User login
Optimal management of postpartum and postoperative pain
LARC prolongs interpregnancy intervals but doesn’t cut preterm birth risk
NASHVILLE, TENN. – when used between a first and second pregnancy, results of a retrospective cohort study suggest.
Of 35,754 women who had a first and second live birth between 2005 and 2015 and who received non-emergent care within 10 years of the first birth, 3,083 (9%) had evidence of interpregnancy LARC exposure and were significantly less likely to have short interpregnancy intervals than were 32,671 with either non-LARC contraceptive use or no record of contraceptive-related care (P less than .0001), Sara E. Simonsen, PhD, reported in a poster at the annual meeting of the American College of Obstetricians and Gynecologists.
Intervals in those with intrapartum LARC use were 12 months or less in 4% of women, 13-18 months in 8%, 19-24 months in 11%, and greater than 24 months in 13%.
However, preterm birth, which occurred in 7% of first births and 6% of second births, was not lower among those with LARC exposure vs. those with no contraceptive encounters after adjustment for interpregnancy interval and a number of demographic factors, including education, presence of father, mother’s age, Hispanic ethnicity, fetal anomalies, and preterm birth history (adjusted odds ratio, 1.13), said Dr. Simonsen, a certified nurse midwife at the University of Utah Hospital, Salt Lake City.
“Preterm birth, a live birth at less than 37 weeks’ gestation, is a major determinant of poor neonatal outcomes,” she and her colleagues wrote. “Short interpregnancy interval, defined as less than 18 months, is an important risk factor for preterm birth.”
Given the increasing number of U.S. women who use highly effective LARCs to space pregnancies, she and her colleagues performed a retrospective cohort study of electronic medical records from two large health systems and linked them with birth and fetal death records to explore the relationship between interpregnancy LARC and both interpregnancy interval and preterm birth in the subsequent pregnancy.
“We did find that women who used LARC between their pregnancies were less likely to have a short interpregnancy interval, but in adjusted models ... we found no association with intrapartum LARC use and preterm birth in the second birth,” Dr. Simonsen said during an e-poster presentation at the meeting.
In fact, preterm birth in the second birth was most strongly associated with a prior preterm birth – a finding consistent with the literature, she and her colleagues noted.
Although the findings are limited by the use of retrospective data not designed for research, the data came from a large population-based sample representing about 85% of Utah births, they said.
The findings suggest that while LARC use may not reduce preterm birth risk, it “may contribute favorably to outcomes to the extent that having optimal interpregnancy interval does,” they wrote.
“‘We feel that these findings support providers counseling women on the full range of contraception options in the postpartum and not pushing [intrauterine devices,]” Dr. Simonsen added.
The related topic of immediate postpartum LARC use was addressed by Eve Espey, MD, in a separate presentation at the meeting.
Dr .Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, reported that immediate postpartum insertion of an intrauterine device (IUD) is highly cost-effective despite an expulsion rate of between 10% and 30%. She also addressed the value of postpartum LARC for reducing rapid-repeat pregnancy rates.
Payment models for immediate postpartum LARC are “very cumbersome,” but at the university, a persistent effort over 4 years has led to success. Immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases, she said, adding that efforts are underway to help other hospitals “troubleshoot the issues.”
The lack of private insurance coverage for immediate postpartum LARC remains a challenge, but Dr. Espey said she remains “super enthusiastic” about its use.
“I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen,” she said, urging colleagues to advocate for that within their American College of Obstetricians and Gynecologists sections when possible.
Dr. Simonsen and Dr. Espey reported having no relevant disclosures.
NASHVILLE, TENN. – when used between a first and second pregnancy, results of a retrospective cohort study suggest.
Of 35,754 women who had a first and second live birth between 2005 and 2015 and who received non-emergent care within 10 years of the first birth, 3,083 (9%) had evidence of interpregnancy LARC exposure and were significantly less likely to have short interpregnancy intervals than were 32,671 with either non-LARC contraceptive use or no record of contraceptive-related care (P less than .0001), Sara E. Simonsen, PhD, reported in a poster at the annual meeting of the American College of Obstetricians and Gynecologists.
Intervals in those with intrapartum LARC use were 12 months or less in 4% of women, 13-18 months in 8%, 19-24 months in 11%, and greater than 24 months in 13%.
