with delayed pushing).3-8,35 An observational study of nearly 44,000 nulliparous women without epidural analgesia found that prolonged second stage was associated with increased chorioamnionitis, third- and fourth-degree lacerations, neonatal sepsis, neonatal asphyxia, and perinatal mortality.35
A pragmatic multicenter randomized clinical trial on the optimal management of second stage of labor across the United States recently was conducted.7 More than 2,000 nulliparous women at term in spontaneous or induced labor with epidural analgesia were randomly assigned at complete dilation to immediate pushing or delayed pushing (1 hour after complete dilation). There was no difference in the rate of vaginal delivery. The rate of postpartum hemorrhage was significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (2.3% vs 4.0%, respectively; relative risk [RR], 0.6; 95% confidence interval [CI], 0.3–0.9; P = .03). Furthermore, rates of chorioamnionitis were significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (6.7% vs 9.1%, respectively; RR, 0.70; 95% CI, 0.66–0.90; P = .005). No significant difference occurred in the composite outcome of neonatal morbidity between the groups. However neonatal acidemia (umbilical cord arterial pH <7.1) and confirmed or suspected sepsis were significantly increased in the delayed-pushing group.
The evidence supports active pushing at the start of the second stage. Women who consider delayed pushing should be informed that delayed pushing has not been shown to increase the likelihood of vaginal birth and that it is associated with increased risks of infection, hemorrhage, and neonatal acidemia.36
Maternal pushing position and technique
Spontaneous pushing (in which women are free to follow their instincts and generally push 3 to 5 times per contraction) versus directed pushing (women are encouraged to take a deep breath at the beginning of a contraction then hold it and bear down throughout the contraction) demonstrated no clear difference in duration of the second stage, perineal laceration, episiotomy, time spent pushing, or number of women with spontaneous vaginal birth. There was no difference in 5-minute Apgar score less than 7 or admission to the NICU.26
With regard to maternal positioning during the second stage, a Cochrane systematic review found benefits for upright posture, including a very small reduction in the duration of the second stage, reduction in episiotomy rates, and reduction in assisted deliveries.37 There was an increased risk of blood loss greater than 500 mL and possibly an increased risk in second-degree tears.37 Compared with women allocated to lying down, women in the upright position during the second stage with epidural analgesia had significantly fewer spontaneous vaginal births. There was no difference in operative vaginal delivery, obstetric anal sphincter injury (OASI), infant Apgar score of less than 4 at 5 minutes, and maternal fecal incontinence at 1 year.28
Continue to: Maternal support person...
Maternal support person
Continuous support during labor may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labor, and decreased cesarean birth.29 In a randomized trial of 412 healthy nulliparous women, women in labor were assigned to either a support group that received continuous support from a doula or an observed group that was monitored by an inconspicuous observer. Continuous labor support significantly reduced the rate of CDs and forceps deliveries.30,36 Indeed, during the COVID-19 pandemic, doulas have found innovative ways to continue to provide this essential support through virtual health.38
Prevention of perineal tears
Evidence suggests that warm compresses, and massage, may reduce third- and fourth-degree perineal tears.31 A meta-analysis of observational studies showed a significant reduction in the risk of OASI.32
Second stage steps: Recap
Throughout the second stage of labor, the decision to continue with expectant management or intervene with either an operative vaginal delivery or a CD is complex and requires consistent assessment and integration of multiple factors. An evidence-based approach to second stage labor management includes active pushing that is either Valsalva pushing or spontaneous, coached or uncoached, but most importantly, at the start of the second stage when a patient reaches complete dilation. Reassessment should occur at regular intervals to determine progress, after ensuring maternal and fetal well-being.
If there has been no advancement in station, an attempt at manual rotation or titration of epidural analgesia should be considered. Importantly, fetal descent with adequate pushing should be demonstrated throughout the second stage.
Additional considerations that improve outcomes include warm compresses or perineal massage to prevent third- and fourth-degree tears and the presence of a continuous support person to reduce the risk for an operative delivery.
Delivery should be expected within 2 hours for multiparous women and 3 hours for nulliparous women in the second stage. Prolonging the second stage beyond these thresholds should be individualized and occur only in the setting of assured maternal and fetal well-being.

CASE An alternative management strategy
Despite Ms. J.’s great active pushing effort for 60 minutes, the presenting part remains at 0 station and occiput transverse. Ms. J. is counseled regarding the risks and benefits of an attempt at manual rotation of the fetal head, and she wishes to proceed. The fetal position remains occiput transverse.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. At this time, Ms. J. is informed that there has been no descent, and she is counseled on the risks and benefits of continued pushing versus CD. Through shared decision-making, she consents to a CD. She undergoes a primary CD without complication. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 8 and 9, respectively. The umbilical cord arterial pH was 7.13.
Ms. J. and her baby were discharged home on postoperative day 4. ●

CASE Woman in second stage with prolonged pushing
Ms. J. is an 18-year-old woman (G1P0) at 39 weeks’ gestation whose cervix is completely dilated; she has been actively pushing for 60 minutes. The estimated fetal weight is 8 lb, and electronic fetal monitoring shows a Category I fetal heart rate (FHR) tracing. The presenting part remains at 0 station and occiput transverse despite great pushing effort.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. During the third hour of the second stage, Ms. J. is diagnosed with chorioamnionitis and the fetus remains at 0 station. She undergoes a primary cesarean delivery (CD) complicated by bilateral lower uterine extensions and postpartum hemorrhage. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 4 and 8, respectively. The umbilical cord arterial pH was 7.03.
Ms. J. and her baby were discharged home on postoperative day 4.
In 2014, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine jointly released a document, “Safe prevention of the primary cesarean delivery,” in response to the sharp rise in cesarean births from 1996 to 2011.1 It described management strategies to safely reduce the most common indications for a primary CD in nulliparous women. Specifically, it recommended that the second stage of labor—defined as the interval from complete cervical dilation through delivery of the neonate—may be prolonged, as “longer durations may be appropriate on an individualized basis (eg, with the use of epidural analgesia or with fetal malposition) as long as progress is being documented.”1
A prolonged second stage was defined as 3 hours of pushing in nulliparous women and 2 hours in multiparous women, with 1 additional hour (or longer) in those receiving epidural analgesia. Indeed, the primary CD rate decreased slightly to 21.7% in 2018, down from 21.9% in 2017.2 More recent evidence, however, has shown an increase in maternal and neonatal morbidity with prolonged second stage.3-8
Efforts to manage the second stage from an evidence-based perspective are critical to balance the desired outcome of a safe vaginal delivery against the risks of prolonged second stage and operative vaginal delivery or CD.
Perspectives on the “ideal” labor duration
It is important to consider the historical context that led to the 2014 change in recommendations for duration of the second stage.9 In 1955, Dr. Emanuel Friedman published a prospective observational study of 622 consecutive primigravid parturients at term, of which 500 were included in the analysis that led to the graphicostatistical labor curve, or the well-known “Friedman’s curve.”10 The mean duration of the second stage was 0.95 hour. The statistical maximum for “ideal labor” for the second stage was set at 2 hours, with an additional hour allotted for patients receiving epidural analgesia.
In 2010, Zhang and colleagues published contemporary labor curves using data from the Consortium on Safe Labor, a multicenter retrospective observational study of 62,415 parturients.11 Among more than 25,000 nulliparous women, the median duration (95th percentile) of the second stage in hours was 1.1 (3.6), respectively. Notably, this analysis included only women with a spontaneous vaginal delivery and normal neonatal outcome.
Prior to the publication of the “Safe prevention of primary cesarean delivery,” multiple investigations examined the relationship between the duration of the second stage and adverse maternal and neonatal outcomes, and the findings have been inconsistent.12-15
For example, Cheng and colleagues noted increased maternal complications that included postpartum hemorrhage, third- and fourth-degree perineal lacerations, and chorioamnionitis, but not neonatal morbidity, with each increasing hour within the second stage.12 By contrast, a large, population-based cohort study among low-risk women showed an increase in low 5-minute Apgar scores, admission to the neonatal intensive care unit (NICU), and composite perinatal morbidity with prolonged second stage.15 Furthermore, a secondary analysis of the Pushing Early or Pushing Late with Epidural (PEOPLE) trial showed that the chances of a vaginal delivery with a newborn without signs of asphyxia decreased significantly every hour after the first hour, and the risk of postpartum hemorrhage and intrapartum fever increased significantly after 2 hours of pushing.14
While these findings may represent the risks inherent with the intervention of operative delivery and not the duration of second stage of labor per se, one could posit that if the intervention were initiated earlier, could it prevent or at least reduce maternal and neonatal morbidity?
Continue to: Factors to assess and monitor in the second stage...