However, preterm birth, which occurred in 7% of first births and 6% of second births, was not lower among those with LARC exposure vs. those with no contraceptive encounters after adjustment for interpregnancy interval and a number of demographic factors, including education, presence of father, mother’s age, Hispanic ethnicity, fetal anomalies, and preterm birth history (adjusted odds ratio, 1.13), said Dr. Simonsen, a certified nurse midwife at the University of Utah Hospital, Salt Lake City.
“Preterm birth, a live birth at less than 37 weeks’ gestation, is a major determinant of poor neonatal outcomes,” she and her colleagues wrote. “Short interpregnancy interval, defined as less than 18 months, is an important risk factor for preterm birth.”
Given the increasing number of U.S. women who use highly effective LARCs to space pregnancies, she and her colleagues performed a retrospective cohort study of electronic medical records from two large health systems and linked them with birth and fetal death records to explore the relationship between interpregnancy LARC and both interpregnancy interval and preterm birth in the subsequent pregnancy.
“We did find that women who used LARC between their pregnancies were less likely to have a short interpregnancy interval, but in adjusted models ... we found no association with intrapartum LARC use and preterm birth in the second birth,” Dr. Simonsen said during an e-poster presentation at the meeting.
In fact, preterm birth in the second birth was most strongly associated with a prior preterm birth – a finding consistent with the literature, she and her colleagues noted.
Although the findings are limited by the use of retrospective data not designed for research, the data came from a large population-based sample representing about 85% of Utah births, they said.
The findings suggest that while LARC use may not reduce preterm birth risk, it “may contribute favorably to outcomes to the extent that having optimal interpregnancy interval does,” they wrote.
“‘We feel that these findings support providers counseling women on the full range of contraception options in the postpartum and not pushing [intrauterine devices,]” Dr. Simonsen added.
The related topic of immediate postpartum LARC use was addressed by Eve Espey, MD, in a separate presentation at the meeting.
Dr .Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, reported that immediate postpartum insertion of an intrauterine device (IUD) is highly cost-effective despite an expulsion rate of between 10% and 30%. She also addressed the value of postpartum LARC for reducing rapid-repeat pregnancy rates.
Payment models for immediate postpartum LARC are “very cumbersome,” but at the university, a persistent effort over 4 years has led to success. Immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases, she said, adding that efforts are underway to help other hospitals “troubleshoot the issues.”
The lack of private insurance coverage for immediate postpartum LARC remains a challenge, but Dr. Espey said she remains “super enthusiastic” about its use.
“I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen,” she said, urging colleagues to advocate for that within their American College of Obstetricians and Gynecologists sections when possible.
Dr. Simonsen and Dr. Espey reported having no relevant disclosures.
NASHVILLE, TENN. – when used between a first and second pregnancy, results of a retrospective cohort study suggest.
Of 35,754 women who had a first and second live birth between 2005 and 2015 and who received non-emergent care within 10 years of the first birth, 3,083 (9%) had evidence of interpregnancy LARC exposure and were significantly less likely to have short interpregnancy intervals than were 32,671 with either non-LARC contraceptive use or no record of contraceptive-related care (P less than .0001), Sara E. Simonsen, PhD, reported in a poster at the annual meeting of the American College of Obstetricians and Gynecologists.
Intervals in those with intrapartum LARC use were 12 months or less in 4% of women, 13-18 months in 8%, 19-24 months in 11%, and greater than 24 months in 13%.
However, preterm birth, which occurred in 7% of first births and 6% of second births, was not lower among those with LARC exposure vs. those with no contraceptive encounters after adjustment for interpregnancy interval and a number of demographic factors, including education, presence of father, mother’s age, Hispanic ethnicity, fetal anomalies, and preterm birth history (adjusted odds ratio, 1.13), said Dr. Simonsen, a certified nurse midwife at the University of Utah Hospital, Salt Lake City.
“Preterm birth, a live birth at less than 37 weeks’ gestation, is a major determinant of poor neonatal outcomes,” she and her colleagues wrote. “Short interpregnancy interval, defined as less than 18 months, is an important risk factor for preterm birth.”
Given the increasing number of U.S. women who use highly effective LARCs to space pregnancies, she and her colleagues performed a retrospective cohort study of electronic medical records from two large health systems and linked them with birth and fetal death records to explore the relationship between interpregnancy LARC and both interpregnancy interval and preterm birth in the subsequent pregnancy.
“We did find that women who used LARC between their pregnancies were less likely to have a short interpregnancy interval, but in adjusted models ... we found no association with intrapartum LARC use and preterm birth in the second birth,” Dr. Simonsen said during an e-poster presentation at the meeting.