Factors to assess and monitor in the second stage
When assessing progress in the second stage of labor, consider:
- maternal factors
- fetal/neonatal factors, and
- modifiable factors.
Maternal factors that influence the second stage of labor include parity, body mass index (BMI), age, and clinical pelvimetry.11,16-19 Fetal/neonatal factors that impact the second stage include the estimated fetal weight, fetal presentation (cephalic, face, and so on), position, and station, as well as the FHR Category.20, 21 Factors that can be modified in the second stage include the effect of epidural analgesia (turning it down to reduce motor blockade while maintaining sensory pain relief so that patients feel the “urge” to push), maternal pushing position and technique, the presence of maternal support person(s), manual rotation for a fetal position that is not optimal, immediate versus delayed pushing, and prevention of perineal tears.22-32 Interestingly, epidural analgesia, parity, birth weight, and station at complete dilation predicted second stage duration but accounted for only 25% of the variability in second stage length, leaving 75% of the variance unexplained.16
A specific absolute maximum length of time spent in the second stage of labor beyond which all women should undergo operative delivery has not been identified.1 Therefore, maternal, fetal/neonatal, and modifiable factors need to be critically assessed and continually monitored to determine whether a prolonged second stage or an operative delivery is warranted to prevent or minimize adverse maternal and neonatal outcomes.
Maternal factors
Maternal age correlates directly with the length of the second stage. That is, the length of the second stage increases with increasing age.17
Multiparous women have a shorter length of the second stage, regardless of epidural analgesia, compared with nulliparous women.11 In the Consortium for Safe Labor, multiparous women had a significantly shorter median second stage compared with nulliparous women.11
In adjusted analyses, maternal obesity was associated with an increased risk for CD, with the risk of CD more than 3 times greater in women with a BMI higher than 40 kg/m2 compared with those who had a BMI less than 25 kg/m2.18 There were no significant differences in the length of the second stage of labor by BMI catgeories.19
Fetal factors
Birth weight greater than 4,000 g was associated with an increased risk for arrest of descent during the second stage.33
Persistent fetal occiput posterior or transverse position may impact the duration of the second stage. A retrospective cohort study in women who underwent a trial of manual rotation compared with expectant management during the second stage of labor with the fetus in occiput posterior or occiput transverse position found that women with manual rotation were less likely to have a CD, severe perineal laceration, postpartum hemorrhage, and chorioamnionitis. However, an increased risk of cervical laceration was associated with manual rotation.20
Regarding FHR status, FHR abnormalities occurred in 91% of second stage labor patterns, with Category II being the most common.21 The fetal status should remain reassuring to allow for continuation of the second stage.
Continue to: Epidural analgesia...
Epidural analgesia
About 60% of women receive neuraxial analgesia in the United States,22 although rates vary widely across different populations. A Cochrane review showed no difference in the duration of the second stage among women who had early versus late initiation of epidural analgesia in labor.23 Epidural analgesia has no impact on the risk of CD; however, women with epidural analgesia experienced more hypotension, motor blockade, fever, and urinary retention.24
One management practice has been to discontinue epidural analgesia to allow resumption of sensory and motor nerve function. Another Cochrane systematic review found no difference in mode of delivery or neonatal outcomes.25 Rather than discontinuing epidural analgesia, which results in a profound increase in inadequate pain relief, one may consider titrating the dose with joint patient decision-making to allow for greater motor capability while maintaining adequate analgesia.34
Immediate vs delayed pushing
The 2 most common approaches to managing the second stage were either to initiate pushing with contractions once complete dilation occurred (immediate pushing) or to allow for a rest period in which the fetus passively rotated and descended while conserving a woman’s energy for pushing efforts (delayed pushing, laboring down, or passive descent). Since the publication of “Safe prevention of primary cesarean delivery,” however, studies have shown a concerning association between maternal and neonatal complications and prolonged second stage (which may occur with delayed pushing).3-8,35 An observational study of nearly 44,000 nulliparous women without epidural analgesia found that prolonged second stage was associated with increased chorioamnionitis, third- and fourth-degree lacerations, neonatal sepsis, neonatal asphyxia, and perinatal mortality.35
A pragmatic multicenter randomized clinical trial on the optimal management of second stage of labor across the United States recently was conducted.7 More than 2,000 nulliparous women at term in spontaneous or induced labor with epidural analgesia were randomly assigned at complete dilation to immediate pushing or delayed pushing (1 hour after complete dilation). There was no difference in the rate of vaginal delivery. The rate of postpartum hemorrhage was significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (2.3% vs 4.0%, respectively; relative risk [RR], 0.6; 95% confidence interval [CI], 0.3–0.9; P = .03). Furthermore, rates of chorioamnionitis were significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (6.7% vs 9.1%, respectively; RR, 0.70; 95% CI, 0.66–0.90; P = .005). No significant difference occurred in the composite outcome of neonatal morbidity between the groups. However neonatal acidemia (umbilical cord arterial pH <7.1) and confirmed or suspected sepsis were significantly increased in the delayed-pushing group.
The evidence supports active pushing at the start of the second stage. Women who consider delayed pushing should be informed that delayed pushing has not been shown to increase the likelihood of vaginal birth and that it is associated with increased risks of infection, hemorrhage, and neonatal acidemia.36
Maternal pushing position and technique
Spontaneous pushing (in which women are free to follow their instincts and generally push 3 to 5 times per contraction) versus directed pushing (women are encouraged to take a deep breath at the beginning of a contraction then hold it and bear down throughout the contraction) demonstrated no clear difference in duration of the second stage, perineal laceration, episiotomy, time spent pushing, or number of women with spontaneous vaginal birth. There was no difference in 5-minute Apgar score less than 7 or admission to the NICU.26
With regard to maternal positioning during the second stage, a Cochrane systematic review found benefits for upright posture, including a very small reduction in the duration of the second stage, reduction in episiotomy rates, and reduction in assisted deliveries.37 There was an increased risk of blood loss greater than 500 mL and possibly an increased risk in second-degree tears.37 Compared with women allocated to lying down, women in the upright position during the second stage with epidural analgesia had significantly fewer spontaneous vaginal births. There was no difference in operative vaginal delivery, obstetric anal sphincter injury (OASI), infant Apgar score of less than 4 at 5 minutes, and maternal fecal incontinence at 1 year.28
Continue to: Maternal support person...
Maternal support person
Continuous support during labor may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labor, and decreased cesarean birth.29 In a randomized trial of 412 healthy nulliparous women, women in labor were assigned to either a support group that received continuous support from a doula or an observed group that was monitored by an inconspicuous observer. Continuous labor support significantly reduced the rate of CDs and forceps deliveries.30,36 Indeed, during the COVID-19 pandemic, doulas have found innovative ways to continue to provide this essential support through virtual health.38
Prevention of perineal tears
Evidence suggests that warm compresses, and massage, may reduce third- and fourth-degree perineal tears.31 A meta-analysis of observational studies showed a significant reduction in the risk of OASI.32
Second stage steps: Recap
Throughout the second stage of labor, the decision to continue with expectant management or intervene with either an operative vaginal delivery or a CD is complex and requires consistent assessment and integration of multiple factors. An evidence-based approach to second stage labor management includes active pushing that is either Valsalva pushing or spontaneous, coached or uncoached, but most importantly, at the start of the second stage when a patient reaches complete dilation. Reassessment should occur at regular intervals to determine progress, after ensuring maternal and fetal well-being.
If there has been no advancement in station, an attempt at manual rotation or titration of epidural analgesia should be considered. Importantly, fetal descent with adequate pushing should be demonstrated throughout the second stage.
Additional considerations that improve outcomes include warm compresses or perineal massage to prevent third- and fourth-degree tears and the presence of a continuous support person to reduce the risk for an operative delivery.
Delivery should be expected within 2 hours for multiparous women and 3 hours for nulliparous women in the second stage. Prolonging the second stage beyond these thresholds should be individualized and occur only in the setting of assured maternal and fetal well-being.

CASE An alternative management strategy
Despite Ms. J.’s great active pushing effort for 60 minutes, the presenting part remains at 0 station and occiput transverse. Ms. J. is counseled regarding the risks and benefits of an attempt at manual rotation of the fetal head, and she wishes to proceed. The fetal position remains occiput transverse.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. At this time, Ms. J. is informed that there has been no descent, and she is counseled on the risks and benefits of continued pushing versus CD. Through shared decision-making, she consents to a CD. She undergoes a primary CD without complication. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 8 and 9, respectively. The umbilical cord arterial pH was 7.13.
Ms. J. and her baby were discharged home on postoperative day 4. ●
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine; Caughey AB, Cahill AG, Guise JM, et al. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179-193. doi:10.1016/j.ajog.2014.01.026.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1-47.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127:667-673. doi:10.1097/aog.0000000000001354.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-6. doi:10.1016/j.ajog.2015.12.042.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8. doi:10.1016/j.ajog.2017.10.007.