In fact, preterm birth in the second birth was most strongly associated with a prior preterm birth – a finding consistent with the literature, she and her colleagues noted.
Although the findings are limited by the use of retrospective data not designed for research, the data came from a large population-based sample representing about 85% of Utah births, they said.
The findings suggest that while LARC use may not reduce preterm birth risk, it “may contribute favorably to outcomes to the extent that having optimal interpregnancy interval does,” they wrote.
“‘We feel that these findings support providers counseling women on the full range of contraception options in the postpartum and not pushing [intrauterine devices,]” Dr. Simonsen added.
The related topic of immediate postpartum LARC use was addressed by Eve Espey, MD, in a separate presentation at the meeting.
Dr .Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque, reported that immediate postpartum insertion of an intrauterine device (IUD) is highly cost-effective despite an expulsion rate of between 10% and 30%. She also addressed the value of postpartum LARC for reducing rapid-repeat pregnancy rates.
Payment models for immediate postpartum LARC are “very cumbersome,” but at the university, a persistent effort over 4 years has led to success. Immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases, she said, adding that efforts are underway to help other hospitals “troubleshoot the issues.”
The lack of private insurance coverage for immediate postpartum LARC remains a challenge, but Dr. Espey said she remains “super enthusiastic” about its use.
“I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen,” she said, urging colleagues to advocate for that within their American College of Obstetricians and Gynecologists sections when possible.
Dr. Simonsen and Dr. Espey reported having no relevant disclosures.
REPORTING FROM ACOG 2019
U.S. travelers to Europe need up to date measles immunization
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
FROM PEDIATRICS
In MS, the challenges for women are unique
SEATTLE – Mitzi Joi Williams, MD.
About three in four people with MS are female – about 750,000 in the United States. And the risk and incidence may be highest in African American women.
In a presentation about the unique needs of women with MS, Dr. Williams, an assistant professor of internal medicine at the Morehouse School of Medicine in Atlanta, offered these tips at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pay attention to sexual dysfunction
Patients with MS often are ashamed to talk about sexual dysfunction, Dr. Williams said, but it is on many minds. “If I have a program on intimacy in MS, people are out the door.”
She urged colleagues to understand that MS can affect sexuality through three routes: primary, secondary, and tertiary dysfunction.
In primary sexual dysfunction, brain and spinal lesions directly related to MS can cause problems such as lack of sensation or abnormal sensations, decreased libido, vaginal dryness, and difficult orgasm.
Secondary sexual dysfunction refers to problems caused by symptoms of MS such as fatigue, which can worsen as the day progresses and affect nighttime intimacy, she said. Bladder dysfunction is another sensitive area in sexuality, with patients – especially women – “concerned that they will lose control of their bladder or they have already lost control.”
Cognitive dysfunction also can disrupt sexual function. “It is important to focus, and certain things cannot happen if you do not. If you are not able to focus and concentrate, it can affect interest,” Dr. Williams said.
Additionally, medications can improve some symptoms while making others worse. For example, a drug may relieve spasticity but boost fatigue. “We have to walk this tightrope,” she said. “But if we are not asking our patients, they may not volunteer this information.”
Finally, she said, MS can spark tertiary sexual dysfunction – poor body image, depression, anxiety, and disruptive changes in familial roles. For example, one partner may become a caregiver, and “it is hard to go from caregiving to sexy time.”
“It is something we have to acknowledge and find ways to deal with,” Dr. Williams said.
To address these issues, she pointed to strategies for symptomatic relief and disease-modifying therapy (DMT) and pinpointed several treatment options.
- Fatigue – stimulants, diet, exercise.
- Spasticity – muscle relaxants, exercise.
- Bladder dysfunction – fluid restriction, medication.
- Paresthesia – antidepressants, anticonvulsants.
- Numbness – vibrators, devices to increase stimulation.
Sexual therapy, couples therapy, and pelvic floor physical therapy also can be helpful.
Be aware of special needs during prepregnancy and pregnancy
“MS itself does not have a lot of effects on fertility, pregnancy, or pregnancy outcomes,” Dr. Williams said. However, “medications cause concern about how we manage pregnancy and fertility.”
In vitro fertilization may increase the risk of relapse, she added, and patients on dimethyl fumarate who experience vomiting or diarrhea may not be able to properly absorb oral contraceptives.
Women with MS may not need to go off DMT when they are trying to conceive, she said. “If patients have very aggressive disease, they may need to be on DMT through conception, through the first trimester, and even the entire pregnancy to prevent long-term disability.”
What about pregnancy itself? “An MS diagnosis alone does not mean that a pregnancy is high risk,” she said. “There are not necessarily additional tests and ultrasounds that are recommended for our patients based on MS diagnosis alone.”
Treatment discontinuation may be warranted during pregnancy, when MS generally improves. However, some MS symptoms – fatigue, bladder dysfunction, and balance – may increase. Corticosteroids can be appropriate if relapses occur during pregnancy.
Menopause and MS symptoms may overlap
Symptoms such as hot flashes, mood changes, sleep disturbance, bladder dysfunction, and decreased energy may be signs of MS, or they could indicate menopause, Dr. Williams said. “Sometimes patients come in and they are getting worse, and we look into it and discover they are premenopausal.”
A decline in estrogen during menopause may worsen MS symptoms, she added, and hormone therapy may be appropriate. A phase 2 study found a benefit in menopausal patients with MS for estriol in conjunction with a DMT, but more studies are needed.
Dr. Williams reported no relevant financial disclosures.
SEATTLE – Mitzi Joi Williams, MD.
About three in four people with MS are female – about 750,000 in the United States. And the risk and incidence may be highest in African American women.
In a presentation about the unique needs of women with MS, Dr. Williams, an assistant professor of internal medicine at the Morehouse School of Medicine in Atlanta, offered these tips at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pay attention to sexual dysfunction
Patients with MS often are ashamed to talk about sexual dysfunction, Dr. Williams said, but it is on many minds. “If I have a program on intimacy in MS, people are out the door.”
She urged colleagues to understand that MS can affect sexuality through three routes: primary, secondary, and tertiary dysfunction.
In primary sexual dysfunction, brain and spinal lesions directly related to MS can cause problems such as lack of sensation or abnormal sensations, decreased libido, vaginal dryness, and difficult orgasm.
Secondary sexual dysfunction refers to problems caused by symptoms of MS such as fatigue, which can worsen as the day progresses and affect nighttime intimacy, she said. Bladder dysfunction is another sensitive area in sexuality, with patients – especially women – “concerned that they will lose control of their bladder or they have already lost control.”
Cognitive dysfunction also can disrupt sexual function. “It is important to focus, and certain things cannot happen if you do not. If you are not able to focus and concentrate, it can affect interest,” Dr. Williams said.
Additionally, medications can improve some symptoms while making others worse. For example, a drug may relieve spasticity but boost fatigue. “We have to walk this tightrope,” she said. “But if we are not asking our patients, they may not volunteer this information.”
Finally, she said, MS can spark tertiary sexual dysfunction – poor body image, depression, anxiety, and disruptive changes in familial roles. For example, one partner may become a caregiver, and “it is hard to go from caregiving to sexy time.”
“It is something we have to acknowledge and find ways to deal with,” Dr. Williams said.
To address these issues, she pointed to strategies for symptomatic relief and disease-modifying therapy (DMT) and pinpointed several treatment options.
- Fatigue – stimulants, diet, exercise.
- Spasticity – muscle relaxants, exercise.
- Bladder dysfunction – fluid restriction, medication.
- Paresthesia – antidepressants, anticonvulsants.
- Numbness – vibrators, devices to increase stimulation.
Sexual therapy, couples therapy, and pelvic floor physical therapy also can be helpful.
Be aware of special needs during prepregnancy and pregnancy
“MS itself does not have a lot of effects on fertility, pregnancy, or pregnancy outcomes,” Dr. Williams said. However, “medications cause concern about how we manage pregnancy and fertility.”
In vitro fertilization may increase the risk of relapse, she added, and patients on dimethyl fumarate who experience vomiting or diarrhea may not be able to properly absorb oral contraceptives.
Women with MS may not need to go off DMT when they are trying to conceive, she said. “If patients have very aggressive disease, they may need to be on DMT through conception, through the first trimester, and even the entire pregnancy to prevent long-term disability.”
What about pregnancy itself? “An MS diagnosis alone does not mean that a pregnancy is high risk,” she said. “There are not necessarily additional tests and ultrasounds that are recommended for our patients based on MS diagnosis alone.”
Treatment discontinuation may be warranted during pregnancy, when MS generally improves. However, some MS symptoms – fatigue, bladder dysfunction, and balance – may increase. Corticosteroids can be appropriate if relapses occur during pregnancy.