- Grantz KL, Sundaram R, Ma L, et al. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet Gynecol. 2018;131:345-353. doi:10.1097/aog.0000000000002431.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454. doi:10.1001/jama.2018.13986.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019;220:191.e1-191.e7. doi:10.1016/j.ajog.2018.10.028.
- Leveno KJ, Nelson DB, McIntire DD. Second-stage labor: how long is too long? Am J Obstet Gynecol. 2016;214:484-489. doi:10.1016/j.ajog.2015.10.926.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567-589. doi:10.1097/00006250-195512000-00001.
- Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281-1287. doi:10.1097/AOG.0b013e3181fdef6e.
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol. 2004;191:933-938. doi:10.1016/j.ajog.2004.05.044.
- Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1-7. doi:10.1016/j.ajog.2009.08.003.
- Le Ray C, Audibert F, Goffinet F, et al. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361.e1-7. doi:10.1016/j.ajog.2009.08.002.
- Allen VM, Baskett TF, O’Connell CM, et al. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113:1248-1258. doi:10.1097/AOG.0b013e3181a722d6.
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. Am J Obstet Gynecol. 1991;165(4 pt 1):976-979. doi:10.1016/0002-9378(91)90452-w.
- Zaki MN, Hibbard JU, Kominiarek MA. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018-1024. doi:10.1097/AOG.0b013e3182a9c92c.
- Kominiarek MA, Vanveldhuisen P, Hibbard J, et al; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264.e1-7. doi:10.1016/j.ajog.2010.06.024.
- Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244.e1-8. doi:10.1016/j.ajog.2011.06.014.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72. doi:10.3109/14767051003710276.
- Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. V. Fetal heart rate patterns in the second stage of labor. Am J Obstet Gynecol. 1981;140:435-439. doi:10.1016/0002-9378(81)90041-7.
- Grant EN, Tao W, Craig M, et al. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG. 2015;122:288-293. doi:10.1111/1471-0528.12966.
- Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation of epidural analgesia for labour. Cochrane Database Syst Rev. 2014;(10):CD007238. doi:10.1002/14651858.CD007238.pub2.
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi:10.1002/14651858.CD000331.pub4.
- Torvaldsen S, Roberts CL, Bell JC, et al. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;(4):CD004457. doi:10.1002/14651858.CD004457.pub2.
- Lemos A, Amorim MM, Dornelas de Andrade A, et al. Pushing/bearing down methods for the second stage of labour. Cochrane Database Syst Rev. 2017;3(3):CD009124. doi:10.1002/14651858.CD009124.pub3.
- Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG. 2011;118:662-670. doi:10.1111/j.1471-0528.2011 .02910.x.
- Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ. 2017;359:j4471. doi:10.1136/bmj.j4471.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7(7):CD003766. doi:10.1002/14651858.CD003766.pub6.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197-2201.
- Aasheim V, Nilsen AB, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6(6):CD006672. doi:10.1002/14651858.CD006672.pub3.
- Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG. 2015;122:1157-1165. doi:10.1111/1471-0528.13431.
- Feinstein U, Sheiner E, Levy A, et al. Risk factors for arrest of descent during the second stage of labor. Int J Gynaecol Obstet. 2002;77:7-14. doi:10.1016/s0020-7292(02)00007-3.
- Cheng YW, Caughey AB. Defining and managing normal and abnormal second stage of labor. Obstet Gynecol Clin North Am. 2017;44:547-566. doi:10.1016/j.ogc.2017.08.009.
- Laughon SK, Berghella V, Reddy UM, et al. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124:57-67. doi:10.1097/aog.0000000000000278.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 766: approaches to limit intervention during labor and birth. Obstet Gynecol. 2019;133:e164-e173. doi:10.1097/aog.0000000000003074.
- Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2017;5(5):CD002006. doi:10.1002/14651858.CD002006.pub4.
- Castaneda AN, Searcy JJ. Practising intimate labour: birth doulas respond during COVID-19. Anthropol Action. 2021;28:21-24. https://www.berghahnjournals.com/view/ journals/aia/28/1/aia280104.xml. Accessed February 8, 2021.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine; Caughey AB, Cahill AG, Guise JM, et al. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179-193. doi:10.1016/j.ajog.2014.01.026.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1-47.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127:667-673. doi:10.1097/aog.0000000000001354.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-6. doi:10.1016/j.ajog.2015.12.042.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8. doi:10.1016/j.ajog.2017.10.007.
- Grantz KL, Sundaram R, Ma L, et al. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet Gynecol. 2018;131:345-353. doi:10.1097/aog.0000000000002431.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454. doi:10.1001/jama.2018.13986.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019;220:191.e1-191.e7. doi:10.1016/j.ajog.2018.10.028.
- Leveno KJ, Nelson DB, McIntire DD. Second-stage labor: how long is too long? Am J Obstet Gynecol. 2016;214:484-489. doi:10.1016/j.ajog.2015.10.926.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567-589. doi:10.1097/00006250-195512000-00001.
- Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281-1287. doi:10.1097/AOG.0b013e3181fdef6e.
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol. 2004;191:933-938. doi:10.1016/j.ajog.2004.05.044.
- Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1-7. doi:10.1016/j.ajog.2009.08.003.
- Le Ray C, Audibert F, Goffinet F, et al. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361.e1-7. doi:10.1016/j.ajog.2009.08.002.
- Allen VM, Baskett TF, O’Connell CM, et al. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113:1248-1258. doi:10.1097/AOG.0b013e3181a722d6.
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. Am J Obstet Gynecol. 1991;165(4 pt 1):976-979. doi:10.1016/0002-9378(91)90452-w.
- Zaki MN, Hibbard JU, Kominiarek MA. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018-1024. doi:10.1097/AOG.0b013e3182a9c92c.
- Kominiarek MA, Vanveldhuisen P, Hibbard J, et al; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264.e1-7. doi:10.1016/j.ajog.2010.06.024.
- Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244.e1-8. doi:10.1016/j.ajog.2011.06.014.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72. doi:10.3109/14767051003710276.
- Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. V. Fetal heart rate patterns in the second stage of labor. Am J Obstet Gynecol. 1981;140:435-439. doi:10.1016/0002-9378(81)90041-7.
- Grant EN, Tao W, Craig M, et al. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG. 2015;122:288-293. doi:10.1111/1471-0528.12966.
- Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation of epidural analgesia for labour. Cochrane Database Syst Rev. 2014;(10):CD007238. doi:10.1002/14651858.CD007238.pub2.
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi:10.1002/14651858.CD000331.pub4.
- Torvaldsen S, Roberts CL, Bell JC, et al. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;(4):CD004457. doi:10.1002/14651858.CD004457.pub2.
- Lemos A, Amorim MM, Dornelas de Andrade A, et al. Pushing/bearing down methods for the second stage of labour. Cochrane Database Syst Rev. 2017;3(3):CD009124. doi:10.1002/14651858.CD009124.pub3.
- Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG. 2011;118:662-670. doi:10.1111/j.1471-0528.2011 .02910.x.
- Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ. 2017;359:j4471. doi:10.1136/bmj.j4471.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7(7):CD003766. doi:10.1002/14651858.CD003766.pub6.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197-2201.
- Aasheim V, Nilsen AB, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6(6):CD006672. doi:10.1002/14651858.CD006672.pub3.
- Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG. 2015;122:1157-1165. doi:10.1111/1471-0528.13431.
- Feinstein U, Sheiner E, Levy A, et al. Risk factors for arrest of descent during the second stage of labor. Int J Gynaecol Obstet. 2002;77:7-14. doi:10.1016/s0020-7292(02)00007-3.
- Cheng YW, Caughey AB. Defining and managing normal and abnormal second stage of labor. Obstet Gynecol Clin North Am. 2017;44:547-566. doi:10.1016/j.ogc.2017.08.009.
- Laughon SK, Berghella V, Reddy UM, et al. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124:57-67. doi:10.1097/aog.0000000000000278.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 766: approaches to limit intervention during labor and birth. Obstet Gynecol. 2019;133:e164-e173. doi:10.1097/aog.0000000000003074.
- Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2017;5(5):CD002006. doi:10.1002/14651858.CD002006.pub4.
- Castaneda AN, Searcy JJ. Practising intimate labour: birth doulas respond during COVID-19. Anthropol Action. 2021;28:21-24. https://www.berghahnjournals.com/view/ journals/aia/28/1/aia280104.xml. Accessed February 8, 2021.
Six pregnancy complications flag later heart disease risk
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Maternal caffeine consumption, even small amounts, may reduce neonatal size
For pregnant women, just half a cup of coffee a day may reduce neonatal birth size and body weight, according to a prospective study involving more than 2,500 women.
That’s only 50 mg of a caffeine day, which falls below the upper threshold of 200 mg set by the American College of Obstetricians and Gynecologists, lead author Jessica Gleason, PhD, MPH, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md, and colleagues reported.
“Systematic reviews and meta-analyses have reported that maternal caffeine consumption, even in doses lower than 200 mg, is associated with a higher risk for low birth weight, small for gestational age (SGA), and fetal growth restriction, suggesting there may be no safe amount of caffeine during pregnancy,” the investigators wrote in JAMA Network Open.
Findings to date have been inconsistent, with a 2014 meta-analysis reporting contrary or null results in four out of nine studies.
Dr. Gleason and colleagues suggested that such discrepancies may be caused by uncontrolled confounding factors in some of the studies, such as smoking, as well as the inadequacy of self-reporting, which fails to incorporate variations in caffeine content between beverages, or differences in rates of metabolism between individuals.
“To our knowledge, no studies have examined the association between caffeine intake and neonatal anthropometric measures beyond weight, length, and head circumference, and few have analyzed plasma concentrations of caffeine and its metabolites or genetic variations in the rate of metabolism associated with neonatal size,” the investigators wrote.
Dr. Gleason and colleagues set out to address this knowledge gap with a prospective cohort study, including 2,055 nonsmoking women with low risk of birth defects who presented at 12 centers between 2009 and 2013. Mean participant age was 28.3 years and mean body mass index was 23.6. Races and ethnicities were represented almost evenly even across four groups: Hispanic (28.2%), White (27.4%), Black (25.2%), and Asian/Pacific Islander (19.2%). Rate of caffeine metabolism was defined by the single-nucleotide variant rs762551 (CYP1A2*1F), according to which, slightly more women had slow metabolism (52.7%) than fast metabolism (47.3%).
Women were enrolled at 8-13 weeks’ gestational age, at which time they underwent interviews and blood draws, allowing for measurement of caffeine and paraxanthine plasma levels, as well as self-reported caffeine consumption during the preceding week.
Over the course of six visits, fetal growth was observed via ultrasound. Medical records were used to determine birth weights and neonatal anthropometric measures, including fat and skin fold mass, body length, and circumferences of the thigh, arm, abdomen, and head.
Neonatal measurements were compared with plasma levels of caffeine and paraxanthine, both continuously and as quartiles (Q1, ≤ 28.3 ng/mL; Q2, 28.4-157.1 ng/mL; Q3, 157.2-658.8 ng/mL; Q4, > 658.8 ng/mL). Comparisons were also made with self-reported caffeine intake.
Women who reported drinking 1-50 mg of caffeine per day had neonates with smaller subscapular skin folds (beta = –0.14 mm; 95% confidence interval, –0.27 to -–0.01 mm), while those who reported more than 50 mg per day had newborns with lower birth weight (beta = –66 g; 95% CI, –121 to –10 g), and smaller circumferences of mid-upper thigh (beta = –0.32 cm; 95% CI, –0.55 to –0.09 cm), anterior thigh skin fold (beta = –0.24 mm; 95% CI, –0.47 to -.01 mm), and mid-upper arm (beta = –0.17 cm; 95% CI, –0.31 to –0.02 cm).
Caffeine plasma concentrations supported these findings.
Compared with women who had caffeine plasma concentrations in the lowest quartile, those in the highest quartile gave birth to neonates with shorter length (beta = –0.44 cm; P = .04 for trend) and lower body weight (beta = –84.3 g; P = .04 for trend), as well as smaller mid-upper arm circumference (beta = -0.25 cm; P = .02 for trend), mid-upper thigh circumference (beta = –0.29 cm; P = .07 for trend), and head circumference (beta = –0.28 cm; P < .001 for trend). A comparison of lower and upper paraxanthine quartiles revealed the similar trends, as did analyses of continuous measures.
“Our results suggest that caffeine consumption during pregnancy, even at levels much lower than the recommended 200 mg per day of caffeine may be associated with decreased fetal growth,” the investigators concluded.
Sarah W. Prager, MD, of the University of Washington, Seattle, suggested that the findings “do not demonstrate that caffeine has a clinically meaningful negative clinical impact on newborn size and weight.”
She noted that there was no difference in the rate of SGA between plasma caffeine quartiles, and that most patients were thin, which may not accurately represent the U.S. population.
“Based on these new data, my take home message to patients would be that increasing amounts of caffeine can have a small but real impact on the size of their baby at birth, though it is unlikely to result in a diagnosis of SGA,” she said. “Pregnant patients may want to limit caffeine intake even more than the ACOG recommendation of 200 mg per day.”
According to Robert M. Silver, MD, of the University of Utah Health Sciences Center, Salt Lake City, “data from this study are of high quality, owing to the prospective cohort design, large numbers, assessment of biomarkers, and sophisticated analyses.”
Still, he urged a cautious interpretation from a clinical perspective.
“It is important to not overreact to these data,” he said. “The decrease in fetal growth associated with caffeine is small and may prove to be clinically meaningless. Accordingly, clinical recommendations regarding caffeine intake during pregnancy should not be modified solely based on this study.”
Dr. Silver suggested that the findings deserve additional investigation.
“These observations warrant further research about the effects of caffeine exposure during pregnancy,” he said. “Ideally, studies should assess the effect of caffeine exposure on fetal growth in various pregnancy epochs as well as on neonatal and childhood growth.”
The study was funded by the Intramural Research Program of the NICHD. Dr. Gerlanc is an employee of The Prospective Group, which was contracted to provide statistical support.
For pregnant women, just half a cup of coffee a day may reduce neonatal birth size and body weight, according to a prospective study involving more than 2,500 women.
That’s only 50 mg of a caffeine day, which falls below the upper threshold of 200 mg set by the American College of Obstetricians and Gynecologists, lead author Jessica Gleason, PhD, MPH, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md, and colleagues reported.
“Systematic reviews and meta-analyses have reported that maternal caffeine consumption, even in doses lower than 200 mg, is associated with a higher risk for low birth weight, small for gestational age (SGA), and fetal growth restriction, suggesting there may be no safe amount of caffeine during pregnancy,” the investigators wrote in JAMA Network Open.
Findings to date have been inconsistent, with a 2014 meta-analysis reporting contrary or null results in four out of nine studies.
Dr. Gleason and colleagues suggested that such discrepancies may be caused by uncontrolled confounding factors in some of the studies, such as smoking, as well as the inadequacy of self-reporting, which fails to incorporate variations in caffeine content between beverages, or differences in rates of metabolism between individuals.
“To our knowledge, no studies have examined the association between caffeine intake and neonatal anthropometric measures beyond weight, length, and head circumference, and few have analyzed plasma concentrations of caffeine and its metabolites or genetic variations in the rate of metabolism associated with neonatal size,” the investigators wrote.
Dr. Gleason and colleagues set out to address this knowledge gap with a prospective cohort study, including 2,055 nonsmoking women with low risk of birth defects who presented at 12 centers between 2009 and 2013. Mean participant age was 28.3 years and mean body mass index was 23.6. Races and ethnicities were represented almost evenly even across four groups: Hispanic (28.2%), White (27.4%), Black (25.2%), and Asian/Pacific Islander (19.2%). Rate of caffeine metabolism was defined by the single-nucleotide variant rs762551 (CYP1A2*1F), according to which, slightly more women had slow metabolism (52.7%) than fast metabolism (47.3%).
Women were enrolled at 8-13 weeks’ gestational age, at which time they underwent interviews and blood draws, allowing for measurement of caffeine and paraxanthine plasma levels, as well as self-reported caffeine consumption during the preceding week.
Over the course of six visits, fetal growth was observed via ultrasound. Medical records were used to determine birth weights and neonatal anthropometric measures, including fat and skin fold mass, body length, and circumferences of the thigh, arm, abdomen, and head.
Neonatal measurements were compared with plasma levels of caffeine and paraxanthine, both continuously and as quartiles (Q1, ≤ 28.3 ng/mL; Q2, 28.4-157.1 ng/mL; Q3, 157.2-658.8 ng/mL; Q4, > 658.8 ng/mL). Comparisons were also made with self-reported caffeine intake.
Women who reported drinking 1-50 mg of caffeine per day had neonates with smaller subscapular skin folds (beta = –0.14 mm; 95% confidence interval, –0.27 to -–0.01 mm), while those who reported more than 50 mg per day had newborns with lower birth weight (beta = –66 g; 95% CI, –121 to –10 g), and smaller circumferences of mid-upper thigh (beta = –0.32 cm; 95% CI, –0.55 to –0.09 cm), anterior thigh skin fold (beta = –0.24 mm; 95% CI, –0.47 to -.01 mm), and mid-upper arm (beta = –0.17 cm; 95% CI, –0.31 to –0.02 cm).