Menopause and MS symptoms may overlap
Symptoms such as hot flashes, mood changes, sleep disturbance, bladder dysfunction, and decreased energy may be signs of MS, or they could indicate menopause, Dr. Williams said. “Sometimes patients come in and they are getting worse, and we look into it and discover they are premenopausal.”
A decline in estrogen during menopause may worsen MS symptoms, she added, and hormone therapy may be appropriate. A phase 2 study found a benefit in menopausal patients with MS for estriol in conjunction with a DMT, but more studies are needed.
Dr. Williams reported no relevant financial disclosures.
SEATTLE – Mitzi Joi Williams, MD.
About three in four people with MS are female – about 750,000 in the United States. And the risk and incidence may be highest in African American women.
In a presentation about the unique needs of women with MS, Dr. Williams, an assistant professor of internal medicine at the Morehouse School of Medicine in Atlanta, offered these tips at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pay attention to sexual dysfunction
Patients with MS often are ashamed to talk about sexual dysfunction, Dr. Williams said, but it is on many minds. “If I have a program on intimacy in MS, people are out the door.”
She urged colleagues to understand that MS can affect sexuality through three routes: primary, secondary, and tertiary dysfunction.
In primary sexual dysfunction, brain and spinal lesions directly related to MS can cause problems such as lack of sensation or abnormal sensations, decreased libido, vaginal dryness, and difficult orgasm.
Secondary sexual dysfunction refers to problems caused by symptoms of MS such as fatigue, which can worsen as the day progresses and affect nighttime intimacy, she said. Bladder dysfunction is another sensitive area in sexuality, with patients – especially women – “concerned that they will lose control of their bladder or they have already lost control.”
Cognitive dysfunction also can disrupt sexual function. “It is important to focus, and certain things cannot happen if you do not. If you are not able to focus and concentrate, it can affect interest,” Dr. Williams said.
Additionally, medications can improve some symptoms while making others worse. For example, a drug may relieve spasticity but boost fatigue. “We have to walk this tightrope,” she said. “But if we are not asking our patients, they may not volunteer this information.”
Finally, she said, MS can spark tertiary sexual dysfunction – poor body image, depression, anxiety, and disruptive changes in familial roles. For example, one partner may become a caregiver, and “it is hard to go from caregiving to sexy time.”
“It is something we have to acknowledge and find ways to deal with,” Dr. Williams said.
To address these issues, she pointed to strategies for symptomatic relief and disease-modifying therapy (DMT) and pinpointed several treatment options.
- Fatigue – stimulants, diet, exercise.
- Spasticity – muscle relaxants, exercise.
- Bladder dysfunction – fluid restriction, medication.
- Paresthesia – antidepressants, anticonvulsants.
- Numbness – vibrators, devices to increase stimulation.
Sexual therapy, couples therapy, and pelvic floor physical therapy also can be helpful.
Be aware of special needs during prepregnancy and pregnancy
“MS itself does not have a lot of effects on fertility, pregnancy, or pregnancy outcomes,” Dr. Williams said. However, “medications cause concern about how we manage pregnancy and fertility.”
In vitro fertilization may increase the risk of relapse, she added, and patients on dimethyl fumarate who experience vomiting or diarrhea may not be able to properly absorb oral contraceptives.
Women with MS may not need to go off DMT when they are trying to conceive, she said. “If patients have very aggressive disease, they may need to be on DMT through conception, through the first trimester, and even the entire pregnancy to prevent long-term disability.”
What about pregnancy itself? “An MS diagnosis alone does not mean that a pregnancy is high risk,” she said. “There are not necessarily additional tests and ultrasounds that are recommended for our patients based on MS diagnosis alone.”
Treatment discontinuation may be warranted during pregnancy, when MS generally improves. However, some MS symptoms – fatigue, bladder dysfunction, and balance – may increase. Corticosteroids can be appropriate if relapses occur during pregnancy.
Menopause and MS symptoms may overlap
Symptoms such as hot flashes, mood changes, sleep disturbance, bladder dysfunction, and decreased energy may be signs of MS, or they could indicate menopause, Dr. Williams said. “Sometimes patients come in and they are getting worse, and we look into it and discover they are premenopausal.”
A decline in estrogen during menopause may worsen MS symptoms, she added, and hormone therapy may be appropriate. A phase 2 study found a benefit in menopausal patients with MS for estriol in conjunction with a DMT, but more studies are needed.