Caffeine plasma concentrations supported these findings.
Compared with women who had caffeine plasma concentrations in the lowest quartile, those in the highest quartile gave birth to neonates with shorter length (beta = –0.44 cm; P = .04 for trend) and lower body weight (beta = –84.3 g; P = .04 for trend), as well as smaller mid-upper arm circumference (beta = -0.25 cm; P = .02 for trend), mid-upper thigh circumference (beta = –0.29 cm; P = .07 for trend), and head circumference (beta = –0.28 cm; P < .001 for trend). A comparison of lower and upper paraxanthine quartiles revealed the similar trends, as did analyses of continuous measures.
“Our results suggest that caffeine consumption during pregnancy, even at levels much lower than the recommended 200 mg per day of caffeine may be associated with decreased fetal growth,” the investigators concluded.
Sarah W. Prager, MD, of the University of Washington, Seattle, suggested that the findings “do not demonstrate that caffeine has a clinically meaningful negative clinical impact on newborn size and weight.”
She noted that there was no difference in the rate of SGA between plasma caffeine quartiles, and that most patients were thin, which may not accurately represent the U.S. population.
“Based on these new data, my take home message to patients would be that increasing amounts of caffeine can have a small but real impact on the size of their baby at birth, though it is unlikely to result in a diagnosis of SGA,” she said. “Pregnant patients may want to limit caffeine intake even more than the ACOG recommendation of 200 mg per day.”
According to Robert M. Silver, MD, of the University of Utah Health Sciences Center, Salt Lake City, “data from this study are of high quality, owing to the prospective cohort design, large numbers, assessment of biomarkers, and sophisticated analyses.”
Still, he urged a cautious interpretation from a clinical perspective.
“It is important to not overreact to these data,” he said. “The decrease in fetal growth associated with caffeine is small and may prove to be clinically meaningless. Accordingly, clinical recommendations regarding caffeine intake during pregnancy should not be modified solely based on this study.”
Dr. Silver suggested that the findings deserve additional investigation.
“These observations warrant further research about the effects of caffeine exposure during pregnancy,” he said. “Ideally, studies should assess the effect of caffeine exposure on fetal growth in various pregnancy epochs as well as on neonatal and childhood growth.”
The study was funded by the Intramural Research Program of the NICHD. Dr. Gerlanc is an employee of The Prospective Group, which was contracted to provide statistical support.
For pregnant women, just half a cup of coffee a day may reduce neonatal birth size and body weight, according to a prospective study involving more than 2,500 women.
That’s only 50 mg of a caffeine day, which falls below the upper threshold of 200 mg set by the American College of Obstetricians and Gynecologists, lead author Jessica Gleason, PhD, MPH, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md, and colleagues reported.
“Systematic reviews and meta-analyses have reported that maternal caffeine consumption, even in doses lower than 200 mg, is associated with a higher risk for low birth weight, small for gestational age (SGA), and fetal growth restriction, suggesting there may be no safe amount of caffeine during pregnancy,” the investigators wrote in JAMA Network Open.
Findings to date have been inconsistent, with a 2014 meta-analysis reporting contrary or null results in four out of nine studies.
Dr. Gleason and colleagues suggested that such discrepancies may be caused by uncontrolled confounding factors in some of the studies, such as smoking, as well as the inadequacy of self-reporting, which fails to incorporate variations in caffeine content between beverages, or differences in rates of metabolism between individuals.
“To our knowledge, no studies have examined the association between caffeine intake and neonatal anthropometric measures beyond weight, length, and head circumference, and few have analyzed plasma concentrations of caffeine and its metabolites or genetic variations in the rate of metabolism associated with neonatal size,” the investigators wrote.
Dr. Gleason and colleagues set out to address this knowledge gap with a prospective cohort study, including 2,055 nonsmoking women with low risk of birth defects who presented at 12 centers between 2009 and 2013. Mean participant age was 28.3 years and mean body mass index was 23.6. Races and ethnicities were represented almost evenly even across four groups: Hispanic (28.2%), White (27.4%), Black (25.2%), and Asian/Pacific Islander (19.2%). Rate of caffeine metabolism was defined by the single-nucleotide variant rs762551 (CYP1A2*1F), according to which, slightly more women had slow metabolism (52.7%) than fast metabolism (47.3%).
Women were enrolled at 8-13 weeks’ gestational age, at which time they underwent interviews and blood draws, allowing for measurement of caffeine and paraxanthine plasma levels, as well as self-reported caffeine consumption during the preceding week.
Over the course of six visits, fetal growth was observed via ultrasound. Medical records were used to determine birth weights and neonatal anthropometric measures, including fat and skin fold mass, body length, and circumferences of the thigh, arm, abdomen, and head.
Neonatal measurements were compared with plasma levels of caffeine and paraxanthine, both continuously and as quartiles (Q1, ≤ 28.3 ng/mL; Q2, 28.4-157.1 ng/mL; Q3, 157.2-658.8 ng/mL; Q4, > 658.8 ng/mL). Comparisons were also made with self-reported caffeine intake.
Women who reported drinking 1-50 mg of caffeine per day had neonates with smaller subscapular skin folds (beta = –0.14 mm; 95% confidence interval, –0.27 to -–0.01 mm), while those who reported more than 50 mg per day had newborns with lower birth weight (beta = –66 g; 95% CI, –121 to –10 g), and smaller circumferences of mid-upper thigh (beta = –0.32 cm; 95% CI, –0.55 to –0.09 cm), anterior thigh skin fold (beta = –0.24 mm; 95% CI, –0.47 to -.01 mm), and mid-upper arm (beta = –0.17 cm; 95% CI, –0.31 to –0.02 cm).
Caffeine plasma concentrations supported these findings.
Compared with women who had caffeine plasma concentrations in the lowest quartile, those in the highest quartile gave birth to neonates with shorter length (beta = –0.44 cm; P = .04 for trend) and lower body weight (beta = –84.3 g; P = .04 for trend), as well as smaller mid-upper arm circumference (beta = -0.25 cm; P = .02 for trend), mid-upper thigh circumference (beta = –0.29 cm; P = .07 for trend), and head circumference (beta = –0.28 cm; P < .001 for trend). A comparison of lower and upper paraxanthine quartiles revealed the similar trends, as did analyses of continuous measures.
“Our results suggest that caffeine consumption during pregnancy, even at levels much lower than the recommended 200 mg per day of caffeine may be associated with decreased fetal growth,” the investigators concluded.
Sarah W. Prager, MD, of the University of Washington, Seattle, suggested that the findings “do not demonstrate that caffeine has a clinically meaningful negative clinical impact on newborn size and weight.”
She noted that there was no difference in the rate of SGA between plasma caffeine quartiles, and that most patients were thin, which may not accurately represent the U.S. population.
“Based on these new data, my take home message to patients would be that increasing amounts of caffeine can have a small but real impact on the size of their baby at birth, though it is unlikely to result in a diagnosis of SGA,” she said. “Pregnant patients may want to limit caffeine intake even more than the ACOG recommendation of 200 mg per day.”
According to Robert M. Silver, MD, of the University of Utah Health Sciences Center, Salt Lake City, “data from this study are of high quality, owing to the prospective cohort design, large numbers, assessment of biomarkers, and sophisticated analyses.”
Still, he urged a cautious interpretation from a clinical perspective.
“It is important to not overreact to these data,” he said. “The decrease in fetal growth associated with caffeine is small and may prove to be clinically meaningless. Accordingly, clinical recommendations regarding caffeine intake during pregnancy should not be modified solely based on this study.”
Dr. Silver suggested that the findings deserve additional investigation.
“These observations warrant further research about the effects of caffeine exposure during pregnancy,” he said. “Ideally, studies should assess the effect of caffeine exposure on fetal growth in various pregnancy epochs as well as on neonatal and childhood growth.”
The study was funded by the Intramural Research Program of the NICHD. Dr. Gerlanc is an employee of The Prospective Group, which was contracted to provide statistical support.
FROM JAMA NETWORK OPEN
COVID-19 maternal antibodies transferred to fetus, newborn from pregnant and lactating vaccine recipients
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Reproductive safety of treatments for women with bipolar disorder
Since March 2020, my colleagues and I have conducted Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital. It has been an opportunity to review the basic tenets of care for reproductive age women before, during, and after pregnancy, and also to learn of extraordinary cases being managed both in the outpatient setting and in the context of the COVID-19 pandemic.