Dr. Williams reported no relevant financial disclosures.
EXPERT ANALYSIS FROM CMSC 2019
Reducing pediatric RSV burden is top priority
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.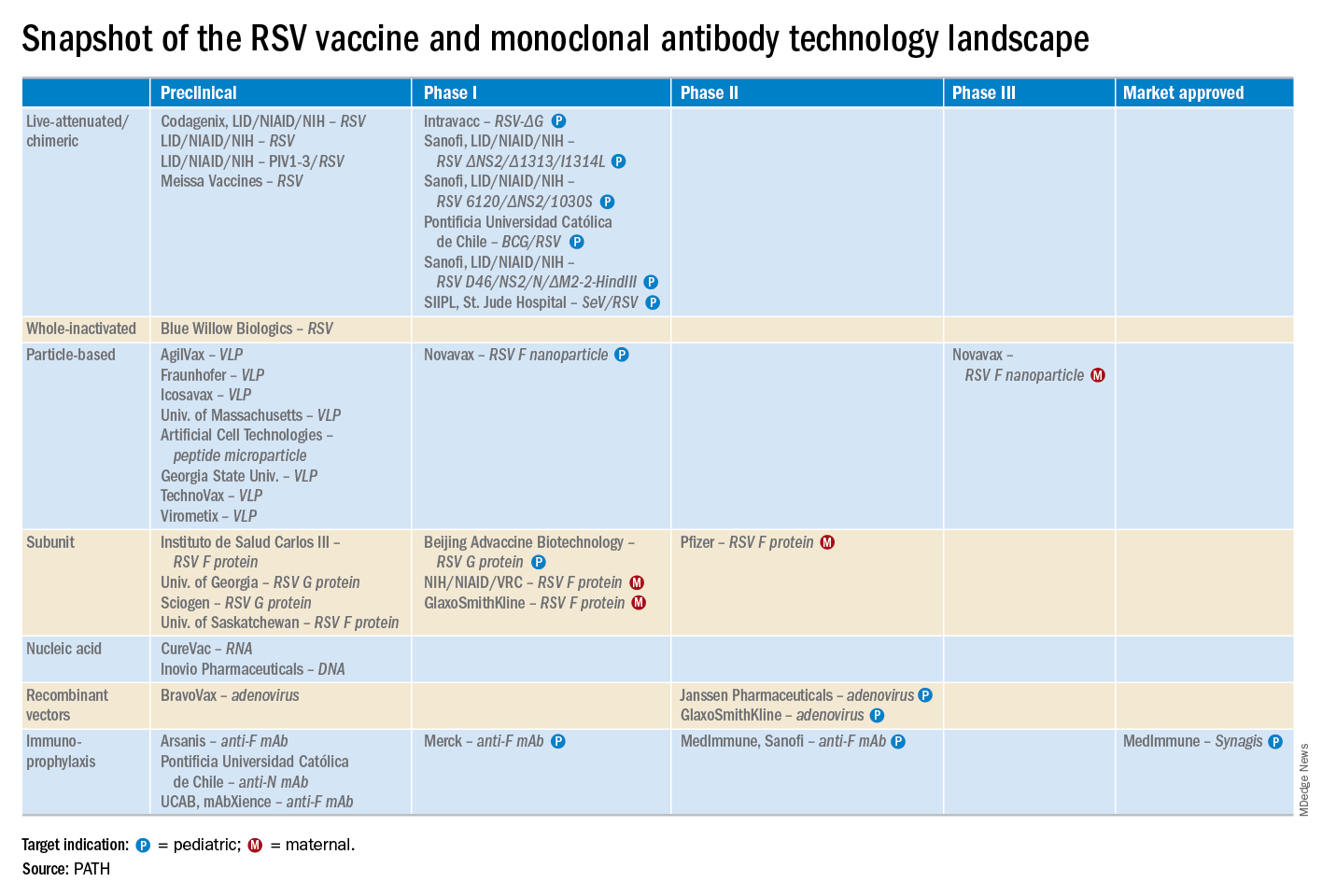
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
EXPERT ANALYSIS FROM ESPID 2019
USPSTF reaffirms HIV screening recommendations
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
FROM JAMA
Postpartum LARC uptake increased with separate payment
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
FROM JAMA
Diverse vaginal microbiome may signal risk for preterm birth
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
FROM NATURE MEDICINE
The benefits of first-trimester fetal heart evaluation
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.
Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.
In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
Considering congenital heart defects early
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.