As I’ve noted in previous columns, we have seen a heightening of symptoms of anxiety and insomnia during the pandemic in women who visit our center, and at the centers of the more than 100 clinicians who join Virtual Rounds each week. These colleagues represent people in rural areas, urban environments, and underserved communities across America that have been severely affected by the pandemic. It is clear that the stress of the pandemic is undeniable for patients both with and without psychiatric or mental health issues. We have also seen clinical roughening in women who have been well for a long period of time. In particular, we have noticed that postpartum women are struggling with the stressors of the postpartum period, such as figuring out the logistics of support with respect to childcare, managing maternity leave, and adapting to shifting of anticipated support systems.
Hundreds of women with bipolar disorder come to see us each year about the reproductive safety of the medicines on which they are maintained. Those patients are typically well, and we collaborate with them and their doctors about the safest treatment recommendations. With that said, women with bipolar disorder are at particular risk for postpartum worsening of their mood. The management of their medications during pregnancy requires extremely careful attention because relapse of psychiatric disorder during pregnancy is the strongest predictor of postpartum worsening of underlying psychiatric illness.
This is an opportunity to briefly review the reproductive safety of treatments for these women. We know through initiatives such as the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications that the most widely used medicines for bipolar women during pregnancy include lamotrigine, atypical antipsychotics, and lithium carbonate.
Lamotrigine
The last 15 years have generated the most consistent data on the reproductive safety of lamotrigine. One of the issues, however, with respect to lamotrigine is that its use requires very careful and slow titration and it is also more effective in patients who are well and in the maintenance phase of the illness versus those who are more acutely manic or who are suffering from frank bipolar depression.
Critically, the literature does not support the use of lamotrigine for patients with bipolar I or with more manic symptoms. That being said, it remains a mainstay of treatment for many patients with bipolar disorder, is easy to use across pregnancy, and has an attractive side-effect profile and a very strong reproductive safety profile, suggesting the absence of an increased risk for major malformations.
Atypical antipsychotics
We have less information but have a growing body of evidence about atypical antipsychotics. Both data from administrative databases as well a growing literature from pregnancy registries, such as the National Pregnancy Registry for Atypical Antipsychotics, fail to show a signal for teratogenicity with respect to use of the medicines as a class, and also with specific reference to some of the most widely used atypical antipsychotics, particularly quetiapine and aripiprazole. Our comfort level, compared with a decade ago, with using the second-generation antipsychotics is much greater. That’s a good thing considering the extent to which patients presenting on a combination of, for example, lamotrigine and atypical antipsychotics.
Lithium carbonate
Another mainstay of treatment for women with bipolar I disorder and prominent symptoms of mania is lithium carbonate. The data for efficacy of lithium carbonate used both acutely and for maintenance treatment of bipolar disorder has been unequivocal. Concerns about the teratogenicity of lithium go back to the 1970s and indicate a small increased absolute and relative risk for cardiovascular malformations. More recently, a meta-analysis of lithium exposure during pregnancy and the postpartum period supports this older data, which suggests this increased risk, and examines other outcomes concerning to women with bipolar disorder who use lithium, such as preterm labor, low birth weight, miscarriage, and other adverse neonatal outcomes.
In 2021, with the backdrop of the pandemic, what we actually see is that, for our pregnant and postpartum patients with bipolar disorder, the imperative to keep them well, keep them out of the hospital, and keep them safe has often required careful coadministration of drugs like lamotrigine, lithium, and atypical antipsychotics (and even benzodiazepines). Keeping this population well during the perinatal period is so critical. We were all trained to use the least number of medications when possible across psychiatric illnesses. But the years, data, and clinical experience have shown that polypharmacy may be required to sustain euthymia in many patients with bipolar disorder. The reflex historically has been to stop medications during pregnancy. We take pause, particularly during the pandemic, before reverting back to the practice of 25 years ago of abruptly stopping medicines such as lithium or atypical antipsychotics in patients with bipolar disorder because we know that the risk for relapse is very high following a shift from the regimen that got the patient well.
The COVID-19 pandemic in many respects has highlighted a need to clinically thread the needle with respect to developing a regimen that minimizes risk of reproductive safety concerns but maximizes the likelihood that we can sustain the emotional well-being of these women across pregnancy and into the postpartum period.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since March 2020, my colleagues and I have conducted Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital. It has been an opportunity to review the basic tenets of care for reproductive age women before, during, and after pregnancy, and also to learn of extraordinary cases being managed both in the outpatient setting and in the context of the COVID-19 pandemic.
As I’ve noted in previous columns, we have seen a heightening of symptoms of anxiety and insomnia during the pandemic in women who visit our center, and at the centers of the more than 100 clinicians who join Virtual Rounds each week. These colleagues represent people in rural areas, urban environments, and underserved communities across America that have been severely affected by the pandemic. It is clear that the stress of the pandemic is undeniable for patients both with and without psychiatric or mental health issues. We have also seen clinical roughening in women who have been well for a long period of time. In particular, we have noticed that postpartum women are struggling with the stressors of the postpartum period, such as figuring out the logistics of support with respect to childcare, managing maternity leave, and adapting to shifting of anticipated support systems.
Hundreds of women with bipolar disorder come to see us each year about the reproductive safety of the medicines on which they are maintained. Those patients are typically well, and we collaborate with them and their doctors about the safest treatment recommendations. With that said, women with bipolar disorder are at particular risk for postpartum worsening of their mood. The management of their medications during pregnancy requires extremely careful attention because relapse of psychiatric disorder during pregnancy is the strongest predictor of postpartum worsening of underlying psychiatric illness.
This is an opportunity to briefly review the reproductive safety of treatments for these women. We know through initiatives such as the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications that the most widely used medicines for bipolar women during pregnancy include lamotrigine, atypical antipsychotics, and lithium carbonate.
Lamotrigine
The last 15 years have generated the most consistent data on the reproductive safety of lamotrigine. One of the issues, however, with respect to lamotrigine is that its use requires very careful and slow titration and it is also more effective in patients who are well and in the maintenance phase of the illness versus those who are more acutely manic or who are suffering from frank bipolar depression.
Critically, the literature does not support the use of lamotrigine for patients with bipolar I or with more manic symptoms. That being said, it remains a mainstay of treatment for many patients with bipolar disorder, is easy to use across pregnancy, and has an attractive side-effect profile and a very strong reproductive safety profile, suggesting the absence of an increased risk for major malformations.
Atypical antipsychotics
We have less information but have a growing body of evidence about atypical antipsychotics. Both data from administrative databases as well a growing literature from pregnancy registries, such as the National Pregnancy Registry for Atypical Antipsychotics, fail to show a signal for teratogenicity with respect to use of the medicines as a class, and also with specific reference to some of the most widely used atypical antipsychotics, particularly quetiapine and aripiprazole. Our comfort level, compared with a decade ago, with using the second-generation antipsychotics is much greater. That’s a good thing considering the extent to which patients presenting on a combination of, for example, lamotrigine and atypical antipsychotics.
Lithium carbonate
Another mainstay of treatment for women with bipolar I disorder and prominent symptoms of mania is lithium carbonate. The data for efficacy of lithium carbonate used both acutely and for maintenance treatment of bipolar disorder has been unequivocal. Concerns about the teratogenicity of lithium go back to the 1970s and indicate a small increased absolute and relative risk for cardiovascular malformations. More recently, a meta-analysis of lithium exposure during pregnancy and the postpartum period supports this older data, which suggests this increased risk, and examines other outcomes concerning to women with bipolar disorder who use lithium, such as preterm labor, low birth weight, miscarriage, and other adverse neonatal outcomes.
In 2021, with the backdrop of the pandemic, what we actually see is that, for our pregnant and postpartum patients with bipolar disorder, the imperative to keep them well, keep them out of the hospital, and keep them safe has often required careful coadministration of drugs like lamotrigine, lithium, and atypical antipsychotics (and even benzodiazepines). Keeping this population well during the perinatal period is so critical. We were all trained to use the least number of medications when possible across psychiatric illnesses. But the years, data, and clinical experience have shown that polypharmacy may be required to sustain euthymia in many patients with bipolar disorder. The reflex historically has been to stop medications during pregnancy. We take pause, particularly during the pandemic, before reverting back to the practice of 25 years ago of abruptly stopping medicines such as lithium or atypical antipsychotics in patients with bipolar disorder because we know that the risk for relapse is very high following a shift from the regimen that got the patient well.
The COVID-19 pandemic in many respects has highlighted a need to clinically thread the needle with respect to developing a regimen that minimizes risk of reproductive safety concerns but maximizes the likelihood that we can sustain the emotional well-being of these women across pregnancy and into the postpartum period.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since March 2020, my colleagues and I have conducted Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital. It has been an opportunity to review the basic tenets of care for reproductive age women before, during, and after pregnancy, and also to learn of extraordinary cases being managed both in the outpatient setting and in the context of the COVID-19 pandemic.
As I’ve noted in previous columns, we have seen a heightening of symptoms of anxiety and insomnia during the pandemic in women who visit our center, and at the centers of the more than 100 clinicians who join Virtual Rounds each week. These colleagues represent people in rural areas, urban environments, and underserved communities across America that have been severely affected by the pandemic. It is clear that the stress of the pandemic is undeniable for patients both with and without psychiatric or mental health issues. We have also seen clinical roughening in women who have been well for a long period of time. In particular, we have noticed that postpartum women are struggling with the stressors of the postpartum period, such as figuring out the logistics of support with respect to childcare, managing maternity leave, and adapting to shifting of anticipated support systems.
Hundreds of women with bipolar disorder come to see us each year about the reproductive safety of the medicines on which they are maintained. Those patients are typically well, and we collaborate with them and their doctors about the safest treatment recommendations. With that said, women with bipolar disorder are at particular risk for postpartum worsening of their mood. The management of their medications during pregnancy requires extremely careful attention because relapse of psychiatric disorder during pregnancy is the strongest predictor of postpartum worsening of underlying psychiatric illness.
This is an opportunity to briefly review the reproductive safety of treatments for these women. We know through initiatives such as the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications that the most widely used medicines for bipolar women during pregnancy include lamotrigine, atypical antipsychotics, and lithium carbonate.
Lamotrigine
The last 15 years have generated the most consistent data on the reproductive safety of lamotrigine. One of the issues, however, with respect to lamotrigine is that its use requires very careful and slow titration and it is also more effective in patients who are well and in the maintenance phase of the illness versus those who are more acutely manic or who are suffering from frank bipolar depression.
Critically, the literature does not support the use of lamotrigine for patients with bipolar I or with more manic symptoms. That being said, it remains a mainstay of treatment for many patients with bipolar disorder, is easy to use across pregnancy, and has an attractive side-effect profile and a very strong reproductive safety profile, suggesting the absence of an increased risk for major malformations.
Atypical antipsychotics
We have less information but have a growing body of evidence about atypical antipsychotics. Both data from administrative databases as well a growing literature from pregnancy registries, such as the National Pregnancy Registry for Atypical Antipsychotics, fail to show a signal for teratogenicity with respect to use of the medicines as a class, and also with specific reference to some of the most widely used atypical antipsychotics, particularly quetiapine and aripiprazole. Our comfort level, compared with a decade ago, with using the second-generation antipsychotics is much greater. That’s a good thing considering the extent to which patients presenting on a combination of, for example, lamotrigine and atypical antipsychotics.
Lithium carbonate
Another mainstay of treatment for women with bipolar I disorder and prominent symptoms of mania is lithium carbonate. The data for efficacy of lithium carbonate used both acutely and for maintenance treatment of bipolar disorder has been unequivocal. Concerns about the teratogenicity of lithium go back to the 1970s and indicate a small increased absolute and relative risk for cardiovascular malformations. More recently, a meta-analysis of lithium exposure during pregnancy and the postpartum period supports this older data, which suggests this increased risk, and examines other outcomes concerning to women with bipolar disorder who use lithium, such as preterm labor, low birth weight, miscarriage, and other adverse neonatal outcomes.
In 2021, with the backdrop of the pandemic, what we actually see is that, for our pregnant and postpartum patients with bipolar disorder, the imperative to keep them well, keep them out of the hospital, and keep them safe has often required careful coadministration of drugs like lamotrigine, lithium, and atypical antipsychotics (and even benzodiazepines). Keeping this population well during the perinatal period is so critical. We were all trained to use the least number of medications when possible across psychiatric illnesses. But the years, data, and clinical experience have shown that polypharmacy may be required to sustain euthymia in many patients with bipolar disorder. The reflex historically has been to stop medications during pregnancy. We take pause, particularly during the pandemic, before reverting back to the practice of 25 years ago of abruptly stopping medicines such as lithium or atypical antipsychotics in patients with bipolar disorder because we know that the risk for relapse is very high following a shift from the regimen that got the patient well.
The COVID-19 pandemic in many respects has highlighted a need to clinically thread the needle with respect to developing a regimen that minimizes risk of reproductive safety concerns but maximizes the likelihood that we can sustain the emotional well-being of these women across pregnancy and into the postpartum period.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
How long is the second stage of labor in women delivering twins?
, researchers say.
Although the analysis found statistically significant differences in second-stage labor lengths for twin and singleton deliveries, “ultimately I think the value in this is seeing that it is not much different,” said Nathan Fox, MD, a maternal-fetal medicine specialist who has studied twin pregnancies and delivery of twins.
Knowledge gap
While most twin births occur by cesarean delivery, vaginal delivery is a preferred method for diamniotic twins with the first twin in vertex presentation, wrote study author Gabriel Levin, MD, and colleagues. Prior studies, however, have not clearly established the duration of the second stage of labor in twin deliveries – that is, the time from 10-cm dilation until delivery of the first twin, they said.
Knowing “the parameters of the normal second stage of labor” for twin deliveries may help guide clinical practice and possibly avoid unnecessary operative deliveries, the researchers wrote.
To establish normal ranges for the second stage of labor in twin deliveries, Dr. Levin, of the department of obstetrics and gynecology at Hadassah-Hebrew University Medical Center, Jerusalem, and coauthors conducted a retrospective cohort study. They analyzed data from three large academic hospitals in Israel between 2011 and June 2020 and assessed the length of the second stage of labor by obstetric history and clinical characteristics.
The researchers included data from women who delivered the first of diamniotic twins spontaneously or delivered a singleton spontaneously. The researchers excluded twin pregnancies with fetal demise of one or both twins, structural anomaly or chromosomal abnormality, monochorionic complications, and first twin in a nonvertex presentation. They did not consider the delivery mode of the second twin.
The study included 2,009 twin deliveries and 135,217 singleton deliveries. Of the women with twin deliveries, 32.6% were nulliparous (that is, no previous vaginal deliveries), 61.5% were parous (one to four previous vaginal deliveries, and no cesarean deliveries), and 5.9% were grand multiparous (at least five previous deliveries).
Of the women with singleton deliveries, 29% were nulliparous.
For nulliparous women delivering twins, the median length of the second stage was 1 hour 27 minutes (interquartile range, 40-147 minutes), and the 95th percentile was 3 hours 51 minutes.
For parous women delivering twins, the median length of the second stage was 18 minutes (interquartile range, 8-36 minutes), and the 95th percentile was 1 hour 56 minutes.
For grand multiparous women, the median length of the second stage was 10 minutes.
In a multivariable analysis, epidural anesthesia and induction of labor were independently associated with increased length of the second stage of labor.
Second-stage labor longer than the 95th percentile based on parity and epidural status was associated with approximately twice the risk of admission to the neonatal intensive care unit (35.4% vs. 16.4%) and need for phototherapy, the researchers reported.
Compared with singleton deliveries, the second stage was longer in twin deliveries. Among nulliparous patients, the median length of the second stage of labor was 1 hour 18 minutes for singleton deliveries, versus 1 hour 30 minutes for twin deliveries. Among parous patients, the median length of the second stage was 19 minutes for twin deliveries, compared with 10 minutes for singleton deliveries.
The study was conducted in Israel, which may limit its generalizability, the authors noted. In addition, the researchers lacked data about maternal morbidity and had limited data about neonatal morbidity. “The exact time that the woman became 10-cm dilated cannot be known, a problem inherent to all such studies,” and cases where doctors artificially ended labor with operative delivery were not included, the researchers added. “More research is needed to determine at what point, if any, intervention is warranted to shorten the second stage in patients delivering twins,” Dr. Levin and colleagues wrote.
Providing a framework
“We always get more concerned if the labor process is happening in a way that is unusual,” and this study provides data that can provide a framework for that thought process, said Dr. Fox, who was not involved in the study.
The results demonstrate that the second stage of labor for twin deliveries may take a long time and “that is not necessarily a bad thing,” said Dr. Fox, clinical professor of obstetrics and gynecology and maternal and fetal medicine at the Icahn School of Medicine at Mount Sinai in New York.
For women having their first child, the second stage of labor tends to take much longer than it does for women who have had children. “That is well known for singletons, and everyone assumes it is the same for twins,” but this study quantifies the durations for twins, he said. “That is valuable, and it is also helpful for women to know what to expect.”
A study coauthor disclosed financial ties to PregnanTech and Anthem AI, and money paid to their institution from New Sight. Dr. Fox works at Maternal Fetal Medicine Associates and Carnegie Imaging for Women in New York and is the creator and host of the Healthful Woman Podcast. He had no relevant financial disclosures.
, researchers say.
Although the analysis found statistically significant differences in second-stage labor lengths for twin and singleton deliveries, “ultimately I think the value in this is seeing that it is not much different,” said Nathan Fox, MD, a maternal-fetal medicine specialist who has studied twin pregnancies and delivery of twins.
Knowledge gap
While most twin births occur by cesarean delivery, vaginal delivery is a preferred method for diamniotic twins with the first twin in vertex presentation, wrote study author Gabriel Levin, MD, and colleagues. Prior studies, however, have not clearly established the duration of the second stage of labor in twin deliveries – that is, the time from 10-cm dilation until delivery of the first twin, they said.
Knowing “the parameters of the normal second stage of labor” for twin deliveries may help guide clinical practice and possibly avoid unnecessary operative deliveries, the researchers wrote.
To establish normal ranges for the second stage of labor in twin deliveries, Dr. Levin, of the department of obstetrics and gynecology at Hadassah-Hebrew University Medical Center, Jerusalem, and coauthors conducted a retrospective cohort study. They analyzed data from three large academic hospitals in Israel between 2011 and June 2020 and assessed the length of the second stage of labor by obstetric history and clinical characteristics.
The researchers included data from women who delivered the first of diamniotic twins spontaneously or delivered a singleton spontaneously. The researchers excluded twin pregnancies with fetal demise of one or both twins, structural anomaly or chromosomal abnormality, monochorionic complications, and first twin in a nonvertex presentation. They did not consider the delivery mode of the second twin.
The study included 2,009 twin deliveries and 135,217 singleton deliveries. Of the women with twin deliveries, 32.6% were nulliparous (that is, no previous vaginal deliveries), 61.5% were parous (one to four previous vaginal deliveries, and no cesarean deliveries), and 5.9% were grand multiparous (at least five previous deliveries).
Of the women with singleton deliveries, 29% were nulliparous.
For nulliparous women delivering twins, the median length of the second stage was 1 hour 27 minutes (interquartile range, 40-147 minutes), and the 95th percentile was 3 hours 51 minutes.
For parous women delivering twins, the median length of the second stage was 18 minutes (interquartile range, 8-36 minutes), and the 95th percentile was 1 hour 56 minutes.
For grand multiparous women, the median length of the second stage was 10 minutes.
In a multivariable analysis, epidural anesthesia and induction of labor were independently associated with increased length of the second stage of labor.
Second-stage labor longer than the 95th percentile based on parity and epidural status was associated with approximately twice the risk of admission to the neonatal intensive care unit (35.4% vs. 16.4%) and need for phototherapy, the researchers reported.
Compared with singleton deliveries, the second stage was longer in twin deliveries. Among nulliparous patients, the median length of the second stage of labor was 1 hour 18 minutes for singleton deliveries, versus 1 hour 30 minutes for twin deliveries. Among parous patients, the median length of the second stage was 19 minutes for twin deliveries, compared with 10 minutes for singleton deliveries.
The study was conducted in Israel, which may limit its generalizability, the authors noted. In addition, the researchers lacked data about maternal morbidity and had limited data about neonatal morbidity. “The exact time that the woman became 10-cm dilated cannot be known, a problem inherent to all such studies,” and cases where doctors artificially ended labor with operative delivery were not included, the researchers added. “More research is needed to determine at what point, if any, intervention is warranted to shorten the second stage in patients delivering twins,” Dr. Levin and colleagues wrote.
Providing a framework
“We always get more concerned if the labor process is happening in a way that is unusual,” and this study provides data that can provide a framework for that thought process, said Dr. Fox, who was not involved in the study.
The results demonstrate that the second stage of labor for twin deliveries may take a long time and “that is not necessarily a bad thing,” said Dr. Fox, clinical professor of obstetrics and gynecology and maternal and fetal medicine at the Icahn School of Medicine at Mount Sinai in New York.
For women having their first child, the second stage of labor tends to take much longer than it does for women who have had children. “That is well known for singletons, and everyone assumes it is the same for twins,” but this study quantifies the durations for twins, he said. “That is valuable, and it is also helpful for women to know what to expect.”
A study coauthor disclosed financial ties to PregnanTech and Anthem AI, and money paid to their institution from New Sight. Dr. Fox works at Maternal Fetal Medicine Associates and Carnegie Imaging for Women in New York and is the creator and host of the Healthful Woman Podcast. He had no relevant financial disclosures.
, researchers say.
Although the analysis found statistically significant differences in second-stage labor lengths for twin and singleton deliveries, “ultimately I think the value in this is seeing that it is not much different,” said Nathan Fox, MD, a maternal-fetal medicine specialist who has studied twin pregnancies and delivery of twins.
Knowledge gap
While most twin births occur by cesarean delivery, vaginal delivery is a preferred method for diamniotic twins with the first twin in vertex presentation, wrote study author Gabriel Levin, MD, and colleagues. Prior studies, however, have not clearly established the duration of the second stage of labor in twin deliveries – that is, the time from 10-cm dilation until delivery of the first twin, they said.
Knowing “the parameters of the normal second stage of labor” for twin deliveries may help guide clinical practice and possibly avoid unnecessary operative deliveries, the researchers wrote.
To establish normal ranges for the second stage of labor in twin deliveries, Dr. Levin, of the department of obstetrics and gynecology at Hadassah-Hebrew University Medical Center, Jerusalem, and coauthors conducted a retrospective cohort study. They analyzed data from three large academic hospitals in Israel between 2011 and June 2020 and assessed the length of the second stage of labor by obstetric history and clinical characteristics.
The researchers included data from women who delivered the first of diamniotic twins spontaneously or delivered a singleton spontaneously. The researchers excluded twin pregnancies with fetal demise of one or both twins, structural anomaly or chromosomal abnormality, monochorionic complications, and first twin in a nonvertex presentation. They did not consider the delivery mode of the second twin.
The study included 2,009 twin deliveries and 135,217 singleton deliveries. Of the women with twin deliveries, 32.6% were nulliparous (that is, no previous vaginal deliveries), 61.5% were parous (one to four previous vaginal deliveries, and no cesarean deliveries), and 5.9% were grand multiparous (at least five previous deliveries).
Of the women with singleton deliveries, 29% were nulliparous.
For nulliparous women delivering twins, the median length of the second stage was 1 hour 27 minutes (interquartile range, 40-147 minutes), and the 95th percentile was 3 hours 51 minutes.
For parous women delivering twins, the median length of the second stage was 18 minutes (interquartile range, 8-36 minutes), and the 95th percentile was 1 hour 56 minutes.
For grand multiparous women, the median length of the second stage was 10 minutes.
In a multivariable analysis, epidural anesthesia and induction of labor were independently associated with increased length of the second stage of labor.
Second-stage labor longer than the 95th percentile based on parity and epidural status was associated with approximately twice the risk of admission to the neonatal intensive care unit (35.4% vs. 16.4%) and need for phototherapy, the researchers reported.
Compared with singleton deliveries, the second stage was longer in twin deliveries. Among nulliparous patients, the median length of the second stage of labor was 1 hour 18 minutes for singleton deliveries, versus 1 hour 30 minutes for twin deliveries. Among parous patients, the median length of the second stage was 19 minutes for twin deliveries, compared with 10 minutes for singleton deliveries.
The study was conducted in Israel, which may limit its generalizability, the authors noted. In addition, the researchers lacked data about maternal morbidity and had limited data about neonatal morbidity. “The exact time that the woman became 10-cm dilated cannot be known, a problem inherent to all such studies,” and cases where doctors artificially ended labor with operative delivery were not included, the researchers added. “More research is needed to determine at what point, if any, intervention is warranted to shorten the second stage in patients delivering twins,” Dr. Levin and colleagues wrote.
Providing a framework
“We always get more concerned if the labor process is happening in a way that is unusual,” and this study provides data that can provide a framework for that thought process, said Dr. Fox, who was not involved in the study.
The results demonstrate that the second stage of labor for twin deliveries may take a long time and “that is not necessarily a bad thing,” said Dr. Fox, clinical professor of obstetrics and gynecology and maternal and fetal medicine at the Icahn School of Medicine at Mount Sinai in New York.
For women having their first child, the second stage of labor tends to take much longer than it does for women who have had children. “That is well known for singletons, and everyone assumes it is the same for twins,” but this study quantifies the durations for twins, he said. “That is valuable, and it is also helpful for women to know what to expect.”
A study coauthor disclosed financial ties to PregnanTech and Anthem AI, and money paid to their institution from New Sight. Dr. Fox works at Maternal Fetal Medicine Associates and Carnegie Imaging for Women in New York and is the creator and host of the Healthful Woman Podcast. He had no relevant financial disclosures.
FROM OBSTETRICS AND GYNECOLOGY
Prenatal dietary folate not enough to offset AEDs’ effect on kids’ cognition
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
THC persists in breast milk 6 weeks after quitting cannabis
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.